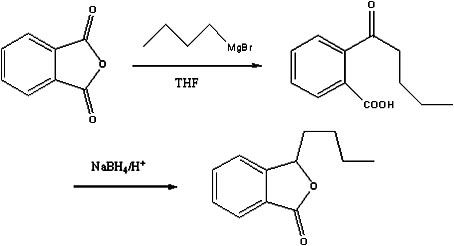
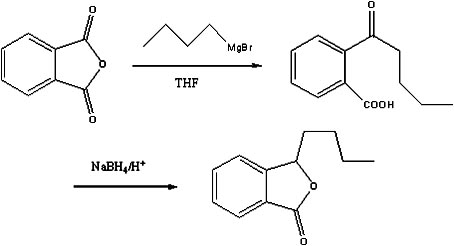
Preparation method of butylphthalide
A technology of apigenin A and phthalic anhydride, applied in the direction of organic chemistry, can solve the problems of unavailable raw materials, many reaction steps, cumbersome operation, etc., and achieve the effect of easy industrial production, few reaction steps, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1), Preparation of Bromobutane Grignard Reagent
[0021] Under the protection of nitrogen, add 200ml of tetrahydrofuran, 6g of magnesium flakes and 0.1g of iodine into a 500ml four-neck flask equipped with electromagnetic stirring, a thermometer and a reflux condenser with a drying tube. 31.5g of bromobutane, control the temperature not to exceed 70°C, after the dropwise addition, continue to stir for 1h to obtain the Grignard reagent of bromobutane;
[0022] (2), preparation of o-valerylbenzoic acid
[0023] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 30g of phthalic anhydride and 2.5g of copper iodide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of bromobutane dropwise, and the dropwise addition time After the dropwise addition, continue to stir for 2 hours, add 1mol / L hydrochloric acid solution to hydrolyze to pH 2, let stand to separate the water layer, extract the water layer twice with methyl tert-butyl e...
Embodiment 2
[0027] (1) Preparation of Chlorobutane Grignard Reagent
[0028] Under the protection of nitrogen, add 200ml of tetrahydrofuran, 6g of magnesium flakes and 0.1g of iodine into a 500ml four-neck flask equipped with electromagnetic stirring, a thermometer and a reflux condenser with a drying tube. 21.3g chlorobutane, the temperature is controlled not to exceed 70°C. After the dropwise addition, continue to stir for 1h to obtain the Grignard reagent of chlorobutane;
[0029] (2), preparation of o-valerylbenzoic acid
[0030] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 30g of phthalic anhydride and 2.5g of copper iodide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of chlorobutane dropwise, and the dropwise addition time After the dropwise addition, continue to stir for 2 hours, add 1mol / L hydrochloric acid solution to hydrolyze to pH 2, let stand to separate the water layer, extract the water layer twice with methyl tert-...
Embodiment 3
[0034] (1), Preparation of Bromobutane Grignard Reagent
[0035] Under the protection of nitrogen, add 200ml of tetrahydrofuran, 6g of magnesium flakes and 0.1g of iodine into a 500ml four-neck flask equipped with electromagnetic stirring, a thermometer and a reflux condenser with a drying tube. 31.5g of bromobutane, the temperature is controlled not to exceed 70°C, after the dropwise addition is completed, continue to stir for 1 hour to obtain the Grignard reagent of bromobutane;
[0036] (2), preparation of o-valerylbenzoic acid
[0037] Under the protection of nitrogen, add 300ml of tetrahydrofuran, 30g of phthalic anhydride and 2.5g of copper bromide into a 1L four-neck flask, cool to -20°C, add the above-mentioned Grignard reagent of bromobutane dropwise, and add the time After the dropwise addition, continue to stir for 2 hours, add 1mol / L hydrochloric acid solution to hydrolyze to pH 2, let stand to separate the water layer, extract the water layer twice with methyl te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com