Polyvinyl thioether ester as well as preparation method and application thereof
A technology of polyvinyl sulfide ester and polyvinyl sulfide, applied in the field of polyvinyl sulfide ester and its preparation, can solve problems such as limiting the application range of luminescent materials, achieve wide substrate applicability, eliminate influence, The effect of good functional group compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
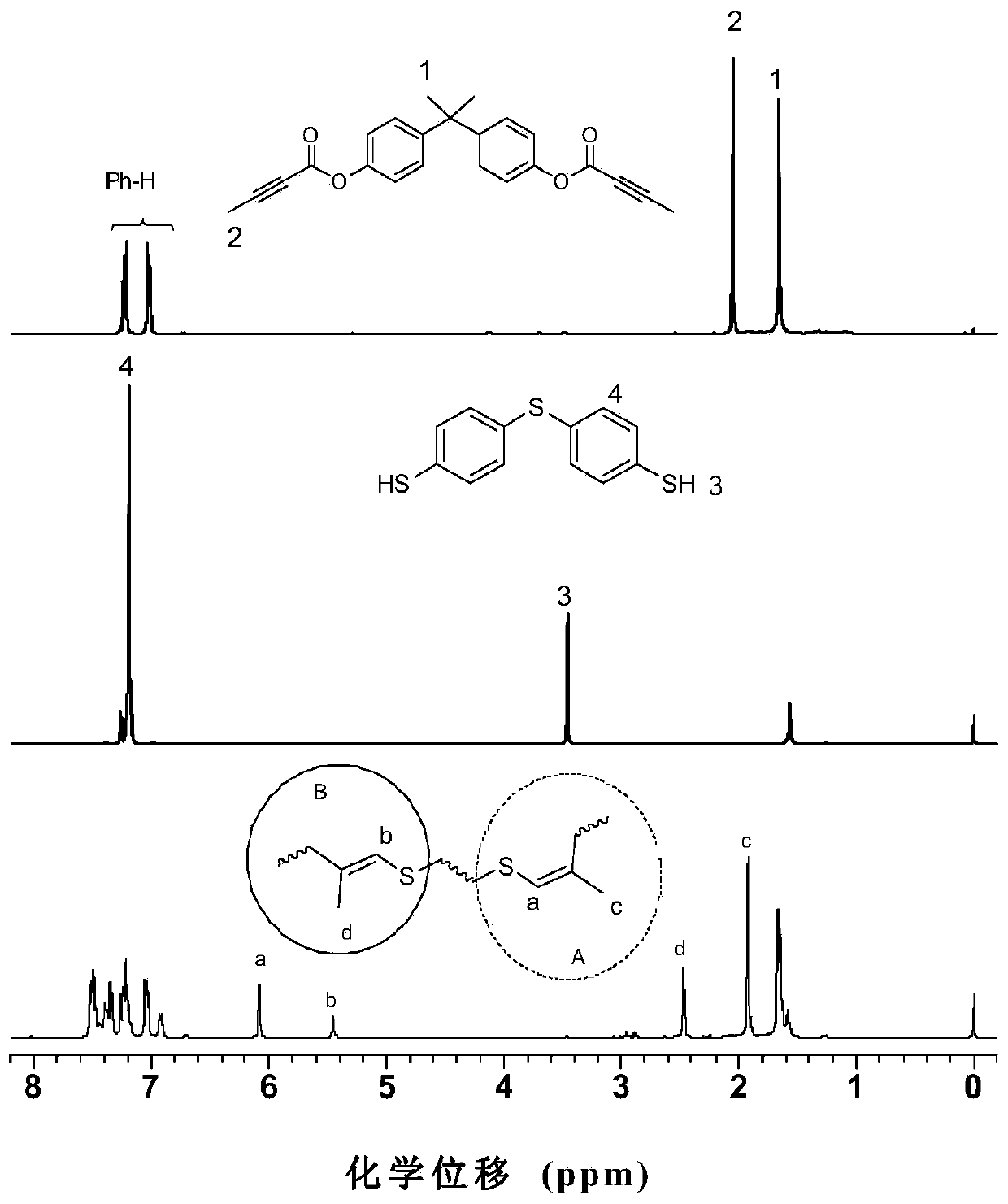
[0052] Synthesis of the first monomer dibasic butynolate monomer: add 2.3 g (10 mmol) bisphenol A, 6.2 g (30 mmol) N,N-dicyclohexylcarbonyl in a 250 mL two-necked flask Amine (DCC), 0.5 g (4 mmol) 4-dimethylaminopyridine (DMAP), 0.8 g (4 mmol) p-toluenesulfonic acid hydrate, vacuum three times with nitrogen, add 80 mL of dry dichloromethane and stir to dissolve , to get the reaction system. Then, at 0°C, 2.0 g (25 mmol) butynoic acid was dissolved in 20 mL of dry dichloromethane, and dropped into the above reaction system through a constant pressure dropping funnel, stirred overnight, filtered, and dichloromethane Washing with methane, spinning the filtrate to get the crude product, which was separated and purified by column chromatography, and dried in vacuum to constant weight to obtain 2.6 g of white solid (72% yield), which is the first monomer dibasic butynoic acid phenol ester monomer. 1 H NMR (400 MHz, CDCl 3 ) δ7.24 (t, J = 9.6 Hz, 4H), 7.04 (t, J = 11.3...
Embodiment 2
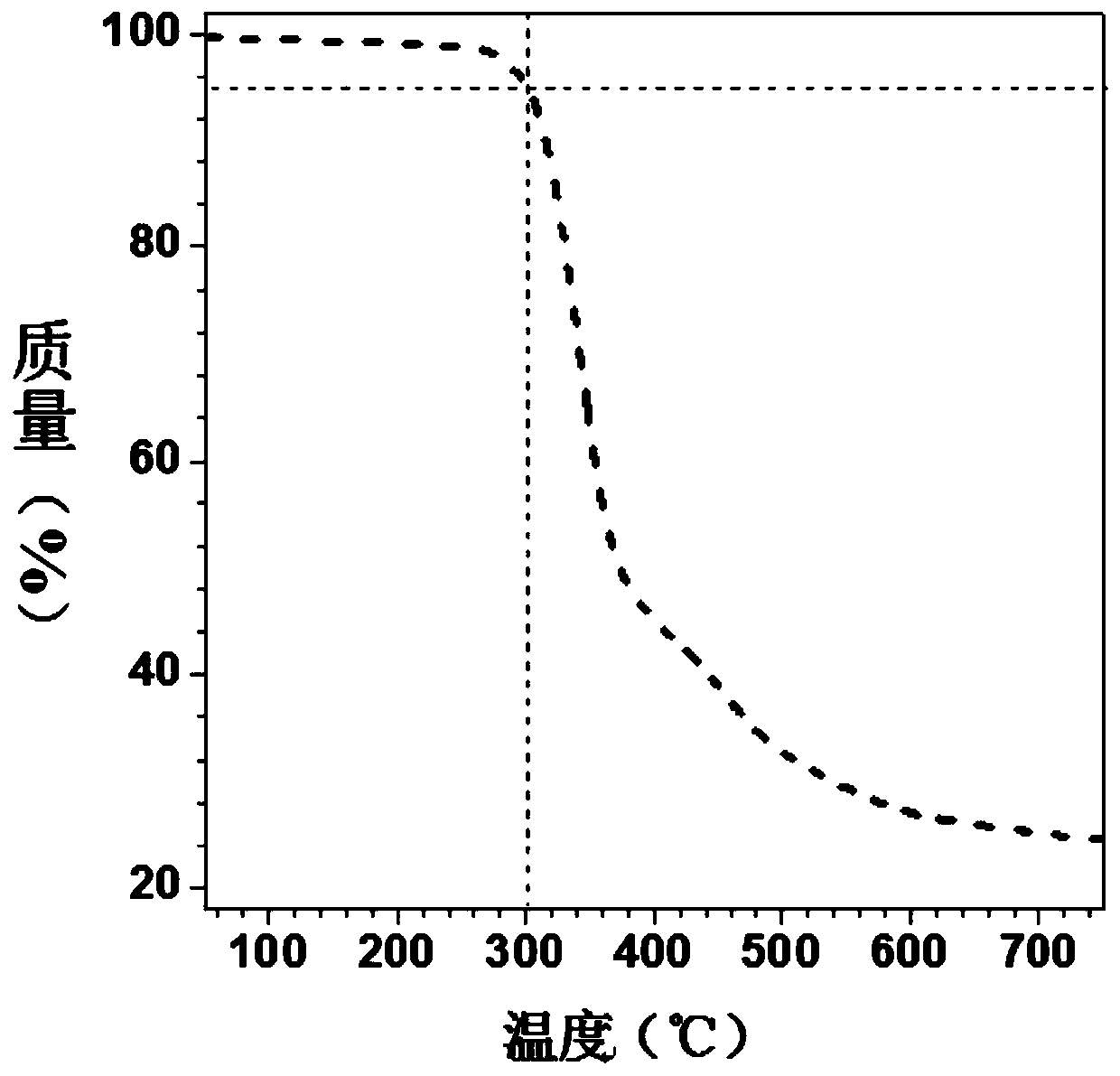
[0057] The dibasic butynolate monomer and the dibasic thiophenol monomer are the same as in Example 1. Add 36.0 mg (0.1 mmol) of the first monomer and 25.0 mg (0.1 mmol) of the second monomer into a 10 mL polymerization tube, vacuumize the system and fill it with nitrogen, repeat three times, then add 2 mL of dry DMF, and wait for a single After the body was completely dissolved, it was reacted at 60 °C for 24 hours. After cooling to room temperature, dilute with 5 mL of chloroform, and drop the solution into 250 mL of vigorously stirred n-hexane through a dropper plugged with cotton, let it stand, filter, and dry to constant weight to obtain a polymer, the product The rate is 82%. GPC results show: M w = 13800, PDI = 2.2. The polymer also has good solubility and thermal stability, and the 5% thermal weight loss temperature is 298 ℃.
Embodiment 3
[0059] The dibasic butynolate monomer and the dibasic thiophenol monomer are the same as in Example 1. Add 36.0 mg (0.1 mmol) of the first monomer and 25.0 mg (0.1 mmol) of the second monomer into a 10 mL polymerization tube, vacuumize the system and fill it with nitrogen, repeat three times, then add 1 mL of dry DMF, and wait for a single After the body was completely dissolved, react at 60 °C for 24 hours. After cooling to room temperature, dilute with 5 mL of chloroform, and drop the solution into 250 mL of vigorously stirred n-hexane through a dropper plugged with cotton, let it stand, filter, and dry to constant weight to obtain a polymer, the product The rate is 77%. GPC results show: M w = 18600, PDI = 2.3. The polymer also has good solubility and thermal stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com