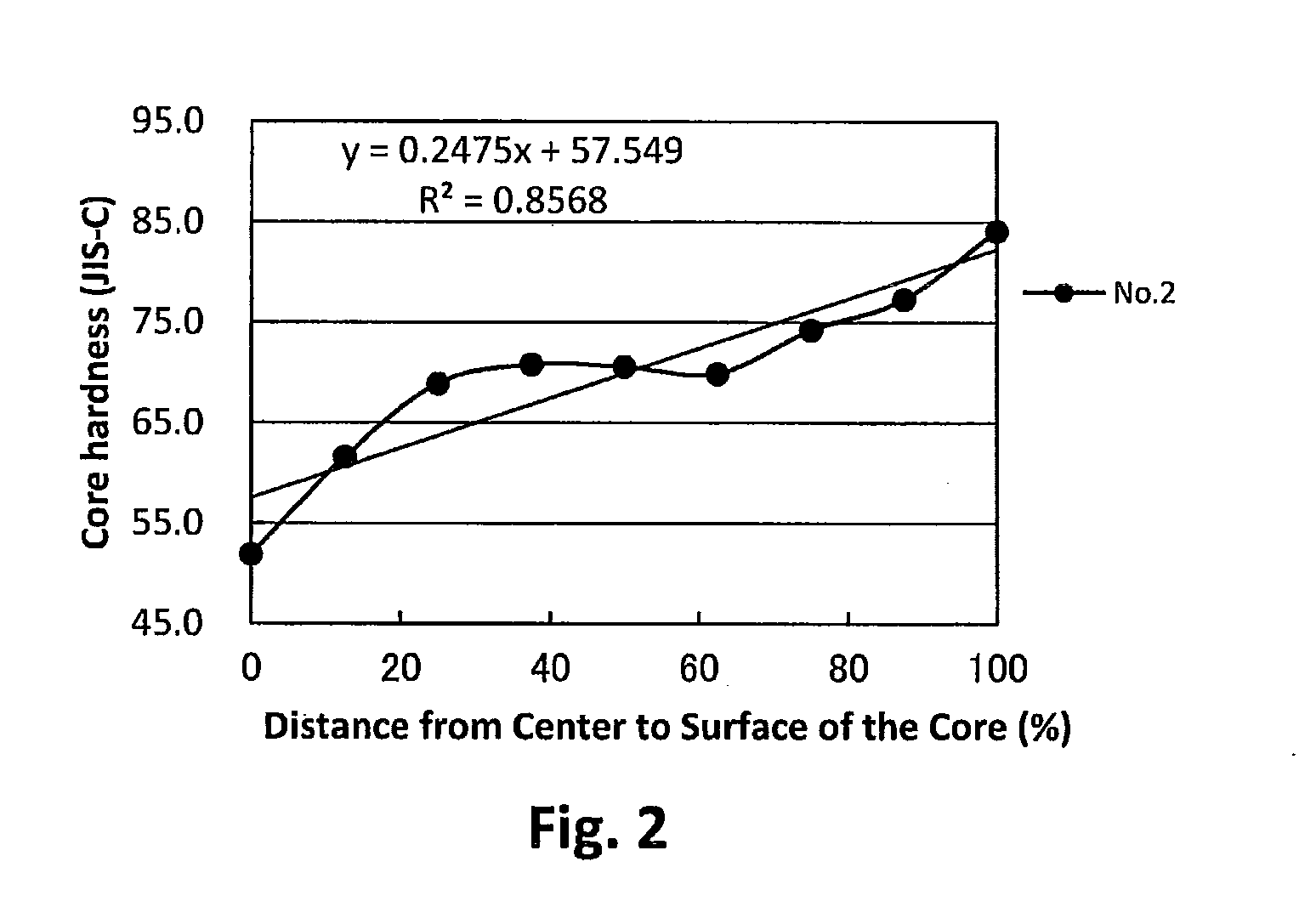
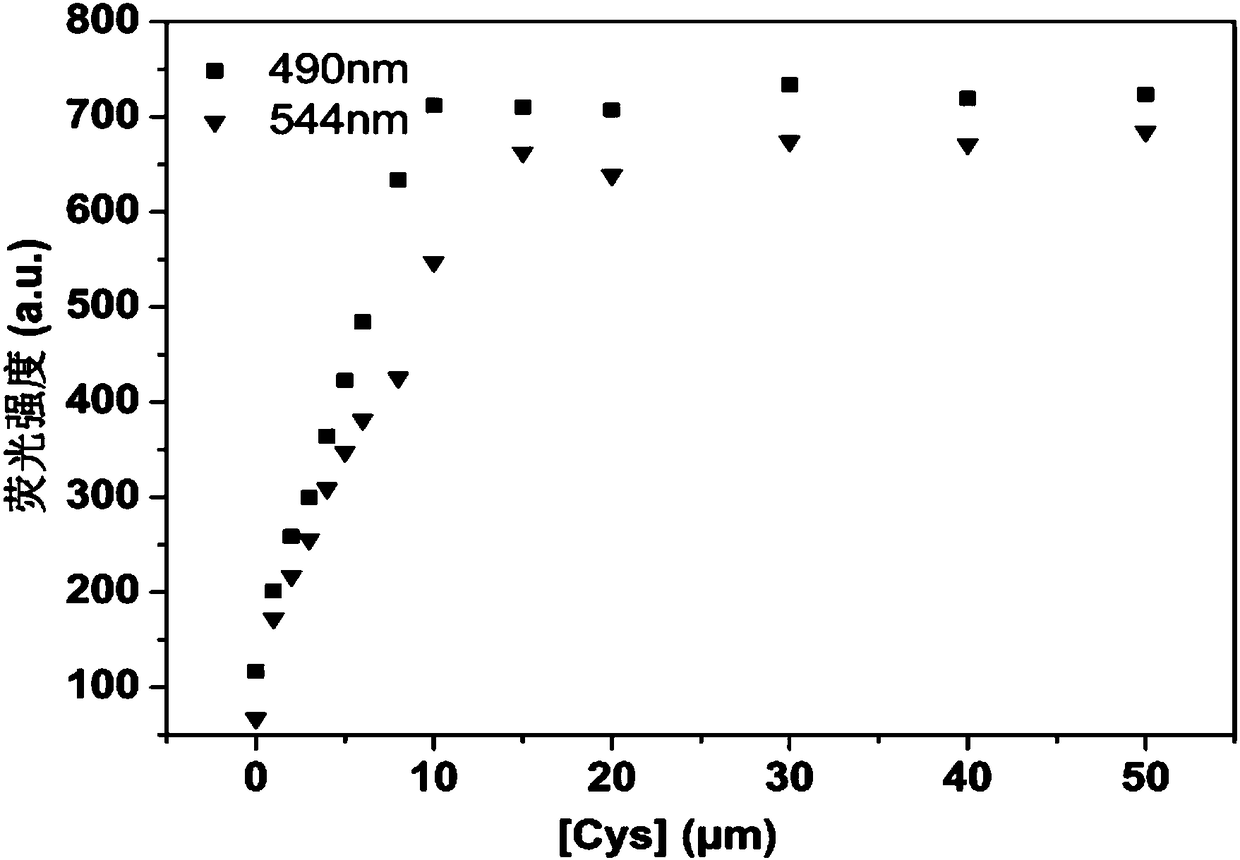
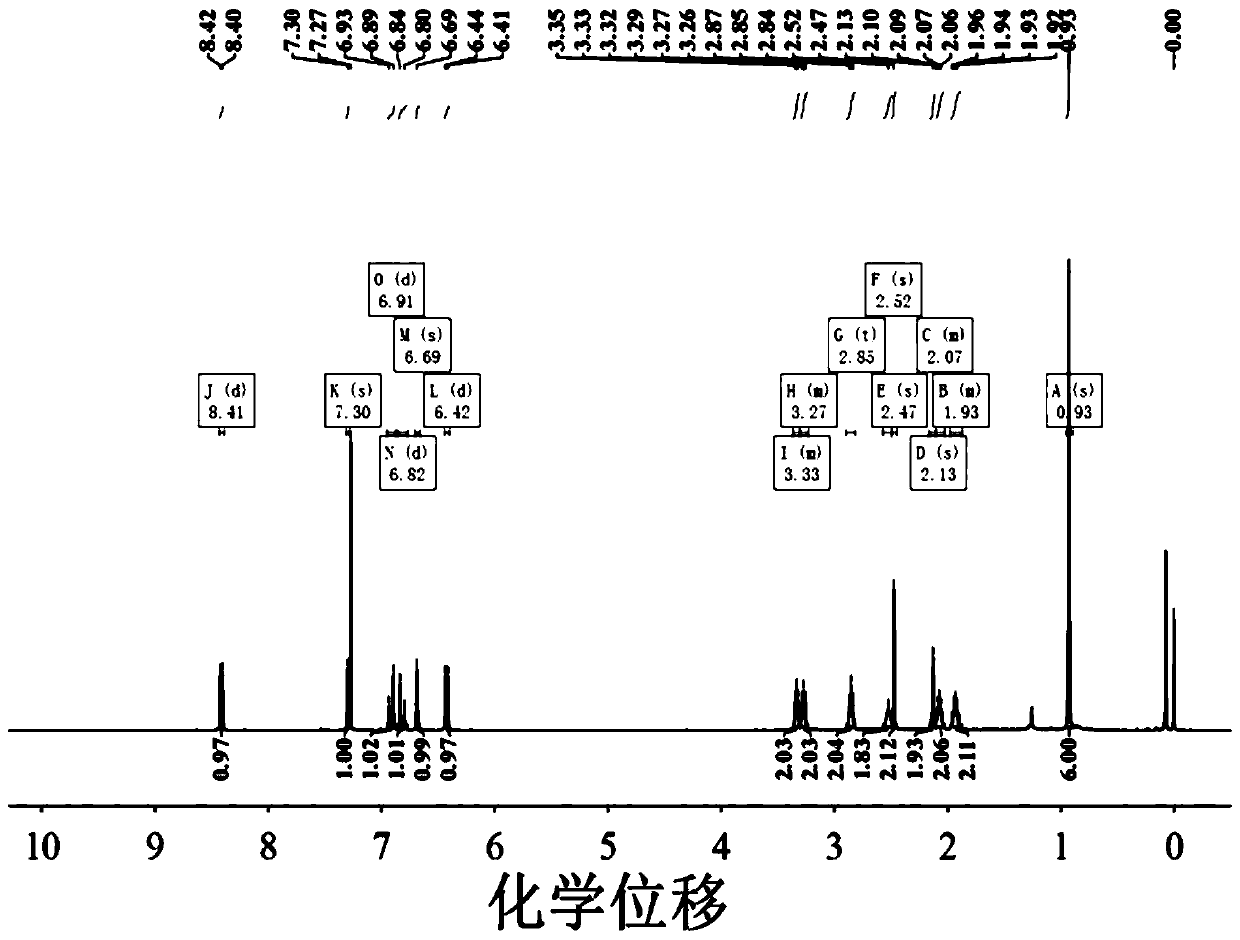
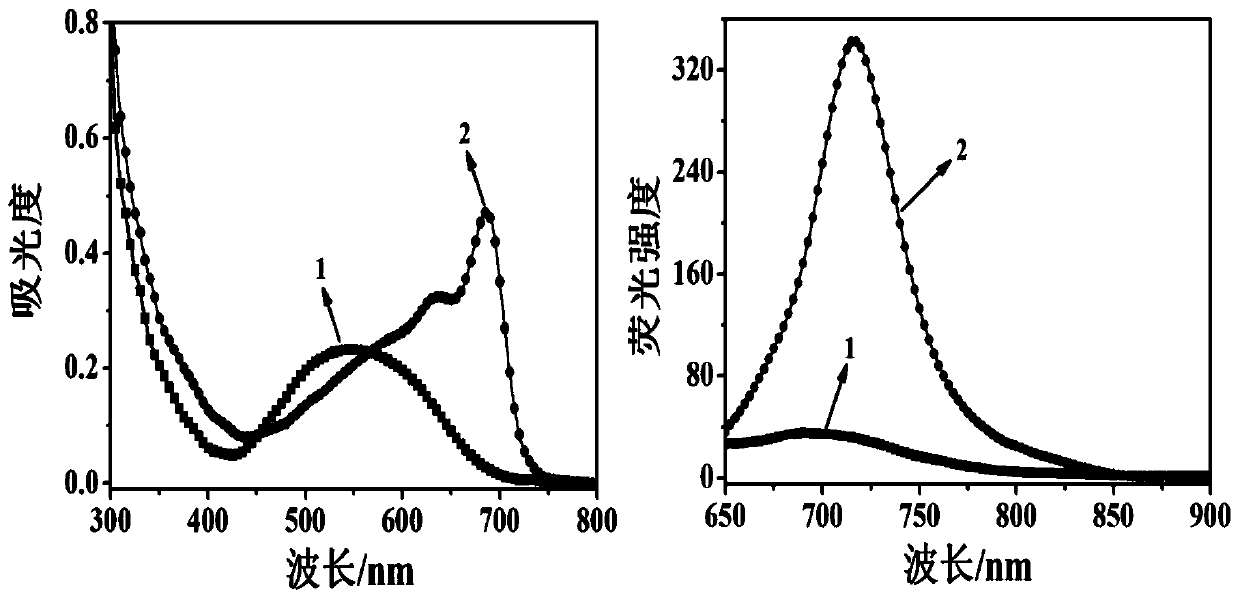
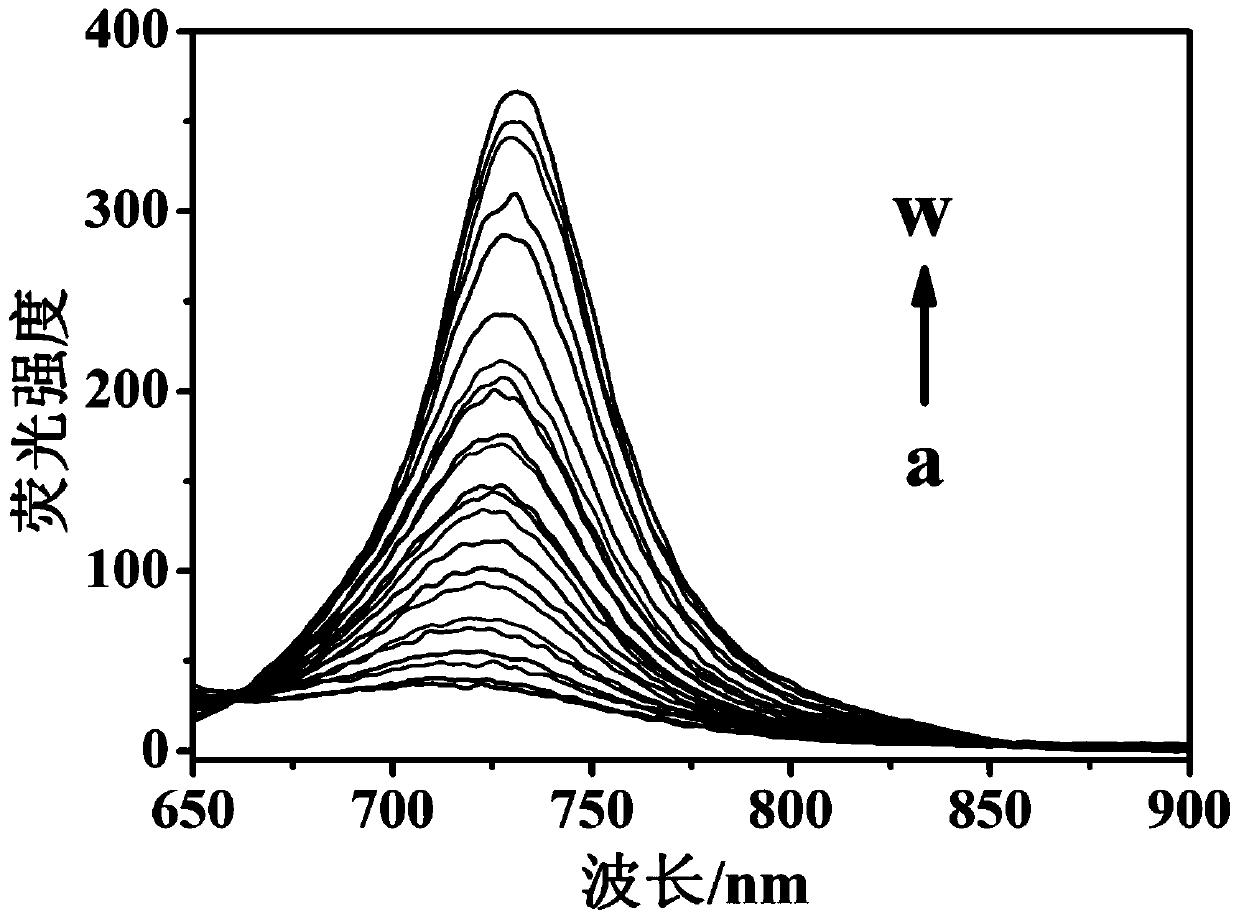
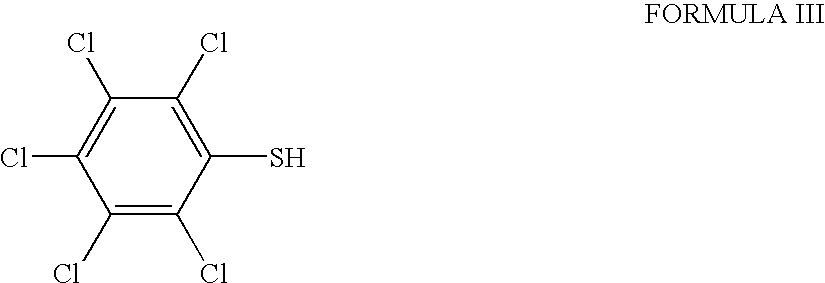Patents
Literature
636 results about "Thiophenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
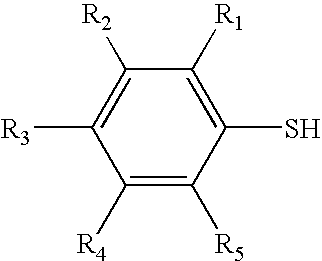
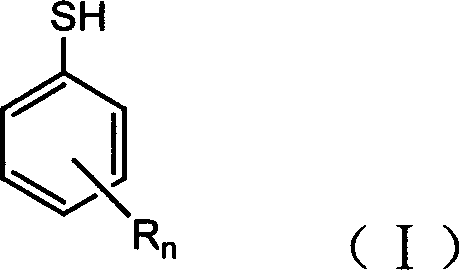
Thiophenol is an organosulfur compound with the formula C₆H₅SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols except the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
Powder coating fluoropolymer compositions with aromatic materials
Provided is a composition comprising an aromatic material selected from a polythiol aromatic compound or resin, a hydroxythiophenol compound or resin, a catechol novolak resin, a catechol cresol novolak resin, a polyhydroxy aromatic resin or compound comprising at least one aromatic ring having at least one hydroxyl group attached directly to the aromatic ring wherein at least one hydroxyl group is a phenolate salt, or a combination thereof, and a salt former compound capable of forming a salt with the aromatic material, a fluoropolymer, and optionally a phase transfer catalyst. Also provided are articles comprising powder-coated fluoropolymers with aromatic compounds, and methods for making the compositions and articles.
Owner:3M INNOVATIVE PROPERTIES CO
Dark blue organic light-emitting material and preparation method and application thereof
InactiveCN110790782AHigh color purityImprove stabilitySilicon organic compoundsSolid-state devicesBond energyULTRAMARINE BLUE
The invention discloses a dark blue organic light-emitting material and a preparation method and application thereof. The dark blue organic light-emitting material contains a structural unit disclosedin the invention, wherein, M is B or Bi; X is O, S or NR4; R1-R4 are independently selected from connecting bonds or groups obtained from H-H, H-F, H-O-H, H-S-H, H-CN, saturated hydrocarbons, unsaturated hydrocarbons, fluorinated hydrocarbons, heterocyclic compounds, organoboron, organosilicone, alcohols, mercaptans, ethers, thioethers, phenols, thiophenol, aldehydes, ketones, amines, amides, nitriles or sulfones losing one or more H; R1-R3 are located at any substitution position on rings of the structural unit where R1-R3 are located, and the bond energy between the ring where R3 is locatedand M is greater than or equal to the bond energy between the ring where R2 is located and M. The dark blue organic light-emitting material containing the B / Bi-N main body structure has very narrow light-emitting spectrum and TADF properties; the color purity is high, and the stability is good.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
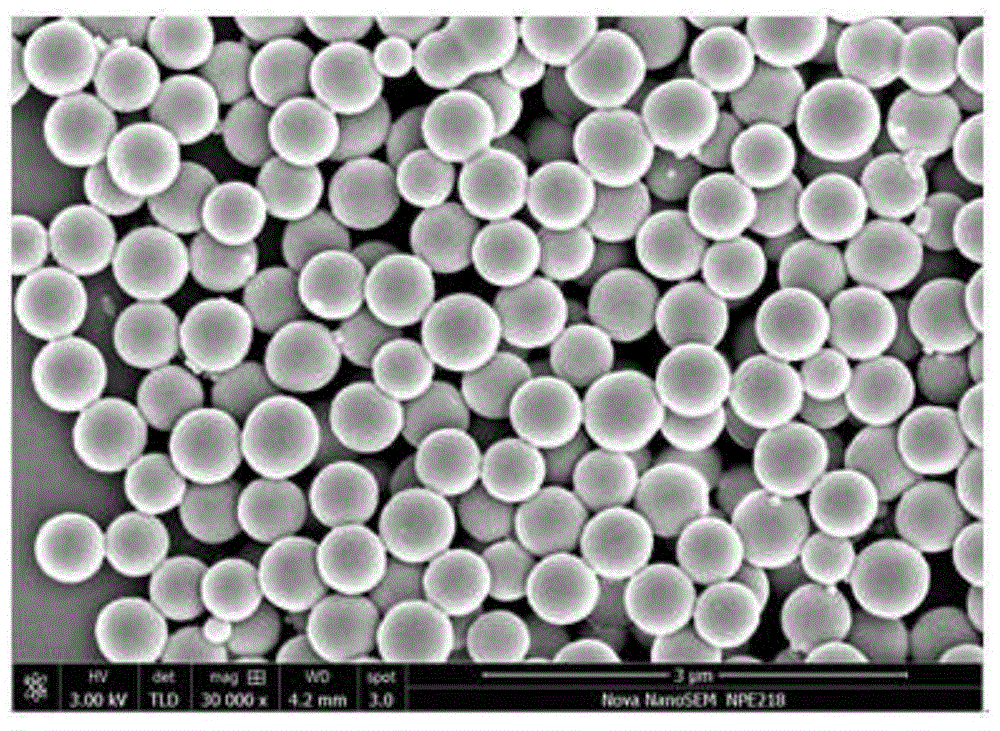
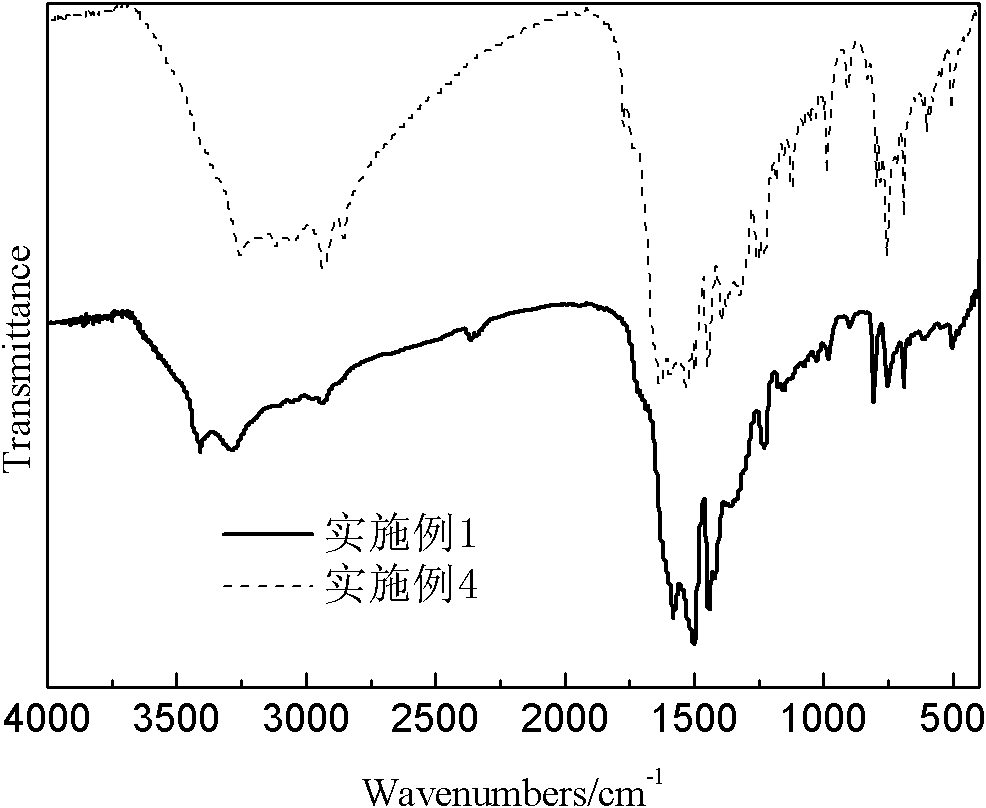
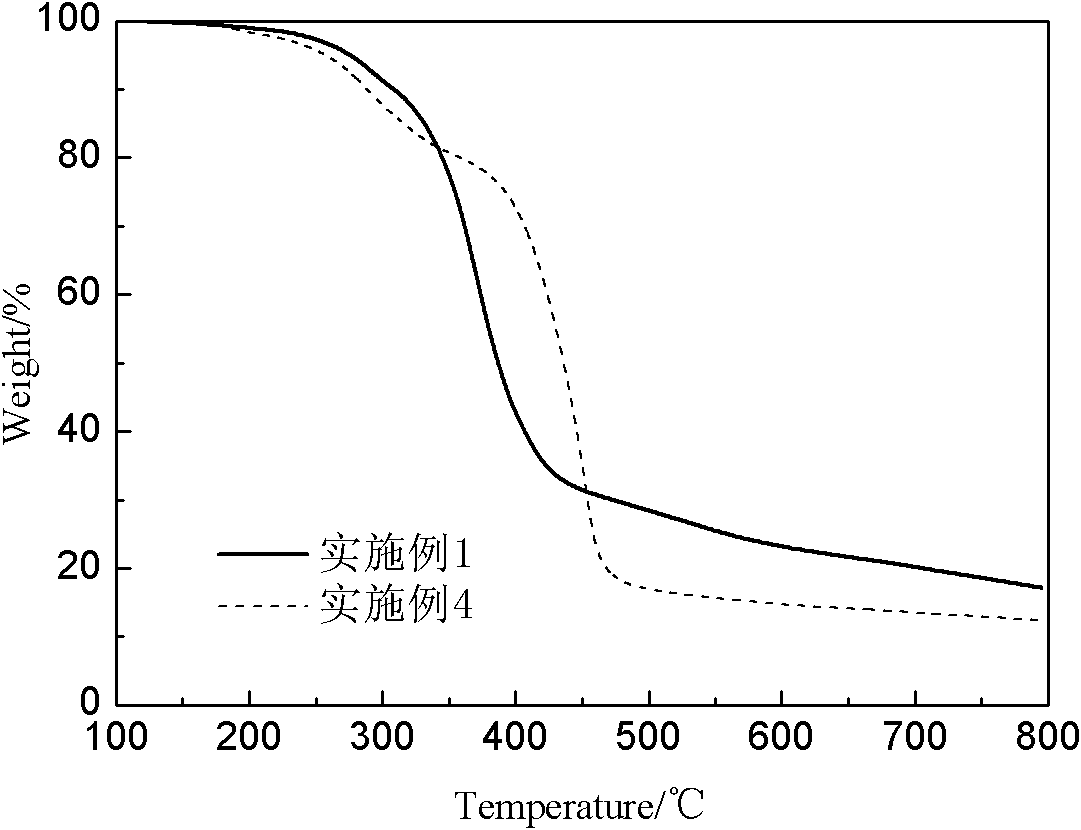
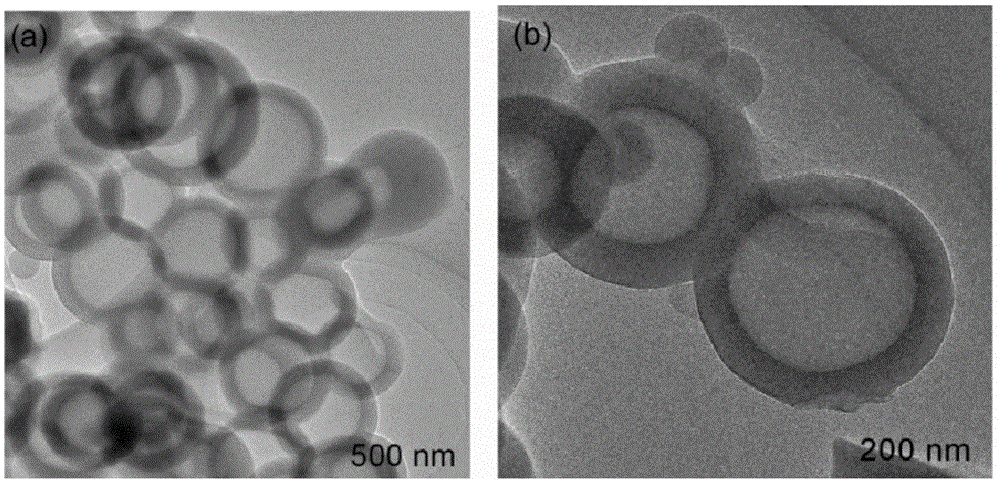
Highly cross-linked polyphosphazene hollow microspheres as well as preparation method thereof
The invention relates to highly cross-linked polyphosphazene hollow microspheres as well as a preparation method thereof, belonging to the technical field of organic micro-nanometer materials. The preparation method comprises the following steps: dissolving 4, 4-disulfydryl dithiophenol, phosphonitrilic chloride trimer and an acid-binding agent in an organic solvent for reaction; and after reaction, carrying out centrifugal separation on a solid product and washing and drying the solid product to obtain the polyphosphazene hollow microspheres. The highly cross-linked polyphosphazene hollow microspheres provided by the invention are prepared by a one-pot method by means of self-assembly of the reactant to a reactive template while a cross-linking agent is introduced, so that the whole preparation method is convenient and concise to implement.
Owner:SHANGHAI JIAO TONG UNIV
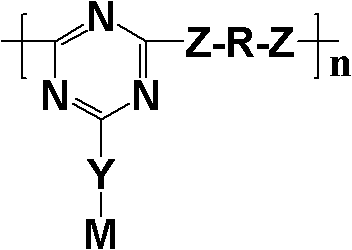
Novel expansion-type charring agent for flame-retardant polyolefine material and synthesis method thereof
InactiveCN102161763AAvoid separation and purificationEasy to separate and purifySynthesis methodsPhenol
The invention discloses a expansion-type charring agent for a flame-retardant polyolefine material and a synthesis method thereof. The synthesis method comprises the following steps: by using cyanuric chloride as an initial raw material, dropwisely adding amine containing benzene ring, phenol or thiophenol and an acid binding agent into an ice bath to obtain a monosubstituted compound; dropwisely adding aliphatic diamine or aliphatic dibasic alcohol and an acid binding agent, heating to 40-60 DEG C to react, thereby obtaining a disubstituted compound; and finally, dropwisely adding the aliphatic diamine or aliphatic dibasic alcohol and the acid binding agent, heating to 80-110 DEG C, refluxing with a condenser, cooling, washing, and drying to obtain the expansion-type charring agent containing benzene ring, triazine ring and diamino or dialkoxy group. The reaction process adopts a one-pot method, and thus, the invention has the advantages of simple technique, short reaction time and environmental protection in the preparation process; the product has the advantages of high thermal stability, favorable charring effect, low water absorptivity and favorable compatibility with alkene polymers; and after being compounded with ammonium polyphosphate (APP), the product is applicable to polyolefin materials, and has favorable flame-retardant effect.
Owner:SUN YAT SEN UNIV
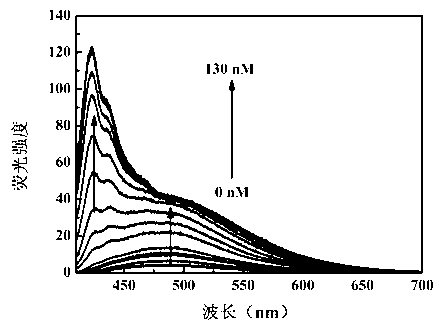
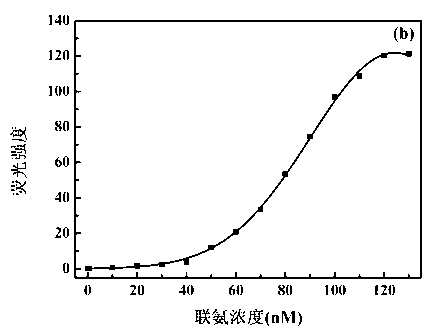
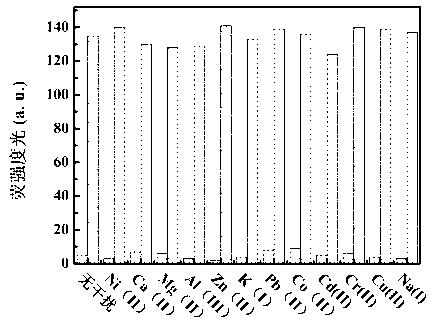
Benzothiazole-cyanophenyl compound serving as hydrazine fluorescence probe as well as preparation method and application method of benzothiazole-cyanophenyl compound
ActiveCN103214428ASimple preparation processConjugate plane largeOrganic chemistryFluorescence/phosphorescenceIndustrial waste waterStructural formula
The invention discloses a benzothiazole-cyanophenyl compound serving as a hydrazine fluorescence probe. The benzothiazole-cyanophenyl compound has a structural formula as shown in (I); the compound is prepared by performing cyclodehydration with bromobenzaldehyde and 2-amino-4-chloro thiophenol serving as the raw materials, then performing coupled reaction in order to connect with a bromobenzaldehyde derivate, and finally performing Knoevenagel reaction with malononitrile. The benzothiazole-cyanophenyl compound has the advantages that the raw materials are low in price and easy to gain, the synthetic route is simple, and the yield is relatively high; rigid structures such as benzothiazole and phenylacetylene groups are introduced into such a fluorescence probe, thus high fluorescence quantum efficiency is realized, and relatively high thermal stability and dissolubility are brought. The probe adopts the photoinduced charge transfer mechanism and the conjugate passivation mechanism, therefore, a response range respect to hydrazine can be expanded; the probe has the characteristics of being fast in response, high in sensitivity and high in selectivity, is suitable for being applied to safety detection of foods as well as safety detection of a laboratory, in particular applied to industrial wastewater monitor; and the probe has a wide application prospect in environment monitoring, ecological protection, disease diagnosis, industrial production and pollution discharge inspection.
Owner:浙江富昇科技有限公司
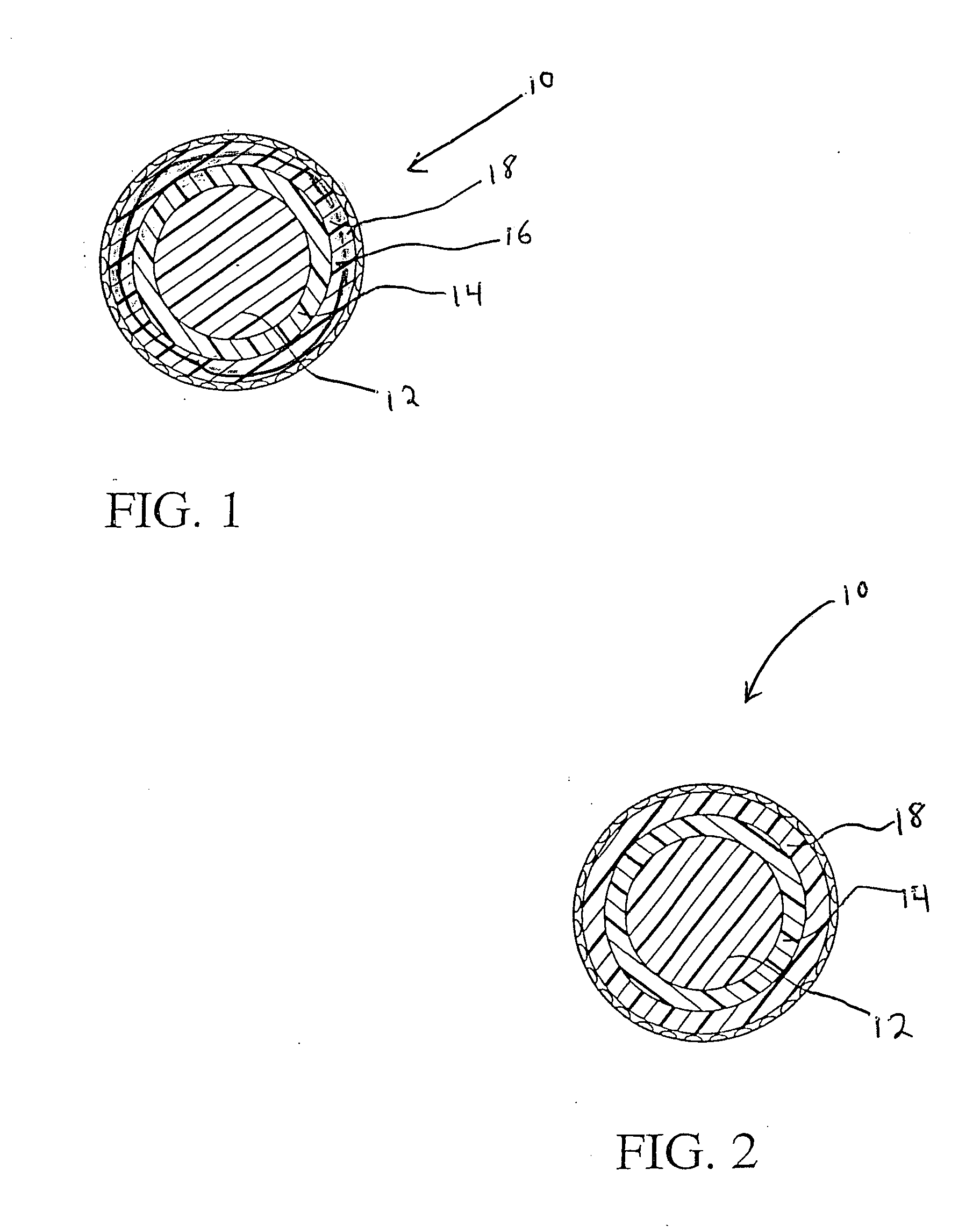
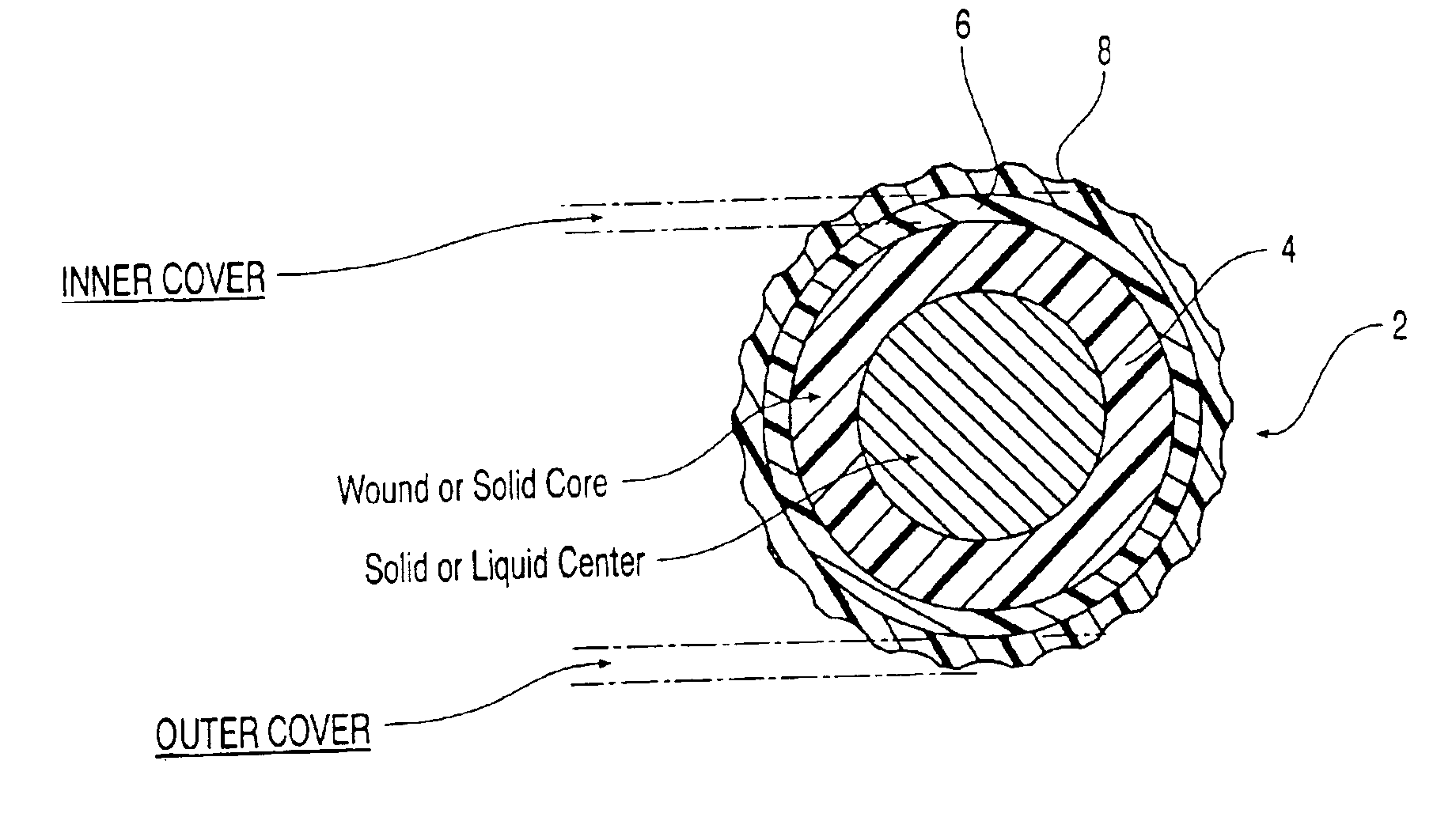
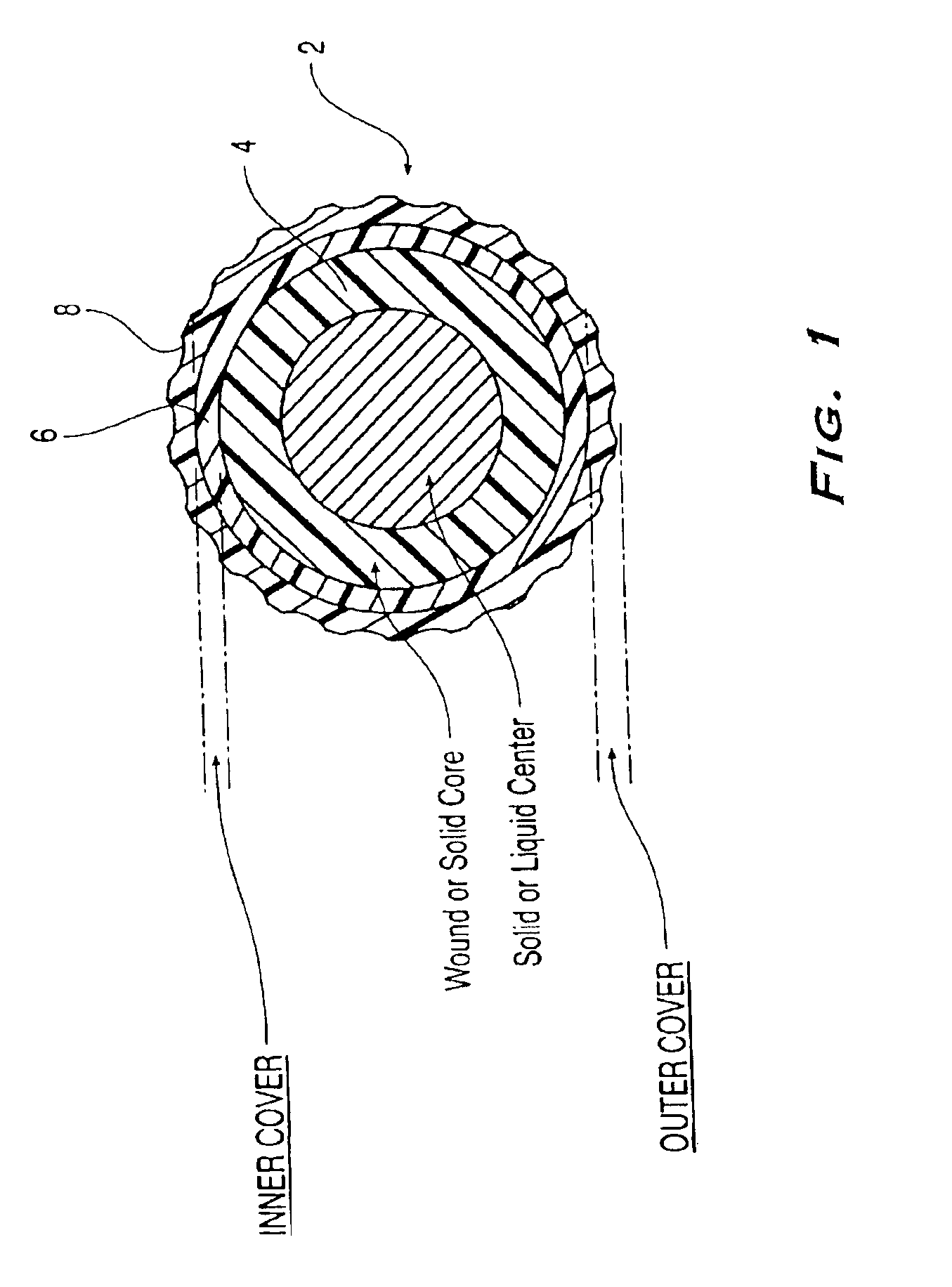
Golf ball
An elastomeric composition for forming a golf ball or a component thereof is disclosed that includes the use of a metal thiosulfate, either alone or in combination with one or more halogenated organic sulfur compounds, such as halogenated thiophenol (HTP), or salts thereof. The composition produces a molded product exhibiting an enhanced combination of increased compression (i.e., softness) and / or resilience (C.O.R.).
Owner:TOPGOLF CALLAWAY BRANDS CORP
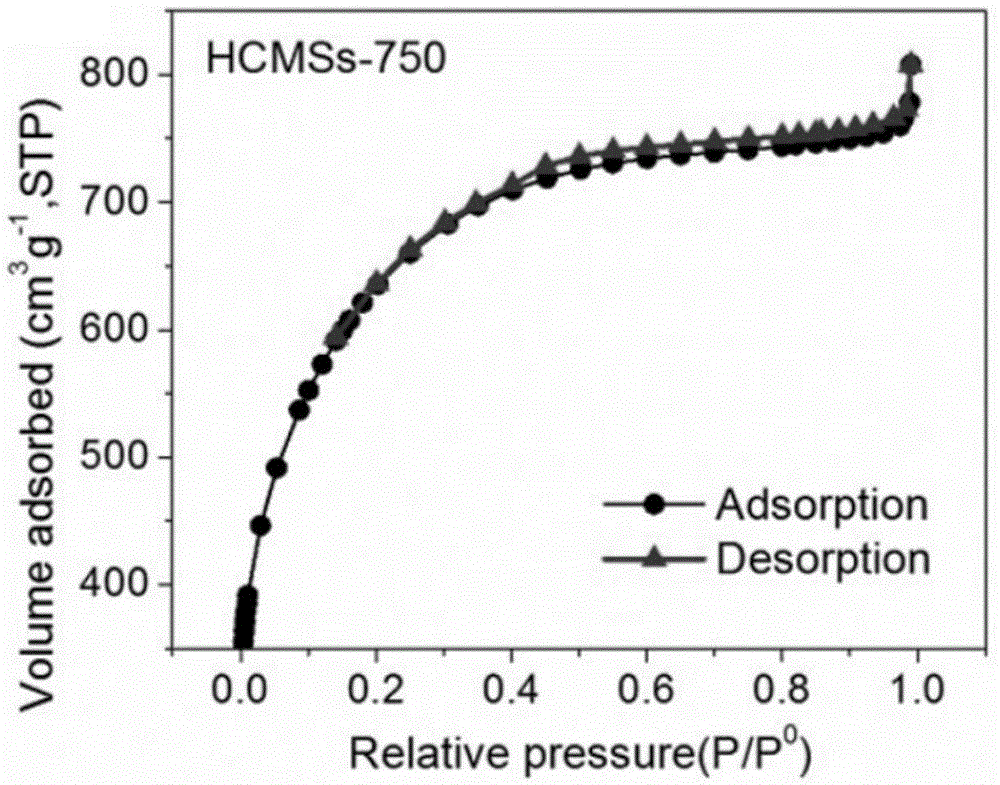
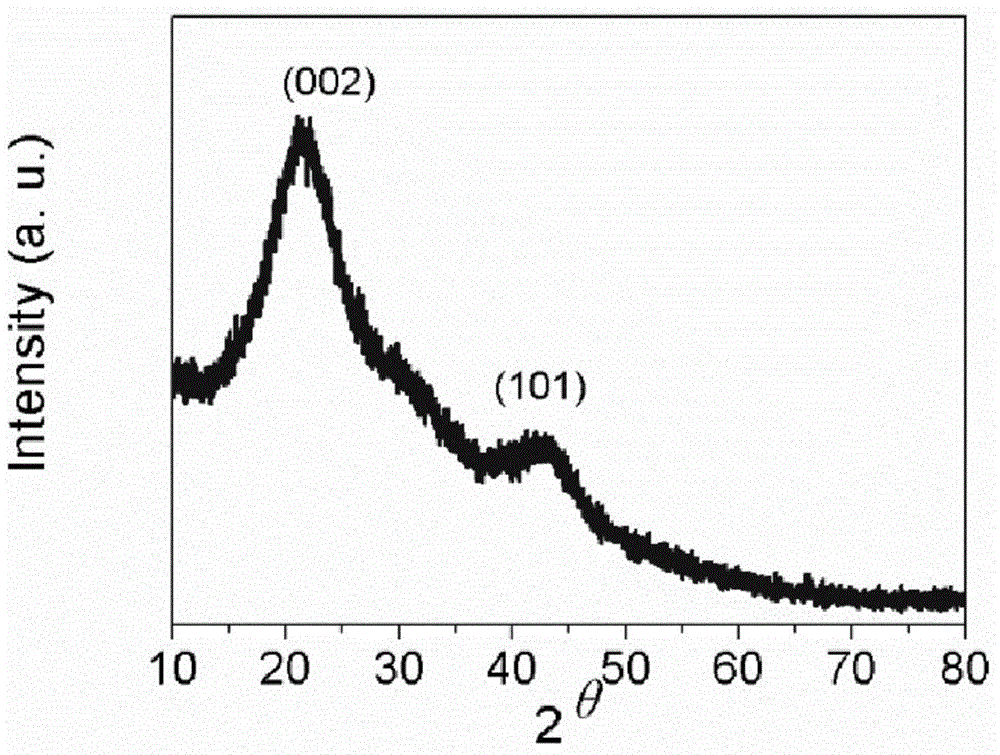
Heteroatom doped hollow porous carbon microspheres and preparation method thereof
The invention provides heteroatom doped hollow porous carbon microspheres and a preparation method thereof. A micro-nano capsule formed by 4,4'-thiobisbenzenethiol and triethylamine is taken as a template, hollow polyphosphazene microspheres are prepared by coating and modifying the surface of the template through polyphophazene, finally, carbonization and activation treatment are performed in an inert atmosphere, and the heteroatom doped hollow porous carbon microspheres are obtained. The following complicated template removal process of a hard template method is avoided; a large quantity of non-carbon atoms in the polyphophazene molecular structure provide a necessary condition for formation of the heteroatom doped structures in the hollow carbon microspheres. According to the preparation method, the yield is high, the material structure is uniform, the specific surface area is large, and the preparation method is an efficient method for preparation of the heteroatom doped hollow porous carbon microspheres.
Owner:上海鸿鹄骥航科技有限公司
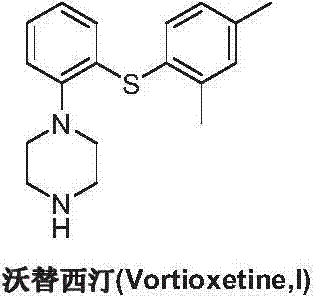
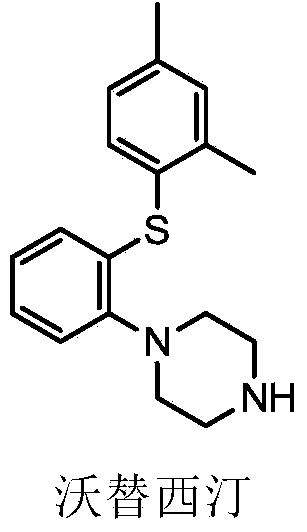
Preparation method of vortioxetine
ActiveCN103788019AEase of industrial productionEco-friendly economyOrganic chemistryNitrobenzeneAniline
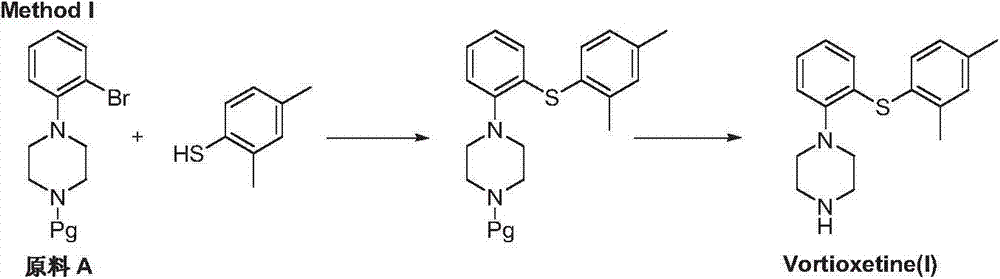
The invention discloses a preparation method of vortioxetine (I). The preparation method comprises the following steps: subjecting 2-substituted thiophenol shown in a formula (II) and 2,4-dimethylphenyl halide shown in a formula (III) to condensation to generate 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV) or 2-(2,4-dimethylphenylthioalkyl)aniline (V) which is obtained by reducing the 2-(2,4-dimethylphenylthioalkyl)nitrobenzene (IV), and subjecting the 2-(2,4-dimethylphenylthioalkyl)aniline (V) and a compound shown in a formula (VI) to cyclization under alkaline conditions to obtain the vortioxetine (I). The preparation method is accessible in raw materials, is concise in process, is economical and environment-friendly and is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
Specific fluorescence probe for identifying thiophenol and application of specific fluorescence probe
ActiveCN104531136AStrong specificityHigh sensitivityOrganic chemistryFluorescence/phosphorescenceChemical synthesisN dimethylformamide
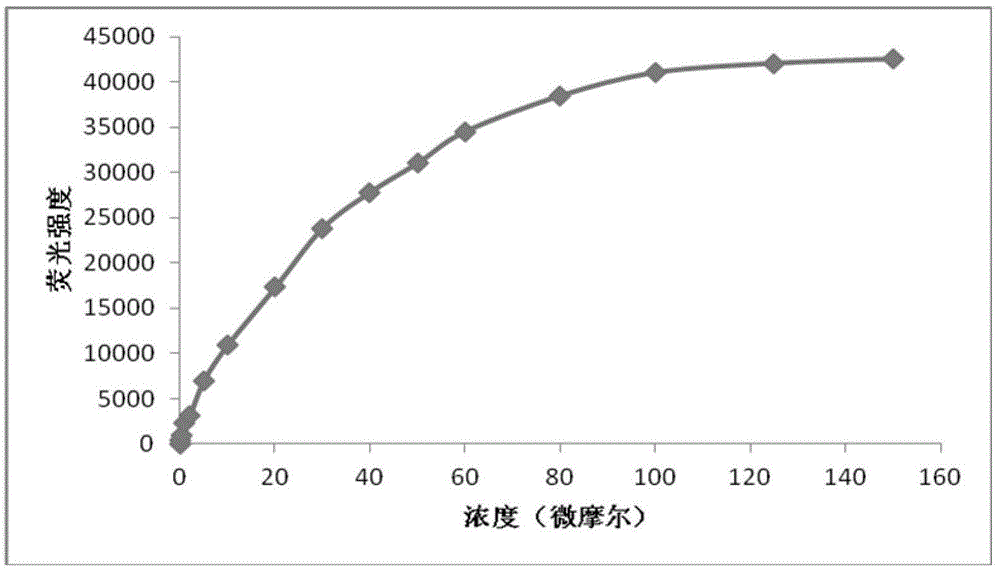
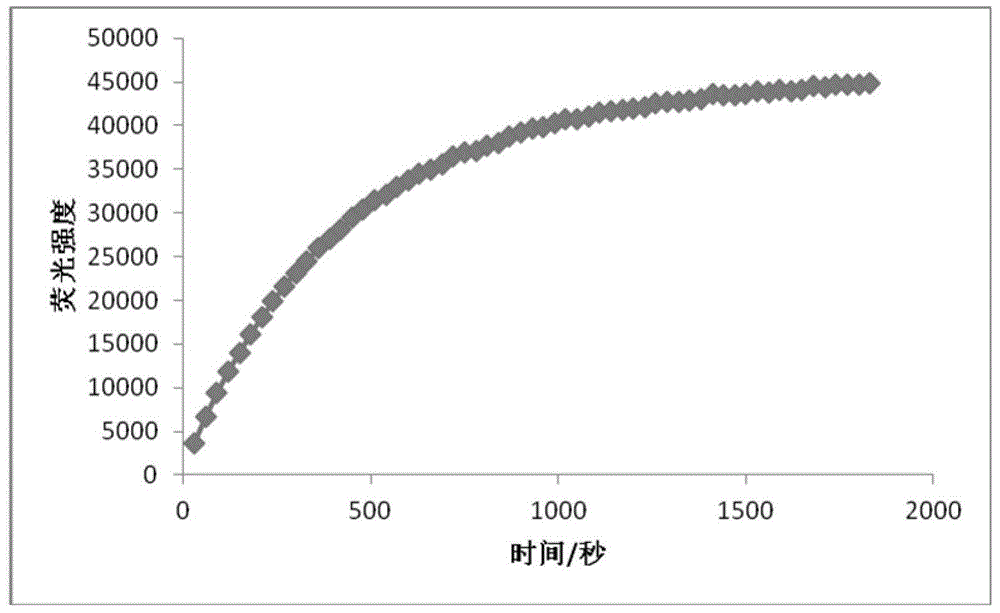
The invention discloses a specific fluorescence probe for identifying thiophenol and application of the specific fluorescence probe and belongs to the field of fine chemical industry. The specific fluorescence probe is a derivative of 2-benzothiazole-6-naphthol and is prepared by the following steps: mixing the 2-benzothiazole-6-naphthol and 2, 4-dinitrobenzene into an N, N-dimethylformamide solution according to a proportion, heating, and finally, purifying by adopting silica gel chromatography to obtain the fluorescence probe. The fluorescence probe and a corresponding thiophenol content detecting process can not be interfered by matrixes and impurities in a biological system and can be used for quantitatively determining the thiophenol content in various biological systems. The specific fluorescence probe is high in specificity, can be hydrolyzed after being acted with the thiophenol, namely an ether bond is fractured; is cheap and is easy in acquisition, can be obtained by chemical synthesis, is simple and feasible in synthetic process; is high in sensitivity, is good in fluorescence attribute. A hydrolysate can be excited by a two-photon laser with 800nm as an excitation light source, a biological sample is weak in background fluorescence, and the specific fluorescence probe is suitable for detecting the thiophenol content in cells and is capable of quantitatively determining the thiophenol by drawing a standard curve.
Owner:CHANGSHU RES INST OF DALIAN UNIV OF TECH CO LTD
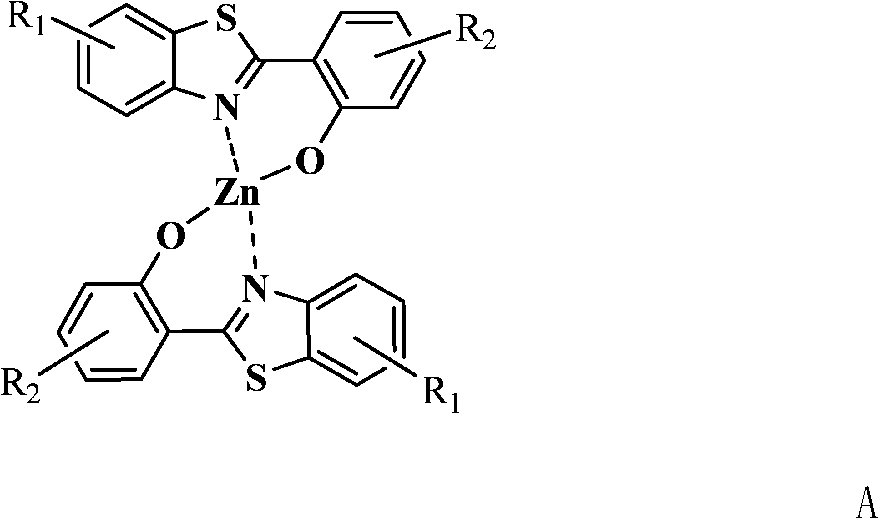
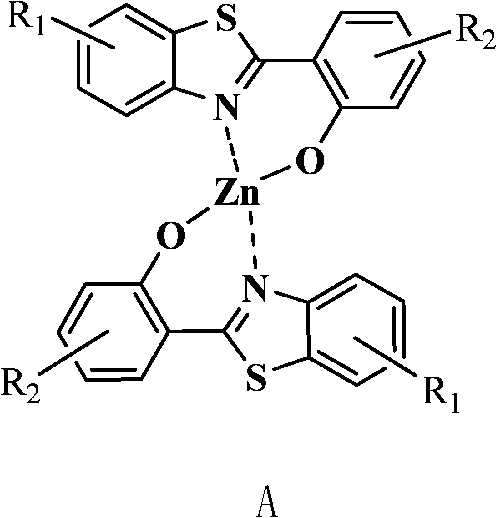
2-(2'-hydroxyphenyl) benzothiazole chelated zinc derivative as well as preparation method and application thereof
InactiveCN101654442AQuality improvementImprove performanceOrganic chemistrySolid-state devicesElectronic transmissionChelated zinc
The invention relates to a 2-(2'-hydroxyphenyl) benzothiazole chelated zinc derivative as well as a preparation method and an application thereof, belonging to the field of organic electroluminescentluminescent materials. The preparation method of the derivative comprises the following steps: firstly, introducing different substituent groups with electron-withdrawing or electron-donating capability in a benzothiazole benzene ring or a hydroxyphenyl benzene ring of a 2-(2'-hydroxyphenyl) benzothiazole matrix to obtain a substituted 2-(2'-hydroxyphenyl) benzothiazole ligand; coordinating with diatomic zinc to form a corresponding complex, and the like. The substituted 2-(2'-hydroxyphenyl) benzothiazole ligand can be prepared by a plurality of reactions, such as substituted aniline acylation, hydroxyl protection, hydroxyl thiocarbonate, Jacobson cyclization while hydroxyl deprotection, and the like or prepared by a direct reaction of O-amino thiophenol and substituted ortho-hydroxybenzoic acid. When applied to an organic electroluminescent luminescent device as an electronic transmission layer, the derivative has favorable electronic transmission performance and has performance superior to the most common electronic transmission material 8-hydroxyquinoline aluminum.
Owner:DALIAN UNIV OF TECH
Stabilized Cross-Linked Polyolefin Compositions
A composition is disclosed that includes at least one polyolefin, at least one organic peroxide, and an antioxidant mixture of a composition of at least one polyolefin, at least one organic peroxide, and an antioxidant mixture. The antioxidant mixture has at least one fast radical scavenger selected from the group consisting of low hindered phenols, low hindered thiophenols, low hindered thiobisphenols, aliphatic amines, aromatic amines, NOR HALS, hydroxylamines, and mixtures thereof, and at least one long term stabilizer selected from the group consisting of low hindered phenols, highly hindered phenols, thiosynergists, aliphatic amines, aromatic amines, HALS, hydroxylamines, and mixtures thereof. The preferred polyolefin is a homopolymer of ethylene or a copolymer of ethylene and the compositions are useful in insulating media for medium and high voltage wire and cable applications.
Owner:ADDIVANT USA
Method for preparing 2-substituted benzoxazole compound
The invention discloses a method for preparing a 2-substituted benzoxazole compound. According to the method, a benzylamine compound or a benzaldehyde compound or a benzyl alcohol compound, and o-toluidine-N-methyl-o-phenylenediamine, ortho-aminophenol and o-aminobenzenethiol serve as raw materials, metals palladium, platinum or ruthenium serves as a catalyst, and N,N-dimethylformamide, N,N-dimethylacetamide or N-methylpyrrolidone serves as a solvent. The method comprises the following preparation steps: (1) mixing the raw materials; (2) reacting; (3) separating and extracting; and (4) drying and concentrating. An oxidant and a hydrogen acceptor are not required in the whole preparation process, and the used partial catalysts can be recycled. The method is high in atom economy, simple in aftertreatment and mild in reaction conditions and has a certain industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
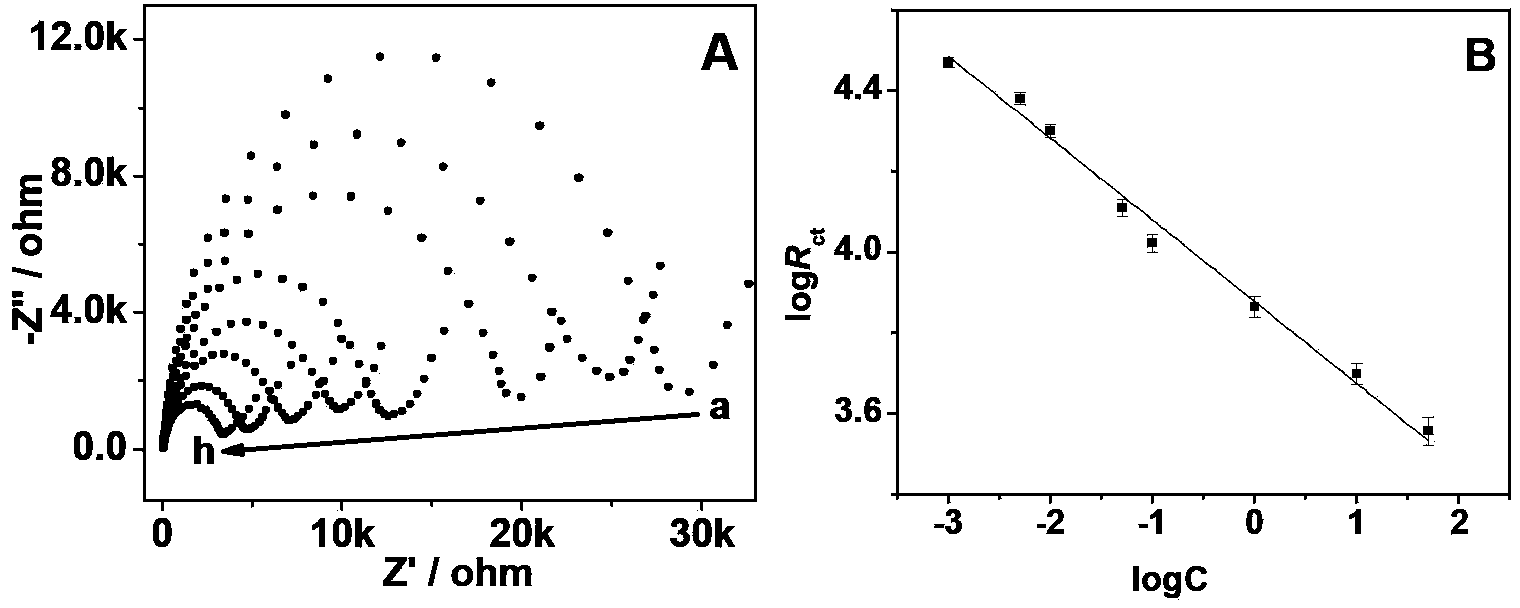
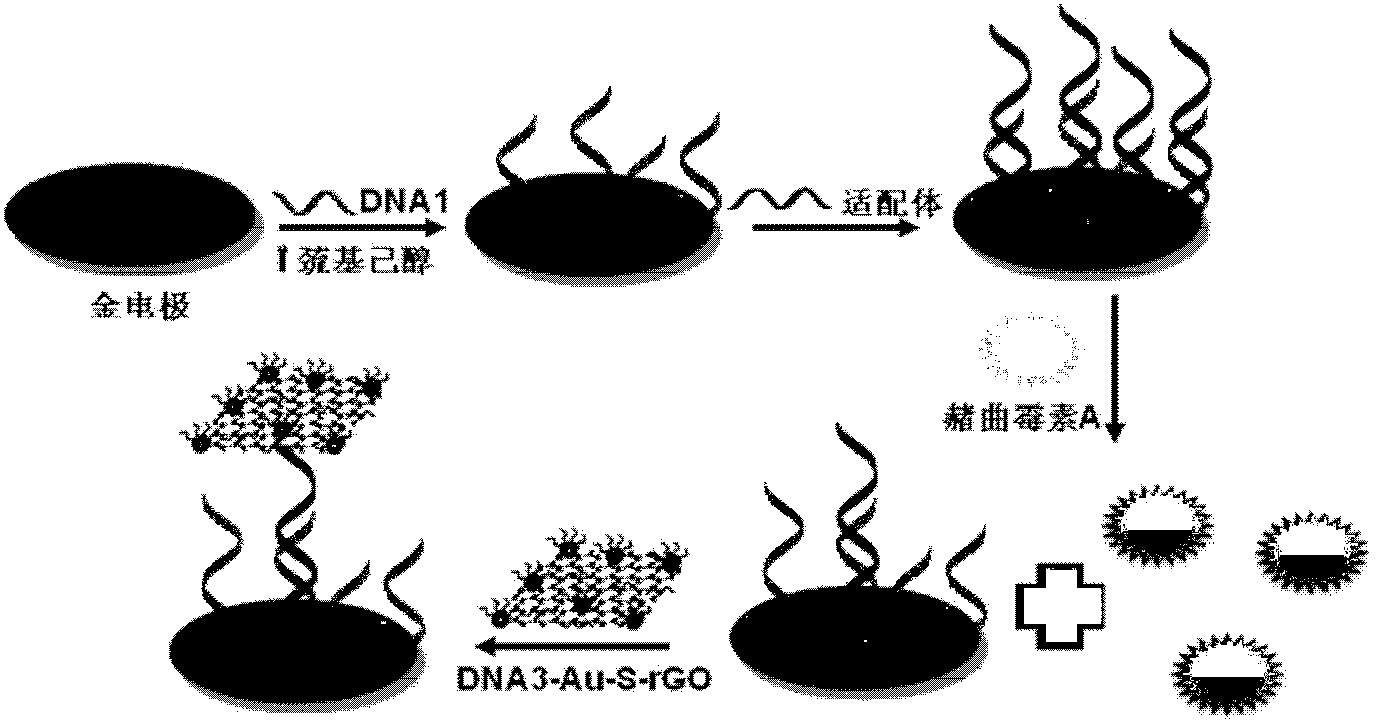
Method for detection of ochratoxin A with ultralow concentration by label-free aptamer sensor
InactiveCN103512931ALow costAchieving Sensitive DetectionMaterial electrochemical variablesAptamerOchratoxin A
The invention relates to a method for detection of ochratoxin A (OTA) with ultralow concentration by a label-free aptamer sensor. The method comprises the following specific steps: using o-amino thiophenol functionalized graphene oxide (GO-SH) and chloroauric acid as starting materials and sodium citrate as a reducing agent to prepare gold nanoparticles modified functionalized reduction graphene oxide (Au-S-rGO) by a one pot method; loading mercapto modified single stranded DNA3 by using the Au-S-rGO as a vector to obtain DNA3-Au-S-rGO; then modifying mercapto modified single stranded DNA1 on a gold electrode surface, and modifying an aptamer (DNA2) to the electrode surface through complementary pairing of bases; and then reacting the sensor interface with OTA of different concentrations. The method for quantitative detection of OTA has the advantages of simple preparation process, high sensitivity, wide measuring range and low cost, and solves the problems of high cost, complex detection scheme, long detection time and low sensitivity in the prior art.
Owner:JIANGSU UNIV
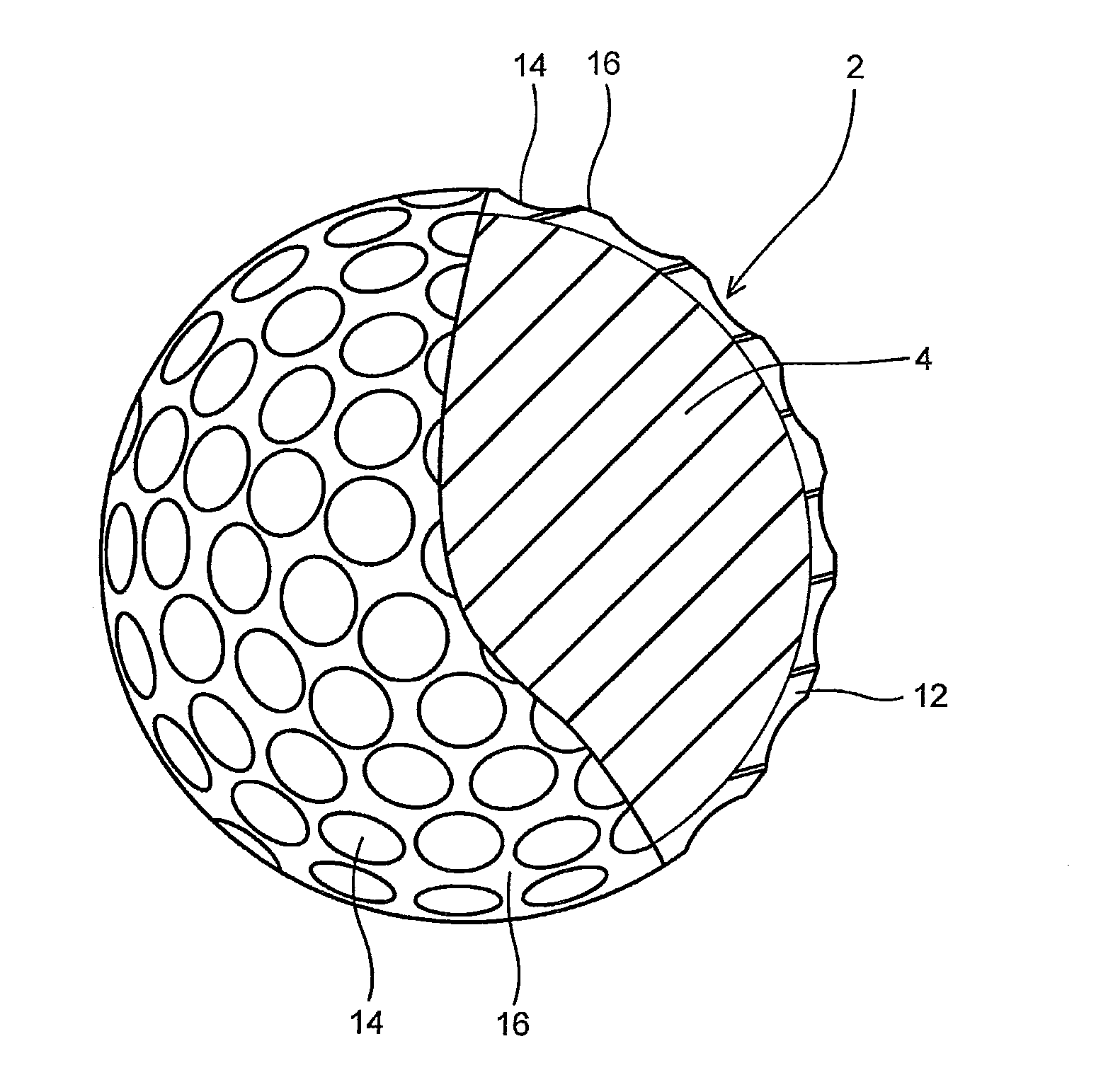
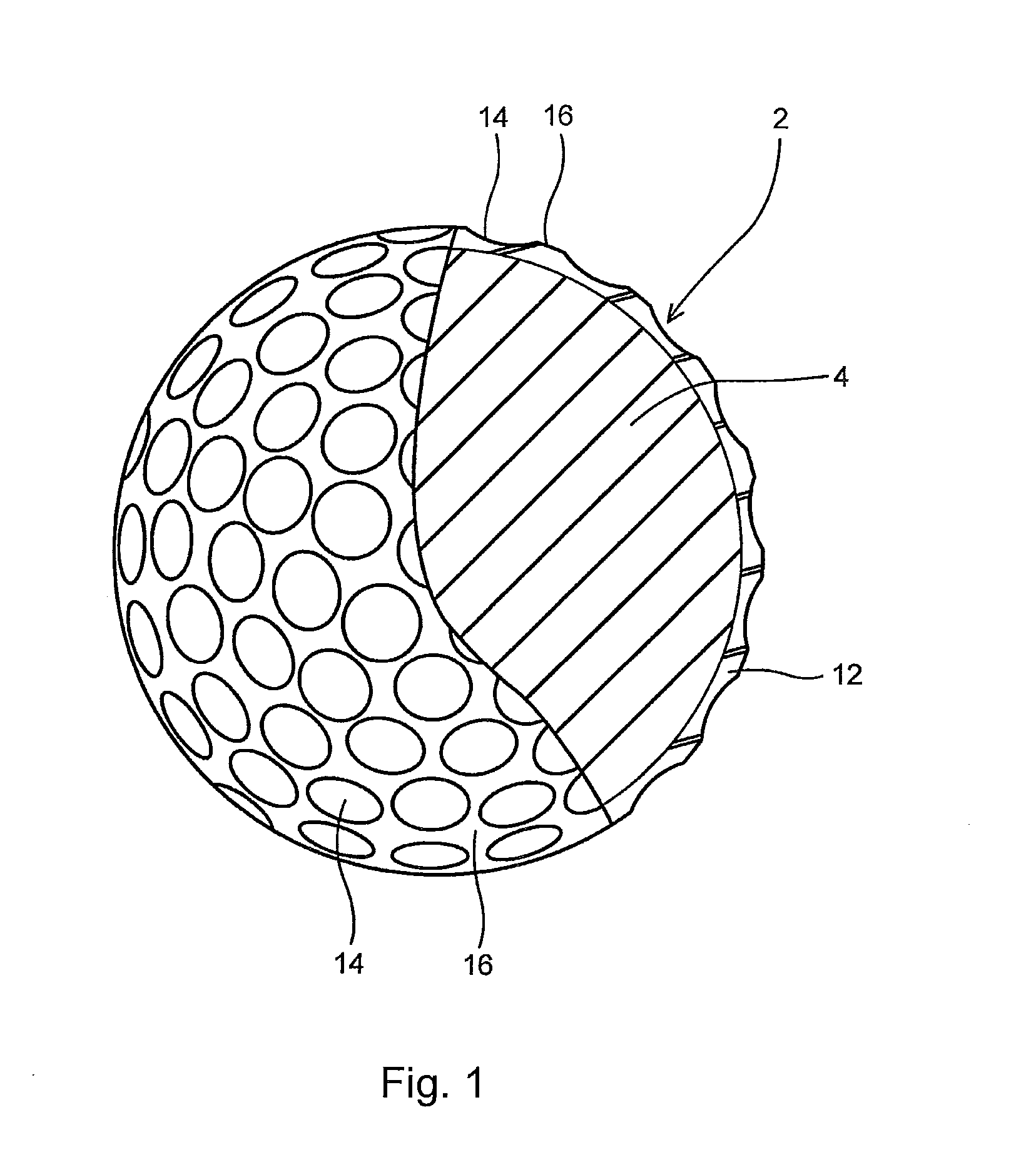
Multi-layer golf ball
A golf ball comprises a molded core, one or more ionomer mantles, and a thermoset polyurethane cover. The core is a high cis-polybutadiene crosslinked with zinc diacrylate and may also comprise a halogenated thiophenol and metal thiosulfate. One or more of the ionomer mantles comprises an ionomer neutralized to 80% or greater.
Owner:CALLAWAY GOLF CO
Golf ball
An object of the present invention is to provide a golf ball traveling a great flight distance on driver shots. The present invention provides a golf ball comprising a spherical core and at least one cover layer covering the spherical core, wherein the spherical core is formed from a rubber composition containing (a) base rubber, (b) an α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms and / or a metal salt thereof as a co-crosslinking agent, (c) a crosslinking initiator, (d) a carboxylic acid having 1 to 14 carbon atoms and / or a salt thereof, and (e) a halogen-substituted thiophenol and / or a metal salt thereof as an organic sulfur compound, provided that the rubber composition further contains (f) a metal compound in the case of containing only (b) the α,β-unsaturated carboxylic acid having 3 to 8 carbon atoms as the co-crosslinking agent.
Owner:SUMITOMO RUBBER IND LTD
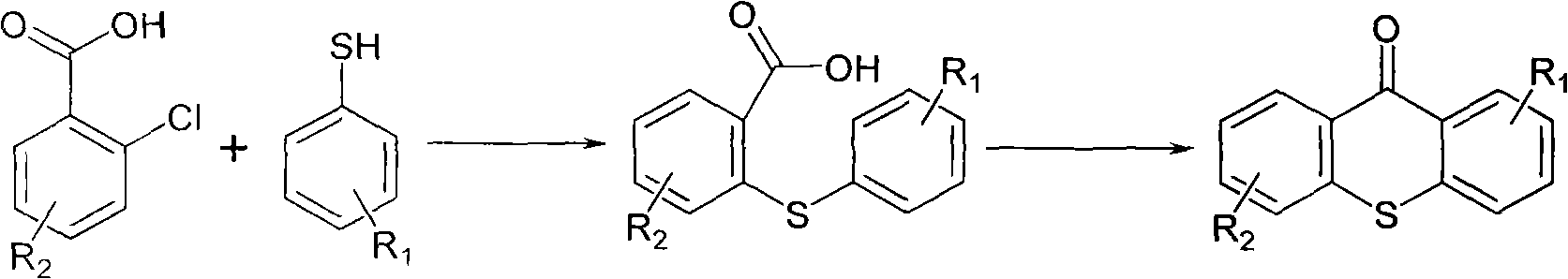
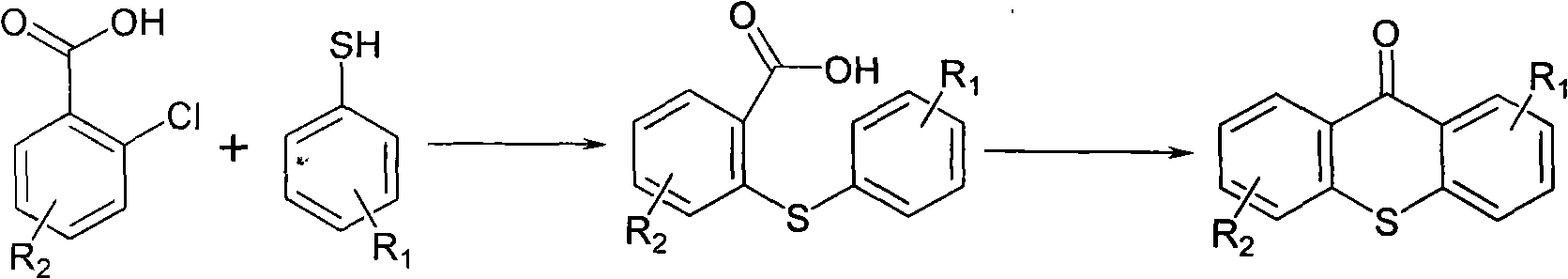
Preparation method of 2-isopropyl thioxanthone and derivatives thereof
The invention relates to a preparation method of 2-isopropyl thioxanthone and derivatives thereof, comprising the following steps of: carrying out condensation reaction on a derivative of o-chlorobenzoic acid (C7H5ClO2) and a derivative of 4-isopropyl thiophenol (C9H12S) in an organic solvent, and carrying out dehydration cyclization reaction on condensation products under the catalysis of concentrated sulfuric acid. The 2-isopropyl thioxanthone prepared by the invention can be used as a high-efficiency radical II-type photo initiator. The invention has easy operation, low cost, environmental protection and high purity; the purity of the product can reach more than 99 percent; the product can be commercially prepared and produced on a large scale to meet the current ever-increasing market demand.
Owner:TIANJIN JIURI NEW MATERIALS CO LTD
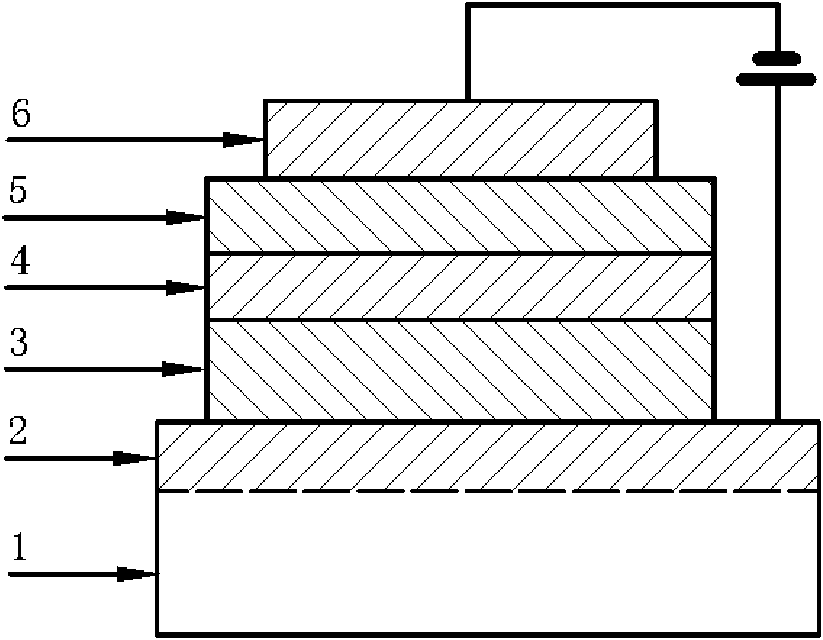
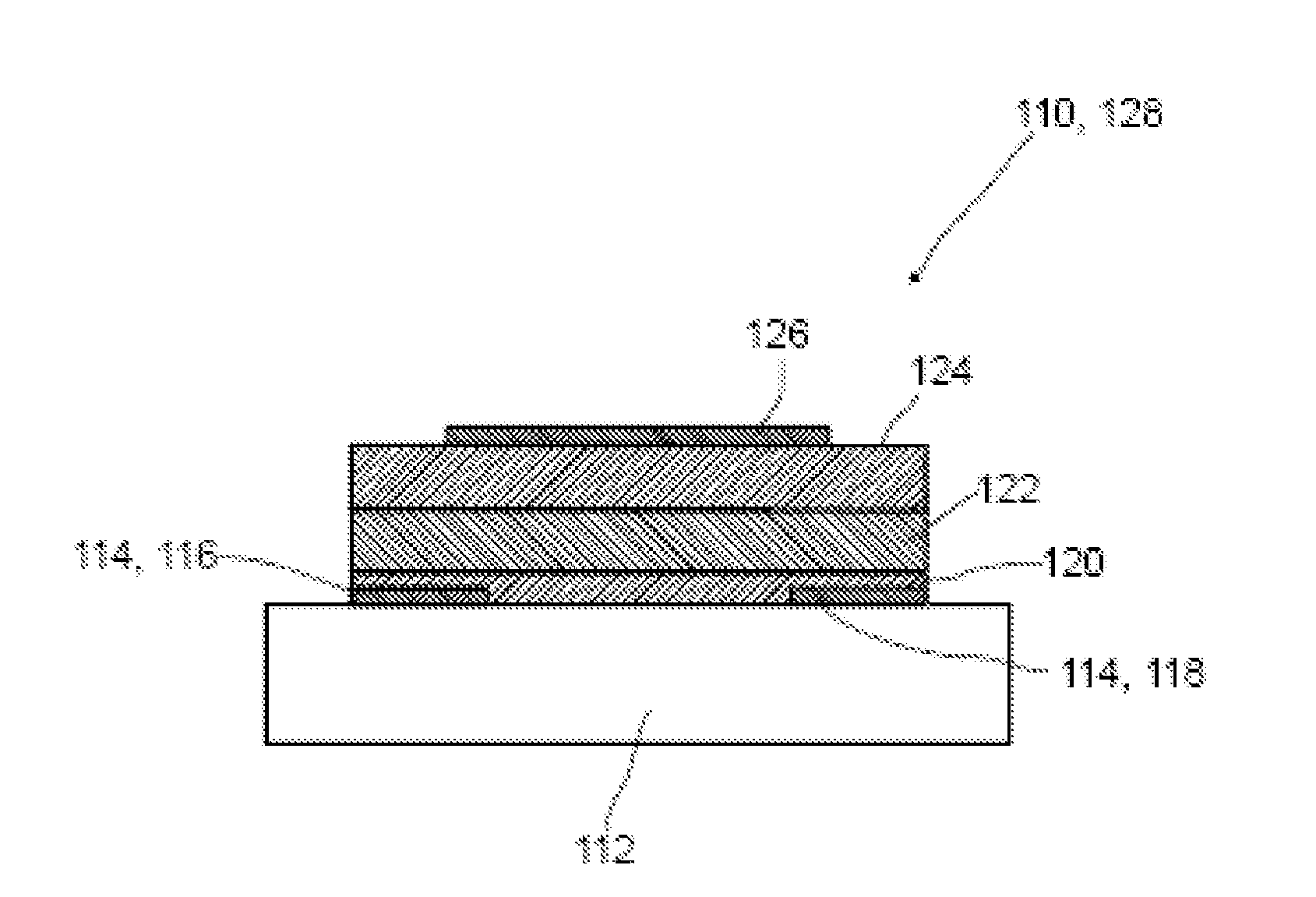
Method for producing an organic semiconductor device
ActiveUS20130200336A1High carrier mobilityHigh currentSolid-state devicesSemiconductor/solid-state device manufacturingSemiconductor materialsCharge carrier
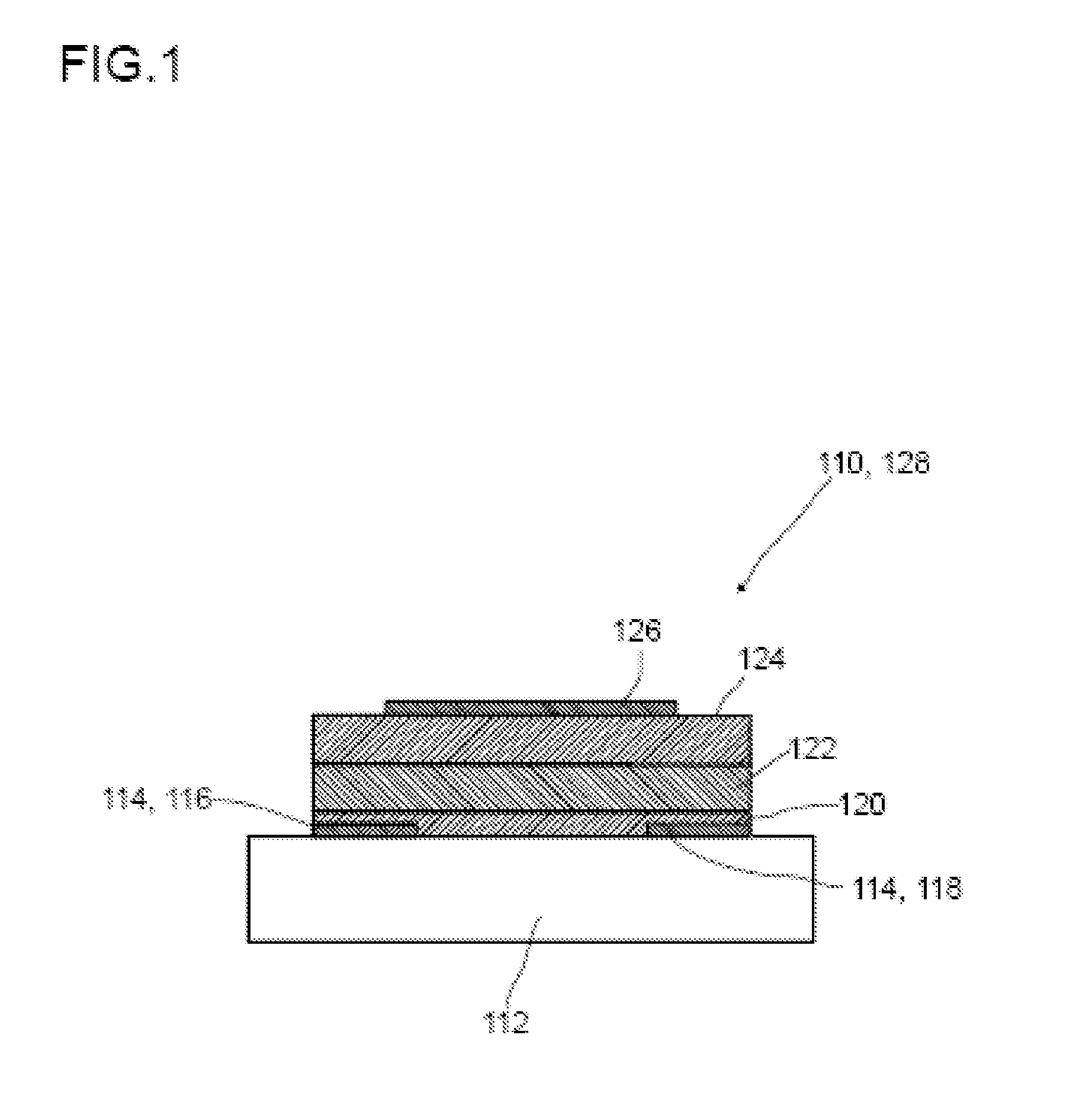
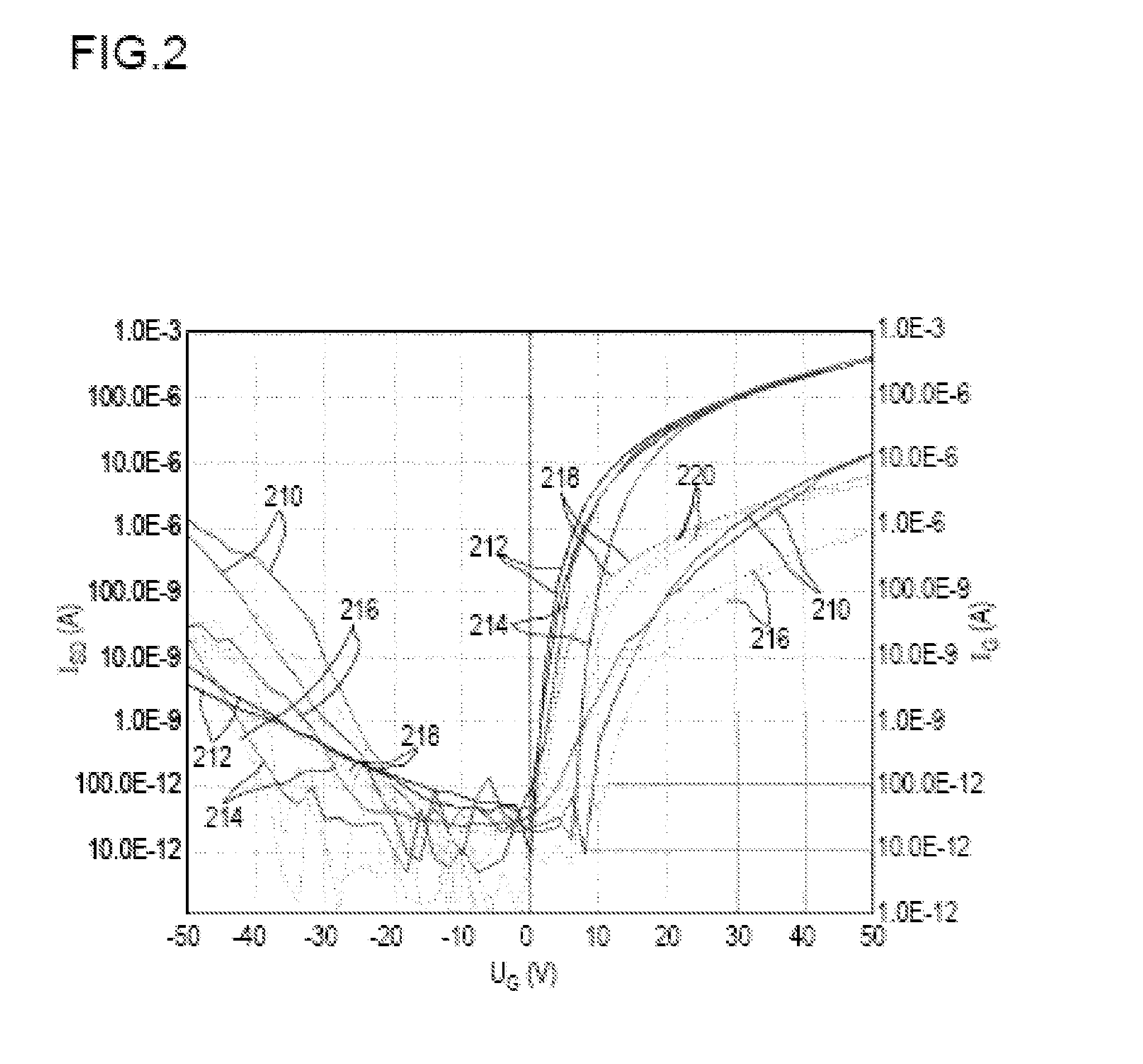
A method for producing an organic semiconductor device (110) having at least one organic semiconducting material (122) and at least two electrodes (114) adapted to support an electric charge carrier transport through the organic semiconducting material (122) is disclosed. The organic semiconducting material (122) intrinsically has ambipolar semiconducting properties. The method comprises at least one step of generating at least one intermediate layer (120) which at least partially is interposed between the organic semiconducting material (122) and at least one of the electrodes (114) of the organic semiconductor device (110). The intermediate layer (120) comprises at least one thiol compound having the general formula HS—R, wherein R is an organic residue. The thiol compound has an electric dipole moment pointing away from the SH-group of the thiol compound. The electric dipole moment has at least the same magnitude as the electric dipole moment in 4-Phenylthiophenol. By the intermediate layer (120) an ambipolar charge carrier transport between the electrodes (114) is suppressed in favor of a unipolar charge carrier transport.
Owner:CLAP CO LTD
Preparation method of 3,5-dimethyl-4-chlorophenol
InactiveCN104326881AImprove conversion rateReduce generationOrganic chemistryOrganic compound preparationAluminium chlorideWater chlorination
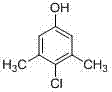
The invention discloses a preparation method of 3,5-dimethyl-4-chlorophenol, which takes tetrachloroethylene as a solvent, benzyl thiophenol and aluminium chloride as cocatalysts, sulfuric chloride as a chloridizing agent, orientation chlorination is carried out through two phases of low-temperature chlorination and high-temperature chlorination, the mass ratio of tetrachloroethylene to MX is 0.5-4: 1; the mass ratio of the cocatalyst to MX is 2.5-6.5:1000; at low temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.9-1.2: 1; at high temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.1-0.2: 1; the temperature at the low temperature chlorination phase is controlled at 30-45 DEG C, chlorination is carried out for 4-6 hours; the temperature at high temperature chlorination phase is controlled at 50-65 DEG C, and chlorination is carried out for 1-2 hours, insulation reaction is carried out after the dropping process of sulfuric chloride is completed, tail gas is removed for 1-2 hours, and steps of water-washing layering, cooling and crystallizing, centrifuging and washing, and drying to obtain the product. According to the method, the conversion rate can reach more than 95%, finished product PCMX yield is increased, and the by-product can be effectively reduced.
Owner:RONGCHENG QINGMU CHEM MATERIALS
Low spin, soft compression, performance golf ball
A golf ball including a core formed from a polybutadiene having Mooney viscosity of about 40 to about 65 and a salt of a halogenated thiophenol, and having a compression of about 30 to about 60; a first cover layer including a highly neutralized ethylene copolymer having a Shore D hardness of less than about 65; and a second cover layer including a thermoset or thermoplastic polyurethane having a Shore D hardness of about 40 to about 55.
Owner:ACUSHNET CO
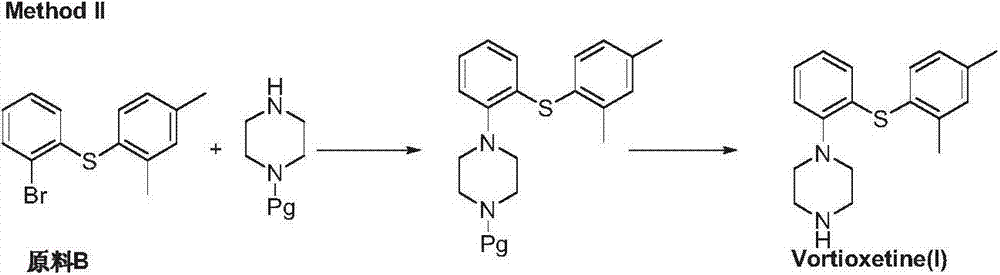
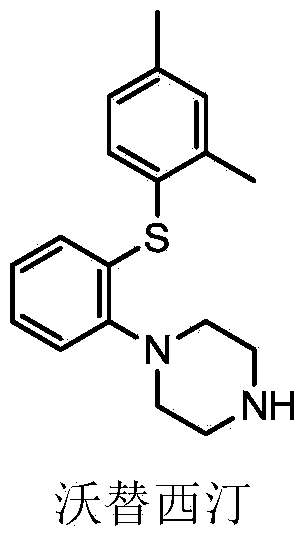
Synthetic method of vortioxetine
The invention discloses a synthetic method of vortioxetine. The synthetic method comprises the following steps of by adopting a compound as shown in a formula (I) as a raw material, carrying out substitution reaction on the compound and 2,4-dimethyl thiophenol (II) to generate 2-(2,4-dimethyl phenyl alkyl sulfide) nitrobenzene (III); reducing 2-(2,4-dimethyl phenyl alkyl sulfide) nitrobenzene (III) to obtain 2-(2,4-dimethyl phenyl alkyl sulfide) phenylamine (IV); cyclizing 2-(2,4-dimethyl phenyl alkyl sulfide) phenylamine (IV) and N,N-bis(2-chloroethyl)-4-methyl benzsulfamide (VI) to obtain Tos-protecting vortioxetine (V); and preparing vortioxetine (VII) from Tos-protecting vortioxetine (V) under the action of a phenol additive. The synthetic method disclosed by the invention has the advantages of easily available raw material, simple process, low cost and high purity, and is suitable for industrialized production.
Owner:SUNDIA MEDITECH COMPANY LTD
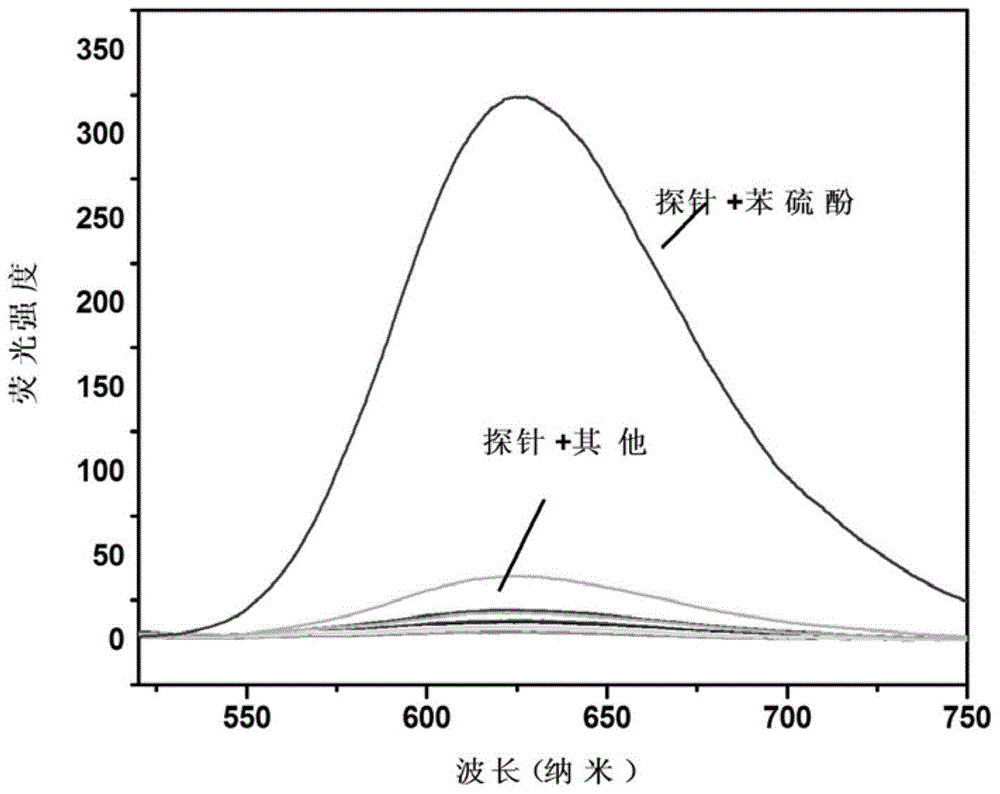
Fluorescent probe containing N, N-diethyl p-thylaminophenol and application thereof in thiophenol detection
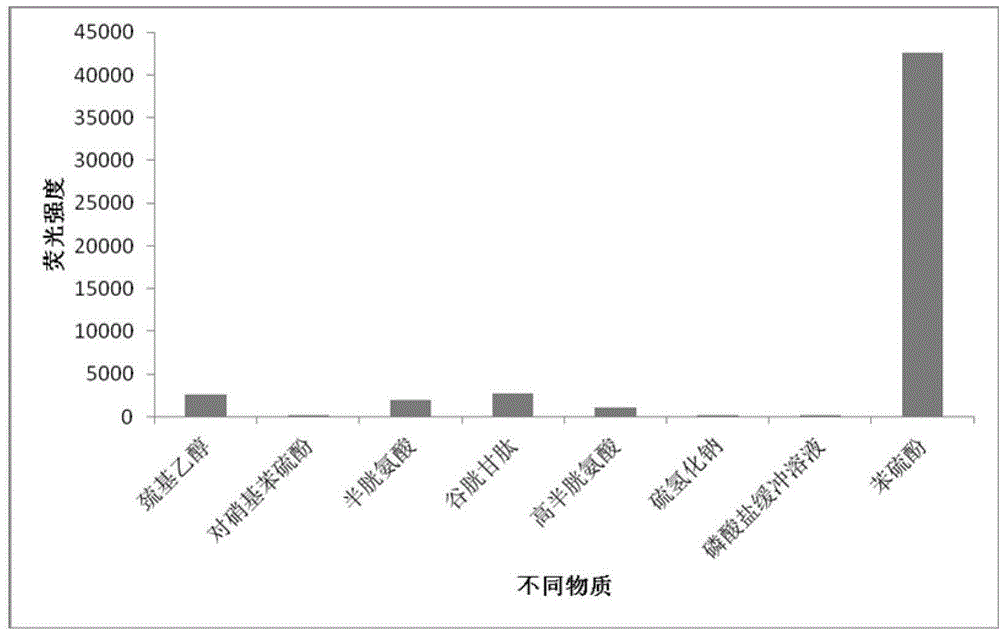
InactiveCN103589423ARealize qualitative and quantitative detectionEasy to operateOrganic chemistryFluorescence/phosphorescenceThiolPhosphate
The invention discloses a fluorescent probe containing N, N-diethyl paramethylaminophenol and application thereof in the thiophenol detection. The structural formula of the fluorescent probe is as follows: in a phosphate buffered solution with pH value of 7.4, the compound can be in nucleophilic substitution reaction with thiophenol for releasing out the strong fluorescent material 3-(N, N-diethyl)-6-hydroxy-fluorane, so that the reaction system has a strong fluorescent signal at the wavelength of 543 nm; experimental results show that the fluorescent probe is used to detect the thiophenol; the detection limit of the thiophenol is 6.0*10<-9>mol / L; the relative standard deviation is 2.6%; the qualitative and quantitative detection of the thiophenol can be realized. The detecting method is simple in operation, high in accuracy and sensitivity and good in selectivity; different thiol compounds have no disturbance to the system of the fluorescent probe detecting the thiophenol; the fluorescent probe can be used for detecting the content of the thiophenol in a water sample.
Owner:陕西省计量科学研究院
Preparation method of baloxavir marboxil intermediate
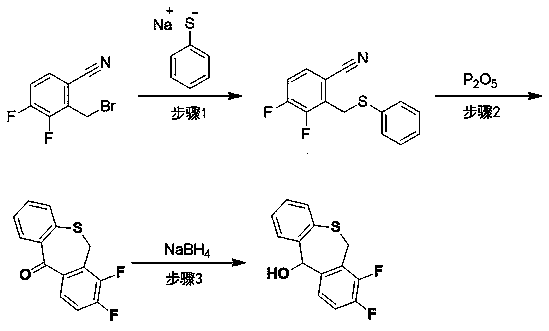
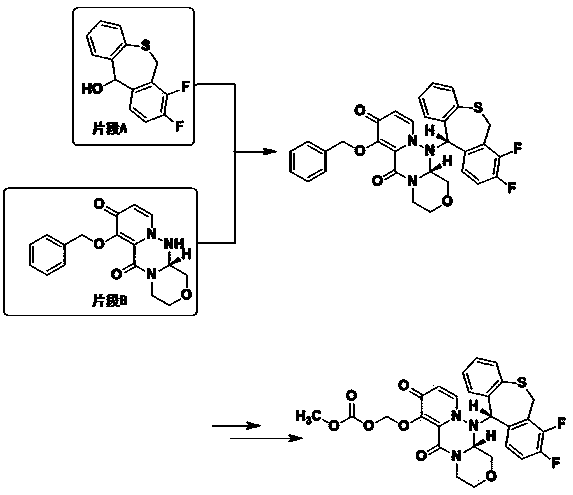
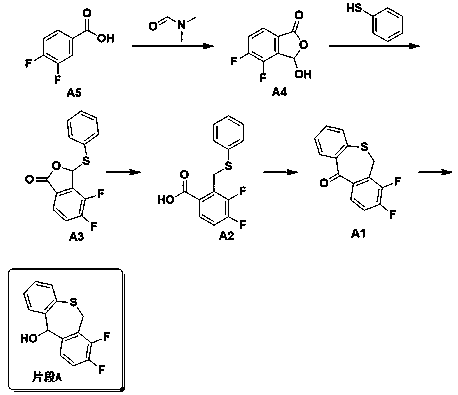
The invention discloses a preparation method of a baloxavir marboxil intermediate. The preparation method is characterized by comprising the following steps: (1) substituting 2-bromomethyl-3,4-difluorophenylacetonitrile through sodium thiophenolate in a solvent A to obtain 3,4-difluoro-2-[(thiophenyl) methyl] benzyl cyanide; (2) reacting the 3,4-difluoro-2-[(thiophenyl) methyl] benzyl cyanide obtained in the step (1) with a cyclizing agent in a solvent B, and carrying out cyclization to obtain 7,8-difluoro dibenzo [b,e] thia heptacyclic-11(6H)-ketone; and (3) reducing the 7,8-difluoro dibenzo[b,e] thia heptacyclic-11(6H)-ketone obtained in the step (2) to obtain 7,8-difluoro dibenzo [b,e] thia heptacyclic-11(6H)-alcohol. The reaction steps in the preparation method are reduced to a six-step reaction from a five-step reaction in the prior art, so that the generation of a side reaction is reduced, and the reaction efficiency is improved. The sodium thiophenolate is used for replacing thiophenol with foul smell and high toxicity, and harm to experimenters during the production is reduced.
Owner:NANJING POLYTECHNIC INSITUTE
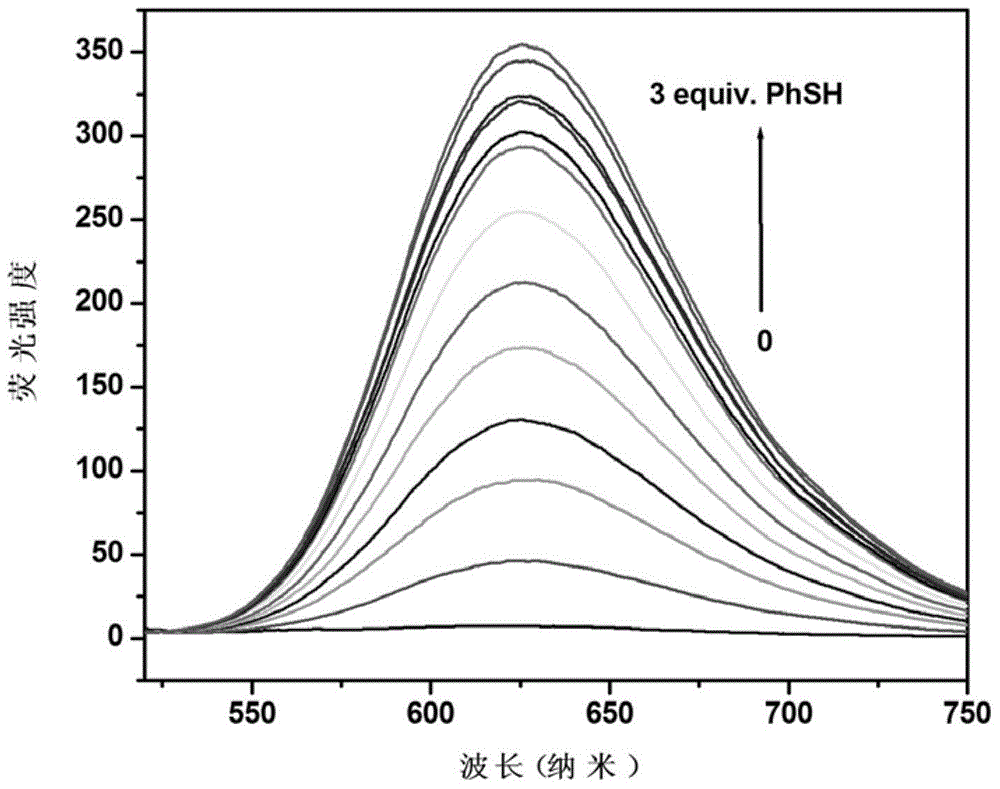
Preparation and application of fluorescence-enhanced thiophenol fluorescence probe
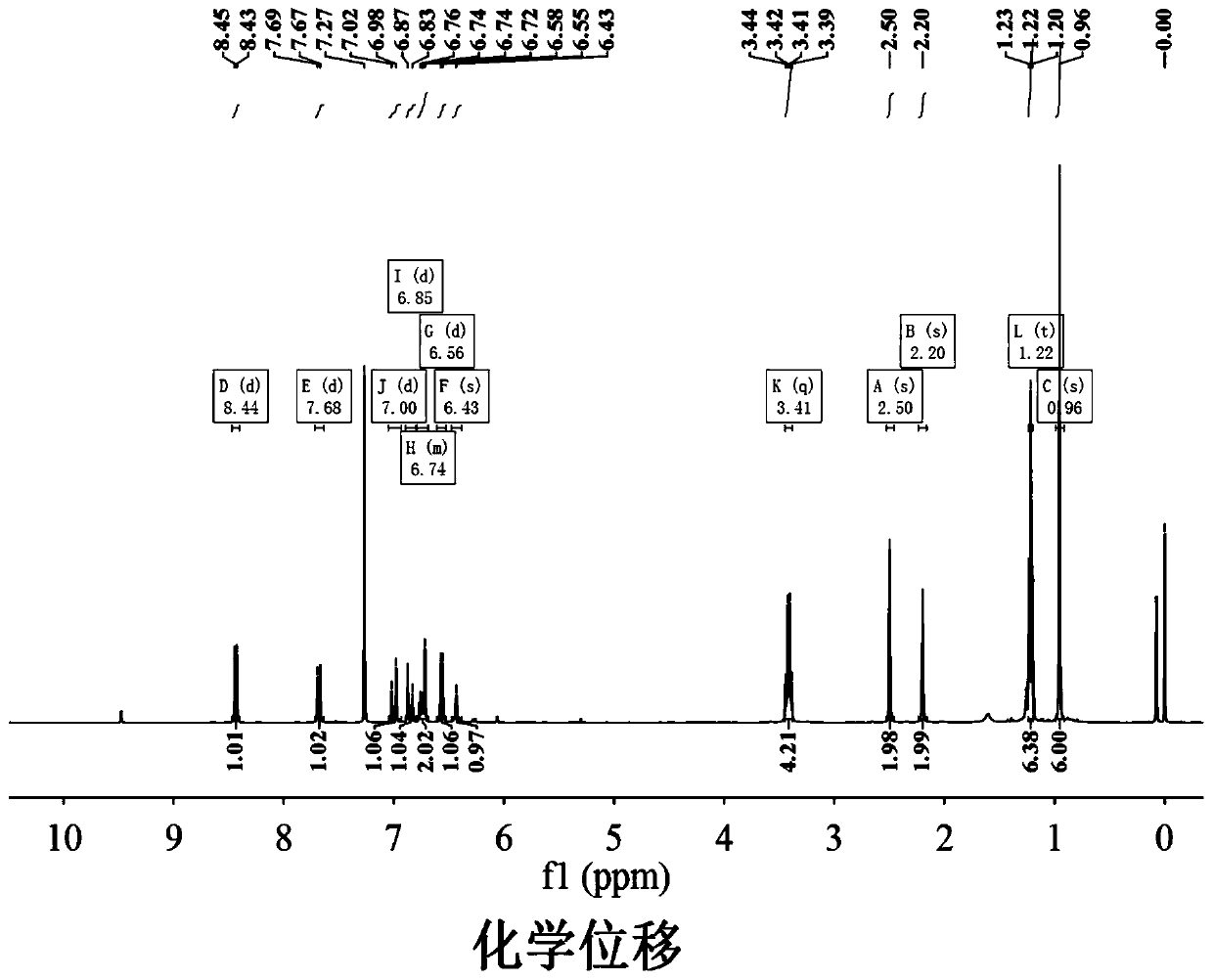
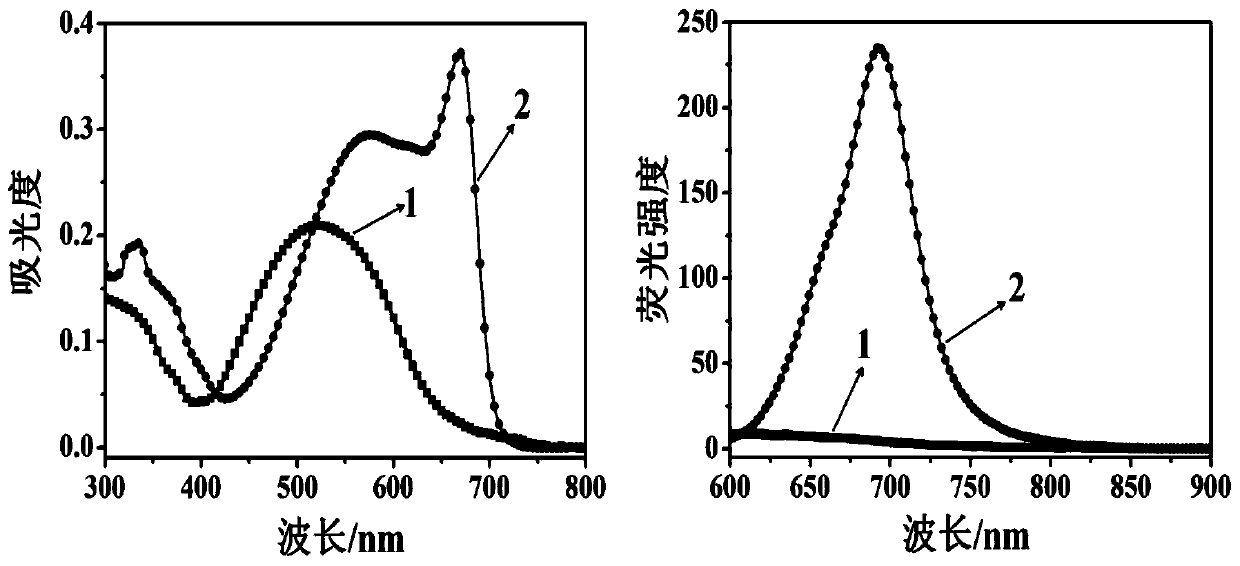
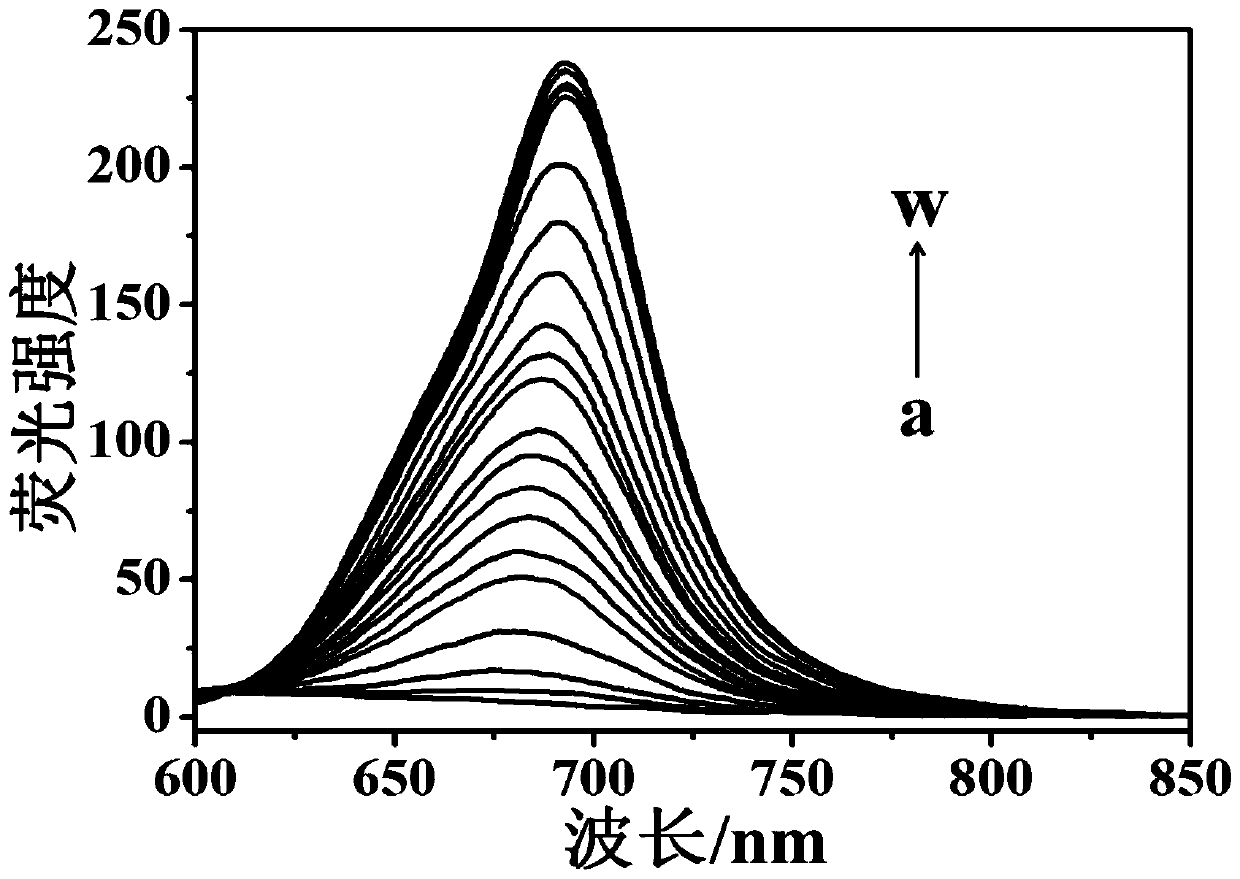
ActiveCN104804728AEasy to synthesizeLow costOrganic chemistryFluorescence/phosphorescenceFluorescenceStructural formula
The invention discloses a novel compound which can be utilized for fluorescence detection of thiophenol, particularly relates to preparation and application of a novel fluorescence probe, and belongs to the technical field of chemical analysis and detection. The novel fluorescence probe has a molecular structural formula shown in the description. The novel fluorescence probe is high in selectivity, anti-interference capability and sensitivity, can be utilized for the fluorescence detection of thiophenol in a biological or environmental sample, and has an excellent application prospect.
Owner:SUZHOU ROWLAND BIOTECH
Fluorescent probe for specifically distinguishing different thiol
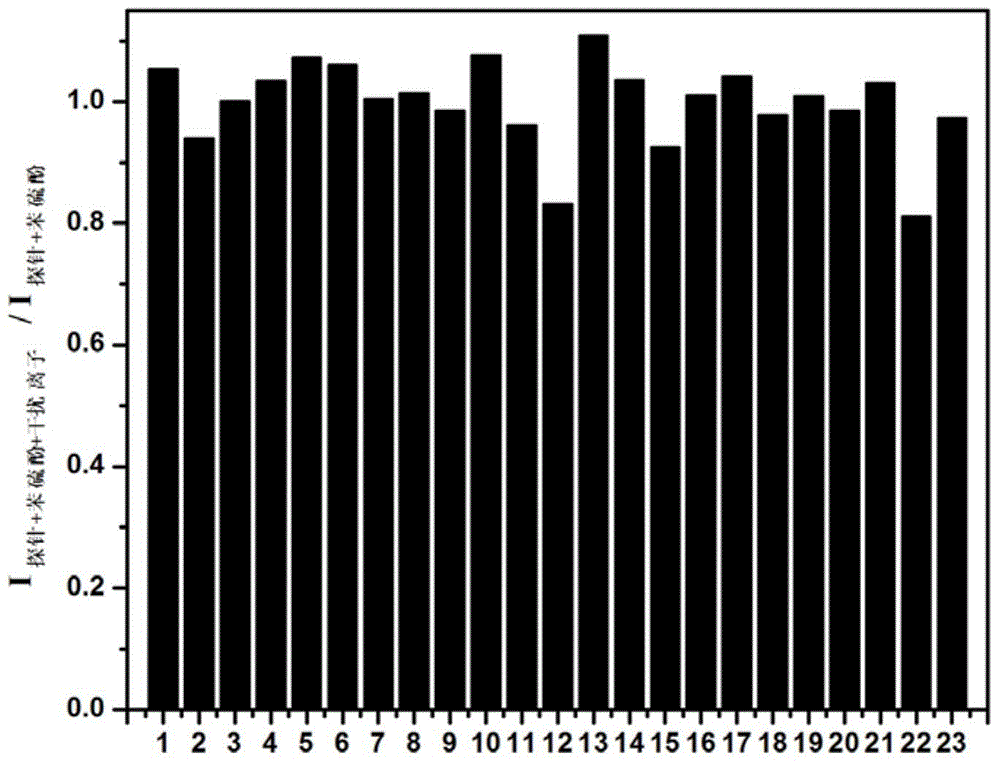
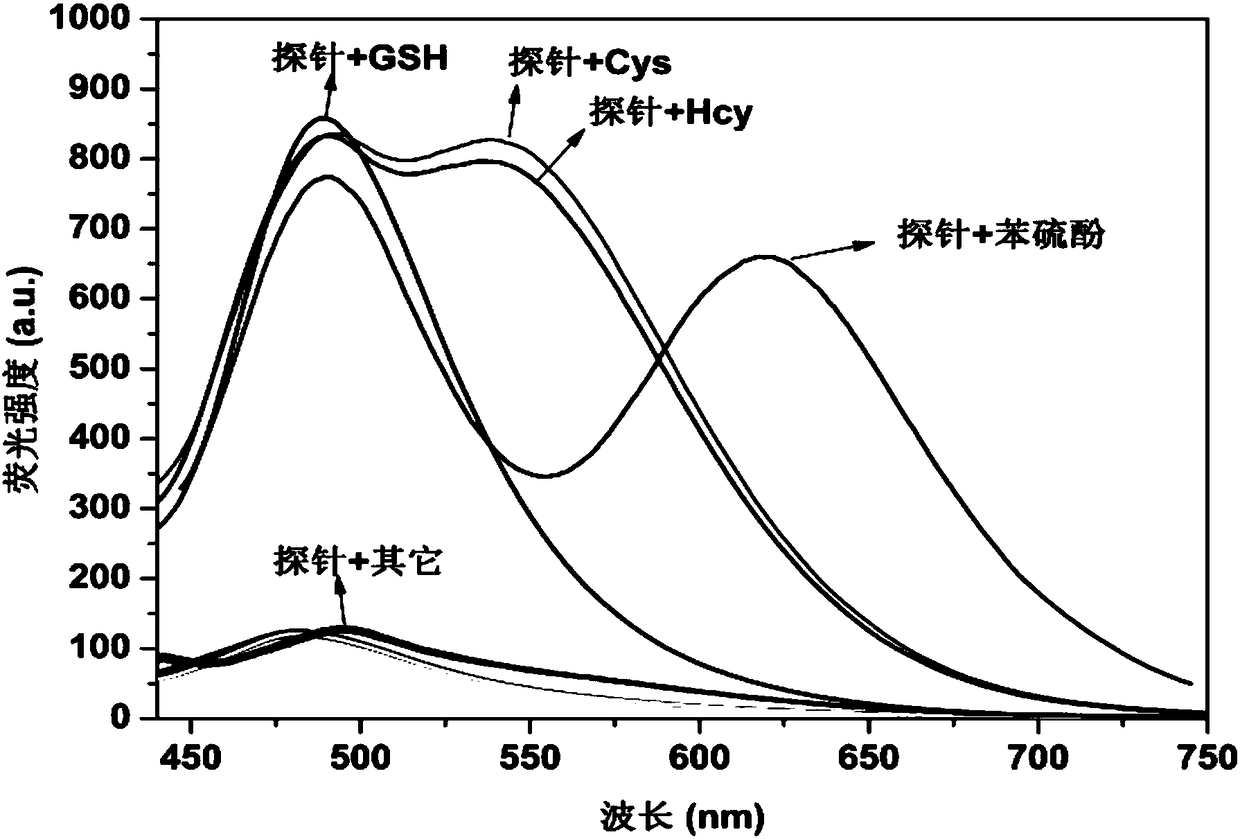
ActiveCN108358906AAchieve qualitativeRealize quantitative detectionOrganic chemistryFluorescence/phosphorescenceThiolFluorescence
The invention relates to a fluorescent probe for specifically distinguishing different thiol and belongs to the field of fluorescent probes. A molecular structure of the fluorescent probe is as follows: a formula is shown in the description. A probe molecule has no fluorescent light; after the probe molecule responds with thiophenol, a solution produces blue light and red light; after the probe molecule responds with GSH (glutathione), the solution produces the blue light; after the probe molecule responds with Hcy / Cys, the solution produces the blue light and green light. The probe molecule provided by the invention can be used for distinguishing different types of the thiol and can be used for rapidly and quantitatively detecting different types of the thiol, and has important application value in the fields of biochemistry, environment monitoring and the like.
Owner:CENT SOUTH UNIV
Thick inner cover multi-layer golf ball
A golf ball including a core including a halogenated thiophenol and having a diameter of from about 1.3 inches to about 1.4 inches, a compression of about 44 or less; and a coefficient of restitution of from about 0.770 to about 0.810; a cast polyurethane or polyurea cover; and an intermediate layer disposed between the core and the cover, the intermediate layer having a thickness of from about 0.11 inches to about 0.12 inches and being formed from a composition including at least two ionomers; wherein a combination of the core and the intermediate layer results in a compression of from about 70 to about 100, and the golf ball has a coefficient of restitution of from about 0.805 to about 0.820 at about 125 ft / s and a compression of from about 75 to about 105.
Owner:ACUSHNET CO
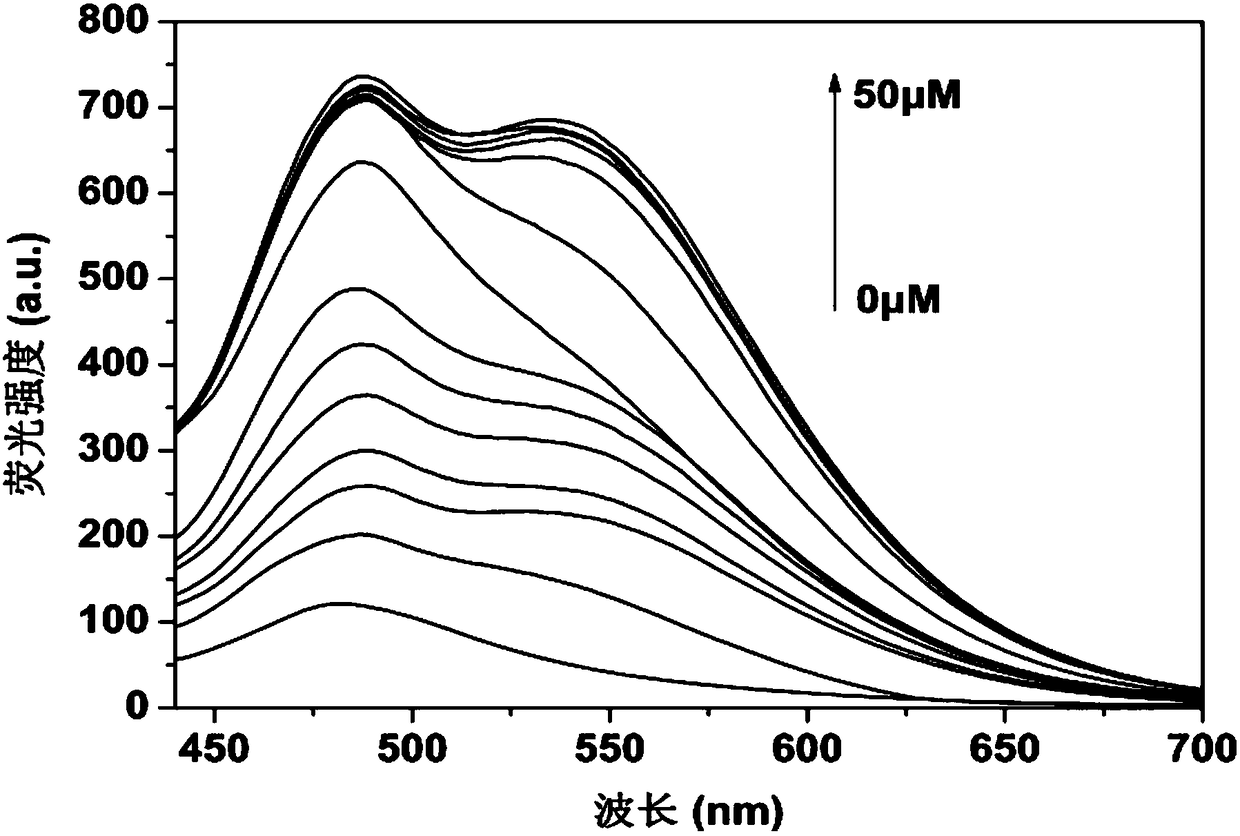
Near-infrared fluorescent probe for detecting thiophenol and synthesis method and application thereof
InactiveCN109761978AImprove stabilityExcellent optical propertiesOrganic chemistryMaterial analysis by observing effect on chemical indicatorSynthesis methodsJulolidine
The invention discloses a near-infrared fluorescent probe for high-selectivity detecting of thiophenol and a synthesis method and application thereof, and belongs to the technical field of chemical analysis and detection. The near-infrared fluorescent probe is obtained by reaction between a dicyanoisophorone-julolidine large pi system and 2,4-dinitrofluorobenzene, and has the following structure (please see the specifications for the structure). Fluorophore of the probe is the dicyanoisophorone-julolidine large pi system, and a response group to thiophenol is 2,4-dinitrophenoxy. Molecules of the probe have high selectivity and sensitivity to thiophenol, the detection range is 1-60 [mu]mol.L<-1>, and the limit of detection is 0.13 [mu]mol.L<-1>. The probe can be used for detecting thiophenol in water bodies, soil and cells.
Owner:SHANGQIU NORMAL UNIVERSITY
Near-infrared fluorescent probe for detecting thiophenol and synthesis method and application thereof
InactiveCN109761853ARaw materials are easy to getHigh synthetic yieldCarboxylic acid nitrile preparationOrganic compound preparationFluorescenceSynthesis methods
The invention discloses a near-infrared fluorescent probe for detecting thiophenol and a synthesis method and application thereof, and belongs to the technical field of chemical analysis and detection. The near-infrared fluorescent probe is obtained by reaction between a dicyano-substituted isophorone large pi system and 2,4-dinitrofluorobenzene, and has the following structure (being defined as in the specification). Fluorophore of the probe is the dicyano-substituted isophorone large pi system, and a response group to thiophenol is 2,4-dinitrophenoxy. Molecules of the probe have high selectivity and sensitivity to thiophenol, the detection range is 0.5-10 [mu]mol.L<-1>, and the limit of detection is 23 nmol.L<-1>. The probe can be used for detecting thiophenol in water bodies, soil and cells.
Owner:SHANGQIU NORMAL UNIVERSITY
Preparation method of substituted thiophenol
A process for preparing substituted benzenethiol includes such steps as reaction between substituted halobenzene and thiohydrogenating reagent in non-protonic polar solvent while stirring, acidifying to pH=1-6, and distilling.
Owner:ZHEJIANG UNIV OF TECH
High-elasticity abrasion-resisting rubber material and preparation method thereof
InactiveCN104212000AIncrease the degree of cross-linkingIncrease elasticityRubber materialCalcium silicate
The invention discloses a high-elasticity abrasion-resisting rubber material and a preparation method thereof. The high-elasticity abrasion-resisting rubber material comprises, by weight, 97-103 parts of natural rubber, 5-8 parts of methyl phenyl vinyl silicone rubber, 10-13 parts of chloroprene rubber, 23-26 parts of modified graphite, 18-22 parts of diatomite, 15-18 parts of talcum powder, 12-15 parts of dolomite powder, 8-11 parts of calcium silicate, 2.4-2.8 parts of silane coupling agent A-172, 12-16 parts of polyarmide fiber, 4.3-6.5 parts of sulfur, 1.3-1.9 parts of accelerant DM, 2.2-2.6 parts of 4,4'-dithio diphenyl maleimide, 3-6 parts of ditolyl thiophenol, 8-12 parts of trioctyl trimellitate, 1.2-2.3 parts of anti-aging agent SP, 1.8-2.5 parts of anti-aging agent 800-A and 7-11 parts of phenyl petroleum sulfonate.
Owner:ANHUI ELECTRIC GRP SHARES
Preparation method of heat-resistant oxygen-resistant polyester film
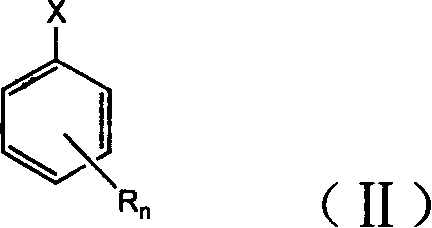
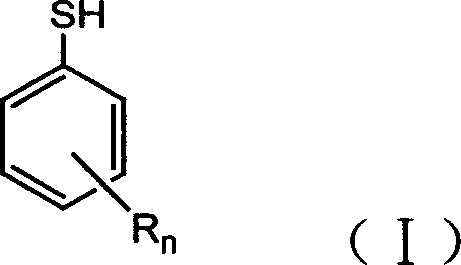
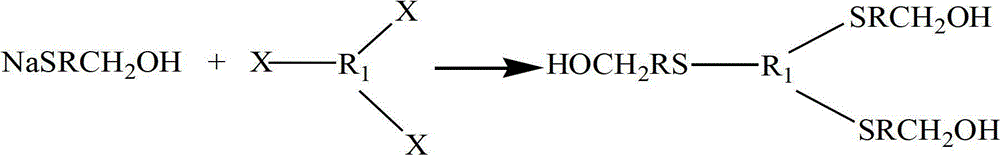
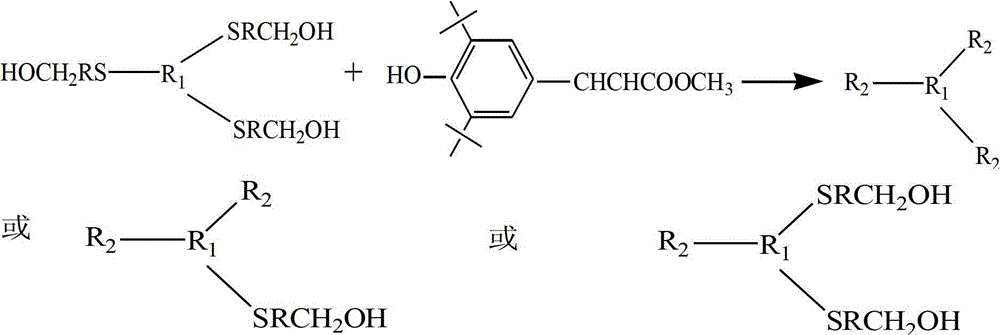
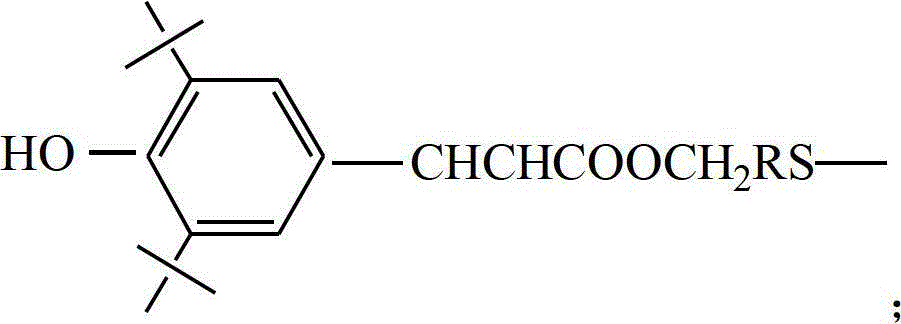
ActiveCN102875980AImprove thermal oxygen resistanceImprove temperature resistanceFlat articlesSulfide preparationOxygenPhenol
The invention discloses a preparation method of a heat-resistant oxygen-resistant polyester film. The preparation method is characterized by including the steps of preparation of hydroxy thiophenol containing compounds, preparation of sulfur-containing hindered phenol antioxidants, polyester synthesis and film processing. The preparation method includes: using 4-hydroxy benzyl thiophenol sodium, 1, 3, 5-trichlorobenzene, 1, 3, 5-tribromobenzene and 2, 4, 6-tri(4-hydroxy benzyl thiophenyl)-1, 3, 5-triazine to synthesize the hydroxy thiophenol containing compounds, namely, 1, 3, 5-tri(4-hydroxy benzyl thiophenyl) benzene and 2, 4, 6-tri(4-hydroxy benzyl thiophenyl)-1, 3, 5-triazine in N, N-dimethyl formamide solvent; synthesizing the 1, 3, 5-tri(4-hydroxy benzyl thiophenyl) benzene, the 2, 4, 6-tri(4-hydroxy benzyl thiophenyl)-1, 3, 5-triazine and 3, 5-di-tert-butyl-4-hydroxy phenylacrylic acid methyl ester to prepare the type A reactive sulfur-containing hindered phenol antioxidant and the type B reactive sulfur-containing hindered phenol antioxidant; and synthesizing the type A reactive sulfur-containing hindered phenol antioxidant, the type B reactive sulfur-containing hindered phenol antioxidant, terephthalic acid and ethylene glycol to prepare heat-resistant oxygen-resistant polyester resin, so that the heat-resistant oxygen-resistant polyester film good in performance is prepared.
Owner:四川东方绝缘材料股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com