Dark blue organic light-emitting material and preparation method and application thereof
A luminescent material, dark blue technology, applied in the direction of luminescent materials, organic chemistry, silicon organic compounds, etc., can solve the problems of poor stability and poor color purity of blue light, and achieve the effect of enhanced stability and improved color purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The embodiment of the present invention provides a method for preparing a deep blue organic light-emitting material as described above, wherein the reaction formula is: According to reaction formula (1), including steps:
[0039] A. Under the inert atmosphere, under the joint catalysis of tris(dibenzylideneacetone)dipalladium and tri-tert-butylphosphorus, R 1 The substituted 1,3-dibromobenzene reacts with the secondary amine compound in the first organic solvent, and is separated by column chromatography to obtain the ditertiary amine compound; the secondary amine compound is
[0040] B. Under inert atmosphere, ditertiary amine compounds and MBr 3 The reaction is carried out in the second organic solvent and purified to obtain a dark blue organic light-emitting material.
[0041] In one embodiment, the inert atmosphere may be, but not limited to, a nitrogen atmosphere or an argon atmosphere.
[0042] In one embodiment, in step A, the first organic solvent may be...
Embodiment 1
[0057] Example 1 Preparation of dark blue organic light-emitting material 1b, the reaction formula is
[0058]
[0059] (1) Preparation of ditertiary amine compound 1a
[0060] According to reaction formula (2), the specific steps are: under nitrogen atmosphere, tris(dibenzylideneacetone)dipalladium (0.4mmol), tri-tert-butylphosphorus (1.2mmol), 1-fluoro-3,5- Dibromobenzene (10 mmol) and diphenylamine (22 mmol) were dispersed in dry toluene (80 mL), heated to reflux (110° C.) and reacted at this temperature for 16 h. After the reaction system was cooled to room temperature, the reaction solution was diluted, and the diluted reaction solution was suction filtered with a Buchner funnel covered with silica gel. Silica gel column chromatography was performed as the eluent to obtain ditertiary amine compound 1a with a yield of 94%.
[0061] (2) Preparation of dark blue organic light-emitting material 1b
[0062] According to reaction formula (2), the specific steps are: under...
Embodiment 2
[0064] (1) Preparation of dark blue organic light-emitting material 1c
[0065] The reaction formula is The specific steps are as follows: under a nitrogen atmosphere, the dark blue organic light-emitting material 1b (5 mmol) and carbazole (6 mmol) are dissolved in N,N-dimethylformamide (30 mL), cesium carbonate (20 mmol) is added, and at 140 The reaction was carried out at ℃ for 16 h. After the reaction was completed, it was extracted with ethyl acetate, washed with water to separate the liquids, dried the organic phase, spin-dried and separated on the column to obtain the dark blue organic light-emitting material 1c with a yield of 83%. The structure of the dark blue organic light-emitting material 1c was identified, and the measured data included: 1 H NMR (CDCl 3 ,400MHz)δ9.04(dd,J=18.9,2.1Hz,2H),8.33(d,J=1.8Hz,1H),8.16(d,J=2.0Hz,1H),7.55–7.36(m,5H ), 7.34–7.10 (m, 11H), 6.65 (d, J=9.0Hz, 1H), 6.20–6.15 (m, 1H); 13 C NMR (CDCl 3 ,100MHz)13C NMR(101MHz,CDCl3)δ151.90,15...
PUM
| Property | Measurement | Unit |
|---|---|---|
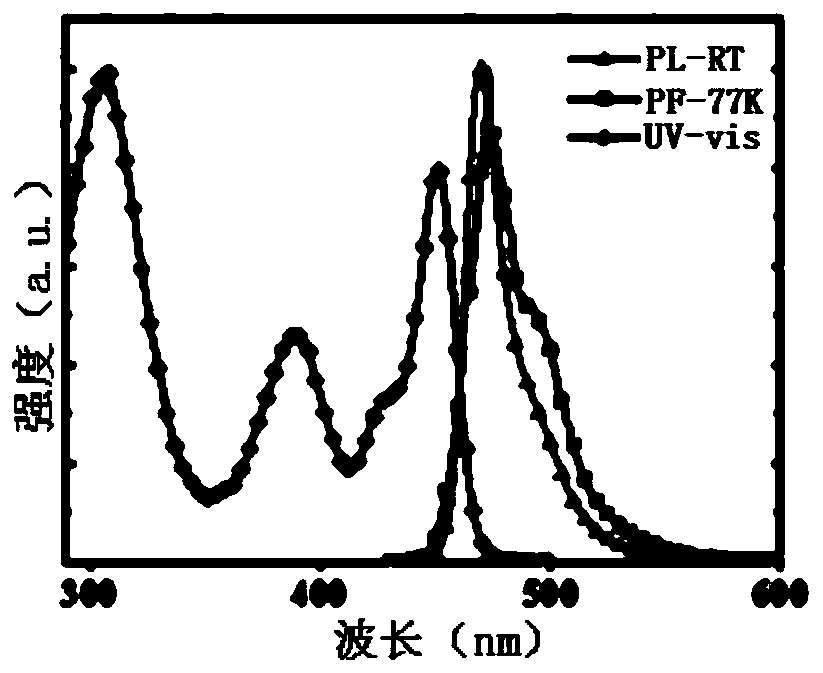
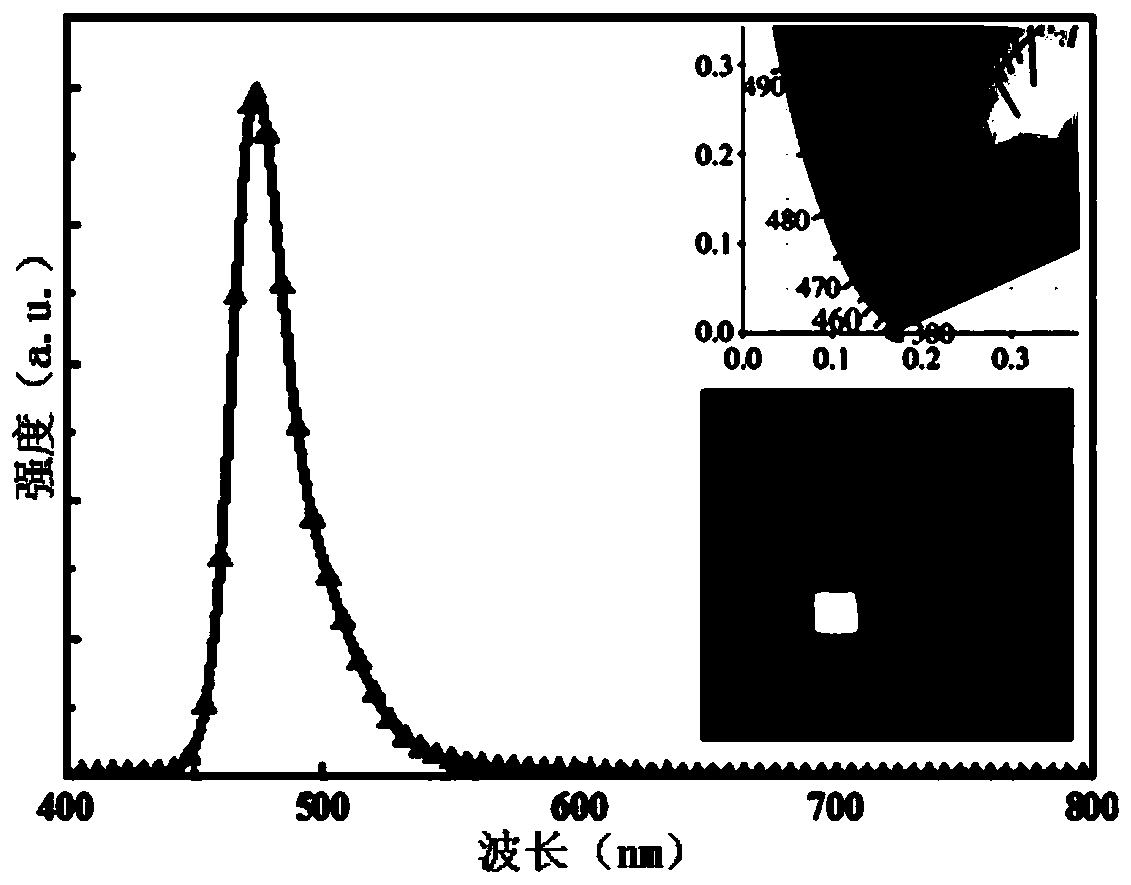
| full width at half maximum | aaaaa | aaaaa |
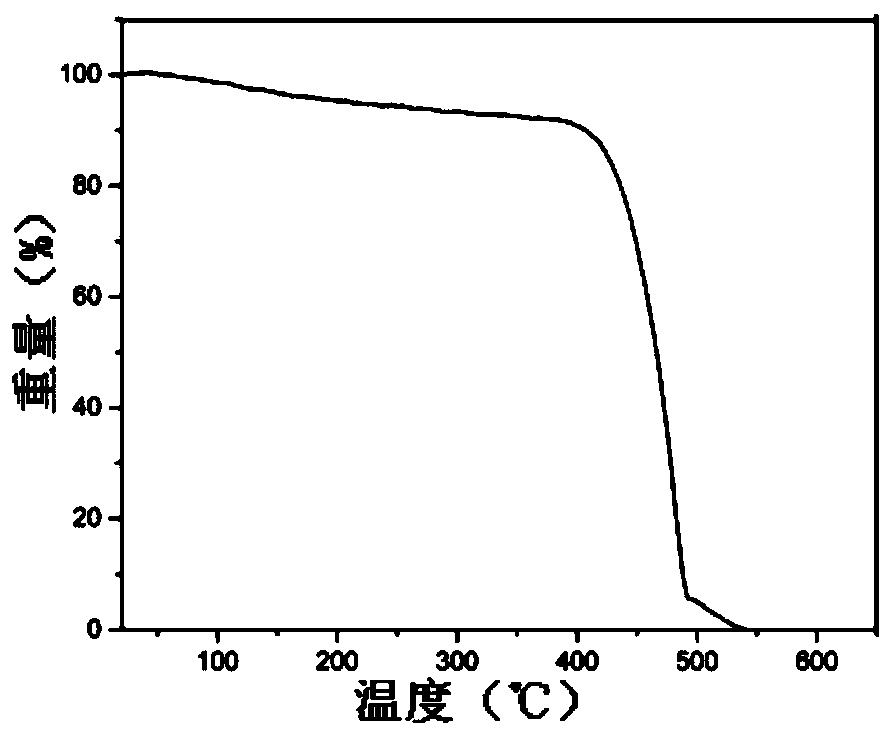
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com