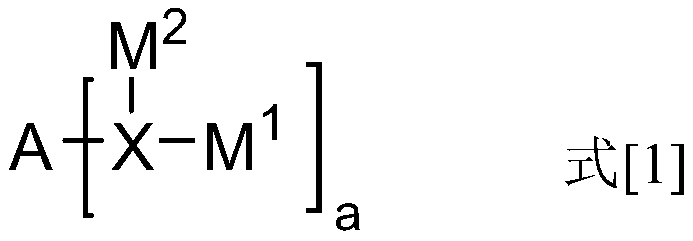Patents
Literature
143results about How to "Narrow emission spectrum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
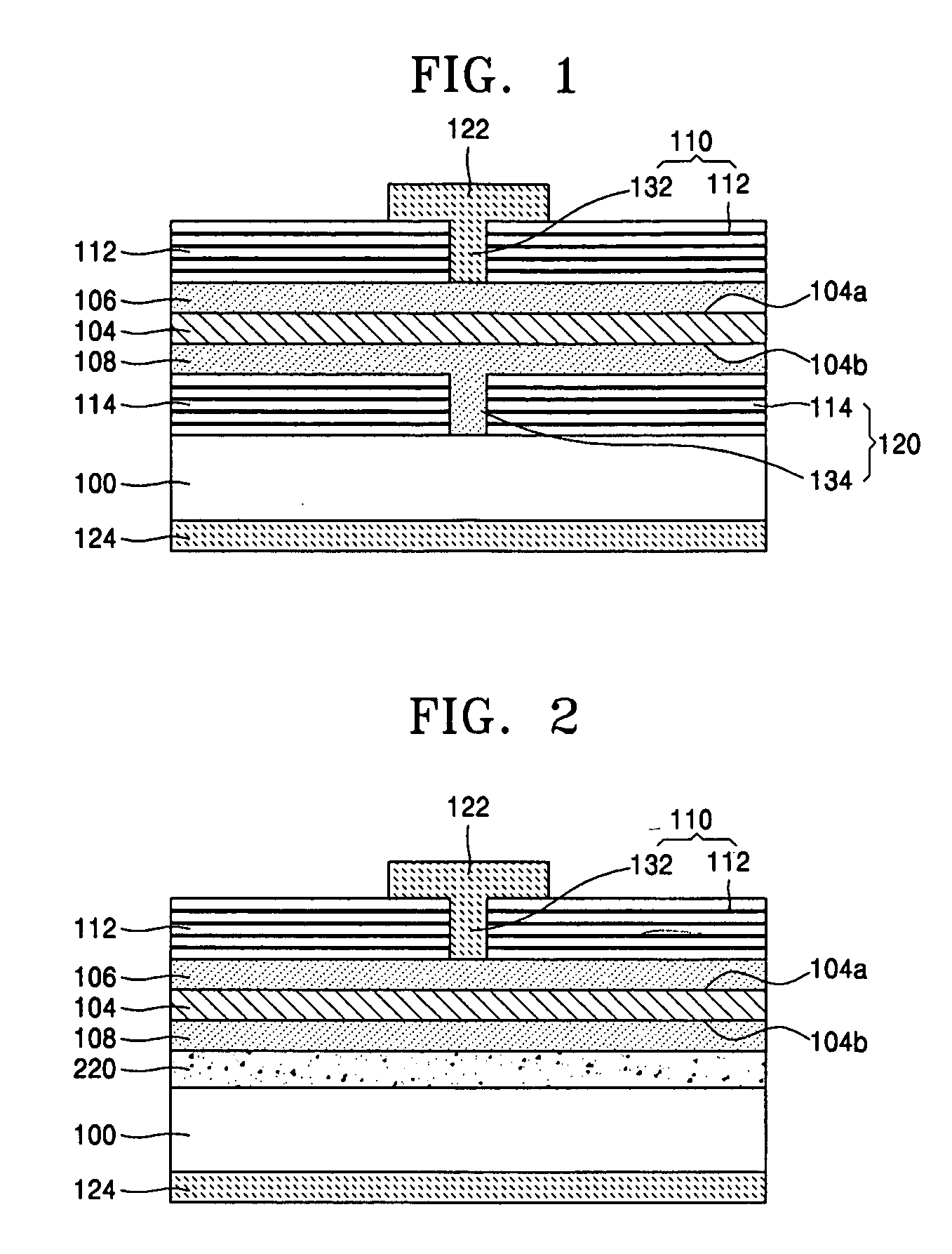
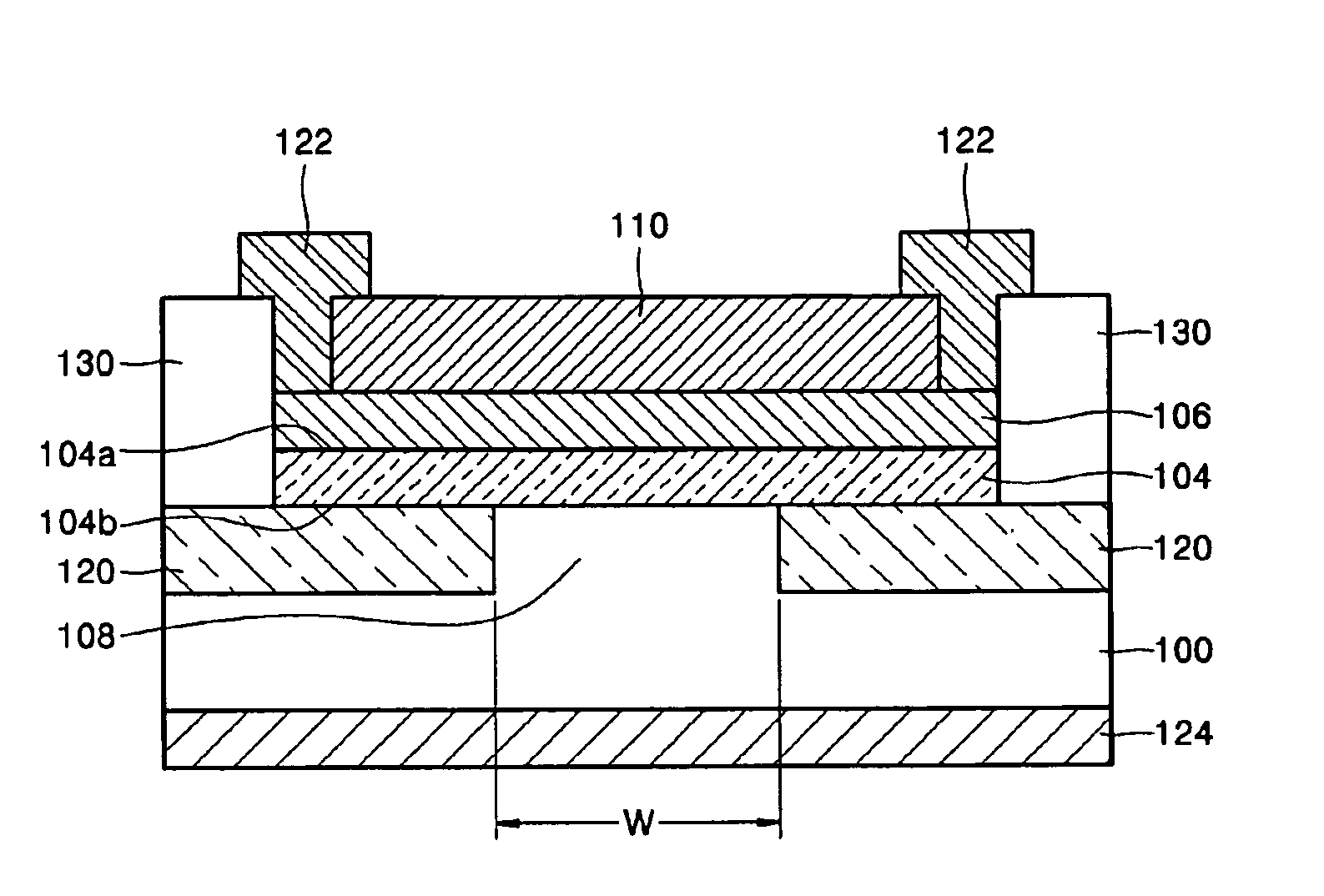
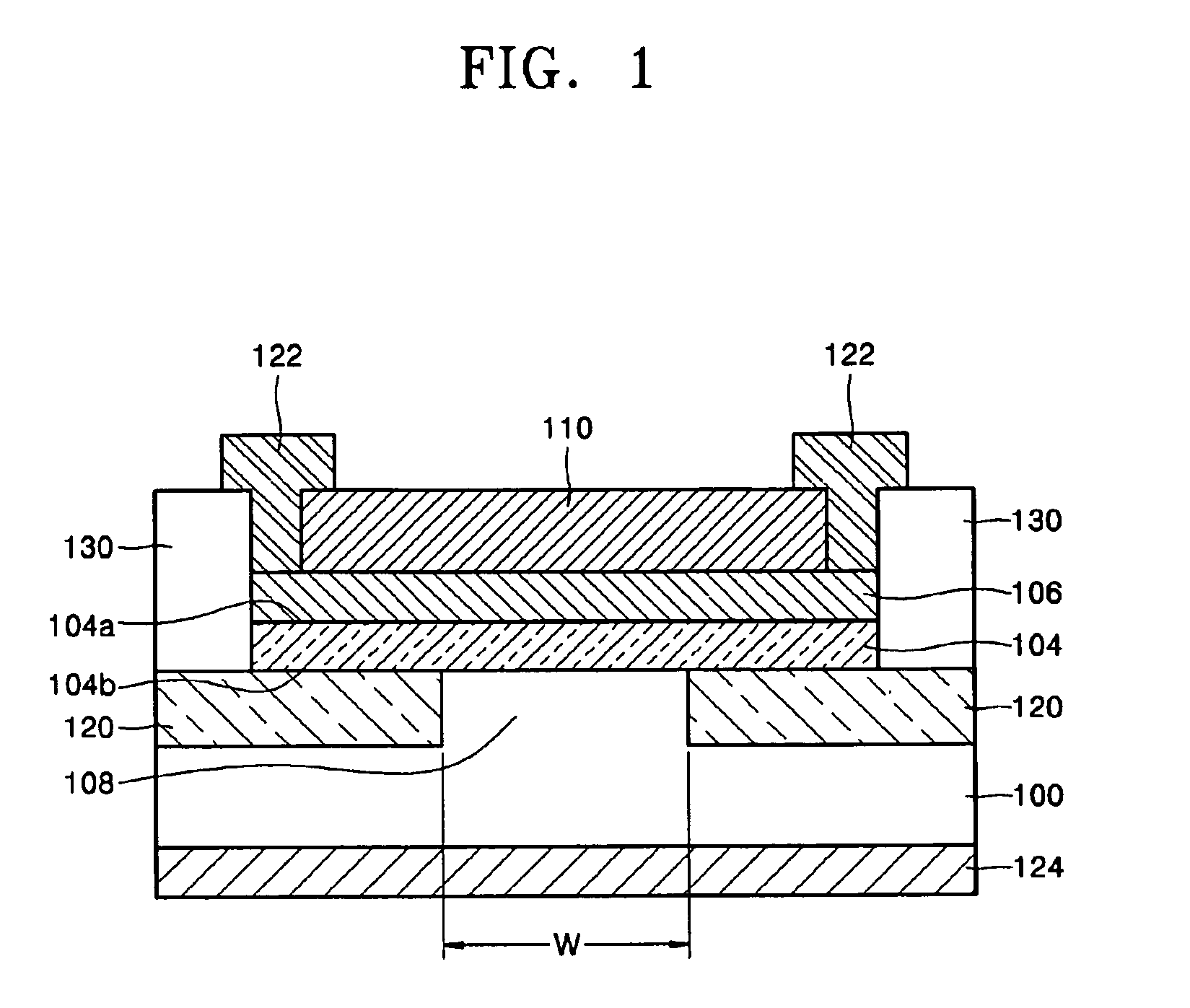
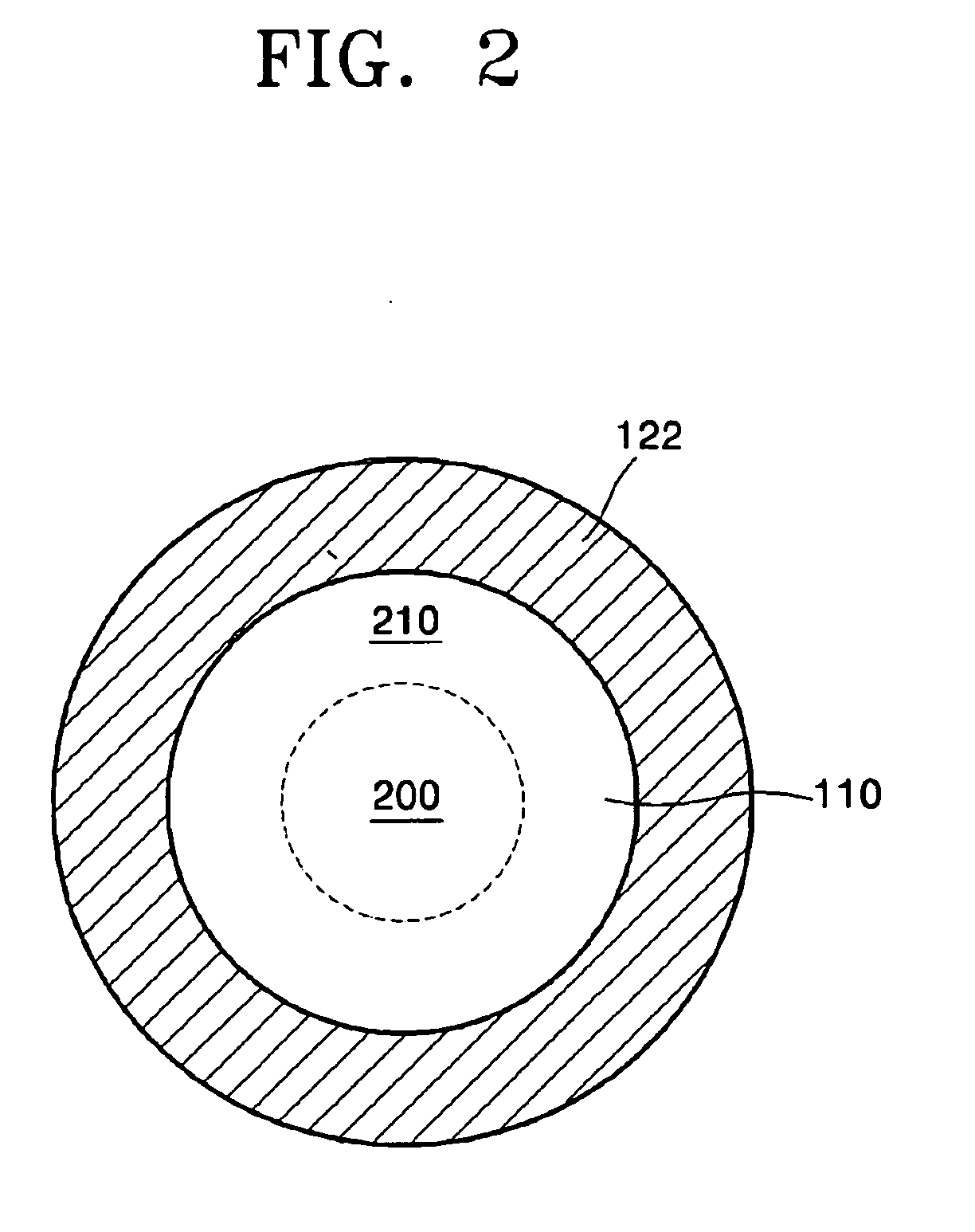
Light source comprising a light emitter arranged inside a translucent outer envelope
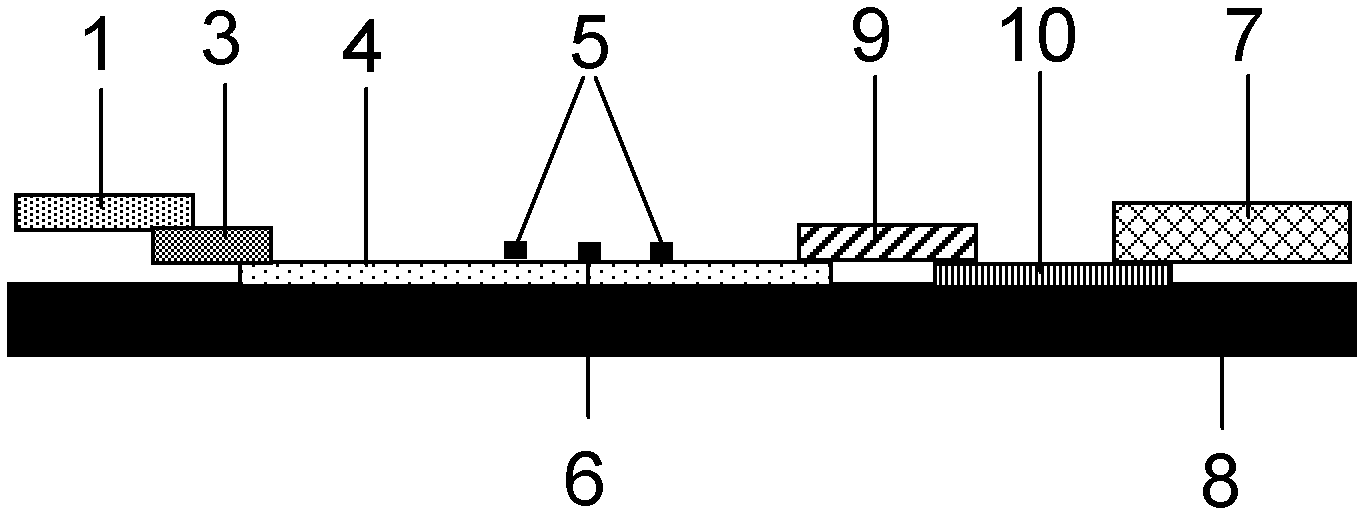
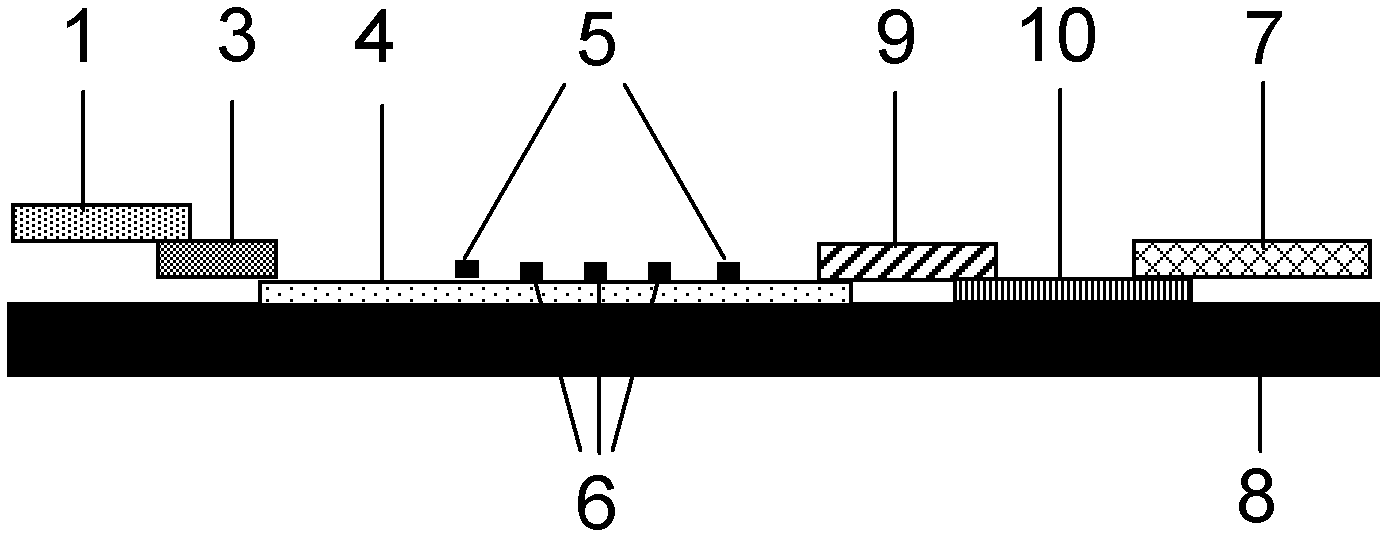
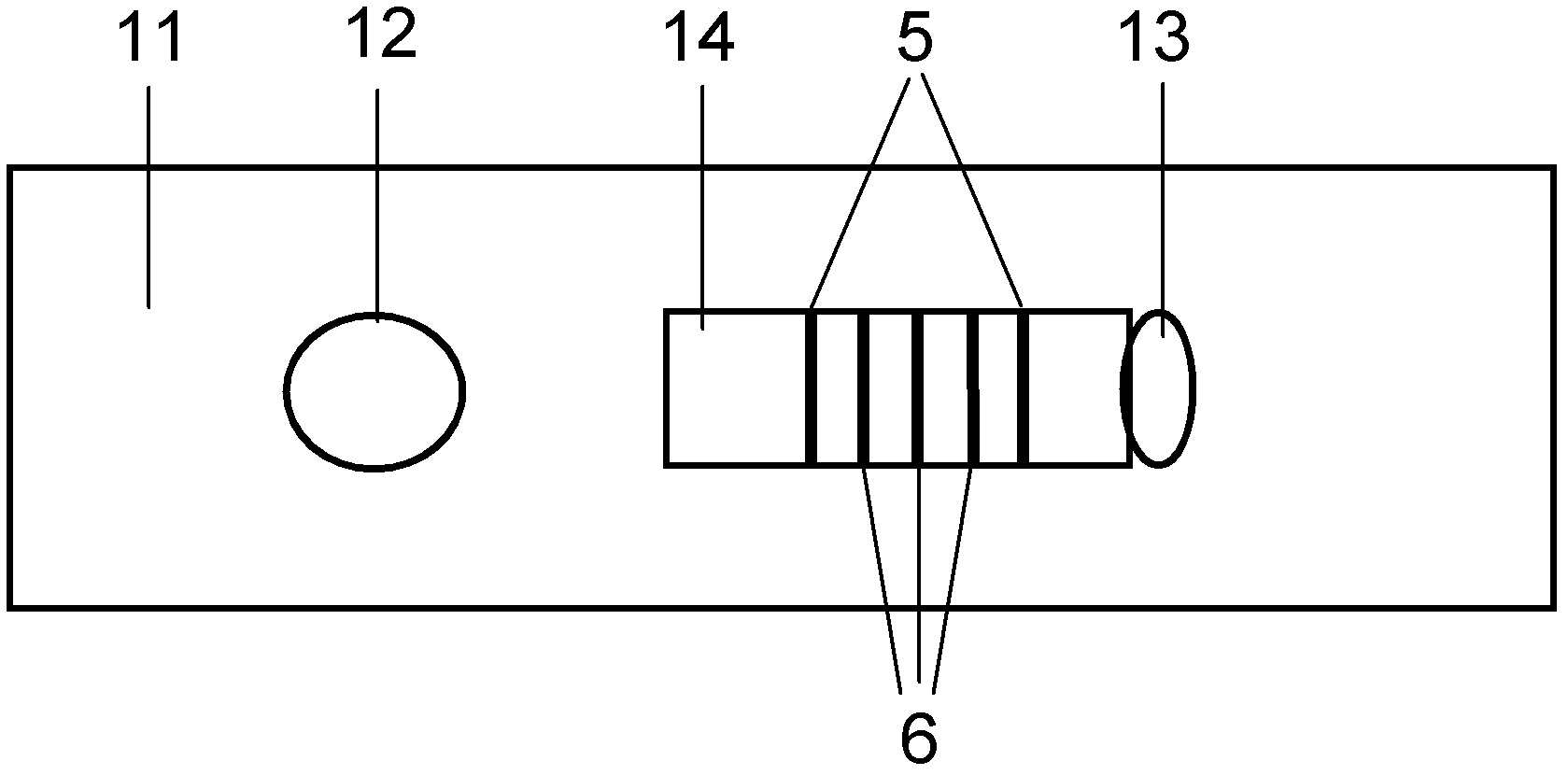
ActiveUS20120155059A1Increasing spatial emission profileSpatial emission profilePoint-like light sourceLighting heating/cooling arrangementsLight emitting deviceInner envelope
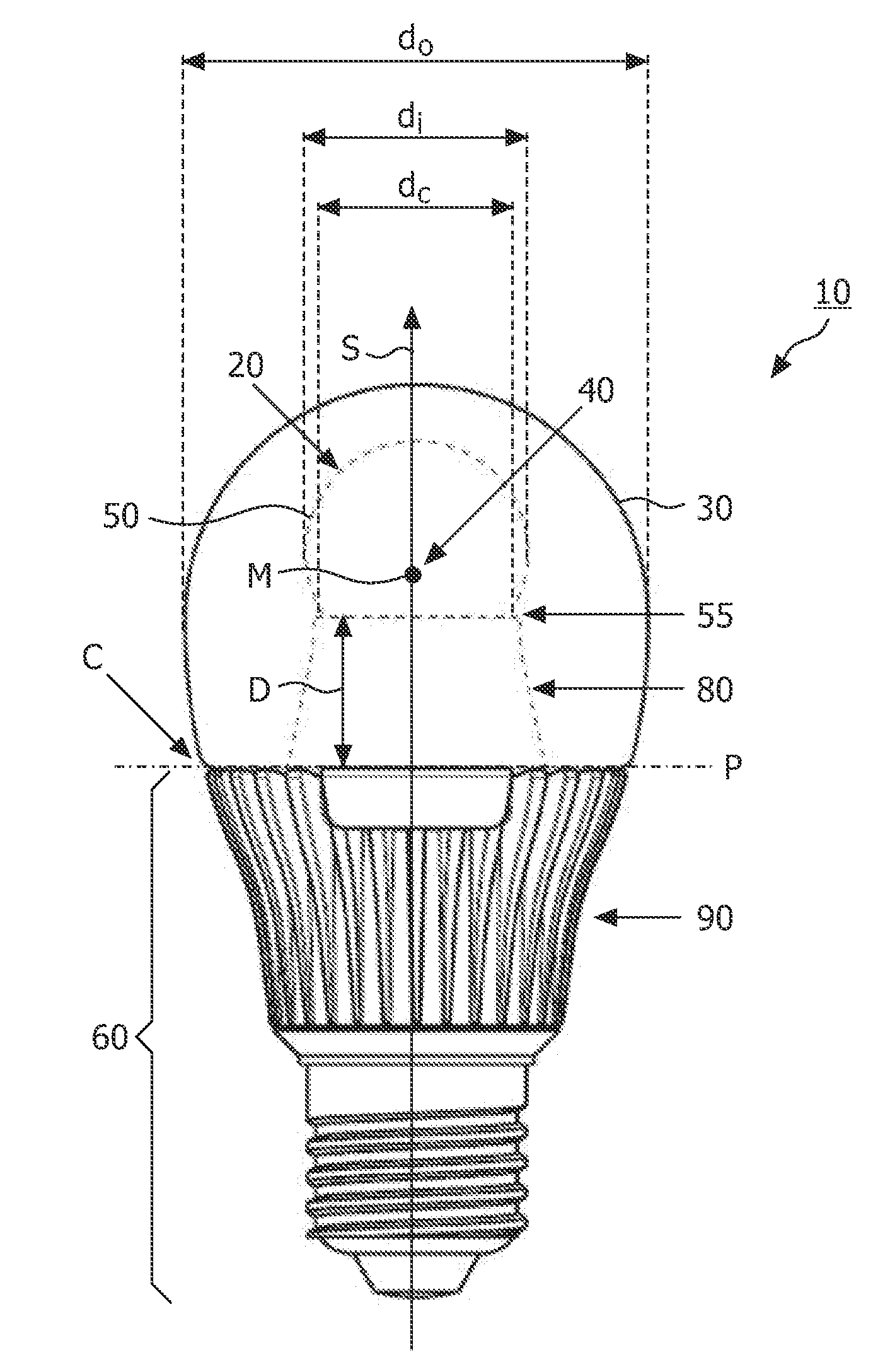
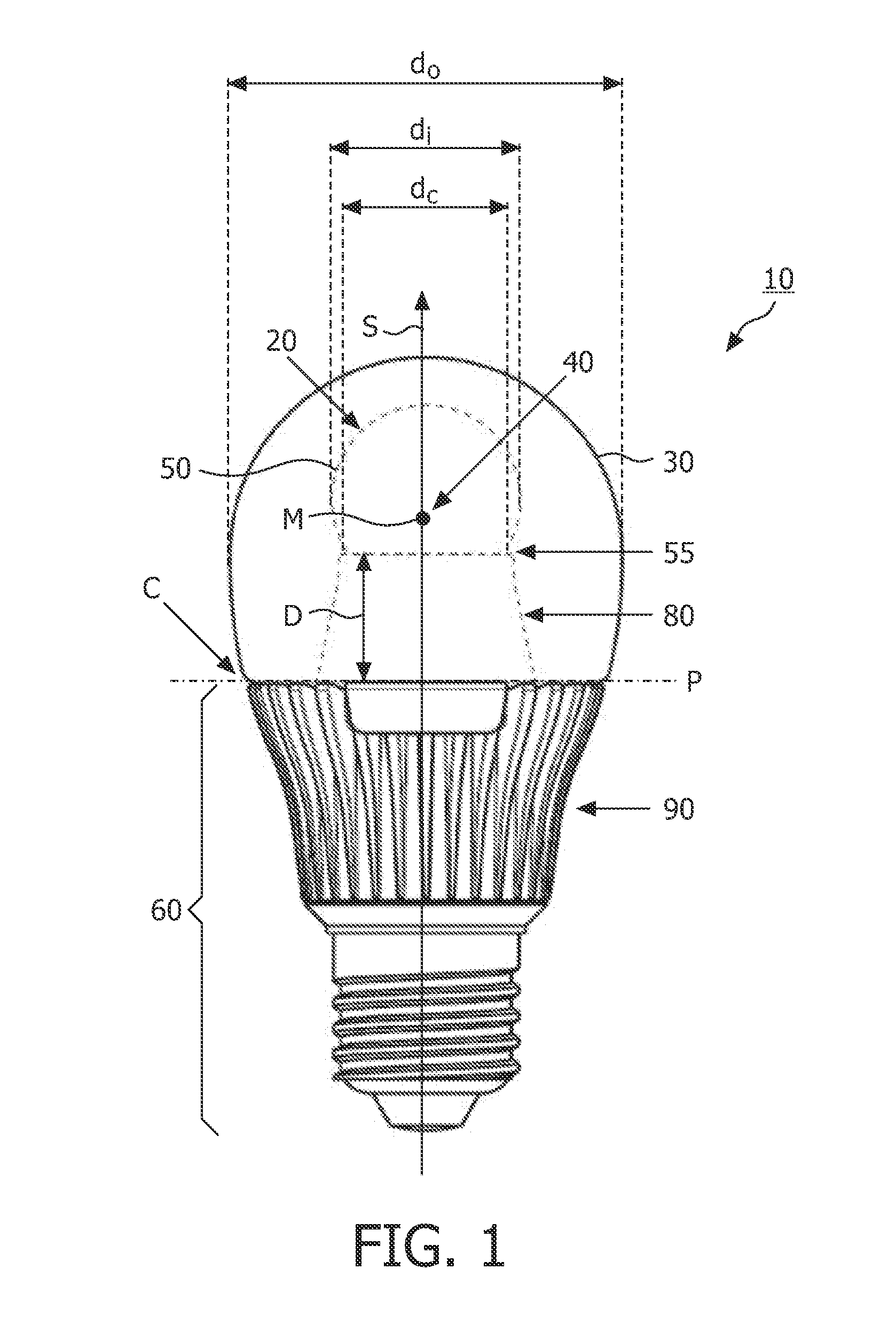
The invention relates to a light source (10, 12) comprising a light emitter (20) arranged inside a translucent outer envelope (30, 32). The light emitter comprising a light emitting device (40) and comprising a translucent inner envelope (50) at least partially surrounding the light emitting device, the translucent inner envelope comprising a diffuser. A diameter (di) of the translucent inner envelope is smaller than a diameter (do) of the translucent outer envelope. The translucent outer envelope is connected to a base (60) not being translucent. The translucent outer envelope further comprises a symmetry axis (S). An imaginary base-plane (P) is defined substantially perpendicular to the symmetry axis (S) and intersects with a connection point (C) being part of the translucent outer envelope. The connection point is a light transmitting part of the translucent outer envelope at an interface between the translucent outer envelope and the base at a furthest distance from a center (M) of the translucent outer envelope. The light emitter is arranged inside the translucent outer envelope at a distance from the imaginary base-plane away from the base.An effect of the light source according to the invention is that the emission profile of the light source according to the invention is increased.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay
ActiveCN102520165ASensitive quantitative detection fastRealize detectionMaterial analysisCritical illnessLinear range
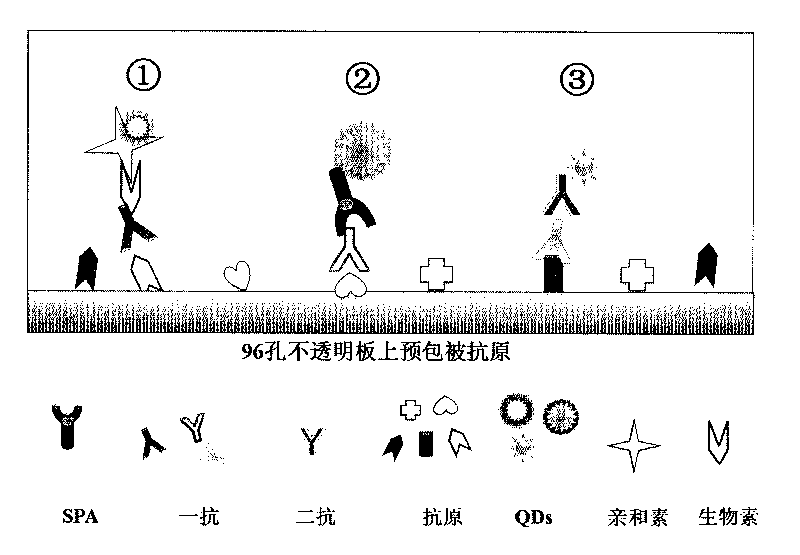
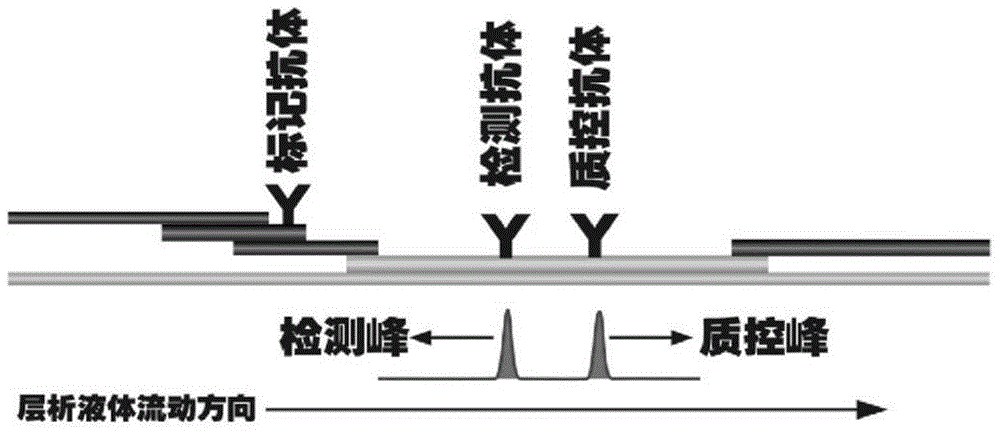
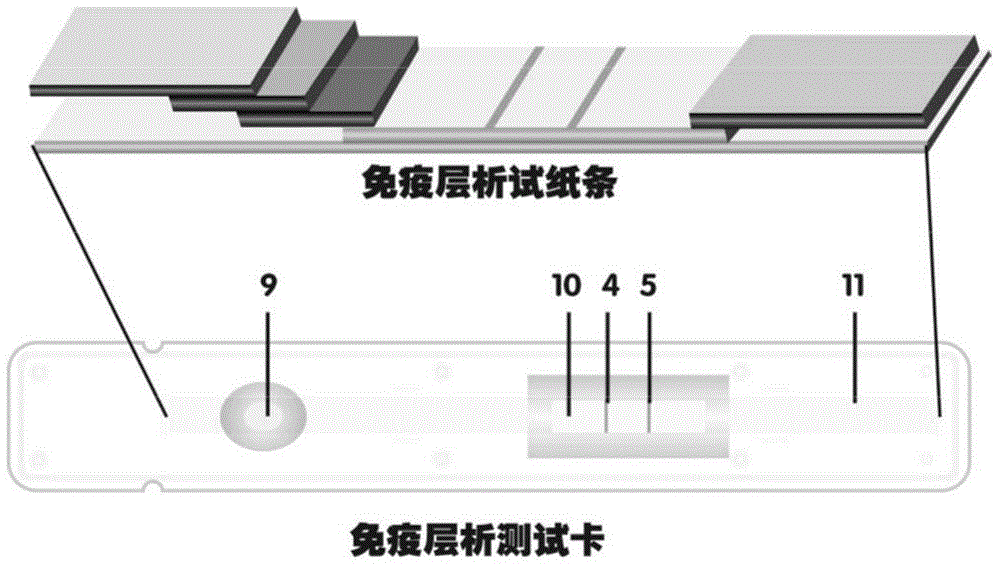
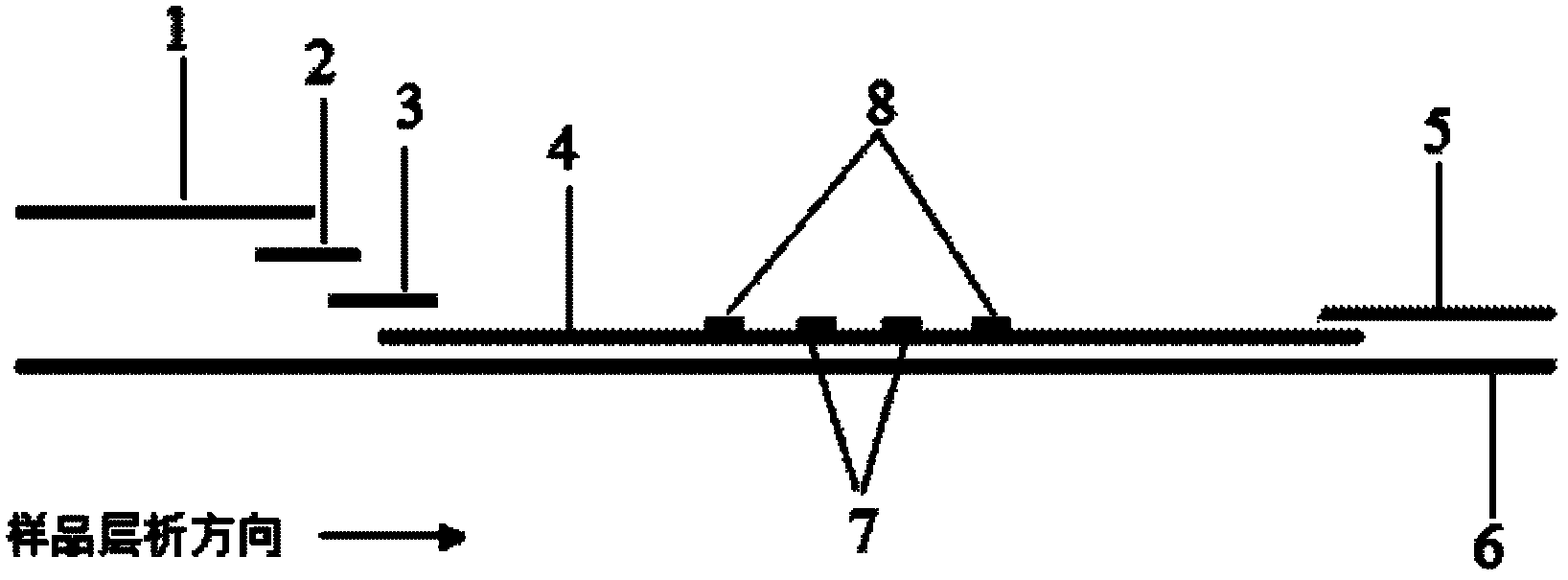
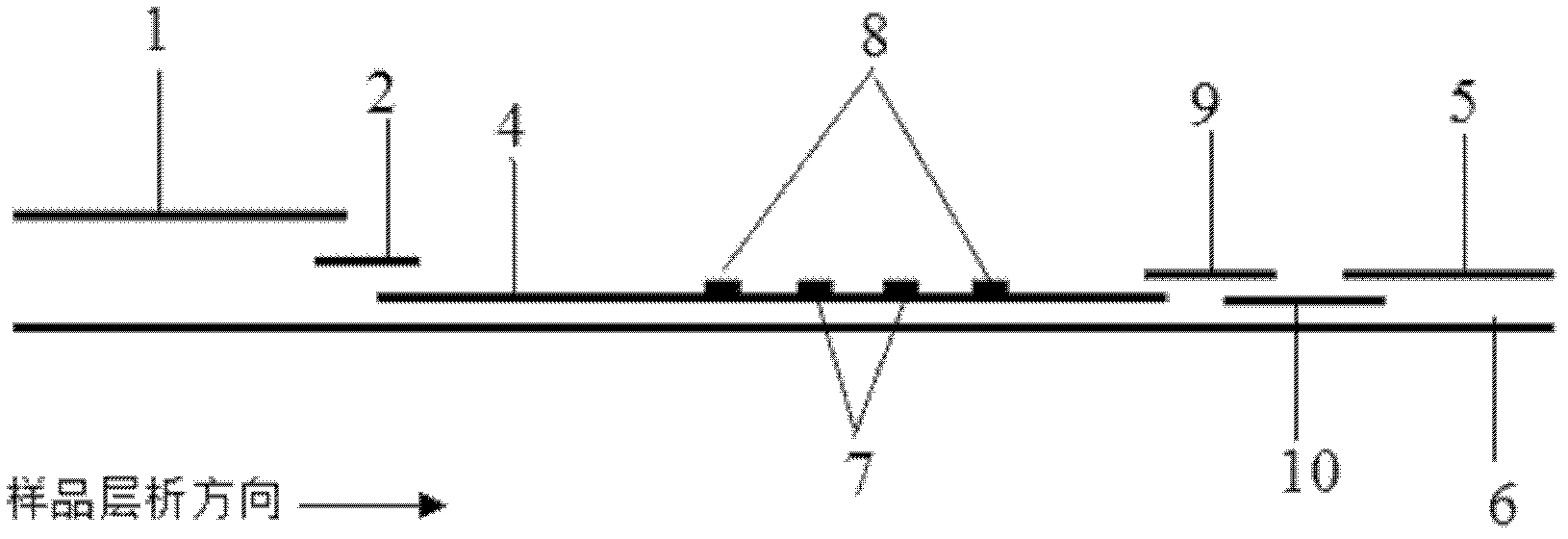
The invention discloses a method for highly sensitive quantitative detection of quantum dot fluorescence immunochromatographic assay. The method includes: building a fluorescence immunochromatographic assay test strip on the basis of optimizing the structure of the test strip and components by the aid of excellent fluorescent characteristics of quantum dots and by means of combining quantum dot fluorescence labeling technology and immunochromatographic assay; detecting fluorescence signal strength of a quantitative belt and a quality control belt by the aid of a fluorescence quantometer and correcting the fluorescence strength of the quantitative belt by the aid of the quality control belt after immunochromatographic assay of the test strip; and further quantitatively detecting analyte according to a standard curve obtained by the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and quite high in sensitivity. Compared with a conventional colloidal gold immunochromatographic assay method, the method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The method is applicable to samples such as blood samples, urine samples, spittle, excrement and the like, and can be applied to detection of critical illness, poison, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Dark blue organic light-emitting material and preparation method and application thereof
InactiveCN110790782AHigh color purityImprove stabilitySilicon organic compoundsSolid-state devicesBond energyULTRAMARINE BLUE
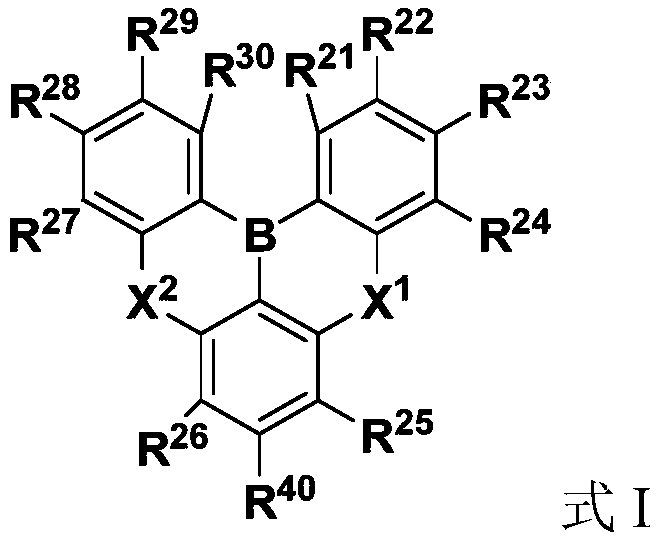
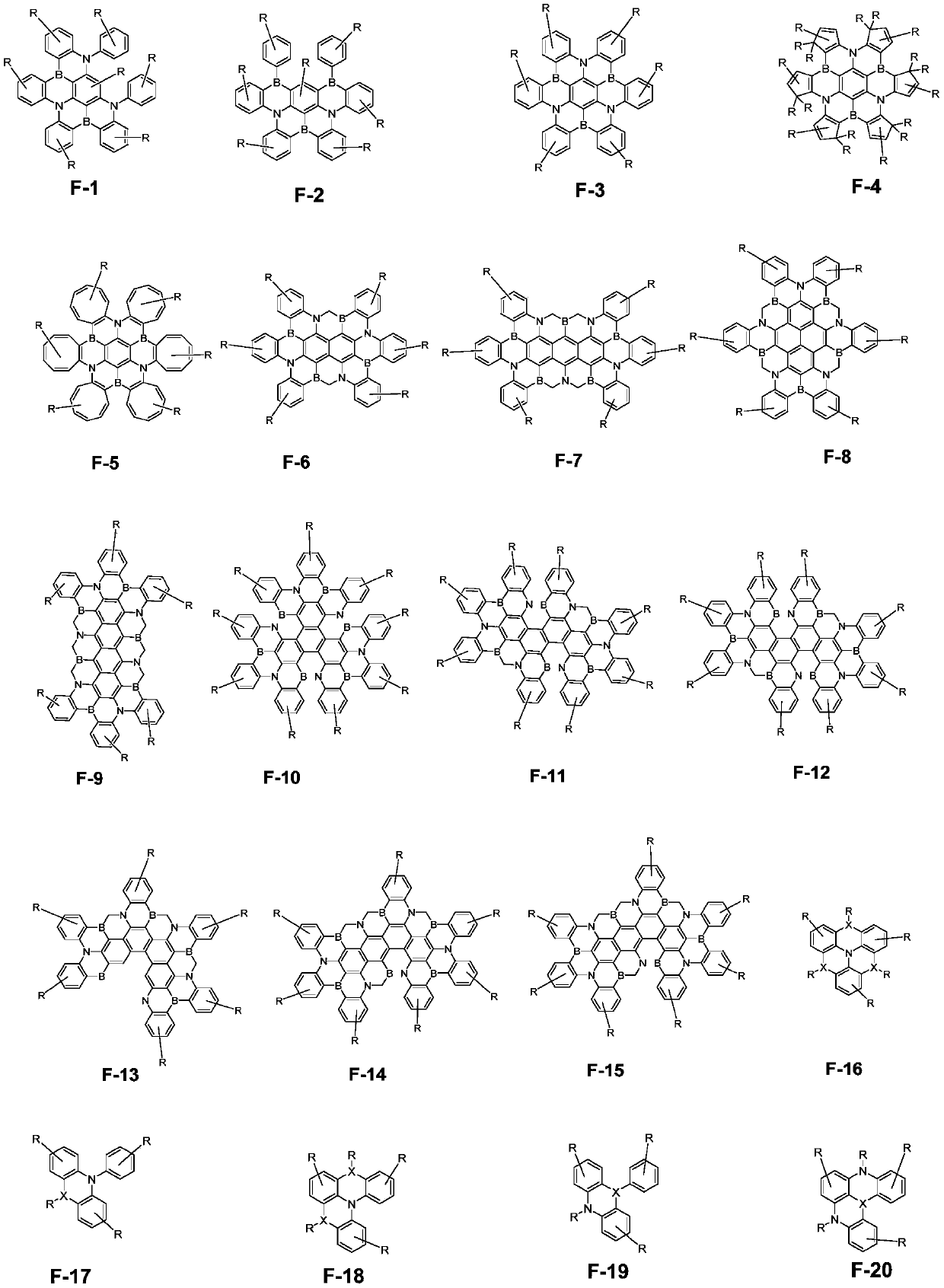
The invention discloses a dark blue organic light-emitting material and a preparation method and application thereof. The dark blue organic light-emitting material contains a structural unit disclosedin the invention, wherein, M is B or Bi; X is O, S or NR4; R1-R4 are independently selected from connecting bonds or groups obtained from H-H, H-F, H-O-H, H-S-H, H-CN, saturated hydrocarbons, unsaturated hydrocarbons, fluorinated hydrocarbons, heterocyclic compounds, organoboron, organosilicone, alcohols, mercaptans, ethers, thioethers, phenols, thiophenol, aldehydes, ketones, amines, amides, nitriles or sulfones losing one or more H; R1-R3 are located at any substitution position on rings of the structural unit where R1-R3 are located, and the bond energy between the ring where R3 is locatedand M is greater than or equal to the bond energy between the ring where R2 is located and M. The dark blue organic light-emitting material containing the B / Bi-N main body structure has very narrow light-emitting spectrum and TADF properties; the color purity is high, and the stability is good.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
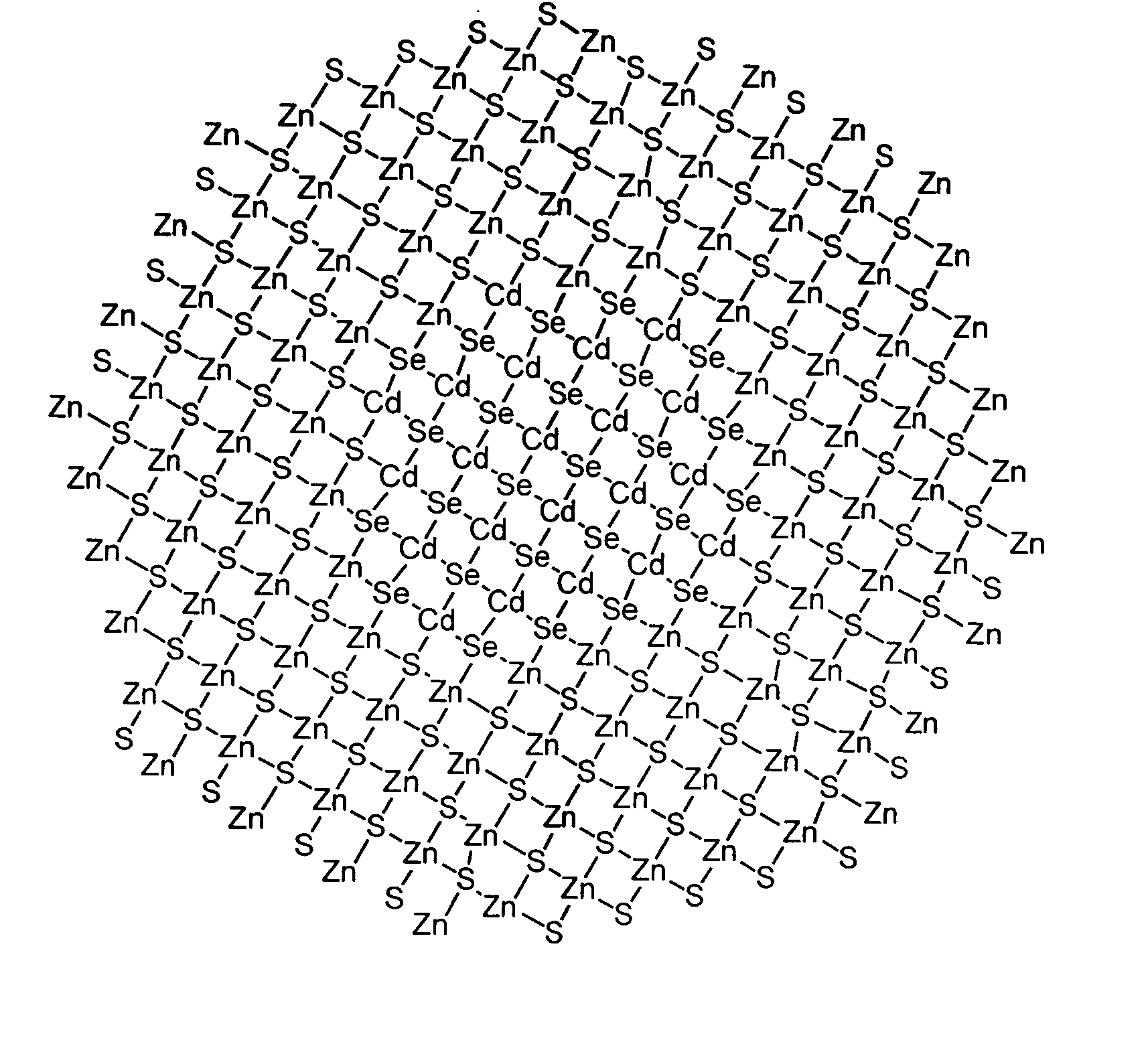
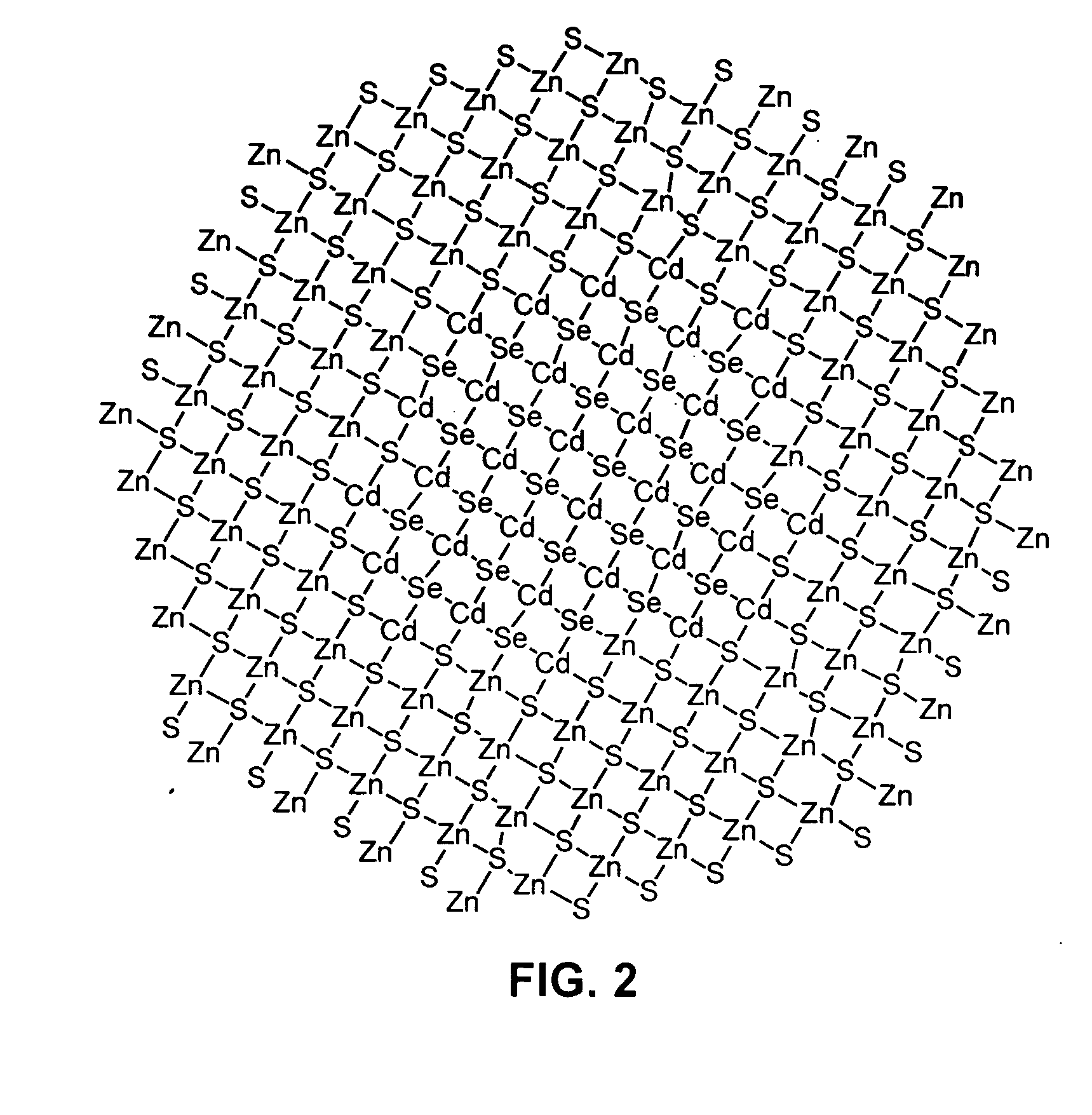
Luminescent nanoparticles and methods for their preparation
InactiveUS20060057382A1Guaranteed accuracyGood colorNanostructure manufacturePretreated surfacesGroup 12 elementNanoparticle
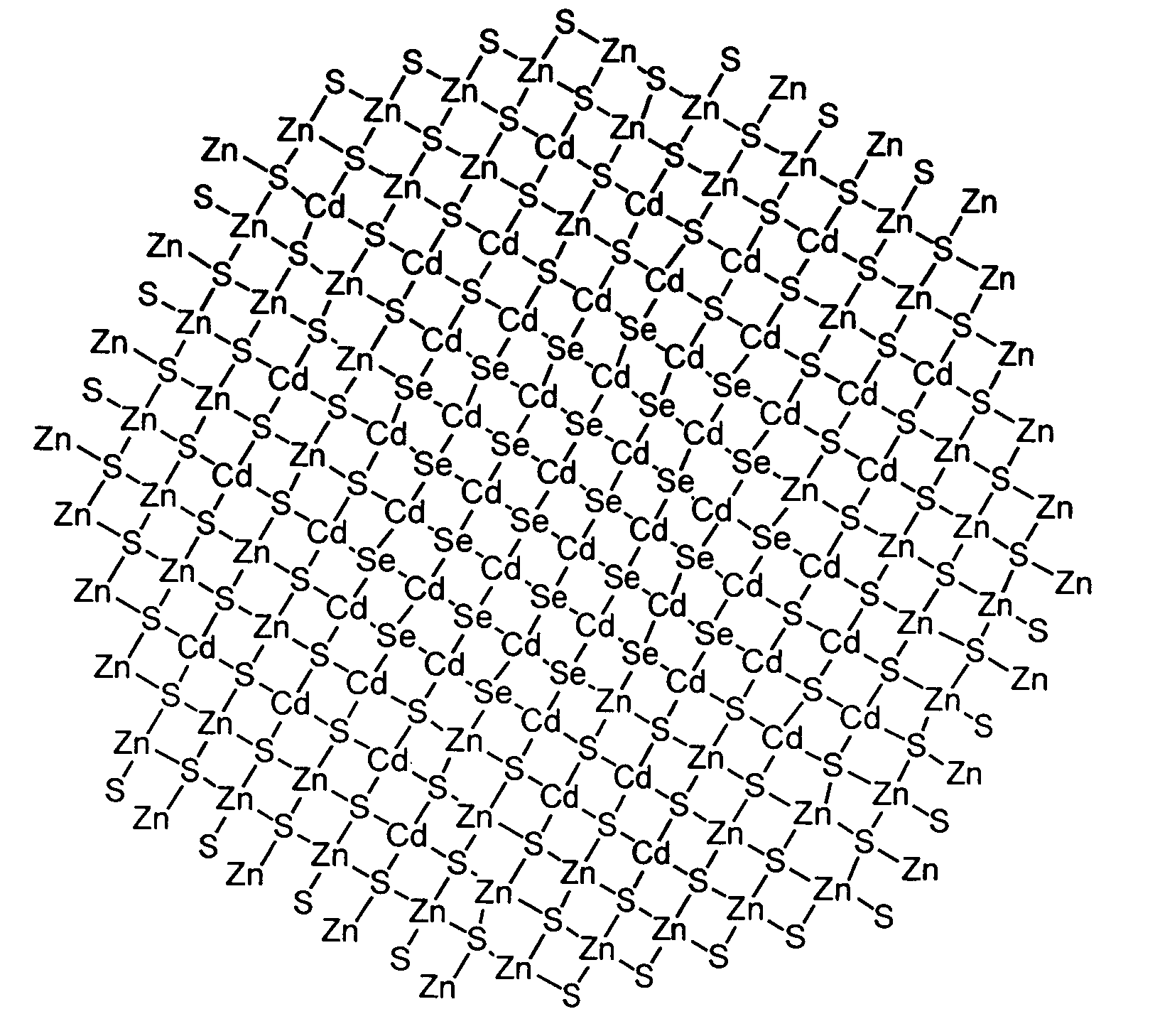
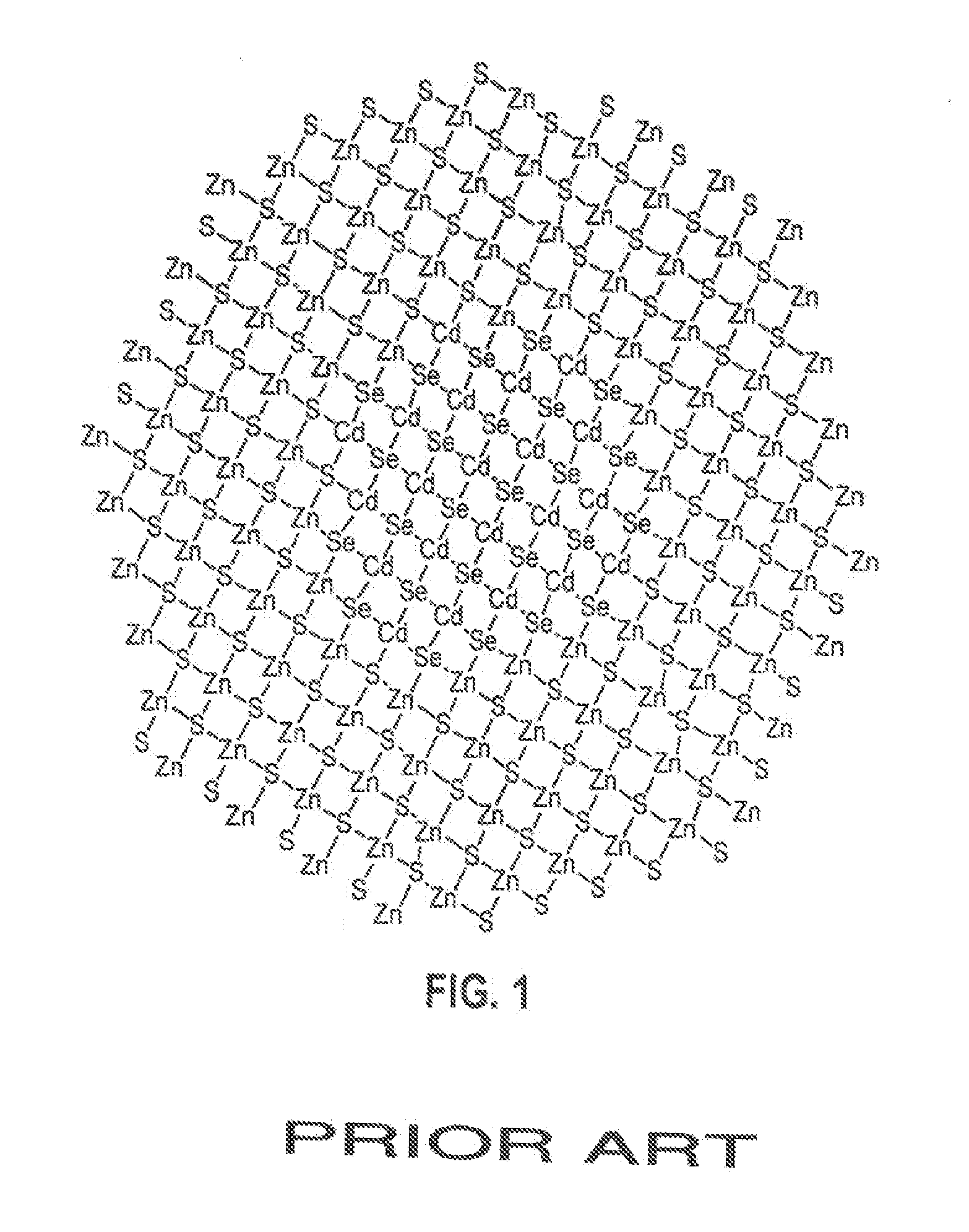
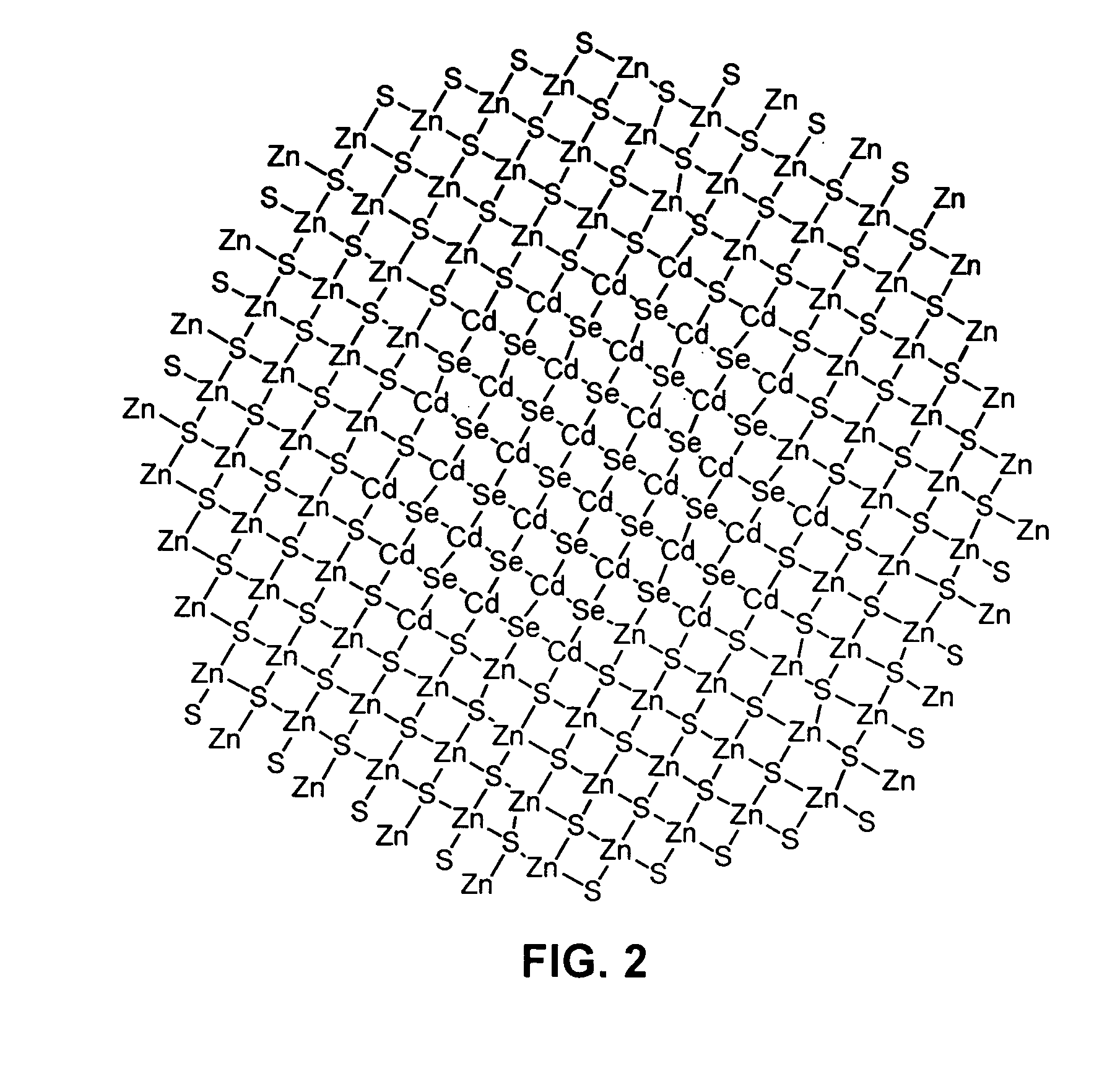
Methods for synthesizing luminescent nanoparticles and nanoparticles prepared by such methods are provided. The nanoparticles are prepared by a method in which an additive is included in the reaction mixture. The additive may be a Group 2 element, a Group 12 element, a Group 13 element, a Group 14 element, a Group 15 element, or a Group 16 element. In additions, a luminescent nanoparticle is provided that comprises a semiconductive core surrounded by an inorganic shell, an interfacial region and an additive present in the interfacial region or both the interfacial region and the shell.
Owner:INVITROGEN
Fluorescence immunochromatographic assay and kit for quantitative detection of troponin I/creatine kinase isoenzyme/myohemoglobin
ActiveCN102520192AHigh sensitivityHigh detection sensitivityBiological testingNon specificImmunochromatographic Assays
The invention discloses a quantum dot multicolor marking method for quantitative detection of various cardiovascular disease markers and a kit of troponin I / creatine kinase isoenzyme / myohemoglobin. The method realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a multicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the method has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range, small cross interference, and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the troponin I, the creatine kinase isoenzyme and the myohemoglobin simultaneously, is suitable for detection of whole blood, blood serum and plasma samples, can provide a reference for cardiovascular and cerebrovascular disease diagnosis, and is widely applied to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
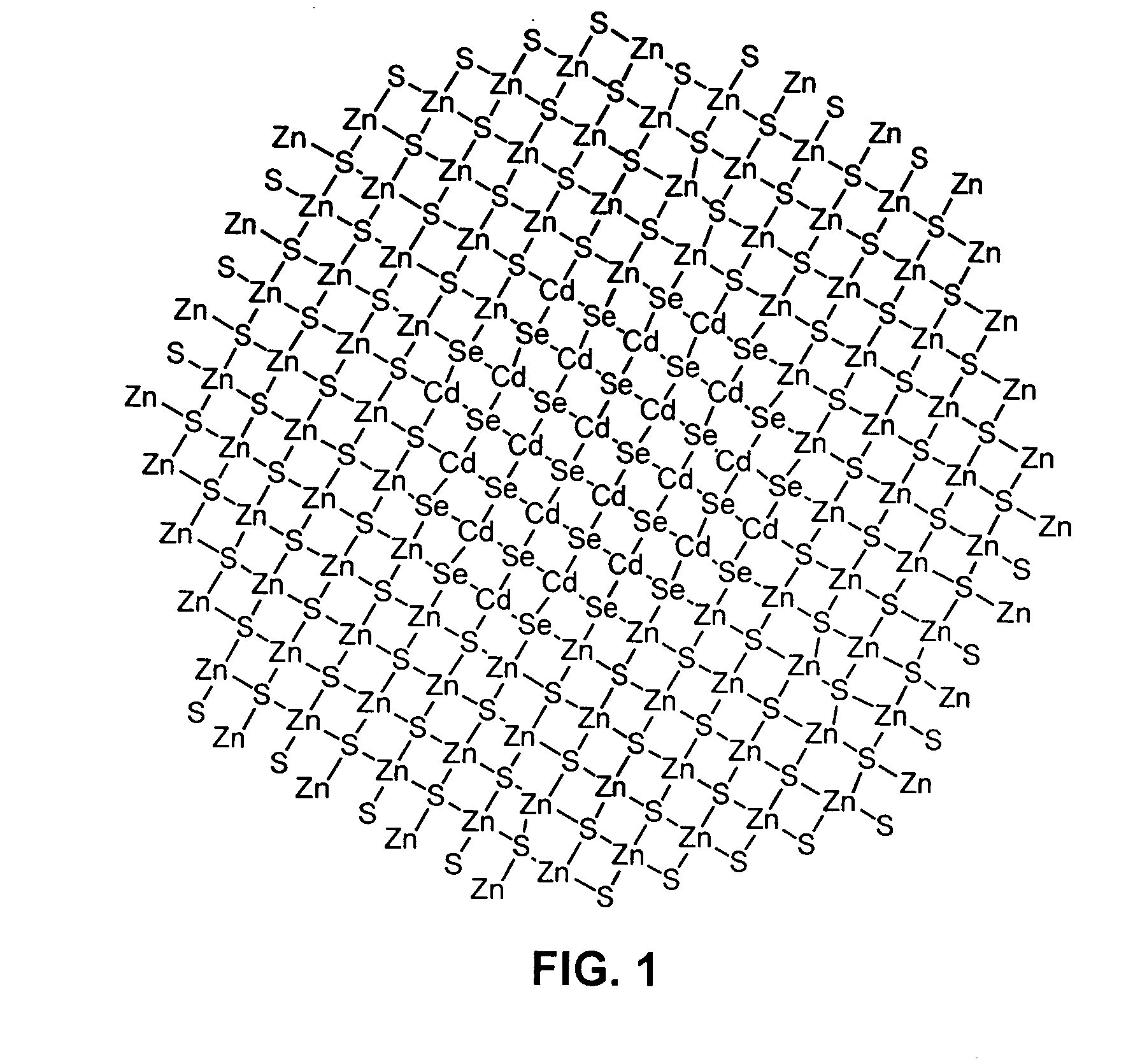
Luminescent nanoparticles and methods for their preparation
InactiveUS7172791B2Good colorNarrow emission spectrumNanostructure manufacturePretreated surfacesGroup 12 elementNanoparticle
Methods for synthesizing luminescent nanoparticles and nanoparticles prepared by such methods are provided. The nanoparticles are prepared by a method in which an additive is included in the reaction mixture. The additive may be a Group 2 element, a Group 12 element, a Group 13 element, a Group 14 element, a Group 15 element, or a Group 16 element. In additions, a luminescent nanoparticle is provided that comprises a semiconductive core surrounded by an inorganic shell, an interfacial region and an additive present in the interfacial region or both the interfacial region and the shell.
Owner:INVITROGEN
Method of detecting residue of small-molecule substance harmful to human body and a special kit
InactiveCN101762706AExcitation spectrum widthNarrow emission spectrumFluorescence/phosphorescenceHuman bodyBiology
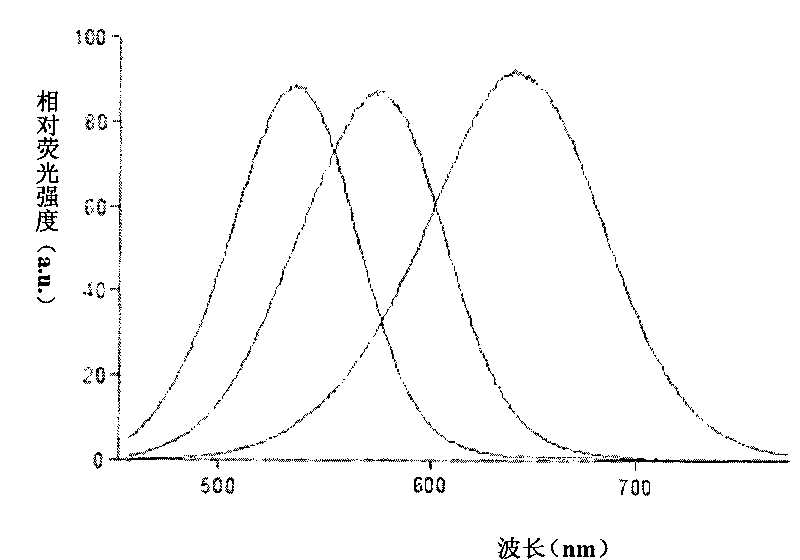
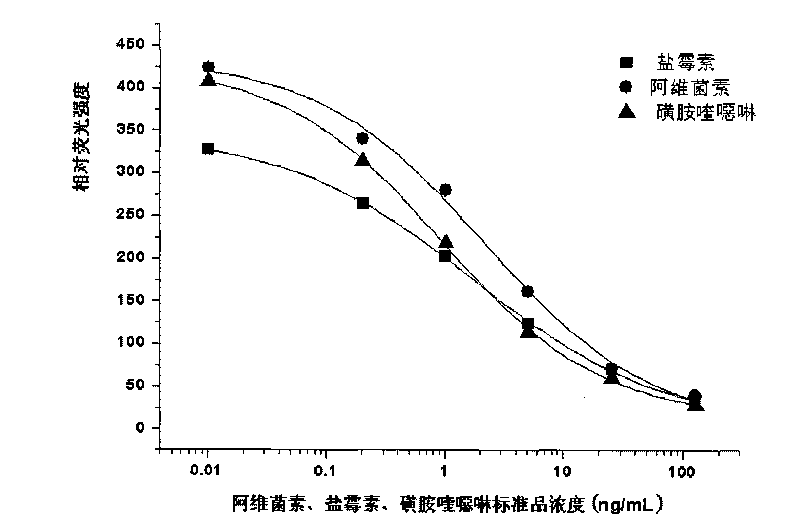
The invention discloses a method of detecting the residue of small-molecule substances harmful to the human body and a special kit. The special kit comprises a non-transparent micro-porous plate and a light-emitting compound, wherein each hole of the non-transparent micro-porous plate is filled with a coat antigen which is simultaneously coated with three kinds of small-molecule substances. The invention makes full use of the multi-color marking function of QDs, establishes a novel kit for simple and rapid detection of the residue and a method thereof, and realizes the multi-color marking through indirect marking of polyclonal antibodies and monoclonal antibodies in the veterinary drug by coupling the QDs with different particle sizes and targets with functional groups (such as an amino) with specific surfaces. The method comprises: obtaining quantum dots with different fluorescent characteristics through separation and purification, namely, multi-color antibody markers, using the multi-color antibody markers as fluorescent probes, and establishing a reaction system for synchronous analysis of various antigen components of different kinds, thereby realizing the synchronous detection of multiple kinds of residues of the veterinary drug in animal food. Moreover, the method has the advantages of simple operation, high fluorescence intensity and long stabilization time.
Owner:CHINA AGRI UNIV
Dual-mode optical coding probe and preparation method thereof
InactiveCN102559190AEnhancing Optical Encoding CapabilitiesCapable of joint codingRaman scatteringFluorescence/phosphorescenceGold nanorodFluorescence
The invention discloses a dual-mode optical coding probe and a preparation method thereof. The probe is in a three-layer core-shell structure, the first-layer core is a gold nano bar, the second-layer shell is silica, the third-layer shell is a cadmium telluride quantum dot, the second-layer shell is wrapped on the outer side of the first-layer core, the third-layer shell covers the outer side ofthe second-layer shell in a sticking mode, and the outer surface of the first-layer core is stuck with Raman molecules which are wrapped by the second-layer shell. The preparation method of the dual-mode optical coding probe comprises the following steps of: 1, preparing an original gold nano bar solution; 2, preparing gold nano bars marked by the Raman molecules; 3, preparing a gold nano bar andsilica metal medium composite nano ball solution; and 4, preparing the dual-mode optical coding probe. The dual-mode optical coding probe has the joint encoding capacity of fluorescence and SERS (Surface Enhanced Raman Scattering), and enhances the optical coding capacity. The preparation method of the dual-mode optical coding probe has a simple process and high repeatability.
Owner:SOUTHEAST UNIV
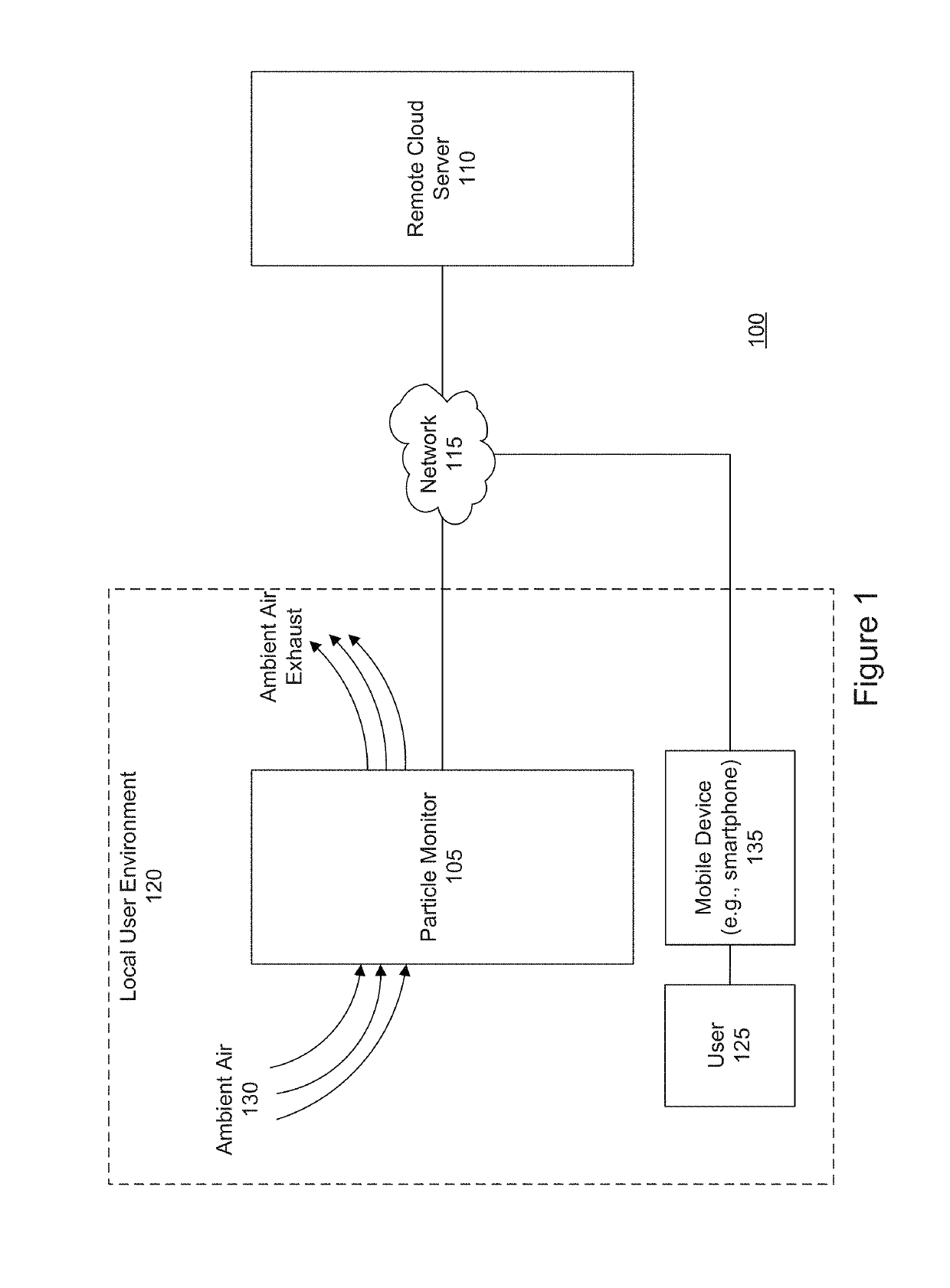
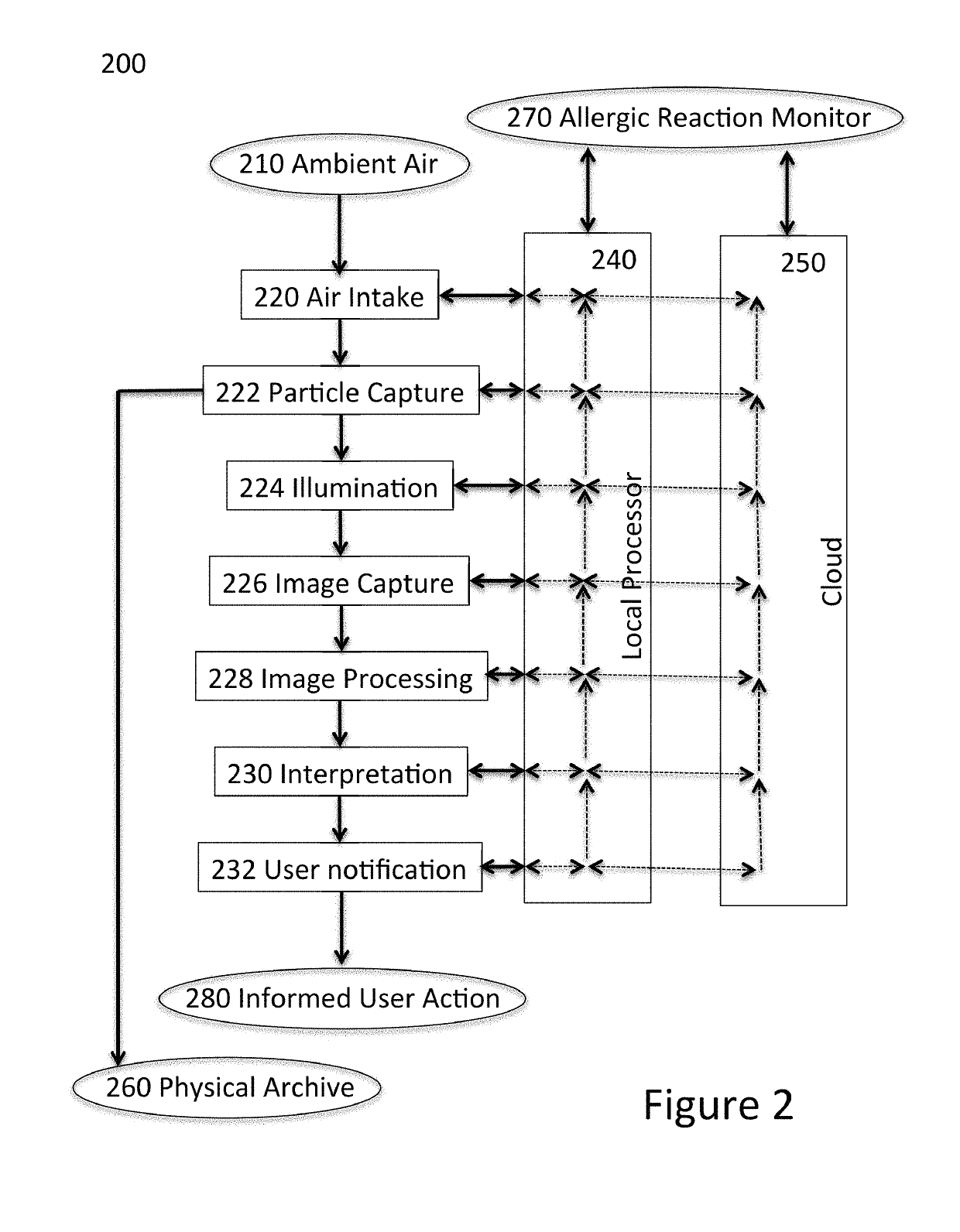
Airborne particle monitor
ActiveUS20190293539A1Narrow emission spectrumWithdrawing sample devicesMaterial analysis by optical meansCollection systemAdhesive
An airborne particle collection system includes a monitoring device and a collection media. The monitoring device includes a housing having an air-intake slot, and a motor. The collection media includes an adhesive-coated tape contained within a removable cartridge inserted into the housing. The removable cartridge includes a particle intake zone, proximate to the air-intake slot, and through which the adhesive-coated tape is exposed to capture airborne particles passing through the air-intake slot, and an inspection zone at which the airborne particles captured at the intake zone are transported for optical imaging. The motor moves the airborne particles captured by the adhesive-coated tape from the particle intake zone to the inspection zone.
Owner:SCANIT TECH INC
Fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and reagent kit thereof
ActiveCN102539785ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceBasic levelQuantum dot
The invention discloses a fluorescent immunochromatography method for whole quantitative detection of C-reactive protein and a reagent kit thereof. The fluorescent immunochromatography method for the whole quantitative detection of the C-reactive protein (CRP) utilizes excellent fluorescent characteristics of quantum dots, and combines double-color marking technology and immunochromatography technology to achieve fluorescent quantitative detection on the basis of optimizing each constituent elements of test paper. Compared with a conventional colloidal gold immunochromatography method, the fluorescent immunochromatography method for the whole quantitative detection of the CRP has the advantages of being good in stability, low in non-specificity, high in flexibility, wide in linear range and accurate in quantifying. The reagent kit of the fluorescent immunochromatography method can perform the whole quantifying and can simultaneously predict and evaluate infectious diseases, antibiotic effects and cardiovascular and cerebrovascular diseases. The fluorescent immunochromatography method for the whole quantitative detection of the CRP and the reagent kit of the fluorescent immunochromatography method are suitable for various-level hospitals, and particularly contribute to wide popularization in basic-level hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
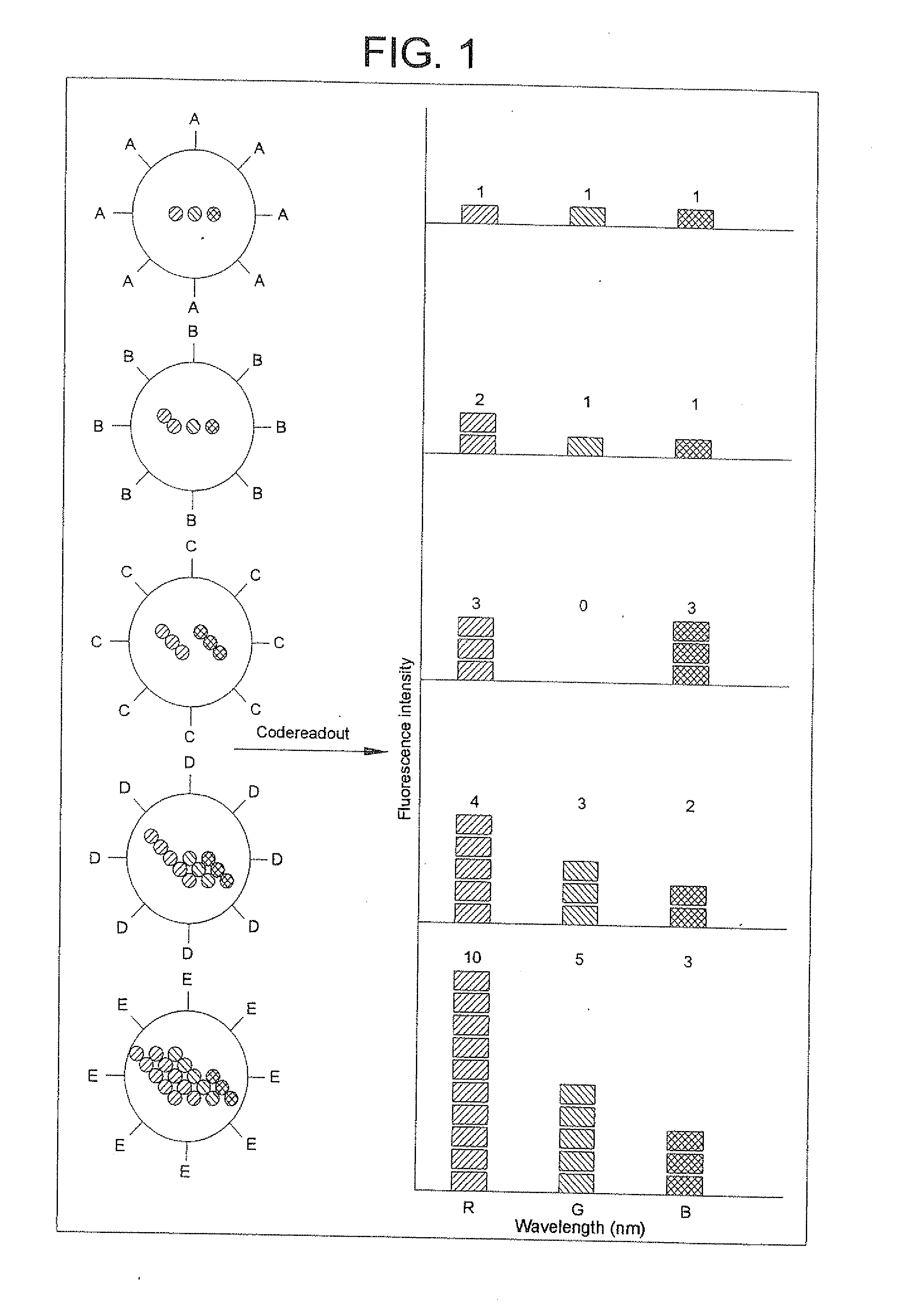
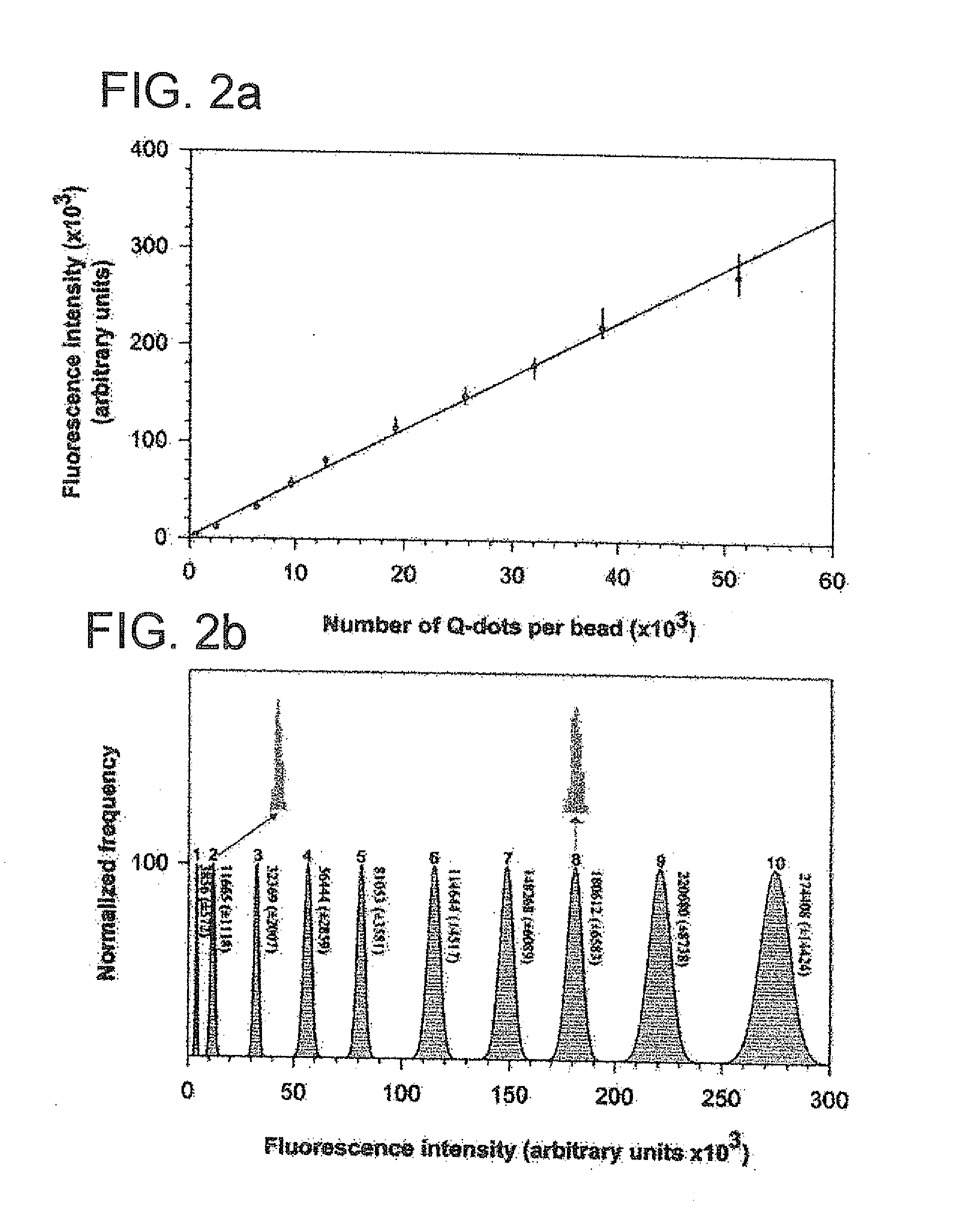
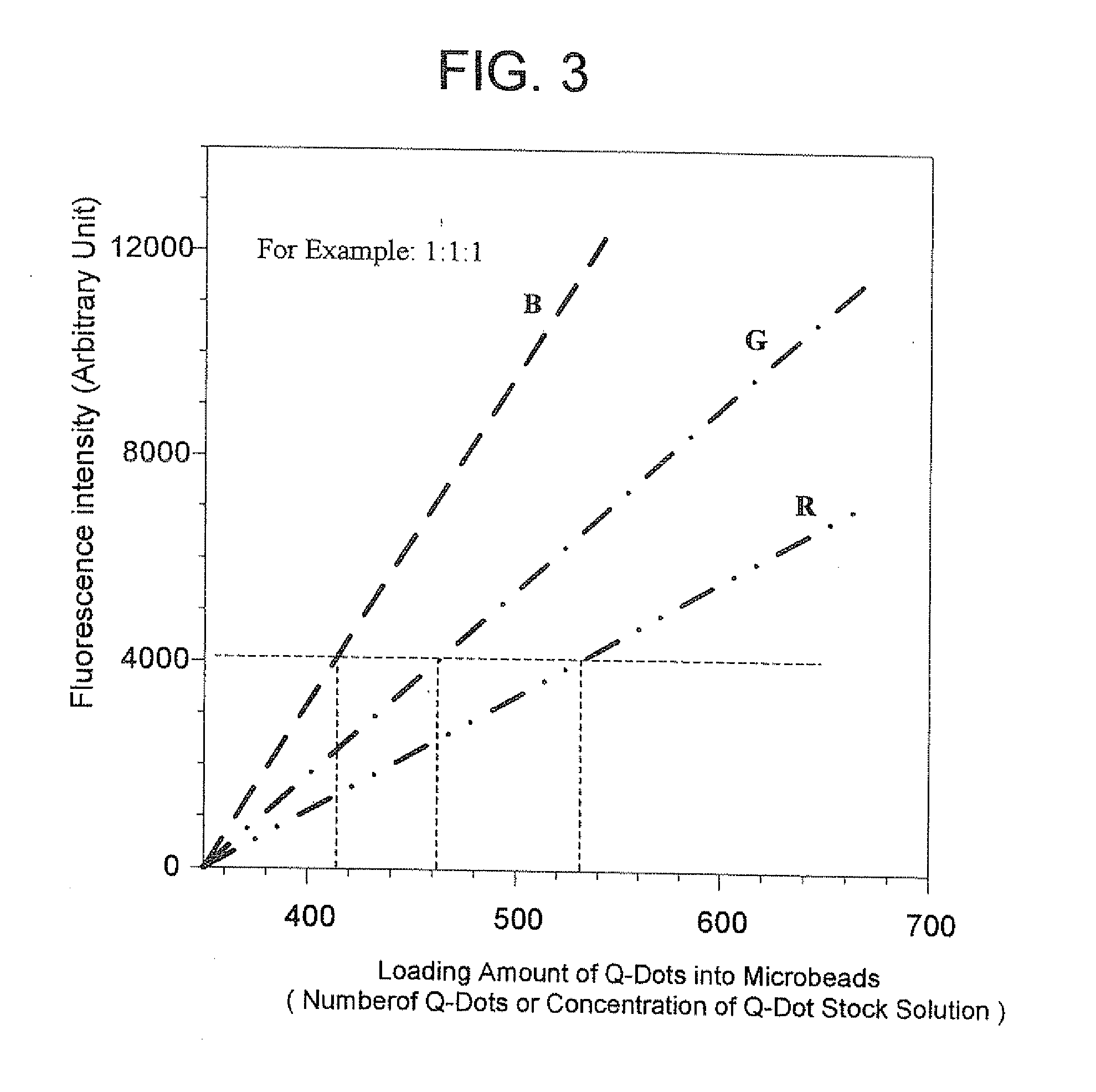
Methods of Preparing Multicolor Quantum Dot Tagged Beads and Conjugates Thereof
InactiveUS20070161043A1Narrow emission spectrumFlexibly selectMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementQuantum dotComputational chemistry
The present invention provides a method of preparing a multicolor quantum dot-tagged bead, a multicolor quantum dot-tagged bead, a conjugate thereof, and a composition comprising such a bead or conjugate. Additionally, the present invention provides a method of making a conjugate thereof and methods of using a conjugate for multiplexed analysis of target molecules.
Owner:ADVANCED RES TECH INST
Display panel and display device
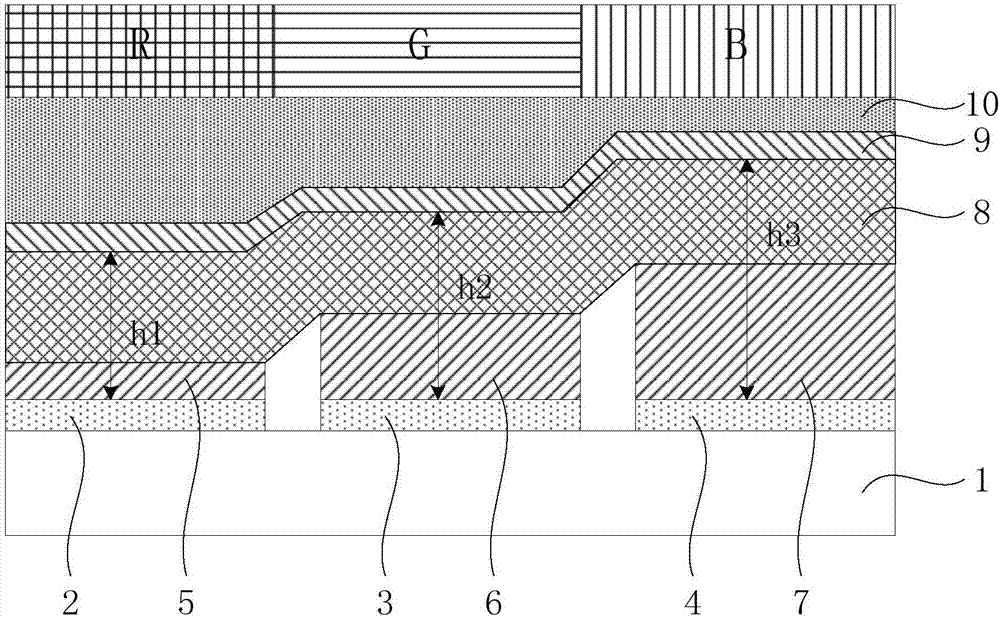
ActiveCN106981504AIncrease brightnessReduce power consumptionSolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceGreen-light
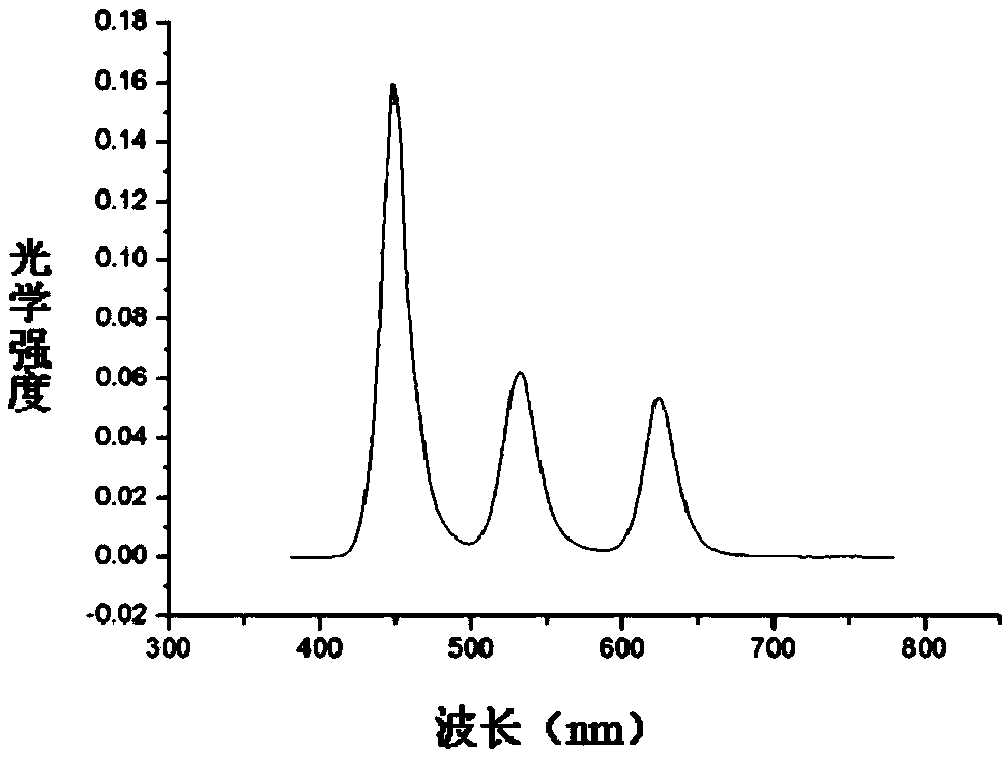
The invention discloses a display panel and a display device. The display panel comprises a reflection electrode layer; a microcavity structure layer formed on the side, away from a substrate, of a first electrode layer and including a first microcavity structure for transmitting red light, a second microcavity structure for transmitting green light, and a third microcavity structure for transmitting blue light; and a semitransparent electrode layer formed on the side, away from the substrate, of the microcavity structure layer. The microcavity structure layer comprises a white light emitting layer including a red light-emitting peak, a green light-emitting peak and a blue light-emitting peak the positions of which satisfy a condition that a difference between the red light-emitting peak and the green light-emitting peak is greater than or equal to the sum of the half-peak width of the red light-emitting peak and the half-peak width of the green light-emitting peak, and a difference between the green light-emitting peak and the blue light-emitting peak is greater than or equal to the sum of the half-peak width of the green light-emitting peak and the half-peak width of the blue light-emitting peak. The process cost and the power consumption of the display device are reduced and the brightness of the display device is increased.
Owner:SOUTH CHINA UNIV OF TECH
Electromodulation MEMS infrared source and fabrication method thereof
InactiveCN103332648AStable structureImprove work performanceDecorative surface effectsSolid-state devicesElectricityWork performance
The invention relates to the technical field of infrared and discloses an electromodulation MEMS (Micro-electro-mechanical Systems) infrared source and fabrication method thereof. According to the infrared source and the method, a four-side fixed support structure is adopted, heating electrodes fixed on a support film are used for generating external infrared radiation energy, so that the electromodulation MEMS infrared source is secure in structure, stable in working performance, and high in duty ratio, a fabrication technology is simple, the cost is low, and massive production with low cost is realized.
Owner:NANJING MOMANSI ELECTRONICS TECH
Organic electroluminescent device, display panel and display device
ActiveCN111029477AHigh color purityReduce displacementSolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceGreen-light
The invention relates to an organic electroluminescent device, a display panel and a display device. The organic electroluminescent device comprises a first electrode, a second electrode and an organic layer located between the first electrode and the second electrode. The organic layer comprises a light-emitting layer, the light-emitting layer contains a main material, a thermal activation delayed fluorescence sensitizer and a green fluorescent dye, and the green fluorescent dye has a structure as shown in a formula I. The thermal activation sensitization fluorescence technology is used, andthe green fluorescent dye with a specific structure, the sensitizing agent and the main material are matched for use so that the effects of narrowing the spectrum of the device and improving the colorpurity of green light are achieved, the device has the efficiency equivalent to that of a phosphorescent green light device, and the display panel comprising the device has a relatively high displaycolor gamut area.
Owner:KUNSHAN GO VISIONOX OPTO ELECTRONICS CO LTD
Fluorescence immunochromatographic assay method for quantitatively detecting heart fatty acid binding protein and kit for quantitatively detecting same
ActiveCN102520194ASolve the backgroundSolve the signal indistinguishableBiological testingBlood plasmaBiology
The invention discloses a fluorescence immunochromatographic assay method for quantitatively detecting hFABP (heart fatty acid binding protein) and a kit for quantitatively detecting the same. The fluorescence immunochromatographic assay method for quantitatively detecting the hFABP realizes quantitative fluorescence detection on the basis of optimizing components of a test strip by the aid of excellent fluorescent characteristics of quantum dots and by means of combining bicolor labeling technique and immunochromatographic assay. Compared with a conventional colloidal gold immunochromatographic assay method, the fluorescence immunochromatographic assay method has the advantages of fine labeling stability, low non-specificity, high sensitivity, wide linear range and accuracy in quantization. The kit is used for quantitatively detecting the hFABP, can be used for simultaneously detecting whole blood, blood serum and plasma samples, serves as a simple, accurate, specific and inexpensive detecting tool for early screening and prognosis evaluation of acute myocardial infarction, is applicable to hospitals at all levels, and is particularly beneficial to wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Fluorescence immunochromatographic assay and kit for quantitatively detecting N-terminal pro brain natriuretic peptide
ActiveCN102565423ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescence/phosphorescenceDiseaseN-terminal pro-Brain Natriuretic Peptide
Owner:SHENZHEN KANGMEI BIOTECH
Silicon-based light emitting diode
ActiveUS20050139847A1High luminous efficiencyNarrow emission spectrumSolid-state devicesSemiconductor/solid-state device manufacturingDistributed Bragg reflectorActive layer
A silicon-based light emitting diode simultaneously adopts doping layers and Distributed Bragg Reflector (DBR). The silicon-based light emitting diode includes an active layer having mutually opposing a first side and a second side. A first reflecting portion faces with the first side of the active layer, and a second reflecting portion faces with the second side of the active layer. A first doping layer is interposed between the active layer and the first reflecting portion. A second doping layer is interposed between the active layer and the second reflecting portion. A first electrode is electrically connectable to the first doping layer, and a second electrode is electrically connectable to the second doping layer. Here, At least one of the first reflecting portion and the second reflecting portion has the DBR that is formed by alternately stacking two kinds of differently composed silicon-containing insulating layers and a gate.
Owner:ELECTRONICS & TELECOMM RES INST
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Silicon based light emitting diode
ActiveUS20090242913A1Emission efficiency is highNarrow emission spectrumSemiconductor/solid-state device manufacturingSemiconductor devicesSilicon basedReflective layer
Provided is a highly efficient silicon-based light emitting diode (LED) including a Distributed Bragg Reflector (DBR), an n-type doping layer, and a p-type substrate structure. The silicon-based LED includes: a substrate having a p-type mesa substrate structure; an active layer that is formed on the substrate and has a first surface and a second surface opposite the first surface; a first reflective layer facing the first surface of the active layer; a second reflective layer that is located on either side of the p-type substrate structure and faces the second surface of the active layer; an n-type doping layer sandwiched between the active layer and the first reflective layer; a first electrode electrically connected to the n-type doping layer; and a second electrode electrically connected to the p-type substrate structure.
Owner:ELECTRONICS & TELECOMM RES INST
Immunofiltration assay fluorescent quantitative detection method based on high-sensitivity quantum dot
ActiveCN102539771AHigh luminous intensityWide excitation spectrumFluorescence/phosphorescenceQuantum dotQuality control
The invention discloses an immunofiltration assay fluorescent quantitative detection method based on a high-sensitivity quantum dot; the immunofiltration assay fluorescent quantitative detection method comprises the following steps of: constructing a fluorescence immunofiltration array device by using the excellent fluorescent characteristic of the quantum dot in combination of a quantum dot fluorescence labeling technology and an immunofiltration array technology on the basis of optimizing constituent parts for the immunofiltration assay; and after immunofiltration array, detecting the strenght of fluorescent signals of the quantum dot and a quality control dot by using a fluorescence quantometer, correcting the fluorescence strenght of the quantum pot by using the quality control dot, and further realizing the quantitative detection of a tested object according to a standard curve obtained by using the fluorescence quantometer. The method is simple, rapid, accurate, low in cost and high in sensitiveness. Comapred with the conventional collodial gold immunofiltration array method, the immunofiltration assay fluorescent quantitative detection method has the advantages of good labeling stability, low non-specificity, high sensitivity, wide linear range and accurate quantification. The method is suitable for samples such as serums, urine, spittle, excrement and the like and can be applicable to the detection of serious illness, poisons, food safety and the like.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Mini-LED liquid crystal display backlight structure based on quantum dots and preparation method of mini-LED liquid crystal display backlight structure
InactiveCN109471298ANarrow emission spectrumAchieve ultra-wide color gamutSolid-state devicesNon-linear opticsLiquid-crystal displayQuantum dot
The invention relates to a mini-LED liquid crystal display backlight structure based on quantum dots and a preparation method of the mini-LED liquid crystal display backlight structure. The mini-LED liquid crystal display backlight structure comprises a mini-LED blue light chip substrate, an adhesive layer, a laminating layer and a quantum dot fluorescent conversion layer in sequence form bottom to top. The mini-LED liquid crystal display backlight structure based on the quantum dots has the advantages that the quantum dots have very narrow emission spectra, ultra-wide color gamut of liquid crystal display can be achieved, a required laminating process is mild, the quantum dot fluorescent conversion layer can be fully, tightly and flatly combined with a mini-LED blue light chip, a luminousstructure of the mini-LED blue light chip cannot be destroyed, the backlight structure can be formed through one-time assembly, and the mini-LED liquid crystal display backlight structure is suitablefor producing backlight sources of liquid crystal displays in large area.
Owner:GUANGDONG POLY OPTOELECTRONICS
Method for detecting early apoptosis of cells
InactiveCN101893572ANarrow emission spectrumReduce distractionsMicrobiological testing/measurementFluorescence/phosphorescenceAlexa FluorApoptosis
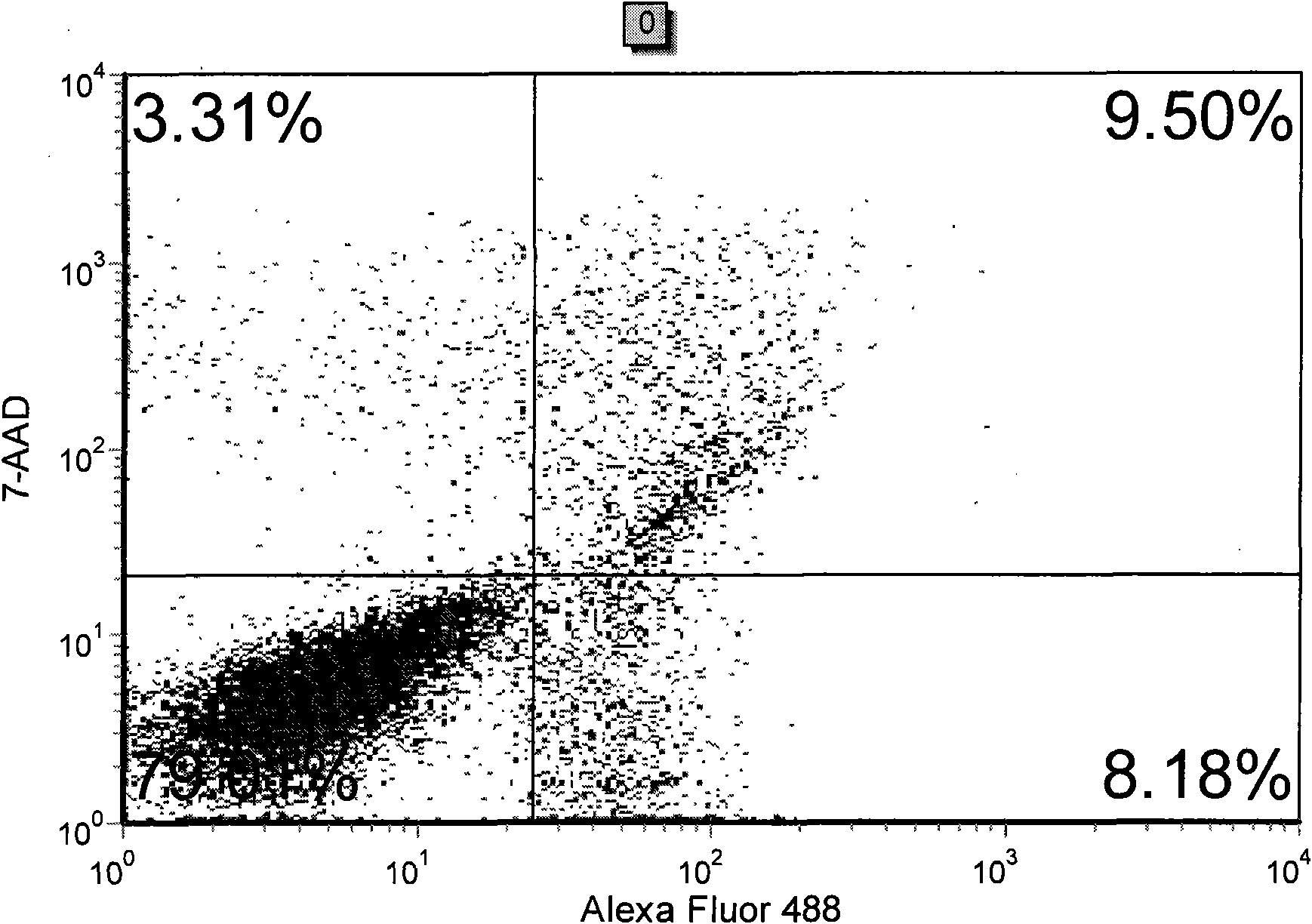
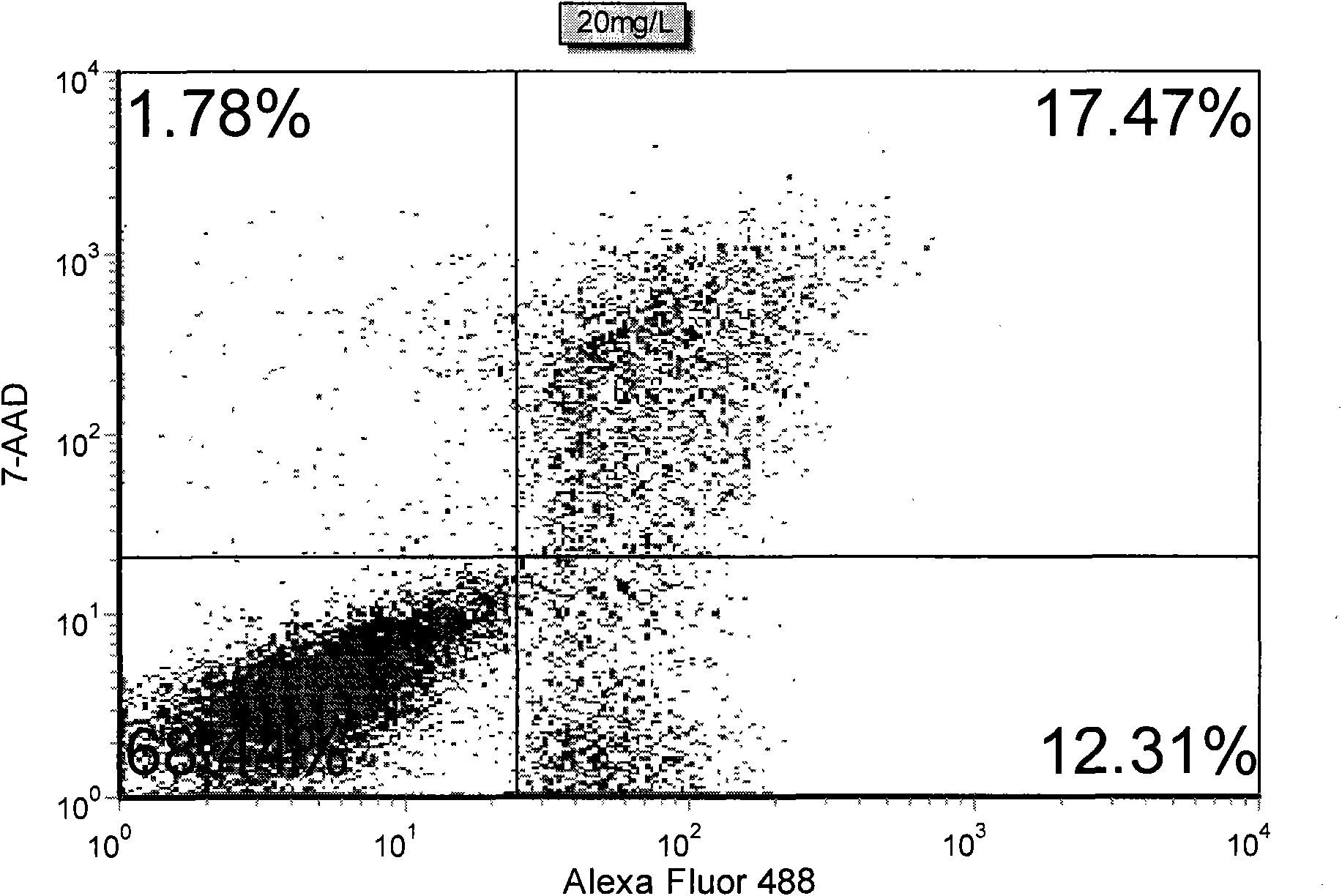
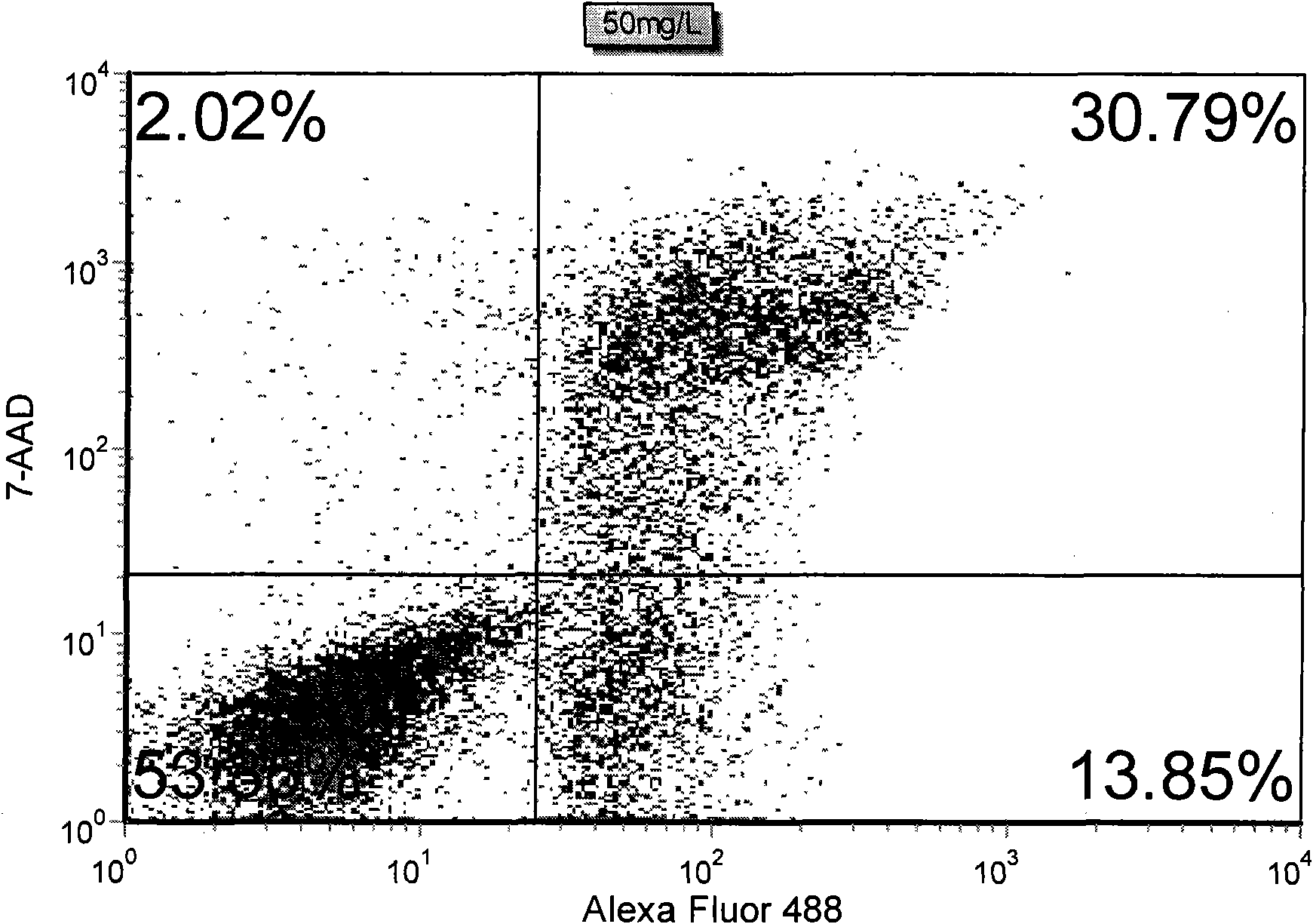
The invention discloses a method for detecting early apoptosis of cells. A phosphatidylserin antibody marked with a fluorescent dye Alexa Fluor 488 and a nucleic acid fluorescent dye 7-AAD are matched to detect the cells together, wherein the phosphatidylserin antibody is used for identifying normal living cells and apoptotic cells, and the nucleic acid fluorescent dye is used for identifying early apoptotic cells and late apoptotic cells in the apoptotic cells. After the two fluorescent dyes, namely the Alexa Fluor 488 and the 7-AAD with different emission wavelengths are detected and analyzed through a flow cytometry, the cells are divided into four subsets, wherein the Alexa Fluor 488+ / 7-AAD- subset is the early apoptotic cells.
Owner:GUANGXI MEDICAL UNIVERSITY
Preparation of colorimetric and fluorescent double-signal nanospheres and application of colorimetric and fluorescent double-signal nanospheres to immunochromatographic quantitative detection
ActiveCN106680496AHigh luminous intensityWide excitation spectrumBiological material analysisNanoopticsFluorescenceQuantum dot
The invention discloses preparation of colorimetric and fluorescent double-signal nanospheres and application of the colorimetric and fluorescent double-signal nanospheres to immunochromatographic quantitative detection, and belongs to the field of medical detection. The preparation comprises the following steps: taking nanospheres as main bodies; embedding fluorescent quantum dots into the nanospheres or assembling the fluorescent quantum dots on surfaces of the nanospheres through an ultrasonic swelling method or an assembling method, so as to construct fluorescent nanospheres with rich carboxyl on surfaces and the grain diameter of 100nm to 500nm; assembling nano-gold on the surfaces of the fluorescent nanospheres through the assembling method, so as to obtain the colorimetric and fluorescent double-signal nanospheres. Visual qualitative and fluorescent quantitative detection of a target object can be simultaneously realized by combining the colorimetric and fluorescent double-signal nanospheres with an immunochromatographic technology. The double-signal nanospheres have fluorescent signal and colorimetric signal amplification effects. An immunochromatographic method, which takes the double-signal nanospheres as markers, is simple, convenient and rapid to use; compared with a traditional colloid gold immunochromatographic test paper strip, the colorimetric and fluorescent double-signal nanospheres has the advantages of high sensitivity, good repeatability, capability of accurately quantifying and the like.
Owner:WUHAN UNIV
Fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T
ActiveCN102565422ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitatively detecting cardiac troponin T (cTnT). The fluorescence immunochromatographic assay for quantitatively detecting cTnT realizes fluorescence quantitative detection on the basis of optimizing various constituent parts of test paper by using excellent fluorescence characteristic of quantum dots and combining a bicolor marking technology and an immunochromatographic technology. Compared with the conventional colloidal gold immunochromatographic assay, the fluorescence immunochromatographic assay disclosed by the invention has the advantages of good marking stability, low non-specificity, high sensitivity, wide linear range and quantifying accuracy. The fluorescence immunochromatographic kit disclosed by the invention is used for carrying out quantitative detection on the cTnT and detecting whole blood, blood serum and blood plasma samples and is suitable for different levels of hospitals and particularly good for wide popularization in primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Organic electroluminescent device and display device
ActiveCN109817818AWiden the compound areaIncrease profitSolid-state devicesSemiconductor/solid-state device manufacturingResonanceOrganic light emitting device
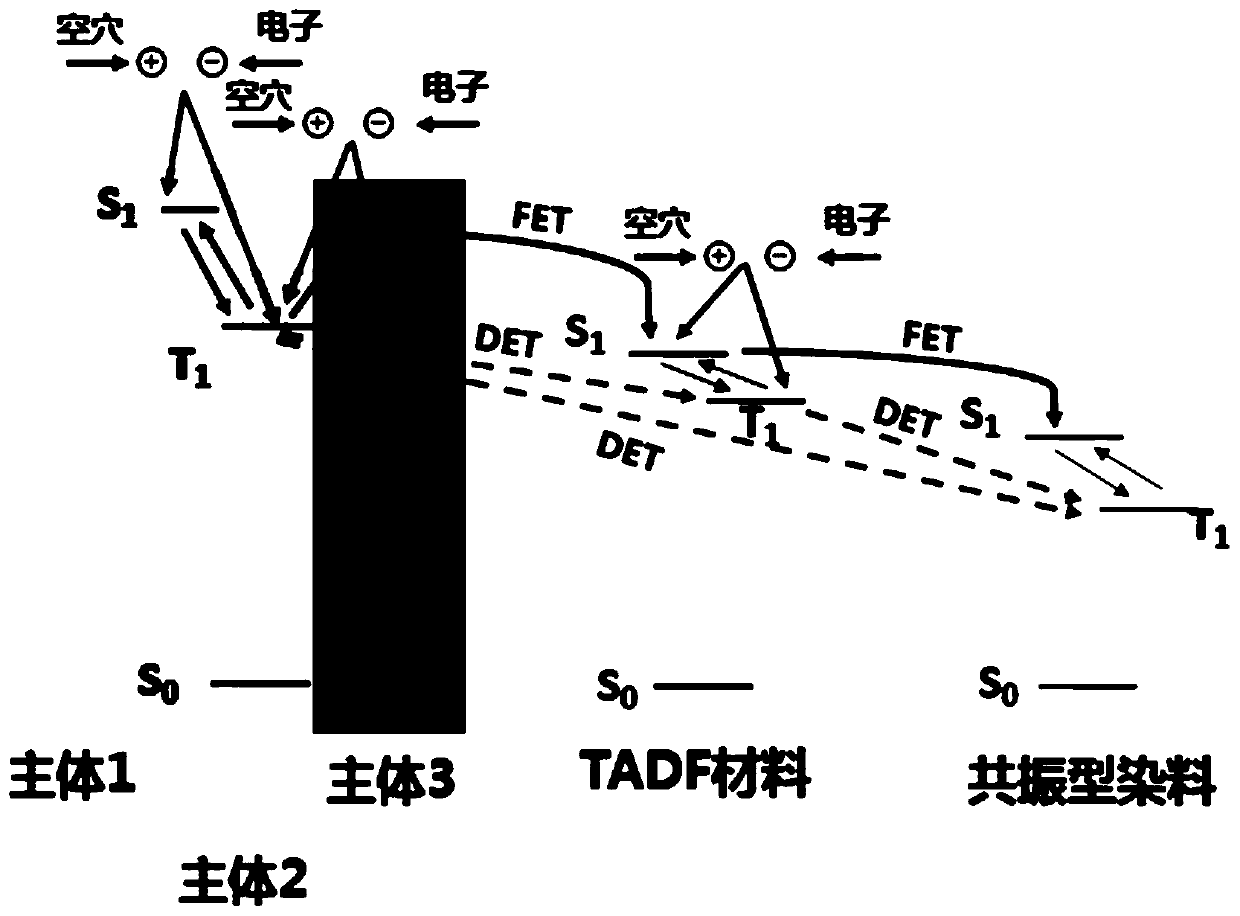
The invention provides an organic electroluminescent device and a display device. The organic light-emitting device comprises a first light-emitting layer and a second light-emitting layer, wherein the material of the first light-emitting layer comprises a first main body material, a first sensitizer and a first dye, and the material of the second light-emitting layer comprises a second main bodymaterial, a third main body material, a second sensitizer and a second dye; the first main body material can form an exciplex with the second main body material or the third main body material, and the second main body material can form an exciplex with the third main body material; the first sensitizer and the second sensitizer are both thermally activated delayed fluorescent materials; the firstdye and the second dye are both resonance type thermal activation delayed fluorescence materials. According to the light-emitting layer of the organic light-emitting device provided by the invention,the interface exciplex and the bulk phase exciplex are used as main body materials, and the TADF material is used as a sensitizer to sensitize the resonant TADF material, so that the efficiency roll-off is remarkably reduced, and the device efficiency and stability are remarkably improved.
Owner:YUNGU GUAN TECH CO LTD
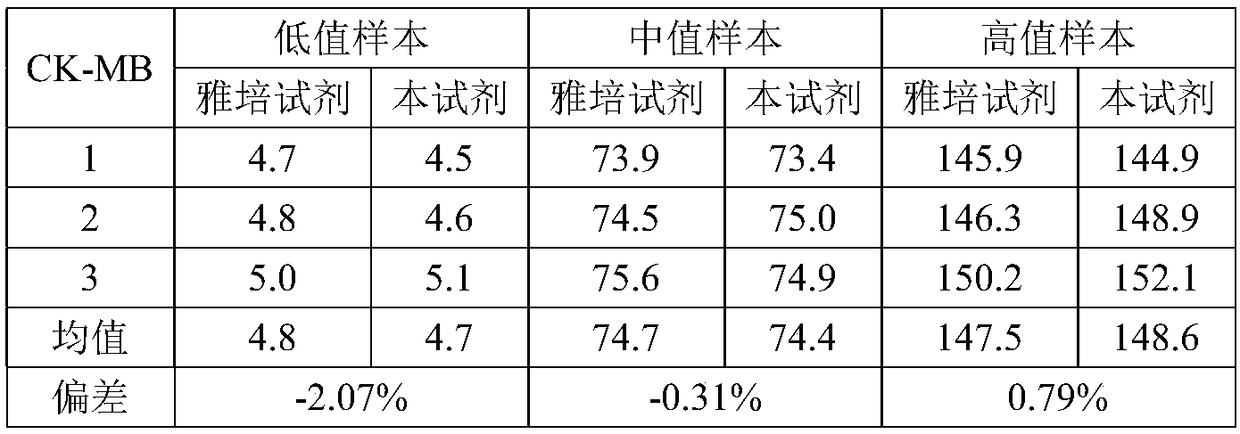
Magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB (creatine kinase-MB) kit
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB kit. The CK-MB kit comprises an immunomagnetic bead coating a CK-MB monoclonal antibody, a CK-MB standardized product solution, a europium-marked CK-MB monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the CK-MB monoclonal antibody isa covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the CK-MB monoclonal antibody. The kit has the high sensibility, the sensibility of CK-MB is 1ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample isless, and only 50 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, no artificial error exists, and labor is saved. The kit reasonably utilizes the space of a reagent strip, the structure of the reagent strip is more compact, the reagent strip can be transported more easily, and used conveniently, the operation is simple, and the stability is good.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Complex whorl aryl fluorene material, preparation and application method thereof
ActiveCN101492443ATest thermal stabilityImprove performanceOrganic chemistryLaser active region structureOrganic laserGlass transition
The invention relates to a complicated spiro-aryl fluorine material and preparation and application methods thereof, which belong to the organic photoelectric material scientific field, in particular to the complicated spiro-aryl fluorine material and the preparation method thereof. And the material is applied to organic electronic fields of organic rectifier diode, organic luminescence display, organic laser, etc. With the structure as is shown on the right, the compound material I has the advantages of: (1) having specific electronic structure and photoelectric properties, such as narrow emission spectrum; (2) maintaining high thermal stability and glass transition temperature, etc. Materials of the kind have commercial application potential in stable dark blue materials.
Owner:NINGBO LUMILAN NEW MATERIAL CO LTD
Fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 and preparation method for fluorescence immunochromatography kit
InactiveCN104655858AHigh luminous intensityWide excitation spectrumDisease diagnosisBiological testingPlasma samplesFluorescence
The invention discloses a fluorescence immunochromatography kit for quantitatively detecting human epididymis secretory protein-4 by taking fluorescent dye as a marker. The fluorescence immunochromatography kit disclosed by the invention realizes fluorescence quantitative detection for the human epididymis secretory protein-4, has the advantages of being good in stability, wide in linear range, good in specificity, accurate to quantify, simple and quick, can be used for simultaneously detecting whole blood, blood serum and plasma samples, and is suitable for hospitals of various levels.
Owner:DEMAIJI BIOTECH BEIJING
Fluorescence immunochromatographic assay and kit for quantitative detection of human cardiac troponin I (cTnI)
ActiveCN102520193ASolve the backgroundSolve the signal indistinguishableBiological testingFluorescenceBlood plasma
The invention discloses a fluorescence immunochromatographic assay and kit for quantitative detection of cardiac troponin I (cTnI). The fluorescence immunochromatographic assay for quantitative detection of the cTnI realizes fluorescent quantitative detection by utilizing excellent fluorescent properties of quantum dots and combining a bicolour marking technology and an immunochromatographic assay on the basis of optimizing each component of a test strip. Compared with the common collaurum immunochromatographic assay, the fluorescence immunochromatographic assay has the advantages of good mark stability, low nonspecificity, high sensitivity, wide linear range and accuracy in quantification. The kit disclosed by the invention is used for carrying out quantification detection on the cTnI, can be used for detecting whole blood, blood serum and plasma samples simultaneously, and is applied to different levels of hospitals and is particularly favored to be widely popularized to primary hospitals and clinics.
Owner:SHENZHEN KANGMEI BIOTECH
Time resolution fluorescent immunochromatographic test strip for detecting carbendazim, and preparation method and application thereof
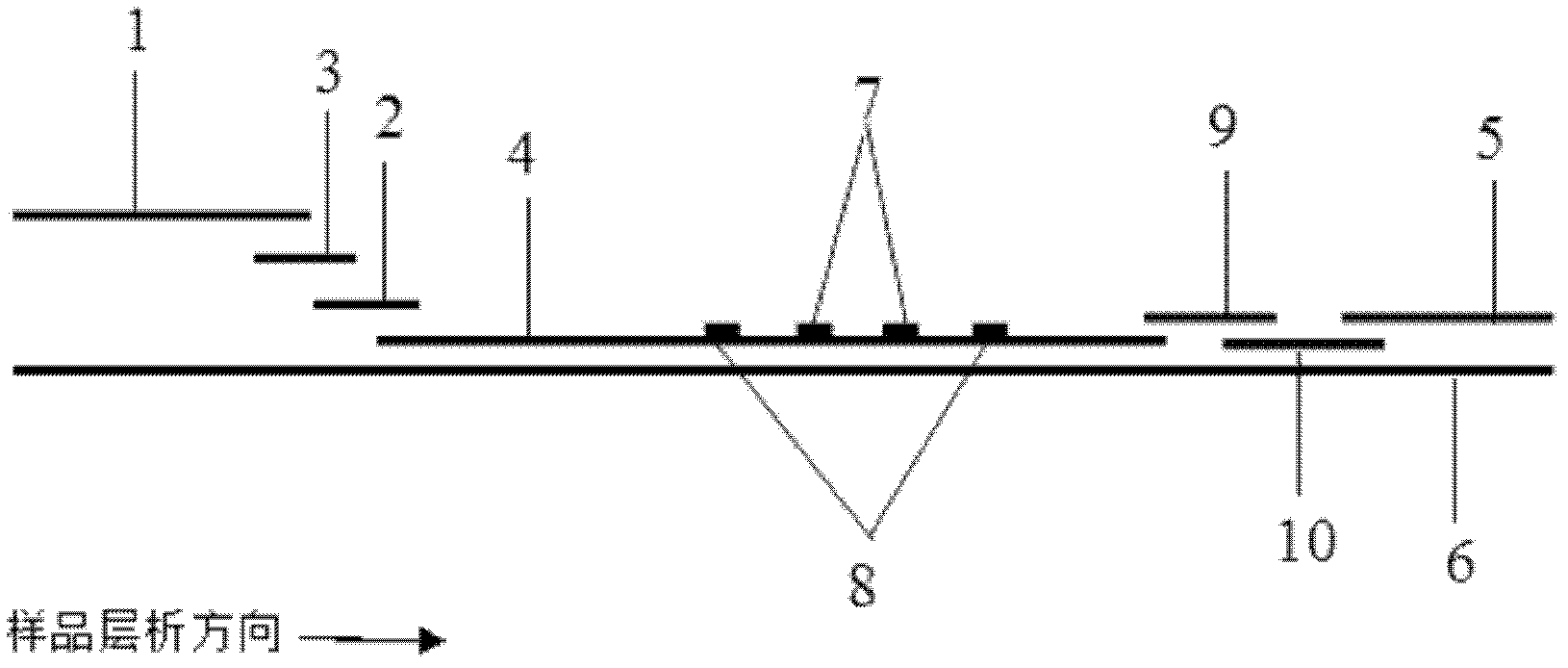
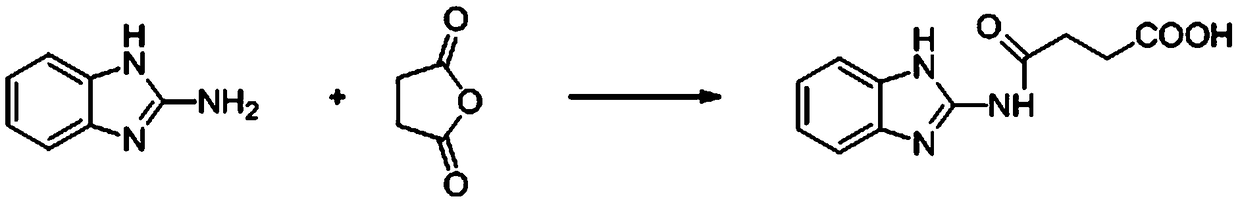
InactiveCN109061152AImprove hydrophilicityRelease fullyBiological testingFluorescence/phosphorescenceNitrocellulose2-aminobenzimidazole
The invention discloses a time resolution fluorescent immunochromatographic test strip for detecting carbendazim, and a preparation method and application thereof. The time resolution fluorescent immunochromatographic test strip comprises a bottom plate and further comprises a sample absorbent pad, a combination releasing pad, a nitrocellulose membrane and a water absorbent pad which are lapped and pasted on the bottom plate in sequence. A carbendazim monoclonal antibody labelled by fluorescent microspheres is embedded into the combination releasing pad, and a detecting area and a quality control area are fixed to the nitrocellulose membrane. A carbendazim hapten-carrier protein conjugate is sprayed in the detecting area, and an anti-sheep and anti-mouse antibody is sprayed in the qualitycontrol area. A carbendazim hapten is obtained in the mode of reacting of 2-aminobenzimidazole with butanedioic anhydride. The invention further provides the preparation method of the test strip and amethod for detecting the carbendazim in samples by applying the test strip. The time resolution fluorescent immunochromatographic test strip and the detecting method provided by the invention have the advantages that operation is easy, sensitivity is high, the detecting speed is high, and the cost is low, and the carbendazim in the large-batch samples can be detected quickly and monitored on site.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com