Patents
Literature
2076 results about "Non specific" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In medical parlance, "non-specific" (often spelled "nonspecific" in the medical world) means not due to any single known cause, and alternatively, not directed at a particular agent but having a general effect.
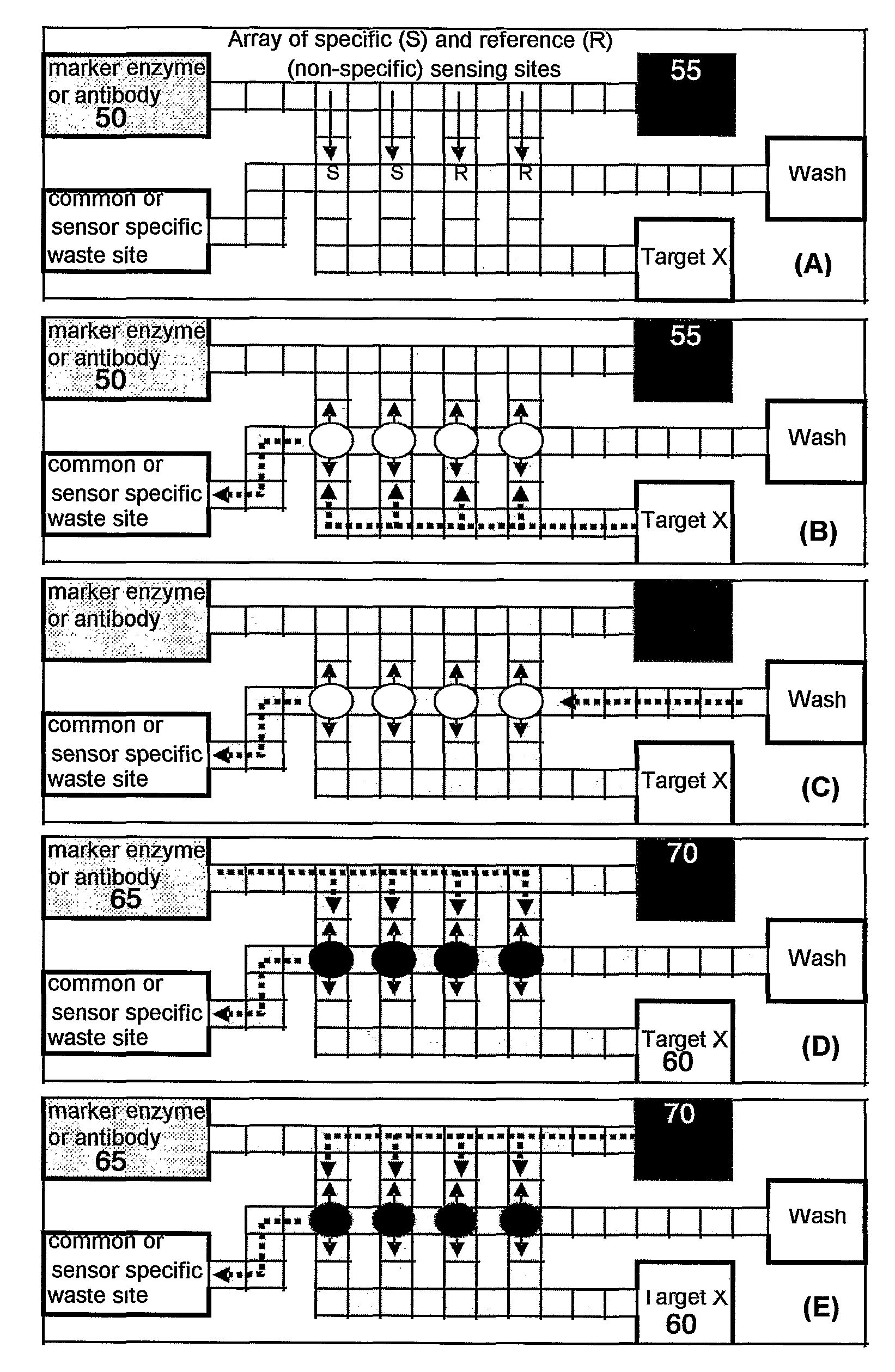
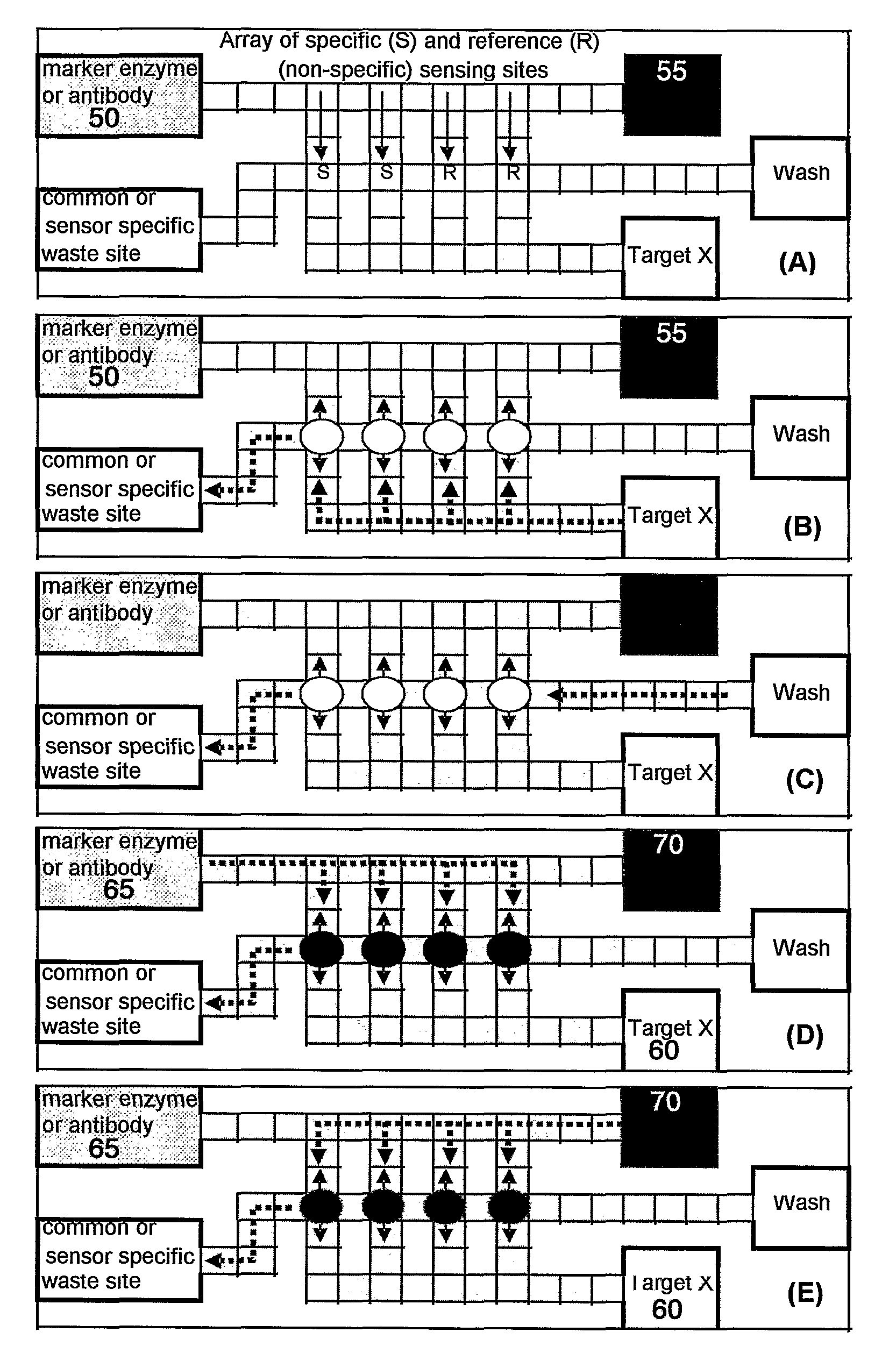
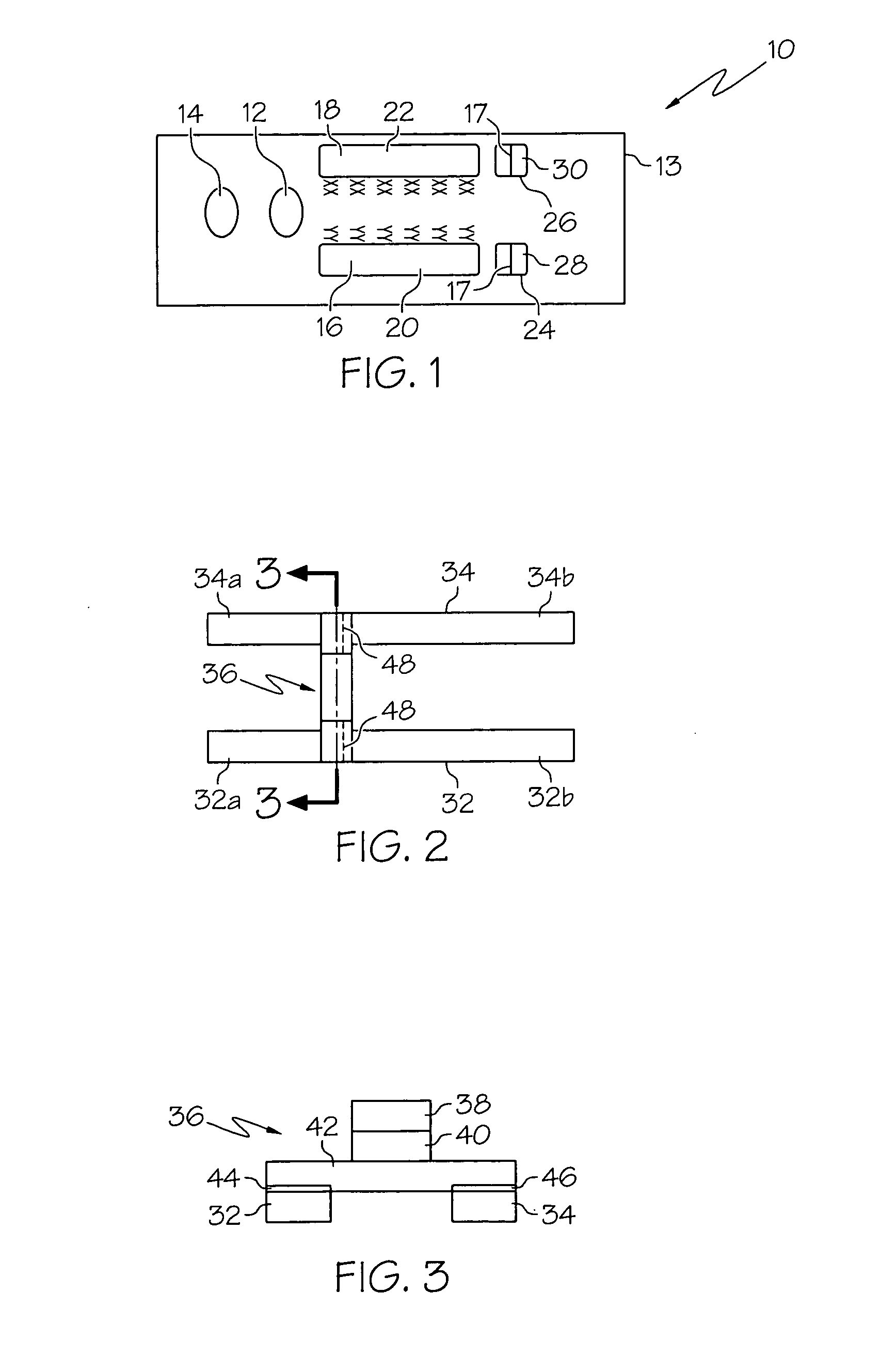
Immunoassay device with immuno-reference electrode
InactiveUS20060160164A1Reduce distractionsIncrease ionic strengthBioreactor/fermenter combinationsBiological substance pretreatmentsImmune profilingImmobilized Antibodies
An electrochemical immunosensor system with reduced interference, comprising: a first immunosensor that generates an electrochemical signal based on the formation of a sandwich between an immobilized antibody, a target analyte and a labeled antibody, wherein a portion of the signal arises from non-specific binding of the labeled antibody in the region of the first immunosensor, and a second immunosensor that acts as an immuno-reference sensor and generates a signal that is the same as or predictably related to the degree of non-specific binding which occurs in the region of the first immunosensor, and has an immunocomplex between an immobilized antibody and an endogenous or exogenous protein that is in the sample and that is not the target analyte.
Owner:ABBOTT POINT CARE
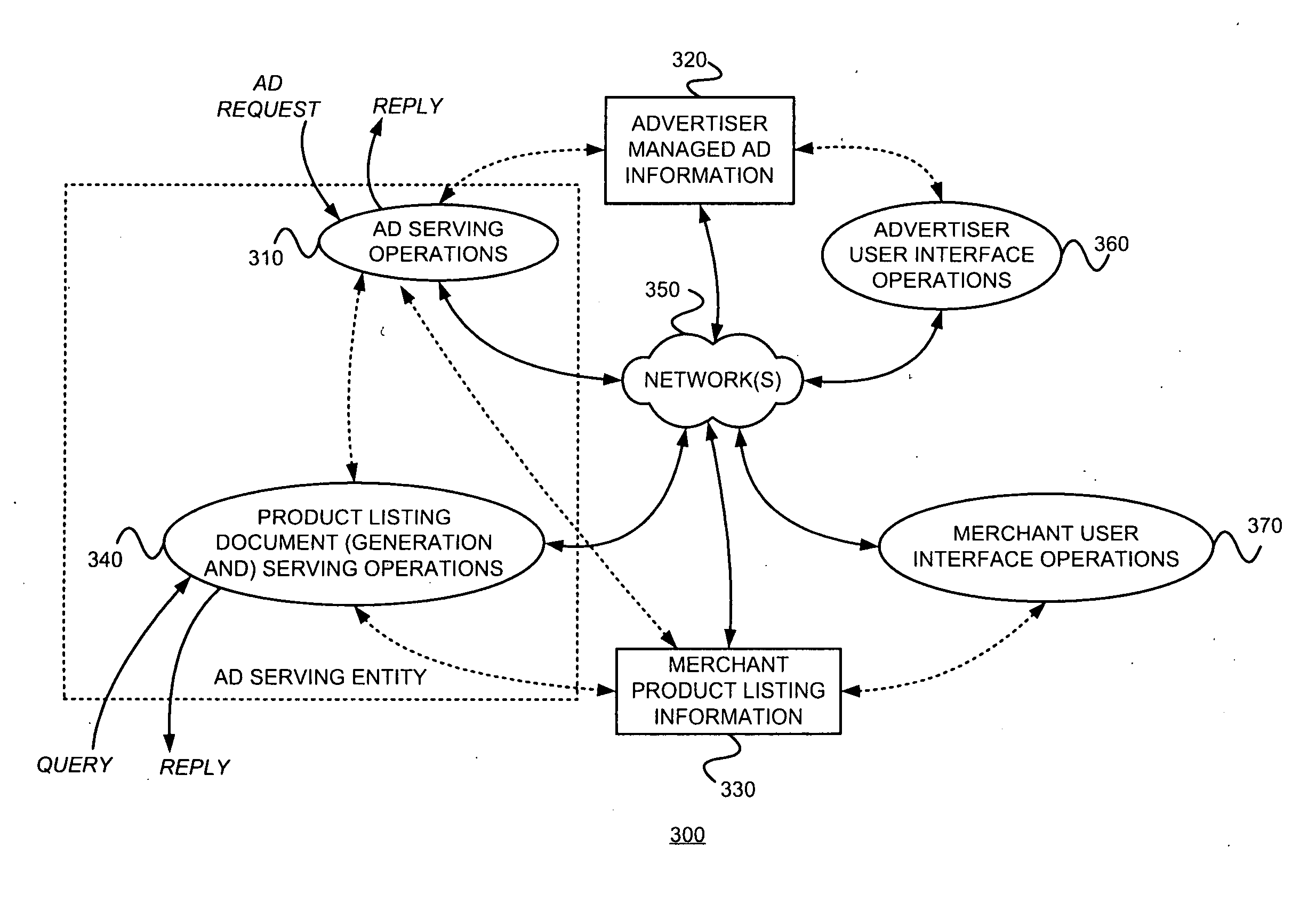
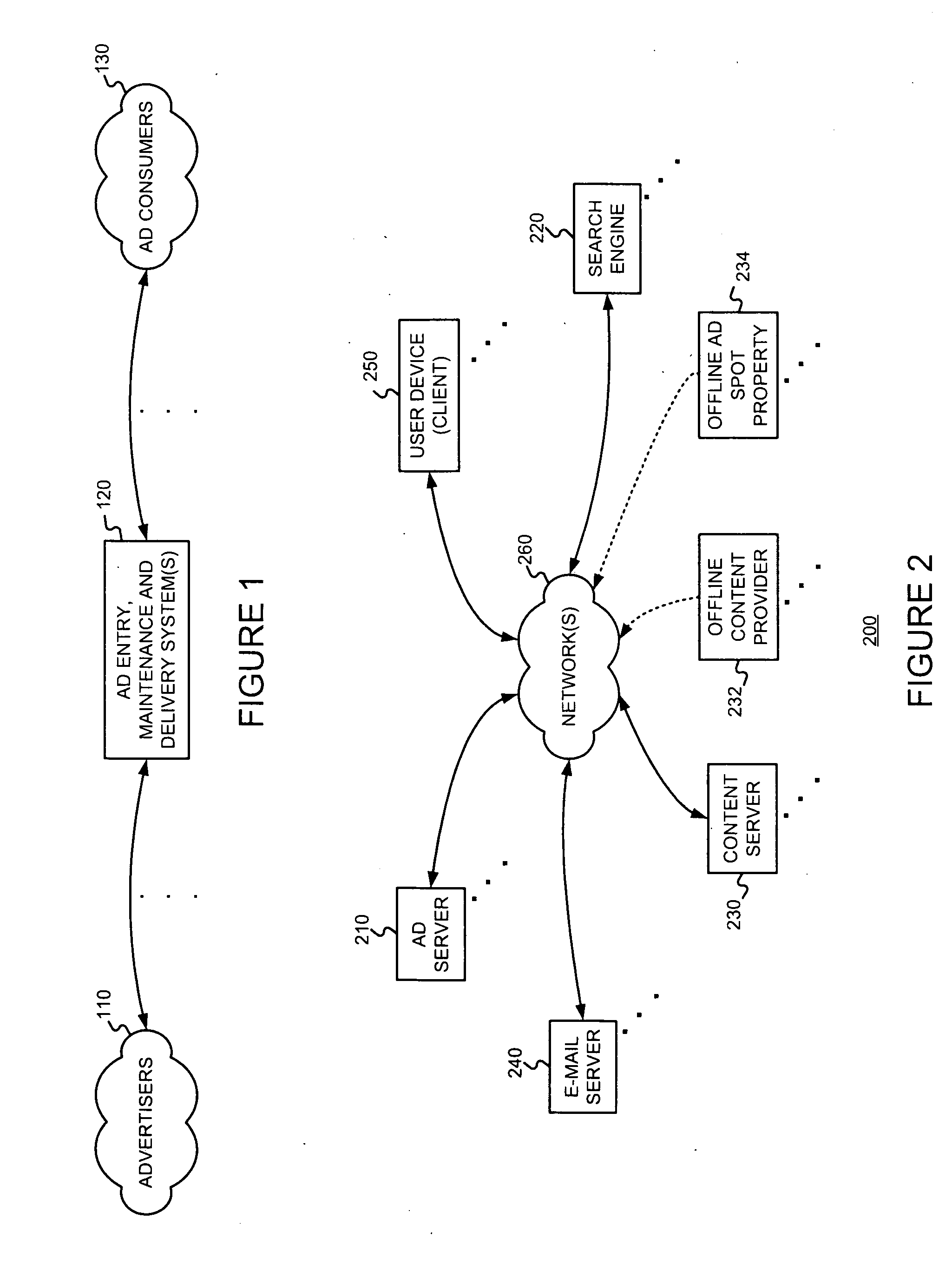
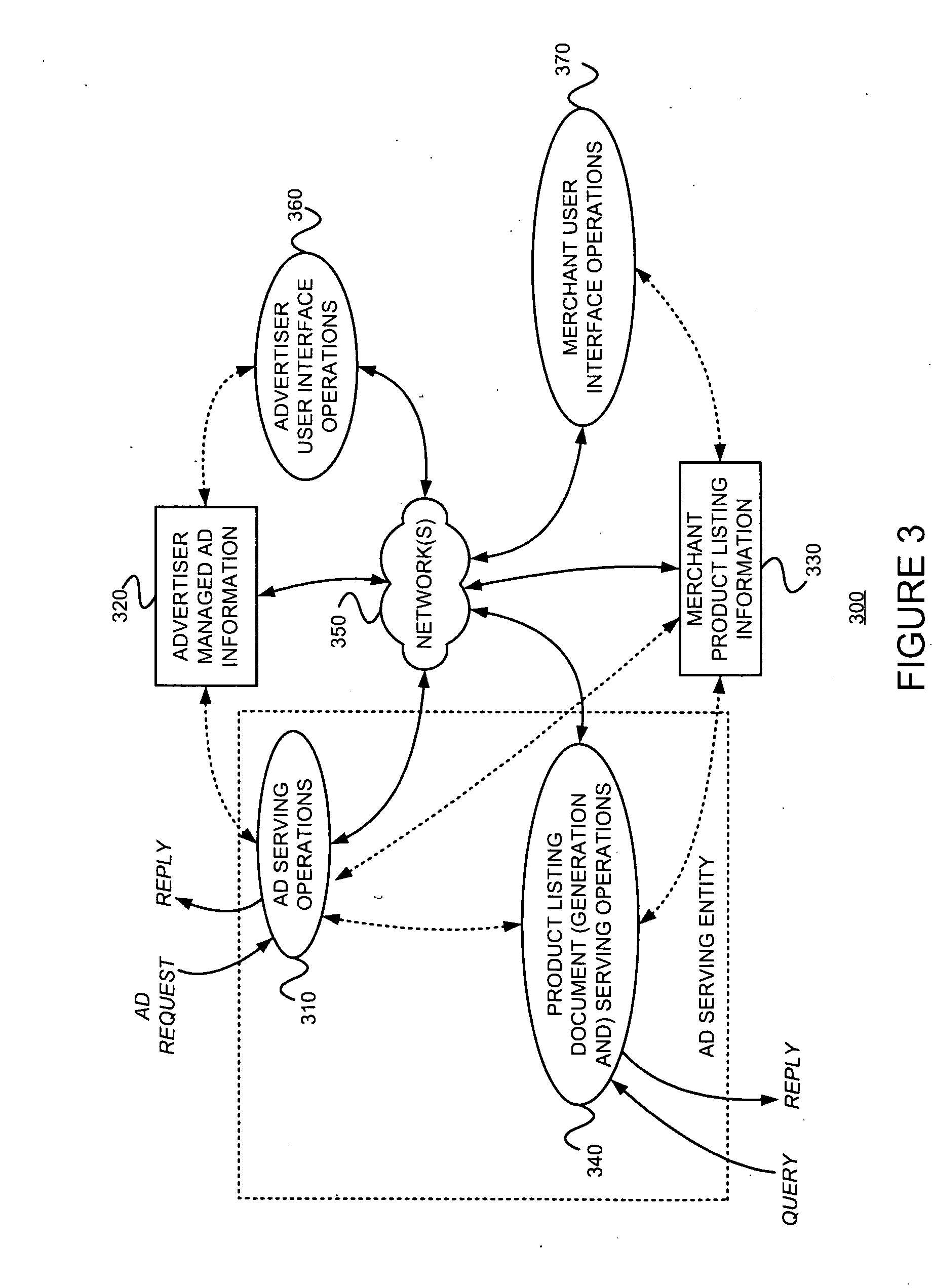
Controlling the serving, with a primary document, of ads from a first source, subject to a first compensation scheme, and ads from a second source, subject to a second compensation scheme
An advertising entity manages determinations, bidding, and / or billing for serving “generic advertisements” on a primary document. Generic ads are not for specific merchants or products; rather they lead users to a secondary document containing more specific information, product listings, and / or merchant listings. Such information and / or listings are determined to be relevant to a generic concept corresponding to the generic ad. For example, when a user selects a generic ad, they may be presented with a secondary document including product listings. If the user selects a merchant's product listing, then that merchant compensates an ad serving entity and / or a publisher of the primary document. Such management might include choosing or generating an appropriate generic advertisement creative for a potential advertising situation. A determination of whether or not to render a generic ad instead of one or more typical advertiser-managed ads may use an expected value of rendering the generic ad versus showing the advertiser-managed ad(s). The result of this determination may be reflected in a bid associated with the generic ad. Generic ads will often be useful for primary documents (e.g., Web pages) that are non-specific.
Owner:GOOGLE LLC
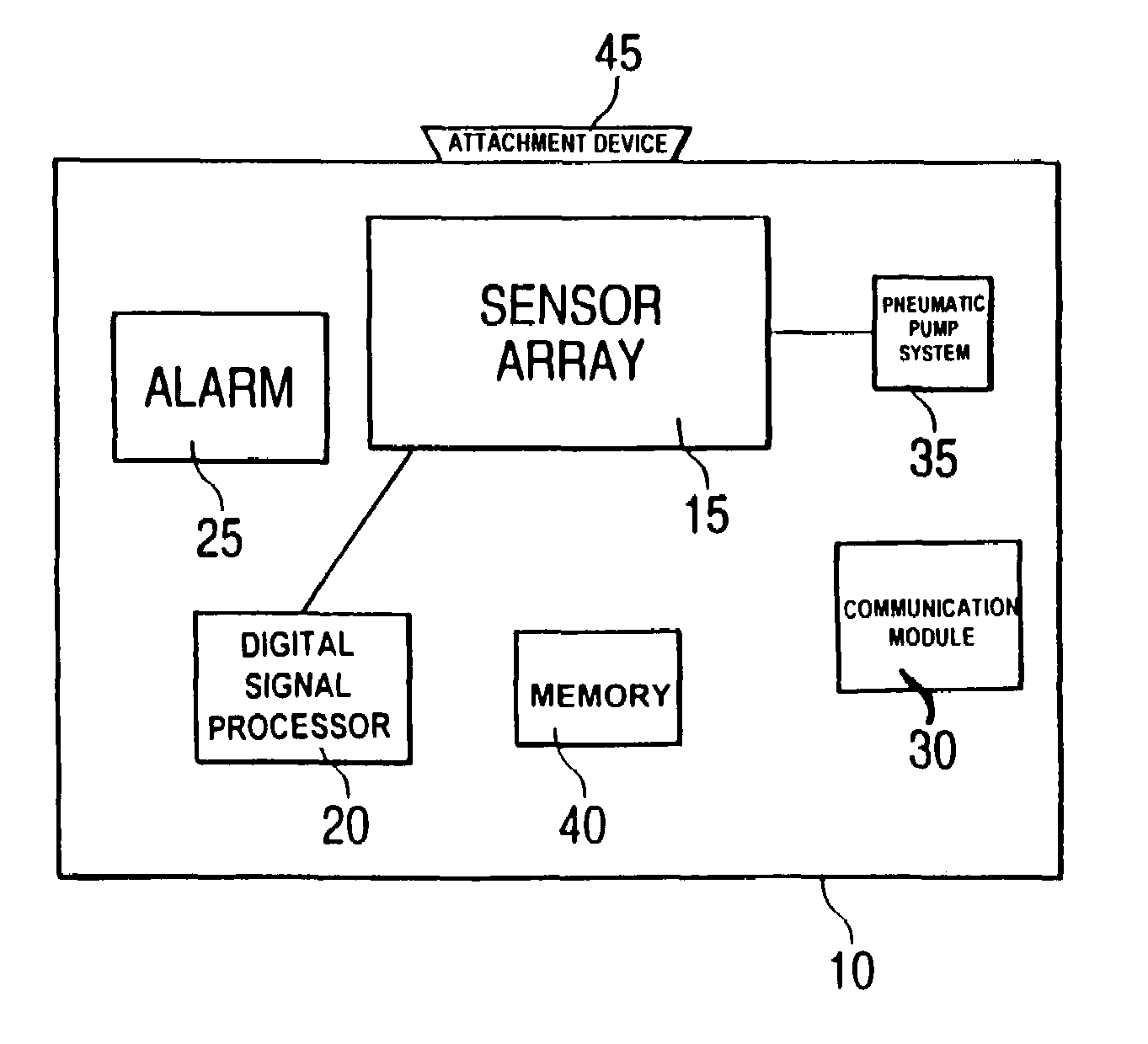
Non-specific sensor array detectors
InactiveUS7034677B2Easy to implementLow-powerMaterial nanotechnologyElectric signal transmission systemsSensor arrayAnalyte
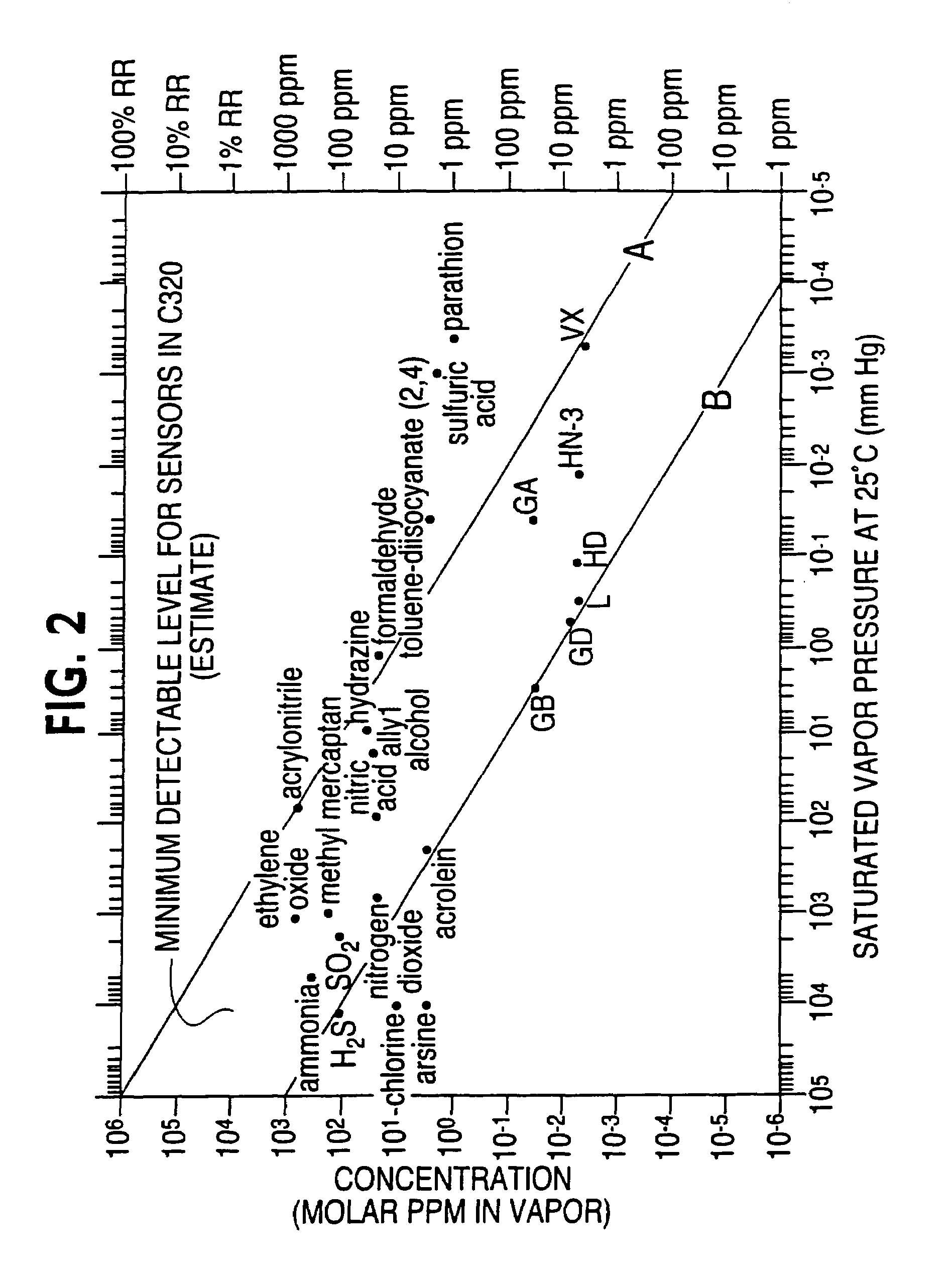
Portable and wearable chemical detector devices, such as badges, that are analyte-general, rather than analyte-specific, and which provide an optimal way to notify and protect personnel against known and unknown airborne chemical hazards. The devices are advantageously low-cost, have low-power requirements, may be wearable and are designed to detect and alarm to a general chemical threat. A sensor device includes two or more sensor devices, a processing module coupled to each of the sensor devices and configured to process signals received from each of the two or more sensor devices to determine an environmental state; and a communication module that communicates information about the environmental state to a user.
Owner:SMITHS DETECTION
Voice recognizer, voice recognizing method and game machine using them
InactiveUS6529875B1Avoid misidentificationImprove recognition rateSpeech recognitionVideo gamesControl signalSpeech input
A voice recognition device used as a peripheral device for a game machine including a voice input device, a voice recognition section for recognizing the player's voice by comparing the voice signal output from the voice input device with data from previously defined voice recognition dictionaries and generating control signals relating to the game on the basis of the recognition result. The voice recognition section includes a non-specific speaker voice recognition dictionary which is previously defined for unspecified speakers, and a specific speaker voice recognition dictionary which is defined by the player.
Owner:SEGA CORP
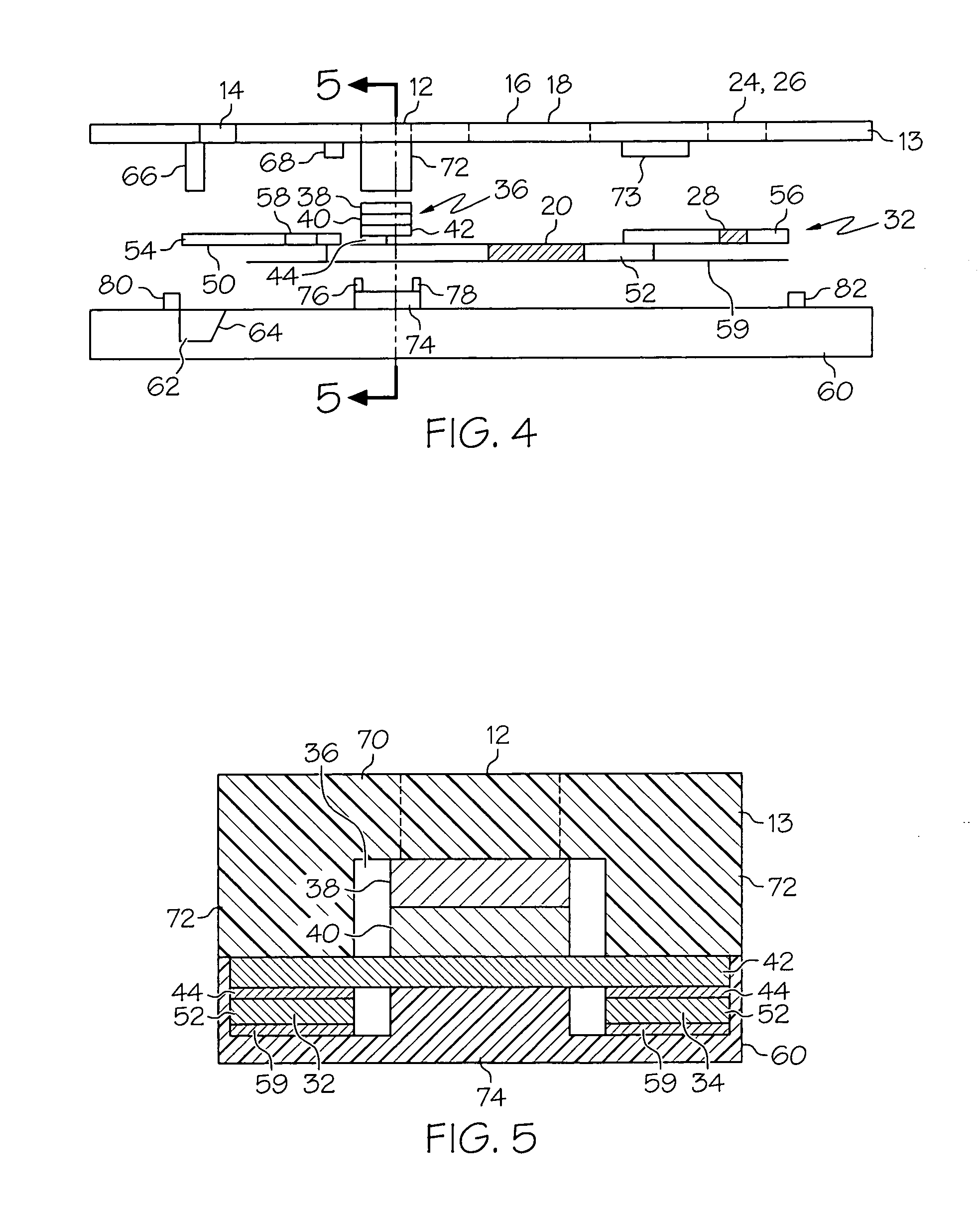
Immunoassay device with immuno-reference electrode
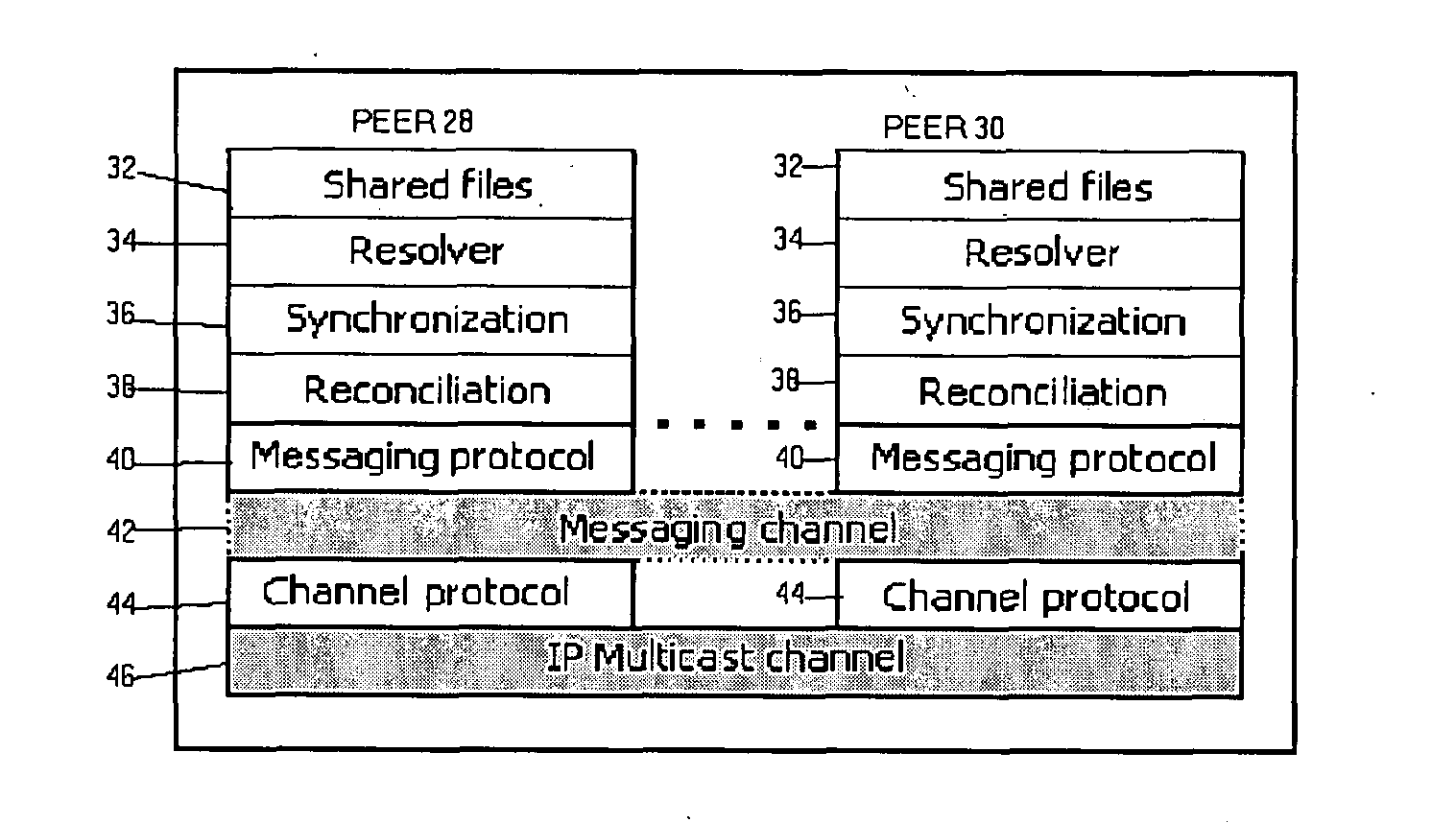
InactiveUS7723099B2Increase ionic strengthReduce distractionsBioreactor/fermenter combinationsBiological substance pretreatmentsProtein insertionNon specific
An electrochemical immunosensor system with reduced interference, comprising: a first immunosensor that generates an electrochemical signal based on the formation of a sandwich between an immobilized antibody, a target analyte and a labeled antibody, wherein a portion of the signal arises from non-specific binding of the labeled antibody in the region of the first immunosensor, anda second immunosensor that acts as an immuno-reference sensor and generates a signal that is the same as or predictably related to the degree of non-specific binding which occurs in the region of the first immunosensor, and has an immunocomplex between an immobilized antibody and an endogenous or exogenous protein that is in the sample and that is not the target analyte.
Owner:ABBOTT POINT CARE
Multi-speech control method suitable for cloud platform
Owner:厦门云聚智能科技有限公司
Functional surface coating
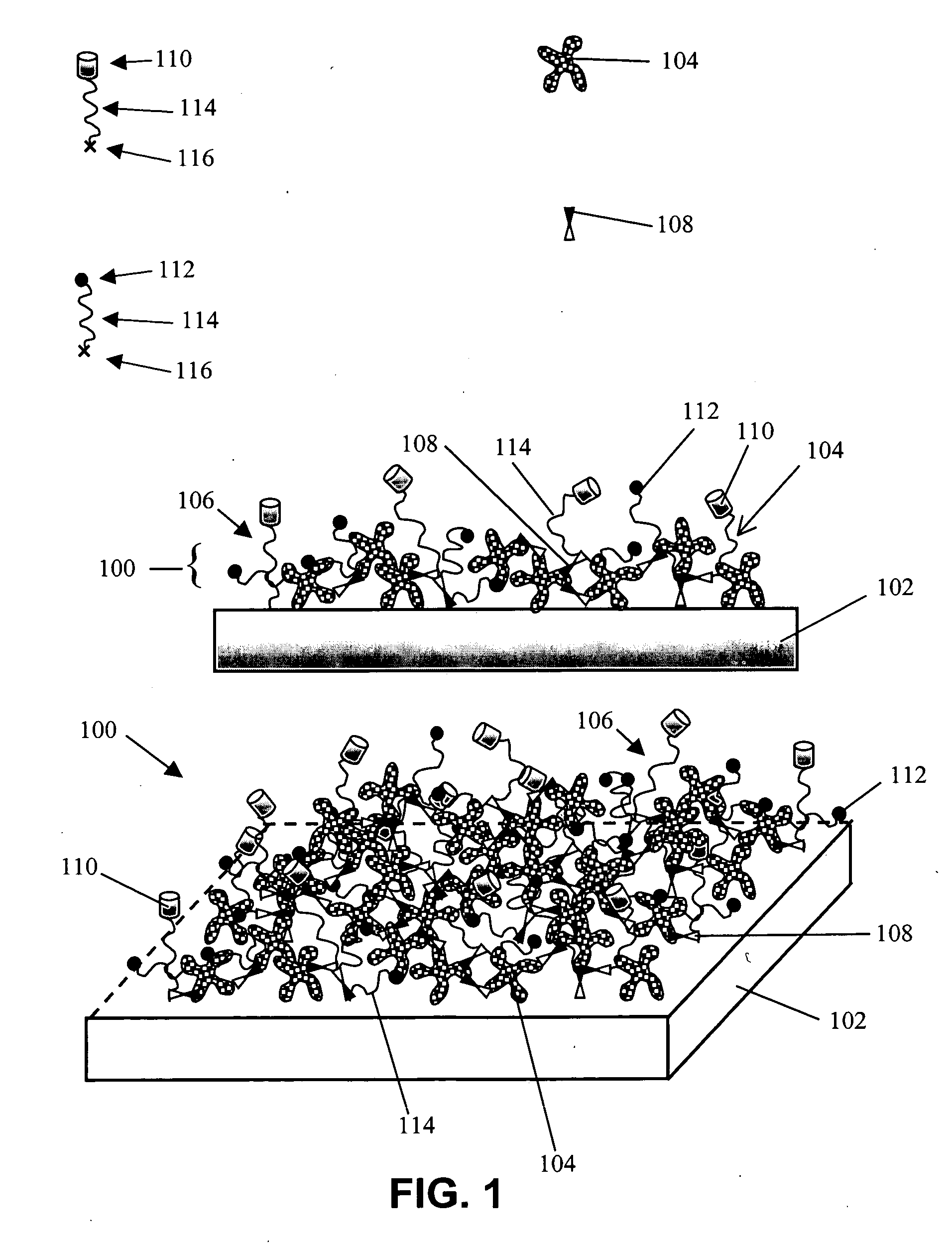
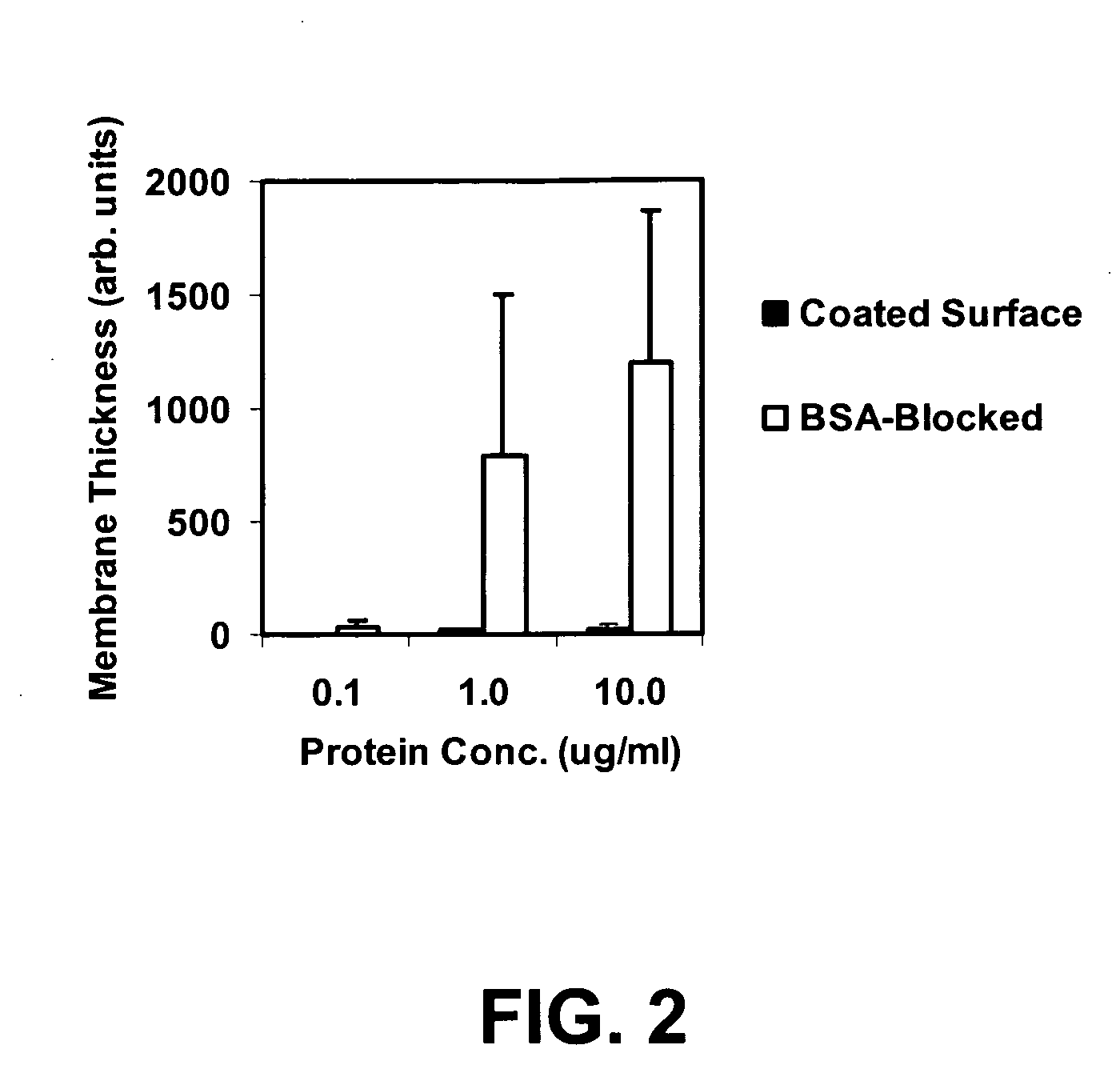
InactiveUS6844028B2Inhibit non-specific bindingImprove propertiesMicrobiological testing/measurementPreparing sample for investigationProtein targetBound property
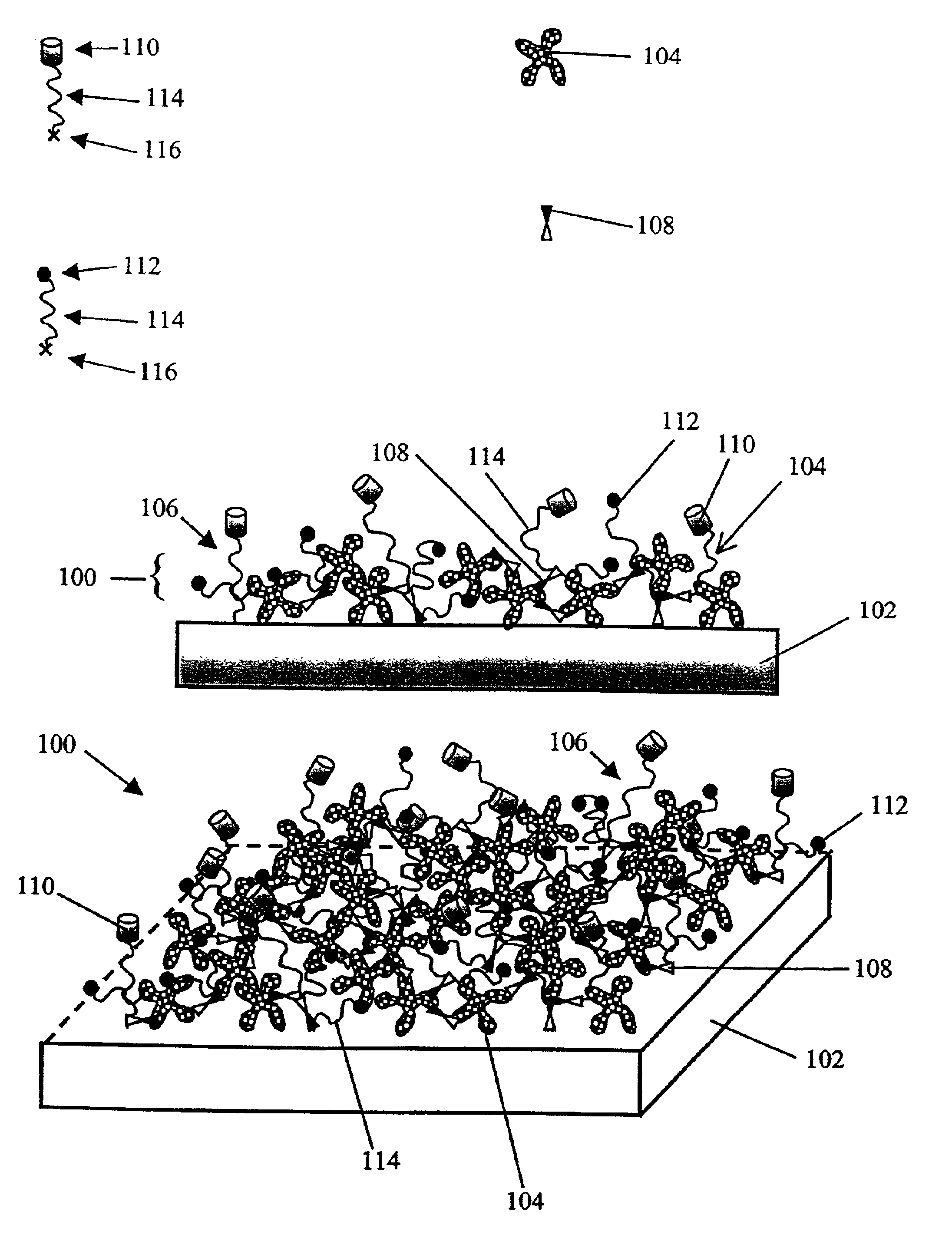
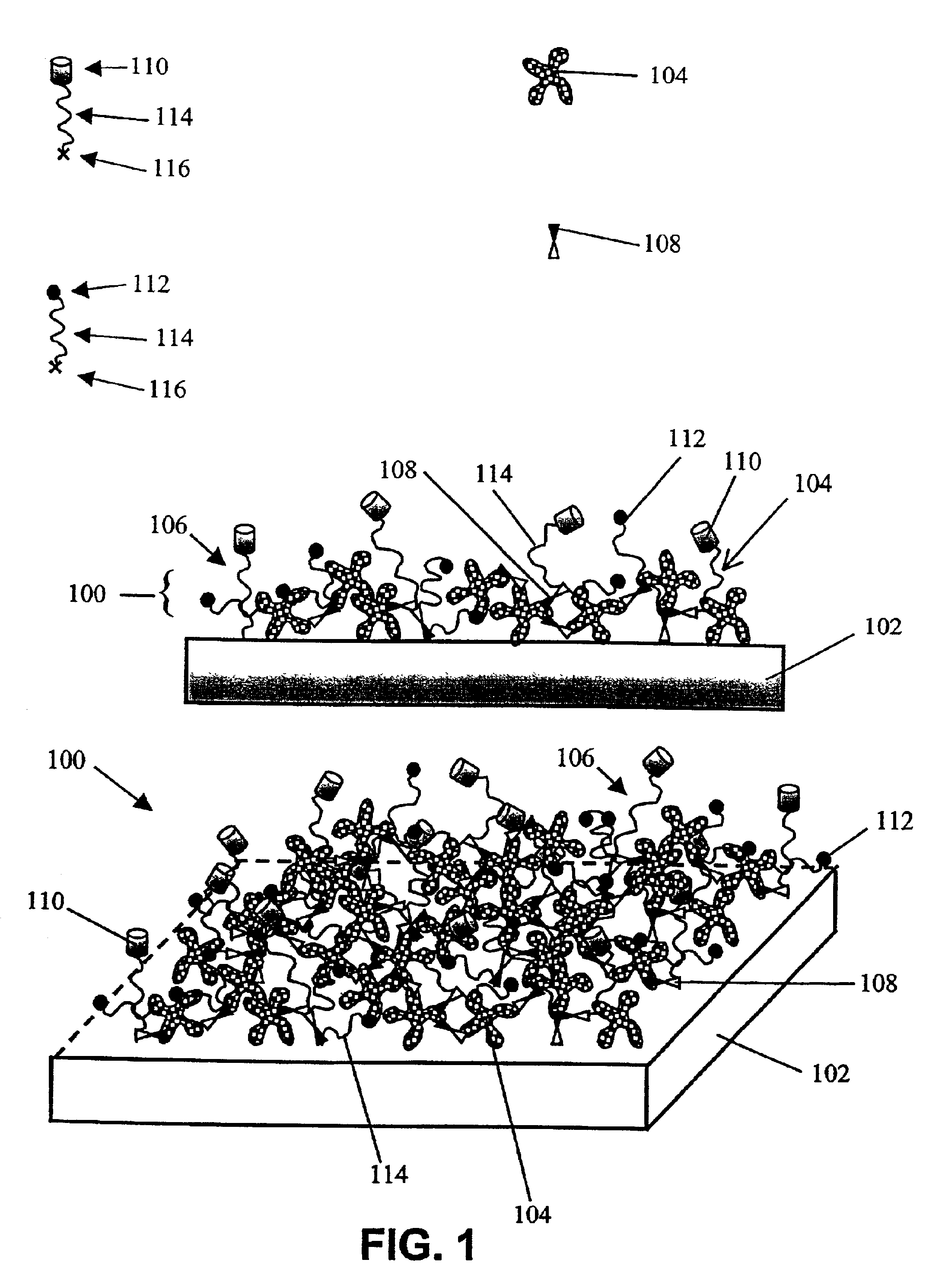
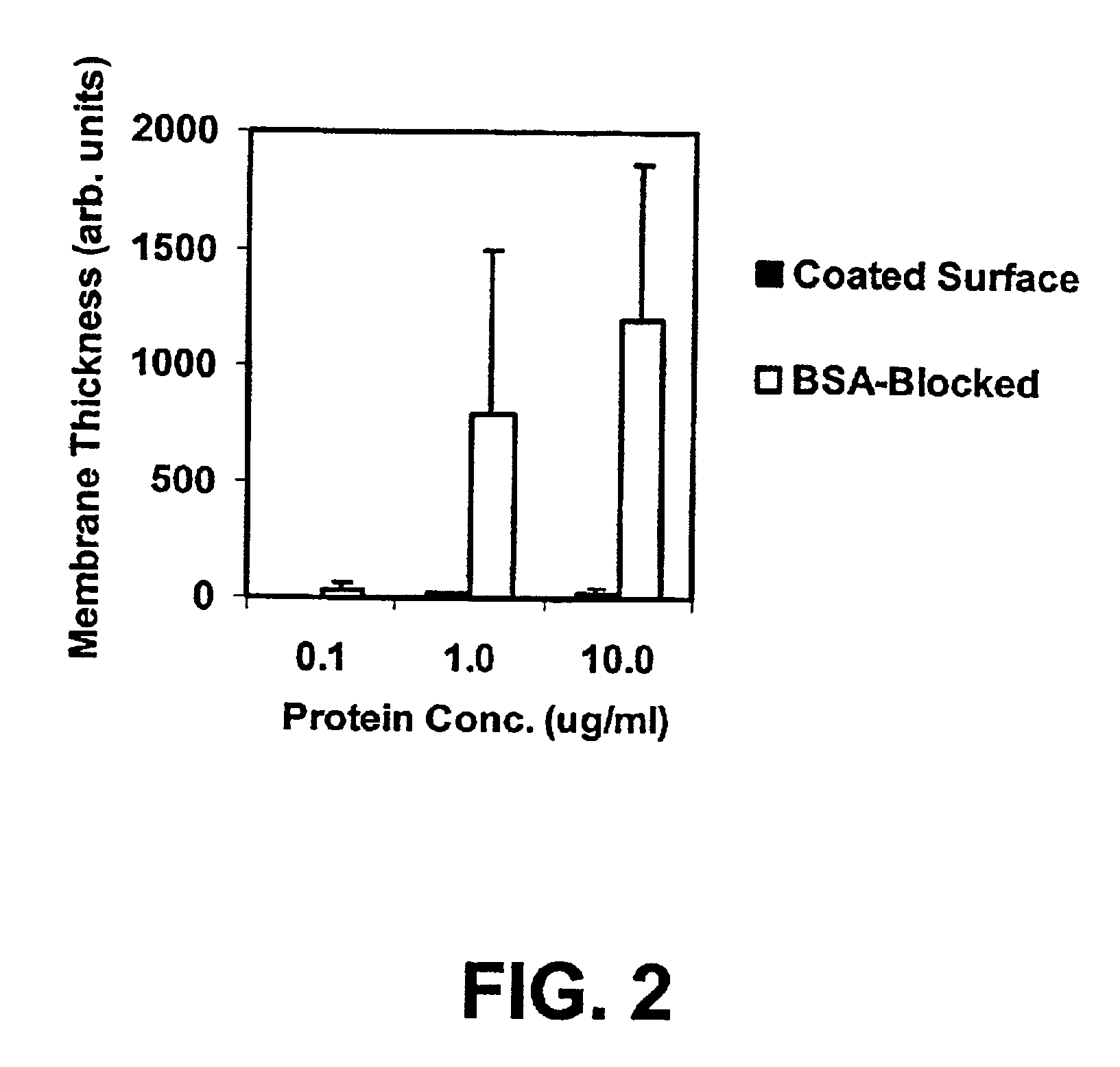
Compositions and methods of preparing functional thin films or surface coatings with low non-specific binding are described. The thin films contain specified functional groups and non-specific binding repellant components. The thin films are either covalently bound to or passively adsorbed to various solid substrates. The specified functional group provides specified activity for the thin film modified solid surfaces and non-specific binding repellant components significantly reduce the non-specific binding to the thin film modified solid surfaces. Non-specific binding repellant components do not affect specified functional group's activity in the thin films. In these methods, specified functional groups are anchored to the solid substrates through a spacer. Surface coatings are also described having both non-specific protein binding properties combined with functional groups for specific binding activity thereby providing surface coating that specifically recognize target proteins but limit binding to non-specific protein.
Owner:ACCELERATED MEDICAL DIAGNOSTICS INC
Non-leaching non-fouling antimicrobial coatings
InactiveUS20090155335A1Solve the lack of flexibilityPromote adequate mobilityAntibacterial agentsPeptide/protein ingredientsDendrimerFiber
Compositions containing one or more types of membrane-targeting antimicrobial agents immobilized on a substrate with activity in relevant biological environments, and methods of making and using thereof, are described herein. The antimicrobial agents retain their activity in the presence of blood proteins and / or in vivo due to improved molecular structures which allow for cooperative action of immobilized agents and hydrophilic chemistries which resist non-specific protein adsorption. Suitable molecular structures include branched structures, such as dendrimers and randomly branched polymers. The molecule structures may also include hydrophilic tethers which provide both flexibility and resistance to non-specific protein adsorption. The membrane targeting antimicrobial agent coatings can be applied to a variety of different types of substrates including medical implants such as vascular grafts, orthopedic devices, dialysis access grafts, and catheters; surgical tools, surgical garments; and bandages. The substrates can be composed of metallic materials, ceramics, polymers, fibers, inert materials such as silicon, and combinations thereof. The compositions described herein are substantially non-leaching, resistant to non-specific protein adsorption, and non-hemolytic.
Owner:ARROW INT INC
Parallel polymorphism scoring by amplification and error correction
InactiveUS20070009954A1Easy to processFusion with DNA-binding domainSugar derivativesPolymerase LBinding domain
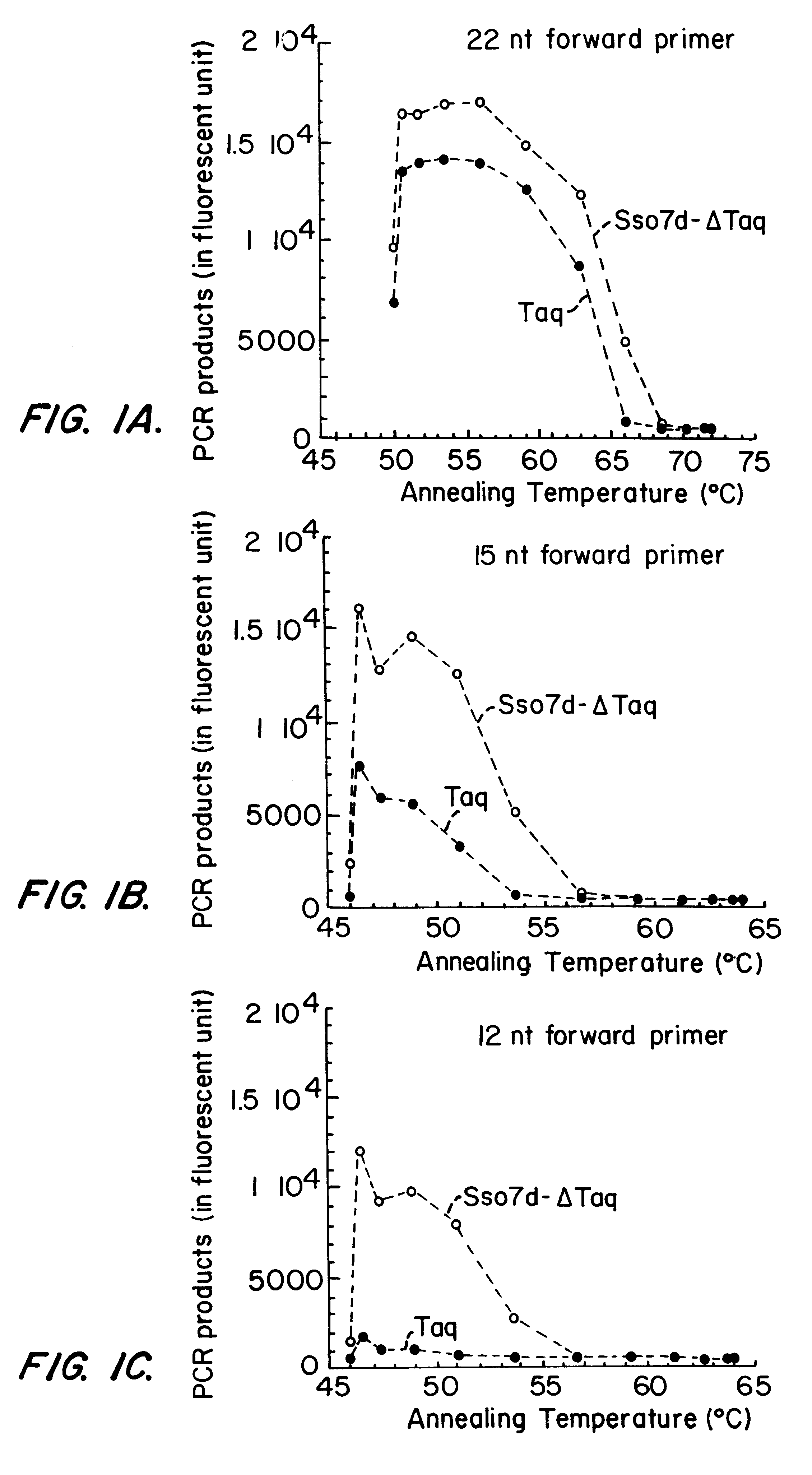
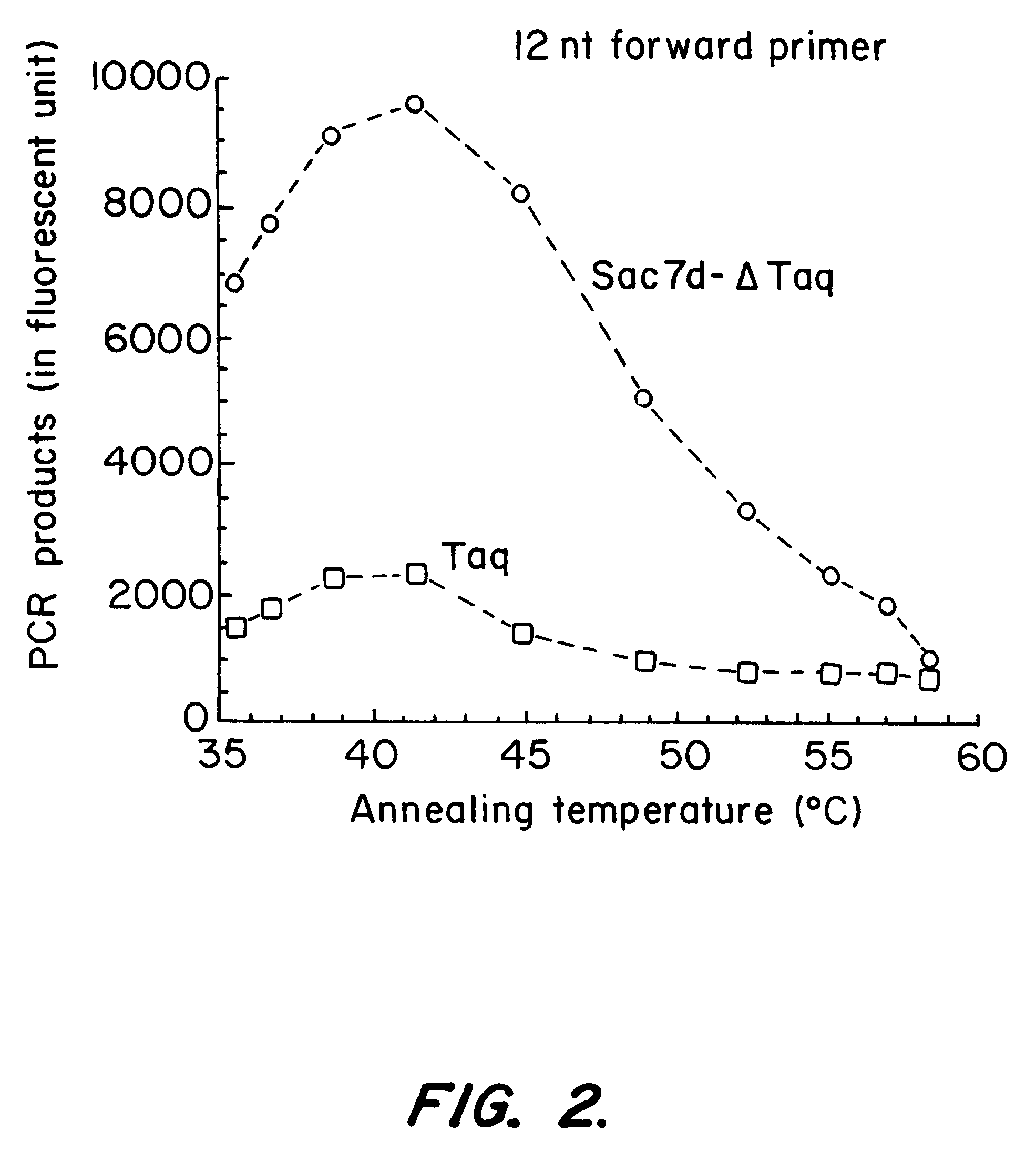
This invention provides a method of detecting polymorphisms, e.g., single nucleotide polymorphisms (SNPs), by amplification and error correction. The invention encompasses methods of performing amplification and error correction using an improved generation of nucleic acid polymerases, and methods of multiplexing the assay. The improvement to the polymerases is the joining of a sequence-non-specific nucleic-acid-binding domain to the enzyme in a manner that enhances the ability of the enzyme to bind and catalytically modify the nucleic acid.
Owner:BIO RAD LAB INC
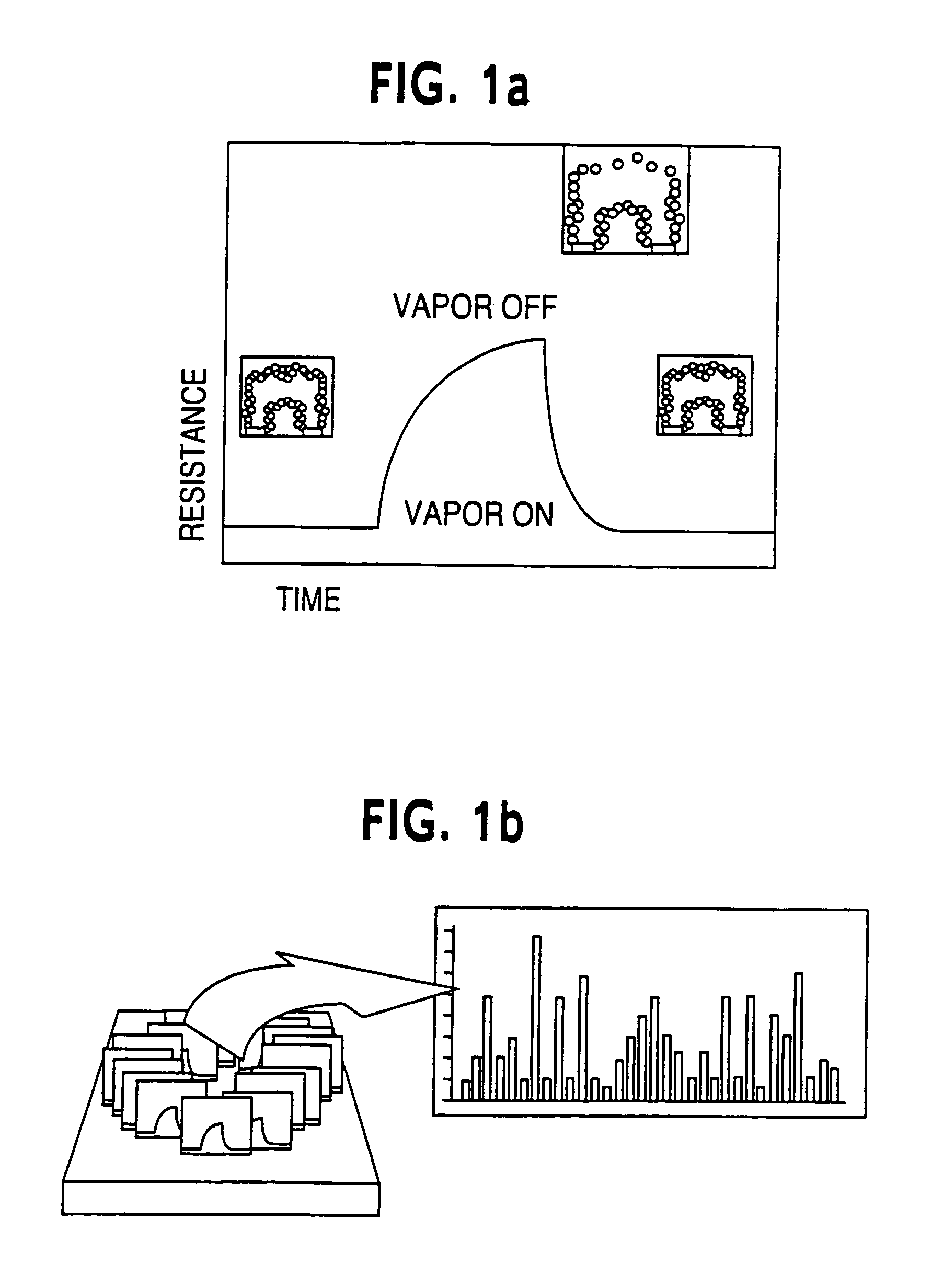
Optical sensor unit and procedure for the ultrasensitive detection of chemical or biochemical analytes
InactiveUS6346376B1High sensitivitySelective retentionBioreactor/fermenter combinationsBiological substance pretreatmentsSpecific detectionNon-covalent interactions
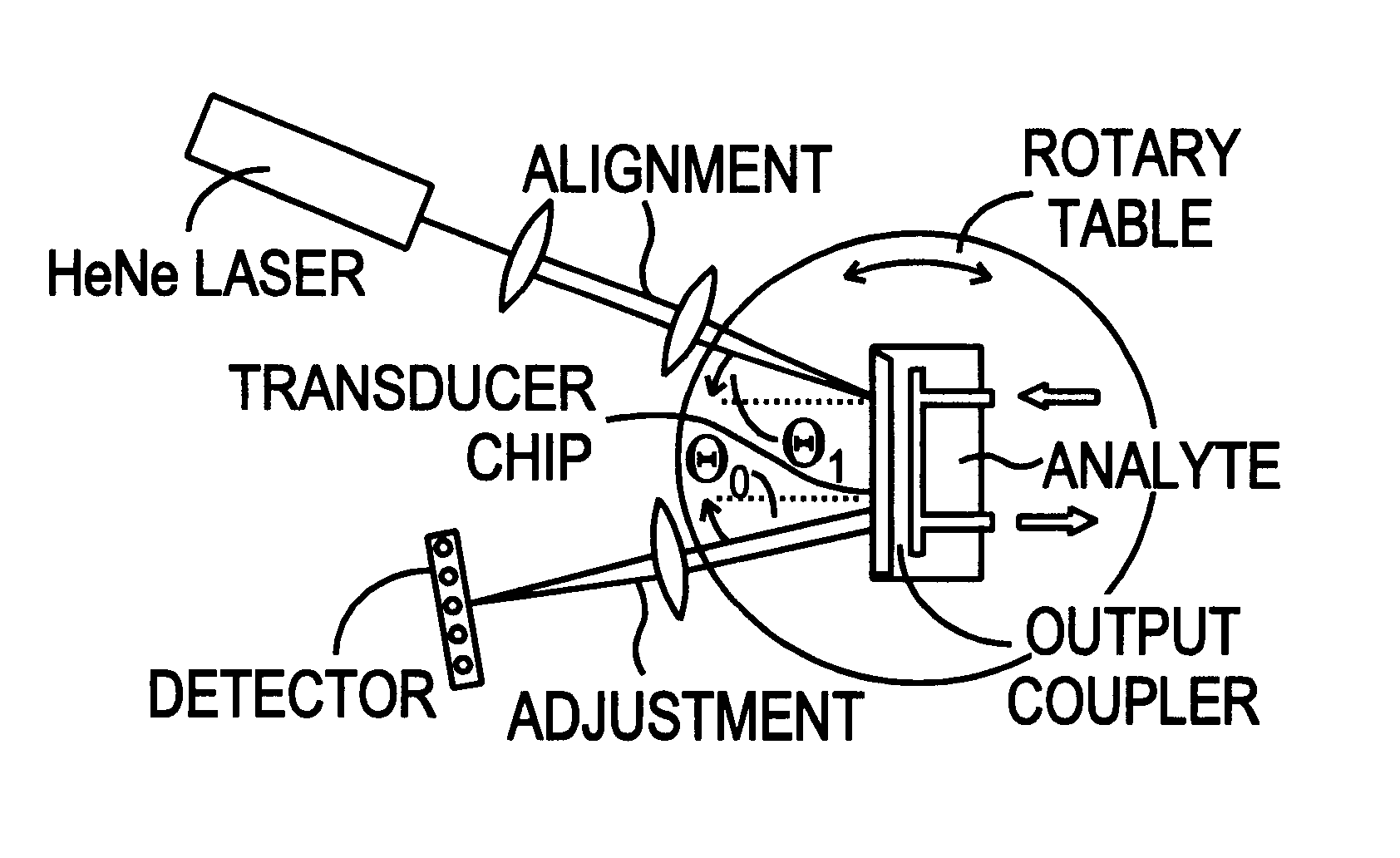
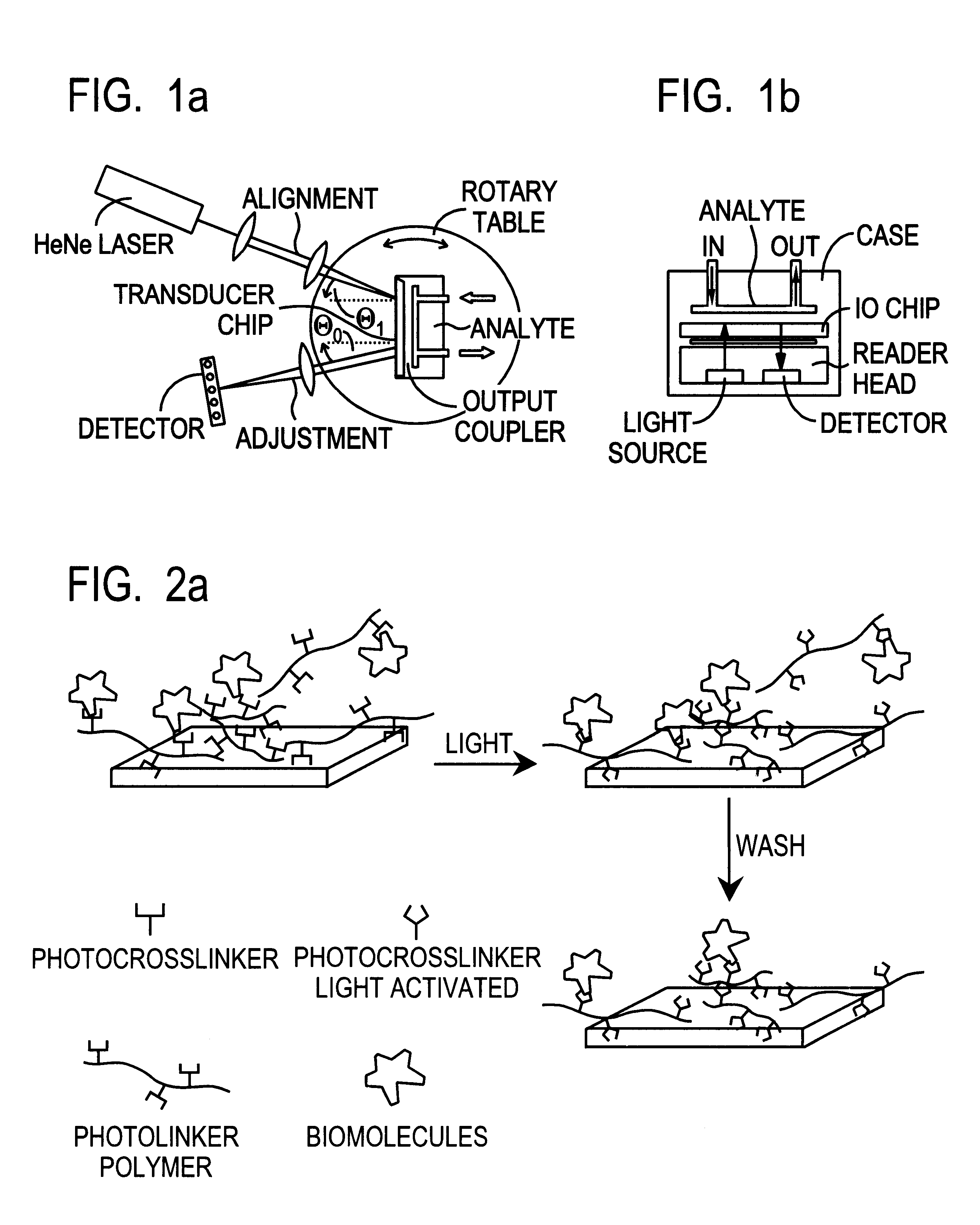
This document describes an optical sensor unit and a procedure for the specific detection and identification of biomolecules at high sensitivity in real fluids and tissue homogenates. High detection limits are reached by the combination of i) label-free integrated optical detection of molecular interactions, ii) the use of specific bioconstituents for sensitive detection and iii) planar optical transducer surfaces appropriately engineered for suppression of non-specific binding, internal referencing and calibration. Applications include the detection of prion proteins and identification of those biomolecules which non-covalently interact with surface immobilized prion proteins and are intrinsically involved in the cause of prion related disease.
Owner:CSEM CENT SUISSE DELECTRONIQUE & DE MICROTECHNIQUE SA RECH & DEV
Diagnostic markers of mood disorders and methods of use thereof
InactiveUS20050176057A1Negative predictive accuracyMaximizing sensitivityBiocideBiostatisticsAssayProper treatment
The present invention relates to methods for the diagnosis, evaluation, and treatment of mood disorders, particularly bipolar disorder. In particular, patient test samples are analyzed for the presence and amount of members of a panel of biallelic markers comprising one or more specific markers for bipolar treatment and one or more non-specific markers for bipolar treatment. A variety of markers are disclosed for assembling a panel of markers for such diagnosis and evaluation. Algorithms for determining proper treatment are disclosed. A diagnostic kit for a panel of said markers is disclosed. In various aspects, the invention provides methods for the early detection and differentiation of mood disorders or bipolar treatment. Methods for screening therapeutic compounds for mood disorders are disclosed. The invention (1) gives methods providing rapid, sensitive and specific assays that can greatly increase the number of patients that can receive beneficial treatment and therapy, thereby reducing the costs associated with incorrect diagnosis, and (2) provides methods for improved therapies.
Owner:BREMER TROY +1
Nucleic acid modifying enzymes
This invention provides for an improved generation of novel nucleic acid modifying enzymes. The improvement is the fusion of a sequence-non-specific nucleic-acid-binding domain to the enzyme in a manner that enhances the ability of the enzyme to bind and catalytically modify the nucleic acid.
Owner:BIO RAD LAB INC
Diagnostic markers of cardiovascular illness and methods of use thereof
InactiveUS20050181386A1Reduce dimensionalityMore confidenceMicrobiological testing/measurementMedical automated diagnosisProper treatmentTest sample
The present invention relates to methods for the diagnosis and evaluation of cardiovascular illness, particularly stroke, myocardial and other cardiovascular damage damage, hypertension treatment. In particular, patient test samples are analyzed for the presence and amount of members of a panel of markers comprising one or more specific markers for cardiovascular illness or hypertension treatment and one or more non-specific markers for cardiovascular illness or hypertension treatment. A variety of markers are disclosed for assembling a panel of markers for such diagnosis and evaluation. Algorithms for determining proper treatment are disclosed. A diagnostic kit for a panel of said markers is disclosed. In various aspects, the invention provides methods for the early detection and differentiation of cardiovascular illness or hypertension treatment. Invention methods provide rapid, sensitive and specific assays that can greatly increase the number of patients that can receive beneficial treatment and therapy, reduce the costs associated with incorrect diagnosis, and provide important information about the prognosis of the patient.
Owner:DIAMOND CORNELIUS +2
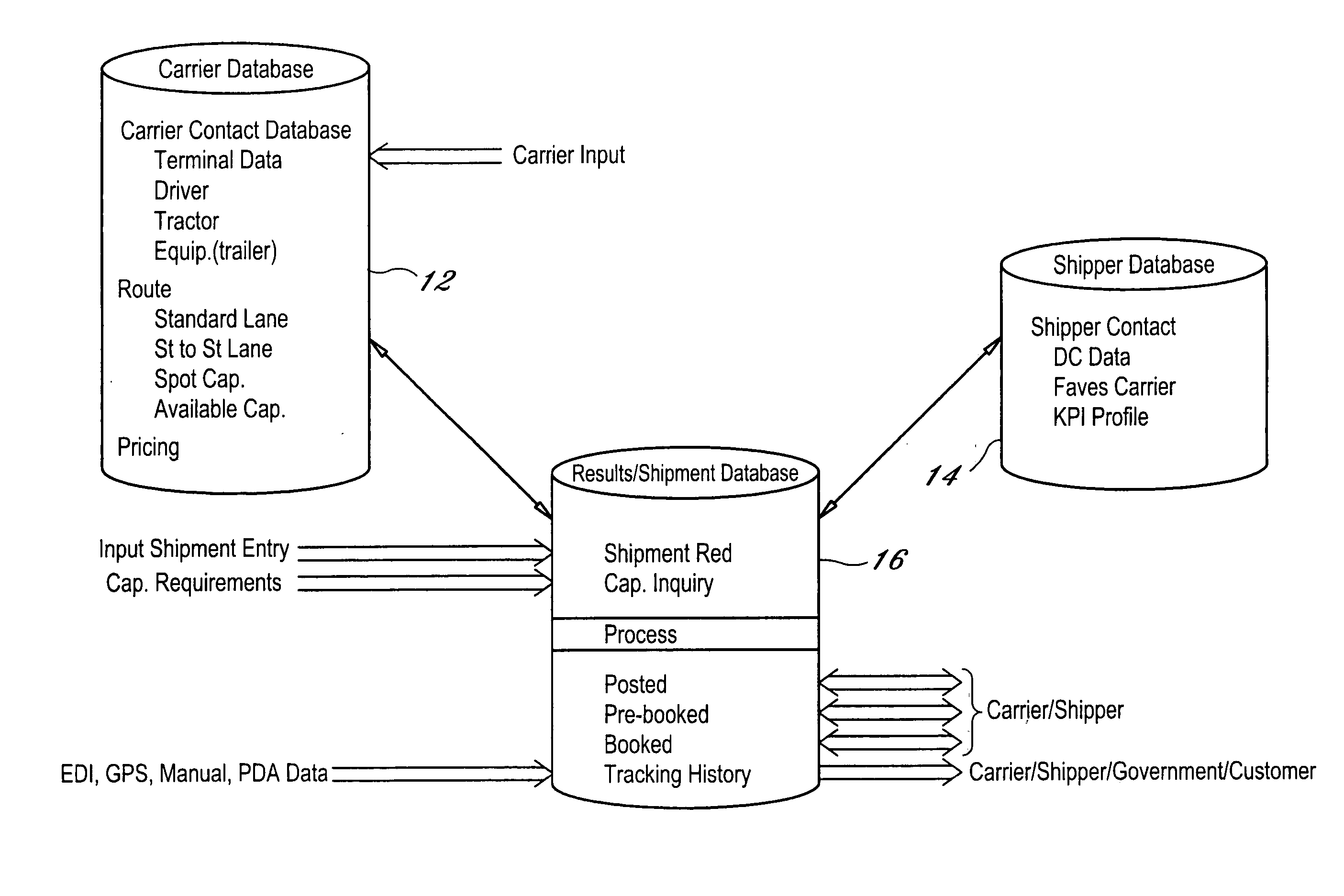
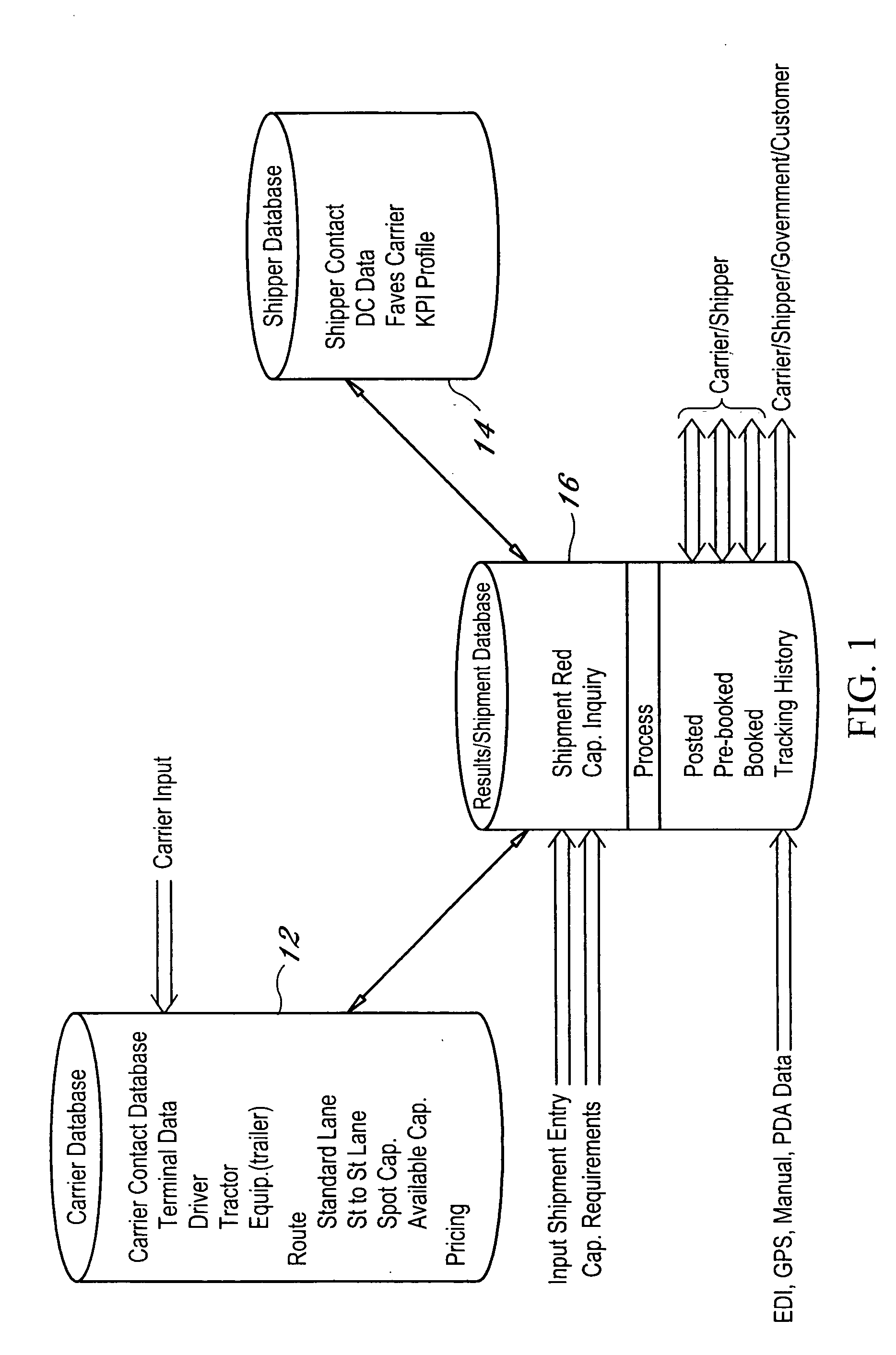
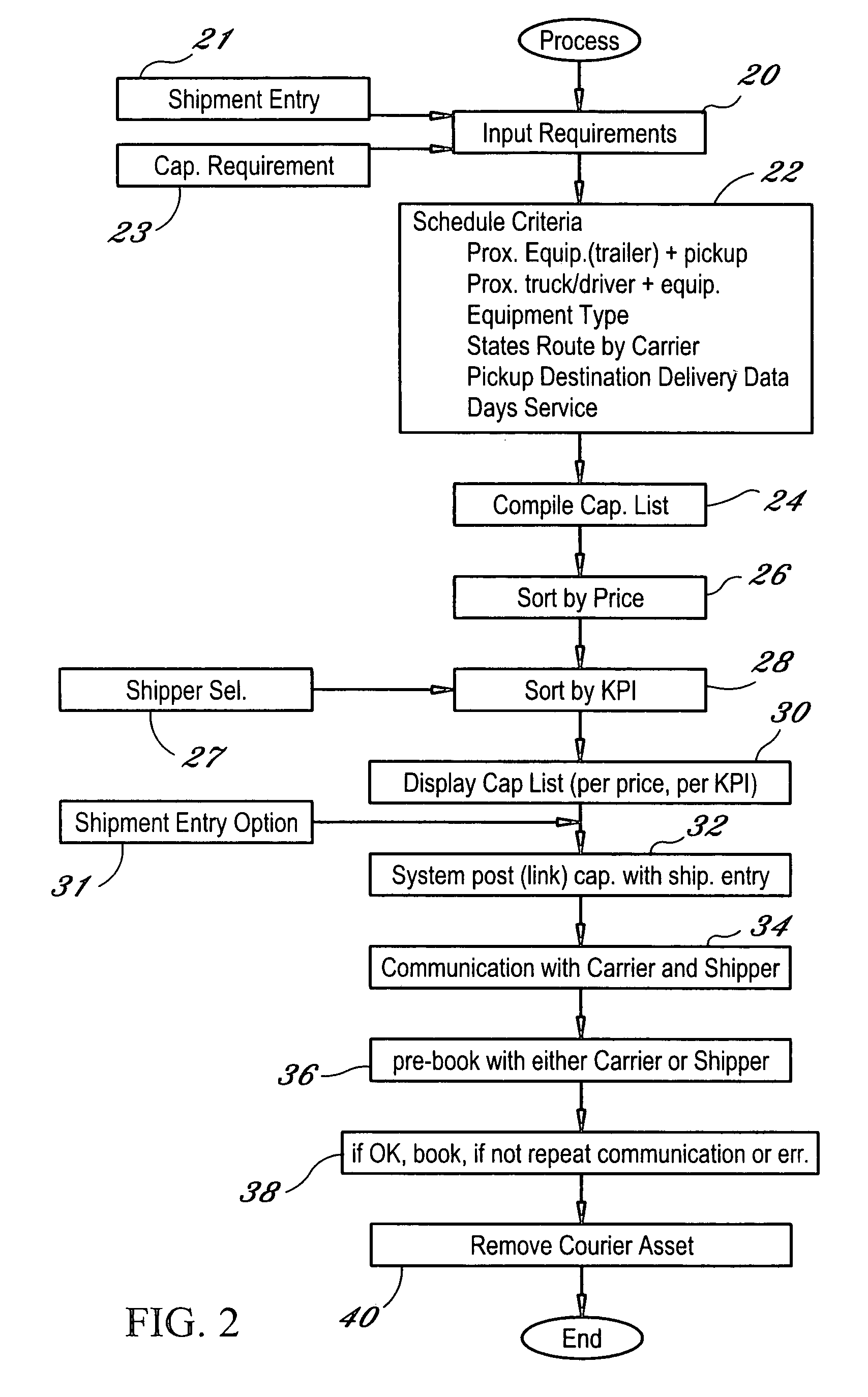
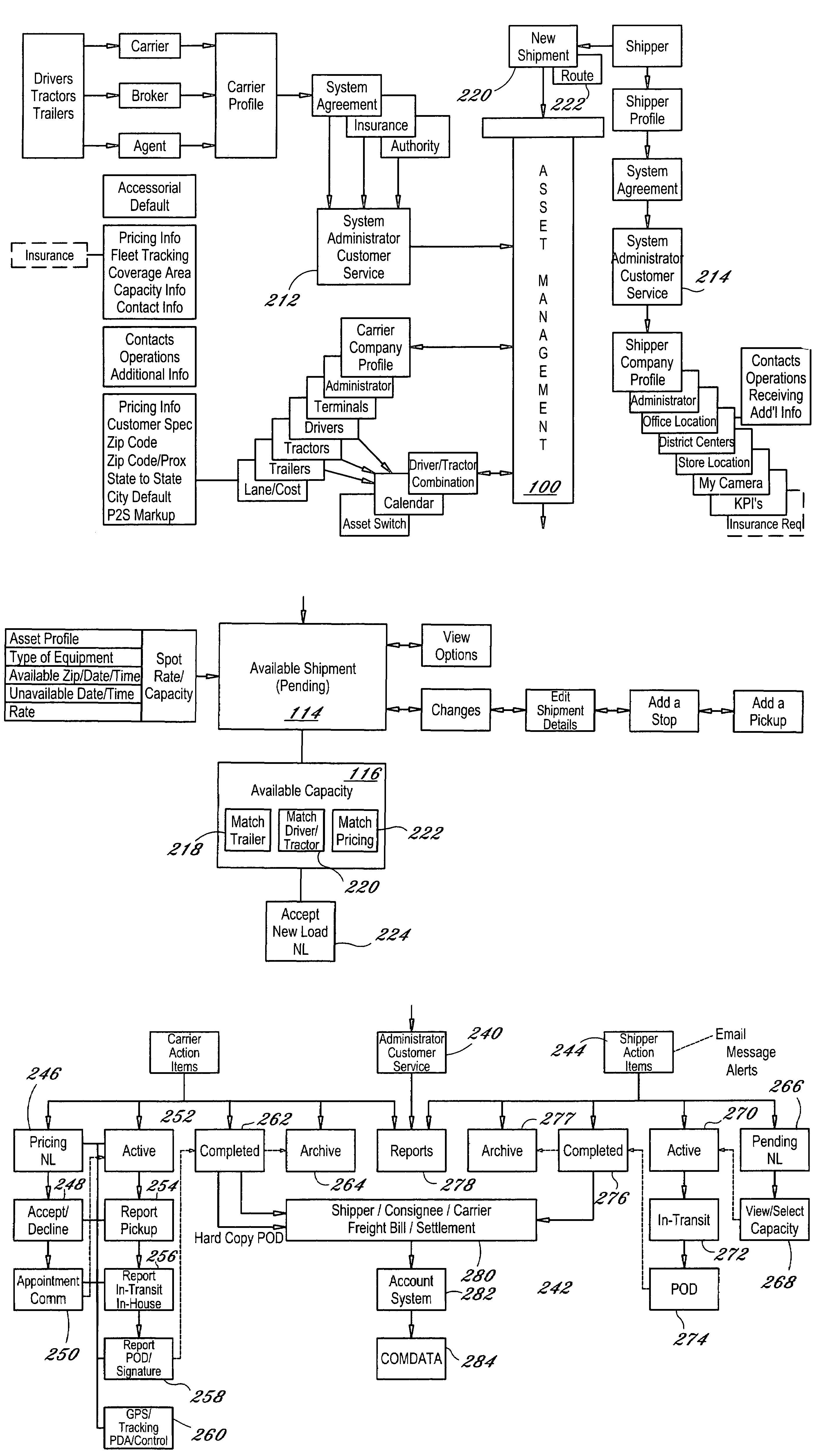
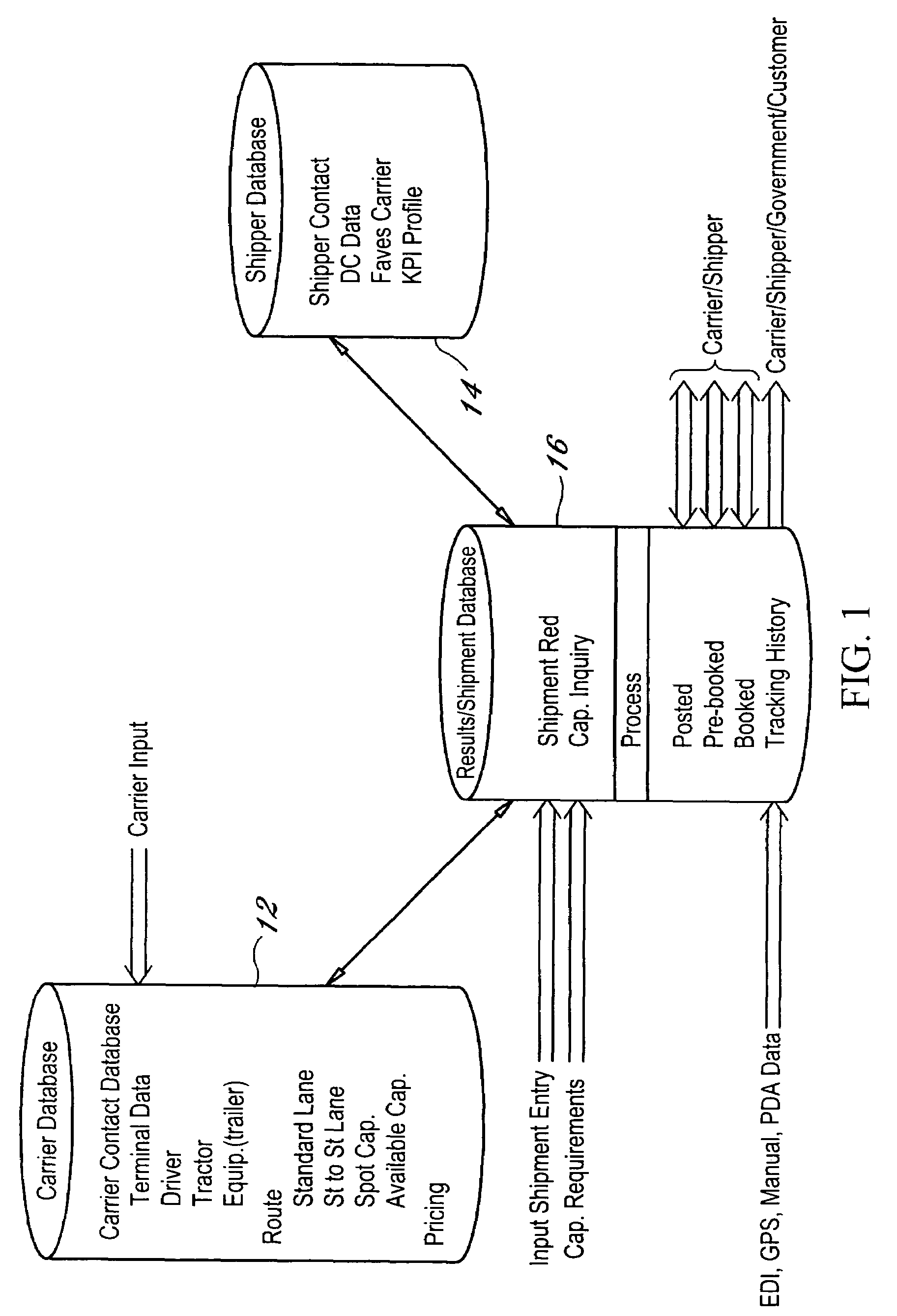
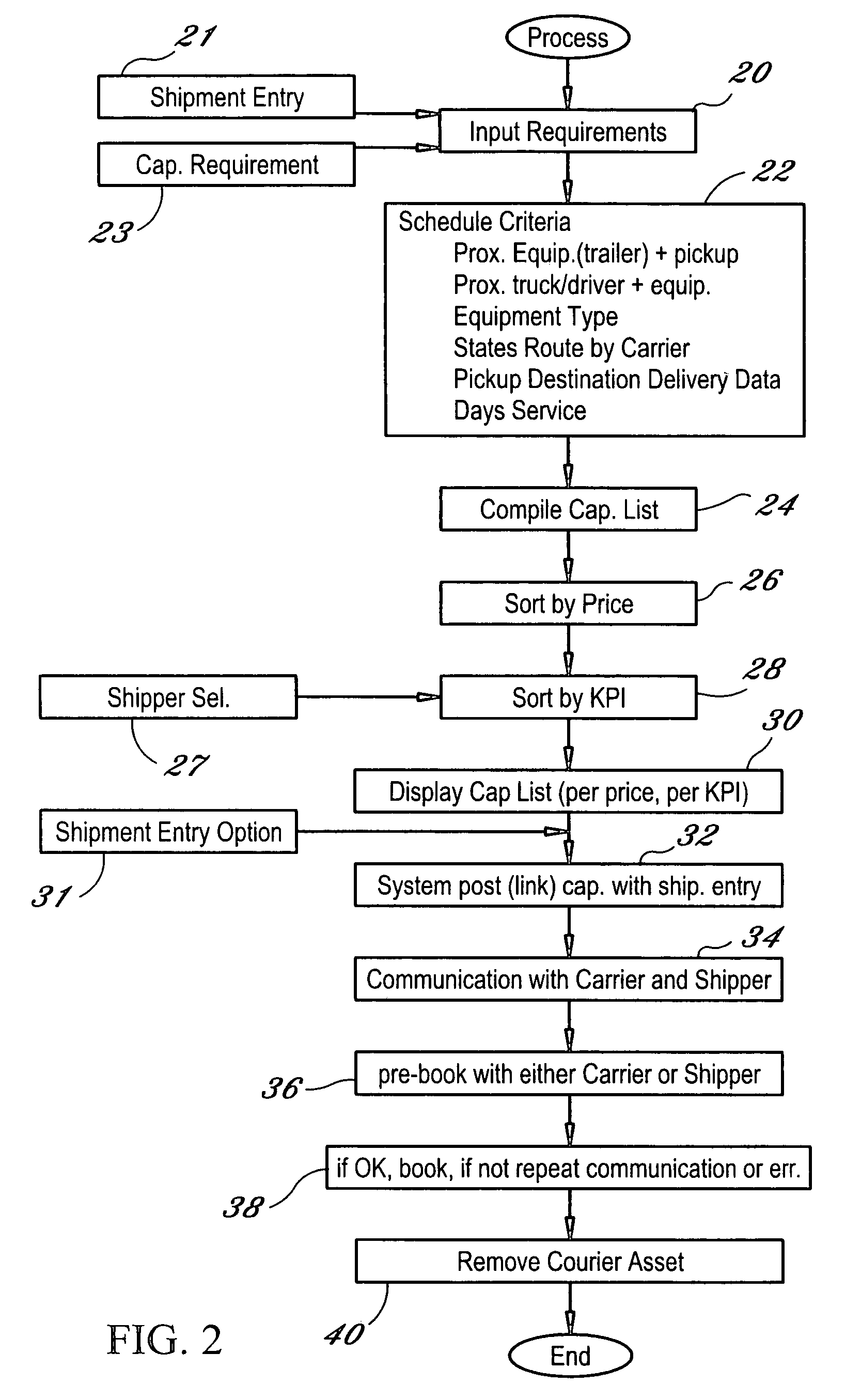
Dynamic and predictive information system and method for shipping assets and transport
InactiveUS20050278063A1Ticket-issuing apparatusRoad vehicles traffic controlDriver/operatorCarrier signal
The dynamic, predictive information system and method assigns shipping assets (drivers-tractors-trailers) from carriers to transport orders by shippers. Computer databases hold shipping asset data. Specific transport orders are electronically joined to specific driver-tractor-trailer combinations. A search and sort routine produces resulting records based upon proximity, trailer type, proximity of the joined driver-trailer combination, carrier service region and pick-up and delivery date constraints. The sort is by price or performance indicators which are pre-selected shipper ranges matched to historical shipping data from carriers. The system books the carrier, the driver-tractor-trailer combination and the shipper to transport order with an electronic communications phase. In a truck lane scenario, the system joins a specific driver and a specific tractor and a non-specific trailer to a specific transport order. GPS data and electronic shipping document data from PDAs with the drivers is logged into the system and is viewable by the participants.
Owner:NUSTATE ENERGY HLDG +1
Enzymatic electrochemical detection assay using protective monolayer and device therefor
InactiveUS20060160100A1Material nanotechnologyMicrobiological testing/measurementOxidation-Reduction AgentRedox
There is provided an electrochemical assay method for detecting a target molecule, for example a protein, in a sample, which involves the use of a protective monolayer and a redox polymer to form a bilayer immobilized on an electrode. The monolayer protects the electrode from non-specific adherence of reagents, particular proteins, to the electrode while simultaneously providing a surface that can be functionalized to immobilize a capture molecule and that can interact with the redox polymer.
Owner:AGENCY FOR SCI TECH & RES
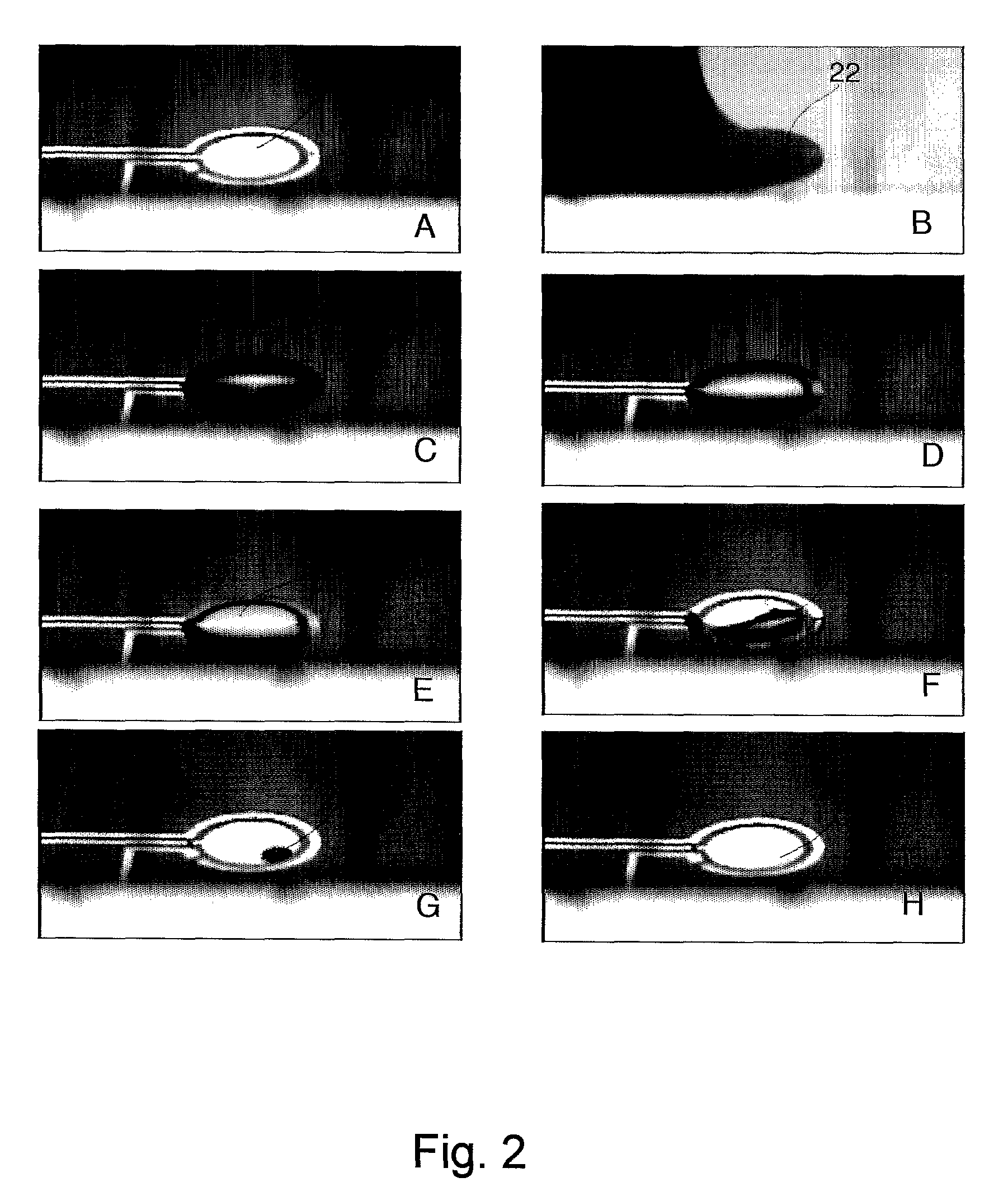
Biosensor Detection By Means Of Droplet Driving, Agitation, and Evaporation
InactiveUS20090042319A1More dataLow costSamplingTransportation and packagingSignal-to-noise ratio (imaging)Analyte
Methods of improving microfluidic assays are disclosed. Assays can be improved (better signal to noise ratio) by using sessile drop evaporation as an analyte concentration step (enhanced signal) and repeated passes of wash droplets as a means to reduce non-specific binding (noise reduction). In addition multiple massively parallel analyses improve the statistical precision of the analyses.
Owner:ADVANCED LIQUID LOGIC
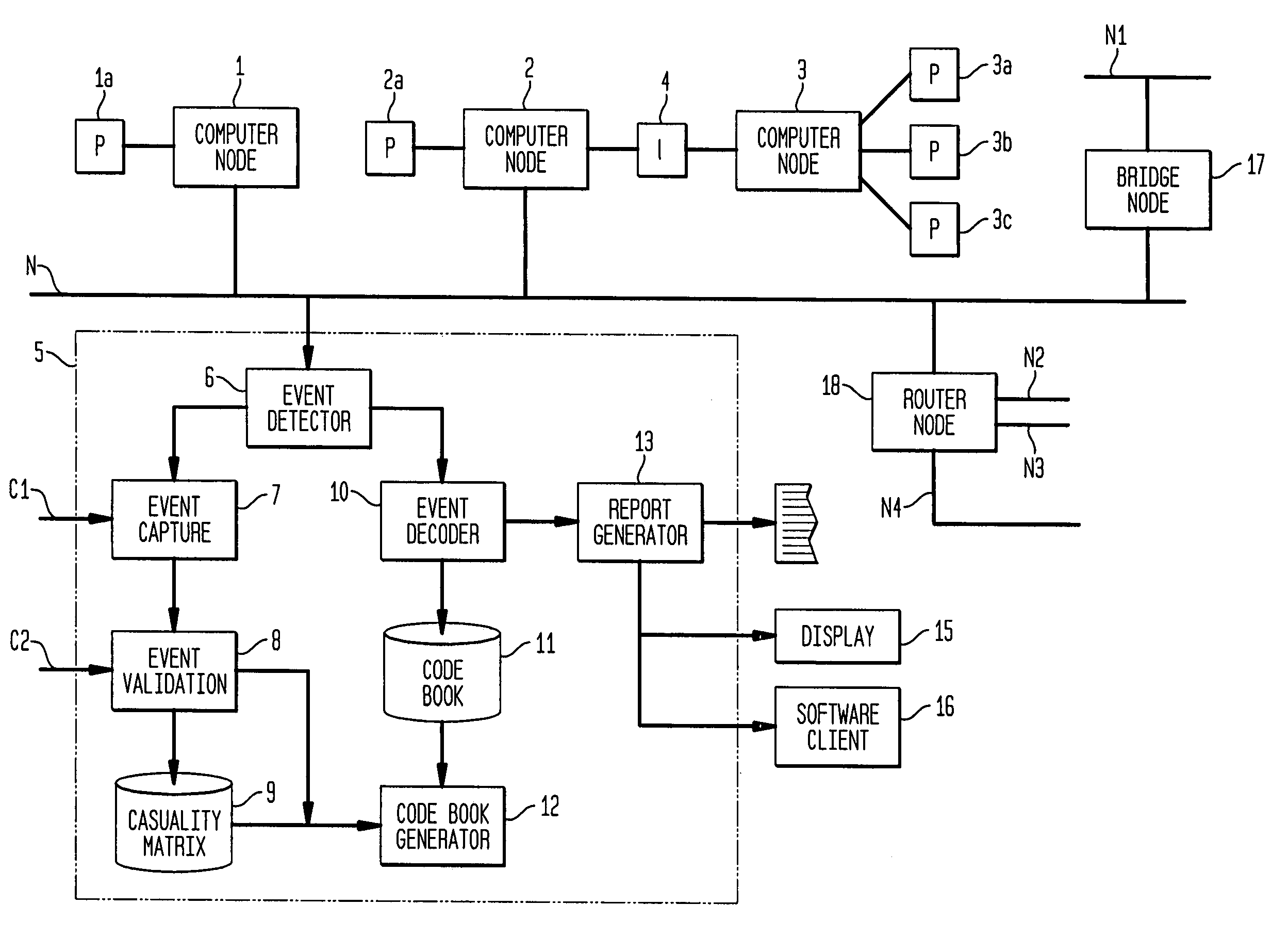
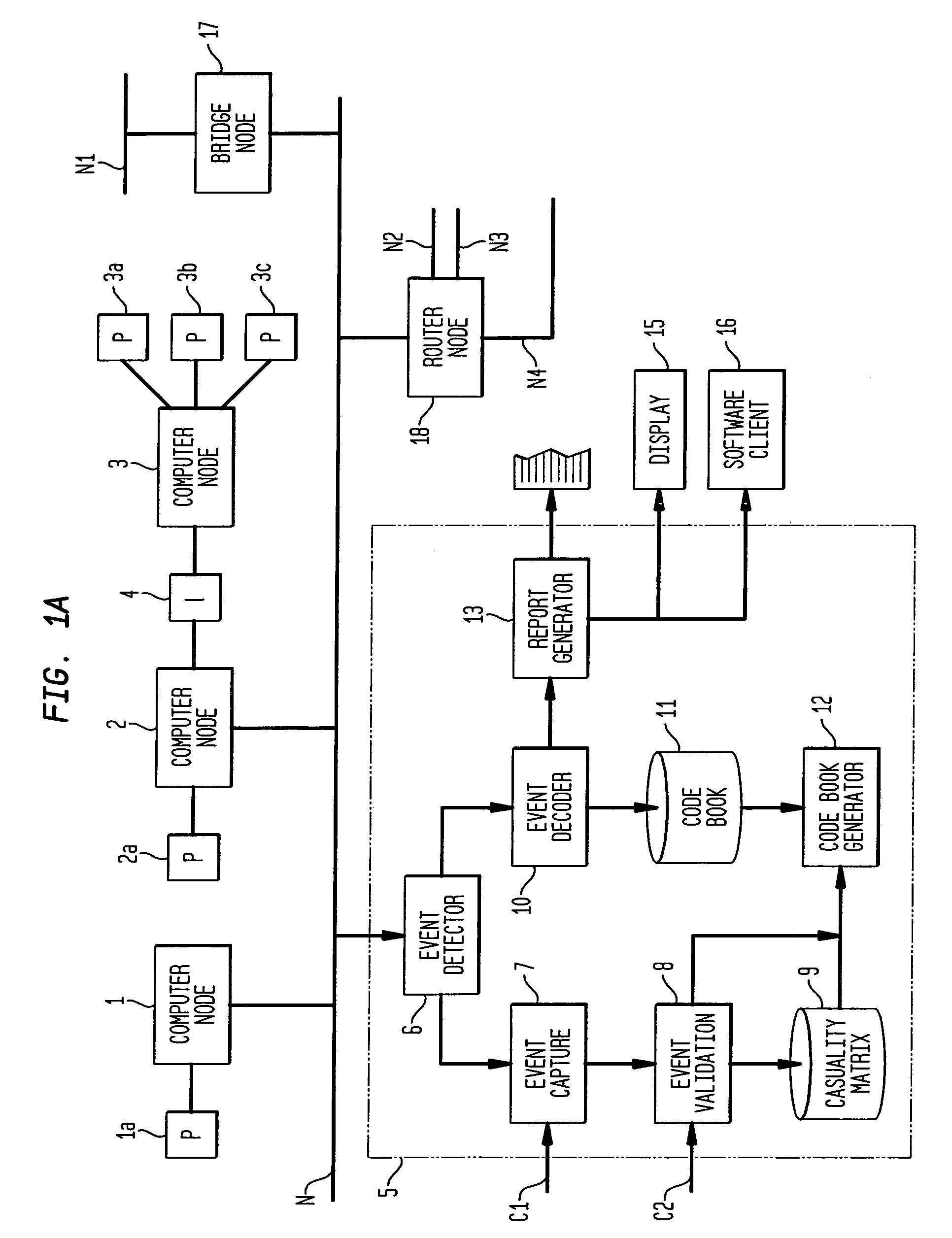
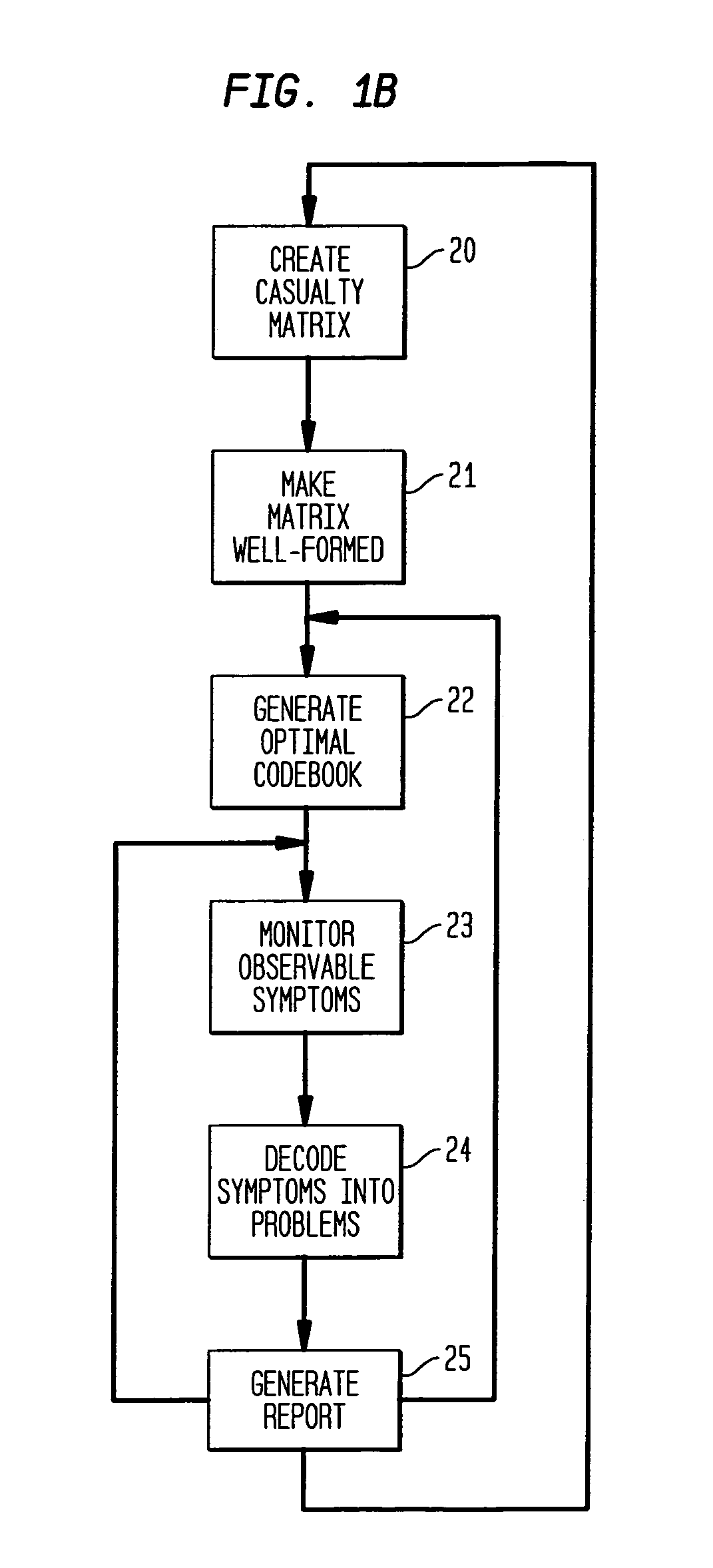
Apparatus and method for event correlation and problem reporting
InactiveUS7107185B1Effective monitoringImprove efficiencyNuclear monitoringDigital computer detailsComplex systemComputer science
A computer implemented method on a computer readable media is provided for determining the source of a problem in a complex system of managed components based upon symptoms. The problem source identification process is split into different activities. Explicit configuration non-specific representations of types of managed components, their problems, symptoms and the relations along which the problems or symptoms propagate are created that can be manipulated by executable computer code. A data structure is produced for determining the source of a problem by combining one or more of the representations based on information of specific instances of managed components in the system. Computer code is then executed which uses the data structure to determine the source of the problem from one or more symptoms.
Owner:VMWARE INC
Method of improving sensor detection of target molcules in a sample within a fluidic system
InactiveUS7919330B2More dataLow costSamplingTransportation and packagingAnalyteSignal-to-noise ratio (imaging)
Owner:ADVANCED LIQUID LOGIC
Inhibitor nucleic acids
InactiveUS20050256071A1Low melting pointQuality improvementOrganic active ingredientsNervous disorderAptamerHalf-life
The present invention provides methods and compositions for attenuating expression of a target gene in vivo. In general, the method includes administering RNAi constructs (such as small-interfering RNAs (i.e., siRNAs) that are targeted to particular mRNA sequences, or nucleic acid material that can produce siRNAs in a cell), in an amount sufficient to attenuate expression of a target gene by an RNA interference mechanism. In particular, the RNAi constructs may include one or more modifications to improve serum stability, cellular uptake and / or to avoid non-specific effect. In certain embodiments, the RNAi constructs contain an aptamer portion. The aptamer may bind to human serum albumin to improve serum half life. The aptamer may also bind to a cell surface protein that improves uptake of the construct.
Owner:CALIFORNIA INST OF TECH
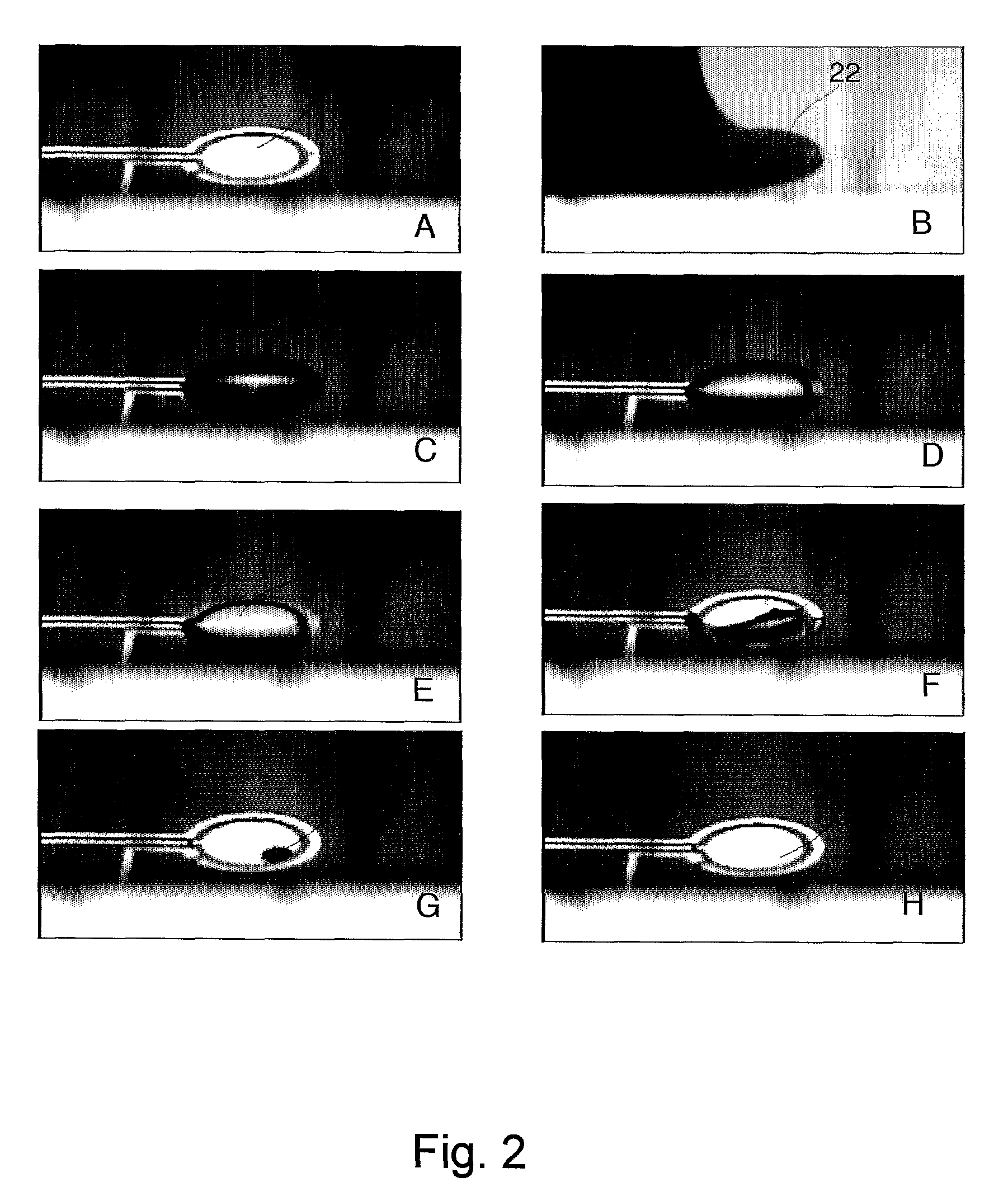
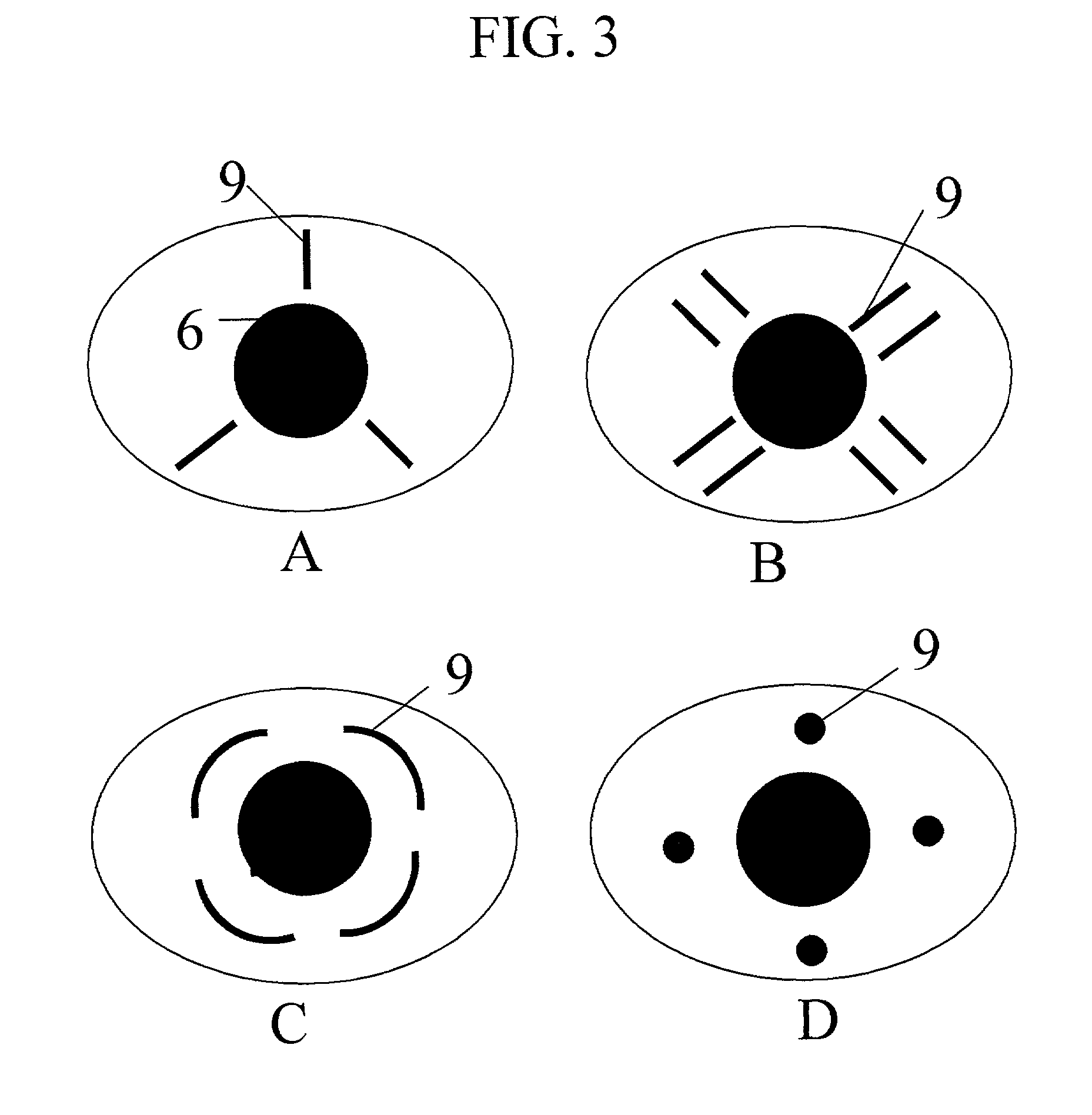
Method and apparatus for treatment of presbyopia by lens relaxation and anterior shift
A surgical method and apparatus for presbyopia correction removal of the sclera tissue are disclosed. Mechanisms based on sub-conjunctiva filled-in of the sclera area and cause the sclera-ciliary-body and zonule "complex" become more flexible (or less rigidity) are proposed. Total accommodation based a lens relaxation and lanes anterior shift is calculated and proposed as the guidance of the parameters for device design and clinical outcomes The preferred embodiments for the ablation patterns include radial lines, curved lines, ring dots or any non-specific shapes in a symmetric geometry. The surgery apparatus includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device. Another preferred embodiment is to use post-operation medication such as pilocarpine (1%-10%) or medicines with similar nature which may cause ciliary body contraction for more stable and enhancement after the treatment.
Owner:LIN J T
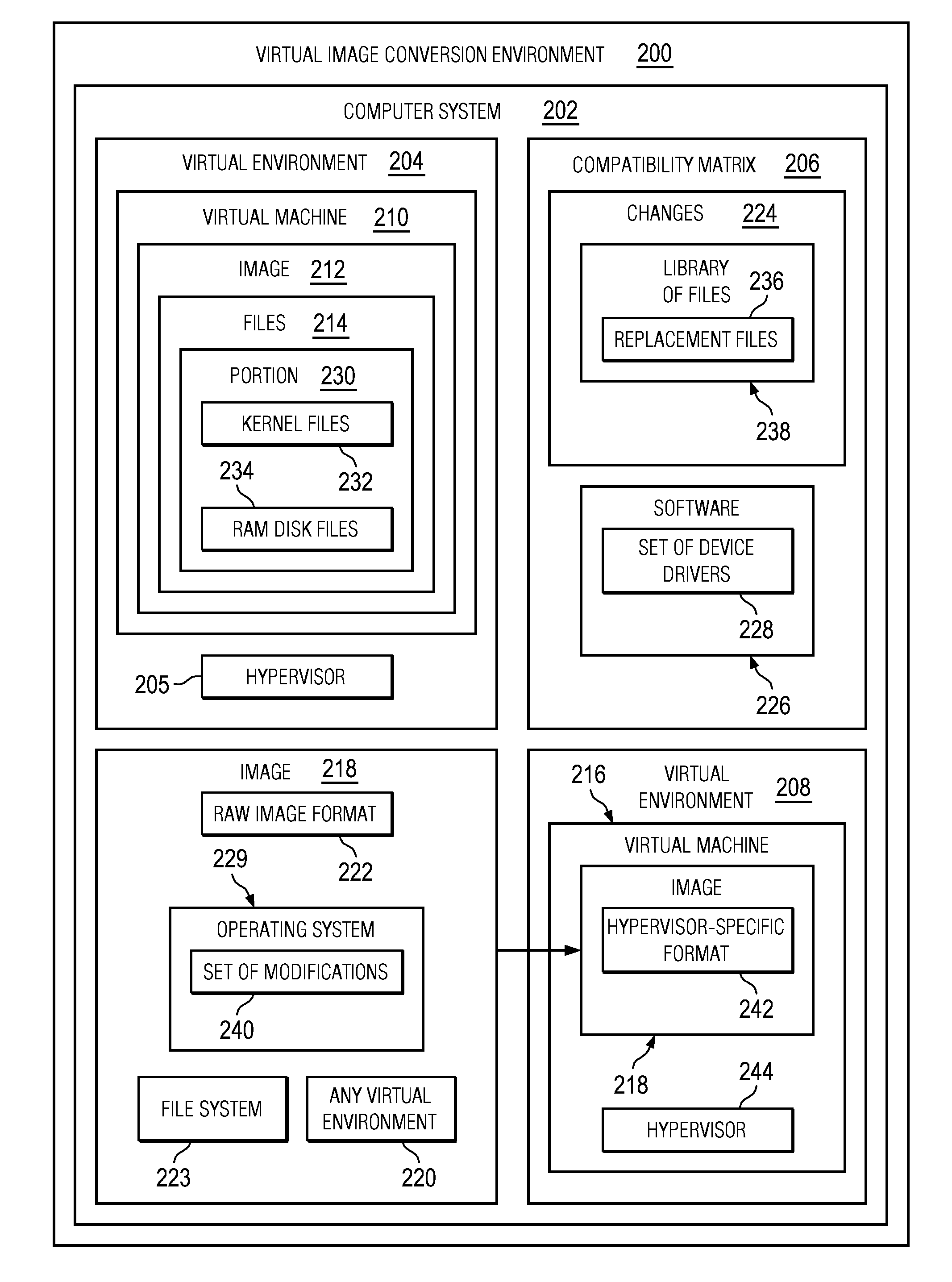
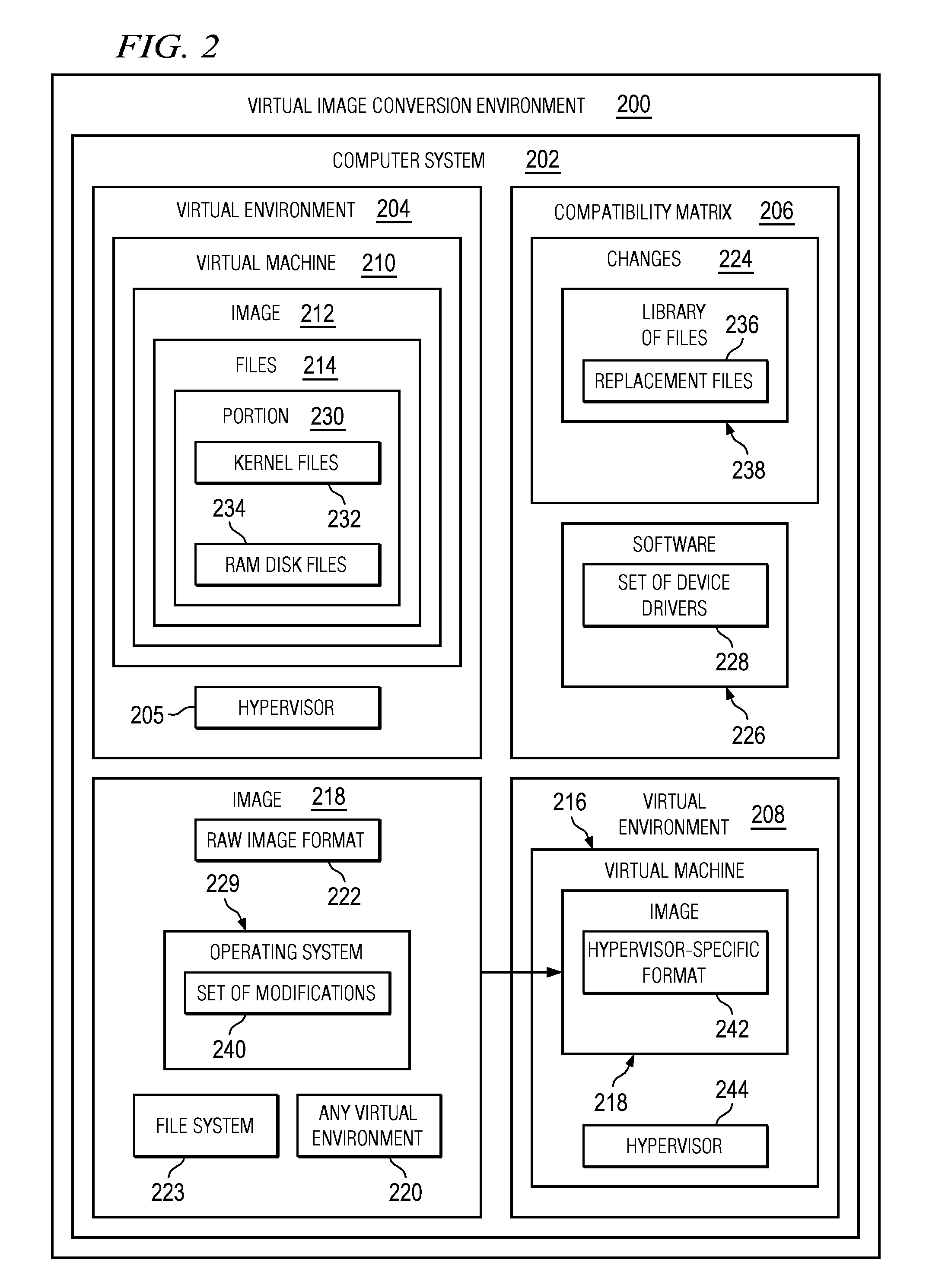
Converting Images in Virtual Environments
The different illustrative embodiments provide a method, computer program product, and apparatus for converting a first image for a virtual machine formatted for a first virtual environment. A second image is created, wherein the second image is non-specific to any virtual environment. A determination is made whether a portion of files to be copied from the first virtual image to the second virtual image should be replaced using a compatibility matrix, wherein the compatibility matrix identifies changes between the first virtual environment and a second virtual environment. A replacement for the portion of the files is copied to the second image using the compatibility matrix responsive to a determination that the portion of the files in the set of files should be replaced. The file is copied to the second image responsive to an absence of a determination that the each file in the set of files should be replaced.
Owner:IBM CORP
Distributed file system
InactiveUS20050015461A1Digital data information retrievalMultiple digital computer combinationsFile systemDistributed File System
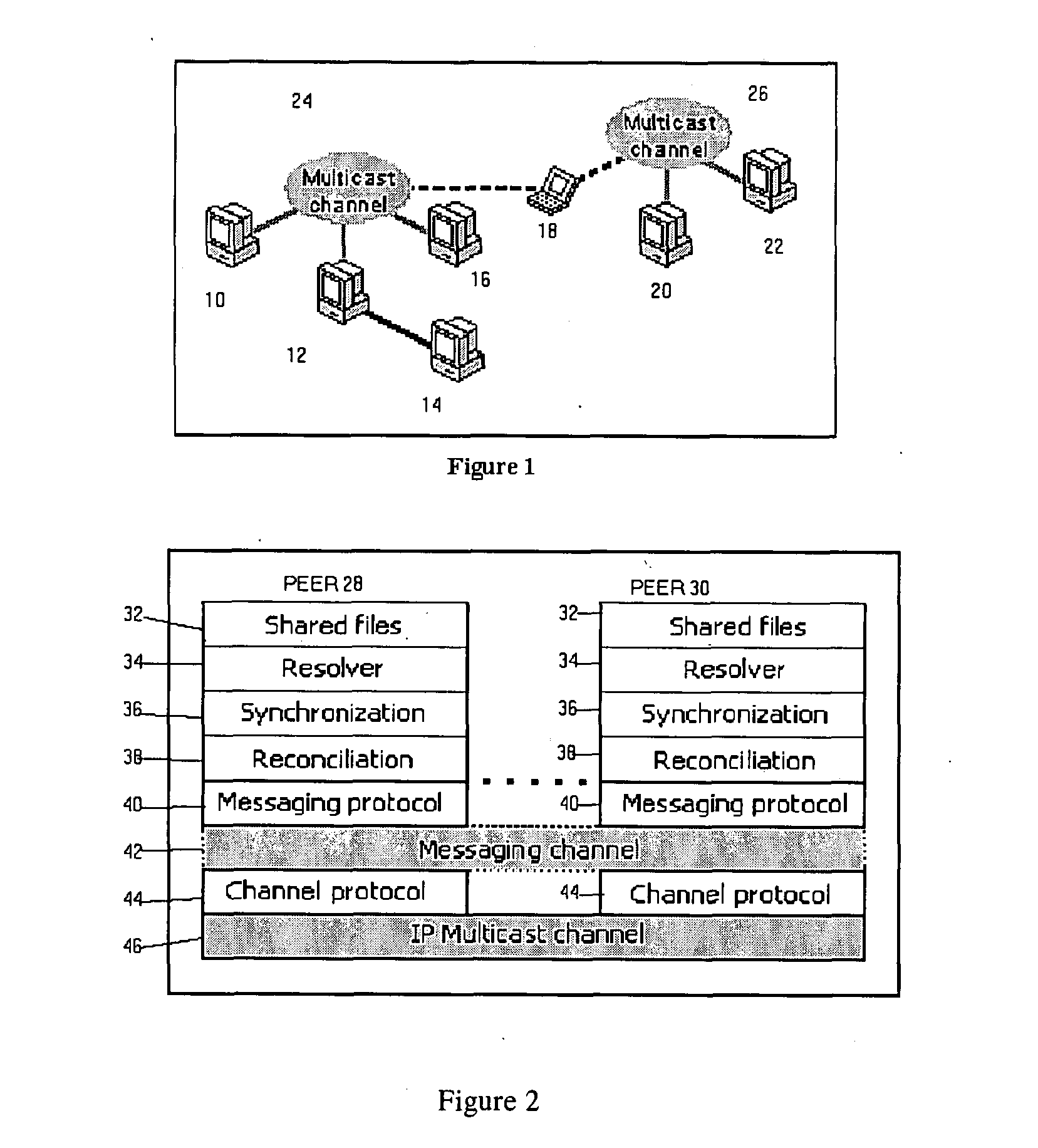
The invention relates to a distributed file system and method for distributing files between computing devices (10-22) of at least one group of computing devices (10-22) in which files are distributed automatically upon the networking of two or more computing devices (10-22). In the preferred embodiment, the user is not required to invoke any file distribution application as this is effected automatically. Advantageously, all files in a shared file directory are distributed to all computers (10-22) in the group and stored in a usable format such that every computer (10-22) is able to read and write to a shared file even when not networked to other computers (10-22) in the group. File distribution is preferably carried out in a non-specific manner and in a one-for-all manner. The system is very suitable for networks with low connectivity patterns between the computers.
Owner:HEWLETT PACKARD DEV CO LP
Dynamic and predictive information system and method for shipping assets and transport
InactiveUS7385529B2Ticket-issuing apparatusDigital data processing detailsDriver/operatorElectronic communication
The dynamic, predictive information system and method assigns shipping assets (drivers-tractors-trailers) from carriers to transport orders by shippers. Computer databases hold shipping asset data. Specific transport orders are electronically joined to specific driver-tractor-trailer combinations. A search and sort routine produces resulting records based upon proximity, trailer type, proximity of the joined driver-trailer combination, carrier service region and pick-up and delivery date constraints. The sort is by price or performance indicators which are pre-selected shipper ranges matched to historical shipping data from carriers. The system books the carrier, the driver-tractor-trailer combination and the shipper to transport order with an electronic communications phase. In a truck lane scenario, the system joins a specific driver and a specific tractor and a non-specific trailer to a specific transport order. GPS data and electronic shipping document data from PDAs with the drivers is logged into the system and is viewable by the participants.
Owner:NUSTATE ENERGY HLDG +1
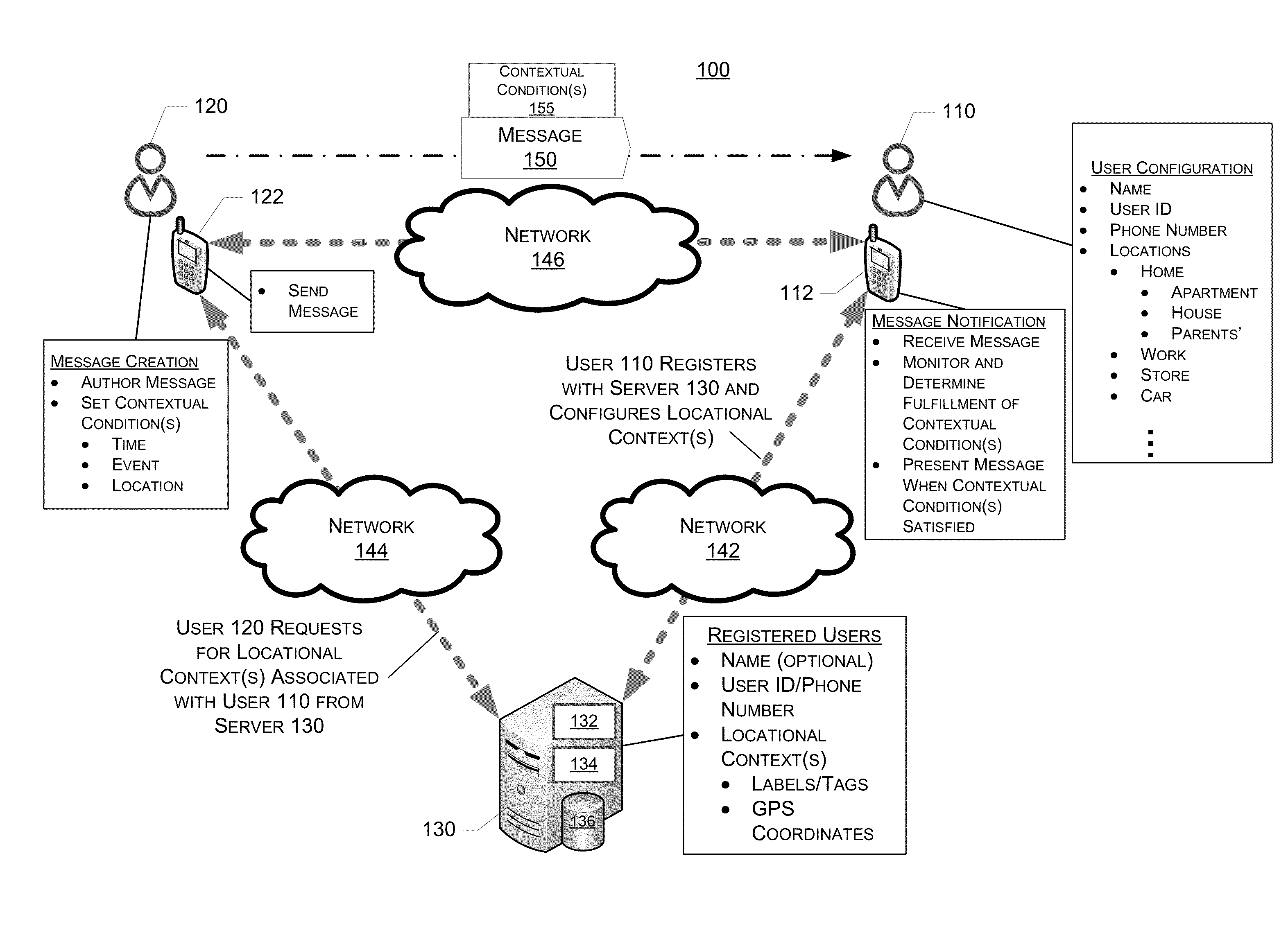
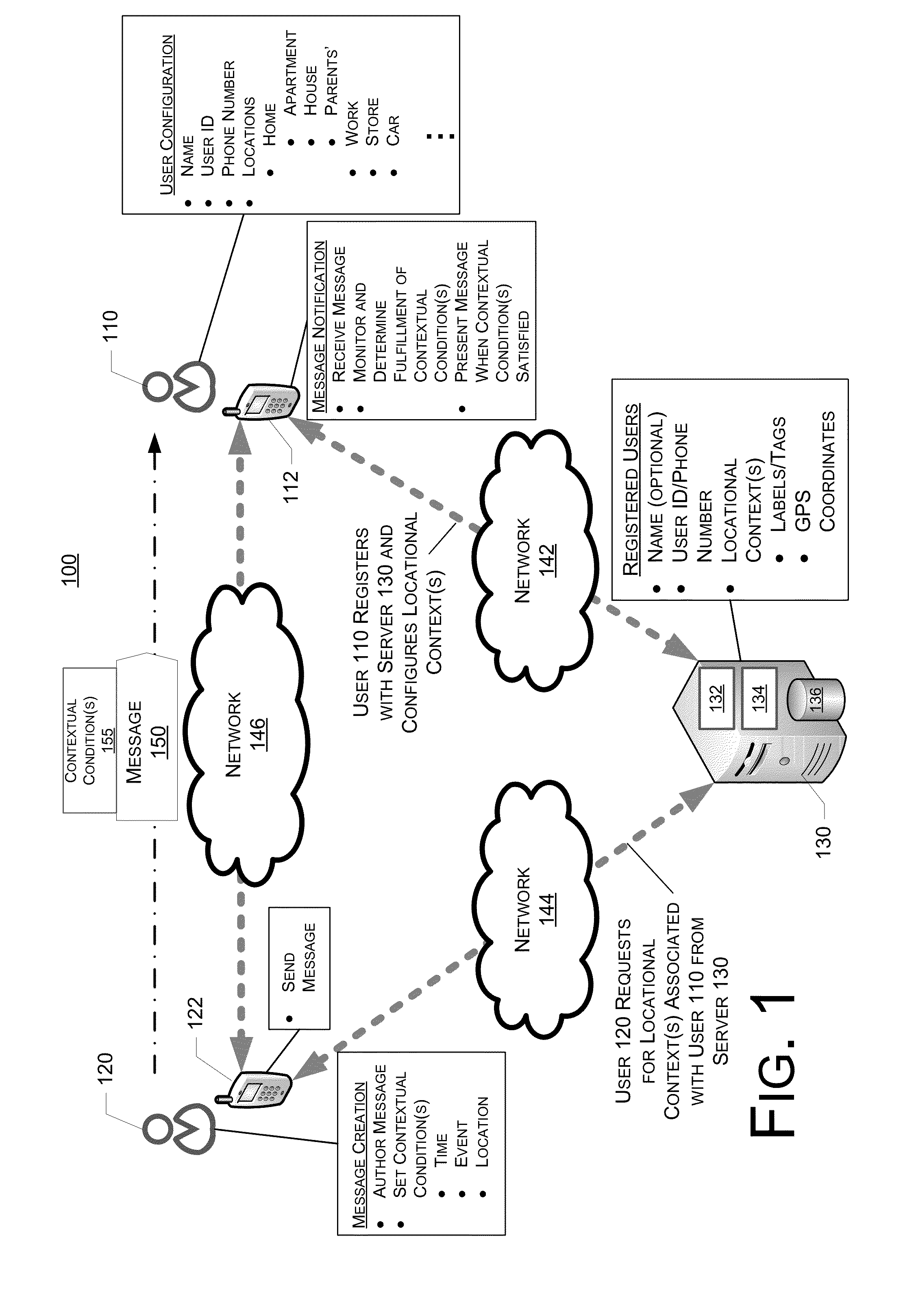
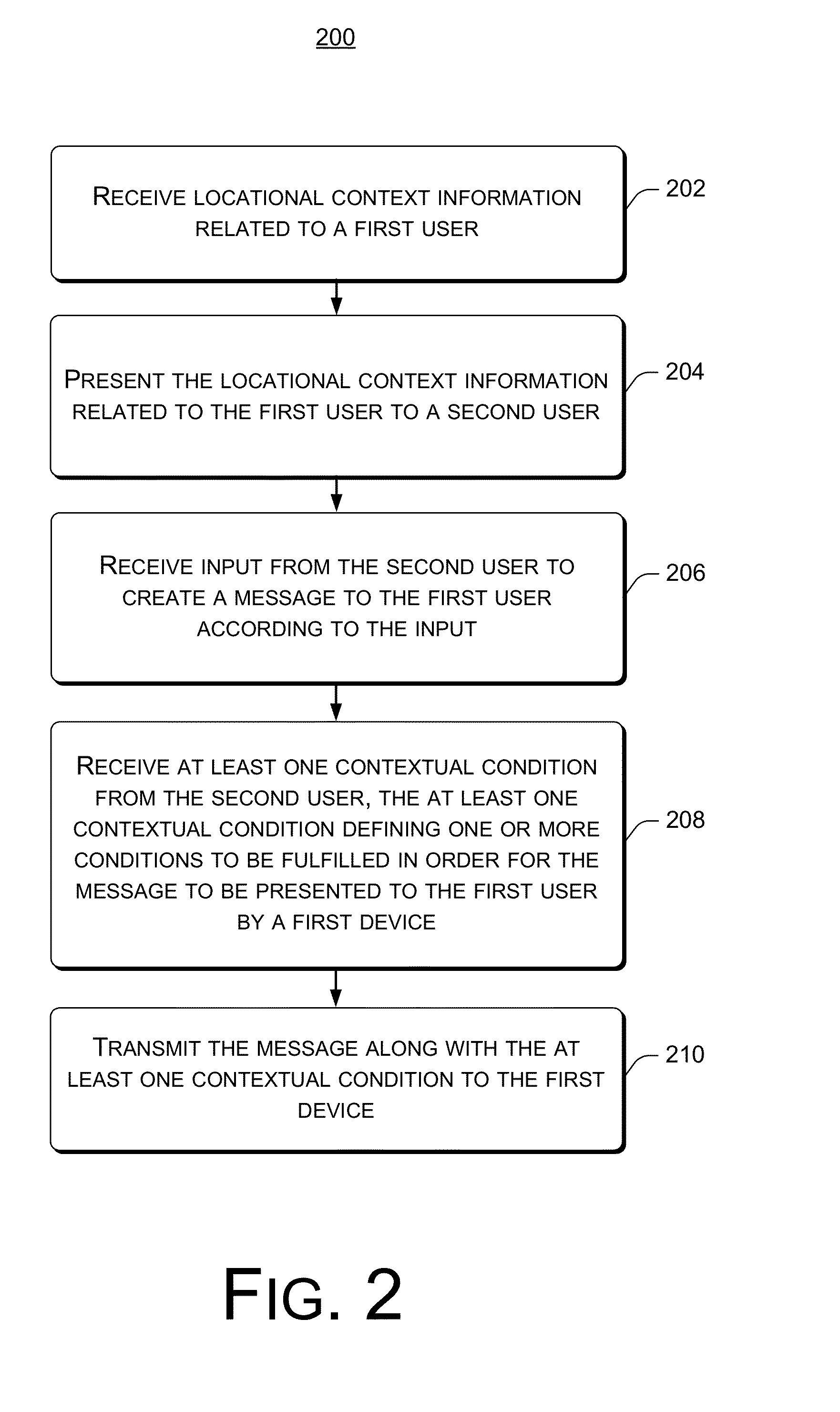
System, Method And Device For Creation And Notification Of Contextual Messages
System, method and device for the creation and notification of contextual messages are described. When creating or authoring a message, a sender can define a context in which the message is to be delivered to a recipient. Accordingly, after receiving the message, the recipient's device does not notify the recipient of the receipt of the message until the context is fulfilled. The recipient's device monitors conditions and determines whether the context is fulfilled, and notifies the recipient of the receipt of the message when the context is fulfilled. The recipient can configure common locations associated with the recipient and the information thereof is available in a non-specific way to a message sender for defining the context.
Owner:GROSSMAN BRENT
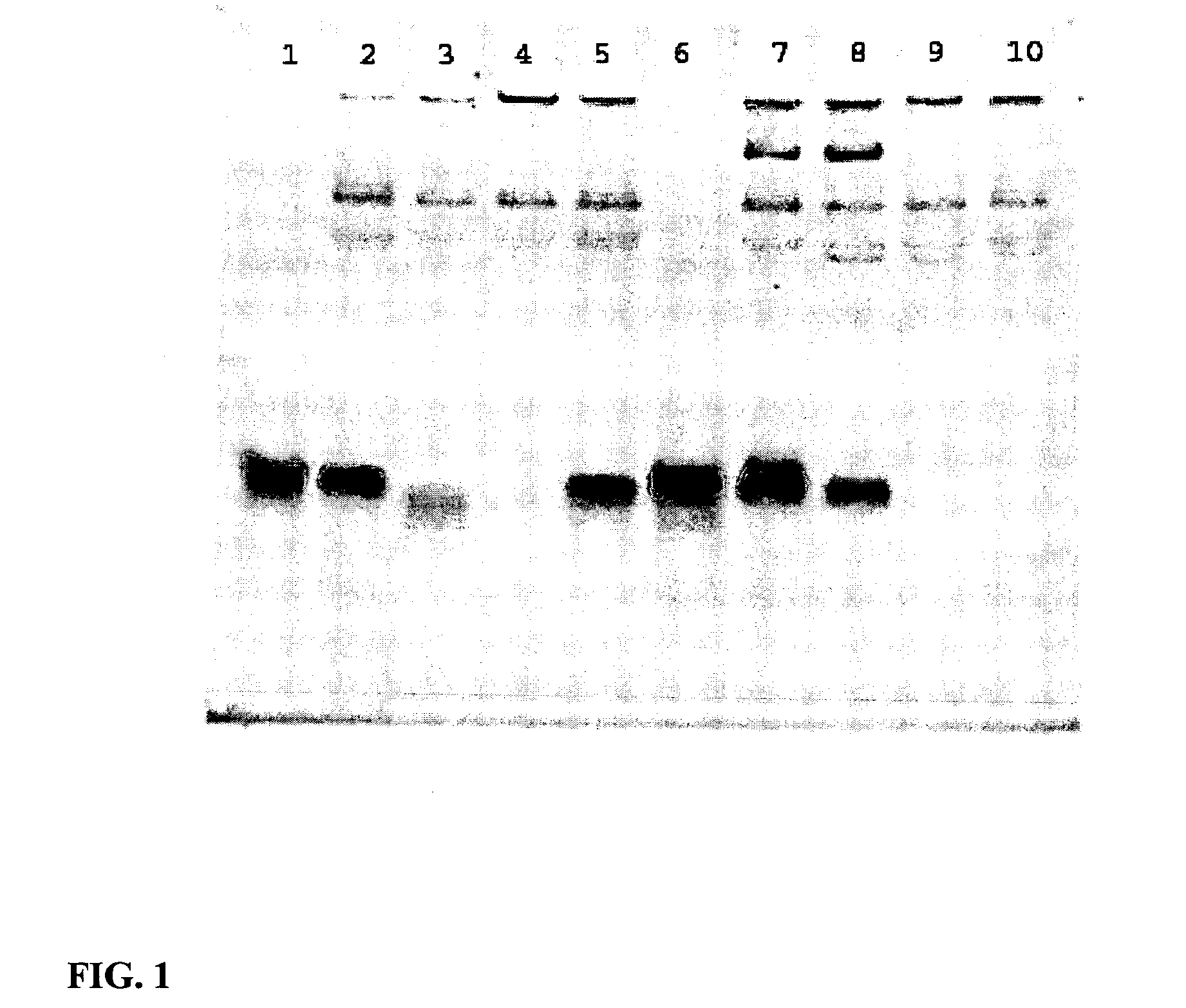
High throughput multiplex DNA sequence amplifications
InactiveUS20060281105A1Efficient and simultaneous amplificationMinimize formationMicrobiological testing/measurementBiological testingHigh throughput genotypingGenome scale
The present invention provides methods of designing PCR primers that allow the efficient and simultaneous amplification of a large number of different desired DNA fragments in a single multiplex PCR and minimize the formation of nonspecific extensions of undesired DNA fragments. The present invention allows a multiplex PCR to use at least 50 pairs of primers and produce at least 50 DNA fragments of interest. The present invention significantly broadens the application of multiplex PCR in the identification of multiple genes related to multifactorial diseases, the genome-scale detection of genetic alterations, the studies in large-scale pharmacogenetic reactions, the genotyping genetic polymorphism in a large population, the gene expression profiling in various samples, and high throughput genotyping technologies.
Owner:LI HONGHUA +1
Two step lateral flow assay methods and devices
ActiveUS20070020768A1Easy to optimizeEasy to detectEnzymologyDisease diagnosisAntigenLateral flow immunoassay
Lateral flow assay devices and methods for detecting a first member of a specific binding pair in a sample which comprises a plurality of nonspecific binding pair members are adapted for two step determinations. In one embodiment, a two step lateral flow assay method for identifying IgE antibodies in a sample comprises applying a sample to a sample port of a device, wherein the device is adapted to deliver the sample to a lateral flow matrix having a plurality of IgE antigen species immobilized at respective positions at a first location The two step method further comprises allowing the sample to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a second location downstream of the first location, applying liquid buffer to the lateral flow matrix to mobilize labeled reagent which is adapted to bind anti-IgE antibody and is dried on the lateral flow matrix at a location upstream of the delivery of the filtered sample to the lateral flow matrix, and allowing labeled reagent mobilized by the liquid buffer to travel along the lateral flow matrix through the immobilized plurality of IgE antigen species to a location downstream of the first location. Further embodiments comprise additional lateral flow immunoassay devices and methods for identifying IgE antibodies in a sample.
Owner:PHADIA AB
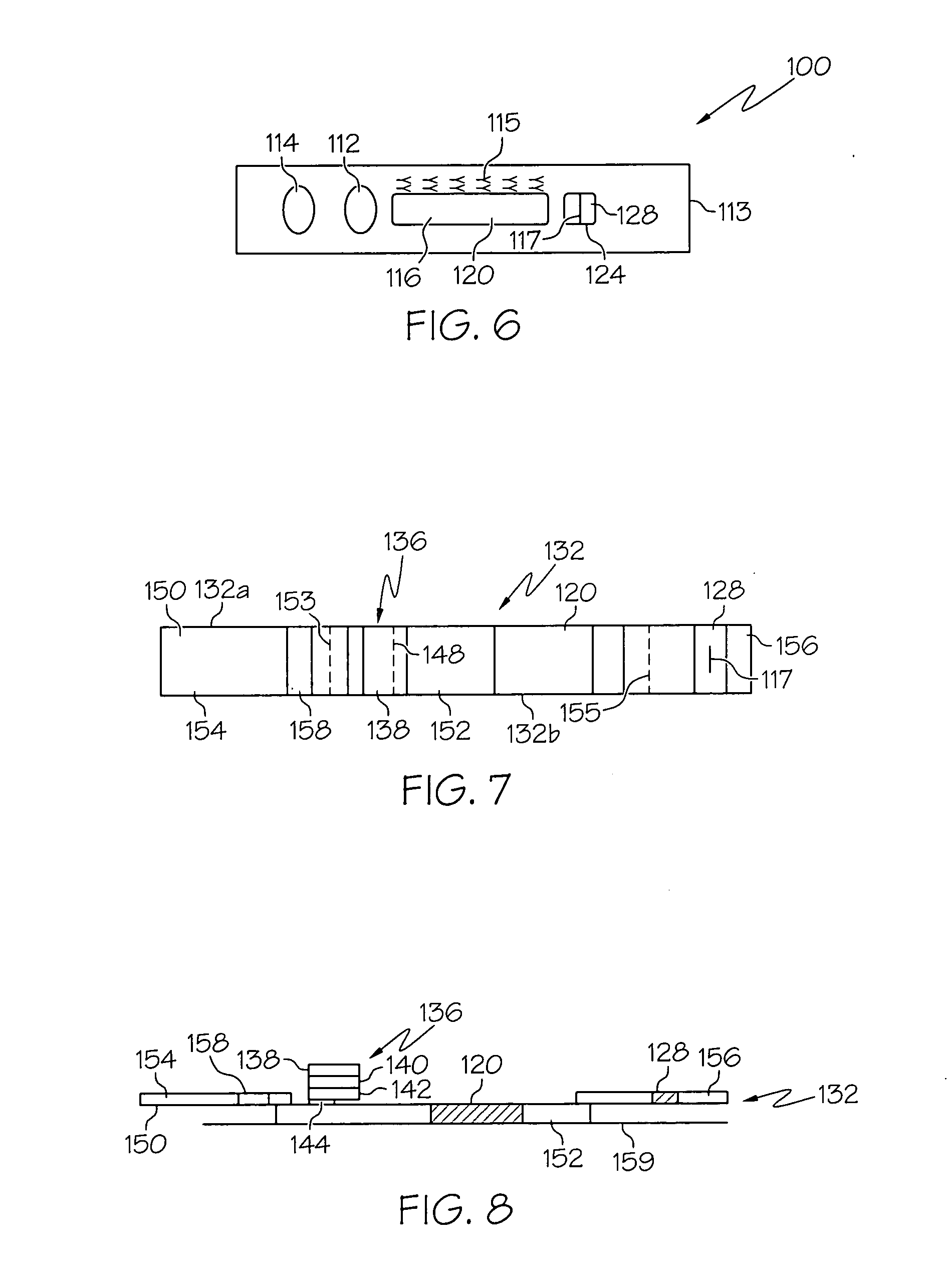
Functional surface coating
InactiveUS20050100675A1Inhibit non-specific bindingRobust attachmentPretreated surfacesBiological testingProtein targetCombinatorial chemistry
Compositions and methods of preparing functional thin films or surface coatings with low non-specific binding are described. The thin films contain specified functional groups and non-specific binding repellant components. The thin films are either covalently bound to or passively adsorbed to various solid substrates. The specified functional group provides specified activity for the thin film modified solid surfaces and non-specific binding repellant components significantly reduce the non-specific binding to the thin film modified solid surfaces. Non-specific binding repellant components do not affect specified functional group's activity in the thin films. In these methods, specified functional groups are anchored to the solid substrates through a spacer. Surface coatings are also described having both non-specific protein binding properties combined with functional groups for specific binding activity thereby providing surface coating that specifically recognize target proteins but limit binding to non-specific protein.
Owner:ACCELERATED MEDICAL DIAGNOSTICS INC
Reduction of the nonspecific animal toxicity of immunotoxins by mutating the framework regions of the Fv to lower the isoelectric point
InactiveUS7521054B2Growth inhibitionPolypeptide with localisation/targeting motifAnimal cellsNon specificFramework region
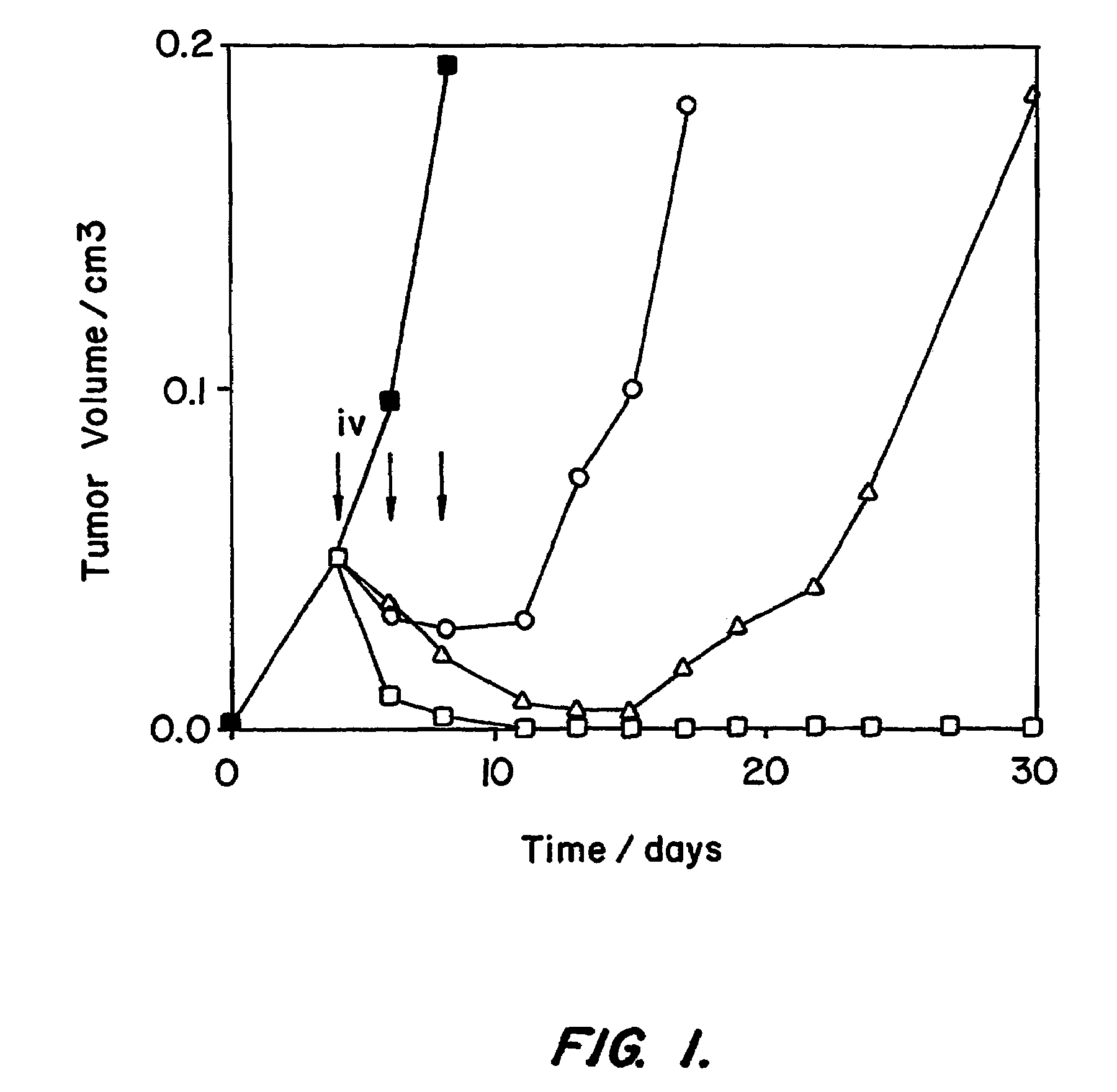
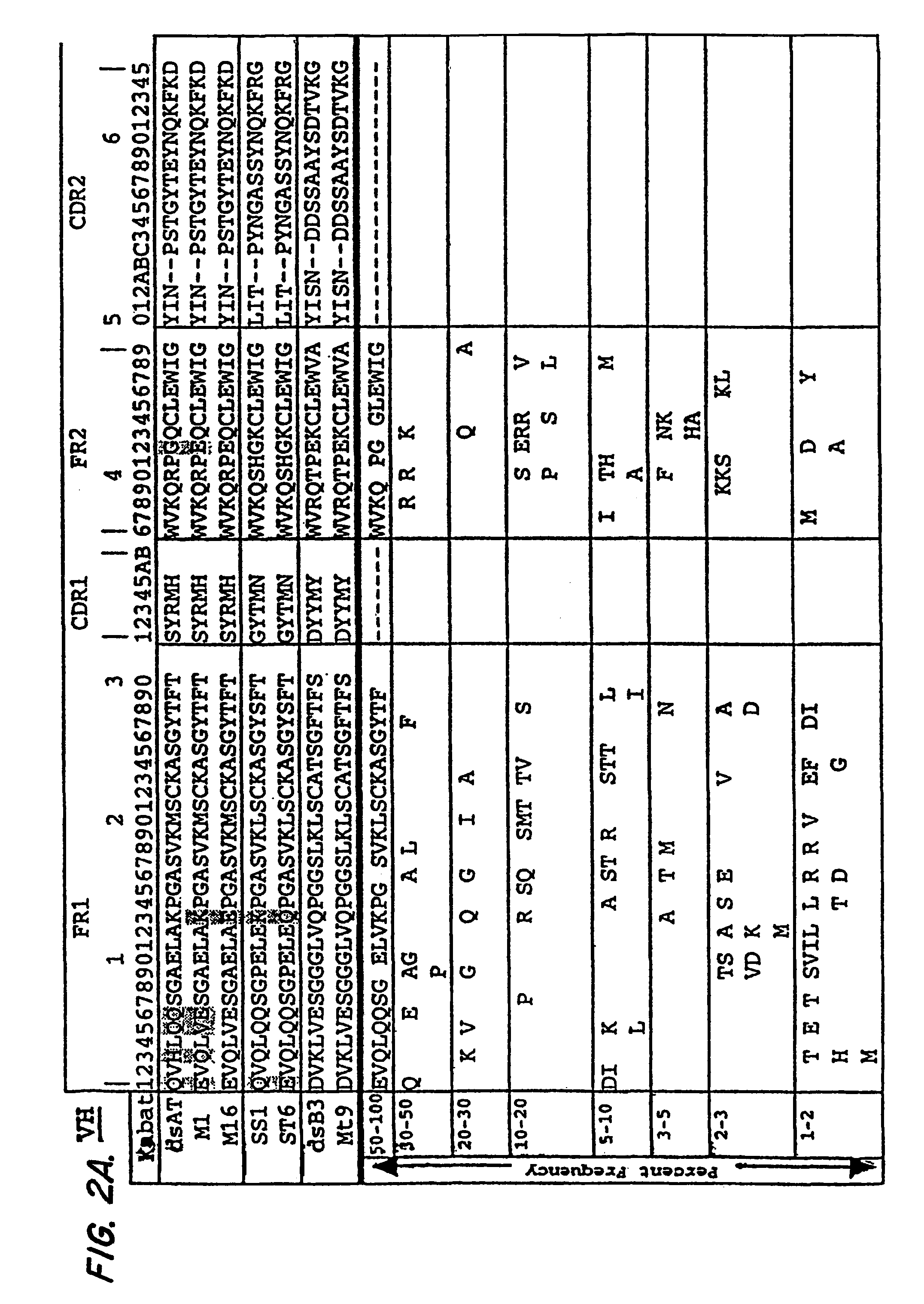
The invention provides recombinant immunotoxins that have been modified from a parental immunotoxin to lower liver toxicity. The immunotoxins are created by specifically mutating charged residues in the framework regions of the heavy chain, the light chain, or both, of the antibody portion or antigen-binding fragment thereof of the parental immunotoxin to reduce the pI of the antibody or fragment. In preferred forms, the antibody portion of the parental is an anti-Tac, anti-mesothelin, or anti-LewisY antigen antibody or antigen-binding fragment, and in particularly preferred forms the antibody portion is an M16 dsFv, a St6 dsFv or a Mt9 dsFv, or a sequence that has at least 90% sequence identity to one of these molecules but retain the particular mutations that lower pI without affecting antibody activity. The invention further provides nucleic acids encoding the recombinant immunotoxins of the invention, expression cassettes comprising the nucleic acids, and host cells comprising the expression cassettes. The invention also provides a method for killing a cell comprising an antigen on the surface of the cell, the method comprising contacting the cell with a recombinant immunotoxin of the invention that has an antibody or antigen-binding fragment thereof that binds specifically to the antigen on the surface of the cell, and uses of immunotoxins of the invention for the manufacture of medicaments.
Owner:HEALTH & HUMAN RESOURCES GOVERNMENT OF THE UNITED STATES THE AS REPRESENTED BY THE SEC OF THE DEPT OF
Methods, Systems, Apparatus, Products, Articles and Data Structures for Cross-Platform Digital Content
InactiveUS20160048606A1Effective machine readabilityNatural language data processingWebsite content managementDigital contentData resources
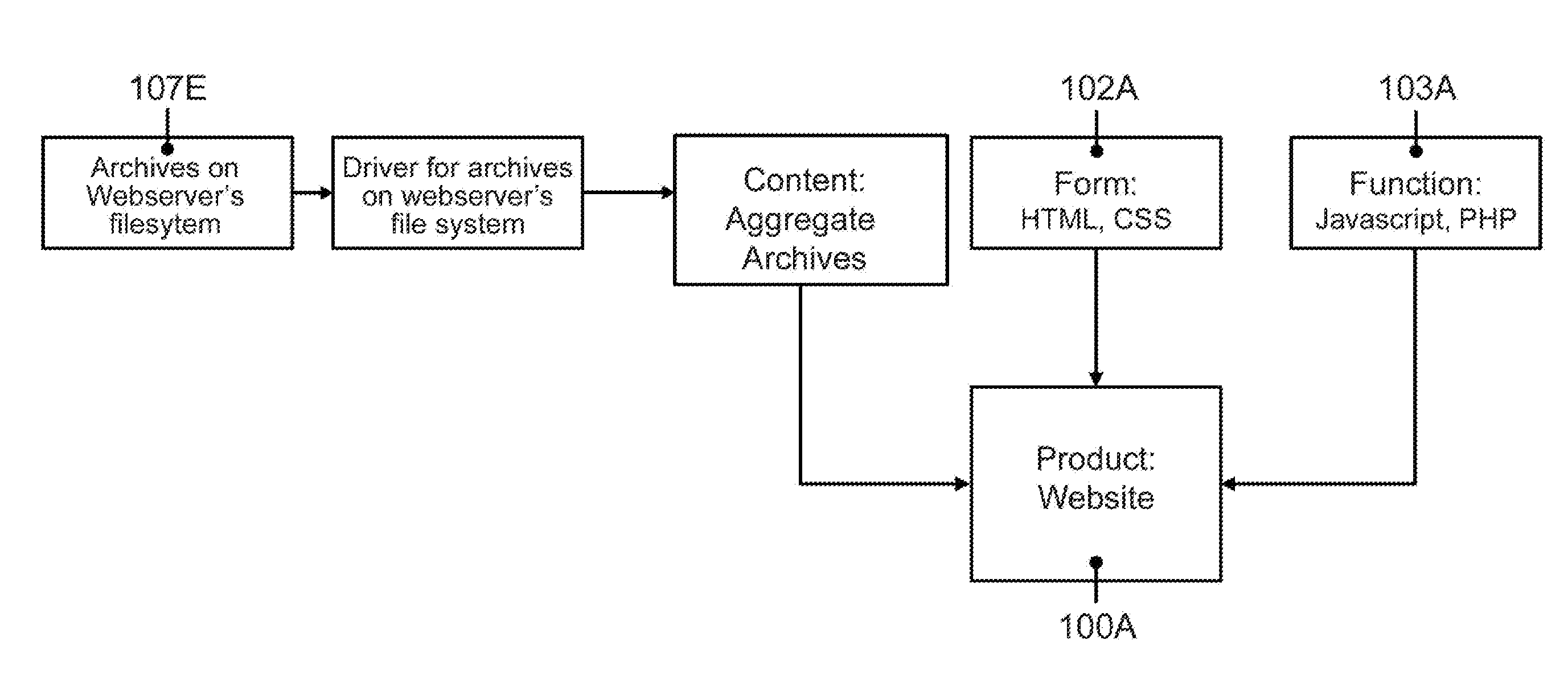
The present invention relates to handling of digital content in cross-platform environments and provides methods, systems, computer readable storage with instructions, and / or products for providing digital content, including the possibility of portions of the content being accessible over a communication network. In one form, the invention provides a functional software product compliant with a predetermined computing platform adapted for cross-platform use that may include: a predetermined form means for presenting content to a user of the product, a predetermined function means for enabling the user of the product to navigate content, and at least one driver means operatively associated with the functional software product for enabling access to content by the predetermined form and function means wherein the driver means is adapted for accessing at least one originating content data resource having a format non-specific to the predetermined computing platform.
Owner:KISS DIGITAL MEDIA
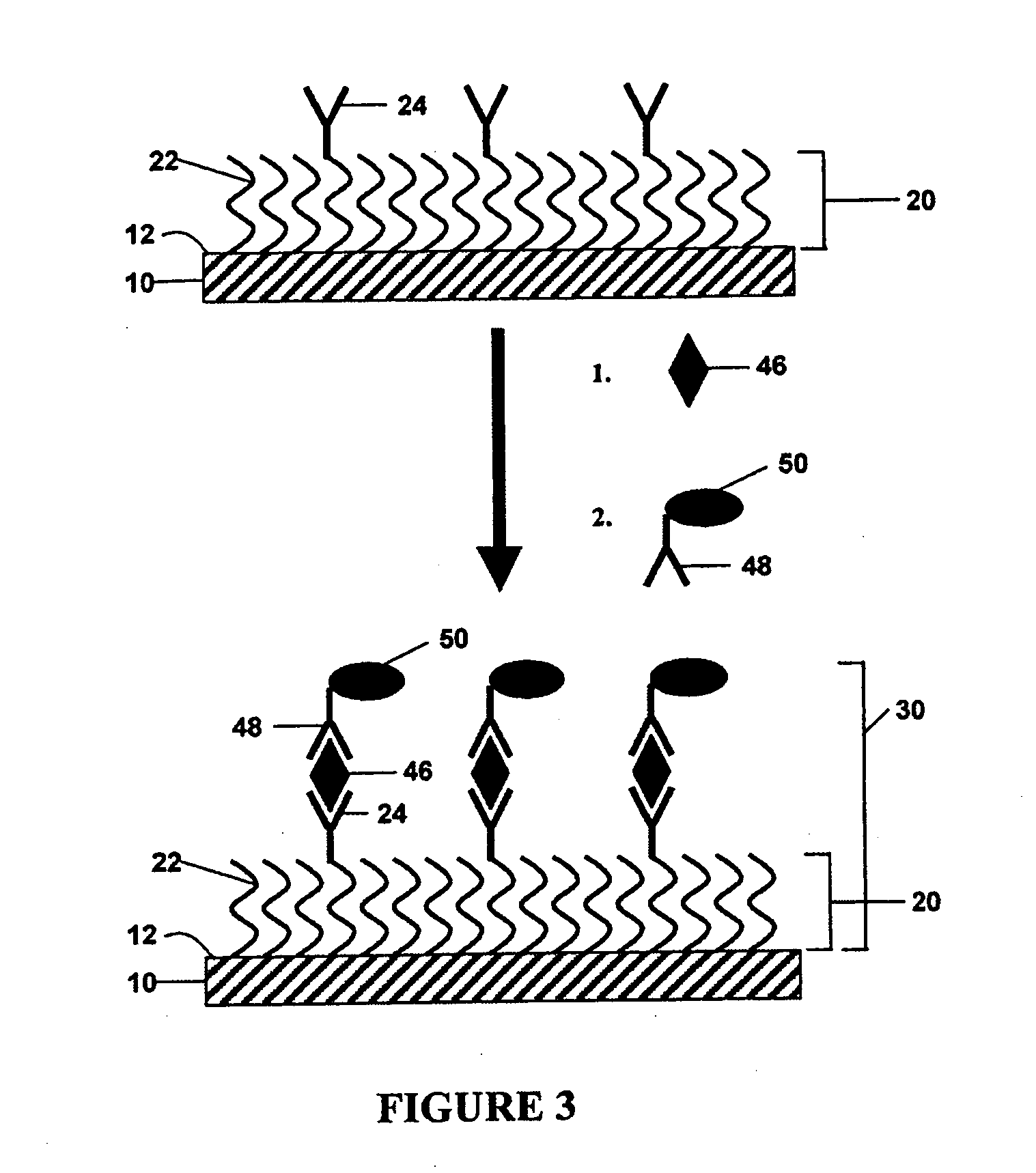
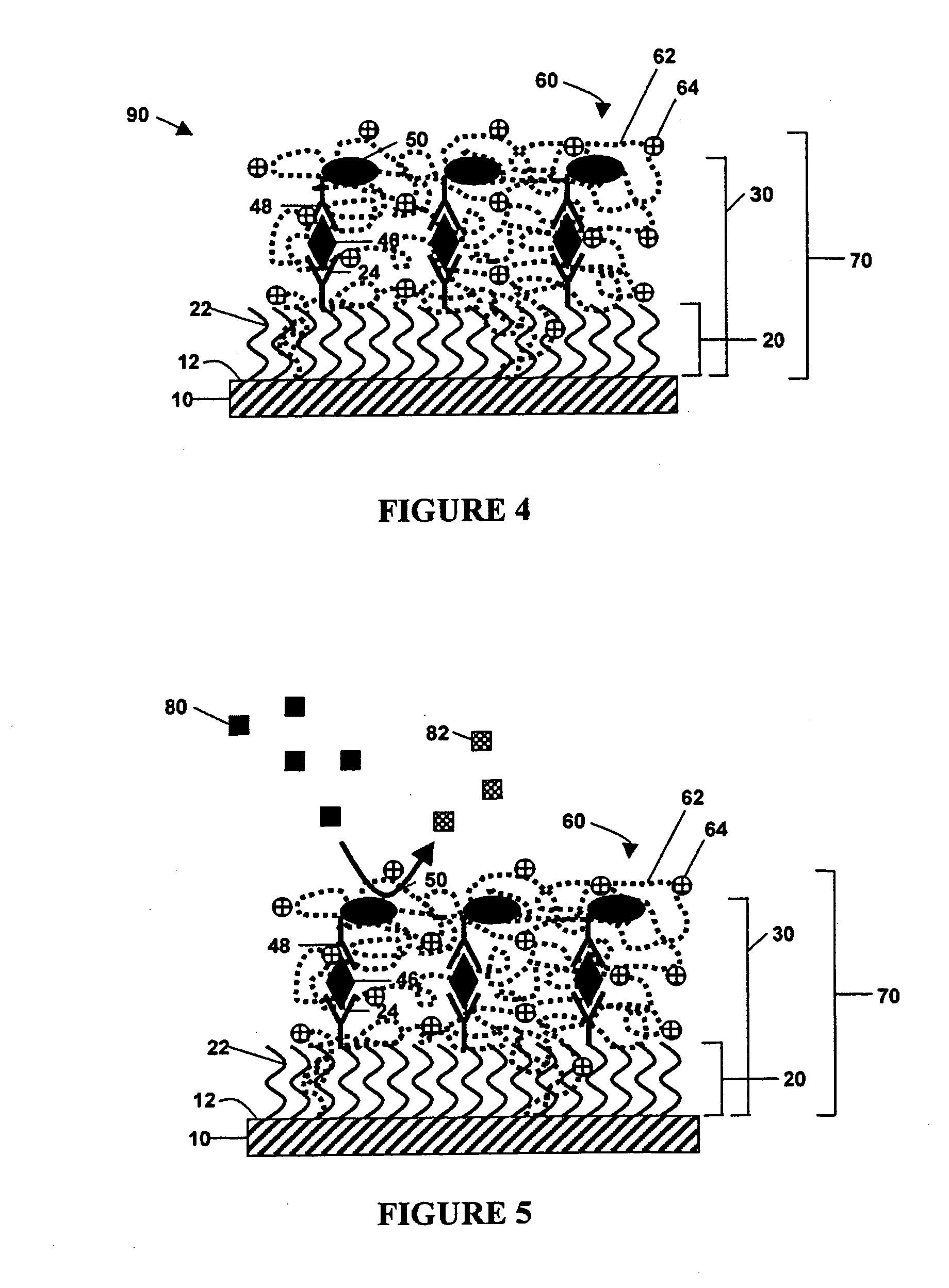
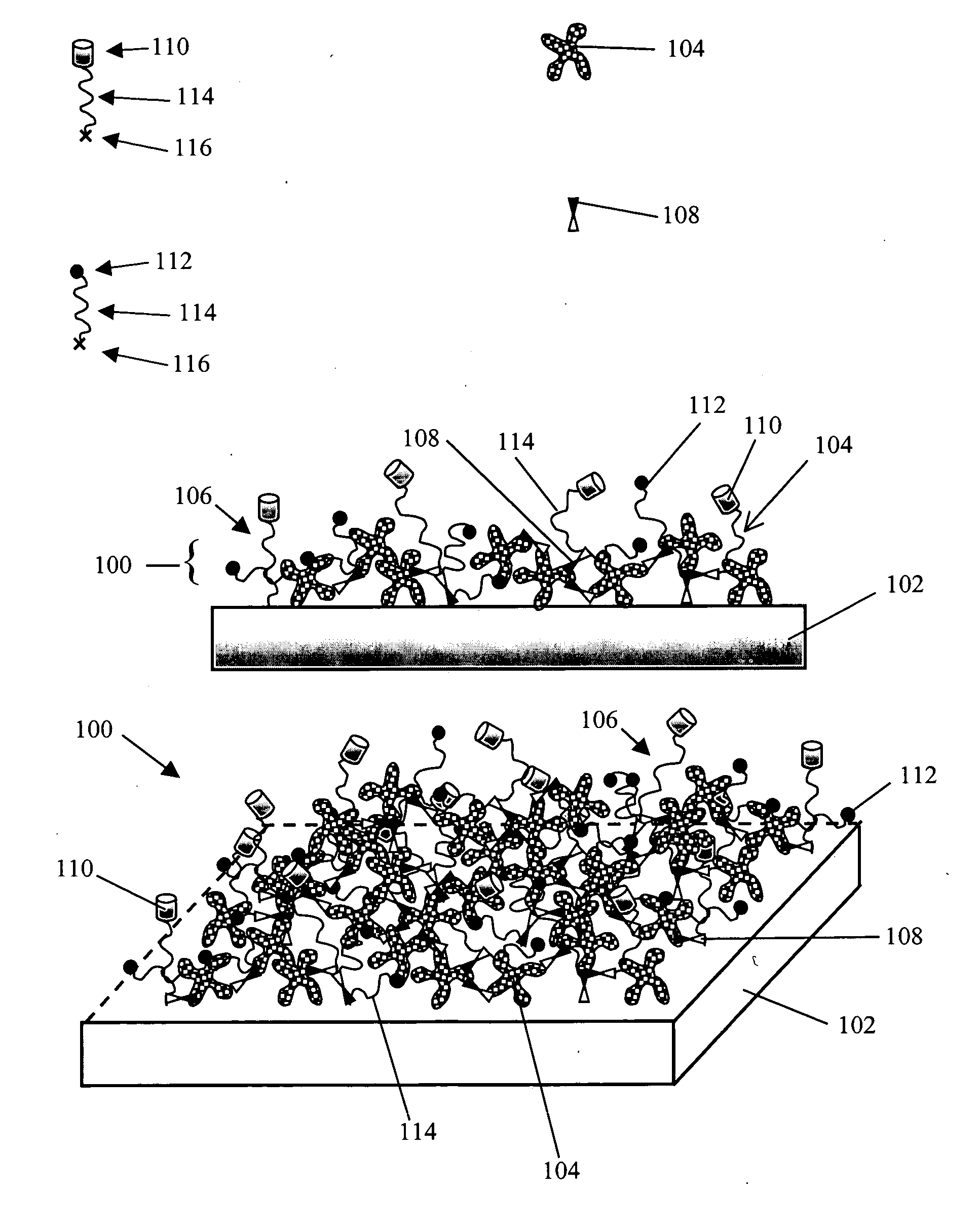
Non-specific binding resistant protein arrays and methods for making the same
InactiveUS6897073B2Effective resistanceMaterial nanotechnologyBioreactor/fermenter combinationsBiological bodyCell biology
Arrays of protein-capture agents useful for the simultaneous detection of a plurality of proteins which are the expression products, or fragments thereof, of a cell or population of cells in an organism are provided. A variety of antibody arrays, in particular, are described. Methods of both making and using the arrays of protein-capture agents are also disclosed. The invention arrays are particularly useful for various proteomics applications including assessing patterns of protein expression and modification in cells.
Owner:ZYOMYX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com