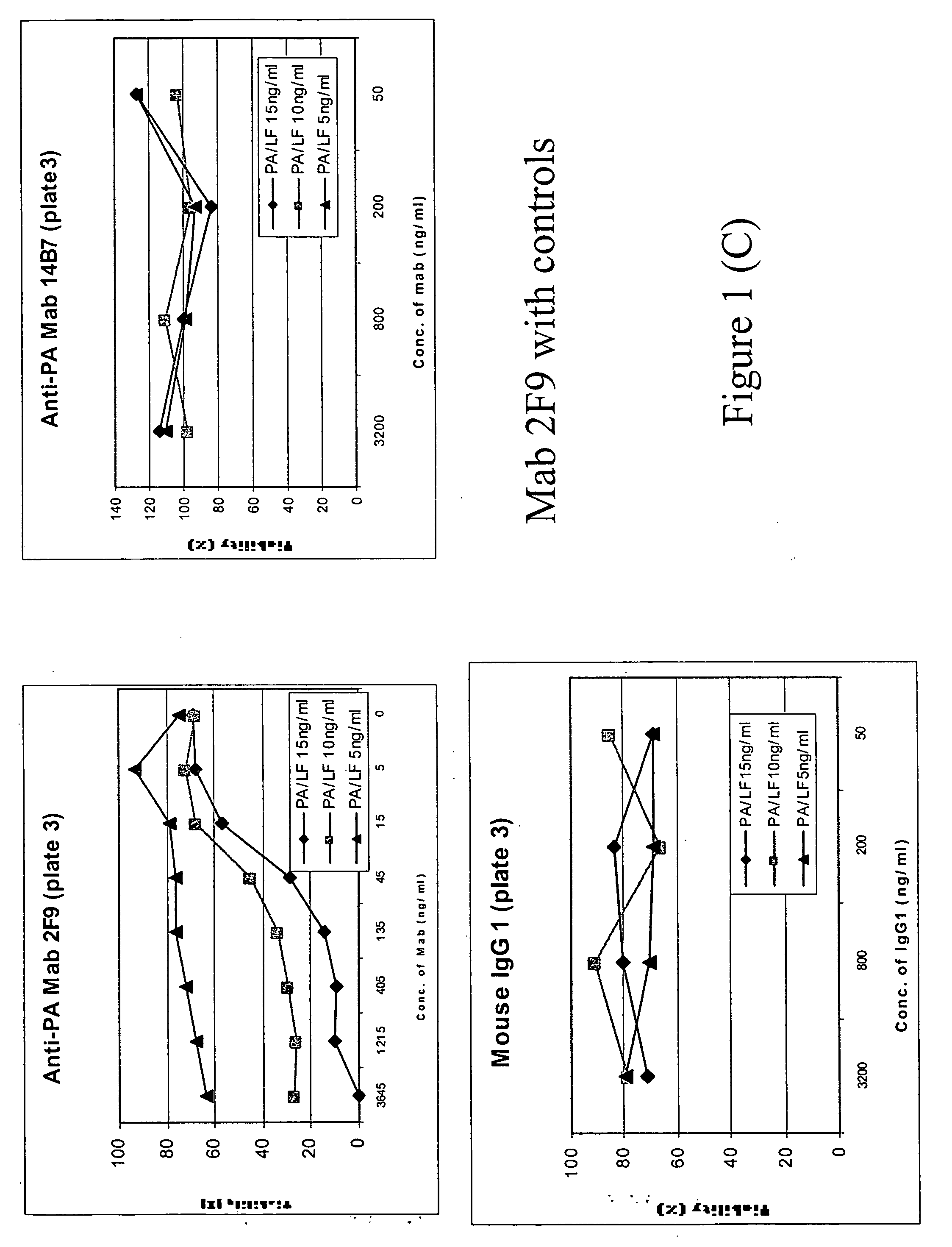
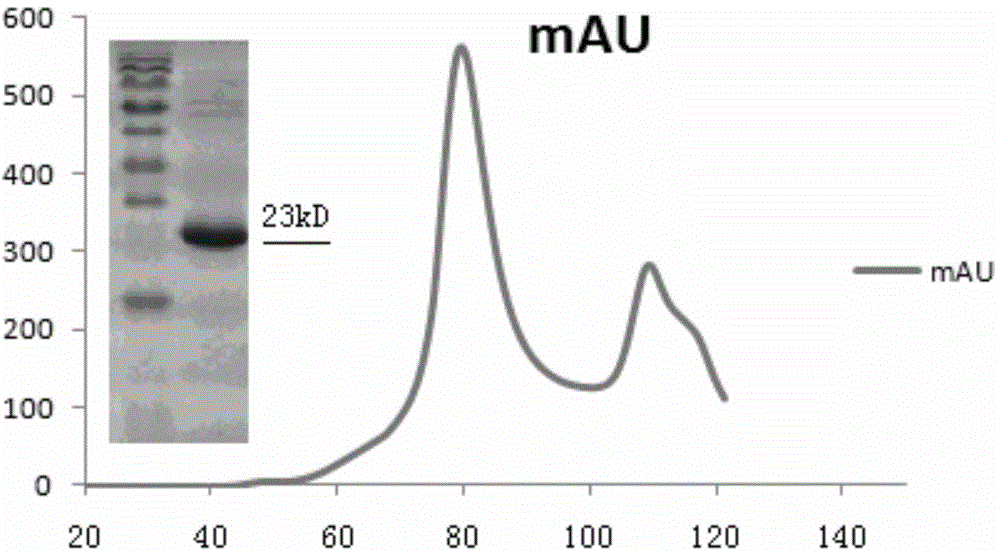
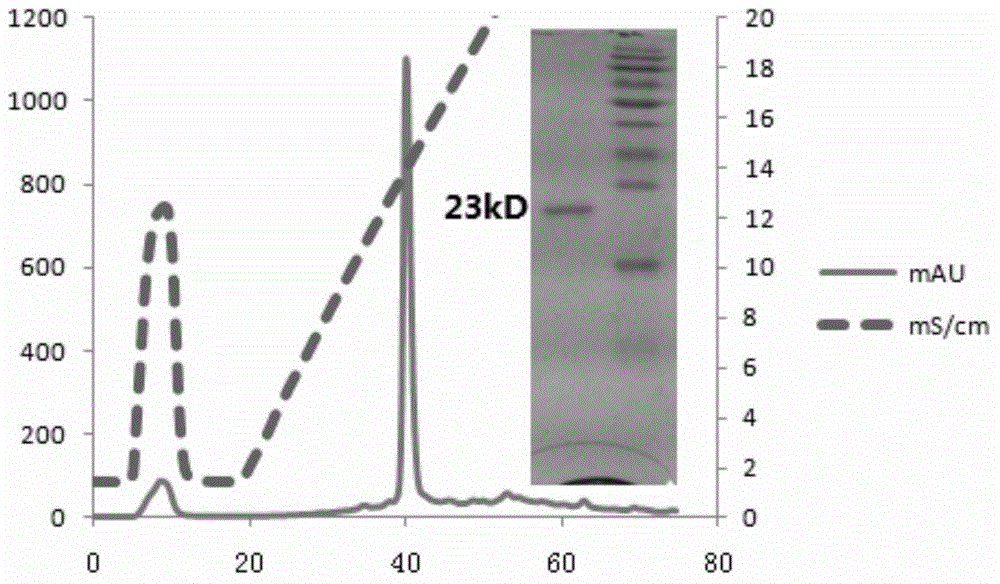
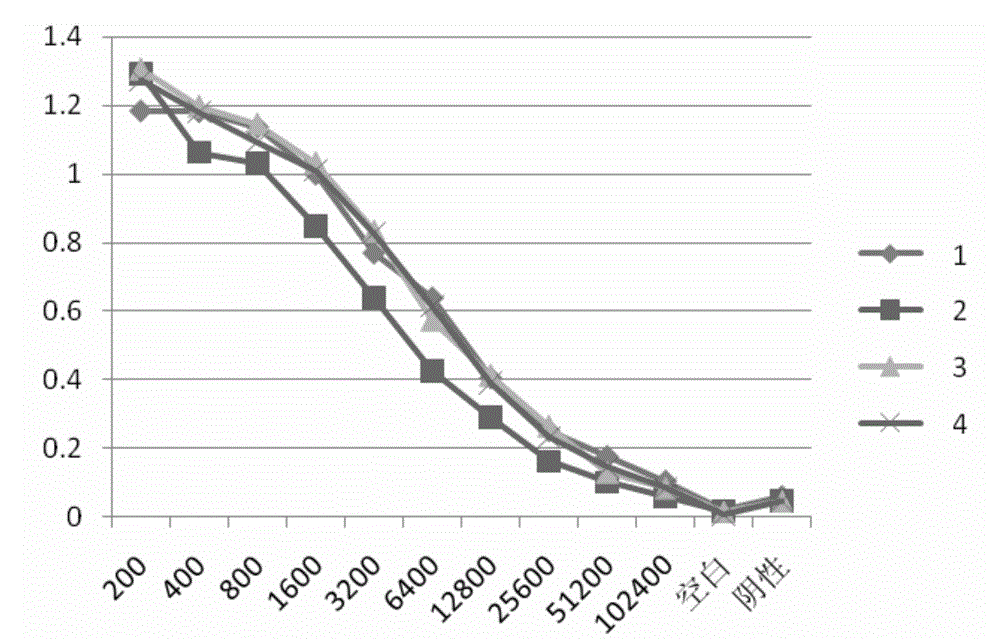
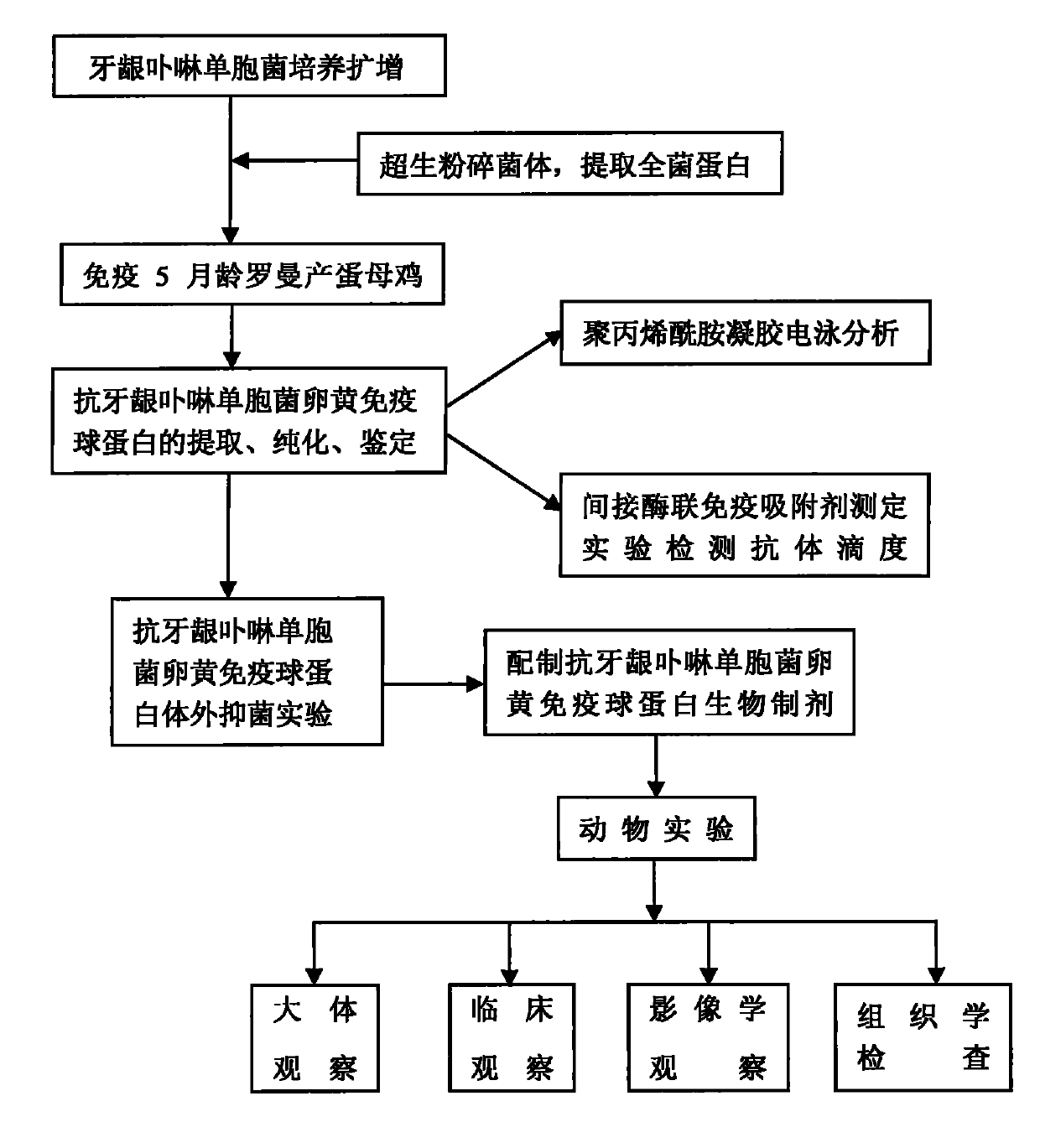
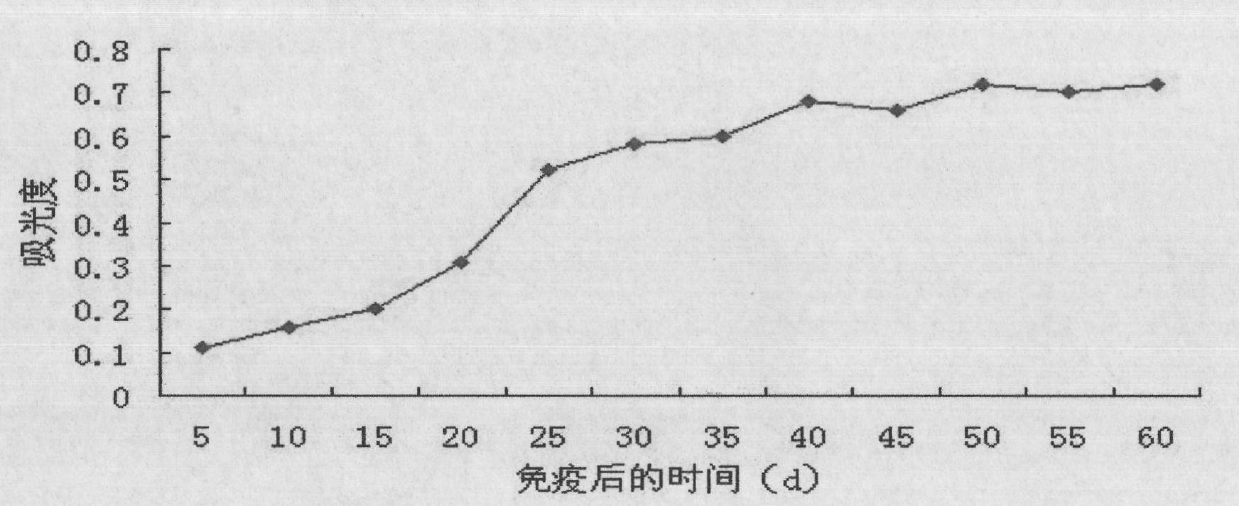
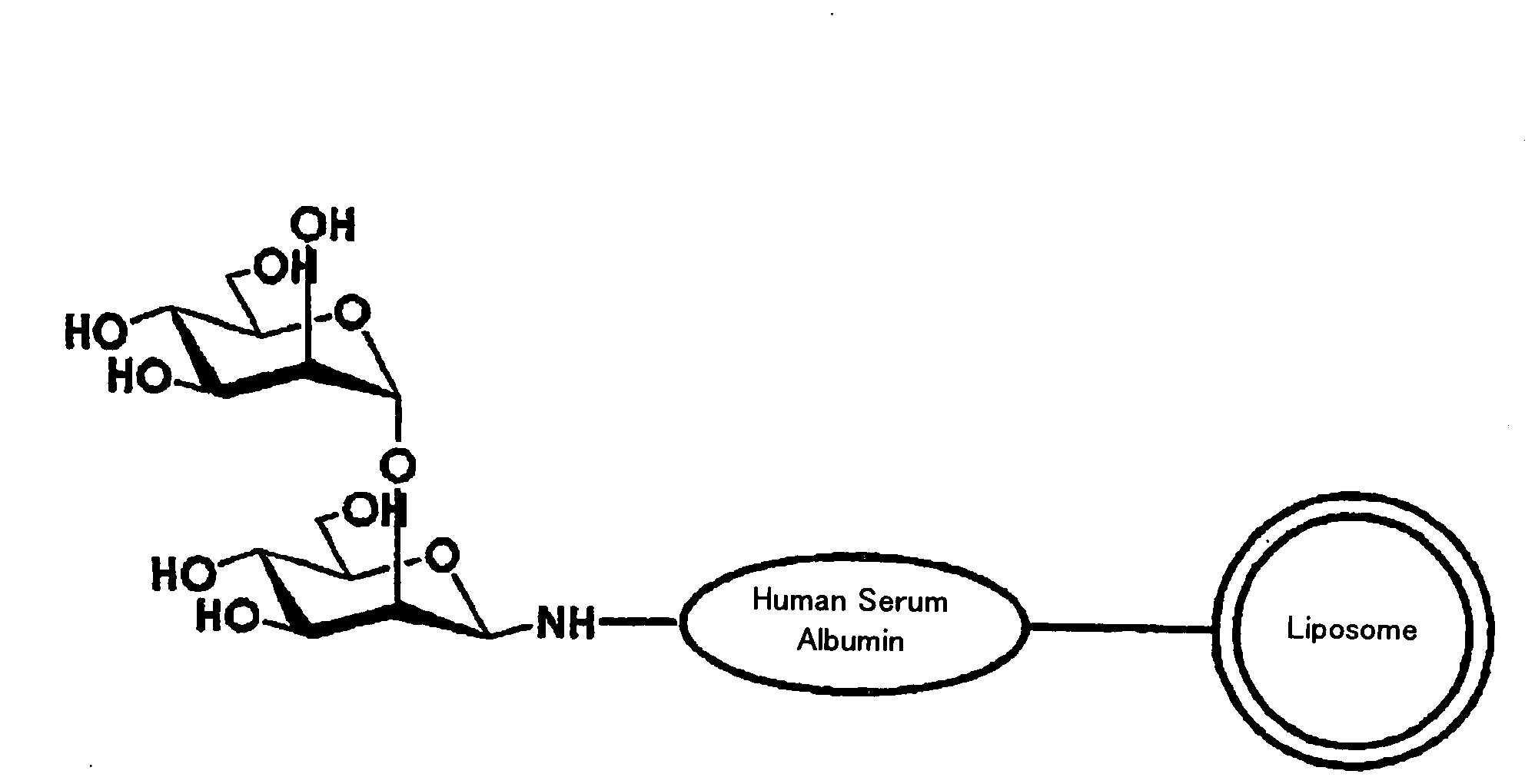
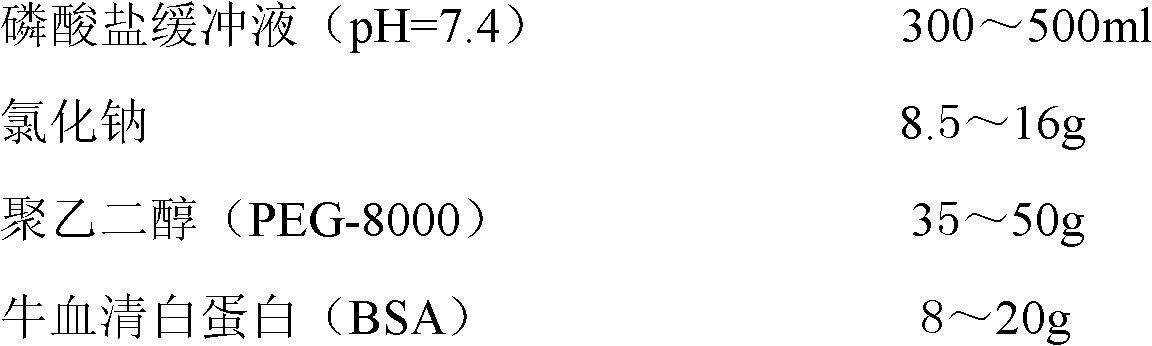Patents
Literature
145 results about "Antibody activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Antibody - any of a large variety of proteins normally present in the body or produced in response to an antigen which it neutralizes, thus producing an immune response active site - the part of an enzyme or antibody where the chemical reaction occurs
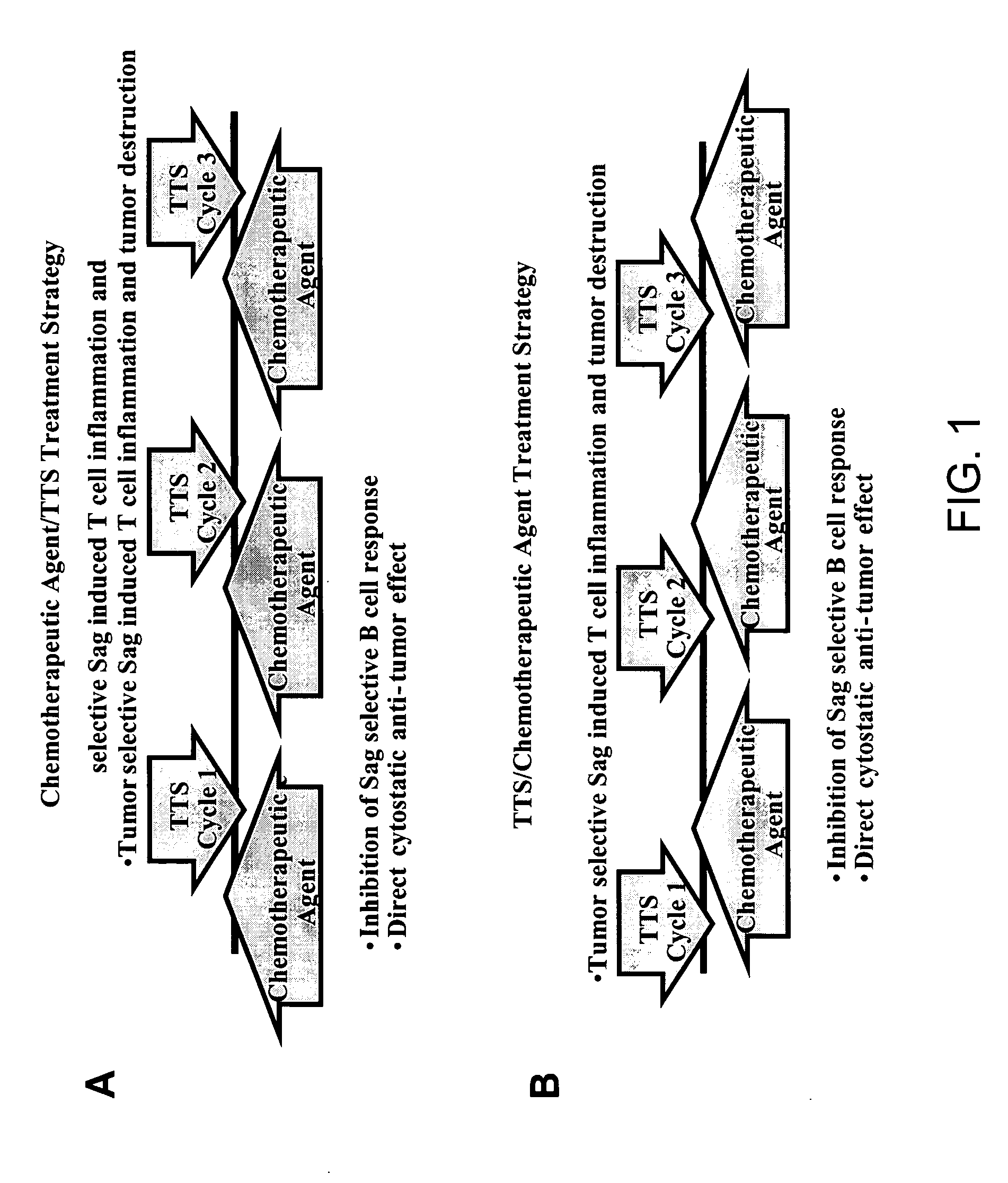
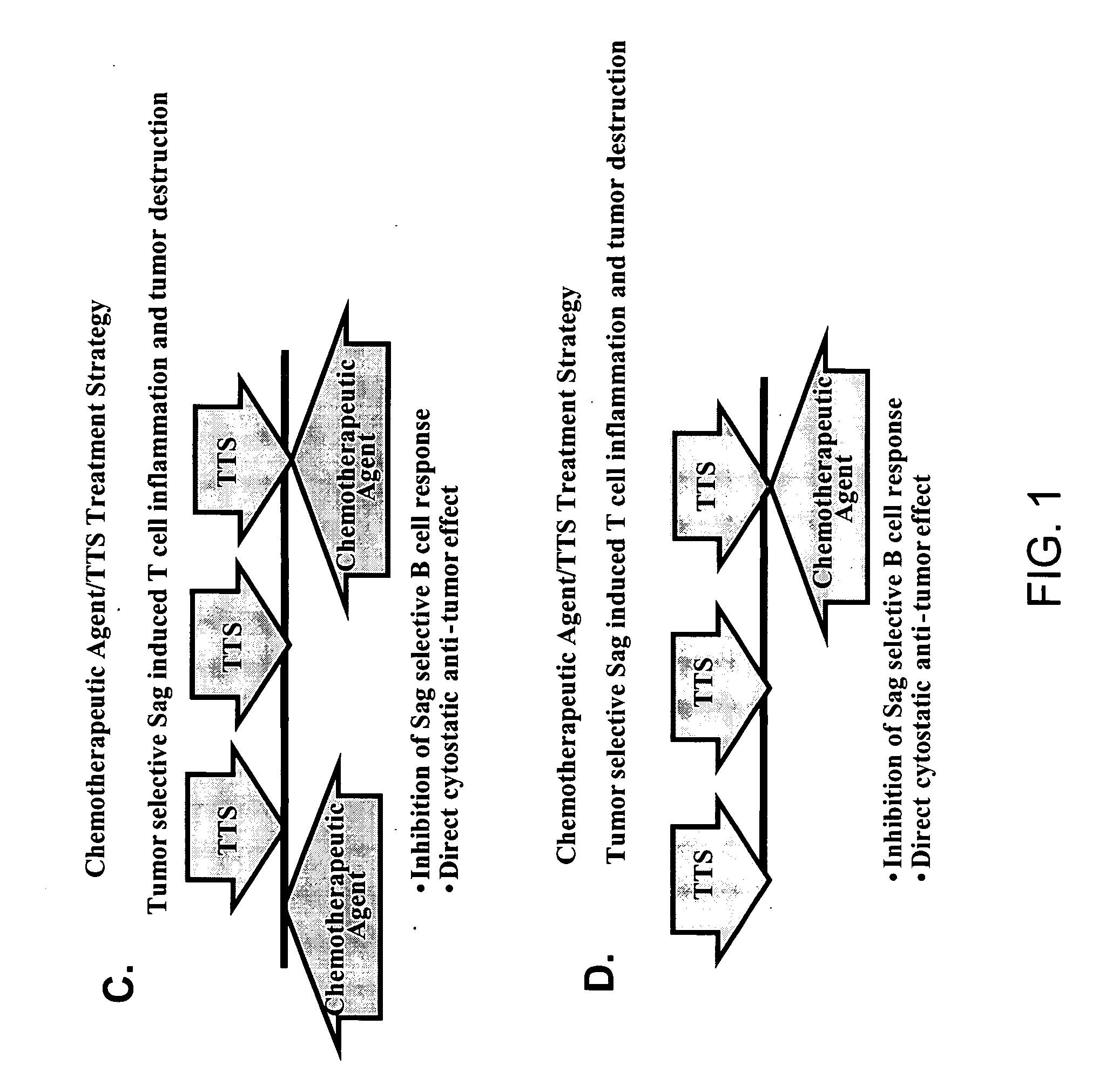
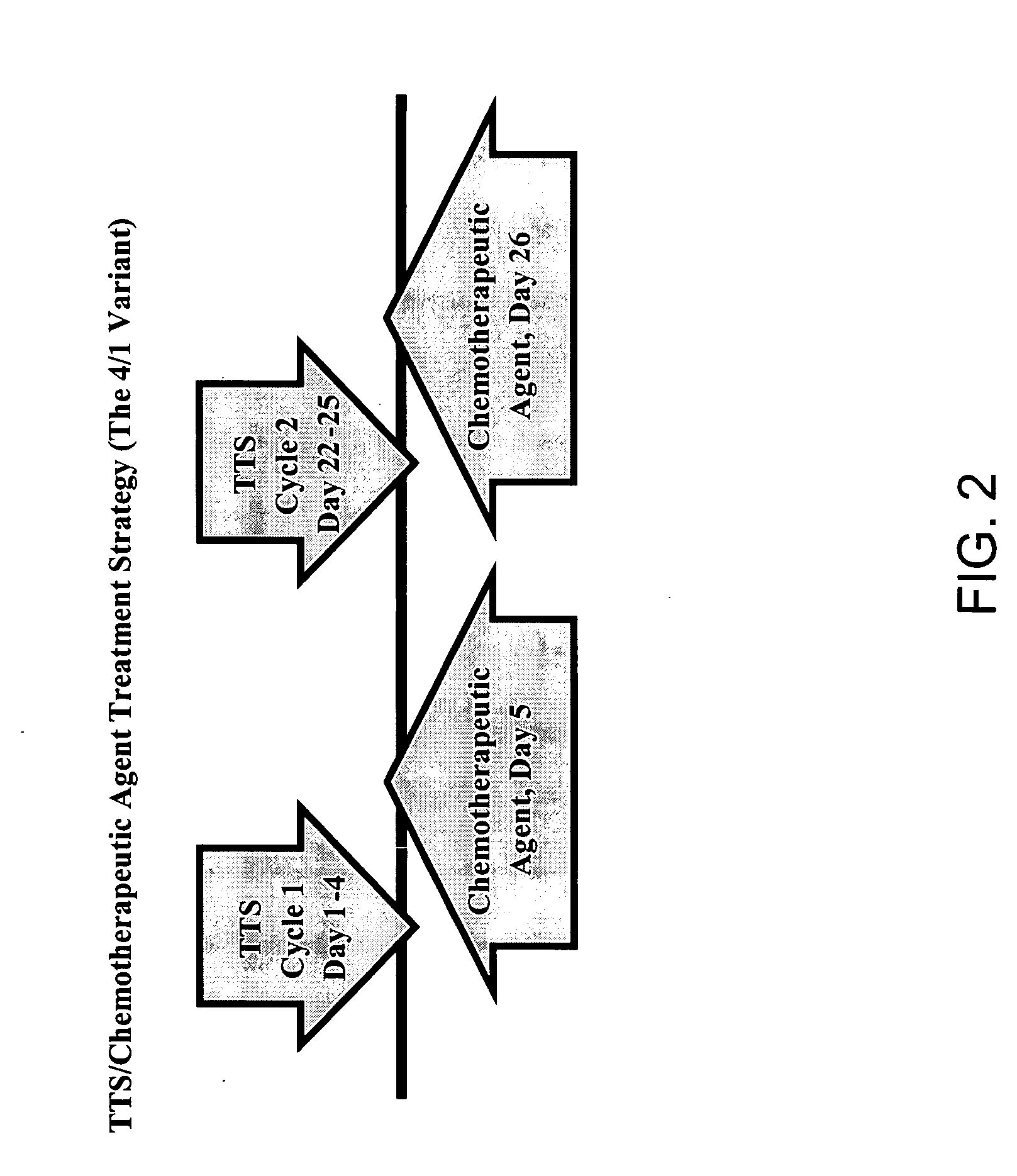
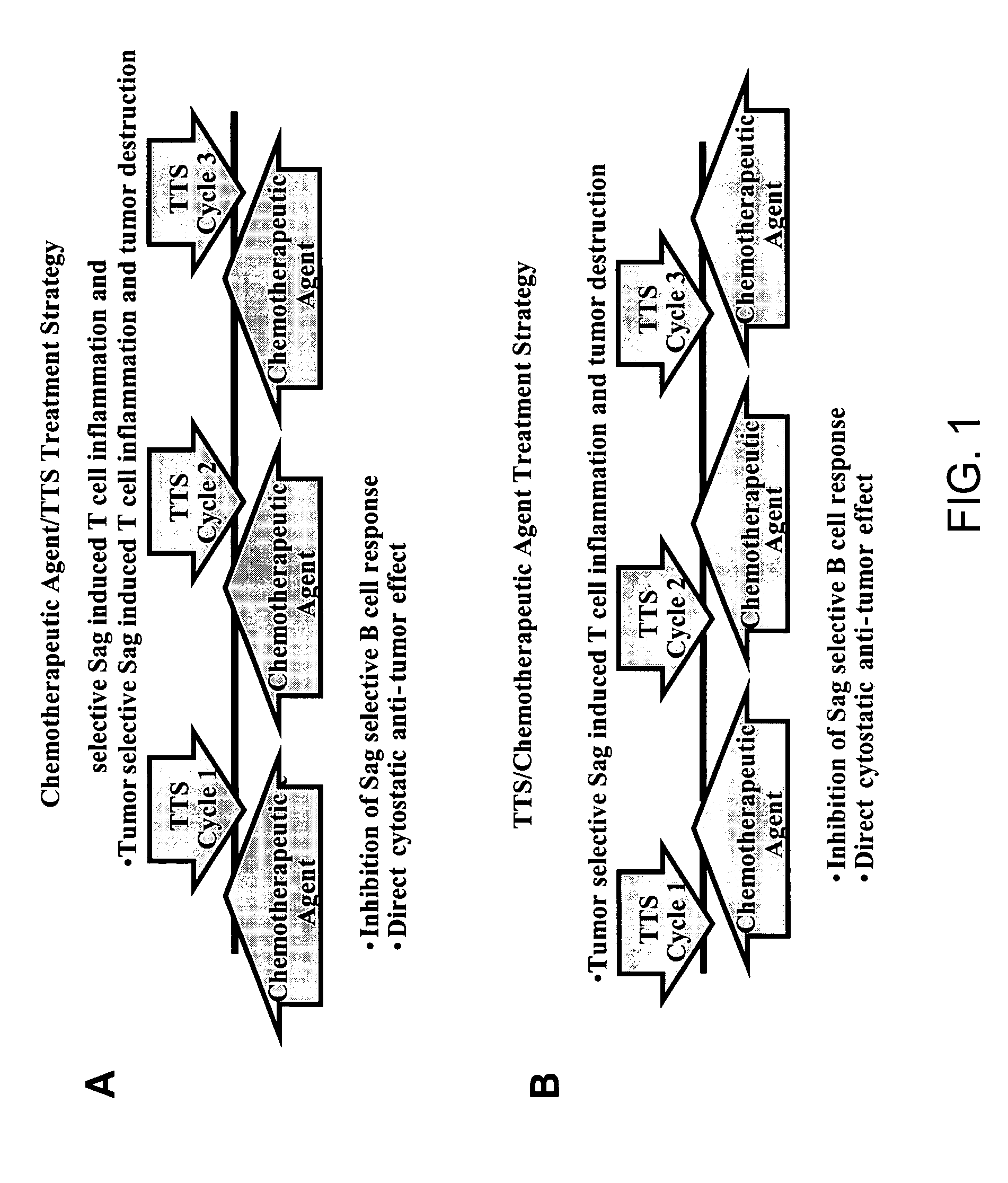
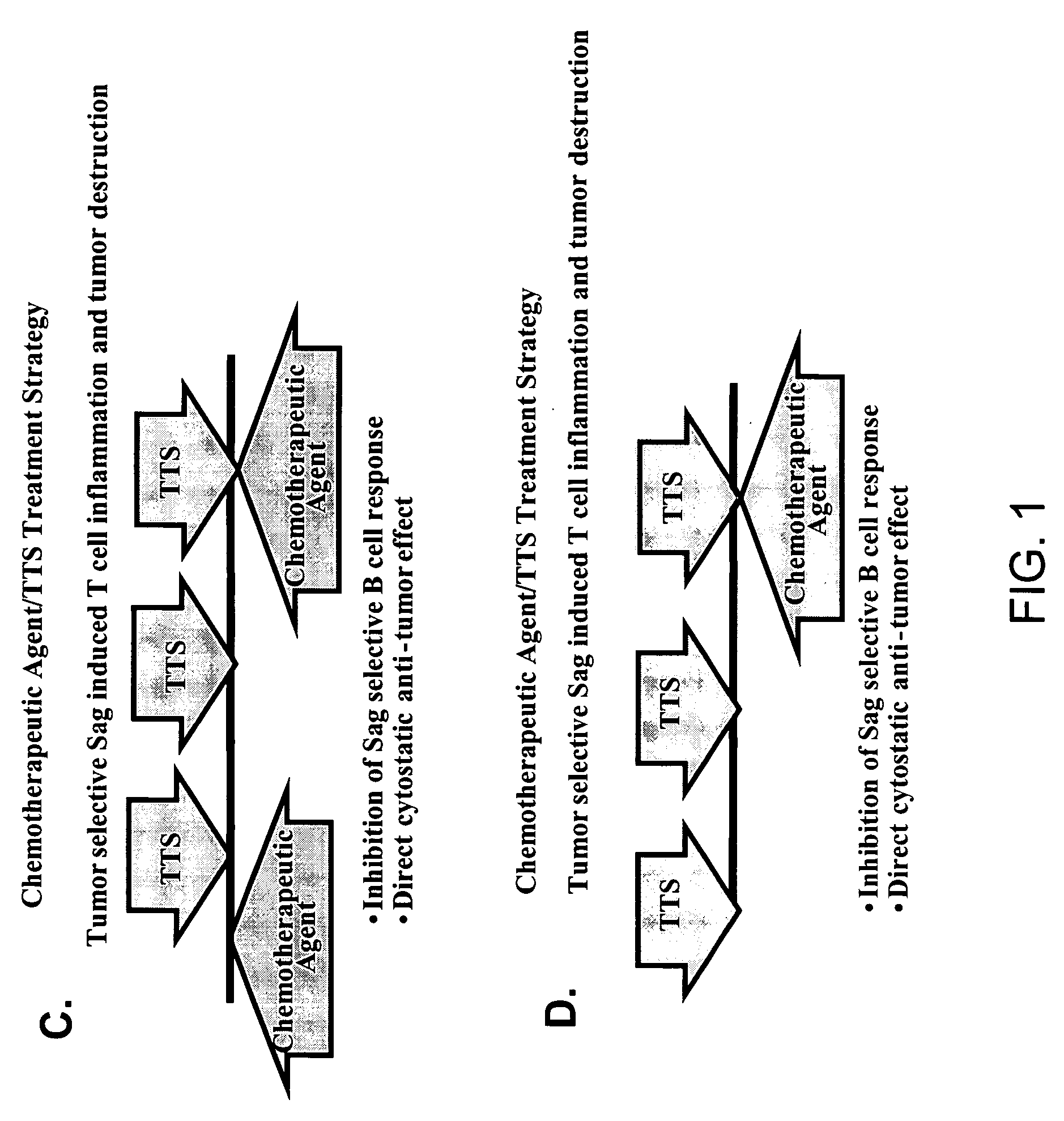
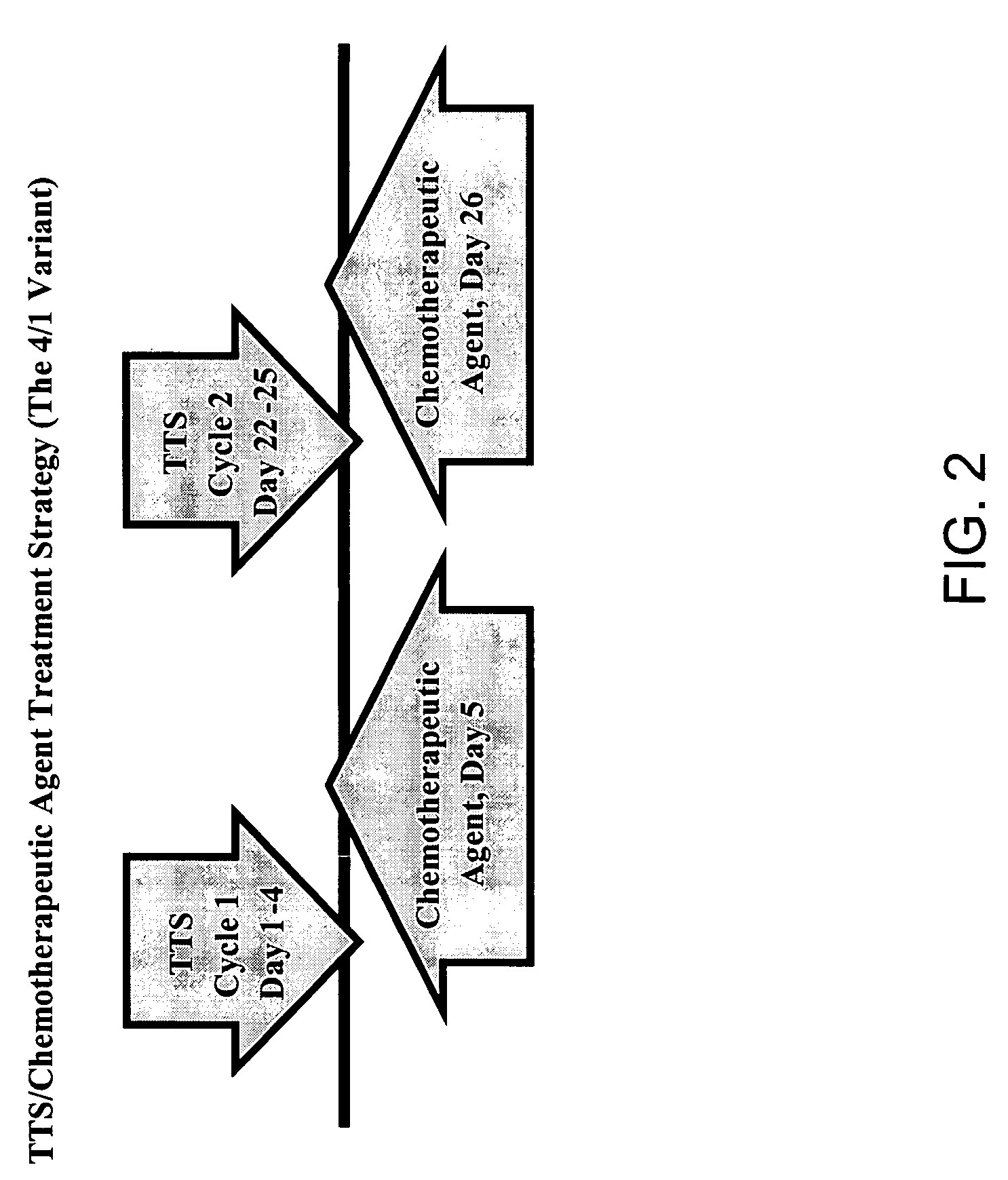
Treatment of hyperproliferative disease with superantigens in combination with another anticancer agent
ActiveUS20060057111A1Reduced antibody productionReduce productionBiocideHeavy metal active ingredientsTumor targetDisease
The present invention relates to methods of treating mammals affected by, for example, a hyperproliferative disease such as cancer, by administering a tumor-targeted superantigen and a chemotherapeutic agent, whereby the administration of the tumor-targeted superantigen and chemotherapeutic agent reduce the antibody response and enhance the T cell response. The superantigen, wild-type or modified, is fused to a target-seeking moiety, such as an antibody or an antibody active fragment. The combined administration of a superantigen and a chemotherapeutic agent provides enhanced therapeutic effects in a treated animal.
Owner:ACTIVE BIOTECH AB
Treatment of hyperproliferative disease with superantigens in combination with another anticancer agent
ActiveUS7763253B2Reduce productionImprove anti-tumor effectOrganic active ingredientsHeavy metal active ingredientsTumor targetAnticarcinogen
The present invention relates to methods of treating mammals affected by, for example, a hyperproliferative disease such as cancer, by administering a tumor-targeted superantigen and a chemotherapeutic agent, whereby the administration of the tumor-targeted superantigen and chemotherapeutic agent reduce the antibody response and enhance the T cell response. The superantigen, wild-type or modified, is fused to a target-seeking moiety, such as an antibody or an antibody active fragment. The combined administration of a superantigen and a chemotherapeutic agent provides enhanced therapeutic effects in a treated animal.
Owner:ACTIVE BIOTECH AB
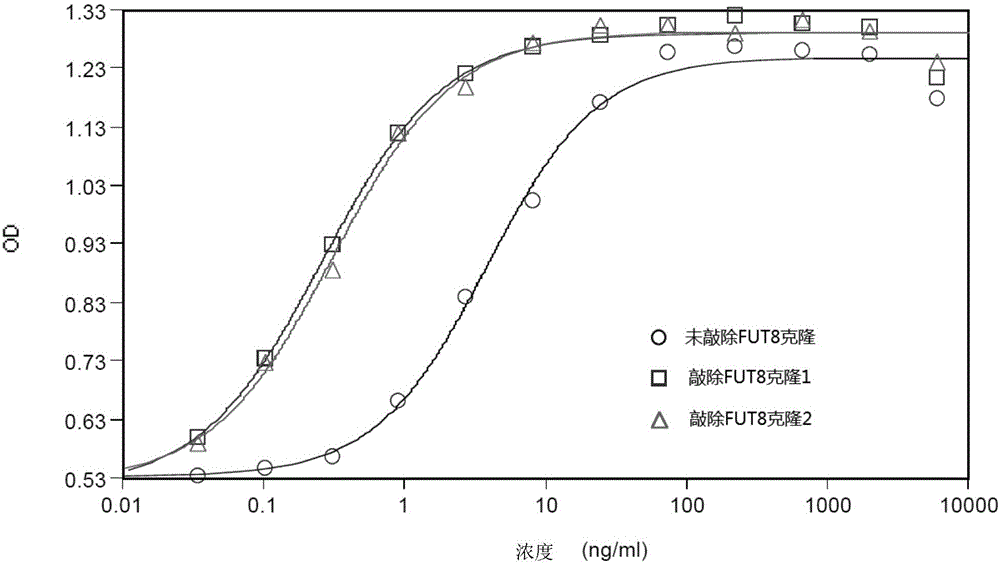
FUT8 gene knockout method based on CRISPR technology
InactiveCN106399360AAchieve the purpose of inactivationImprove ADCC activityVector-based foreign material introductionHeterologousDouble stranded
The invention discloses a FUT8 gene knockout method based on a CRISPR technology. The FUT8 gene knockout method comprises: 1) designing a sgRNA sequence; 2) recombining the sgRNA sequence into a first vector to obtain a second vector; 3) transfecting the second vector into cells, and extracting DNA; 4) carrying out PCR amplification; 5) carrying out denaturation and annealing on the PCR product to form a heterologous hybrid double-stranded DNA; 6) cutting the heterologous hybrid double-stranded DNA, and analyzing the product to obtain the NHEJ occurring proportion in each cell population; and 7) selecting the cell population having the high NHEJ occurring proportion, carrying out limiting dilution method cloning, screening the monoclonal cell, and carrying out expanded culture to obtain the FUT8 gene knockout antibody. The invention further discloses the sgRNA sequence for the method. According to the present invention, the sgRNA capable of recognizing the FUT8 gene specific sequence is encoded, and the sgRNA and the sequence encoding the endonuclease are transfected into the cells, such that the FUT8 gene inactivation purpose is achieved, and the activity of the antibody ADCC is improved.
Owner:SHANGHAI WUXI BIOLOGIC TECH CO LTD +1
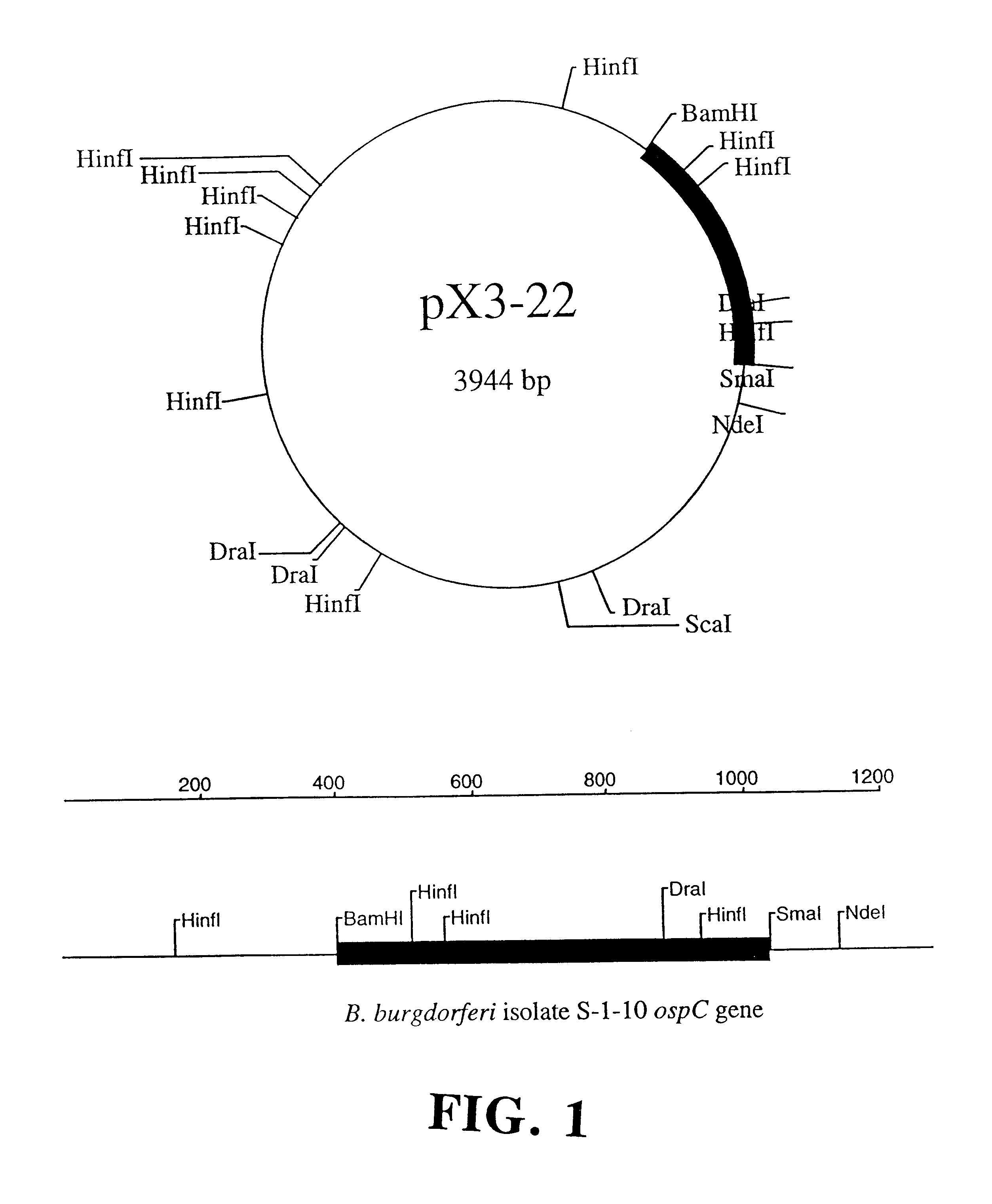
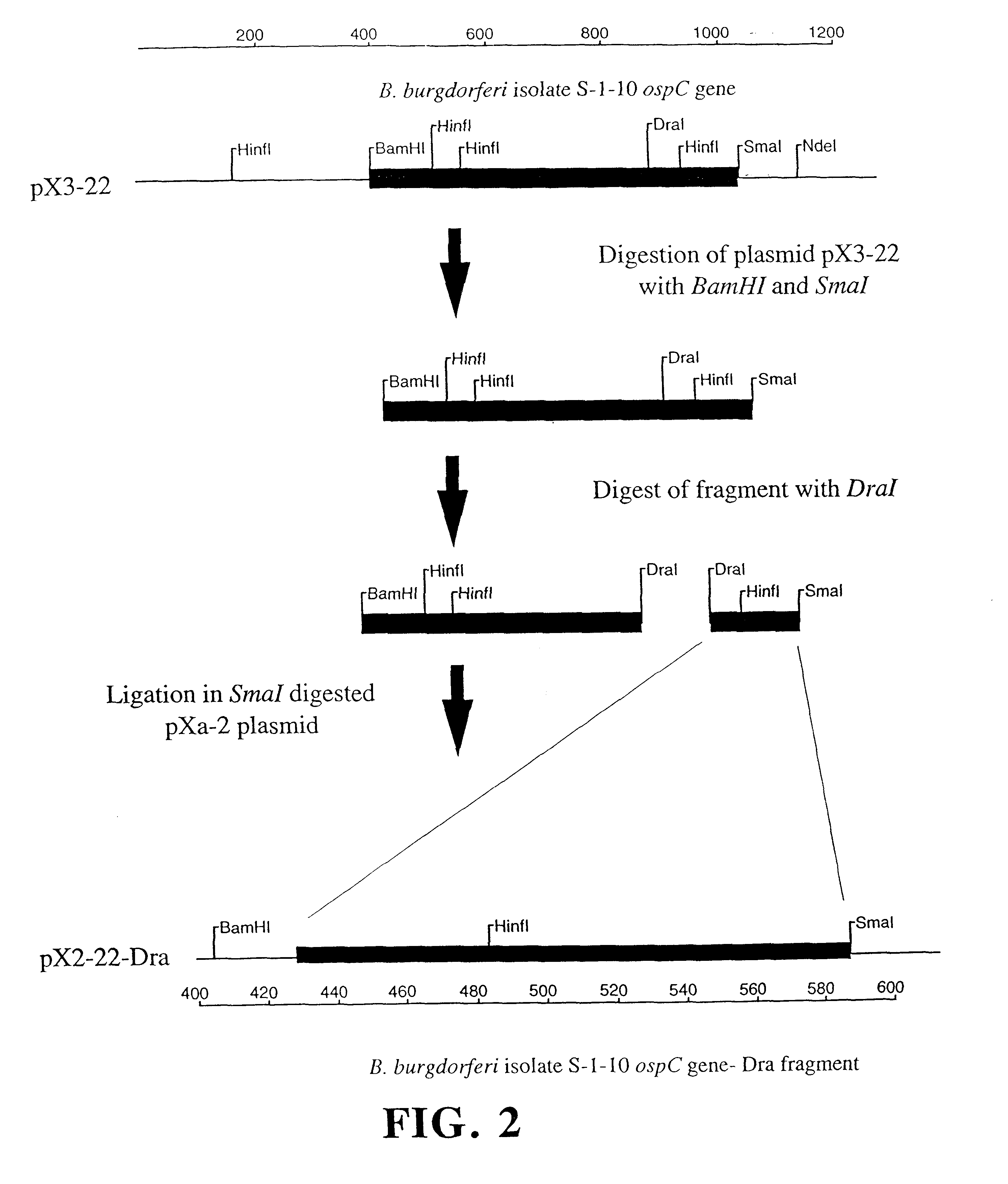
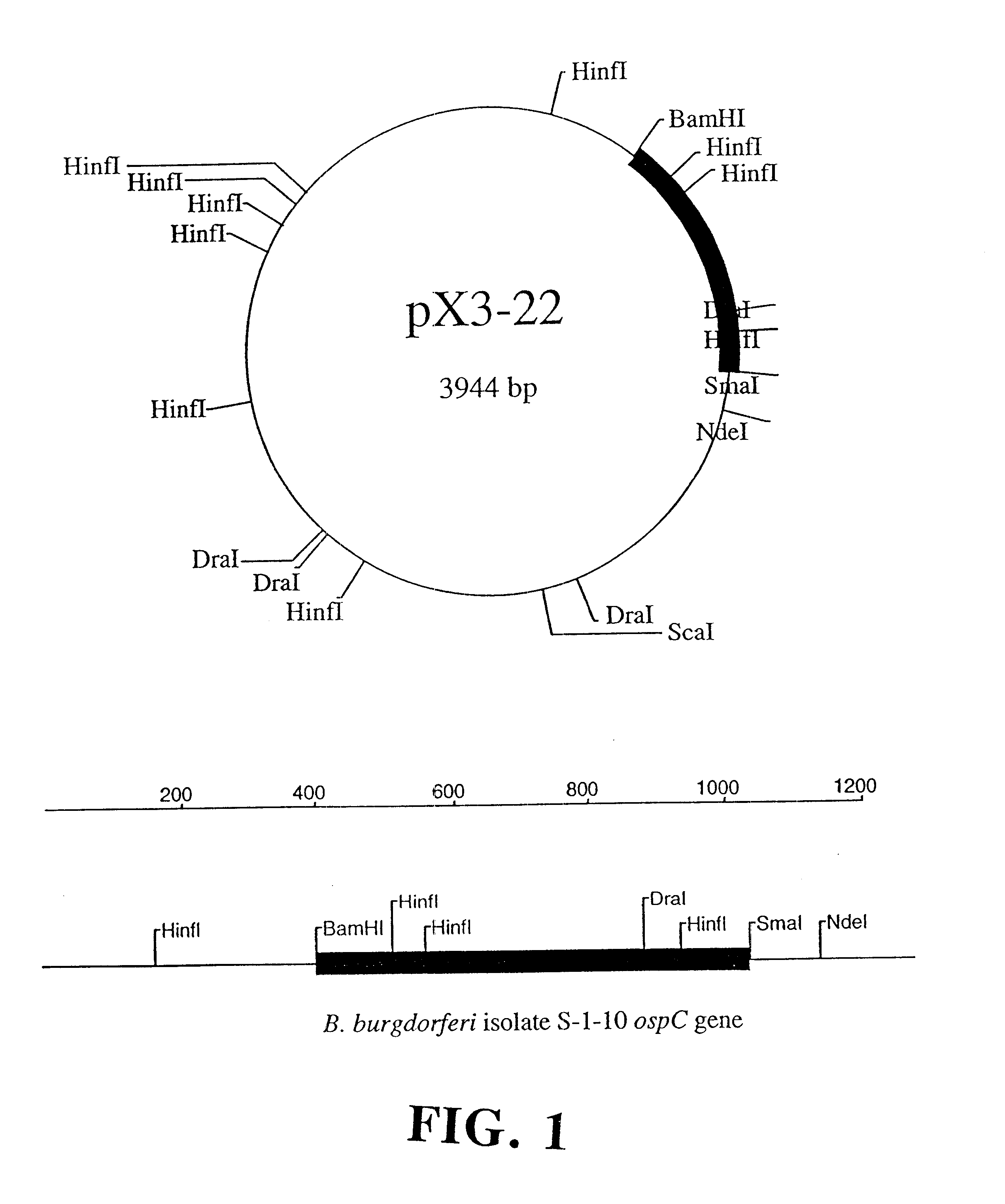
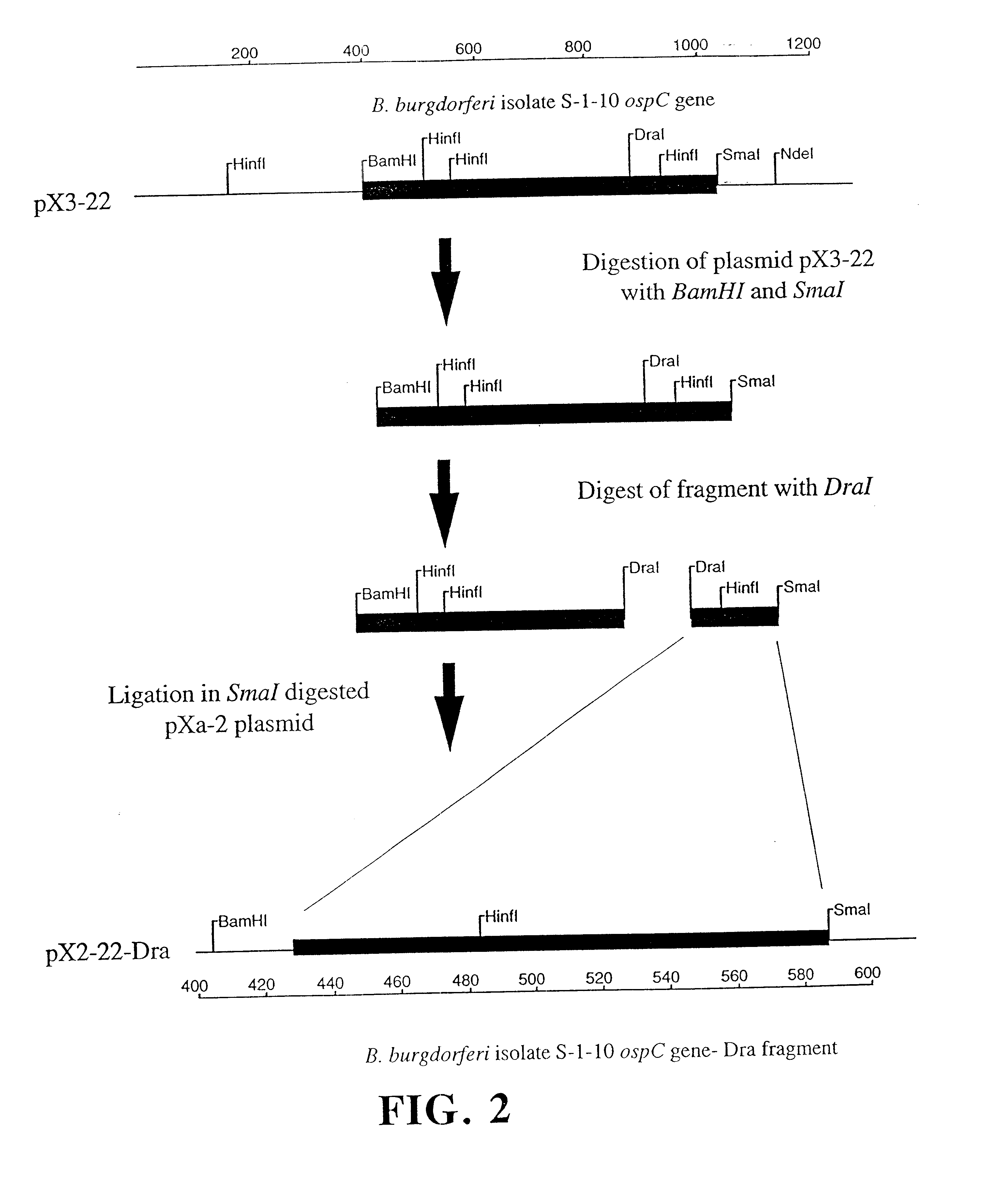
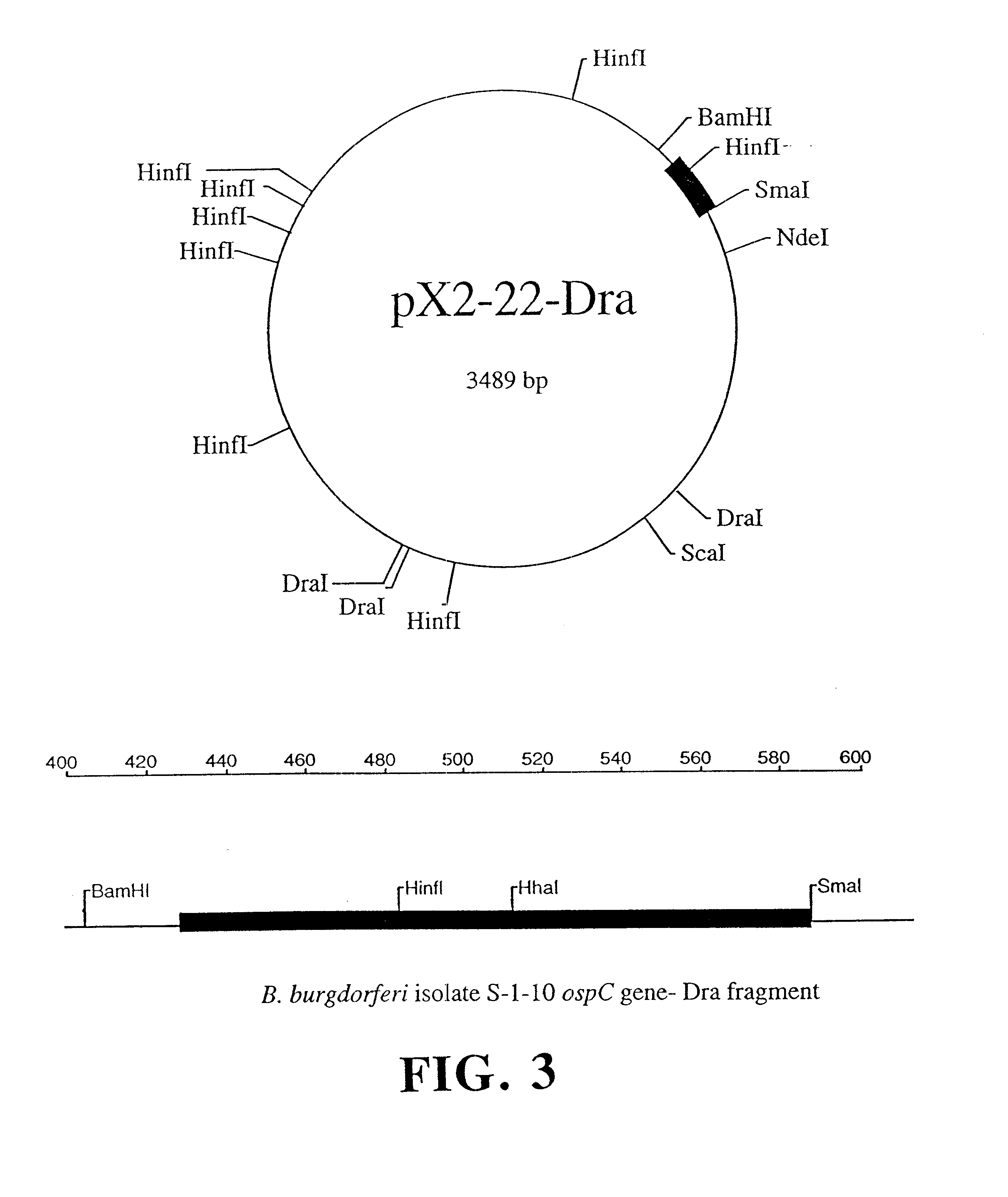
Compositions and methods using the borreliacidal epitope(s) of borrelia burgdorferi outer surface protein C (OspC) for the diagnosis and prevention of lyme disease
Owner:GUNDERSEN LUTHERAN MEDICAL FOUND
Antihuman TNF-alpha antibody activity lowering inhibitor
InactiveUS20060292148A1Inhibit inflammationAntibacterial agentsHydroxy compound active ingredientsSide effectAnti-CEA Antibody
The present invention provides an antihuman TNF-α antibody activity lowering inhibitor comprising a protein source(s) and / or carbohydrate source(s), in the treatment of inflammatory bowel syndrome with repeated administration of anti-TNF-α antibody; and a kit preparation wherein a freeze-dried antihuman TNF-α antibody and the activity lowering inhibitor in the above repeated administration of the anti-TNF-α antibody are separately contained in a plastic container so that they can communicate with each other. According to the present invention, in the drug therapy to the patients with inflammatory bowel syndrome, therapeutic agents which inhibit the inflammation for long periods without accompanying serious side effects can be provided.
Owner:EA PHARMA CO LTD
Preparation method of cystatin c detection kit
InactiveCN102279269AImmunoglobulins against animals/humansBiological testingAntibody conjugateHybrid antibody
The invention relates to a preparation method of cystatin C antibody and a preparation method of a detection kit. Cystatin C (Cys C) is currently one of the most sensitive diagnostic markers for clinical kidney disease. The antibody preparation of the present invention is extracted and purified from human normal serum, and then a highly sensitive antigen is obtained by artificial modification. Animals were immunized multiple times to obtain hyperimmune serum containing Cys C antibody, and high-purity Cys C antibody was obtained by affinity chromatography. During the preparation process, the loss of antibody activity is small, and it is easy to produce in batches, and the antibody has high affinity and high titer, and does not generate cross-immune reaction. After the antibody is coupled with polystyrene latex balls, the prepared detection kit has higher sensitivity and linear range, good repeatability, low detection limit, easy automatic analysis, fast and simple operation. Due to the high purity of the antibody, the content of heteroantibody is very small, so there is less interference and the matrix is stable.
Owner:王贤俊
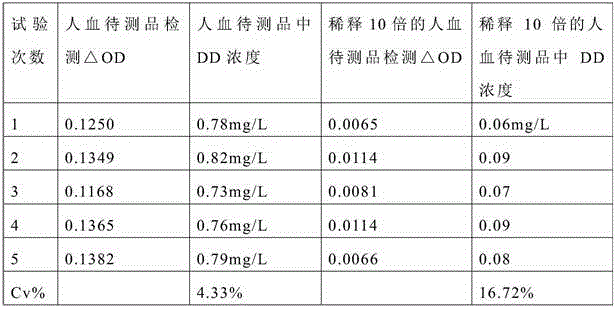
Preparation method and application of D-dimer immuno latex microspheres
ActiveCN106290822AGuaranteed Coupling EfficiencyAvoid damageMaterial analysisMicrospherePolystyrene microsphere
The invention discloses a preparation method and application of D-dimer immuno latex microspheres. In the preparation method of D-dimer immuno latex microspheres, a chemical crosslinking method is optimized, the carboxyl of polystyrene microsphere is activated in a low-pH condition and then coupled with amino of an antibody dissolved in a high-pH condition, and the mixture of the two is neutral and is coupled for 12-24h at a low temperature, so that not only can the coupling efficiency of the antibody be guaranteed, but also the damage of the antibody activity caused by an activator is reduced to a great extent. A latex enhanced turbidimetric immunoassay D-dimer test kit prepared from the immuno latex microspheres prepared by the method has the characteristics of high sensitivity, accurate quantification, good repeatability and stable property, and the lowest detection limit can reach 0.01mg / L; the latex enhanced turbidimetric immunoassay D-dimer test kit can be applied to a full-automatic biochemical analyzer or coagulation analyzer, is quick and easy to operate, only needs 5-10 minutes from detection to result collection and has relatively good clinical application prospect.
Owner:WUHAN KING DIAGNOSTIC TECH CO LTD
Compositions and methods using the borreliacidal epitope(s) of Borrelia burgdorferi outer surface protein C (OspC) for the diagnosis and prevention of lyme disease
An OspC Dra fragment fusion peptide isolated from Borrelia burgdorferi is described herein for the prevention, treatment and early diagnosis of Lyme disease in humans and other animals. This invention also relates to a screening method detecting anti-Osp borreliacidal antibody activity, and antibodies reacting with a protein fragment encoded by a DraI-SmaI DNA fragment of OspC.
Owner:GUNDERSEN LUTHERAN MEDICAL FOUND
Stable protein solution formulation containing high concentration of an Anti-vegf antibody
ActiveUS20160340420A1Low to undetectable levelSenses disorderImmunoglobulins against growth factorsProtein solutionAntiendomysial antibodies
The present invention provides anti-VEGF antibodies formulated as high concentration, aqueous pharmaceutical compositions, suitable for an injection, preferably an intravitreal injection. The aqueous pharmaceutical compositions are useful for delivery of a high concentration of the antibody active ingredient to a patient without high levels of antibody aggregation and without a high level of sub-visible particulate matter. An aqueous composition of the invention comprises an antibody having a concentration of at least 50 mg / ml. An aqueous pharmaceutical composition of the invention includes a sugar, a buffering agent, and a surfactant.
Owner:NOVARTIS AG
Aav vector and assay for Anti-aav (adeno-associated virus) neutralizing antibodies
ActiveUS20160123990A1Microbiological testing/measurementGenetic material ingredientsAnti virusViral antibody
Virus vectors, virus particles, and methods and uses of screening for, detecting, analyzing and determining amounts of virus antibody, or neutralizing antibody activity of samples are provided. Such virus vectors, virus particles, and methods and uses are applicable to a broad range of virus types, such as lentiviruses, adenovirus, and adeno-associated virus (AAV) serotypes. Methods and uses include virus antibody screening, such as anti-virus immunoglobulins screened for, detected, analyzed and amounts determined
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
A novel method of preparing agarose magnetic microspheres and uses of the agarose magnetic microspheres in separation and purification of an IgG antibody
InactiveCN104475041AGood spherical shapeRounded sphereOther chemical processesBiological testingMicrosphereNational standard
The invention relates to a novel method of preparing agarose magnetic microspheres and application of the prepared agarose magnetic microspheres to separation and purification of an IgG antibody by utilization of an immunomagnetic separation technique. In specific, active amino is introduced to surfaces of the agarose magnetic microspheres, glutaraldehyde is adopted as a crosslinking arm to crosslink a ligand, and the ligand is staphylococcus aureus protein A capable of being specifically combined with the IgG antibody, thus preparing immunomagnetic microspheres capable of specifically adsorbing the IgG antibody. In addition, lysate of bacteria generating the IgG antibody by fermentation is separated and purified by utilization of a magnetic separation and purification device, the activity of the prepared antibody can reach 1.5*10<8> g IU / mg, the corresponding activity recovery rate is more than 90%, the purity of the antibody is almost 100% by purification analysis, and each index meets the national standards.
Owner:HARBIN INST OF TECH
Methods for stabilizing proteins
ActiveUS20060287508A1Increase valueQuality improvementImmunoglobulins against blood coagulation factorsAnimal cellsGlycineAntiendomysial antibodies
The present inventors revealed that deamidation of an antibody can be suppressed without influencing its activity by substituting a glycine that is located adjacent to an asparagine with another amino acid.
Owner:CHUGAI PHARMA CO LTD
Preparation method for single-chain antibody based on hybridoma cell
ActiveCN103160515AAvoid distraction puzzlesEfficient and Accurate AcquisitionTissue cultureImmunoglobulinsSingle-Chain AntibodiesStructural homology
The invention discloses a preparation method for a single-chain antibody based on a hybridoma cell. The preparation method comprises the following steps: carrying out inverse transcription with total mRNA of the hybridoma cell as a template to obtain cDNA and selecting and using universal primers with low degeneracy for amplification of a complete set of heavy chain variable region and light chain variable region genes of the antibody; inputting sequences of the amplified heavy chain and light chain variable region genes into an IMGT database for analysis and eliminating antibody genes and nonsense genes originated from the hybridoma cell so as to obtain candidate heavy chain and light chain variable region genes; translating the genes into amino acid sequences, carrying out three dimensional structural homology modeling to obtain a three dimensional simulation structure of the single-chain antibody and carrying out molecular docking analysis on the three dimensional simulation structure and a corresponding antigen target molecule; and splicing a candidate heavy chain and a candidate light chain with correct analysis results into a single-chain antibody gene by using an overlap extension PCR method and carrying out expression and identification of antibody activity. The preparation method provided by the invention is applicable to preparation of all the single-chain antibodies originated from monoclonal hybridoma cells; the process of the preparation method is simple and rapid, and a prepared product has high quality.
Owner:SOUTH CHINA AGRI UNIV
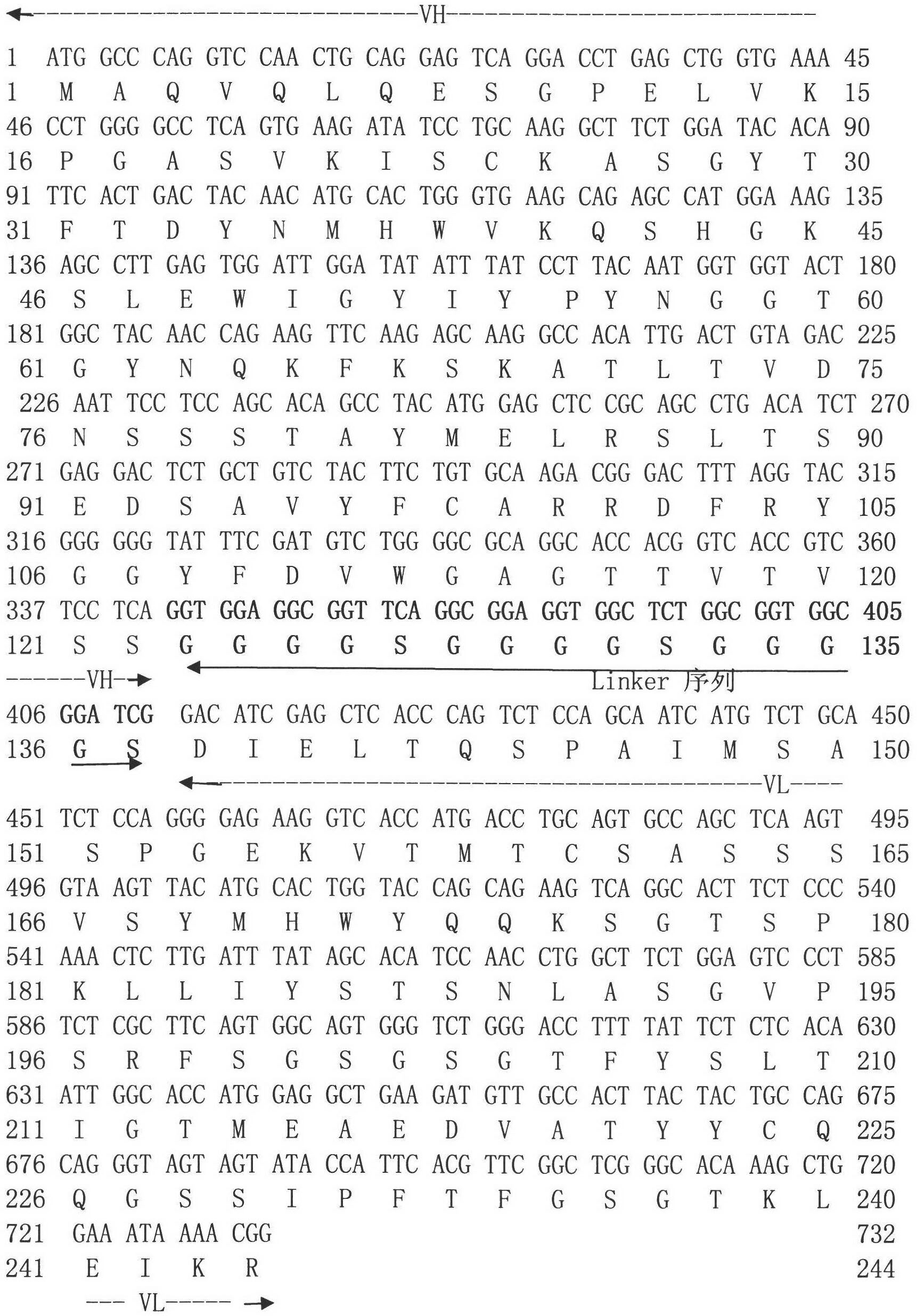
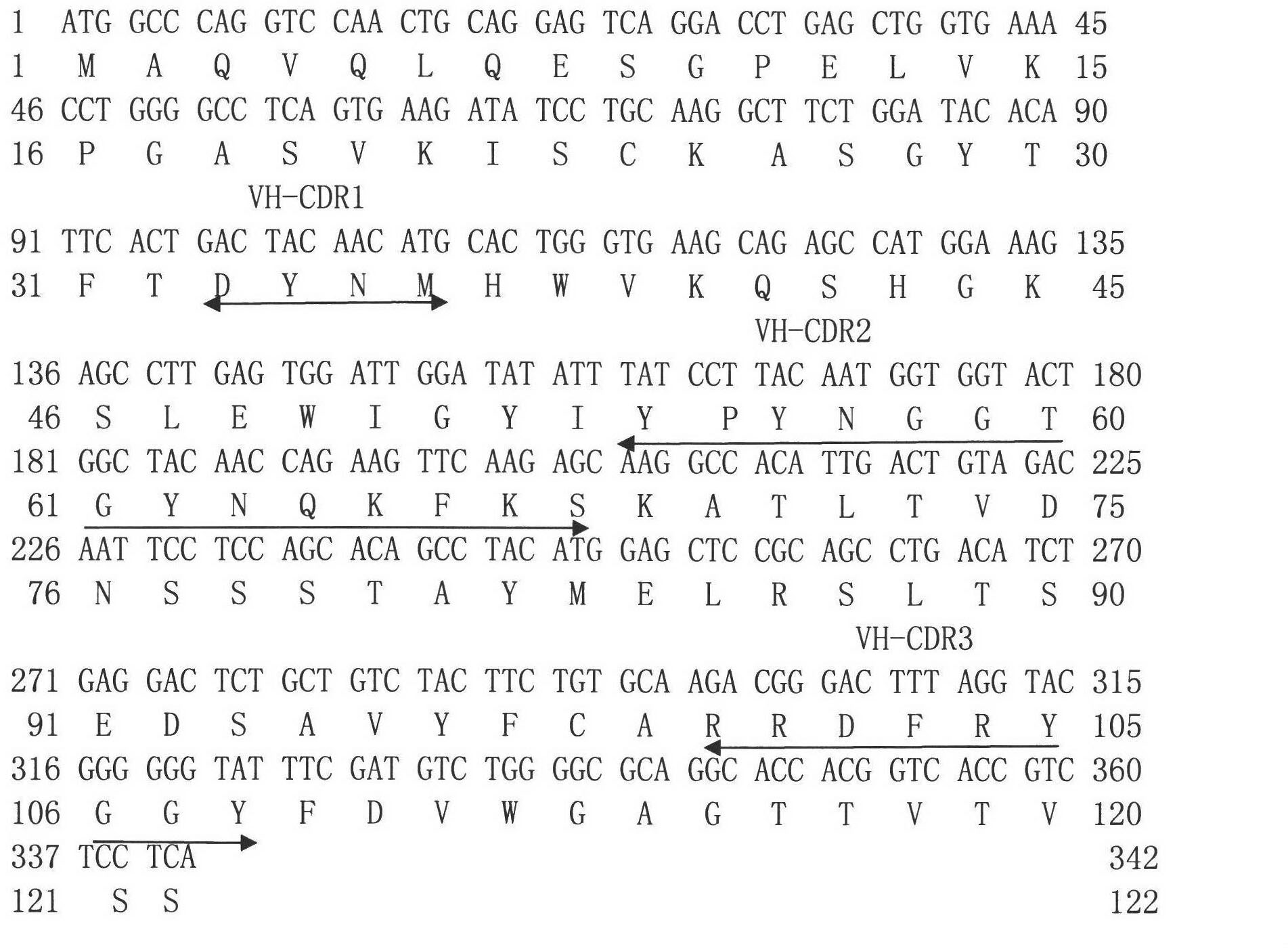
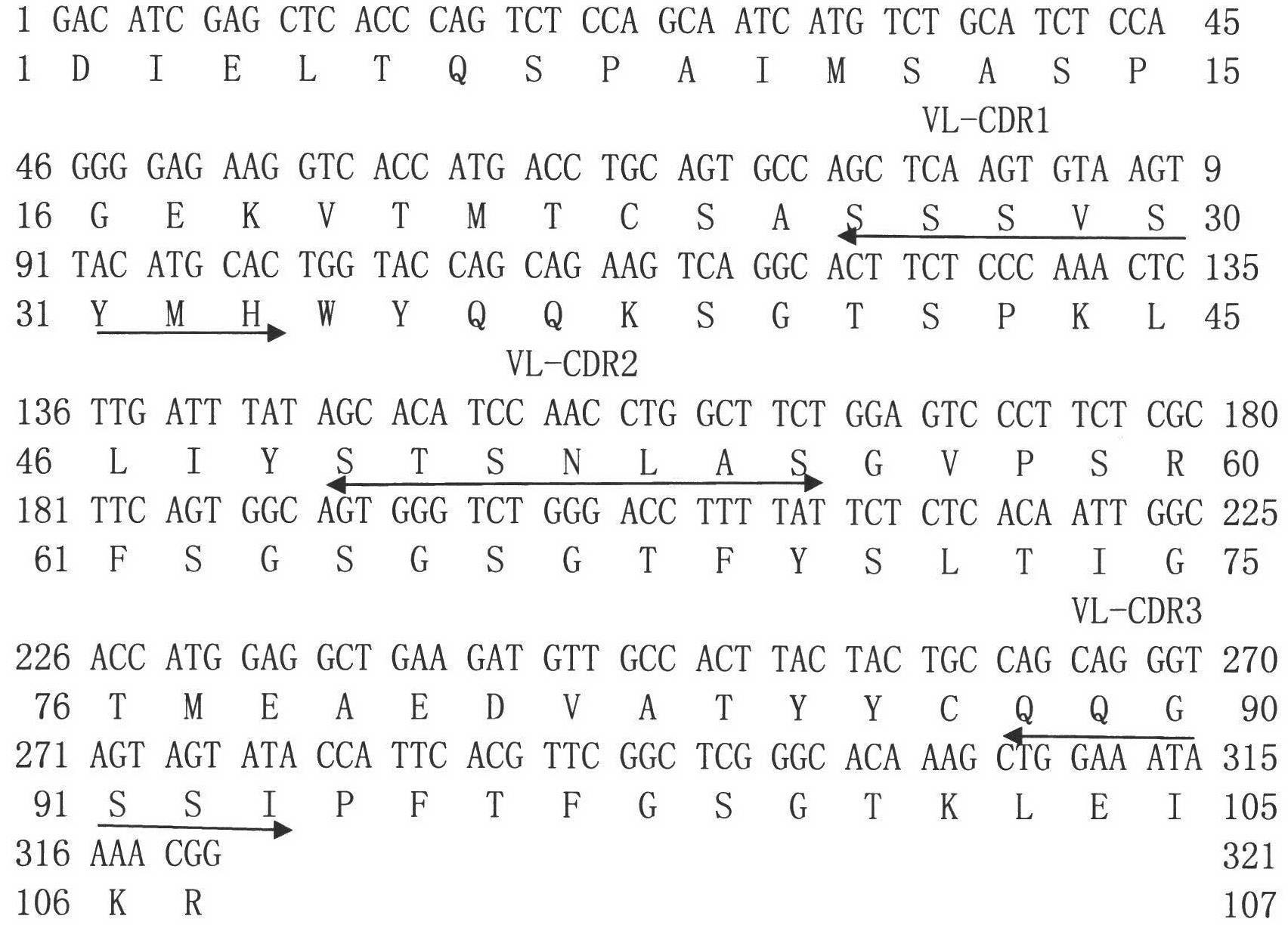
Gene engineering monoclonal antibody combined with A-beta oligomer specificity
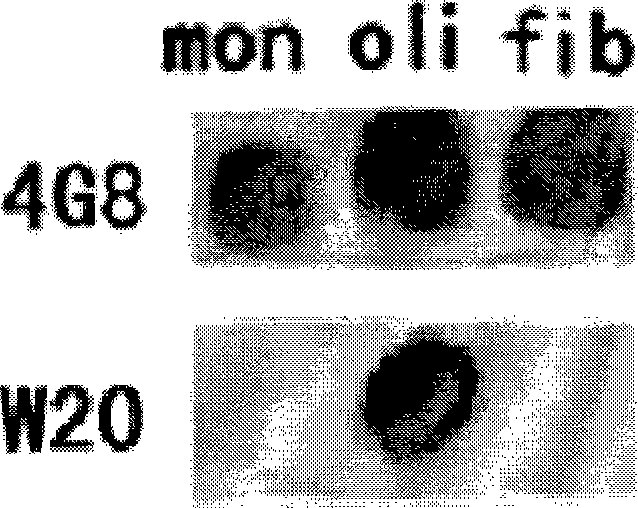
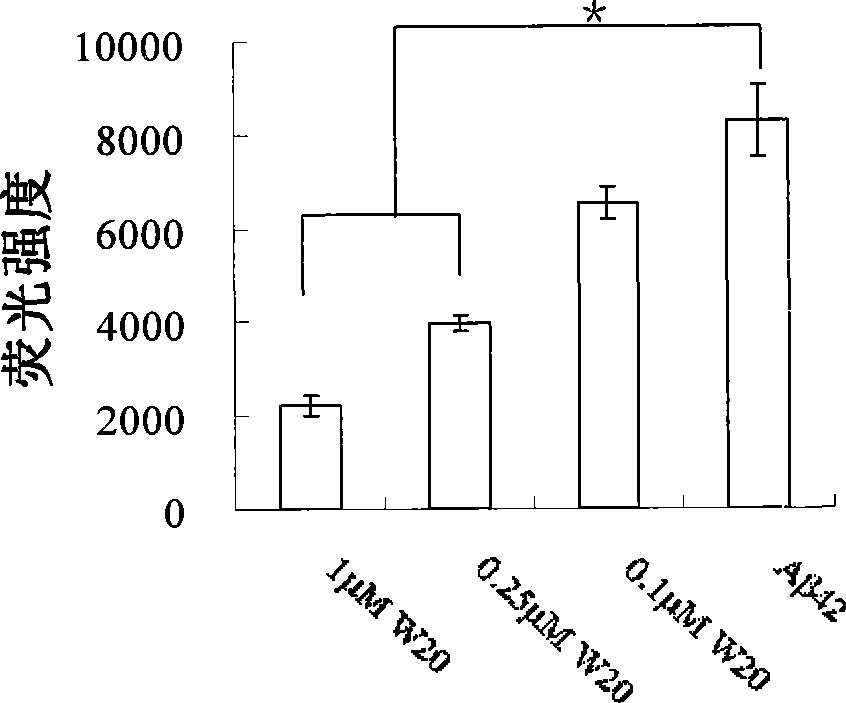
ActiveCN101463082AStrong binding specificityImprove playbackNervous disorderBacteriaEscherichia coliSingle-Chain Antibodies
The invention relates to the technical field of genetic engineering antibody and provides a monoclonal antibody. The variable region of heavy chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.1, SEQ ID NO.2 and SEO ID NO.3; the variable region of light chain of the monoclonal antibody contains the amino acid sequences shown in SEQ ID NO.4, SEQ ID NO.5 and SEQ ID NO.6. The invention also specifically provides a humanization single-chain antibody generated by the recombinant strain of enterotoxigenic Escherichia coli with the accession number CGMCC No.2821 and the amino acid sequence of the humanization single-chain antibody is shown in SEQ ID NO.7. The antibody of the invention can be specifically bound with the A-beta oligomer, effectively inhibit the fibrosis aggregation of A-beta and obviously alleviate the toxic effect of the A-beta on cells. The invention also relates to a pharmaceutical composite containing the antibody. The antibody of the invention has strong activity, good specificity, easy preparation and wide prospect of experiment application and clinical application.
Owner:TSINGHUA UNIV
Methods and compositions for conversion of antibody activity
InactiveUS20050031625A1Reducing and preventing symptomRelieve symptomsAntibacterial agentsAntimycoticsAntigen bindingEnhancing Antibodies
The present invention provide a bispecific molecule comprising an antibody that binds a C3b-like receptor linked to one or more non-neutralizing antigen-binding antibodies or fragments thereof. The present invention also provides methods to identify non-neutralizing antibodies, and particularly, to identify enhancing antibodies. Methods of producing such bispecific molecules and their therapeutic and / or prophylactic uses are also provided by the present invention
Owner:ELUSYS THERAPEUTICS
Monoclonal antibody for detecting porcine C-reactive protein (CRP)
InactiveCN104630151AHigh purityHigh antibody activityImmunoglobulins against animals/humansTissue cultureRational useTreatment effect
The invention provides a monoclonal antibody for detecting porcine C-reactive protein (CRP), and preparation and application thereof. A gene engineering process is performed to obtain high-purity CRP recombinant protein; and the recombinant protein is utilized to screen out the hybridoma cell strain with the highest stability and highest antibody activity for secreting CRP protein antibody, wherein the collection number is CGMCC NO.9345. The monoclonal antibody generated by the hybridoma cell strain has the advantages of high specificity, high affinity and simple and efficient preparation method, and can monitor the CRP content in porcine serum, perform differential diagnosis on bacterial and virus diseases and perform auxiliary observation on treatment effects, thereby promoting the rational use of antibiotics, reducing the drug residues and ensuring the safety of animal food.
Owner:CHINA AGRI UNIV
Method for preparing yolk immunoglobulin vaccine for resisting porphyromonas gingivalis
ActiveCN101791405ALow priceReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsYolkMycoprotein
The invention relates to a method for preparing a yolk immunoglobulin vaccine for resisting porphyromonas gingivalis, which comprises the following operating steps of: (1) culturing and separating an international standard strain of the porphyromonas gingivalis to obtain the porphyromonas gingivalis; (2) obtaining mycoprotein of the porphyromonas gingivalis; (3) mixing a whole bacterium soluble protein antigen of the porphyromonas gingivalis with a freunds incomplete adjuvant uniformly to prepare an intravenous injection preparation, performing an intravenous injection under a chicken wing of a laying hen, and collecting eggs laid by the laying hen; and (4) extracting yolk immunoglobulin for resisting the porphyromonas gingivalis in the eggs. The method has a low production cost and a high yield because 100 milligrams of the yolk immunoglobulin can be obtained from one egg; and the method is simple, convenient and quick to operate and has the advantages of heat resistance, acid resistance, good stability and the like. Under an acid condition of which the temperature is not over 75 DEG C and the pH is more than 4, the yolk immunoglobulin vaccine can still well maintain the biological activity, can be stored for about 3 months at the normal temperature, and can be stored for 6 to 12 months at 4 DEG C with non-decreased antibody activity.
Owner:ANHUI MEDICAL UNIV
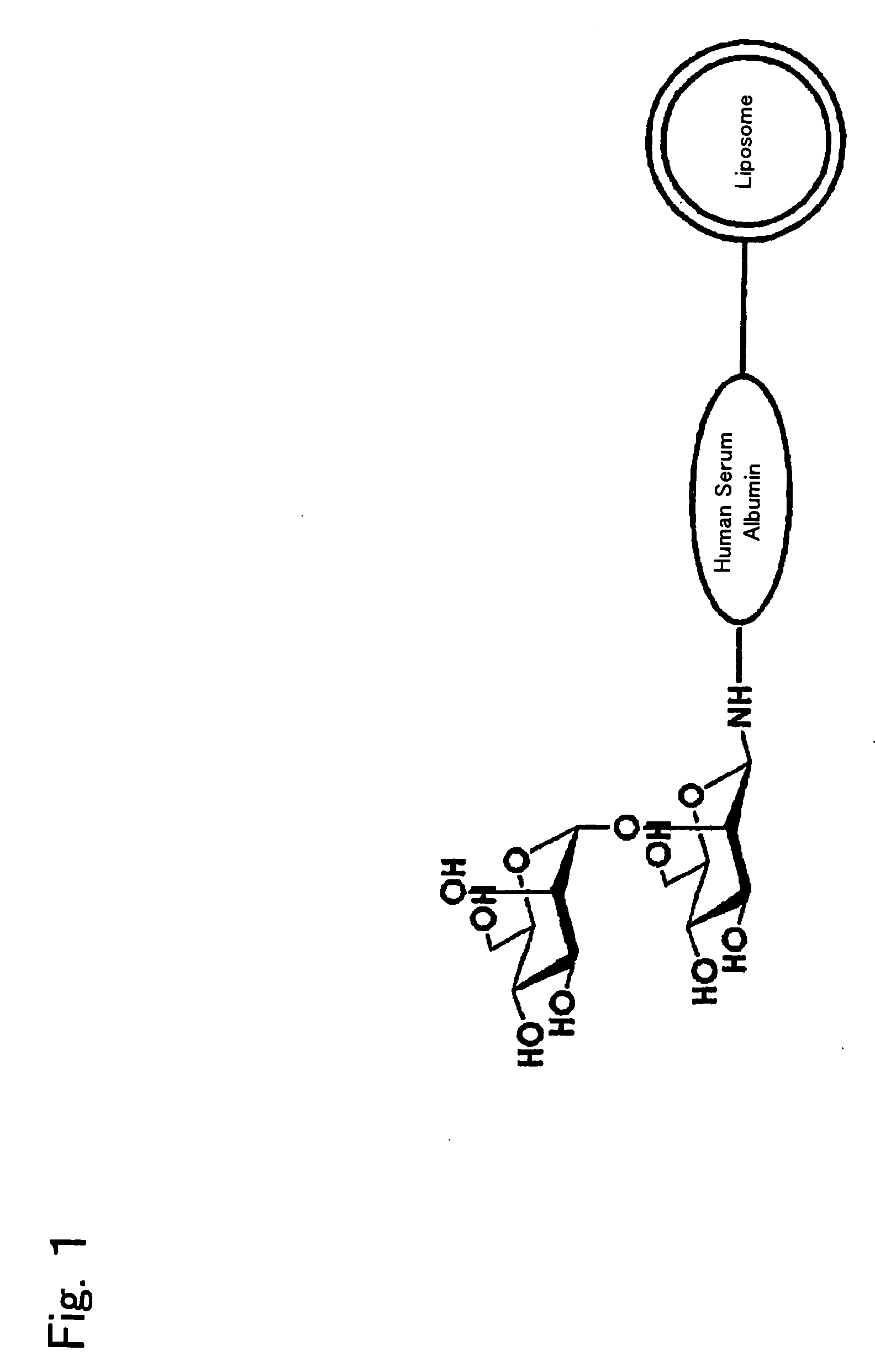
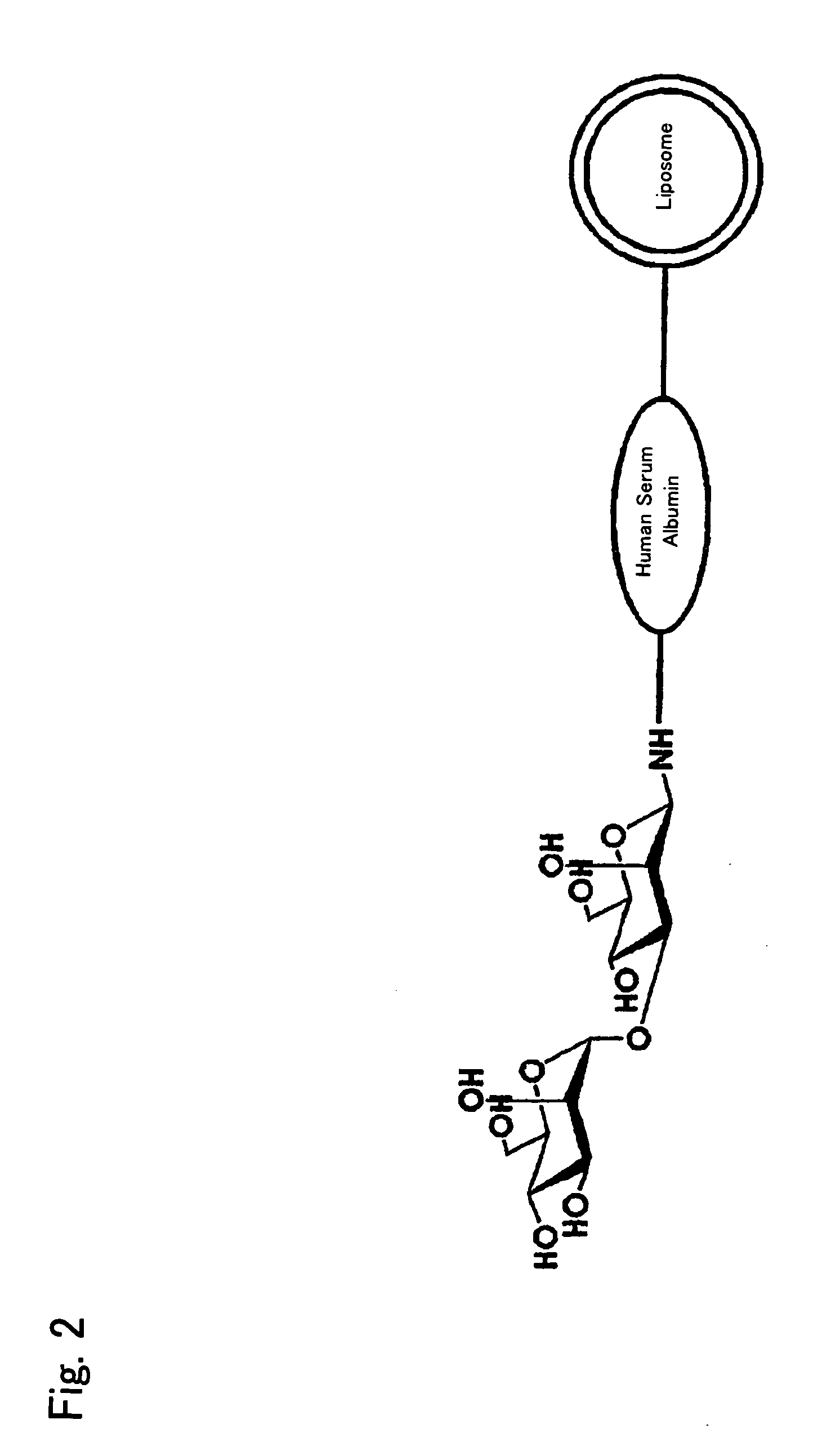
Liposome Preparation
InactiveUS20090169610A1Improve targetingReduce drug doseOrganic active ingredientsAntibody ingredientsGnRH AntagonistLiposome
The present invention provides cancer treatment preparations of excellent targetability. The sugar chain-modified liposomes of the present invention, which contain an aromatase inhibitor, anti-androgenic agent, lyase inhibitor, GnRH agonist, GnRH antagonist, anti-angiogenic agent, tyrosine kinase inhibitor, serine-threonine kinase inhibitor, antibody having an anticancer activity, ansamitocin, capecitabine, celmoleukin, docetaxel hydrate, gemcitabine hydrochloride, oxaliplatin, prednisolone, tegafur-uracil mixtures, zinostatin stimalamer or arsenic trioxide may be used as cancer treatment preparations having an excellent targetability.
Owner:SIEMENS AG +1
Single-chain antibody for resisting influenza viruses, preparation method for single-chain antibody, application of single-chain antibody
InactiveCN102558348ABlock bindingNeutralizing activityBacteriaMicroorganism based processesSerum igeSingle-Chain Antibodies
The invention discloses a single-chain antibody ScFv for resisting influenza viruses, a preparation method for the single-chain antibody ScFv, application of the single-chain antibody ScFv, a gene for encoding the single-chain antibody ScFv, a carrier containing the gene, a host cell and the like. The single-chain antibody ScFv for resisting the influenza viruses is one of the following proteins: 1) a single-chain antibody formed by connecting a heavy chain variable region and a light chain variable region of the antibody through a linker peptide, wherein an amino acid sequence of the light chain variable region and an amino acid sequence of the heavy chain variable region are shown as SEQ ID NO.1 and SEQ ID NO.2 in a sequence table respectively; and 2) a derived antibody obtained by improving the single-chain antibody in step 1), wherein the improvement comprises the deletion, substitution or insertion of amino acid, and the derived antibody has the antibody activity of resisting H1N1 influenza viruses. The molecular weight of the single-chain antibody is about 27kD; and the single-chain antibody can specifically identify the H1N1 influenza viruses and block the combination of the viruses and natural serum. The single-chain antibody can be used for diagnosing, preventing, controlling and treating the infection of the H1N1 influenza viruses, or is used for antiviral breeding of transgenic animals.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
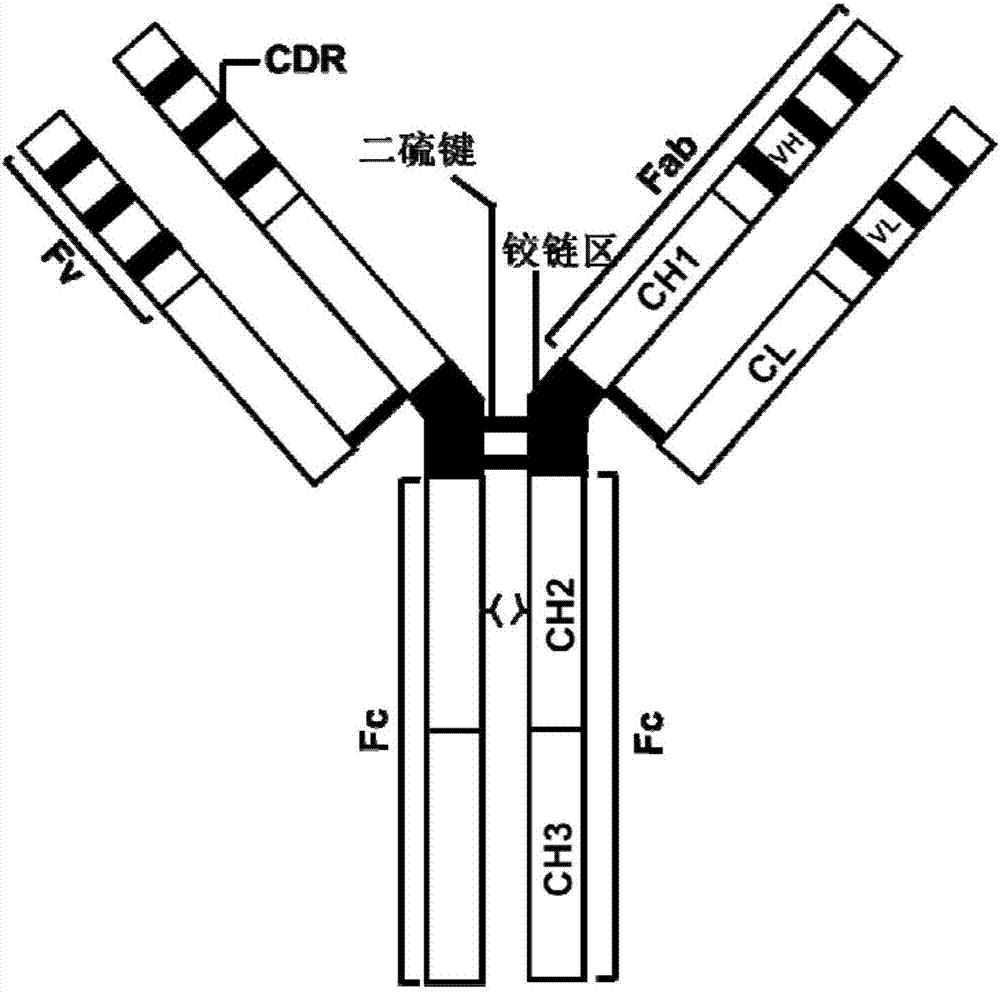
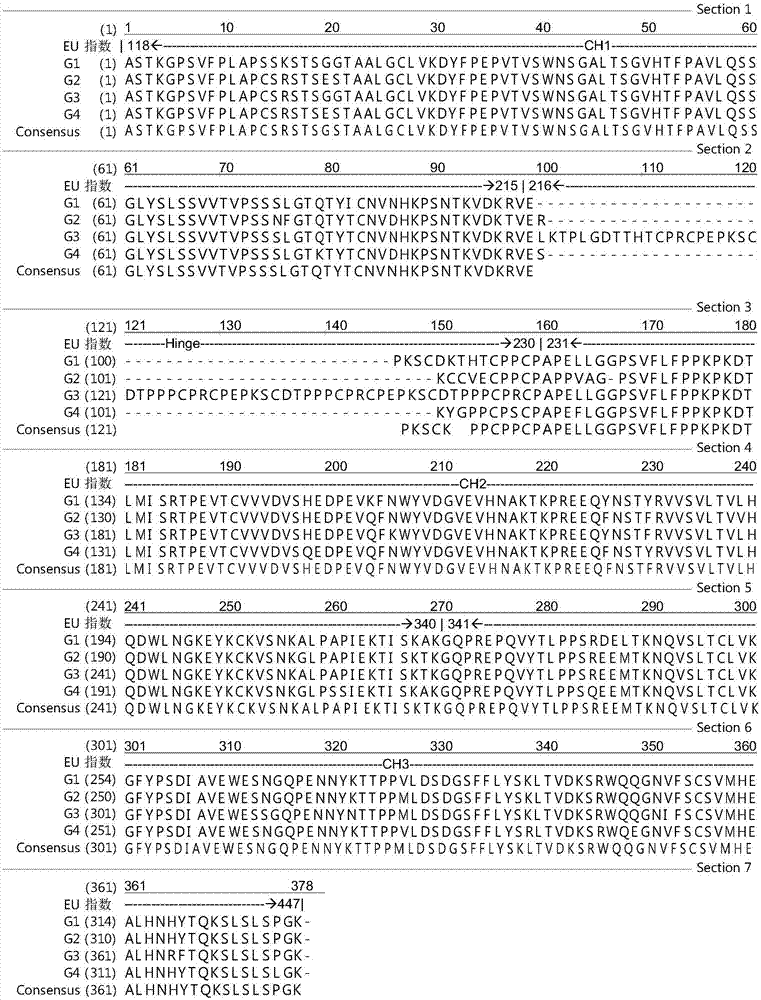
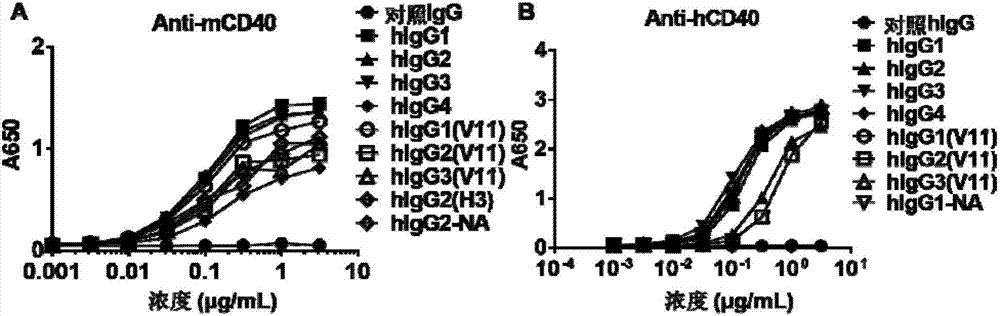
An antibody heavy chain constant region sequence enhancing activity of an agonistic antibody
ActiveCN107474136AHigh activityImprove securityHybrid immunoglobulinsAntipyreticDiseaseAutoimmune responses
The invention provides a heavy chain constant region. The heavy chain constant region includes a CH1 structural domain, a hinge region, a CH2 structural domain and a CH3 structural domain. Sequences of the CH1 structural domain and the hinge region are sequences derived from a CH1 structural domain and a hinge region of human IgG2. Sequences of the CH2 structural domain and the CH3 structural domain are sequences derived from a CH2 structural domain and a CH3 structural domain of human IgG. Affinity between the antibody heavy chain constant region to human Fc[gamma]IIB is equal to or higher than affinity between human IgG1 and the human Fc[gamma]IIB. The I / A ratio of the antibody heavy chain constant region is equal to or higher than the I / A ratio of the human IgG1. An antibody or fusion protein based on the heavy chain constant region is also provided. The antibody heavy chain constant region can significantly enhance agonist activity of the antibody or the fusion protein, and can improve treatment effects of the antibody or the fusion protein on tumor, autoimmunity, and other diseases.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Method for preparing micro-cantilever beam modified with antibody
InactiveCN101407548ALittle loss of activityShort reaction timeCarrier-bound/immobilised peptidesCantileverHydrochloride
The invention discloses a method for preparing a micro-cantilever decorated with an antibody, which uses hydrochloride mercaptan imine as a sulfhydrylation agent, and the antibody is sulfhydrylized, and the sulfhydrylation antibody is fixed on a gold membrane of the micro-cantilever to obtain the micro-cantilever decorated with the antibody. The result shows that by introducing the sulfhydrylation agent-hydrochloride mercaptan imine, the method for preparing the micro-cantilever decorated with the antibody has the advantages of short reaction time, simple operation, few loss of antibody activity and the like, compared with the existing method.
Owner:CHINA AGRI UNIV
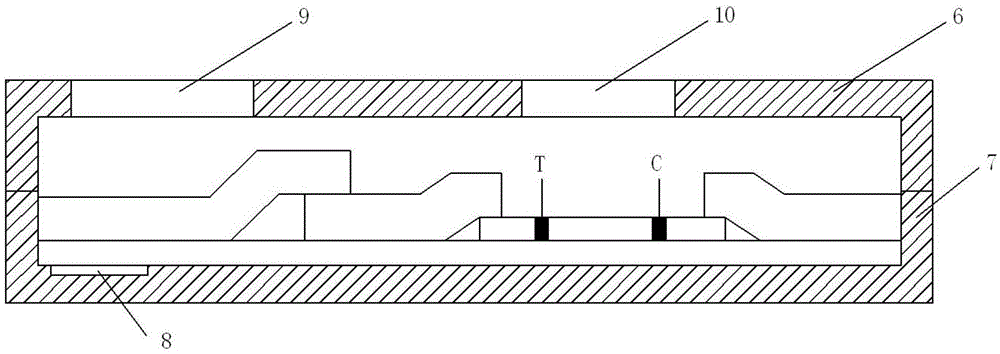
Convenient procalcitonin detection kit
InactiveCN105467116AAvoid interferenceAvoid being copied and tampered withPreparing sample for investigationBiological testingCelluloseLap joint
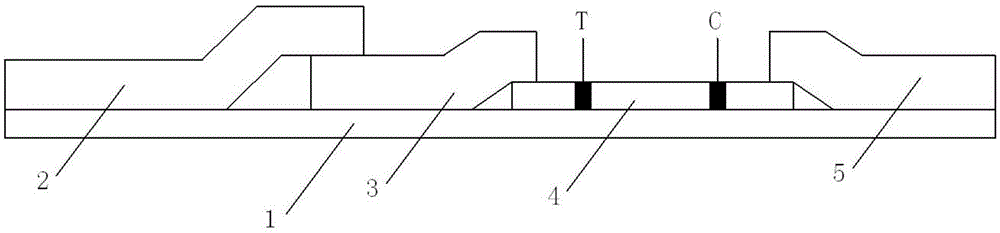
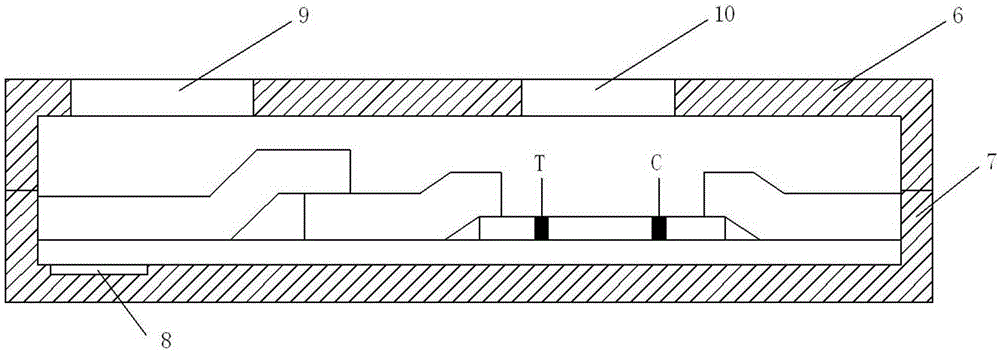
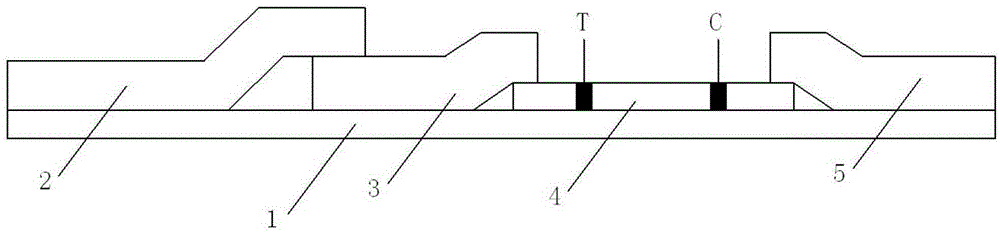
A convenient procalcitonin detection kit comprises a test strip, an upper clamping groove, a lower clamping groove and an RFID (radio frequency identification) chip, wherein the test strip comprises a plastic rubber slab; a sample pad, a first antibody bearing pad, a cellulose membrane and a water absorption pad are arranged sequentially on the plastic rubber slab; the first antibody bearing pad and the water absorption pad are in lap joint at two ends of the cellulose membrane; one end of the sample pad is in lap joint on the first antibody bearing pad; a detection band T for immobilizing a second C-reactive protein antibody and a quality control band C for immobilizing a goat-anti-mouse antibody are arranged on the cellulose membrane; the test strip is arranged in a cavity formed by splicing the upper clamping groove and the lower clamping groove; a sampling hole and a detection window are formed in the upper clamping groove; a sample diluent adopts a buffer containing rheumatoid factors and a heterophil antibody blocker; the RFID chip is arranged in the lower clamping groove close to the left end. The detection kit has the advantages that the antibody activity can be reflected accurately, an error detection result can be avoided, the kit is simple to use, and the like.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
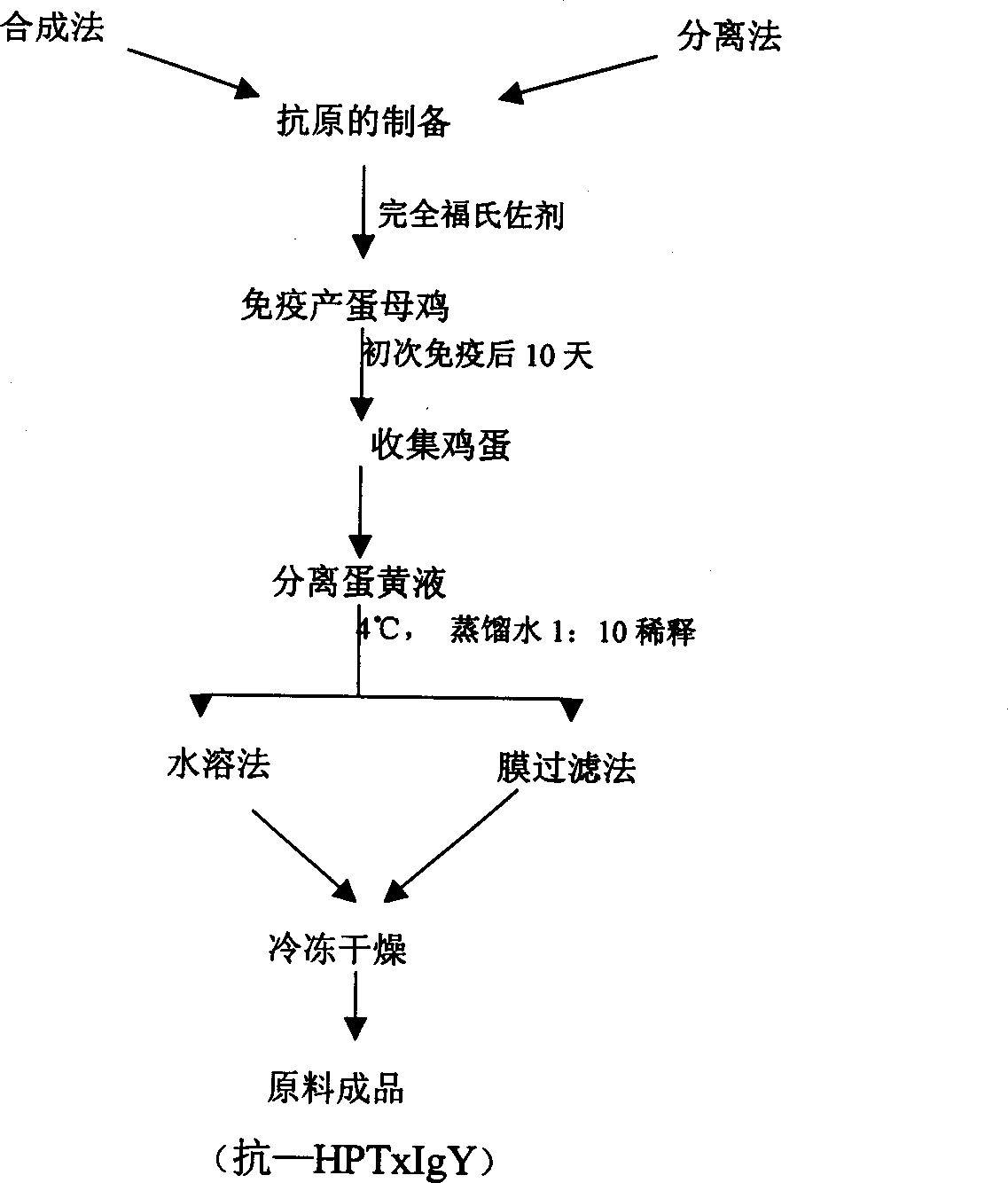
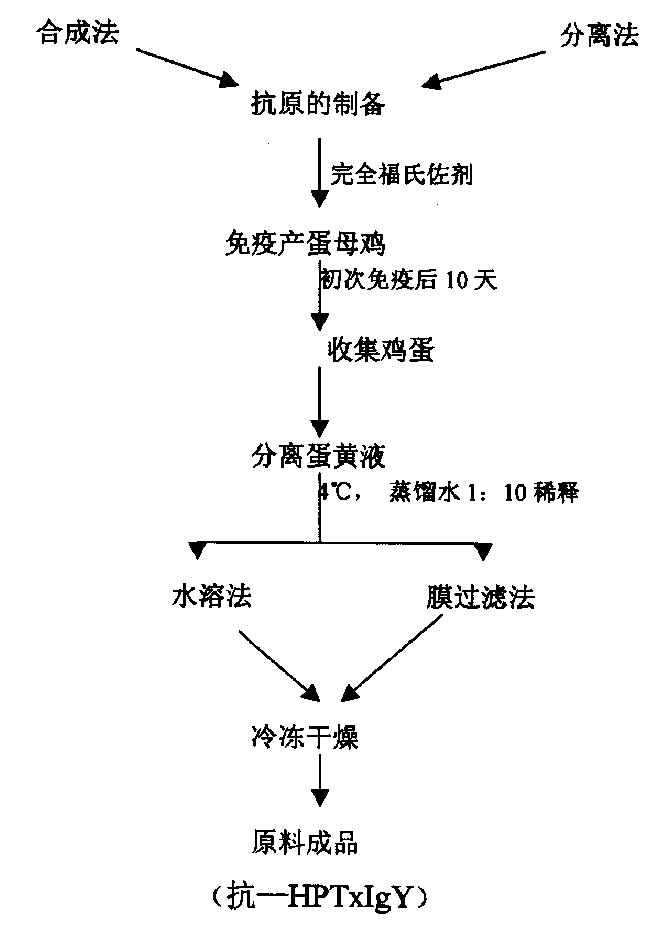
Hen egg yolk antibody for resisting pyloric helicobacterium cytotoxin, its preparation and application
InactiveCN1354187ASpecificGood effectMicrobiological testing/measurementNitro compound active ingredientsElectrophoresisHelicobacter pylori infection
The present invention relates to a chicken vitellus antibody (anti-HPTxIgY) with antibody activity for resisting pyloric helicobacterium cytotoxin, its preparation method and application in the preparation of medicine and health-care product for curing related diseases resulted from pyloric helicobacterium infection and preparation for detecting these related diseases. The above-mentioned antibody is undergone the process of IgY purity checking treatment. PAGE electrophoressi can be used for detecting purified vitallus antibody purity. Only one electrophoretic band is produced in the electrophoresis, and an UV spectrophotometer is used for determination so as to calculate the albumen concentration (mg / ml) which is (1.45XOD 280nm-0.74XOD 260nm)X dilute multiple. The above-mentioned method is as follows: preparing pyloric helicobacterium cytotoxin antigen, immunizing health hen with said antigen, collecting egg and extracting anti-HPTxIgY from the egg.
Owner:重庆和润实业(集团)有限公司
Fusion protein based on anti-EGFR single-chain antibody and arginine nonamer, and applications of fusion protein
ActiveCN103910799AReduce sizeGuaranteed targetingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsSingle-Chain AntibodiesArginine
The invention discloses a fusion protein based on anti-EGFR single-chain antibody and arginine nonamer, and applications of the fusion protein. The fusion protein comprises a heavy chain variable region and a light chain variable region which are connected via connection peptides; the terminal of the light chain variable region is connected with the arginine nonamer; and amino acid sequence of the arginine nonamer is represented by SEQ ID No.3. According to the fusion protein, the heavy chain variable region and the light chain variable region of the anti-EGFR single-chain antibody are connected via the selected connection peptides, so that specificity and affinity of combination of the antibody with EGFR are maintained, and targeting performance of the constructed fusion protein on EGFR is ensured; the size of the fusion protein is reduced, so that on the one hand construction, expression and purification of the fusion protein are convenient, and cost is reduced, and on the other hand, combination of the fusion protein on EGFR is ensured, demands on molecular size and antibody activity during cellular internalization are ensured, and feasibility and accuracy of the combination of the fusion protein on EGFR and targeting tumor cellular internalization are ensured.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Portable C reaction protein detection kit
InactiveCN105334318AAvoid interferenceAvoid copying and tamperingMaterial analysisCelluloseProtein detection
A portable C reaction protein detection kit comprises a test strip, an upper clamp slot, a lower clamp slot and a radio frequency identification chip; the test strip comprises a plastic cement plate, on which a sample pad, a first antibody bearing pad, a cellulose membrane and a water absorption pad are arranged in sequence; the first antibody bearing pad and the water absorption pad are respectively overlapped at two ends of the cellulose membrane; one end of the sample pad is overlapped on the first antibody bearing pad; the cellulose membrane is provided with a detection belt T for fixing a C reaction protein second antibody and a quality control belt for fixing a sheep anti-mouse antibody; the test strip is arranged in a cavity formed by splicing the upper clamp slot and the lower clamp slot; the upper clamp slot is provided with a sampling hole and a detection window; a sample diluent is a buffer solution containing a rheumatoid factor and heterophil antibody blocking agent; the lower clamp slot is provided with the radio frequency identification chip close to the left end. The kit has the advantages of being capable of accurately reflecting antibody activity, capable of avoiding wrong detection results, simple to use, and the like.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
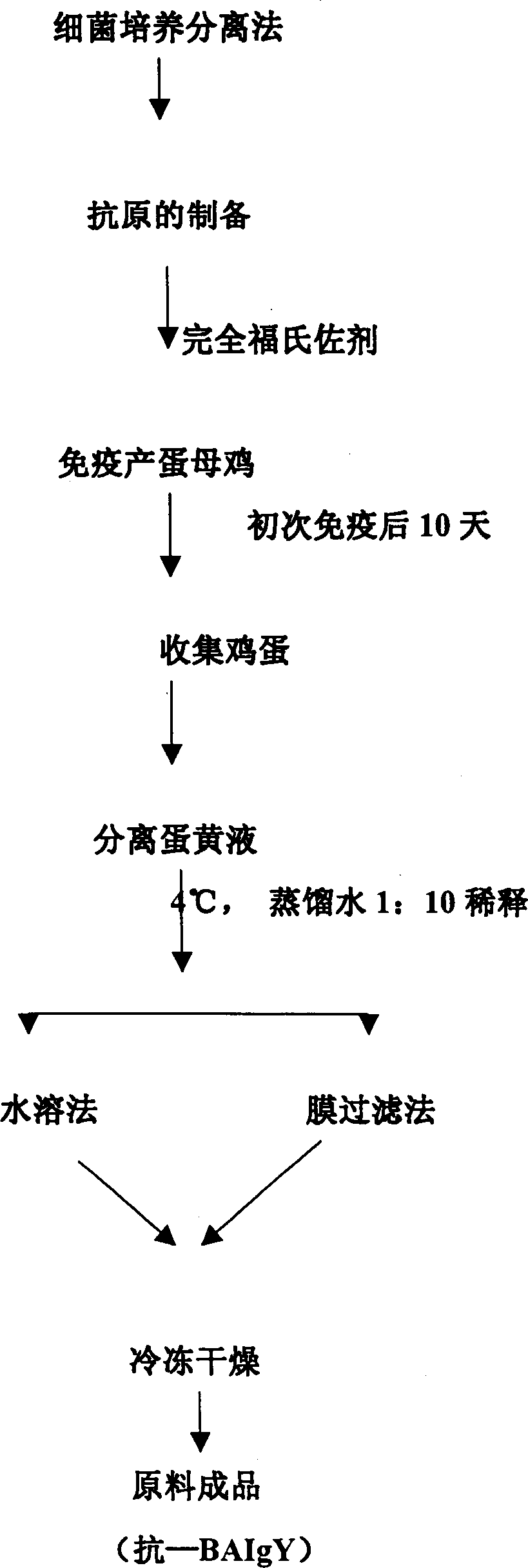
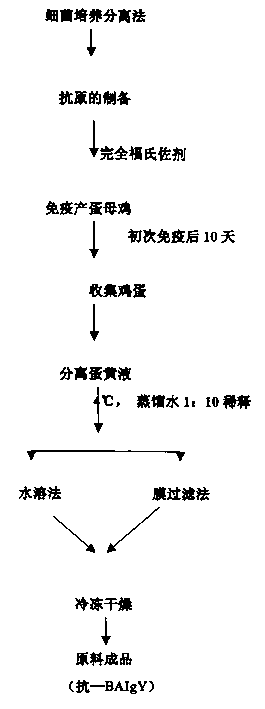
Compound yolk antibody capable of resisting fowl bacterial blight and its preparation and use
The present invention relates to a compound vitelline antibody (anti-BAIgY) with antibody activity for resisting salmonella gallinarum, bacterium multocidum and chicken colibacillus, method for preparing said antibody and application of said antibody in preparation of medicine or feed additive for curing relatied diseases which can be infected by above-mentioned bacteria. The preparation method of said compound vitelline antibody includes the following steps: preparing compound antigen of the above-mentioned bacteria, immunizing health hen with said antigen, collecting egg and extracting anti-BA IgY from egg.
Owner:重庆和润实业(集团)有限公司
Connection method of antibody and microsphere
InactiveCN103159854AAvoid structural damageHigh activityCarrier-bound/immobilised peptidesHydrazoneMicrosphere
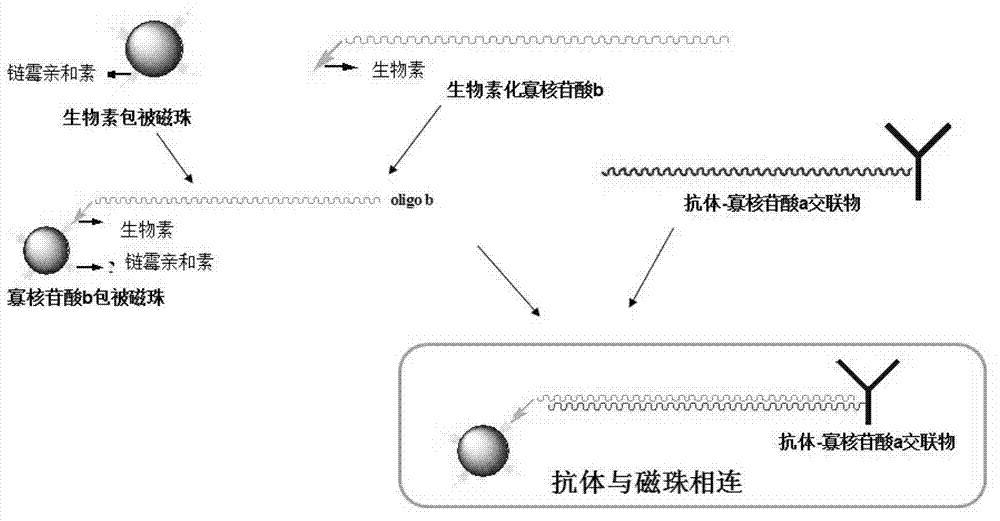
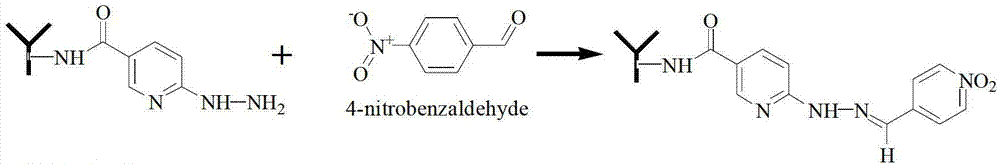
The invention discloses a connection method of antibody and microsphere, which comprises crosslinking of the antibody and nucleotide, combination of nucleotide and microsphere, and hybridization of a nucleotide complementary chain. The crosslinking of the antibody and nucleotide comprises modification and desalination of the antibody, modification and desalination of nucleotide, crosslinking and purifying of the modified antibody and nucleotide, and the like. The connection method takes a double functional group of nucleotide and the antibody as modification, and the nucleotide and the antibody are combined through an aldehyde group and an amino group to form hydrazone bond combination. According to the invention, the combination of the antibody and microsphere is completed by microsphere coated with avidin and biotin which is modified on a nucleotide chain through specifically adsorption; the hybridization of the nucleotide complementary chain is completed under certain temperature condition through a specific hybridization liquid; the incubation and identification for whether the reaction on the microsphere successes or not can be carried out under the specific temperature condition through Cy3 labeled secondary antibody and the like. The connection method takes oligonucleotide as a support arm for separating the antibody and the magnetic bead for a certain distance, and is in favor of avoiding the destroy of a structure of the antibody, and thereby the antibody activity can be protected.
Owner:ZHEJIANG UNIV
HIV positive serum surrogate
Substitute of HIV positive blood serum is cross-linking object obtained from cross-linking reaction between unhuman anti HIV antibody or its treating fluid and normal person immunoglobulin or its treating fluid. Essence of the invention is to combine unhuman anti HIV antibody activity (i.e. activity reacting to HIV antigen) with antigen activity of normal person immunoglobulin (i.e. activity reacting to the conjugate of anti abzyme of human immunoglobulin) so as to eliminate risk of containing pathogenesis factor possibly in blood serum (or blood plasma), which is positive in human anti antibody of pathogenesis factor, but possess reactivity on blood serum, which is positive in anti antibody of pathogenesis factor. Since the substitute is from unhuman anti HIV antibody and normal person immunoglobulin, thus it is essential to prevent risk infected by positive serum potentially.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST
Method for fixing antibody on the surface of medical instrument
ActiveUS20100112189A1Improve smoothnessHigh antibody activitySurgeryPharmaceutical containersBare-metal stentMicro arc oxidation
A method for fixing antibody on the surface of medical instrument, mainly includes: 1) pre-treating the instrument surface; 2) preparing holes: preparing multicrystal phase structure which has same size holes in the surface of the instrument by chemical corrosion, electrochemical corrosion, anodic oxidation, micro-arc oxidation, micro-arc nitridation; 3) post-treating the instrument surface; 4) fixing the antibody: immerging the bare metal scaffold which has holes in surface into a buffer solution containing antibody, adjusting the pH value of the antibody buffer solution, fixing the antibody on the surface of the instrument by the attraction between positive and negative charge and hole effect; and 5) confirming the effectiveness of the fixed antibody by artificial simulation hemodynamics and detecting method of antibody activity in scaffold surface. The method can promote the firm degree of the antibody which is fixed on the surface of the instrument, and keep high activity of the antibody on the surface of medical instrument.
Owner:LEPU MEDICAL TECH (BEIJING) CO LTD
Anti-Blys monoclonal antibody and pharmaceutical composition containing anti-Blys monoclonal antibody
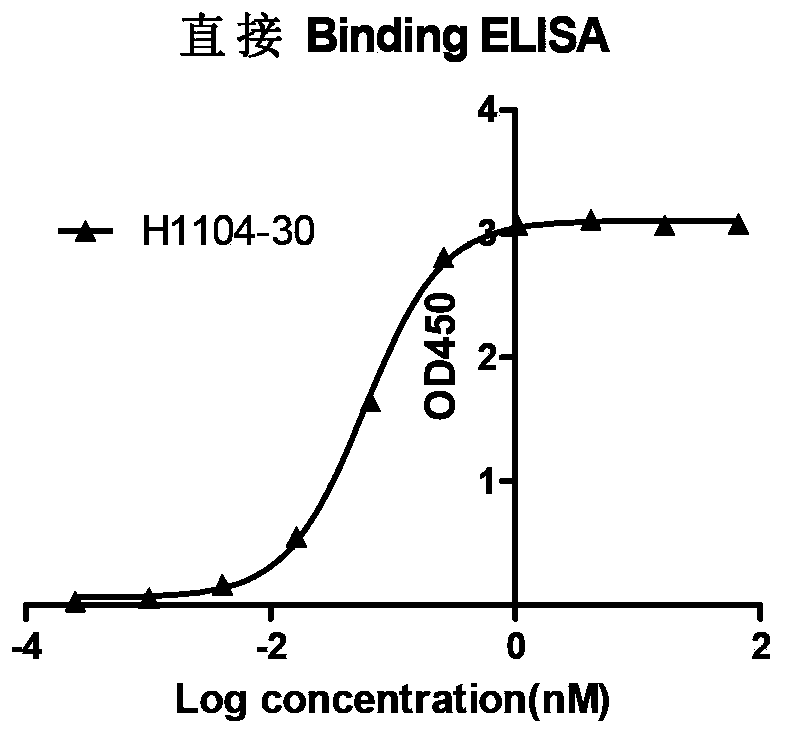
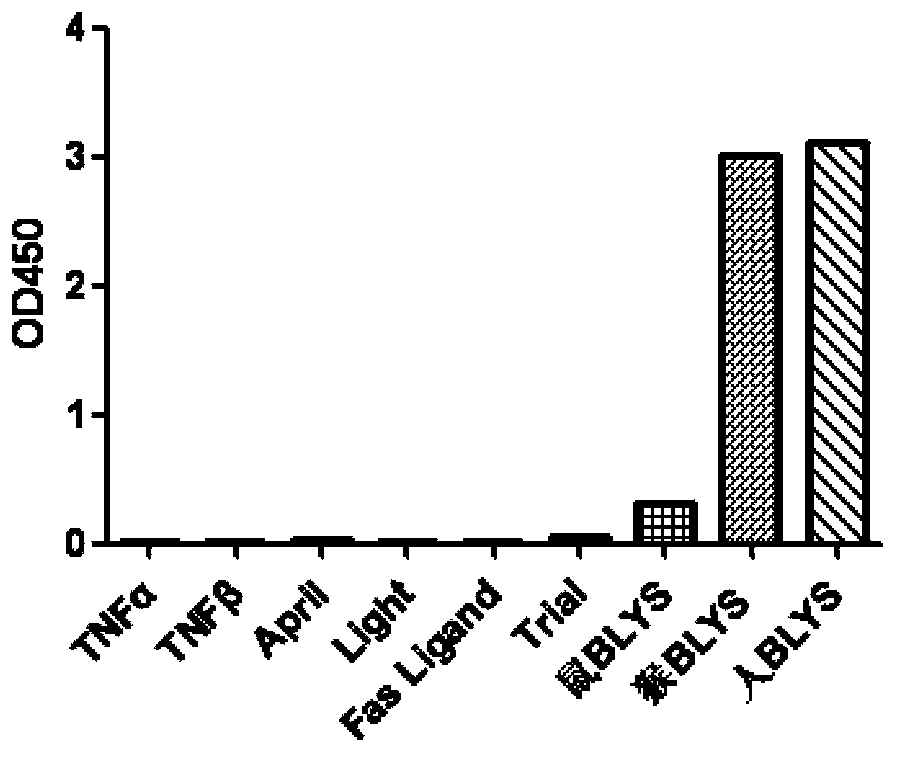
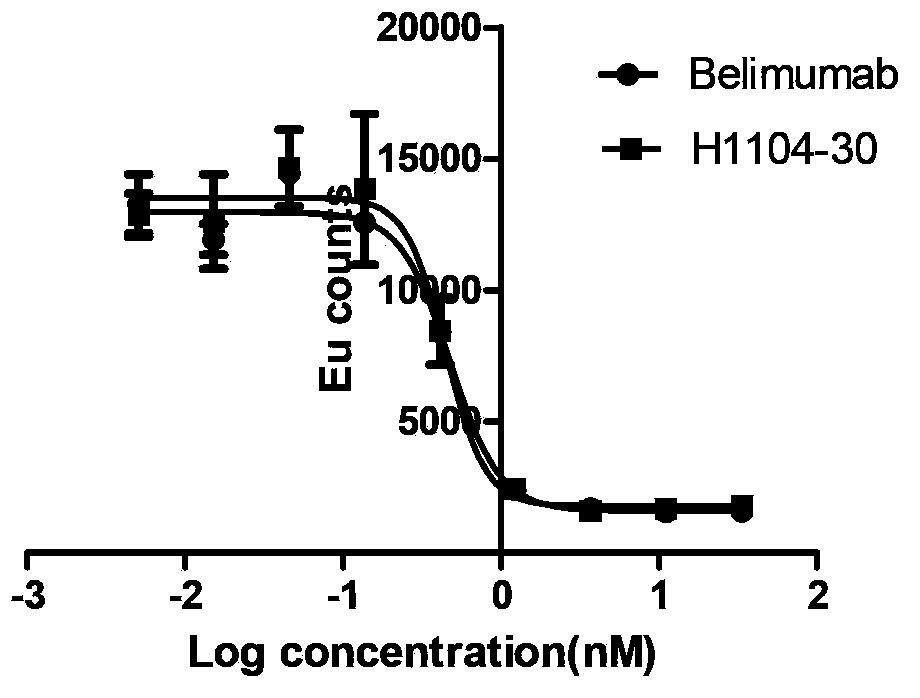
A genetic engineering antibody combined with a specificity of B lymphocyte stimulator (Blys), a pharmaceutical composition containing the antibody, and a kit. A heavy chain variable region of the genetic engineering antibody contains amino acid sequences shown by SEQ ID NO. 1, SEQ ID NO. 2, and SEQ ID NO. 3, or a light chain variable region of the genetic engineering antibody contains amino acid sequences shown by SEQ ID NO. 4, SEQ ID NO. 5, and SEQ ID NO. 6. The present invention further provides an anti-Blys monoclonal antibody, generated by a cell line with a preservation number being CGMCC No.7352.
Owner:JIANGSU NOVOMAB BIOPHARM INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com