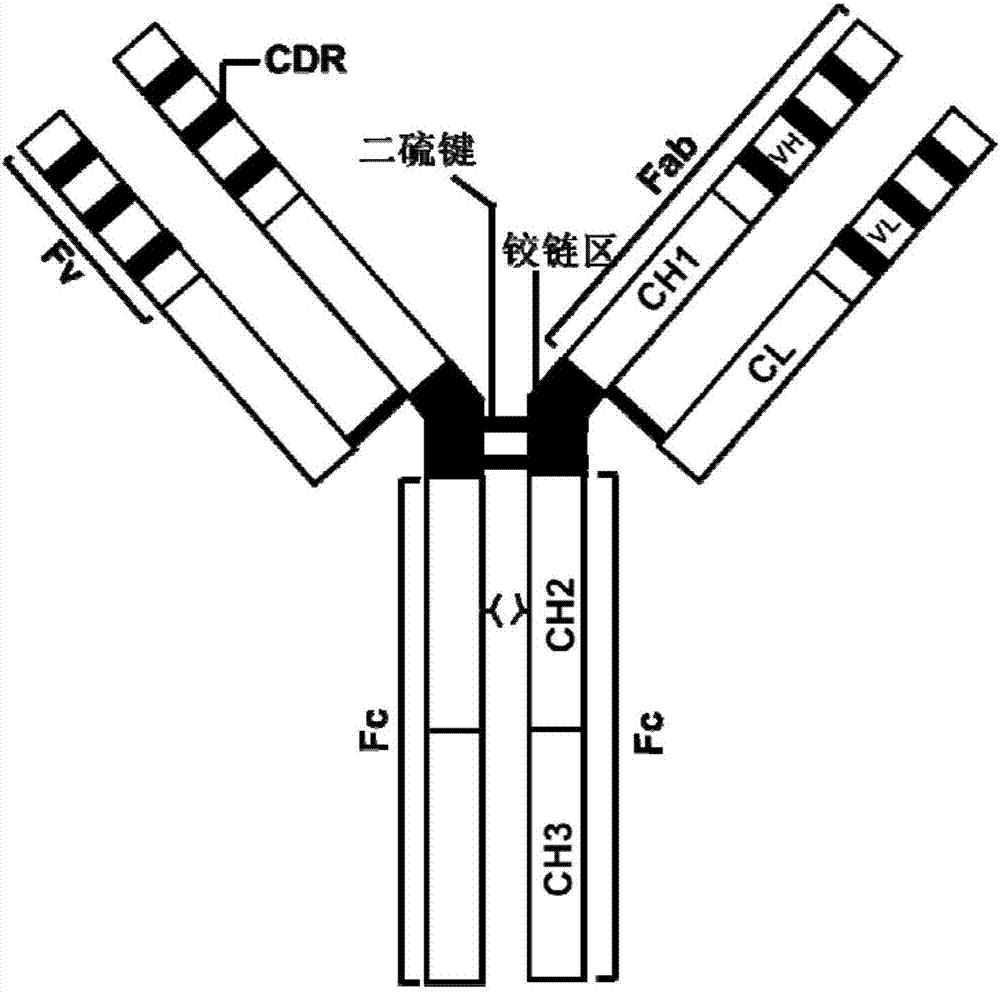
An antibody heavy chain constant region sequence enhancing activity of an agonistic antibody
A heavy chain constant region and constant region technology, which is applied in the direction of antibodies, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody medical components, etc., can solve the problems that antibody research and development has not been successful, and achieve good development Prospect, good safety profile, enhanced activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1. The heavy chain constant region of the present invention and the agonistic antibody comprising the sequence of the heavy chain constant region of the present invention
[0138] Through the above-mentioned gene cloning and expression purification techniques, the present invention has constructed a series of specific examples of different heavy chain constant regions, and constructed anti-human CD40 or anti-mouse CD40 antibodies based on these heavy chain constant regions and antibodies against mouse DR5.
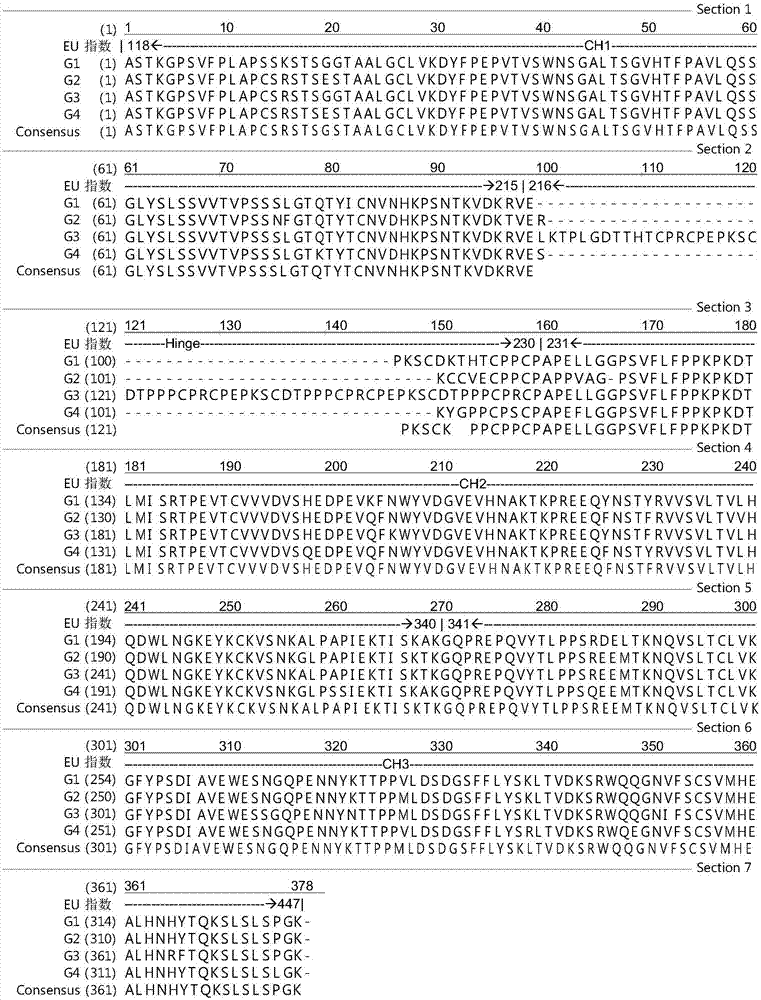
[0139] In general, in a specific embodiment of the present invention, the heavy chain constant region is divided into two parts for research and optimization, including the CH1-hinge region part and the CH2-CH3 domain part. The CH1-hinge region part means a segment consisting of an antibody CH1 domain and a hinge region, and the CH2-CH3 domain part means a segment consisting of an antibody CH2 domain and a CH3 domain. In a specific embodiment, the CH1-hinge...
Embodiment 2
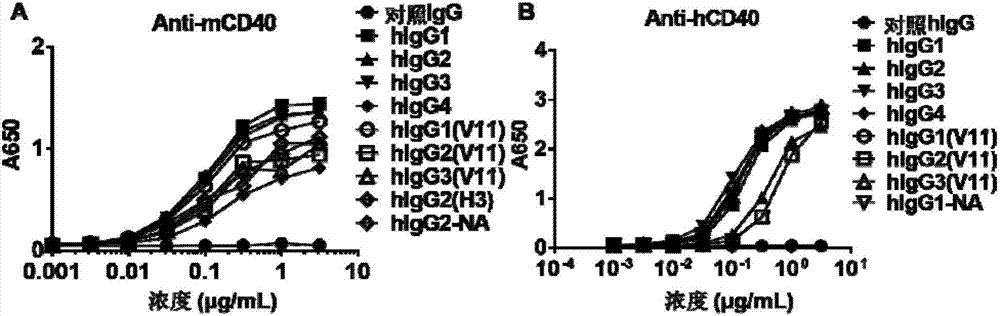
[0156] Example 2. The activity of human IgG agonistic antibodies is dependent on the interaction of antibody Fc with Fcγ receptors
[0157] In order to investigate whether the activity of human IgG agonistic antibodies is regulated by the interaction between antibody Fc and Fcγ receptors, agonistic anti-mouse CD40 antibodies (Anti-mCD40-hIgG1, Anti-mCD40-hIgG2, Anti-mCD40-hIgG3, Anti-mCD40-hIgG4) were first used as model antibodies to study whether their immune activation activity was affected by the expression of Fcγ receptors.
[0158] Since agonistic CD40 has immune activation activity that promotes the activation of antigen-presenting cells and induces the activation and expansion of antigen-specific CD8-positive T cells, agonistic human anti-mouse CD40 antibody (Anti-mCD40-hIgG) promotes OVA pattern antigen-specific CD8 The ability to activate and expand positive T cells in OVA-immunized mice was used to evaluate their immune activation activity (Science. 2011A μg 19; 333...
Embodiment 3
[0161] Example 3. Specific interaction of human inhibitory Fcγ receptor (hFcγRIIB) with antibody Fc promotes activity of agonistic human anti-CD40 antibody.
[0162] To analyze the specific Fcγ receptors in the Fcγ receptor family that specifically provide human IgG agonistic antibody activity-dependent Fc-Fcγ receptor interactions, the contribution of the human inhibitory Fcγ receptor hFcγRIIB was further analyzed. Inhibitory Fcγ receptor humanized mice (Fcgr2b - / - hFCGR2B Tg ) and control mice (Fcgr2b - / - ), Anti-mCD40-hIgG2 antibody in Fcgr2b - / - No activity in mice ( Figure 4 C), while in inhibitory Fcγ receptor humanized mice (Fcgr2b - / - hFCGR2B Tg ) in the activity (approximately nearly 80% OT-I CD8 + , Figure 4 C) and in humanized mice expressing all human Fcγ receptors (hFCGR Tg ) Medium activity (approximately nearly 60-80% OT-I CD8 + , Figure 4 A) Comparable, indicating that human inhibitory Fcγ receptors are sufficient to provide support for Anti-mCD40-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com