Mild catalytic steam gasification process
a gasification process and steam technology, applied in the field of low temperature catalytic gasification of carbonaceous materials, can solve the problems of coal gasification systems and designs, non-catalytic, and high cost of process operation, and achieve the effect of little additional treatment and greater carbon conversion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Low Temperature Steam Gasification Results
[0052] Steam gasification of Illinois #6 coal was studied at elevated pressures and low temperatures. In the absence of a catalyst at 500° C. and elevated pressure (500-1000 psig—i.e. ˜34 to 68 atm), no coal conversion was observed. When the temperature was increased to 700° C., a significant amount of conversion was observed. Apparently the lower temperature is insufficient to overcome the activation energy barrier. Gas analyses at 700° C. showed no or substantially no methane formation for de-mineralized coal samples. A small amount of methane was detected for the raw coal gasification. These observations are in agreement with the findings that significant amounts of methane cannot be generated in the absence of catalysts, and that minerals in coal can contribute to catalysis.
[0053] The catalytic effects of iron, nickel and potassium in steam gasification were also studied. In the presence of these catalysts a substantial amount of Illin...
example 2
MCCG in Accordance with an Embodiment of the Invention
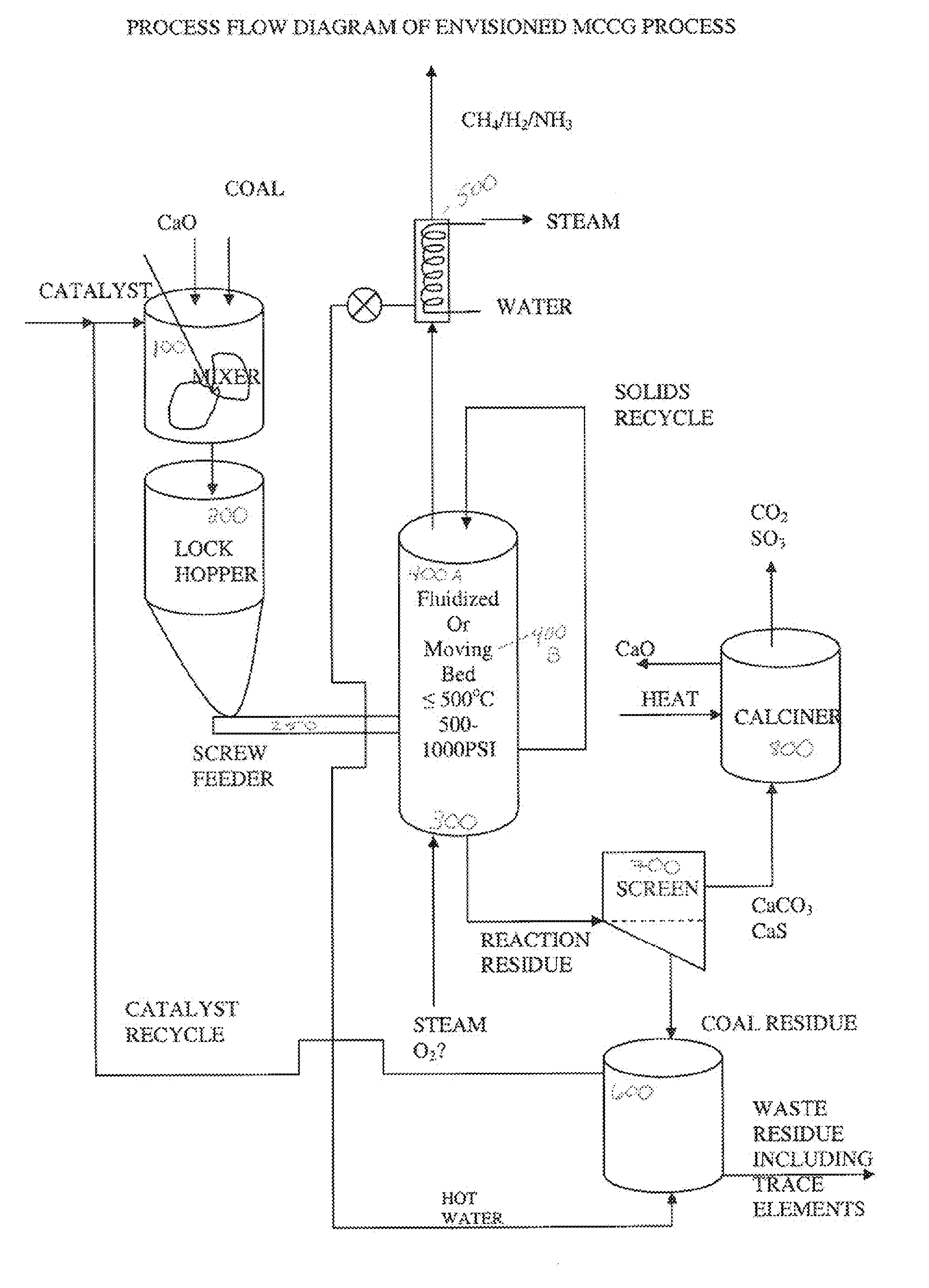
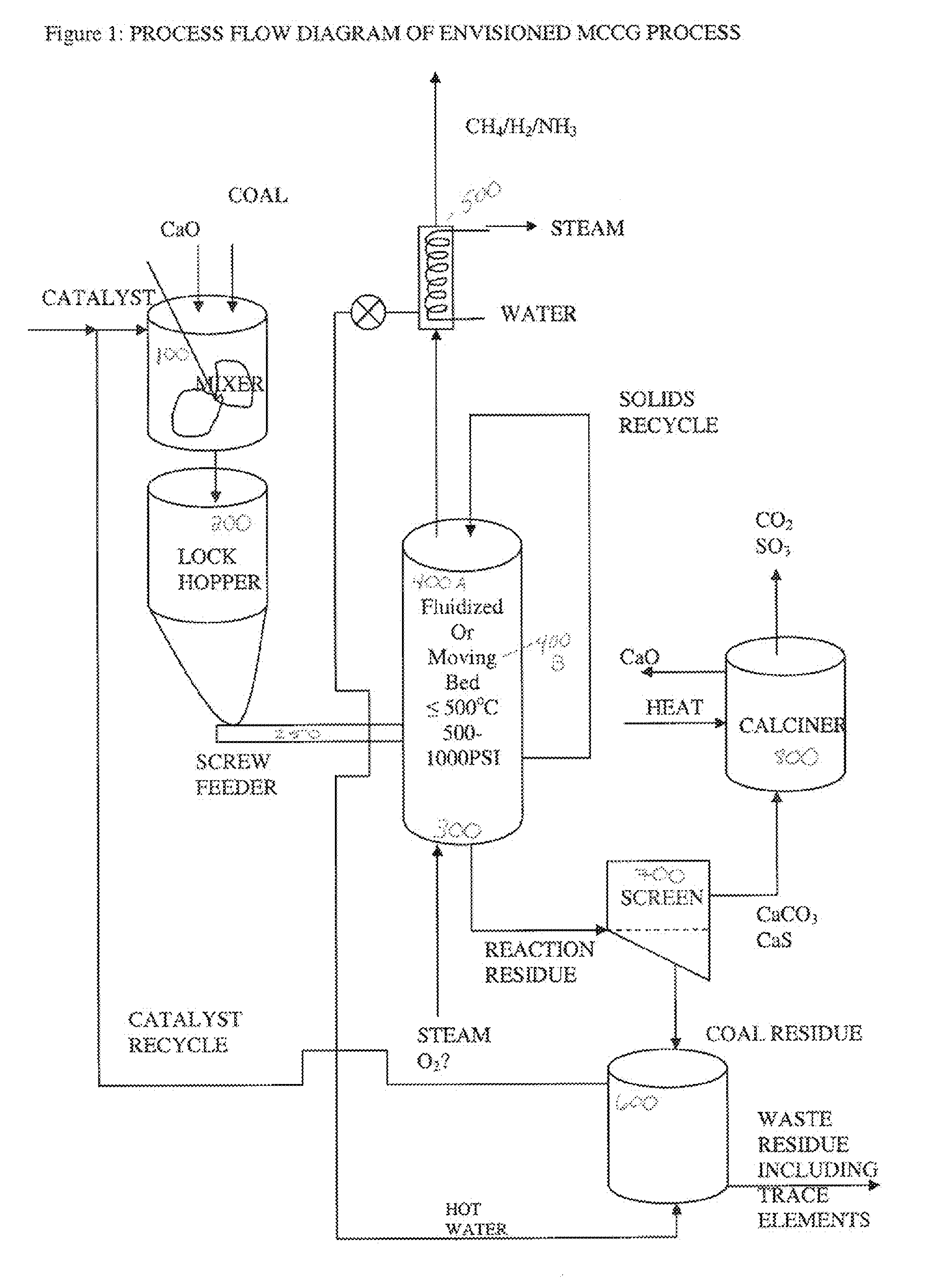
[0057] A process flow diagram for the envisioned low temperature steam gasification process, mild catalytic coal gasification (MCCG), is shown in FIG. 1. Among the advantages for this process is, as discussed above, that it is a simple process. Particulate coal or other carbonaceous material, particles of CO2 trap material and / or mineral binder material, and an alkali metal catalyst solution, can be combined and mixed in mixer 100 to form a feed stream and fed to one or more lock hoppers shown generally as lock hopper 200. Said particulate streams can be fed separately to mixer 100 or combined (not shown) before being fed to mixer 100. From lock hopper 200, the feed stream can be fed to gasifier 300 by a screw feeder 250, which alternatively can be a star feeder, or a mechanism that feeds the carbonaceous material as a liquid slurry, or any other feed mechanism known in the art which allows carbonaceous material to be fed to a g...
example 3
MCCG in Accordance with Another Embodiment
[0063] In other particular embodiments, coal or other carbonaceous material; a CO2 trap material such as CaO or Ca(OH)2 particles; and an alkali metal catalyst solution, are mixed in mixer 100, fed to lock hopper 200, and fed to gasifier 300 as described above. Mixer 100 can comprise an impeller and means to heat the contents such that the carbonaceous particles can become impregnated with alkali catalyst therein.
[0064] Gasifier 300 can be operated in a fluid bed 400A or a moving bed 400B mode, as described, and is operated at a temperature between about 300° C. about 700° C. and a pressure from about 12 to about 40 atm. As described in Example 2, CaO or Ca(OH)2 can be used as a trap for CO2 and sulfur gases, and CaO, Ca(OH)2, CaCO3, or other alkaline earth metal salts can react with alumina, silica, or other mineral constituents of the coal.
[0065] The remainder of the process follows that described for Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com