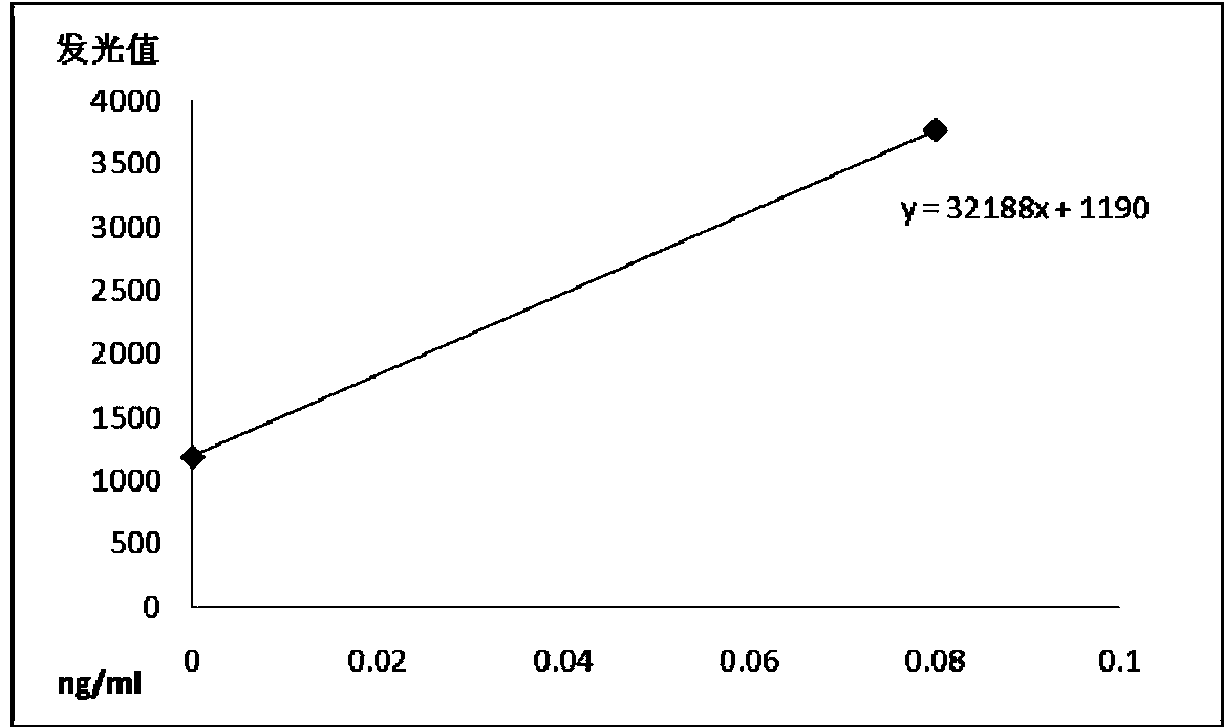
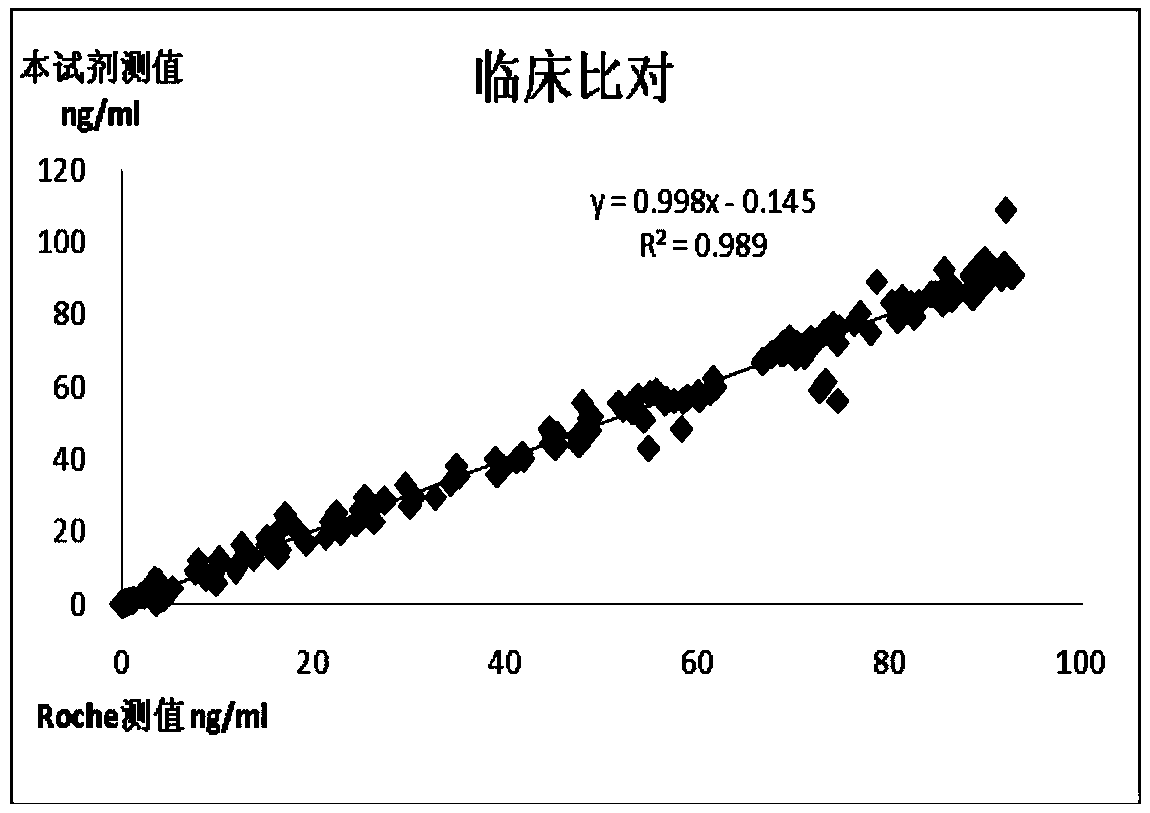
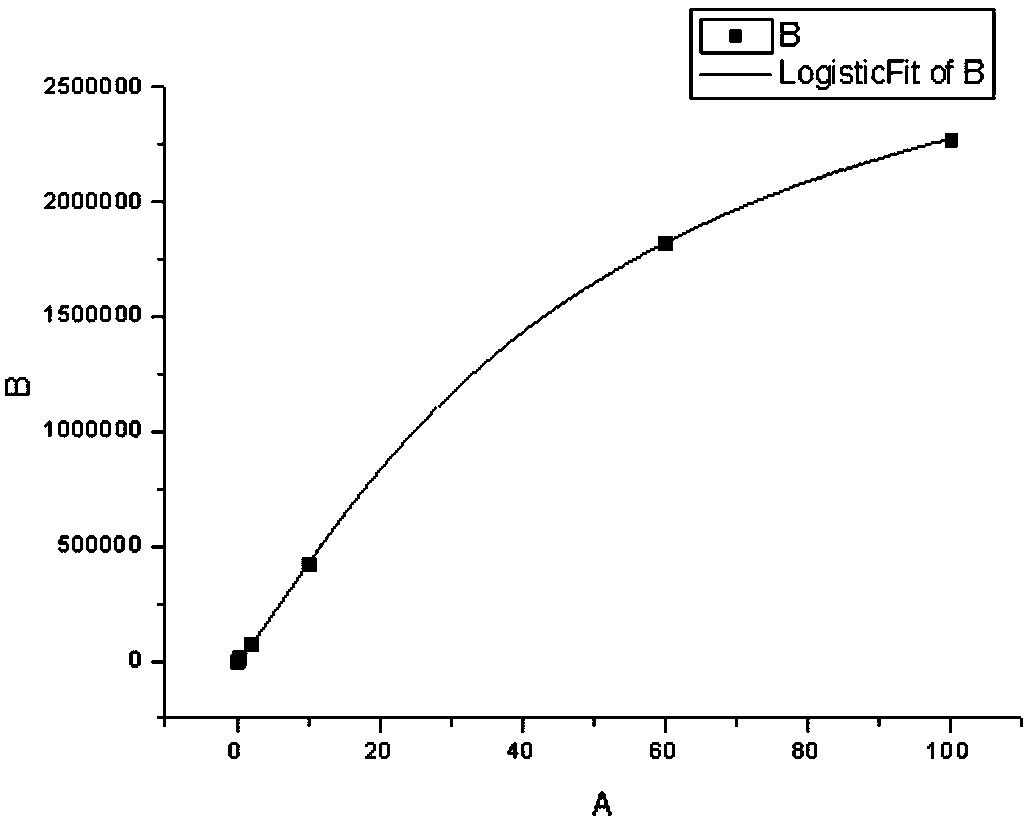
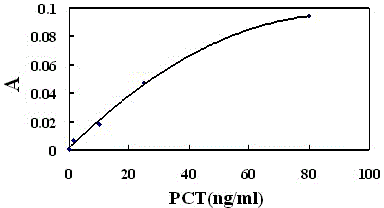
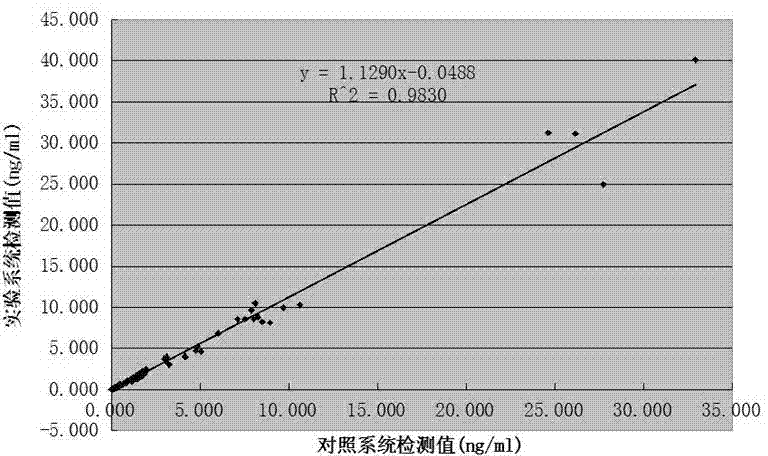
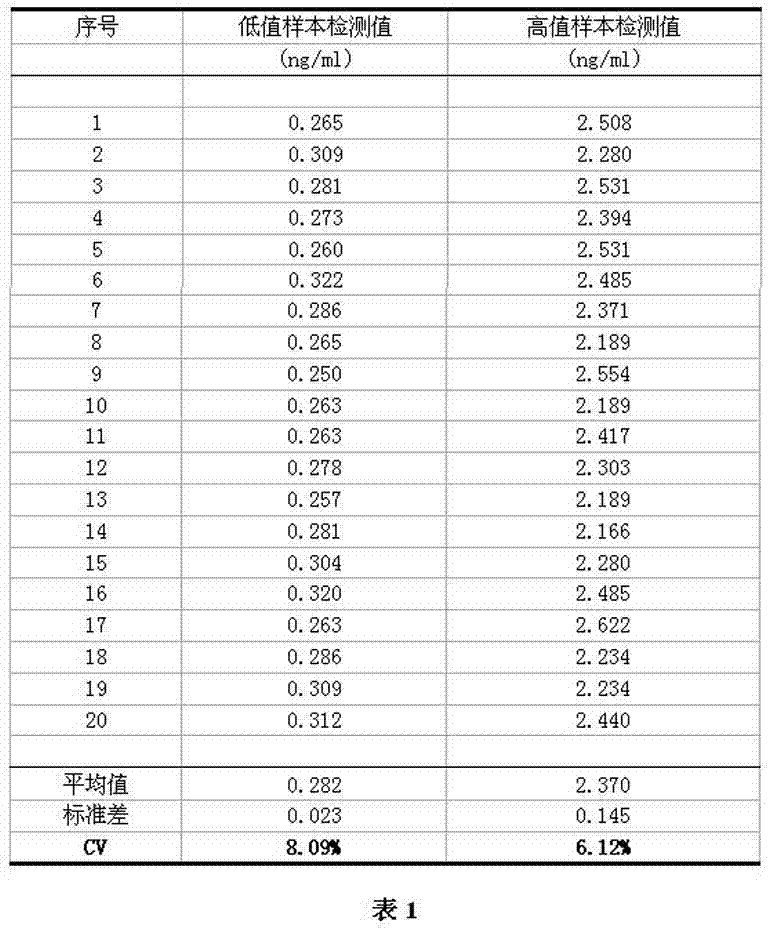
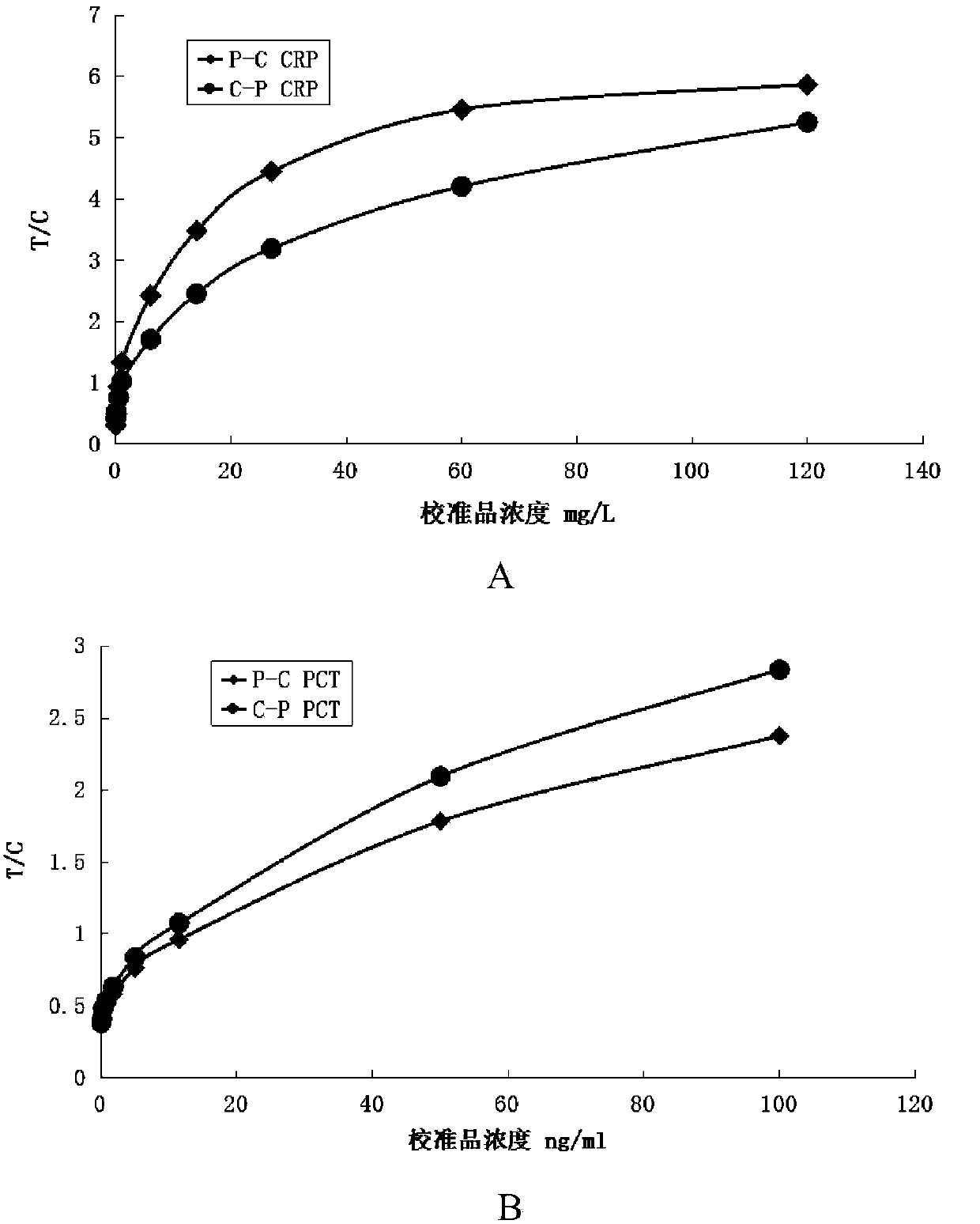
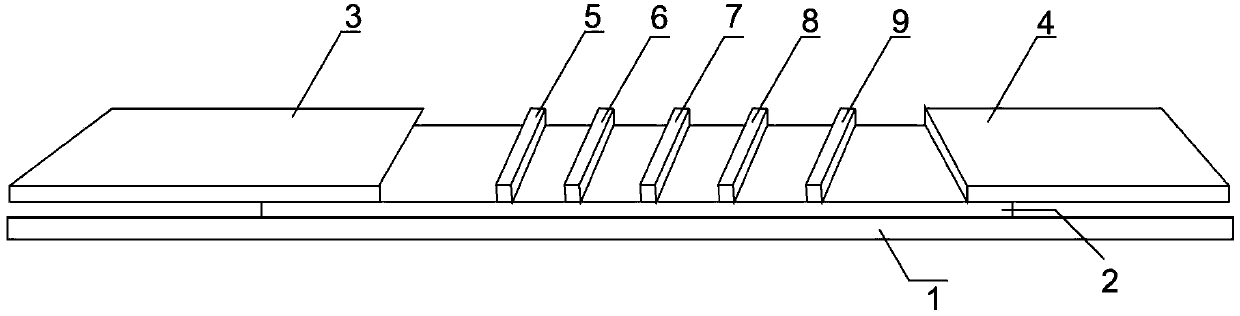
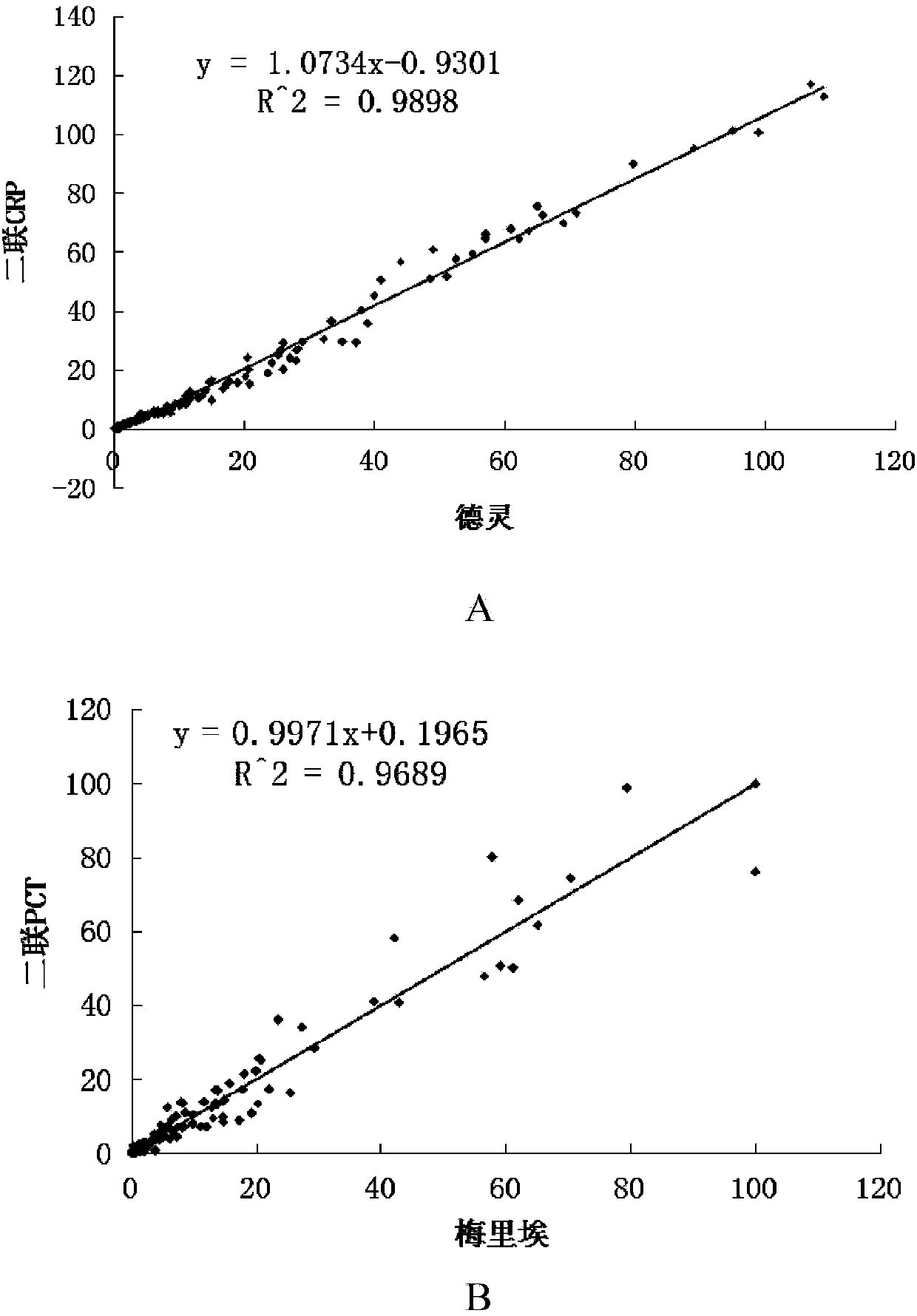
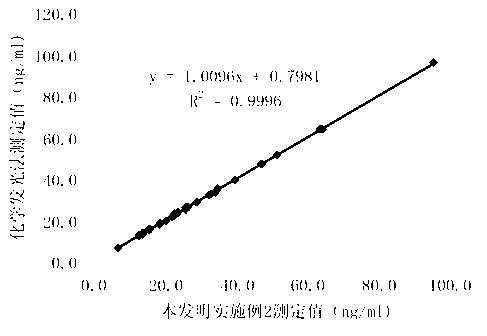
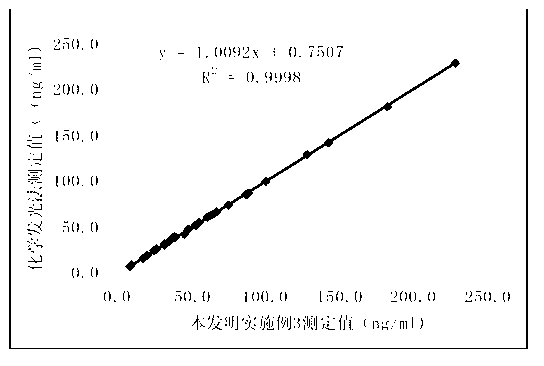
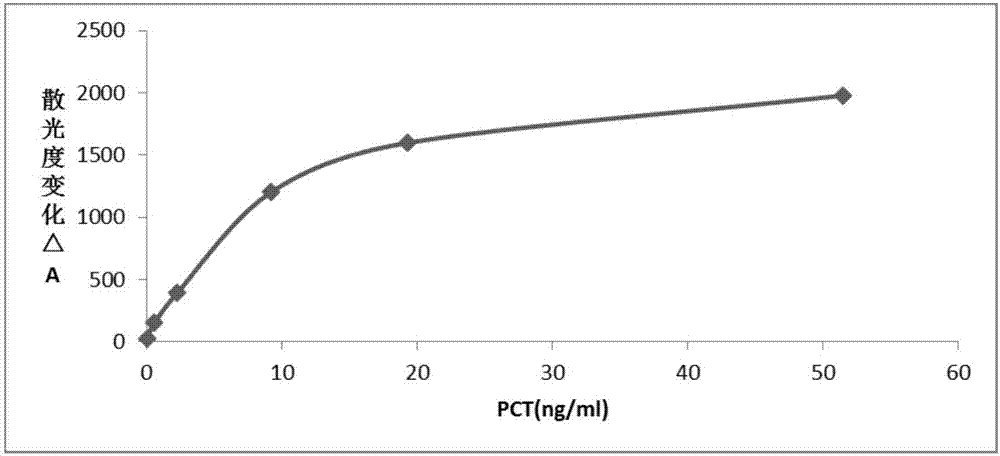
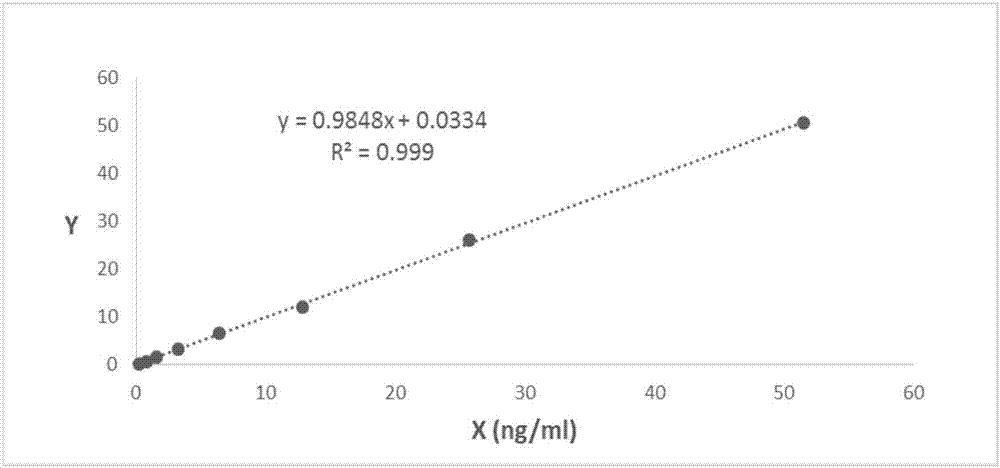
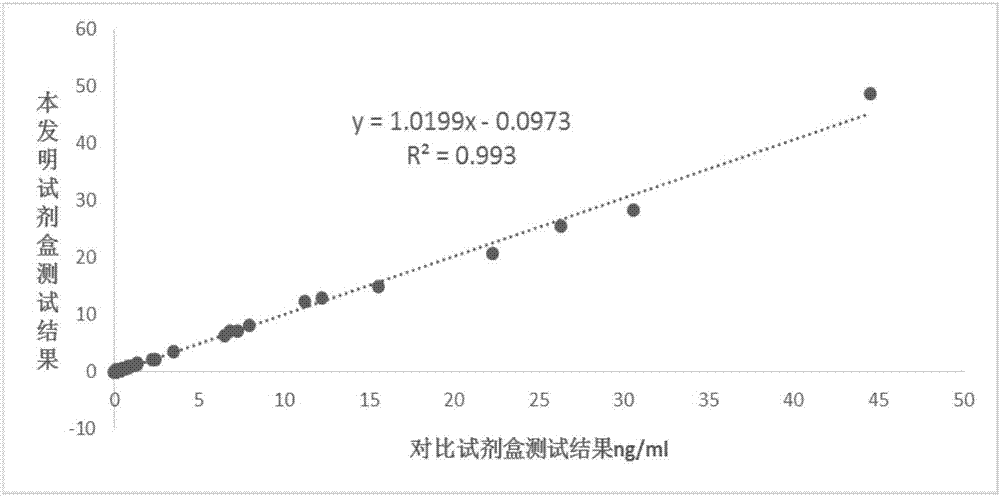
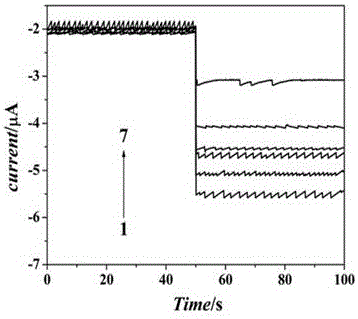
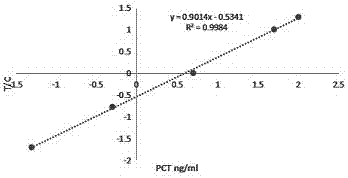
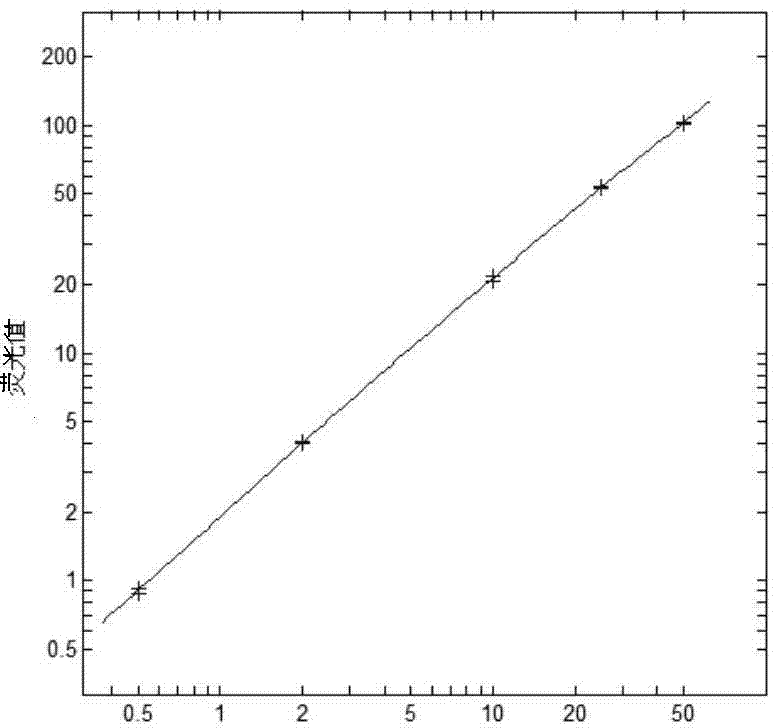
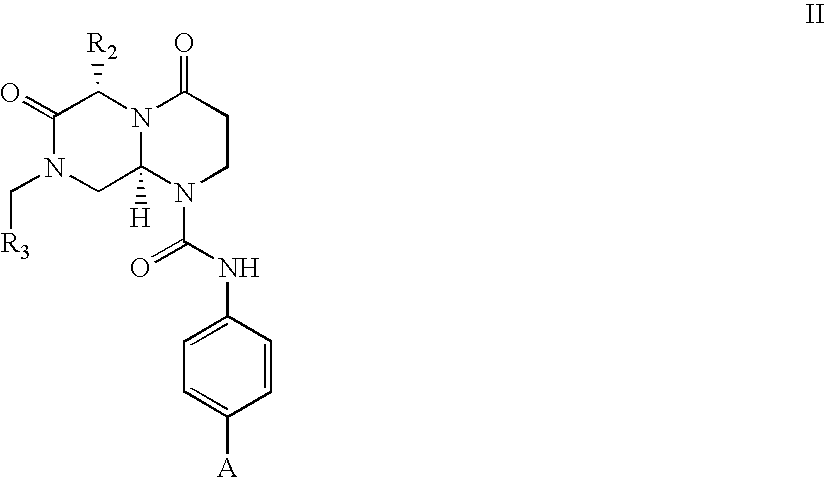
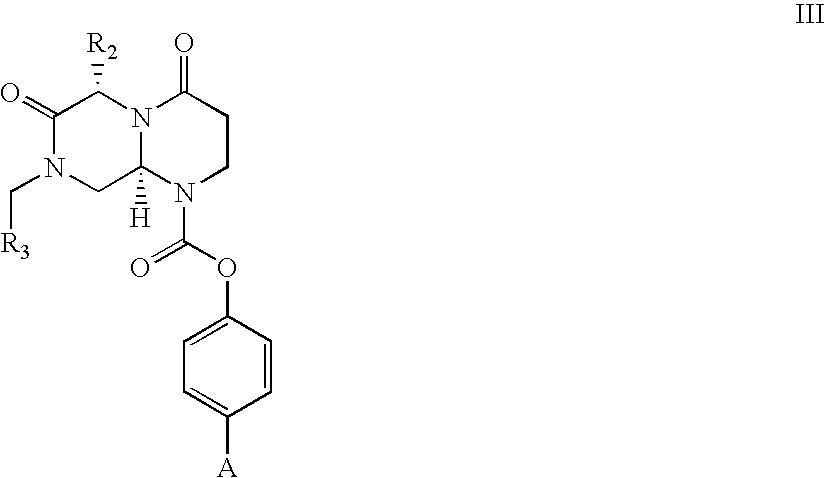
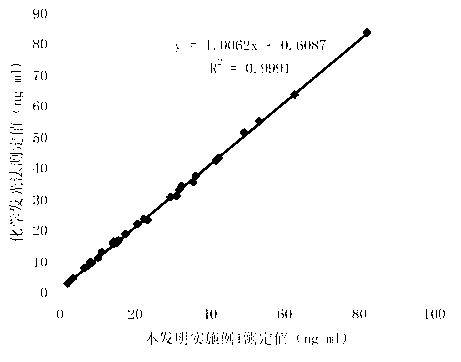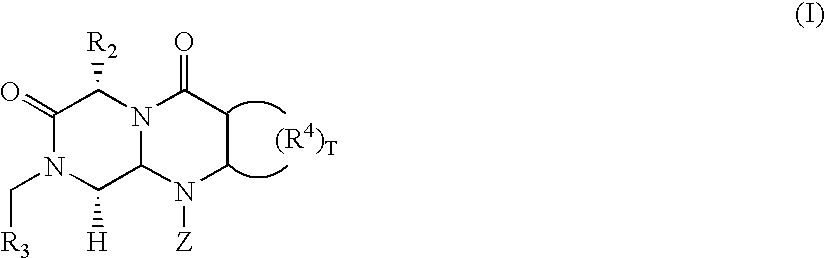Patents
Literature
190 results about "Procalcitonin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
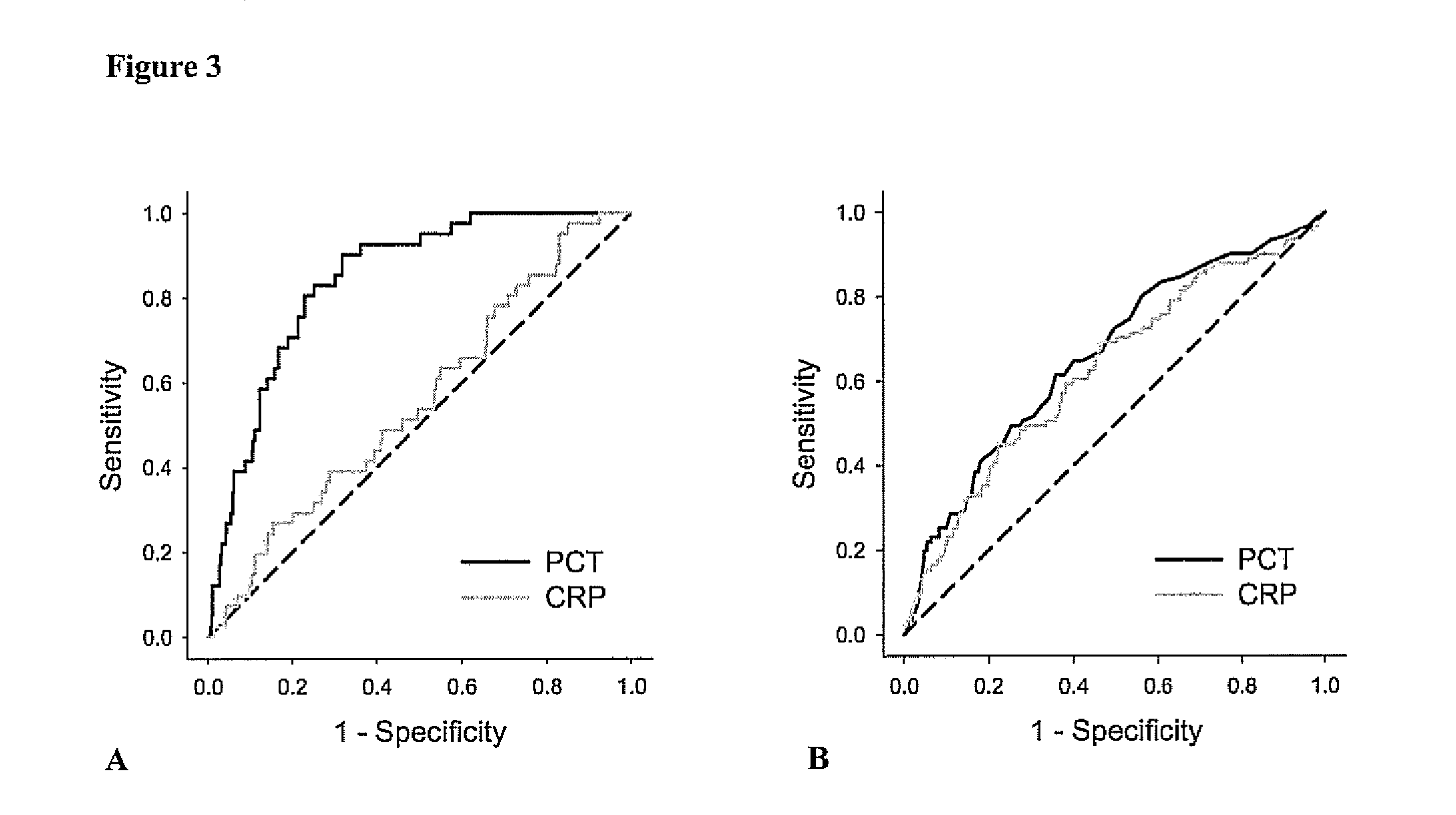
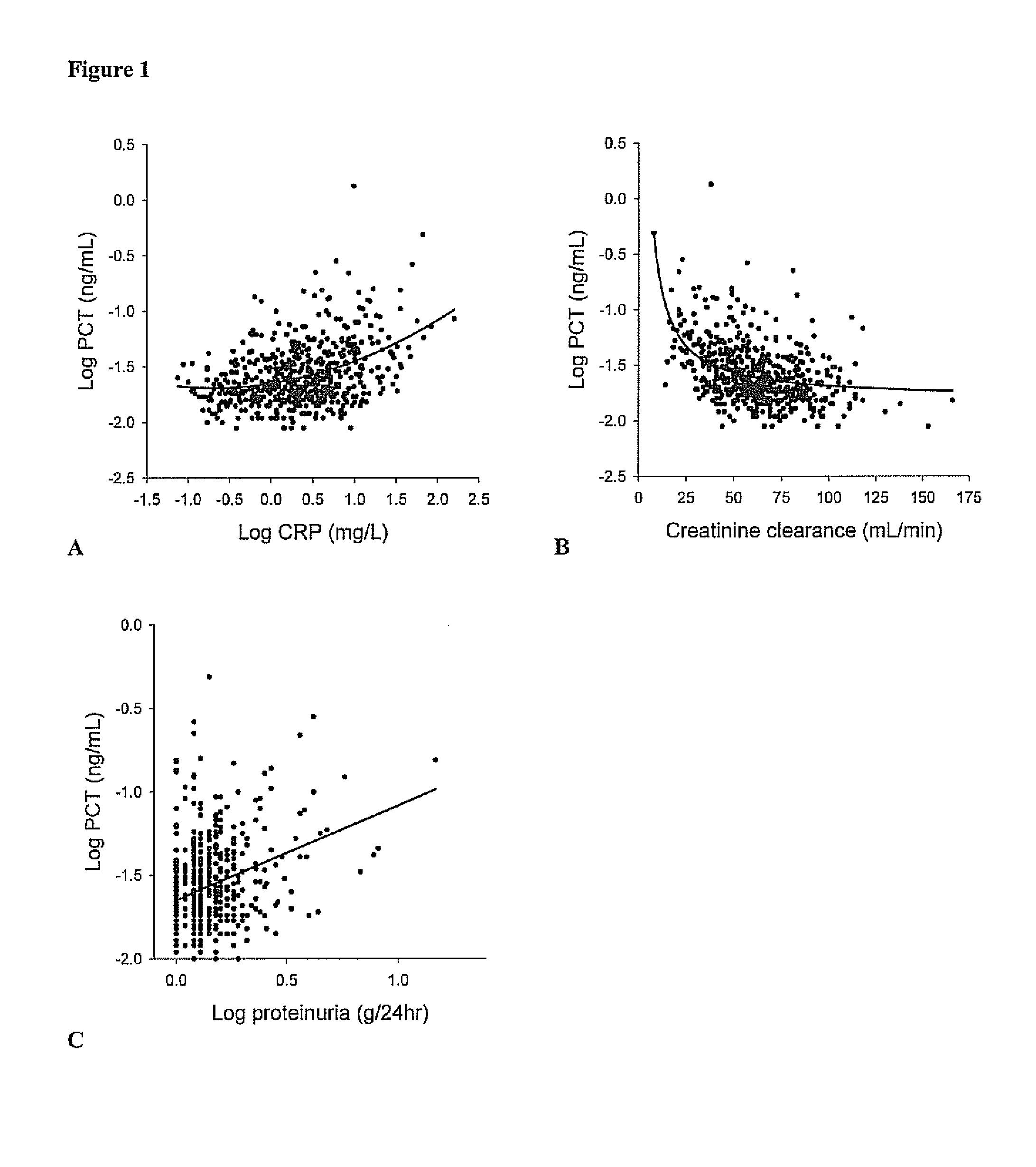
Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by endopeptidase. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s. It is composed of 116 amino acids and is produced by parafollicular cells (C cells) of the thyroid and by the neuroendocrine cells of the lung and the intestine.
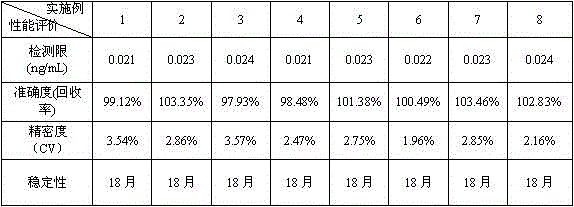
Chemiluminescence quantitative detection kit for procalcitonin, and preparation method and detection method thereof
ActiveCN103901203ALow cross-reactivityImprove accuracyChemiluminescene/bioluminescenceBiotin-streptavidin complexN-Hydroxysuccinimide
The invention relates to a chemiluminescence quantitative detection kit for procalcitonin, and a preparation method and detection method thereof. The kit comprises a procalcitonin series standard substance, a magnetic separation reagent (magnetic particle suspension coupled with streptavidin), a first reagent (anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label) and a second reagent (anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the magnetic particle suspension coupled with streptavidin, anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label and anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label is up to 0.008ng / ml; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
Procalcitonin latex enhanced immunoturbidimetry detection kit
The invention discloses a procalcitonin (PCT) latex enhanced immunoturbidimetry detection kit and a preparation method thereof. The procalcitonin latex enhanced immunoturbidimetry detection kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 mainly comprises a buffer solution 1, a stabilizing agent 1, a preservative 1, a coagulation increasing agent, a protective agent 1 and EDTA (Ethylene Diamine Tetraacetic Acid); the reagent R2 mainly comprises a buffer solution 2, a stabilizing agent 2, a preservative 2, a polystyrene latex microballon sphere, a PCT antibody and a protective agent 2; the particle size of the polystyrene latex microballon sphere in the reagent R2 is 100-600nm, the PCT antibody is one or more of a mouse anti-human PCT antibody, a goat anti-human procalcitonin antibody or a rabbit anti-human procalcitonin antibody, and the PCT antibody is connected with the polystyrene latex microballon sphere in a covalent coupling or physical absorption manner. Compared with the prior art, the PCT detection kit provided by the invention has the advantages of low preparation cost, good stability, high detection sensitivity and strong specificity, is easy to store, and is easily popularized in clinical.
Owner:CHONGQING ZHONGYUAN BIOLOGICAL TECH
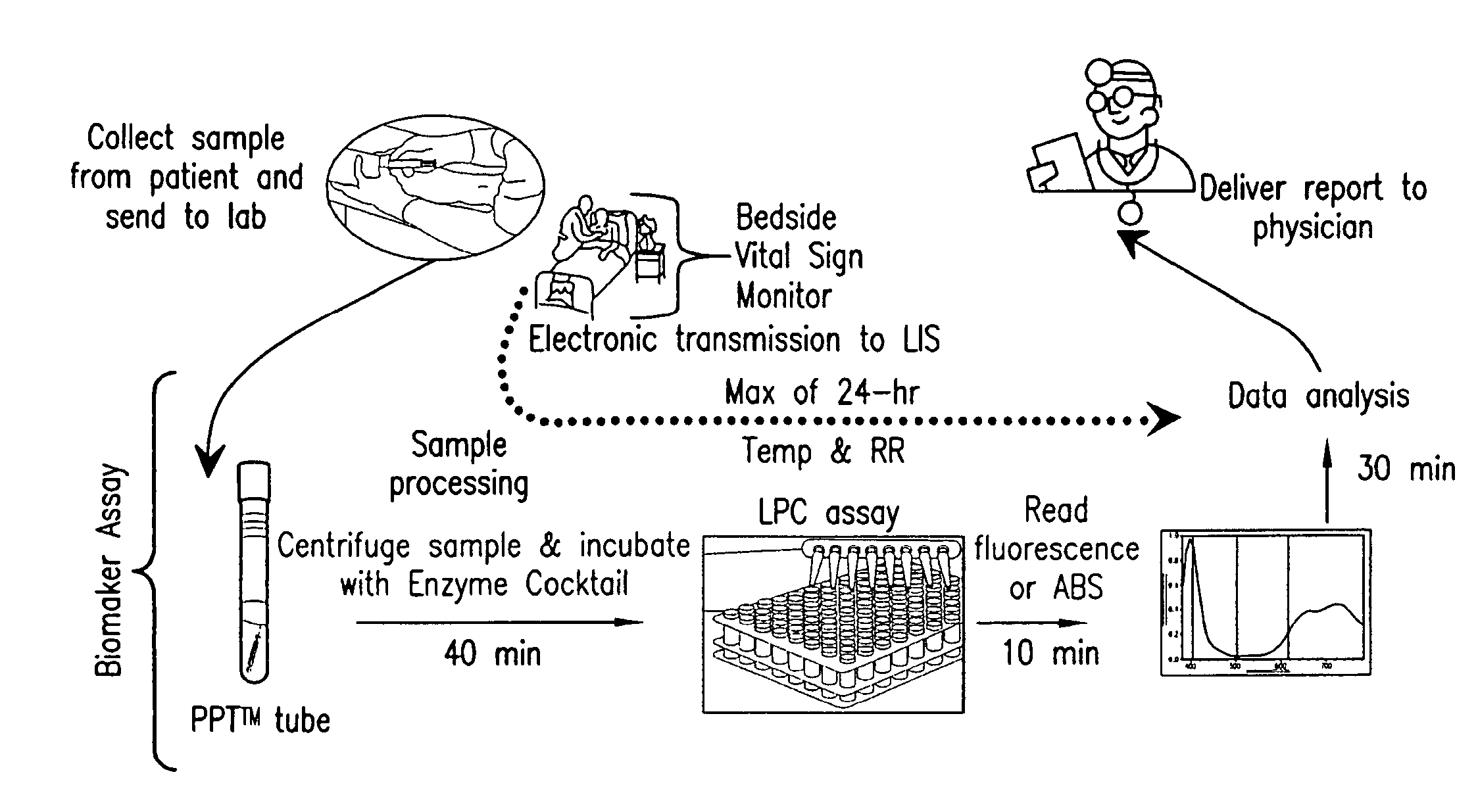
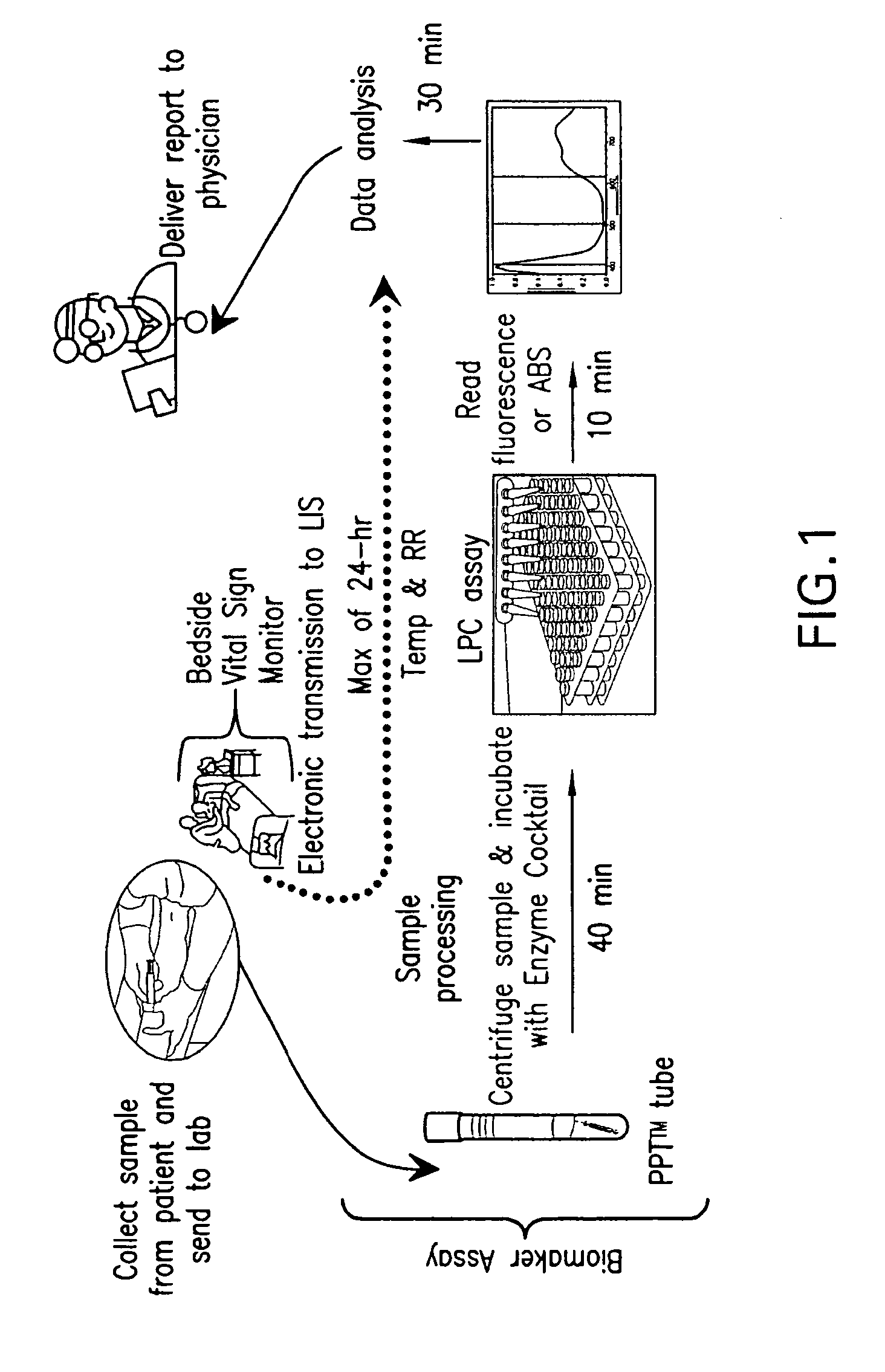
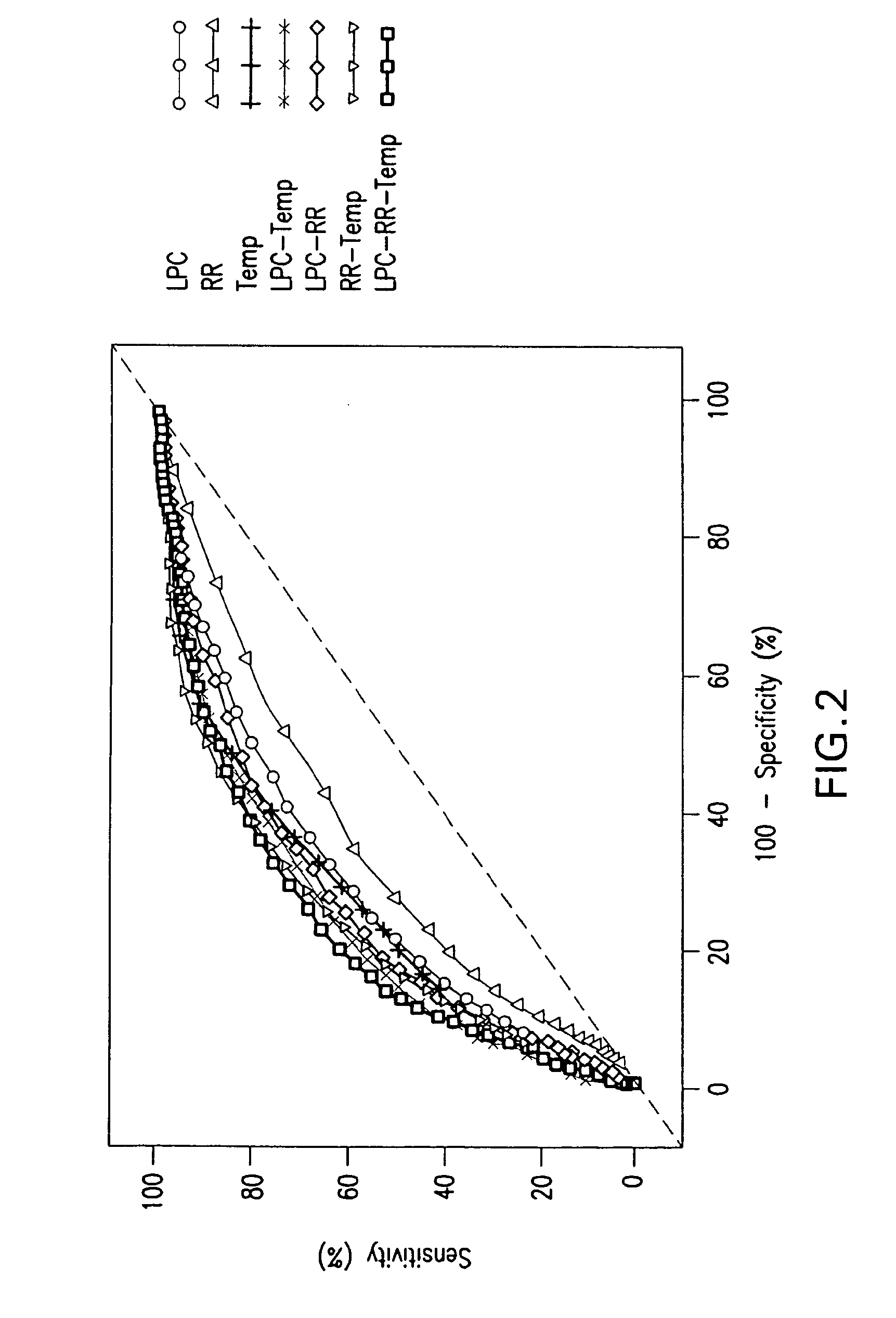
Advanced Detection of Sepsis
ActiveUS20110118569A1Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsPhospholipinClinical marker
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
Biomarkers and methods for diagnosing, predicting and/or prognosing sepsis and uses thereof
InactiveUS20100292131A1Peptide librariesPeptide/protein ingredientsHealthy subjectsBiomarker (petroleum)
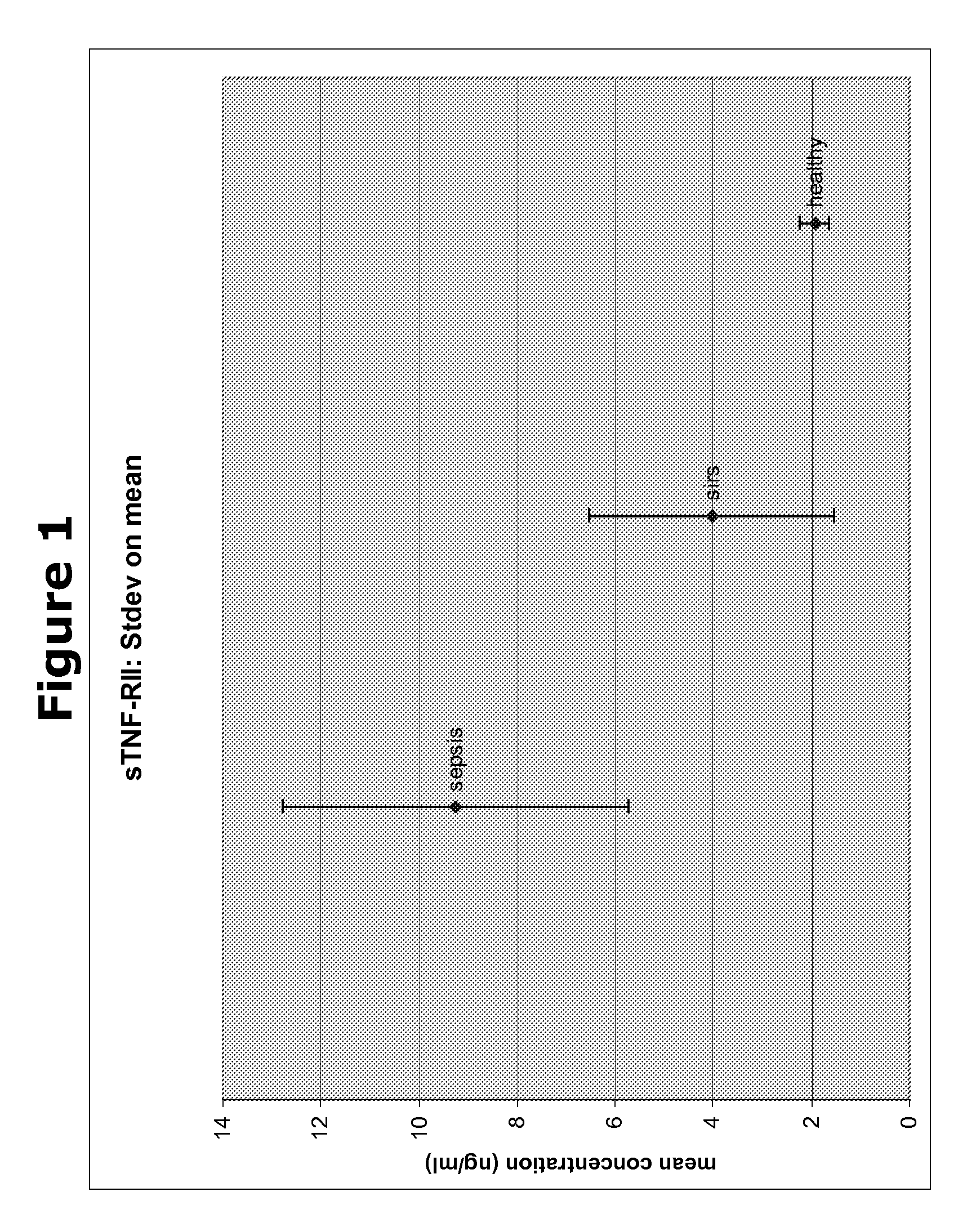
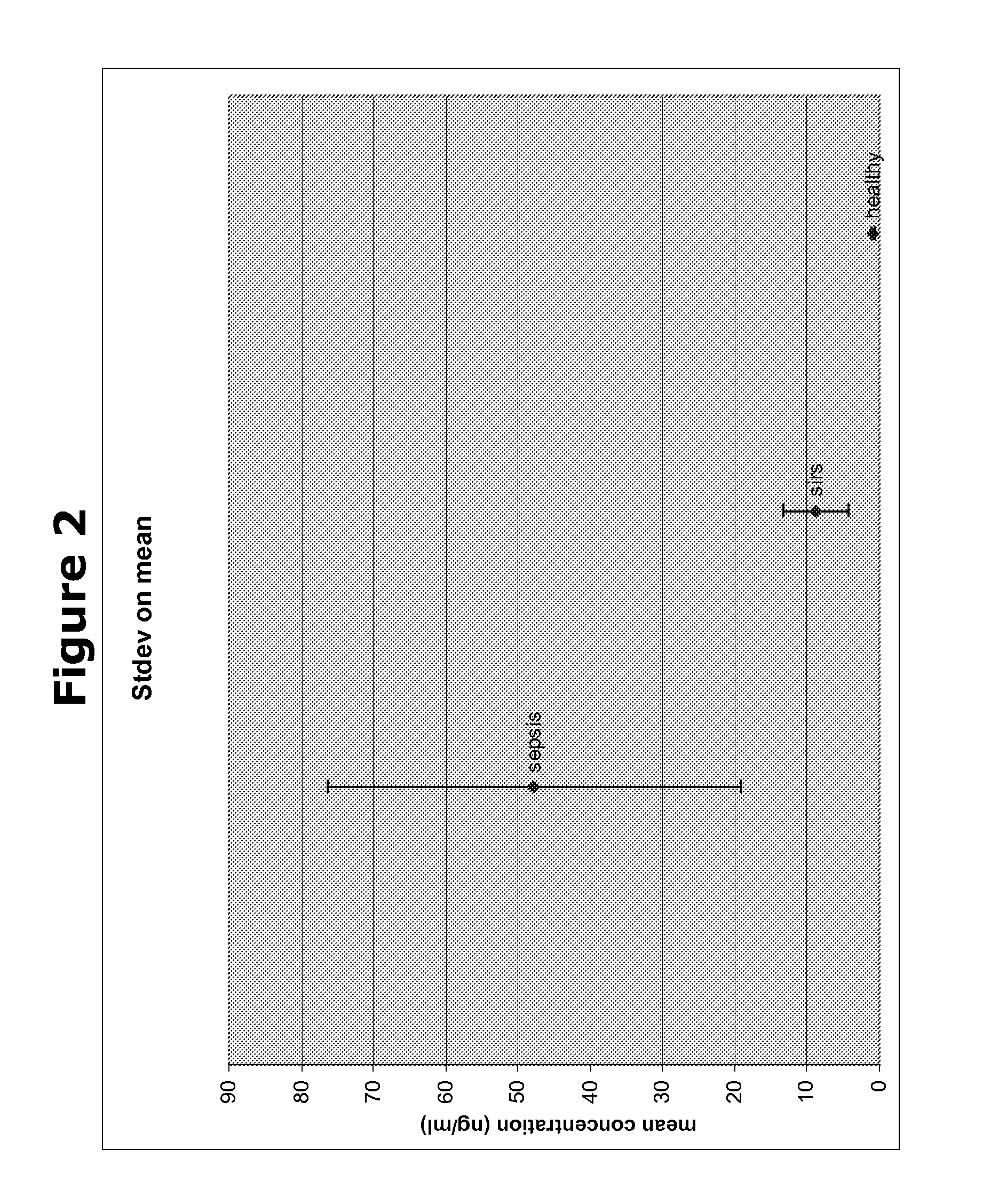
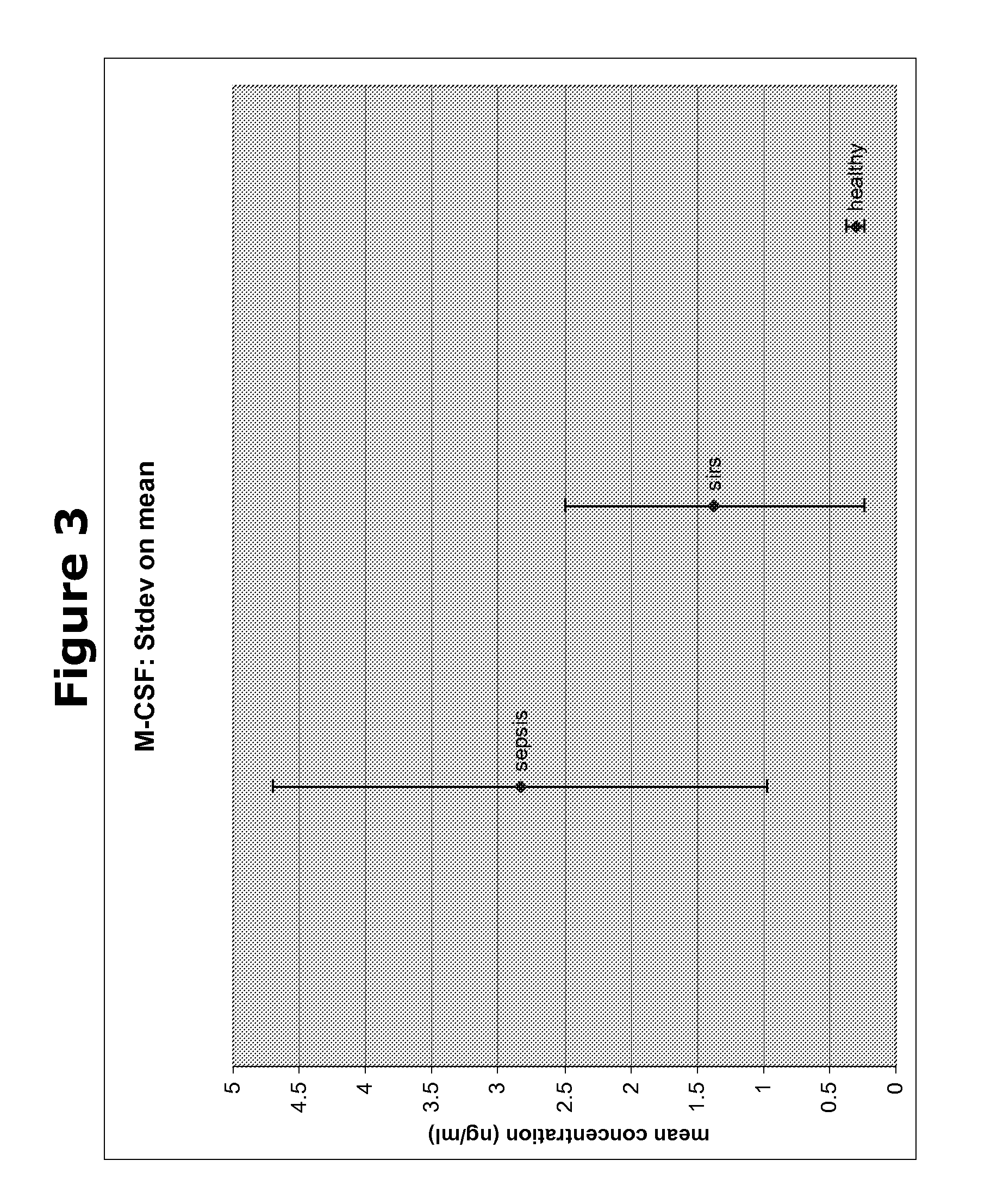
The present invention provides kits and methods for the diagnosis, prognosis and prediction of sepsis in a subject or for the differentiation between sepsis and SIRS in a subject, the method comprising(a) measuring the level of pro-hepcidin (pro-HEPC) in a biological sample taken from said subject, (b) measuring the level of at least one further biomarker selected from the group consisting of soluble TNF-receptor 2 (sTNFR2), Pentraxin-3 (PTX-3), Macrophage Colony-Stimulating Factor (MCSF), pro-Brain Natriuretic Protein (pro-BNP), one or more members of the Histone protein family, Procalcitonin (PCT) and c-Reactive Protein (CRP) in a biological sample from said subject, (c) using said measurements obtained in steps (a) and (b) to create a profile for said biomarkers and (d) comparing said profile with a reference biomarker profile obtained form a patient having SIRS or from a healthy subject.
Owner:BIOCARTIS NV
Procalcitonin detection kit and detection method
ActiveCN104714033ARapid responseSuitable for detectionImmunoglobulins against hormonesBiological testingAntigen epitopeAntigen
The invention relates to the field of fluorescence immunochromatography technique in medical immunology, specifically to a procalcitonin detection kit and a procalcitonin detection method. The detection kit is provided with a test cassette and is characterized in that the test cassette is successively provided with, from bottom to top, a PVC plate, a sample pad, a combination pad, a cellulose nitrate film and a water-absorbing pad, wherein a procalcitonin monoclonal antibody labeled by a rare earth fluorescent microsphere is adsorbed on the combination pad, the rare earth fluorescent microsphere has a diameter of 60 to 120 nm, is doped by rare earth lanthanide, is stable in a ground state and emits fluorescent light with a wavelength in a range of 540 to 600 nm under the action of an excitation light source in a wavelength range of 340 to 380 nm, and the monoclonal antibody is a purified mixed monoclonal antibody and is originated from monoclonal antibody cell strains directed at 2 to 6 different procalcitonin antigen epitopes. The procalcitonin detection kit has the advantages of simple operation, rapid reaction, high sensitivity, strong specificity, etc.
Owner:WEIHAI NEOPROBIO
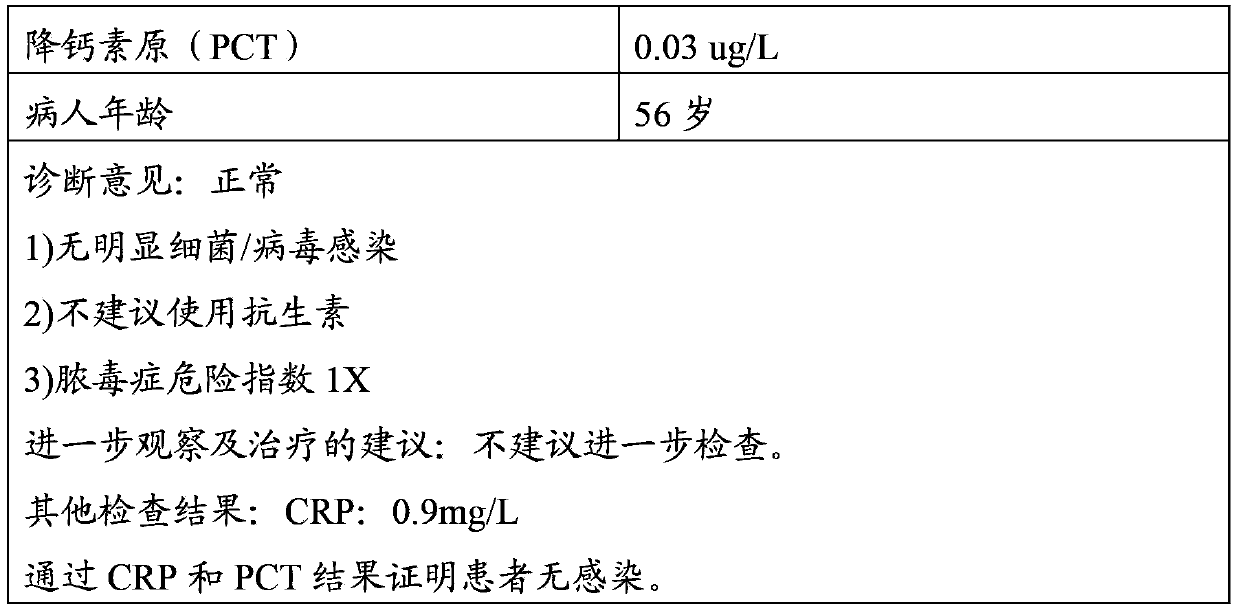
PCT (procalcitonin) and CRP quantitative joint inspection chromatography test strip and preparation method thereof
The invention discloses a PCT (procalcitonin) and CRP quantitative joint inspection chromatography test strip and preparation method thereof. The test strip comprises a bottom lining and a coating film on the bottom film, wherein the coating film is provided with a sample pad, a coating line region and a water-absorbing paper, the coating line region comprises a labeled line for coating a fluorescence latex labeled antibody, a quality control line of coating chicken IgY antibody, a CRP detection line for coating another CRP monoclonal antibody capable of being combined with a to-be-detected antigen CRP specificity, and a PCT detection line capable of coating another PCT monoclonal antibody capable of being combined with to-be-detected antigen PCT specificity, which are arranged in parallel from the part close to a sample pad end to the part close to a water-absorbing paper end. The test strip disclosed by the invention simplifies the structure of the normal test strip, a combination pad is solidified by removing the fluorescence latex labeled antibody in the traditional test strip, the antibody is solidified on the coating film, the precision of the test is improved, and the test strip cost is reduced.
Owner:GUANGZHOU WONDFO BIOTECH
Reagent for determining procalcitonin and preparation method of reagent
ActiveCN103018464ASimple and fast operationRaw materials are easy to getMaterial analysis by observing effect on chemical indicatorBiological testingMicrosphereCalcitonin
The invention relates to a reagent for determining procalcitonin and a preparation method of the reagent, in order to provide the reagent which has the characteristic of high accuracy degree, and the provided preparation method has the characteristic of convenience in preparation. The technical scheme is that the reagent for determining procalcitonin comprises the following components: a. a procalcitonin reagent 1, b. a procalcitonin reagent 2, and c. a liquid-type procalcitonin reference calibration item. The preparation method of the reagent for determining procalcitonin comprises the following steps of: (1) uniformly mixing the procalcitonin reagent 1; (2) removing the procalcitonin reagent 2, the removing process specifically comprises the following steps of: a. taking a suspension, b. carrying out reaction on a mixture, c. acquiring a carboxylic latex microsphere suspension, d. regulating the concentration, e. taking the suspension in step (3), and adding into the step (4), f. reacting, g. adding cholamine, and h. carrying out centrifugal treatment; and (3) treating the liquid-type procalcitonin reference calibration product: mixing according to the amount of a formula, and ranking according to the content of the procalcitonin or adding a procalcitonin pure product in the mixed liquid.
Owner:YESEN BIOTECH SHANGHAI
Multi-antibody marked quick procalcitonin detecting kit
ActiveCN102928606AMaximum captureHigh detection sensitivityBiological testingTreatment effectCommon carrier
The invention relates to a multi-antibody marked quick procalcitonin detecting kit in the technical field of detection. The kit comprises a common carrier, a monoclonal or polyclonal capturing body which is fixed on the common carrier and corresponding to different procalcitonin areas, and a monoclonal or polyclonal capturing body which is compatible with the capturing body and is suitable for a plurality of same or different areas for the procalcitonin marked by a marker. According to the multi-antibody marked quick procalcitonin detecting kit, different distinguishing areas with multi-antibody pair coated are marked and the spaces are subjected to compatibility of medicines, the procalcitonin in a sample can be captured to the greatest extent, and the sensitivity in detection can be improved; the detection sensitivity of PCT (procalcitonin) is improved to 0.1ng / ml by the kit, so that the application value and the application range of a quick PCT detecting agent in diagnosis of bacterial infection, differential diagnosis and treatment can be increased and expanded; and the kit can be applied to diagnosis of bacterial infection, differential diagnosis, treatment effect monitoring and post-curing assessment.
Owner:WUHAN EASYDIAGNOSIS BIOMEDICINE
Immunofluorescence chromatography kit for quantitative detection of SAA (serum amyloid A), CRP (C-reactive protein) and PCT (procalcitonin) and preparation method of immunofluorescence chromatography kit
The invention discloses an immunofluorescence chromatography kit for quantitatively detecting SAA, CRP, and PCT and a preparation method thereof, which includes a fluorescent immunochromatography test strip and a fluorescent substance. Nitrocellulose membrane, one end of the nitrocellulose membrane is connected with a sample pad, and the other end of the nitrocellulose membrane is connected with an absorption pad; the nitrocellulose membrane is provided with detection lines for detecting the contents of SAA, CRP, and PCT in parallel. ; The fluorescent substance includes a fluorescent substance coupled to a rabbit IgG polyclonal antibody, a fluorescent substance coupled to a paired SAA monoclonal antibody, a fluorescent substance coupled to a paired CRP monoclonal antibody, and a paired PCT monoclonal antibody. of fluorescent substances. This kit can simultaneously detect the contents of SAA, CRP, and PCT conveniently, accurately, and with high sensitivity.
Owner:深圳市惠安生物科技有限公司
Kit for detecting procalcitonin
ActiveCN102305858AReduce dosageImprove responseChemiluminescene/bioluminescenceBiotin-streptavidin complexProtein molecules
The invention discloses a kit for detecting procalcitonin. The kit comprises a magnetic particle separation reagent, a PCT (procalcitonin) detection antibody, an enzyme conjugate and a luminescent substrate, wherein the protein molecule coupled with the magnetic particles in the magnetic particle separation reagent is goat anti-mouse immunoglobulin G or streptavidin; the PCT detection antibody isa mouse anti-PCT monoclonal antibody; and the enzyme conjugate is a horseradish peroxidase-labeled goat anti-PCT polyclonal antibody. The kit has the beneficial effects of high detection sensitivity and low cost.
Owner:SHENZHEN GOLDSITE DIAGNOSTICS
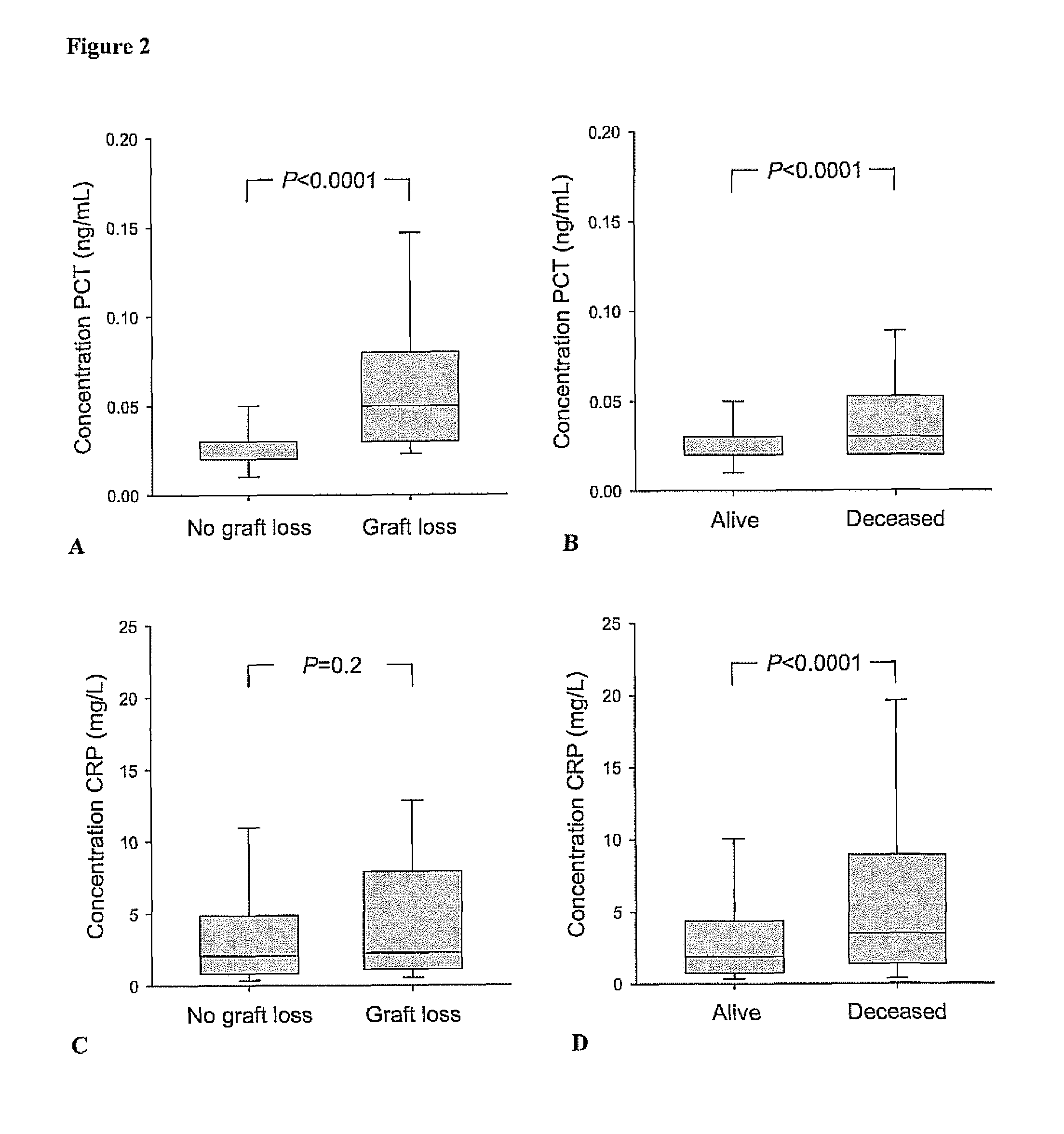
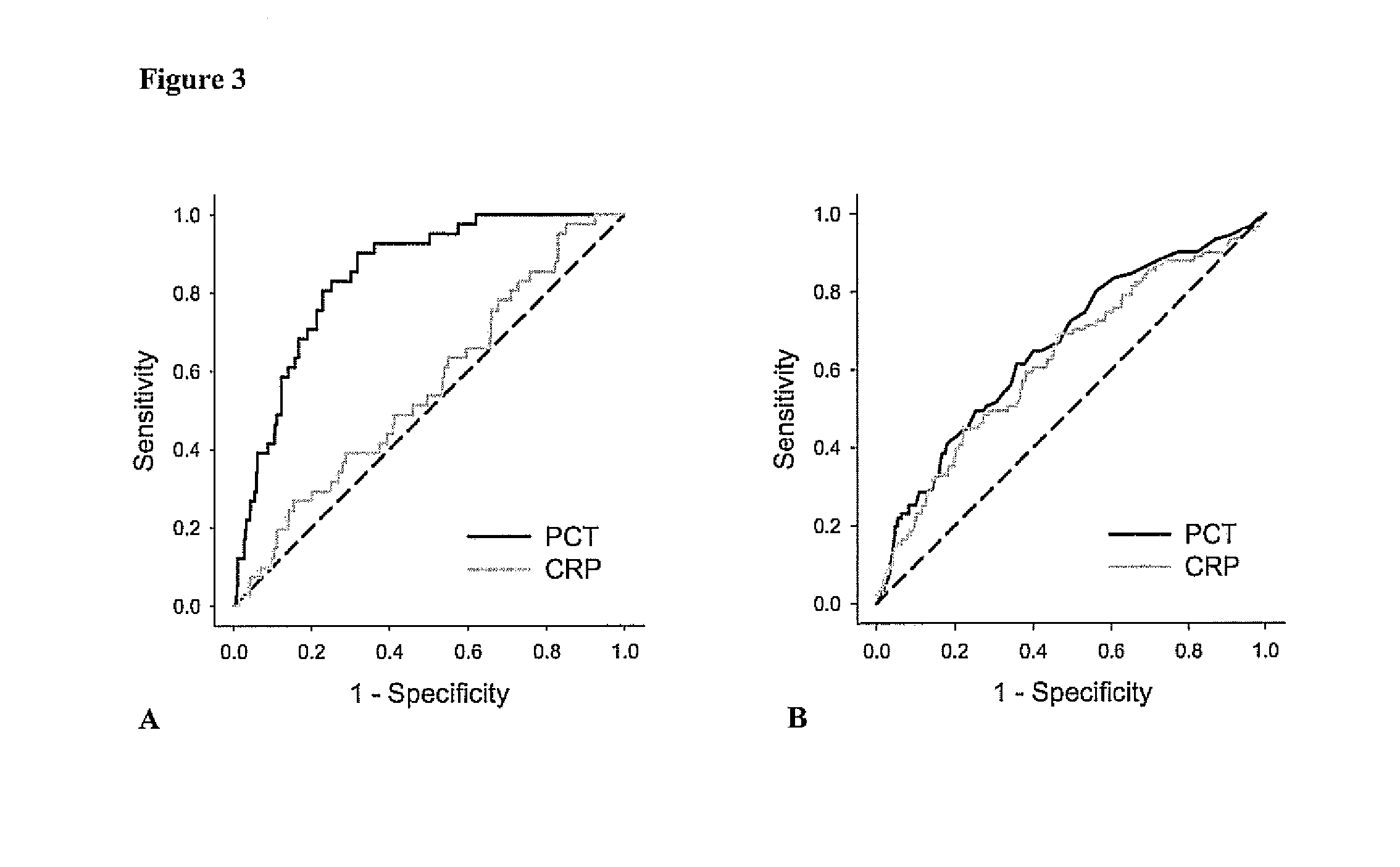
Marker for graft failure and mortality
ActiveUS8889366B2Lower levelAnalysis using chemical indicatorsPeptide/protein ingredientsOrgan transplantationMortality rate
Subject of the present invention is a biomarker for graft failure and / or mortality after organ transplantation. Procalcitonin was found to be a useful marker for the prediction or risk stratification for graft failure and / or mortality of a subject who has received an organ transplant and monitoring and therapy guidance of such subject.
Owner:BRAHMS GMBH
Latex enhanced immuno-nephelometry kit for determining procalcitonin and preparation method and application of latex enhanced immuno-nephelometry kit for determining procalcitonin
ActiveCN104237525AMaterial analysis by observing effect on chemical indicatorBiological testingDiseaseMedicine
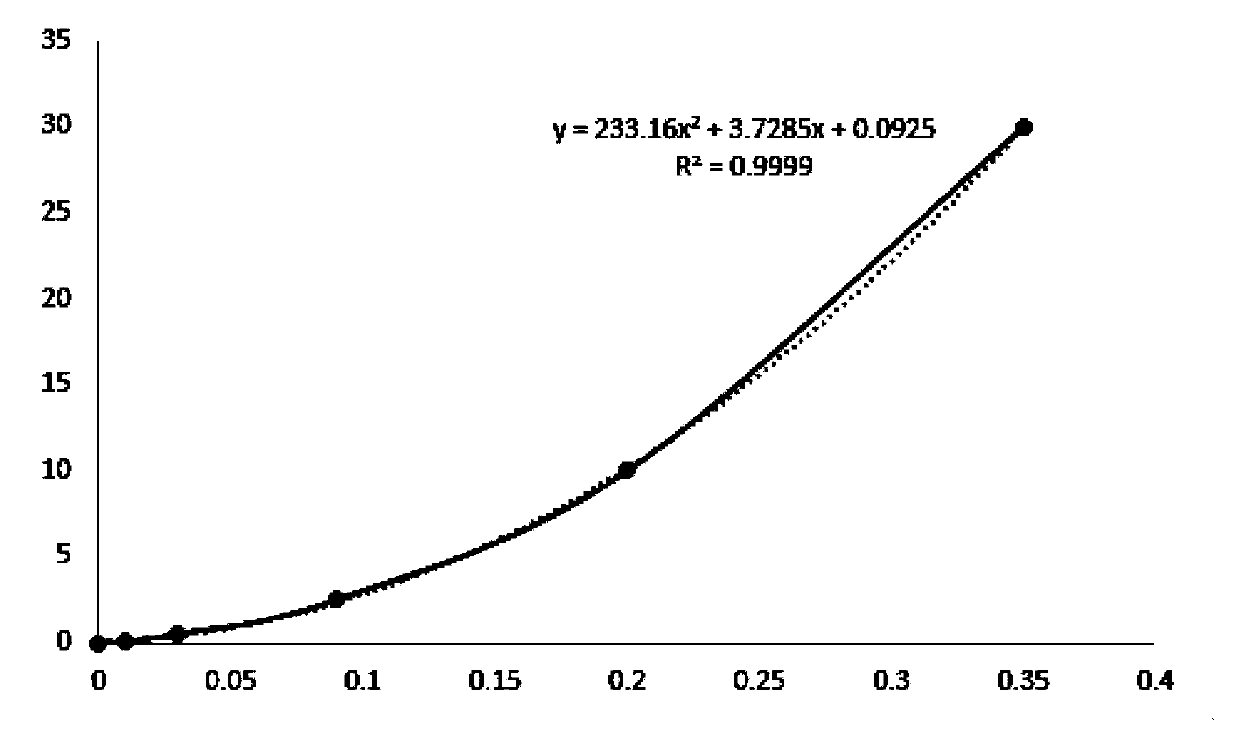
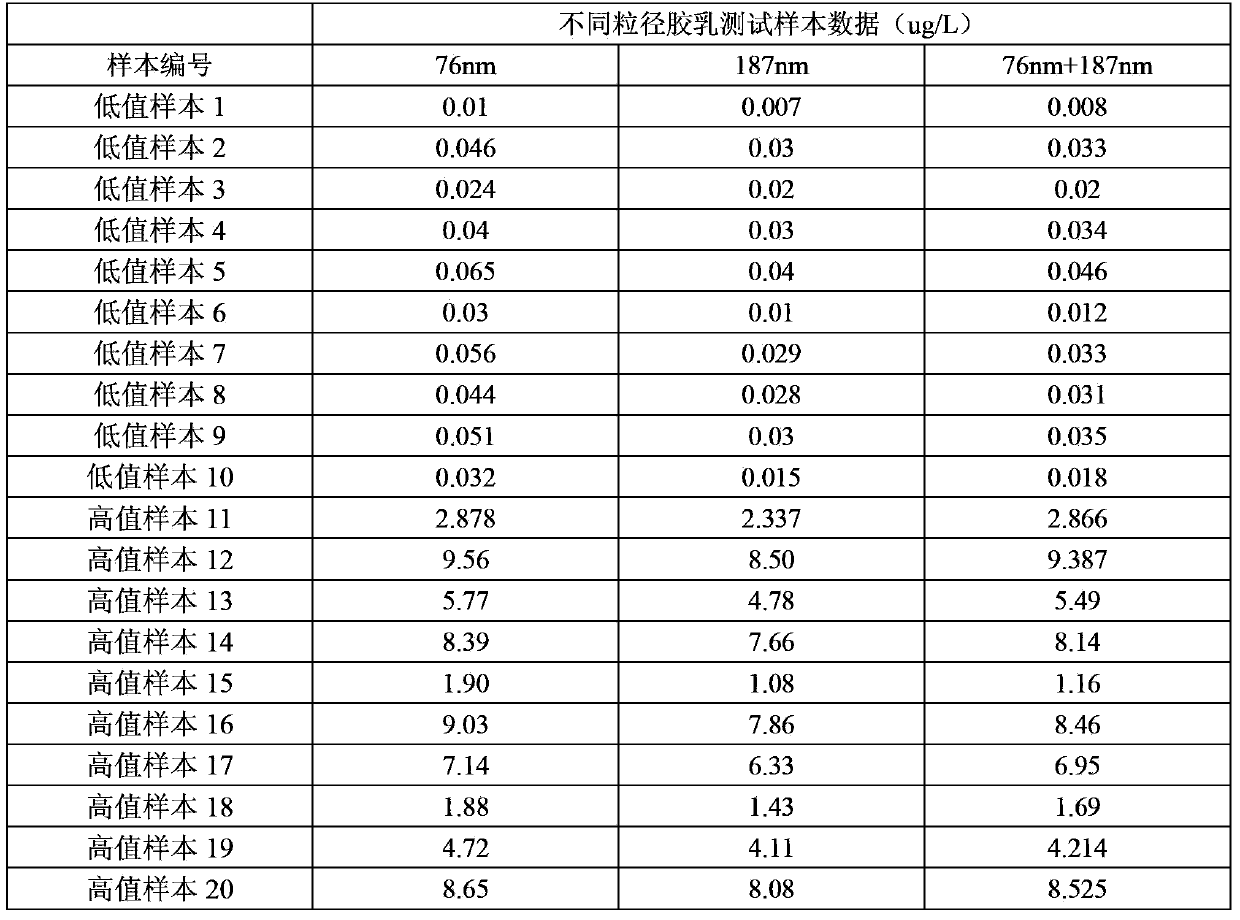
The invention discloses a latex enhanced immuno-nephelometry kit for determining procalcitonin and a preparation method and application of the latex enhanced immuno-nephelometry kit for determining procalcitonin, and belongs to the technical field of disease diagnosis and detection. The latex enhanced immuno-nephelometry kit for determining the procalcitonin comprises a diluent, a latex preparation, a blank liquid, a calibrator and a quality control material, wherein the latex preparation contains carboxylated polystyrene latex with different particle sizes and coupled with a PCT monoclonal antibody and a PCT polyclonal antibody respectively. According to the method, the PCT monoclonal antibody and the PCT polyclonal antibody are marked respectively, the latex with the large particle size can improve the detection sensitivity, and the latex with the small particle size can expand the linearity range, and therefore, the composite latex preparation can improve the detection sensitivity, can expand the detection linearity range, and has the advantages of high detection speed, high sensitivity, strong specificity and good accuracy for detection on the procalcitonin.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Immunoturbidimetric kit for detecting procalcitonin
InactiveCN107340395AReduce dosageHigh detection sensitivityScattering properties measurementsBiological testingHemolytic AgentsMicrosphere
The present invention relates to the field of medical immunology, particularly to an immunoturbidimetric kit for detecting procalcitonin. The kit comprises a reagent R1 and a reagent R2, wherein the reagent R1 comprises a buffer liquid, a stabilizer, a protection agent, a coagulant, a hemolytic agent and a preservative, the reagent R2 comprises a buffer liquid, a stabilizer, a protection agent, a preservative and a polystyrene latex microsphere-procalcitonin antibody complex, and the polystyrene latex microspheres comprise two types of microspheres such as microspheres having a particle size of 80-150 nm and microspheres having a particle size of 150-400 nm. According to the present invention, the test results show that the kit has advantages of good stability and long storage period, can directly detect the PCT in whole blood, and further has advantages of high detection result accuracy, high sensitivity and wide linear detection range.
Owner:SONOSCAPE MEDICAL CORP
Magnetic particle chemiluminescence detection kit for calcitonin and preparation method thereof
InactiveCN107543932ASmooth connectionReduce yieldChemiluminescene/bioluminescenceBiological testingCalcitoninRepeatability
The invention discloses a calcitonin magnetic particle chemiluminescent detection kit and a preparation method. The kit includes: magnetic particles coupled with calcitonin capture antibody, acridinium ester-labeled calcitonin detection antibody, calcitonin calibrator, chemiluminescence pre-excitation solution A, chemiluminescence excitation solution B and cleaning solution. The kit of the invention uses the magnetic separation chemiluminescence technology as the detection means, and simultaneously combines the acridinium ester labeling technology. The direct chemiluminescence method established by the invention has high sensitivity, strong specificity, accuracy and rapidity, short detection time and higher accuracy and repeatability of detection results, and the kit can be applied to various luminescence detection instruments.
Owner:太原瑞盛生物科技有限公司
Preparation method and application of MoS2/Au-Pd compound-based procalcitonin electrochemical immunosensor
InactiveCN106568973AEasy to manufactureImprove catalytic performanceBiological material analysisBiological testingAntigenElectrochemistry
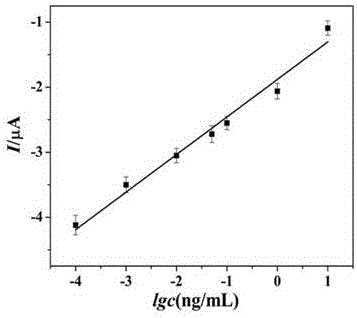
The invention relates to the field of electrochemical immunosensing technique, especially to a preparation method and application of a MoS2 / Au-Pd compound-based label-free electrochemical immunosensor. H2O2 is used as an electrochemical probe, and a MoS2 / Au-Pd compound is used as a substrate material. Based on good film forming capability and large specific surface area and excellent catalytic performance to H2O2, stability and sensitivity of an immunosensor are remarkably enhanced. The testing principle is to utilize current change before and after an antigen-antibody immunoreaction. When an antigen reacts with an antibody modified on an electrode, the formed immunocomplex is a non-electroactive substance and severely obstructs electron transfer, and current signal is reduced. The sensor is used for detection of procalcitonin. Linearity range for detection is 0.0001 ng / mL- 10 ng / mL, and detection limit is 0.05 pg / mL.
Owner:UNIV OF JINAN
Human procalcitonin immunodetection kit, and preparation method and application thereof
Owner:DYNAMIKER BIOTECH TIANJIN
Chemluminescent detection kit for procalcitonin and preparation method of chemluminescent detection kit
InactiveCN107807240AHigh sensitivityImprove featuresMaterial analysisMonoclonal antibodyChemiluminescent immunoassay
The invention discloses a chemiluminescent detection kit for procalcitonin and a preparation method thereof. The kit includes: sample diluent, magnetic particles coated with procalcitonin monoclonal antibody, procalcitonin monoclonal antibody labeled with acridinium ester, procalcitonin series standard solution, chemiluminescence excitation solution A, Chemiluminescence excitation solution B, cleaning solution. The kit of the invention combines the chemiluminescent technology with the immunomagnetic particle, and provides a nearly homogeneous reaction system. Compared with the prior art, the kit of the invention has the advantages of high sensitivity, strong specificity, short reaction time and the like.
Owner:太原瑞盛生物科技有限公司
Procalcitonin chemiluminescence detection reagent based on nanometer antibodies and detection method thereof
ActiveCN108445230ASimple and fast operationStrong specificityChemiluminescene/bioluminescenceBiological material analysisQuality controlEnzyme
The invention relates to the technical field of biomedicine and biological detection, in particular to a reagent for testing procalcitonin in blood serum and a detection method thereof, in particularto a procalcitonin chemiluminescence detection reagent based on nanometer antibodies, a detection method thereof and application thereof to diagnosis. The detection reagent is prepared from a luminoussubstrate solution A, a luminous substrate solution B, a coating plate, enzyme conjugates, concentrated washing liquid, a calibration product and a quality control product, wherein the procalcitoninnanometer antibodies are covered on the coating plate; enzyme labelling procalcitonin nanometer antibodies are contained in the enzyme conjugates. Experiments show that the detection reagent and the detection method have the characteristics that the operation is simple and convenient; the specificity is high; the sensitivity is high; the detection speed is high; the detection result obtaining timeis short; the detection result is accurate and reliable, and the like. The detection accuracy in the experiment reaches 100 percent.
Owner:北京科卫临床诊断试剂有限公司
Procalcitonin monoclonal antibody hybrid tumor 2H4 and monoclonal antibody
ActiveCN104745534AImprove featuresHigh sensitivityMicroorganism based processesTissue cultureProtein detectionMonoclonal antibody
The invent9ion discloses a procalcitonin monoclonal antibody hybrid tumor 2H4 and monoclonal antibody. The procalcitonin monoclonal antibody hybrid tumor 2H4 is preserved in the China Center for Type Culture Collection at 16, Dec, 2014, and the preservation number is CCTCC NO: C2014232. The monoclonal antibody prepared aiming at procalcitonin compound oligopeptide has high specificity and sensitivity to the procalcitonin protein detection and lays a foundation for the function study and development of the procalcitonin and the research of corresponding diagnostic reagents.
Owner:SOUTHERN MEDICAL UNIVERSITY
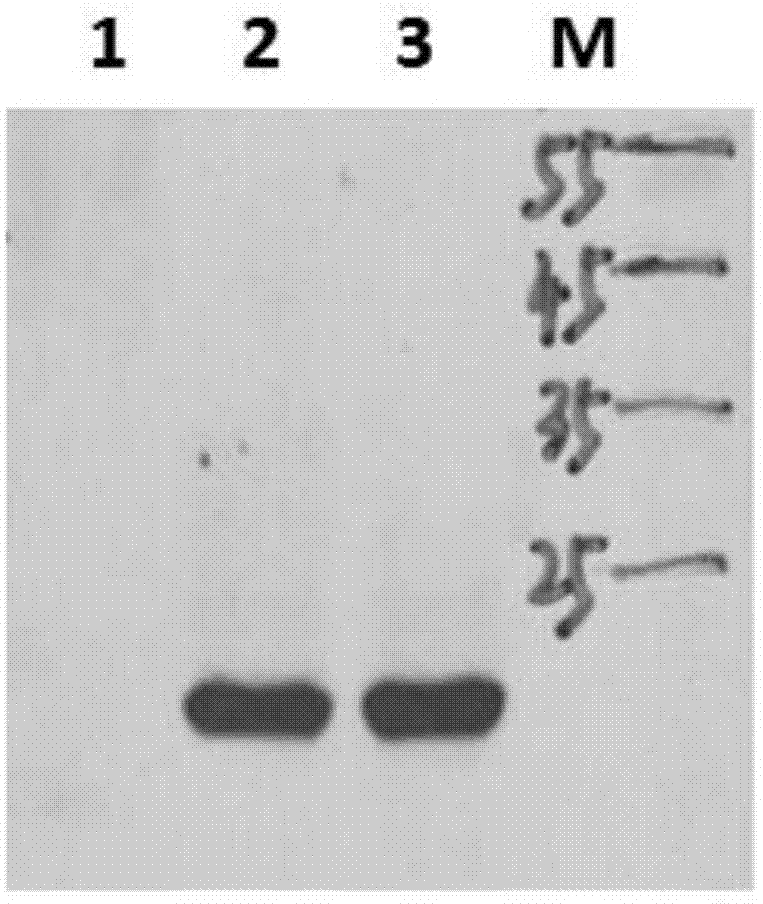
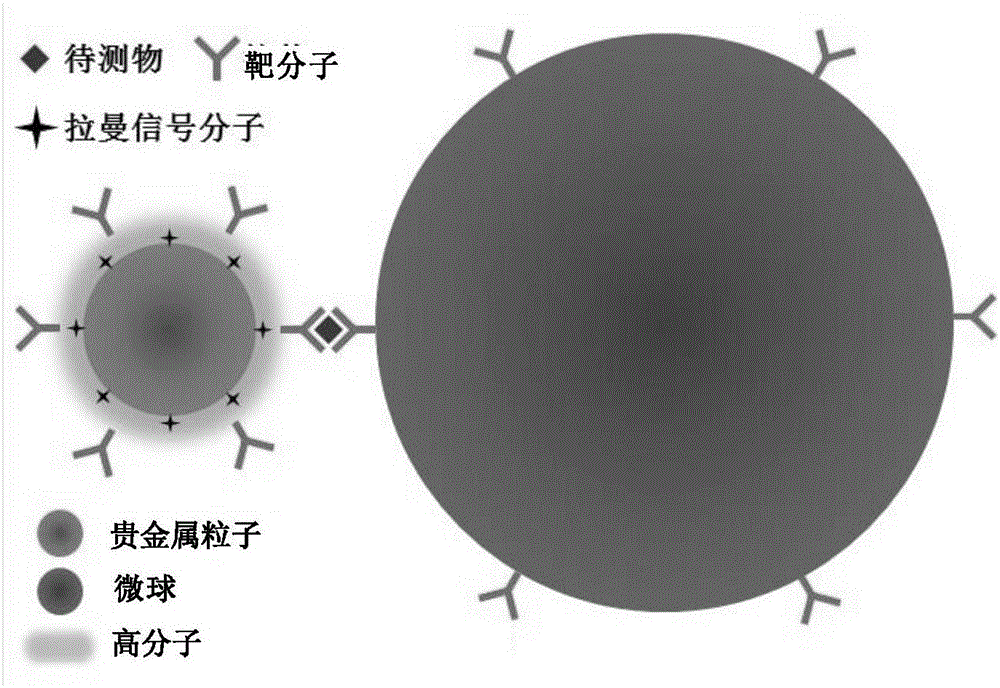
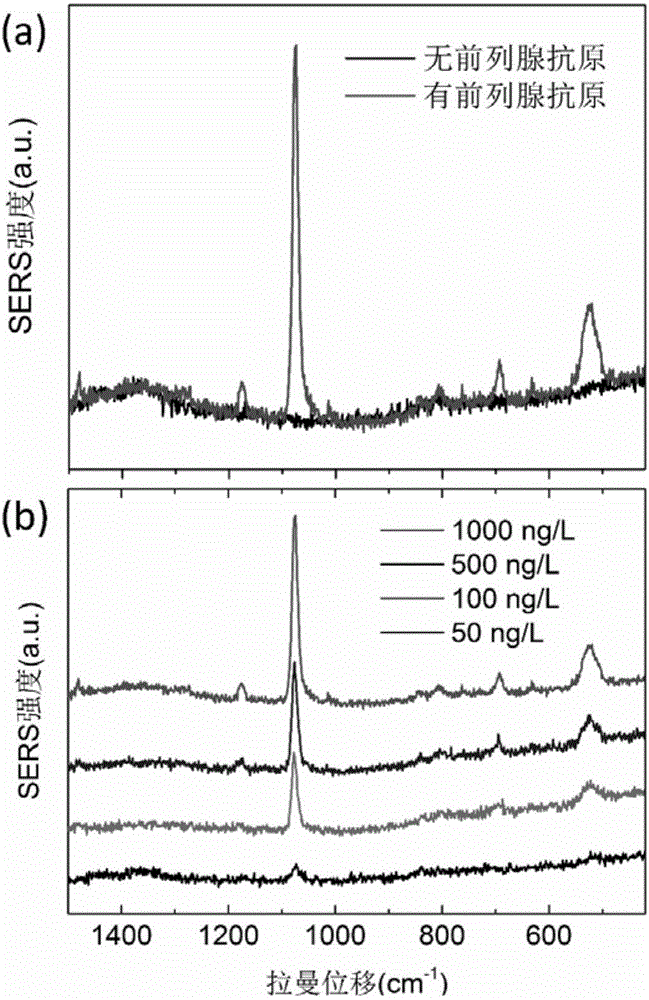
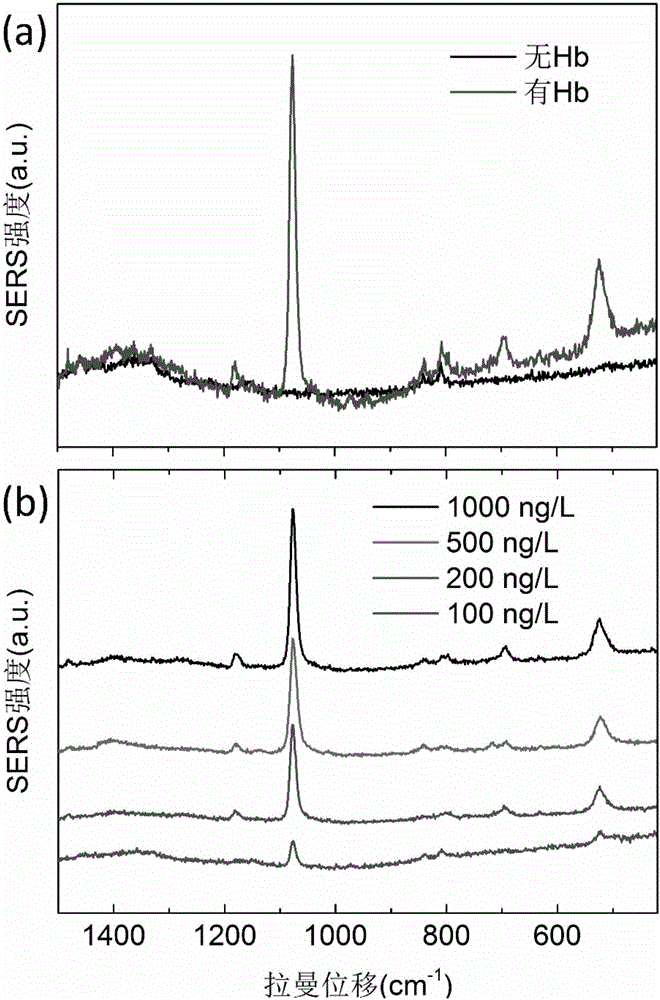
Surface enhanced Raman scattering technology-based composite material and preparation method thereof
The invention relates to a surface enhanced Raman scattering technology-based composite material and a preparation method thereof. The composite material comprises microspheres modified with first target molecules on the surfaces and a second component modified with Raman signal molecules and second target molecule-modified polymers on the surface. In a solution containing a substance to be detected, the substance to be detected, the microspheres and the second component are bonded to the second target molecules through the first target molecules so that the composite material which can be easily precipitated through centrifugation is obtained. The invention also discloses the preparation method and a use of the composite material. The composite material can be used for detecting a human prostate-specific antigen (PSA), a human ferrohemoglobin (Hb), a procalcitonin (PCT) or disease markers (such as tumor markers, cardiovascular disease markers, senile dementia markers, mycoplasma and chlamydia), can effectively reduce false positive and false negative effects and has advantages of high sensitivity, simpleness, fastness and low cost.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Oral administration of a calcitonin
InactiveUS20100210526A1Promote oral absorptionIncreases systemic bioavailabilityBiocideOrganic active ingredientsWhole bodyBuccal administration
The invention is directed to a method of administering pharmaceutical compositions comprising peptide drugs such as a calcitonin in combination with one or more oral delivery agents, together with an amount of a liquid, and method of treatment of disorders responsive to the action of peptide drugs such as a calcitonin employing such method of administration so as to enhance the oral bioavailability of a calcitonin. The methods of the invention increase the oral absorption and systemic bioavailability of peptide drugs, such as a calcitonin.
Owner:NOVARTIS AG
Marker for graft failure and mortality
ActiveUS20110171750A1Lower levelDisease diagnosisBiological testingOrgan transplantationMortality rate
Subject of the present invention is a biomarker for graft failure and / or mortality after organ transplantation. Procalcitonin was found to be a useful marker for the prediction or risk stratification for graft failure and / or mortality of a subject who has received an organ transplant and monitoring and therapy guidance of such subject.
Owner:BRAHMS GMBH
Method for preparing salmon calcitonin acetate by fragment condensation
ActiveCN104177490AHigh purityHigh yieldCalcitoninsPeptide preparation methodsCrystallographyRecombinant salmon calcitonin
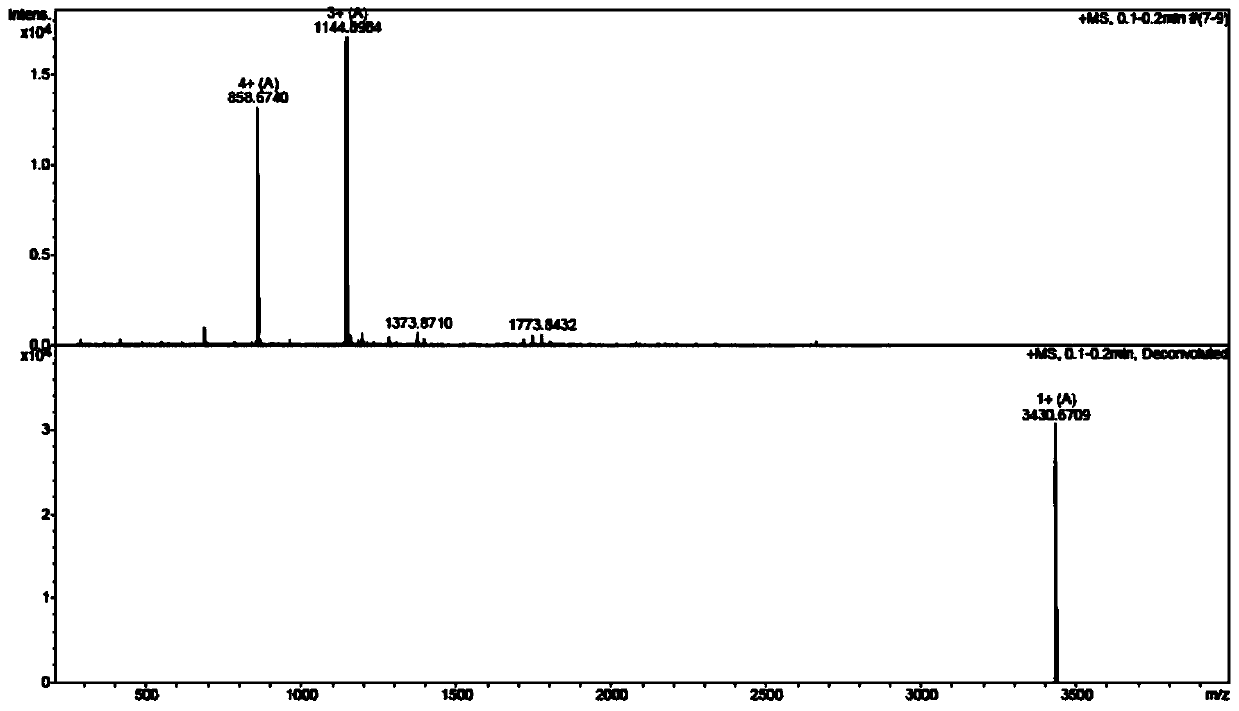
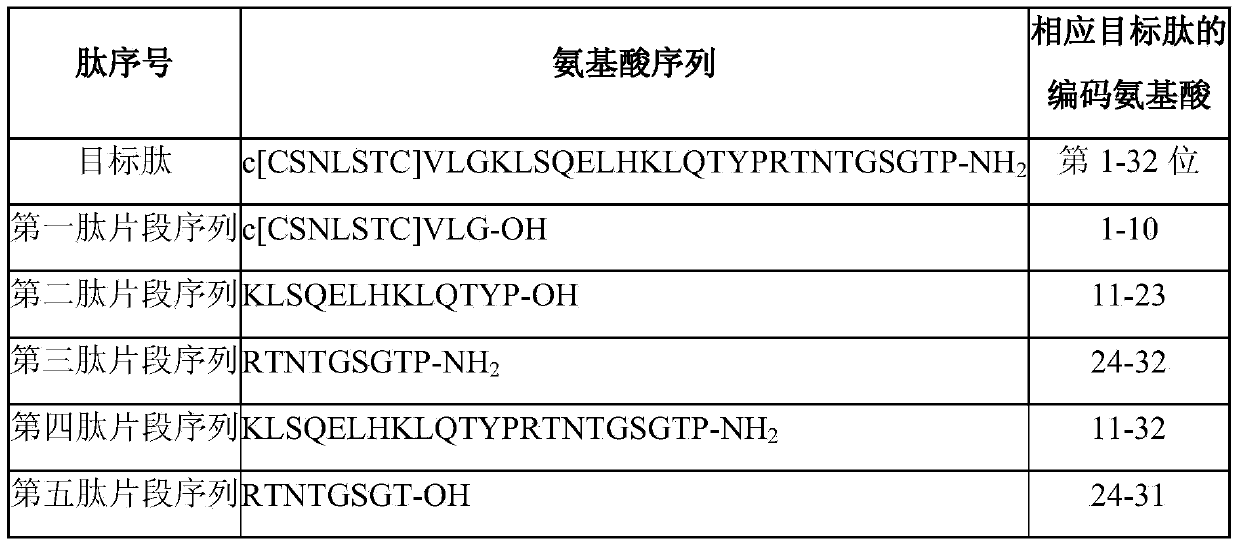
The invention discloses a method for preparing salmon calcitonin acetate by fragment condensation, which comprises the following steps: 1) synthesizing fragment sequences of salmon calcitonin (1st-10th, 11th-23rd, 24th-32nd or 24th-31st amino acids) on a solid-phase vector by solid-phase synthesis; 2) sequentially coupling the fragment in the liquid phase to form full-protection salmon calcitonin; and 3) cracking the full-protection salmon calcitonin to obtain salmon calcitonin crude peptides, and carrying out high-efficiency liquid-phase purification salt exchange to obtain the salmon calcitonin acetate pure peptides. The method for preparing salmon calcitonin acetate by combining solid / liquid phase and fragment condensation enhances the yield and purity, and is low in cost and beneficial to large-scale production.
Owner:HAINAN JIANKE PHARMA
Procalcitonin for the prognosis of adverse events
The present invention relates to an in vitro method for the prognosis of an adverse event in asymptomatic subjects comprising the determination of the level of Procalcitonin (PCT) or a fragment thereof or a precursor or fragment thereof having at least 12 amino acid residues in a sample of a bodily fluid from said subject and the correlation of the determined level to a potential risk of sustaining an adverse event.
Owner:BRAHMS GMBH
Procalcitonin detection test strip and preparation method thereof
The invention relates to the field of biotechnology and discloses a procalcitonin detection test strip and a preparation method thereof. The procalcitonin detection test strip comprises a sample feeding region, a conjugate releasing region, a reaction region and a water absorbing region which are arranged in sequence; the conjugate releasing region comprises a conjugate releasing cushion of a procalcitonin monoclonal antibody covered by a quantum dot mark; the reaction region is divided into a testing region and a control region; and the reaction region is characterized in that the testing region is covered by procalcitonin and the control region is covered by a solid support of an anti-rat IgG antibody. The procalcitonin detection test strip disclosed by the invention takes a novel fluorescent dye quantum dot to replace traditional colloidal gold so as to accurately, quantitatively, rapidly and sensitively detect the procalcitonin in human blood serum; and a needed instrument is different from a large-sized dear instrument of a previous clinical laboratory, and only a small-sized fluorescent quantitative analyzer is needed to realize real-time detection.
Owner:SHENZHEN BLOT BIOTECH
PCT (Procalcitonin) fluorescent microsphere immunochromatographic assay reagent card and preparation method thereof
The invention discloses a PCT (Procalcitonin) fluorescent microsphere immunochromatographic assay reagent card and a preparation method thereof, aims to provide a PCT fluorescent microsphere immunochromatographic assay reagent card which is convenient in operation, and is capable of detecting low-concentration and high-concentration PCT antigens at the same time and of which the detection result is reliable, and belongs to the technical field of bioassay. The PCT fluorescent microsphere immunochromatographic assay reagent card is characterized is that the PCT fluorescent microsphere immunochromatographic assay reagent card comprises a bottom plate, wherein the bottom plate is sequentially connected with a sample pad, a combination pad, a coating membrane and an absorbing pad; the combination pad is sprayed with two kinds of PCT antibodies labeled by fluorescent microspheres in different particle diameters and fluorescent microsphere-labeled chicken IgY (Immunoglobulin of Yolk); a T-line position of the coating membrane is coated with an anti-PCT antibody, and a C-line position is coated with a goat anti-chicken IgY.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
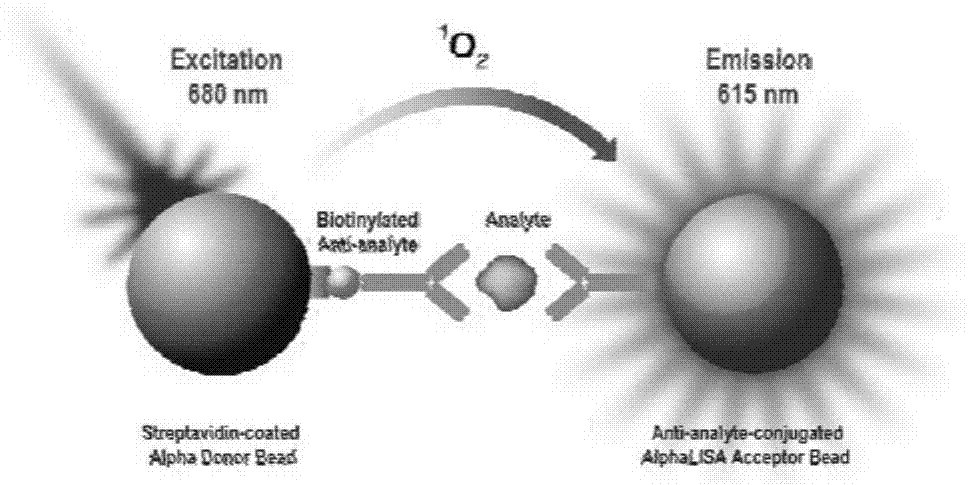
Procalcitonin light-initiated chemiluminescence immunoassay kit and preparation method thereof
The invention discloses a procalcitonin light-initiated chemiluminescence immunoassay kit and a preparation method thereof. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention is composed of a white opaque 96-pore plate, a procalcitonin calibrating product, a receptor microsphere coated with an anti-procalcitonin monoclonal antibody, a biotinylated anti-procalcitonin monoclonal antibody, and a streptavidin biotinylated donor microsphere. The procalcitonin light-initiated chemiluminescence immunoassay kit disclosed by the invention has the advantages of rapidness, high sensitivity, wide measuring range, simplicity in operation, and the like, and has higher sensitivity and wider detectability in comparison with an enzyme immunoassay, can be used for diagnosing and identifying individual infectious diseases, and has an application value.
Owner:GUANGZHOU DARUI BIOTECH
Application of B cell epitope peptide of human PCT (Procalcitonin) and monoclonal antibody of B cell epitope peptide
ActiveCN102643332AHigh purityHigh potencyTissue cultureImmunoglobulins against hormonesAssayB-Cell Epitopes
The invention belongs to the field of immunology in medicines and particularly relates to application of a B cell epitope peptide of a human PCT (Procalcitonin) and a monoclonal antibody of the B cell epitope peptide. The epitope peptide is shown as SEQ ID NO:3 and can be used for preparing a hybridoma cell and secreting a corresponding monoclonal antibody. The monoclonal antibody can be used for preparing a diagnostic reagent for detecting the procalcitonin and has the advantages of purity as high as 98 percent, valence as high as 1:512000, favorable specificity, capability of being prepared in a large batch and the like. The monoclonal antibody and a polyclonal antibody which are prepared by the invention can be used for detecting the content of the PCT in the blood of a patient, for example, a double antibody sandwich ELISA (Enzyme-Linked Immuno Sorbent Assay) reaction mode, namely enzyme-labelled anti-human PCT antibody and an elisa plate to used to coat an anti-PCT polyclonal antibody and a measured sample PCT antigen to form a double antibody sandwich structure for measuring.
Owner:重庆业为基生物科技集团有限公司
Novel beta-turn mimetics as calcitonin gene related peptide receptor antagonists
InactiveUS20050209256A1BiocideOrganic active ingredientsNK1 receptor antagonistProphylactic treatment
Conformationally constrained compounds which mimic the secondary structure of reverse-turn regions of biologically active peptides are disclosed. The compounds of the present invention of Formula I have valuable pharmacological properties based on their ability to selectively antagonize calcitonin gene-related peptide (CGRP) receptor for acute and prophylactic treatment of headaches, particularly migraines.
Owner:MYRIAD GENETICS
Test paper for procalcitonin and C reactive protein as well as preparation method and detection method thereof
InactiveCN104122401AAccurate diagnosisConvenient for clinical operationBiological material analysisBiological testingQuantum dotSpecific antibody
The invention discloses test paper for procalcitonin and C reactive protein, which is quantum dot labeled immunity quantitative detection test paper. The test paper comprises a bottom lining and a sample mat, a filter mat, a bonding mat, a chromatographic membrane and a water absorption mat which are sequentially stuck on the bottom lining by lapping so as to form multiple layers of membrane structures, wherein the membrane structures are partially superposed; the bonding mat is coated with a quantum dot labeled procalcitonin specific antibody I, a quantum dot labeled C reactive protein specific antibody I and a quantum dot labeled rabbit IgG; and a detection line coated with a procalcitonin specific antibody II, a detection line coated with a C reactive protein specific antibody II and a quality control line coated with a goat anti-rabbit IgG antibody are formed on the chromatographic membrane. The test paper can be used for conveniently, quickly, accurately, quantitatively and simultaneously detecting the procalcitonin and the C reactive protein.
Owner:SHENZHEN POLYTECHNIC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com