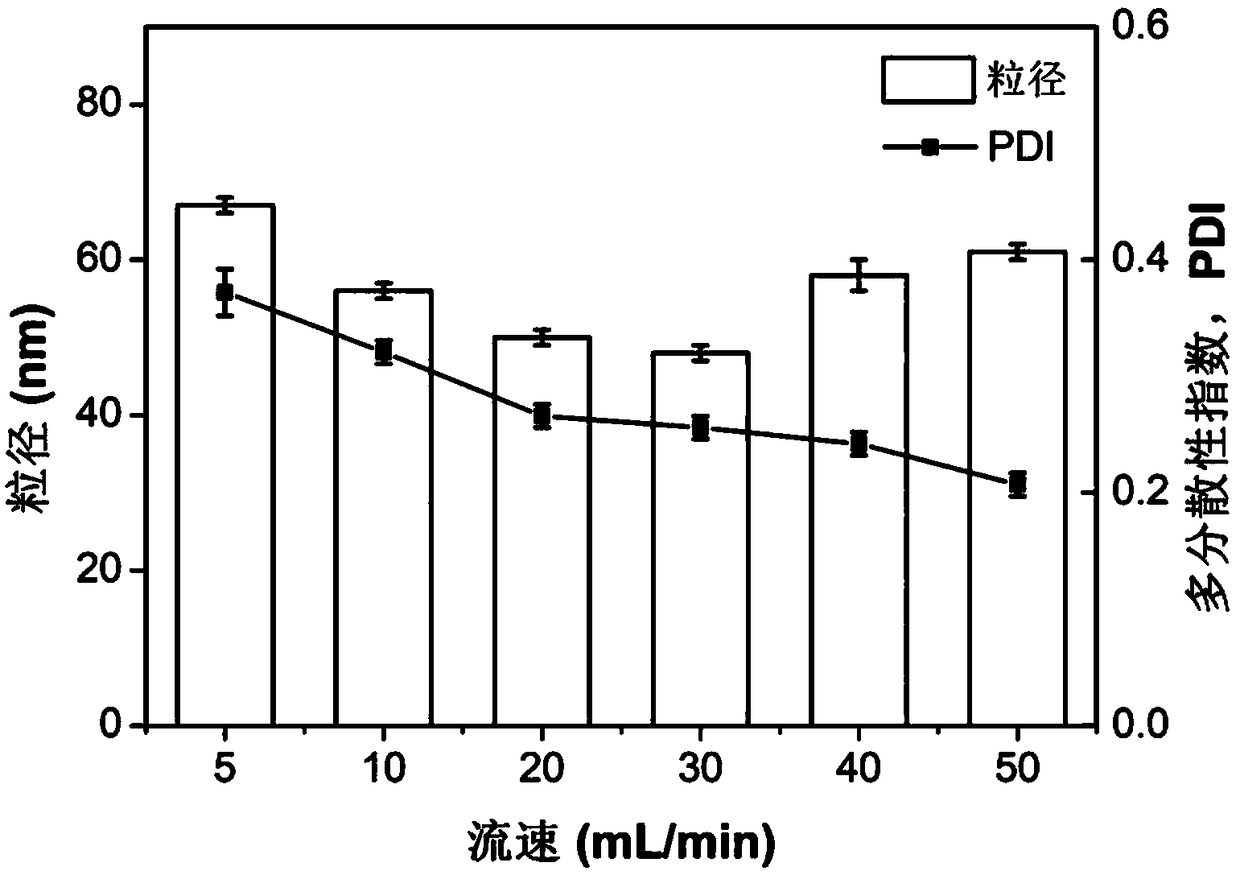
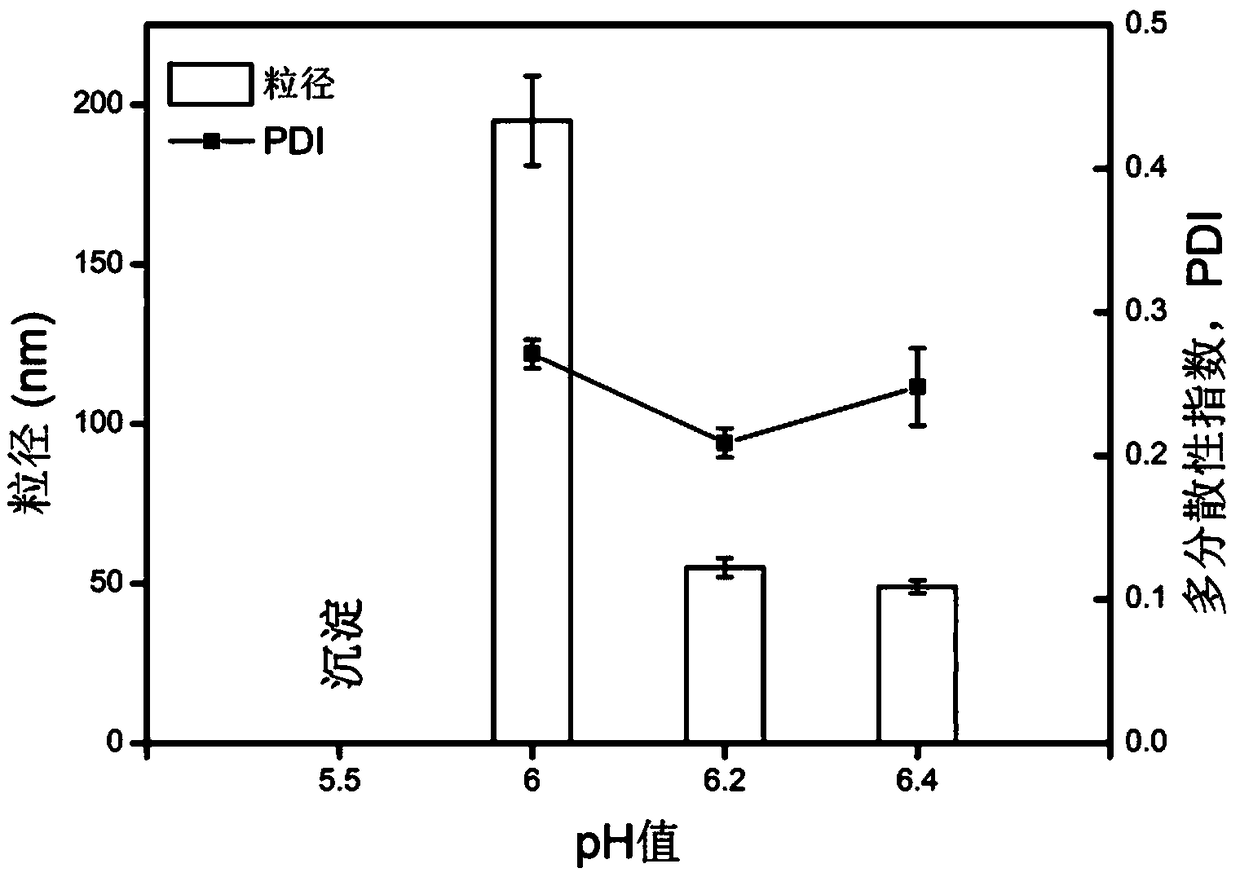
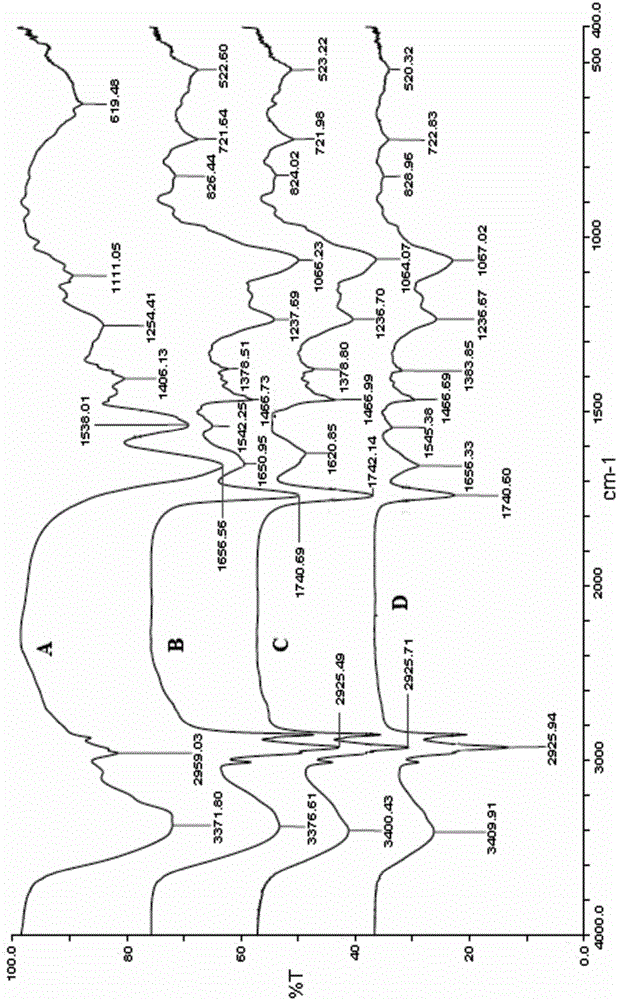
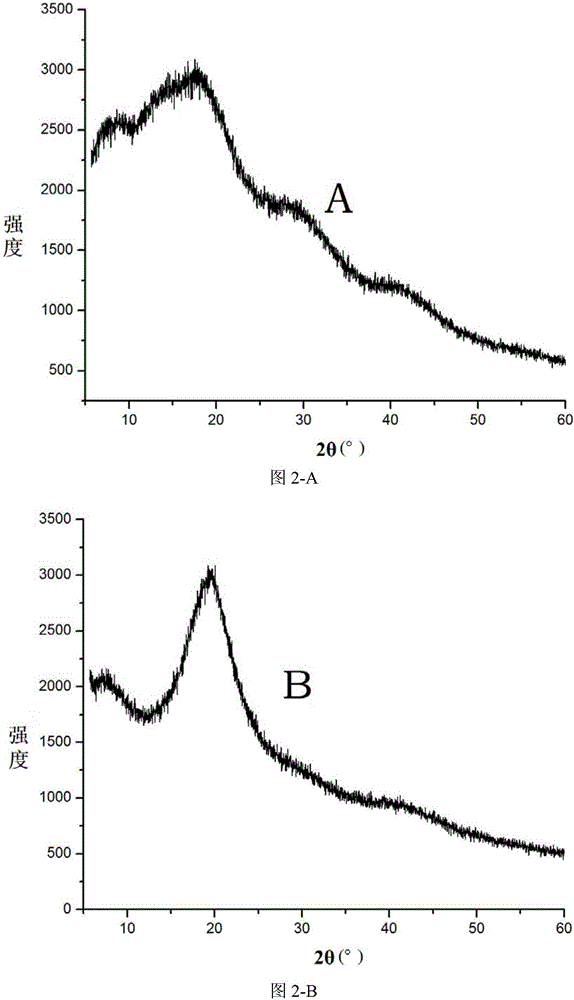
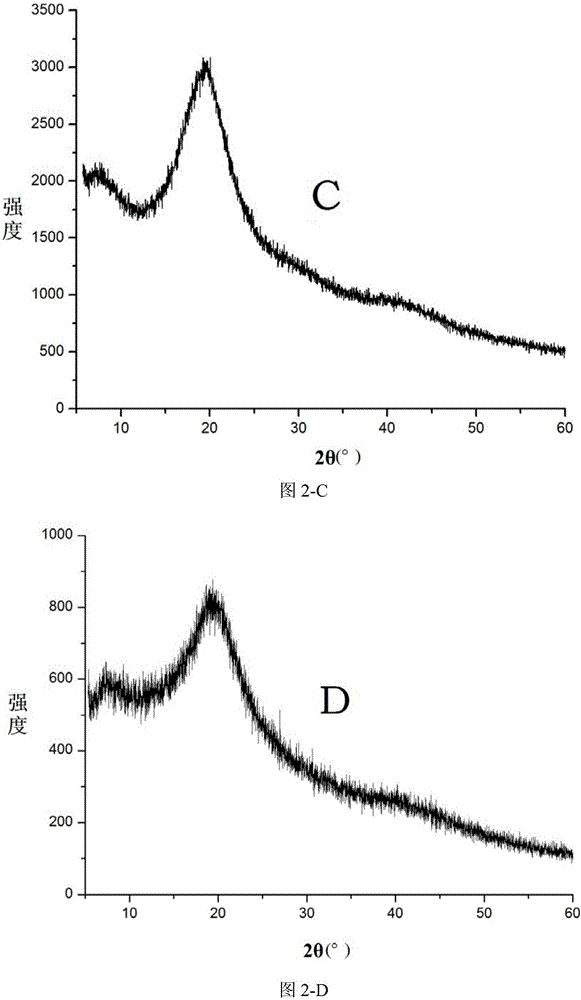
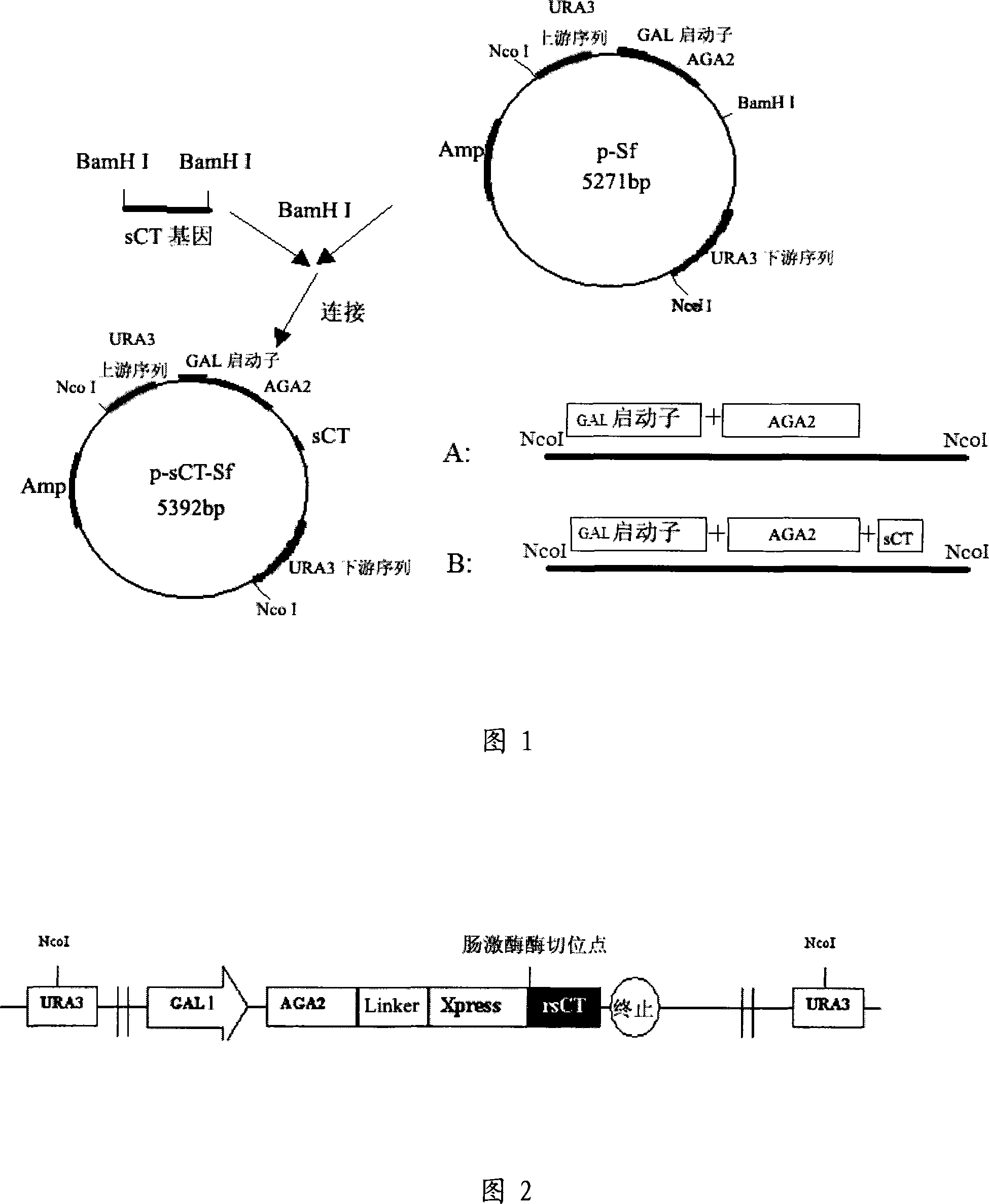
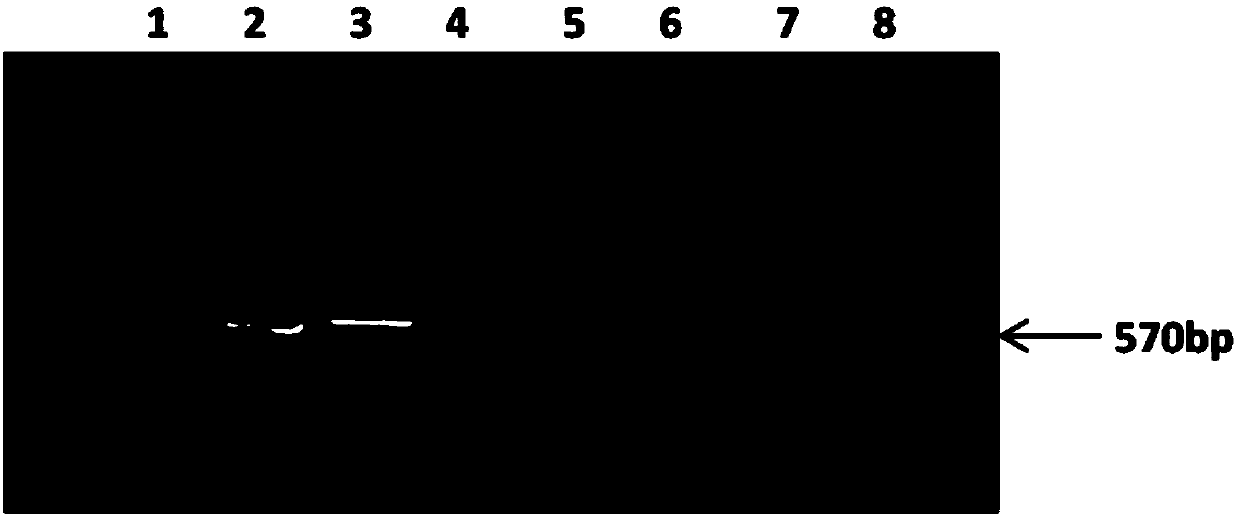
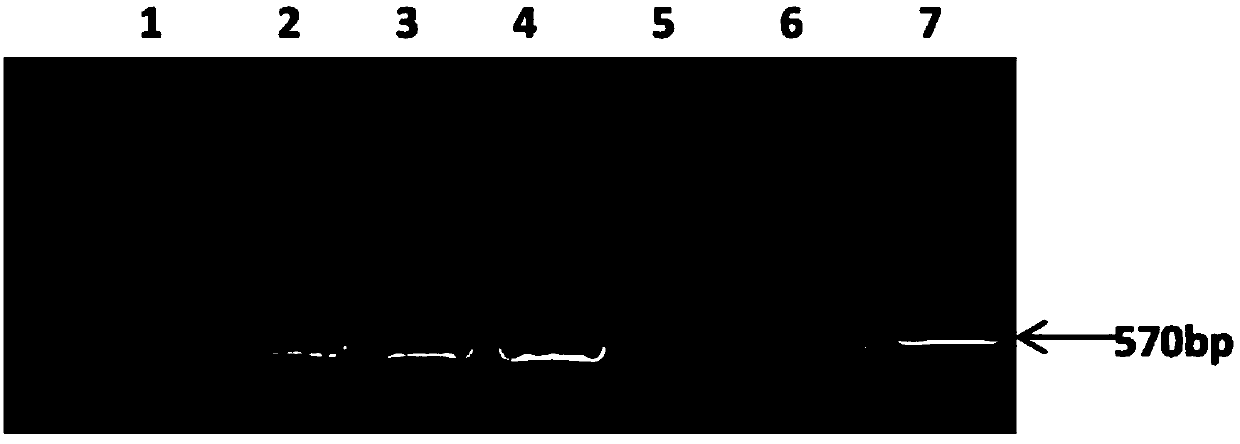
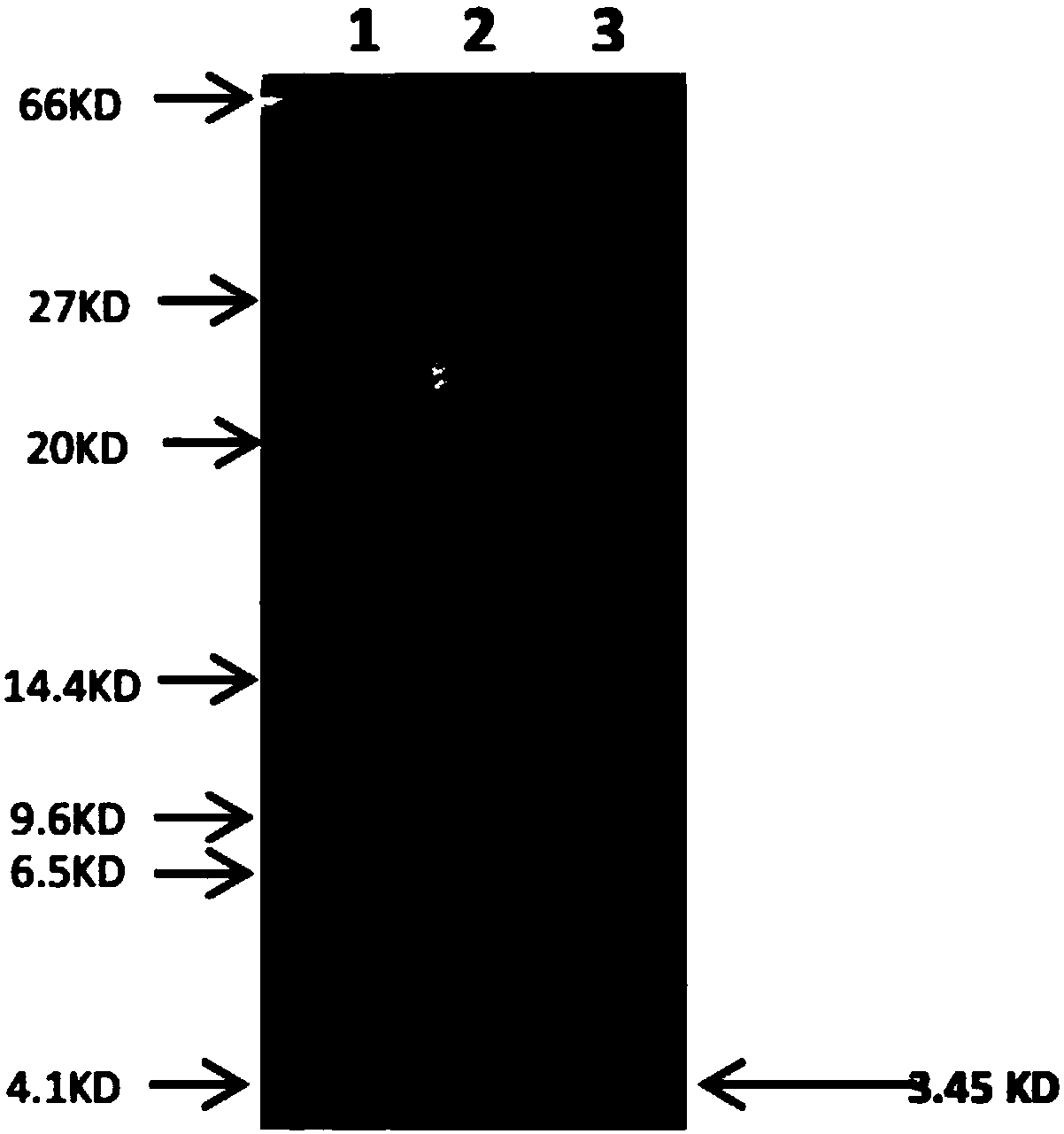
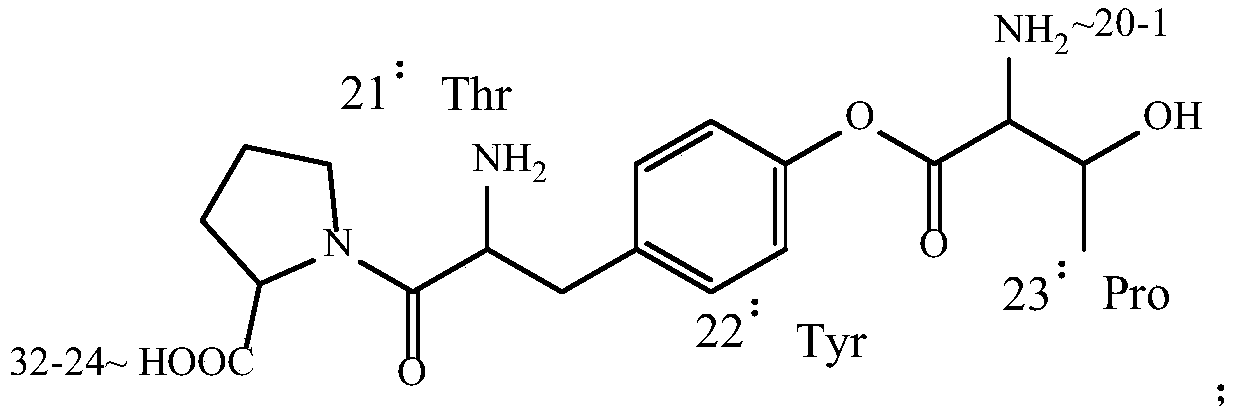
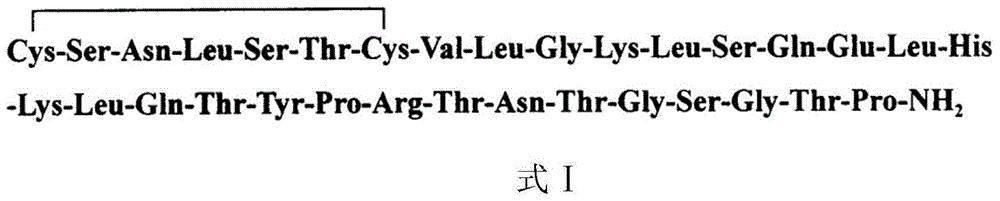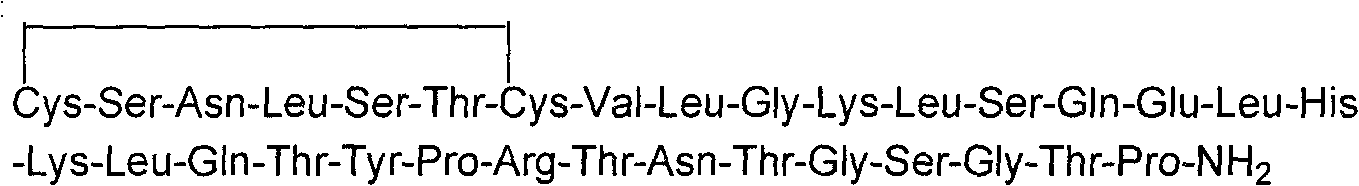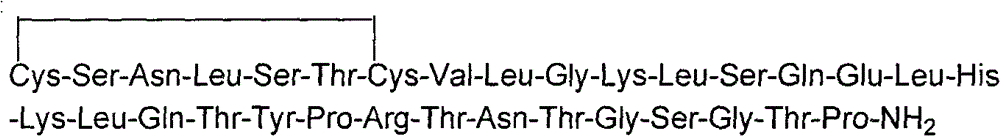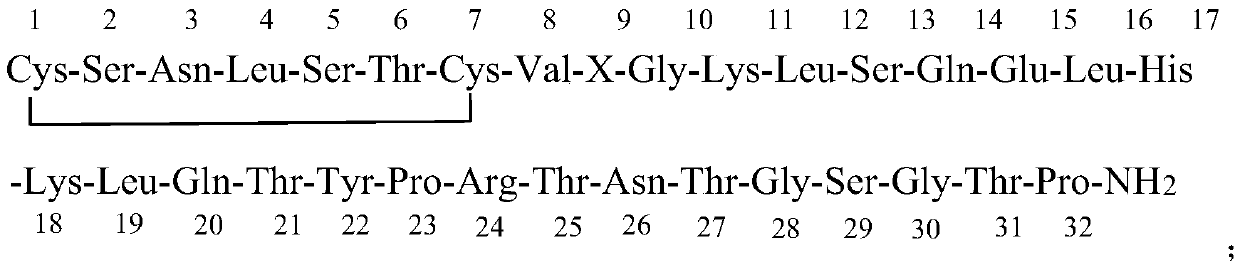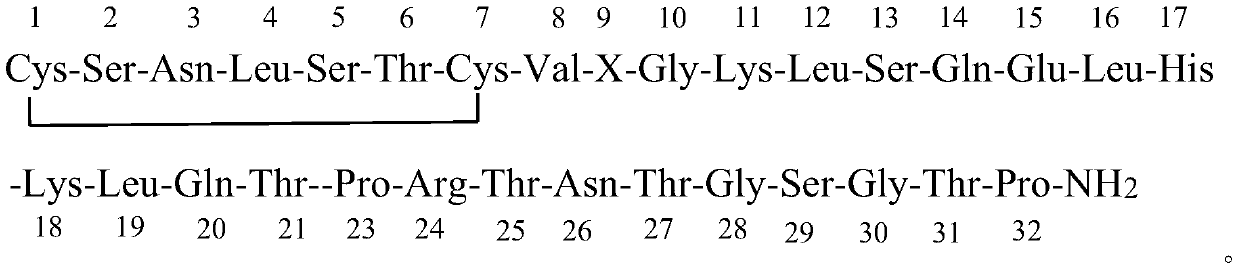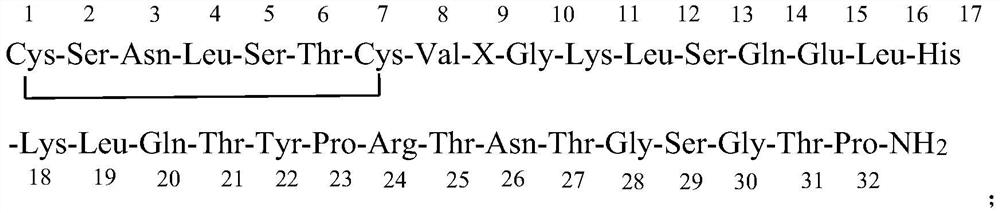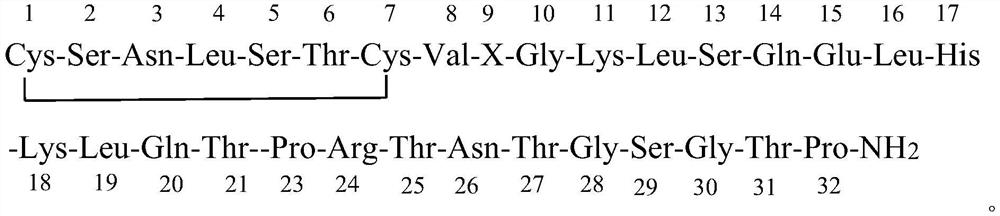Patents
Literature
52 results about "Recombinant salmon calcitonin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tarsa Therapeutics announced that the Phase 3 ORACAL trial of its oral recombinant salmon calcitonin in the treatment of postmenopausal osteoporosis was successfully concluded and yielded statistically significant, positive top-line results.
Oral peptide pharmaceutical dosage form and method of production
InactiveUS7316819B2Improve bioavailabilityAvoid contactPeptide/protein ingredientsPharmaceutical non-active ingredientsRecombinant salmon calcitoninWater soluble
A pharmaceutical composition for oral delivery of a peptide is in the form of a lamination having at least two layers. The first layer of the lamination includes at least one pharmaceutically acceptable pH-lowering agent. The second layer includes a therapeutically effective amount of the peptide. The composition also includes at least one absorption enhancer effective to promote bioavailability of the peptide, which is preferably in the second layer, and an enteric coating surrounding the lamination. In a preferred dosage form of a tablet, a water-soluble coating is applied between the lamination and enteric coating which substantially prevents contact between the pH-lowering agent and the enteric coating. In a preferred embodiment, the peptide is salmon calcitonin, the pH-lowering agent is citric acid, and the absorption enhancer is lauroyl l-carnitine.
Owner:ENTERIS BIOPHARMA
Polypeptide or protein nanoparticles based on hydrogen-bonded complexation, and preparation method and application thereof
InactiveCN109224081AKeep alive functionImprove encapsulation efficiencyPowder deliveryPeptide/protein ingredientsRecombinant salmon calcitoninPolyvinylpyrrolidone
The invention discloses a polypeptide or protein nanoparticle based on hydrogen-bonded complexation and a preparation method and application thereof. The nanoparticles comprise a polyphenol compound carrier such as tannic acid as a hydrogen bond donor, a polypeptide or a protein drug such as a glucagon-like peptide as a hydrogen bond acceptor 1 analog (exenatide), salmon calcitonin or insulin, etc., and chitosan, polyvinylpyrrolidone or hyaluronic acid, etc. coated on that surface of the particle as stabilizer and / or functional molecule. The nanoparticles of the invention have extremely high encapsulation efficiency and drug loading capacity, and the prepared therapeutic polypeptide or protein drug nanoparticles are suitable for oral delivery or subcutaneous injection application, and havegreat application prospect.
Owner:SUN YAT SEN UNIV
Method for preparing salmon calcitonin acetate by fragment condensation
ActiveCN104177490AHigh purityHigh yieldCalcitoninsPeptide preparation methodsCrystallographyRecombinant salmon calcitonin
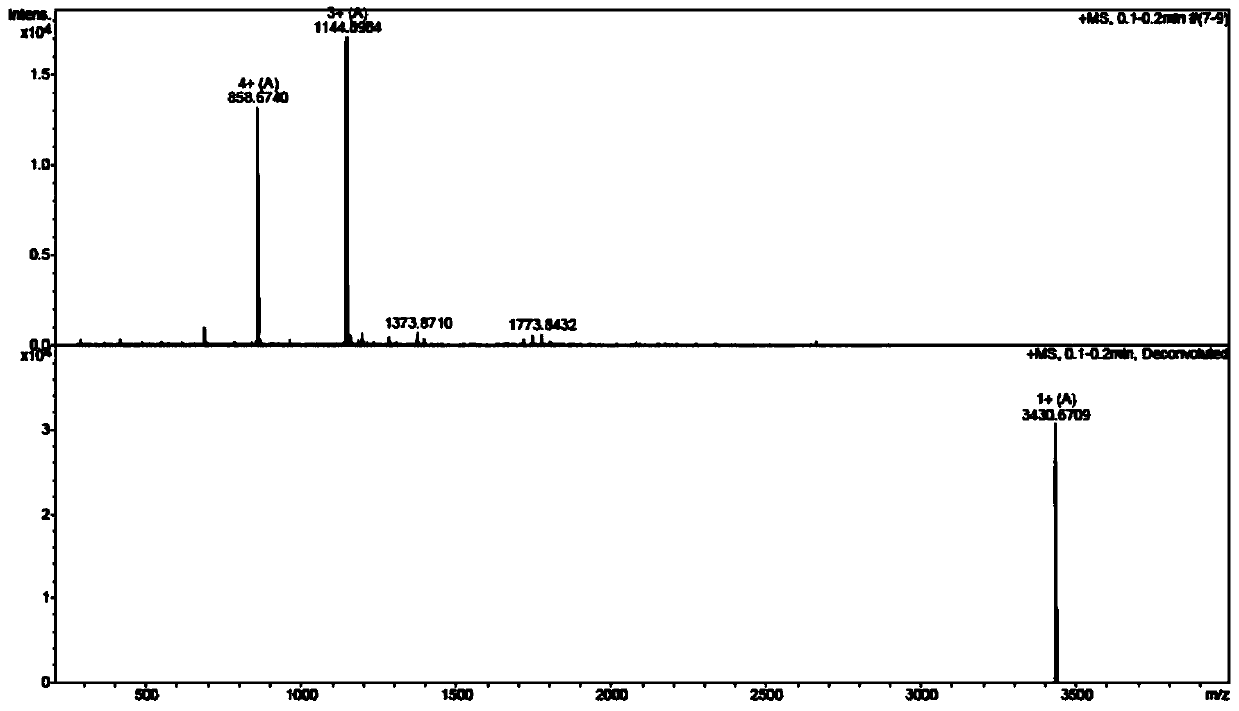
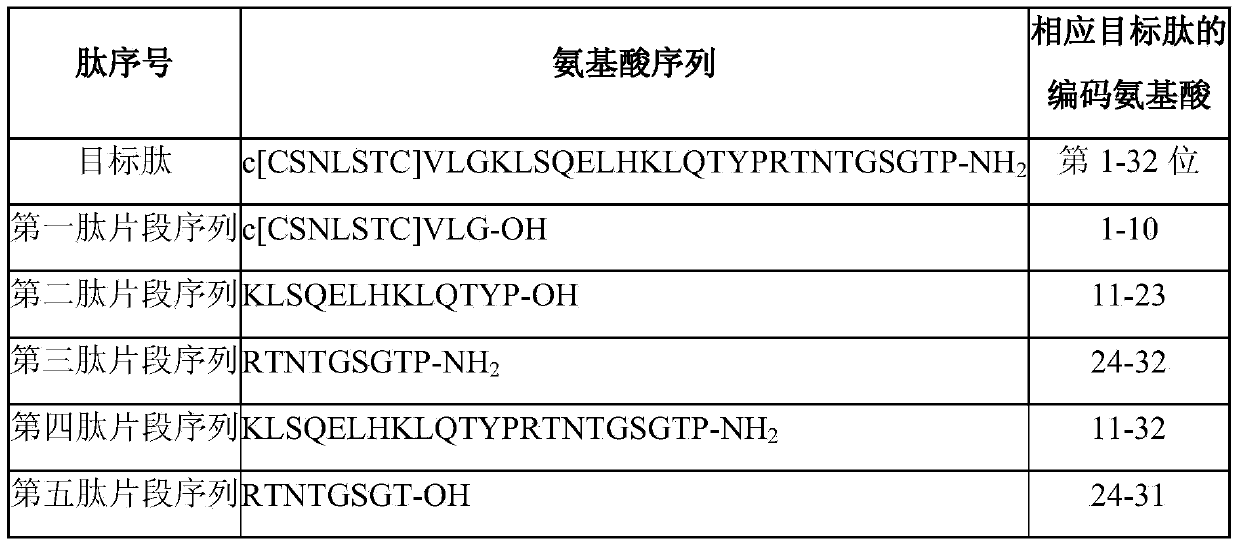
The invention discloses a method for preparing salmon calcitonin acetate by fragment condensation, which comprises the following steps: 1) synthesizing fragment sequences of salmon calcitonin (1st-10th, 11th-23rd, 24th-32nd or 24th-31st amino acids) on a solid-phase vector by solid-phase synthesis; 2) sequentially coupling the fragment in the liquid phase to form full-protection salmon calcitonin; and 3) cracking the full-protection salmon calcitonin to obtain salmon calcitonin crude peptides, and carrying out high-efficiency liquid-phase purification salt exchange to obtain the salmon calcitonin acetate pure peptides. The method for preparing salmon calcitonin acetate by combining solid / liquid phase and fragment condensation enhances the yield and purity, and is low in cost and beneficial to large-scale production.
Owner:HAINAN JIANKE PHARMA
Calcitonin-gene-related peptide and trout calcitonin amalgamation polypeptide transgenic sequence, and transgenic engineering bacterial strain
InactiveCN101319223AEffective ELISAInexpensive and effective in field cultivationBacteriaMicroorganism based processesRecombinant salmon calcitoninEscherichia coli
The invention provides a fusion polypeptide transgene sequence for calcitonin-gene-related peptide and salmon calcitonin and a transgene engineering strain thereof, and relates to a fusion polypeptide transgene sequence and a transgene engineering strain. The invention solves the problem that: the prior calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide are not suitable for industrialized production with microbes and animals as reactors. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene sequence is shown as SEQ ID NO.1. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene engineering strain is an agrobacterium containing fusion gene plasmids, wherein the fusion genes in the plasmids are shown as SEQ ID NO.1. The calcitonin-gene-related peptide and salmon calcitonin fusion polypeptide transgene engineering strain can be used for mediating a plant with the cost of the engineering strain being 1 / 10 to 1 / 50 of that of bacillus coli.
Owner:HEILONGJIANG UNIV
Fusion protein and method for preparing same
InactiveCN1721446AGood antigenicityLow antigenicityHybrid peptidesDNA/RNA fragmentationRecombinant salmon calcitoninCancer research
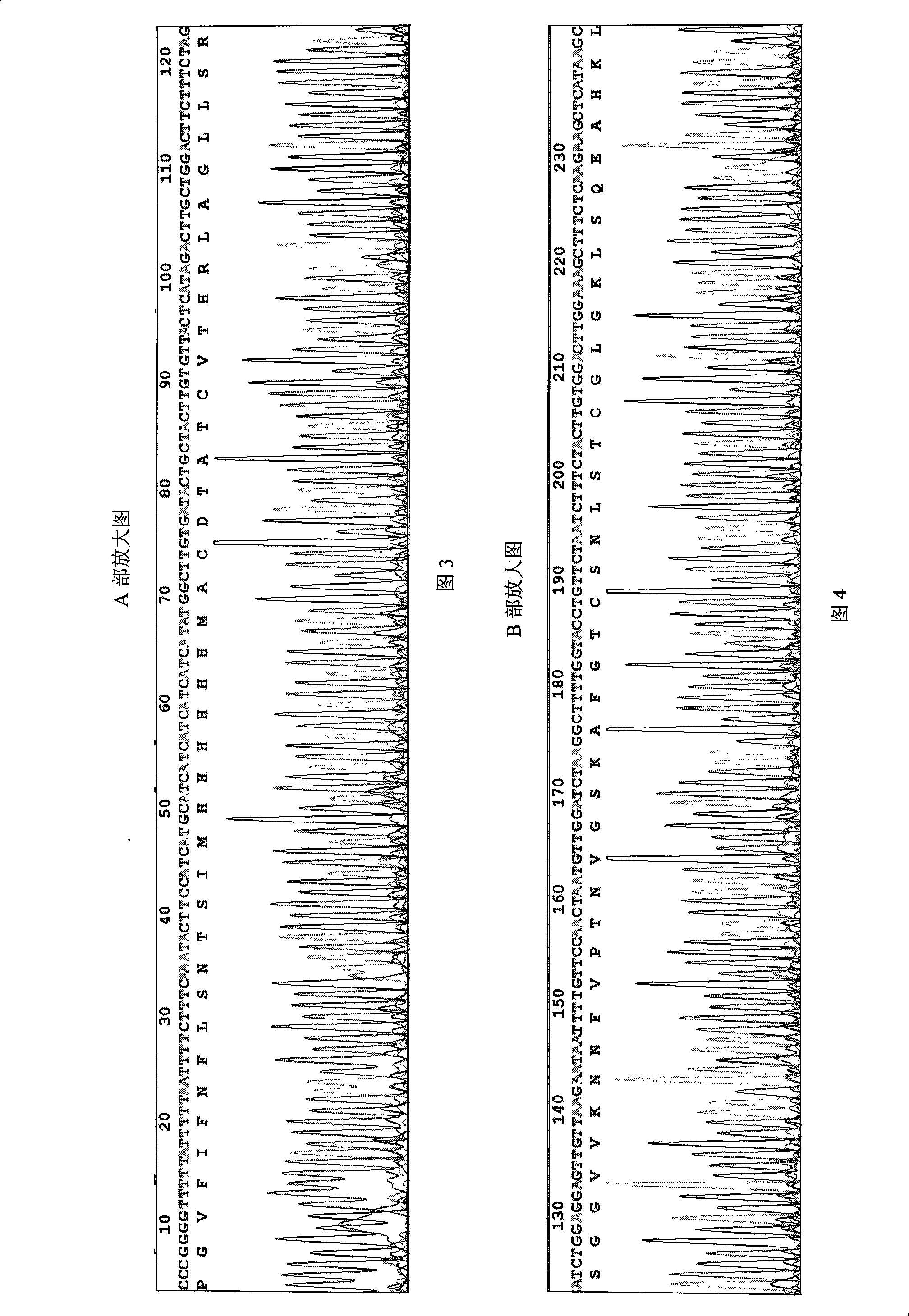
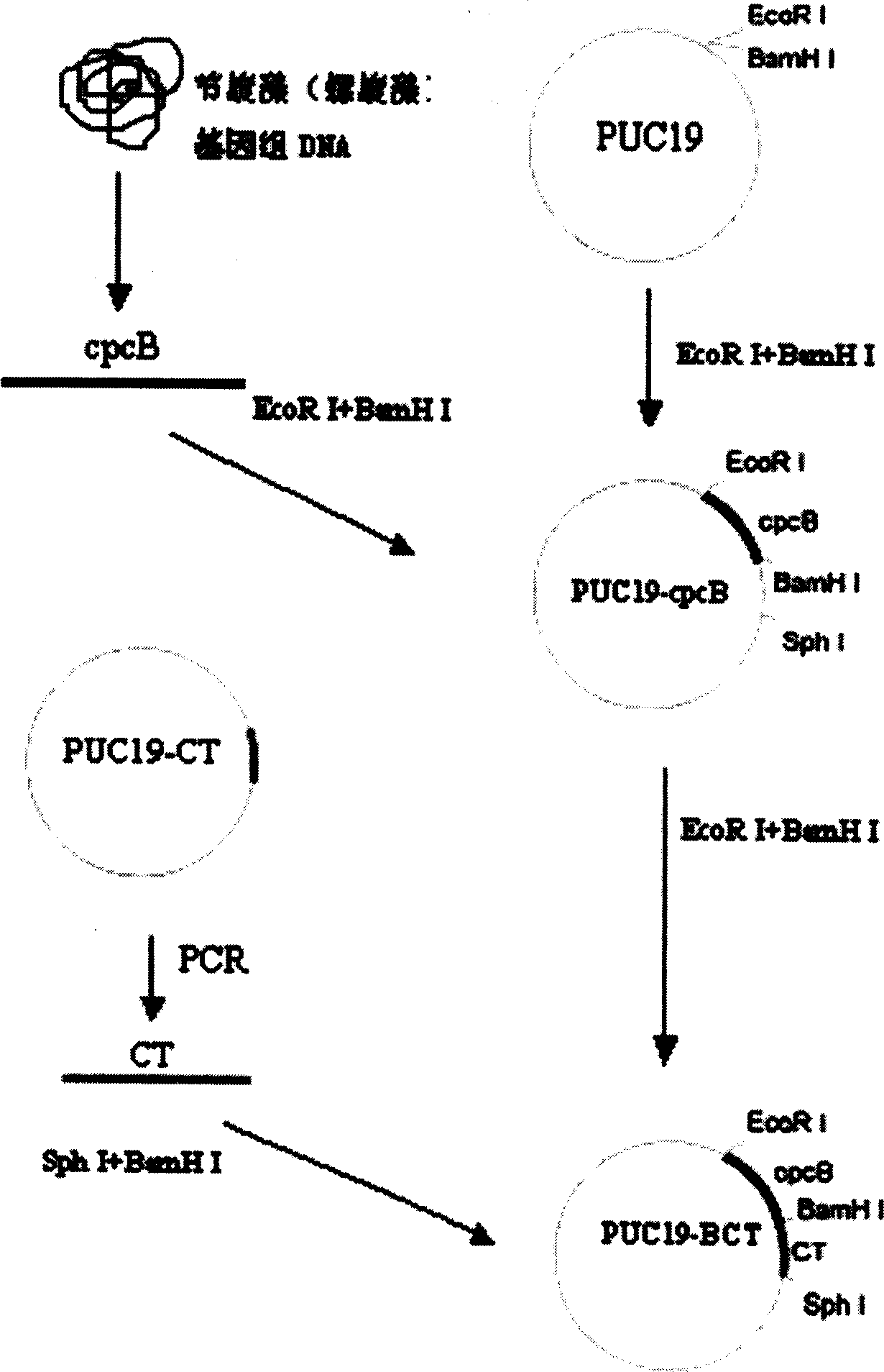
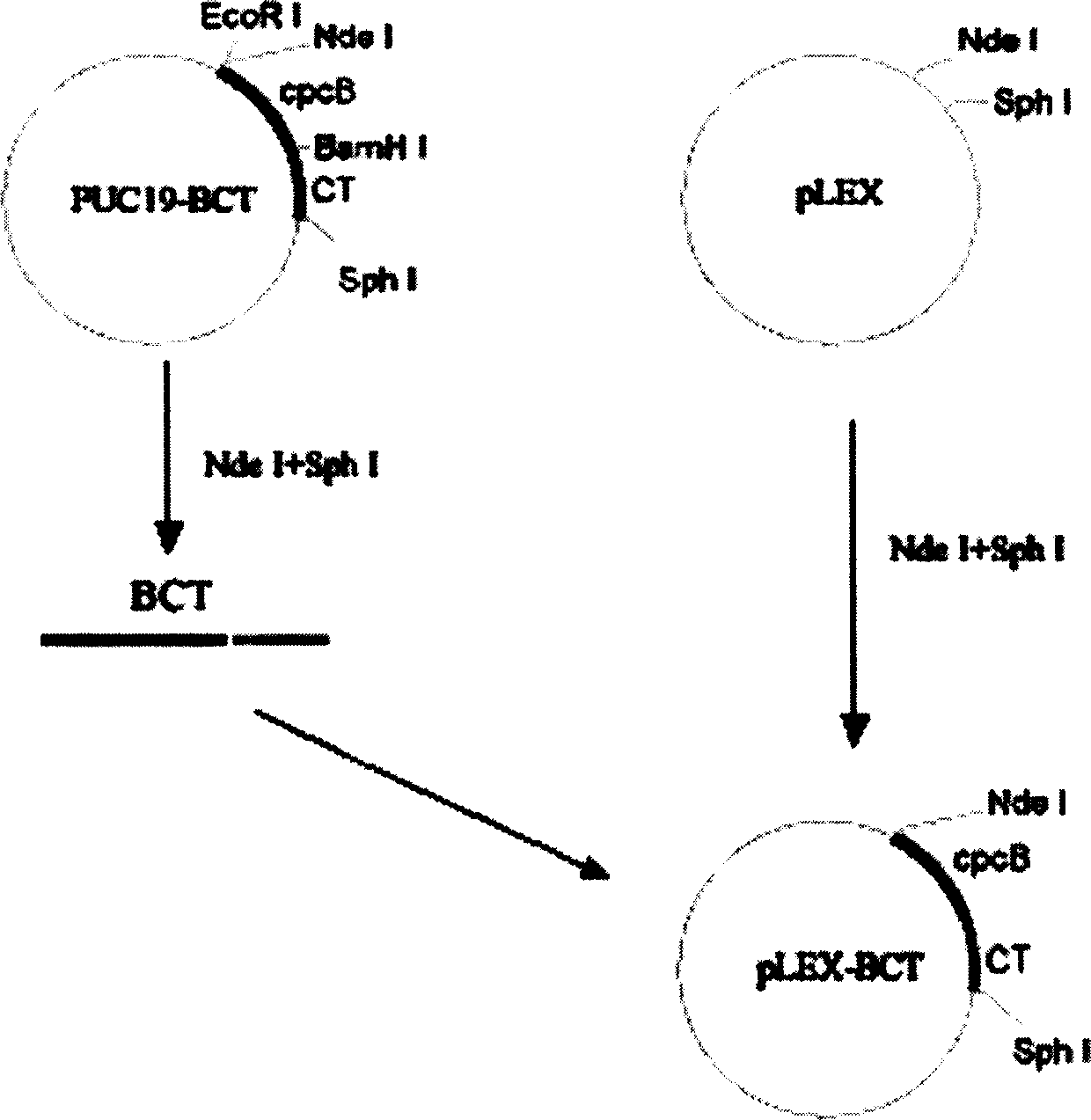
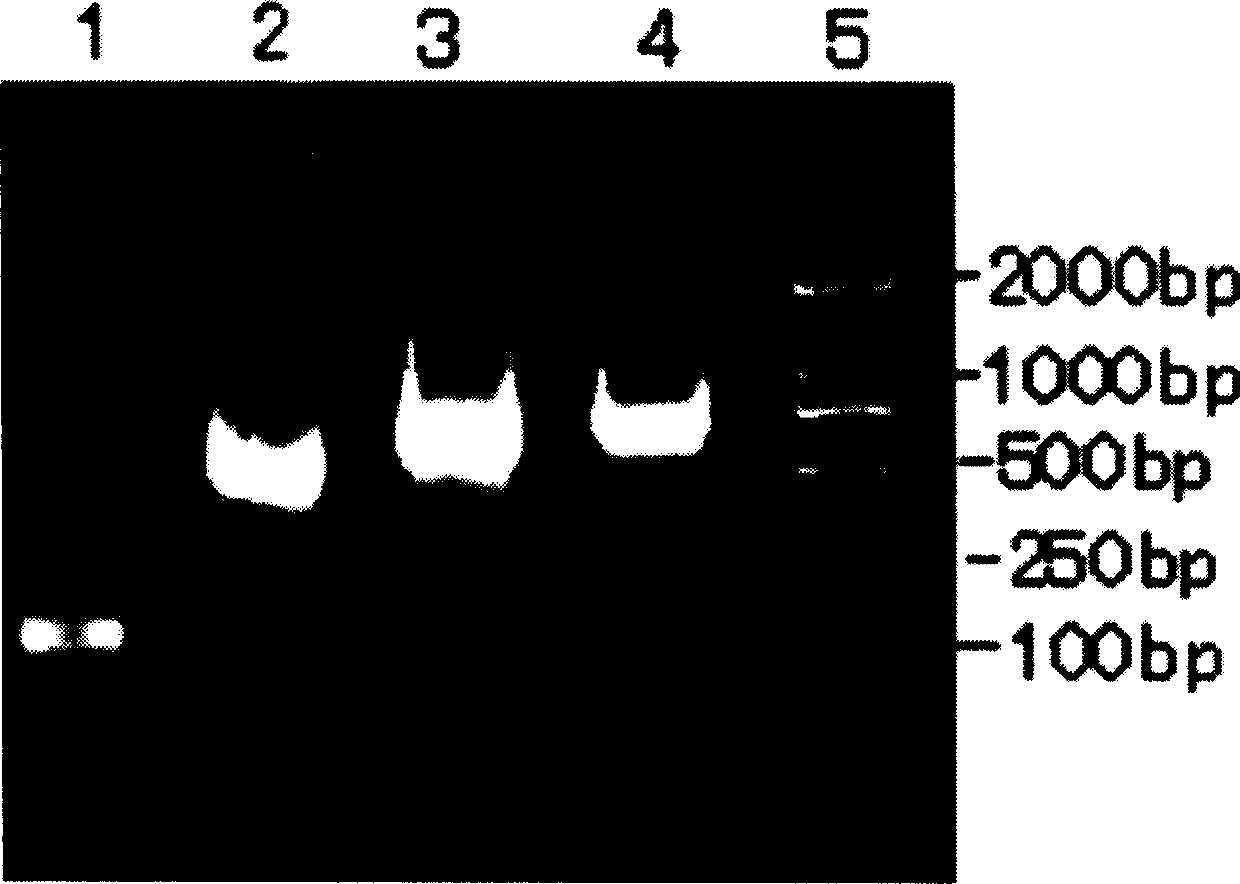
The present invention is fusion protein constituted through fusing phycocyanin beta-subunit gene in the upstream of human and salmon calcitonin chimera gene. The fusion protein has length 648 bp, molecular weight 23 KDa, amino acid sequence with 100 % homology with the sequence shown in SEQ No. 2 and polypeptide with 213 residues; and has prokaryotic expression vector with preservation number of CCTCC1261. The calcitonin chimera gene has length 102 bp; and the phycocyanin beta-subunit gene is cpcB gene of length 519 bp and polypeptide coding 171 amino acid residues. The constitution of the fusion protein needs no processing and purification, and this ensures the high expression amount of the calcitonin, increases the stability in clinical application, lowers the antigenicity of calcitonin and raises its bioactivity.
Owner:OCEAN UNIV OF CHINA
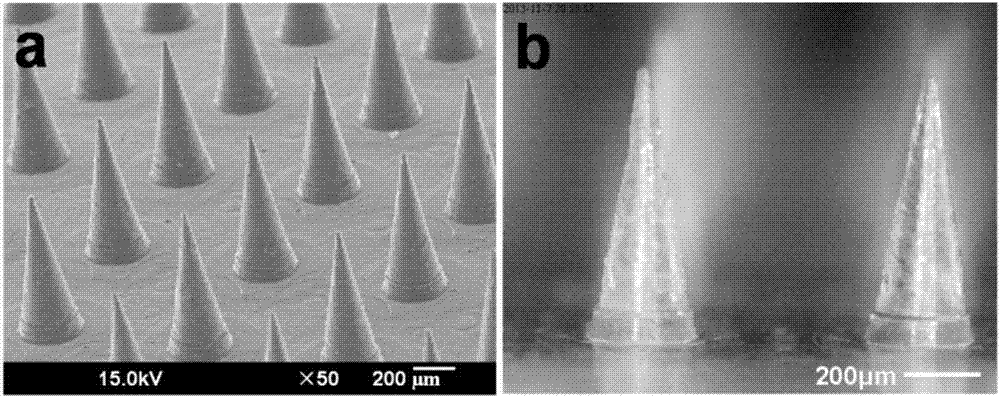
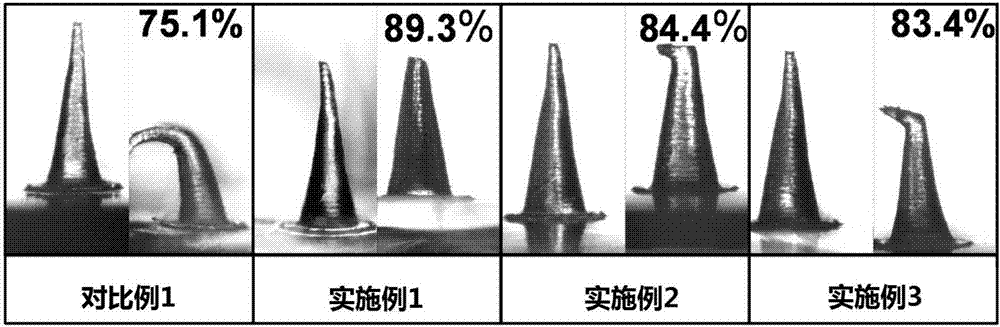
Salmon calcitonin soluble microneedle patch and preparation method thereof
ActiveCN107096013AImprove in vivo delivery efficiencyImprove mechanical propertiesPeptide/protein ingredientsMicroneedlesRecombinant salmon calcitoninWater soluble
The invention relates to a salmon calcitonin soluble microneedle patch and a preparation method thereof. The salmon calcitonin soluble microneedle patch is composed of a substrate and a needle body, the needle body is prepared from a mixed solution of salmon calcitonin, dextran and trehalose, the mass ratio of salmon calcitonin to dextran to trehalose is 1:5-20:4-8; and the substrate is prepared from a solution of a water soluble polymer material. The salmon calcitonin soluble microneedle patch has the advantages of good mechanical strength, good stability, good effect of reducing blood calcium and high bioavailability. The addition of the trehalose can facilitates the improvement of the stability of the salmon calcitonin and the transdermal drug release efficiency of a microneedle, the bioavailability of the salmon calcitonin is improved, and a higher effect of reducing the blood calcium can be achieved.
Owner:广州新济生物医药研究院有限公司 +1
Calcitonin-gene-related peptide and trout calcitonin amalgamation polypeptide
The invention provides calcitonin gene-related peptide and salmon calcitonin fusion polypeptide, relating to fusion polypeptide. In order to generate a product containing both calcitonin and the calcitonin gene-related peptide, the invention provides the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide. The amino acid sequence of the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide is shown in SEQ ID No:1; moreover, C-terminal amidation is carried out to the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide. Experiments verify that the calcitonin gene-related peptide and the salmon calcitonin fusion polypeptide preserve respective activities of the calcitonin gene-related peptide and salmon calcitonin.
Owner:HEILONGJIANG UNIV
Biomarkers for the efficacy of calcitonin and parathyroid hormone treatment
InactiveCN1905894APeptide/protein ingredientsMicrobiological testing/measurementRecombinant salmon calcitoninAnabolic Effect
A mufti-organ gene profiling analysis of the results of an administration to a subject of salmon calcitonin or a parathyroid hormone analogue provides biomarkers of calcitonin treatment efficacy and parathyroid hormone or parathyroid hormone analogue treatment efficacy. Among the biomarkers are the expression profiles of the genes for Y-box binding protein, BMPs, FGFs, IGFs, VEGF, &x3B1;-2-HS glycoprotein (AHSG), OSF, nuclear receptors (steroid / thyroid family) and others. The results obtained support the anabolic effect of salmon calcitonin on bone metabolism.
Owner:NOVARTIS AG
Preparation method of salmon calcitonin acetate
ActiveCN105111301AReduce effective utilizationReduce manufacturing costCalcitoninsPeptide preparation methodsRecombinant salmon calcitoninLysis
The invention discloses a preparation method of salmon calcitonin acetate. The method comprises the steps that Fmoc-Pro-OH is coupled with resin to obtain Fmoc-Pro-resin; the Fmoc-Pro-resin is coupled with protected amino acid and protected peptide fragments one by one to obtain salmon calcitonin acetate linear peptide resin; the salmon calcitonin acetate linear peptide resin is lysed in a lysis solution to obtain a reducing crude product; the reducing crude product is oxidized through an oxidizing agent to obtain an oxidized crude product; the oxidized crude product is purified to obtain the refined salmon calcitonin acetate.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
Method for ultrahigh-level and targeting expression of salmon calcitonin protein in oleosin of transgenic rape by using chromosome substitution technology
The invention provides a method for realizing targeting substitution by comprehensively using specific expression and gene targeting of plant oleosin and for ultrahigh-level expression of salmon calcitonin protein in transgenic rapeseeds. According to the invention, rape 5' UTR sequence is obtained by cloning 1900 bp with Race; an inherent endogenous sequence in the genome of rape is substituted by 5' UTR sequence of rape oleosin gene--sesame oleosin gene+CT fusion protein gene'--Pnos-NPTII-Tnos--3' UTR sequence of rape oleosin gene; a transgenic rape plant and a transgenic rape line are obtained by using the method of inplanta. According to detection results, the endogenous sequence in the genome of rape is substituted by a new sequence, which enables targeting expression of a target gene to be successfully realized and ultrahigh-level expression of salmon calcitonin protein in transgenic rapeseeds to be achieved. The method has the following remarkable advantages: the expression level of target protein is high, and the expressed protein is easy to store and purify.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Use of Calcitonin for the Treatment of RA
InactiveUS20120219603A1Organic active ingredientsPowder deliveryRecombinant salmon calcitoninFree form
The present invention relates to a novel use of calcitonin in rheumatoid arthritis, and to methods of treating and / or preventing rheumatoid arthritis and conditions associated therewith in mammals, particularly humans.In particular, a method is provided of preventing or / and treating rheumatoid arthritis in a patient in need thereof comprising administering to said patient a therapeutically effective amount of calcitonin, e.g. salmon calcitonin in free form or salt form, in a pharmaceutically acceptable oral delivery form, wherein the therapeutically effective amount of a calcitonin is delivered orally in a composition comprising the calcitonin and a delivery agent for calcitonin.
Owner:AZRIA MOISE +1
Salmon calcitonin nano liposome injection and preparation method thereof
InactiveCN102327239ASmall toxicityReduce dosagePowder deliveryNervous disorderRecombinant salmon calcitoninMedicine
The invention relates to the technical field of medicine, and discloses a cloxacillin sodium liposome preparation for injection and a preparation method thereof. The cloxacillin sodium liposome preparation is a powder injection, and is a sterile nano freeze-drying preparation which is prepared by coating salmon calcitonin with a pharmaceutically-acceptable biological carrier. The cloxacillin sodium liposome preparation comprises the following raw materials in parts by weight: 1 part of salmon calcitonin, 4-15 parts of pharmaceutically-acceptable biological carrier, 0.5-2 parts of stabilizer and 2-10 parts of freeze-drying protective agent. The product disclosed by the invention has high stability and a low side effect, can not be cracked as a result of dehydration, fusion, generation of ice crystals and the like in the freeze-drying process, and can keep a good entrapment rate after hydration and refusion, thereby facilitating the transportation and storage of the product.
Owner:傅苗青
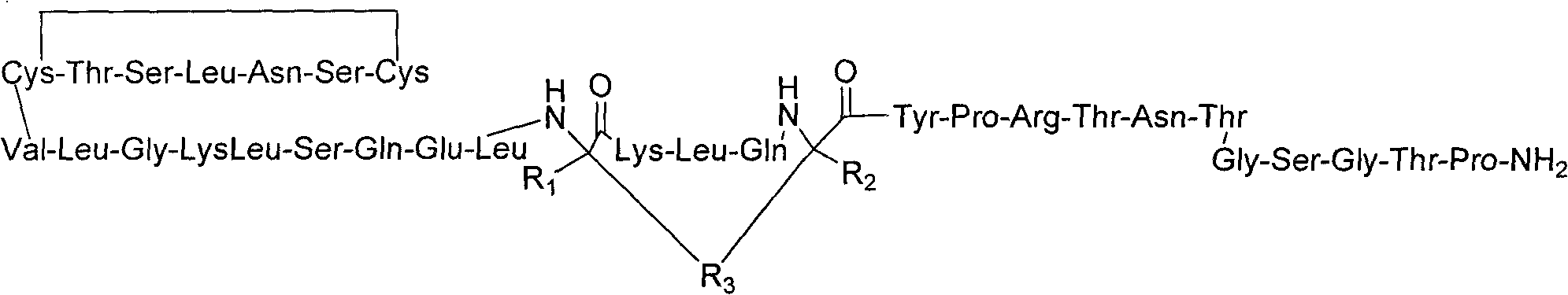
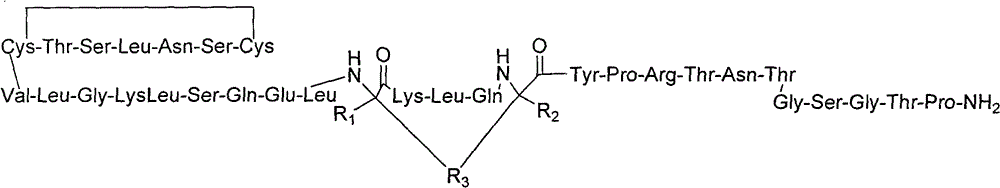
Derivatised hybrid peptides of amylin and salmon calcitonin
InactiveUS20110152183A1Enhances albumin binding effectTo/enhance the albumin binding effect of the substituentNervous disorderPeptide/protein ingredientsRecombinant salmon calcitoninPeptide sequence
Described are derivatives of hybrid peptides and pharmaceutical compositions comprising such, wherein said hybrid peptides comprise the C-terminal end of the human amylin peptide sequence, the middle portion of the salmon calcitonin peptide sequence and the N-terminal end of the human amylin peptide sequence, and wherein an albumin binding moiety is attached to the hybrid peptide, optionally via a linker.
Owner:NOVO NORDISK AS
Nucleic acid encoding recombinant salmon calcitonin, expression vector thereof, and method for producing recombinant salmon calcitonin therewith
Nucleic acid encoding recombinant salmon calcitonin, expression vector thereof, and method for producing recombinant salmon calcitonin therewith. The nucleic acid encoding recombinant salmon calcitonin comprises the sequence of SEQ ID No. 2
Owner:IND TECH RES INST
Calcitonin-gene-related peptide and trout calcitonin amalgamation polypeptide
Owner:HEILONGJIANG UNIV
Salmon calcitonin-phospholipid complex and its lipid nanoparticle and preparation method
ActiveCN105919974AImprove long-term stabilityFor long-term storageNervous disorderPeptide/protein ingredientsRecombinant salmon calcitoninMembrane permeability
The invention belongs to the technical field of medicines and relates to a salmon calcitonin-phospholipid complex and its lipid nanoparticle and preparation method. The salmon calcitonin-phospholipid complex and its lipid nanoparticle can improve drug lipid solubility, have a certain intestinal adhesion, can be easily absorbed through intestinal mucosa and can prevent drug degradation caused by gastrointestinal tract protease. The lipid nanoparticle of the salmon calcitonin-phospholipid complex comprises the salmon calcitonin-phospholipid complex, a lipid material, a surfactant and water and is prepared through a film dispersion method. The water-soluble drug nanoparticle obtained through the preparation method has a high drug oil-liquid distribution coefficient and good membrane permeability and improves oral bioavailability. The preparation method has simple processes.
Owner:CHENGDU UNIV
Transgenic salmon calcitonin gene yeast as well as preparation method and uses thereof
InactiveCN101182474AEasy to useAchieve oral administrationFungiSugar derivativesRecombinant salmon calcitoninOral medication
The invention discloses transgenic salmon calcitonin yeast, a preparation method and the application. The salmon calcitonin is expressed in a eukaryotic system of Saccharomyces cerevisiae. The fermentation technology of Saccharomyces cerevisiae is mature with a higher expression and the Saccharomyces cerevisiae can be eaten directly; the obtained transgenic salmon calcitonin Saccharomyces cerevisiae can be taken orally without purification. The invention reduces the production cost and realizes oral administration of calcitonin.
Owner:OCEAN UNIV OF CHINA
Salmon's calcitonin gene synthesized by plant preference codon and its application
A salmon's calcitonin gene synthesized by plant preference codon, its fusion gene configurator, the recombinant expression carrier carrying said configurator, the plant cell converted by said expression carrier, the transgenic plant and its descendant generated by said converted cells for expressing said salmon's calcitonin, and the process for detecting the nucleic acid sequence and polypeptide of salmon's calcitonin in specimen are disclosed.
Owner:FUDAN UNIV
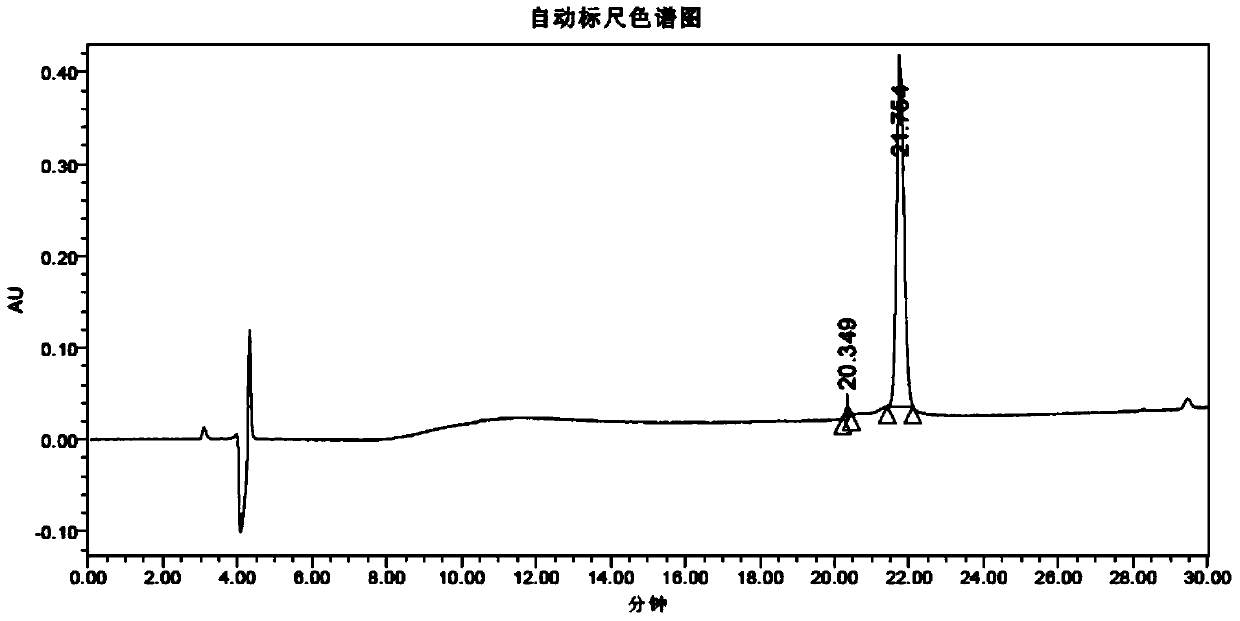
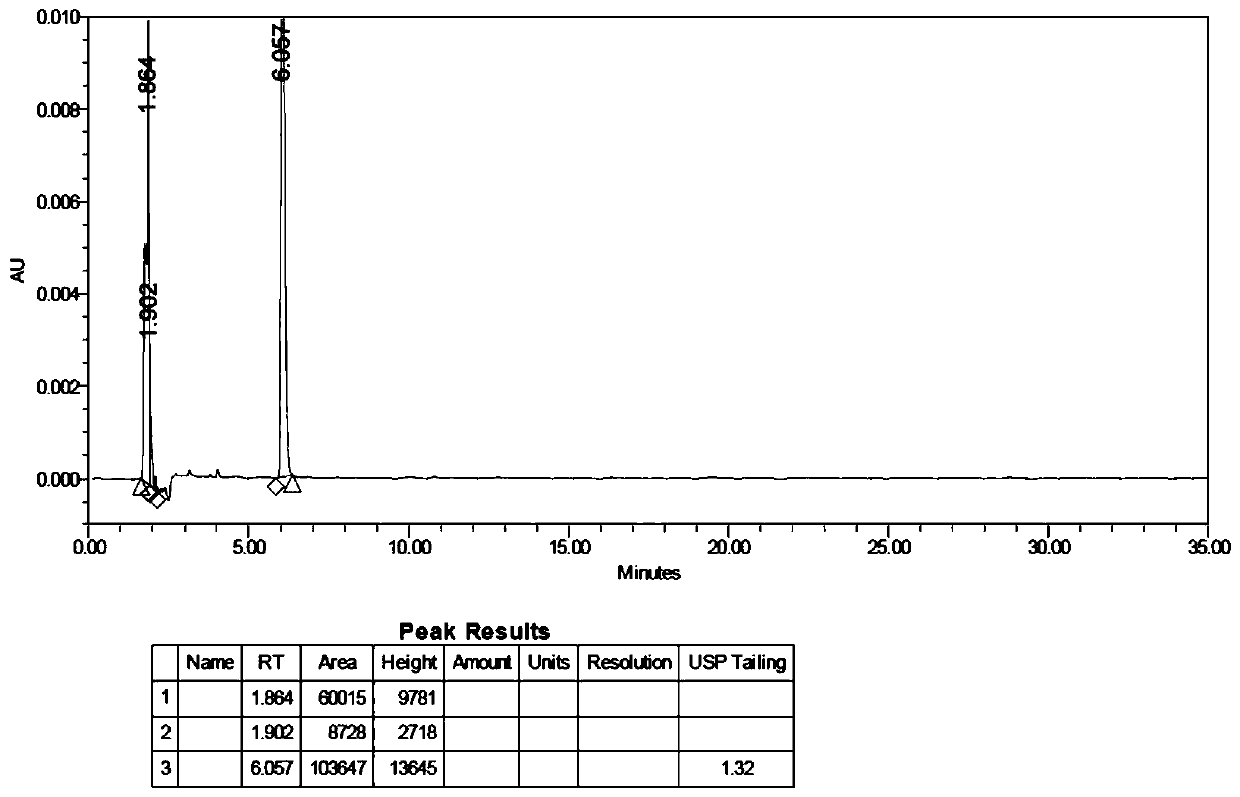
Method for determining content of benzalkonium chloride in salmon calcitonin nasal spray by HPLC method
PendingCN110031584ADetection pertinenceEasy to detectComponent separationRecombinant salmon calcitoninHplc method
The invention belongs to the field of analytical chemistry, and relates to a method for determining the content of benzalkonium chloride in a salmon calcitonin nasal spray by an HPLC method. A chromatographic column adopted in the method adopts octadecyl silane chemically bonded silica as filler, and an optimized mobile phase A and a mobile phase B are adopted for isocratic elution; and a drug isa polypeptide nasal spray, and a bacteriostatic agent is benzalkonium chloride. According to the method, the content of the bacteriostatic agent in the salmon calcitonin nasal spray of polypeptide drugs can be conveniently and accurately determined; and the method is easy and convenient to operate, rapid, accurate, high in sensitivity, good in repeatability, mild in condition and small in trailingfactor, through systematic methodology verification, it is proved that the method is applied to the determination of the content of the benzalkonium chloride in the salmon calcitonin nasal spray, theinspection results are accurate and reliable, and great significance to the quality control of the salmon calcitonin nasal spray is achieved.
Owner:YINGU PHARMA
Site-specific pegylated linear salmon calcitonin analogues
InactiveUS20100227815A1Reduced calcium levelPeptide/protein ingredientsCalcitoninsDiseaseRecombinant salmon calcitonin
The present invention relates to site-specific PEGylated linear salmon calcitonin analogues, or pharmaceutically acceptable salts thereof, process for their preparation, pharmaceutical compositions comprising them, and their use for the preparation of a medicament for the treatment or prevention of diseases associated with bone metabolism, e.g., osteoporosis.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Biomarkers for the efficacy of calcitonin and parathyroid hormone treatment
InactiveUS20070099828A1Peptide/protein ingredientsCalcitoninsRecombinant salmon calcitoninAnabolic Effect
A mufti-organ gene profiling analysis of the results of an administration to a subject of salmon calcitonin or a parathyroid hormone analogue provides biomarkers of calcitonin treatment efficacy and parathyroid hormone or parathyroid hormone analogue treatment efficacy. Among the biomarkers are the expression profiles of the genes for Y-box binding protein, BMPs, FGFs, IGFs, VEGF, &x3B1;-2-HS glycoprotein (AHSG), OSF, nuclear receptors (steroid / thyroid family) and others. The results obtained support the anabolic effect of salmon calcitonin on bone metabolism.
Owner:BOBADILLA MARIA
Novel salmon calcitonin analogue and preparation method as well as use thereof
ActiveCN102911266ARetain pharmacological activityGood chemical stabilityPeptide/protein ingredientsCalcitoninsChemical structureRecombinant salmon calcitonin
The invention relates to a novel salmon calcitonin analogue and a preparation method thereof. Compared with a natural salmon calcitonin, the chemical stability of a compound provided by the invention is improved and the pharmacological activity of the natural salmon calcitonin is kept. The salmon calcitonin analogue provided by the invention has the following chemical structure.
Owner:CHINESE PEPTIDE CO
Method for preparing salmon calcitonin
ActiveCN106755085AImprove biological activityCalcitoninsFermentationRecombinant salmon calcitoninProtein tag
The invention discloses a method for preparing salmon calcitonin. The method provided by the invention comprises the following steps: (1) importing a recombinant vector containing an expression cassette into a receiver plant, so as to obtain a transgenic plant, wherein the expression cassette comprises the following elements from top to bottom in sequence: a promoter of a plant oil body protein gene, a fusion gene and a termination sequence; the fusion gene comprises a segment III used for coding a protein tag, a segment I used for coding a plant oil body protein, a digestion identification sequence of protease and a nucleic acid molecule of a precursor used for coding salmon calcitonin; (2) taking seeds of the transgenic plant for extraction of oil bodies; (3) purifying a fusion protein from the oil bodies by utilizing the protein tag; (4) digesting the fusion protein with protease; (5) separating salmon calcitonin; (6) acetifying; (7) performing desalting. Salmon calcitonin with comparatively high biological activity can be obtained by utilizing the method provided by the invention. Therefore, the method provided by the invention has an important application value.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Novel salmon calcitonin analogue and its preparation method and use
Owner:CHINESE PEPTIDE CO
Method for preparing salmon calcitonin
ActiveCN107699588AImprove biological activityCalcitoninsVector-based foreign material introductionRecombinant salmon calcitoninPlanting seed
The invention discloses a method for preparing salmon calcitonin. The method provided by the invention sequentially comprises the following steps: (1) introducing a recombinant vector containing an expression cassette into a receptor plant, thus obtaining a transgenic plant, wherein the expression cassette sequentially comprises the following elements from the upstream to the downstream: a promoter of a plant seed specific expression protein gene, a fusion gene and a termination sequence; the fusion gene comprises a section c for coding a protein label, an identification sequence of formic acid, and nucleic acid molecules encoding the precursor of the salmon calcitonin; (2) taking the seeds of the transgenic plant, and extracting crude protein after degreasing; (3) purifying the fusion protein from the oil body by utilizing the protein label; (4) carrying out cutting by using formic acid; (5) separating salmon calcitonin; (6) carrying out acetification; and (7) carrying out desalting.The experiment proves that with the method provided by the invention, salmon calcitonin with high biological activity is obtained. Therefore, the method provided by the invention has an important application value.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
A kind of preparation method of salmon calcitonin
ActiveCN105111301BReduce effective utilizationReduce manufacturing costCalcitoninsPeptide preparation methodsRecombinant salmon calcitoninLysis
The invention discloses a preparation method of salmon calcitonin acetate. The method comprises the steps that Fmoc-Pro-OH is coupled with resin to obtain Fmoc-Pro-resin; the Fmoc-Pro-resin is coupled with protected amino acid and protected peptide fragments one by one to obtain salmon calcitonin acetate linear peptide resin; the salmon calcitonin acetate linear peptide resin is lysed in a lysis solution to obtain a reducing crude product; the reducing crude product is oxidized through an oxidizing agent to obtain an oxidized crude product; the oxidized crude product is purified to obtain the refined salmon calcitonin acetate.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
Salmon calcitonin phospholipid complex, its lipid nanoparticles and preparation method
ActiveCN105919974BIncrease fat solubilityFacilitate transmembrane absorptionNervous disorderPeptide/protein ingredientsRecombinant salmon calcitoninWater soluble drug
The invention belongs to the technical field of medicines and relates to a salmon calcitonin-phospholipid complex and its lipid nanoparticle and preparation method. The salmon calcitonin-phospholipid complex and its lipid nanoparticle can improve drug lipid solubility, have a certain intestinal adhesion, can be easily absorbed through intestinal mucosa and can prevent drug degradation caused by gastrointestinal tract protease. The lipid nanoparticle of the salmon calcitonin-phospholipid complex comprises the salmon calcitonin-phospholipid complex, a lipid material, a surfactant and water and is prepared through a film dispersion method. The water-soluble drug nanoparticle obtained through the preparation method has a high drug oil-liquid distribution coefficient and good membrane permeability and improves oral bioavailability. The preparation method has simple processes.
Owner:CHENGDU UNIV
Preparation method for salmon calcitonin, conjugated preparation of salmon calcitonin and use of conjugated preparation in drugs for osteoporosis
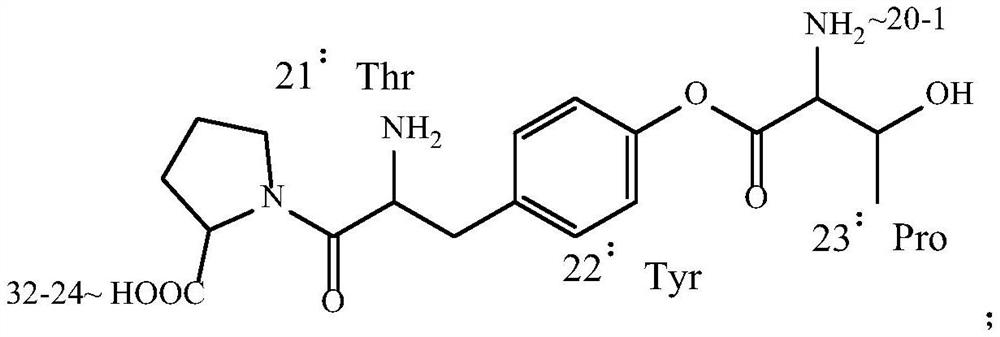
ActiveCN111303273ALower C and ERapid responsePeptide/protein ingredientsCalcitoninsRecombinant salmon calcitoninPharmaceutical drug
The invention discloses a preparation method for salmon calcitonin, a preparation of the salmon calcitonin and use of the preparation in drugs for osteoporosis. According to the preparation method, when amino acids of 22 and 23 positions of a fragment of the salmon calcitonin prepared by an Fmoc-policy solid-phase method are condensed, a condensation agent system DFIH / EEDQ is employed, and thus, generation of an impurity E and an impurity C of the salmon calcitonin can be lowered; and when amino acids of 17 and 18 positions of a fragment of the salmon calcitonin prepared by the Fmoc-policy solid-phase method are condensed, DMSO is used as a condensation agent, a weight ratio of the DMSO to Fmoc-His-OH is (10 to 15): 1, and thus, generation of an impurity F of the salmon calcitonin can be lowered. The calcitonin prepared by the preparation method is good in stability and few in impurities; and the calcitonin biological vector conjugated preparation disclosed by the invention has new usein treatment of osteoporosis of the male.
Owner:广东金城金素制药有限公司
A preparation method of salmon calcitonin, its combined preparation and the application of the combined preparation in medicine for osteoporosis
ActiveCN111303273BLower C and ERapid responsePeptide/protein ingredientsCalcitoninsRecombinant salmon calcitoninMedicine
The invention discloses a preparation method for salmon calcitonin, a preparation of the salmon calcitonin and use of the preparation in drugs for osteoporosis. According to the preparation method, when amino acids of 22 and 23 positions of a fragment of the salmon calcitonin prepared by an Fmoc-policy solid-phase method are condensed, a condensation agent system DFIH / EEDQ is employed, and thus, generation of an impurity E and an impurity C of the salmon calcitonin can be lowered; and when amino acids of 17 and 18 positions of a fragment of the salmon calcitonin prepared by the Fmoc-policy solid-phase method are condensed, DMSO is used as a condensation agent, a weight ratio of the DMSO to Fmoc-His-OH is (10 to 15): 1, and thus, generation of an impurity F of the salmon calcitonin can be lowered. The calcitonin prepared by the preparation method is good in stability and few in impurities; and the calcitonin biological vector conjugated preparation disclosed by the invention has new usein treatment of osteoporosis of the male.
Owner:广东金城金素制药有限公司
Method for expressing salmon calcitonin and special expression cassette for method
ActiveCN106636189AImprove biological activityCalcitoninsNucleic acid vectorRecombinant salmon calcitoninWAS PROTEIN
The invention discloses a method for expressing salmon calcitonin and a special expression cassette for the method. The method provided by the invention sequentially comprises the following steps: (1) introducing a recombinant vector into a receptor plant so as to obtain a transgenic plant; and (2) cultivating the transgenic plant so as to obtain the salmon calcitonin, wherein the recombinant vector comprises the expression cassette; the expression cassette, from upstream to downstream, sequentially comprises the following elements: a promoter of a plant seed specific expression protein gene, a fusion gene and a terminator sequence; the fusion gene contains a section B; the section B is in charge of coding a precursor of the salmon calcitonin; and the precursor of the salmon calcitonin is protein with an amino acid sequence shown as the 158th-190th sites from N terminal of a sequence 5 in a sequence list. Experiments prove that with the application of the method provided by the invention, the obtained salmon calcitonin is relatively high in bioactivity. Therefore, the method provided by the invention, in the expression of the salmon calcitonin, has an important application value.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com