Use of Calcitonin for the Treatment of RA
a technology of rheumatoid arthritis and calcitonin, which is applied in the field of rheumatoid arthritis treatment with calcitonin, can solve the problems of premature death and substantial disability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Formulation 1 (3 Batches)
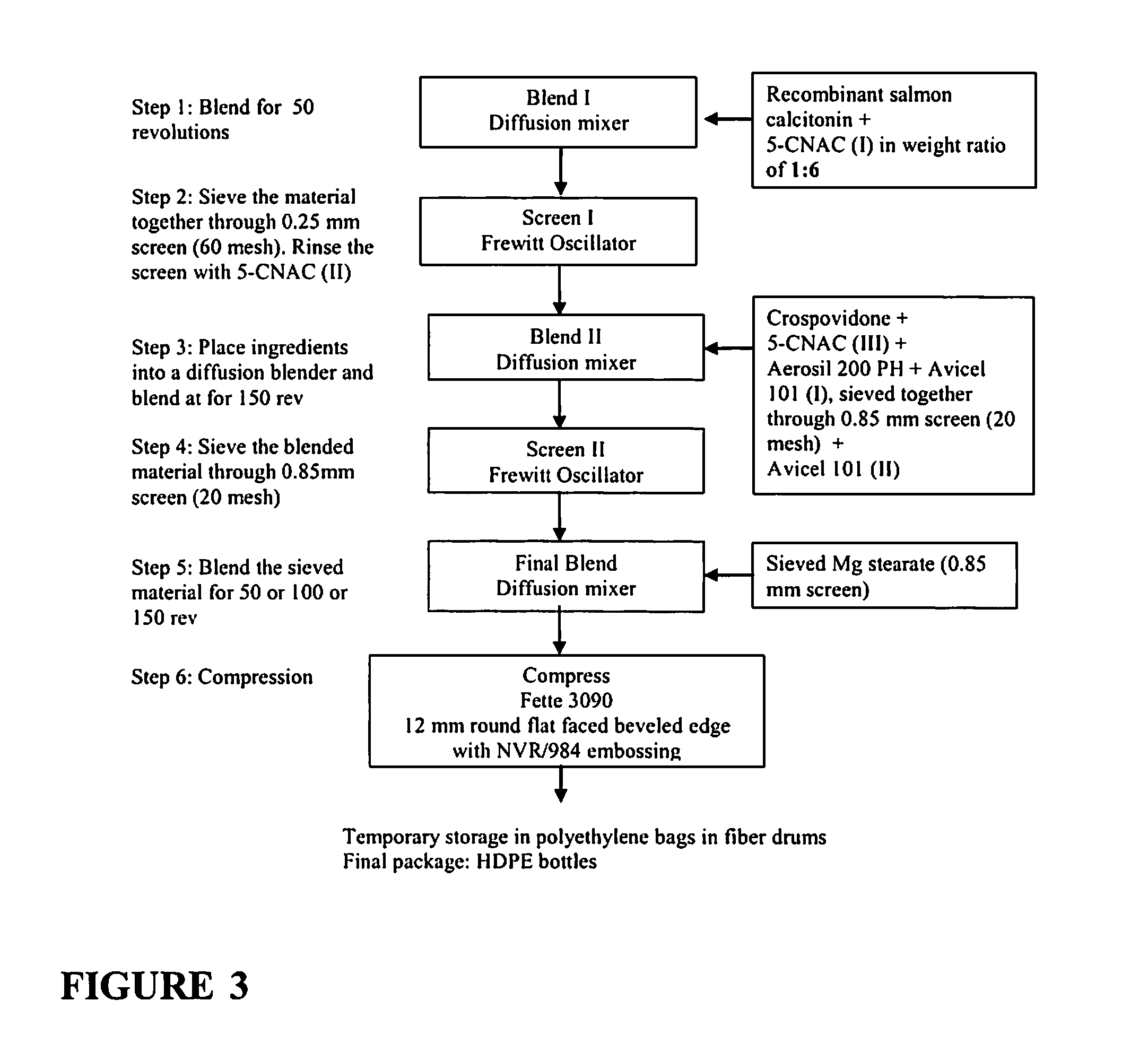
[0087]Preparation of Micronized 5-CNAC: Coarse 5-CNAC, which is to be micronized, is added to a jet mill (Air Jet Mill Gem T® Copley Scientific, Ltd., Nottingham, UK) using a 80 ceramic pan cake jet mill, 8 cm diameter, 6 bar N2, 0.5 mm nozzles with manual feed of about 700 g / h. The coarse 5-CNAC is jet milled and periodically sampled under microscope with reference ruler measurements to identify when the average desired micronized particle size is obtained. Three different batches are ground to create a mean particle size, i.e. D50=6 um, 35 um, and 46 um batches. Individual sieving of the separate micronized batches is then done by using a conical sieve mill (Quadro Comil, Quadro Engineering Incorporated 613 Colby Drive, Waterloo, Ontario, Canada N2V 1A1) with a U10, 813 um conical sieve, round beater, operating at 1500 rpm with throughput of about 150 kg / h.
[0088]Formulation 1-3. Salmon Calcitonin Formulation with 5-CNAC of Different Particle Size
Ingredient...
example 2
Preparation of Formulations 2-3
[0090]Alternatively, there are further formulations provided:
Formulation 2:
[0091]
Ingredient% for batchBatch mg per tabletRecombinant 0.120.6Salmon calcitonin5-CNAC (I)0.24a1.25-CNAC (II)45.36b226.8Avicel PH 101 (I)3a15aAvicel PH 101 (II)44.9b224.9bCrospovidone525Aerosil 200 PH0.31.5Magnesium stearate1.05Total tablet weight (mg)100500Unit weight (a + b) listed as 5-CNAC disodium salt, corresponding to combined weight of 200 mg 5-CNAC free acid.Unit weight (a + b) of Avicel PH 101 (I) and (II) corresponds to combined weight of Avicel PH 101.
Formulation 3
[0092]
Ingredient% for batchBatch mg per tabletRecombinant0.160.8Salmon calcitonin5-CNAC (I)2.1a4.8a5-CNAC (II)2.1b4.8b5-CNAC (III)41.4c218.4cAvicel PH 101 (I)3a15aAvicel PH 101 (II)44.9b224.7bCrospovidone525Aerosil 200 PH0.31.5Magnesium stearate1.05Total tablet weight (mg)100500Unit weight (a + b + c) listed as 5-CNAC disodium salt, corresponding to combined weight of 200 mg 5-CNAC free acid.Unit weight (...
example 3
Preparation of Formulation 4
[0104]The above mentioned formulation 2 is compressed into tablets with various hardness using Manesty Beta press or Fette 3090 using different compression forces. Resultant tablets having different dissolution profiles were tested in Rhesus Monkeys for oral absorption of sCT. Tablets and their physical properties are listed in Table 1 and 2 below:
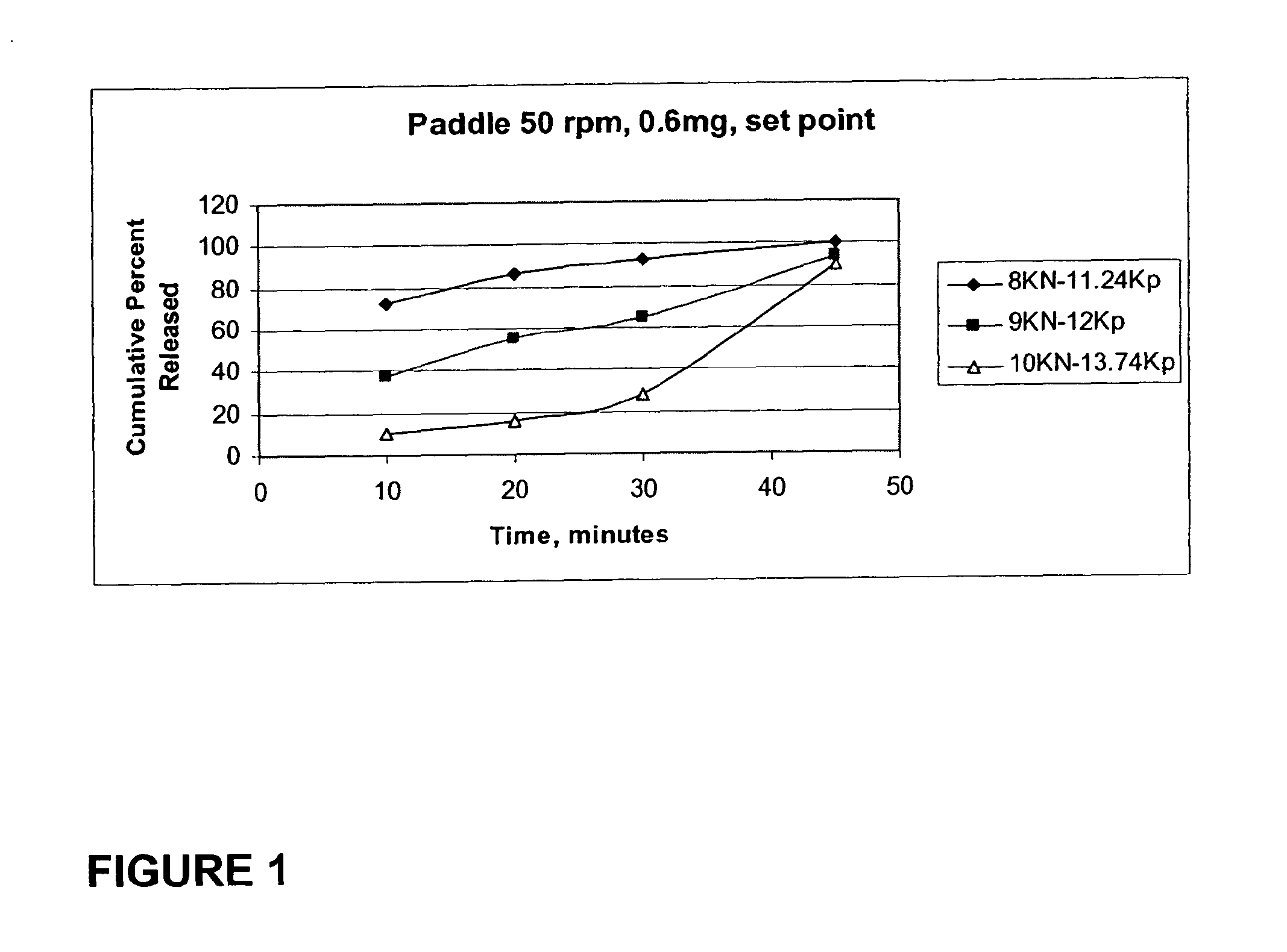
TABLE 10.6 mgSpeed: 197600 tab / hrwhich is 27 rpmForceWeightWeightThicknessHardnessHardness(KN)(mg)RSD(mm)(Kp)rangeDTFriability5.5500.580.584.955.885.7-6.130 s6504.150.894.866.795.7-7.740 s0.737.1503.681.014.69.41 8.3-10.42 m 30 s-2 m 15 s0.258499.680.694.5210.24 9.8-10.93 m 40 s-5 m 35 s0.528.5502.040.934.4711.711.2-12.84 m 30 s-5 m 46 s0.259505.740.624.431211.7-12.66 m 15 s-7 m 55 s0.1410.2504.80.574.3113.7412.8-14.67 m 19 s-8 m 8 s
TABLE 20.8 mgSpeed: 329400 tab / hrwhich is 45 rpmForceWeightWeightThicknessHardnessHardness(KN)(mg)RSD(mm)(Kp)rangeDTFriability5.1497.010.674.882.942.4-3.120 s1.1 (severechipping)6.44...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com