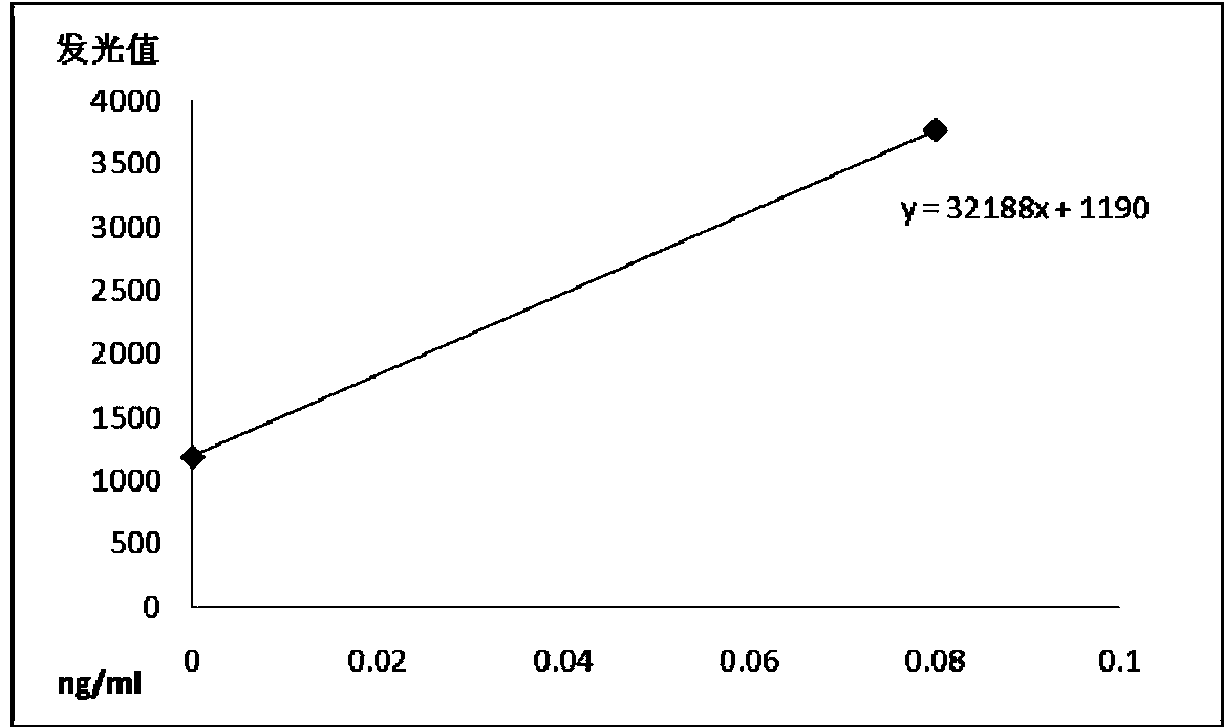
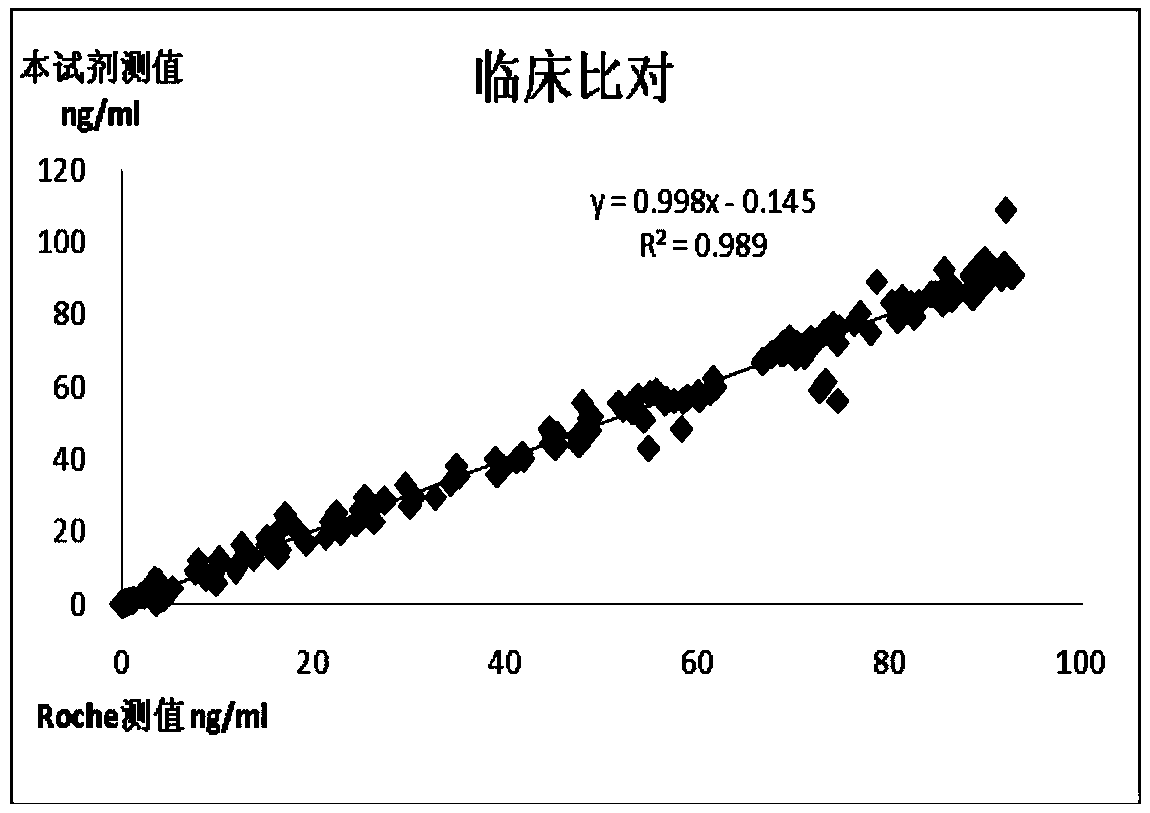
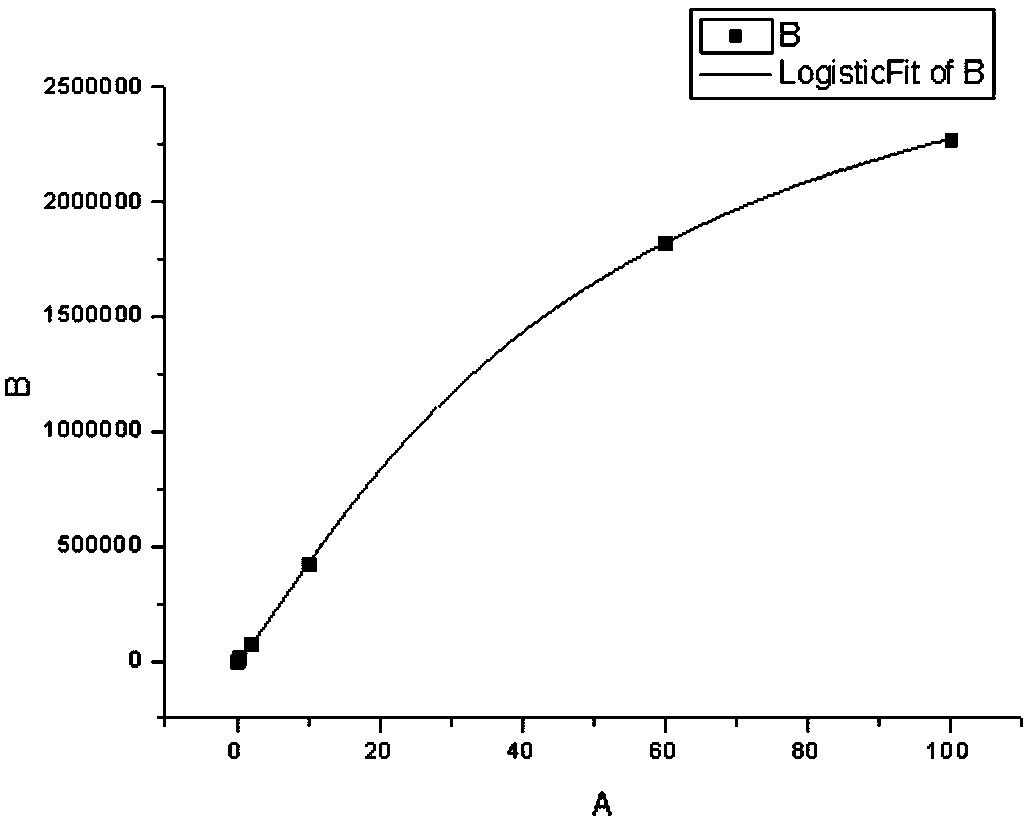
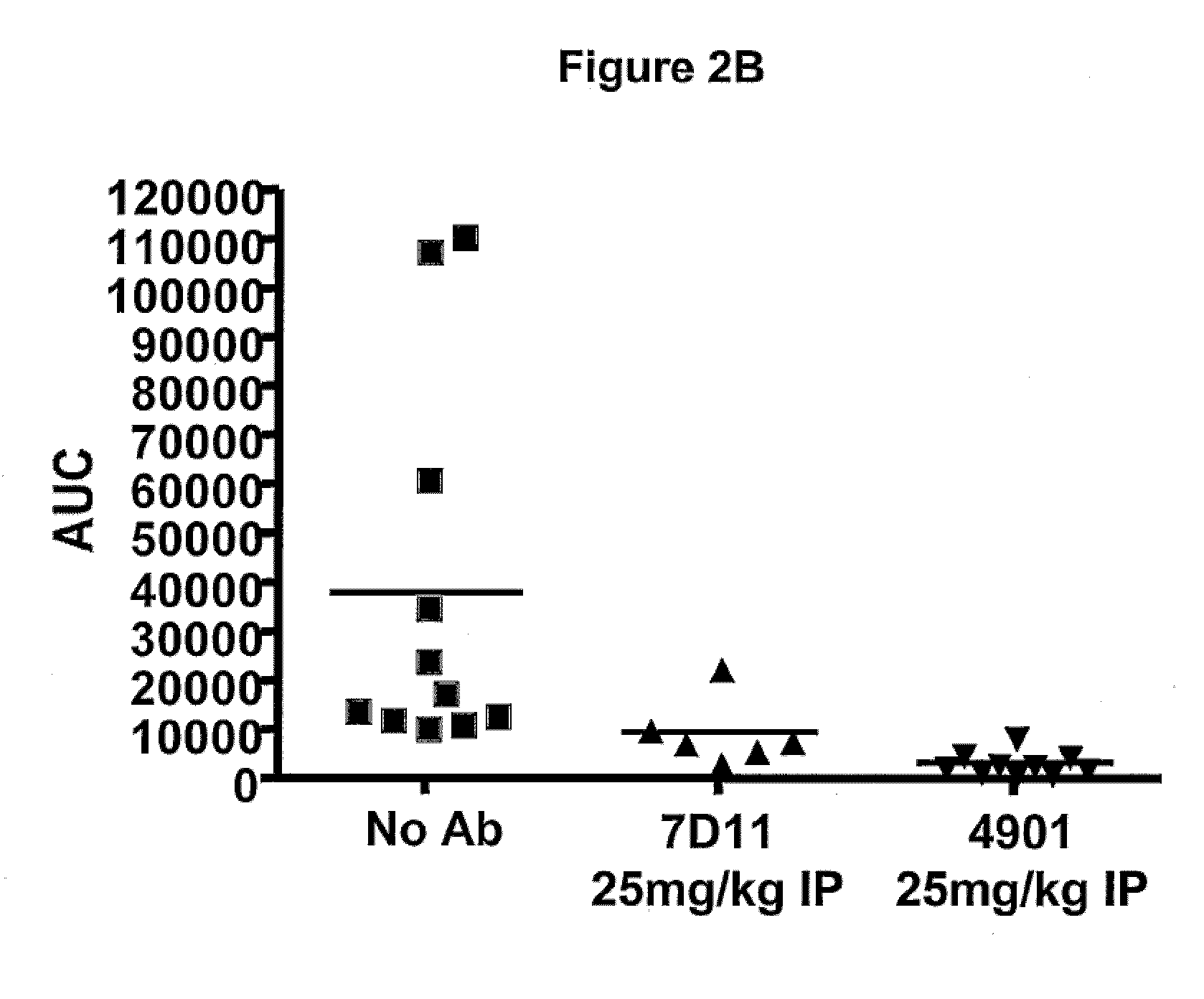
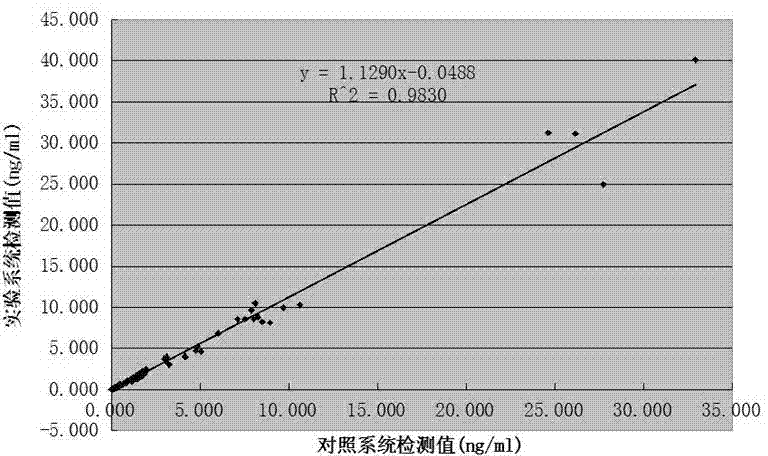
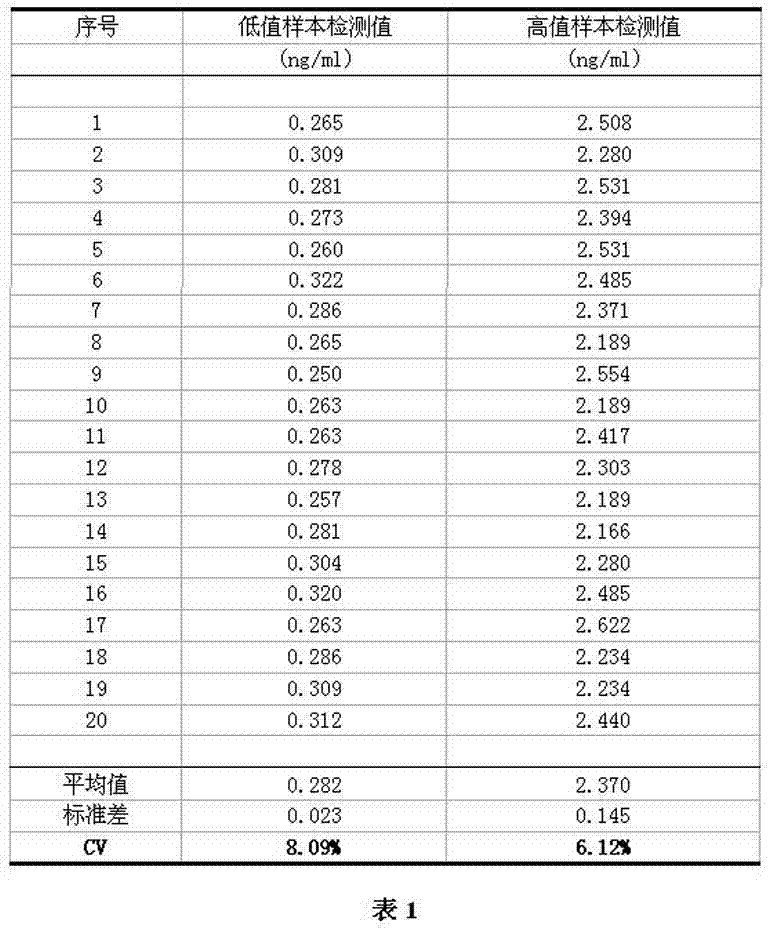
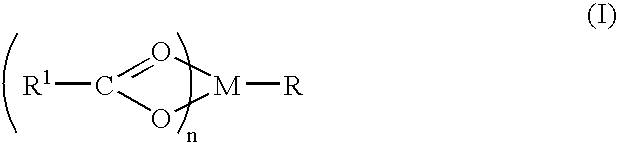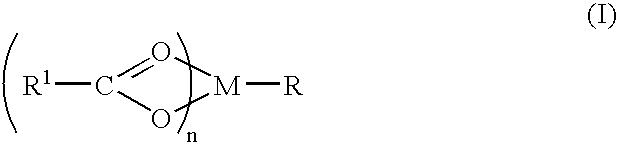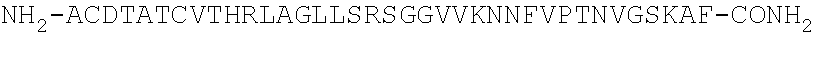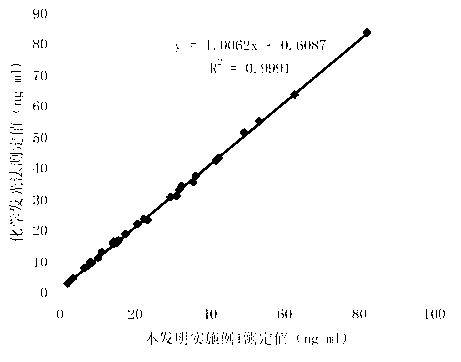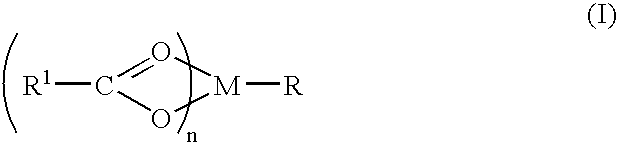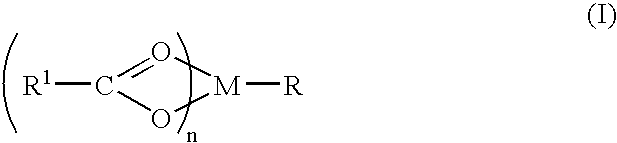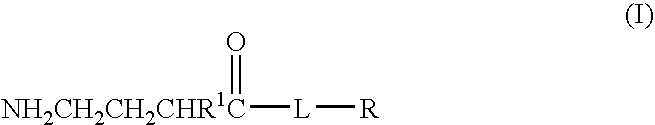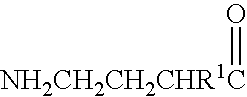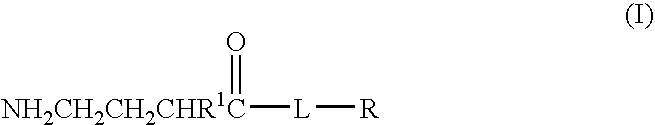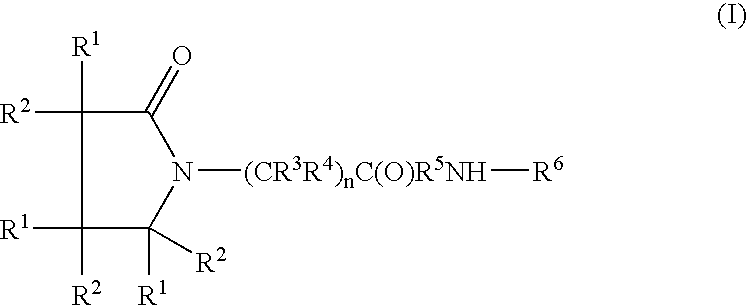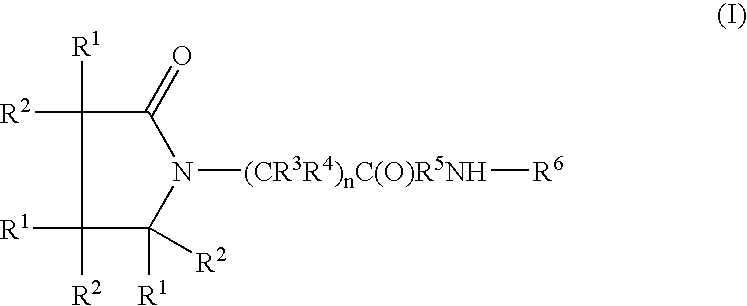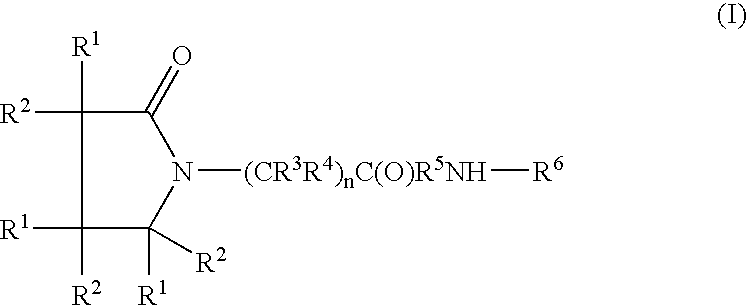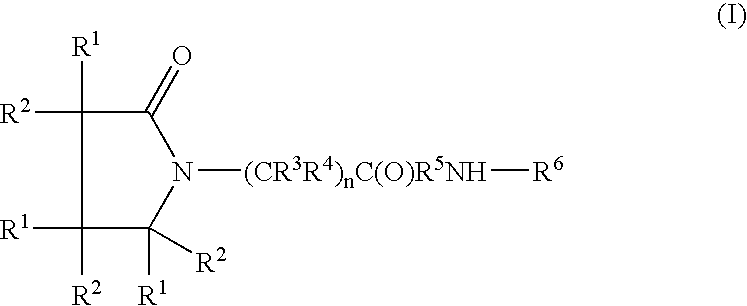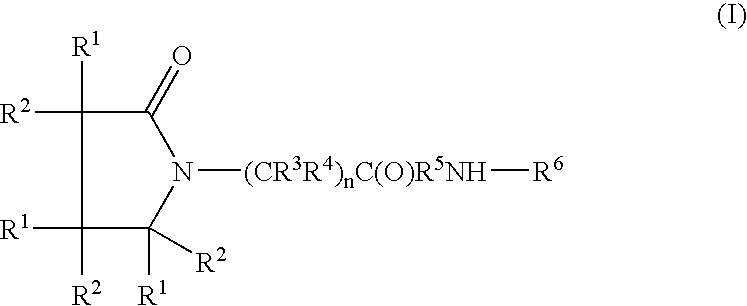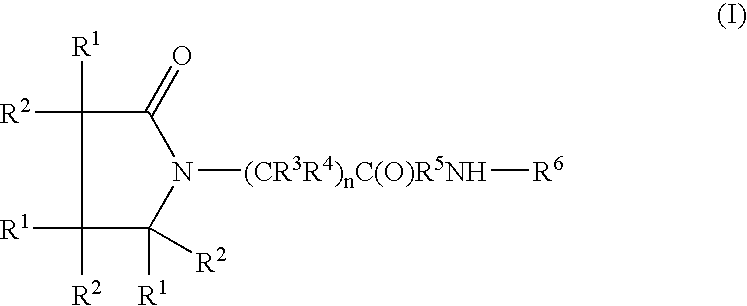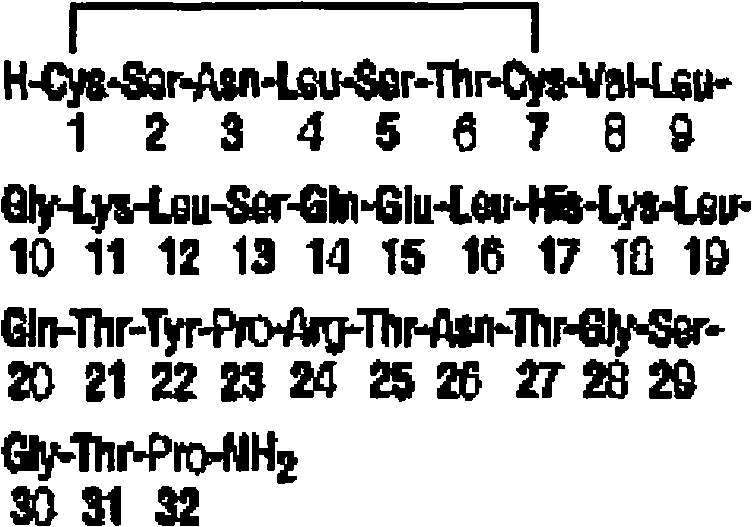Patents
Literature
254 results about "Calcitonin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcitonin is a 32 amino acid peptide hormone secreted by parafollicular cells (also known as C cells) of the thyroid gland in humans, and in many other animals in the ultimopharyngeal body. It acts to reduce blood calcium (Ca²⁺), opposing the effects of parathyroid hormone (PTH).
Anti-diabetic peptides
InactiveUS6087334AInhibiting gastric emptyingSmall sizeHormone peptidesPeptide/protein ingredientsMetabolic derangementMammal
Compounds of formula I which act as amylin agonists with respect to certain desired amylin activities and as calcitonin agonists with respect to certain desired calcitonin activities are provided. Such compounds are useful in treating disturbances in fuel metabolism in mammals, including, but not limited to diabetes mellitus, including Type I diabetes and Type II diabetes. The present invention also relates to methods of treating Type I diabetes, treating Type II diabetes and to methods of beneficially regulating gastrointestinal motility comprising administration of a therapeutically effective among of one of the compounds. Also provided are pharmaceutical composition comprising a compound of formula I and a pharmaceutically acceptable carrier.
Owner:ASTRAZENECA PHARMA LP
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
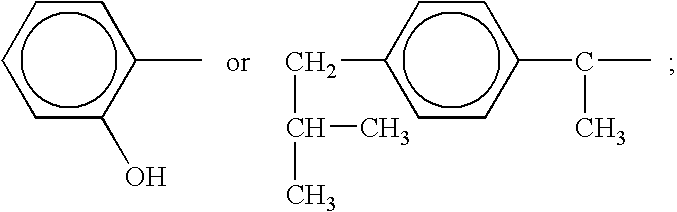
A complex is provided for the treatment of neurogenic conditions having the formula: where R1 is M is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium (II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium (II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium (II):transporter ligand.
Owner:MILLER LANDON C G
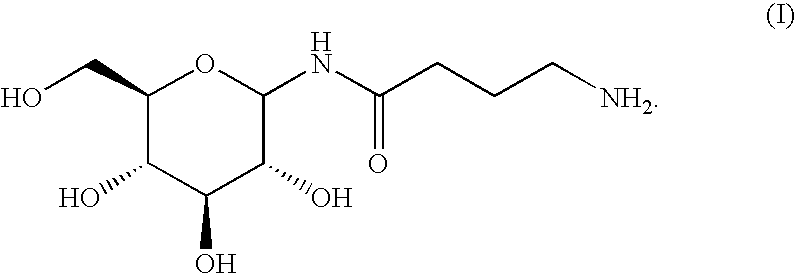
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS20060058219A1Improve efficiencyEliminate side effectsBiocidePeptide/protein ingredientsTryptophanSaccharin
A compound is provided that has the formula NH2CH2CH2CH2C(O)N—R (I) where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
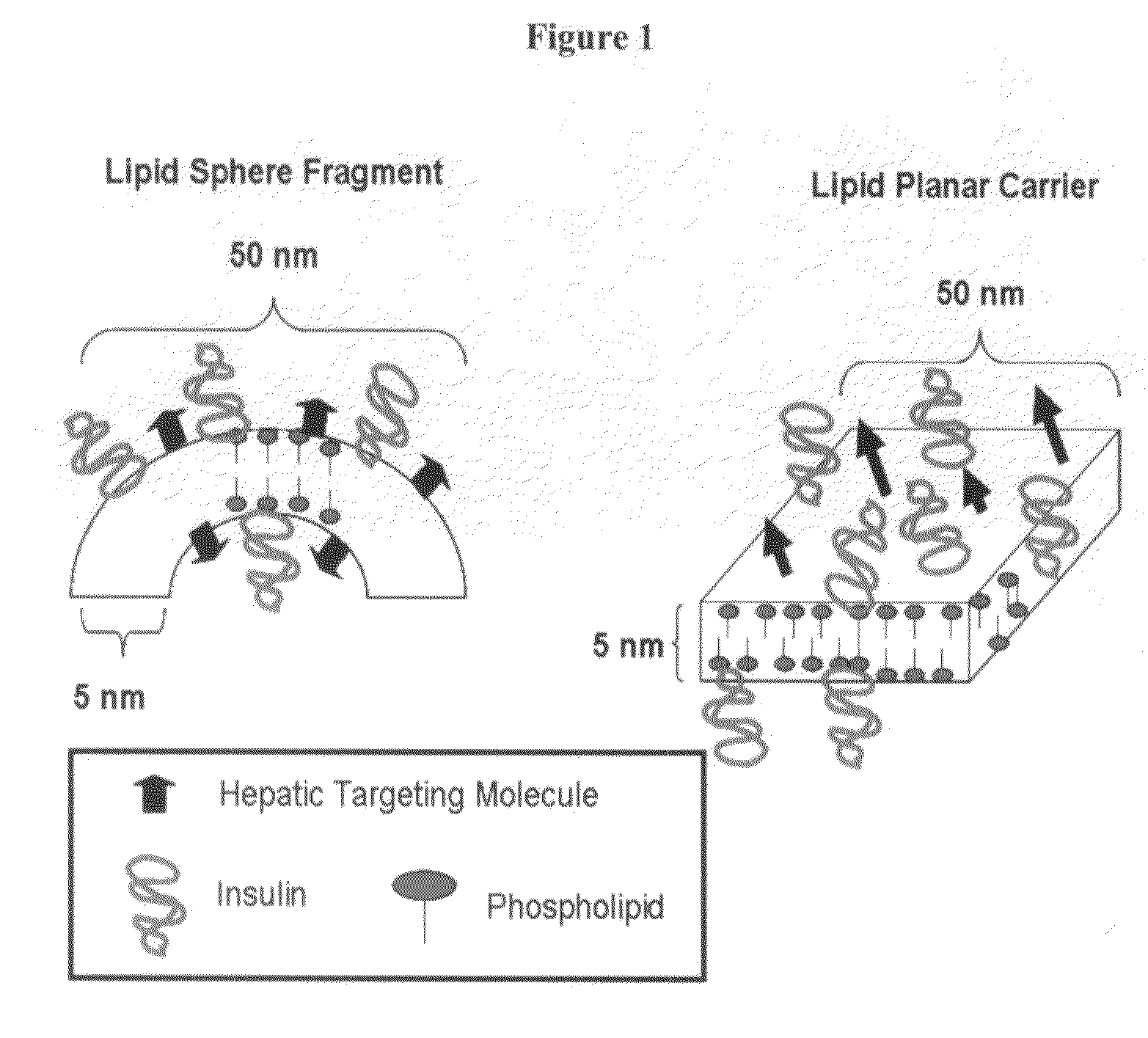
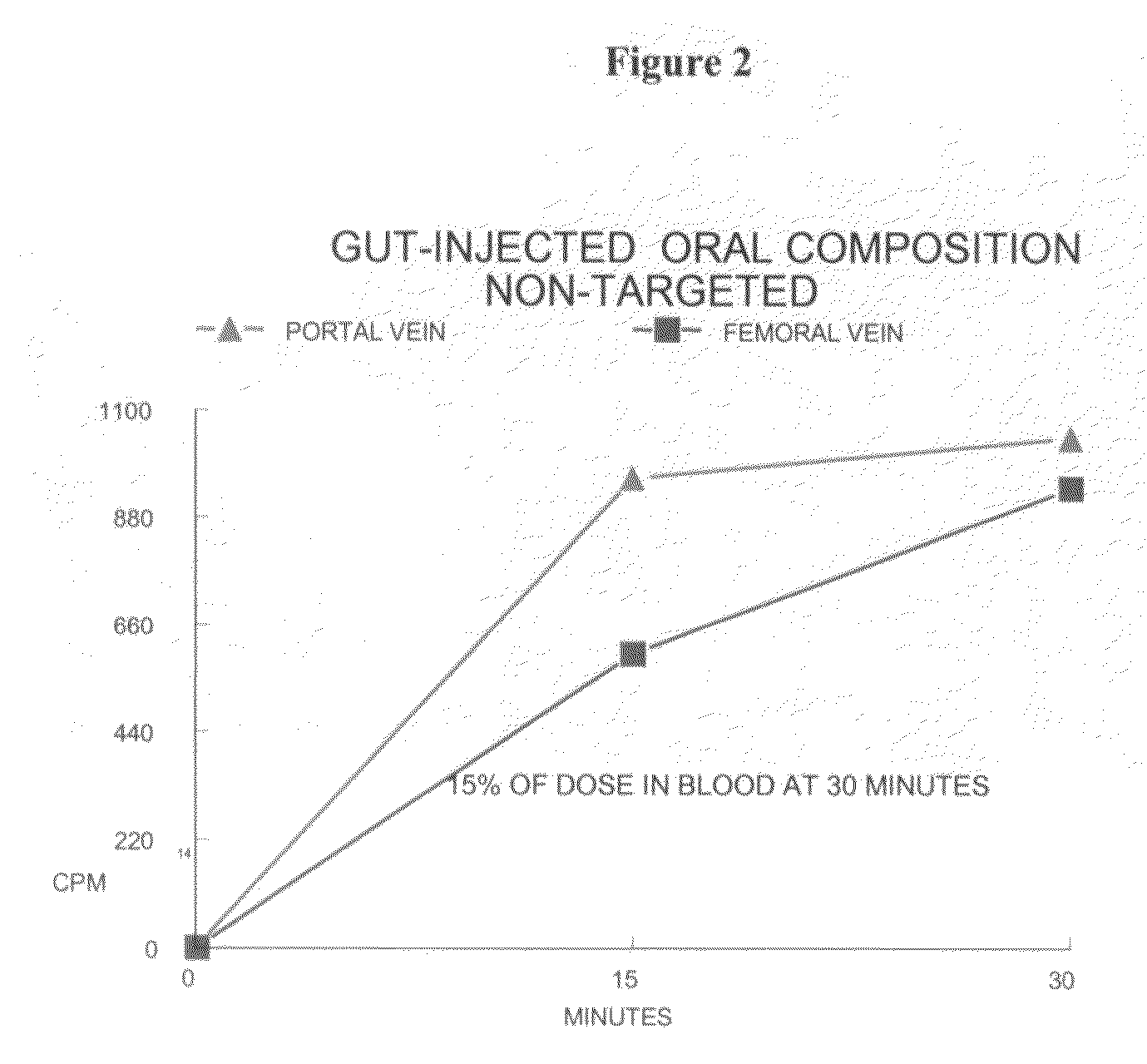
Orally bioavailable lipid-based constructs
ActiveUS20090087479A1Organic active ingredientsPeptide/protein ingredientsLipid formationDiagnostic agent
The present invention is embodied by a composition capable of chaperoning a typically non-orally available therapeutic or diagnostic agent through the environment of the digestive tract such that the therapetucic or diagnostic agent is bioavailable. The composition may or may not be targeted to specific cellular receptors, such as hepatocytes. Therapeutic agents include, but are not limited to, insulin, calcitonin, serotonin, and other proteins. Targeting is accomplished with biotin or metal based targeting agents.
Owner:SDG INC (US)
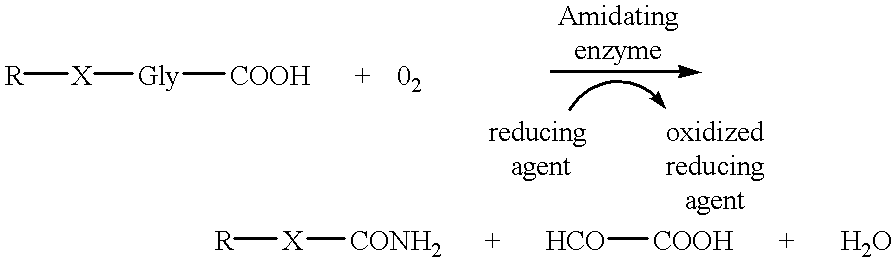
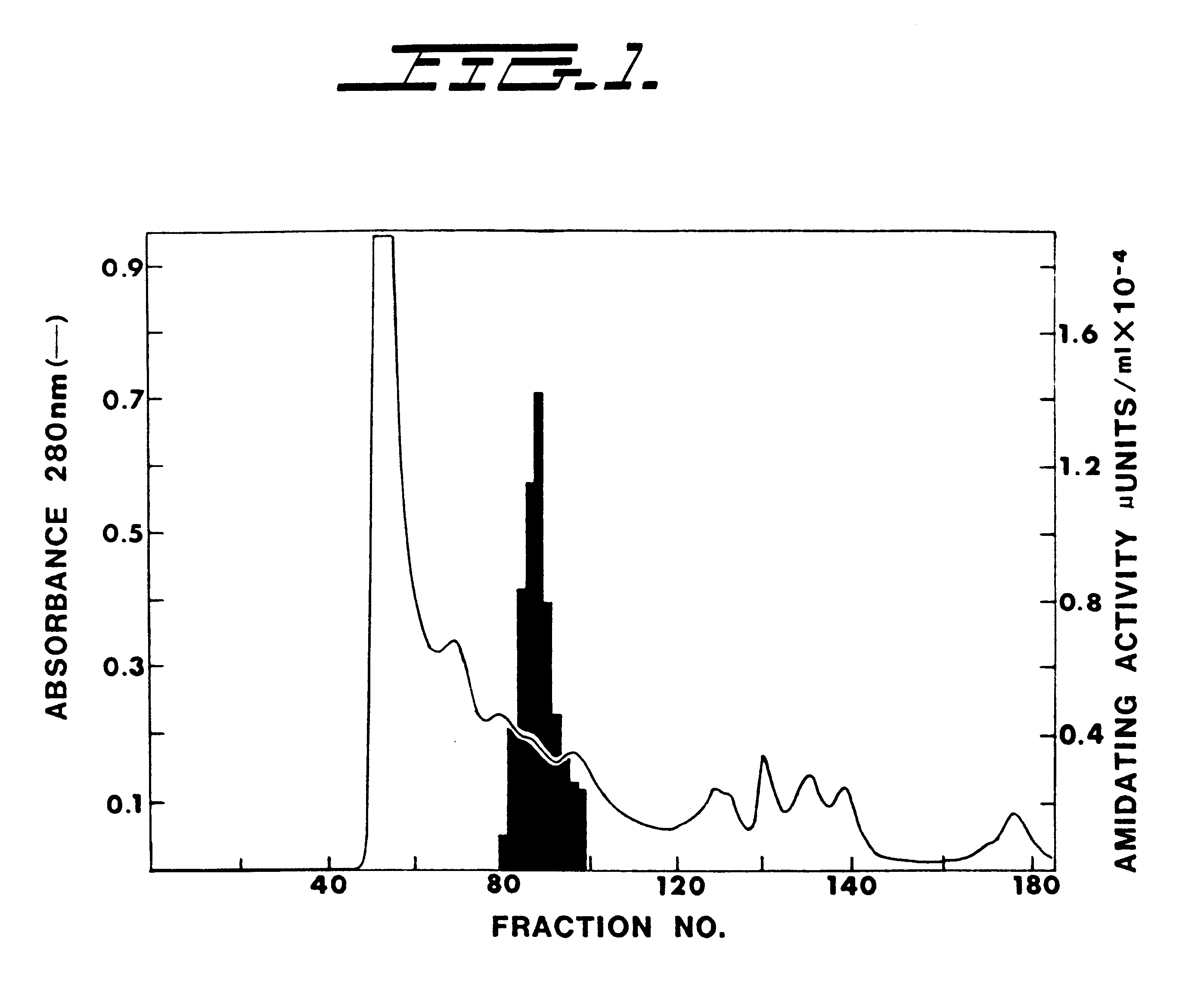
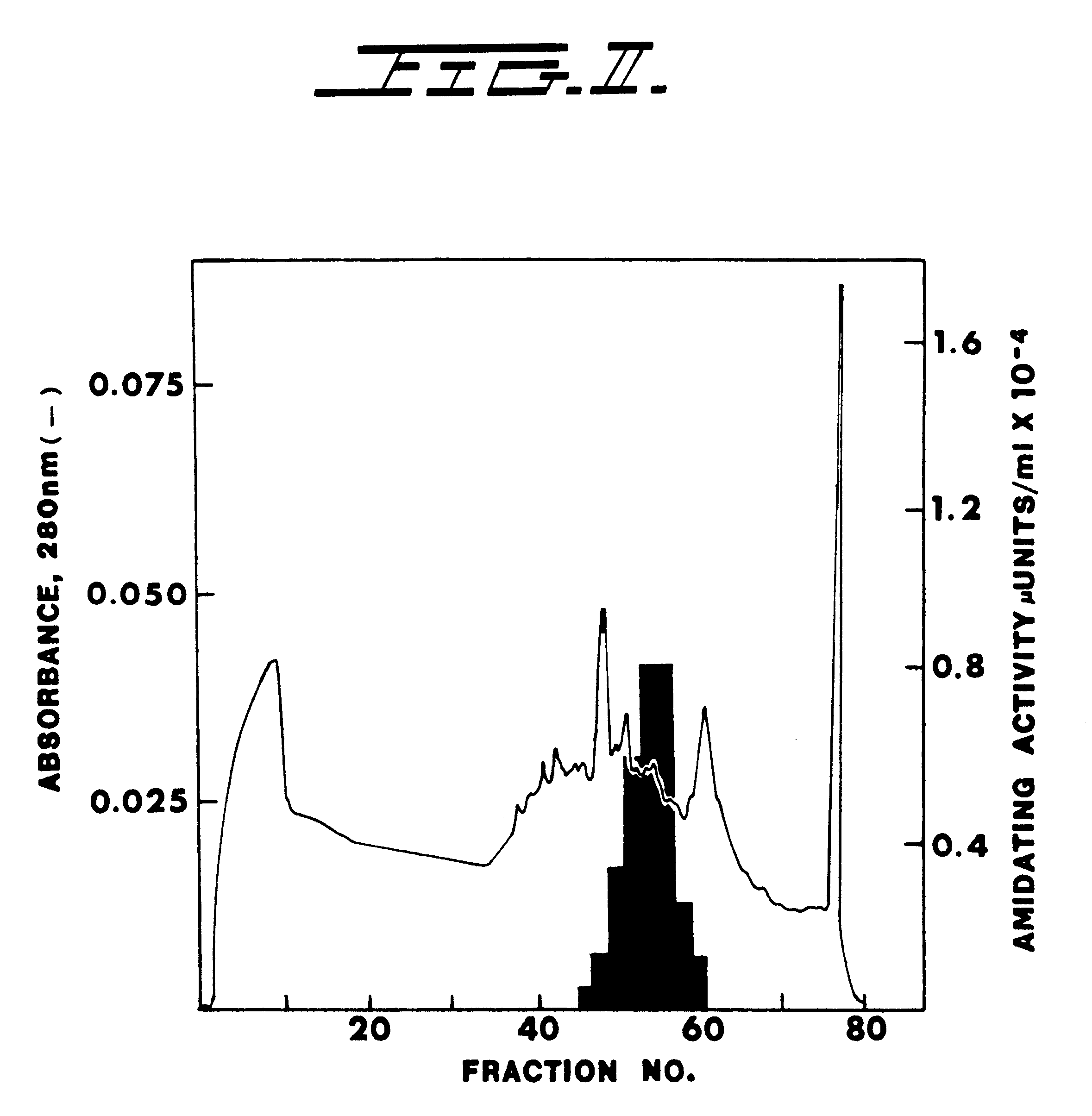
Alpha-amidating enzyme compositions and processes for their production and use
InactiveUS6319685B1Efficient productionImprove efficiency and reusabilityImmobilised enzymesBacteriaADAMTS ProteinsSize-exclusion chromatography
Purified enzymatic compositions are provided having alpha-amidating enzymes capable of catalyzing the conversion of a peptidyl compound having a C-terminal glycine residue to a corresponding peptidyl amide having an amino group in place of the C-terminal glycine. The purified compositions have specific activities above 25 mU per mg protein and are sufficiently free of proteases to allow effective catalysis of even peptidyl compounds having L-amino acids. Biologically important alpha-amidated products such as calcitonin and other regulatory hormones are efficiently produced using the alpha-amidation reaction catalyzed by the enzymes. Purification by size exclusion chromatography in combination with strong anion exchange chromatography results in homogeneous enzyme species which are used to prepare antibodies specific for the alpha-amidating enzyme. A gene capable of expressing the alpha-amidating enzyme is ligated into an expression vector and transformed into a host cell capable of expressing the gene.
Owner:MICROCAP FUND INC THE A CORP OF MD
Pharmaceutical compositions for the oral delivery of pharmacologically active agents
InactiveUS7049283B2Improve oral bioavailabilityBiocidePeptide/protein ingredientsCrospovidonesActive agent
Solid pharmaceutical compositions suitable for the oral delivery of pharmacologically active agents, e.g. peptides, comprising a therapeutically-effective amount of a pharmacologically active agent; a crospovidone or povidone; and a delivery agent for said pharmacologically active agent are disclosed. The compositions provide excellent oral bioavailability of pharmacologically active agents, particularly calcitonin.
Owner:NOVARTIS AG
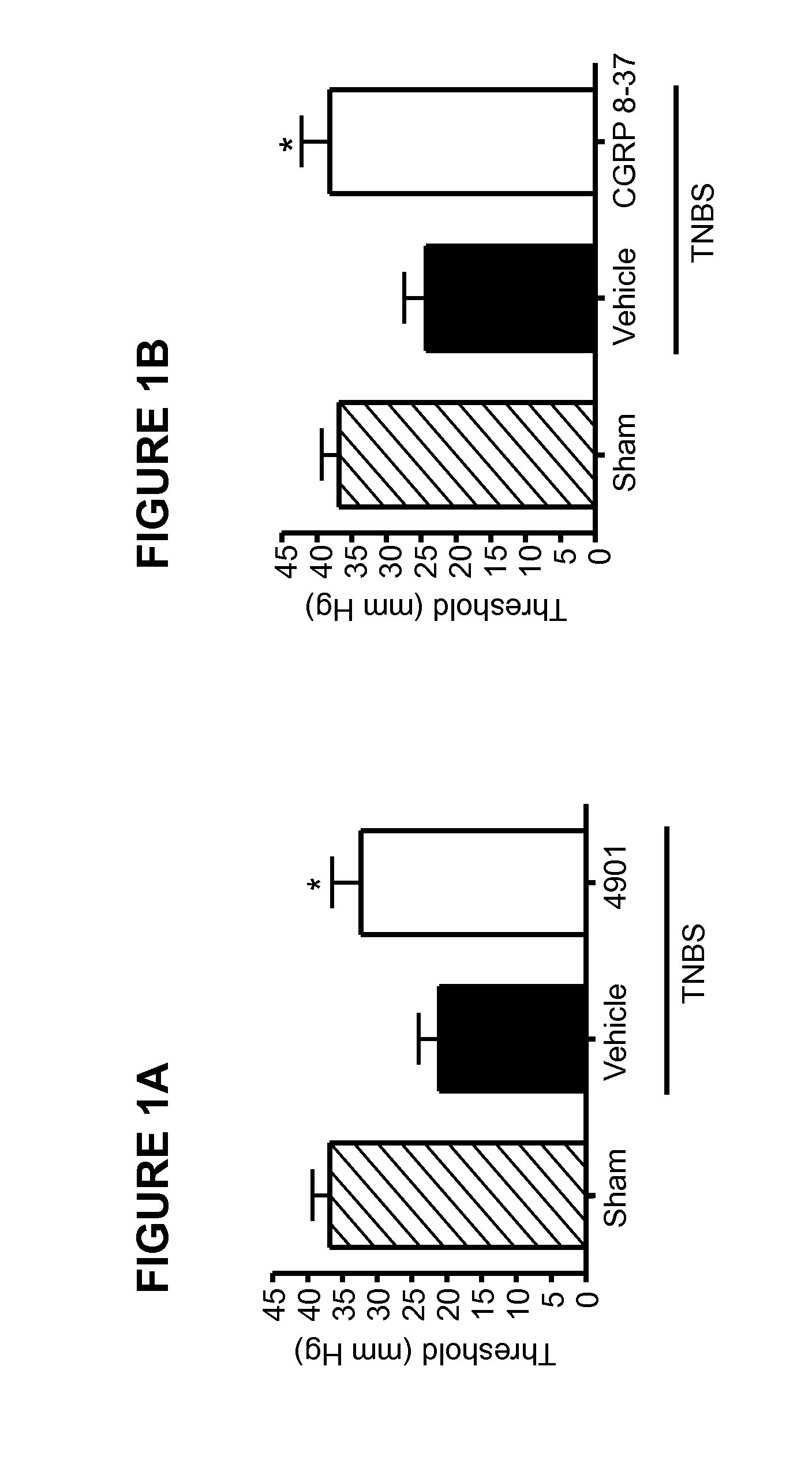
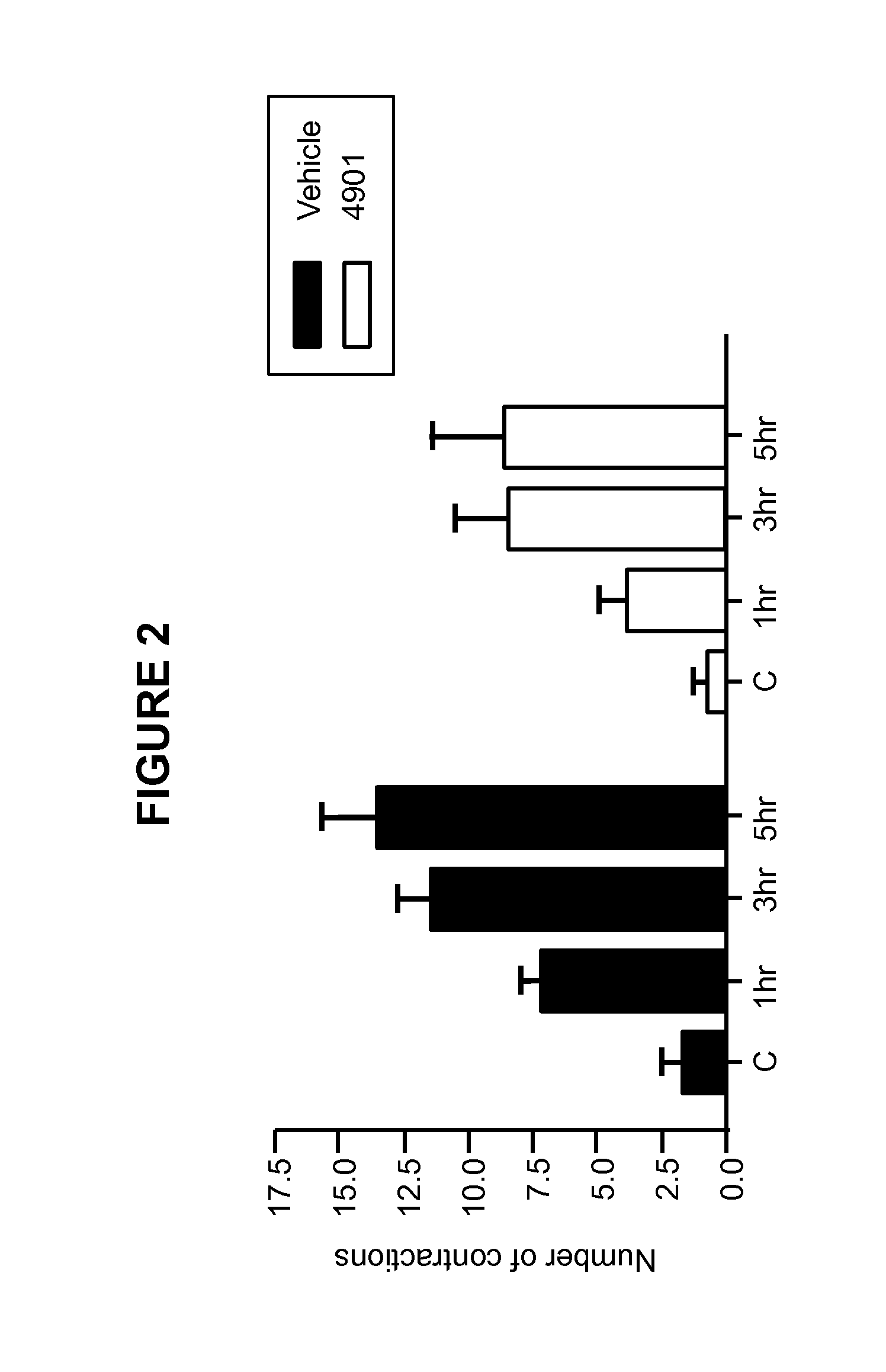
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
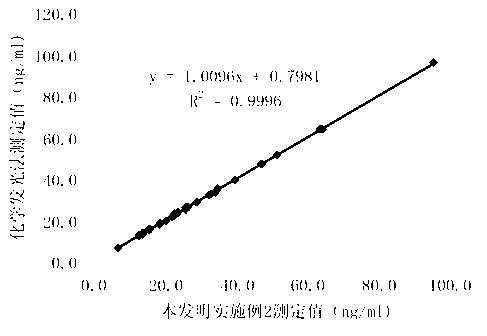
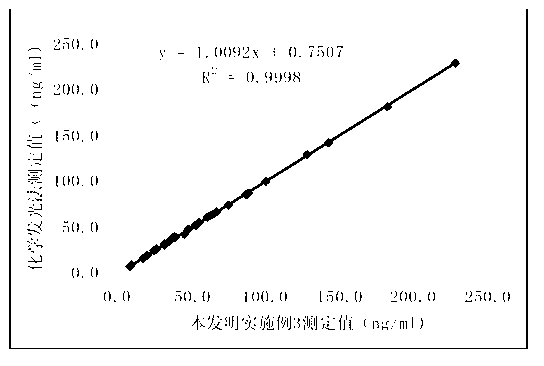
Chemiluminescence quantitative detection kit for procalcitonin, and preparation method and detection method thereof
ActiveCN103901203ALow cross-reactivityImprove accuracyChemiluminescene/bioluminescenceBiotin-streptavidin complexN-Hydroxysuccinimide
The invention relates to a chemiluminescence quantitative detection kit for procalcitonin, and a preparation method and detection method thereof. The kit comprises a procalcitonin series standard substance, a magnetic separation reagent (magnetic particle suspension coupled with streptavidin), a first reagent (anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label) and a second reagent (anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label). The sensitivity of the kit prepared from the magnetic particle suspension coupled with streptavidin, anti-procalcitonin monoclonal antibody solution containing biotin N-hydroxysuccinimide ester label and anti-procalcitonin monoclonal antibody solution containing alkaline phosphatase label is up to 0.008ng / ml; and the kit has the advantages of high accuracy, high precision, no need of prediluting the sample, and wide detection range, and is simple and time-saving to operate.
Owner:SUZHOU HAOOUBO BIOPHARML
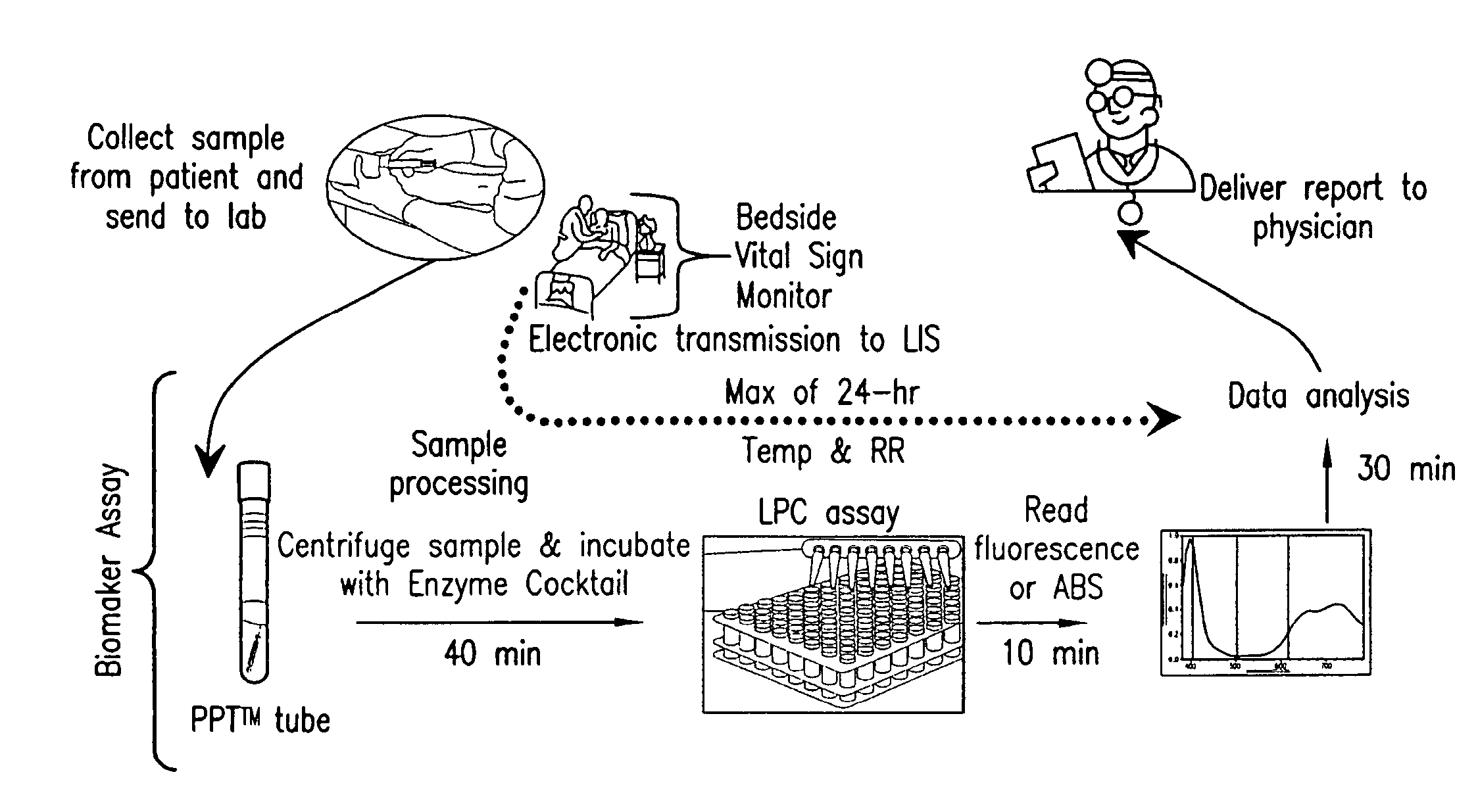
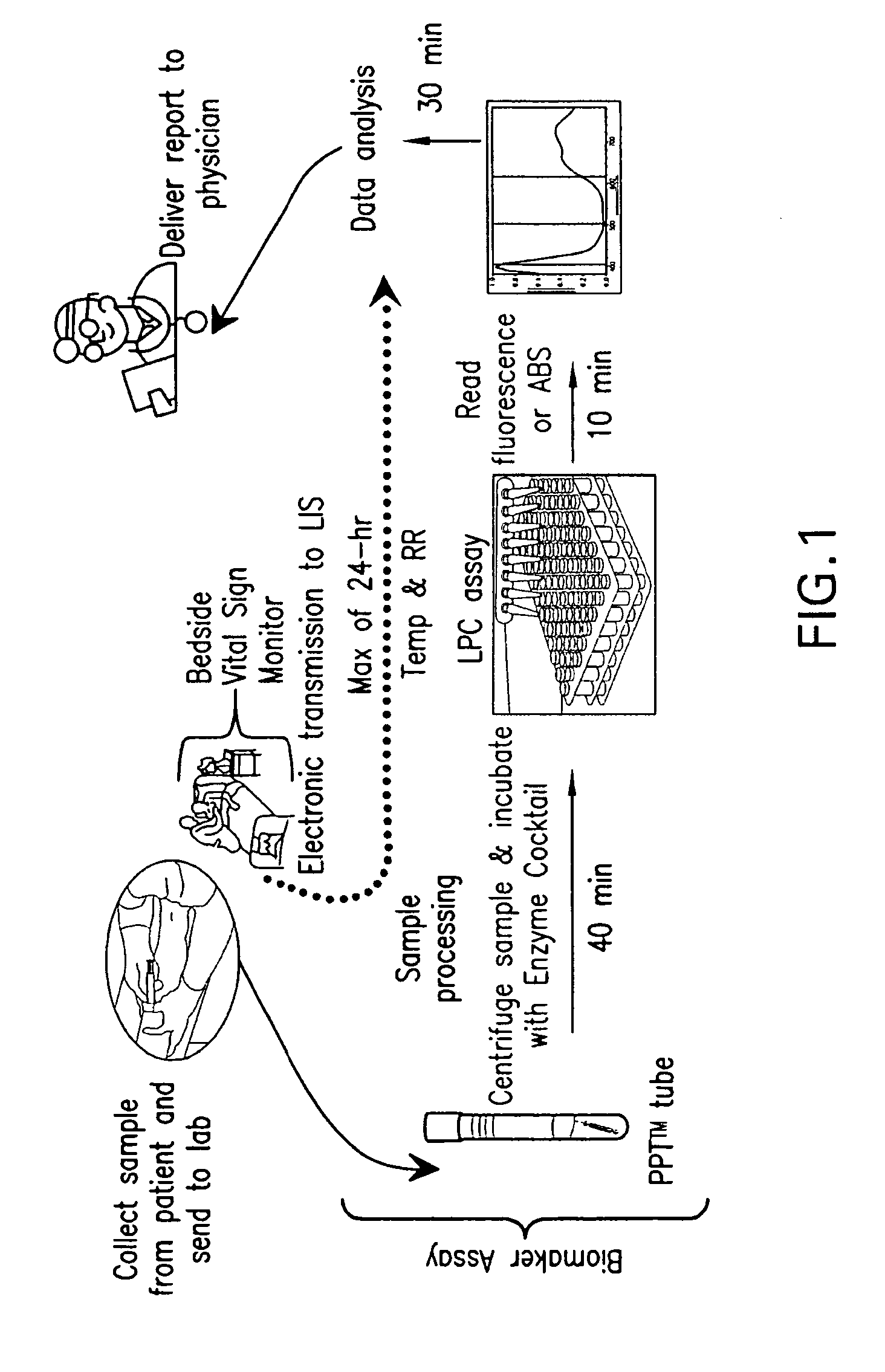
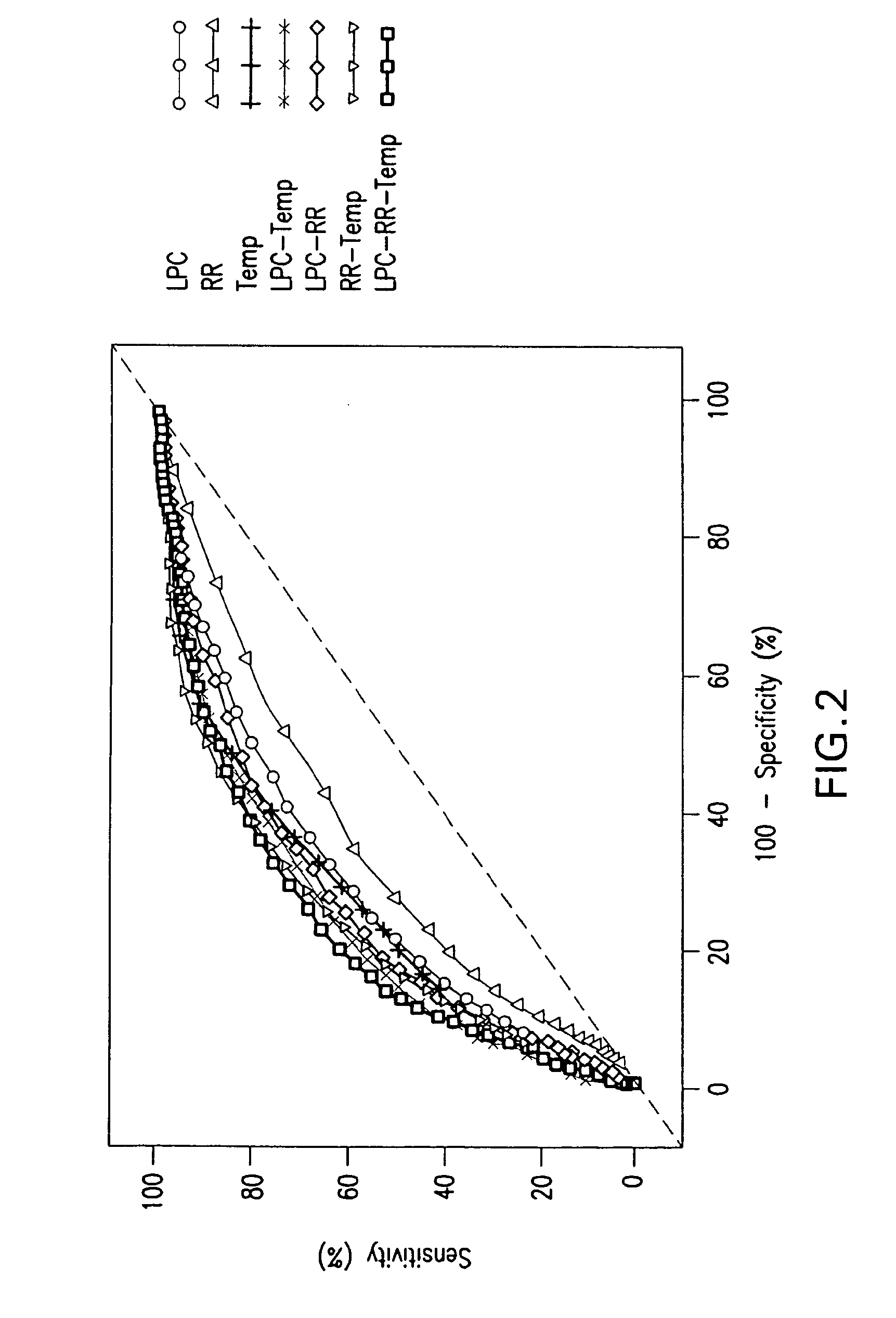
Advanced Detection of Sepsis
ActiveUS20110118569A1Raise the possibilityBioreactor/fermenter combinationsElectrolysis componentsPhospholipinClinical marker
The present invention relates to methods, monitors and systems measuring lysophosphatidylcholine, its derivatives and / or procalcitonin as well as at least one clinical marker (e.g. temperature or respiration rate) and / or at least one biomarker for the early detection of sepsis in a subject.
Owner:BECTON DICKINSON & CO
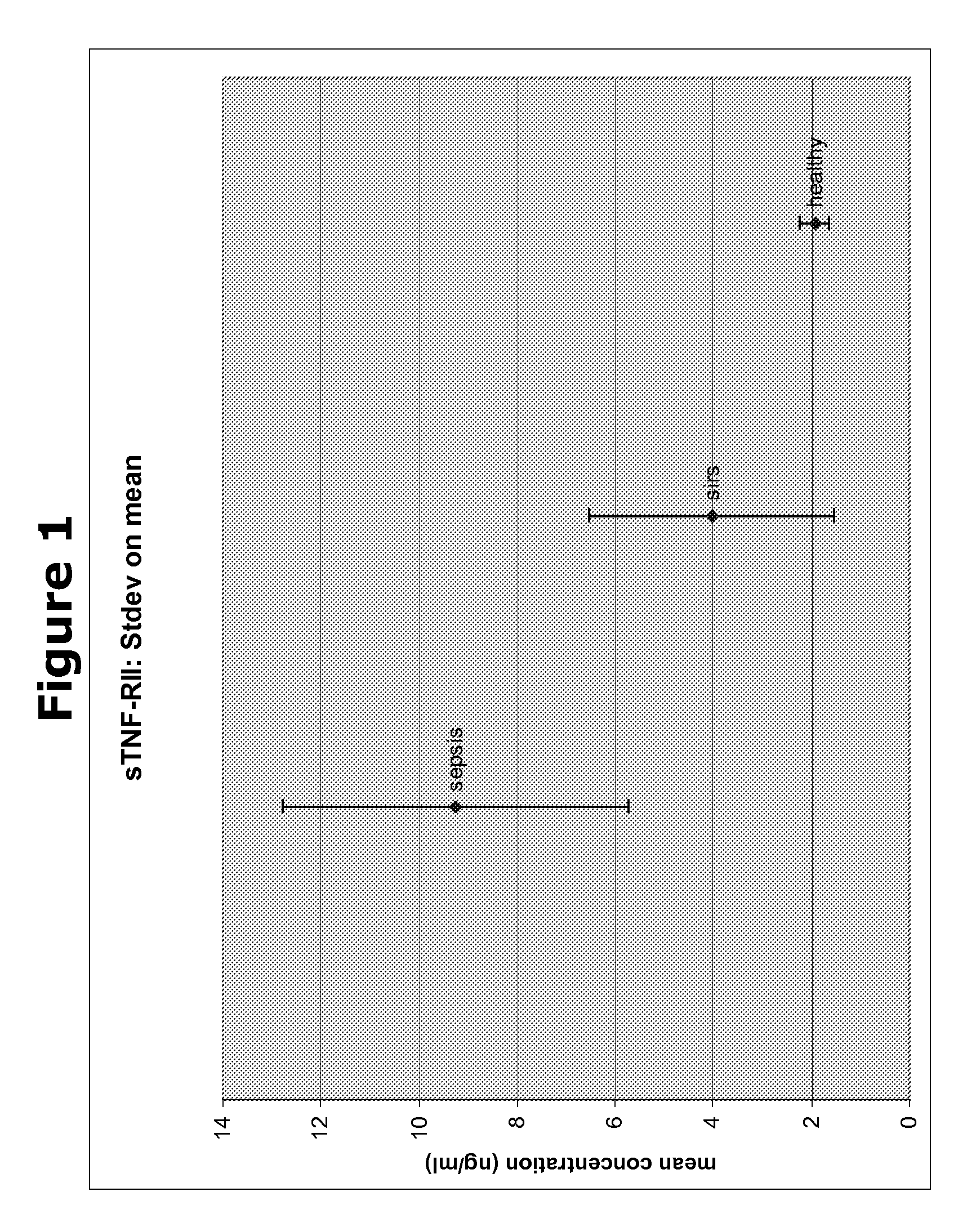
Biomarkers and methods for diagnosing, predicting and/or prognosing sepsis and uses thereof
InactiveUS20100292131A1Peptide librariesPeptide/protein ingredientsHealthy subjectsBiomarker (petroleum)
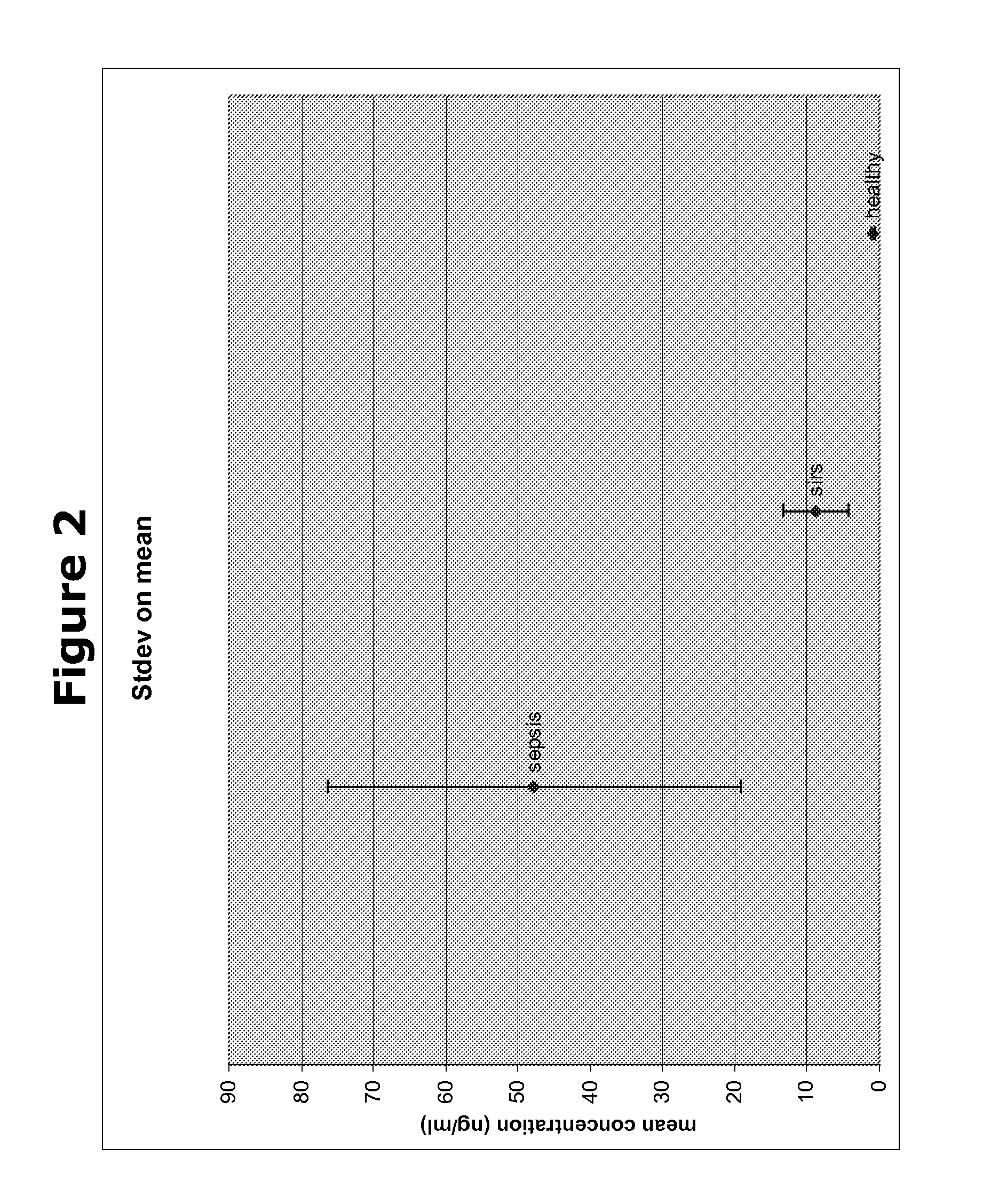
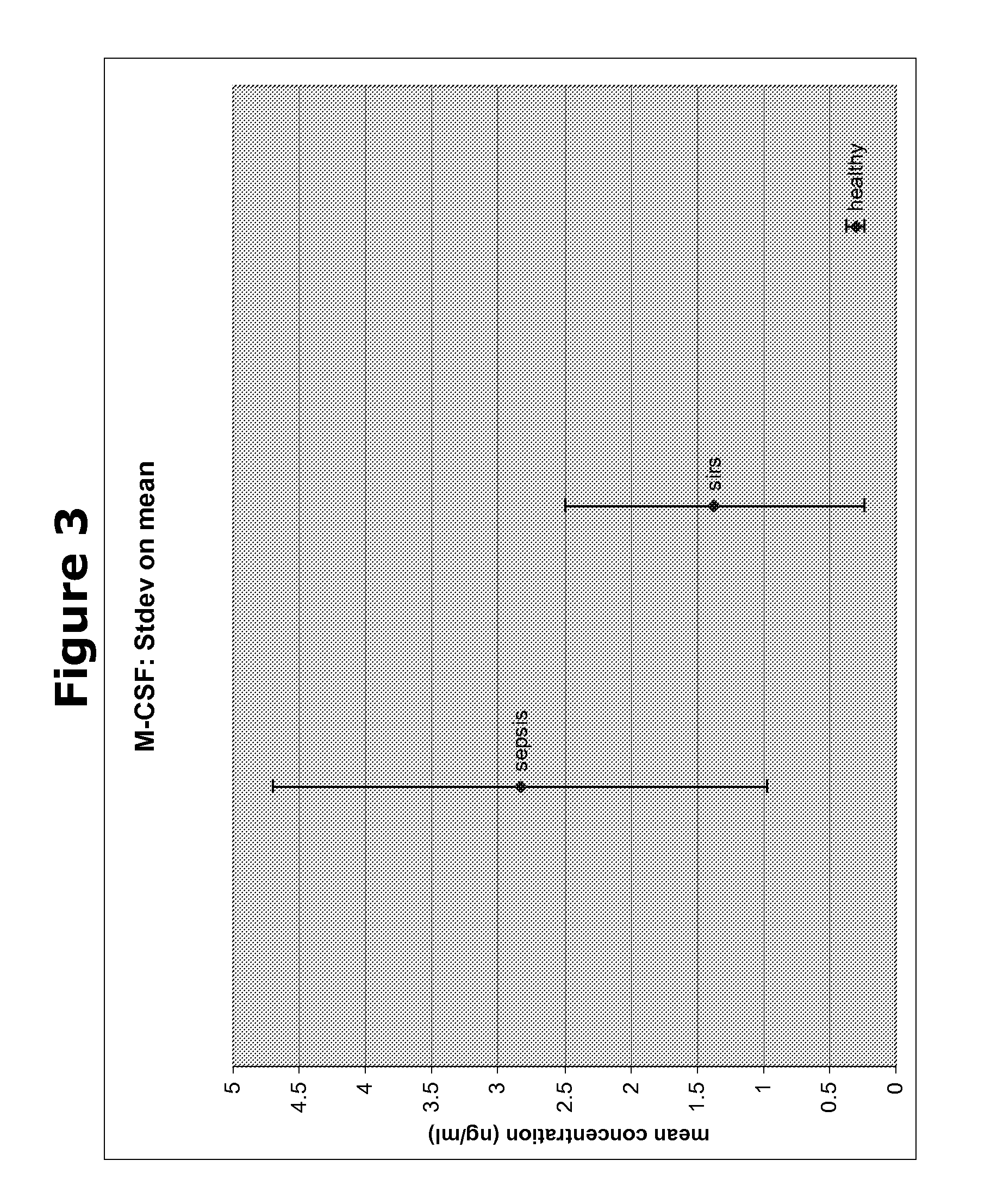
The present invention provides kits and methods for the diagnosis, prognosis and prediction of sepsis in a subject or for the differentiation between sepsis and SIRS in a subject, the method comprising(a) measuring the level of pro-hepcidin (pro-HEPC) in a biological sample taken from said subject, (b) measuring the level of at least one further biomarker selected from the group consisting of soluble TNF-receptor 2 (sTNFR2), Pentraxin-3 (PTX-3), Macrophage Colony-Stimulating Factor (MCSF), pro-Brain Natriuretic Protein (pro-BNP), one or more members of the Histone protein family, Procalcitonin (PCT) and c-Reactive Protein (CRP) in a biological sample from said subject, (c) using said measurements obtained in steps (a) and (b) to create a profile for said biomarkers and (d) comparing said profile with a reference biomarker profile obtained form a patient having SIRS or from a healthy subject.
Owner:BIOCARTIS NV
Antagonist antibodies directed against calcitonin gene-related peptide and methods using same
ActiveUS20120009192A1Reduce developmentDelay progressOrganic active ingredientsFungiVasomotor symptomCalcitonin
The invention features methods for preventing or treating CGRP associated disorders such as vasomotor symptoms, including headaches (e.g., migraine, cluster headache, and tension headache) and hot flushes, by administering an anti-CGRP antagonist antibody. Antagonist antibody G1 and antibodies derived from G1 directed to CGRP are also described.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Procalcitonin detection kit and detection method
ActiveCN104714033ARapid responseSuitable for detectionImmunoglobulins against hormonesBiological testingAntigen epitopeAntigen
The invention relates to the field of fluorescence immunochromatography technique in medical immunology, specifically to a procalcitonin detection kit and a procalcitonin detection method. The detection kit is provided with a test cassette and is characterized in that the test cassette is successively provided with, from bottom to top, a PVC plate, a sample pad, a combination pad, a cellulose nitrate film and a water-absorbing pad, wherein a procalcitonin monoclonal antibody labeled by a rare earth fluorescent microsphere is adsorbed on the combination pad, the rare earth fluorescent microsphere has a diameter of 60 to 120 nm, is doped by rare earth lanthanide, is stable in a ground state and emits fluorescent light with a wavelength in a range of 540 to 600 nm under the action of an excitation light source in a wavelength range of 340 to 380 nm, and the monoclonal antibody is a purified mixed monoclonal antibody and is originated from monoclonal antibody cell strains directed at 2 to 6 different procalcitonin antigen epitopes. The procalcitonin detection kit has the advantages of simple operation, rapid reaction, high sensitivity, strong specificity, etc.
Owner:WEIHAI NEOPROBIO
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formula NH2CH2CH2CHR1C(O)N—R (I) where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Reagent for determining procalcitonin and preparation method of reagent
ActiveCN103018464ASimple and fast operationRaw materials are easy to getMaterial analysis by observing effect on chemical indicatorBiological testingMicrosphereCalcitonin
The invention relates to a reagent for determining procalcitonin and a preparation method of the reagent, in order to provide the reagent which has the characteristic of high accuracy degree, and the provided preparation method has the characteristic of convenience in preparation. The technical scheme is that the reagent for determining procalcitonin comprises the following components: a. a procalcitonin reagent 1, b. a procalcitonin reagent 2, and c. a liquid-type procalcitonin reference calibration item. The preparation method of the reagent for determining procalcitonin comprises the following steps of: (1) uniformly mixing the procalcitonin reagent 1; (2) removing the procalcitonin reagent 2, the removing process specifically comprises the following steps of: a. taking a suspension, b. carrying out reaction on a mixture, c. acquiring a carboxylic latex microsphere suspension, d. regulating the concentration, e. taking the suspension in step (3), and adding into the step (4), f. reacting, g. adding cholamine, and h. carrying out centrifugal treatment; and (3) treating the liquid-type procalcitonin reference calibration product: mixing according to the amount of a formula, and ranking according to the content of the procalcitonin or adding a procalcitonin pure product in the mixed liquid.
Owner:YESEN BIOTECH SHANGHAI
Compound and method of treating neurogenic conditions using non-steroidal anti-inflammatory drug complexes
InactiveUS7151084B2Nervous disorderPeptide/protein ingredientsMembrane TransportersPancreatic hormone
A complex is provided for the treatment of neurogenic conditions having the formula:where R1 isM is a metal ion Ca(II), Mg(II), Cu(II) or Ni(II); n is an integer 1 or 2; R is BBB peptide, transferrin, membrane transporter peptide, TAT peptide, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, L-lactate, L-leucine, L-tryptophan, and L-glutamate; and R is coupled to M through a carboxylate moiety. Magnesium(II) represents the preferred metal ion as magnesium is known to have neuroprotective effects. The metal ion is in part chelated by a non-steroidal anti-inflammatory drug that does not inhibit platelet activity and includes salicylate and ibuprofenate. The complex also includes a ligand operative in transport across the blood brain barrier. A process for making an inventive complex includes the stoichiometric addition of ligands containing carboxylate groups to a solution of the metal ion. In instances where the metal ion is magnesium(II), a stoichiometric ratio of 1:1:1 is found between the non-steroidal anti-inflammatory ligand:magnesium(II):transporter ligand.
Owner:MILLER LANDON C G
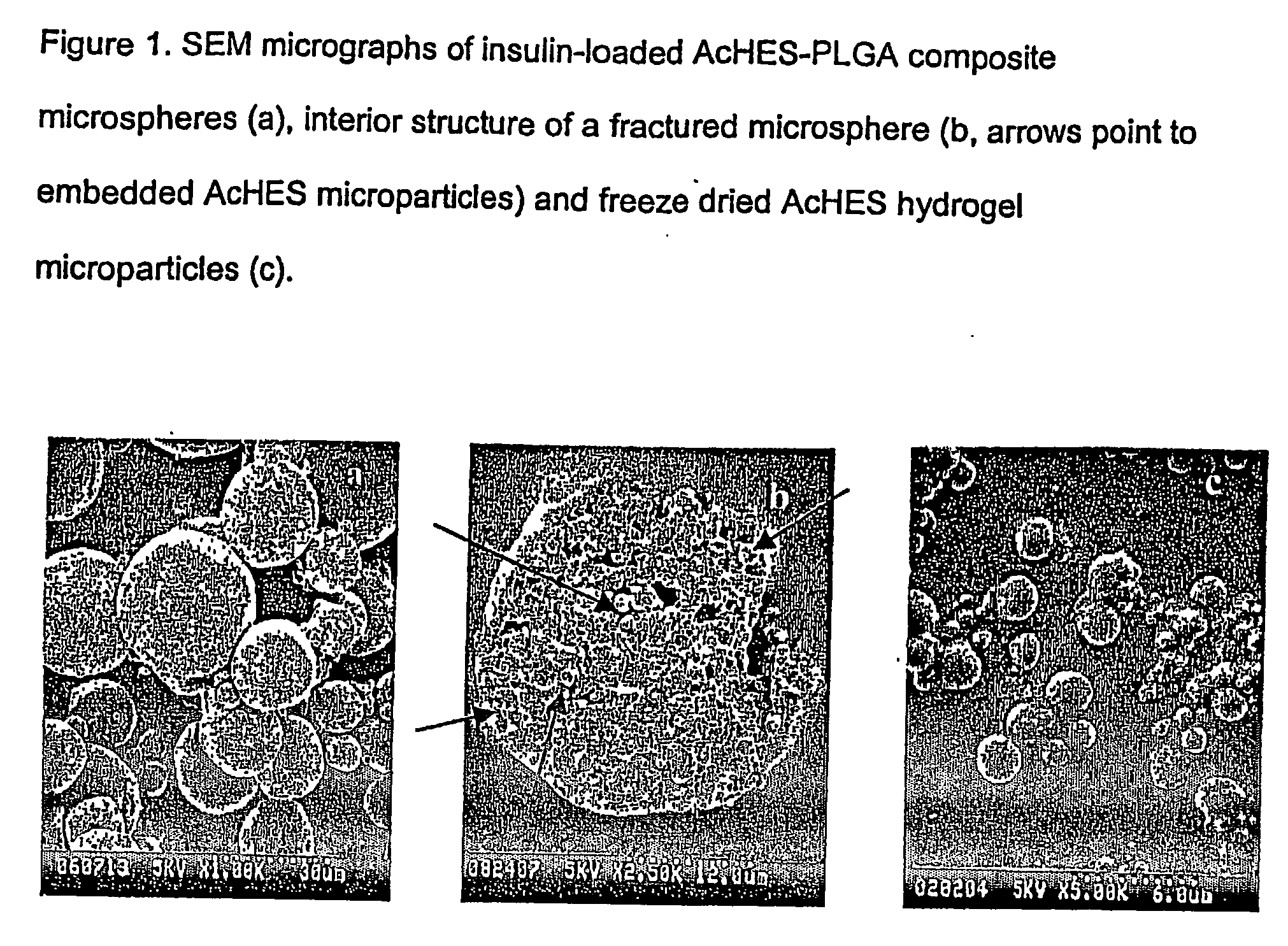
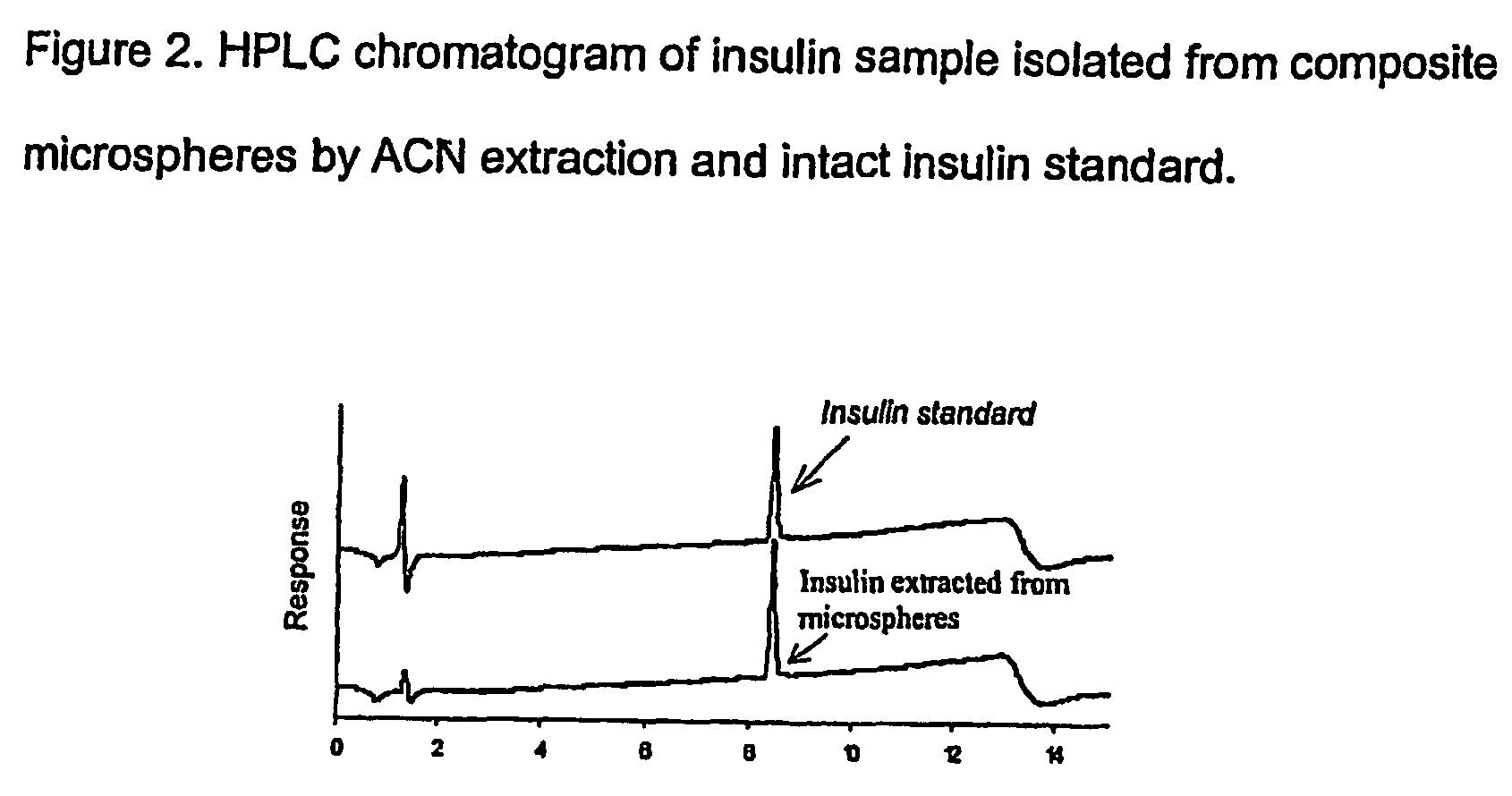
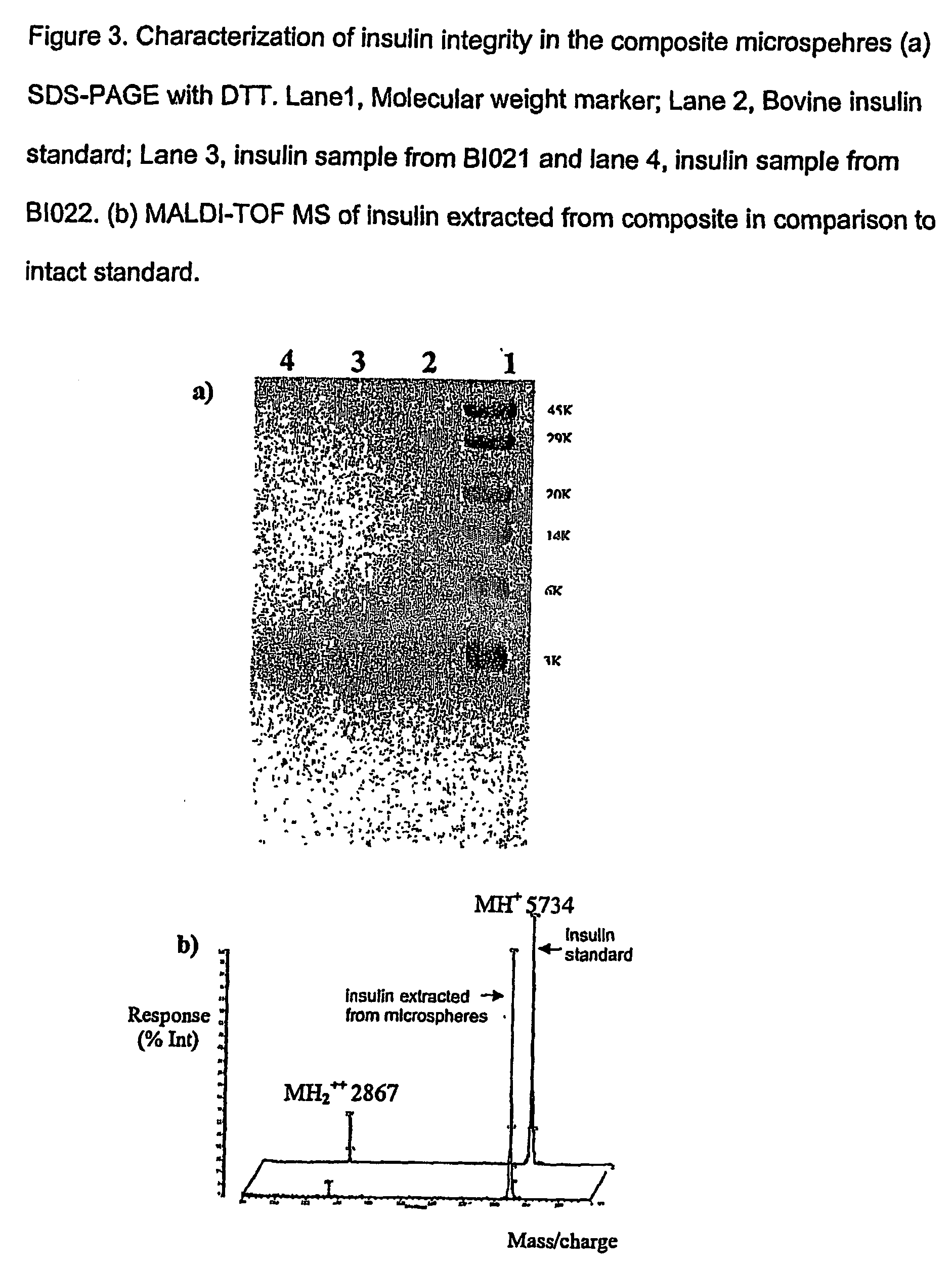
(Poly(acryloyl-hydroxyethyl starch)-plga composition microspheres
The present invention relates to a composite microsphere system comprising poly(D,L-lactide-co-glycolide) (PLGA), poly(acryloyl hydroxyethyl starch) (AcHES), and a pharmaceutically effective amount of a biologically active compound. The active compound may be, for example, an insulin, an interferon, a luteinizing hormone-releasing hormone (LHRH) analog, a somatostatin and / or derivatives thereof, a calicitonin, a parathyroid hormone (PTH), a bone morphogenic protein (BMP), an erythropoietin (EPO), an epidermal growth factor (EGF) or a growth hormone. This invention also relates to methods of using the composite microspheres, and methods of preparing same.
Owner:UNIV OF KENTUCKY RES FOUND
Aminobutyramide conjugate and a pharmaceutical composition for treatment of neuronal disorders
ActiveUS7074775B2Improve efficiencyEliminate side effectsBiocideDipeptide ingredientsTryptophanSecretin
A compound is provided that has the formulaNH2CH2CH2CH2C(O)N—R (I)where R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidetransferrin, glucosylamnine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Baclofen conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound is provided that has the formulaNH2CH2CH2CHR1C(O)N—R (I)where R1 is p-chlorophenyl, R is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine blood brain barrier (BBB) peptide, membrane translocating protein, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptide transferrin, glucosylamine, amino saccharin, lactylamine, leucine, tryptophan, glutamate and amino cholines.
Owner:MILLER LANDON C G
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formulawhere R1 is H, C1–C4 alkyl and OH; R2 in is H, C1–C4 alkyl and OH; R3 is H and C1–C4 alkyl; R4 is H and C1–C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2–C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
Prognosis and risk assessment in stroke patients by determining the level of marker peptides
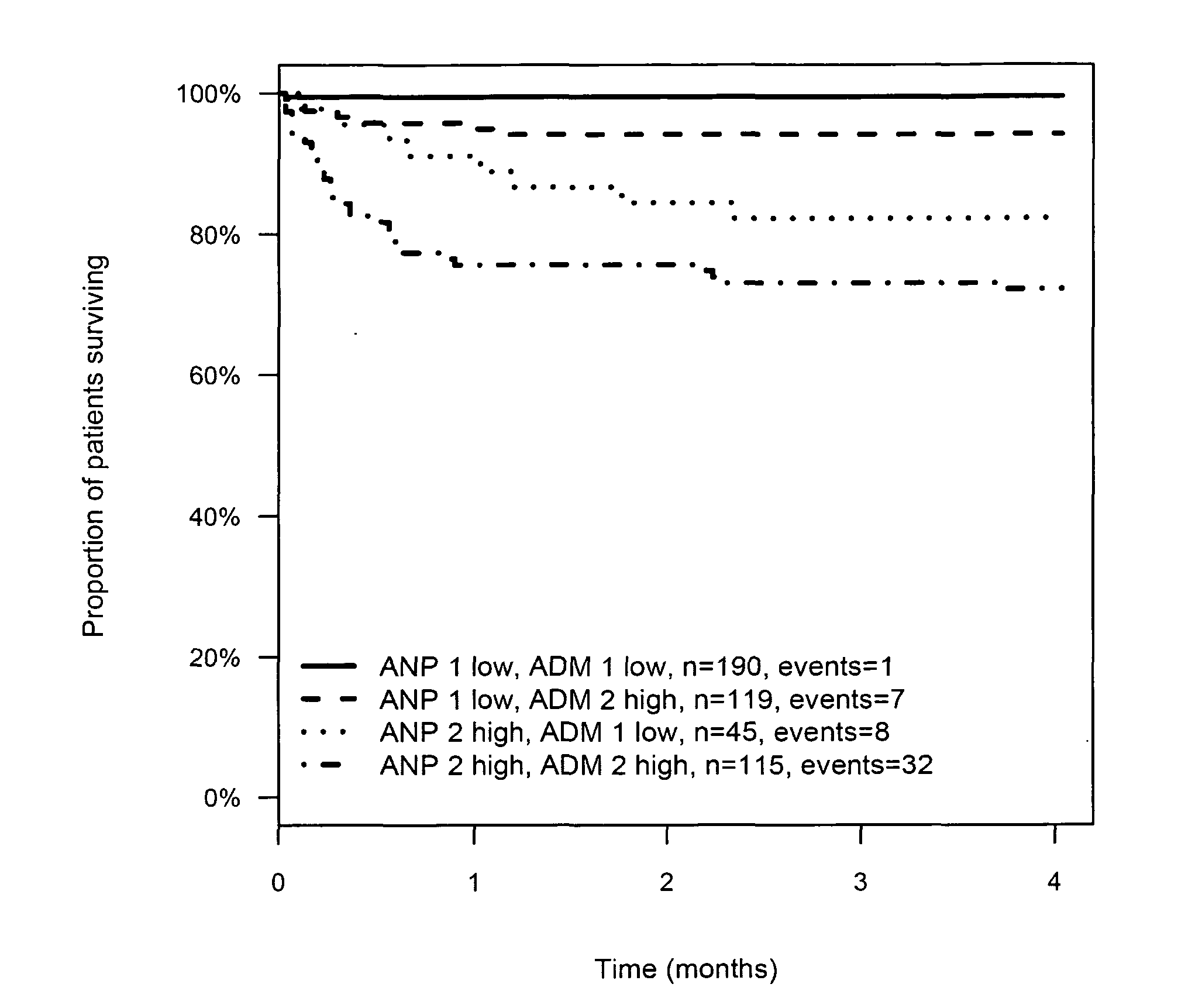
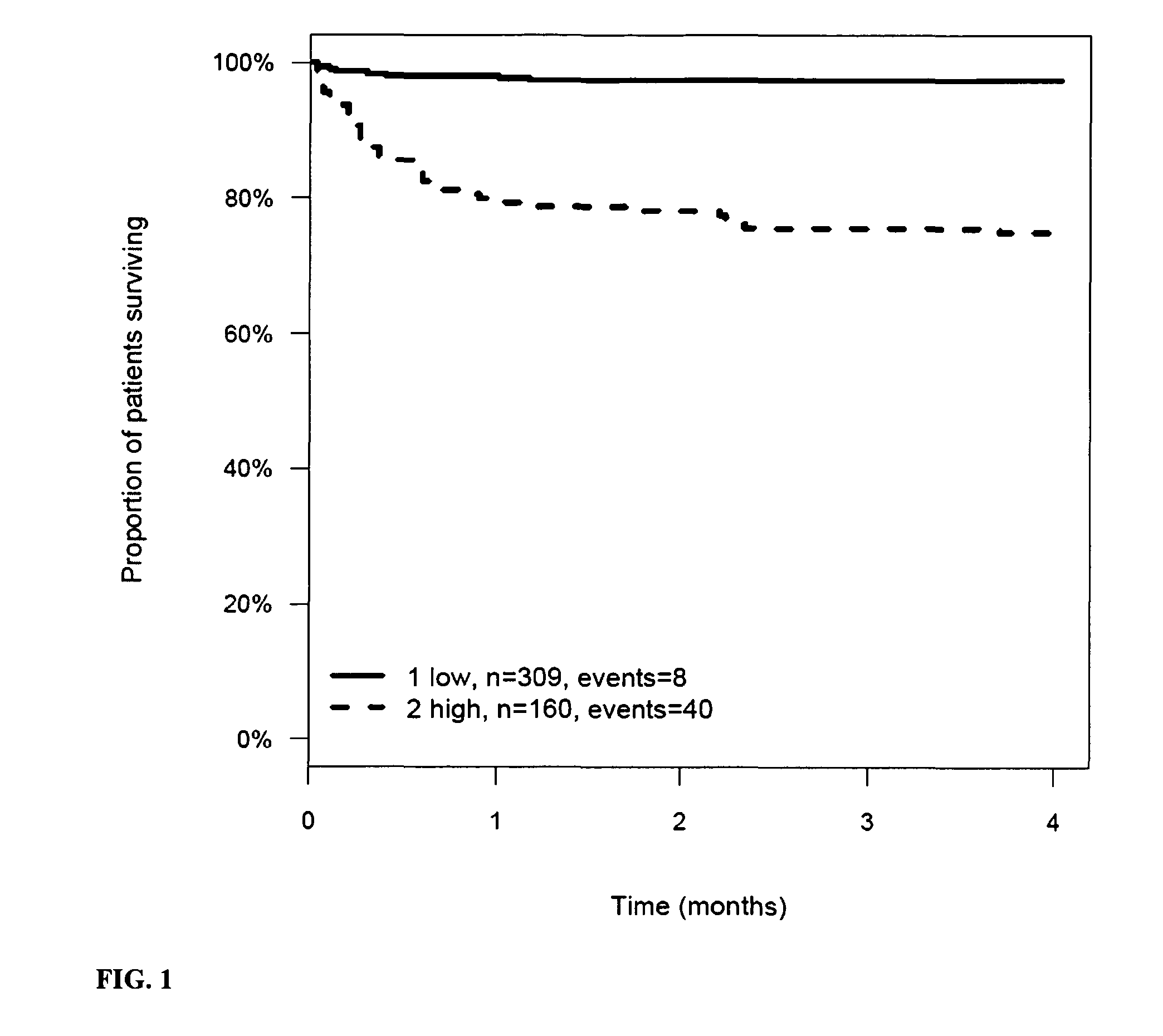
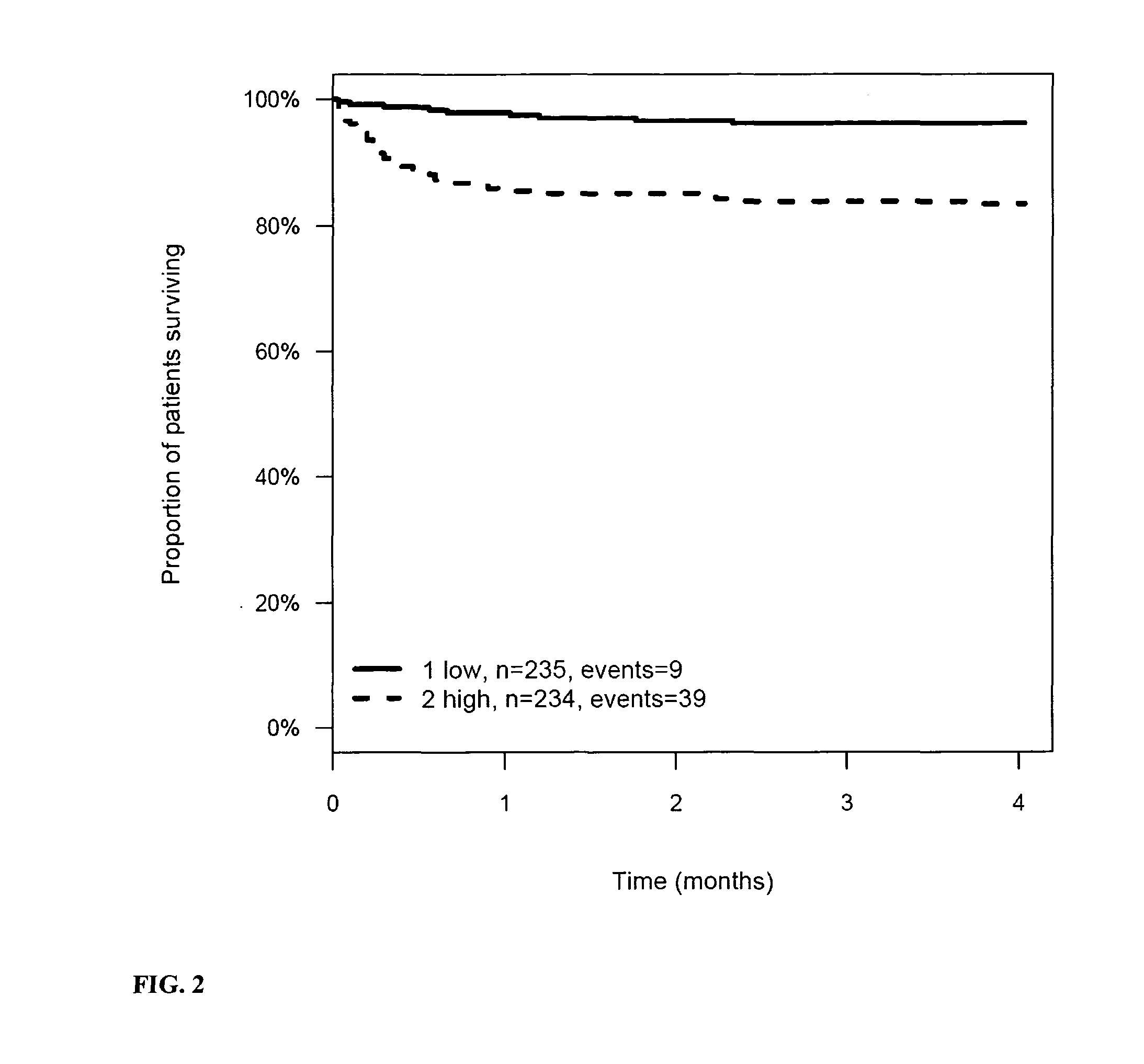
InactiveUS20110263821A1Increase probabilityChemiluminescene/bioluminescenceLibrary screeningCalcitoninCancer risk assessment
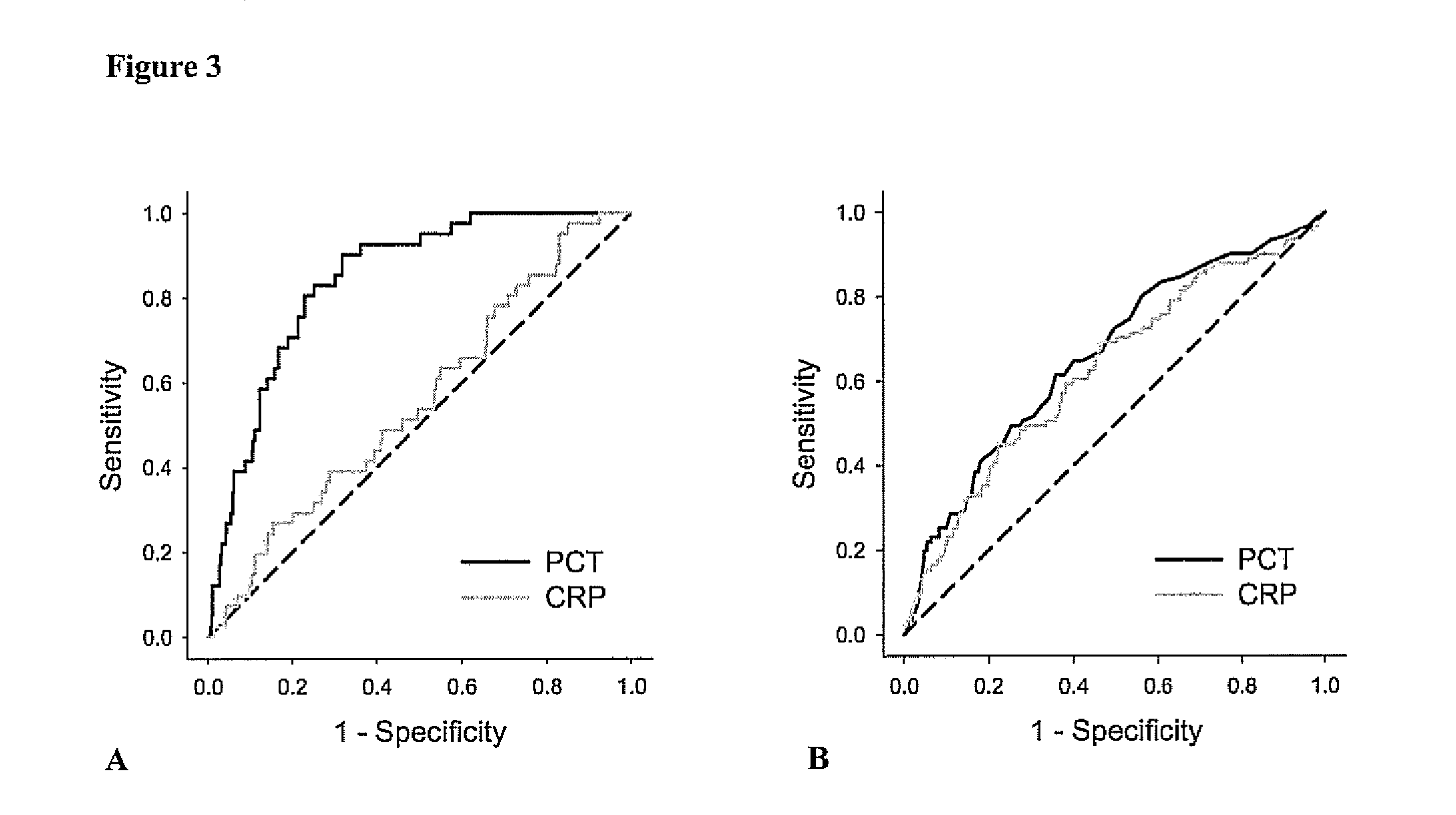
The present invention relates to a method for prognosis of an outcome or assessing the risk of a patient having suffered a stroke or a transient ischemic attack, comprising the determination of the level of at least one marker peptide in said sample said marker peptide selected from the group comprising ANP, AVP, ADM, ET-1, troponin, CRP, calcitonin and hGH or fragments thereof or its precursor or fragments thereof and attributing the level of said at least one marker peptides its precursor or fragments thereof with the prognosis of an outcome or assessing the risk for said patient.
Owner:BRAHMS GMBH
Piracetam and piracetam analog conjugate and a pharmaceutical composition for treatment of neuronal disorders
A compound of the formula where R1 is H, C1-C4 alkyl and OH; R2 in is H, C1-C4 alkyl and OH; R3 is H and C1-C4 alkyl; R4 is H and C1-C4 alkyl; n is an integer between 0 and 2 inclusive; R5 is a nullity, NHR7C(O)—, C6H4—, C6H4—O—; R7 is C2-C6 alkyl; and R6 is a moiety capable of crossing the blood brain barrier and is as a free compound serotonin, dopamine, blood brain barrier (BBB) peptide, membrane translocating peptide, TAT peptides, bradykinin, beta-endorphin, bombesin, calcitonin, cholecystokinin, an enkephalin, dynorphin, insulin, gastrin, substance P, neurotensin, glucagon, secretin, somatostatin, motilin, vasopressin, oxytocin, prolactin, thyrotropin, an angiotensin, galanin, neuropeptide Y, thyrotropin-releasing hormone, gonadotropnin-releasing hormone, growth hormone-releasing hormone, luteinizing hormone, vasoactive intestinal peptidegluconate, transferrin, glucosylamine, amino saccharin, saccharin ester, lactylamine, leucine, tryptophan, amino glutamate and amino cholines.
Owner:MILLER LANDON C G
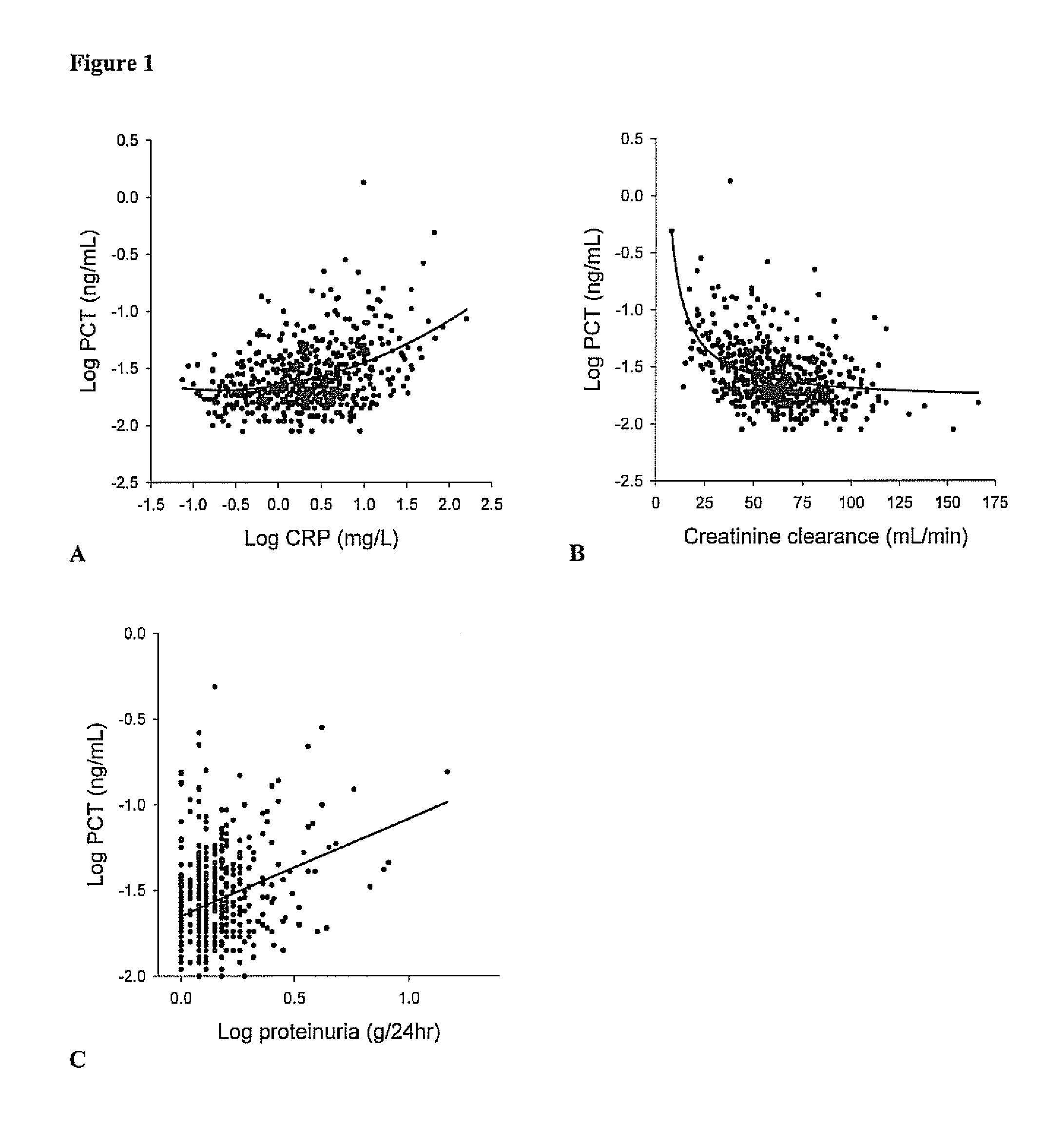
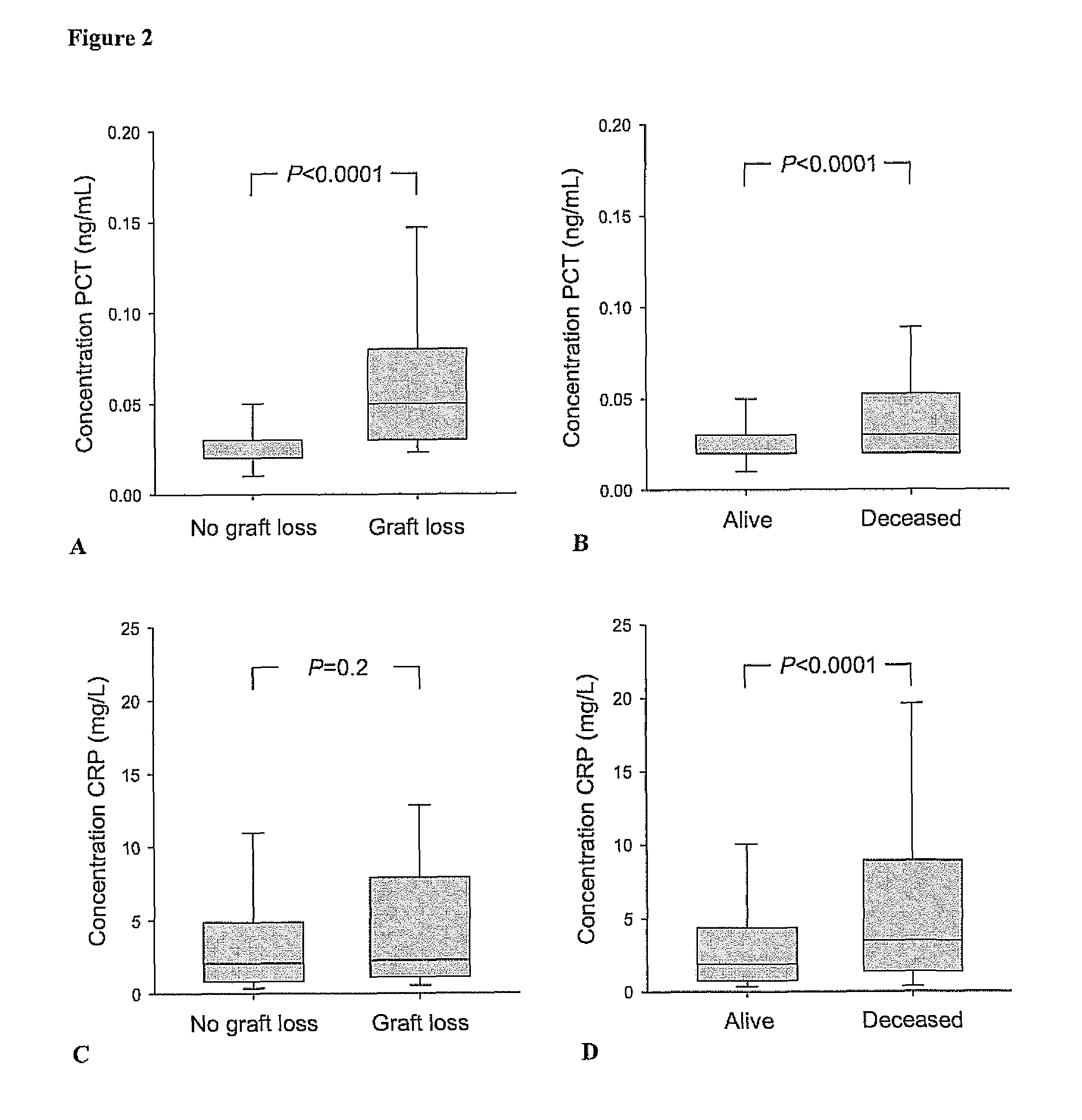
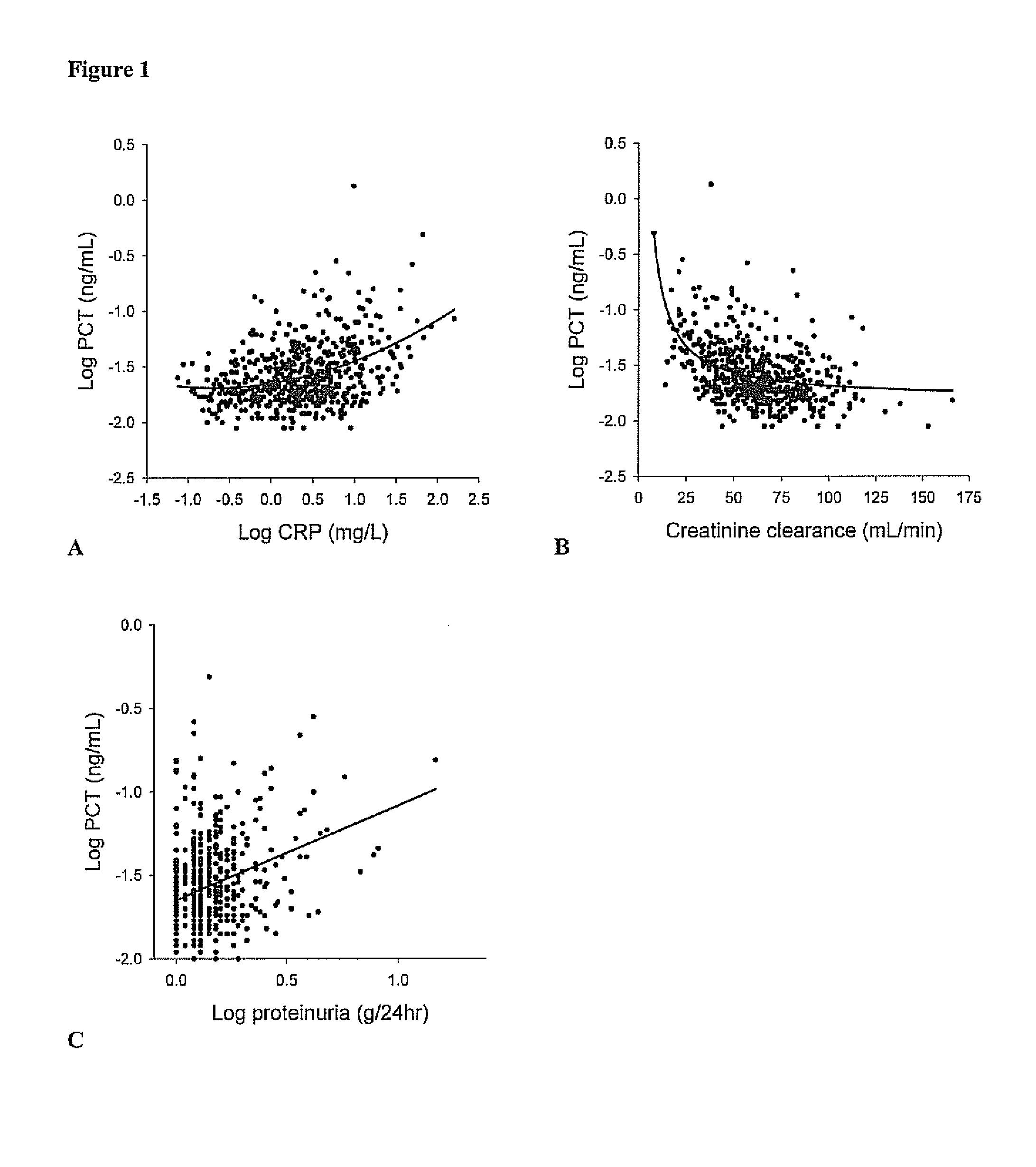
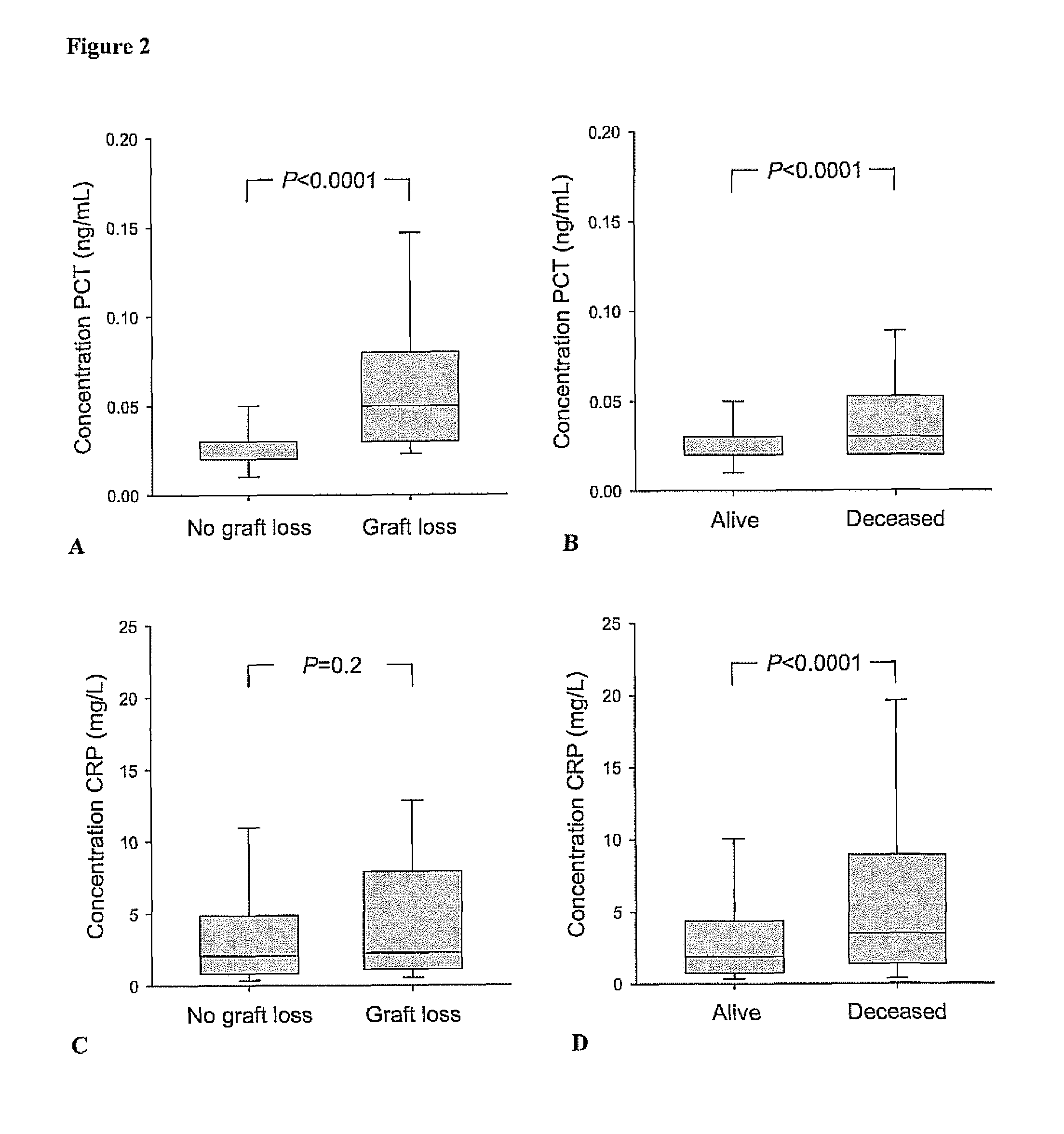
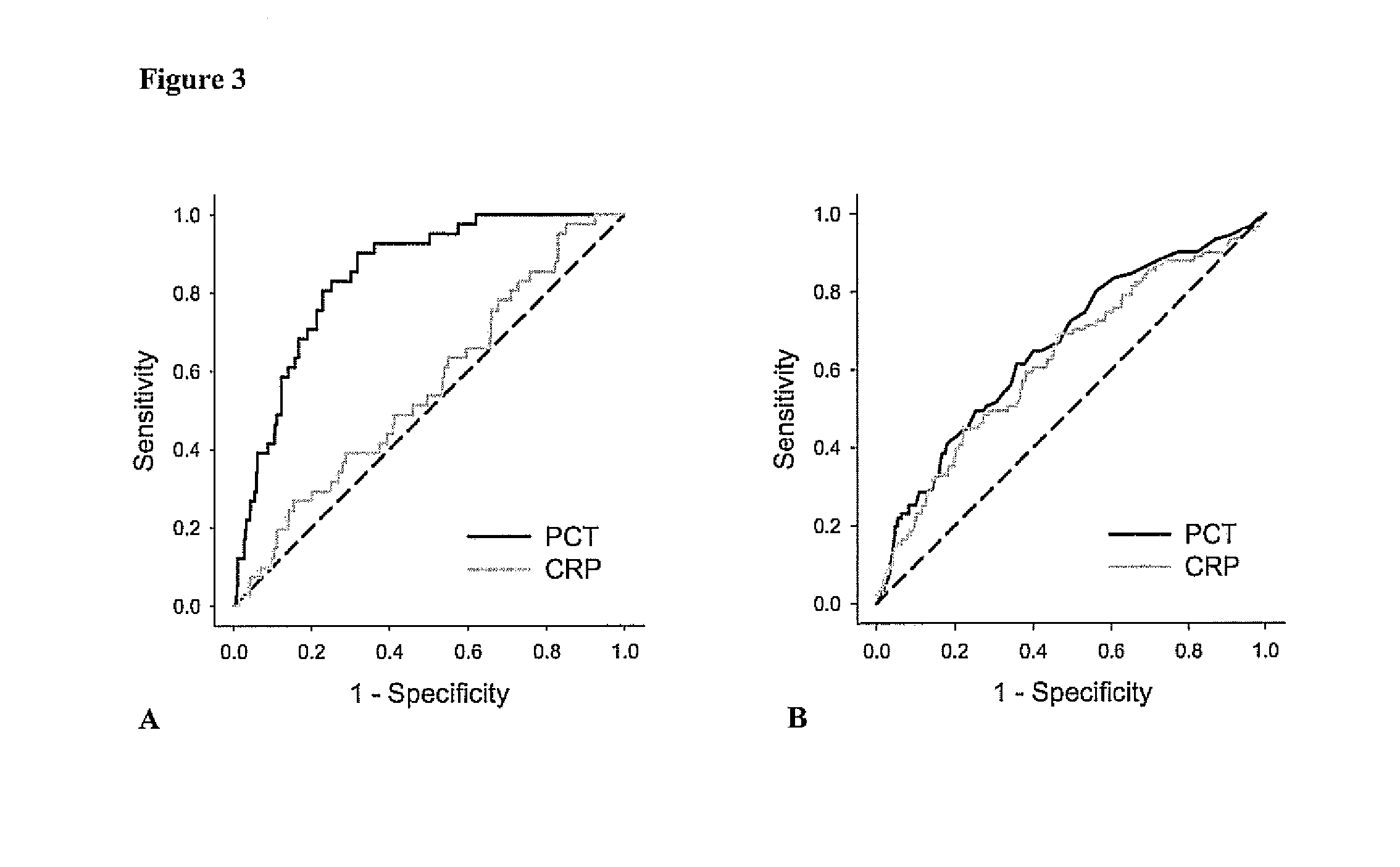
Marker for graft failure and mortality
ActiveUS8889366B2Lower levelAnalysis using chemical indicatorsPeptide/protein ingredientsOrgan transplantationMortality rate
Subject of the present invention is a biomarker for graft failure and / or mortality after organ transplantation. Procalcitonin was found to be a useful marker for the prediction or risk stratification for graft failure and / or mortality of a subject who has received an organ transplant and monitoring and therapy guidance of such subject.
Owner:BRAHMS GMBH
Therapeutic agents for the treatment of migraine
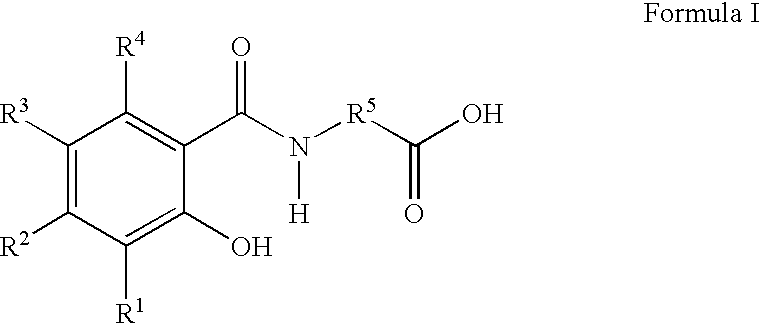
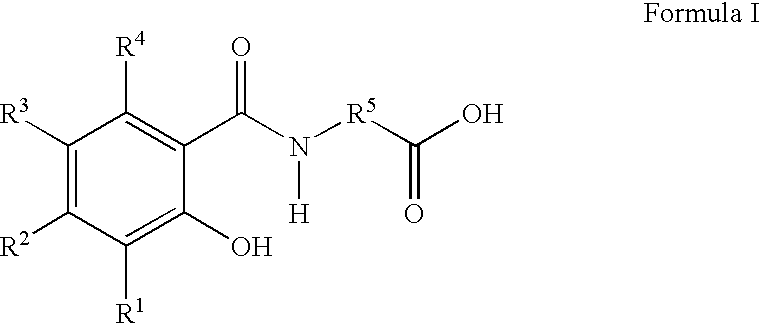
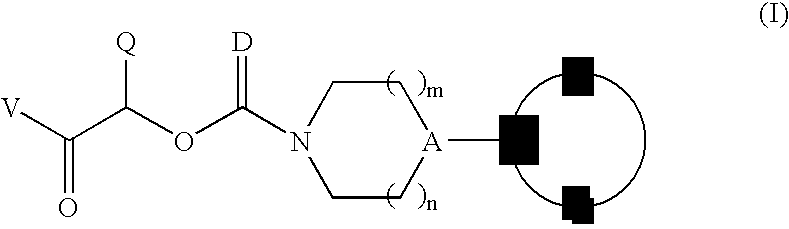
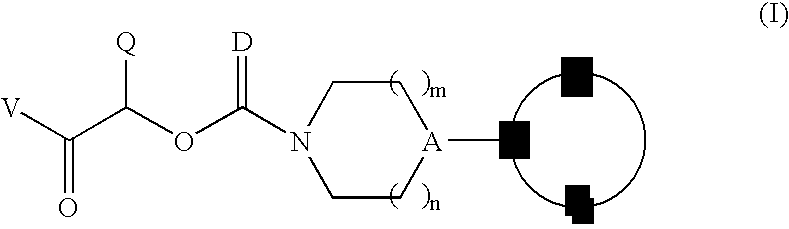
The present invention relates to compounds of Formula (I)as antagonists of calcitonin gene-related peptide receptors (“CGRP-receptor”), pharmaceutical compositions comprising them, methods for identifying them, methods of treatment using them and their use in therapy for treatment of neurogenic vasodilation, neurogenic inflammation, migraine and other headaches, thermal injury, circulatory shock, flushing associated with menopause, airway inflammatory diseases, such as asthma and chronic obstructive pulmonary disease (COPD), and other conditions the treatment of which can be effected by the antagonism of CGRP-receptors.
Owner:BRISTOL MYERS SQUIBB CO
Magnetic particle chemiluminescence detection kit for calcitonin and preparation method thereof
InactiveCN107543932ASmooth connectionReduce yieldChemiluminescene/bioluminescenceBiological testingCalcitoninRepeatability
The invention discloses a calcitonin magnetic particle chemiluminescent detection kit and a preparation method. The kit includes: magnetic particles coupled with calcitonin capture antibody, acridinium ester-labeled calcitonin detection antibody, calcitonin calibrator, chemiluminescence pre-excitation solution A, chemiluminescence excitation solution B and cleaning solution. The kit of the invention uses the magnetic separation chemiluminescence technology as the detection means, and simultaneously combines the acridinium ester labeling technology. The direct chemiluminescence method established by the invention has high sensitivity, strong specificity, accuracy and rapidity, short detection time and higher accuracy and repeatability of detection results, and the kit can be applied to various luminescence detection instruments.
Owner:太原瑞盛生物科技有限公司
Oral solid lipid nano-particle preparation of calcitonin and preparation method thereof
ActiveCN101569608AReasonable blood calcium concentrationPeptide/protein ingredientsSolution deliveryLipid formationMicroparticle
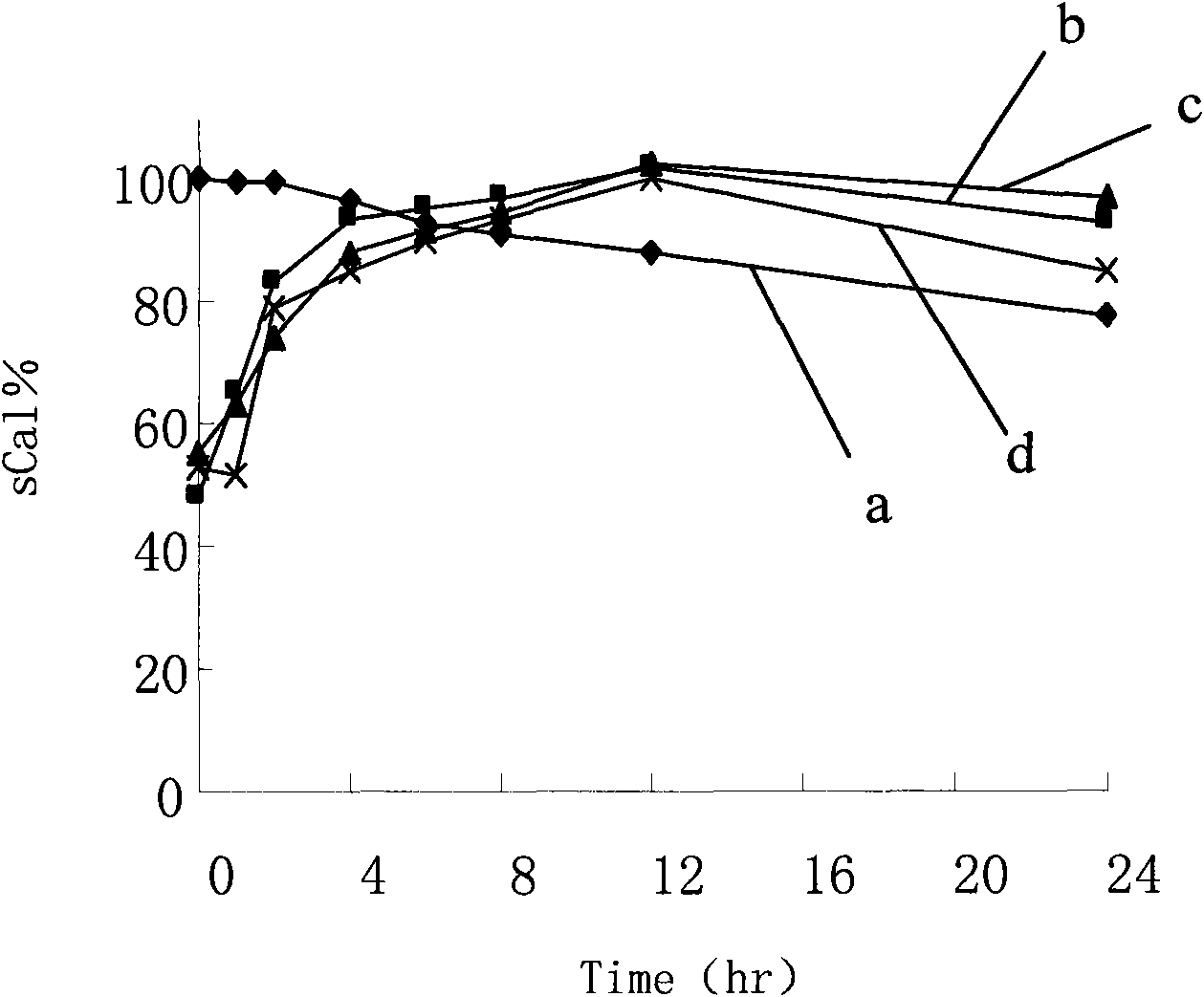
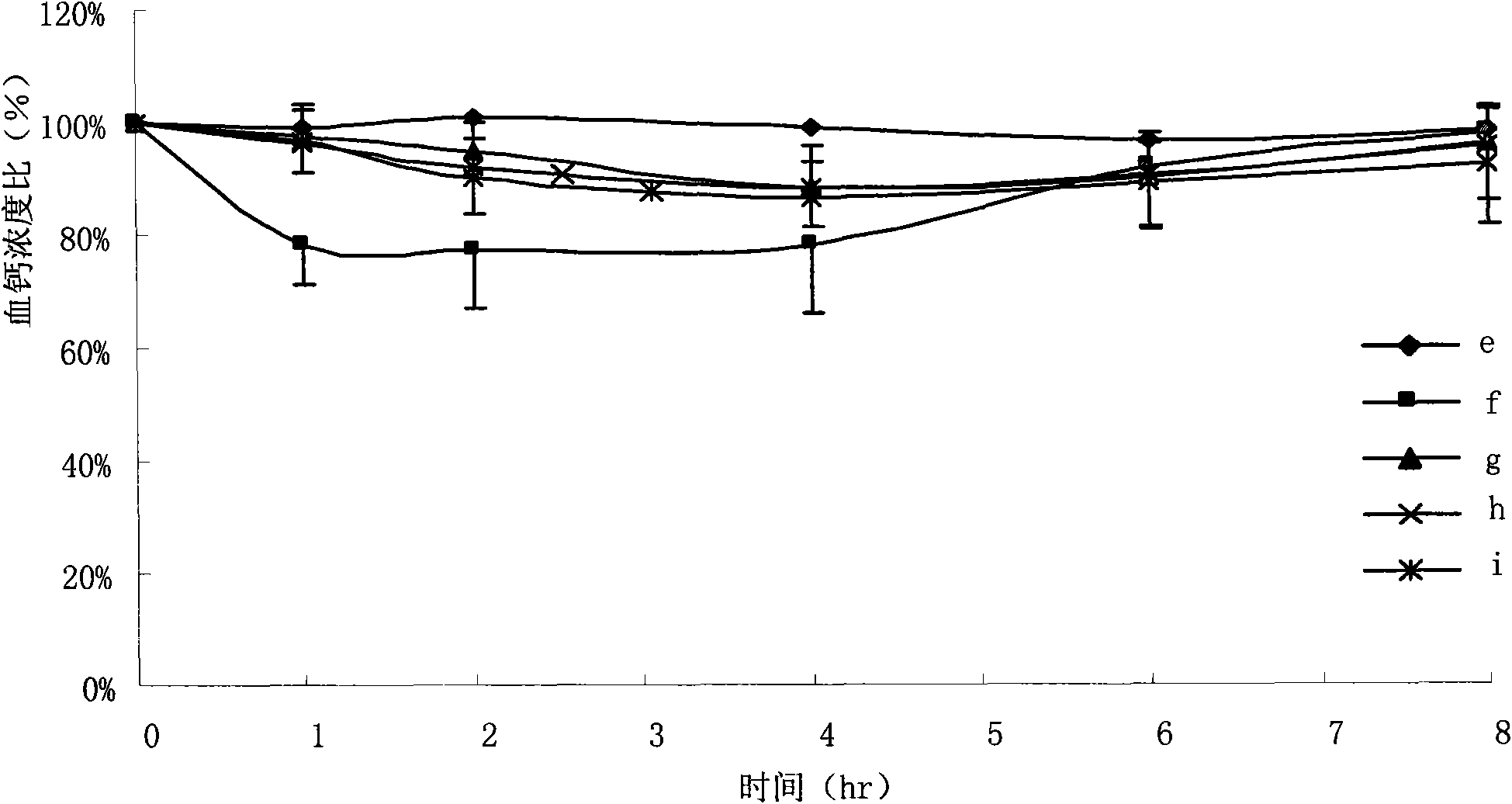
The invention discloses an oral solid lipid nano-particle preparation of calcitonin, which is a particle suspension containing 0.01 percent sodium cholate in a water phase, wherein lipid nano-particles comprise an active medicament component and a lipid material forming the particles. Simultaneously, the preparation method comprises the following steps: dissolving or melting a medicament and a carrier in an organic solvent phase together; quickly infusing the organic phase into the water phase stirred at a low temperature to form a lipid nano-particle dispersion liquid; standing, melting and performing high-speed centrifugal separation on the lipid nano-particle dispersion liquid to obtain a nano-particle deposition; and dispersing the deposition to obtain a target oral calcitonin lipid nano-particle dispersion liquid. The oral solid lipid nano-particle preparation uses the lipid material as a structural matrix material, and the oral solid lipid nano-particle preparation of the calcitonin is prepared through reasonable component proportion to prevent the medicament from leaking in an aqueous medium and release the medicament in modes of in vivo esterase degradation and the like, thus the aim that the medicament contained in a nano-carrier can adjust the serum calcium concentration more reasonably and more safely through biomembranes of mammals is achieved.
Owner:上海宝龙药业股份有限公司
Chemluminescent detection kit for procalcitonin and preparation method of chemluminescent detection kit
InactiveCN107807240AHigh sensitivityImprove featuresMaterial analysisMonoclonal antibodyChemiluminescent immunoassay
The invention discloses a chemiluminescent detection kit for procalcitonin and a preparation method thereof. The kit includes: sample diluent, magnetic particles coated with procalcitonin monoclonal antibody, procalcitonin monoclonal antibody labeled with acridinium ester, procalcitonin series standard solution, chemiluminescence excitation solution A, Chemiluminescence excitation solution B, cleaning solution. The kit of the invention combines the chemiluminescent technology with the immunomagnetic particle, and provides a nearly homogeneous reaction system. Compared with the prior art, the kit of the invention has the advantages of high sensitivity, strong specificity, short reaction time and the like.
Owner:太原瑞盛生物科技有限公司
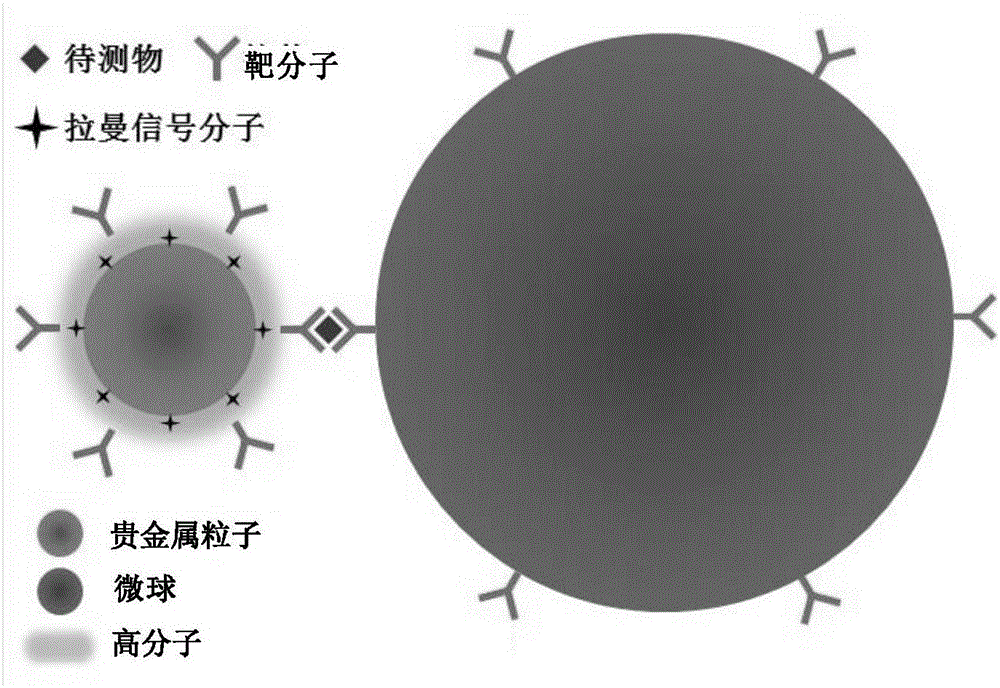
Surface enhanced Raman scattering technology-based composite material and preparation method thereof
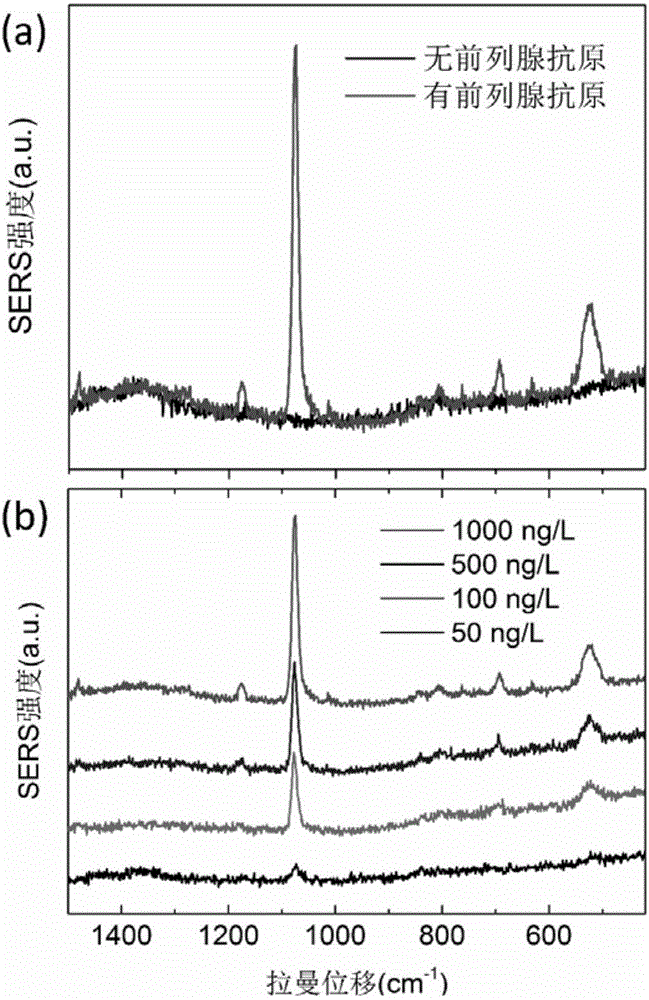
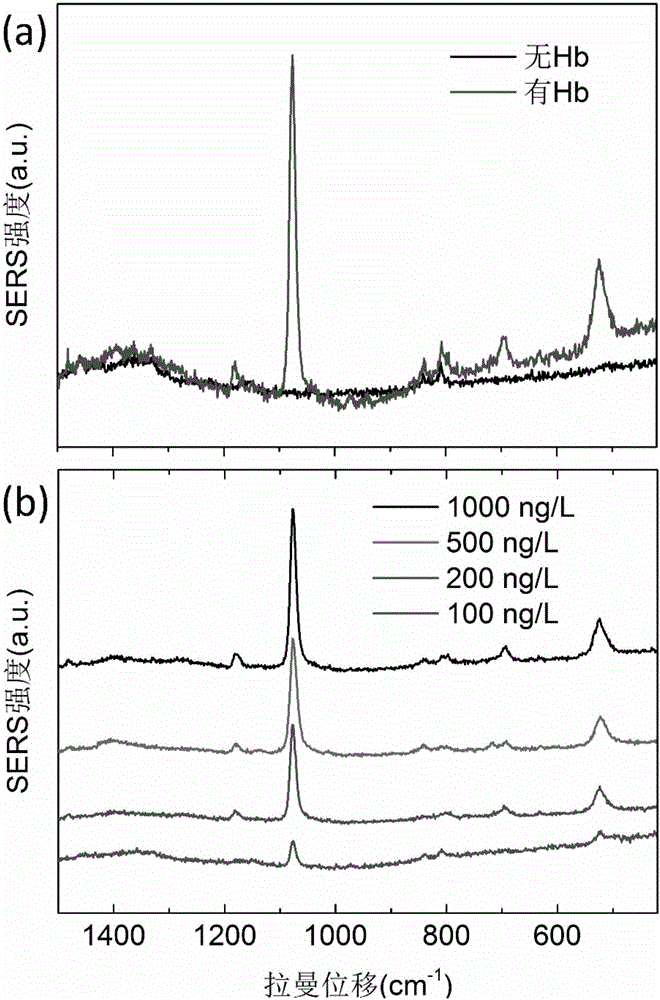
The invention relates to a surface enhanced Raman scattering technology-based composite material and a preparation method thereof. The composite material comprises microspheres modified with first target molecules on the surfaces and a second component modified with Raman signal molecules and second target molecule-modified polymers on the surface. In a solution containing a substance to be detected, the substance to be detected, the microspheres and the second component are bonded to the second target molecules through the first target molecules so that the composite material which can be easily precipitated through centrifugation is obtained. The invention also discloses the preparation method and a use of the composite material. The composite material can be used for detecting a human prostate-specific antigen (PSA), a human ferrohemoglobin (Hb), a procalcitonin (PCT) or disease markers (such as tumor markers, cardiovascular disease markers, senile dementia markers, mycoplasma and chlamydia), can effectively reduce false positive and false negative effects and has advantages of high sensitivity, simpleness, fastness and low cost.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Procalcitonin monoclonal antibody hybrid tumor 2H4 and monoclonal antibody
ActiveCN104745534AImprove featuresHigh sensitivityMicroorganism based processesTissue cultureProtein detectionMonoclonal antibody
The invent9ion discloses a procalcitonin monoclonal antibody hybrid tumor 2H4 and monoclonal antibody. The procalcitonin monoclonal antibody hybrid tumor 2H4 is preserved in the China Center for Type Culture Collection at 16, Dec, 2014, and the preservation number is CCTCC NO: C2014232. The monoclonal antibody prepared aiming at procalcitonin compound oligopeptide has high specificity and sensitivity to the procalcitonin protein detection and lays a foundation for the function study and development of the procalcitonin and the research of corresponding diagnostic reagents.
Owner:SOUTHERN MEDICAL UNIVERSITY
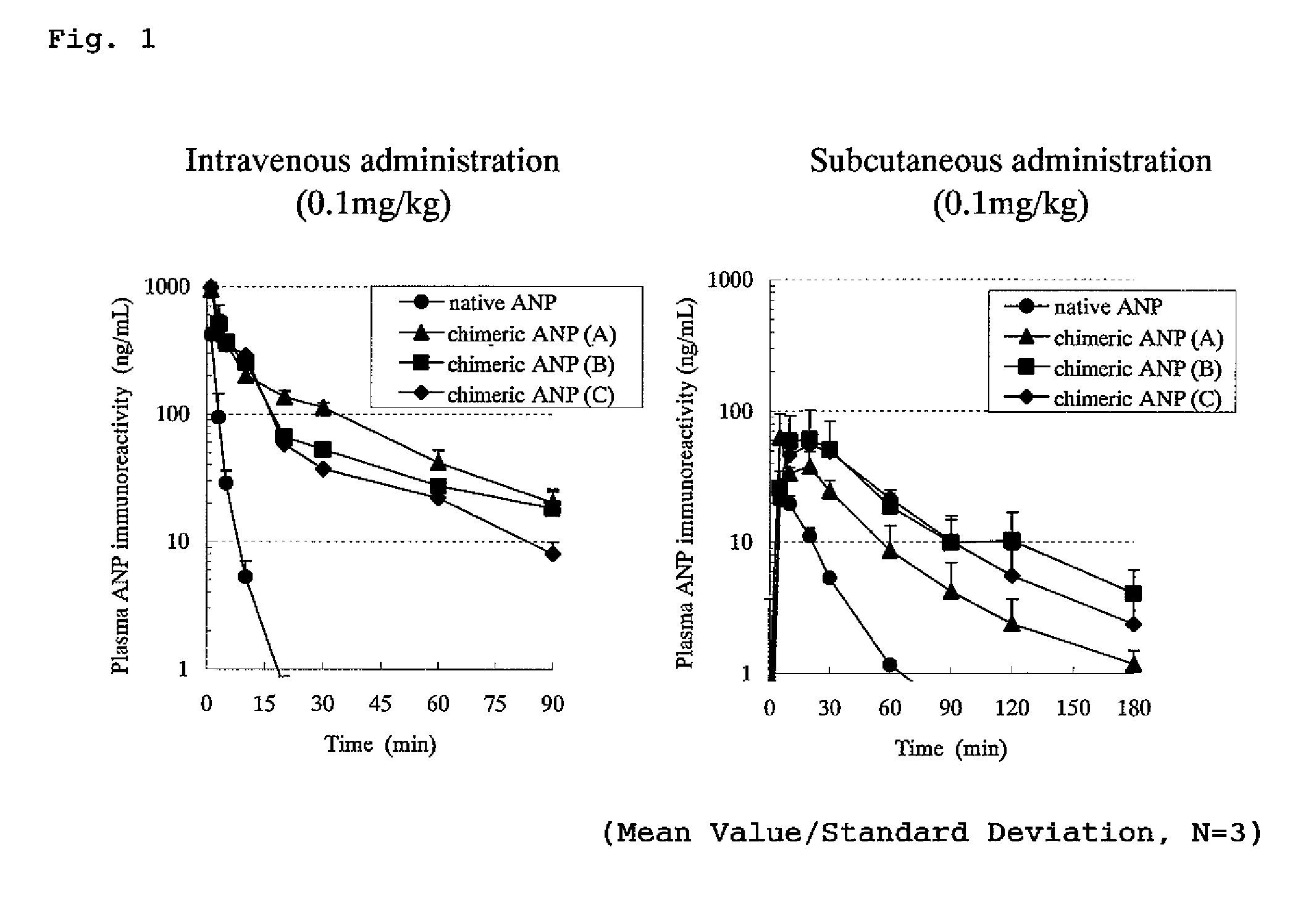
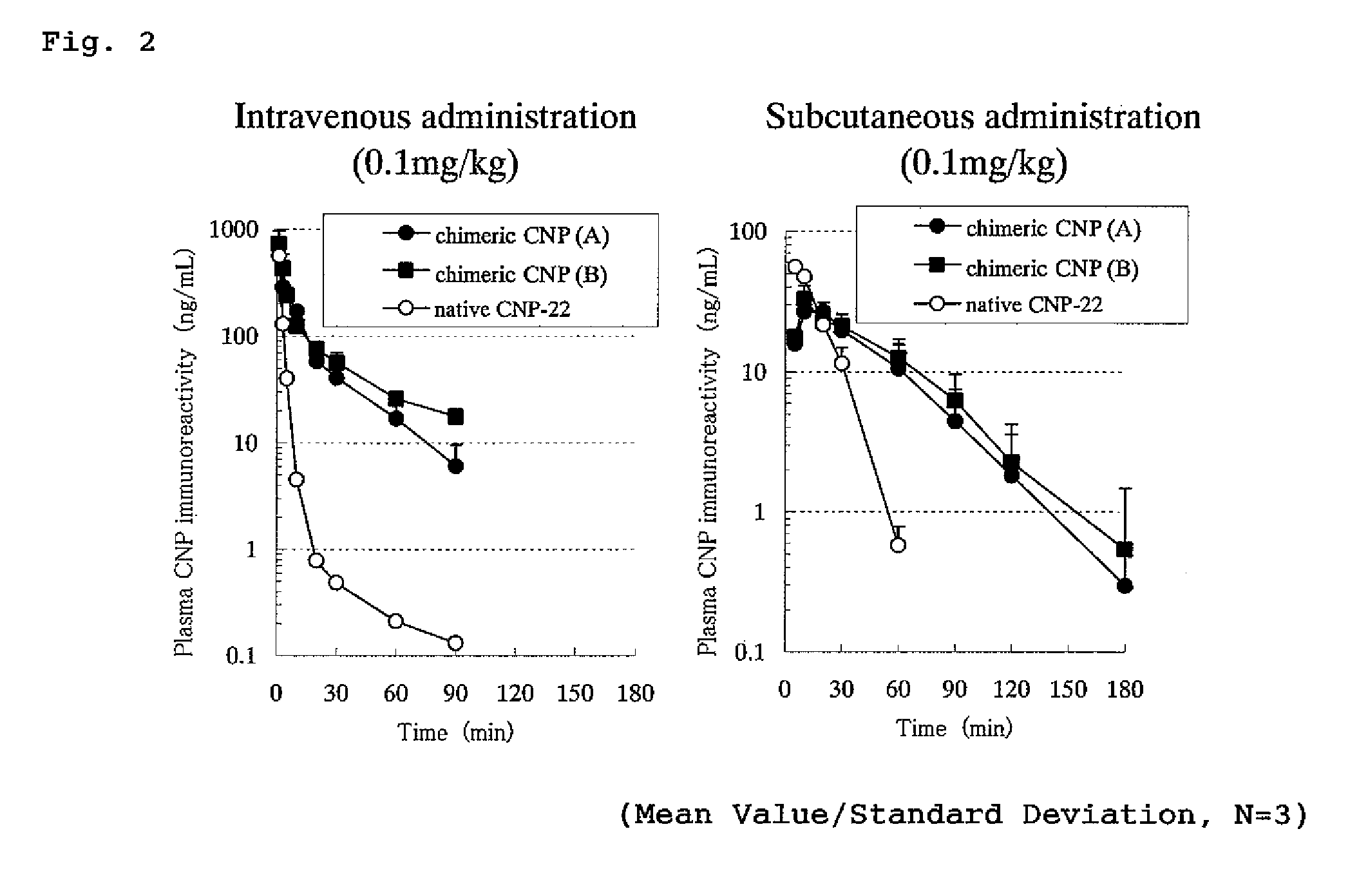
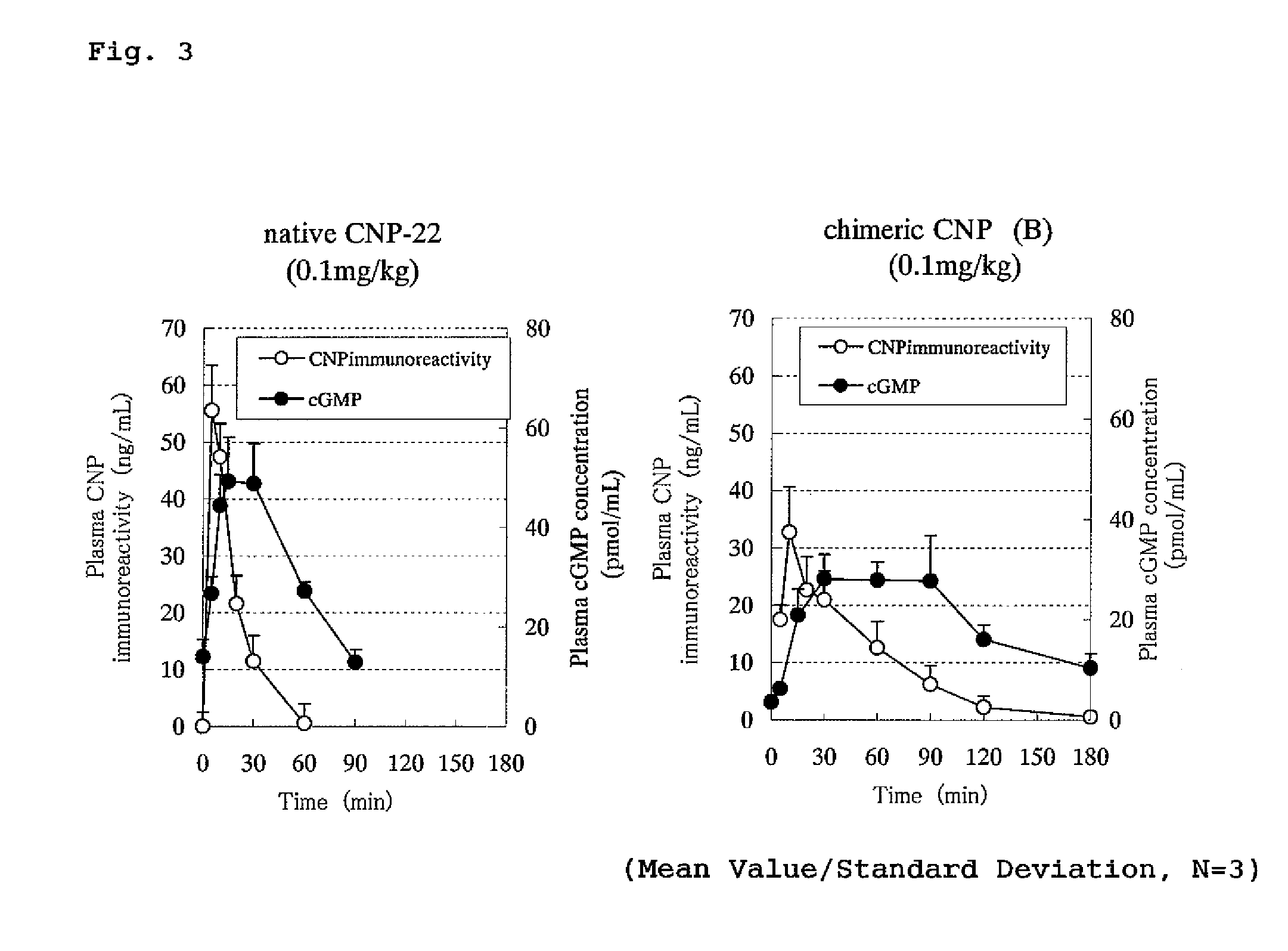
Peptide having an extending action for half-life of object peptide in plasma
ActiveUS8551937B2Improve complianceImprove securityPeptide/protein ingredientsAntipyreticC-type natriuretic peptideHalf-life
An isolated chimeric peptide consisting of one or two added peptides and an object peptide wherein the added peptide is bonded to the N-terminus, the C-terminus or both of the object peptide, wherein if the added peptides are bound to both terminals, the two added peptides may be the same or different; and physiological activity of the object peptide is still retained, wherein the object peptide is a natural physiologically active peptide selected from the group consisting of an atrial natriuretic peptide, a brain natriuretic peptide, a C-type natriuretic peptide, motilin, a glucagon-like peptide 1, parathyroid hormone, and calcitonin, or a derivative of any thereof, wherein the derivative has one or more amino acid(s) deleted from the amino acid sequence of a natural physiologically active peptide and has the desired physiological activity.
Owner:DAIICHI SANKYO CO LTD
Marker for graft failure and mortality
ActiveUS20110171750A1Lower levelDisease diagnosisBiological testingOrgan transplantationMortality rate
Subject of the present invention is a biomarker for graft failure and / or mortality after organ transplantation. Procalcitonin was found to be a useful marker for the prediction or risk stratification for graft failure and / or mortality of a subject who has received an organ transplant and monitoring and therapy guidance of such subject.
Owner:BRAHMS GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com