Patents
Literature
226 results about "Troponin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
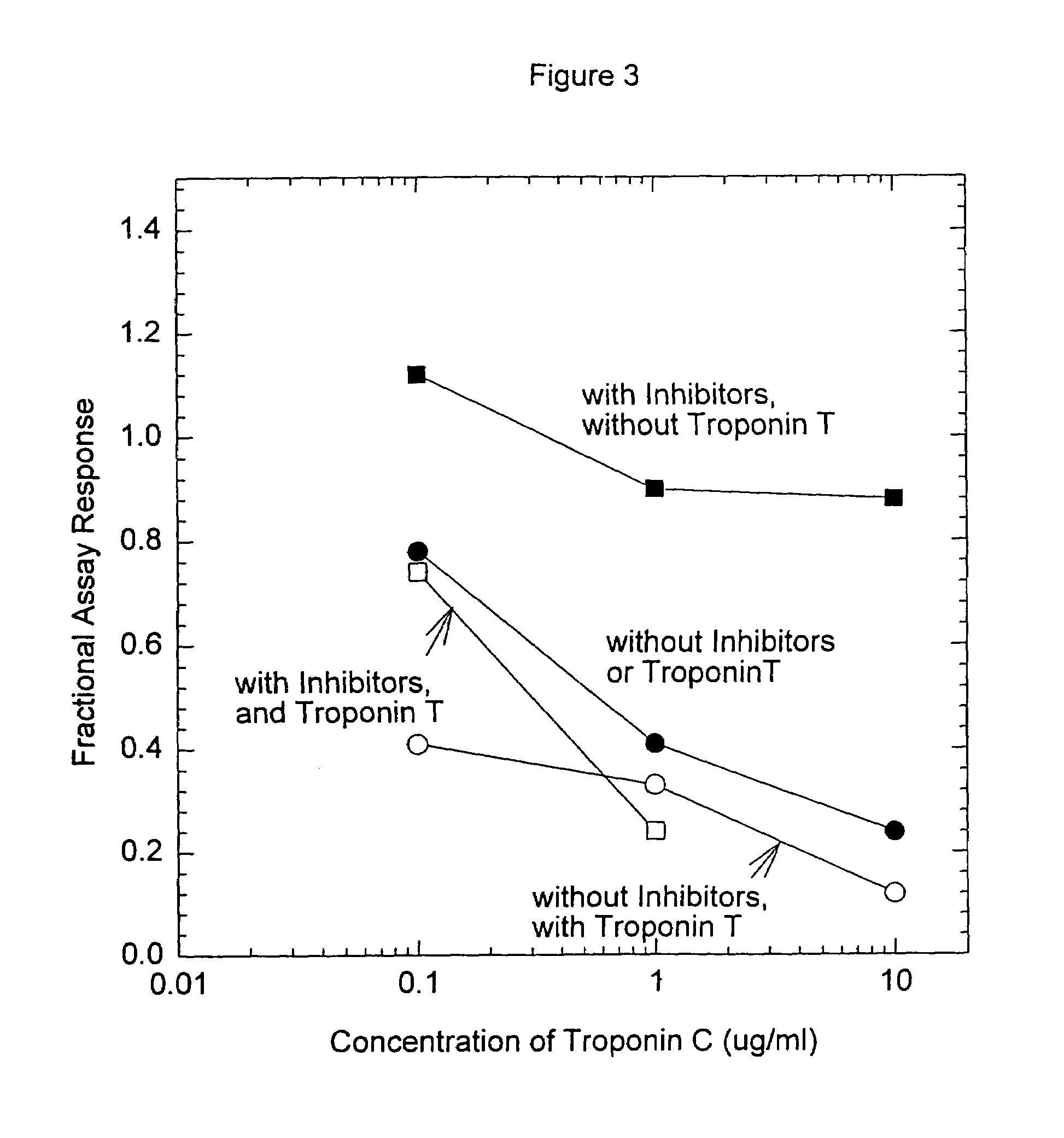
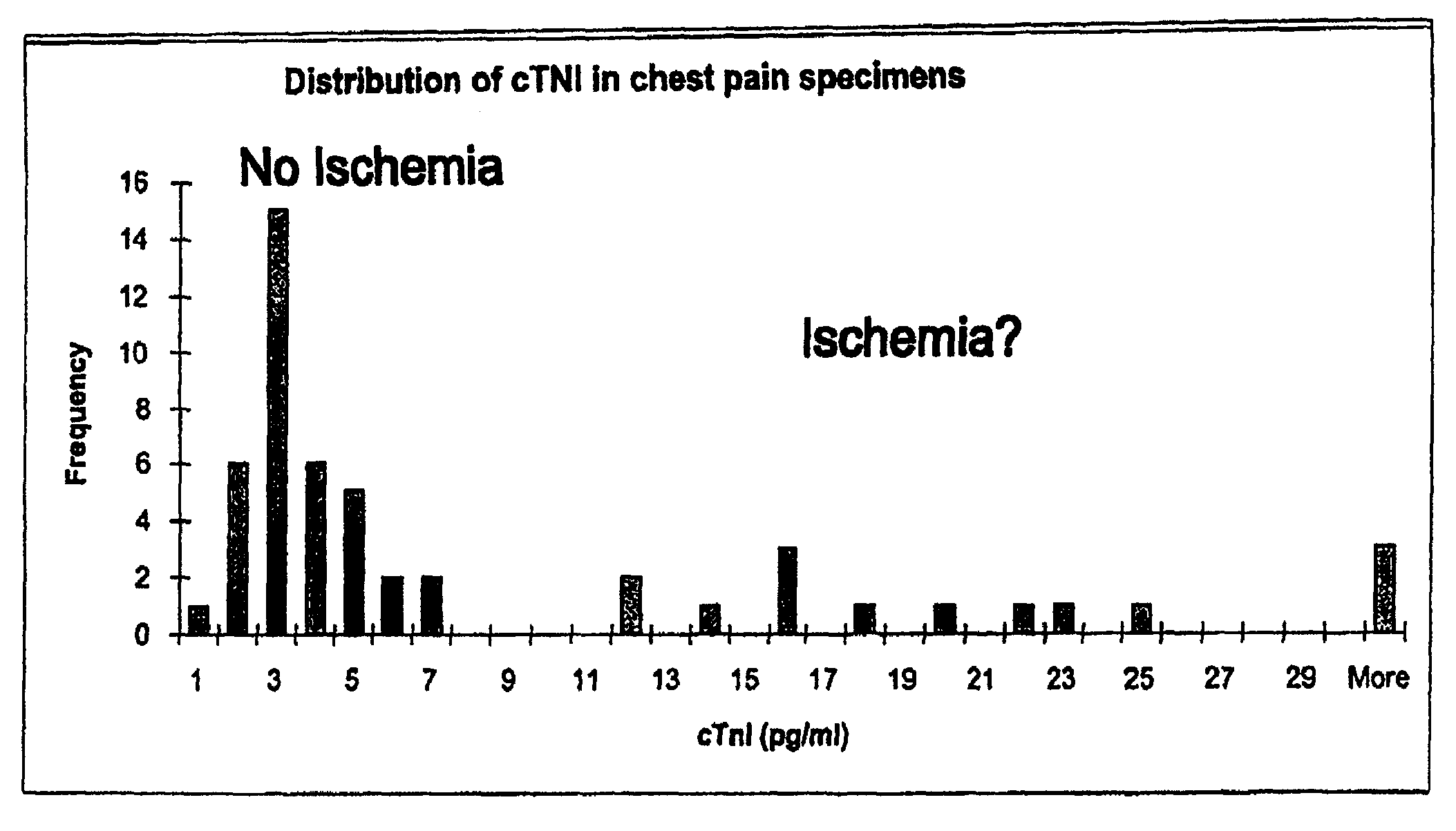
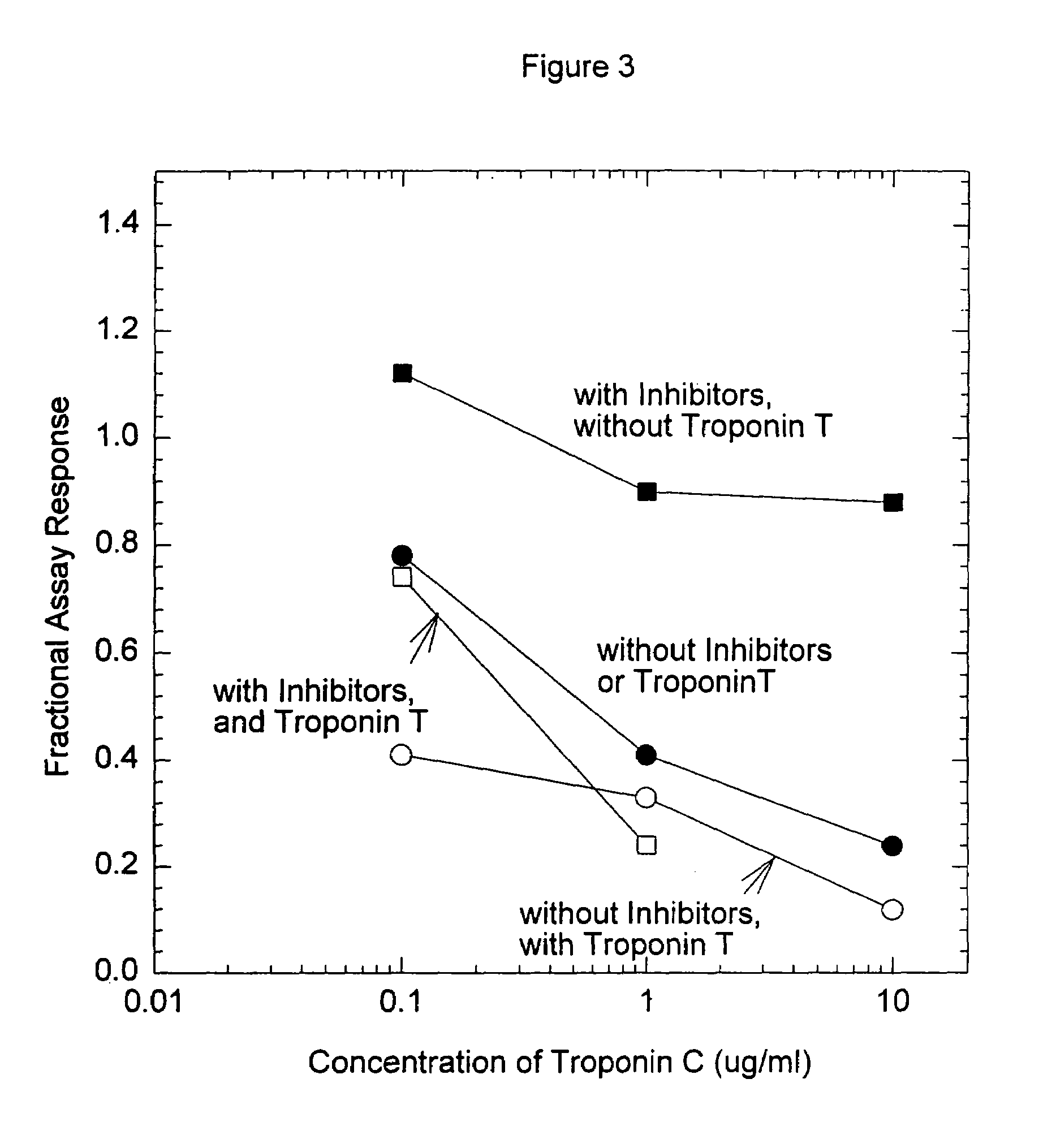
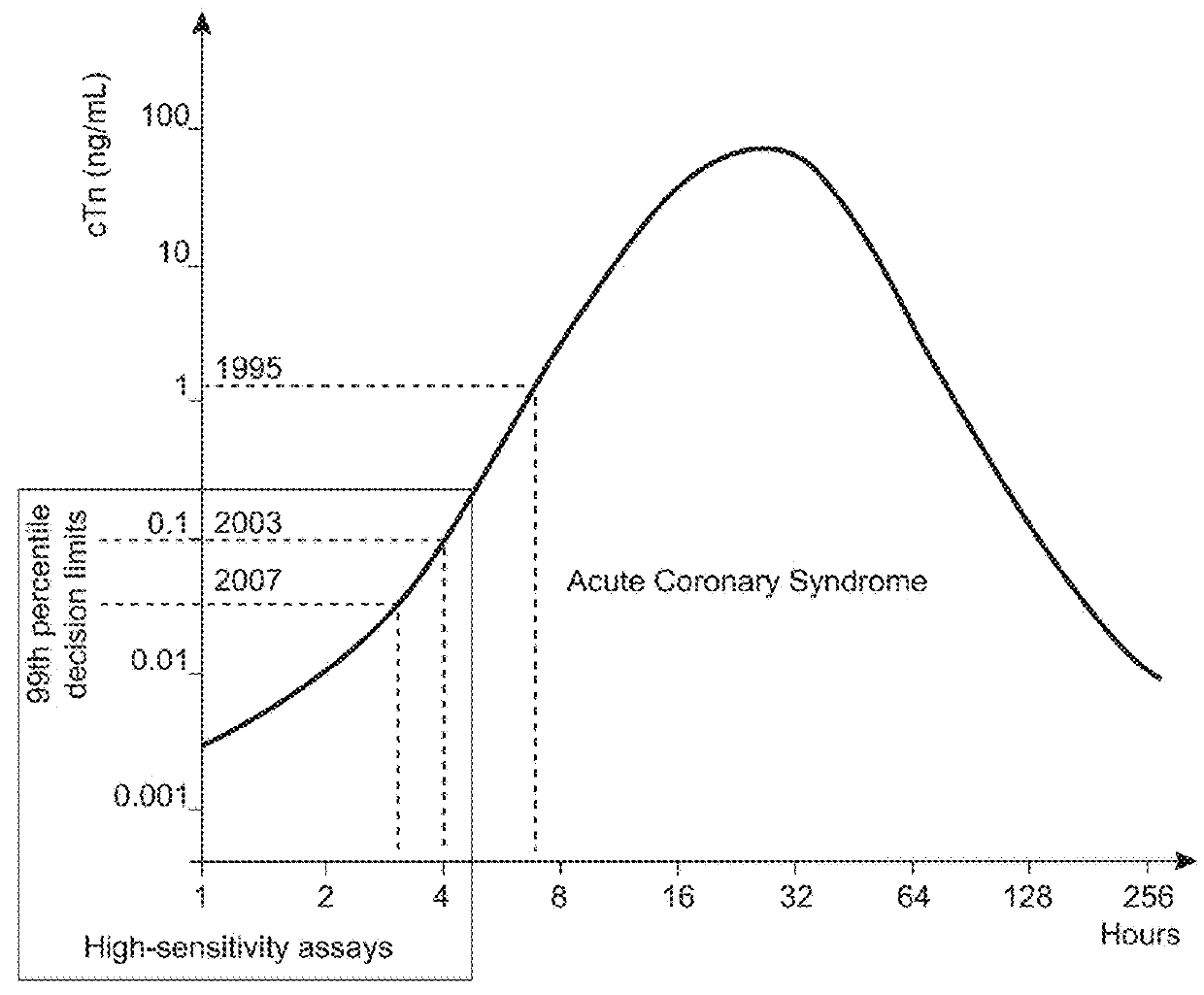
Troponin, or the troponin complex, is a complex of three regulatory proteins (troponin C, troponin I, and troponin T) that is integral to muscle contraction in skeletal muscle and cardiac muscle, but not smooth muscle. Blood troponin levels may be used as a diagnostic marker for stroke, although the sensitivity of this assay is low. Assays of cardiac-specific troponins I and T are extensively used as diagnostic and prognostic indicators in the management of myocardial infarction and acute coronary syndrome.
Muscle-specific expression vectors
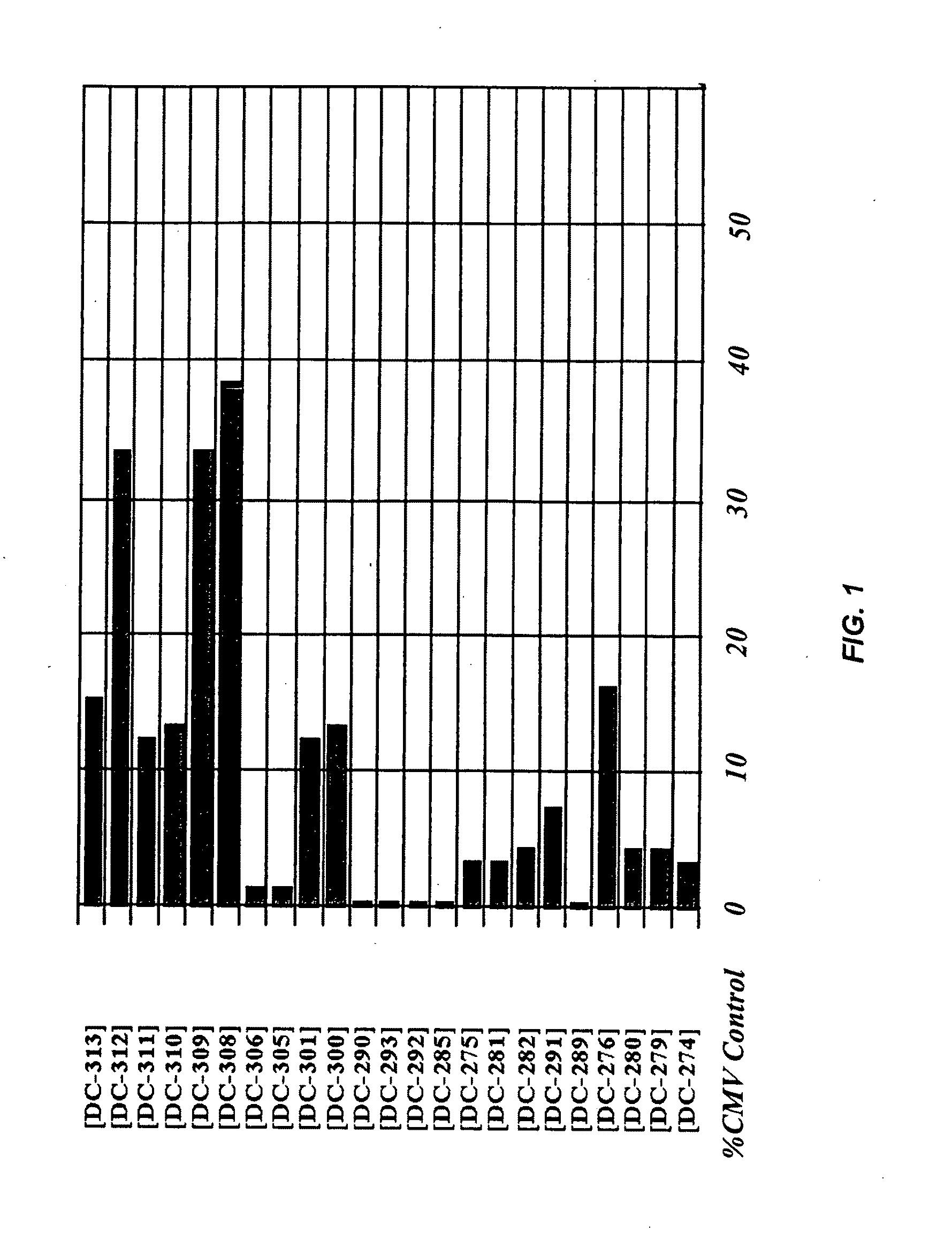
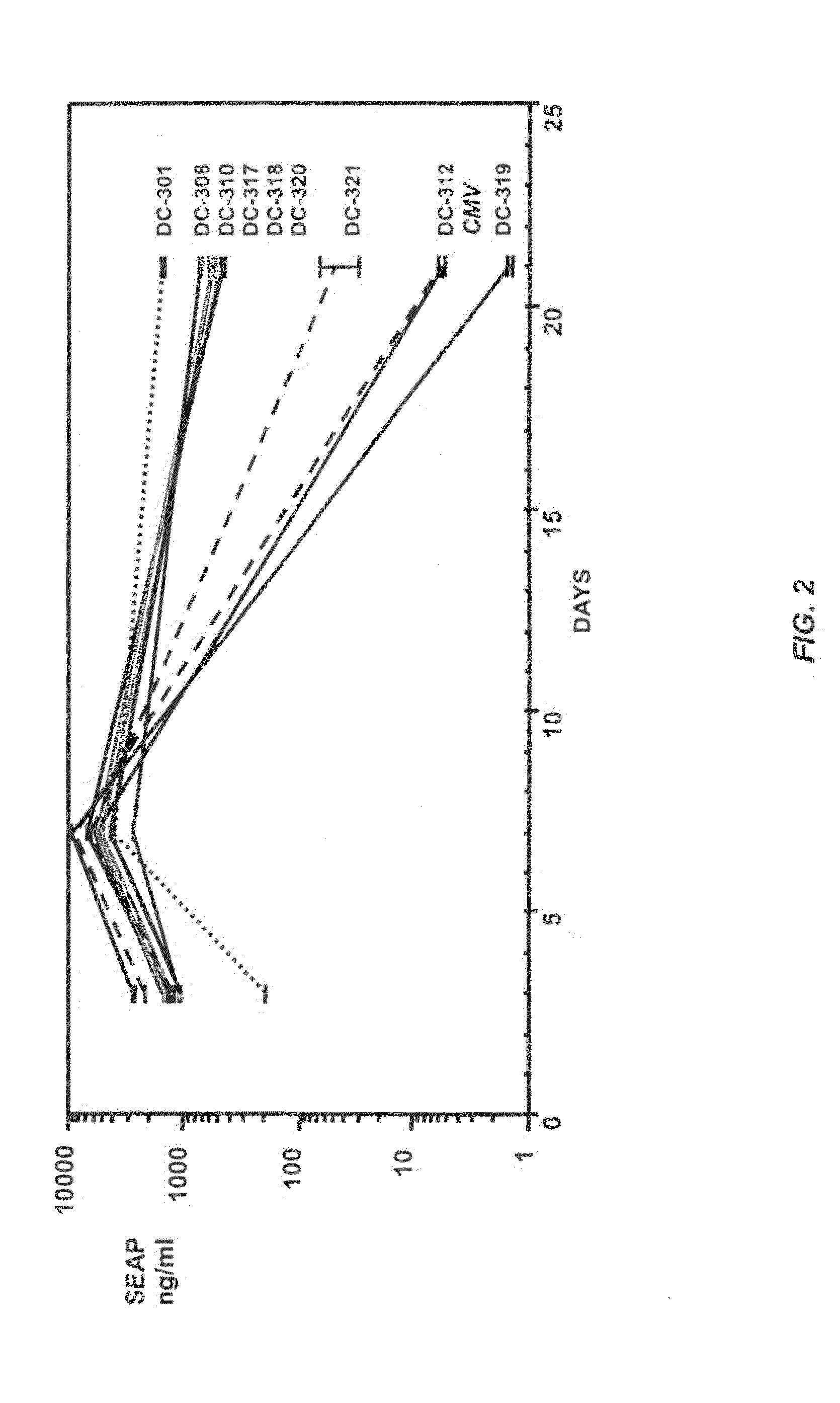
The invention is directed to novel combinations of muscle-specific enhancers and promoter elements useful for achieving persistent expression in the muscle or myocyctes. The muscle-specific promoter elements are derived from a muscle creatine kinase promoter, a troponin I promoter, a skeletal alpha-actin promoter, or a desmin promoter. The muscle-specific enhancer elements are derived from either troponin I internal regulatory elements, muscle creatine kinase enhancers, or desmin enhancers.
Owner:SOUZA DAVID +1
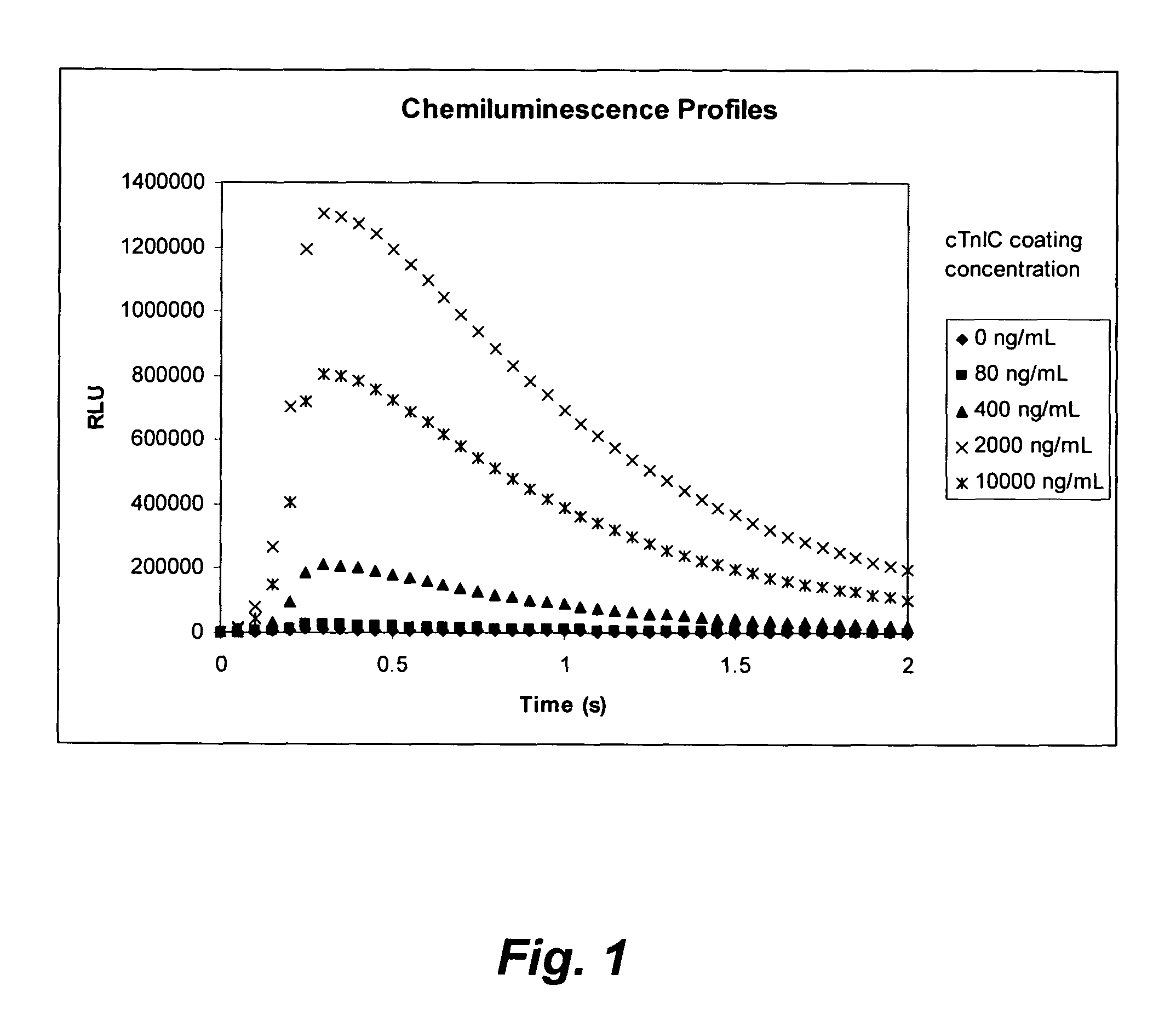
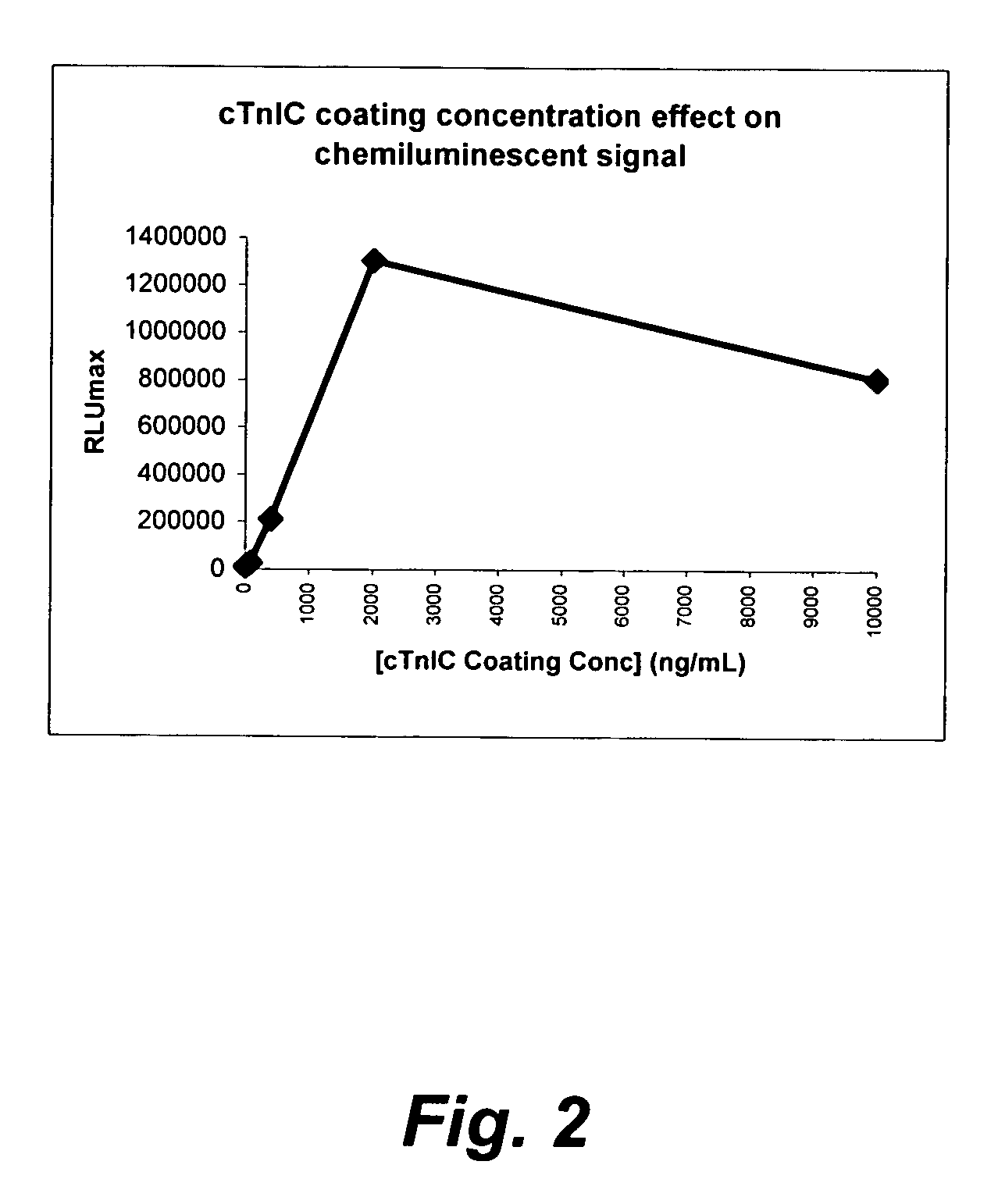
Methods for the assay of troponin I and T and complexes of troponin I and T and selection of antibodies for use in immunoassays
InactiveUS6991907B1Promote recoveryChemiluminescene/bioluminescenceEnzymologyHeart diseaseClinical state
Antibodies and methods are described for the detection and quantitation of cardiac specific troponin I in samples. Cardiac-specific troponin isoforms exist in various forms in the blood, including free and complexed forms. By selecting antibodies that are insensitive and / or sensitive to these various forms, the present invention can provide immunoassays that more accurately reflect the clinical state of an individual. These described antibodies and methods can be used for providing indicators of myocardial infarction and other cardiac pathologies.
Owner:BIOSITE INC
Method and compositions for highly sensitive detection of molecules
InactiveUS20090234202A1Detection moreImprove the level ofElectrocardiographyMicrobiological testing/measurementVascular endothelial growth factorPhysiological markers
The present invention discloses methods for the detection and monitoring of a condition in a subject using highly sensitive detection of molecules. The invention provides a method for detecting or monitoring a condition in a subject, comprising detecting a first marker in a first sample from the subject and detecting a second marker, wherein the first marker comprises a biomarker, e.g., Cardiac Troponin-I (cTnI) or Vascular Endothelial Growth Factor (VEGF), and wherein the limit of detection of the first marker is less than about 10 pg / ml. The second marker can be a biomarker, physiological marker, a molecular marker or a genetic marker.
Owner:SINGULEX
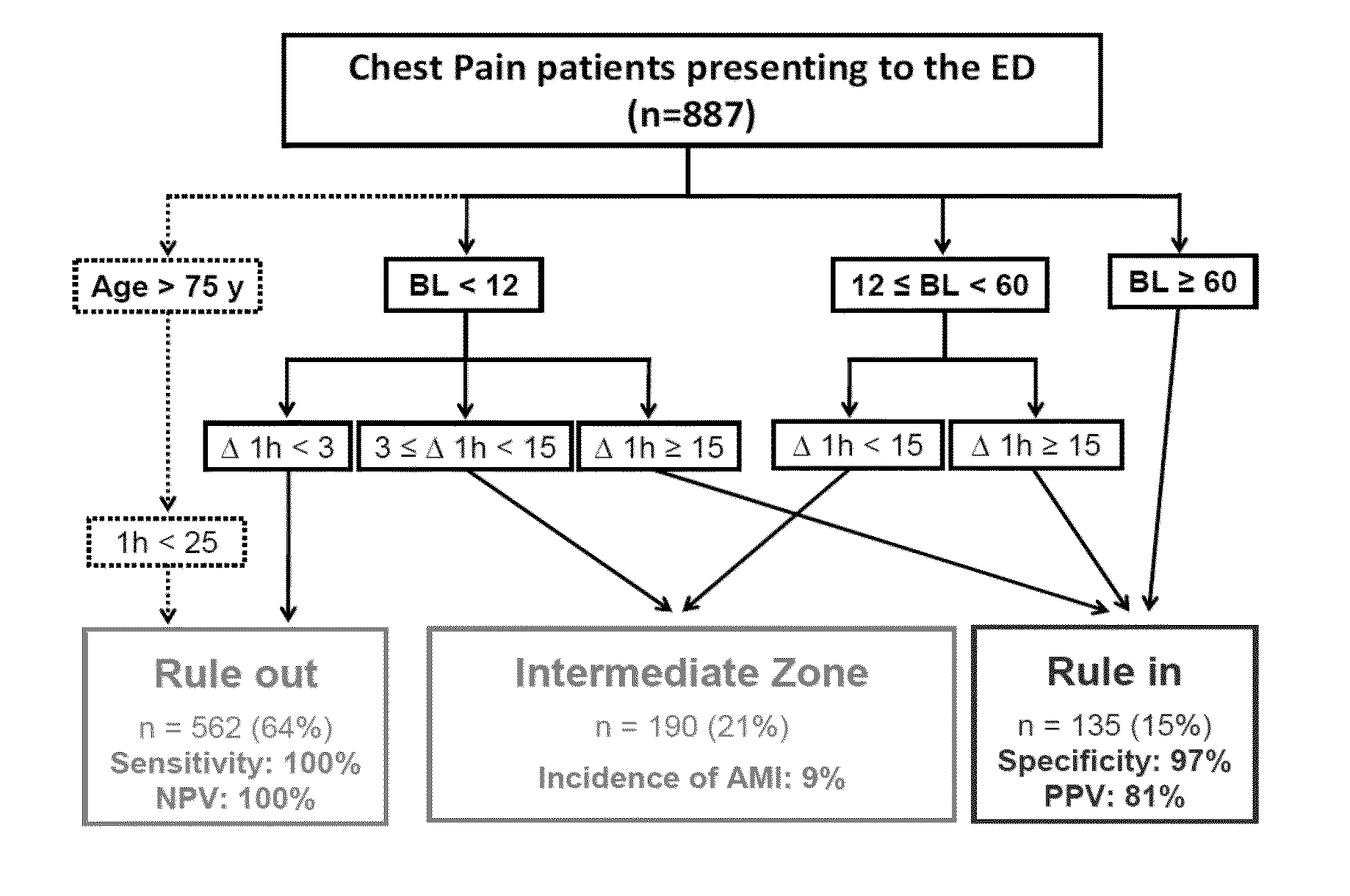
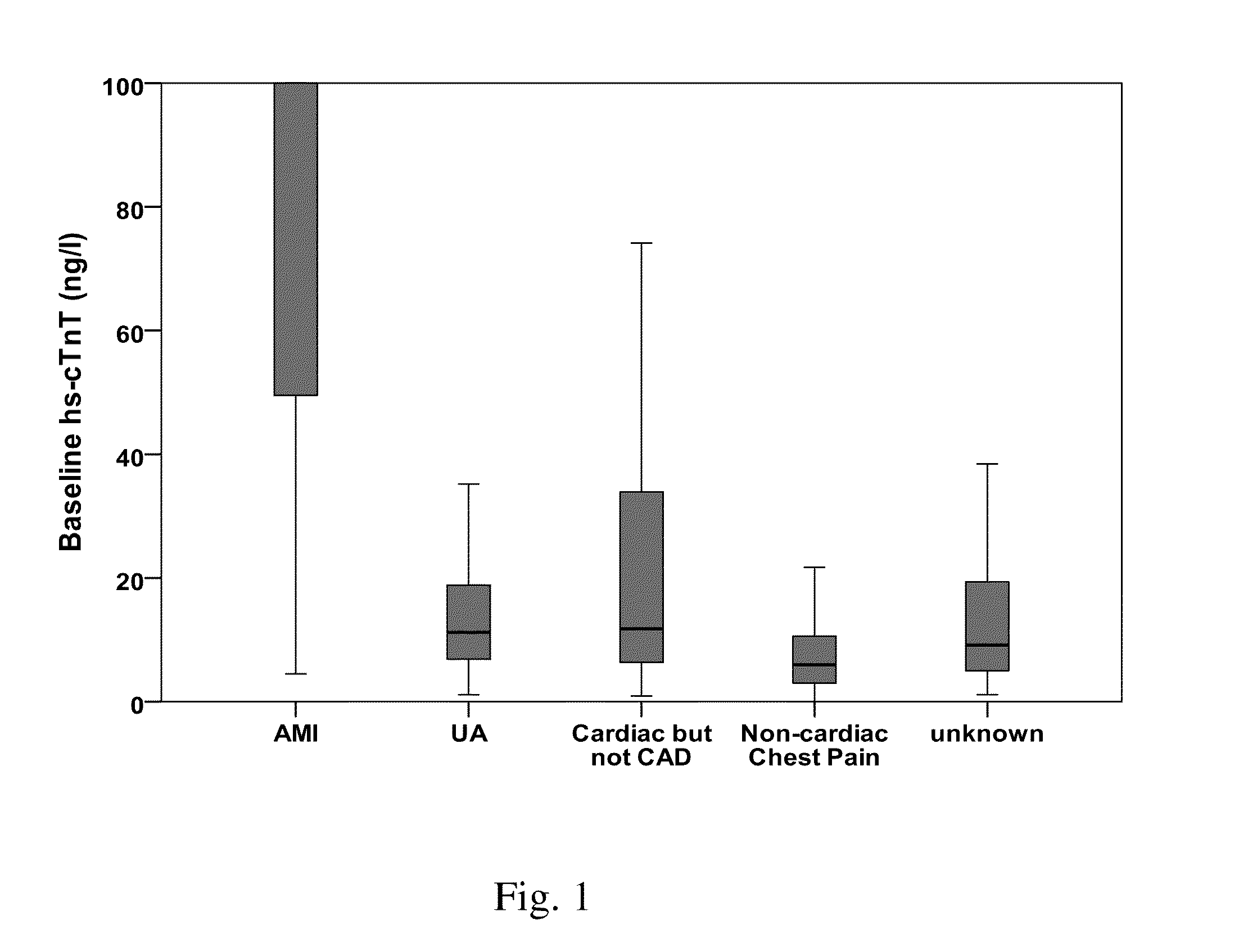
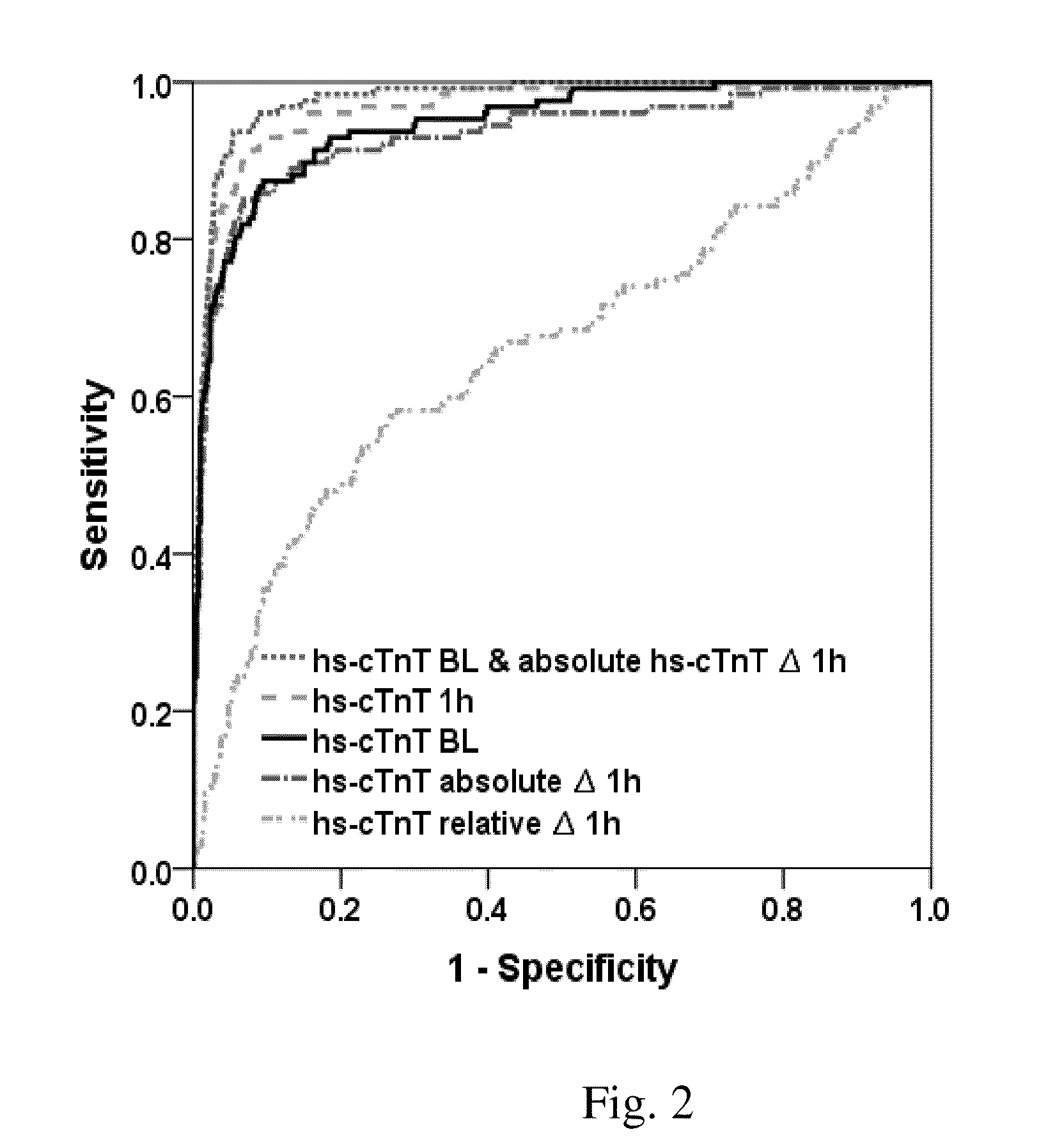
Troponin based rule-in and rule-out algorithm of myocardial infarction
InactiveUS20130035603A1Eliminate needPositive predictive valueCatheterDisease diagnosisChest painCardiac troponin
The present invention relates to a method for diagnosing myocardial infarction in a subject presenting with chest pain. The method is based on the determination of an amount of a cardiac troponin in a first sample from the subject obtained at presentation to a physician, and in a second sample obtained within one hour after the first sample. Moreover, the present invention envisages a method for ruling in myocardial infarction and a method for ruling out myocardial infarction. The said methods are also based on the determination of the amount of a cardiac troponin in a first sample from the subject obtained at presentation to a physician, and in a second sample obtained within one hour after the first sample.
Owner:JARAUSCH JOCHEN +6
Highly sensitive system and methods for analysis of troponin
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for the assay of troponin I and T and complexes of troponin I and T and selection of antibodies for use in immunoassays
InactiveUS6939678B1Promote recoveryChemiluminescene/bioluminescenceEnzymologyCysteine thiolateBlood plasma
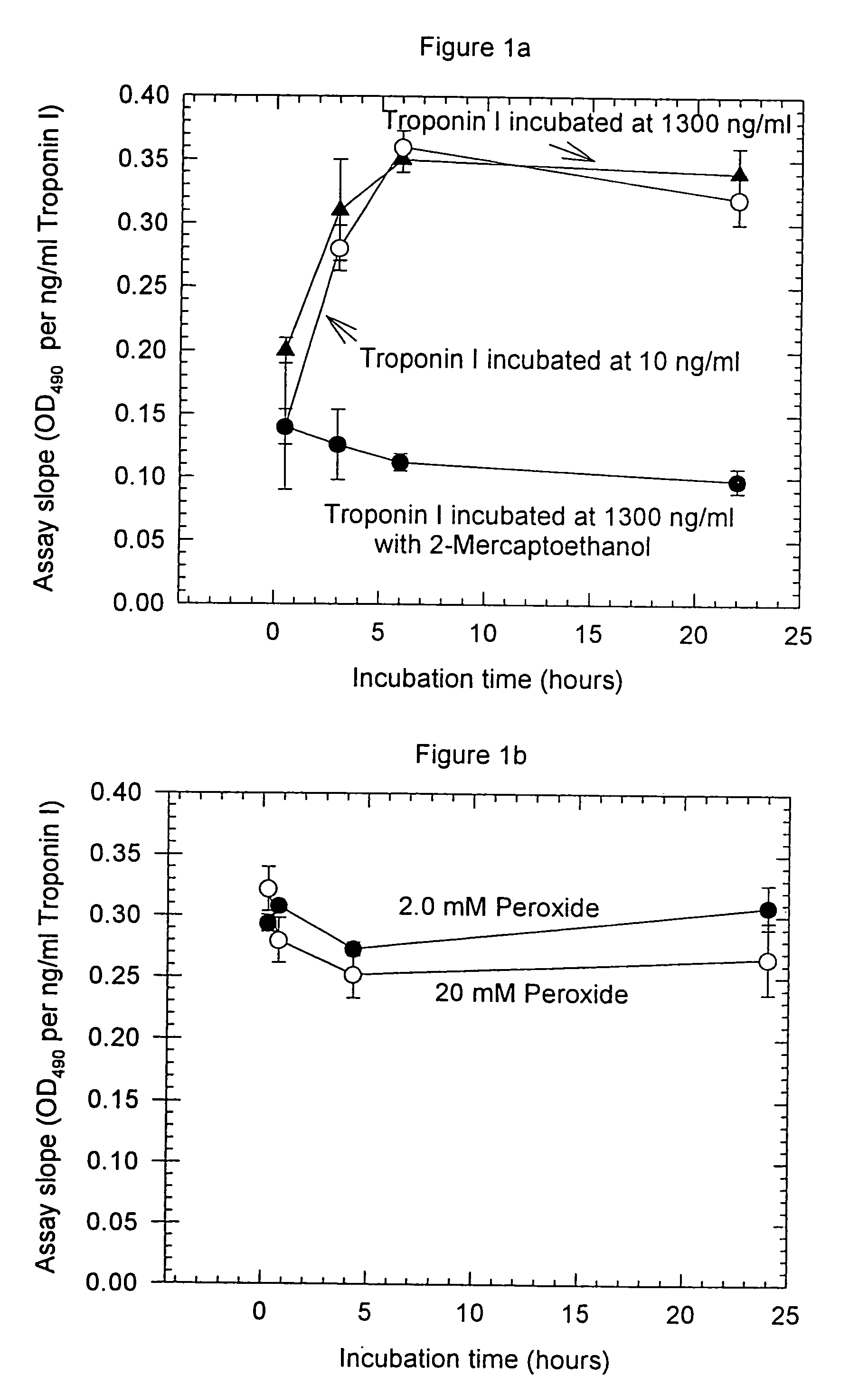
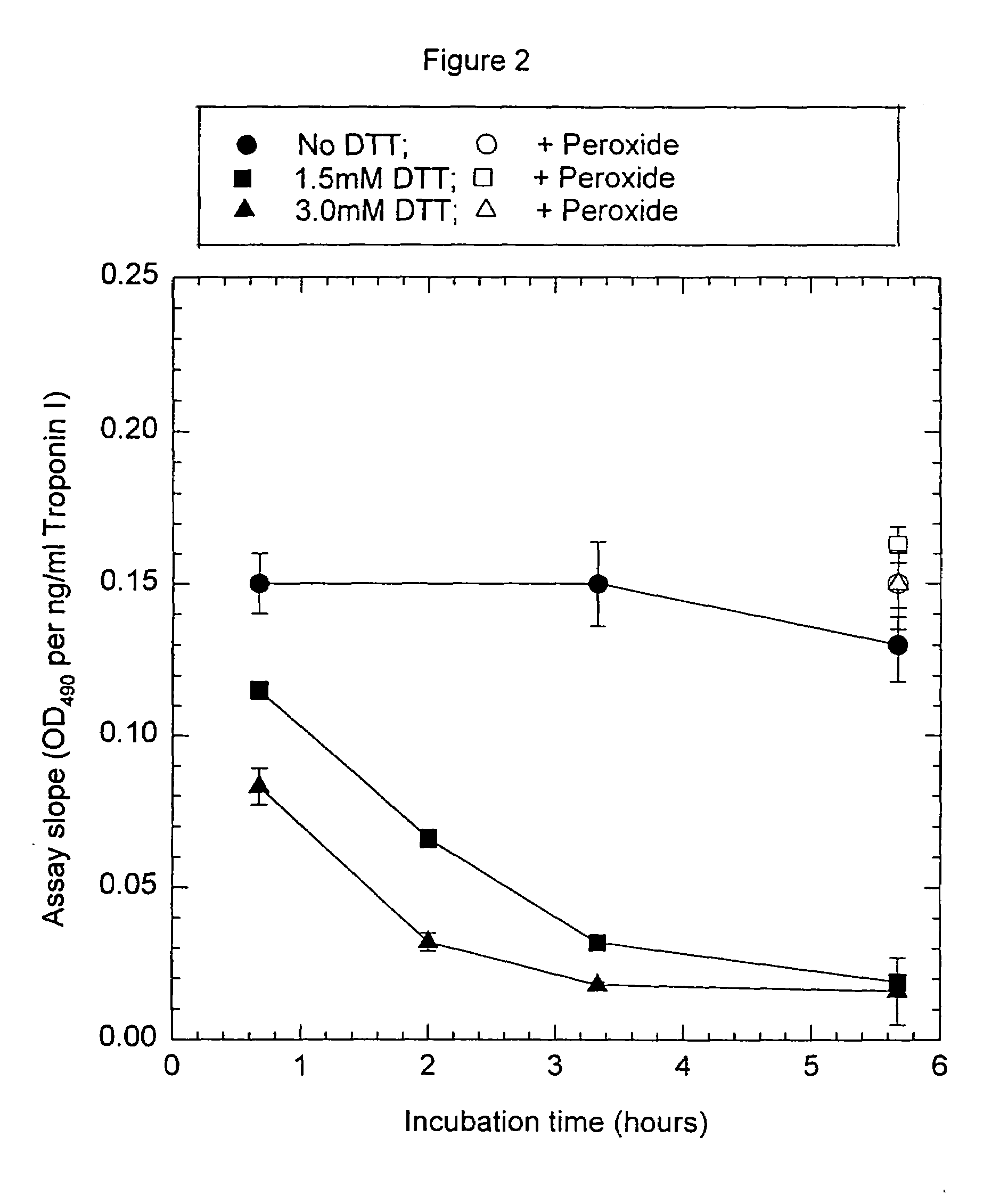
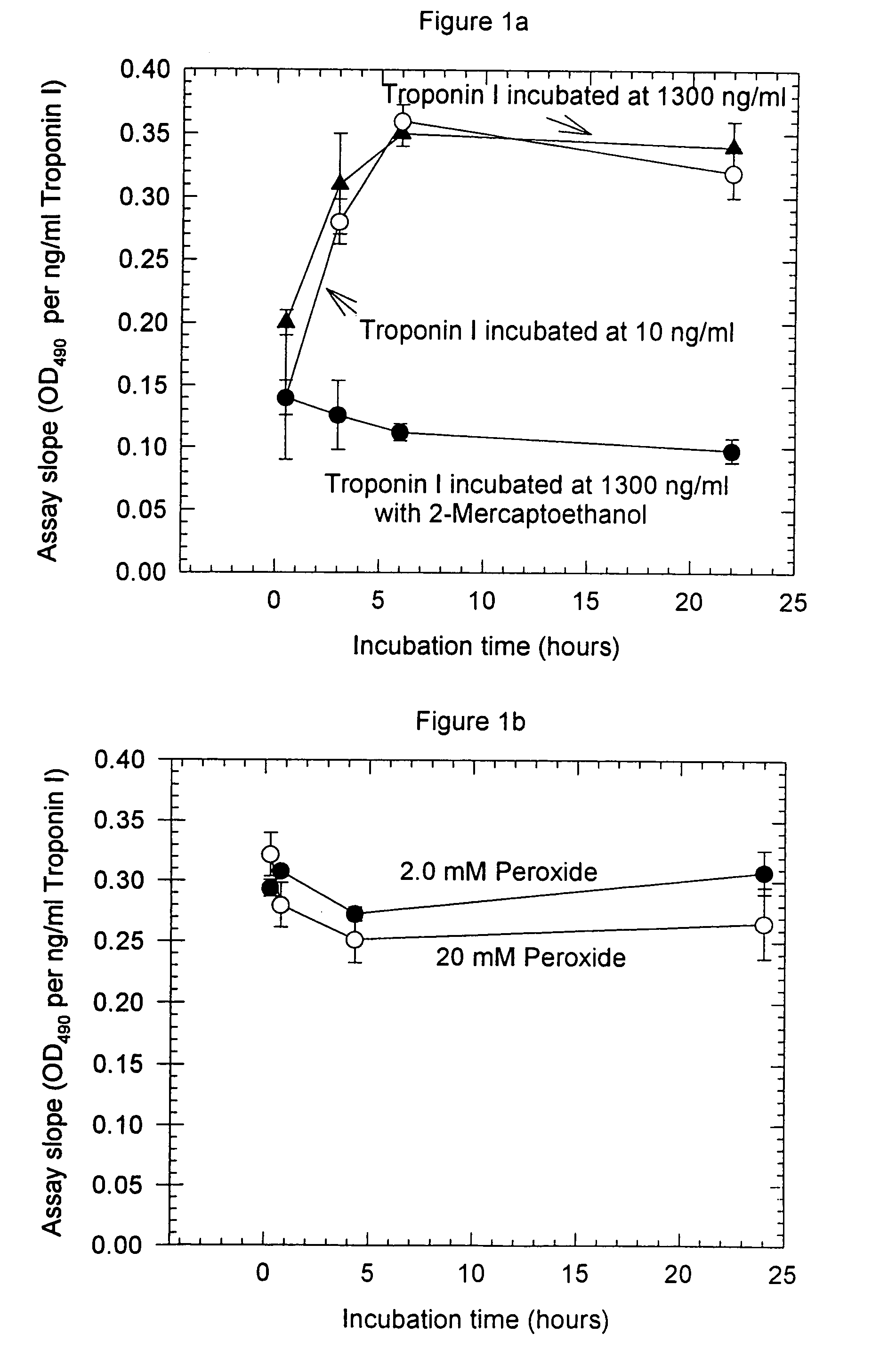
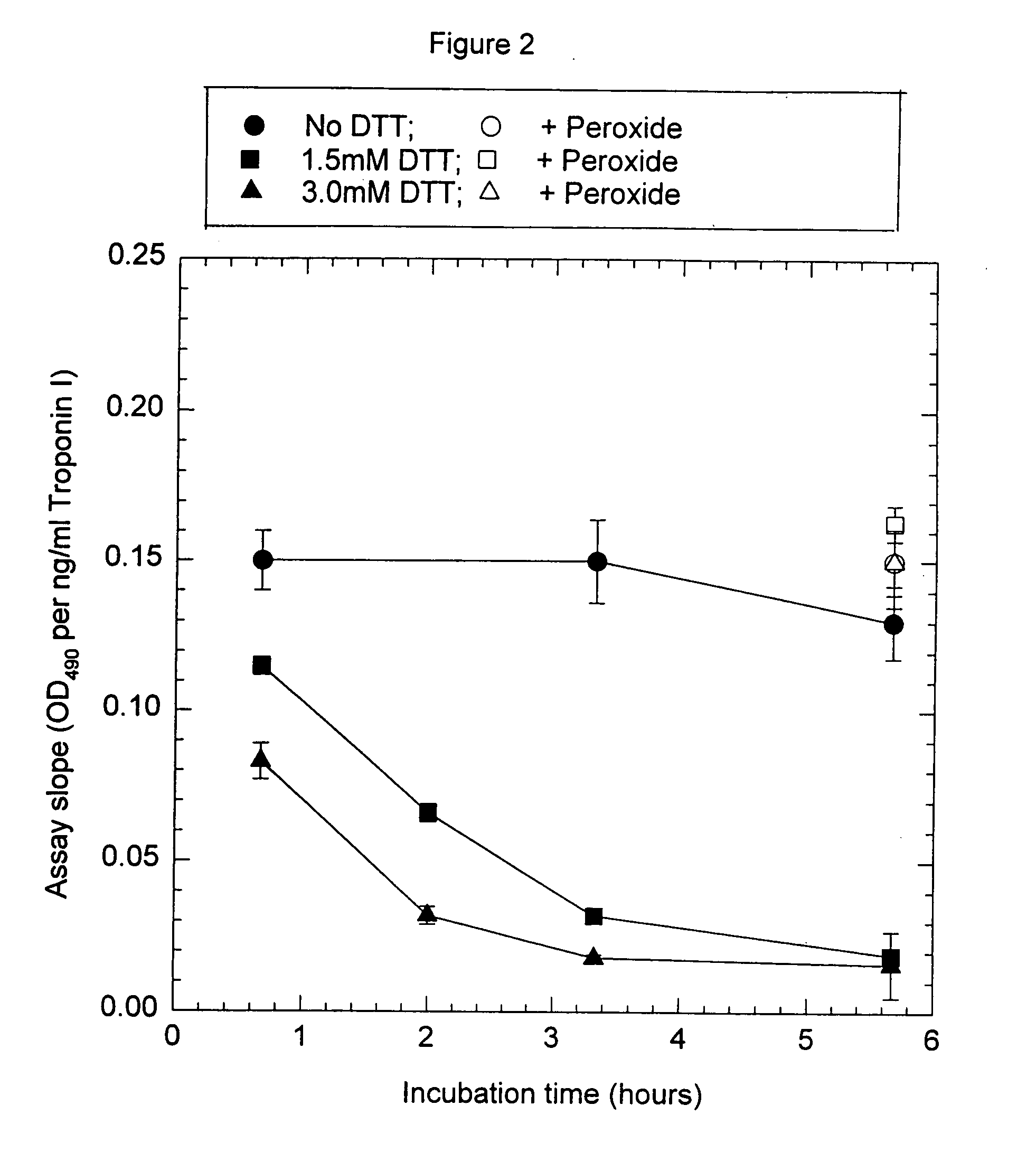
Assay systems and specialized antibodies for the detection and quantitation of troponin I and troponin T in body fluids as an indicator of myocardial infarction. Since troponin I and T exist in various conformations in the blood, the ratios of the monomeric troponin I an T and the binary and ternary complexes, as well as which form of troponin present in the blood, may be related to the metabolic state of the heart. Disclosed is a system to determine the presence of a troponin form or a group of troponin forms in a sample of whole blood, serum or plasma.Disclosed is a stabilized composition of troponin; the stabilized composition can comprise a stabilized composition of troponin I, wherein the troponin I is oxidized, the troponin I can be unbound or the troponin I can be in a complex.Disclosed is a method for improving the recovery of troponin I or T from a surface used in immunoassays.Also disclosed are antibodies which recognize, unbound troponin forms, the forms of troponin in binary complexes, the ternary complex of troponin I, T and C, and the conformations of troponin I having intramolecularly oxidized and reduced cysteines.
Owner:BIOSITE INC
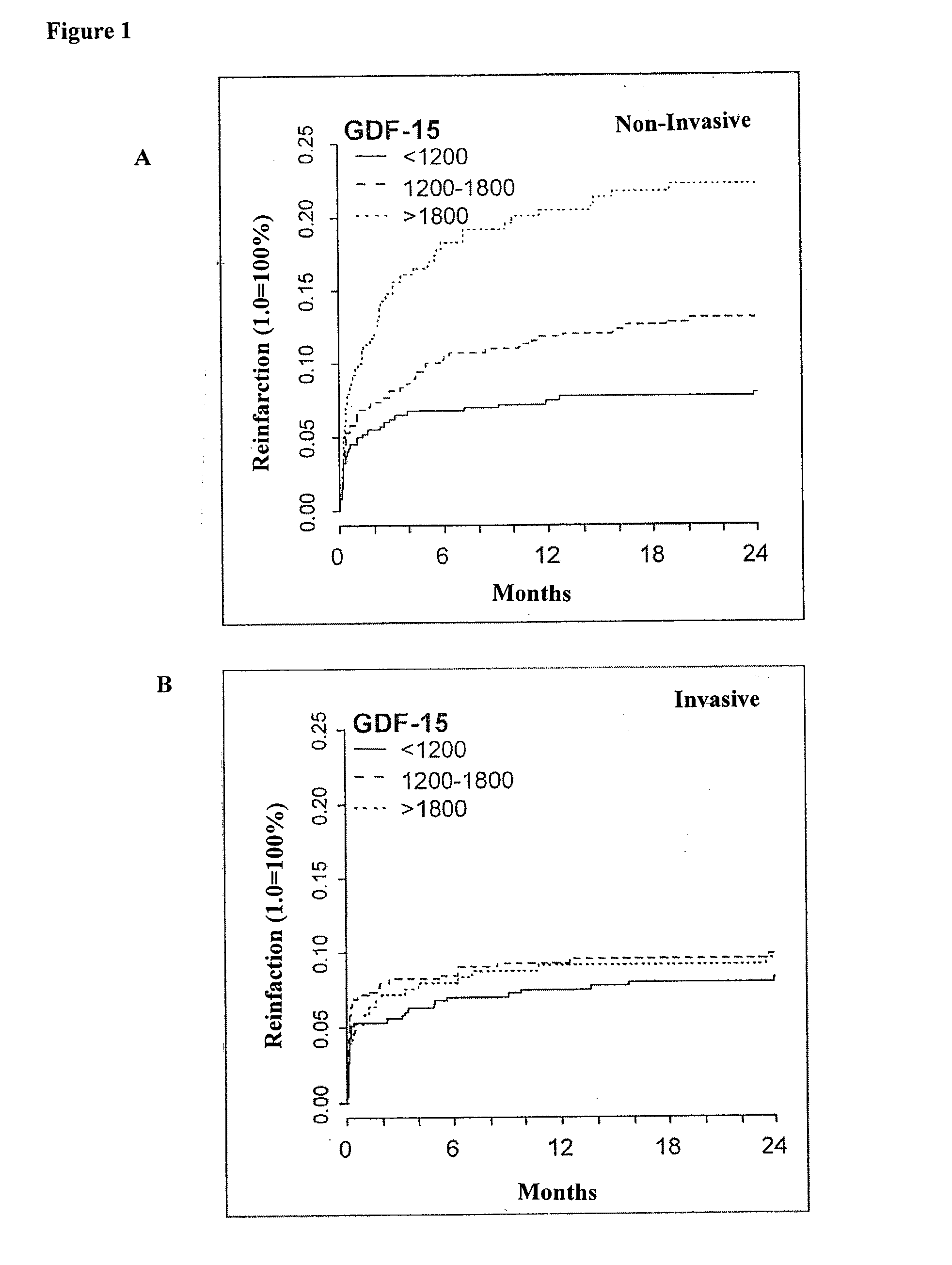
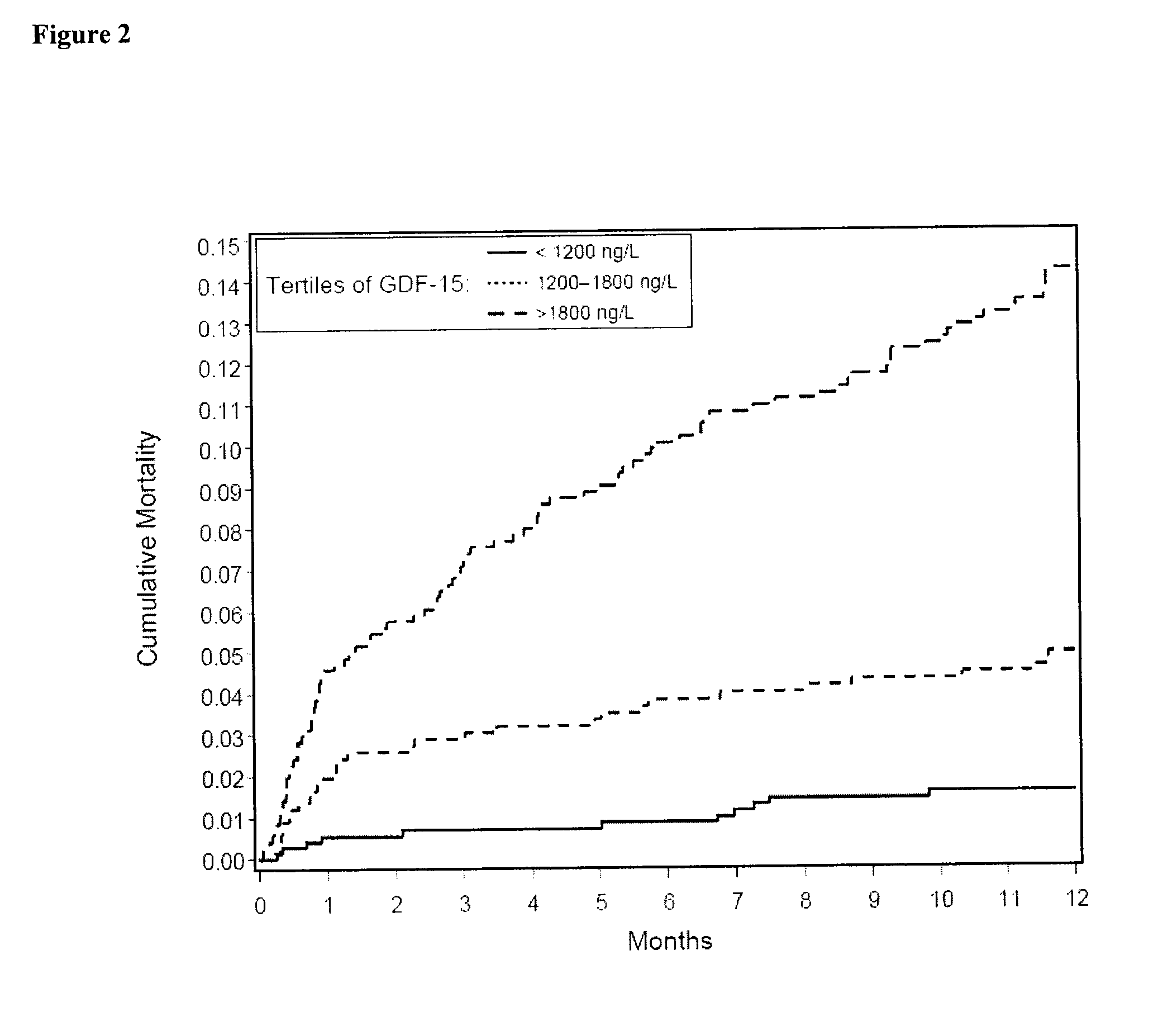
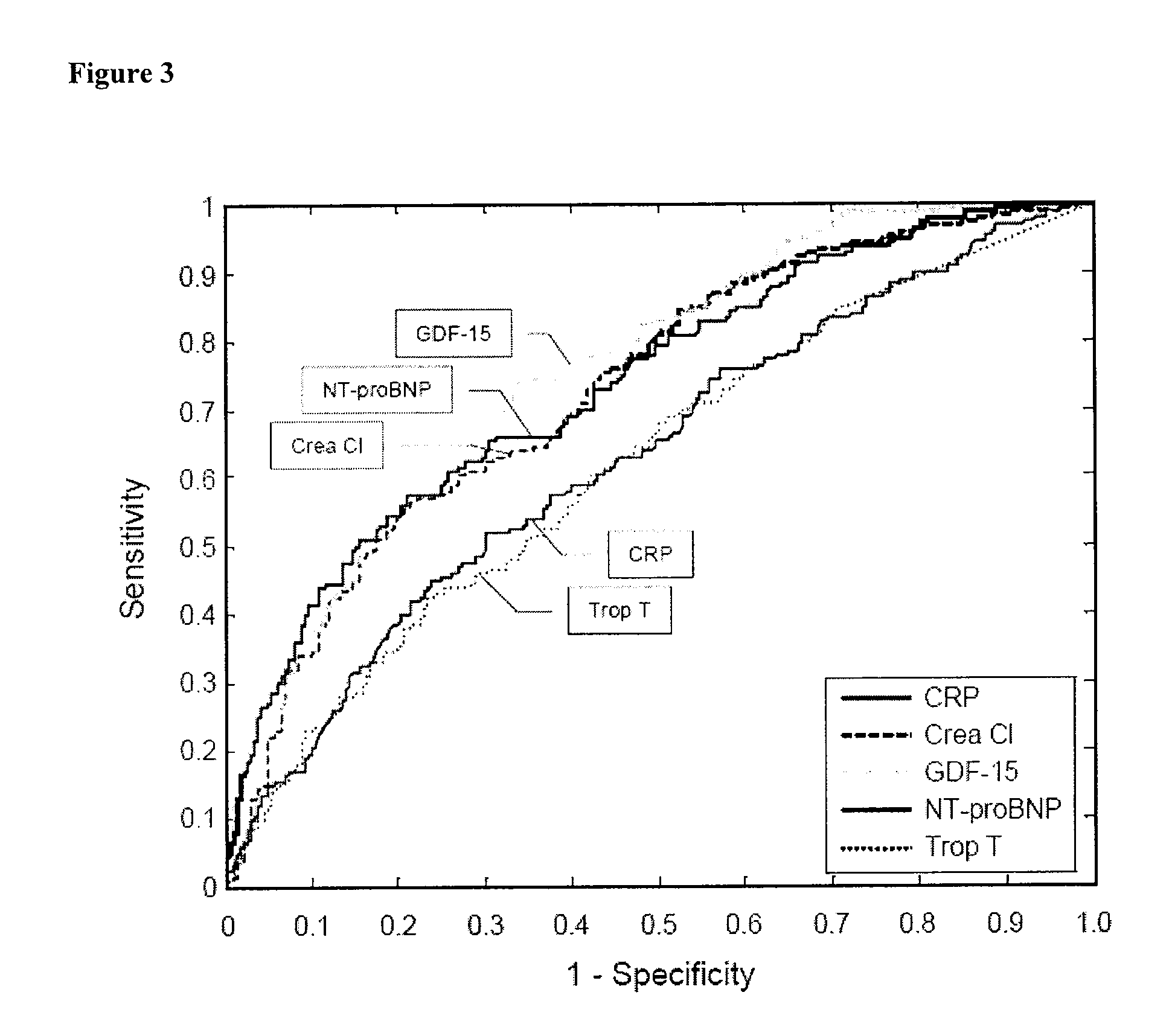
Assessing risk of cardiac intervention based on gdf-15
ActiveUS20110065204A1Analysis using chemical indicatorsComponent separationRisk of mortalityNatriuretic peptide
The present invention relates to a method of identifying a subject being susceptible to a cardiac intervention based on the determination of GDF-15 in a sample of a subject in need of a cardiac intervention. Moreover, the present invention pertains to a method for predicting the risk of mortality or a further acute cardiovascular event for a subject suffering from a cardiovascular complication based on the determination of GDF-15 and a natriuretic peptide and / or a cardiac troponin in a sample the said subject. Also encompassed by the present invention are devices and kits for carrying out the aforementioned methods.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
Highly Sensitive System and Methods for Analysis of Troponin
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
Measuring troponin antibodies to assess cardiovascular risk
This invention relates to the field of myocardial disorders. It discloses that antibodies to a cardiac troponin found in a sample obtained from an individual can be used as a diagnostic marker, especially in the assessment of an individual's risk of developing a myocardial disorder. A method aiding in the assessment of an individual's risk of developing a myocardial disorder, comprising measuring in vitro antibodies to a cardiac troponin and optionally one or more other marker useful in assessing an individual's risk of developing a myocardial disorder, and correlating the value or the values obtained to the individual's risk of developing a myocardial disorder is decribed.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Preparing method of photoelectrochemical sensor based on sandwich cardiac troponin T marked by Ag2Se@CdSe and application
InactiveCN104849331AGood photoelectric conversion characteristicsImprove photoelectric signal responseBiological testingMaterial electrochemical variablesCardiac muscleEngineering
The invention relates to a preparing method of a photoelectrochemical sensor based on sandwich cardiac troponin T marked by Ag2Se@CdSe and application, and belongs to the technical field of novel functional materials and biosensing detection. According to concrete contents of the preparing method and the application, CeO2-TiO2 composite nanometer materials with photoelectrocatalytic activity are used as optical activity substrate materials; Ag2Se@CdSe is used as an antibody marker; the sandwich photoelectrochemical sensor is prepared through the signal amplification effect of the marker Ag2Se@CdSe on the substrate materials, and is used for the high-sensitivity detection of the cardiac troponin T of myocardial damage specific marker. The method has the important significance on the early diagnosis and treatment of acute myocardial infarction.
Owner:UNIV OF JINAN
Highly sensitive system and methods for analysis of troponin
InactiveUS20100255518A1Analysis using chemical indicatorsMicrobiological testing/measurementMedicineTroponin T
The invention provides methods, compositions, kits, and systems for the sensitive detection of cardiac troponin, Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of cardiac troponin.
Owner:RGT UNIV OF CALIFORNIA +1
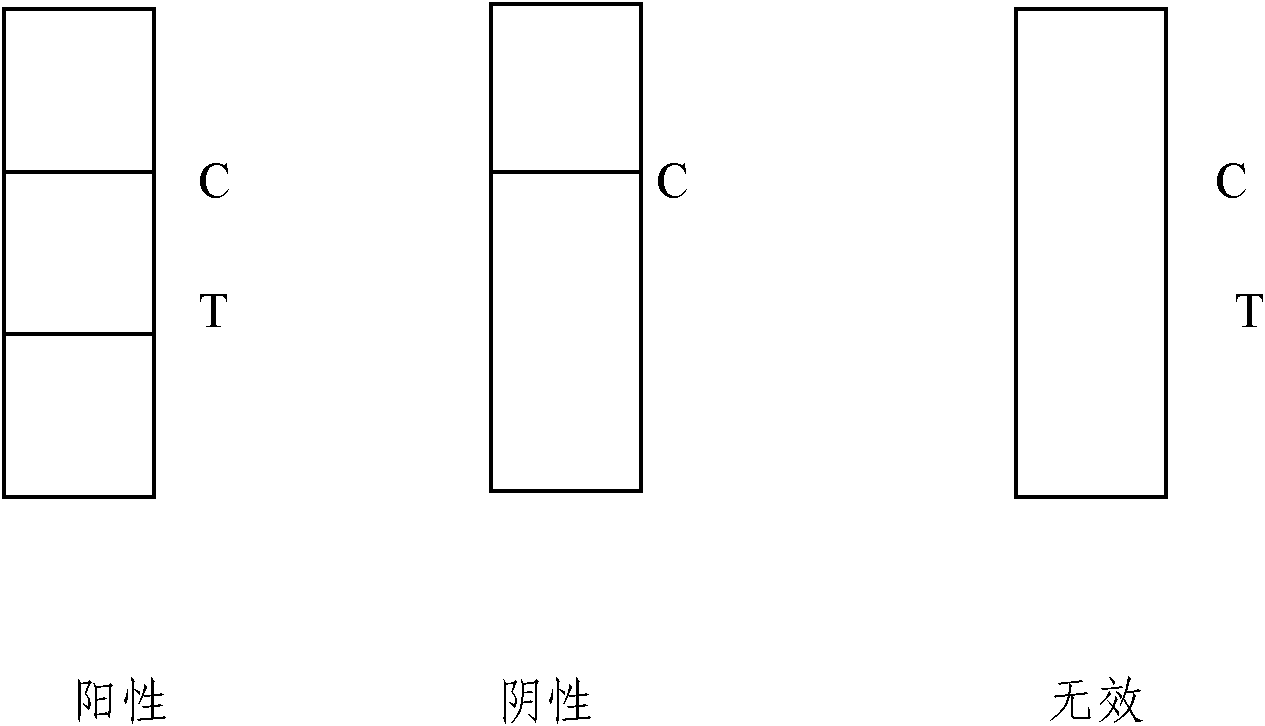
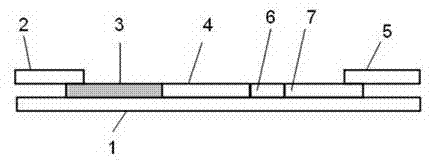
Fluorescent immune chromatographic test strip for quantitively detecting troponin I and preparation method thereof
InactiveCN102841207AHigh sensitivity detectionShorten detection timeBiological testingEpitopeClinical immunology
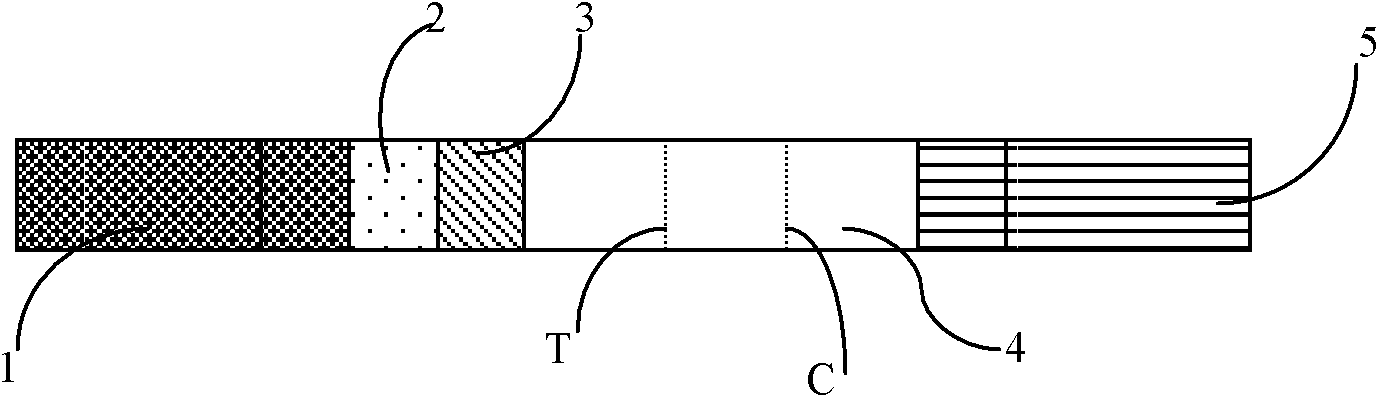
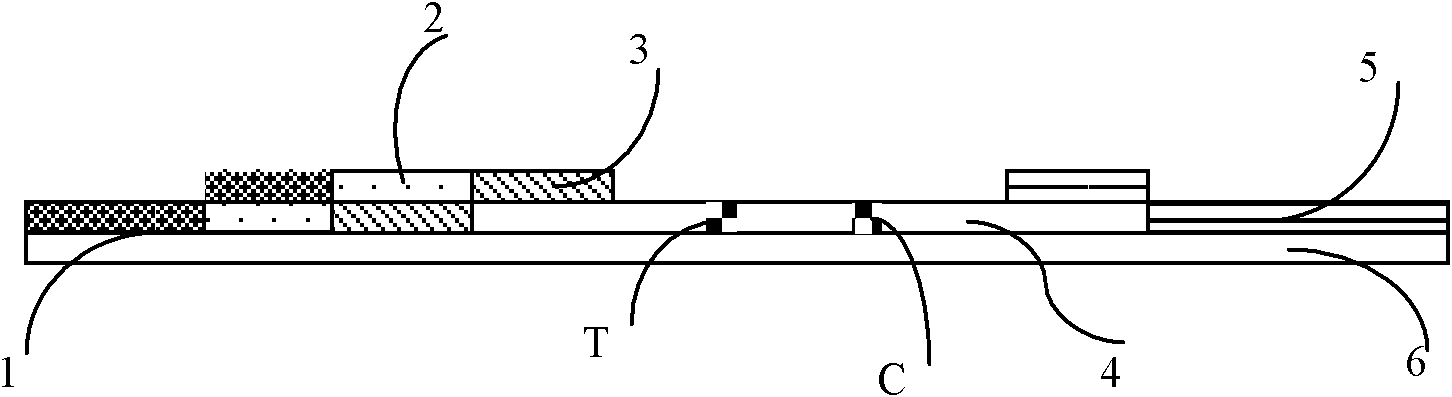
The invention relates to the field of detection in clinical immunology, in particular to a fluorescent immune chromatographic test strip for quantitively detecting troponin I and a preparation method thereof. The test strip provided by the invention comprises a bottom plate, sample absorbing pads attached to the bottom plate and staggered to each other sequentially, a labeled antibody pad, a coating film and a water absorbing pad. The labeled antibody pad is a troponin monoclonal antibody pad I coated with a fluorescent micro-spherical label and a troponin monoclonal antibody pad II coated with a biotin label for identifying different epitopes. The coating film is provided with a detection strip and a quality control strip. Avidin is fixedly arranged on the detection strip. A rabbit antimouse IgG antibody is fixedly arranged on a quality control line. By improving the test strip, a biotin-avidin amplifying system and a fluorescent immune chromatographic technology are introduced into detection of troponin I. Combined with a fluorescent detector, individual quantitive detection of troponin I is realized, therefore, extreme convenience is provided for clinical use.
Owner:北京乐普诊断科技股份有限公司
Turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement and use method thereof
InactiveCN103185798ANo special training requiredNo need to waitMaterial analysis by observing effect on chemical indicatorBiological testingDisease courseBiomarker (petroleum)
The invention provides a turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement. The turbidimetric rapid detection kit comprises a reaction-detection integrated device, and a detection kit arranged in the reaction device, wherein a reagent R1, a reagent R2 and a calibrator are contained in the detection kit; simultaneously, quantitative detection for five important myocardial infarction-related biomarkers in one sample can be realized, and the five important biomarkers include content of troponin-I, content of D-dimer, content of myoglobin, content of hypersensitive C-reactive protein (hs-CRP) and content of heart-type fatty acid binding protein (FABP); and the turbidimetric rapid detection kit has an important clinical significance in the aspects of early diagnosis and disease course monitoring for myocardial infarction, treatment monitoring for medicines, and the like. The turbidimetric rapid detection kit provided by the invention realizes the integration of the reagents and the reaction device, and is simple, rapid and accurate to operate, high in sensitivity, strong in specificity, and low in detection cost; and the turbidimetric rapid detection kit is a detection / reagent measurement integrated kit suitable for outpatient and emergency treatment / clinical detection, and wide in instrument application range.
Owner:SUZHOU DIAGVITA BIOTECH
Immune chromatography fluorescence reagent strip for detecting cardiac troponin and preparation method thereof
InactiveCN104237537AShorten detection timeImprove featuresBiological testingReagent stripMonoclonal antibody
The invention discloses an immune chromatography fluorescence reagent strip for detecting cardiac troponin and a preparation method thereof, belonging to the field of clinical medicine detection. The reagent strip comprises a baseboard, a sample mat, a tracer particle combining mat, a nitrocellulose membrane and a water absorption mat; the sample mat, the tracer particle combining mat, the nitrocellulose membrane and the water absorption mat are orderly connected and fixed on the baseboard in the horizontal direction. The tracer particle combining mat is provided with the cTnI monoclonal antibody-fluorescence particle compound adsorbed layer covered on the surface of the nanocrystalline metal particle; the nitrocellulose membrane is provided with the detection line composed of the cTnI monoclonal antibody or polyclonal antibody capture molecule and the quality control line composed of the rabbit antimouse IgG antibody. The immune chromatography fluorescence reagent strip for detecting cardiac troponin is short in detection time, high in specificity and sensitivity and more accurate in detection result by improving the fluorescence intensity through the nanocrystalline metal particle.
Owner:GETEIN BIOTECH
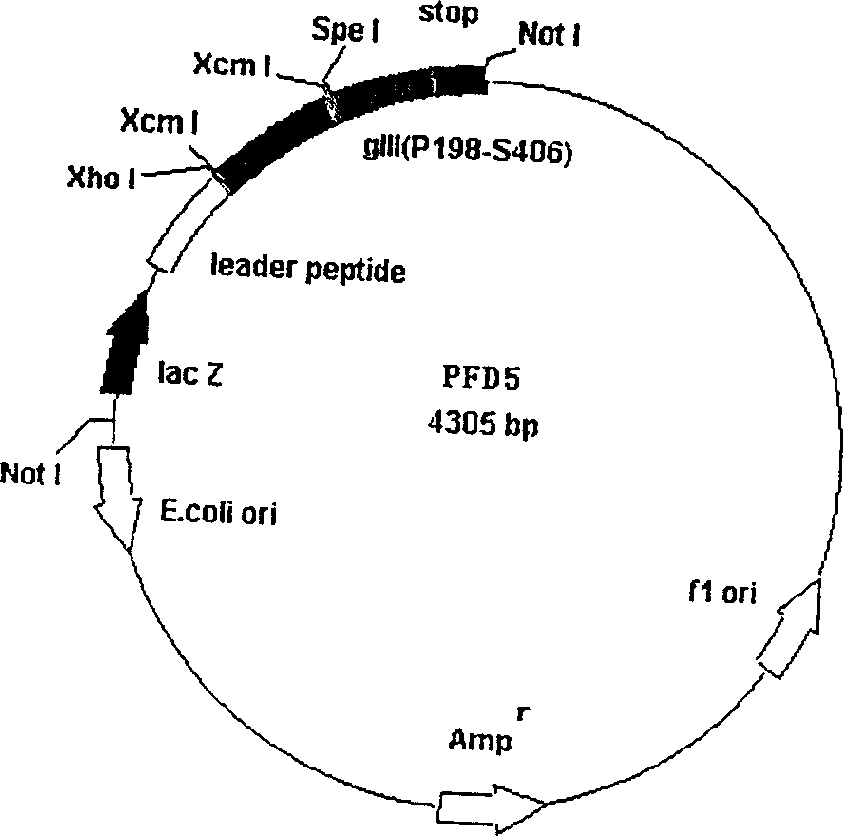
Exhibiting method of preparing antibody by antigen utilizing bacteriophage exhibiting technique
InactiveCN1485432AImproving immunogenicityHigh titerImmunoglobulins against animals/humansOther foreign material introduction processesPolyclonal antibodiesTroponin
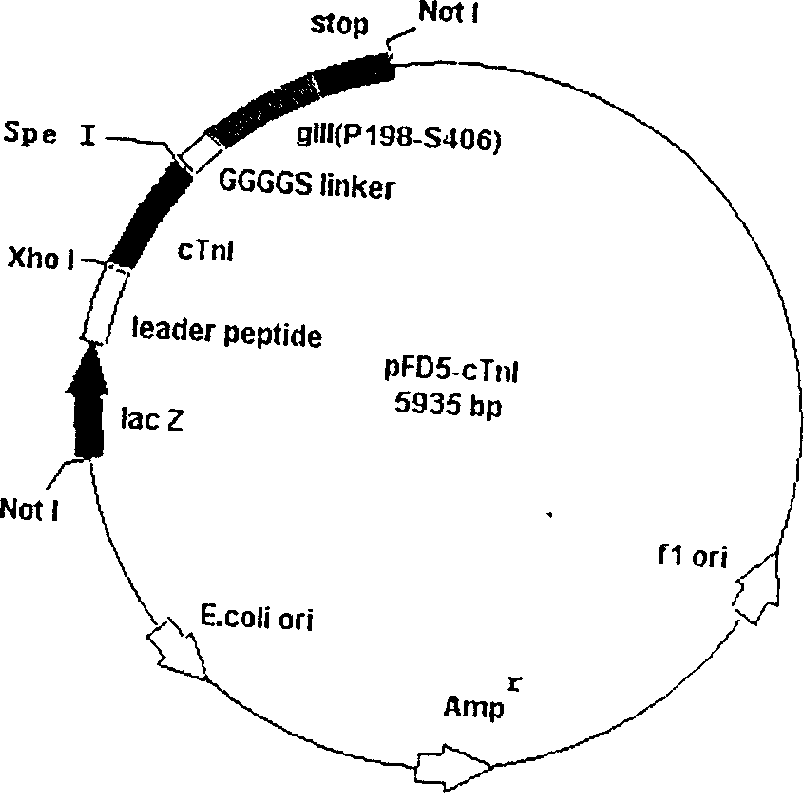
The invention comprises, fusing exogenous antigen genes and filobactivirus III gene proteins and expressed onto the tail of filobactivirus, fusing antigen epitopes and filobactivirus III gene proteins and expressed onto the surface of filobactivirus, preparing antibodies taking filobactivirus with exogenous antigen and antigen epitopes as the immunogen. The invention claims:1)a method of preparing monoantibodies and polyantibodies based on filobactivirus showing technology;2) a method of preparing cTnI monoantibody and polyantibody based on filobactivirus showing technology showing antigen and antigen epitopes of human troponin; 3) a PIII filobactivirus showing carrier pFD5; 4) a filobactivirus showing fusion expressing carrier pFD5-cTnI;5) a PIII filobactivirus showing fusion expressing carrier PC89-cTnI[95??08];6)taking filobactivirus particles showing cTnI and cTnI[95í½108] as immunogens, immunizing new zealand white rabbit to get cTnI antibody.
Owner:CHINA PHARM UNIV
Prognosis and risk assessment in stroke patients by determining the level of marker peptides
InactiveUS20110263821A1Increase probabilityChemiluminescene/bioluminescenceLibrary screeningCalcitoninCancer risk assessment
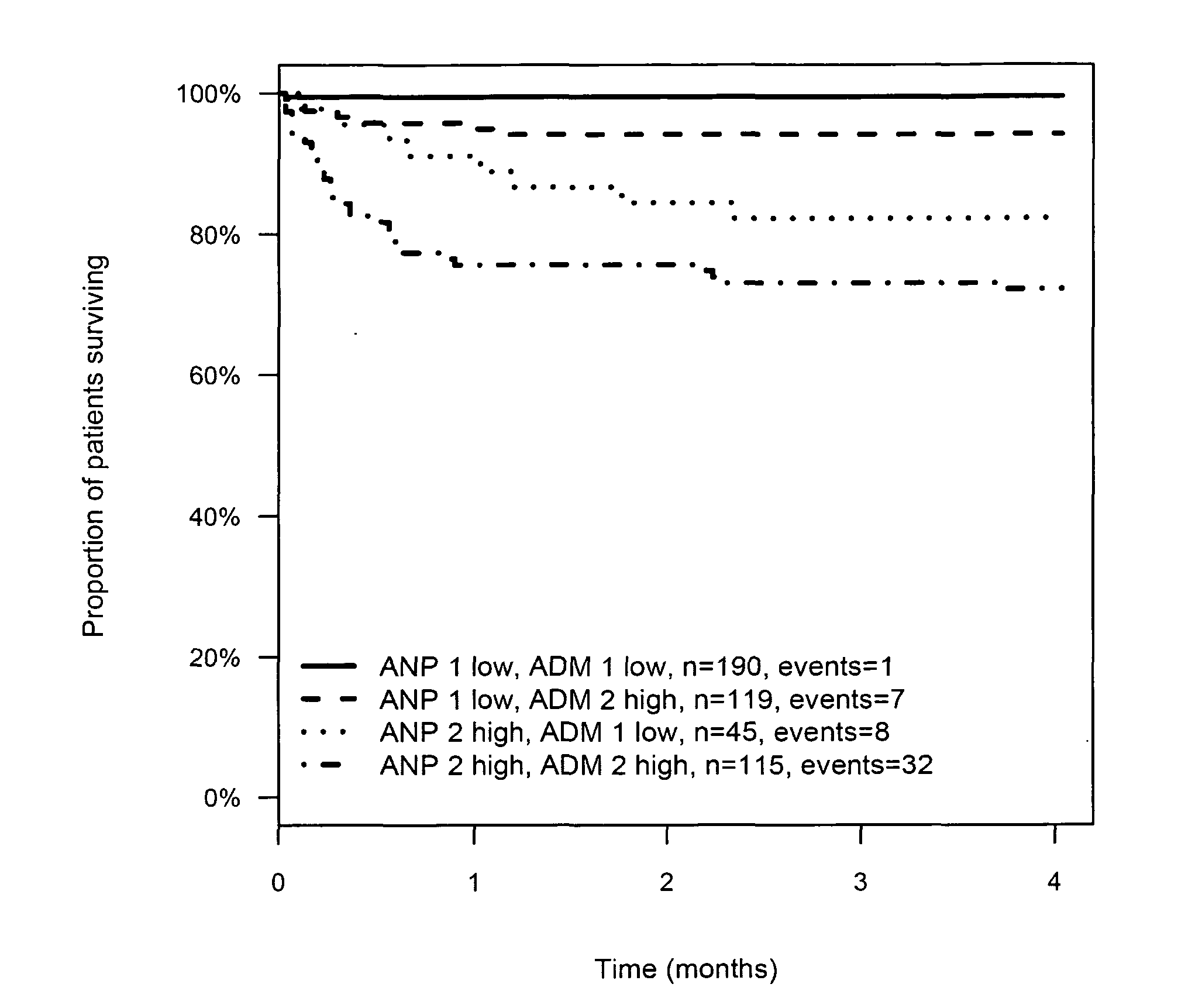
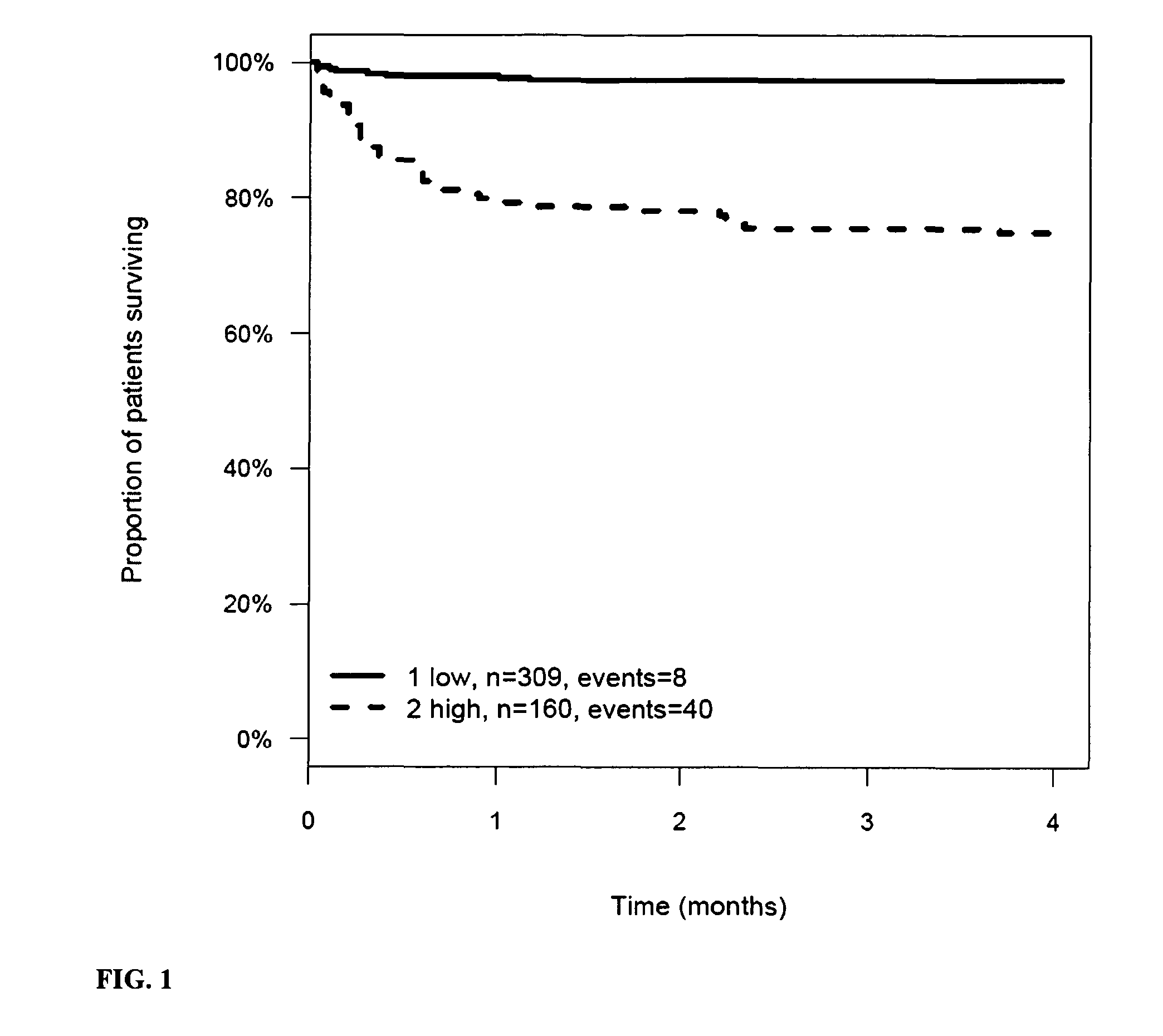
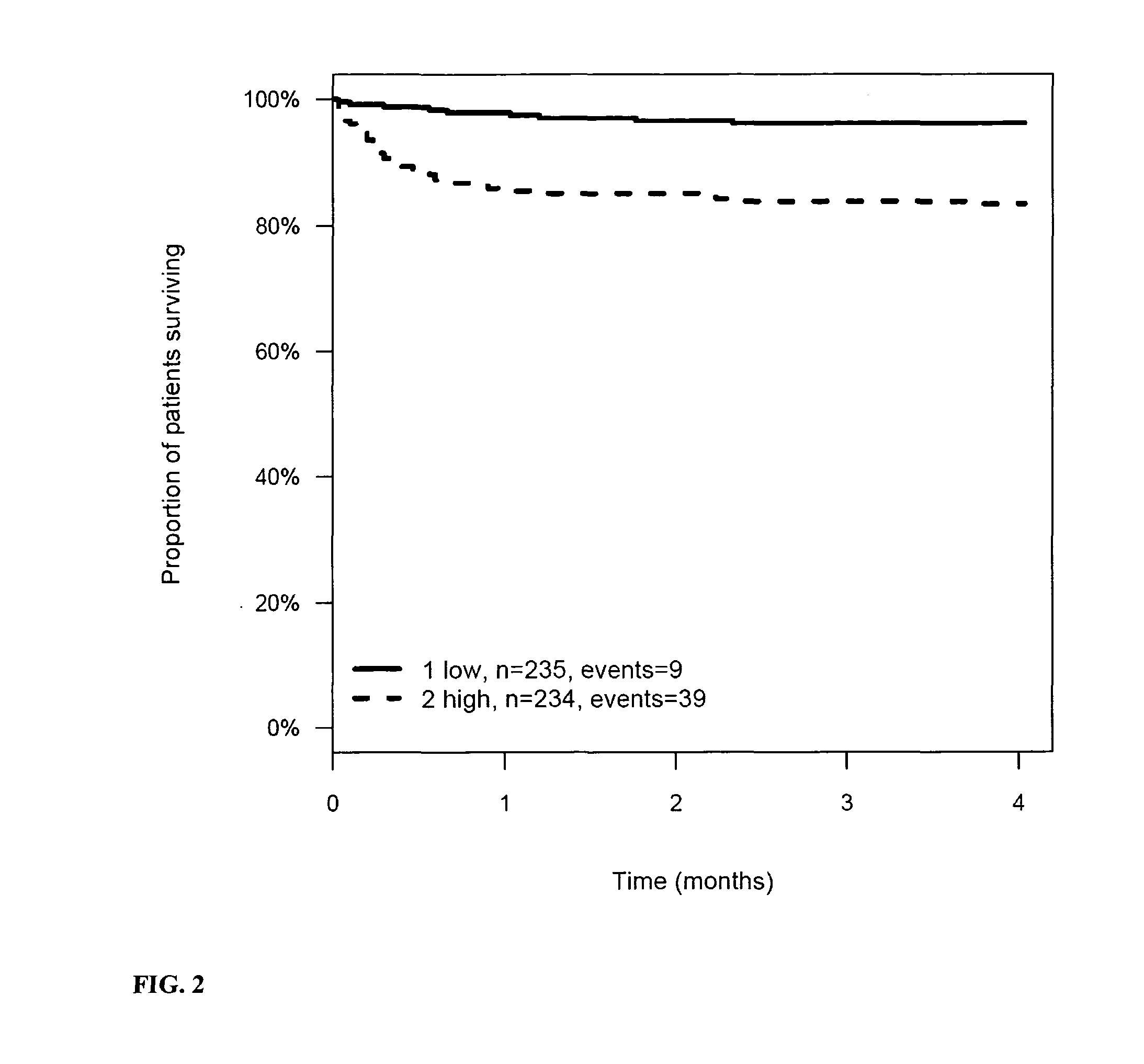
The present invention relates to a method for prognosis of an outcome or assessing the risk of a patient having suffered a stroke or a transient ischemic attack, comprising the determination of the level of at least one marker peptide in said sample said marker peptide selected from the group comprising ANP, AVP, ADM, ET-1, troponin, CRP, calcitonin and hGH or fragments thereof or its precursor or fragments thereof and attributing the level of said at least one marker peptides its precursor or fragments thereof with the prognosis of an outcome or assessing the risk for said patient.
Owner:BRAHMS GMBH
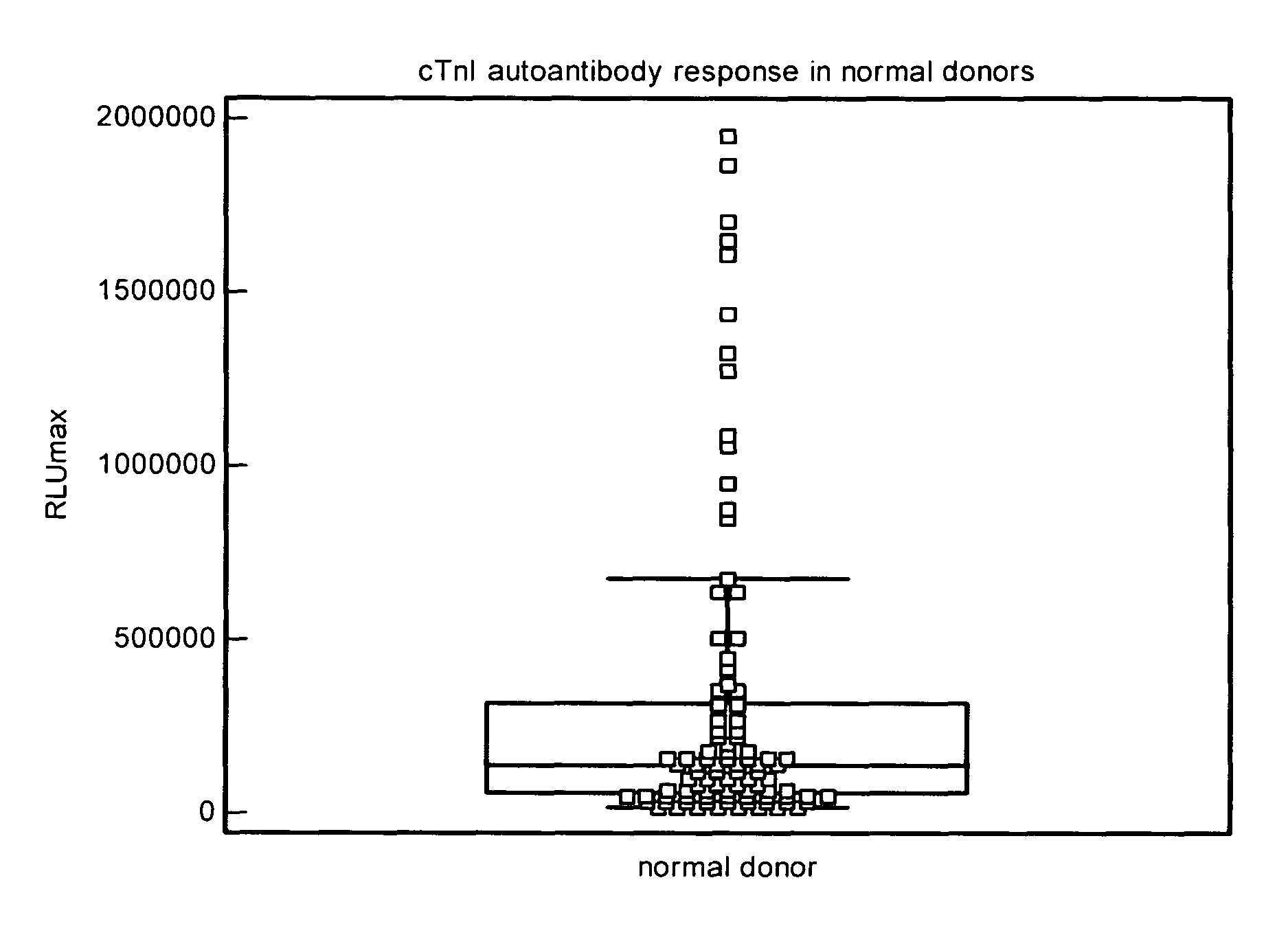
Assay for cardiac troponin autoantibodies
The invention provides among other things methods and kits based on assaying for cardiac troponin autoantibodies, either in conjunction with an assay for cardiac troponin and / or as an independent indicator of cardiac pathology, such as myocarditis, cardiomyopathy, and / or ischemic heart disease. Assay methods of the invention can be employed among other things to identify cardiac pathology, or risk thereof, in subjects who have an autoimmune disease or who are related to an individual with an autoimmune disease. In particular embodiments, the invention also provides a method of determining whether a subject having, or at risk for, a cardiac pathology is a candidate for immunosuppressive therapy or immunoabsorption therapy. The invention also provides kits and kit components that are useful for performing the methods of the invention.
Owner:ABBOTT LAB INC
Troponin-T determination kit
ActiveCN102841206AHigh detection sensitivityEasy to operateMaterial analysis by observing effect on chemical indicatorBiological testingMicrosphereFully automatic
The invention relates to a kit for determining troponin-T (TNT) content of serum, aims to overcome the deficiencies of the above background technology, and provides a kit for detecting troponin-T by an immunoturbidimetry method, wherein the kit does not require sample dilution, has simple operation, high accuracy and good repeatability, and is suitable for various types of fully automatic biochemical analyzers and special protein instruments. A technical scheme is as below: the kit comprises: A. a reagent R1 containing a buffer solution, a preservative, an accelerating agent, inorganic salts, a surfactant and the balance of purified water; b. a reagent R2 containing a buffer solution, latex microspheres with diameter of 60-150 nm combined with anti-human troponin antibodies and an antiseptic; and c. a reference calibrator containing a buffer solution, a stabilizer, a preservative, a certain amount of recombinant human protein troponin-T pure product determined by concentration requirements and the balance of purified water.
Owner:NANJING NORMAN BIOLOGICAL TECH
Method of determining time of death
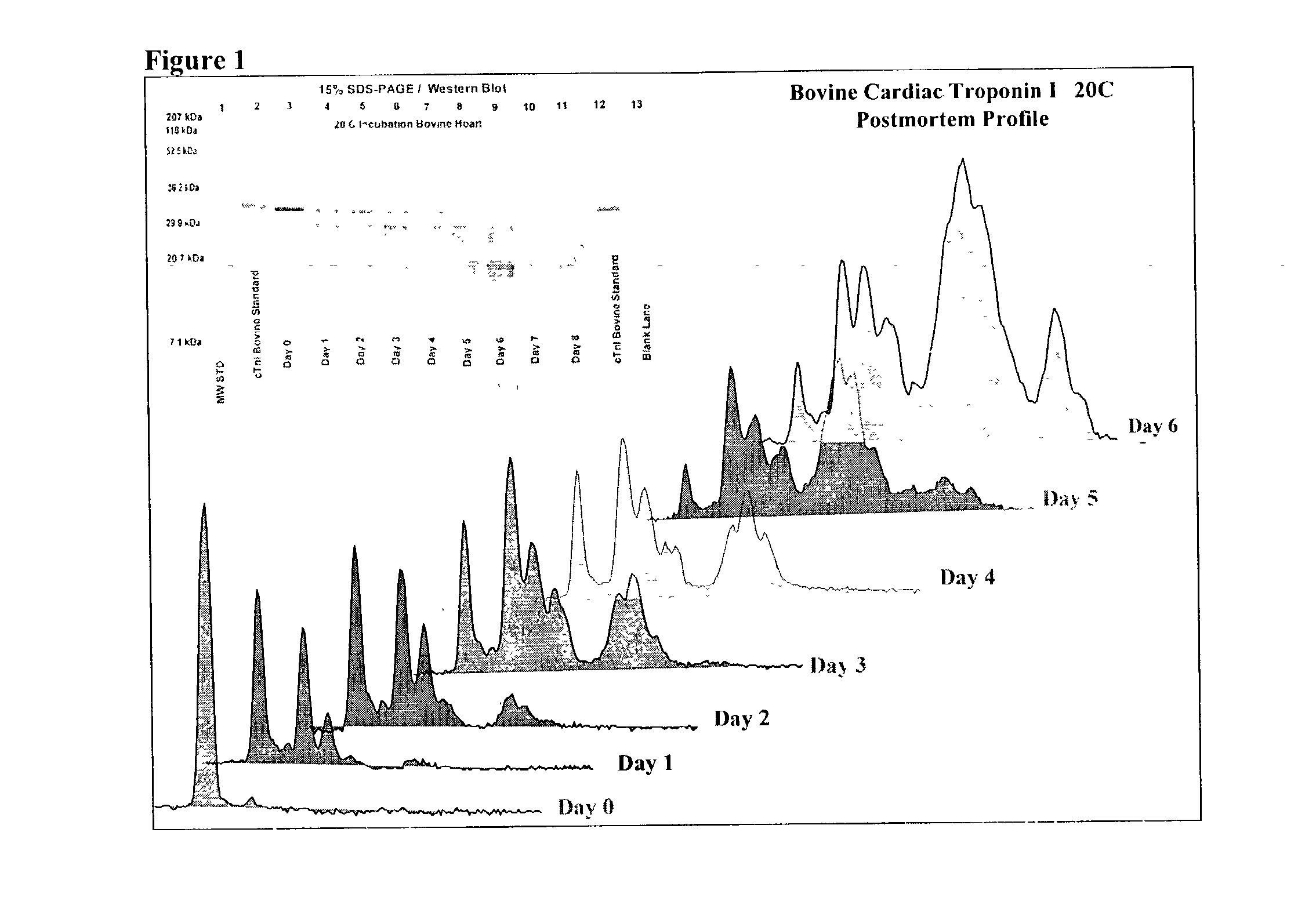
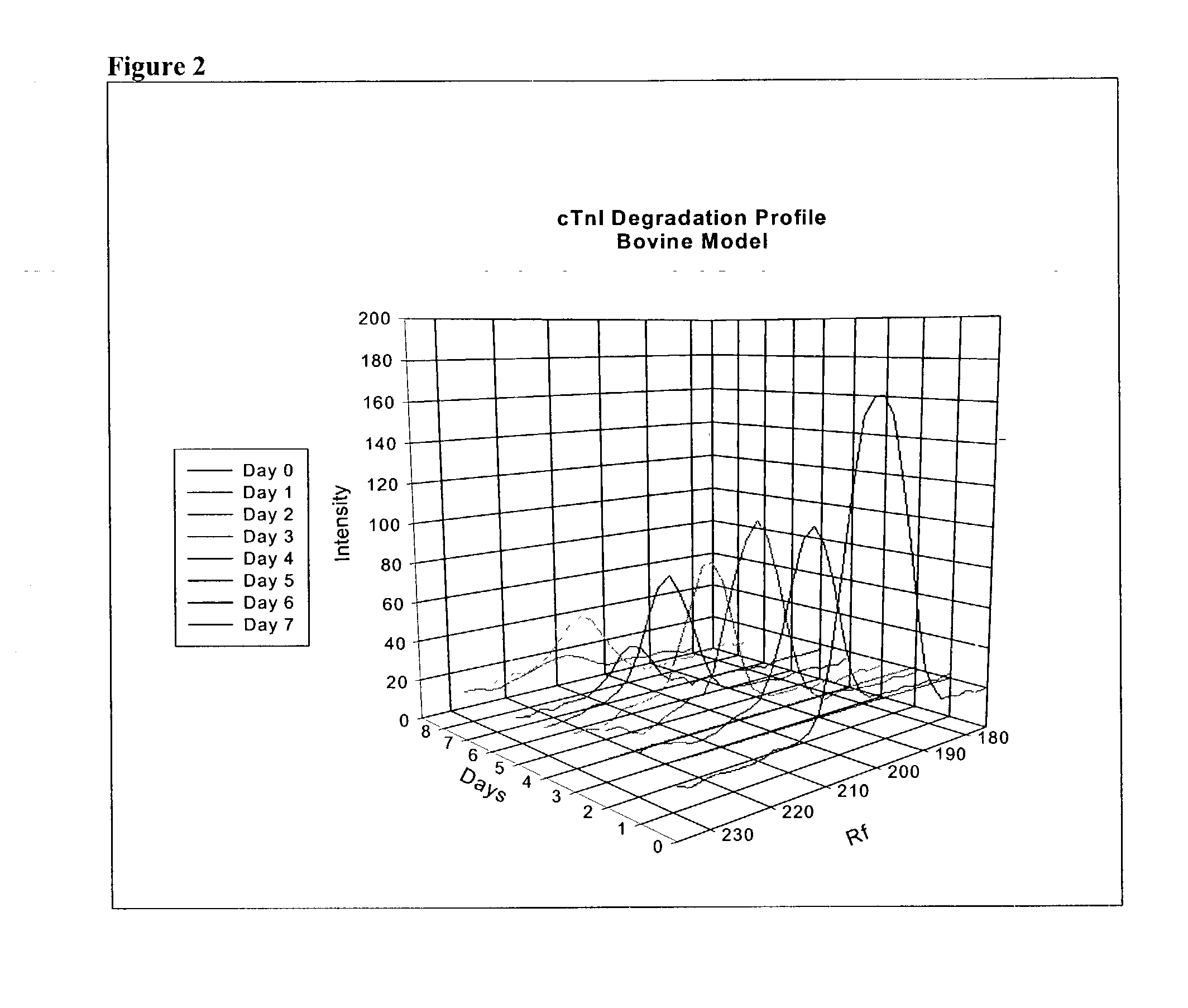
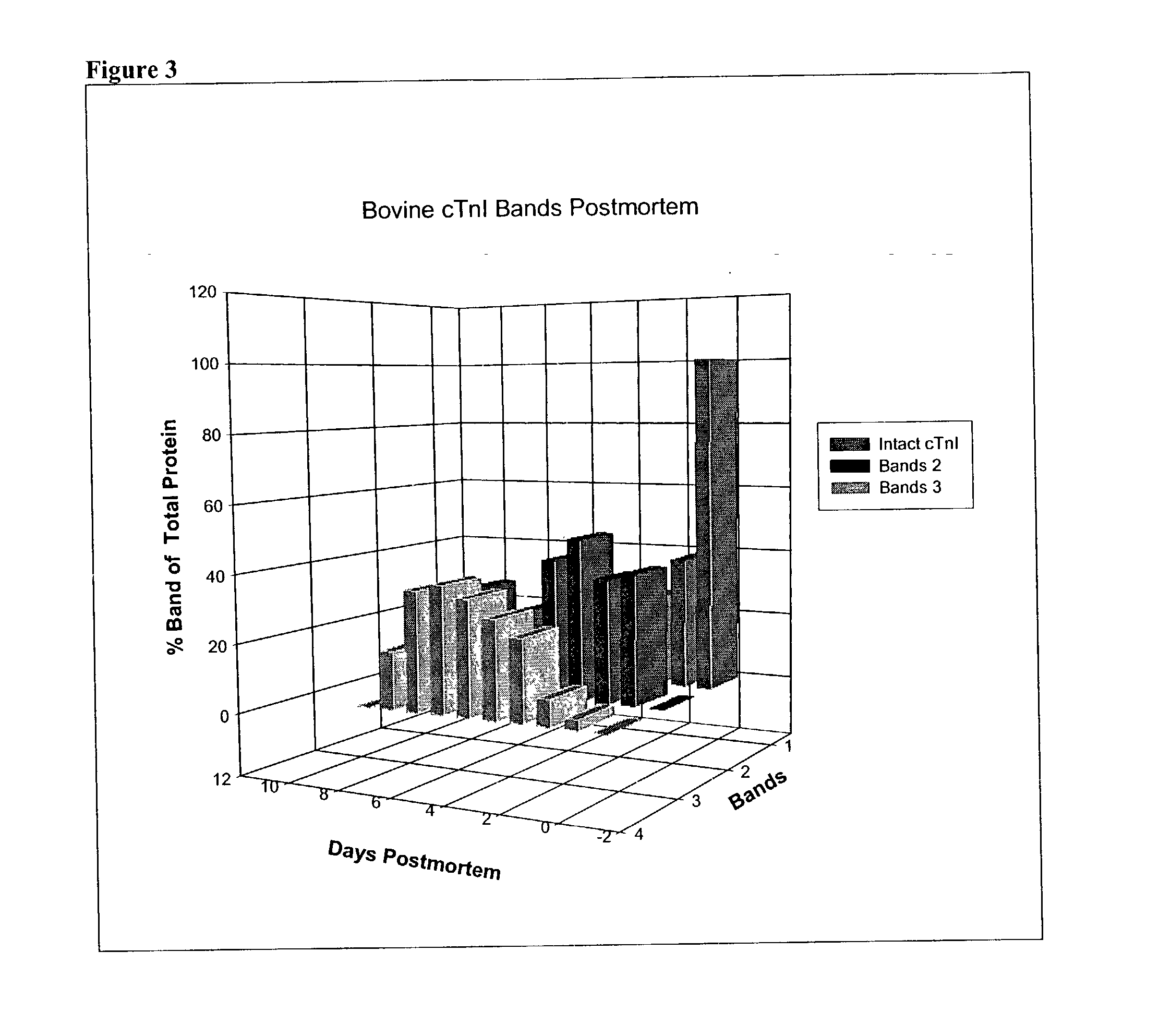
A method of determining time of death is disclosed. Cardiac troponin I (cTnI) is the most specific marker for the determination of myocardial infarction (MI). Cardiac troponin I has a distinctive temporal degradation profile after death, which is key to its use as a time of death marker in forensic medicine. The method consists of sampling cardiac tissue, extracting a cardiac protein from a homogenate via methods which may include the magnetic microparticles or magnetic microparticles linked to anti-cTnI antibodies, eluting the proteins, separating proteins by electrophoresis, transferring by western blot the different bands of proteins to paper. A comparison / analysis of the concentration and kinetics of degradation of the protein at a given temperature(s) against known standards after time of death provides an accurate measurement of the time of death. The present invention is more accurate and reliable than the rudimentary time of death techniques currently used by medical examiners.
Owner:SABUCEDO ALBERT J +1
Assay for cardiac troponin autoantibodies
The invention provides among other things methods and kits based on assaying for cardiac troponin autoantibodies, either in conjunction with an assay for cardiac troponin and / or as an independent indicator of cardiac pathology, such as myocarditis, cardiomyopathy, and / or ischemic heart disease. Assay methods of the invention can be employed among other things to identify cardiac pathology, or risk thereof, in subjects who have an autoimmune disease or who are related to an individual with an autoimmune disease. In particular embodiments, the invention also provides a method of determining whether a subject having, or at risk for, a cardiac pathology is a candidate for immunosuppressive therapy or immunoabsorption therapy. The invention also provides kits and kit components that are useful for performing the methods of the invention.
Owner:ABBOTT LAB INC
Method for the prediction of vascular events and the diagnosis of acute coronary syndrome
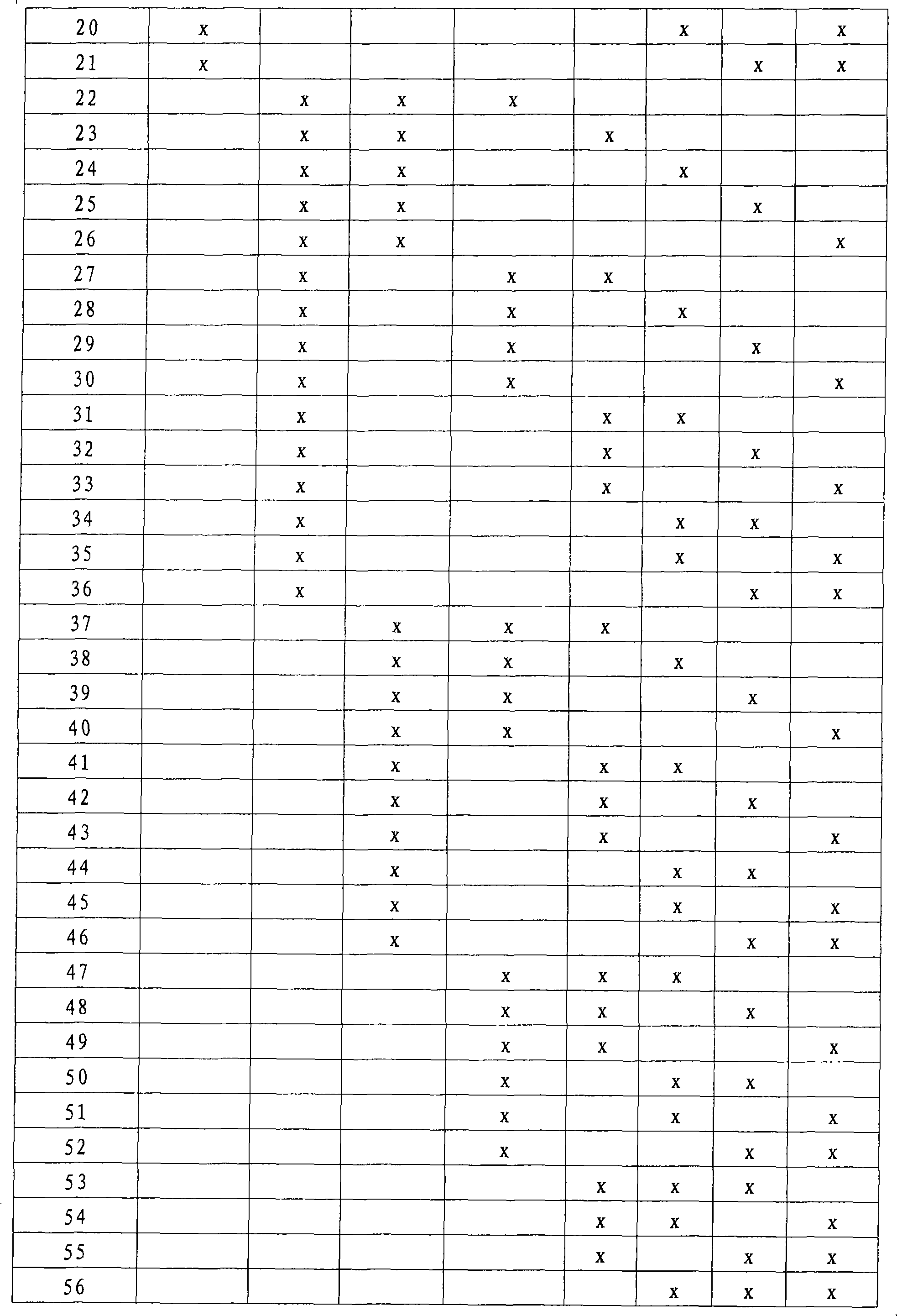
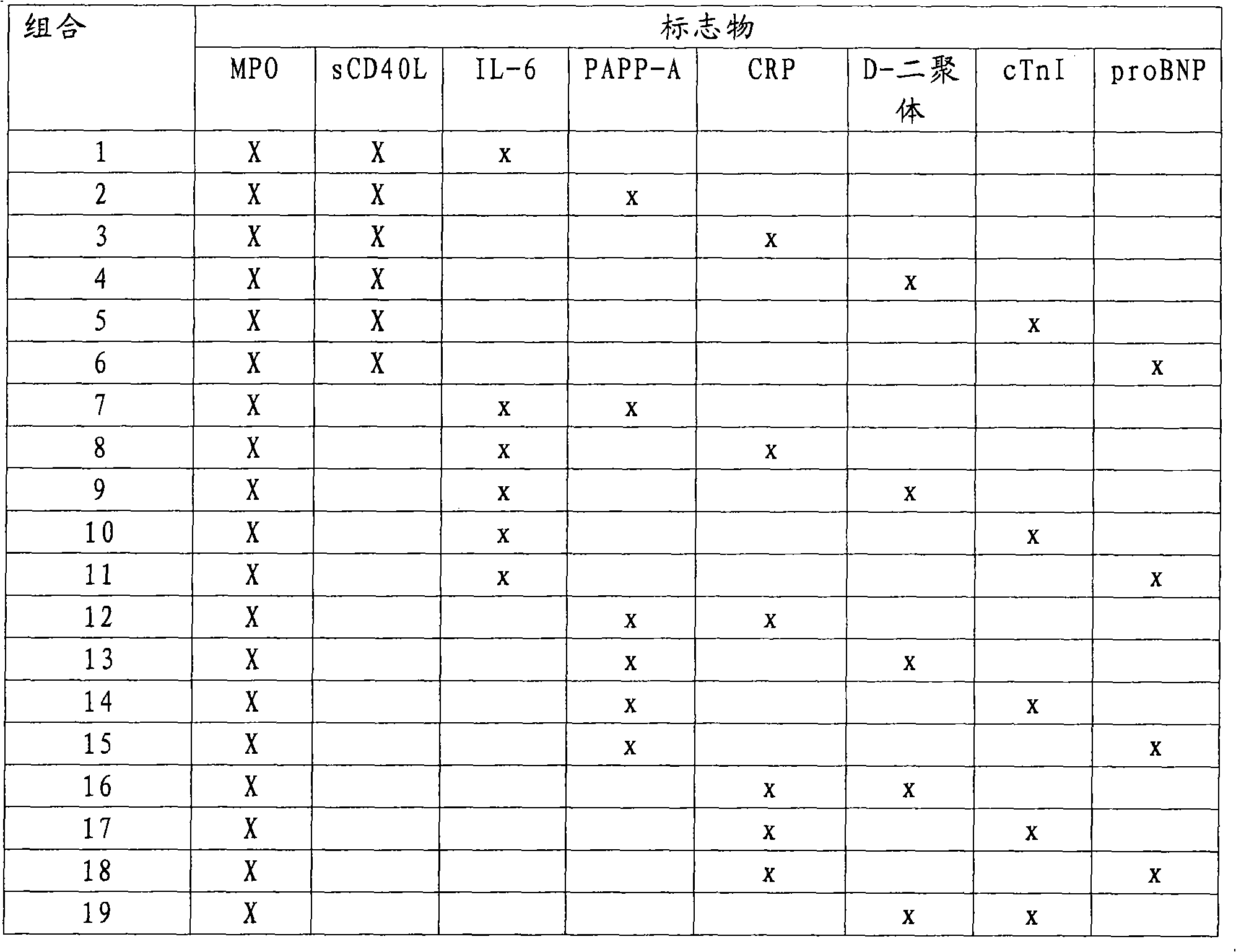
The present invention relates to a method for the prognosis of a vascular event in a patient suspected of being at risk for a vascular event, said patient presenting: - no elevation of the ST segment as seen on an electrocardiogram, and / or - a normal level of at least one myocardial necrosis marker, wherein the presence and / or levels of at least two different biochemical markers are measured in a biological sample of said patient, whereby the probability that the patient will experience a vascular event is deduced from the measured presence and / or levels of the biochemical markers such as MPO, sCD40L, IL-6, PaPP-A, CRP, D-dimer, troponin, and proBNP.
Owner:CENT NAT DE LA RECHERCHE SCI
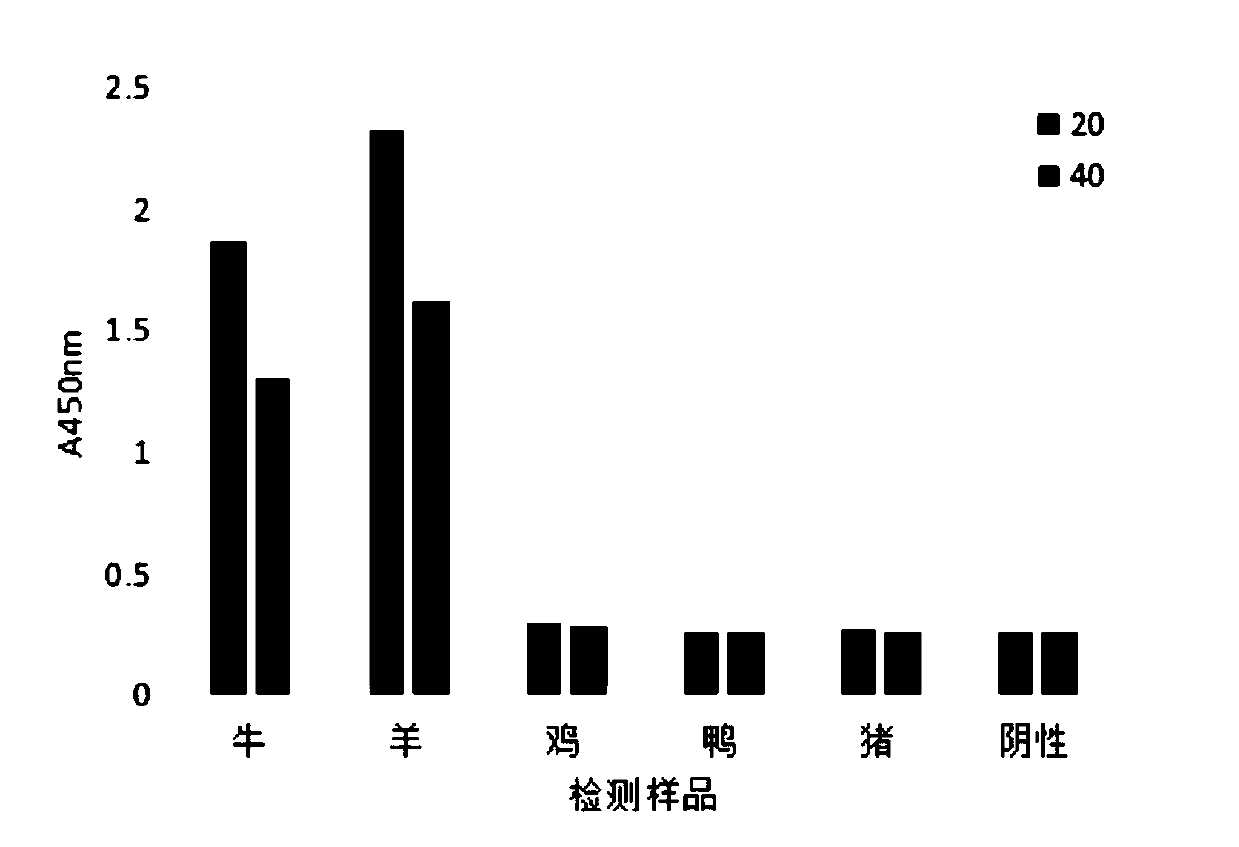
Enzyme-linked immunosorbent assay kit for detecting skeletal muscle troponin I of cattle or sheep, and preparation method thereof and application thereof
The invention, which belongs to the technical fields of immunology and food safety analysis, relates to an enzyme-linked immunosorbent assay kit for detecting skeletal muscle troponin I of cattle or sheep, and a preparation method thereof and application thereof. The kit comprises an enzyme label plate coated with a capture antibody, a horseradish-peroxidase-labeled detection antibody, a skeletalmuscle troponin I standard solution of cattle or sheep, a substrate coloured solution, a stop solution, and a concentrated washing solution. The capture antibody is obtained by secretion of a hybridoma cell line 3A8 with the preservation number of CCTCC NO:C2018217; and the detection antibody is obtained by secretion of a hybridoma cell line 3D3 with the preservation number of CCTCC NO:C2018218. The kit has advantages of high sensitivity, high precision, high accuracy, and low cross-reaction rate and is suitable for large-scale sample detection.
Owner:BIOLOGY INST OF HEBEI ACAD OF SCI
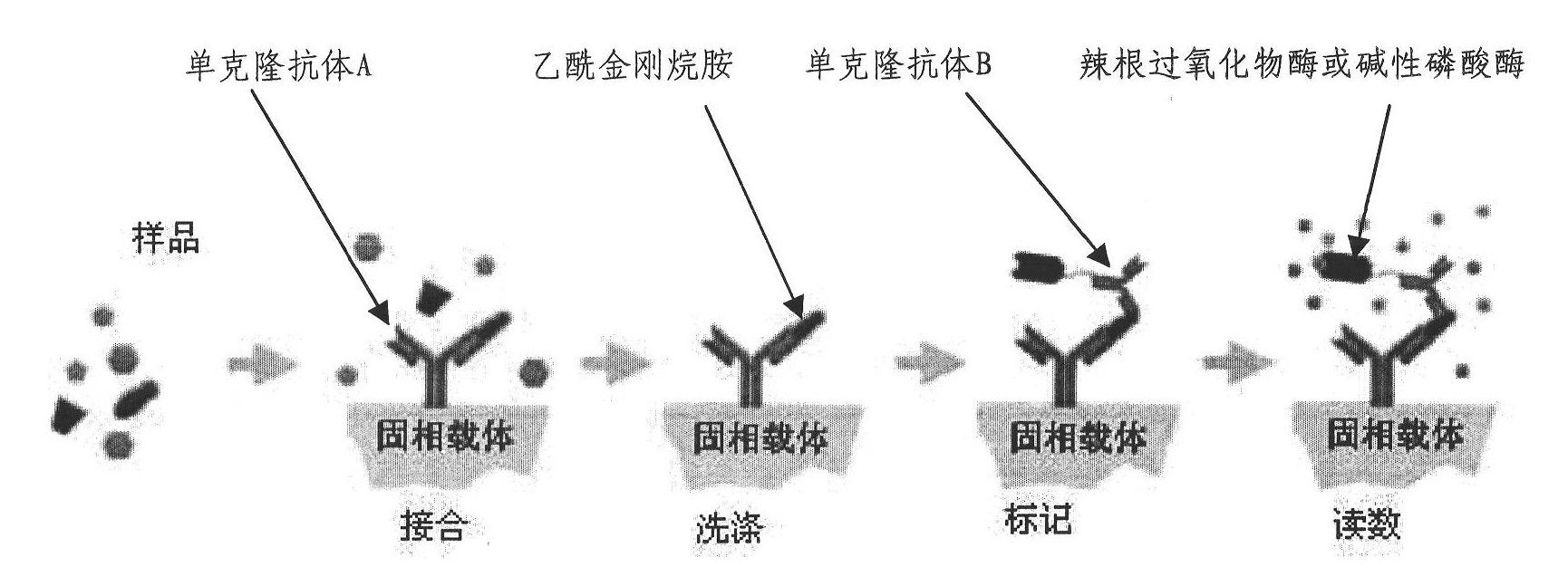
Detection kit of acetyl amantadine for predicting tumors
InactiveCN102043058ALow costEffectively eliminate false positive judgmentsMaterial analysisDiseaseMonoclonal antibody
The invention provides a detection kit of acetyl amantadine for predicting tumors. The kit comprises a carrier coated with an acetyl amantadine-resisting monoclonal antibody A and an acetyl amantadine-resisting monoclonal antibody B with a marker. The kit detects the acetyl amantadine in a sample on the basis of an immunological principle. The kit is used for detecting the acetyl amantadine for predicting the tumors, has the advantages of simplicity, fastness, high sensitivity and low cost and is easy to popularize and use on a large scale. In addition, the detection kit jointly applies an immunological method for detecting the acetyl amantadine and a biochemical method for detecting C-reactive protein (CRP) and troponin so as to eliminate false positive interference to tumor prediction due to diseases such as internal inflammation and myocardial ischemia and make the specificity of a method for early predicting cancers by detecting the acetyl amantadine higher.
Owner:BIOSINO BIO TECH & SCI
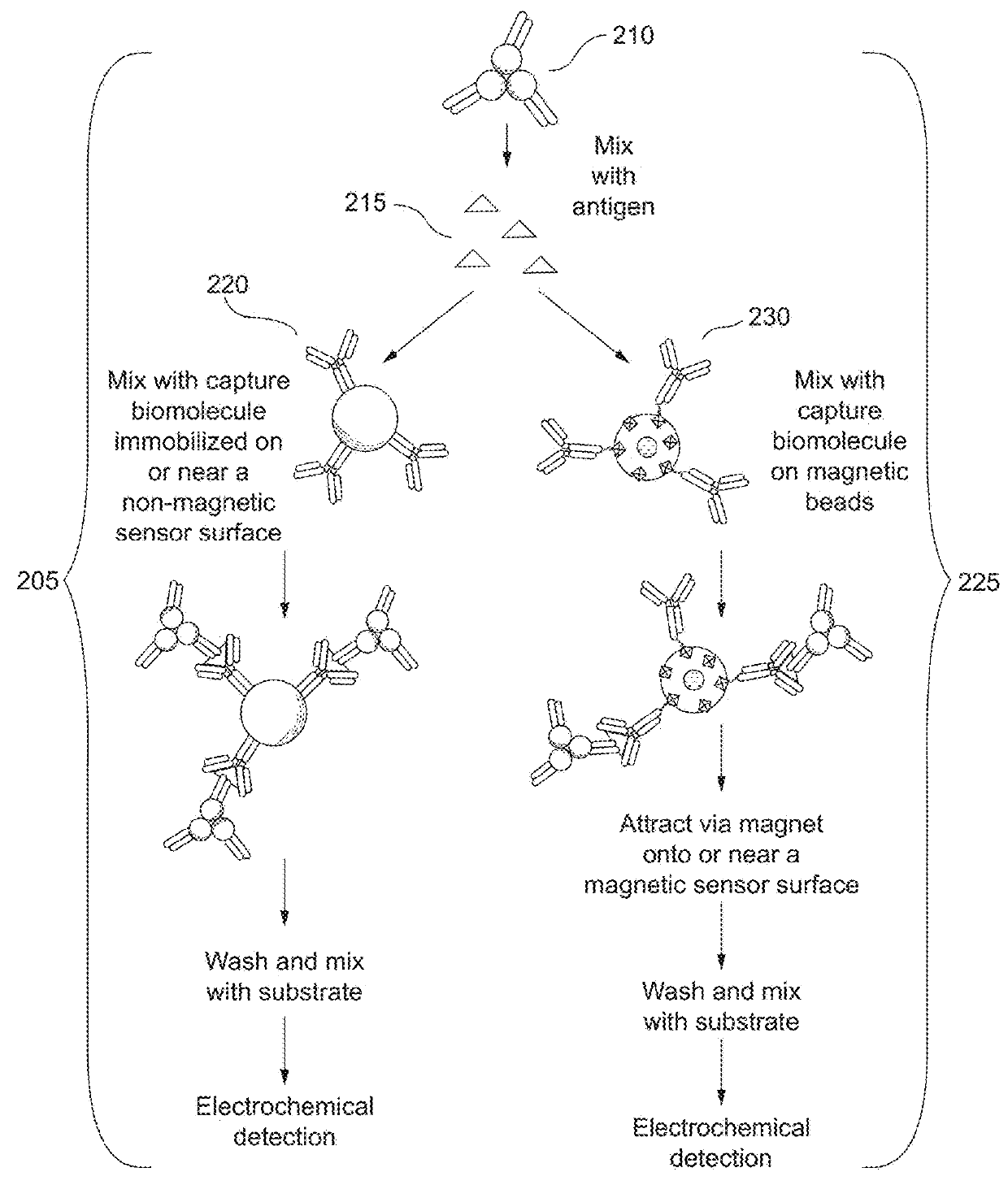
Dual range cardiac troponin immunoassay devices and methods using immunosensor and magnetic immunosensor
The present invention relates to systems and methods that utilize a combination of immunoassay and magnetic immunoassay techniques to detect an analyte within an extended range of specified concentrations. In particular, a device includes a first immunosensor including an immobilized layer of capture antibodies configured to bind to a first complex of signal antibodies and cardiac troponin such that a second complex of the first complex and the immobilized layer of capture antibodies is localized on or near the first immunosensor. The device further includes a second immunosensor having a magnetic field disposed locally around the second immunosensor. The magnetic field is configured to attract magnetic beads such that a third complex of the first complex and capture antibodies immobilized on the magnetic beads is localized on or near the second immunosensor sensor.
Owner:ABBOTT POINT CARE
Immune fluorescent test strip component for quickly quantitatively detecting troponin I, detection card component comprising immune fluorescent test strip component and preparation methods for immune fluorescent test strip component and detection card component
The invention discloses an immune fluorescent test strip component for quickly quantitatively detecting troponin I, a detection card component comprising the immune fluorescent test strip component and preparation methods for the immune fluorescent test strip component and the detection card component. The test strip component comprises a test strip and an independently packaged platinum porphyrin labelled specific antibody, wherein the test strip comprises a substrate, an absorbent pad, an enveloping analysis film and a sample pad; the enveloping analysis film is provided with a detection line and a quality control line; a specific antibody enveloped by the detection line is a troponin I resistant monoclonal antibody; and a specific antibody enveloped by the quality control line is a rabbit IgG antibody. The detection card component comprises the test strip, a card box consisting of a cover plate and a back plate, and an independently packaged platinum porphyrin labelled specific antibody. An immune fluorescent rapid detection technology is adopted for detecting the level of the troponin I in a human body, the cost is low, the operation is simple, rapid and sensitive, the specificity is high, and the invention has significance for preventing cardiovascular and cerebrovascular diseases.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Detection kit and preparation method thereof and detection method of troponin T
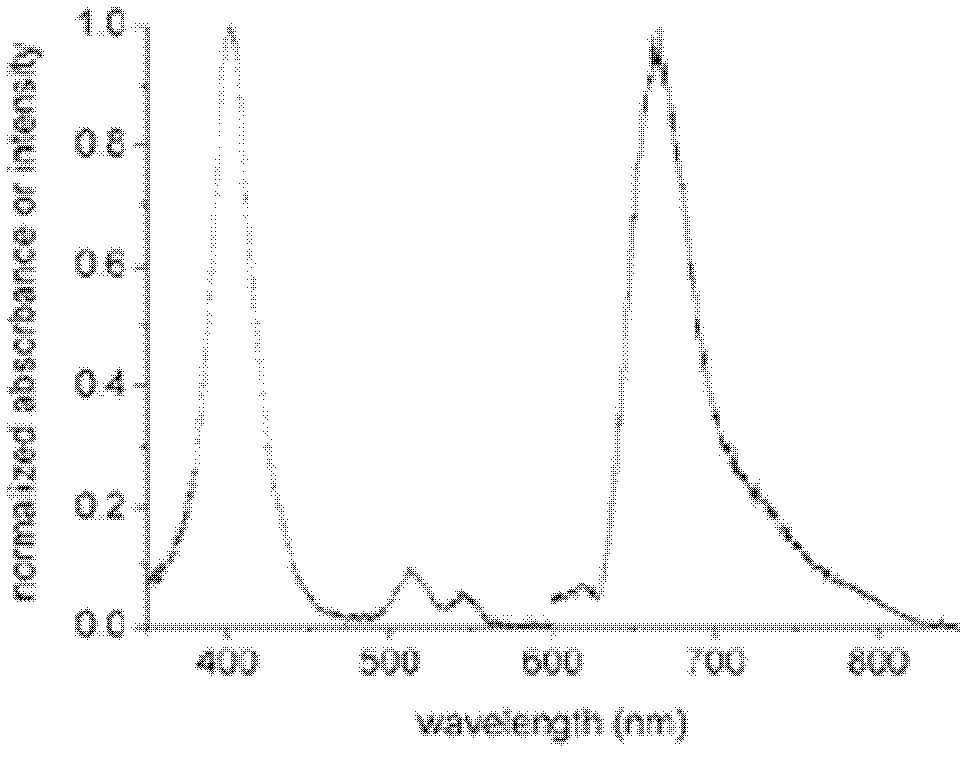
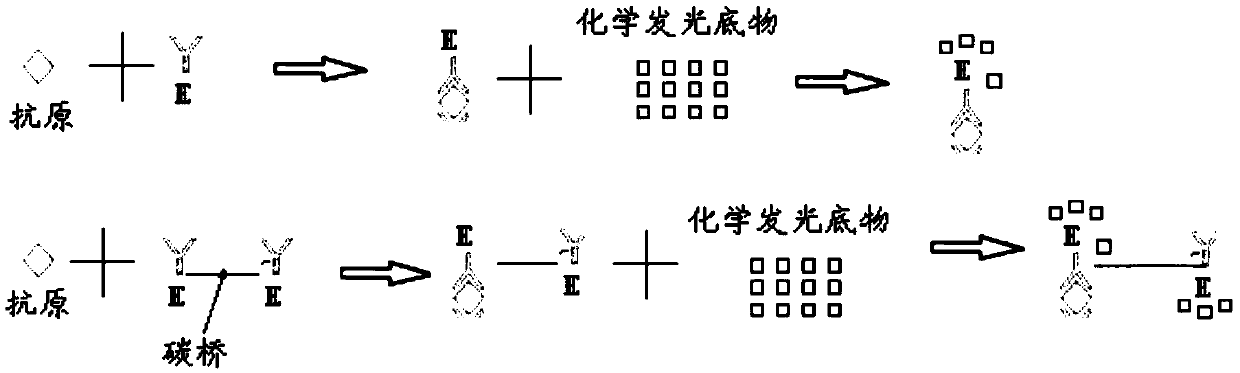
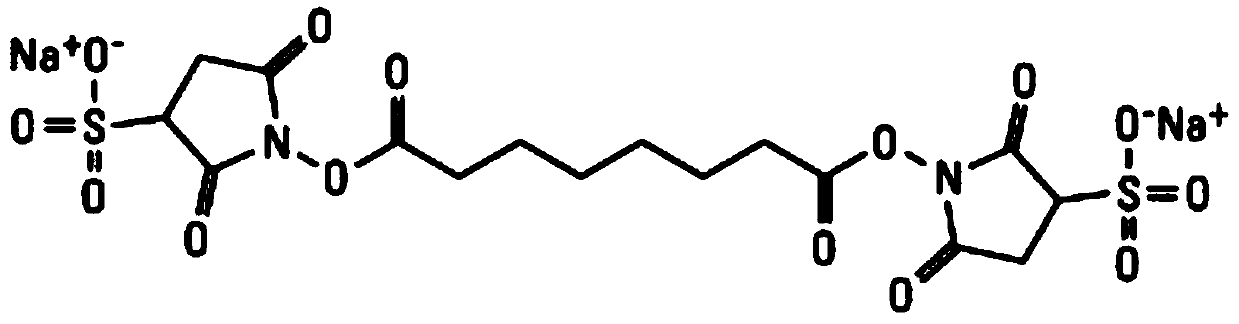
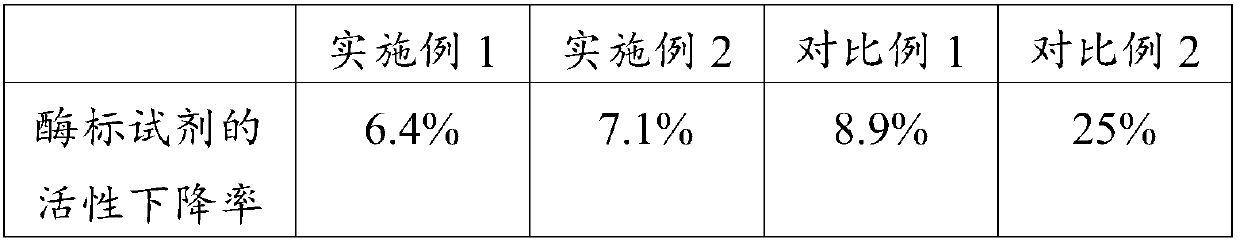
ActiveCN109596835AImprove coating efficiencyDoes not affect activityBiological testingBiotin-streptavidin complexAntigen
The invention belongs to the technical field of in-vitro detection, and particularly relates to a detection kit and a preparation method and application thereof. The detection kit comprises a magneticseparation reagent and an enzyme-labeled reagent; the magnetic separation reagent is prepared from troponin T antibody-coated gold magnetic particles or prepared from streptavidin-coated gold magnetic particles and a biotin-labeled troponin T antibody; and the enzyme-labeled reagent is prepared from an enzyme-labeled antibody polymer, wherein the enzyme-labeled antibody polymer is prepared from at least two enzyme-labeled antibodies and carbon bridges; the carbon bridges connect any two or more, adjacent or non-adjacent enzyme-labeled antibodies, and each carbon bridge has at least two connecting loci for connecting the enzyme-labeled antibodies; and each enzyme-labeled antibody is prepared from a detection antibody and a labeling enzyme coupled to the detection antibody, and the connecting loci are connected with the detection antibodies and / or the labeling enzymes. When chemiluminescence detection is conducted, a reaction signal value of a chemiluminescence method is amplified, thesensitivity of an antigen captured by the detection reagent can be enhanced, and the detection sensitivity is improved.
Owner:深圳天辰医疗科技有限公司
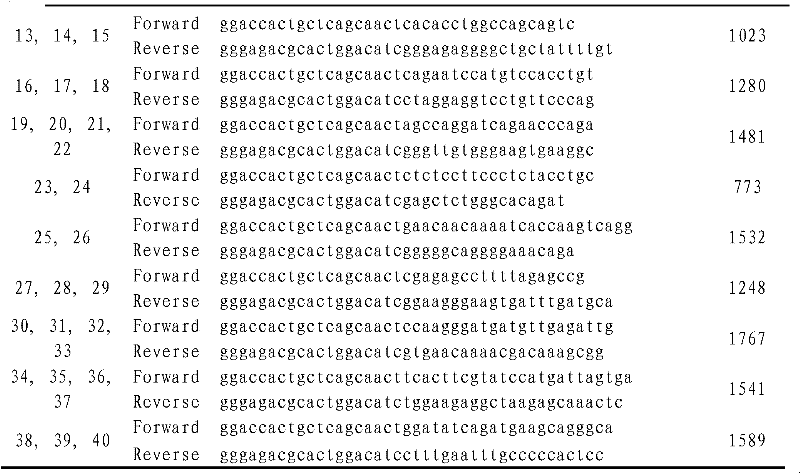
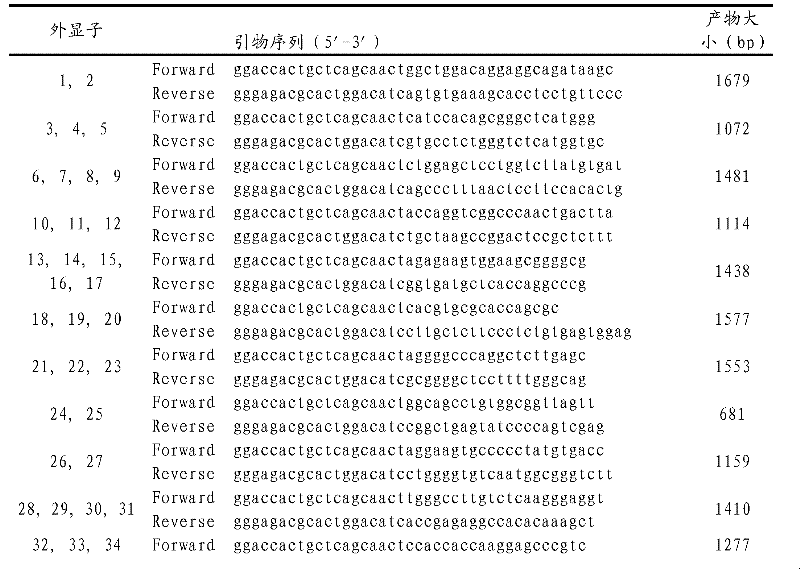
HCM (Hypertrophic Cardiomyopathy) genotyping method and kit
The invention provides a technical scheme to provide an HCM (Hypertrophic Cardiomyopathy) genotyping method and a kit. The method comprises the following steps of: a, carrying out a gene mutation test on genes MYH7 (Myosin Heavy Chain 7), MYBPC3 (Myosin Binding Protein C 3) and TNNT2 (Troponin 2) in a sample DNA (Deoxyribonucleic Acid), wherein the gene mutation test comprises PCR (Polymerase Chain Reaction) amplification sequencing of MYH7, MYBPC3 and TNNT2 gene exons; and b, comparing sequences obtained by amplification in the step a with a standard sequence of a database to determine a genotyping result. The invention has the beneficial effects of: (1) assisting the early diagnosis: finding out asymptomatic generation sufferers and non-generation sufferers in family members; and (2) assisting to guide the selective birth so as to eliminate the spread of the HCM in the family.
Owner:泰普生物科学(中国)有限公司
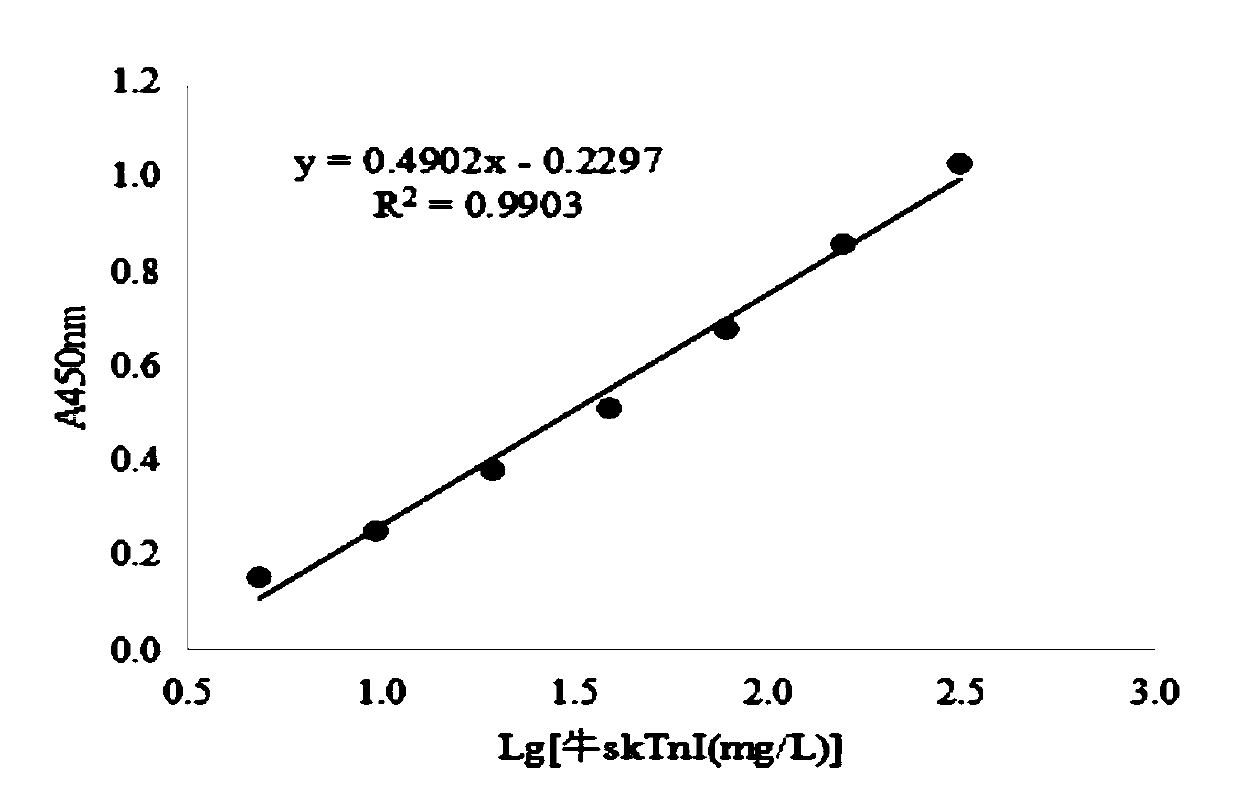
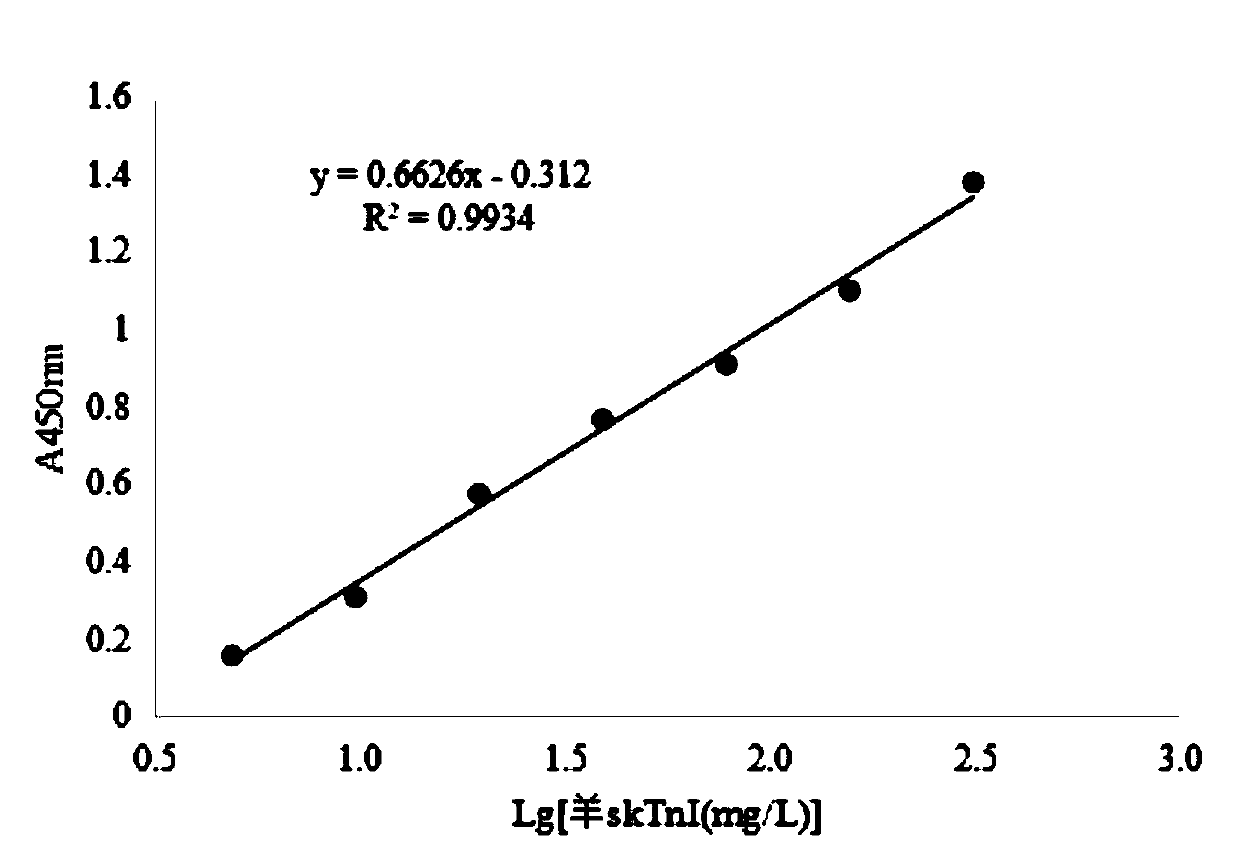
Human rapid contraction of skeletal muscle troponin I production and activity detection method
InactiveCN1570105AInhibition of newbornsThe process is simple and convenientPeptide/protein ingredientsPeptide preparation methodsDiseaseAbnormal tissue growth
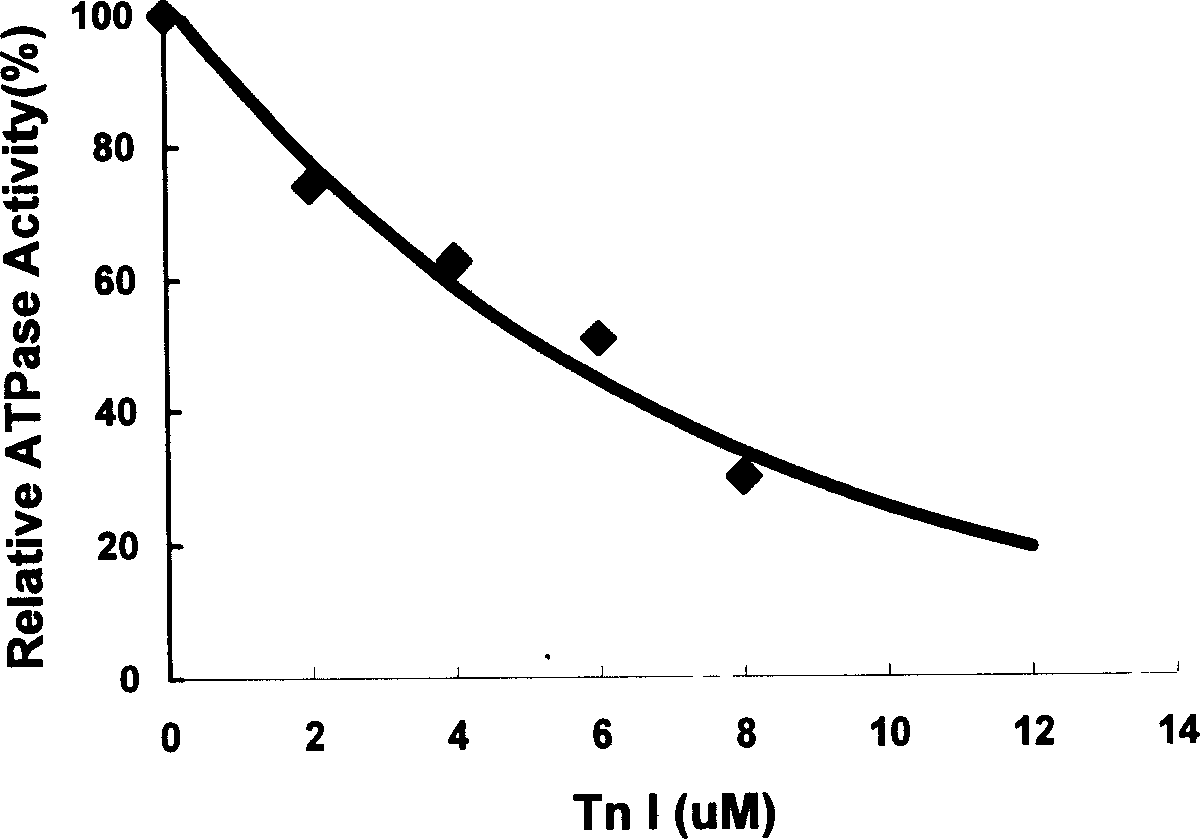
The invention belongs to gene engineering field. The invention establish a production method of human skeletal muscle troponin I for quick contract: recombing DNA to construct expression plasmid, refining expression products with inclusion bodies, cation exchange resin column chromatography, cation exchange resin column chromatography, renaturing inclusion bodies, at last purifying and obtaining recombinant human troponin I for suppressing blood vessel endothelial cell propagation and angiogenesis. The invention also establishes a method for determing the biological activity index of troponin I by suppressing Acto-S1 Mg-ATPase activity. The troponin I can prepare drugs of the tumor and angiogenesis related disease
Owner:NANJING UNIV
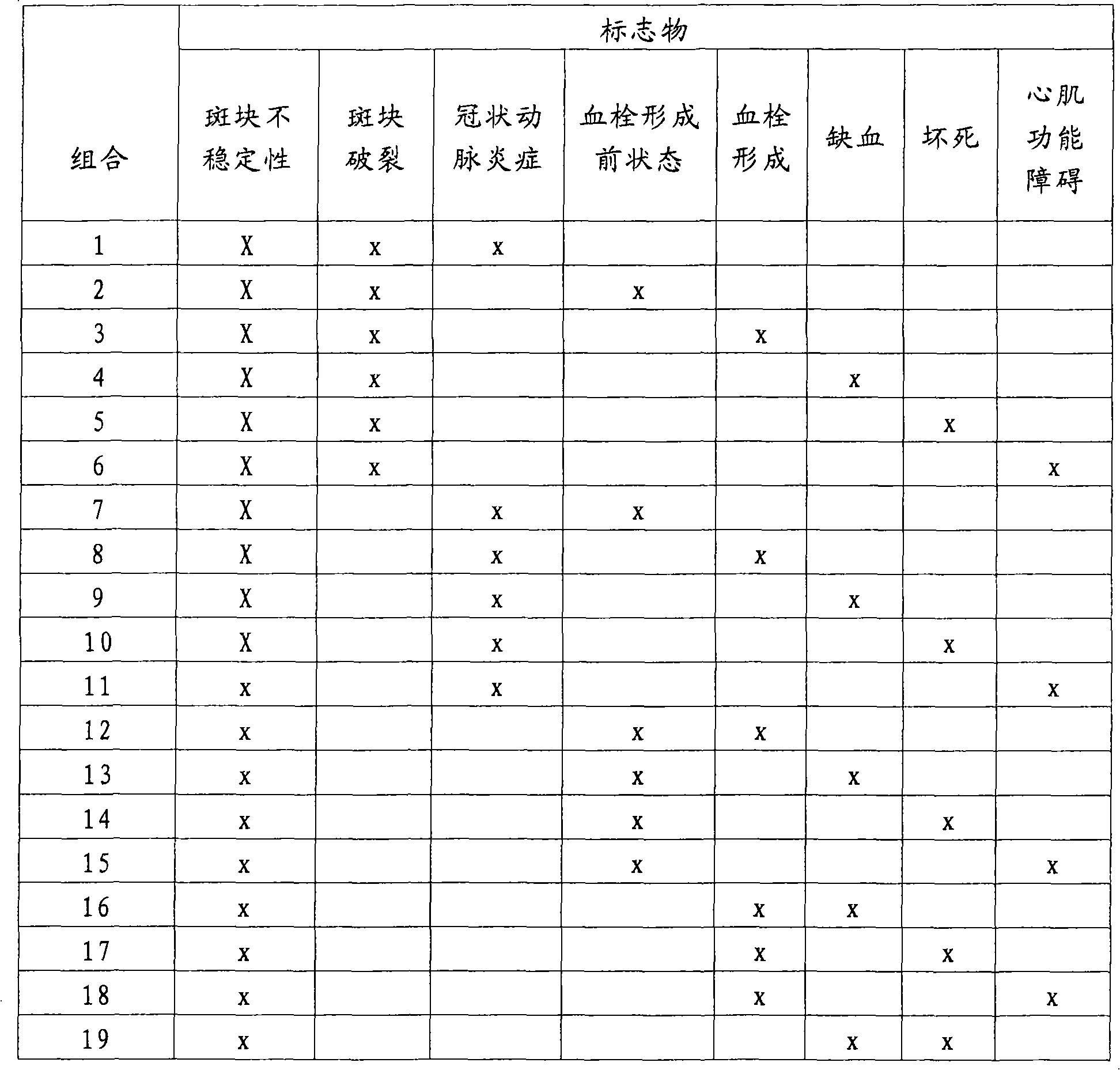
Detection and determination of the stages of coronary artery disease
A method having clinically sufficient degree of diagnostic accuracy for detecting the presence of coronary artery disease in a human patient from the general population and for distinguishing between the stages of the disease in that patient is disclosed. The stages are, first, the non-acute stage, which is either asymptomatic coronary artery disease or stable angina, second, the acute stage known as unstable angina, and, third, the acute stage known as acute myocardial infarction. The diseased state (as opposed to the non-diseased state) is indicated by the clinically significant presence of a first marker in a sample from the patient. The presence of one of the two acute stages, unstable angina or acute myocardial infarction, is indicated by the clinically significant presence of a second marker in a sample from the patient. The presence of the more severe acute stage known as acute myocardial infarction is indicated by the clinically significant presence of a third marker in a sample from the patient. Preferably the first marker comprises OxLDL, the second marker comprises MDA-modified LDL, and the third marker is a troponin. Preferably the OxLDL and MDA-modified LDL are detected using monoclonal antibodies that can detect the presence of those markers in undiluted human plasma at concentrations as low as 0.02 milligrams / deciliter.
Owner:LEUVEN RES & DEV VZW
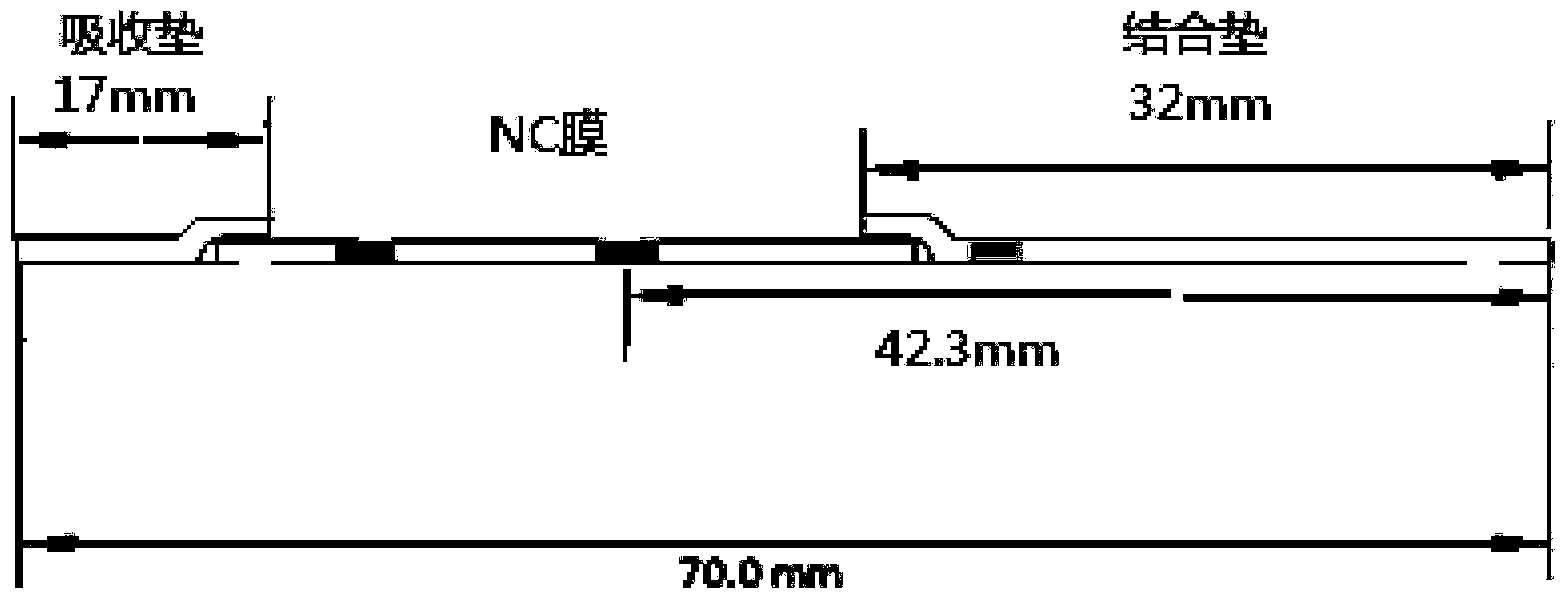
Troponin diagnosis test paper strip for coupled immunomagnetic beads
The invention relates to a troponin diagnosis test paper strip for coupled immunomagnetic beads. The structure of the troponin diagnosis test paper strip is that an NC (nitrocellulose) film is arranged between a combined pad and an absorption pad and the NC film is an area in which a blood sample to be detected is dropped. The troponin diagnosis test paper strip is characterized in that the immunomagnetic beads are accommodated in the NC film; the immunomagnetic beads are magnetic beads with carboxyl on the surfaces, and are antibody-coupled immunomagnetic beads formed after coupling with a monoclonal antibody in an activated buffer solution. In an optimal scheme, the using amount of the antibody-coupled immunomagnetic beads and the blood sample to be detected are as follows: 4-5 microliters of 16A11 antibody-coupled immunomagnetic beads, 4-5 microliters of 810 antibody-coupled immunomagnetic beads, 2.5-3.5 microliters of blood sample to be detected, and 142-152 microliters of mixed buffer solution. The troponin diagnosis test paper strip for coupled immunomagnetic beads disclosed by the invention overcomes the defects in the prior art, so that troponin can be simply and quickly detected, the detection result is more accurate, the application field is wider, and the utilization rate for antibody raw materials is higher.
Owner:南京爱思唯志生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com