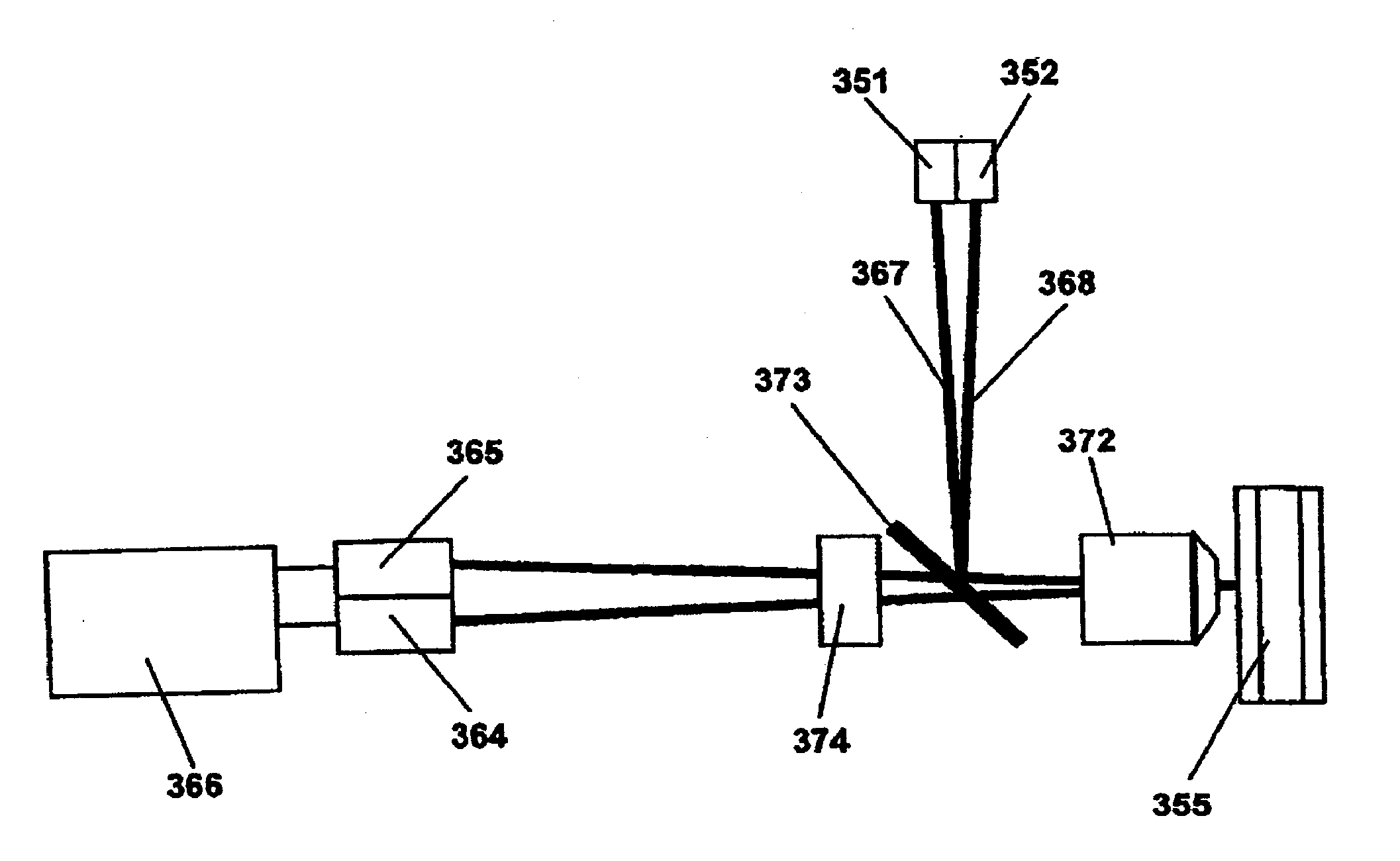
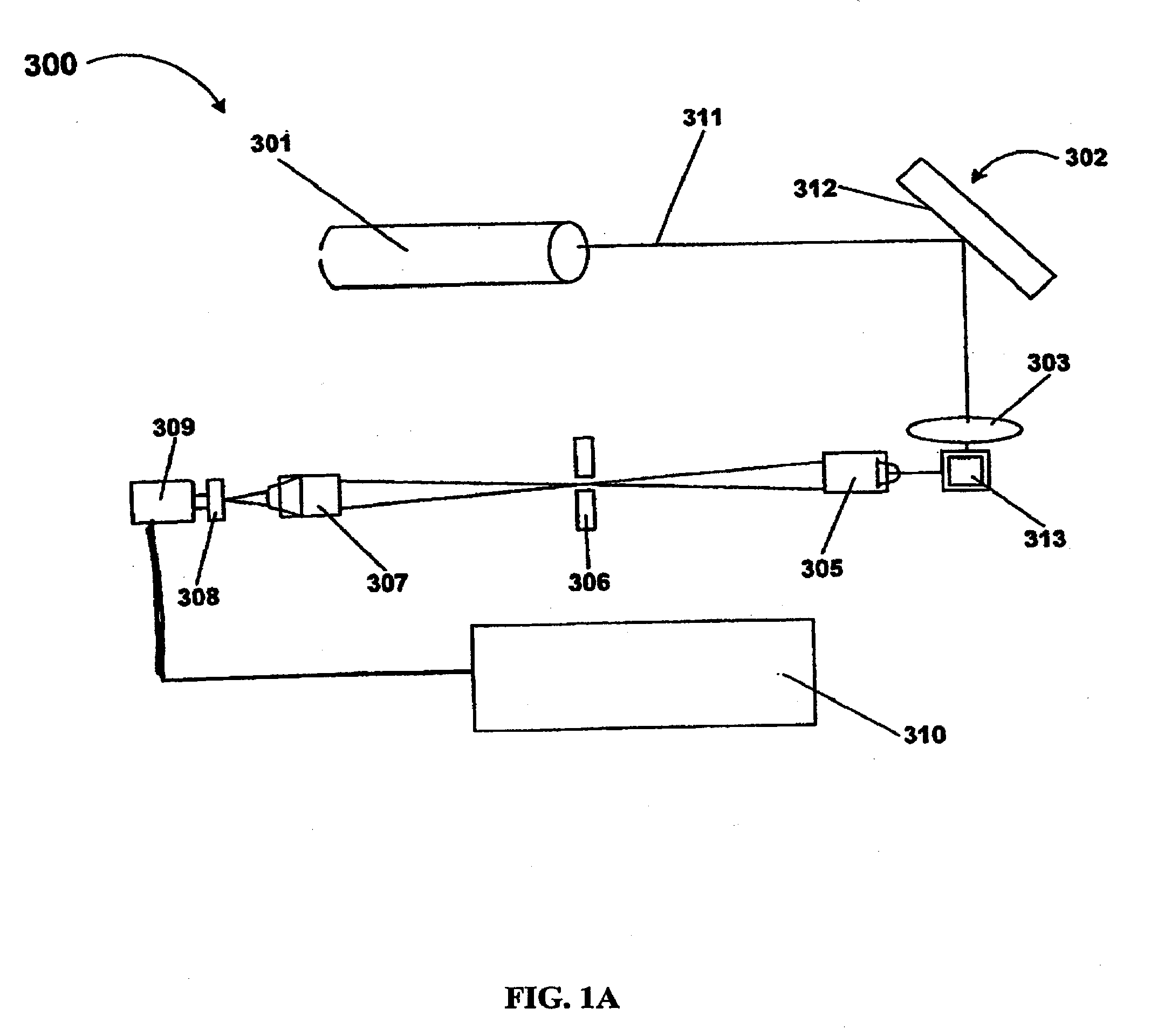
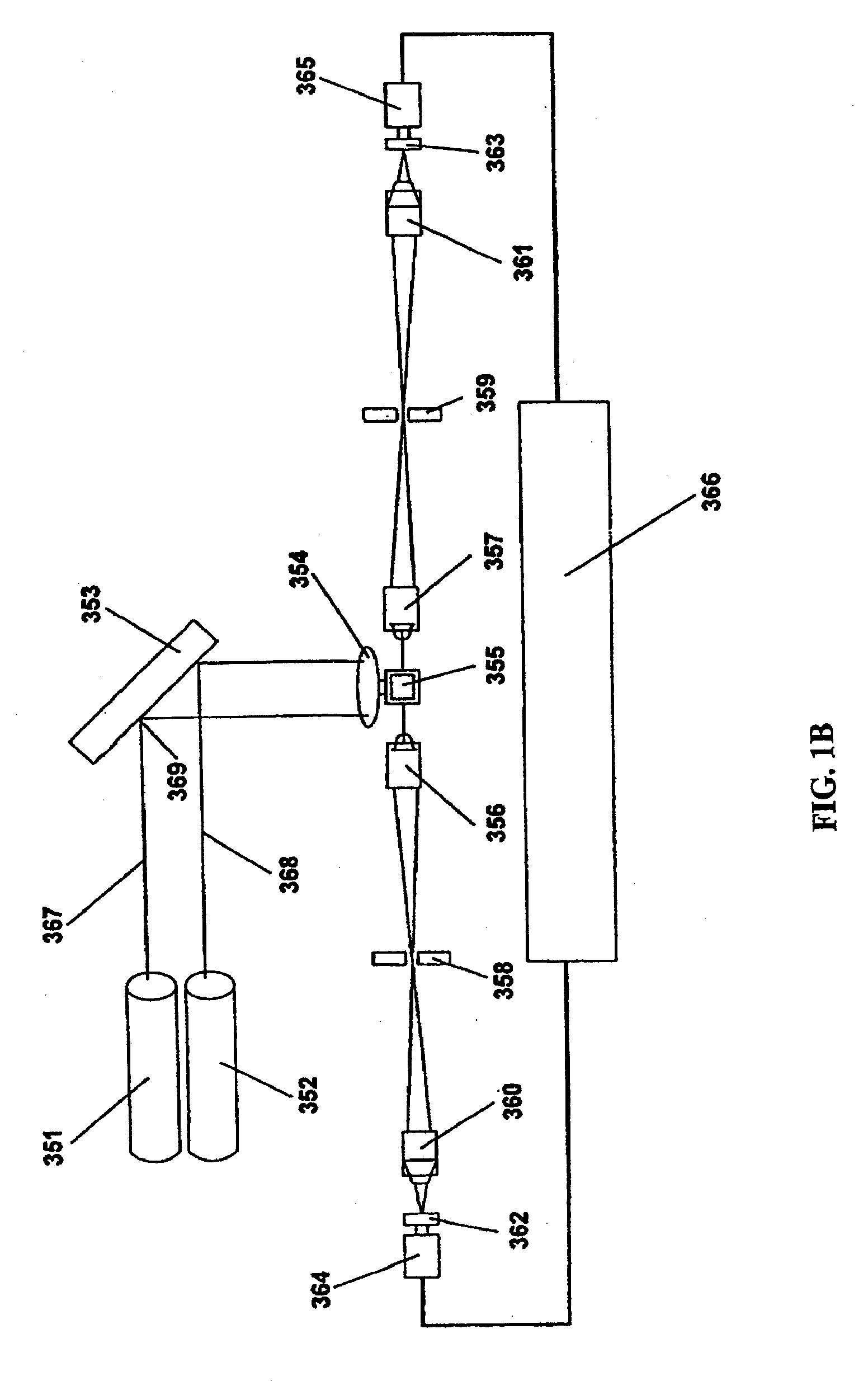
Highly Sensitive System and Methods for Analysis of Troponin
a troponin and sensitive technology, applied in the field of high-sensitivity system and method for analysis of troponin, can solve problems such as cardiac toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Sandwich Assays for Biomarkers: Cardiac Troponin I (cTnI)
[0273]The assay: The purpose of this assay was to detect the presence of cardiac Troponin I (cTnI) in human serum. The assay format was a two-step sandwich immunoassay based on a mouse monoclonal capture antibody and a goat polyclonal detection antibody. Ten microliters of sample were required. The working range of the assay is 0-900 pg / ml with a typical analytical limit of detection of 1-3 pg / ml. The assay required about four hours of bench time to complete.
[0274]Materials: the following materials were used in the procedure described below: Assay plate: Nunc Maxisorp, product 464718, 384 well, clear, passively coated with monoclonal antibody, BiosPacific A34440228P Lot # A0316 (5 μg / ml in 0.05 M sodium carbonate pH 9.6, overnight at room temperature); blocked with 5% sucrose, 1% BSA in PBS, and stored at 4 oC. For the standard curve, Human cardiac Troponin I (BiosPacific Cat # J34000352) was used. The diluent for the standard...
example 2
Sandwich Bead-Based Assays for TnI
[0288]The assays described above use the same microtiter plate format where the plastic surface is used to immobilize target molecules. The single particle analyzer system also is compatible with assays done in solution using microparticles or beads to achieve separation of bound from unbound entities.
[0289]Materials: MyOne Streptavidin C1 microparticles (MPs) are obtained from Dynal (650.01-03, 10 mg / ml stock). Buffers use in the assay include: 10× borate buffer saline Triton Buffer (BBST) (1.0 M borate, 15.0 M sodium chloride, 10% Triton X-100, pH 8.3); assay buffer (2 mg / ml normal goat IgG, 2 mg / ml normal mouse IgG, and 0.2 mg / ml MAB-33-IgG-Polymer in 0.1 M Tris (pH 8.1), 0.025 M EDTA, 0.15 M NaCl, 0.1% BSA, 0.1% Triton X-100, and 0.1% NaN3, stored at 4 C); sand elution buffer (BBS with 4 M urea, 0.02% Triton X-100, and 0.001% BSA, stored at 2-8 C). Antibodies used in the sandwich bead-based assay include: Bio-Ab (A34650228P (BiosPacific) with 1-...
example 3
Concentration Range for cTnI in a Population of Normal Non-Diseased Subjects
[0297]A reference range or normal range for cTnI concentrations in human serum was established using serum samples from 88 apparently healthy subjects (non-diseased). A sandwich immunoassay as described in Example 1 was performed and the number of signals or events as described above were counted using the single particle analyzer system of the invention. The concentration of serum troponin I was determined by correlating the signals detected by the analyzer with the standard curve as described above. All assays were perfumed in quadruplicate.
[0298]In accordance with recommendations by the current European and American Cardiology Societies (ESC / ACC) troponin assays should quantify accurately the 99th percentile of the normal range with an assay imprecision (CV) of less than 10% in order to distinguish reliably between patients with ACS and patients without ischemic heart disease, and risk stratification for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com