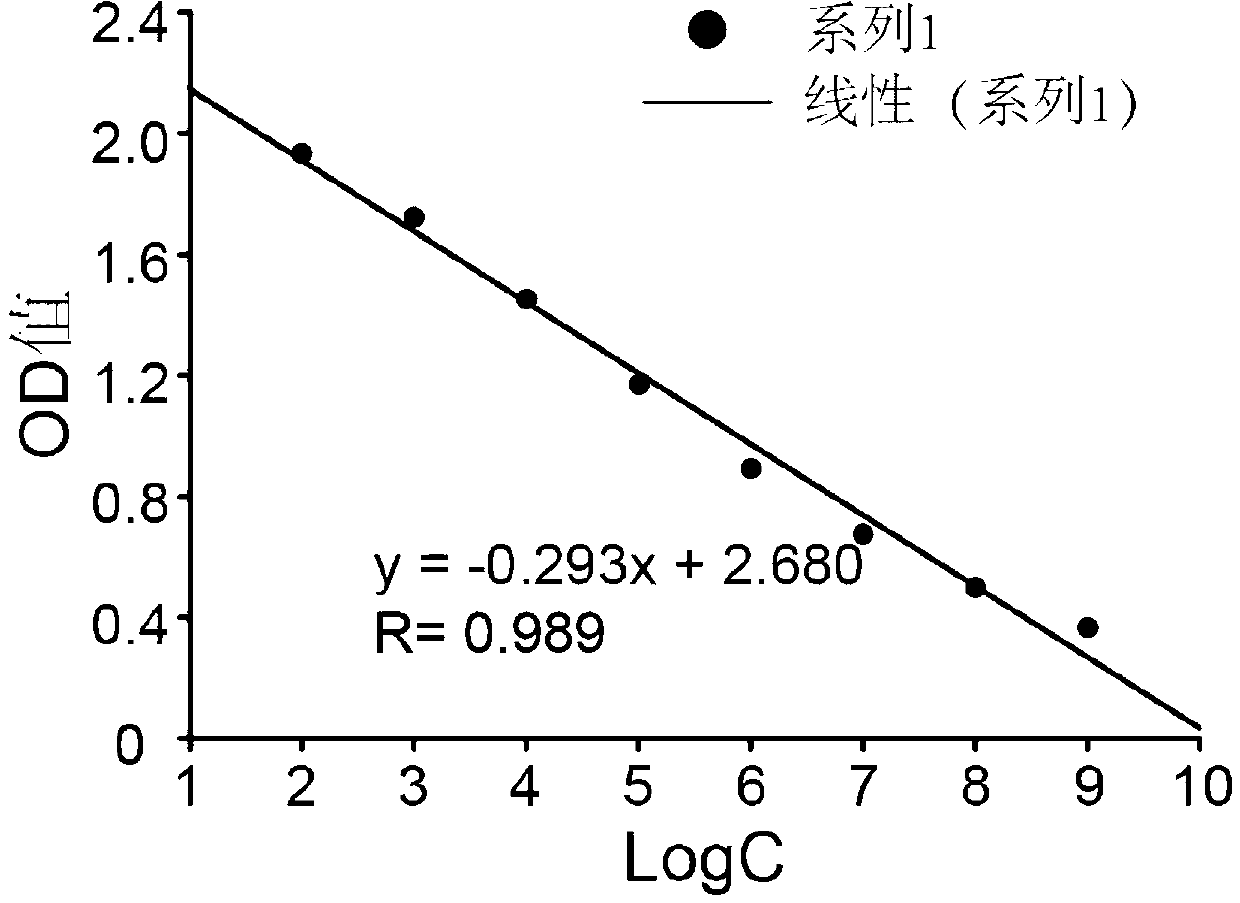
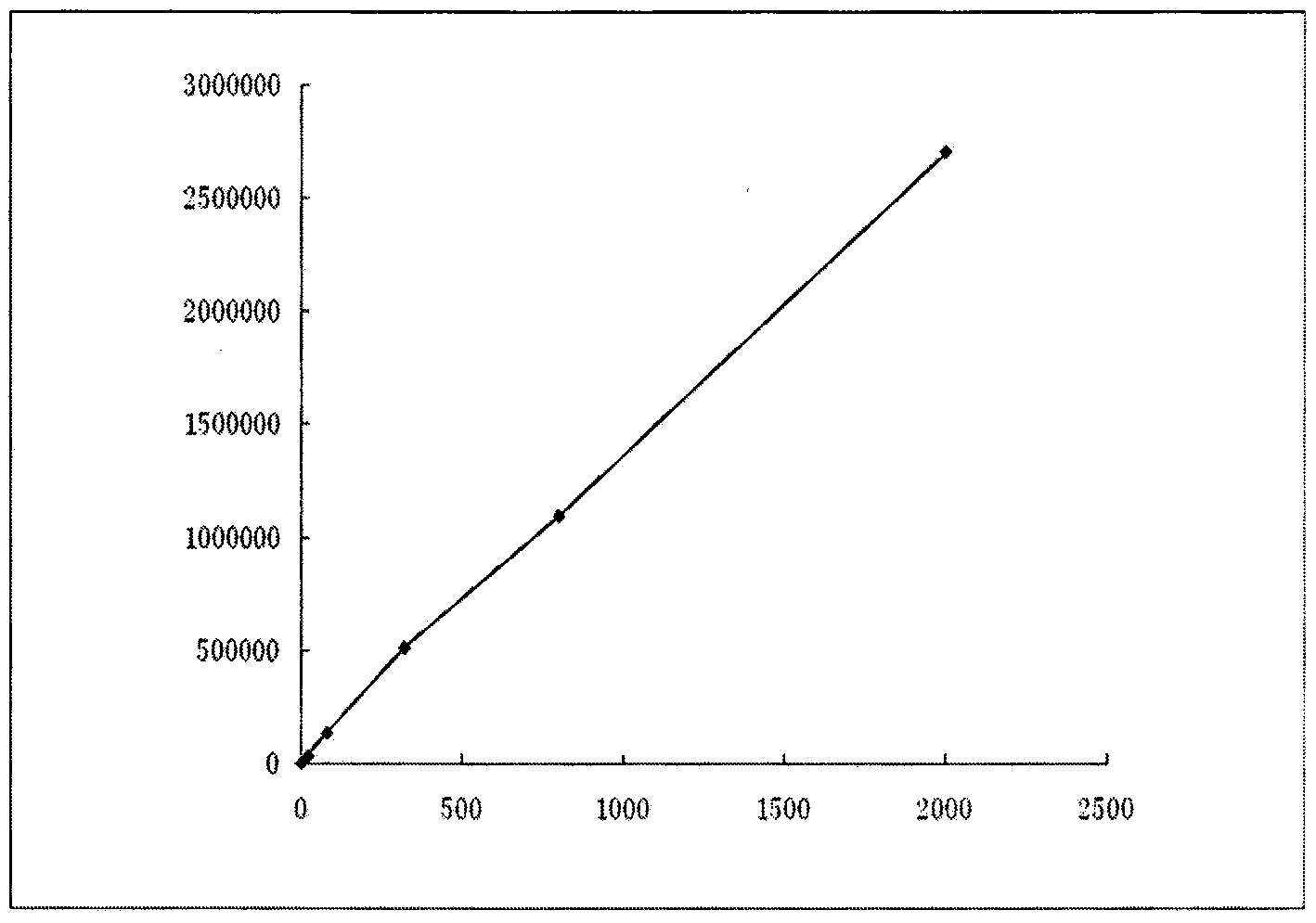
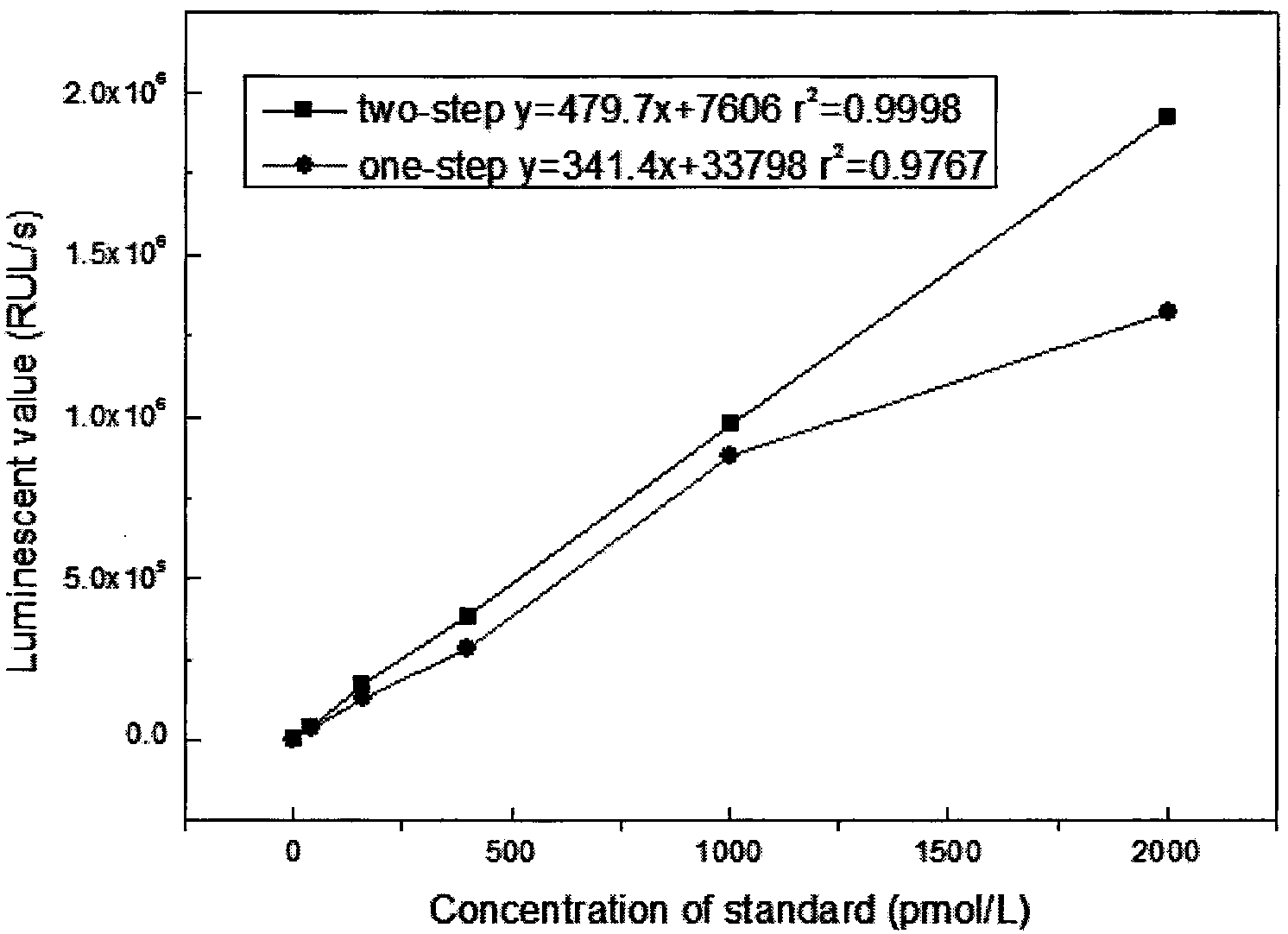
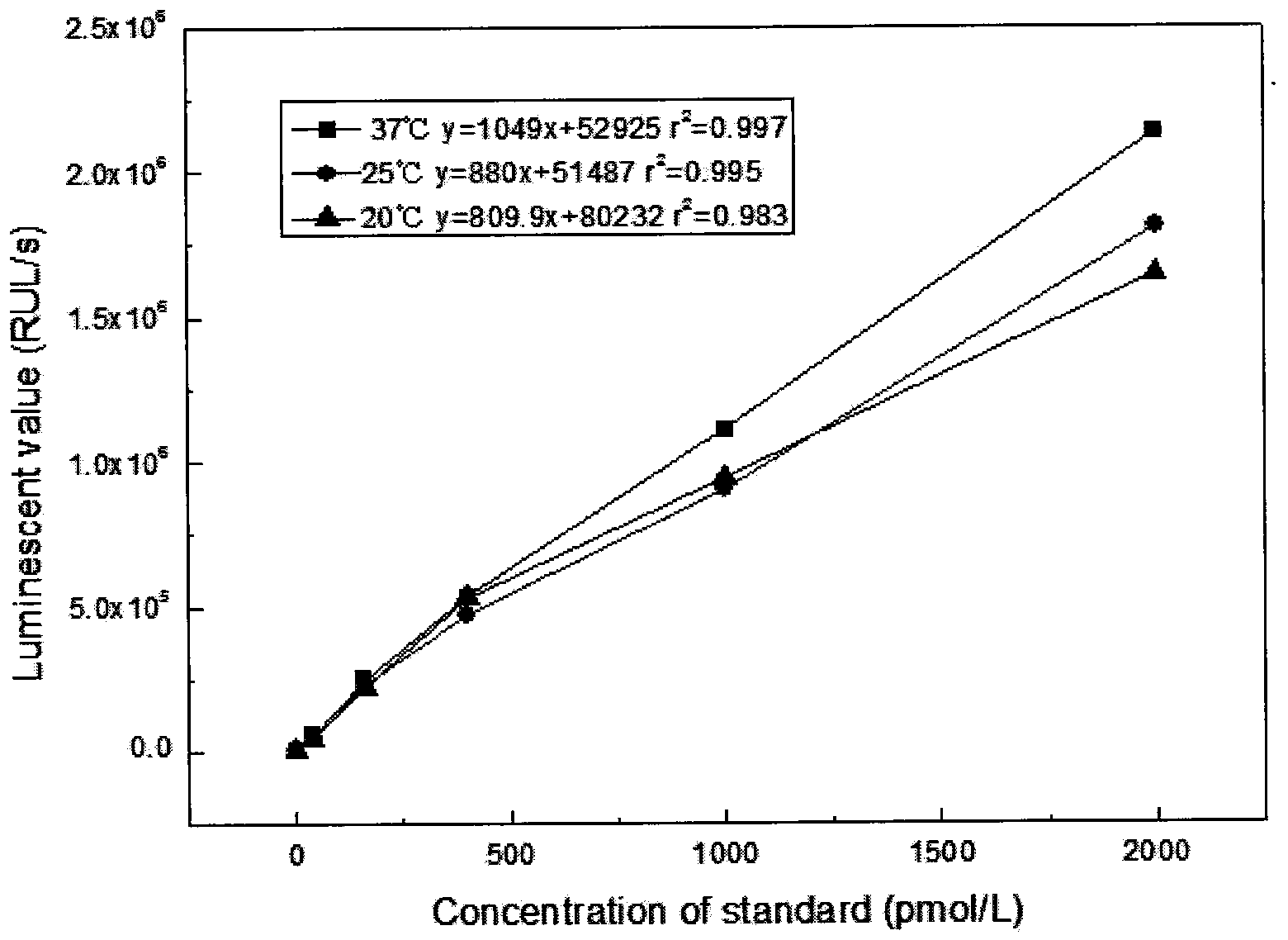
Patents
Literature
81 results about "Sandwich immunoassay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
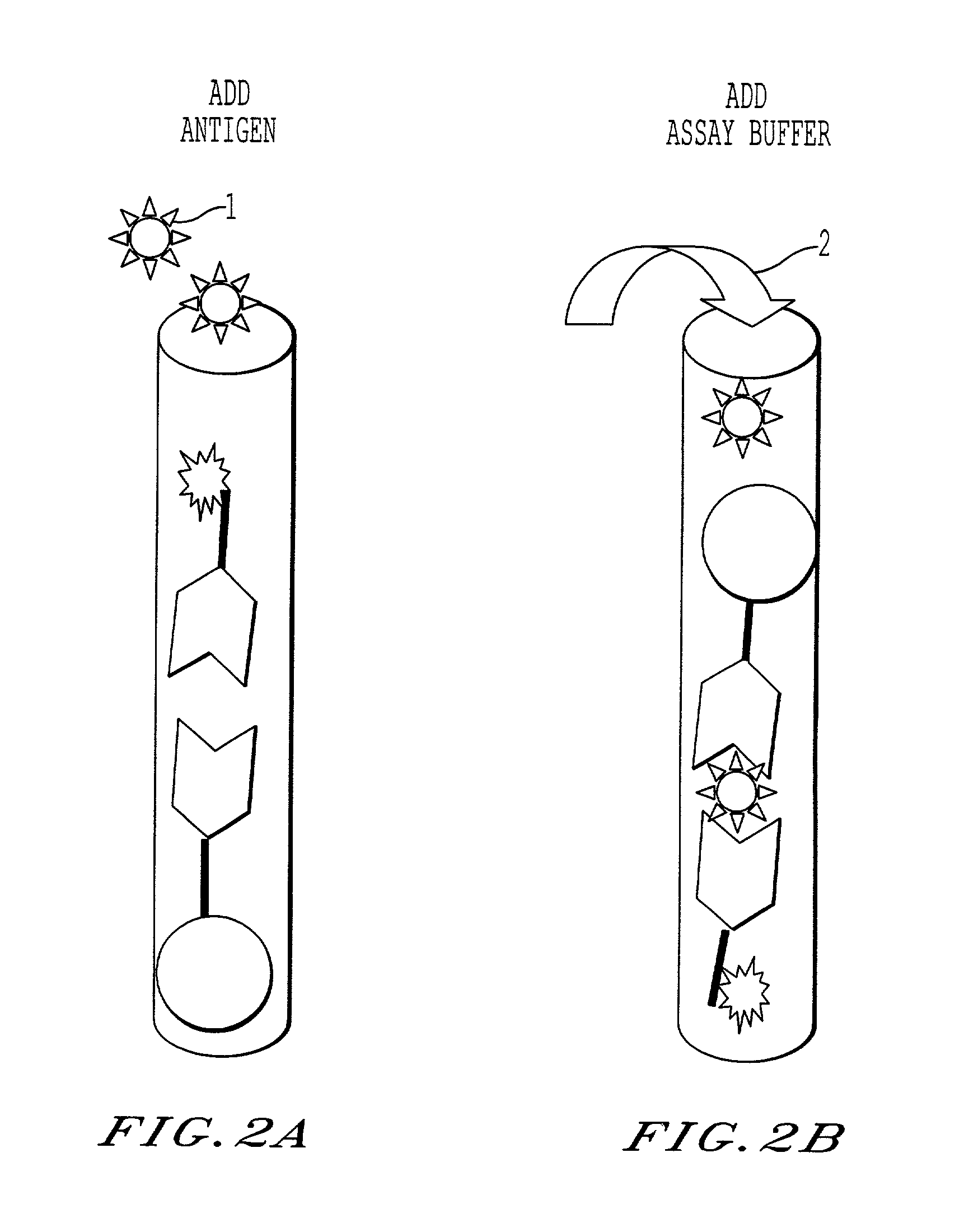
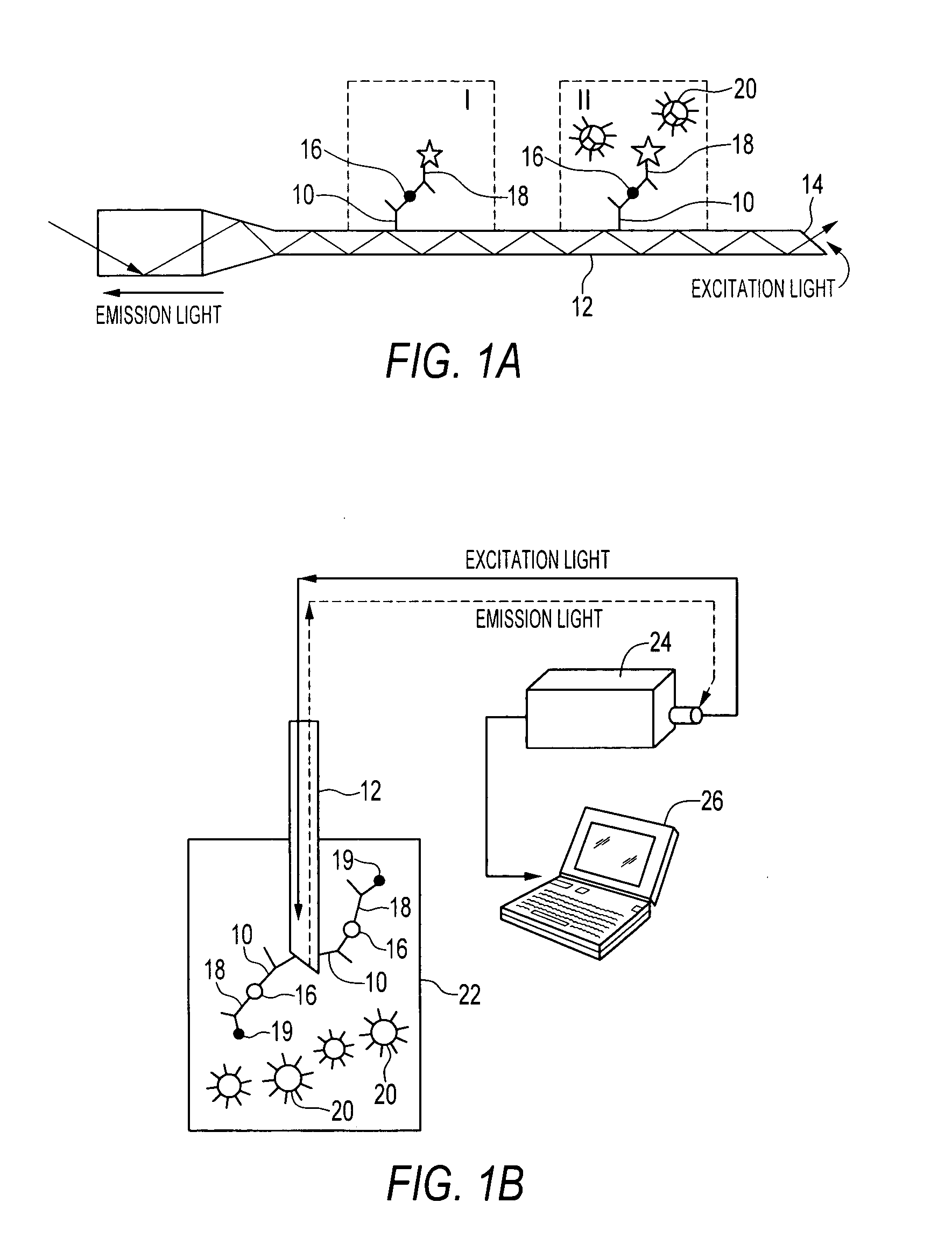
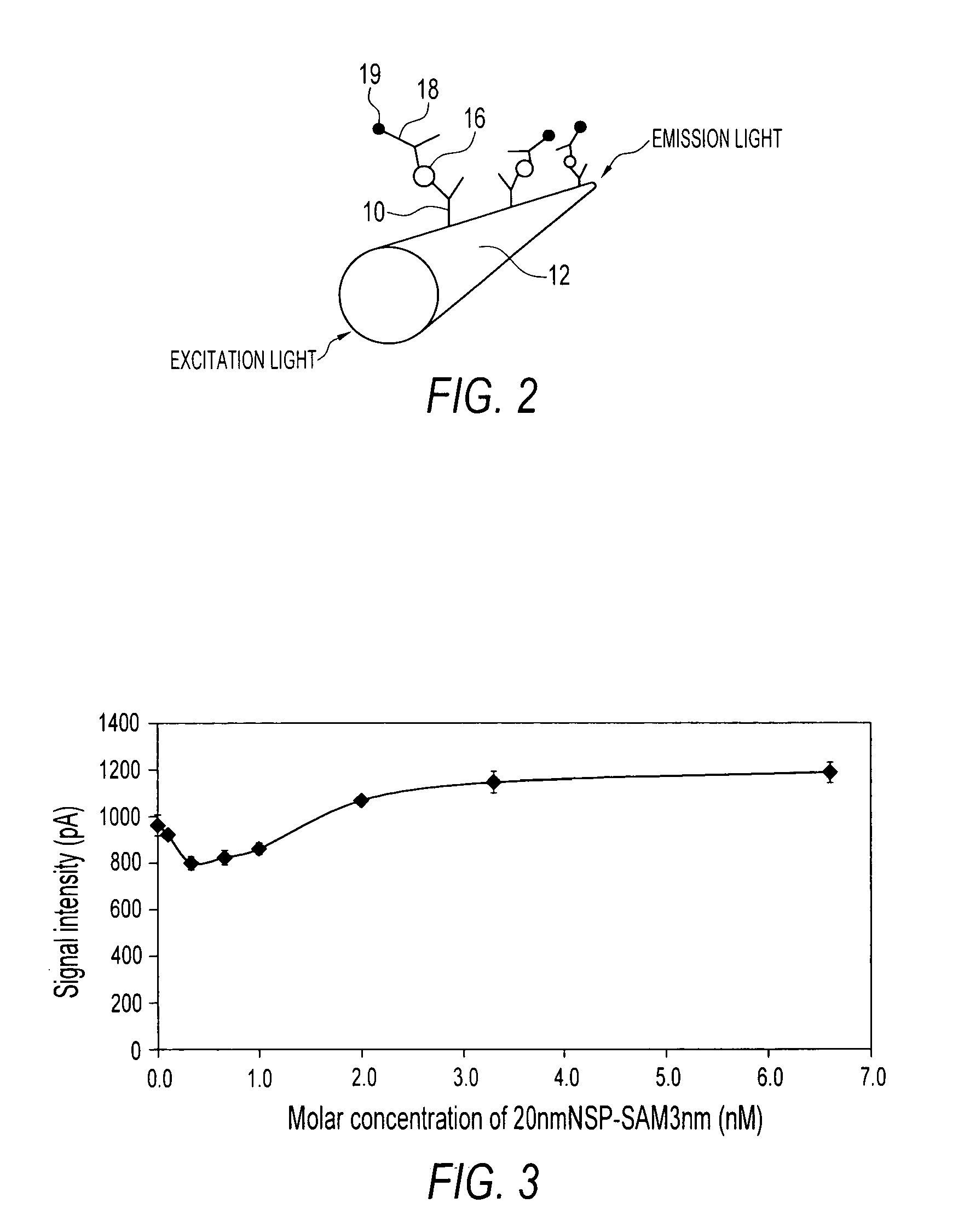
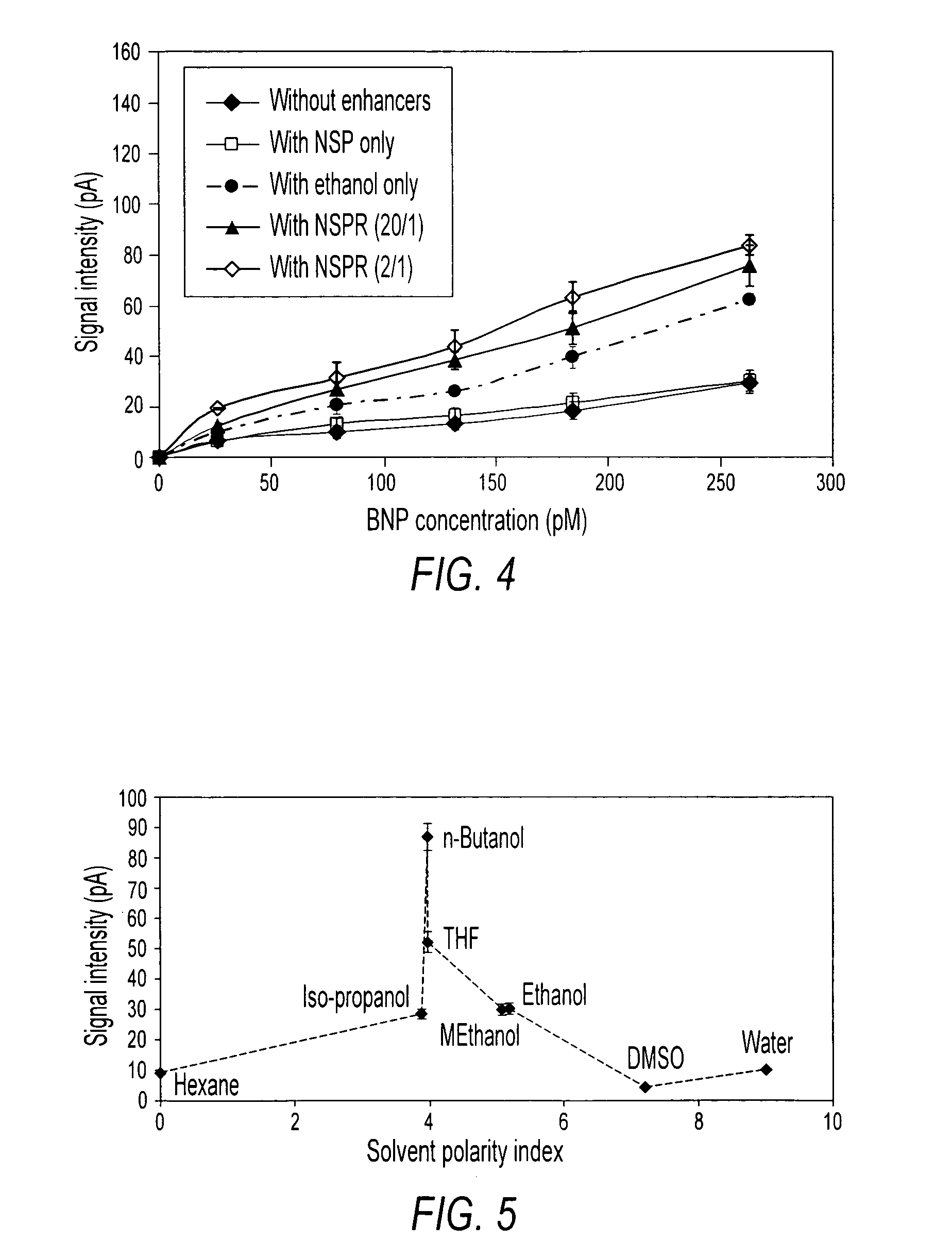
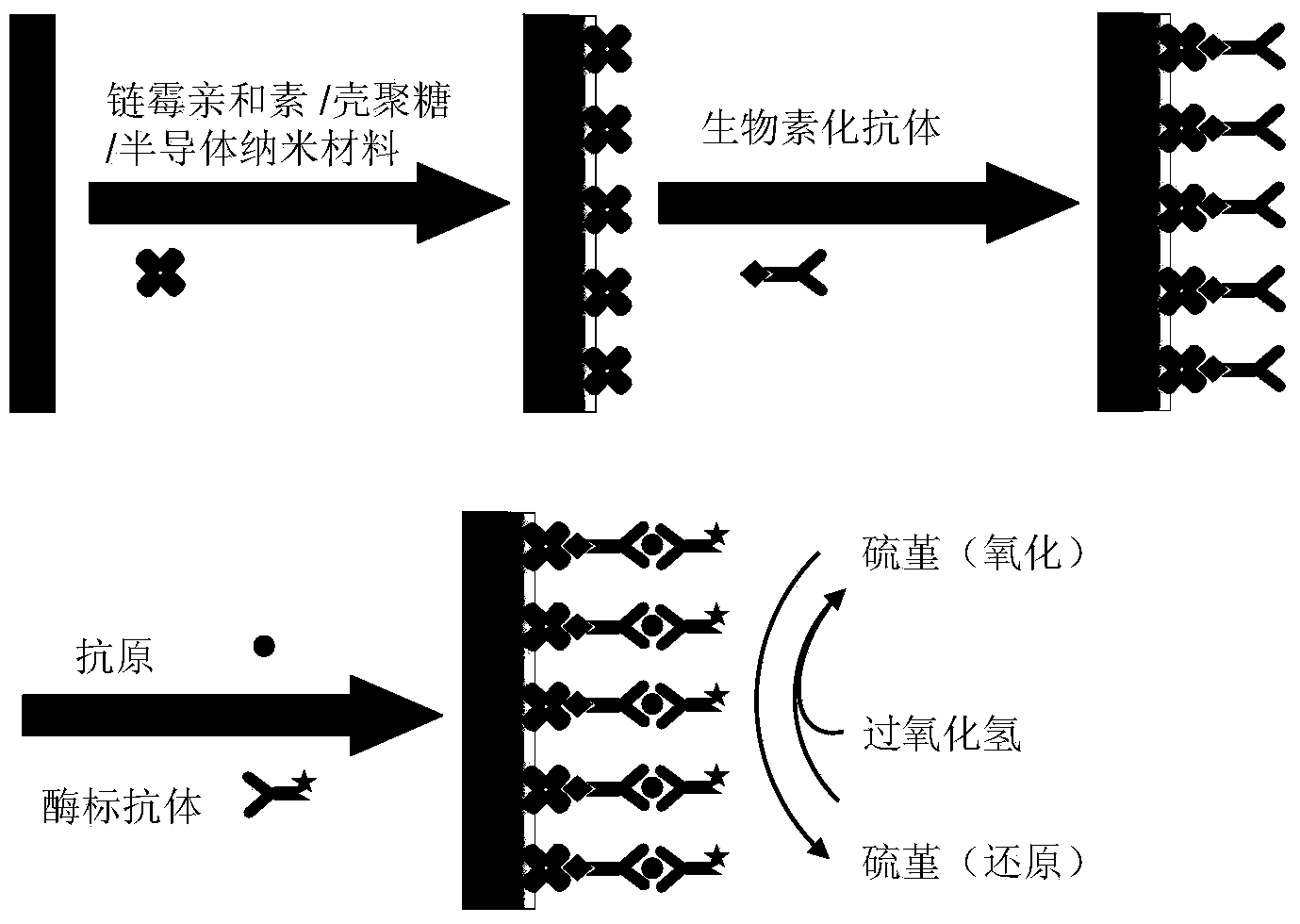
Sandwich immunoassay. An immunoassay in which the analyte is bound to a solid phase and a labeled reagent subsequently bound immunochemically to the analyte.
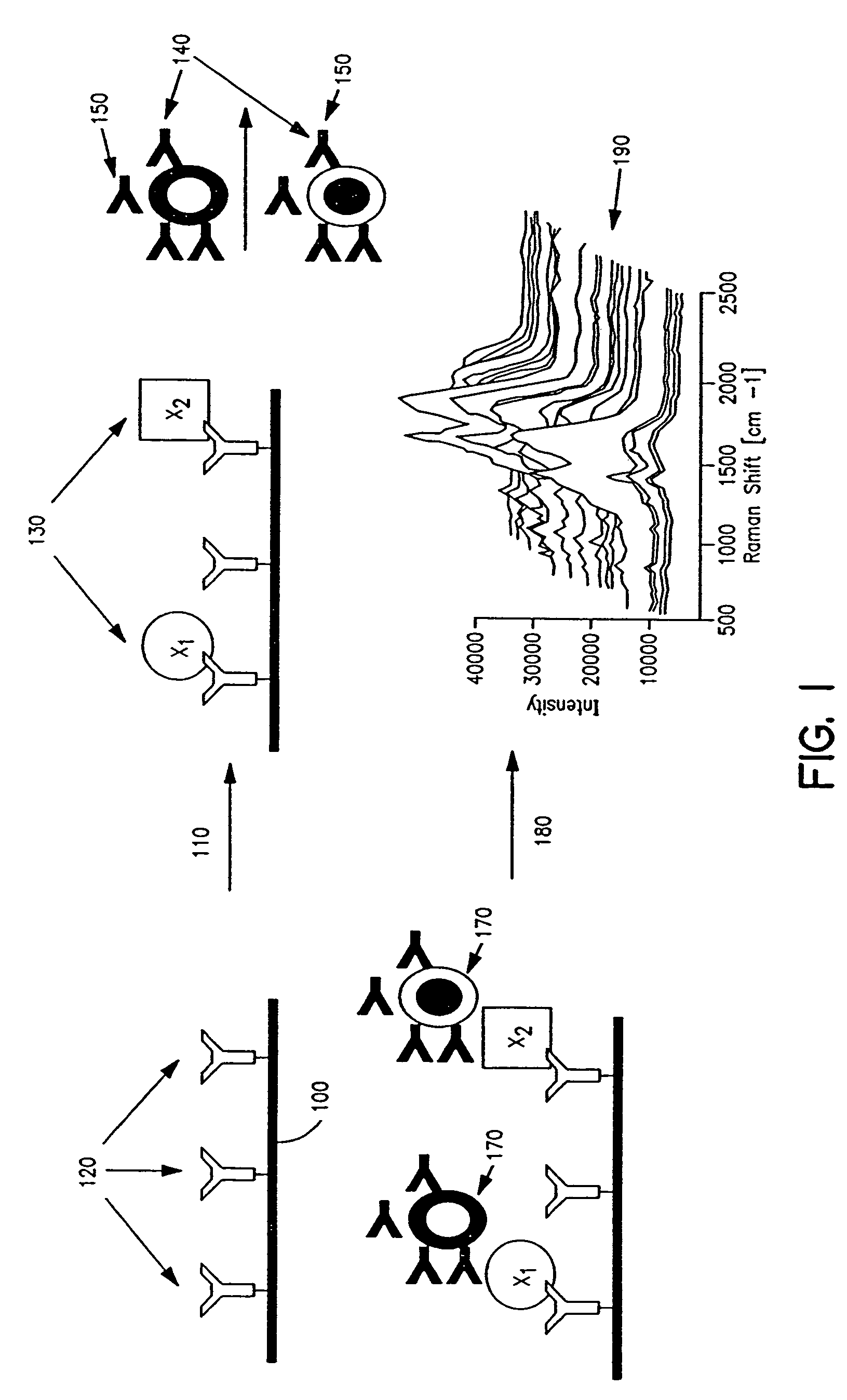
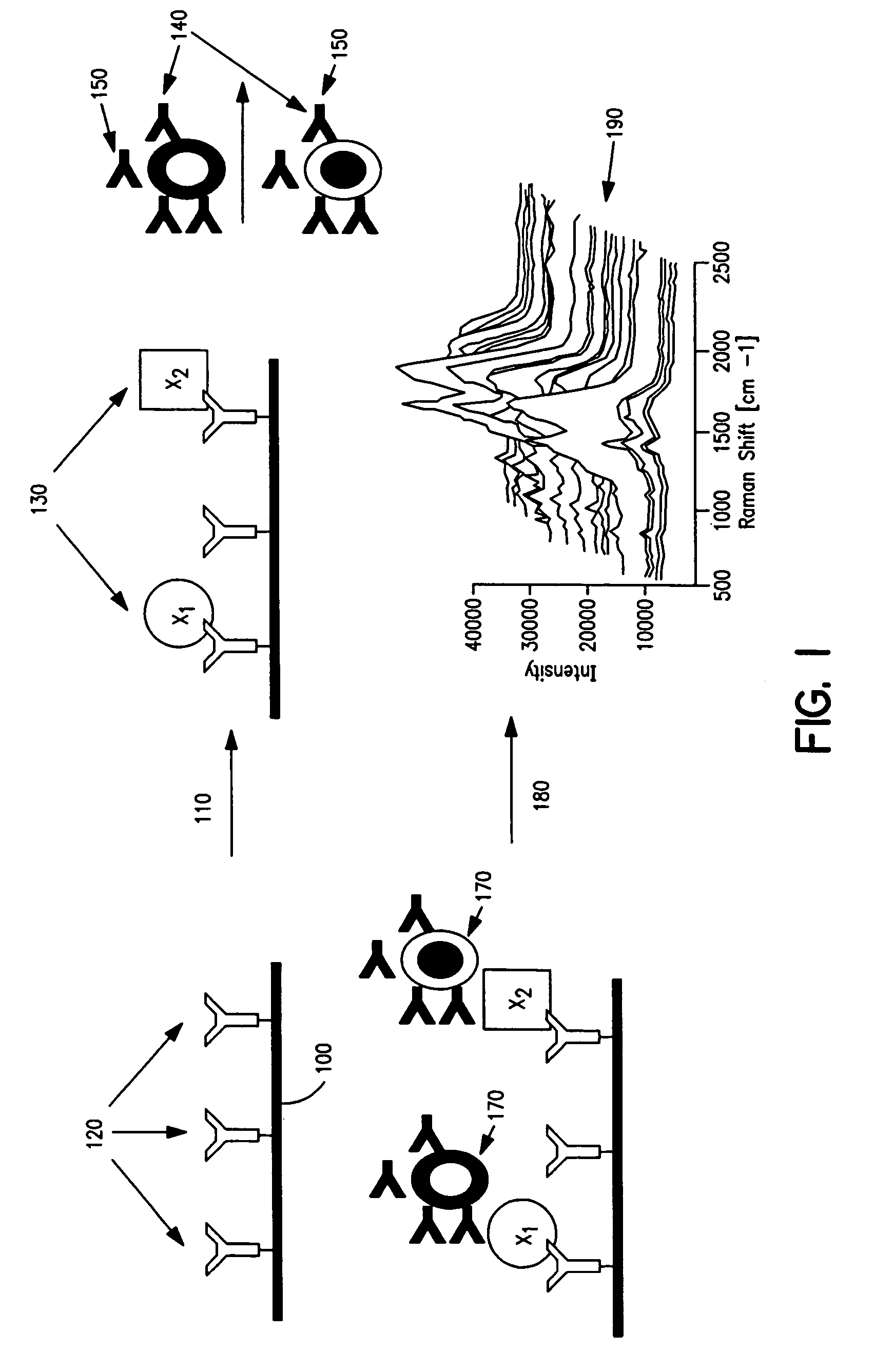
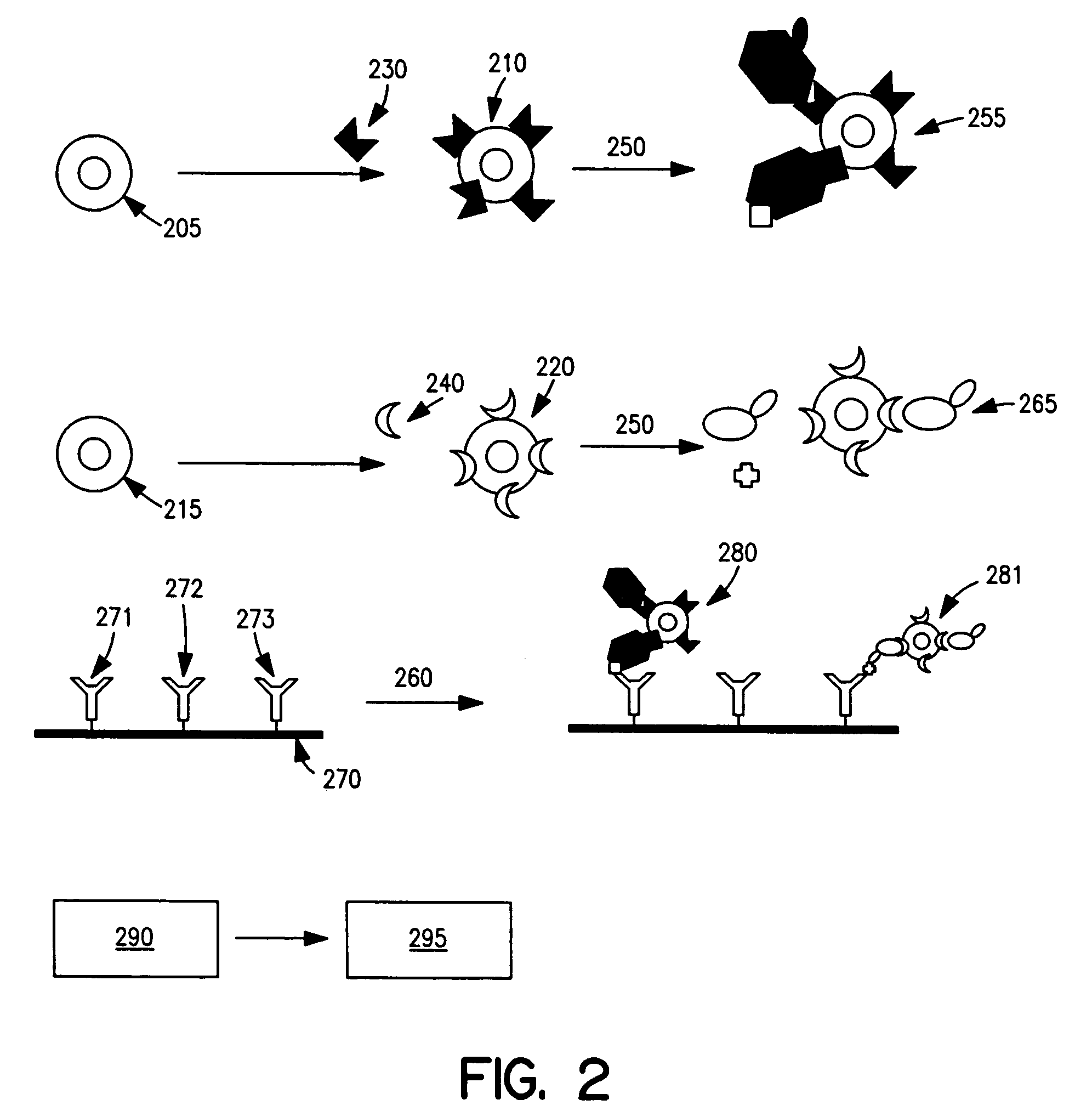
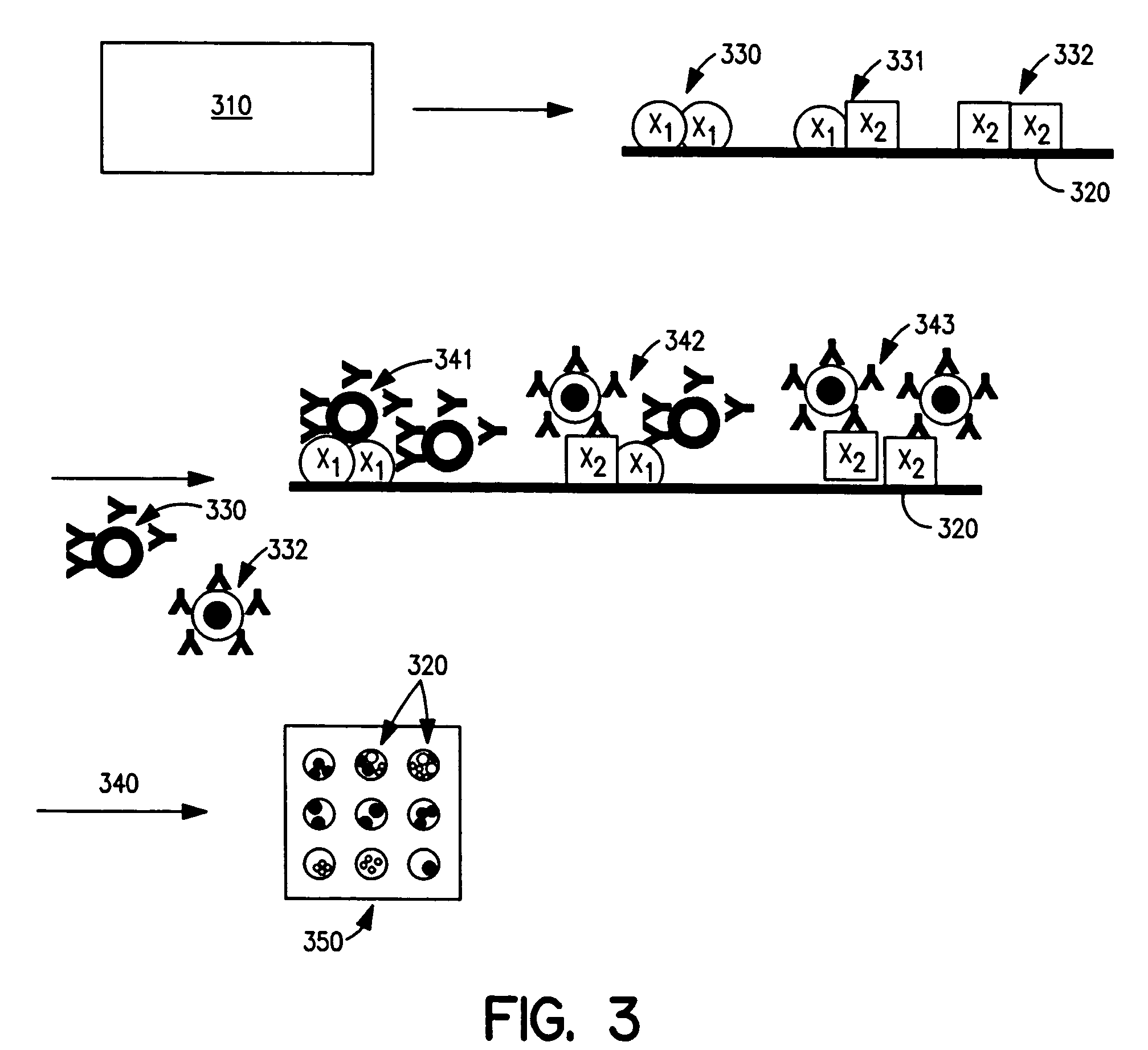
Cellular analysis using Raman surface scanning
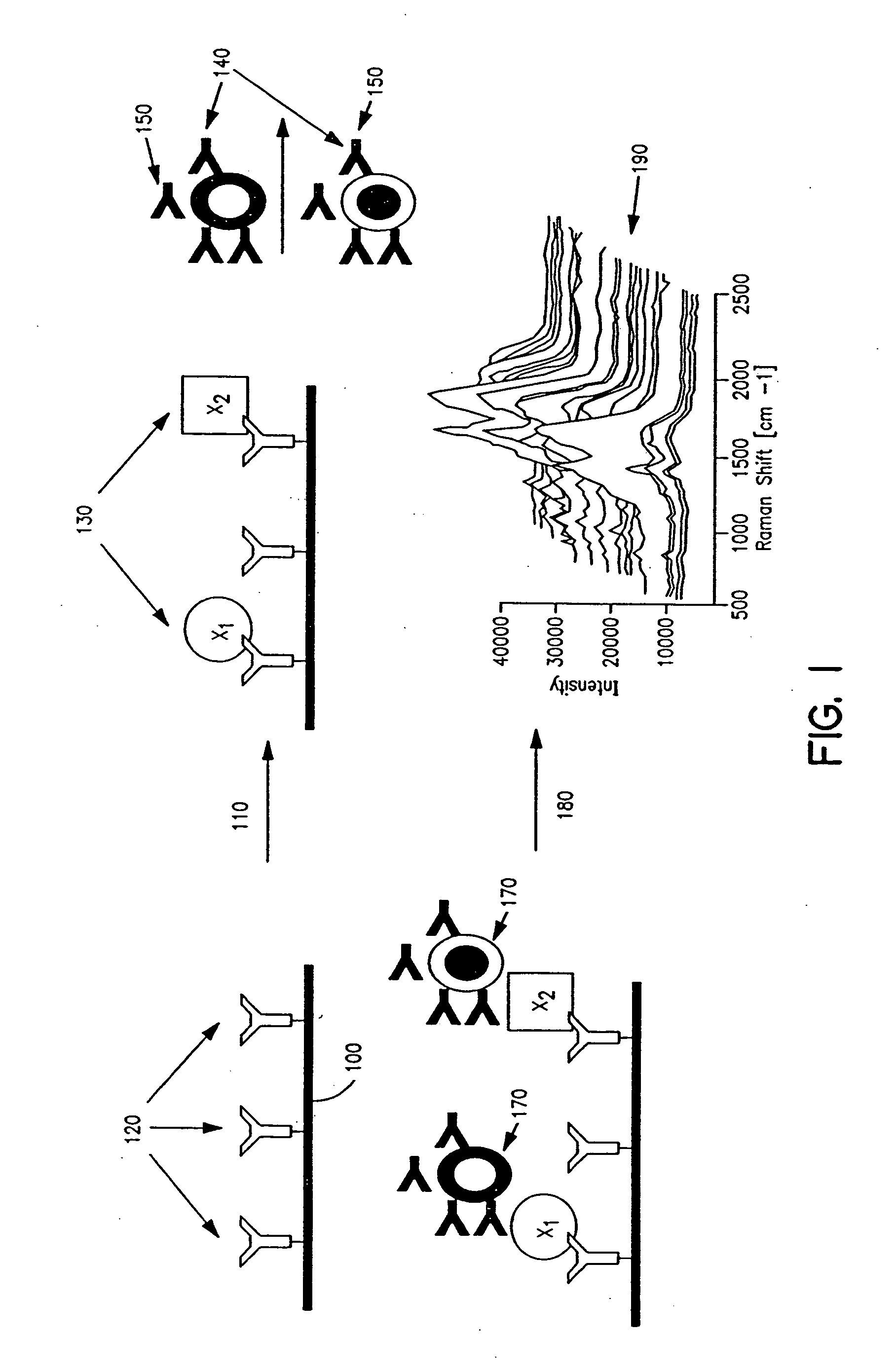
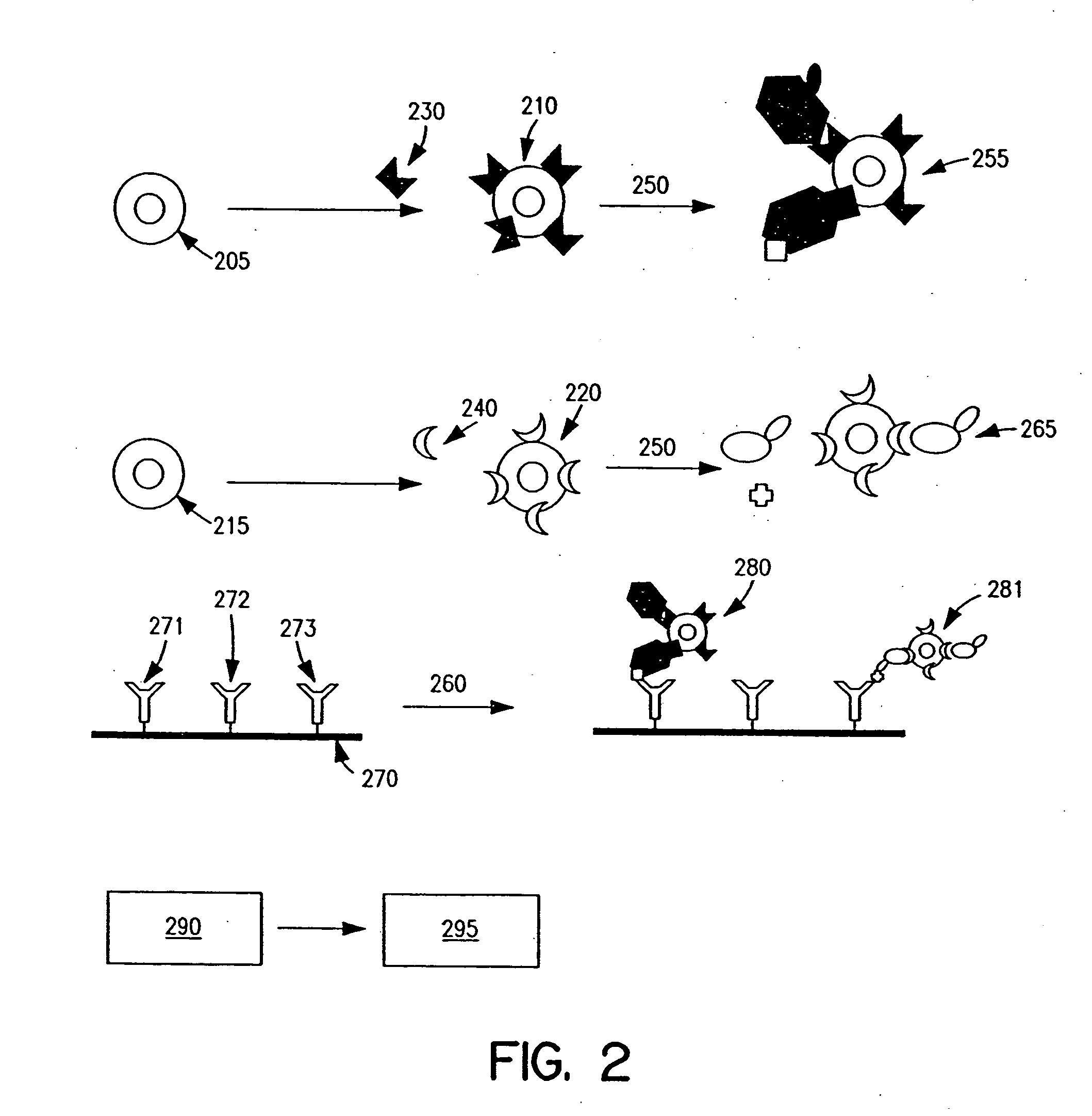
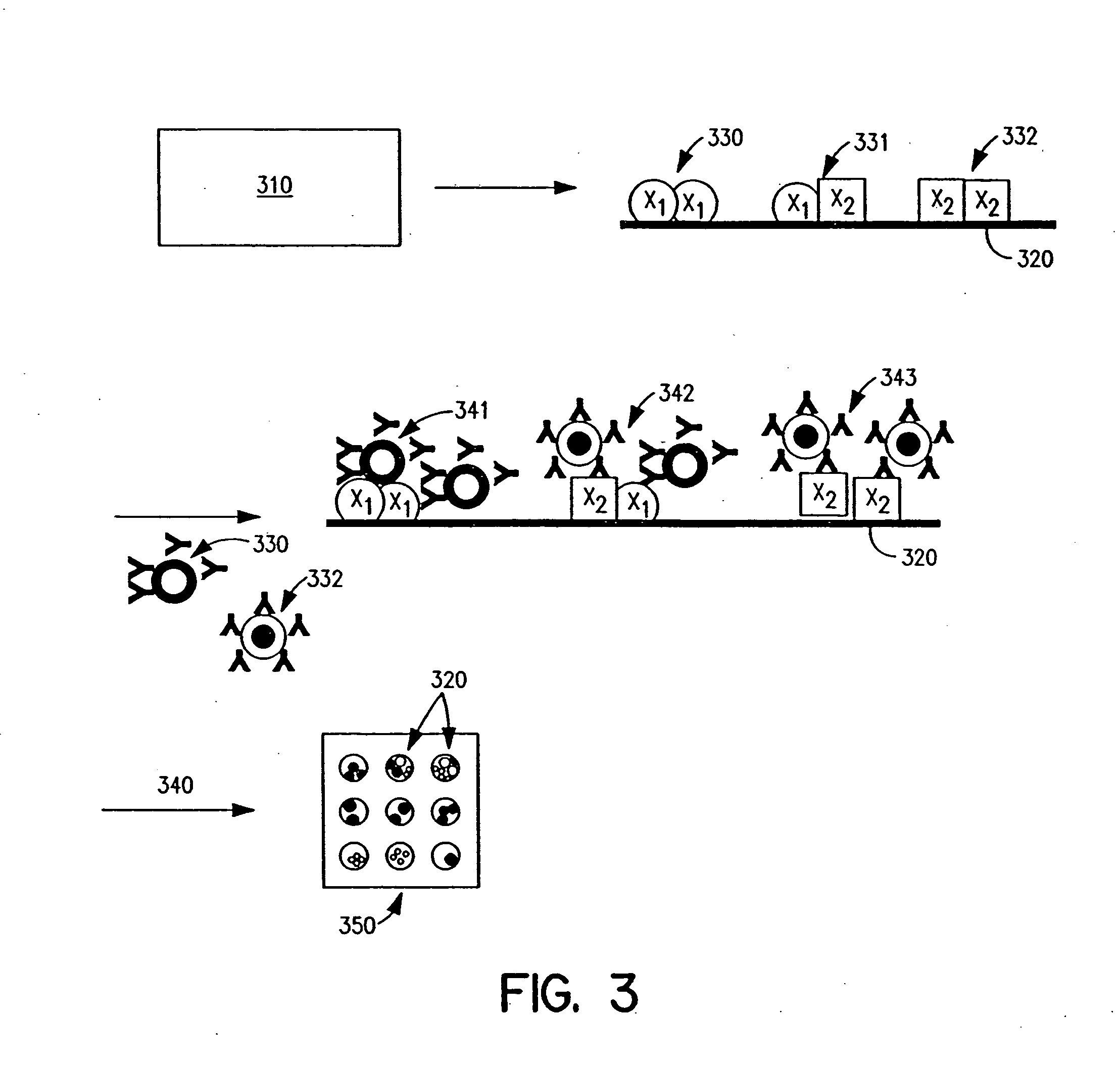
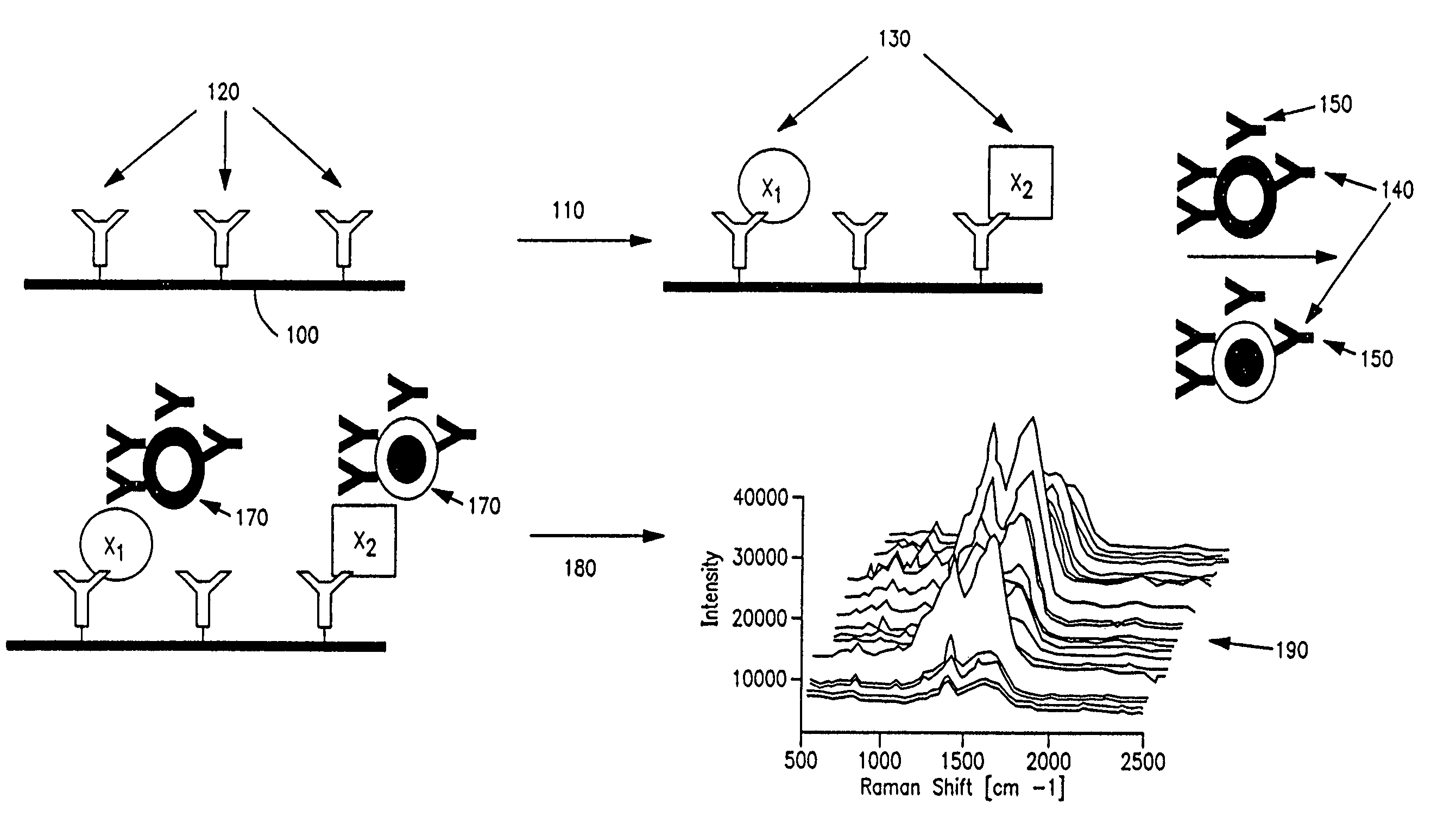
Methods and apparatus are provided for assaying cell samples, which may be living cells, using probes labeled with composite organic-inorganic nanoparticles (COINs) and microspheres with COINs embedded within a polymer matrix to which the probe moiety is attached. COINs intrinsically produce SERS signals upon laser irradiation, making COIN-labeled probes particularly suitable in a variety of methods for assaying cells, including biological molecules that may be contained on or within cells, most of which are not inherently Raman-active. The invention provides variations of the sandwich immunoassay employing both specific and degenerate binding, methods for reverse phase assay of tissue samples and cell microstructures, in solution displacement and competition assays, and the like. Systems and chips useful for practicing the invention assays are also provided.
Owner:INTEL CORP
Cellular analysis using Raman surface scanning
Methods and apparatus are provided for assaying cell samples, which may be living cells, using probes labeled with composite organic-inorganic nanoparticles (COINs) and microspheres with COINs embedded within a polymer matrix to which the probe moiety is attached. COINs intrinsically produce SERS signals upon laser irradiation, making COIN-labeled probes particularly suitable in a variety of methods for assaying cells, including biological molecules that may be contained on or within cells, most of which are not inherently Raman-active. The invention provides variations of the sandwich immunoassay employing both specific and degenerate binding, methods for reverse phase assay of tissue samples and cell microstructures, in solution displacement and competition assays, and the like. Systems and chips useful for practicing the invention assays are also provided.
Owner:INTEL CORP
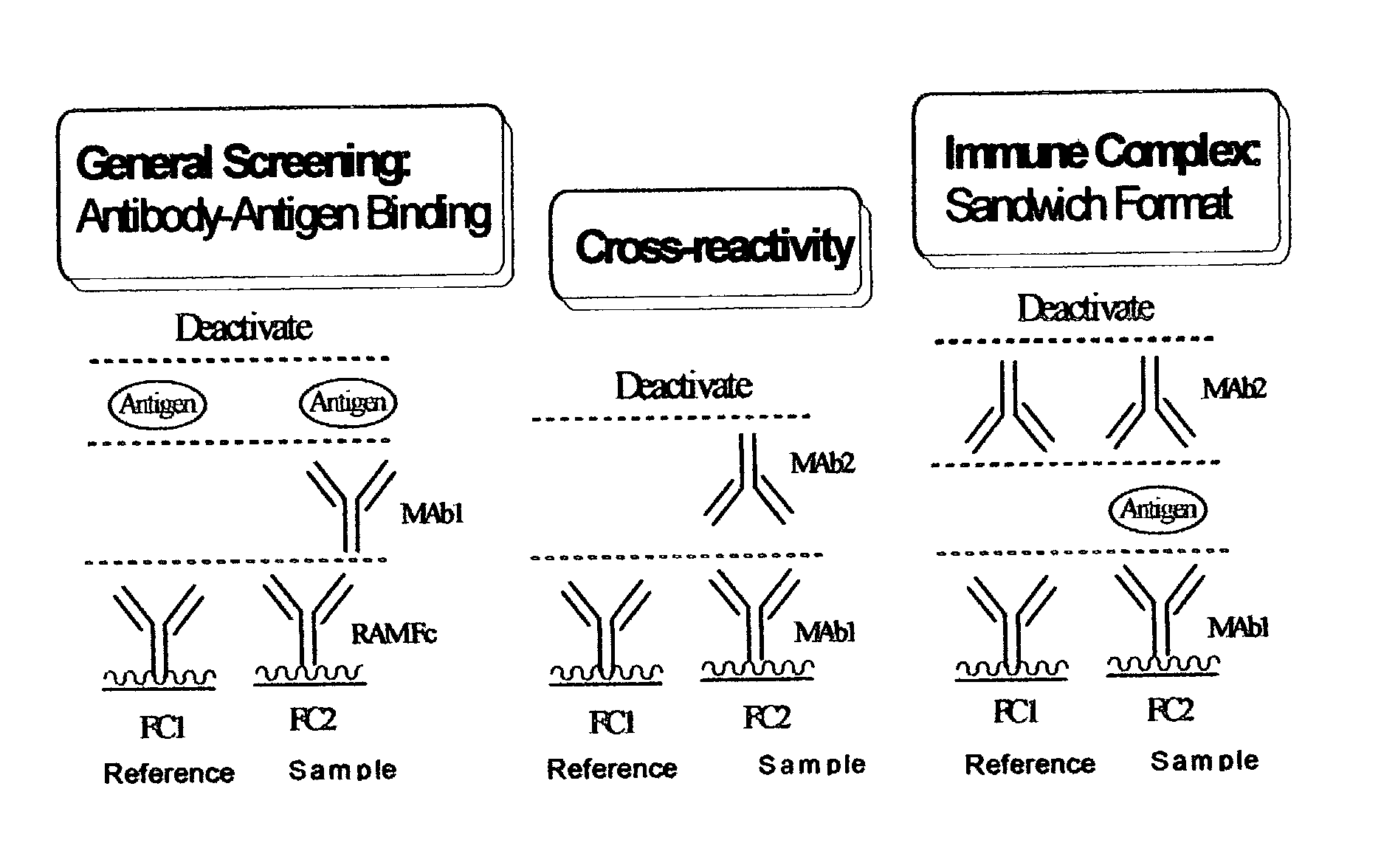
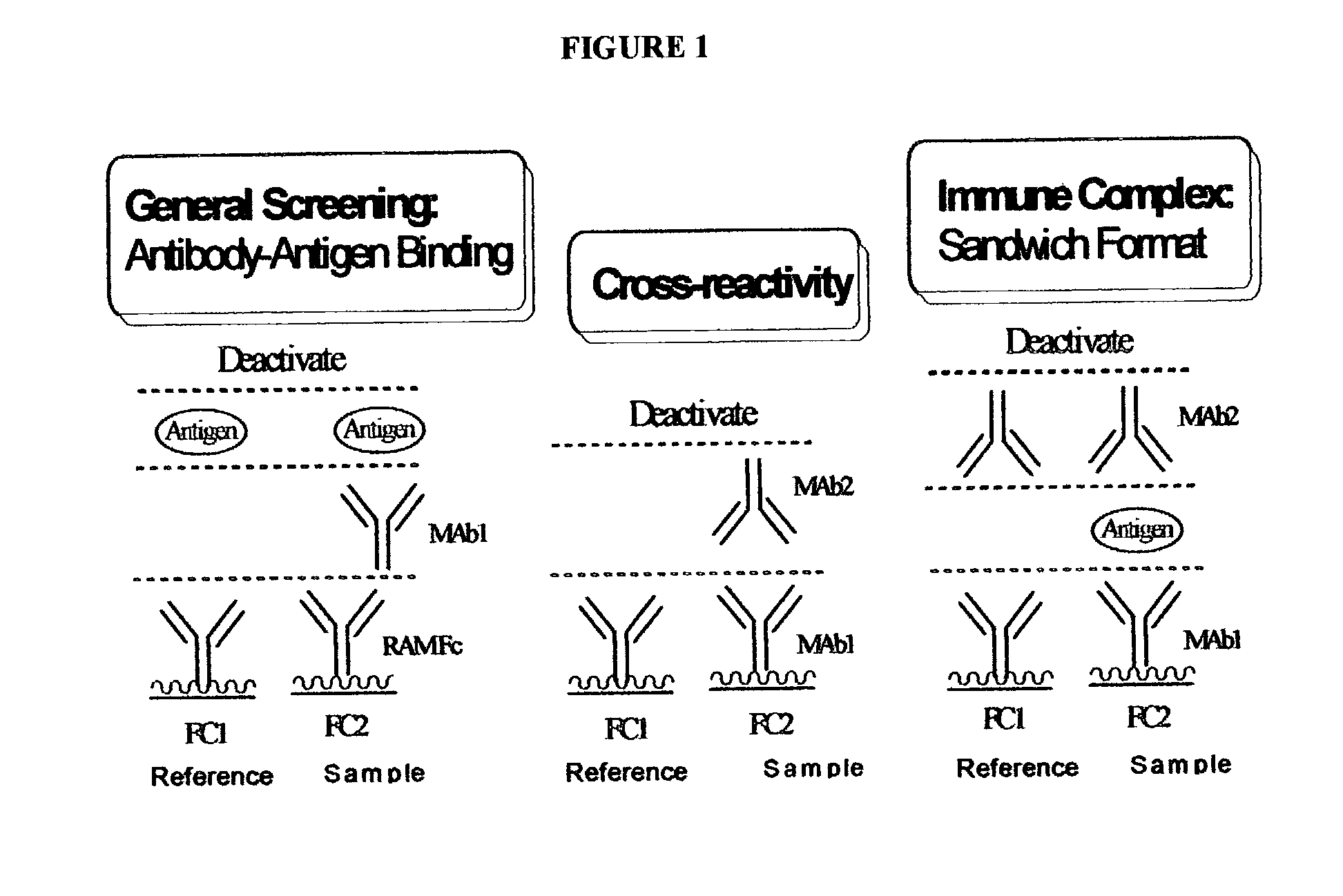
Antibody pair screening methods
The invention provides methods for identifying antibody preparations that can form a pair of antibodies that optimally detect a target antigen, for example, in a sandwich immunoassay. These methods provide high affinity and epitope-specific antibodies.
Owner:KIMBERLY-CLARK WORLDWIDE INC
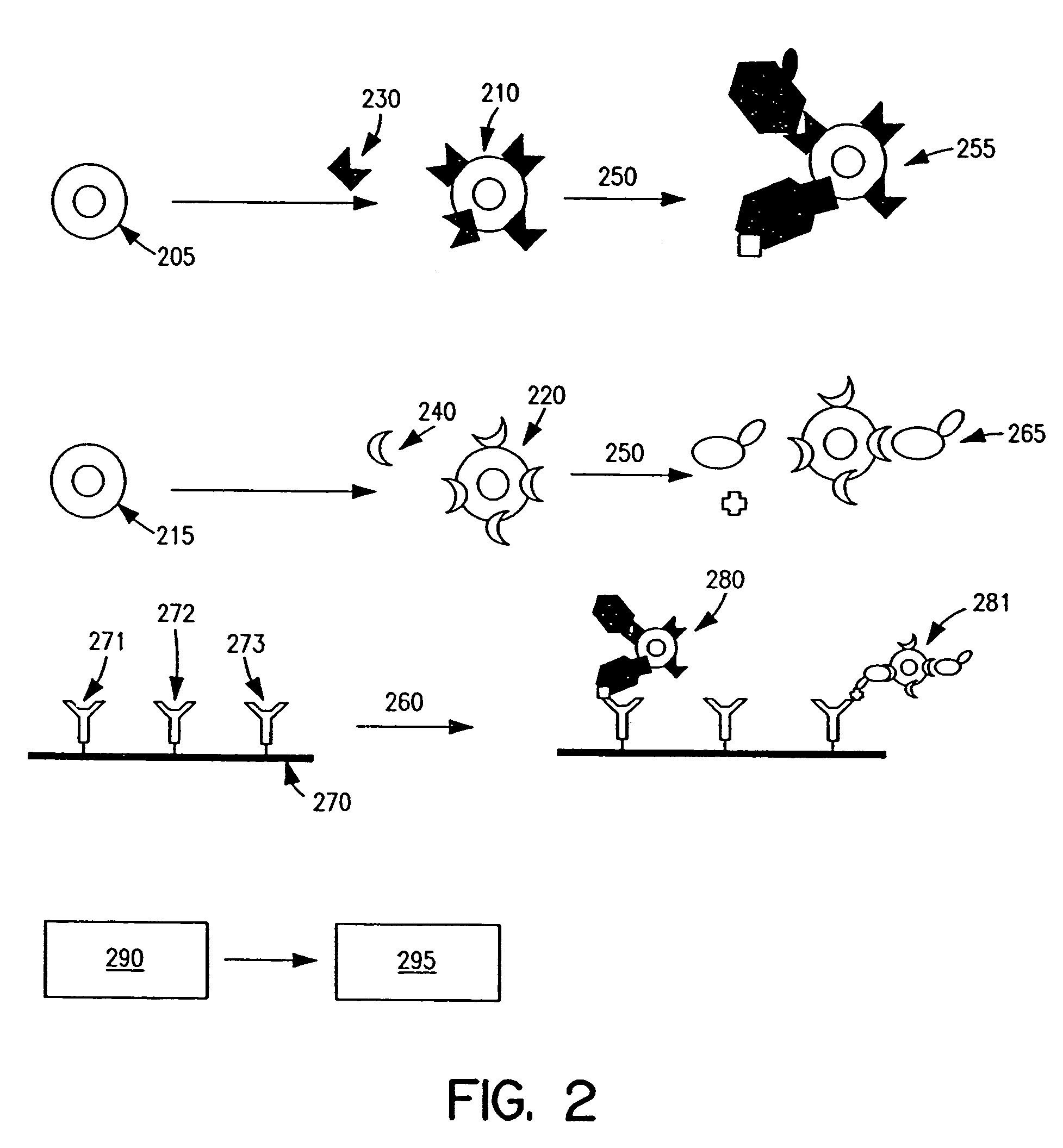
Biomolecule analysis using Raman surface scanning
Methods and apparatus are provided herein for assaying biological samples using probes labeled with composite organic-inorganic nanoparticles (COINs) and microspheres with COINs embedded within a polymer matrix to which the probe moiety is attached. COINs are Raman-active nanoparticles made up of aggregated primary metal crystal particles with Raman-active organic compounds adsorbed on the surface in the junctions of aggregated primary metal crystal particles or embedded in the crystal lattice of the primary metal particles. Since COINs intrinsically produce SERS signals upon laser irradiation, COIN-labeled probes are particularly suitable in a variety of methods for assaying biological molecules, most of which are not inherently Raman-active. The invention provides variations of the sandwich immunoassay employing both specific and degenerate binding, methods for reverse phase assay of tissue samples and cell microstructures, in solution displacement and competition assays, and the like. Kits and chips useful for practicing the invention assays are also provided.
Owner:INTEL CORP
Device and method for integrated diagnostics with multiple independent flow paths
InactiveUS20030040021A1Avoid flowHigh sensitivityBioreactor/fermenter combinationsBiological substance pretreatmentsWater insolubleHydrophobic polymer
Devices and methods for performing assays to determine the presence or quantity of a specific analyte of interest in a fluid sample. In devices according to this invention two separate flow paths are established sequentially in the device with a single user activation step. The first flow path delivers the analyte of interest (if present in the sample) and conjugate soluble binding reagents to the solid phase. If analyte is present, an analyte:conjugate complex is formed and immobilized. The volume of sample delivered by this first path is determined by the absorbent capacity of the solid phase, and not by the amount of sample added to the device, relieving the user from the necessity of measuring the sample. The sample / conjugate mixture is prevented from entering the second flow path because the capillarity and the surface energy of the second flow path prevent it from being wetted by this mixture. The second flow path allows a wash reagent to remove unbound conjugate and sample from the solid phase to the absorbant, and optionally to deliver detection reagents. The invention may be adapted to many assay formats including, sandwich immunoassays, colloidal gold, or sol particle assays, heterogeneous generic capture assays and competitive assays. In one embodiment, sandwich assays can be performed by immobilizing an analyte binding reagent on the solid phase, and drying a labeled analyte binding reagent in the first flow path. In a competitive assay embodiment, the first flow path would contain labeled analyte that is dissolved by the sample, and the analyte binding reagent is immobilized on the solid phase. In each of these embodiments, the assay can be further modified to run in a "generic capture" format, where the solid phase binding reagent is instead conjugated to a generic ligand such as biotin, and dried in the first flow path (either together or separately from the other assay reagents), and a generic ligand binding reagent (such as avidin) is immobilized on the solid phase. Another aspect of the present invention includes a subassembly for the immunoassay device that is comprised of a plastic housing and a means for delivering fluid and / or wash solution. This subassembly comprises a structure formed from a hydrophobic polymer selectively treated with a water insoluble surface active agent that has been applied as a solution in an organic solvent rendering portions of the surface hydrophilic. When the surface is contacted with an aqueous liquid, it flows only along the treated areas, creating a defined fluid flow path, thereby delivering sample / conjugate solutions to said solid phase.
Owner:CLARK SCOTT M +4
Electrochemical immunosensor for detecting toxoplasma gondii IgM antibody and preparation method thereof
InactiveCN102914648AImprove conductivityEasy to passMaterial electrochemical variablesMedical diagnosisElectronics
The invention belongs to the technical field of analytical chemistry and chemical sensors and discloses an electrochemical immunosensor for detecting a toxoplasma gondii IgM (Immunoglobulin m) antibody (Tg-IgM) of a gravida and a preparation method of the electrochemical immunosensor. The immunosensor is prepared by sequentially modifying graphene, polythionine, gold nanoparticles and capture antigen to the surface of a glassy carbon electrode. An enzyme-functionalized nano-composite detection probe with an electrical signal amplifying function is prepared by assembling enzyme and a second antibody with high proportions on an Au-Fe3O4 surface. According to the sandwich immunoassay principle, the concentration of Tg-IgM is determined by using an electrochemical signal generated by catalysis of enzyme to a substrate. According to the electrochemical immunosensor, the specificity of immunoreaction is combined with the sensitivity of electrochemical detection; the transmission of electronics is promoted by using the graphene, the polythionine, the gold nanoparticles, Au-Fe3O4 and other material; and the sensitivity of the detection is improved. The electrochemical immunosensor has the advantages of simplicity and convenience for operation, favorable regeneration performance and detection cost reduction. The electrochemical immunosensor prepared on the basis can be also used for detecting other immunological markers and has favorable application prospect in medical diagnosis.
Owner:CHONGQING MEDICAL UNIVERSITY
Disposable multi-channel electrochemical immunosensor with high sensitivity
InactiveCN102053161AEasy to operateHigh detection throughputBiological testingElectronic transmissionCarbon nanotube
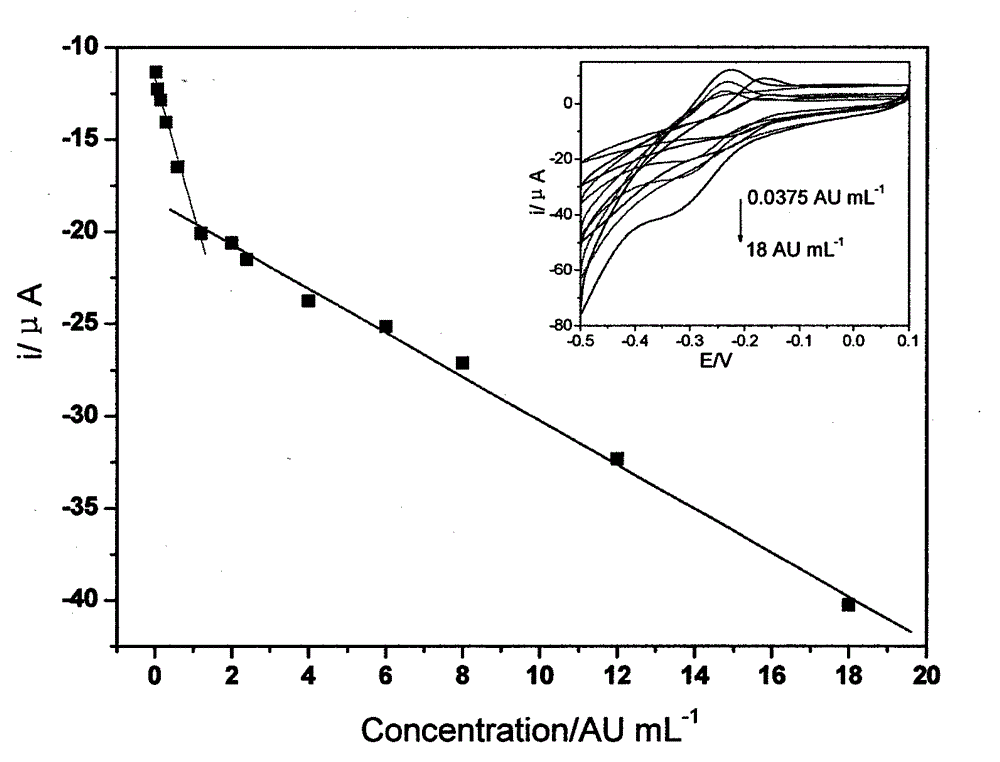
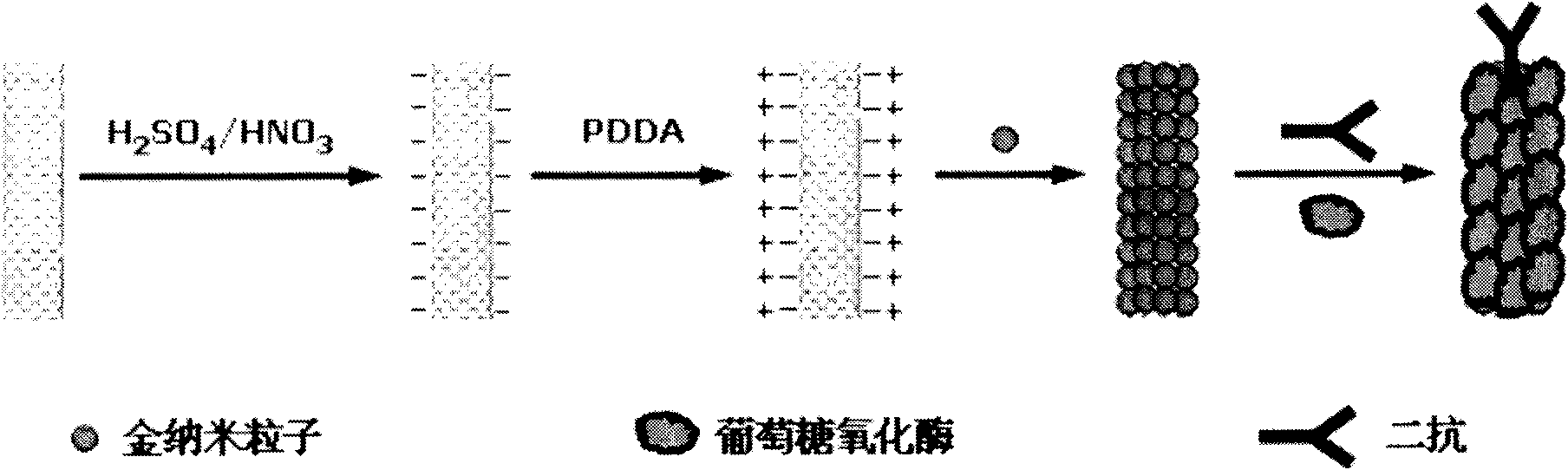
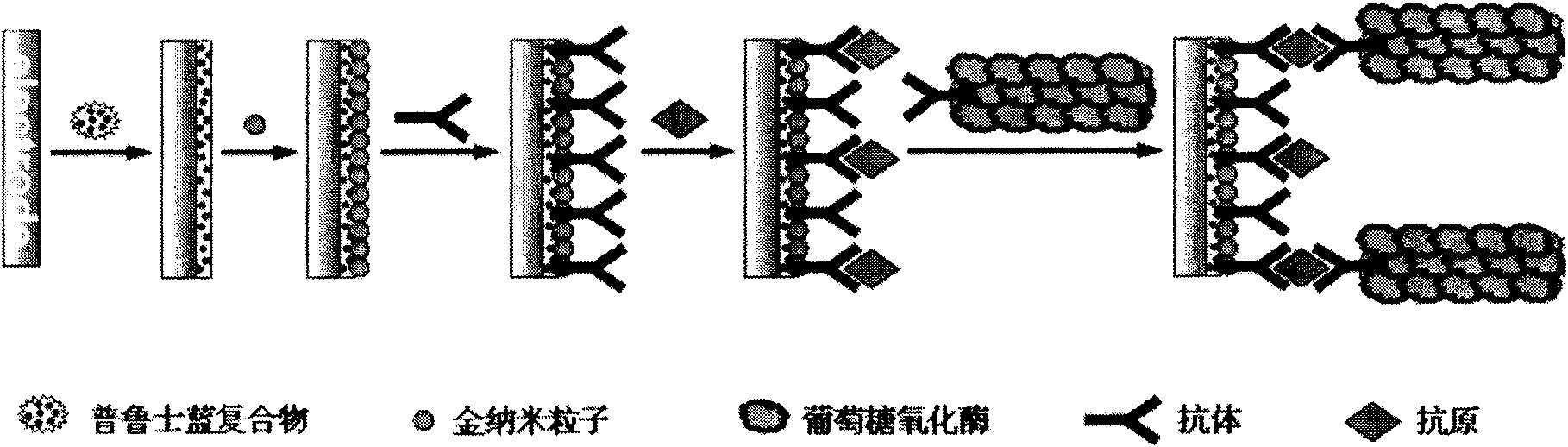
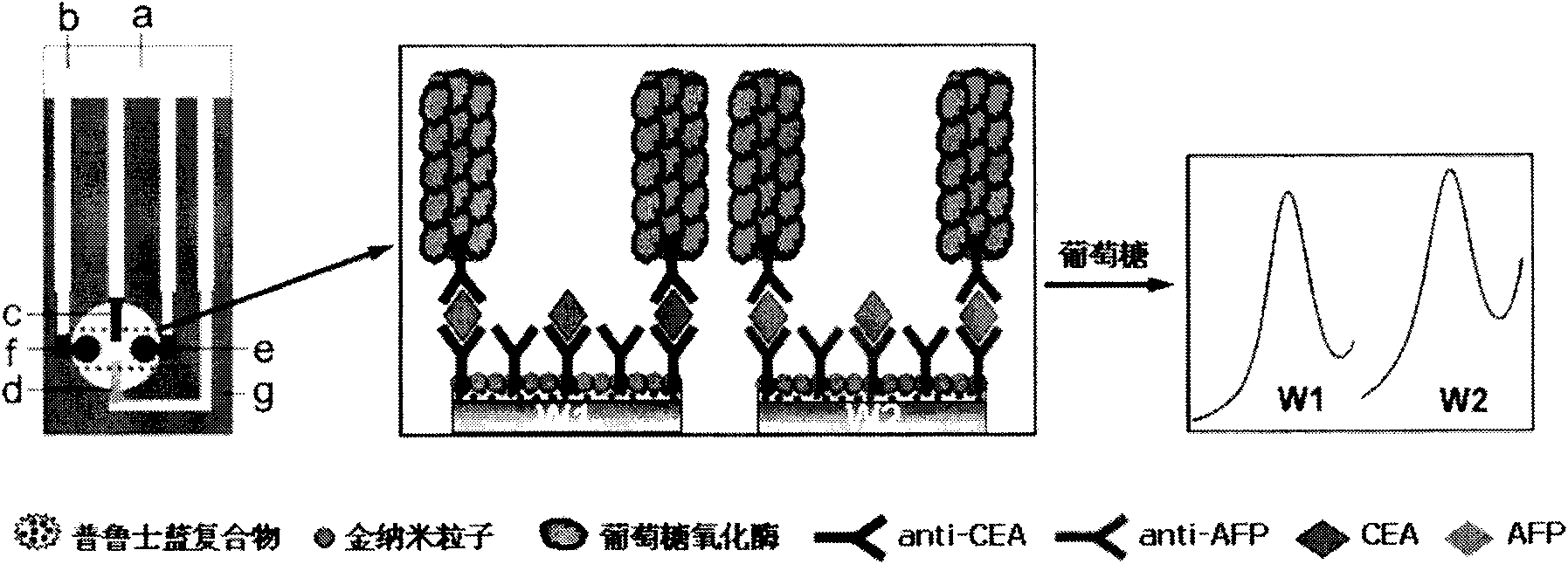
The invention relates to a disposable multi-channel electrochemical immunosensor with high sensitivity. A prussian blue composite, nanogold and a capture antibody are assembled layer by layer on a disposable printing electrode array to obtain a multi-channel immunosensor. Enzyme and secondary antibody in great proportion are assembled on a carbon nano tube loading gold nanoparticles and a novel glucose oxidase functional nano composite probe is designed for sandwich immunoassay. The nano composite probe is combined with the multi-channel immunosensor to realize double signal amplification and high sensitive immunodetection of protein. The prussian blue, as electronic transmission media, catalyzes and reduces hydrogen peroxide generated by glucose oxidation under the catalysis of glucose oxidase on oxygen so as to obtain current signal. The method avoids the interference of dissolved oxygen in the detection solution and deoxidization is not required in process of amperometric detection. The invention has the advantages of wide detection concentration range, good repeatability, accurate results and the like and has a certain clinical application value.
Owner:NANJING UNIV +2
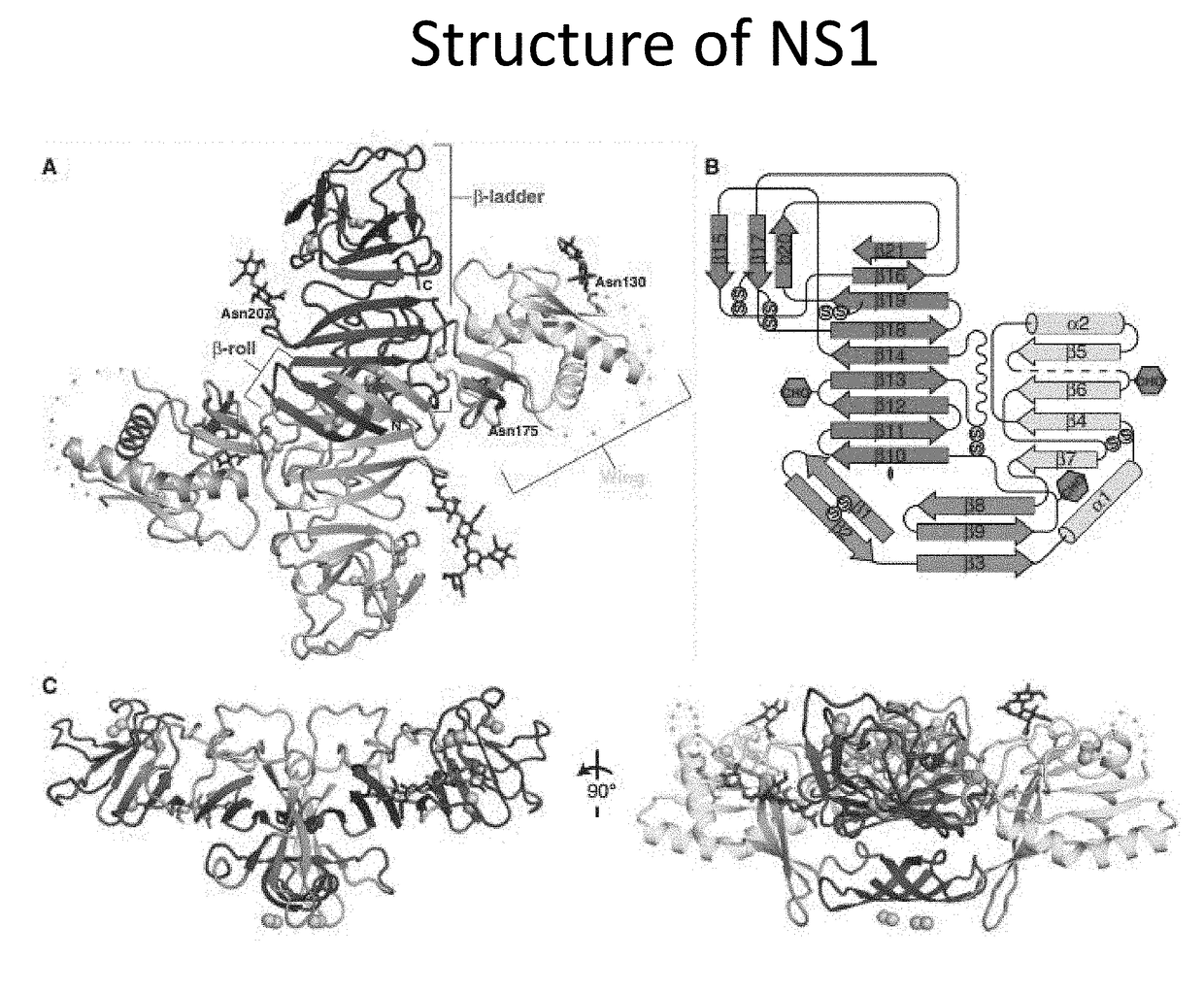
Anti-Dengue Virus NS1 Protein Monoclonal Antibodies
ActiveUS20170233460A1Improve developmentUseful in therapyImmunoglobulins against virusesAntibody ingredientsProtein.monoclonalSpecific detection
The present invention provides matched antibody pairs for the specific detection of one or more of the four dengue virus serotypes in a biological sample that may contain one or more of such dengue virus serotypes. Each matched antibody pair is capable of detecting not more than one serotype of dengue virus NS1 protein that may be present in the sample and will not cross react with other serotypes that may be present in the sample. Multiple matched pairs may be used to detect one or more dengue virus serotypes that may be present in a sample. Such matched pair antibodies, facilitate the development of confirmatory in vitro diagnostic tests such as sandwich immunoassays, that detect and distinguish the presence of one or more dengue virus serotypes in a biological sample, preferably a sample derived from human subject. The invention also provides kits comprising the matched antibody pairs of the invention and methods for using the kits for immunoassays for the specific detection of one or more serotypes of dengue virus in a patient population. The present invention also provides monoclonal antibodies specific for the NS1 protein of dengue virus and therapeutic compositions and methods for treating dengue virus infection.
Owner:THE FOOD & DRUG ADMINISTATION +1
Use of cytokines secreted by dendritic cells
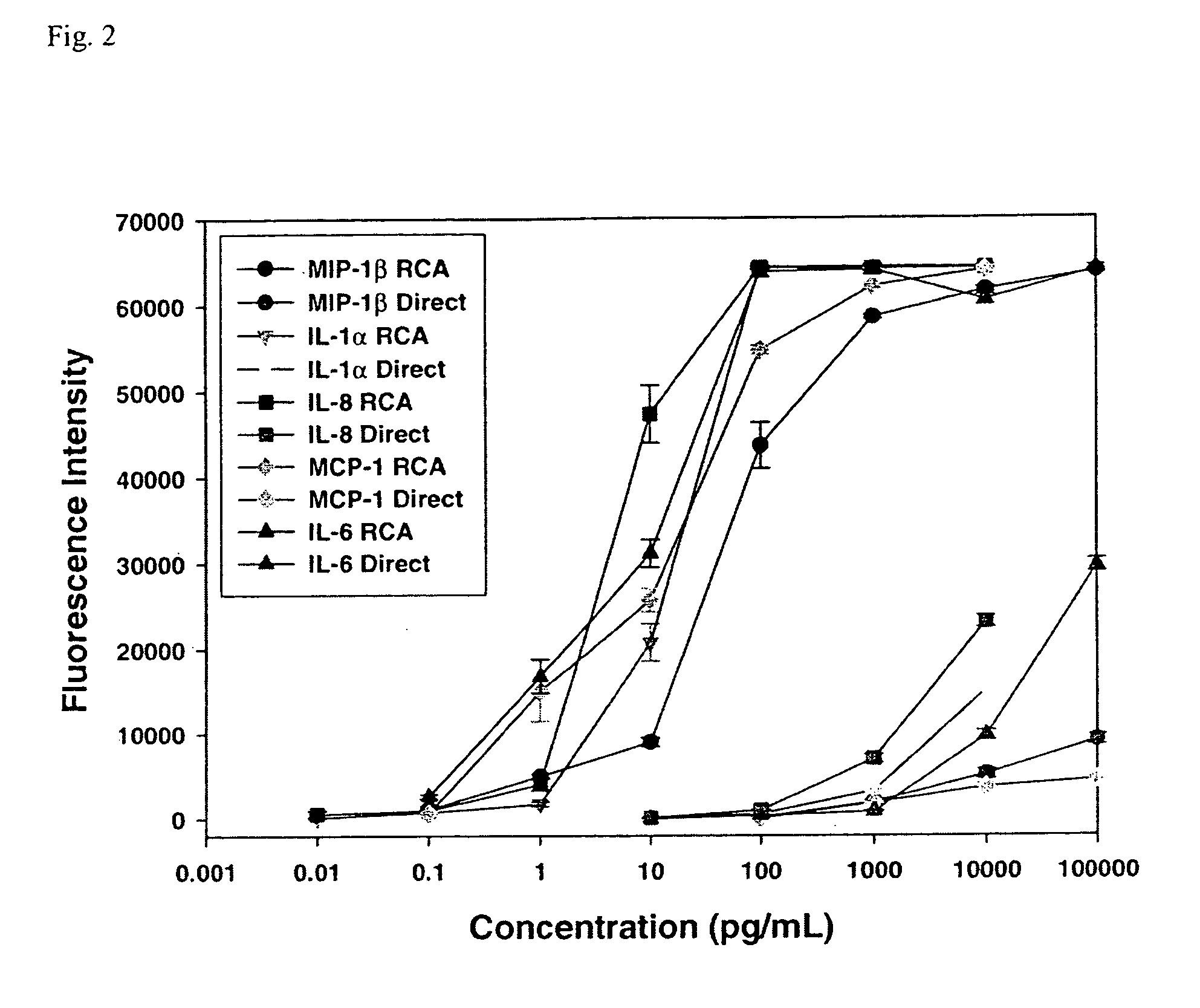
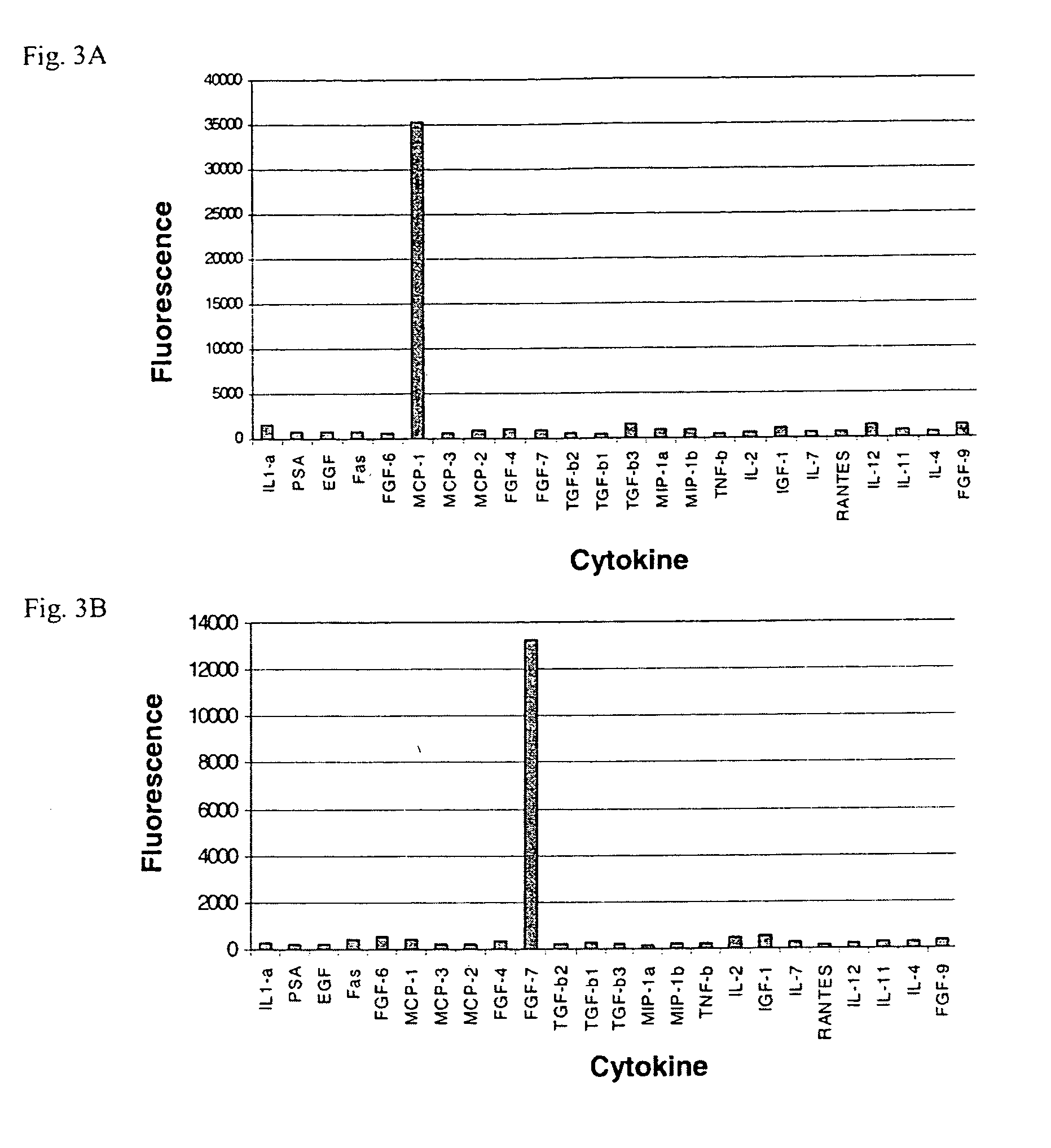
A prerequisite of proteomics is the ability to quantify many selected proteins simultaneously. Fluorescent sandwich immunoassays on microarrays hold appeal for such studies, since equipment and antibodies are readily available, and assays are simple, scalable and reproducible. To attain adequate sensitivity and specificity, however, a general method of immunoassay amplification is required. Coupling of isothermal rolling circle amplification (RCA) to universal antibodies can be used for this purpose: RCA on a synthetic DNA circle is initiated by a complementary oligonucleotide attached to an anti-biotin antibody; single-stranded RCA product remains attached to the antibody, and is detected by hybridization of complementary, fluorescent oligonucleotides. 51 cytokines were measured simultaneously on microarrays with signal amplification by RCA with high specificity, femtomolar sensitivity and 4 log quantitative range. This cytokine microarray was used to measure secretion from human Dendritic cells (DCs) induced by lipopolysaccharide (LPS) or tumor necrosis factor-alpha (TNF-(alpha). Rapid secretion of inflammatory cytokines such as MIP-1beta, IL-8, and IP-10 was induced by LPS. Eotaxin-2 and I-309 were found to be induced by LPS, and MDC, TARC, sIL-6R, and sTNF-RI were found to be induced by TNF-alpha. Since microarrays can accommodate ~1000 sandwich immunoassays of this type, a relatively small number of RCA microarrays appears to offer a tractable approach for proteomic surveys.
Owner:MOLECULAR STAGING
Method for determining contents of biological marker and DNA through direct-reading portable glucometer
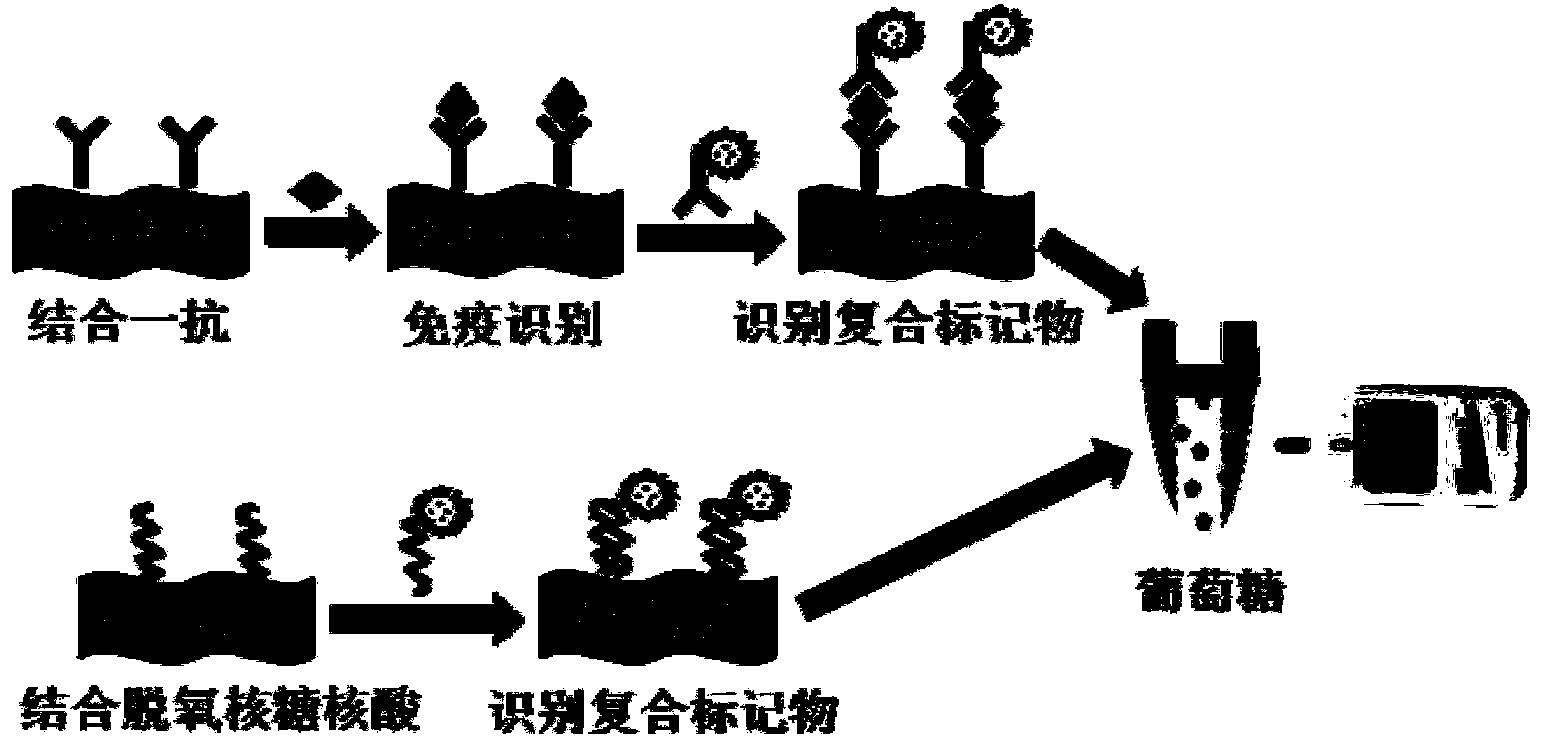
A method for determining the contents of a biological marker and DNA through a direct-reading portable glucometer is characterized in that a glucose molecule embedded carrier liposome, hollow metal ball, porous carbon ball, porous silicon ball or polymer is introduced into the surface of a sensor as a composite marker through a sandwich immunization reaction or a DNA reaction, so the detection of the biological marker or DNA is converted into the detection of a final product glucose, the contents of the biological marker and DNA for detection are in direct proportion to the content of the embedded glucose according to the sandwich immunization analysis principle, and the contents of the biological marker and DNA can be obtained by detecting the concentration of glucose through the glucometer. The glucometer is developed into a portable and universal biological marker and DNA tester, and the glucometer detection technology based biological marker and DNA analysis new method is constructed, and can be used for the real-time, online, simple and sensitive determination of various biological markers and DNA. An apparatus used in the method is simple, so the analysis cost is low.
Owner:HUAZHONG NORMAL UNIV
Immunoassay
ActiveUS7348157B2Accurate detectionSamplingChemiluminescene/bioluminescenceCapture antibodySandwich immunoassay
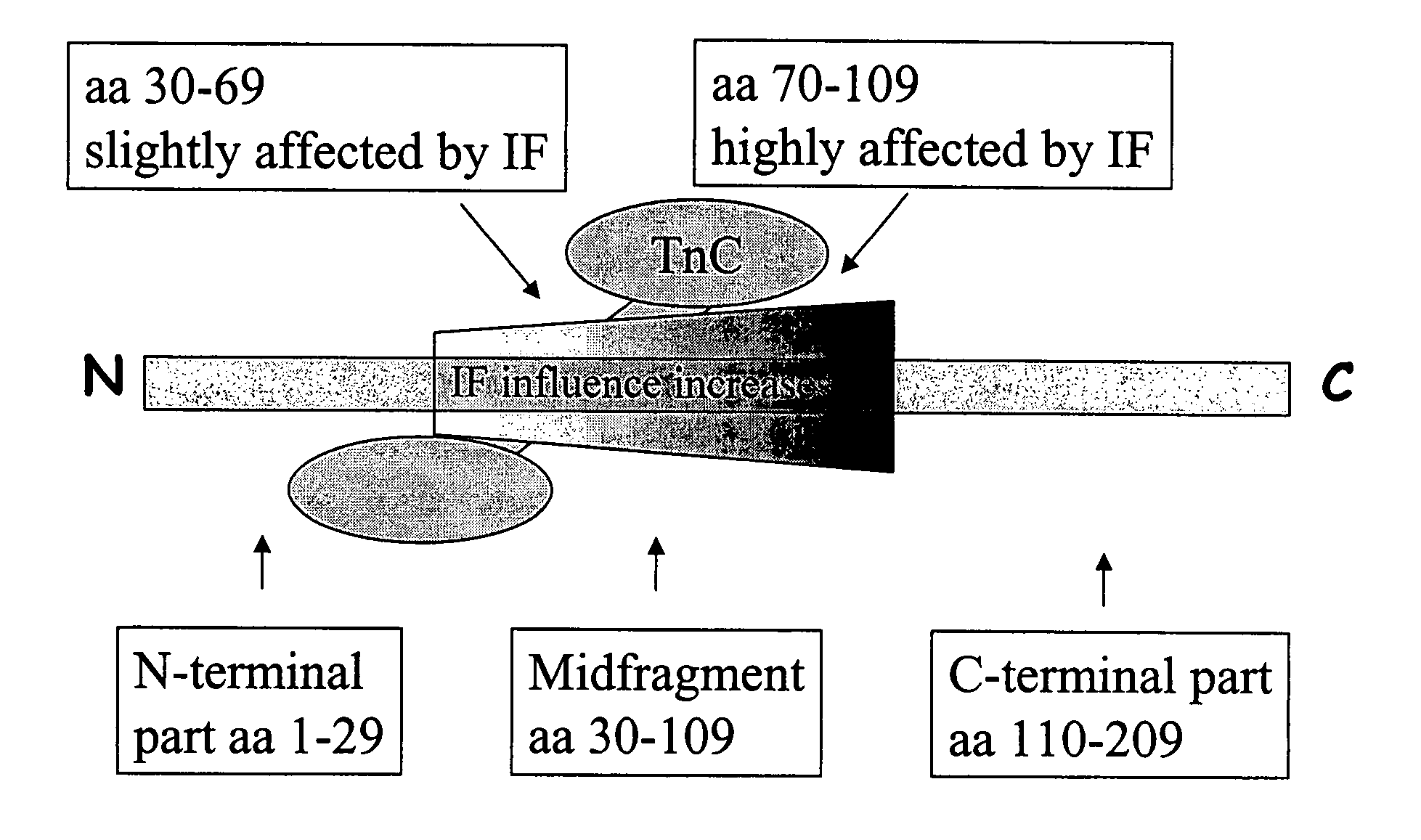
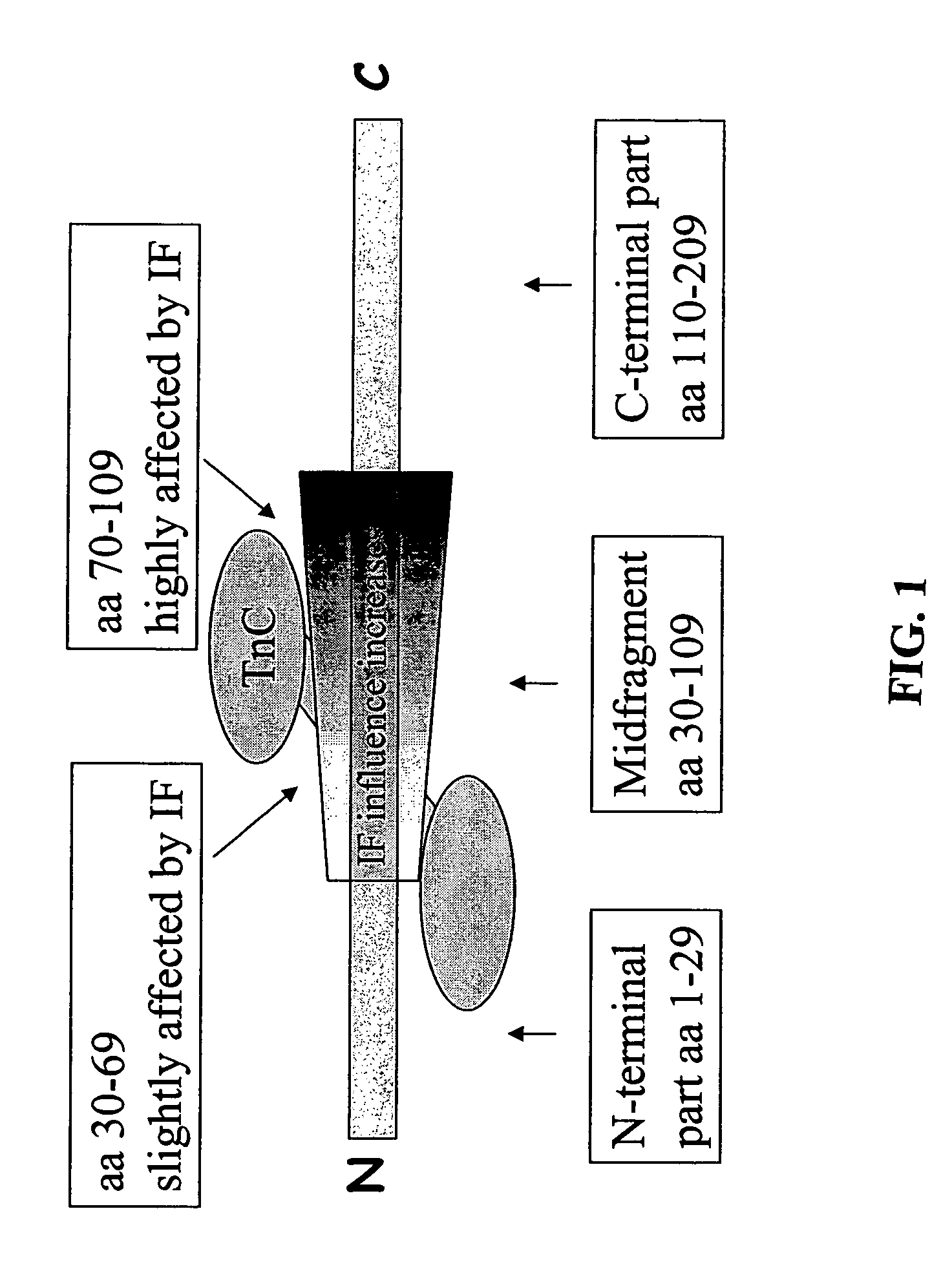
A method for detection and / or quantification of cardiac troponin I (cTnI) in a sample derived from an individual's blood, the method being based on a sandwich immunoassay employing at least two capture antibodies and at least one tracer antibody, in which a first capture antibody is directed to the N-terminal part of cTnI, to the C-terminal part of cTnI or to the part of the cTnI midfragment, which is slightly affected by the interfering factor, and a second capture antibody is directed to another of these parts, and a tracer antibody is directed to the N-terminal part of cTnI, to the C-terminal part of cTnI or to TnC, which is complexed with cTnI.
Owner:TURUN YLIOPISTO
High sensitive and jettisonable multicomponent chemiluminescent imaging immunosensor
InactiveCN103575896AAchieving Simultaneous High Sensitivity Image ImmunoassaysEasy to makeMaterial analysis by optical meansBiological testingSensor arrayPeroxidase
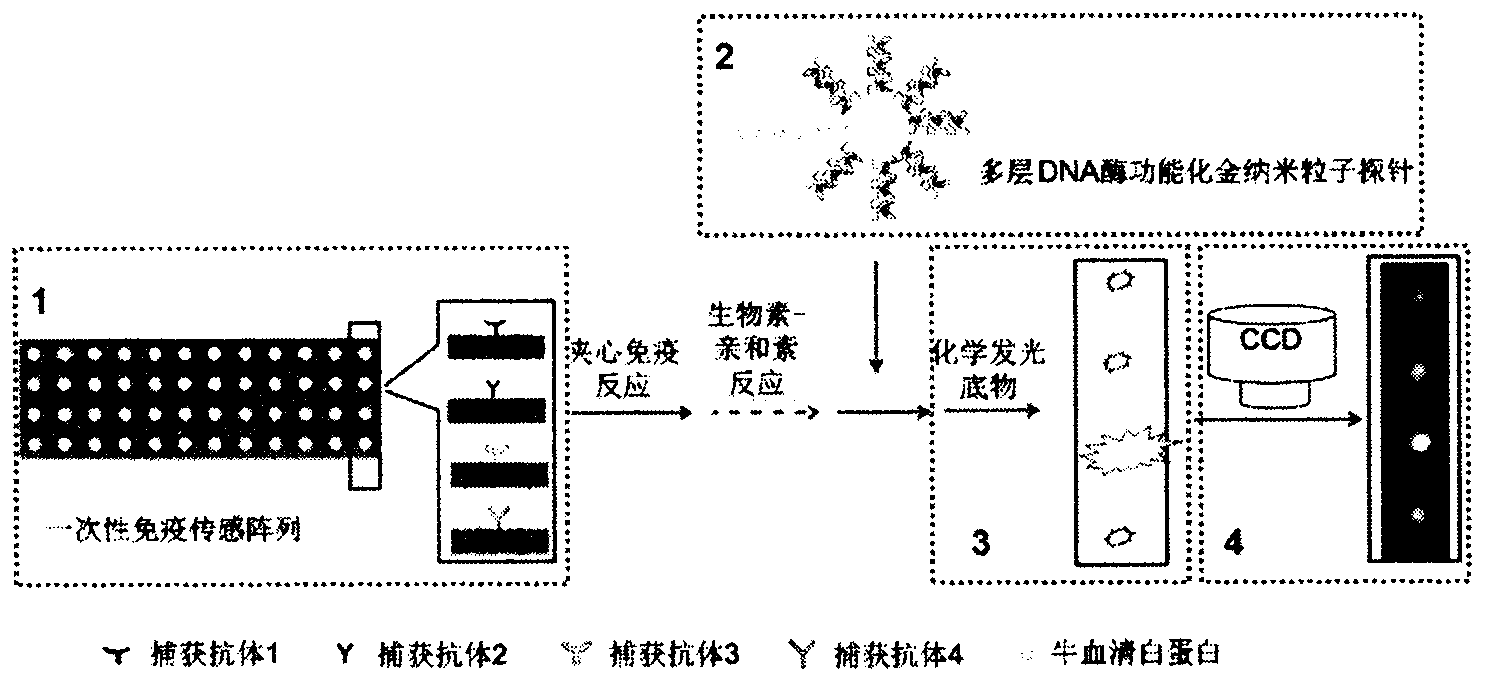
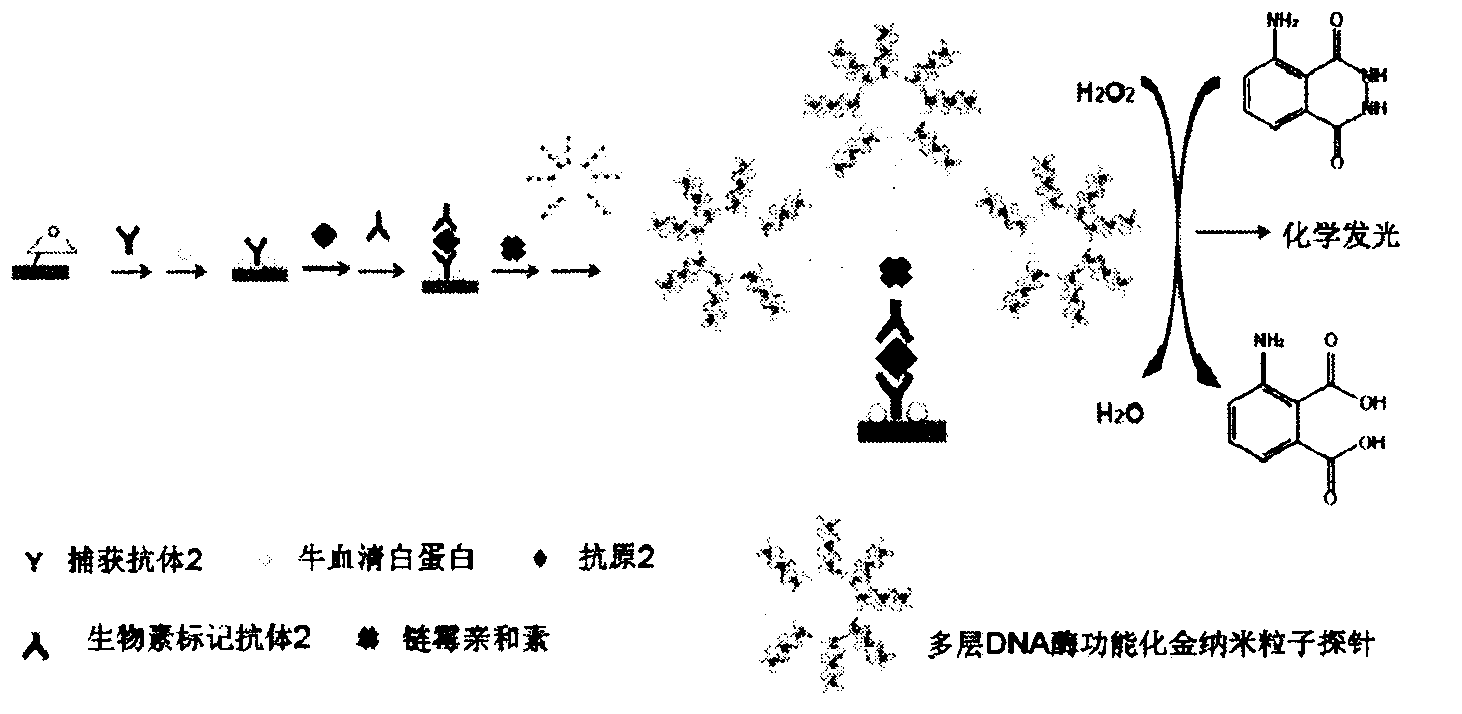
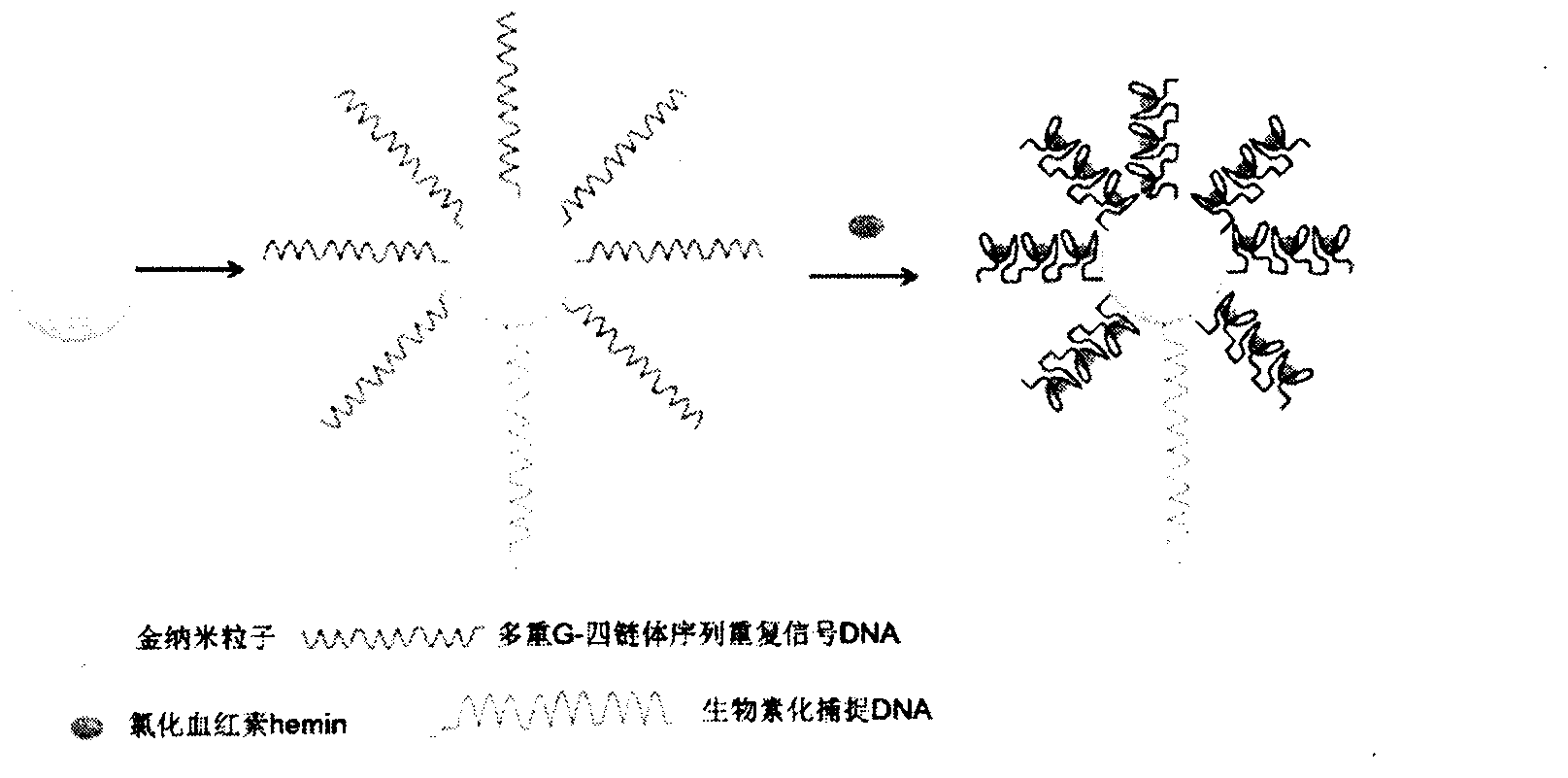
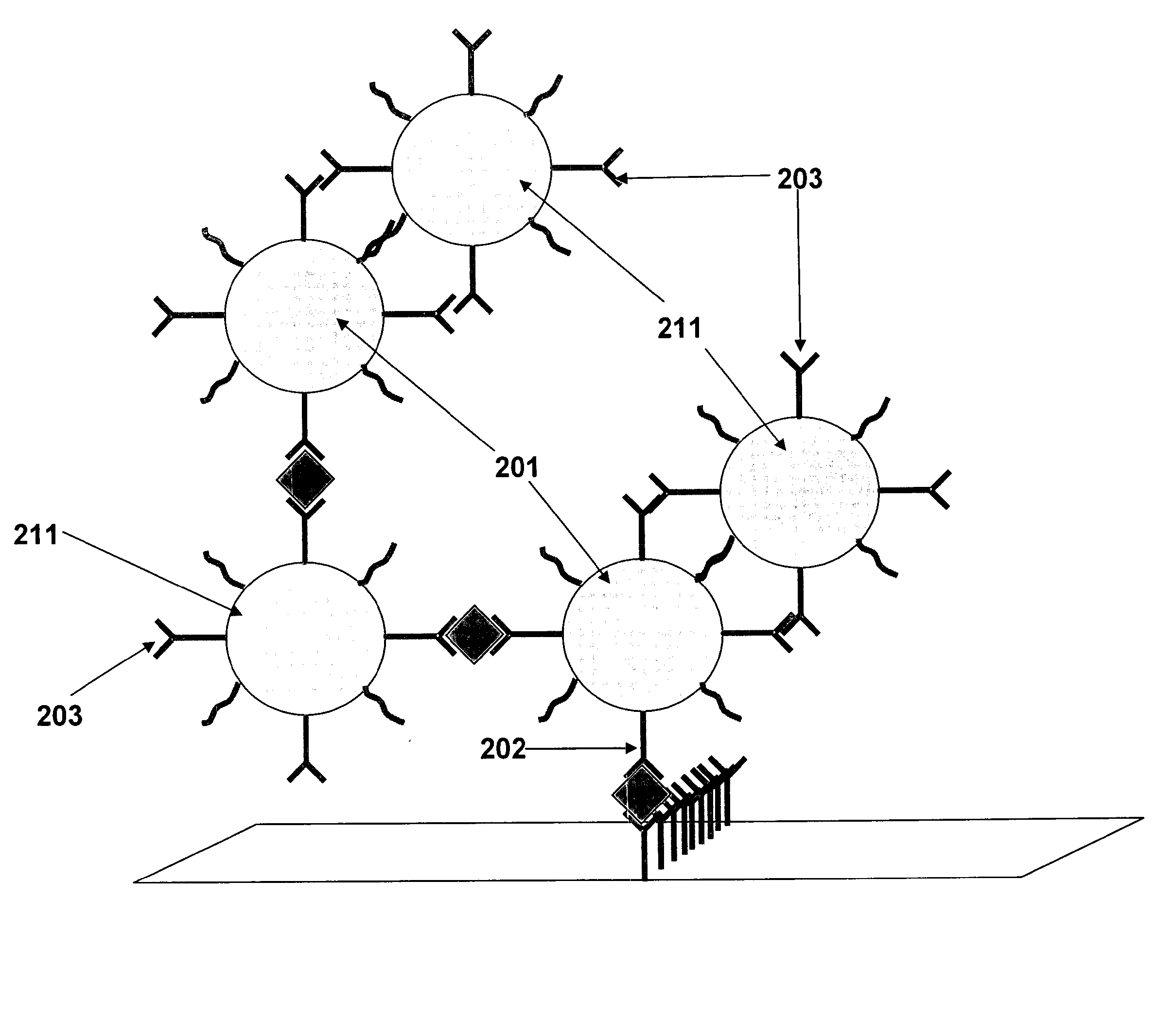
The invention relates to a high sensitive and jettisonable multicomponent chemiluminescent imaging immunosensor. The immunosensor is prepared by: constructing a 4*12 array on a silanized glass slide by a screen printing technique, and coating the array points with different capture antibodies to construct a jettisonable multicomponent immune sensor array; immobilizing biotinylated capture DNA and multiple G-quadruplex sequence repeat signal DNA on gold nanoparticle surfaces simultaneously, combining G-quadruplex signal DNA with heme to form DNA enzyme so as to prepare a multilayer DNA enzyme functionalized gold nanoparticle probe; and based on sandwich immunoassay, forming a sandwich immune complex on the sensor array, carrying out biotin-avidin reaction, labeling different immune complexes with the multilayer DNA enzyme functionalized gold nanoparticle probe; and making use of the peroxidase characteristics of DNA enzyme to catalyze reaction between chemiluminescent substrate H2O2-luminol to obtain a sensitive chemiluminescent signal, thus realizing high sensitive image immunoassay of a variety of protein. The immunosensor has the advantages of simple design, low cost, high sensitivity, high throughput and good repeatability, etc., and has certain clinical application value.
Owner:JIANGSU CANCER HOSPITAL +1
Rapid immunochromatographic detection by amplification of the colloidal gold signal
InactiveUS20100068727A1Improve rapid immunochromatographic detection of targetHigh sensitivityComponent separationPreparing sample for investigationColloidSpecific antibody
The present invention relates to a method for rapid immunochromatographic detection of a target in a sample by double sandwich immunoassay detection, wherein the target is an antibody and / or an antigen, using different colloidal gold conjugates conjugated with a first and a second specific antibody or antigen and with at least one oligonucleotide and its at least one complementary oligonucleotide and / or at least one non-specific antibody and its related non-specific antigen, to a rapid immunochromatographic detection device, to uses of the method for detecting diseases or specific conditions, and to a method for the manufacture of the device as well as to a kit which comprises the device.
Owner:ARAGEN BIOTECH
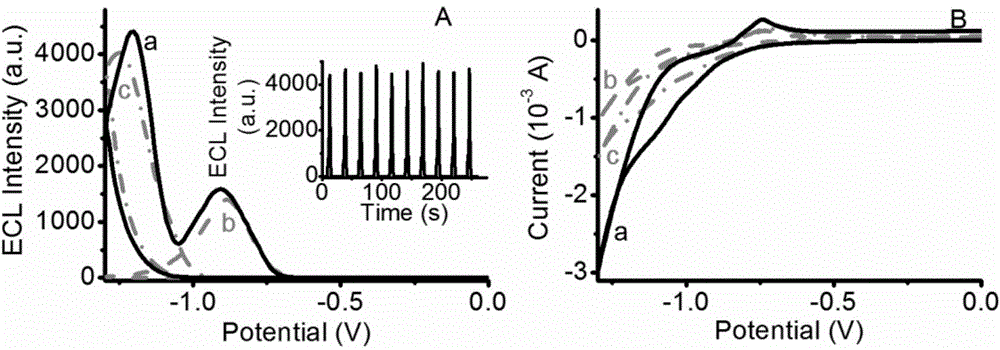
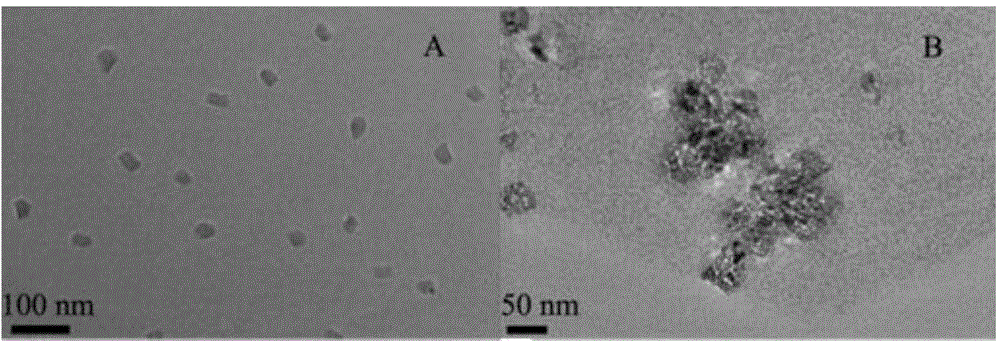
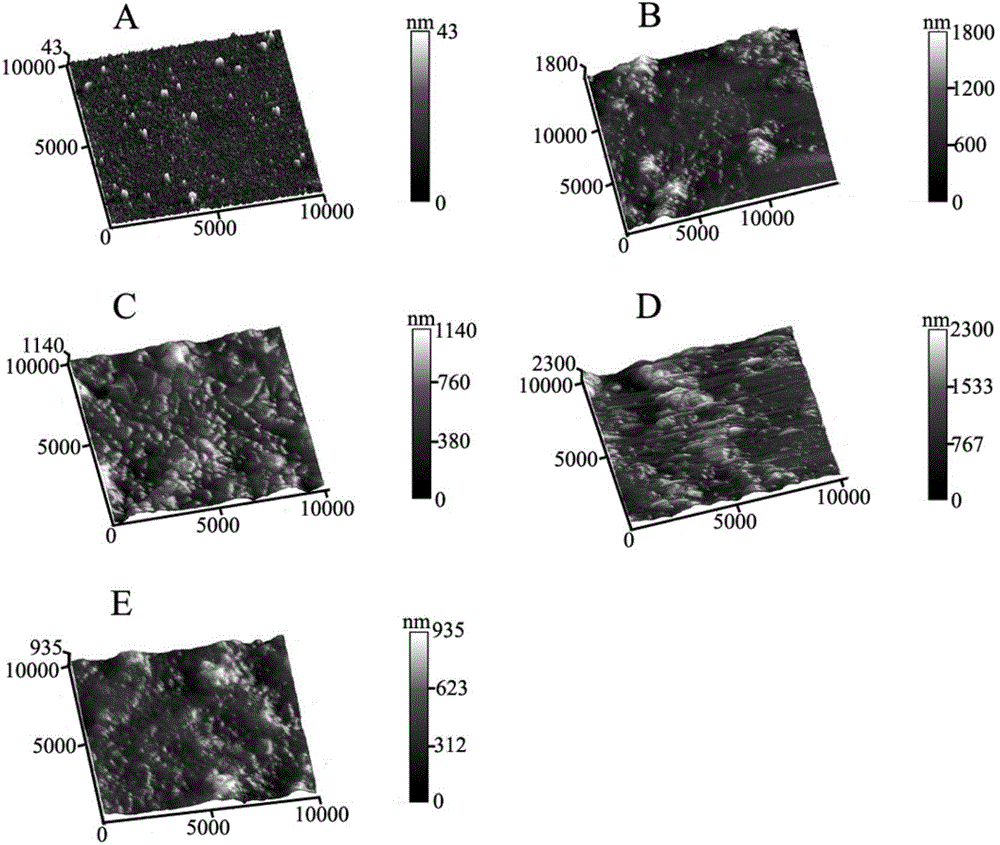
Electrochemical luminescence immune sensor built by mesoporous material loading quantum dots and coated by nanogold, and detection method of HIV (human immunodeficiency virus)
InactiveCN103048314AIncrease loadIncreased biocompatibilityChemiluminescene/bioluminescenceImmune complex depositionBiocompatibility Testing
The invention relates to the field of analysis tests, and discloses an electrochemical luminescence immune sensor built on the basis of a mesoporous material which loads quantum dots and is coated by nanogold, and a sandwich immunoassay method for detecting an HIV (human immunodeficiency virus) antibody. The electrochemical luminescence immune sensor takes the mesoporous material which loads the quantum dots and is coated by the nanogold as a signal label; and immune complex is formed by a biological immune method and decorated to the surface of an electrode, so as to form the electrochemical luminescence immune sensor. By adopting the mesoporous material which loads the quantum dots and is coated by the nanogold, a lot of quantum dots are loaded on the basis of the mesoporous material; a substance of a signal source is increased; the coated nanogold can be used for a method signal, and also can increase the biocompatibility; and the detection method is simple in sample pretreatment, and can adapt to high-sensitivity detection and analysis of the content of the HIV antibody in a serum sample.
Owner:NINGBO UNIV
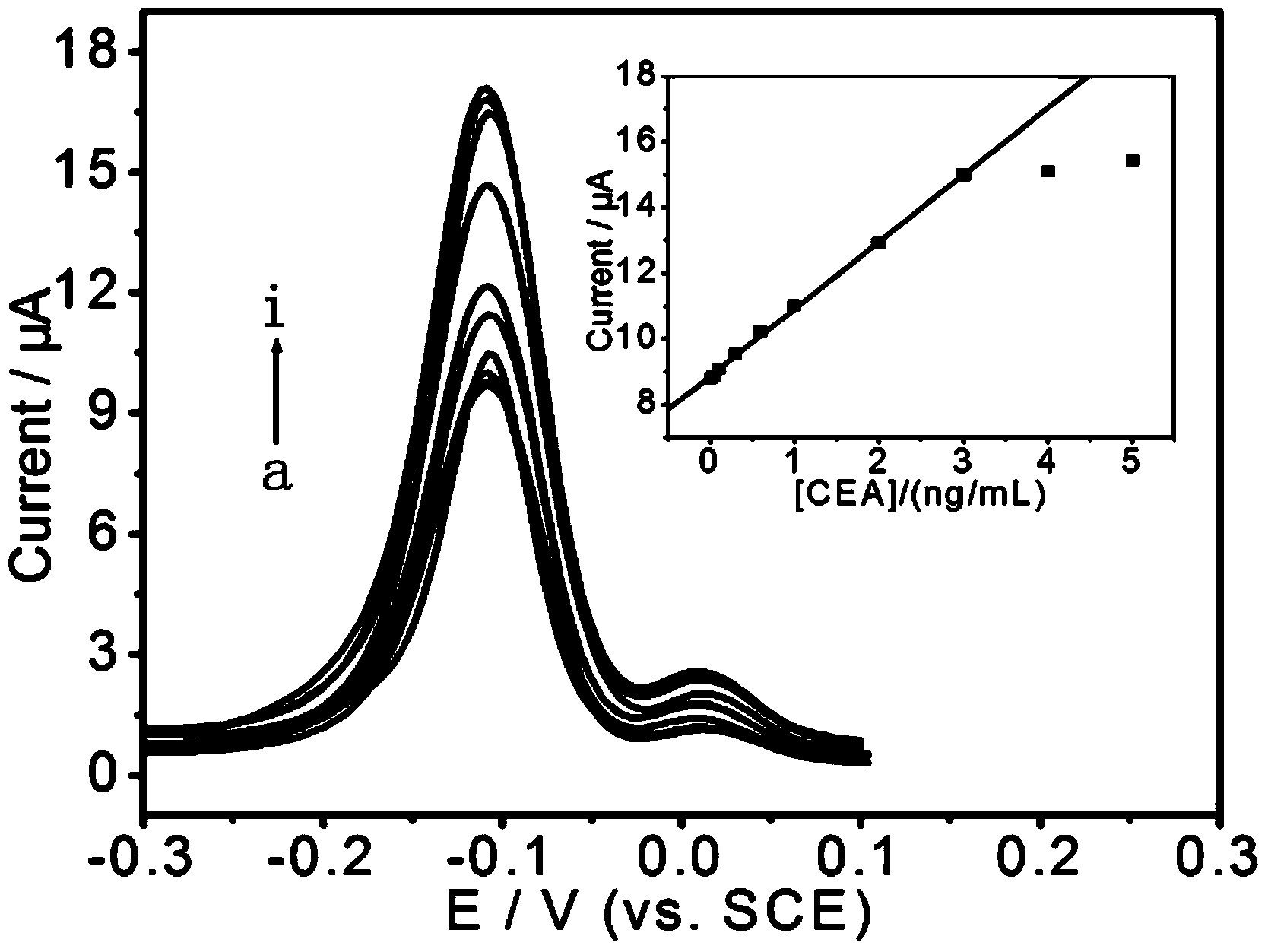
In-vitro sandwich immunoassay process with magnetic separation for detecting alpha fetoprotein
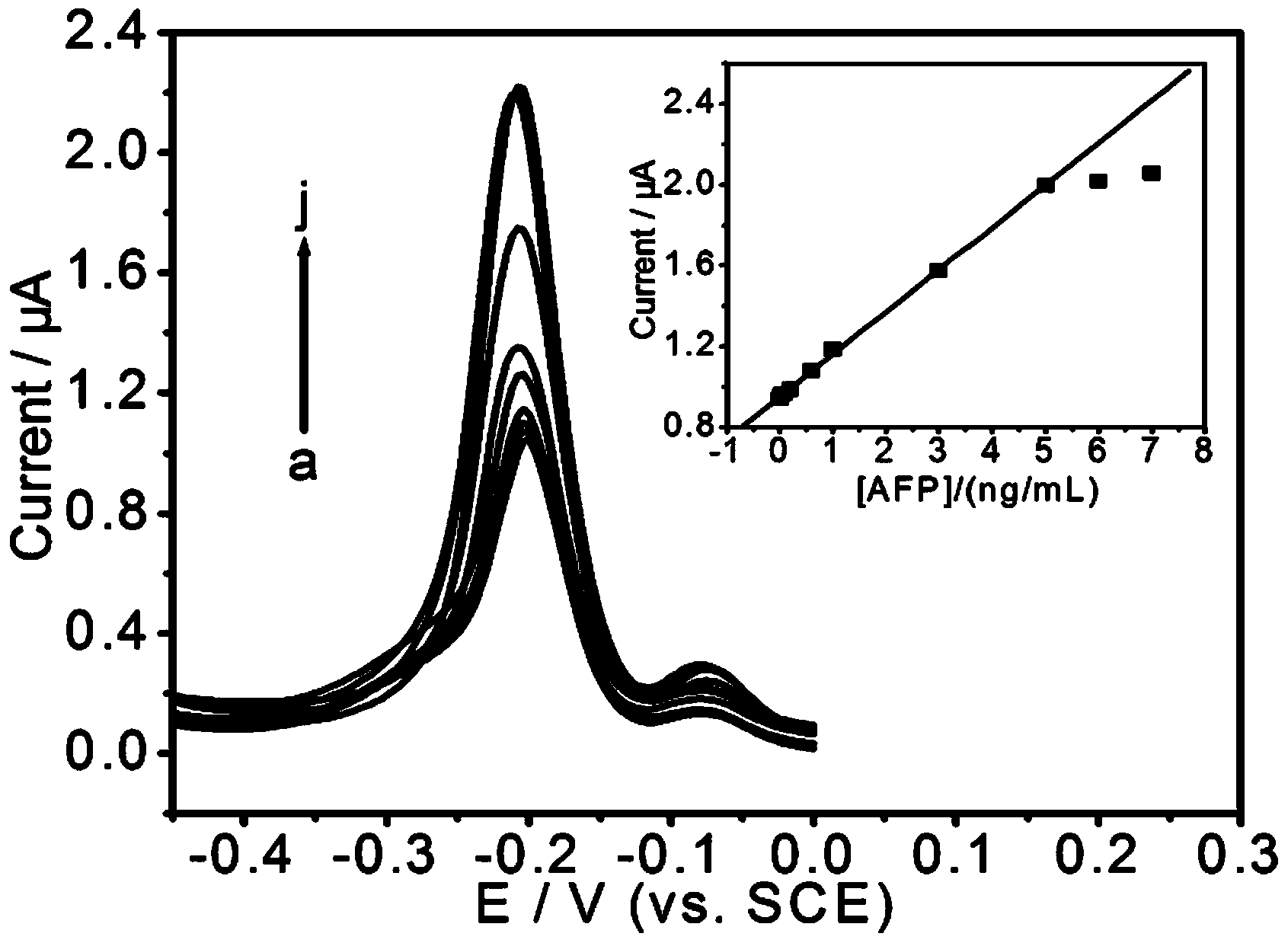
The invention relates to the field of analysis and testing, and an in-vitro sandwich immunoassay process with magnetic separation for detecting alpha fetoprotein is built. The in-vitro sandwich immunoassay process is that a sandwich immune compound with a magnetic separation characteristic is obtained by catching an alpha fetoprotein antigen and a signal probe sandwich fixed by a quantum dot through a magnetic probe. The in-vitro sandwich immunoassay process for detecting the alpha fetoprotein builds an immunosensor to implement the detection of a target substance. The built immunosensor comprises a substrate electrode and modifies a prepared magnetic sandwich immune compound on the surface of the substrate electrode; and the relative electrogenerated chemiluminescent signals are different due to the different quantities of quantum dots relative to the alpha fetoprotein antigens with the different concentrations, so that the quantitative analysis of the alpha fetoprotein can be implemented. The in-vitro sandwich immunoassay process based on magnetism provided by the invention is good in anti-interference performance; and the detection method of the alpha fetoprotein is easy, the sample preprocessing is simple, and the detection and the analysis of content of the alpha fetoprotein in a serum sample with high flexibility can be adapted.
Owner:NINGBO UNIV
Double sandwich immunoassay test kit labeled by HE4 quantum dots and application thereof
InactiveCN103344761AWide excitation spectrumWeak autofluorescenceBiological testingMonoclonal antibodyQuantum dot
The invention discloses a double sandwich immunoassay test kit labeled by HE4 quantum dots. The kit comprises HE4 protein standards, an ELISA plate coated with HE4 specific polyclonal antibodies, and CdTe quantum dots labeled monoclonal antibodies in specific binding with HE4 proteins. The invention further discloses an application of the kit in diagnosis of ovarian tumor. According to the invention, the test kit can directly determine test results through fluorescent ELISA, emitted fluorescence spectral peaks are narrow, autofluorescence is weak, and a sensitivity is high. The kit is high in fluorescence intensity and long in stable time, and can relatively effectively facilitate early detection and risk assessment of the ovarian tumor.
Owner:深圳市柏明胜医疗器械有限公司 +1
Quantitative determination kit for human epididymis secretory protein 4
InactiveCN104237532AHigh sensitivityStrong specificityDisease diagnosisBiological testingChemical reactionMicrosphere
The invention provides a quantitative determination kit for human epididymis secretory protein 4 (HE4), which is applicable to a full-automatic chemiluminescence immunoassay analyzer, in particular relates to a preparation process of a kit for quantitatively detecting the concentration level of HE4 in human serum by adopting a dual-antibody sandwich acridinium ester-marked tubular chemiluminescence method. The kit adopts the dual-antibody sandwich immunoassay and utilizes a chemiluminescence magnetic microsphere immune technology, magnetic microspheres which are coated with anti-HE4 antibodies are specifically combined with the HE4 in a standard product / sample in a reaction cup and then react with another anti-HE4 antibody marked by the acridinium ester to form an immune complex, and the immune complex has the acid-alkali chemical reaction in a pre-trigger solution and a trigger solution, so that relative light unit (RLU / s) of the chemiluminescence reaction can be measured; the content of the HE4 in the standard product / sample is in direct proportion to the relative light unit (RLU / s) measured by an optical system, and the content of HE4 in a serum sample can be quantitatively measured by virtue of standard curve fitting. The quantitative determination kit has advantages of high sensitivity, high specificity, good stability, simplicity and convenience in operation, low cost and the like.
Owner:GUANGZHOU DARUI BIOTECH
Electrochemical immune sensor for phosphating protein
InactiveCN102253221AIncrease fixed amountGood biocompatibilityBiological testingMaterial electrochemical variablesEpoxySignalling molecules
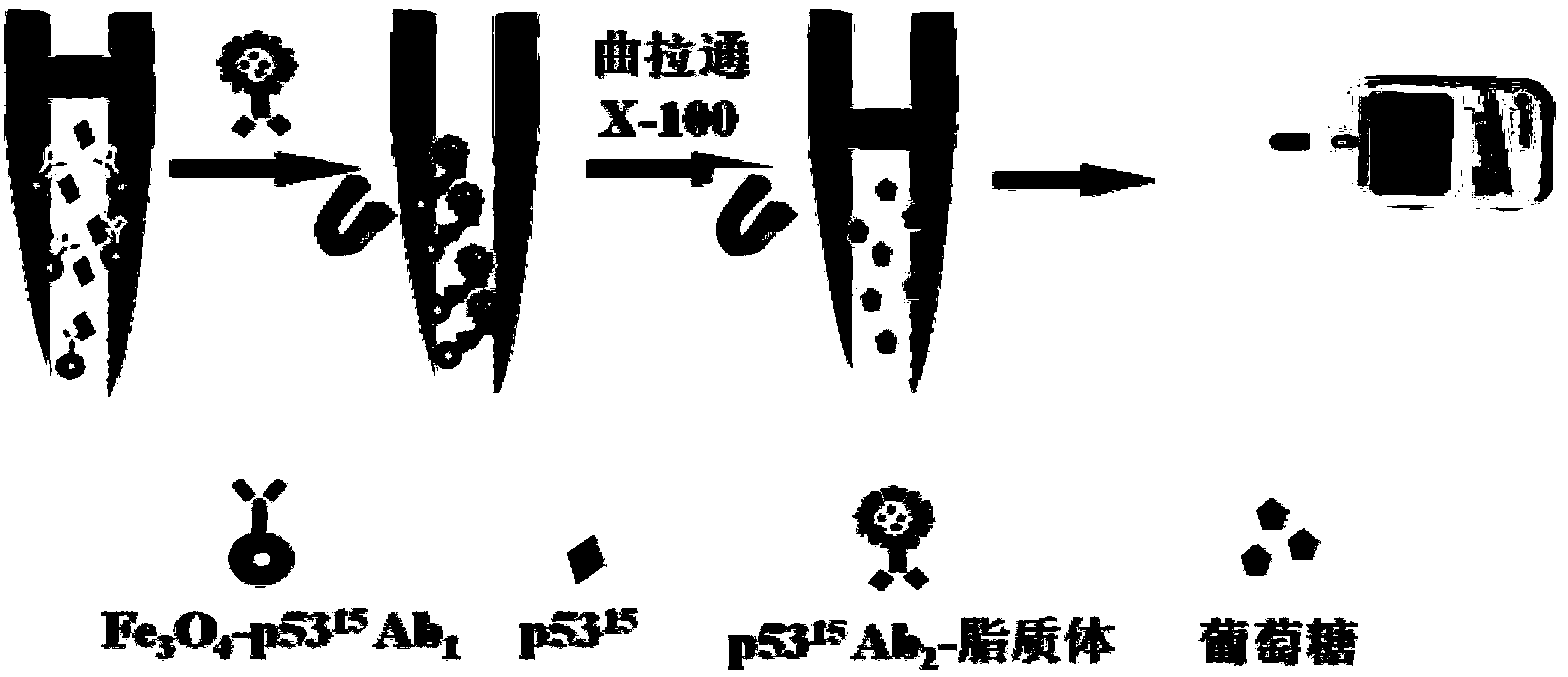
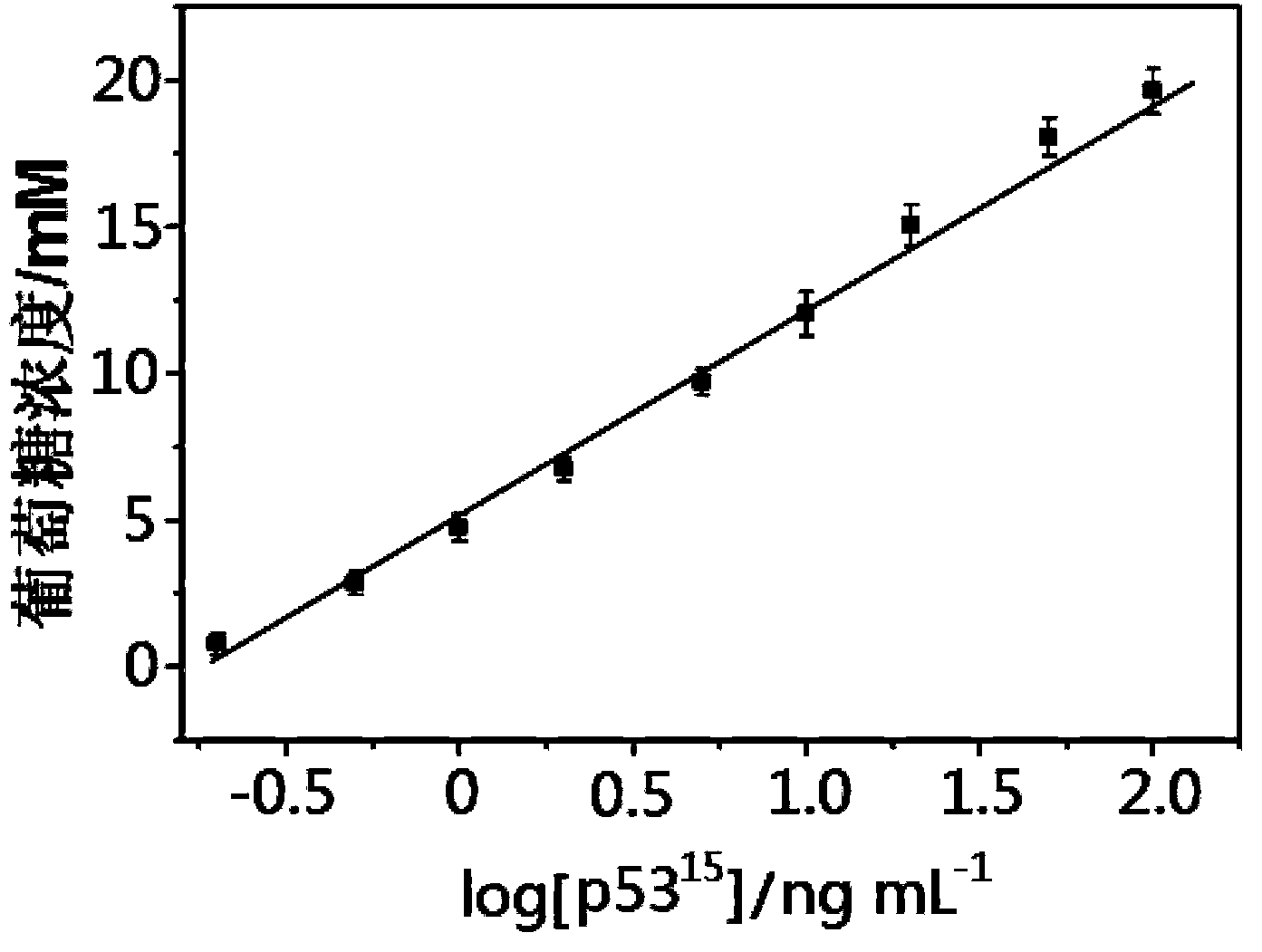
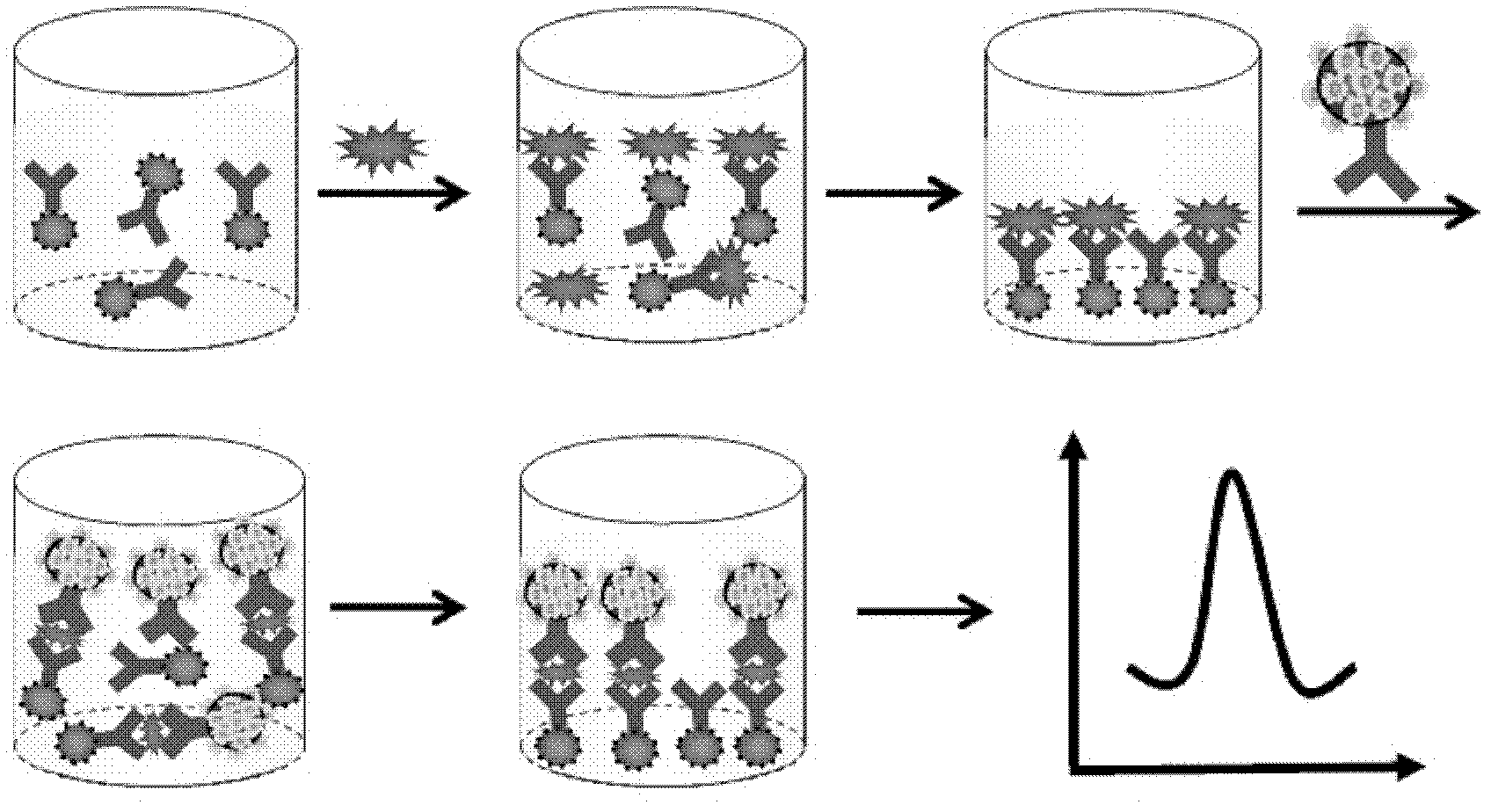
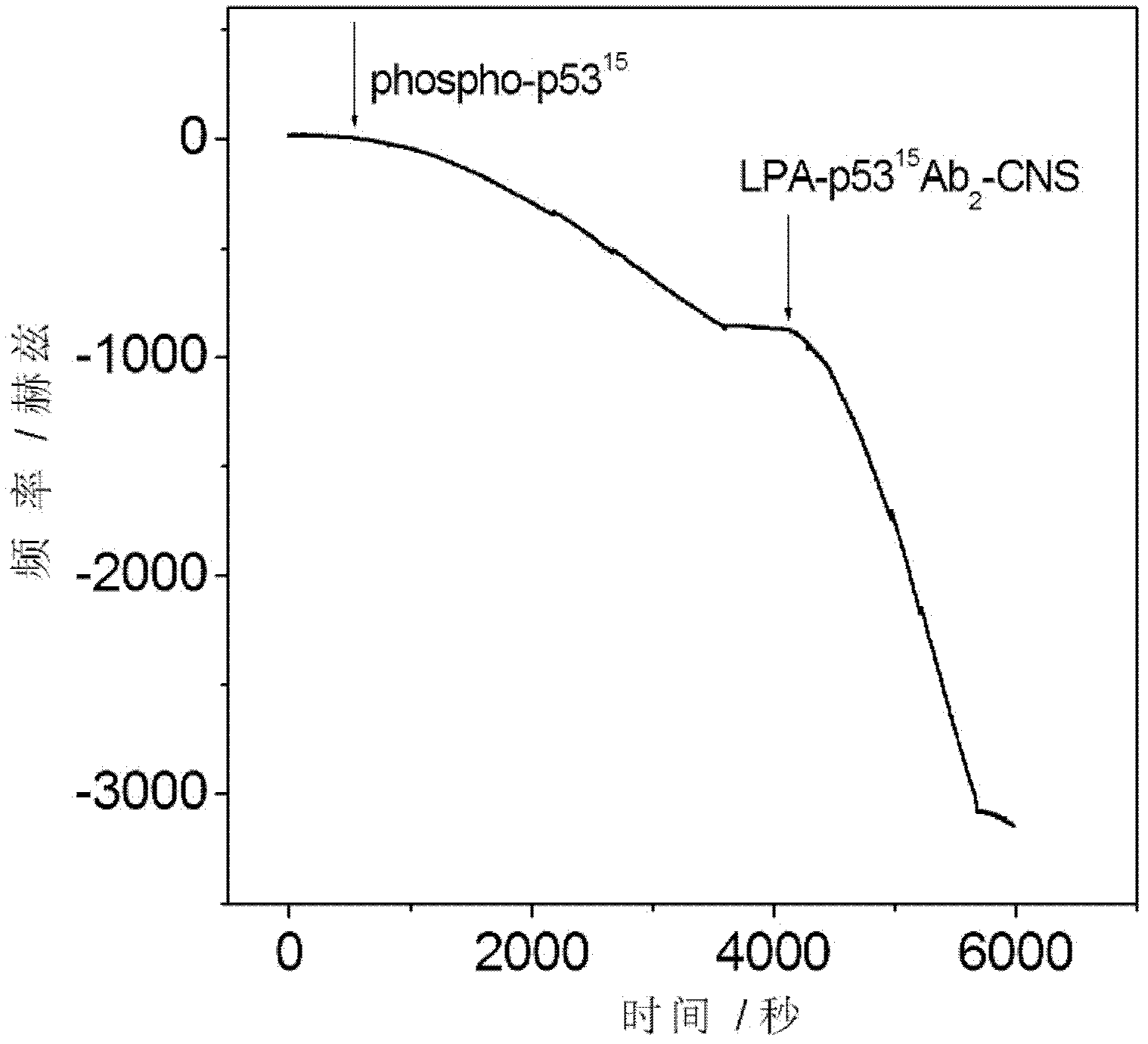
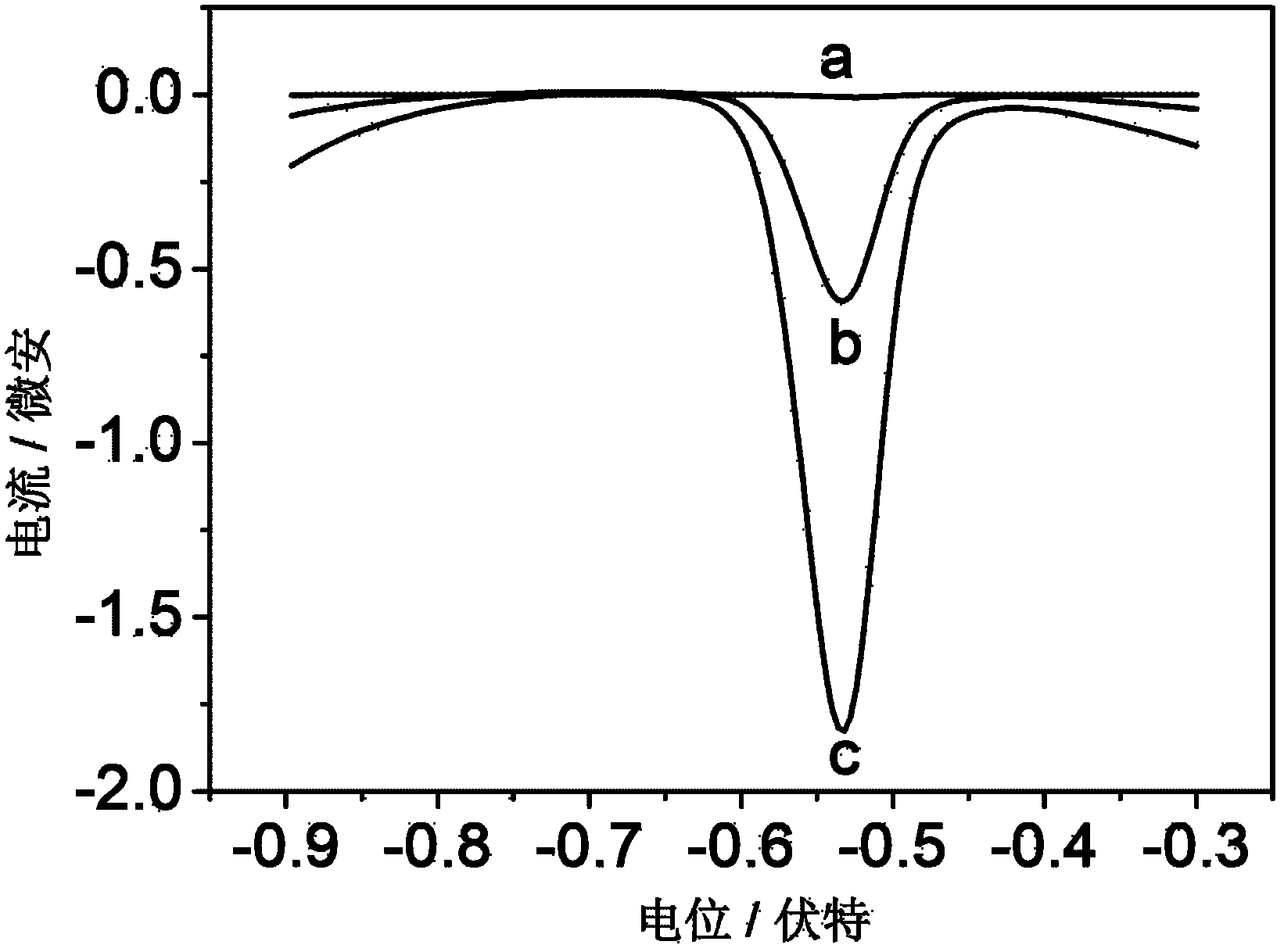
The invention relates to an electrochemical immune sensor for phosphating protein. In the electrochemical immune sensor, a magnetic nanometer-antibody composition MPs-p53<15>Ab1 is used as an adsorbent to separate the phosphating protein, a carbon nanometer sphere CNS is used as a carrier, lead phosphate-apoferritin (LPA) and a phosphating protein antibody p5315Ab2 are modified on the surface of the CNS, and thus, LPA-p53<15>Ab2-CNS nanometer complexing agent is prepared for detecting signal amplification. According to the sandwich immunoassay principle, the content of the phosphating protein to be detected is in proportion to the captured LPA-p5315Ab2-CNS, and the content of lead ions encapsulated in LPA is detected according to a dissolving volt-ampere method to measure the concentration of the phosphating protein in a sample. The method has the advantages of simplicity and convenience for operation and simple and efficient separation, and the advantage that an integral immunologic reaction is finished in an epoxy (EP) tube; a large number of LPA signal molecules are introduced by taking the CAN as the carrier, and each LPA molecule contains a large number of Pb<2+>, so the sensitivity of detection is improved; and the dissolved Pb<2+> is used as a detection signal without adding an enzyme substrate, so the electrochemical immune sensor is a reagent-free sensor. In the electrochemical immune sensor for the phosphating protein, the linear concentration range of the phosphating protein phosphor-p53<15> is from 0.02 to 20 ng mL<-1>, and the detection limit is 0.01 ng mL<-1>.
Owner:HUAZHONG NORMAL UNIV
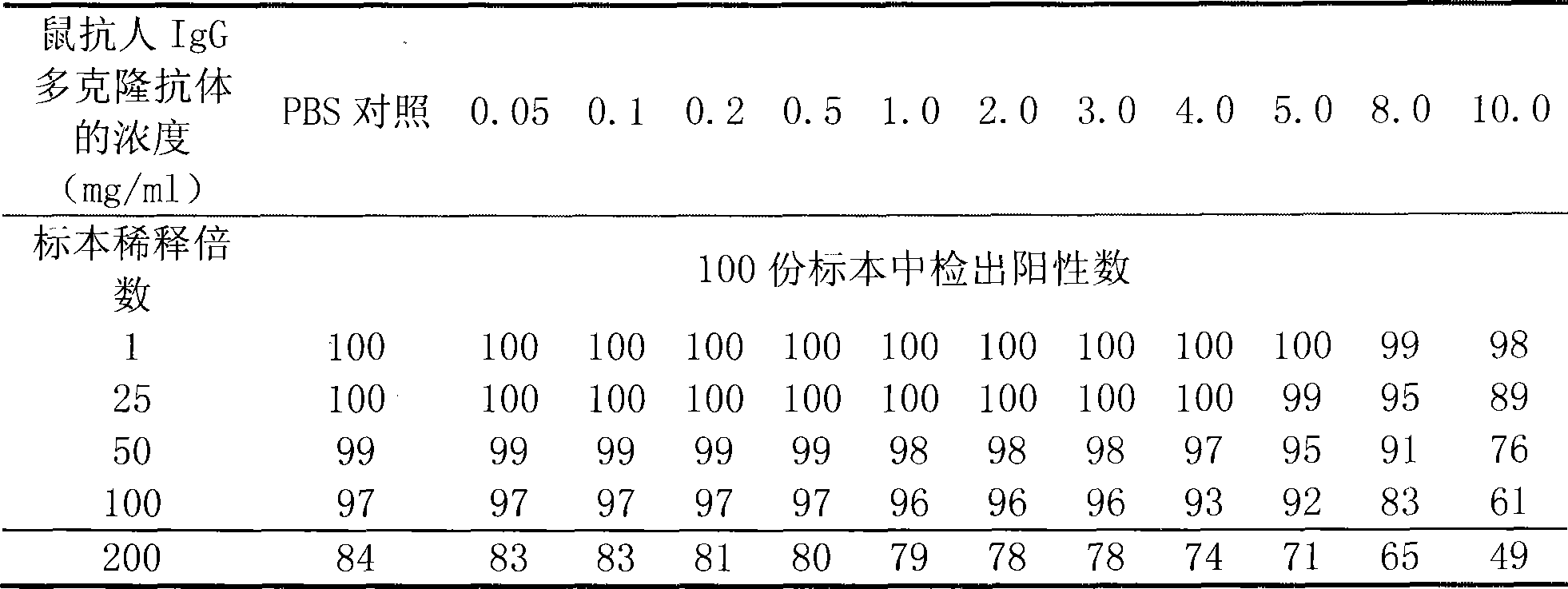
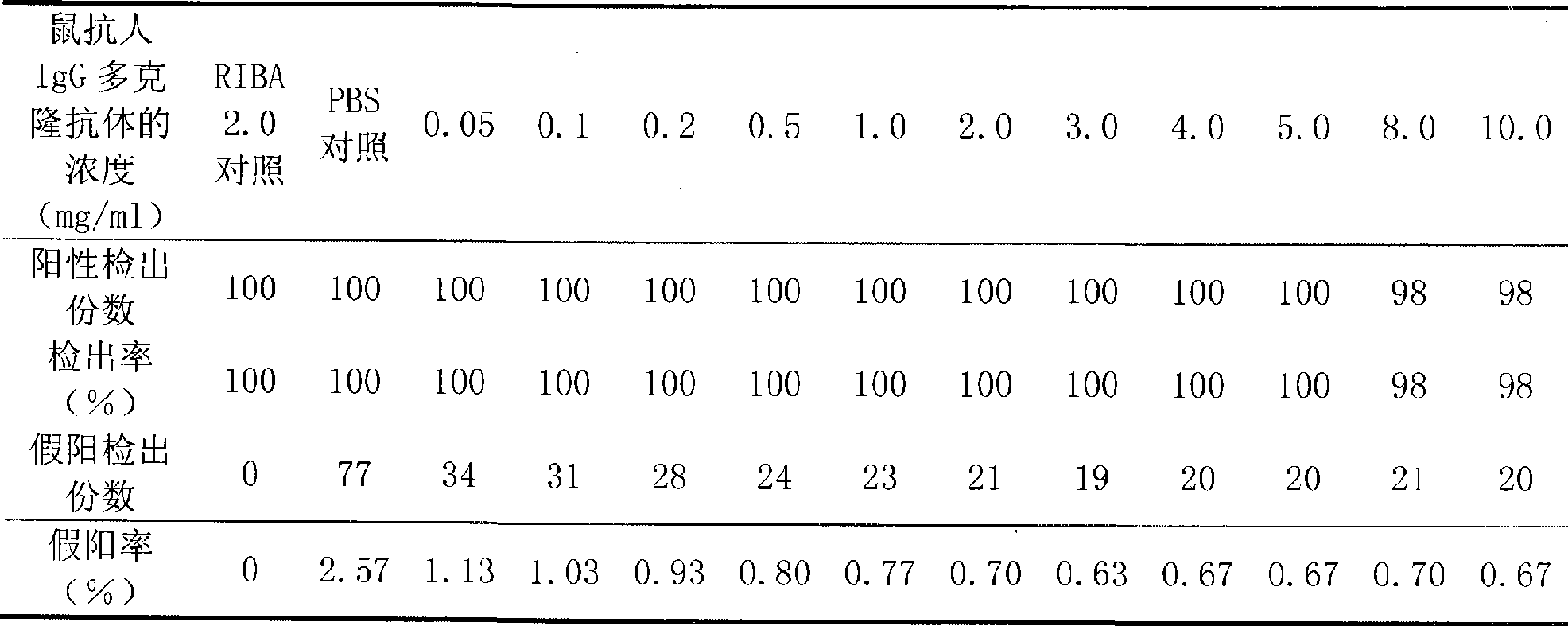
Improved double-antigen sandwiching immunity detection method
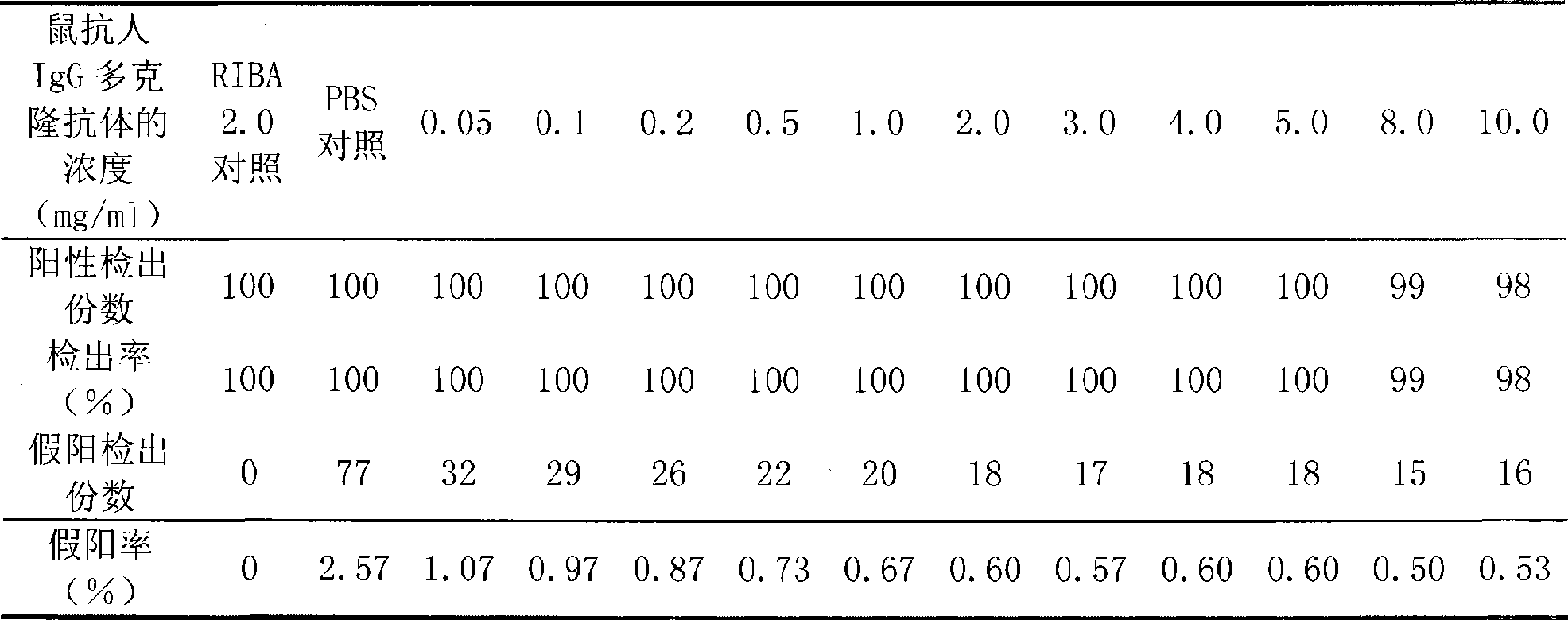
ActiveCN101498730AImproved Double Antigen Sandwich MethodStrong specificityMaterial analysisPolyclonal antibodiesIgG.monoclonal
An improved double antigen sandwich immunoassay method aims to lower the false positive rate of the prior double antigen sandwich immunoassay method and improve the specificity of detection. The method can be applied to various detection methods such as rapid diagnosis, enzyme linked immune detection, chemiluminescent detection, and the like through adding an antihuman IgG monoclonal antibody or polyclonal antibody in a reaction system of the double antigen sandwich immunoassay method according to a certain proportion to participate in detecting. Compared with the prior double antigen sandwich immunoassay method, the double antigen sandwich immunoassay method of the invention remarkably lowers the detection background and the false positive rate and greatly improves the specificity.
Owner:GUANGDONG WESAIL BIOTECH CO LTD
Sandwith immunoassay method and method for detecting antigen by using the same
The invention provides a sandwich immunoassay method comprising steps of, (a) forming a complex of an antigen and a first antibody by contacting the antigen with the first antibody which recognizes the antigen and is labelled by a detectable labelling substance; and (b) fixing the complex formed in the step (a) to a solid phase by using a second antibody which recognizes the antigen and is capable of binding to the solid phase, as well as a method for detecting an antigen in an analyte by using a method of sandwich immunoassay.
Owner:SYSMEX CORP
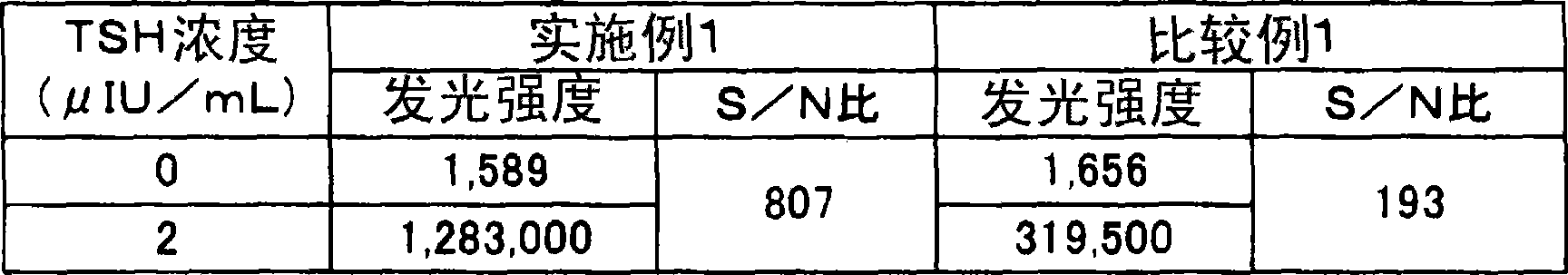
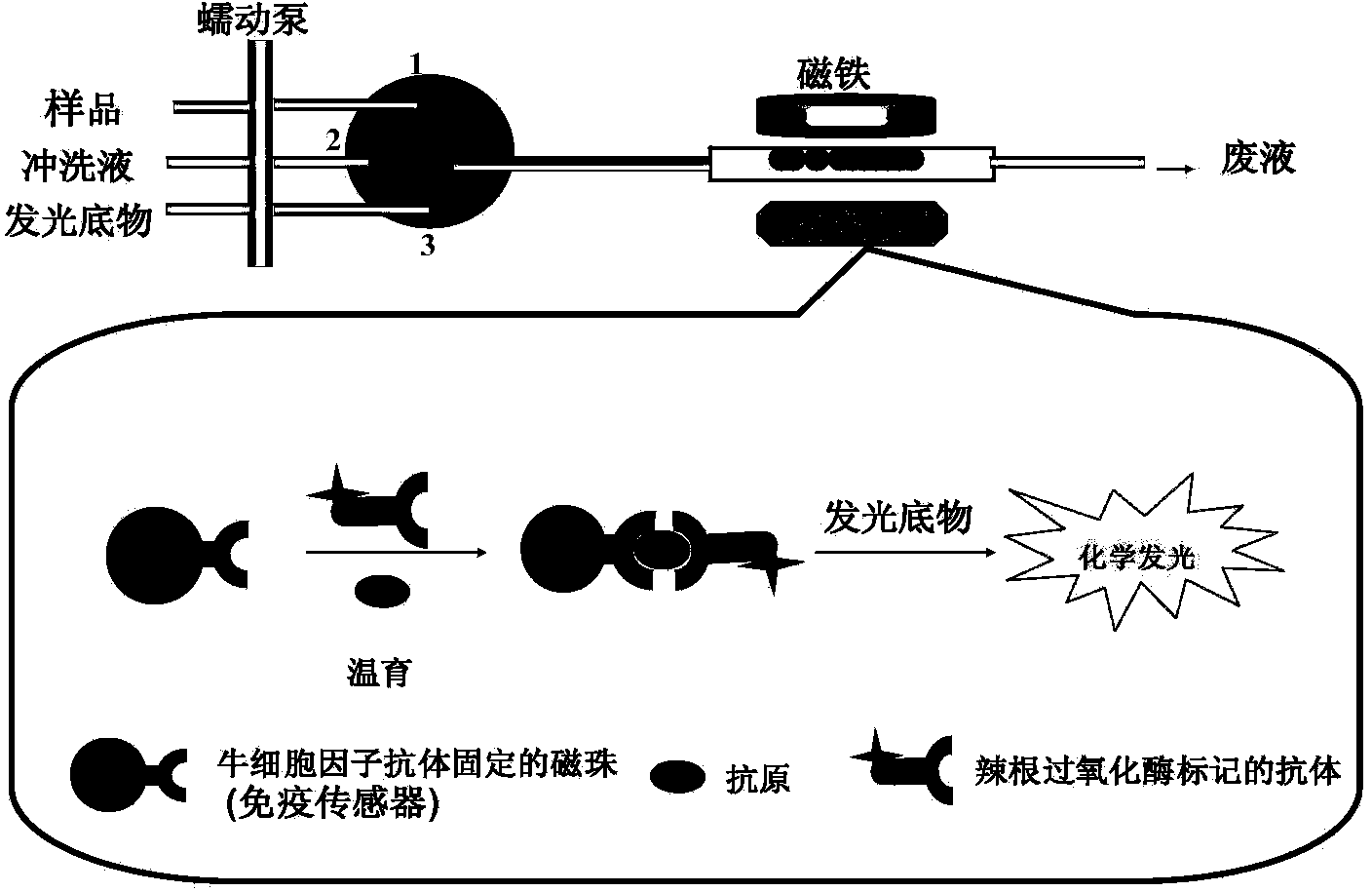
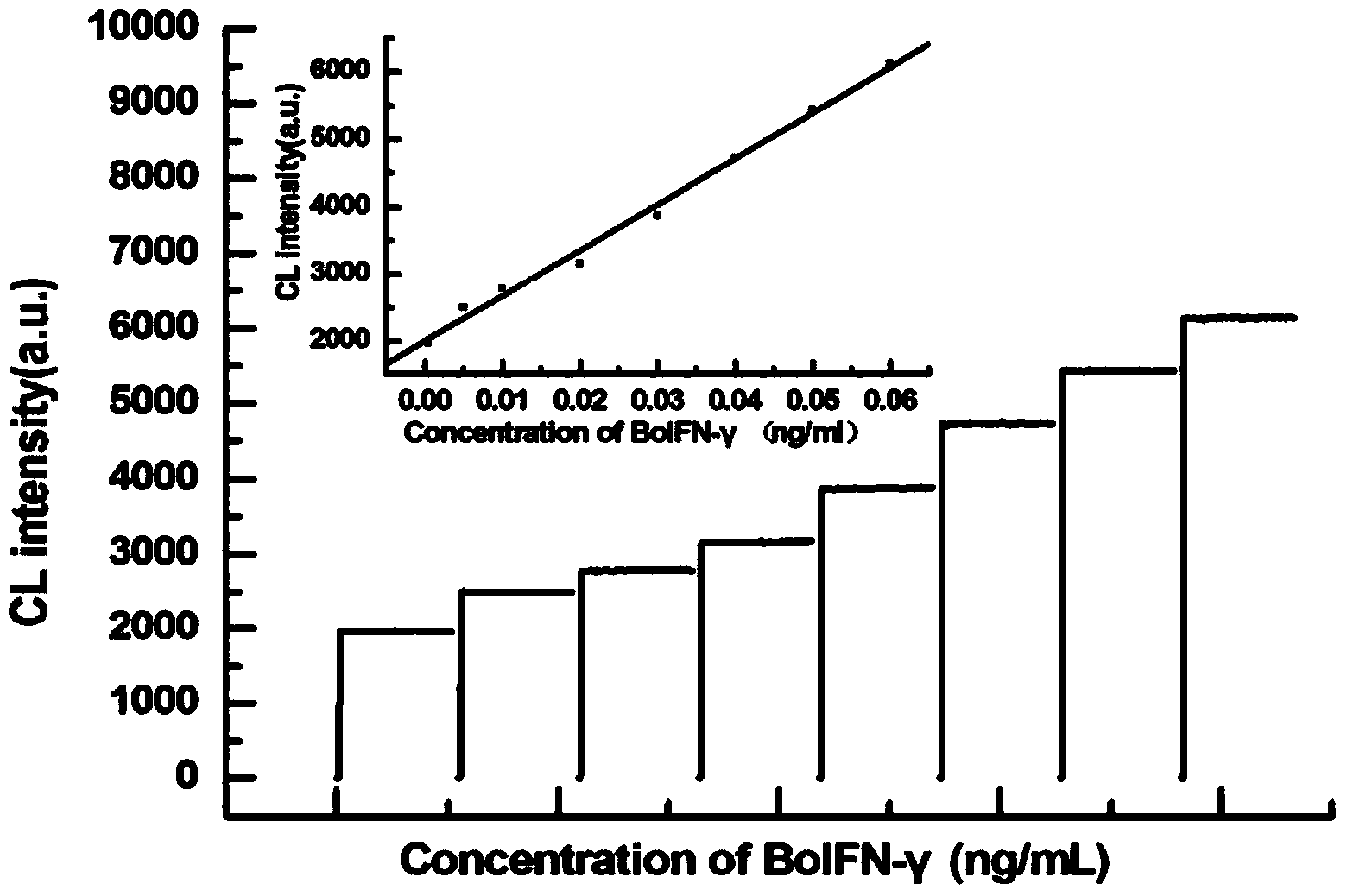
Automatic bovine cell factor chemiluminescence immunoassay detection method based on magnetic particles
Owner:YANGZHOU UNIV
Immunoassay and reagents and kits for performing the same
InactiveUS7141436B2Easy to carryMinimize number of stepBioreactor/fermenter combinationsCapsAntigenAnalyte
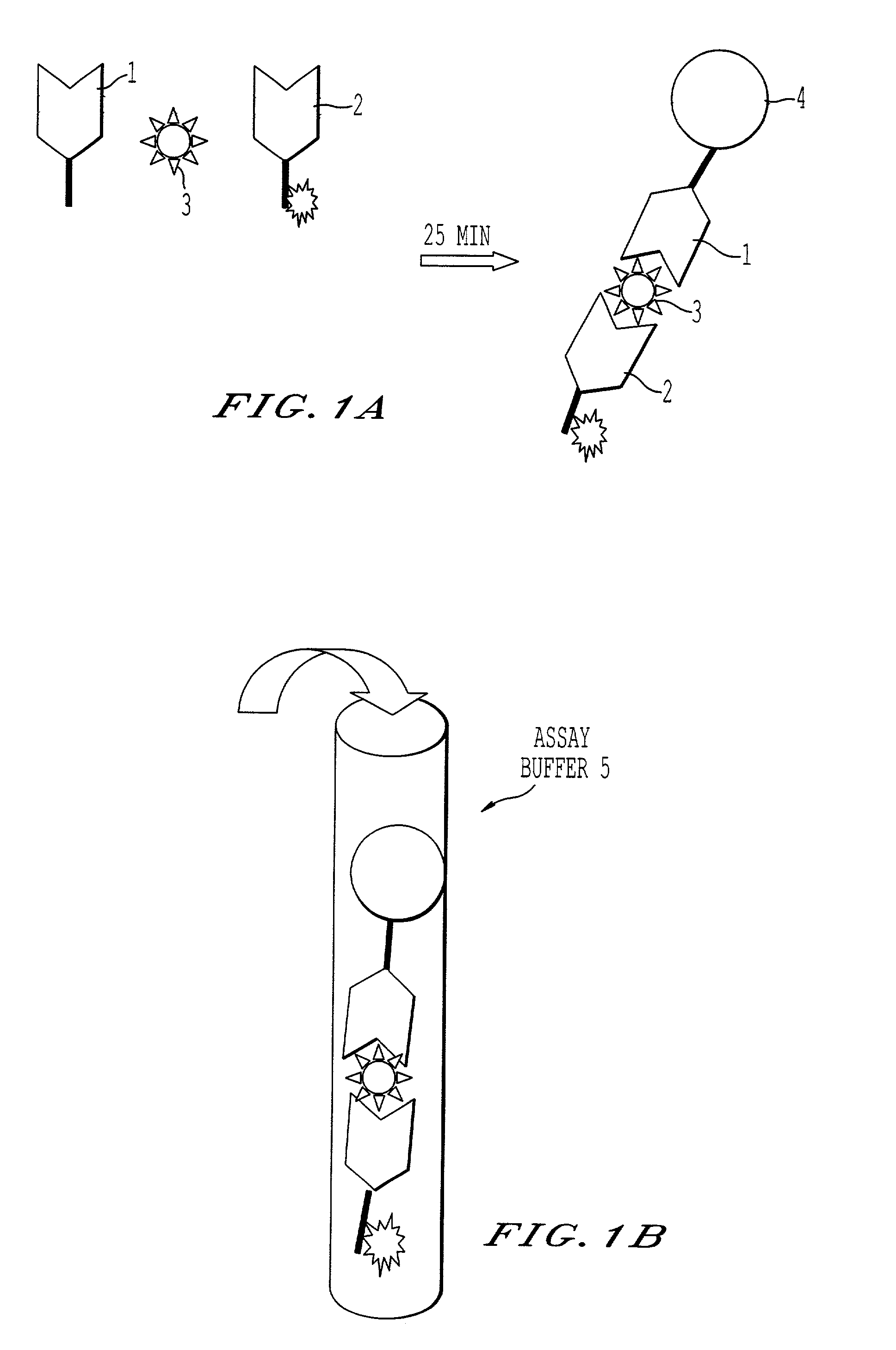
A sandwich immunoassay is disclosed that provides simple to perform yet sensitive identification of analytes in samples. All assay constituents needed (except analyte to be detected) for one assay are dried. Upon reconstitution with sample, a 10 to 15 minute incubation gives a rapid and convenient detection assay capability. The method incorporates the capture of antigen to an immobilized capture antibody. A labeled reporter antibody with the molecule, binds to the antigen to form an immunocomplex capable of generating a detectable signal.
Owner:STC UNM
Disposable nanometer electrogenerated chemiluminescence two-component immune sensor and preparation method thereof
InactiveCN104865240ALow detection limitAchieving potential-area dual resolutionChemiluminescene/bioluminescenceAnalysis by electrical excitationElectricityPotential difference
The invention discloses a disposable nanometer electrogenerated chemiluminescence two-component immune sensor and a preparation method thereof. A monothiol compound and a bisthiol compound wrapped QDs electrogenerated chemiluminescence potential difference distinguish and ITO electrode space distinguish are used to achieve object detection. The sensor has a three-electrode structure; Ag / AgCl or saturated calomel electrode is used as a reference electrode, Pt electrode is used as an auxiliary electrode, and two pieces of modified ITO conductive glass capable of specifically identifying the two components are placed back to back as a working electrode for the two-component immune sensor; and an object to be measured is crosslinked to the surface of the working electrode by a sandwich immunoassay reaction, and finally crosslinked with horseradish peroxidase.
Owner:THE SECOND HOSPITAL OF NANJING
Enhancement of sensitivity of fluorophore mediated biosensing and bioimaging
InactiveUS20060024771A1Enhanced fluorescence emissionBioreactor/fermenter combinationsMaterial nanotechnologyTarget analysisAnalyte
Owner:UNIV OF LOUISVILLE RES FOUND INC
Streptavidin functionalized semiconductor nano material-based tumor marker electrochemical immunosensor and preparation method thereof
InactiveCN103645316ALarge specific surface areaGood biocompatibilityNanosensorsMaterial analysisBiotin-streptavidin complexAntigen
The invention relates to a streptavidin functionalized semiconductor nano material-based tumor marker electrochemical immunosensor and a preparation method thereof. The immunosensor is prepared by the following steps: firstly, synthesizing or screening the semiconductor nano structure material with excellent performance, carrying out bio-functionalization by using the streptavidin, and then specifically combining with a biotinylation antibody. The prepared sensor is incubated in a mixed solution containing an enzyme-labeled antibody and an antigen, thionine and hydrogen peroxide are taken as enzyme reaction substrates, and the tumor marker is detected by using a sandwich immunoassay method. An ampere response of an electrode is increased along with an increase of antigen concentration in an incubation solution. The detection method is high in sensitivity, low in detection limit, and good in reproducibility and stability, and can be applied to quantitative determination of the tumor marker in serum.
Owner:YANGZHOU UNIV
Immunoassay for soluble PD-l1
The present disclosure describes two matched antibody pairs for use in a sandwich immunoassay for detecting and quantifying soluble PD-L1 in liquid samples, as well as an electrochemiluminescence sandwich immunoassay that has been optimized and validated with one of the matched pairs.
Owner:MERCK SHARP & DOHME LLC
Anti-human calcitonin monoclonal antibodies and an immunoassay utilizing said antibodies
Monoclonal antibodies having a high affinity for human calcitonin, particularly monoclonal antibodies suitable for a sandwich immunoassay are disclosed. Also disclosed are hybridomas producing said monoclonal antibodies and a sandwich immunoassay utilizing said antibodies for determining human calcitonin in blood.
Owner:OSAKA GAS CO LTD
Immunodetection assay for mycobacterium tuberculosis complex
ActiveUS20100285506A1Improve accuracyReduce riskBacterial antigen ingredientsSnake antigen ingredientsBacteroidesSecretory protein
The present invention provides a method for specifically detecting a Mycobacterium tuberculosis complex-specific secretory protein MPT64 antigen in a biological sample, whereby diagnosis of infection with Mycobacterium tuberculosis is carried out rapidly and safely with higher accuracy than before. An antibody that recognizes an epitope for MPB64 located in any one of amino acid sequences of SEQ ID NOS: 2 to 4, particularly a monoclonal antibody was obtained. Thus, an immunoassay using the antibody, particularly a sandwich immunoassay using first and second antibodies to MPB64, particularly an immunochromatographic assay and an immunochromatographic test strip are provided. A biological sample can be rapidly subjected to the immunoassay without culturing or after culturing for a time before Mycobacterium tuberculosis complex bacteria in the sample substantially start to grow. The biological sample may be pretreated by treatment for inactivation of Mycobacterium tuberculosis, or treatment by dispersion or solubilization.
Owner:TAUNS CO LTD
Method and composition for the diagnosis of equine infectious anemia virus disease by using the recombinant capsid protein virus (p26)
InactiveUS6596846B2Microbiological testing/measurementPeptide preparation methodsSerum igeEquine infectious anemia
The present invention relates to a method and kit for detecting antibodies in clinical samples of animals infected with equine infectious anemia virus using the immunodiagnosis with the recombinant viral antigen p26. The antigen was bound to solid supports (microtitter plates, tubes, beads or nitrocellulose papers or nylon) and reacted with the test serum. After incubation with conjugated anti-equine immunoglobulin-enzyme the reaction was revealed with a solution composed of the substrate of the enzyme used in the conjugate (cromogene). After development of the reaction (color formation) it was stopped with acid solution and measured. The immunoassay may be a direct second antibody immunoassay, a one or two step sandwich immunoassay.
Owner:UNIVERSIDADE FEDERAL DE MINAS GERAIS
Immunoassays for beta2-microglobulin
InactiveUS20030180811A1Biological material analysisPeptide preparation methodsBeta-2 microglobulinMonomer
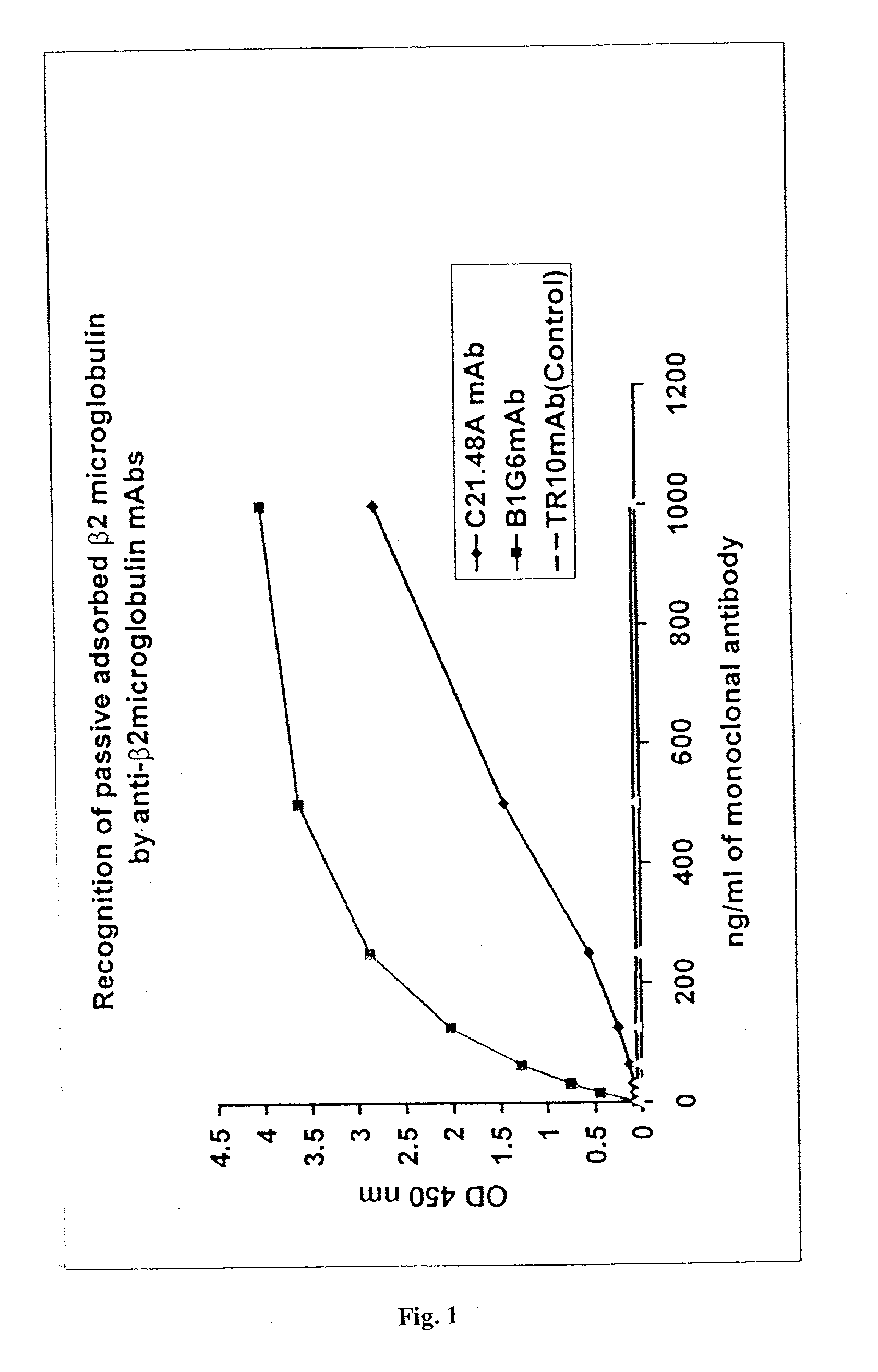
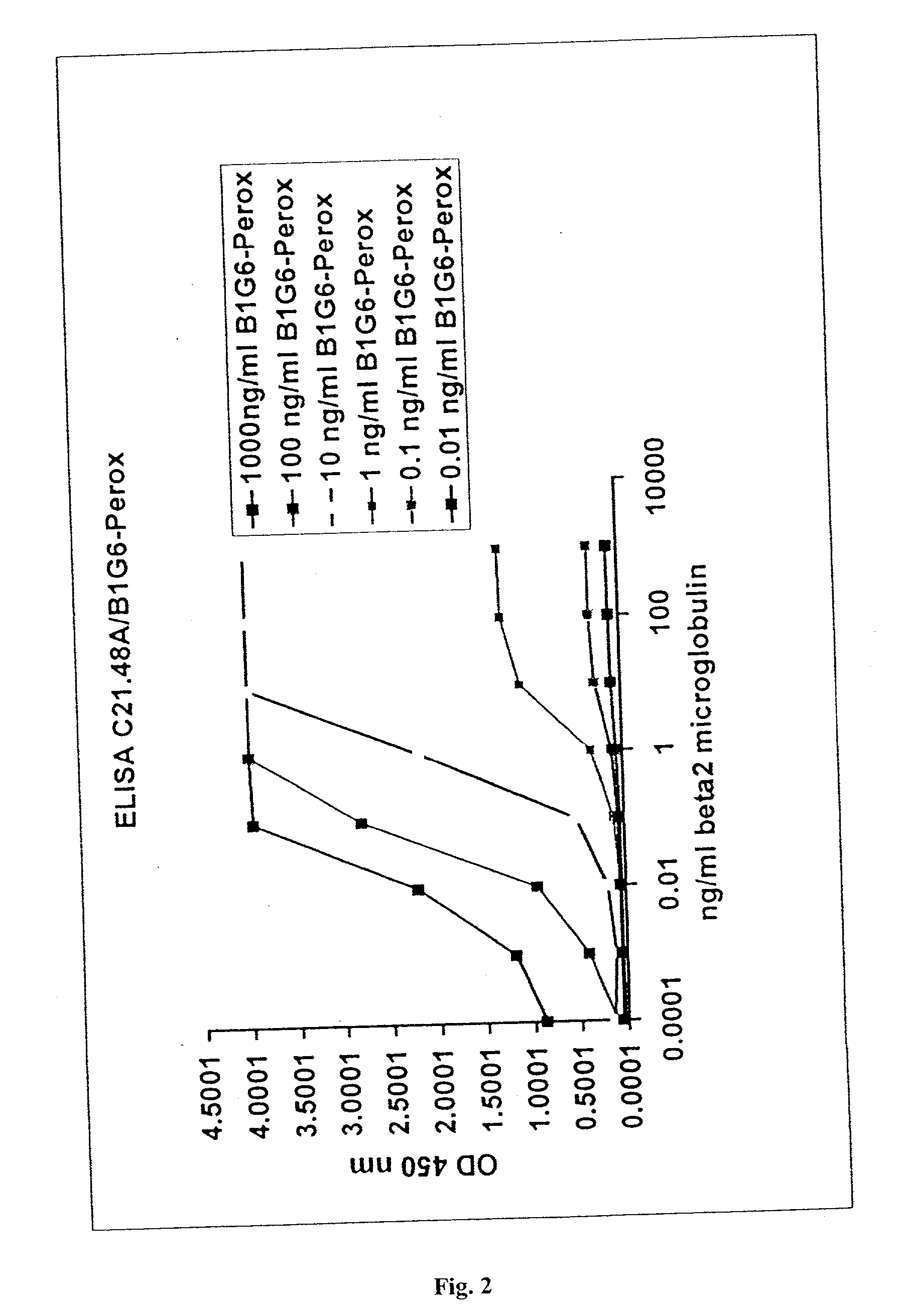
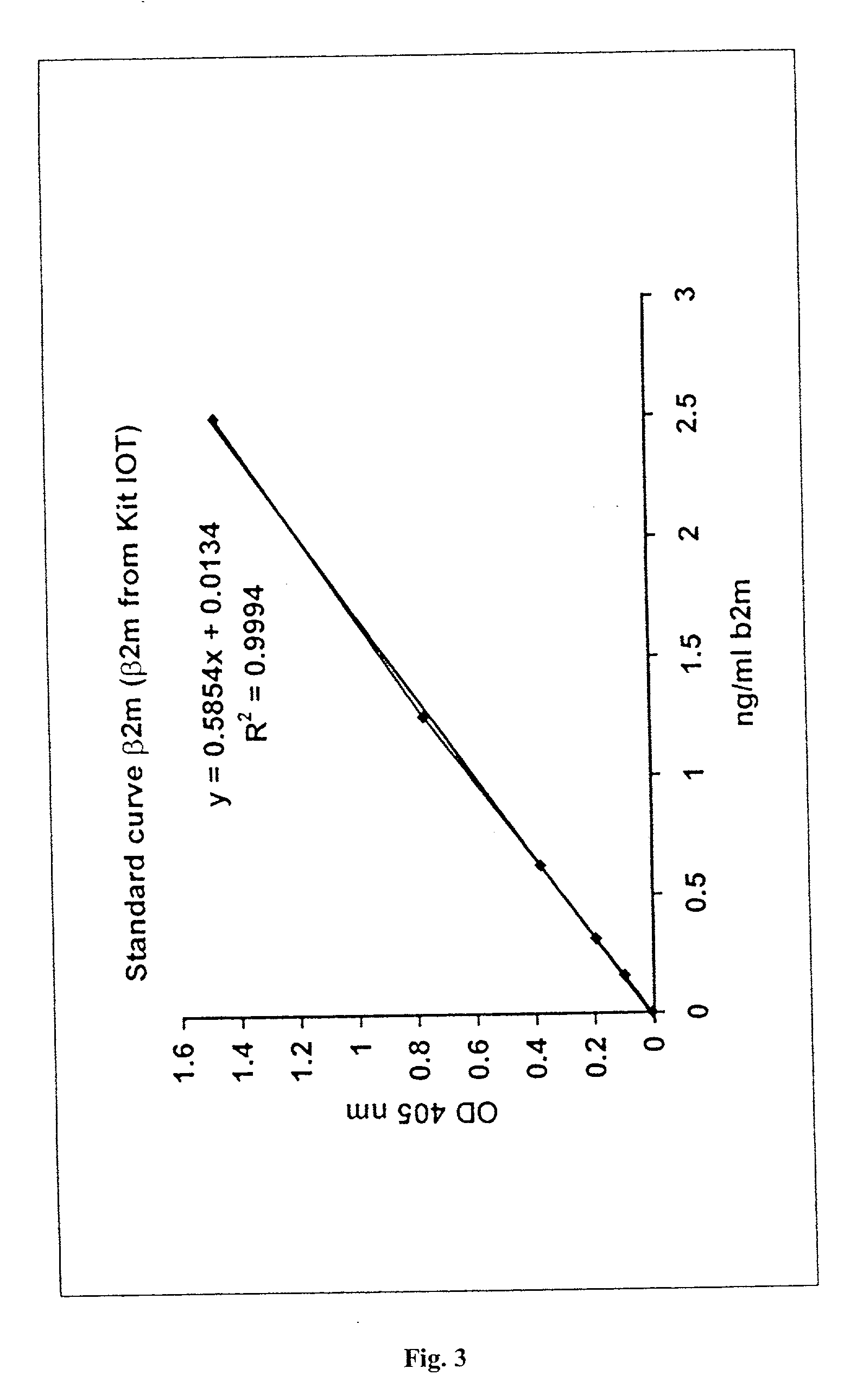
Immunoassays useful for detecting free beta2-microglobulin in a sample containing beta2-microglobulin / beta2-microglobulin associated protein complexes are provided. Also provided are a sandwich immunoassay and a competition immunoassay for detecting free beta2-microglobulin in a sample containing MHC monomers or MHC tetramers. Kits for performing such immunoassays also are provided.
Owner:BECKMAN COULTER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com