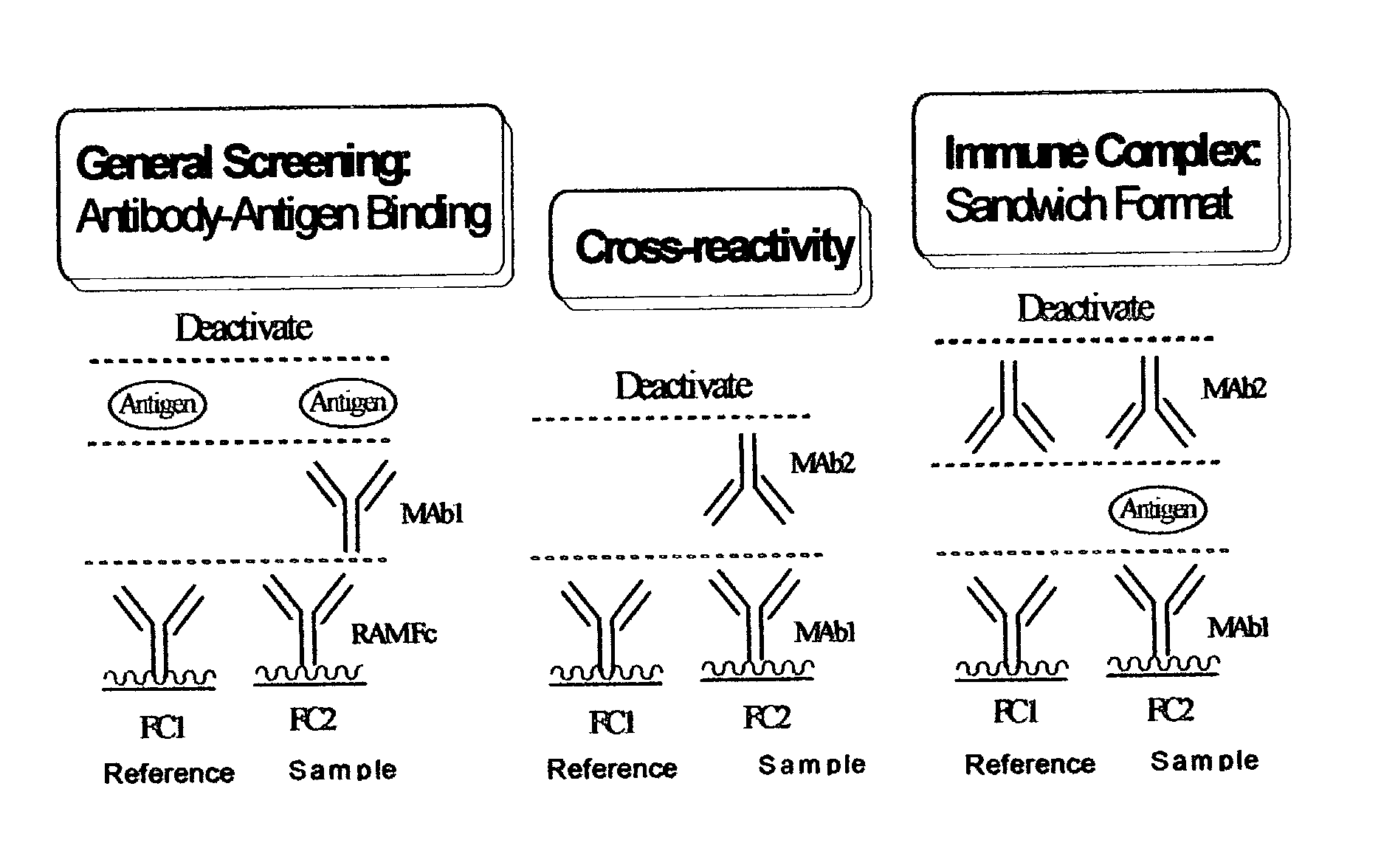
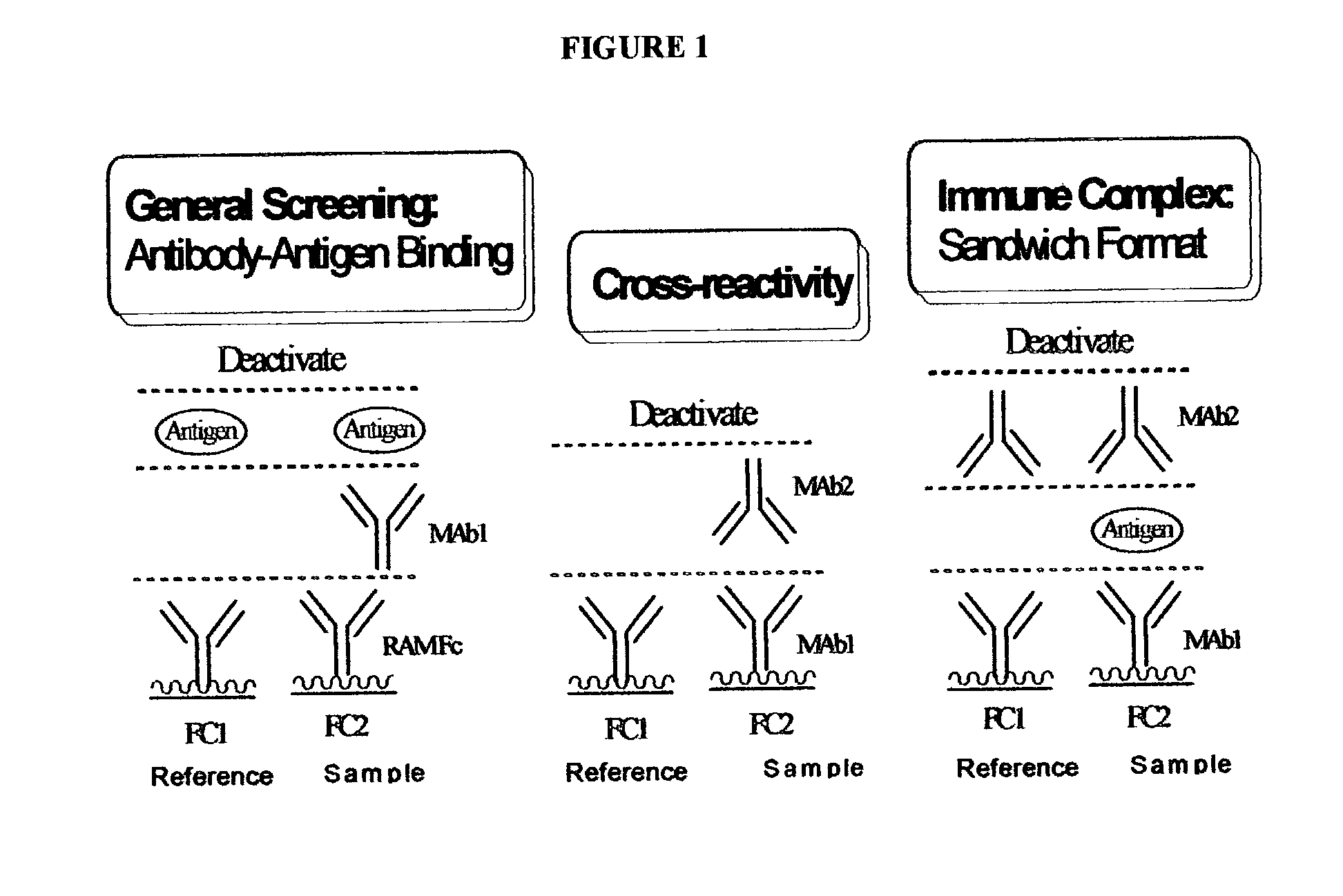
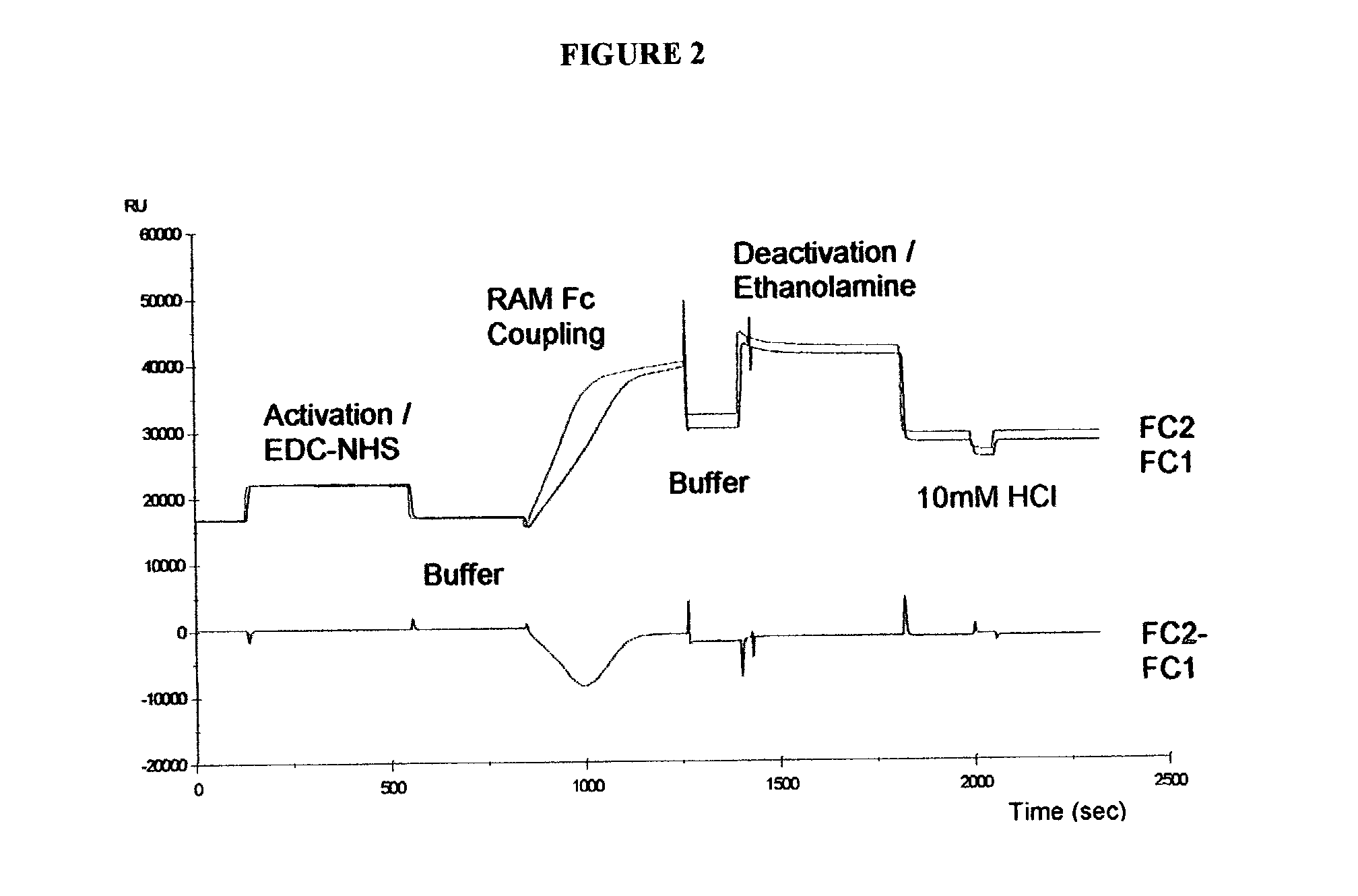
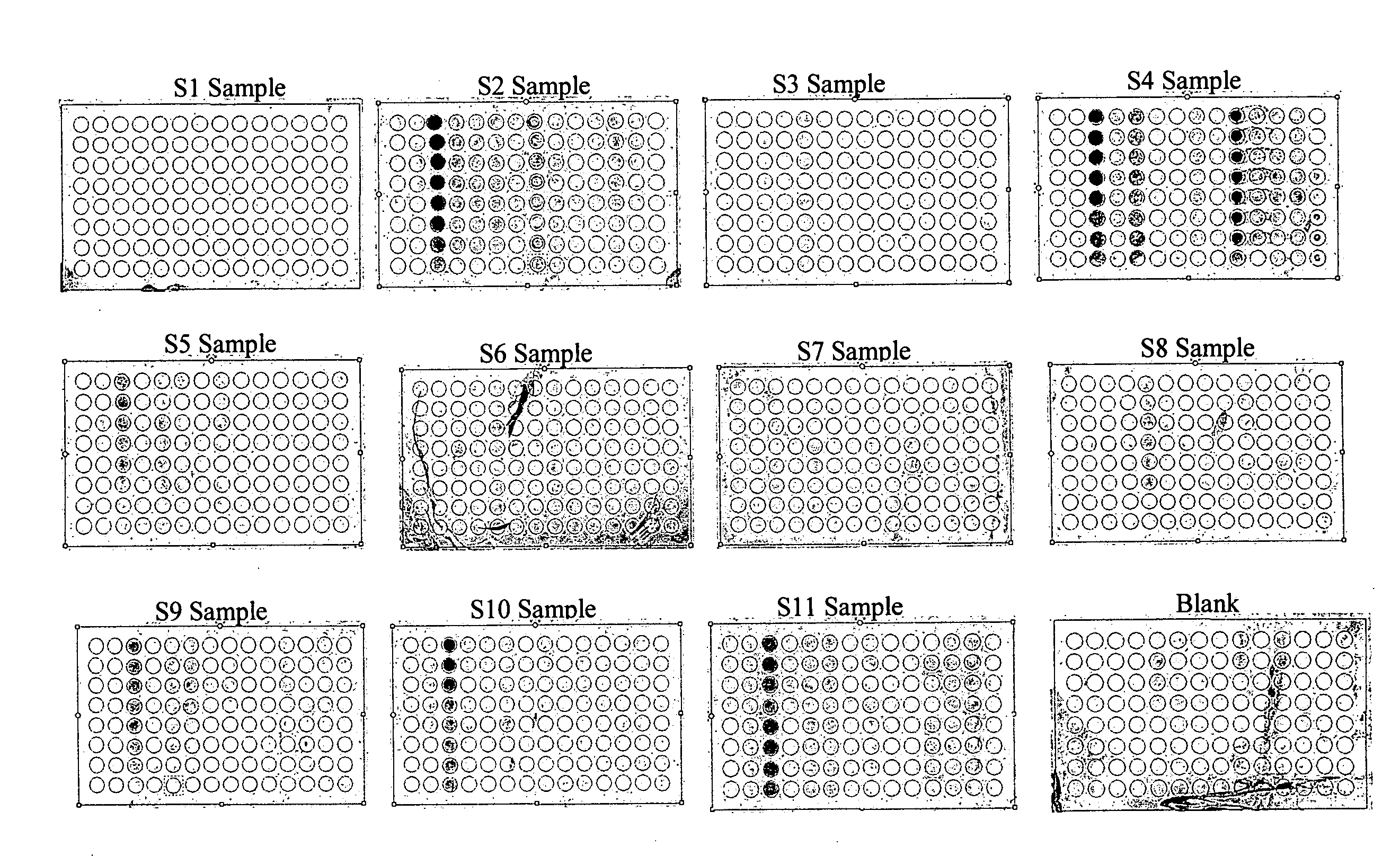
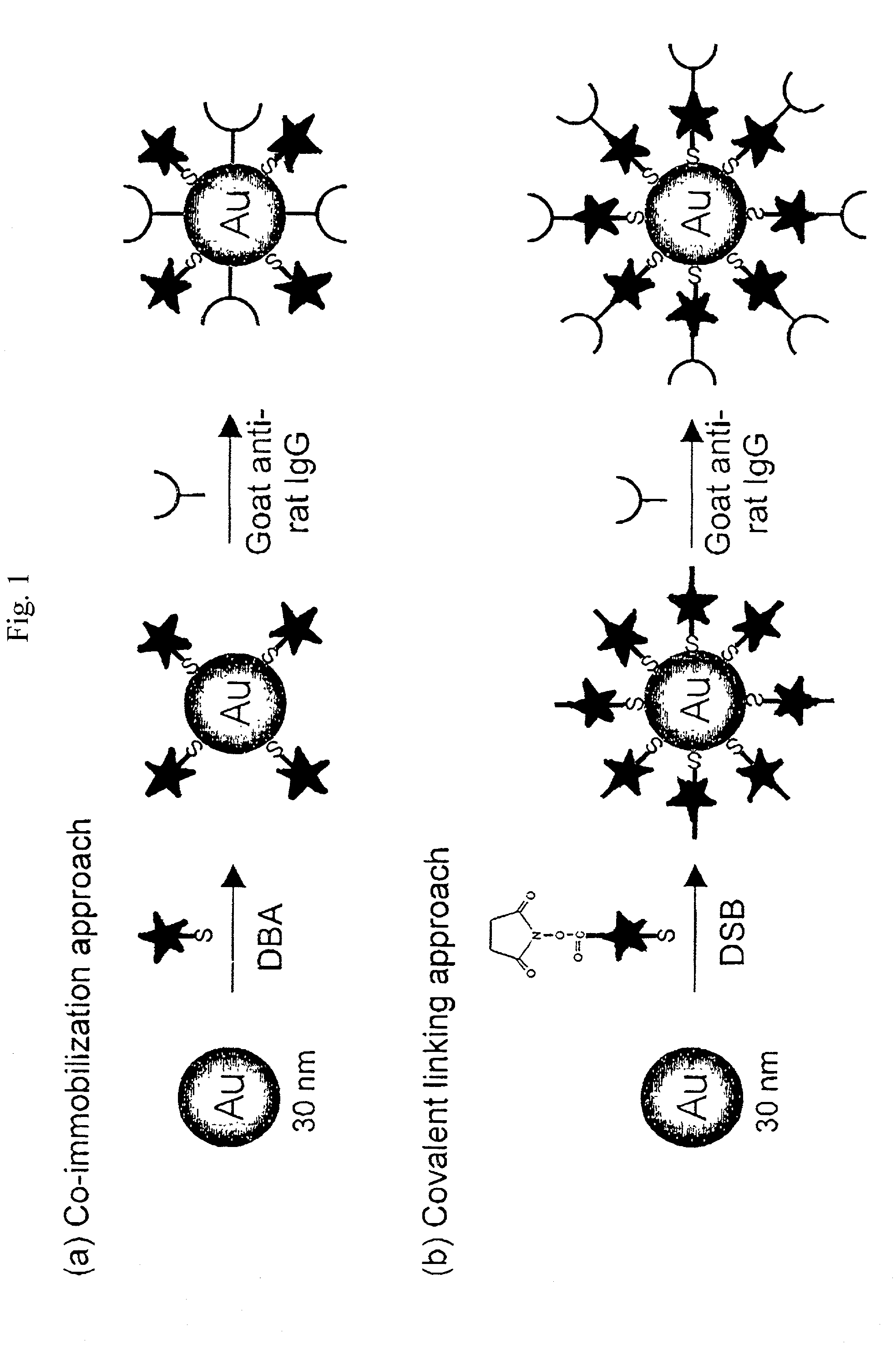
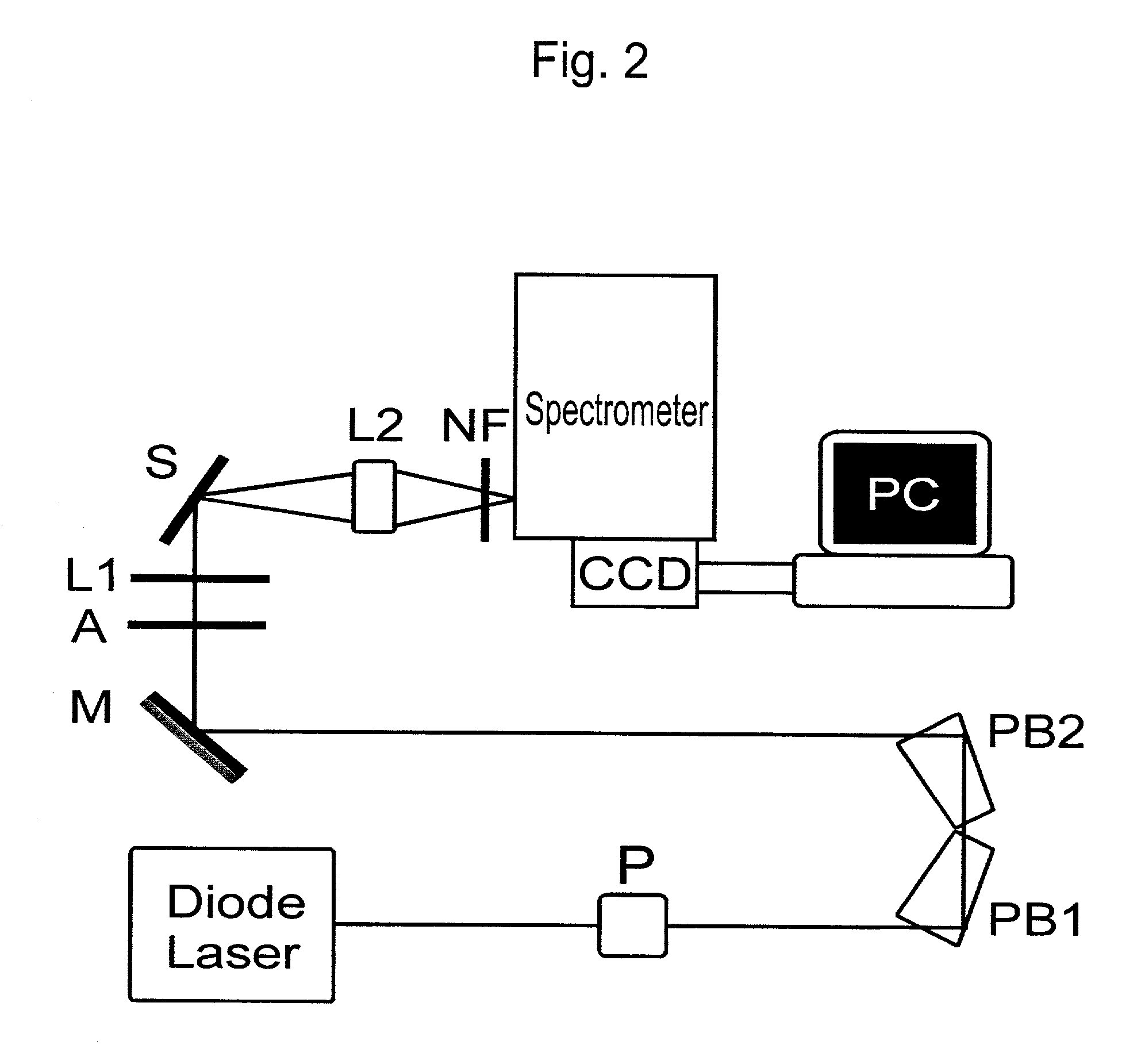
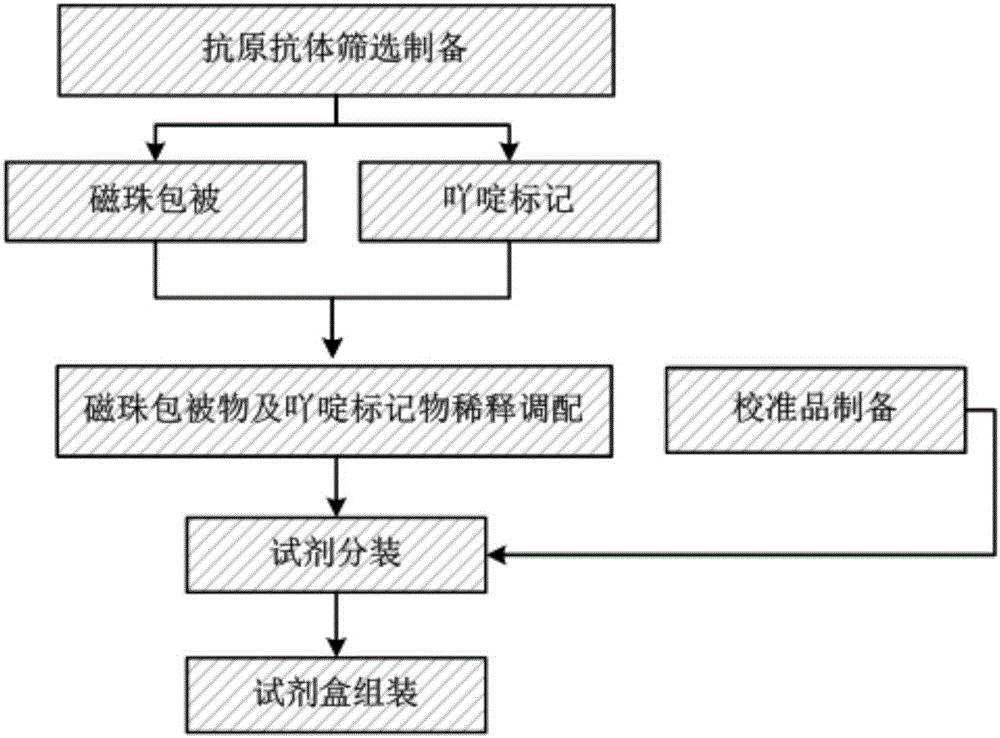
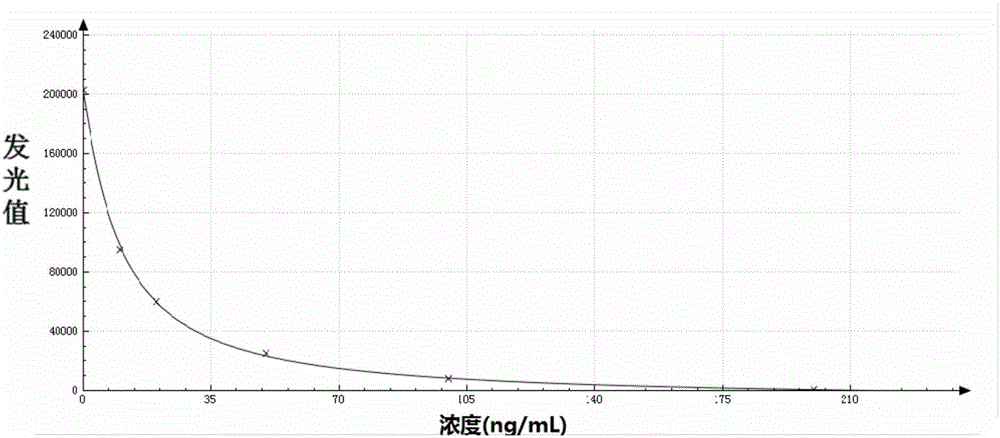
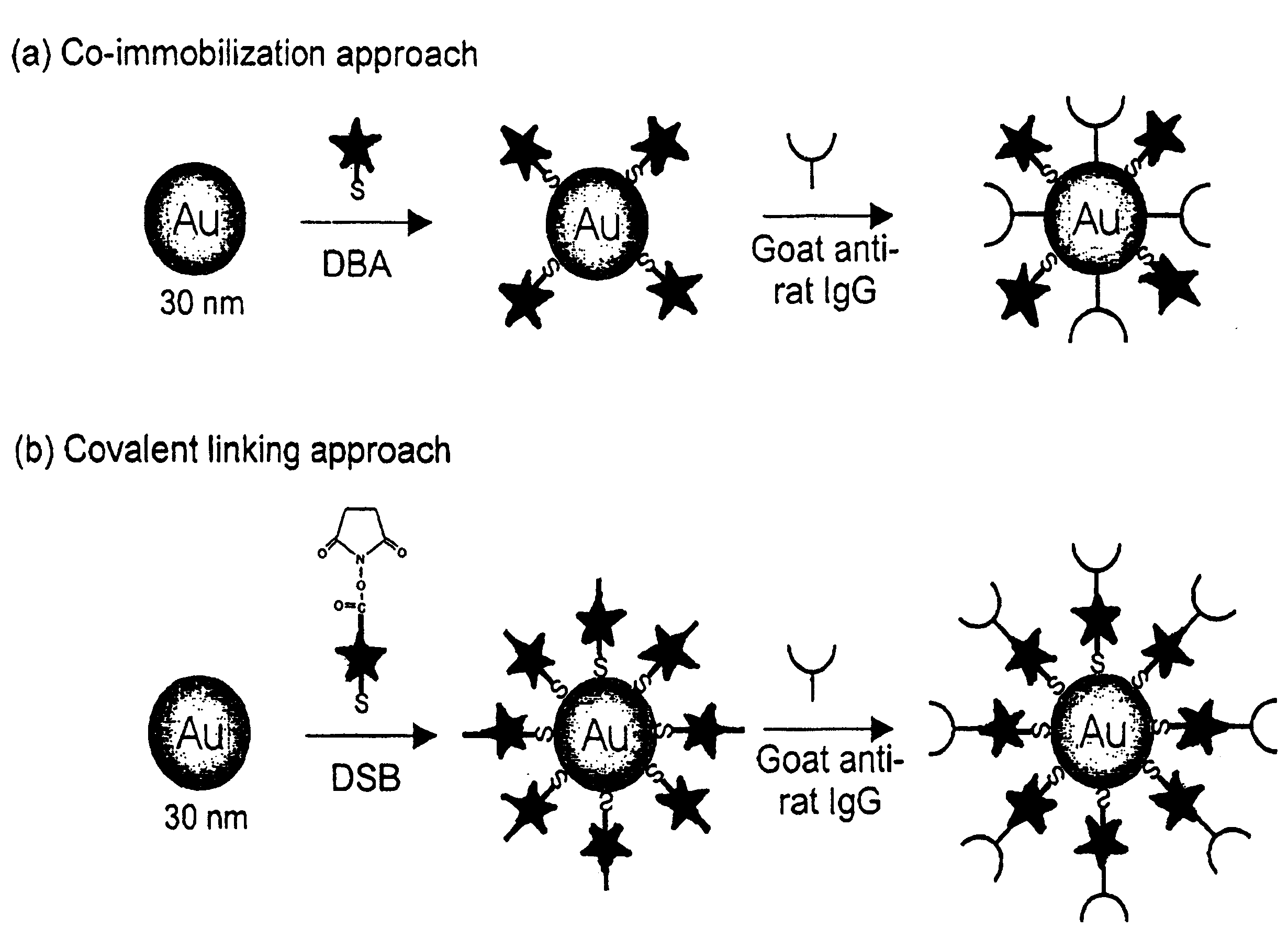
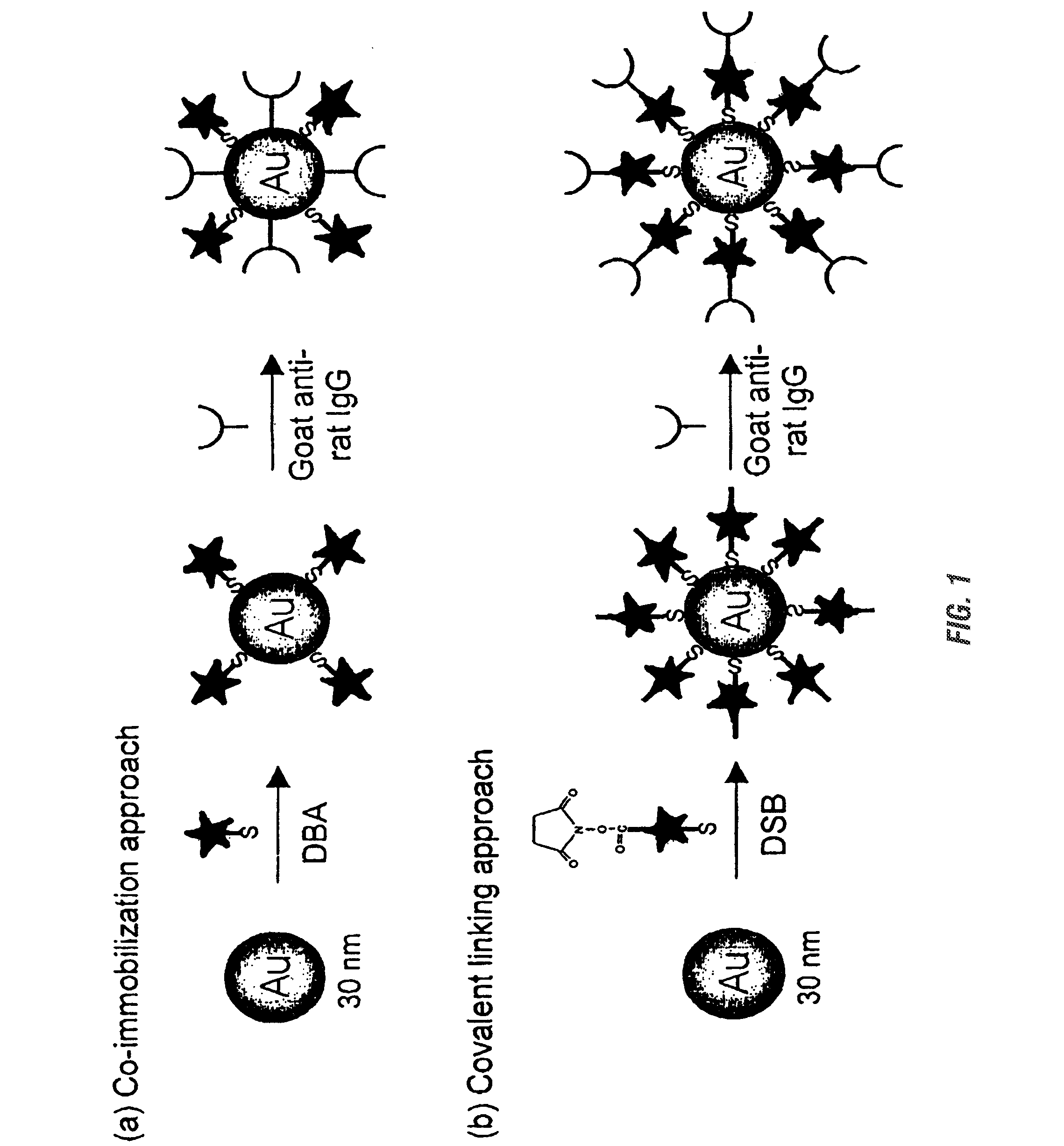
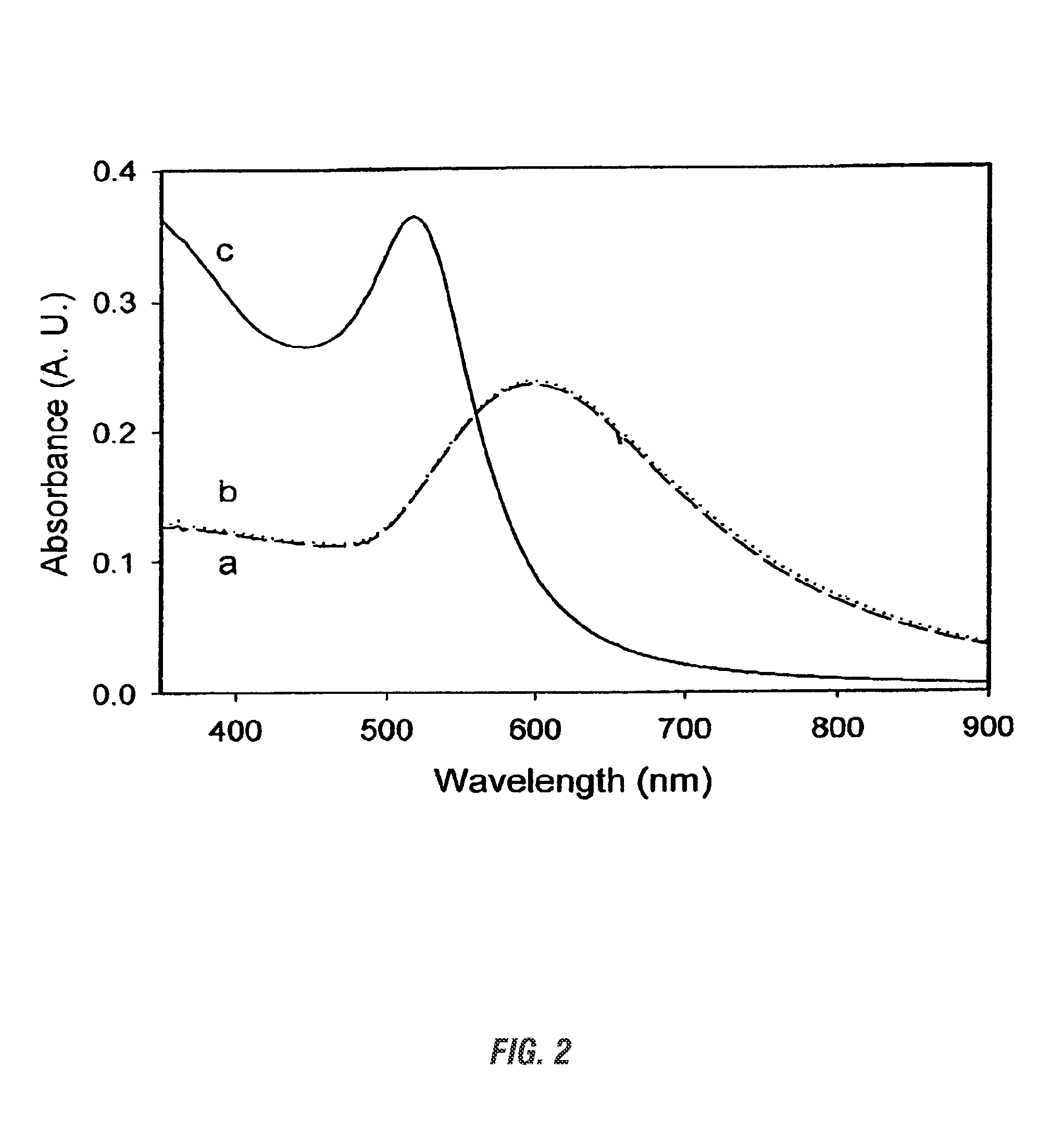
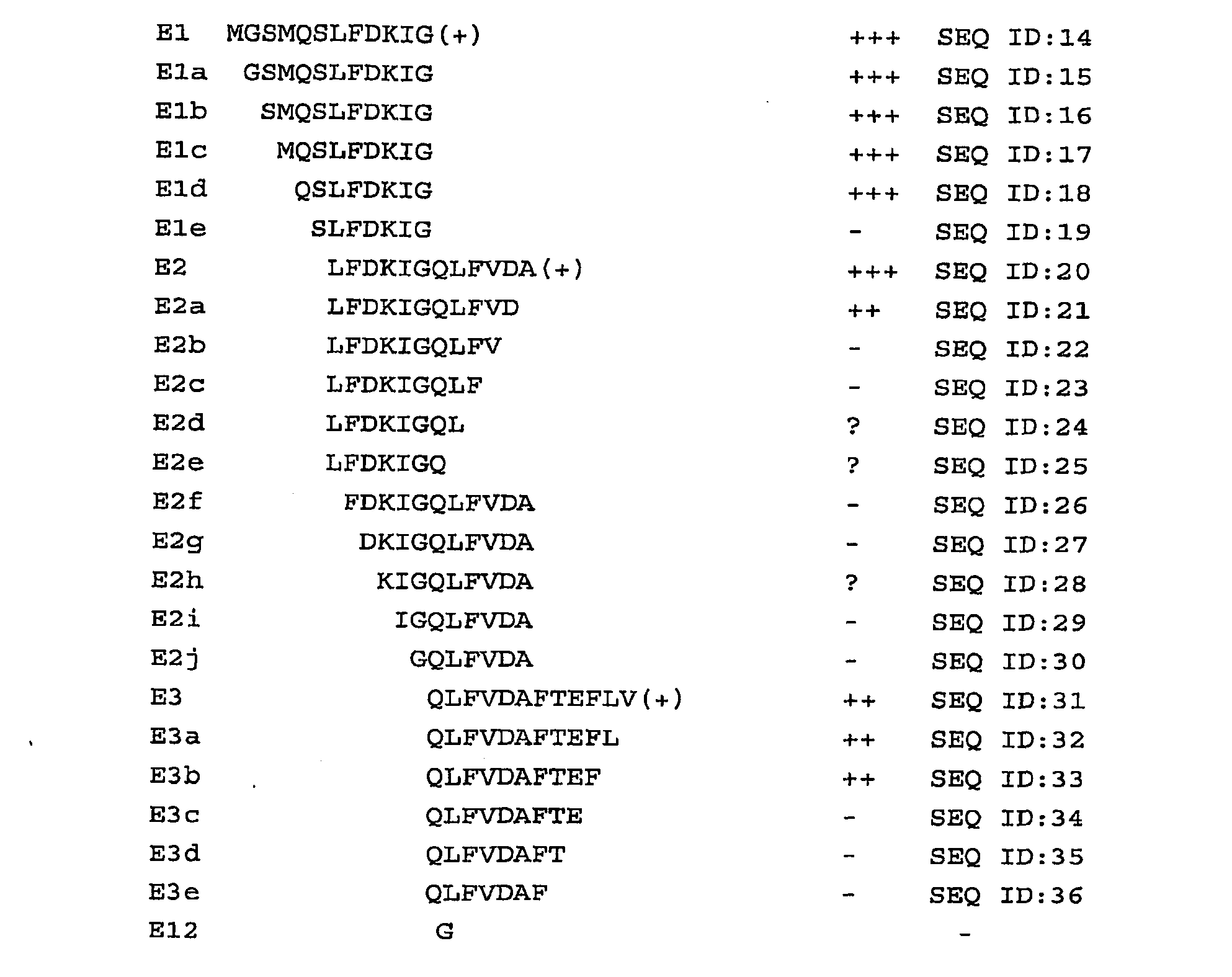
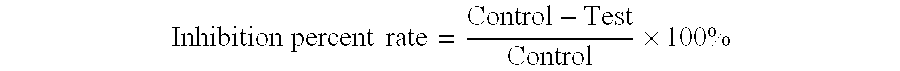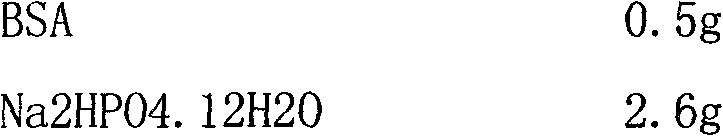Patents
Literature
104 results about "Immuno assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of immunoassay. : a technique or test (as the enzyme-linked immunosorbent assay) used to detect the presence or quantity of a substance (as a protein) based on its capacity to act as an antigen or antibody.
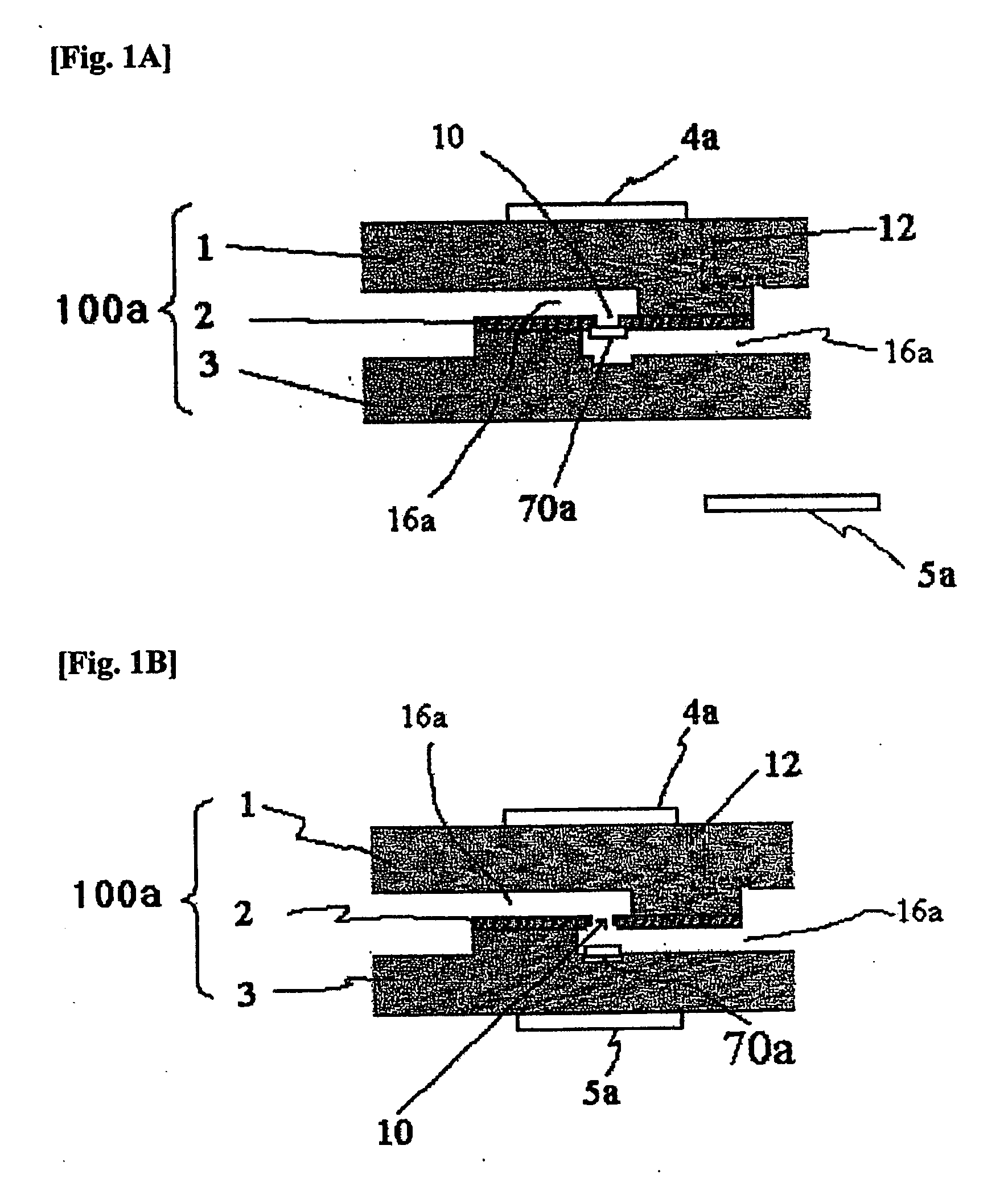
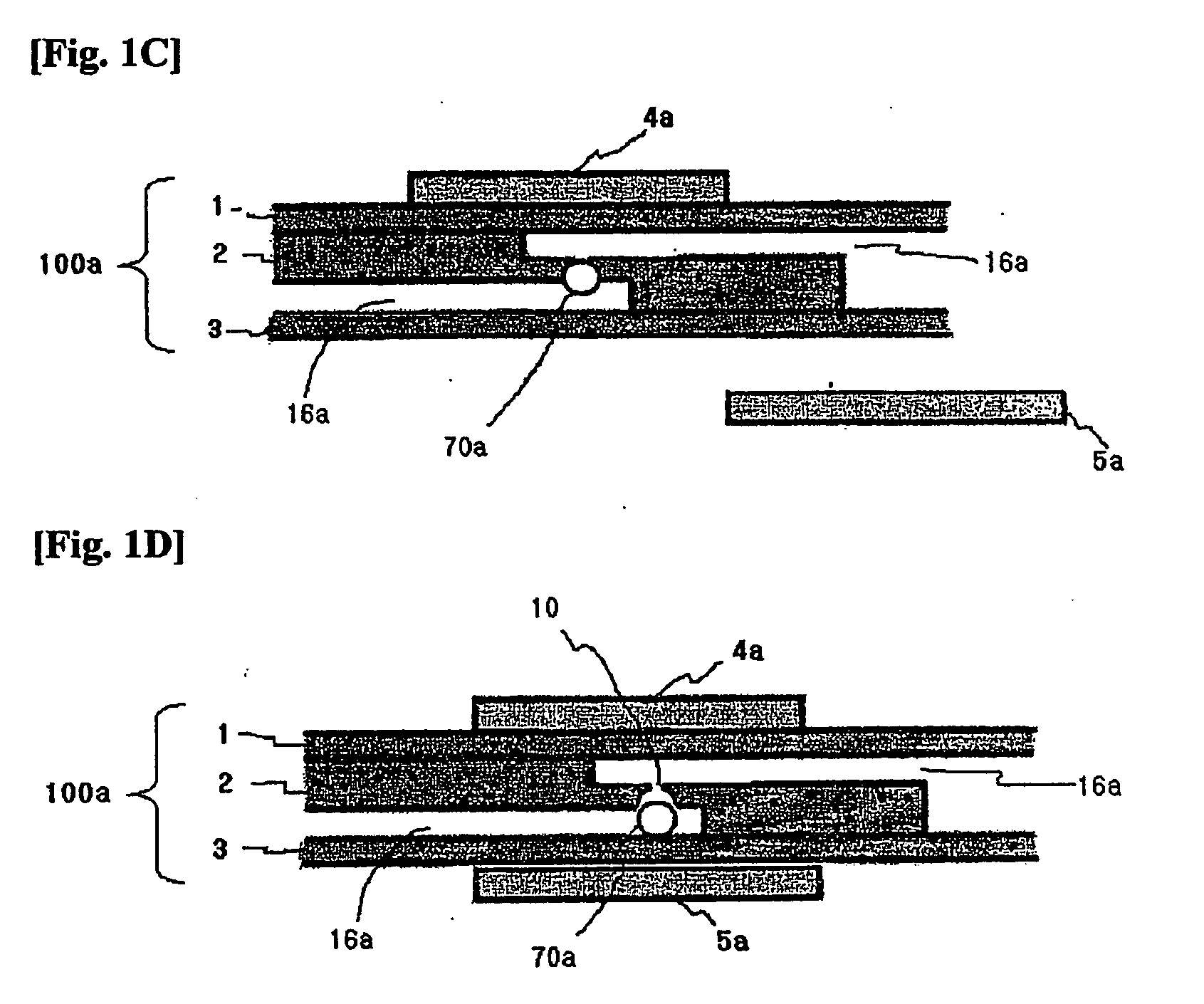
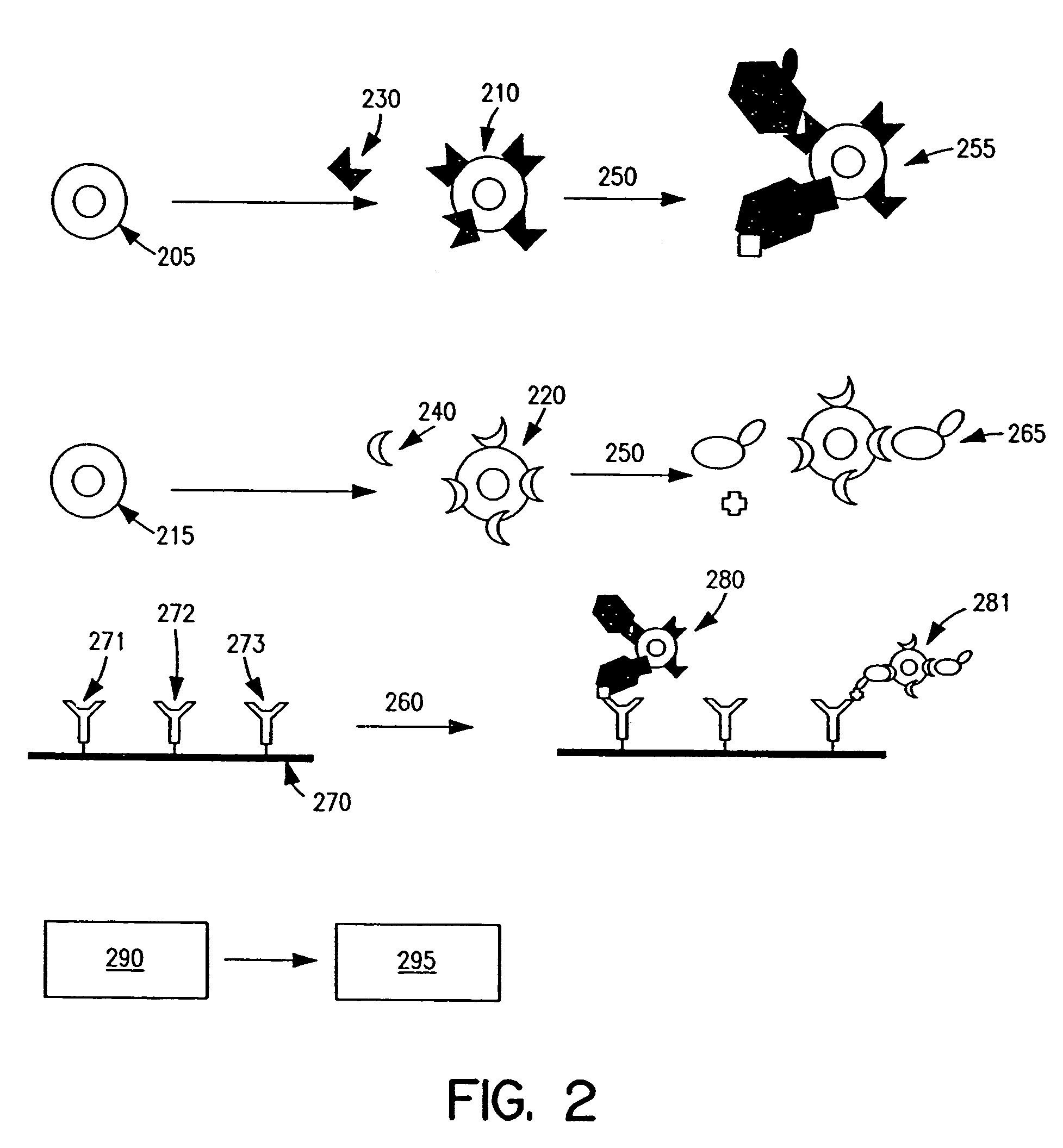
Apparatus and methods for analyte measurement and immuno assay
ActiveUS20030170881A1Avoid disadvantagesBioreactor/fermenter combinationsBiological substance pretreatmentsPoint of careOrganism
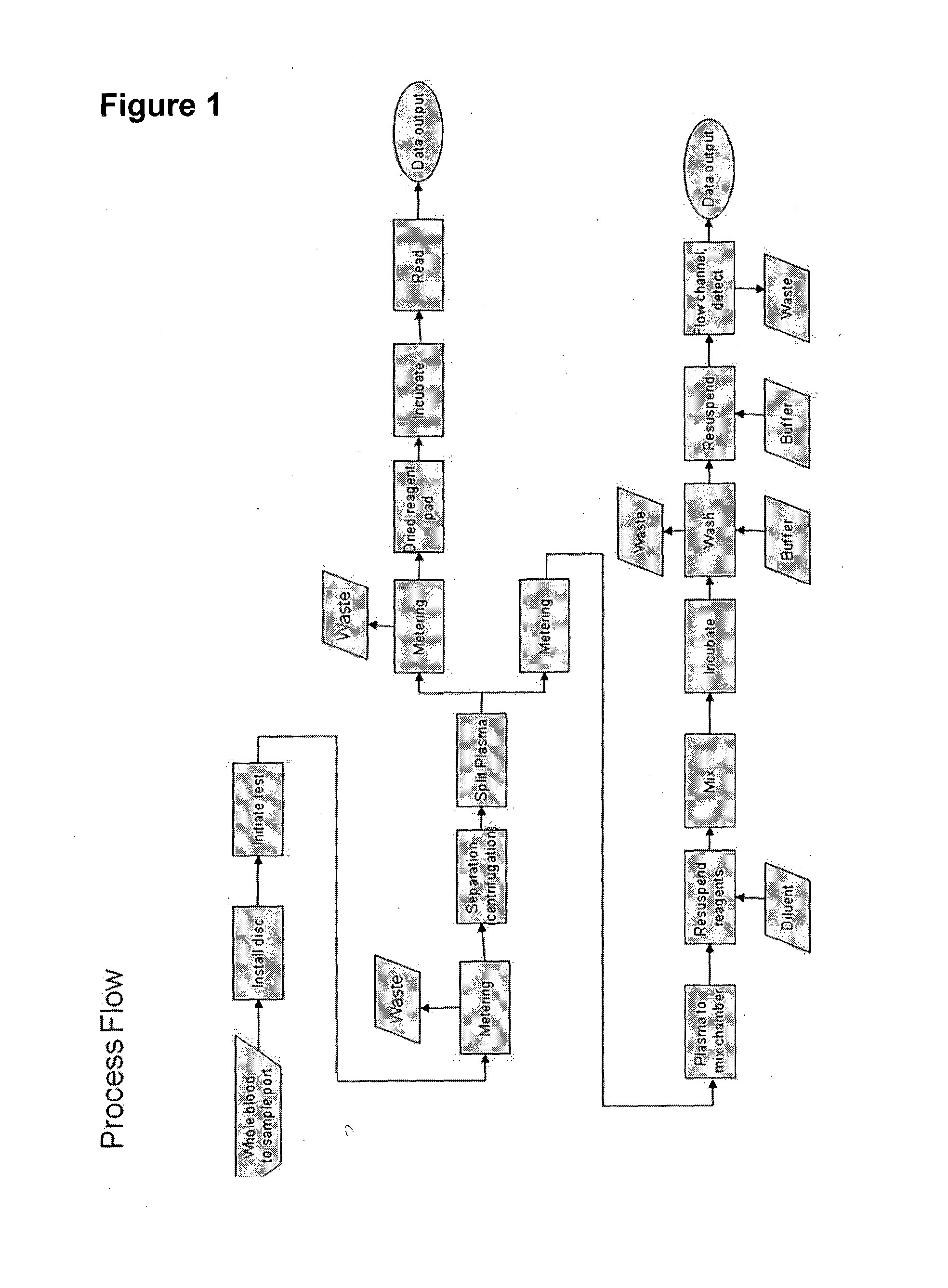
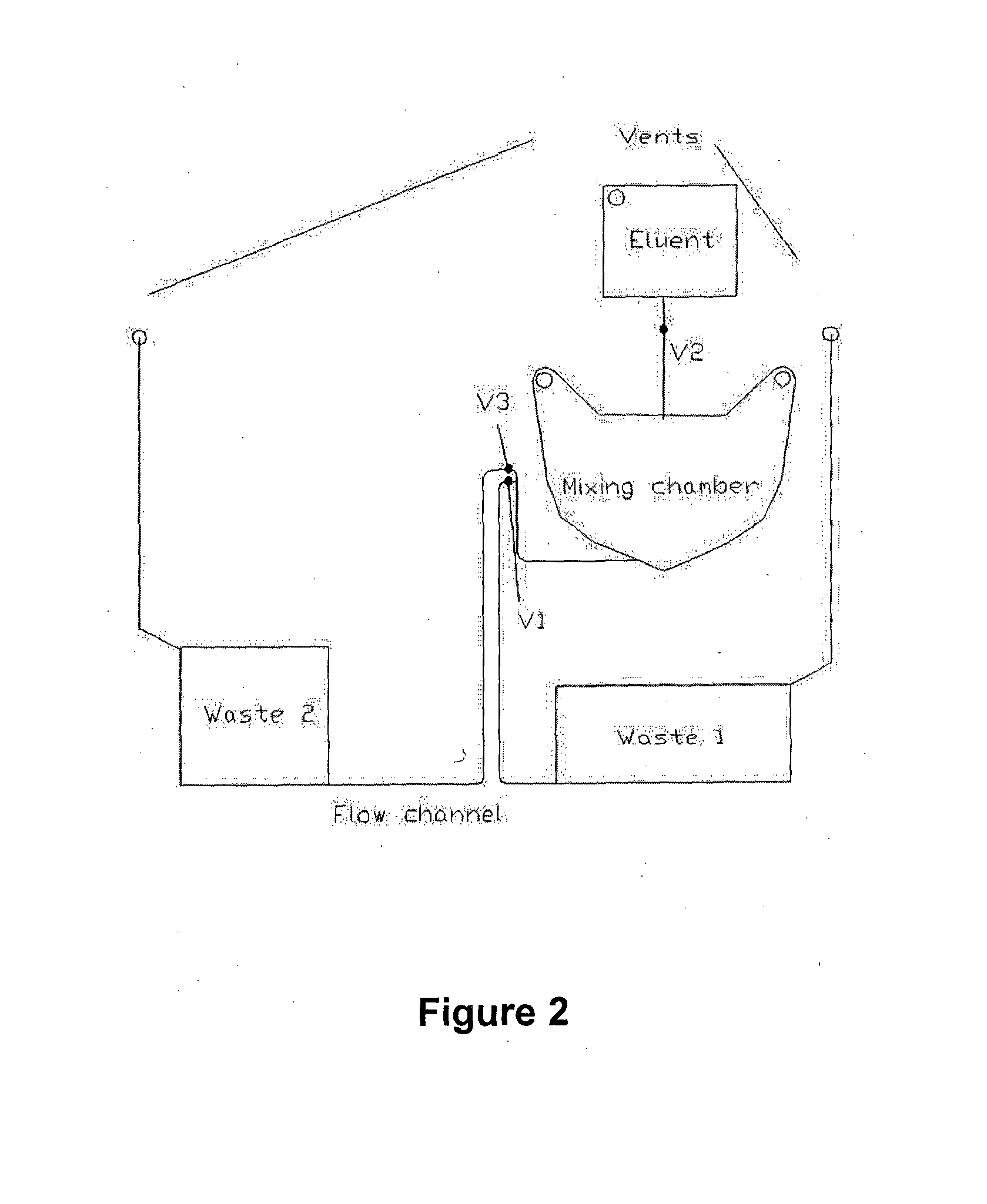
The present invention relates to an apparatus for conducting a variety of assays for the determination of analytes in liquid samples, and relates to the methods for such assays. In particular, the invention relates to a single-use cartridge designed to be adaptable to a variety of real-time assay protocols, preferably assays for the determination of analytes in biological samples using immunosensors or other ligand / ligand receptor-based biosensor embodiments. The cartridge provides novel features for processing a metered portion of a sample, for precise and flexible control of the movement of a sample or second fluid within the cartridge, for the amending of solutions with additional compounds during an assay, and for the construction of immunosensors capable of adaptation to diverse analyte measurements. The disclosed device and methods of use enjoy substantial benefits over the prior art, including simplicity of use by an operator, rapid in situ determinations of one or more analytes, and single-use methodology that minimizes the risk of contamination of both operator and patient. The disclosed invention is adaptable to the point-of-care clinical diagnostic field, including use in accident sites, emergency rooms, surgery, nursing homes, intensive care units, and non-medical environments.
Owner:ABBOTT POINT CARE
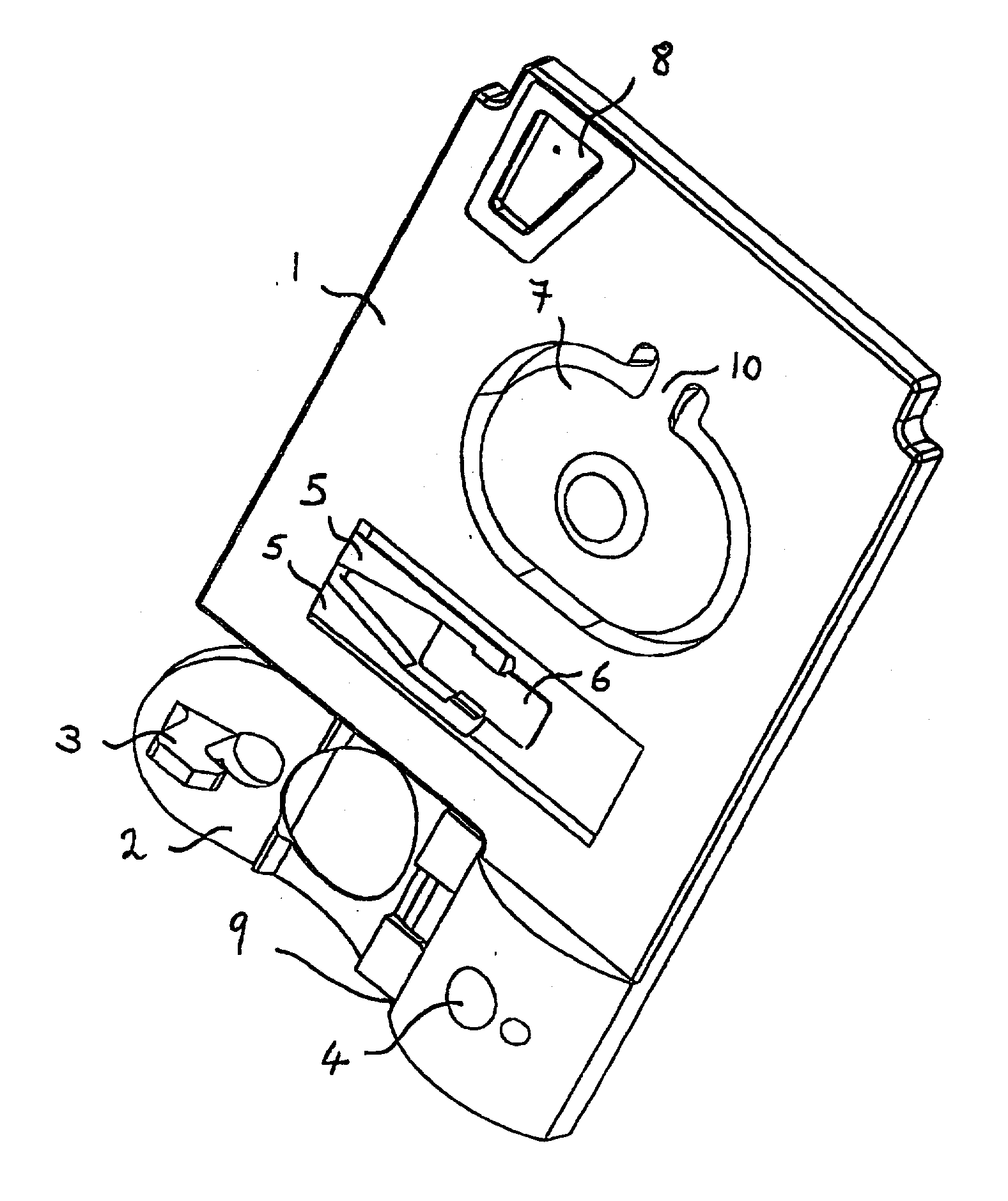
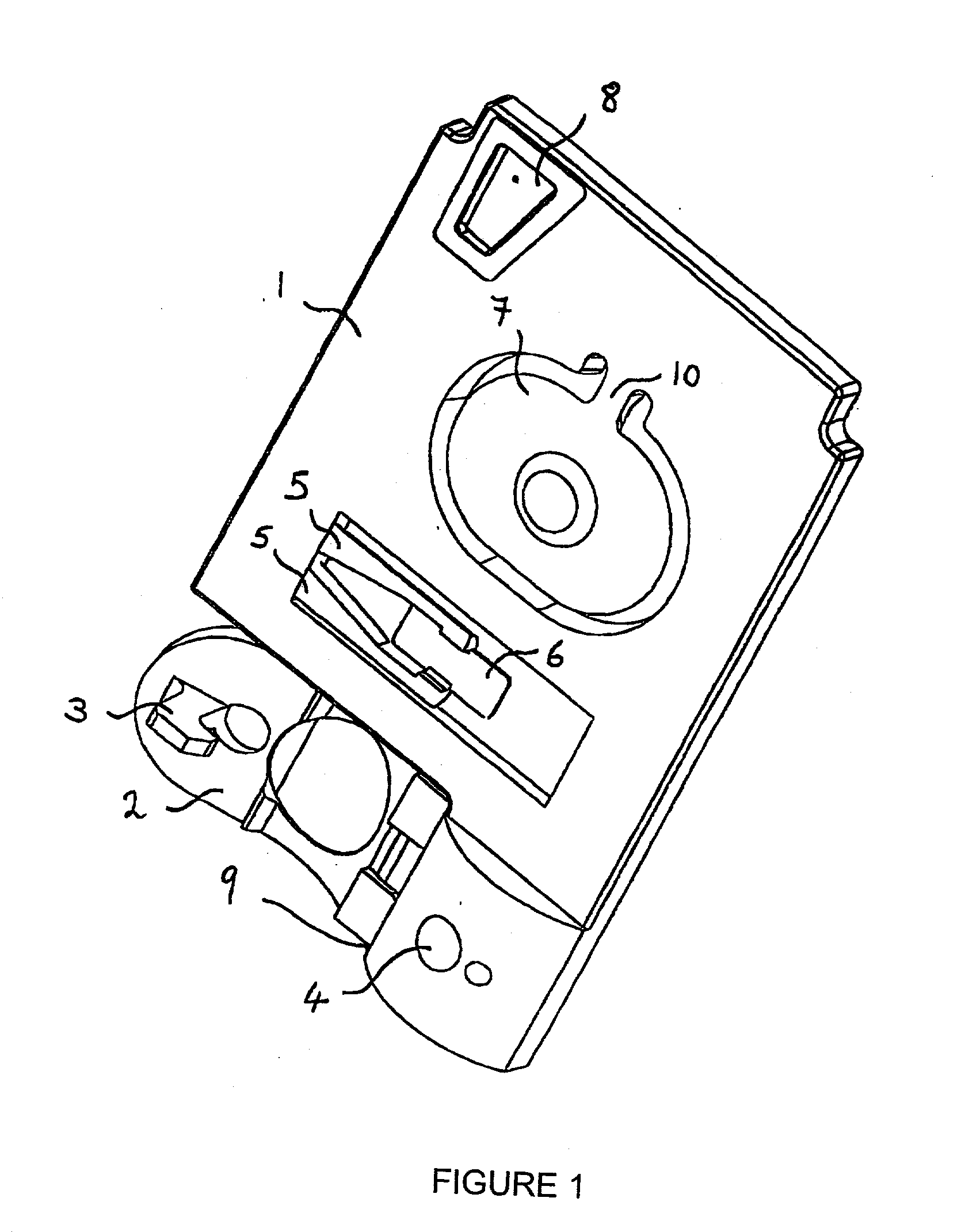
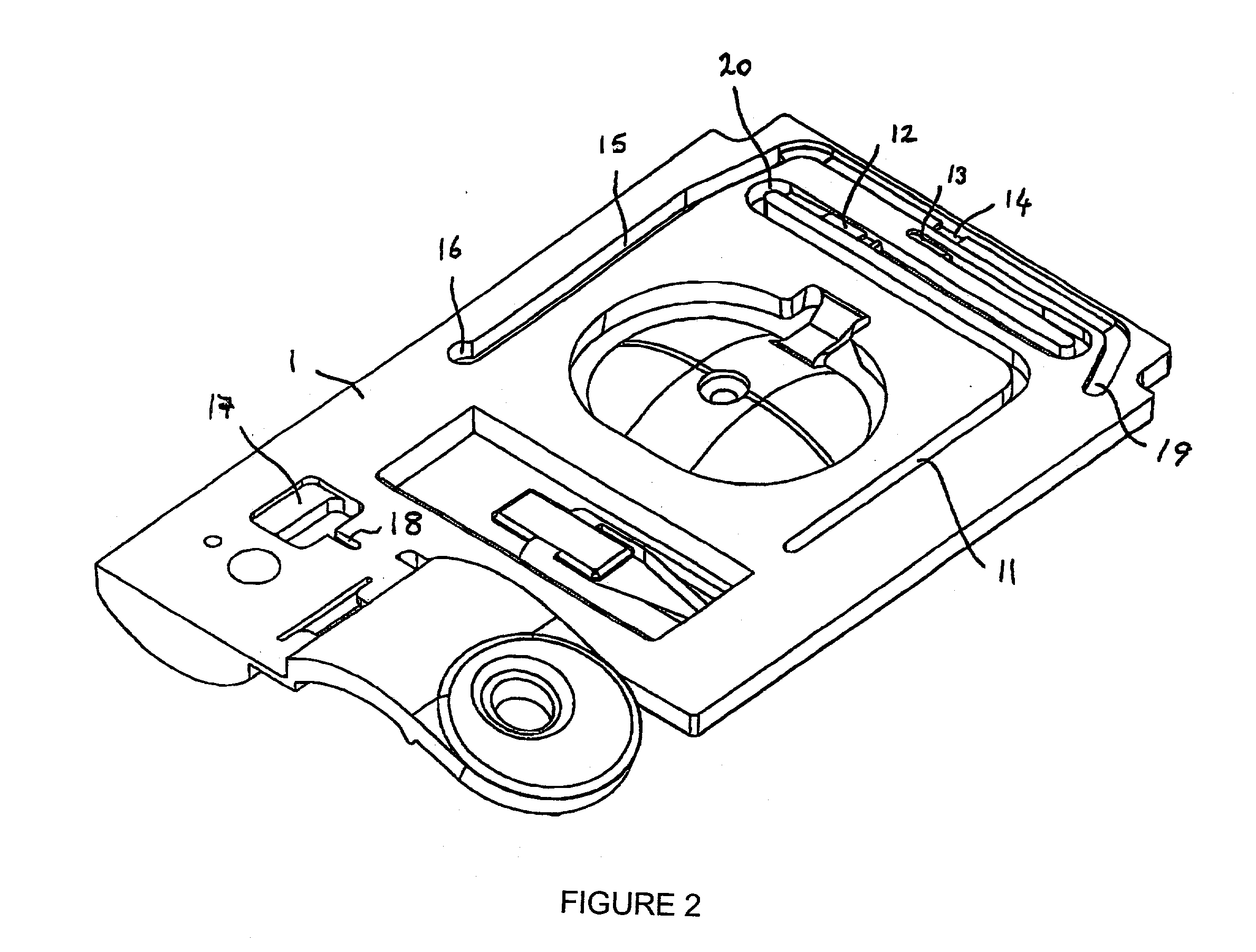
Bio memory disc and bio memory disc drive apparatus, and assay method using the same
ActiveUS20090253130A1Easy to implementPrevent leakageBioreactor/fermenter combinationsBiological substance pretreatmentsProcess systemsImmuno assay
The present invention provides a bio memory disc where a lab-on-a-chip process system including an assay-diagnosis unit, a nucleic acid hybridization assay unit, or an immuno-assay unit and a semiconductor memory is disposed, a bio memory disc drive apparatus including a controller for controlling and driving an optical disc including CD or DVD and the bio memory disc and an assay method using the same.
Owner:PRECISIONBIOSENSOR INC
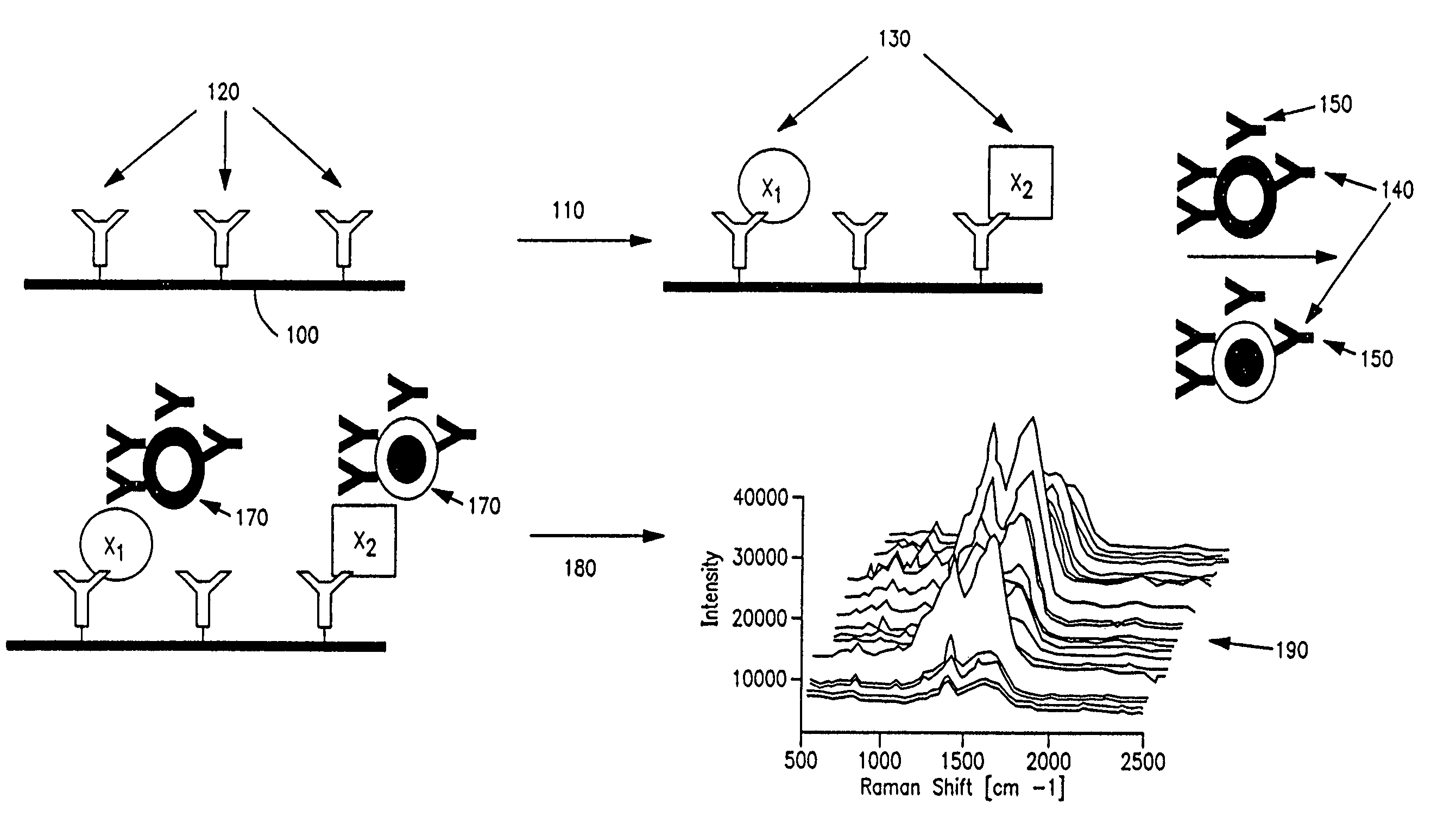
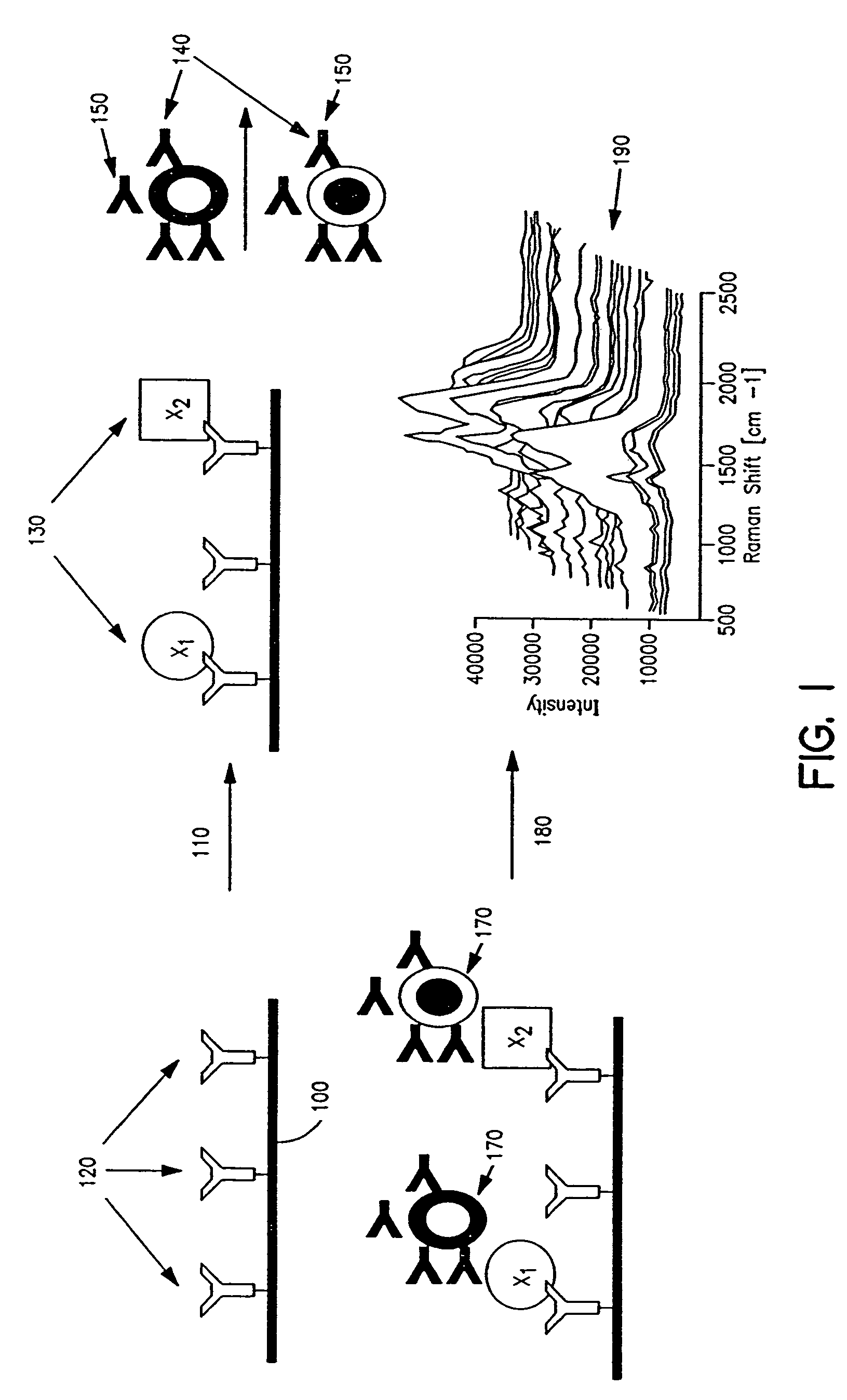
Cellular analysis using Raman surface scanning
Methods and apparatus are provided for assaying cell samples, which may be living cells, using probes labeled with composite organic-inorganic nanoparticles (COINs) and microspheres with COINs embedded within a polymer matrix to which the probe moiety is attached. COINs intrinsically produce SERS signals upon laser irradiation, making COIN-labeled probes particularly suitable in a variety of methods for assaying cells, including biological molecules that may be contained on or within cells, most of which are not inherently Raman-active. The invention provides variations of the sandwich immunoassay employing both specific and degenerate binding, methods for reverse phase assay of tissue samples and cell microstructures, in solution displacement and competition assays, and the like. Systems and chips useful for practicing the invention assays are also provided.
Owner:INTEL CORP
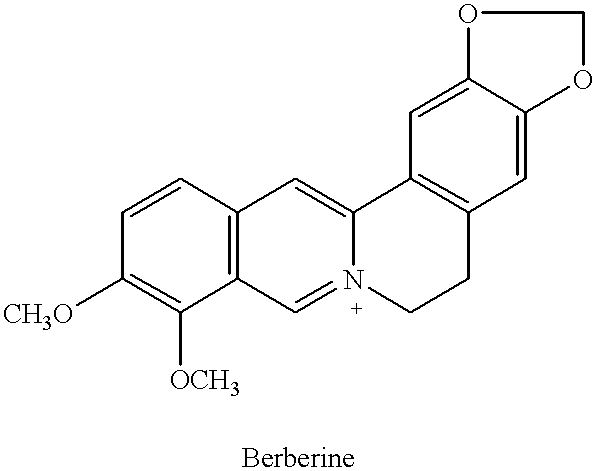
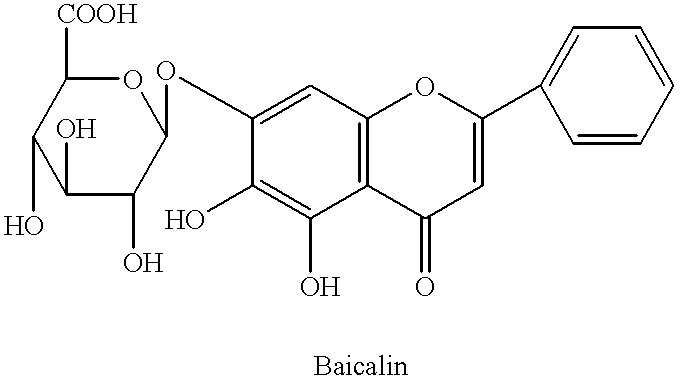
Plant drug for preventing cancer II
InactiveUS6290995B1Efficient analysisAccurate measurementBiocideUnknown materialsRadioimmunoassayBerberine
This invention relates to new safe natural drug, which is prevention of cancer and control of cancer cells. Specifically, this invention provides methods for producing of Berberine and Baicalin.Also, the present invention proved a new radioimmunoassay (RIA) method for precise determination of Berberine and Baicalin. The RIA is an efficient analytical method for large clinical programs including double blind analysis (DBA), and good clinical practice (GCP).
Owner:XINXIAN ZHAO
Antibody pair screening methods
The invention provides methods for identifying antibody preparations that can form a pair of antibodies that optimally detect a target antigen, for example, in a sandwich immunoassay. These methods provide high affinity and epitope-specific antibodies.
Owner:KIMBERLY-CLARK WORLDWIDE INC
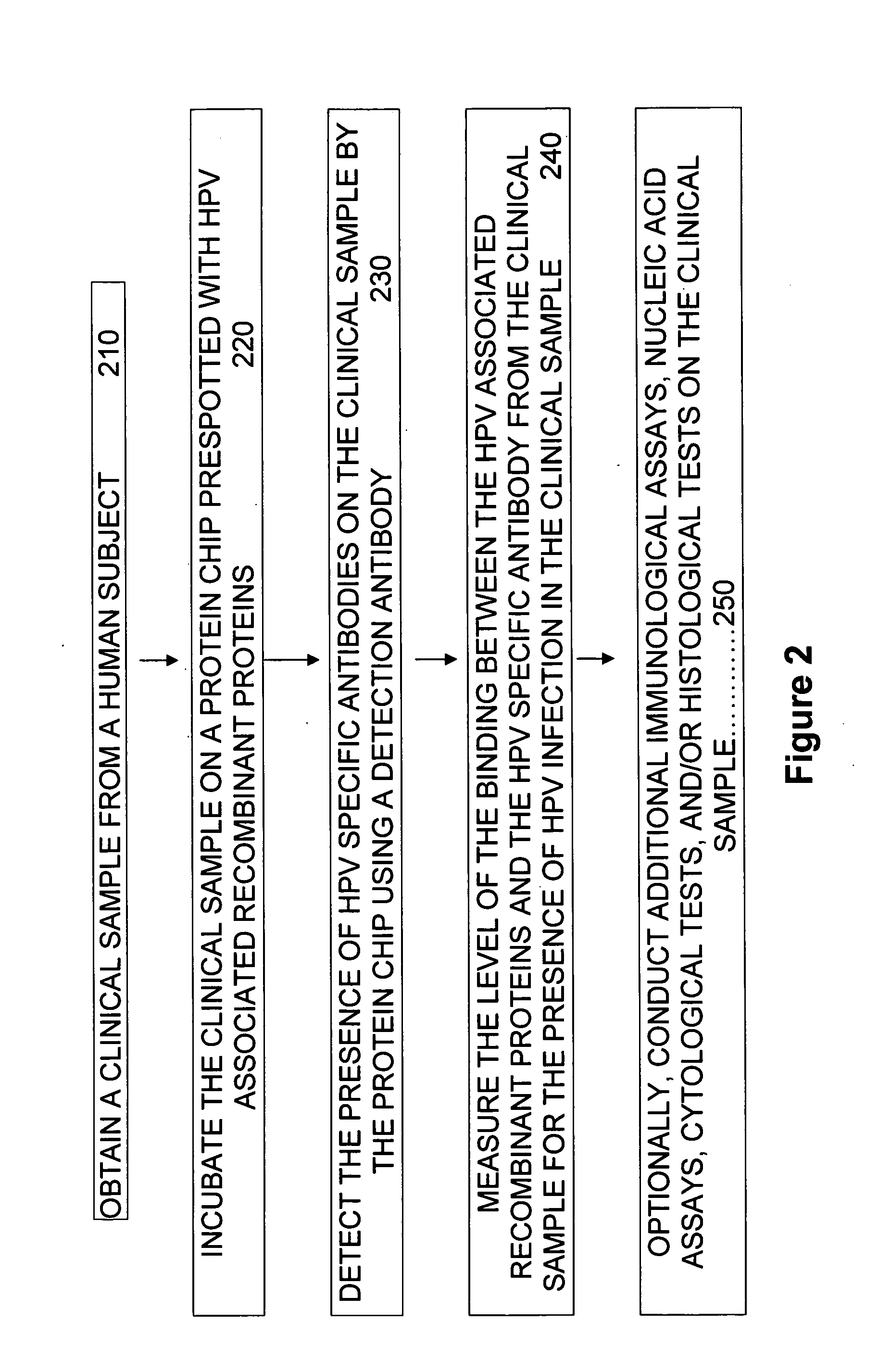
Protein chips for HPV detection
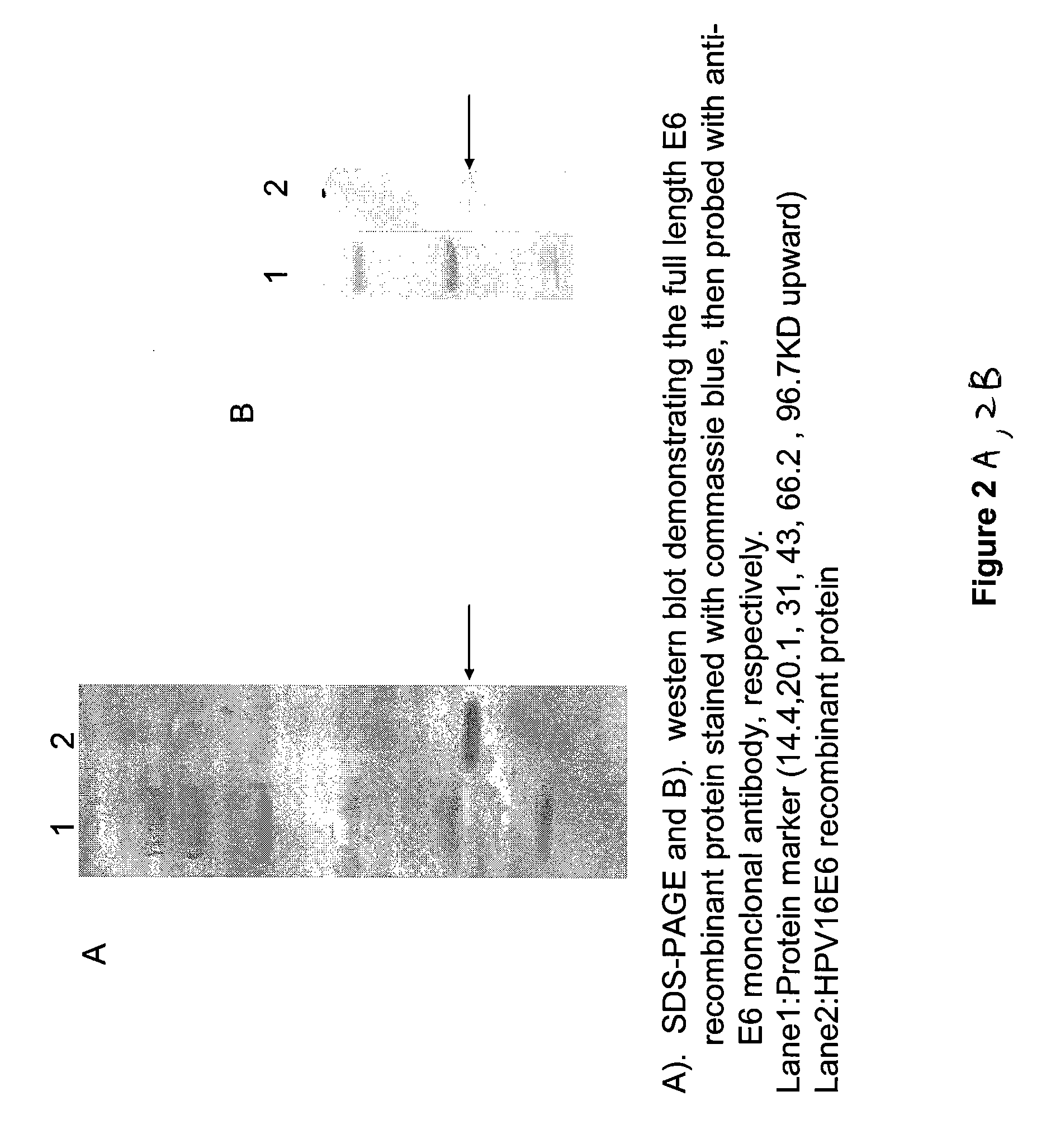
Embodiments of the invention provide methods, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Detection of HPV DNAs, genomes, and / or oncoproteins by protein chips immunological assays can be used in early clinical screening for HPV infection and general diagnosis for cervical cancer and can be advantageous performed in a multiplexed test. Comparative detection of altered levels of HPV proteins and host proteins can performed in one or more assays. The polypeptides, recombinant proteins, antibodies, nucleic acids, and various detection methods thereof are particularly useful for diagnosing carcinomas of the uterine cervix and those at risk of developing cervical cancer.
Owner:HEER MEDICAL TECH DEV CO LTD
Assay Devices with Integrated Sample Dilution and Dilution Verification and Methods of Using Same
ActiveUS20120142026A1Bioreactor/fermenter combinationsShaking/oscillating/vibrating mixersPoint of careAnalyte
The invention is to devices and method for rapid determination of analytes in liquid samples by various assays including immunoassays incorporating a sample dilution feature for forming a diluted sample for analysis. The devices and methods also include a dilution verification feature for verifying the degree of dilution of the diluted sample. The devices preferably are capable of being used in the point-of-care diagnostic field is provided.
Owner:ABBOTT POINT CARE
Sensor device for and a method of sensing particles
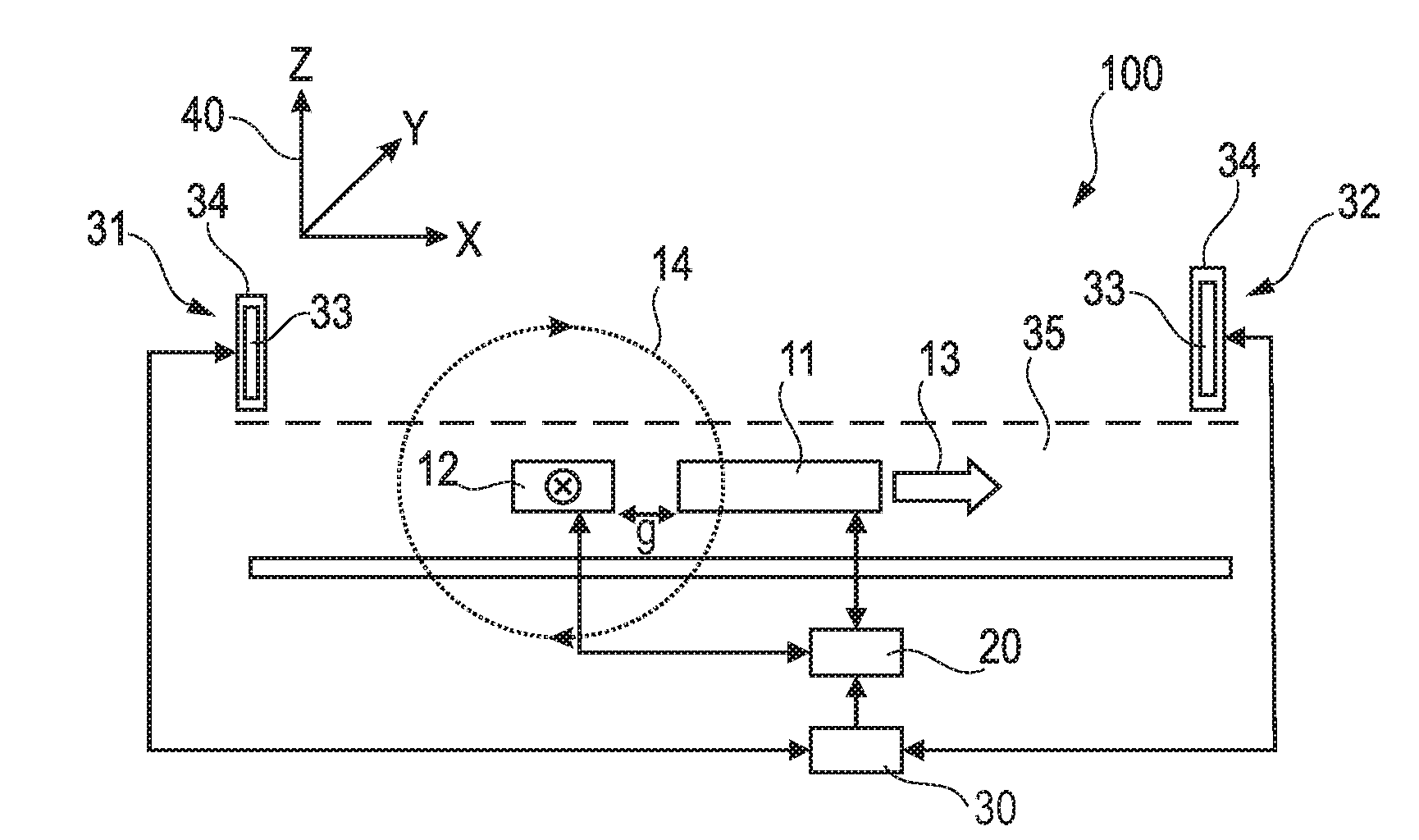
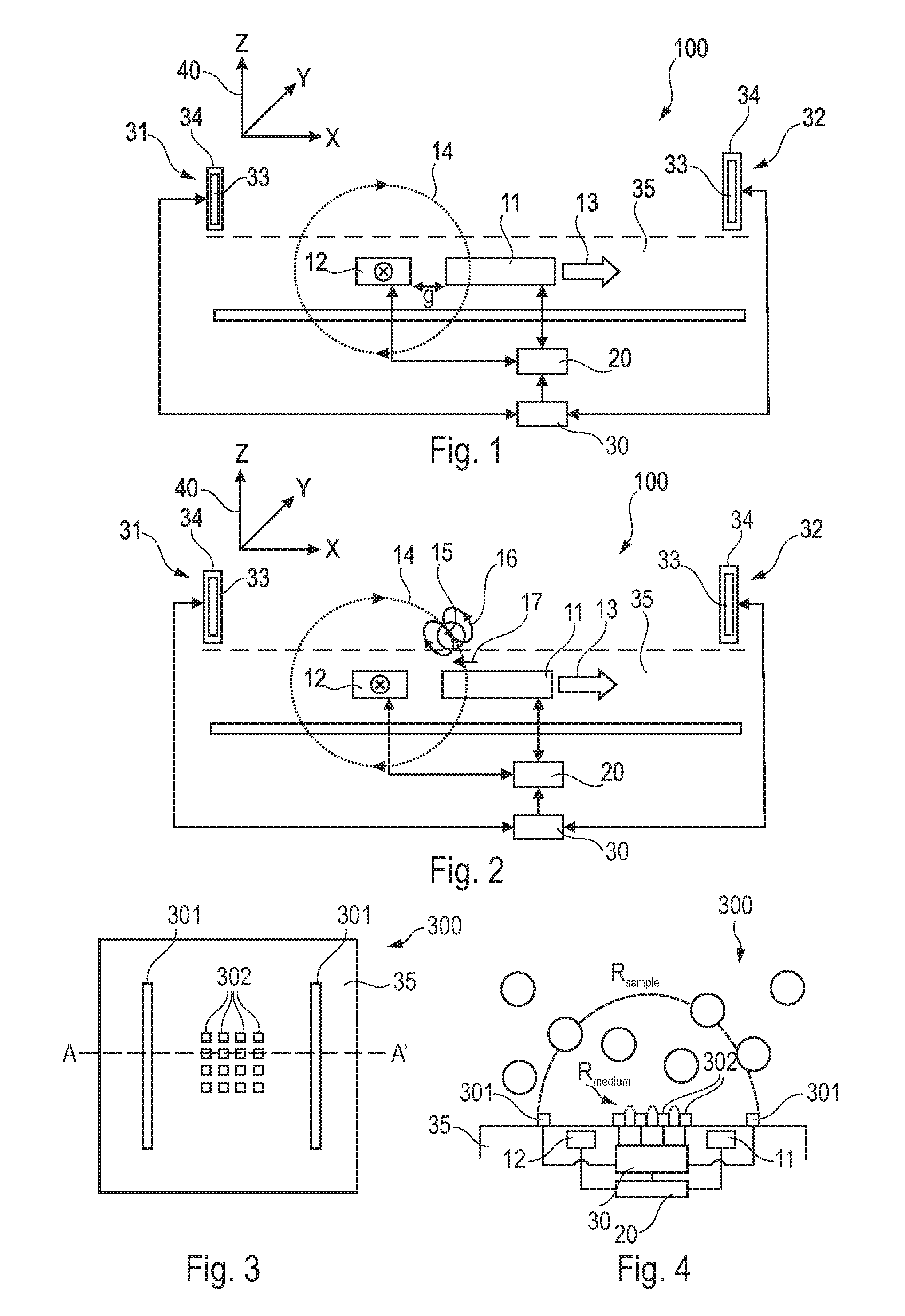
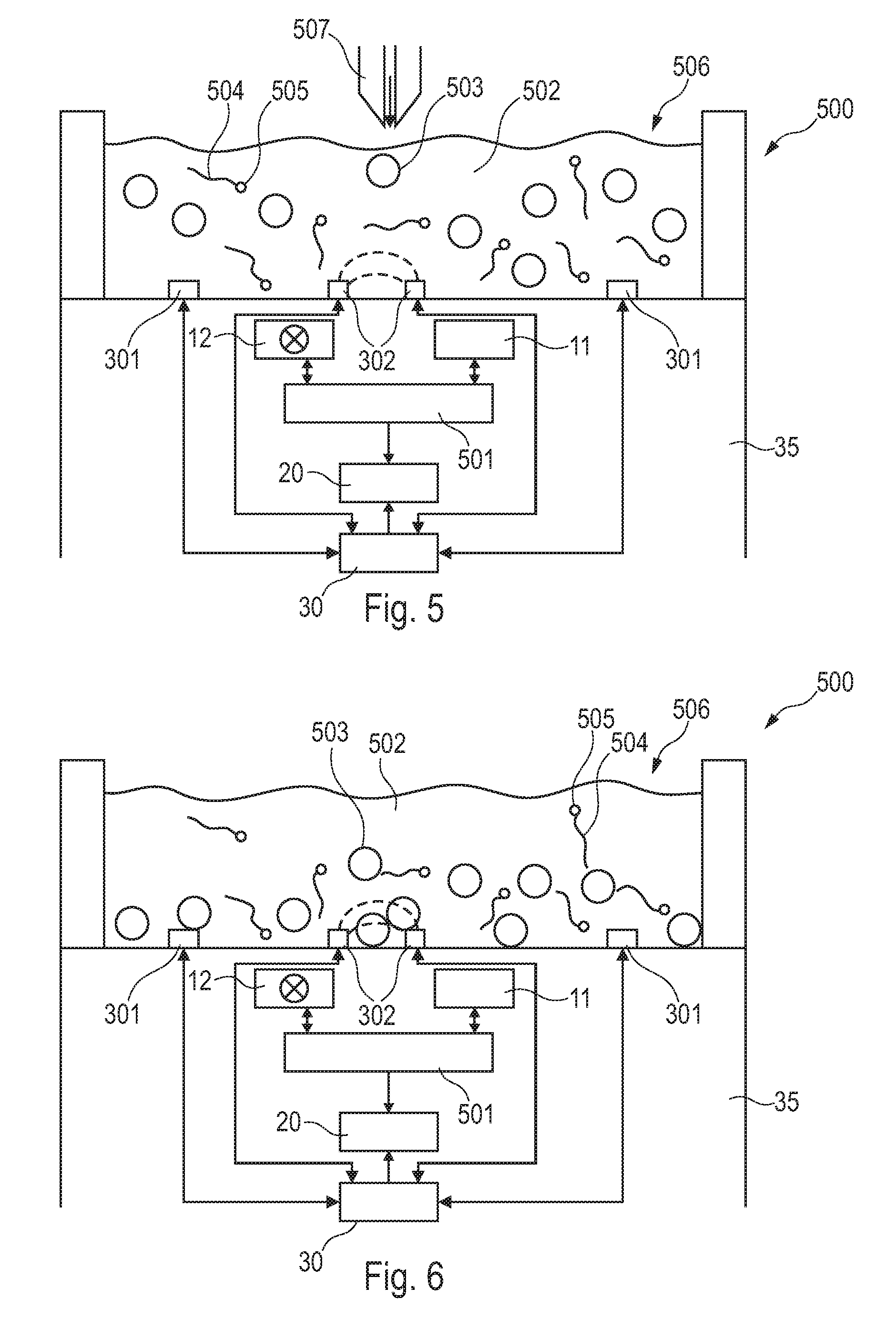
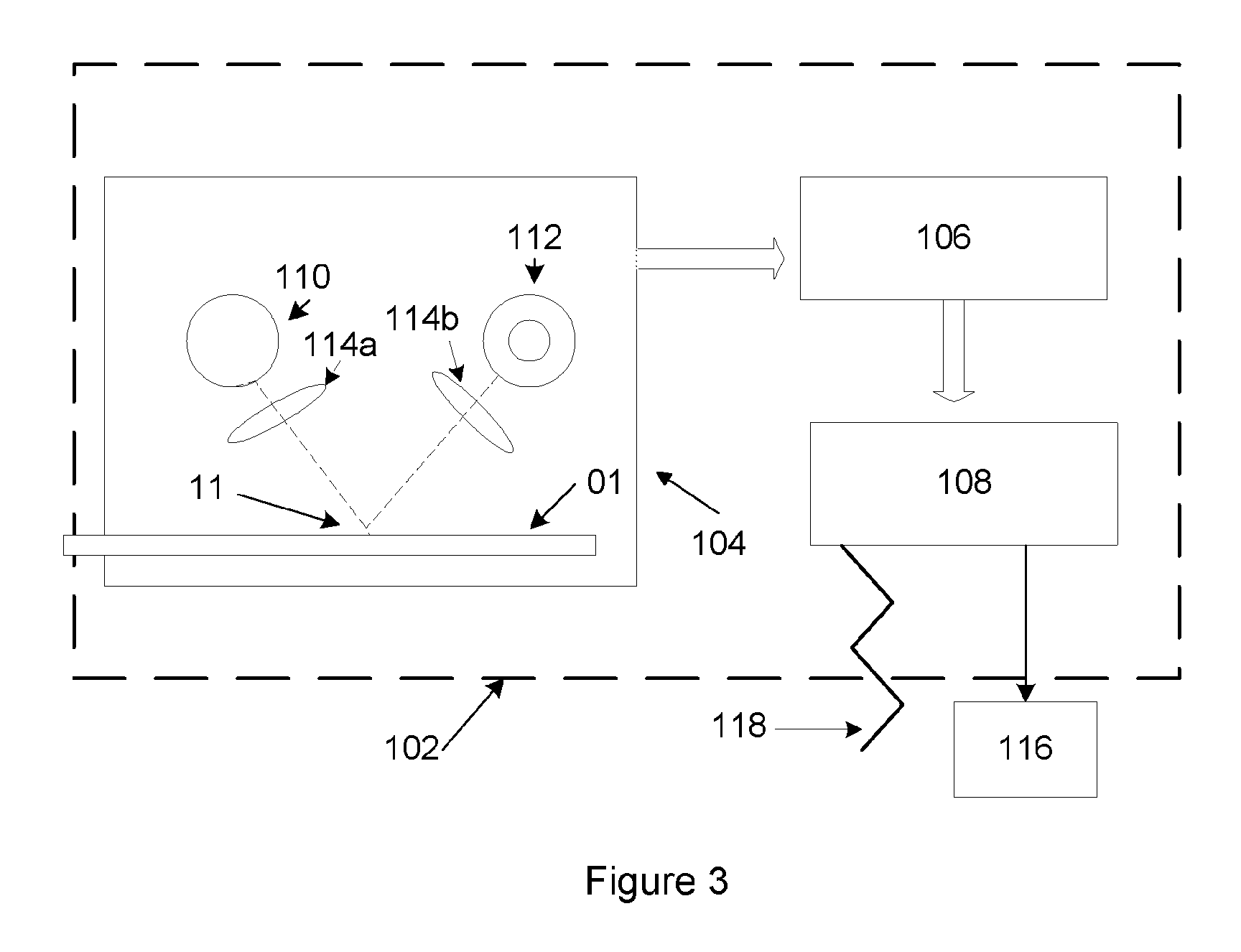
InactiveUS20090314066A1Simplifies cartridgeReduce and minimize durationResistance/reactance/impedenceIndividual particle analysisImmuno assayRed blood cell
A GMR based sensor device (100) for sensing first particles (504, 505) e.g. magnetic beads for immuno assay of a sample comprising the first particles (504, 505) and second particles (503) e.g. red blood cells, the sensor device (100) comprising a detection unit (11, 12) adapted to detect a signal which depends on a quantity of the first particles (504, 505) and which depends on a quantity of the second particles (503)″ based on a measurement performed with the sample comprising the first particles (504, 505) and the second particles (503), an estimation unit (30) for estimating information indicative of the quantity of the second particles (503) e.g. haematocrit based on an impedance measurement, and a determining unit (20) adapted for determining the quantity of the first particles (504, 505) based on the detected signal under consideration of the estimated information. The advantage of this arrangement is that whole blood samples may be used.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Microfluidic Disc For Use In With Bead-Based Immunoassays
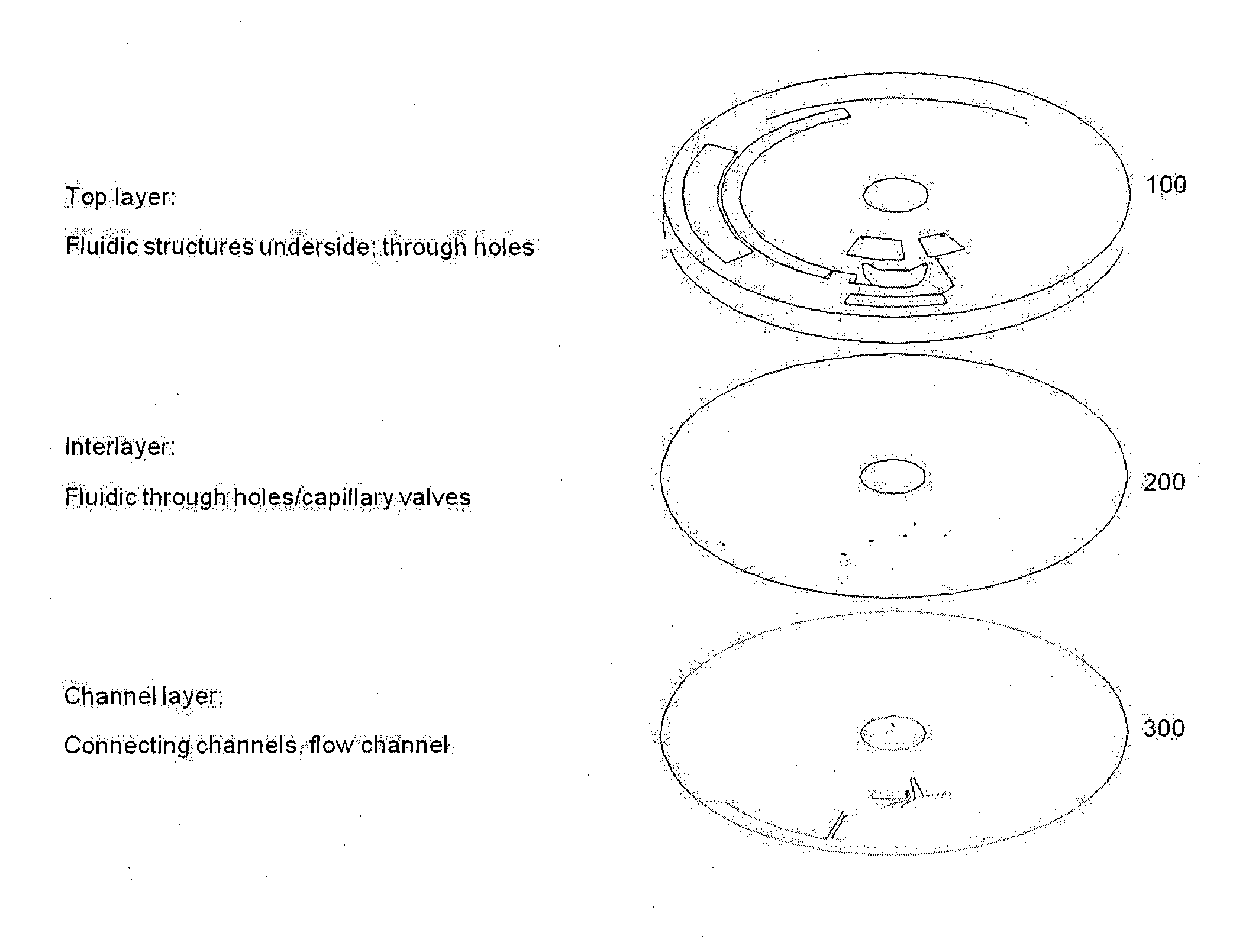
ActiveUS20140242721A1Remove complexityCost-effectiveComponent separationLaboratory glasswaresImmunofluorometric AssaysImmuno assay
The invention relates to a microfluidic system for processing biological samples comprising a rotary motor; a means for controlling said motor; a platform coupled to the rotary motor and adapted to provide at least one particle-washing structure and one particle receiving structure for receiving washed particles; and a detection zone for detection of particles of the sample in the particle receiving structure while the platform rotates. The invention provides a sample processing system that is both automated and prone to fewer errors than manual processing. This is accomplished using a centrifugal microfluidic platform that can process raw biological samples (e.g., blood, sputum, urine,) in order to perform high-quality bead-based immunofluorescent assays. The invention uses a simple rotary motor and custom-designed plastic disc to perform the sample preparation steps outlined above.
Owner:RADISENS DIAGNOSTICS
Nonseparation assay methods
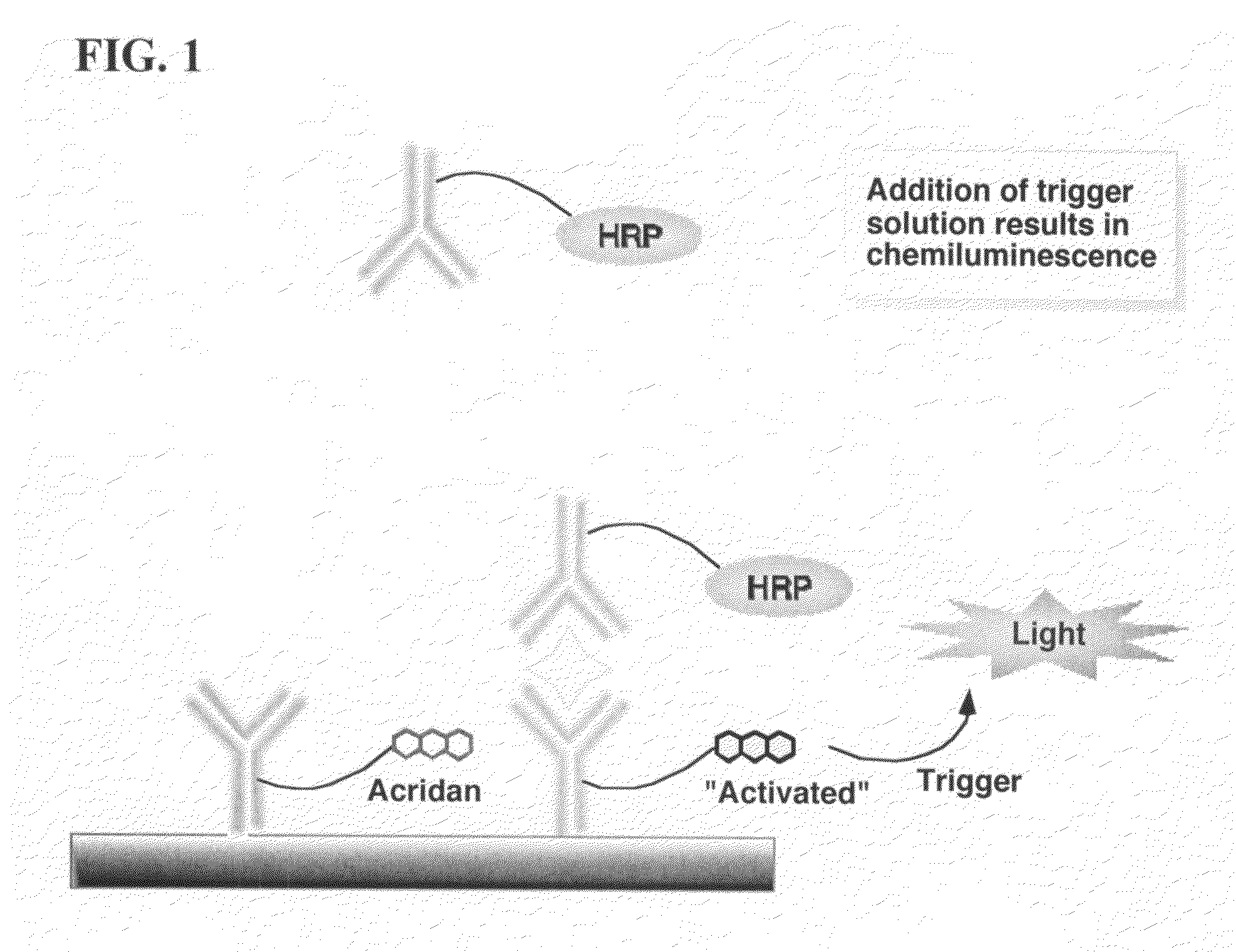
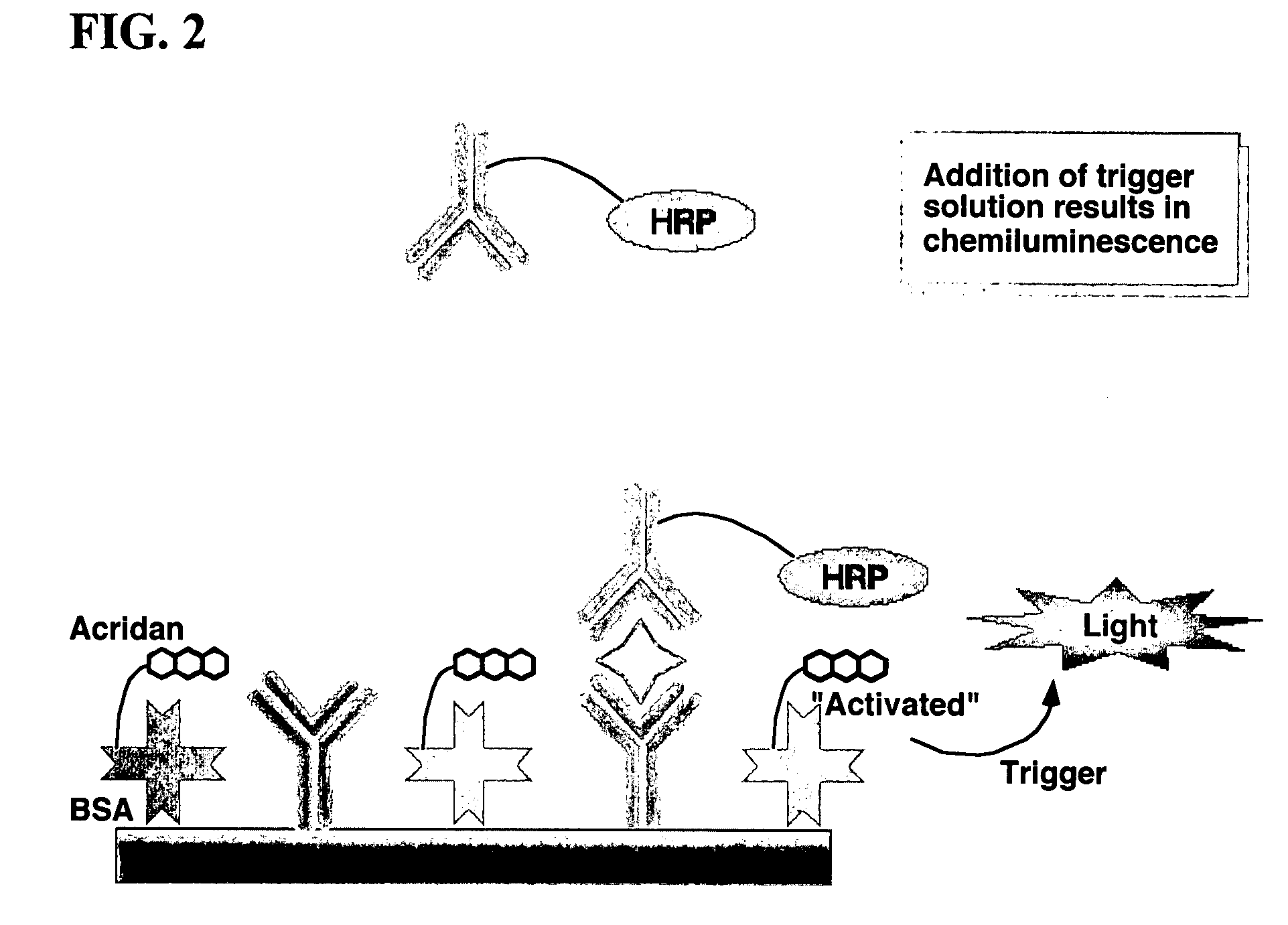
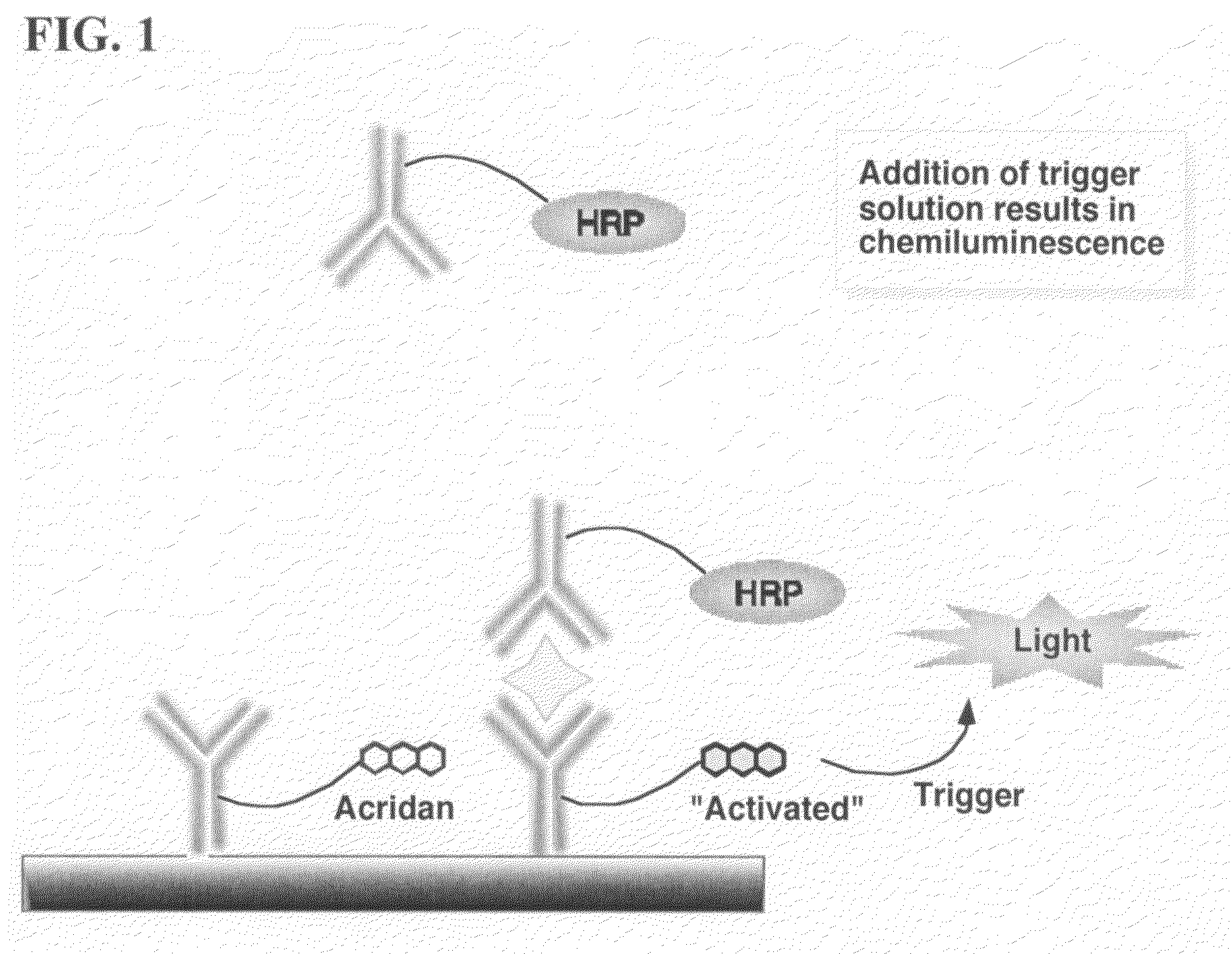
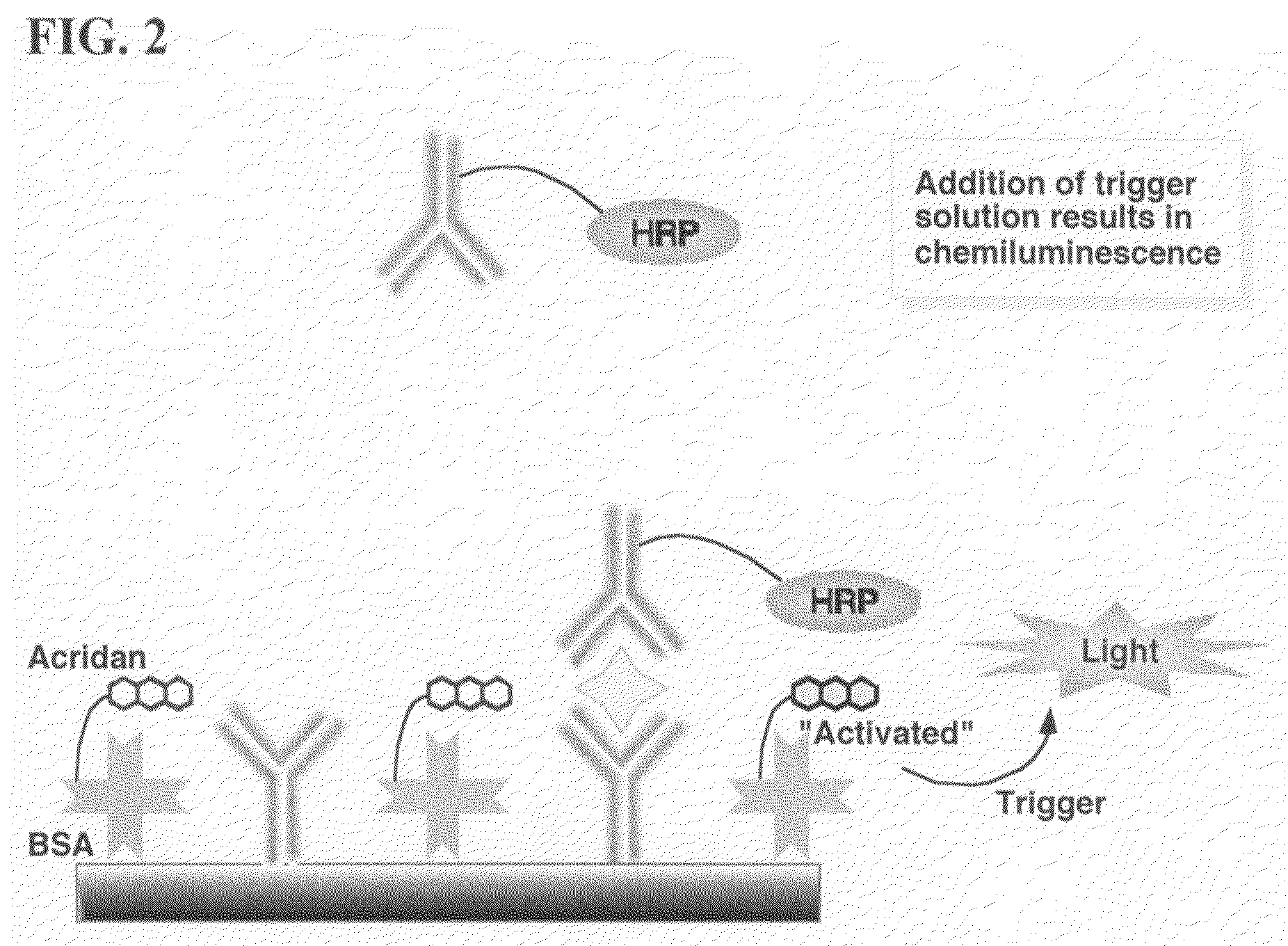
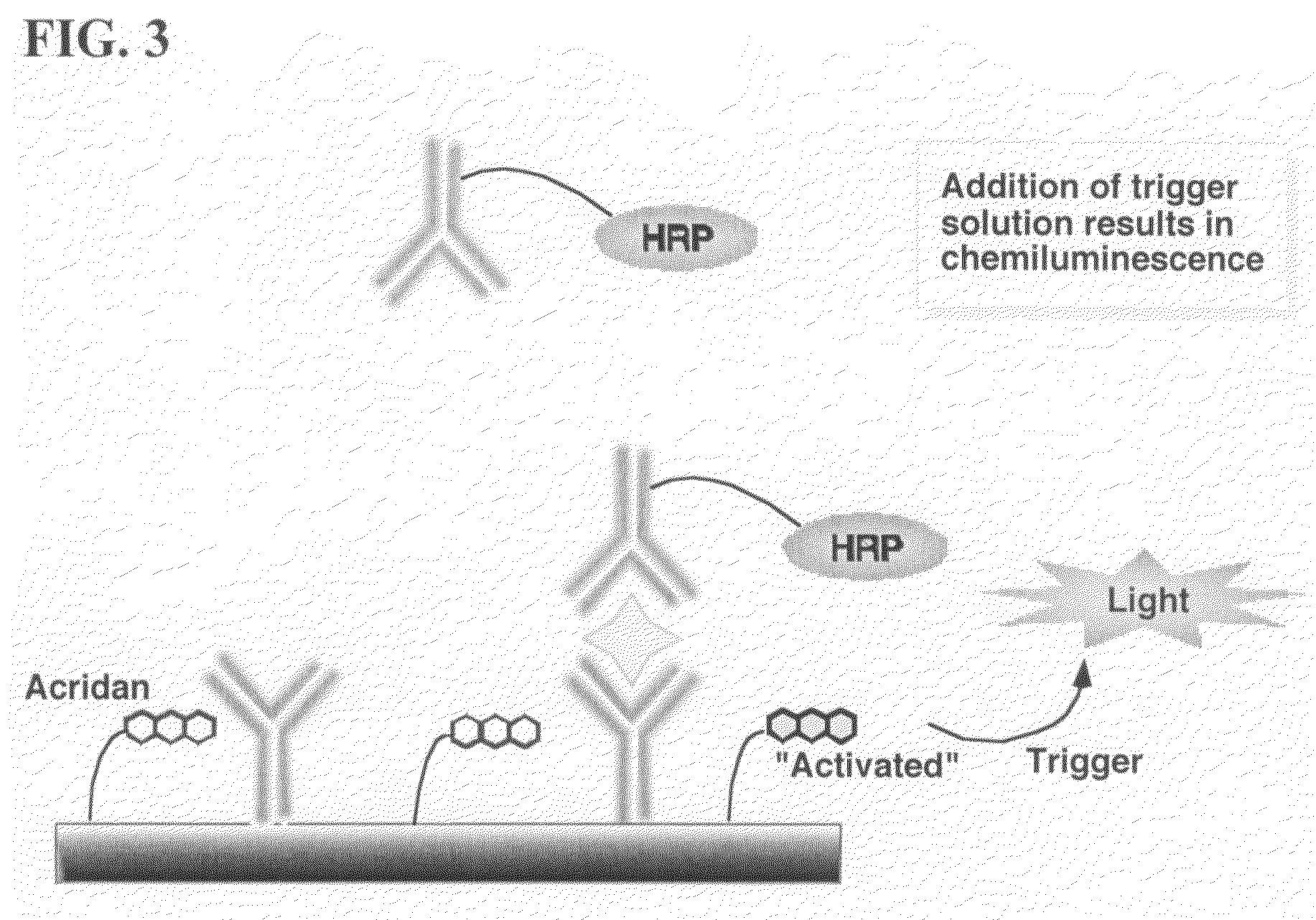
ActiveUS20070264664A1Simple methodSimplifying assayPeptide/protein ingredientsChemiluminescene/bioluminescenceAnalyteAssay
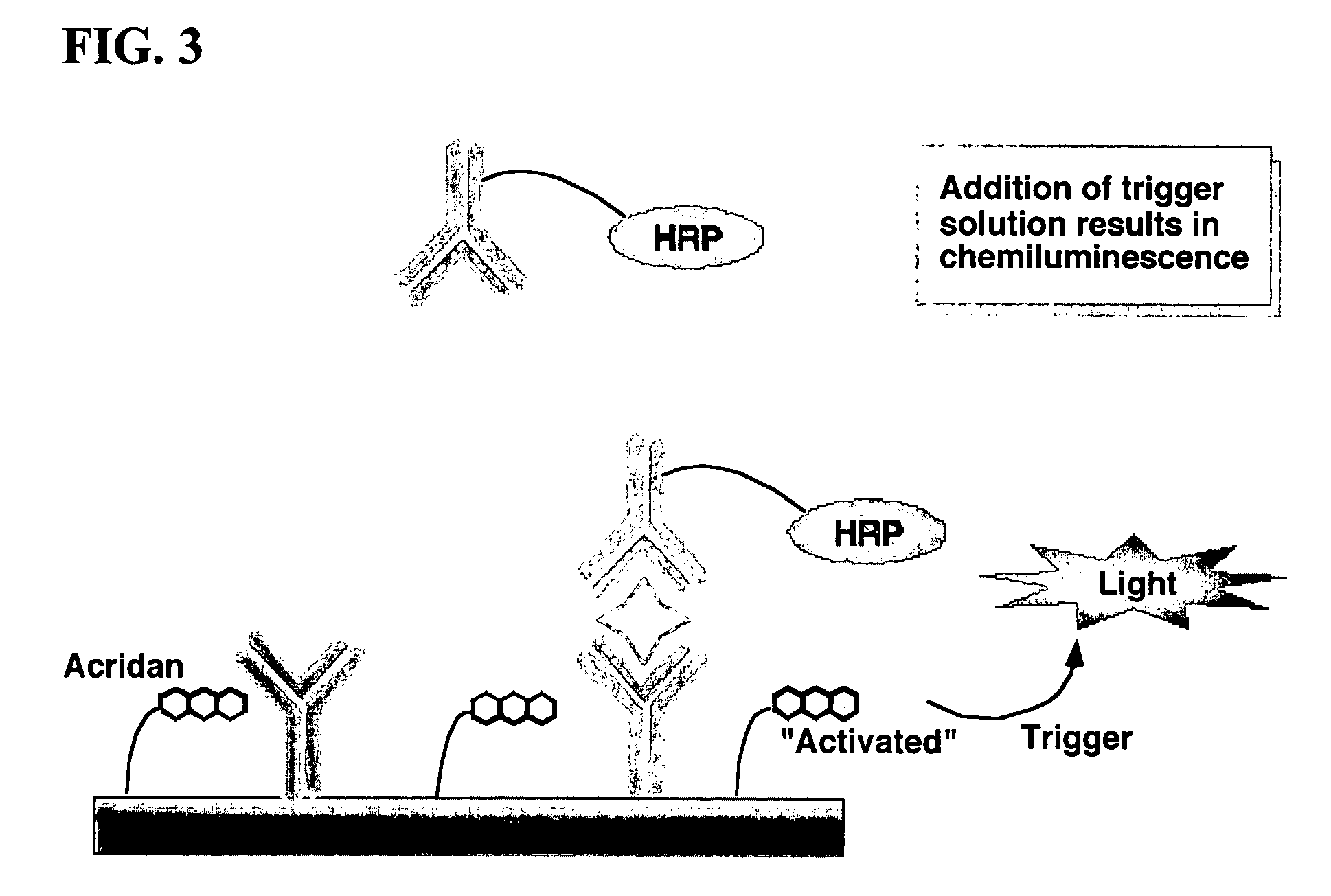
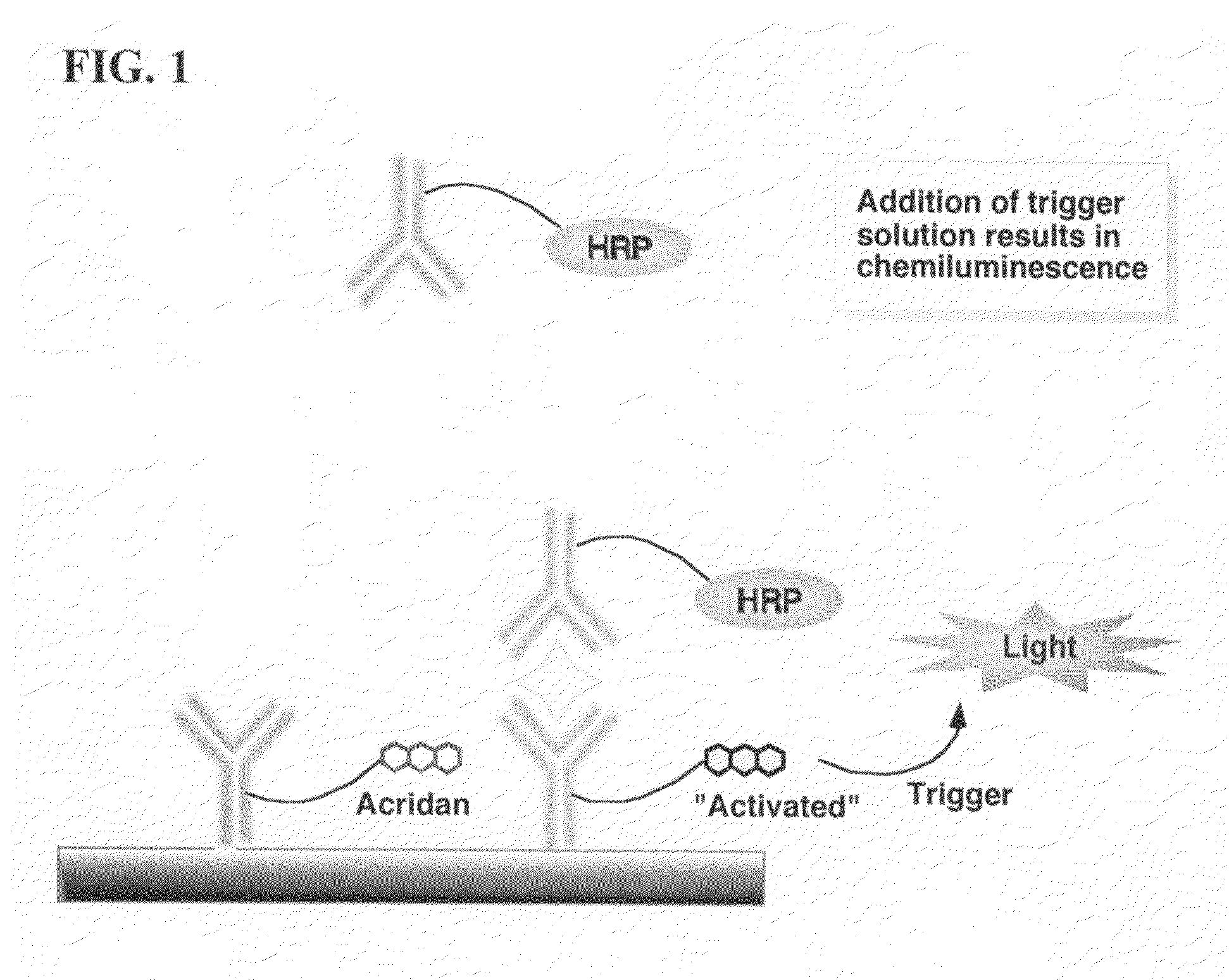
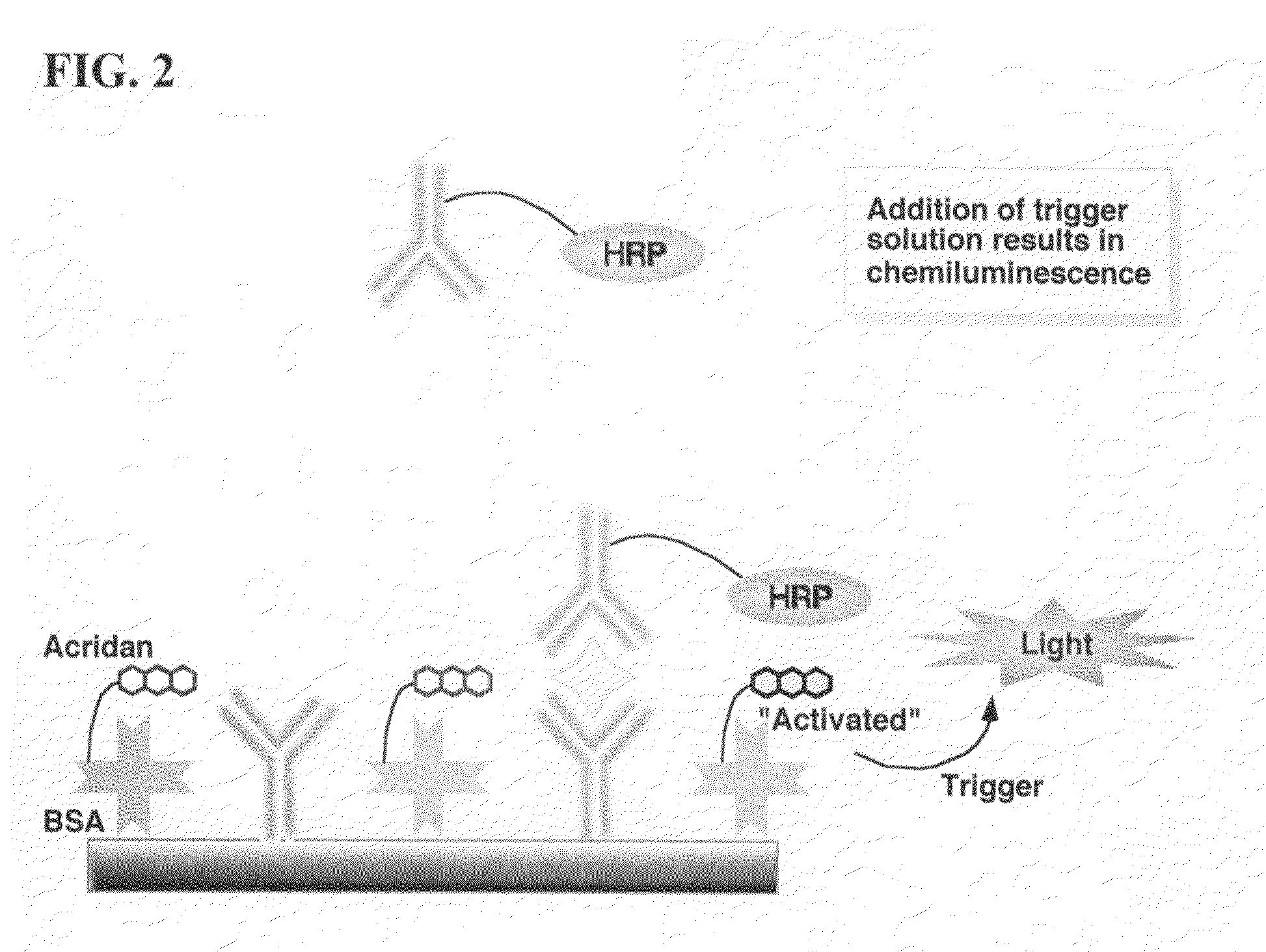
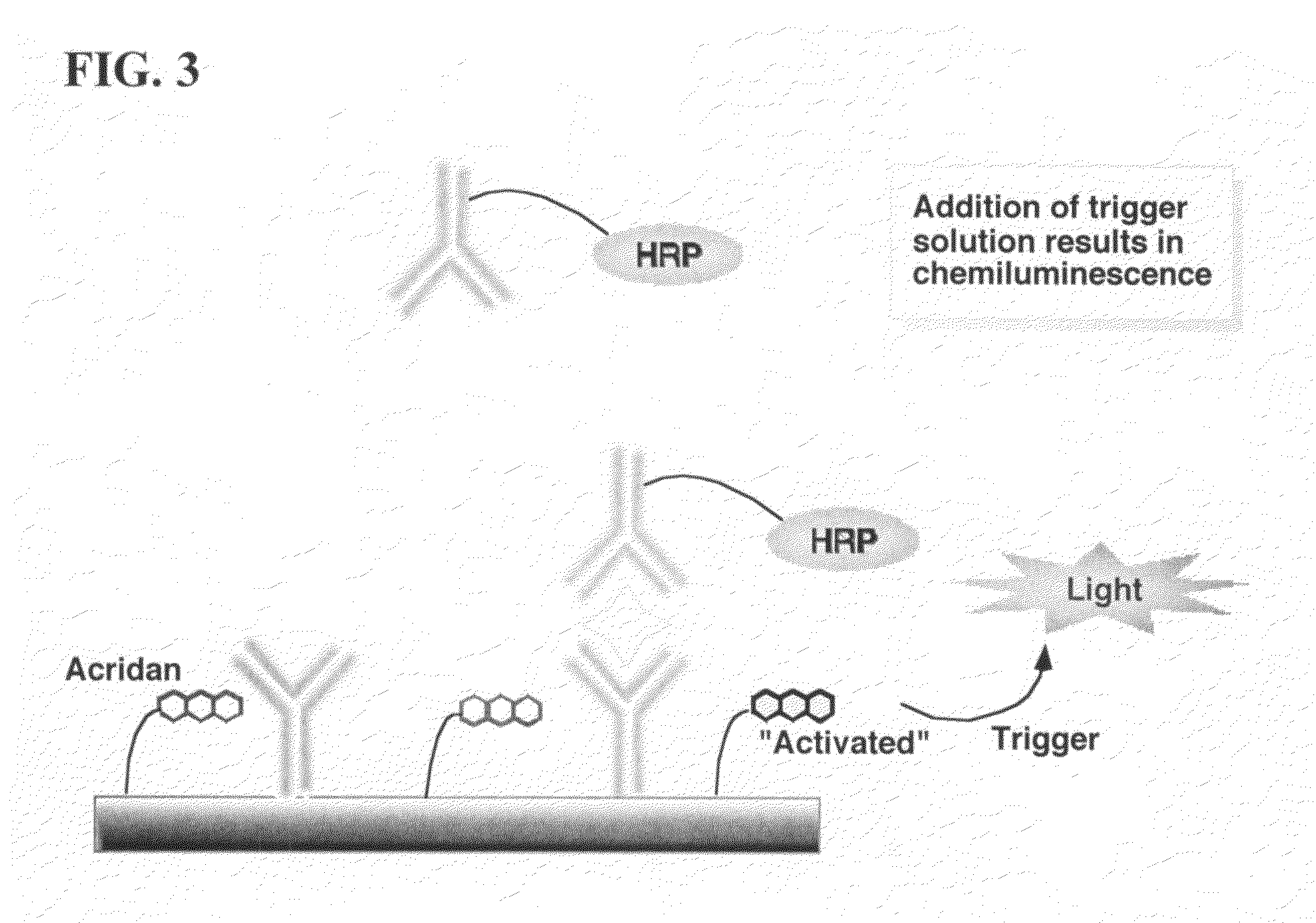
Assay methods are disclosed involving specific binding reactions which are simplified compared to known methods. A compound capable of producing chemiluminescence is immobilized on a solid support as is a member of a specific binding pair for capturing an analyte from a sample. An activator compound that activates the chemiluminescent compound and is conjugated to a specific binding pair member is added in excess along with the sample to the solid support. Addition of a trigger solution causes a chemiluminescent reaction at the sites where the activator conjugate has been specifically bound. The assay methods are termed non-separation assays because they do not require removal or separation of excess detection label (activator conjugate) prior to the detection step. The methods are applicable to various types of assays including immunoassays, receptor-ligand assays and nucleic acid hybridization assays.
Owner:BECKMAN COULTER INC
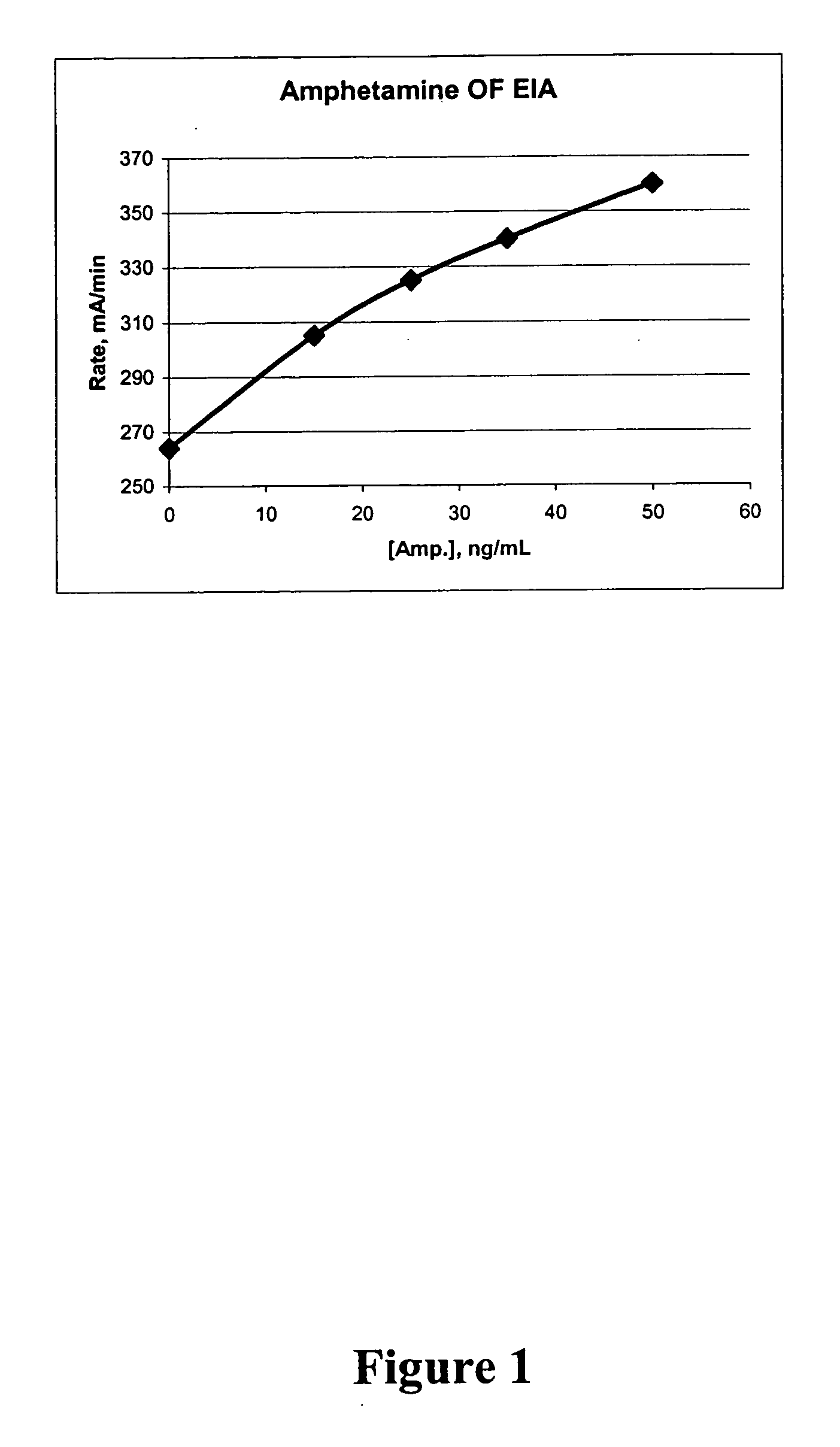
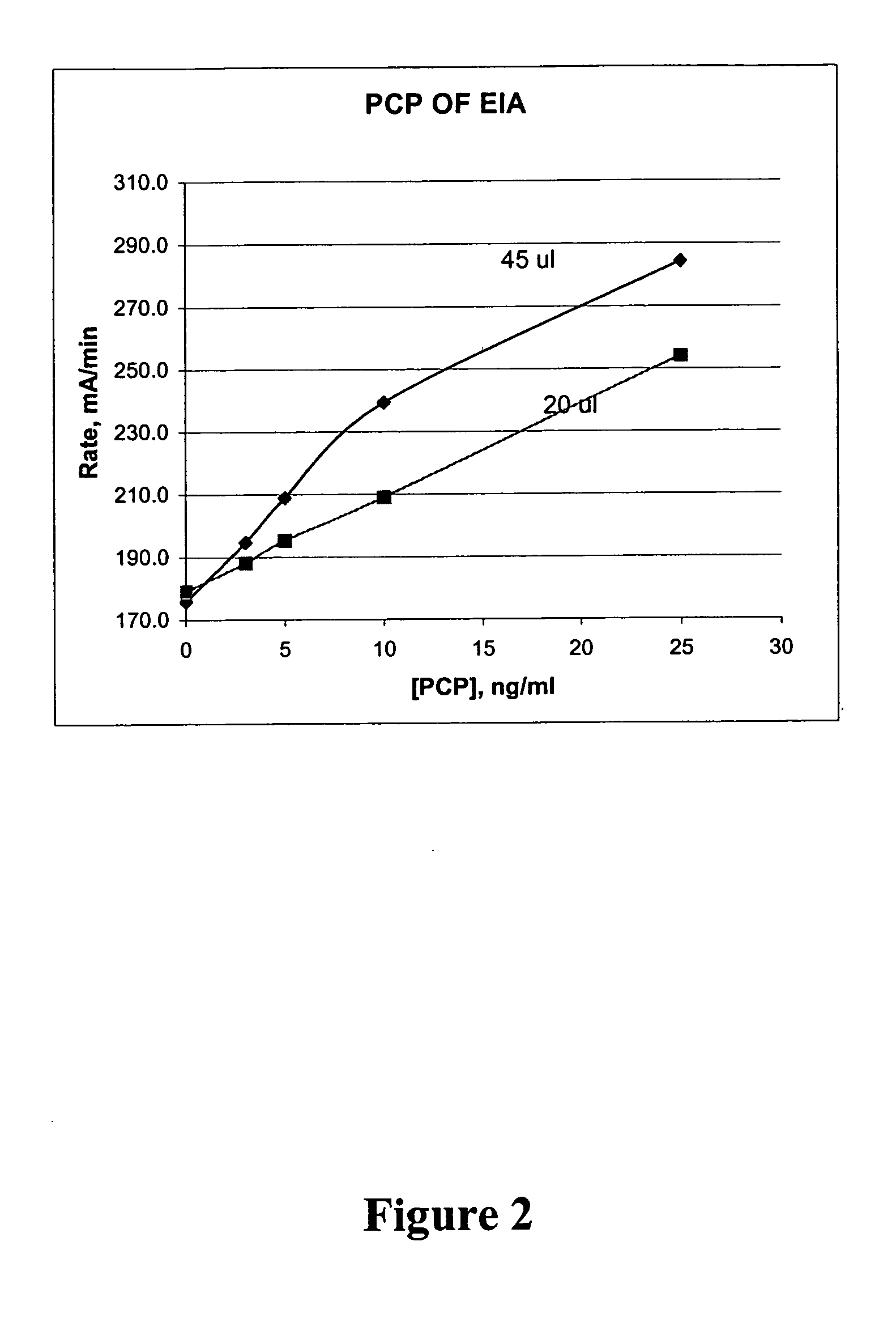
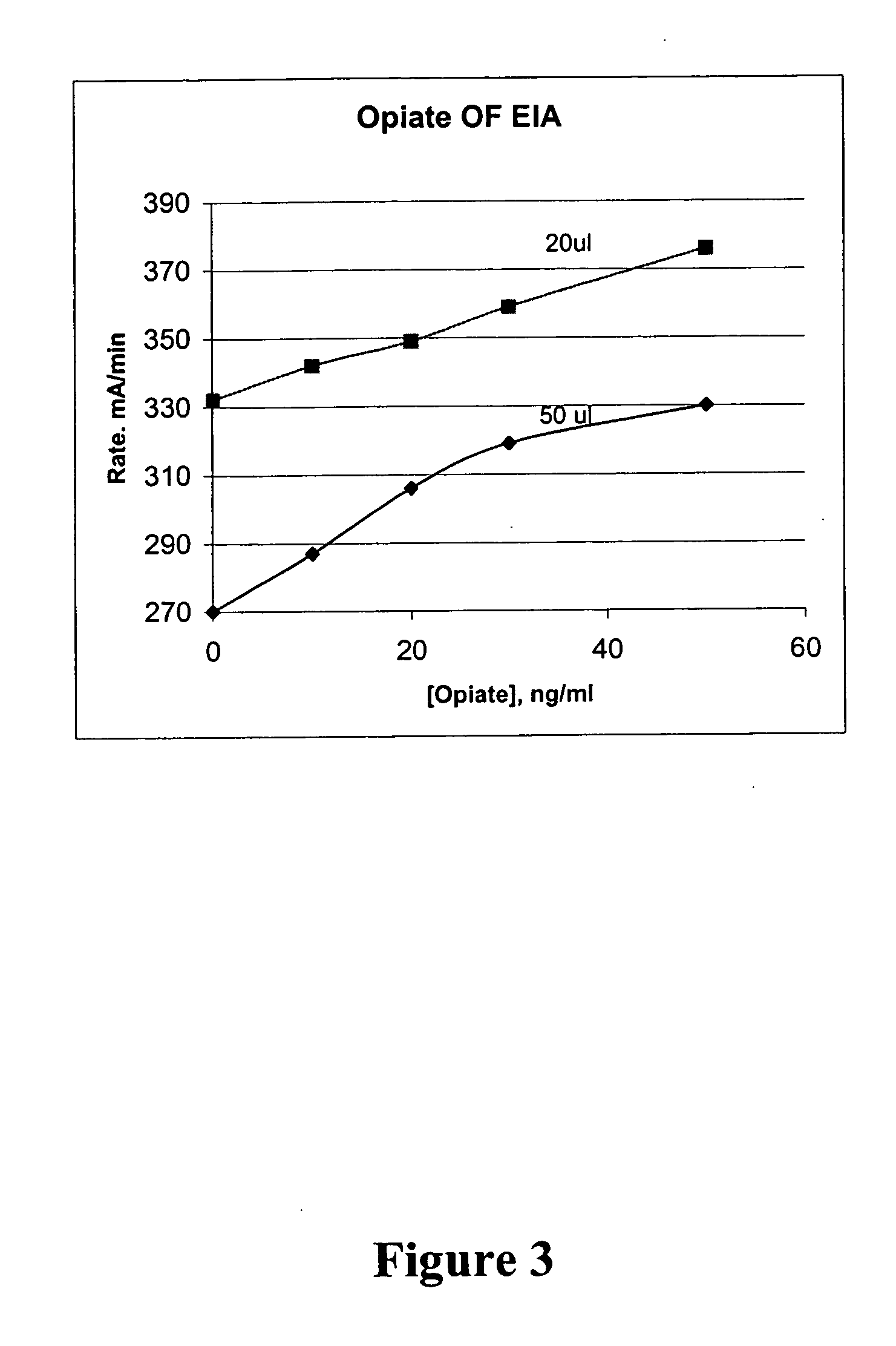
Homogeneous enzyme immunoassay for oral fluid
The present invention discloses homogeneous enzyme immunoassay systems, methods and kits useful for the qualitatively and quantitatively determination of analytes in oral fluid samples. The system involves a competitive enzyme immunoassay employing a conjugate comprising glucose-6-phosphate dehydrogenase (G6PDH) and an analyte. The methods and kits are particularly useful in the detection of recent drug use and for fast determination of analytes using auto-analyzers.
Owner:LIN ZHI INT
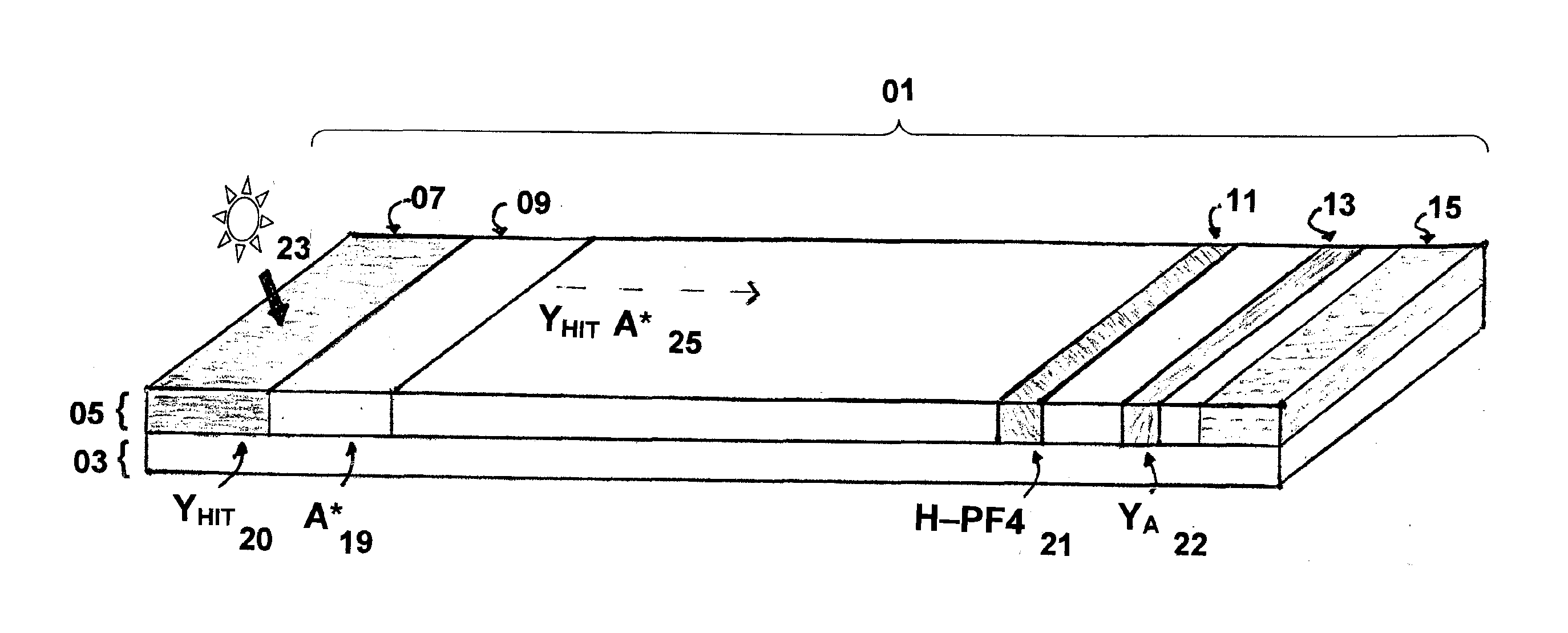
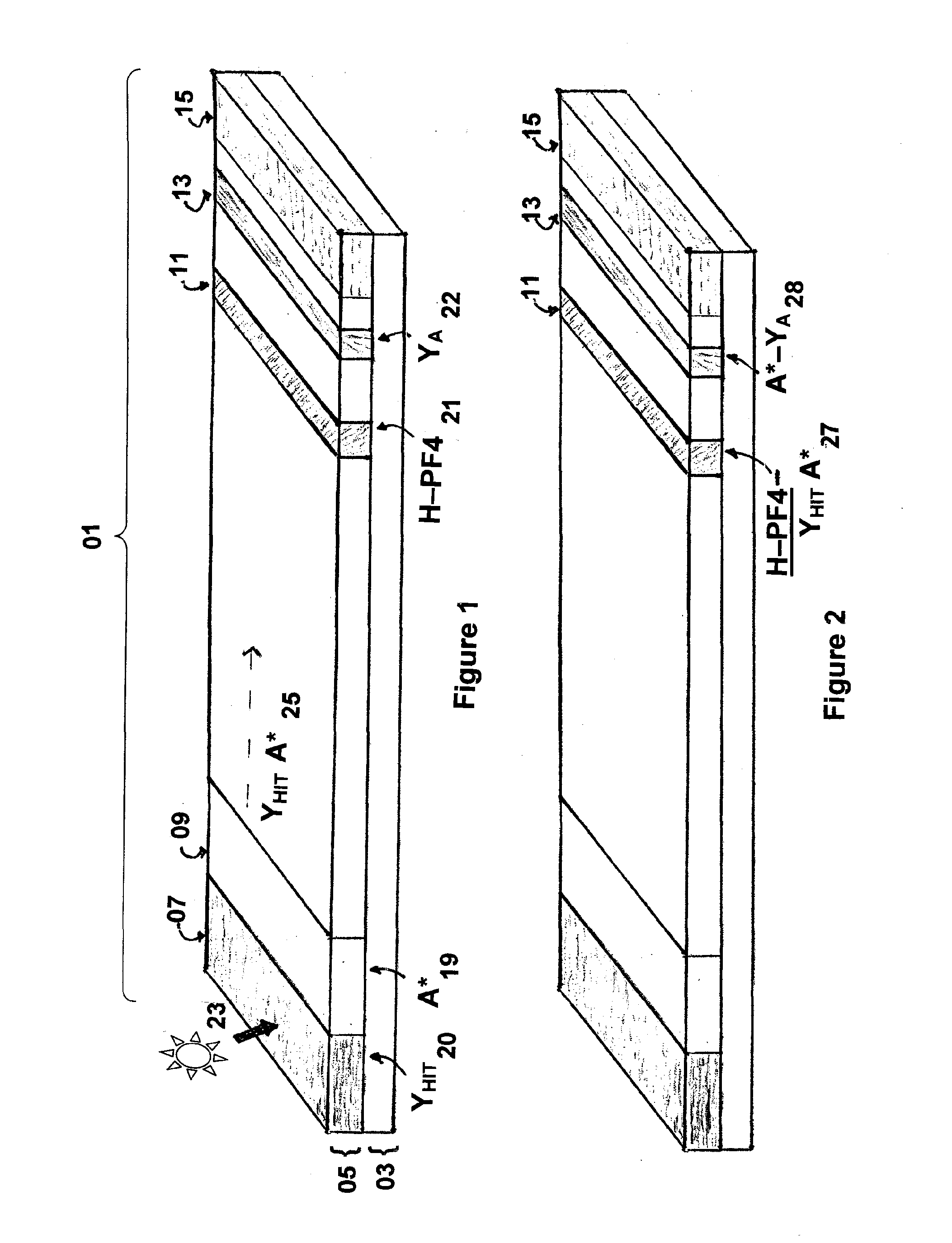
Rapid and sensitive method for quantitative determination of the level of heparin - pf4 complex induced immunoglobulin antibodies
ActiveUS20100255510A1Bioreactor/fermenter combinationsBiological substance pretreatmentsImmuno assayQuantitative determination
Disclosed herein is a lateral flow immuno-assay system capable of rapidly, cost effectively, and quantitatively detecting and assessing the level of HIT antibodies in body fluids of a patient. Also taught are methods for employing the system to assist in diagnosis of HIT, and for screening or detecting a changing titer of HIT antibodies in the body fluids of a patient to determine susceptibility toward HIT.
Owner:BIOMEDOMICS
Assay procedure using fluorogenic tracers
Fluorescent energy transfer dyes capable of moving between a more stacked configuration to exhibit fluorescent quenching and a more spaced configuration to exhibit fluorescence can be conjugated to a peptide epitope or nucleic acid for use in the detection of an unknown antibody in bulk solution. The resulting labeled peptide reagent can be used in an immunoassay procedure by placing it in bulk solution along with the unknown antibody to be detected. When the antibody binds to the peptide epitope, the pair of dyes carried by the peptide epitope will have their configuration altered from a stacked to an unstacked configuration and will exhibit a fluorescent increase in response thereto.
Owner:UNIV OF UTAH RES FOUND
Automated Diagnostic Workstation
InactiveUS20110071039A1Reduce errorsBioreactor/fermenter combinationsBiological substance pretreatmentsImmuno assayPre analytical
A flexible diagnostic workstation comprises is equipped to read label information on well strips of loaded well plates and on loaded reagent kit holders, automatically perform the pre-analytical steps of an immuno-assay which consists of a sequence of operations in accordance with scheduled test requirements for each microwell, read the results according to either a standard singleplex ELISA or multiplex test format as indicated by the well strip label, and report the results.
Owner:IMMCO DIAGNOSTICS
Nonseparation assay methods
ActiveUS7799534B2Analysis using chemical indicatorsMicrobiological testing/measurementImmuno assayAnalyte
Assay methods are disclosed involving specific binding reactions which are simplified compared to known methods. A compound capable of producing chemiluminescence is immobilized on a solid support as is a member of a specific binding pair for capturing an analyte from a sample. An activator compound that activates the chemiluminescent compound and is conjugated to a specific binding pair member is added in excess along with the sample to the solid support. Addition of a trigger solution causes a chemiluminescent reaction at the sites where the activator conjugate has been specifically bound. The assay methods are termed non-separation assays because they do not require removal or separation of excess detection label (activator conjugate) prior to the detection step. The methods are applicable to various types of assays including immunoassays, receptor-ligand assays and nucleic acid hybridization assays.
Owner:BECKMAN COULTER INC
Digital bio disc (DBD), DBD driver apparatus, and assay method using the same
InactiveUS8263386B2Prevent leakageEasy to separateBioreactor/fermenter combinationsValve arrangementsImmuno assayLab-on-a-chip
Owner:SAMSUNG ELECTRONICS CO LTD
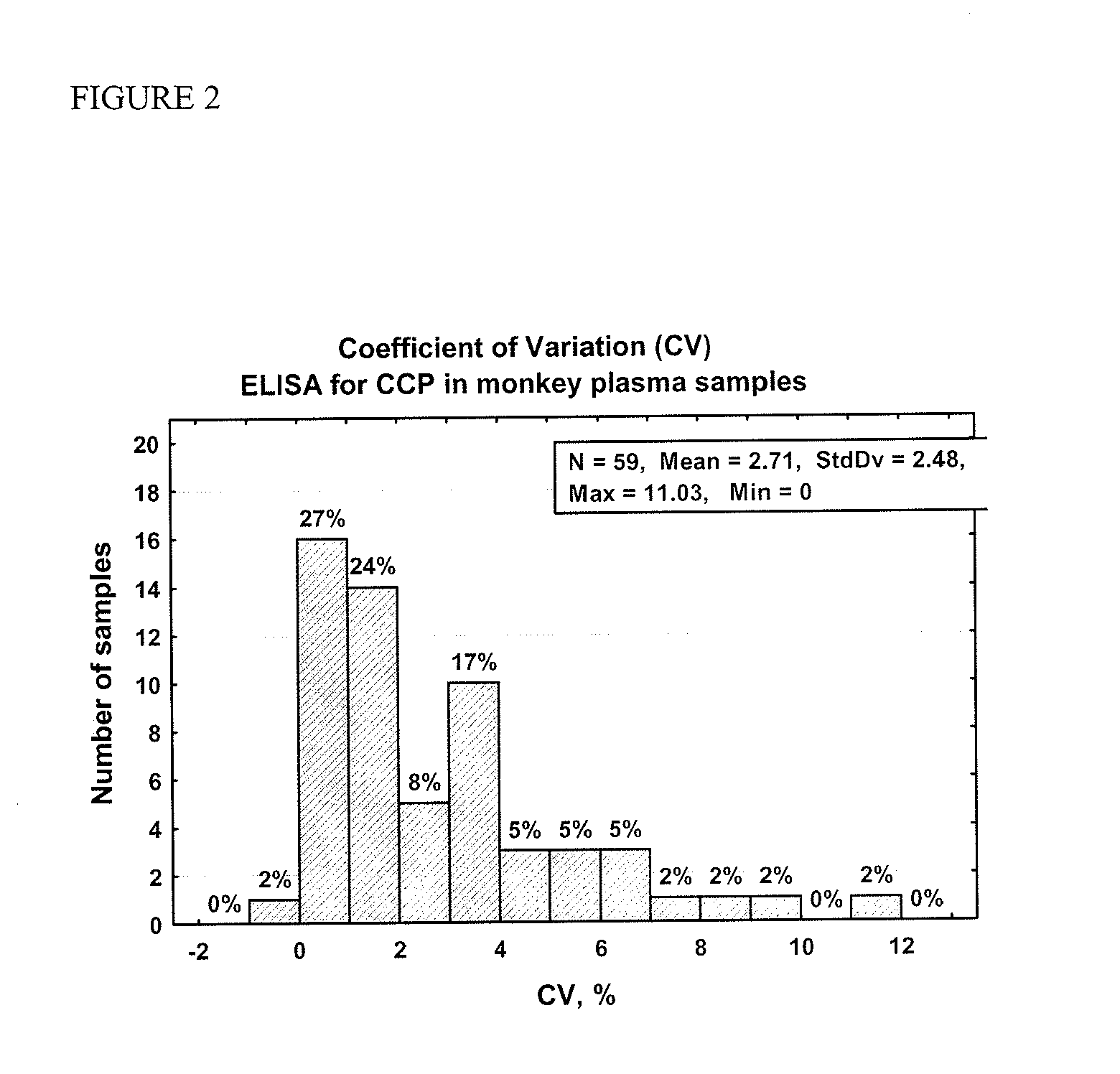
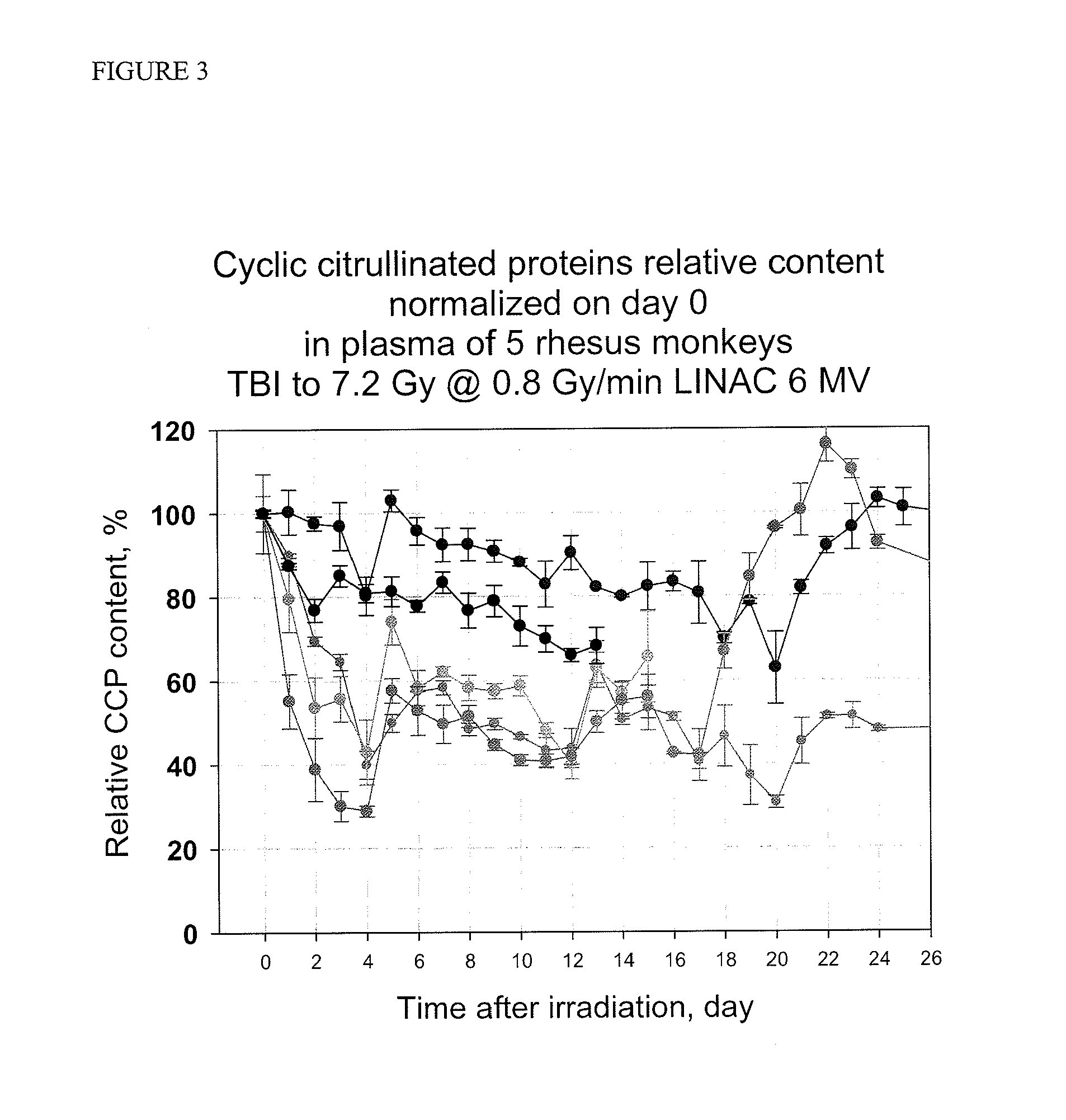
Immunoassays for citrullinated proteins
ActiveUS20110244492A1Reduce in quantityEnhanced radiationDisease diagnosisBiological testingRadiation injuryImmuno assay
Methods and kits are provided for assessing radiation injury and exposure in a mammal. The methods comprise the steps of: obtaining one or more test samples from the mammal, contacting the test samples with an antibody immunoreactive with a citrullinated protein to form an immunocomplex; and detecting the immunocomplex with an ELISA; wherein a decrease in the quantity of the immunocomplex in the test samples, as compared to the quantity of immunocomplexes formed under identical conditions with the same antibody and a control sample from one or more mammals known to have a lower degree of radiation injury or exposure, indicates a higher degree of radiation injury and exposure to the mammal. The information obtained from such methods can be used by a clinician to accurately assess the extent of radiation injury / exposure in the mammal, and thus will provide a valuable tool for determining treatment protocols on a subject by subject basis.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
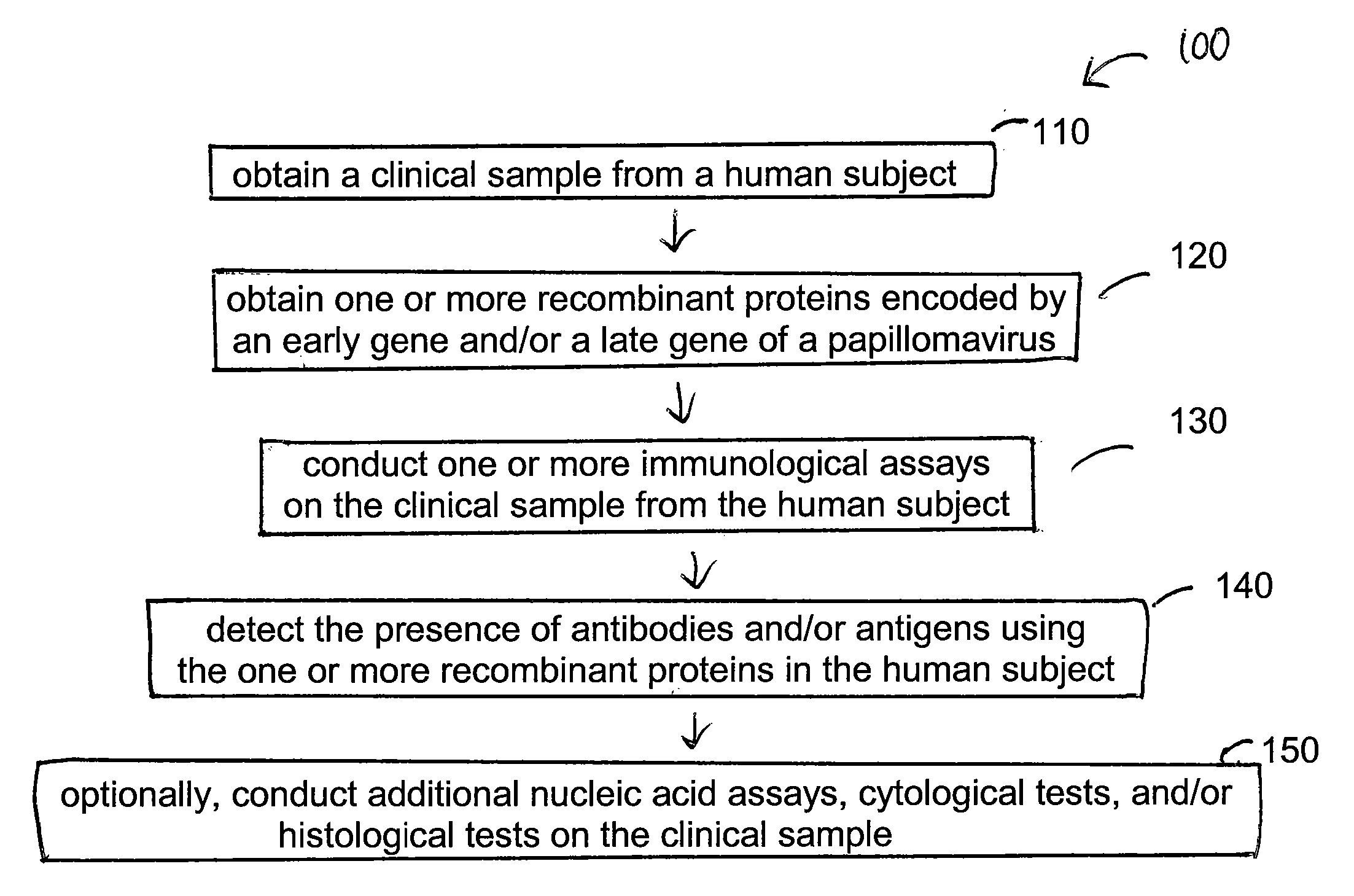
Detection method for human pappilomavirus (HPV) and its application in cervical cancer
InactiveUS20080044809A1High riskMicrobiological testing/measurementVirus peptidesEpithelial cell abnormalityHistone antibody
Embodiments of the invention provide methods, assays, and kits for detecting HPV infection and HPV associated epithelial cell abnormalities, most notably those associated with pre-malignant and malignant epithelial cell lesions. Detection of HPV DNAs, genomes, and / or oncoproteins by nucleic acid hybridization assays and immunological assays can be used in early clinical screening for HPV infection and diagnosis for cervical cancer. The polypeptides, recombinant proteins, antibodies, nucleic acids, and various detection methods thereof are particularly useful for diagnosing carcinomas of the uterine cervix and those at risk of developing cervical cancer.
Owner:HEER MEDICAL TECH DEV CO LTD
Methods of Detecting Conjugation Site-Specific and Hidden Epitope/Antigen
ActiveUS20150241450A1Reduce non-specific bindingReduce non-specific bindingsMicrobiological testing/measurementImmunoglobulins against animals/humansEpitopeImmuno assay
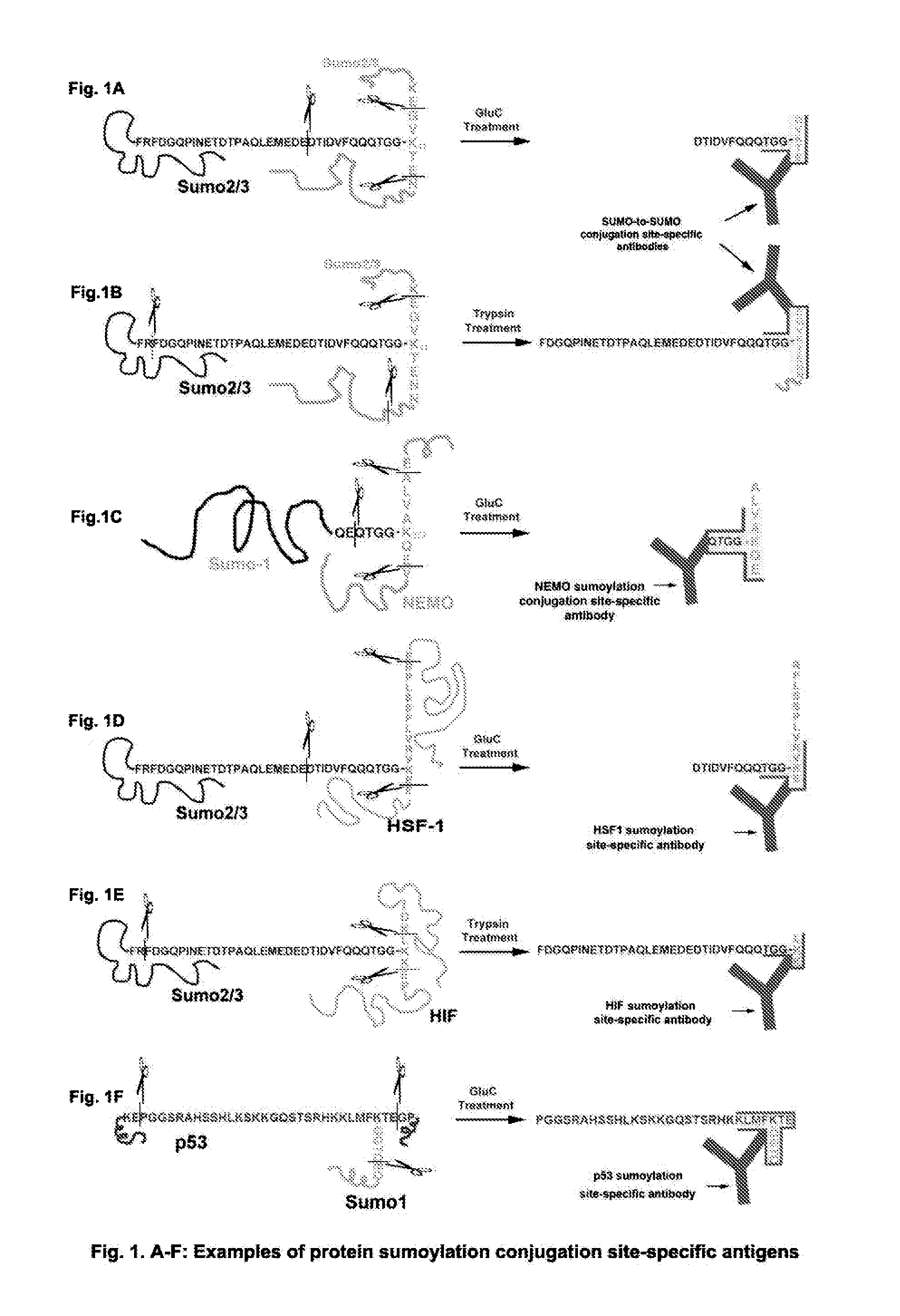
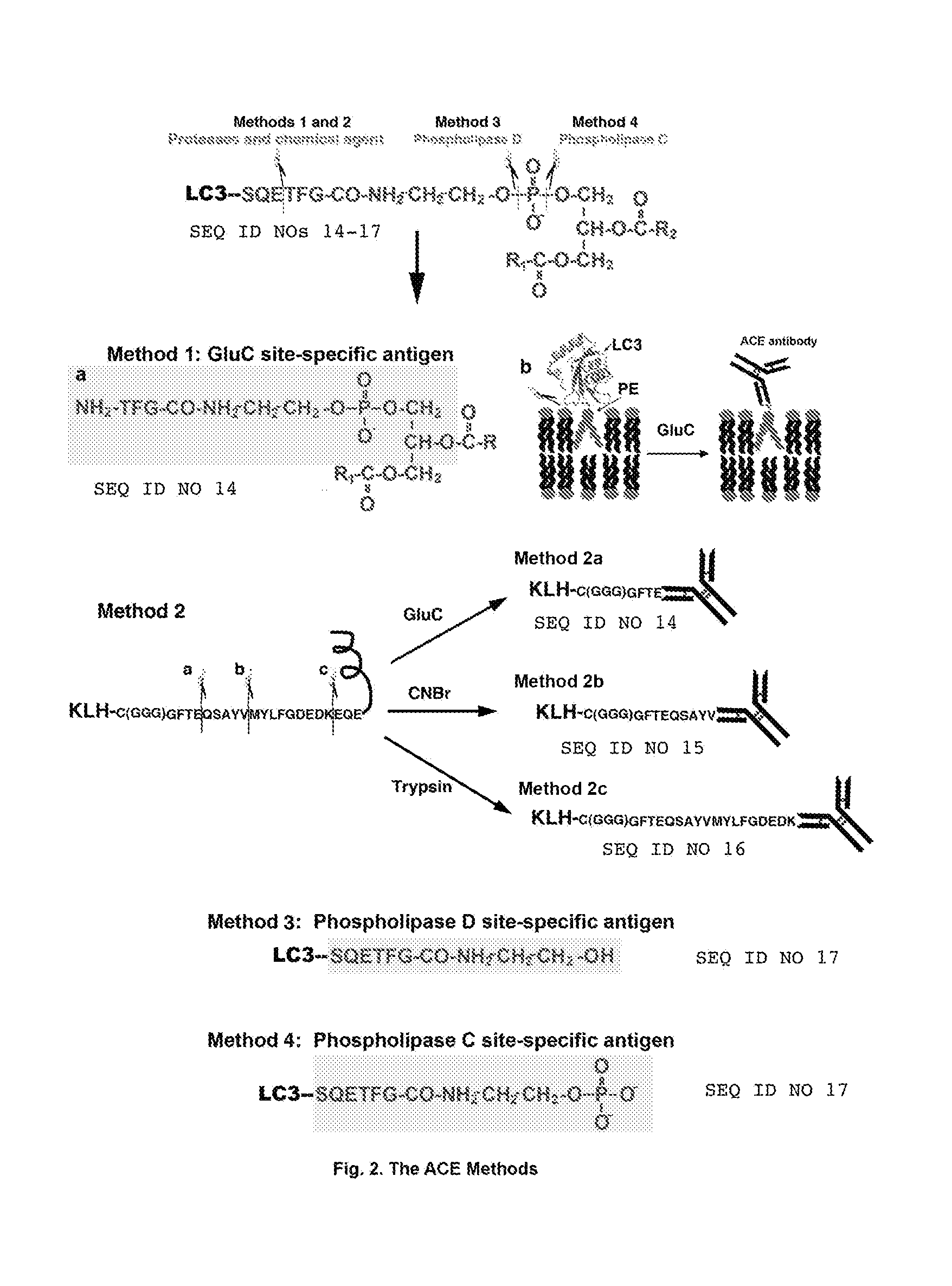
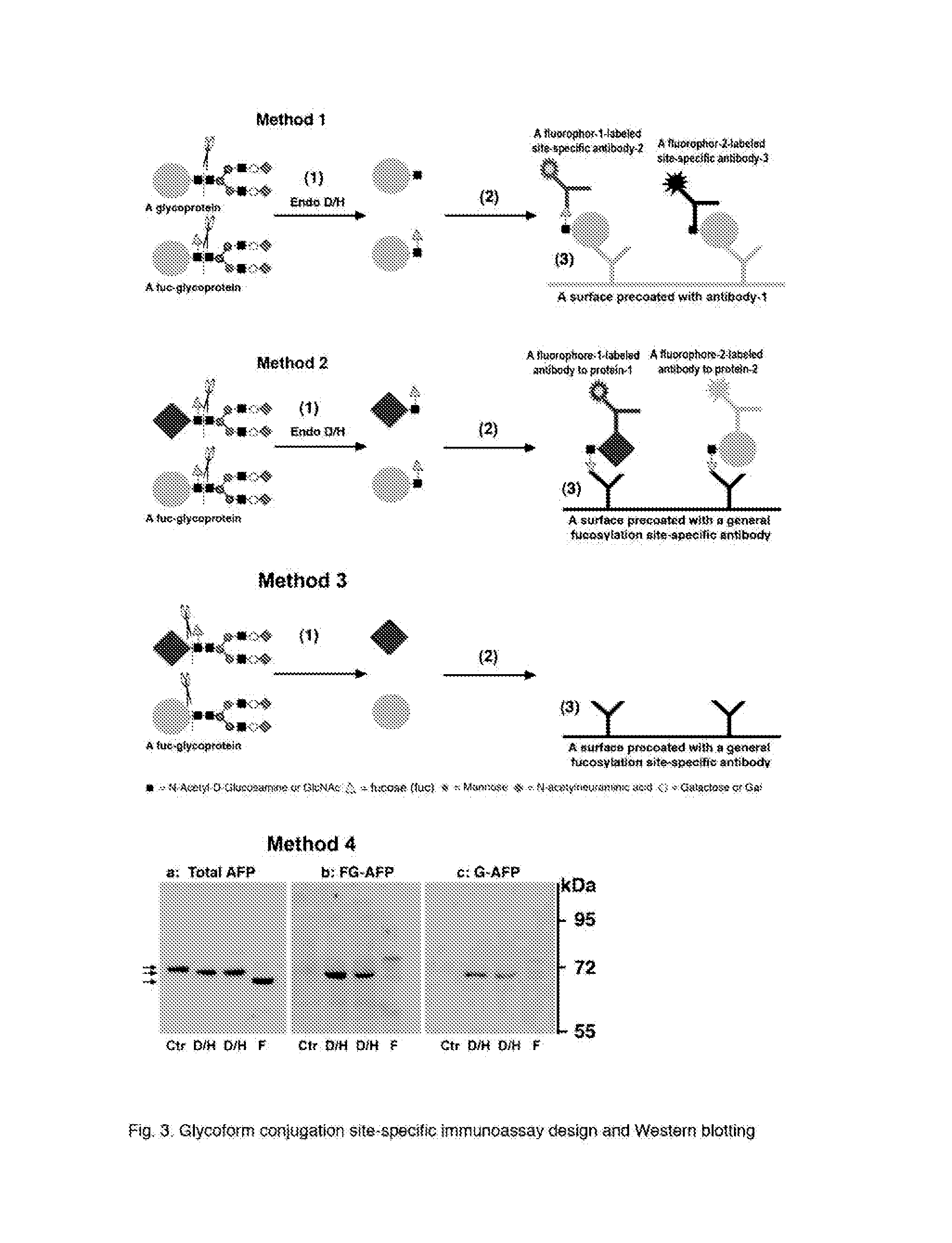
This invention discloses “Artificially Cleaved Epitope (ACE)” methods, antibodies, reagents, immunoassays, and kits for designing and detecting hidden epitopes / antigens. The ACE methods can detect epitopes that are either absent or poorly accessible naturally to antibodies, and thus must be specifically and artificially created (free terminals) and / or exposed in samples and sample preparations for antibody detection. The ACE structures include, but are not limited to, macromolecule-to-macromolecule conjugation sites, and any types of linear hidden epitopes. The ACE methods comprise ACE antigen design and ACE antigen detection. The ACE methods, antibodies, reagents, immunoassays, and kits are useful in research and discovery, diagnostic, and therapeutic applications. In another aspect, the ACE methods can artificially and specifically expose hidden antigens while reducing the antibody non-specific bindings in all antibody-based applications.
Owner:LIU CHUNLI +1
Nonseparation assay methods
ActiveUS7732153B2Assays are simplifiedPeptide/protein ingredientsChemiluminescene/bioluminescenceImmuno assayAnalyte
Assay methods are disclosed involving specific binding reactions which are simplified compared to known methods. A compound capable of producing chemiluminescence is immobilized on a solid support as is a member of a specific binding pair for capturing an analyte from a sample. An activator compound that activates the chemiluminescent compound and is conjugated to a specific binding pair member is added in excess along with the sample to the solid support. Addition of a trigger solution causes a chemiluminescent reaction at the sites where the activator conjugate has been specifically bound. The assay methods are termed non-separation assays because they do not require removal or separation of excess detection label (activator conjugate) prior to the detection step. The methods are applicable to various types of assays including immunoassays, receptor-ligand assays and nucleic acid hybridization assays.
Owner:BECKMAN COULTER INC
Raman-active reagents and the use thereof
ActiveUS7824926B1Faster analysis speedReduce labor costsAnalysis by subjecting material to chemical reactionBiological testingAntigenImmuno assay
The present invention provides a new class of Raman-active reagents for use in biological and other applications, as well as methods and kits for their use and manufacture. Each reagent includes a Raman-active reporter molecule, a binding molecule, and a surface enhancing particle capable of causing surface enhanced Raman scattering (SERS). The Raman-active reporter molecule and the binding molecule are affixed to the particle to give both a strong SERS signal and to provide biological functionality, i.e. antigen or drug recognition. The Raman-active reagents can function as an alternative to fluorescence-labeled reagents, with advantages in detection including signal stability, sensitivity, and the ability to simultaneously detect several biological materials. The Raman-active reagents also have a wide range of applications, especially in clinical fields (e.g., immunoassays, imaging, and drug screening).
Owner:PORTER MARC D
Chemiluminiscence immuno-assay kit for 25-hydroxyl vitamin D and preparation method thereof
InactiveCN106199016AImprove detection accuracyHigh detection sensitivityChemiluminescene/bioluminescenceBiological testingImmuno assayMedicine
The invention discloses a chemiluminiscence immuno-assay kit for 25-hydroxyl vitamin D and a preparation method thereof. The chemiluminiscence immuno-assay kit herein includes: carboxylated magnetic micro-particles that are coated by a 25-hydroxyl vitamin D monoclonal antibody and a chemiluminiscence marker that is marked by a vitamin D derivative. The chemiluminiscence immuno-assay kit, with a full-auto chemiluminiscence immuno-analysis instrument as a detection tool, completes the detection on the 25-hydroxyl vitamin D. A test proves that the kit reaches 0.2 ng / ml in detection sensitivity, which is improved by at least 10 times than that of a conventional detection method of the 25-hydroxyl vitamin D. The chemiluminiscence immuno-assay kit has high detection precision.
Owner:SHENZHEN YHLO BIOTECH
Heparin binding protein detection kit
The invention provides a heparin binding protein detection kit. A double-antibody sandwich method is adopted and is characterized by comprising the following steps: coating micropores of an ELISA plate with HBP monoclonal antibodies, diluting HBP in plasma as antigen substance by using a sample diluting solution, and combining the antigen substance with the HBP monoclonal antibodies coating a solid phase to form a solid phase-antibody-antigen complex in a first incubation period; fully washing uncombined components by using a washing buffer solution, adding an enzyme conjugate for incubation, and combining the enzyme conjugate with the solid phase-antibody-antigen complex; further washing, removing uncombined components, adding a substrate for incubation, catalyzing the substrate via enzyme to prepare a coloured product, adding a stop solution for stopping reacting, and detecting optical density values of various pores via an enzyme labeling instrument, wherein the size of the optical density values is in positive correlation with the concentration of HBP in the plasma. The heparin binding protein detection kit is a novel immune detection kit for quantitatively detecting heparin binding protein in human plasma in vitro and can contribute strength to medical examination and human health.
Owner:安徽同致生物工程股份有限公司
Nucleic acid aptamer for recognizing liver cancer cells, and screening method and purpose thereof
InactiveCN108866061AStrong specificityImprove performanceOrganic active ingredientsAntineoplastic agentsAptamerFluorescence
The invention discloses a nucleic acid aptamer for recognizing liver cancer cells, and a screening method and a purpose thereof. The nucleotide sequence of the nucleic acid aptamer comprises the feature sequence part shown as Seq ID No.1. The purpose comprises the purpose of the nucleic acid aptamer to the design and preparation of anti-liver-cancer medicine or detection reagents. The detection reagents comprise enzyme-linked immunosorbent assay, immunoradiometric assay or fluorescence method. The invention also comprises a target preparation and a medicine composition. The target preparationcomprises the nucleic acid aptamer and a medically acceptable carrier or a shaping agent. The medicine composition comprises the nucleic acid aptamer, at least one antitumor medicine and a medically acceptable carrier. Compared with the prior art, the nucleic acid aptamer can be used for specifically recognizing the liver cancer cells; good application prospects are realized.
Owner:THE FIFTH AFFILIATED HOSPITAL SUN YAT SEN UNIV
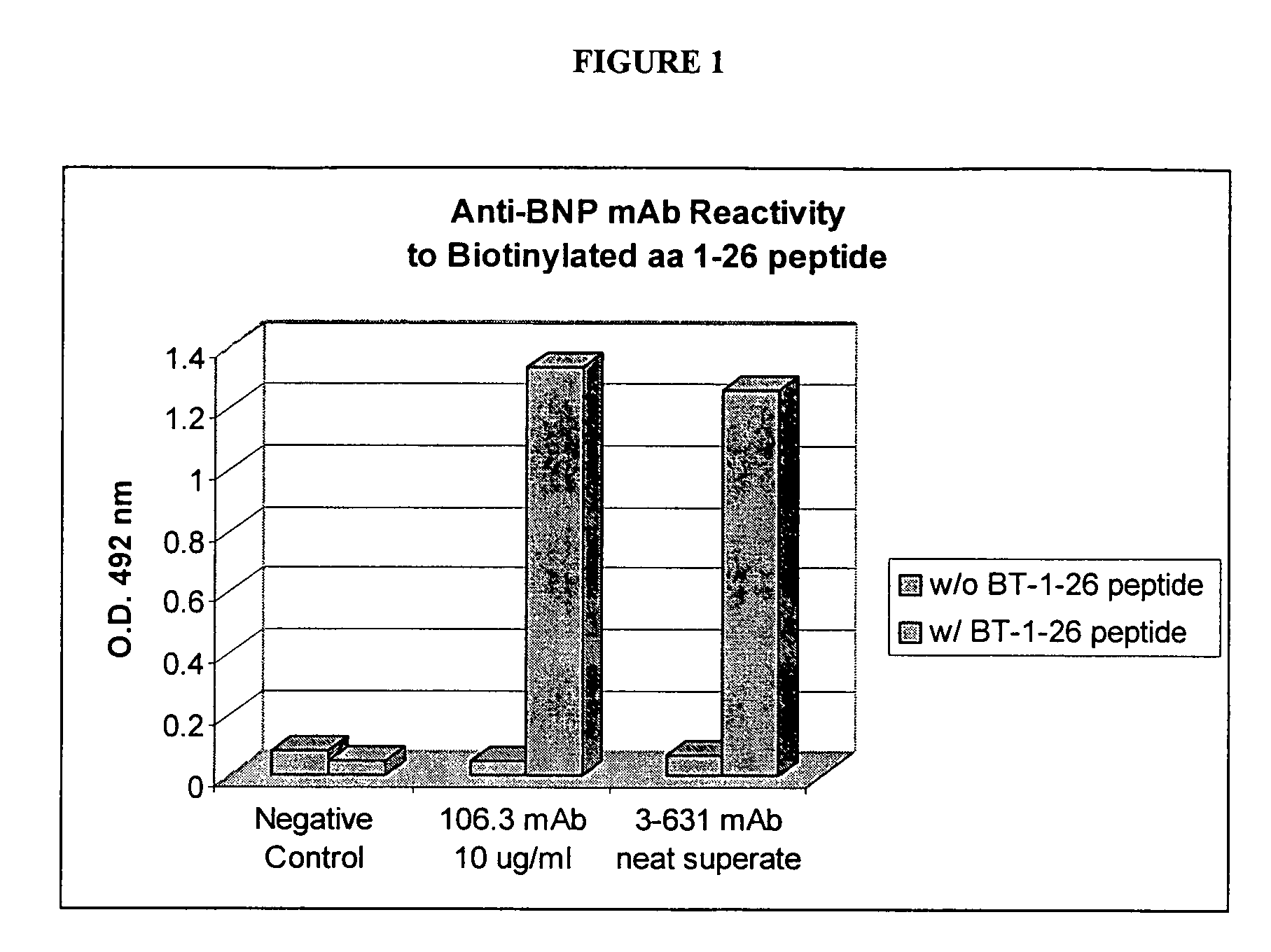
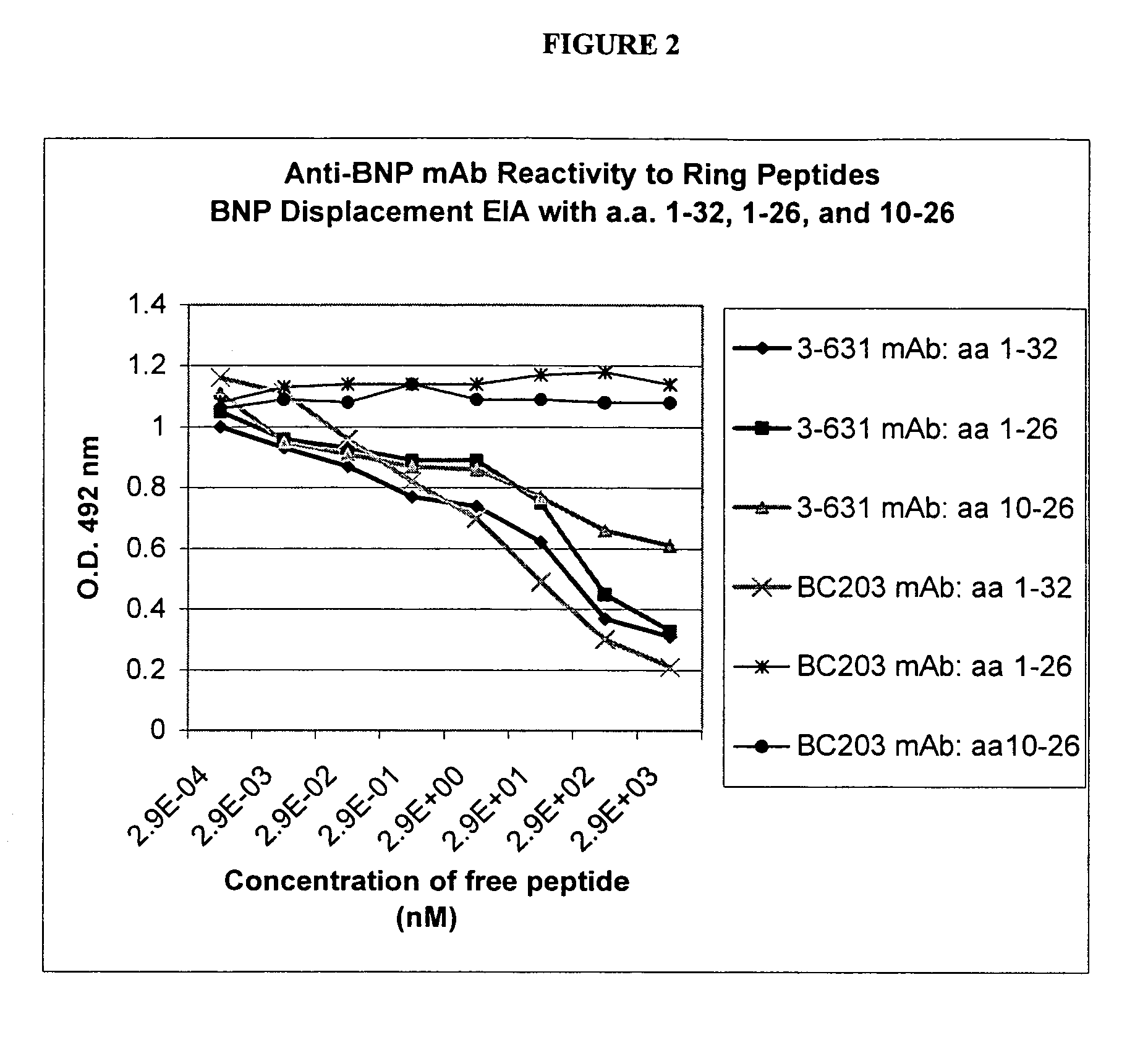
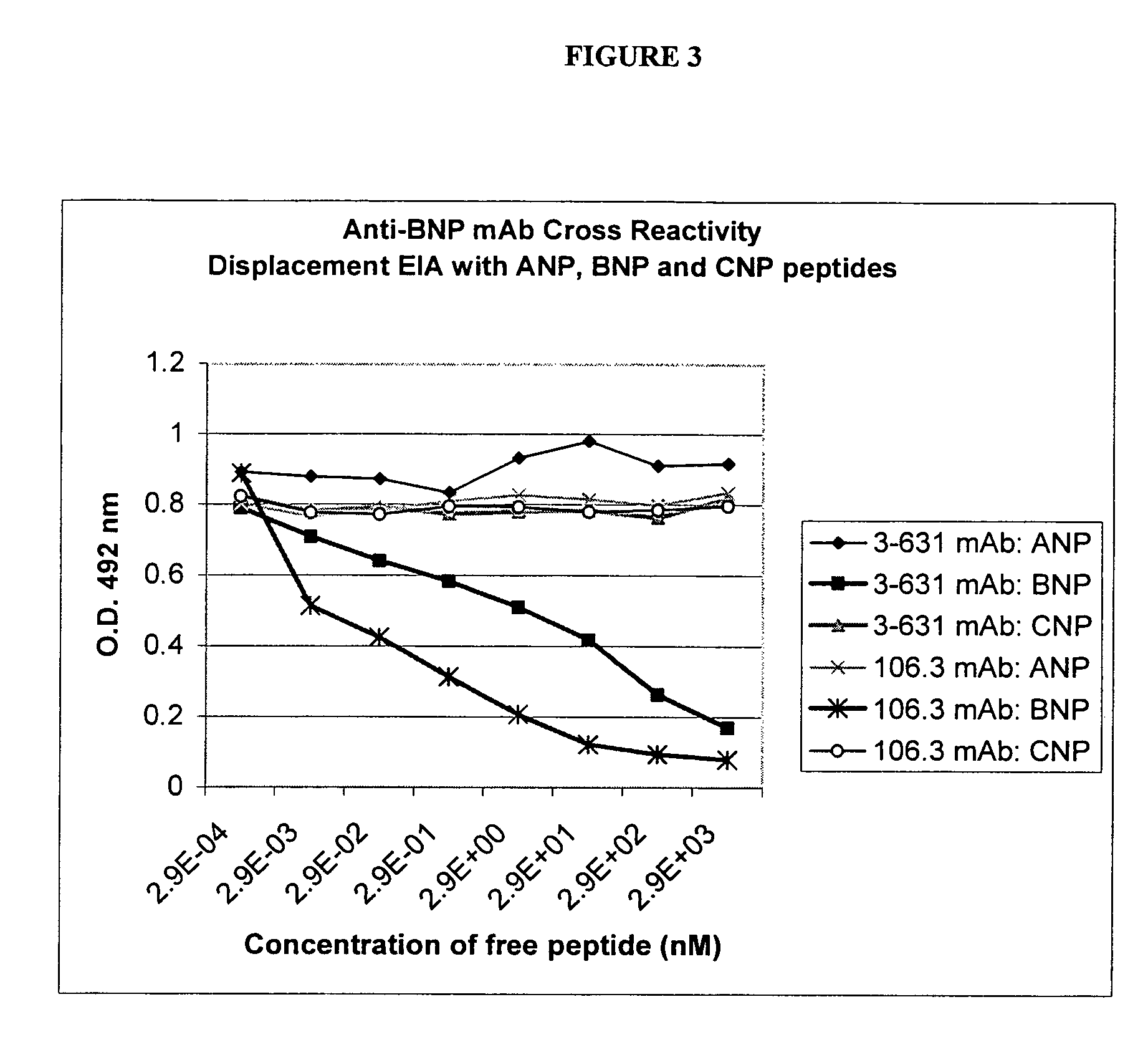
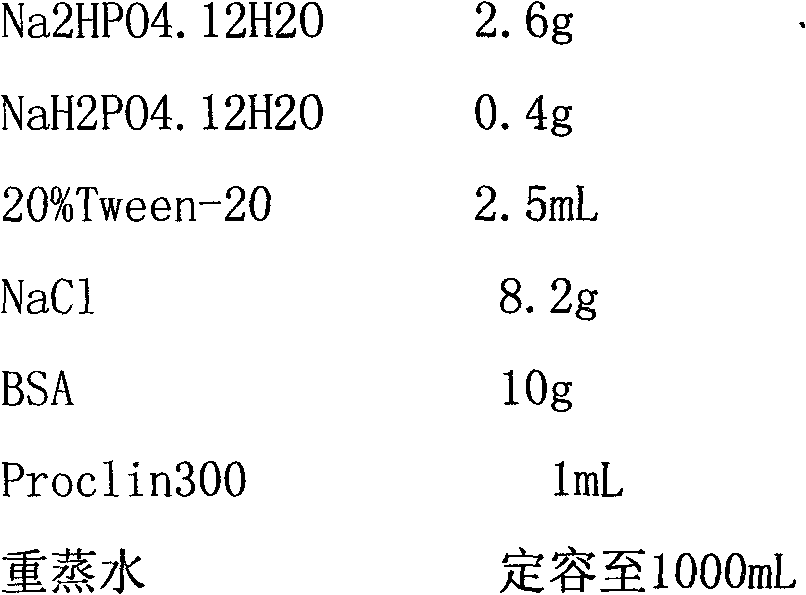
Human ring specific BNP antibodies
The present invention relates to antibodies that specifically bind to human BNP and immunoassays using said antibodies in the quantification of human BNP or a fragment of human BNP in a test sample.
Owner:ABBOTT LAB INC
Hepatitis B virus PreS1 antigen enzyme-linked immunoassay kit employing one-step method
InactiveCN103048456ASimple and fast operationGood match rateMaterial analysisAntigenPositive control
The invention provides a hepatitis B virus PreS1 antigen enzyme-linked immunoassay kit employing a one-step method. The concentration of HBs-Ag-PreS1 protein in blood serum of a sufferer can be exclusively detected. The kit comprises an elisa plate enveloped reaction strip, an enzyme labeling pre-S1 antibody, a positive control solution, a negative control solution, a bottle of 20-fold wash buffer, a bottle of substrate buffer A, a bottle of substrate buffer B and a bottle of stop buffer (2NH2SO4), wherein the elisa plate enveloped reaction strip is prepared from a primary ingredient avidin which is enveloped in advance and then added to a biotin anti-hepatitis B virus PreS1 monoclonal antibody or an anti-HBS antibody. The kit is good in uniformity, specificity and sensitivity, rapid and convenient to collect, can obtain an experiment result about 30 minutes by the one-step method, and is time-saving (the experiment can be fastest finished by 6-8 hours in a PCR (polymerase chain reaction) method) compared with the PCR detection method. The positivity detected by clinical PreS1 protein and the HBV-DNA positivity detected by PCR have good coincidence rate.
Owner:WUHAN KANGZHU BIOTECH
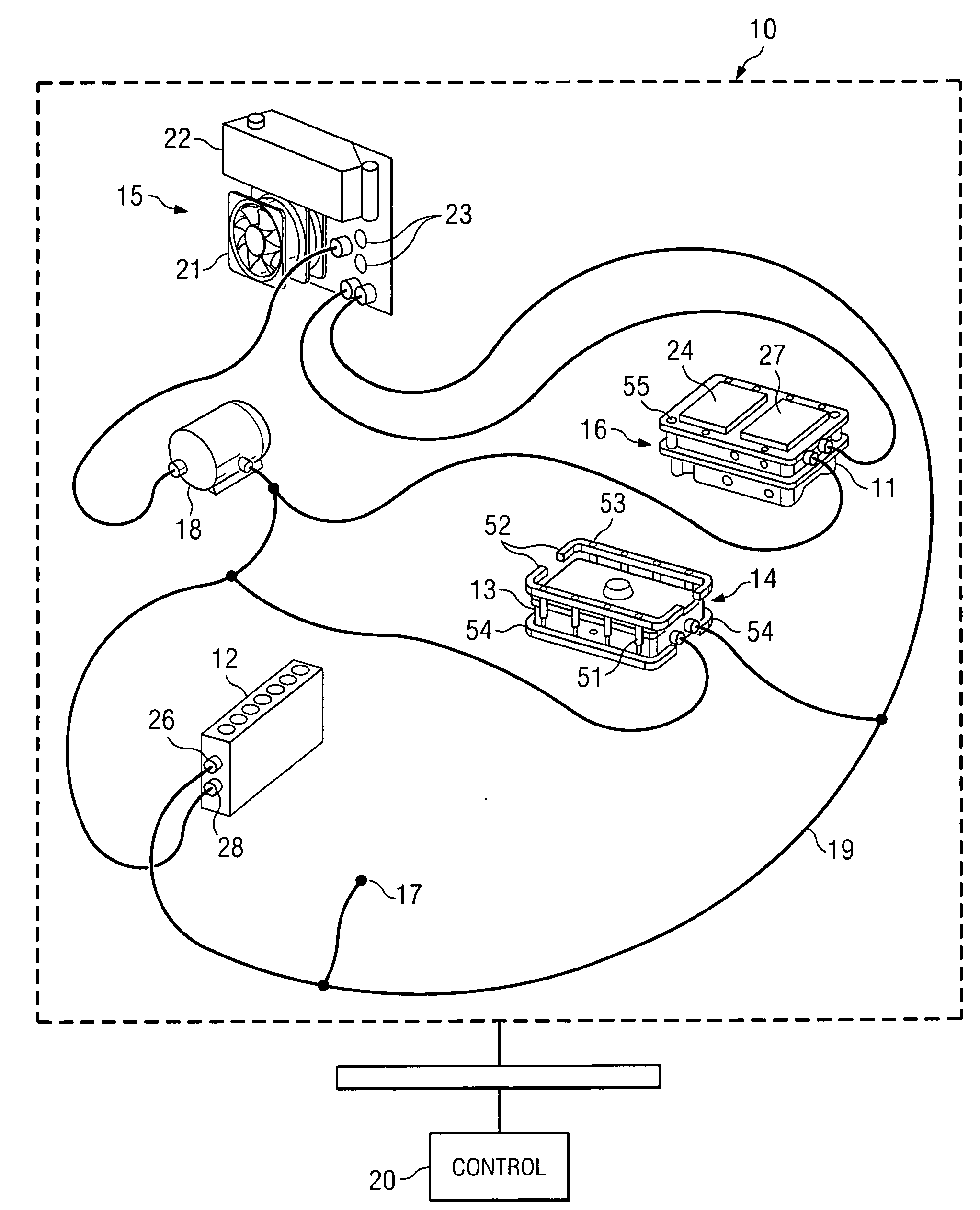
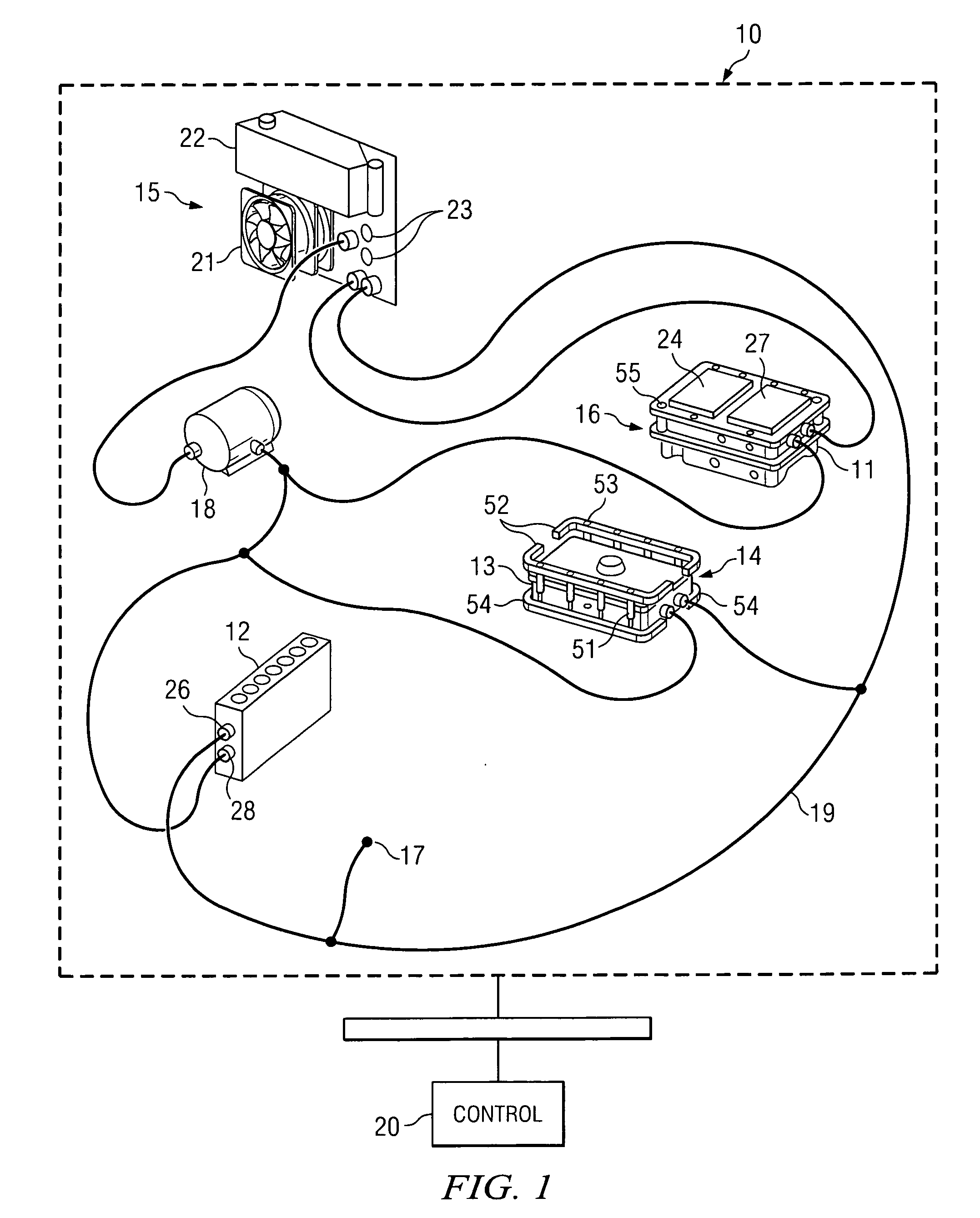
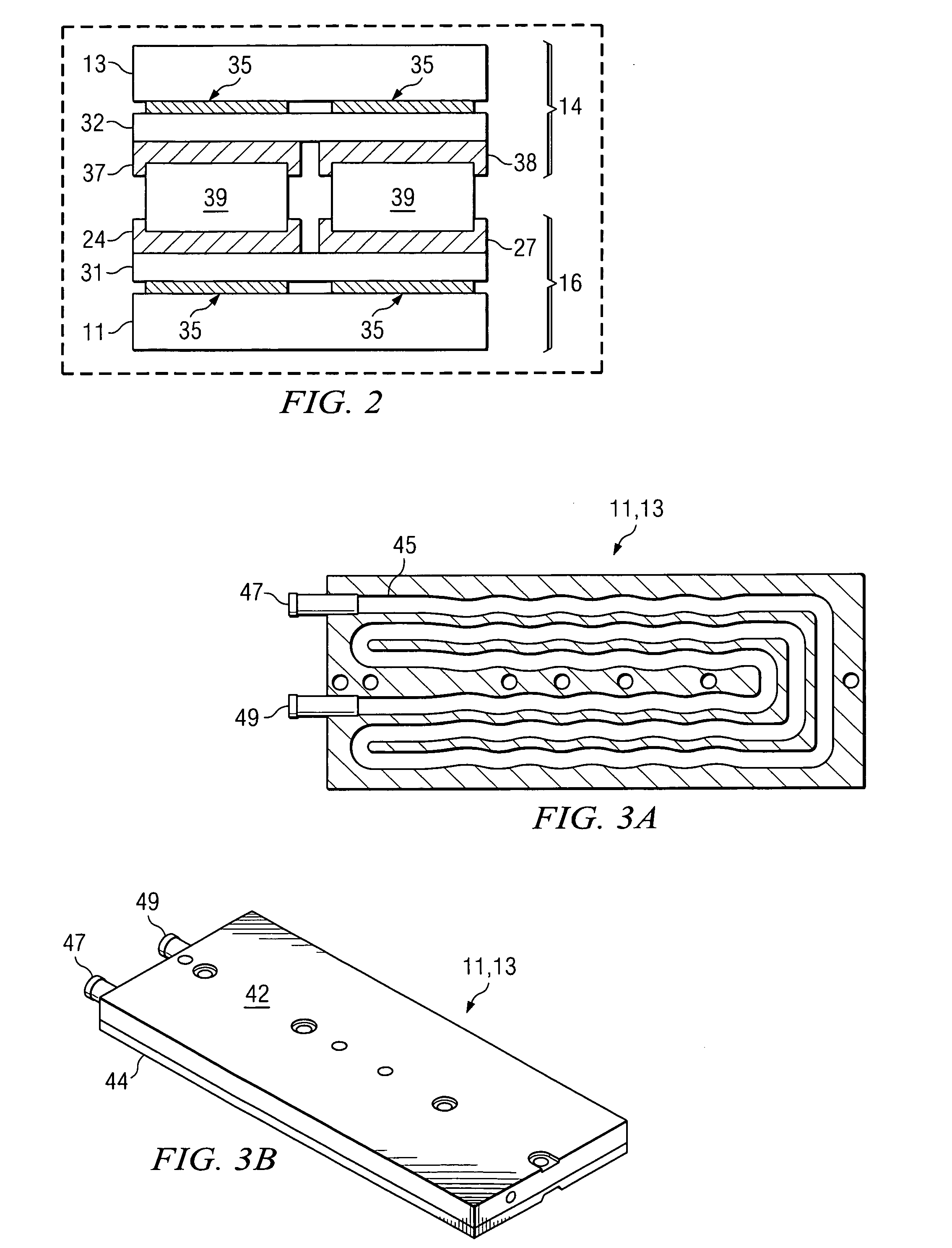
Active, micro-well thermal control subsystem
ActiveUS20080227186A1Bioreactor/fermenter combinationsHeating or cooling apparatusImmuno assayChemiluminescent immunoassay
Devices and systems for active thermal control of sample holding devices for bDNA testing, polymerase chain reaction testing, chemiluminescent immuno-assay testing, and so forth. The thermal control subsystem includes a fluidic circuit, first and second heater assemblies, a centrifugal pump, and a heat exchange device. The first and second heater assemblies include a heat removal device and a controllable thermo-electric device. One or both of the heater assemblies can include a heat spreader. A controller actively controls the pump, the heat removal device, and the thermo-electric devices, to thermally-control sample-containing vessels retained in the holding device.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Raman-active reagents and the use thereof
InactiveUS20110070662A1Minimize nonspecific bindingExtended shelf lifeSugar derivativesAnalysis by subjecting material to chemical reactionAntigenImmuno assay
The present invention provides a new class of Raman-active reagents for use in biological and other applications, as well as methods and kits for their use and manufacture. Each reagent includes a Raman-active reporter molecule, a binding molecule, and a surface enhancing particle capable of causing surface enhanced Raman scattering (SERS). The Raman-active reporter molecule and the binding molecule are affixed to the particle to give both a strong SERS signal and to provide biological functionality, i.e. antigen or drug recognition. The Raman-active reagents can function as an alternative to fluorescence-labeled reagents, with advantages in detection including signal stability, sensitivity, and the ability to simultaneously detect several biological materials. The Raman-active reagents also have a wide range of applications, especially in clinical fields (e.g., immunoassays, imaging, and drug screening).
Owner:IOWA STATE UNIV RES FOUND
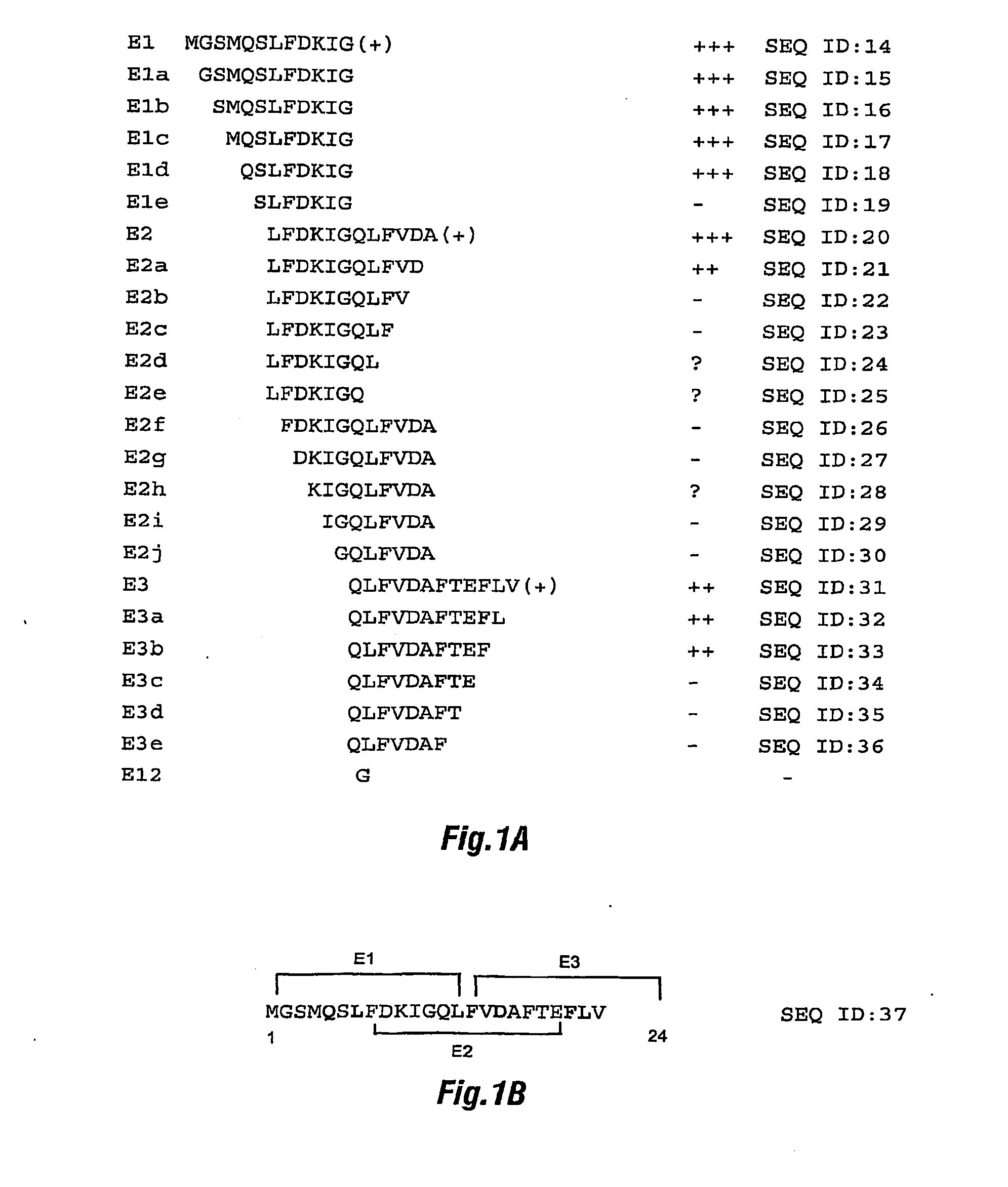
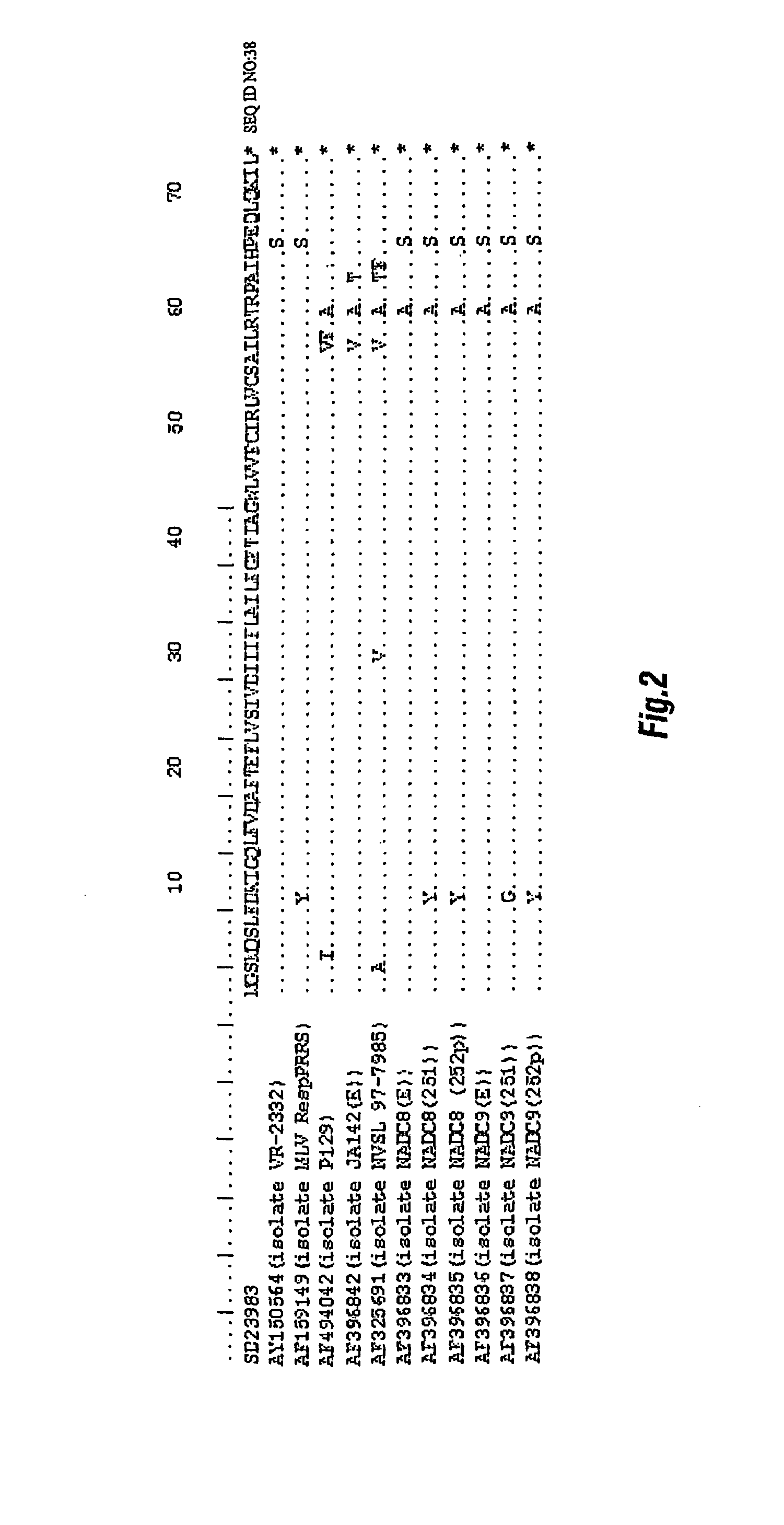
Differential immunoassay for prrs vaccine antibody
The present invention relates to immunoassays for serologically differentiating animals naturally infected with PRRS virus from animals vaccinated against PRRS. The immunoassays provide detection of at least a portion of the N terminal region of the 2b portion of PRRSV. The immunoassay is preferably an enzyme-linked immunosorbent assay (ELISA).
Owner:IOWA STATE UNIV RES FOUND
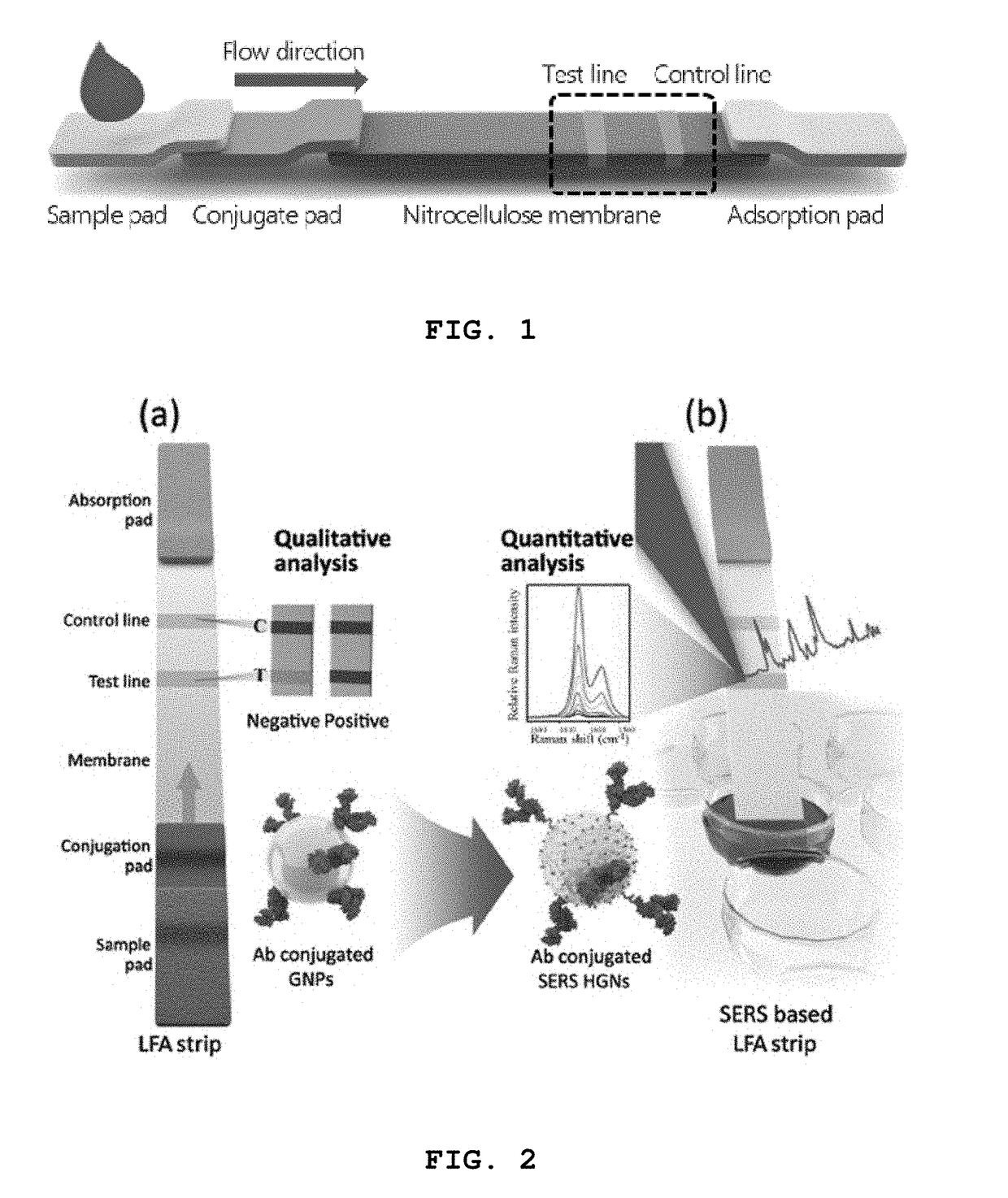
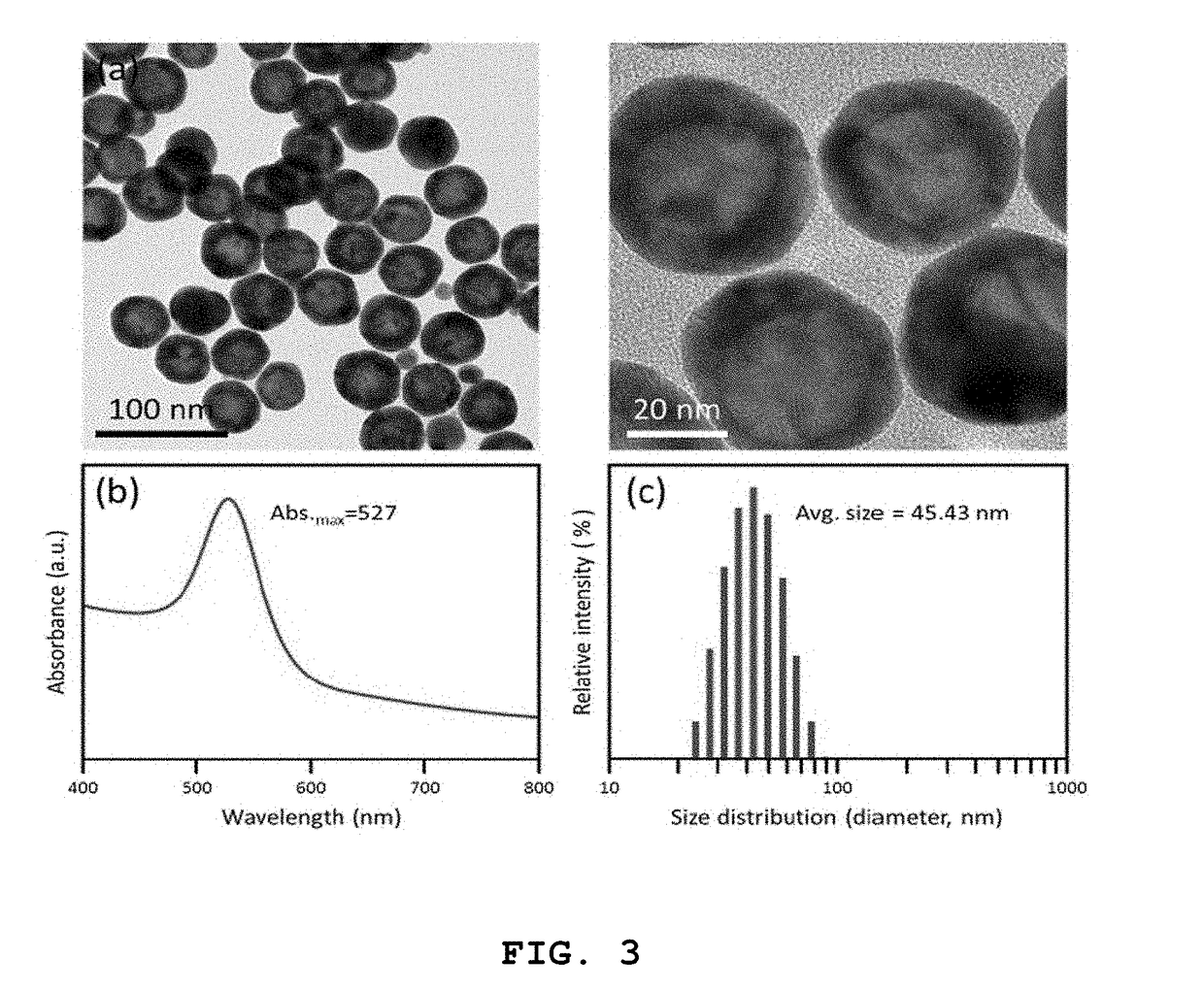
High-sensitivity lateral flow immunoassay strip based on surface-enhanced raman scattering and detection method using the same
InactiveUS20190049384A1Good reproducibilityMaterial analysis by observing effect on chemical indicatorRaman scatteringImmuno assayLateral flow immunoassay
The present disclosure relates to a surface-enhanced Raman scattering (SERS) lateral flow immunoassay strip containing: a sample pad into which a sample containing a target material is introduced; a conjugate pad containing a hollow metal nanoprobe for surface-enhanced Raman scattering, on which an antibody that can be coupled to the target material and a Raman marker are immobilized; and a detection pad including a detection region to which a secondary antibody that can be coupled to the target material coupled to the hollow metal nanoprobe is immobilized. Use of the SERS-based lateral flow immunoassay strip according to the present disclosure enables high-sensitivity quantitative analysis and qualitative analysis of the target material from Raman signal measurement depending on the concentration of the target material.
Owner:IND UNIV COOPERATION FOUND HANYANG UNIV ERICA CAMPUS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com