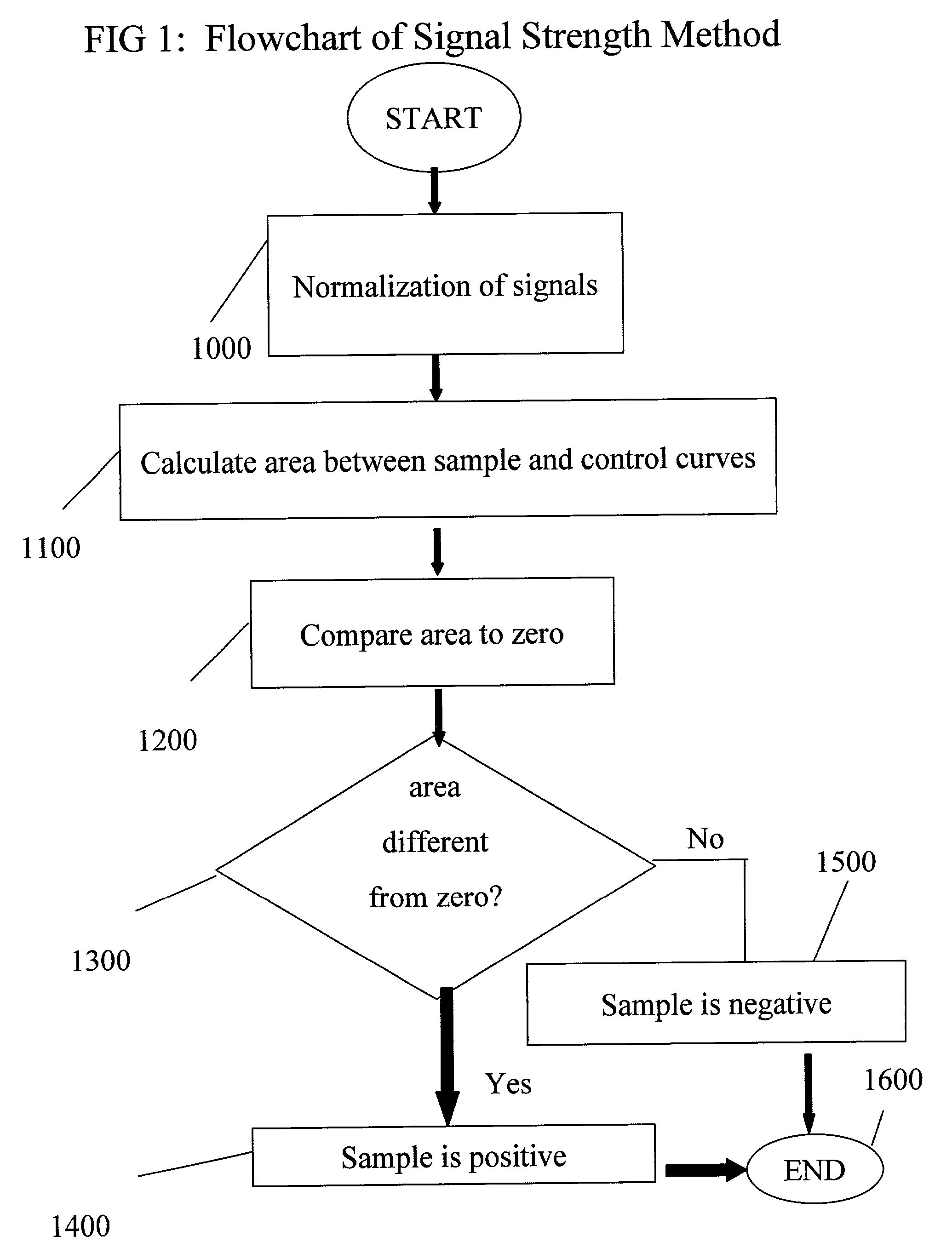
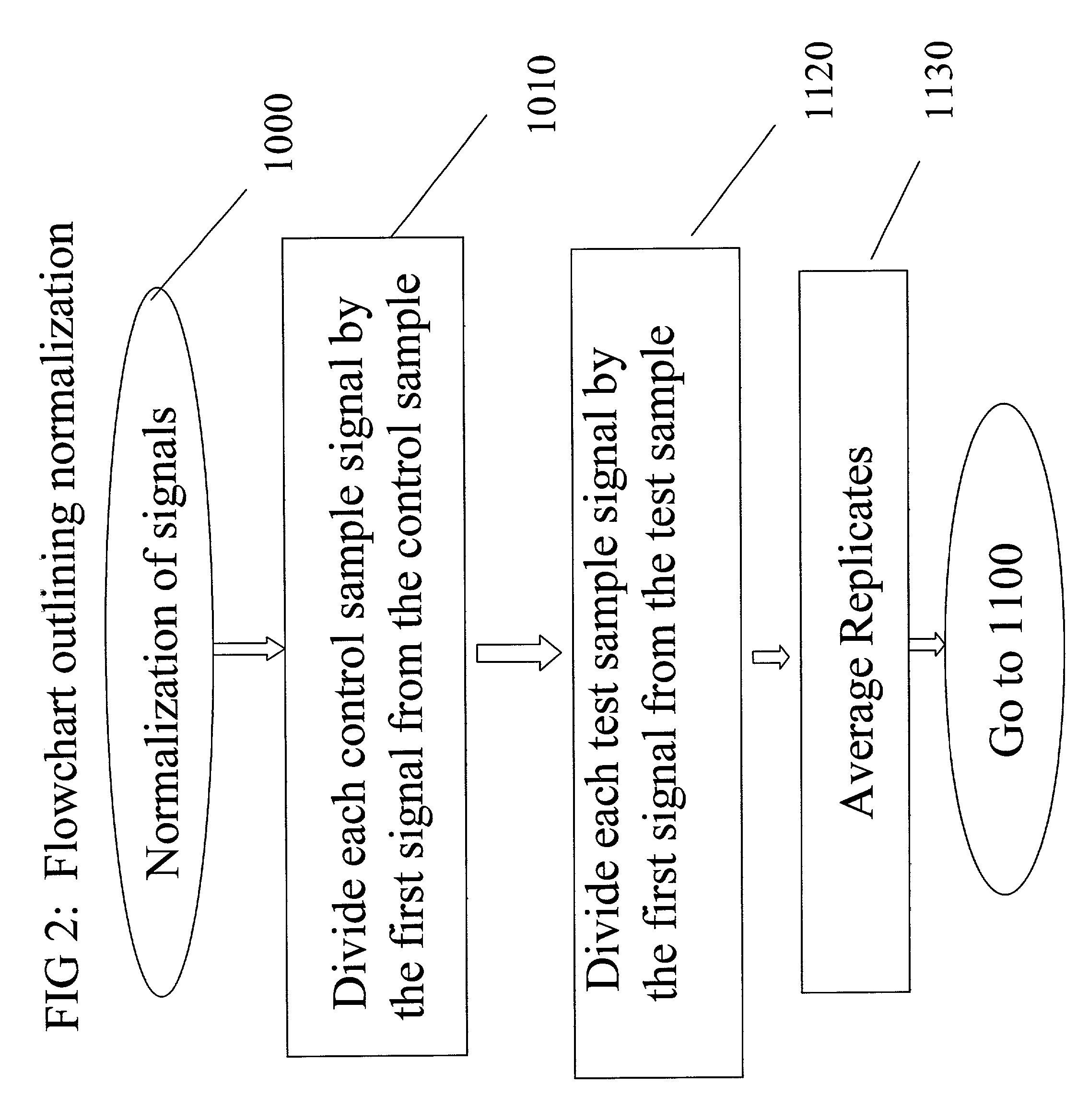
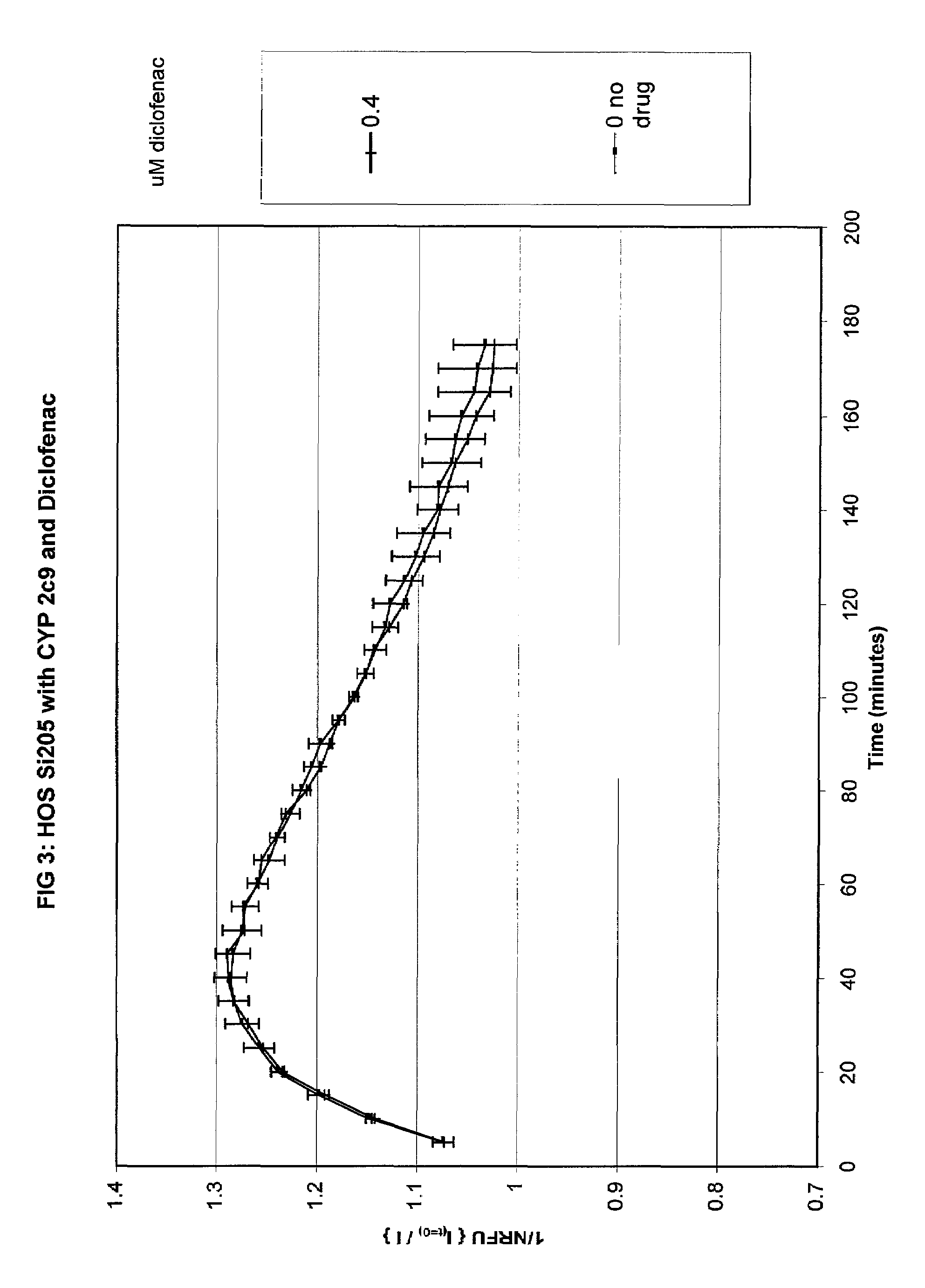
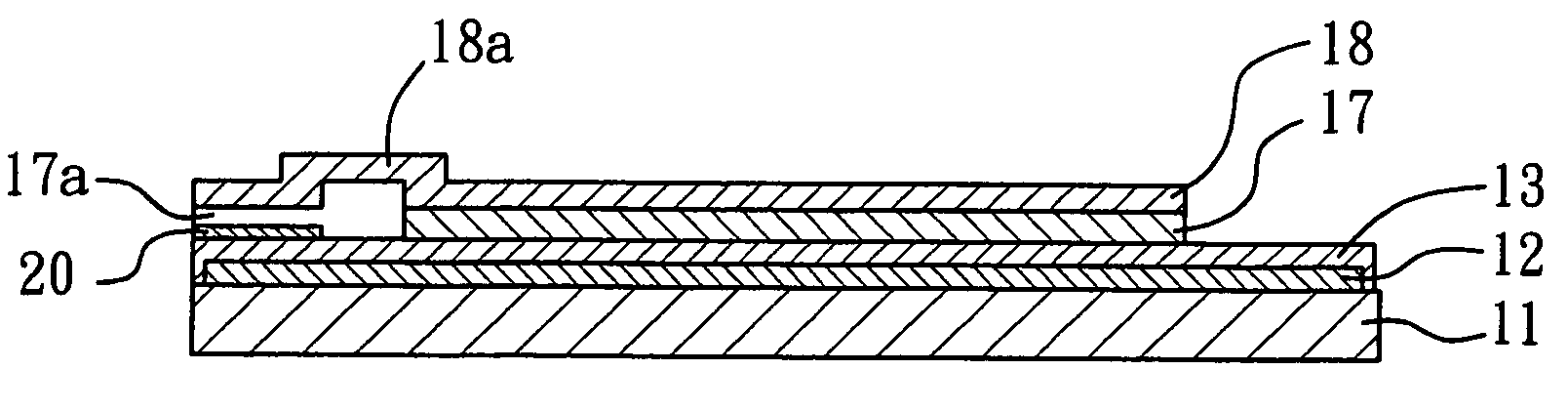
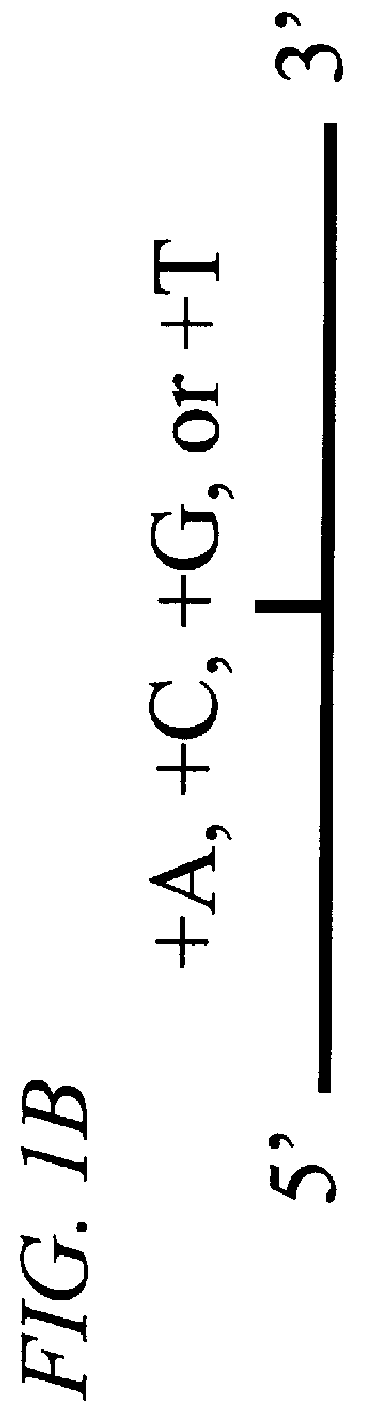Patents
Literature
707 results about "Control sample" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A control sample is an important part of the scientific method in experimental procedures. Using a control group allows the person conducting the experiment to isolate the effect of the experimental treatment. If there are no control groups or the control group is imperfect,...
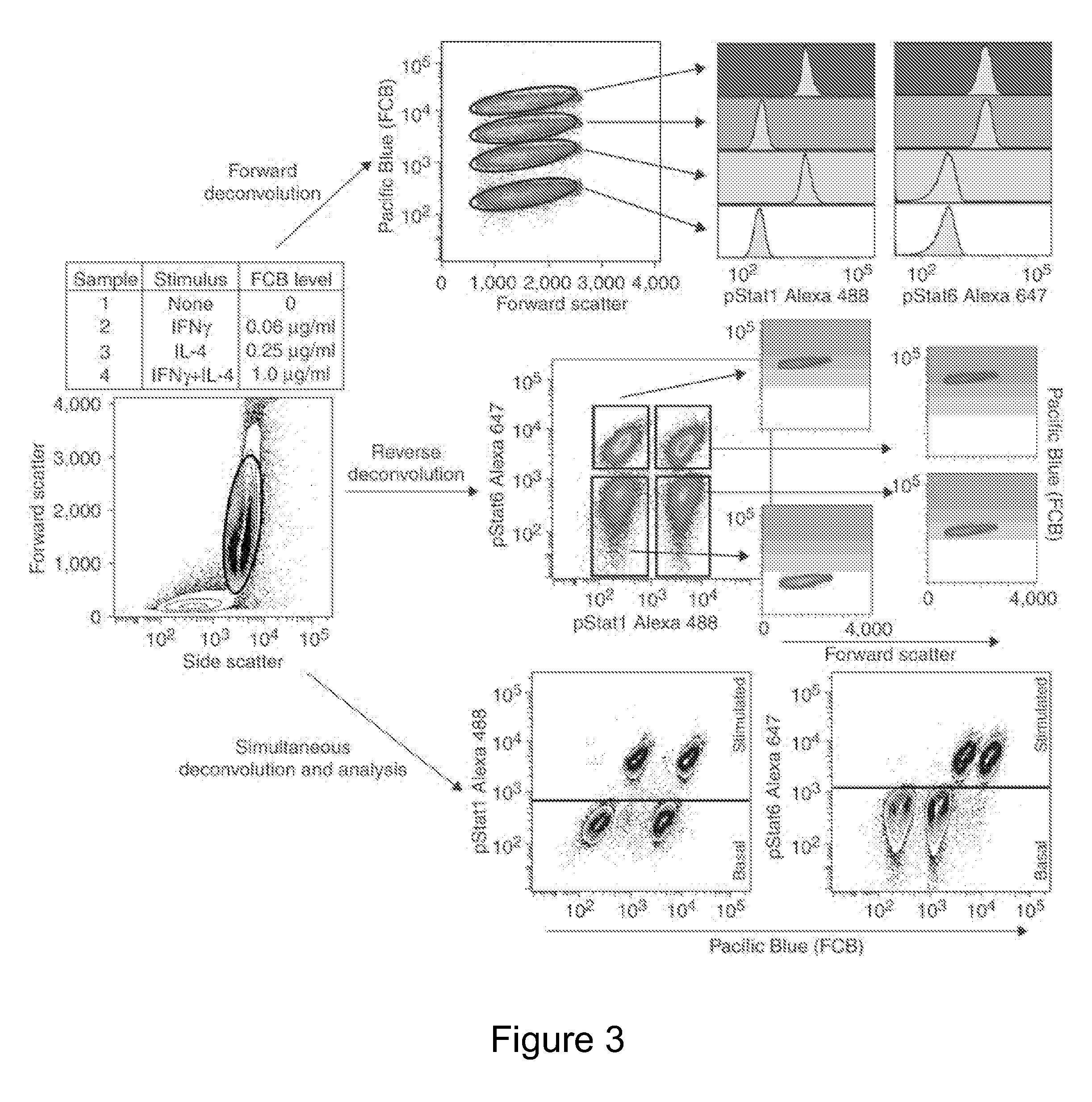
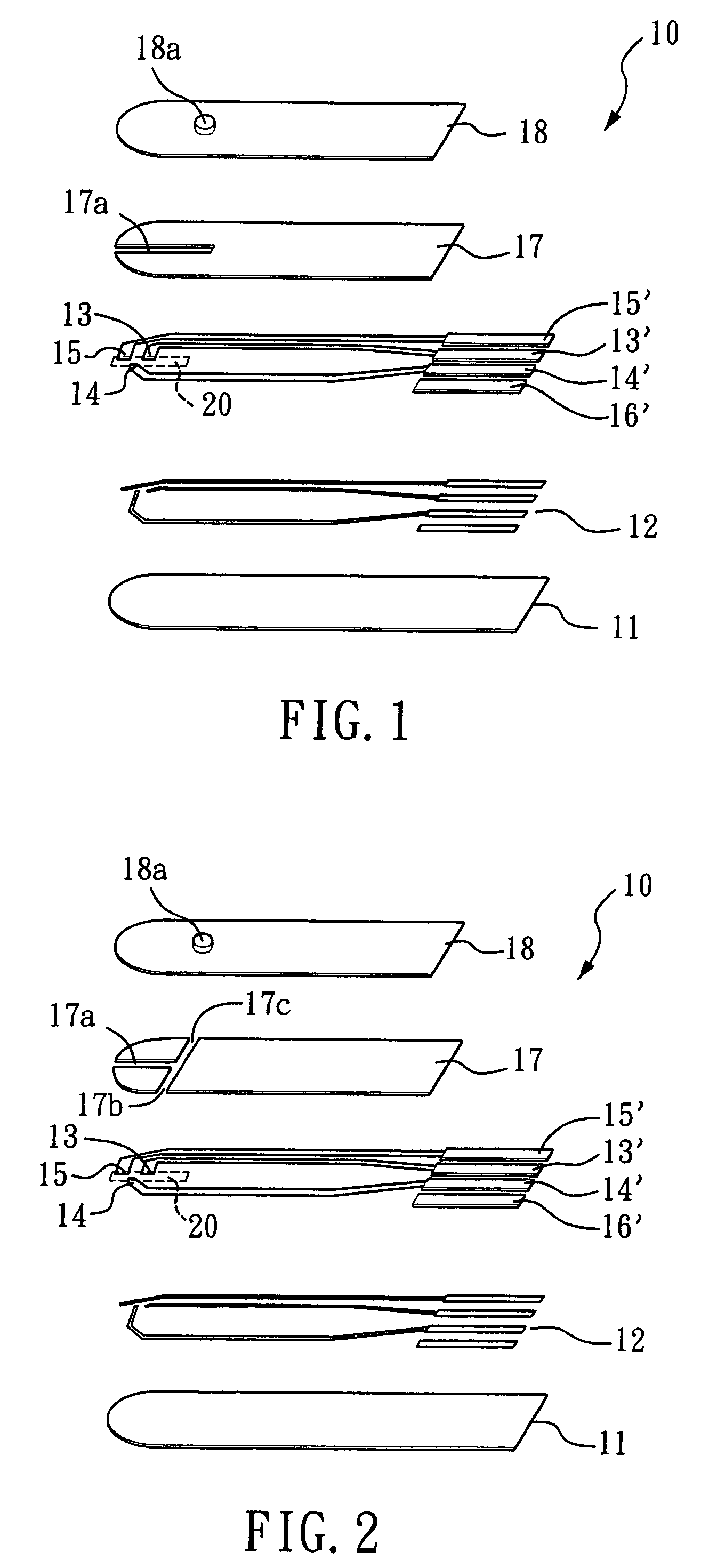
Probe-based analysis of heterozygous mutations using two-color labelling
InactiveUS6013449ASugar derivativesMicrobiological testing/measurementVariant alleleHeterozygous mutation
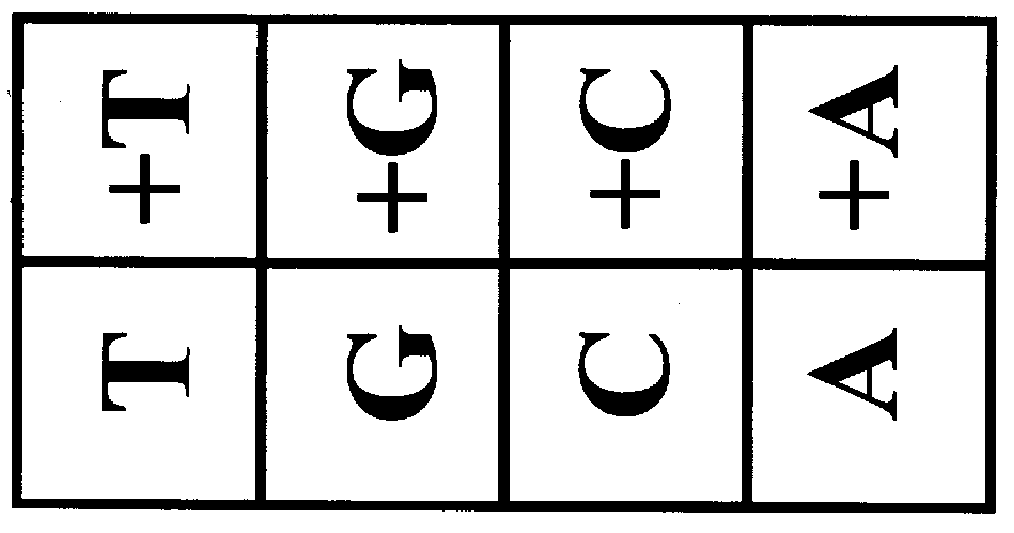
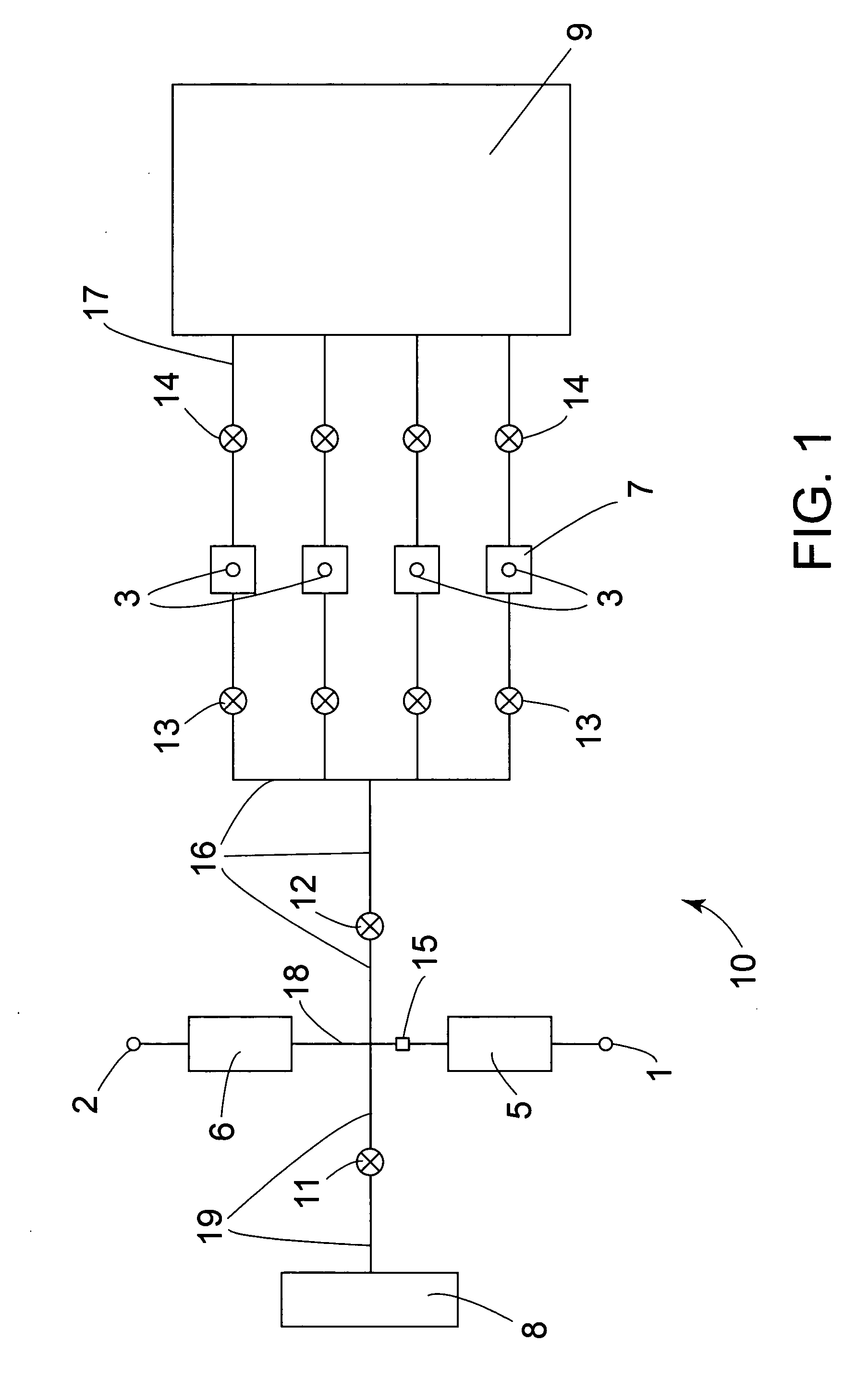
The invention provides methods of analyzing a nucleic acid in a target sample for variant alleles. In such methods, a first-labelled control sample and a second-labelled target sample are hybridized to at least one set of probes. The control sample comprises a homozygous reference allele. The target sample comprises the homozygous reference allele, or variant alleles differing from the reference allele at a locus, or one variant allele differing from the reference allele at the locus and one reference allele. The probes in the probe set span the locus and are complementary to the reference allele. After hybridization the intensity of first and second label bound to each probe in the set is measured. This information is then used to indicate the presence of one variant allele and one reference allele, or the presence of two variant alleles in the target sample.
Owner:HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA REPRESENTED BY THE +1
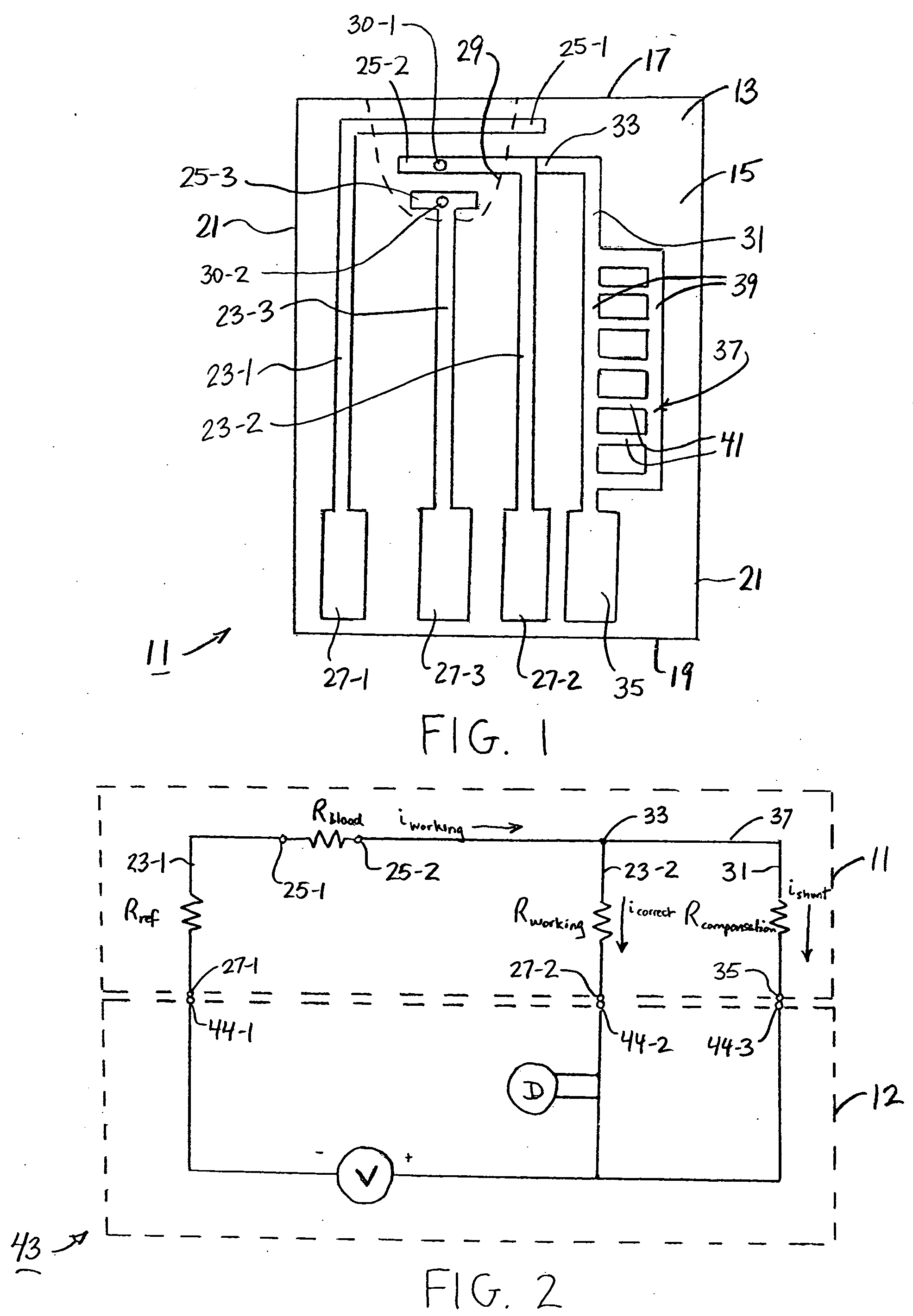
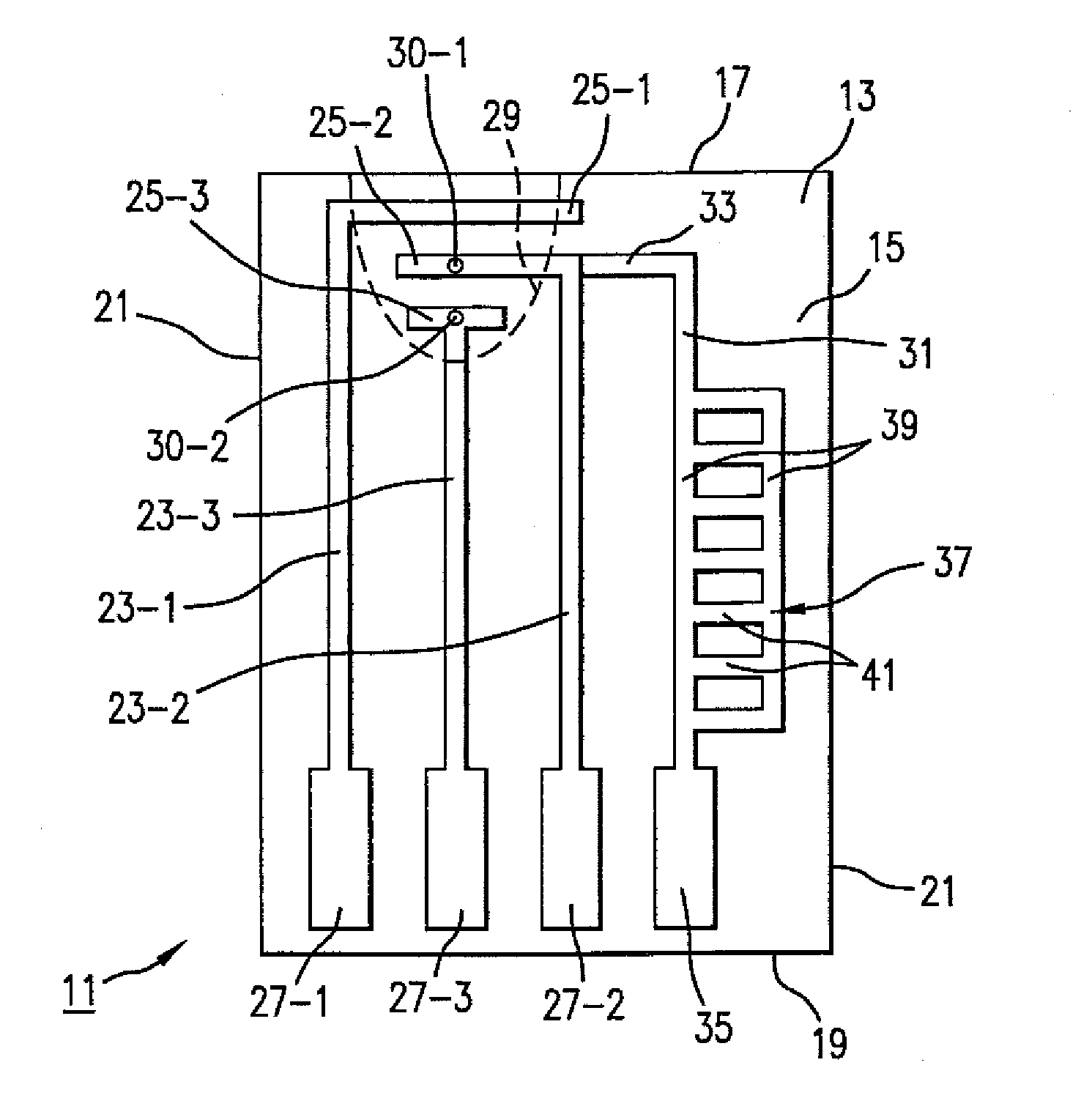
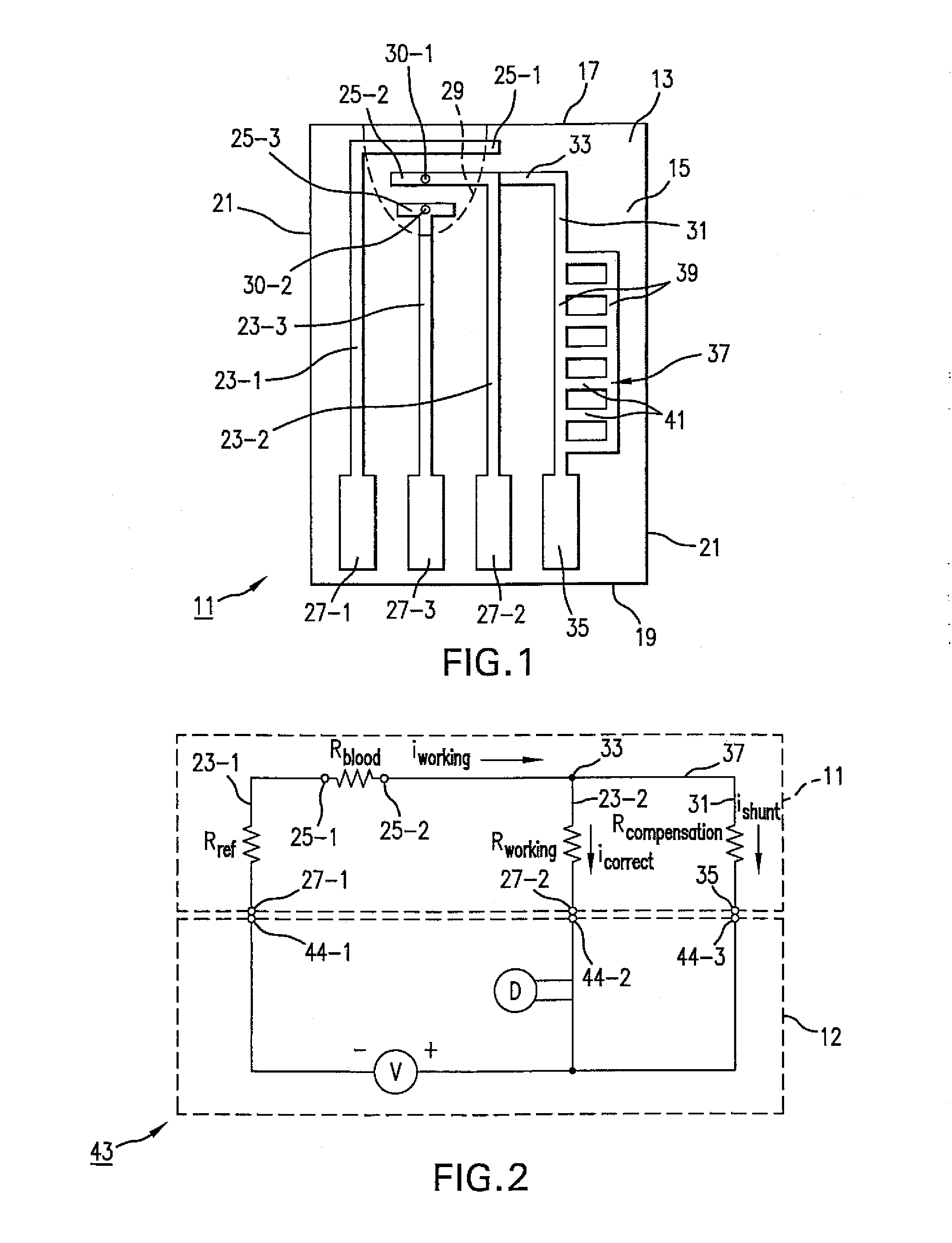
Analyte test sensor and method of manufacturing the same
InactiveUS20060144704A1Reduce manufacturing costEasy to useImmobilised enzymesBioreactor/fermenter combinationsElectrical resistance and conductanceAnalyte
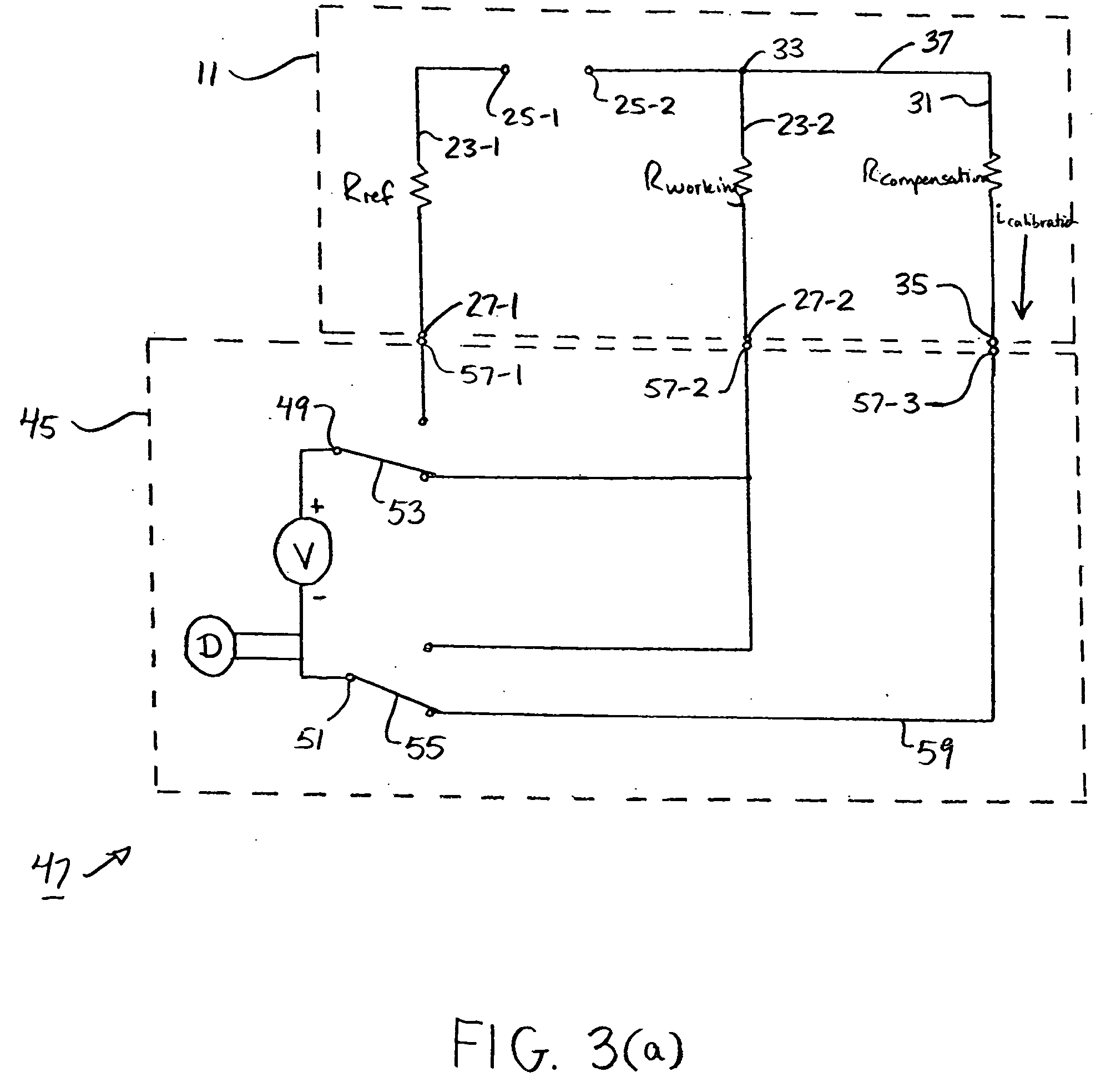
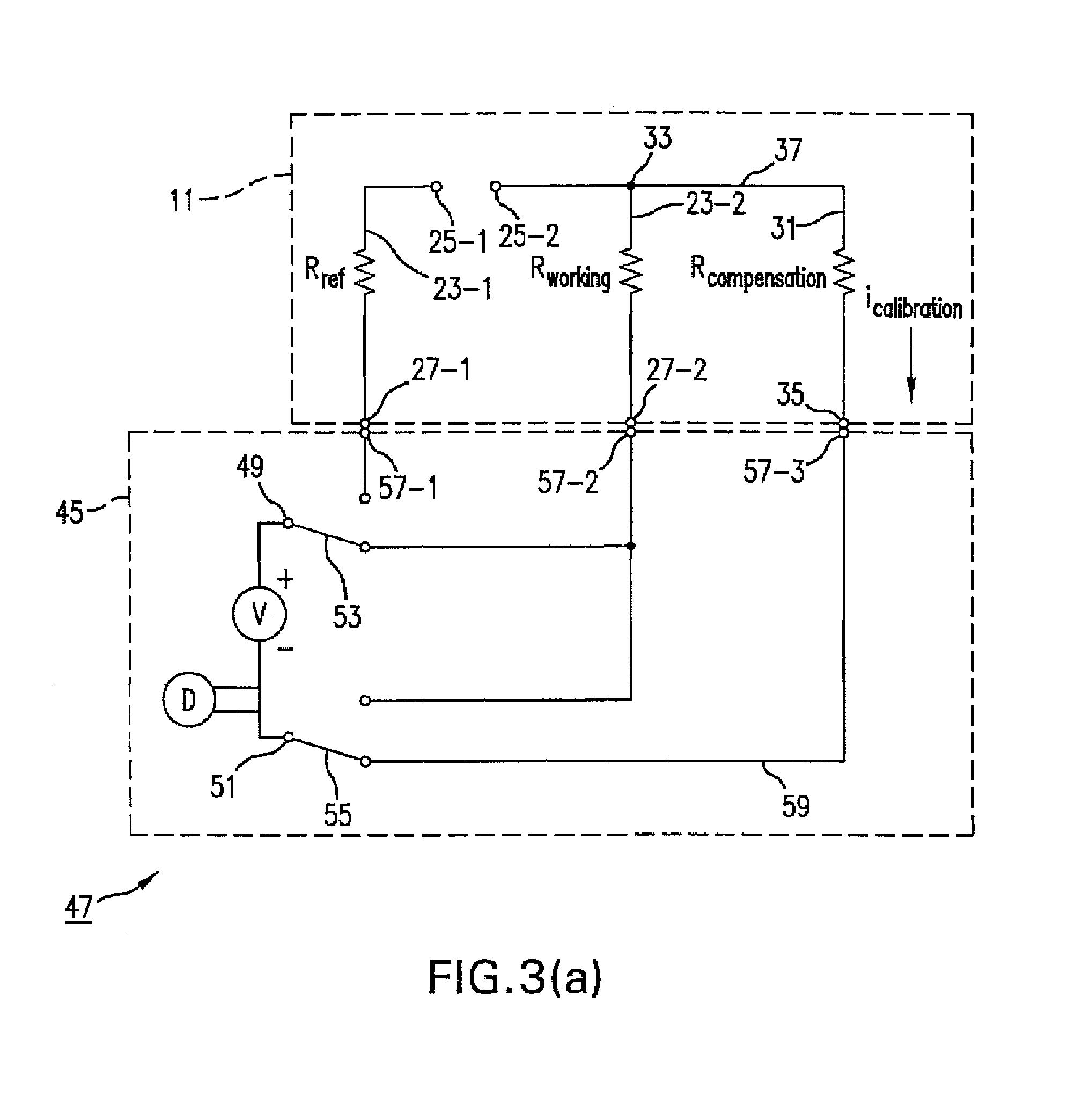
An analyte test sensor for use in measuring the concentration of a particular analyte in a test sample includes a non-conductive substrate, a reference electrode deposited on the substrate, a working electrode deposited on the substrate and a compensation electrode deposited on the substrate. The compensation electrode is provided with a resistive ladder and is designed to correct for test result inaccuracies which are the result of variances in the manufacturing of the test sensor. Specifically, in one embodiment, the compensation electrode corrects for test result inaccuracies in an analog manner by shunting a portion of the working current away from working electrode. In another embodiment, the compensation electrode corrects for test result inaccuracies in a digital manner by providing a calibration code which is proportional its resistance value. A batch of analyte test sensors are preferably manufactured in the following manner. An initial batch of the test sensors is constructed. Then, a limited sampling of the sensors is tested for accuracy using a control sample. Based on the test results, the resistance value of the compensation electrode for each remaining sensor in the batch is adjusted accordingly.
Owner:ABBOTT DIABETES CARE INC
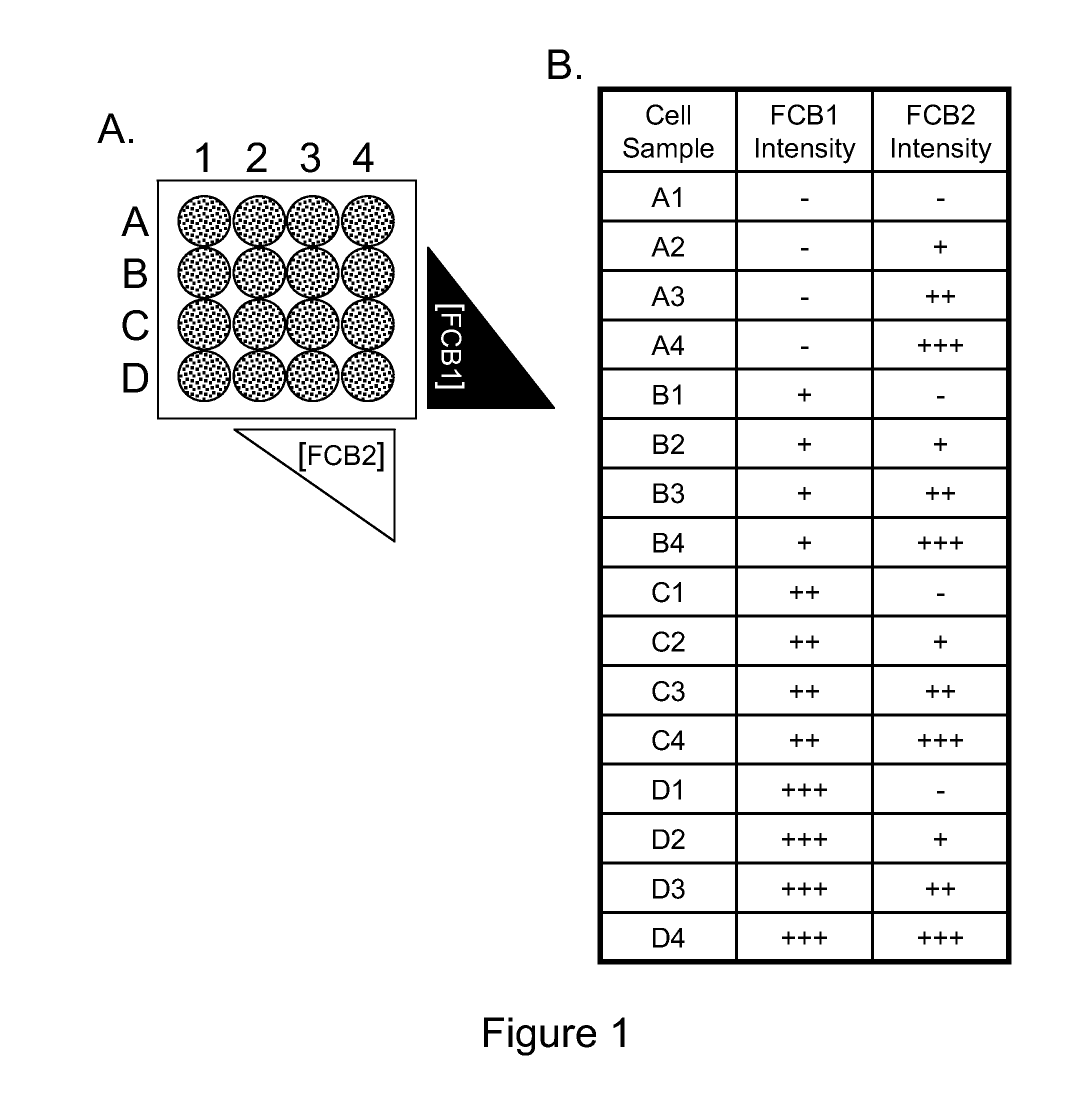
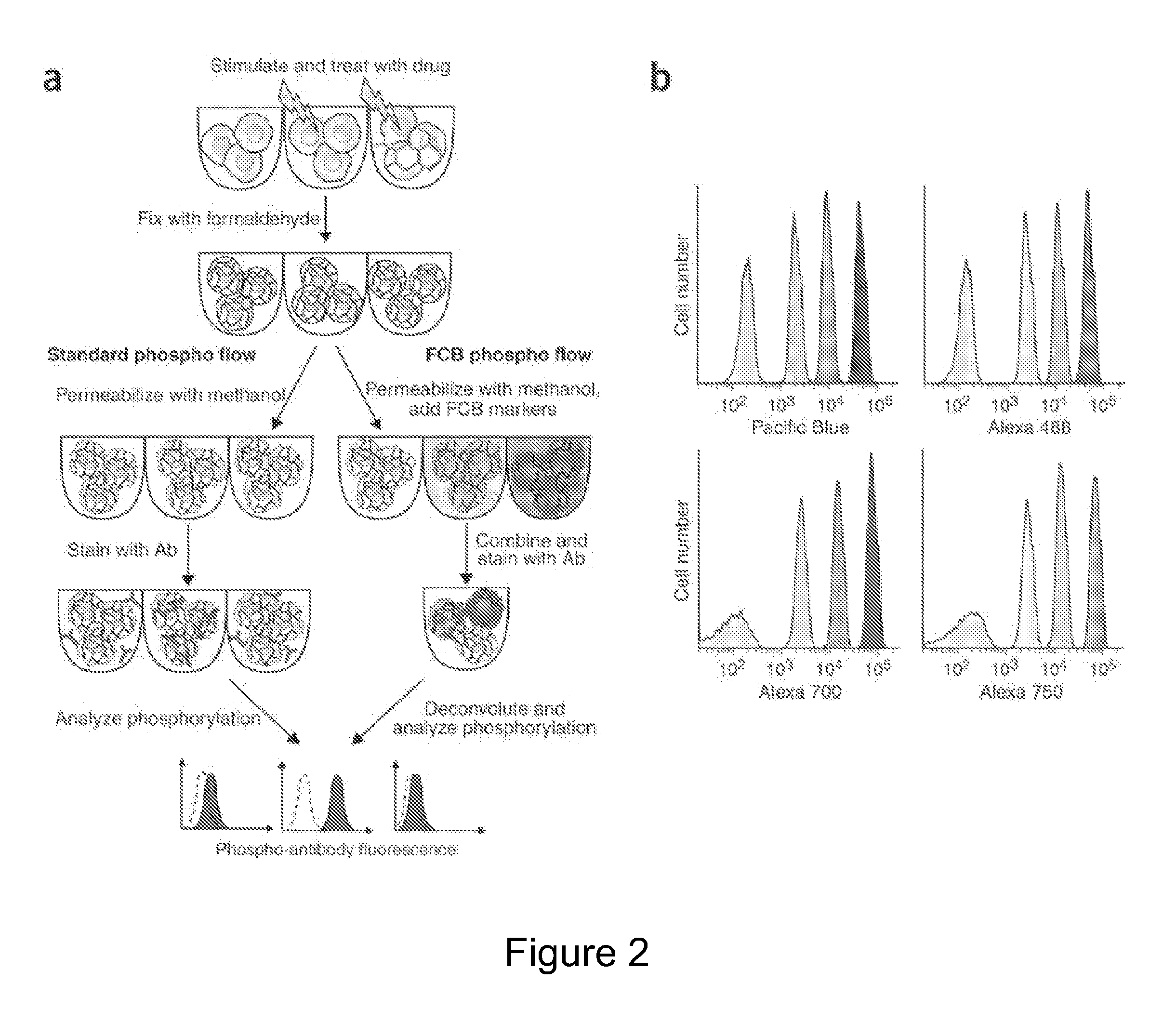
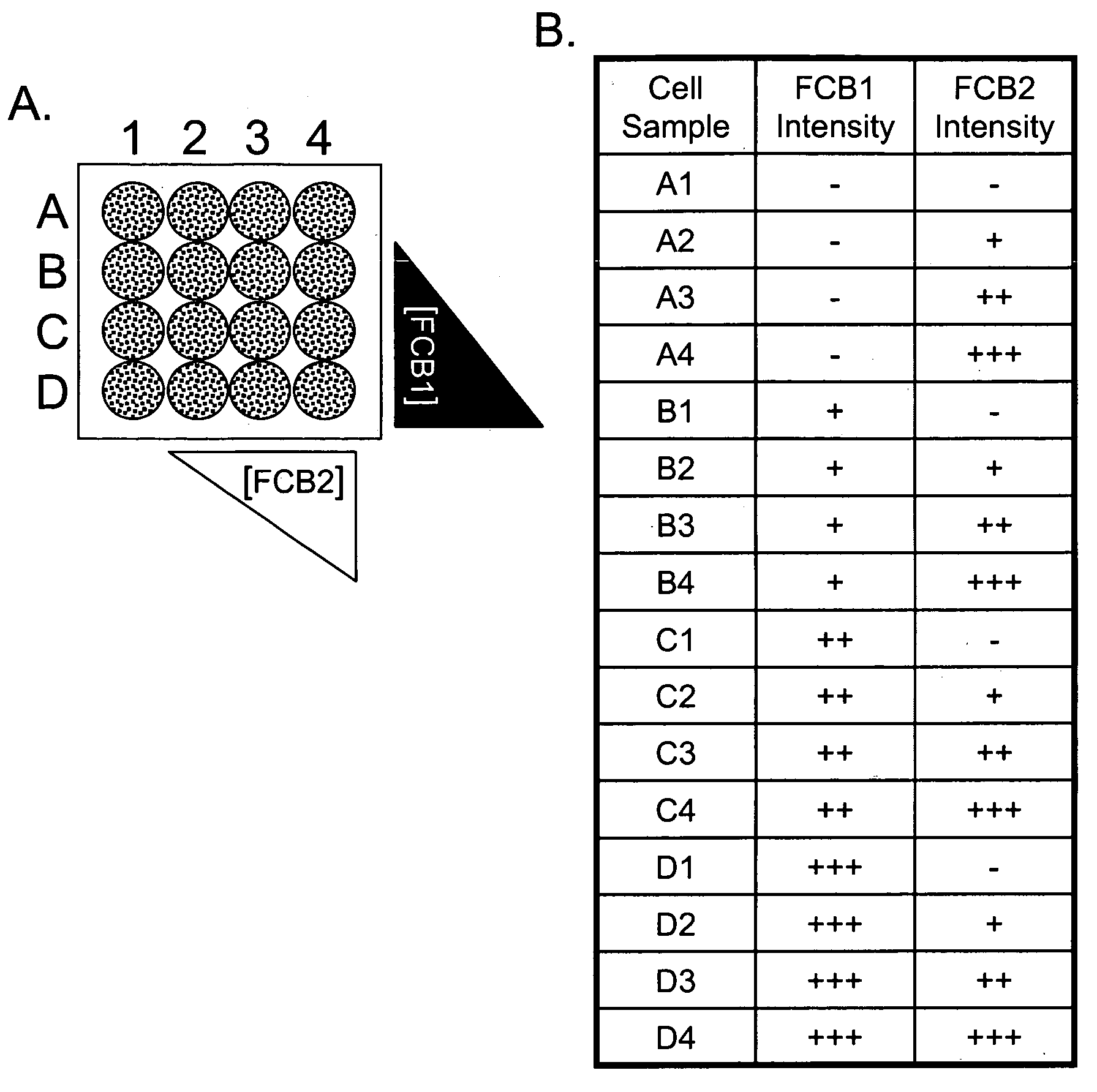
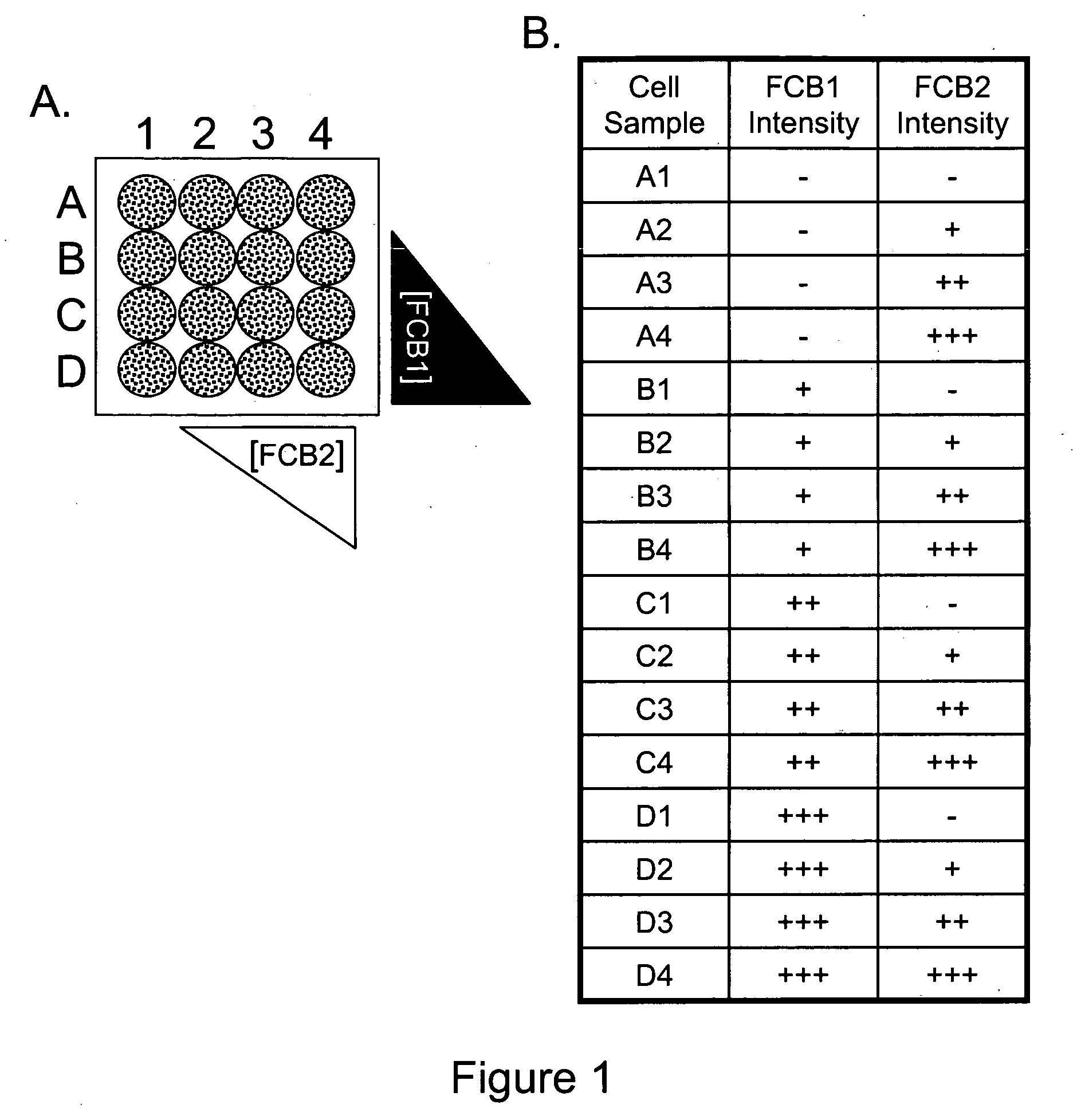
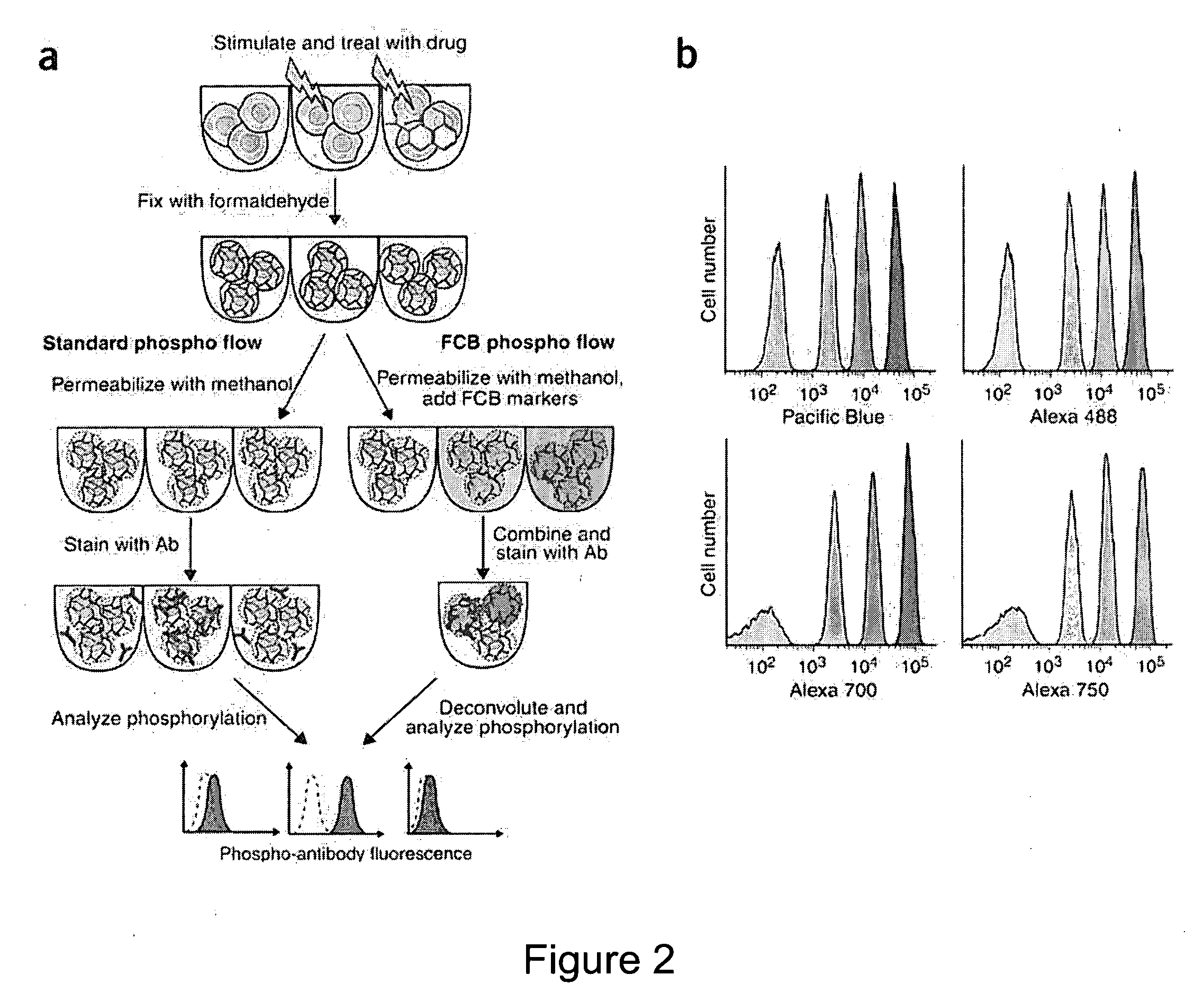
Multiplex Cellular Assays Using Detectable Cell Barcodes
InactiveUS20110263457A1Improve throughputReduce consumptionOrganic chemistryMicrobiological testing/measurementMultiplexingAnalyte
We describe herein a cell-based multiplexing technique called detectable cell barcoding (DCB). In DCB, each individual sample is labeled with a different DCB signature that distinguishes each sample by one or both of detected intensity or type of detection characteristic. The samples are then combined and analyzed for a detectable characteristic of interest (e.g., presence of an analyte). By employing multiple distinct DCB labels at varying concentrations, one can perform multiplex analyses on up to hundreds or thousands (or more) of cell samples in a single reaction tube. DCB reduces reagent consumption by factors of 100-fold or more, significantly reduces data acquisition times and allows for stringent control sample analysis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
System for cell enrichment
Owner:ARTEMIS HEALTH INC +2
Methods for improving sensitivity of oxygen biosensors
InactiveUS6991918B2Microbiological testing/measurementBiological testingTest samplePhysical chemistry
A method for detecting oxygen in a test sample is provided, the method involving exposing a test sample and a control sample to sensor compositions having a luminescent compound capable of generating a signal indicative of oxygen depletion. The method further involves determining the strengths of such signals for the test sample versus the control sample, and using the difference to determine whether oxygen in the test sample is being consumed.
Owner:BECTON DICKINSON & CO
Electrochemical biosensor by screen printing and method of fabricating same
ActiveUS7138041B2Rapid sample inflowSmall volumeImmobilised enzymesBioreactor/fermenter combinationsReaction layerSiphon
An electrochemical biosensor formed by screen printing and method of fabricating such biosensor is disclosed in the present invention. The biosensor can quickly absorb a sample to be measured therein, effectively control volume of the sample fed and “fill-and-position” the sample therein. The biosensor includes an electrode layer (electrode area) comprising two or three electrodes, which are a working electrode, a reference electrode and an auxiliary electrode (tri-electrode) on an insulating substrate. An active reaction layer containing reactant, reaction catalyst, mediator, wetting agent and surfactant is spread on the surface of the electrode layer. A sample inflow area is formed above the electrode area by adding an upper cover on top of a middle insulating layer with a U-shaped opening formed therein. Sample solution with a minute amount about 0.8 to 1 μl can be rapidly introduced into the electrode area and the active reaction layer via the inflow area by siphon or capillary, where the ingredient of the sample can be analysed by measuring reaction between the sample, reaction catalyst and mediator in the reaction layer using electrochemical potentiometric or amperometric method. An upwardly extended closed space formed within the upper cover above the electrode area adjacent to the front of conductive wires can be effectively used to control sample volume and “fill-and-position” the sample.
Owner:GENERAL LIFE BIOTECHNOLGOY
Probe-based analysis of heterozygous mutations using two-color labelling
InactiveUS6342355B1Bioreactor/fermenter combinationsBiological substance pretreatmentsVariant alleleGenomic clone
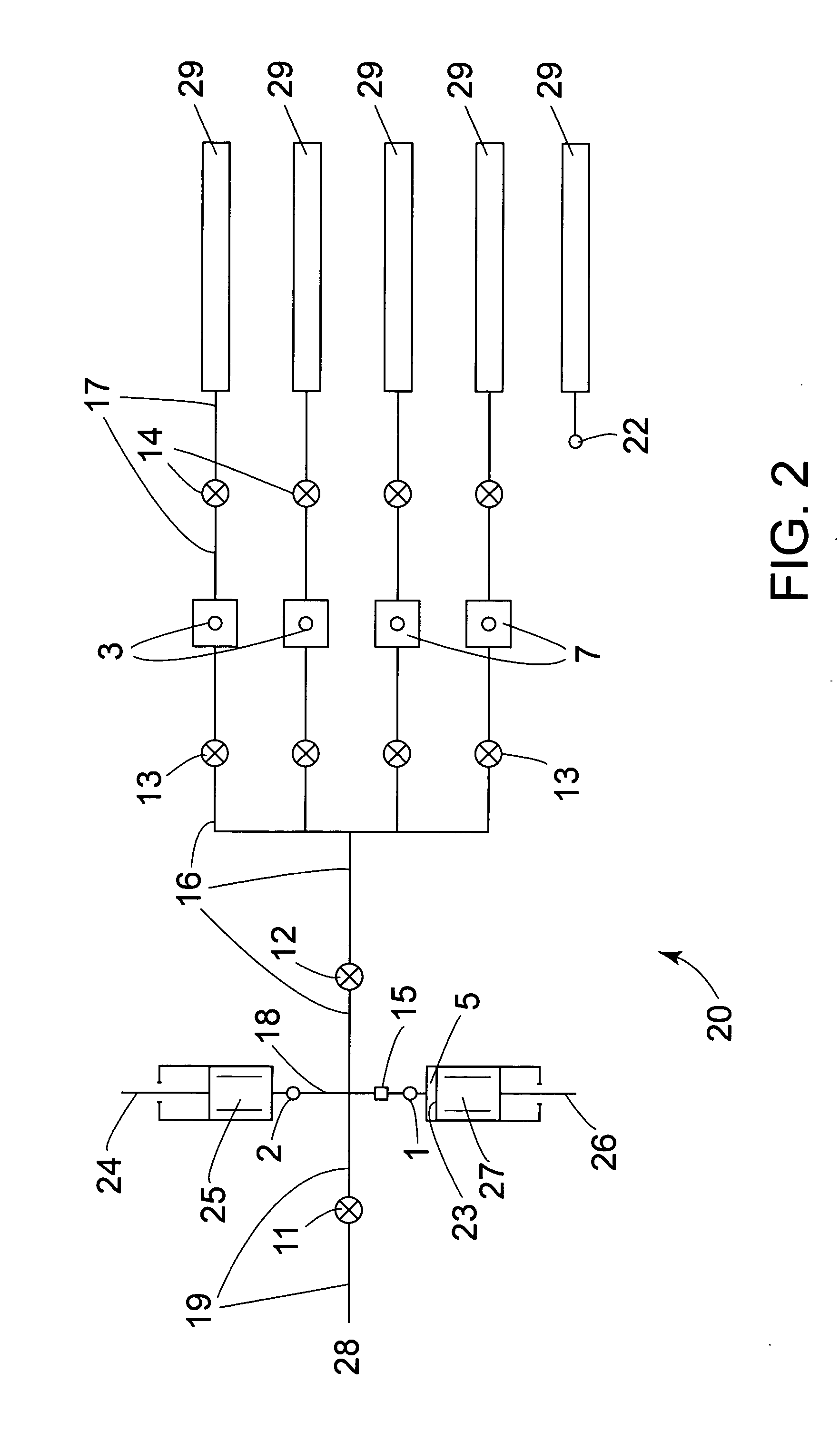
The invention provides methods of analyzing a nucleic acid in a target sample for variant alleles. In such methods, a first-labelled control sample and a second-labelled target sample are hybridized to at least one set of probes. The control sample comprises a homozygous reference allele. The target sample comprises the homozygous reference allele, or variant alleles differing from the reference allele at a locus, or one variant allele differing from the reference allele at the locus and one reference allele. The probes in the probe set span the locus and are complementary to the reference allele. After hybridization the intensity of first and second label bound to each probe in the set is measured. This information is then used to indicate the presence of one variant allele and one reference allele, or the presence of two variant alleles in the target sample.
Owner:UNITED STATES OF AMERICA +1
Multiplex cellular assays using detectable cell barcodes
ActiveUS20080241820A1Reduces regent consumptionImprove throughputMicrobiological testing/measurementBiological testingMultiplexingAnalyte
We describe herein a cell-based multiplexing technique called detectable cell barcoding (DCB). In DCB, each individual sample is labeled with a different DCB signature that distinguishes each sample by one or both of detected intensity or type of detection characteristic. The samples are then combined and analyzed for a detectable characteristic of interest (e.g., presence of an analyte). By employing multiple distinct DCB labels at varying concentrations, one can perform multiplex analyses on up to hundreds or thousands (or more) of cell samples in a single reaction tube. DCB reduces reagent consumption by factors of 100-fold or more, significantly reduces data acquisition times and allows for stringent control sample analysis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Analyte test sensor and method of manufacturing the same
InactiveUS7418285B2Reduce manufacturing costEasy to useImmobilised enzymesBioreactor/fermenter combinationsElectrical resistance and conductanceAnalyte
An analyte test sensor for use in measuring the concentration of a particular analyte in a test sample includes a non-conductive substrate, a reference electrode deposited on the substrate, a working electrode deposited on the substrate and a compensation electrode deposited on the substrate. The compensation electrode is provided with a resistive ladder and is designed to correct for test result inaccuracies which are the result of variances in the manufacturing of the test sensor. Specifically, in one embodiment, the compensation electrode corrects for test result inaccuracies in an analog manner by shunting a portion of the working current away from working electrode. In another embodiment, the compensation electrode corrects for test result inaccuracies in a digital manner by providing a calibration code which is proportional its resistance value. A batch of analyte test sensors are preferably manufactured in the following manner. An initial batch of the test sensors is constructed. Then, a limited sampling of the sensors is tested for accuracy using a control sample. Based on the test results, the resistance value of the compensation electrode for each remaining sensor in the batch is adjusted accordingly.
Owner:ABBOTT DIABETES CARE INC
Multiple label fluorescence polarization assay system and method
InactiveUS20010033374A1Accurate calculationAccurate valueSpectrum investigationChemiluminescene/bioluminescenceSpectral bandsFluorescence
A sample having a plurality of probe molecules is illuminated with at least one beam of excitation light that is linearly polarized along a first axis, thereby effecting fluorescence emission in a plurality of spectral bands. The intensity of a first component of fluorescence emission that is polarized along the first axis, as well as the intensity of a second component of fluorescence emission that is polarized along an orthogonal second axis, is measured for each of said plurality of spectral bands. These measurements are represented as a measurement vector M. Since each probe emits some limited amount of light in the characteristic band of another probe, this results in cross-talk between probes. The measurement vector is therefore corrected using an instrument response matrix A, which is generated by measuring the flux output of control samples which each have only a single probe species. A flux vector S is calculated according to S=A.sup.-1M, and the fluorescence polarization FP is calculated from the S values.
Owner:CAMBRIDGE RES & INSTR
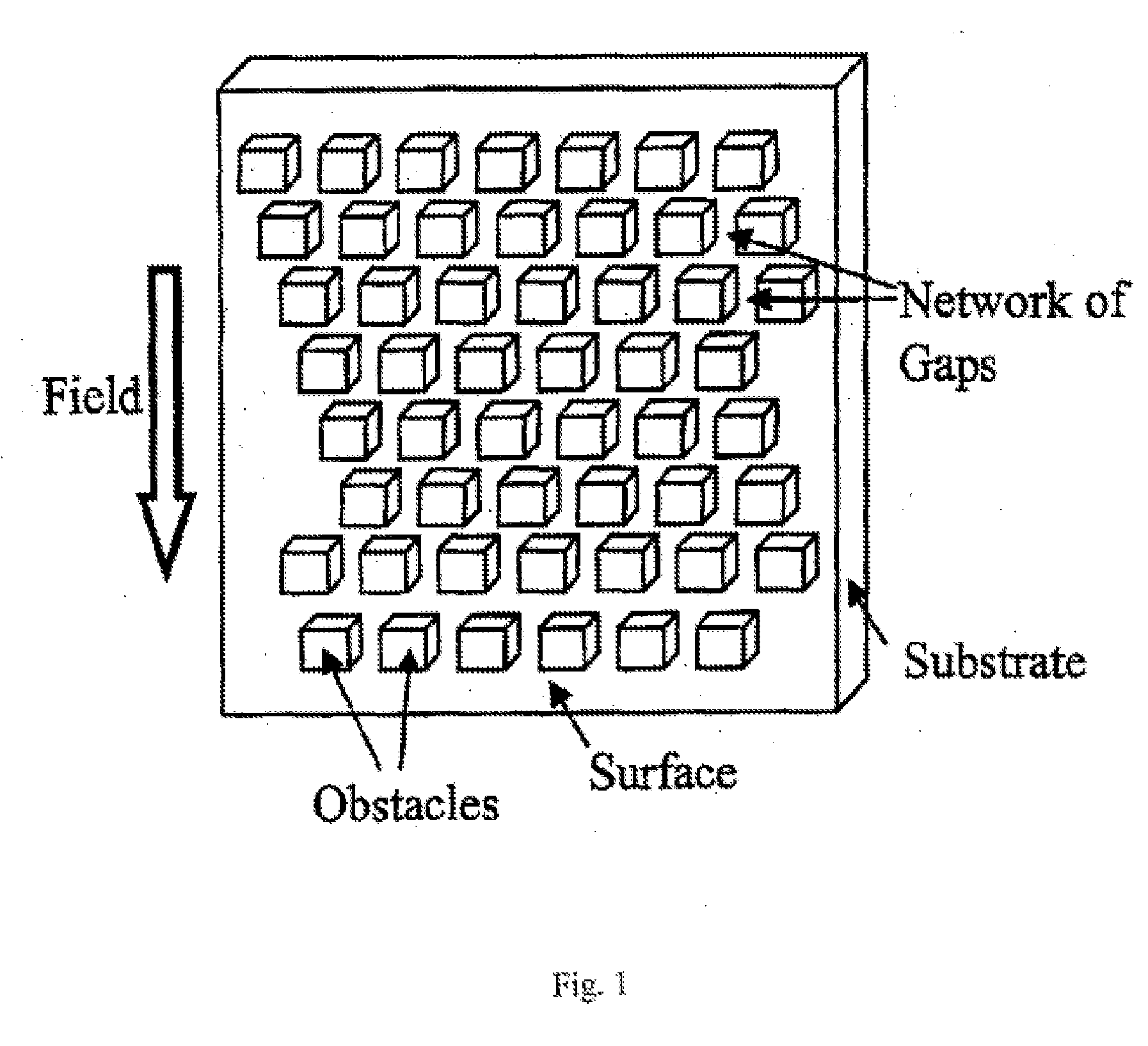
Process for preparing control samples of particles such as microorganisms and cells
Processes for preparing controlled samples of particles, including microorganisms and cells are described. A sample of particles is provided and separated into a predetermined number of desired particles by particle separation means. The predetermined number of particles is dispensed into a receptacle or onto a surface in accordance with a sorting instruction, with the receptacle or surface being positioned by collecting means so as to receive the dispensed particles. A sorting instruction from the particle dispensing means activates the collecting means such that when a sorting instruction has been actuated, so as to deliver a predetermined number of particles into a receptacle or onto a surface which is positioned accurately for sufficient time to collect all sorted particles, the collecting means advances and positions a subsequent surface or receptacle for receipt of particles, the collector means thereafter signaling the particle separation means to commence the next sorting instruction.
Owner:BTF
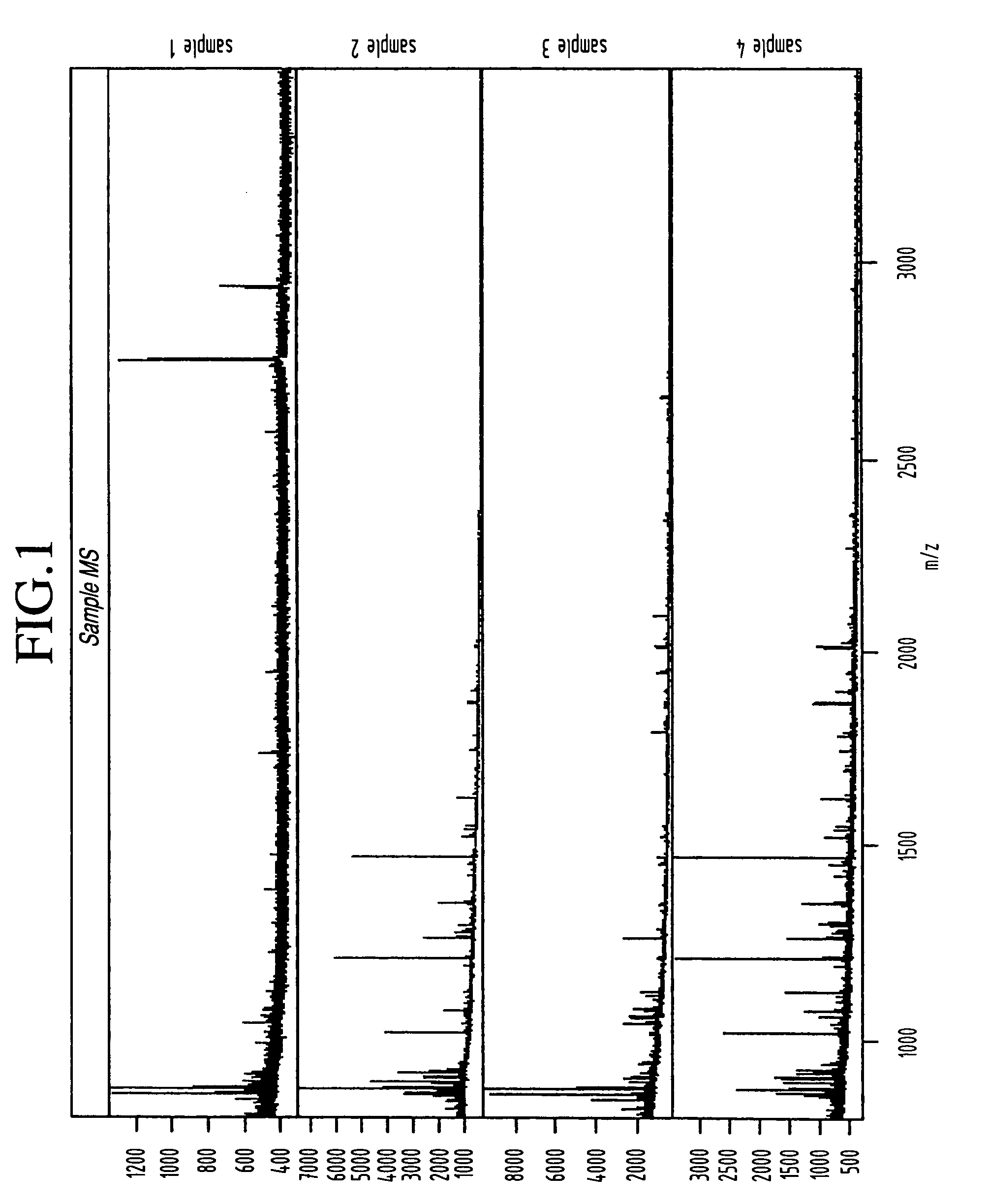
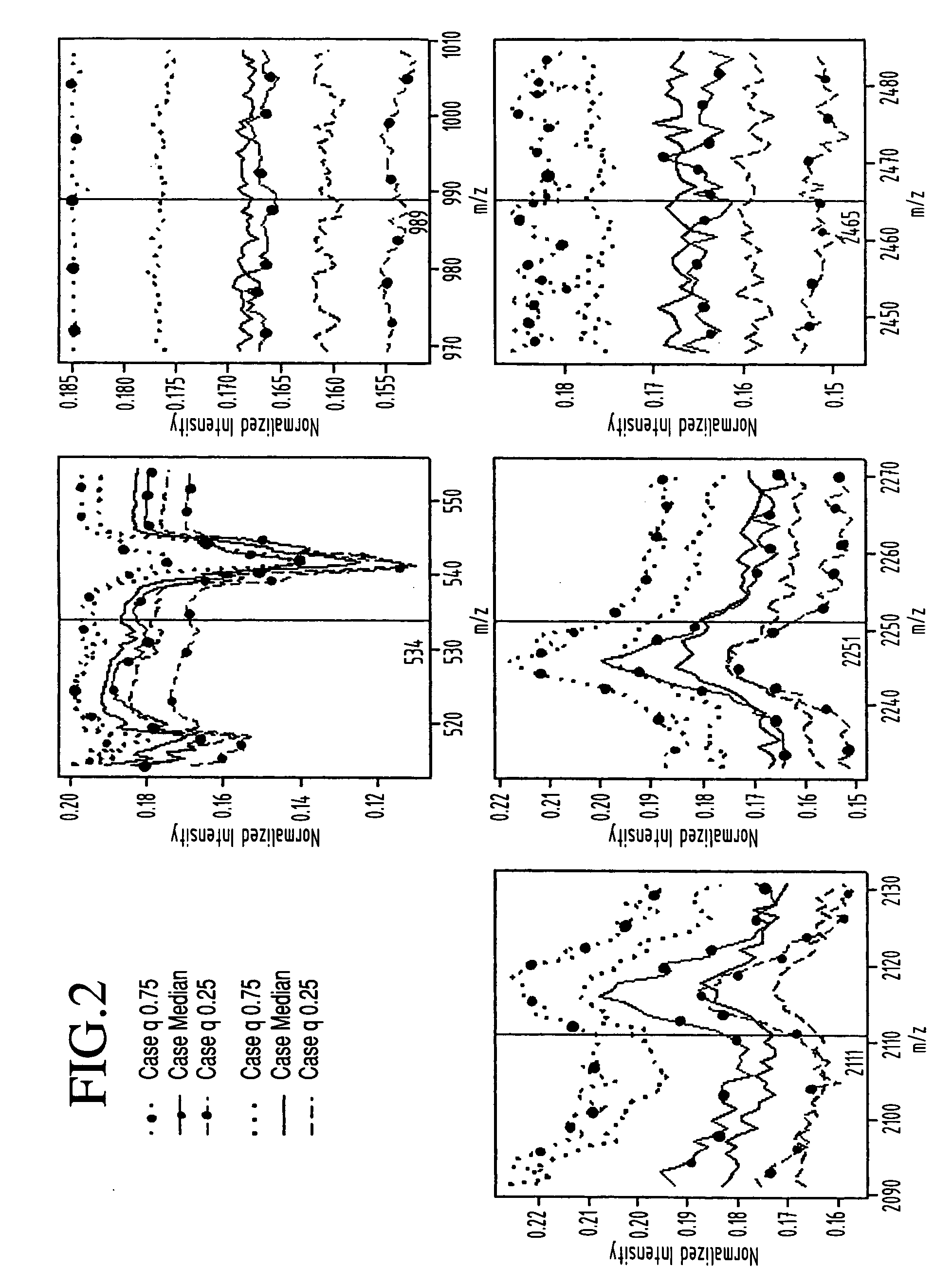
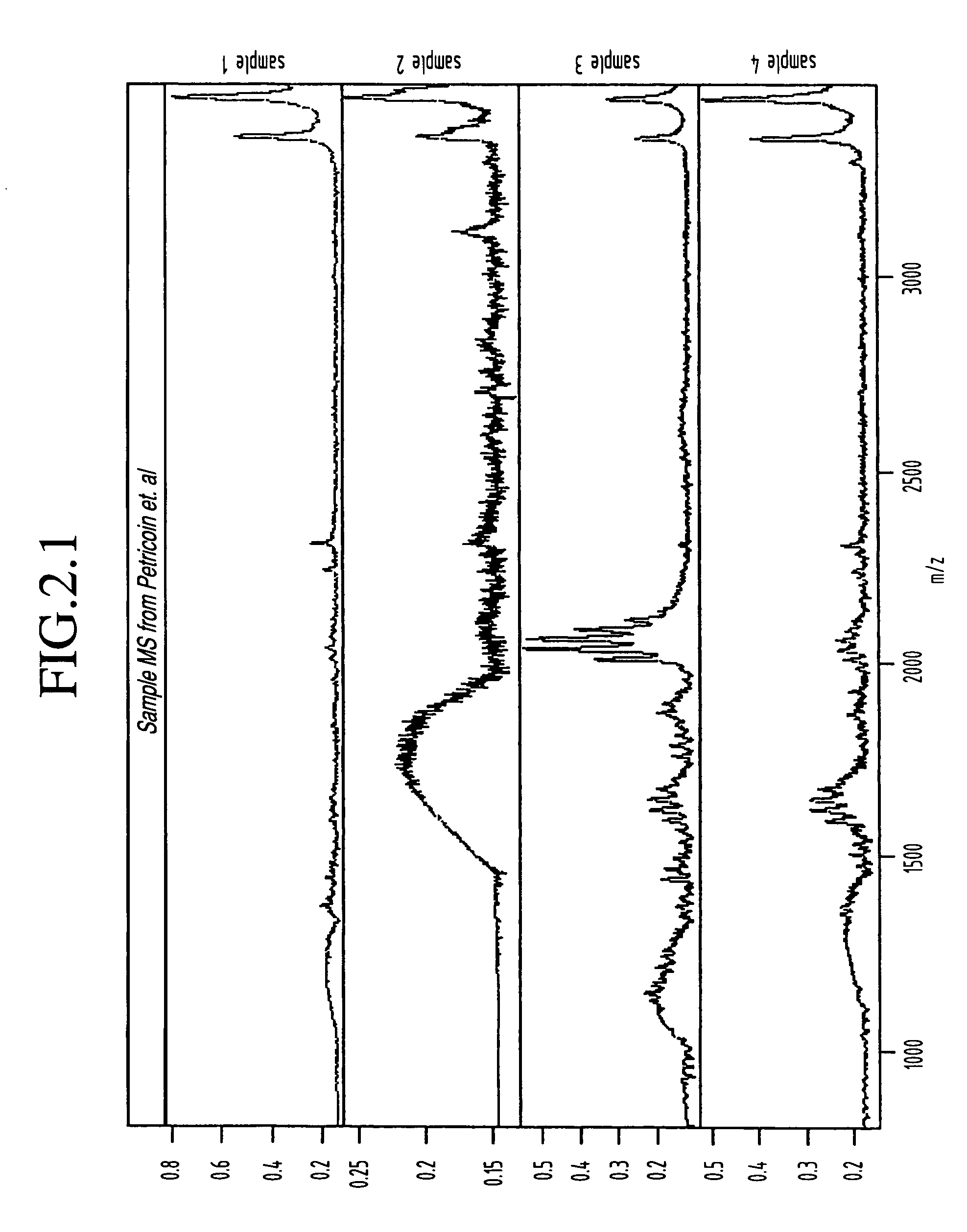
Classification of disease states using mass spectrometry data
InactiveUS20050048547A1Improve discriminationSamples introduction/extractionMicrobiological testing/measurementData setSpectroscopy
A method for identification of biological characteristics is achieved by collecting a data set relating to individuals having known biological characteristics and analyzing the data set to identify biomarkers potentially relating to selected biological state classes. A system for identification of biological characteristics is also provided. A methodology is also provided for utilizing mass spectroscopy data to identify peptide and protein biomarkers that can be used to optimally discriminate experimental from control samples—where the experimental samples may, for instance, be derived from patients with various diseases such as ovarian cancer.
Owner:ZHAO HONGYU +5
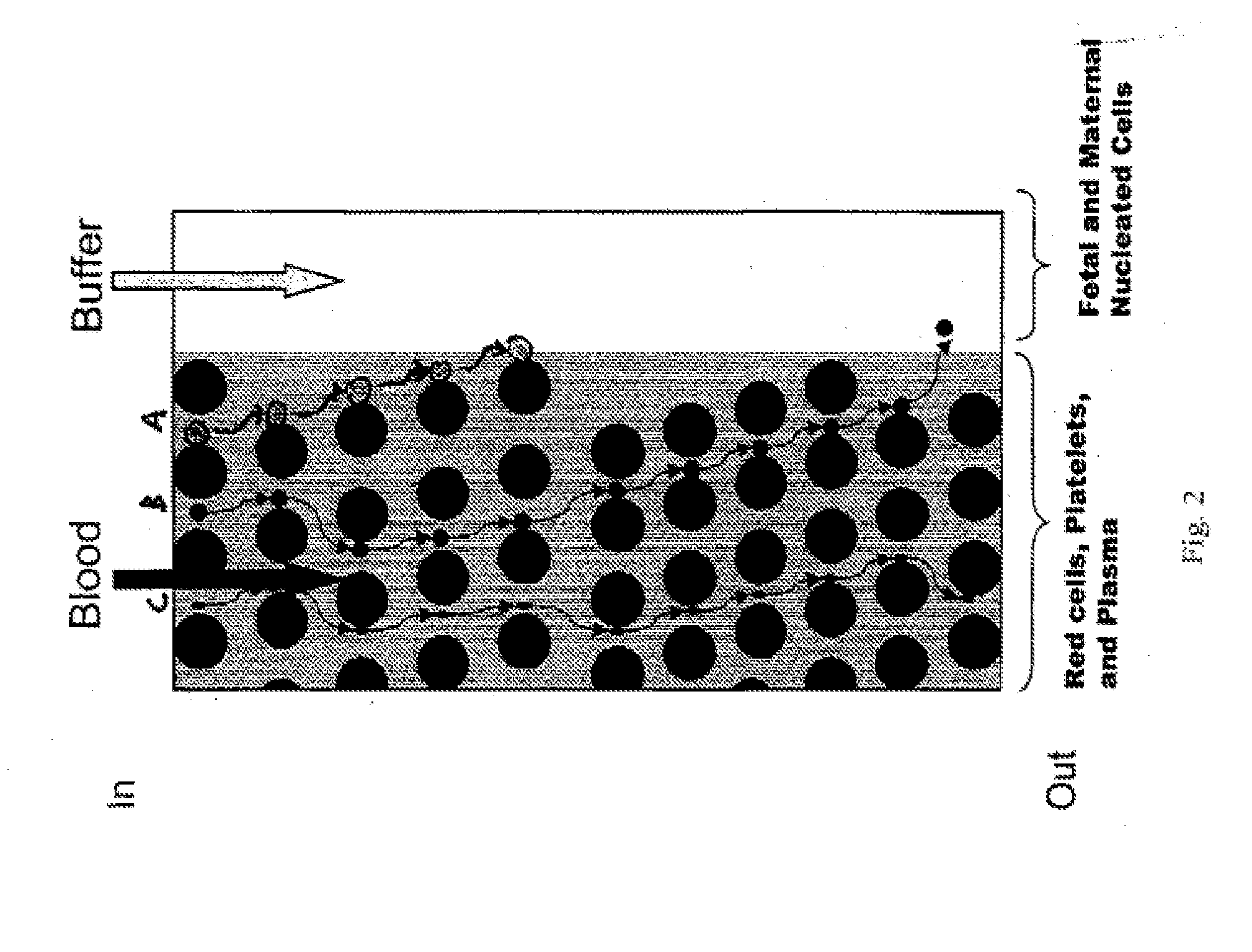
Cassette for isolation, amplification and identification of DNA or protein and method of use
InactiveUS20050176135A1Bioreactor/fermenter combinationsBiological substance pretreatmentsElectrophoresisDigestion chambers
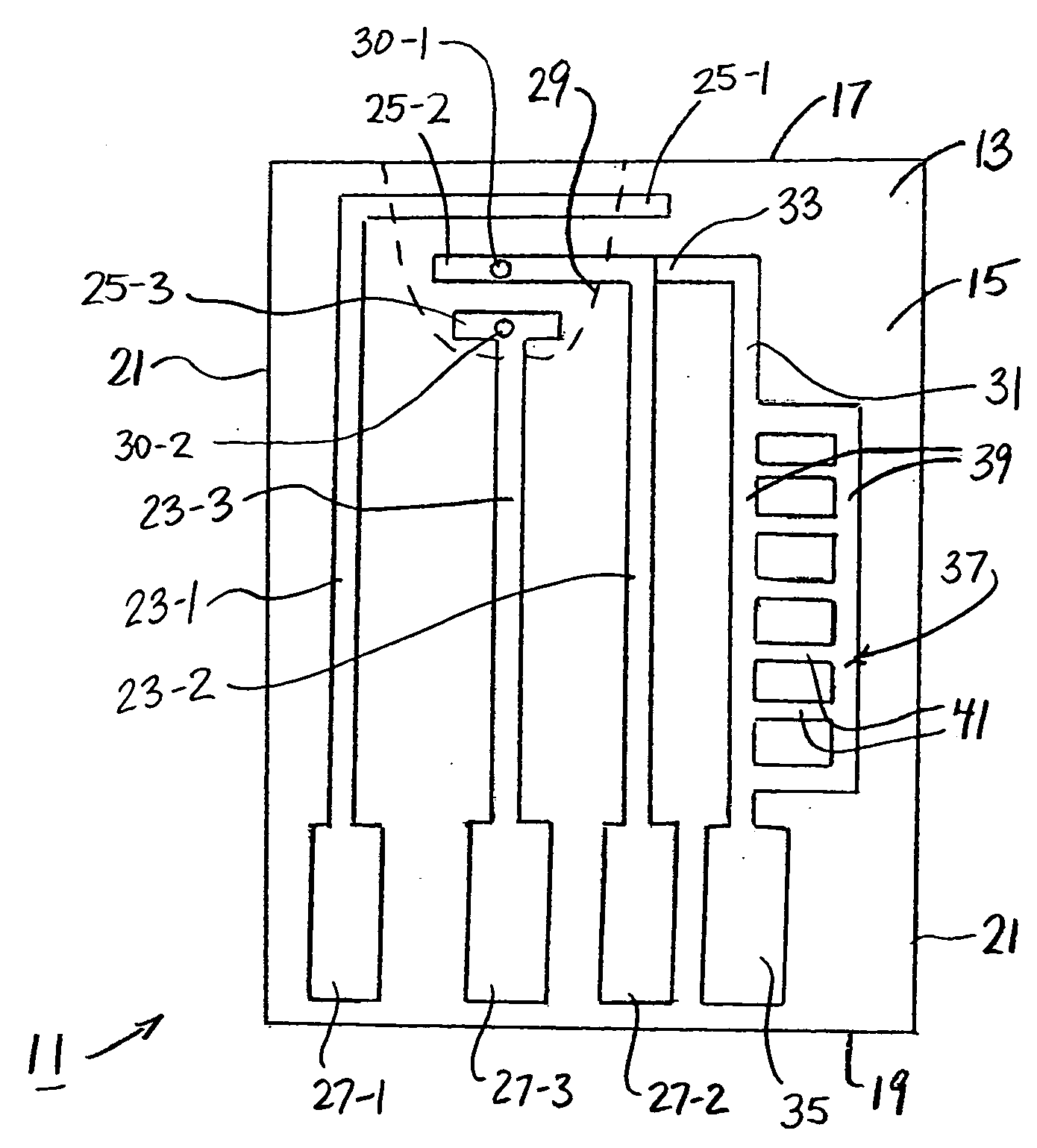
The present invention is directed to a device for DNA analysis. More particularly, the present invention is directed to a cassette which comprises a first chamber suitable for isolating DNA from a biological sample that is suspected of containing a target DNA, one or more second chambers suitable for amplifying any target DNA found in the sample, a chamber suitable for digesting the target DNA with restriction endonucleases, a medium for separation of the digestion fragments, and a channel connecting the digestion chamber to the separation medium and being of suitable size for transferring at least a portion of the contents of the digestion chamber to the separation medium. Typically, the medium for separation of the digestion fragments is an electrophoretic medium. The cassette of the present invention can also be used for the separation and identification of a protein of interest in a biological sample. It is within the scope of the present invention that the cassette also contains one or more internal waste chambers into which used reagents and biological sample can be directed and stored for disposal with the cassette. It is also within the scope of the present invention that the cassette contains a chamber for storage or receipt of a biological control sample.
Owner:JONES BRIAN
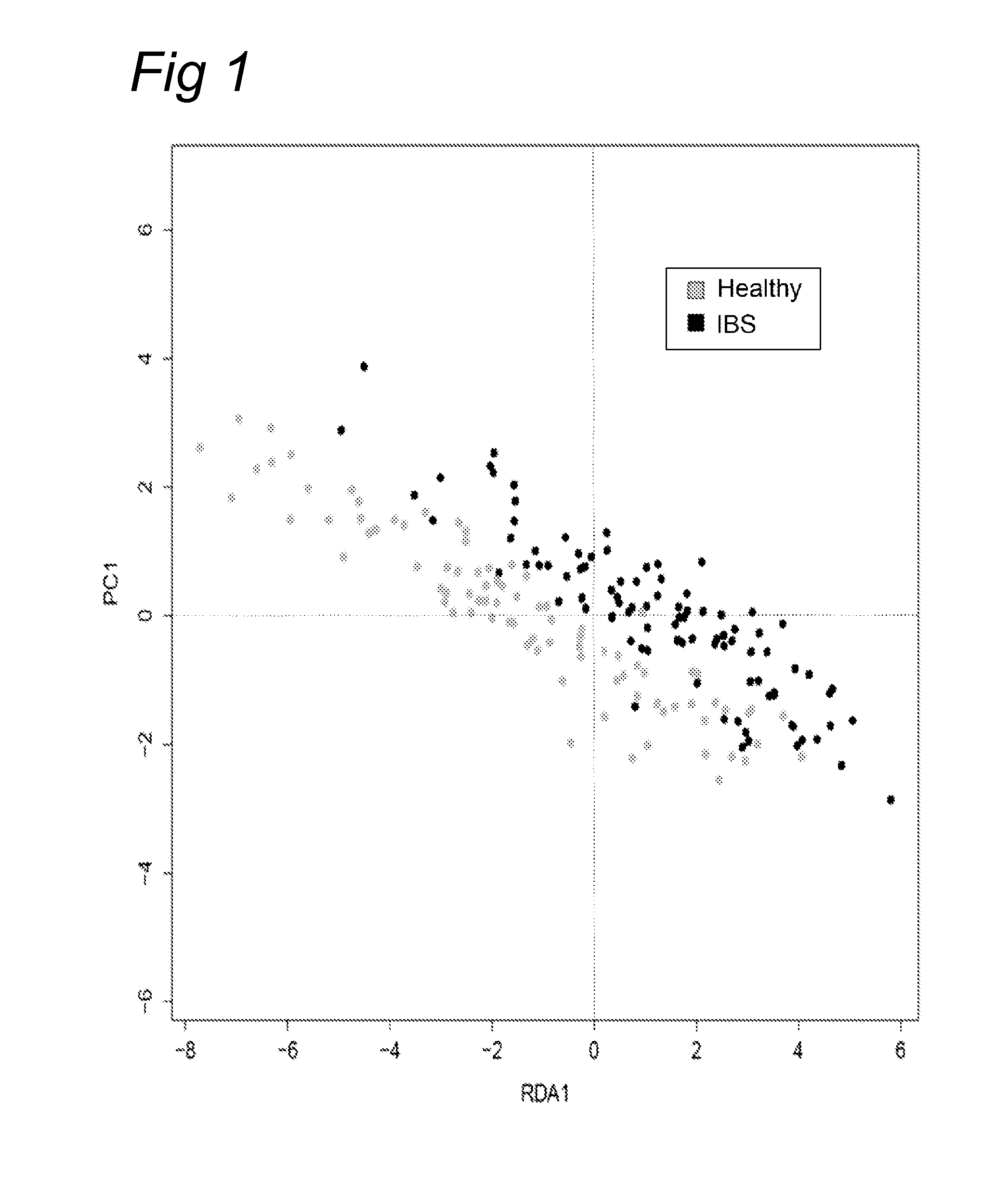
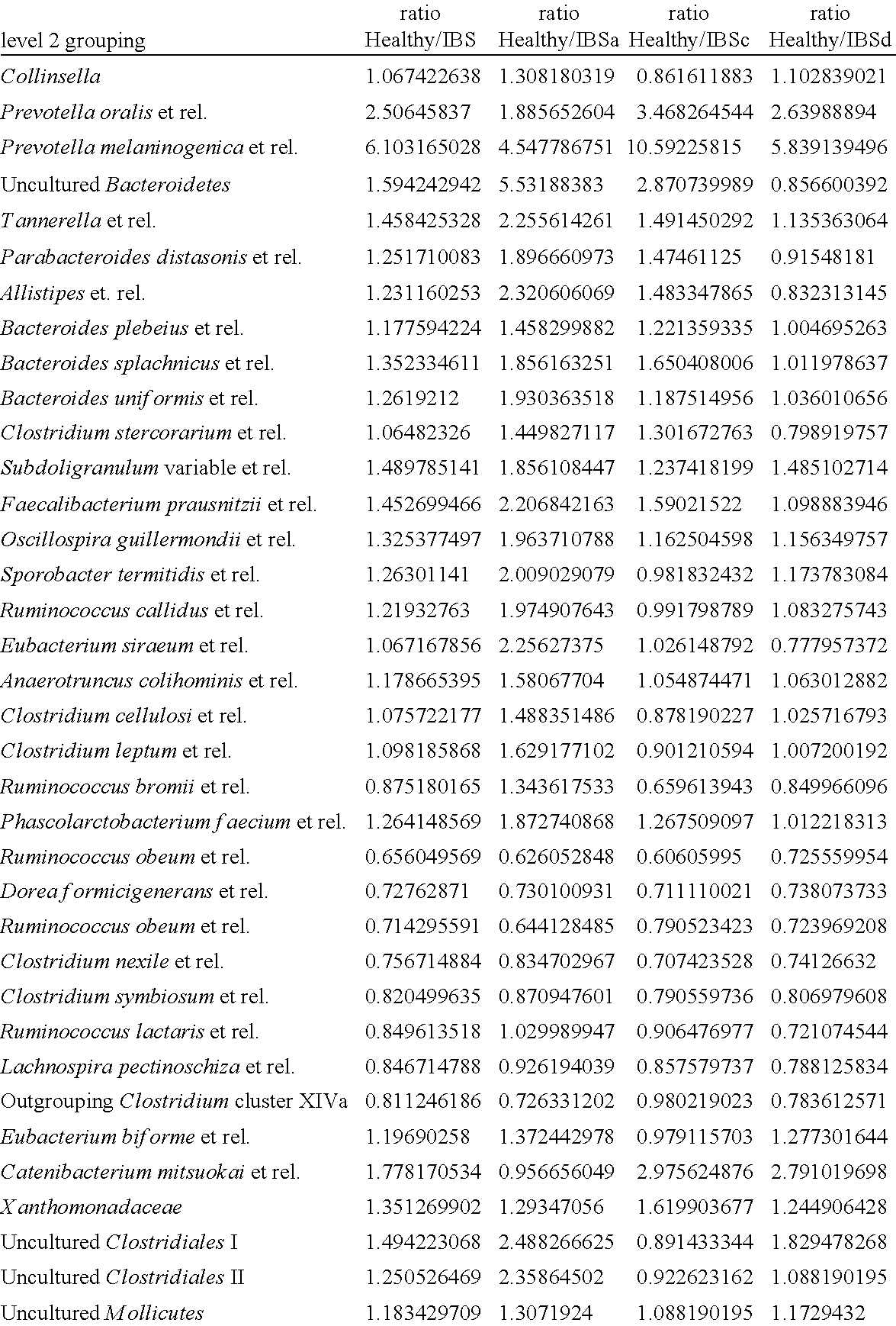
Methods for diagnosing irritable bowel syndrome
InactiveUS20120238468A1Improve the level ofNucleotide librariesMicrobiological testing/measurementTest sampleBacilli
The present invention discloses a method for diagnosing Irritable Bowel Syndrome (IBS) in a test sample by determining the level of several bacterial taxa in the test sample, comparing this level with the levels of those bacterial taxa in a control sample, and relating the level to a diagnosis of IBS. Additionally, the present invention provides a method for treatment of IBS based on said diagnosis. Also, the invention provides a method for subtyping IBS in a test sample.
Owner:AAK PATENT
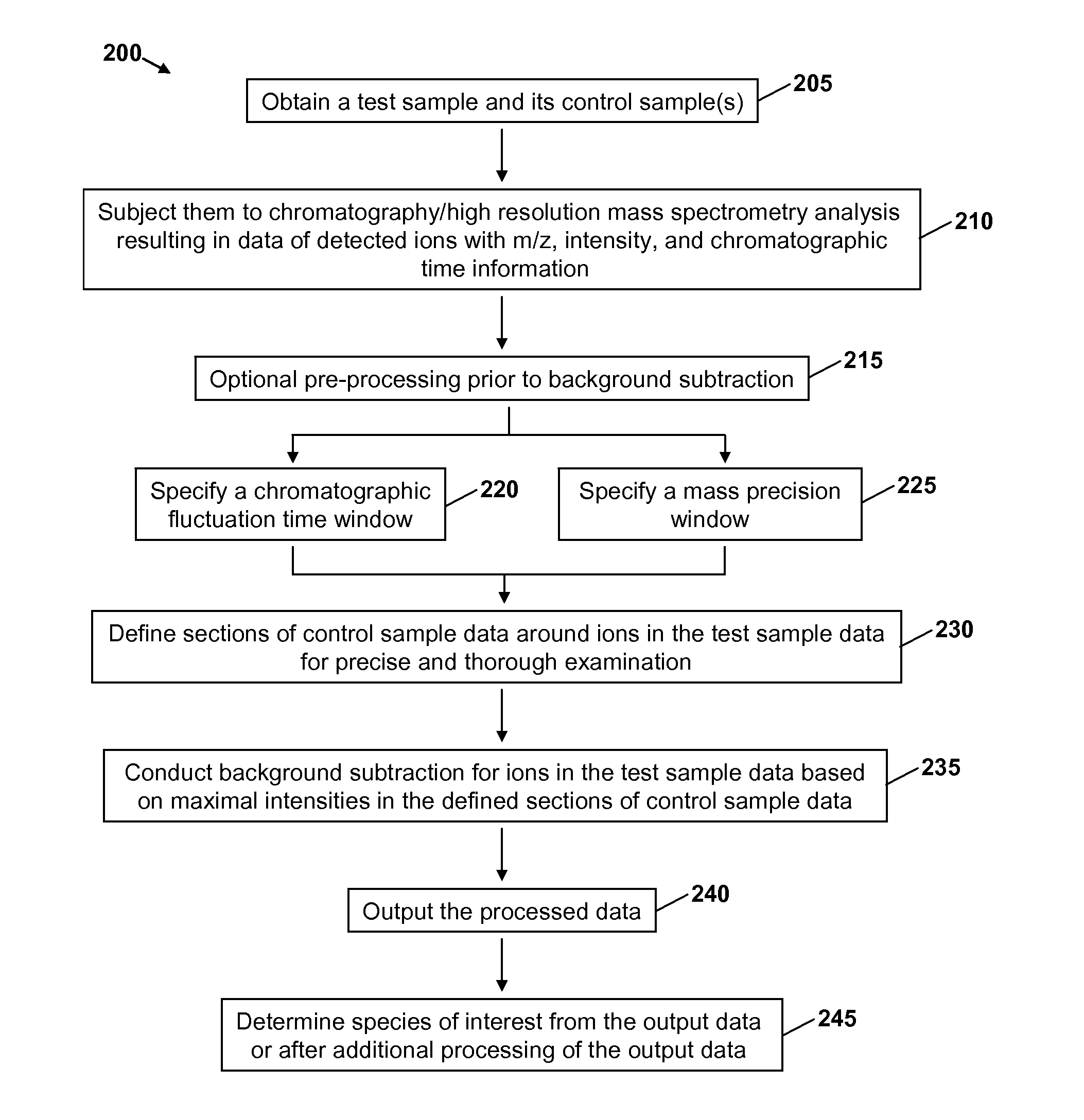
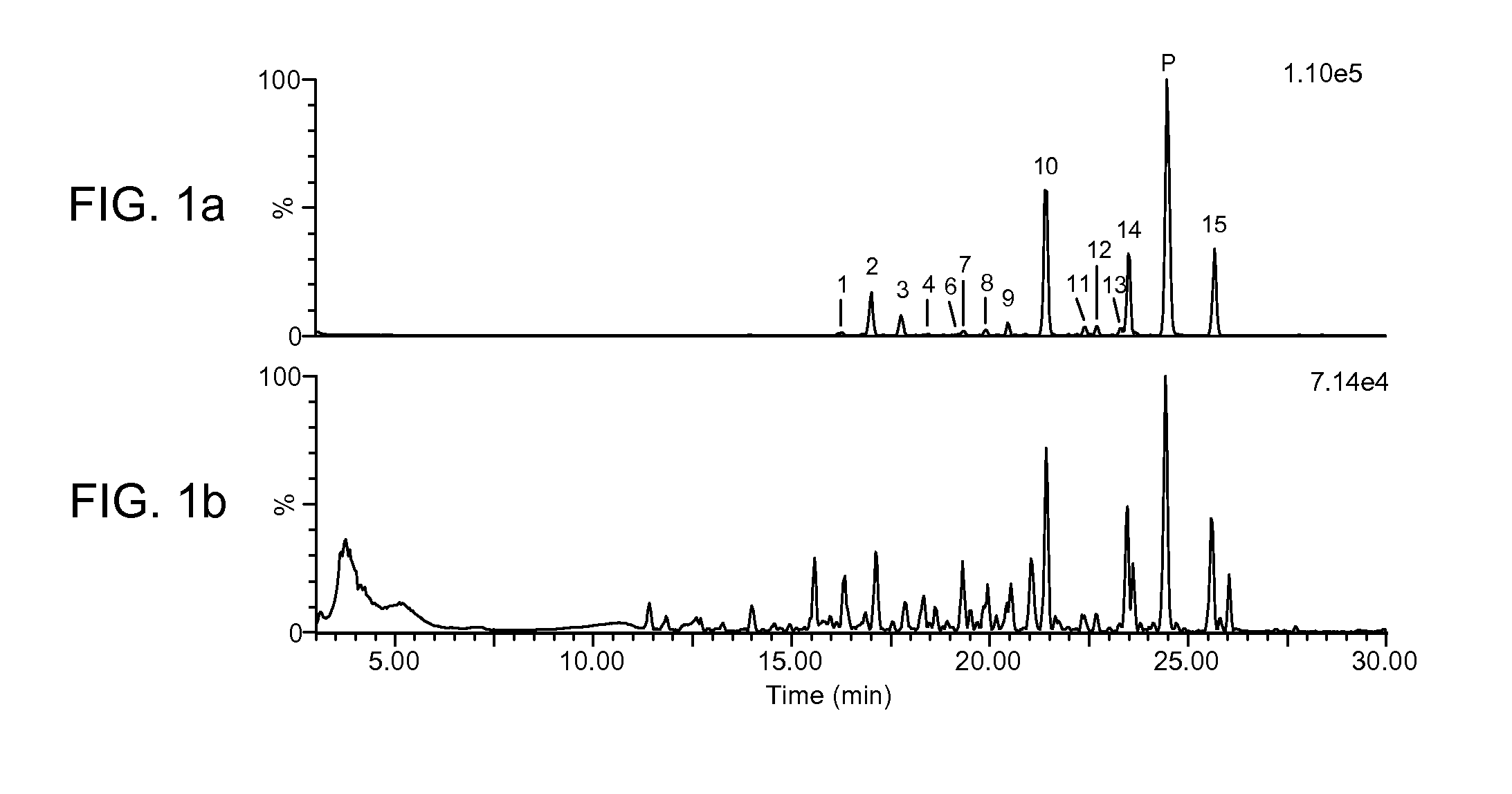
Precise and thorough background subtraction
ActiveUS20100213368A1Avoid enteringIsotope separationMass spectrometersTest sampleMass Spectrometry-Mass Spectrometry
A method for identifying and characterizing components of interest in complex samples includes subjecting both a sample and its control samples to chromatography / high resolution mass spectrometry analysis to detect ions of the samples. The method includes defining sections of control sample data within specified chromatographic fluctuation time and mass precision windows around each ion or each group of the same ions of question in the test sample data. The defined sections of the control sample data are examined and the maximal intensities are subtracted from respective ions in the test sample. Components of interest are determined from the resultant data of the test sample. The method can be used for identifying molecular ions and / or their fragment ions for components of interest in complex samples.
Owner:MASSDEFECT TECH
New rice and old rice quality detection device and detection method based on visual light, near-infrared and terahertz integrated spectrum technology
InactiveCN106124435ANo pollution in the processExpand the effective spectral band rangeColor/spectral properties measurementsInfraredTime domain
The invention discloses a new rice and old rice quality detection device and detection method based on a visual light, near-infrared and terahertz integrated spectrum technology. The detection device is composed of a halogen light source, a visual light-near-infrared hyperspectrum sensor, a transmission-type terahertz time-domain spectrum system, a rotation-type sample carrying device, a groove-type sample table, an electronic control detection rocker arm, electronic control sample table rocker arms, a computer, a data acquisition card, a control module, a mode switching button, a shading hood, an instrument rack and the like. When detection is conducted, visual light-near-infrared hyperspectra and terahertz spectra of a sample are collected through the mode switching button. An optimal characteristic wave band combination of the related visual light, near-infrared and terahertz integrated spectra which are stored in experiments is extracted and substituted into a related quantitative detection model, and qualitative results of the related new rice and old rice quality of the rice sample and related protein and amino acid are obtained and output. Accordingly, limitation generated when the new rice and old rice quality is detected through a single spectrum technique is broken through, and the detection device and detection method can be used for real-time storage quality detection on warehouse rice.
Owner:JIANGSU UNIV
Electrochemical biosensor by screen printing and method of fabricating same
ActiveUS20050183953A1Rapid sample inflowSmall volumeImmobilised enzymesBioreactor/fermenter combinationsReaction layerSiphon
An electrochemical biosensor formed by screen printing and method of fabricating such biosensor is disclosed in the present invention. The biosensor can quickly absorb a sample to be measured therein, effectively control volume of the sample fed and “fill-and-position” the sample therein. The biosensor includes an electrode layer (electrode area) comprising two or three electrodes, which are a working electrode, a reference electrode and an auxiliary electrode (tri-electrode) on an insulating substrate. An active reaction layer containing reactant, reaction catalyst, mediator, wetting agent and surfactant is spread on the surface of the electrode layer. A sample inflow area is formed above the electrode area by adding an upper cover on top of a middle insulating layer with a U-shaped opening formed therein. Sample solution with a minute amount about 0.8 to 1 μl can be rapidly introduced into the electrode area and the active reaction layer via the inflow area by siphon or capillary, where the ingredient of the sample can be analysed by measuring reaction between the sample, reaction catalyst and mediator in the reaction layer using electrochemical potentiometric or amperometric method. An upwardly extended closed space formed within the upper cover above the electrode area adjacent to the front of conductive wires can be effectively used to control sample volume and “fill-and-position” the sample.
Owner:GENERAL LIFE BIOTECHNOLGOY
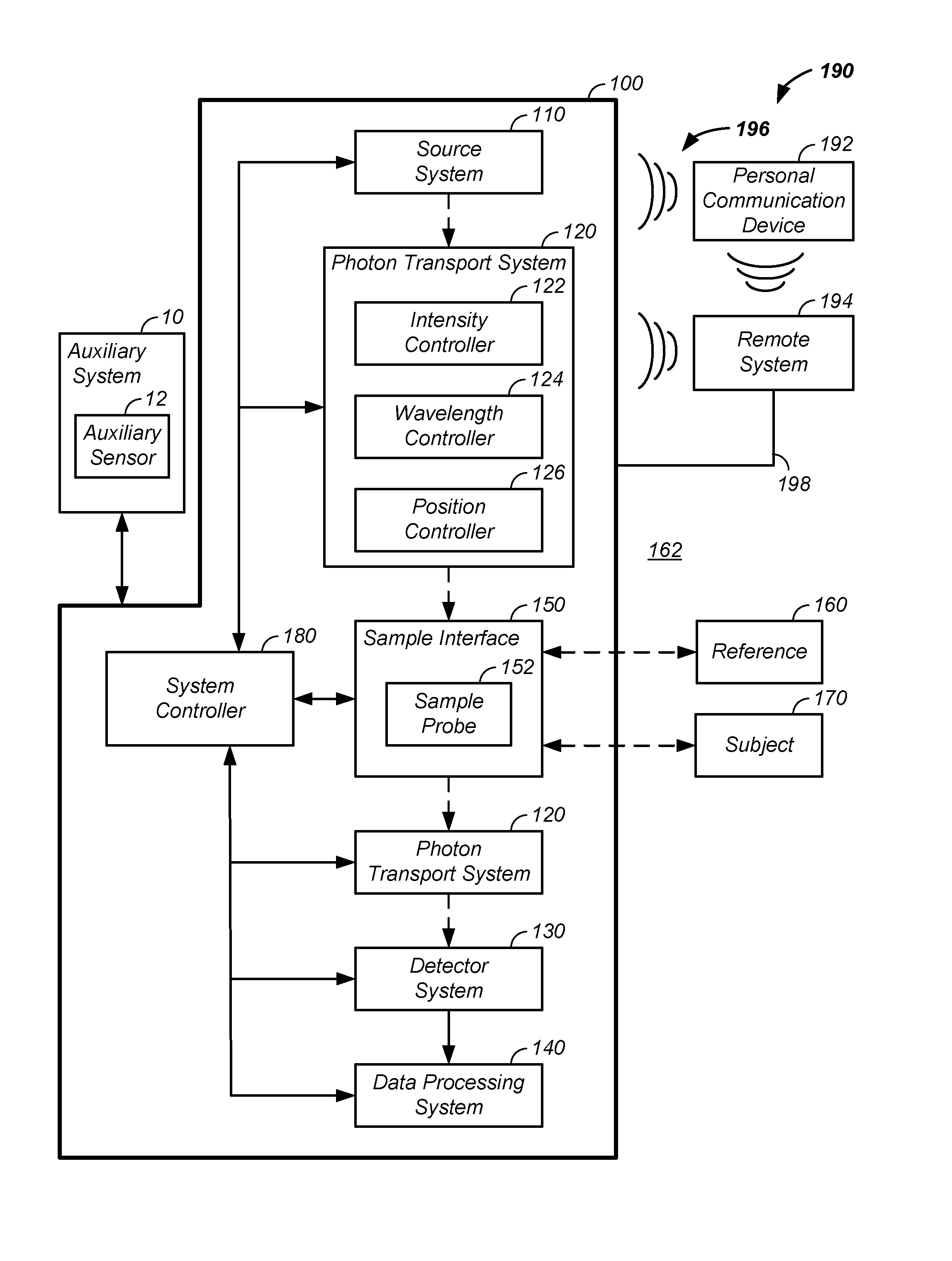
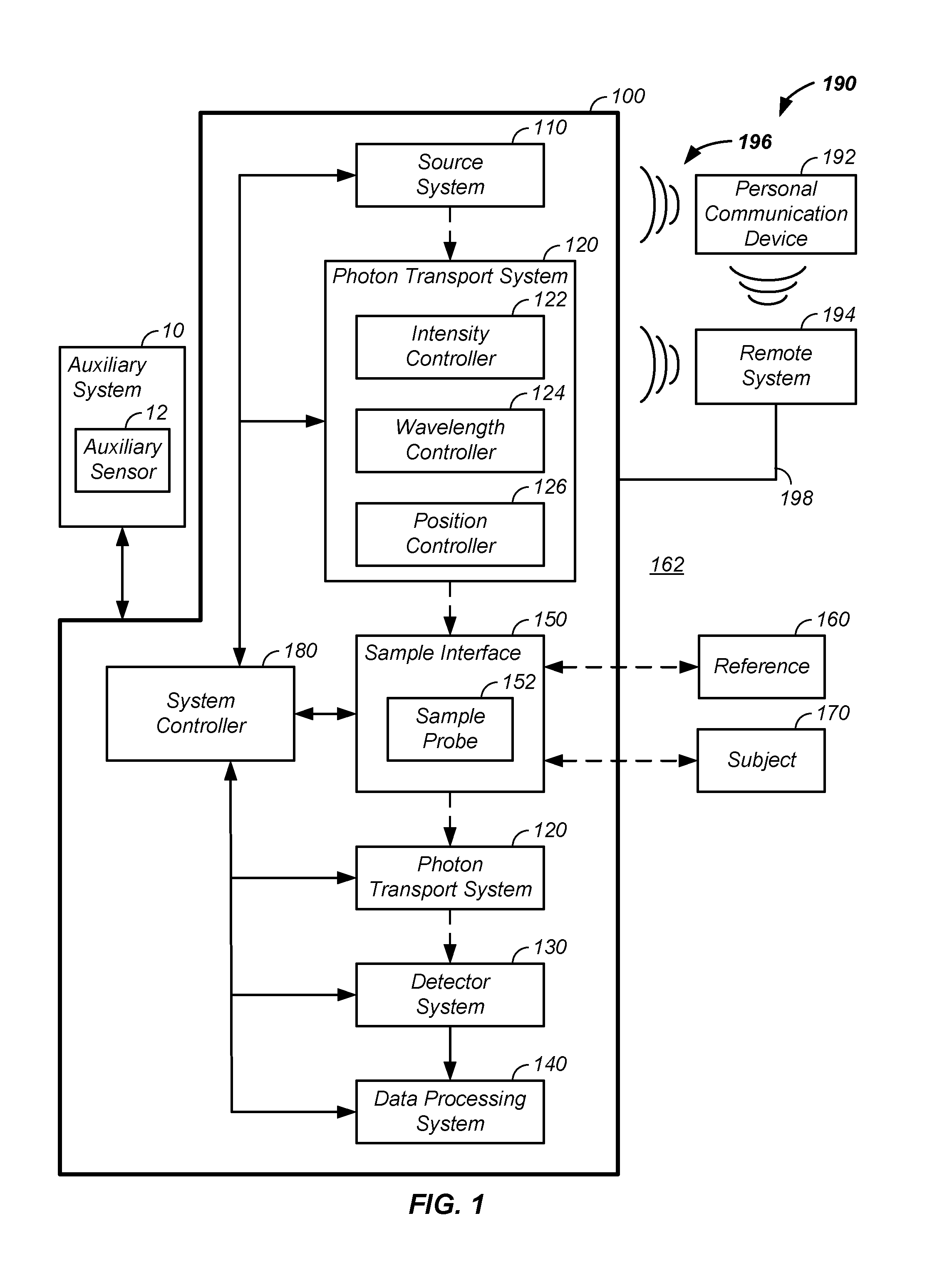
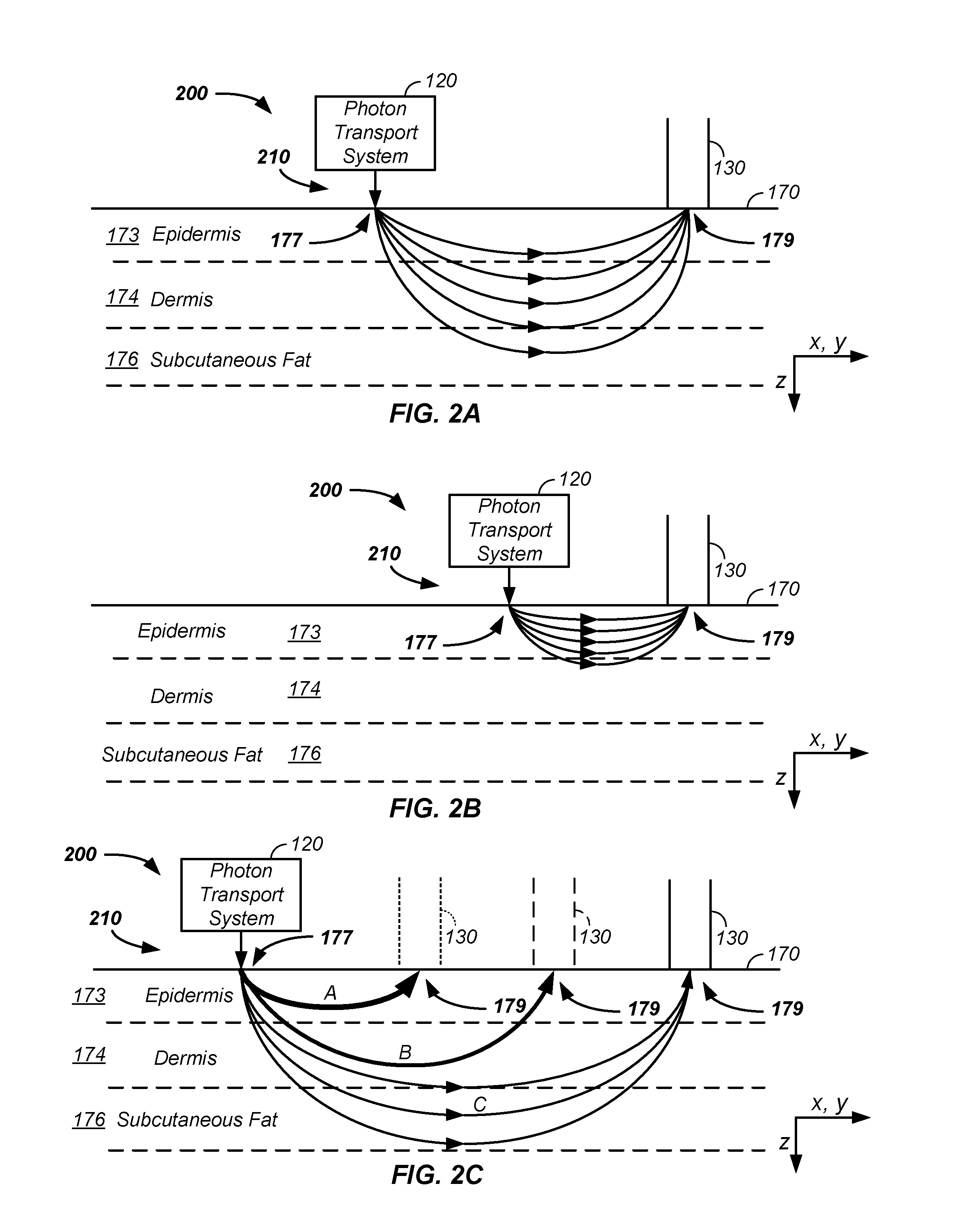
Sample optical pathlength control using a noninvasive analyzer apparatus and method of use thereof
InactiveUS20160249836A1Improve accuracyImprove propertiesMaterial analysis by optical meansAbsorption/flicker/reflection spectroscopyAnalyteDetector array
A noninvasive analyzer apparatus and method of use thereof is described for spatially separating light for use in noninvasively determining an analyte concentration of a subject through use of detectors linked to multiple controlled sample illumination zone to sample detection zone distances. The controlled radial separation of illumination and detection zones yields reduced deviation in total observed optical pathlength and / or control of pathlengths in a desired tissue volume for each element of a set of detector elements. Performance using the discrete detection zones is enhanced using a combination of segmented spacers, arcs of detector elements, use of micro-optics, use of optical filters associated with individual detector elements, control of detector response shapes, and / or outlier analysis achievable through use of multiple separate and related observed signals of a detector array.
Owner:ZYOMED
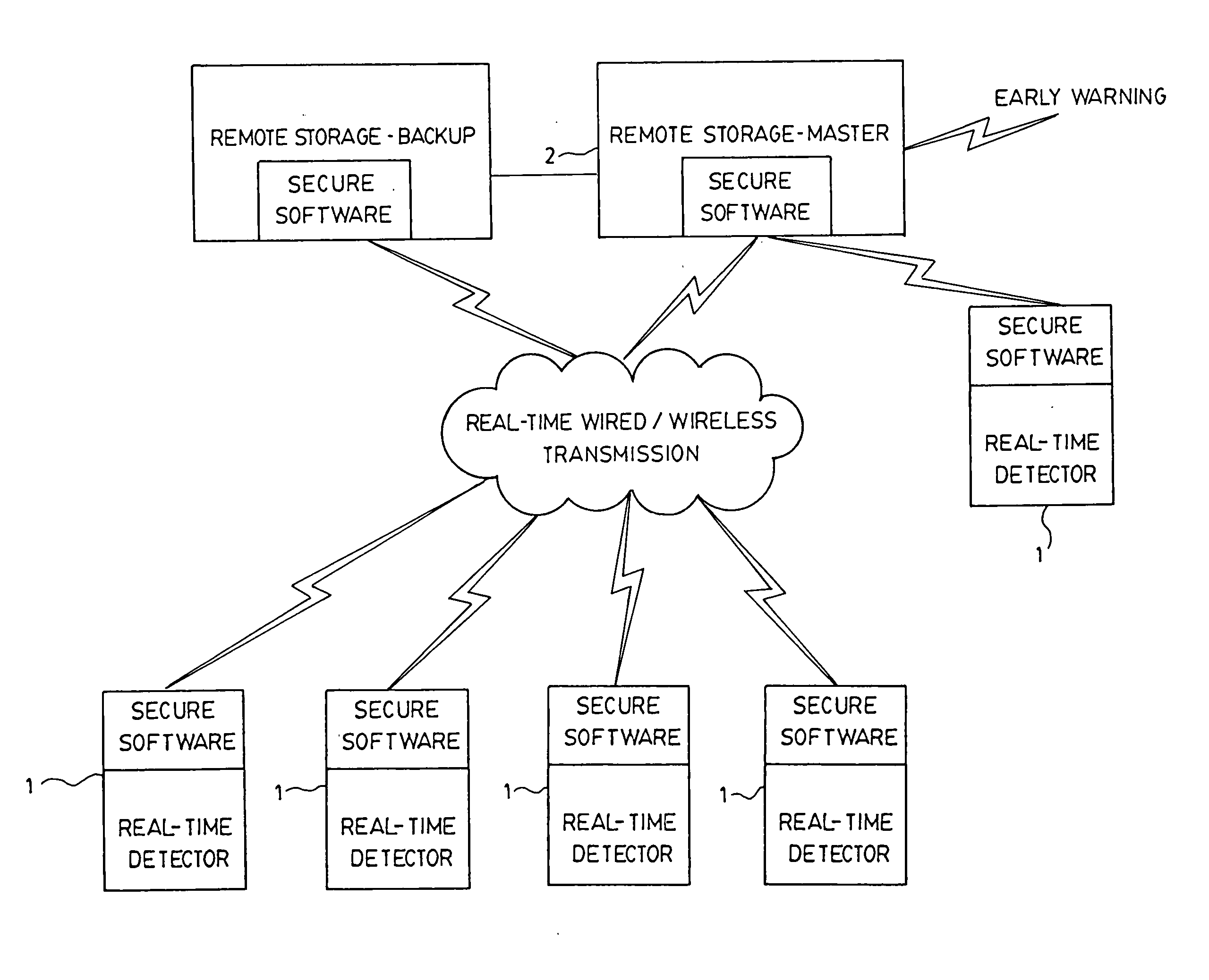
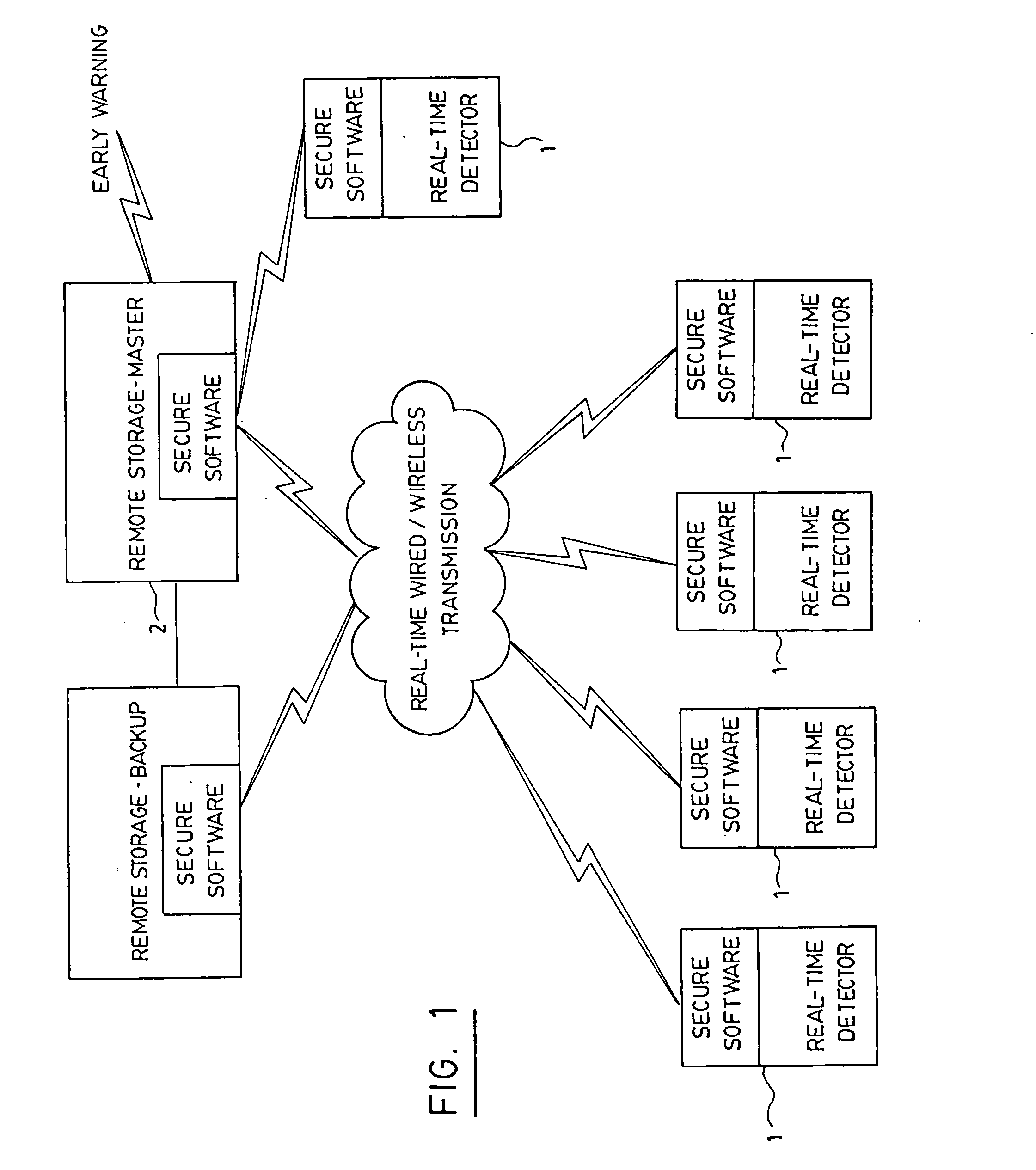
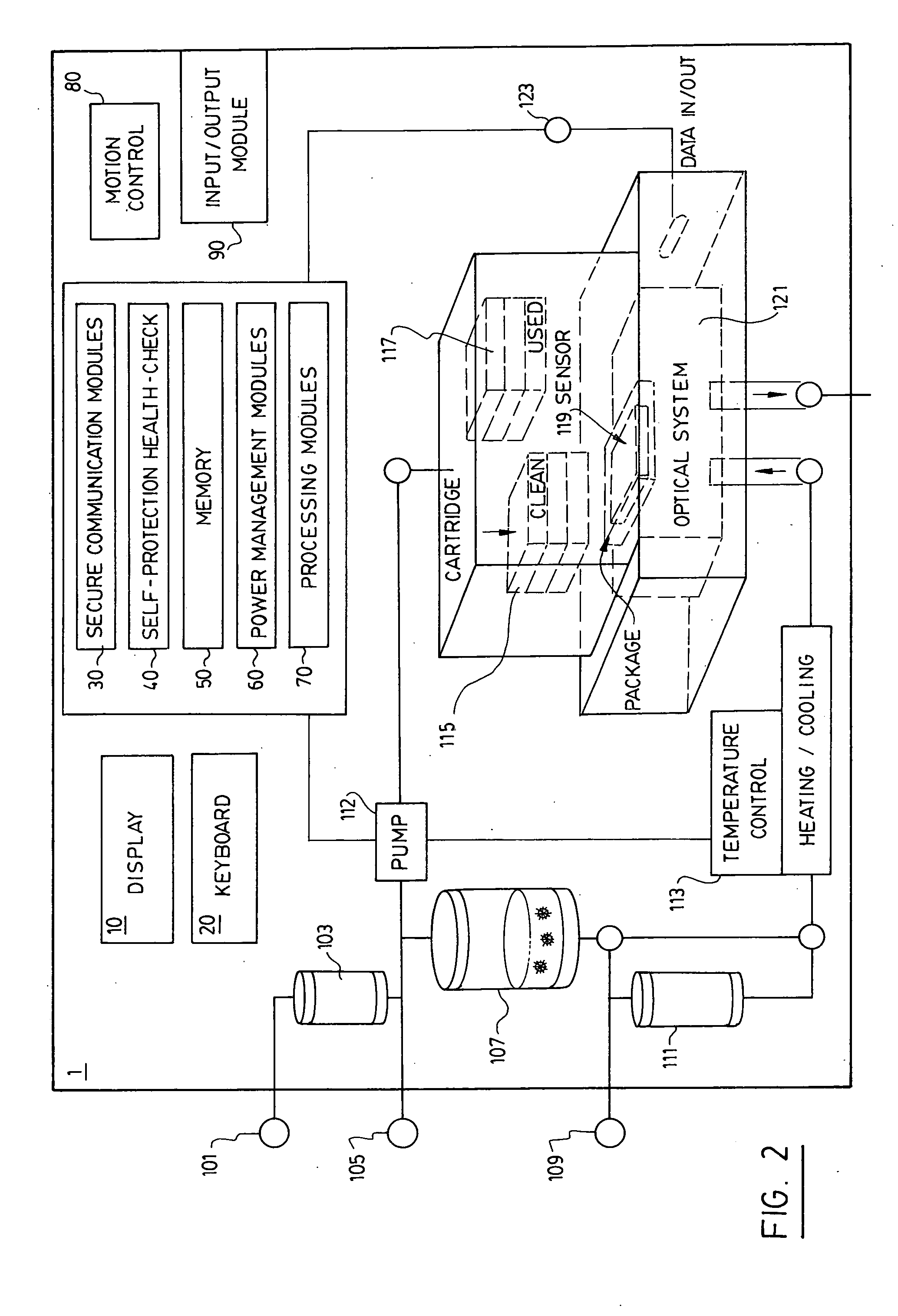
System and method for real-time detection and remote monitoring of pathogens
InactiveUS20050118704A1Improve accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsSecure communicationOptical Module
A real-time continuous detector device for detection of contaminants in a sample includes at least one sample management module, including a mechanism to control sample flow management to place the sample on a sample area. At least one electronic module for data processing and control is provided, and there is at least one optical module consisting of at least one real-time replaceable sensor cartridge containing a plurality of sensors, and at least one real-time optical pathogen detector connected to the electronic module for data processing. Power is provided and the device is equipped with at least one secure communication module adapted to transmit encrypted information over a secure link to a remote location and for receiving information. The invention also discloses a system making use of detectors for real-time detection of contaminants and for early warning capability, among others.
Owner:TELAURA
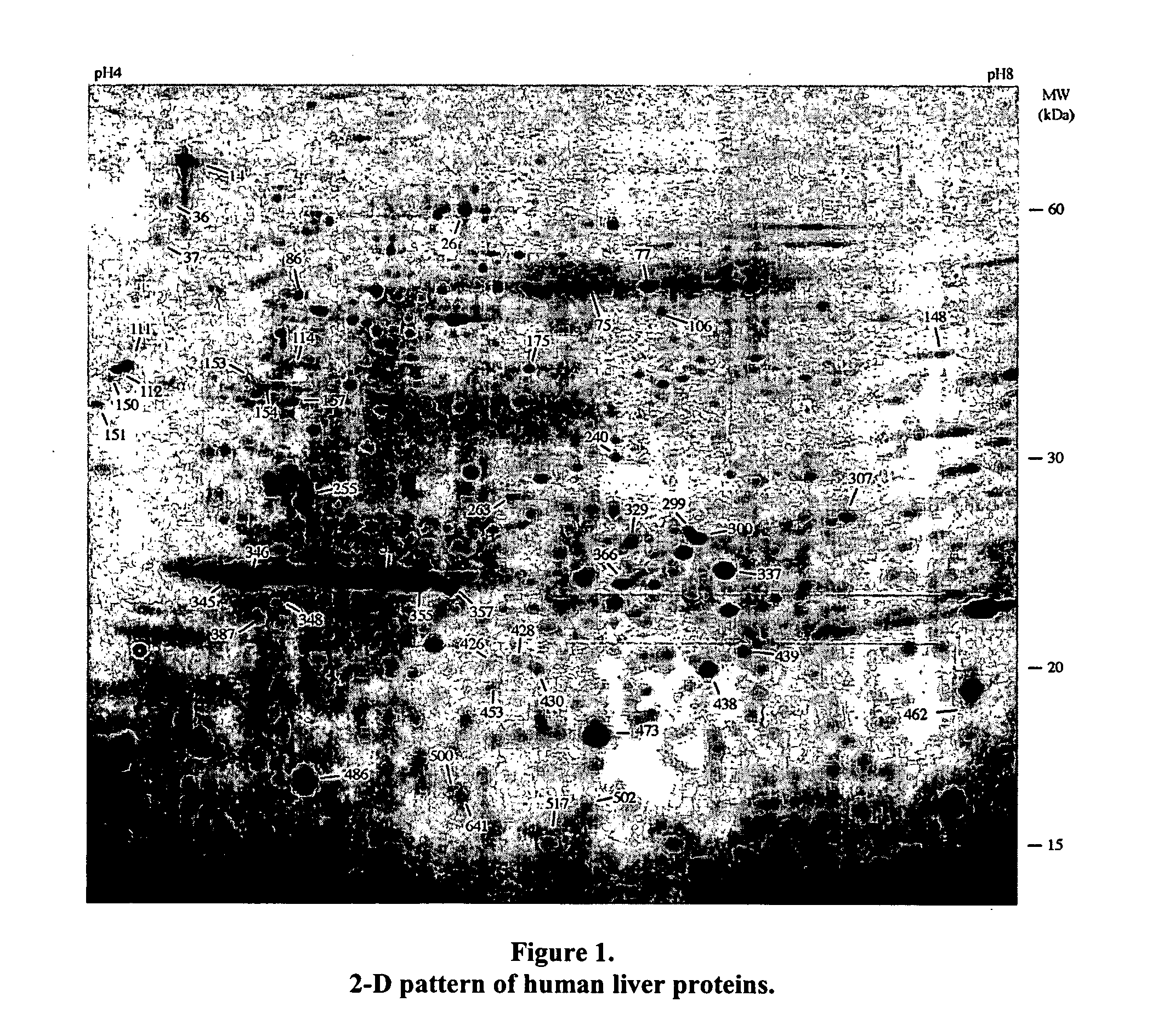
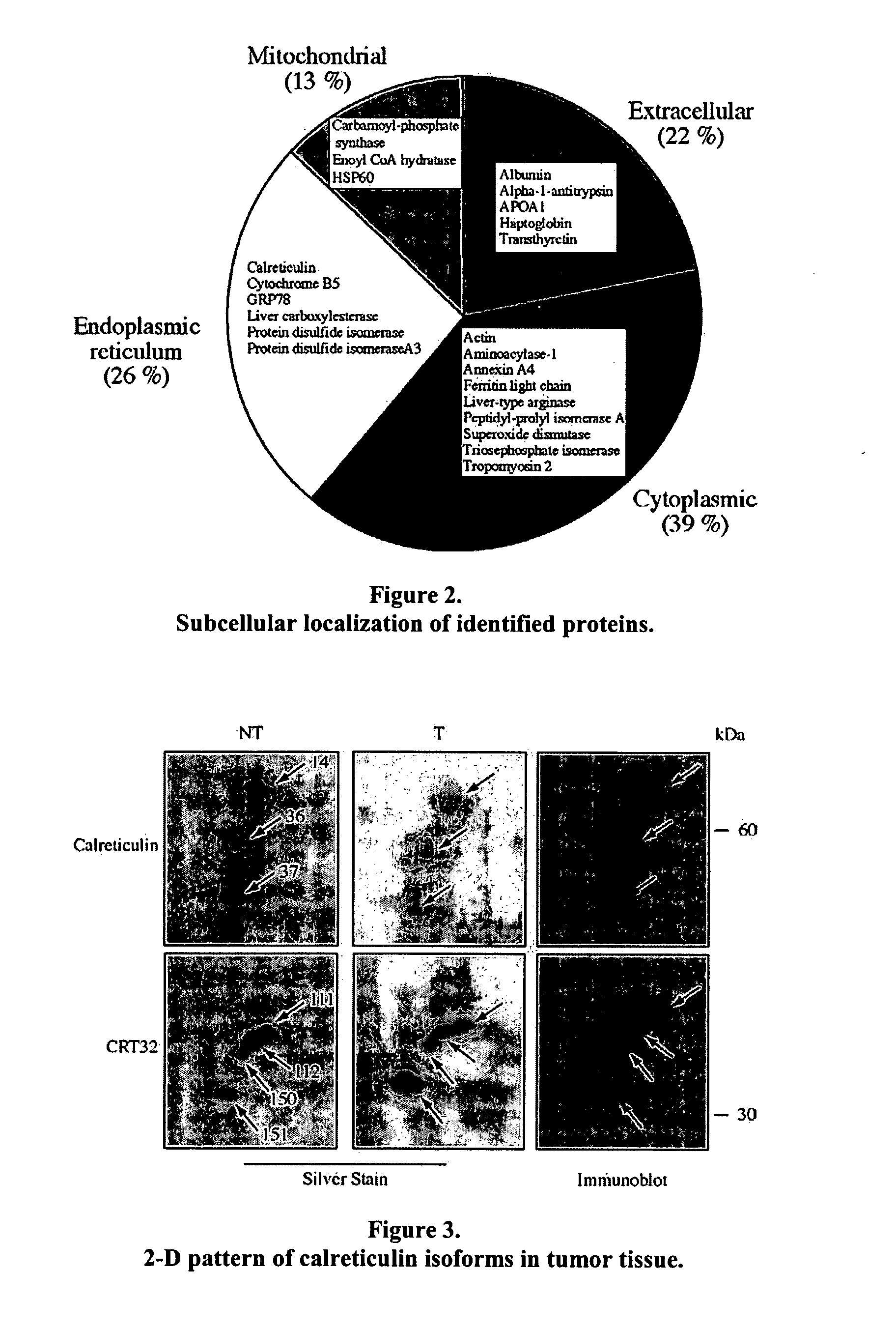
Liver cancer biomarkers
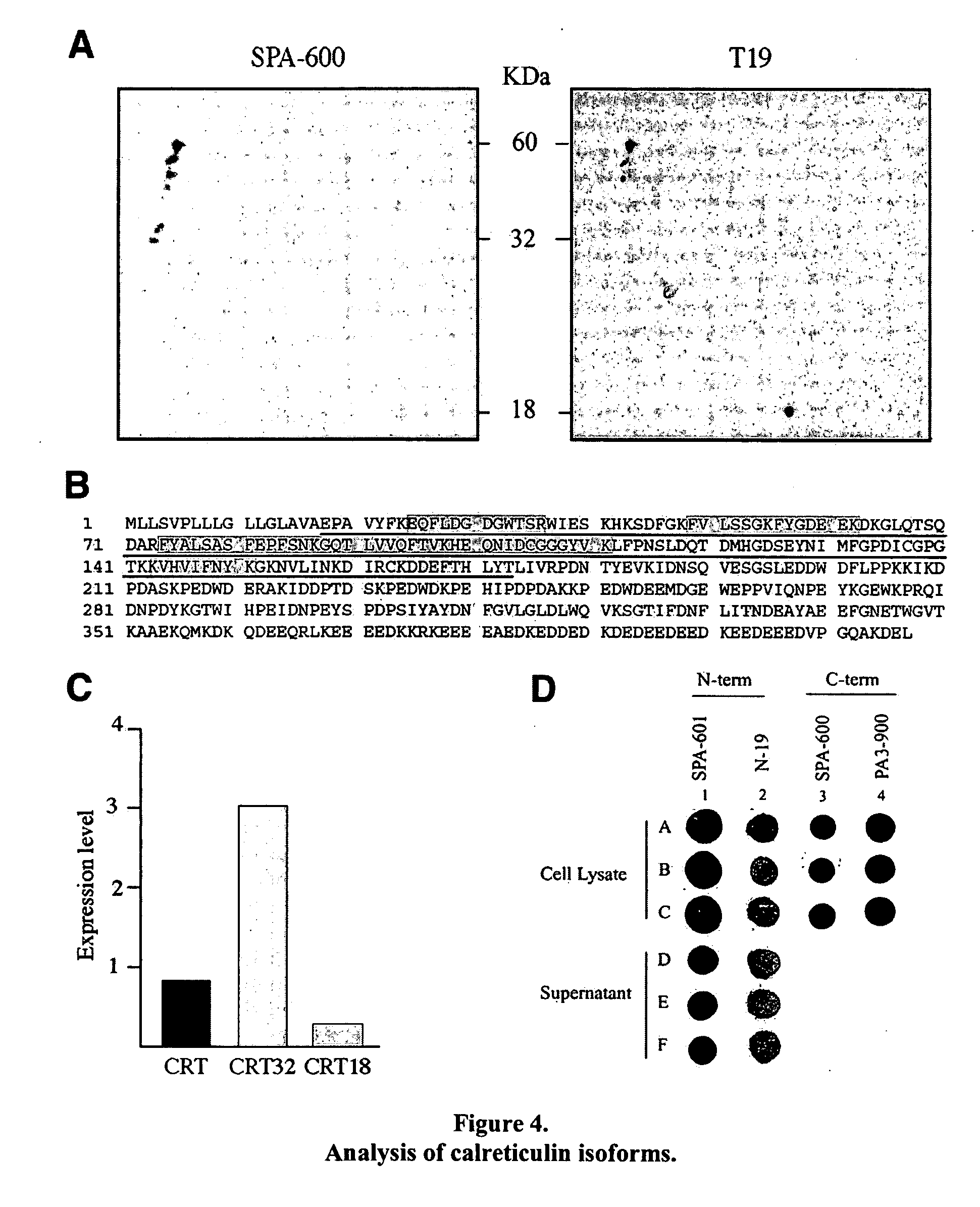
InactiveUS20070202496A1Microbiological testing/measurementBiological testingCalreticulinIncreased risk
A method of detecting whether a subject is afflicted with or at increased risk of developing liver cancer is carried out by detecting cleavage of a marker in a sample such as a blood sample from the subject. Suitable markers include but are not limited to, calreticulin, calreticulin precursor, protein disulfide isomerase family A member 3 (PDIA3), and cleavage products thereof. The cleavage, or extent of cleavage, can be as compared to that found in a control sample.
Owner:FRED HUTCHINSON CANCER RES CENT
Mass spectrometer
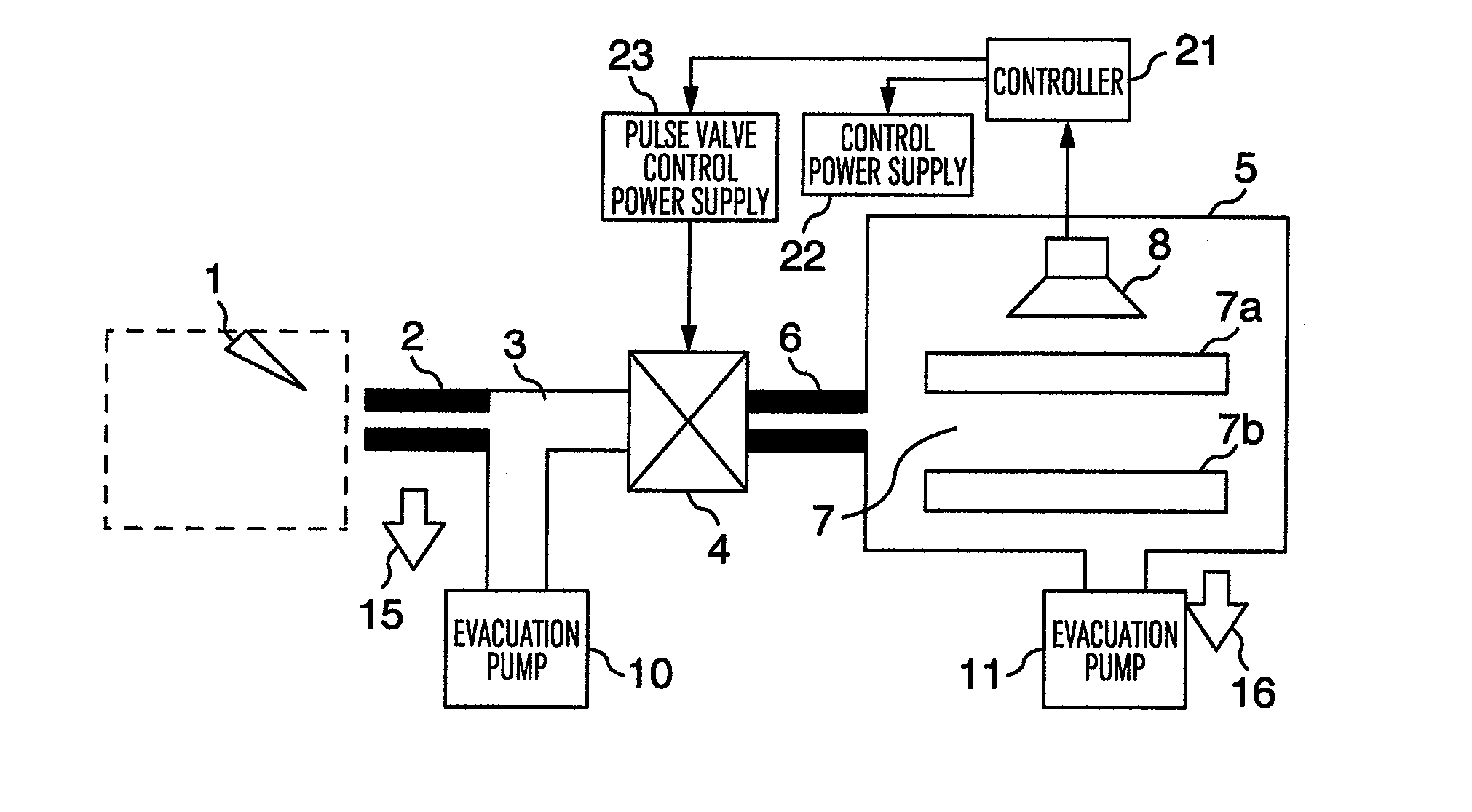
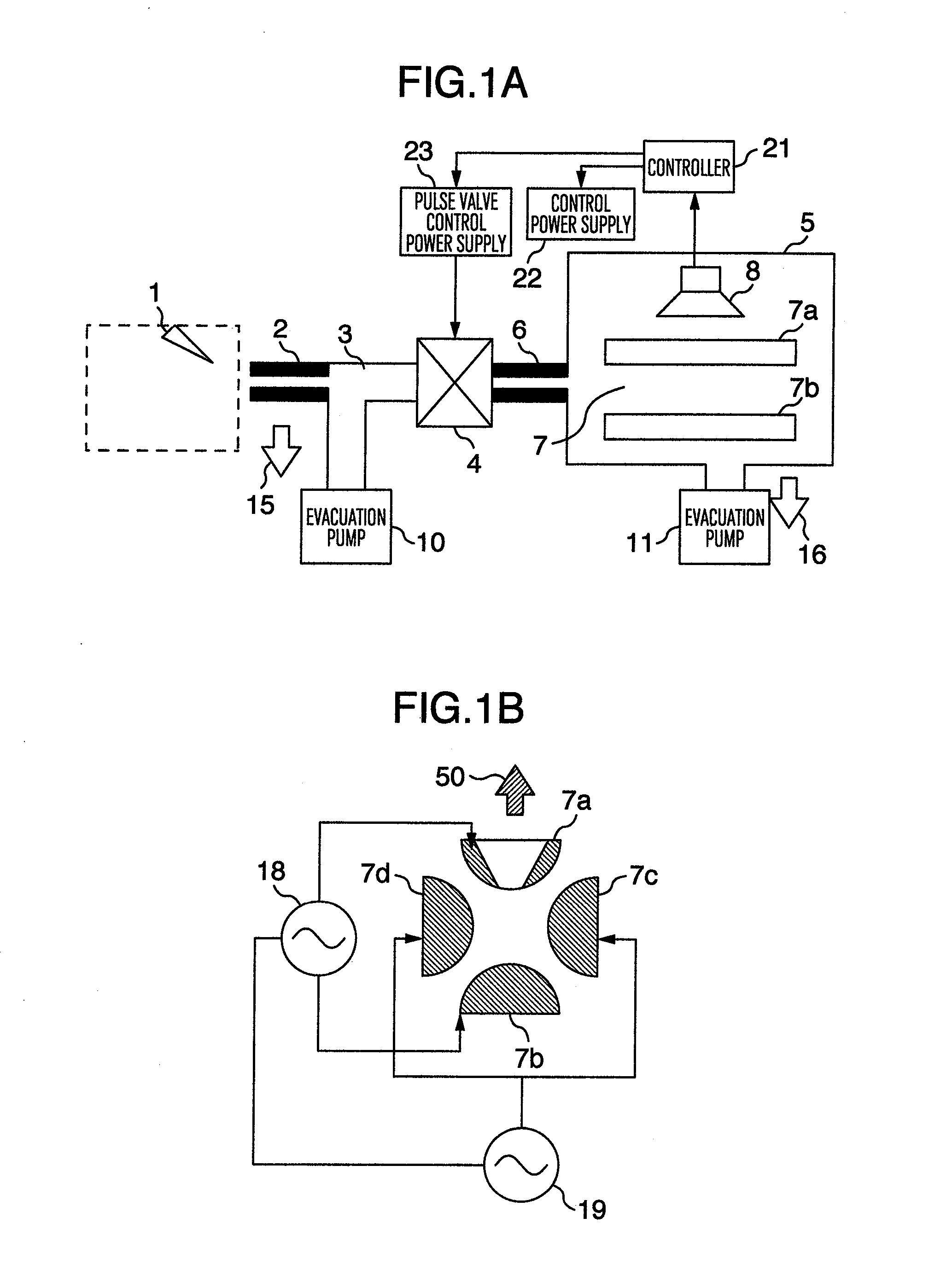
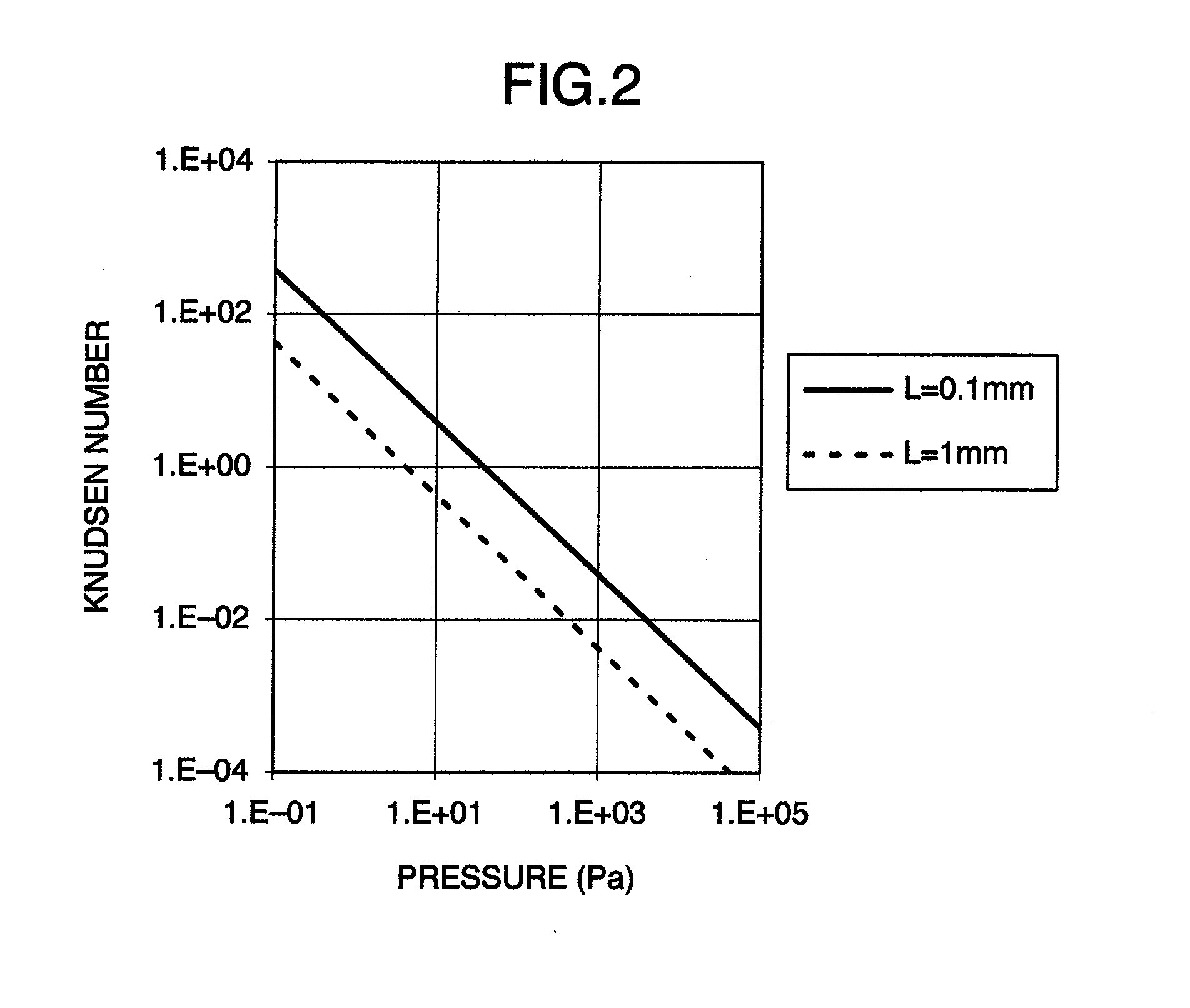
InactiveUS20130056633A1Increase the amount of gasSmall pumping speedSamples introduction/extractionMiniaturised spectrometersMass Spectrometry-Mass SpectrometryHigh pressure
Owner:HASHIMOTO YUICHIRO +3
Charged Particle Beam Apparatus
InactiveUS20080203299A1Easy to operateImprove precisionMaterial analysis using wave/particle radiationElectric discharge tubesOperabilityCondensed matter physics
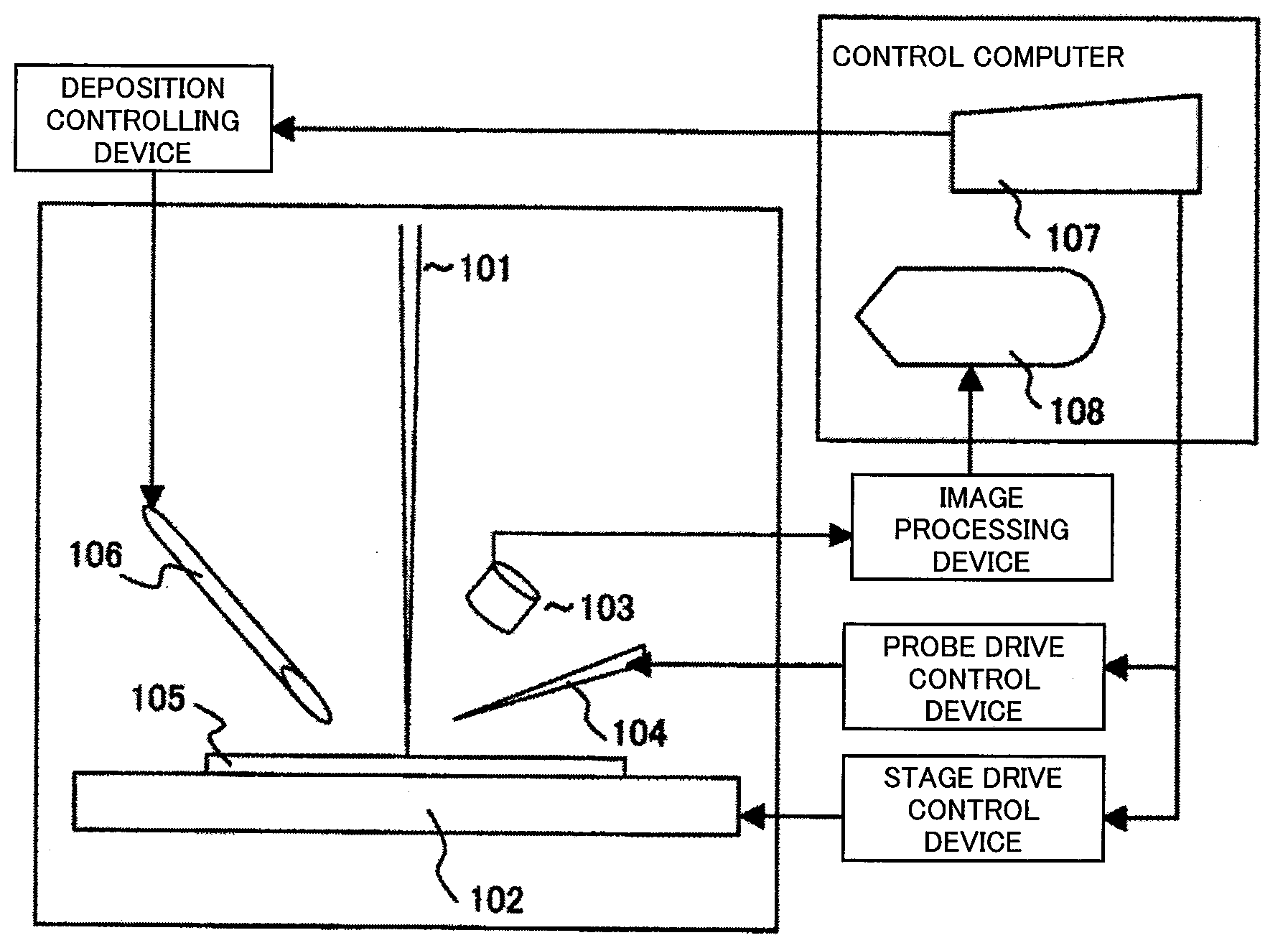
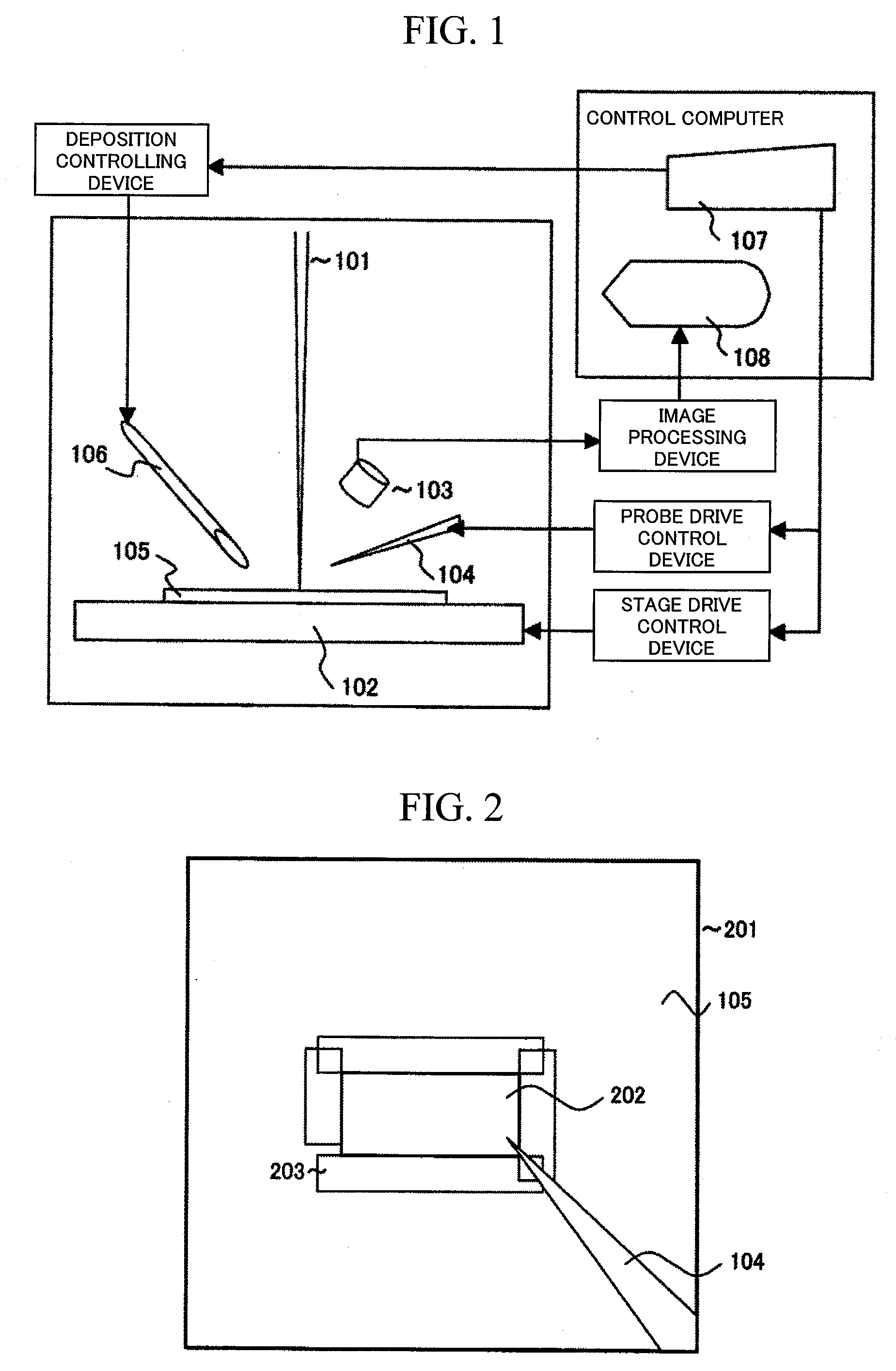
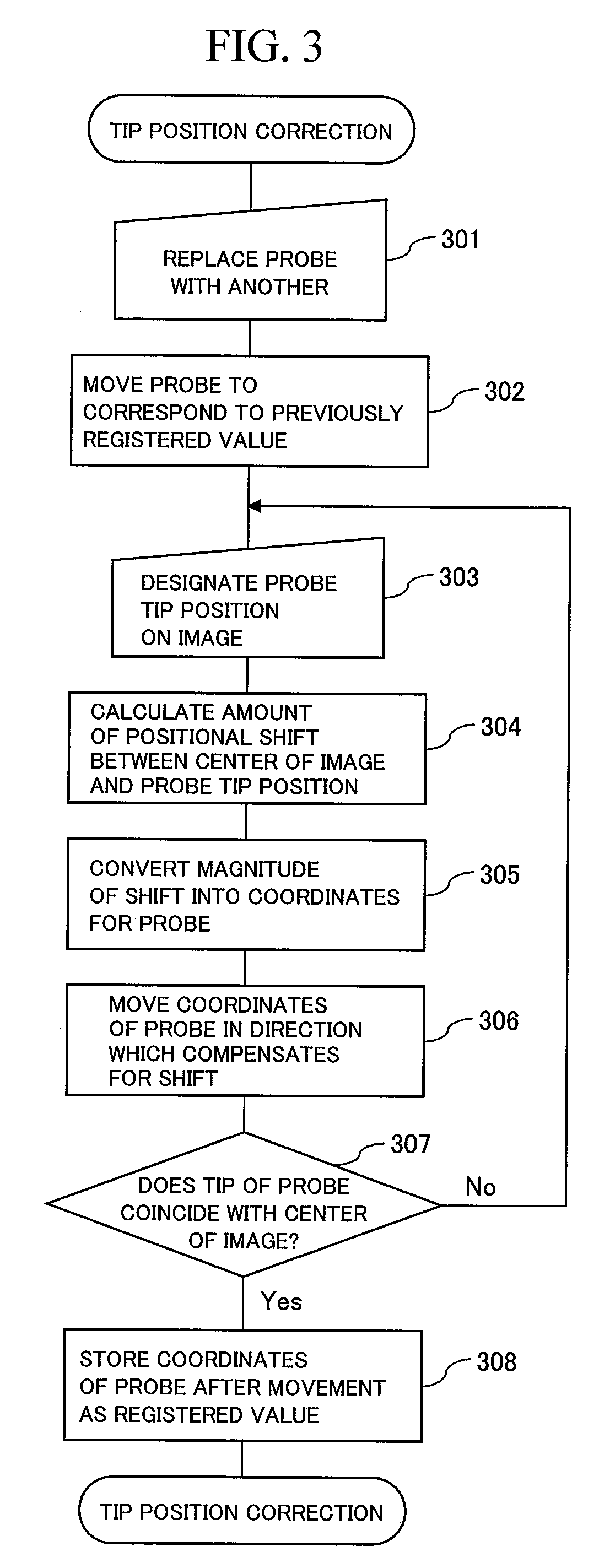
It is an object of this invention to improve contact precision and probe operability. This invention controls sample stage movement and probe movement on an observation image using a single coordinate system, thereby allowing positioning using a sample stage stop error as a probe control movement amount. This invention also figures out the position of the tip of a probe using the observation image and stores the coordinates of the probe at a reference position on the image. This invention facilitates precise probe contact operation to a sample position of the order of microns.
Owner:HITACHI HIGH-TECH CORP
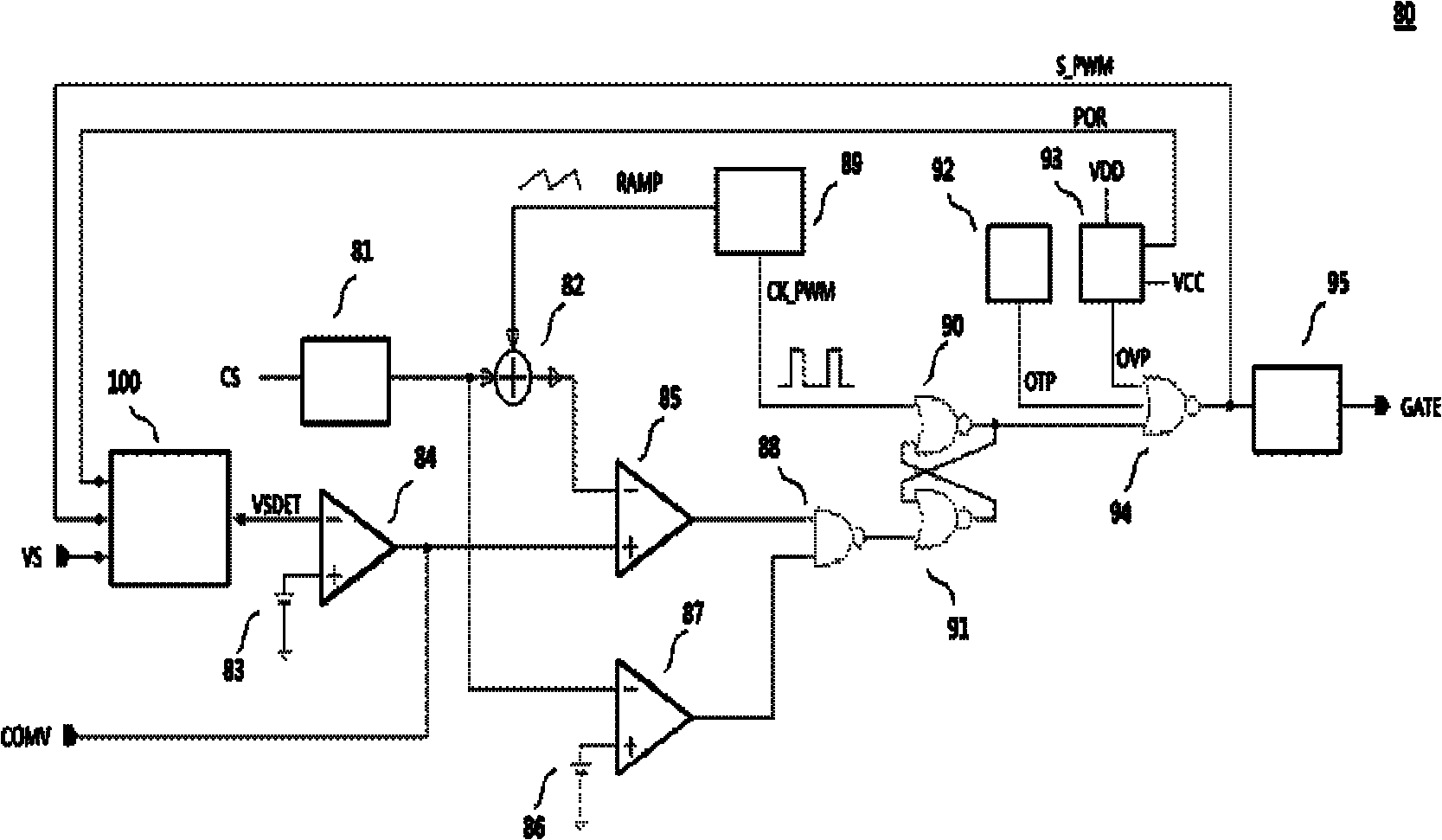
Circuit and method for switch power supply feedback voltage detection and sampling and holding
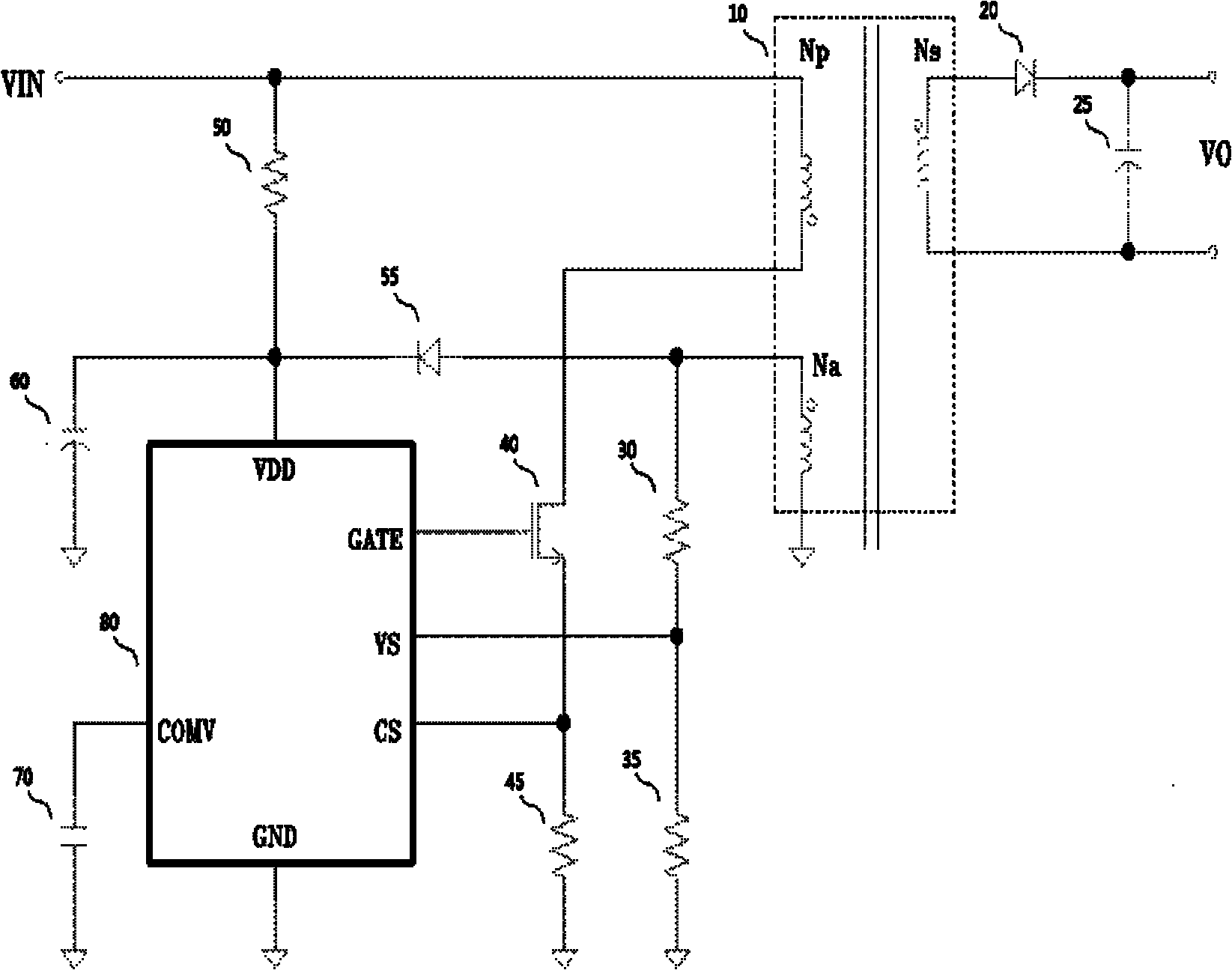
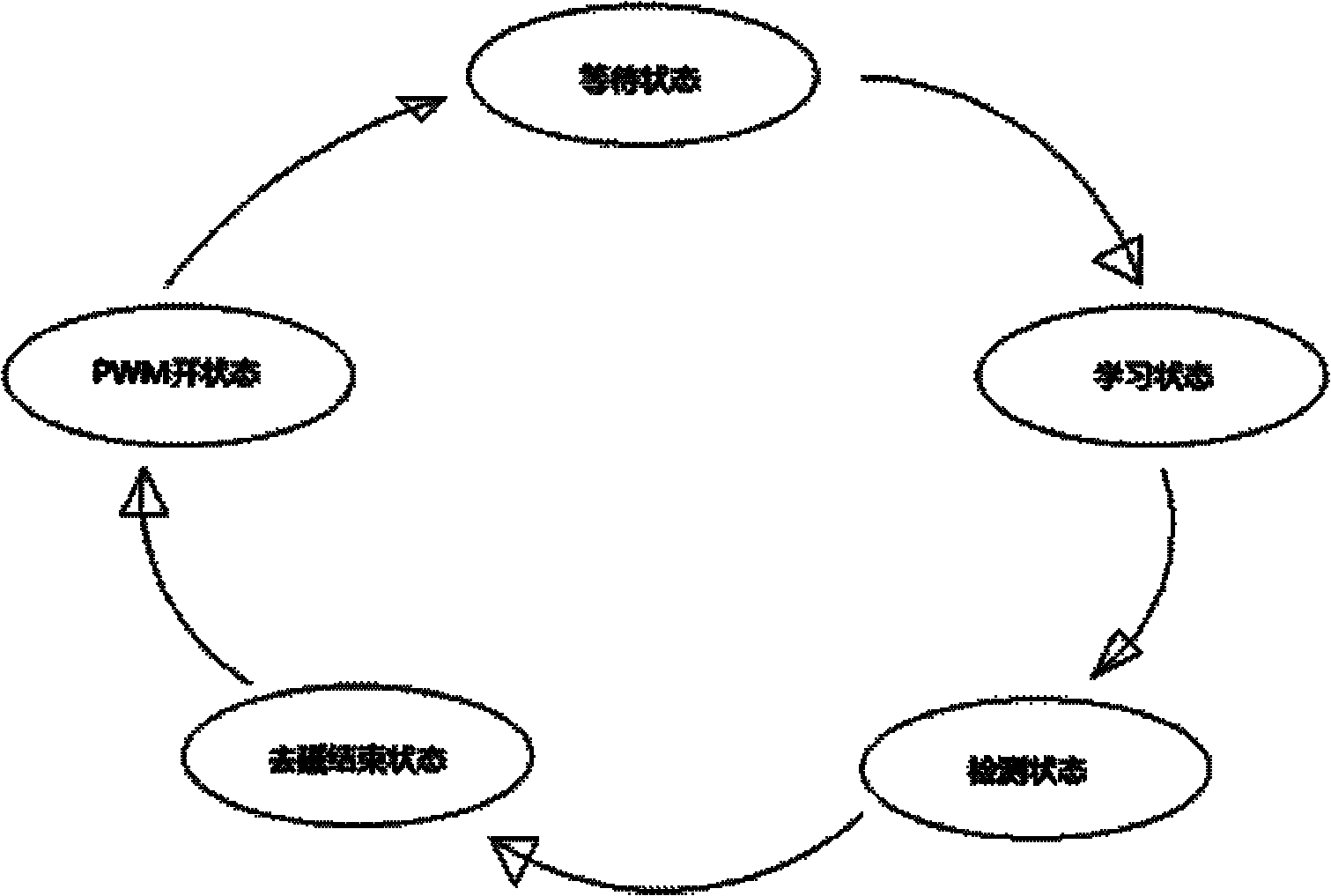
InactiveCN101867299AReduce parameter requirementsLow costDc-dc conversionElectric variable regulationWave shapePower switching
The invention provides a circuit and a method for switch power supply feedback voltage detection and sampling and holding. The circuit provided by the invention comprises a clock control circuit, a control sampling clock, a sampling and holding circuit, a waiting state control circuit, a learning state control circuit, an increment control circuit, a system control circuit and a delay circuit, wherein the sampling and holding circuit samples an input voltage at different time in two paths and selects a larger value to be output; the waiting state control circuit controls sampling and waits till a waveform is stable; the learning state control circuit controls the time sequence of a learning state; the increment control circuit controls an increment circuit which realizes two adjacent times of sampling; the system control circuit controls a finite state machine together with other circuits; and the delay circuit realizes required delay. In the method of the invention, an entire switching period of a switch power supply is divided into five states in turn: a PWM open state, a waiting state, a learning state, a detection state and a demagnetization ending state; the five states form the finite state machine; and the detection and sampling and holding of a feedback voltage can be realized by controlling the state switching and output of the finite state machine.
Owner:深圳市矽湾微电子有限公司
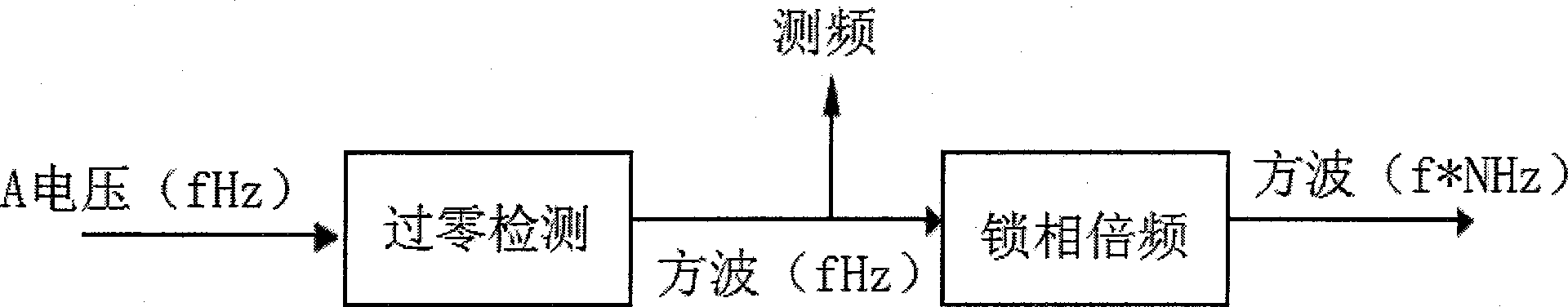
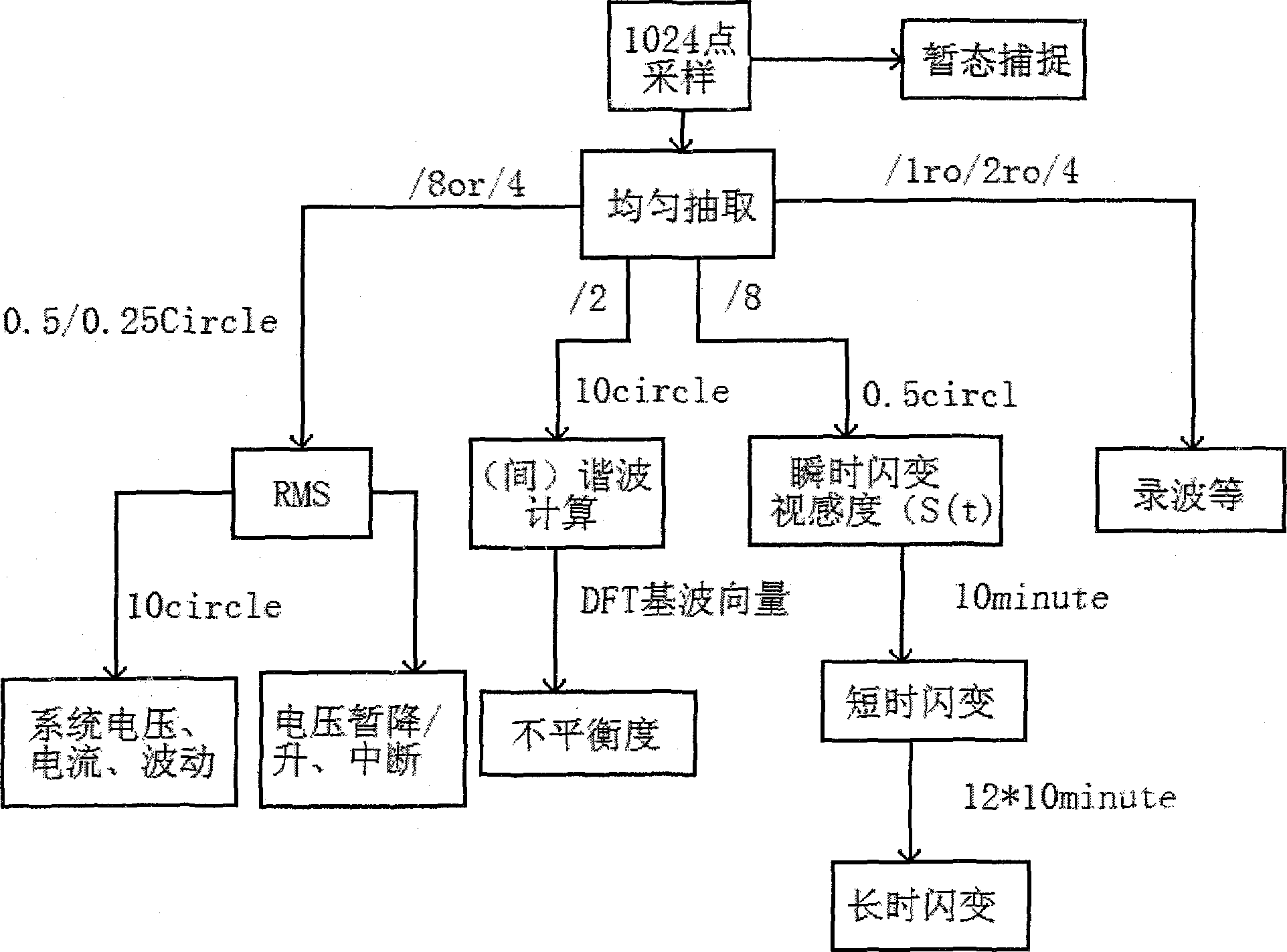
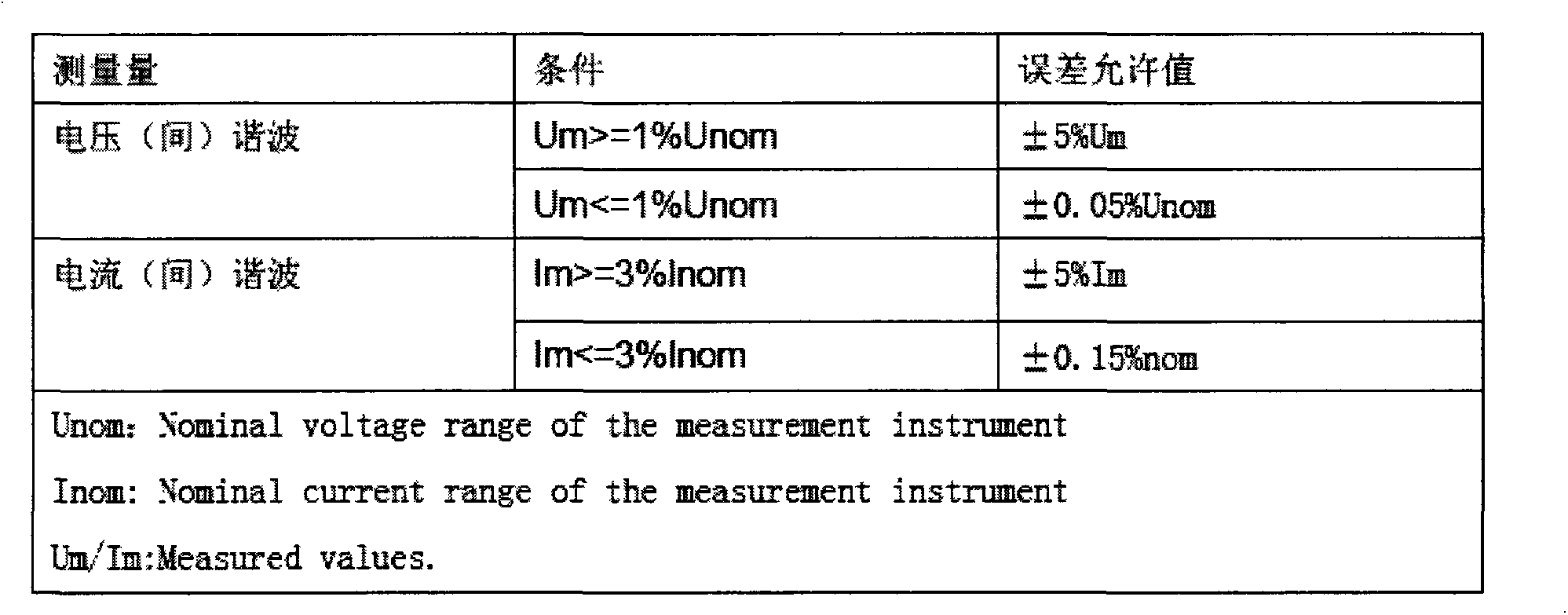
Power quality monitoring method
ActiveCN103353558AImprove real-time performanceGood followabilityProgramme controlComputer controlPower qualityFrequency measurements
The invention discloses a power quality monitoring method. A FPGA is employed in the method to realize zero-crossing detection function and compared with traditional voltage-controlled oscillators, the method exhibits better real-time performance and following performance. On frequency measurement and sampling control, three-phase voltage signal is introduced as a standard of the frequency measurement and the sampling control so that, compared with one-phase voltage signal introduced as the standard, the method is more reliable and flexible; that software controls sample interval is as a supplement of hardware sampling control, so that sampling accuracy is improved; on algorithm input points, accuracy requirement of the algorithm and the calculated quantity are balanced; and under the condition that accuracy is guaranteed, calculated quantity is lessened, so that cost performance of the actual device is improved.
Owner:SHENZHEN KANGBIDA CONTROL TECH
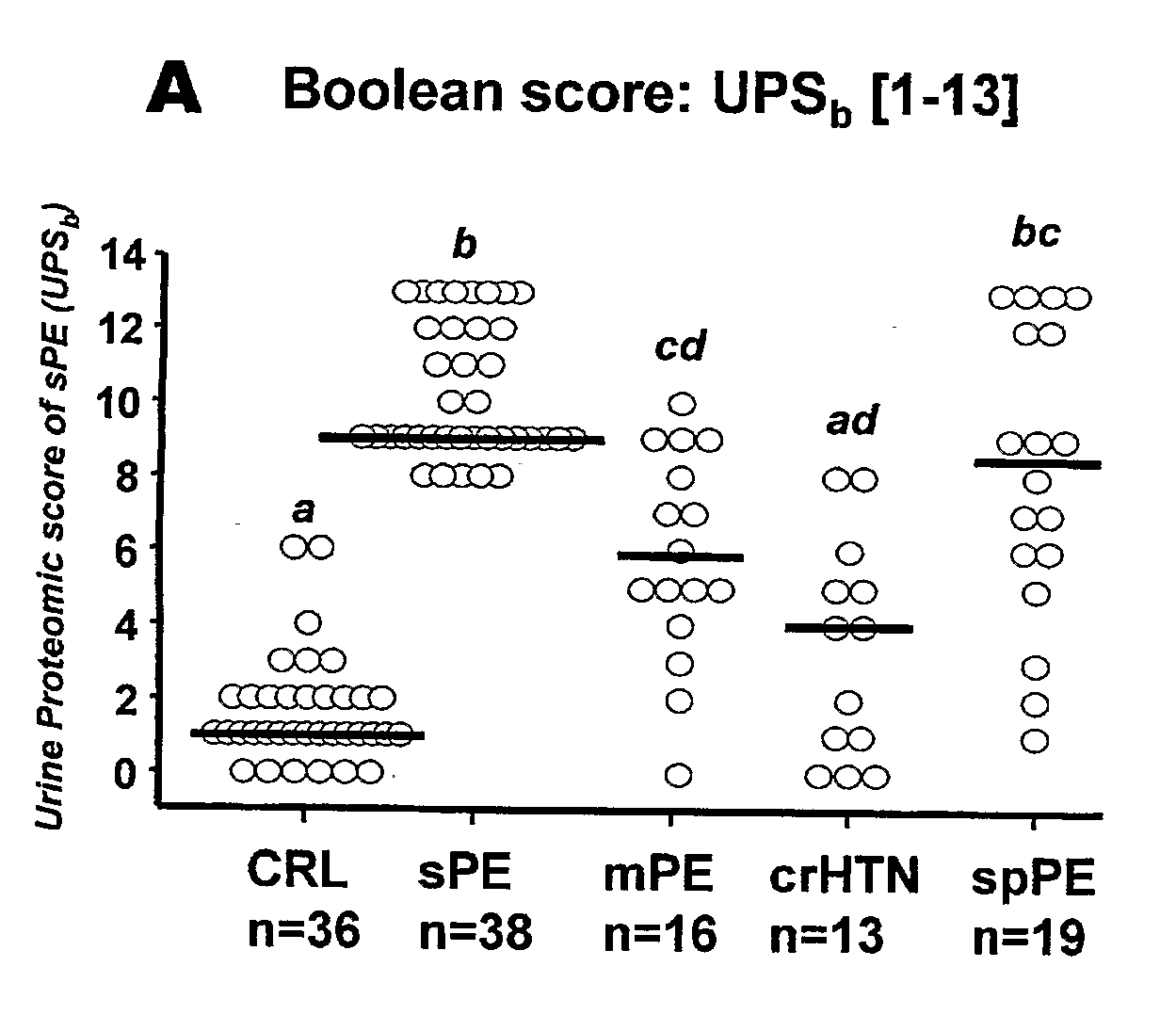
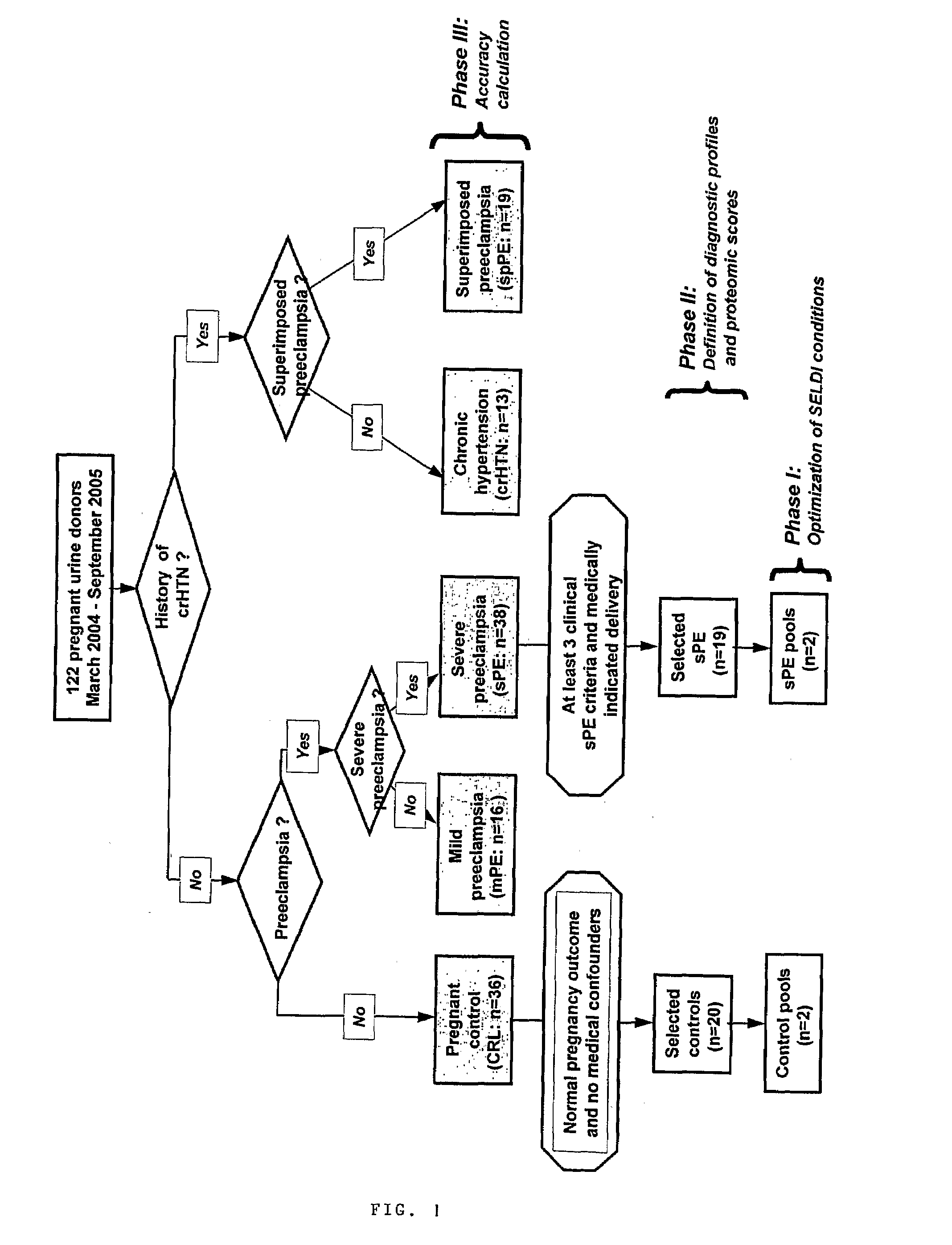
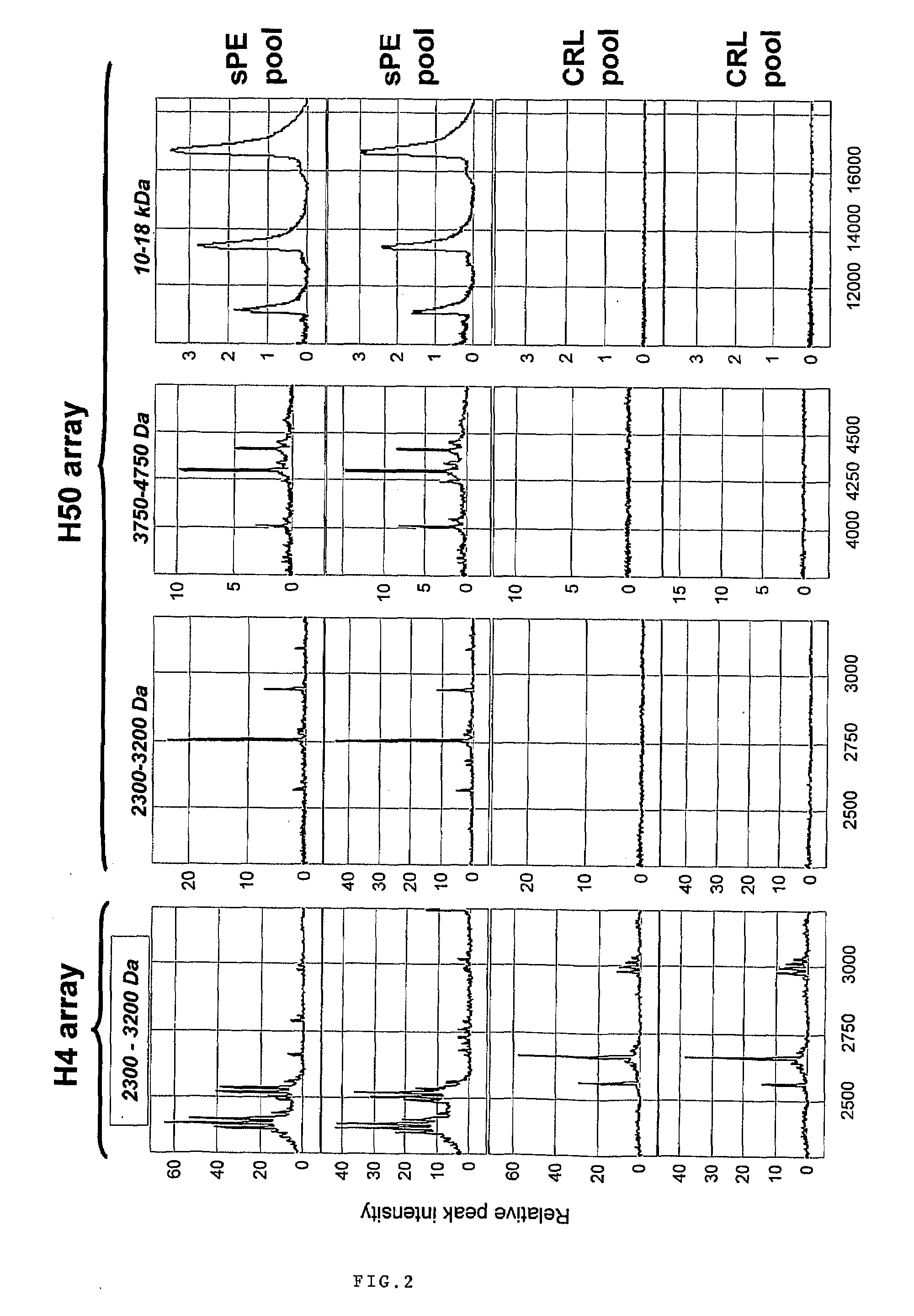
Urinary Proteomic Biomarker Patterns in Preeclampsia
The invention relates, in part, to methods of using proteomic biomarkers to diagnose preeclampsia. In some aspects the invention, in part, relates to the detection of serpina-1 polypeptide and / or albumin polypeptide in samples from pregnant subjects. Samples from subjects may be compared to control samples to diagnose preeclampsia and / or to determine the onset, progression, or regression of preeclampsia in a subject. The invention also relates, in part, to screening methods to identify agents that can be used to treat preeclampsia and to determine the efficacy of a preeclampsia treatment. The invention, in part, also includes kits that are useful to diagnose and assess preeclampsia in a subject.
Owner:YALE UNIV
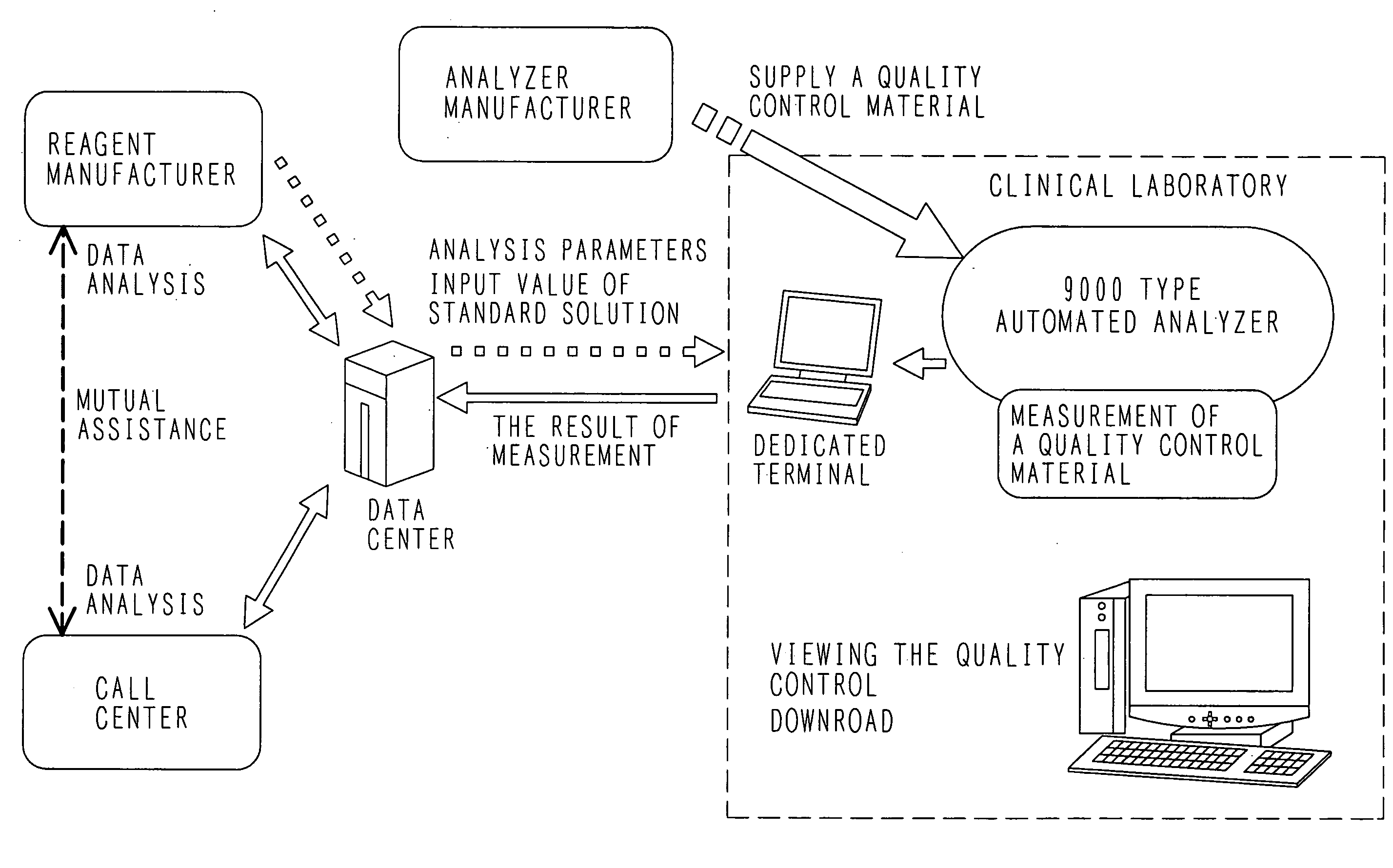
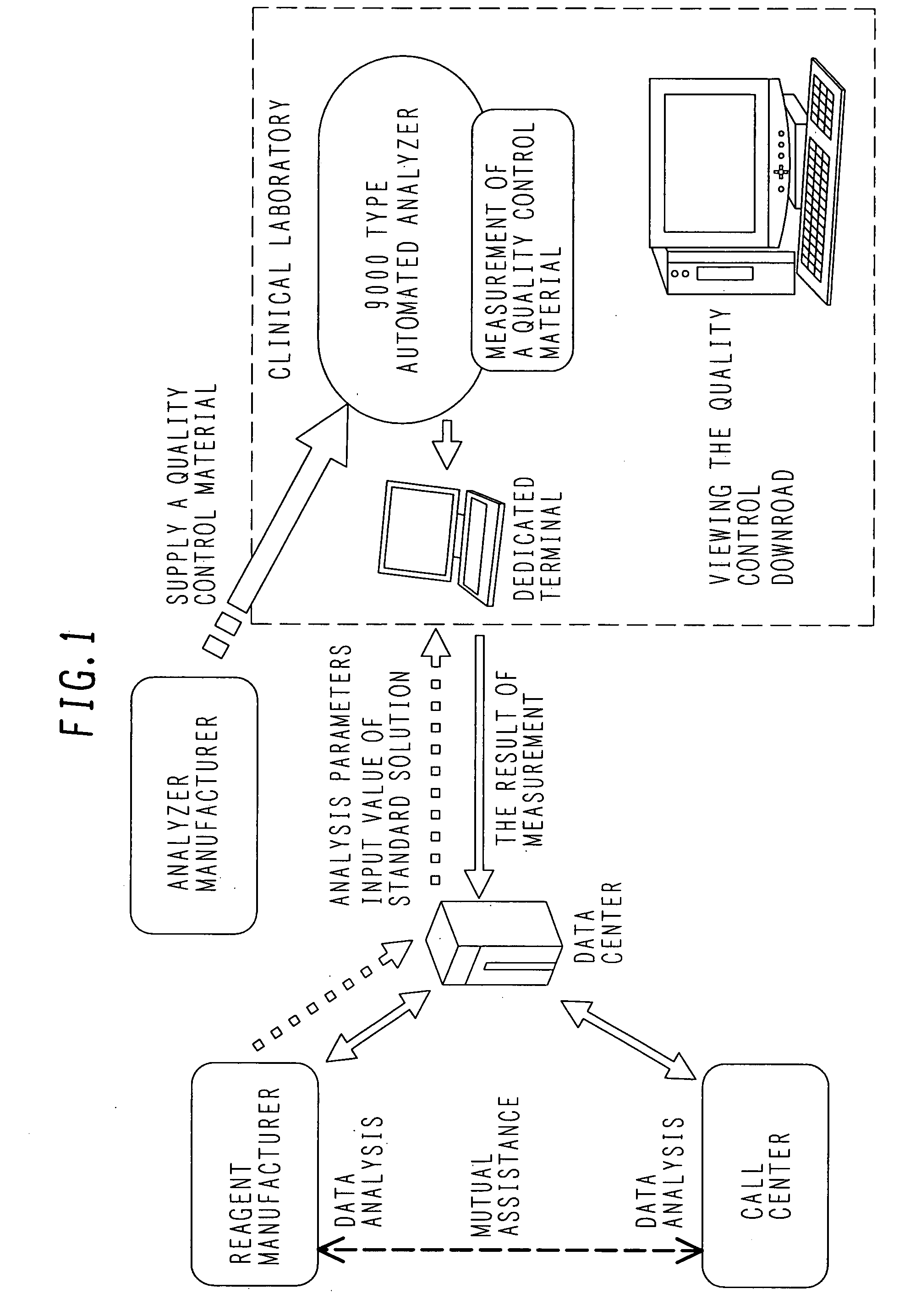
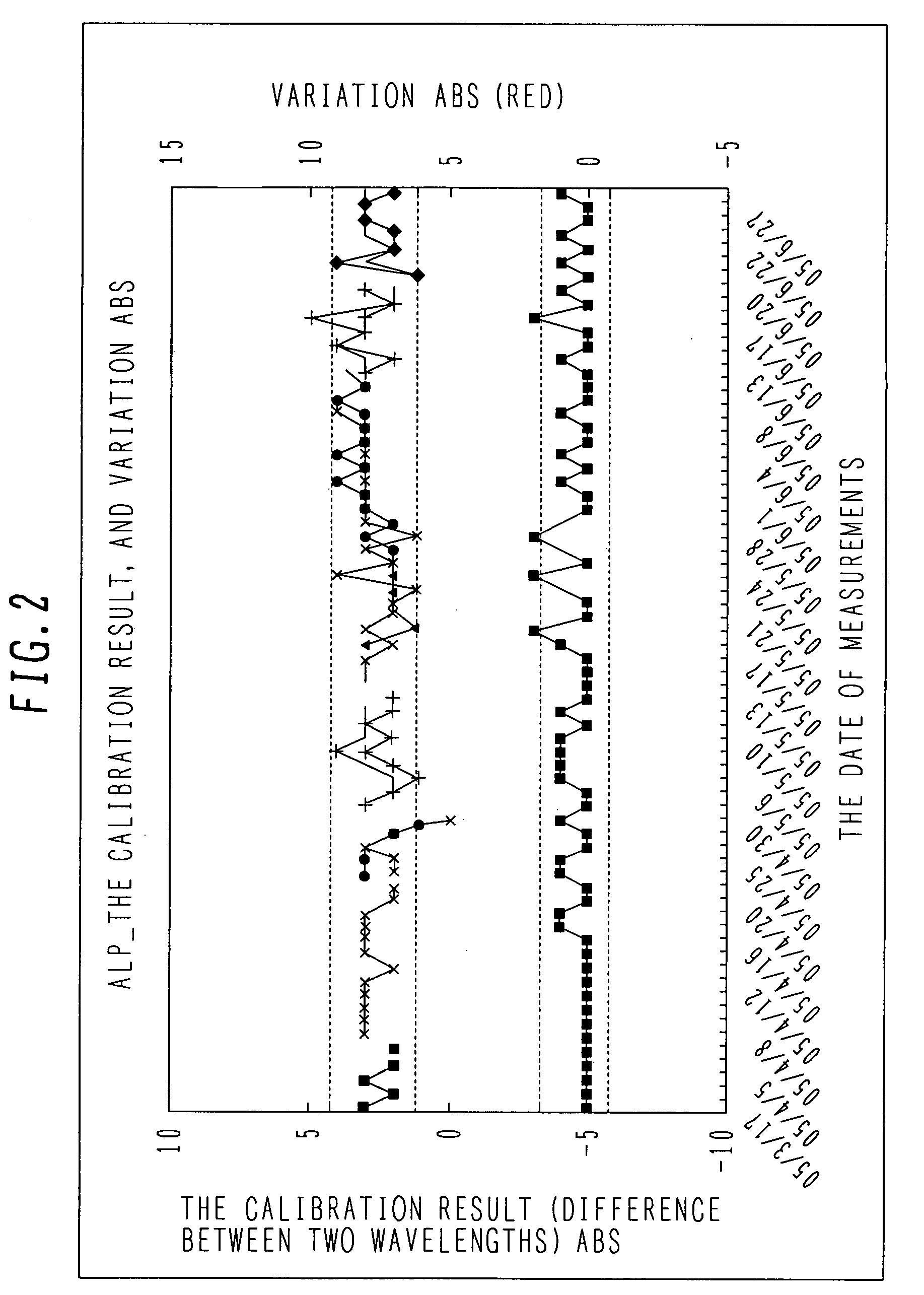
Quality control system
ActiveUS20070217949A1Reduce workloadReduce retrieval timeLevel controlMaterial analysis by optical meansQuality control systemQuality control
In a clinical laboratory of a hospital, an enormous amount of effort has been required to maintain the quality of an analyzer, standard solution and control samples. An object of the present invention is to provide a control method for controlling a clinical laboratory with reduced cost, and a control apparatus using the same.In order to control data of an analyzer, standard solution, and a control sample, a support center is connected to each analyzer located in each hospital through a network line. Various kinds of analysis parameters and the result of measurements are exchanged so as to provide each clinical laboratory with a control situation in real time.
Owner:HITACHI HIGH-TECH CORP
Biomarkers
InactiveUS20150141273A1Conducive to screeningUseful in detectionCompound screeningApoptosis detectionCvd riskBiomarker (petroleum)
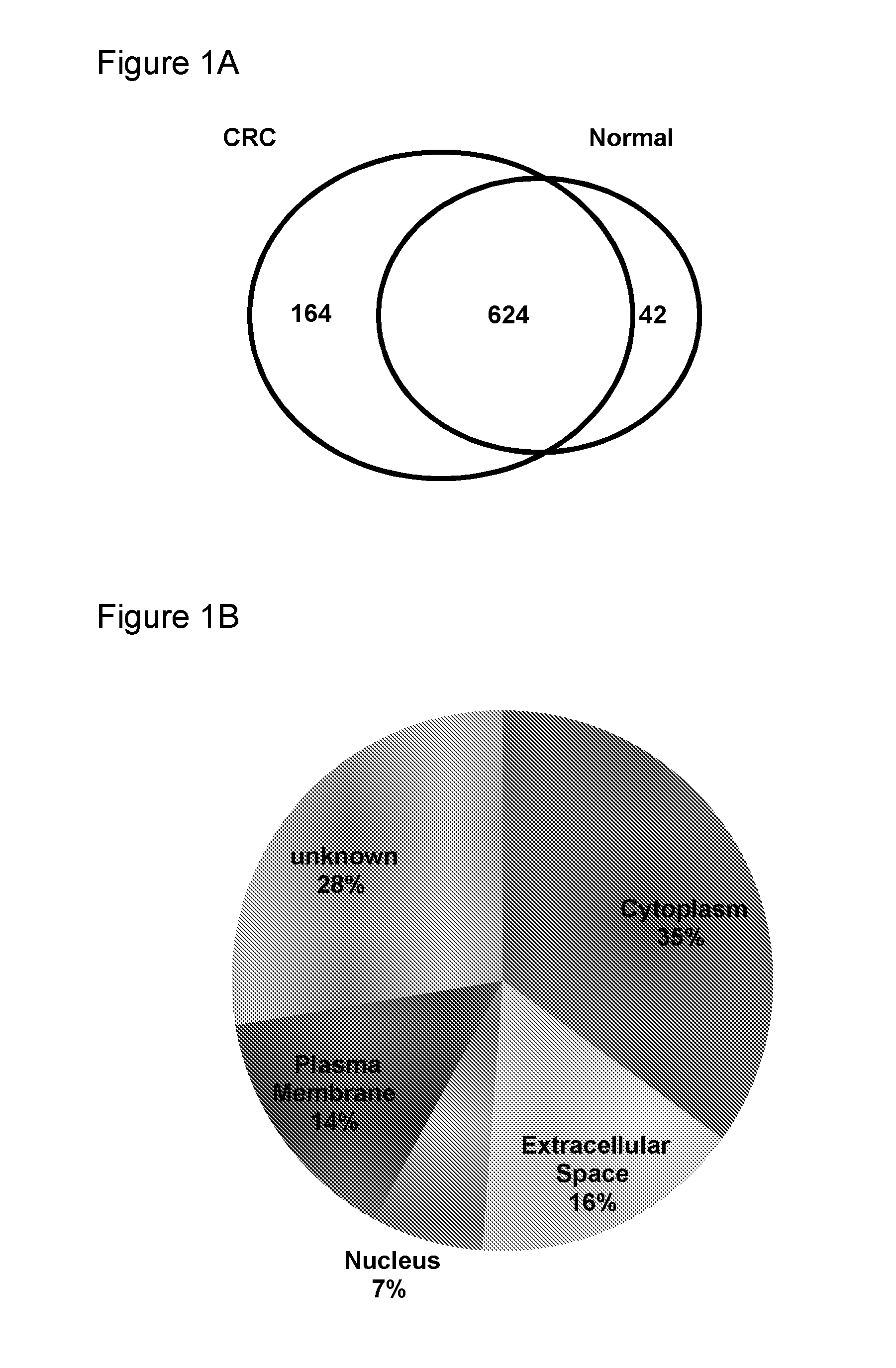
The invention provides a method for screening for colorectal cancer, the method comprising: screening a biological sample from an individual for one or more biomarkers selected from the group defined in Table 1 and / or Table 6, wherein the presence of or increased expression of the one or more biomarkers relative to a control sample is indicative that the individual is at risk of suffering from or is suffering from colorectal cancer. The invention also provides an array and kit suitable for use in the methods of the invention, methods of treating colorectal cancer and therapeutic agents for use in methods of treating cancer.
Owner:STICHTING VU VUMC
Differential diagnosis of neurodegeneration
InactiveUS20040014142A1Specific detectionSpecific quantificationDisease diagnosisBiological testingSpecific detectionSynuclein
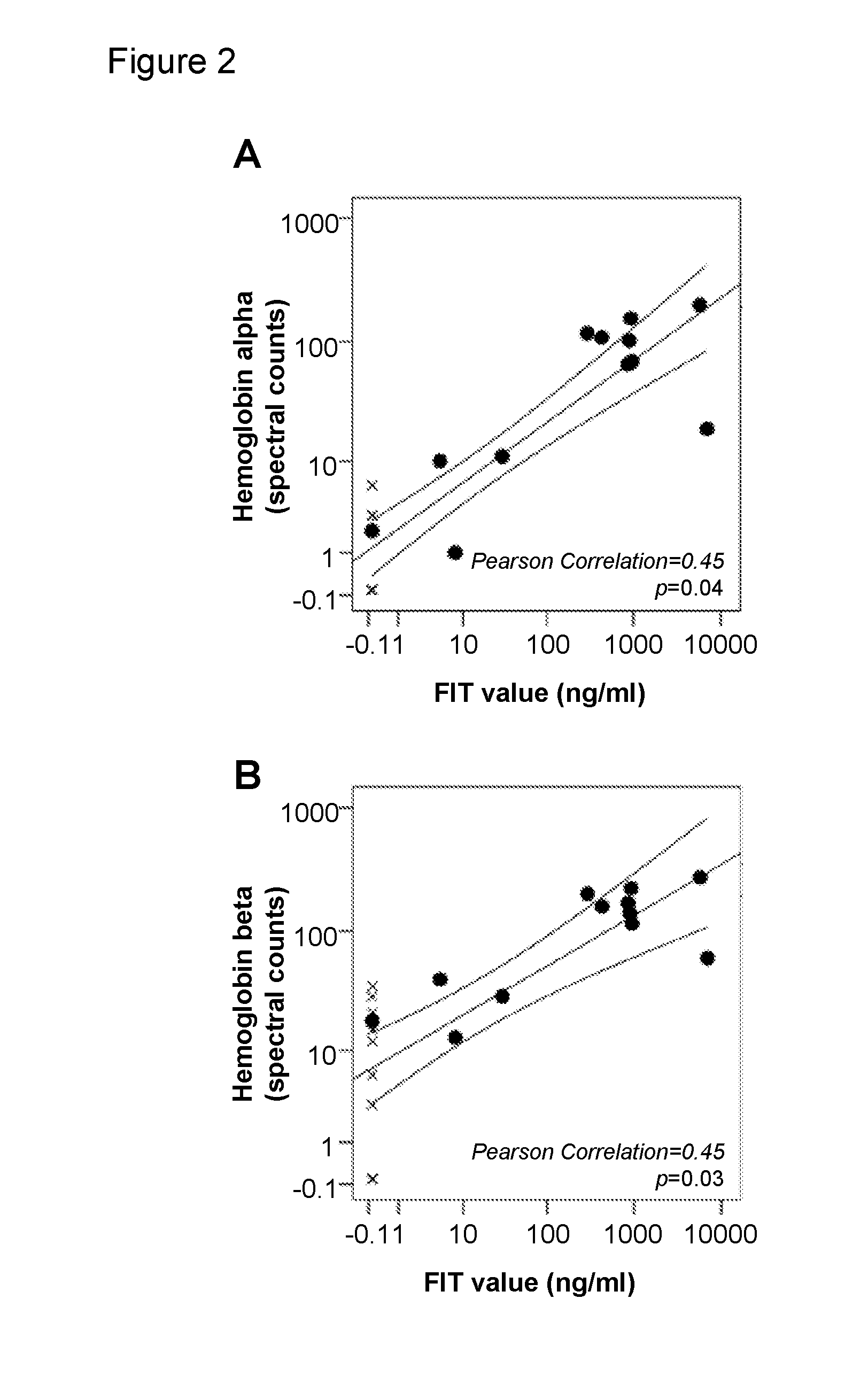
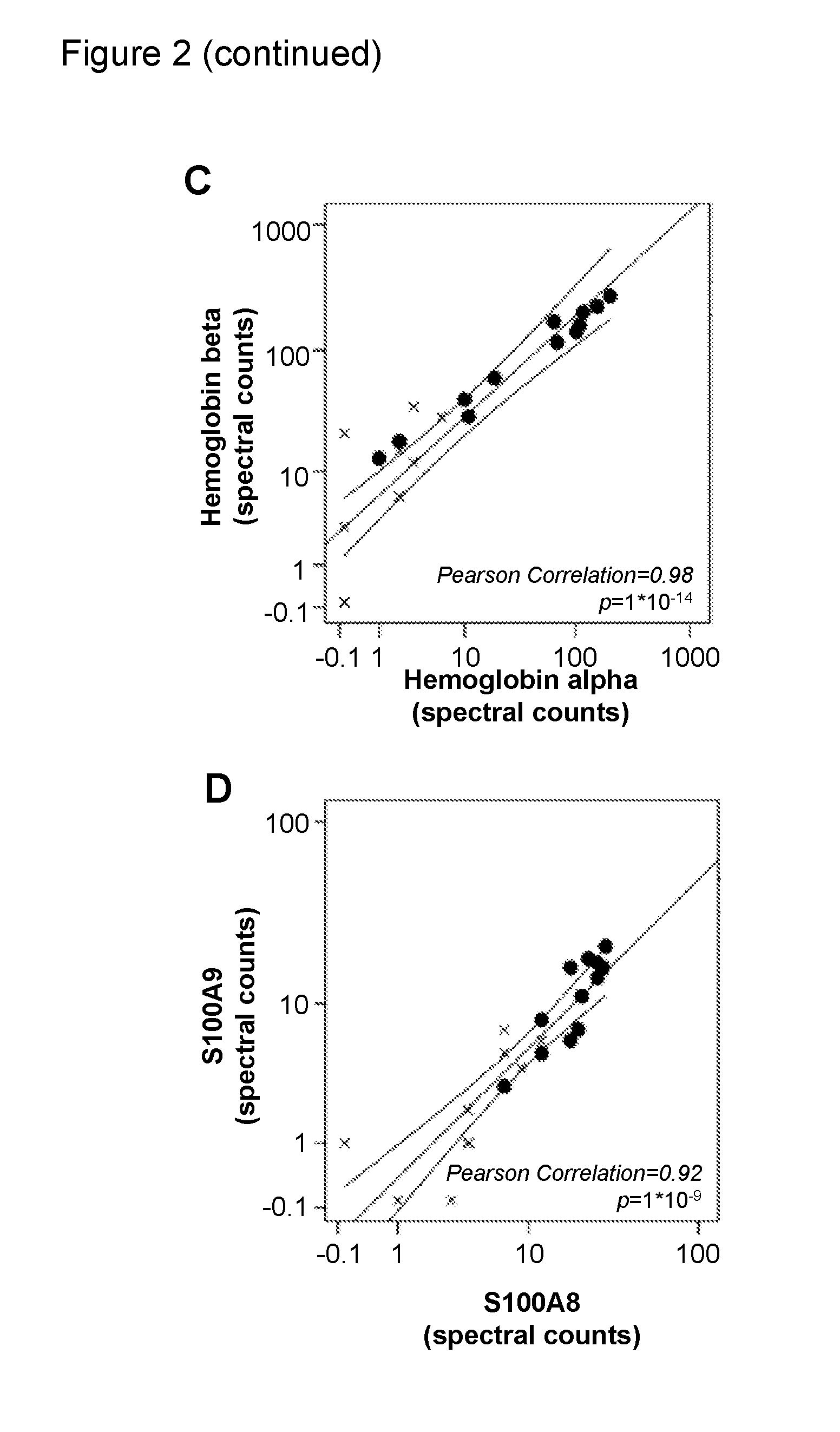
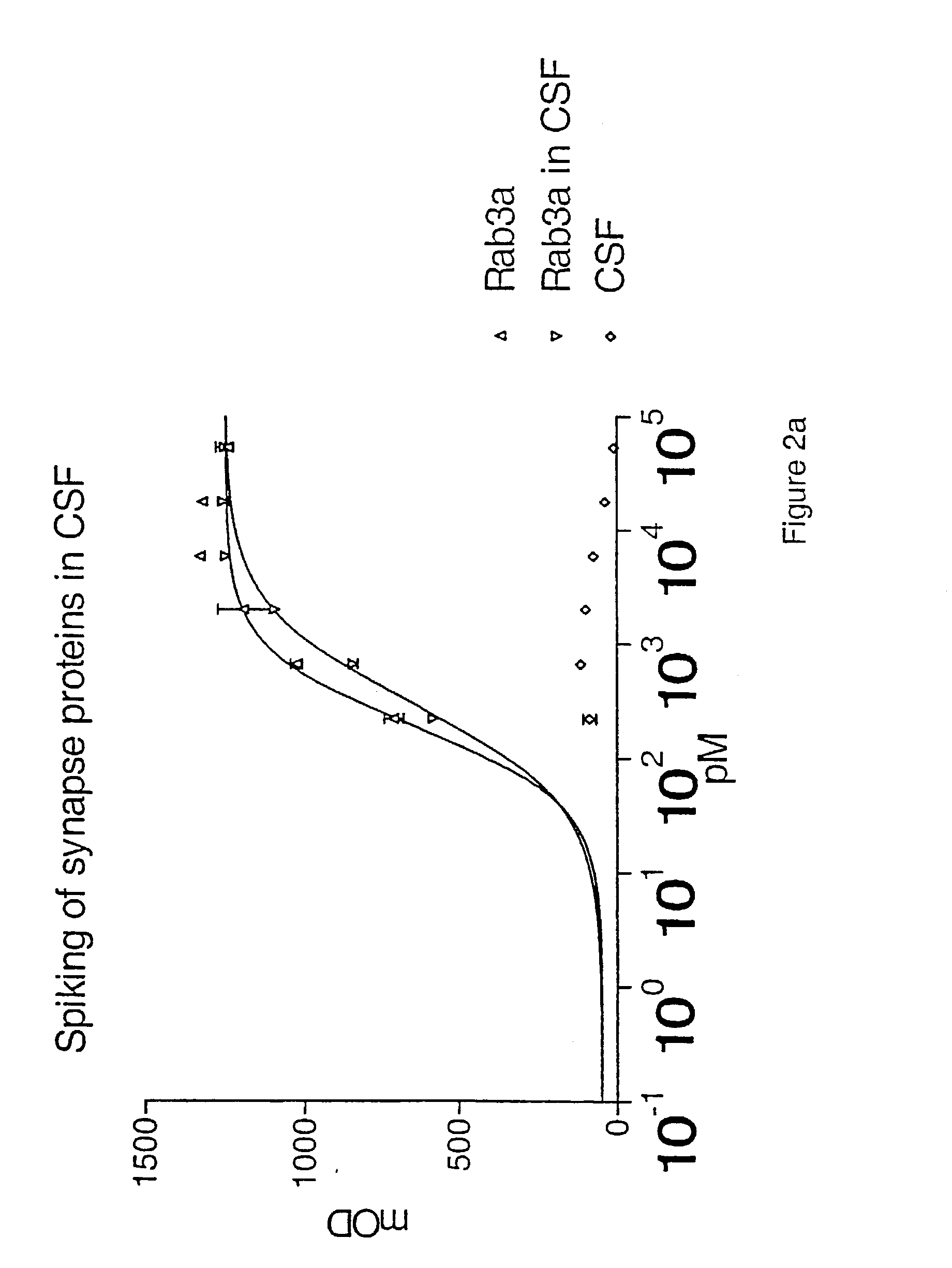
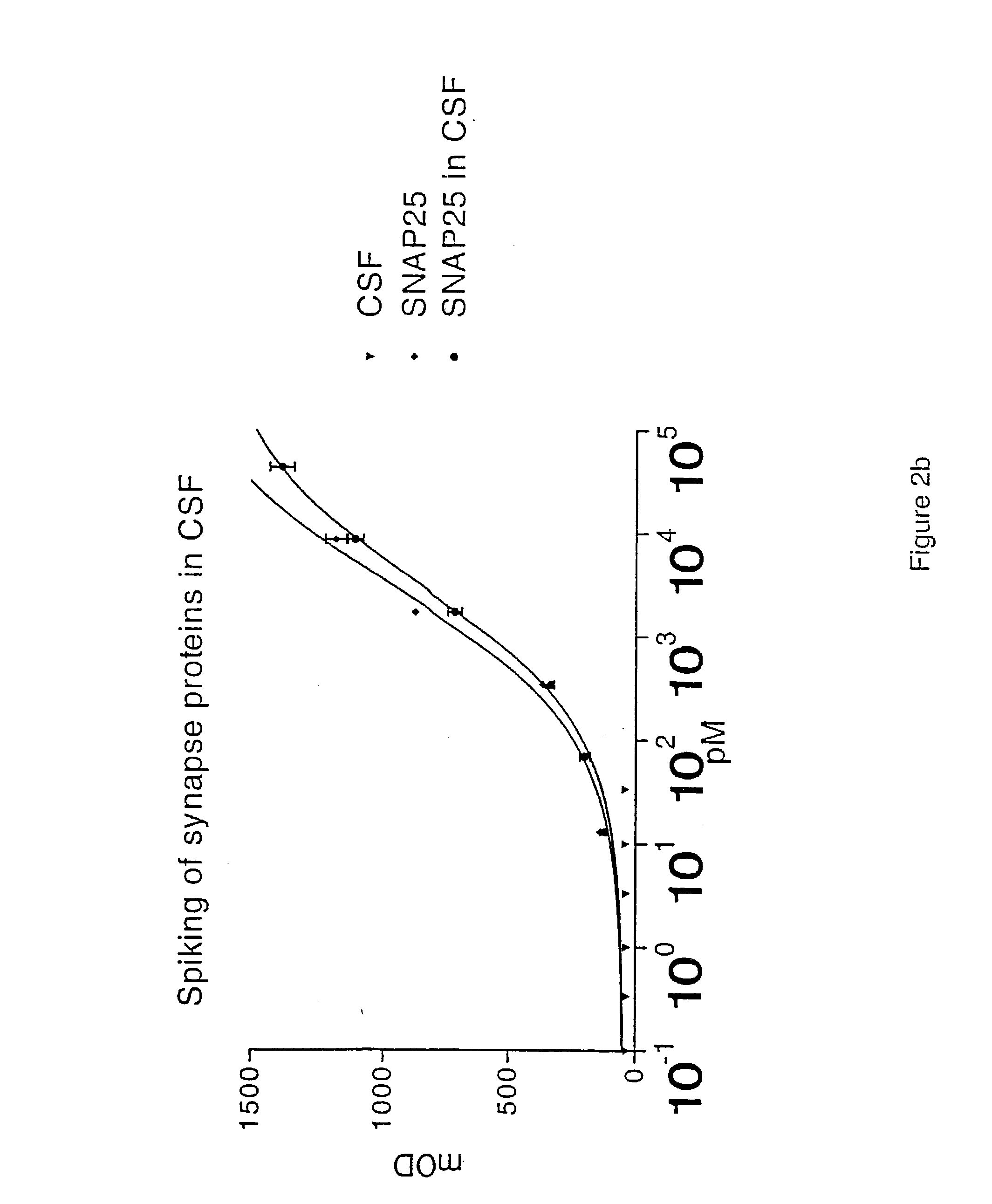
The present invention relates to new methods for the specific detection, quantification and / or differential diagnosis of neurodegeneration in an individual making use of a combination assay detecting at least three neurological markers in one or more body fluids of said individual, the type and degree of neurodegeneration being reflected in the quantitative changes in the level of all of said neurological markers compared to the control sample. The present invention also relates to methods for the detection of Rab3a, SNAP25 and alpha-synuclein in cerebrospinal fluid and to the use of these methods in a combination assay for specific detection, quantification and / or differential diagnosis of neurodegeneration.
Owner:INNOGENETICS NV
Diagnostic Testing Process and Apparatus Incorporating Controlled Sample Flow
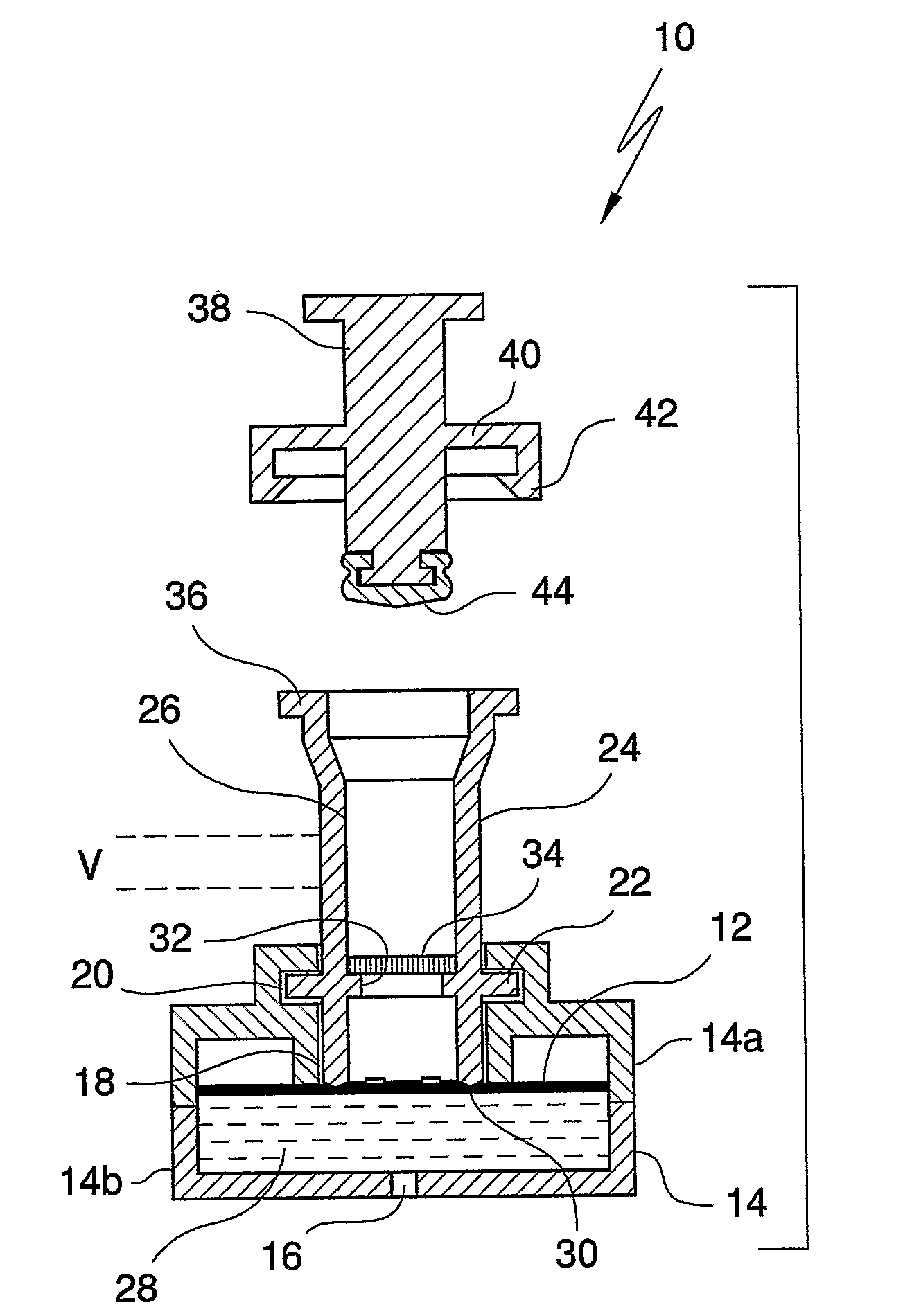
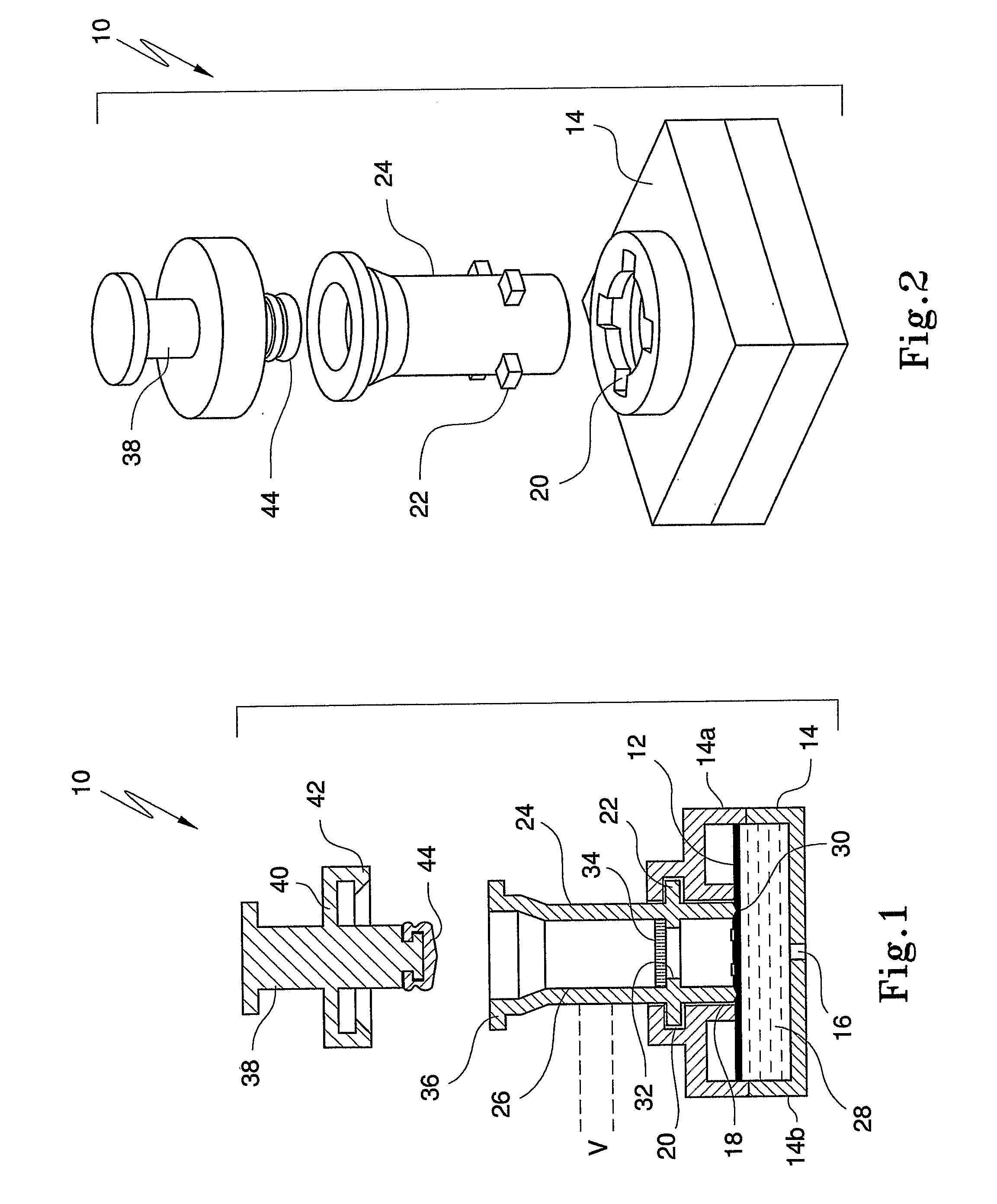
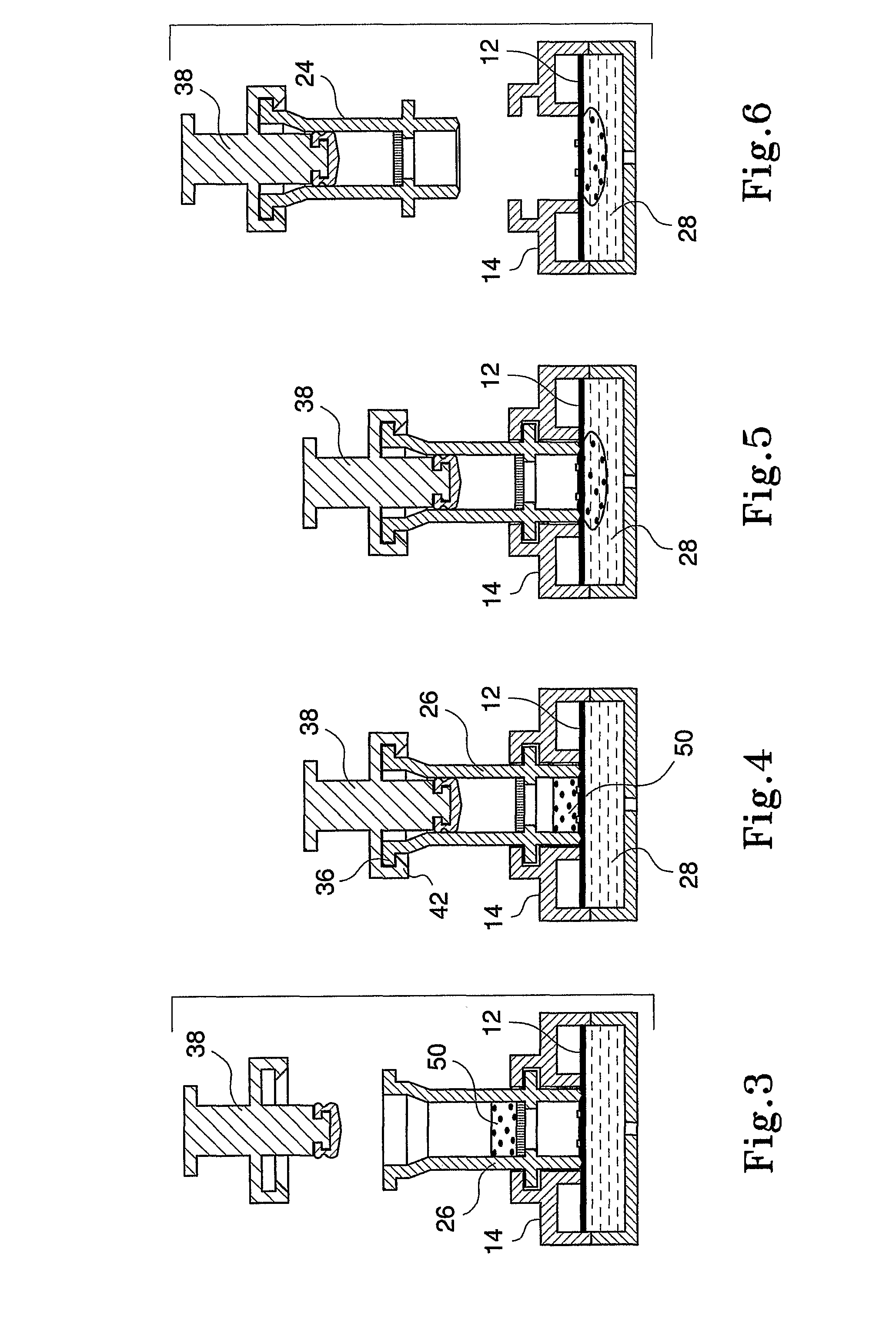
InactiveUS20080318342A1Avoid accumulationPrevents wickingLaboratory glasswaresBiological testingActuatorPiston
An apparatus (10) and method for use in a vertical flow-through assay process is characterised by applying pressure to a sample to force the sample through a reaction / capture membrane (12), to which one or more ligands are bound, at a controlled rate. Typically, the method includes a pre-incubation step in which the sample and a detection analyte typically an antibody bound to colloidal gold or a fluorescent tag bind together The pre-incubation step typically takes place in a chamber (26) spaced above the capture membrane (12). The base of the chamber is defined by a porous hydrophobic frit (34) typically formed from polyethylene. It is preferred that the chamber (26) is defined by the upper part of a cylinder (24) extending from a seal (30) compressing the reaction membrane (12) against an absorbent pad (28). The seal (30) has the effect of compressing tie reaction membrane and preventing wicking of the sample in the lateral direction outside of the circular seal. A piston (28) compresses air located in the chamber above atmospheric pressure to farce the sample to pass through the hydrophobic frit (34). Alternatively, a hydraulic actuator (60) may directly act on the sample to force the sample through the frit and reaction membrane at a predetermined rate.
Owner:PROTEOME SYST LTD
Detection of STRP, such as fragile X syndrome
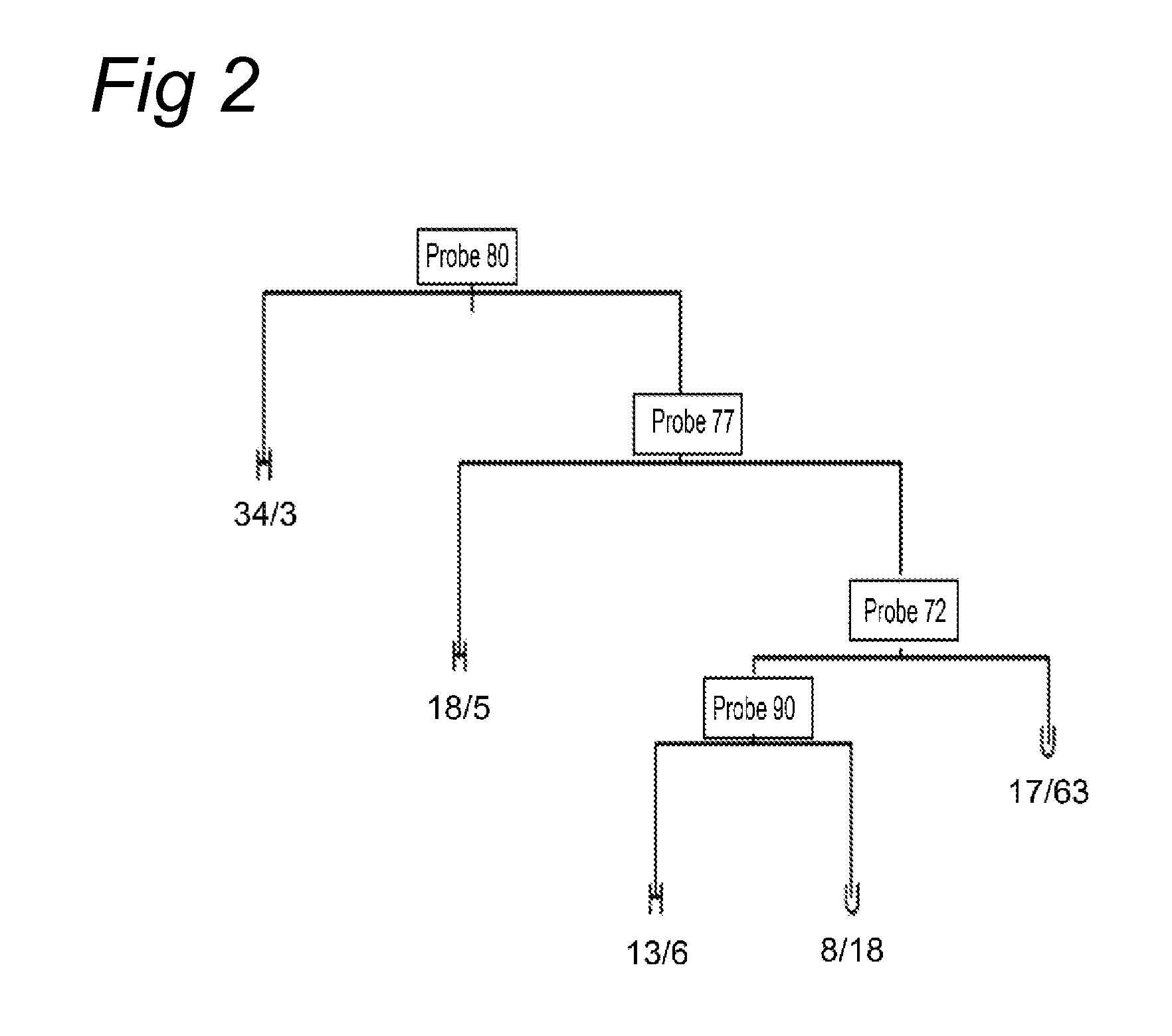
InactiveUS20050191636A1Accurate estimateQuantitative precisionMicrobiological testing/measurementBiological testingFragile X chromosomeGenomic DNA
Methods for detecting a short tandem repeat polymorphism (STRP), such as fragile X syndrome, wherein PCR is used to amplify nucleic acid along the chromosome in the genomic DNA which includes all of the STRs of interest plus a substantial contiguous segment of the nucleic acid adjacent to the STRs. Single-stranded product is then obtained, and colorimetric-labeled oligonucleotides which target for (i) STRs and (ii) the contiguous DNA segment are hybridized with this single-stranded product which is then bound to a solid phase and separated from the remainder of the target material. The labeled oligonucleotide target material is recovered by treatment with base and then hybridized to a microarray having a plurality of spots containing suitable oligonucleotide probes complementary thereto. Following hybridization, colorimetric intensities of the hybridized labeled target material present at specific spots on the microarray are measured to obtain individual values which are compared with results from known control samples to accurately quantify the number of STRs in the region of interest of the DNA being analyzed.
Owner:BIOCEPT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com