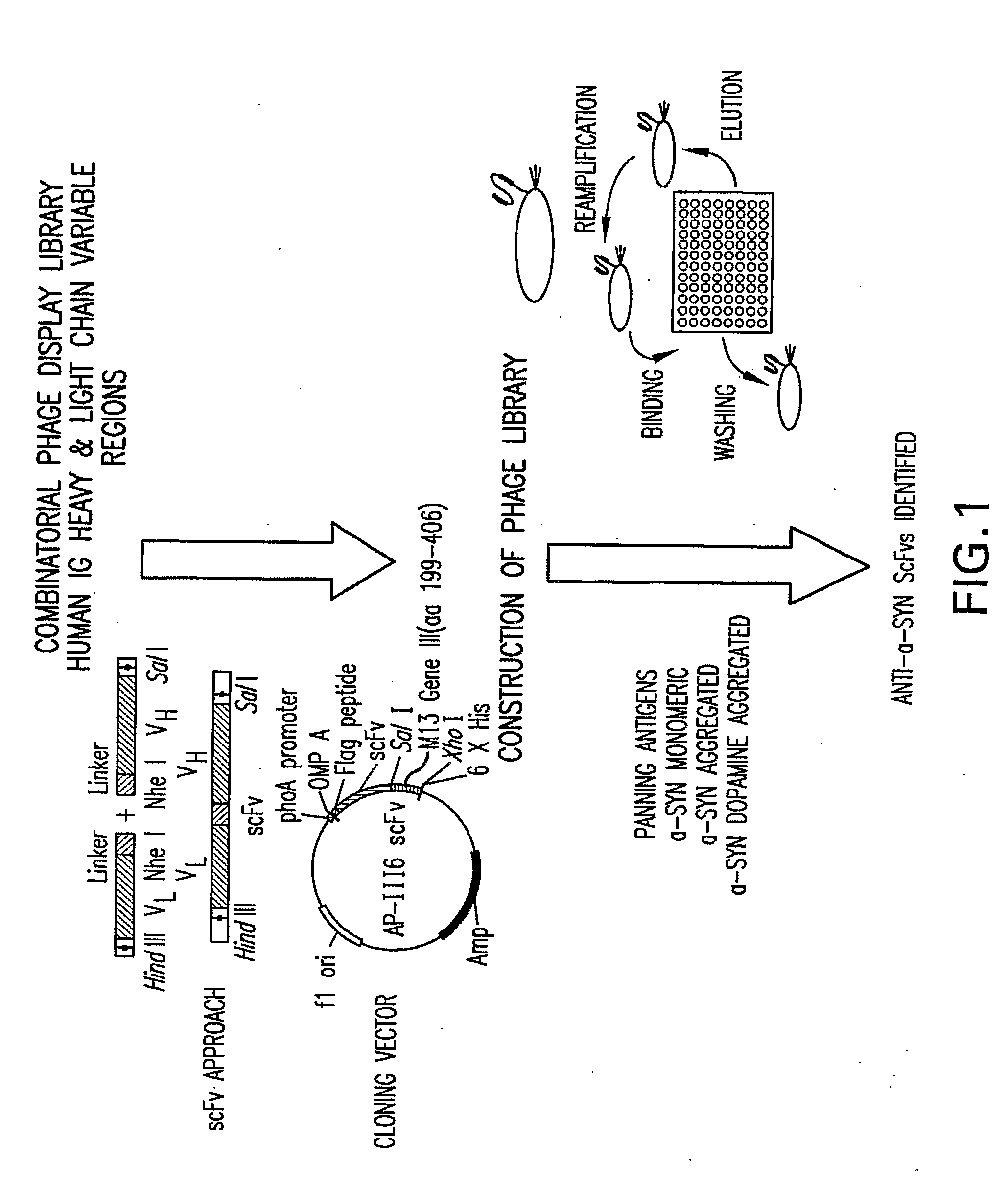
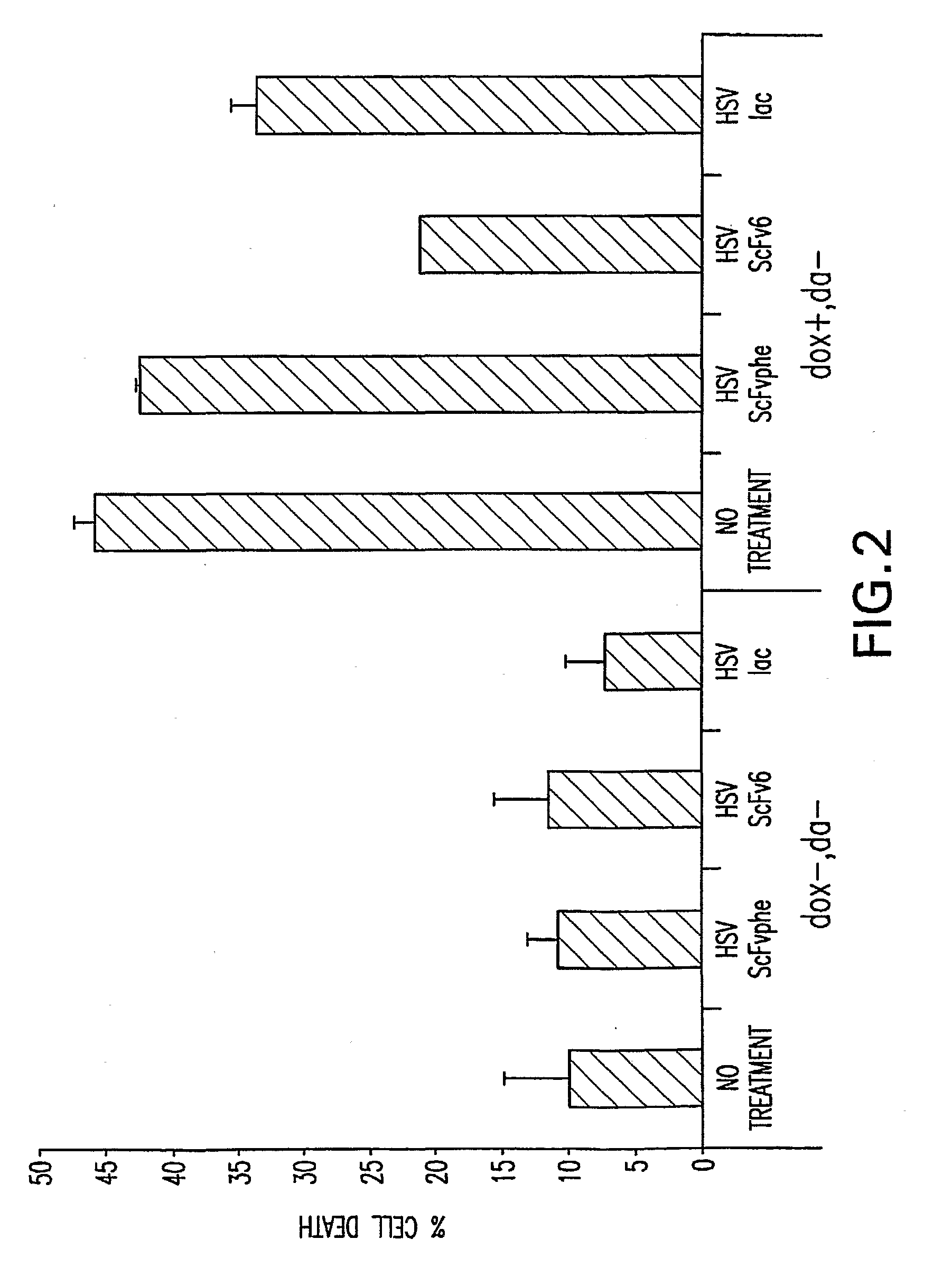
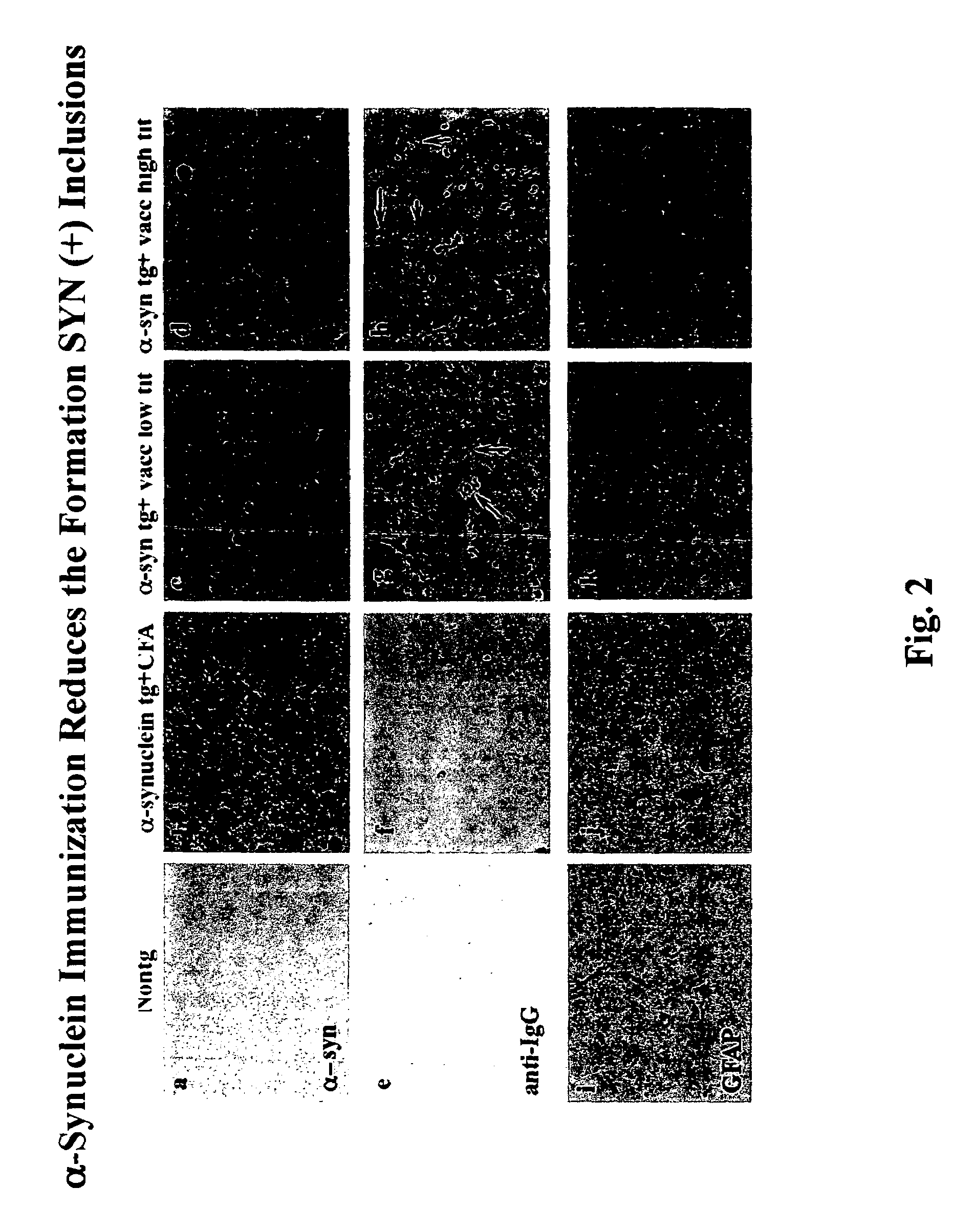
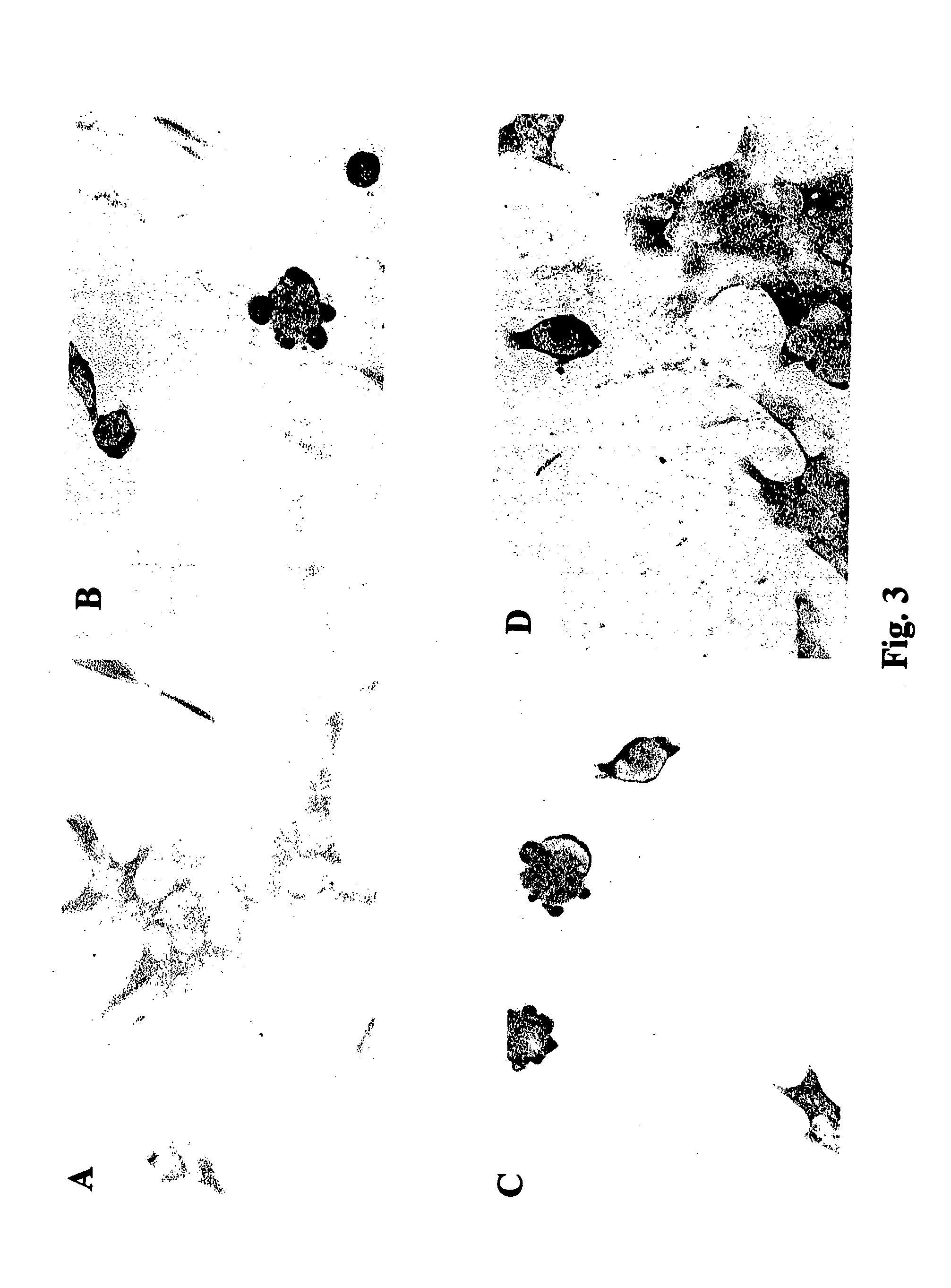
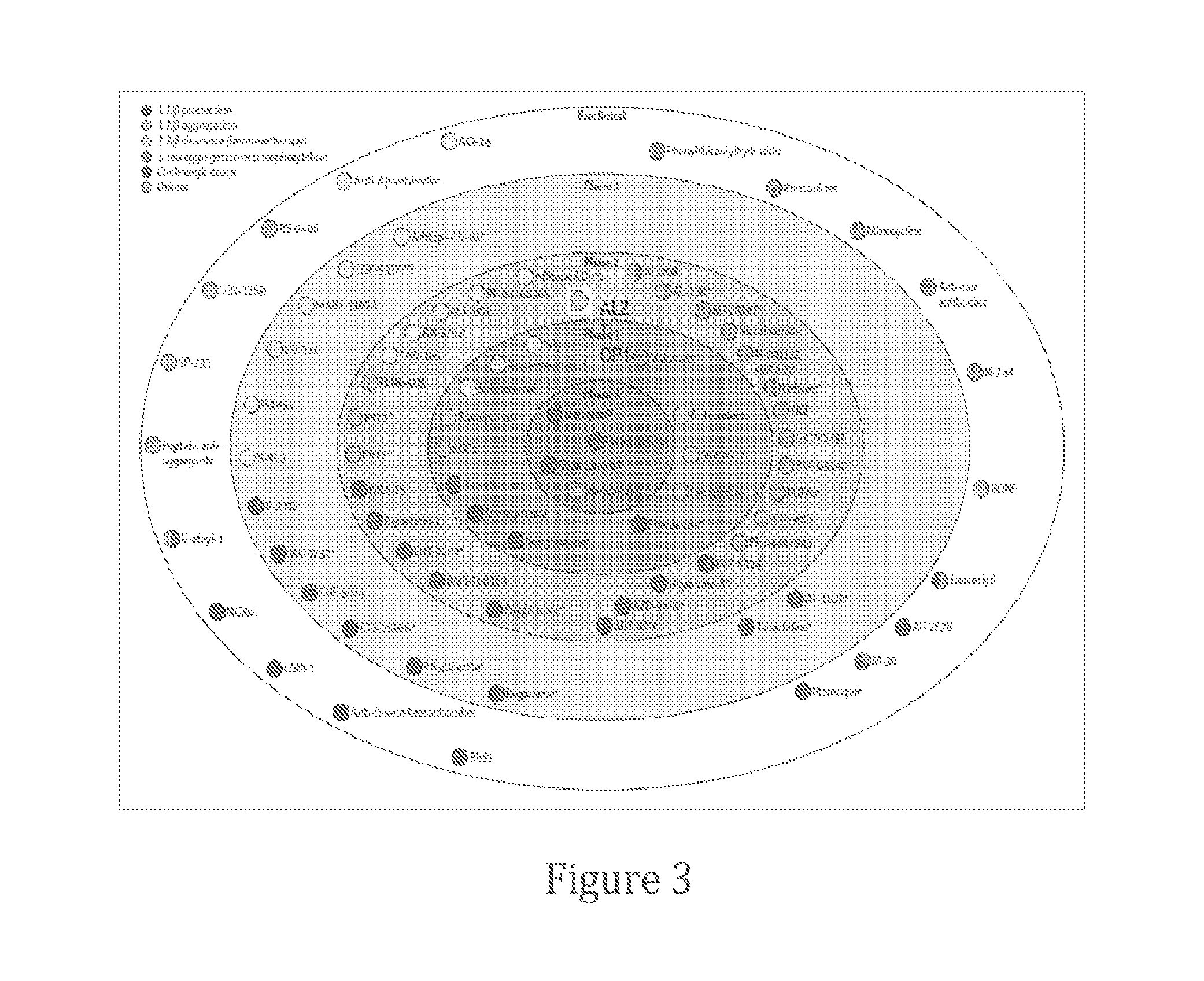
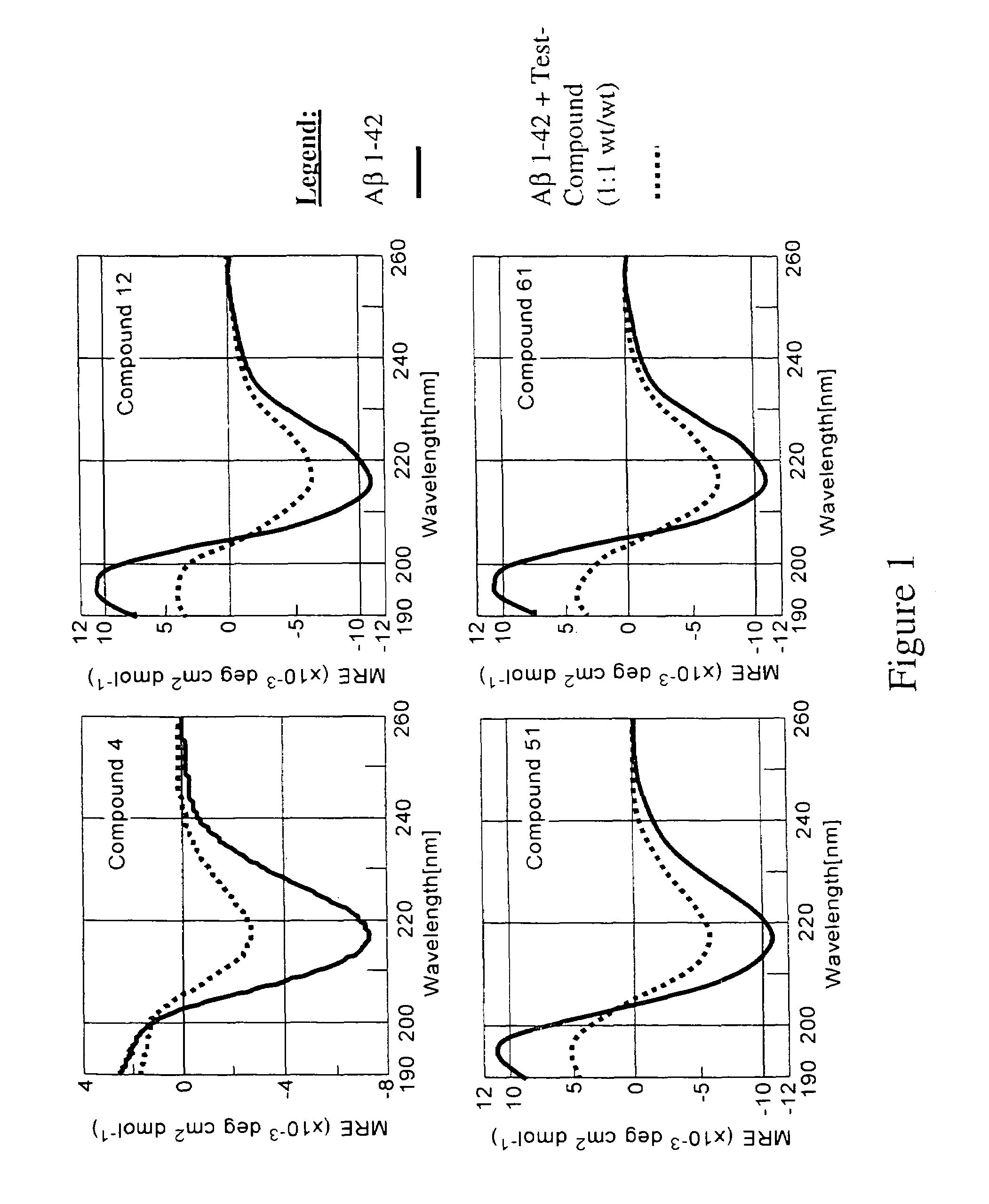
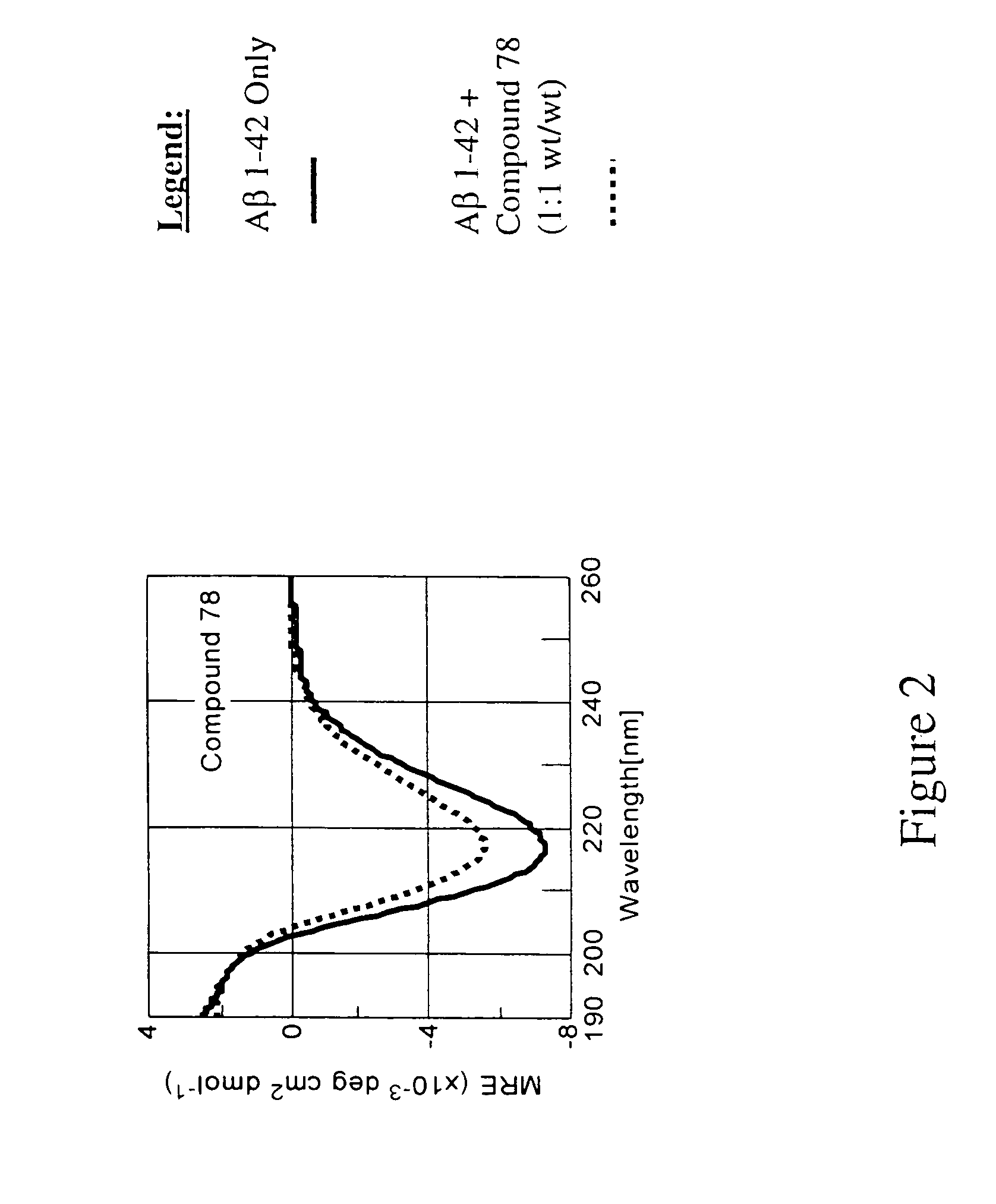
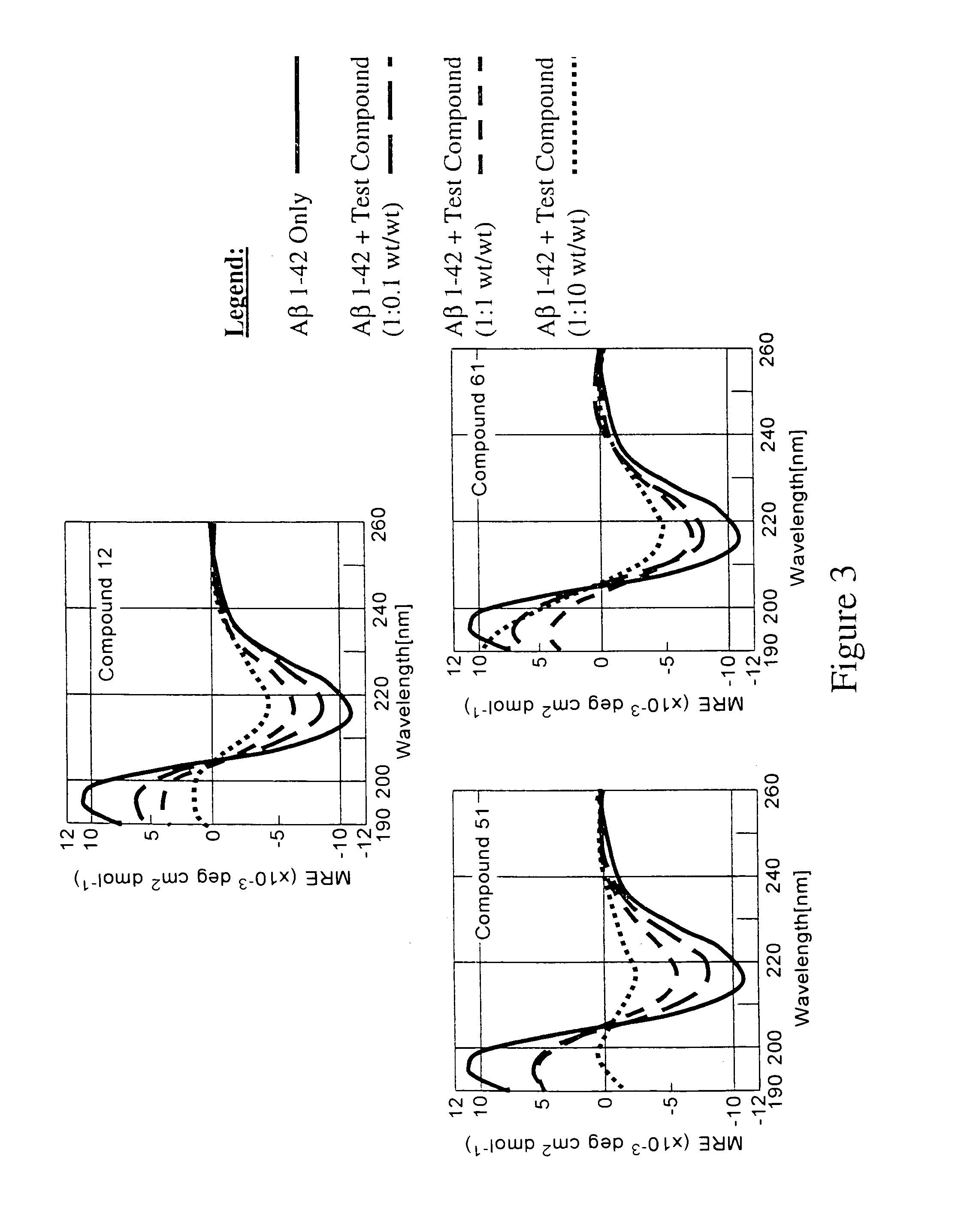
Patents
Literature
413 results about "Synuclein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synucleins are a family of soluble proteins common to vertebrates, primarily expressed in neural tissue and in certain tumors.
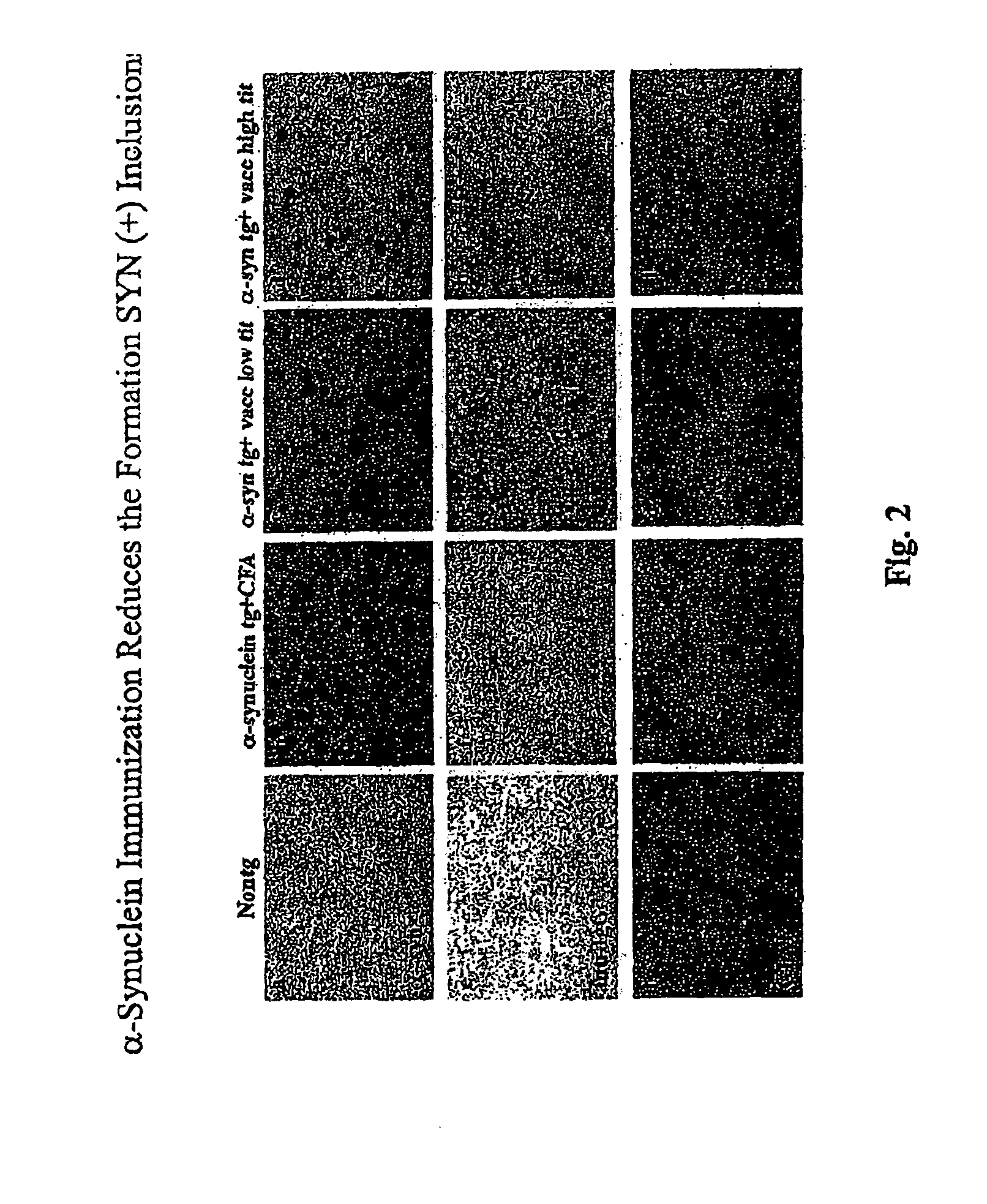
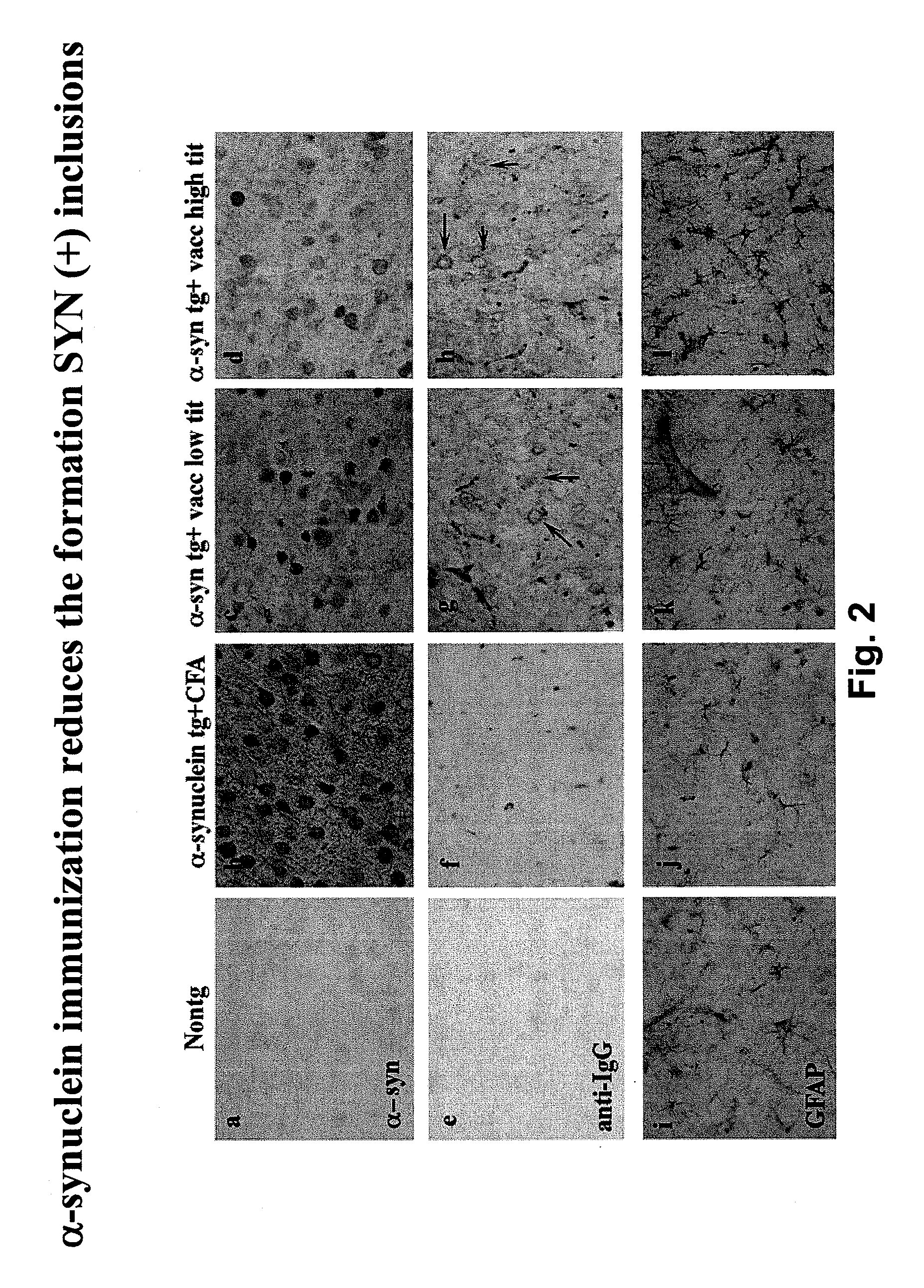
Prevention and treatment of synucleinopathic and amyloidogenic disease
ActiveUS20090208487A1Improves motor characteristicInhibit deteriorationAnimal cellsImmunoglobulins against animals/humansSynucleinLewy body
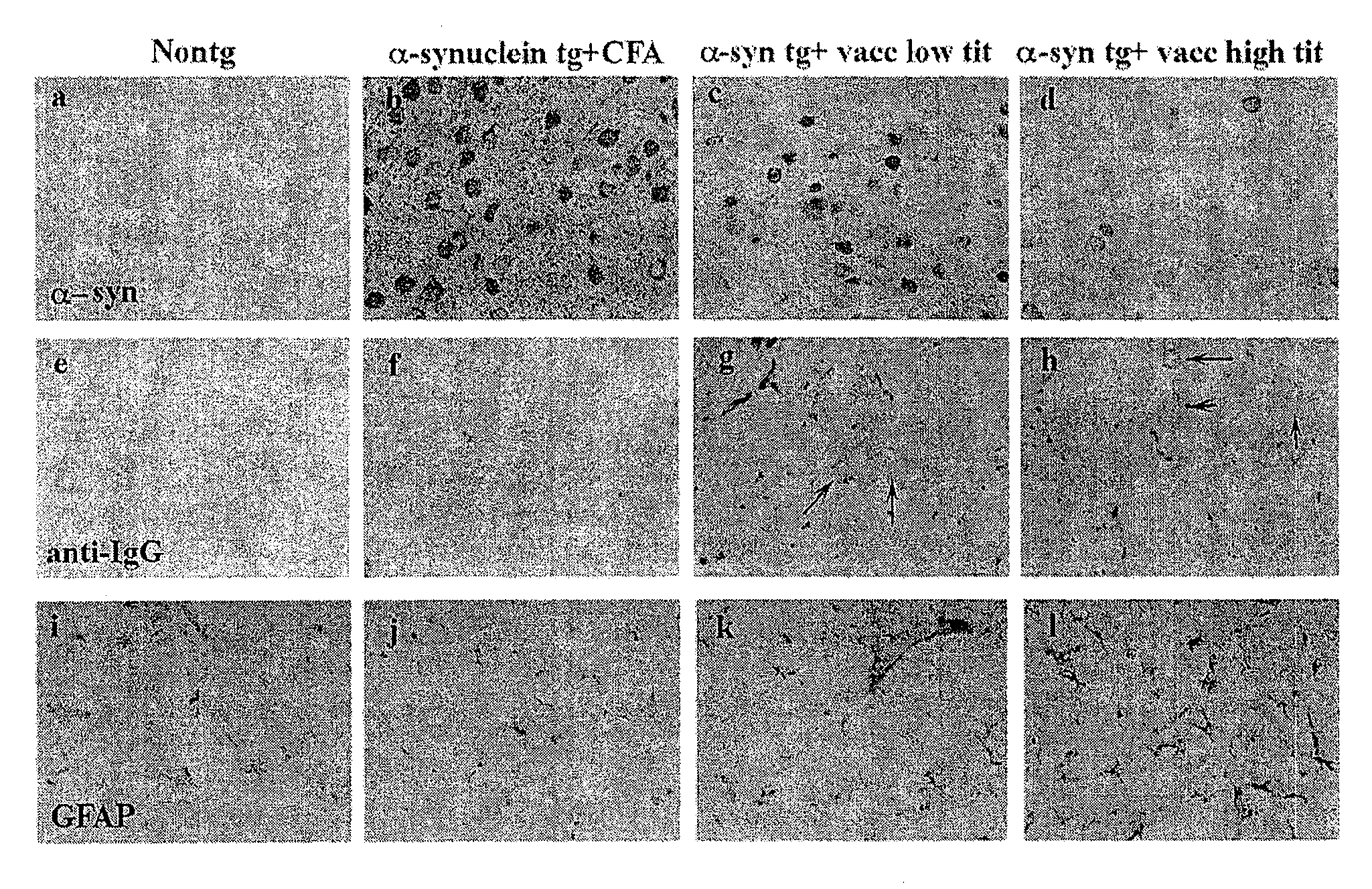
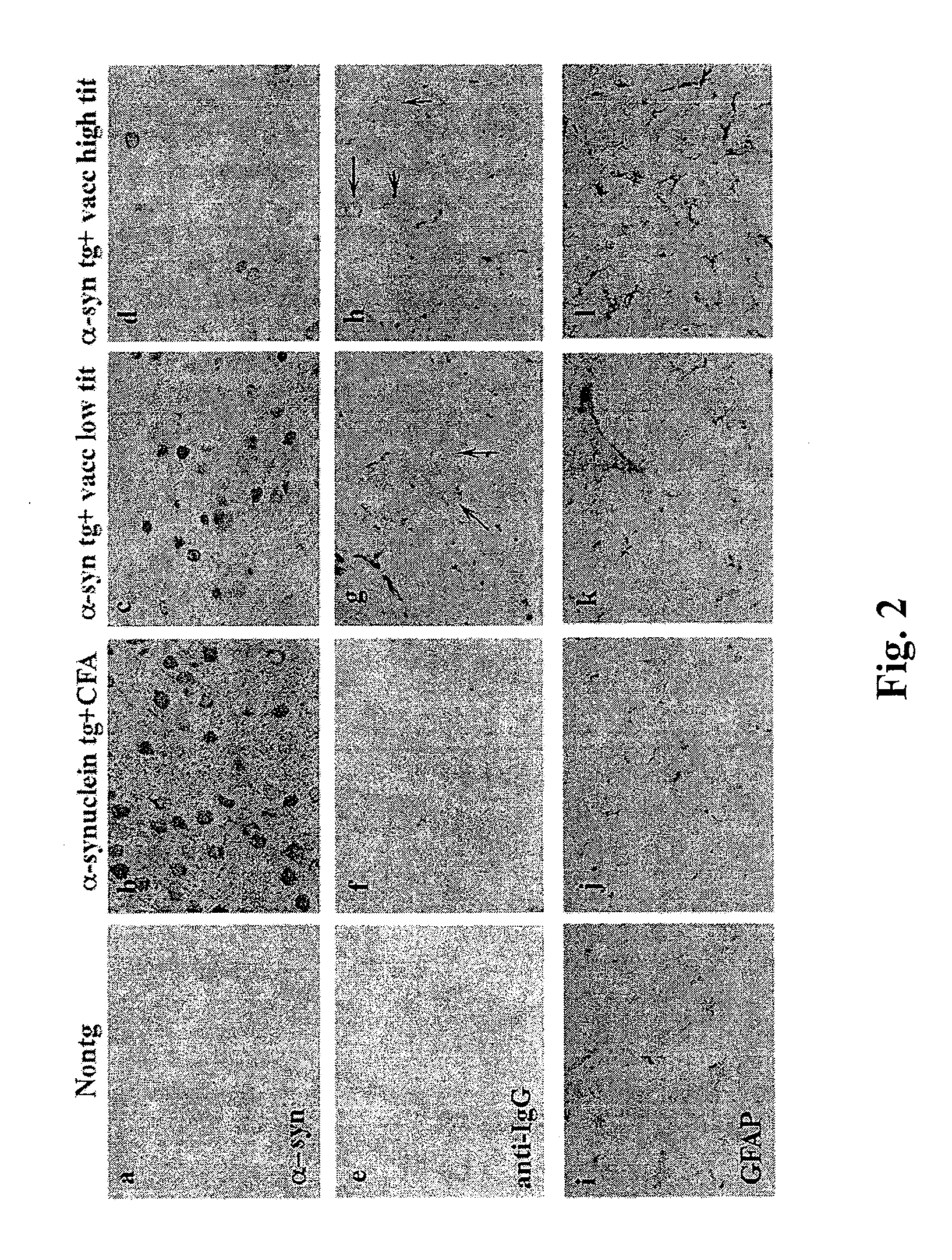
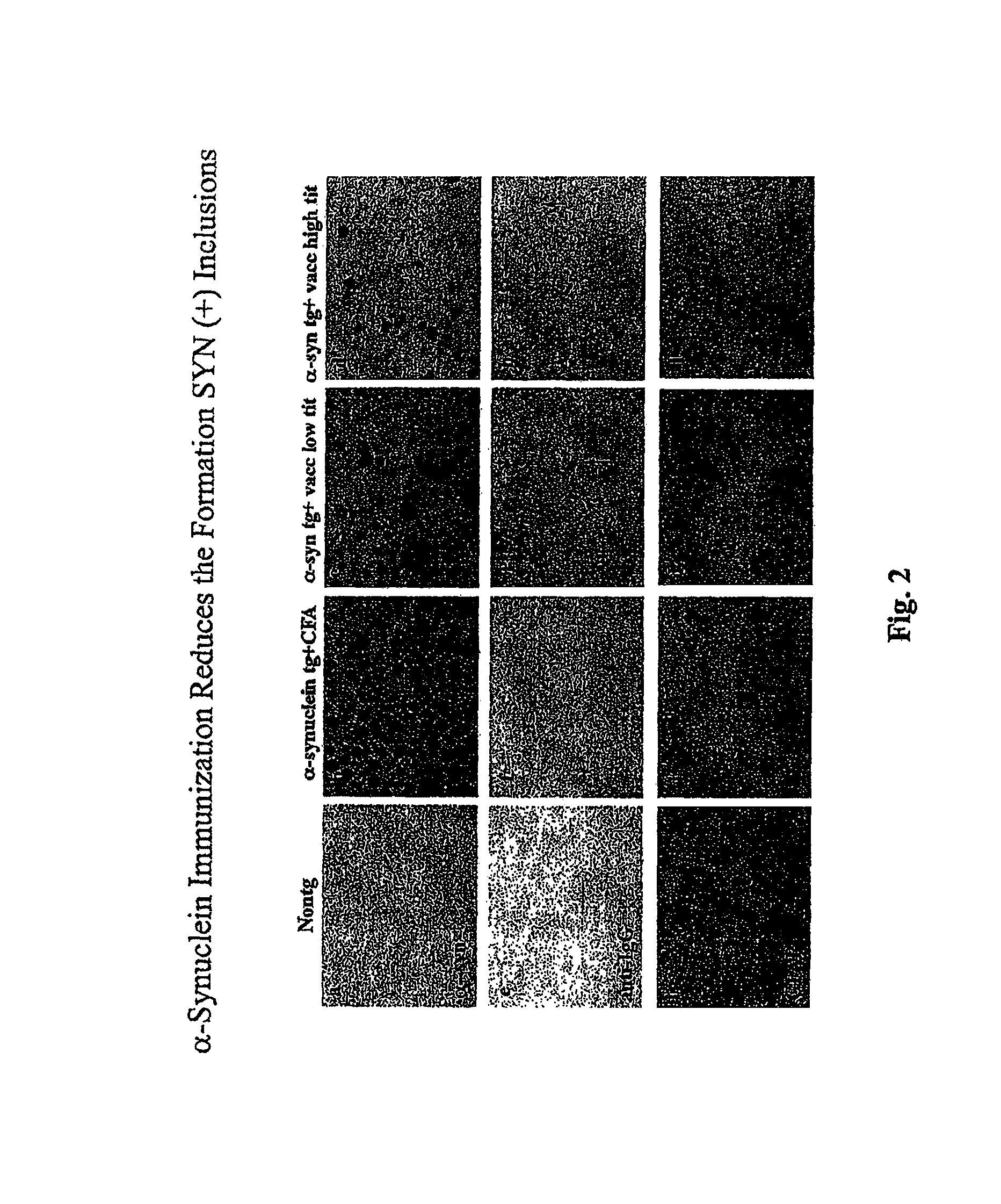
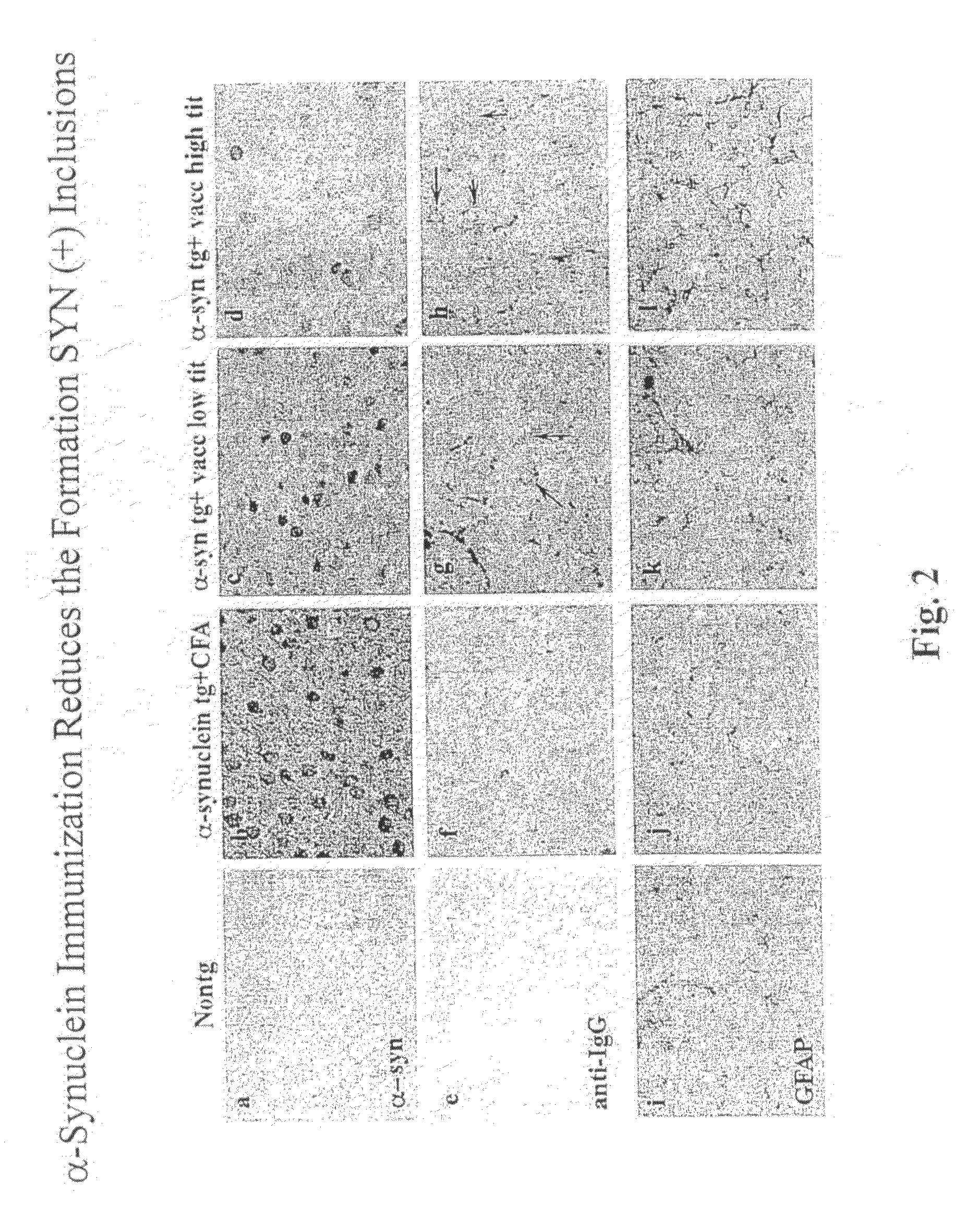
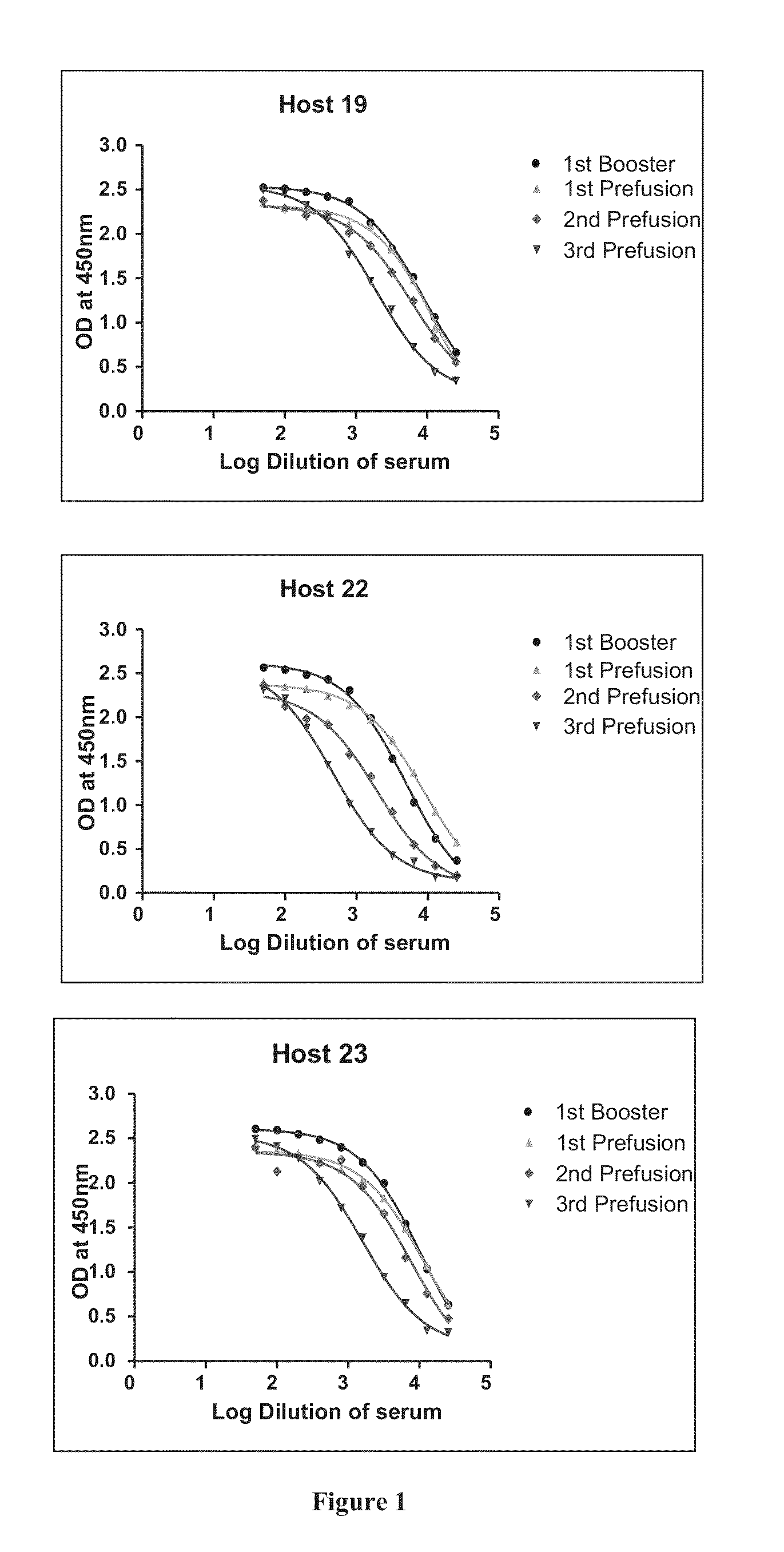
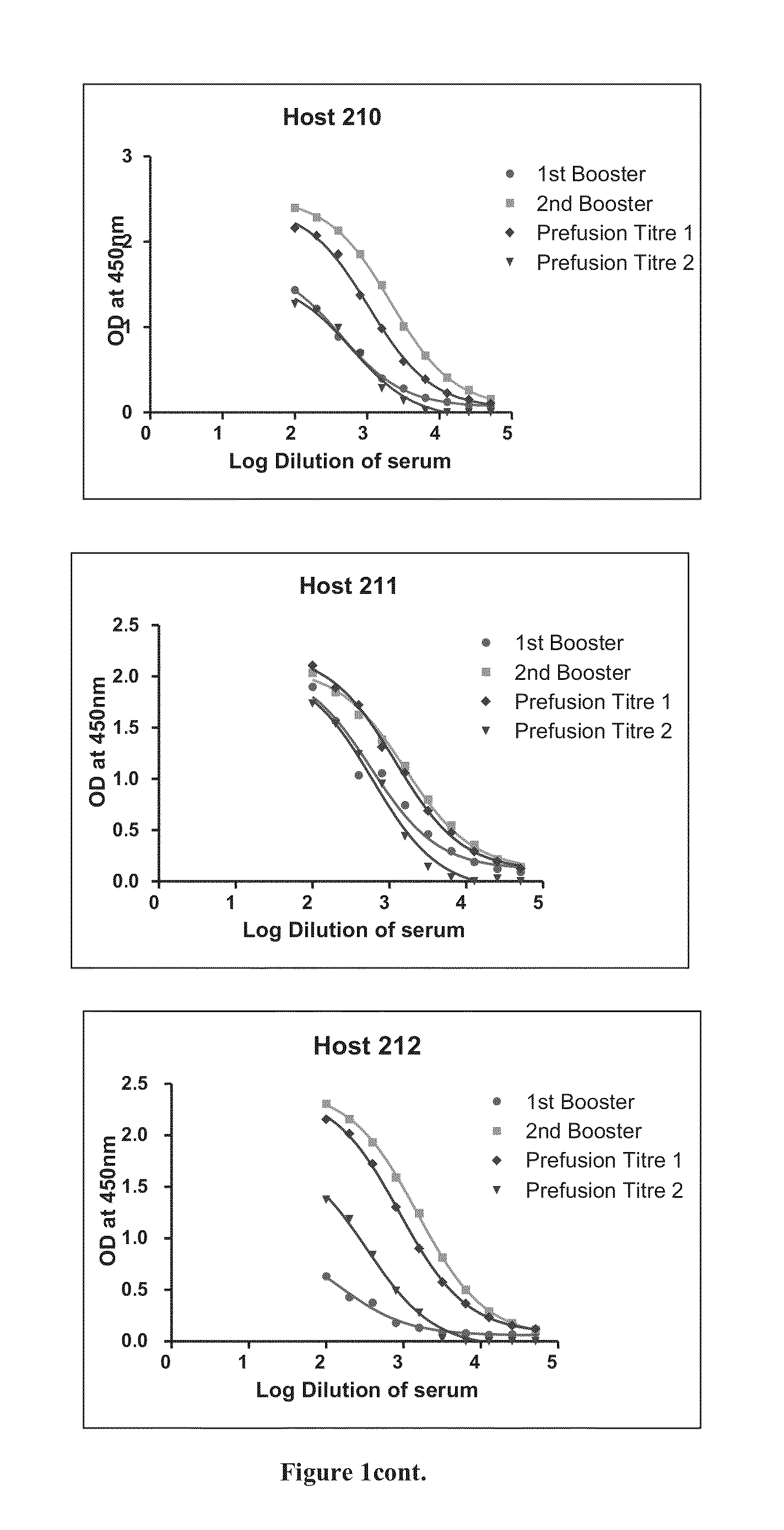
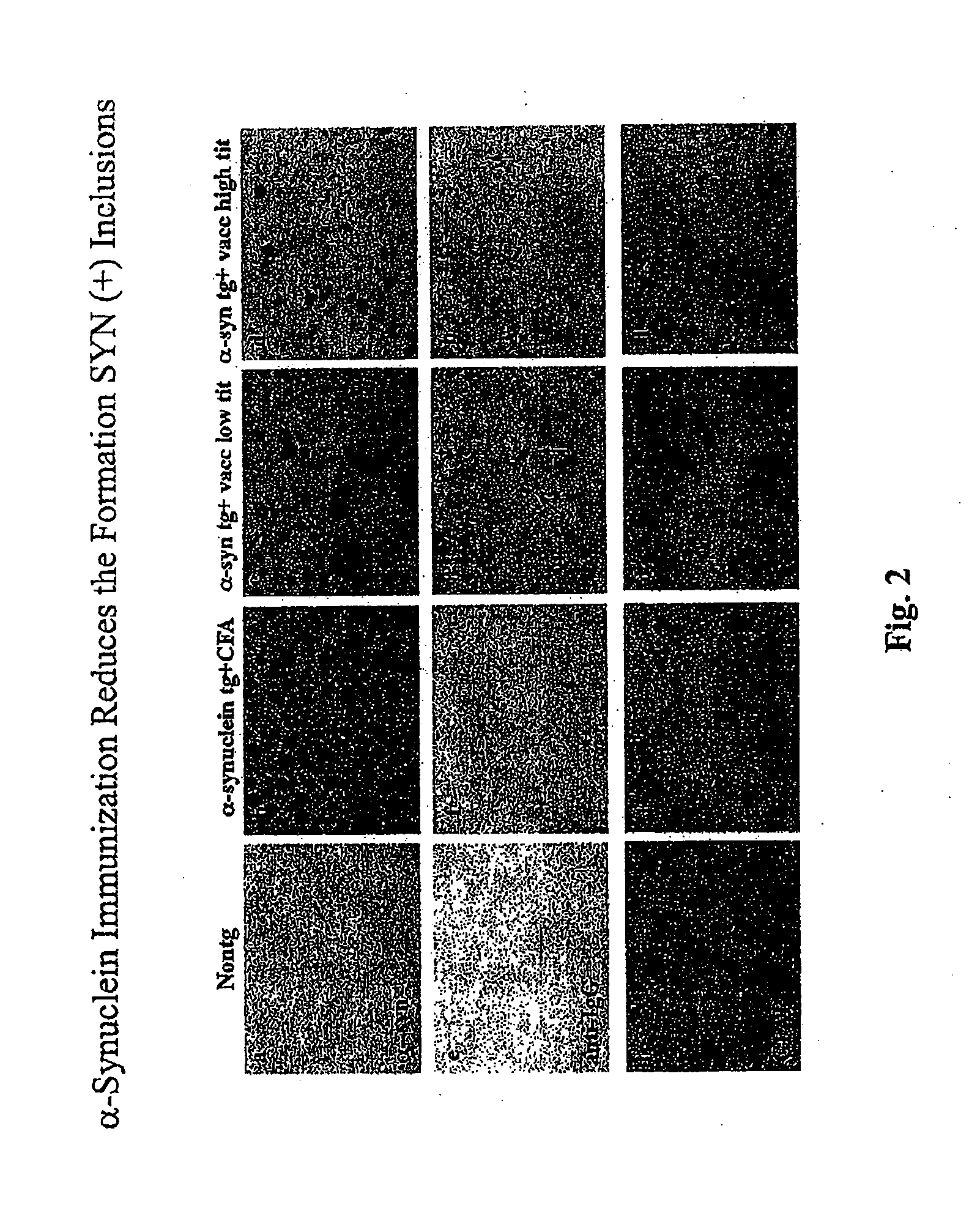
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:RGT UNIV OF CALIFORNIA +1
Prevention and Treatment of Synucleinopathic and Amyloidogenic Disease
InactiveUS20080014194A1Improves motor characteristicInhibit deteriorationNervous disorderImmunoglobulins against animals/humansTherapeutic treatmentAmyloid
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
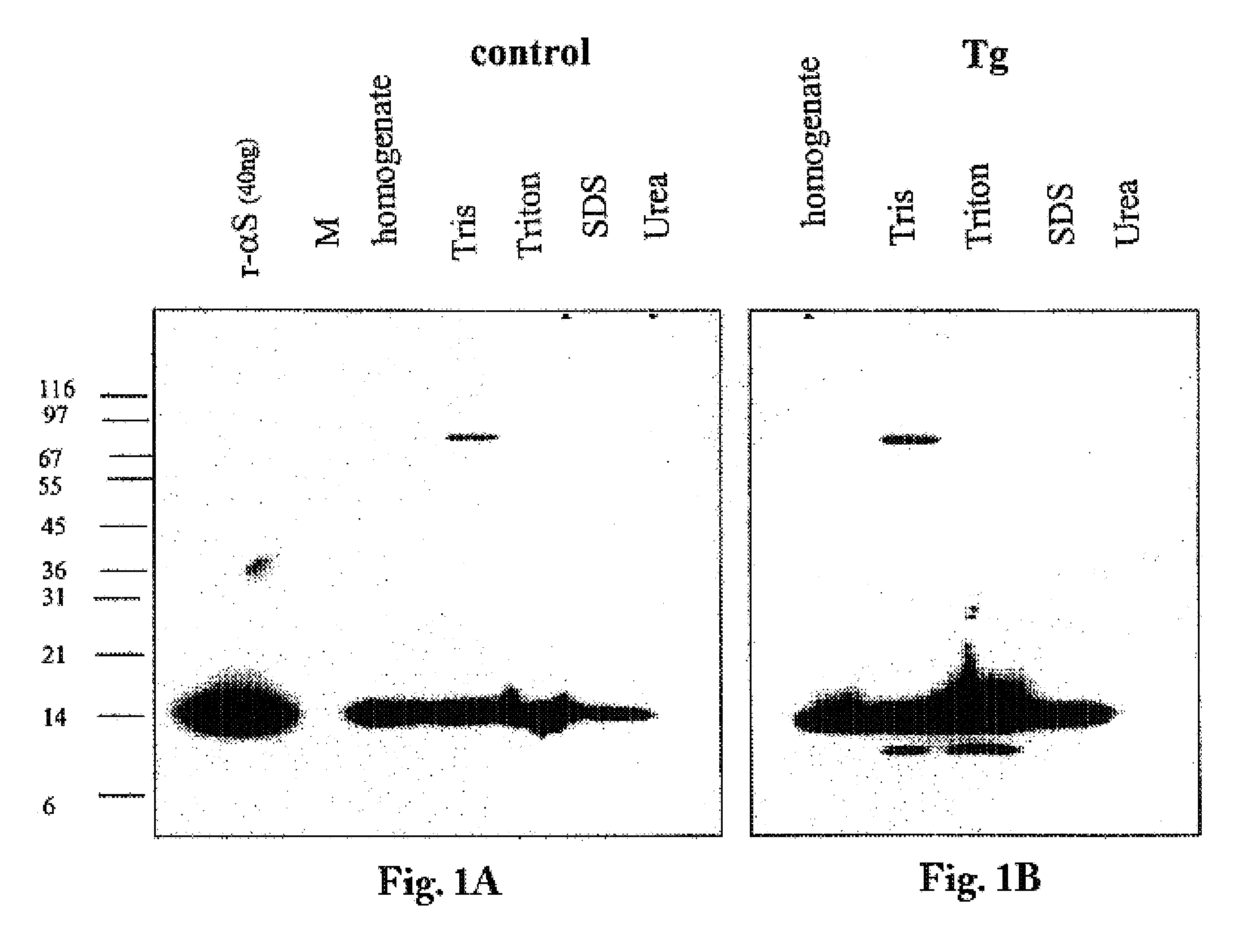
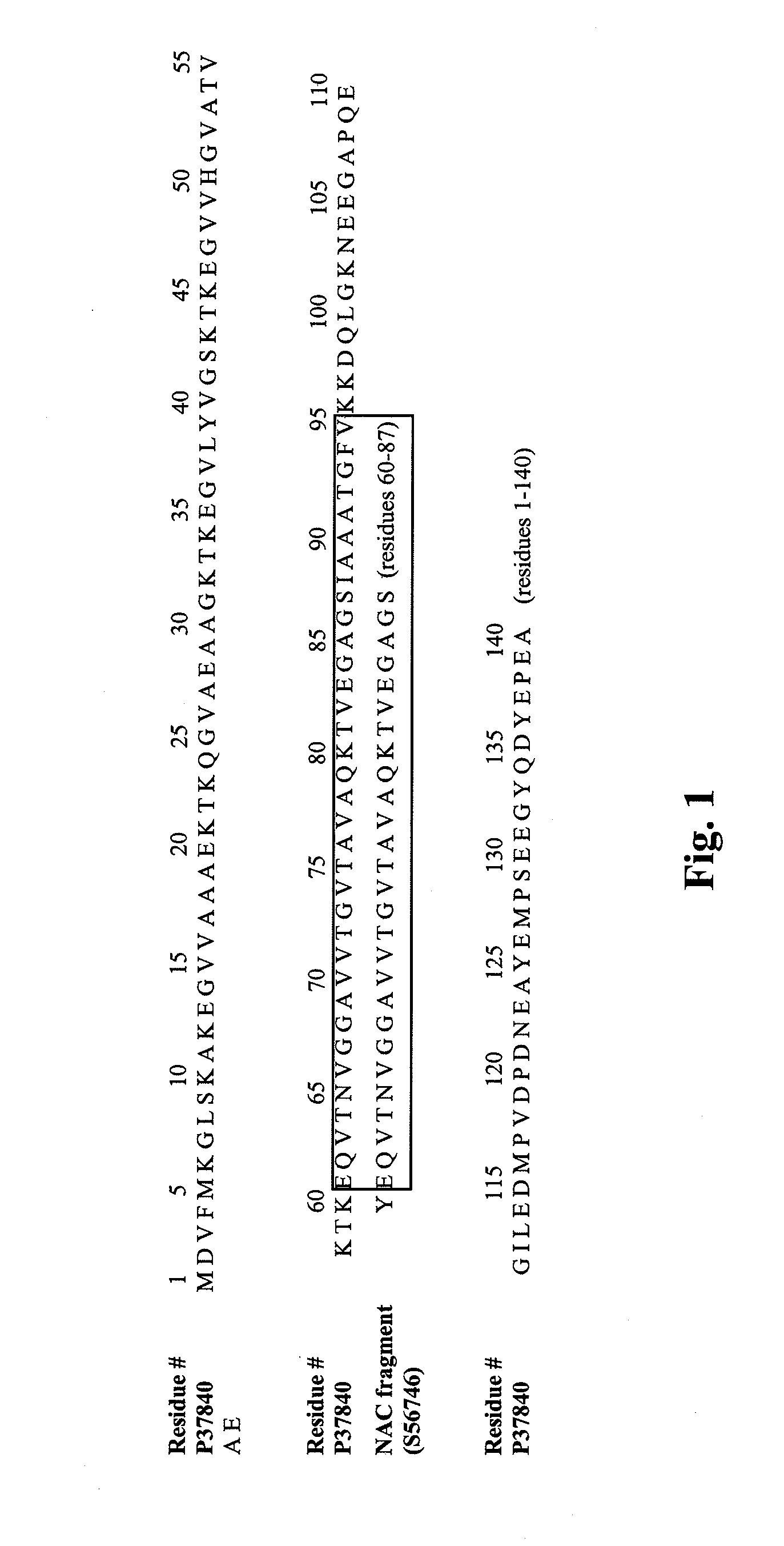
Truncated fragments of alpha-synuclein in Lewy body disease
ActiveUS20050198694A1Useful pharmacological activityBiocideNervous disorderGel electrophoresisC-terminus
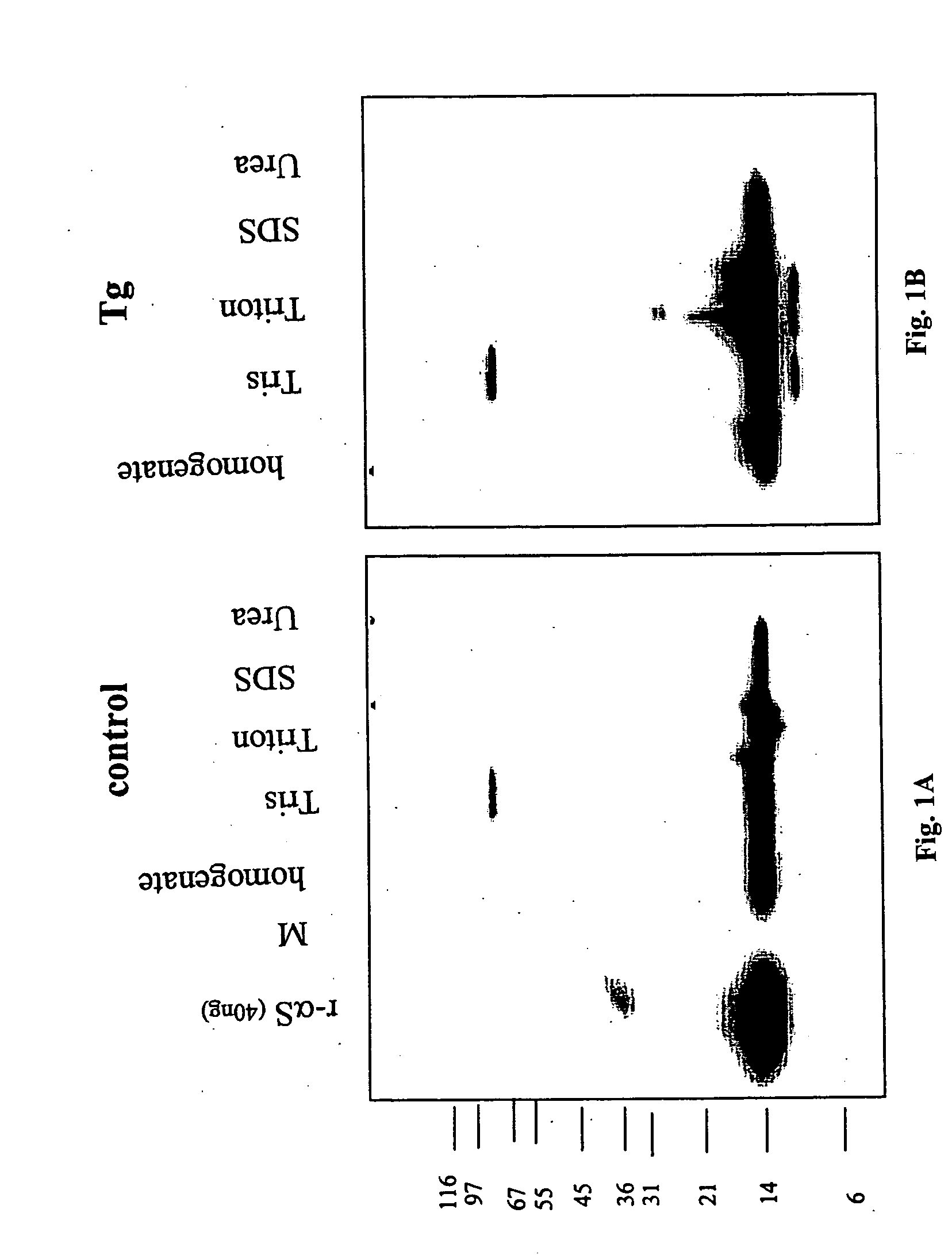
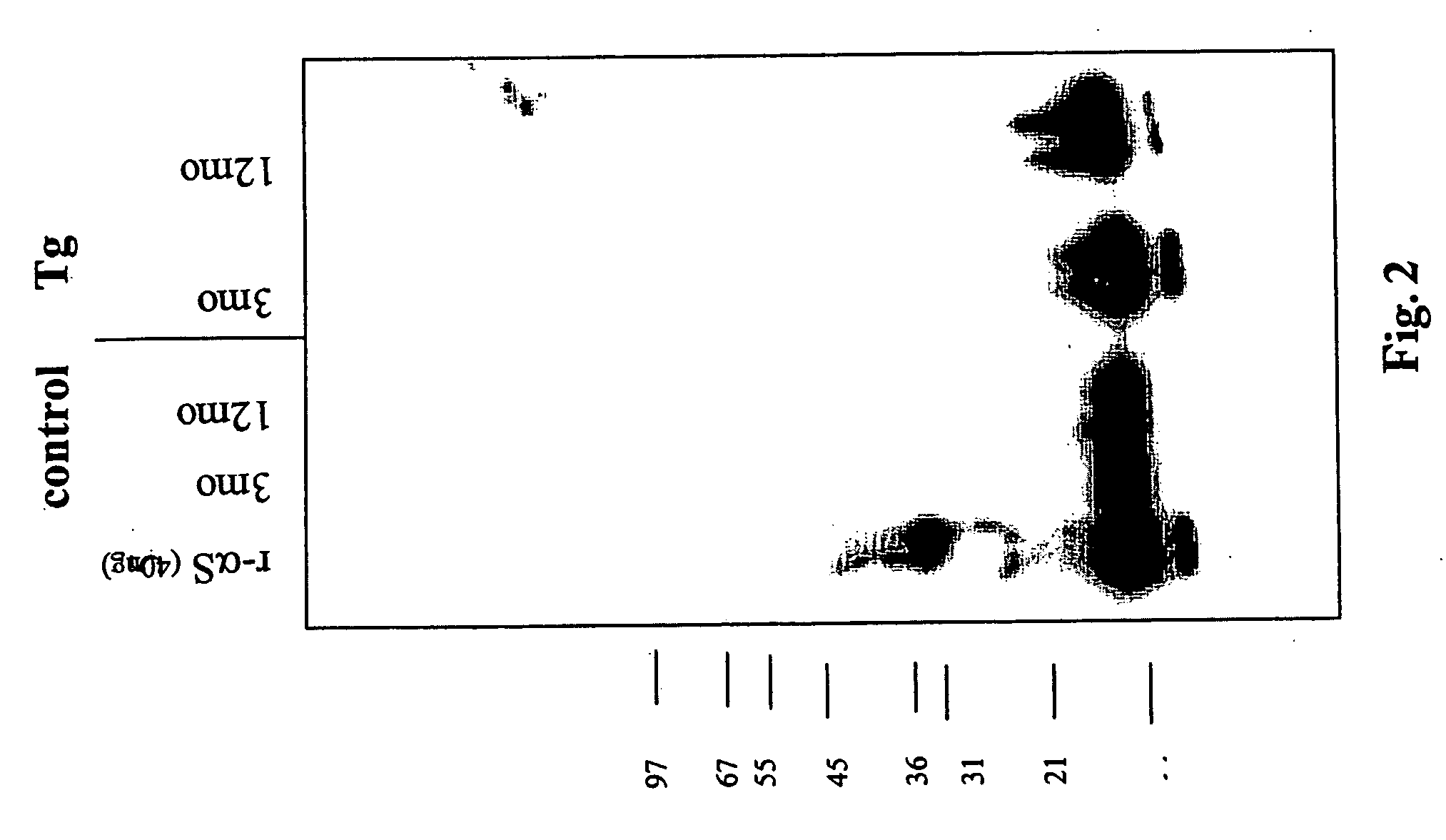
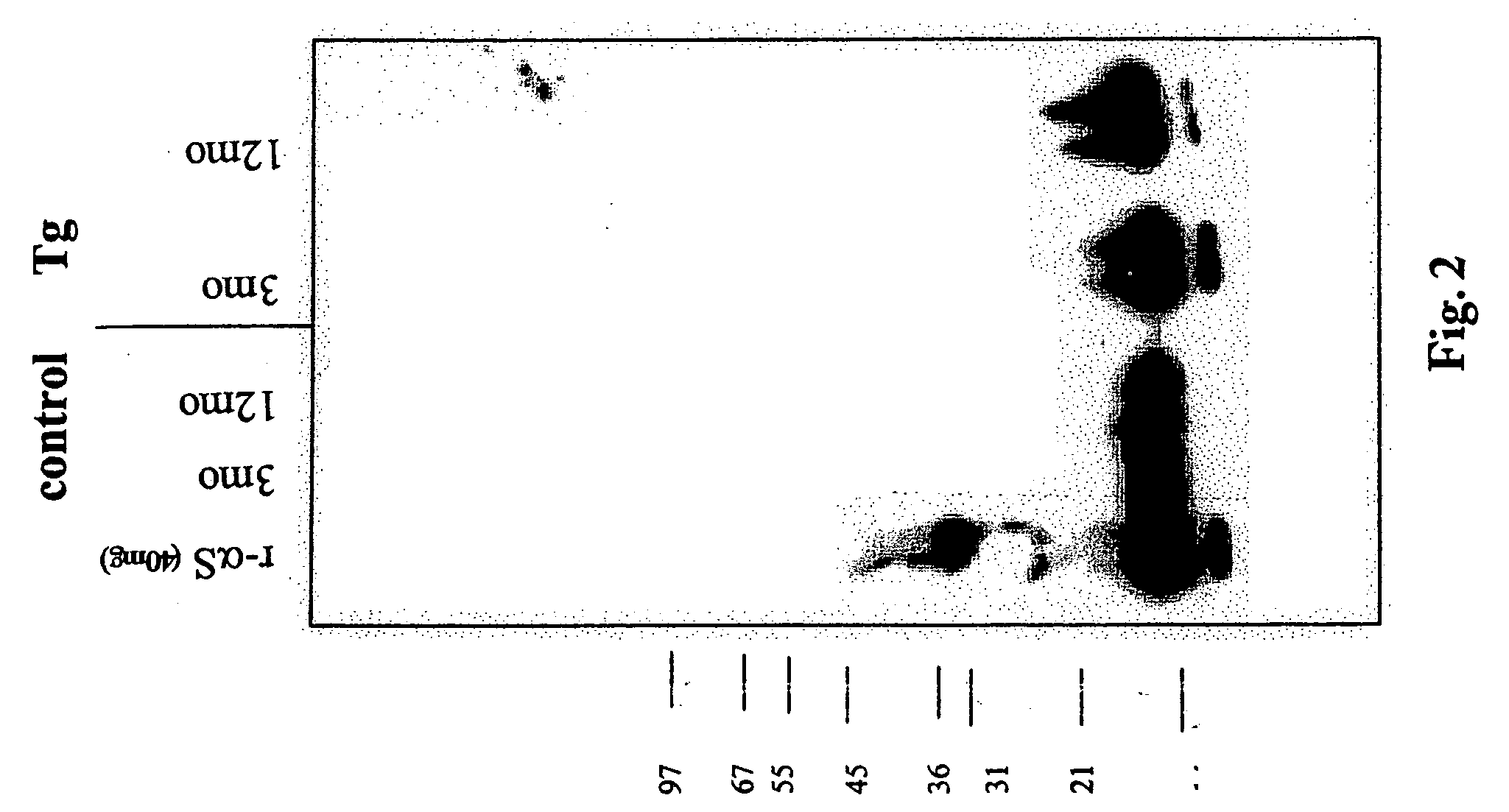
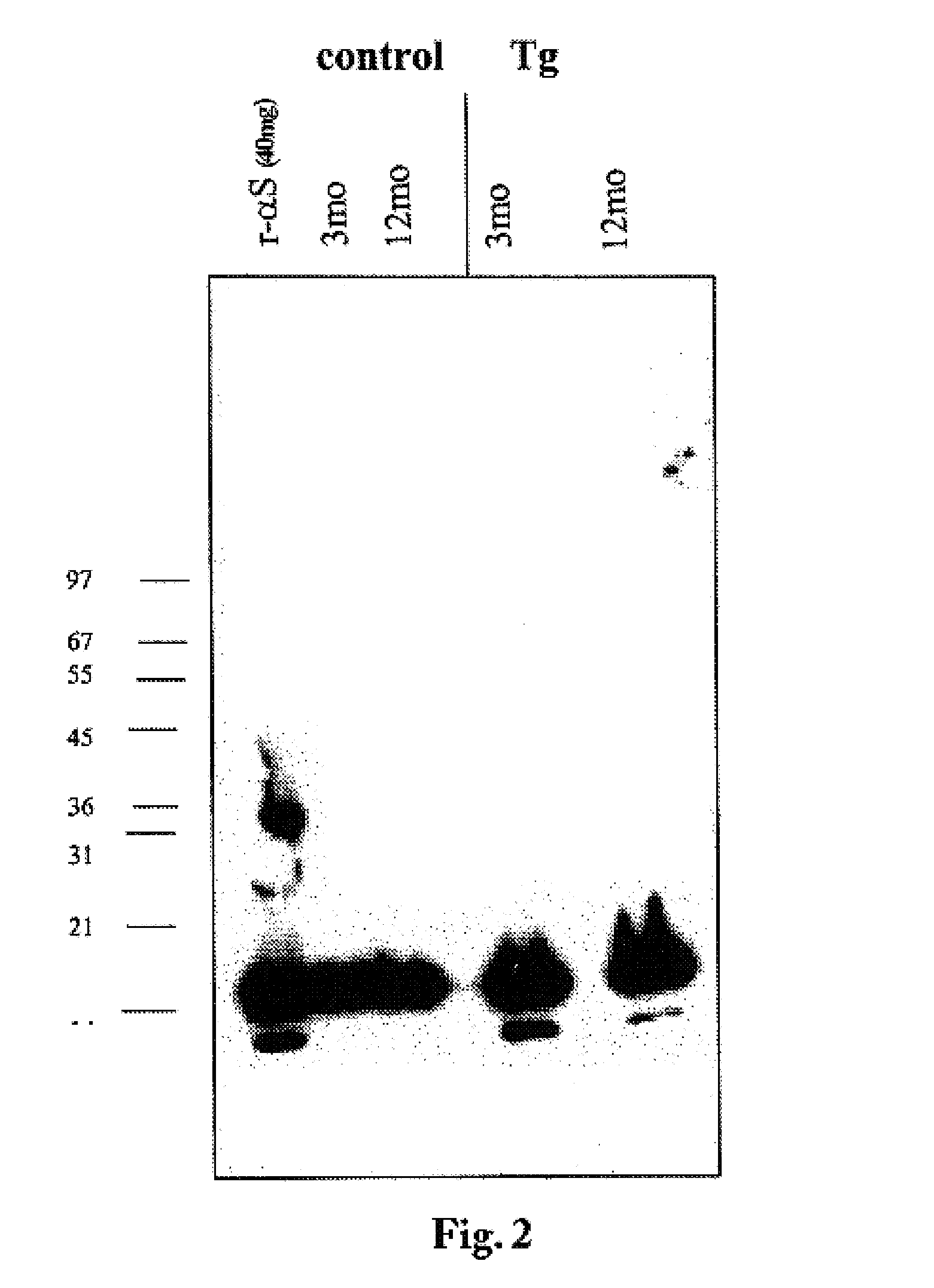
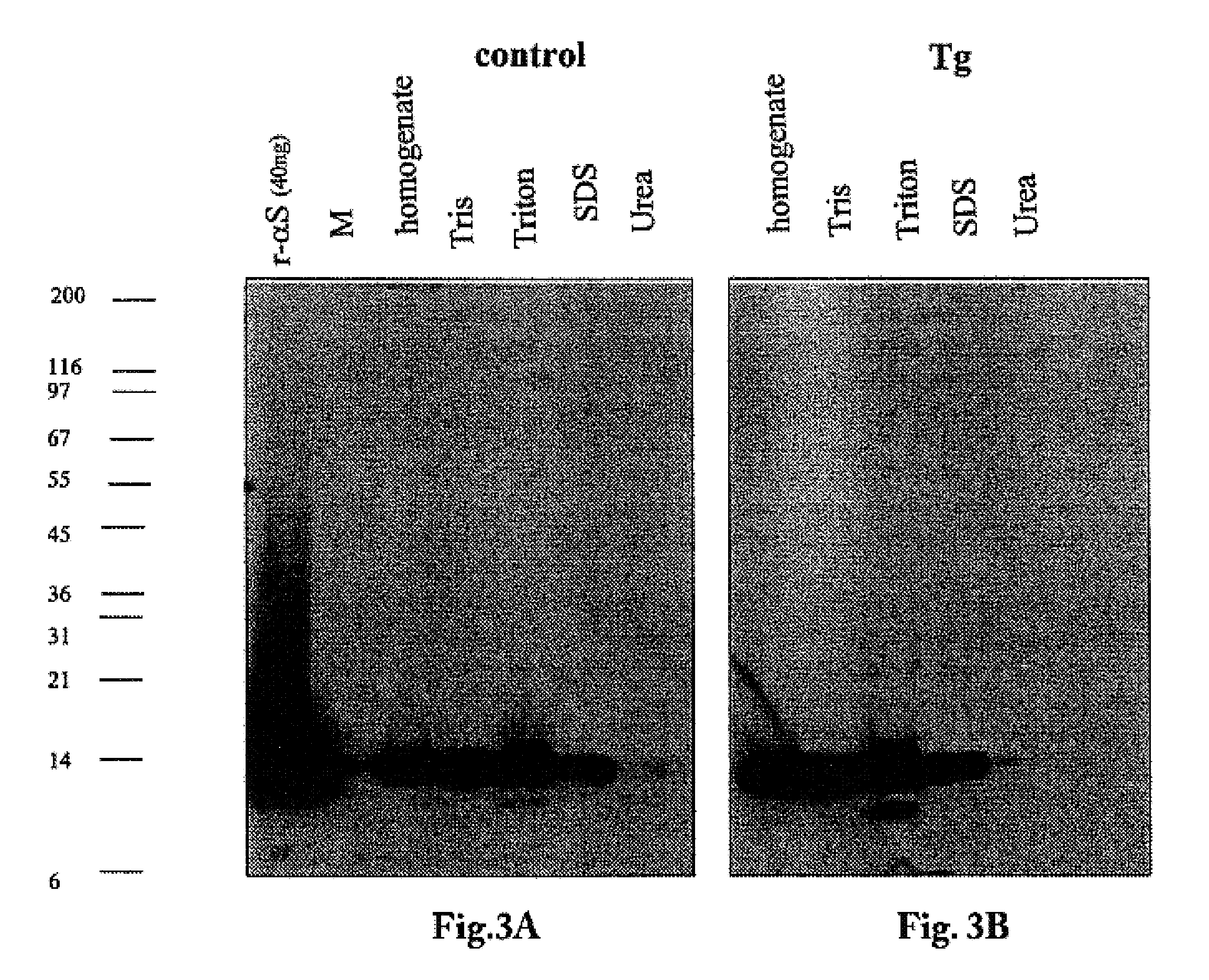
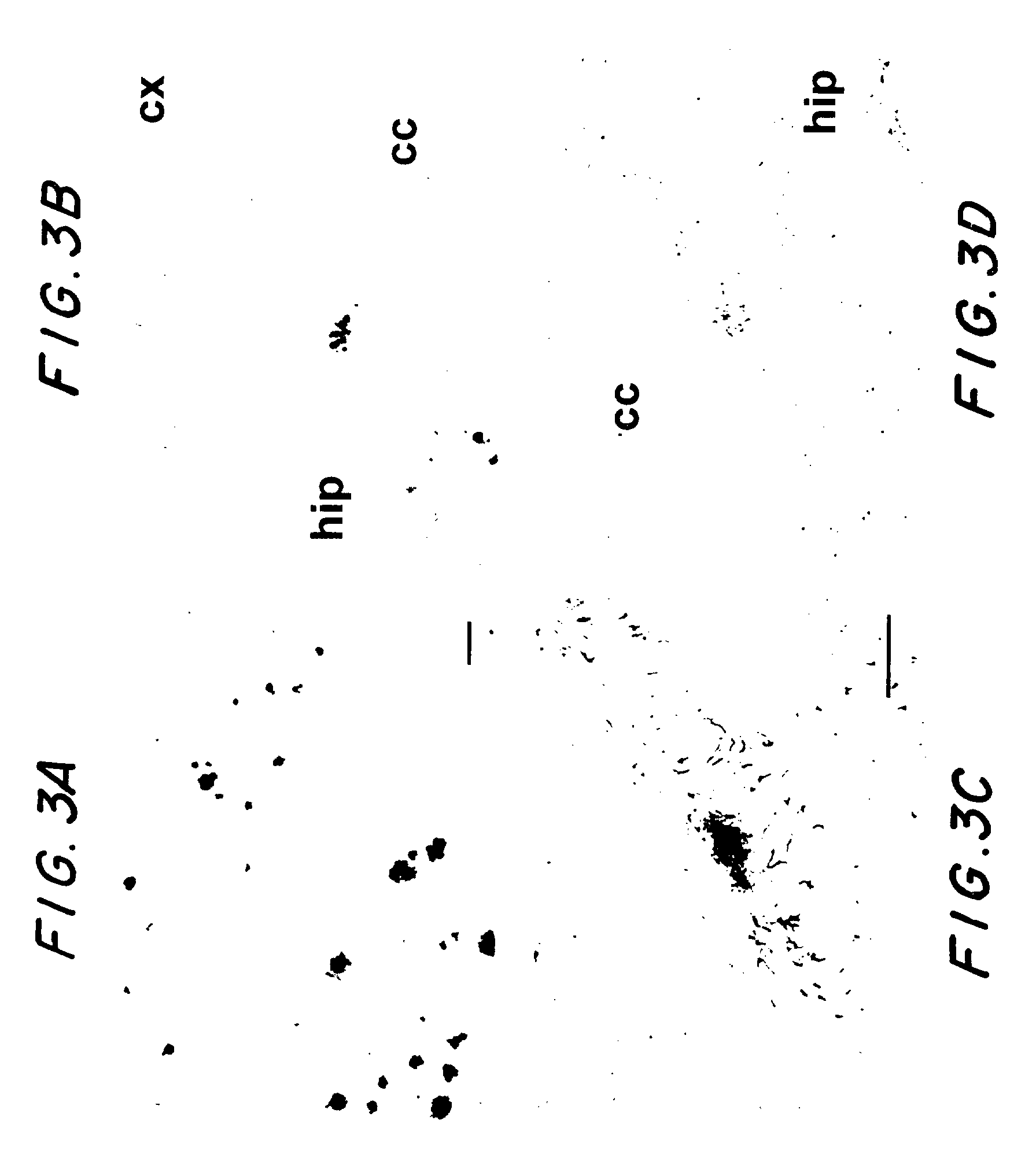
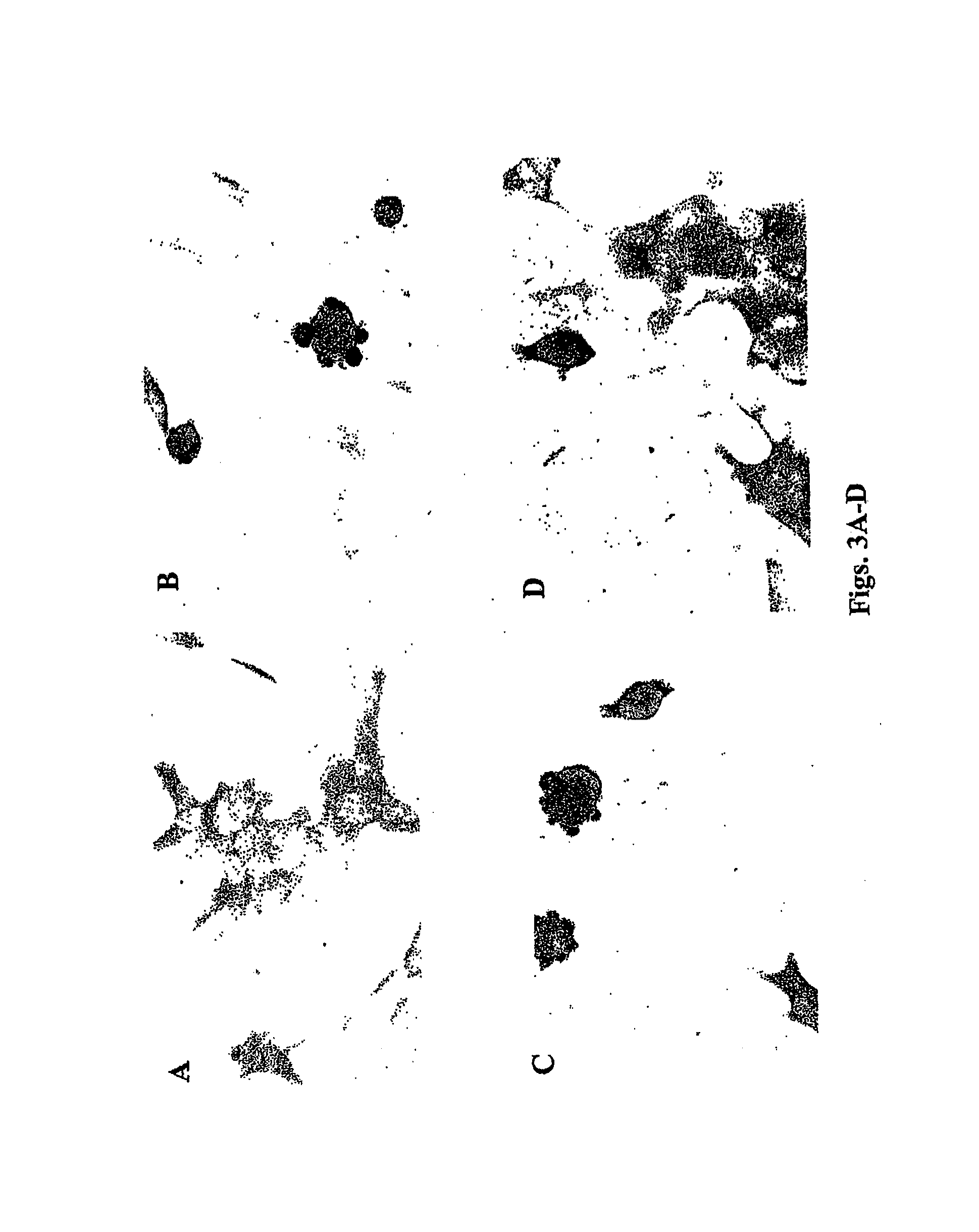
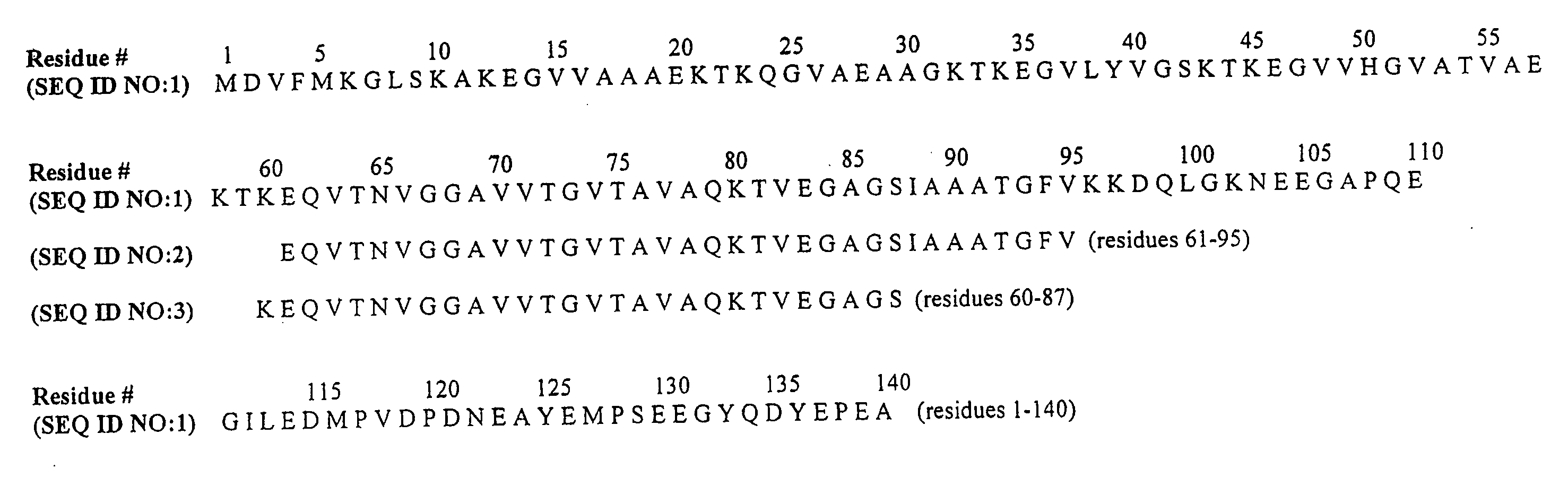
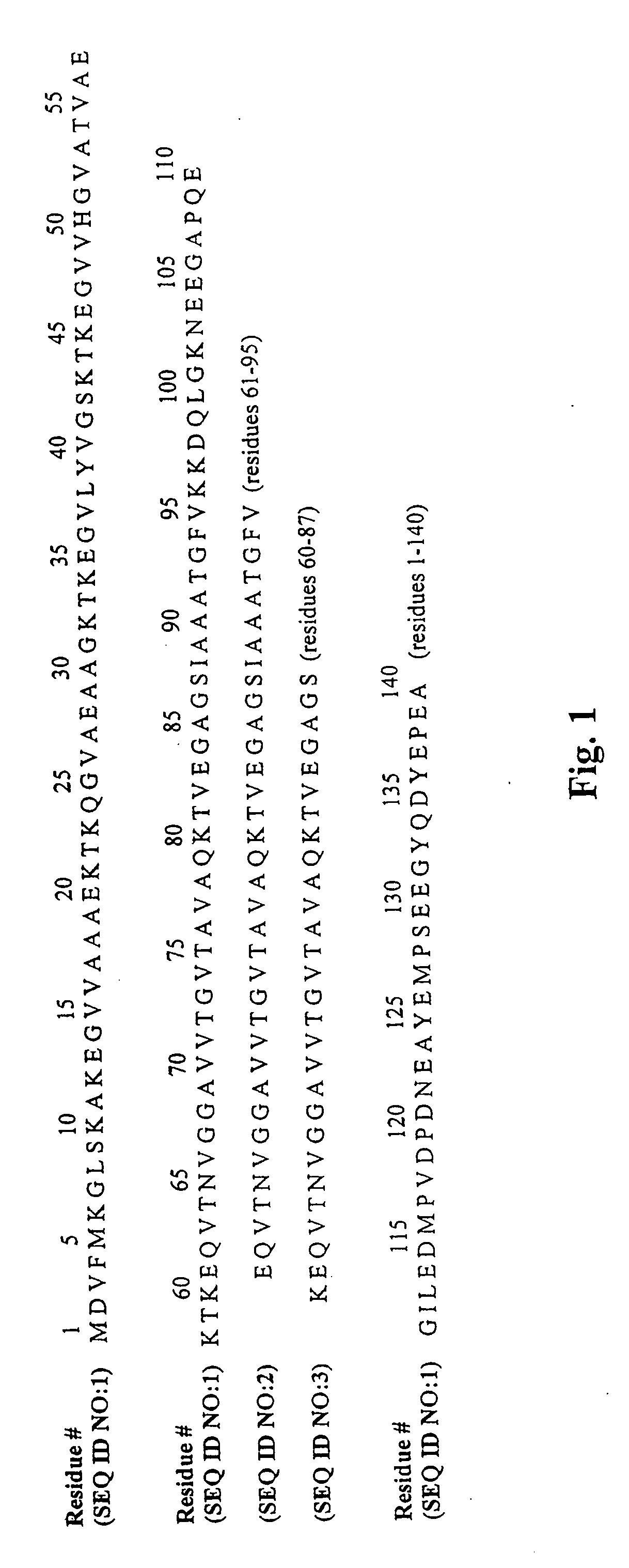
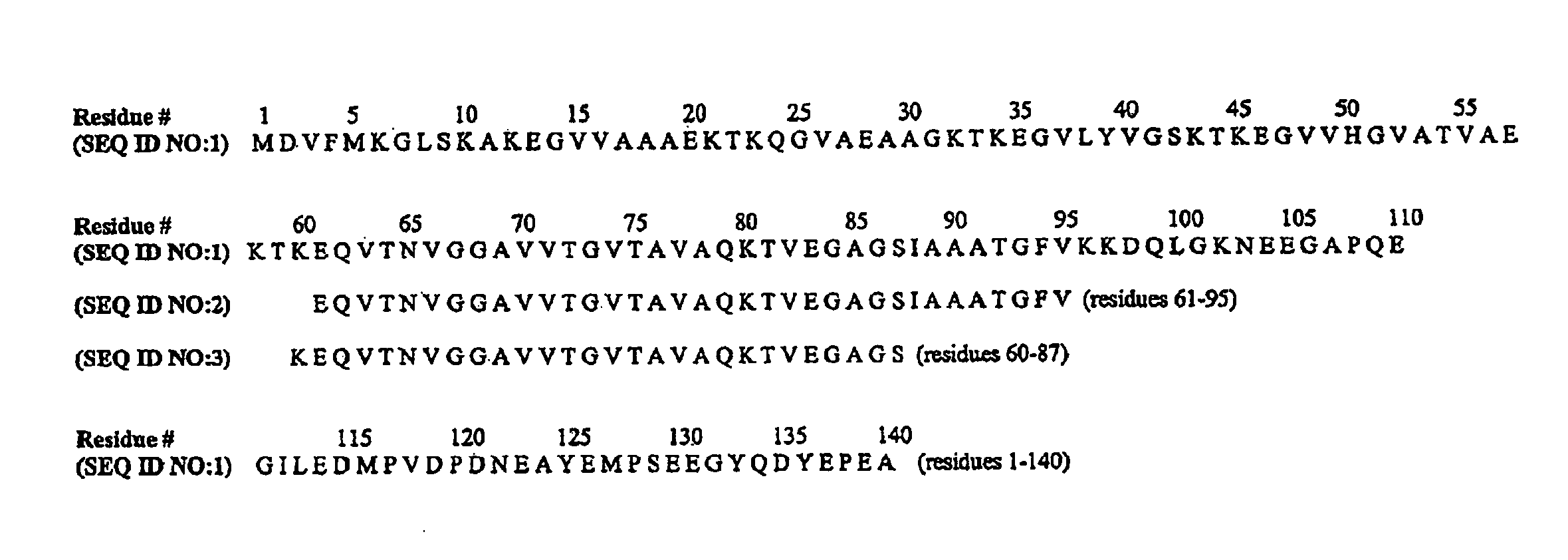
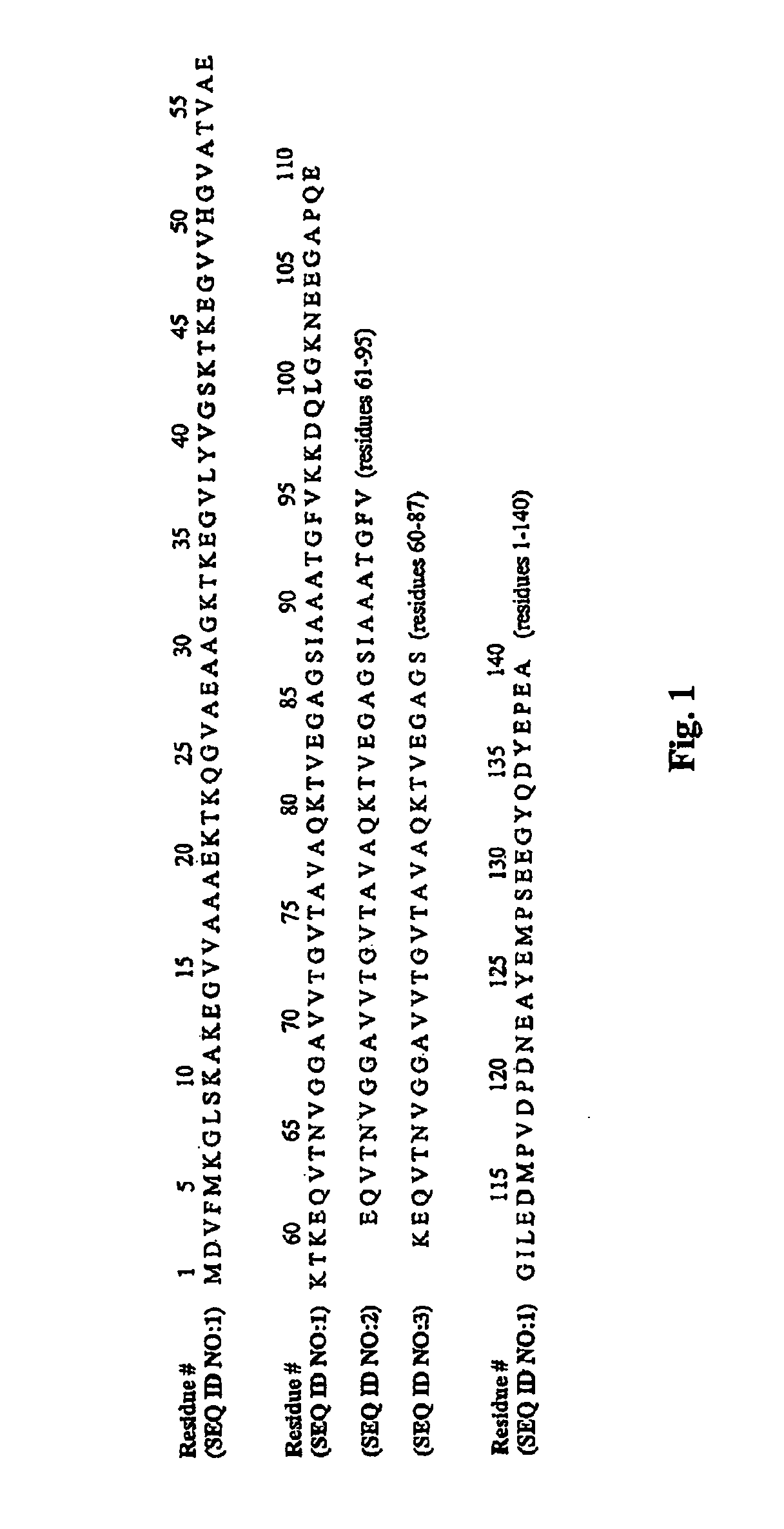
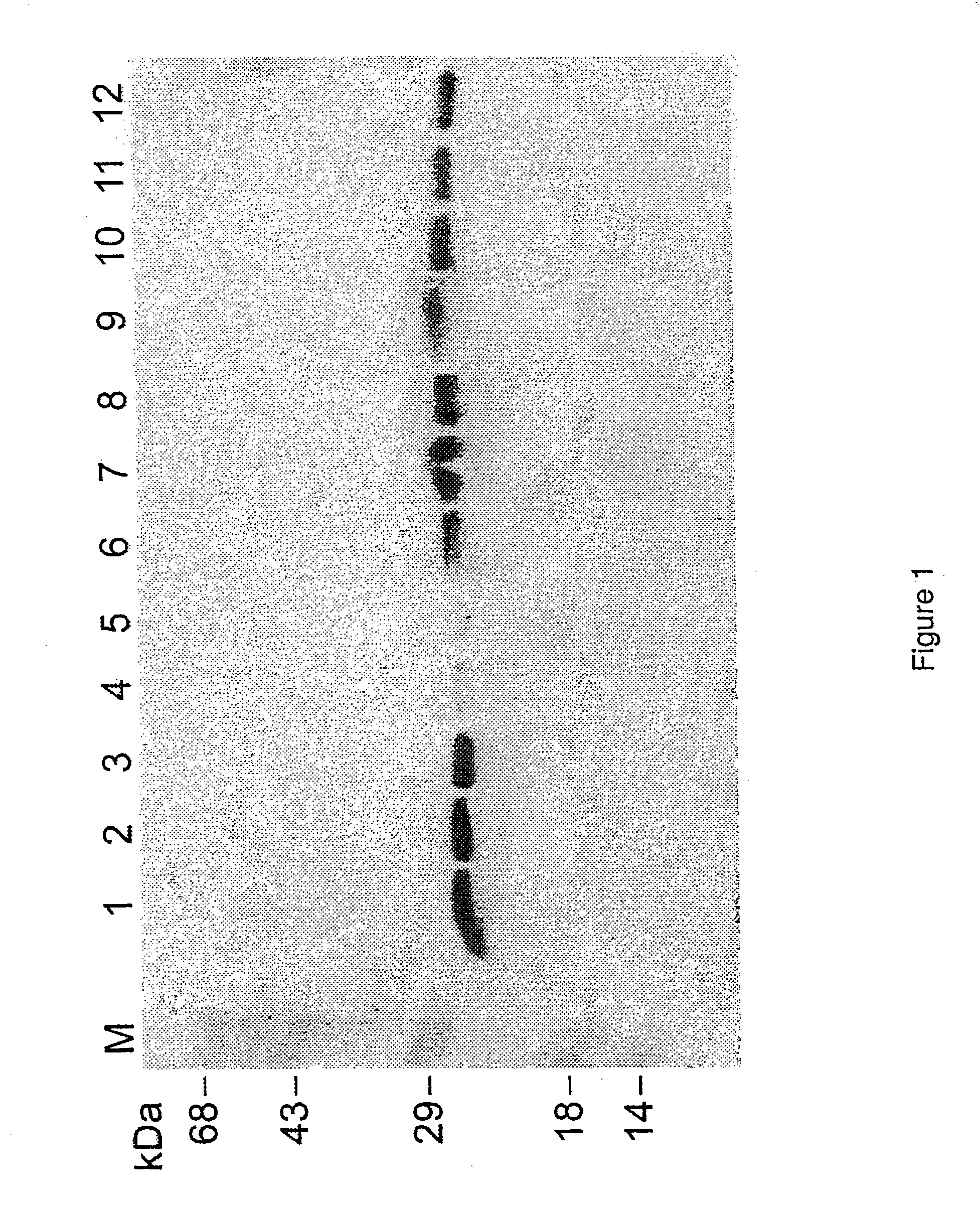
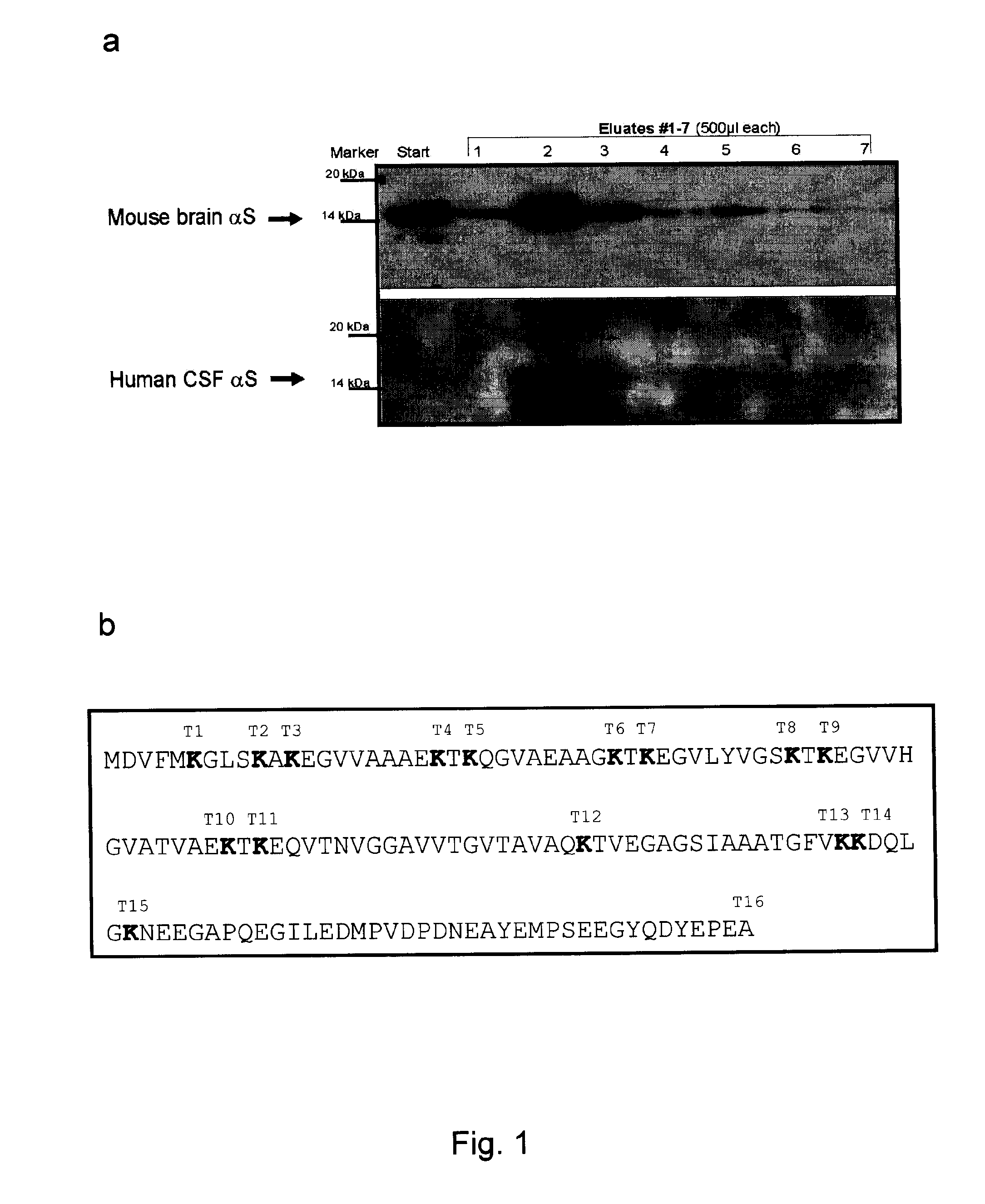
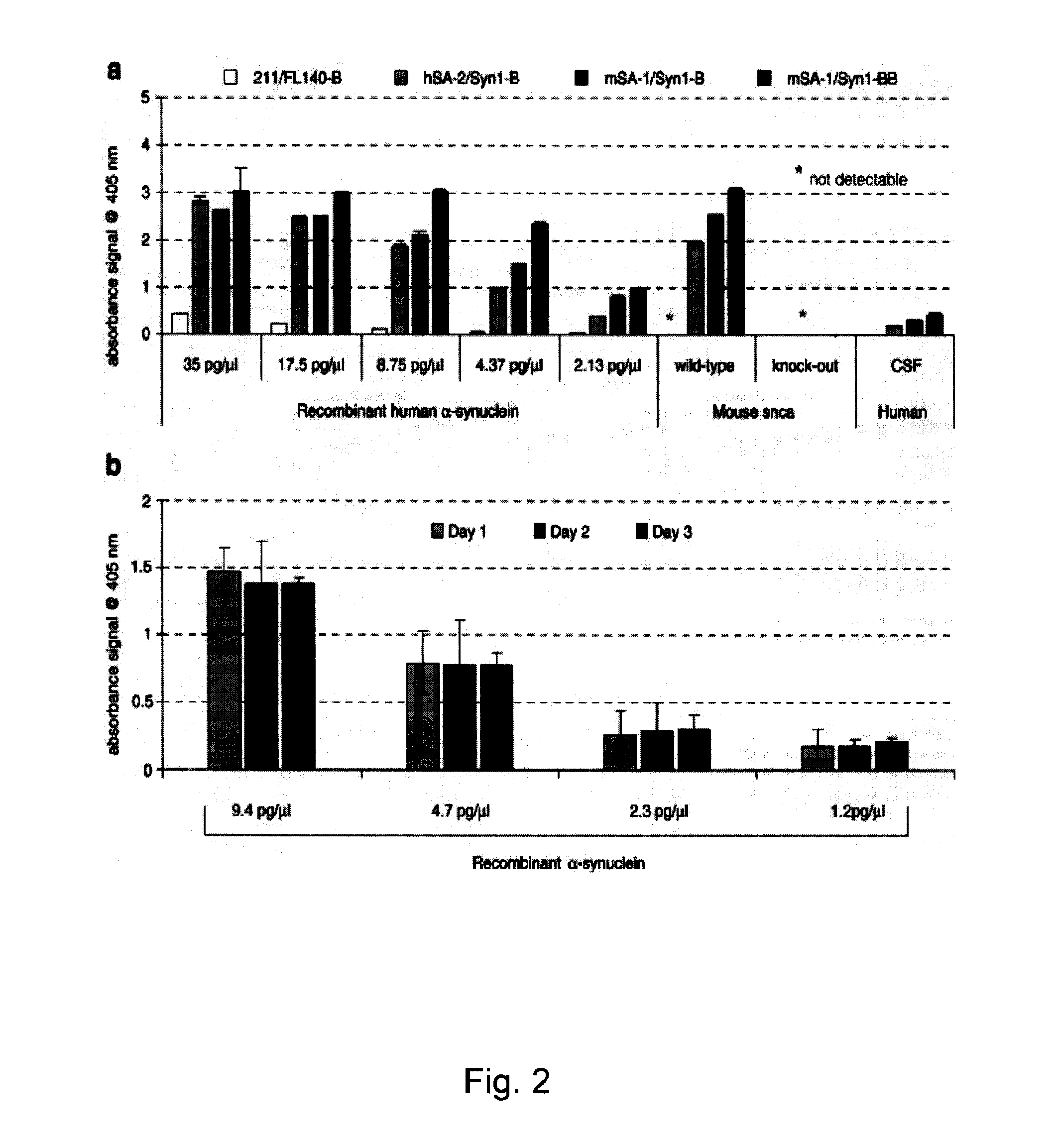
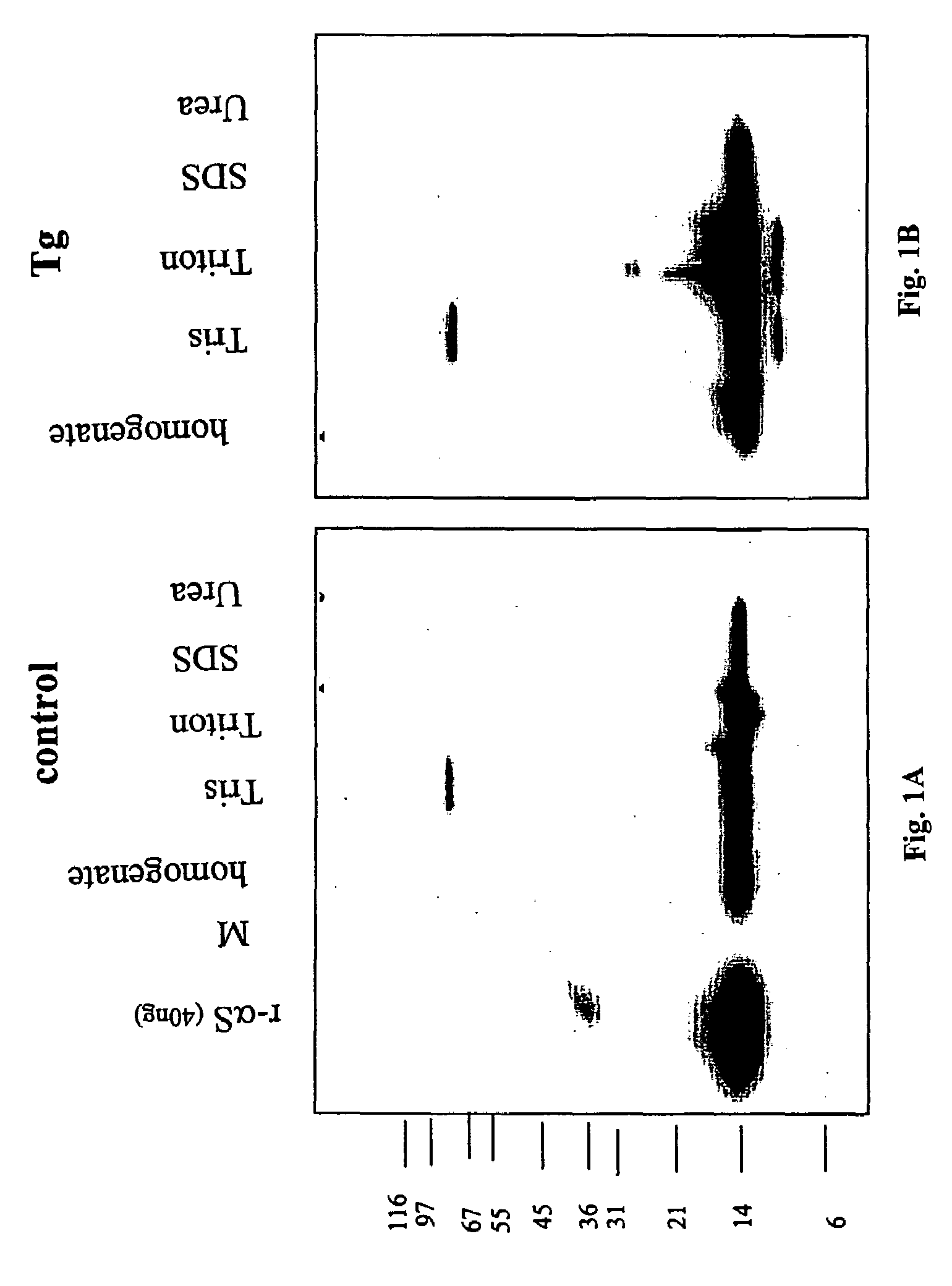
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to fill-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:TRUSTEES OF FLINDERS UNIV +2
Compositions for treatment of cancer
ActiveUS20070238667A1Reduces SNCG-mediated resistanceInhibit cancer cell proliferationPeptide/protein ingredientsAntibody mimetics/scaffoldsAnkyrin Repeat ProteinCancer therapy
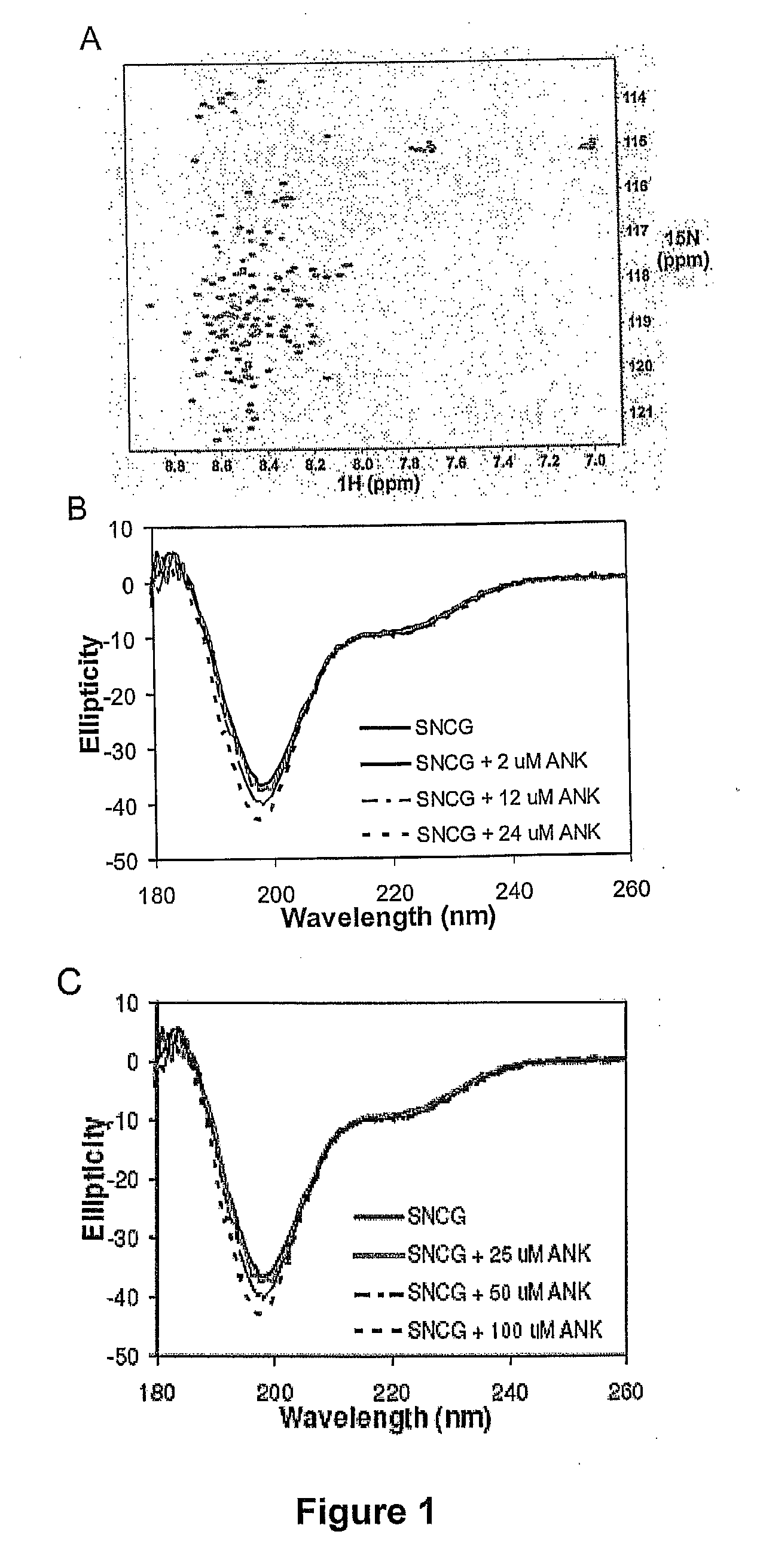
Synthetic peptides containing an ankyrin repeat-like motif or portion thereof and mimetics thereof which interact with synuclein-gamma (SNCG) and reduce SNCG-mediated resistance of SNCG-expressing cancer cells to treatment with anticancer drugs or inhibit tumorigenesis and cancer cell proliferation are provided. Compositions containing these peptides, portions thereof or mimetics thereof are also provided. Methods for use of these peptides or portions thereof, compositions, and mimetics thereof in potentiating efficacy of anticancer drugs, in particular microtubule inhibitors and hormonal cancer therapies, and in treating cancer are also provided.
Owner:SINGH VINAY K +1
Truncated fragments of alpha-synuclein in Lewy Body Disease
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:PROTHENA BIOSCI LTD
Prevention And Treatment of Synucleinopathic And Amyloidogenic Disease
ActiveUS20080175838A1Improves motor characteristicInhibit deteriorationAnimal cellsNervous disorderSynucleinLewy body
Owner:PROTHENA BIOSCI LTD +1
Alpha-Synuclein Antibodies and Methods Related Thereto
Disclosed are antibodies specific for alpha-synuclein conformers and methods related thereto. For example, disclosed are methods of diagnosing a neurodegenerative monitoring a neurodegenerative disease treatment using the disclosed antibodies. Assays, kits, and solid supports related to alpha-synuclein and antibodies specific for alpha-synuclein are also disclosed.
Owner:UNIVERSITY OF ROCHESTER
Treatment and delay of outset of Parkinson's disease
ActiveUS7727957B2Improves motor characteristicInhibit deteriorationNervous disorderPeptide/protein ingredientsTherapeutic treatmentSynuclein
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:RGT UNIV OF CALIFORNIA +1
Antibodies and Vaccines for Use in Therapeutic and Diagnostic Methods for Alpha-Synuclein-Related Disorders
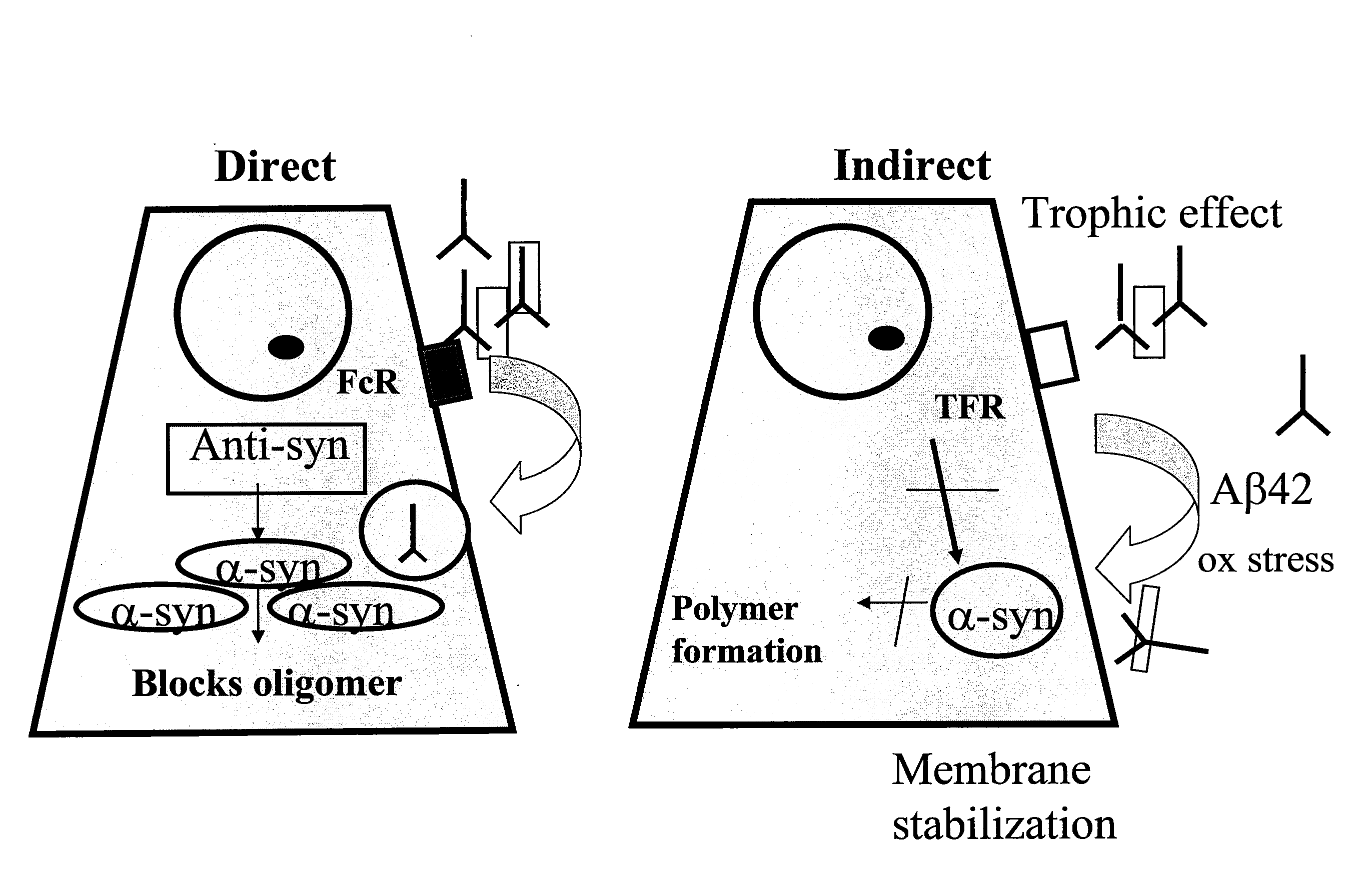
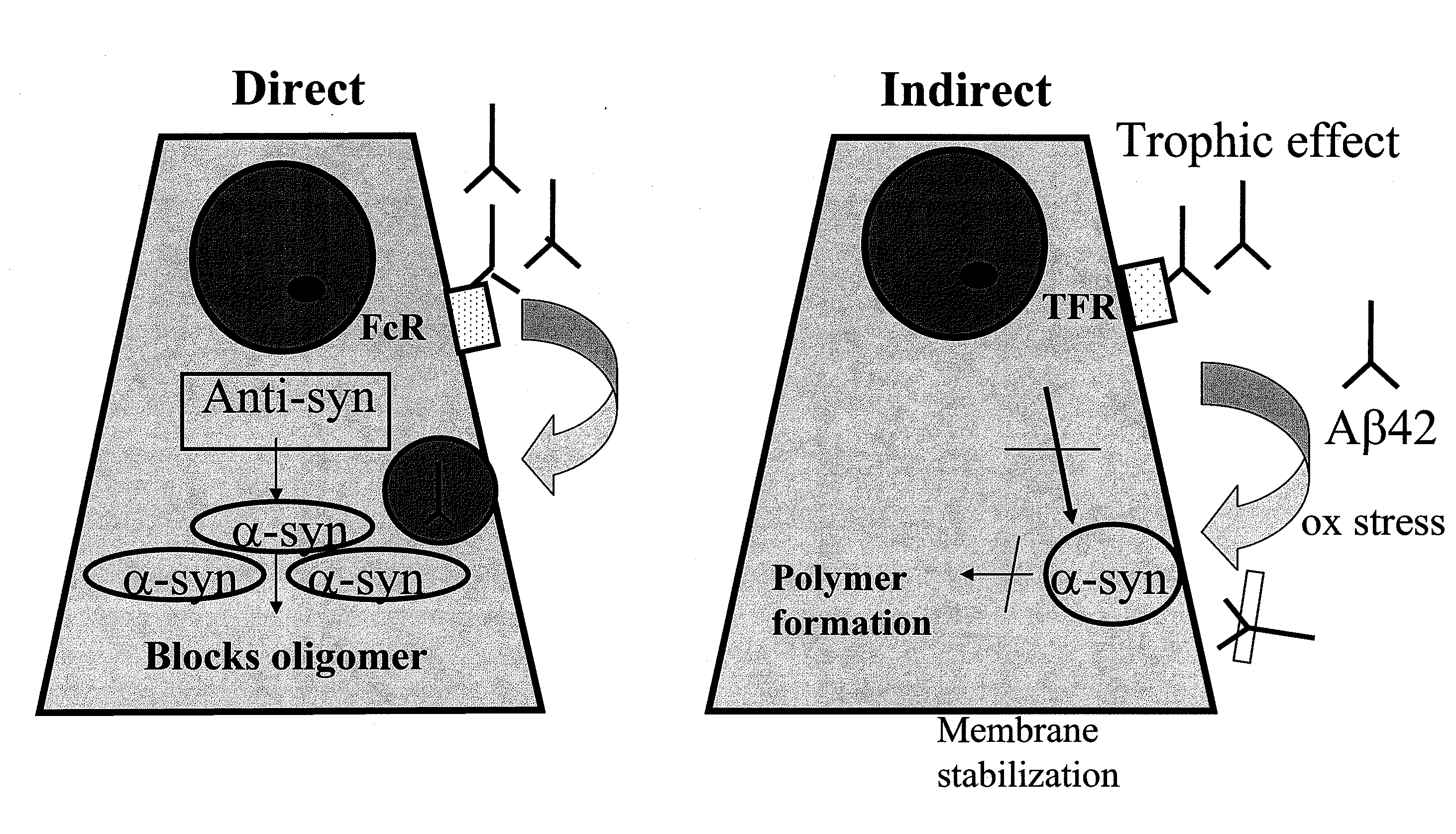
A vaccine for delaying an onset of or for treatment of an α-synuclein-related disorder in an individual comprises a therapeutically effective amount of isolated stabilized soluble α-synuclein oligomer having a lower formation rate to a non-soluble aggregated form than a non-stabilized oligomer of the α-synuclein. An antibody for delaying an onset of or for treatment of an α-synuclein-related disorder in an individual binds soluble α-synuclein. Methods for delaying an onset of for treatment or for prevention of an α-synuclein-related disorder employ the vaccine or antibody. Methods of detecting α-synuclein oligomers employ the antibody.
Owner:BIOARCTIC NEUROSCI AB
Truncated fragments of alpha-synuclein in Lewy body disease
The application identifies novel fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:PROTHENA BIOSCI LTD
Synthetic immunogenic but non-deposit-forming polypeptides and peptides homologous to amyloid beta, prion protein, amylin, alpha-synuclein, or polyglutamine repeats for induction of an immune response thereto
InactiveUS7479482B2Reduce formationAvoid formingHormone peptidesNervous disorderPassive ImmunizationsAmyloid beta
The present invention relates to immunogenic but non-depositing-forming polypeptides or peptides homologous to amyloid β, prion, amylin or α-synuclein which can be used alone or conjugated to an immunostimulatory molecule in an immunizing composition for inducing an immune response to amyloid β peptides and amyloid deposits, to prion protein and prion deposits, to amylin and amylin deposits, to α-synuclein and deposits containing α-synuclein, or to polyglutamine repeats and deposits of proteins containing polyglutamine repeats. Described are also antibodies directed against such peptides, their generation, and their use in methods of passive immunization to such peptides and deposits.
Owner:NEW YORK UNIV
Prevention and treatment of synucleinopathic and amyloidogenic disease
ActiveUS8092801B2Improves motor characteristicInhibit deteriorationNervous disorderNervous system antigen ingredientsSynucleinTherapeutic treatment
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:RGT UNIV OF CALIFORNIA +1
Prevention and treatment of synucleinopathic and amyloidogenic disease
InactiveUS20100031377A1Improves motor characteristicInhibit deteriorationAnimal cellsNervous disorderSynucleinLewy body
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
Prevention and Treatment of Synucleinopathic and Amyloidogenic Disease
ActiveUS20100086545A1Improves motor characteristicInhibit deteriorationAnimal cellsNervous disorderSynucleinLewy body
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:RGT UNIV OF CALIFORNIA +1
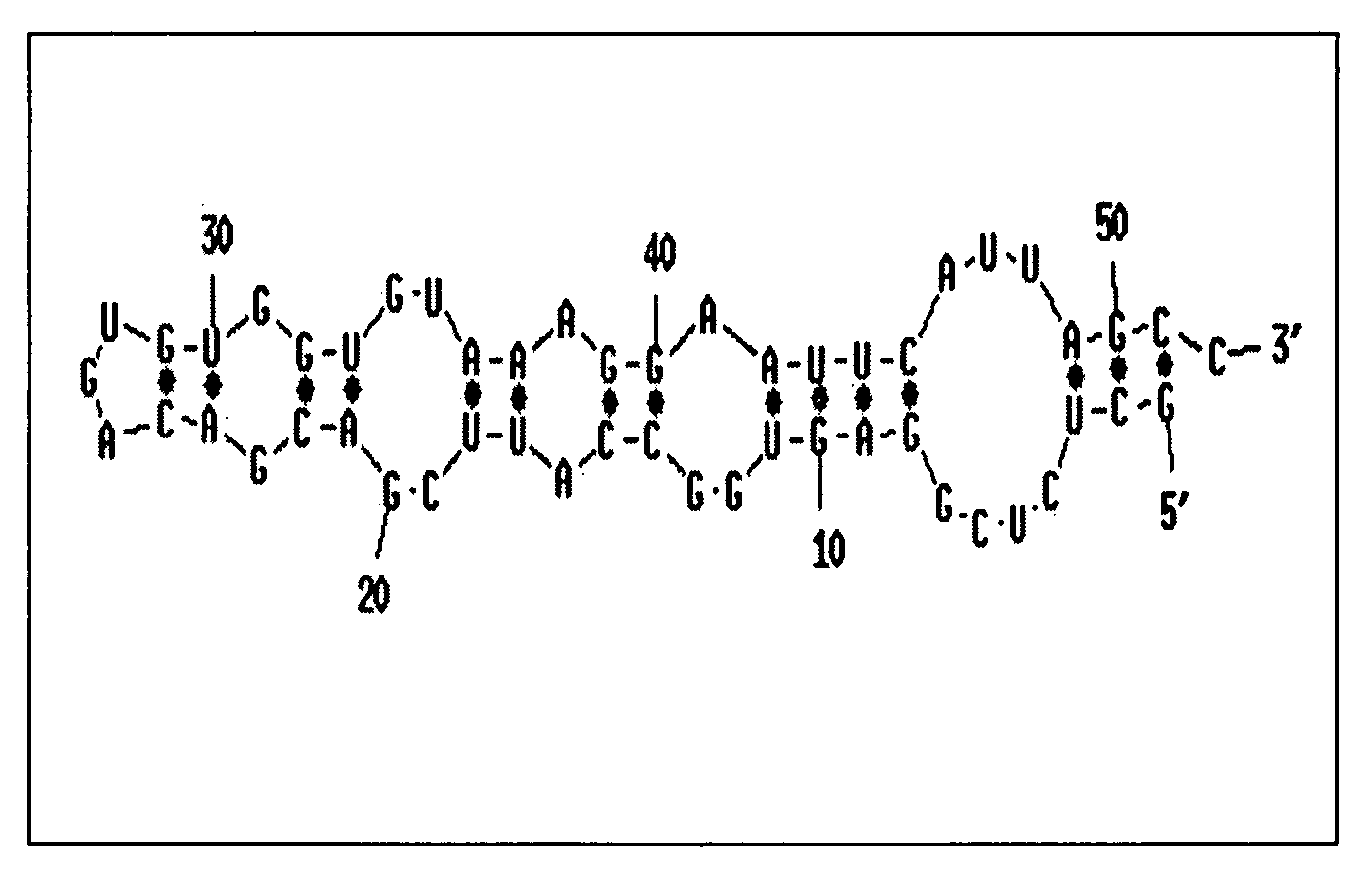
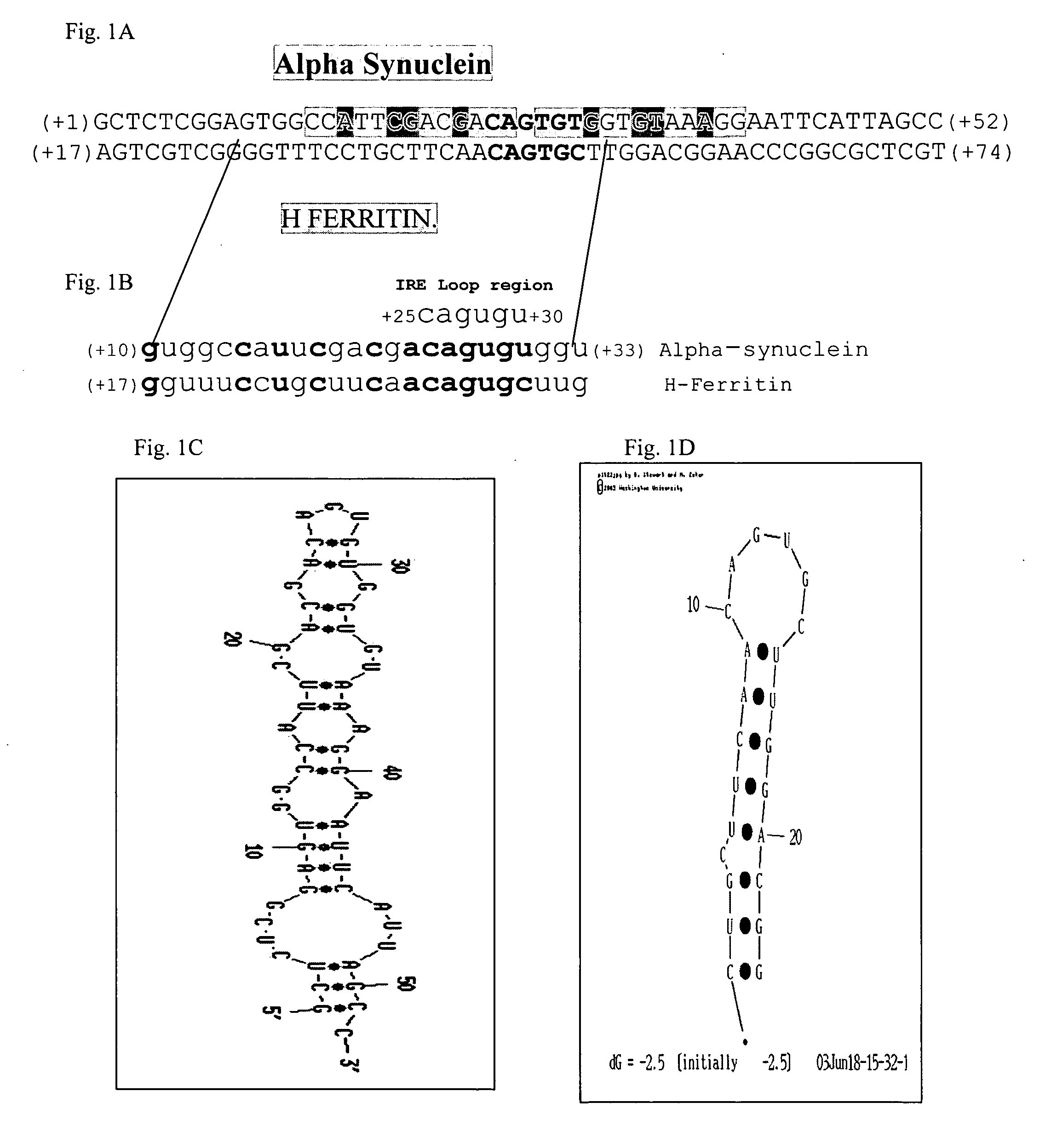
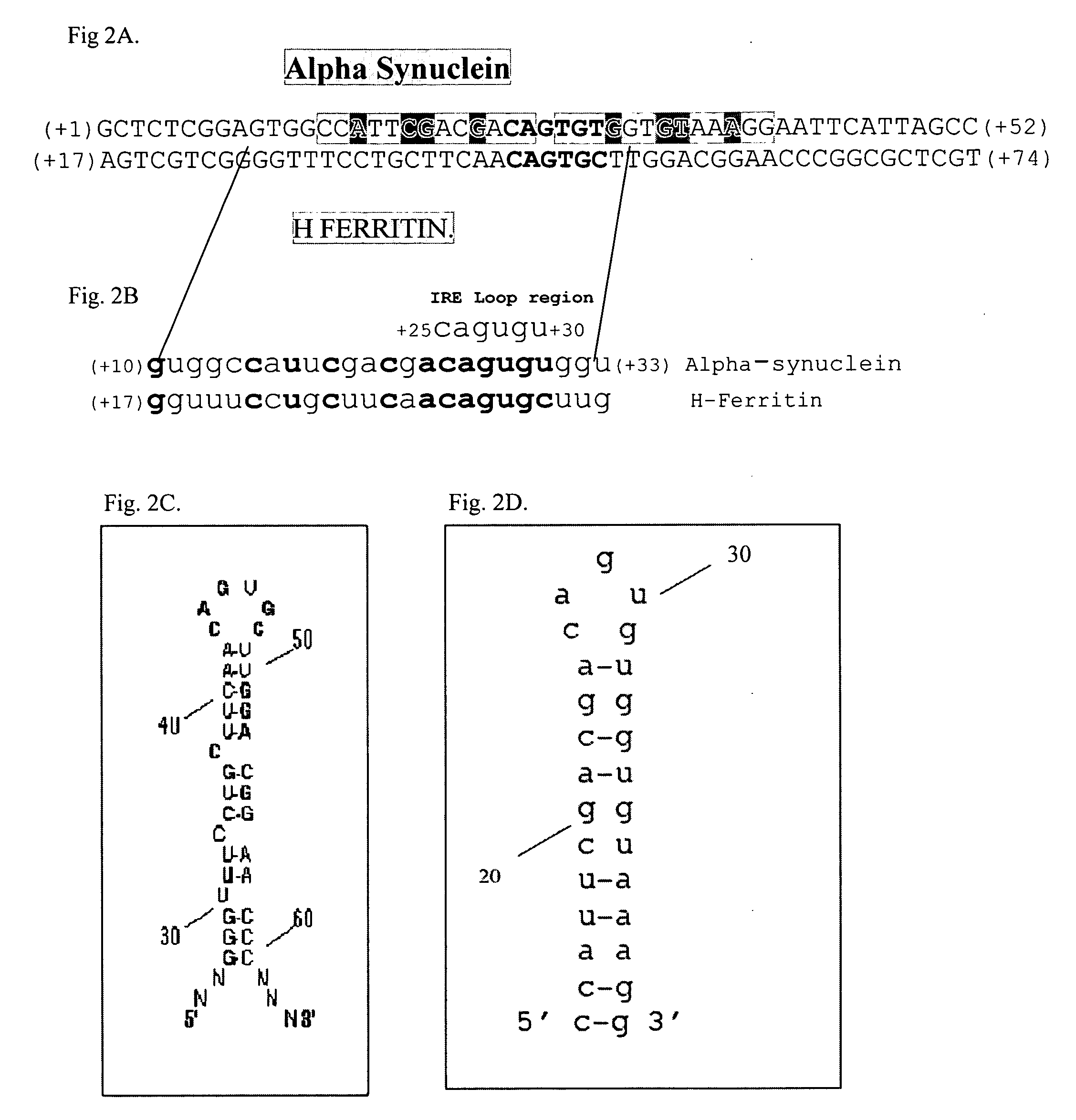
Translation enhancer elements of genes encoding human Tau protein and human alpha-synuclein protein
InactiveUS20080003570A1Sugar derivativesPeptide/protein ingredientsNeuro-degenerative diseaseEnhancer Elements
The invention relates to translation enhancer elements that enhance translation of the gene encoding the human microtubule-associated tau protein and nucleic acid molecules that enhance translation of the gene encoding the human α-synuclein protein. The translation enhancer elements of the invention are useful in compositions and methods for identifying compounds for the prevention and / or treatment of neurodegenerative disease. The invention also includes in some aspects, vectors that include a translation enhancer element of the invention. The invention also includes the use of enhancer element containing vectors in methods to produce recombinant protein and in assays to identify compounds that modulate expression of tau protein or α-synuclein protein.
Owner:THE GENERAL HOSPITAL CORP
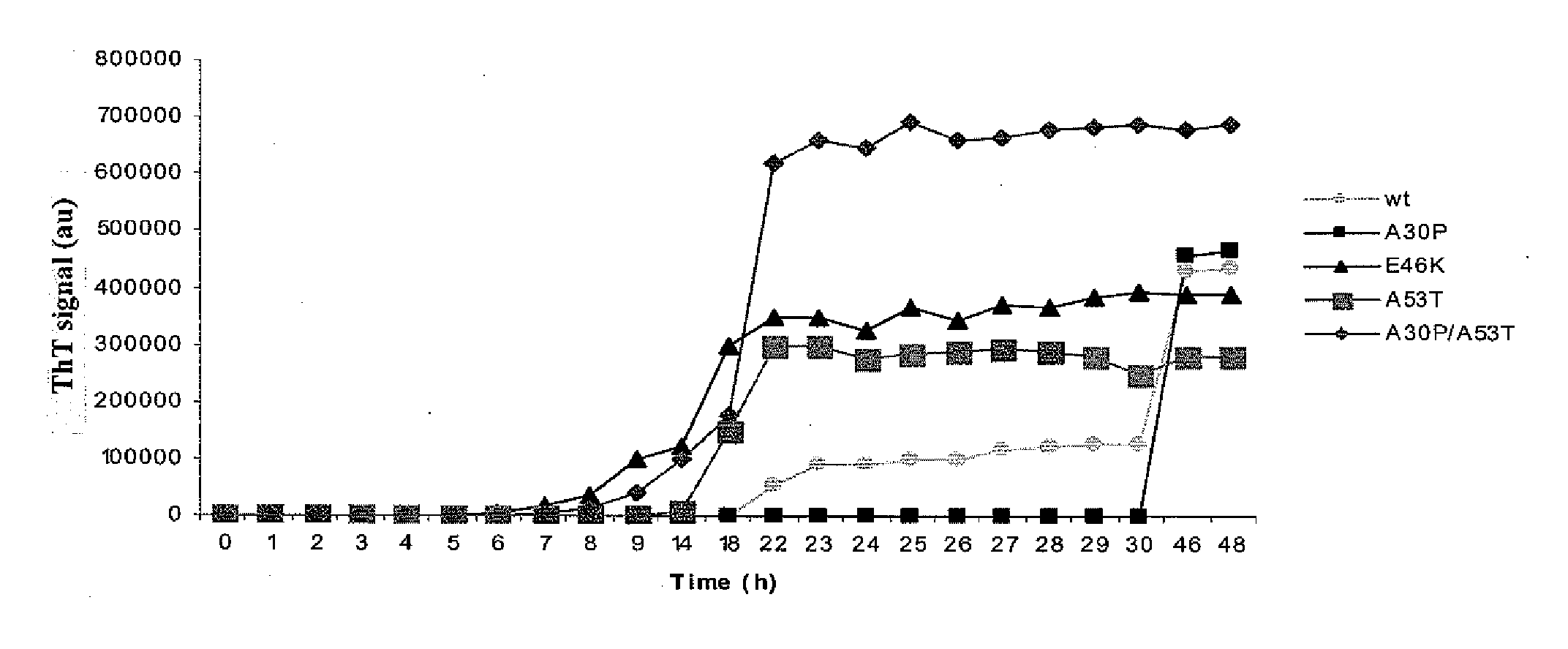
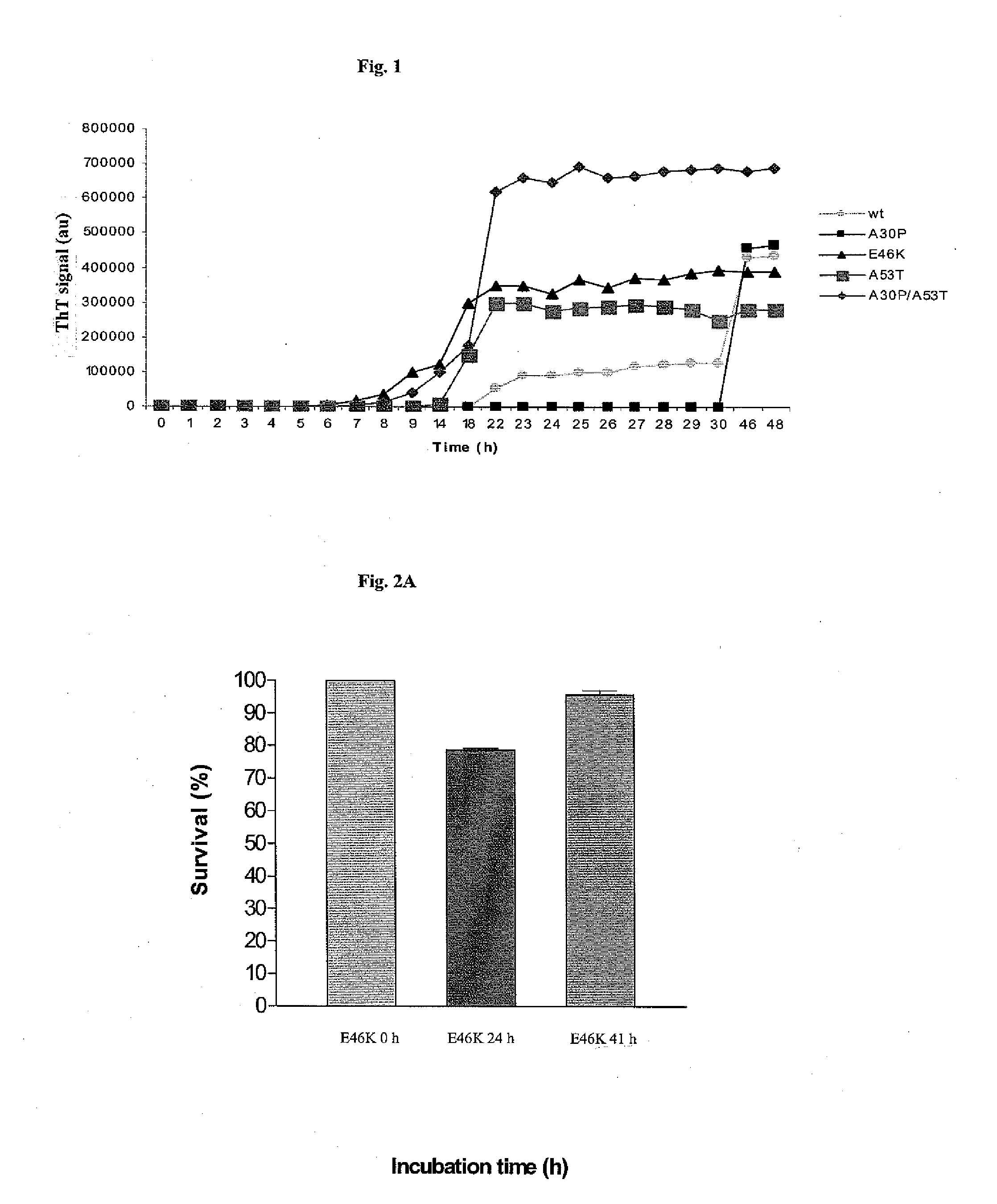
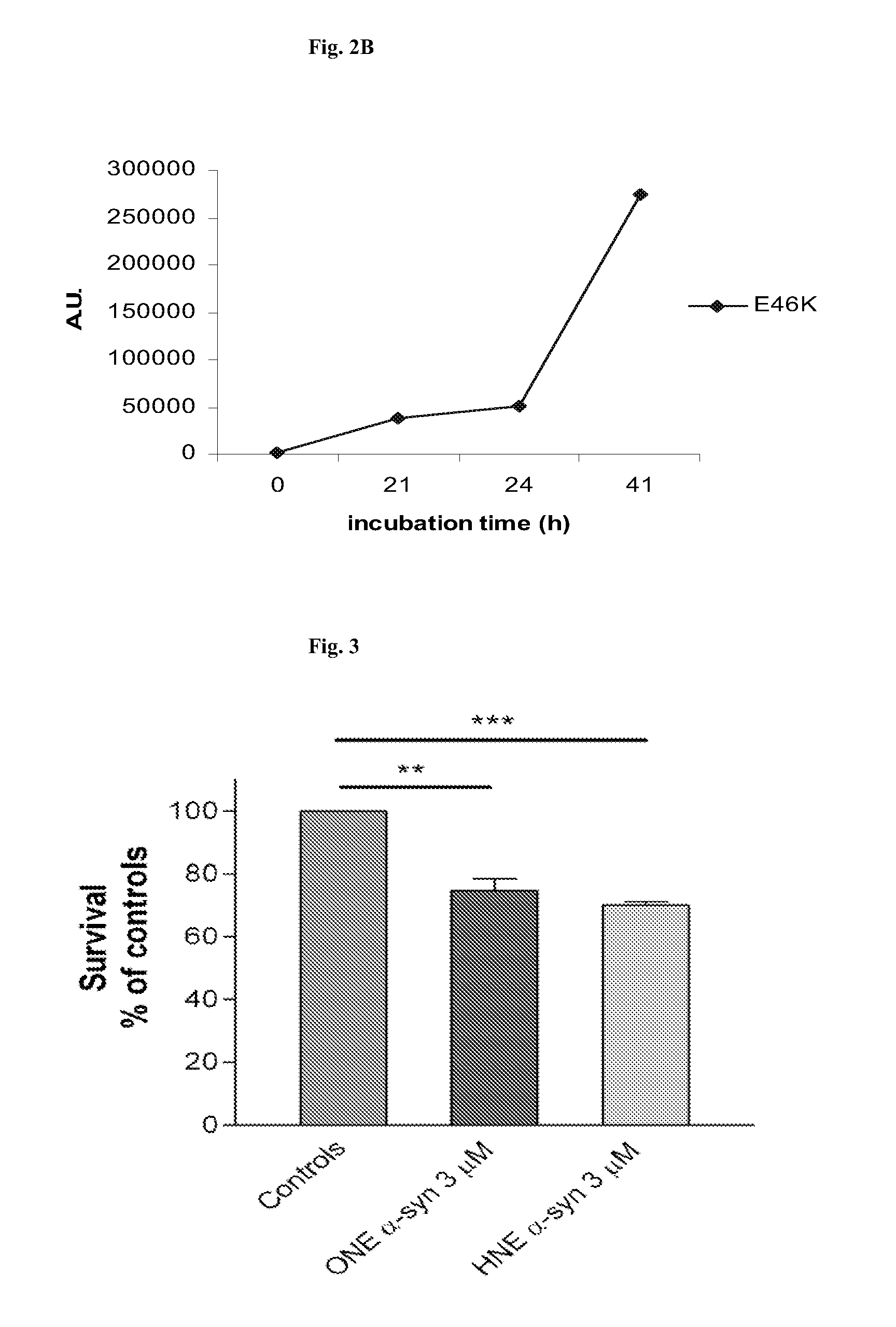
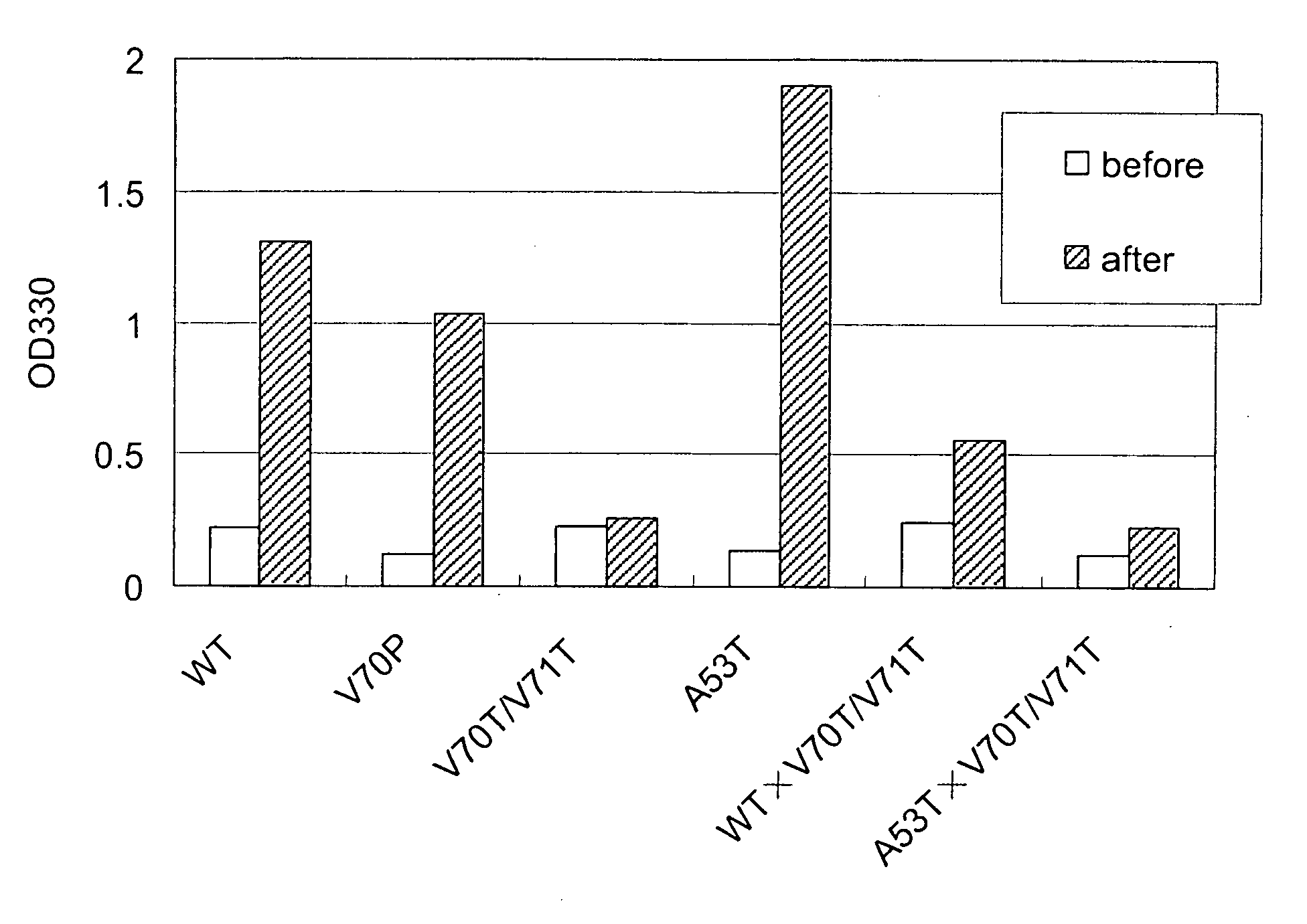
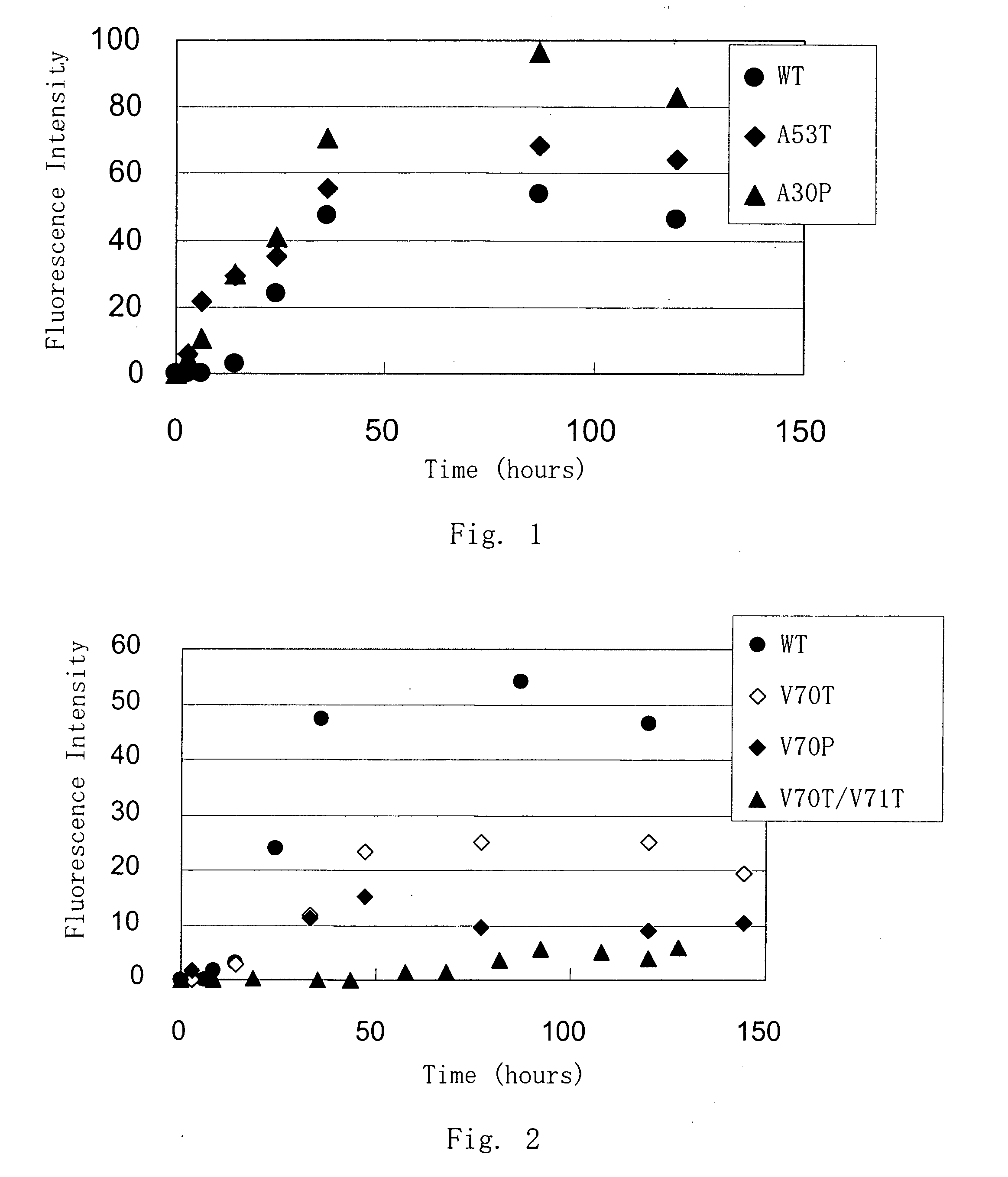
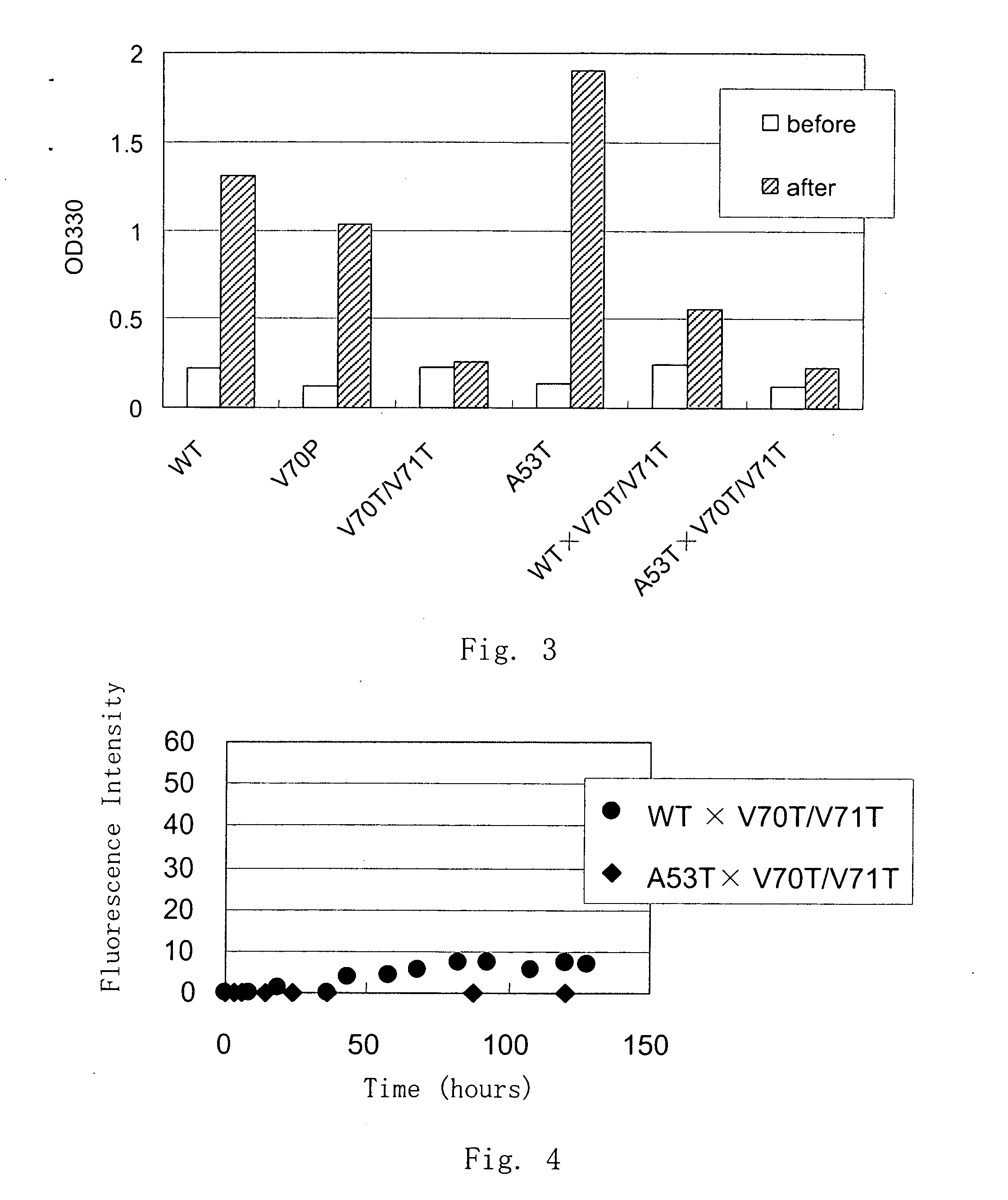
Synuclein Mutant Having Aggregation-Inhibitory Activity
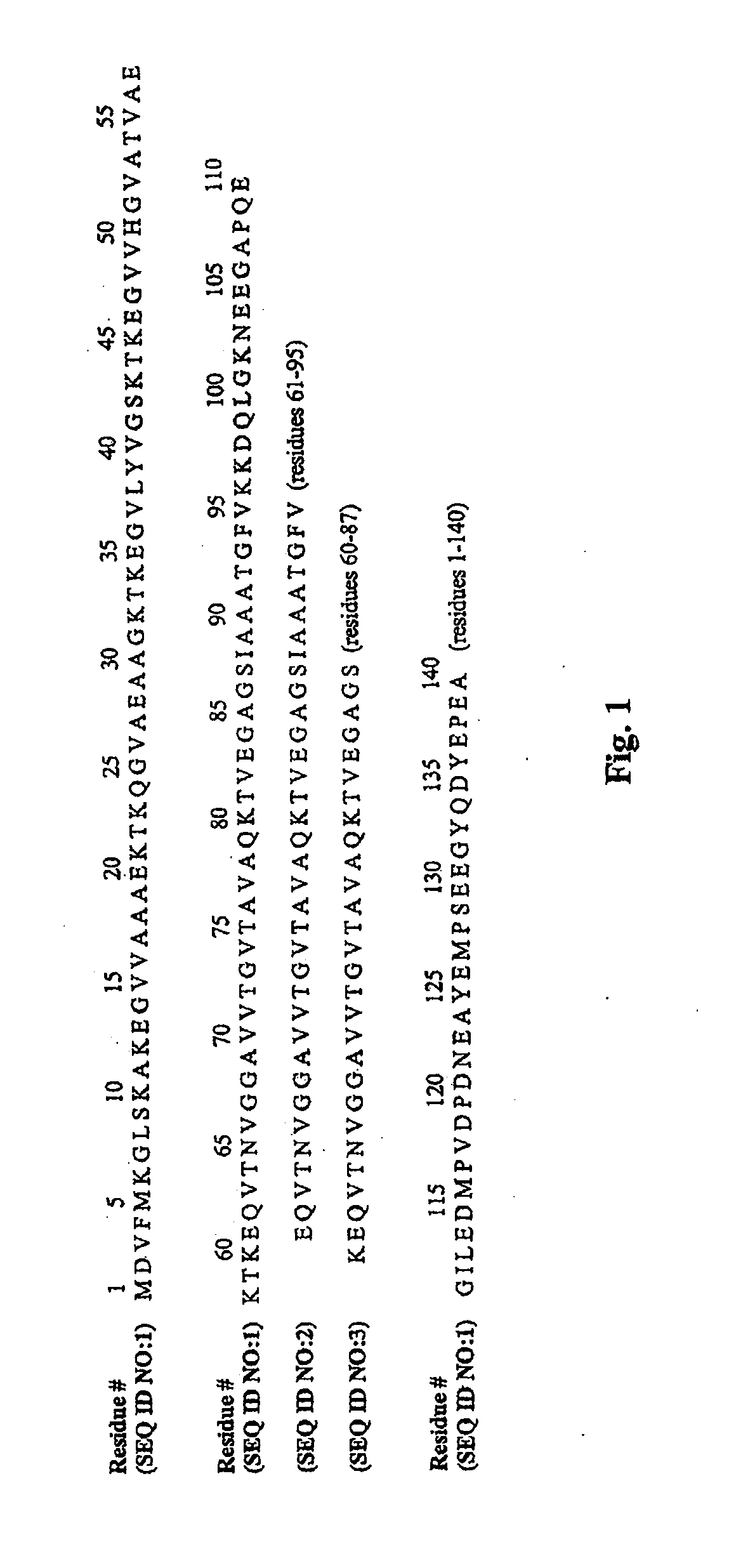
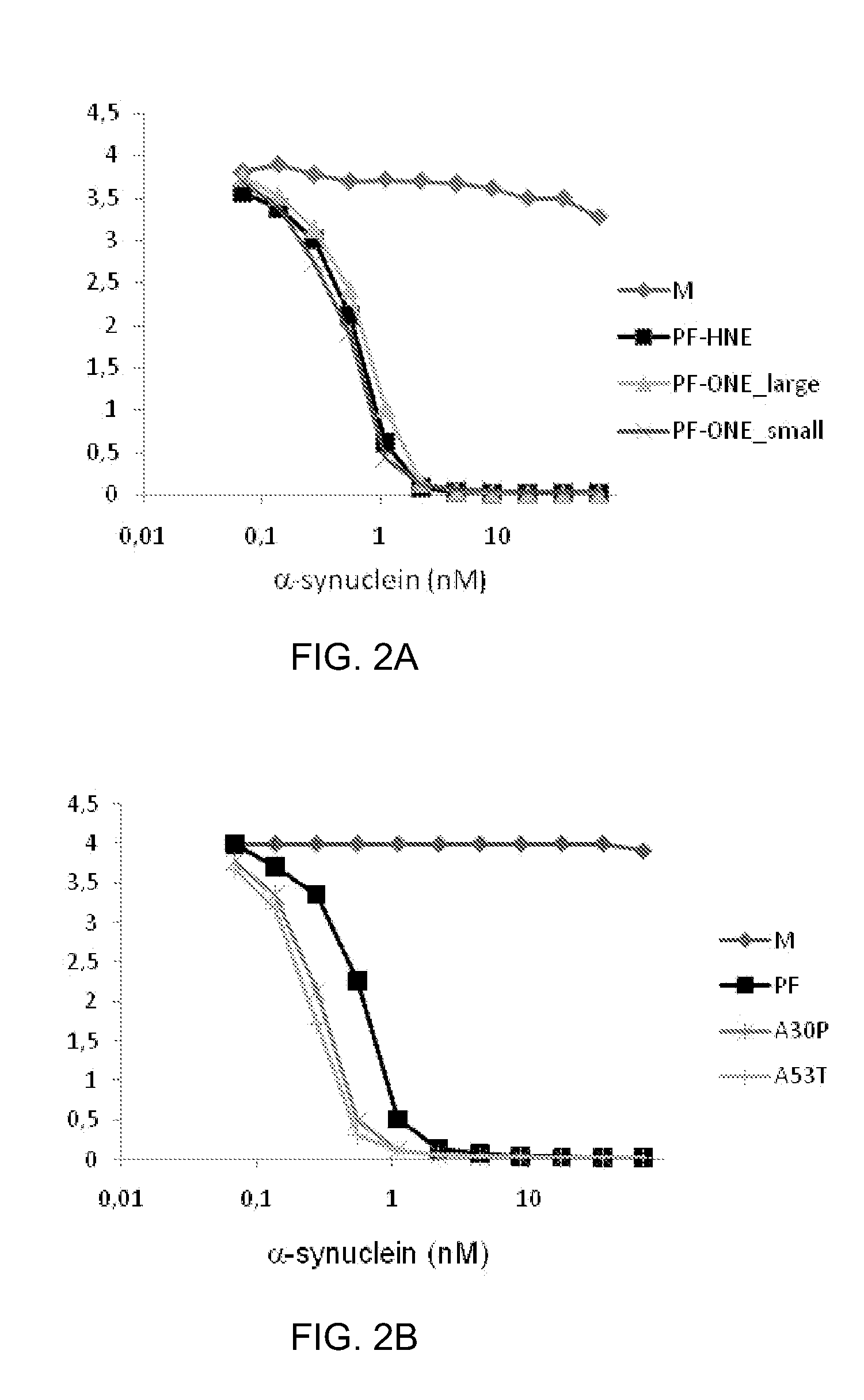
Disclosed is a mutant human α-synuclein with decreased ability of forming aggregation. The mutant human α-synuclein of the invention is able to inhibit aggregation of the wild type human α-synuclein, Ala53Thr mutant human α-synuclein or Ala50Pro mutant human α-synuclein, thus is useful for investigation of pathology and treatment of Parkinson's disease and for research and development of gene therapy. Also disclosed is a partial structure peptide of human α-synuclein comprising amino acid substitutions as taught by the invention.
Owner:SODE
Differential diagnosis of neurodegeneration
InactiveUS20040014142A1Specific detectionSpecific quantificationDisease diagnosisBiological testingSpecific detectionSynuclein
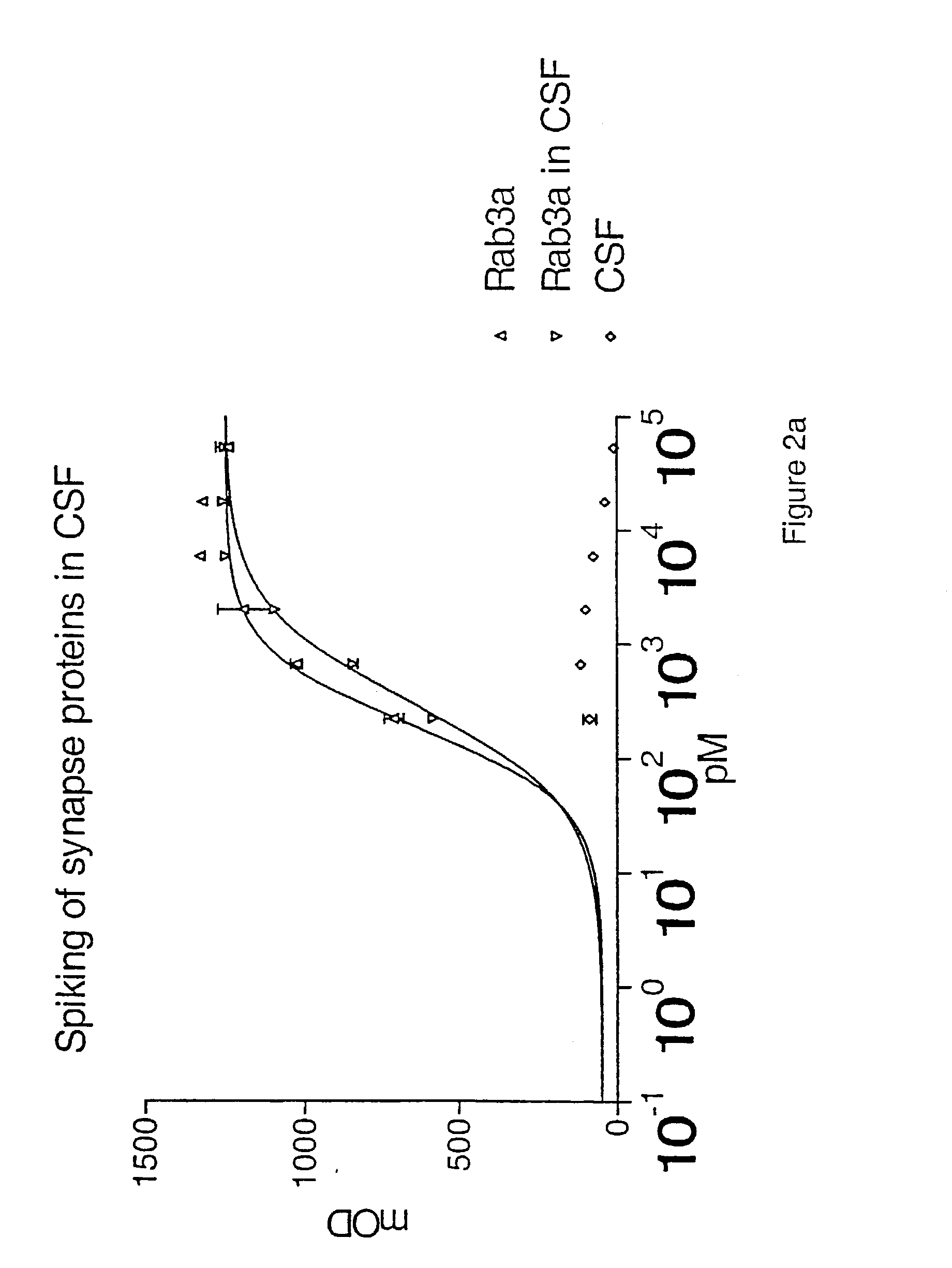
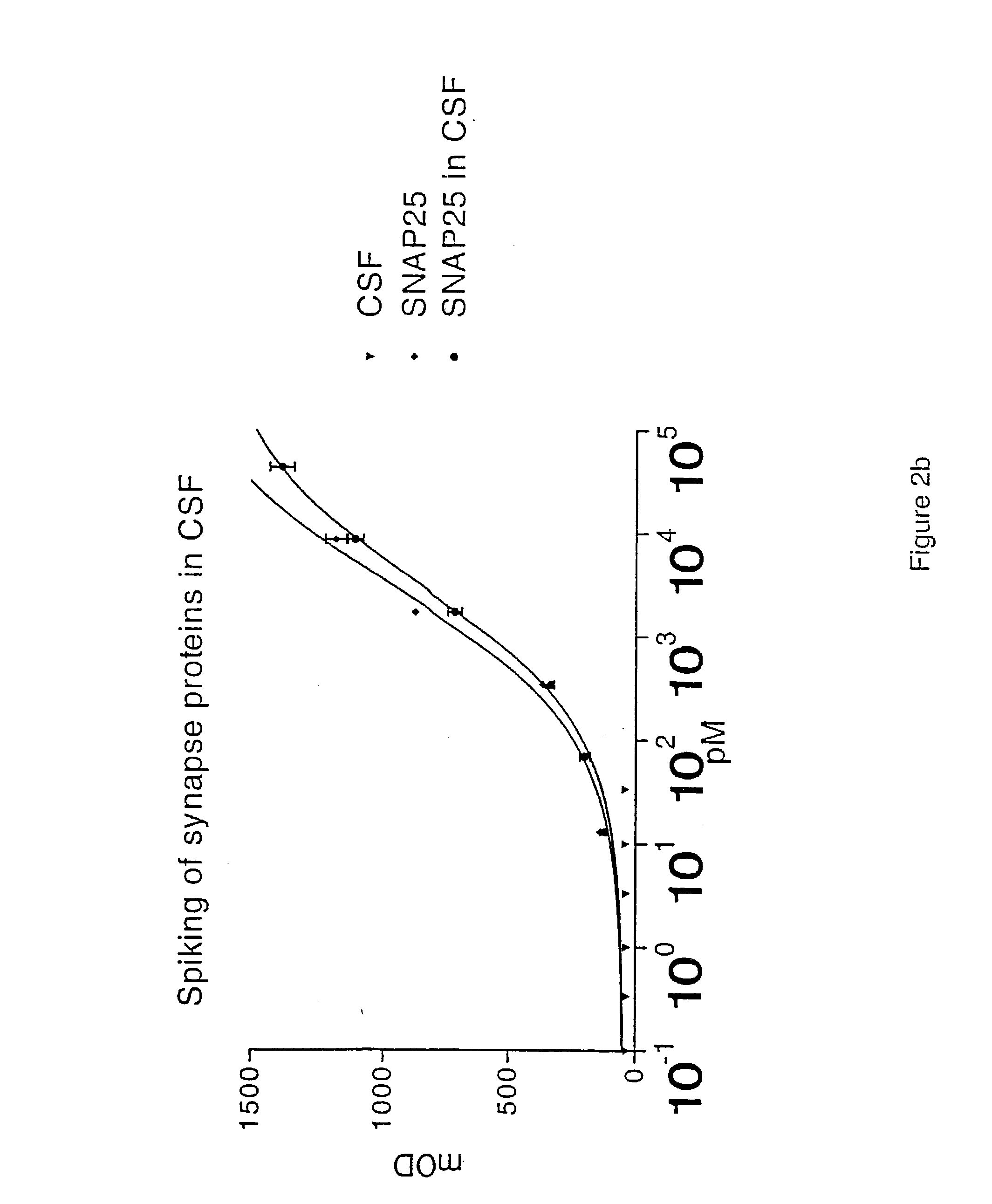
The present invention relates to new methods for the specific detection, quantification and / or differential diagnosis of neurodegeneration in an individual making use of a combination assay detecting at least three neurological markers in one or more body fluids of said individual, the type and degree of neurodegeneration being reflected in the quantitative changes in the level of all of said neurological markers compared to the control sample. The present invention also relates to methods for the detection of Rab3a, SNAP25 and alpha-synuclein in cerebrospinal fluid and to the use of these methods in a combination assay for specific detection, quantification and / or differential diagnosis of neurodegeneration.
Owner:INNOGENETICS NV
Methods and kits for diagnosing neurodegenerative disease
InactiveUS20110159527A1Prevent slaughterRaise the ratioMicrobiological testing/measurementDisease diagnosisDisease freeTotal protein
Methods and diagnostic kits for determining whether a subject may develop a or for diagnosing a neurodegenerative disease. The method includes quantitating the amount of alpha-synuclein and total protein in a cerebrospinal fluid (CSF) sample obtained from the subject and calculating a ratio of alpha-synuclein to total protein content; comparing the ratio of alpha-synuclein to total protein content in the CSF sample with the alpha-synuclein to total protein content ratio in CSF samples obtained from healthy neurodegenerative disease-free subjects; and (c) determining from the comparison whether the subject has a likelihood to develop neurodegenerative disease or making a diagnosis of neurodegenerative disease in a subject. A difference in the ratio of alpha-synuclein to total protein content indicates that the subject has a likelihood to develop a neurodegenerative disease or has developed a neurodegenerative disease.
Owner:SCHLOSSMACHER MICHAEL GEBHARD +2
Alpha-synuclein antibodies and uses thereof
The invention describes antibodies having a high affinity for aggregated forms of α-synuclein and a low affinity for monomeric forms of α-synuclein. The antibodies are useful in the diagnosis of neurodegenerative diseases.
Owner:UNITED ARAB EMIRATES UNIVERSITY
Prevention and treatment of synucleinopathic and amyloidogenic disease
ActiveUS20100278814A1Improves motor characteristicInhibit deteriorationNervous disorderNervous system antigen ingredientsLewy bodySynuclein
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:RGT UNIV OF CALIFORNIA +1
Prevention and treatment of synucleinopathic disease
InactiveUS20110135660A1Improves motor characteristicInhibit deteriorationNervous disorderPeptide/protein ingredientsSynucleinLewy body
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
Owner:JANSSEN ALZHEIMER IMMUNOTHERAPY +1
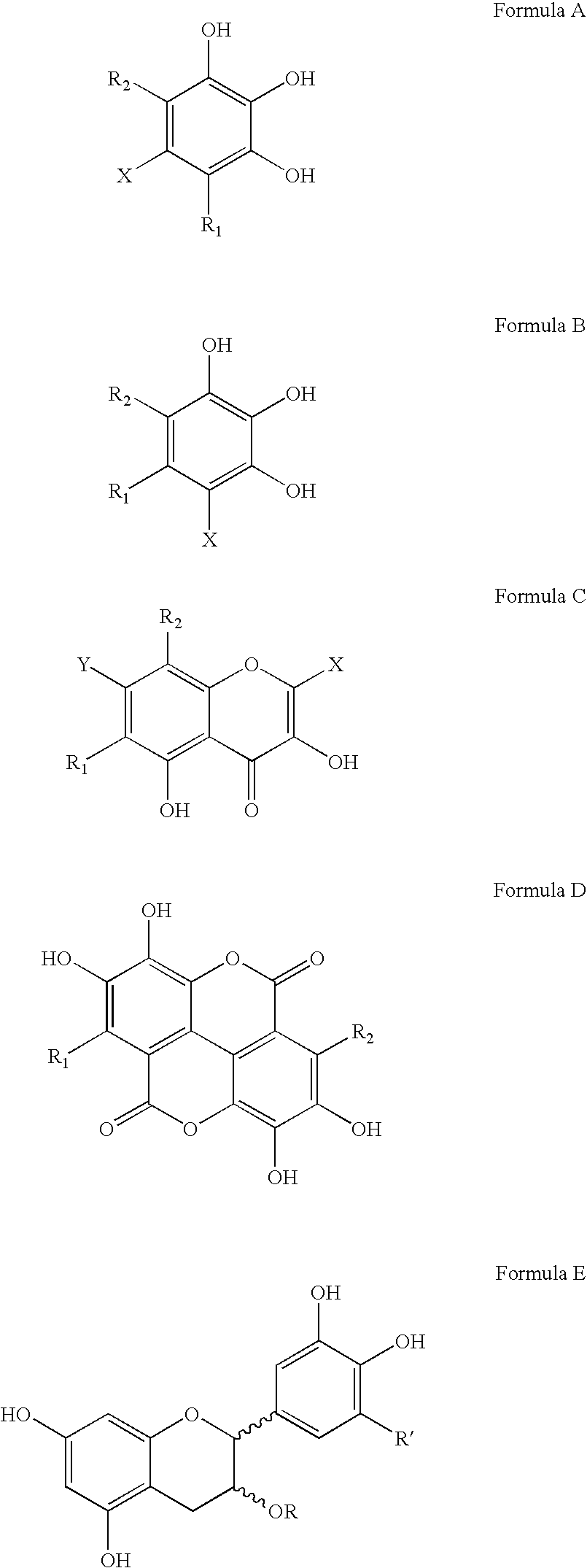
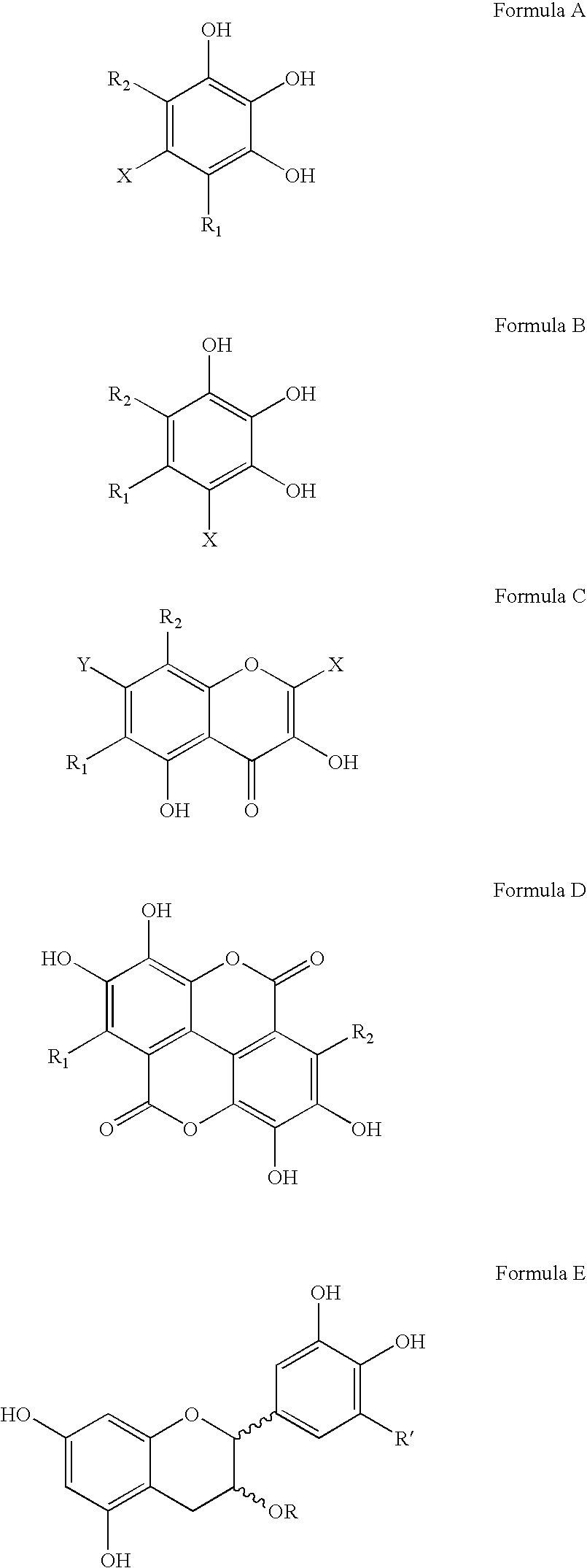
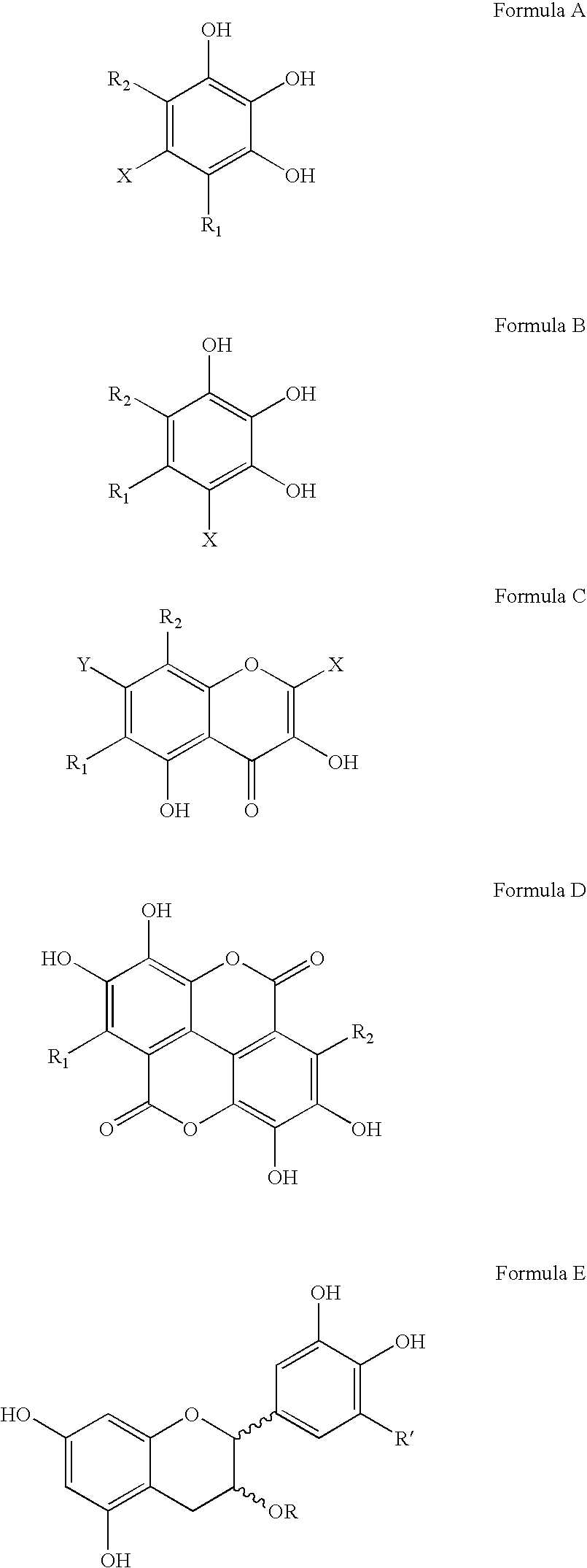
Polyhydroxylated aromatic compounds for the treatment of amyloidosis and alpha-synuclein fibril diseases
Owner:COGNITIVE CLARITY INC
Truncated fragments of alpha-synuclein in Lewy body disease
The application identifies fragments of alpha-synuclein in patients with Lewy Body Disease (LBD) and transgenic animal models thereof. These diseases are characterized by aggregations of alpha-synuclein. The fragments have a truncated C-terminus relative to full-length alpha-synuclein. Some fragments are characterized by a molecular weight of about 12 kDa as determined by SDS gel electrophoresis in tricine buffer and a truncation of at least ten contiguous amino acids from the C-terminus of natural alpha-synuclein. The site of cleavage preferably occurs after residue 117 and before residue 126 of natural alpha-synuclein. The identification of these novel fragments of alpha-synuclein has a number of application in for example, drug discovery, diagnostics, therapeutics, and transgenic animals.
Owner:TRUSTEES OF FLINDERS UNIV +2
Protofibril-Binding Antibodies and Their Use in Therapeutic and Diagnostic Methods for Parkinson's Disease, Dementia with Lewy Bodies and Other Alpha-Synucleinopathies
ActiveUS20120308572A1Increase delayEasy diagnosisCompounds screening/testingNervous disorderDementia with Lewy bodiesNerve degeneration
Antibodies and fragments thereof have high affinity for human α-synuclein protofibrils and low binding of α-synuclein monomers, wherein the antibodies or fragments have specified Complementarity Determining Region (CDR) sequences. Compositions comprise such an antibody or fragment and methods of detecting α-synuclein protofibrils use such an antibody or fragment. In further embodiments, methods of preventing, delaying onset of or treating a neurodegenerative disorder with α-synuclein pathology comprise administering such an antibody or fragment, and such an antibody or fragment is used in the manufacture of a pharmaceutical composition for treatment of a neurodegenerative disorder with α-synuclein pathology. Such an antibody or fragment is used in the diagnosis or monitoring of the development of a neurodegenerative disorder with α-synuclein pathology, and in methods for reducing or inhibiting α-synuclein aggregation by administration of such an antibody or fragment.
Owner:BIOARCTIC NEUROSCI AB
Combination therapies for the treatment of alzheimer's disease and related disorders
The present invention relates to combination therapies for treating Alzheimer's disease or an amyloidosis-associated pathological condition comprising co-administering a therapeutically effective amount of a first compound, and a therapeutically effective amount of a second compound. In certain embodiments, the first compound or the second compound inhibits AB peptide polymerization; is an anti-inflammatory; improves cognitive function, mood, or social behavior; is associated with Tau or alpha-synuclein; or regulates amyloid peptide washout.
Owner:THE GENERAL HOSPITAL CORP
Methods of identifying agents that diminish cellular toxicity associated with an α-synuclein polypeptide of Parkinson's disease in yeast
ActiveUS7452670B2Low toxicityIncreased toxicityBiocideCompound screeningHuntingtons choreaCytotoxicity
Methods of screening candidate agents to identify lead compounds for the development of therapeutic agents for the treatment of a neurodegenerative disease, such as Huntington's Disease and Parkinson's Disease and methods for identifying a mutation in, or changes in expression of, a gene associated with neurodegenerative disease, such as Huntington's Disease and Parkinson's Disease, are provided.
Owner:UNIV OF WASHINGTON +1
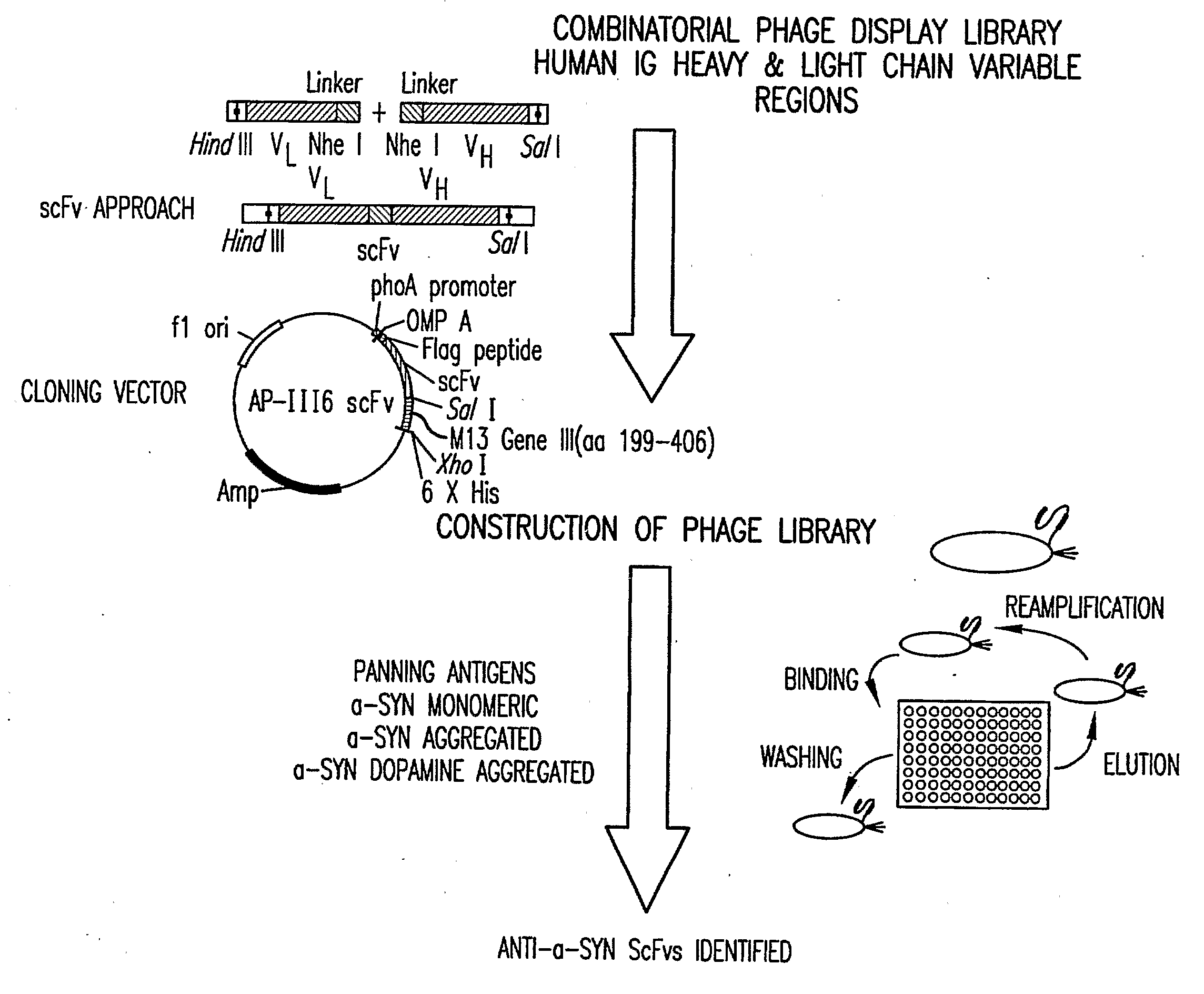
Human anti-alpha-synuclein antibodies
Provided are human alpha-synuclein-specific autoantibodies as well as fragments, derivatives and variants thereof as well as methods related thereto. Assays, kits, and solid supports related to antibodies specific for α-synuclein are also disclosed. The antibody, immunoglobulin chain(s), as well as binding fragments, derivatives and variants thereof can be used in pharmaceutical and diagnostic compositions for α-synuclein targeted immunotherapy and diagnosis, respectively.
Owner:BIOGEN INT NEUROSCI +1
Compounds, compositions and methods for the treatment of amyloid diseases and synucleinopathies such as alzheimer's disease, type 2 diabetes, and parkinson's disease
Bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable esters, their synthesis, pharmaceutical compositions containing them, and their use in the treatment of amyloid diseases, especially Aβ amyloidosis, such as observed in Alzheimer's disease, IAPP amyloidosis, such as observed in type 2 diabetes, and synucleinopathies, such as observed in Parkinson's disease, and the manufacture of medicaments for such treatment.
Owner:PROTAMED
Agents, Uses and Methods for the Treatment of Synucleinopathy
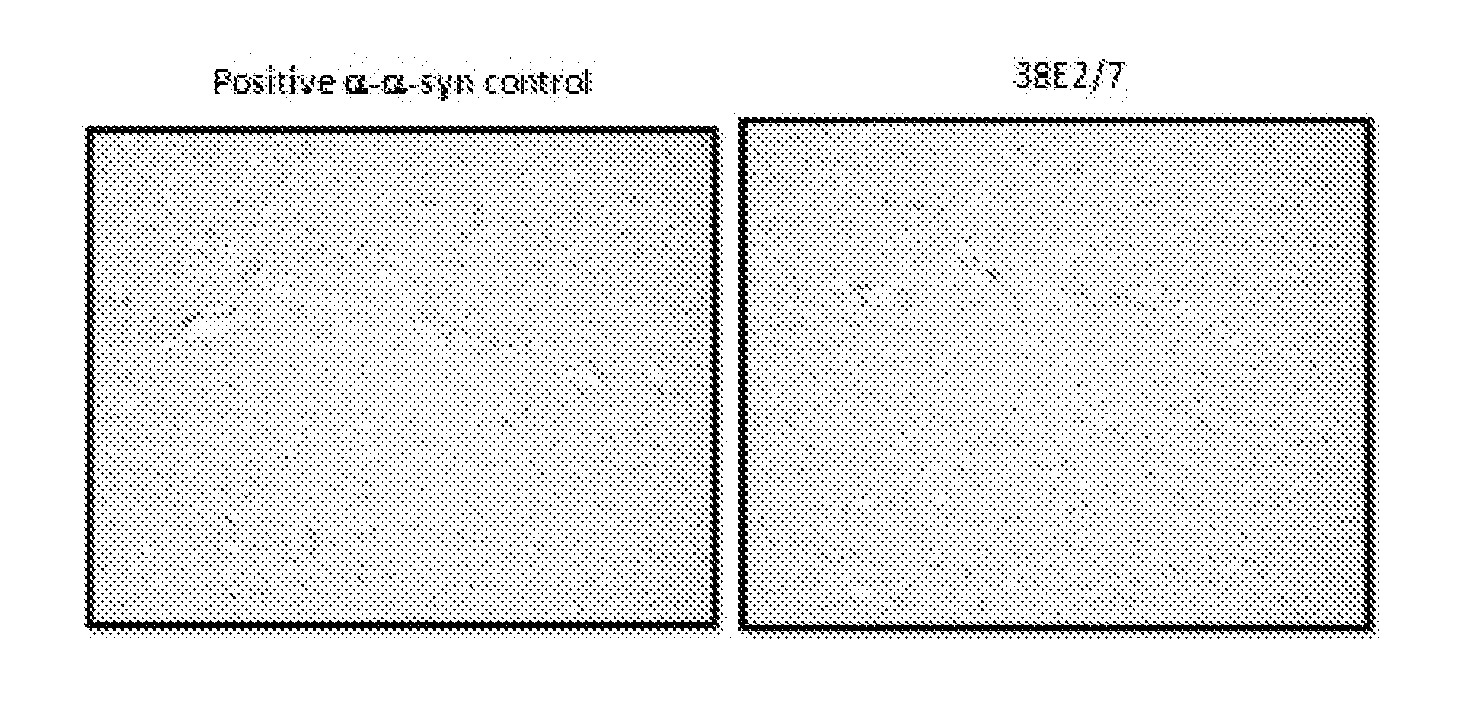
The invention relates to novel monoclonal anti-alpha-synuclein antibodies. The antibodies can be used for treating a synucleinopathy such as Parkinson's disease (including idiopathic and inherited forms of Parkinson's disease), Diffuse Lewy Body Disease (DLBD), Lewy body variant of Alzheimer's disease (LBV), Combined Alzheimer's and Parkinson disease, pure autonomic failure and multiple system atrophy.
Owner:H LUNDBECK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com