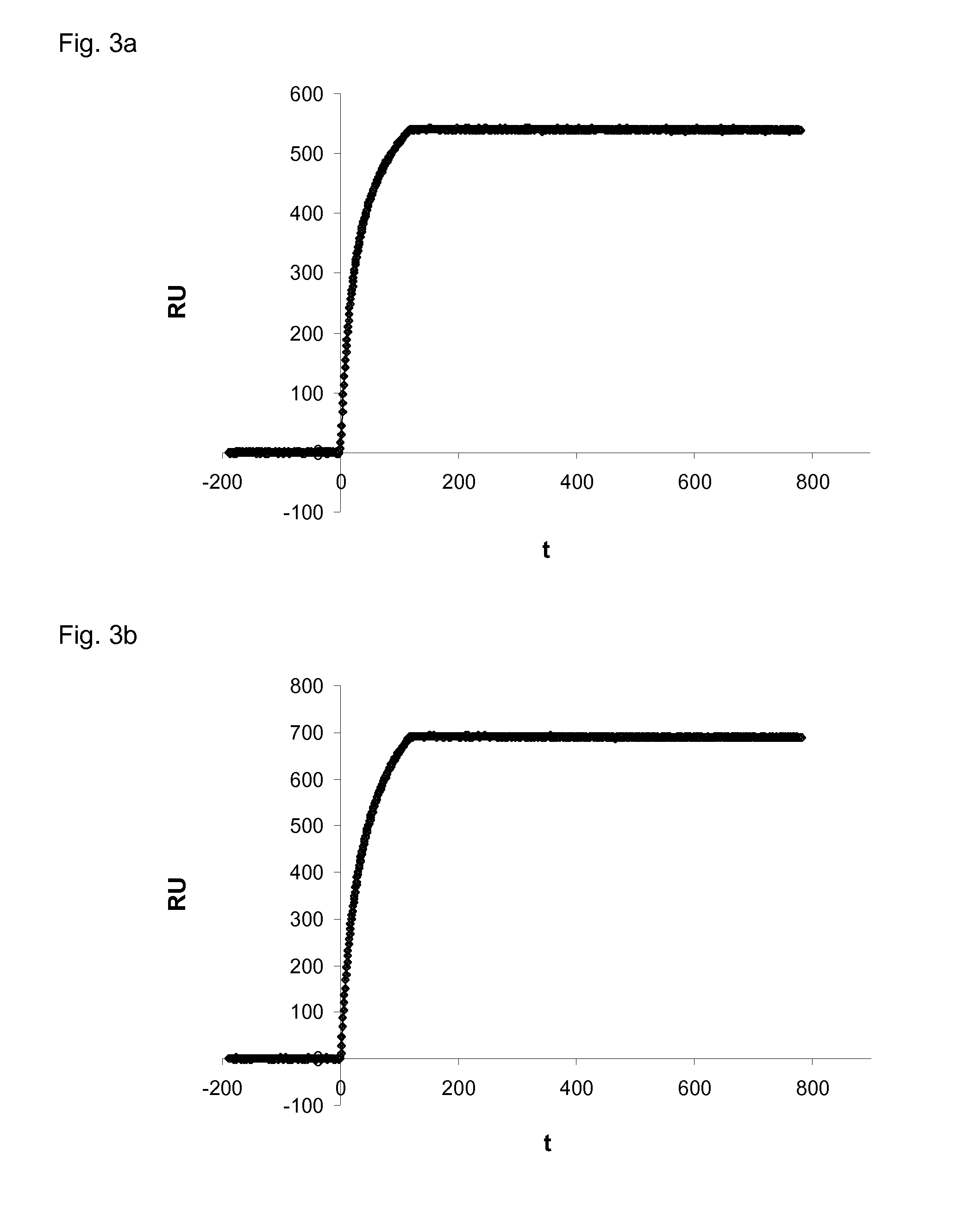
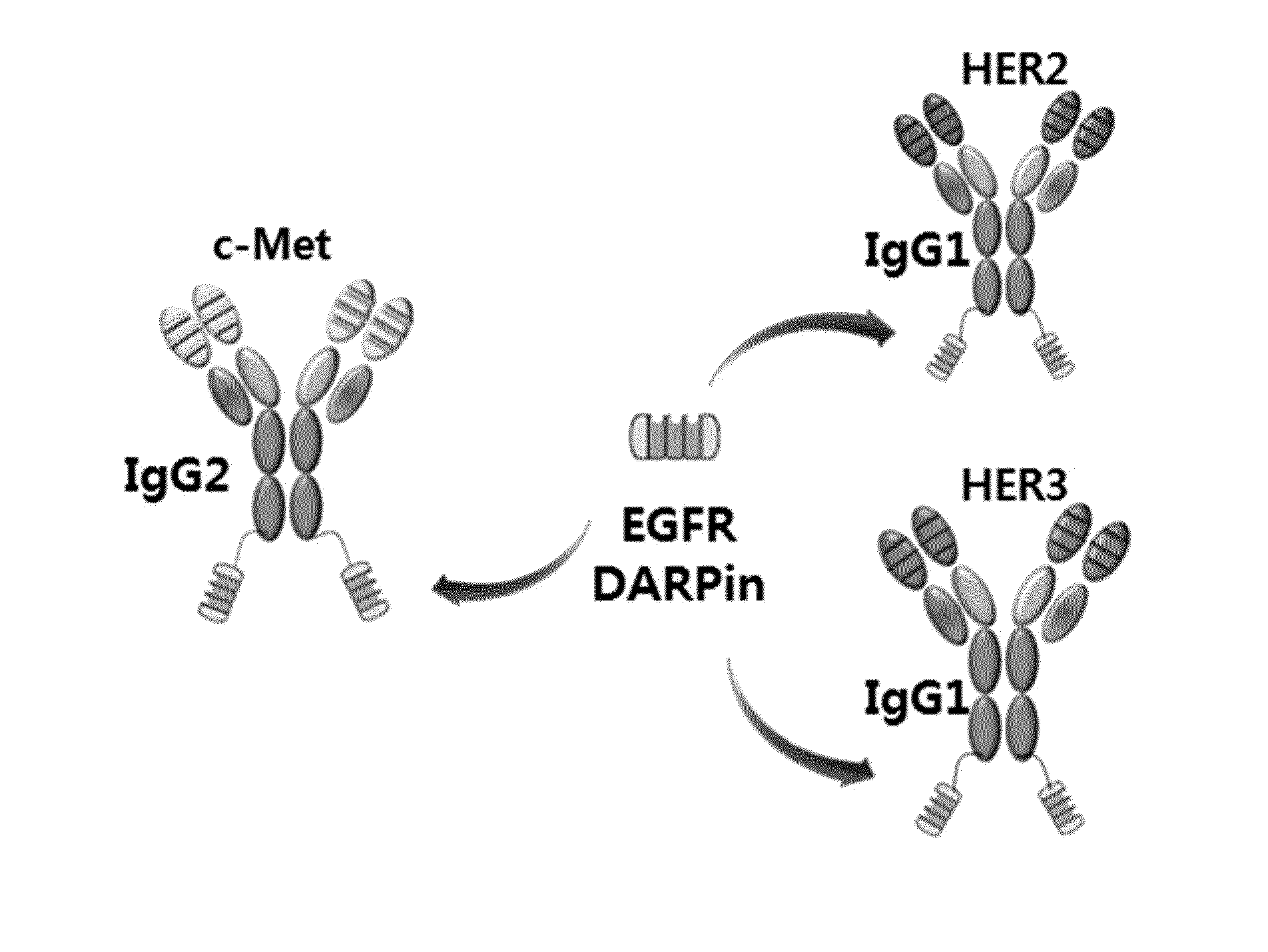
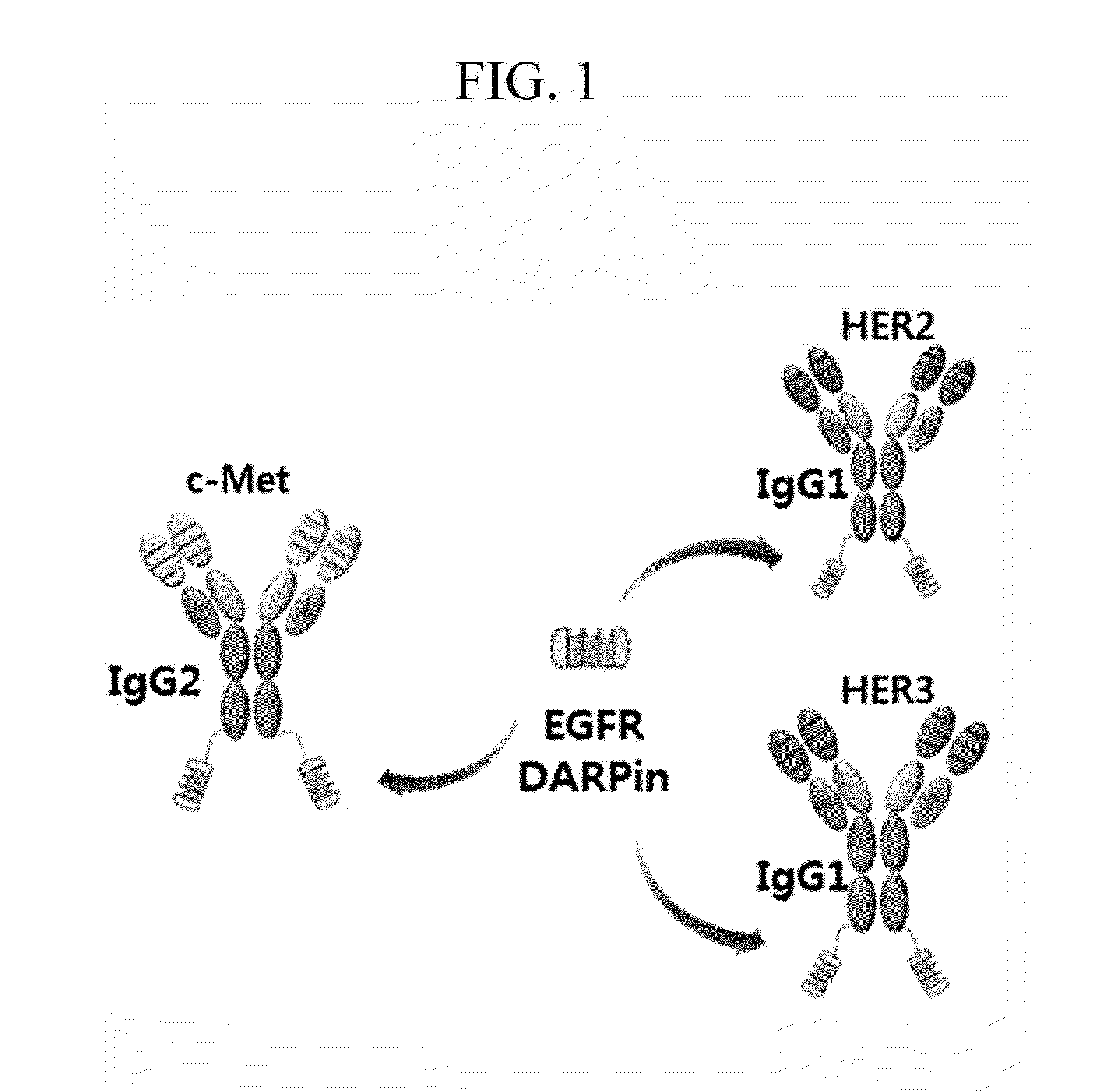
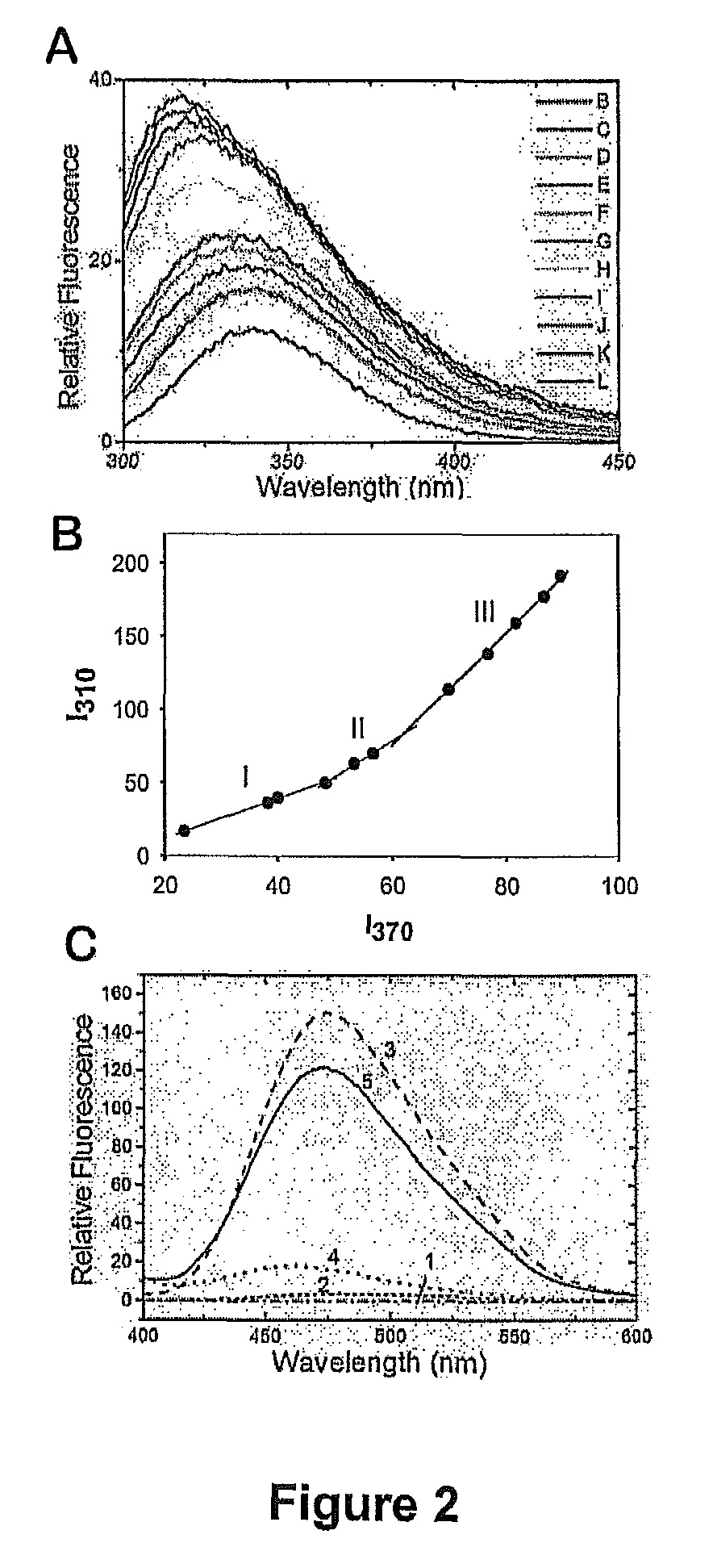
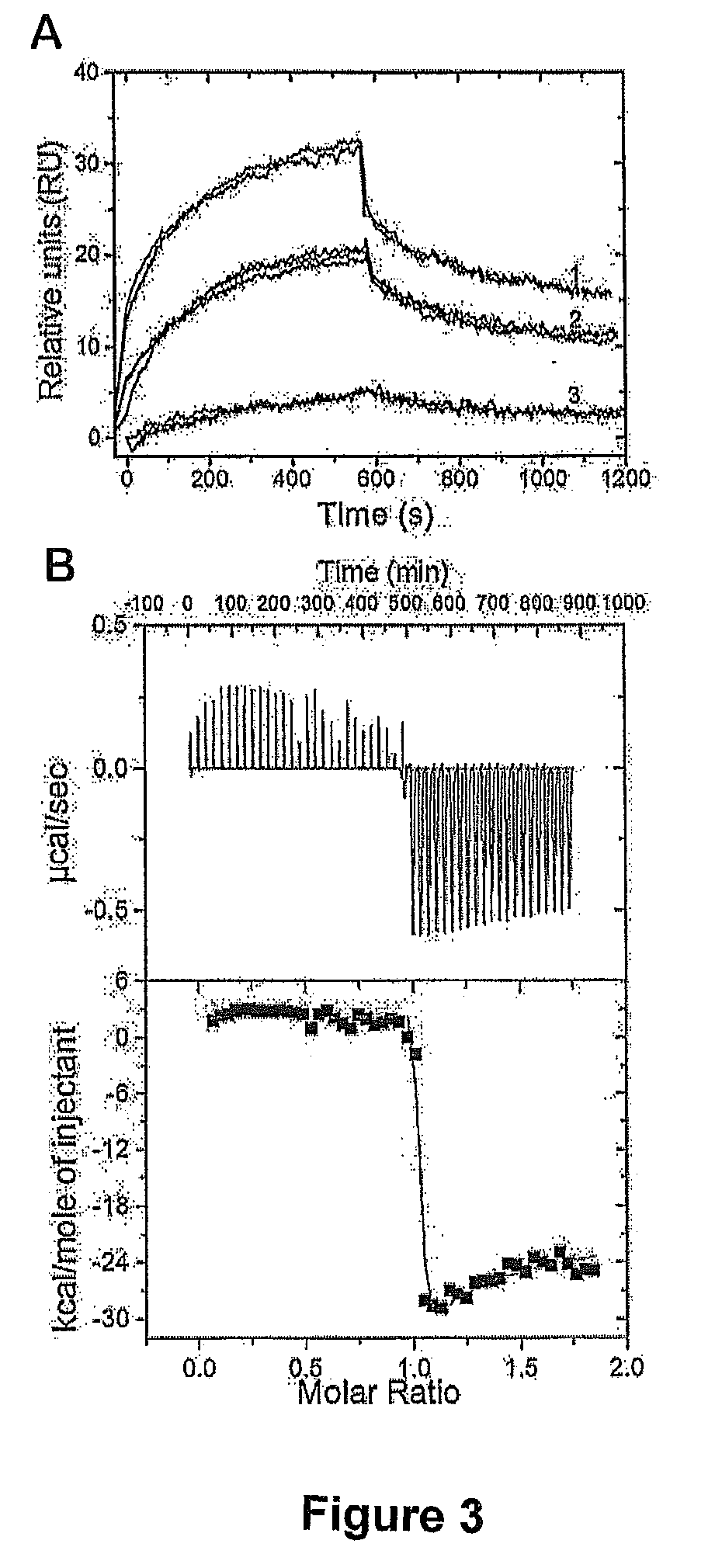
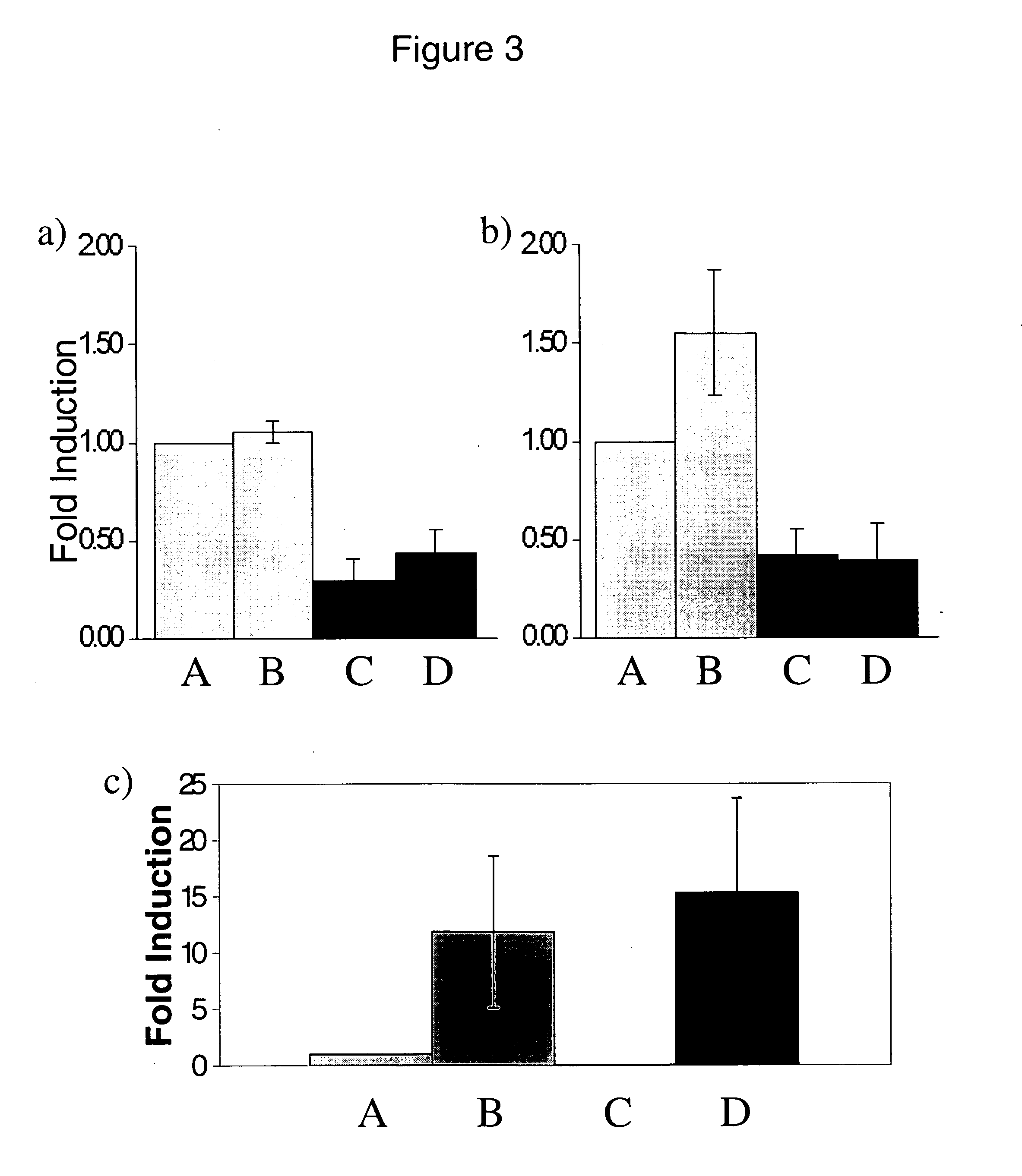
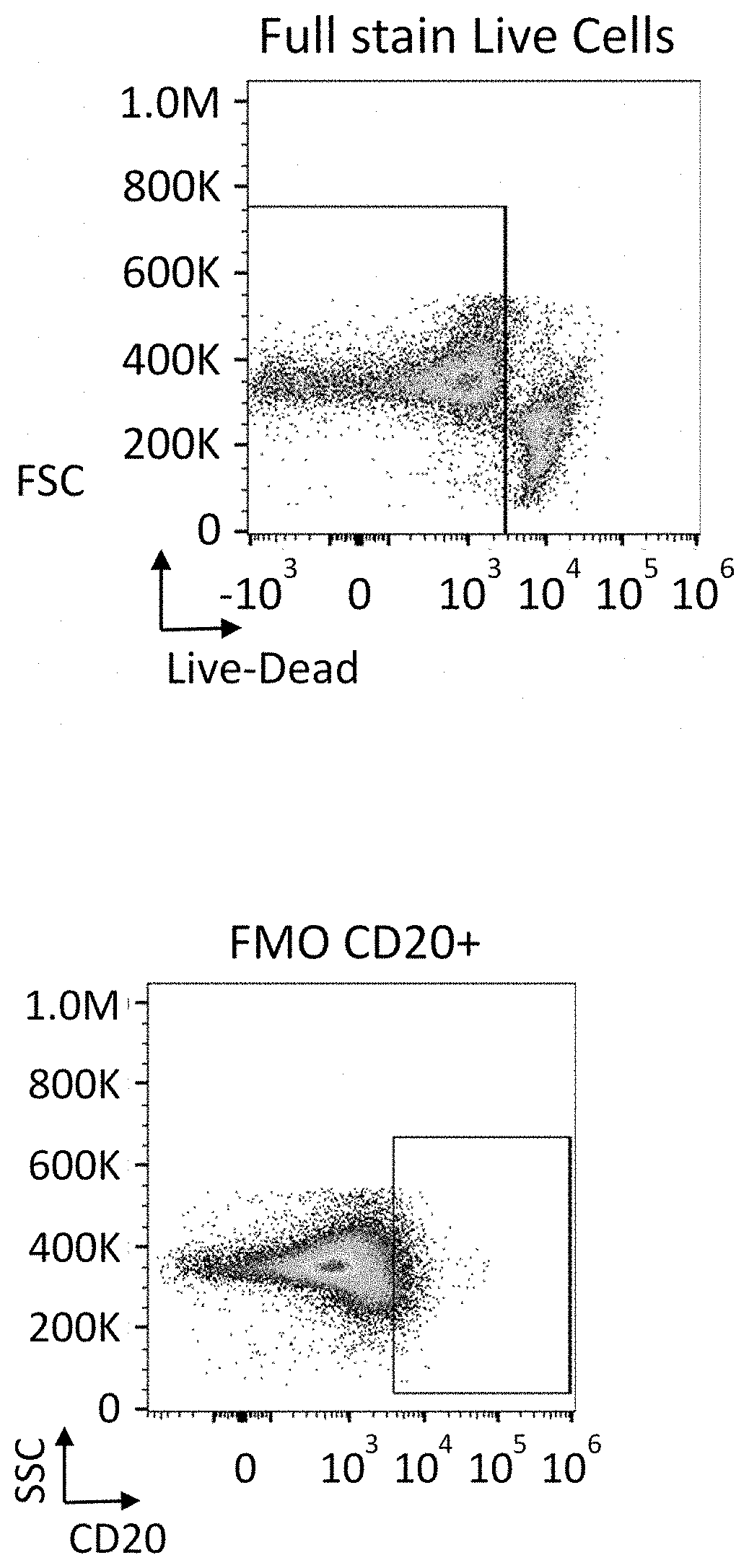
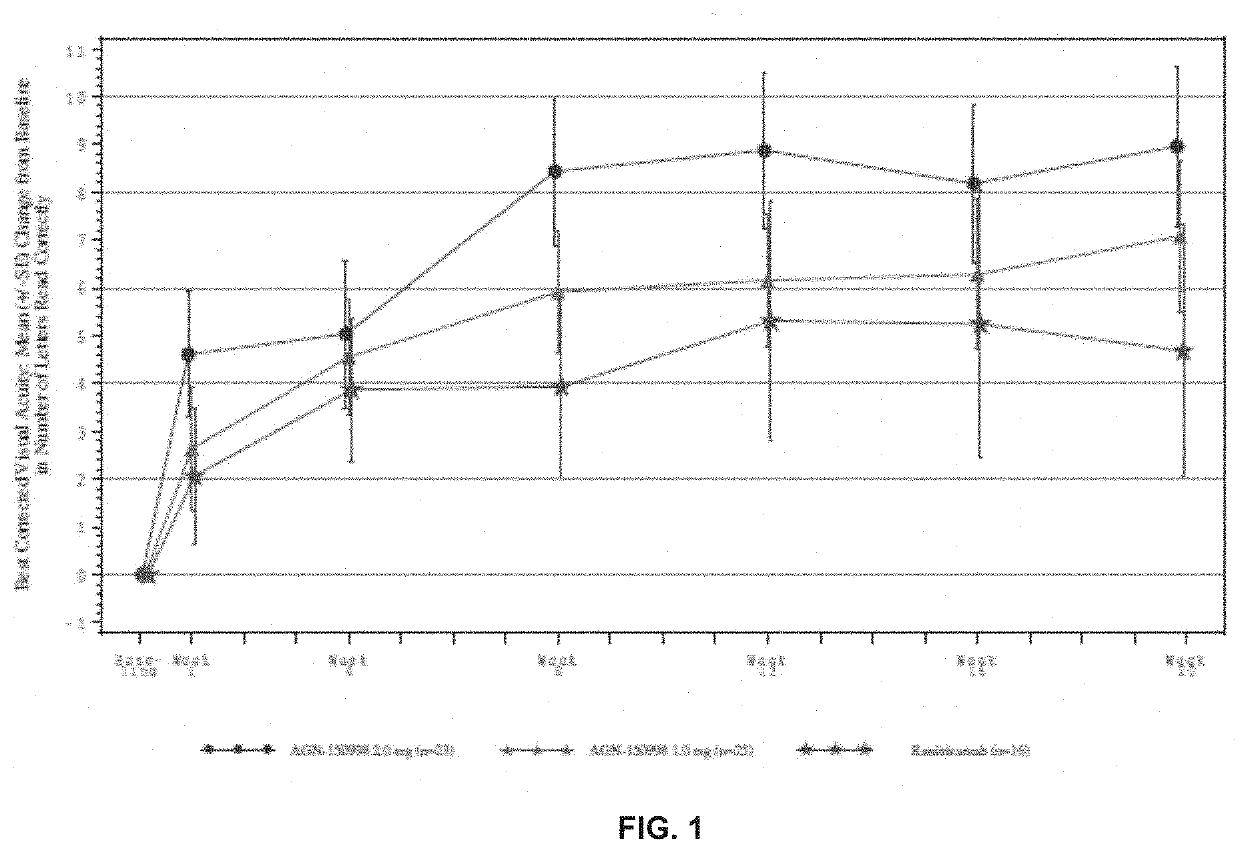
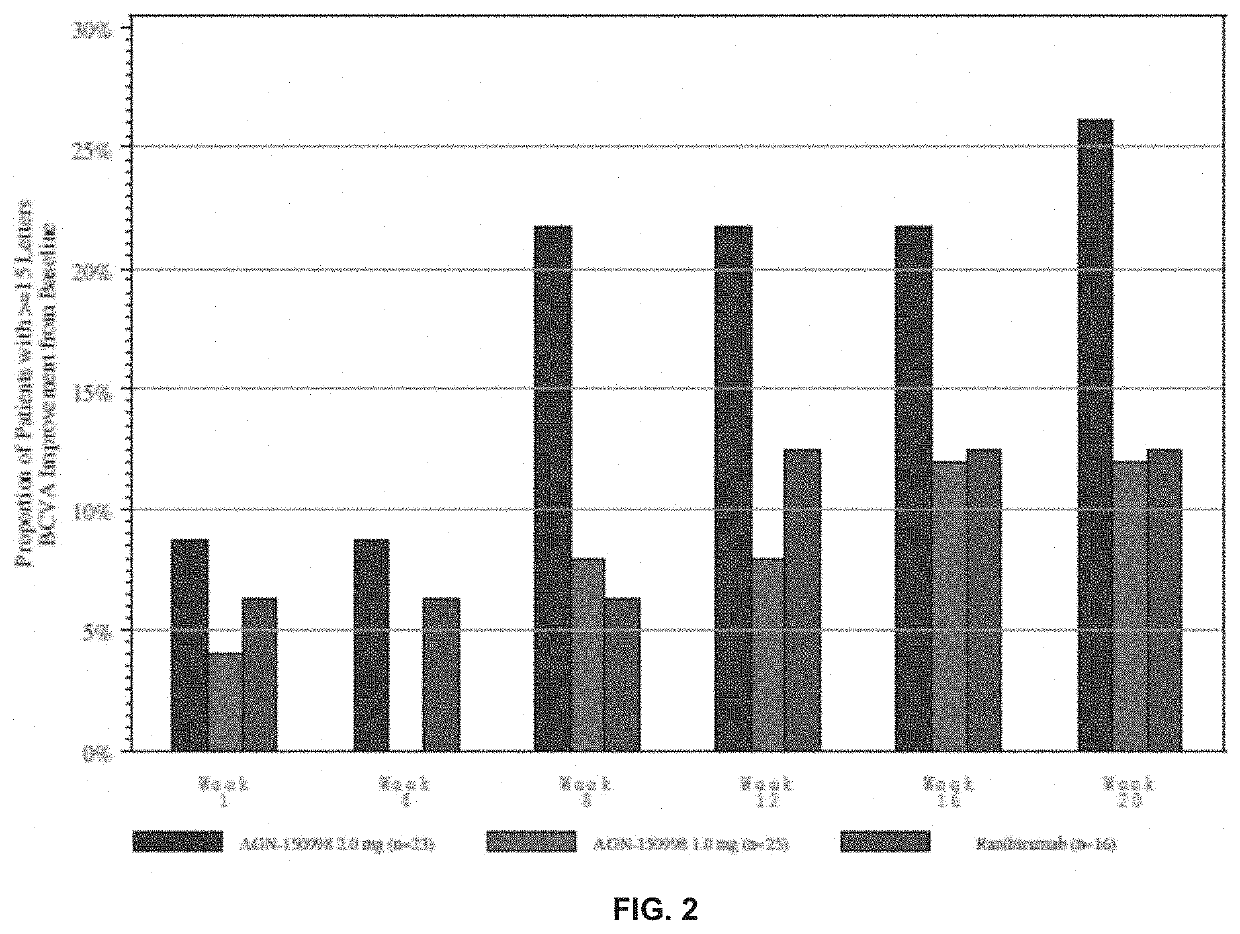
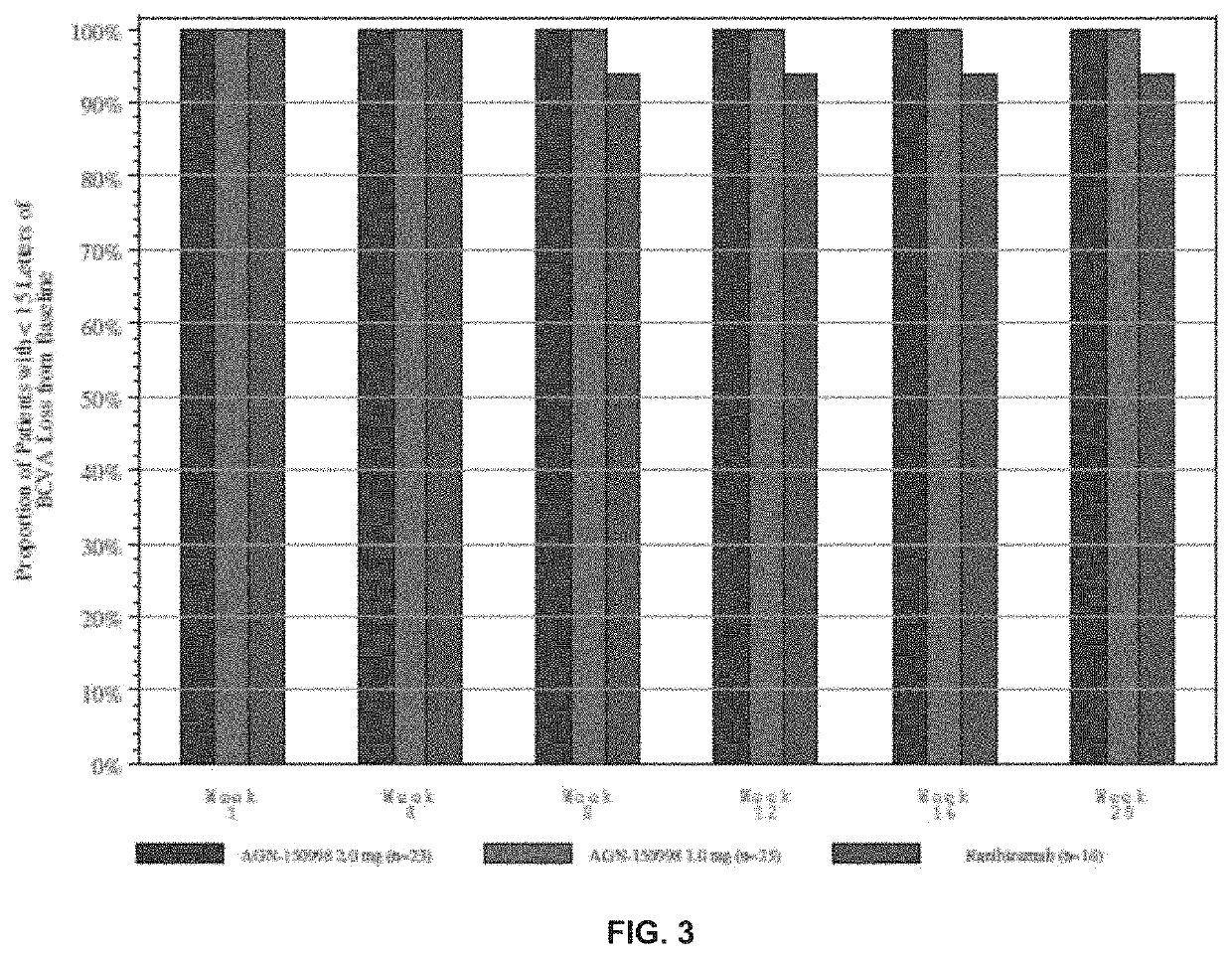
Patents
Literature
46 results about "Ankyrin Repeat Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The ankyrin repeat is a 33-residue motif in proteins consisting of two alpha helices separated by loops, first discovered in signaling proteins in yeast Cdc10 and Drosophila Notch. Domains consisting of ankyrin repeats mediate protein–protein interactions and are among the most common structural motifs in known proteins.
Compositions for treatment of cancer
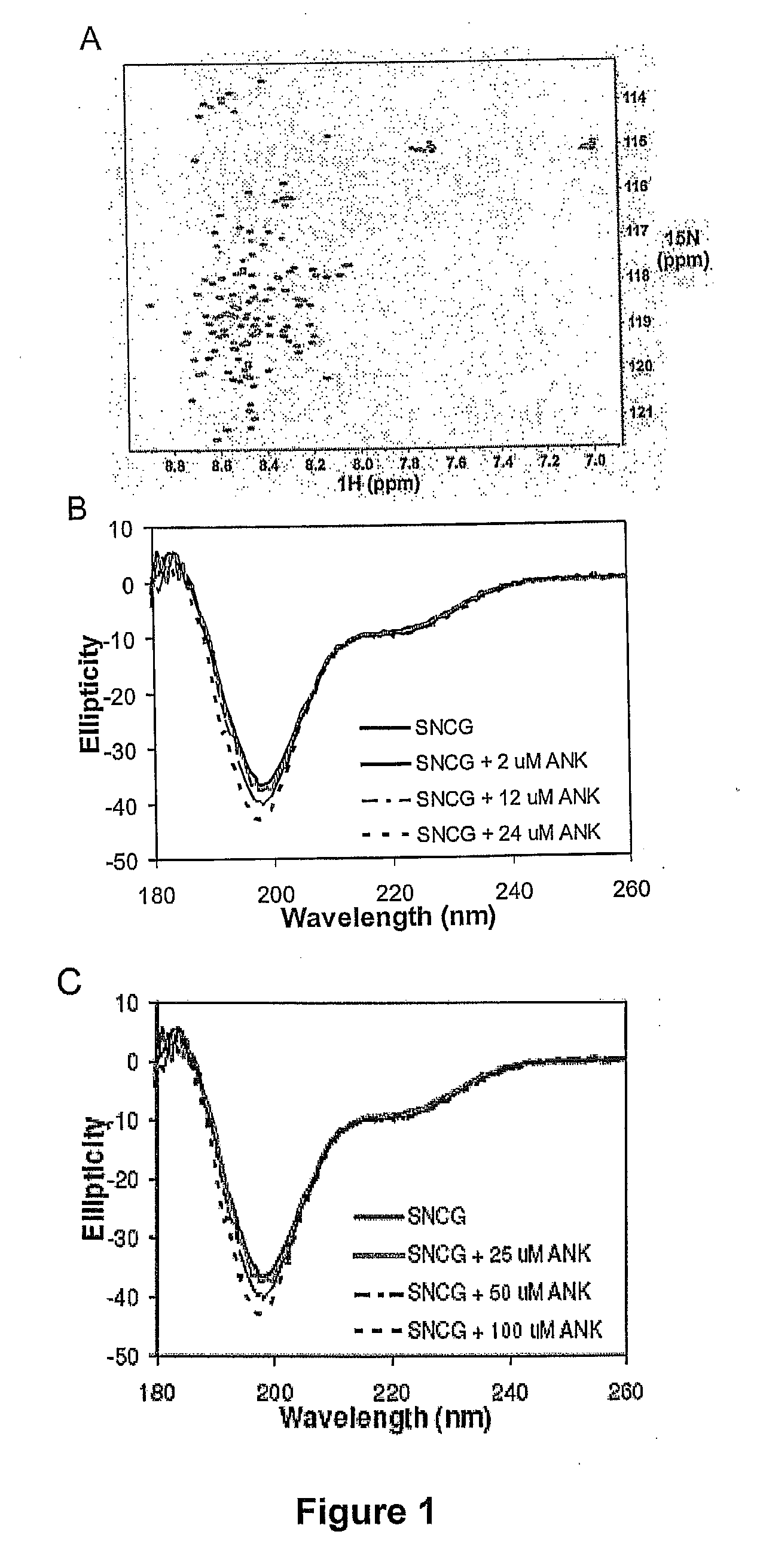
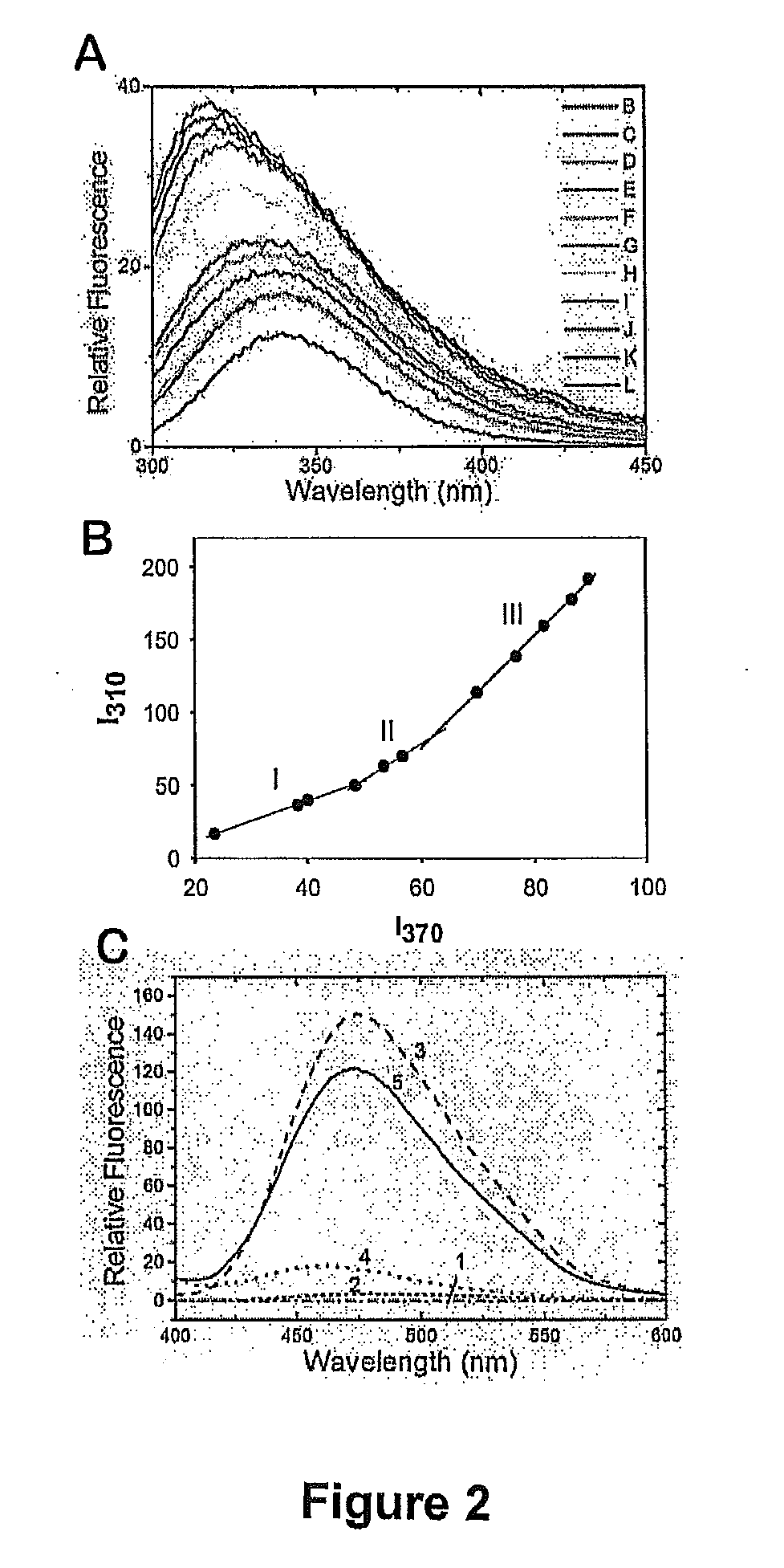
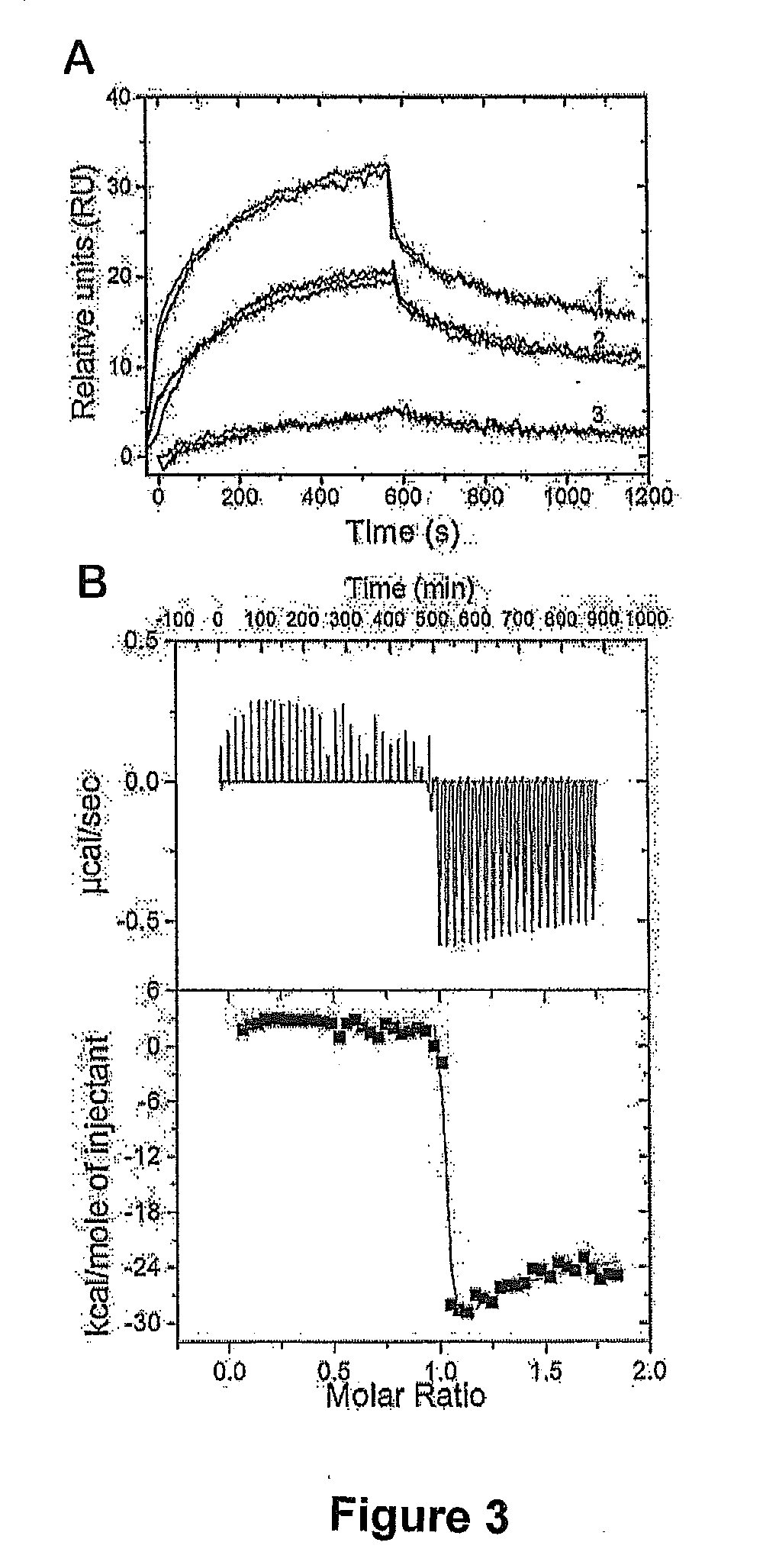
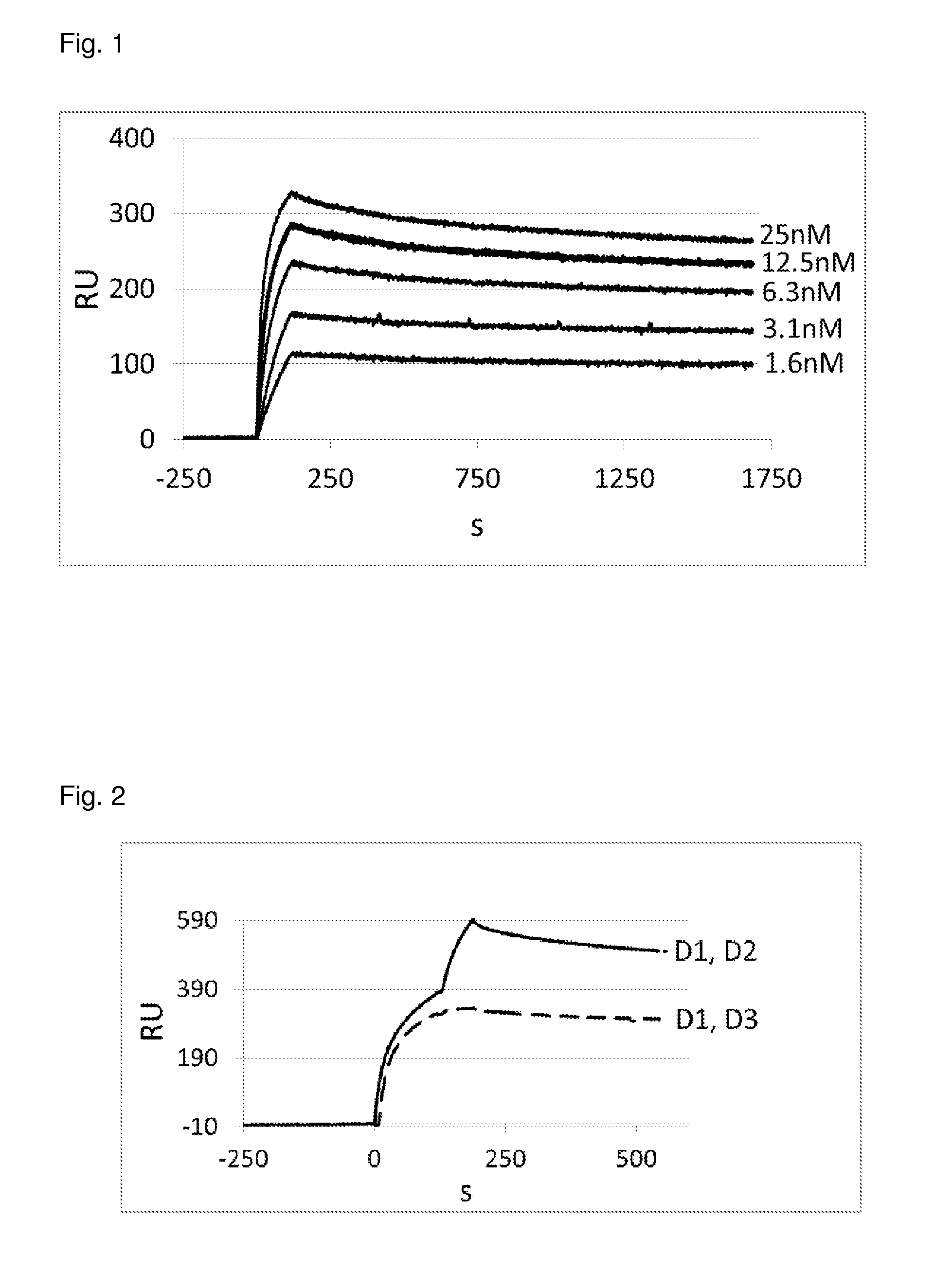
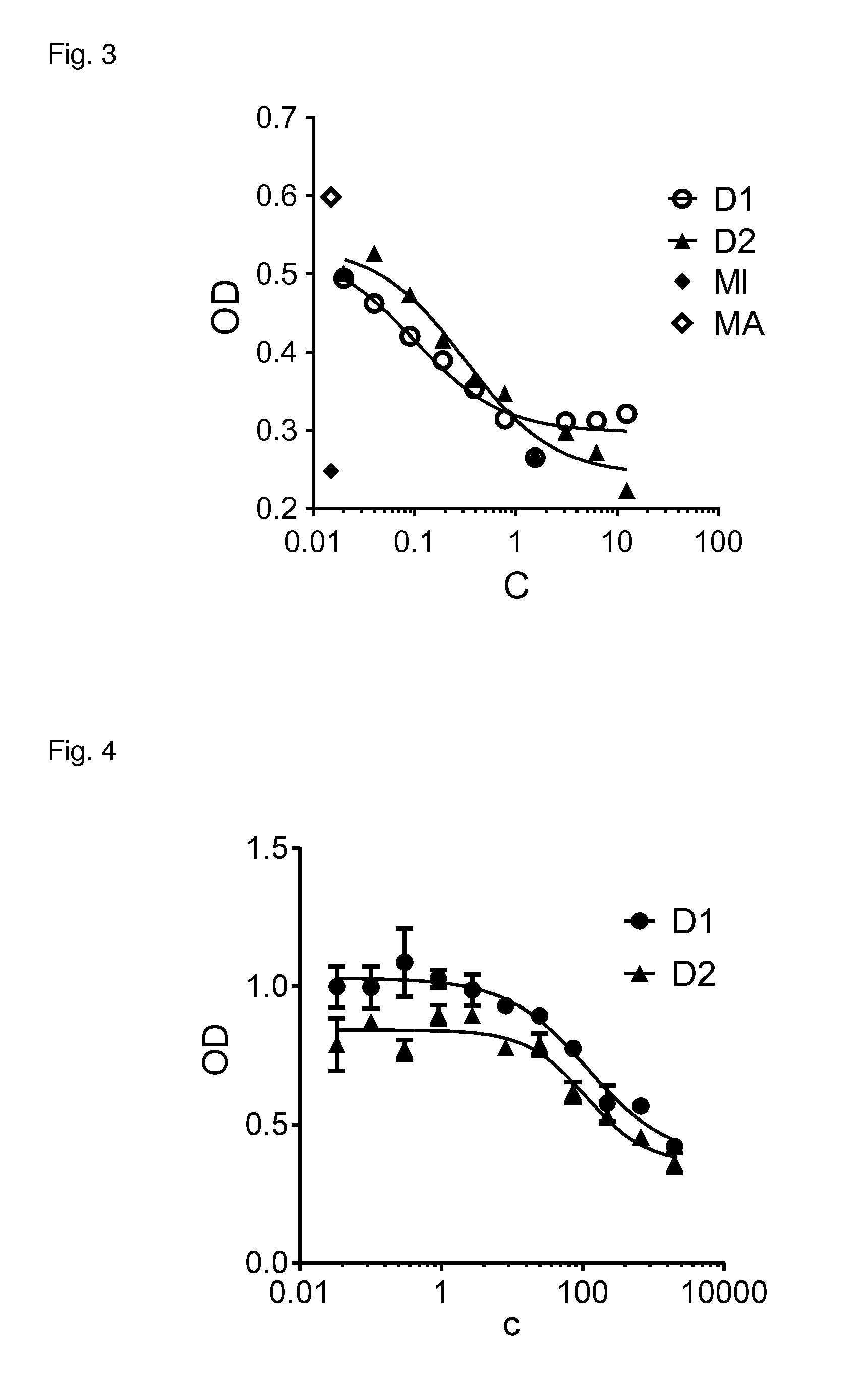
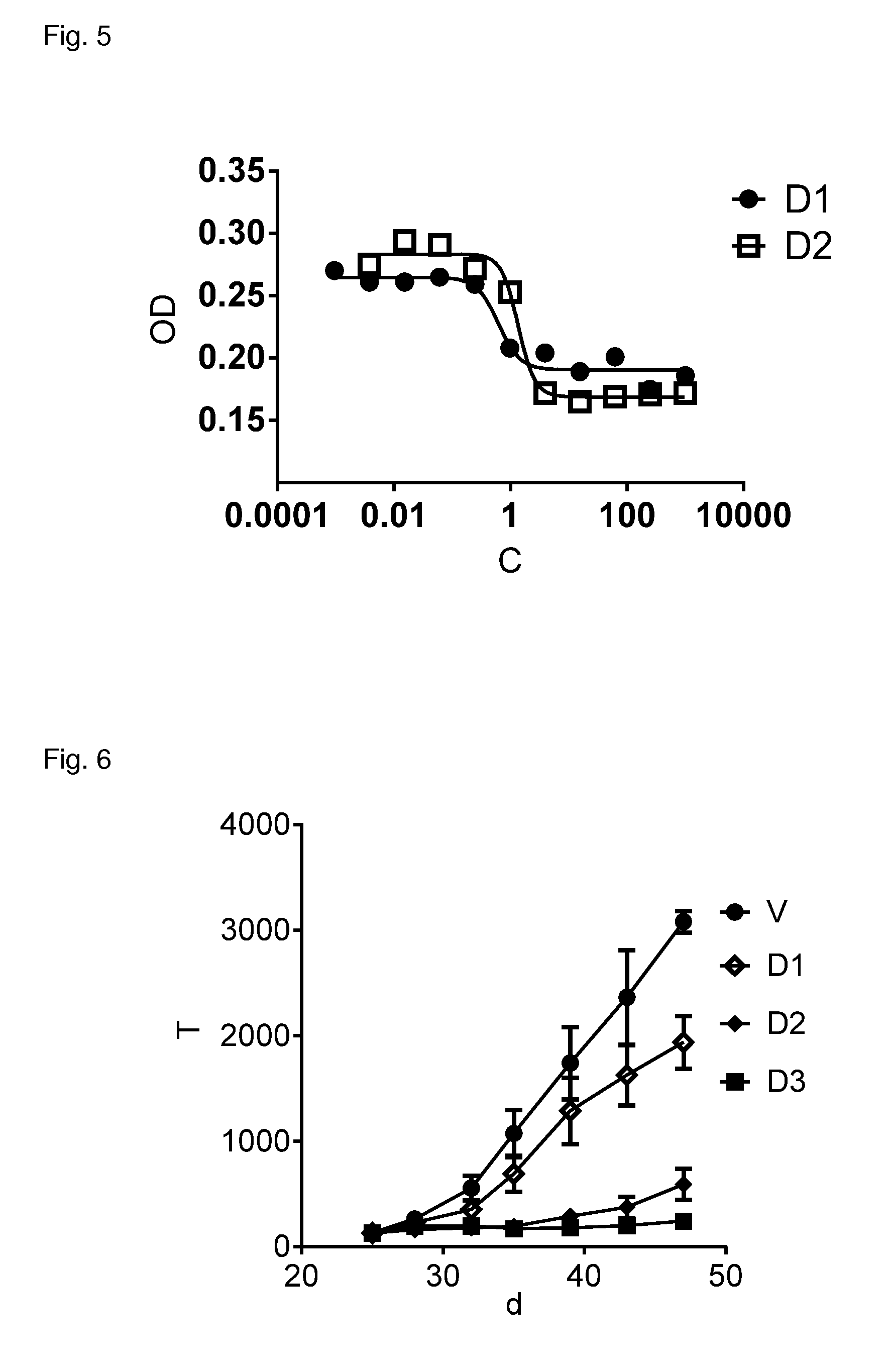
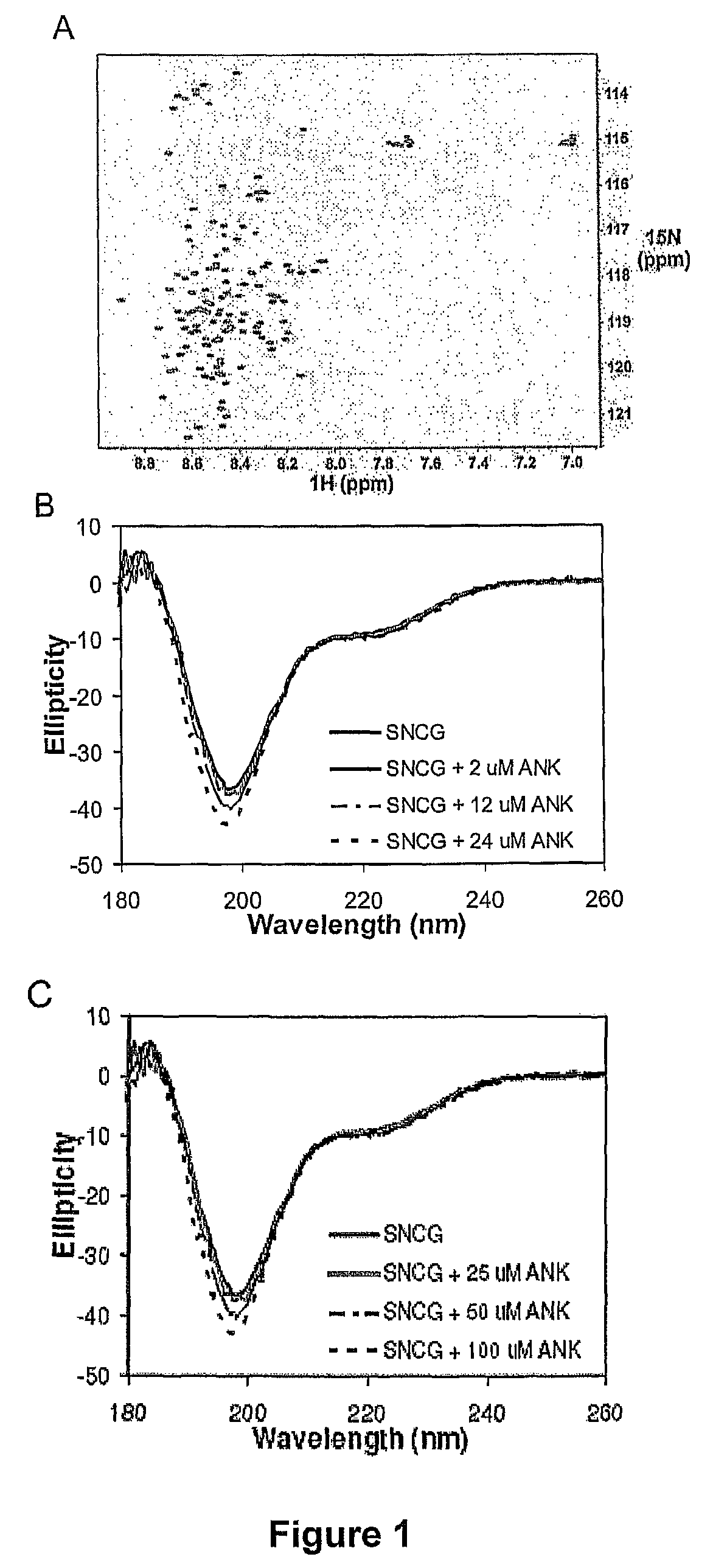
ActiveUS20070238667A1Reduces SNCG-mediated resistanceInhibit cancer cell proliferationPeptide/protein ingredientsAntibody mimetics/scaffoldsAnkyrin Repeat ProteinCancer therapy
Synthetic peptides containing an ankyrin repeat-like motif or portion thereof and mimetics thereof which interact with synuclein-gamma (SNCG) and reduce SNCG-mediated resistance of SNCG-expressing cancer cells to treatment with anticancer drugs or inhibit tumorigenesis and cancer cell proliferation are provided. Compositions containing these peptides, portions thereof or mimetics thereof are also provided. Methods for use of these peptides or portions thereof, compositions, and mimetics thereof in potentiating efficacy of anticancer drugs, in particular microtubule inhibitors and hormonal cancer therapies, and in treating cancer are also provided.
Owner:SINGH VINAY K +1
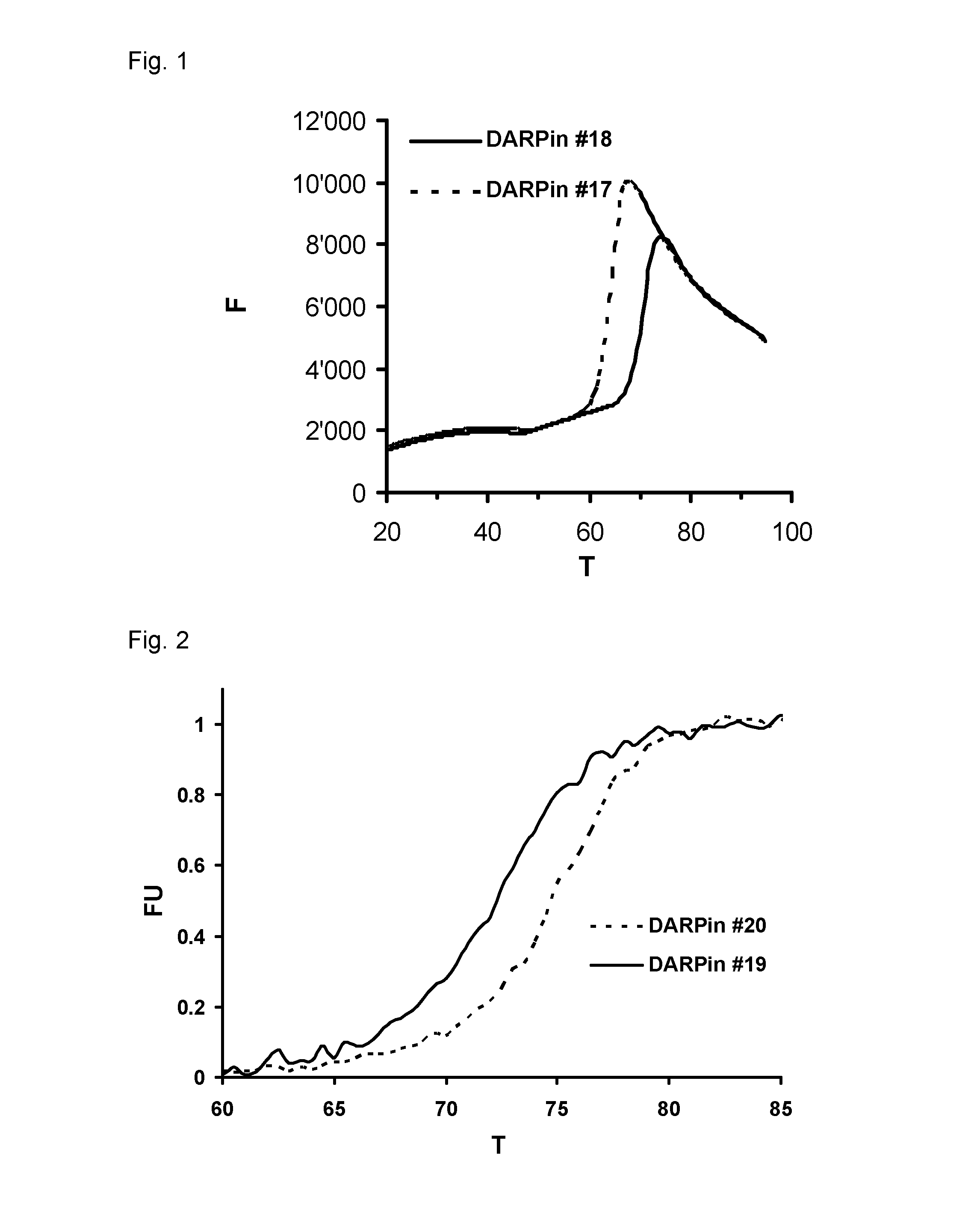
Improved capping modules for designed ankyrin repeat proteins
ActiveUS20130296221A1Improve thermal stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsBiotechnologyDisease
Improved N-terminal capping modules for designed ankyrin repeat proteins (DARPins) conferring improved thermal stability to the DARPins are described, as well as nucleic acids encoding such proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Binding proteins inhibiting the vegf-a receptor interaction
ActiveUS20110207668A1Inhibit bindingSenses disorderPeptide/protein ingredientsWAS PROTEINAnkyrin Repeat Protein
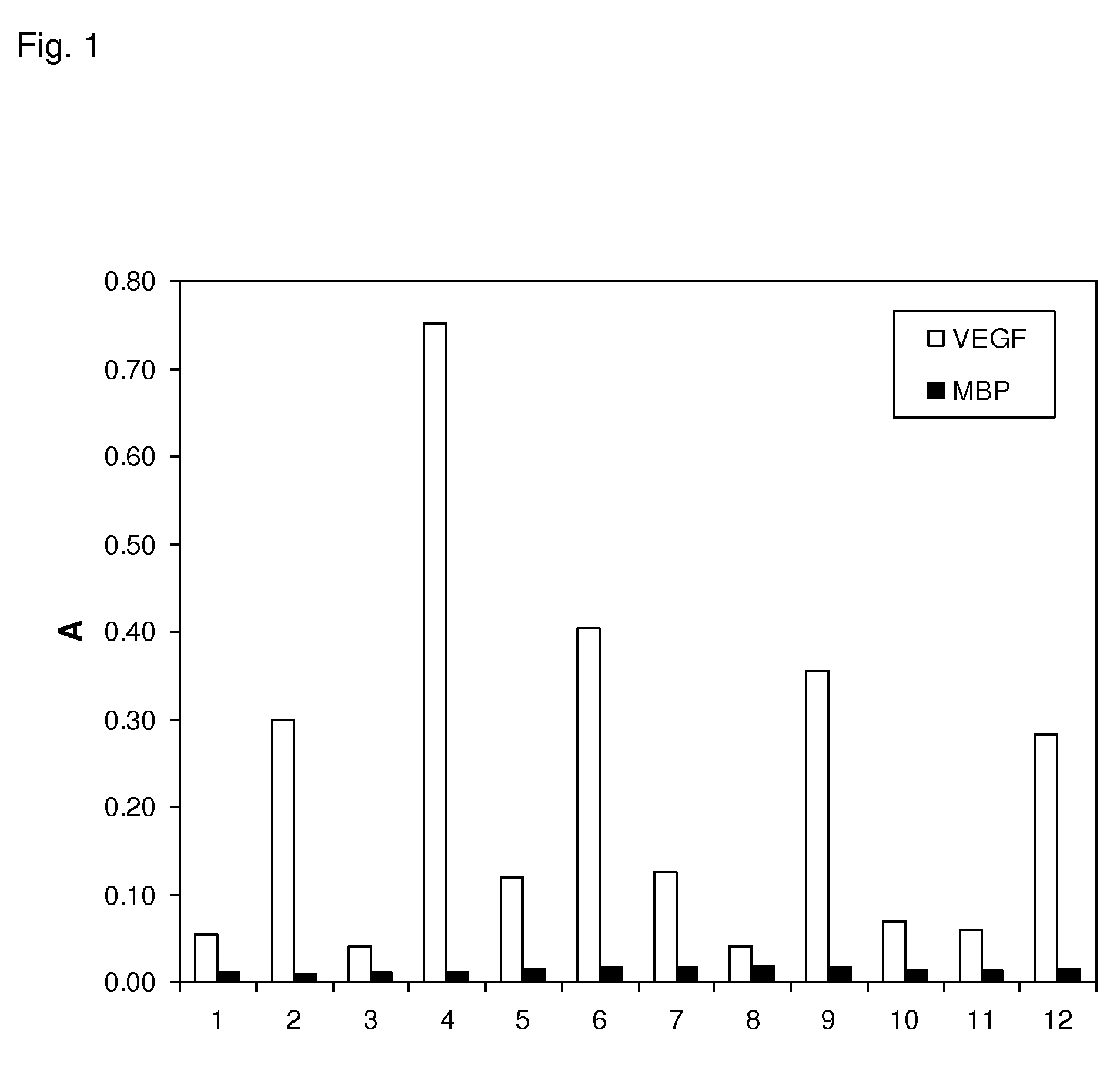
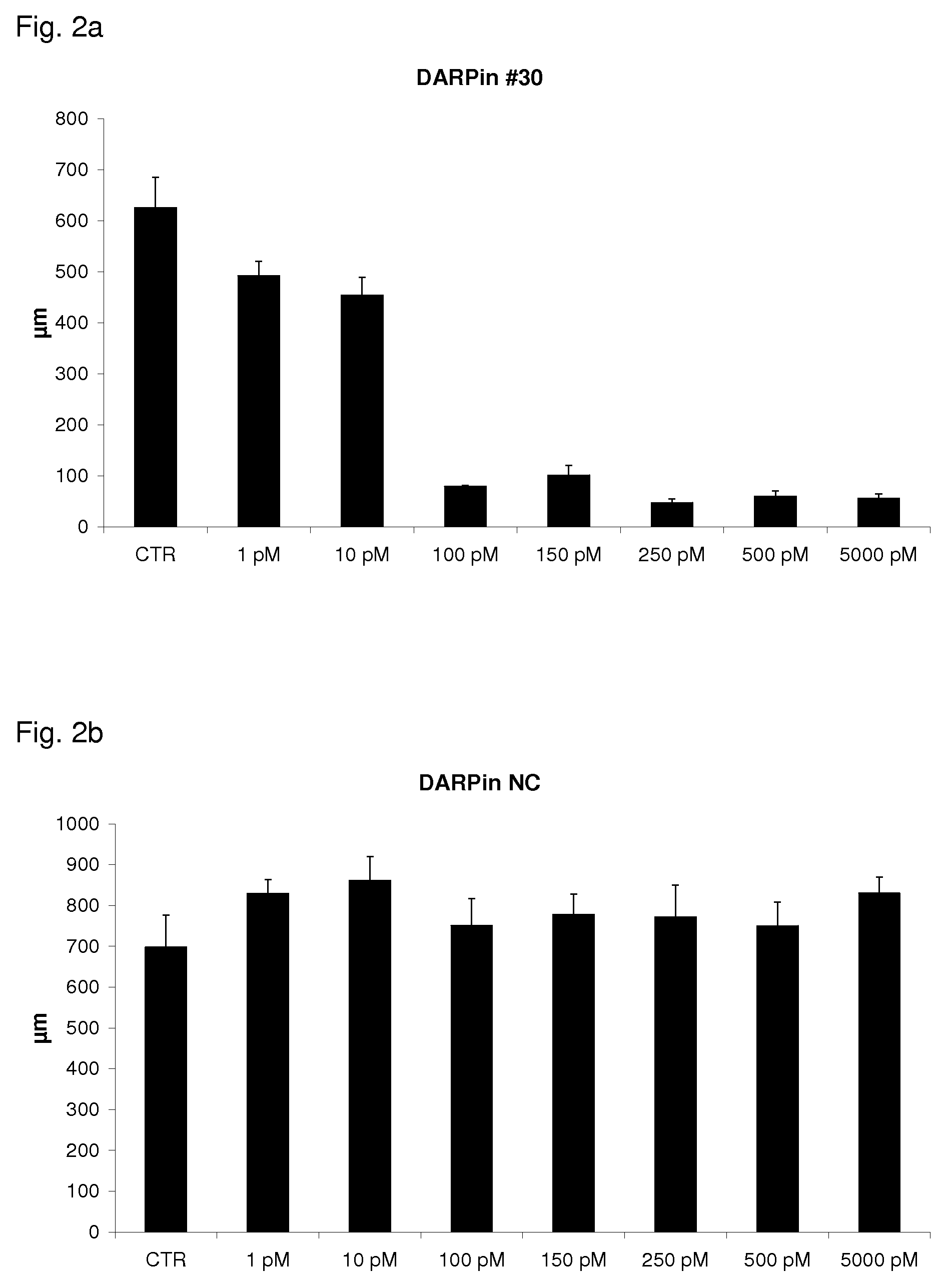
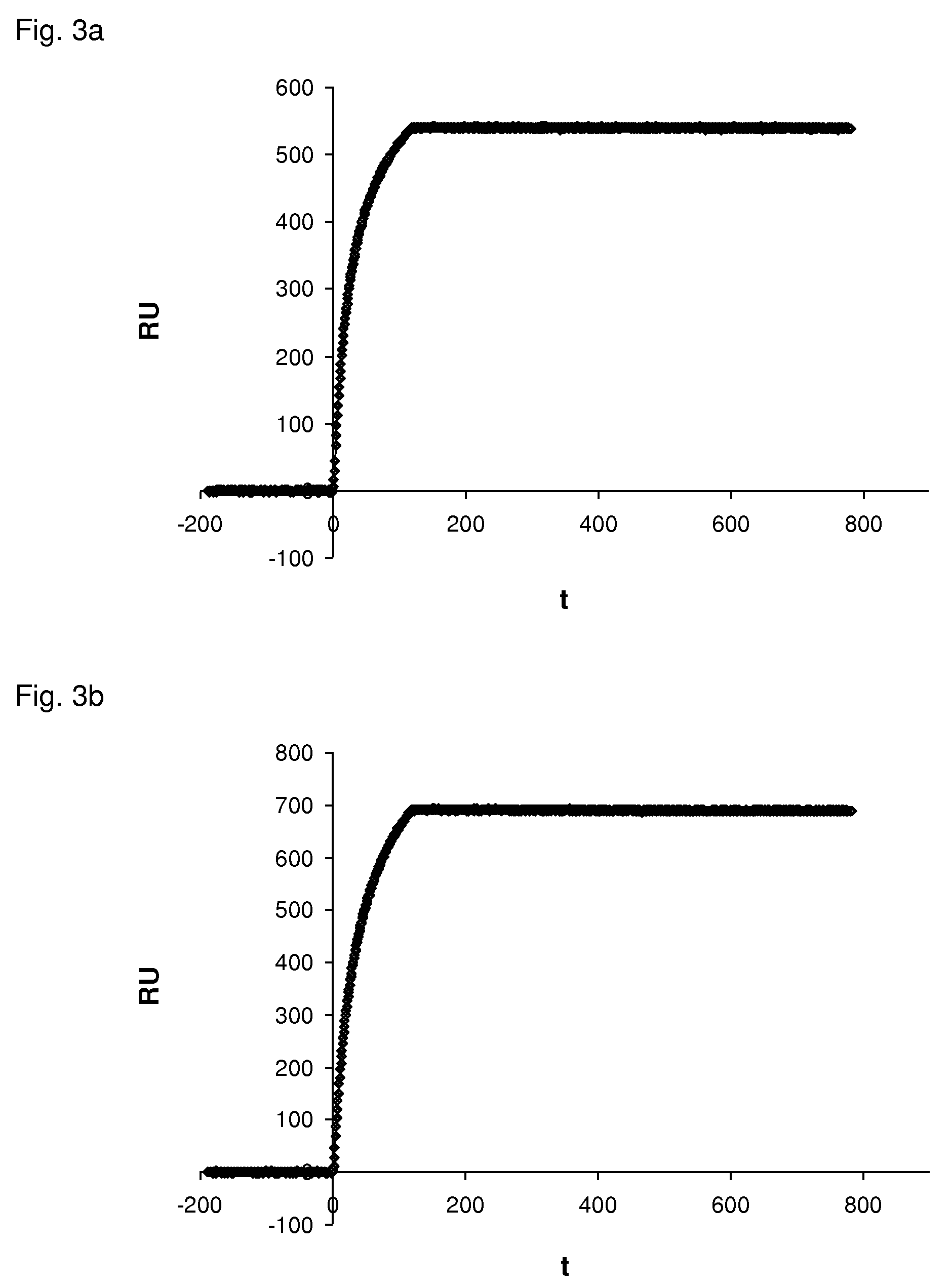
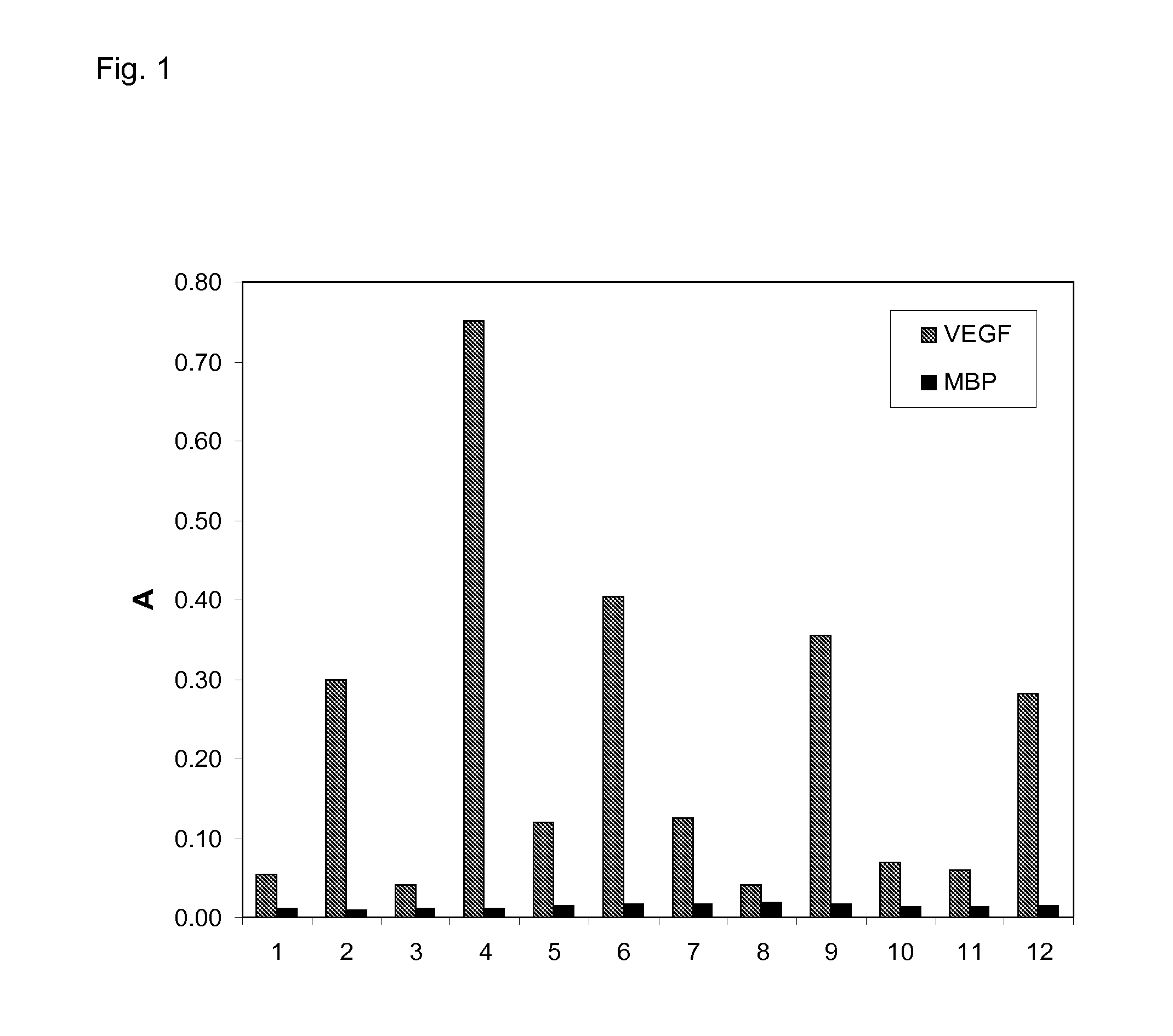
The present invention relates to binding proteins specific for VEGF-A, in particular to recombinant binding proteins comprising a binding domain, which inhibits VEGF-Axxx binding to VEGFR-2. Examples of such binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
Recombinant proteins that simultaneously bind HGF, VEGF-A and serum albumin, comprising ankyrin repeat domains
ActiveUS9458211B1Good storage stabilitySenses disorderNervous disorderSerum protein albuminAnkyrin Repeat Protein
New designed ankyrin repeat domains with binding specificity for serum albumin, recombinant binding proteins comprising at least two designed ankyrin repeat domains with binding specificity for serum albumin, as well as recombinant binding proteins comprising at least one designed ankyrin repeat domain with binding specificity for hepatocyte growth factor (HGF), at least one designed ankyrin repeat domain with binding specificity for vascular endothelial growth factor (VEGF-A), and at least two designed ankyrin repeat domain with binding specificity for serum albumin are described, as well as nucleic acids encoding such designed ankyrin repeat domains and recombinant binding proteins, pharmaceutical compositions comprising such designed ankyrin repeat domains, recombinant binding proteins or nucleic acids and the use of such designed ankyrin repeat domains, recombinant binding proteins, nucleic acids or pharmaceutical compositions in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Designed ankyrin repeat proteins binding to platelet-derived growth factor
New designed ankyrin repeat proteins with binding specificity for PDGF-BB are described, as well as nucleic acids encoding such PDGF binding proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Modified binding proteins inhibiting the VEGF-A receptor interaction
ActiveUS8710187B2Senses disorderPeptide/protein ingredientsAnkyrin Repeat ProteinPolyethylene glycol
The present invention relates to binding proteins specific for Vascular Endothelial Growth Factor A (VEGF-A), in particular to recombinant binding proteins comprising a polyethylene glycol moiety and a binding domain, which inhibits VEGF-Axxx (wherein xxx denotes the amino acid length of the VEGF-A mature protein) binding to Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2). Examples of such recombinant binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity, and a polyethylene glycol moiety. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
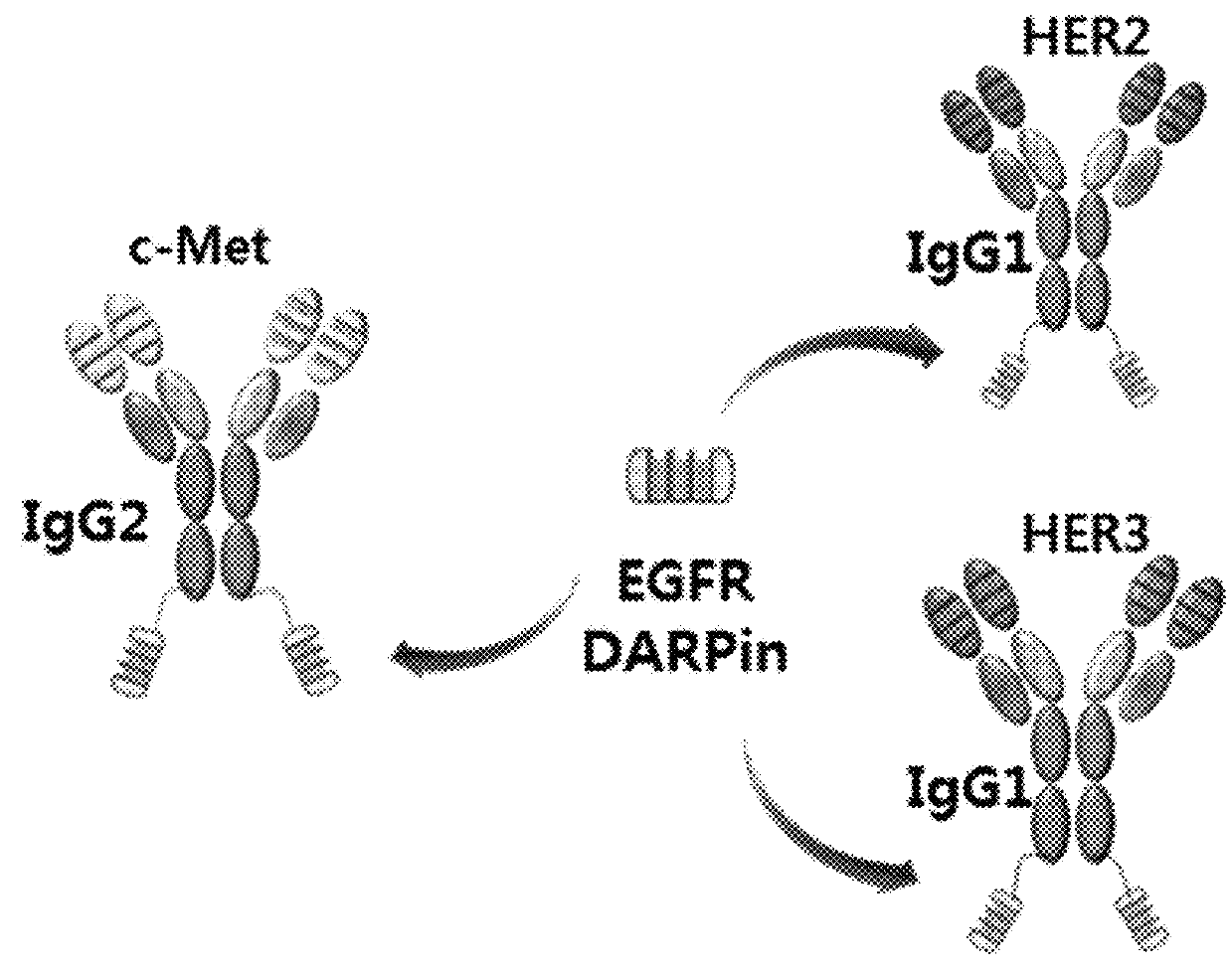
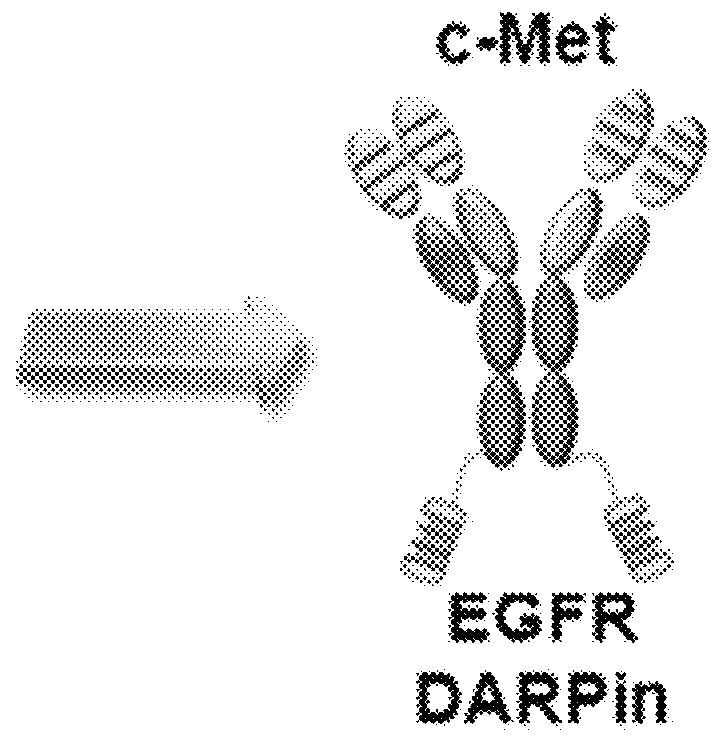
BISPECIFIC CHIMERIC PROTEINS WITH DARPins
ActiveUS20150030596A1Animal cellsCell receptors/surface-antigens/surface-determinantsAnkyrin Repeat ProteinDARPin
Owner:SAMSUNG ELECTRONICS CO LTD
Binding proteins inhibiting the VEGF-A receptor interaction
The present invention relates to binding proteins specific for VEGF-A, in particular to recombinant binding proteins comprising a binding domain, which inhibits VEGF-Axxx binding to VEGFR-2. Examples of such binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
Modified binding proteins inhibiting the vegf-a receptor interaction
ActiveUS20130116197A1Senses disorderPeptide/protein ingredientsAnkyrin Repeat ProteinPolyethylene glycol
The present invention relates to binding proteins specific for VEGF-A, in particular to recombinant binding proteins comprising a polyethylene glycol moiety and a binding domain, which inhibits VEGF-Axxx binding to VEGFR-2. Examples of such recombinant binding proteins are proteins which comprise an ankyrin repeat domain with the desired binding specificity, and a polyethylene glycol moiety. The binding proteins are useful in the treatment of cancer and other pathological conditions, e.g. eye diseases such as age-related macular degeneration.
Owner:MOLECULAR PARTNERS AG
Recombinant newcastle disease virus
InactiveCN101056646ASsRNA viruses negative-senseCosmetic preparationsPeptide ligandAnkyrin Repeat Protein
The goal of the invention is to increase the therapeutical activity of oncolytic NDV. This issue is solved by a Newcastle Disease Virus comprising a recombinant nucleic acid, wherein the nucleic acid codes for a binding protein that has a therapeutic activity when expressed by the virus-infected tumor cell. Binding proteins belong to the following group: A natural ligand or a genetically modified ligand, a recombinant soluble domain of a natural receptor or a modified version of it, a peptide-ligand, an antibody molecule and derivatives thereof or antibody-like molecules like ankyrin repeat molecules or derivatives thereof.
Owner:BAYER SCHERING PHARMA OY
Human diacylglycerol kinase iota
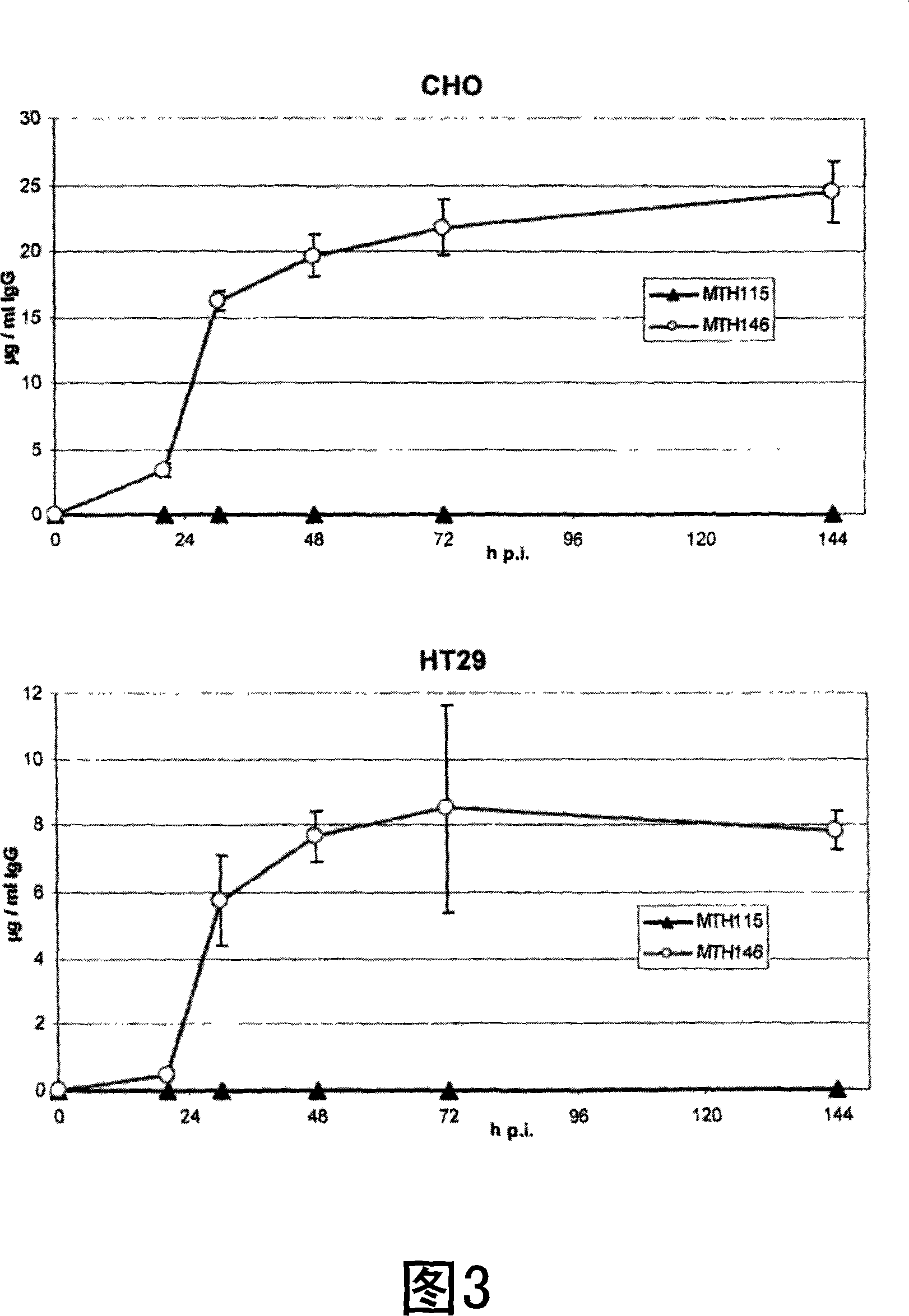
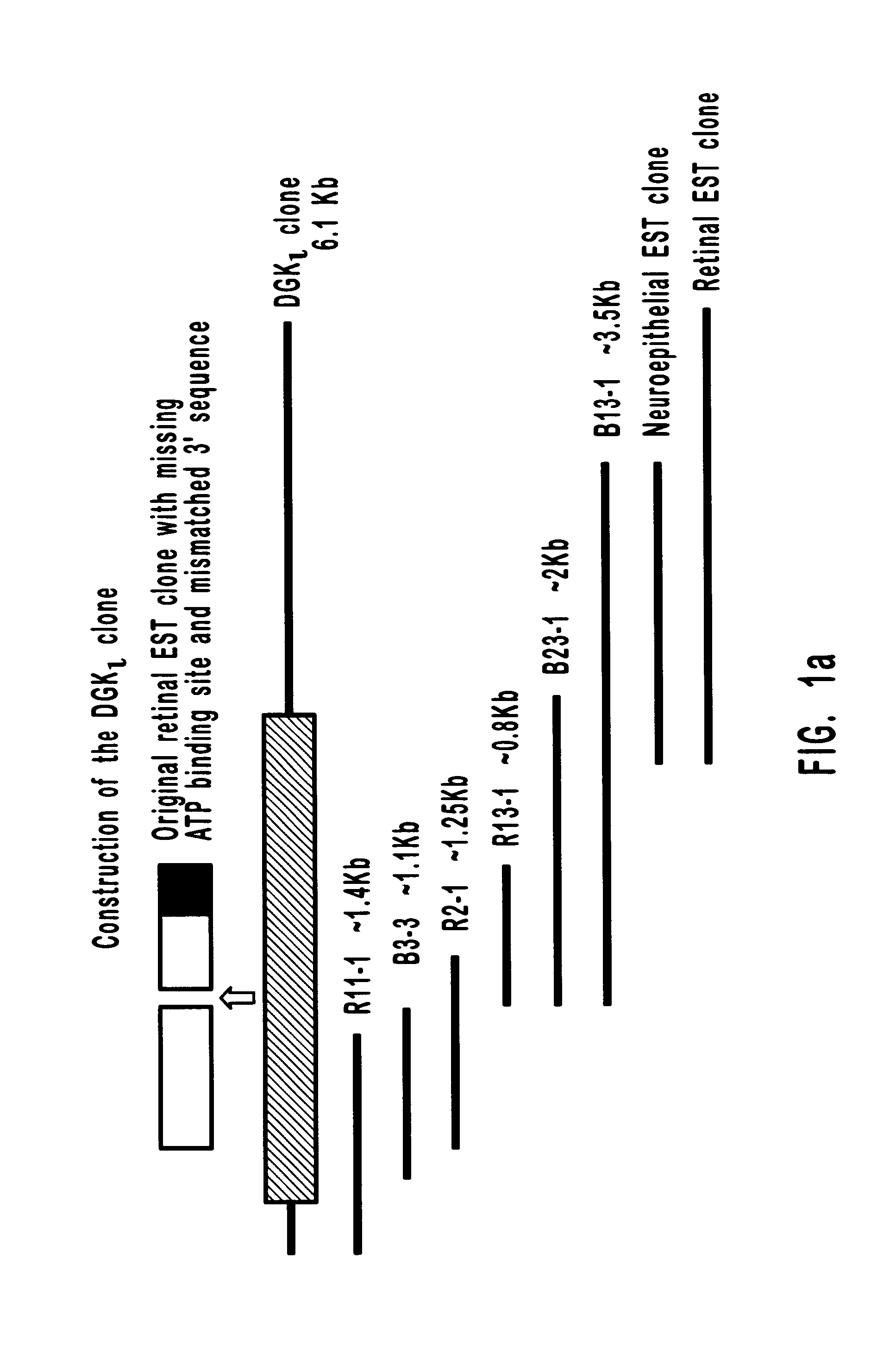
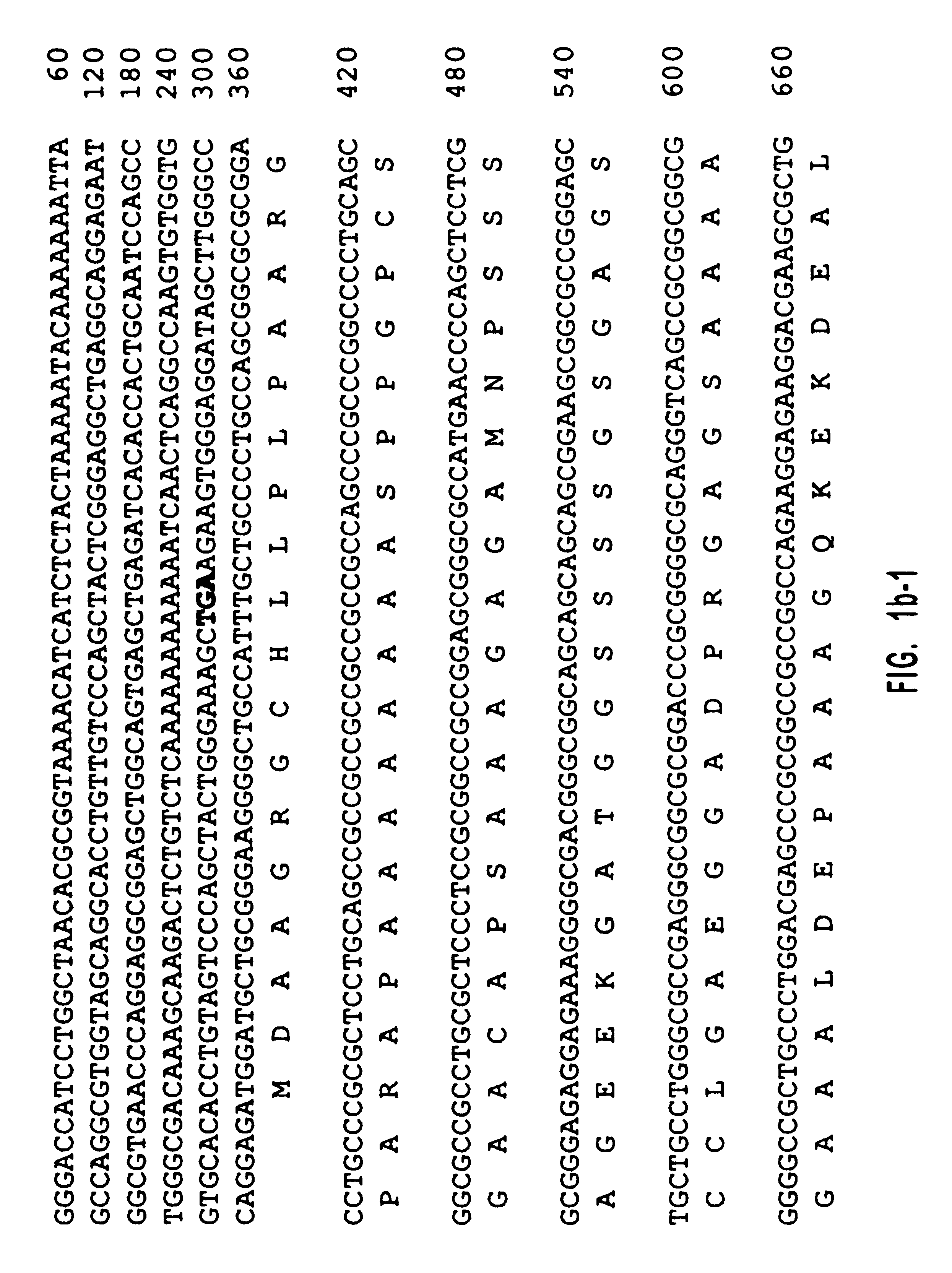
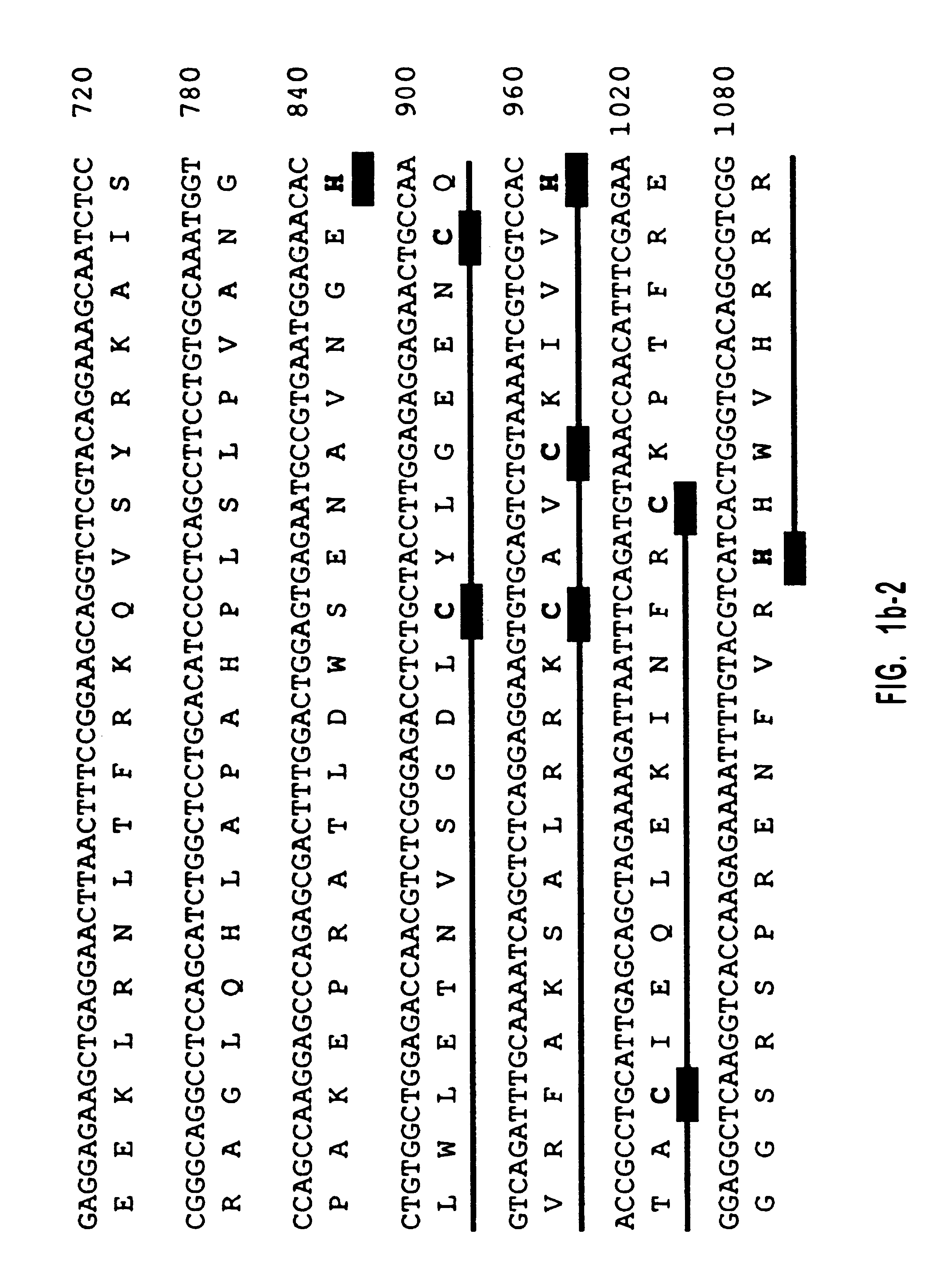
Diacylglycerol (DAG) plays a central role in both the synthesis of complex lipids and in intracellular signaling; diacylglycerol kinase (DGK) catalyzes the phosphorylation of DAG, which yields phosphatidic acid. A family of DGKs has been identified in multicellular organisms over the past few years, but the physiological function(s) of this diversity is not clear. One clue has come from the Drosophila DGK2, rdgA, since mutations in this gene cause retinal degeneration. The present invention relates to a novel DGK, designated DGKι, which was isolated from human retina and brain libraries. DGKι contains two cysteine-rich repeats, a region similar to the phosphorylation site domain of MARCKS, a conserved catalytic domain, and four ankyrin repeats at its C-terminus. By primary structure, DGKι is most similar to human DGKζ and Drosophila rdgA. A>12 kb mRNA for DGKι was detected only in brain and retina among the tissues examined. In cells transfected with the DGKι cDNA, an approximately 130 kDa protein was detected by immunoassay, and activity assays demonstrated that it encodes a functional DAG kinase. The protein was found to be in both the cytoplasm and nucleus, with this localization controlled by PKC isoforms alpha and gamma. The gene encoding DGKι was localized to human chromosome 7q32.3-33, which is known to be a locus for an inherited form of retinitis pigmentosa. These results have defined a novel isoform of DAG kinase, which may have important cellular functions in the retina and brain.
Owner:THE UNIV OF UTAH
Designed ankyrin repeat proteins binding to hepatocyte growth factor
ActiveUS20160251404A1Peptide/protein ingredientsHepatocyte-growth/scatter/tumor-cytotoxic factorDiseaseAnkyrin Repeat Protein
New designed ankyrin repeat proteins with binding specificity for HGF are described, as well as nucleic acids encoding such HGF binding proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Compositions for treatment of cancer
ActiveUS7897568B2Reduces SNCG-mediated resistanceReduces toxicity and side effectPeptide/protein ingredientsAntibody mimetics/scaffoldsCancer cell proliferationDrug treatment
Synthetic peptides containing an ankyrin repeat-like motif or portion thereof and mimetics thereof which interact with synuclein-gamma (SNCG) and reduce SNCG-mediated resistance of SNCG-expressing cancer cells to treatment with anticancer drugs or inhibit tumorigenesis and cancer cell proliferation are provided. Compositions containing these peptides, portions thereof or mimetics thereof are also provided. Methods for use of these peptides or portions thereof, compositions, and mimetics thereof in potentiating efficacy of anticancer drugs, in particular microtubule inhibitors and hormonal cancer therapies, and in treating cancer are also provided.
Owner:SINGH VINAY K +1
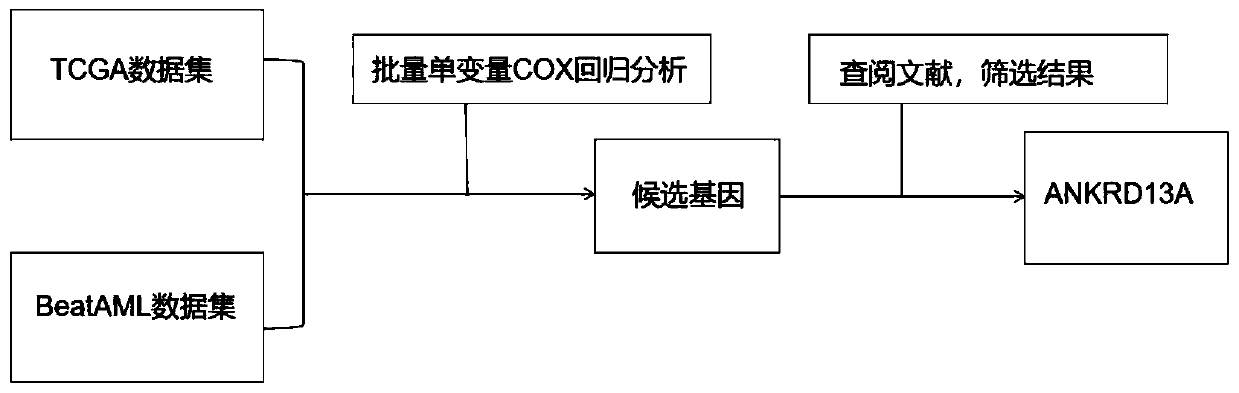
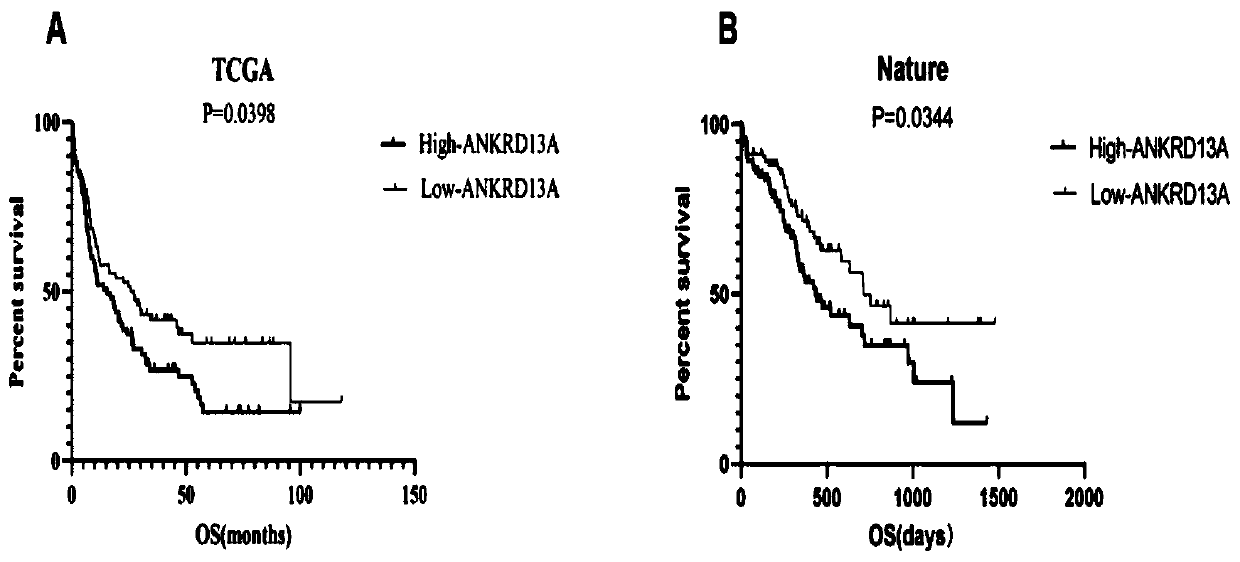
Novel application of ankyrin repeat domain-containing 13A gene and/or protein encoded by the same
PendingCN110938695AImprove relevancePeptide/protein ingredientsMicrobiological testing/measurementAnkyrin Repeat ProteinAcute myeloid leukemias
The invention discloses novel application of an ankyrin repeat domain-containing 13A gene and / or a protein encoded by the same. An acute myeloid leukemia (AML) diagnosis and prognosis related molecular marker is screened to obtain an ankyrin repeat domain-containing 13A gene(ANKRD13A) closely related to AML patient prognosis; the high expression of ANKRD13A in the body of an AML patient warns poorprognosis; the ANKRD13A can be used as an independent factor for influencing the prognosis of the AML patient; the ANKRD13A is related to the FAB typing and the cytogenetics risk stratification of the AML patient; and the ANKRD13A may participate in the disease progression of AML through a variety of pathways. The ANKRD13A can be used for preparing a therapeutic drug or a prognosis detection reagent for the acute myeloid leukemia.
Owner:SHANDONG UNIV QILU HOSPITAL
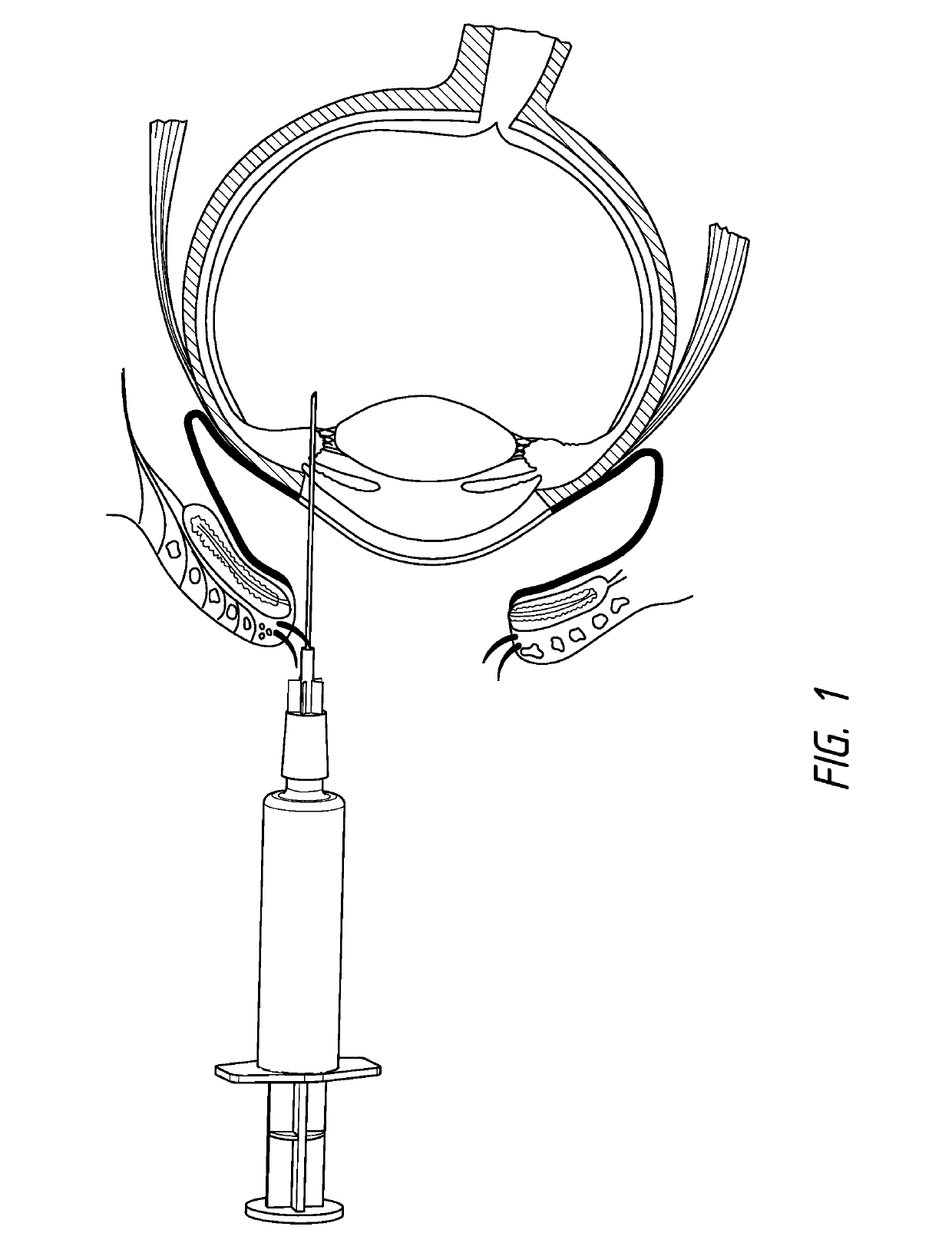
Method of treating AMD in patients refractory to Anti-vegf therapy
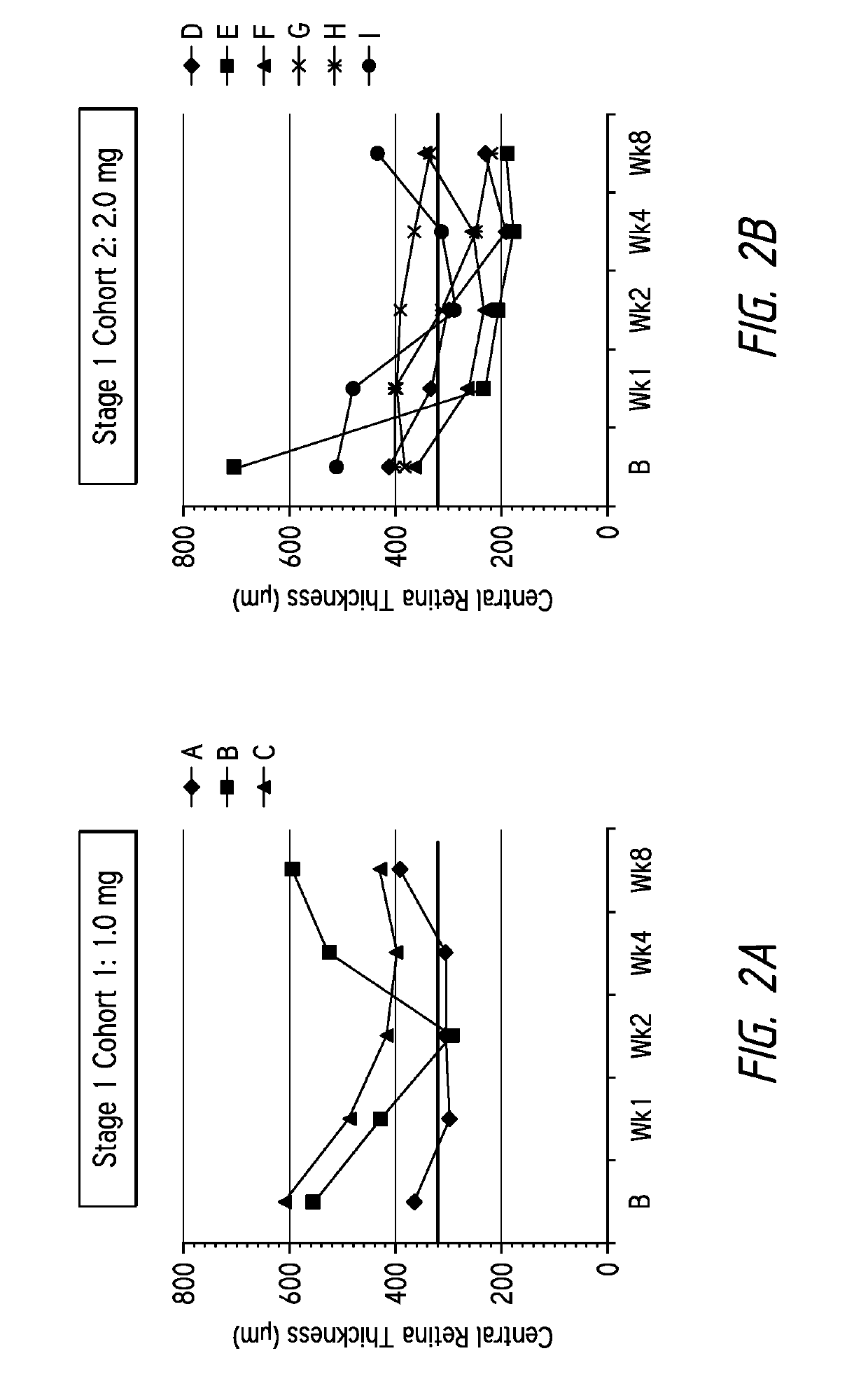
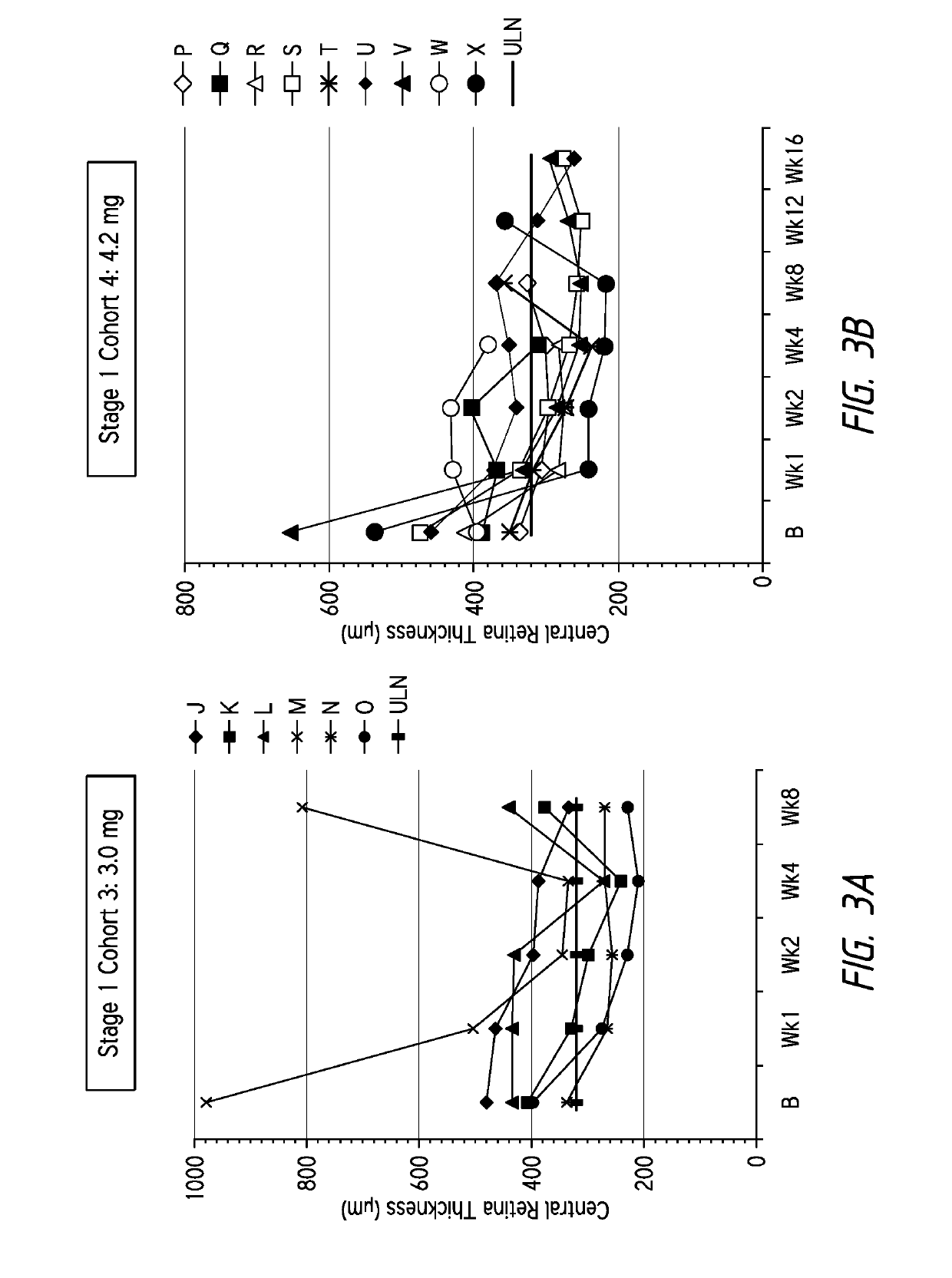
ActiveUS20130296238A1Few injectionTreated conditionSenses disorderPeptide/protein ingredientsAnkyrin Repeat ProteinAnti vegf
Disclosed herein are methods for the treatment of a patient having an ocular conditions such as age-related macular degeneration or diabetic macular edema through administration of a recombinant binding protein comprising an ankyrin repeat domain. In some embodiments, the composition may be administered every 8 weeks to every 16 weeks. In some embodiments, the patient being treated may be refractory to existing anti-VEGF therapies.
Owner:ALLERGAN INC
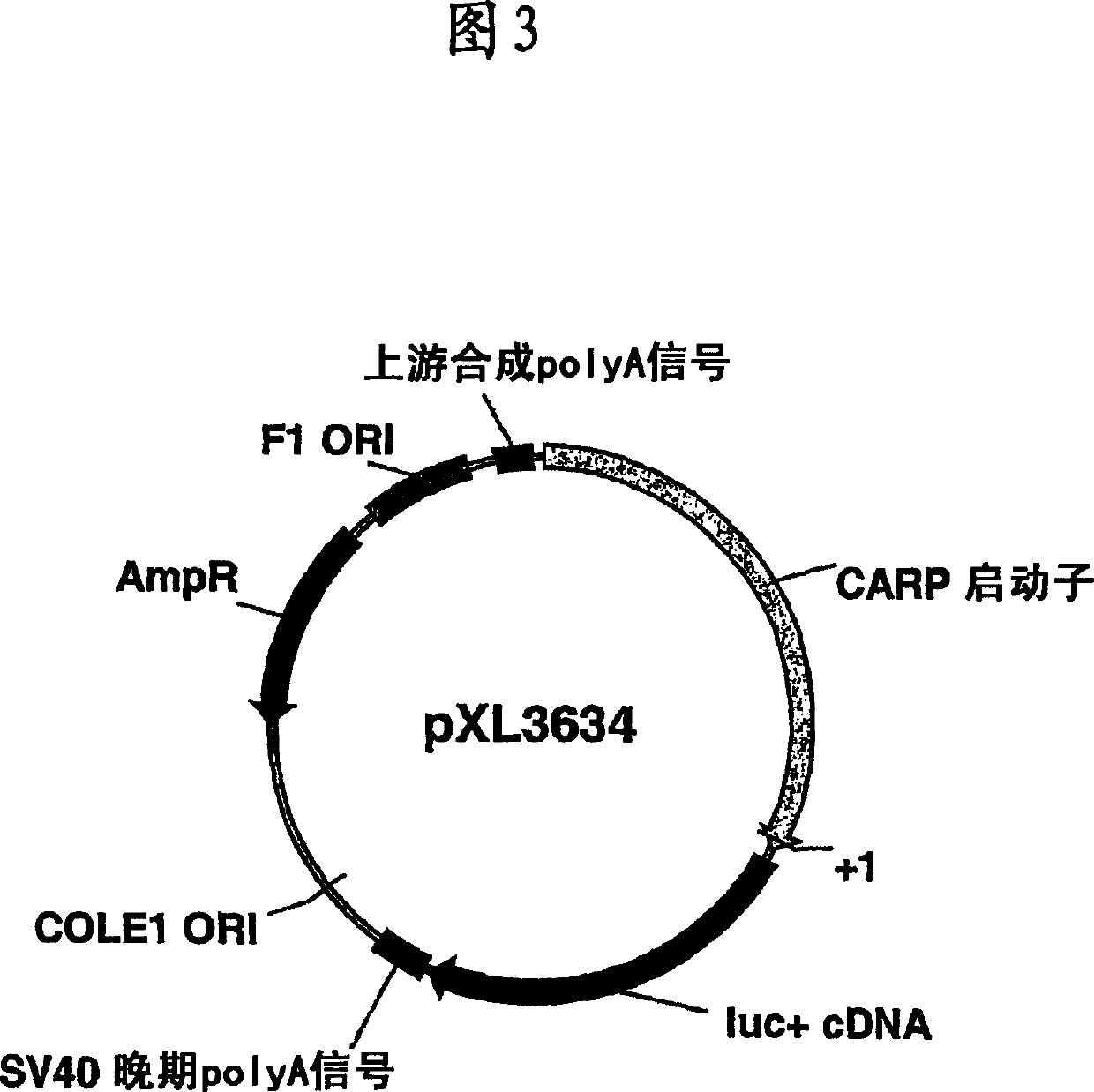
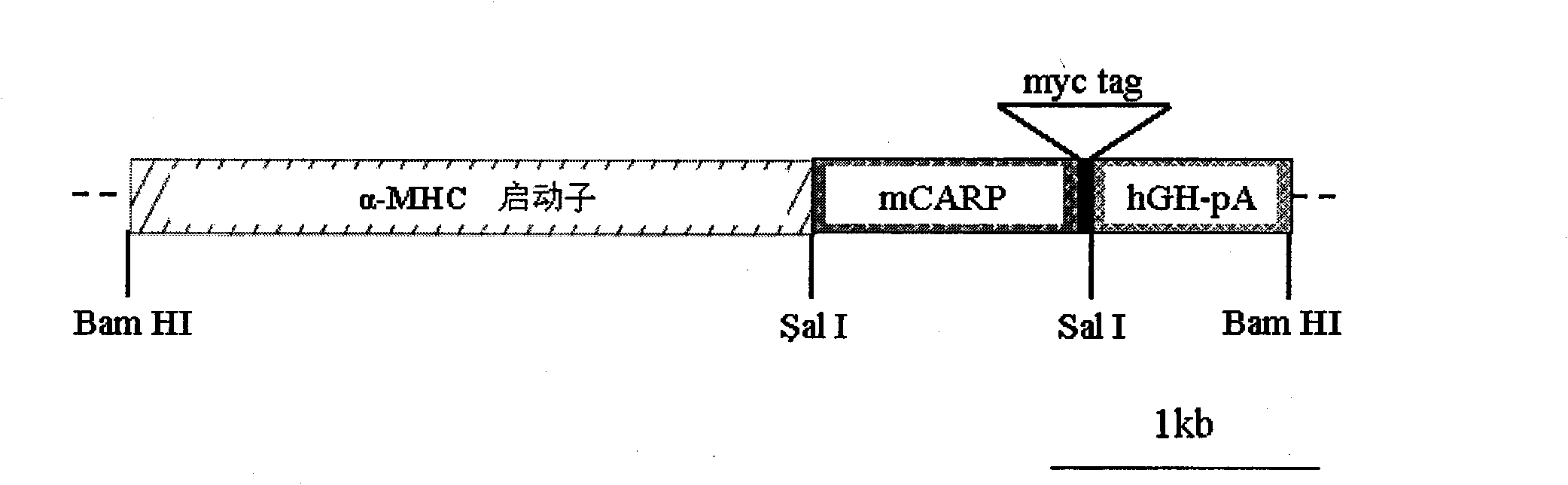
Heart ankyrin repeat protein gene upstream sequence, carrier containing the same sequence and usage thereof
The invention relates to novel promoter sequences derived from a portion upstream of the coding sequence of the gene for the CARP protein (Cardiac Ankyrin Repeat Protein), and which are capable of controlling the level and the specificity of expression of a transgene in vivo in cardiac muscle cells. The invention thus describes novel compositions, constructs, vectors and their uses in vivo for the transfer and expression of a nucleic acid in vivo in cardiac muscle cells. The subject of the present invention is also the use of the promoter sequences for generating transgenic animals which constitute models for studying certain cardiac pathologies.
Owner:GENCELL SA +1
Designed ankyrin repeat proteins binding to platelet-derived growth factor
New designed ankyrin repeat proteins with binding specificity for PDGF-BB are described, as well as nucleic acids encoding such PDGF binding proteins, pharmaceutical compositions comprising such proteins and the use of such proteins in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
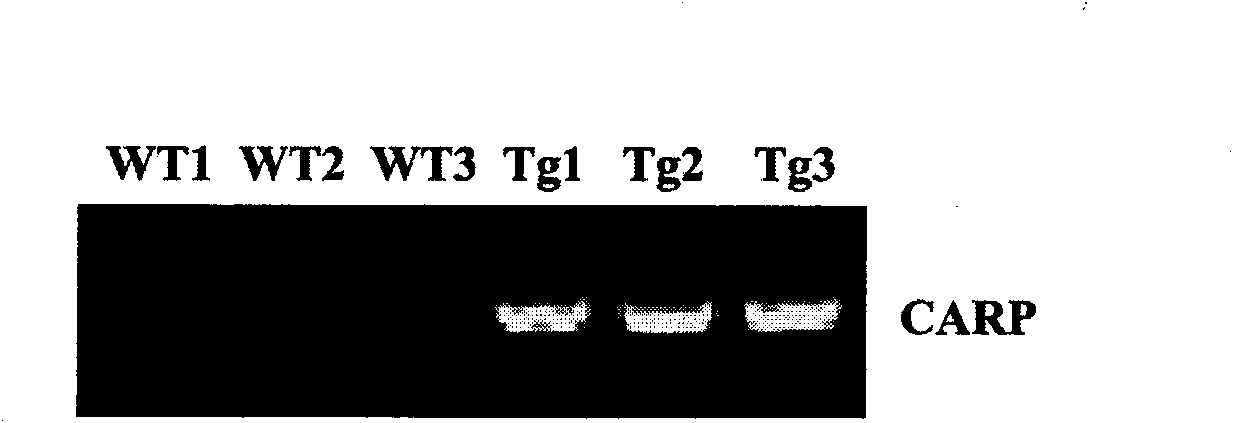
Transgenic animal over-expressing cardiac ankyrin repeat protein as well as production method and application thereof
InactiveCN101953319APeptide/protein ingredientsVector-based foreign material introductionAnkyrin Repeat ProteinCardiac muscle
The invention relates to a transgenic animal over-expressing a cardiac ankyrin repeat protein as well as a production method and application thereof. The transgenic animal specifically over-expresses the cardiac ankyrin repeat protein in a heart tissue can partially inhibit the cardiac hypertrophy induced by isoprenaline and / or the cardiac hypertrophy induced by heat aortic coarctation.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Diagnostic application of mail
The present invention provides nucleic acids. Polypeptides, antibodies and cells related to MAIL (Molecule possessing Ankyrin repeats Induced by LPS; or IκB-ζ), a novel member of the IκB family, expressed in LPS stimulated monocytes and uses thereof. The isolated nucleic acids, polypeptides, antibodies and cells of the invention can be used in detection assays, screening methods and drug discovery assays.
Owner:JANSSEN PHARMA NV
Designed ankyrin repeat domains with binding specificity for serum albumin
New designed ankyrin repeat domains with binding specificity for serum albumin, recombinant binding proteins comprising at least two designed ankyrin repeat domains with binding specificity for serum albumin, as well as recombinant binding proteins comprising at least one designed ankyrin repeat domain with binding specificity for hepatocyte growth factor (HGF), at least one designed ankyrin repeat domain with binding specificity for vascular endothelial growth factor (VEGF-A), and at least two designed ankyrin repeat domain with binding specificity for serum albumin are described, as well as nucleic acids encoding such designed ankyrin repeat domains and recombinant binding proteins, pharmaceutical compositions comprising such designed ankyrin repeat domains, recombinant binding proteins or nucleic acids and the use of such designed ankyrin repeat domains, recombinant binding proteins, nucleic acids or pharmaceutical compositions in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Bispecific chimeric proteins comprising DARPins
ActiveUS9359440B2Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsAntibody fragmentsDARPin
Owner:SAMSUNG ELECTRONICS CO LTD
Method of treating AMD in patients refractory to anti-VEGF therapy
Disclosed herein are methods for the treatment of a patient having an ocular conditions such as age-related macular degeneration or diabetic macular edema through administration of a recombinant binding protein comprising an ankyrin repeat domain. In some embodiments, the composition may be administered every 8 weeks to every 16 weeks. In some embodiments, the patient being treated may be refractory to existing anti-VEGF therapies.
Owner:ALLERGAN INC
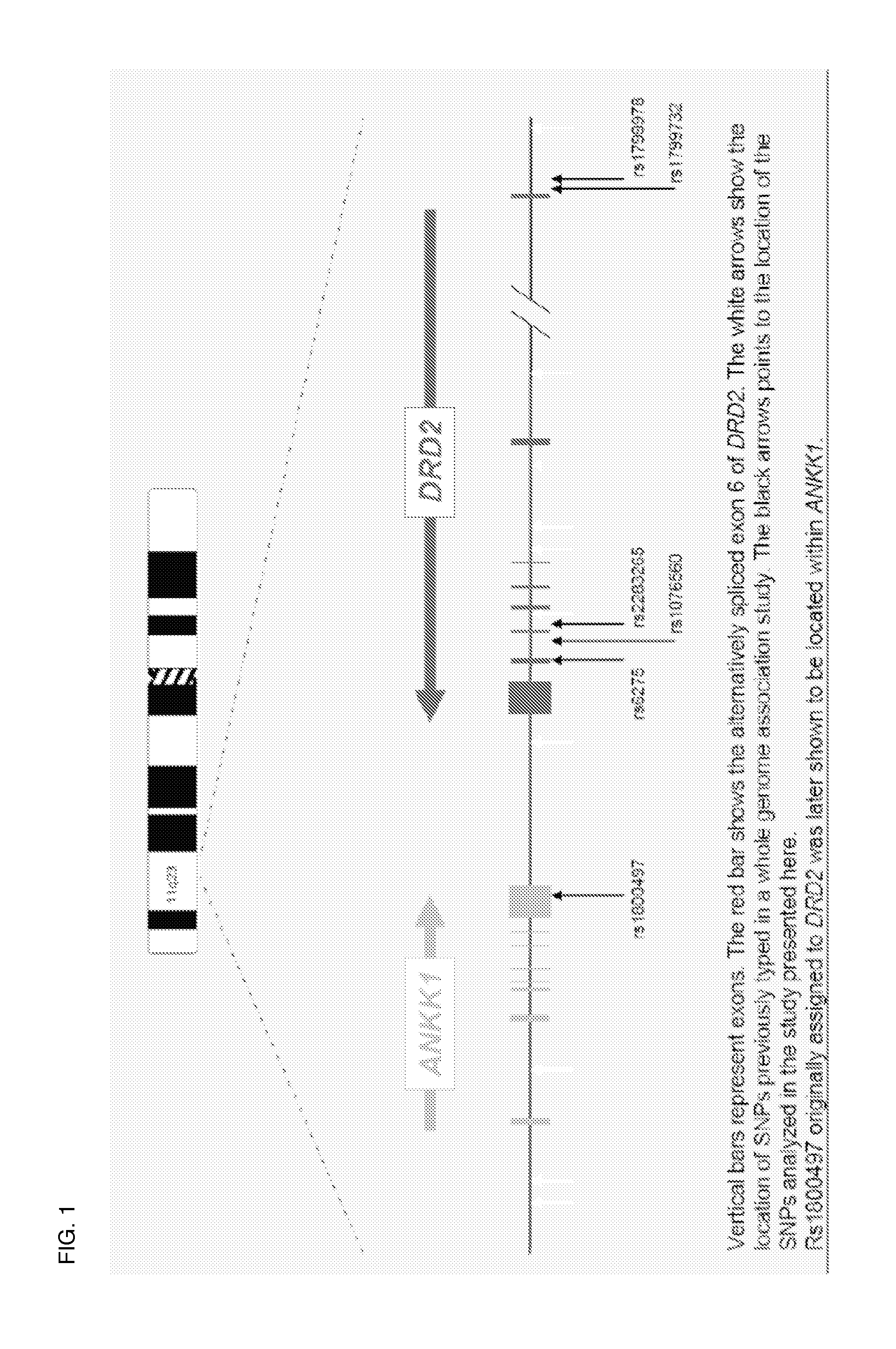
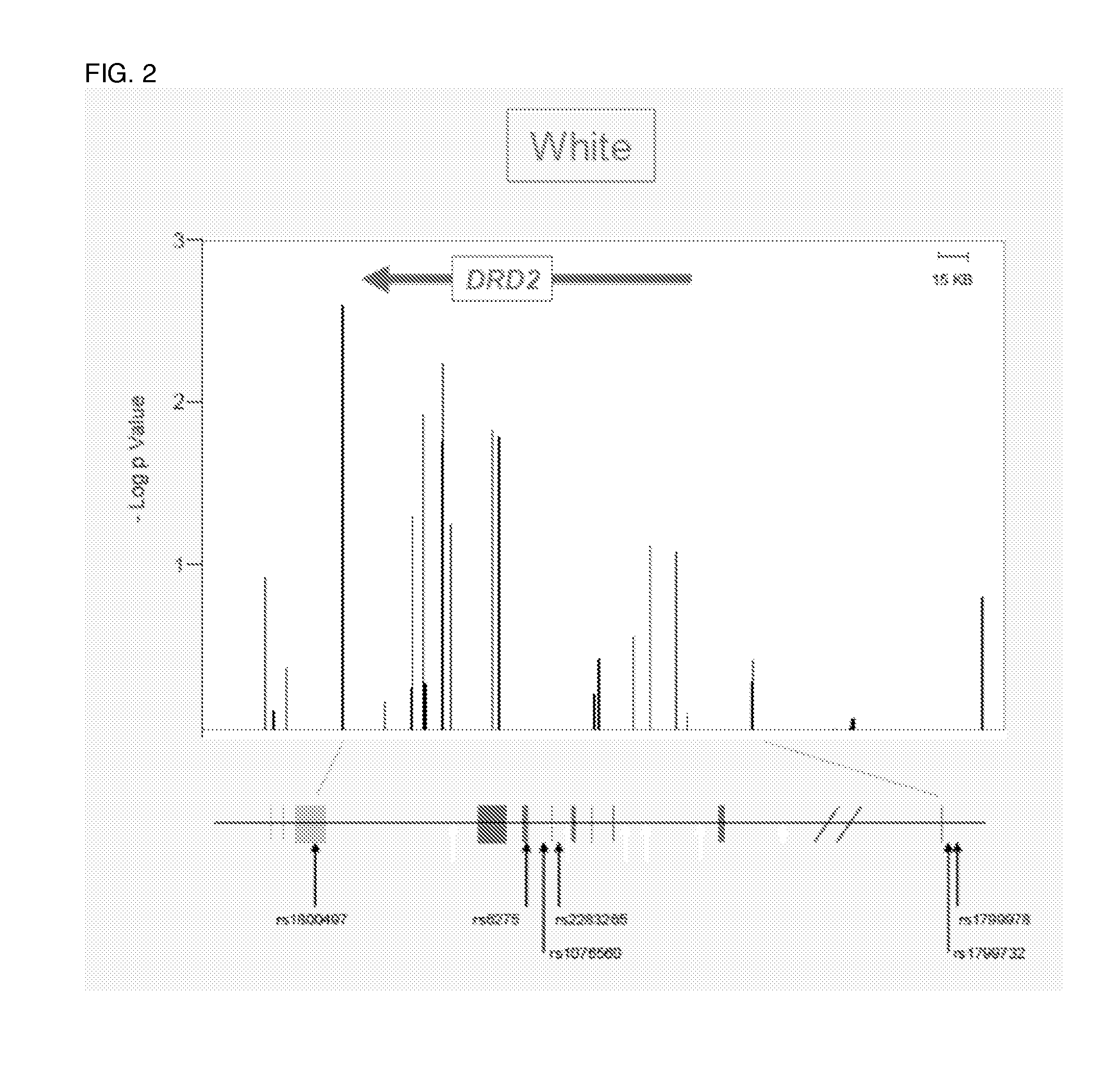
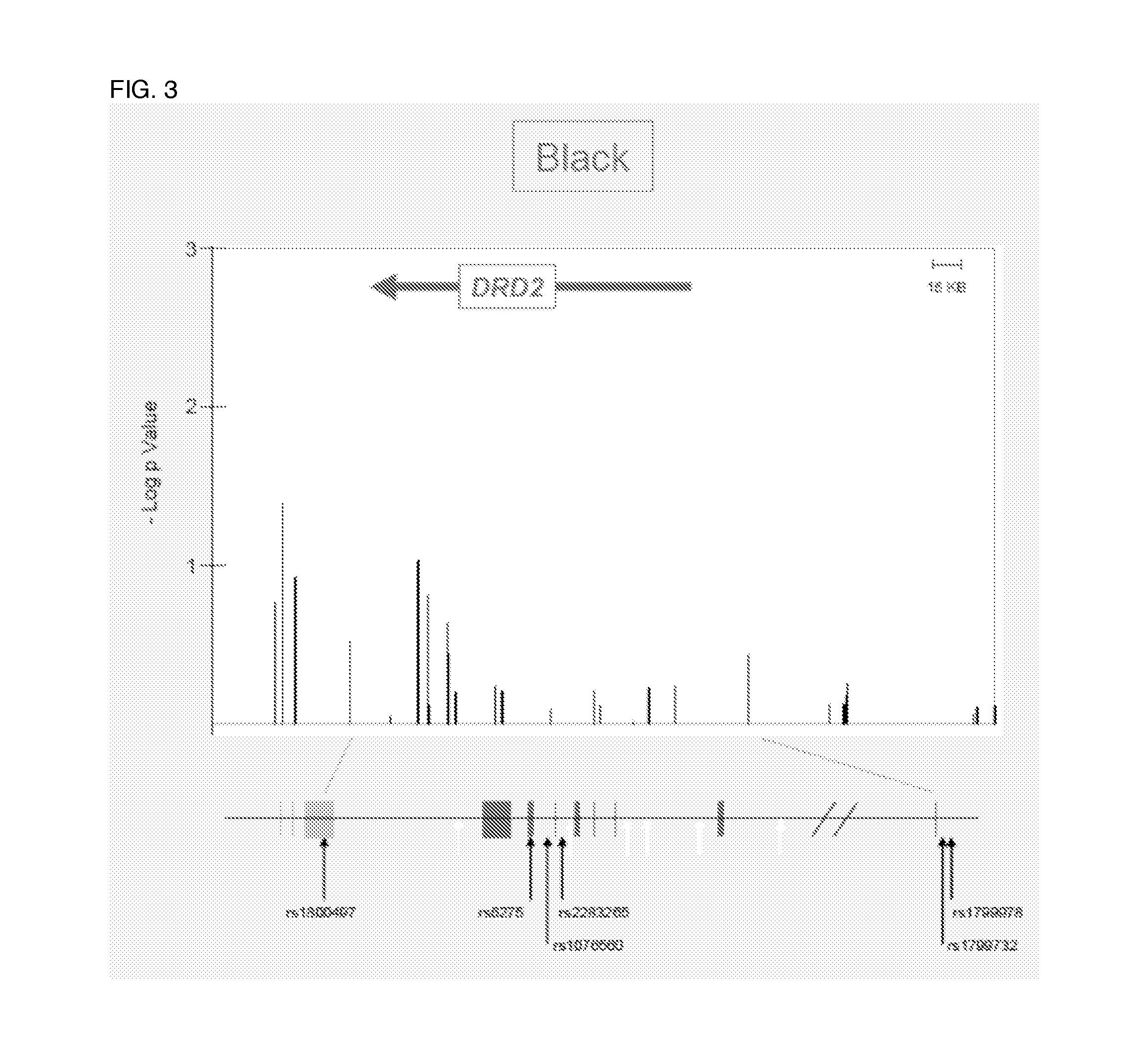
Antipsychotic treatment based on drd2 or ankk1 SNP genotype
The present invention relates to the treatment of an individual with an antipsychotic based on individual's genotype at one or more single nucleotide polymorphism (SNP) associated with the dopamine receptor D2 (DRD2) and / or ankyrin repeat and kinase domain containing 1 (ANKK1) genes.
Owner:VANDA PHARMA INC
Novel ankyrin repeat binding proteins and their uses
ActiveUS20210347835A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAnkyrin Repeat ProteinAnkyrin
The present invention relates to recombinant binding proteins comprising one or more designed ankyrin repeat domains with binding specificity for coronavirus spike proteins, nucleic acids encoding such proteins, pharmaceutical compositions comprising such proteins or nucleic acids, and the use of such proteins, nucleic acids or pharmaceutical compositions in the treatment of coronavirus diseases, particularly diseases caused by SARS-CoV-2.
Owner:MOLECULAR PARTNERS AG
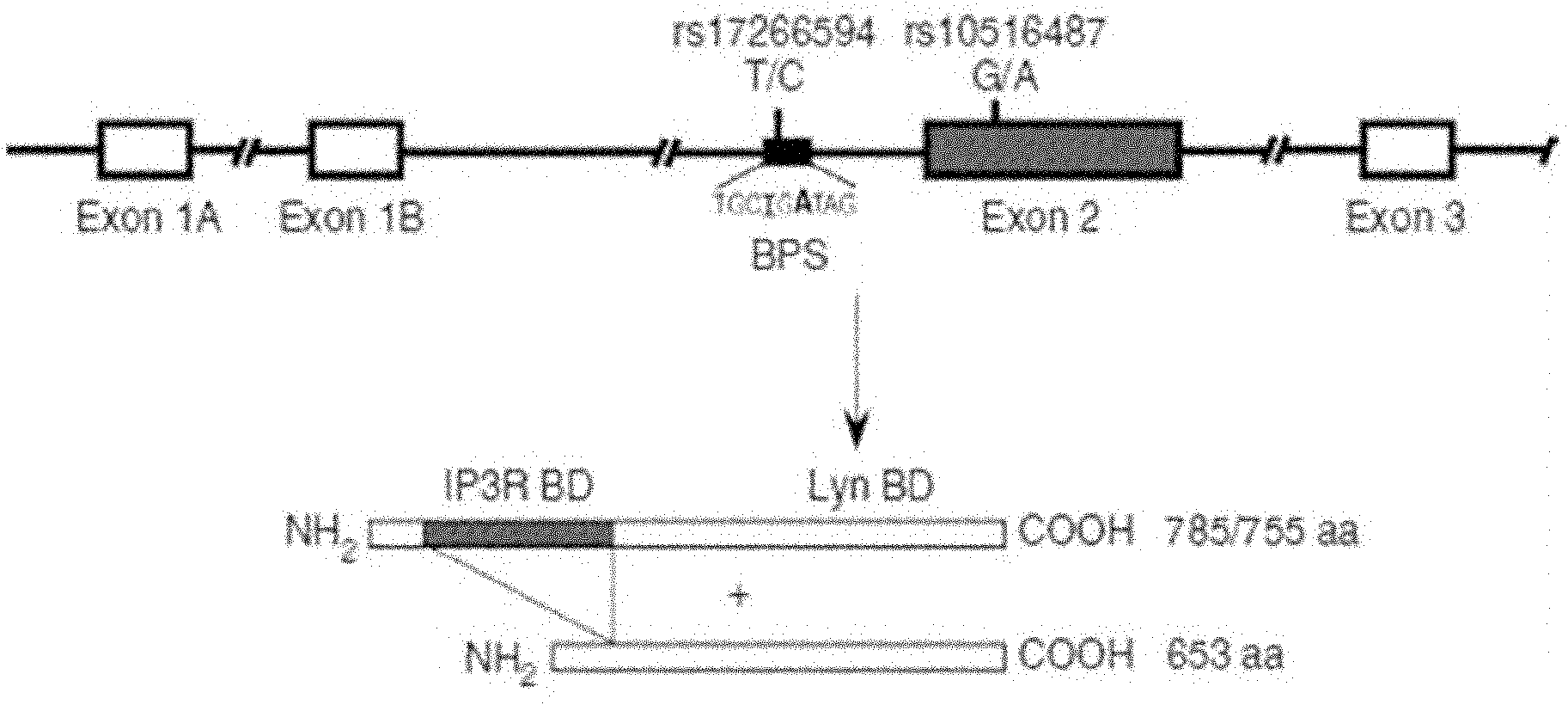
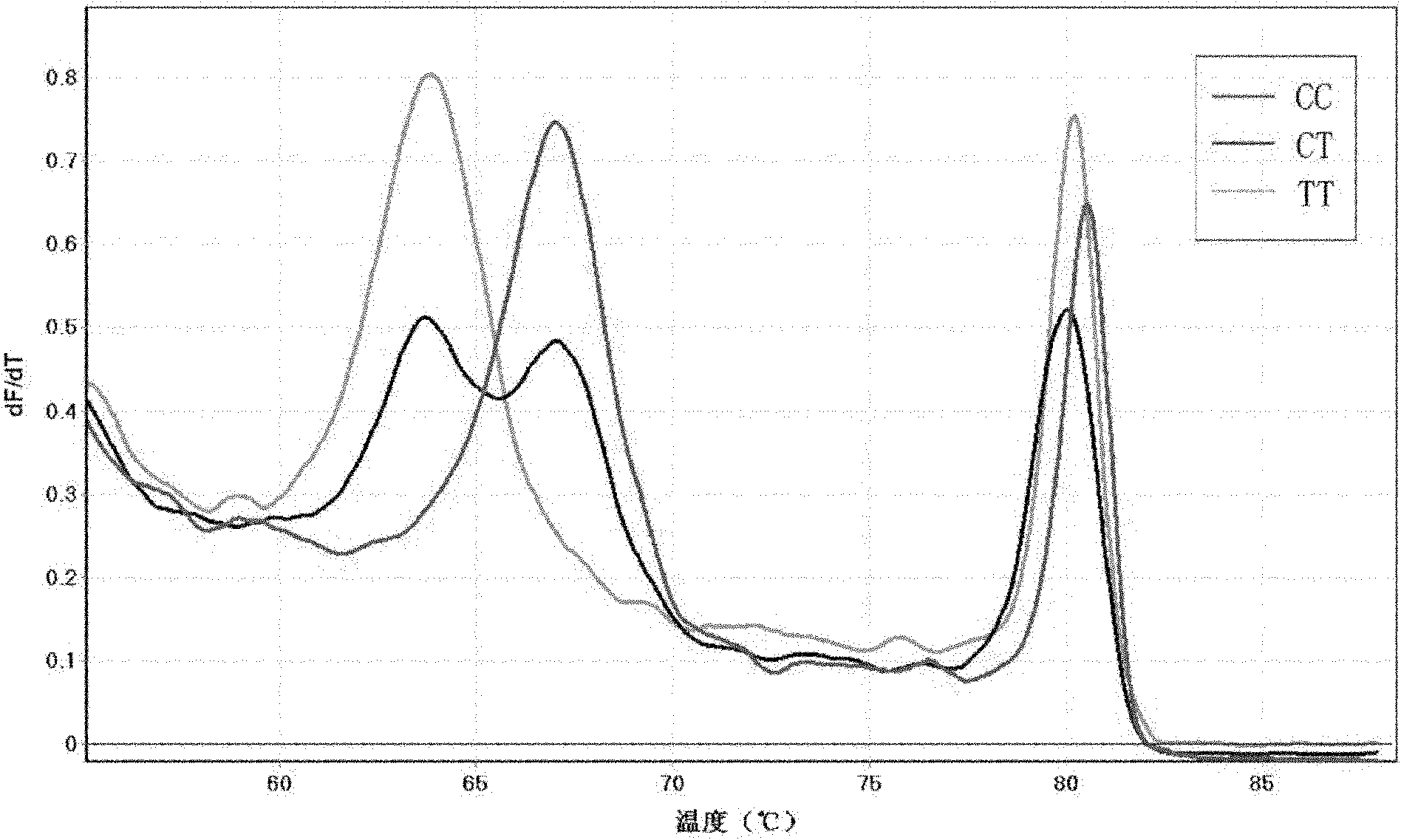
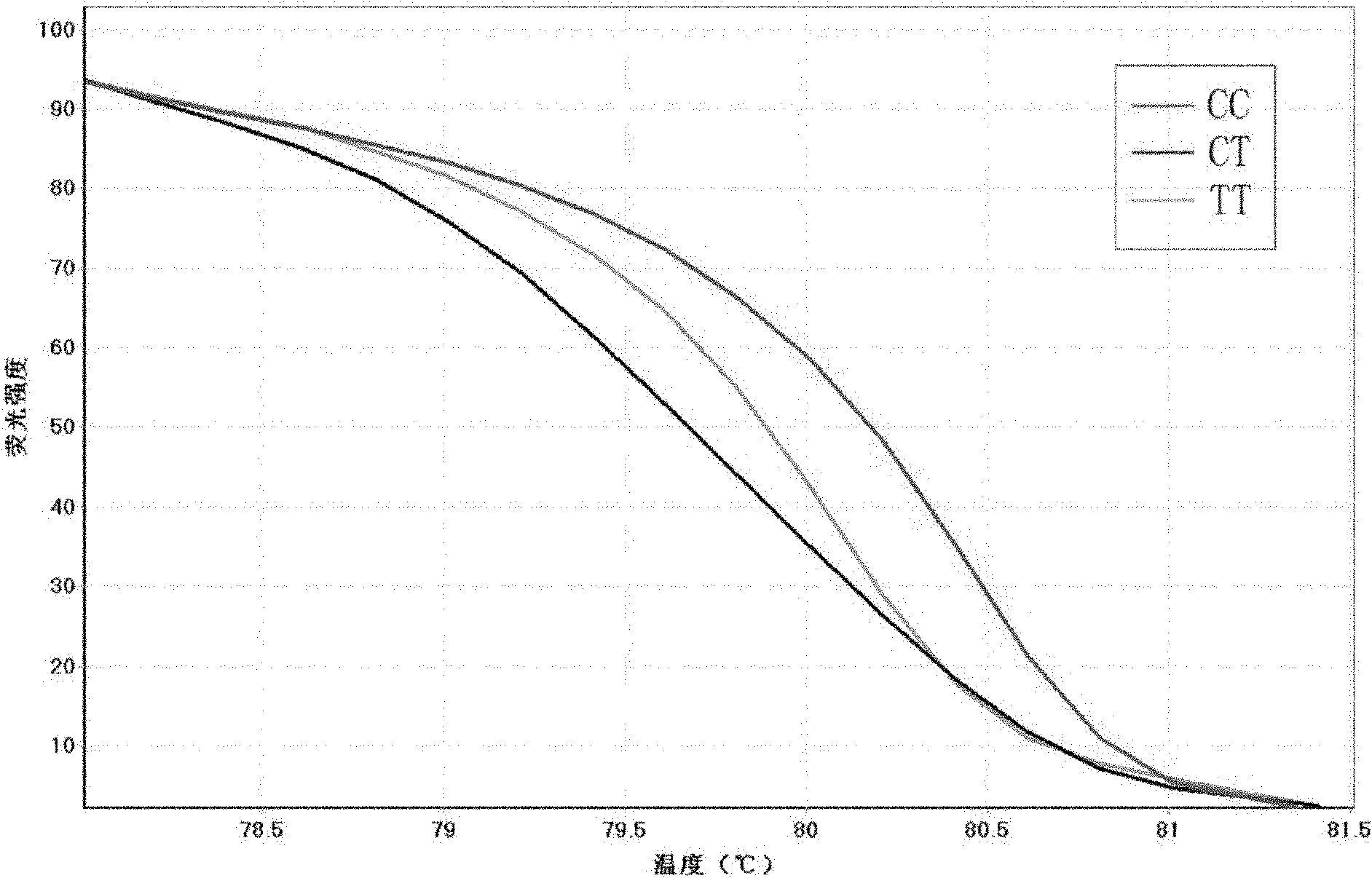
Method for detecting single nucleotide polymorphism (SNP) of BANK1 (B-cell scaffold protein with ankyrin repeats 1) gene
InactiveCN102465170AHigh resolutionEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceDiseaseDiffuse scleroderma
The invention which belongs to the fields of gene engineering and gene diagnosis provides a method for detecting SNP of a BANK1 gene. The method comprises the following steps: 1, designing a primer and a probe for amplifying an rs10516487 site or an rs17266594 site; 2, carrying out PCR amplification by utilizing the primer and the probe with sample DNA as a template, wherein concentrations of the upstream primer and the downstream primer in the primer are different, and the concentration of one is not less than five times the concentration of other one; and 3, adding a saturated dye, analyzing a melting degree curve, and determining the SNP. The method which can detect the polymorphism of the rs10516487 site and the rs17266594 site of the BANK1 gene in peripheral blood or a body fluid can be used to determine the attack possibility of autoimmtme diseases of systemic lupus erythematosus, rheumatoid arthritis, systemic chorionitis and the like. The method has the advantages of rapidness, simplicity, no pollution, and high detection result resolution. The invention also provides a corresponding detection kit.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Novel ankyrin repeat binding proteins and their uses
PendingUS20220235103A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAnkyrin Repeat ProteinAnkyrin
The present invention relates to recombinant binding proteins comprising one or more designed ankyrin repeat domains with binding specificity for coronavirus spike proteins, nucleic acids encoding such proteins, pharmaceutical compositions comprising such proteins or nucleic acids, and the use of such proteins, nucleic acids or pharmaceutical compositions in the treatment of coronavirus diseases, particularly diseases caused by SARS-CoV-2.
Owner:MOLECULAR PARTNERS AG
Multispecific proteins
PendingUS20210395318A1Peptide/protein ingredientsAntibody mimetics/scaffoldsAnkyrin Repeat ProteinAnkyrin
The present disclosure relates to multispecific proteins comprising designed ankyrin repeat domains with binding specificity for different targets, such as, e.g. CD40 and FAP. In addition, the present disclosure relates to nucleic acids encoding such multispecific proteins, pharmaceutical compositions comprising such multispecific proteins or nucleic acids, and the use of such binding proteins, nucleic acids or pharmaceutical compositions in methods for treating or diagnosing diseases, such as cancer, in a mammal, including a human.
Owner:MOLECULAR PARTNERS AG
Method of treating conditions of the eye with an Anti-vegf darpin
InactiveUS20210008158A1Improve eyesightReduce abnormal fluid in the retinaSenses disorderPeptide/protein ingredientsDiseaseAnkyrin Repeat Protein
Disclosed herein are methods for the treatment of a patient having an exudative age-related macular degeneration and other conditions of the retina by administering a binding protein comprising an ankyrin repeat domain, wherein the binding protein is first administered in 2 to 5 doses, with an interval of 25 to 35 days between each dose, and then is administered in additional doses with a longer interval between doses.
Owner:ALLERGAN INC
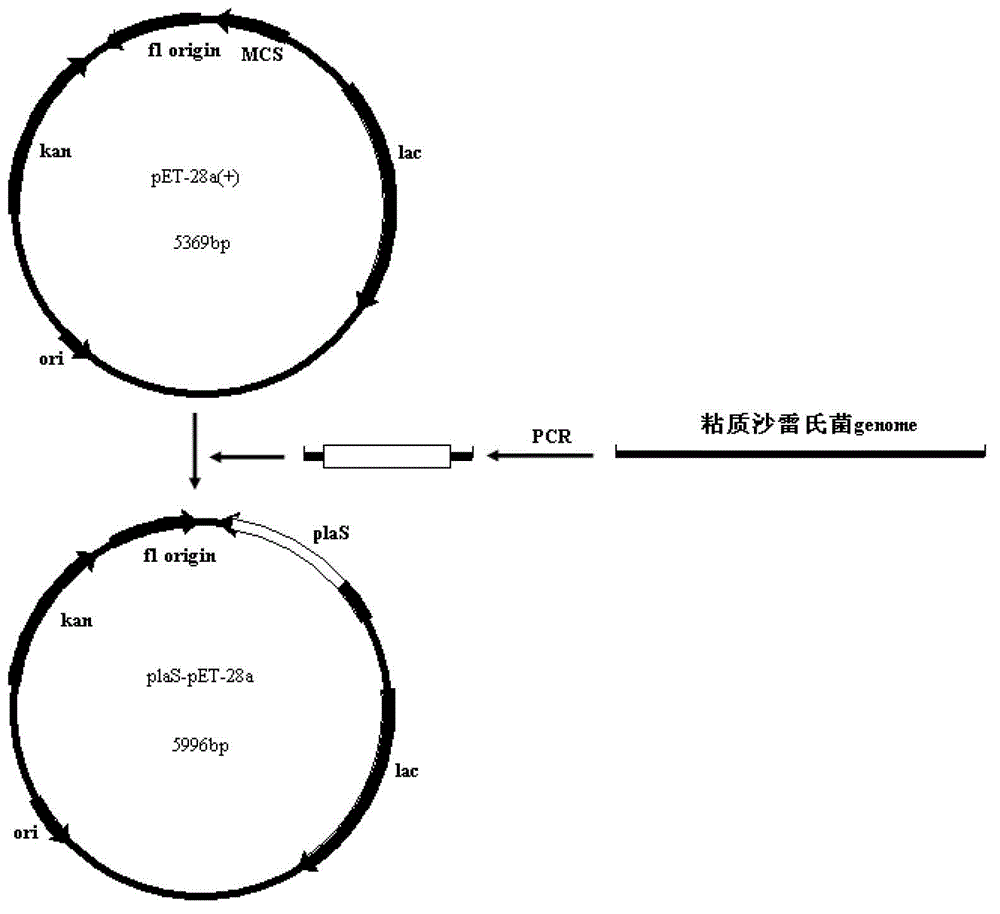
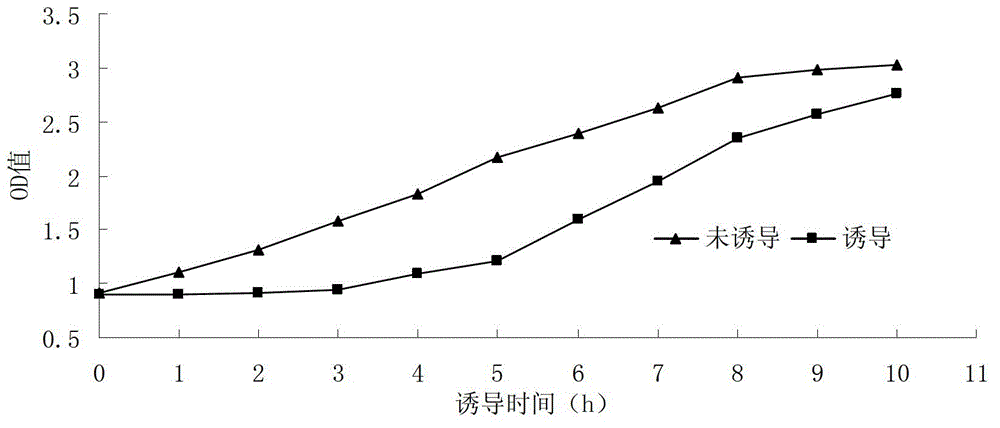
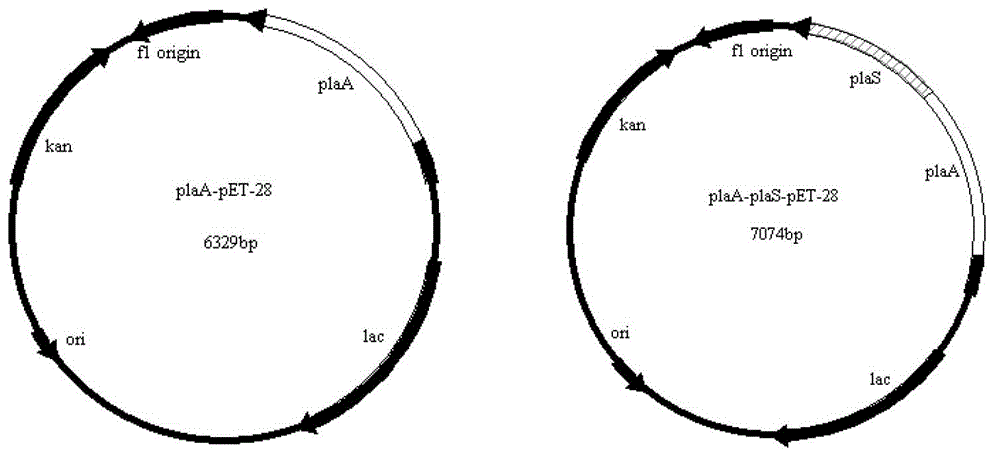
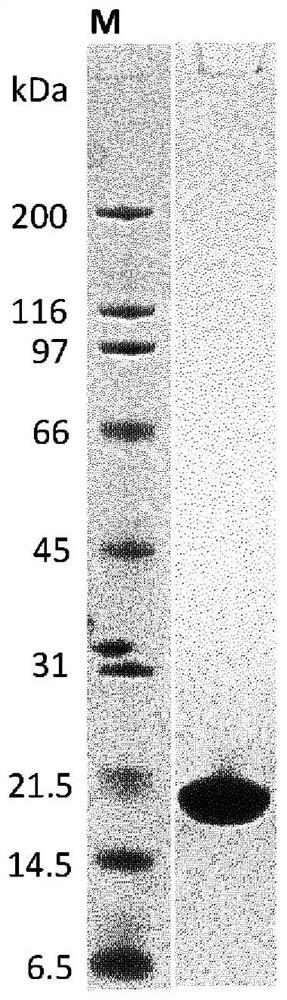
Serratia marcescens ankyrin repeat and its application
ActiveCN103333229BHigh activityImprove convenienceBacteriaMicroorganism based processesEscherichia coliAnkyrin Repeat Protein
The invention provides a serratia marcescens anchorin duplicon and its use in host bacteria. The serratia marcescens anchorin duplicon has a sequence shown in the formula of SEQ ID NO: 2. The invention also provides an inhibition effect of the serratia marcescens anchorin duplicon in host bacteria. The invention also provides a use of the serratia marcescens anchorin duplicon in promotion of expression of phospholipase A1 in escherichia coli. The invention also provides a recombinant vector and a recombinant strain containing the serratia marcescens anchorin duplicon. An experiment proves that through the expression of the serratia marcescens anchorin duplicon, host bacteria can be inhibited obviously. The serratia marcescens anchorin duplicon can greatly promote high-activity expression of the phospholipase A1 in escherichia coli.
Owner:ANHUI POLYTECHNIC UNIV
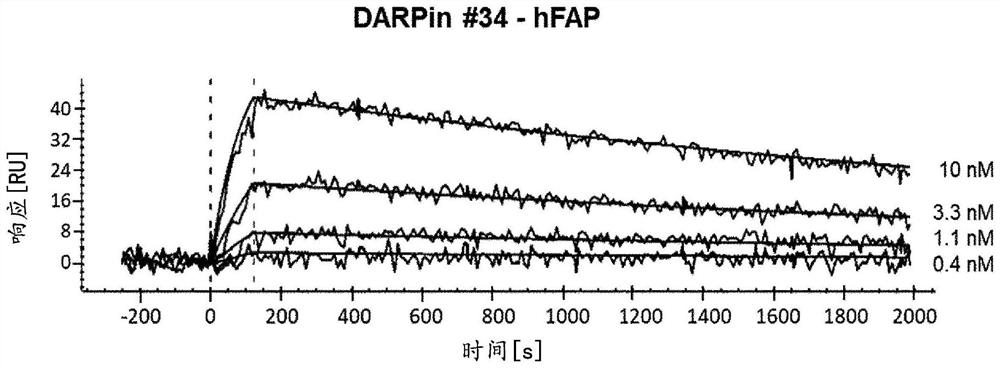
Recombinant FAP binding proteins and uses thereof
PendingCN114269366APeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseAnkyrin Repeat Protein
The present invention relates to recombinant binding proteins comprising a designed ankyrin repeat domain having binding specificity for fibroblast activation protein (FAP). Furthermore, the invention relates to nucleic acids encoding such binding proteins, pharmaceutical compositions comprising such binding proteins or nucleic acids and such binding proteins, use of nucleic acids or pharmaceutical compositions in methods for localization or delivery of bioactive molecules in FAP-expressing tissues, such as tumor tissues, and for treatment, diagnosis or imaging of diseases, such as cancer, in mammals, including humans.
Owner:MOLECULAR PARTNERS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com