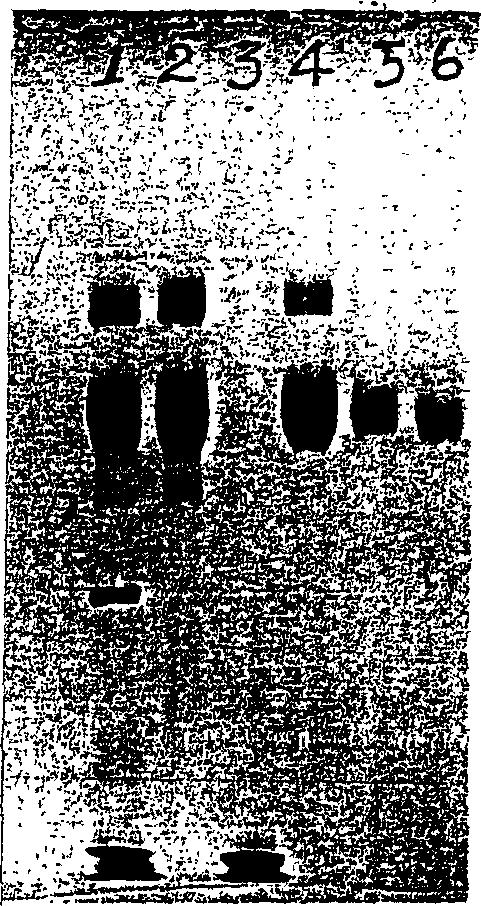Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
211 results about "Serum protein albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Serum albumin, often referred to simply as blood albumin, is an albumin (a type of globular protein) found in vertebrate blood. Human serum albumin is encoded by the ALB gene. Other mammalian forms, such as bovine serum albumin, are chemically similar.
Pharmaceutically acceptable composition comprising an aqueous solution of paclitaxel and albumin
InactiveUS20050282734A1Prevent restenosisFree of organic solventsBiocideOrganic active ingredientsOrganic solventSerum protein albumin
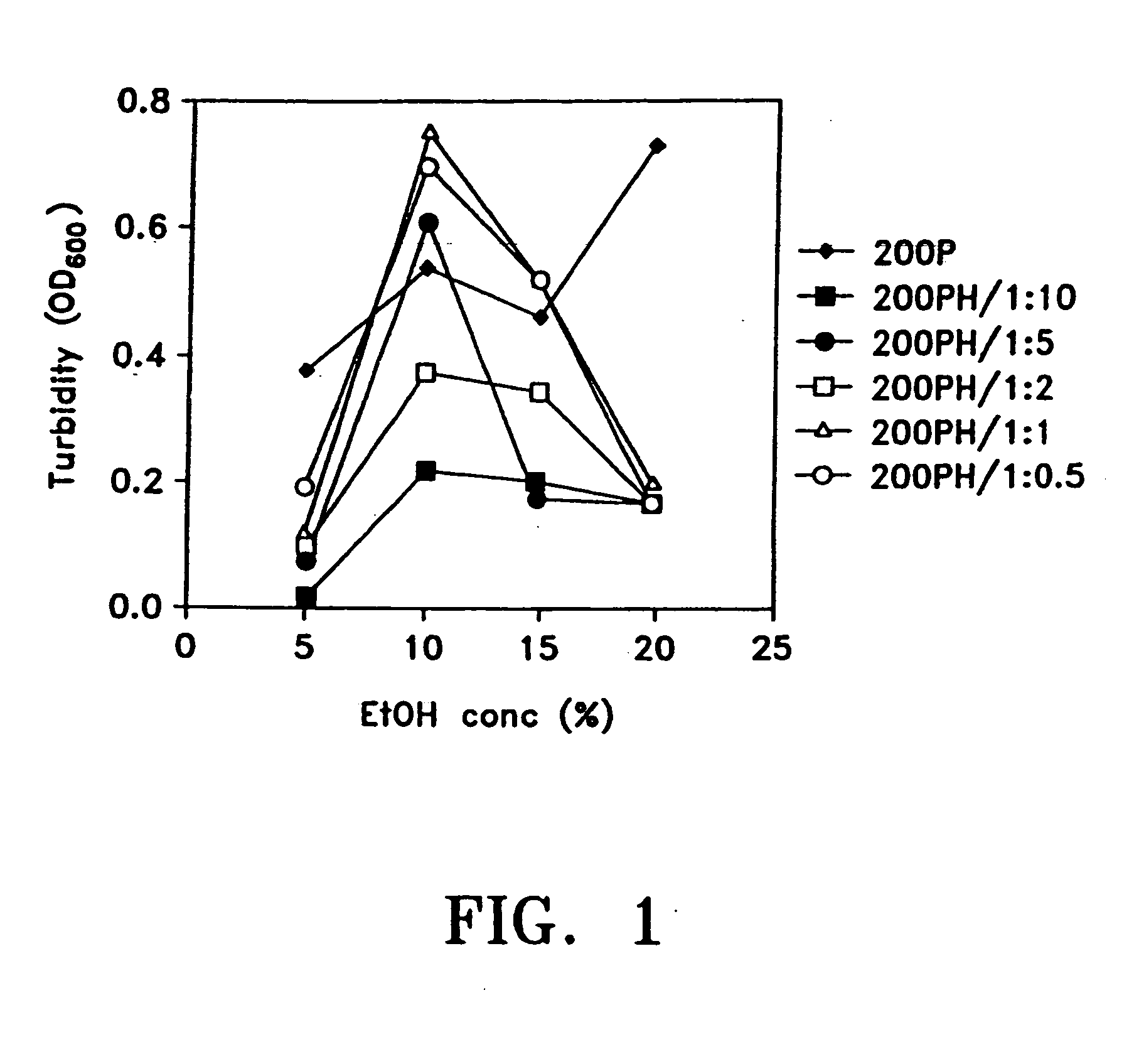
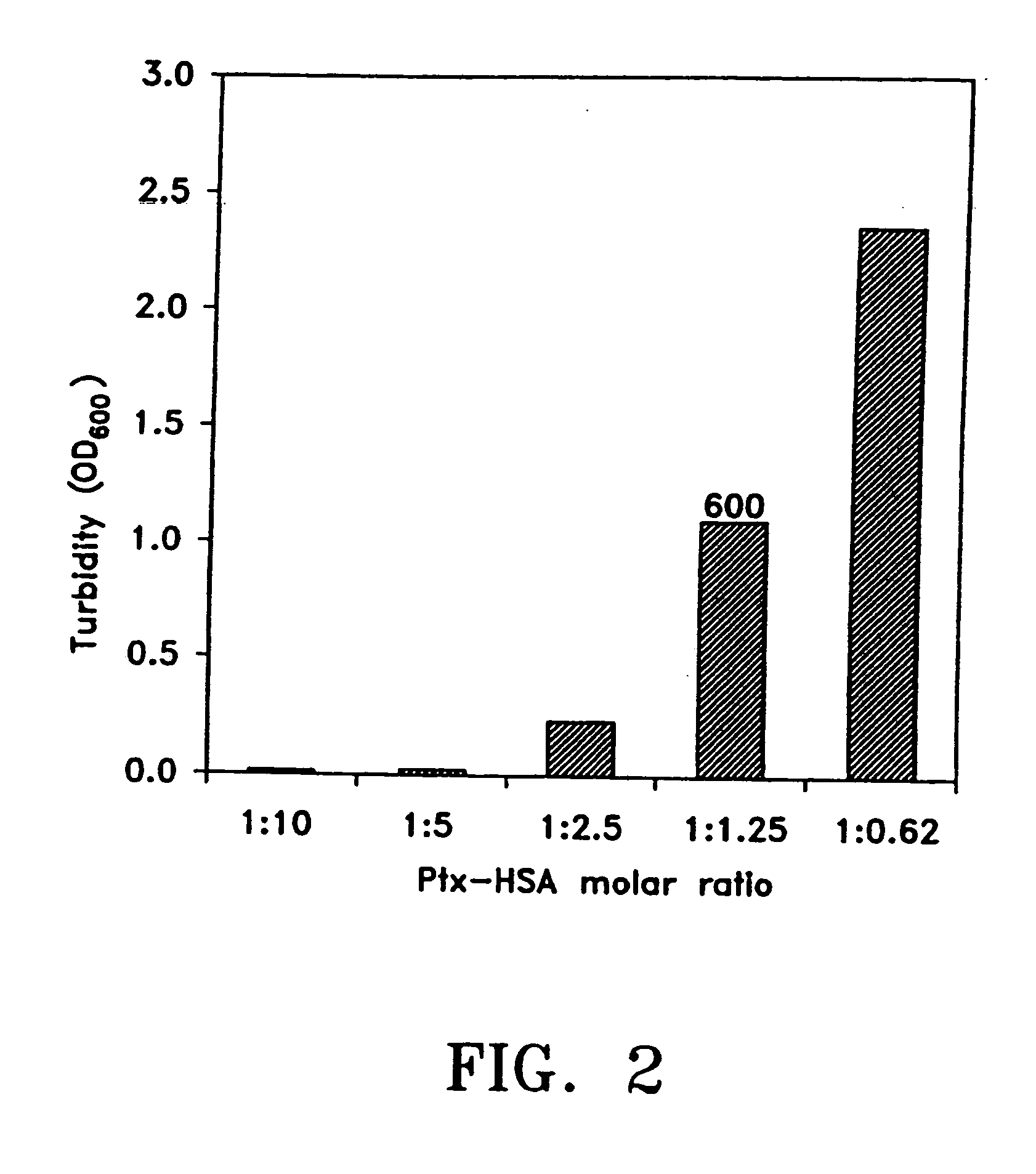
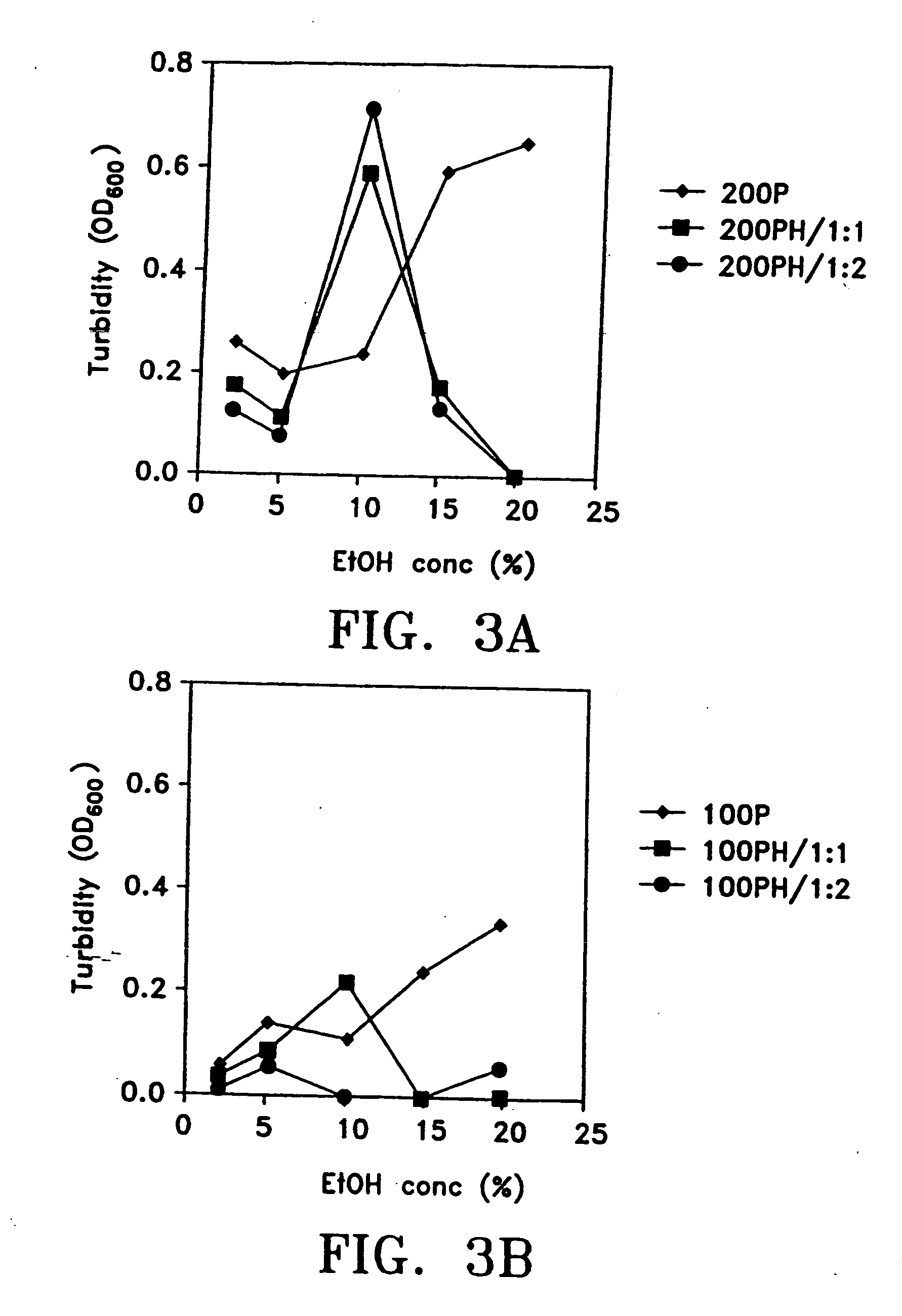
An optically clear, pharmaceutically acceptable aqueous composition comprising paclitaxel or a derivative thereof, serum albumin and a pharmaceutically acceptable vehicle, wherein the composition comprises no more than 10% organic solvent and has a pH of about 3.0 to about 4.8, is described. The serum albumin can be fatted or defatted, and the composition can optionally be lyophilized or optionally lyophilized and reconstituted. At least 70% of the paclitaxel is bound to serum albumin, the ratio of paclitaxel to albumin is at least about 1:5, and the concentration of paclitaxel is at least about 25 μg / ml. Methods of making and using this composition an also provided.
Owner:KADIMA TENSHUK A +6
Test for the rapid evaluation of ischemic states and kits
The present invention relates to rapid methods for the detection of ischemic states and to kits for use in such methods. Provided for is a rapid method of testing for and quantifying ischemia based upon methods of detecting and quantifying the existence of an alteration of the serum protein albumin which occurs following an ischemic event; methods for detecting and quantifying this alteration include evaluating and quantifying the cobalt binding capacity of circulating albumin, analysis and measurement of the ability of serum albumin to bind exogenous cobalt, detection and measurement of the presence of endogenous copper in a purified albumin sample and use of an immunological assay specific to the altered form of serum albumin which occurs following an ischemic event. Also taught by the present invention is the detection and measurement of an ischemic event by measuring albumin N-terminal derivatives that arise following an ischemic event, including truncated albumin species lacking one to four N-terminal amino acids or albumin with an acetylated N-terminal Asp residue.
Owner:ISCHEMIA TECH
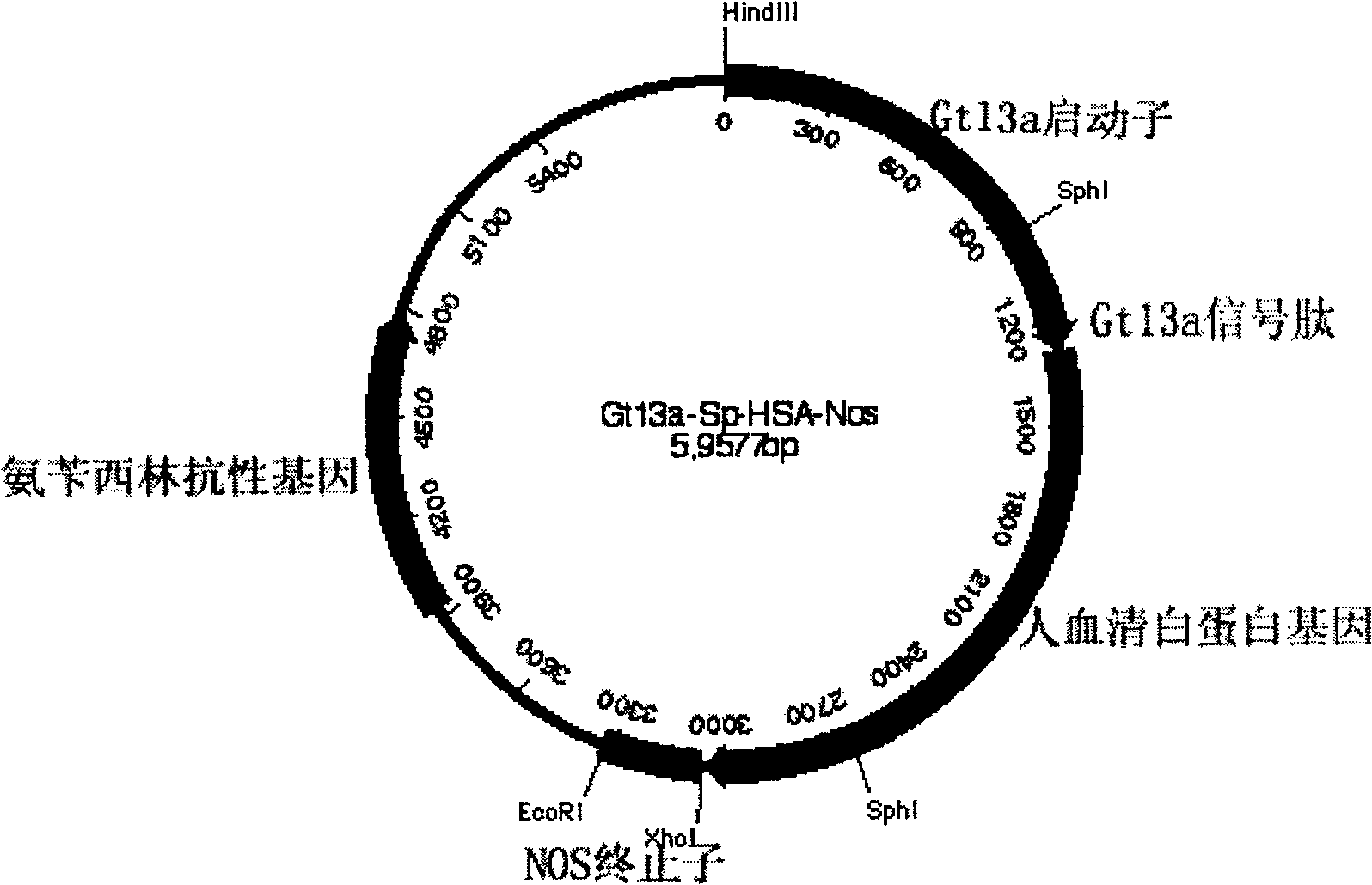
Production of recombinant human serum albumin with rice-embryo milk cell as biological reactor
ActiveCN1896239ANo pollution in the processEfficient expressionSerum albuminFermentationSerum protein albuminEmbryo
Production of recombinant human serum albumin with rice albuminous cell as biological reactor is carried out by taking rice albuminous cell protein as storage site for recombinant protein, taking rice albuminous specific expression starter and signal peptide, entering inductive recombinant human serum albumin into inner-film system of rice serum albumin cell and storing it in rice albuminous protein. It is cheap, has more yield and higher expression.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Serum albumin binding molecules
ActiveUS20110305663A1Improve solubilityReduce aggregationBacteriaPeptide/protein ingredientsSerum igeSerum protein albumin
The present invention relates to an antibody-like protein based on the tenth fibronectin type III domain (10Fn3) that binds to serum albumin. The invention further relates to fusion molecules comprising a serum albumin-binding 10Fn3 joined to a heterologous protein for use in diagnostic and therapeutic applications.
Owner:BRISTOL MYERS SQUIBB CO
Processes for clonal growth of hepatic progenitor cells
A method of propagating mammalian endodermally derived progenitors such as hepatic progenitors, their progeny, or mixtures thereof is developed which includes culturing mammalian progenitors, their progeny, or mixtures thereof on a layer of embryonic mammalian feeder cells in a culture medium. The culture medium can be supplemented with one or more hormones and other growth agents. These hormones and other growth agents can include insulin, dexamethasone, transferrin, nicotinamide, serum albumin, beta-mercaptoethanol, free fatty acid, glutamine, CUSO4, and H2SeO3. The culture medium can also include antibiotics. Importantly, the culture medium does not include serum. The invention includes means of inducing the differentiation of the progenitors to their adult fates such as the differentiation of hepatic progenitor cells to hepatocytes or biliary cells by adding, or excluding epidermal growth factor, respectively. The method of producing mammalian progenitors is useful in that the progenitors can be used subsequently in one or more of the following processes: identification of growth and differentiation factors, toxicological studies, drug development, antimicrobial studies, or the preparation of an extracorporeal organ such as a bioartificial liver.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Albumin-Free Botulinum Toxin Based Pharmaceutical Compositions Containing a Hyaluronidase and Methods of Use
InactiveUS20090324647A1Relieve symptomsReduce secretionBacterial antigen ingredientsNervous disorderSerum protein albuminMedicine
The present invention provides compositions that contain botulinum toxin and a hyaluronidase, and that lack human or recombinant serum albumin. The present invention also provides methods of administering the pharmaceutical composition to a subject in need thereof.
Owner:REVANCE THERAPEUTICS INC
Methods and Compositions for Isolating, Maintaining and Serially Expanding Human Mesenchymal Stem Cells
InactiveUS20100015710A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSodium bicarbonateLipid formation
Compositions and methods for isolating and expanding human mesenchymal stem / progenitor cells through multiple passages in defined serum-free environments are provided. The culture media compositions includes a basal medium supplemented with a nutrient mixture such as Ham's F12 nutrient mixture, glutamine, buffer solutions such as sodium bicarbonate and hepes, serum albumin, a lipid mixture, insulin, transferrin, putrescine, progesterone, fetuin, hydrocortisone, ascorbic acid or its analogues such as ascorbic acid-2-phosphate, fibroblast growth factor and transforming growth factor β, and are free of serum or other undefined serum substitutes such as platelet lysate. Methods employing these compositions and protein-coated surfaces for the isolation of mesenchymal stem / progenitor cells from human bone marrow and other tissues such as adipose tissue are also provided. Finally, methods are also provided for serially expanding these cells through multiple passages without losing mesenchymal stem cell-specific proliferative, phenotypical and differentiation characteristics.
Owner:UTI LLP
Systemic insulin-like growth factor-1 therapy reduces diabetic peripheral neuropathy and improves renal function in diabetic nephropathy
InactiveUS20100216709A1Prevents subsequent hyposensitivityEasy maintenanceOrganic active ingredientsNervous disorderInsulin-like growth factorHyperglycemic disorder
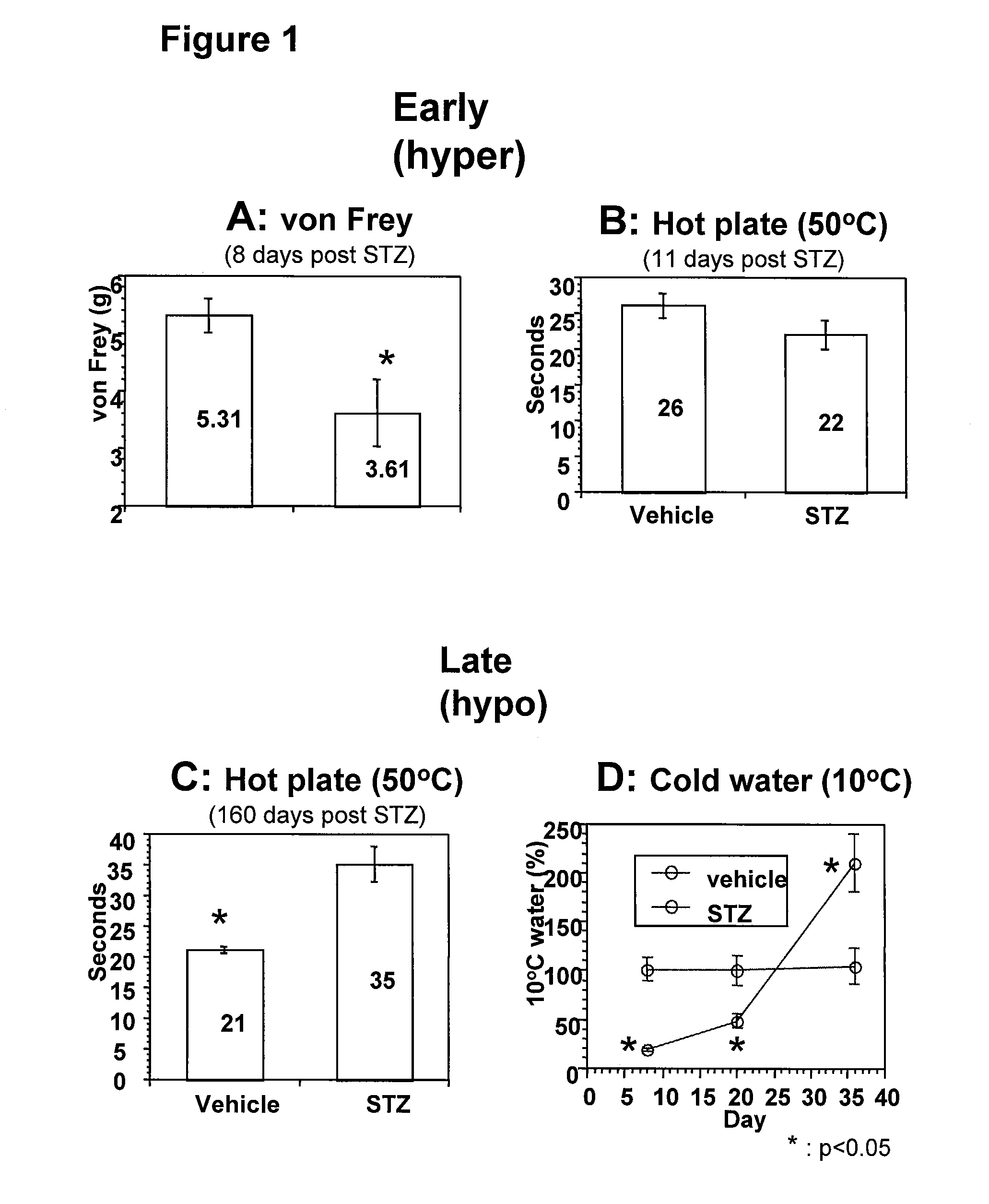
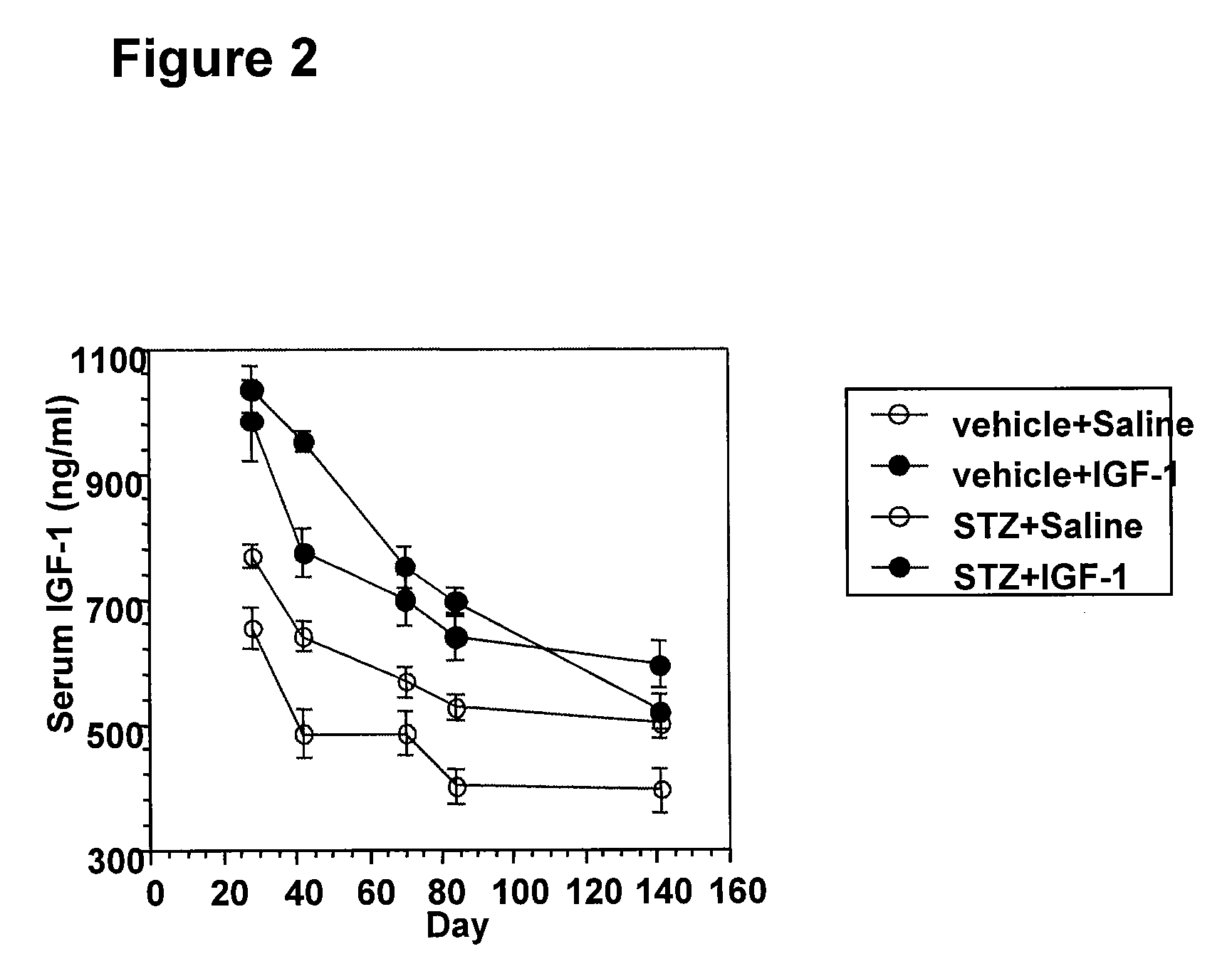
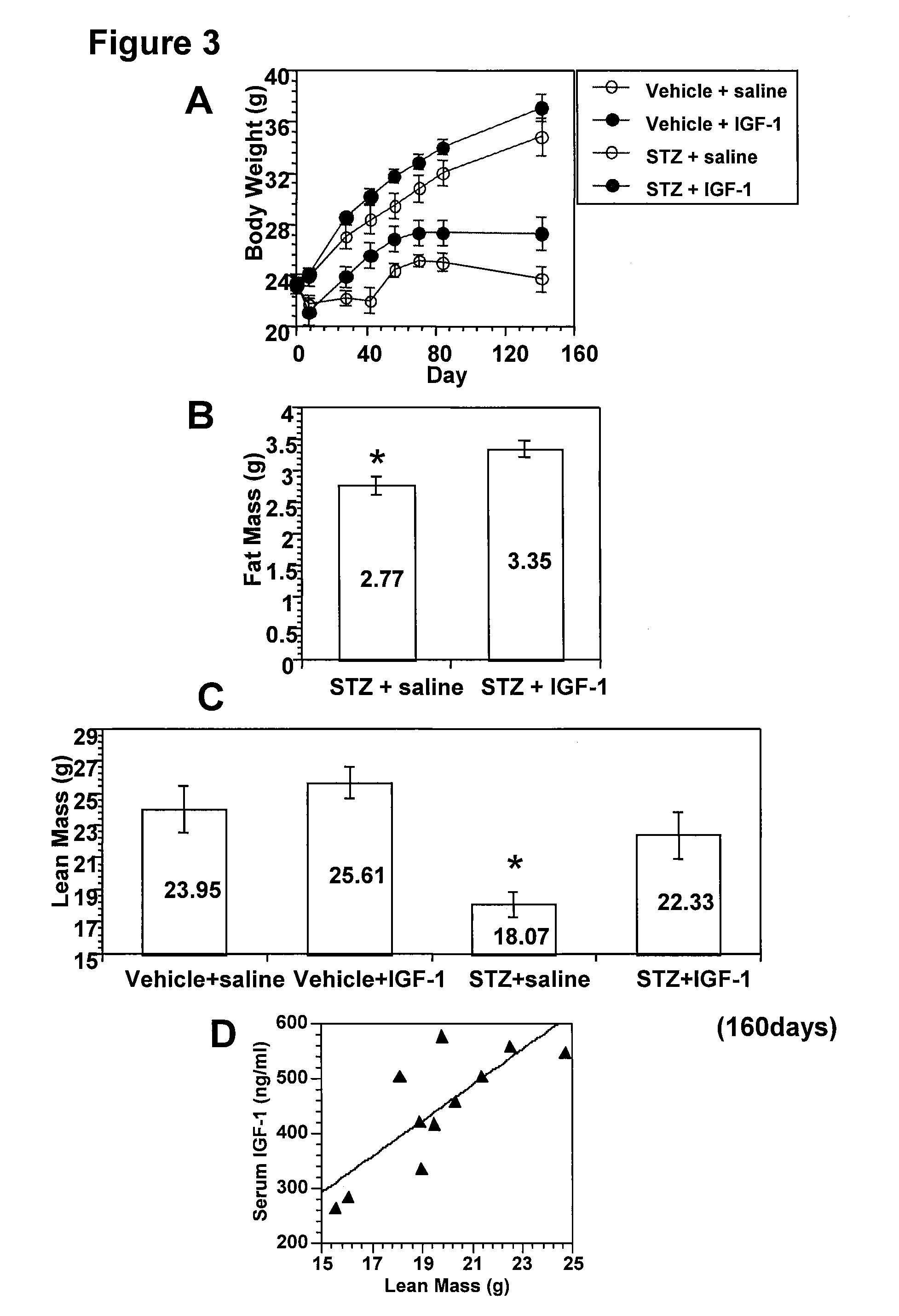
The present invention provides methods of treatment of patients suffering from the complications of blood sugar disorders: diabetic peripheral neuropathy and diabetic nephropathy by administration of IGF-1 via protein therapy or gene therapy. It relates to methods of treating an individual having a diabetic disorder or a hyperglycemic disorder, comprising administering to the individual an effective amount of a DNA vector expressing IGF-1Eb or IGF-1Ec in vivo or an effective amount of at the IGF-1Eb or IGF-1Ec protein in the early hyperalgesia stage or in patients that have advanced to the hyposensitivity stage. Treatment at the early hyperalgesia stage prevents subsequent hyposensitivity with increases or maintenance of sensory nerve function. IGF-1Eb or IGF-1Ec treatment also increases muscle mass and improves overall mobility, which indicates a treatment-related improvement in motor function. Treatment with IGF-1Eb or IGF-1Ec at the hyposensitivity stage reverses hyposensitivity and improves muscle mass and overall health. Systemic IGF-1 provides a therapeutic modality for treating hyposensitivity associated with DPN. In addition, IGF-1Eb or IGF-1Ec provides a therapeutic modality for treating diabetic nephropathy. IGF-1Eb or IGF-1Ec improves renal function as evidenced by a modulation in serum albumin concentration and a reduction in urine volume and protein levels. IGF-1Eb or IGF-1Ec also reduces diabetic glomerulosclerosis.
Owner:GENZYME CORP
Methods for modulating topical inflammatory response
InactiveUS20060228416A1Reduce inflammationRestoring osmolarityBiocidePowder deliveryInterleukin 6Interleukin 1β
A method of modulating topical inflammatory response is disclosed. The method generally includes the selective removal of certain proteins, e.g., one or more cytokines such as interleukin-1β (IL-1β) and / or interleukin-6 (IL-6), from a topical site without substantially altering the local concentrations of other proteins that may be present at or near the topical site. Other proteins that are present at or near the topical site can include serum albumin, fibrinogen, and immunoglobin G (IgG). Hydrogel compositions that can be used to practice the methods of the invention are provided. The invention further provides methods of preparing such hydrogel compositions.
Owner:BIOARTIFICIAL GEL TECH
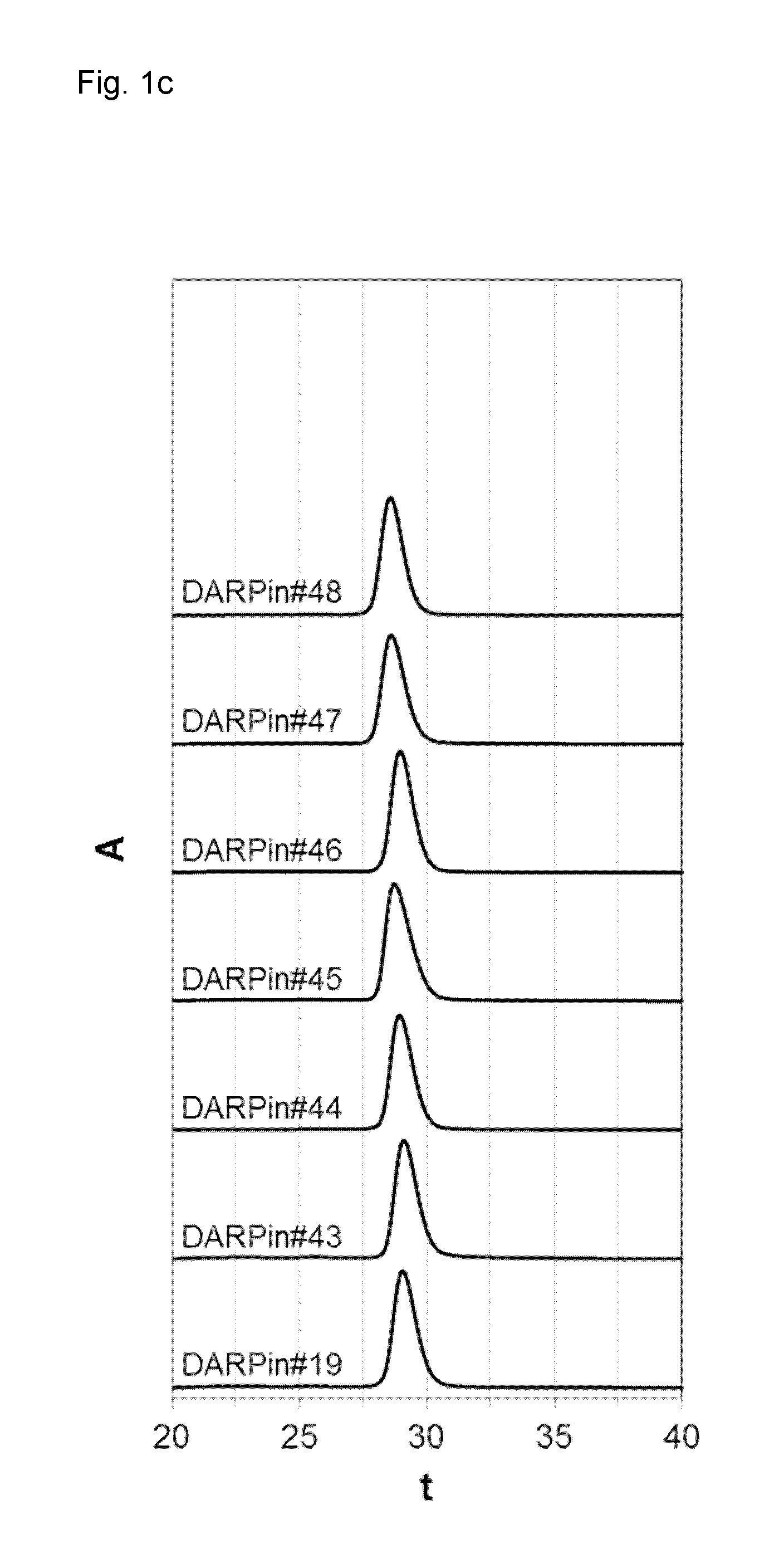
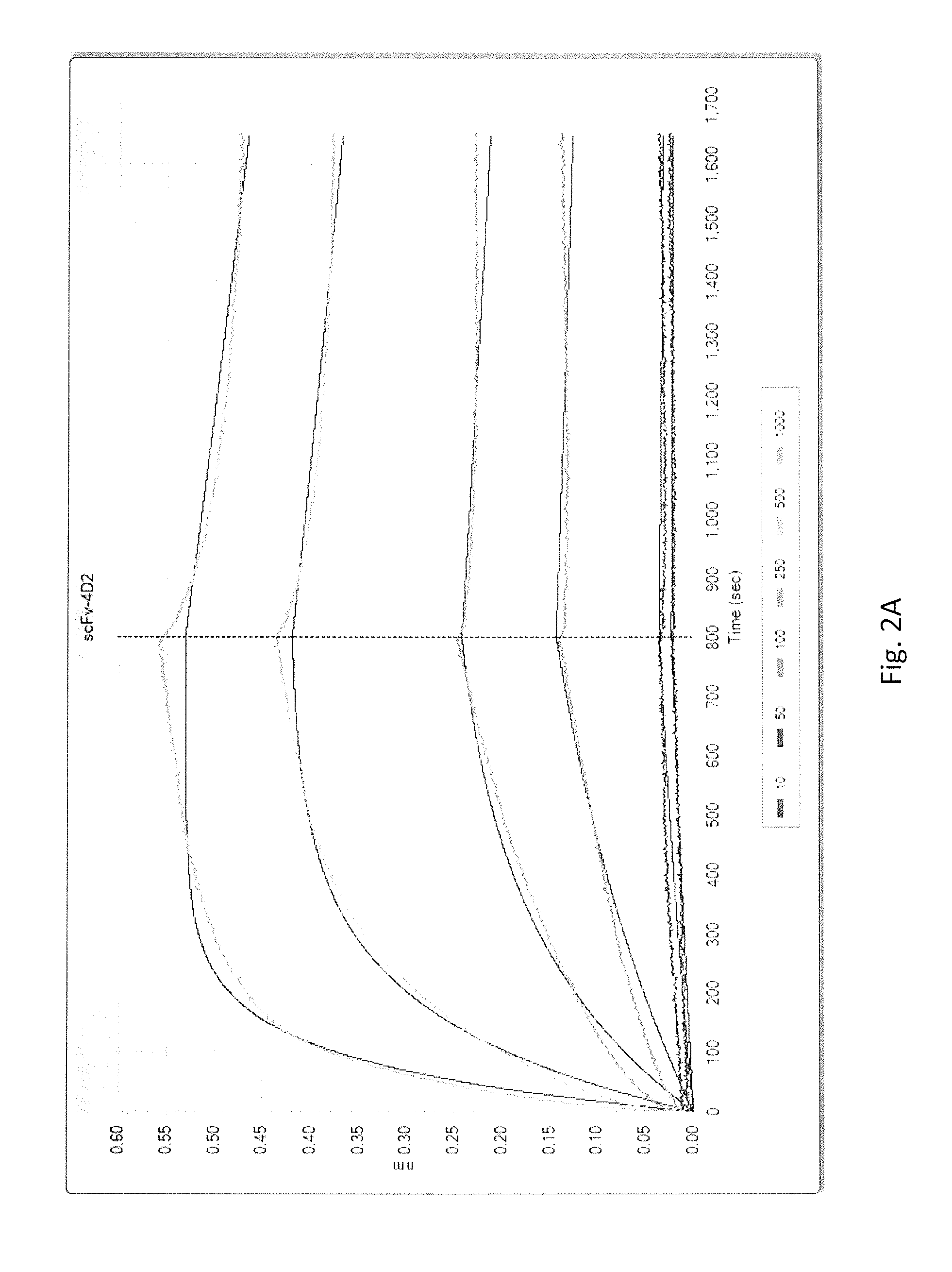
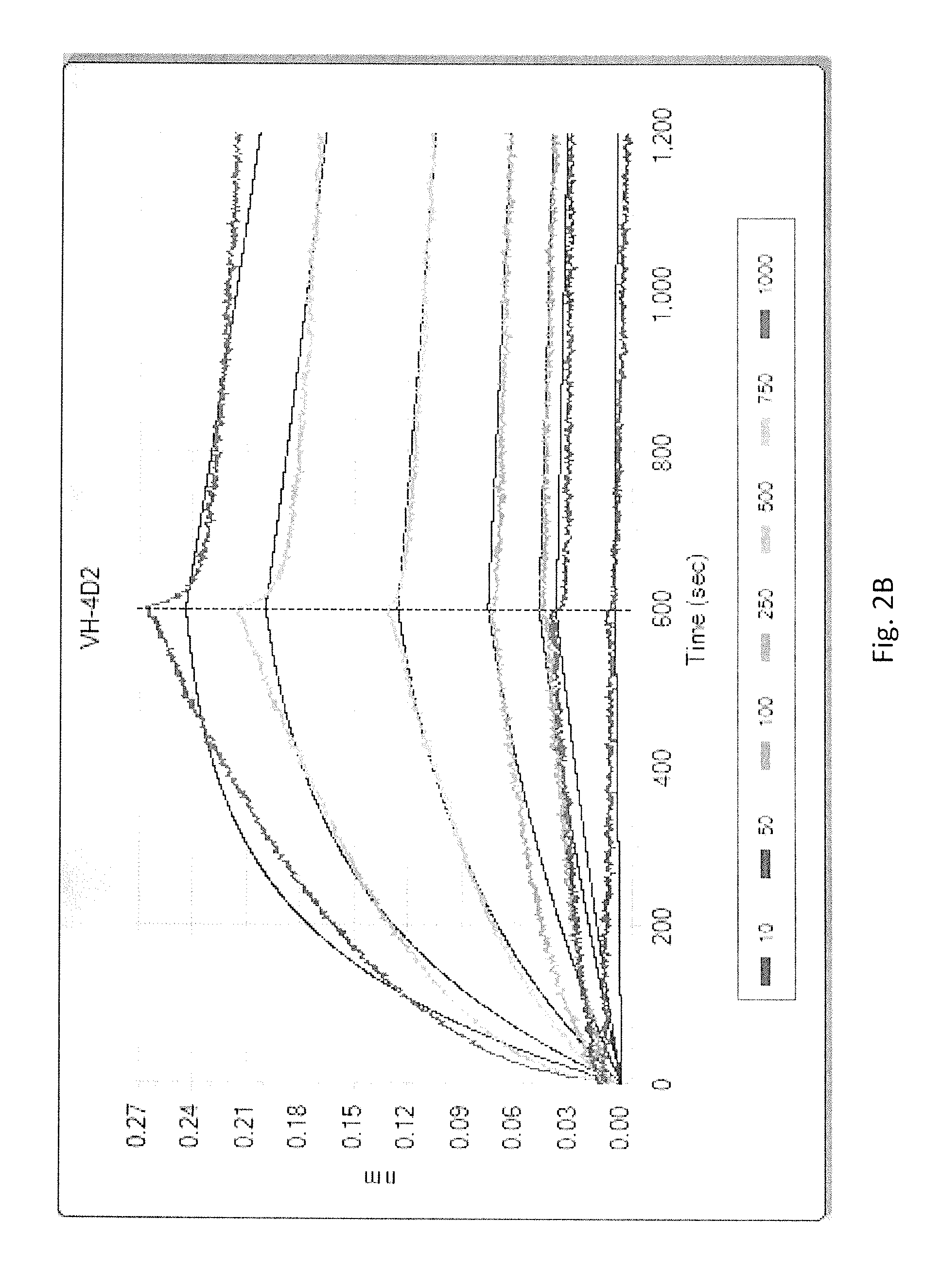
Recombinant proteins that simultaneously bind HGF, VEGF-A and serum albumin, comprising ankyrin repeat domains
ActiveUS9458211B1Good storage stabilitySenses disorderNervous disorderSerum protein albuminAnkyrin Repeat Protein
New designed ankyrin repeat domains with binding specificity for serum albumin, recombinant binding proteins comprising at least two designed ankyrin repeat domains with binding specificity for serum albumin, as well as recombinant binding proteins comprising at least one designed ankyrin repeat domain with binding specificity for hepatocyte growth factor (HGF), at least one designed ankyrin repeat domain with binding specificity for vascular endothelial growth factor (VEGF-A), and at least two designed ankyrin repeat domain with binding specificity for serum albumin are described, as well as nucleic acids encoding such designed ankyrin repeat domains and recombinant binding proteins, pharmaceutical compositions comprising such designed ankyrin repeat domains, recombinant binding proteins or nucleic acids and the use of such designed ankyrin repeat domains, recombinant binding proteins, nucleic acids or pharmaceutical compositions in the treatment of diseases.
Owner:MOLECULAR PARTNERS AG
Methods and systems for increasing protein stability
InactiveUS20130129727A1High expressionEasy to purifyBacteriaAntibody mimetics/scaffoldsSerum protein albuminHalf-life
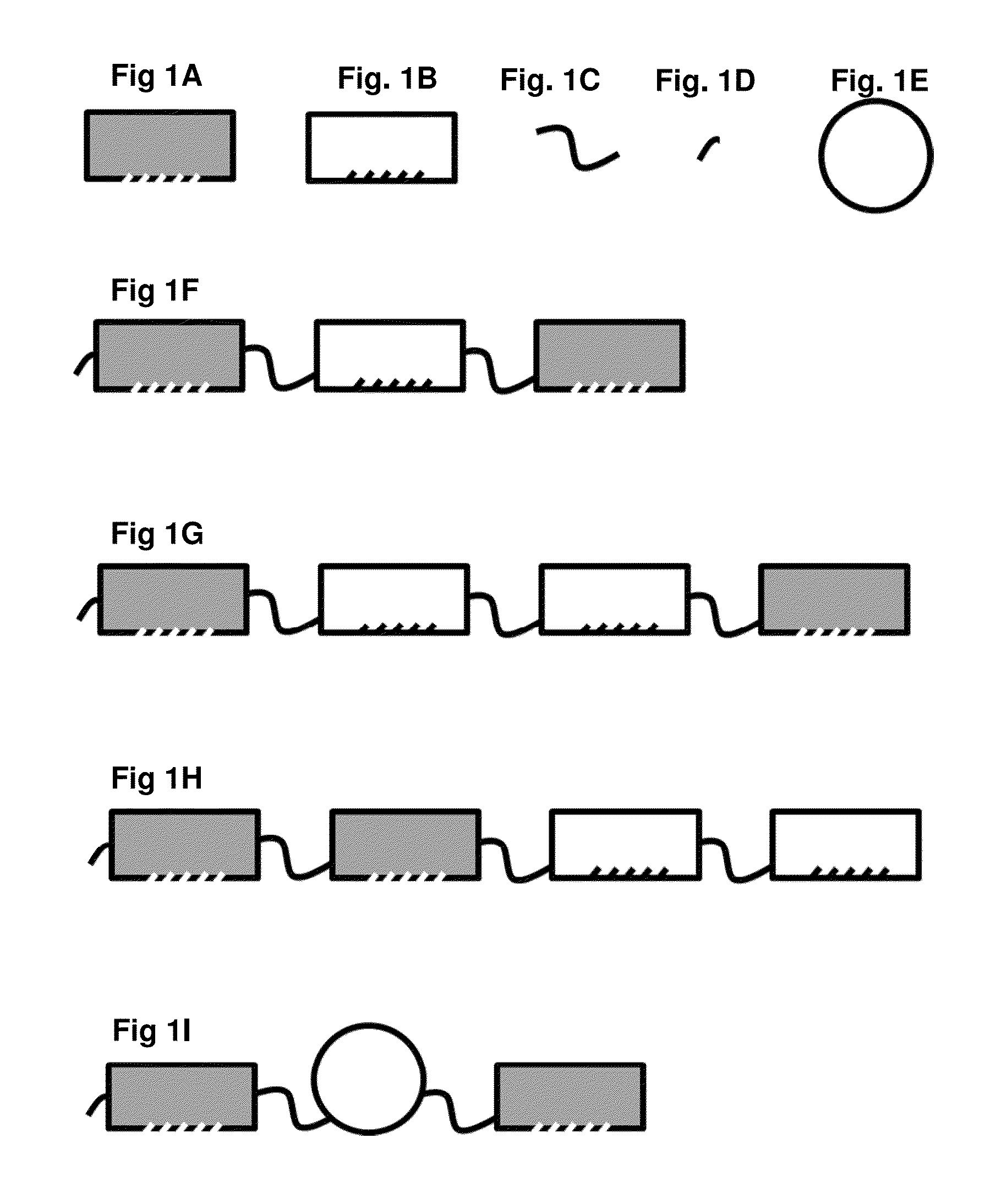
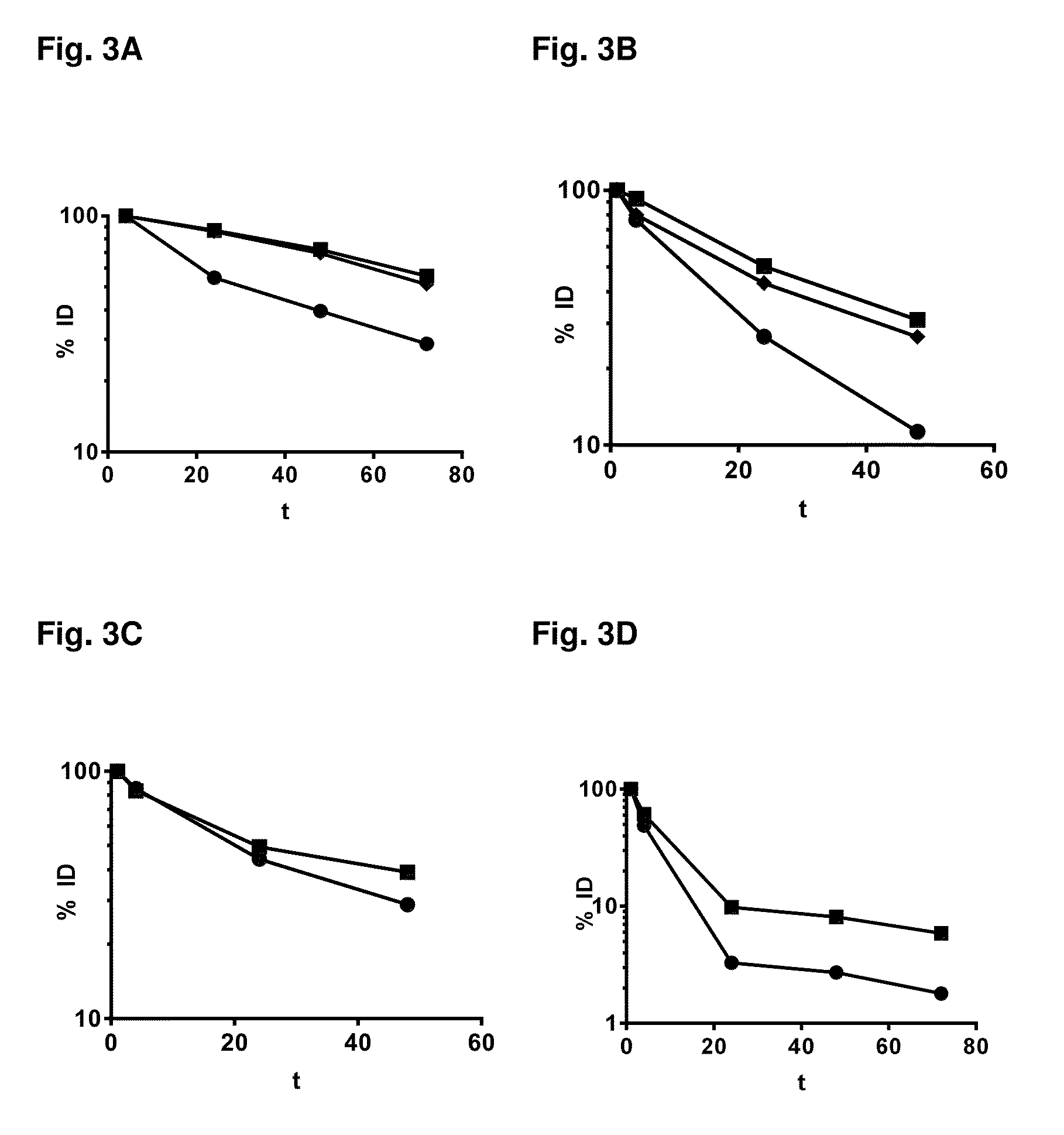
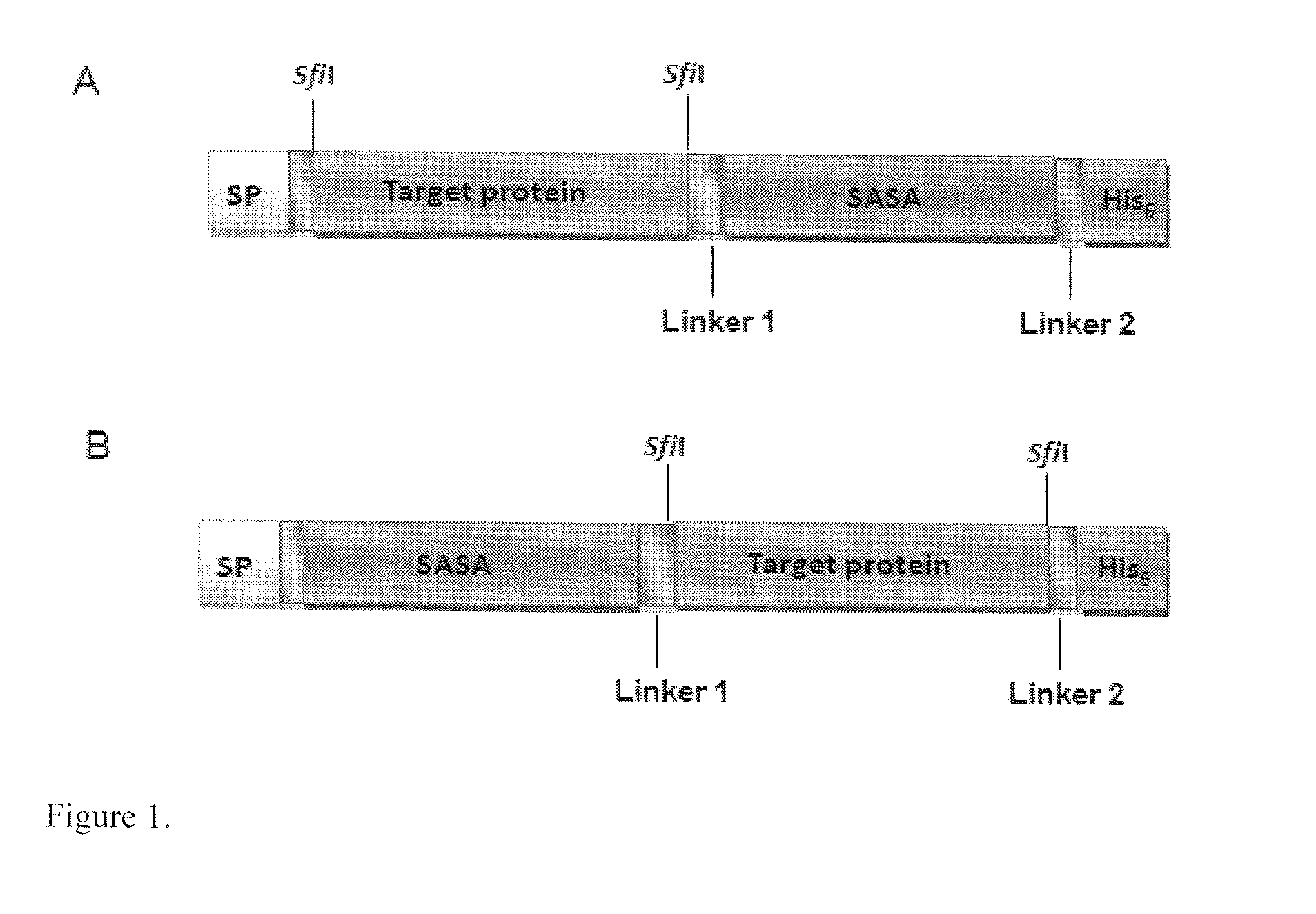
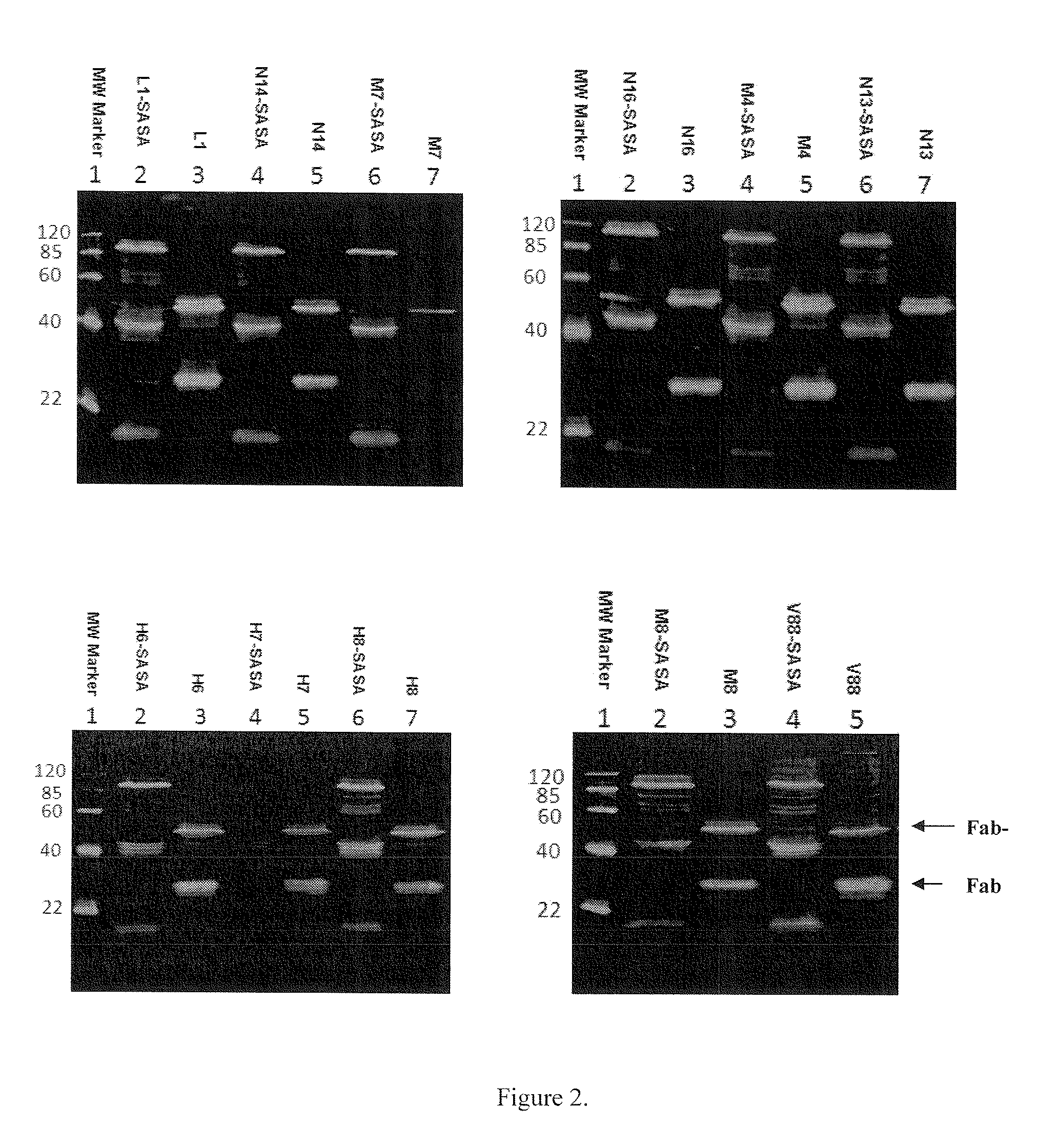
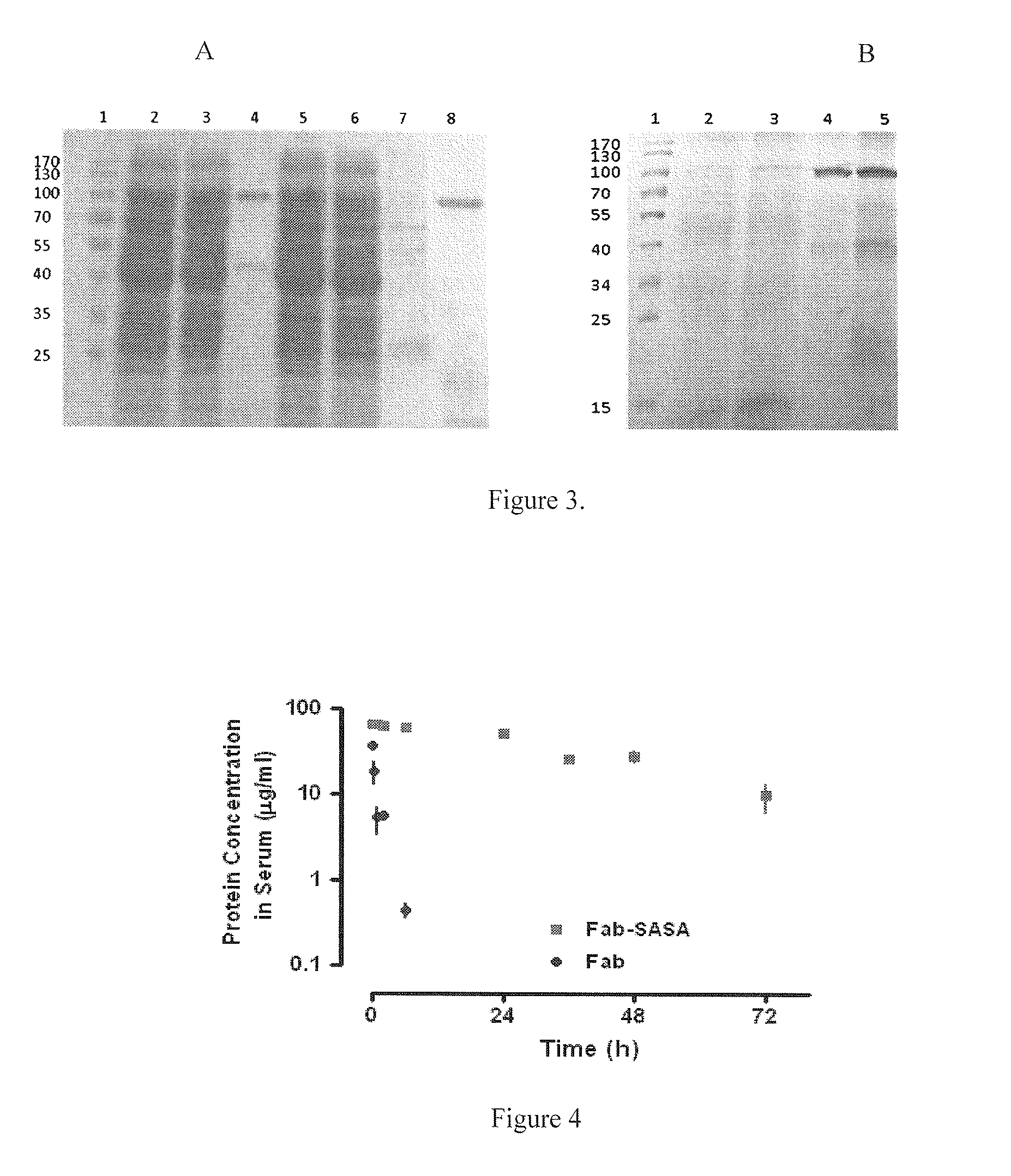
Methods and systems for increasing stability of a target polypeptide in a serum are described. The methods and systems utilize a fusion protein comprising a single-domain antibody against a serum albumin (SASA), the target polypeptide and optionally a linker. The fusion protein has a significantly prolonged serum half life in comparison with the target polypeptide alone. The SASA fusion tag also facilitates the expression and purification of the fusion protein. This allows direct in vivo screening or utilization of the target polypeptide for its biological activity or efficacy regardless of its intrinsic serum half life, which has significantly increased the number of candidates for the development of novel protein based diagnosis or treatment.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Designed repeat proteins binding to serum albumin
ActiveUS20130244940A1Extended half-lifePeptide/protein ingredientsAntibody mimetics/scaffoldsSerum igeSerum protein albumin
New designed repeat proteins with binding specificity for serum albumin are described, as well as nucleic acids encoding such serum albumin binding proteins, pharmaceutical compositions comprising such proteins, the use of such proteins to modify the pharmacokinetics of therapeutic relevant polypeptides and the use of such proteins in the treatment of diseases. The repeat proteins of the invention have a substantially increased half-life in plasma compared to proteins not binding serum albumin.
Owner:MOLECULAR PARTNERS AG
Antibodies that specifically bind to serum albumin without interfering with albumin's capability to interact with the fcrn
Antibodies that specifically bind to an epitope on the serum albumin, including human and / or mouse serum albumin are provided. Nucleic acids encoding such antibodies and cells capable of expressing such antibodies are also provided.
Owner:INST FOR CANCER RES D B A THE RES INSTITUE OF FOX CHASE CANCER CENT +1
Production of recombinant human serum albumin with rice-embryo milk cell as biological reactor
ActiveCN100540667CNo pollution in the processEfficient expressionSerum albuminFermentationSerum protein albuminEmbryo
Production of recombinant human serum albumin with rice albuminous cell as biological reactor is carried out by taking rice albuminous cell protein as storage site for recombinant protein, taking rice albuminous specific expression starter and signal peptide, entering inductive recombinant human serum albumin into inner-film system of rice serum albumin cell and storing it in rice albuminous protein. It is cheap, has more yield and higher expression.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Method for the removal/purification of serum albumins and means for use in the method
InactiveUS6613884B1Simple methodHigh selectivityCell receptors/surface-antigens/surface-determinantsSerum albuminSerum protein albuminAqueous medium
A method for removing a serum albumin from a mixture of other compounds by contacting said mixture with a ligand a) having affinity for and enabling selective binding of the serum albumin and b) being attached to a base matrix insoluble in the aqueous media used or being possible to attach to such a matrix after having become bound to the serum albumin, characterized in that said ligand is derived from an albumin binding bacterial cell surface receptor and that the ligand lacks the IgG-binding and / or alpha2-macroglobulin-binding ability found in native forms of these type of bacterial receptors. An albumin-binding ligand derived from a cell surface bacterial receptor and attached covalently to a carrier matrix, characterized in that the ligand is monovalent with respect to ability to bind a serum albumin. A method for removal of serum albumin from a sample that is to be assayed for non-serum albumin components. The characteristic feature is to subject the sample to affinity adsorption by an albumin-binding ligand derived from an albumin-binding receptor.
Owner:GE HEALTHCARE BIO SCI CORP
Serum-free medium of human epidermis cell and its cultivating method and application
InactiveCN1431299AClear structurePromote growthArtificial cell constructsVertebrate cellsSerum free mediaSerum protein albumin
A non-serum culture medium for human epidermal cells is based on IMDM culture medium and features additional use of hydrogen peroxidase, bovin serum albumin, human transferrin, cholesterin, insulin, hydrocortisone, epidermal cell growth factor, ox hypophysis extract and ethanolamine. Its advantages are no interference and high correctness and precision. It can be used for medicine research and medical purpose.
Owner:江西医学院
Processes for clonal growth of hepatic progenitor cells
A method of propagating mammalian endodermally derived progenitors such as hepatic progenitors, their progeny, or mixtures thereof is developed which includes culturing mammalian progenitors, their progeny, or mixtures thereof on a layer of embryonic mammalian feeder cells in a culture medium. The culture medium can be supplemented with one or more hormones and other growth agents. These hormones and other growth agents can include insulin, dexamethasone, transferrin, nicotinamide, serum albumin, β-mercaptoethanol, free fatty acid, glutamine, CuSO4, and H2SeO3. The culture medium can also include antibiotics. Importantly, the culture medium does not include serum.The invention includes means of inducing the differentiation of the progenitors to their adult fates such as the differentiation of hepatic progenitor cells to hepatocytes or biliary cells by adding, or excluding epidermal growth factor, respectively.The method of producing mammalian progenitors is useful in that the progenitors can be used subsequently in one or more of the following processes: identification of growth and differentiation factors, toxicological studies, drug development, antimicrobial studies, or the preparation of an extracorporeal organ such as a bioartificial liver.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Screening method for Chinese-medicine effective ingredient
InactiveCN1939348AAffect accuracyQuick searchBiological testingPlant ingredientsSerum protein albuminAdditive ingredient
A method for screening the active components of Chinese-medicinal material includes such steps as mixing the liquid extract of the Chinese-medicinal material to be screened with serum albumin solution, sampling by equilibrium dialysis or microdialysis to obtain reactive dialytic liquid, mixing the liquid extract of the Chinese-medicinal material to be screened with the blank solution not containing serum albumin, sampling by equilibrium dialysis or microdialysis to obtain blank dialytic liquid, respective efficient liquid-phase chromatography analysis for said reactive dialytic liquid and blank dialytic liquid, and calculating the albumin combining percentage of each component.
Owner:CHINA PHARM UNIV
VEGF slowly releasing injection microsphere support and its prepn and use
InactiveCN1973828AImprove survival ratePromote formationPeptide/protein ingredientsGranular deliverySerum protein albuminMicrosphere
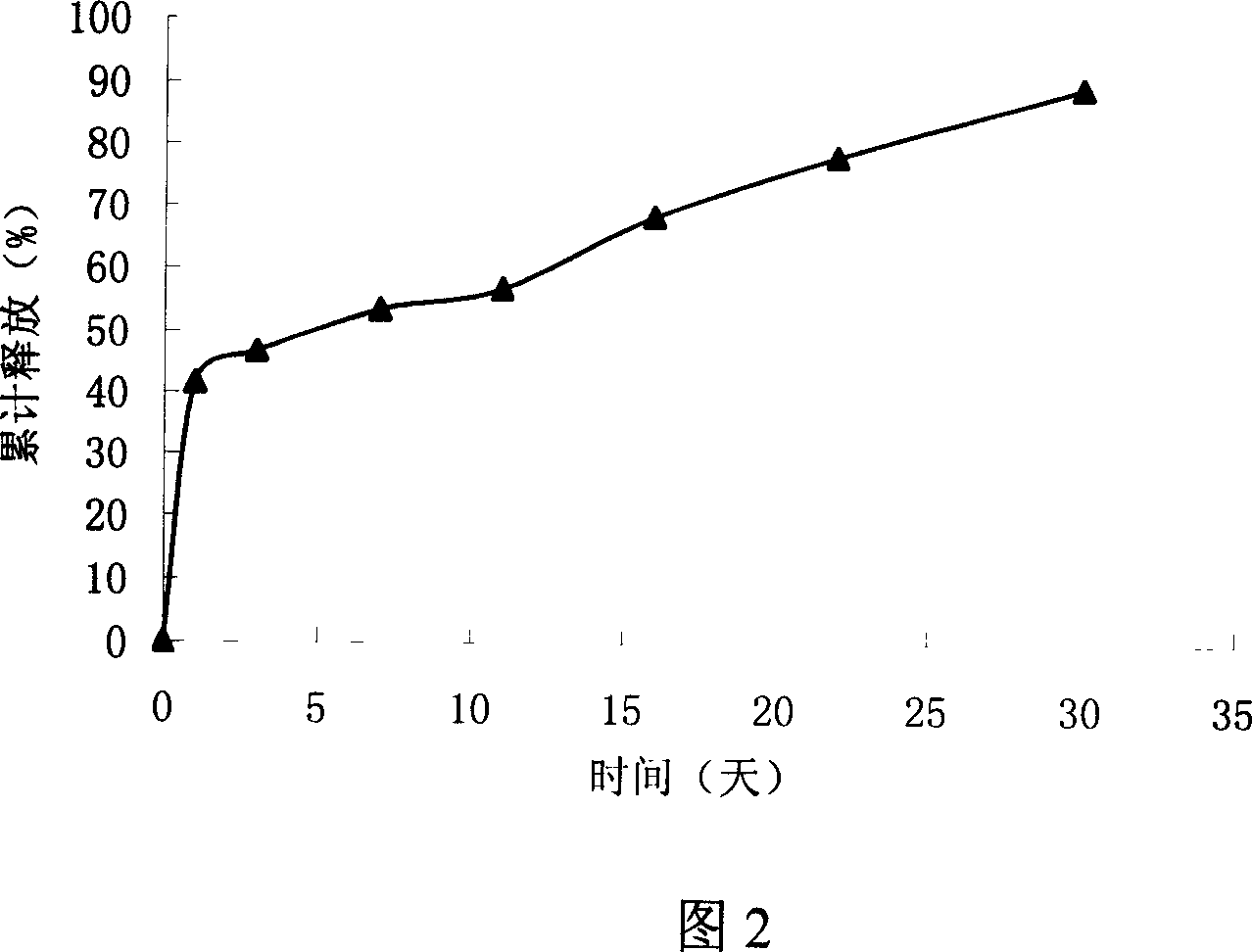
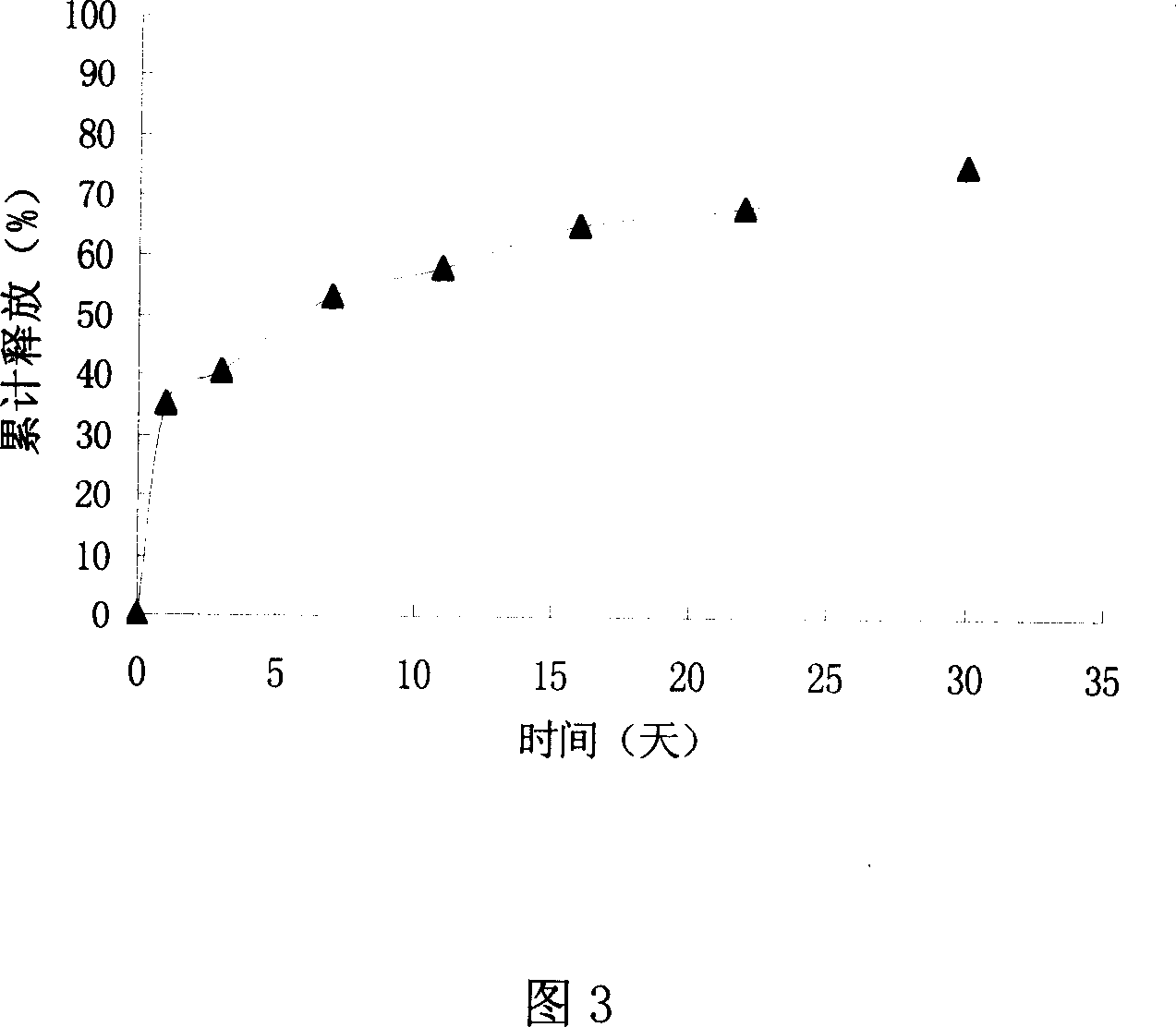
The VEGF slowly releasing injection microsphere support includes matrix, VEGF, protectant and ammonium bicarbonate. It features the matrix of polylactic acid-hydroxyl acetate copolymer of polylactic acid / hydroxyl acetate ratio 25:75 to 75:25; the protectant comprising zinc carbonate, serum albumin, mycose and mannitol; the weight ratio between VEGF and polylactic acid-hydroxyl acetate copolymer of 1:100000 to 1:10; and the microsphere size of 50-1000 microns. It is prepared through one W / O / W solvent volatilizing process or one fine medicine powder adding W / O / W solvent volatilizing process. The preparation process is simple, easy in operation and high in repeatability. In the microsphere support, protein medicine VEGF may be in vitro released for over 4 weeks in approached zero order kinetic mode. The present invention may be used in the repair and treatment of different kinds of tissue and blood vessel damage.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hemostatic
The present invention is one kind snake venom cytozyme hemostatic containing blood coagulating factor, activator RVV-X and synergist snake venom thrombase. Preferably, the snake venom cytozyme hemostatic contains also serum albumin, for example human serum albumin. In addition, the present invention also discloses the preparation process and hemostatic use of high purity RVV-X.
Owner:北京诺维康医药科技有限公司
Expression human seralbumin carrier and engineering bacterium
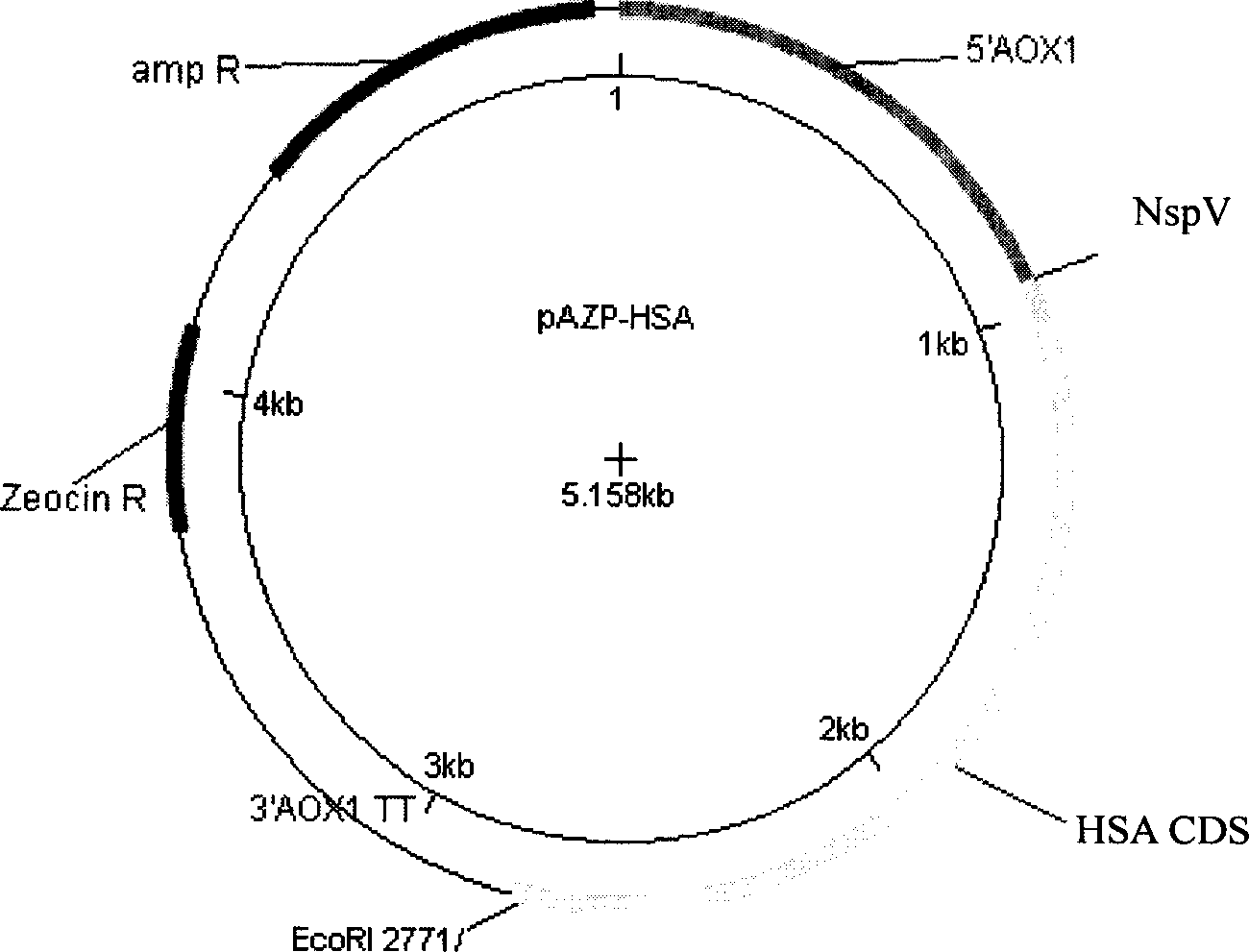
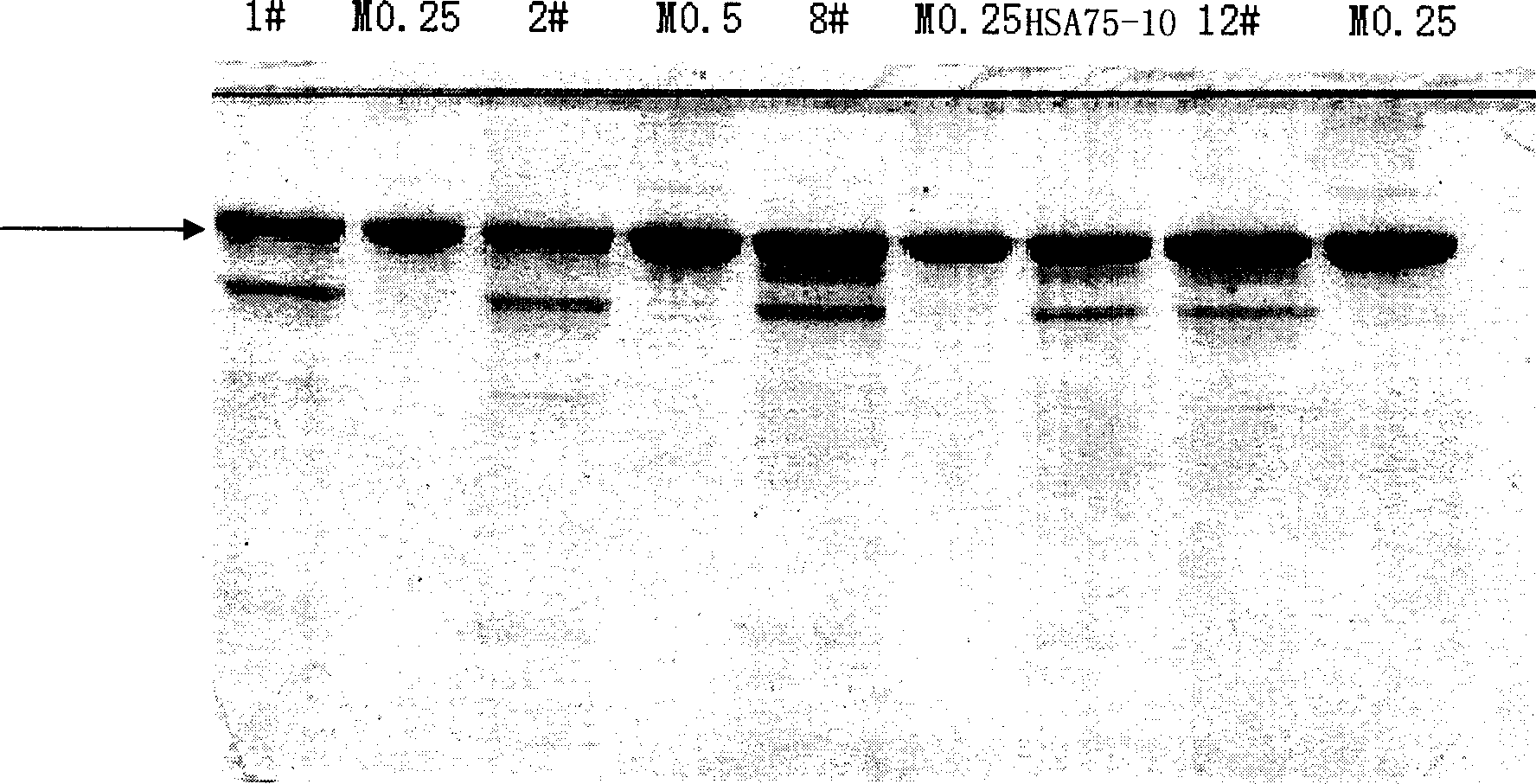
ActiveCN1854301AIncrease productionEasy to separateFungiMicroorganism based processesPichia pastorisSerum protein albumin
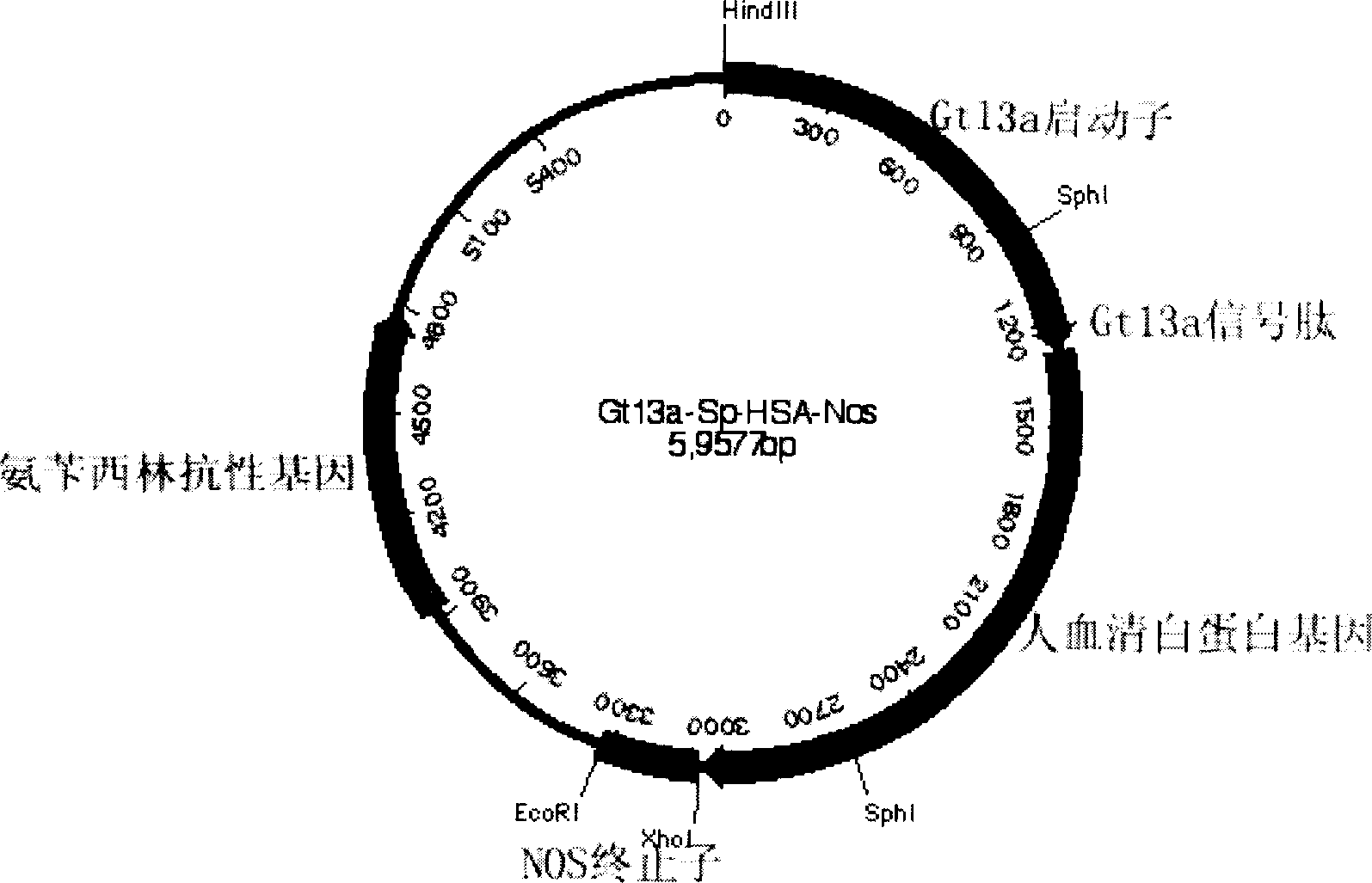
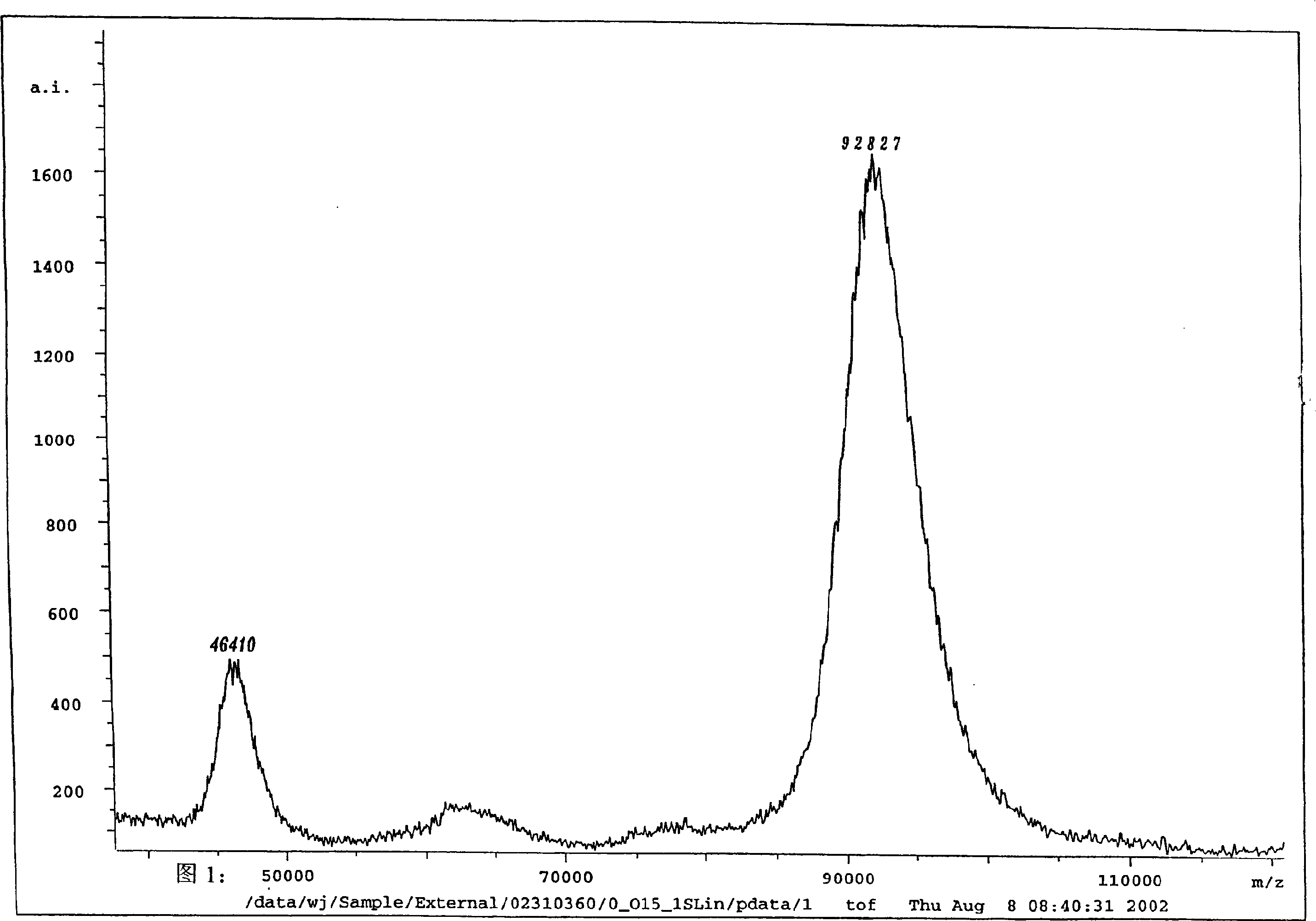
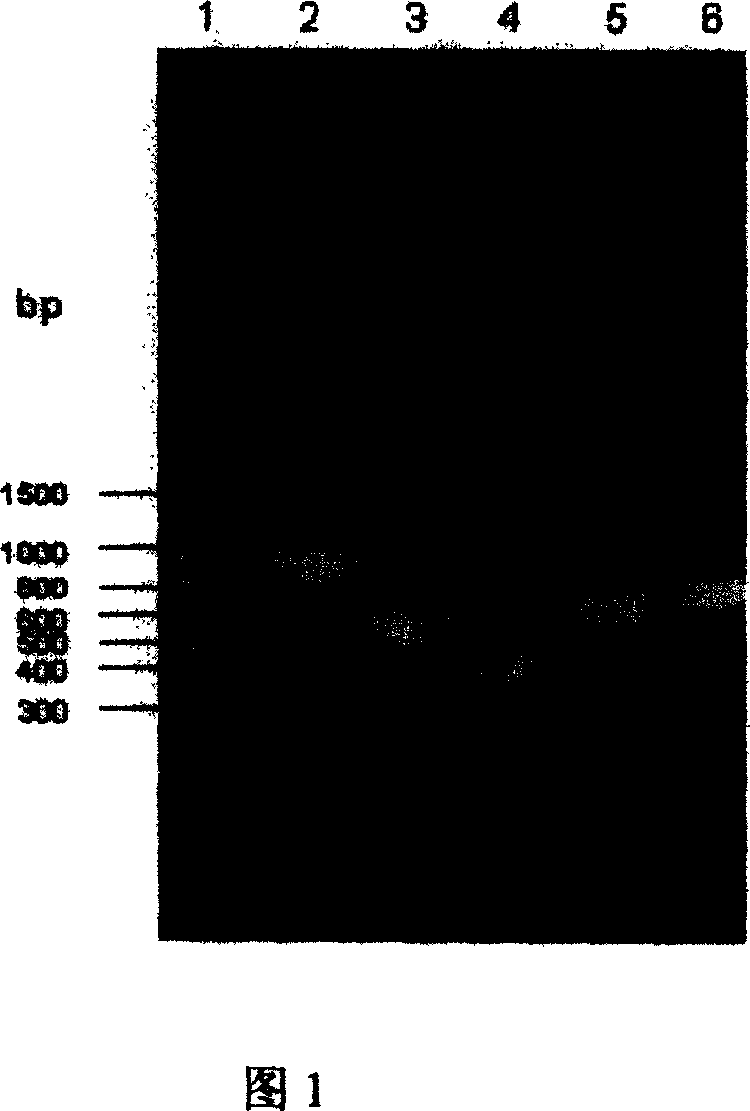
The invention discloses a carrier and engineering bacteria to express the human serum albumin. The carrier is the plasmid which inserts the encoding human serum albumin sequence into the multi clone locus of the Pichia pastoris expression carrier. The 5'end of the encoding sequence of the serum albumin is connected to the encoding sequence of the secretory signal peptide and the leading peptide which the 5' end is connected to the sequence GAAACG of the AOX1 gene for the Pichia pastoris. The bacteria including the expression carrier especially the Pichia pastoris HSA75-10 CGMCCNo.1360 can be cultured to get the high yield of the human serum albumin (10g / L of the supernate) which is 80% of the secretory protein.
Owner:NCPC NEW DRUG RES & DEV
Serum-free culture medium, preparation method thereof and culture method of mesenchymal stem cells
ActiveCN110923196AImprove proliferative abilityImprove performanceCulture processSkeletal/connective tissue cellsPolyvinyl alcoholHydrocortisone
Owner:GUANGZHOU SALIAI STEMCELL SCI & TECH CO LTD
Stabilization of protein preparations
InactiveUS20050119172A1Satisfactory stabilityComposition is stablePowder deliveryFactor VIISerum protein albuminProtein
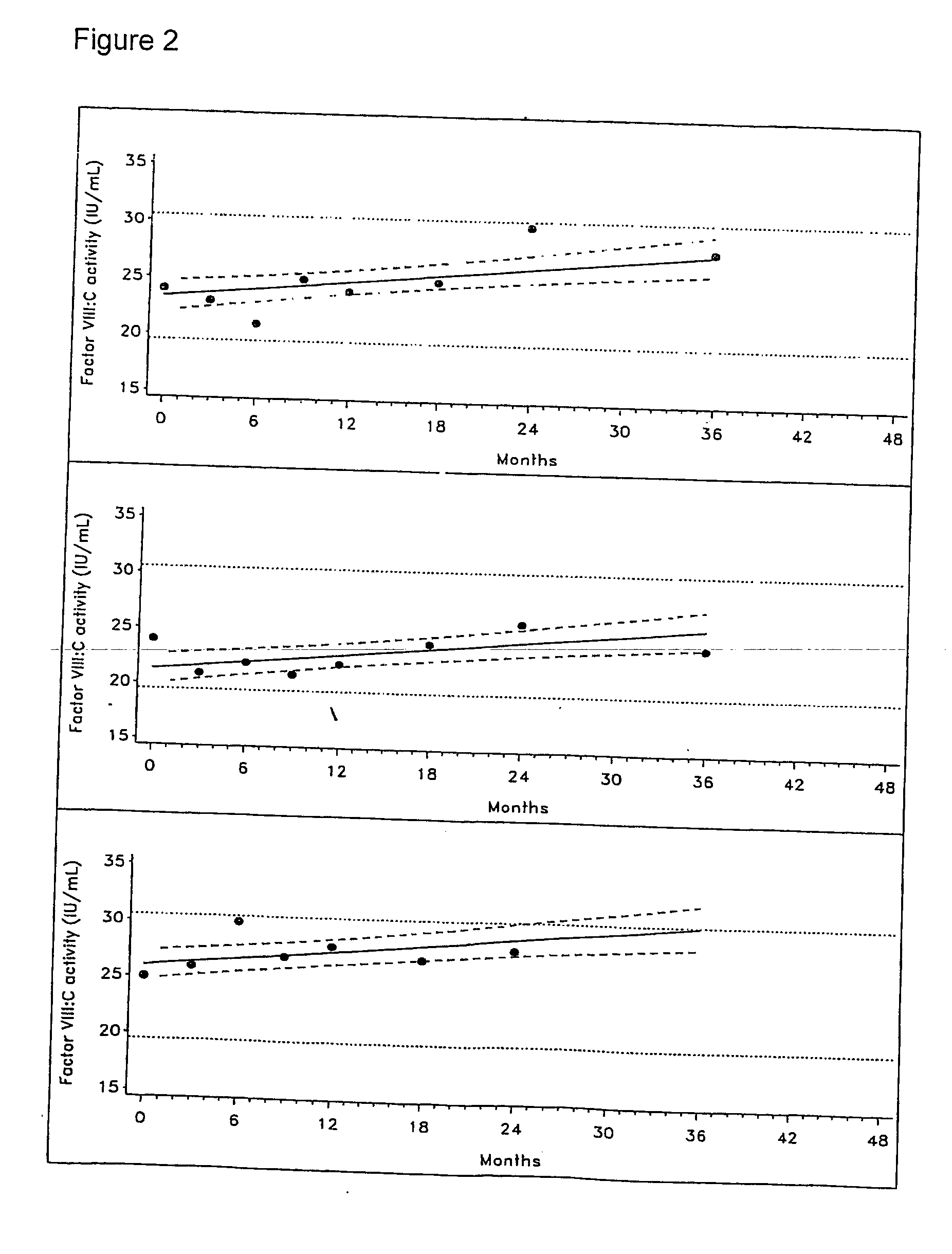
A composition comprising a non-albumin protein is stabilised by the addition of a highly purified recombinant human serum albumin. The non-albumin protein may be Factor VIII.
Owner:NOVOZYMES BIOPHARMA DK AS
Freeze-dried powder, essence and skincare set for repairing acne marks and scars
InactiveCN105456050AImprove microcirculationImprove health statusCosmetic preparationsToilet preparationsSerum protein albuminFiltration
The invention discloses freeze-dried powder for repairing acne marks and scars. The freeze-dried powder is prepared by the steps of: A, preparing raw materials; B, dissolving mannitol, oligopeptide-3, disodium hydrogen phosphate and oligopeptide-2 in water at normal temperature, placing the solution in a 121 DEG C autoclave, and performing sterilization at a constant temperature for 20 minutes to form a solution A; C, sequentially adding sodium dihydrogen phosphate and serum albumin into the solution A, shaking the mixture uniformly, and performing filtration by using a filter membrane to obtain a solution B; D, standing the solution B for 18-36h, and then performing filling; E, performing freeze drying on the solution B after filling; and F, packaging the product after drying. The freeze-dried powder prepared by the invention can achieve multiple repair and protection of a skin damaged by acnes, and can promote skin microcirculation and improve the acne marks to ensure that the skin can recovered to a healthy state.
Owner:QINGDAO MEIBO BIOTECH CO LTD
Kit for quantitatively detecting bovine milk serum albumin in milk or dairy product
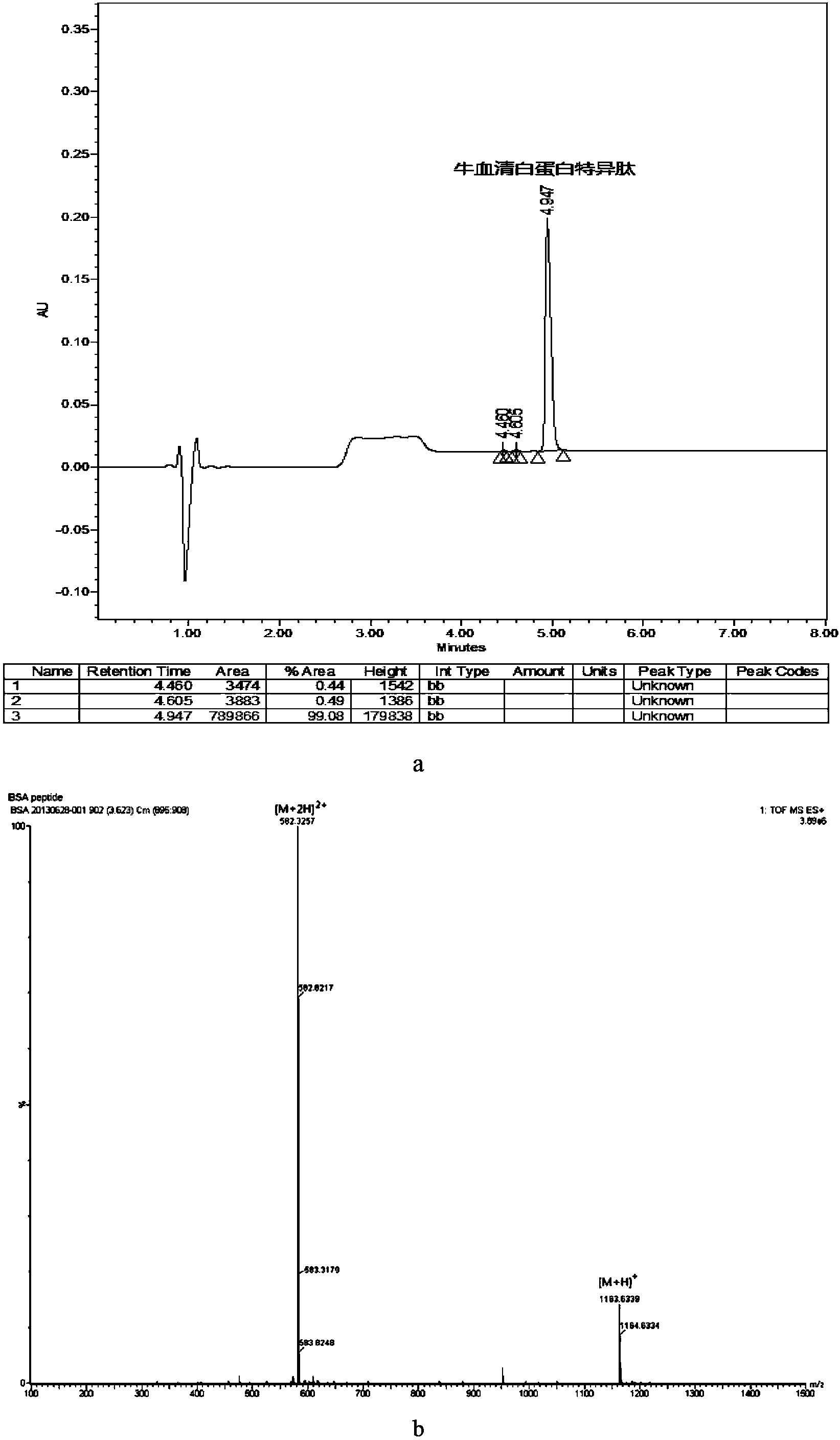
ActiveCN104345107AEasy to prepareReduce dosageComponent separationSerum protein albuminBovine serum albumin
The invention provides a kit for quantitatively detecting bovine milk serum albumin. The kit comprises a bovine serum albumin specific peptide, an isotope labeled bovine milk serum albumin specific peptide, an isotope labeled bovine serum albumin internal standard, a proteolytic reagent and a quality control sample. The amino acid sequence of the bovine serum albumin specific peptide is LVNELTEFAK; the amino acid sequence of the isotope labeled bovine milk serum albumin specific peptide is LV *NEL*TEFAK; and the amino acid sequence of the isotope labeled bovine serum albumin internal standard is QCPFDEHVKLV*NEL*TEFAKTCVADESHAGC, wherein V* and L* are carbon and nitrogen isotope labeled amino acids. The kit can accurately quantitatively detect the bovine milk serum albumin in various milk-containing products by adopting the isotope labeled internal standard synthesized through researches and designing, ensures the result reliability, and also has the advantages of less reagent consumption and low detection cost.
Owner:SHANGHAI IMPACT SCI INSTR
Bispecific fusion antibodies with enhanced serum half-life
Drug compositions, fusions and conjugates are provided. The drug fusions and conjugates contain a therapeutic or diagnostic agent that is fused or conjugated to an antigen-binding fragment of an antibody that binds serum albumin. The drug compositions, fusions and conjugates have a longer in vivo half-life in comparison with the unconjugated or unfused therapeutic or diagnostic agent.
Owner:DORMANTIS LTD
Helicobacter pylori urease B subunit B cell antigen epitope polypeptide, identification method and application
InactiveCN101033468AGood immunogenicityClear Helicobacter pylori infectionBacterial antigen ingredientsMicrobiological testing/measurementAnti-Helicobacter pylori IgGCarrier protein
This invention provides a B cell epitope peptide and its identification method to neutralize helicobacterpylori urease B subunit, determines the antigen epitope corresponding with the monoclonal antibody 6E6 of helicobacterpylori urease B subunit, and confirms this B cell epitope is specific for the 6E6. The invention also provides a chimeric epitope vaccine and its preparation method containing B cell epitope and carrier protein BSA, and the B cell epitope has strong antigenicity.
Owner:ARMY MEDICAL UNIV
Serum albumin binding moieties
InactiveUS7211395B2Increased serum half-lifeExtended half-lifePeptide/protein ingredientsComponent separationSerum igeSerum protein albumin
Compositions comprising non-naturally occurring serum albumin binding moieties are described, together with methods of use thereof, e.g., for detecting or isolating serum albumin molecules in a solution, for blood circulation imaging, and for linking therapeutics or other molecules to albumin. Preferred serum albumin binding peptides having a high affinity for human serum albumin are particularly disclosed.
Owner:MORFOZIS AG
Method for detecting vestigial clenbuterol
InactiveCN101231293AEasy to operateLow costMaterial analysisSerum protein albuminMonoclonal antibody
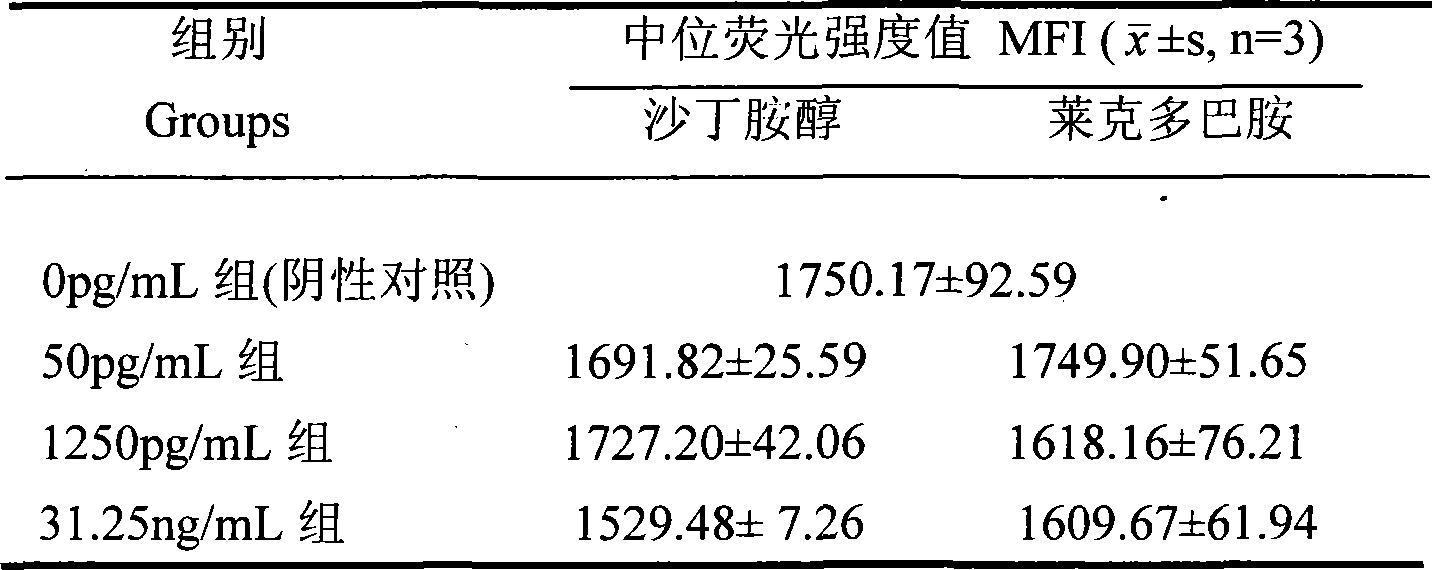
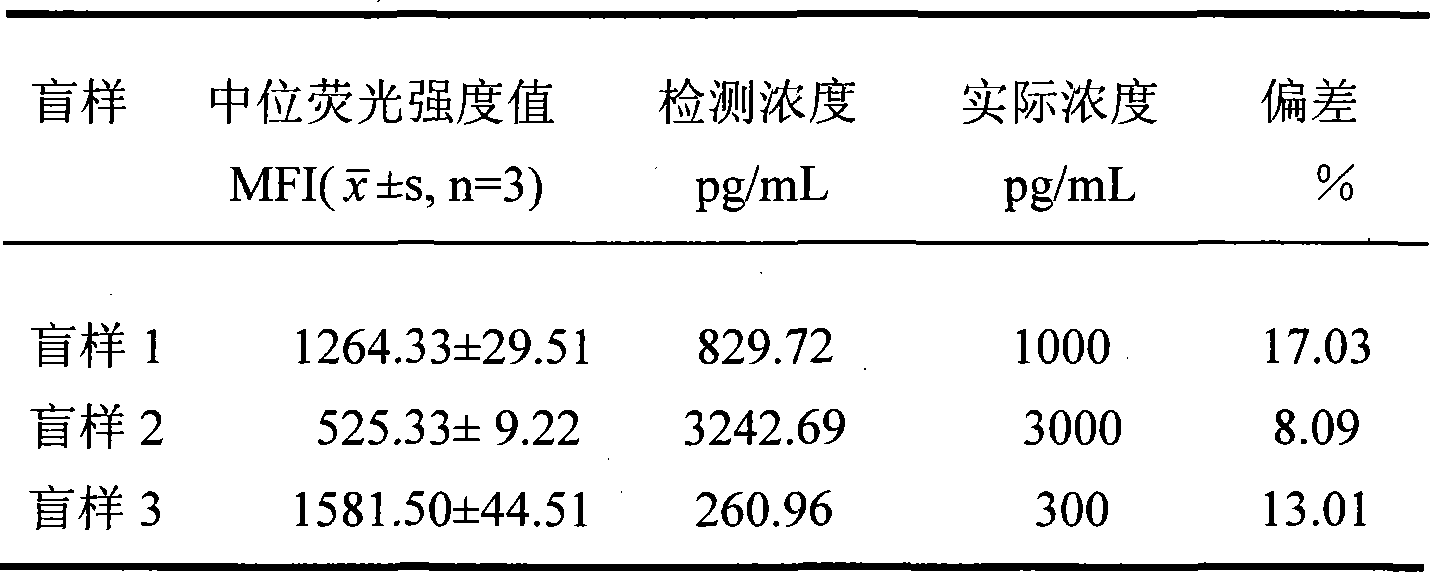
The invention discloses a method for detecting clenbuterol residues, which comprises the following steps: (1) preparing, identifying and biotinylating clenbuterol-calf serum albumin conjugate; (2) coupling anti-clenbuterol monoclonal antibody on fluorescent microspheres; (3) drawing standard curve for suspension chip detection of clenbuterol residues; (4) testing specificity of suspension chip detection of clenbuterol residues; and (5) measuring the concentration of clenbuterol residues sample. The method of the invention for detecting clenbuterol residues has the advantages of simple operation, rapidness, sensitivity and low cost; and the overall detection process only takes 1-2h.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
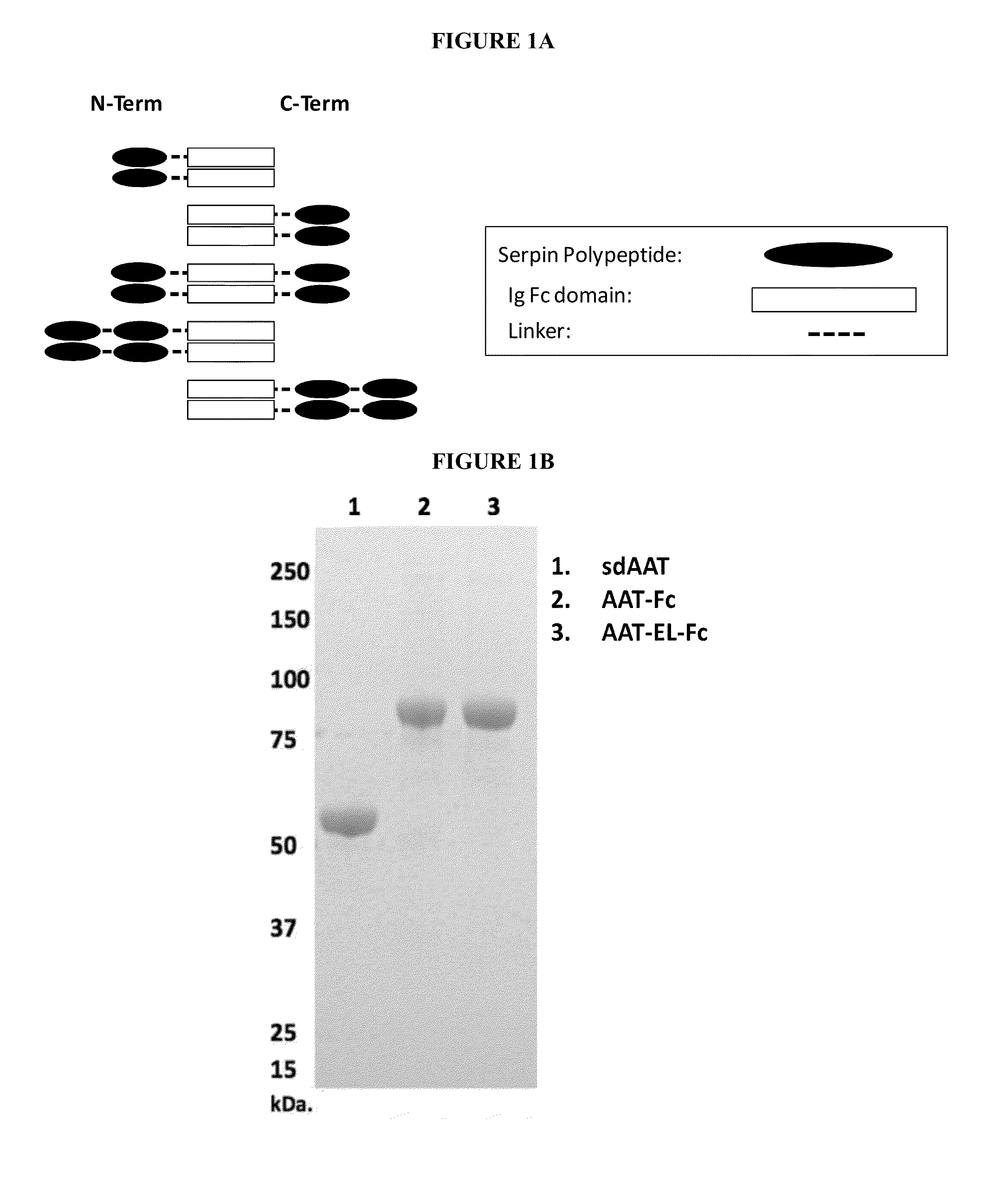
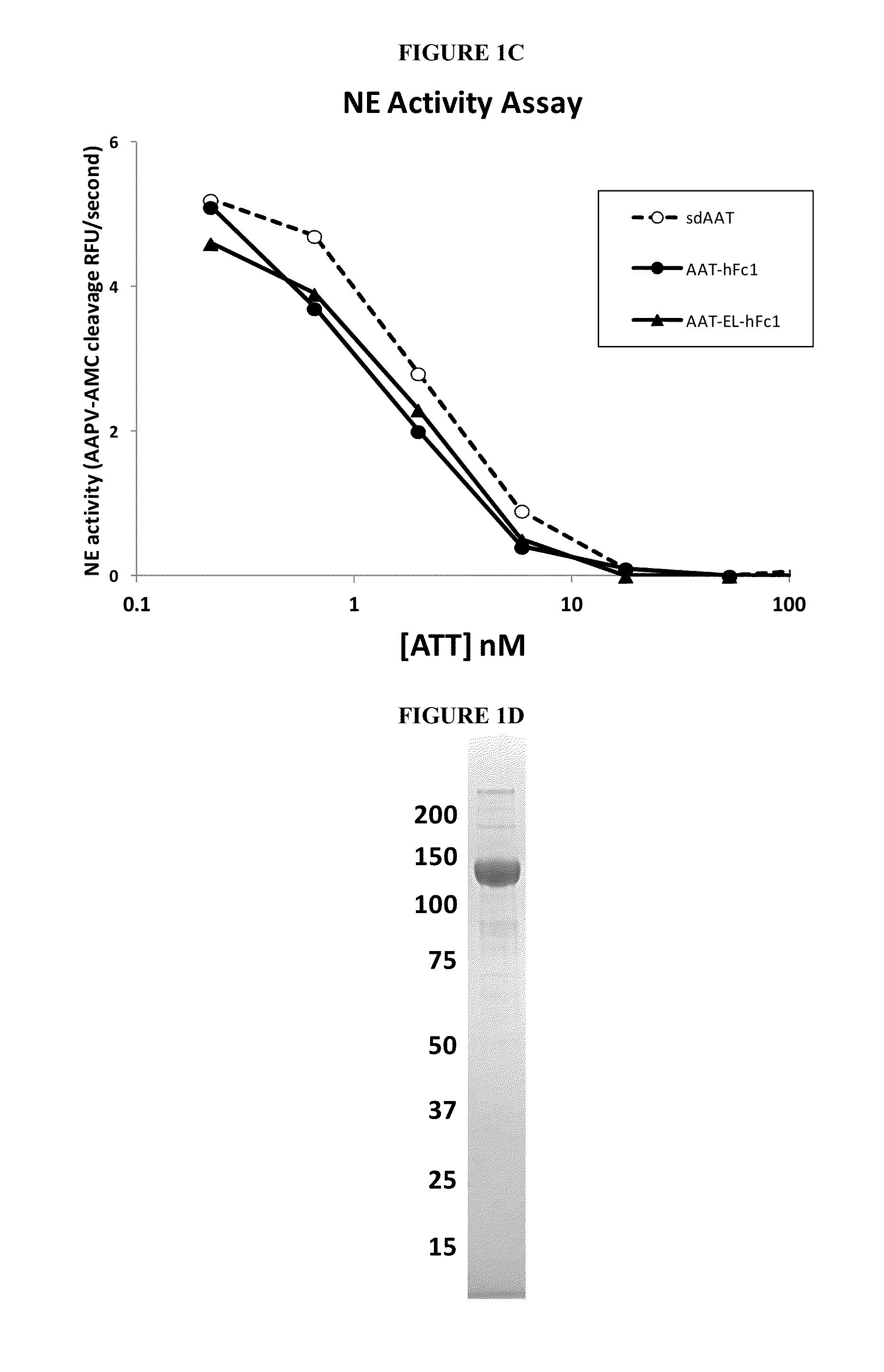
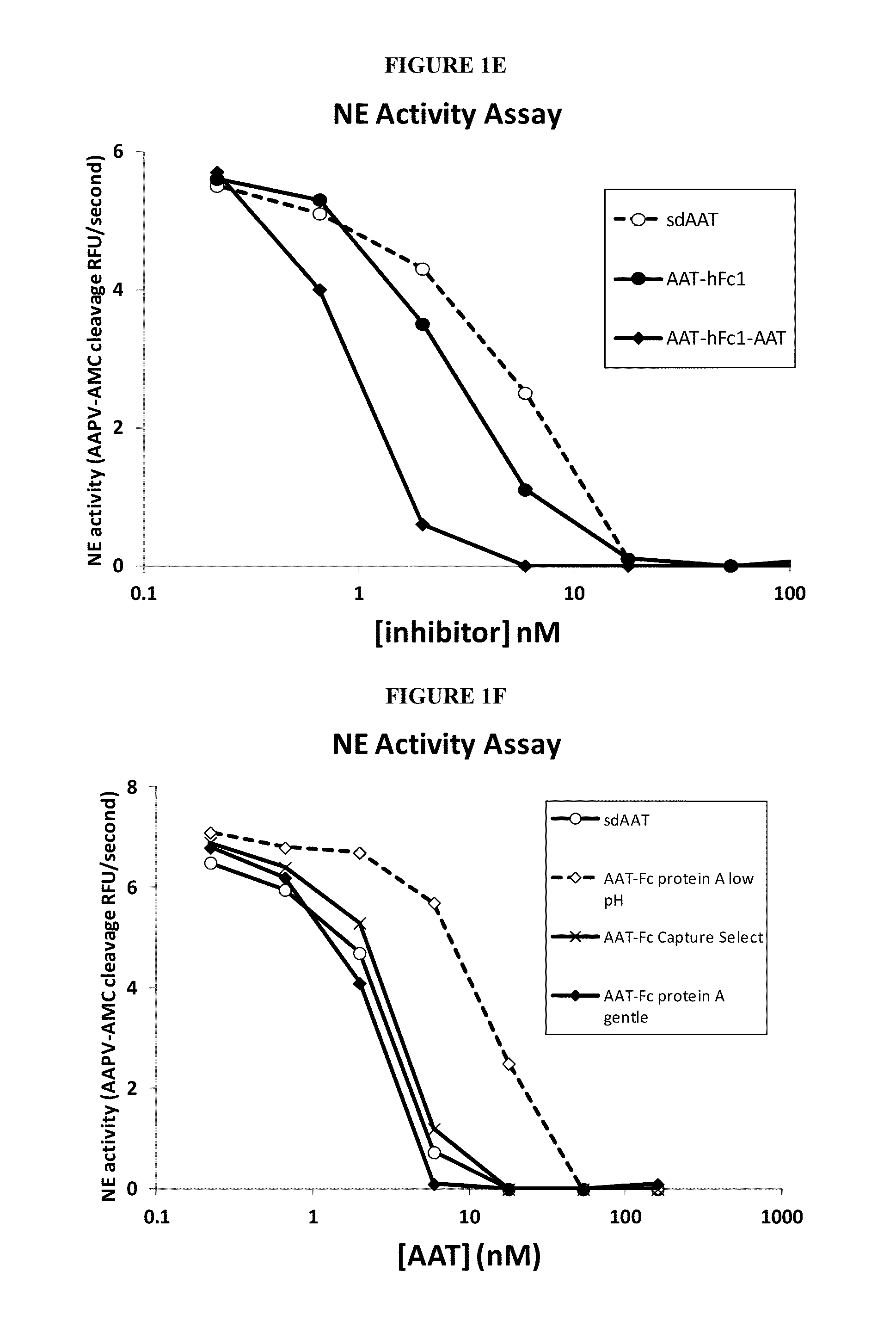
Serpin Fusion Polypeptides and Methods of Use Thereof
ActiveUS20130011398A1Enhance FcRn bindingInhibition releaseAntibacterial agentsAntimycoticsSerum protein albuminCytokine
This invention relates to molecules, particularly polypeptides, more particularly fusion proteins that include a serpin polypeptide or an amino acid sequence that is derived from a serpin and second polypeptide comprising of at least one the following: an Fc polypeptide or an amino acid sequence that is derived from an Fc polypeptide; a cytokine targeting polypeptide or a sequence derived from a cytokine targeting polypeptide; a WAP domain containing polypeptide or a sequence derived from a WAP containing polypeptide; and an albumin polypeptide or an amino acid sequence that is derived from a serum albumin polypeptide. This invention also relates to methods of using such molecules in a variety of therapeutic and diagnostic indications, as well as methods of producing such molecules.
Owner:INHIBRX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com