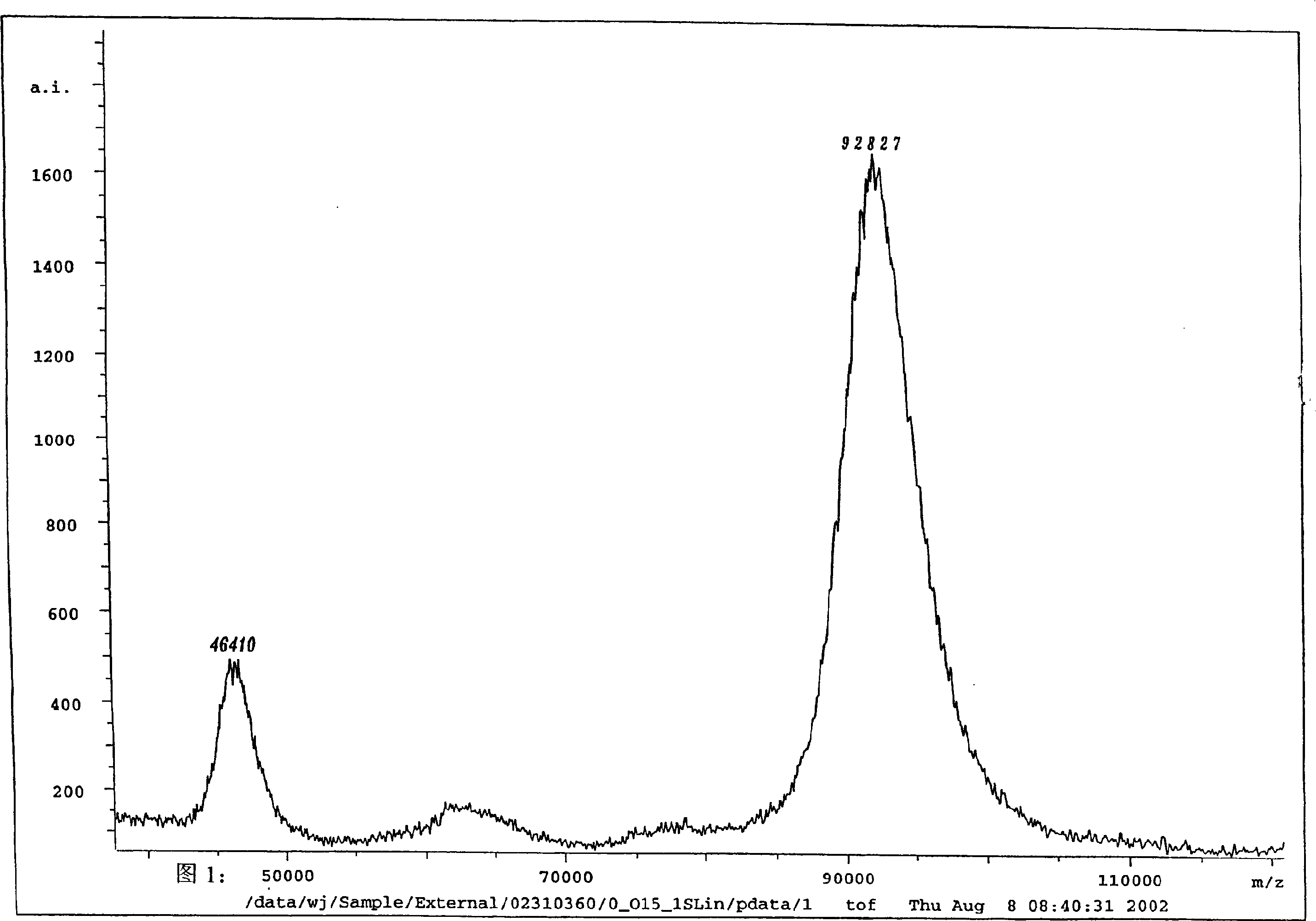
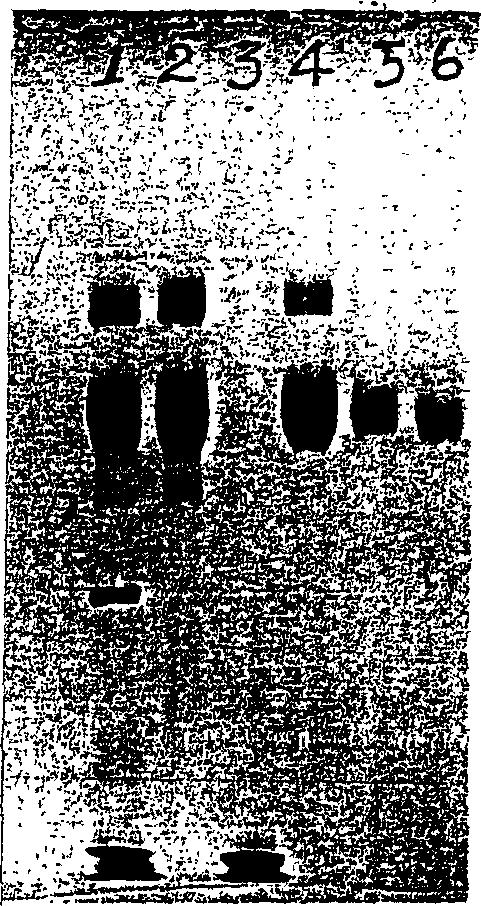Hemostatic
A drug and coagulation factor technology, applied in the field of snake venom kinase hemostatic drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Prepare snake venom coagulase hemostatic drug according to the composition of prescription 2 in the above table: take coagulase 1U / ml, RVV-X 5U / ml, human serum albumin 20mg, mannitol 30mg according to the conventional method, and make it into freeze-dried Powder injection, the snake venom coagulase hemostatic drug for injection with a specification of 1ml / bottle.
Embodiment 2
[0089] Prepare snake venom coagulase hemostatic drug according to the composition of prescription 2 in the above table: take coagulase 0.8U / ml, RVV-X 1U / ml, human serum albumin 10mg, mannitol 30mg according to the conventional method, and make it into frozen Dry powder injection, the snake venom coagulase hemostatic drug for injection with a specification of 1ml / bottle.
Embodiment 3
[0091] According to the composition of prescription 2 in the above table, the snake venom coagulase hemostatic drug was prepared: take coagulase 0.01U / ml, RVV-X 0.1U / ml, human serum albumin 1mg, and mannitol 30mg according to the conventional method, and make it into Freeze-dried powder injection, the snake venom coagulase hemostatic drug for injection with a specification of 1ml / bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com