Patents
Literature
57 results about "VIPeR" patented technology
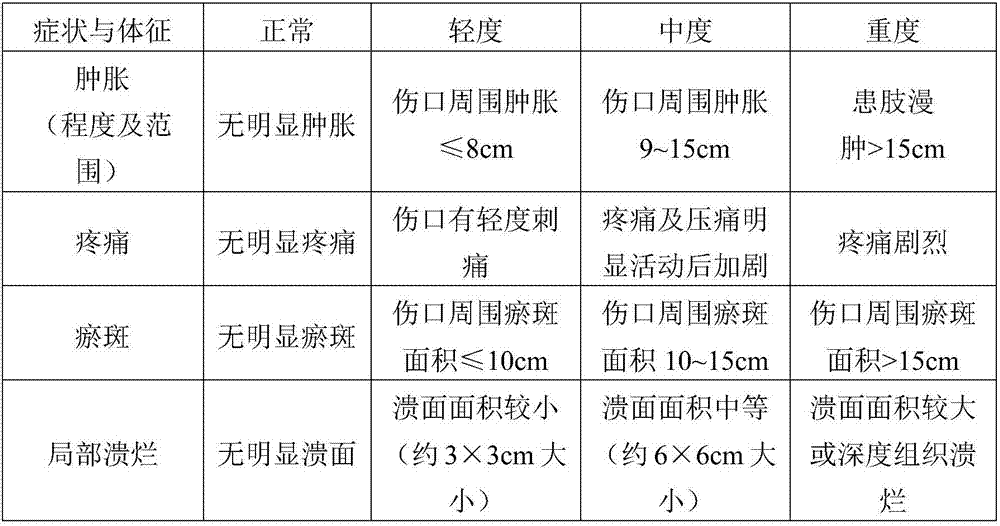
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
VIPeR is a military robot developed by the Israeli company Elbit Systems and intended for use in warfare. It was unveiled in March, 2007.
Viper crescent wrench device
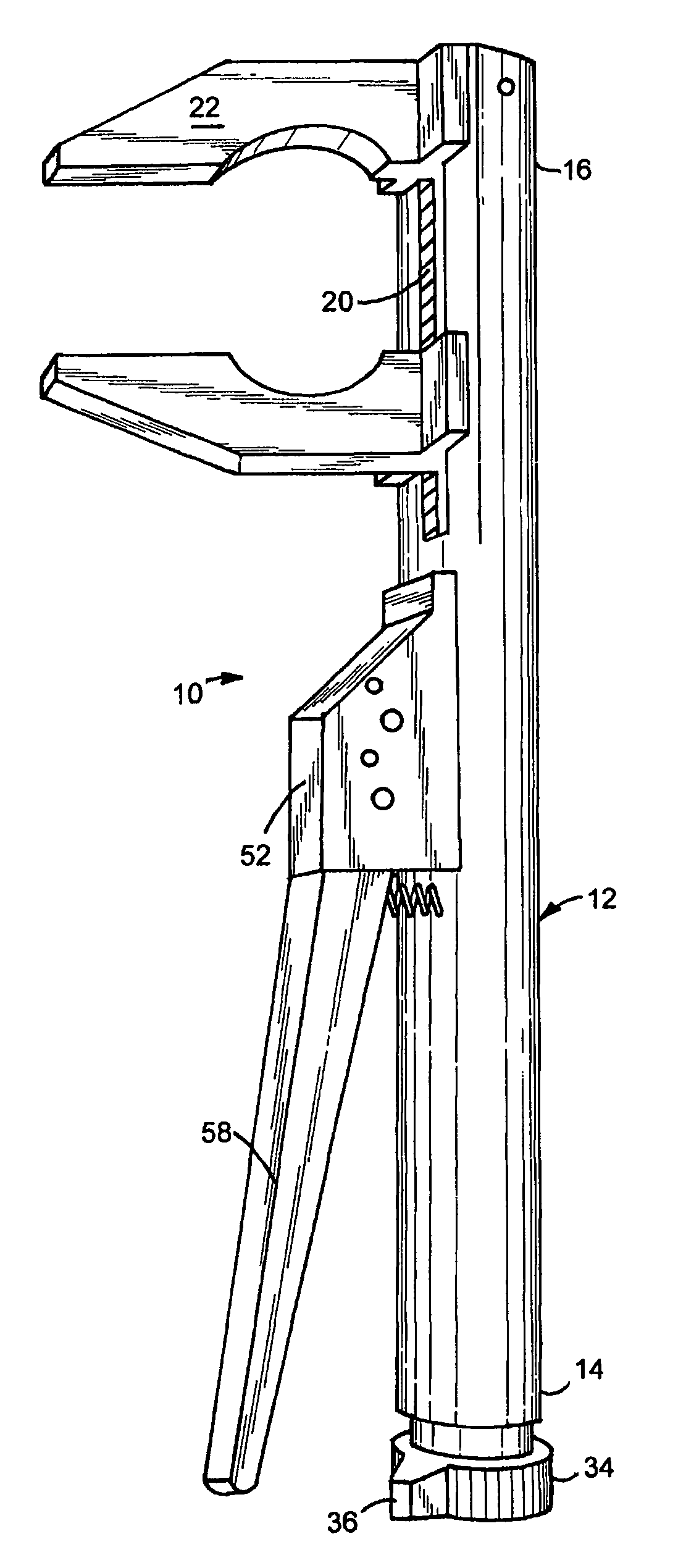
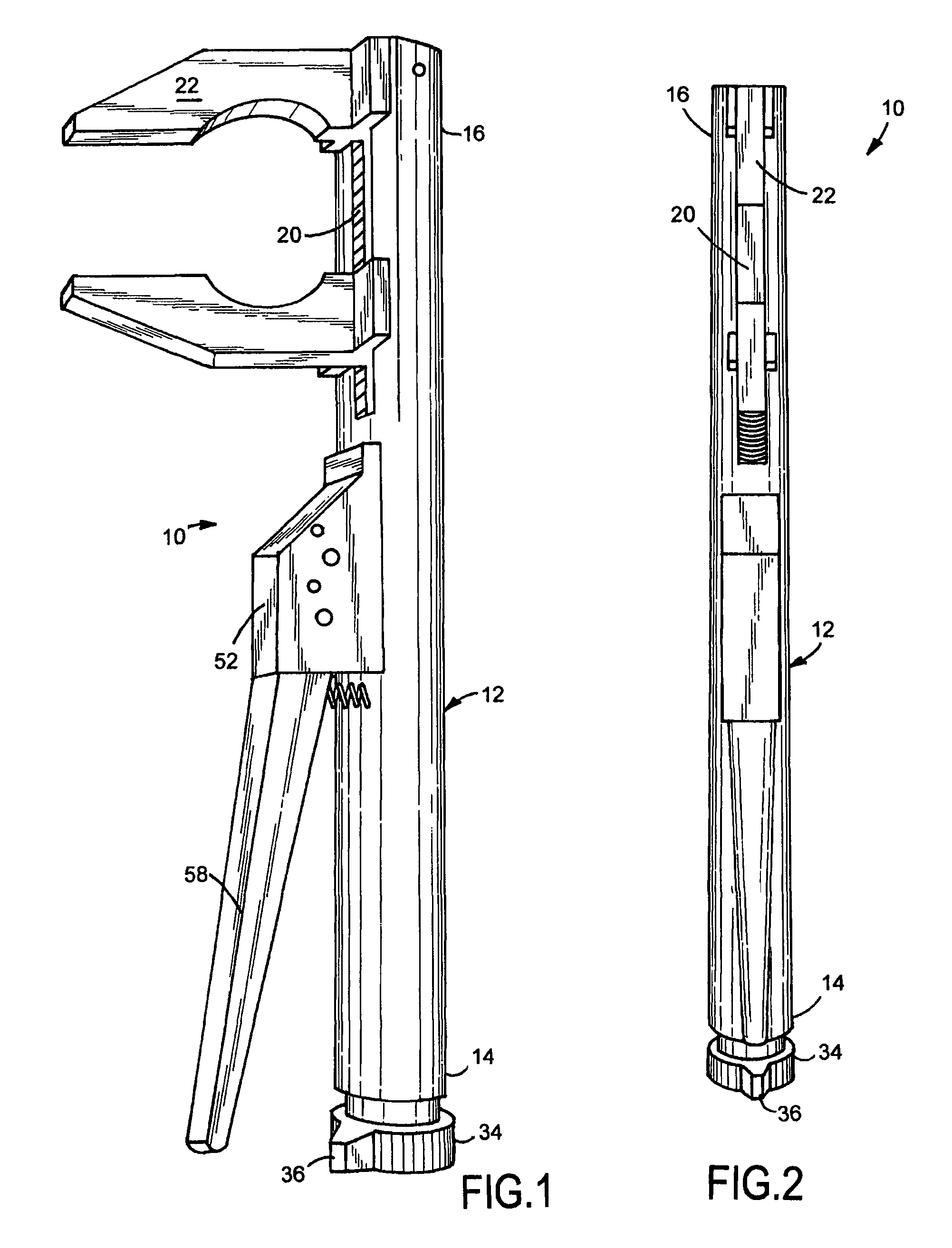
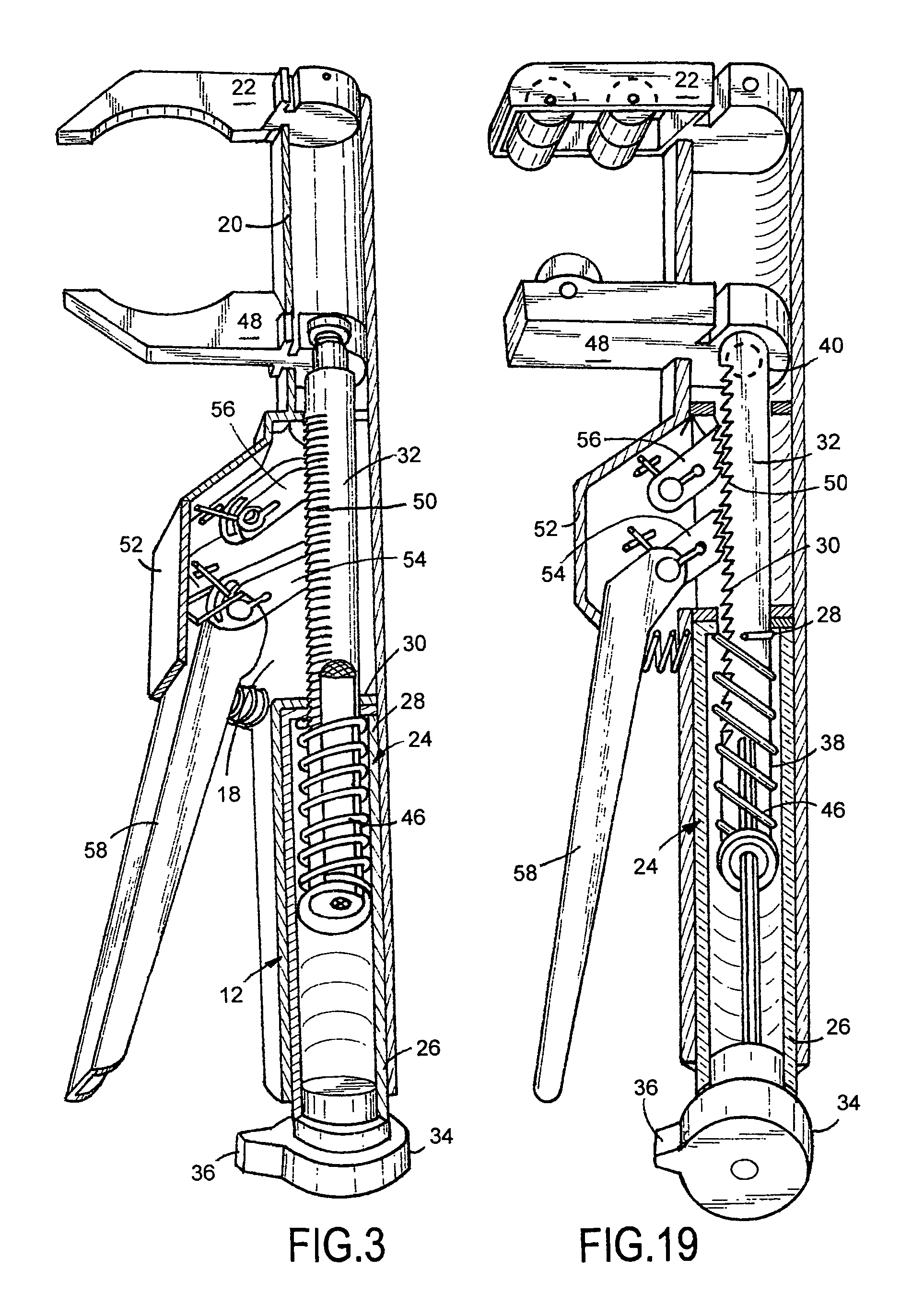
A viper crescent wrench device for holding a work piece is provided. Upon squeezing a second handle toward a first handle, a first pawl and a second pawl move an adjusting bar toward the first handle thereby moving a second jaw portion toward a first jaw portion. Upon releasing the second handle, the first pawl and the second pawl releasably lock into place and the second handle readjusts relative to the first pawl thereby repositioning to do the same action again when the second handle is squeezed again until the work piece is secured between the first jaw portion and the second jaw portion. Upon rotation of a release mechanism, toothed notches are rotated out of contact with the first pawl and the second pawl such that the second jaw portion moves away from the first jaw portion under bias of a first spring thereby releasing the work piece.
Owner:LOPEZ SERGIO
Lyophilized viper antivenin and preparation method thereof
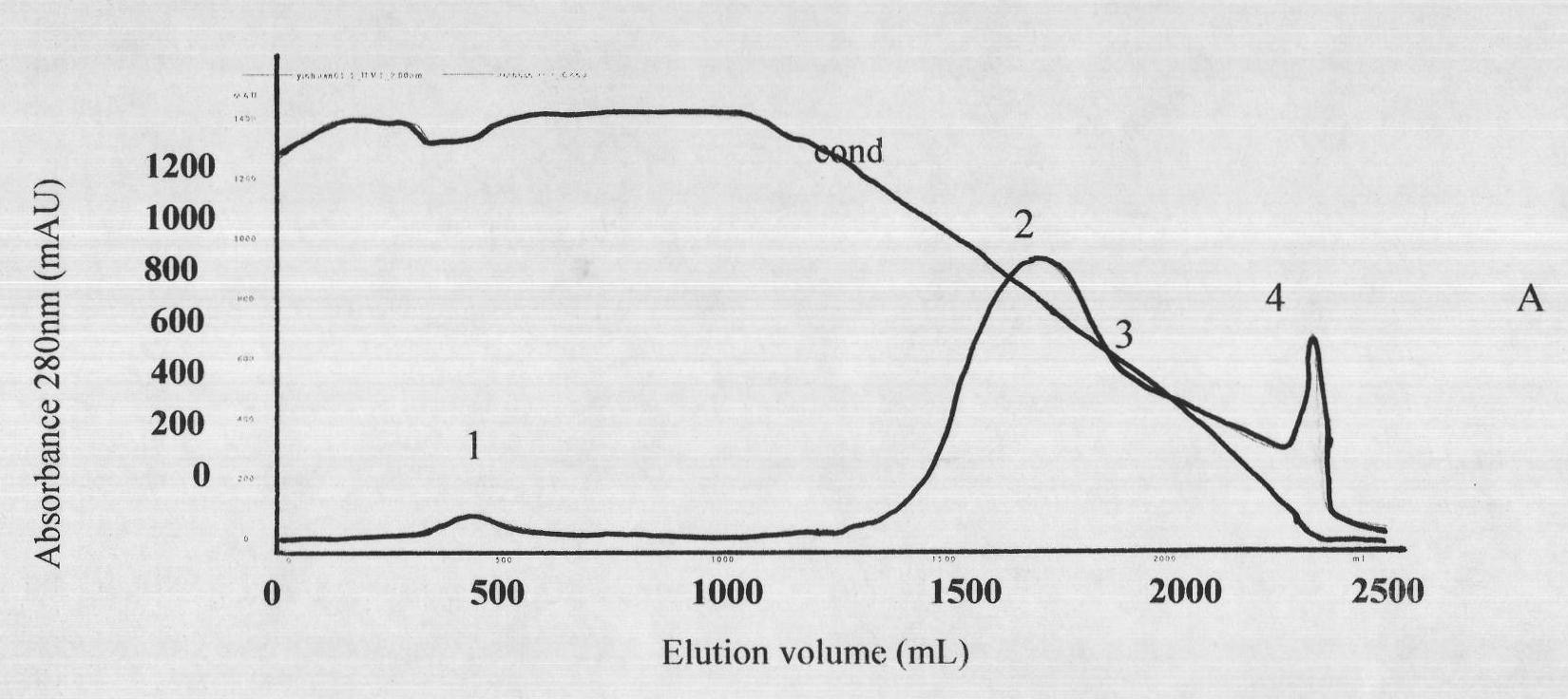
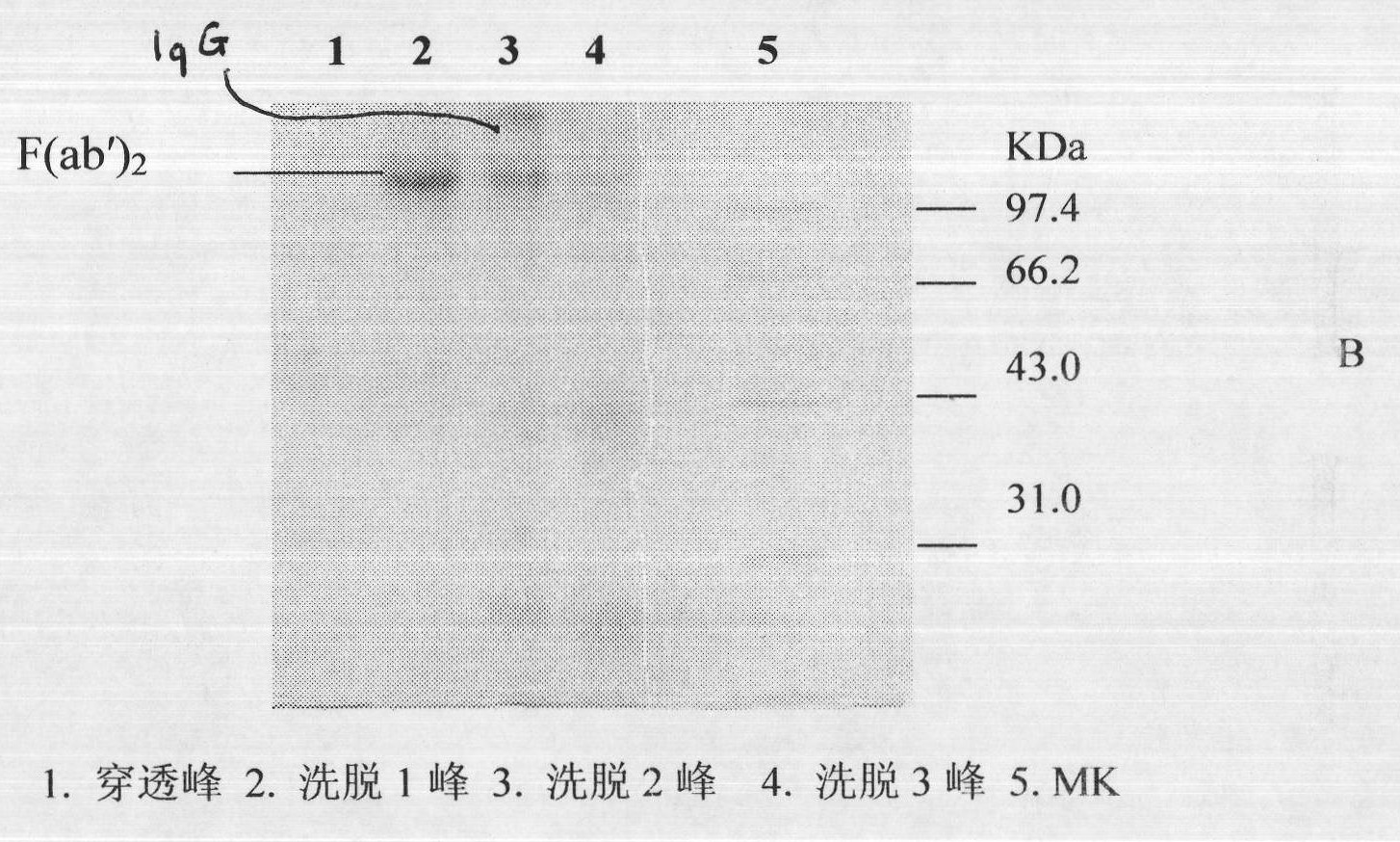
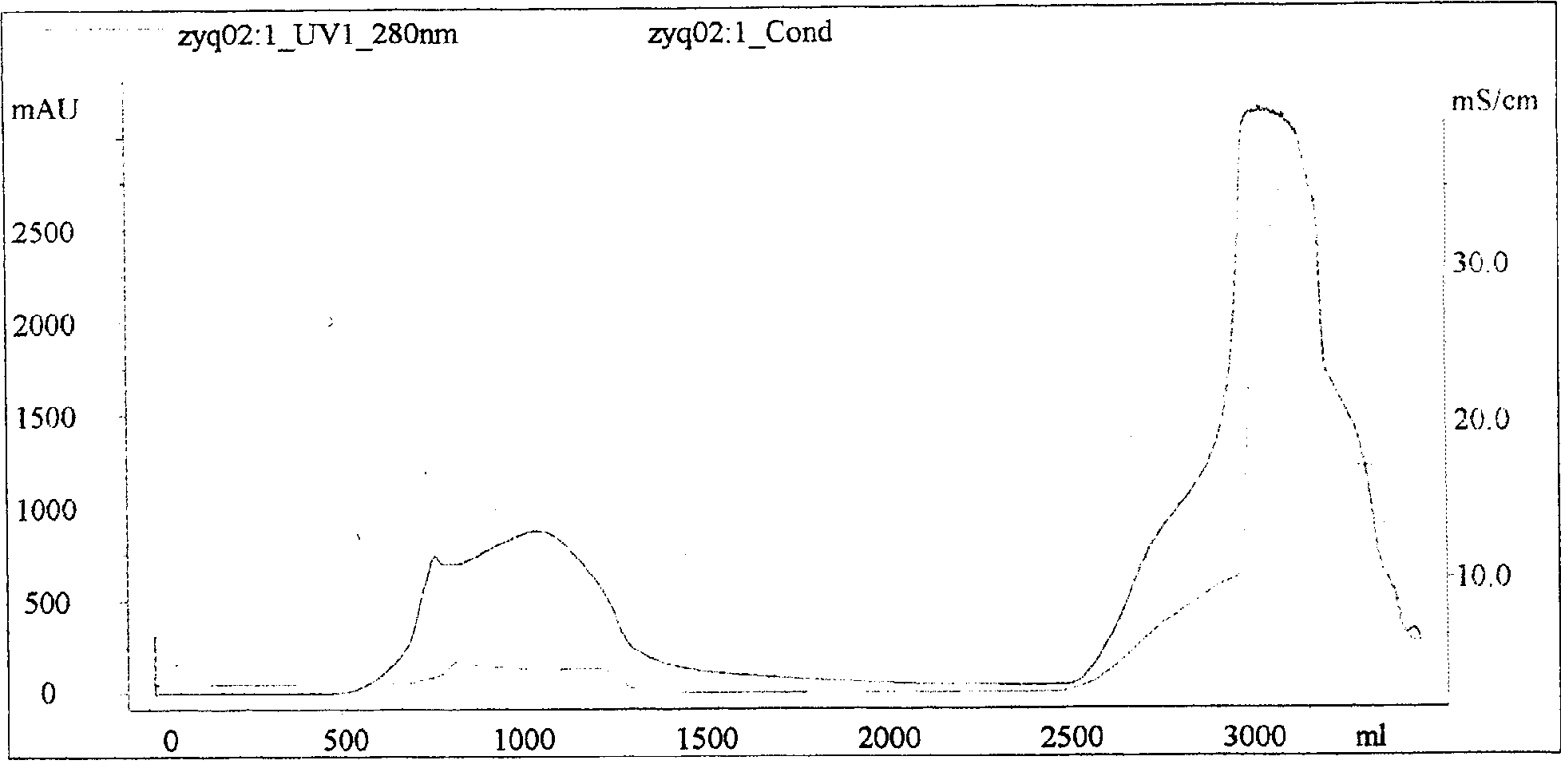
ActiveCN101816789AHigh potencyGuaranteed curative effectAntinoxious agentsMammal material medical ingredientsMass ratioBlood plasma
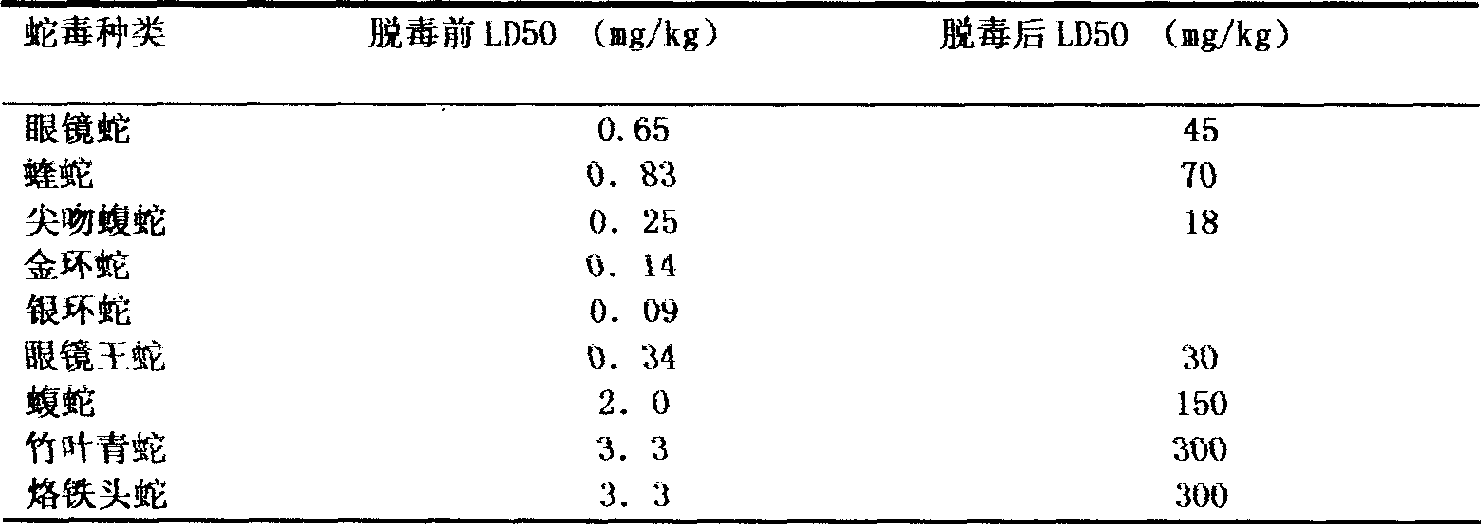
The invention discloses a lyophilized viper antivenin and a preparation method thereof, belonging to biochemical products, more particularly relating to an antivenin and a preparation technology thereof. The mass ratio of the antivenin and viper venom is 15:1, which can specifically neutralize the viper venom injected to mouse; as determined by immunodiffusion, the immunoprecipitation line appears in case that the ratio of viper antivenin F(ab')2 in lyophilized form to viper venom is 8:1; and other detected items conform to the quality standard of antivenin in Chinese Pharmacopoeia 2010. The detection of Phenyl-Sepharose (low-sub) FF column chromatography result shows that: activity is centralized at eluting peak 1, micromolecule impurity proteins are centralized at penetration peal, eluting peak 2 and eluting peak 3. According to the technology of the invention, immune blood plasma is resulted from viper venom immune horse, IgG is prepared by salting out the immune blood plasma, and F(ab')2 active fragment is obtained after the IgG is subject to enzymolysis and purification by a hydrophobic column. The lyophilized viper antivenin has strong specificity, high potency and more than 85% of the purity of antivenin F(ab')2 in lyophilized form.
Owner:浙江健博生物科技股份有限公司
Fine purification method of polyvalent anti-snake venom freeze-dry blood serum
InactiveCN101385855AImprove immunityQuality improvementAntibody ingredientsDermatological disorderVirulent characteristicsFreeze-drying
The invention discloses a method for refining a polyvalent ASV (anti-snake venom) lyophilized serum, pertaining to an immune serum containing antibody which aims at least two kinds of antigens. The serum takes the venom of Ancistrodon acutus, Agkistrodon halys, Pit-vipers, bamboo snakes, adders and the like as the immune antigen which is inoculated into the bodies of animals after degerming and dialysis, and then titer immune serum after purification is used for collecting the antibody which is then desalted and lyophilized; mice are used for evaluating the LD50 of the virulence of the venom and the titer of the ASV serum; the antibody inoculated into the bodies of the animals is one of the venom, toxoid or snake venom or the composition thereof, and glutaraldehyde or formaldehyde is used for toxin detoxication; before immunity, anti-tetanic toxoid condensed antibody for human beings is used for carrying out minim gradual immunization on the animals, or an Emuade adjuvant is used for immunization purpose. The polyvalent ASV lyophilized serum complies with the biological product standards upon the tests and inspections of purity and specific activity, safety, pyrogen, hypersusceptibility, dissolution and the like; moreover, the serum can cure sufferers who are bitten by vipers with the advantages of high purity, low foreign protein, easy preservation and quick dissolving capacity.
Owner:KUNMING INST OF MILITARY MEDICINE CHENGDU MILITARY REGIONS CENT FOR DISEASE CONTROL & PREVENTION
Viper wind-dispelling plaster
The invention relates to a plaster for expelling wind by vipers, which is a drug for treating various osteoarthropathies caused by rheumatism and overcomes the defects of other methods. The plaster is prepared by the following traditional Chinese medicine materials with weight proportion according to certain technique: fructus chaenomelis, clematis chinensis, cortex periplocae, common clubmoss herb, safflower, centipede, parasitism, cassia twig, obscured homalomena rhizome, paniculate swallowwort root, mulberry twig, sappan wood, kadsura pepper stem, notopterygium root, akebia stem, hiraute shiny bugleweed herb, peach seed, long-noded pit viper, whole worm, garter snake, jiapian, panax pseudoginseng, dragon's blood, tortoise plastron, nux vomica, Chinese angelica, ground beetle, earthworm, garden balsam stem, common monkshood mother root, kusnezoff monkshood root, eucommia bark, rhizoma cibotii, rhizoma corydalis, sesame oil and yellow lead.
Owner:马明灿
Refined polyvalent anti-snake poison lyophilized blood serum and using method
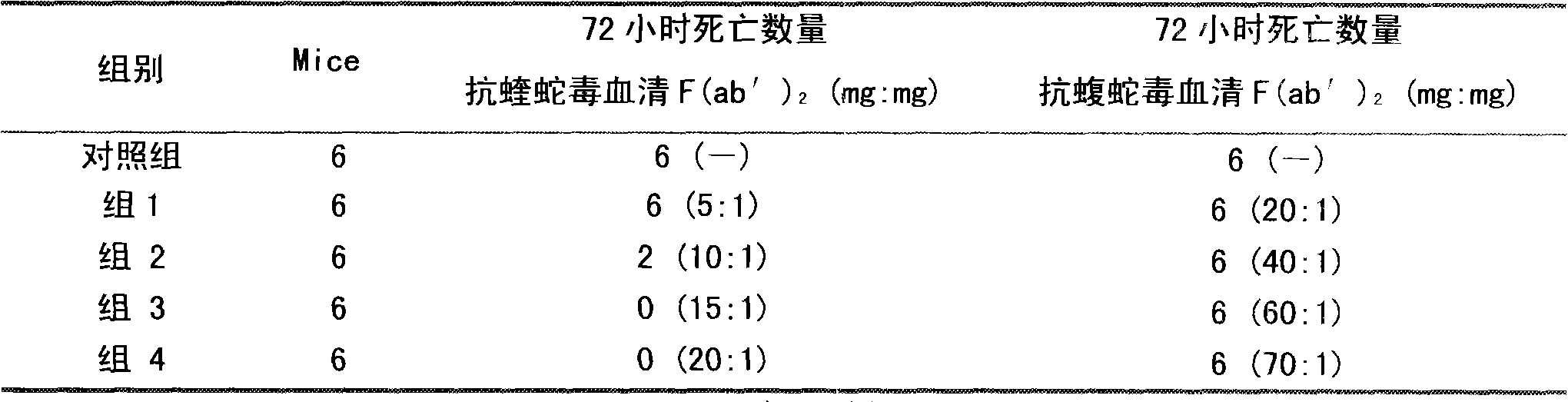
ActiveCN101347617ATimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentSnake venom
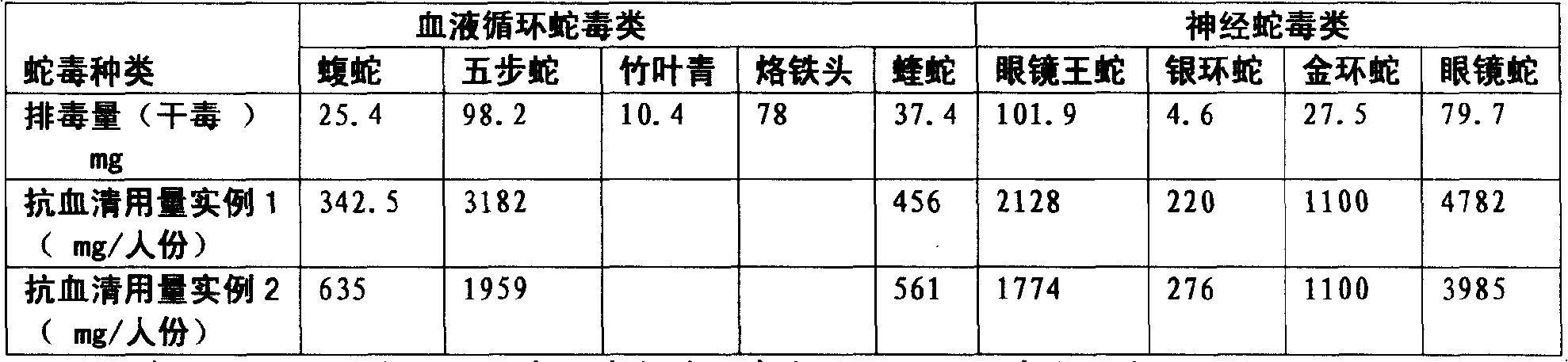
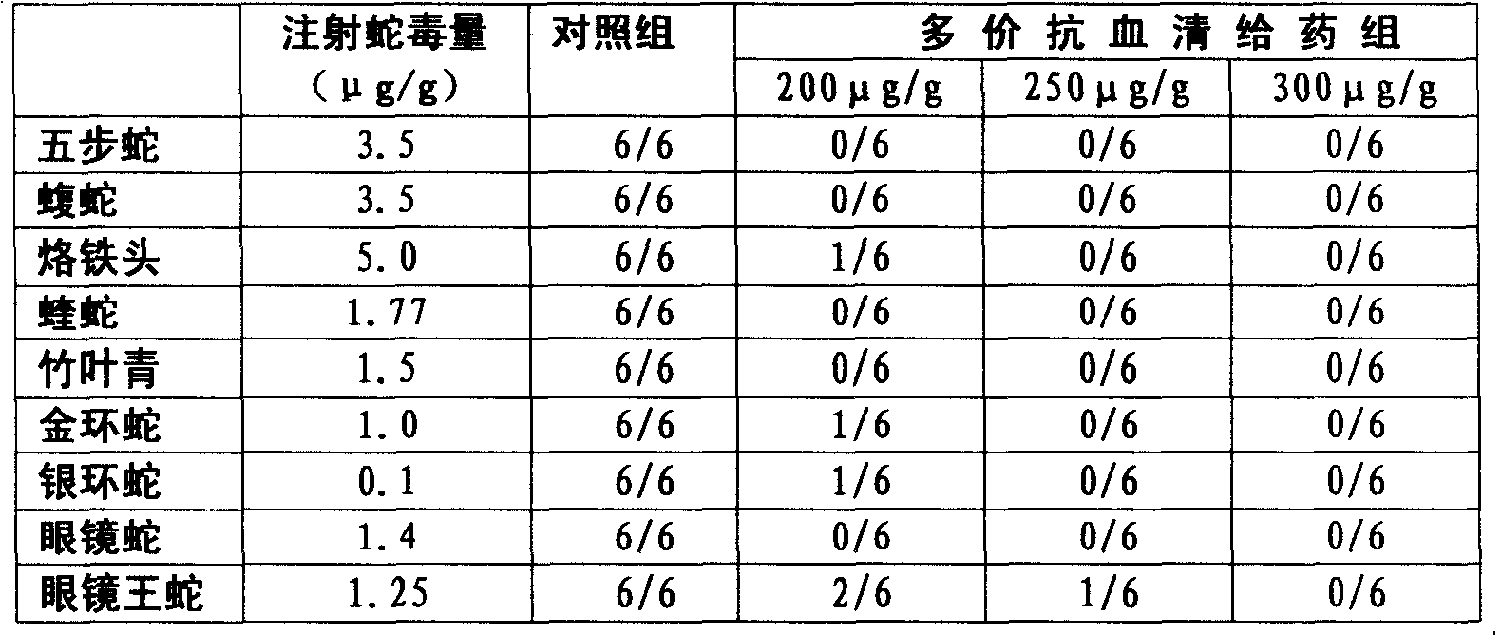
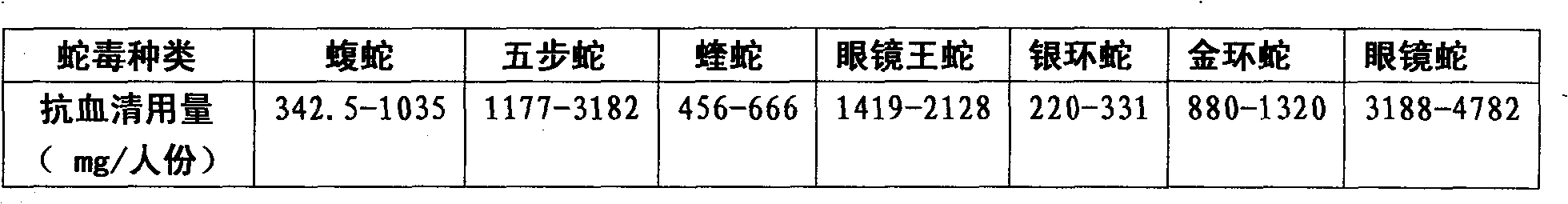
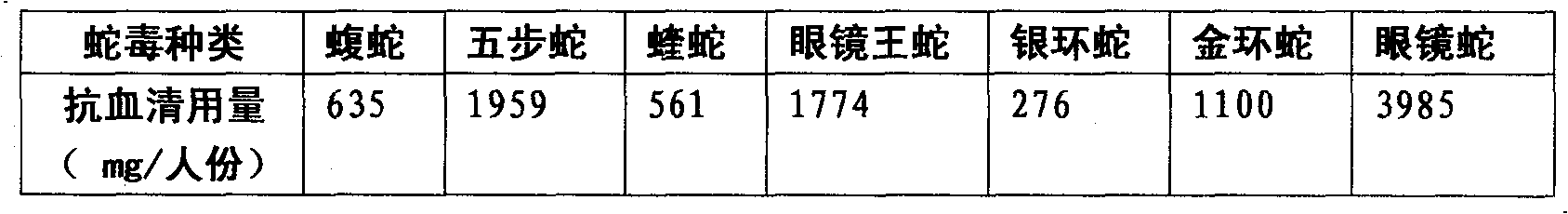
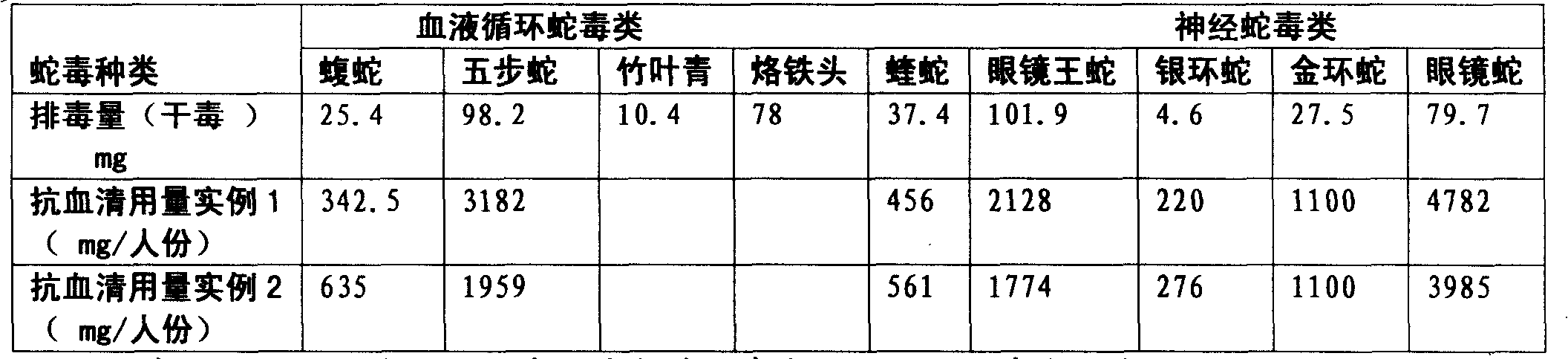
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
Five-poison chicken for preventing and treating cancer and preparation method
InactiveCN1437956AImprove immunityPrevention of recurrence and metastasisAmphibian material medical ingredientsAnthropod material medical ingredientsToad VenomTreatment effect
The preparation method of five-venom chicken for preventing and curing cancers includes: respectively extracting snake venom, scorpion venom, centipede venom, house lizard venom and toad venom, mixing them and injecting them into poisonous snake body, using said poisonous snake as carrier of venom, feeding it into the crop of live cock, after the poisonous snake is digested and absorbed by said live cock and the venom is bio-converted, the cock can be choked to death, processed and eaten by cancer's pateint. Said invention can raise immunity of patient, can be used for curing cancer of lung, carcinoma of esophagus, carcinoma of stomach and intestinal cancer, etc..
Owner:贾玉山
Broad-specturm medicine for treating snake venom poisoning
A broad-spectrum Chinese medicine for treating the biting injury of various vipers, carbuncle, subcutaneous ulcer and furuncle is prepared from 27 Chinese-medicinal materials including coptis root, phellodendron bark, scutellaria root, notoginseng, etc. Its advantage is high curative effect.
Owner:李章雄
Snake venom wine for treating cardiovascular diseases and preparation method thereof
InactiveCN101623484ATake care of yourselfRecovery functionAnthropod material medical ingredientsAntipyreticSide effectCervical spondylosis
The invention discloses a snake venom wine for treating cardiovascular diseases and a preparation method thereof. The snake wine is prepared by mixing a snake liquid obtained by soaking live garters and vipers in white spirit, a medicine liquid obtained by soaking 24 traditional Chinese medicines such as codonopsis pilosula, astragalus root, medlar, desertliving cistanche and epimedium herb and the like in white sprit, as well as viper venom powder and honey. The snake venom wine is capable of getting through the micro-circulation of a human body, solving the micro-circulatory disturbance of the body and fundamentally treating cardiovascular diseases, such as hemiplegic stroke, cervical spondylosis, scapulohumeral periarthritis, lumbar, arthritis, rheumatism, breast hyperplasia and gout, resulting from the micro-circulatory disturbance. Further, the invention has no poison or side effect and has nutrition and healthcare functions.
Owner:何龙
Refined polyvalent anti-snake poison lyophilized blood serum and using method
ActiveCN101347617BTimely treatmentEffective treatmentAntinoxious agentsAntibody ingredientsAntigen Binding FragmentNaja
Purified multivalent and lyophilized antivenin and a use method belong to antibody immunity serums that aim at more than two antigens. The invention aims at preparing the multivalent antivenin by a plurality of lyophilized antivenins and the use method. The invention is characterized in that: the antigen-binding fragment F(ab)2 has 60 to 80 percent of lyophilized antiserum, the unit is mg per person, 342.5 to 1035 of vipers, 1177 to 3182 of agkistrodon acutus, 456 to 666 of adders, 1419 to 2128 of king cobras, 220 to 331 of coral snakes, 880 to 1320 of gold banded kraits, 3188 to 4782 of cobras are combined; the optimized combination is as follows: 635 of vipers, 1959 of agkistrodon acutus, 561 of adders, 1774 of king cobras, 276 of coral snakes, 1100 of gold banded kraits and 3985 of cobras. Blood circulation snake venom antiserum and nerves snake venom antiserum are respectively filled and measured. The use method is as follows: the antivenin that is diluted by sodium chloride and an anti-allergic agent, such as chlorphenamine maleate, are injected into muscle or a vein. The antivenin is prepared by only seven antivenins, and can effectively and in time treat injuries caused by various poisonous snakes in our country when the antiserum is directly injected in condition of the undetermined variety of poisonous snakes, and the invention reaches the advanced world level of the antivenin.
Owner:浙江健博生物科技股份有限公司
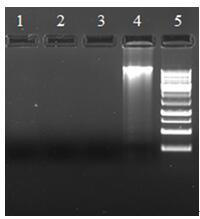
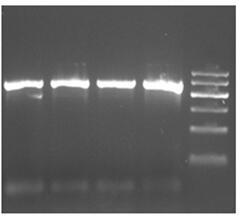
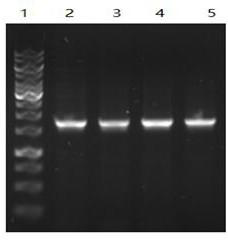
Primer for amplifying COI sequence of DNA code bar of viper species, PCR method and kit
InactiveCN104164426AEfficient amplificationAccurate amplificationMicrobiological testing/measurementFermentationMolecular identificationElectrophoresis
The invention belongs to the technical field of viper species and traditional Chinese herbal identification, and discloses a primer for amplifying the COI sequence of DNA code bar of viper species, a PCR method, and a kit; wherein the method comprises the steps of DNA extraction, PCR amplification, and electrophoresis detection. The identification method has the characteristics of convenience, little DNA using amount, and good universality; and overcomes the shortages of COI sequence universal primers (LCO1490 and HCO2198) in viper gene amplification and traditional Chinese herbal identification in the prior art. The primer can be applied to the amplification of COI sequence of viper, and establishes a foundation for molecular identification of viper.
Owner:晁志
Application of snake venom L-amino acid oxidase in preparing AIDS treating medicine
InactiveCN1526445ASignificant anti-HIV activityIncrease vitalityPeptide/protein ingredientsAntiviralsPeroxidaseCytotoxicity
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Snake catcher
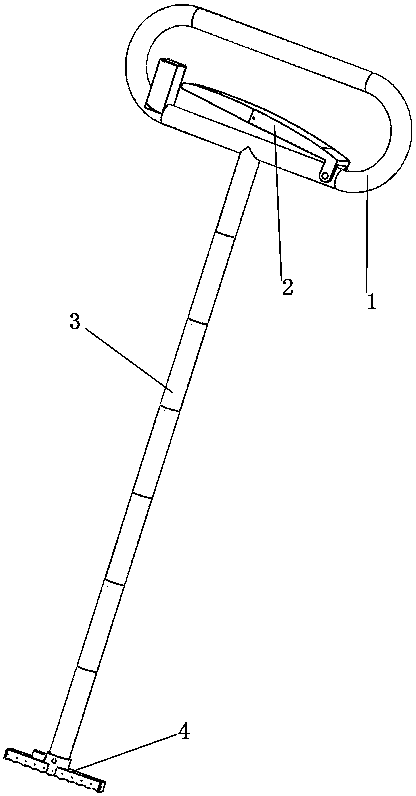
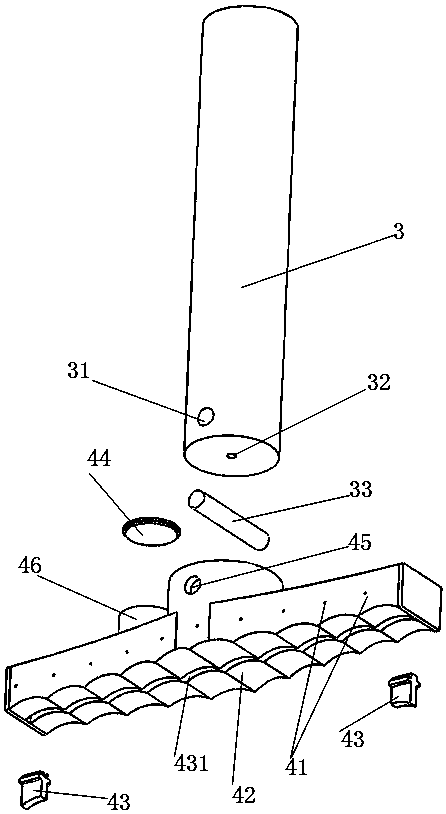
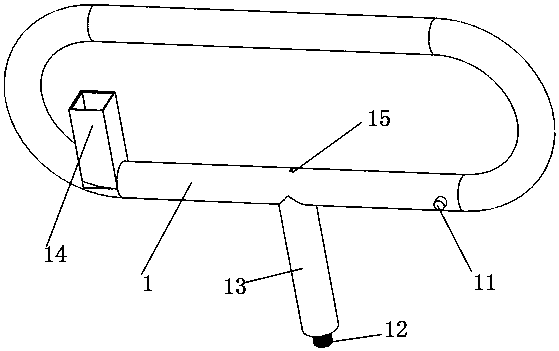
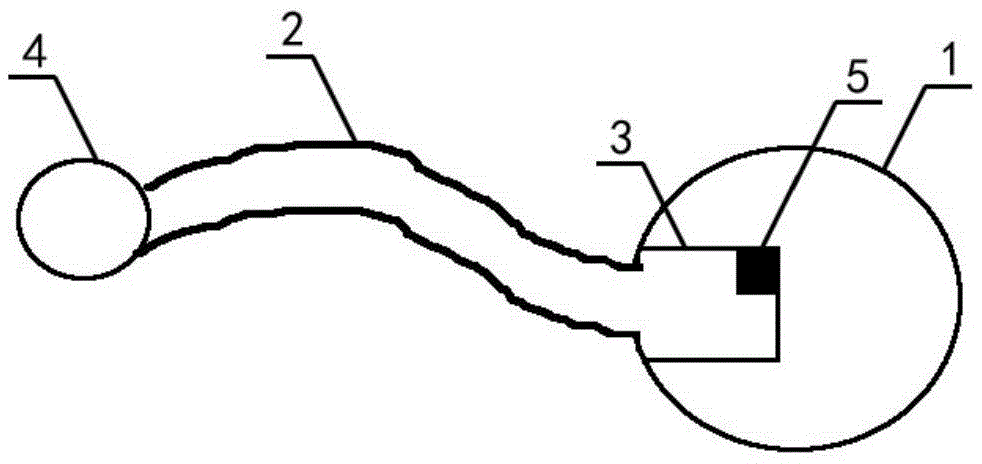
A snake catcher comprises a holder (1), a handle (2) and a working head (4). The snake catcher is characterized in that the snake catcher is divided into a sectional snake catcher and an integrated snake catcher according to the fact whether a rod body is sectioned or not, the sectional snake catcher comprises a holder (1), a handle (2), a rod body and a working head (4), and the integrated snakecatcher comprises a holder (1), a handle (2) and a working head (4). The holder (1) serves as a handheld portion of the snake catcher, the handle (2) is mounted on the holder (1), the rod body (3) isprovided with an elongated rod-shaped object, and the working head (4) is used for catching and repelling snakes and positioned at the lowermost end of the snake catcher. According to the sectional snake catcher, the working head (4) is connected with the rod body (3). According to the integrated snake catcher, the working head is connecting with a holder rod (13). The snake catcher integrates poisonous snake identification, snake repelling, bite treatment and snake catching and is simple in structure, easy to operate and complete in function.
Owner:ZHANGJIAJIE INST OF AERONAUTICAL ENG
Medicine made from root of dahuriae angelica, atractylodes and so on for treating snakebite
Disclosed is a medicament for treating snake bites, which is prepared from atractylodes rhizome, dahurian angelica root, centipede 2 pieces, silver flower, capsule of weeping forsythia, ledebouriella root, scrophularia root and pollen.
Owner:方阳
Viper antidote
InactiveCN101543572AWith active meridiansEasy to carryAnthropod material medical ingredientsAntinoxious agentsMedicinal herbsSide effect
A viper antidote, prepared with the following raw materials: 2-6g of scorpion, 2-4 centipedes, 8-12g of paris polyphylla, 0.2-0.6g of musk, 3-6g of bezoar, 8-12g of radix aristolochiae, 12-17g of radix rehmanniae, 18-22g of dandelion, 12-18g of achyranthis bidentatae, 10-14g of angelica dahurica, and 13-17g of Lobelia chinensis. The present invention is prepared with pure Chinese medicinal herbs, has the efficacy of clearing heat and toxics, diminishing inflammation and swelling, stopping pain, and clearing and activating the channels and collaterals, and doesn't have toxic or side effect. The viper antidote has good curative effect and high curative ratio. The viper antidote is economical.
Owner:彭焕庭
External-use liniment for treating poisonous snake bit
InactiveCN105168776AHeat-clearing and detoxifyingDisperses blood stasis and clears swellingAnthropod material medical ingredientsHydroxy compound active ingredientsToxic materialSmilax glabra
The invention discloses an external-use liniment for treating poisonous snake bit. The external-use liniment is prepared from the following raw materials in parts by weight: 10-13 parts of wild chrysanthemum flower, 4-7 parts of achyranthes aspera, 6-8 parts of Indian strawberries, 5-9 parts of Japanese stephania roots, 8-11 parts of andrographis paniculata, 5-6 parts of rhizoma arisaematis, 3-5 parts of cantharis, 4-7 parts of periostracum cicada, 5-8 parts of smilax glabra, 4-7 parts of cortex phellodendri, 6-9 parts of pseudo-ginseng, 5-7 parts of realgar, 4-8 parts of smilax china, 8-10 parts of clematis chinensis, 6-7 parts of rhizoma corydalis, 5-8 parts of unibract fritillary bulbs, 3-5 parts of paris polyphylla, 6-8 parts of loquat leaves, 6-9 parts of blackend swallowwort roots, 4-8 parts of borneol, 5-7 parts of humulus japonicus, 1.5-2.5 parts of indigoplant fruits, 2-3 parts of narrowbract Indian kalimeris herbs, 3.5-4.5 parts of oriental bittersweet roots and 4-6 parts of sarcopyramis bodinieri. The external-use liniment disclosed by the invention has the functions of clearing away heat and toxic materials, eliminating stagnant blood and reducing swelling, diminishing inflammation and relieving pain as well as removing slough and promoting growth of tissue regeneration, is rapid in taking effects, short in treatment course, convenient to use, safe and free of toxic or side effects and has the high cure rate of 96.5% through clinical verification.
Owner:马鞍山德宏堂生物科技有限公司
Three-snake capsule powder and preparation method thereof
InactiveCN102824370AImprove autoimmunityGood curative effectAntipyreticAnalgesicsSide effectAdditive ingredient
The invention belongs to the technical field of pharmaceuticals, and in particular relates to a three-snake capsule powder and a preparation method thereof. The three-snake capsule powder which is disclosed by the invention consists of the following ingredients in percentage by weight: 10%-65% of viper powder, 15%-70% of zaocys dhumnades powder and 20%-75% of dinodon rufozonatum. The three-snake capsule powder is capable of basically improving the immunity of a human body, also has the functions of clearing and activating the channels and collaterals, diminishing inflammation and relieving pain, is suitable for treating rheumatoid arthritis in a treatment course manner, is capable of treating both manifestation and root courses of the disease, has an obvious curative effect, is safe, and does not have toxic or side effects.
Owner:DEQING MOGANSHAN SNAKES INDAL
Health care feed without vipers
InactiveCN102805259AImprove meat qualityHigh nutritional valueAnimal feeding stuffAnguilliformesBiology
The invention relates to a health care feed without vipers and a preparation method of the health care feed. The health care feed is composed of ricefield eels, earthworms, ground beeltles, corns, soybeans, oats, field mints, lean pork, eggs, kelps, coptis chinensis, silkworm chrysalis, lotus leaves and burdocks.
Owner:WUJIANG YISHENG TEXTILE
Traditional Chinese medicine for female acyesis and aphoria and preparation method thereof
InactiveCN101366878ASmall side effectsEasy to manufacturePowder deliveryPill deliveryBULK ACTIVE INGREDIENTBlood stasis
The invention relates to a traditional Chinese medicine for treating female sterility. The crude drugs of the medicine consist of viper, ass hide glue, angelica, red peony root, peach kernel, safflower, fructus liquidambaris, prepared rhubarb, prepared pangolin, fried cow soapwortseed, Chinese violet, prepared Chinese honeylocust spine, radix semiaquilegiae and prepared carapax amydae. According to the traditional Chinese medicine principles of clearing away heat evil, promoting diuresis and dispelling sputum, activating blood circulation to dissipate blood stasis, and unblocking the orifices, the traditional Chinese medicine composition treats the female sterility caused by fallopian tube obstruction, irregular menses, amenorrhea and the like through the synergic action of the active ingredients of the crude drugs. The invention also relates to a preparation method for the traditional Chinese medicine composition.
Owner:李继田 +3
Snake venom sucking apparatus
InactiveCN104906641APrevent proliferationQuick suctionIntravenous devicesSuction drainage systemsSnake bite venomMedicine
A snake venom sucking apparatus comprises a suction ball (1). The suction ball (1) is of a circular structure and is connected with a blood drawing pipeline (2), a blood collecting basket (3) is arranged in the suction ball (1), a sucker (4) is arranged at the front end of the blood drawing pipeline and is fixed to the poisonous bite part of a human body during use, and venom is extracted from the human body through the blood drawing pipeline (2) and flows to the blood collecting basket (3) when a user pinches the suction ball (1); a sensing unit (5) is arranged in the blood collecting basket (3) and issues prompt information when blood in the basket is to be full; the information is promoted by means of sounding or LED lamp displaying; the sucker (4) is made of transparent plastics so as to fixedly suck any parts of the human body in an all-closed manner. The bite part of the human body can be sucked quickly, the venom can flow out, snake venom can be effectively prevented from spreading, simpleness and convenience in operation are achieved, and convenience is brought to treatment of snakebite.
Owner:ANQING XINGBOTE ELECTRONICS TECH
Traditional Chinese medicine for treating poisonous snake bites
InactiveCN104435842AImprove biteQuick resultsAntinoxious agentsUnknown materialsMedicinal herbsChrysanthemum Flower
The invention discloses a traditional Chinese medicine for treating poisonous snake bites. Each dose of the medicine is prepared from the following medicinal materials by mass: 6g to 9g of centipede, 6g to 9g of gold scorpion, 12g to 18g of plantain herb, 12g to 18g of radix angelicae, 12g to 18g of spica prunellae, 12g to 18g of wild chrysanthemum flower, 12g to 18g of dandelion, 20g to 30g of circium japonicum, 20g to 30g of lalang grass rhizome and 45g to 55g of Chinese lobelia. The traditional Chinese medicine has a good effect of treating the poisonous snake bites, has a curative rate of 100%, can take effect rapidly and is easy to prepare, low in cost and especially applicable to most of rural areas.
Owner:黄平县兴且民族民间中草医药科学发展有限责任公司
Liver-protecting and lipid-lowering scented tea and making method thereof
InactiveCN106561882ABalanced nutritionGood beautyOrganic active ingredientsDispersion deliverySemenLipid lowering
The invention discloses a liver-protecting and lipid-lowering scented tea and a making method thereof. The liver-protecting and lipid-lowering scented tea comprises the following raw materials by weight: 15-25 parts of marigolds, 15-20 parts of Hangzhou white chrysanthemum, 10-15 parts of white plum blossoms, 8-12 parts of sorghum powder, 7-10 parts of chestnut kernel, 6-9 parts of longan, 5-7 parts of gynostemma pentaphyllum, 4-6 parts of ganoderma lucidum, 5-7 parts of phellodendron Chinese schneid, 4-6 parts of eucommia ulmoides oliver, 3-5 parts of semen sinapis, 5-7 parts of black Chinese wolfberry, 4-6 parts of Medoggreenpit-viper, 3-5 parts of liquorice, 5-7 parts of laver, 4-6 parts of pachyrhizus erosus starch, 5-7 parts of grape seed extract, 4-6 parts of tomato extract, 5-7 parts of cashew nuts, 3-5 parts of hawthorn, 5-8 parts of Pu'er tea, 2-4 parts of rice nectar, and 5-10 parts of composite powder. The health care scented tea of the invention is fragrant and tasty, and exquisite and silky. Through scientific compatibility, the scented tea is nutritionally balanced. Through collocation of a variety of medicinal and edible traditional Chinese herbal medicines, the scented tea has good effects of beautifying and skin nourishing, kidney nourishing and eyesight improving, and the like, and can reduce the content of blood lipid and balance blood lipid. Moreover, the scented tea can effectively prevent and treat the symptoms of patients with cardiovascular disease, obesity, hyperlipidemia, or the like.
Owner:ANHUI PROVINCE TIANXU TEA
Snake gall capsules and preparation method thereof
InactiveCN102824369AGood therapeutic effectSolve residual problemsDigestive systemAntinoxious agentsMedicineCurative effect
The invention belongs to the technical field of medicaments, and particularly relates to snake gall capsules and a preparation method thereof. The invention discloses snake gall capsules which consist of the following components in percentage by weight: 30-65% of viper gall powder and 35-70% of zaocys dhumnade gall powder. The snake gall capsules provided by the invention have the pharmacological functions of clearing heat and removing toxin, resolving phlegm and relieving cough and clearing the liver to improve vision, and realize obvious curative effects on acne, oral ulcer, live protection and hangover alleviation, cough and the like; the processing technology solves the problem of parasite residues; and thus the snake gall capsules are snake therapy treasures for treating and preventing the diseases.
Owner:DEQING MOGANSHAN SNAKES INDAL
Traditional Chinese medicine composition for treating viper bite
InactiveCN101380392ASignificant effectLow costAntinoxious agentsPlant ingredientsDesmodium microphyllumSide effect
The invention discloses a traditional Chinese medicine composite which treats viper bite wound and comprises the components with the parts by weight as follows: 50 to 150 parts of desmodium microphyllum (Thunb.) DC., 15 to 30 parts of Chinese lobelia herb, 15 to 30 parts of scabrous doellingeria herb, 10 to 15 parts of ford dutchmanspipe root, 15 to 30 parts of root of assam treebine, 10 to 20 parts of radix angelicae formosanae and 10 to 30 parts of angelica. The invention also discloses a traditional Chinese medicine composite for external application, which can be used by matching the traditional Chinese medicine composite which treats viper bite wound and comprises the components with the parts by weight as follows: 55 to 65 parts of arisaema heterophyllum, 15 to 25 parts of elm bark and 15 to 25 parts of quassia wood. The traditional Chinese medicine composite has obvious curative effect on the treatment of patients with viper bite wound, has no toxic and side effect and is suitable for clinical popularization and application.
Owner:肇庆市第三人民医院
Medicine for giving up toxin and its preparation method
A Chinese medicine for dropping drug and treating the viper bite and poisoning is prepared from 6 Chinese-medicinal materials including barbat skullcap, mockstrawberry herb, polygala root, etc. Its advantages are high curative effect, no dependency, and short course of treatment (7 days).
Owner:陈桂敏 +1
Traditional Chinese medicine for treating poisonous snake bite
ActiveCN107213238AGood for snake bitesAchieve detoxificationAntipyreticAerosol deliveryMedicineFlos chrysanthemi
Owner:AFFILIATED HOSPITAL OF JIANGXI UNIV OF TCM
Thanatophidia bite common andrographis herb health tea
InactiveCN102895412AEasy to makeEasy to carryPre-extraction tea treatmentAntinoxious agentsMedicinal herbsMedicine
The present invention relates to a thanatophidia bite common andrographis herb health tea. According to the thanatophidia bite common andrographis herb health tea, 28 parts by weight of common andrographis herb, 16 parts by weight of oldenlandia, 17 parts by weight of ophioglossum vulgatum, and 17 parts by weight of lysimachia are cleaned and cut into strips, and then dried; the drug strips and 22 parts by weight of green tea are uniformly mixed and dried, and then are sub-packaged into the bagged thanatophidia bite common andrographis herb health tea. The thanatophidia bite common andrographis herb health tea has characteristics of simple preparation, convenient carrying, safe drinking, and good effect, and has effects of heat clearing, detoxifying, and blood cooling. With the thanatophidia bite common andrographis herb health tea, pain of people bit by poisonous insects and snake can be well relieved.
Owner:潘亚琴
Extracting micro viper gene and specific detection method thereof
InactiveCN110387370ARapid identificationStrong specificityMicrobiological testing/measurementDNA preparationSpecific detectionToxin
The invention discloses an extracting micro viper gene and a specific detection method thereof, and belongs to the technical field of biology. According to extracting micro viper gene and the specificdetection method thereof, by extracting genes from viper tissue and toxin, different primer pairs are used as specific primers to obtain PCR amplification products, and then through agarose gel electrophoresis detection, the PCR products are used as specific gene sequences of a viper after being sequenced. The detection method has the advantages of high specificity, good stability, rapid detection, easy operation and the like, is conducive to large-scale and automatic detection and analysis, and is a fast and specific method for identifying the viper and the toxin of the viper.
Owner:SHANGHAI SERUM BIOTECH
Snake venom cytotoxin, preparation method and application thereof
ActiveCN101269092ALittle side effectsHigh affinityReptile material medical ingredientsRespiratory disorderCancer cellSide effect
The invention discloses a snake poison cytotoxin, the preparation method and the application. The method of preparing the snake poison cytotoxin in the invention includes the following steps: firstly, the snake poison is conducted an ion-exchange chromatography and then the eluent between a first absorption peak and a second absorption peak on the absorbance curve with a detection wavelength of 280nm in the eluent is gathered; secondly, the eluent obtained in the first step is conducted an exclusion chromatography, water is used as a moving phase, and the second absorption peak on the absorbance curve with a detection wavelength of 280 nm in the eluent is gathered to obtain the snake poison cytotoxin. The saw-scale viper poison cytotoxin in the invention can induce the human laryngeal cancer cell Hep-2 to an apoptosis and the capability of inducing Cell Hep-2 to an apoptosis is stronger than Pingyangmycin with a viscosity of 100 Mug / ml when the snake poison cytotoxin has a concentration of 0.01 mg per milliliter. In addition, experiments of undue toxicity prove that the cytotoxin has slight side effects. The saw-scale viper poison cytotoxin in the invention can be used as an anti-cancer drug to turn biological anti-cancer drugs into a reality.
Owner:JINZHOU AHON PHARM CO LTD
Chinese herbal medicine for treating venomous snake bite
InactiveCN101797313AQuick Pain ReliefNo rednessAntinoxious agentsPlant ingredientsToxic materialB1 Vitamin
The invention discloses a Chinese herbal medicine for treating venomous snake bite, which is characterized by being prepared from the following components in part by weight: 35 to 60 parts of arrowhead, 10 to 20 parts of sweet wormwood herb and 10 to 20 parts of philippine violet herb. According to the research results of the traditional Chinese medicine and the modern pharmacology, the medicament formula of the invention selects arrowhead which contains vitamin B1 and gallbladder-pancreas endoenzyme suppressant and can treat mad dog bit to be matched with sweet wormwood herb with effcts of clearing deficient heat, stopping toxic blood and increasing choleresis and philippine violet herb which has effects of clearing away heat and toxic material and eliminating edema pain and treats all malignant sores and erase poison, sufficiently uses the effect of effectively neutralizing the chemical components, such as neurotoxin, cytotoxin and hemotoxin, in the toxins of the poisonous snakes of the Chinese herbal medicines to rapidly inhibit and neutralize the toxins of the poisonous snakes and also relieve pain and swell, and completely achieves the aim of effectively inhibiting and neutralizing the snake toxins, being suitable for various venomous snake bite and saving precious treatment time for the wounded person.
Owner:马正华
Toner containing agkistrodon halys tissue extract
InactiveCN107669614ARegulate acid-base balanceEnsure balanceCosmetic preparationsToilet preparationsDiseaseTissue extracts
The body lotion of Agkistrodon halys tissue extract of the present invention belongs to the technical field of preparation of cosmetics production enterprises. The body lotion made of traditional Chinese medicines such as chrysanthemum, woody, gentian, chrysanthemum, magnolia, and natural mineral medicine medical stone, tourmaline and other nanotechnology, can be applied, absorbed, and penetrated deep into the subcutaneous area through the skin 3— 5mm, automatically heats up, activates, organizes, promotes metabolism, automatically and continuously generates negative ion far infrared, effectively promotes skin blood circulation, prevents various skin diseases and skin aging and aging, skin care and beauty.
Owner:NANTONG SNAKEBITE THERAPY INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com