Snake venom cytotoxin, preparation method and application thereof
A technology of cytotoxin and snake venom, applied in the field of snake venom cytotoxin and its preparation, to achieve the effect of light side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the preparation of snake venom cytotoxin
[0021] In this example, the lyophilized powder of viper venom (purchased from Jingshi Kaitai Pharmaceutical Co., Ltd.) was used as the raw material.
[0022] The extracted experimental steps are as follows:
[0023] 1. Snake venom dissolving: Dissolve 8 g of Viper saw scales lyophilized powder in 100 ml of Tris-HCl buffer solution (pH 8.2, 0.05 mol / L).
[0024] 2. Remove impurities: Add 6ml of saturated ammonium sulfate solution to the snake venom solution obtained in step 1, mix and place at room temperature for 1 hour, centrifuge at 3000rpm for 10 minutes, discard the precipitate, and keep the supernatant solution.
[0025] 3. Desalination by dialysis: seal all the supernatant obtained in step 2 in a dialysis bag with a molecular weight cut-off of 14000D, then put the dialysis bag into distilled water for dialysis for 15 hours, change the water every 3 hours, and the volume of distilled water is 1200ml. The di...
Embodiment 2
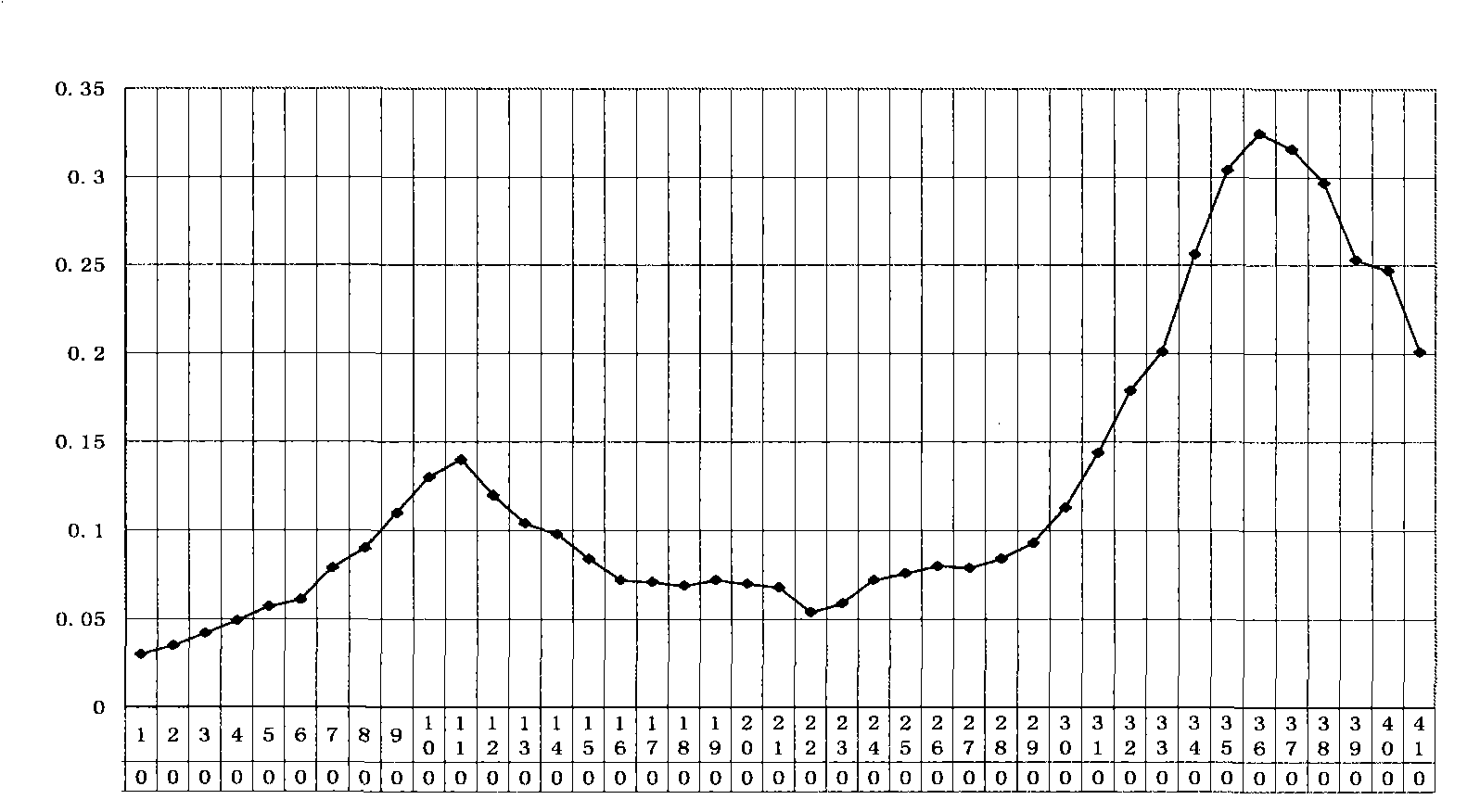
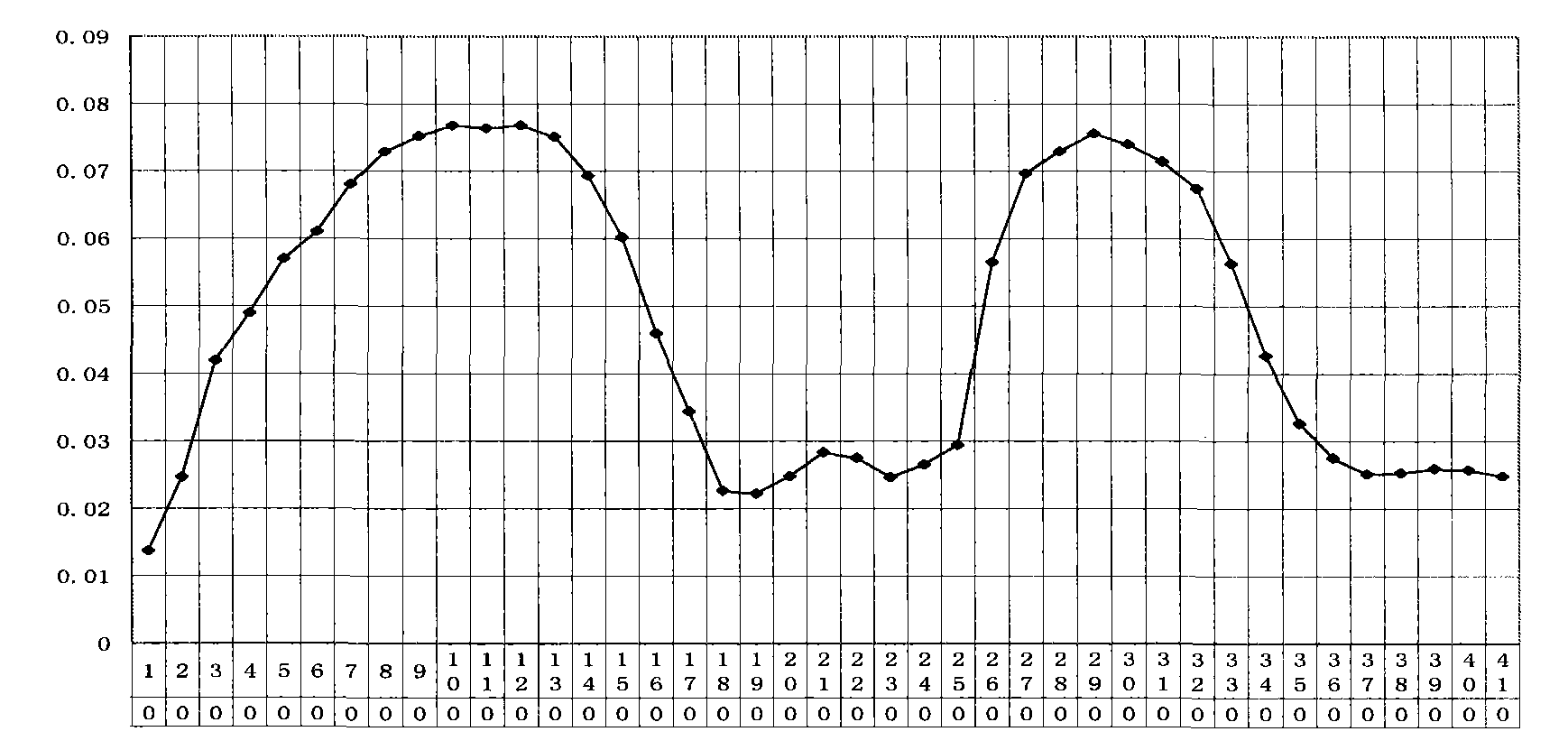
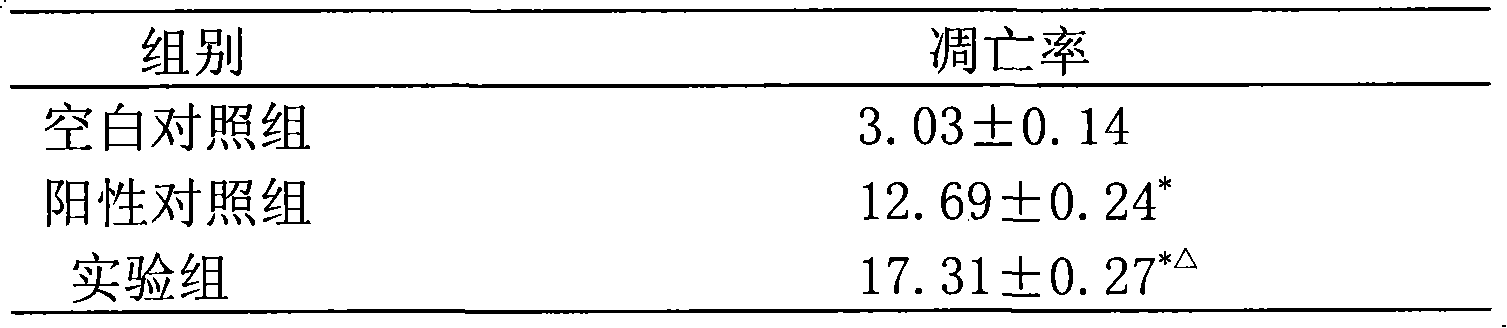
[0032] Example 2, Functional verification of viper venom cytotoxin
[0033] 1. Validation of anti-cancer function of viper venom cytotoxin
[0034] Taking the human laryngeal cancer cell line Hep-2 (provided by the Institute of Head and Neck Surgery, Liaoning Medical College) as the target cell, the efficiency comparison of the snake venom cytotoxin and pingyangmycin inducing the apoptosis of cancer cells was observed. The experimental method refers to the literature (Tai Jun Wang Xuefeng Pingyangmycin-induced apoptosis of human laryngeal carcinoma Hep-2 cells "Journal of Liaoning Medical College" 2004Aug.25 (4) 5-7 pages), as follows:
[0035] Cultivation of the human laryngeal carcinoma cell line Hep-2 cell line: the cells were inoculated in the complete culture medium of RPMI-1640 (fetal bovine serum 10%, penicillin 100u / ml, streptomycin 100ug / ml), placed at 37°C, containing 5%CO 2 Cultured in an incubator.
[0036] Take 20ml of the viper venom cytotoxin stock solution p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com