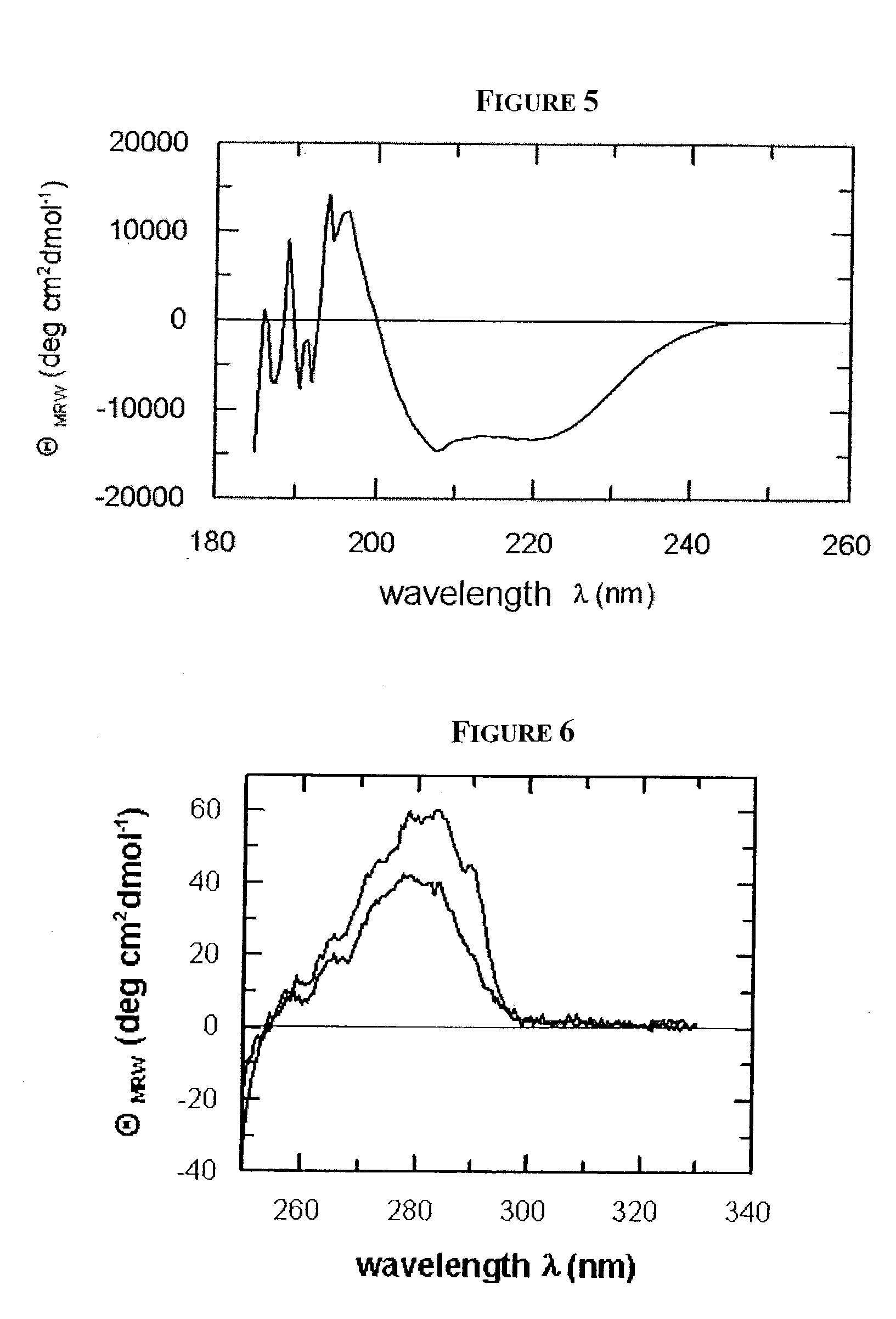
Patents
Literature
819 results about "Foreign protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foreign protein. 1. a protein that differs from any protein normally found in the organism in question.
Recombinant negative strand RNA virus expression systems and vaccines
Owner:MEDIMMUNE VACCINES
Mutiple gene expression for engineering novel pathways and hyperexpression of foreign proteins in plants
InactiveUS20030041353A1High expressionHigh copy numberClimate change adaptationDepsipeptidesPosition effectOperon
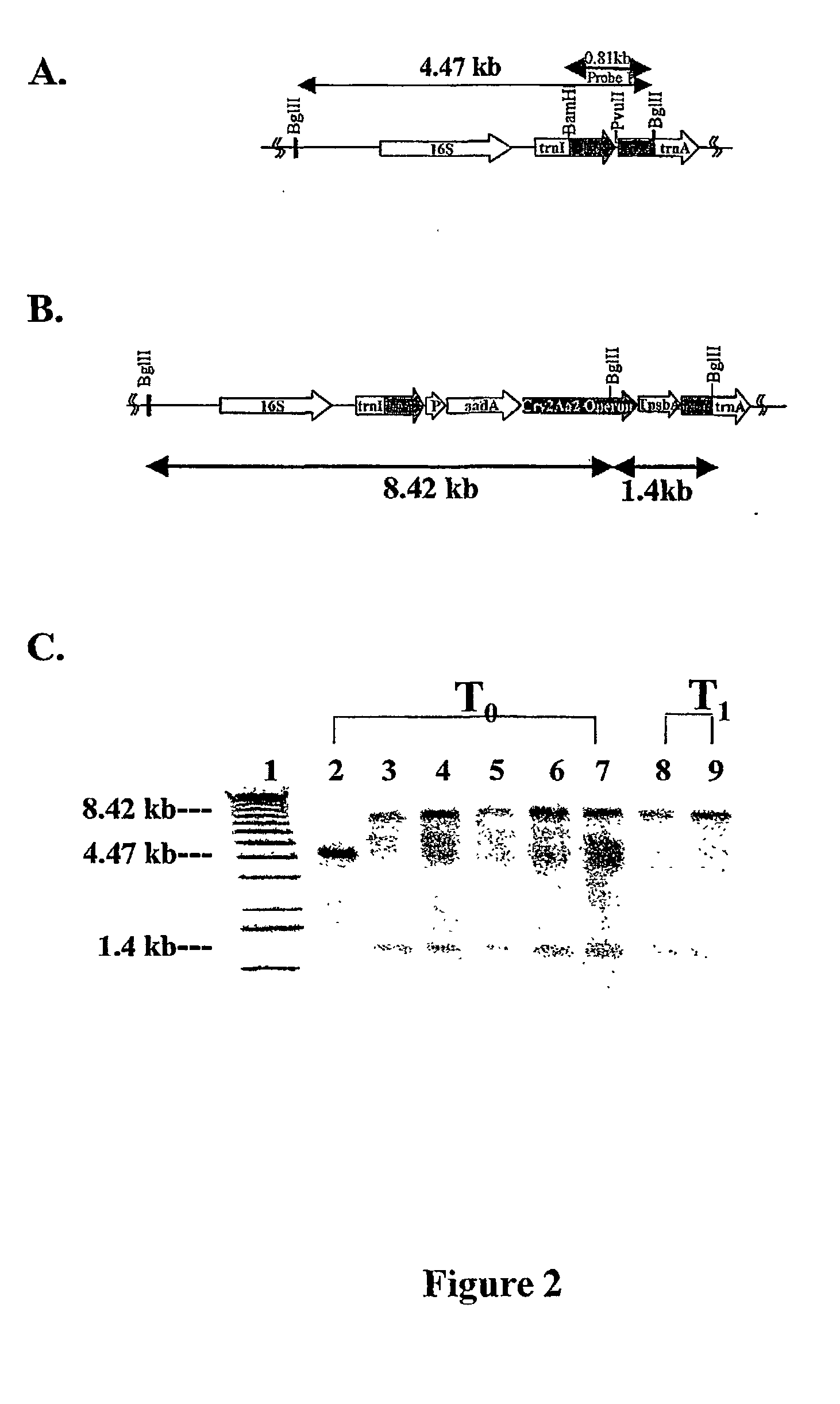
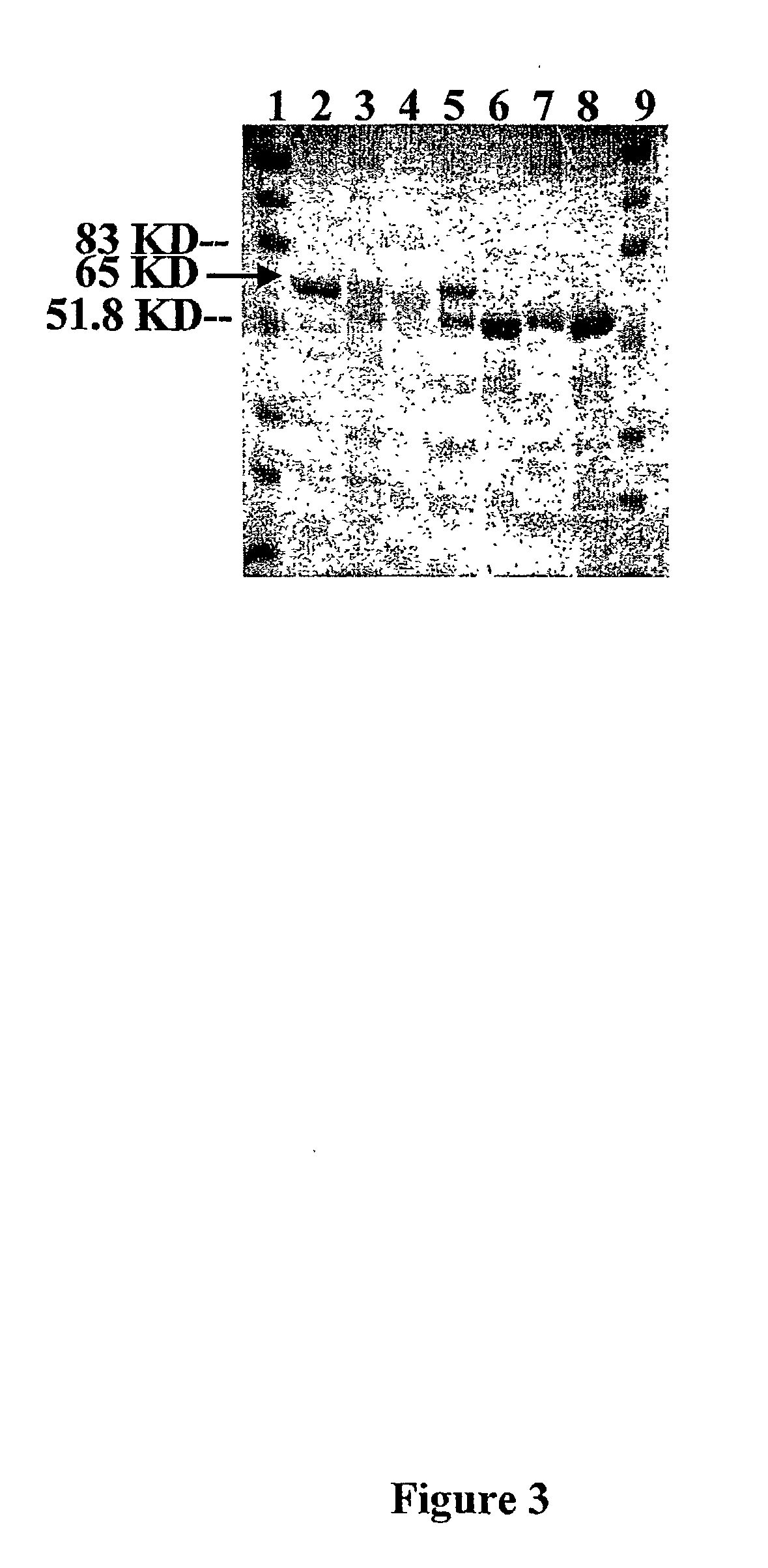
Introducing blocks of foreign genes in a single operon would avoid complications such as position effect and gene silencing inherent in putting one gene at a time into random locations in the nuclear genome. Cloning several genes into a single T-DNA does not avoid the compounded variable expression problem encountered in nuclear transgenic plants. This disclosure shows that a bacterial operon can be expressed in a single integration event as opposed to multiple events requiring several years to accomplish. Expression of multiple genes via a single transformation event opens the possibility of expressing foreign pathways or pharmaceutical proteins involving multiple genes. Expressing the Cry2aA2 operon, including a putative chaperonin to aid in protein folding, in the chloroplast via a single transformation event leads to production of crystalized insecticidal proteins. Expressing the Mer operon via a single transformation event leads to a phytoremediation system.
Owner:UNIV OF CENT FLORIDA RES FOUND INC +1
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
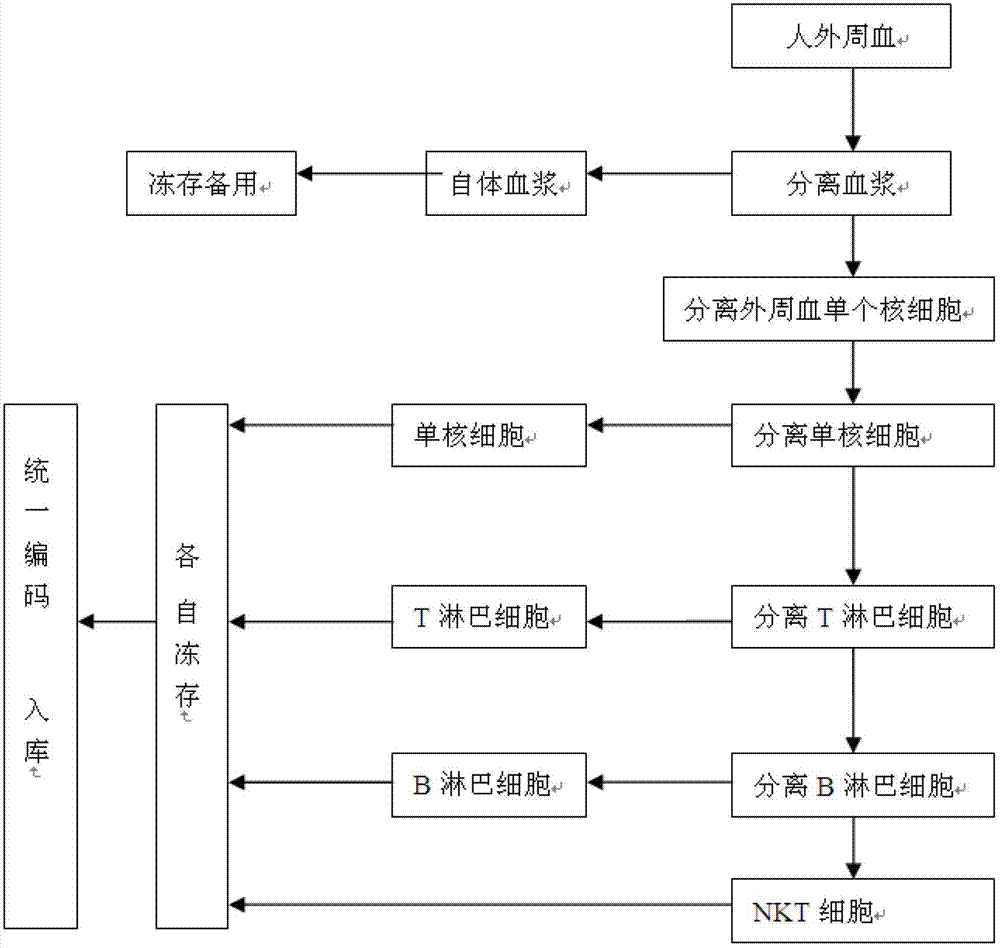
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
Method for screening CHO cell line high-expression site
InactiveCN107557390AEasy to integrateHigh expression siteFermentationVector-based foreign material introductionForeign proteinFluorescence
The invention discloses a method for screening a CHO cell line high-expression site. The method comprises the following steps: integrating slow virus with a green fluorescence gene into a CHO cell genome, collecting monoclonal cells with high fluorescence expression quantity by a flow sorting method, performing expanding culture, and then screening out a corresponding high-expression integration site by using a chromosome walking technology. According to the method, a CRISPR / Cas9-mediated site-specific integration technology is combined, a to be expressed foreign gene can be quickly and accurately into a found site, and a foreign protein high-expression cell line can be quickly and efficiently obtained.
Owner:JIANGNAN UNIV
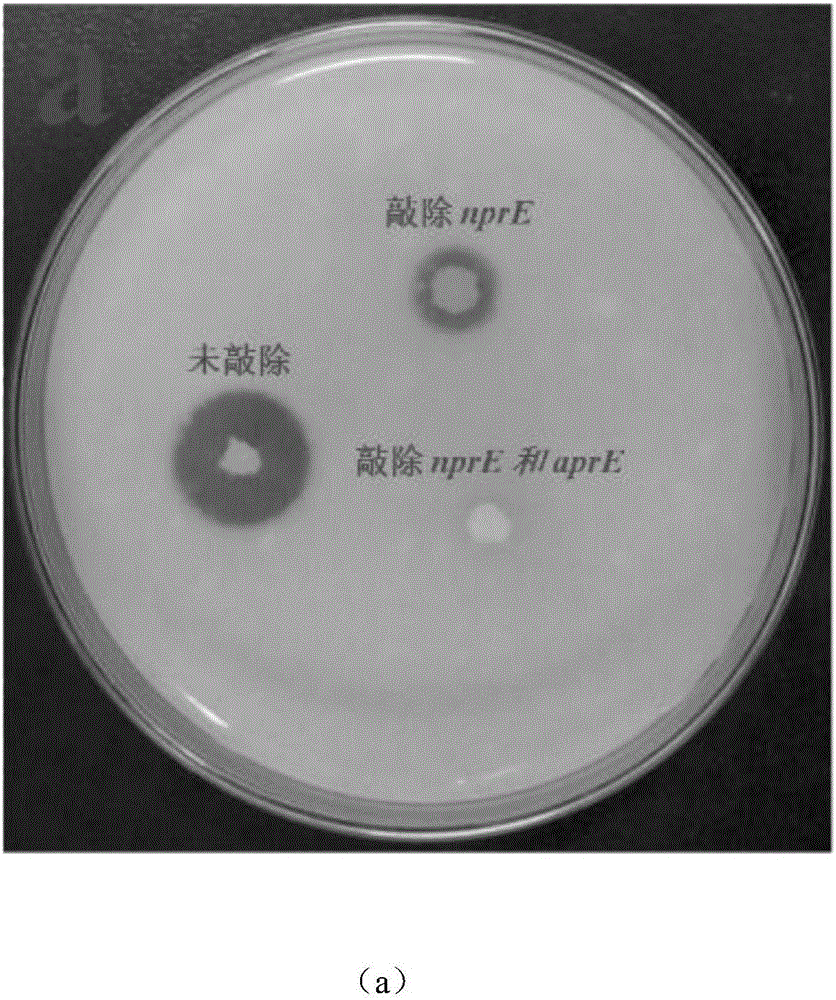
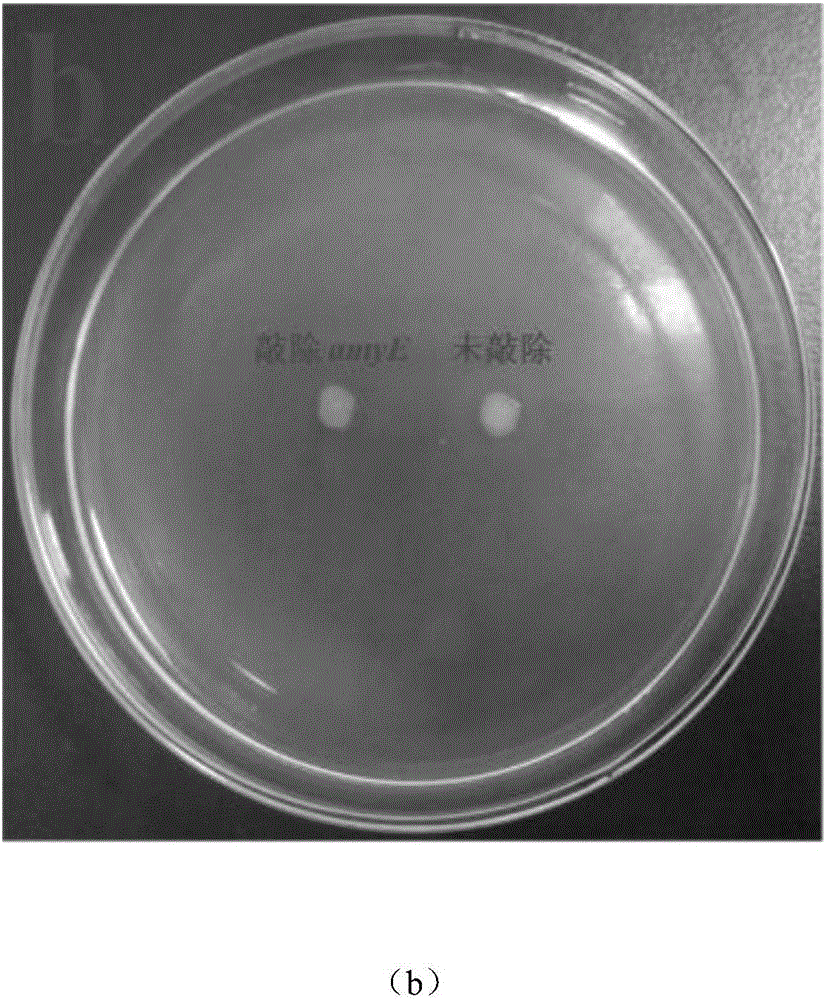
Bacillus subtilis for efficient exogenous protein expression and high density culture
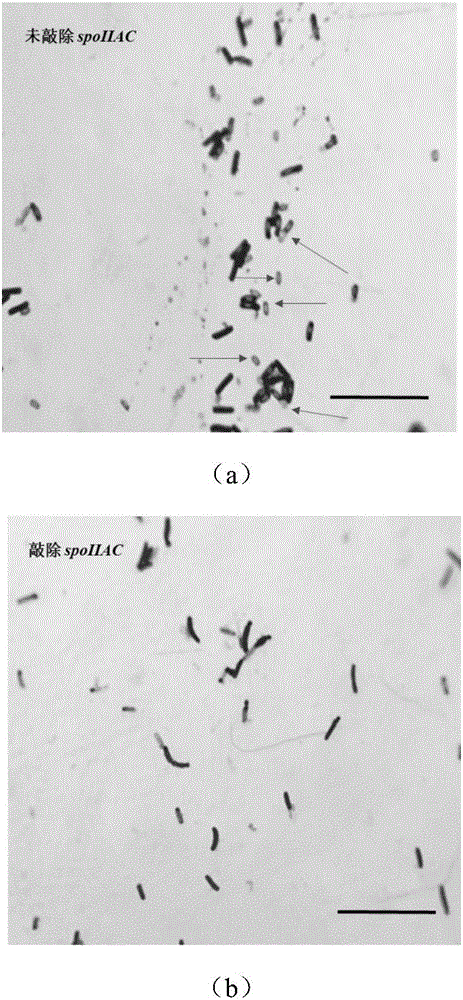
Belonging to the technical field of bioengineering, the invention discloses a bacillus subtilis for efficient exogenous protein expression and high density culture. According to the invention, a screening and separation method is employed to acquire a bacillus subtilis with high protein expression quantity, and the six genes srfC, spoIIAC, amyE, nprE, aprE and bamA are knocked out to obtain B. subtilis WS5 with a preservation number of CCTCC M 2016536. The bacillus subtilis WS5 is adopted as the expression host for construction of a beta-CGTase genetically engineered bacterium. The beta-CGTase genetically engineered bacterium is subjected to 3L fermentor culture, foams produced in the fermentation process are obviously decreased, a mirror does not detect spore production, almost no extracellular amylase and protease exist in the culture process, after 70h of fermentation culture, the beta-CGTase enzyme activity reaches 270U / ml, and DCW reaches 70g / L.
Owner:JIANGNAN UNIV
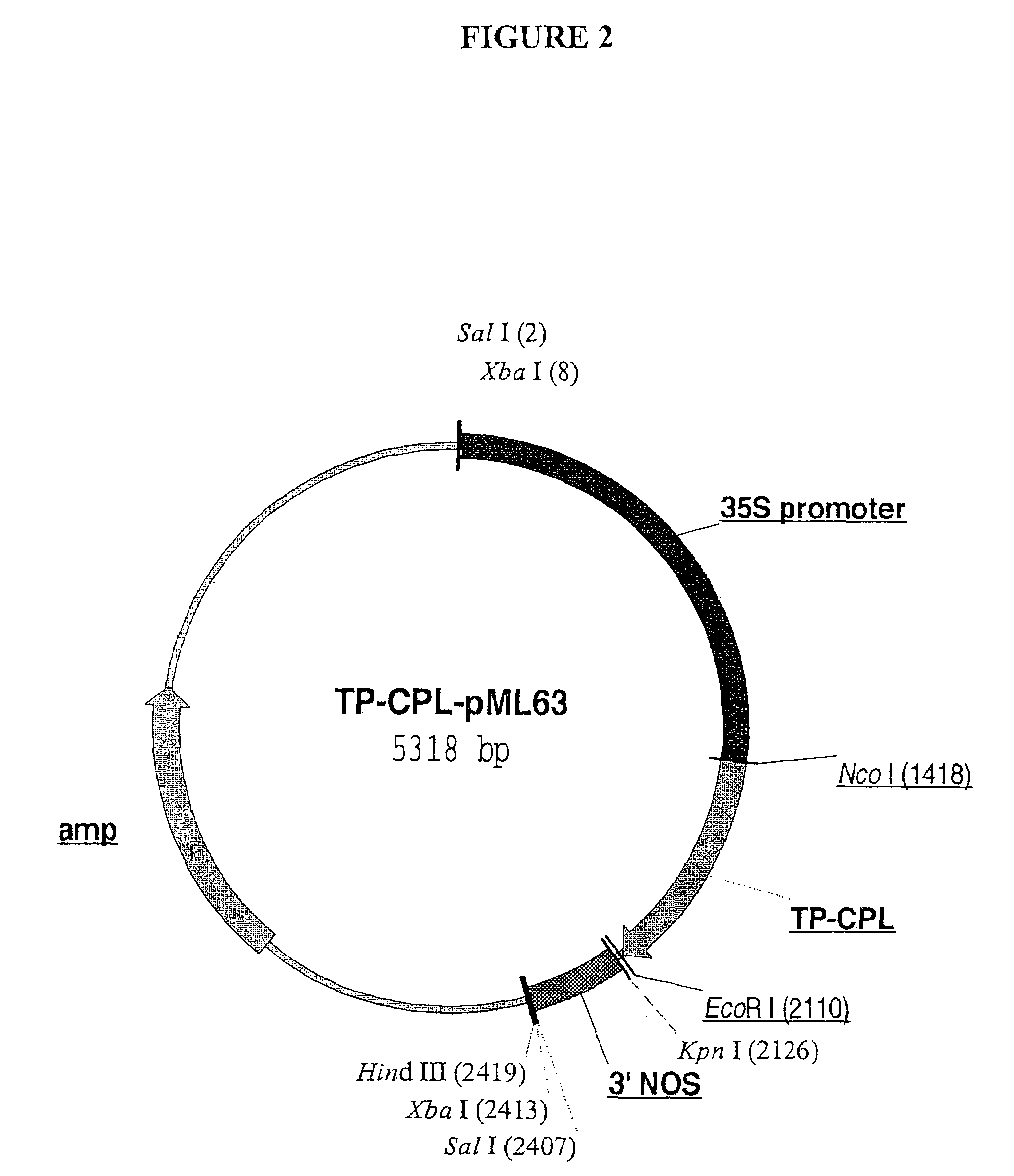
High level production of p-hydroxybenzoic acid in green plants
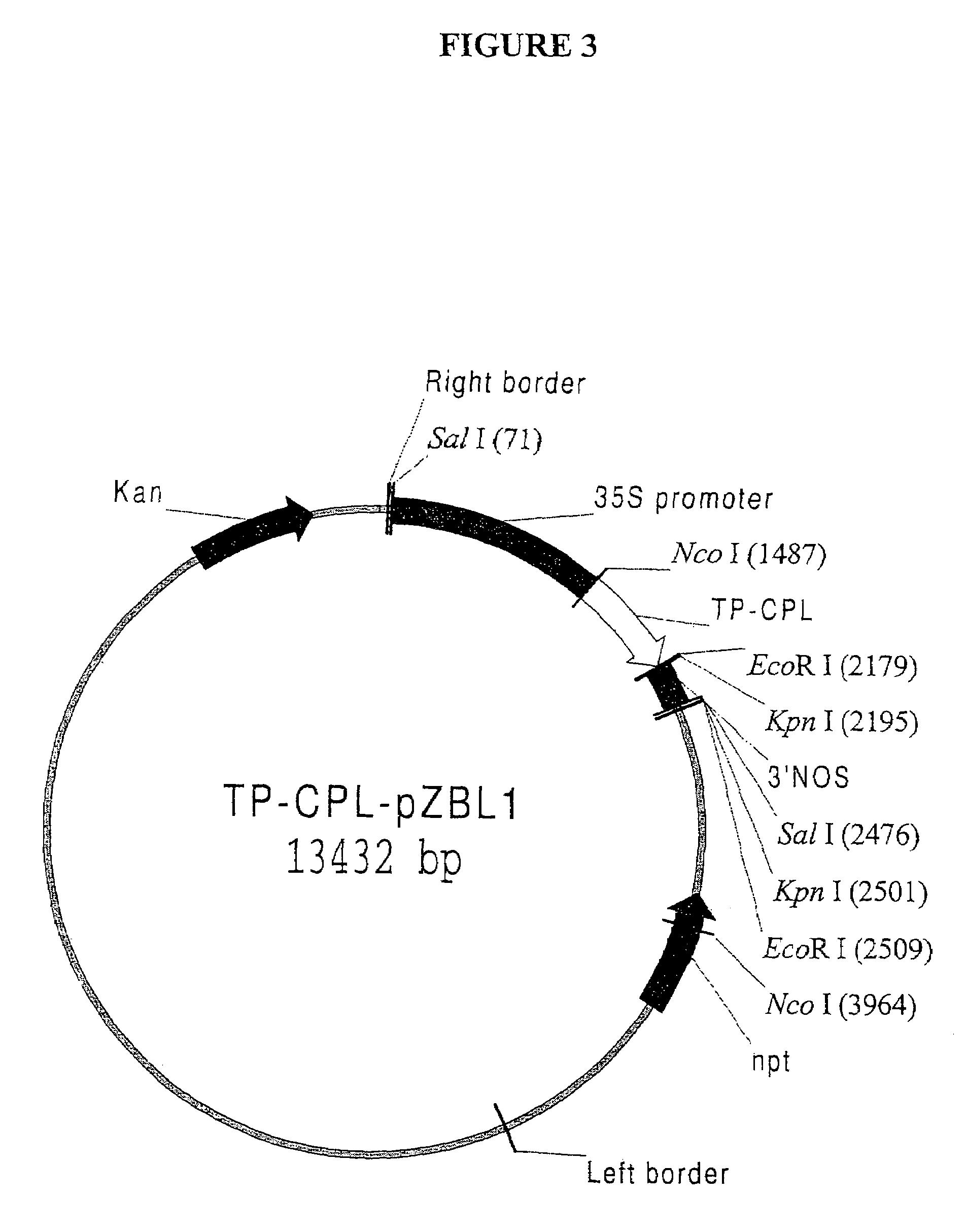
The invention relates to high-level production of pHBA in green plants using a unique expression cassette. The latter comprises a chorismate pyruvate lyase (CPL) coding sequence operably linked to a suitable promoter capable of driving protein expression in higher plants. Additionally, the CPL cassette comprises a sequence encoding a chloroplast transit peptide, its natural cleavage site, and a small portion of the transit peptide donor protein fused to the N-terminus of CPL. The chloroplast targeting sequence targets the foreign protein to the chloroplast compartment and aids in its uptake into the organelle. The cleavage site is unique to the transit peptide, and cleavage of the chimeric protein encoded by the cassette at this site releases a novel polypeptide that has full enzyme activity, comprising the mature CPL enzyme and a small portion of the transit peptide donor.
Owner:UNIVERSITY OF NORTH TEXAS
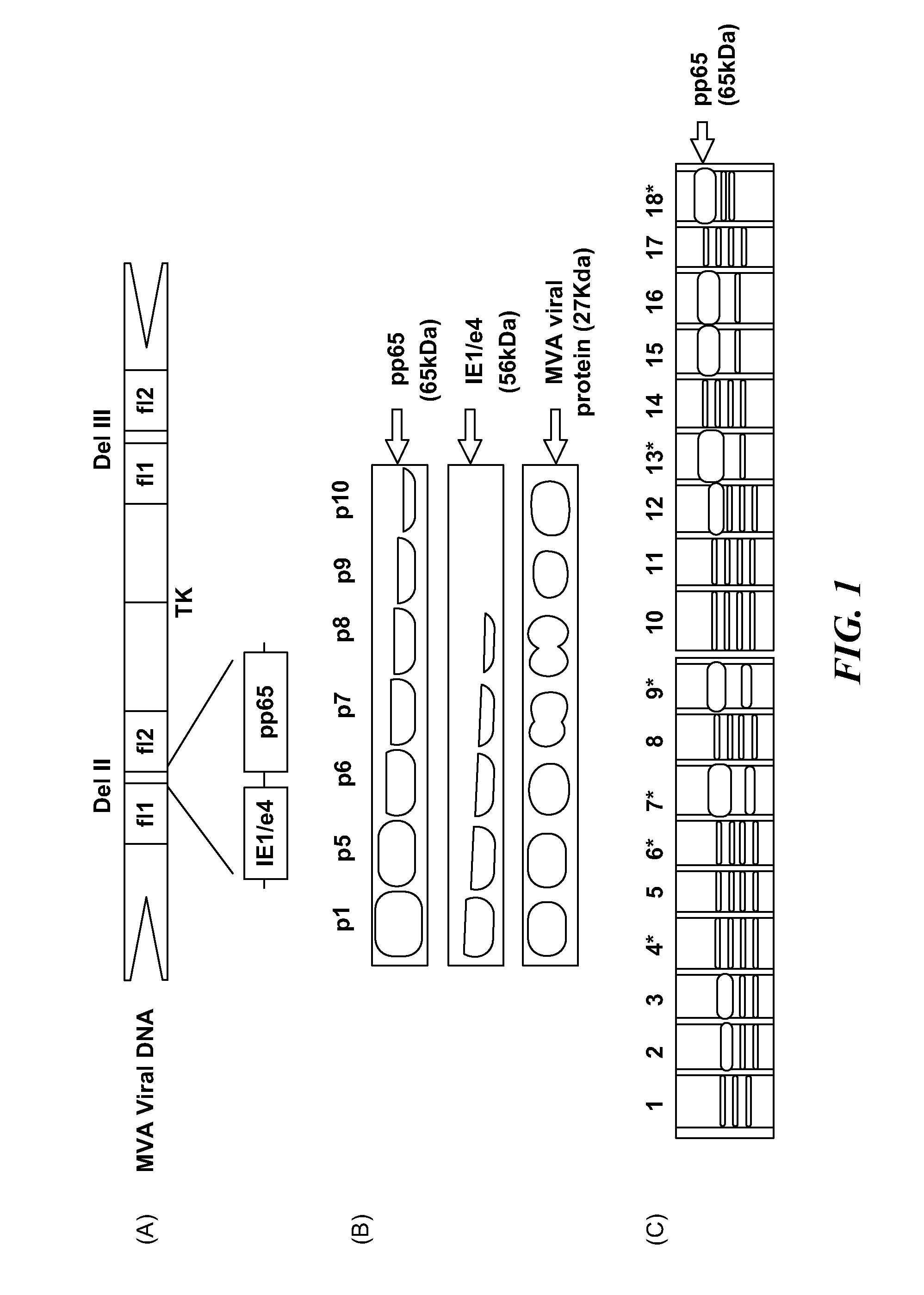
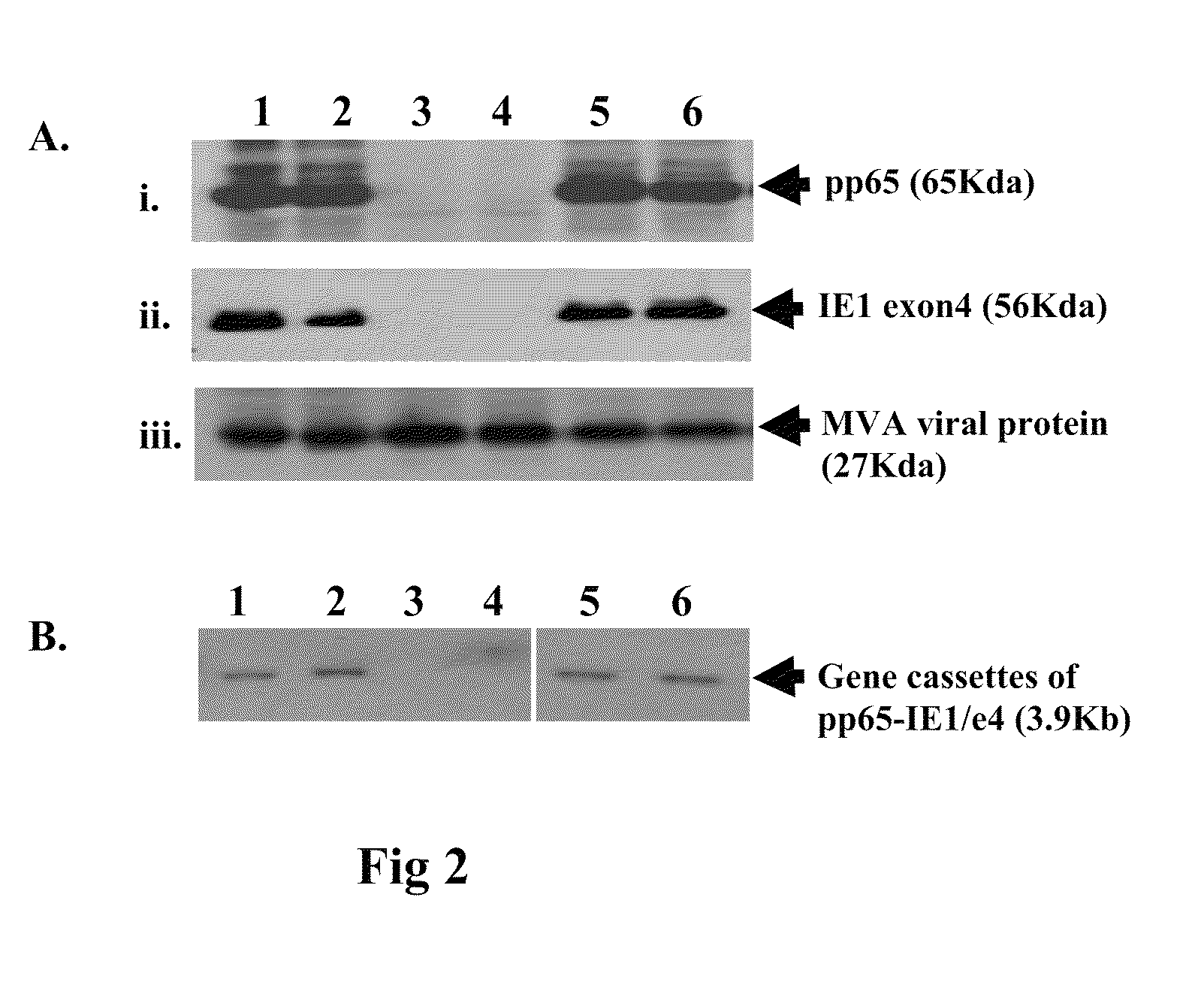
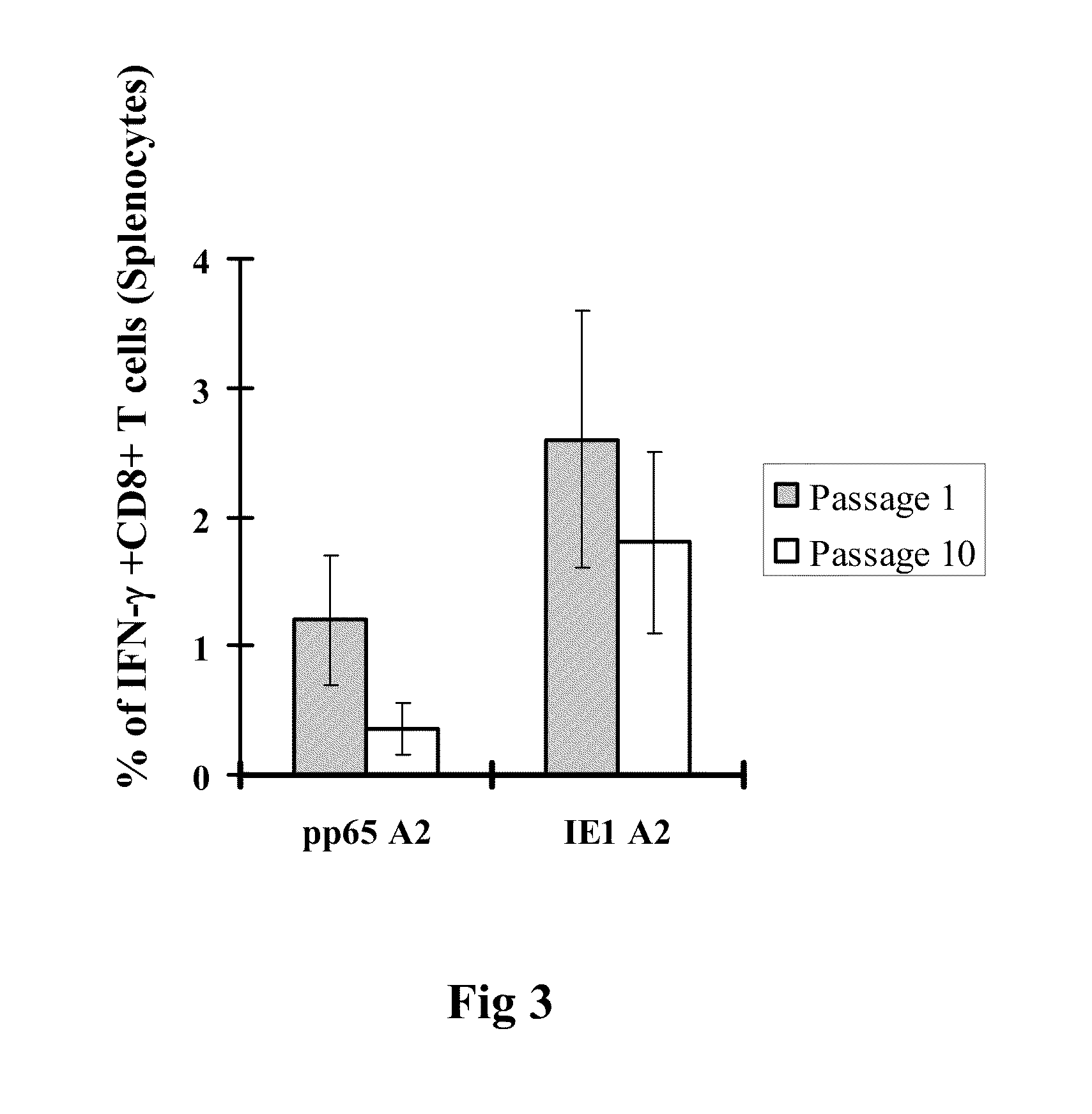
Genetically stable recombinant modified vaccinia ankara (RMVA) vaccines and methods of preparation thereof
ActiveUS20100316667A1Elicit immune responseVectorsSugar derivativesHeterologousModified vaccinia Ankara
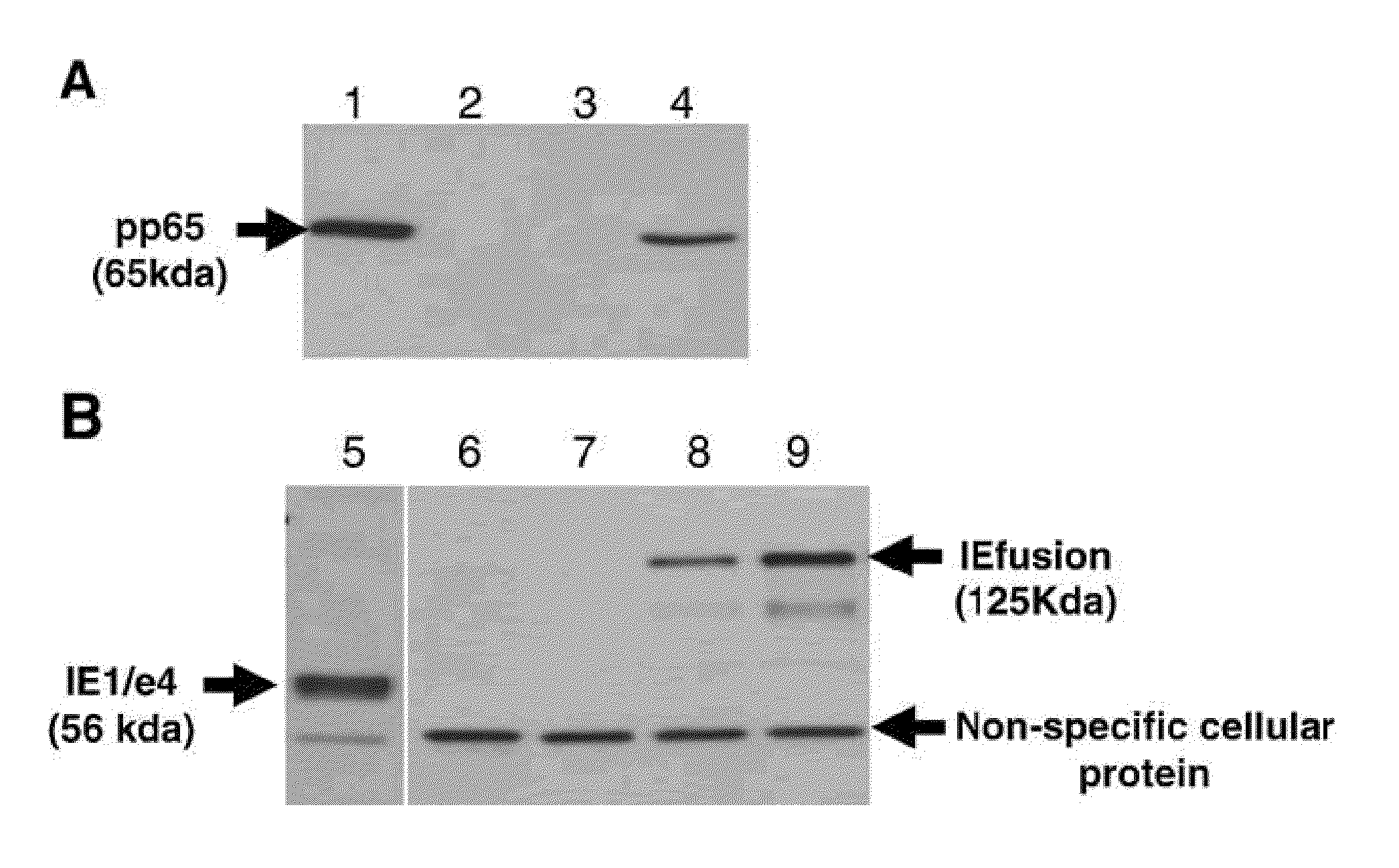
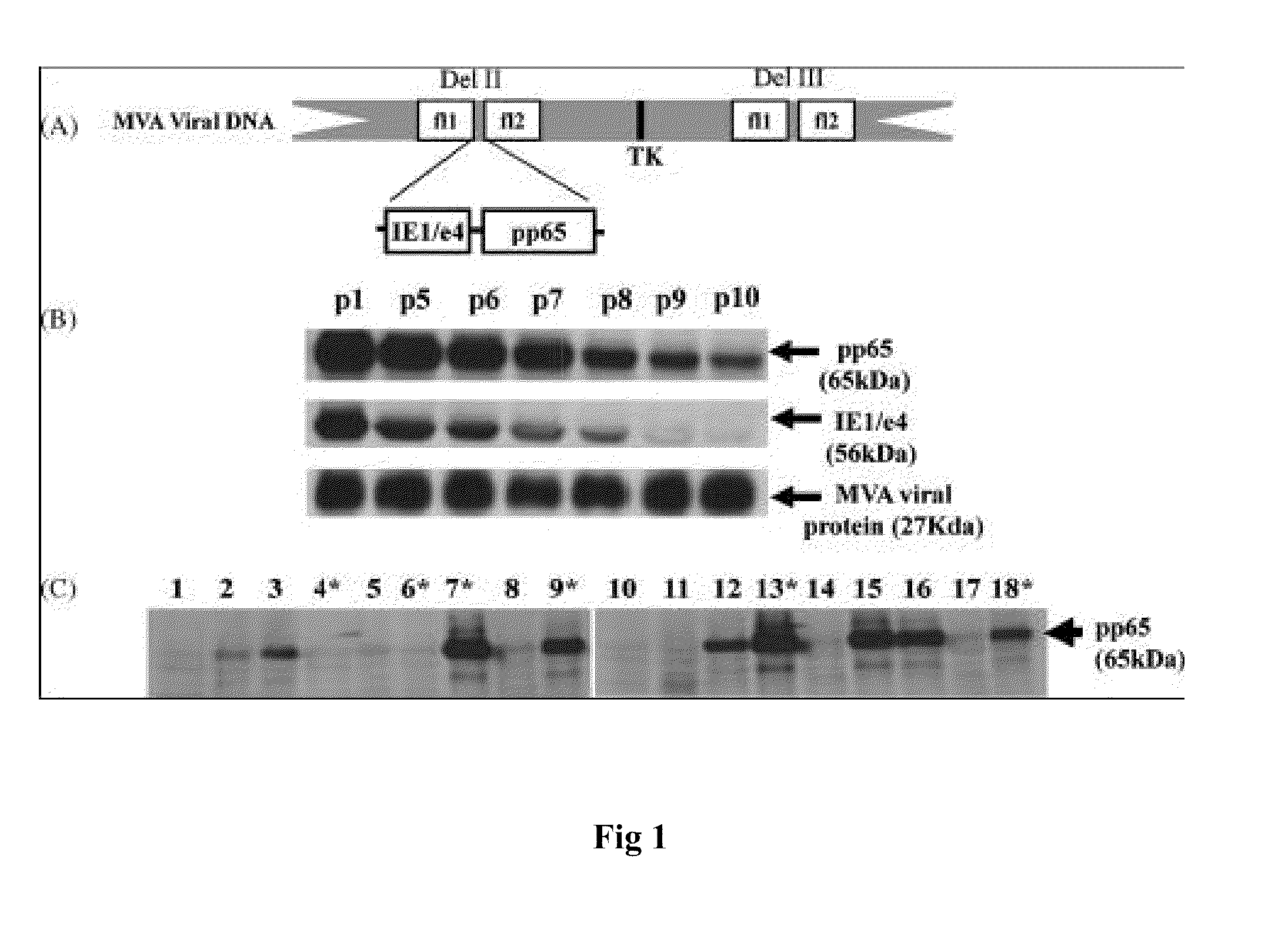
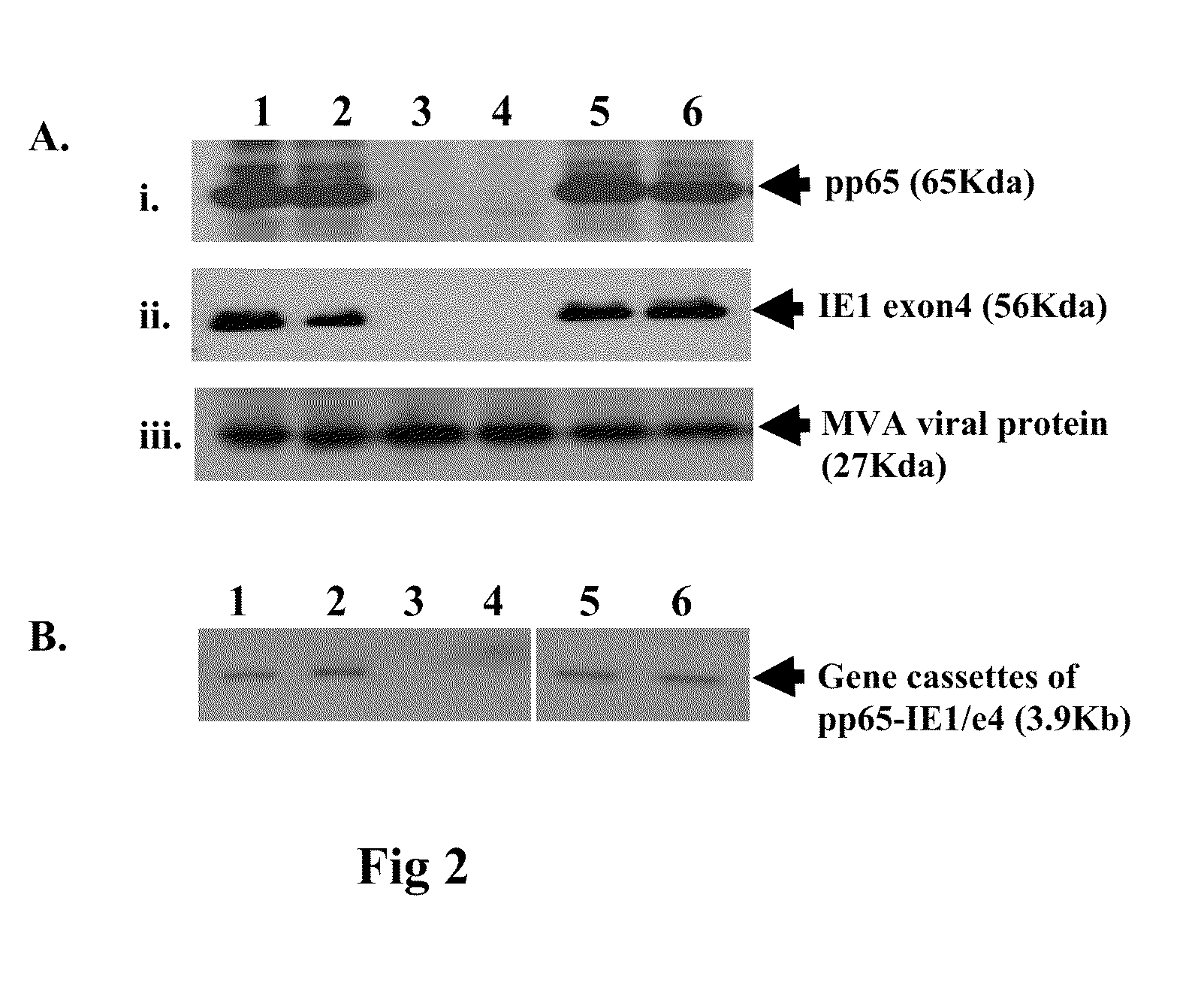
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
Antigen-free collagen aggregate and preparation method thereof
The invention discloses an antigen-free collagen aggregate and a preparation method thereof. The preparation method is characterized by comprising the following steps: with traceable animal skin or tendo calcaneus as a raw material, performing operations by the process such as fleshing, stripping fascia, degreasing, removing foreign protein and decellularizing; performing fine purification on the animal skin or tendo calcaneus, and then carrying out separation and purification on the collagen aggregate by the methods of acid fluffing, homogenizing, salting out for a plurality of times, centrifuging for a plurality of times and the like, thereby finally obtaining the antigen-free collagen aggregate. The collagen aggregate is a mixture of a collagen fiber and a collagen bundle, and has a periodic light and shade horizontal grain structure; and the space between the horizontal grains is about 67nm. The material has good biocompatibility, biodegradability, mechanical property and hemostatic performance, also has the characteristics of low antigenicity, biological activity and the like, and can be widely applied to preparation of biomedical materials such as a hemostatic material, a tissue engineering material, a biological dressing, a biodegradable medical suture and a plastic material.
Owner:SICHUAN UNIV
Protein expression system
ActiveUS20100287670A1Improve expression levelBoost protein levelsVirus peptidesVaccinesStart codonForeign protein
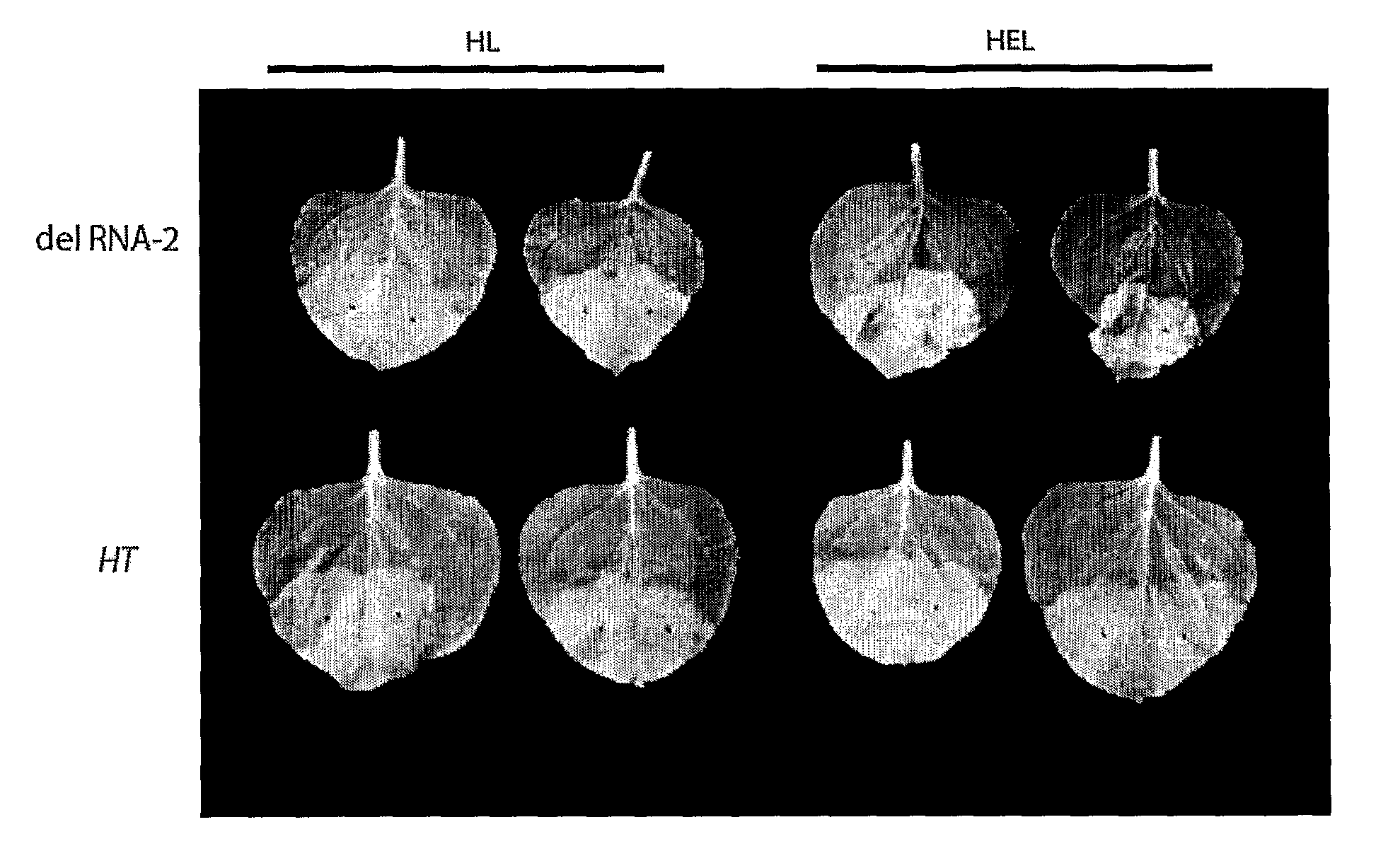
The inventions is based on an expression enhancer sequence derived from the RNA-2 genome segment of a bipartite RNA virus, in which a target initiation site in the RNA-2 genome segment has been mutated. Deletion of appropriate start codons upstream of the main RNA2 translation initiation can greatly increase in foreign protein accumulation without the need for viral replication. Also provided are methods, vectors and systems, including the ‘hyper-translatable’ Cowpea Mosaic Virus (‘CPMV-HT’) based protein expression system.
Owner:PLANT BIOSCI LTD
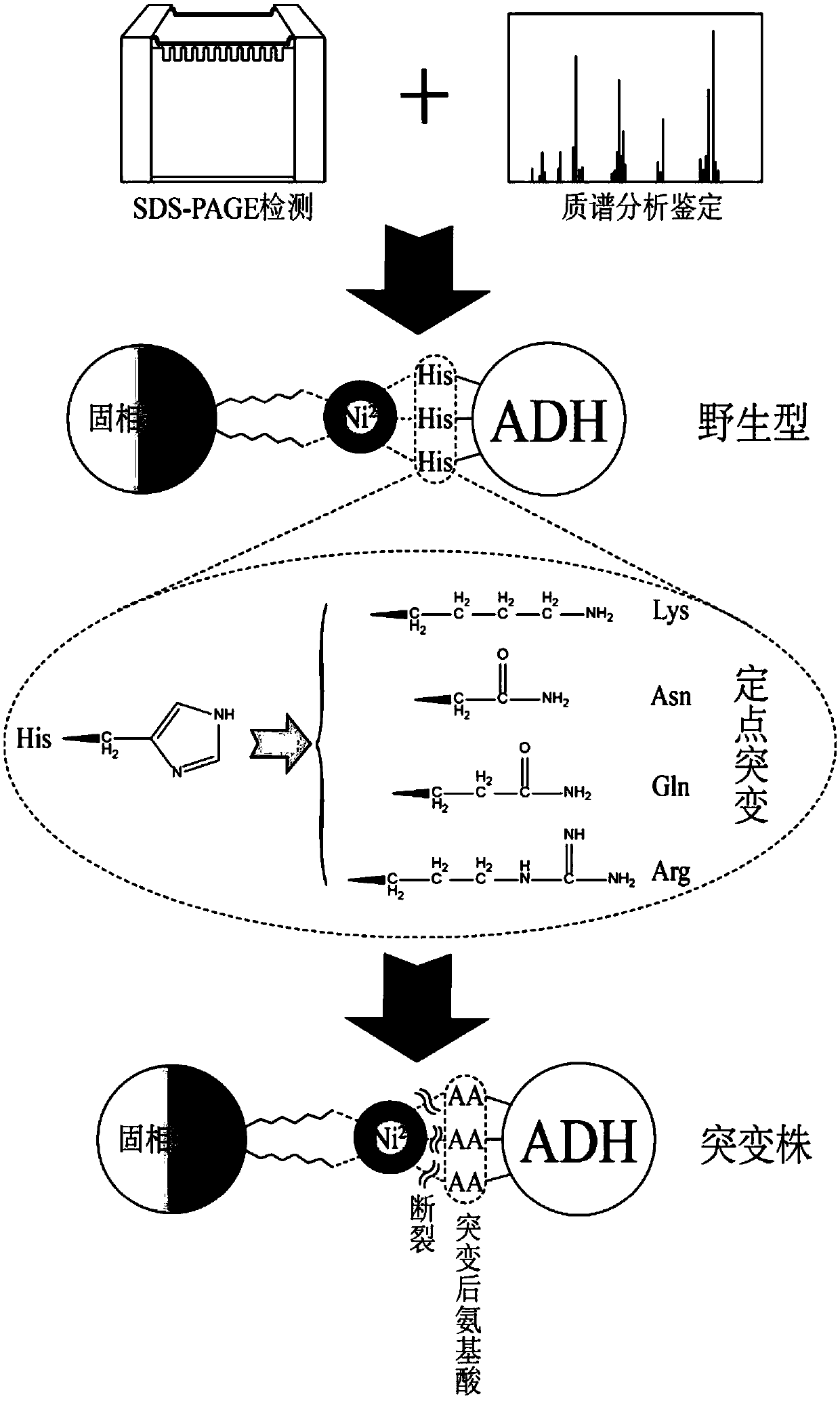
ADH (ethanol dehydrogenase) protein family mutant and application thereof
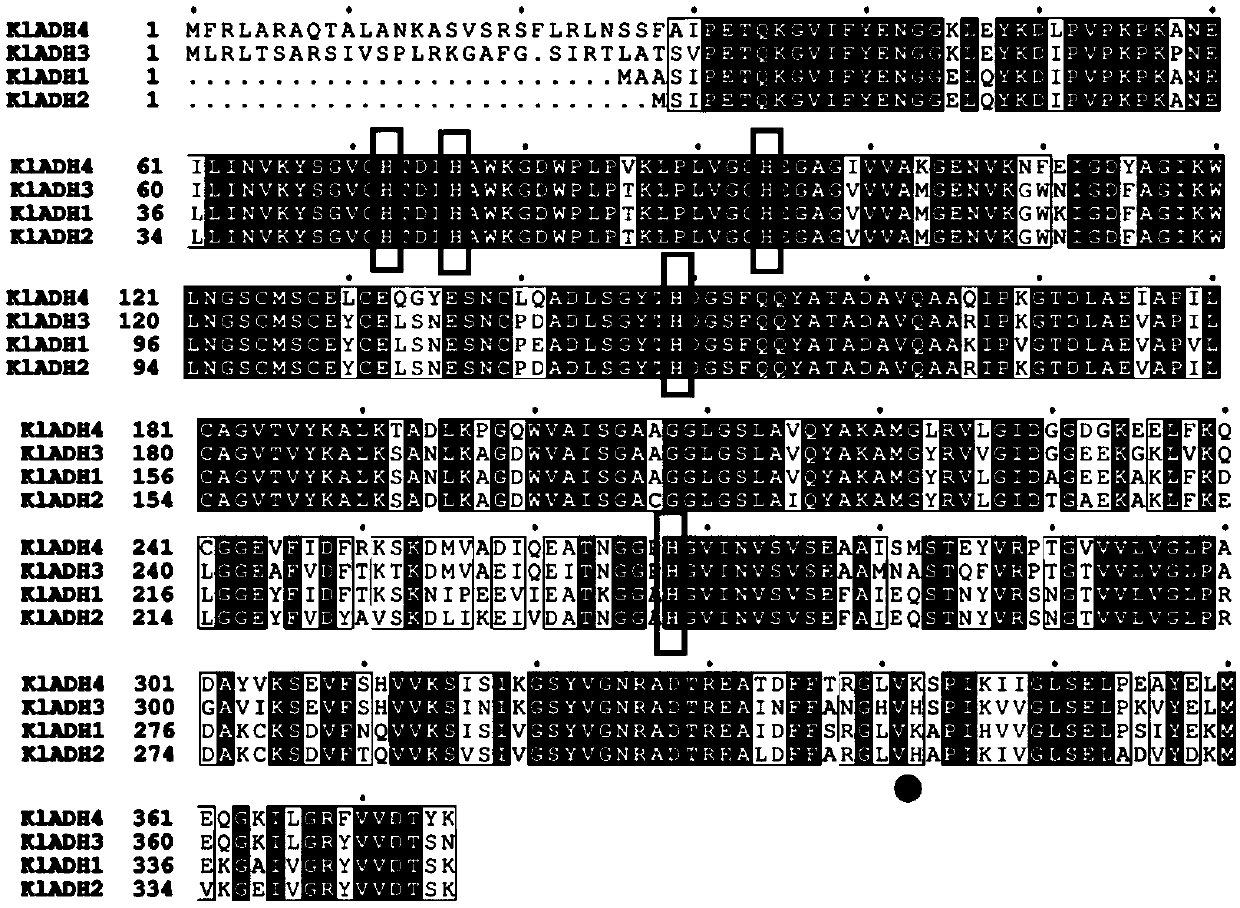
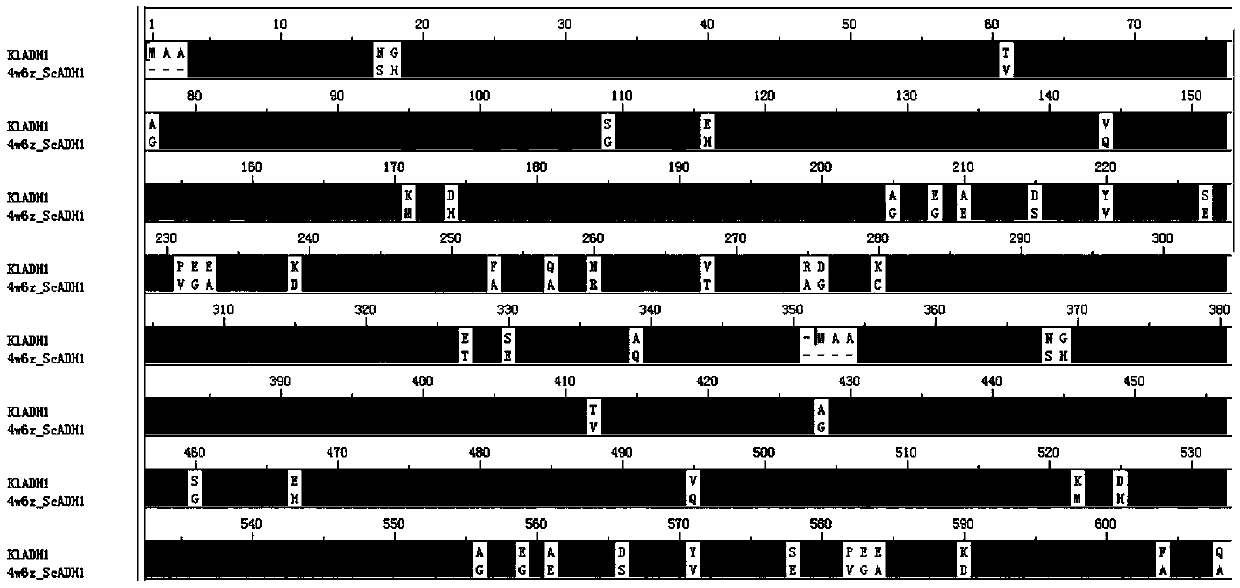
The invention relates to an ADH (ethanol dehydrogenase) protein family mutant and an application thereof, and particularly provides a mutant of an ADH protein. Compared with wild type ADH proteins, the ADH protein mutant is capable of remarkably increasing expression purity, efficiency and yield of foreign proteins in a cell-free synthesis system in vitro, and / or reducing binding capacity of the mutant protein and the Ni medium.
Owner:KANGMA SHANGHAI BIOTECH LTD
Fusion polypeptides, vaccines and compositions of FKBP chaperones and target polypeptides
The present invention relates to the cloning and expression of foreign protein or polypeptides in bacteria, such as Escherichia coli. In particular, this invention relates to expression tools comprising a FKBP-type peptidyl prolyl isomerase selected from the group consisting of FkpA, SlyD, and trigger factor; methods of recombinant protein expression, the recombinant polypeptides thus obtained, as well as to the use of such polypeptides.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
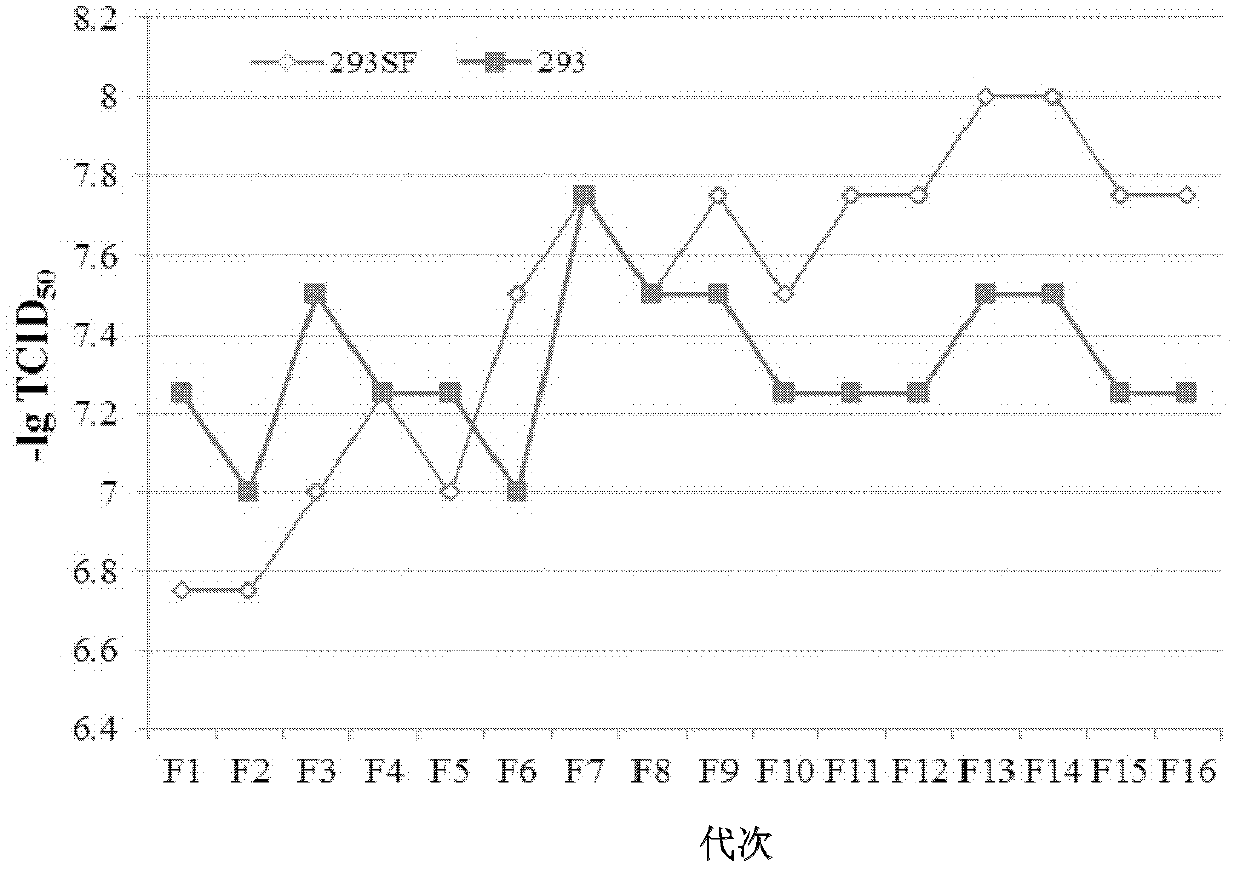
HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and application thereof
InactiveCN102604889AMicroorganism based processesViruses/bacteriophagesHEK 293 cellsVirulent characteristics
The invention discloses an HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and an application thereof. According to the invention, the HEK 293 cell (293SF) applicable to serum-free culture is obtained by adopting a culture solution progressive substitution method, wherein the preservation number of the HEK 293 cell is CGMCC (China General Microbiological Culture Collection Center) No.5824. Detection shows that the 293SF cell has stable adenovirus proliferation capacity and foreign protein expressing ability; average lesion time is 97 hours and is reduced by 12.9% compared with the HEK 293 cell; virus virulence reaches up to 107.48TCID50 / mL and is improved by 43.3% compared with the HEK293 cell; and stability is good while generation number is improved, and the state and susceptibility of the 293SF cell are not changed after the 293SF cell is continuous passage is carried out for sixteen times. The cell line disclosed by the invention can be used for serum-free production of an adenovirus vector vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
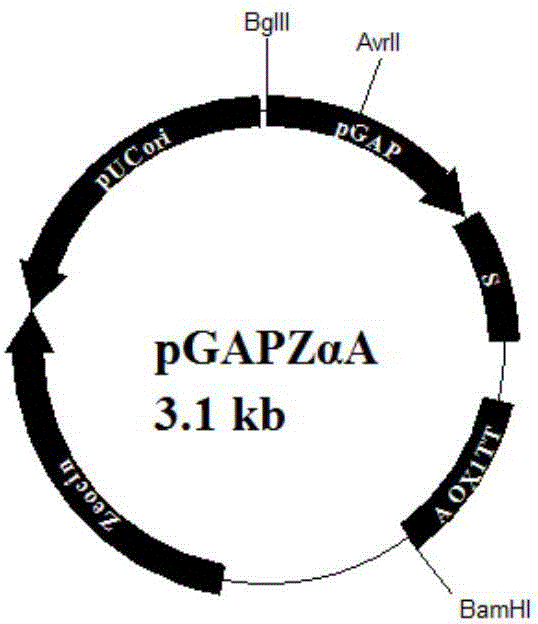
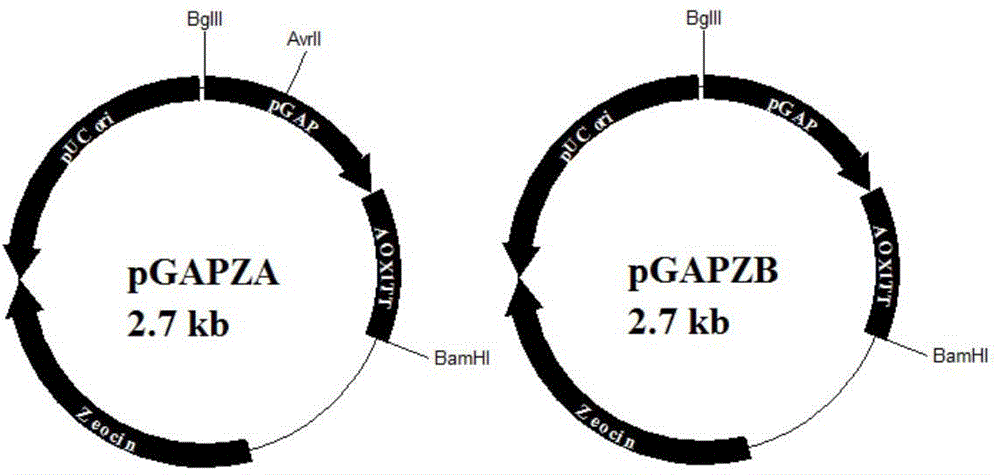
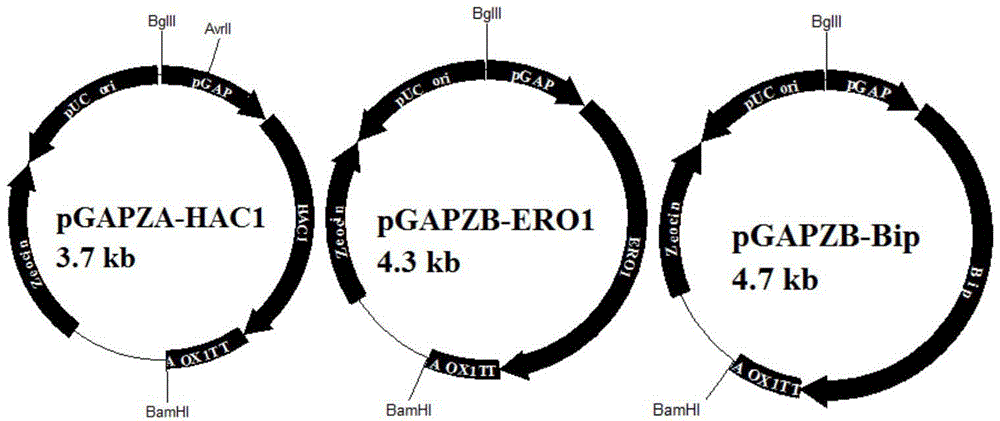
Method for improving expression amount of secretory foreign protein in pichia pastoris
ActiveCN104152484AHigh expressionPromotes proper foldingFungiMicroorganism based processesForeign proteinSecretion expression
The invention aims to provide a method for improving the expression amount of secretory foreign protein in pichia pastoris. Byintracellular co-expression of double genes of HAC1 and ERO1 or three genes of HAC1, ERO1 and BIP in pichia pastoris, the expression amount of secretory foreign protein in pichia pastoris is effectively improved. According to the invention, double genes of HAC1 and ERO1 or three genes of HAC1, ERO1 and BIP are transformed into pichia pastoris secreting and expressing exogenous xylanase to obtain recombinant pichia pastoris strains, thereby significantly increasing the expression amount of the exogenous xylanase. During co-expression of double genes of HAC1 and ERO1 in pichia pastoris, the secretion expression amount of exogenous xylanase is generally increased by 15%-25% compared with the initial level; and during the co-expression of three genes HAC1, ERO1 and BIP, the secretion expression amount of xylanase is increased by 45%-57% compared with the initial level.
Owner:QINGDAO VLAND BIOTECH GRP
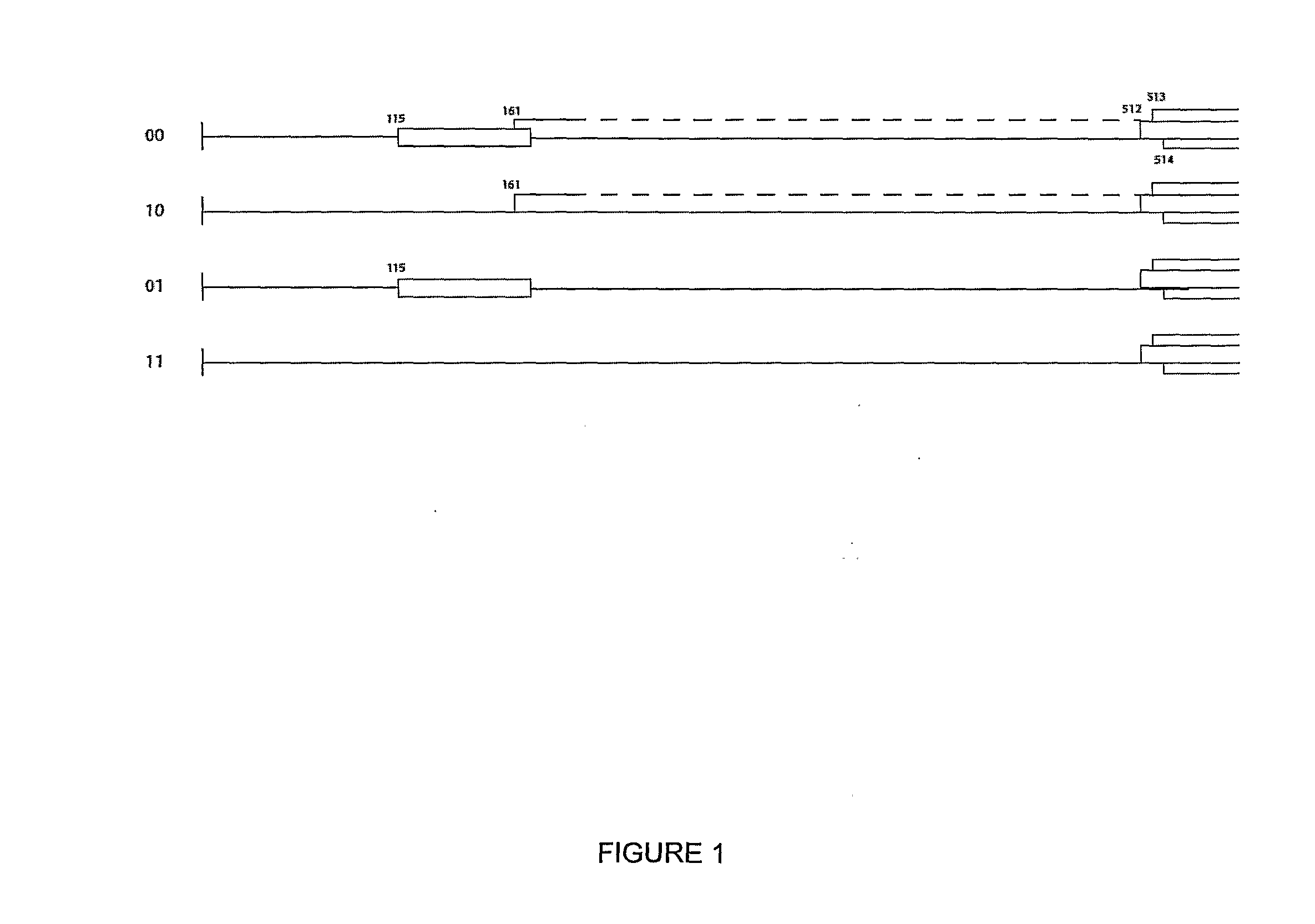
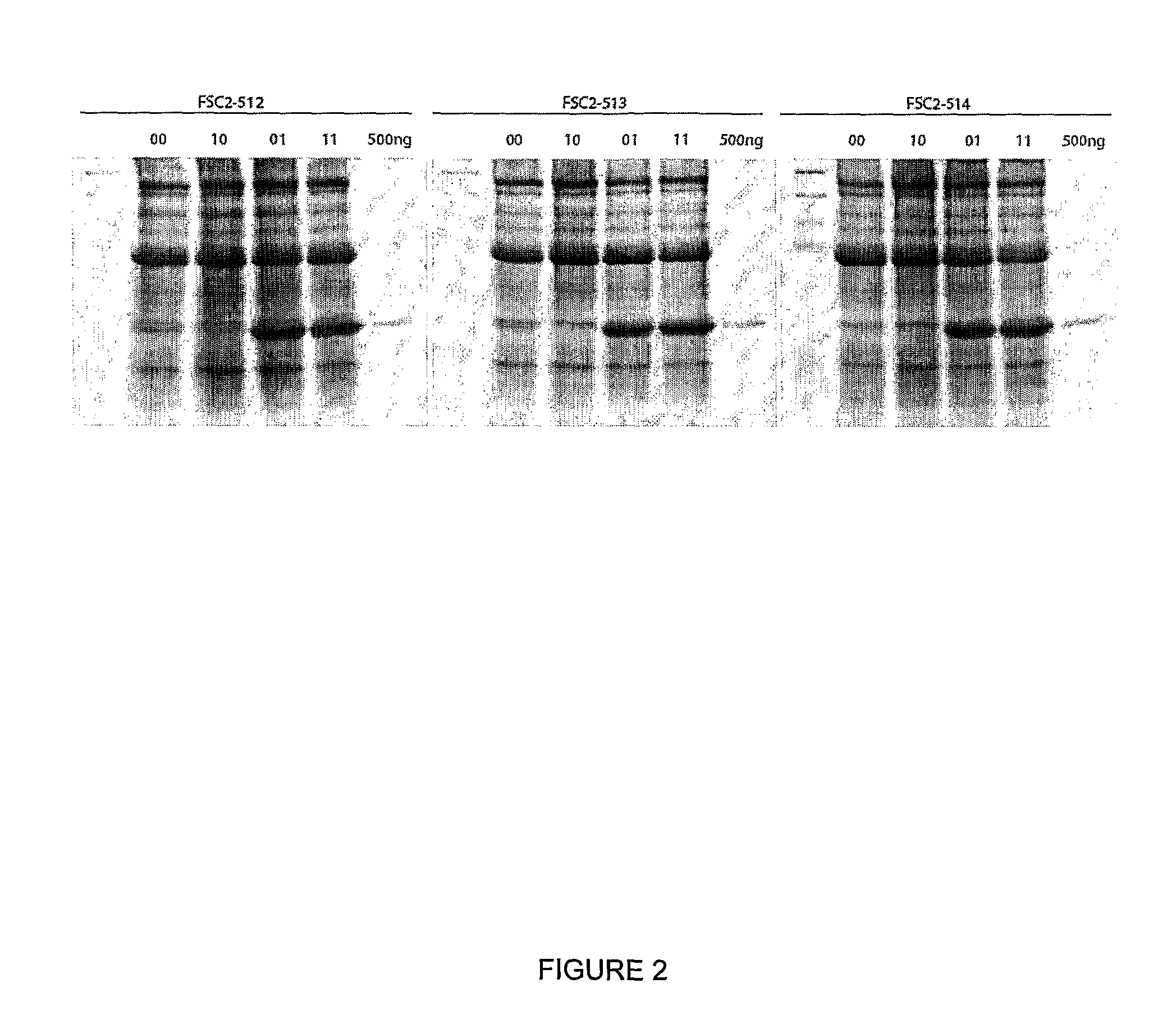
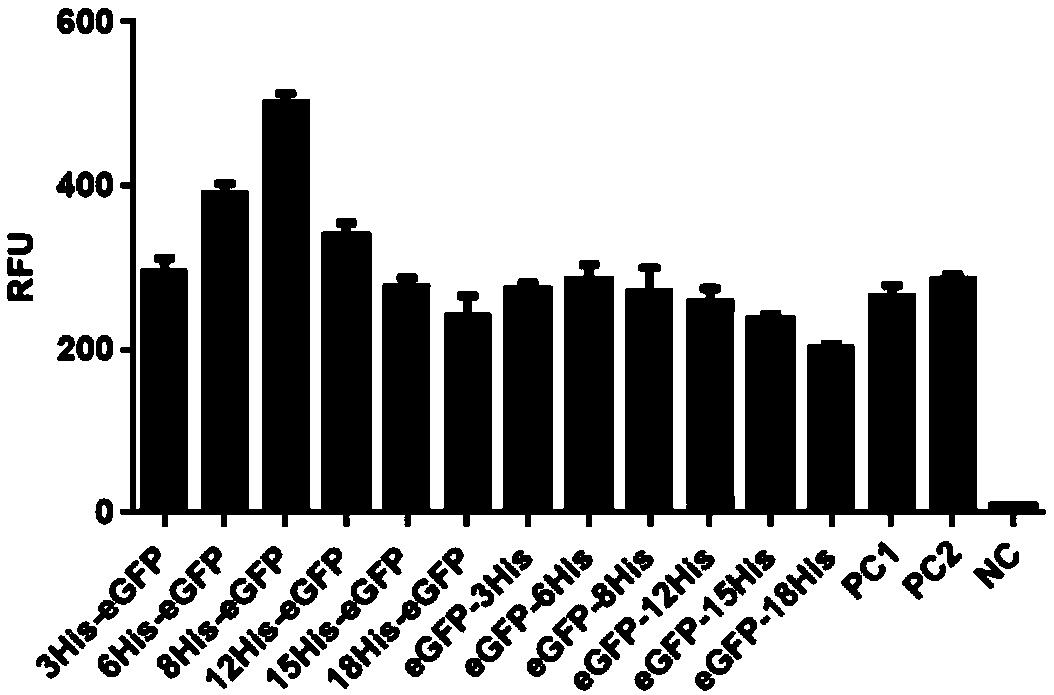
Multiple His sequence tag and application of multiple His sequence tag to protein expression and purification
The invention provides a multiple His sequence tag and application of the multiple His sequence tag to protein expression and purification. Specifically, a nucleic acid builder is composed of coding sequences of nHis, catenation sequences (including the codon optimized catenation sequence and the un-optimized catenation sequence) and coding sequences of foreign proteins. By applying the nucleic acid builder to a protein synthesis system, the translation efficiency of target proteins can be improved, and the foreign proteins can be expressed and purified.
Owner:KANGMA SHANGHAI BIOTECH LTD
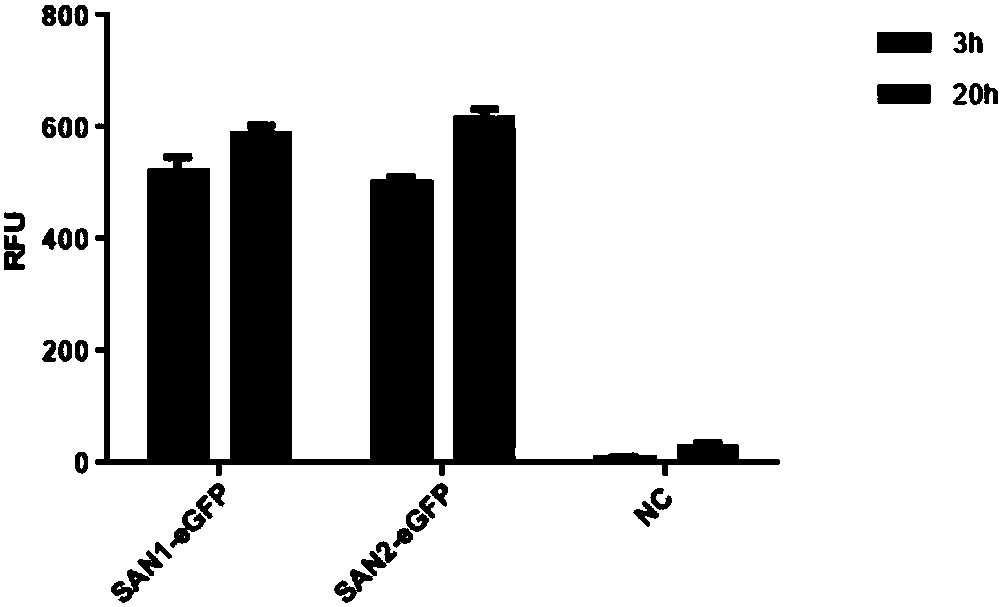
Application of nucleic acid construct containing streptavidin elements to protein expression and purification
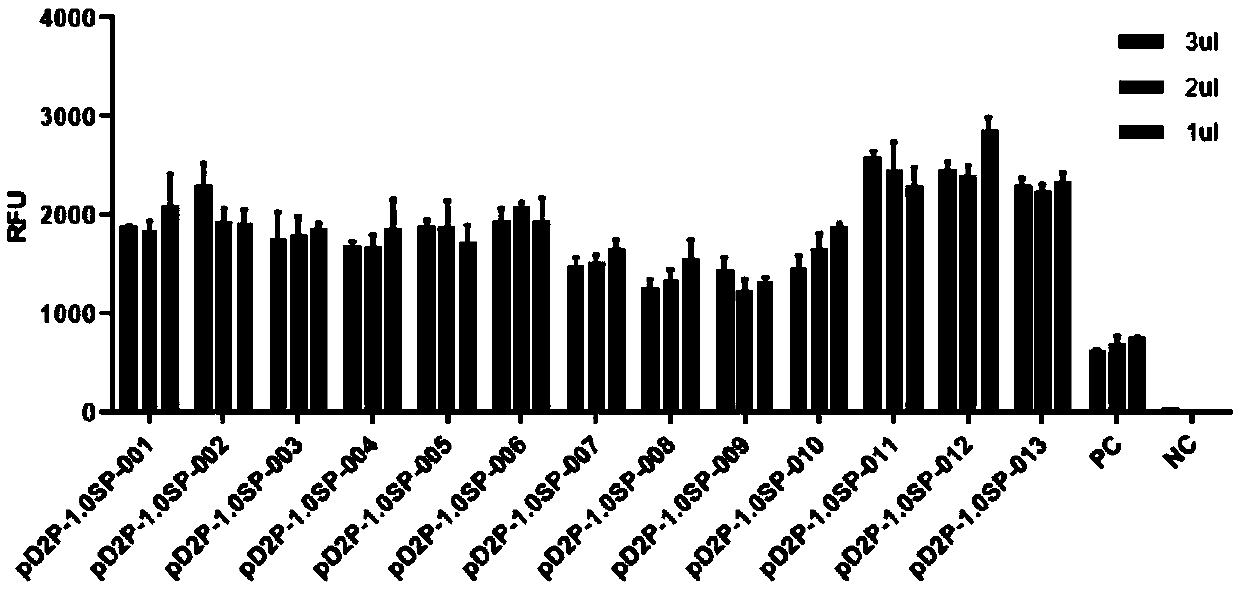
ActiveCN110408635APolypeptide with affinity tagHybrid peptidesBiotin-streptavidin complexForeign protein
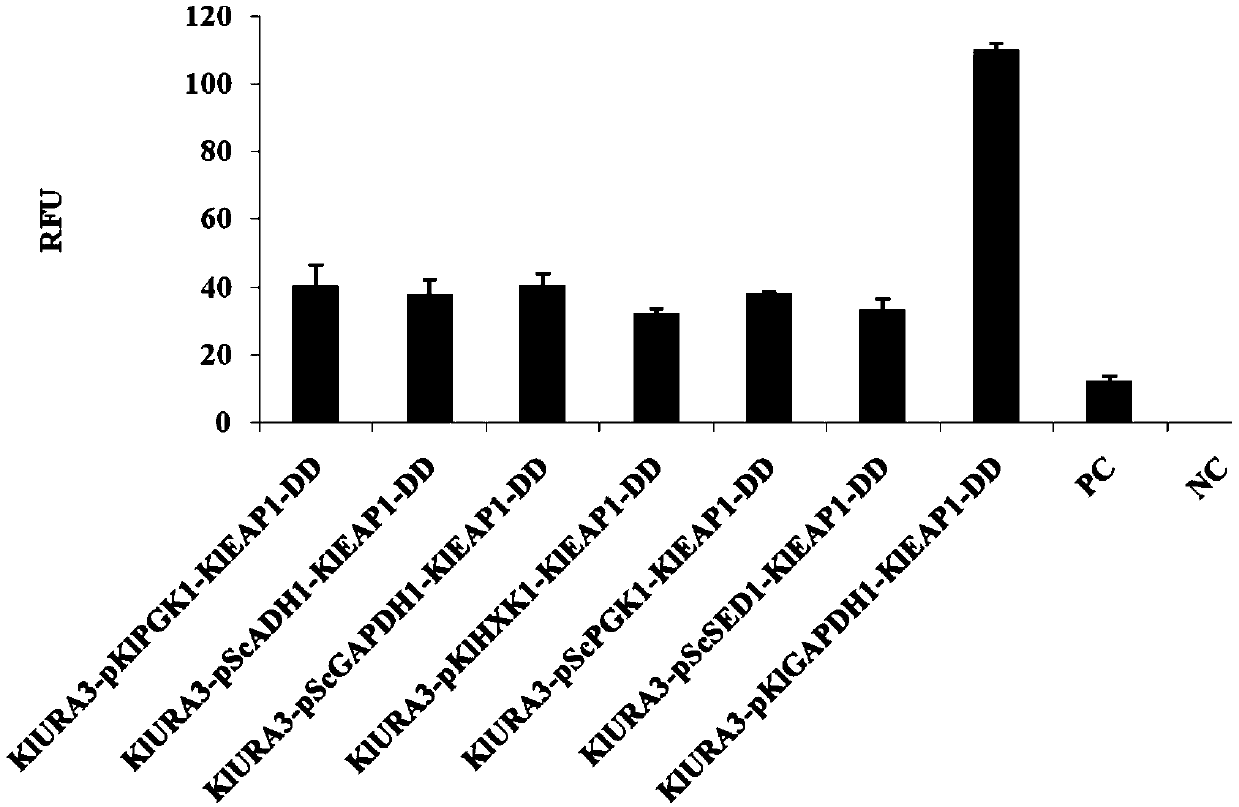
The invention provides application of a nucleic acid construct containing streptavidin elements to protein expression and purification, and particularly provides the nucleic acid construct. The nucleic acid construct has a formula I structure from 5' to 3', and the formula I is as follows: Z1-Z2-Z3 (I); and in the formula I, Z1, Z2 and Z3 are the elements used for constituting the construct correspondingly, all '-' are bonds or nucleotide connection sequences independently, Z1 represents a coding sequence of a tag protein, Z2 represents a connection sequence, and Z3 represents a coding sequence of a passive protein or a foreign protein. By applying the nucleic acid construct to a protein synthesis system (especially an in-vitro protein synthesis system), expression and purification of theforeign protein can be completed, and an RFU value of the synthesized foreign protein is increased.
Owner:KANGMA SHANGHAI BIOTECH LTD
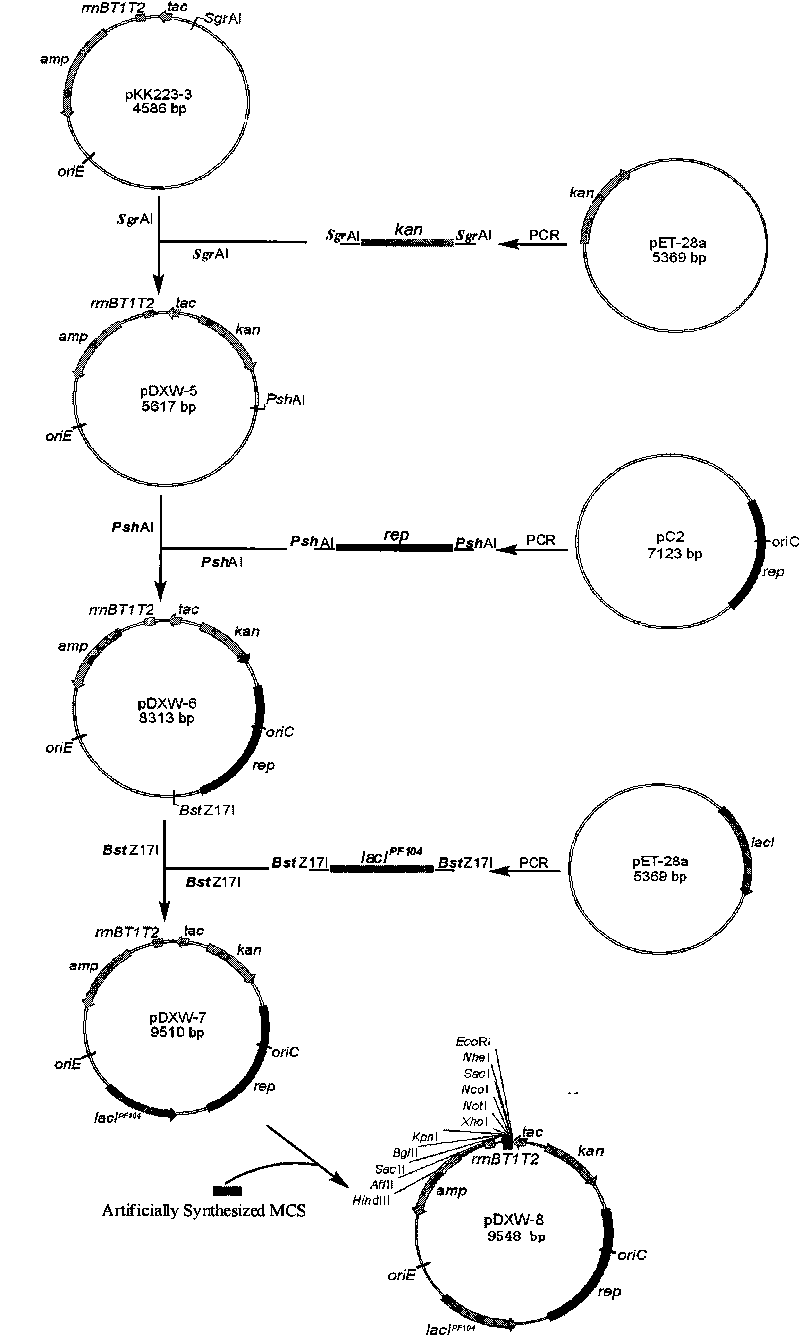
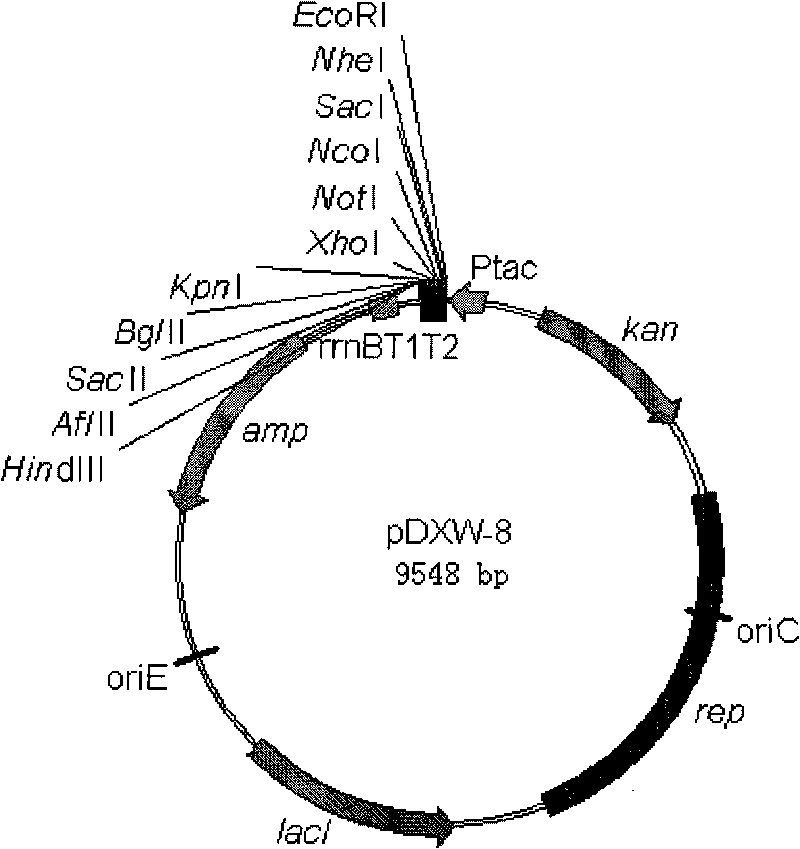
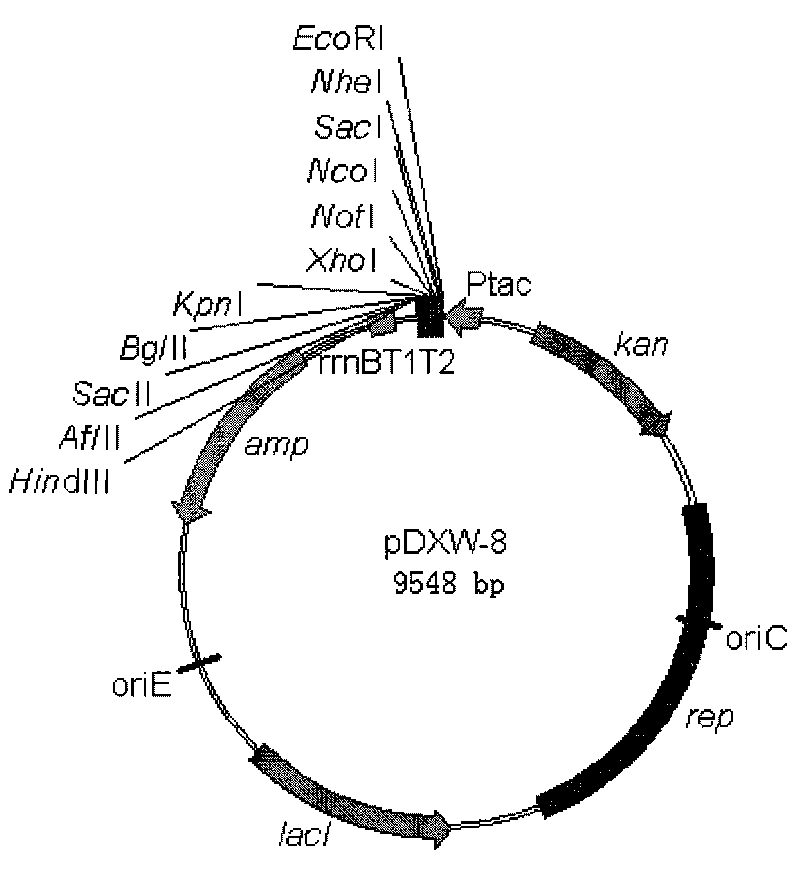
Colibacillus-corynebacterium inducible expression carrier pDXW-8 and building method thereof
ActiveCN101693901AEffective cloningReduced activityMicroorganism based processesVector-based foreign material introductionCorynebacterium efficiensEscherichia coli
A colibacillus-corynebacterium inducible expression carrier pDXW-8 and a building method thereof belong to the technical field of genetic engineering. The inducible expression carrier pDXW-8 can be copied and stably exists in colibacillus and corynebacterium and comprises a tac promoter, a terminator rrnBT1T2, a resistance marker card kanamycin mark resistance gene, a negative regulation gene laclPE104 and a multi-cloning site, wherein the tac promoter plays a transcript promoting function in the colibacillus and the corynebacterium, the terminator rrnBT1T2 has a transcript stopping function, the resistance marker card kanamycin mark resistance gene is used for selecting transformants of the corynebacterium, the negative regulation gene is used for severely controlling expression of the tac promoter, and the multi-cloning site includes 11 signal limited incision enzyme sites. In the corynebacterium, the carrier is moderate in foreign protein expression, and is particularly adaptable to study on corynebacterium metabolic engineering.
Owner:JIANGNAN UNIV
Alteration of cell membrane with B7
InactiveUS7238360B2Systemic effectInhibitionOrganic active ingredientsPeptide/protein ingredientsForeign proteinApoptosis
Methods and compositions are provided for the persistent modification of cell membranes with exogenous proteins so as to alter the function of the cell to achieve effects similar to those of gene therapy, without the introduction of exogenous DNA. DNA sequences, the proteins and polypeptides embodying these sequences are disclosed for modulating the immune system. The modulations include down-regulation, up-regulation and apoptosis.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Immune cell cryopreservation solution and application thereof
ActiveCN105638642ABiological Property GuaranteeReduced Possibility of ContaminationDead animal preservationPolyethylene glycolDrug biological activity
The invention relates to an immune cell cryopreservation solution and application thereof. The immune cell cryopreservation solution is prepared from 250-200 U / mL of recombinant human interleukin-2, 0.1-0.4 g / mL of polyethylene glycol, 0.1-0.4 g / mL 1,2-propylene glycol and 90-99% basal culture medium or sodium chloride for injection by volume. By using the immune cell cryopreservation solution for immune cell cryopreservation, the survival rate of recovery cells can reach 93% or above, the biological property of the cells is not changed, and the biological activity of the immune cells is ensured; in addition, due to the fact that no animal serum is contained in the immune cell cryopreservation solution, foreign protein cannot be introduced, the possibility of animal pathogeny contamination is reduced, and the problems that immune cells cannot be stored and transported for a long time are effectively solved.
Owner:居李生物科技(北京)有限公司
Protein synthesis system for increasing expression amount of foreign protein and application method of protein synthesis system
The invention provides a protein synthesis system for increasing the expression amount of a foreign protein and an application method of the protein synthesis system. Specifically, a poly-nucleotide sequence or vector constructed by a strong promoter sequence is inserted in front of a nucleotide sequence for encoding an eIF4E binding protein, over-expression of eIF4E binding proteins such as Eap1and p20 can be achieved, and foreign protein synthesis is implemented by using the improved cell lysis buffer. The invention provides the protein synthesis system for increasing the expression amountof the foreign protein, and by adopting the synthesis system provided by the invention, the efficiency of protein translation can be remarkably improved.
Owner:KANGMA SHANGHAI BIOTECH LTD
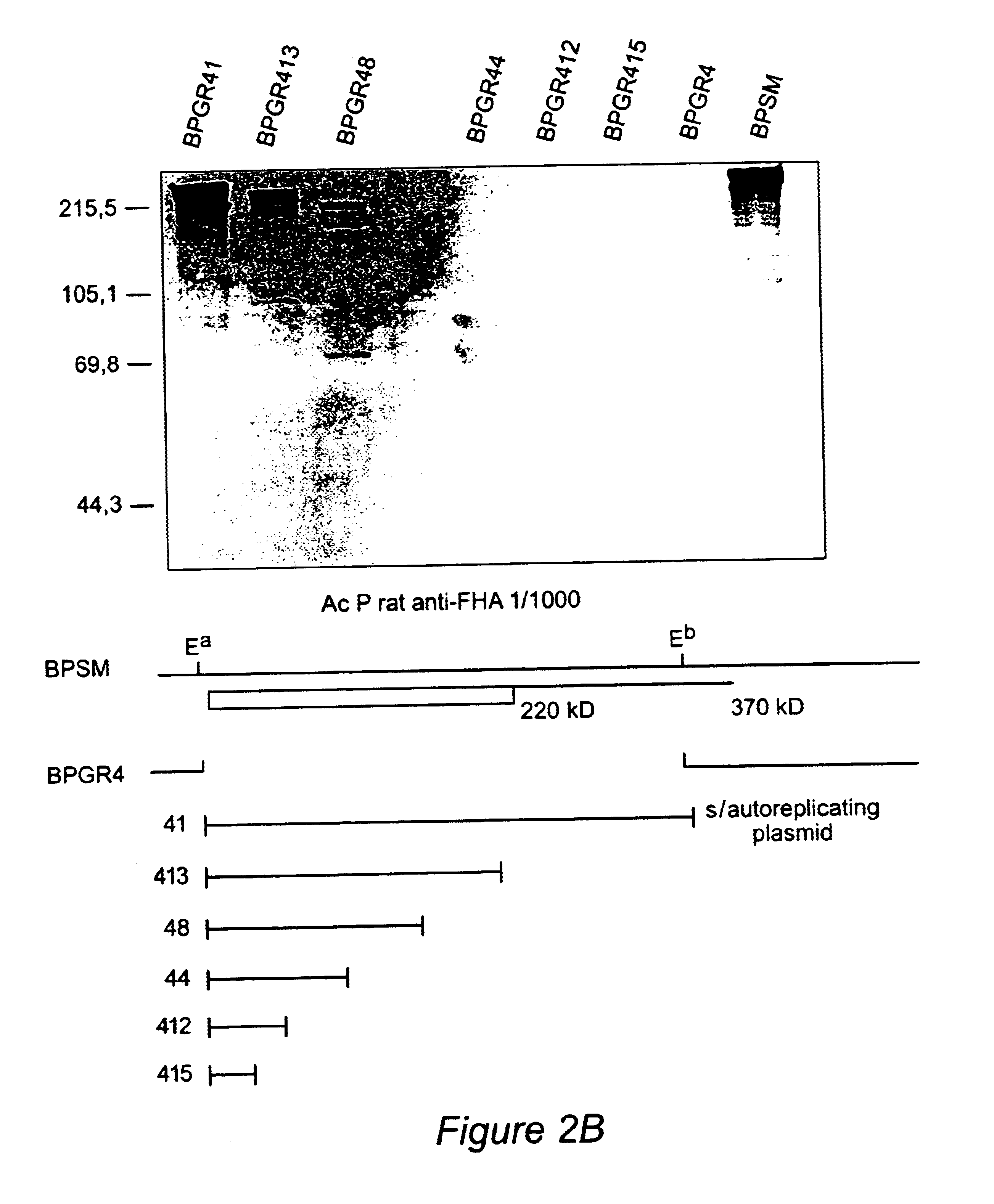
Recombinant proteins of filamentous haemagglutinin of bordetella, particularly bordetella pertussis, method for producing same, and uses thereof for producing foreign proteins of vaccinating active principles
InactiveUS6841358B1Easy to separateEasy to demonstrateBiocideBacterial antigen ingredientsHeterologousPolymerase L
A recombinant DNA containing a sequence (1) coding for a polypeptide heterologous to a filamentous haemagglutinin of Bordetella (Fha) fused within the reading frame to a sequence (2) located upstream from the first sequence. Sequence (2) codes for at least part of the Fha precursor, which part comprises at least the N-terminal region of a truncated mature Fha protein, which contains the interaction site of Fha and heparin and the secretion domain. This Fha protein is under the control of a promoter recognized by the cell polymerases of B. pertussis and is inserted into a B. pertussis cell culture, is expressed in the culture and excreted into the cell culture medium. The invention uses both the abilities of Bordetella and particularly B. pertussis to secrete or surface expose the heterologous polypeptide fused to the Fha portion corresponding to sequence (2), which does not appear to produce extracellular proteases, and the ease with which filamentous haemagglutinins can be isolated from other Bordetella proteins.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
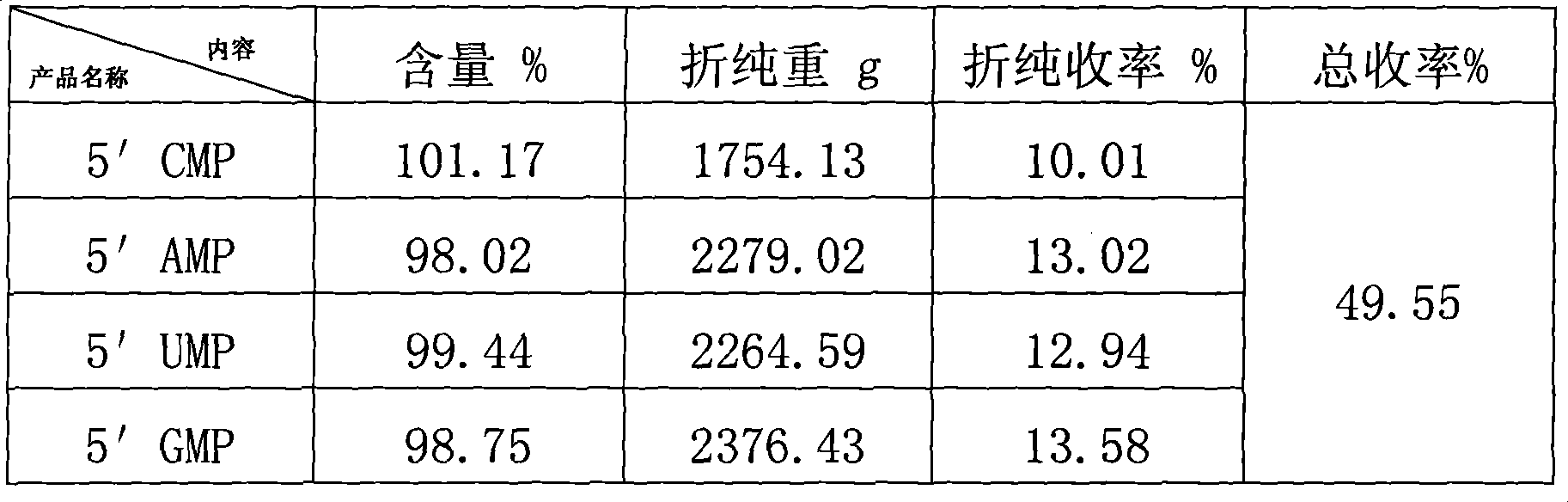
Novel production process of high-purity 5' nucleotide
ActiveCN101418327AEasy to separateIncrease loading volumeMicroorganism based processesFermentationUltrafiltrationNucleotide
The invention relates to a process for producing 5'nucleotide. A penicillium citrinum strain M-71 is cultured by submerged fermentation to generate extracellular nuclease P1, 2.5 to 5 percent ribonucleic acid solution is degraded, of which, the degradation rate is more than 80 percent; foreign protein and residual ribonucleic acid are removed by using a high frequency vibrating screen, clean 5' nucleotide solution after impurity removal is subjected to ultrafiltration by a ceramic membrane to remove the macromolecular residual matters; and four columns filled with strong alkaline anion exchange resin are connected in series to one-time adsorb and elute and collect in multiple steps, four mononucleotide solutions are collected, and a nano filter membrane with the molecular weight of 300 daltons is selected for cutting, desalting, concentration, decoloration by active carbon, crystallization and drying to obtain the product. The process has the advantages of better separating four nucleotides, removing inorganic phosphorus by elution and ensuring the preparation of high-purity 5'nucleotide product, and according to HPLC tests, the purity of the product can reach between 98 and 102 percent.
Owner:大连珍奥生物技术股份有限公司
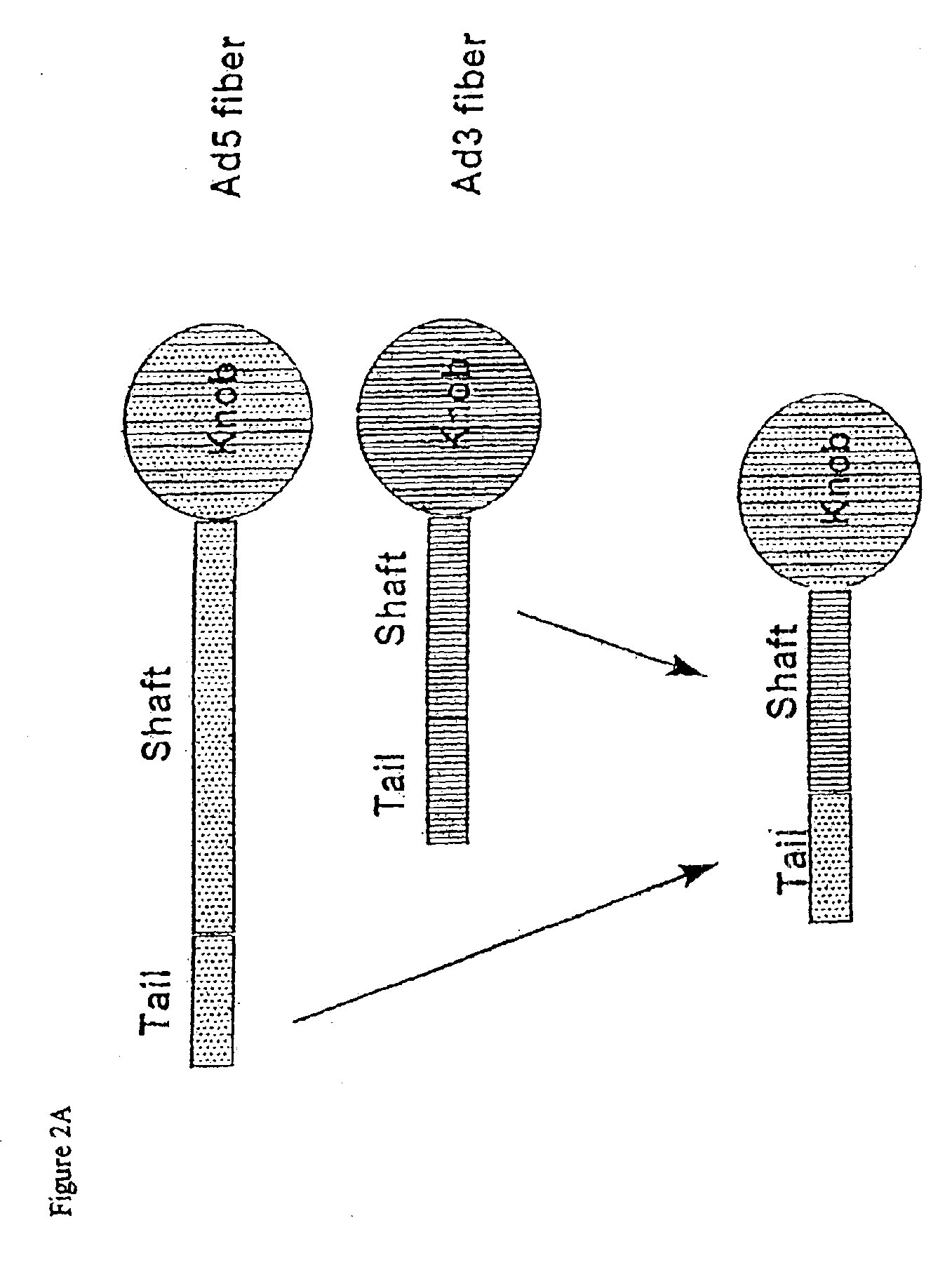
Targeted adenovirus vectors for delivery of heterologous genes
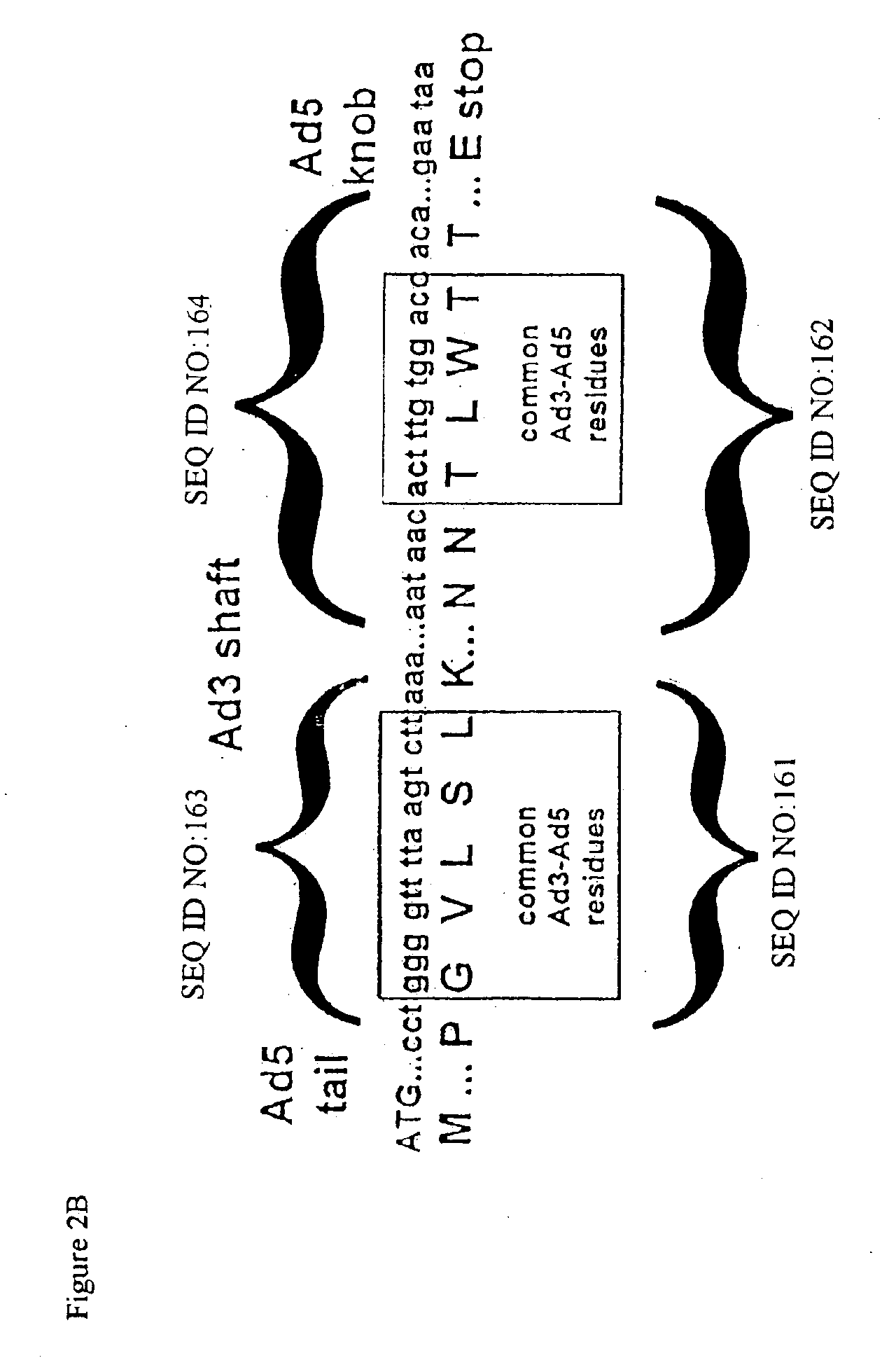
Modification of internal sites of the adenovirus fiber protein and hexon protein permit effective targeting of adenovirus vectors. Accessible sites to redirect adenovirus targeting were identified. The HVR5 loop of the hexon protein and the HI loop of the fiber protein (knob) were highly permissive for the insertion of foreign protein sequences, which apparently did not impact on the viability and productivity of corresponding viruses. Accessibility and functionality of the epitope strongly depend on the size of the neighboring spacers. Other results suggest that short targeting peptides can be effectively fused to the C-terminus of the fiber protein. In a specific embodiment, a series of adenovirus vectors modified at the HVR5 site, the fiber protein HI loop, or the fiber protein C-terminus to target urokinase-type plasminogen activator receptor bearing cells were prepared. Such vectors are particularly useful for targeting the vasculature, e.g., for gene therapy of cancers or cardiovascular conditions.
Owner:CENTELION SAS
Novel vectors for animal cells and use thereof
InactiveUS20060078992A1Decrease productivityFold preciselyMicroorganismsMicroorganism based processesBiotechnologyForeign protein
Protein synthesis inhibitor resistance genes (typified by a cycloheximide resistance gene) are capable of imparting resistance to a protein synthesis inhibitor (typified by cycloheximide) to animal cells sensitive to the inhibitor. The genes have a sequence mutated by substitution in a gene encoding a ribosome-constituting protein derived from an animal. The genes may be placed in recombinant vectors, including expression vectors containing the gene together with a foreign protein structural gene.
Owner:KIRIN HOLDINGS KK
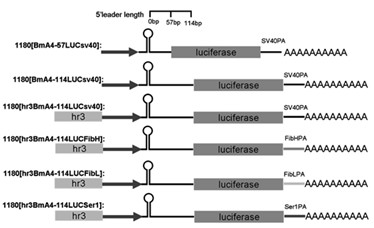
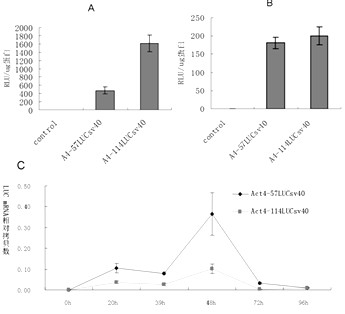
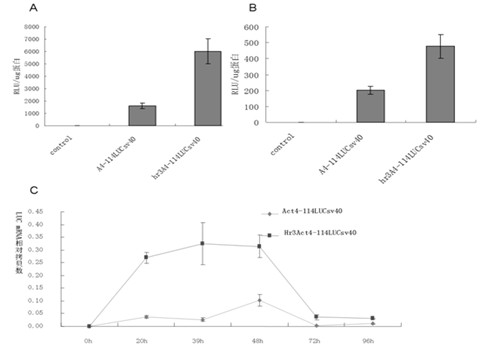
Enhancer Hr3
ActiveCN102492692AIncrease transcriptional activityImprove expression levelVector-based foreign material introductionDNA preparationRestriction sitePromoter
The invention relates to enhancer Hr3 adopting the nucleotide sequence shown in the SEQIDNO: 1. The enhancer Hr3 can be used for preparing and recombining heterologous protein by bombyx mori. The invention further relates to a recombination carrier containing the enhancer Hr3, and the recombination carrier is prepared in such a manner that the enhancer Hr3 is connected with a Nco1 restriction site at the upstream of a promoter of a carrier containing no enhancer through the Nco1 restriction site of the enhancer Hr3. In the invention, functional elements such as the first grade enhancer, activating transcription factor IE1, untranslated region sequences (5' UTR and 3' UTR) and the like which are expressed by intensifier are identified in the cellular level, and an efficient and stable sericin I expression system is built by integrating optimum elements on the basis, so that efficient expression recombination heterologous protein can be made of middle silk gland of transgenic bombyx mori.
Owner:SOUTHWEST UNIVERSITY
Genetically stable recombinant modified vaccinia ankara (rMVA) vaccines and methods of preparation thereof
A vaccine comprising an immunologically effective amount of recombinant modified vaccinia Ankara (rMVA) virus which is genetically stable after serial passage and produced by a) constructing a transfer plasmid vector comprising a modified H5 (mH5) promoter operably linked to a DNA sequence encoding a heterologous foreign protein antigen, wherein the expression of said DNA sequence is under the control of the mH5 promoter; b) generating rMVA virus by transfecting one or more plasmid vectors obtained from step a) into wild type MVA virus; c) identifying rMVA virus expressing one or more heterologous foreign protein antigens using one or more selection methods for serial passage; d) conducting serial passage; e) expanding an rMVA virus strain identified by step d); and f) purifying the rMVA viruses from step e) to form the vaccine. One embodiment is directed to a fusion cytomegalovirus (CMV) protein antigen comprising a nucleotide sequence encoding two or more antigenic portions of Immediate-Early Gene-1 or Immediate-Early Gene-2 (IEfusion), wherein the antigenic portions elicit an immune response when expressed by a vaccine.
Owner:CITY OF HOPE
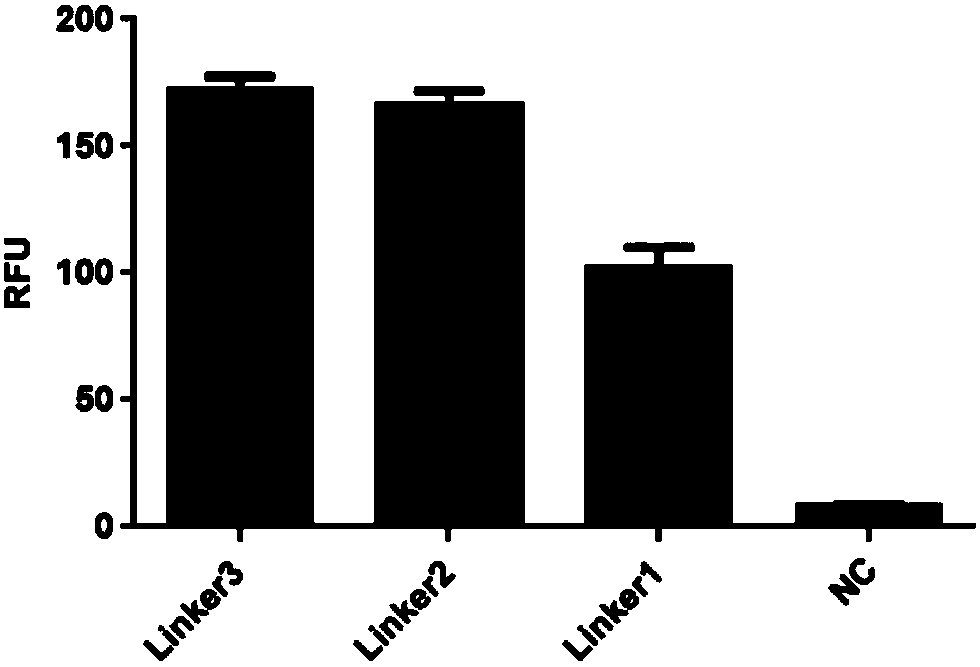
Signal peptide related sequences and application thereof in protein synthesis
InactiveCN110819647APolypeptide with localisation/targeting motifFungiForeign proteinEngineered genetic
The present invention provides signal peptide related sequences and an application thereof in protein synthesis, and specifically provides a signal peptide with a protein expression improvement effectand a coding sequence thereof, a nucleic acid construct is formed by linking the signal peptide coding sequences with foreign protein coding sequences in an operating manner, the efficiency of foreign protein synthesis can be significantly improved, and the expression and purification process of the target foreign protein is simplified. At the same time, the present invention provides a corresponding vector or a combination of the vector, a genetically engineered cell, and a kit, which can be applied in the protein synthesis.
Owner:KANGMA SHANGHAI BIOTECH LTD
Porcine circovirus type 2 recombinant cap protein and subunit vaccine
ActiveCN102174086AImprove securityNot pathogenicBacteriaViral antigen ingredientsAntigenEscherichia coli
The invention belongs to the field of molecular biology, and discloses a porcine circovirus type 2 recombinant cap protein and a subunit vaccine. The porcine circovirus type 2 cap protein expressed by recombinant Escherichia coli is obtained by steps of cloning a porcine circovirus type 2 cap protein in a nuclear localization signal area of which the N terminal is cut and which is rich in arginine into a prokaryotic expression vector to obtain a recombinant expression vector, transfecting the recombinant expression vector into Escherichia coli BL21(DE3), and expressing by using the recombinant Escherichia coli BL21(DE3). Tests prove that the constructed recombinant strain expresses a foreign protein stably. When the subunit vaccine is prepared from the expressed recombinant protein, an antigen has high purity and safety, does not have pathogenicity on animals such as pigs and the like, and passes safety evaluation easily.
Owner:NANJING AGRICULTURAL UNIVERSITY
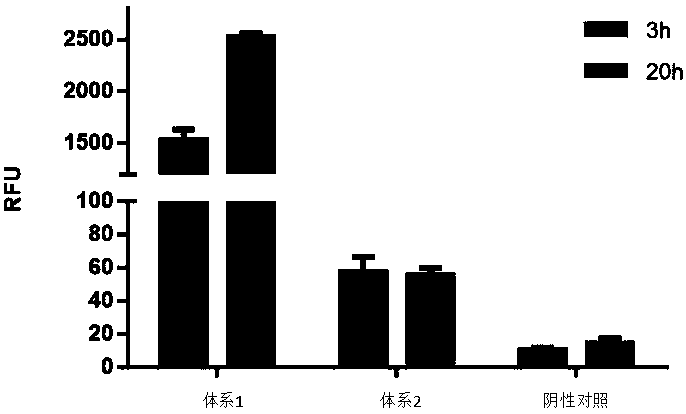
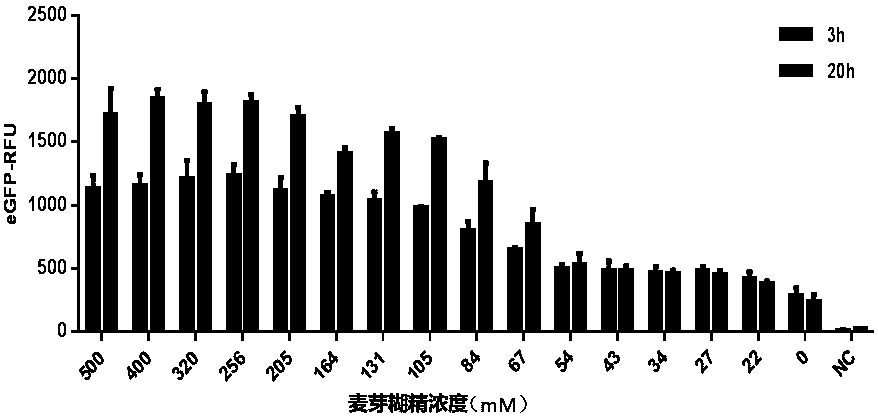
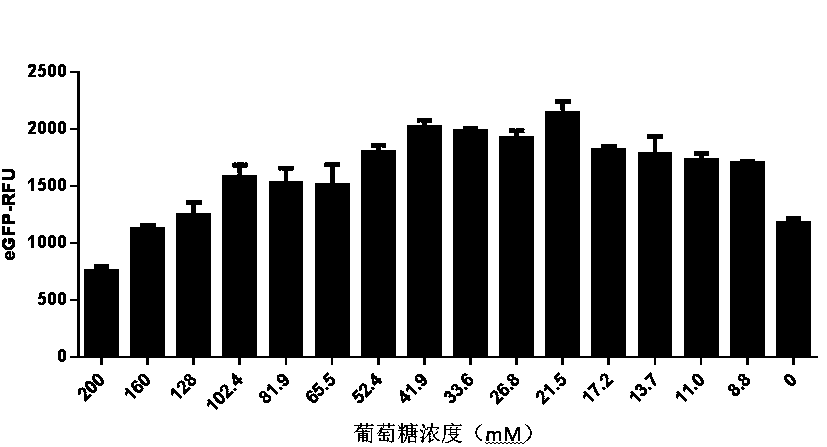
Optimized in vitro cell-free protein synthesis system and application
ActiveCN111378708AIncrease productionLow costFermentationVector-based foreign material introductionProtein targetCell free
The invention discloses an optimized in vitro cell-free protein synthesis system. The system comprises a cell extract, a carbohydrate material, a phosphate compound, a buffering agent and a DNA molecular template for encoding an exogenous protein, wherein the cell extract is a yeast cell extract inserted into a T7 RNA polymerase gene; the carbohydrate material is a mixture of glucose and maltodextrin; the buffering agent is a trihydroxymethyl aminomethane buffering agent; and the DNA molecule template is prepared by a nucleic acid isothermal amplification method, and a sequence as shown in SEQID NO.1 is inserted into the upstream of the coding sequence of the exogenous protein in the DNA molecular template. By optimizing, the cost of in vitro protein synthesis is reduced and the yield ofthe target protein is increased.
Owner:KANGMA SHANGHAI BIOTECH LTD
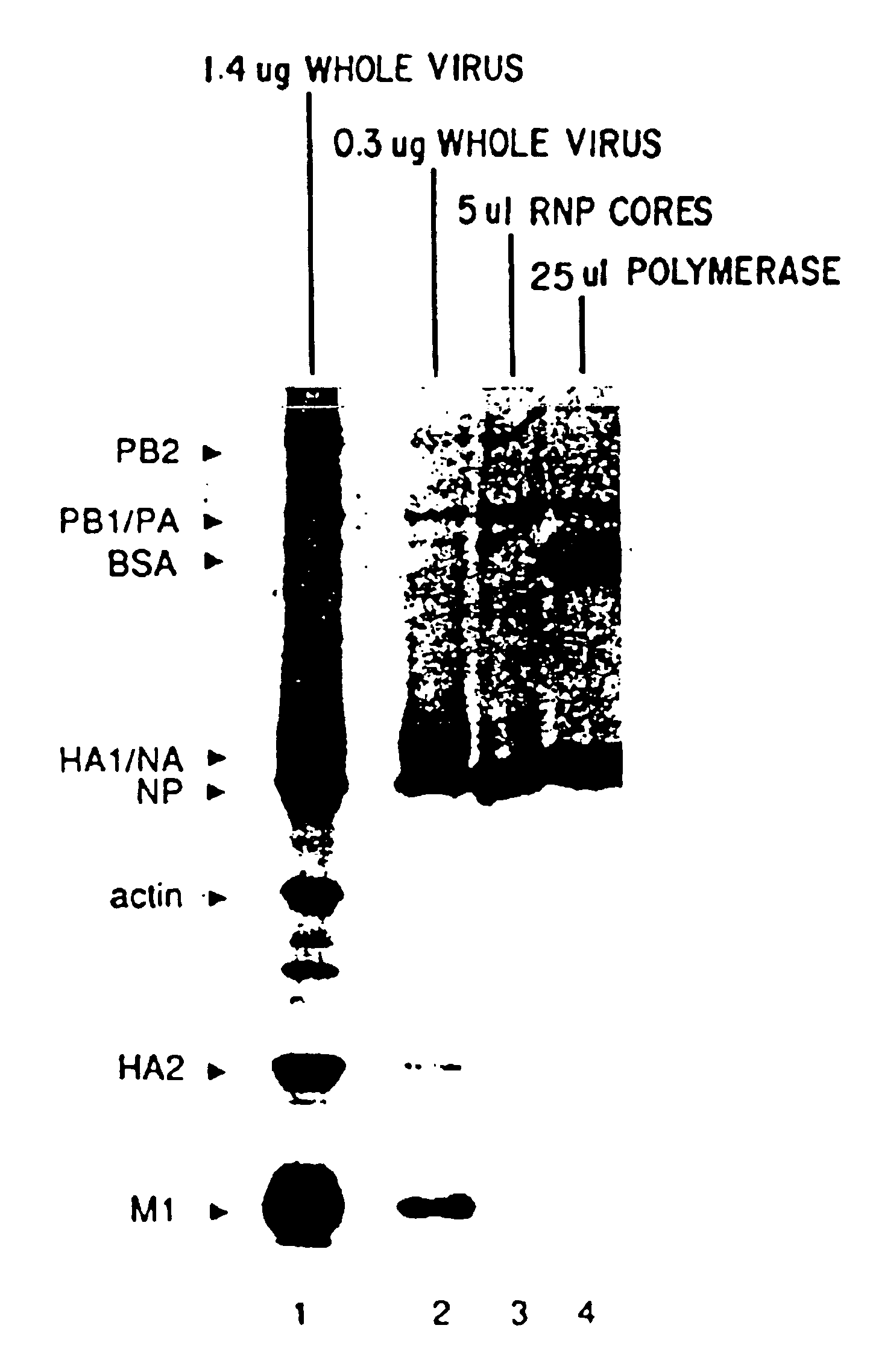
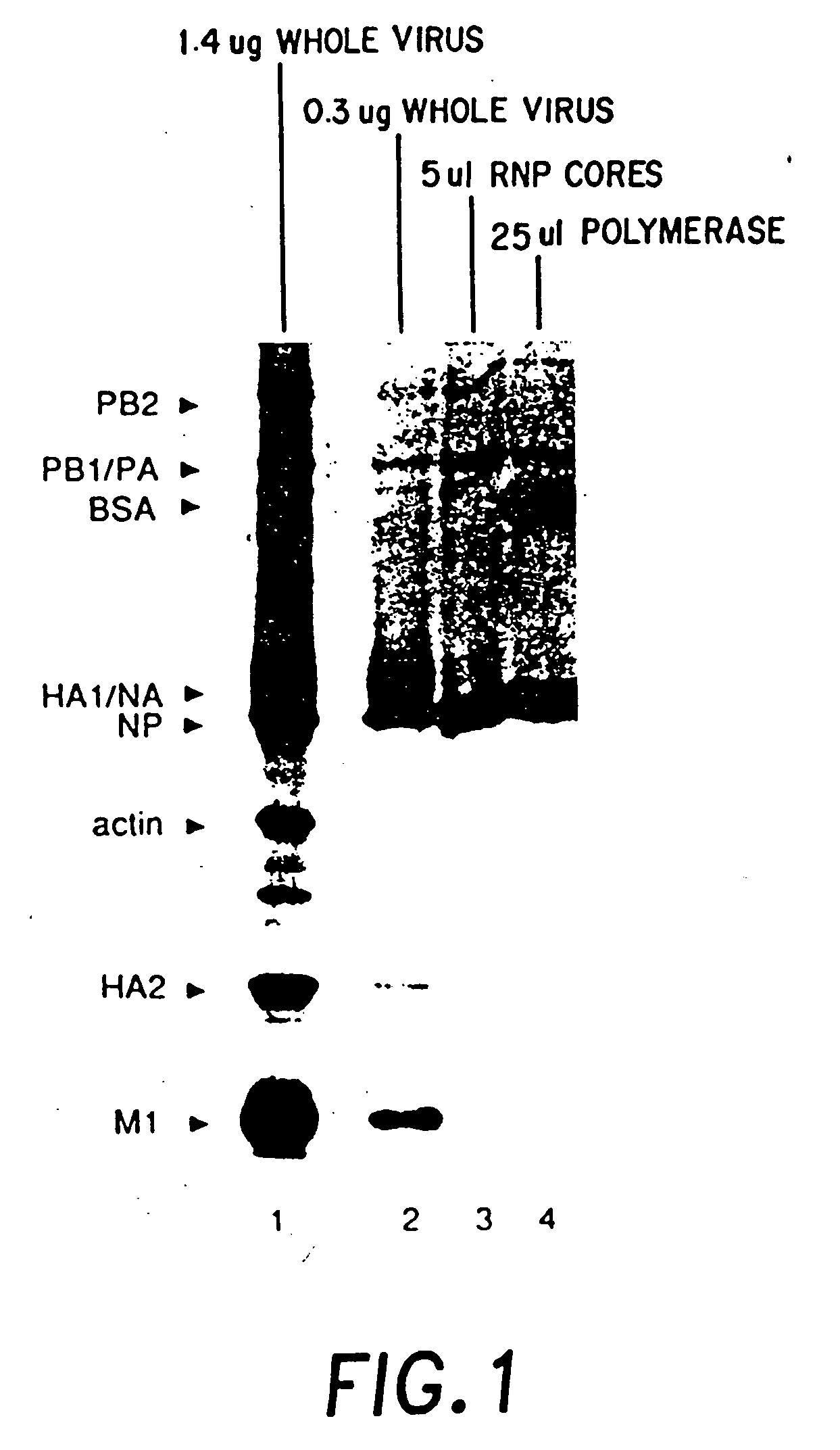
Recombinant negative strand RNA virus expression systems and vaccines
InactiveUS20050032043A1Equal efficiencySsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
Recombinant negative-strand viral RNA templates are described which may be used with purified RNA-directed RNA polymerase complex to express heterologous gene products in appropriate host cells and / or to rescue the heterologous gene in virus particles. The RNA templates are prepared by transcription of appropriate DNA sequences with a DNA-directed RNA polymerase. The resulting RNA templates are of the negative-polarity and contain appropriate terminal sequences which enable the viral RNA-synthesizing apparatus to recognize the template. Bicistronic mRNAs can be constructed to permit internal initiation of translation of viral sequences and allow for the expression of foreign protein coding sequences from the regular terminal initiation site, or vice versa.
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com