ADH (ethanol dehydrogenase) protein family mutant and application thereof
A technology of mutant protein and carrier, applied in the biological field, can solve problems such as interference of target protein purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1K
[0260] Example 1 K. lactis middle ADH Analysis and specific modification of family genes
[0261] 1.1 K. lactis middle ADH Analysis of family genes
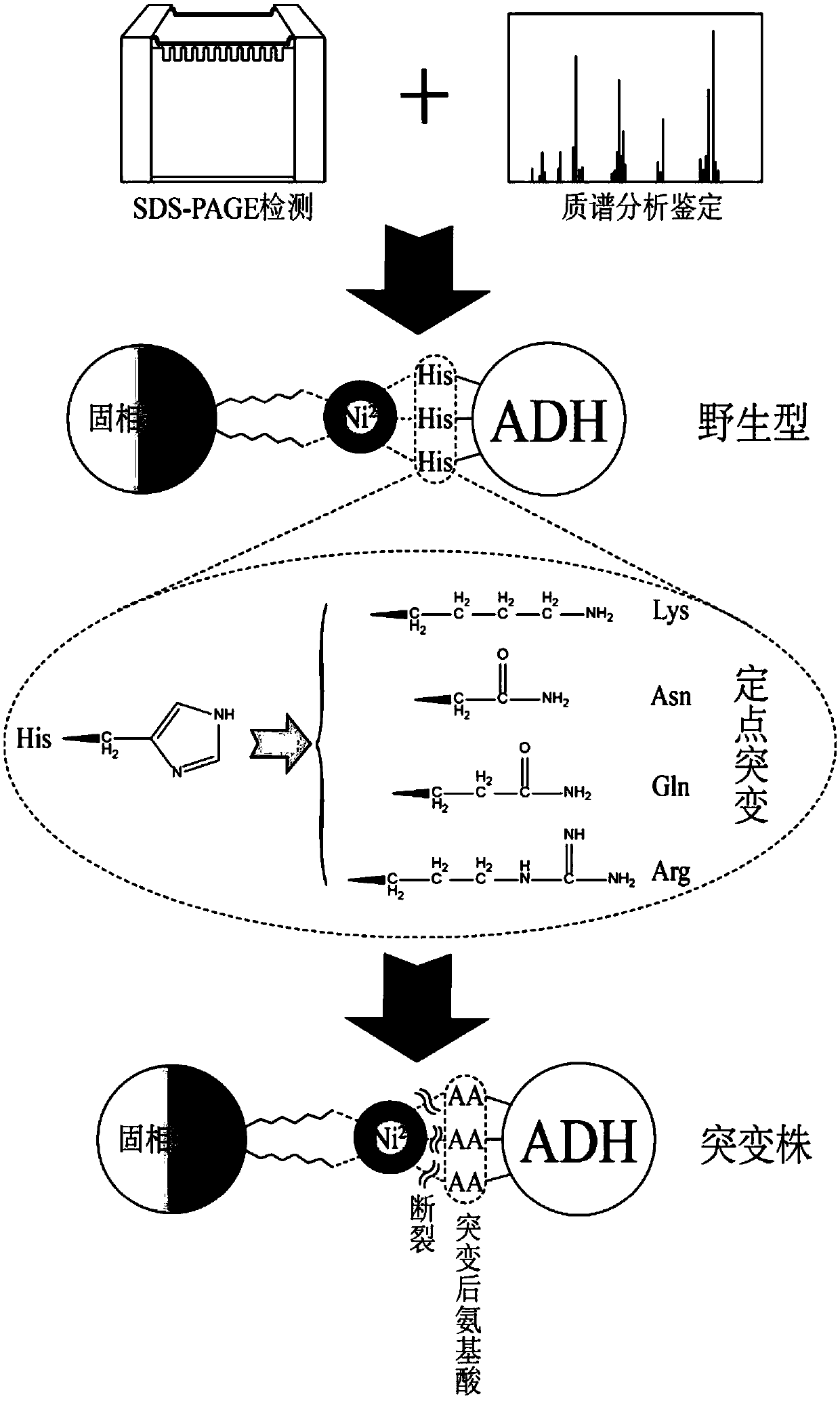
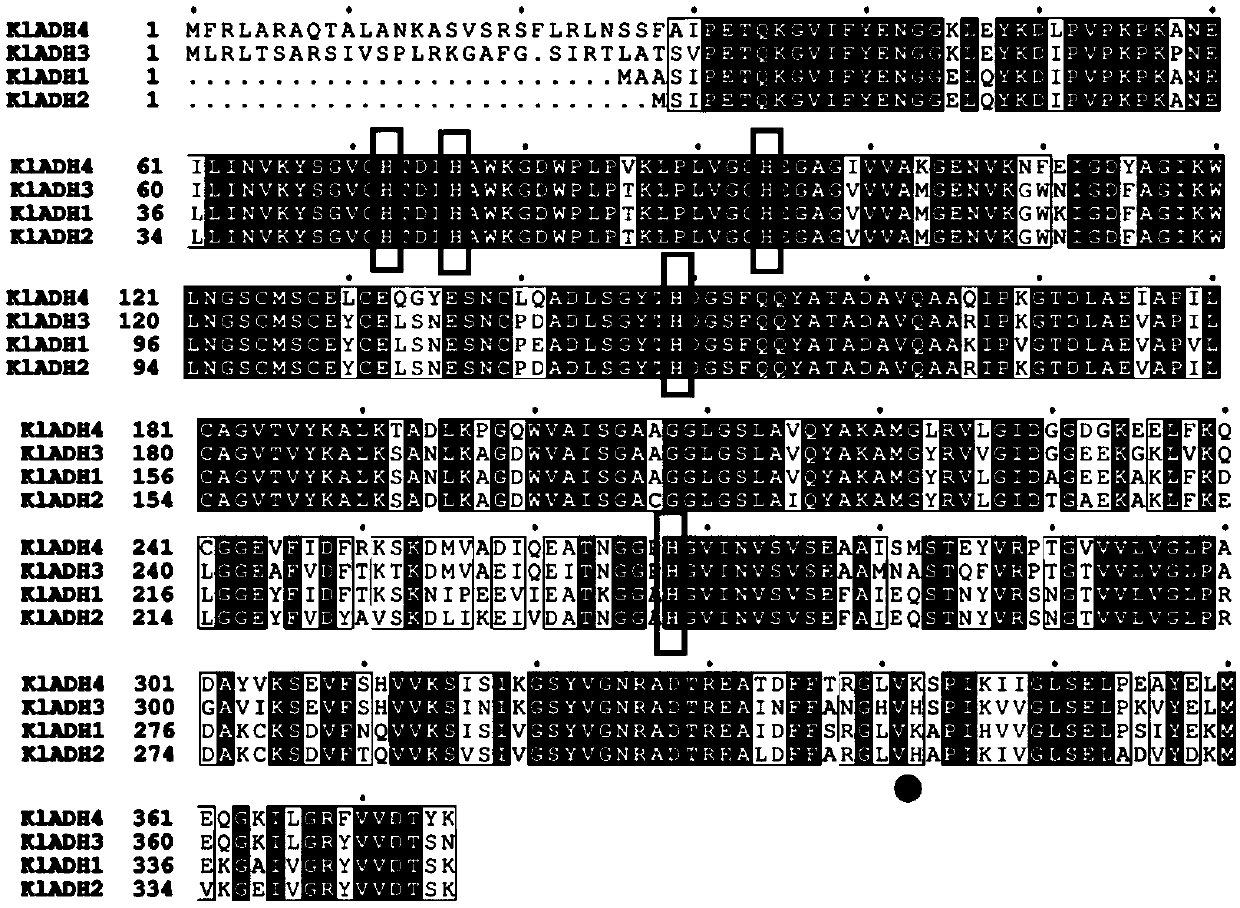
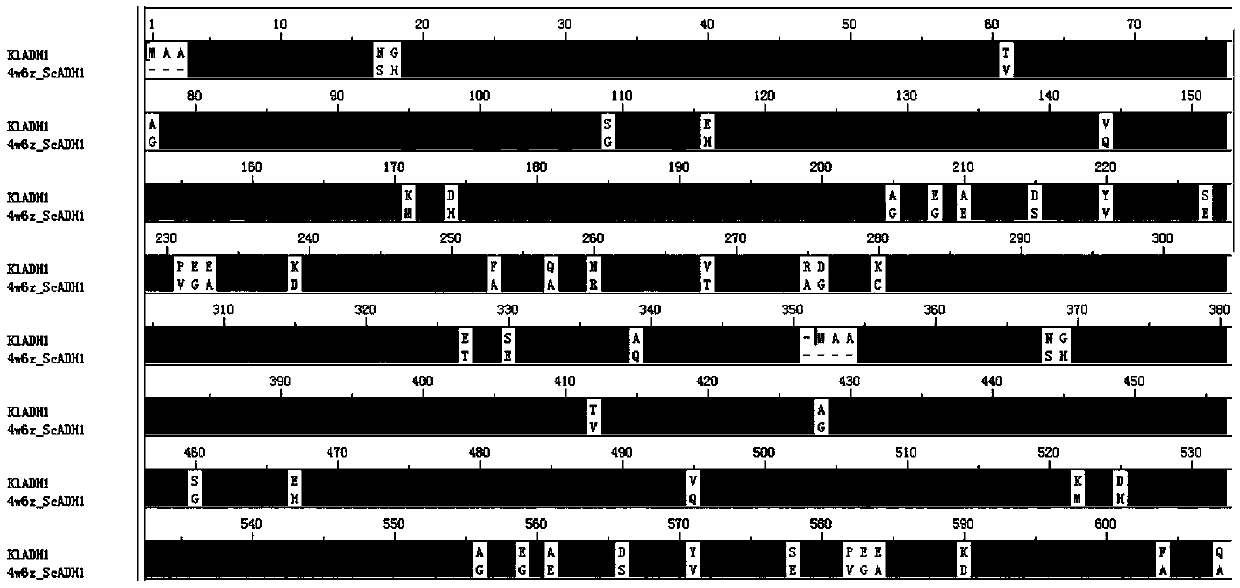
[0262] When Ni medium is used for K. lactis When the target protein containing the His tag expressed in the affinity purification, there is an obvious non-specific band at 35-50kDa. The results of mass spectrometry showed that the non-specific protein was mainly derived from K. lactis ADH family proteins in (Table 1). ADH is a NAD(P)-dependent oxidoreductase present in almost all organisms that catalyzes the reversible oxidation of primary and secondary alcohols to aldehydes and ketones, respectively. K. lactis ADH family proteins in include Kl ADH1, Kl ADH2, Kl ADH3, Kl There are four types of ADH4, all encoded by chromosomal DNA. Wherein, ADH1 gene sequence (SEQ ID NO.164), KEGG database coding is Kl LA0F21010g, located on F chromosome; ADH2 gene sequence (SEQ ID NO.29), KEGG database code is Kl LA0F18260g, lo...
Embodiment 2
[0273] Example 2 Targeted knockout by CRISPR / Cas9 ADH1 Gene
[0274] 2.1 Kl ADH1 CRISPR gRNA sequence determination
[0275] According to Kl ADH1 designed point mutation, select PAM sequence (NGG), and determine the corresponding gRNA sequence. The principle of gRNA selection in this example is: moderate GC content (40%-60%), and avoid the existence of poly T structure. In this example, KlADH1 The gRNA1 sequence is TGGGTGAAAACGTCAAGGGC (SEQ ID NO.: 45).
[0276] Plasmid construction and transformation methods are as follows: use primer pKMCas9- Kl ADH1-gRNA1-PF: TGGGTGAAAACGTCAAGGGCGTTTTAGAGCTAGAAATAGC (SEQ ID NO.: 46) and pCas9- Kl ADH1-gRNA1-PR: GCCCTTGACGTTTTCACCCAAAAGTCCCATTCGCCACCCG (SEQ ID NO.: 47), using the pCAS plasmid as a template for PCR amplification. Take 17 µL of the amplification product, add 1 µL of Dpn I, 2 µL of 10 × digestion buffer, mix well for 37 o C warm bath for 3 h. Take 10 µL of Dpn I-treated product and add it to 50 µL DH5α competent cel...
Embodiment 3
[0288] Example 3 Site-directed mutation of ADH1 H47 site by CRISPR / Cas9
[0289] 3.1 Kl ADH1 CRISPR gRNA sequence determination
[0290] According to Kl ADH1 designed point mutation, select PAM sequence (NGG), and determine the corresponding gRNA sequence. The principle of gRNA selection in this example is: close to the designed mutation site, moderate GC content (40%-60%), and avoid the existence of poly T structure. In this example, KlADH1 The gRNA1 sequence is TGGGTGAAAACGTCAAGGGC (SEQ ID NO.:56).
[0291] Plasmid construction and transformation methods are as follows: use primer pCas9- Kl ADH1-gRNA1-PF: TGGGTGAAAACGTCAAGGGCGTTTTAGAGCTAGAAATAGC (SEQ ID NO.:57) and pCas9- Kl ADH1-gRNA1-PR: GCCCTTGACGTTTTCACCCAAAAGTCCCATTCGCCACCCG (SEQ ID NO.: 58), using the pCAS plasmid as a template for PCR amplification. Take 17 µL of the amplification product, add 1 µL of Dpn I, 2 µL of 10 × digestion buffer, mix well for 37 o C warm bath for 3 h. Take 10 µL of Dpn I-treated pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com