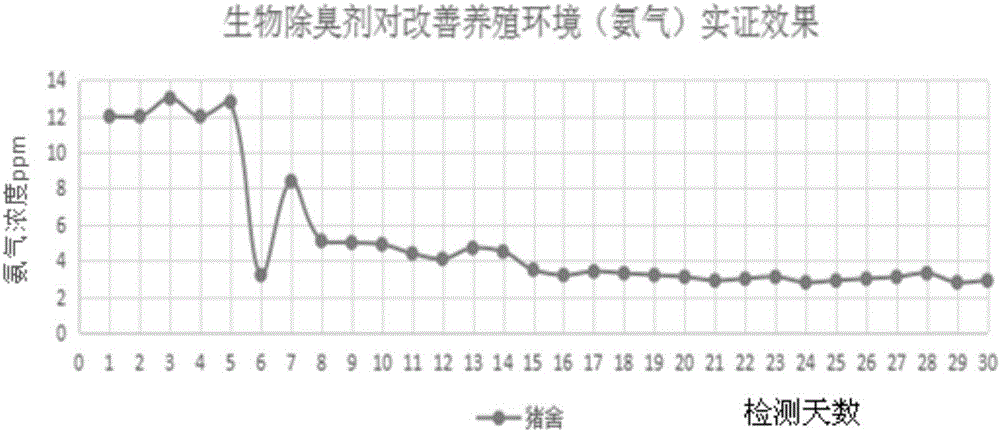
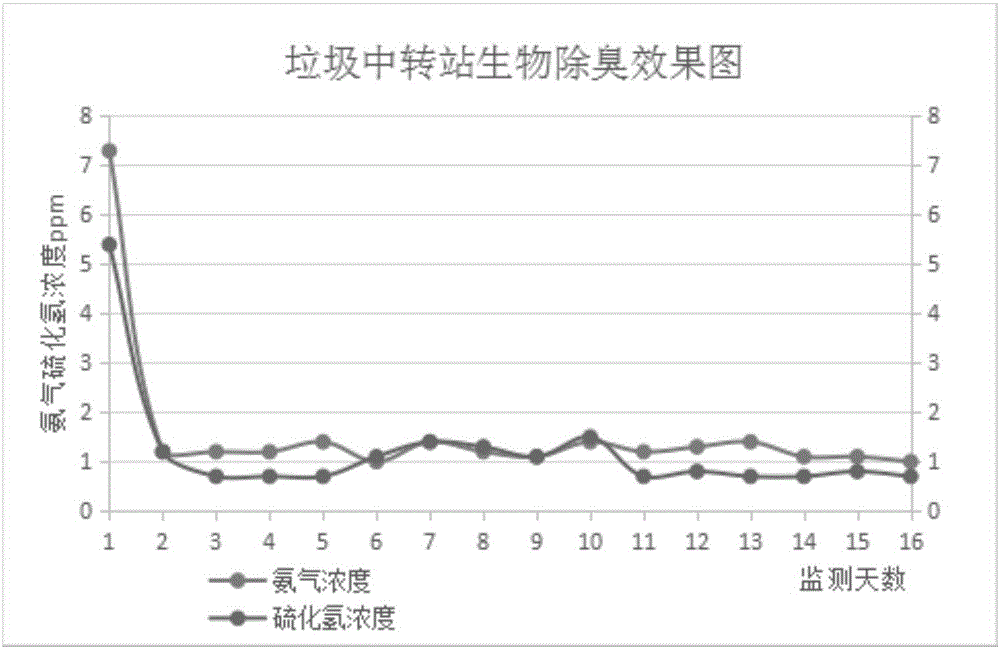
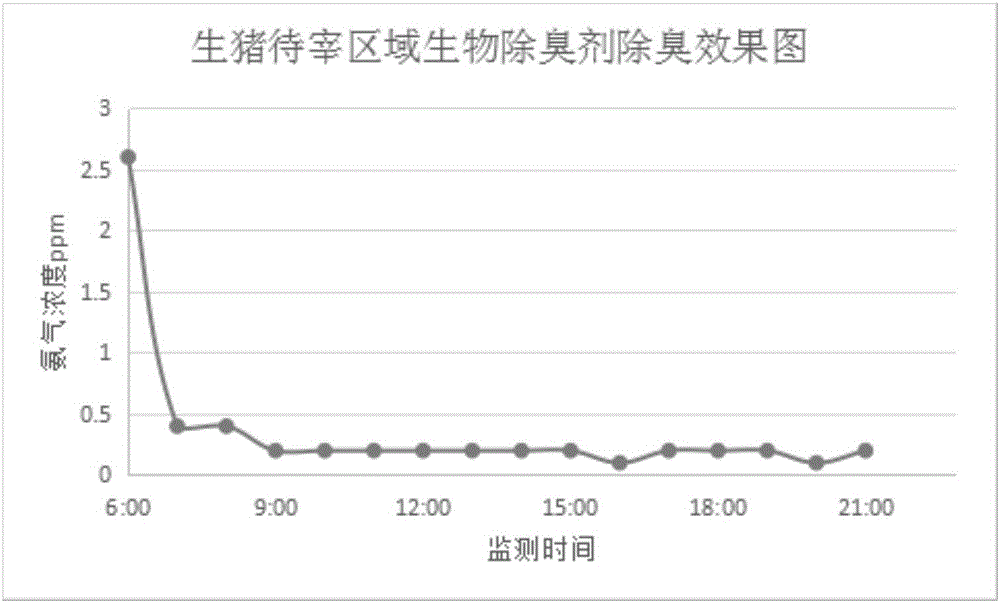
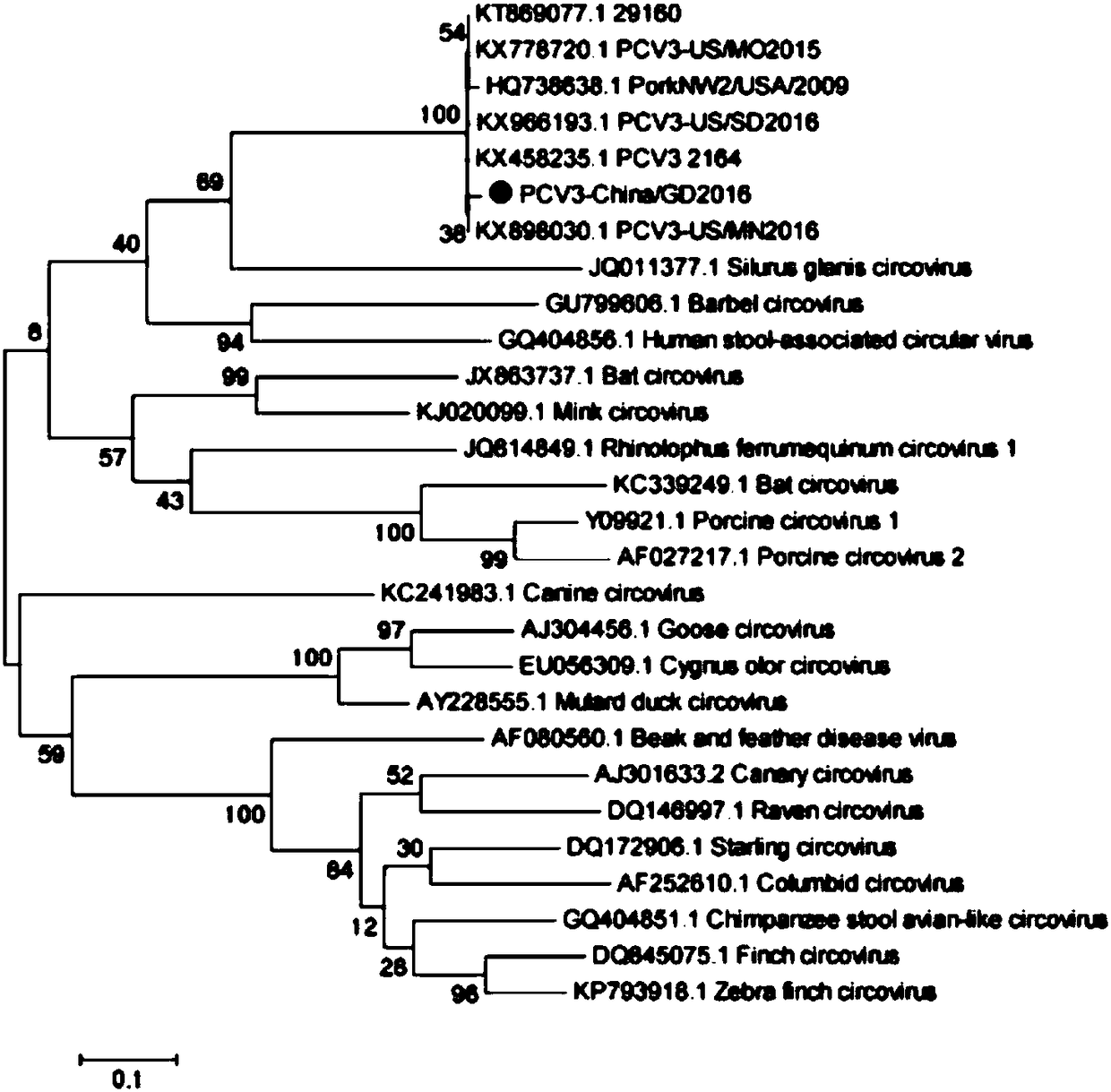
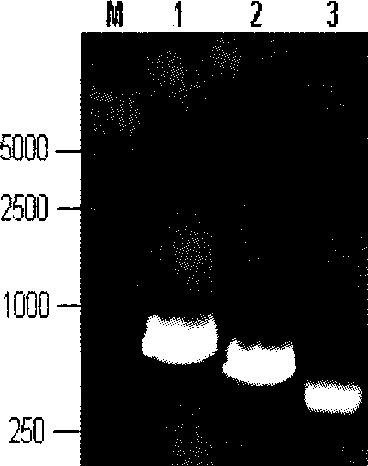Patents
Literature
94results about How to "Not pathogenic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof
ActiveCN106946995AHigh antigen contentImprove securitySsRNA viruses negative-senseViral antigen ingredientsDiseaseInclusion bodies
A fowl adenovirus group I serum type 4 genetic engineering subunit vaccine, and a preparing method and applications thereof are disclosed. The sequence of an antigen protein in the vaccine is shown as SEQ ID NO:1. The antigen protein has advantages of high safety, high immunity, no pathogenicity for chickens or other animals, and the like. The subunit vaccine can prevent chicken hydropericardium syndrome, inclusion body hepatitis and other diseases which are caused by infection of fowl adenovirus group I serum type 4.
Owner:苏州沃美生物有限公司
Asia1 type foot-and-mouth disease recombinant virus and preparation method and application thereof
The invention relates to an Asia1 type foot-and-mouth disease recombinant virus without pathogenicity for a host and a preparation method and application thereof. A saving system is efficient eukaryotic plasmids which are constructed by gene engineering and can express exact foot-and-mouth disease virus genome RNA (Ribonucleic Acid), and therefore the foot-and-mouth disease recombinant virus can be constructed and prepared; vaccine strains with high titer and good antigen matching property can be prepared by using the plasmids, can be prepared into live vaccines or inactivated vaccines and can effectively stimulate bodies to produce immune response after being used for immunizing pigs and cattle, provide an immune protective effect on the pigs and the cattle and effectively protect GV and GII prevalent strains, the immune protection rate can reach 100 percent, and the median protective dose (PD50) is 6.34 to 13.59; and the recombinant virus has the advantages of high titer, high antigen matching property with the prevalent strains, wide antigen spectrum and high immune protection rate, does not have pathogenicity for pig and cattle hosts, does not form toxemia or expel toxin, and can be applied to prevention and control of Asia1 type foot-and-mouth disease viruses of China and neighboring countries.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Porcine reproductive and respiratory syndrome bivalence recombinant adenovirus vaccine and preparation method thereof
InactiveCN101380468AImmediately exert cellular immune functionNot pathogenicViral antigen ingredientsAntiviralsEukaryotic plasmidsAttenuated vaccine
The invention discloses a porcine reproductive and respiratory syndrome divalent recombination adenovirus vaccine and the preparation method thereof. The invention belongs to the technical field of biological vaccine preparation. The vaccine can be prepared by the following steps: a GP5-2A-M fusion protein gene can be constructed by inserting an FMDV2A gene with self craking between PRRSV GP5 and M protein; homologous recombination is carried out on the GP5-2A-M fusion protein gene and adenovirus backbone plasmid pAdEasy-1; recombination adenovirus rAd-GP5-2A-M is prepared by restriction enzyme and HEK-293A cells transfection, and the divalent recombination adenovirus vaccine is prepared by the technology and the steps such as purification, amplification, and the like. After expression, the aggregate protein GP5-2A-M constructed by the invention is self cracked into GP5 and M protein, as well as exerts the viral neutralization of GP5 and the immune function of the M protein; the vaccine has stable titer with the virulent valence being 10<10.43>TCID<50> / 1.0ml, as well as has both the duplication characteristic of a routine attenuated vaccine and the safety of an inactivated vaccine; the divalent recombination adenovirus vaccine can be popularized in and applied to the control work of porcine reproductive and respiratory syndrome.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Composite efficient microbial deodorizer and preparation method thereof
The invention belongs to the technical field of microorganisms, and particularly relates to a composite efficient microbial deodorizer and a preparation method thereof. The microbial deodorizer comprises candida utilis, bacillus subtilis, bacillus licheniformis, bacillus coagulans and enterococcus lactobacilli, viable count is 5.3*10<9>-8.3*10<9>cfu / g, viable count of the candida utilis is 1.8*10<8>-3.6*10<8>cfu / g, viable count of the bacillus subtilis is 3.4*10<8>-4.2*10<8>cfu / g, viable count of the bacillus licheniformis is 3.7*10<8>-7.4*10<8>cfu / g, viable count of the bacillus coagulans is 2.4*10<8>-3.6*10<8>cfu / g, and viable count of the enterococcus lactobacilli is 4.2*10<9>-6.4*10<9>cfu / g. The microbial deodorizer has strange deodorization effects and can rapidly and effectively remove odorous gas of pollution sources.
Owner:湖北凌卓生物工程有限公司
Genetic engineering subunit vaccine for porcine circovirus as well as preparation method and application of genetic engineering subunit vaccine
PendingCN108619503AReduce virus contentHigh antigen purityViral antigen ingredientsAntiviralsSolubilityEscherichia coli
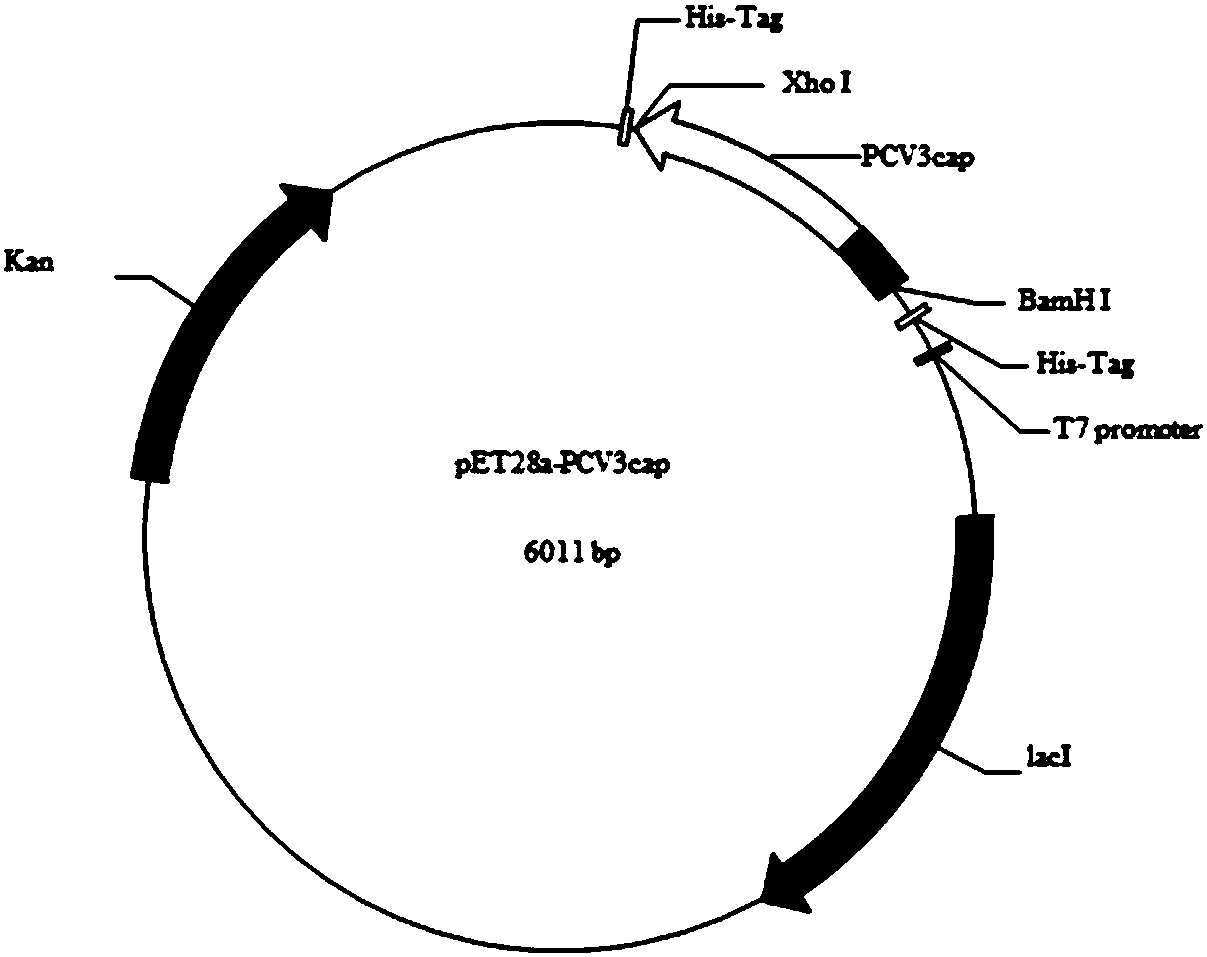
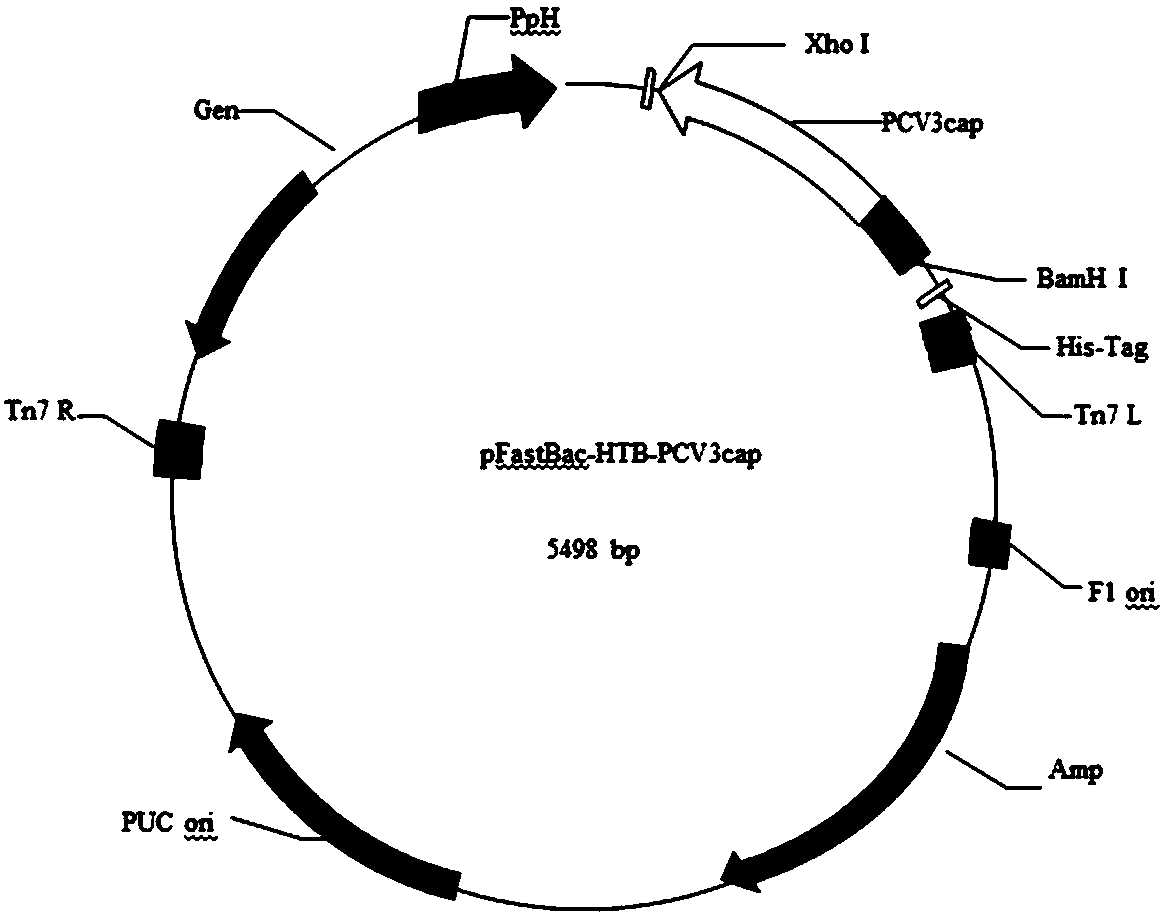
The invention discloses a genetic engineering subunit vaccine for a porcine circovirus as well as a preparation method and application of the genetic engineering subunit vaccine. By cloning the nucleocapsid protein of the novel porcine circovirus 3 (PCV3), a PCV-Cap protein with higher purity is successfully expressed by using an escherichia coli or baculovirus expression system. The subunit vaccine for the PCV3 is successfully developed for the first time by using the PCV-Cap protein; the prepared vaccine is high in antigen purity, good in safety and strong in immunogenicity, and has no pathogenicity to pigs and other animals; the antigen has good solubility in a neutral PH buffer solution; furthermore, the preparation method is simple and low in cost, thus being suitable for large-scaleindustrial production; an effective and powerful means is provided for the prevention and control of novel PCV, and the genetic engineering subunit vaccine has a wide application prospect in the fieldof the prevention and control of the PCV3.
Owner:SOUTH CHINA AGRI UNIV
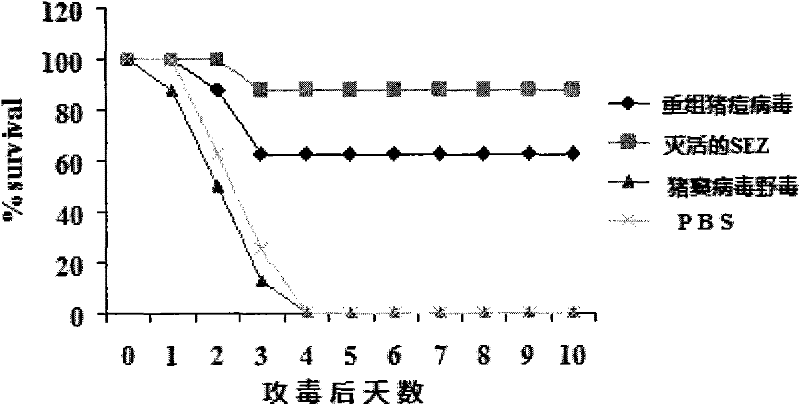
Recombinant Swine pox virus (SPV) vector vaccine for the expression of Streptococcus equi subsp zooepidemicus (SEZ) M-like protein (SzP)
InactiveCN102198268ANot pathogenicEasy Security EvaluationAntibacterial agentsBacterial antigen ingredientsProtein targetAdjuvant
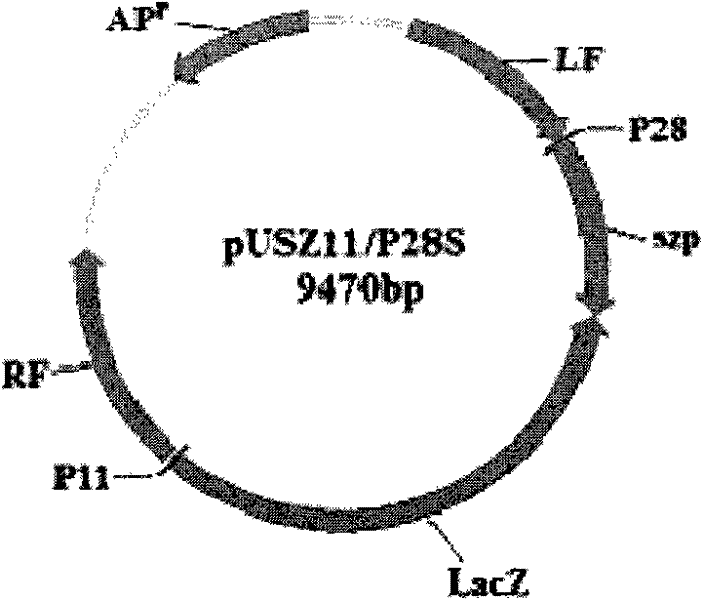
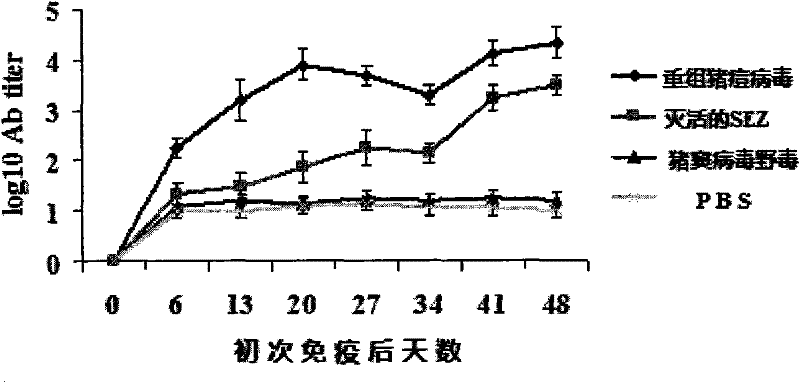
The invention belongs to the field of biological pharmacy. The invention provides a recombinant vaccine, comprising a SPV and one pharmaceutically acceptable vector and / or adjuvant or a plurality of such vectors and / or adjuvants. The SPV comprises an SPV vector and the encoding genes of SzP. The recombinant SPV vaccine provided in the invention can proliferate in large quantities in immune animalbodies, express target protein SzP and induce the generation of high titer antibodies in animal bodies, and exerts a good protective effect on immune animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
Novel genetic engineering vaccine of porcine Seneca virus as well as preparation method and application of novel genetic engineering vaccine
ActiveCN110279855AEasy to assembleGood antigenicitySsRNA viruses positive-senseVirus peptidesVaccine ProductionImmunogenicity
The invention discloses an immunological composition which comprises porcine Seneca virus structural protein VP3 and VP1 proteins, as well as porcine Seneca virus structural protein VP2 and / or VP4 protein. Further, the immunological composition can further comprise a porcine Seneca virus structural protein VP0. The immunological composition can be used for preparing a novel genetic engineering subunit vaccine of porcine Seneca virus, the antigenicity, immunogenicity and function of the vaccine are similar to those of natural proteins, the expression level is relatively high, the immunogenicity is strong, and no pathogenicity is caused to animals; the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, thereby greatly reducing the cost of vaccine production.
Owner:苏州世诺生物技术有限公司
Virus-like particle vaccine for PCV2 (porcine circovirus 2) as well as preparation method and application of virus-like particle vaccine
InactiveCN105999255AComplete epitopeImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliPorcine circovirus
The invention discloses a virus-like particle vaccine for PCV2 (porcine circovirus 2) as well as a preparation method and an application of the virus-like particle vaccine, and belongs to the technical field of bio-pharmaceuticals. The virus-like particle vaccine comprises soluble protein which is obtained through expression of a PCV2 complete capsid protein gene in escherichia coli, wherein the capsid protein gene is screened from capsid protein genes of domestic PCV2 prevalent strains at present and is subjected to codon optimization, therefore, the capsid protein gene can be expressed substantively in the escherichia coli, and the expressed soluble protein can form virus-like particles. The PCV2 subunit vaccine prepared with the method has characteristics of high antigen purity, high immunogenicity and the like; besides, the vaccine preparation process is simple, the production cost is low, and quite good application prospect is realized.
Owner:SOUTH CHINA AGRI UNIV
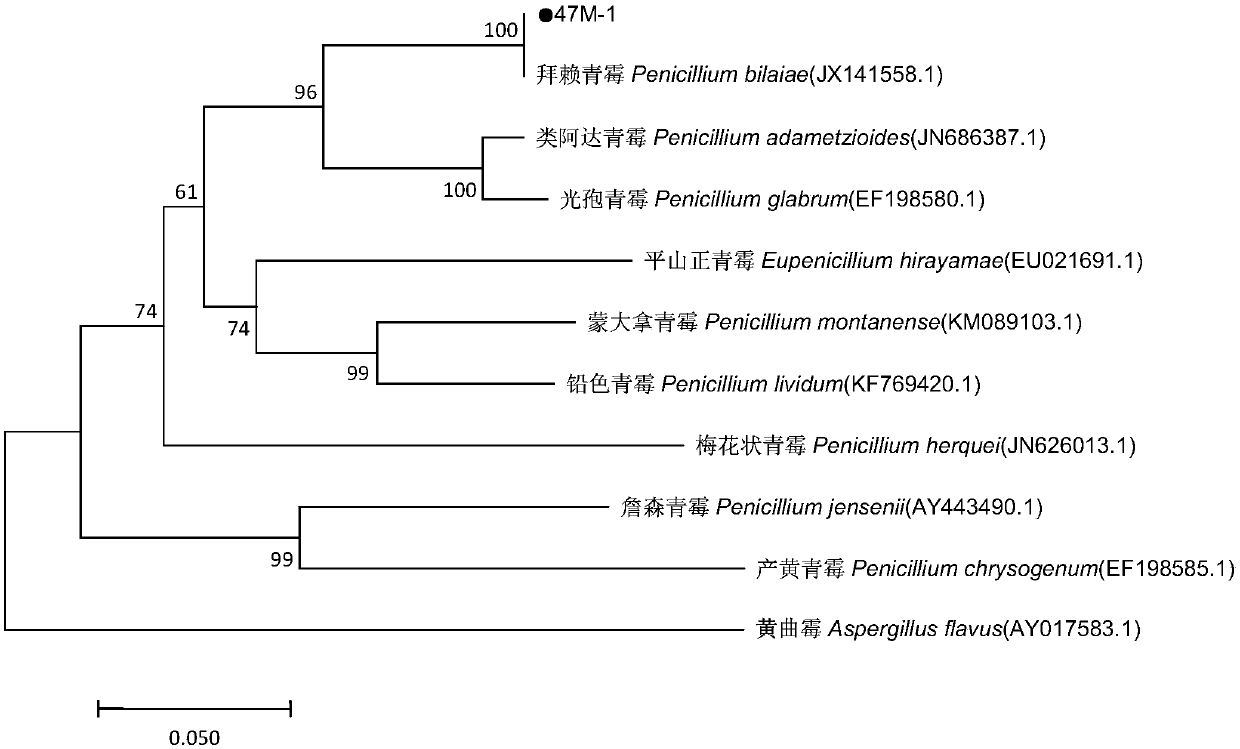
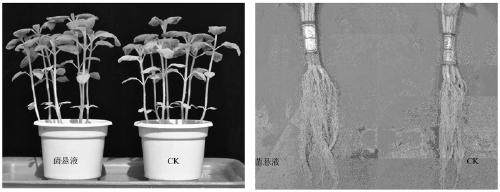
Penicillium bilaiae capable of preventing sesame blight and promoting and guiding resistance and screening method and application thereof
ActiveCN109628328AReduce morbidityPromote growthBiocidePlant growth regulatorsBiotechnologyPesticide residue
The invention discloses a penicillium bilaiae capable of preventing sesame blight and promoting and guiding resistance and a screening method and application thereof. The penicillium bilaiae is penicillium bilaiae 47M-1 (Penicillium bilaiae) which is preserved in the China Center for Type Culture Collection, the preservation address is Wuhan University center for Collection, the preservation codeis CTCC M: 2018899, and the preservation data is December 17 , 2018. a strain has strong inhibition effect on sesame fusarium oxysporum, broad-spectrum bacterial inhibiting activity on pathogenicbacteria of other primary diseases of sesame is achieved, the growth of crops can be promoted to guide the crops to generate resistance, the penicillium bilaiae has no pathogenicity on the crops, common problems of chemical pesticides of the resistance to drugs, pesticide residues and environmental pollution are prevented, the penicillium bilaiae is healthy towards human and livestock and friendlyto the environment, and the demands on ecological agriculture of people are conformed.
Owner:INST OF PLANT PROTECTION HENAN ACAD OF AGRI SCI
Soluble recombinant protein and expression and purification methods and application thereof
InactiveCN107034226ASimple and efficient operationHigh purityBacteriaViral antigen ingredientsSalting outPorcine circovirus
The invention provides a soluble recombinant protein. The soluble recombinant protein is obtained through prokaryotic expression of a gene of a porcine circovirus II type capsid protein. The amino acid sequence of the porcine circovirus II type capsid protein is shown in SEQ ID NO:1. During prokaryotic expression of the Cap protein, a gene for expressing the Cap protein is optimized through a codon, so that soluble expression of the Cap protein in escherichia coli can be improved remarkably. Target protein is purified with a two-step gradient salting-out precipitation method, operation is easy, purity is improved greatly, the production cost is low, the immunogenicity of the obtained protein is high, and large-scale industrial production can be achieved. A PCV2 virus-like particle vaccine does not contain viral nucleic acid and has high safety. The prepared PCV2 virus-like particle vaccine has no pathogenicity to experiment animals, and after animal immunization, a porcine circovirus antibody can be generated quickly at a high level, and lasting time is long.
Owner:SOUTH CHINA AGRI UNIV
Preparation method of microbial fertilizer
InactiveCN105819921APromote growthImprove fertilityMagnesium fertilisersAnimal corpse fertilisersCitrate sodiumFertilizer
A preparation method of a microbial fertilizer is characterized in that the microbial fertilizer is prepared through mixing 400-800kg of charcoal, 10-20kg of potassium fulvate, 30-60kg of a mixed bacterial liquid, 80-105kg of cow hair powder, 10-25kg of Chinese chestnut barbed shell particles, 10-20kg of banana skins, 10-20kg of sweet potato skins, 2-3kg of walnut skins, 10-20kg of bentonite, 5-6kg of sodium citrate and 3-4kg of magnesium stearate. The fertilizer prepared in the invention has a good yield increasing effect.
Owner:SHENZHEN XIANKANGDA BIOTECH CO LTD
Porcine circovirus type II gene engineering subunit vaccine, and preparation method and application thereof
ActiveCN103204942AAntigen highHigh purityBacteriaViral antigen ingredientsSite-directed mutagenesisImmunogenicity
The invention discloses a porcine circovirus type II gene engineering subunit vaccine, and a preparation method and application thereof. The porcine circovirus type II vaccine comprises a soluble fusion protein composed of a protein obtained by expressing a porcine circovirus type II capsid protein gene by Escherichia coli; and the porcine circovirus type II capsid protein gene is subjected to positioning signal zone shear and site-directed mutagenesis, and the site-directed mutagenesis comprises mutation of codon AGA or AGG to CGC. A porcine circovirus type II vaccine prepared by the technical scheme provided by the invention has high antigen purity, good safety, strong immunogenicity, and no pathogenicity to animals like pig. Furthermore, the vaccine antigen in the invention is expressed by Escherichia coli, so a preparation process is relatively simple and low-cost.
Owner:YEBIO BIOENG OF QINGDAO
Two endophytic fungi strains having control effect on soft rot disease of dendrobium plants
The invention discloses two endophytic fungi strains which have biological control effect on soft rot disease of orchid family dendrobium plants. The endophytic fungi strains can be colonized at a root of a dendrobium plant and used for seedling growing or cultivation of the dendrobium plant to effectively control soft rot disease of the dendrobium plant.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Preparation method of special fertilizer for golden camellia
InactiveCN107759398AHigh carbon contentImprove compactnessMagnesium fertilisersBacteriaBanana peelCamellia chrysantha
The invention relates to a preparation method of a special fertilizer for golden camellia. The preparation method is characterized in that the special fertilizer for the golden camellia can be prepared by mixing 280 kg of charcoal, 20 kg of potassium fulvic acid, 30 kg of mixed bacteria liquid, 80 kg of cow hair powder, 10 kg of modified sweet potato straw particles, 10 kg of banana peels, 10 kg of sweet potato peels, 2 kg of walnut peels, 10 kg of bentonite, 5 kg sodium citrate and 3 kg of magnesium stearate to obtain a product, pulverizing with a plant pulverizing machine and sieving with 40-mesh sieve; the prepared fertilizer has a good absorption promoting effect.
Owner:福建鸿丰现代生态农业开发有限公司
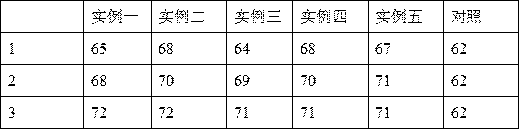
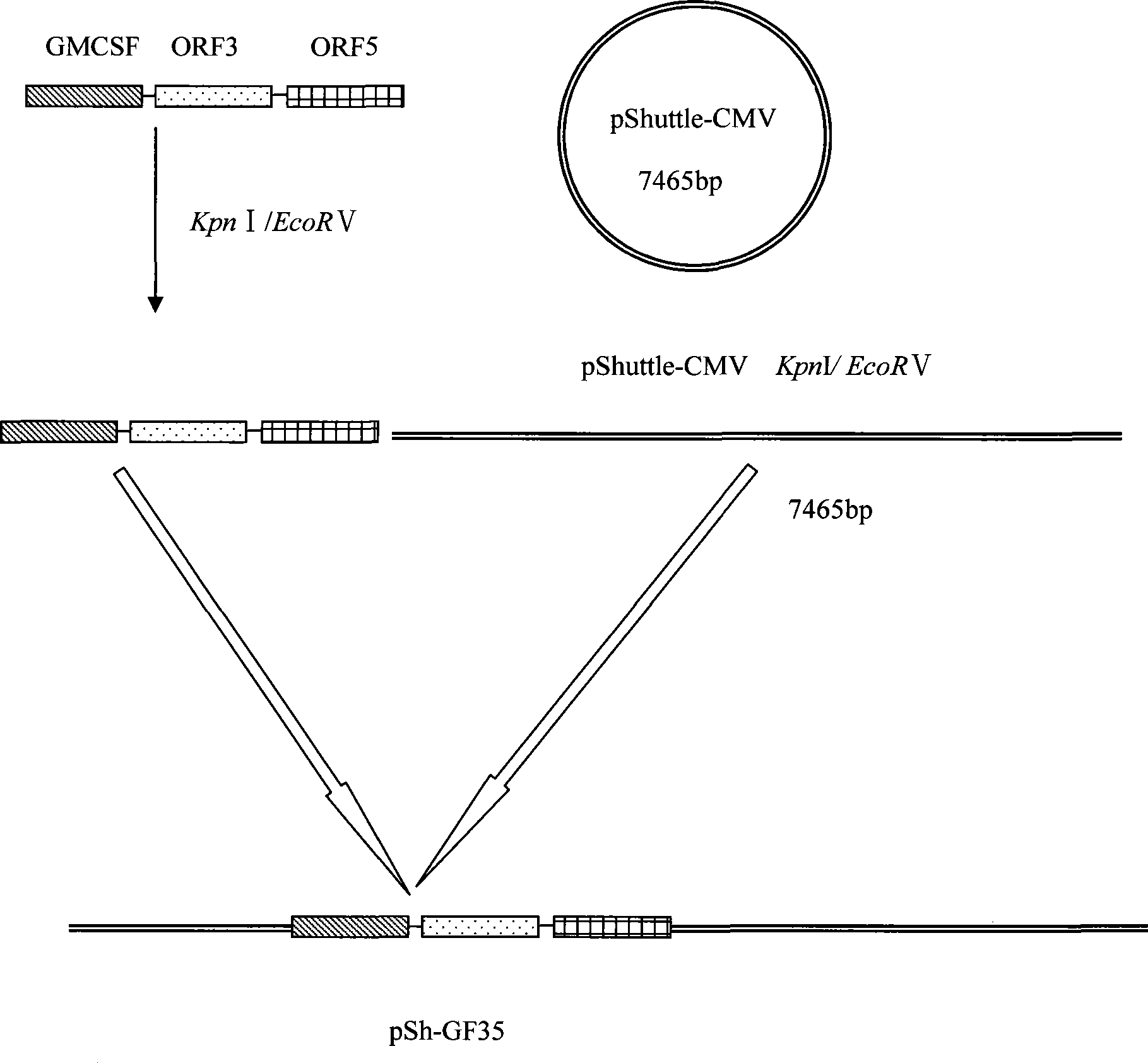
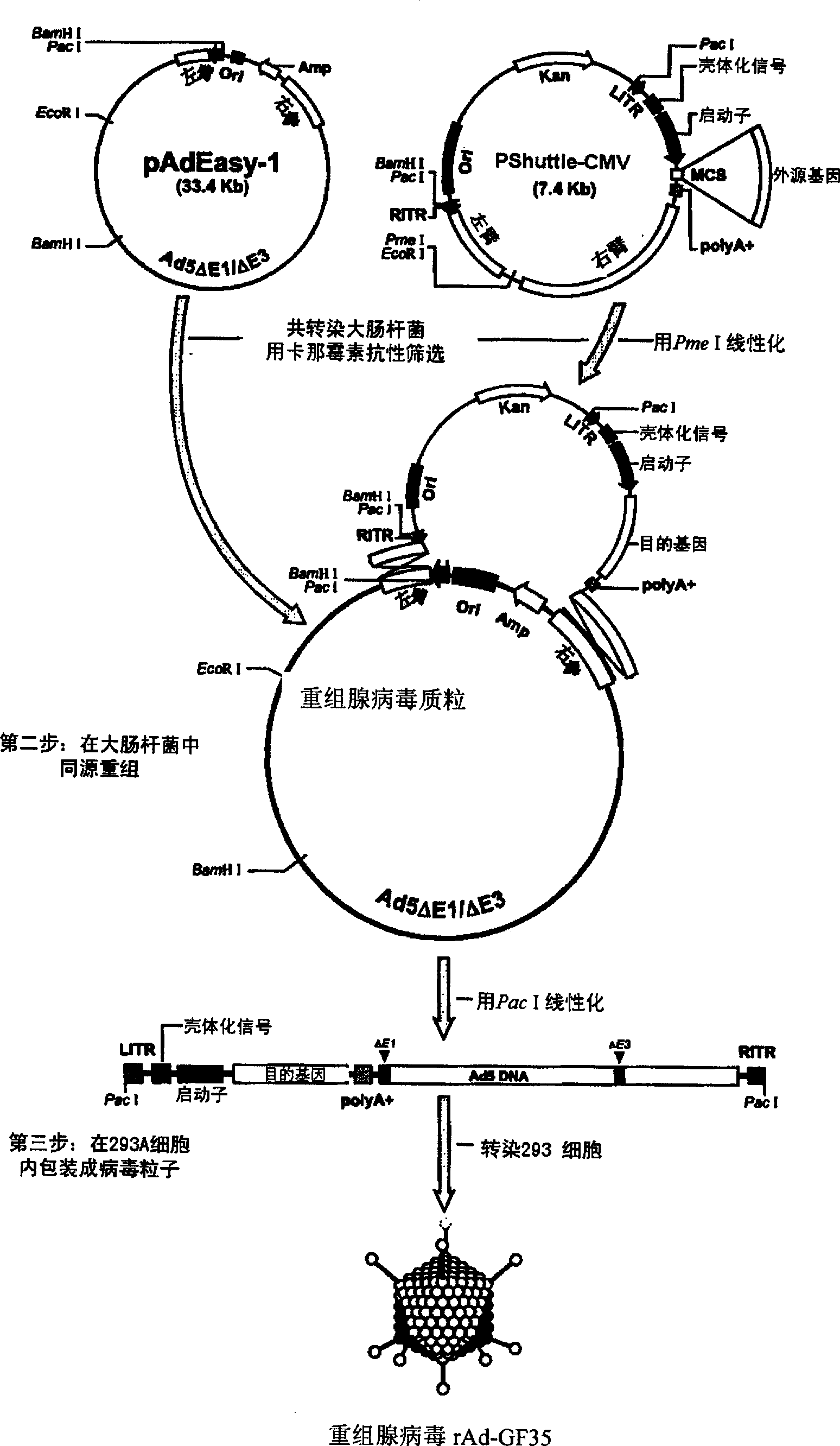
Porcine reproductive and respiratory syndrome recombinant adenovirus rAd-GF35
InactiveCN101423823AHas the copy featureIncreased effect of bioactive adjuvantsViral antigen ingredientsAntiviralsEscherichia coliAntigen
The invention discloses a virus (PRRSV) recombinant adenovirus rAd-GF35 of porcine reproduction and respiratory syndrome, which belongs to the technical field of high-tech biotechnology. Highly pathogenic PRRSV SY0608 separation strains of GP3 and GP5 protein genes and porcine colony sell stimulating factor (GMCSF) whole gene sequences are amplified, connected in series and cloned into pShuttle-CMV to be transformed into escherichia coli BJ5183 together with pAdEasy-1 so as to obtain recombinant plasmids; and HEK293A cells are transfected to obtain the recombinant adenovirus so as to successfully express PRRSV SY0608 strains GP3 and GP5 and a GMCSF protein. The recombinant virus can express the GMCSF correctly, and play a role of an adjuvant so as to improve the immune activity of the PRRSV GP3 and GP5 proteins and stimulate the immune protective reaction of an organism more effectively. The recombinant adenovirus vaccine has the safety of a subunit vaccine and the antigen proliferating ability of an attenuated vaccine, thereby having wide development and application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
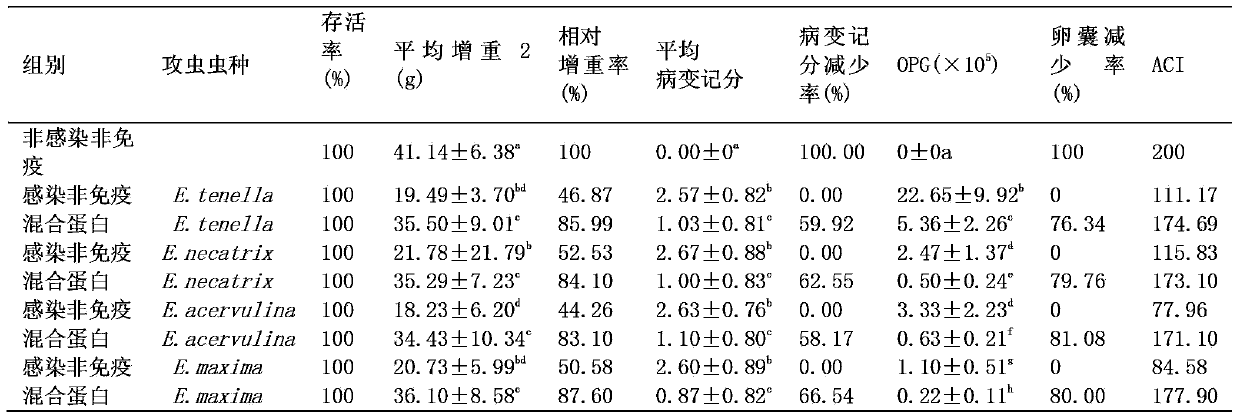
Multivalent recombinant protein subunit vaccine in chicken coccidiosis and application thereof
ActiveCN103386124ANot pathogenicFree from infectionAntiparasitic agentsAntibody medical ingredientsProtein subunitVeterinary Drugs
The invention relates to a technical filed of biological drugs and a multivalence recombinant protein subunit vaccine in chicken coccidiosis. The vaccine comprises four recombinant proteins in the chicken coccidiosis: TA4 (E.tenella), NA4 (E.necatrix), LDH (E.acervulina) and Em8 (E.maxima). It is proved through an animal immunity protective experiment that the vaccine can effectively resist four chicken coccidiosis infections and can be used in preparation of drug formulations for prevention against the chicken coccidiosis.
Owner:NANJING AGRICULTURAL UNIVERSITY
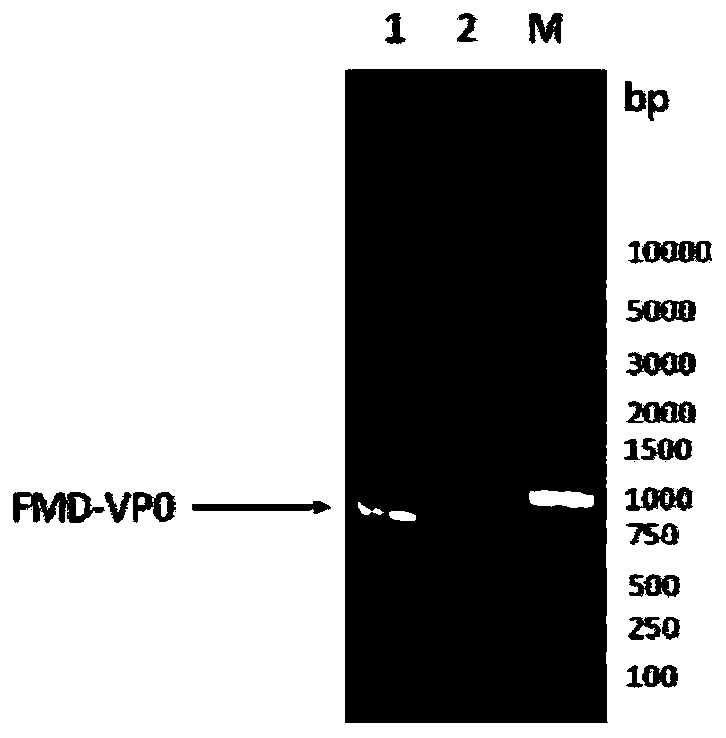
Novel genetically-engineered subunit vaccine to foot-and-mouth disease virus O
ActiveCN110256539AReduce manufacturing costNot pathogenicSsRNA viruses positive-senseViral antigen ingredientsVaccine ProductionStructural protein
The invention discloses an immunization composition, comprising foot-and-mouth disease virus O structural proteins VP3 and VP1, and foot-and-mouth disease virus O structural proteins VP2 and / or VP4. Further, the immunization composition also comprises a foot-and-mouth disease virus O structural protein VP0. The immunization composition is applicable to the preparation of a novel genetically-engineered subunit vaccine to foot-and-mouth disease virus O; the novel genetically-engineered subunit vaccine is similar to natural proteins in terms of antigenicity, immunogenicity and functionality, the expression level is high, the immunogenicity is high, and the novel genetically-engineered subunit vaccine is non-pathogenic to an animal. In addition, the novel genetically-engineered subunit vaccine is suitable for large-scale serum-free suspension culture through biosensors; vaccine production cost is reduced greatly.
Owner:苏州沃美生物有限公司
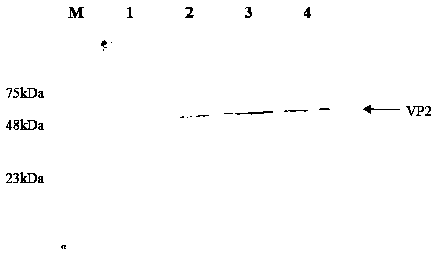
Chicken infectious bursal disease virus VP2 gene, expression product and subunit vaccine thereof, and application of vaccine
InactiveCN108823218AHigh expressionIncrease productionBacteriaViral antigen ingredientsNucleic acid sequencingDisease
The invention discloses a chicken infectious bursal disease virus VP2 gene, an expression product and a subunit vaccine thereof, and an application of the vaccine. The invention further provides a nucleic acid sequence of the chicken infectious bursal disease virus VP2 gene, a primer for cloning the nucleic acid sequence, an expression vector containing the nucleic acid sequence, an engineering bacterium obtained through conversion of the expression vector, a chicken infectious bursal disease virus VP2 protein encoded by the nucleic acid sequence, expressed by the expression vector or separated from the engineering bacterium, and a chicken infectious bursal disease virus-like particle formed through self-assembling the chicken infectious bursal disease virus VP2 protein. The invention further provides the subunit vaccine prepared from the chicken infectious bursal disease virus VP2 protein. The subunit vaccine is prepared from the chicken infectious bursal disease virus-like particle.The subunit vaccine is applied to prevent chicken related diseases caused by the chicken infectious bursal disease virus. The subunit vaccine has the advantages of low production cost, simplicity in operation, good biological security, and effectiveness in prevention of infection of the chicken infectious bursal disease virus to chickens.
Owner:HENAN ACAD OF AGRI SCI +1
Preparation method of seaweed biological fertilizer
ActiveCN103848652AReduce manufacturing costEfficient use ofOrganic fertilisersResource utilizationLarge intestine
The invention discloses a preparation method of seaweed biological fertilizer. The preparation method comprises the following steps: taking schizochytrium limacinum dregs and a functional bacterium strain as preparation raw materials, and implementing liquid culture of the functional bacterium strain in a fermentation tank until a fermentation broth contains 108-1010cfu / ml of bacteria for later use; inoculating the schizochytrium limacinum dregs to the cultivated functional bacterium strain, and implementing aerobic fermentation to obtain a fermentation product; sequentially pelletizing, drying, cooling, sieving and packaging the fermentation product to obtain the finished seaweed biological fertilizer. Organic matter content, the number of effective live bacteria and the like in the produced bio-organic fertilizer exceed national standard (NY / 884-2012) of the bio-organic fertilizer, and water content, pH value, roundworm ova death rate and the number of fecal coliforms are consistent with the national standard (NY / 884-2012). Moreover, the preparation method disclosed by the invention is low in investment, low in energy consumption, rapid to become effective and light in pollution; the preparation method can be used for creating social and economic values, and realizing resource utilization of waste in enterprises.
Owner:QINGDAO KEHAI BIOLOGICAL
Method for expressing ORF2 protein of porcine circovirus 3 by using Escherichia coli and application thereof
InactiveCN109609534AThe method is simple and fastShort expression periodBacteriaVirus peptidesEscherichia coliFusion Protein Expression
The invention discloses a method for expressing an ORF2 protein of a porcine circovirus 3 (PCV3) by using Escherichia coli and application thereof. The method is as below: 1) cloning a solubility-enhancing tag GST gene, a solubility-enhancing tag His6-SUMO gene and a PVC3-ORF2 gene respectively; 2) ligating the obtained three genes to obtain a fusion gene; 3) ligating the obtained fusion gene to an E. coli expression vector to obtain a recombinant expression vector containing the fusion gene; 4) transforming the recombinant expression vector into E. coli expressing strain competent cells to obtain recombinant E. coli expressing strains containing the fusion gene; 5) culturing the obtained recombinant expression strains, and inducing expression of the fusion protein by IPTG; and 6) recovering the recombinant expression bacteria obtained by culture, breaking and purifying the recombinant expression bacteria to obtain the ORF2 protein of the porcine circovirus 3. The method not only obtains soluble protein with high expression, but also achieves high immunoreduction.
Owner:镇江瑞华生物科技有限公司
Lactobacillus helveticus strain and application thereof
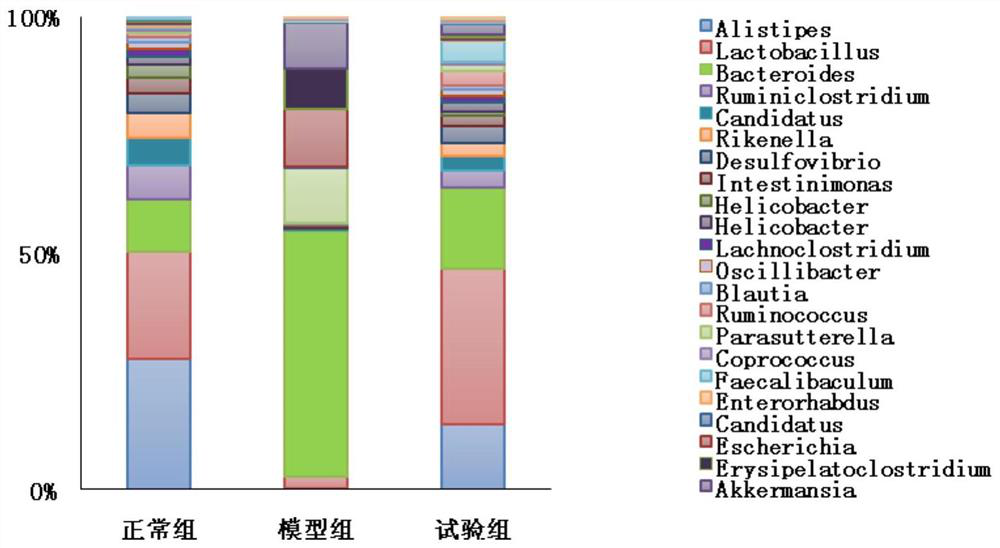
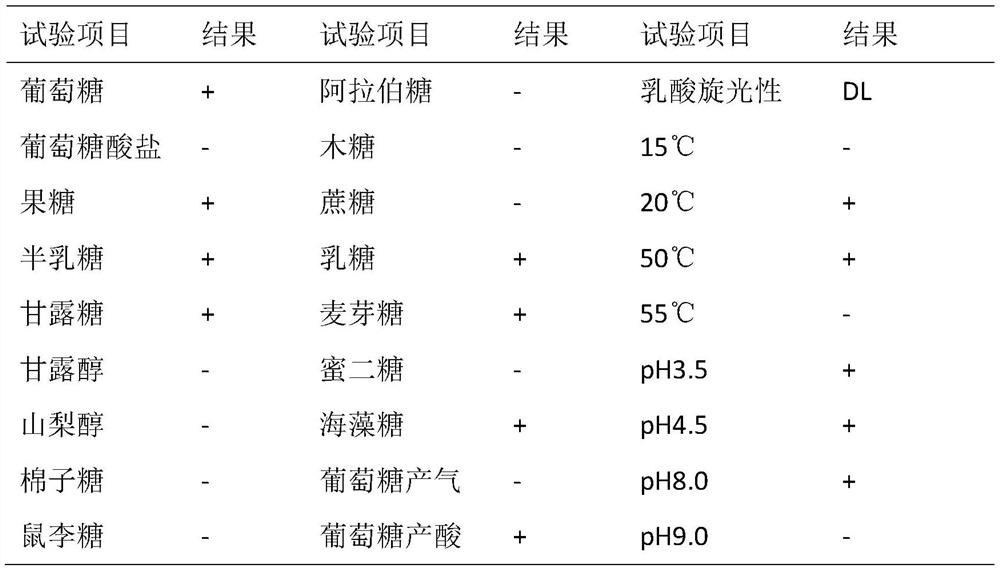
ActiveCN112877233AImprove securityReduce drug resistanceBacteriaDigestive systemLactobacillus helveticumGut flora
The invention provides a strain of probiotics from an environment, and through analysis and identification of morphological characteristics, physiological and biochemical characteristics and molecular biological characteristics of the strain, the strain is Lactobacillus helveticus and is named as Lactobacillus helveticus-S. The lactobacillus helveticus-S can resist acid and bile salt, so that the lactobacillus helveticus-S can be colonized in the digestive tract through oral administration; the lactobacillus helveticus-S has no hemolytic activity and high sensitivity to common antibiotics, so that the lactobacillus helveticus-S has no intestinal pathogenicity and does not diffuse antibiotic tolerance genes; animal model tests show that the lactobacillus helveticus-S can improve the structure and function of intestinal flora, improve the diversity of the intestinal flora and reduce the abundance of inflammation-related flora, and has the effects of treating diarrhea and loose stool and improving the symptom of spleen deficiency.
Owner:江苏斯卡露生物科技有限公司
Novel gene engineering subunit vaccine for mycoplasma gallisepticum
ActiveCN109999191ANot pathogenicReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsNucleotideVaccine Production
The invention provides an immunological composition and a subunit vaccine. The immunological composition comprises a protein which is selected from one or an arbitrary combination of two or more of mycoplasma gallisepticum-associated proteins encoded with nucleic acid molecules of SEQ ID NO: 1 or 3 or 5 or 7 or 9 or nucleic acid molecules which are 95% or above identical to the nucleotide sequenceof SEQ ID NO: 1 or 3 or 5 or 7 or 9. The vaccine adopts eukaryotic expression, the antigenicity and immunogenicity of the product are similar to those of a natural protein, the expression level is high, the immunogenicity is strong, the protective effect is good, and the vaccine has no pathogenicity to chickens; besides, large-scale serum-free suspension culture preparation of the vaccine can berealized through a bioreactor, and meanwhile the vaccine production cost is greatly reduced.
Owner:苏州沃美生物有限公司
Preparation method and application of porcine pseudorabies virus subunit vaccine
ActiveCN110327461AReduce manufacturing costProcess safetyViral antigen ingredientsVirus peptidesSuspension culturePseudorabies
The invention discloses a preparation method and application of a porcine pseudorabies virus subunit vaccine. The porcine pseudorabies virus subunit vaccine is prepared from porcine pseudorabies virusrelated proteins encoded by nucleic acid molecules of SEQ ID NO:1 or SEQ ID NO:3 or SEQ ID NO:5 or SEQ ID NO:7 or SEQ ID NO:9 or encoded by nucleic acid molecules with more than 95% of nucleotide sequences the same as SEQ ID NO:1 or SEQ ID NO:3 or SEQ ID NO:5 or SEQ ID NO:7 or SEQ ID NO:9. The vaccine is expressed by eukaryotes, the antigenicity and immunogenicity of the porcine pseudorabies virus subunit vaccine are similar to that of natural proteins, the expression level is high, the immunogenicity is high, the protective effect is good, no pathogenicity is caused to animals, the vaccine can be prepared by large-scale serum-free suspension culture in a bioreactor, and meanwhile, the cost of vaccine production is greatly reduced.
Owner:苏州世诺生物技术有限公司
Endophytic fungus with preventive and treatment effects on soft rot of dendrobiums
The invention discloses an endophytic fungus with preventive and treatment effects on soft rot of dendrobiums. The fungus can be subjected to field planting at the root of dendrobiums, and is used for seedling raising or cultivation of dendrobiums. The endophytic fungus with preventive and treatment effects on soft rot of dendrobiums can effectively prevent the soft rod of dendrobiums from happening.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Bovine herpes virus antigen composition and application thereof
ActiveCN111808176AImproving immunogenicityImprove expression levelViral antigen ingredientsVirus peptidesBovine herpesvirusImmunogenicity
The invention relates to a bovine herpes virus antigen composition and an application thereof. The bovine herpes virus antigen composition comprises at least two of bovine herpes virus recombinant gBprotein having an amino acid sequence as shown in SEQ ID NO: 1, bovine herpes virus recombinant gD protein having an amino acid sequence as shown in SEQ ID NO: 2, bovine herpes virus recombinant gH protein having an amino acid sequence as shown in SEQ ID NO: 3 and bovine herpes virus recombinant gL protein with an amino acid sequence as shown in SEQ ID NO: 4. Sequence information and space structures of gB protein, gD protein, gH protein and gL protein of bovine herpes virus are comprehensively analyzed, site-specific mutagenesis is performed on the four proteins respectively, respective immunogenicity of the proteins is improved, neutralizing antibody can be better stimulated, the immune effect is better, and the immune period is longer.
Owner:苏州世诺生物技术有限公司
Replication-defective recombinant influenza virus for simultaneously expressing HA and HEF
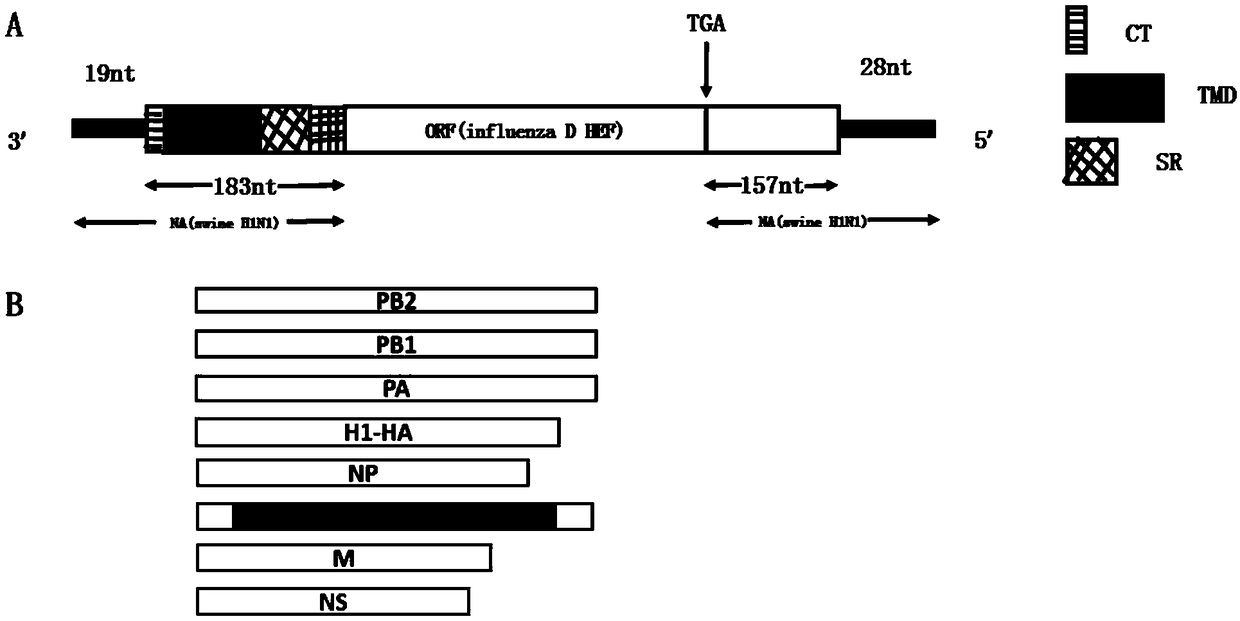
ActiveCN109097341AGood gene stabilityNot pathogenicSsRNA viruses negative-senseViral antigen ingredientsMucosal Immune ResponsesHemagglutinin
The invention provides a preparation method of a replication-defective A and D divalent influenza virus attenuated live vaccine. The invention constructs a recombinant virus strain; on the premise ofnot changing gene stability, the constructed recombinant virus can stably and simultaneously express surface protein hemagglutinin HA of A type influenza virus H1N1 subtype and unique surface proteinhemagglutinin esterase fusion protein HEF of D type influenza virus. The constructed attenuated strain has good gene stability and cannot be replicated in an experimental animal body, so the constructed attenuated strain has no pathogenicity; meanwhile, the constructed attenuated strain also can induce a body to produce strong mucosal immune response and cellular immune response level and keeps intense and durable immunogenicity. The key is that a vaccine candidate strain can produce an immunoprotection action on the A type influenza virus H1N1 subtype and the D type influenza virus. Therefore, the replication-defective A and D divalent influenza virus attenuated live vaccine has huge social significance in preventing and controlling A type and D type influenza.
Owner:QINGDAO AGRI UNIV
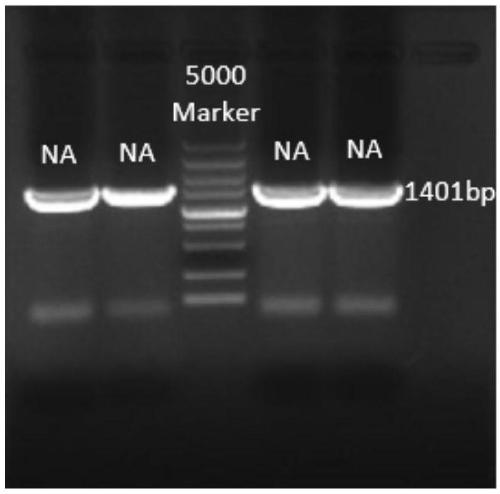
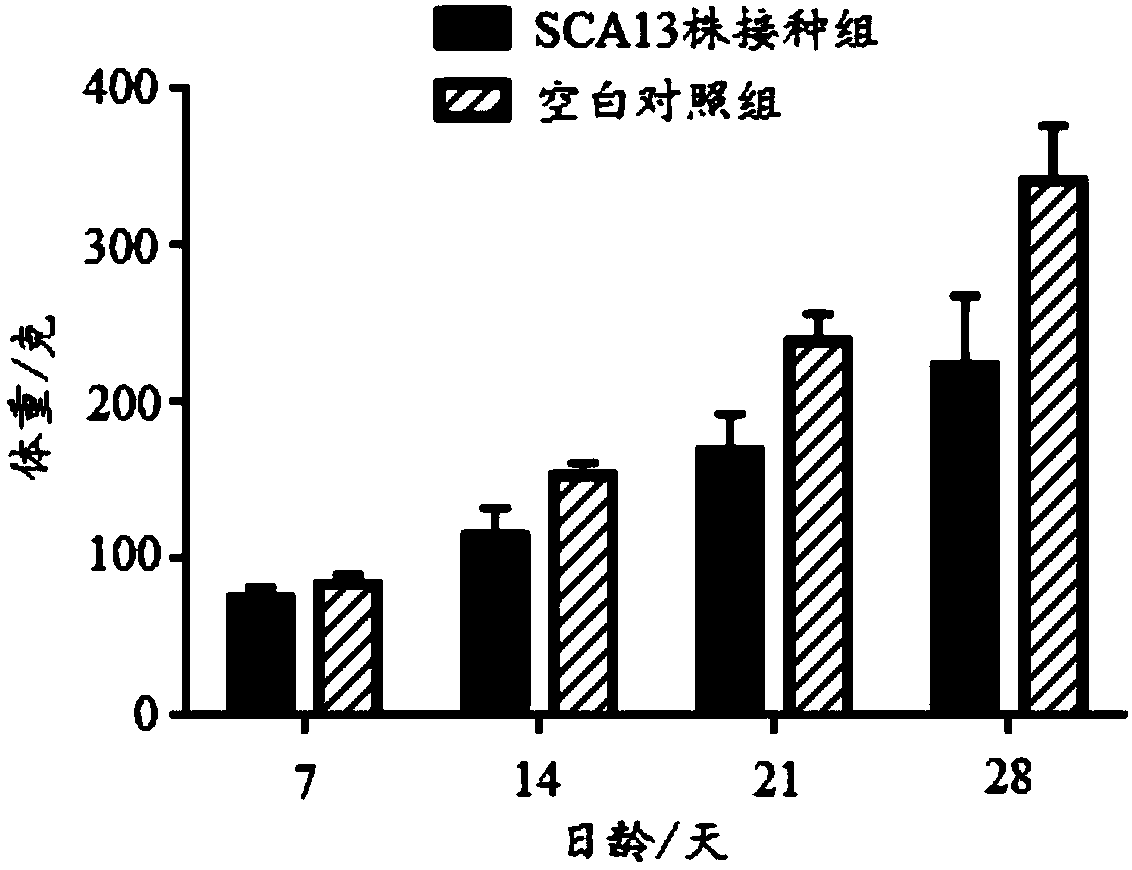
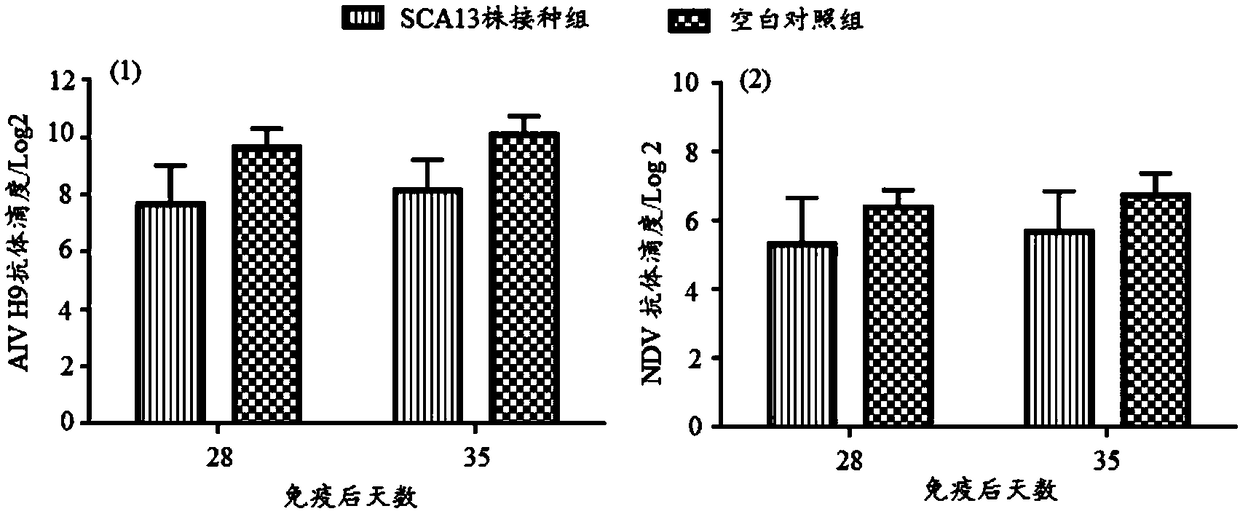
Recombinant Marek's disease virus strain SCA13 strain and application thereof
ActiveCN108342367ANon-tumorigenicGood immune protectionViral antigen ingredientsMicrobiological testing/measurementBac cloneHorizontal transmission
The invention discloses a recombinant Marek's disease virus strain SCA13 strain. The biological preservation number of the strain is CGMCC NO.15285, and an SCA13 strain genome is integrated with ALV LTR and can exist stably. On the basis of BAC clone, and by means of the phenomenon that a Red E / T recombinant technology lacks meq and vTR genes, a deletion strain of the meq and vTR genes of the MDVSCA13 strain is built, the biological preservation number of the strain is CGMCC NO.15286, a double gene deletion strain built on the basis of the SCA13 strain completely loses lethality and tumorigenicity to chickens, the immunosuppressive property is lost, the strain has better biosecurity and better horizontal transmission capacity, the problem that in the MD vaccine immunity process, due to immunity leakage of the chickens, diseases occur can be solved, and the chickens are subjected to better immunoprotection in clinic.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
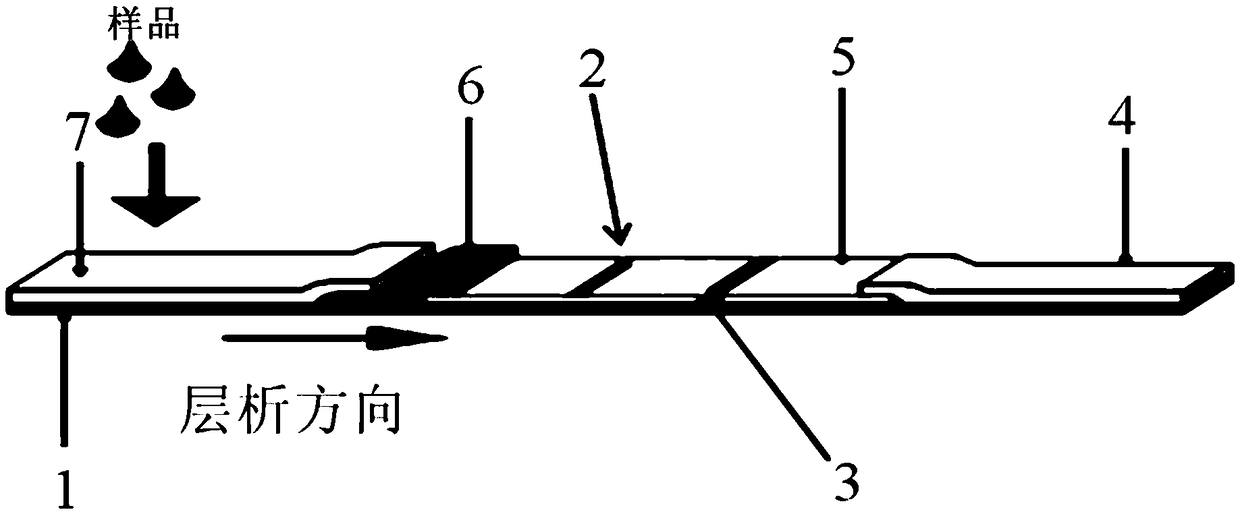
Test paper strip/test paper card for rapidly detecting HPV (Human Papilloma Virus) antibody by using double-antibody sandwich method and preparation method thereof
PendingCN108761091ASolve the problem of prone to non-specific bindingAvoid captureBiological testingHuman bodyEngineering
The invention discloses a test paper strip / test paper card for rapidly detecting an HPV (Human Papilloma Virus) antibody by using a double-antibody sandwich method and a preparation method thereof. The test paper strip (test paper card) comprises a sample pad, a conjugate pad, an analysis membrane and a water sucking pad which are sequentially arranged in a chromatography direction, wherein a nanoparticle marker is arranged on the conjugate pad; a detection zone and a quality control line are arranged on the analysis membrane; the nanoparticle marker is selected from colloidal gold or latex marked anti-human IgG antibody; the detection zone comprises a main HPV capsid protein L1; or the nanoparticle marker is selected from the colloidal gold or latex marked main HPV capsid protein L1; thedetection zone comprises the anti-human IgG antibody. The test paper strip (test paper card) disclosed by the invention is not only convenient and rapid to use and low in cost, but also high in detection accuracy, security and sensitivity, is capable of carrying out on-site rapid detection on HPV16 / 18 Li IgG antibodies in human bodies, is applicable to detection in hospitals, disease control institutes, basis health units and families, and is applicable to popularization.
Owner:陕西医药控股医药研究院有限公司
Mycoplasma hyopneumoniae genetic engineering subunit vaccine as well as preparation method and application thereof
ActiveCN111925452ANot pathogenicReduce manufacturing costAntibacterial agentsAntibody mimetics/scaffoldsProtein compositionImmunogenicity
The invention discloses a mycoplasma hyopneumoniae genetic engineering subunit vaccine as well as a preparation method and an application thereof. The vaccine comprises a protein composition and a pharmaceutically acceptable carrier, wherein the protein composition comprises two fusion proteins with sequences shown in SEQ ID NO: 2 and SEQ ID NO: 6 respectively. The vaccine provided by the invention has no toxicity; the vaccine is high in safety, good in immunogenicity and capable of generating strong humoral immunity in pig bodies, immunized animals can resist poison attacking of strong poisonand are comprehensively protected, and the vaccine can be prepared through large-scale serum-free suspension culture of a bioreactor and has the advantages of being easy to control in quality, stablebetween batches, low in production cost and the like.
Owner:苏州世诺生物技术有限公司 +1
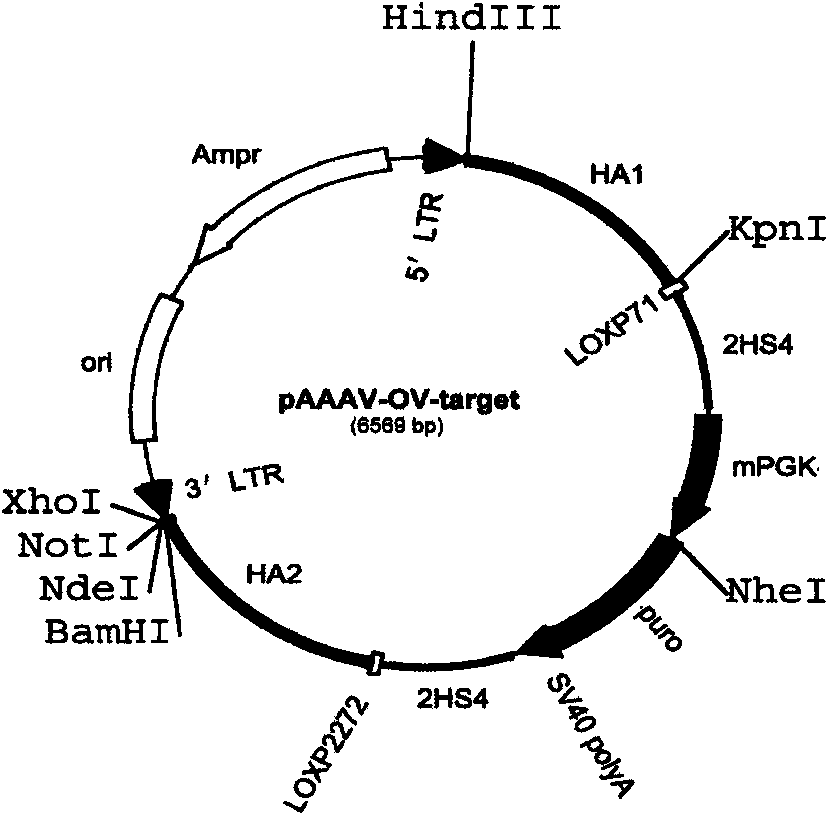
Method for genetic targeting of stem spermatogonium of poultry
InactiveCN101575614AEfficient gene targetingEasy vector constructionMicroorganism based processesGenetic engineeringTransgeneVaccine Immunogenicity
The invention discloses a method for genetic targeting of stem spermatogonium of poultry. By using an adenovirus of poultry as a targeting vector, the method performs targeting operation on the stem spermatogonium of the poultry; the obtained genetically modified stem spermatogonium can be used for transplanting testis and obtaining a transgenetic gene; and by mating the normal poultry, the stem spermatogonium can be further used for breeding the transgenetic poultry. The method has the advantages that: the method can effectively realize the high-efficiency genetic targeting of the stem spermatogonium of the poultry; the used homologous arm is short, and the vector is easily established; and after the exogenous objective albumen is directly integrated in an albumin expression albumen high-efficiency promotor, the expressional specificity of the exogenous albumen of the transgenetic offspring is high, the yield of albumen is high. Moreover, the method also has the advantages of simple operation, safety, no pathogenicity, low immunogenicity and less cell toxins.
Owner:JINLING INST OF TECH +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com