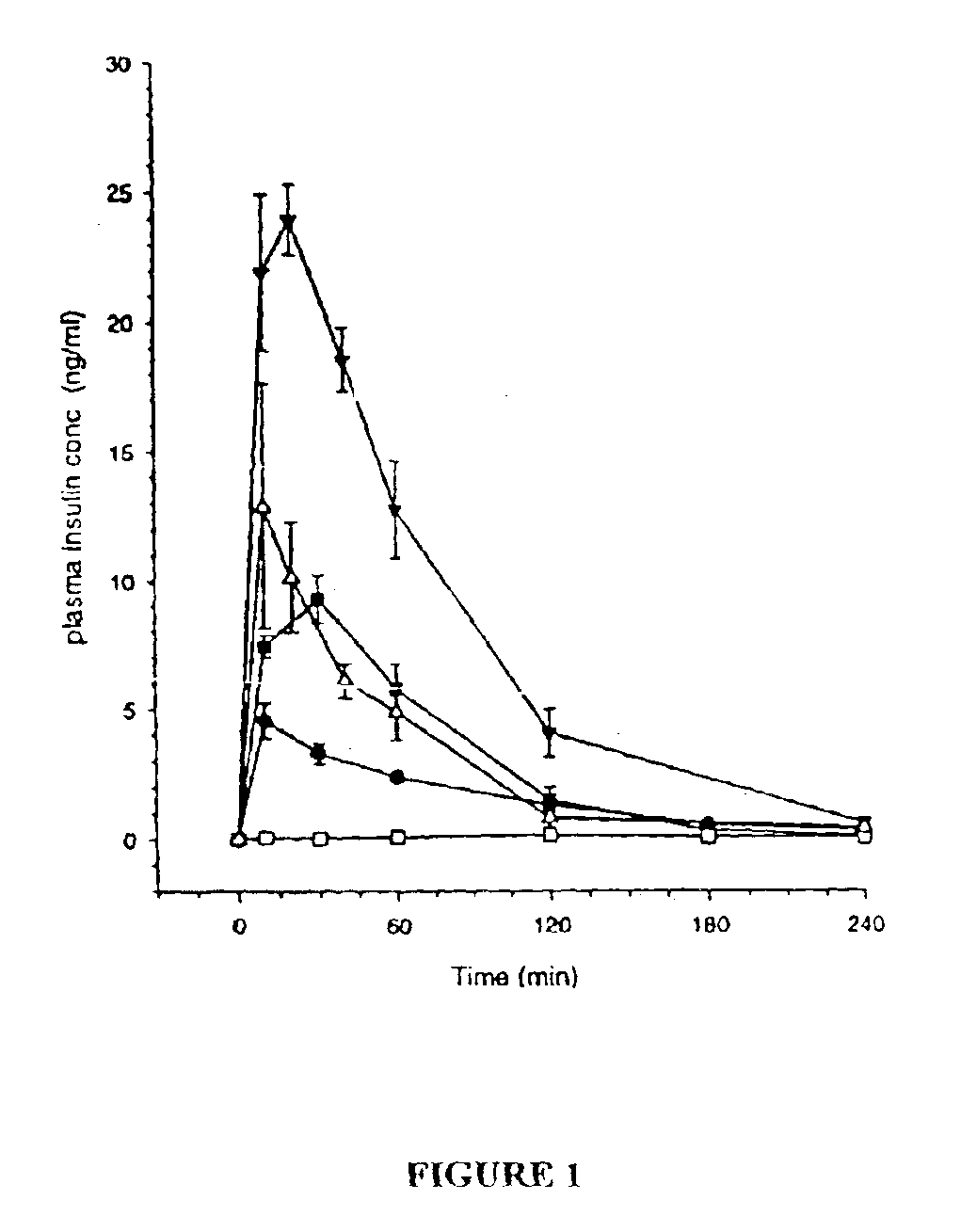
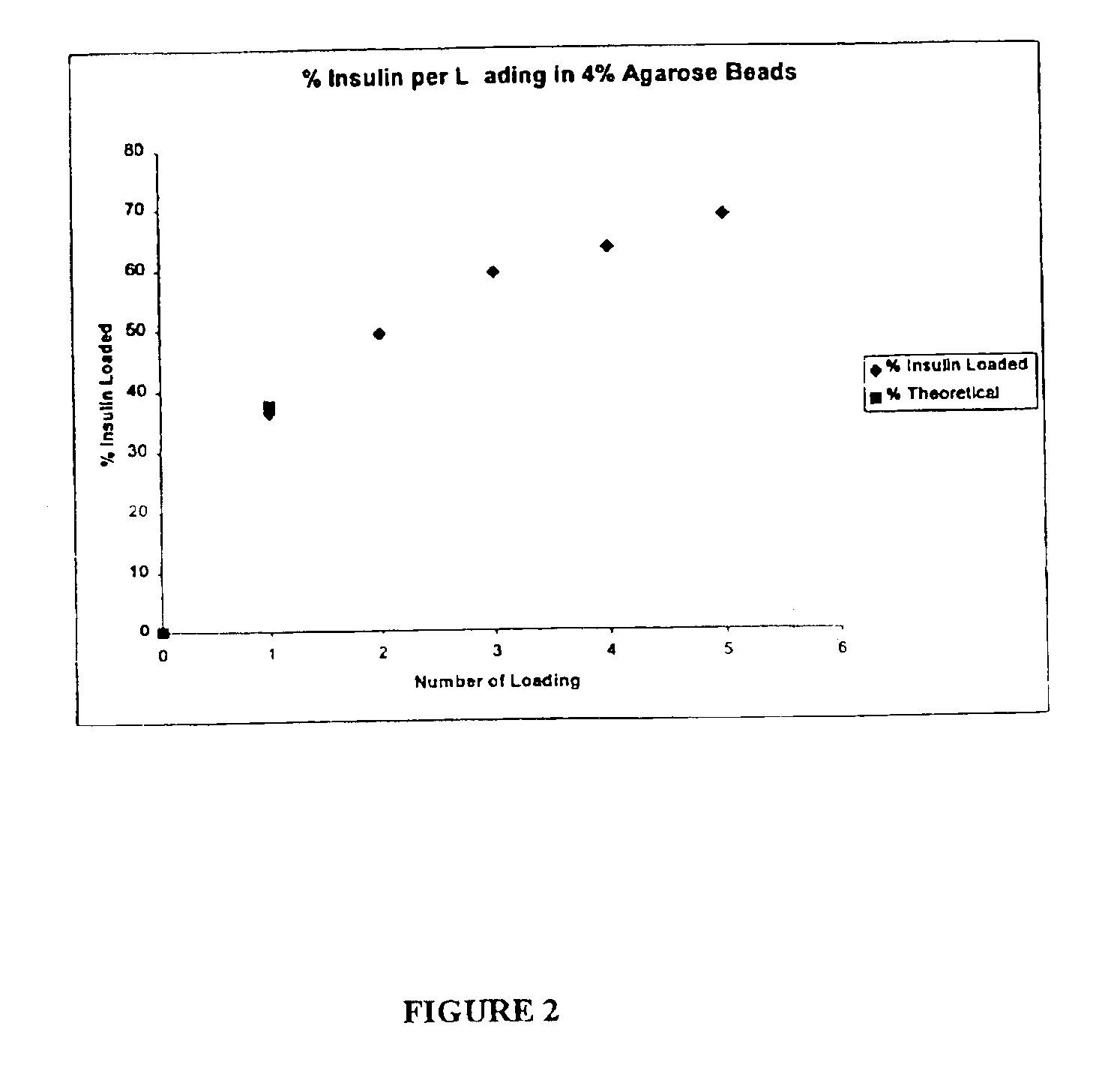
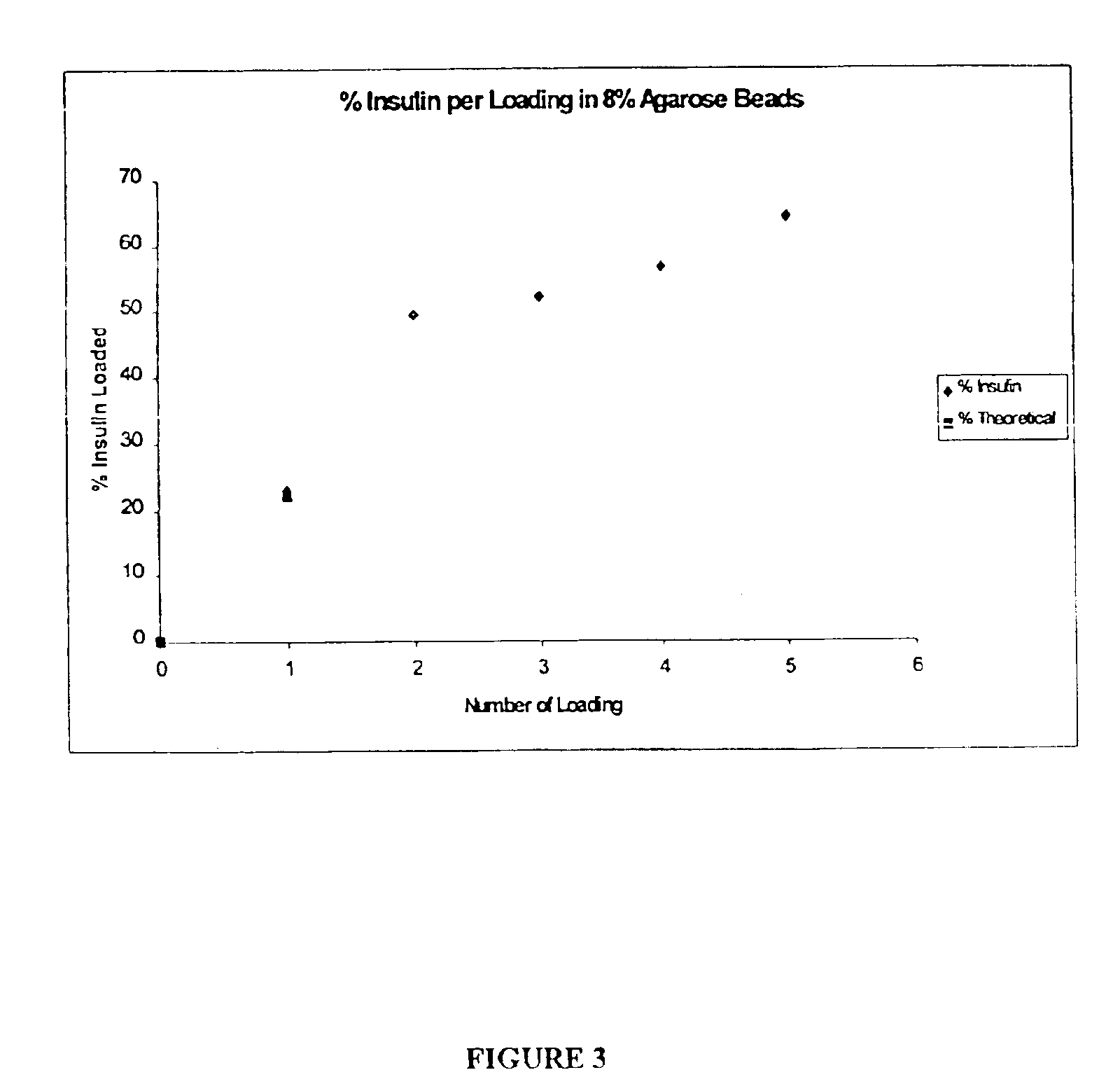
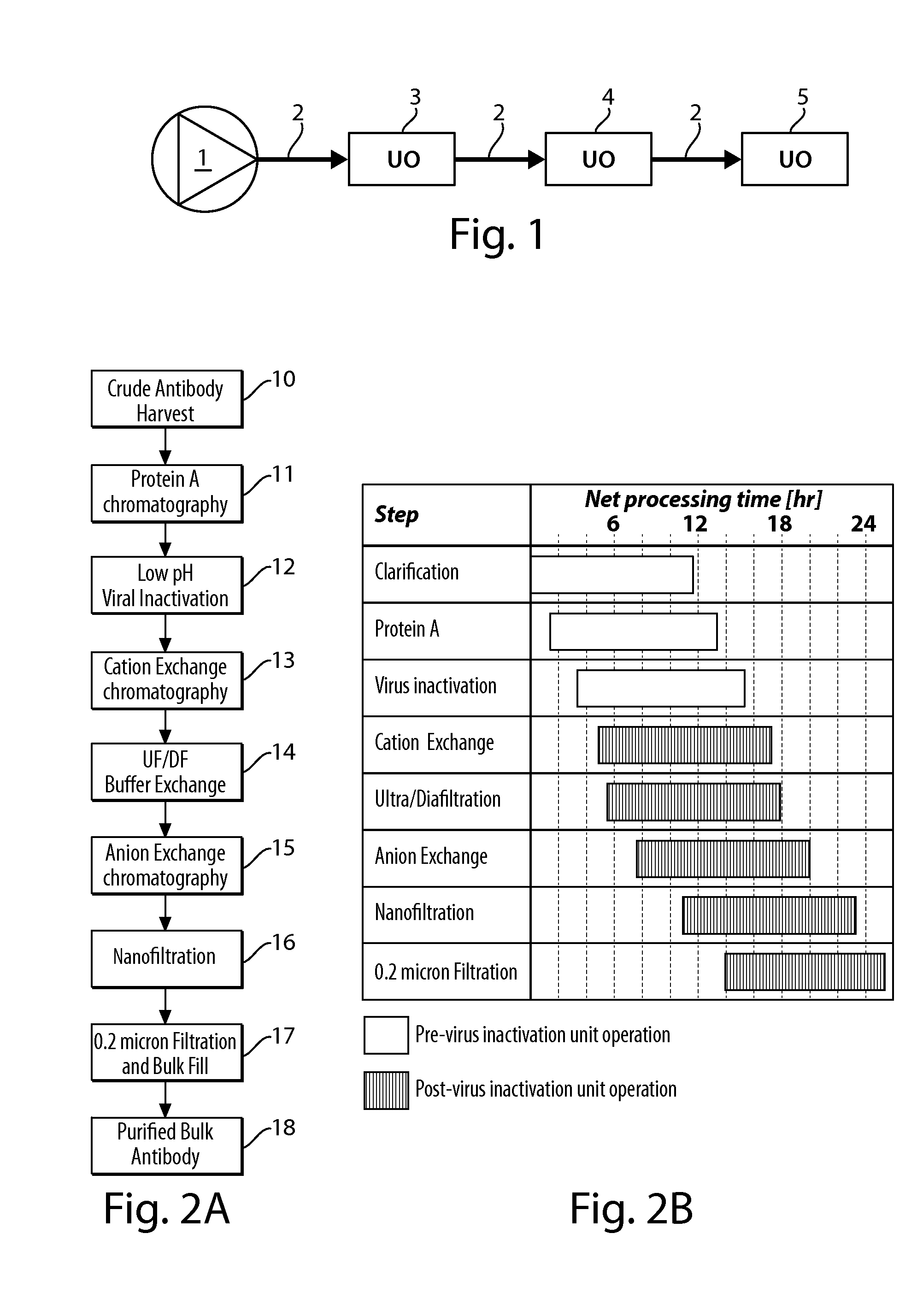
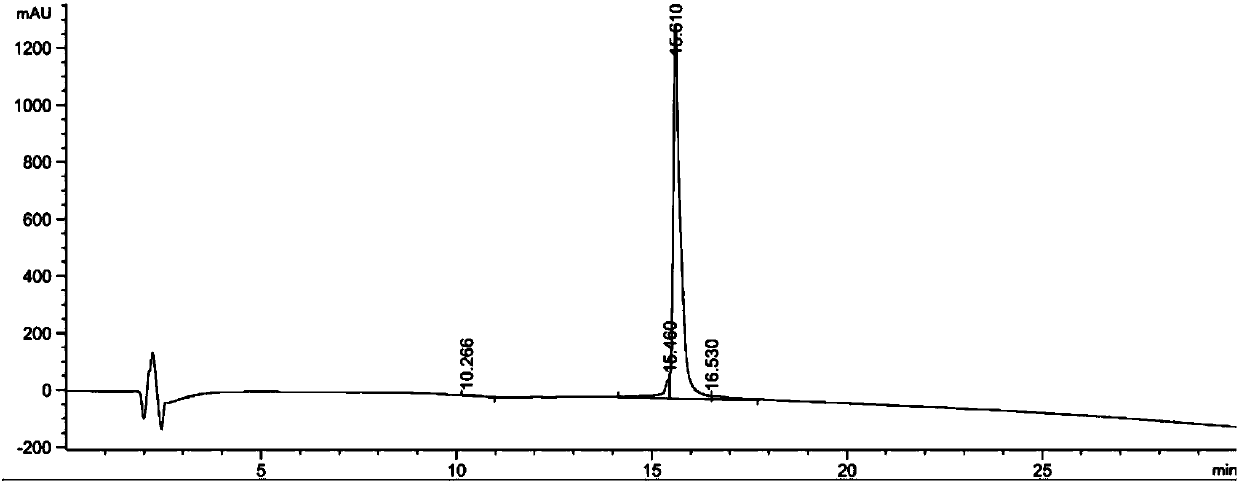
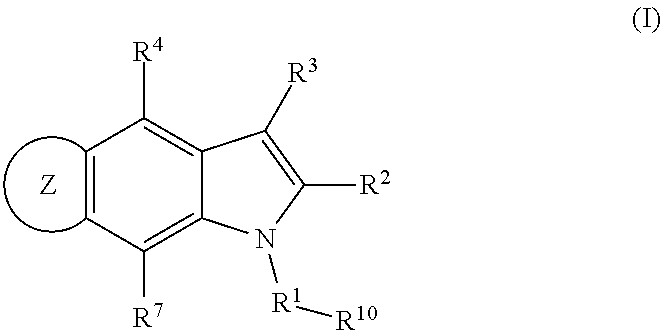
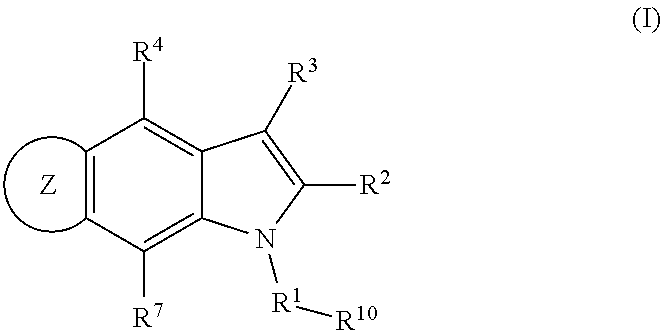
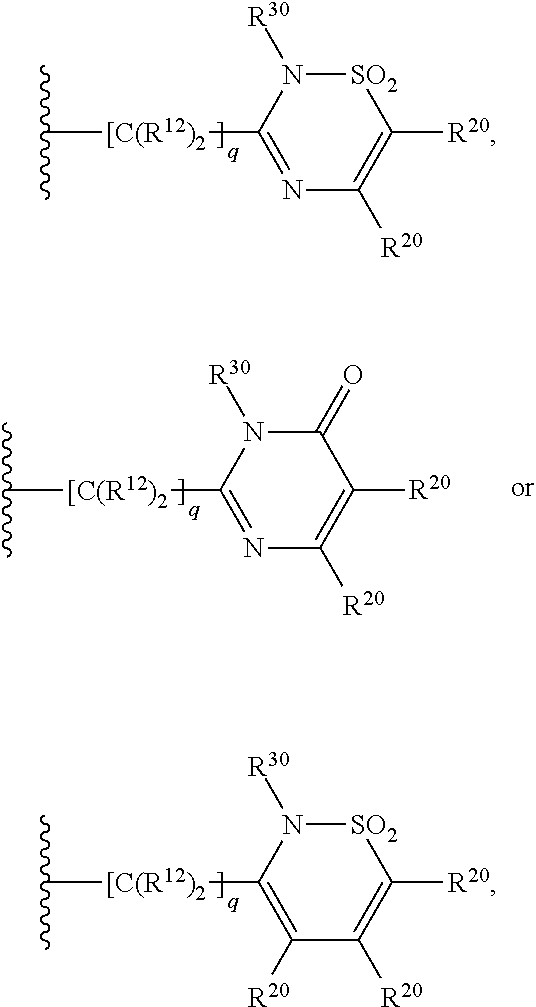
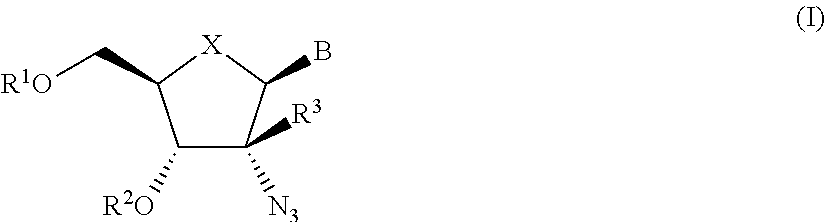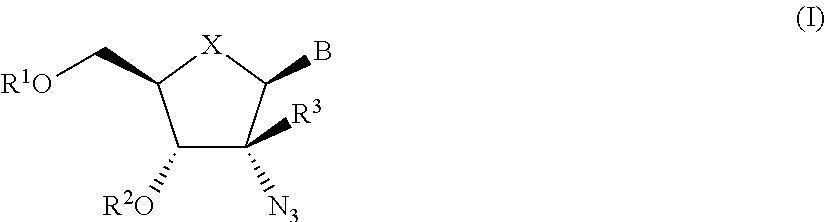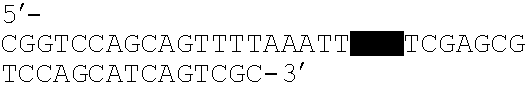Patents
Literature
551 results about "Biological drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A biopharmaceutical, also known as a biologic(al) medical product, biological, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources.
Preservation by Vaporization
ActiveUS20080229609A1Easy to controlImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
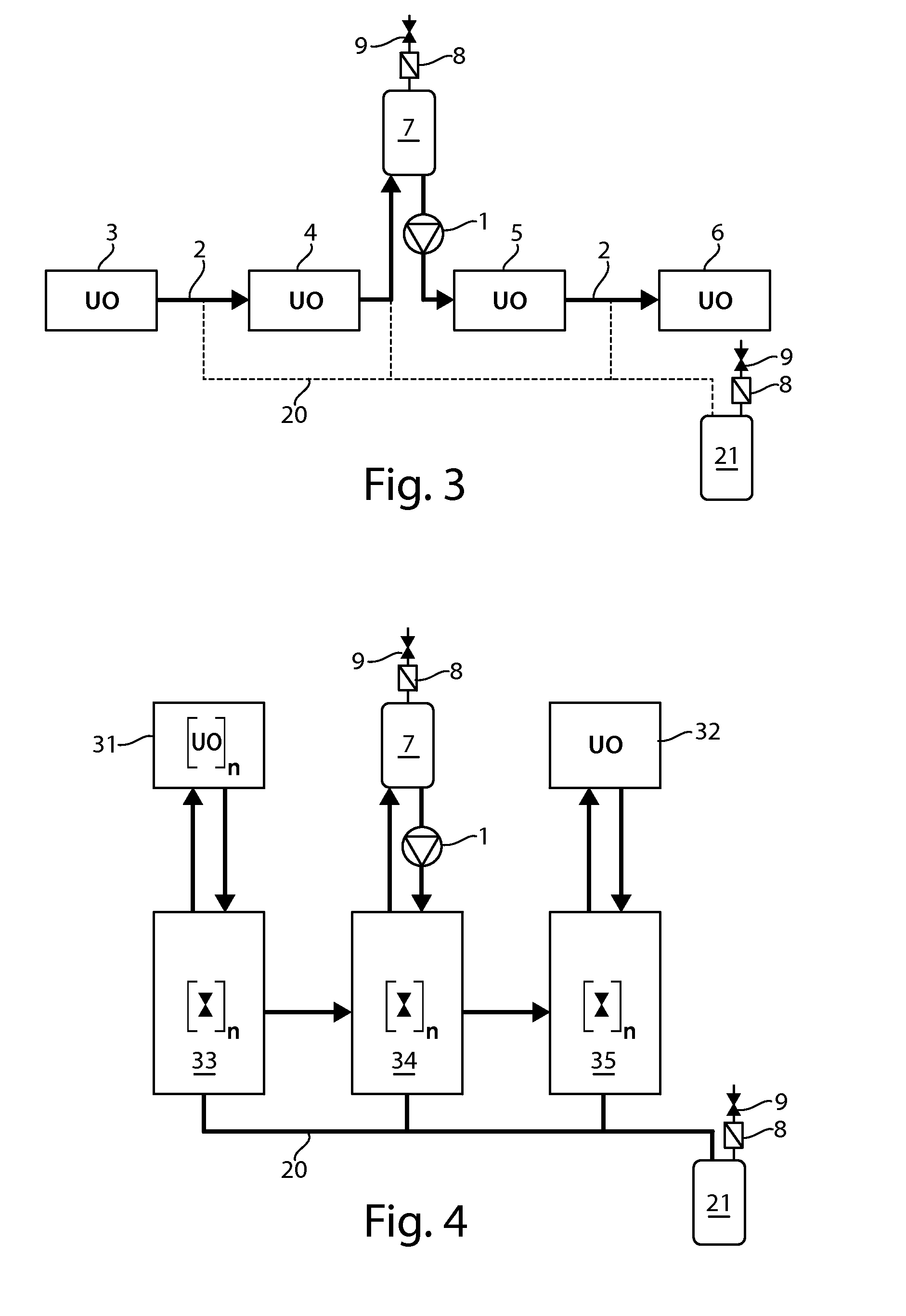
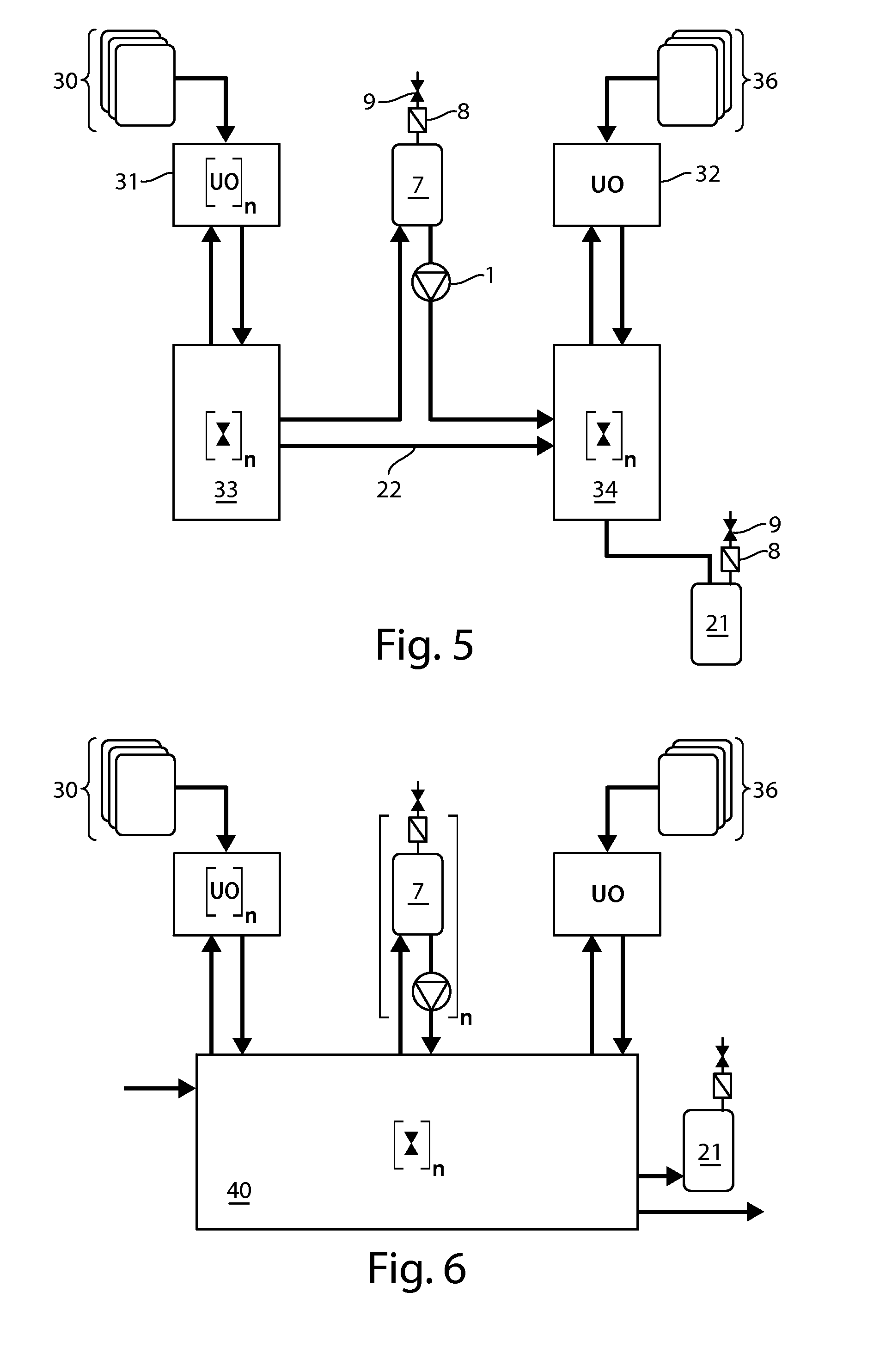
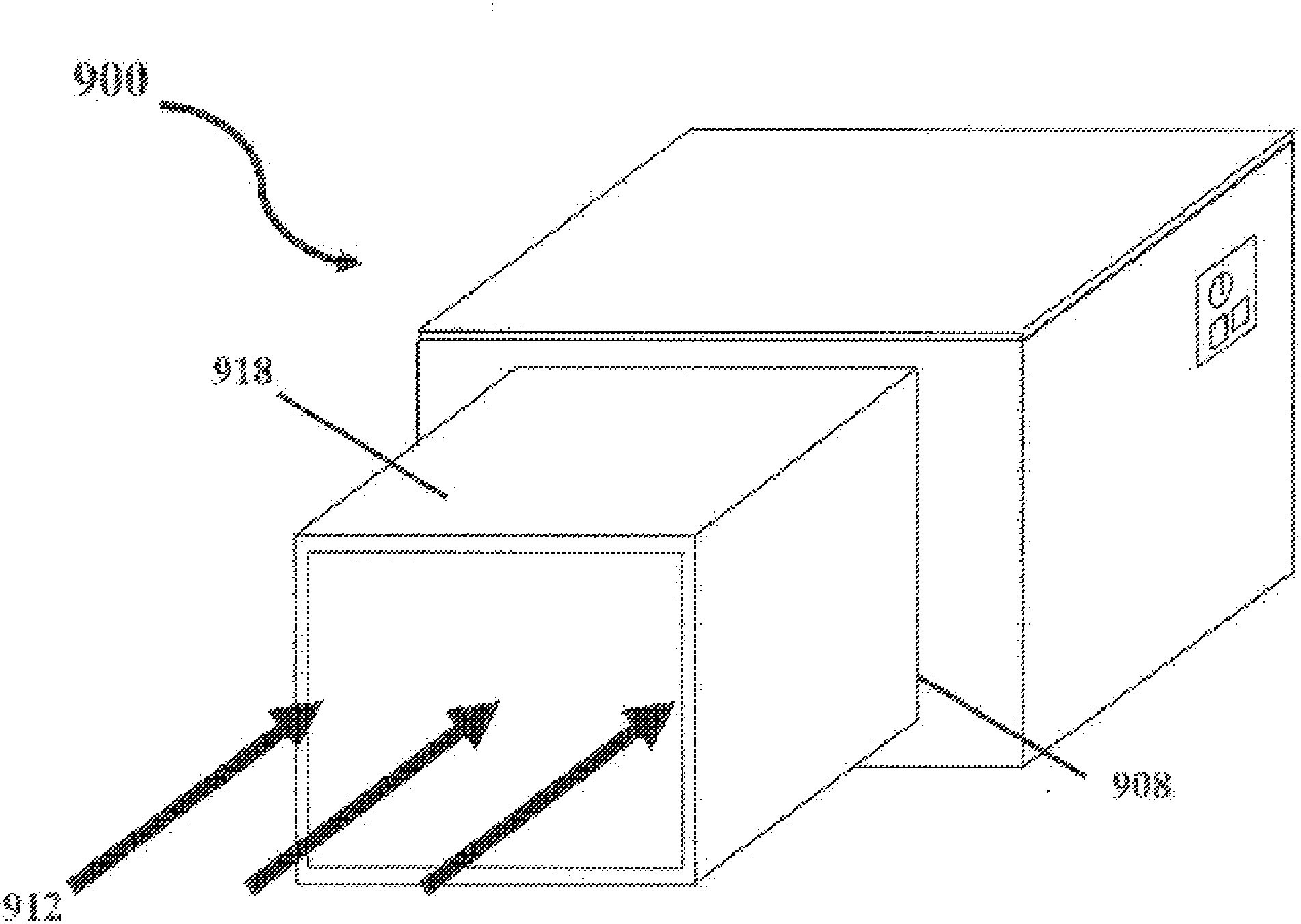
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
Hydrogel particle formulation
InactiveUS7022313B2Avoid lostEasy to customizePowder deliveryPeptide/protein ingredientsDiagnostic agentParticle injection
New compositions formed from the combination of an active substance with a hydrogel carrier moiety are provided. The compositions are suitable for use in high-velocity transdermal particle injection techniques. Methods of providing the new compositions are also provided. In addition, methods for administering pharmacologically active agent to a subject are provided. These methods are useful for delivering drugs, biopharmaceuticals, vaccines and diagnostics agents.
Owner:POWDERJECT RES LTD OXFORD (GB)
Continuous processing methods for biological products
InactiveUS20130260419A1Improve manufacturing productivityImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyMonoclonal antibody
The present invention is directed to the development of continuous processing technology for the purification of biopharmaceuticals and biological products, such as monoclonal antibodies, protein therapeutics, and vaccines. Methods for continuous processing of a biological product in a feed stream toward formulation of a purified bulk product are described.
Owner:SARTORIUS STEDIM CHROMATOGRAPHY SYST LTD
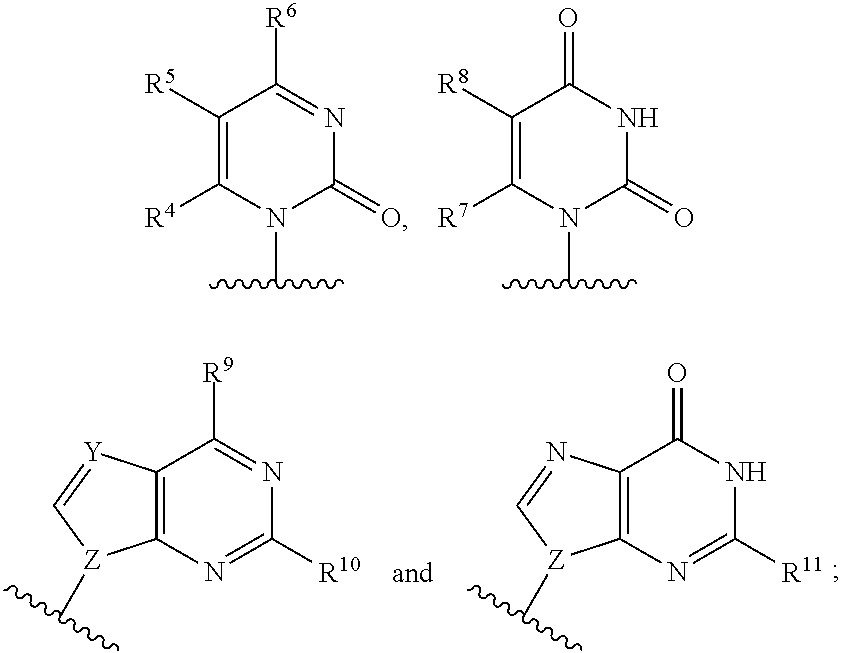
2'-azido substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases
ActiveUS20140206640A1Preventing HCV infectionTreating and preventing HCV infectionOrganic active ingredientsSugar derivativesDiseaseMedicine
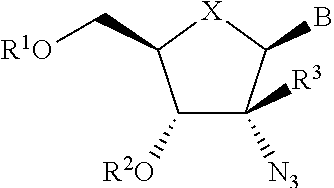
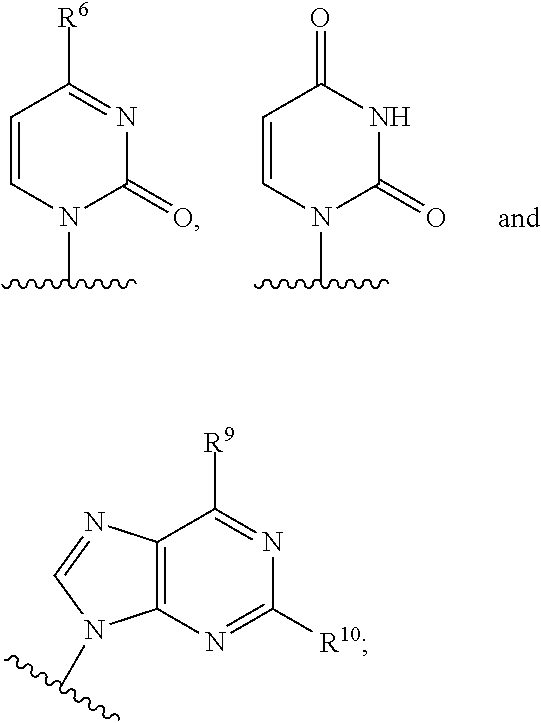
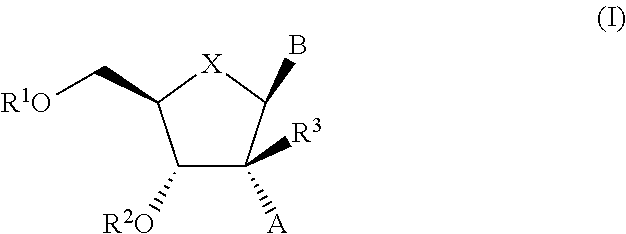
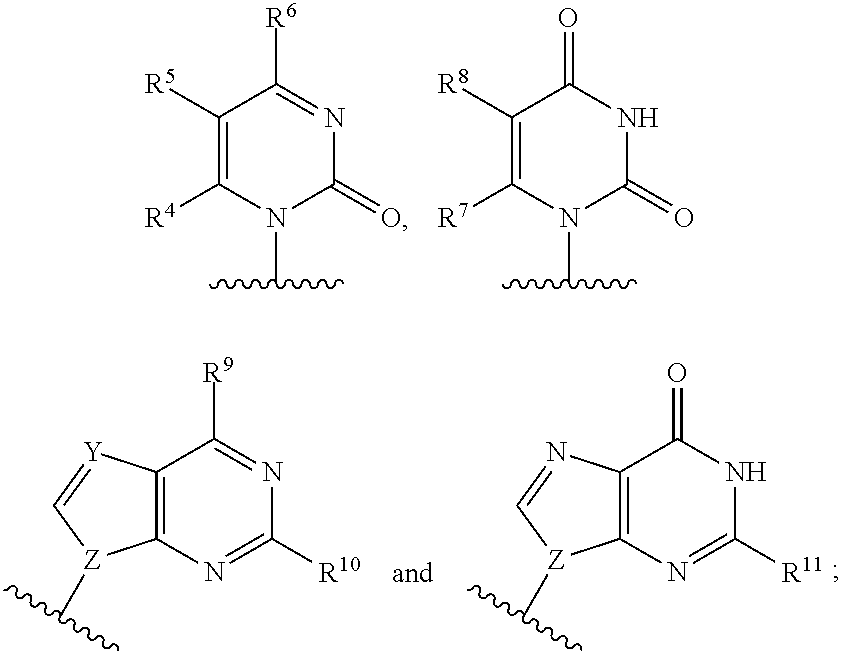
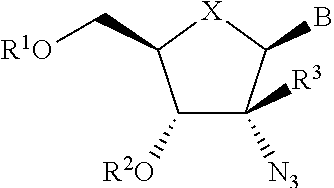
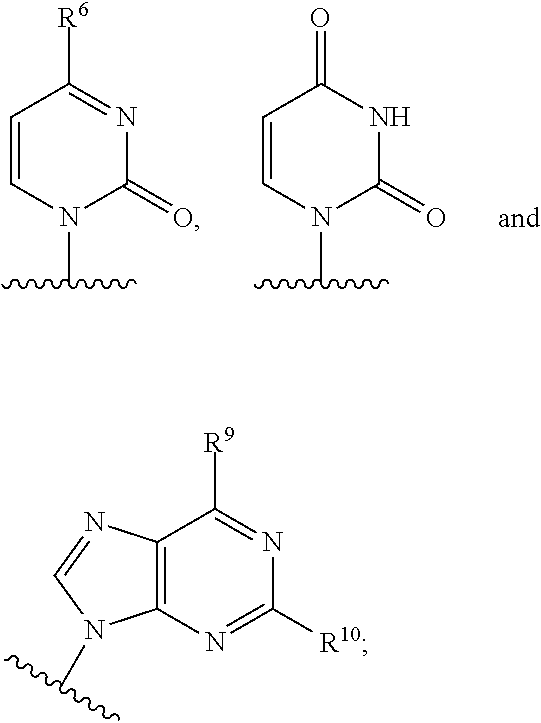
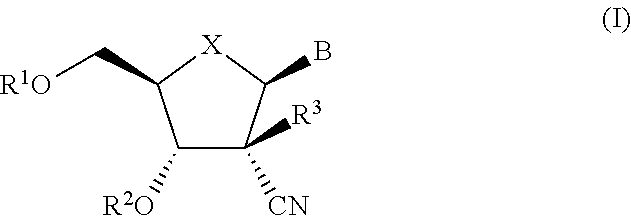
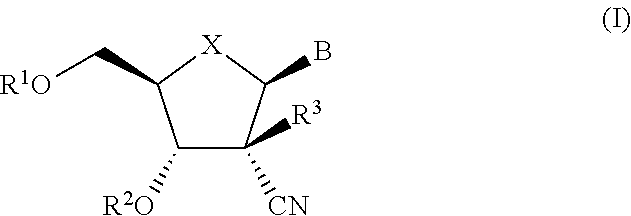
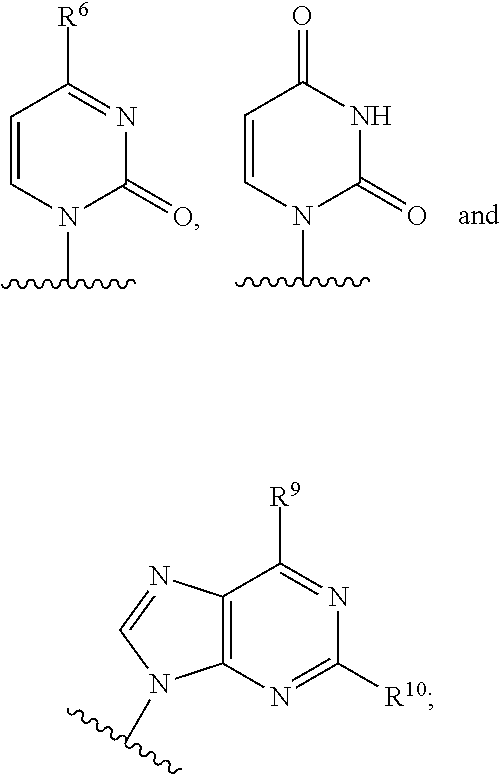
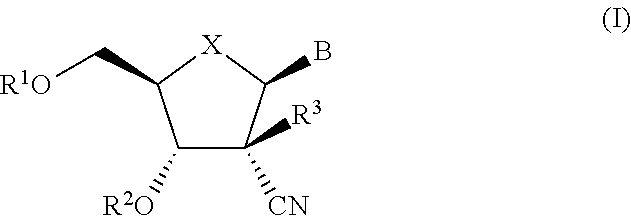
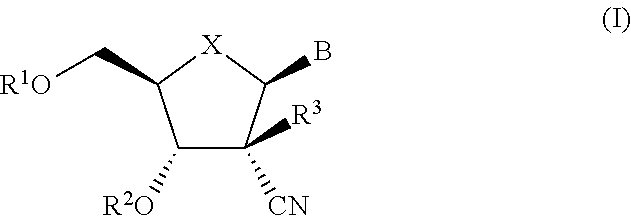
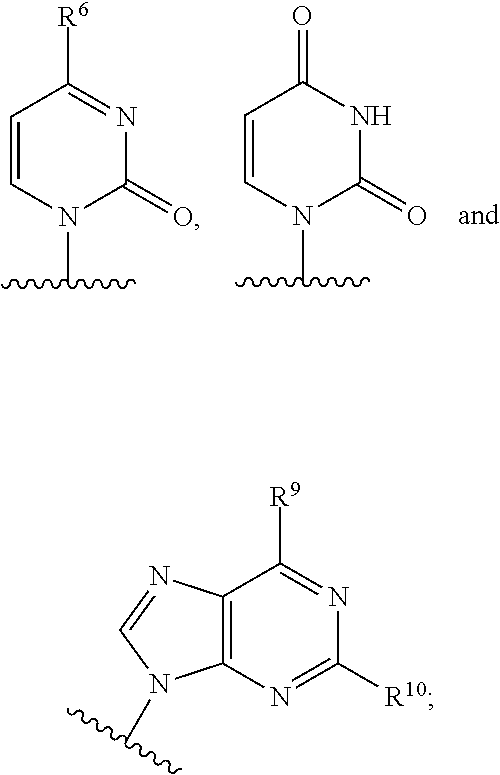
The present invention relates to 2′-Azido Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Azido Substituted Nucleoside Derivative, and methods of using the 2′-Azido Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
2′-substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases
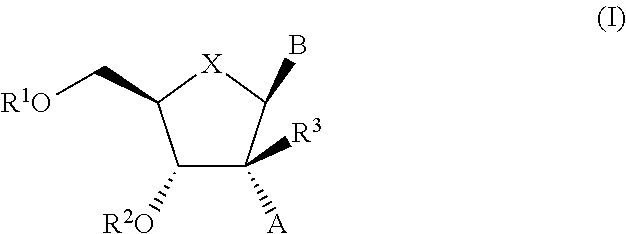
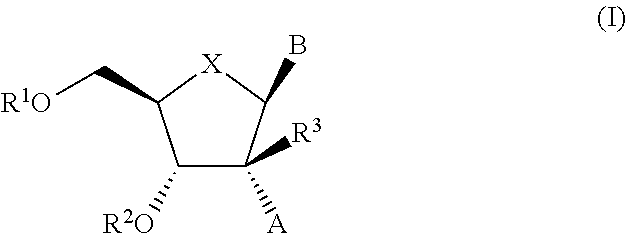
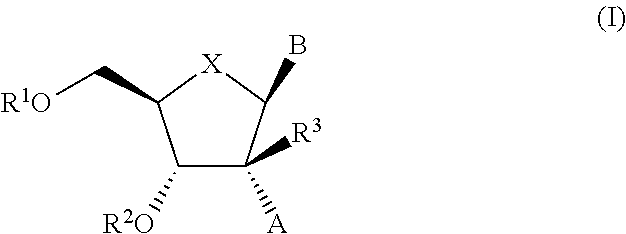
The present invention relates to 2′-Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein A, B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Substituted Nucleoside Derivative, and methods of using the 2′-Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
2′-azido substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases
The present invention relates to 2′-Azido Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Azido Substituted Nucleoside Derivative, and methods of using the 2′-Azido Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
Interferon tau mutants and methods for making them
InactiveUS6833256B1High activityLow cytotoxicityPeptide/protein ingredientsDepsipeptidesADAMTS ProteinsAutoimmune disease
The present invention is directed to the field of animal and human health, and more particularly to pharmacological uses of analogs or mutants of interferon-tau (IFN-tau) that differ from native IFN-tau because of substitutions of amino acids near the amino terminus of the IFN-tau molecule that impart improved biological activity. The IFN-tau mutants described in this disclosure have low toxicity, retain the same or slightly reduced antiviral activity compared with highly effective IFN-alpha, and have enhanced antiproliferative activity compared to native IFN-tau, making them useful in treating viral infections, cancer, and immune system diseases including autoimmune diseases. The present invention is also directed to a method for making novel recombinant proteins, especially interferons, interleukins, and cytokines, polypeptide hormones and other biopharmaceuticals that have improved biological activity over known proteins and / or lower toxicity and / or increased stability.
Owner:UNIV OF MARYLAND
Active biological medical fertilizer and preparation method thereof
InactiveCN101139233AGrowth spurtAmazing growth abilityBiocideBio-organic fraction processingMonkshoodsDisease
The present invention discloses an active biological drug fertilizer, which is characterized in that: the present invention is prepared with the fermenting process by photosynthetic bacteria, yellow corrupted acid and the extraction from Azadirachtin, Langdu Root and kusnezoff monkshood. The present invention is with an especial function for killing insect and preventing disease as well as accelerating the growth for the plant, protecting flower and fruit, enhancing the output and improving the integrative performance for the product quality and so on. The present invention is adaptable to the crops including rice, wheat, cotton, cole, bean, earthpea, corn, bast fibre plants, watermelon, fruit tree, vegetable, tobacco leaf, tea leaf, medicinal materials, flower, and pasture and seeding wood and so on. Besides, the present invention has also the function for accelerating the output for the pecan. The prevention and cure objects include: lepidopterans, orthopteran, Diptera, Coleoptera, ring vein, Hymenoptera and acarid and so on. The present invention has the much higher sensitivity to the following pests including: prodenia litura, asparagus caterpillar, cabbage butterfly, tea geometrid, tea smaller green leaf hopper, pecan peachblossom maggot and locust and so on.
Owner:袁进
Preservation by vaporization
ActiveUS9469835B2Maximize potencyMaximize viabilityBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
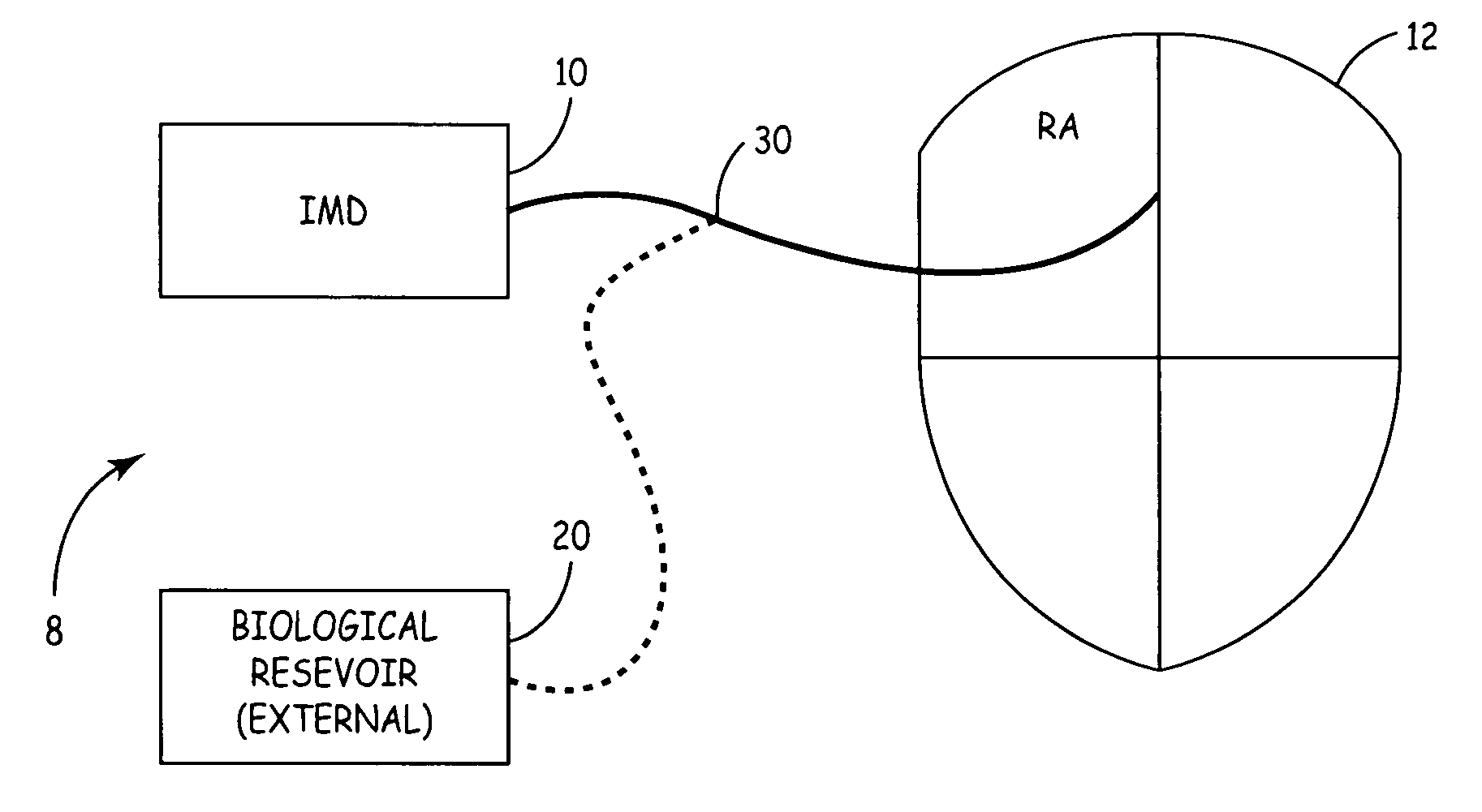
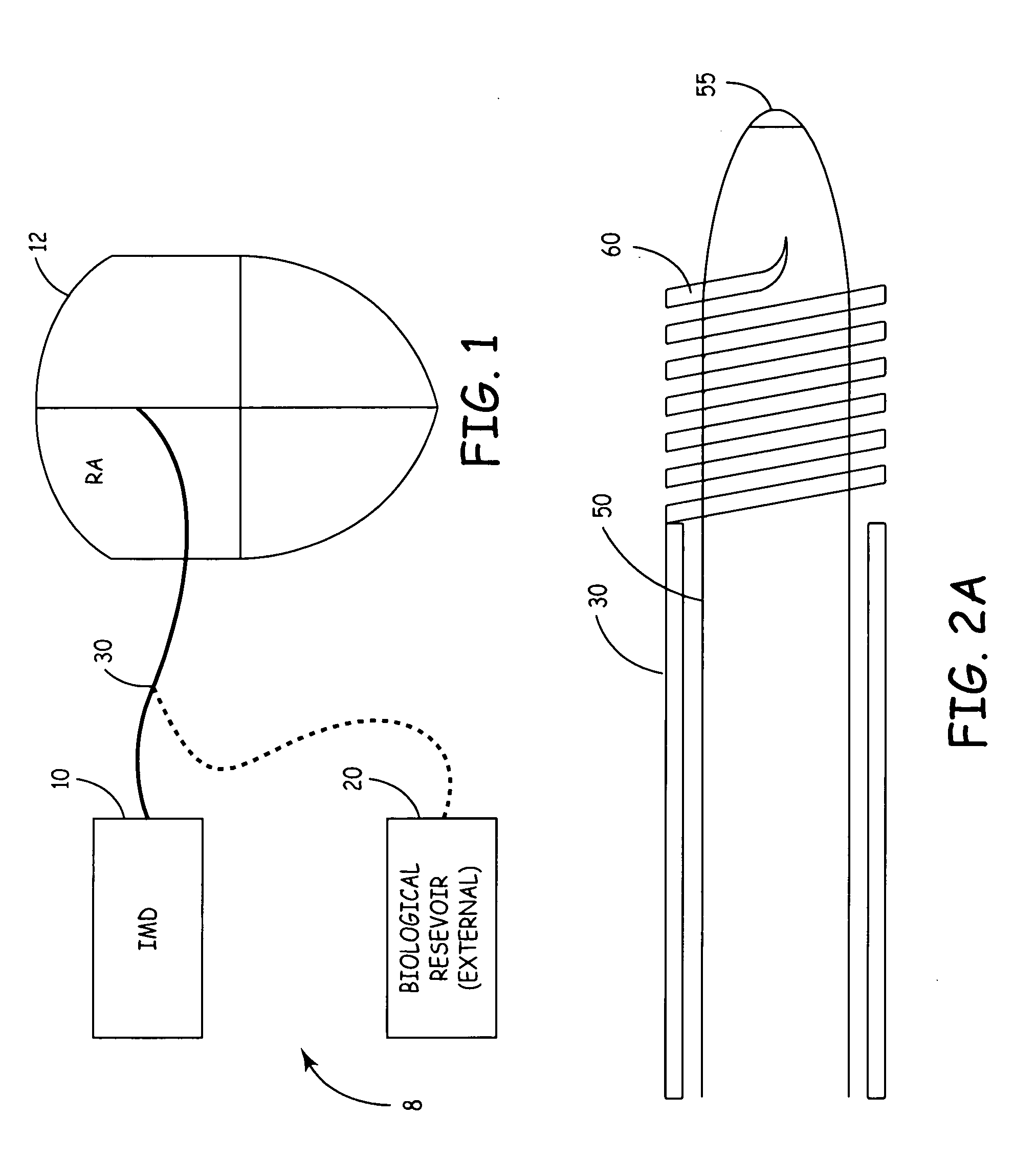
System for the delivery of a biologic therapy with device monitoring and back-up
InactiveUS20050021091A1ElectrocardiographyGenetic material ingredientsCardiac monitoringMedical device
An implantable medical device (IMD), such as a cardiac monitor and / or pulse generator is implanted during a procedure where a biologic is introduced into a targeted area of the heart. The IMD monitors cardiac performance to determine the efficacy of the biologic. Based on the achieved efficacy, the IMD will either take no action, provide device based therapy, and / or ablate the tissue to destroy the biologic and its effects if the biologic proves unsuccessful.
Owner:MEDTRONIC INC
Protein tyrosine kinase modulators and methods of use
InactiveCN105377835AOrganic active ingredientsOrganic chemistryKinase activityTyrosine Protein Kinases
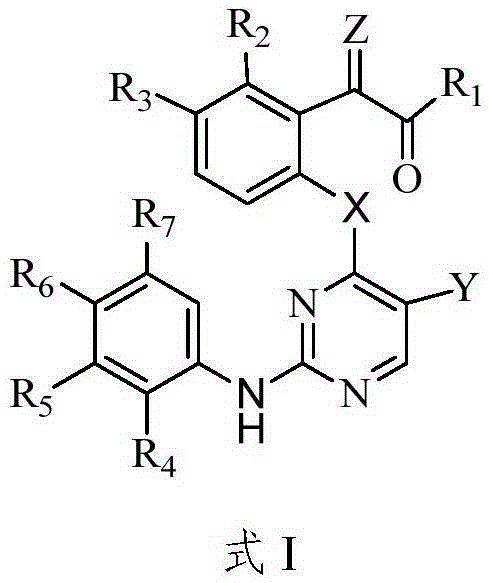
Heterocyclic pyrimidine compounds that modulate mutant-selective epidermal growth factor receptor (EGFR) and ALK kinase activity are disclosed. More specifically, the invention provides pyrimidines which inhibit, regulate and / or modulate kinase receptor, particularly in selectively modulation of various EGFR mutant activity and ALK kinase activity have been disclosed. Pharmaceutical compositions comprising the pyrimidine derivative,and methods of treatment for diseases associated with protein kinase enzymatic activity, particularly EGFR or ALK kinase activity including non-small cell lung cancer comprising administration of the pyrimidine derivative are disclosed.
Owner:BETTA PHARM CO LTD
2'-cyano substituted nucleoside derivatives and methods of use thereof useful for the treatment of viral diseases
ActiveUS20140161770A1Preventing HCV infectionTreating and preventing HCV infectionOrganic active ingredientsSugar derivativesDiseaseMedicine
The present invention relates to 2′-Cyano Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Cyano Substituted Nucleoside Derivative, and methods of using the 2′-Cyano Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
Biological drug for organic vegetables for preventing plant diseases and insect pests, and preparation method thereof
The invention proposes a biological drug for organic vegetables for preventing plant diseases and insect pests, wherein the biological drug comprises the following components by weight: main ingredients, including 3-6kg of radishes, 3-6kg of onions, 3-6kg of garlic, 3-6kg of hot pepper, 3-6kg of pepper and 3-6kg of pine needles, 8-12kg of plant ashes, auxiliary ingredients, including 3-5kg of cacumen biotae, 3-5kg of quispualis indica, 3-5kg of fructus cnidii, 3-5kg of fructus kochiae, 3-5kg of aloes, 3-5kg of rheum officinale, 3-5kg of white alum, 3-5kg of stone-like omphalia, 3-5kg of radix stemonae, 3-5kg of ginger and 3-5kg of cinnamon, and assistants, including 8-12kg of 10% soduim chloride water solution, 8-12kg of 10% magnesium chloride water solution and 8-12kg of 8% sodium carboxymethylcellulose water solution. The biological drug is simple in proportioning, and convenient to use, and a plurality of plant diseases and insect pests can be effectively prevented and treated.
Owner:QINGDAO DONGYI JINHE AGRI TECH
2'-substituted nucleoside derivatives and methods of use thereof for the treatment of viral diseases
The present invention relates to 2′-Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein A, B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Substituted Nucleoside Derivative, and methods of using the 2′-Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
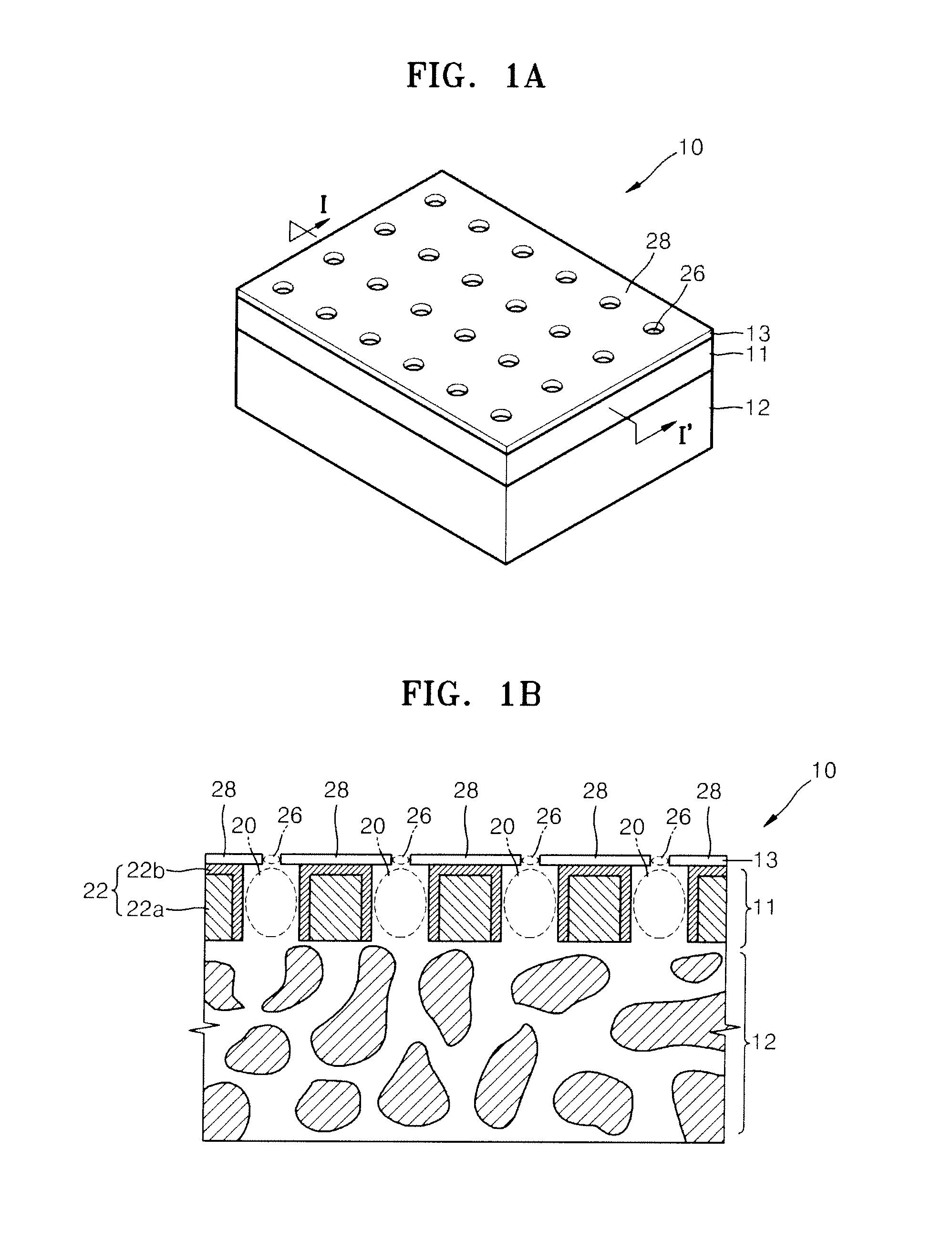
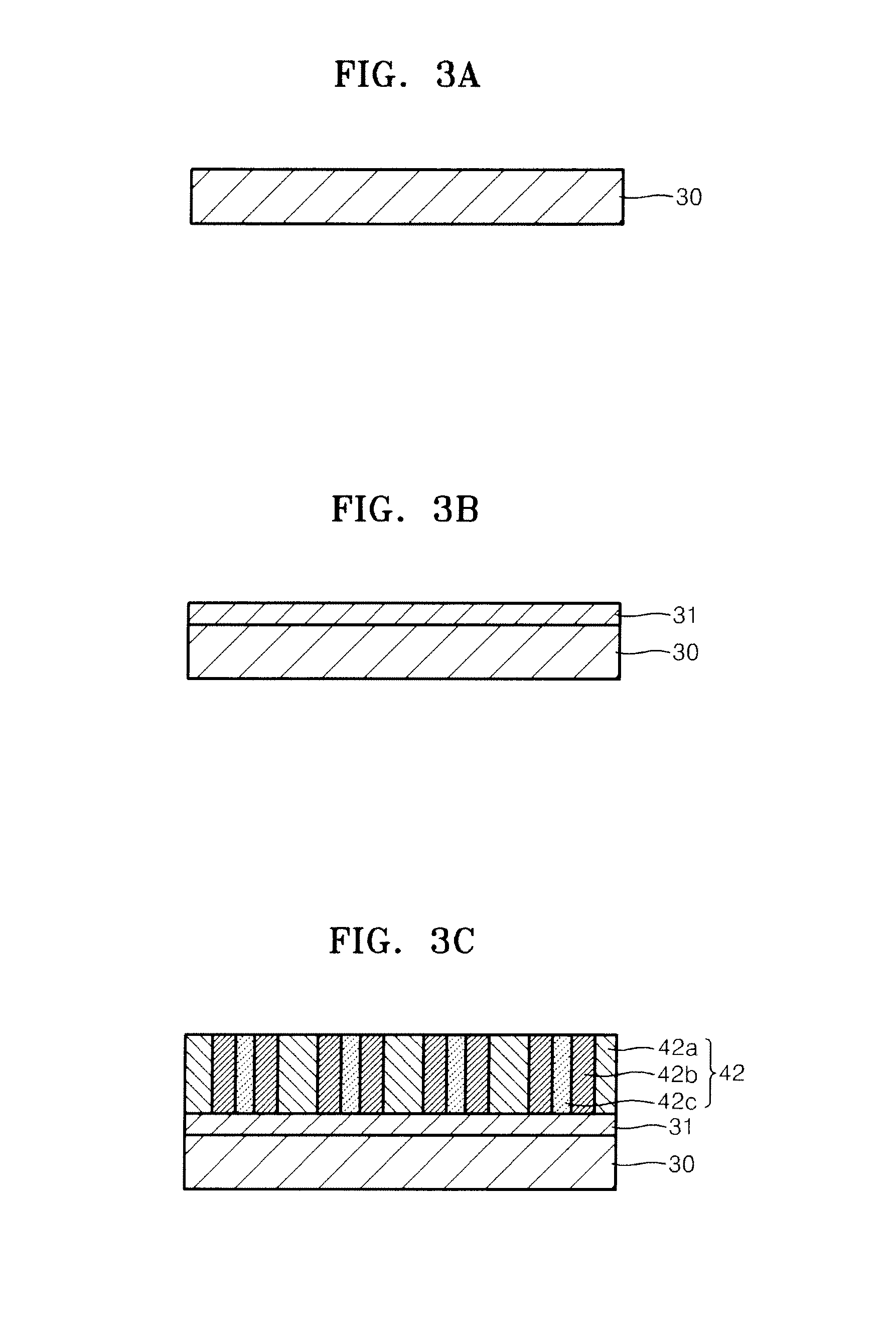
Nanoporous membrane, process of fabricating the same and device for controlled release of biopharmaceuticals comprising the same
InactiveUS7935416B2High hole densityUniform pore sizeSemi-permeable membranesMembranesControl releaseNanoporous membrane
Provided are a nanoporous membrane including a support; a first separation layer with a plurality of first nano-sized pores and a first matrix; and a second separation layer having a plurality of second pores respectively corresponding to the plurality of first pores of the first separation layer and a second matrix, and formed on the first separation layer, wherein a density of the plurality of the first pores and the second pores is equal to or greater than 1010 / cm2, and a diameter of each of the second pores is less than that of the corresponding first pore, a process of fabricating the same, and a device for a controlled release of biopharmaceuticals including the nanoporous membrane. The device for a controlled release of biopharmaceuticals including the nanoporous membrane can release biopharmaceuticals at a constant rate for a long period of time regardless of the concentration of the biopharmaceuticals including in pharmaceuticals, and high flex and selectivity.
Owner:POSTECH ACAD IND FOUND
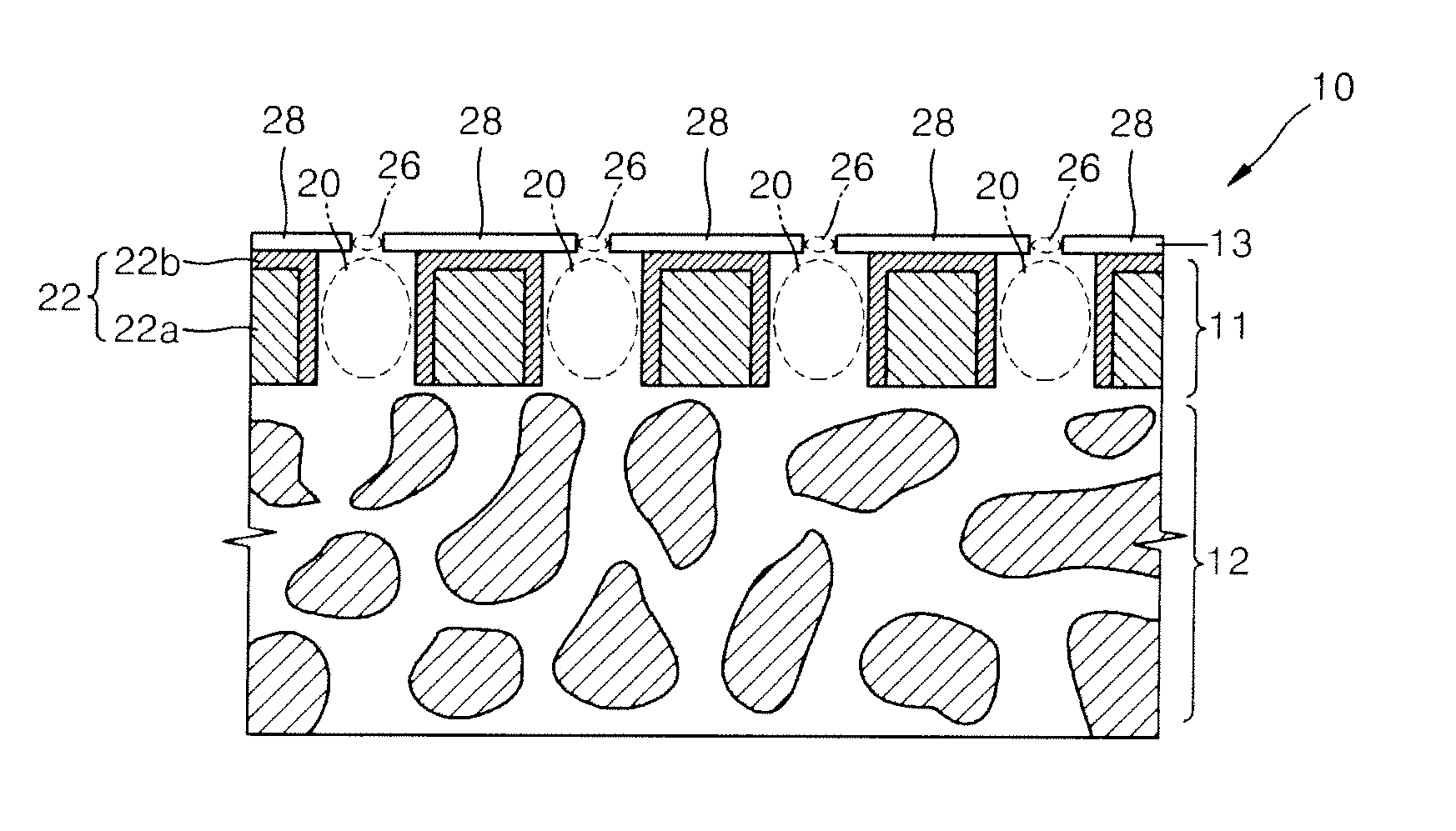
Bulk freezing of biopharmaceuticals
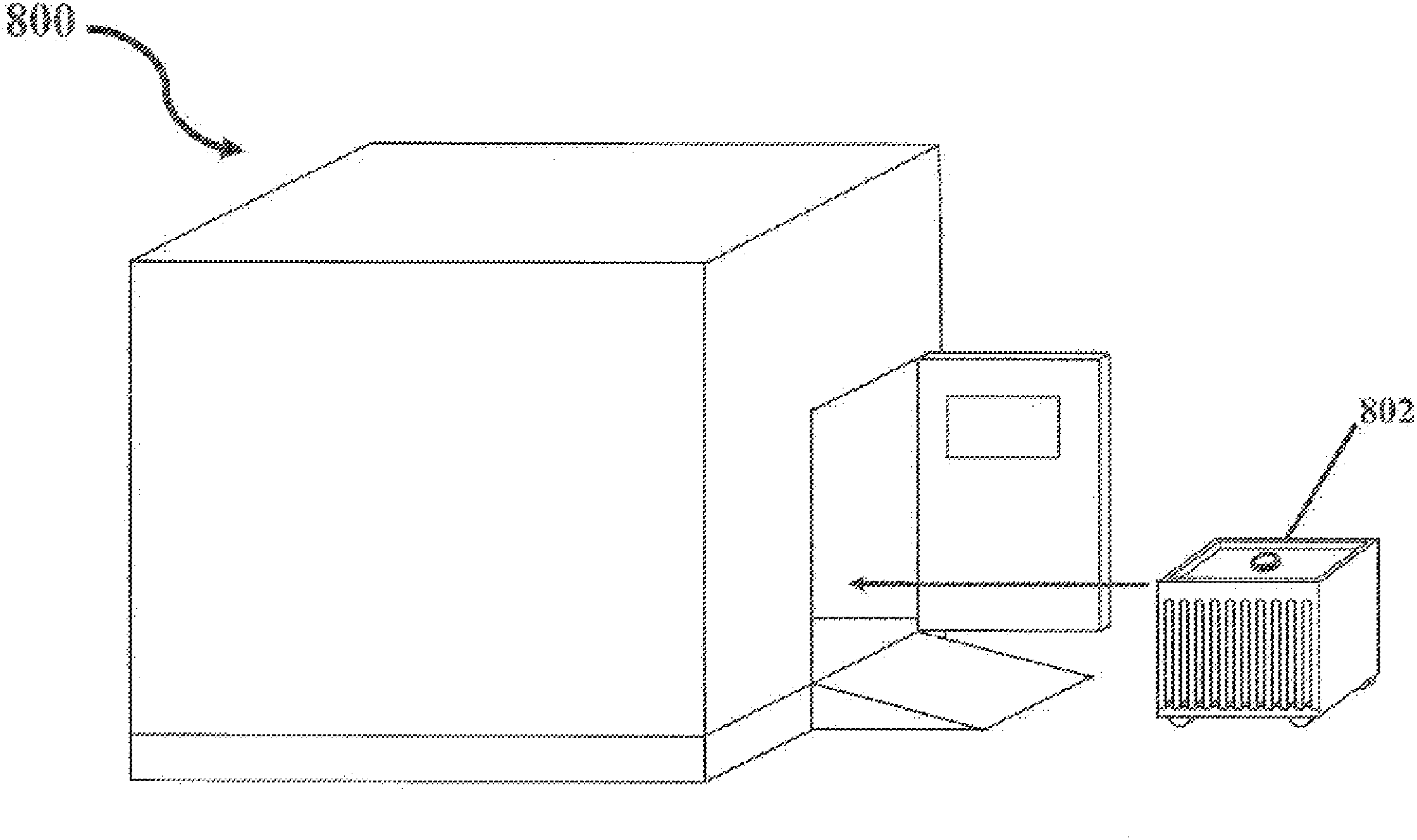
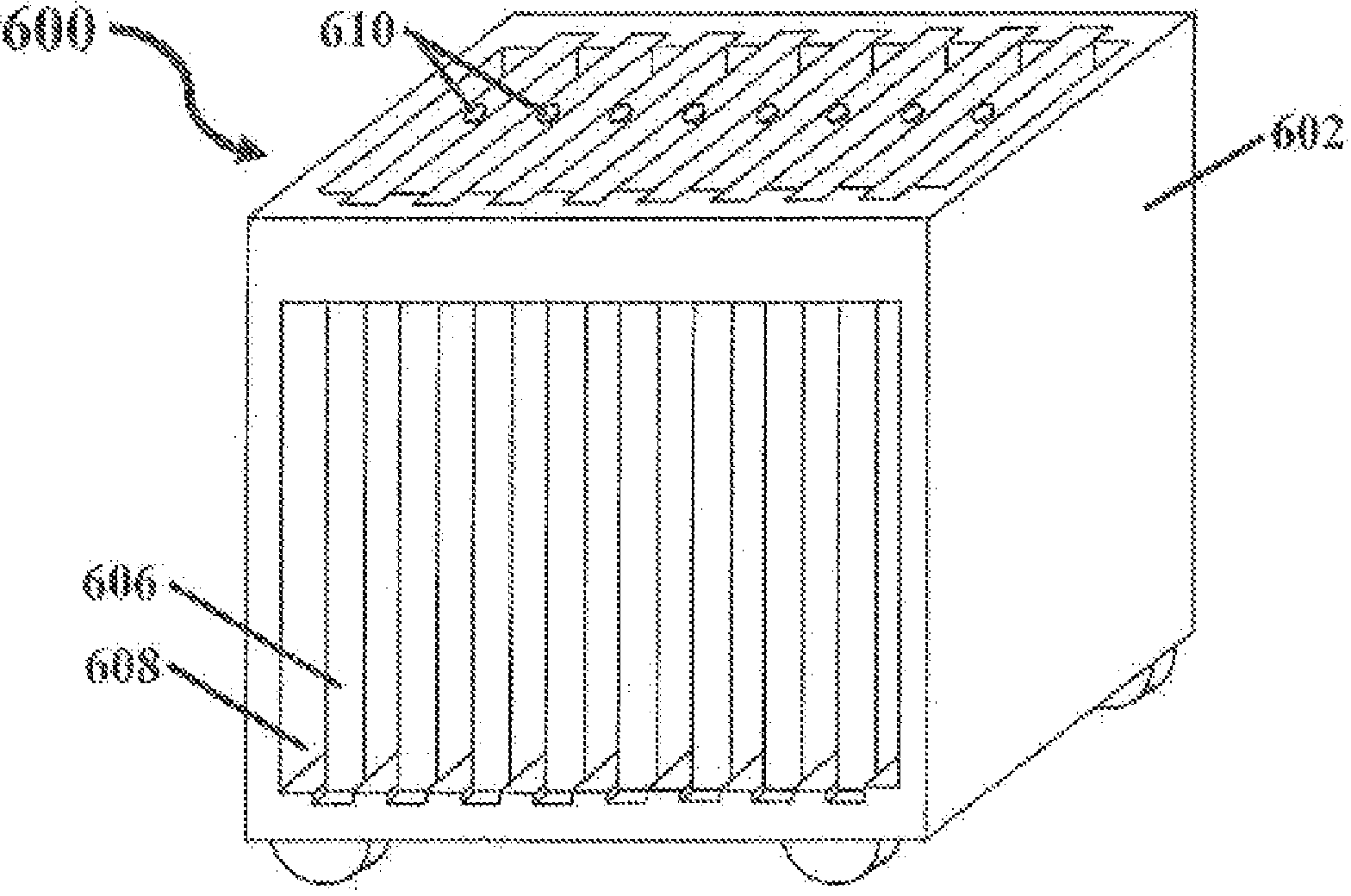
A system and method for bulk freezing is provided. In one embodiment, the system and method for bulk freezing includes a bulk freezing container adapted to hold at least one and preferably a plurality of bags holding a biopharmaceutical liquid. The bulk freezing container includes at least a first and second shelf having corrugations, wherein the second shelf is vertically arranged above the first shelf with the bags disposed between the shelves. The bags and the corrugations of the first and second shelves define a plurality of substantially parallel flow channels through which a cryogenic cold fluid or a warming fluid is passed to freeze and / or thaw the biopharmaceutical fluid. In another embodiment, the bulk freezing system and method includes a bulk freezing container with a plurality of adjacent elongated chambers adapted for holding the biopharmaceutical fluid.
Owner:PRAXAIR TECH INC
Method for preparing nano-porous light silicon oxide microspheres
ActiveCN104556057ASimple preparation processLow priceMaterial nanotechnologySilicaChromatographic separationPtru catalyst
Owner:泉州三欣新材料科技有限公司
Hydrogel particle formation
InactiveUS20050191361A1Improve performanceAvoid lostPowder deliveryGenetic material ingredientsDiagnostic agentActive agent
New compositions formed from the combination of an active substance with a hydrogel carrier moiety are provided. The compositions are suitable for use in high-velocity transdermal particle injection techniques. Methods of providing the new compositions are also provided. In addition, methods for administering pharmacologically active agent to a subject are provided. These methods are useful for delivering drugs, biopharmaceuticals, vaccines and diagnostics agents.
Owner:POWEDERJECT RES
Method and system for discovering and integrating rectal cancer related genes by utilizing public data resources and analyzing functions of rectal cancer related genes, and application
ActiveCN107066835AReduce research costsImprove analysis efficiencyProteomicsGenomicsWeaknessDifferentially expressed genes
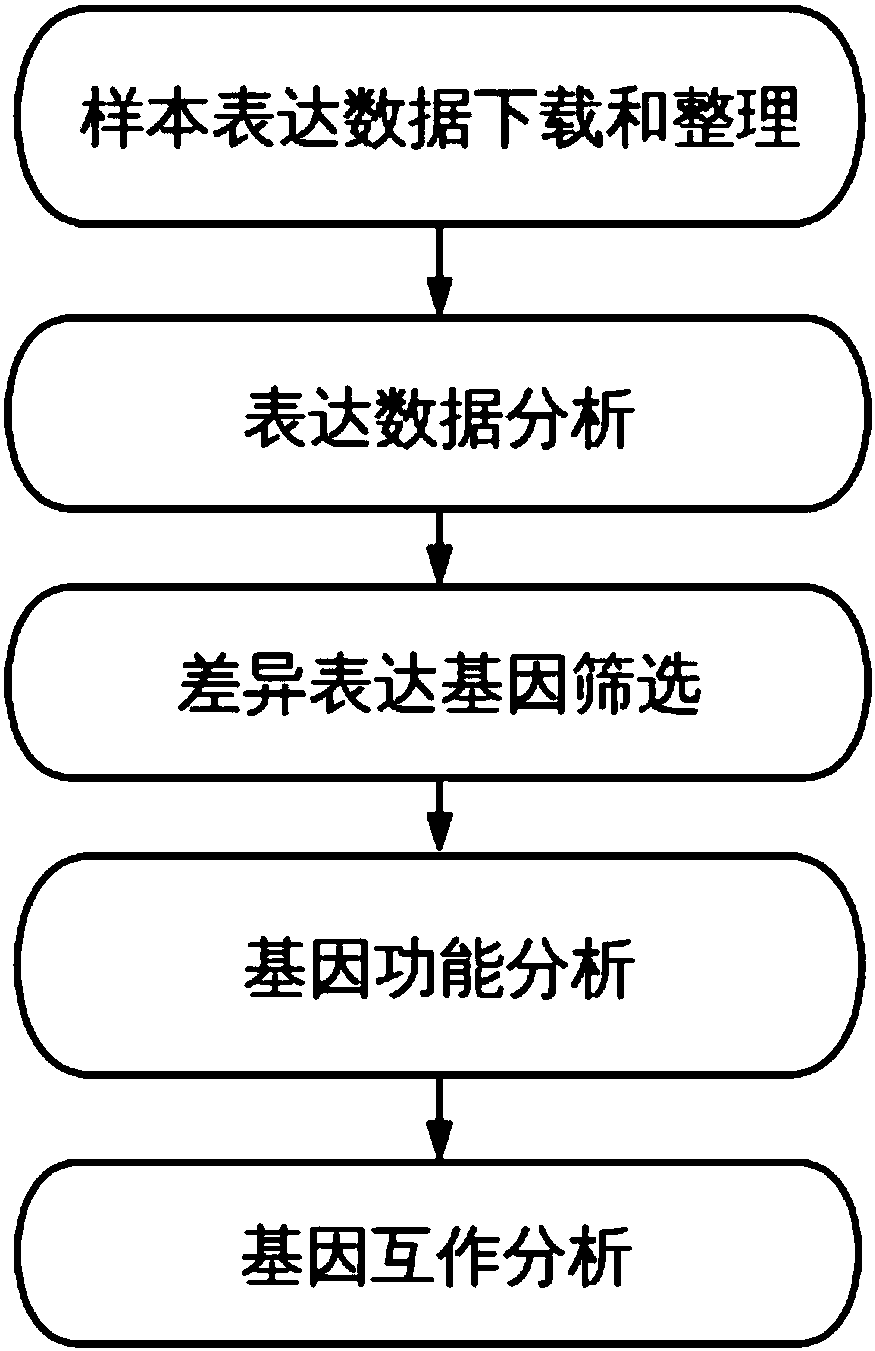
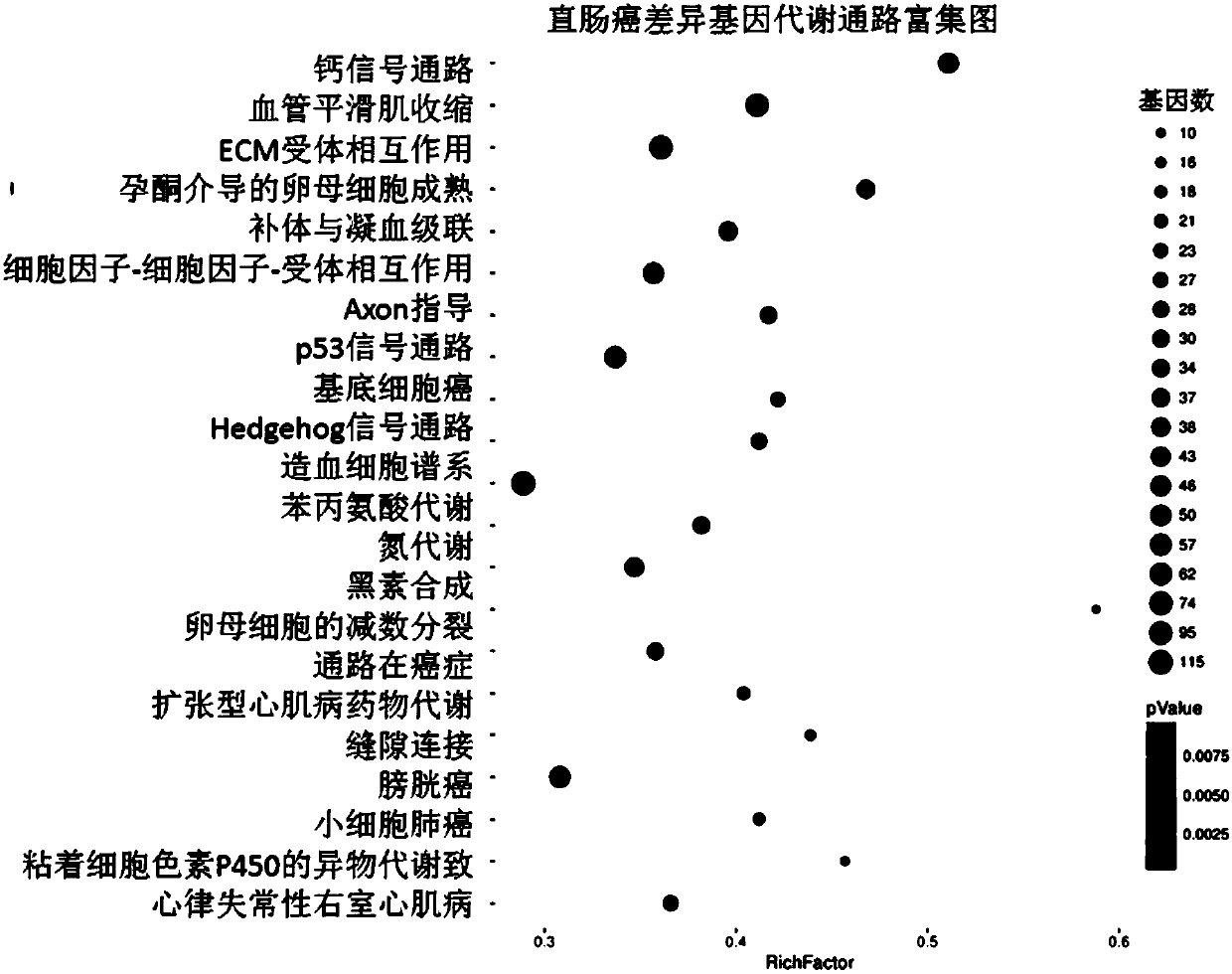
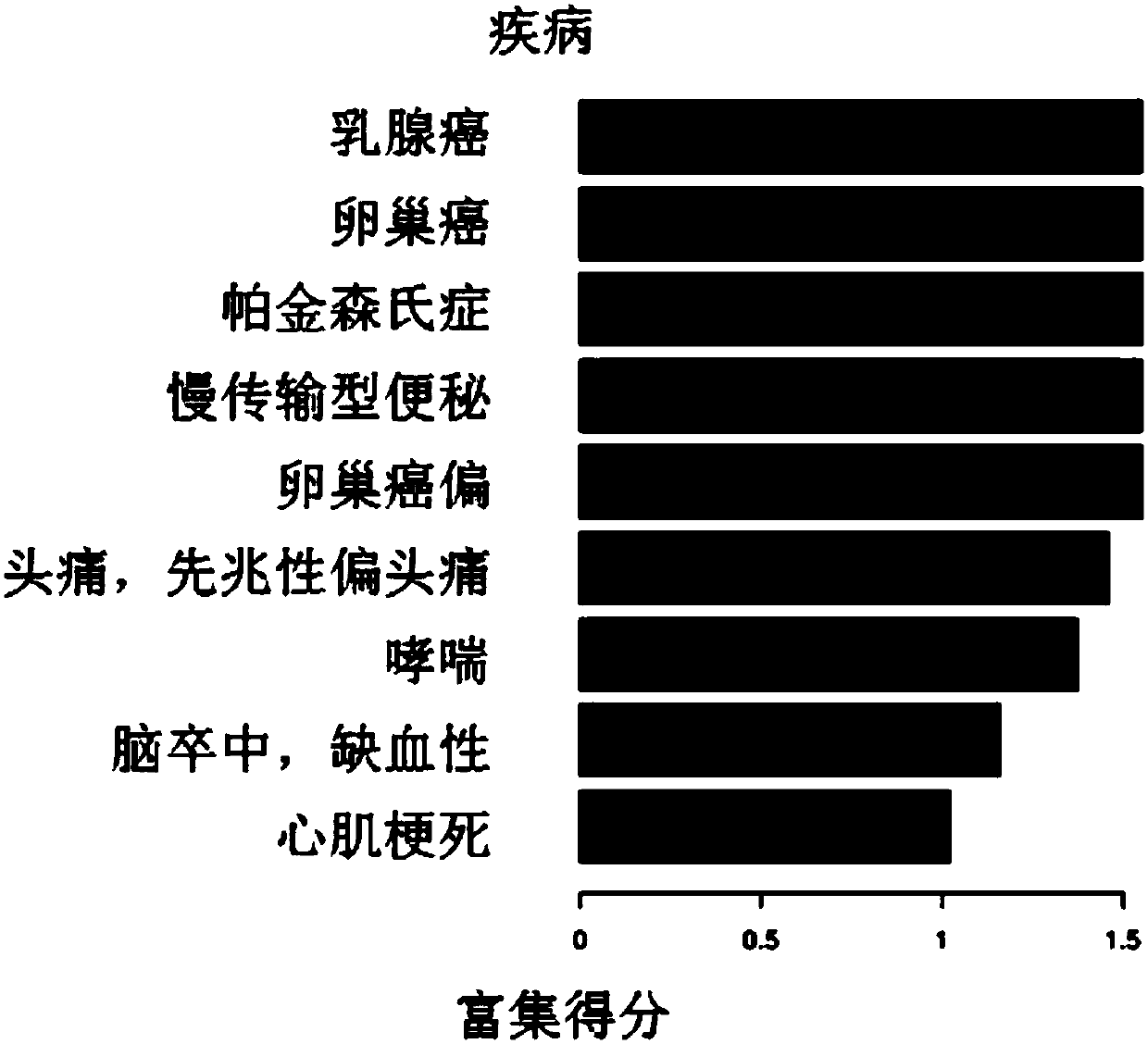
The invention discloses a method and a system for discovering and integrating rectal cancer related genes by utilizing public data resources and analyzing functions of the rectal cancer related genes, and an application. Based on the public data resources, open big data resources and diversified bioinformatics analysis means are reasonably used for performing analysis processing on mRNA expression data, and important genes related to complex diseases and functions of the important genes are identified. The method comprises the steps of sample data downloading and management; gene expression data analysis; difference expression gene screening; and gene function analysis and protein interaction analysis. According to the method and the system, the problems of weakness in integrating existing network resources, unfamiliarity with mRNA related most common databases and frontal analysis methods, incapability of independently finishing mRNA expression spectrum related bioinformatics analysis and the like can be solved; a plurality of risk pathways and genes related to the complex diseases such as the rectal cancer and the like can be discovered; and the method and the system are of important significance for biological targeted treatment of the complex diseases, biological drug research and development, pathogenesis explanation and risk prediction.
Owner:SOUTHEAST UNIV
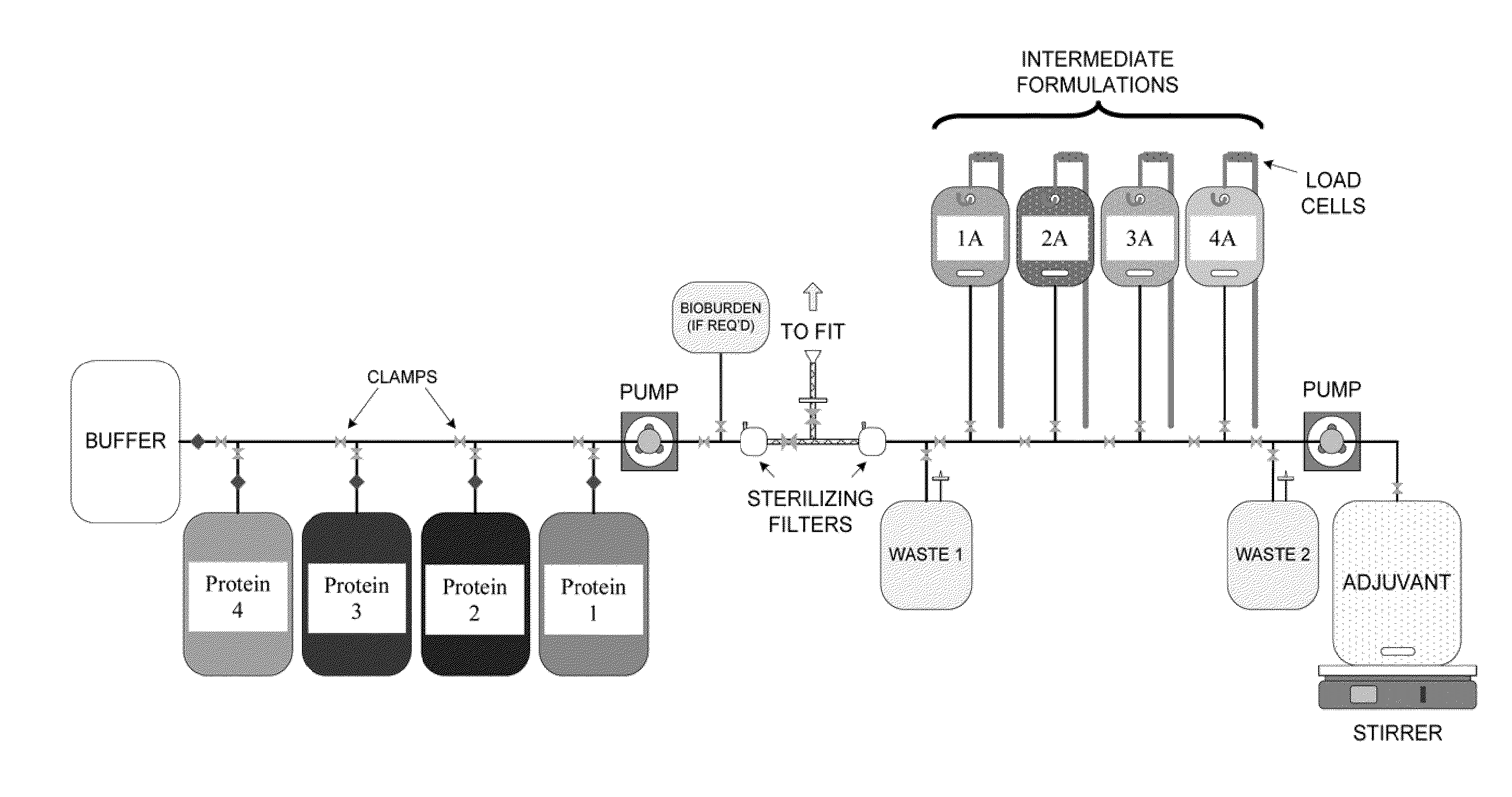
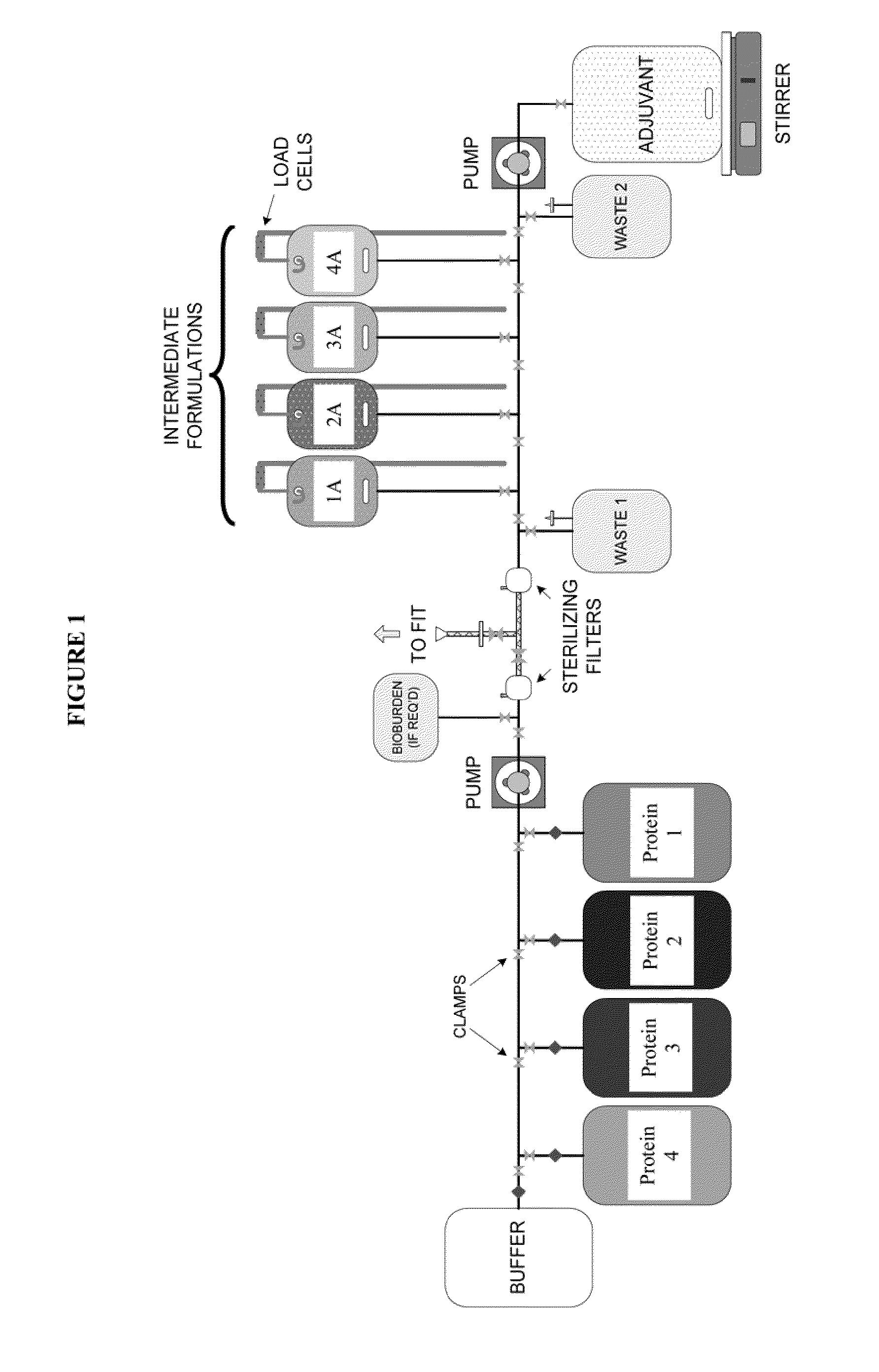
System and process for producing multi-component biopharmaceuticals
A sterile, closed, disposable system for formulating biopharmaceutical compositions containing multiple active agents is described herein.
Owner:SANOFI PASTEUR LTD
2′-cyano substituted nucleoside derivatives and methods of use thereof useful for the treatment of viral diseases
The present invention relates to 2′-Cyano Substituted Nucleoside Derivatives of Formula (I): and pharmaceutically acceptable salts thereof, wherein B, X, R1, R2 and R3 are as defined herein. The present invention also relates to compositions comprising at least one 2′-Cyano Substituted Nucleoside Derivative, and methods of using the 2′-Cyano Substituted Nucleoside Derivatives for treating or preventing HCV infection in a patient.
Owner:MERCK SHARP & DOHME LLC
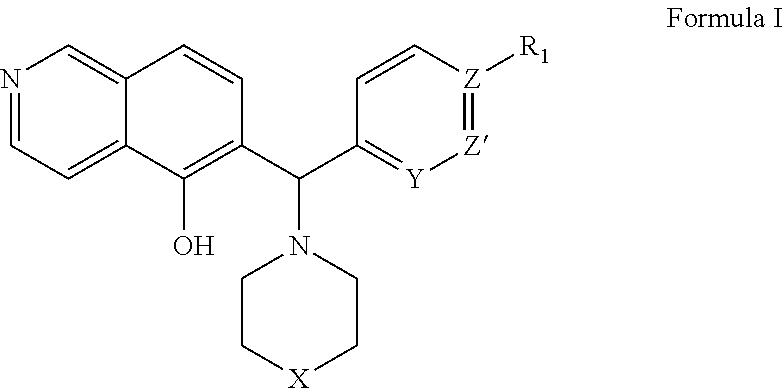
Methods and compositions useful for treating diseases involving bcl-2 family proteins with isoquinoline and quinoline derivatives
InactiveUS20160038503A1BiocideBoron compound active ingredientsAbnormal tissue growthAutoimmune condition
The present invention relates to a compositions for and methods for cancer treatment, for example, hematopoietic cancers (e.g. B-cell Lymphoma). In other aspects, the invention provides methods for treating particular types of hematopoietic cancers, such as B-cell lymphoma, using a combination of one or more of the disclosed compounds and, for example, 26S proteasome inhibitors, such as, for example, Bortezomib. In another aspect the present invention relates to autoimmune treatment with the disclosed compounds. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have unique in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
Antiviral and antimicrobial compounds
Disclosed are guanidine and biguanidine derivatives which have anti-viral and antibacterial activity. Also disclosed are pharmaceutical compositions containing such compounds as an active ingredient, and anti-viral and anti-bacterial methods utilizing such compounds. Methods of treating infections using the guanidine and biguanidine derivatives are also disclosed.
Owner:VYMED
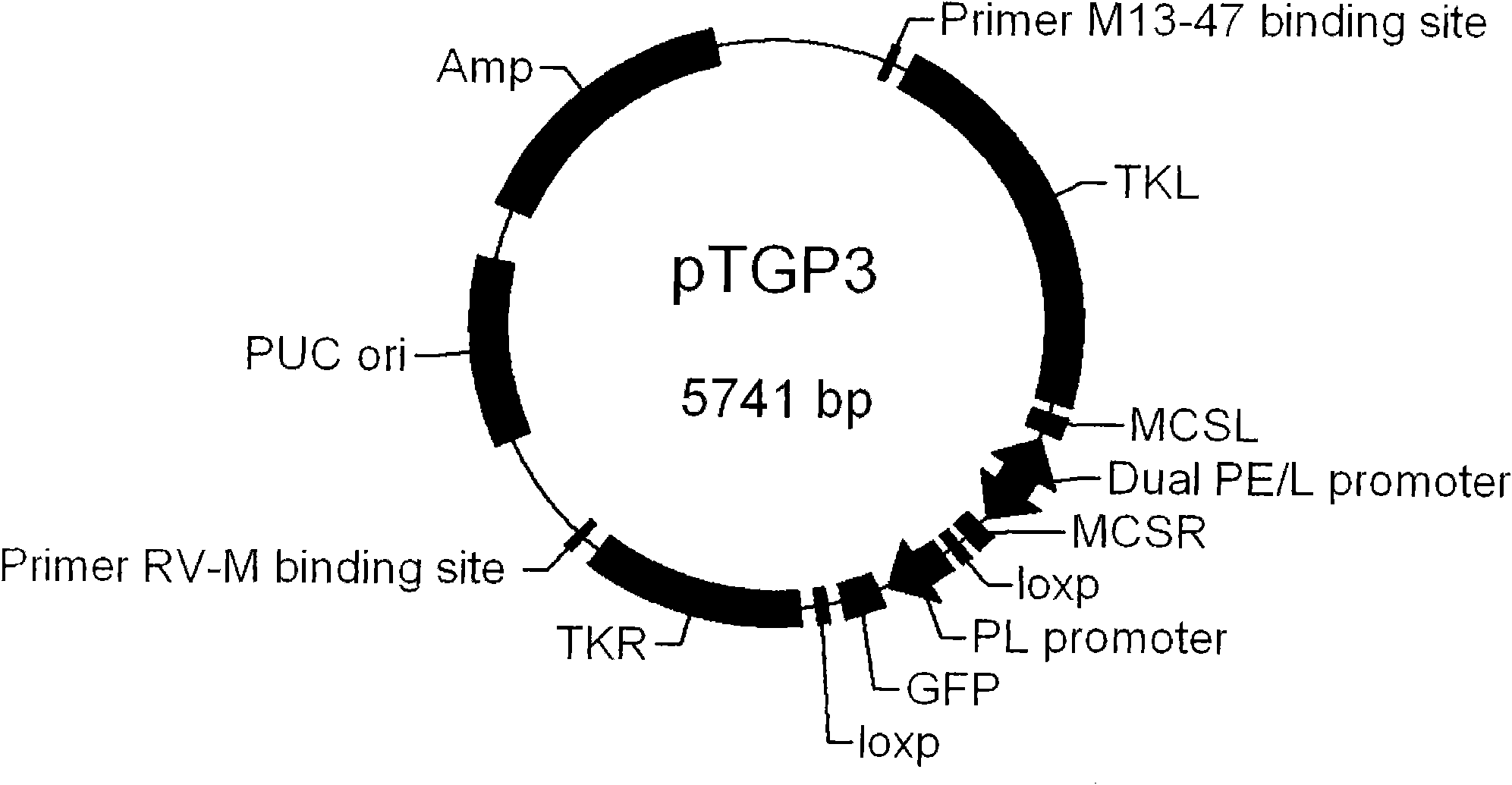
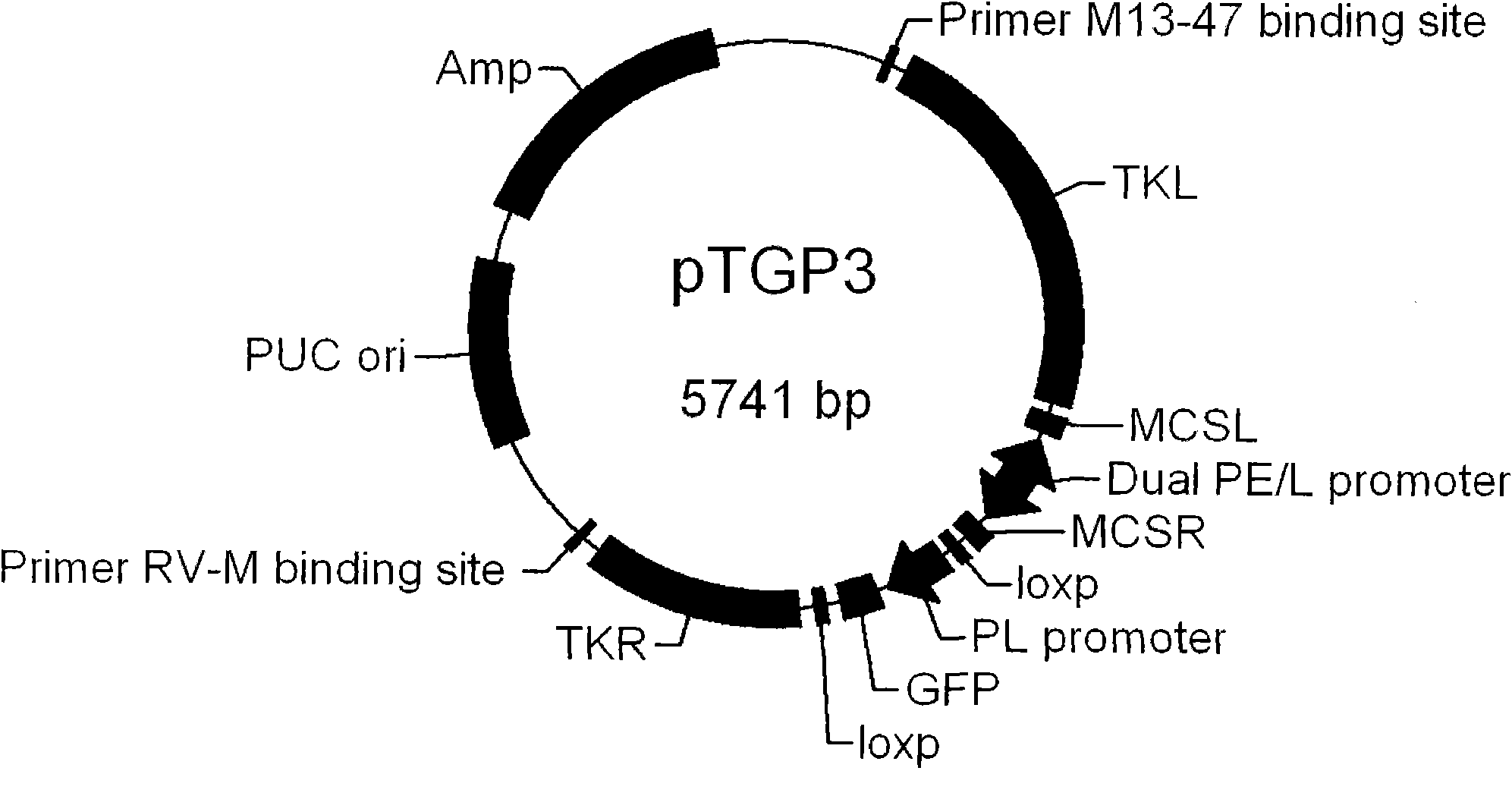
Fowlpox virus vector shuttle plasmid and application thereof
InactiveCN101775410APreserve immune efficiencyRetain the ability to replicateGenetic material ingredientsGenetic engineeringShuttle plasmidFowlpox virus
The invention provides fowlpox virus vector shuttle plasmid pTGP3 which comprises recombinant arms TKL and TKR, a bidirectional promoter PE / L, a fluorescent protein expression cassette, and a resistant marker gene and replication origin ori; the upstream and the downstream of the bidirectional promoter PE / L are respectively provided with cloning sites MCSL and MCSR; and both ends of the fluorescent protein expression cassette are provided with loxp sequences. The plasmid of the invention has two different screening markers, and the recombinant fowlpox virus prepared with the plasmid can express 1 to 3 types of gene with different meshes in the whole processes of the early and the later periods; the strong composite promoter with expression activity in the early and the later periods is applied so as to realize the all-process high-efficiency expression of a target gene; and the loxp sequences are introduced into both ends of the fluorescent protein expression cassette, so as to knock out the exogenous recombinant fowlpox virus screening markers. The invention lays foundation for the series and the scale application of the recombinant fowlpox virus in vaccine and biological drug research and development fields.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
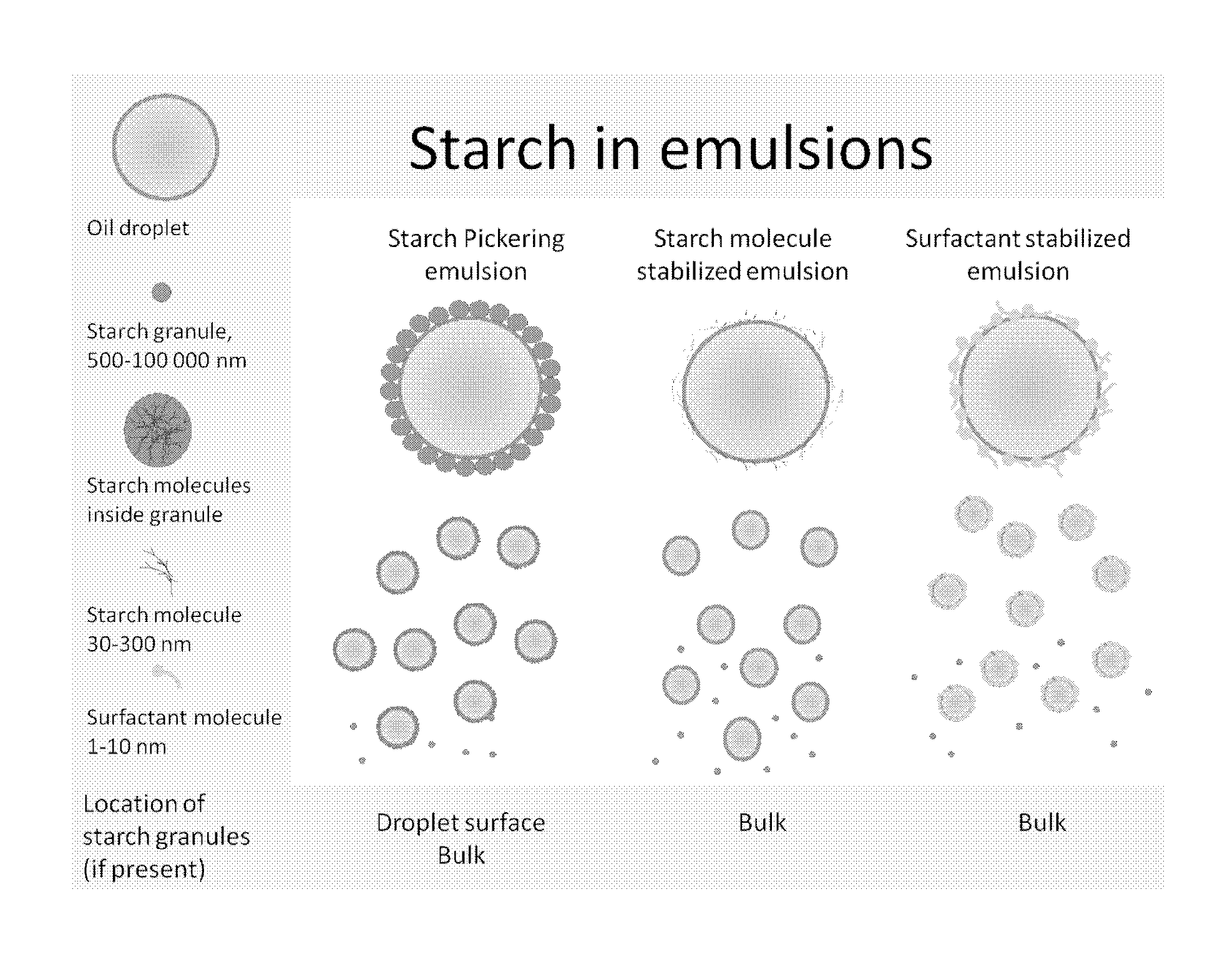
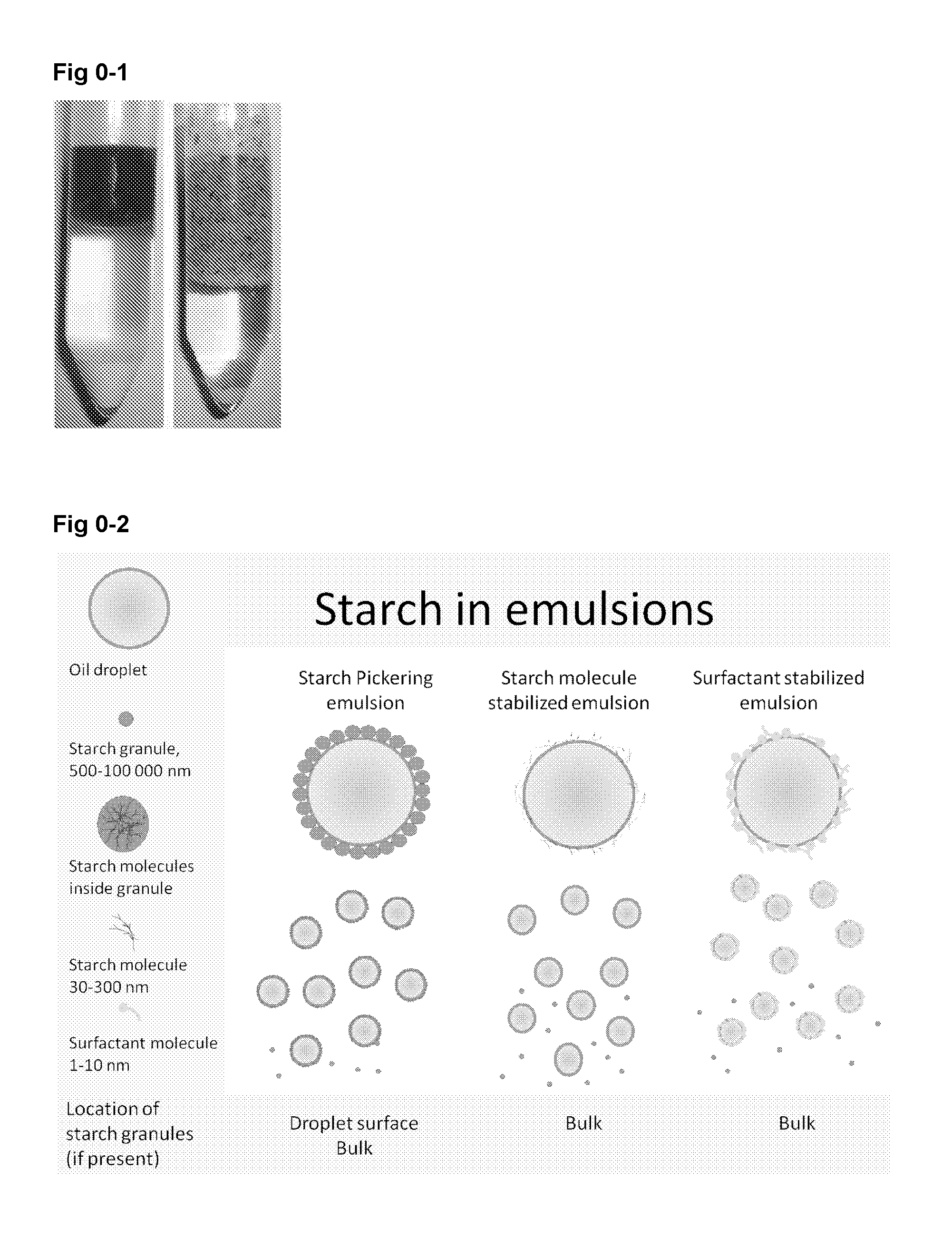
New particle stabilized emulsions and foams
InactiveUS20150125498A1Novel and useful emulsifying propertyFlexibility of systemBiocideCosmetic preparationsSolid particlePharmaceutical formulation
The present invention relates to a particle stabilized emulsion or foam comprising at least two phases and solid particles, wherein said solid particles are starch granules and said starch granules or a portion thereof are situated at the interface between the two phases providing the particle stabilized emulsion or foam. The invention further relates to the use of said particle stabilized emulsion or foam for encapsulation of substances chosen from biopharmaceuticals, proteins, probiotics, living cells, enzymes and antibodies, sensitive food ingredients, vitamins, and lipids in food products, cosmetic products, skin creams, and pharmaceutical formulations.
Owner:SPEXIMO
Preparation of ultra-dispersed nano diamond hydrosol
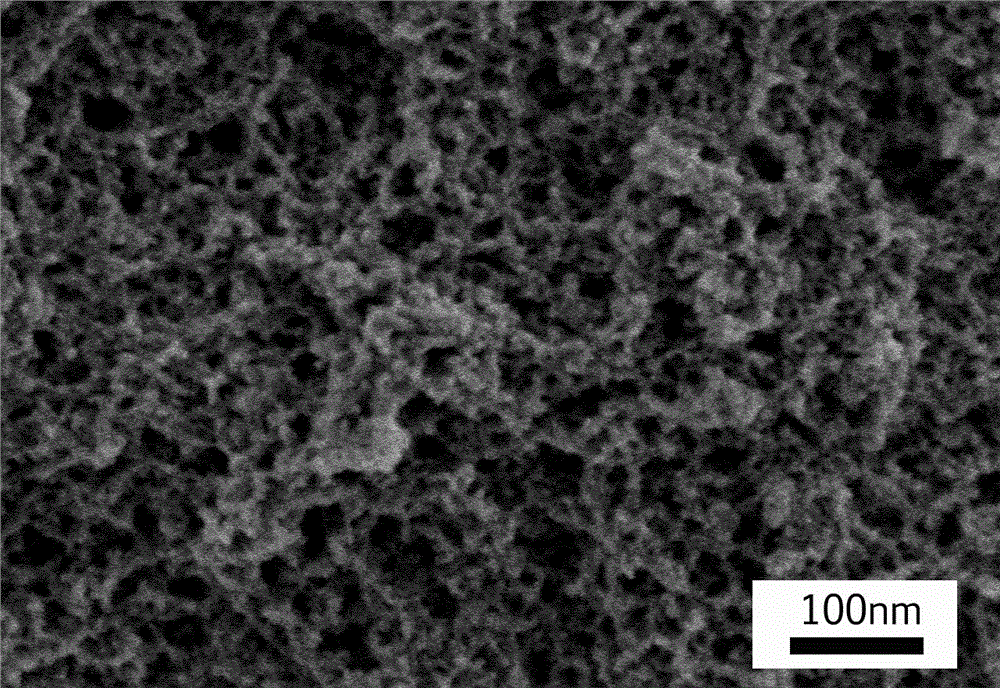
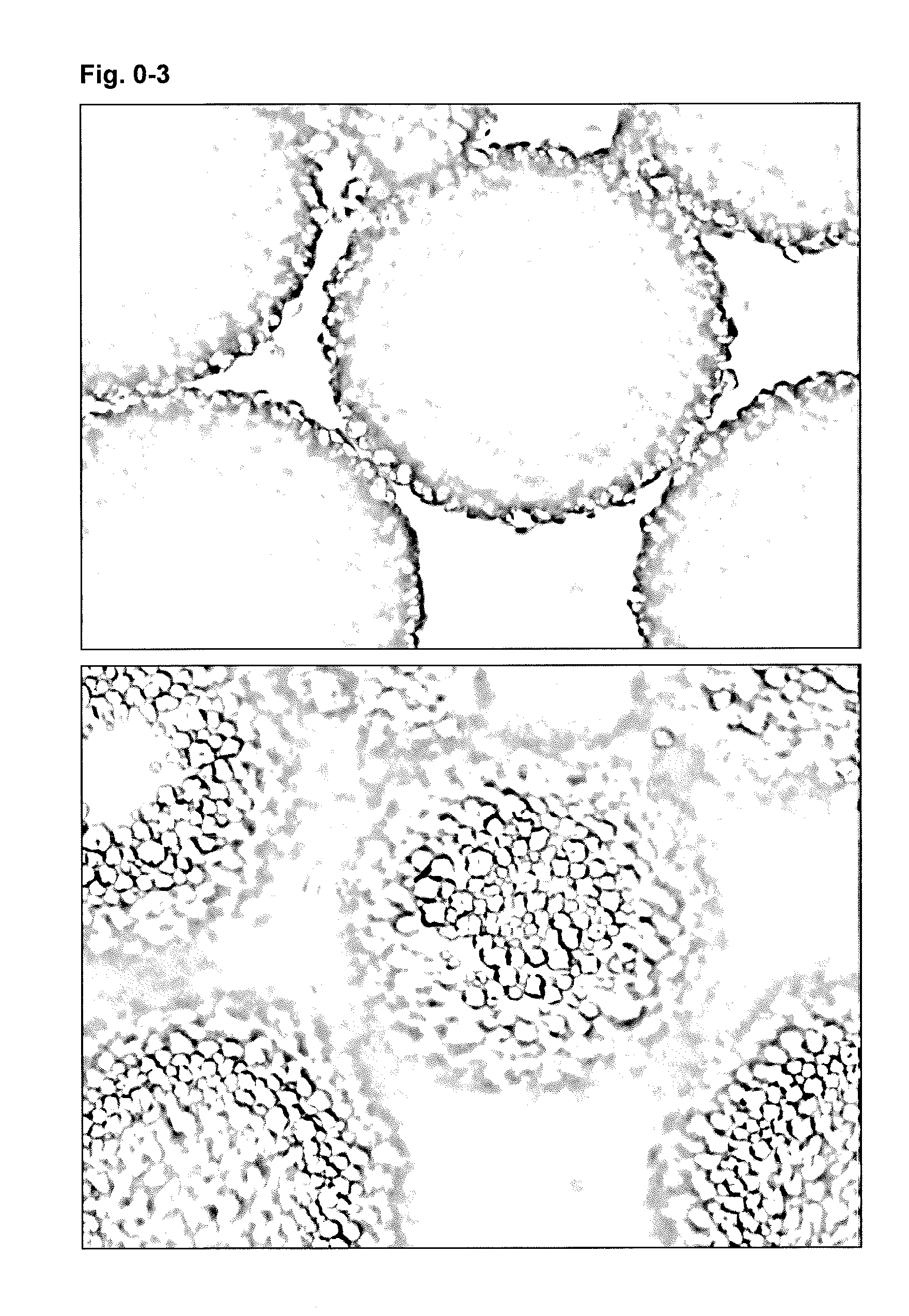
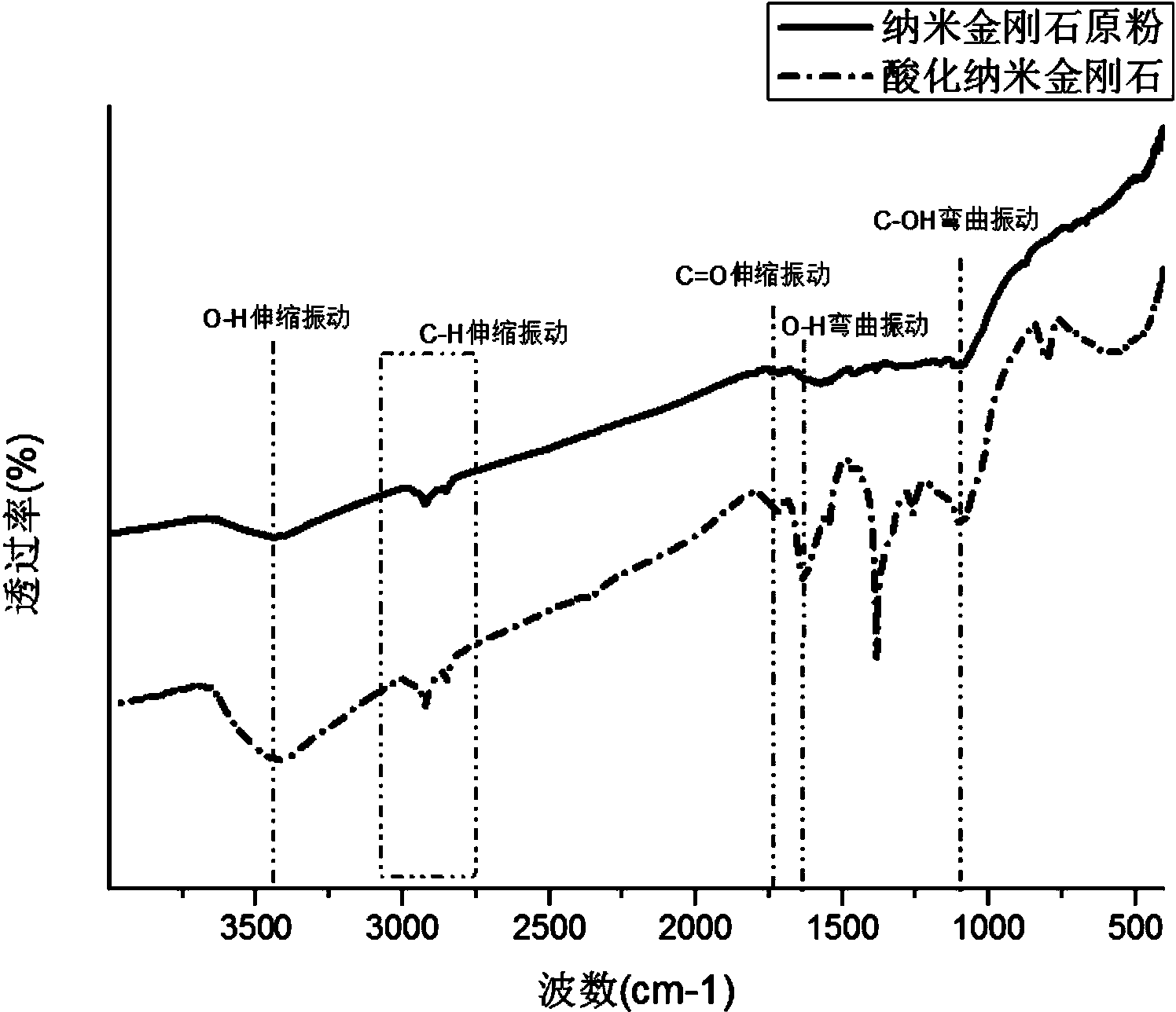
ActiveCN104261404AUniform and stable dispersionSimple technical processMaterial nanotechnologyPharmaceutical non-active ingredientsPerformance enhancementBiological drugs
The invention discloses a method for de-agglomerating a nano diamond, and particularly relates to a method for preparing nano diamond hydrosol. Due to a ball-milling mode, under the condition without any additive, stably dispersed nano diamond hydrosol is obtained; the method comprises the following technical processes: acidizing nano diamond raw powder, adding a certain amount of ball-milling beads and deionized water to carry out wet ball-milling; carrying out ultrasonic dispersion on the obtained nano diamond suspension and ball-milling beads; and finally standing and centrifugally processing the obtained suspension to remove impurities and large particles, so as to obtain nano diamond hydrosol of about 10nm. The hydrosol prepared by the method can be applied to the field of precision polishing; and with respect to agglomerated large-grained nano diamond, the nano diamond hydrosol has higher polishing accuracy, can be applied to mechanical performance enhancement application of composite materials as an additive, and simultaneously can also be applied to research of the field of biological drug transportation.
Owner:UNIV OF SCI & TECH OF CHINA
Glucagon analog for treatment of metabolic diseases
ActiveCN109836488AGood enzyme resistance and stabilityLong half-life in vivoPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseDipeptidyl peptidase
The present invention relates to the field of biopharmaceuticals, and in particular to a glucagon analog for treating metabolic diseases. The structural formula is: H-X2-X3-GTFTSD-X10-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-X<z> or H-S-Q-GTFTSD-Y-SKYLD-X16-X17-AAQ-DFVQWLMN-X29-Xz-NH2. The described glucagon analogue has a GLP-1 / GCG / GIP triple receptor agonist activity and better enzyme resistant stabilityfor neutral endopeptidase (NEP) and dipeptidyl peptidase-4(DPP-4), and has a longer half-life in vivo and duration of action compared with natural glucagon, GLP-1 and GIP.
Owner:ZHEJIANG DOER BIOLOGICS CO LTD
5, 6-ring annulated indole derivatives and use thereof
ActiveUS20100322901A1Avoid virus infectionBiocideOrganic chemistryViral infectionMedicinal chemistry
The present invention relates to 5,6-ring annulated indole derivatives of the formula (I), compositions comprising at least one 5,6-ring annulated indole derivatives, and methods of using the 5,6-ring annulated indole derivatives for treating or preventing a viral infection or a virus-related disorder in a patient.
Owner:MERCK SHARP & DOHME CORP
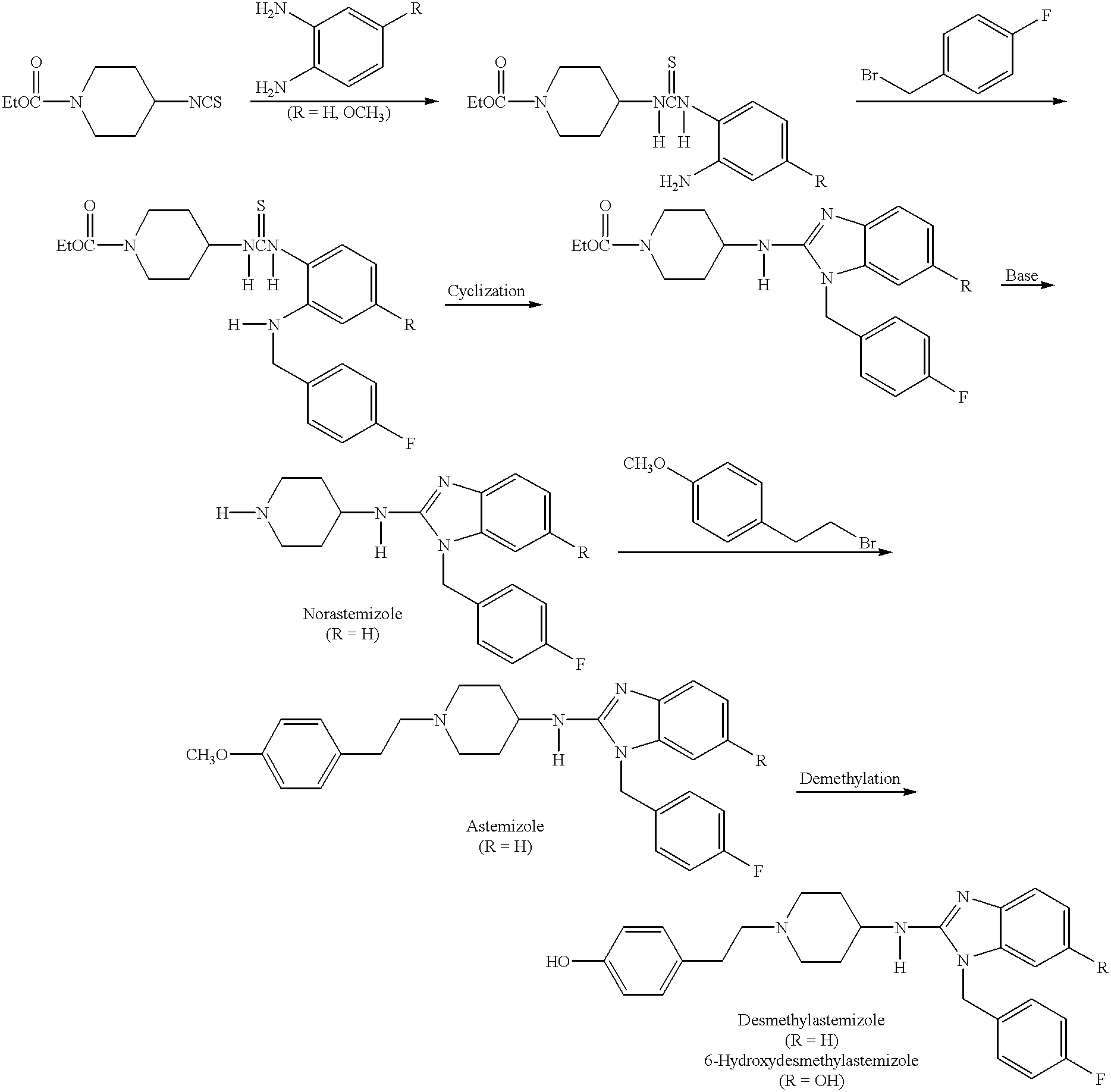
Compositions for treating allergic and other disorders using norastemizole in combination with other active ingredients
InactiveUS6303632B1Reduce adverse effectsUseful in treatmentBiocideOrganic chemistryMotion sicknessWhole body
Methods and compositions are disclosed utilizing metabolic derivatives of astemizole for the treatment of allergic disorders while avoiding the concomitant liability of adverse effects associated with the astemizole. The metabolic derivatives of astemizole are also useful for the treatment of retinopathy and other small vessel disorders associated with diabetes mellitus and such other conditions as may be related to the antihistamine activity of astemizole. For example, the metabolic derivatives of astemizole are useful for the treatment of asthma, motion sickness, and vertigo, without the concomitant liability of adverse effects associated with astemizole. Furthermore, the metabolic derivatives of astemizole, in combination with non-steroidal anti-inflammatory agents or other non-narcotic analgesics, or in combination with a decongestant, cough suppressant / antitussive or expectorant, are useful for the treatment of cough, cold, cold-like, and / or flu symptoms and the discomfort, headache, pain, fever, and general malaise associated therewith, without the concomitant liability of adverse effects associated with astemizole.
Owner:SEPACOR INC
Non-spherical polymer particles uniform in particle size as well as preparation method and application of non-spherical polymer particles
ActiveCN105832704AUniform particle sizeParticle size controllableSsRNA viruses negative-sensePeptide/protein ingredientsPolymer scienceBiological drugs
The invention provides non-spherical polymer particles uniform in particle size as well as a preparation method and an application of the non-spherical polymer particles. The non-spherical polymer particles are in the form of ellipsoids, short rods or fibers, and the interiors of the non-spherical polymer particles are of solid, hollow or porous structures; the short diameter of the non-spherical polymer particles ranges from 100nm to 30[micron]m, the long diameter of the non-spherical polymer particles is 1-60[micron]m, the ratio of the long diameter to the short diameter is 2-40, and the particle size distribution coefficient of the particles is less than 20%. The non-spherical polymer particles disclosed by the invention, which are uniform and controllable in particle size, are prepared by taking disodium hydrogen phosphate and / or sodium dihydrogen phosphate as a deformation inducing agent and by taking the influence of such conditions as the concentration of the disodium hydrogen phosphate and / or sodium dihydrogen phosphate, self-properties of a polymer, mass concentration of the polymer in an oil phase, the size of emulsion drops and the like into comprehensive consideration; the non-spherical polymer particles can be applied to various fields such as biological drug delivery, vaccine adjuvants, enzymatic catalysis, bio-separation, human tissue engineering field and the like; and the preparation method is simple to operate, mild in condition and easy for industrial enlarged production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com