Continuous processing methods for biological products
a technology of biological products and processing methods, applied in the field of continuous processing technology for the production of biopharmaceutical and biological products, can solve the problems of inherently inefficient batch processing techniques, equipment designs for disposable downstream processing, and processing of cultures, and achieve the effect of increasing manufacturing productivity and efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
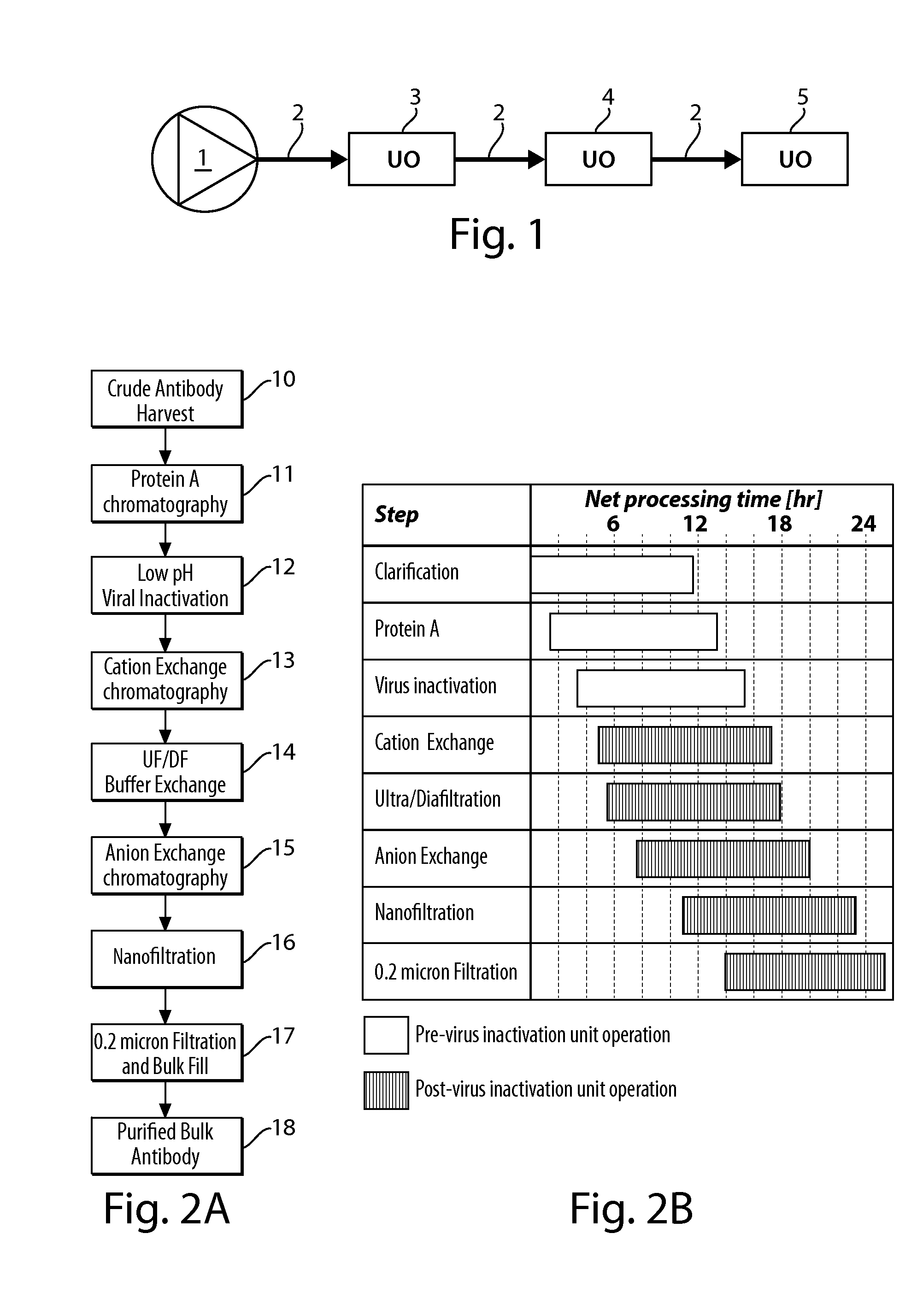
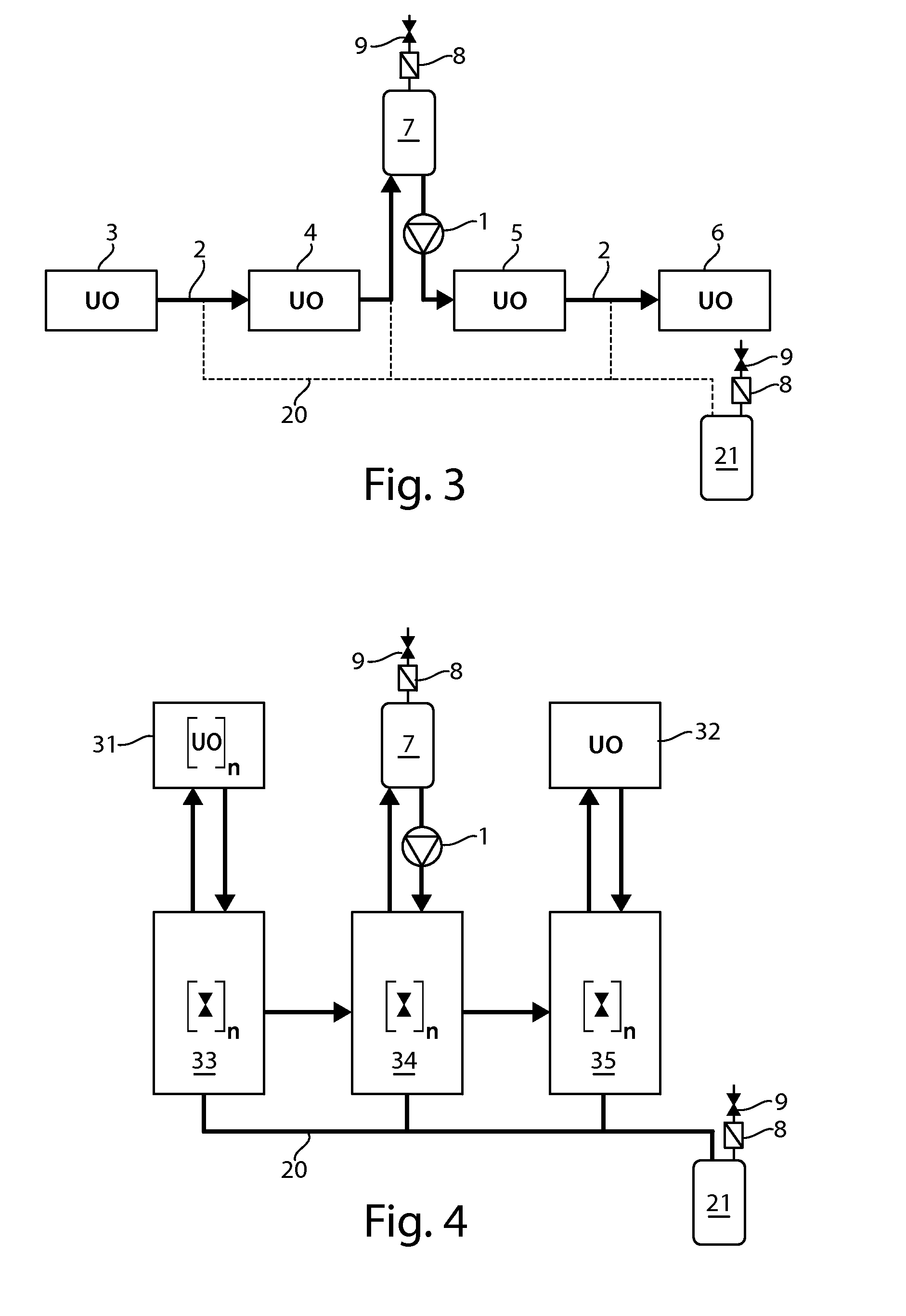
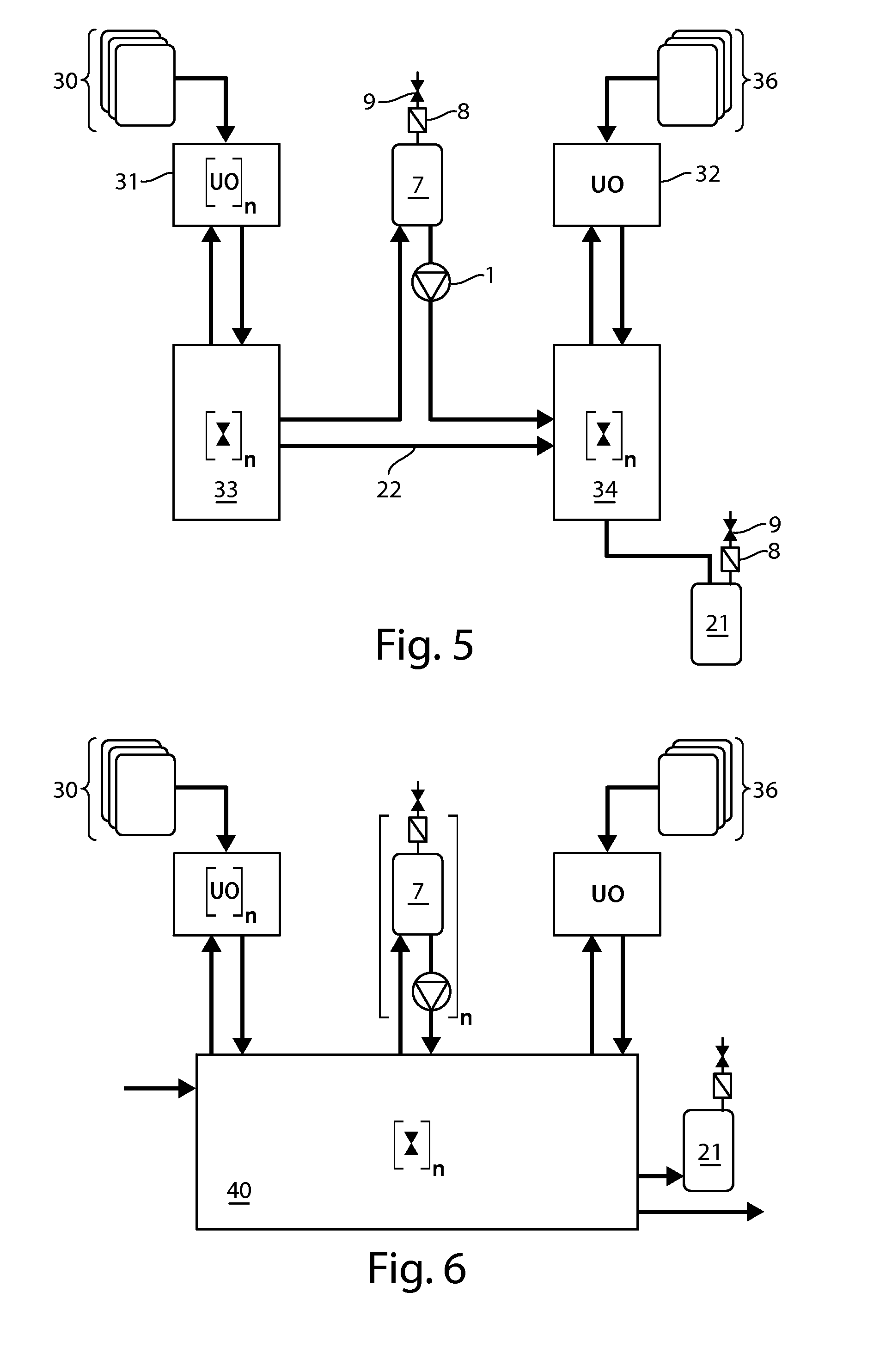
[0085]The present invention provides processes that can be adapted to the manufacture of a biological product which make at least two or more steps of the processing of the biological product continuous. Continuous processing allows the manufacturer to conduct the manufacturing process using less material and relying on equipment of a reduced scaled compared to the equipment that would be required for batch operations. Continuous processing reduces downtime in the overall manufacturing process and thus reduces cycle times from the beginning of a manufacturing run to production of bulk drug substance or packaging of the final product.
[0086]In order that the invention may be more clearly understood, the following abbreviations and terms are used as defined below.
[0087]The term “biological product” as used herein refers to a product of interest created via biological processes or via the chemical or catalytic modification of an existing biological product. Biological processes include ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com