Patents
Literature
1931 results about "Biological product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicinal preparations made from living organisms or their products; venoms, enzymes may be indexed with this term; typically antibiotics and chemically pure biochemicals should not be indexed with this term, also narrower terms animal extract, plant extract, immunological substance or tissue/cell preparation should be considered.
Defined media for pluripotent stem cell culture
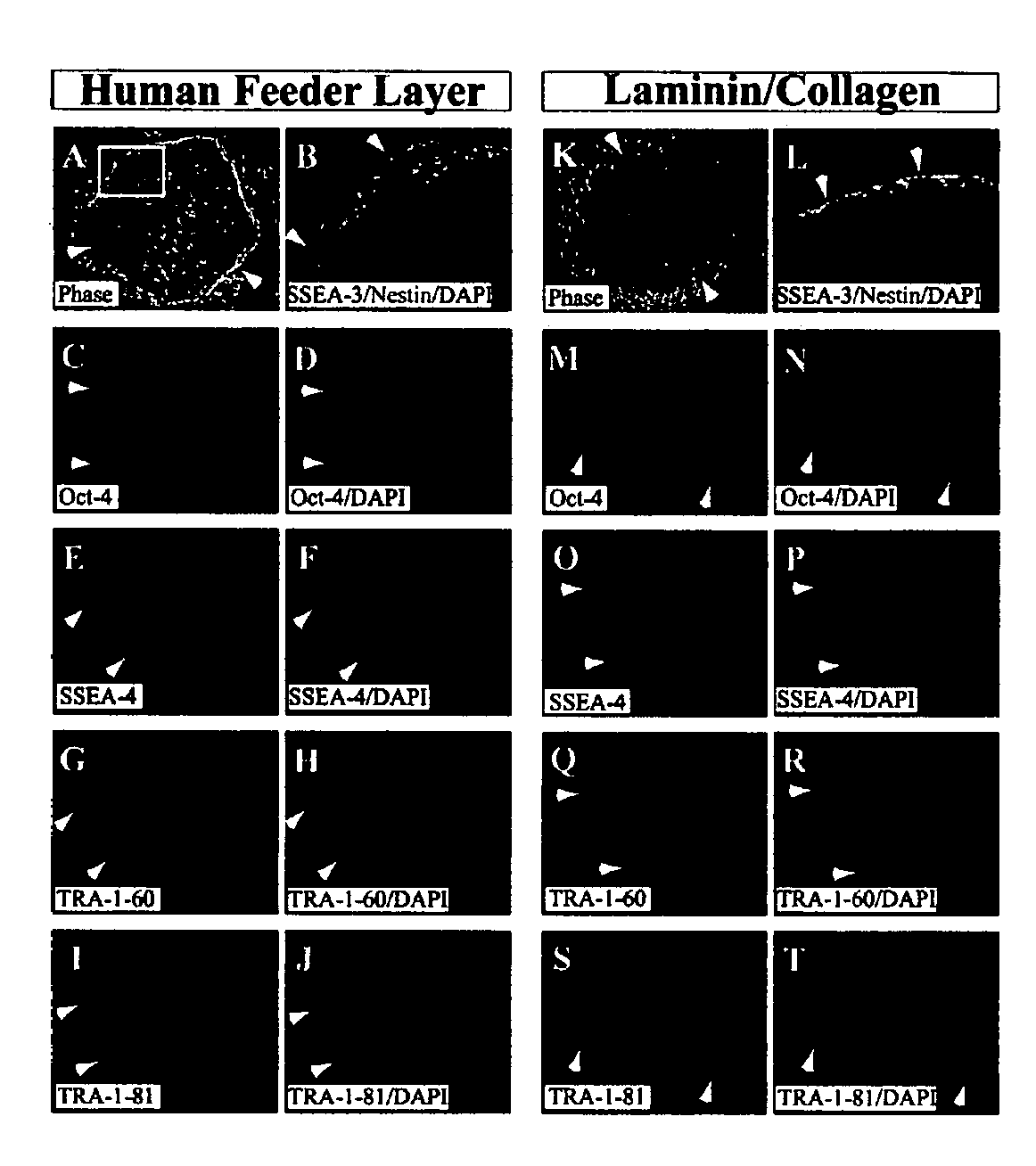
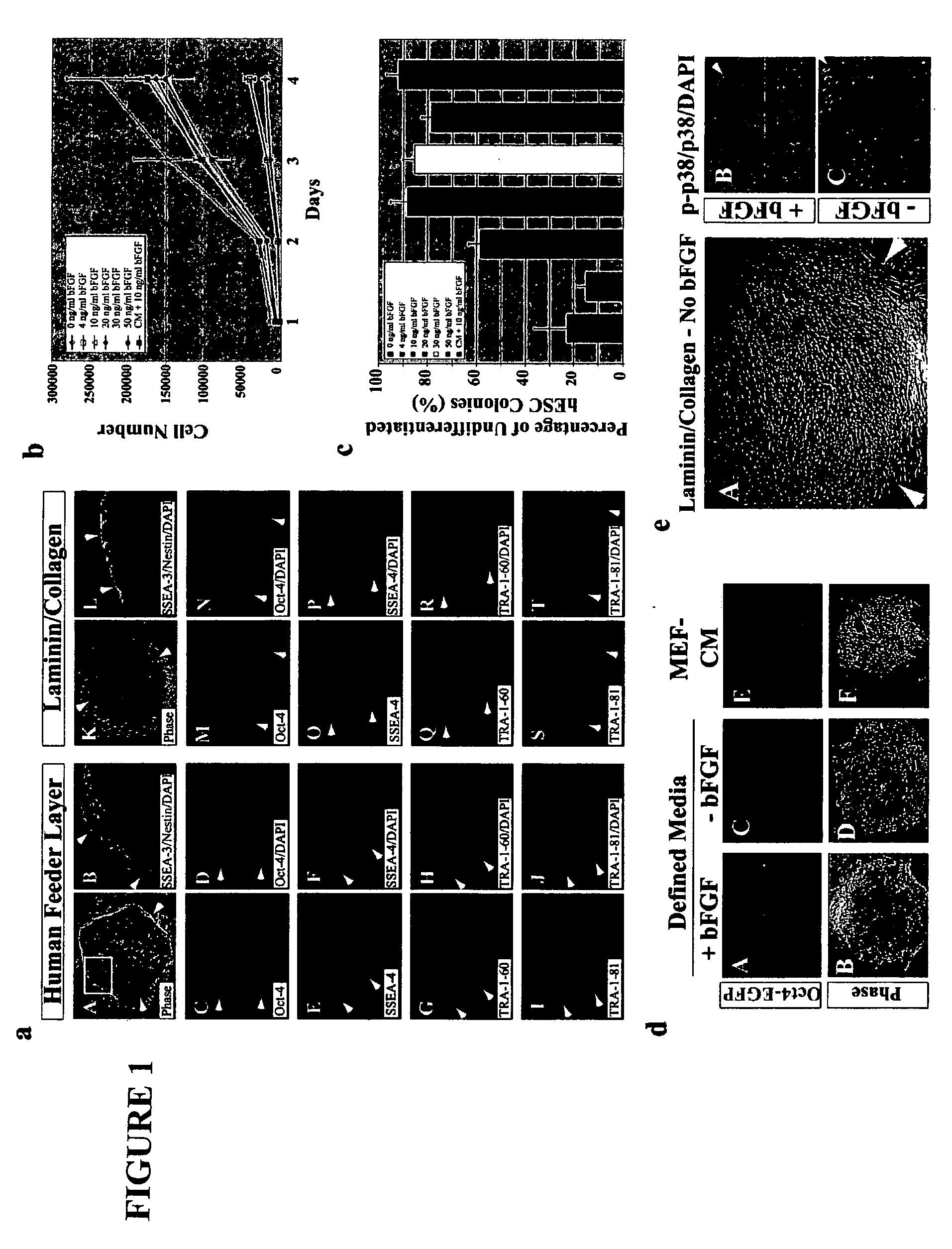
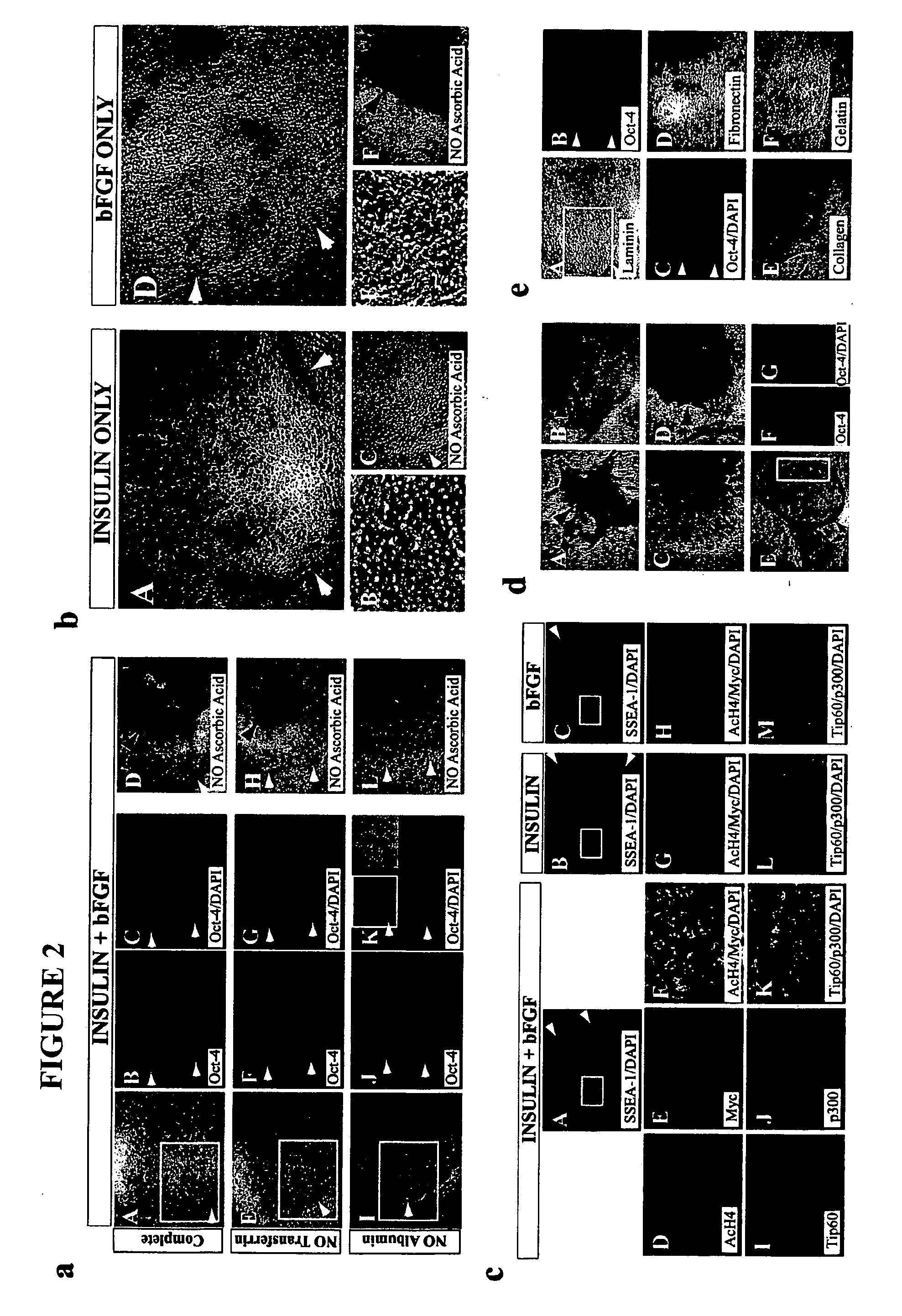
Stem cells, including mammalian, and particularly primate primordial stem cells (pPSCs) such as human embryonic stem cells (hESCs), hold great promise for restoring cell, tissue, and organ function. However, cultivation of stem cells, particularly undifferentiated hESCs, in serum-free, feeder-free, and conditioned-medium-free conditions remains crucial for large-scale, uniform production of pluripotent cells for cell-based therapies, as well as for controlling conditions for efficiently directing their lineage-specific differentiation. This instant invention is based on the discovery of the formulation of minimal essential components necessary for maintaining the long-term growth of pPSCs, particularly undifferentiated hESCs. Basic fibroblast growth factor (bFGF), insulin, ascorbic acid, and laminin were identified to be both sufficient and necessary for maintaining hESCs in a healthy self-renewing undifferentiated state capable of both prolonged propagation and then directed differentiation. Having discerned these minimal molecular requirements, conditions that would permit the substitution of poorly-characterized and unspecified biological additives and substrates were derived and optimized with entirely defined constituents, providing a “biologics”-free (i.e., animal-, feeder-, serum-, and conditioned-medium-free) system for the efficient long-term cultivation of pPSCs, particularly pluripotent hESCs. Such culture systems allow the derivation and large-scale production of stem cells such as pPSCs, particularly pluripotent hESCs, in optimal yet well-defined biologics-free culture conditions from which they can be efficiently directed towards a lineage-specific differentiated fate in vitro, and thus are important, for instance, in connection with clinical applications based on stem cell therapy and in drug discovery processes.
Owner:THE BURNHAM INST
Nano-Chinese medicinal biological product and its preparation
A nano-class biologic product of Chinese-medicinal material for higher biological utilization rate and target nature, lower toxic by-effect and improved curative effect is prepared through preparing the nanoparticles of Chinese-medicinal materials, and preparing nano-class (50-80 nm) biologic product.
Owner:陈永丽
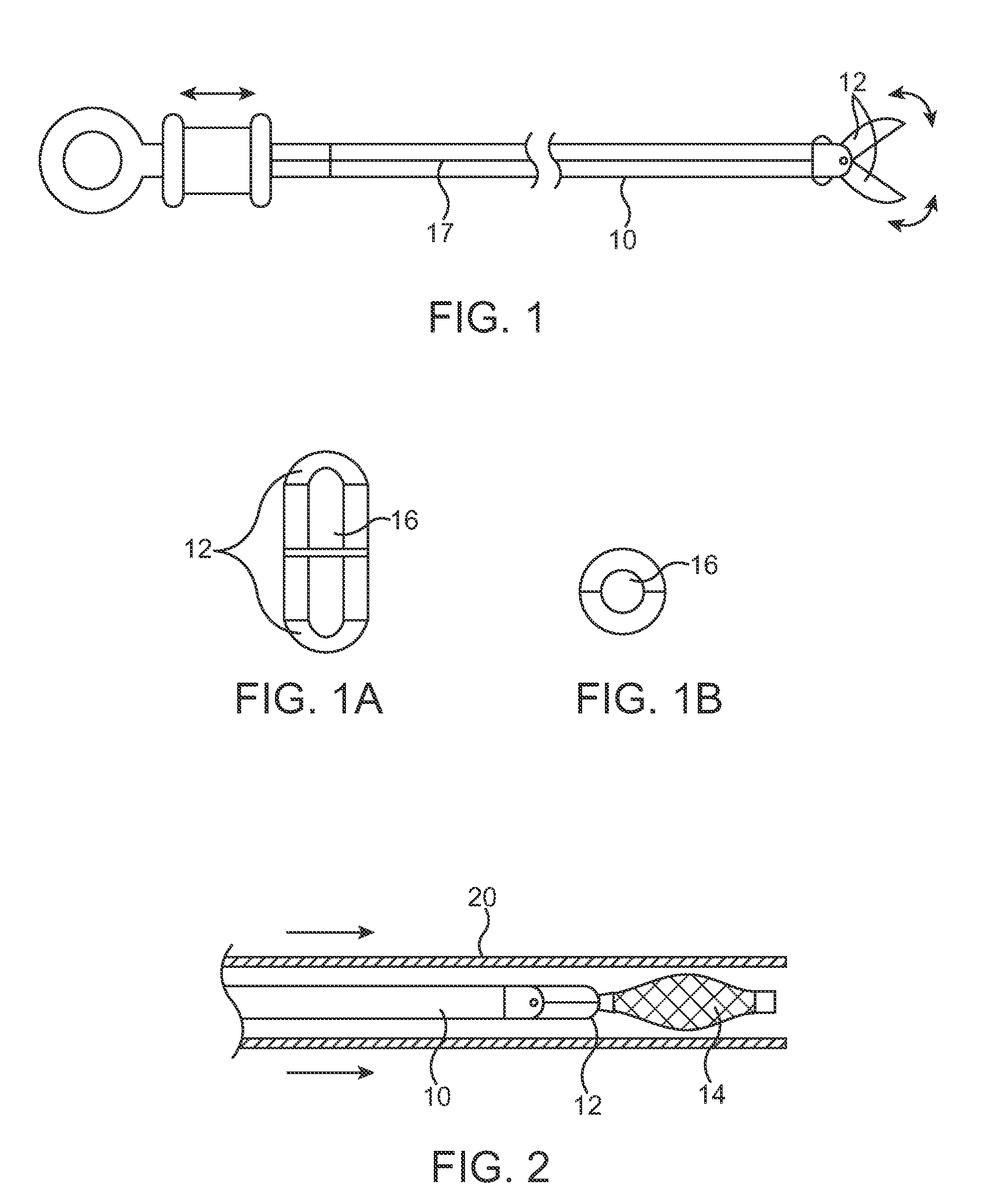
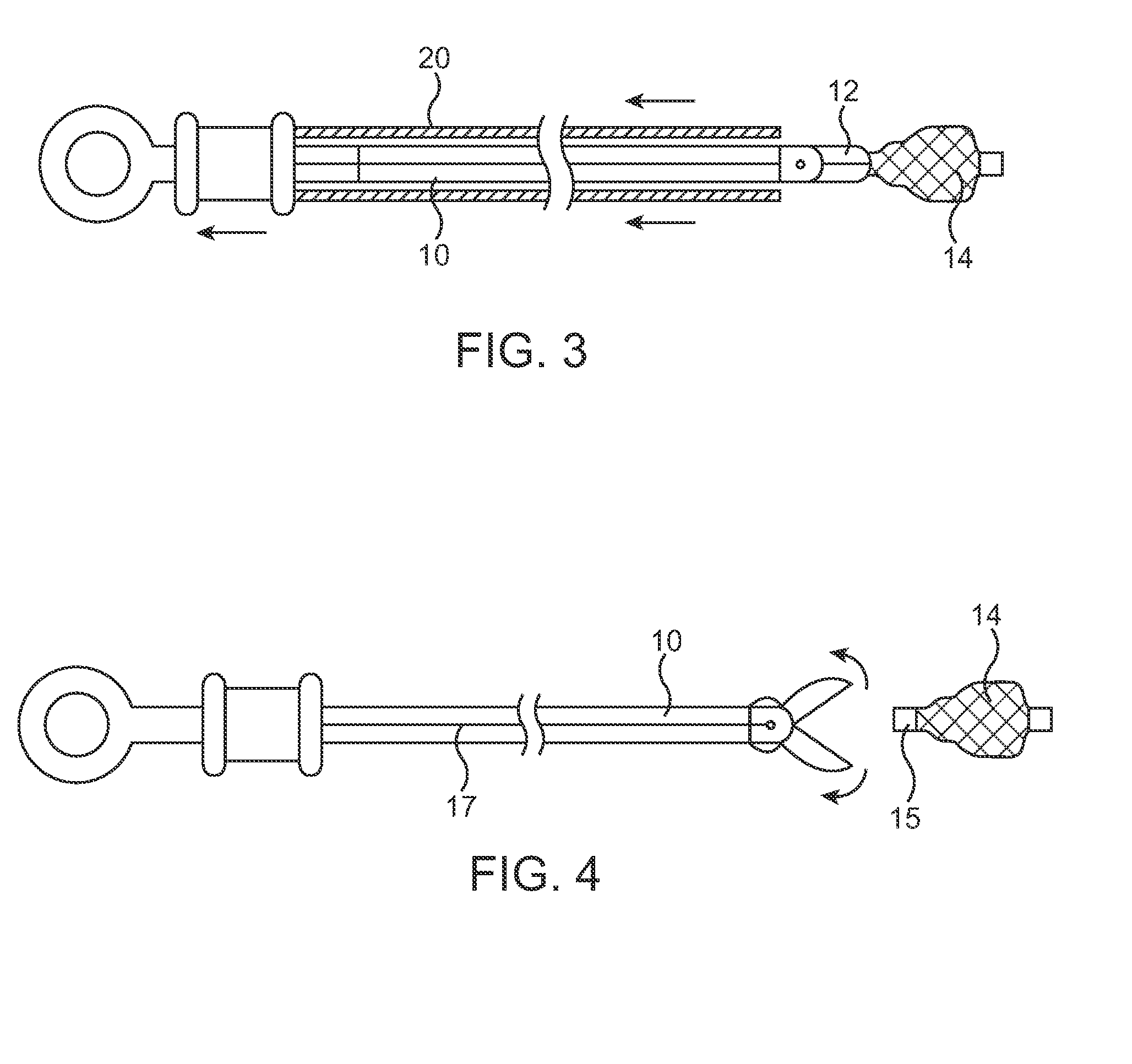
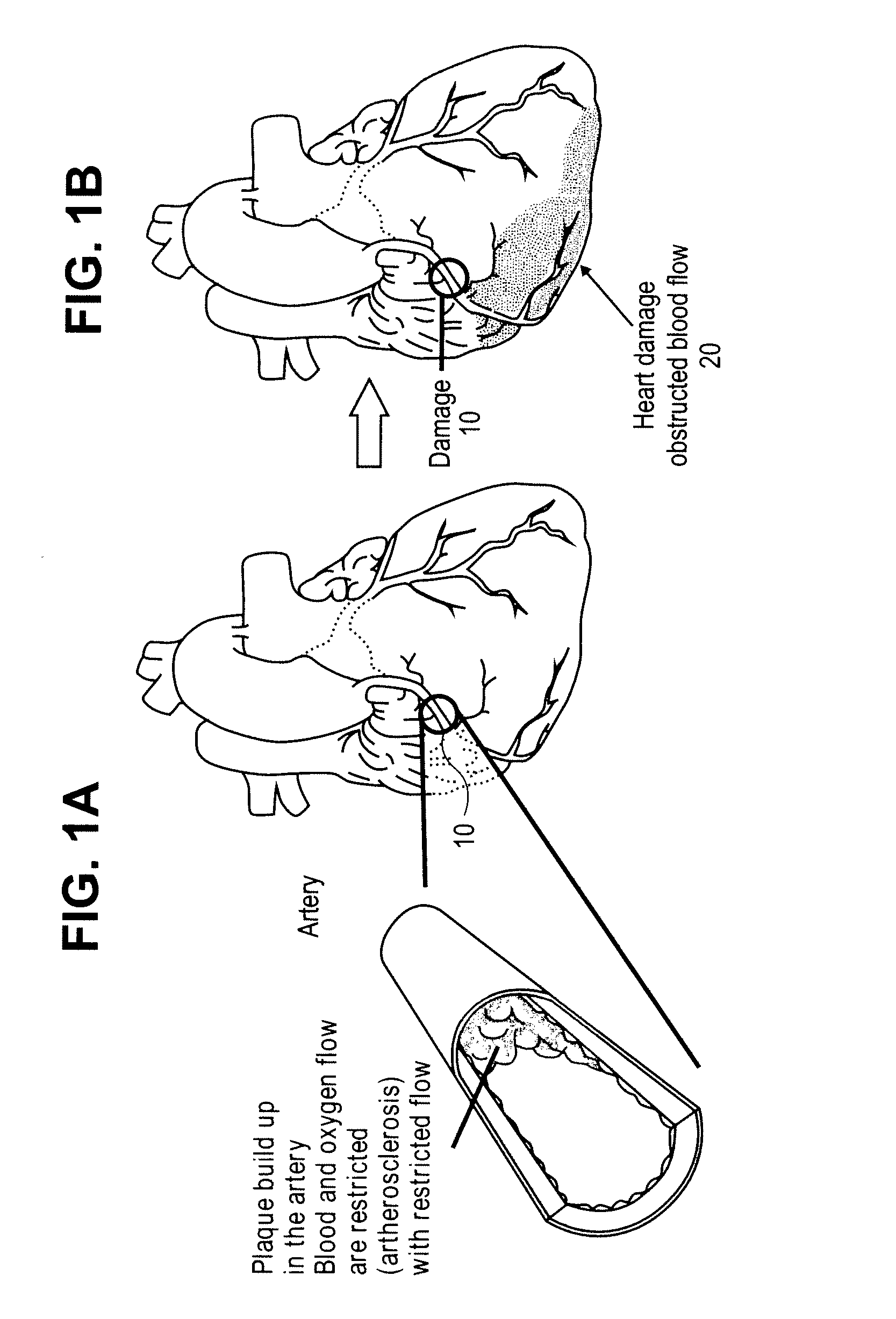
Systems and methods for delivering flow restrictive element to airway in lungs
A method and system for catheter-based delivery of implants in the body. Implants can include stents, plugs, coils, baskets, filters, valves, grafts, prosthesis', drugs, drug reservoirs, biologics, or pumps. The catheter system comprises a uniquely configured grasper mechanism that allows holding the implant during the unsheathing delivery step prior to full release. With this delivery system, the implant can be unsheathed, positioned, and the position can be evaluated prior to releasing the implant from the catheter. Upon evaluation of the position of the implant, if it is found to be inaccurately placed, then removal of the implant can be done easily and without a device exchange. If the implant is found to be positioned correctly, the grasper mechanism can be actuated to release the implant from the catheter.
Owner:PULMONX
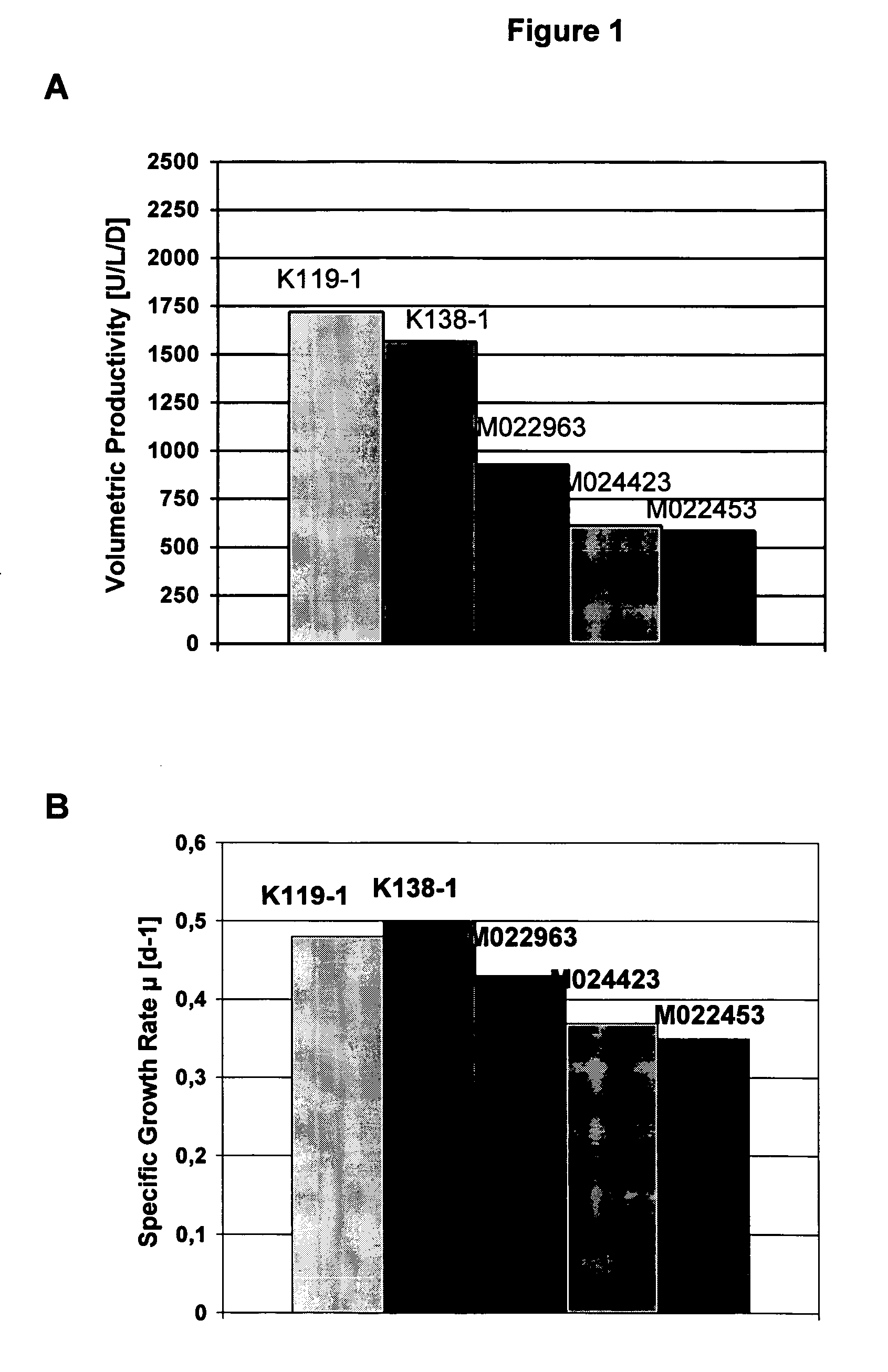
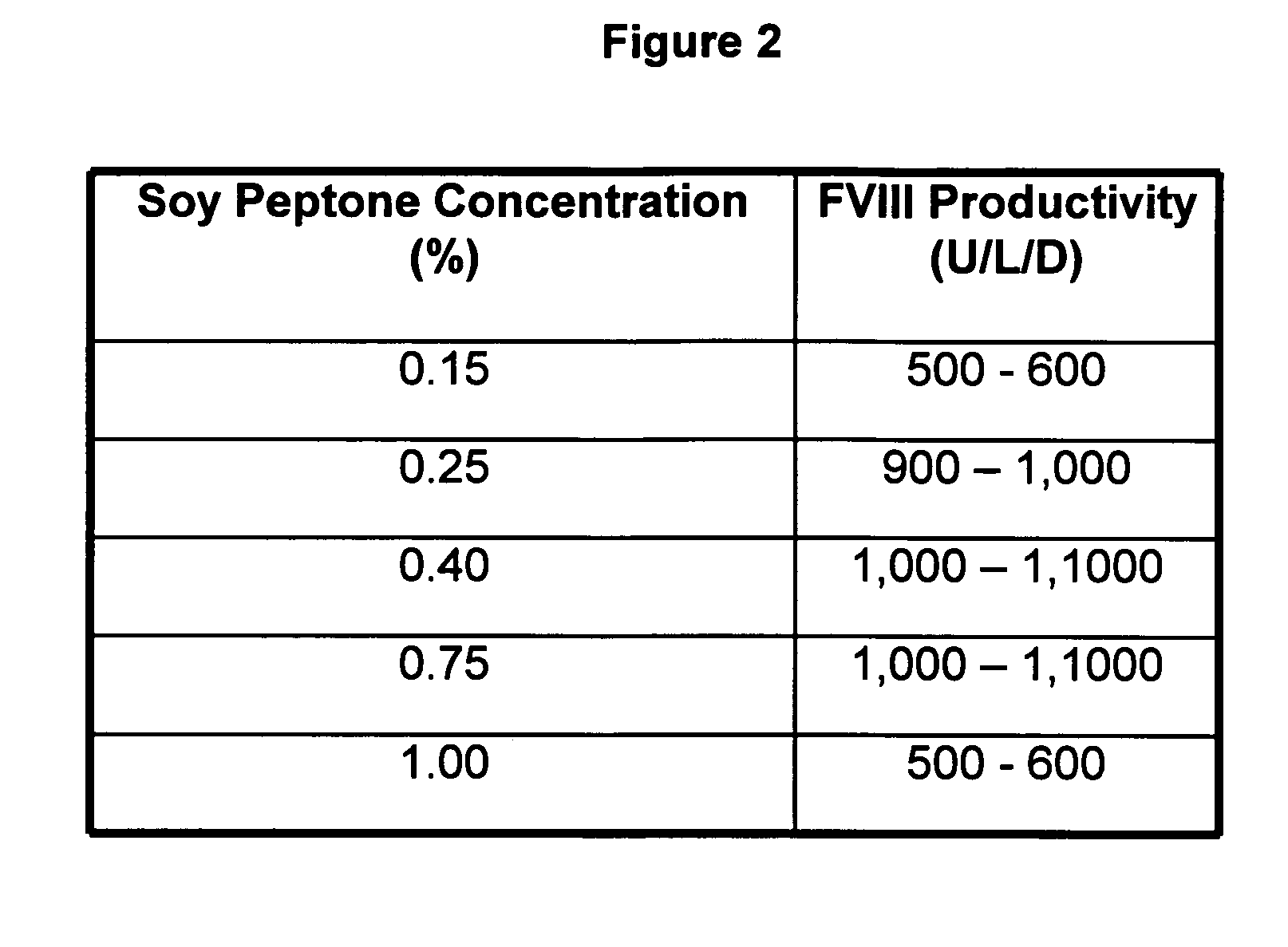
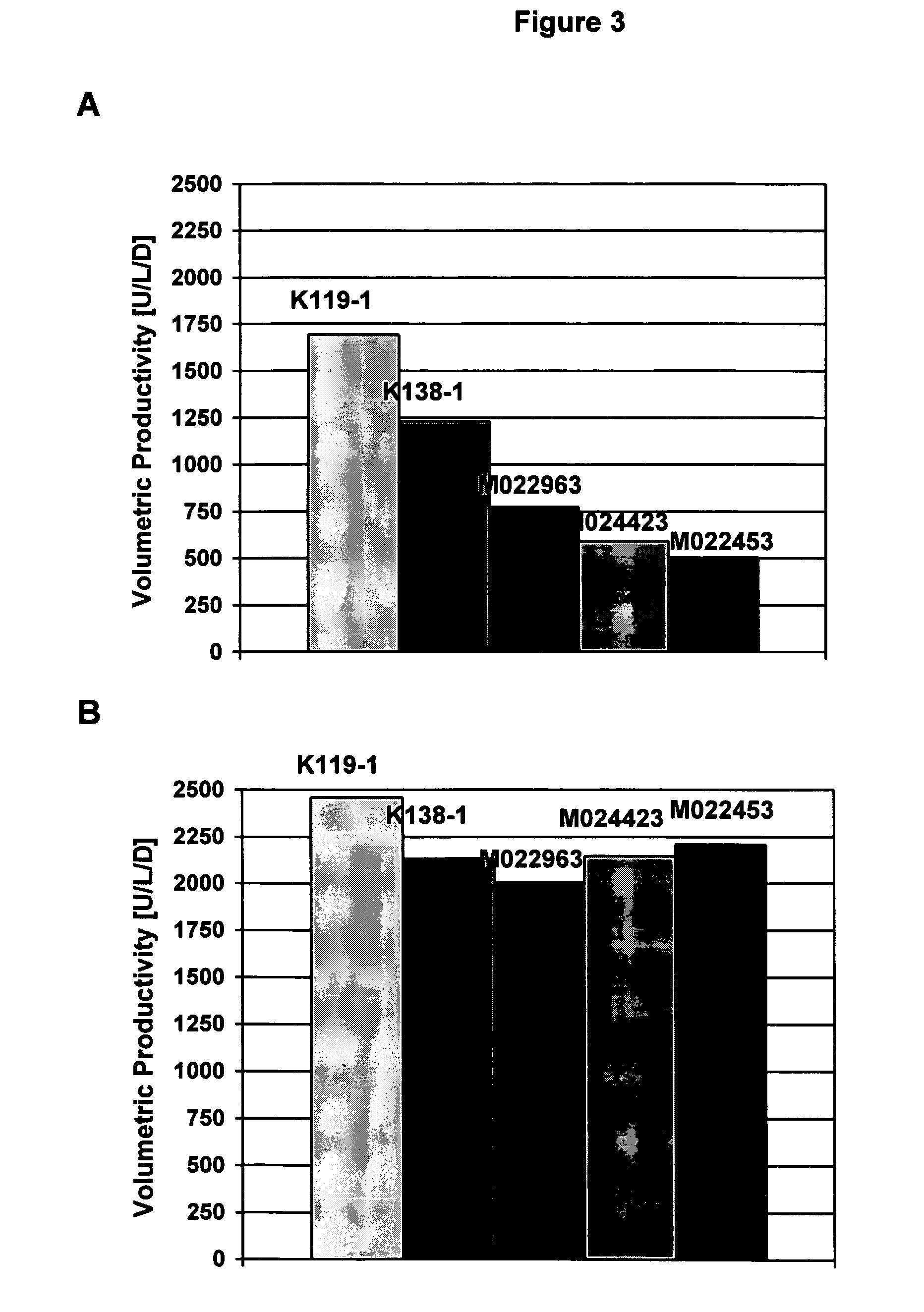
Animal protein-free media for cultivation of cells
InactiveUS20080009040A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processHydrolysateCell culture media
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant- and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
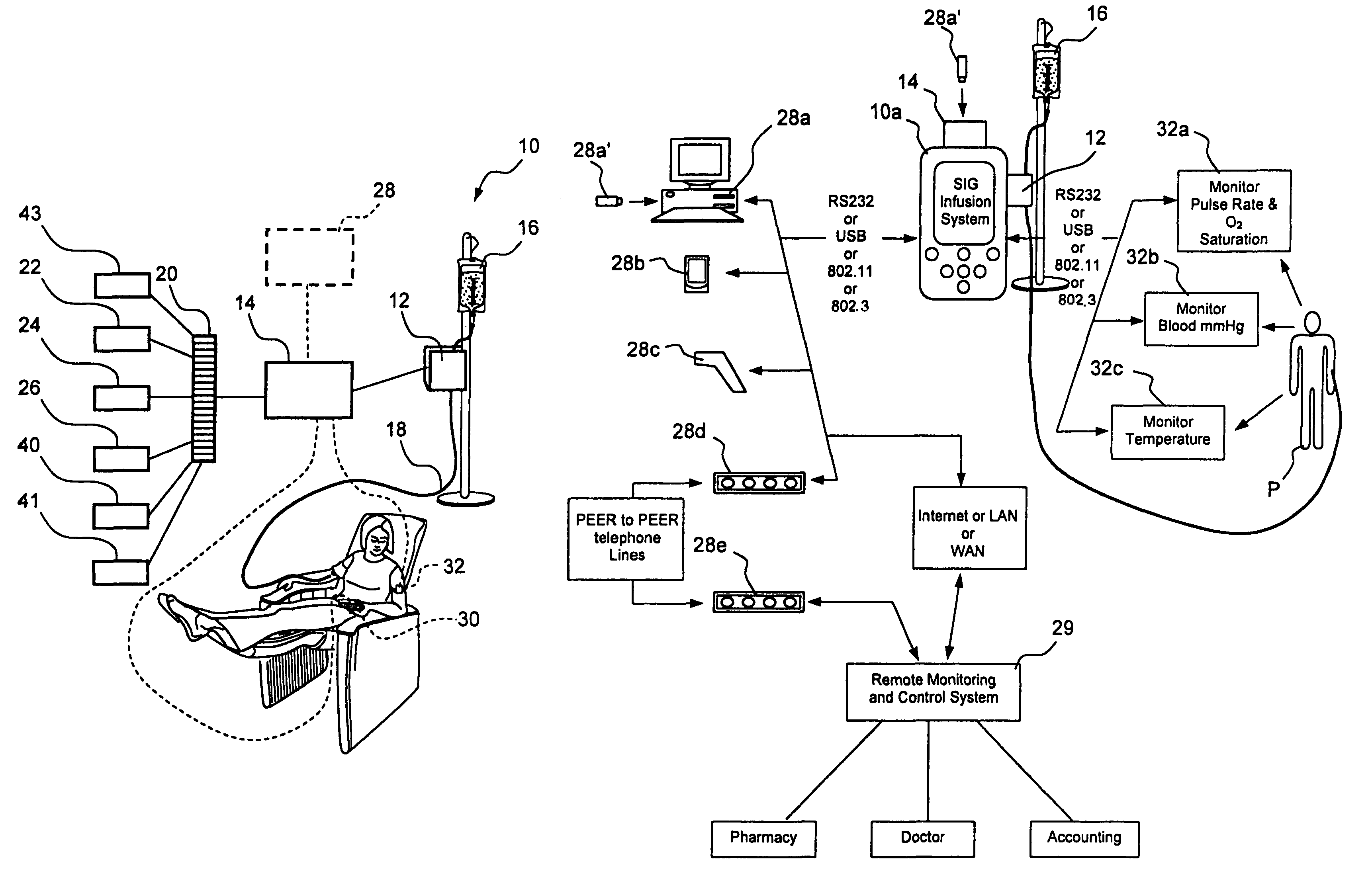
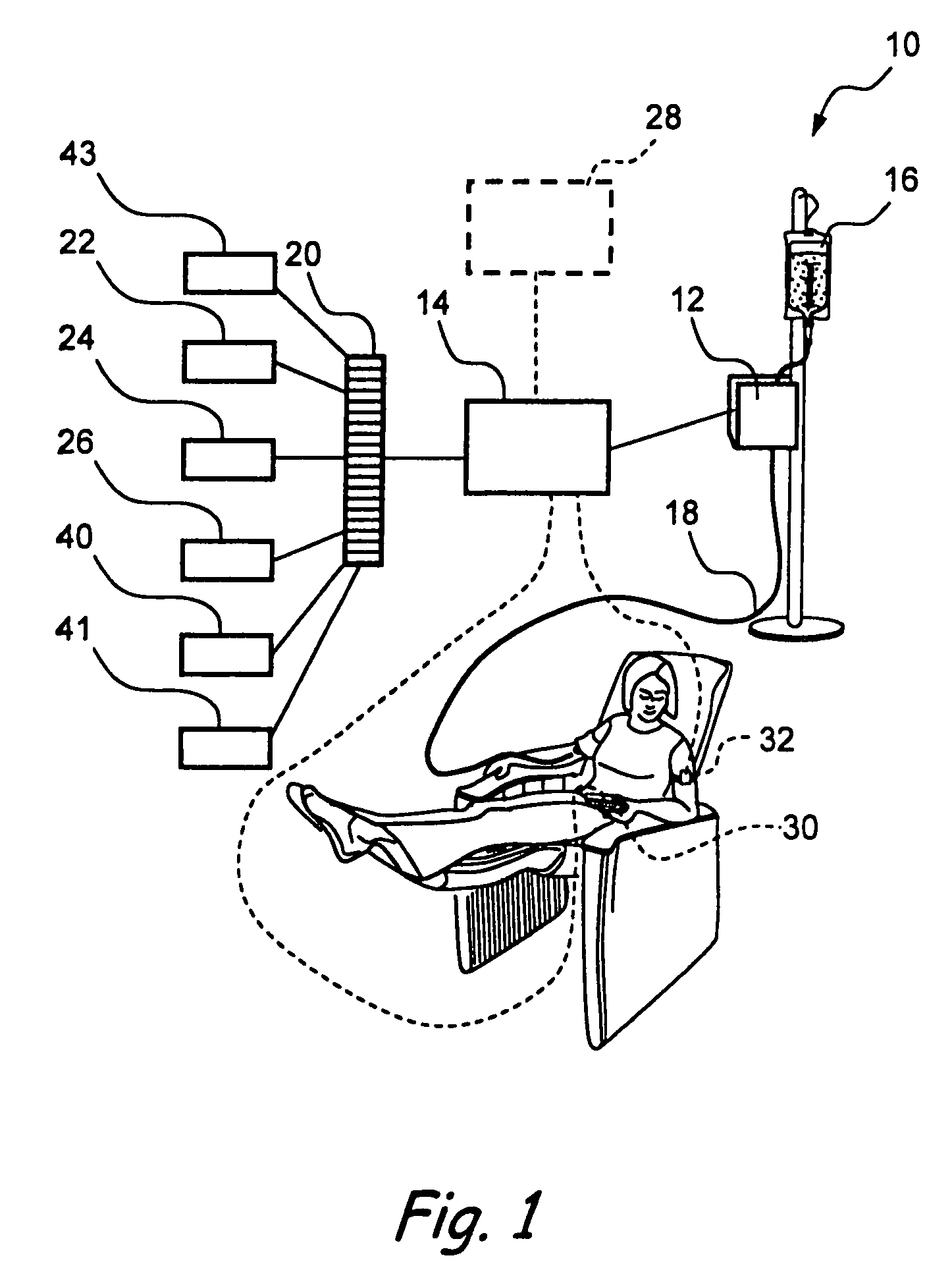
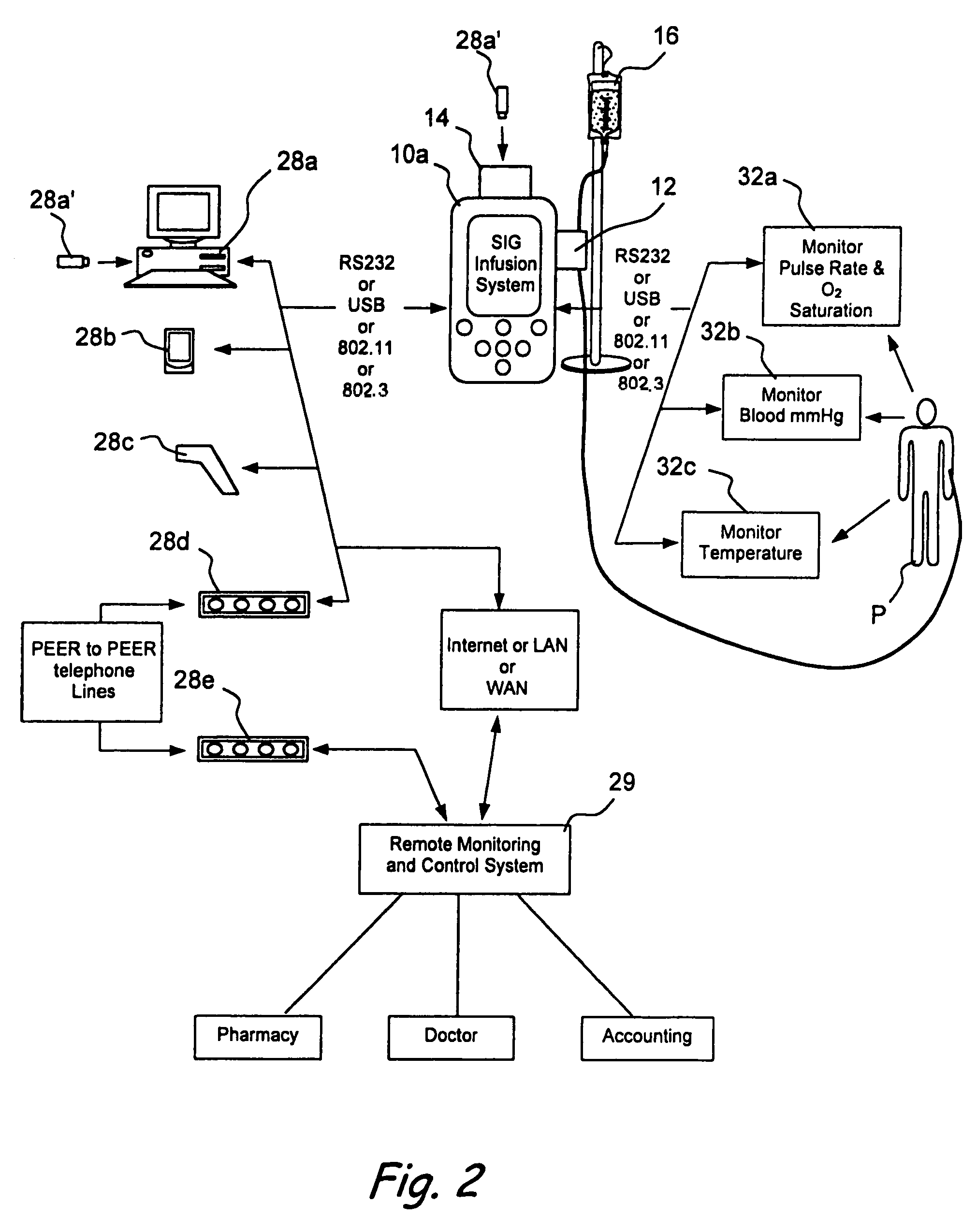
Method and system for controlled infusion of therapeutic substances
Programmable infusion systems and method for controlled infusion of diagnostic or therapeutic substances (e.g., drugs, biologics, fluids, cell preparations, etc.) into the bodies of human or animal subjects.
Owner:BAXTER INT INC +1
Carbon nano-dot, and preparation method and application thereof
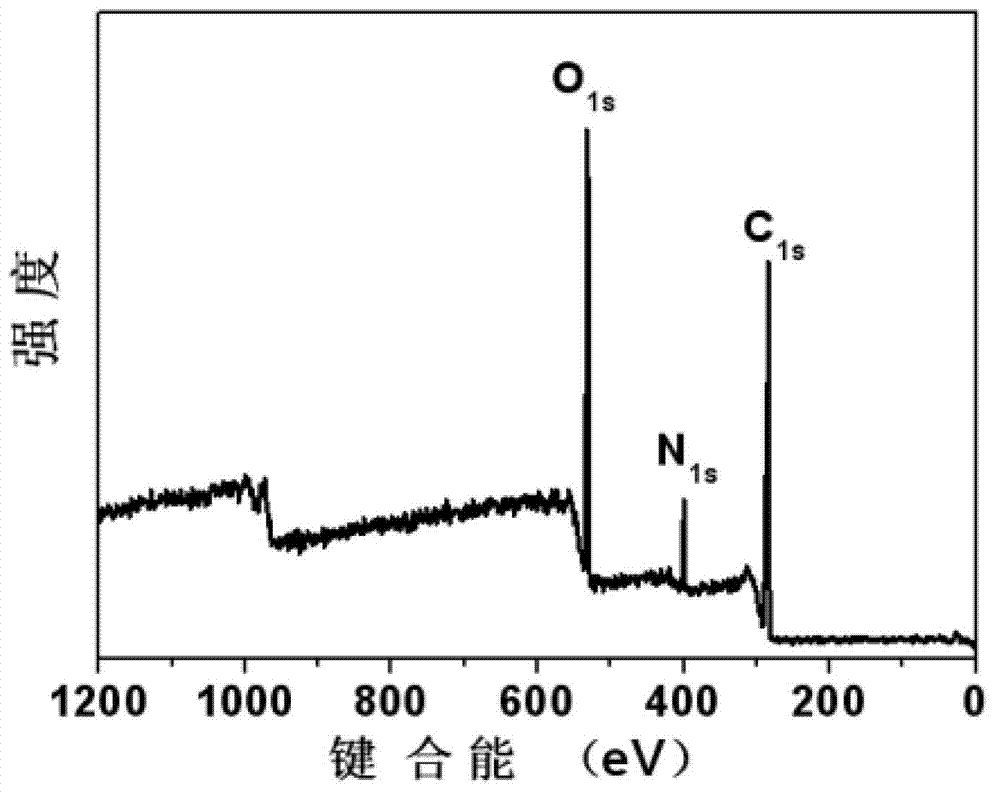
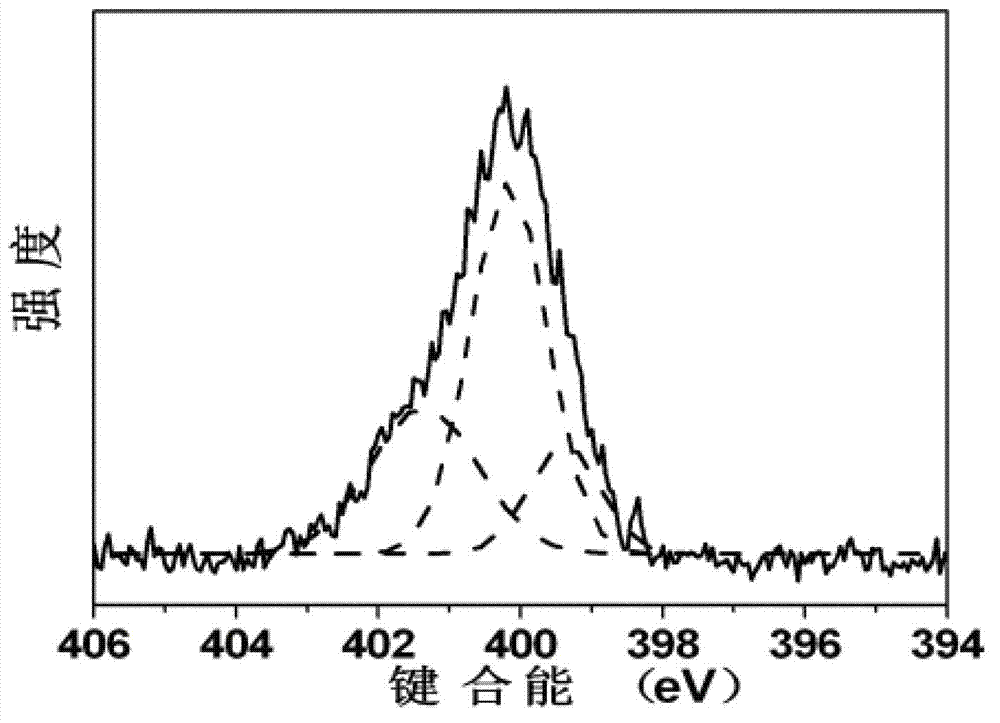
ActiveCN102849722AOvercome costsOvercoming the problem of easy fluorescence quenching in the aggregated stateNanotechnologyNano-carbonBiological imagingOrganic compound
The invention discloses a carbon nano-dot, and a preparation method and an application thereof, and solves a problem that the application of present nano-dots is restricted because of high preparation cost and easy fluorescent quenching appearance of an aggregate state. According to the invention, the carbon nano-dot having a high fluorescence quantum efficiency is prepared through adopting a polycarboxyl or polyhydroxy contained organic compound, or an amino acid as a raw material, and urea as a surface passivation modification agent, and through a microwave process, and a carbon nano-dot fluorescent ink is prepared through using the carbon nano-dot. The preparation method disclosed in the invention has the advantages of simplicity, low cost and convenient large-scale production; the fluorescent quenching of the prepared carbon nano-dot on the surface of a biological product does not appear, and the highest fluorescence quantum efficiency is 42%; and the prepared carbon nano-dot fluorescent ink is nontoxic, does not generate a precipitate after long-time dispose, and can be applied to the biological imaging field, the biological product identification field, the information storage field, the information encryption field, the false proof field, the illumination display field, the photovoltaic device field and the like.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
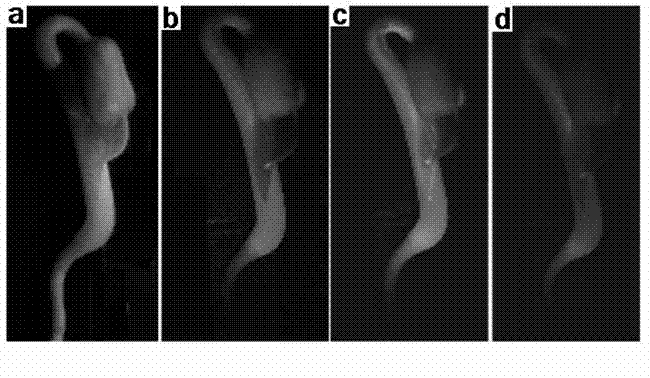
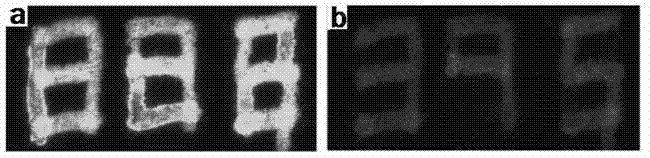
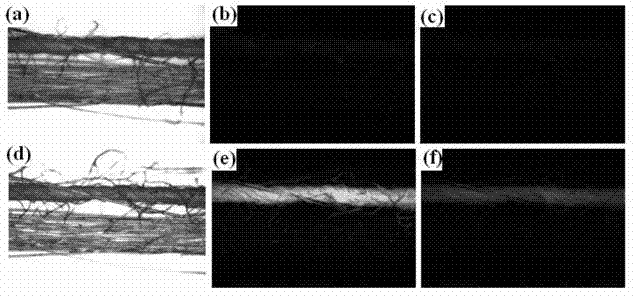
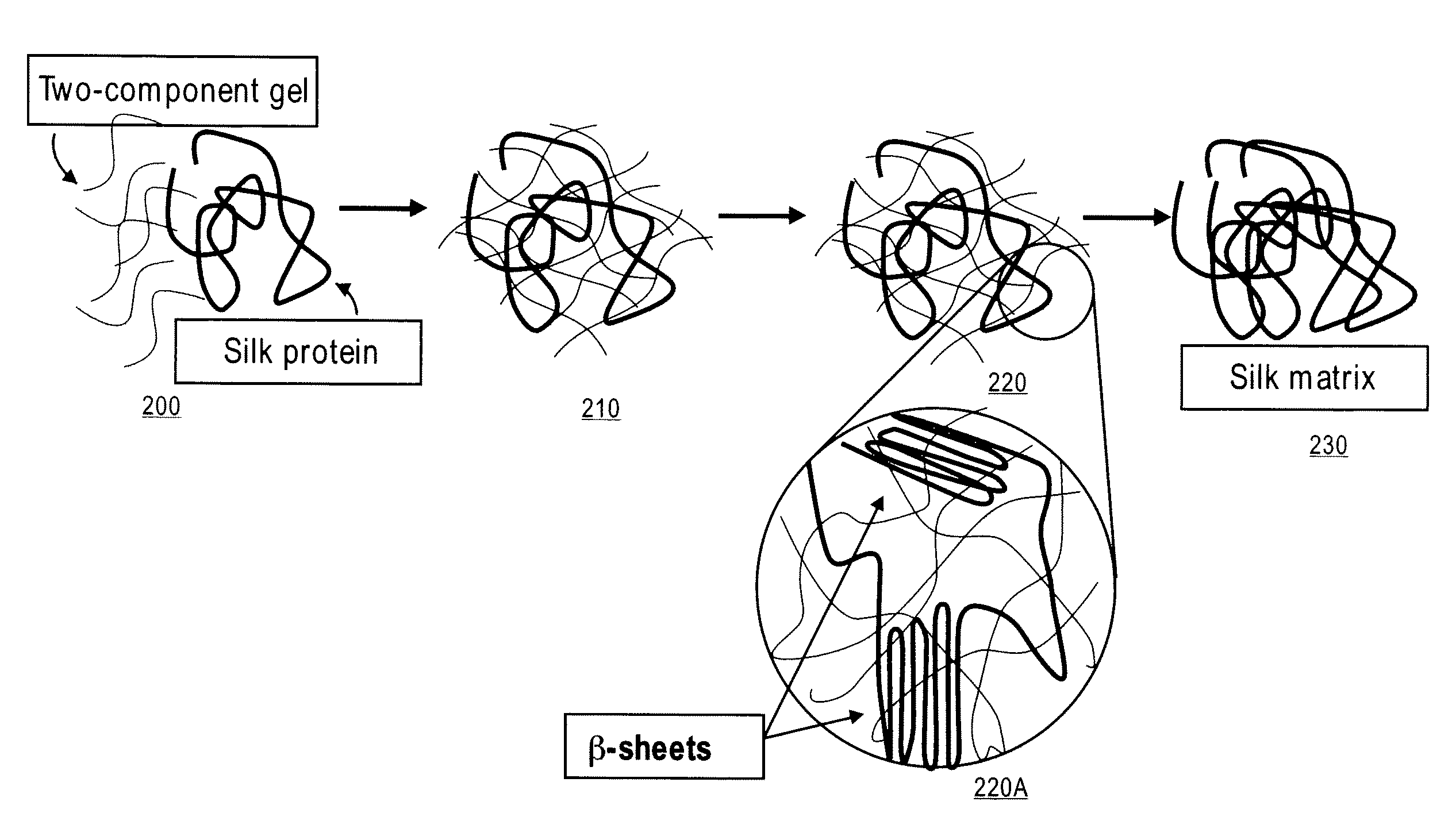
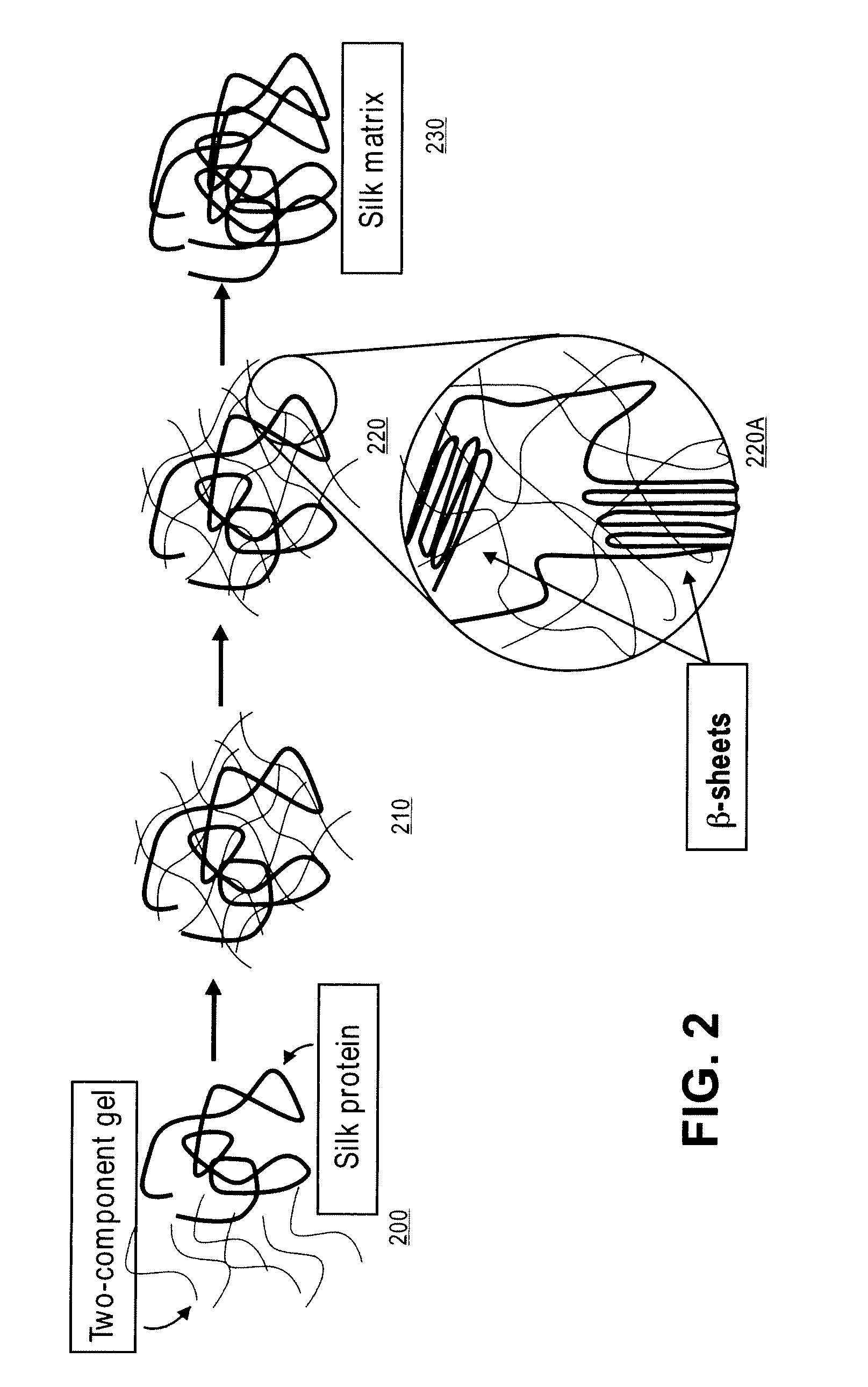
Methods and Compositions for Treating Tissue Using Silk Proteins
Compositions for forming a self-reinforcing composite biomatrix, methods of manufacture and use therefore are herein disclosed. Kits including delivery devices suitable for delivering the compositions are also disclosed. In some embodiments, the composition can include at least three components. In one embodiment, a first component can include a first functionalized polymer, a second component can include a second functionalized polymer and a third component can include silk protein or constituents thereof. In some embodiments, the composition can include at least one cell type and / or at least one growth factor. In some embodiments, the composition can include a biologic encapsulated, suspended, disposed within or loaded into a biodegradable carrier. In some embodiments, the composition(s) of the present invention can be delivered by a dual lumen injection device to a treatment area in situ, in vivo, as well as ex vivo applications.
Owner:ABBOTT CARDIOVASCULAR
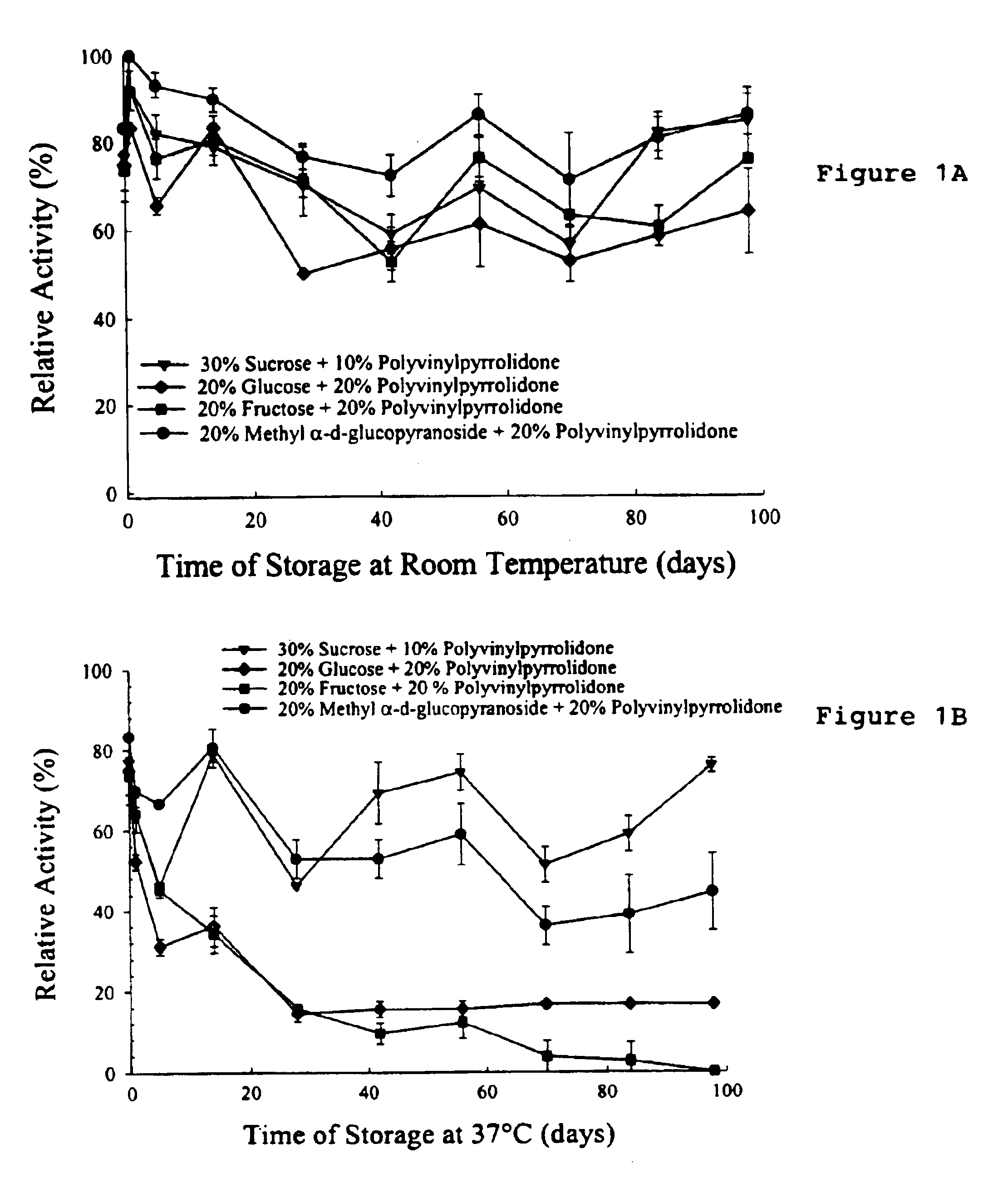
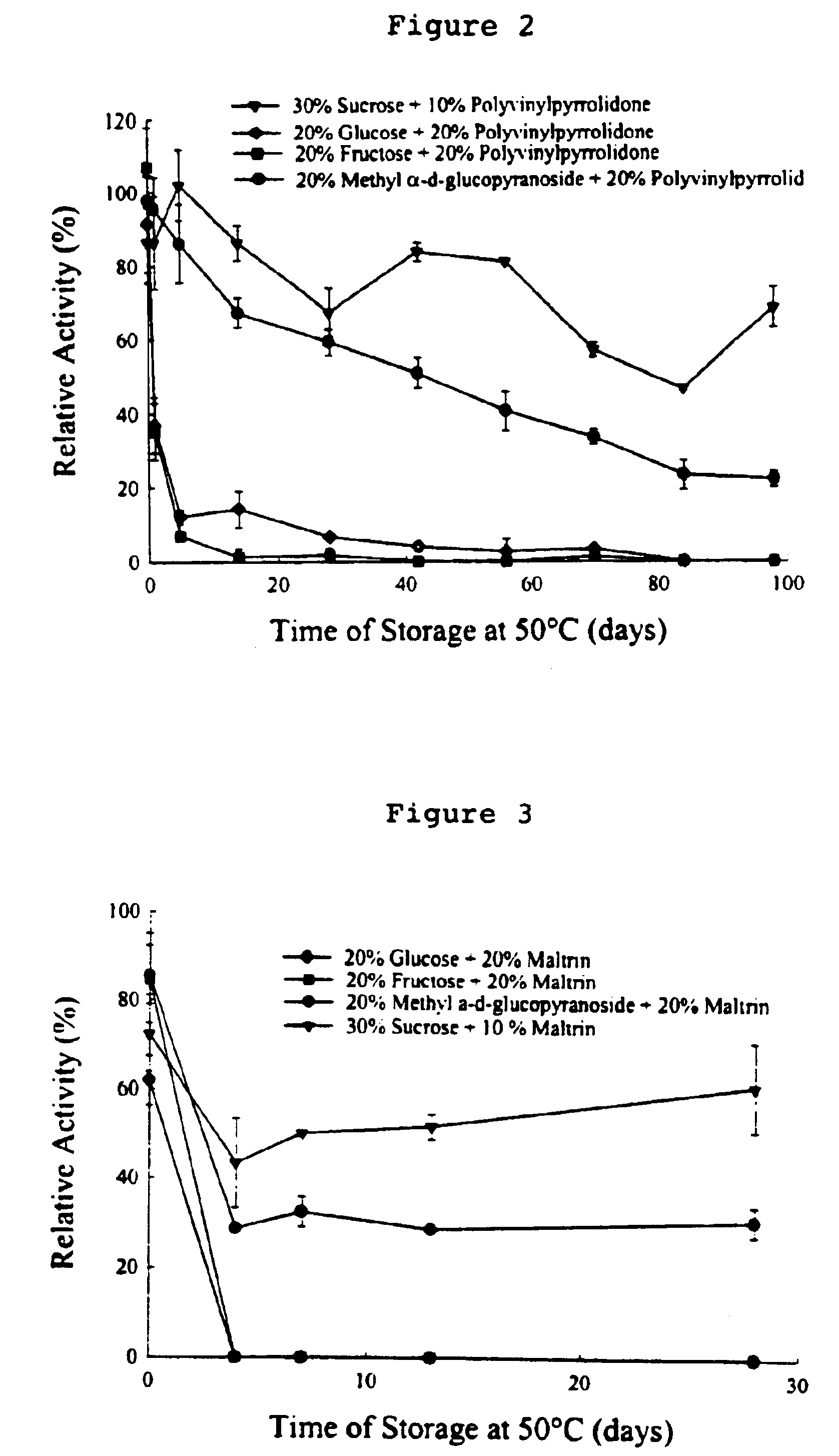
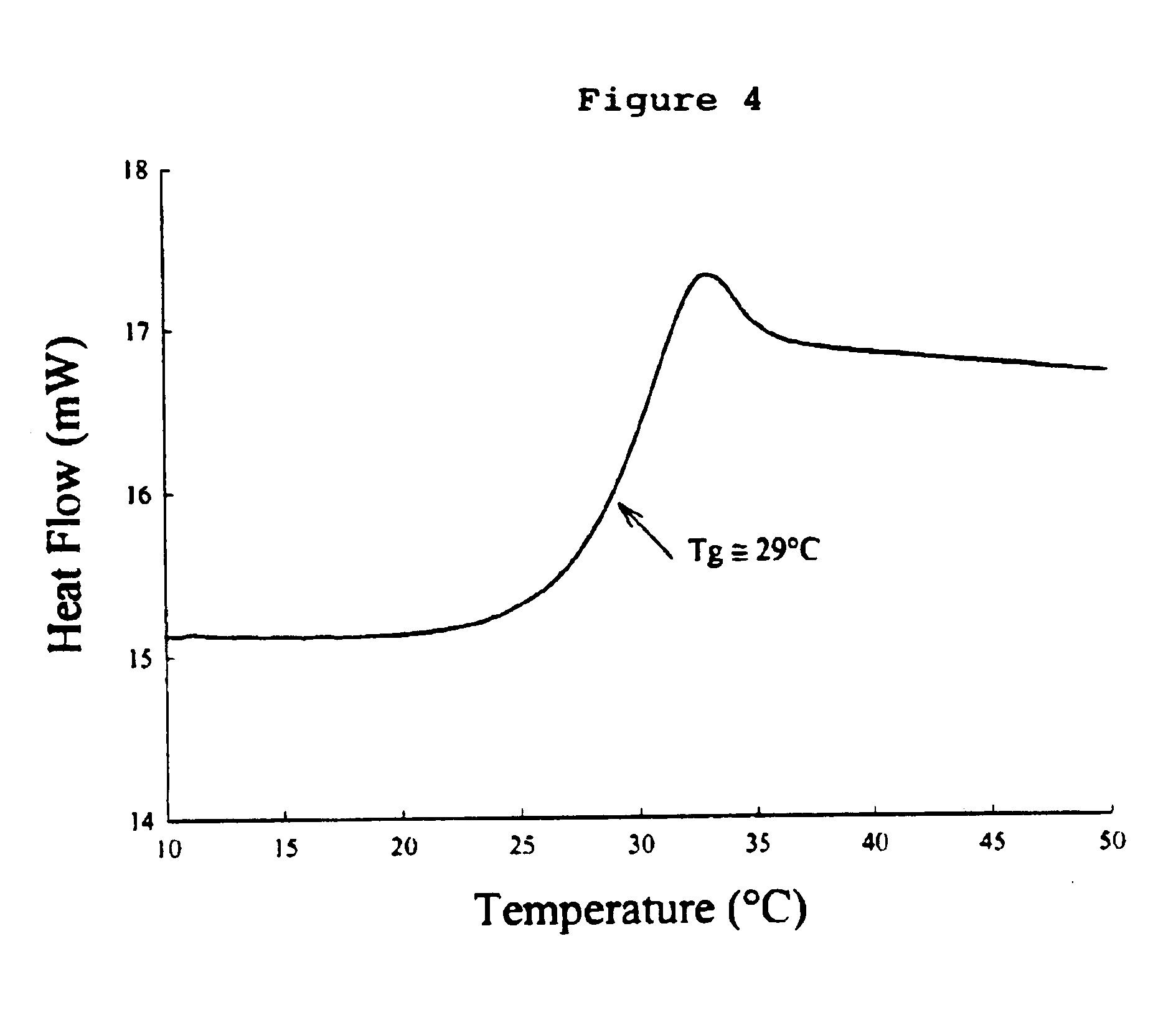
Formulation of preservation mixtures containing sensitive biologicals to be stabilized for ambient temperature storage by drying
This invention relates to formulations and methods for preserving sensitive biologicals, viruses, bacteria and eukaryotic cells by drying. More particularly, the invention relates to preservation mixtures containing viruses or cells and protectants, including a combination of a methylated monosaccharide and a disaccharide, or oligosaccharide, wherein the mixtures are adapted to stabilize these during dehydration and subsequent storage at ambient and higher temperature.
Owner:AVANT IMMUNOTHERAPEUTICS
Separative Bioreactor
InactiveUS20110198286A1Improve abilitiesLow costSolvent extractionLighting and heating apparatusInclusion bodiesPerfusion
A bioreactor that combines the steps of recombinant expression and separation of a biological product by binding the secreted biological product with a resin, discarding the nutrient medium and eluting the biological product as a concentrated solution, eliminating the steps of sterile filtration and volume reduction. The method also allows loading of resin for column-purification, eliminating all steps of perfusion process and maintaining a sink condition of a toxic product in nutrient medium to optimize productivity of host cells. The instant invention also allows harvesting of solubilized inclusion bodies after the cells have been lysed and refolding of proteins inside the bioreactor.
Owner:NIAZI SARFARAZ K
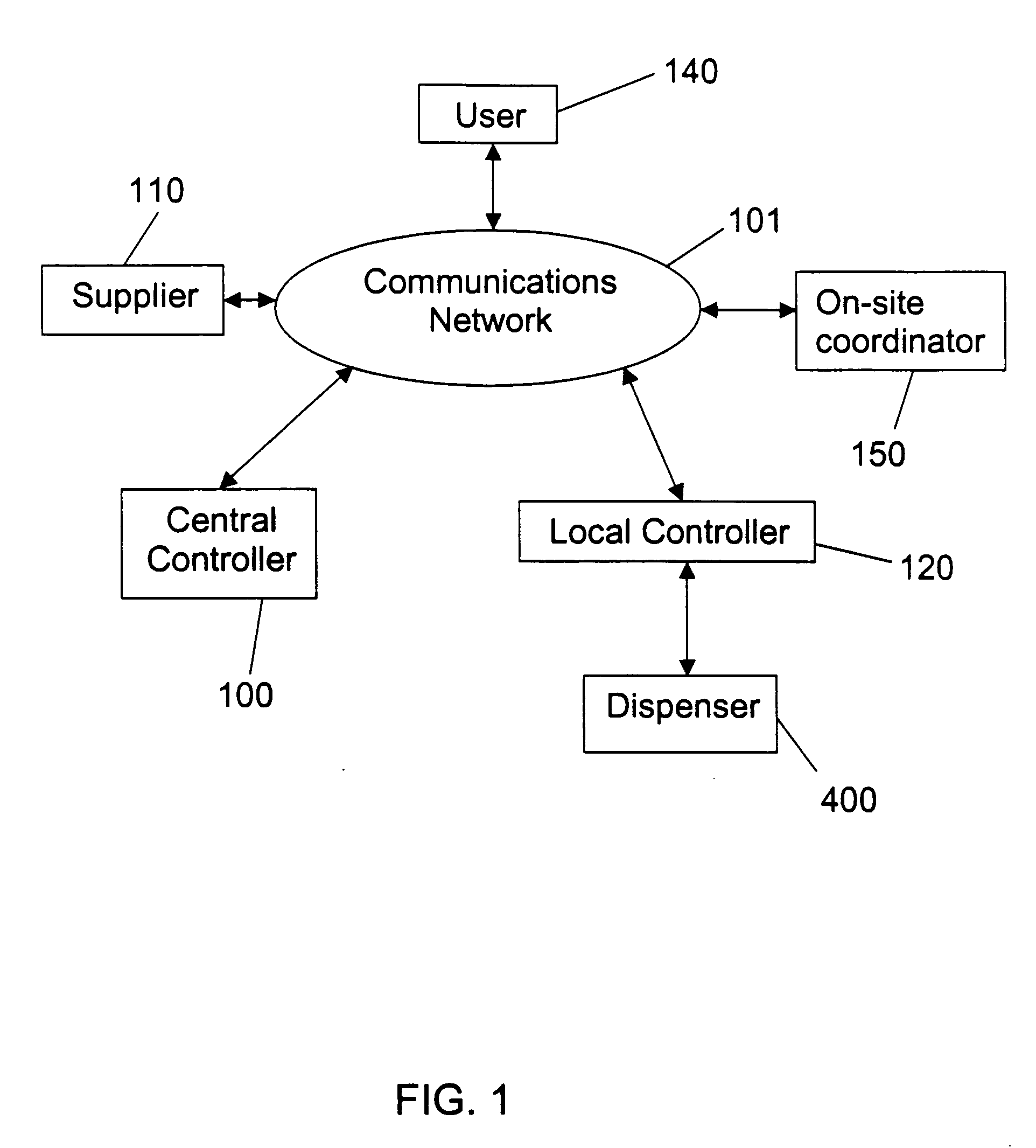
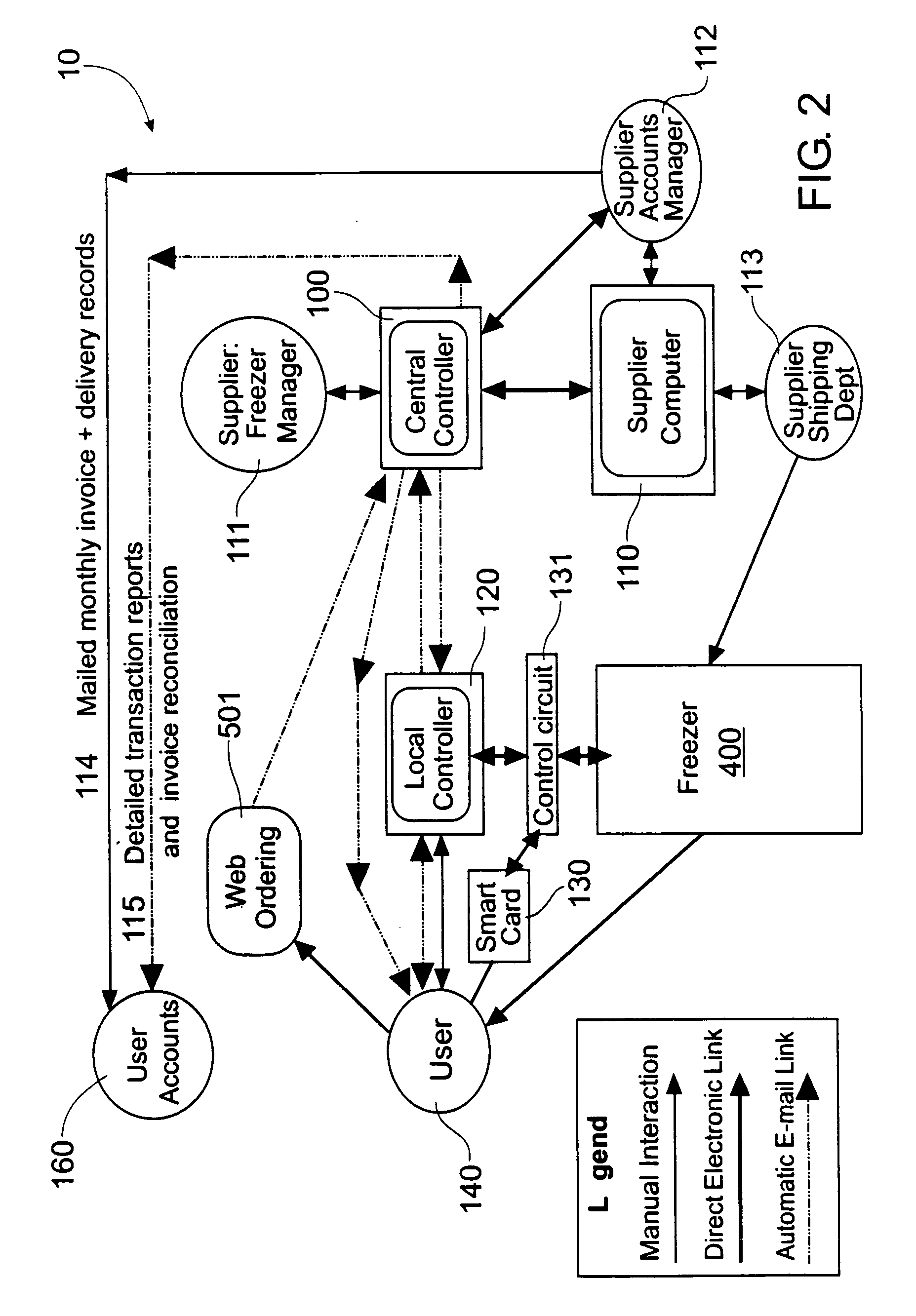
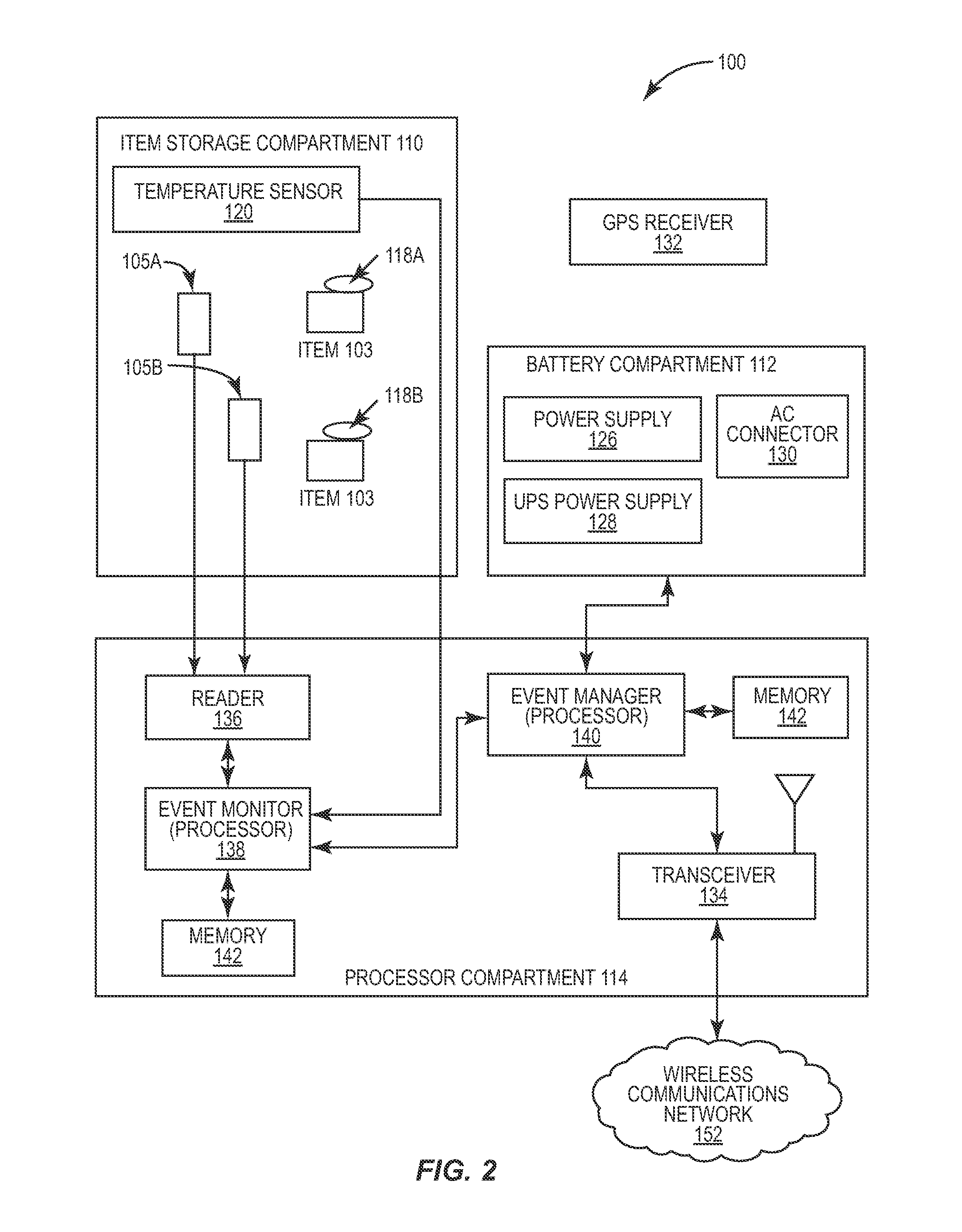
Automated item dispensing systems
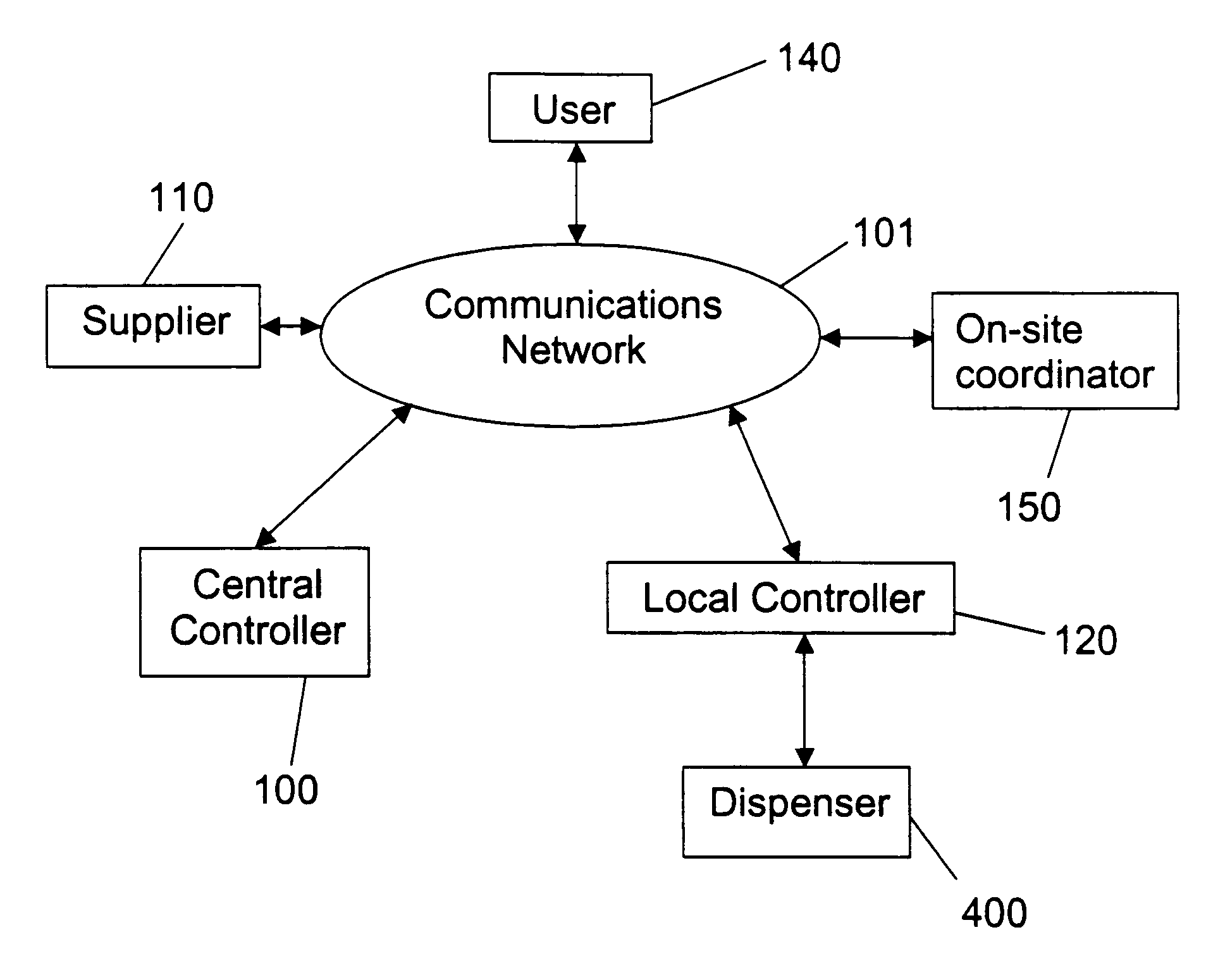
InactiveUS20050060063A1Controlling coin-freed apparatusCoin-freed apparatus detailsBiological productBiomedical engineering
The invention relates to an automated item dispensing system that is capable of dispensing biological products. The system preferably comprises: (a) at least one dispenser (preferably a temperature controlled dispenser) capable of dispensing an item; (b) at least one local controller for controlling and monitoring a respective dispenser; (c) a central controller for receiving and processing electronic item orders from at least one user; and (d) a communication network for providing communication between said central controller, said local controller(s), said user(s) and at least one supplier that is capable of stocking and restocking items in the dispenser. The dispenser preferably comprises: (i) at least one rotatable carousel; (ii) an access hatch aligned with each carousel for accessing an interior of an item cell located within the carousel; and (iii) a motor for rotating the carousel.
Owner:GENESEARCH
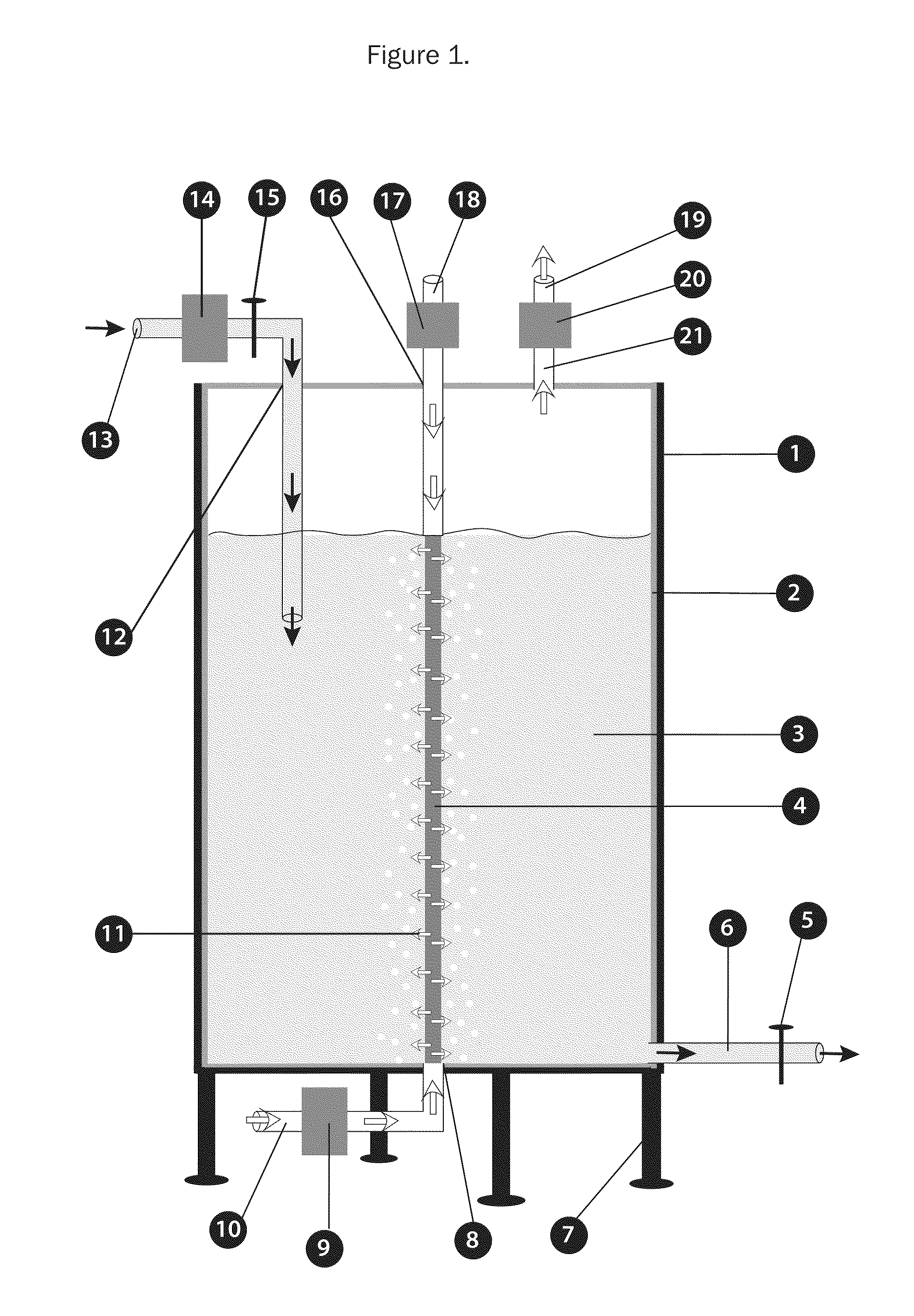
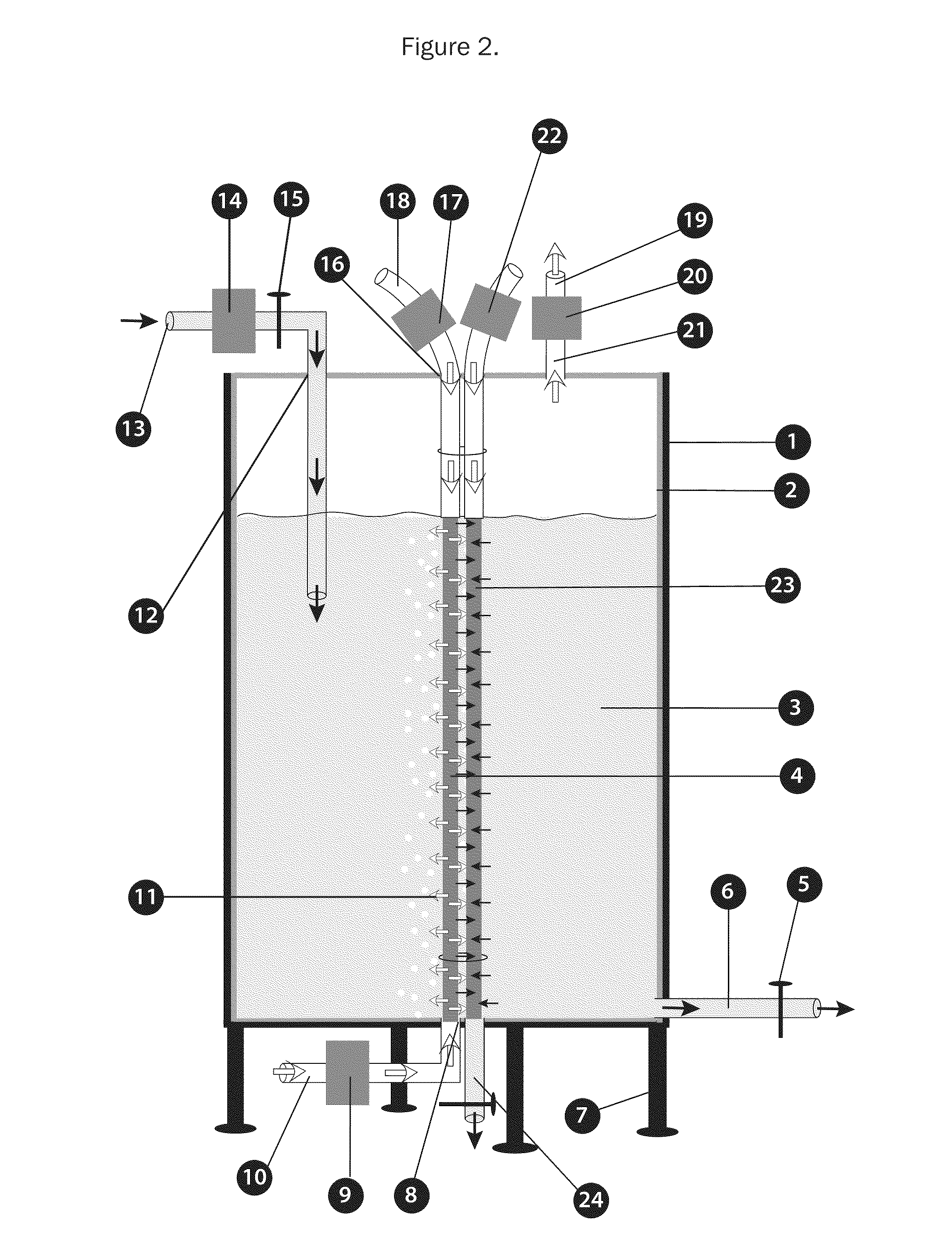
Bioreactors for fermentation and related methods
InactiveUS20110117538A1Suitable for useEvenly distributedBioreactor/fermenter combinationsBiological substance pretreatmentsFermentationBiologic Products
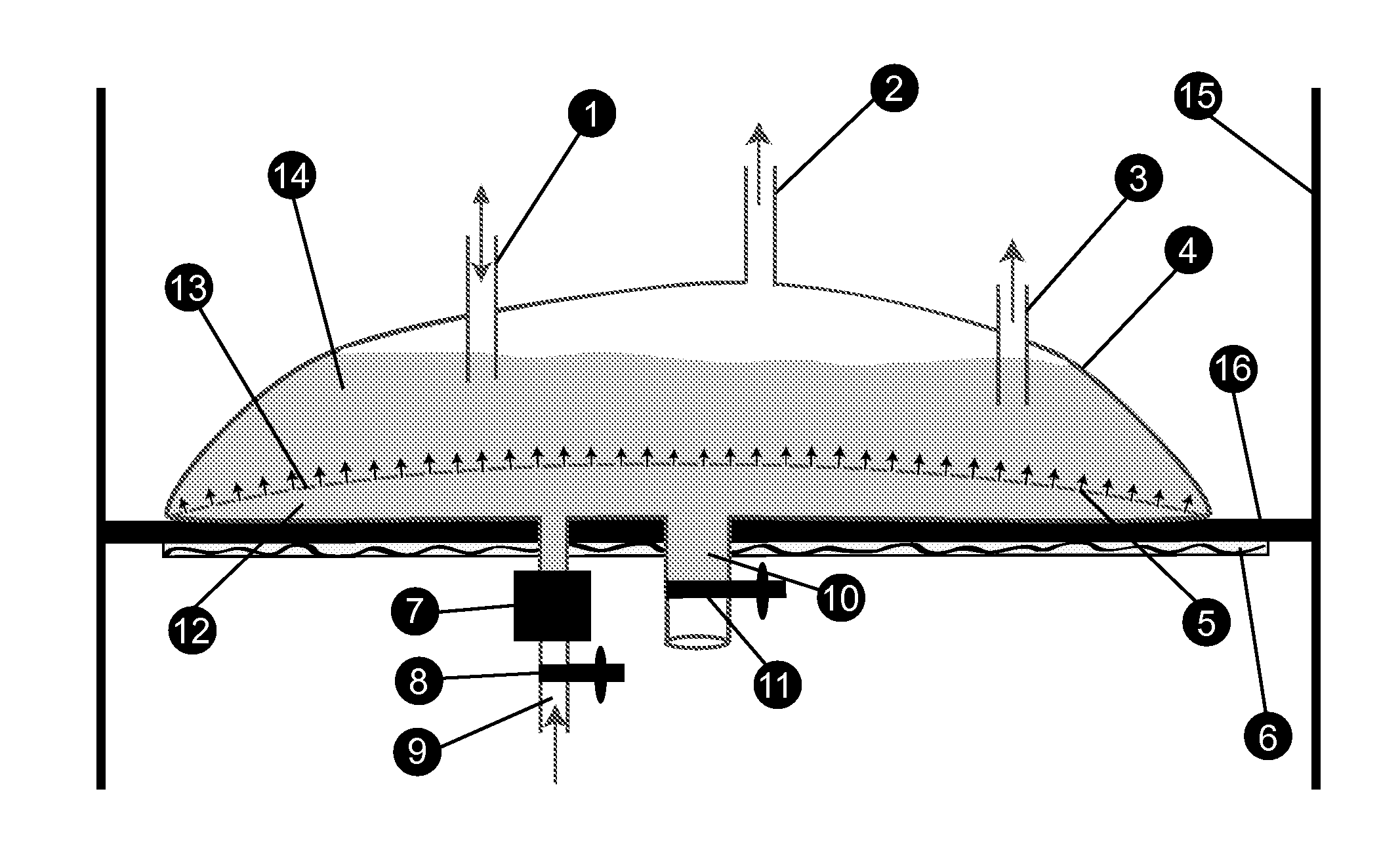
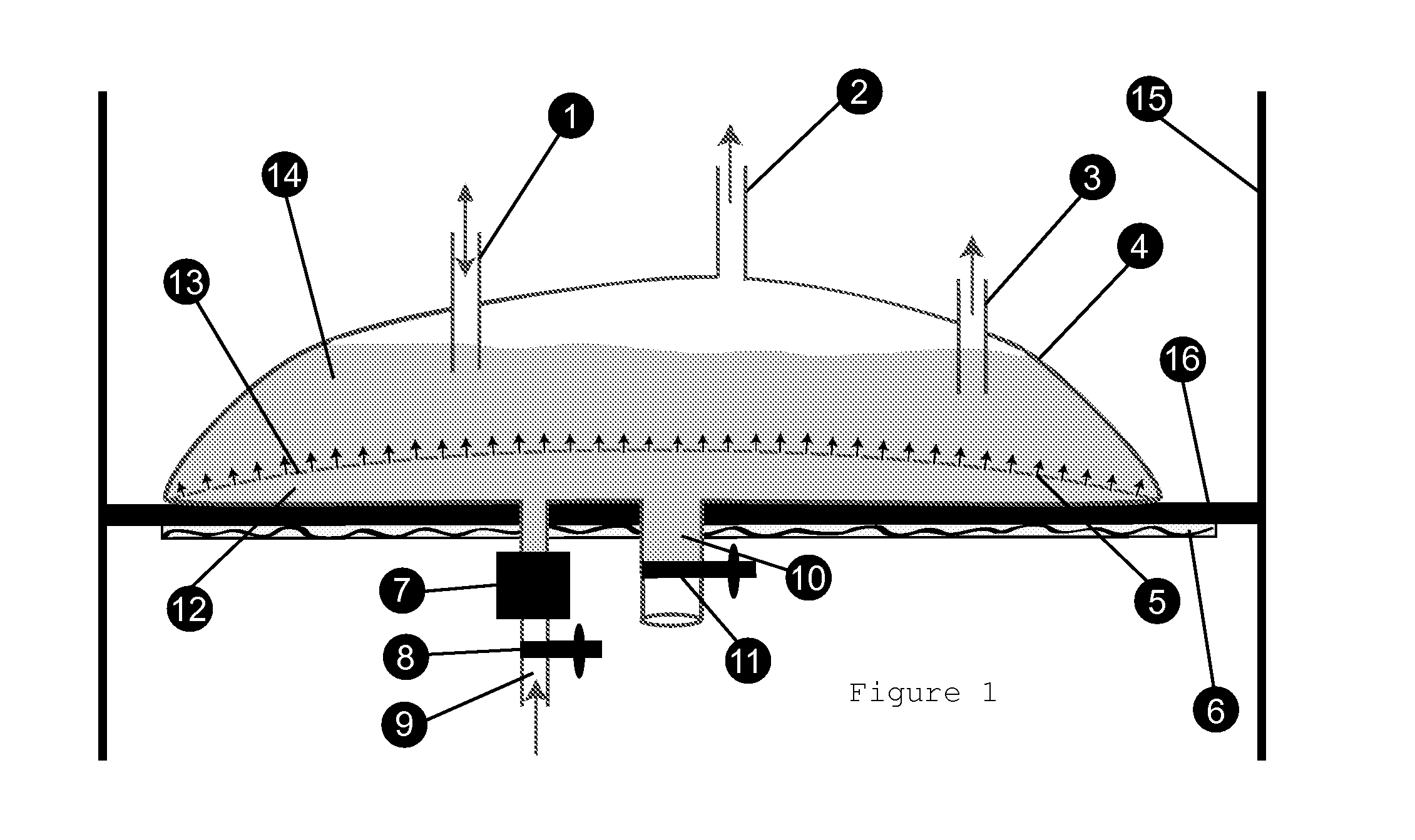
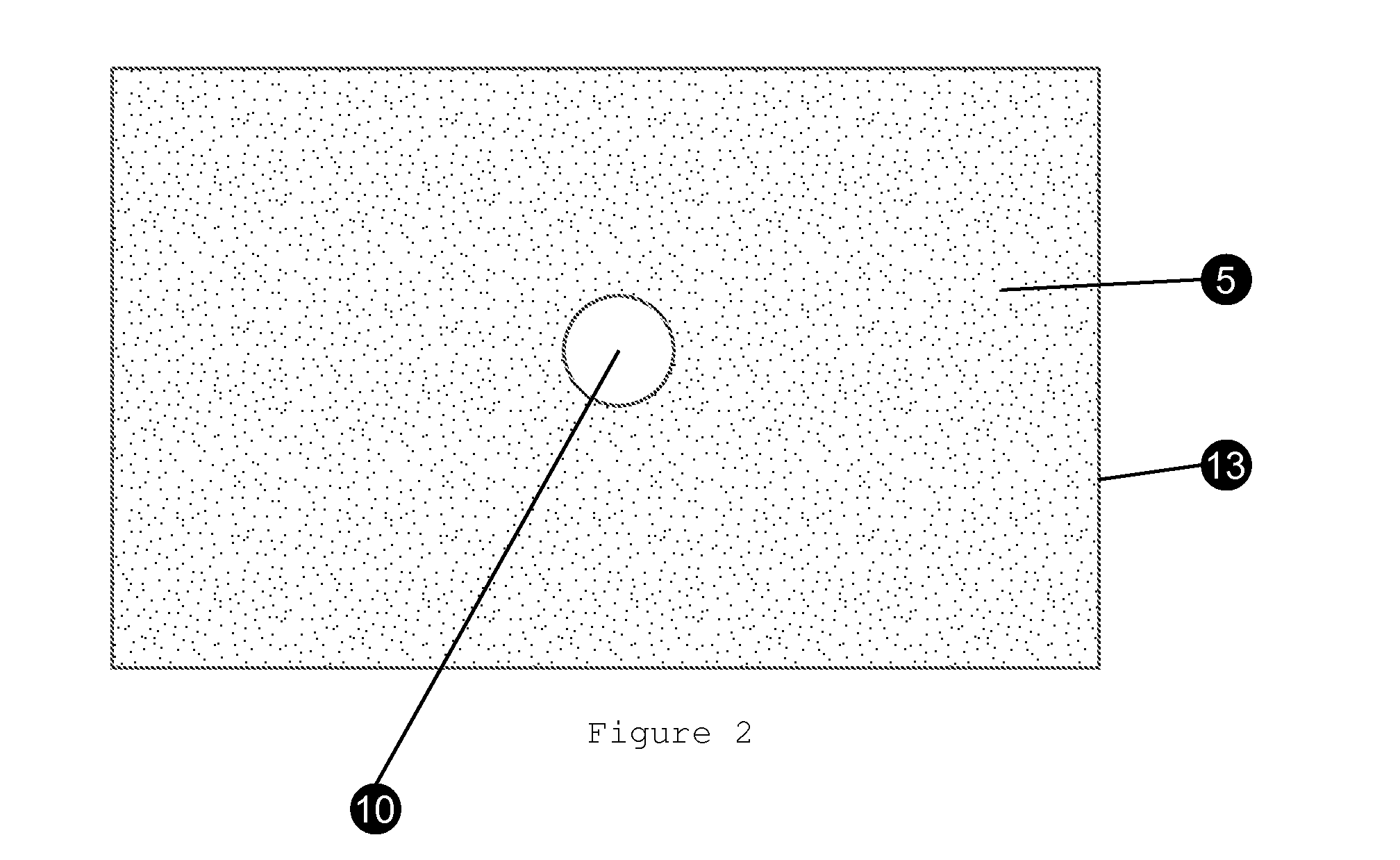
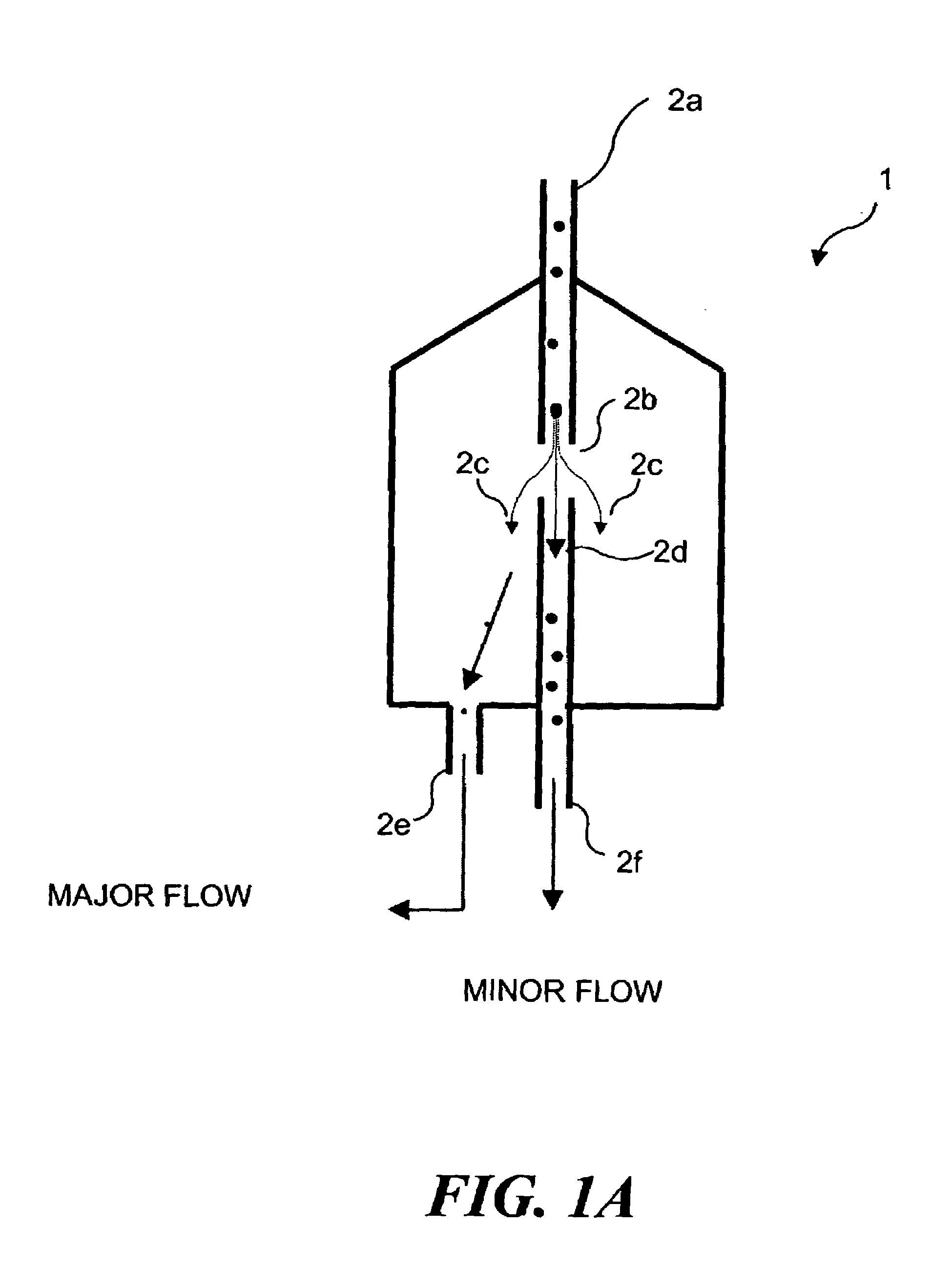
Bioreactors suitable for housing a predetermined volume of liquid comprising nutrient medium and biological culture comprising: (a) a container having at least one interior wall; (b) at least one nutrient medium inlet; (c) at least one liquid outlet; (d) at least one gas inlet; (e) at least one gas outlet; and (f) at least one cylindrical sparging filter attached to the at least one gas inlet, wherein the sparging filter comprises a plurality of pores along its axis which permit gas to be emitted radially from the sparging filter into the liquid, wherein the diameter of the plurality of pores does not exceed about 50 μm, and wherein the orientation of the at least one sparging filter within the container provides for immersion of the plurality of pores within the liquid and substantially uniform distribution of emitted gas throughout the liquid, and related methods of using said bioreactors to prepare various biological products.
Owner:THERAPEUTIC PROTEINS +1
Multiuse reactors and related methods
InactiveUS20120231504A1Bioreactor/fermenter combinationsPeptide/protein ingredientsUnit operationProduct gas
Owner:THERAPEUTIC PROTEINS INT
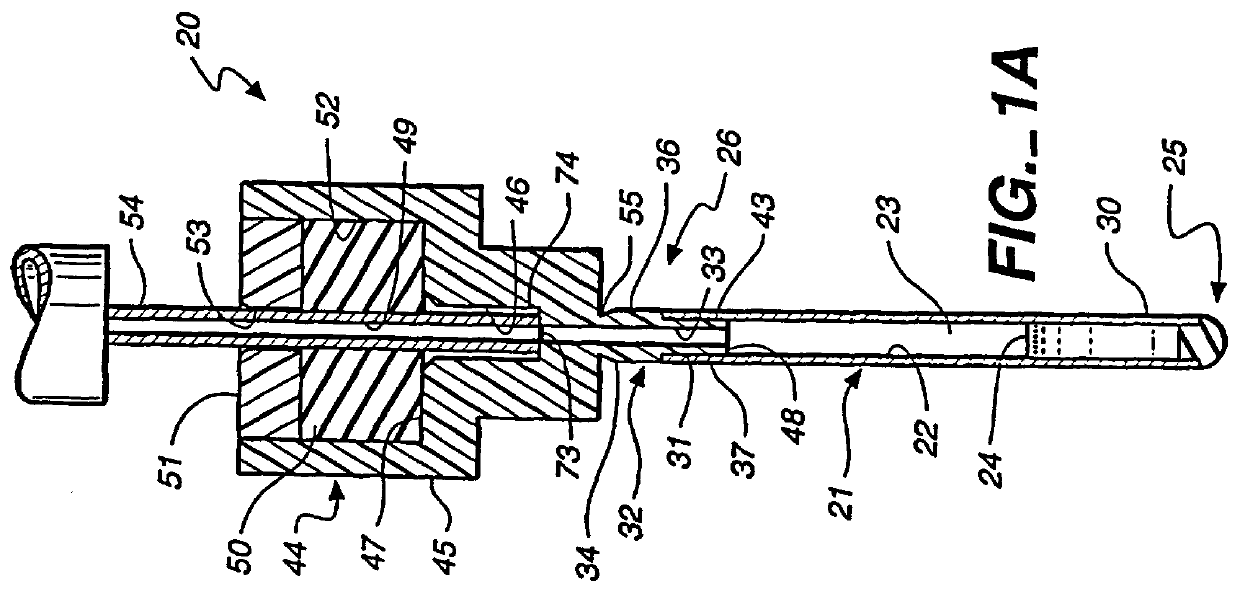
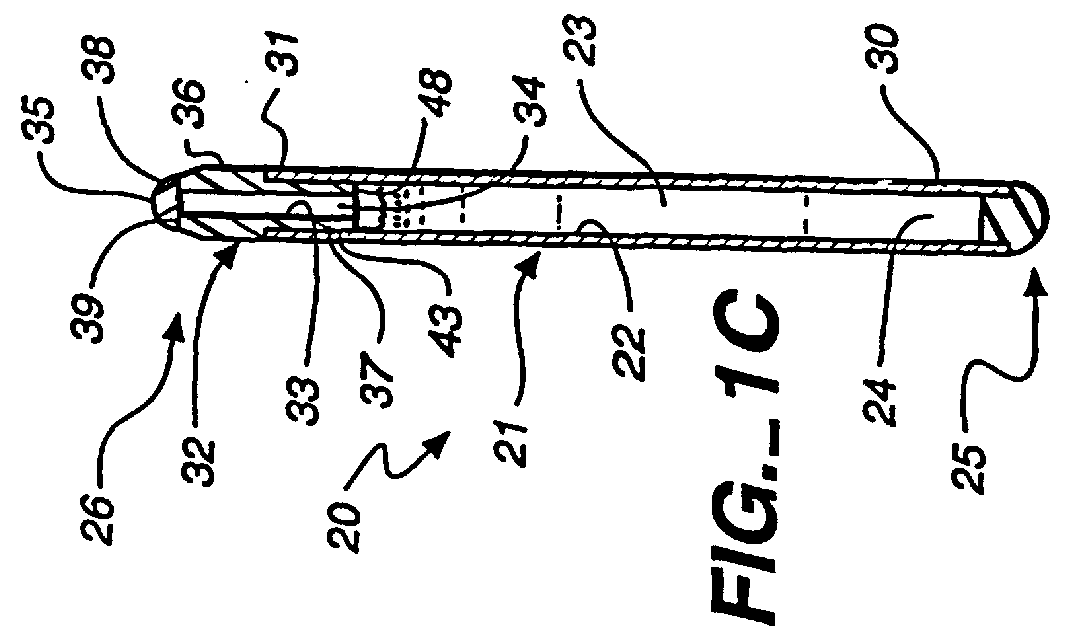
Method and apparatus for sealing implantable membrane encapsulation devices
InactiveUS6123700AImprove adhesionEasy maintenancePharmaceutical delivery mechanismMedical devicesLiving cellBiomedical engineering
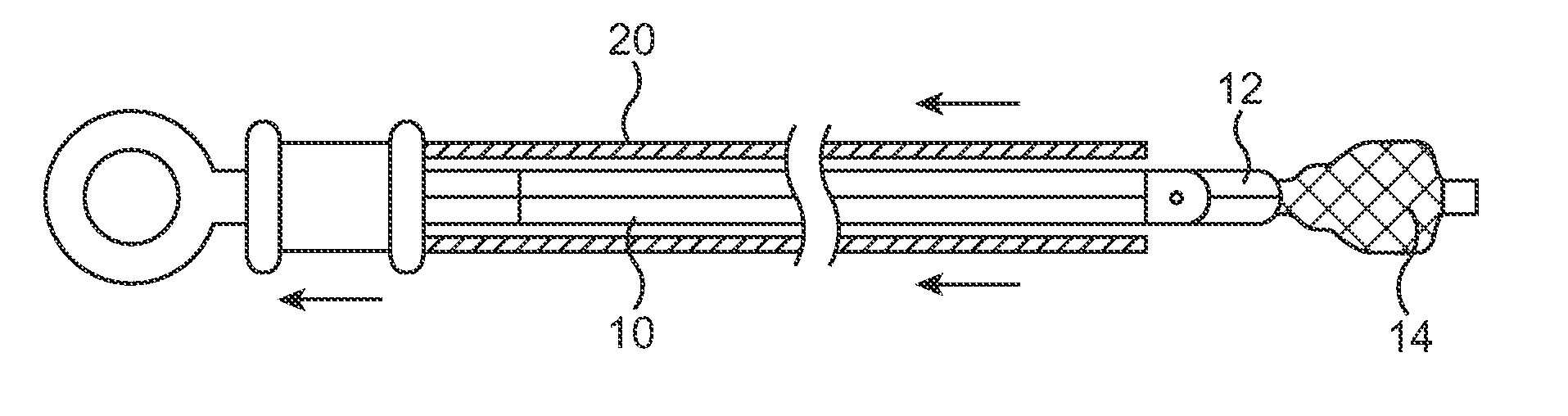
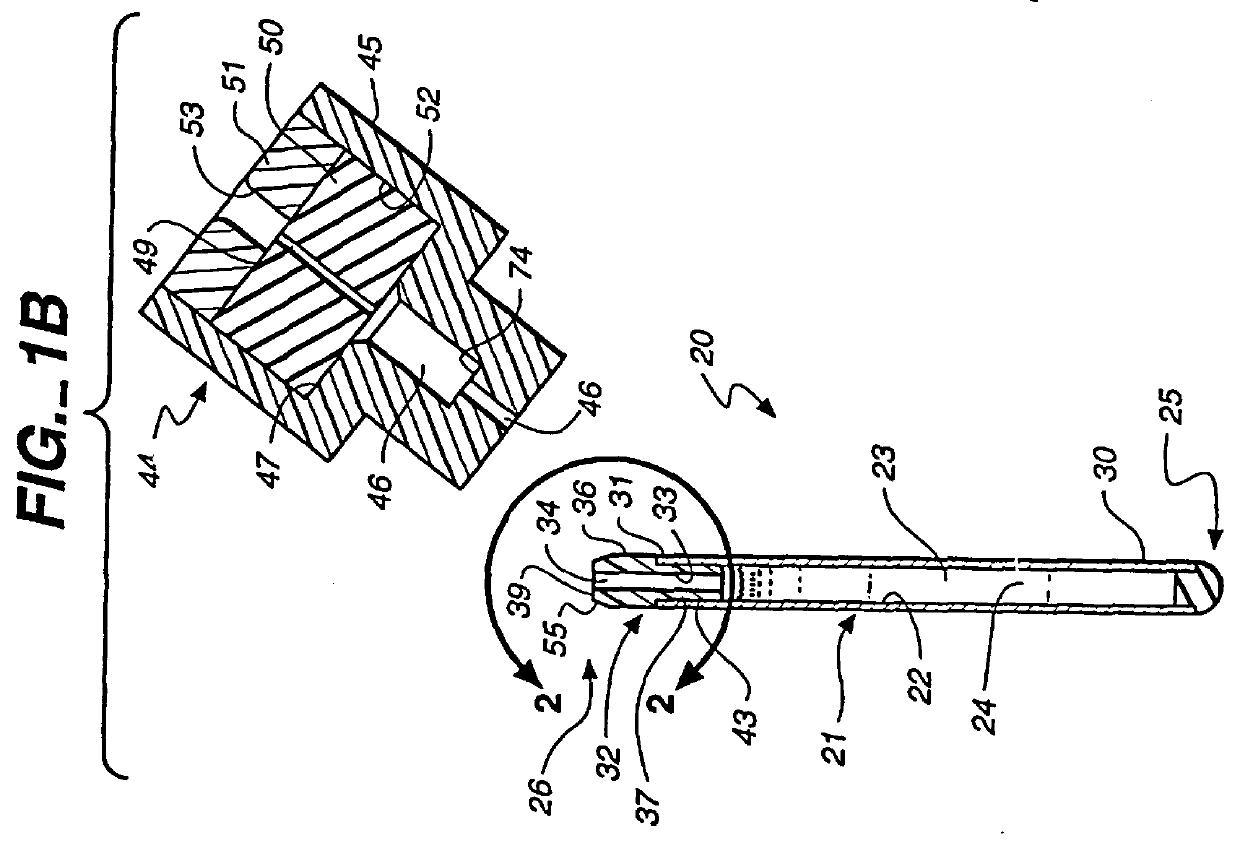
A sealed, implantable, encapsulation device (20) for diffusing a biologically active product or function to an individual which includes a substantially non-porous fitting (32) including an inner surface (33) defining an access port (34). A permselective, porous, membrane (21), having an interior surface (22), cooperates with the fitting inner surface (33) to form a storage cavity (23) therebetween. The membrane interior surface (22) is in substantially cell-tight dry sealing engagement with fitting (32) to seal cavity (23). Living cells (24) are disposed in the cavity (23) which are capable of secreting the biologically active product to an individual. The membrane (21) is of a material capable of permitting the passage of substances between the individual and cells required to provide the biological product or function. A plug member (35) is positioned in the access port (34) and seated in cell-tight sealing engagement with the fitting inner surface (33). A method for sealing the implantable encapsulation device (20) is also provided.
Owner:NEUROTECH SA
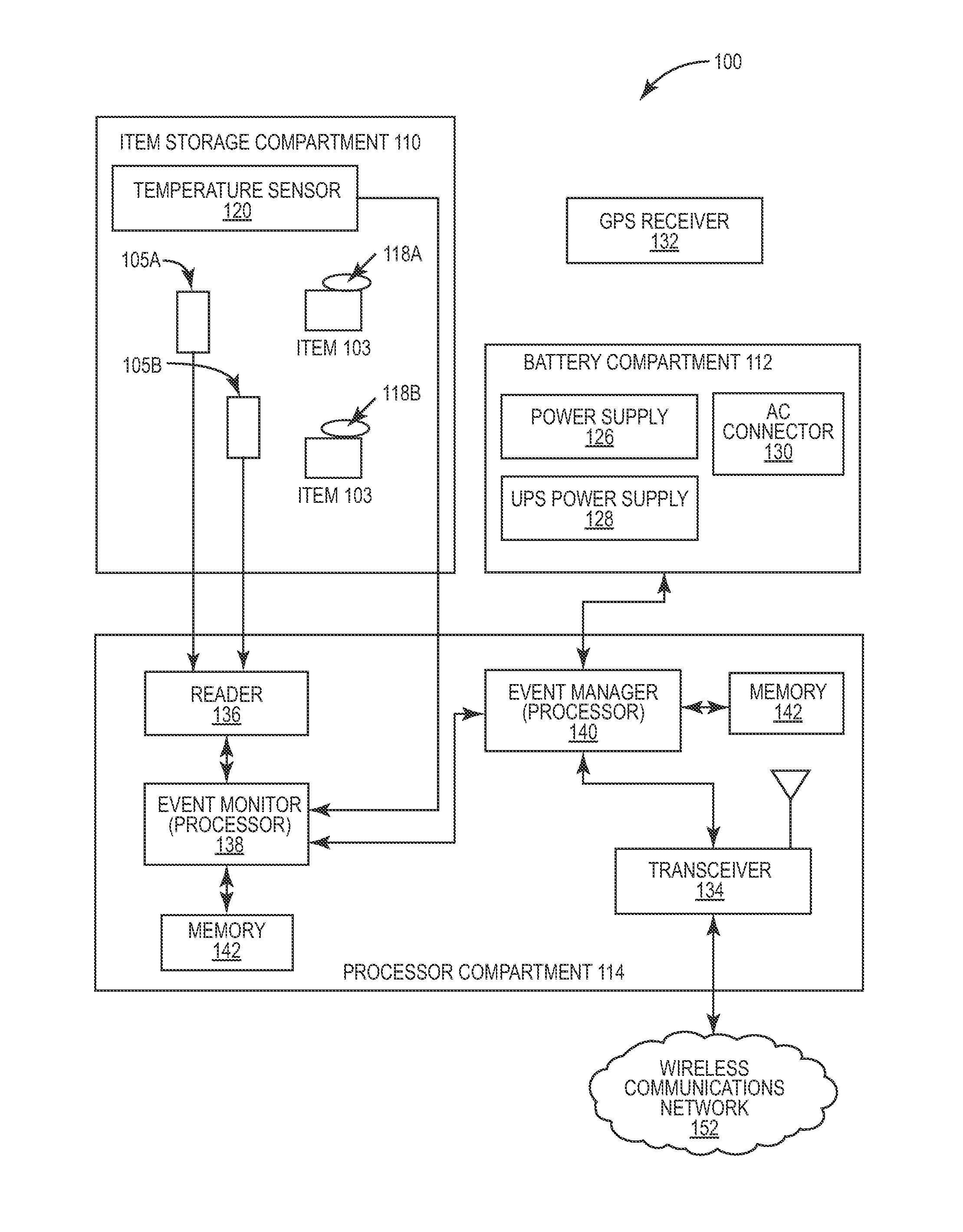
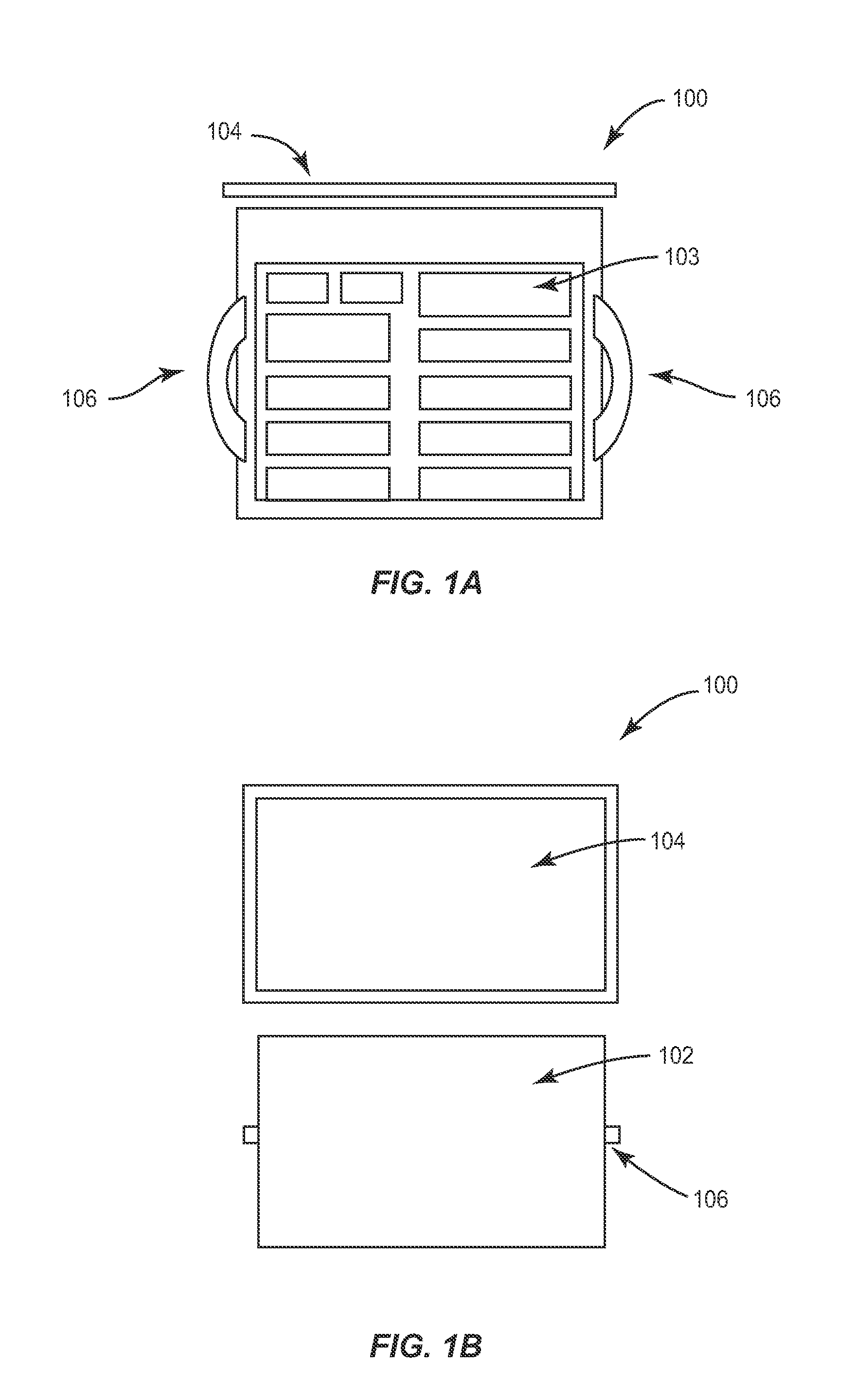
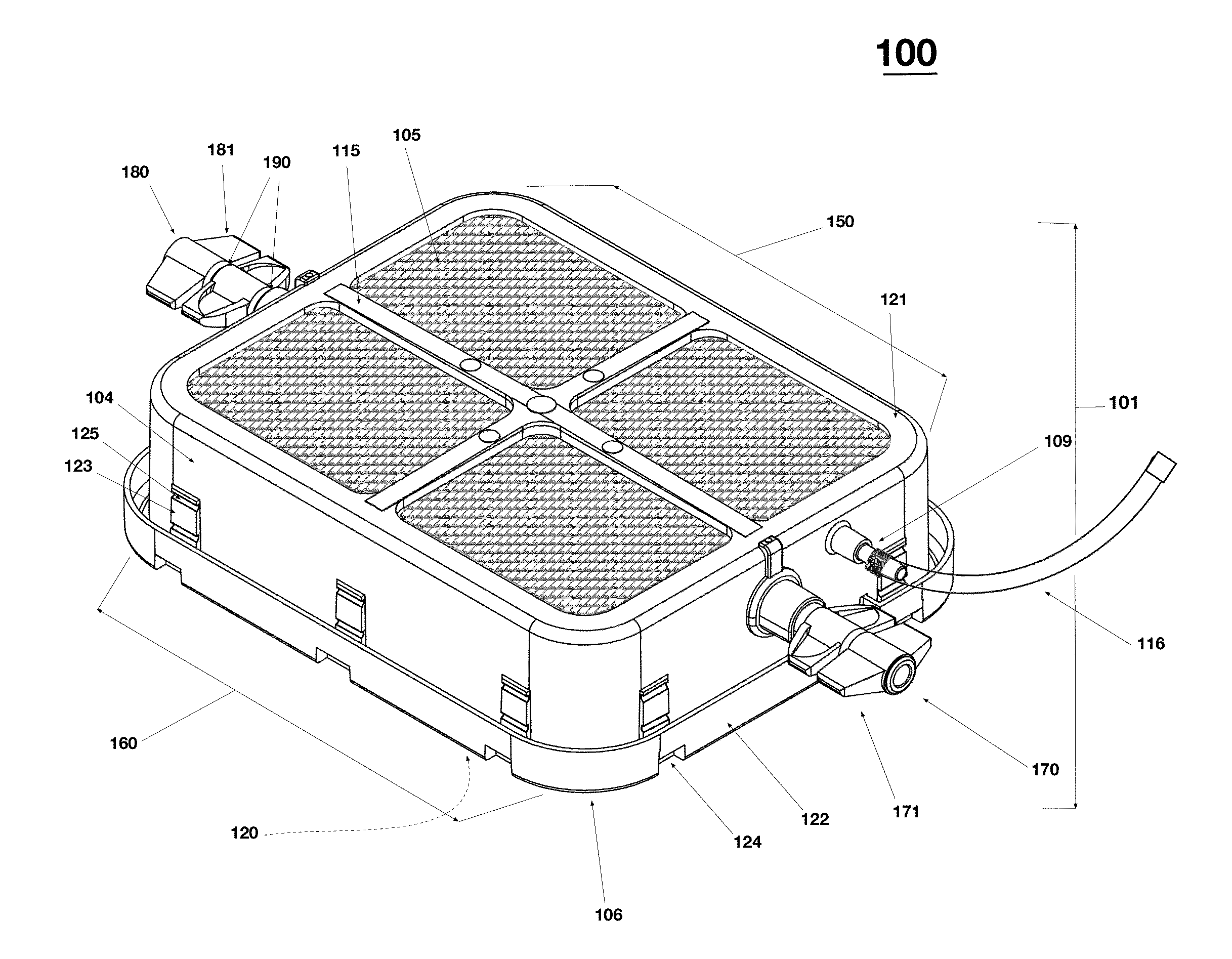
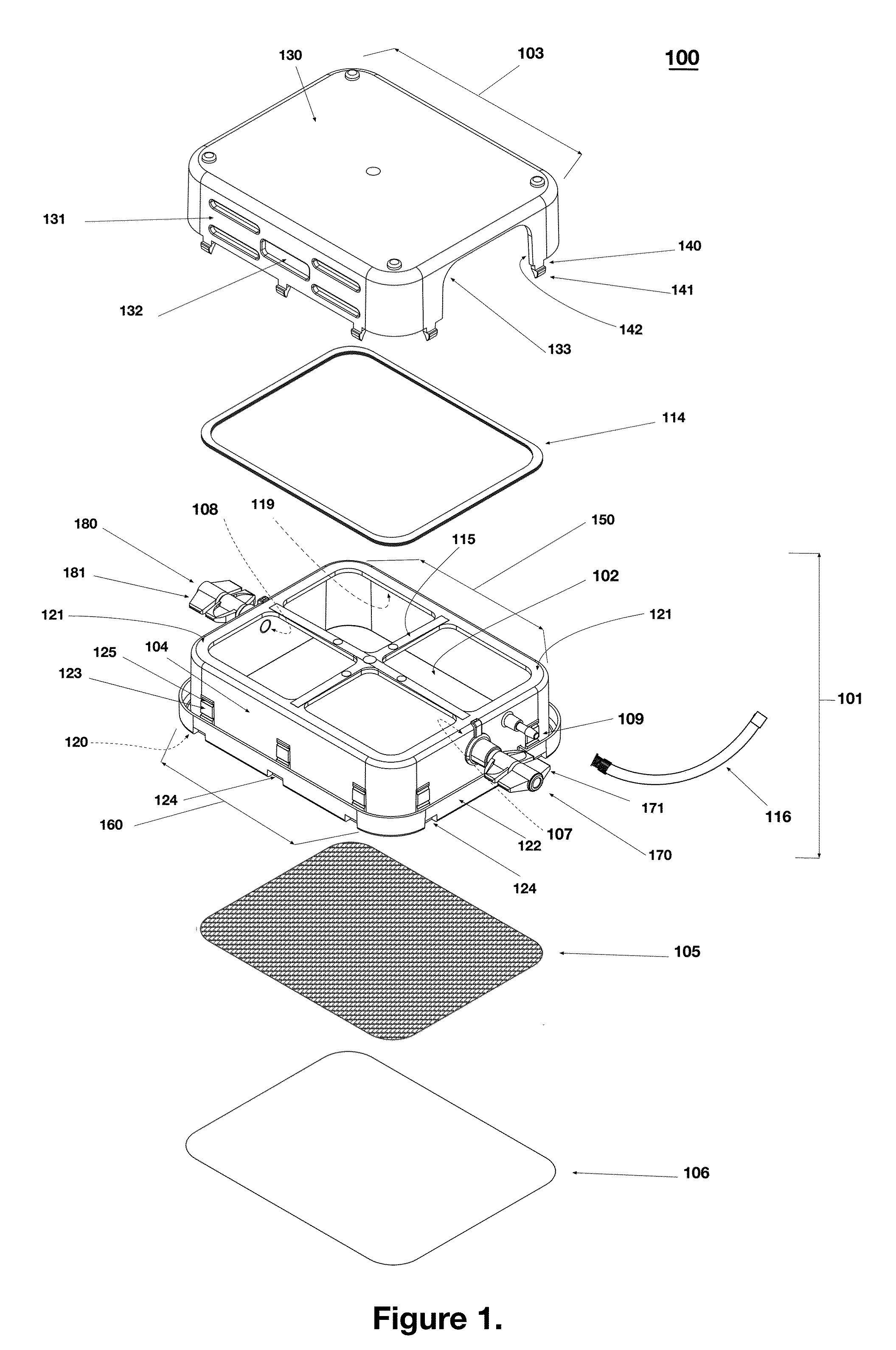
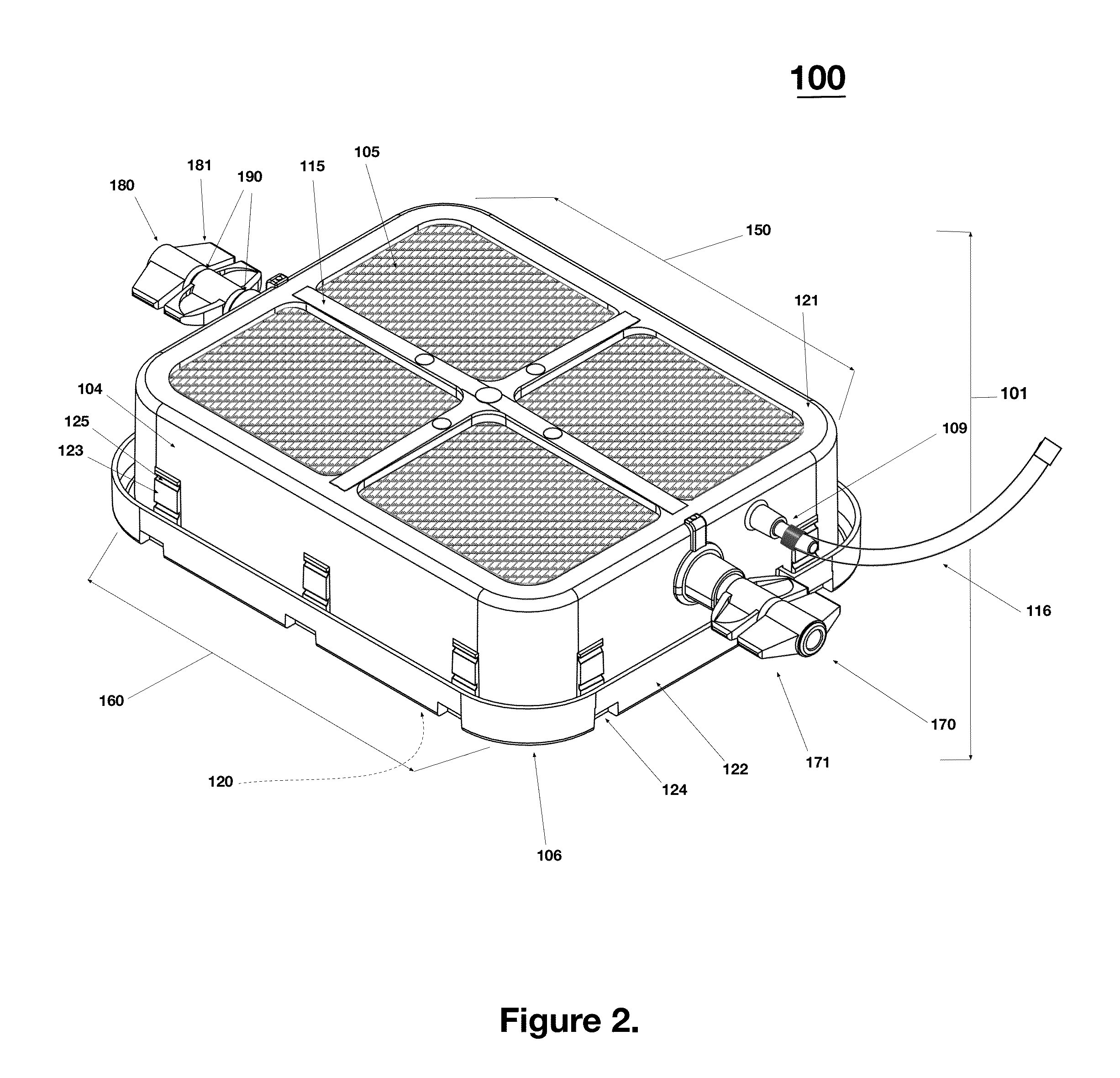
Portable RFID tagged carrier for sterile implants and biological products
ActiveUS20130106607A1Light weightVaccination/ovulation diagnosticsDead animal preservationEngineeringRadio frequency
Intelligent portable carrier device for supporting movement in product tracking and monitoring of regulated products, such as tissue and biologics. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify the regulated products as they are added to or removed from the intelligent portable carrier device. Embodiments of the invention may also be configured to monitor and report temperature and other environmental conditions associated with the intelligent portable carrier device.
Owner:WARSAW ORTHOPEDIC INC
Flexible manufacturing system
ActiveUS20110258837A1High capacity supplyDischarging wasteTreatment involving filtrationChemoinformaticsFlexible manufacturing systemEngineering
Owner:XOMA US
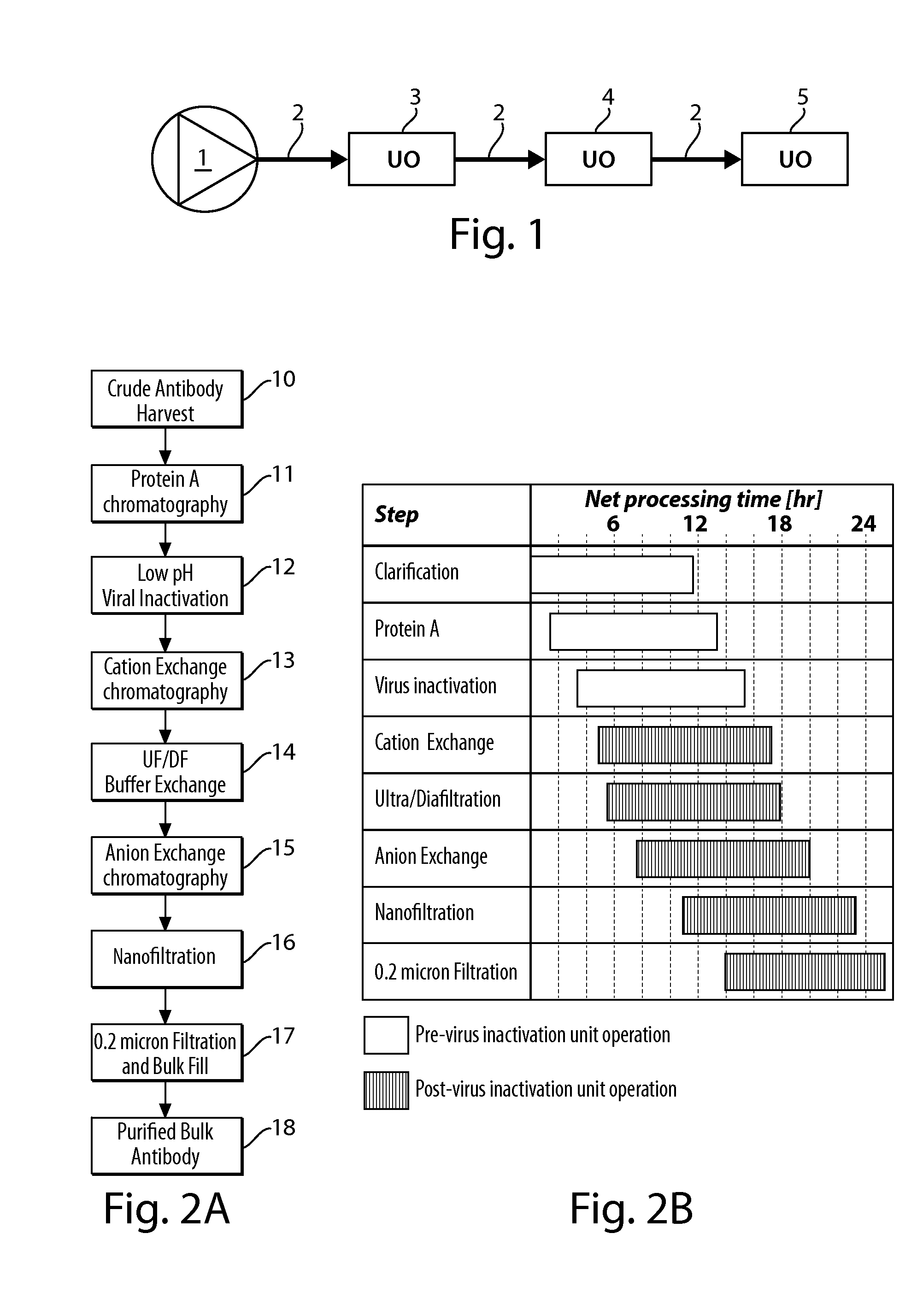
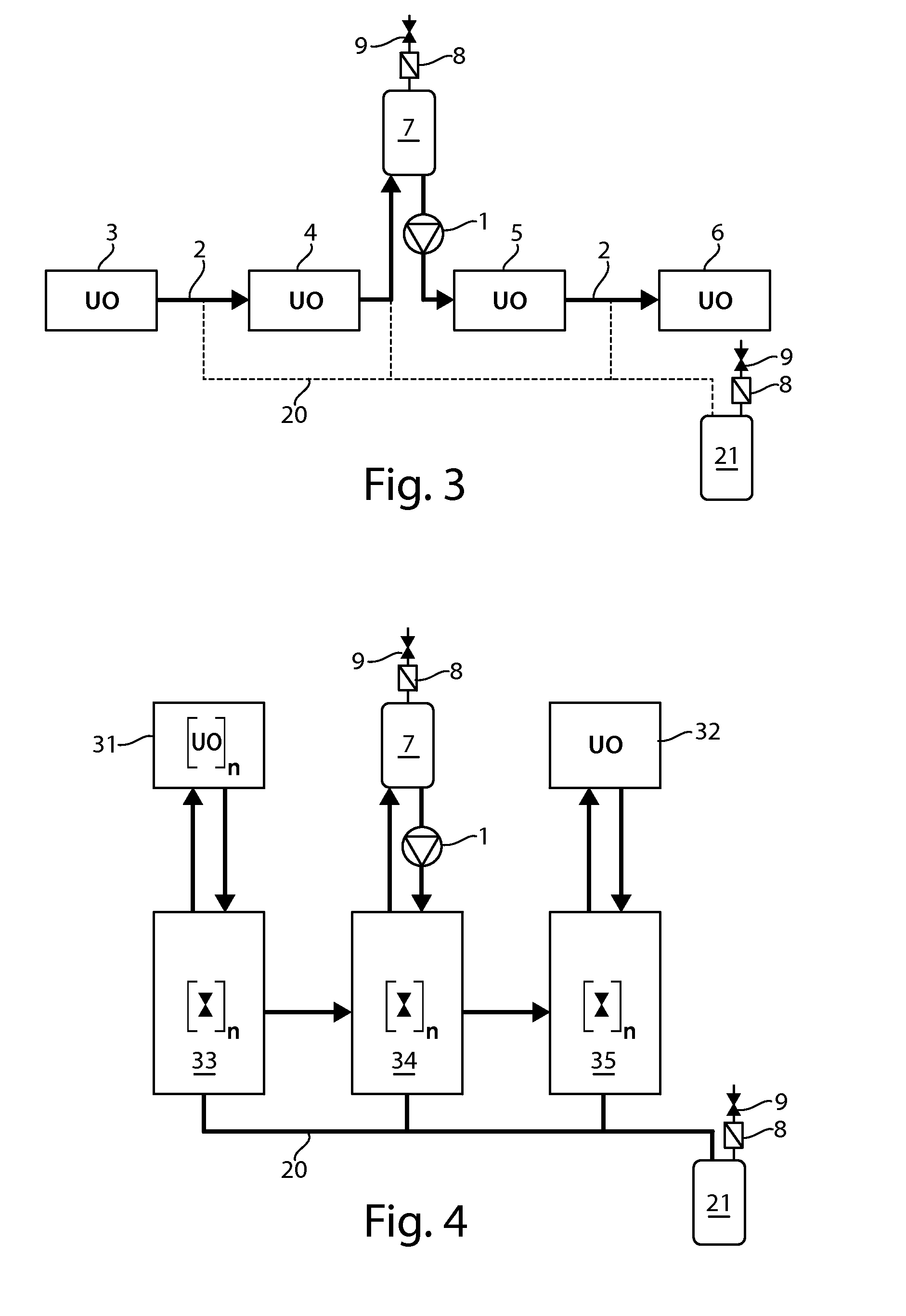
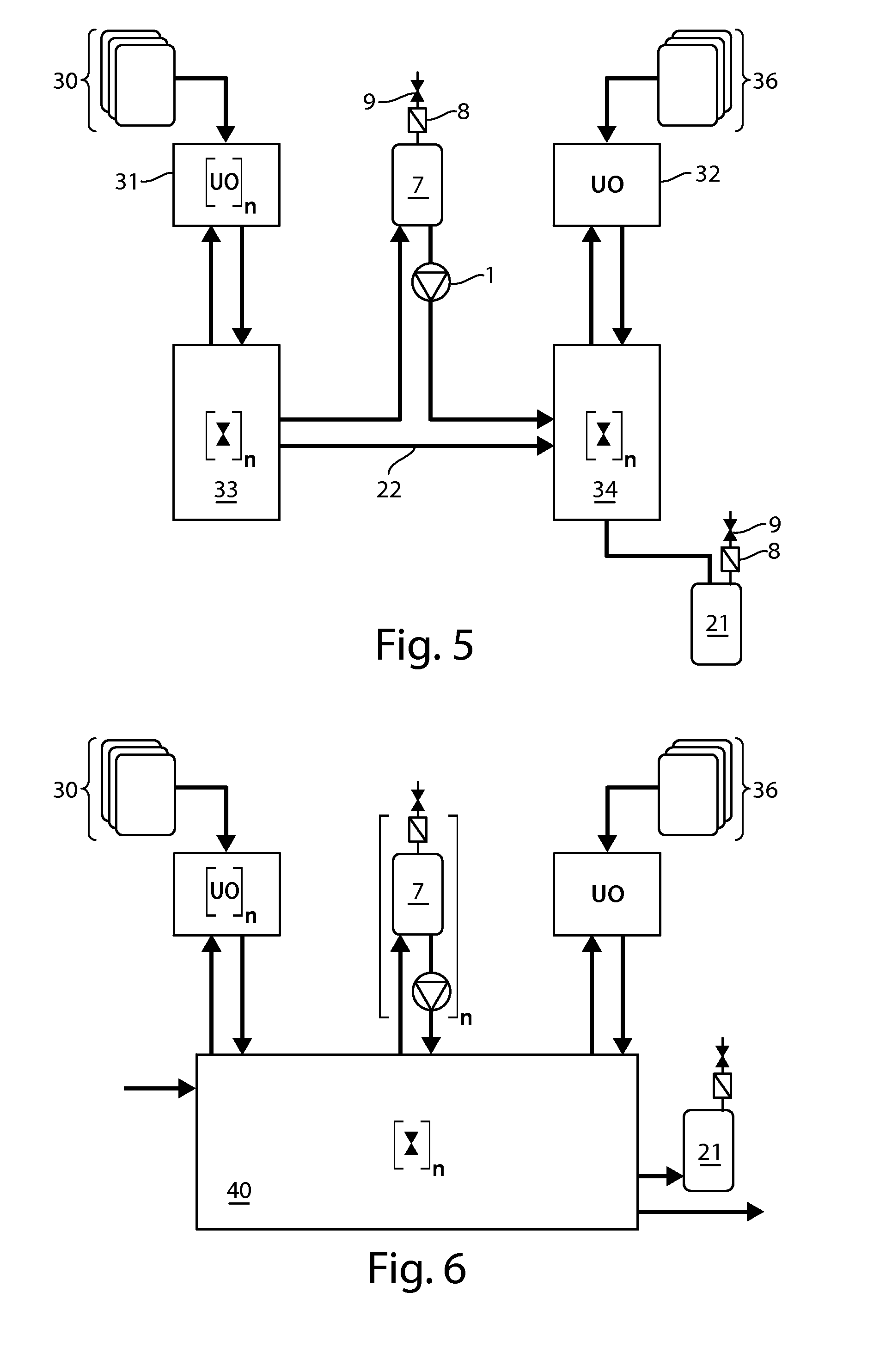
Continuous processing methods for biological products
InactiveUS20130260419A1Improve manufacturing productivityImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyMonoclonal antibody
The present invention is directed to the development of continuous processing technology for the purification of biopharmaceuticals and biological products, such as monoclonal antibodies, protein therapeutics, and vaccines. Methods for continuous processing of a biological product in a feed stream toward formulation of a purified bulk product are described.
Owner:SARTORIUS STEDIM CHROMATOGRAPHY SYST LTD
Introducing a biological material into a patient
A biological preparation is provided and includes a biological material and a purified, natural or recombinant, extracellular matrix degrading enzyme being externally adhered thereto.
Owner:INSIGHT STRATEGY & MARKETING
Method and apparatus for controlling sulfide generation
ActiveUS20050224409A1Water treatment parameter controlOther chemical processesSulfide generationNitrate
Systems and methods for reducing odors utilizes oxidation-reduction potential to characterize the quality of water or wastewater and control addition of a species thereto that reduces or inhibits biological sulfide generation. Oxidation-reduction potential is measured at a downstream station and, based on the measurement, a nitrate compound is added at an upstream station to promote inoffensive biological products instead of foul-smelling products.
Owner:EVOQUA WATER TECH LLC
Polyurethane foam products with controlled release of agents and additives
InactiveUS6706775B2High densityLow densityPowder deliveryBiocideSuperabsorbent polymerIndividual animal
Owner:H H BROWN SHOE TECH
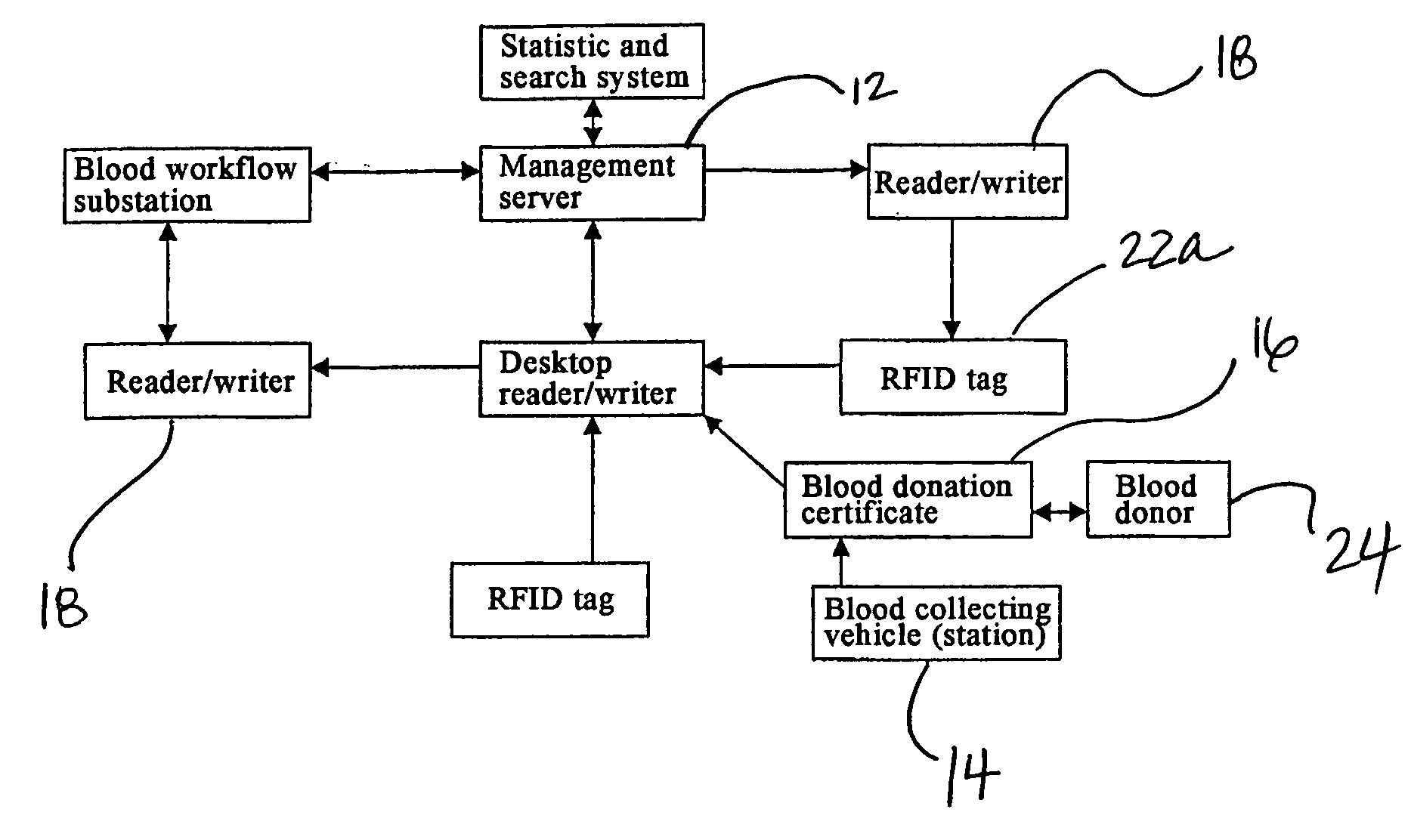
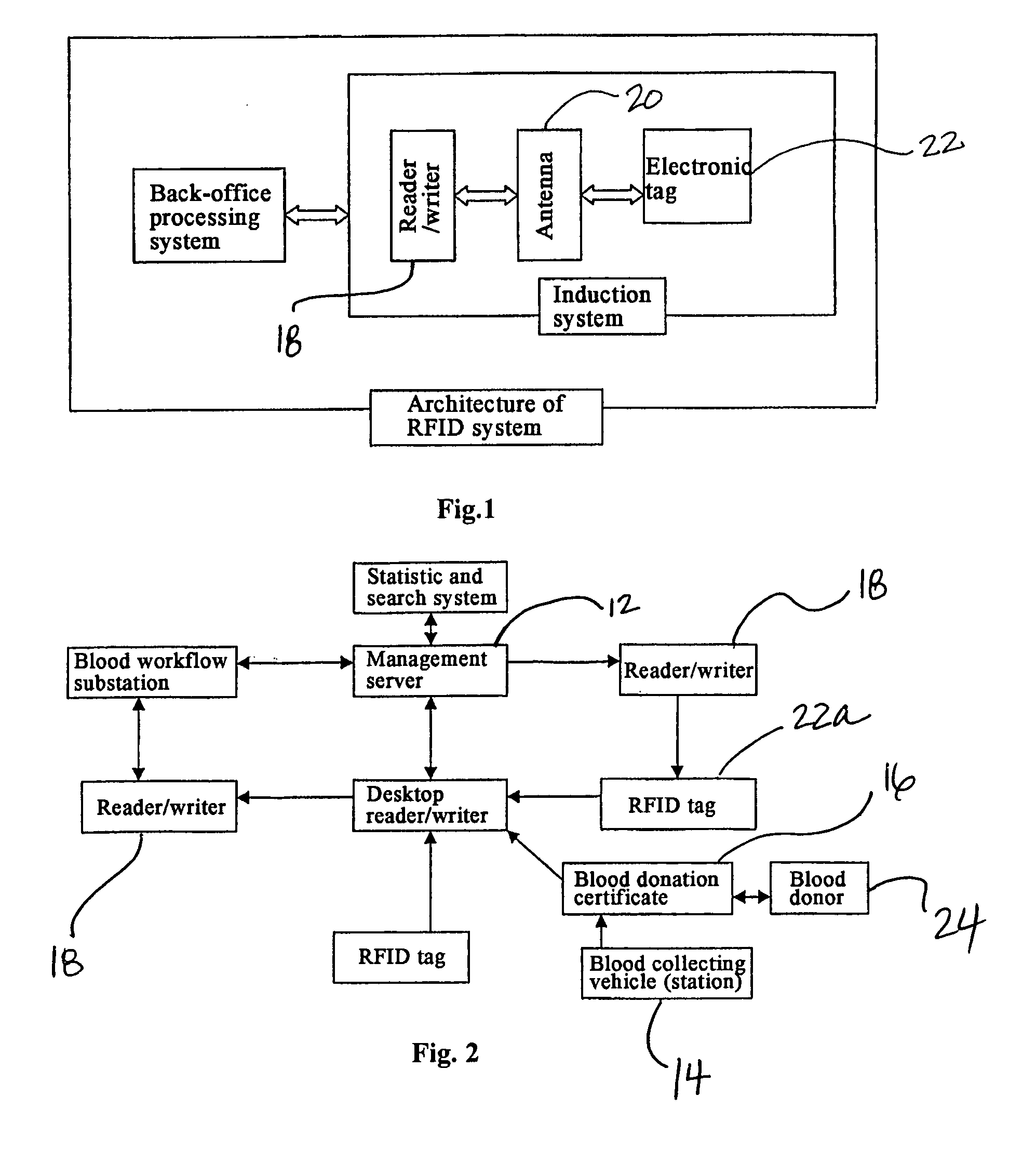
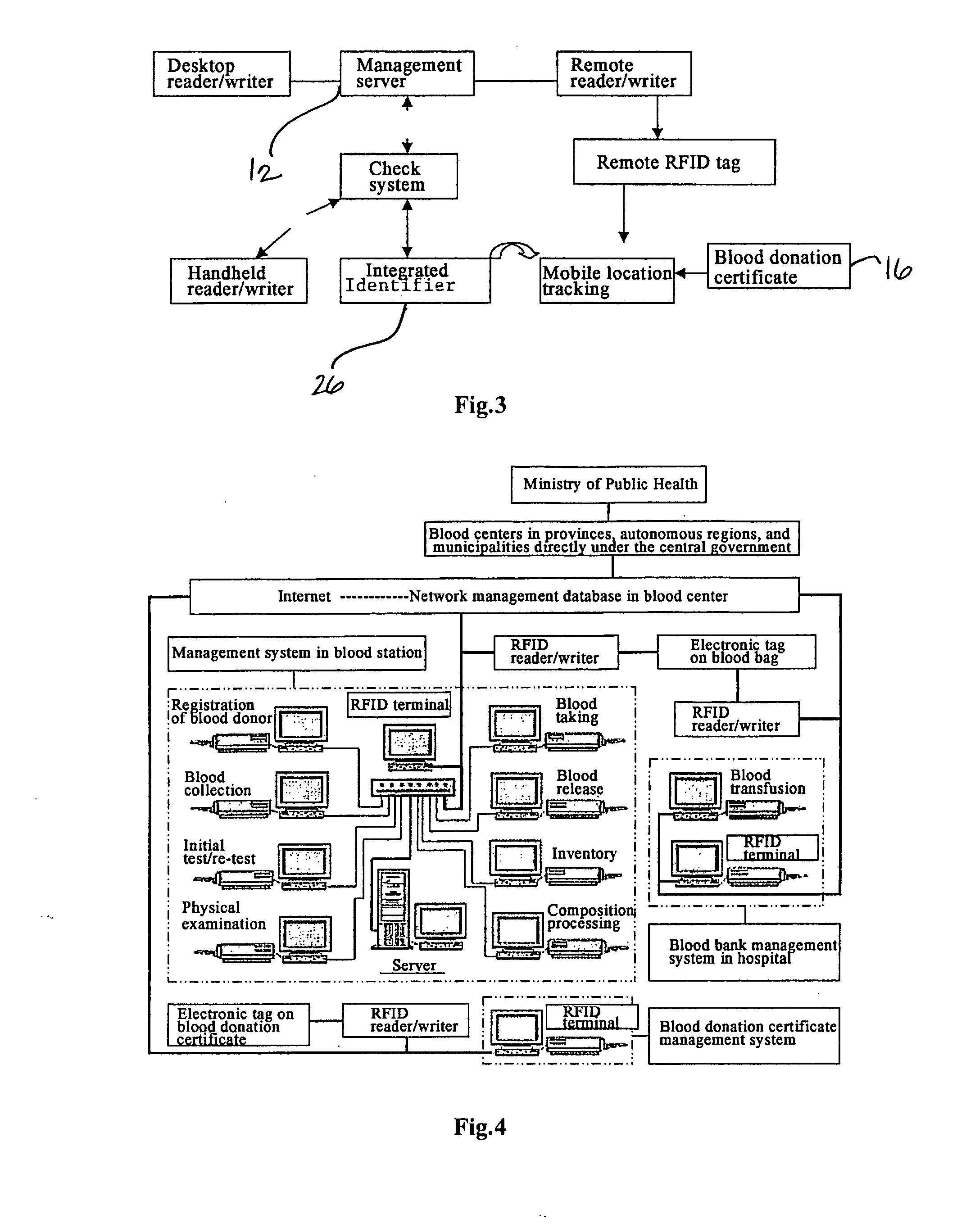
Method and System of Using Rfid in the Workflow of Blood Center
InactiveUS20080208750A1Improve abilitiesReduce probabilityData processing applicationsComputer-assisted medical data acquisitionCustomer relationship managementBlood center
A method and a system for using RFID (Radio Frequency Identification) in the workflow of a blood center and a medical institution from a network Customer Relationship Management (CRM) information system. The CRM comprises an RFID technique system, a computer database system, and a computer information network. The information transferred between an integrated circuit and a reader-writer occurs through the radio waves in the workflow of, for example, a blood center or other medical institution. The related information ranges from identity information to biological product information. In each procedure of the blood collecting and supply workflow, the information is read / written by the computer into electronic tag and through the computer information network into the service management information system. The work flow of blood centers (stations), such as blood test reports before and after blood transfusions and the desired information of people and objects involved in blood transfusions, can thereby be recorded completely, integrally, precisely, and comprehensively by the RFID system.
Owner:CHEN YAN
Low Aspect Ratio Staged Closure Devices, Systems, and Methods for Freeze-Drying, Storing, Reconstituting, and Administering Lyophilized Plasma
InactiveUS20140259724A1Easy to keepAvoid cross contaminationDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingEngineering
The inventive device and methods described herein address the introduction of a safe and effective freeze-dried biological product, and particularly a plasma product, to a subject in need thereof. The present invention relates to a multifunctional, staged closure device, which also is described as a lyophilization container for plasma (LCP). The device and methods described herein address how to reproducibly achieve a low moisture and substantially oxygen-free atmosphere within a finally hermetically sealed biocompatible low aspect plastic vessel within a standard shelf-stoppering freeze dryer. The present inventive device and methods provide a freeze-dried plasma product that is fully traceable, preserves the constituent plasma activity, is readily prepared in a sterile fashion, is stable, ensures ease of storage and permits rapid reconstitution and delivery to a patient.
Owner:HEMCON MEDICAL TECH
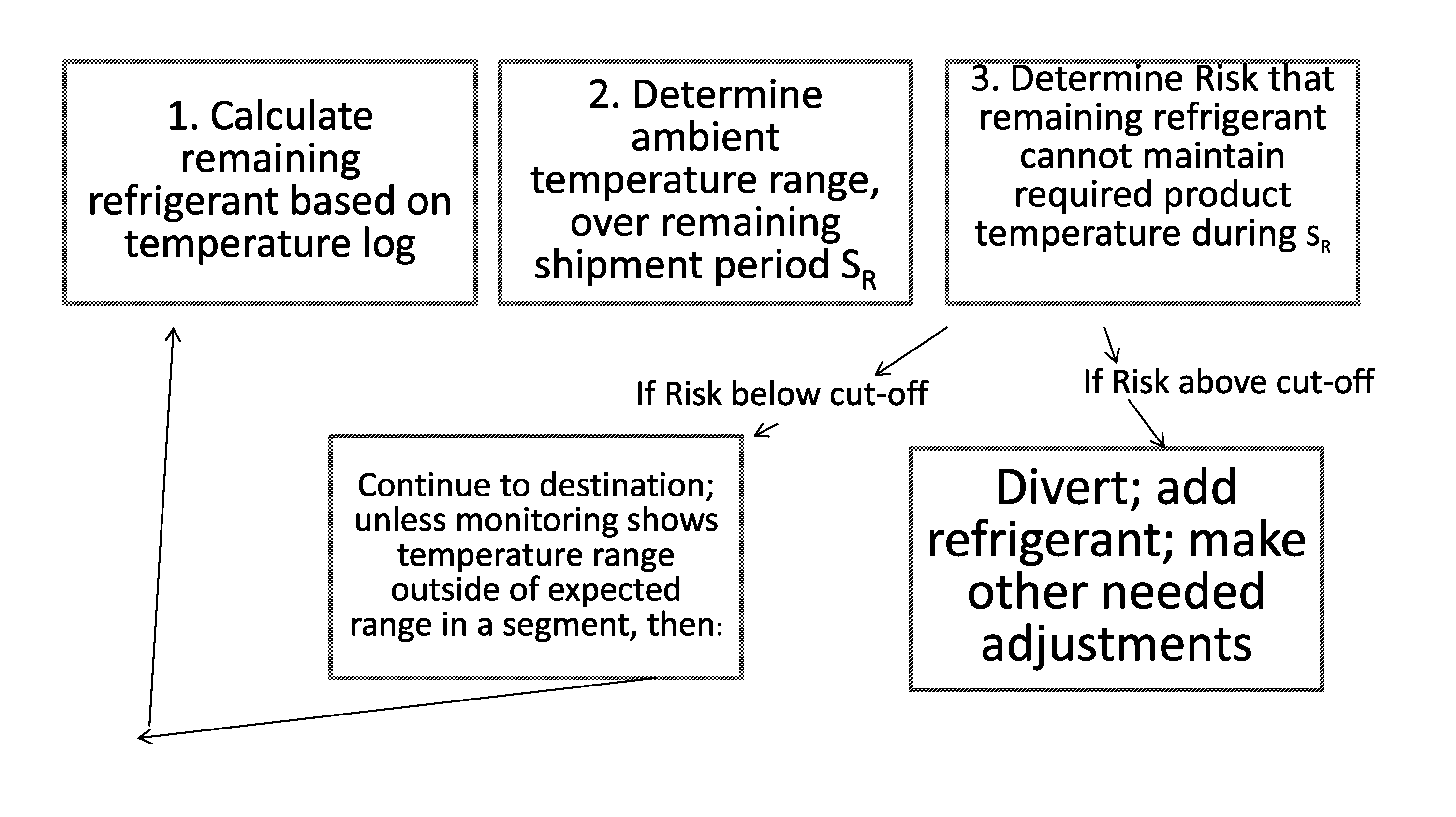
Monitoring Temperature-Sensitive Cargo with Automated Generation of Regulatory Qualification
ActiveUS20150120597A1Reduces effective lifeRetain valueThermometers using mean/integrated valuesLighting and heating apparatusMonitoring temperatureBiologic Products
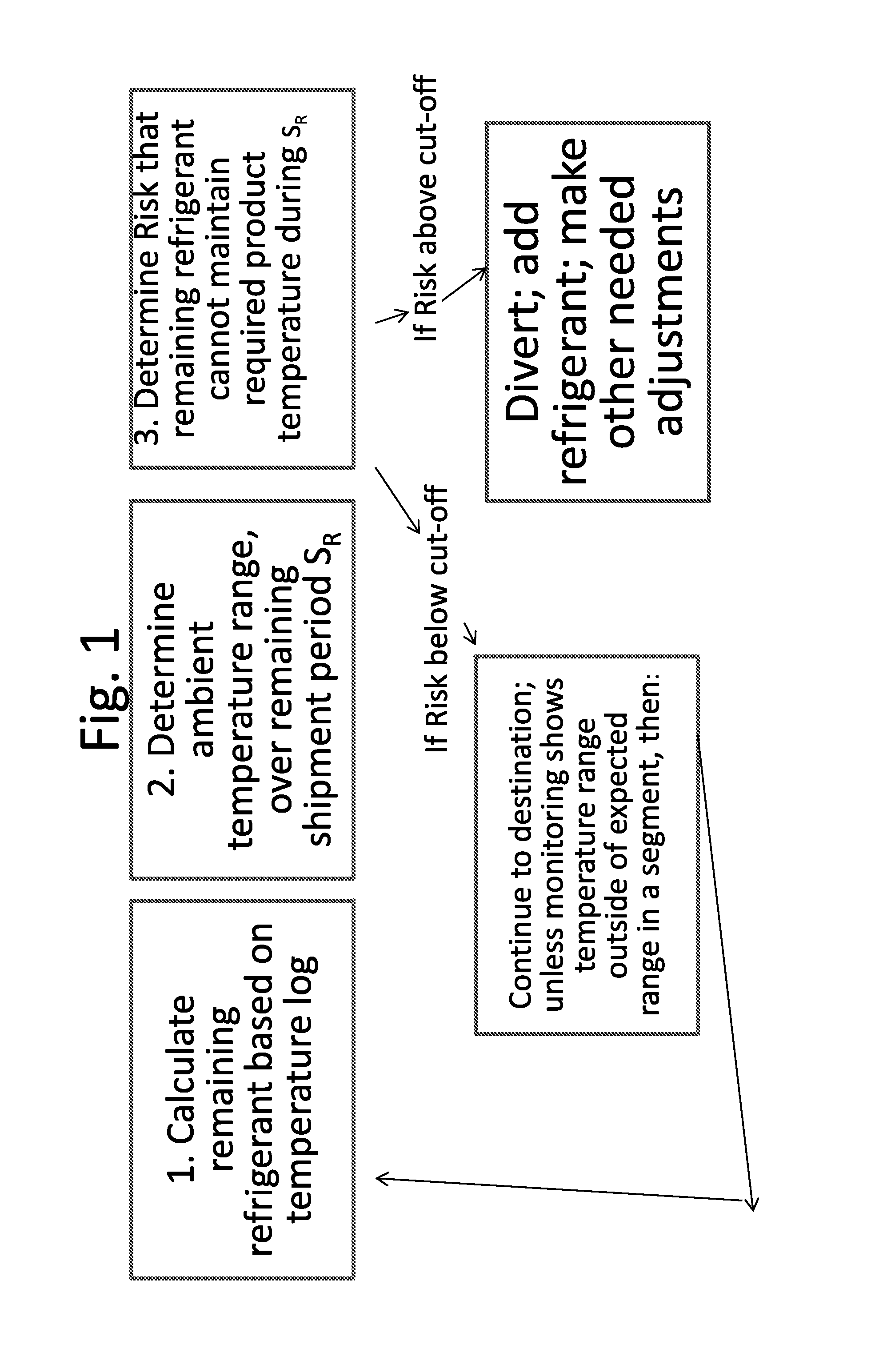
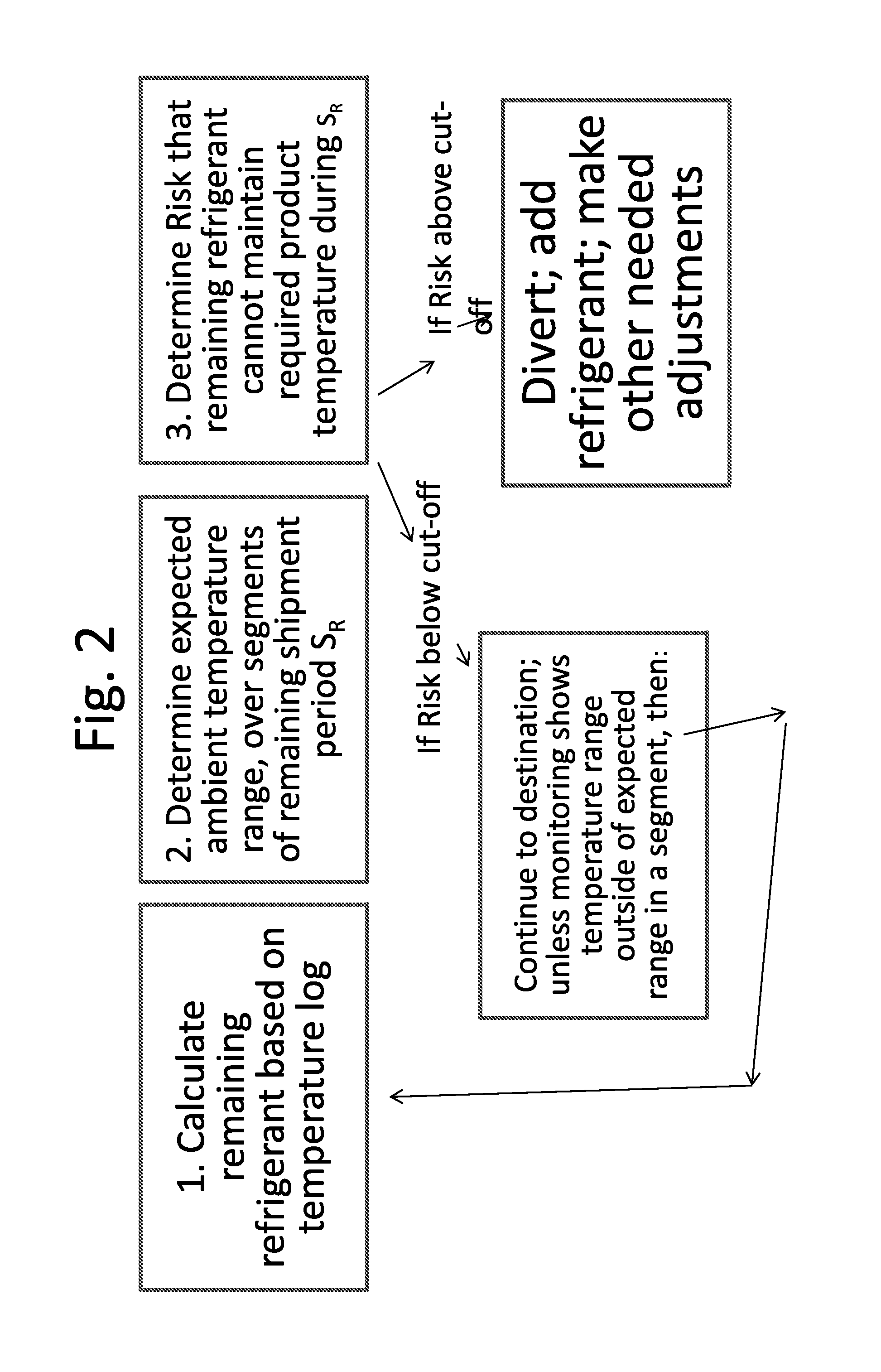
Disclosed is a process of determining, at a point during shipment, whether the solid phase refrigerant in a shipment is sufficient to preserve the shipment cargo, which is blood or other biological products, for the remaining shipment period, by: monitoring the temperatures encountered to said point and estimating the temperatures likely to be encountered during the remaining shipment period; determining the likelihood that the remaining refrigerant can maintain the shipment cargo within a specified temperature range during the remaining shipment period; and if the risk that the remaining refrigerant cannot maintain the shipment cargo within said range during the remaining shipment period is above a cut-off level, then taking action to preserve the value of the cargo.
Owner:INTEGREON GLOBAL INC
Implantable biocompatible immunoisolatory vehicle for delivery of selected therapeutic products
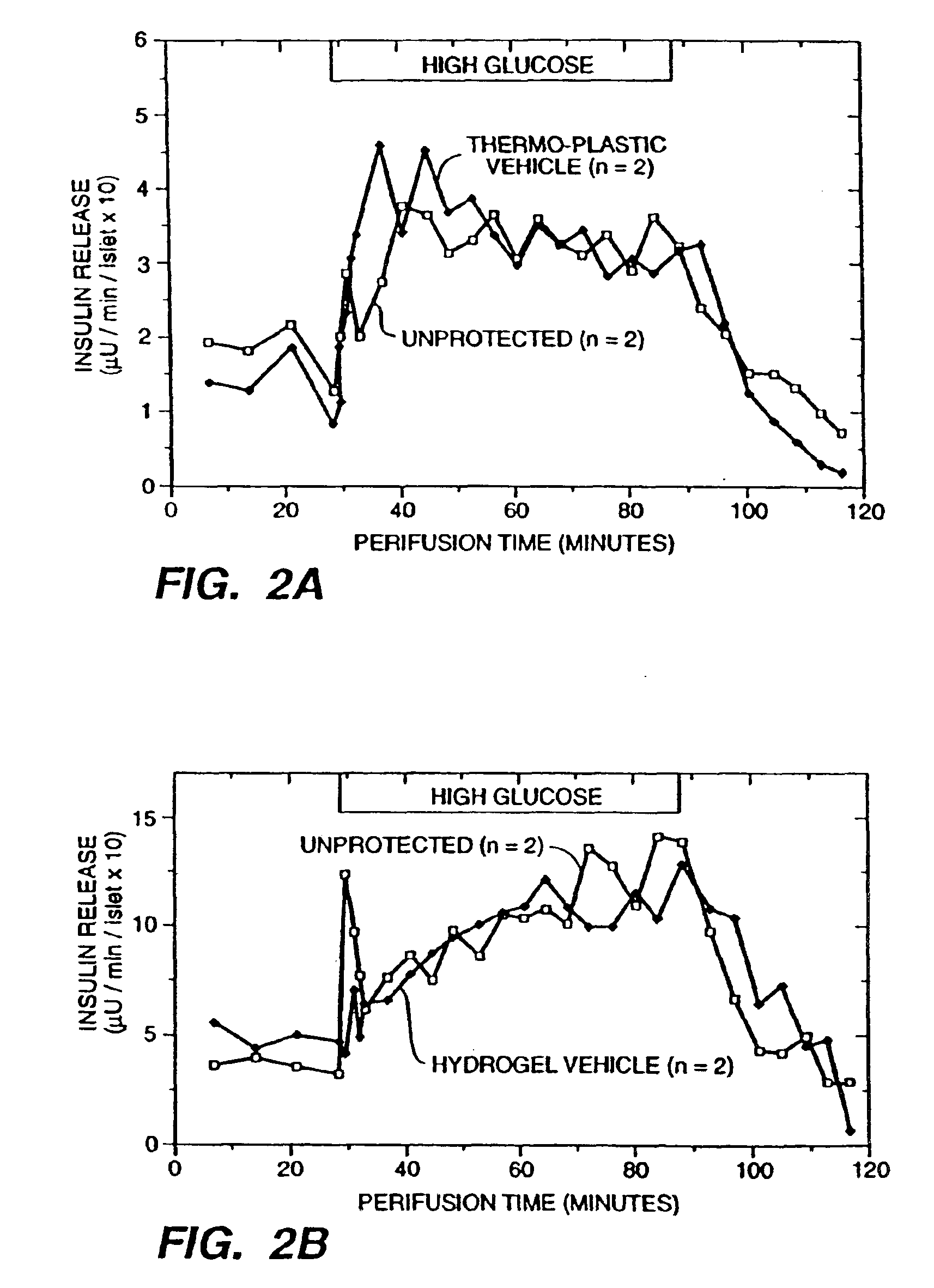
InactiveUS6960351B2Sufficient protectionProtection attackNervous disorderPancreatic cellsBiologyBiological product
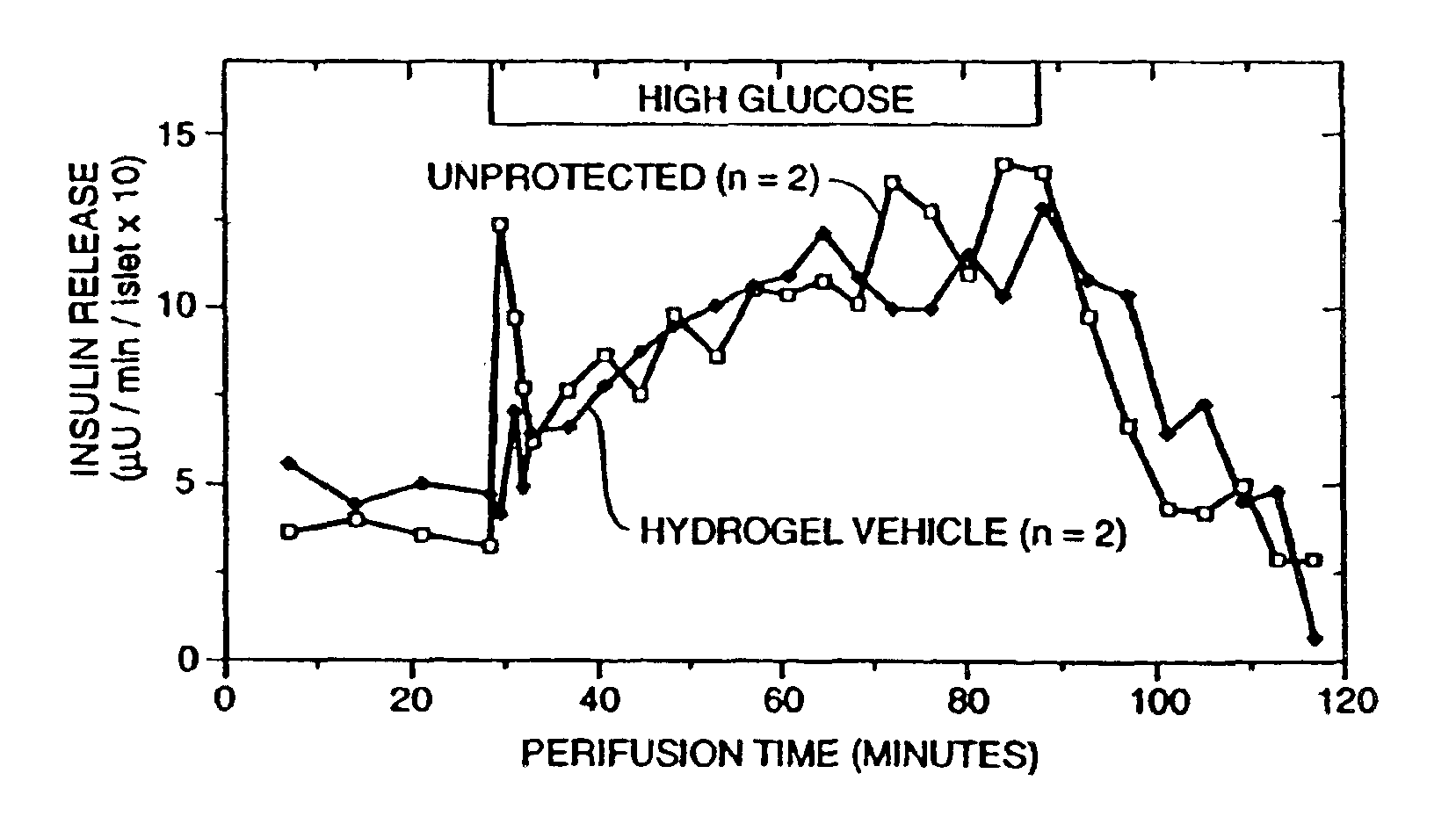
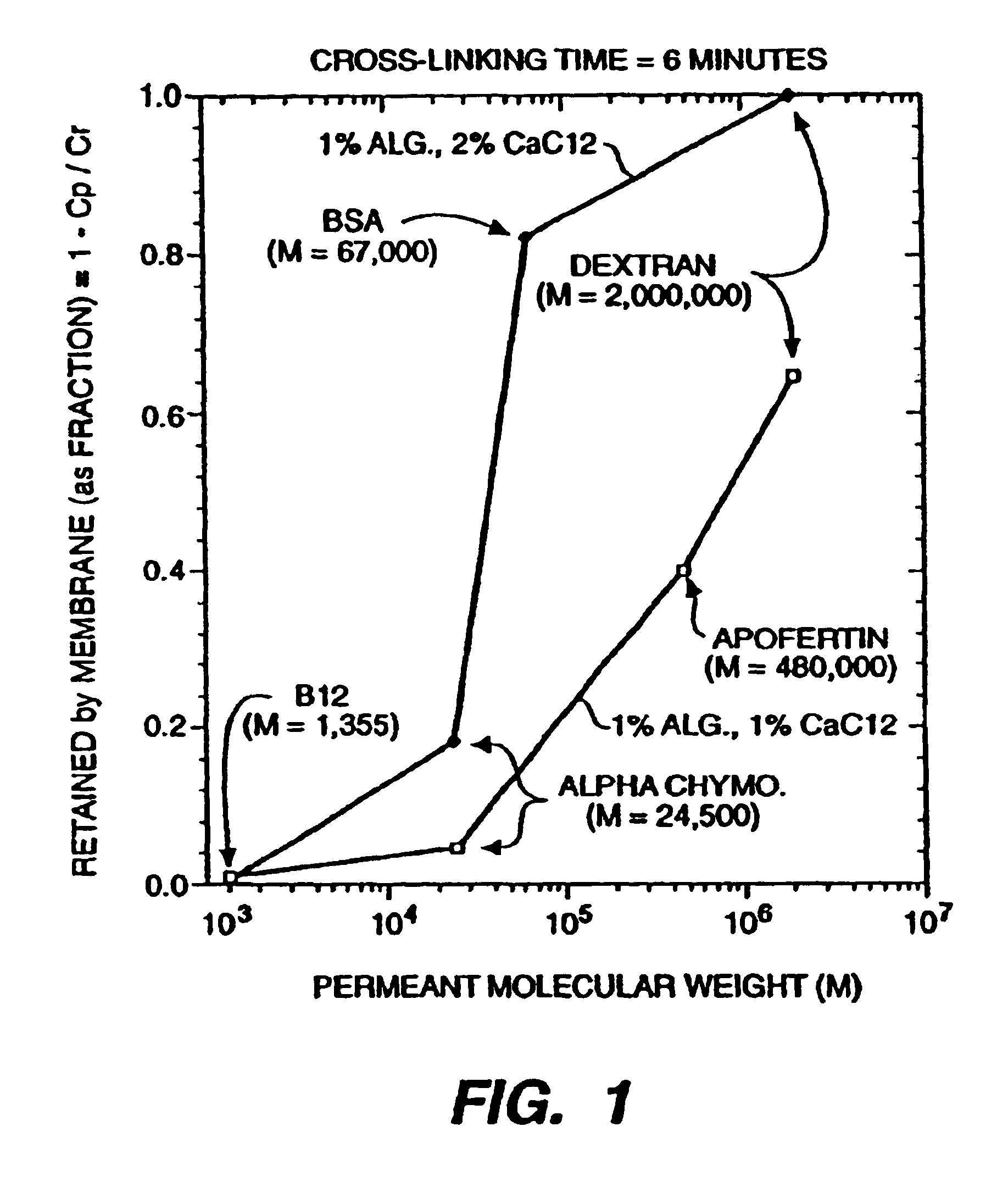
An immunoisolatory vehicle for the implantation into an individual of cells which produce a needed product or provide a needed metabolic function. The vehicle is comprised of a core region containing isolated cells and materials sufficient to maintain the cells, and a permselective, biocompatible, peripheral region free of the isolated cells, which immunoisolates the core yet provides for the delivery of the secreted product or metabolic function to the individual. The vehicle is particularly well-suited to delivery of insulin from immunoisolated islets of Langerhans, and can also be used advantageously for delivery of high molecular weight products, such as products larger than IgG. A method of making a biocompatible, immunoisolatory implantable vehicle, consisting in a first embodiment of a coextrusion process, and in a second embodiment of a stepwise process. A method for isolating cells within a biocompatible, immunoisolatory implantable vehicle, which protects the isolated cells from attack by the immune system of an individual in whom the vehicle is implanted. A method of providing a needed biological product or metabolic function to an individual, comprising implanting into the individual an immunoisolatory vehicle containing isolated cells which produce the product or provide the metabolic function.
Owner:BROWN UNIV RES FOUND INC
Serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture
ActiveCN101760442ASupports adherent growthReduce the burden of separation and purification in the later stageVertebrate cellsArtificial cell constructsLipid formationSerum free media
The invention relates to the culture medium research and development technical field of modern biological technology and provides a serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture, which comprises 21 amino acids, 6 vitamins, 8 salts, 8 lipids, 4 trace elements, 2 buffers, 1 protein hydrolysate, 1 acid-base indicator and 6 other additives. The serum-free medium can be prepared by the conventional preparation method, and an application method thereof is the conventional method. The serum-free medium has the beneficial effects that: the serum-free medium does not contain serum, has clear components, is beneficial for separating and purifying the product and improves the product quality; the serum-free medium supports long-term subculture of MDCK cells and does not require long-term and complex adaptation process; and the serum-free medium can well support the adherent growth and single-cell suspension growth of the MDCK cells, has clear components and easy preparation and utilization, and is suitable for mass production of biological products.
Owner:EAST CHINA UNIV OF SCI & TECH
Devices for continuous sampling of airborne particles using a regenerative surface
InactiveUS7578973B2Good removal effectEasy to disassembleFixed microstructural devicesVolume/mass flow measurementBuilding automationBiologic Products
Airborne particles are impacted on a collection surface, analyzed, and then the collection surface is regenerated. Thus, the same collection surface can be used in numerous cycles. The analysis can be focused on one or more properties of interest, such as the concentration of airborne biologicals. Sensors based on regenerative collection surfaces may be incorporated in many networks for applications such as building automation.
Owner:FLIR DETECTION
Flexible manufacturing system
ActiveUS8584349B2Increase supplyDischarging wasteSpace heating and ventilationTreatment involving filtrationFlexible manufacturing systemProcess engineering
Owner:XOMA (US) LLC
Centrifuge Tube Assembly for Separating, Concentrating and Aspirating Constituents of a Fluid Product
ActiveUS20160367982A1Simpler and efficient and yet highly reliableManufactured much less intricatelyLaboratory glasswaresMaterial analysisEngineeringMechanical engineering
A centrifuge tube assembly includes an elongate tubular receptacle having upper and lower ends and a side wall that extends between the ends. A cap attached to the receptacle carries a common inlet / outlet port formed therethrough for communication with an interior chamber of the tubular receptacle. A liquid impermeable piston is longitudinally movable through the receptacle chamber and sealably interengages an interior wall of the receptacle. The piston separates upper and lower regions of the chamber, which respectively communicate with the inlet / outlet port and a pressure neutralizing vent. Biological products are introduced into and aspirated from the receptacle through the inlet / outlet port.
Owner:EMCYTE CORP
Animal protein-free media for cultivation of cells
InactiveUS20060094104A1Efficient expressionGuaranteed efficient growthMicroorganismsCulture processBiotechnologyHydrolysate
The present invention relates to animal protein-free cell culture media comprising polyamines and a plant and / or yeast-derived hydrolysate. The invention also relates to animal protein-free culturing processes, wherein cells can be cultivated, propagated and passaged without adding supplementary animal proteins in the culture medium. These processes are useful in cultivating cells, such as recombinant cells or cells infected with a virus, and for producing biological products by cell culture processes.
Owner:BAXTER INT INC +1
Nitrogen-doped carbon nanometer particle as well as preparation method and application thereof
InactiveCN103113886AOvercoming the technical problem of easy fluorescence quenchingEasy to prepareNon-macromolecular adhesive additivesInksMicrowave methodSolvent
The invention discloses a nitrogen-doped carbon nanometer particle as well as a preparation method and application thereof, belongs to the field of nanometer material science and is used for solving the technical problems that fluorescence quenching is easily caused to the aggregative state of the carbon nanometer particle due to surface passivation modifier which is added for the preparation of existing carbon nanometer particles. The nitrogen-doped carbon nanometer particle is prepared through a microwave method by using organic compounds containing polycarboxyl or polyhydroxy as materials and using ammonia water as a solvent and a nitrogen doping source. The invention further provides the application of the nitrogen-doped carbon nanometer particle as fluorescent ink and fluorescent glue. The preparation method disclosed by the invention is simple, low in cost, and convenient to realize large-scale production; the maximal fluorescent quantum efficiency of the solid film formed by the prepared fluorescent glue is as high as 84%; the prepared fluorescent ink is non-toxic, generates no precipitates after being placed for a long time, is strong in fluorescence characteristic and can be applied to various fields such as bio-imaging, biological product identification, information storage, information encryption, counterfeiting prevention, illumination and display, sensing and photovoltaic devices.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
Freeze-drying microscope stage apparatus and process of using the same
InactiveUS20060053652A1Microbiological testing/measurementDrying solid materials without heatFreeze-dryingDisease cause
The invention concerns methods, systems, and devices for screening arrays comprising hundreds, thousands, or hundreds of thousands of samples. These methods are useful to optimize, select, and discover compositions, or conditions for cost-effective freeze-drying of preparations and freezing of biologicals while maintaining structural integrity and / or viability. Such freeze-dried compositions are easily reformulated for treating or preventing diseases, the cause of the diseases, or the symptoms of the diseases. Moreover, optimized freezing of biological samples enables viable preservation of a wide variety of biologicals.
Owner:TRANSFORM PHARMACEUTICALS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com