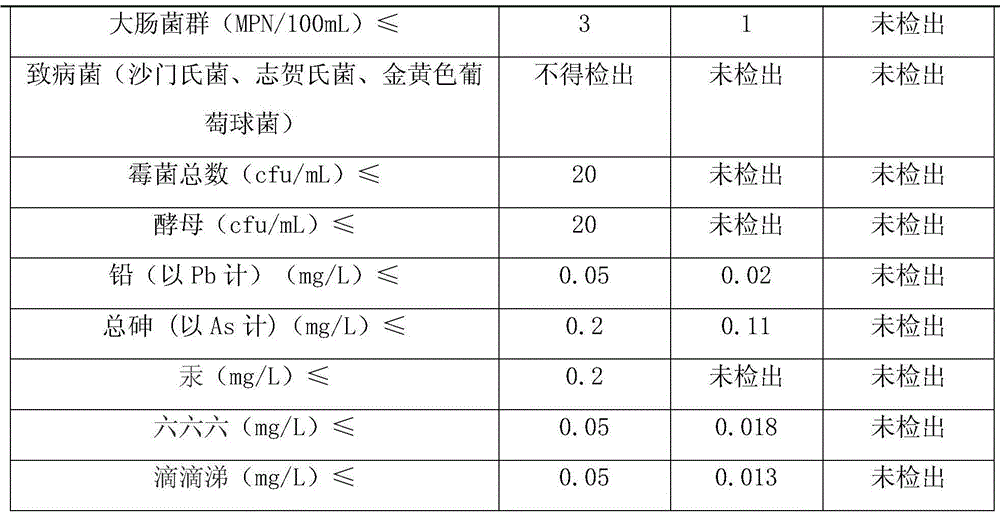
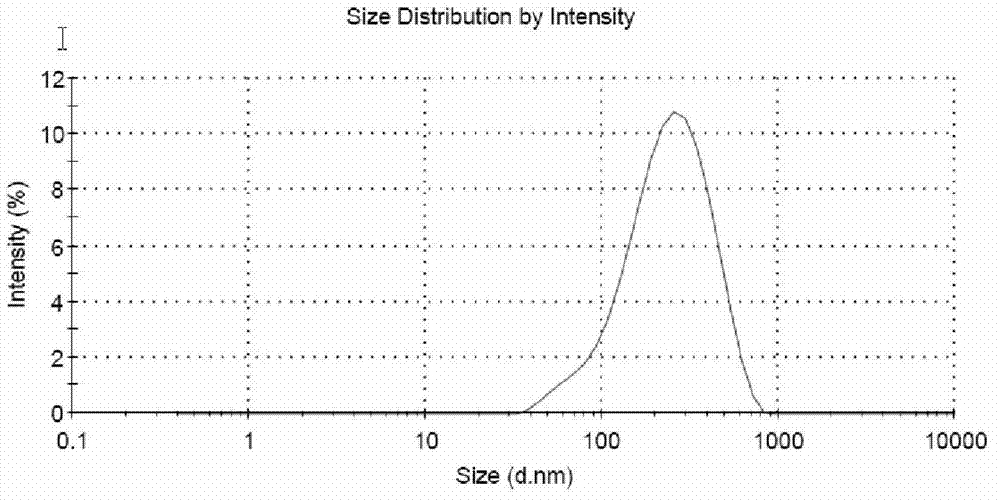
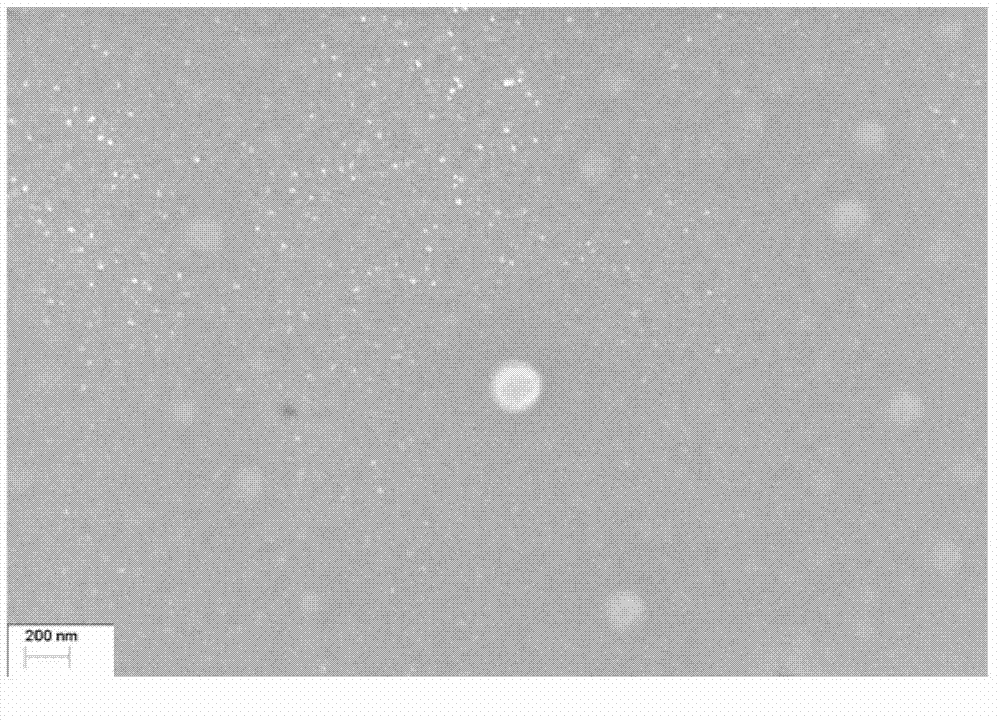
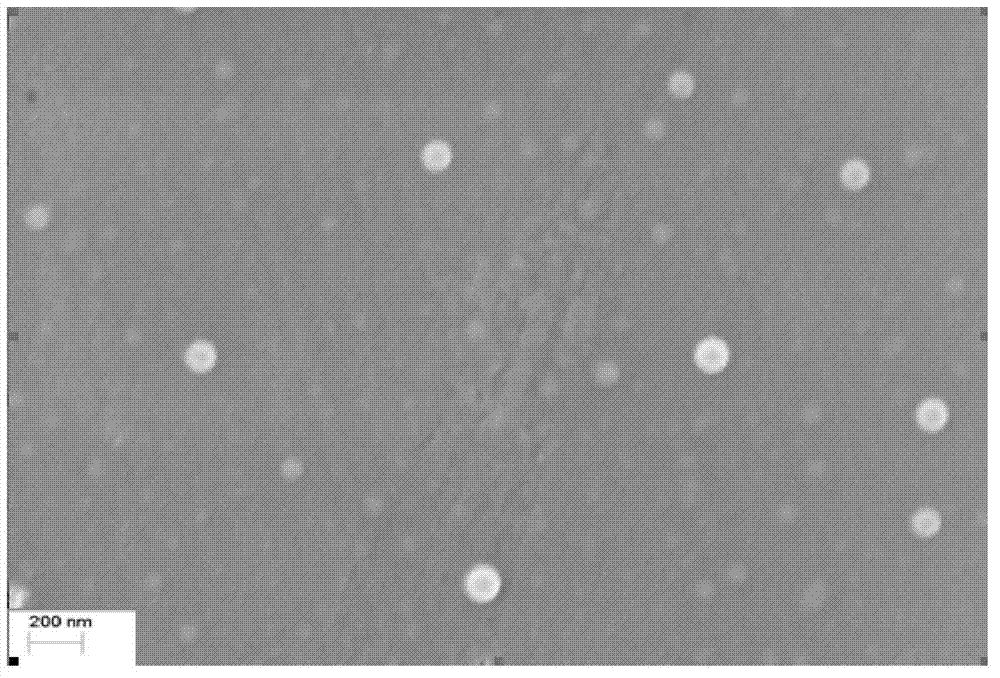
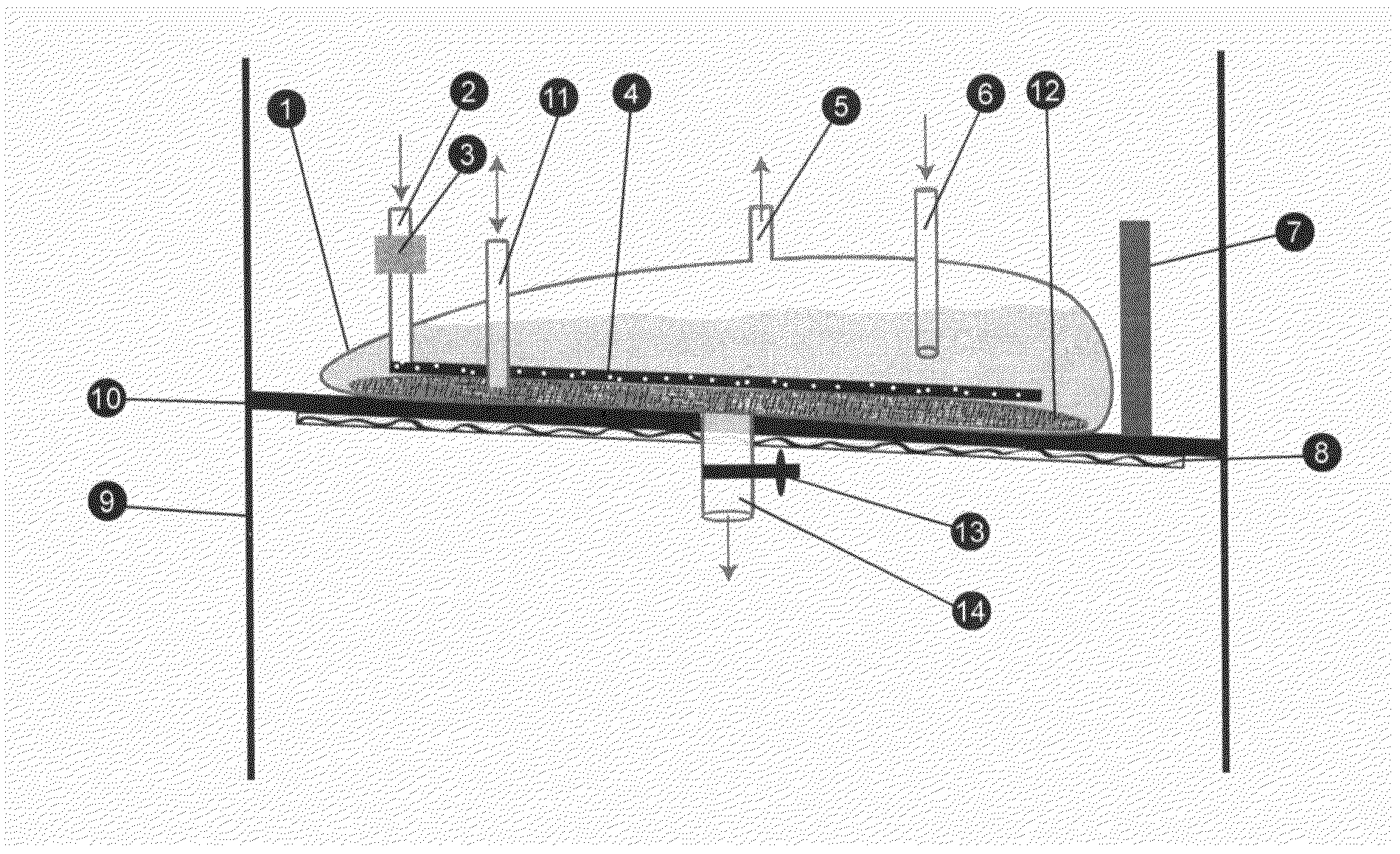
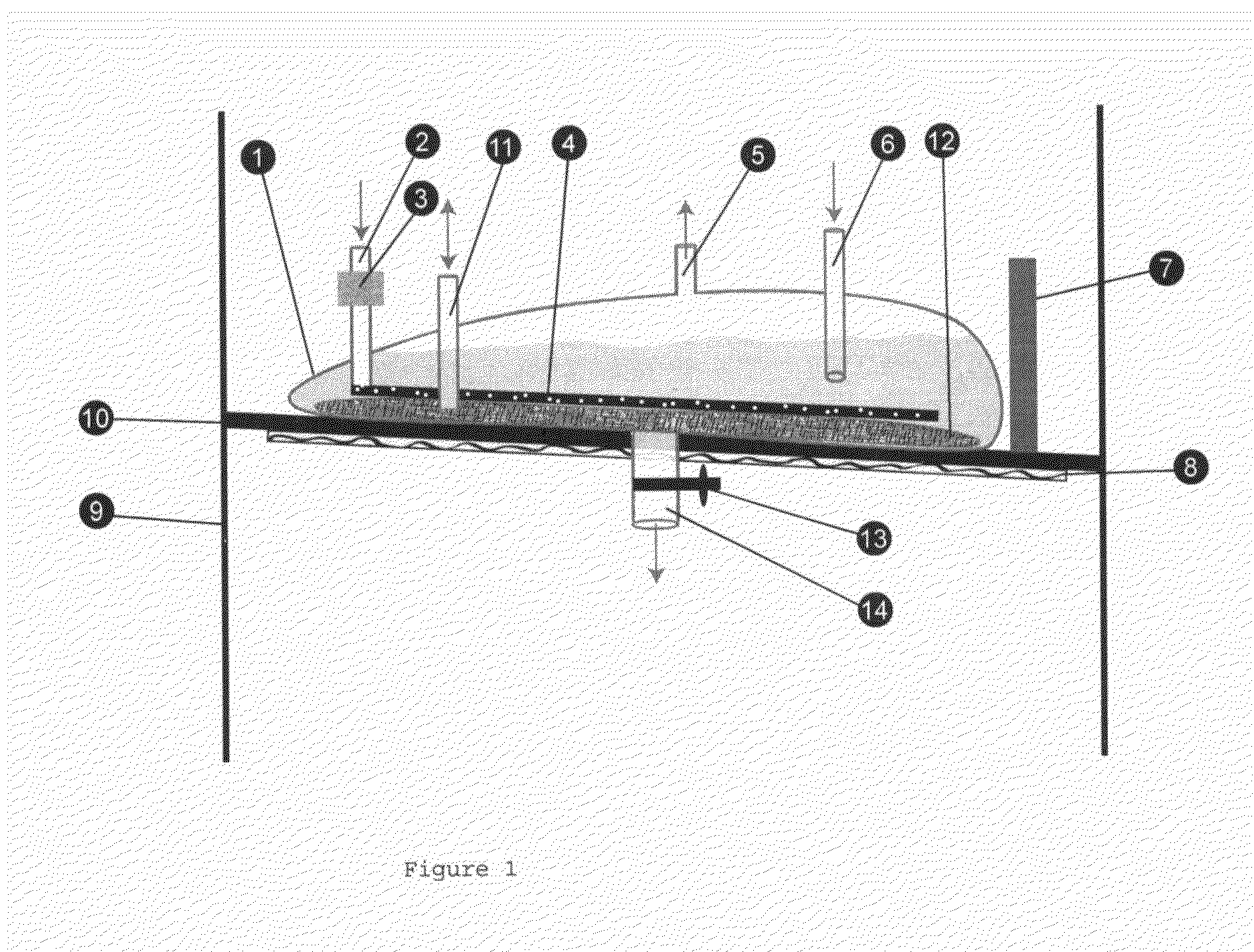
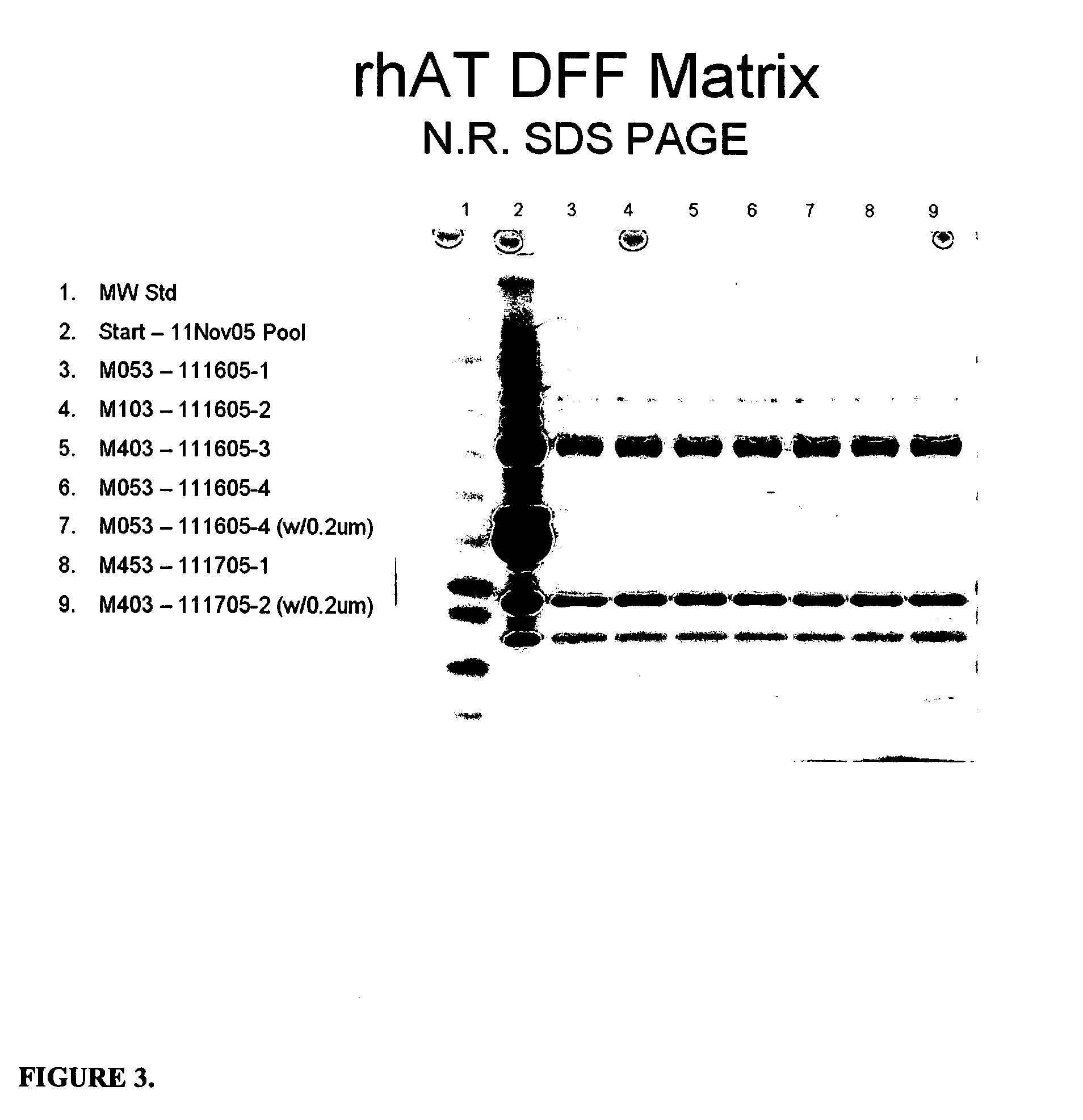
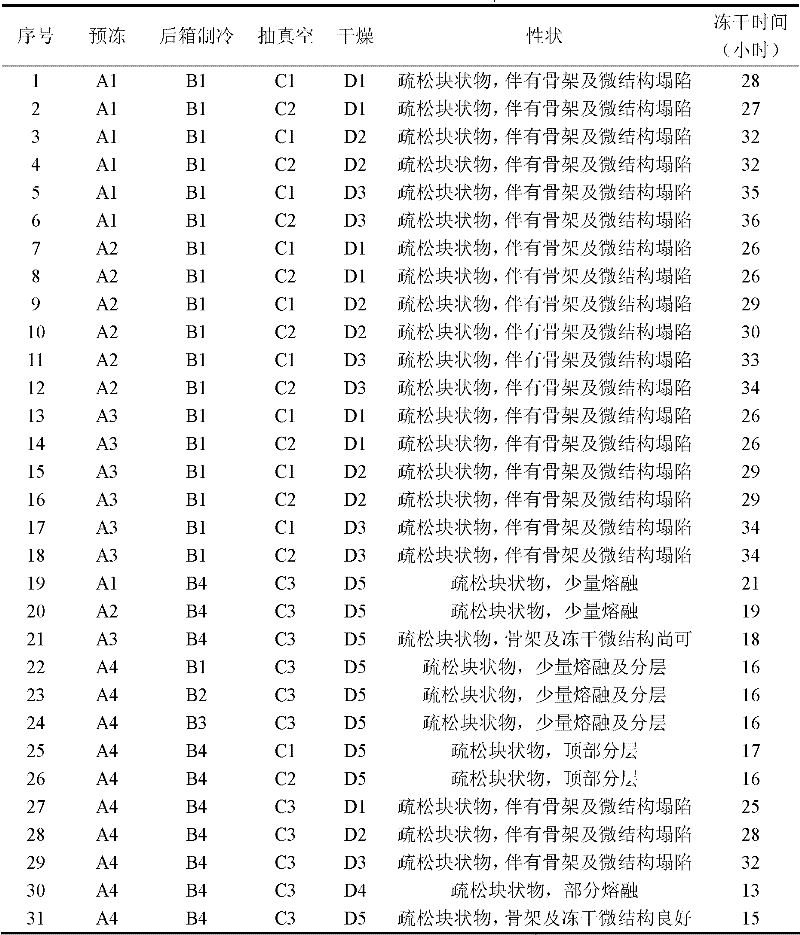
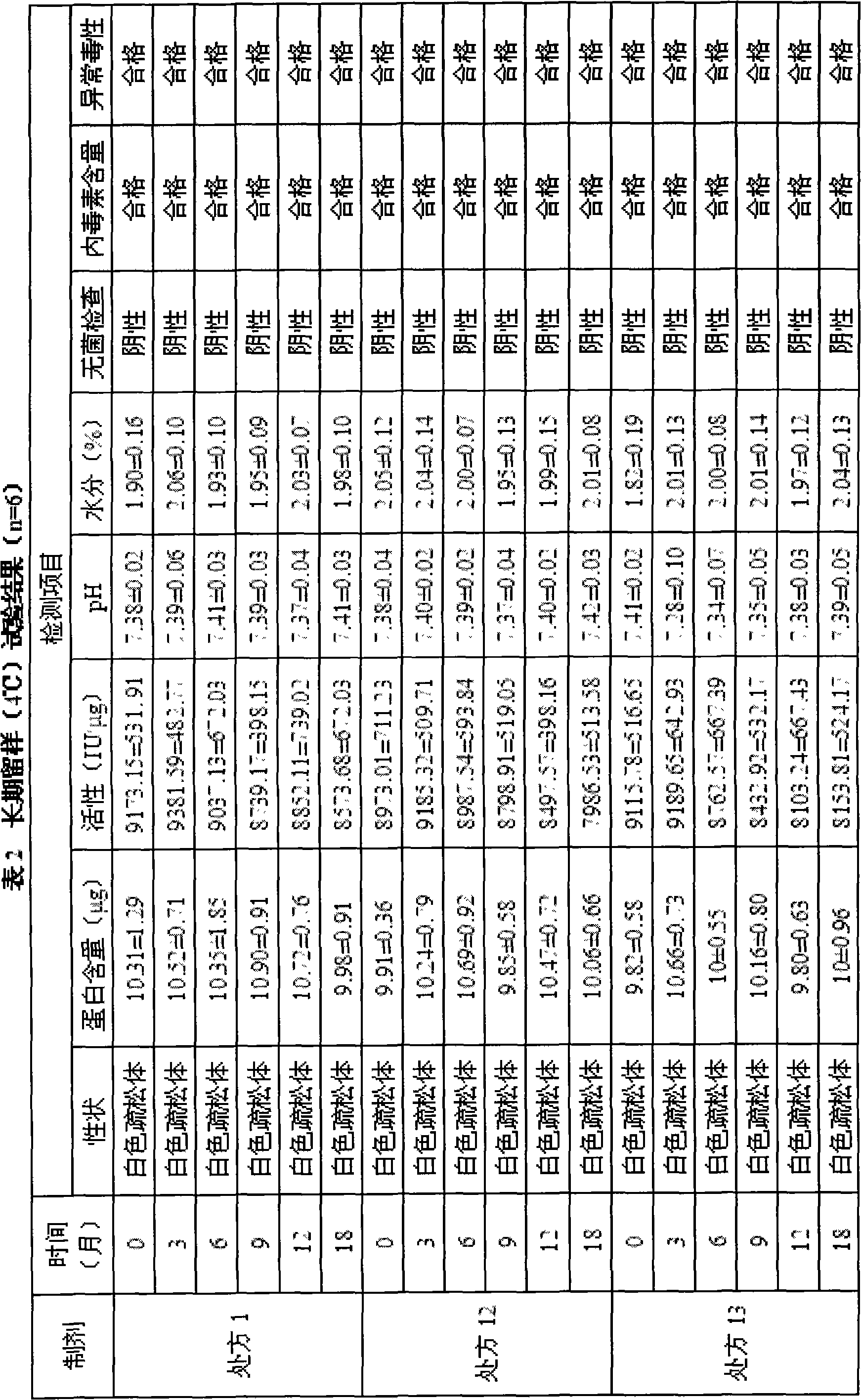
Patents
Literature
167 results about "Sterile filtration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sterile filtration, a form of filtration fine enough to remove spoilage organisms. For beer, “sterile filtration” is understood to reduce yeast and beer spoilage organisms to extremely low levels, such that the packaged product will last for its intended shelf life, which may vary by brand, region, or market.
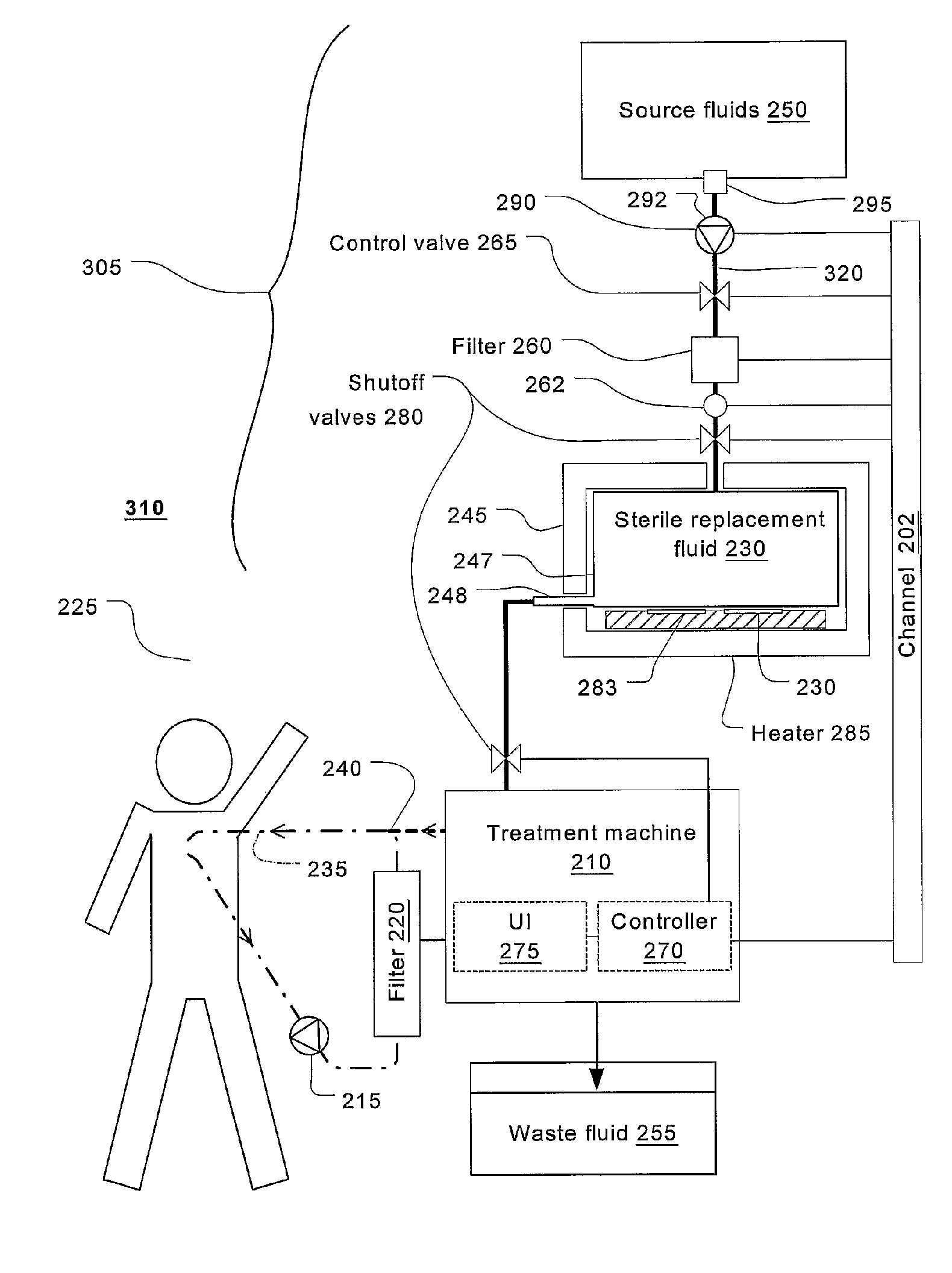
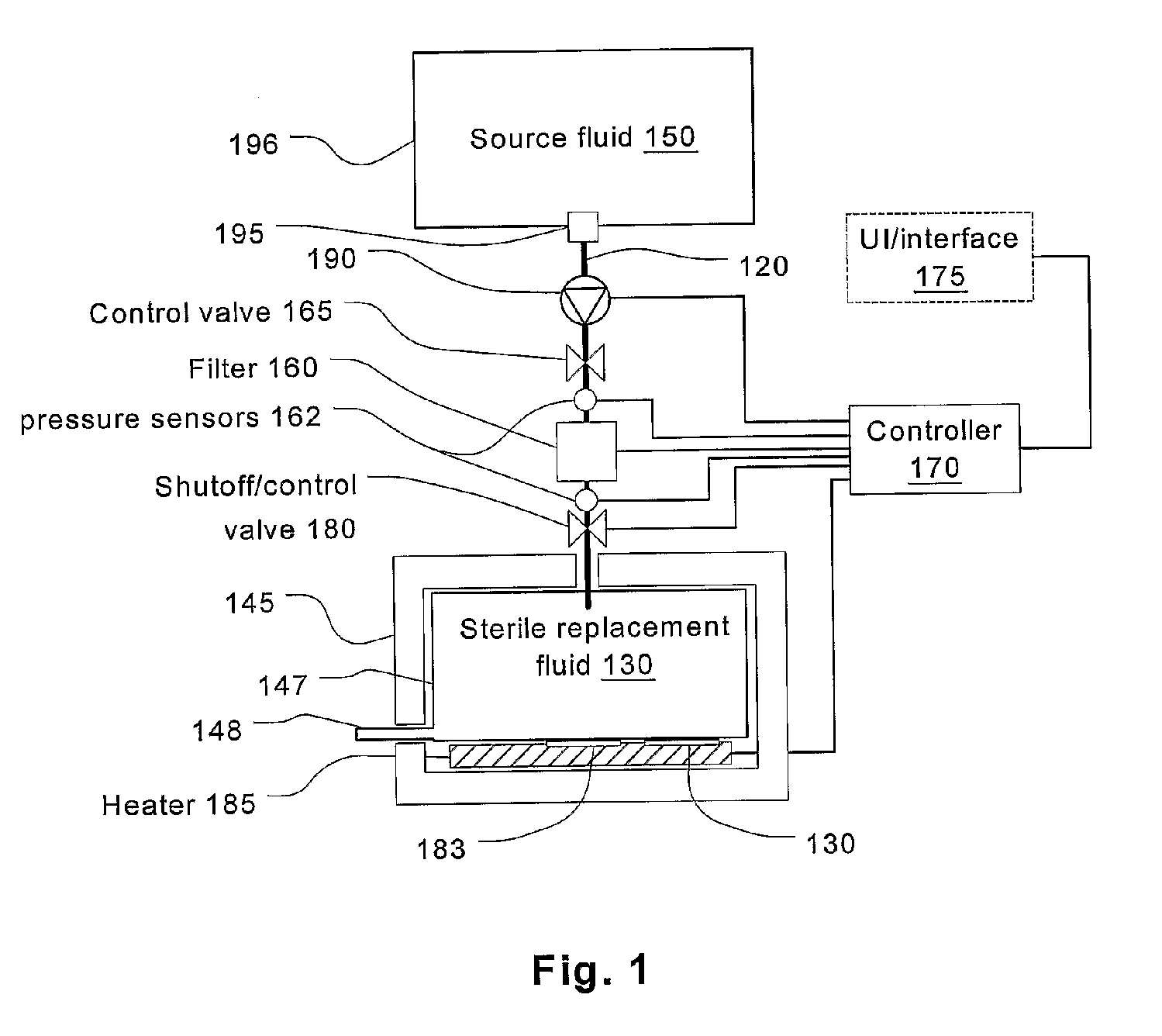
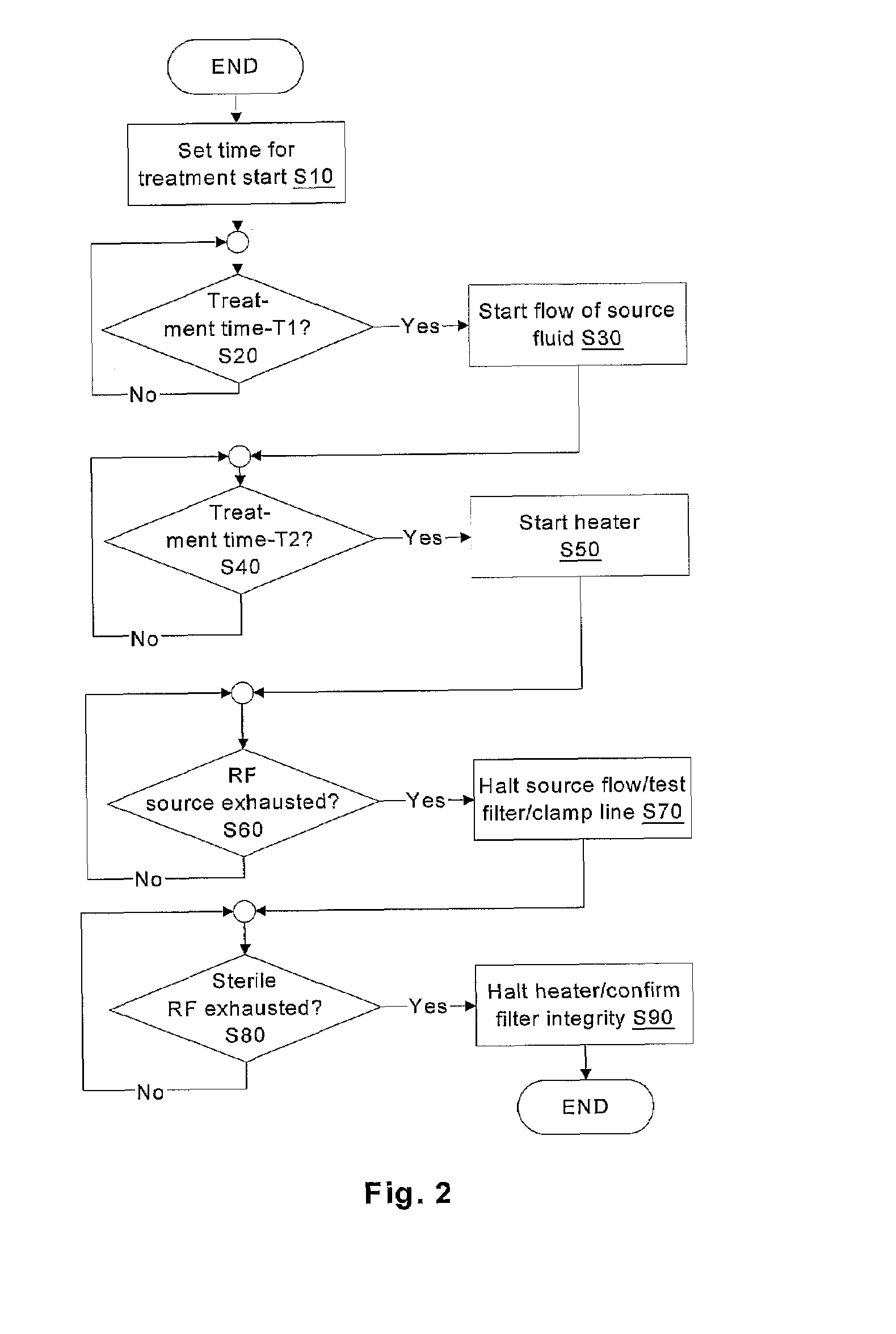
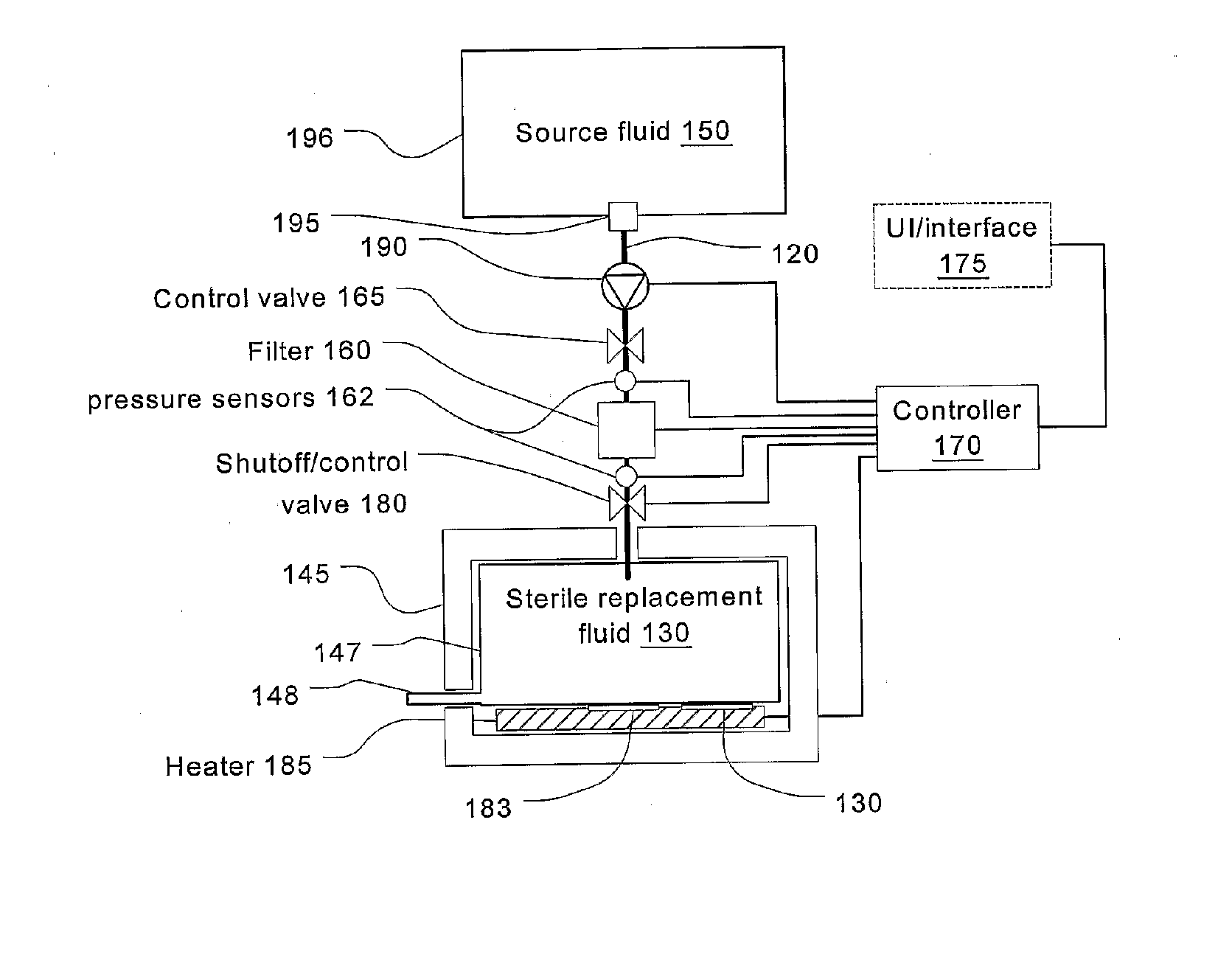
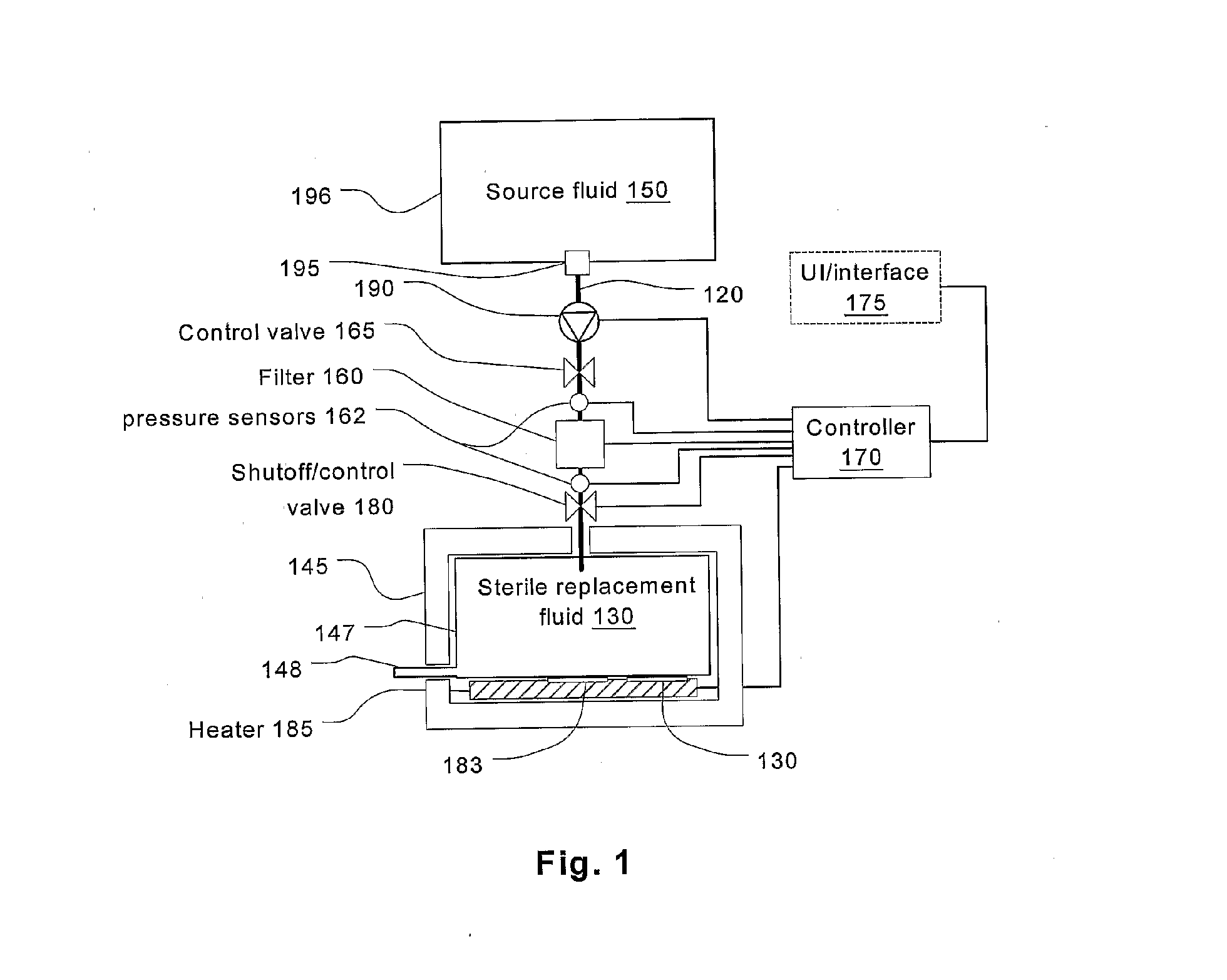
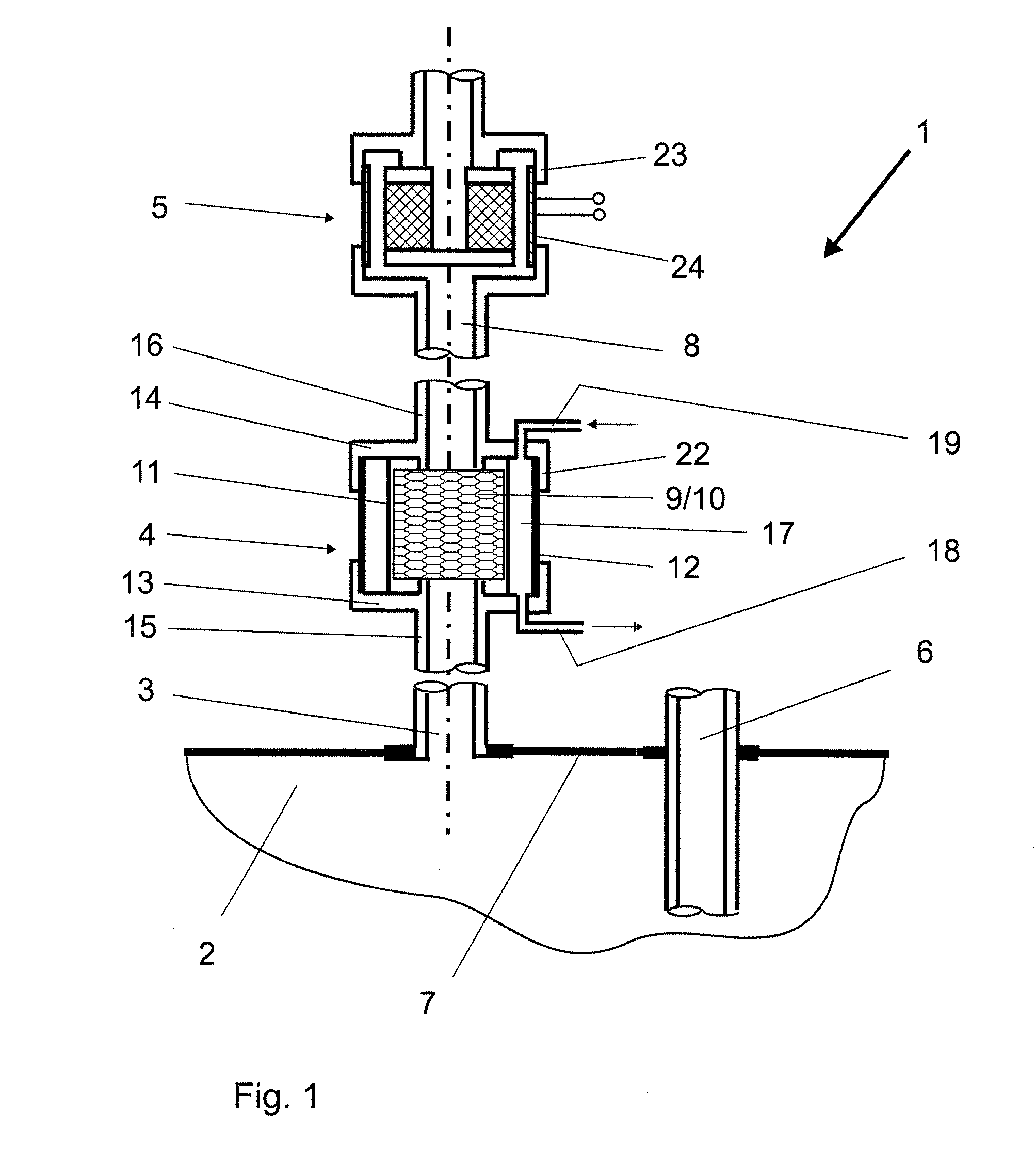
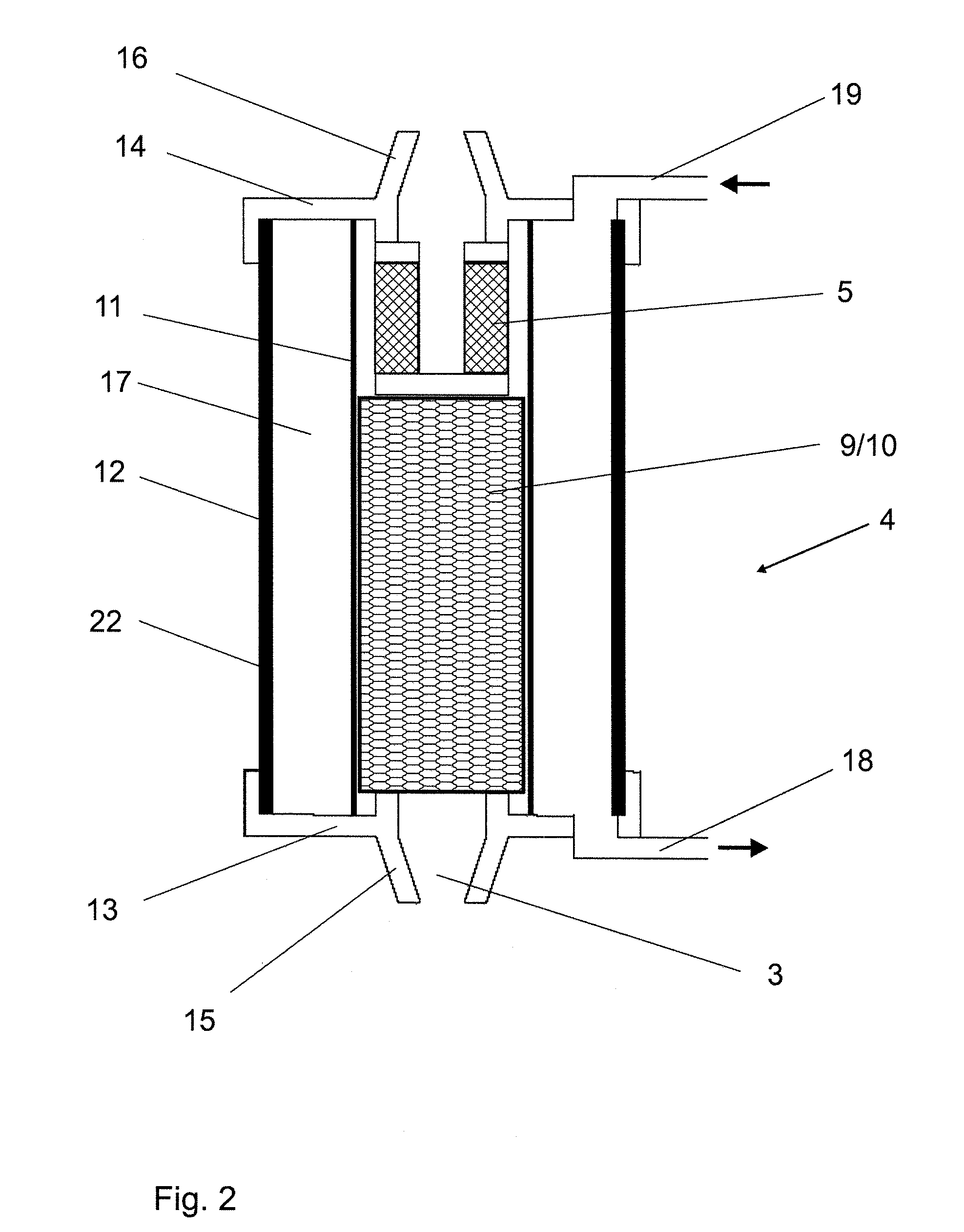
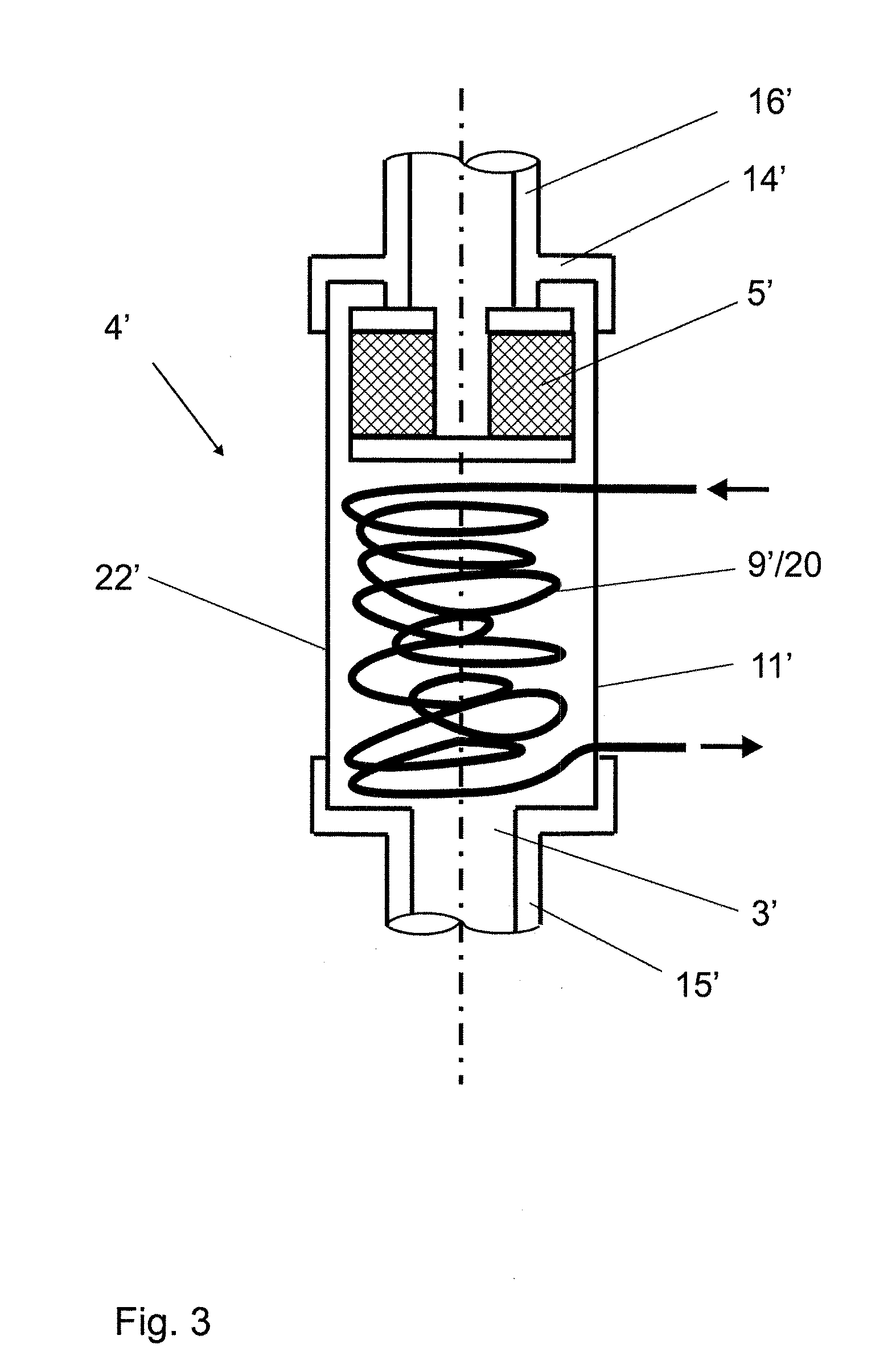
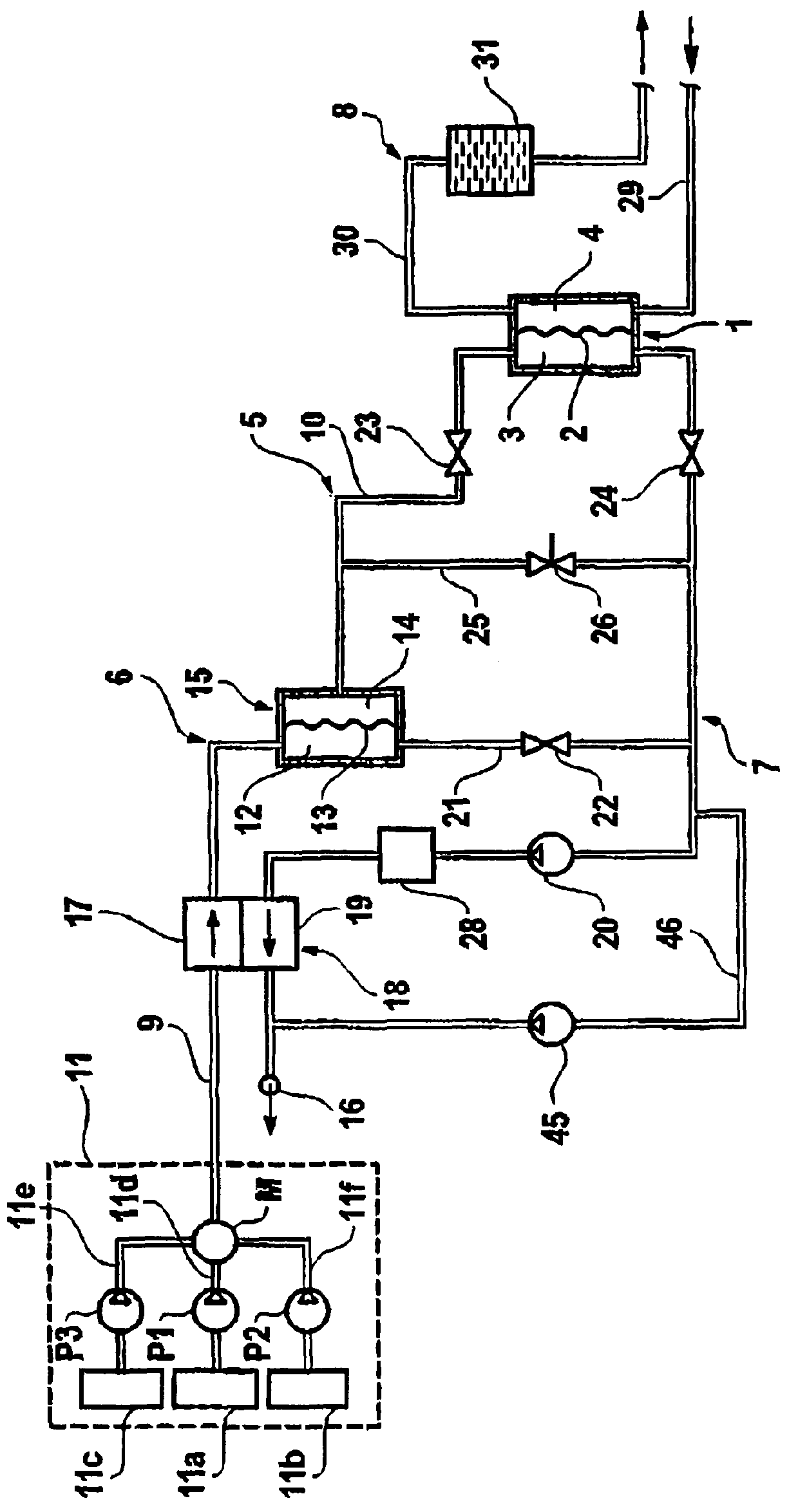
Batch filtration system for preparation of sterile fluid for renal replacement therapy
InactiveUS7544300B2Solve insufficient capacityPrevent heat lossSolvent extractionSettling tanks feed/dischargeBlood treatmentsDialysis fluid
A method and device for blood treatments that use fluids such as dialysate and replacement fluid for renal replacement therapy. In an embodiment, fluid is passed either by pump or passively by gravity feed, through a microporous sterilization filter from a fluid source to a replacement fluid container. The latter forms a batch that may be used during treatment. The advantage of forming the batch before treatment is that the rate of filtering needn't match the rate of consumption during treatment. As a result, the sterilization filter can have a small capacity. In another embodiment, a filter is placed immediately prior to the point at which the sterile fluid is consumed by the treatment process. The latter may be used in combination with the former embodiment as a last-chance guarantee of sterility and / or that the fluid is free of air bubbles. It may also be used as the primary means of sterile-filtration.
Owner:NXSTAGE MEDICAL INC +1
Batch filtration system for preparation of sterile fluid for renal
ActiveUS20080053905A9Solve insufficient capacityPrevent heat lossSolvent extractionMedical devicesBlood treatmentsSterile filtration
A method and device for blood treatments that use fluids such as dialysate and replacement fluid for renal replacement therapy. In an embodiment, fluid is passed either by pump or passively by gravity feed, through a microporous sterilization filter from a fluid source to a replacement fluid container. The latter forms a batch that may be used during treatment. The advantage of forming the batch before treatment is that the rate of filtering needn't match the rate of consumption during treatment. As a result, the sterilization filter can have a small capacity. In another embodiment, a filter is placed immediately prior to the point at which the sterile fluid is consumed by the treatment process. The latter may be used in combination with the former embodiment as a last-chance guarantee of sterility and / or that the fluid is free of air bubbles. It may also be used as the primary means of sterile-filtration.
Owner:NXSTAGE MEDICAL INC +1
Separative Bioreactor
InactiveUS20110198286A1Improve abilitiesLow costSolvent extractionLighting and heating apparatusInclusion bodiesPerfusion
A bioreactor that combines the steps of recombinant expression and separation of a biological product by binding the secreted biological product with a resin, discarding the nutrient medium and eluting the biological product as a concentrated solution, eliminating the steps of sterile filtration and volume reduction. The method also allows loading of resin for column-purification, eliminating all steps of perfusion process and maintaining a sink condition of a toxic product in nutrient medium to optimize productivity of host cells. The instant invention also allows harvesting of solubilized inclusion bodies after the cells have been lysed and refolding of proteins inside the bioreactor.
Owner:NIAZI SARFARAZ K
Bioreactor with condenser
ActiveUS20110076759A1Avoid cloggingGuaranteed heat exchange effectBioreactor/fermenter combinationsBiological substance pretreatmentsBioreactorWaste management
Bioreactor with a vessel having at least one gas dissipation duct for gas discharge, the orifice of the gas dissipation duct being connected to a hydrophobic sterile filter and to a condenser arranged between them and having condensation surfaces, a turbulence generator for generating a turbulent flow is arranged in the gas dissipation duct in the region of the condenser.
Owner:SARTORIUS STEDIM BIOTECH GMBH
Liquorice vegetable drink for improving gastrointestinal function and enhancing immunity and preparation method of liquorice vegetable drink
The invention discloses a liquorice vegetable drink for improving a gastrointestinal function and enhancing the immunity and a preparation method of the liquorice vegetable drink. Herb of liquorice is used as a main raw material; liquorice concentrated juice, an edible mushroom extract, wolfberry fruit concentrated juice, modified dietary fibers, a corn bee pollen extract, pectin hydrolyzate and the like are scientifically compounded; the raw materials are treated by adopting a modern low-temperature and biological extraction technology, so that the content of effective components of the raw materials is greatly increased; various functional components are organically mixed, so that the optimal synergistic effect is achieved; meanwhile, by adoption of methods for thermal hybrid concentration of unstable concentrated liquid, jointed removal of cold and heat, sterile filtration, and the like, the flavor of the liquorice vegetable drink is greatly enhanced, and the expiration date of a product is prolonged; the liquorice vegetable drink with the effects of improving the gastrointestinal function and enhancing the whole immunity of a human body is prepared.
Owner:邵素英
Cabazitaxel drug composition and preparation method thereof
ActiveCN103393632AMeet treatment needsImprove stabilityOrganic active ingredientsGranular deliveryZeta potentialMass ratio
The invention provides a drug composition of cabazitaxel and a pharmaceutically acceptable biological carrier and a preparation method thereof. The cabazitaxel drug composition is actually a nanoparticle colloid dispersing system containing cabazitaxel. Cabazitaxel is encapsulated in a polymer shell made of proteins or is associated with the proteins by way of association to form nanoparticles, wherein the mass ratio of the cabazitaxel to the proteins is 1:(8-15); the pH value ranges from 5.0 to 7.0; the average diameter of the particles is not more than 200nm; the Zeta potential ranges from minus 10mv to minus 30mv; and the particles can be subjected to sterile filtration. The composition can be prepared by a high-pressure homogenating method or a protein denaturation and renaturation method. The composition prepared by the invention can be transformed to re-dispersable cakes or powder, can maintain stability for at least 48 hours at 37 DEG C after being re-dispersed in an aqueous medium, and can meet the requirements of intravenous drip therapy.
Owner:QILU PHARMA HAINAN
Separative harvesting device
InactiveUS20130143313A1Low costReduce manufacturing costBioreactor/fermenter combinationsBiological substance pretreatmentsInclusion bodiesBiochemistry
A harvesting device for capturing a biological product directly by binding the secreted biological product with a resin, discarding the nutrient medium and eluting the biological product as a concentrated solution, eliminating the steps of sterile filtration and volume reduction, thus allowing one to combine the steps of recombinant expression and separation of a biological product. The method allows loading of resin for column-purification, eliminating all steps of perfusion process and maintaining a sink condition of a toxic product in nutrient medium to optimize productivity of host cells. The instant invention also allows harvesting of solubilized inclusion bodies after the cells have been lysed and refolding of proteins inside the bioreactor.
Owner:THERAPEUTIC PROTEINS INT
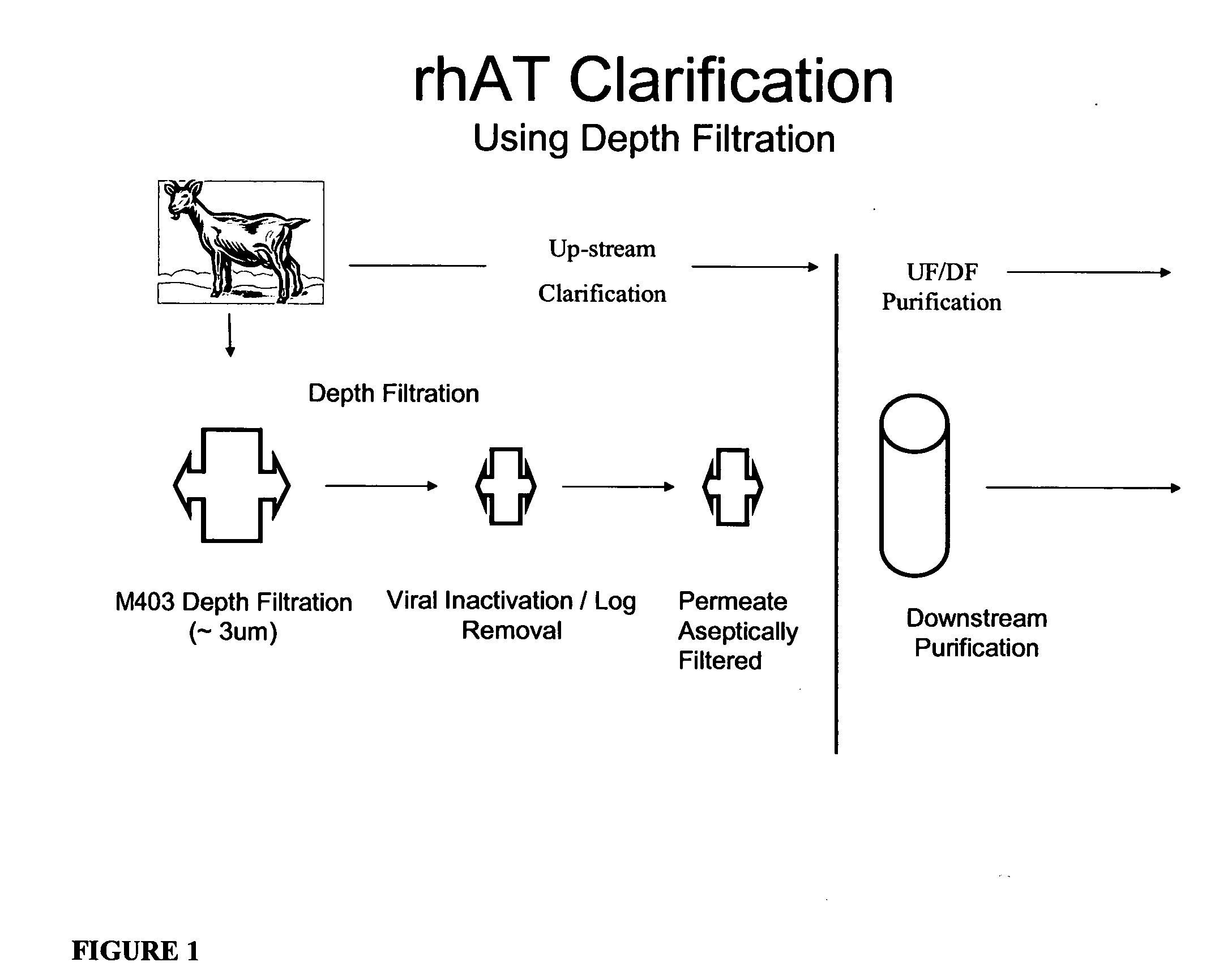
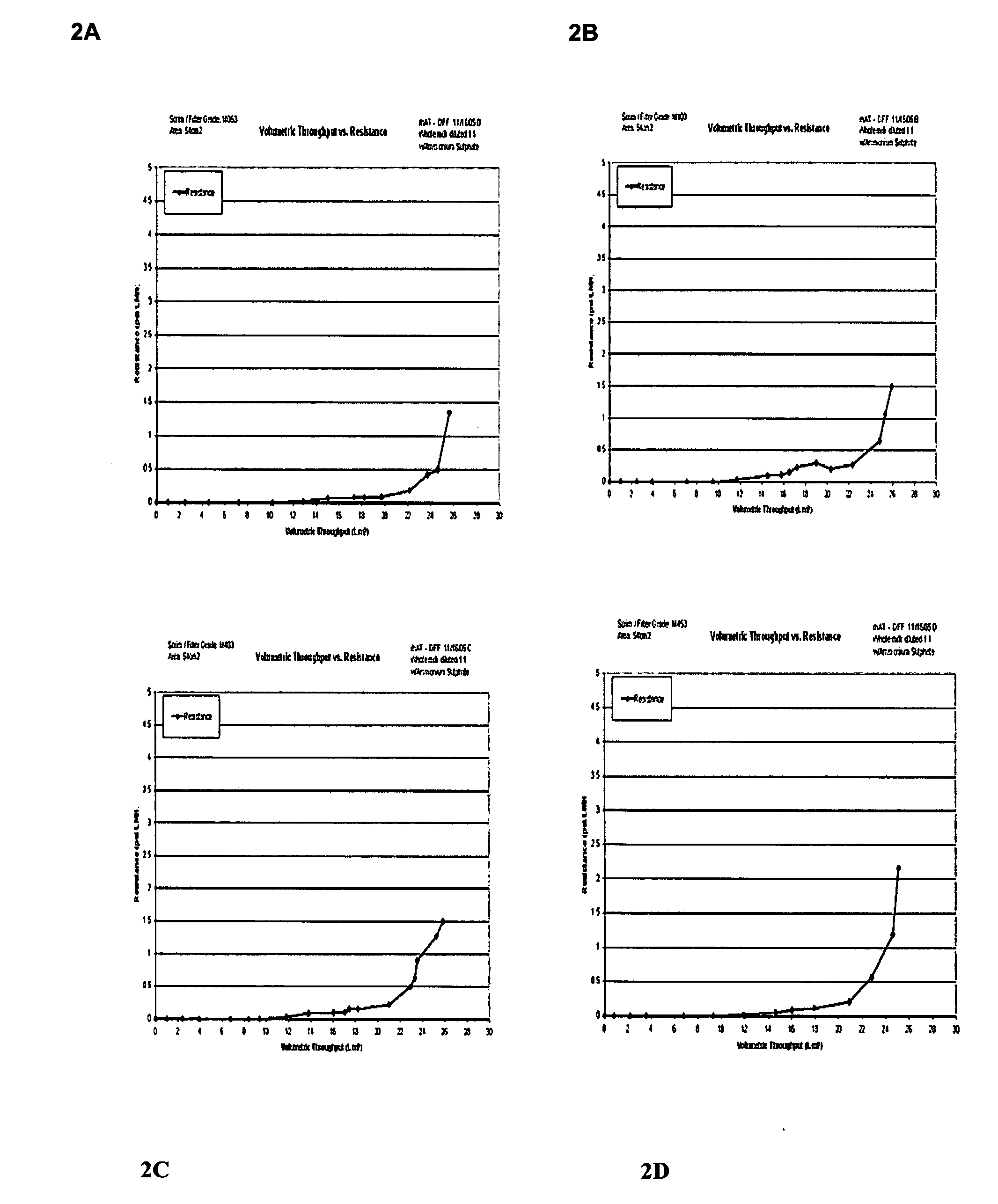
Clarification of transgenic milk using depth filtration
ActiveUS20070192878A1Good clarificationImprove fractionationPeptide/protein ingredientsHydrolasesBacteriaAmount of substance
Processes and apparati are provided for separating molecules of interest from a mixture by depth filtration (DF). The DF of the invention is useful in the clarification and processing of various feedstreams for the removal of a molecule of interest. According to a preferred embodiment, a transgenic milk feedstream is stabilized and particulate matter such as fat, casein miscelles and bacteria are removed. An aseptic filtration step was also developed to remove any bacteria remaining in a clarified transgenic milk feedstream.
Owner:LFB USA
Method for brewing onion grape wine
ActiveCN104087464AHigh content of active ingredientsHigh nutritional valueMicroorganism based processesWine preparationNutritive valuesVitis vinifera
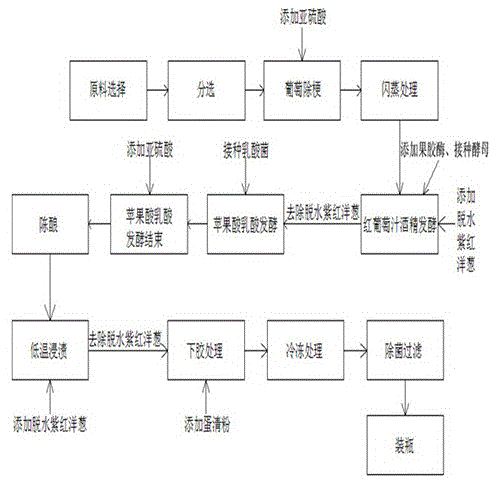
The invention discloses a method for brewing onion grape wine. The method for brewing onion grape wine comprises the following steps: (1) selecting raw materials; (2) sorting; (3) removing stems of grape and uniformly adding 0.2-0.4L / Ton of sulphurous acid on line; (4) carrying out flash evaporation; (5) carrying out alcoholic fermentation by using red grape juice; (6) carrying out malo-lactic fermentation; (7) ending malo-lactic fermentation; (8) ageing for 8-14 months; (9) dipping at the low temperature; (10) fining; (11) freezing; and (12) carrying out sterile filtration. By virtue of the onion grape wine, the onion smell in the wine is greatly reduced under the premise that the effective components of the onion are not damaged; the onion grape wine shows the fragrance similar to green onion pancakes, so that consumers accept the onion grape wine more easily; the onion grape wine is relatively easy to popularize; since the problem of thick onion smell in the wine is solved, the use amount ratio of the onion raw material in the wine brewing process can be higher; the onion grape wine is relatively high in contents of effective components of the onion, and the nutritive value and the health effect of the onion grape wine are improved.
Owner:内蒙古汉森葡萄酒销售有限公司
Polyimide nanofiber flocculus, preparation method and application thereof
InactiveCN105019141AImprove filtration efficiencyImprove breathabilityHeating/cooling textile fabricsFiltration separationAir filtrationFiber
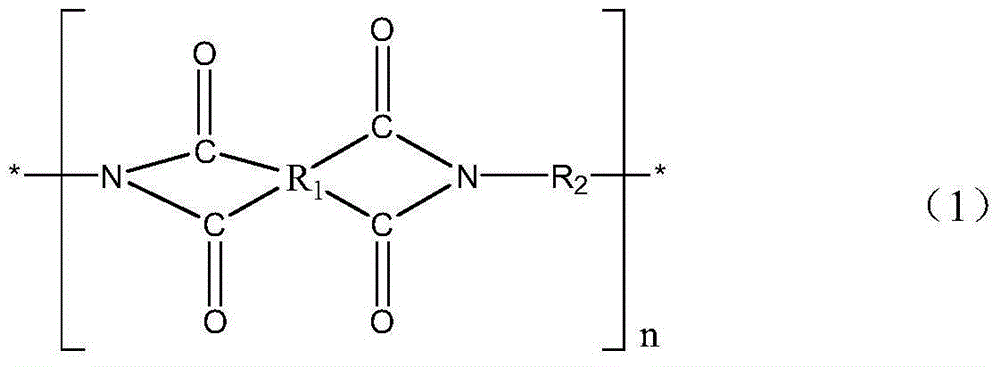
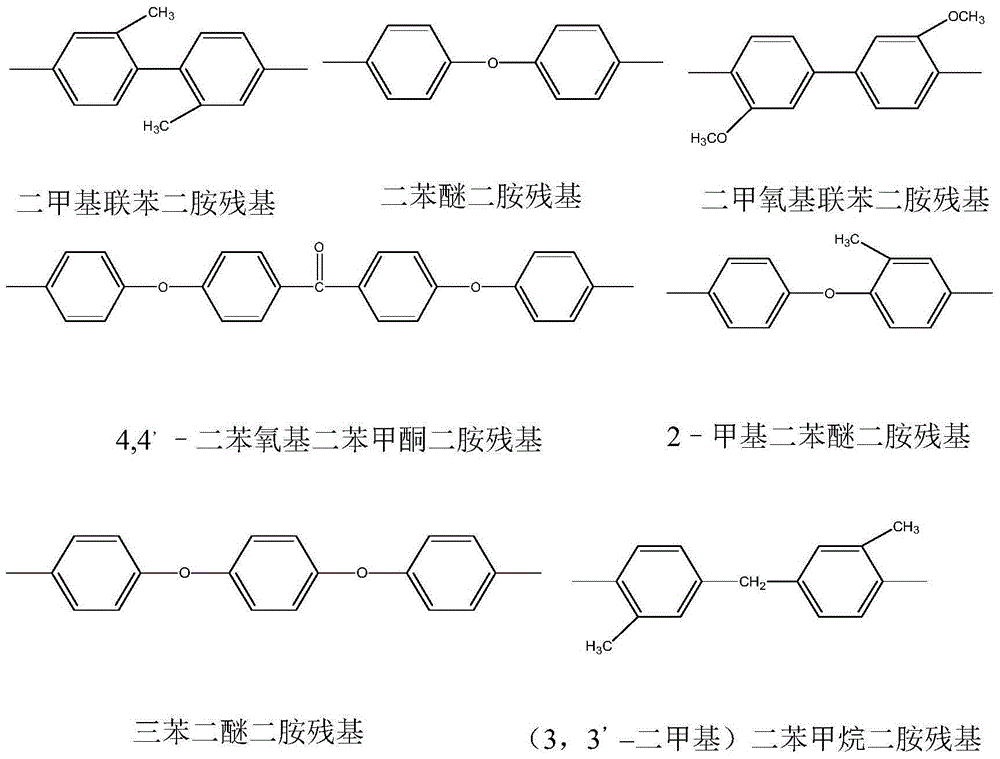
The invention provides a polyimide nanofiber flocculus, a preparation method and application thereof. The structure of the polyimide nanofiber flocculus is described in the following formula (1). In the formula (1), R1 denotes dianhydride residues of aromatic-containing rings. R2 denotes diamine residues of romatic-containing rings. N is an integer larger than 100. The polyimide nanofiber flocculus provided by the invention is featured by being high in filtration efficiency, good in air permeability, permanent in flame retardance and endurable in high temperature and is less susceptible to deformation and has anti-bacteria, mould-proof and electrostatic adsorption functions and has an extensive application prospect in air filtrations fields such as high-temperature filtration, high-end filtration and sterile filtration.
Owner:JIANGXI ADVANCED NANOFIBER S&T CO LTD
Sterile injection water production technique and sterile compressed air preparation method
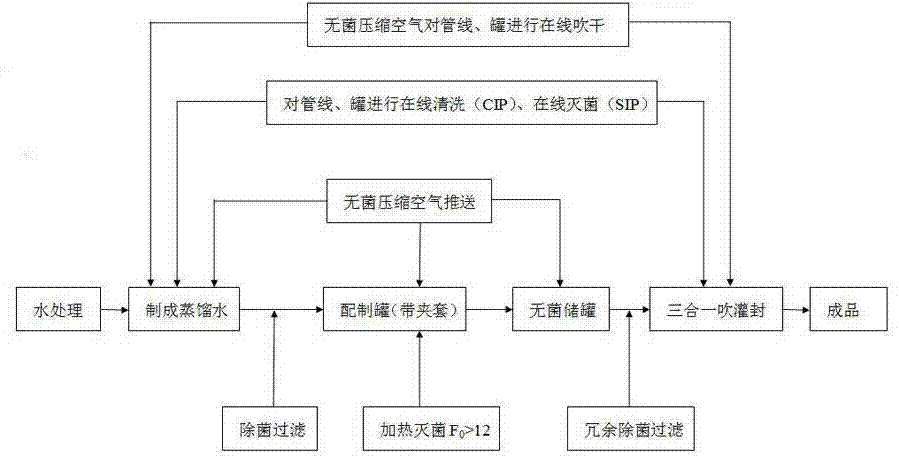
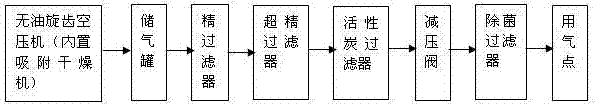
ActiveCN103693791AImproving Sterility Assurance LevelsControl heat source contentMultistage water/sewage treatmentPharmaceutical non-active ingredientsAntioxidantDistilled water
The invention discloses a sterile injection water production technique and a sterile compressed air preparation method. The sterile injection water production technique comprises the following steps: firstly, performing online cleaning CIP (Cleaning in Place) and online SIP (Sterilization in Place) and online drying to all pipelines, a preparation tank and a storage tank used in the production of sterile injection water through distilled water or pure steam, and delivering distilled water into the preparation tank; heating and sterilizing the distilled water, and leading into the storage tank and cooling; and finally, performing sterile filtration to the distilled water through a redundant filtration system, and injecting in a blowing-pouring-sealing three-in-one pouring machine through a sterile pipeline system for sterile pouring and sealing, thus obtaining the product. Through the production technique, the injection water is strictly sterilized before pouring and sealing, heat source content can be effectively controlled, an additive and an antioxidant of a plastic bottle can be prevented from diffusing into the injection water and no impurities of scraps and the like do not exist when terminal high-temperature sterilizing is carried out, therefore, the sterile injection water can provide powerful guarantee for the clinical use safety.
Owner:ZHONGQI PHARMA IND HENGSHUN ZHONGQI
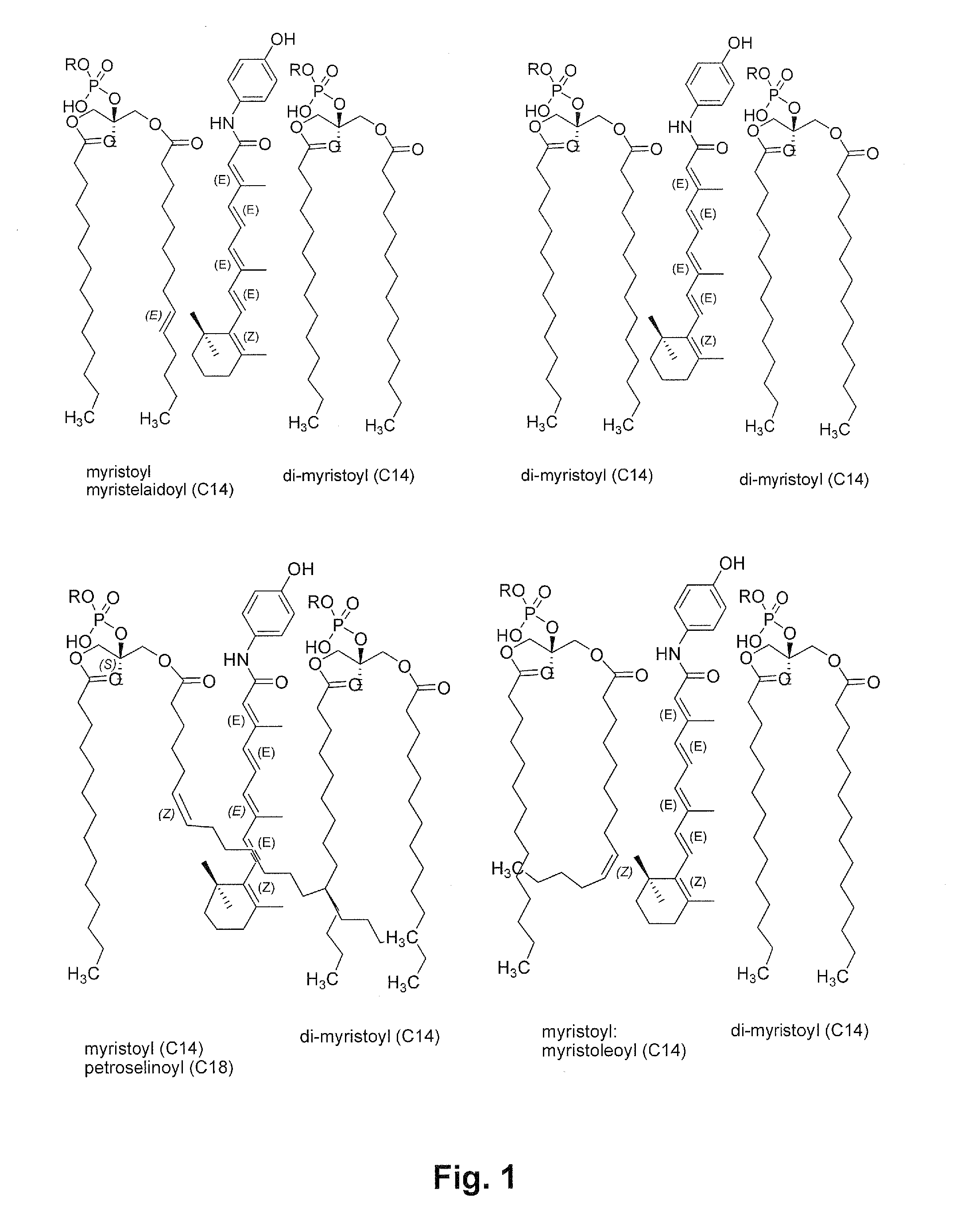
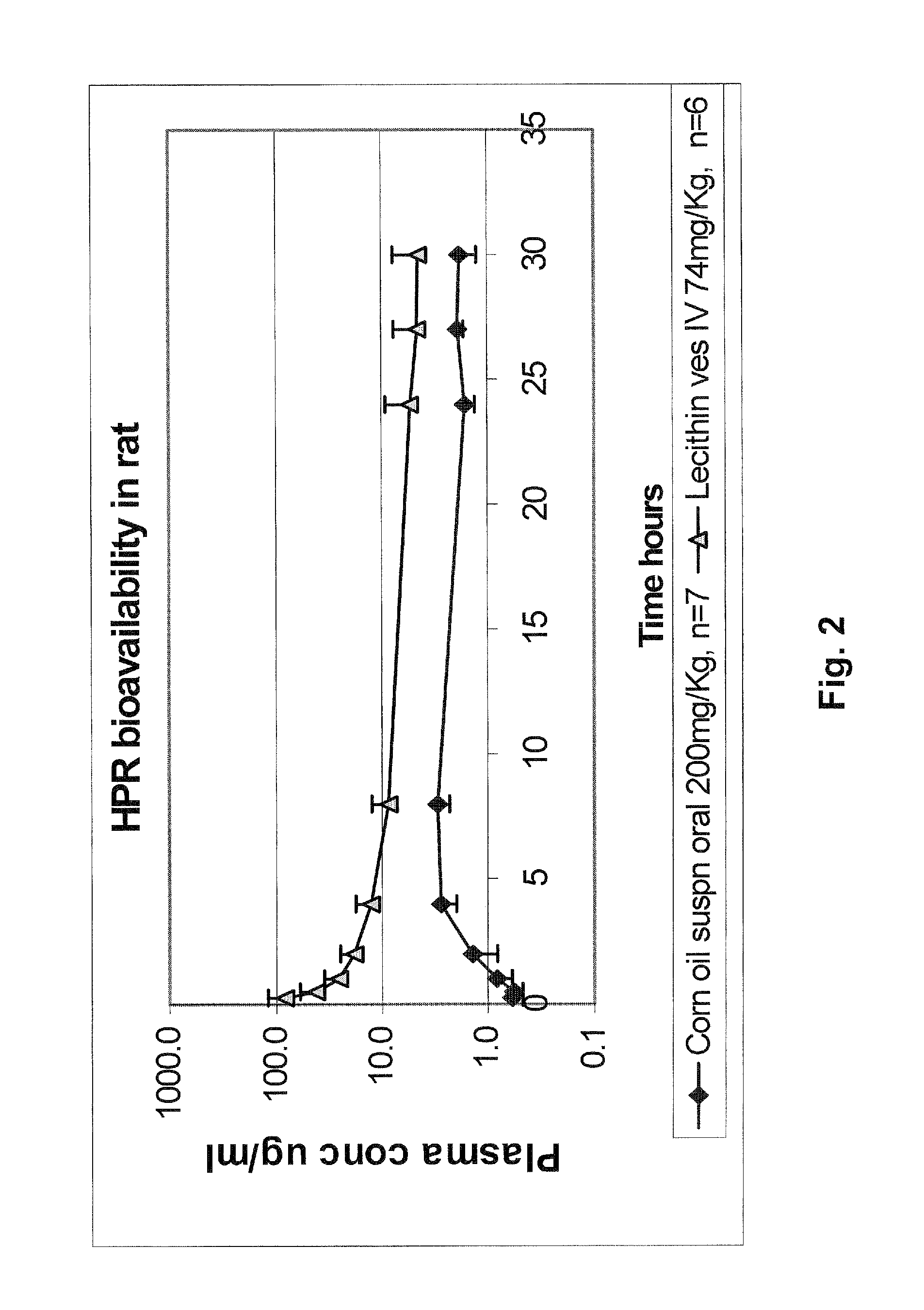
Liposomal nanoparticles and other formulations of fenretinide for use in therapy and drug delivery
ActiveUS20120093718A1Improve securityImprove treatment outcomesUltrasonic/sonic/infrasonic diagnosticsBiocideLipid formationPhospholipid
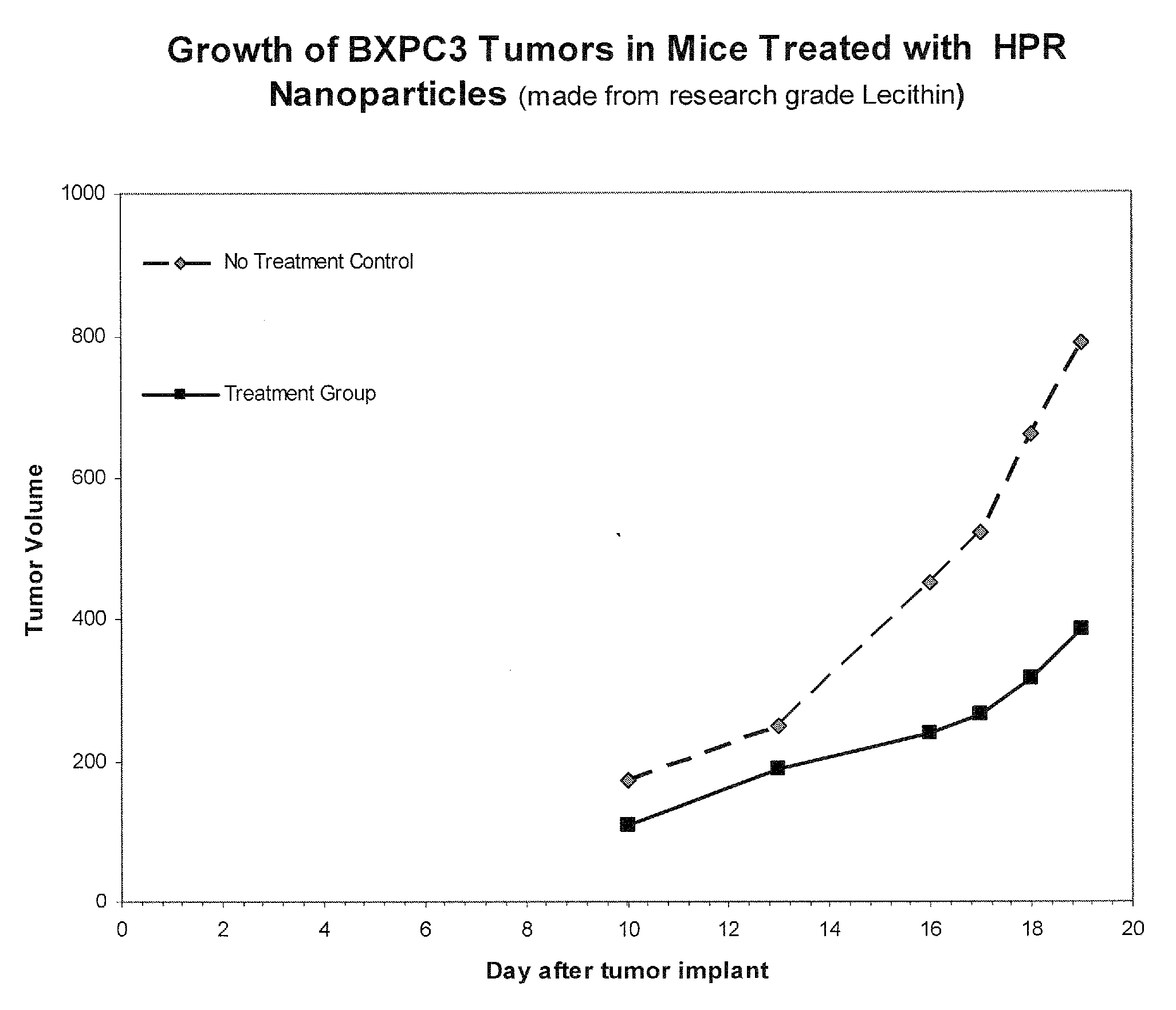
Formulations of neutral retinoids, in particular fenretinide (HPR) in the form of lipid nanoparticles, solid dipersions and emulsions are disclosed. These compositions are used to treat diseases that are amenable to treatment by HPR, such as neoplastic diseases by achieving higher and more prolonged concentrations of HPR in the subject. The key steps for preparing lipid nanovesicles of HPR include mixing and sonication, sterile filtration, without or without lyophilization for long-term stable storage, and employ processes and materials that are scalable from the laboratory to the manufacturing level. The formulation are suitable for injection into human or animal patients without causing allergic or hypersensitivity responses by avoiding chemical surfactants and animal sources of phospholipids in their manufacture.
Owner:WAYNE STATE UNIV +1
Apparatus for extracorporeal blood treatment with a device for checking a sterile filter, and method of checking a sterile filter of an extracorporeal blood treatment apparatus
ActiveUS8182691B2Reduce equipmentReduce spendingSolvent extractionUltrafiltrationBlood treatmentsSterile water
An apparatus for extracorporeal blood treatment has a dialysis fluid circuit and a blood circuit which are separated by a dialyzer. Arranged in the dialysis fluid circuit there is a sterile filter for producing a sterile dialysis fluid which flows into the dialyzer. To check the sterile filter, a chemical and / or physical property of the dialysis fluid, for example the conductivity, is changed upstream of the sterile filter, and the change in the property of the dialysis fluid is detected downstream of the sterile filter. From the time shift between the initiation of the conductivity impulse and the detection of the latter, it is possible to tell whether the blood treatment apparatus is fitted with a sterile filter. Moreover, the volume of the sterile filter can be inferred from the length of the time shift.
Owner:FRESENIUS MEDICAL CARE DEUTSCHLAND GMBH
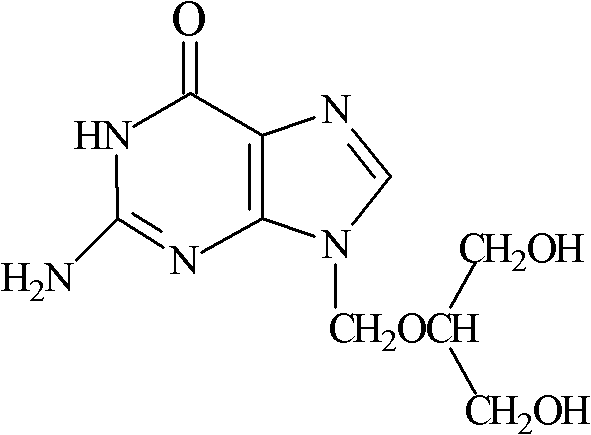
A kind of ganciclovir composition for injection and preparation method thereof
ActiveCN102274197AExcellent freeze-dried structureParticle size andPowder deliveryInorganic non-active ingredientsPorosityFreeze-drying
The invention relates to a ganciclovir composition for injection, which is freeze-dried powder composed of ganciclovir and sodium hydroxide, wherein the weight ratio of the ganciclovir to the sodium hydroxide is (6.6-7.0):1, the average particle size of the freeze-dried powder is 80-100nm, and the porosity is 94-98%. The preparation method comprises the following steps: 1) preparation: weighing ganciclovir and sodium hydroxide, putting the ganciclovir and sodium hydroxide into a preparation tank, adding water for injection, and stirring until the ganciclovir and sodium hydroxide are completely dissolved and evenly mixed; 2) sterile filtration and packaging; and 3) vacuum freeze drying. The invention has the advantages of simple formula, advanced technique, uniform quality and high stability, and has higher redissolution performance and clinical application safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of sterile preparation of lipoalprostadil
InactiveCN101596161AMeet sterility requirementsOrganic active ingredientsLiposomal deliveryMicrometerSterile filtration
The invention aims at providing a preparation method of a sterile preparation of lipoalprostadil. A sterile filtration method is adopted in a preparation technology of the lipoalprostadil for the first time, a microporous filtering film of 0.22 micrometer or a bag type filter is used for degerming and filtering the lipoalprostadil having an average grain diameter ranging from 100 nm to 300 nm at the temperature between 50 DEG C and 80 DEG C, and the sterile preparation of the lipoalprostadil is filled in hundred-level laminar flow environment. The preparation method can completely eliminate microorganisms in the preparation, ensure that the lipoalprostadil is completely filtered, effectively avoid the main medicine degradation of the lipoalprostadil for the traditional sterilizing technology and ensure that all the lipoalprostadils pass through the filtering film of 0.22 micrometer so as to reach the sterile requirement of the preparation.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG
Sterile container with sterile filter
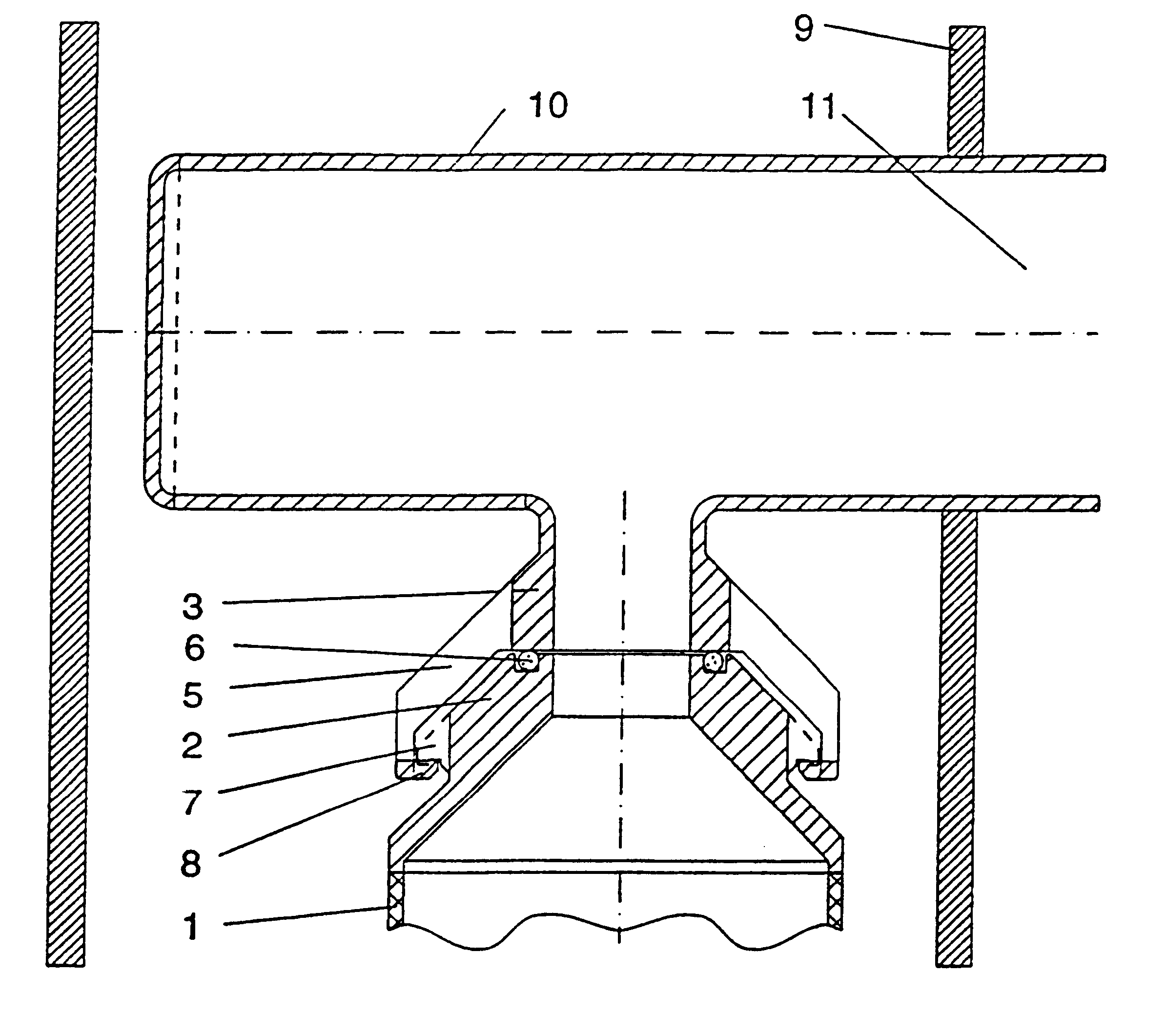
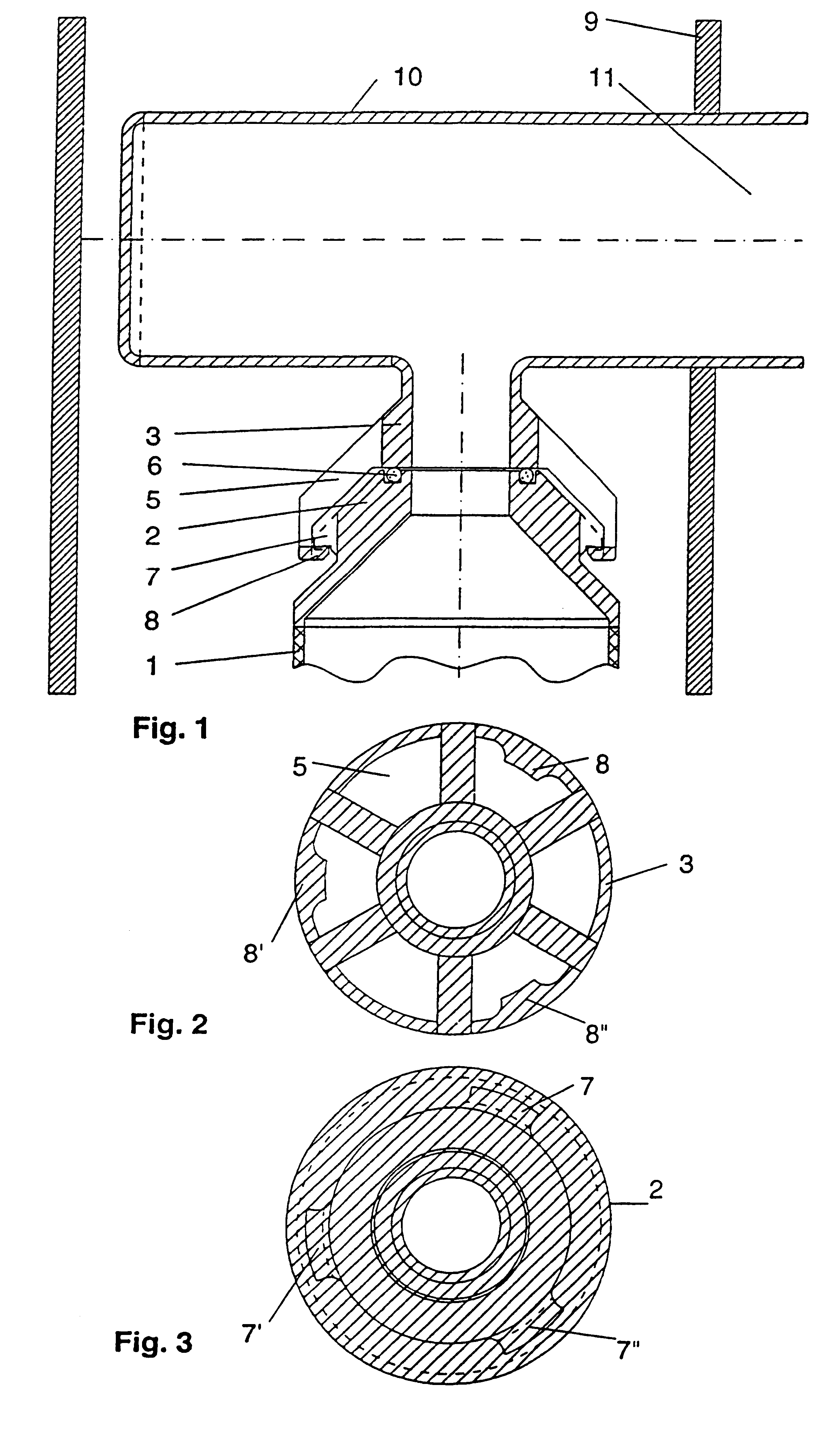
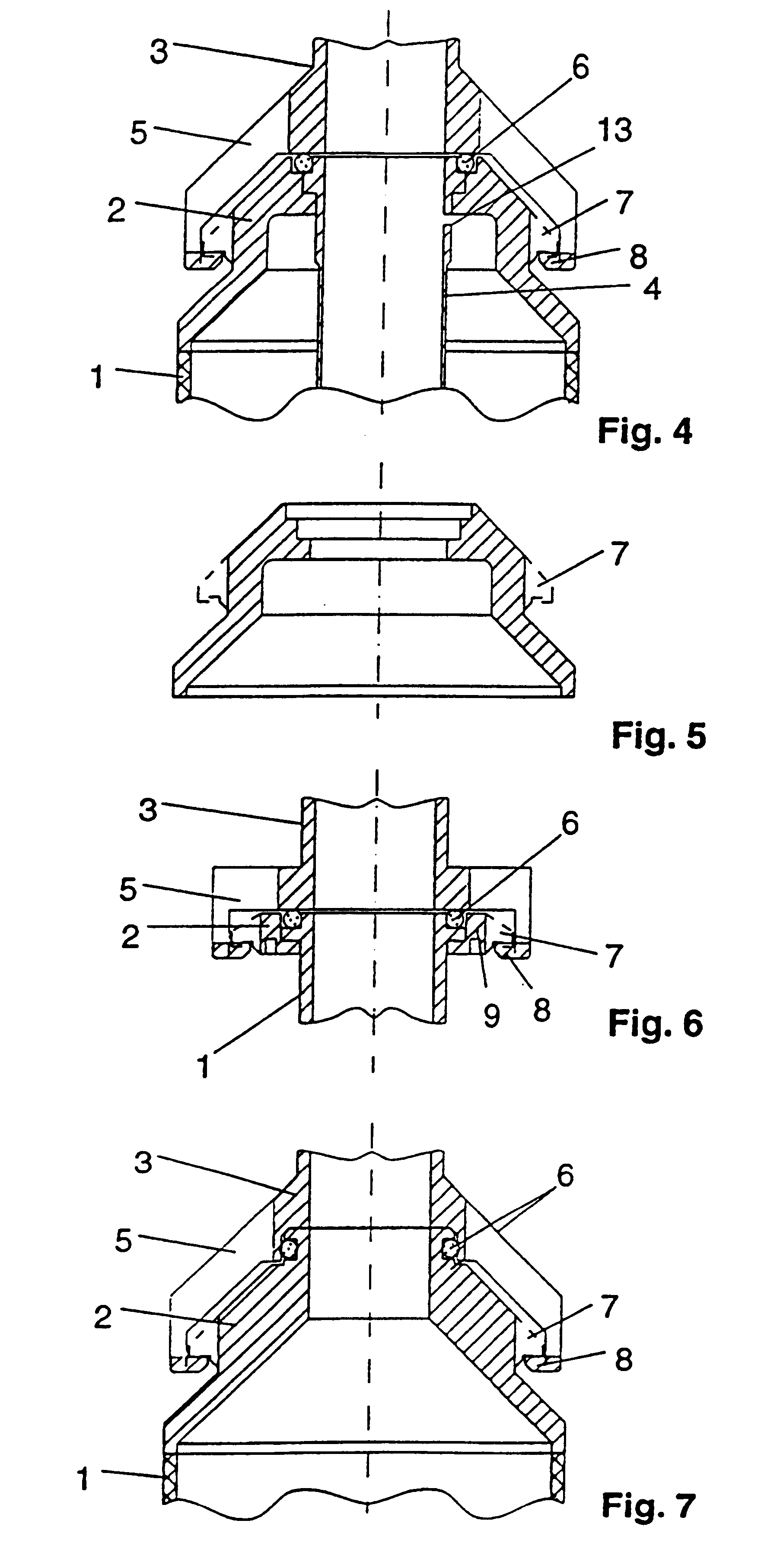
To improve a sterile container, in particular, for receiving and storing surgical instruments or surgical material under sterile conditions, comprising a receiving space formed by a container bottom and container walls, a lid for closing the receiving space, a gas exchange opening for providing a fluid connection between the receiving space and an environment outside of the sterile container, a filter holding device including a first holding element and a second holding element, and a sterile filter, wherein, in a sterile position in which the sterile filter closes the gas exchange opening, the sterile filter is held between the first holding element and the second holding element, so that the design thereof is simplified and damage to the sterile filter is avoided, it is proposed that the sterile filter be constructed so as to have no openings, that the first holding element have at least one first holding surface, that the second holding element have at least one second holding surface, and that in the sterile position the sterile filter be held clamped between the first holding surface and the second holding surface.
Owner:AESCULAP AG
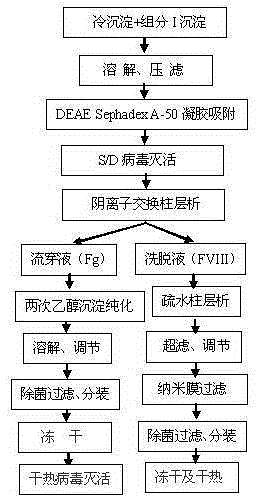
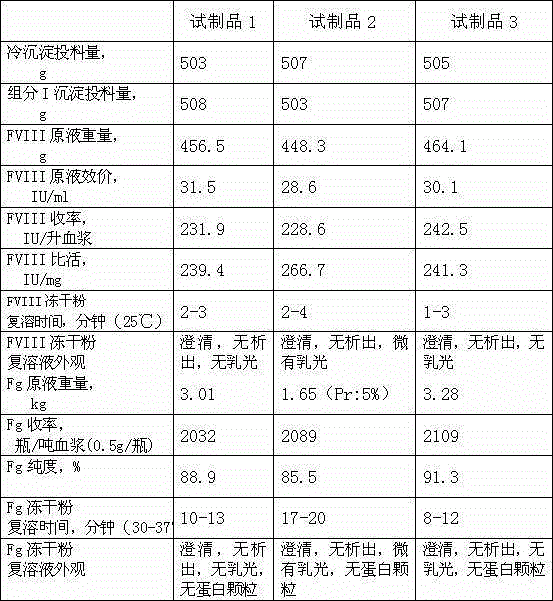
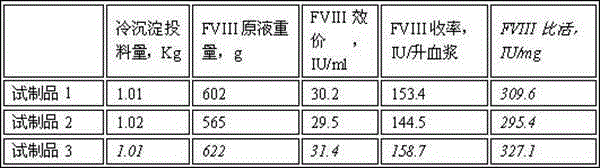
Method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen
InactiveCN105315360AHigh yieldIncrease productivityFactor VIIFibrinogenDEAE SephadexVirus inactivation
The invention discloses a method for simultaneously preparing high-purity human coagulation factor VIII and human fibrinogen by cryoprecipitate and component I precipitation, mixing and feeding. The method comprises the following steps: (1) simultaneous feeding and dissolution of a cryoprecipitate and a component I; (2) DEAE Sephadex A-50 gel adsorption; (3) S / D virus inactivation; (4) anion exchange column chromatography; (5) two-step low-temperature ethanol precipitation and purification, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a chromatographic penetration liquid to obtain a human fibrinogen; (6) further hydrophobic column chromatography of a chromatographic eluant; (7) ultrafiltration, nanofilm filtration, sterile filtration, subpackage, freeze-drying and dry heat virus inactivation of a hydrophobic eluant to obtain a high-purity human coagulation factor VIII. By the adoption of the process, FVIII and Fg in the two raw materials are extracted simultaneously, so that the yields of the two products are greatly improved, the yield of the human coagulation factor VIII can reach 200,000 IU / ton plasmas, the yield of the human fibrinogen exceeds 2,000 bottles / ton plasmas, and the yields are both far higher than those of a traditional process.
Owner:上海洲跃生物科技有限公司
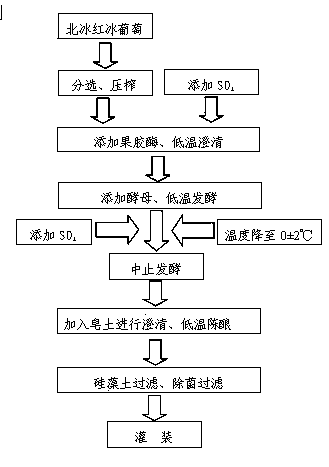
Making method of vitis amurensis ice wine
InactiveCN103436399AHigh in nutrientsThe preparation method is scientific and reasonableWine preparationPectinaseYeast
The invention relates to the technical field of grape wine making and particularly relates to a making method of a vitis amurensis ice wine having the characteristics that the fragrance is strong, the mouth feel is mellow and full, the nutrients are rich and varied, and requirements of high-end consumer groups can be met. The vitis amurensis ice wine is made through the following steps: picking fruits of naturally frozen Beibinghong (a grape variety); carrying out selection and squeezing; adding sulfur dioxide; adding pectinase, and carrying out low-temperature clarification; adding yeast, and carrying out low-temperature fermentation; stopping the fermentation; adding bentonite to carry out clarification; carrying out low-temperature ageing; carrying out kieselguhr filtration and sterile filtration; carrying out bottling; and drinking.
Owner:吉林三快科技有限公司 +2
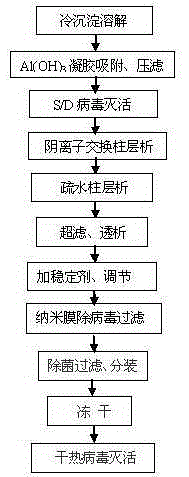
Method for preparing high-purity human coagulation factor VIII
ActiveCN105348382AAvoid damageNo precipitationFactor VIIPeptide preparation methodsUltrafiltrationBlood plasma
The invention discloses a method for preparing a high-purity human coagulation factor VIII from a cryoprecipitate of a human plasma fraction. The method comprises the following steps: (1) cryoprecipitate dissolution; (2) aluminum hydroxide gel adsorption and filter pressing; (3) S / D virus inactivation; (4) anion exchange resin column chromatography; (5) hydrophobic column chromatography; (6) ultrafiltration dialysis and concentration; (7) addition of one or more stabilizers and titer adjustment; (8) nano-membrane virus-removing filtration; (9) sterile filtration and sub-packaging; (10) freeze-drying; (11) dry-heat virus inactivation. The method has the advantages that a human coagulation factor VIII is purified through the two column chromatography steps, so that the prepared high-purity product can reach a specific activity of about 300 IU / mg, considerably higher than about 50 IU / mg in the prior art, and the product appearance and the heat stability are obviously improved; in the preparation process, three virus removing modes are adopted, so that the clinical use safety of the product can be greatly improved.
Owner:上海洲跃生物科技有限公司
Preparation of cefazolin sodium sterilized raw medicine
InactiveCN101463040ALow clarityQuality improvementAntibacterial agentsOrganic active ingredientsCLARITYCefazolin Sodium
The invention provides a preparation method of a cefazolin sodium sterile bulk drug. The preparation method comprises the following steps: suspending cefazolin in deionized water, performing a neutralization reaction with alkali solution containing sodium ions in a hermetic stirring container under depressurized condition, and obtaining the cefazolin sodium sterile bulk drug by rough filtration, sterile filtration and lyophilization of reaction liquid.. The preparation method solves the technical problems of the existing production process that the clarity and the water content of the cefazolin sodium are difficult to be controlled and the contents of related materials are too much higher, improves the product quality and is suitable for large-scale industrialized production.
Owner:YAOPHARMA CO LTD
Large-scale preparation method of recombinant staphylococcus aureus vaccine
PendingCN110343633AEasy to prepareRealize industrial scale productionAntibacterial agentsBacteriaContinuous flow centrifugationStaphylococcus aureus
The invention discloses a large-scale preparation method of a recombinant staphylococcus aureus vaccine. The method comprises the following steps: opening a protein working seed lot, inoculating the protein working seed lot strain in a conical flask, inoculating first-generation production strain in a seeding tank, performing continuous second-generation cultivation, inoculating the second-generation production strain in a fermentation tank, and performing continuous third-generation cultivation; centrifugally removing supernate from a bacterial solution through a continuous flow after fermentation is completed; and performing bacterium body dissolving and bacterium disruption; performing combination, washing the mixture after combination is completed, performing digestion, purifying the protein digestion solution by adopting a chromatographic column and performing desalination, endotoxin removing, sterile filtration and storage. The preparation method is simple and can be used for preparing recombinant staphylococcus aureus vaccine in a large scale.
Owner:CHENGDU OLYMVAX BIOPHARM
Method for producing raspberry icewine
InactiveCN103114015ACrystal clear wineInterest in wine tastingMicroorganism based processesAlcoholic beverage preparationBiotechnologyPectinase
The invention provides a method for producing raspberry icewine. The method comprises the following steps of: picking up ice-covered raspberry fruits in ice ball shapes at -10 to -14 DEG C; selecting out raspberry fruits perfect in appearance and free of corrosion caused by diseases and pests, and putting the selected raspberry fruits in a sealed fermentation tank; pressing at the temperature below -8 DEG C and only remaining 10%-15% of pure raspberry pulp after pressing; heating the raspberry pulp pressed previously to the range of 7 to 14 DEG C; adding 20 mg / L of pectinase to the raspberry pulp for enzymolysis, and then standing for clarification; adding yeast to the fermentation tank for fermentation at 8-15 DEG C; when the alcohol content reaches 9-13%, stopping fermentation to obtain raw raspberry icewine; pumping the raw raspberry icewine into an oak barrel for ageing; adding diatomite to the aged raspberry icewine for fining, performing clarifying treatment, standing for cold storage after sterile filtration; and filling after filtering out the precipitates, thereby obtaining the finished product.
Owner:SHENYANG GUOTIAN AGRI SCI & TECH DEV
A kind of clindamycin phosphate composition for injection and preparation method thereof
ActiveCN102258488AExcellent freeze-dried structureExcellent average particle sizeAntibacterial agentsPowder deliveryPorosityClindamycin Phosphate
The invention relates to a clindamycin phosphate composition for injection. The composition is freeze-dried powder which consists of clindamycin phosphate and sodium hydroxide, wherein the weight ratio of the clindamycin phosphate to the sodium hydroxide is (24-26):1; the average grain diameter of the freeze-dried powder is 70-130nm; and the porosity is 92-98 percent. A preparation method of the composition comprises the following steps of: (1) preparing: weighing the clindamycin phosphate and the sodium hydroxide, filling in a preparation tank, adding water for injection, stirring to fully dissolve the clindamycin phosphate and the sodium hydroxide and uniformly mixing; (2) decarbonizing and performing sterile filtration; (3) performing sterile subpackaging; and (4) freeze-drying under vacuum. The composition has the advantages of simple formula, advanced process, uniform quality and superior stability and meanwhile has better redissolving performance and clinical medication safety.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of arginine aspirin and powder-injection of arginine aspirin
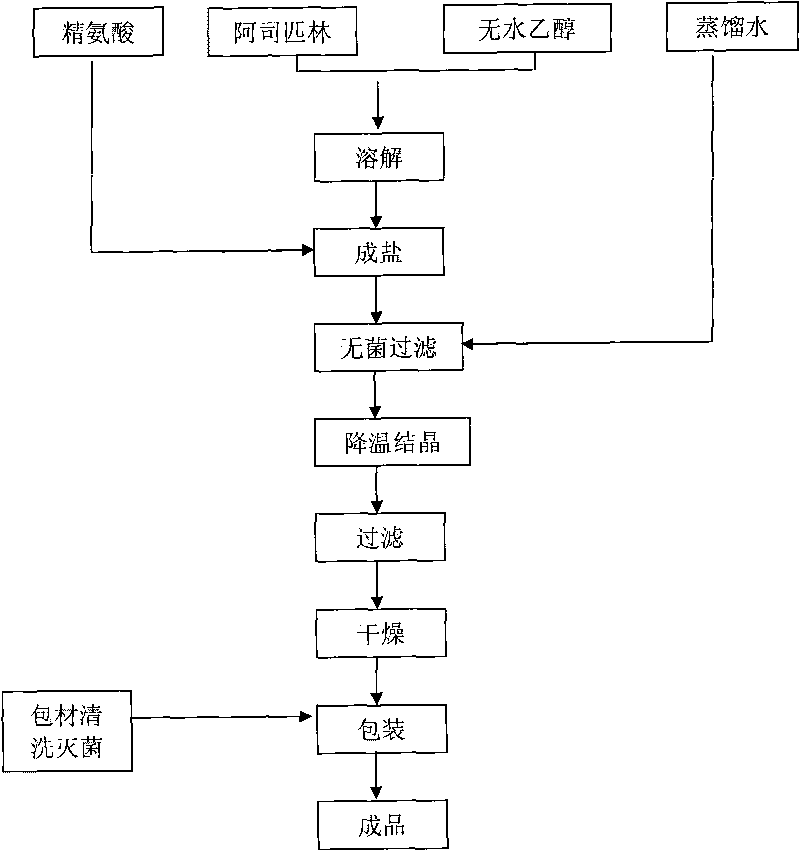
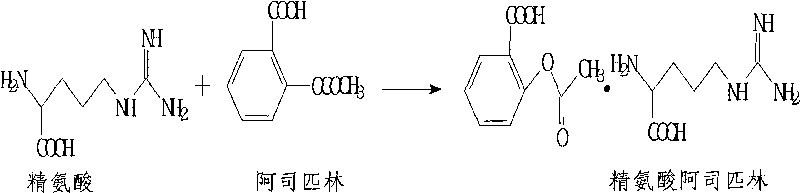
ActiveCN101704766ANo need to dissolveShort reaction timeOrganic active ingredientsPowder deliveryAspirinAlcohol
The invention relates to a preparation method of arginine aspirin and powder-injection of arginine aspirin. The preparation method of arginine aspirin comprises the following steps: 1) dissolving aspirin in absolute alcohol, adding arginine power at 30-40 DEG C to react; 2) adding distilled water to ensure the solution to be transparent, performing sterile filtration; 3) reducing temperature for crystallization, filtrating; and 4) drying to obtain sterile arginine aspirin, and then performing sterile subpackage of the sterile arginine aspirin to obtain the powder-injection of arginine aspirin. Compared with the prior art, the preparation method of the invention has short reaction process and simple operation, the reaction temperature is not high, the method is applicable to industrialized production; and the reaction yield is high, the product quality is good (especially the content of free salicylic acid is low), and the stability is good.
Owner:蚌埠丰原涂山制药有限公司
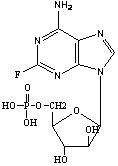
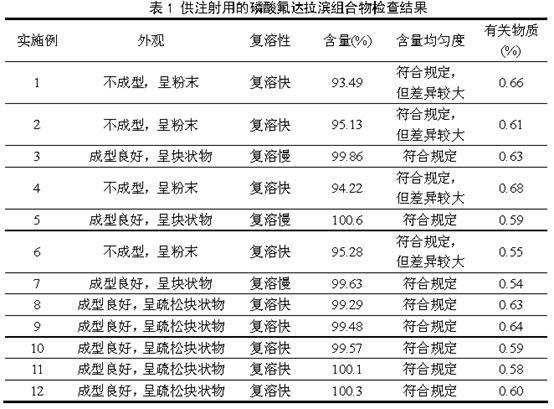
Fludarabine phosphate composition for injection and preparation method thereof
ActiveCN101947208AGood molding effectUniform and stable contentOrganic active ingredientsPowder deliveryPhosphoric acidMannitol
The invention relates to a fludarabine phosphate composition for injection, which contains fludarabine phosphate and mannitol. A preparation method for the fludarabine phosphate composition comprises the following steps of: 1) preparation: putting the fludarabine phosphate and the mannitol in a preparation tank in a weight ratio of 1:1, adding water for injection into the tank, stirring the mixture, adjusting the pH to 7.7 by using 0.1mol / L phosphoric acid solution and 0.1mol / L sodium hydroxide solution to fully dissolve the mixture, and continuously stirring the solution for uniform mixing; 2) sterile filtration, packing and half plugging; and 3) vacuum freezing drying. The fludarabine phosphate composition has the advantages of simple formula, advanced process, good appearance formation, uniform and stable quality, uniform and accurate content, thorough moisture drying and better stability.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Integrated zero-leakage gas positive-negative pressure compatible microorganism isolation operation cabinet
InactiveCN103436443AAvoid interferenceSolving the scientific puzzle of injuryApparatus sterilizationTissue/virus culture apparatusMicroorganismEnvironmental engineering
The invention provides an integrated zero-leakage gas positive-negative pressure compatible microorganism isolation operation cabinet which comprises a positive pressure cabin with a positive pressure fan and a sterile filter at the top and a glass sliding door, wherein the positive pressure cabin is surrounded by a glass front partition plate and a glass side partition plate to form a negative pressure cabin; two sealed isolation sleeves formed by overlapping and interacting an outer partition soft plate and an inner partition soft plate are arranged on the glass front partition plate; on a working table of the negative pressure cabin, a circle of negative-pressure air grid ports are communicated with a negative pressure filtering cabin with a gas filtering material; and the negative pressure fan is arranged in a fan chamber communicated on the lower side of the negative pressure filtering cabin to extract the filtered waste gas to an exhaust connector to be discharged. The zero-leakage gas positive-negative pressure compatible microorganism isolation operation cabinet provided by the invention has the beneficial effects that the device can ensure that the cells and bacteria cultured in a microorganism experiment process are free from the contamination and interference of other bacteria in a sterile positive pressure environment, and harmful microorganisms and gases inside can be isolated and shielded by the negative pressure cabin so as to prevent leakage and consequent environmental pollution and occupational injury; and the device is widely applied to the scientific research fields such as environmental protection, medical scientific research, military security and the like.
Owner:TIANJIN HOPE IND & TRADE
Candle filter elements and method for fixing same in a pressure vessel
A candle filter element adapted to be located inside a pressure vessel and fixed in a hanging position to a collector tube for aseptic and / or sterile filtration of liquids, has a coupling with conical upper coupling part and a conical lower coupling part, with the conical upper coupling part provided with a rinsing opening and a pair of cams.
Owner:DRM DR MULLER
Recombinant human interleukin-12 preparation for injection and preparation method thereof
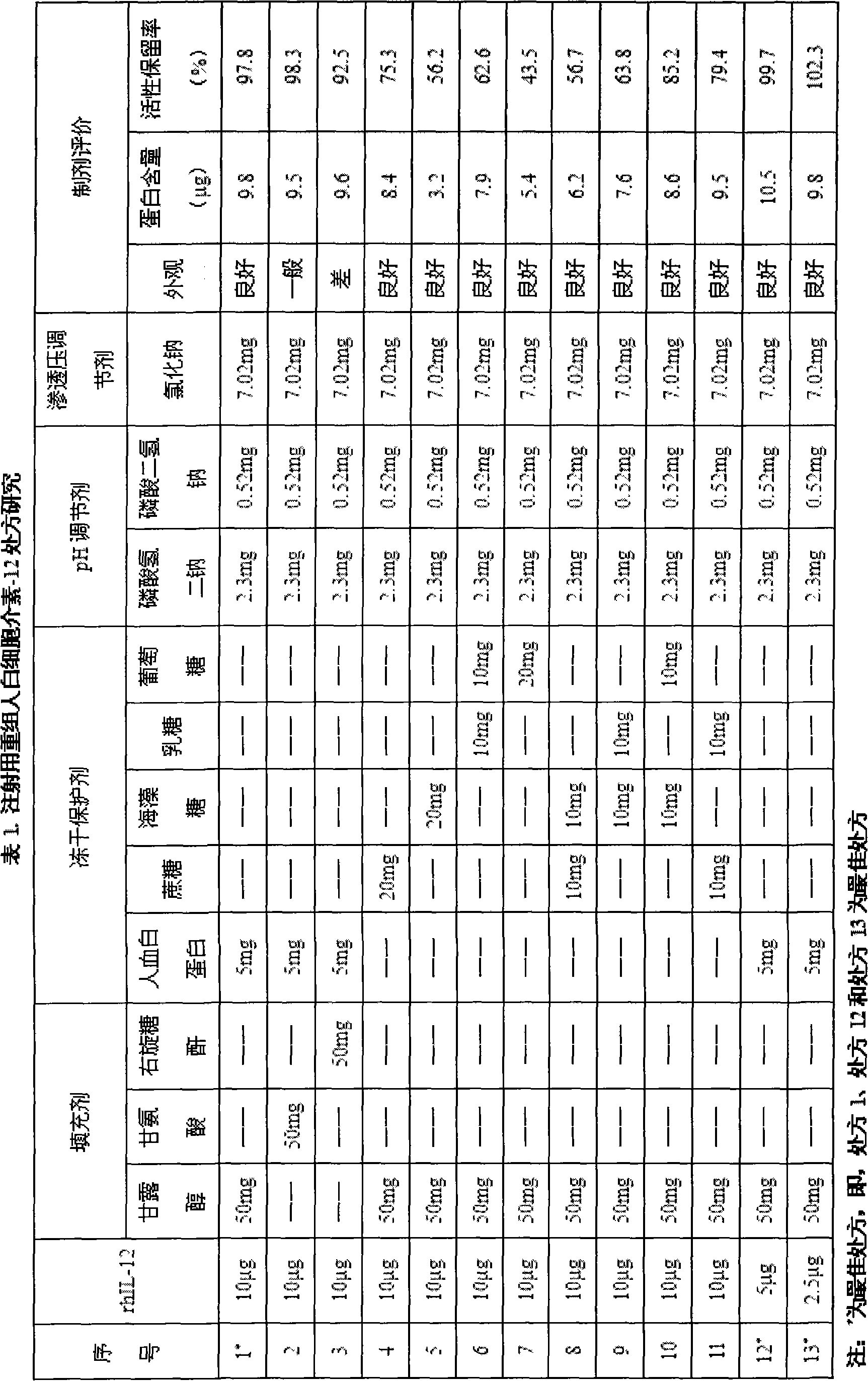
ActiveCN102178931ADoes not reduce protein contentDoes not reduce biological activityPowder deliveryPeptide/protein ingredientsWhite blood cellFreeze-drying
The invention discloses a recombinant human interleukin-12 preparation for injection and a preparation method thereof, and aims to provide a preparation which has the advantages of good stability and low moisture content. The invention has the technical point that each milliliter of preparation contains 2.5-10mu g of recombinant human interleukin-12, 50mg of filling agent, 5-20mg of freeze-dried protective agent, 2.30mg of disodium hydrogen phosphate, 0.52mg of sodium dihydrogen phosphate and 7.02mg of sodium chloride. The preparation method comprises the following steps of: (1) weighing disodium hydrogen phosphate, sodium dihydrogen phosphate and sodium chloride according to the formula proportions, adding water for injection, stirring for dissolving, regulating the pH value to 6.8-7.8 with sodium hydroxide or hydrochloric acid, and metering the volume to the formula proportion; (2) taking out the solution, adding the formula proportion of the filling agent, stirring for dissolving, then adding the formula proportions of the freeze-dried protective agent and recombinant human interleukin-12, stirring until the materials are uniform, carrying out sterile filtration, and respectively packaging in bottles of 1ml; and (3) freeze-drying. The invention belongs to the technical field of medicine preparation.
Owner:广州市茵良强生物科技有限公司
In-vitro separation method of porcine teschovirus
The invention provides an in-vitro separation method of porcine teschovirus. The in-vitro separation method comprises the following steps of: (1) acquiring specimen supernatant which is identified to be positive by nRT-PCR (nested Reverse Transcription-Polymerase Chain Reaction) and subjected to sterile filtration from a swine specimen which is clinically diagnosed to be doubtful porcine teschovirus infection; (2) infecting a swine kidney passage cell line with the acquired specimen supernatant, and carrying out continuous passage culture until more than 90% cells are subjected to pathologic change, wherein supernatant is taken and subjected to nRT-PCR detection when viruses are collected in each generation, and the PCR-positive virus liquid is maintained for keeping passage; and (3) purifying the porcine teschovirus after passage culture in the step (2) by use of a plaque purification method. The separation purification method provided by the invention is high in specificity and strong in sensitivity and can be used for quickly and specifically separating and purifying porcine teschovirus.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
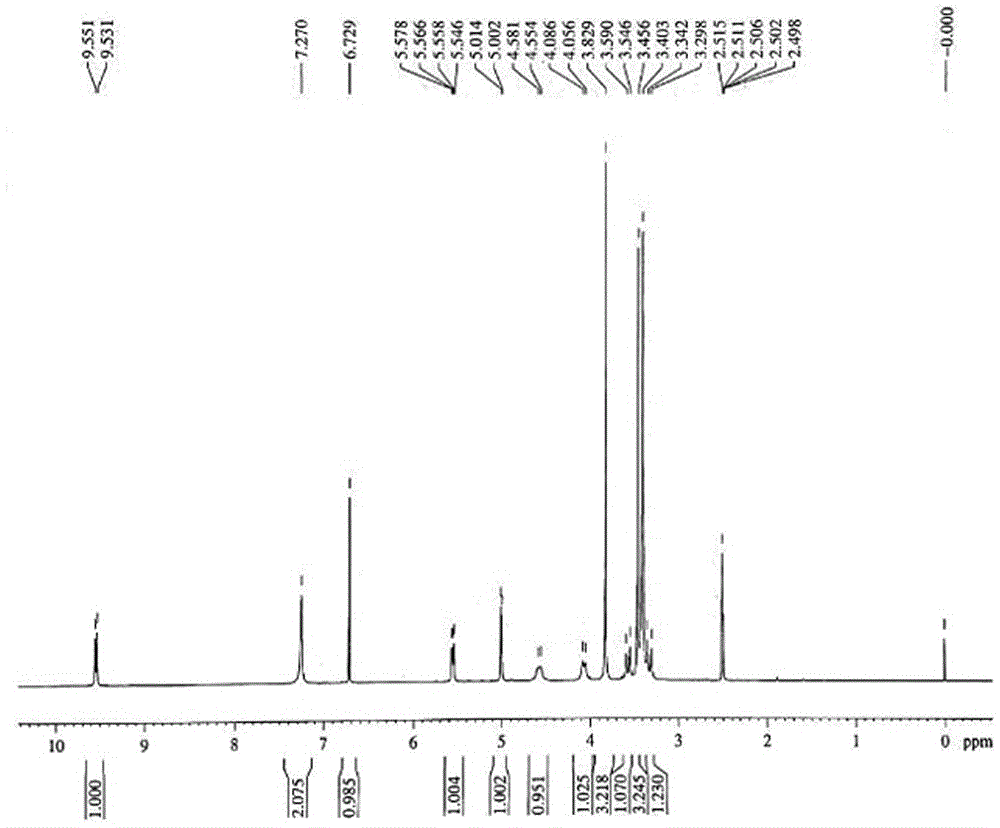
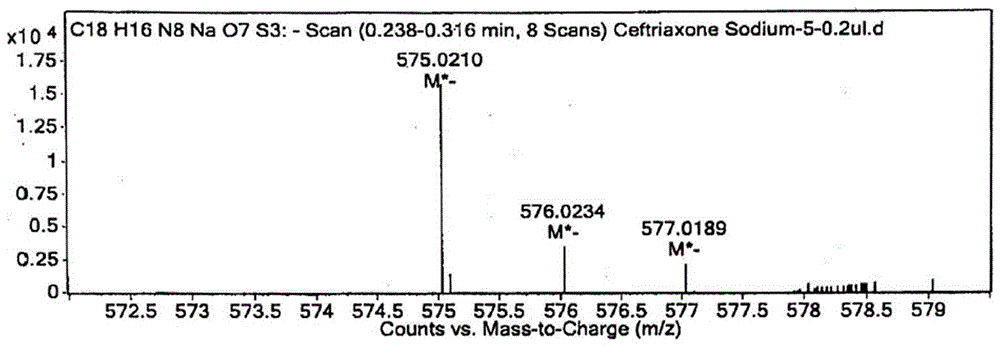
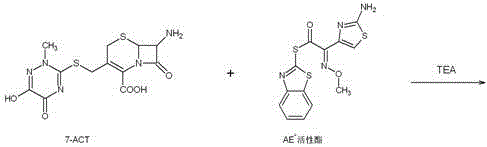
Preparation method and product of ceftriaxone sodium sterile powder
InactiveCN106008554AAvoid open loopAvoid degradationAntibacterial agentsPowder deliveryCeftriaxonumSodium ceftriaxone
The invention discloses a preparation method of ceftriaxone sodium sterile powder, and belongs to the technical field of medicine. The method comprises the following steps of mixing methylene dichloride and alcohol; lowering the temperature; sequentially adding antioxidizers, 7-ACT and AE-active esters; dissolving the materials to obtain a mixed solution; dripping triethylamine into the mixed solution within 1h for reaction; performing extraction after the reaction is completed; after the pressure reduction suction filtration on a water phase, adding a salt forming agent for reaction and salt forming; after a decoloring agent is added into a salt forming reaction solution, performing sterile filtration; adding a solvating agent into filter liquid for crystallization; performing post-treatment to obtain the ceftriaxone sodium sterile powder. The preparation method has the advantages that the operation is simple; the reaction conditions are mild; the control is easy; the ceftriaxone sodium sterile powder is prepared in one step; the refining process is omitted; the production period is shortened; the cost is greatly reduced; the quality yield reaches 165 percent or higher; the product purity can reach 99.5 percent or higher.
Owner:HENAN KANGDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com