Cabazitaxel drug composition and preparation method thereof
A technology of cabazitaxel and composition, which is applied in the field of cabazitaxel pharmaceutical composition, can solve the problems of protein nanoparticle stability damage, poor stability of protein nanoparticle, and impossibility, and achieve the effect of meeting the needs of intravenous drip therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 High-pressure homogeneous emulsification method uses water-compatible solvent as organic phase solvent—control group
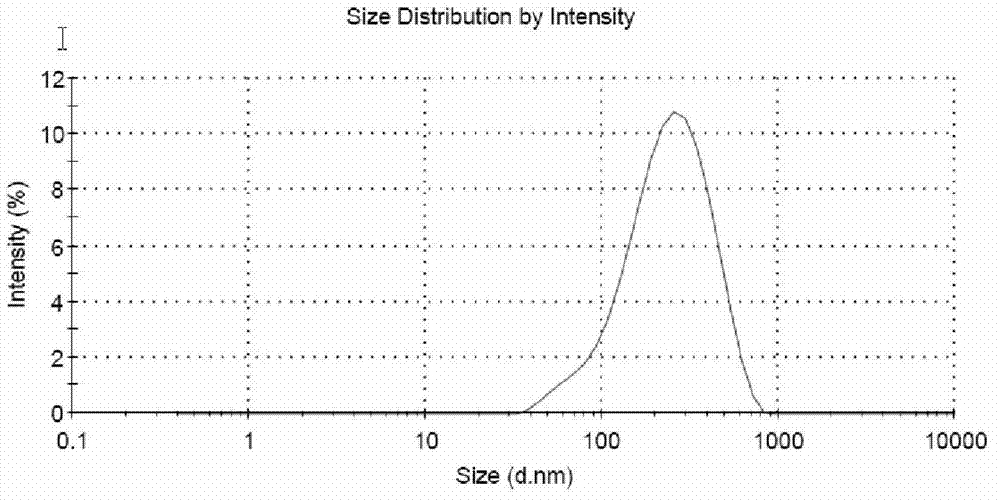
[0065] 600 mg of cabazitaxel was dissolved in 5 ml of absolute ethanol as the organic phase. 90ml of human serum albumin (6%, w / v) aqueous solution is the water phase. The mixture of aqueous and organic phases was homogenized at low speed for 2 minutes To prepare a crude emulsion, the crude emulsion was transferred to a high-pressure homogenizer (Microfulidics), homogenized for 5 cycles under the pressure of 3,000-30,000 psi, and the obtained liquid was transferred to a rotary evaporator, and reduced pressure at 40 °C (10-15KPa) evaporated for 30 minutes to remove absolute ethanol. The average diameter of cabazitaxel particles in the resulting dispersion was greater than 300 nm (Malvern Zetasizer).
Embodiment 2
[0066] Example 2 Preparation of nanoparticles smaller than 200 nm by high pressure homogeneous emulsification
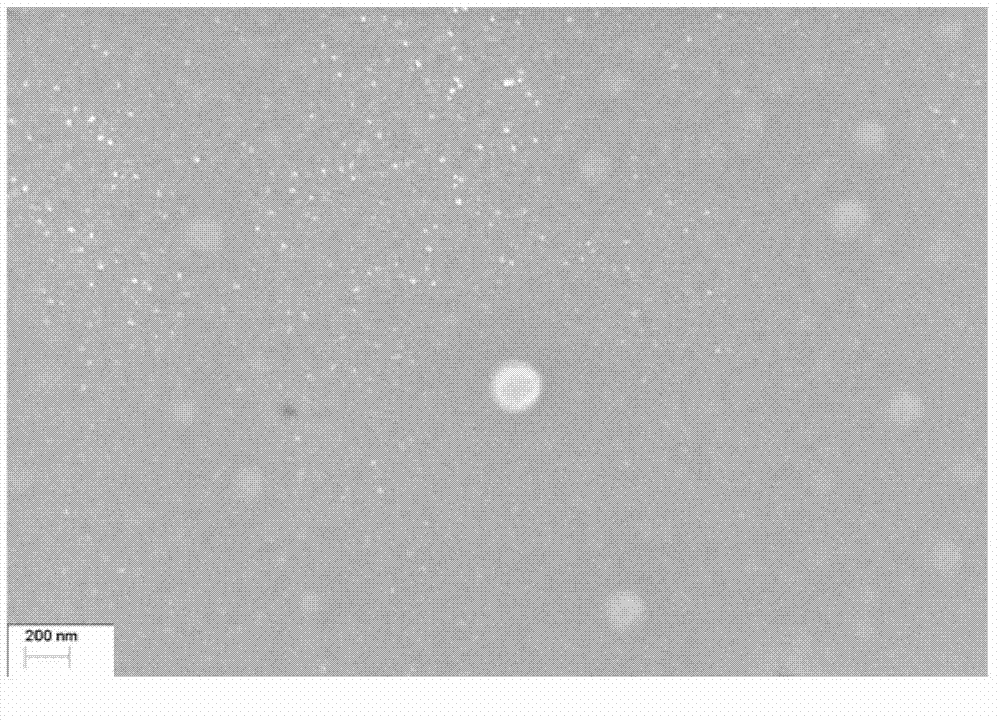
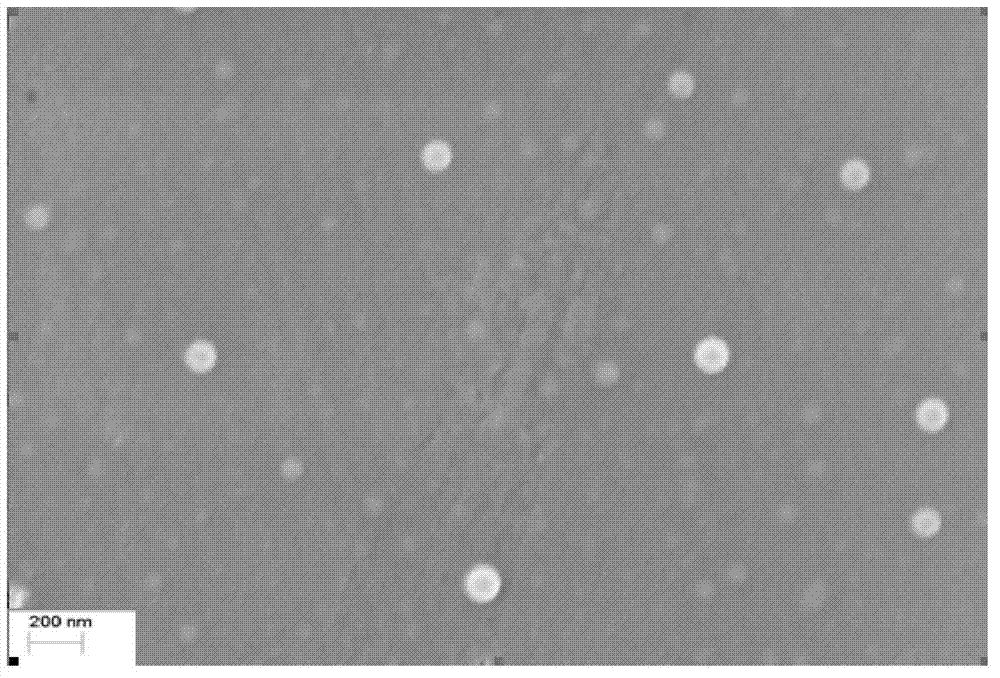
[0067] This example describes the preparation of sterile filterable cabazitaxel particles. 300mg of cabazitaxel was dissolved in 1.35ml of chloroform and 0.15ml of absolute ethanol, the organic phase was added to 28.5ml (9.47%, w / v) of human albumin aqueous solution, and the mixture was heated at low speed. Shear and homogenize for 3 minutes A coarse emulsion was formed, transferred to a high pressure homogenizer (Microfulidics), and homogenized for 7 cycles at a pressure of 3,000-30,000 psi. The obtained medicinal solution was transferred to a rotary evaporator, and evaporated under reduced pressure (10-15KPa) for 30 minutes at 40°C to remove the organic solvent. The obtained dispersion suspension is translucent, the pH is 6.4, the Zeta potential is -26mv, and the average diameter of cabazitaxel particles is generally 120-160nm (Malvern Zetasizer). The dispersion...
Embodiment 3
[0070] Example 3 Nanoparticles smaller than 200 nm that can be sterile filtered by high pressure homogeneous emulsification method
[0071] 300mg of cabazitaxel was dissolved in 1.5ml of chloroform and 0.15ml of absolute ethanol, the organic phase was added to 23.35ml (12.85%, w / v) of human albumin aqueous solution, and the mixture was heated at low speed. Shear and homogenize for 3 minutes A coarse emulsion was formed, transferred to a high pressure homogenizer (Microfulidics), and homogenized for 8 cycles at 3,000-30,000 psi pressure. The obtained medicinal solution was transferred to a rotary evaporator, and evaporated under reduced pressure (10-15KPa) for 30 minutes at 40°C to remove absolute ethanol and chloroform. The obtained dispersion suspension is translucent, the pH is 6.5, the Zeta potential is -25mv, and the average diameter of the cabazitaxel particles is generally 120-160 nm (Malvern Zetasizer). The dispersion suspension was passed through a 0.22 μm microporo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com