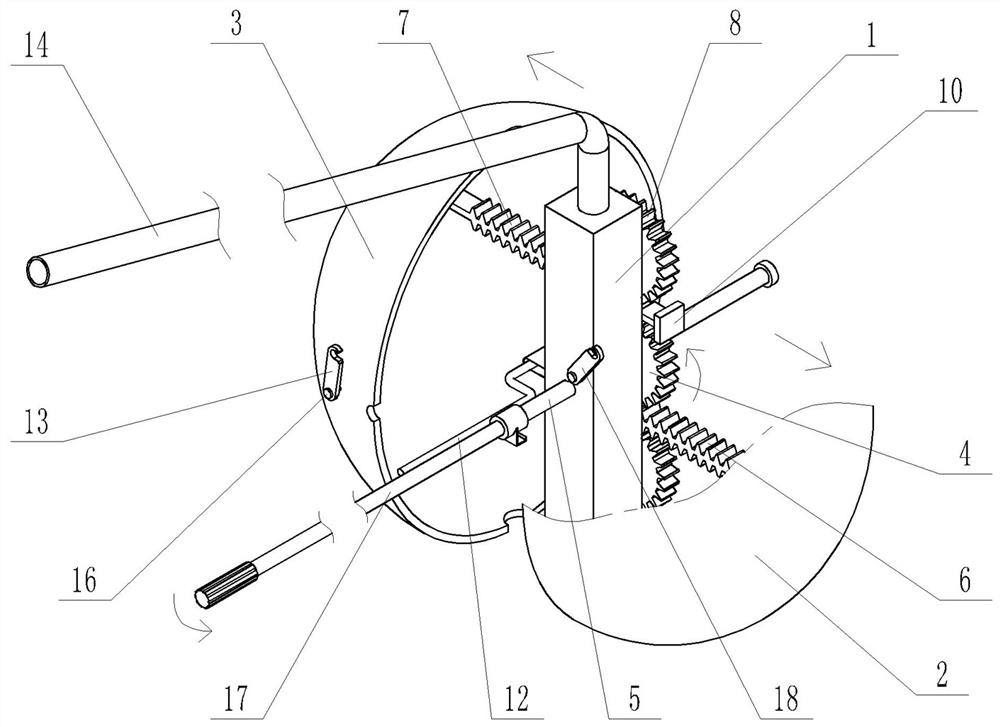
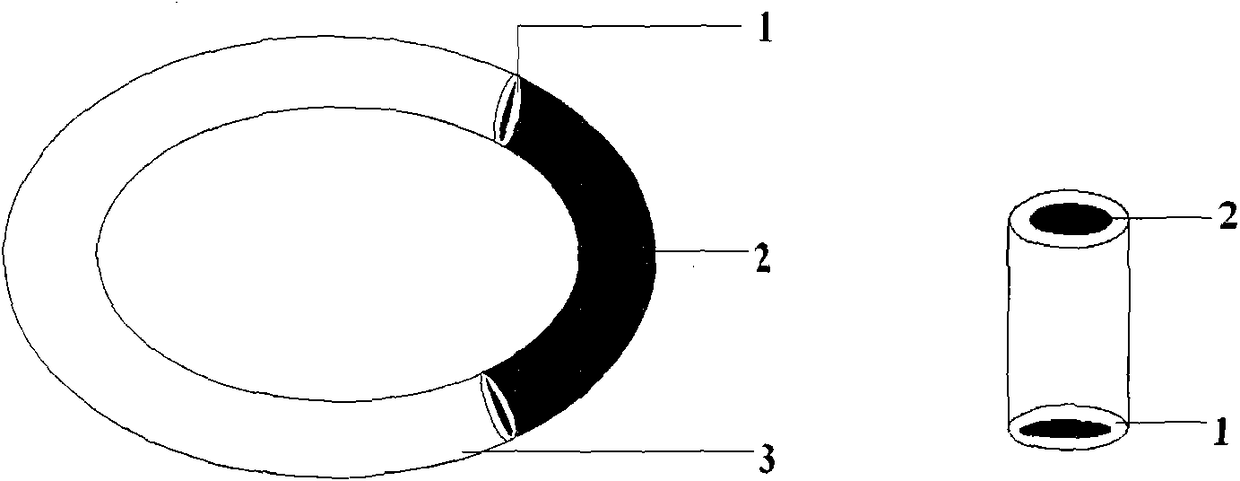
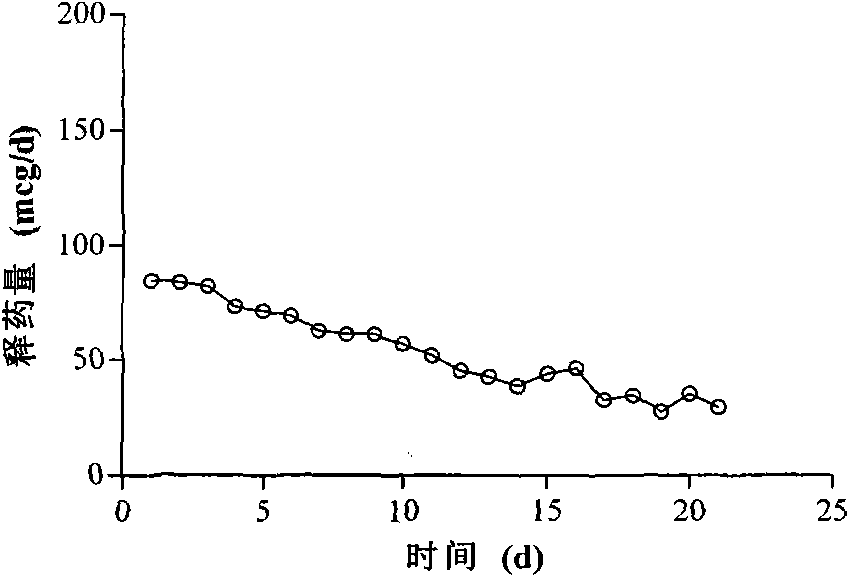
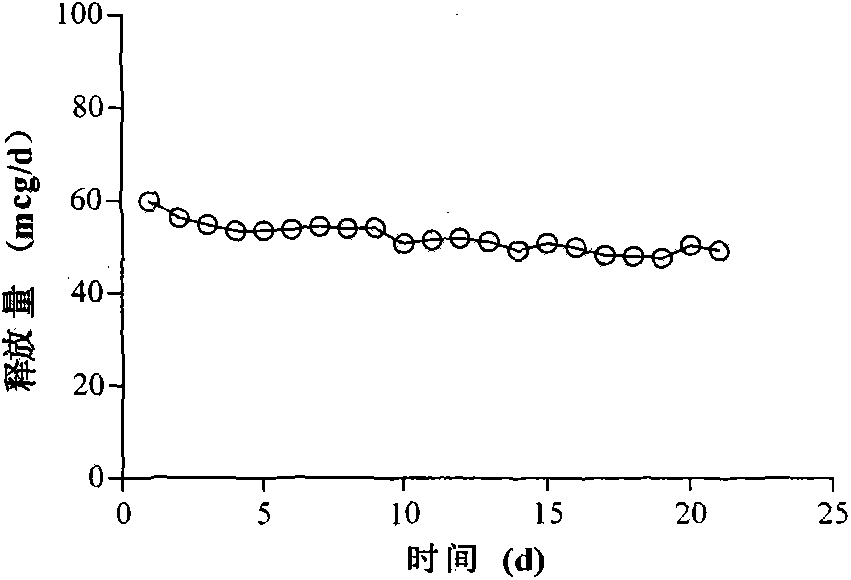
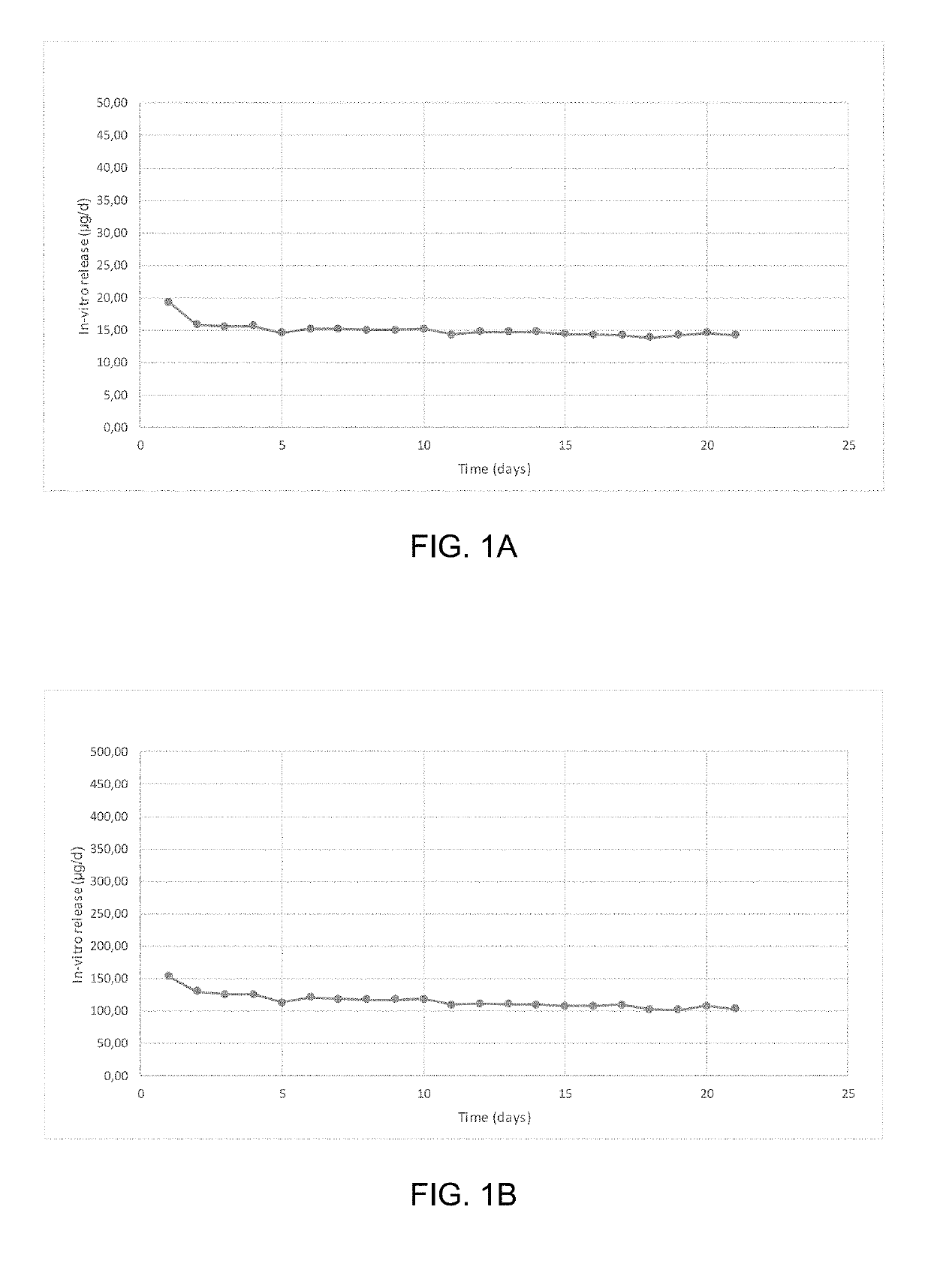
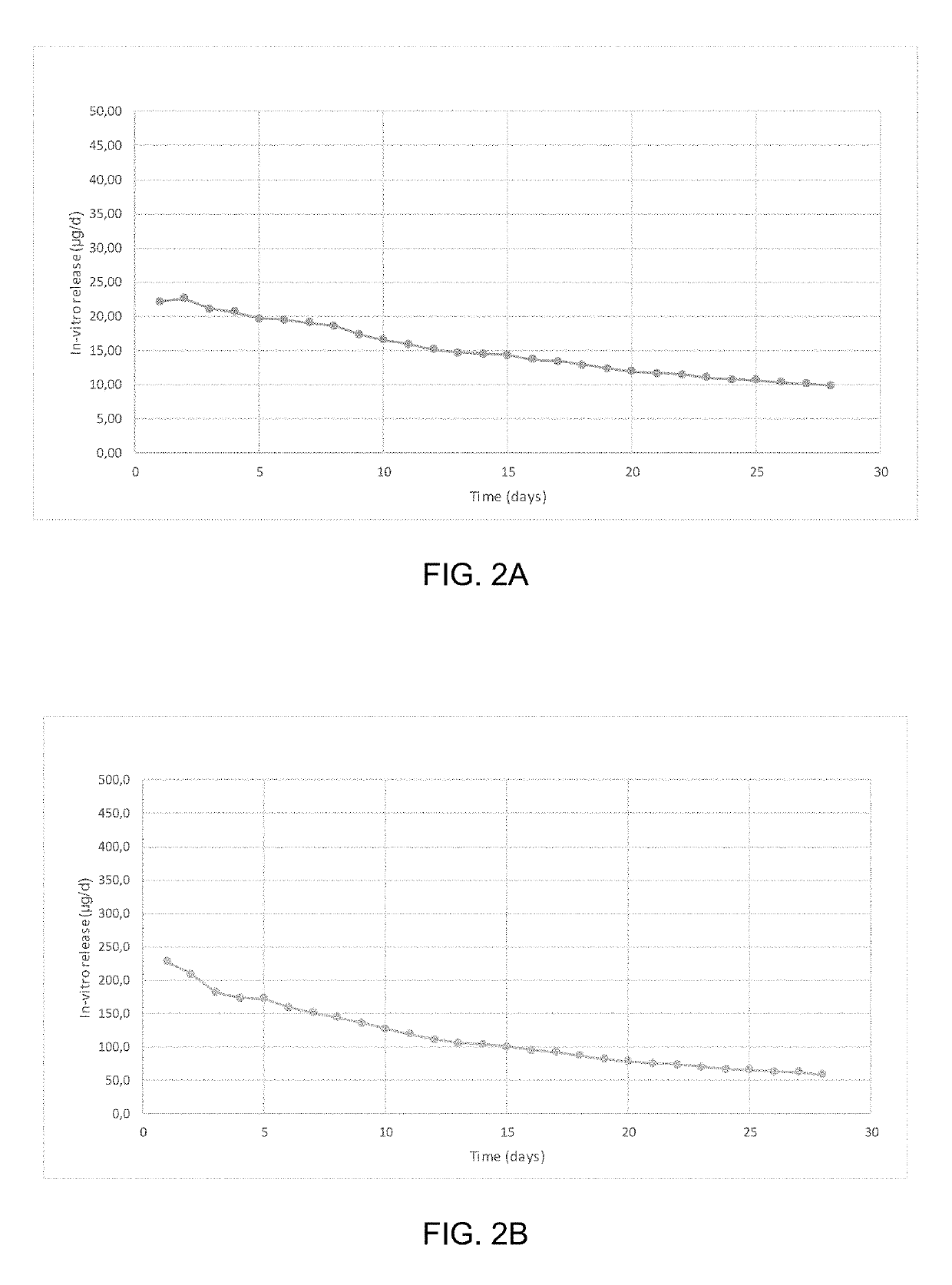
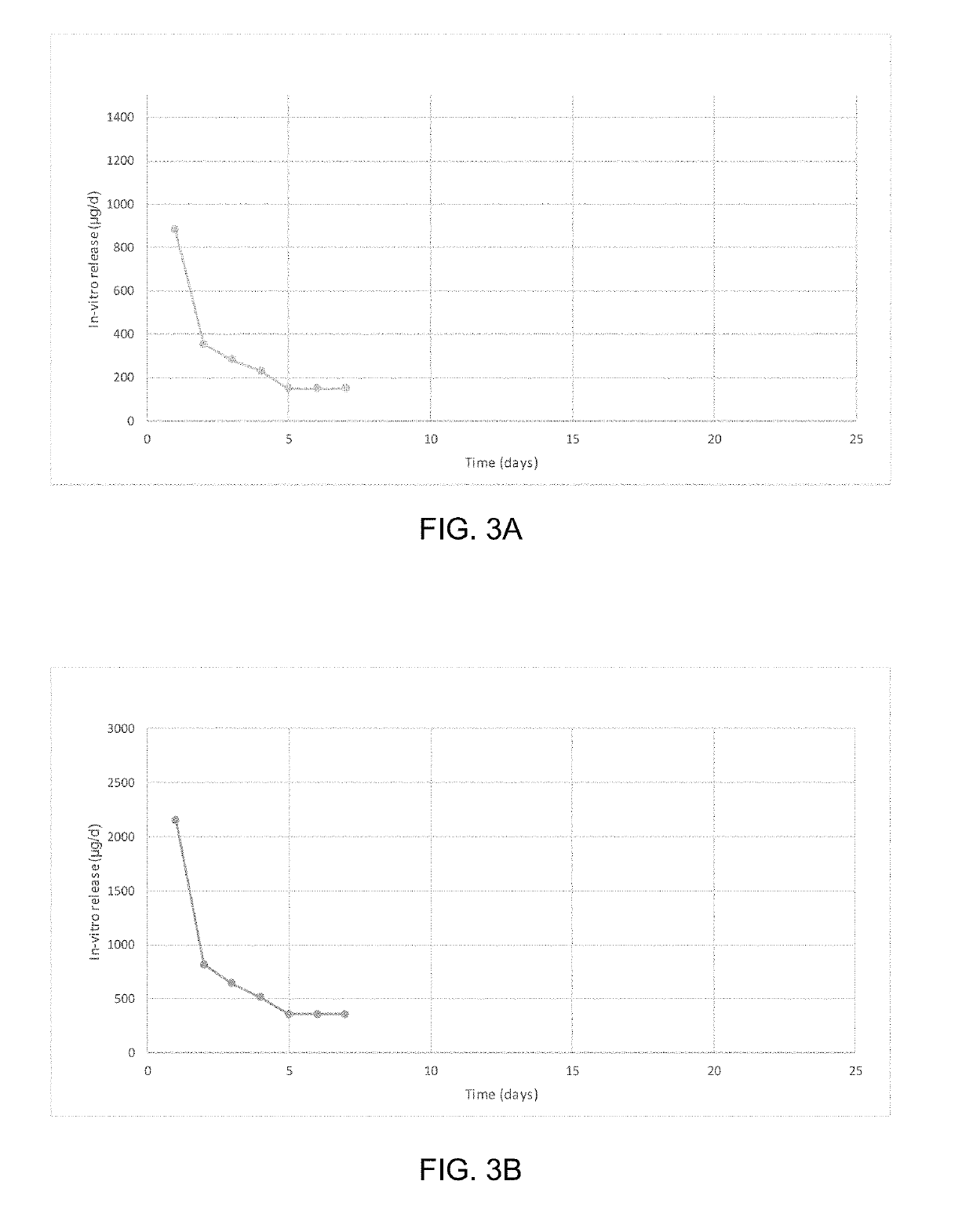
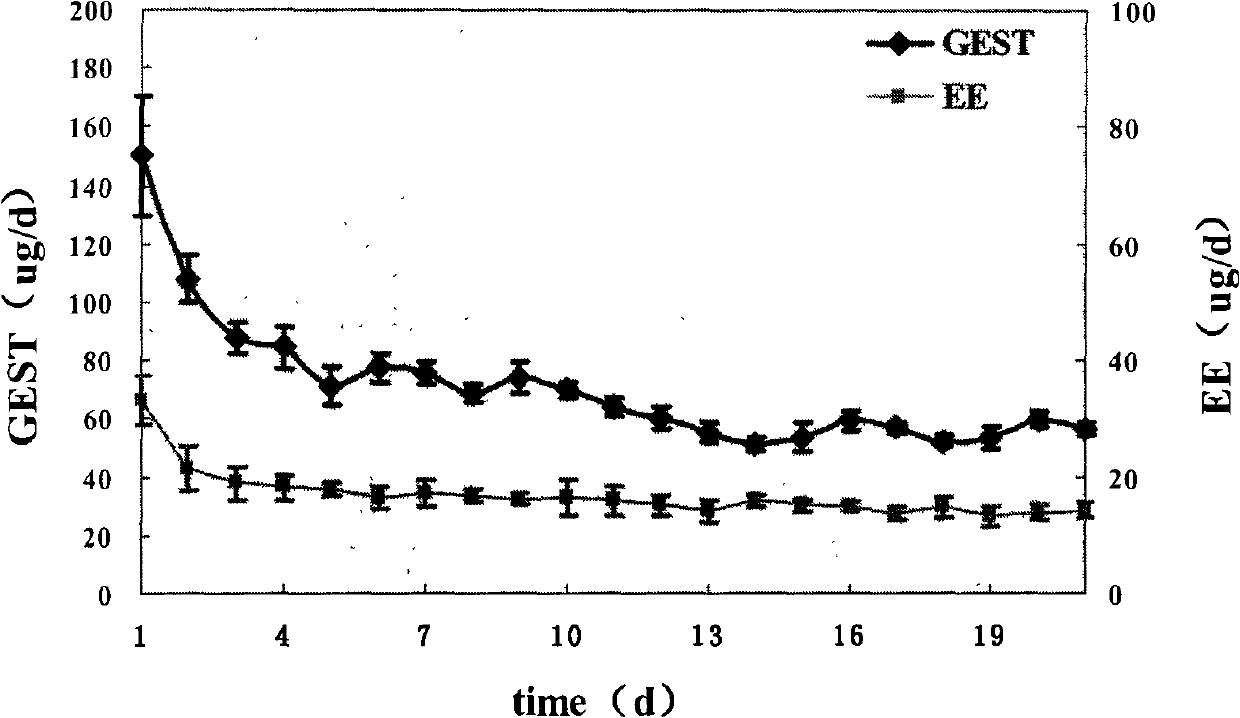
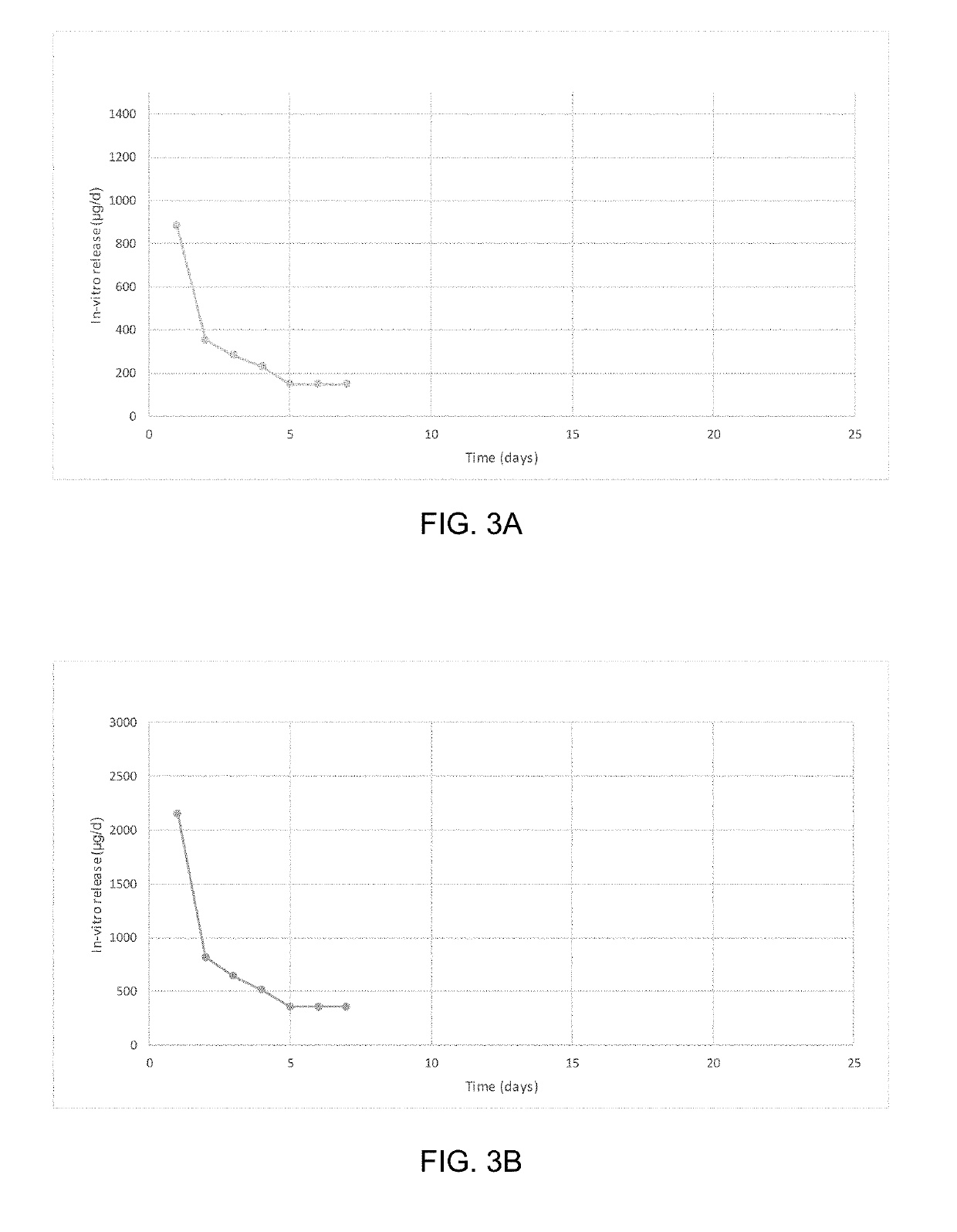
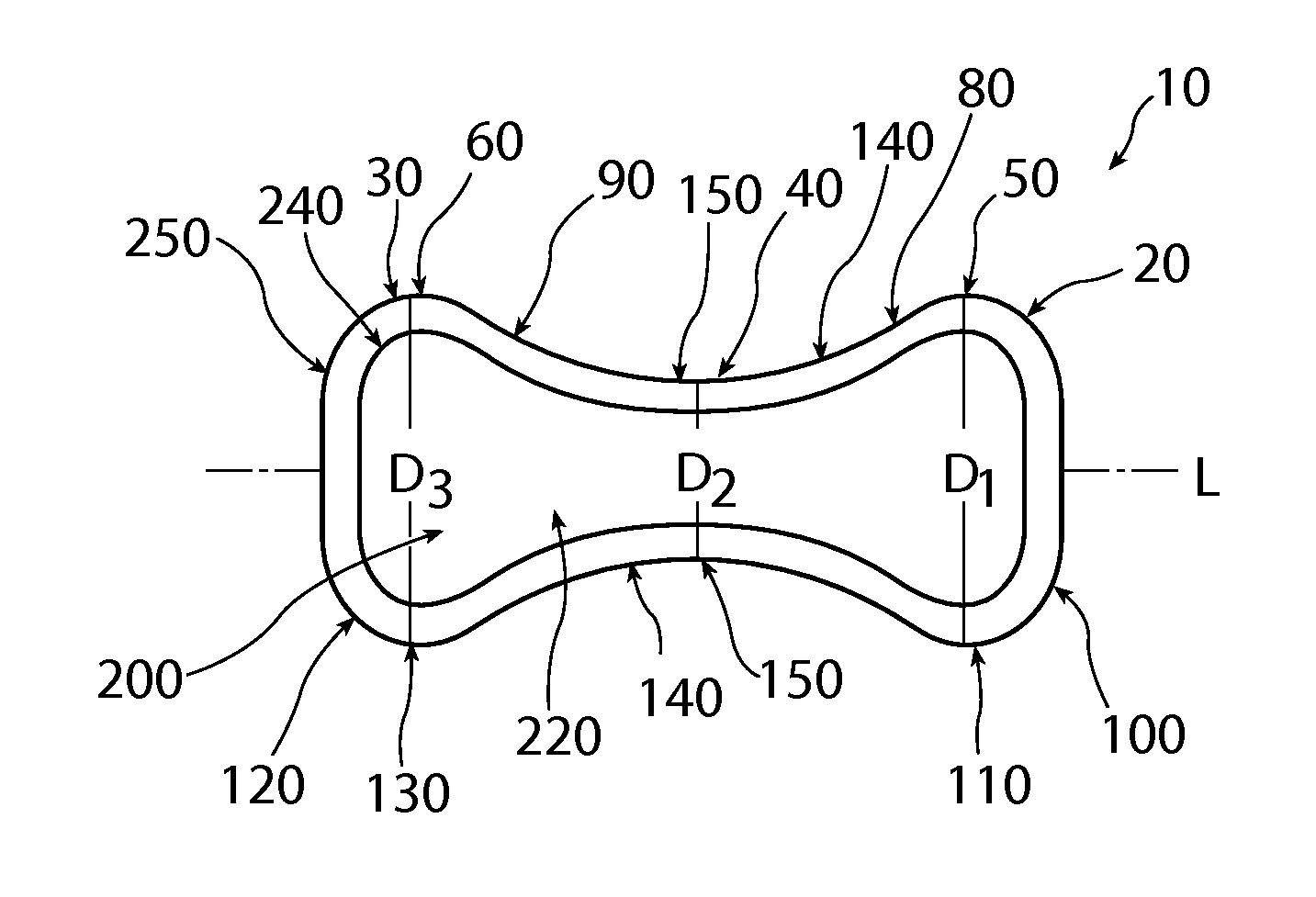
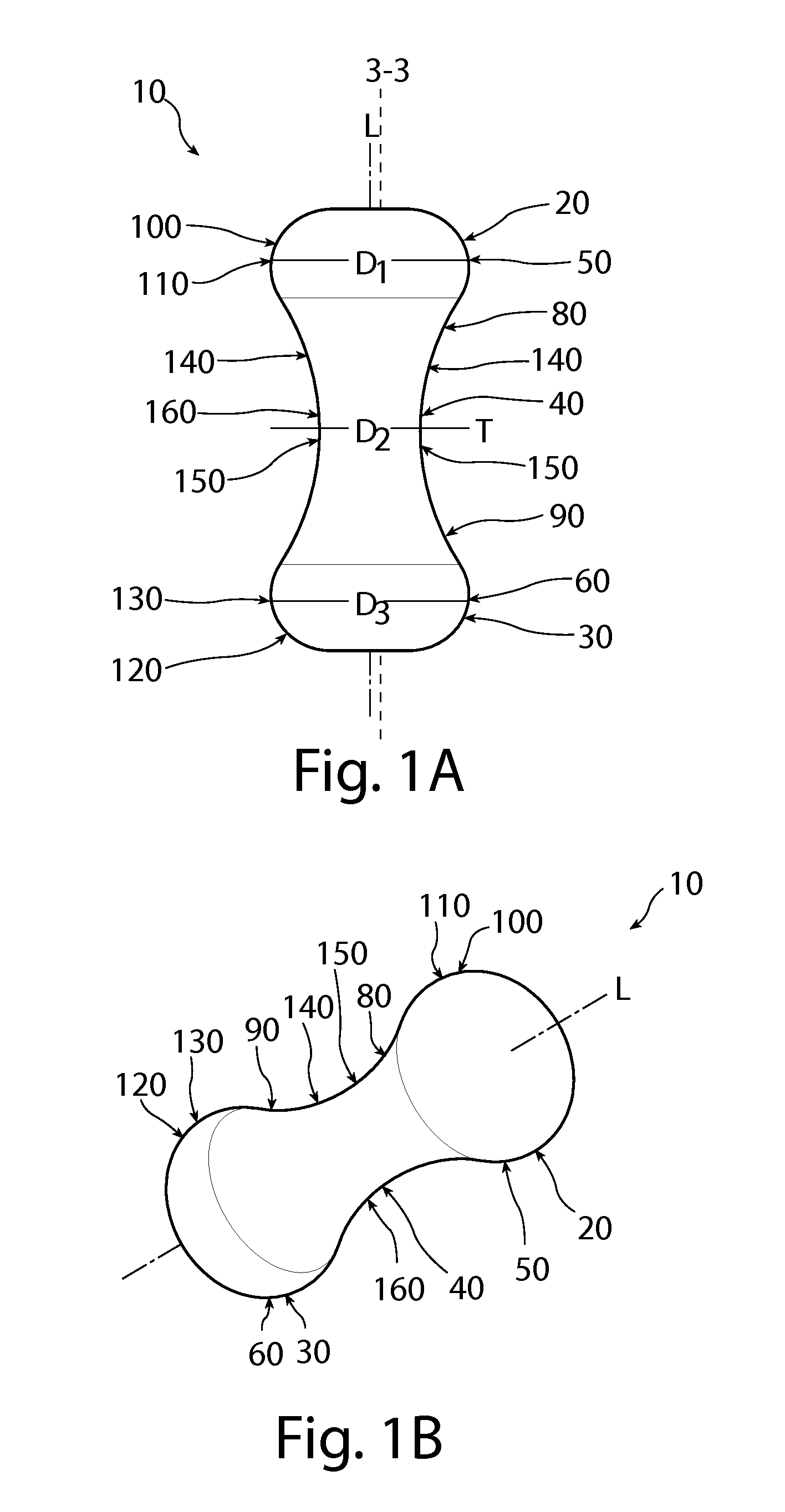
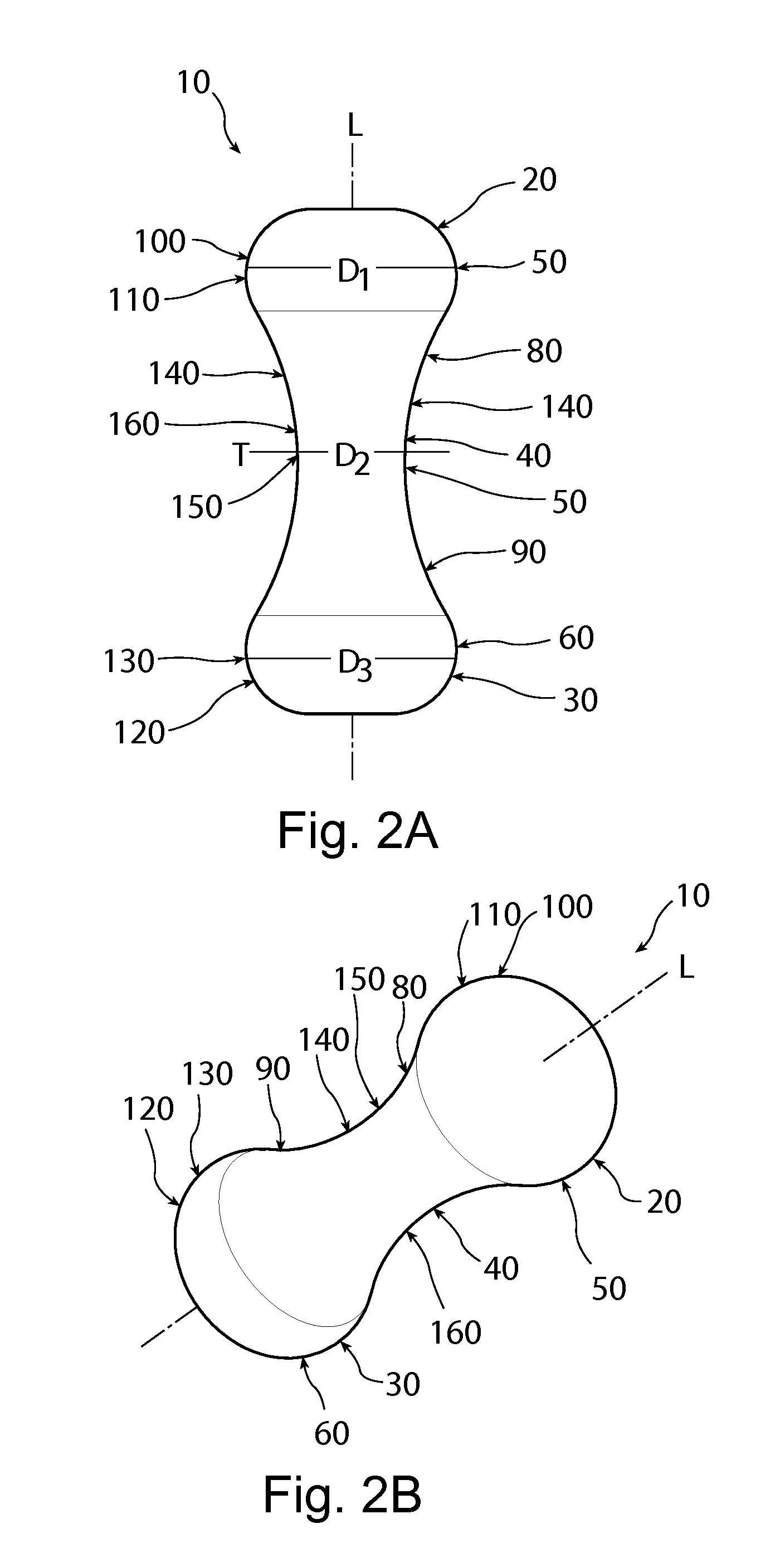
Patents
Literature
51 results about "Vaginal ring" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaginal rings (also known as intravaginal rings, or V-Rings) are polymeric drug delivery devices designed to provide controlled release of drugs for intravaginal administration over extended periods of time. The ring is inserted into the vagina and provides contraception protection. Vaginal rings come in one size that fits most women.
Controlled release compositions of estradiol metabolites
The present invention provides improved sustained release formulations of estradiol metabolites, including 2-hydroxyestradiol, 2-methoxyestradiol, 4-hydroxyestradiol and 4-methoxyestradiol, useful for therapeutic treatments. The invention also provides methods of producing sustained release forms of estradiol metabolites. The compositions of the present invention include microparticles, nanoparticles, patches, crystals, gels, rods, stints, pallets, discs, lozenges, wafers, capsules, films, microcapsules nanocapsules, hydrogels, liposomes, implants and vaginal rings. Compositions also include formulations for transdermal and intravenous delivery of estradiol metabolites. The present invention provides numerous improvements over previous forms of estradiol metabolites, such advantages including the sustained release of normally short half-life compounds to maintain therapeutic blood levels.
Owner:PR PHARMA
Slow-release mifepristone vaginal ring preparation and application thereof
InactiveCN102600001APromote dissolutionImprove bioavailabilityOrganic active ingredientsFemale contraceptivesPharmacyHepatic first pass effect
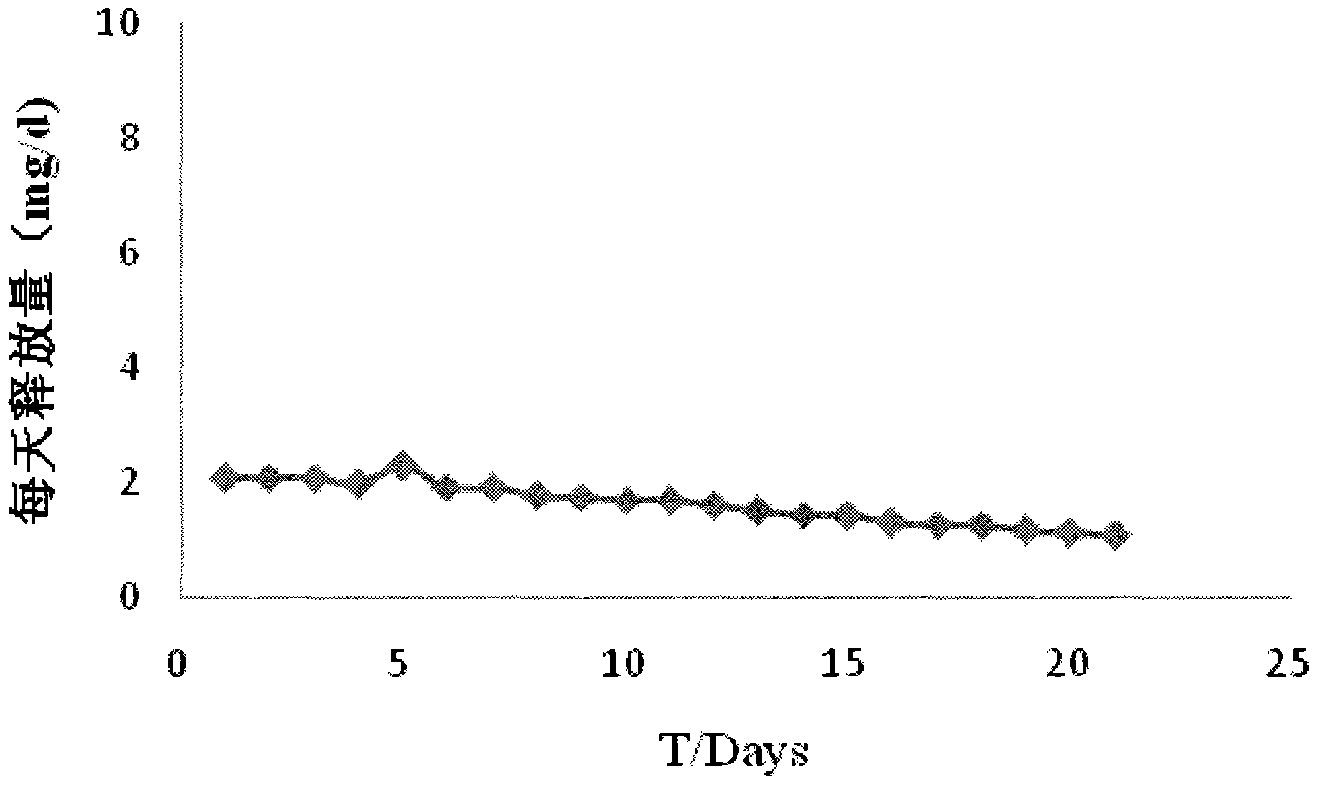
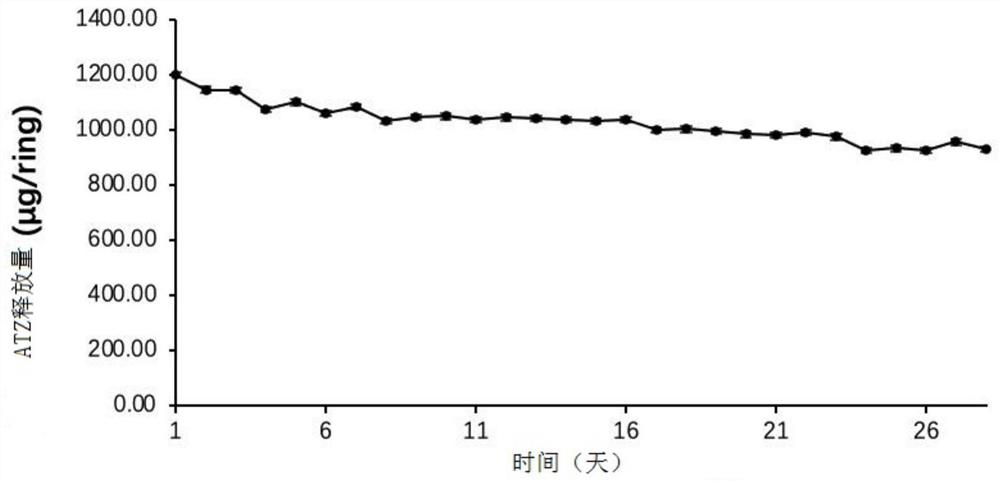
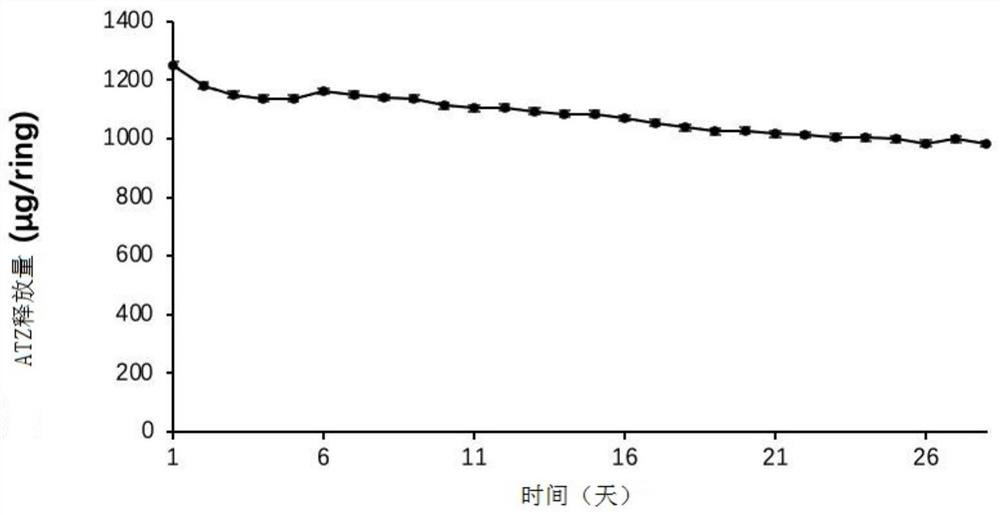
The invention relates to a preparation method for two types of slow-release mifepristone vaginal ring preparations. The first type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant and from 62 to 97.45wt% of medical high polymer release control materials. The second type of slow-release mifepristone vaginal ring preparation includes from 1.0 to 4.5 wt% of mifepristone, from 1.5 to 13.5 wt% of drug scattered carrier, from 0.05 to 20 wt% of surfactant, from 62 to 97.45wt% of medical high polymer release control materials, and a thin release control membrane covered on the outside of each slow-release mifepristone vaginal ring preparation. Due to the preparation method, a slow-release mifepristone vaginal ring can slowly release the mifepristone within seven days, a first pass effect and gastrointestinal reaction of mifepristone orally taken drug are avoided, bioavailability of the drug and pharmacy compliance of a patient are improved.
Owner:NAT RES INST FOR FAMILY PLANNING
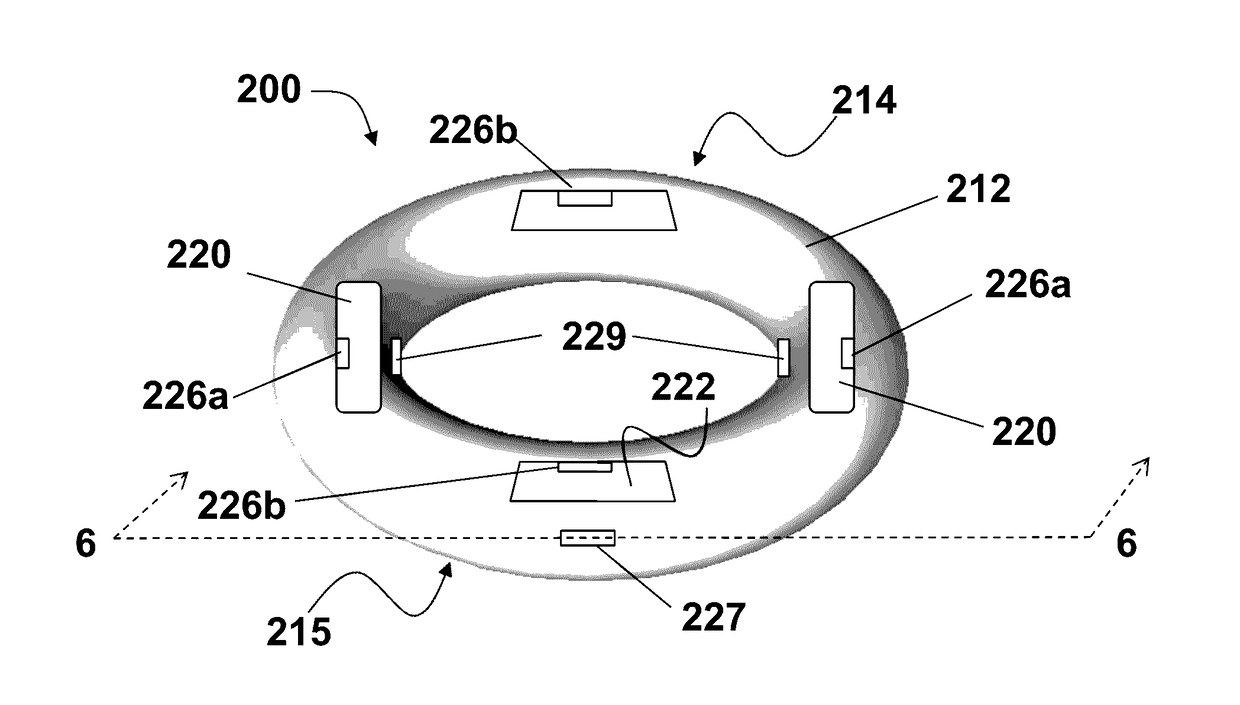
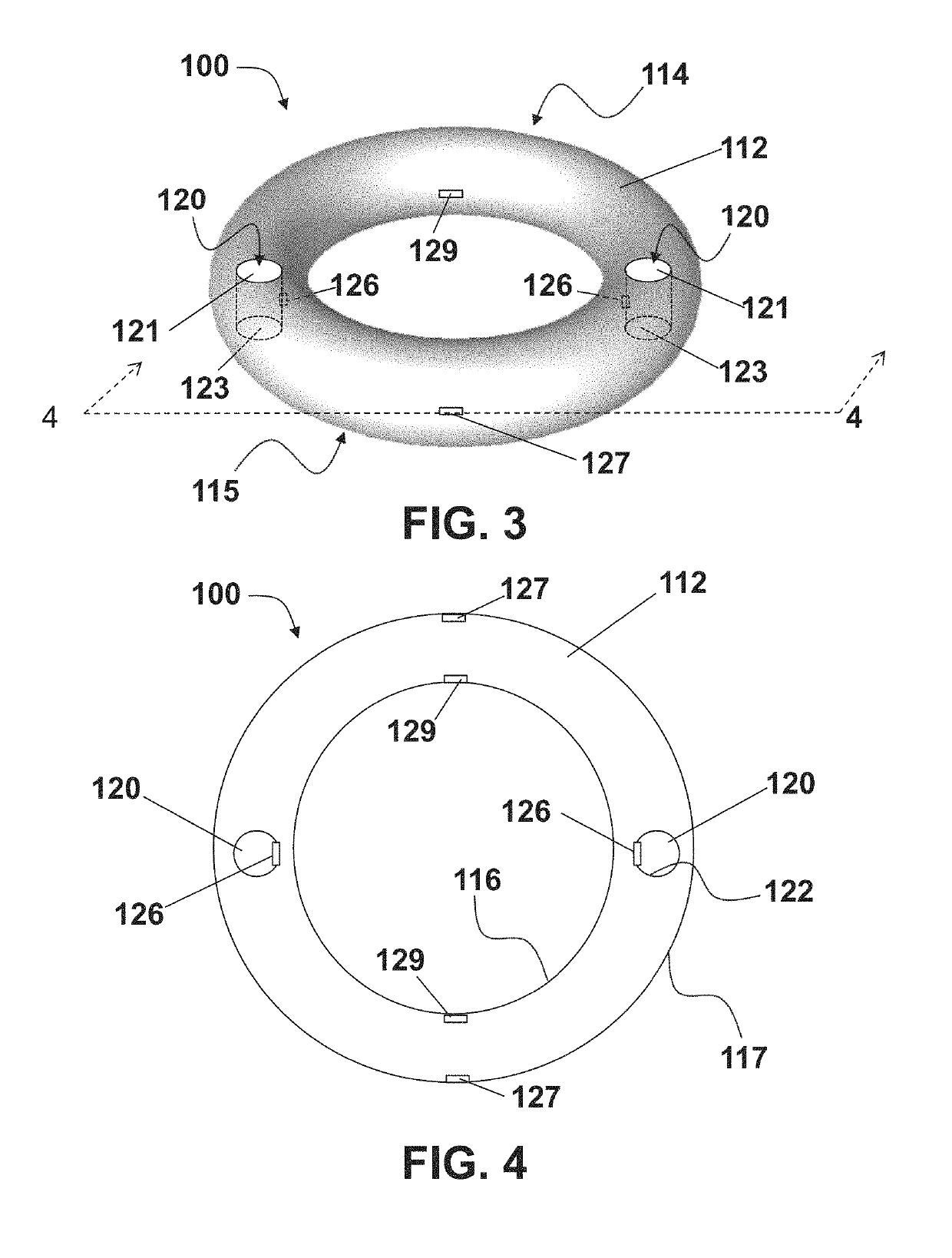
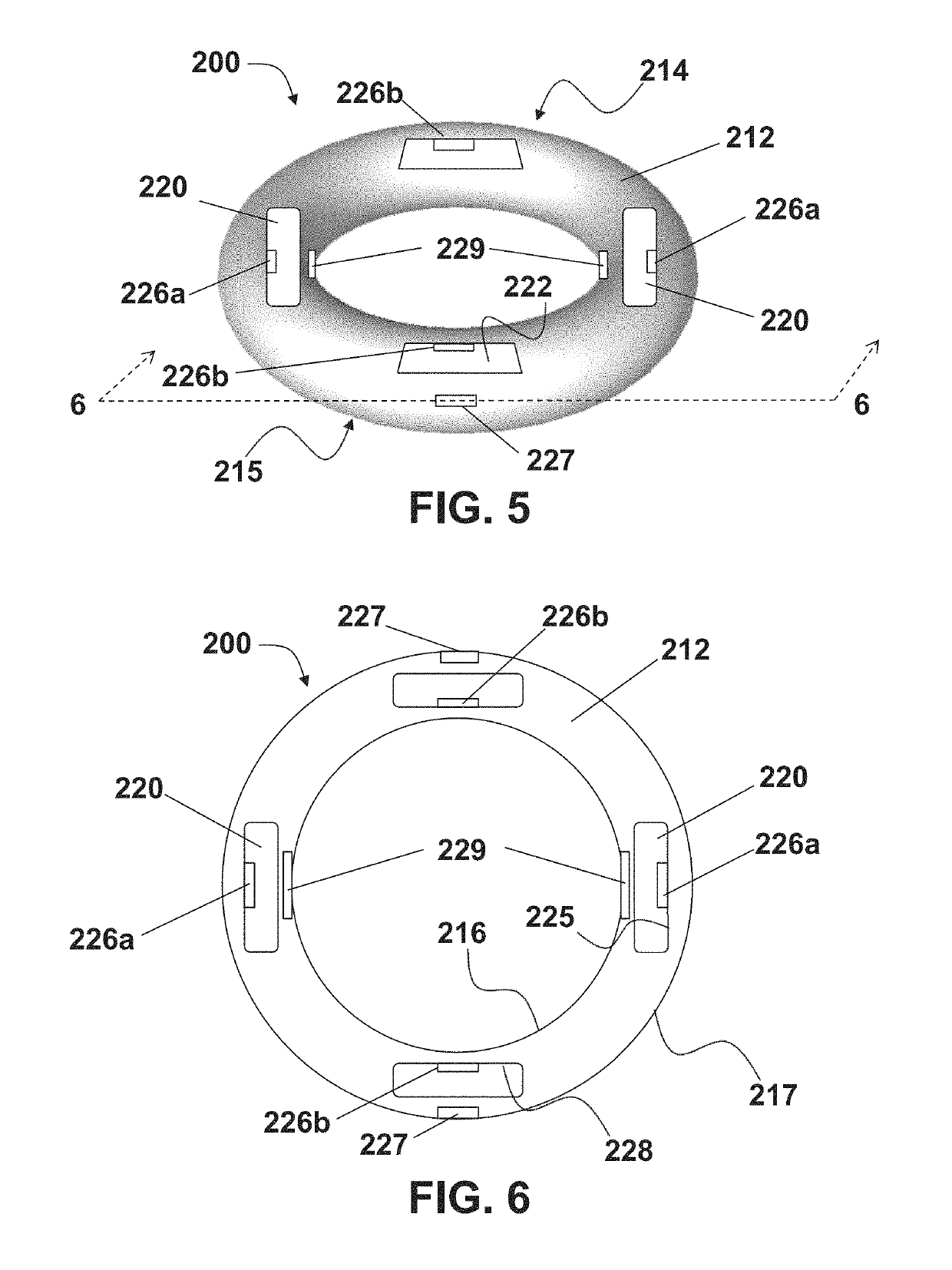
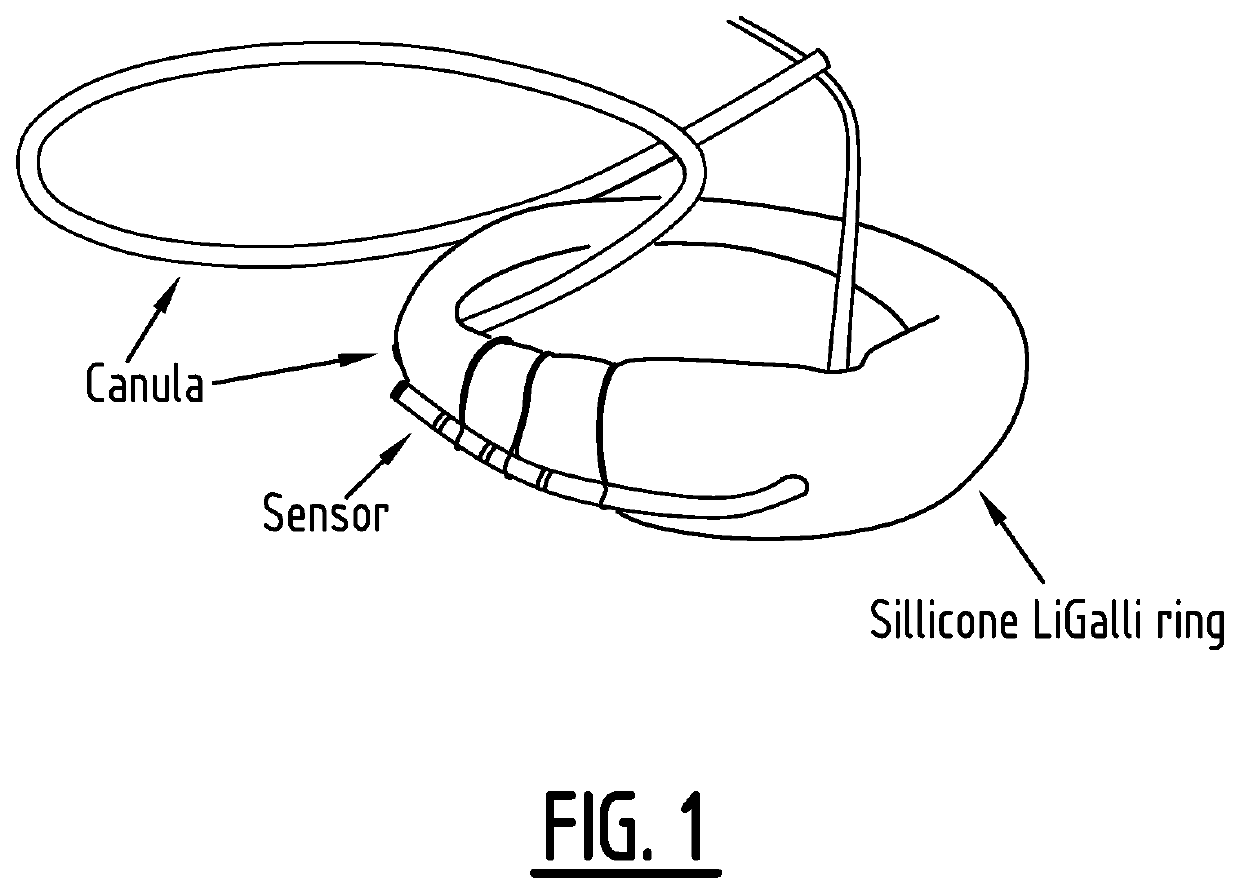
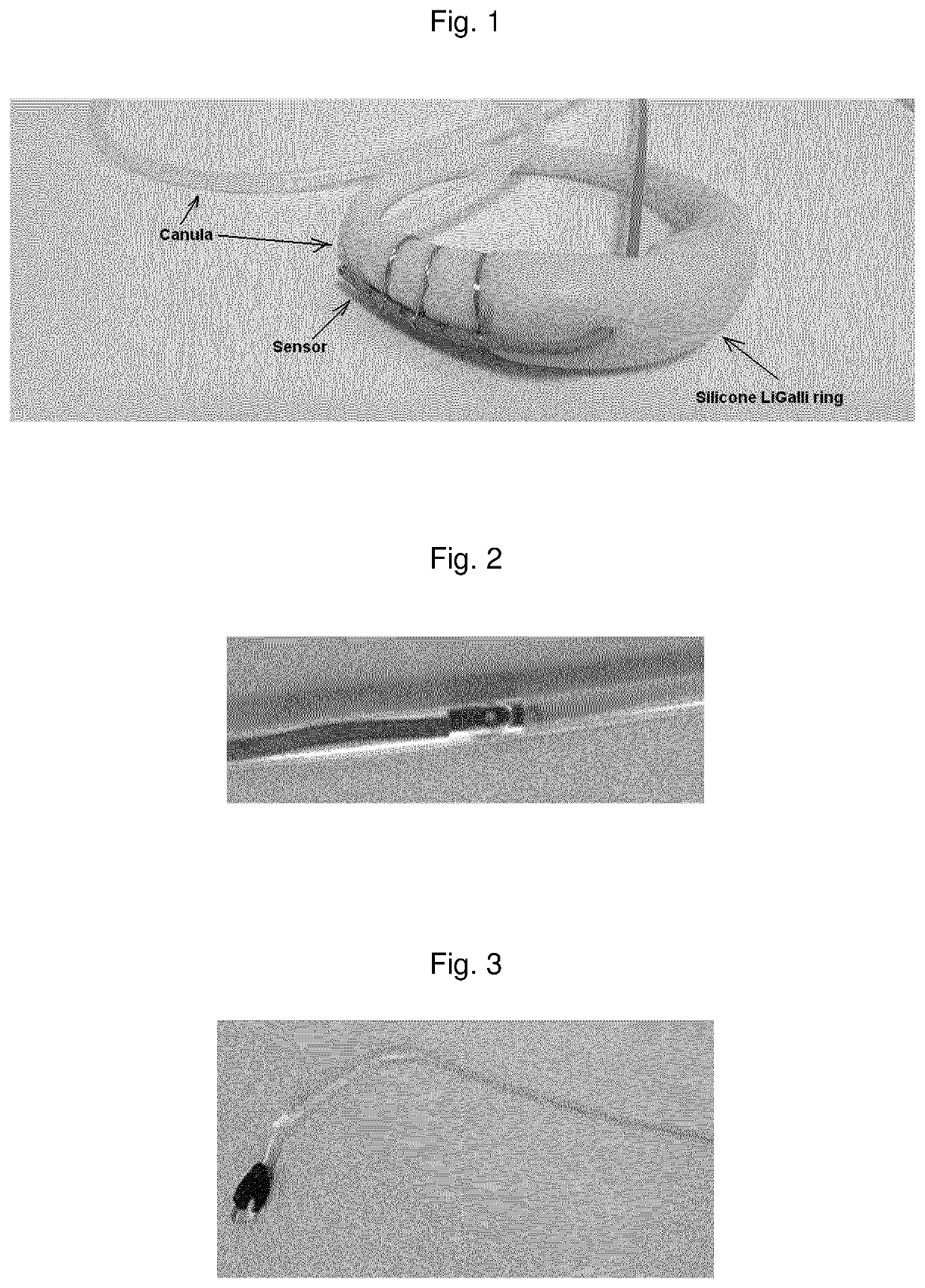
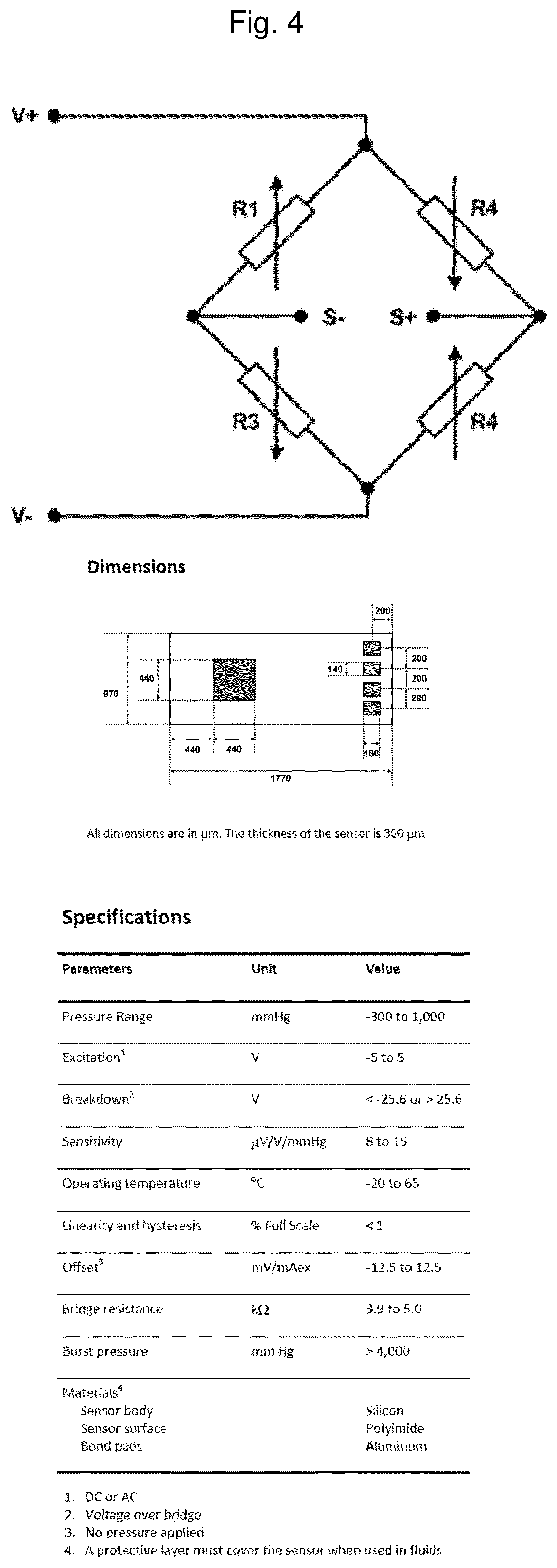
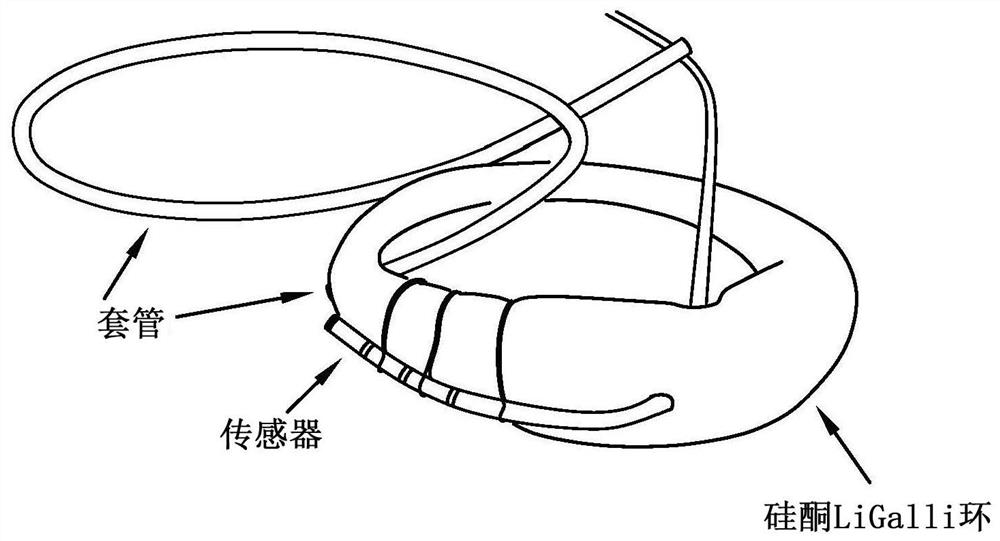
Vaginal ring sensor
A vaginal ring sensor device adapted to be placed within the vaginal vault of a user, the device including a ring body, at least one through hole that passes through the ring body, and at least one biosensor structured and arranged to sense and / or measure a parameter of vaginal fluid as such fluid passes through the at least one through hole.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
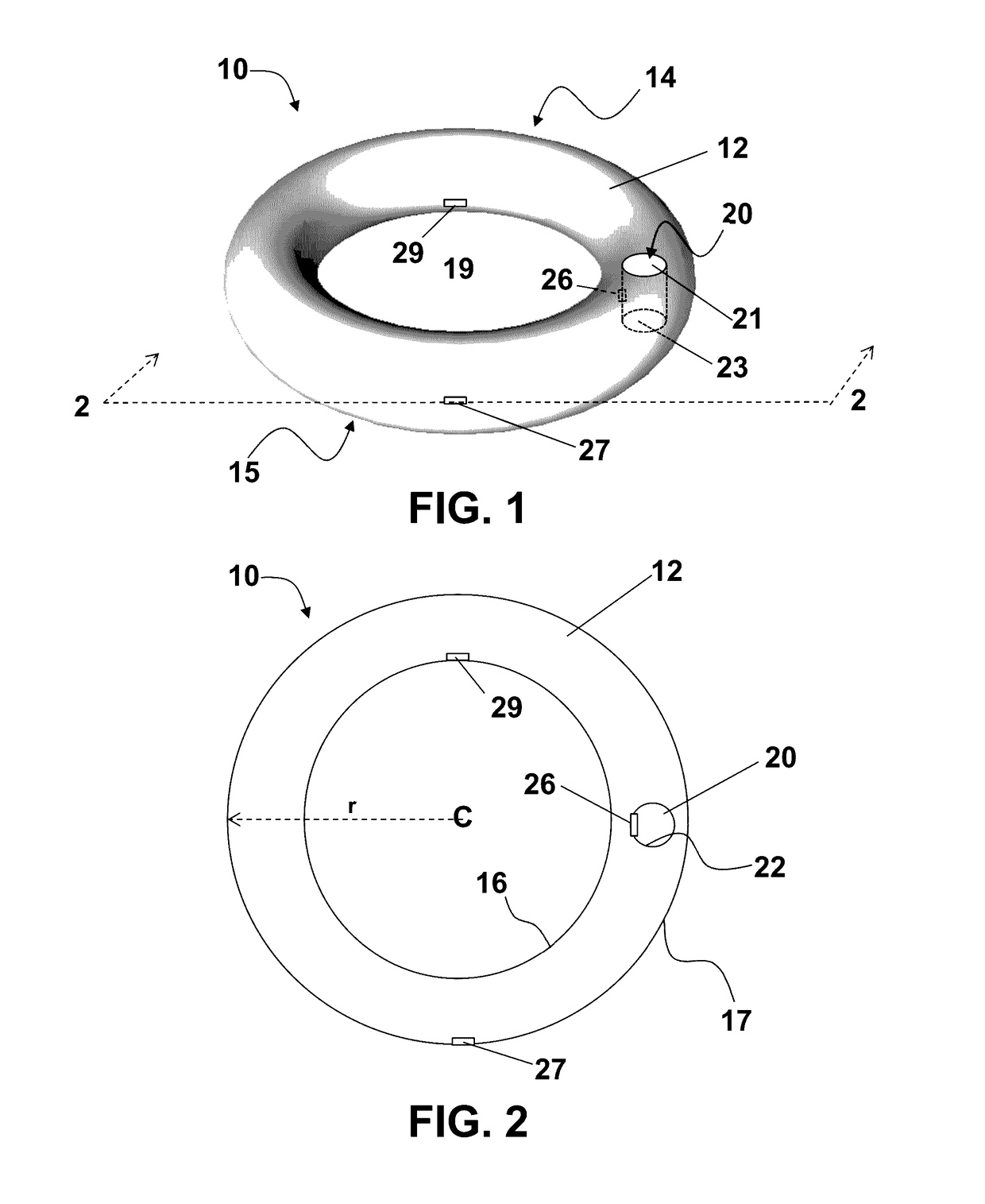
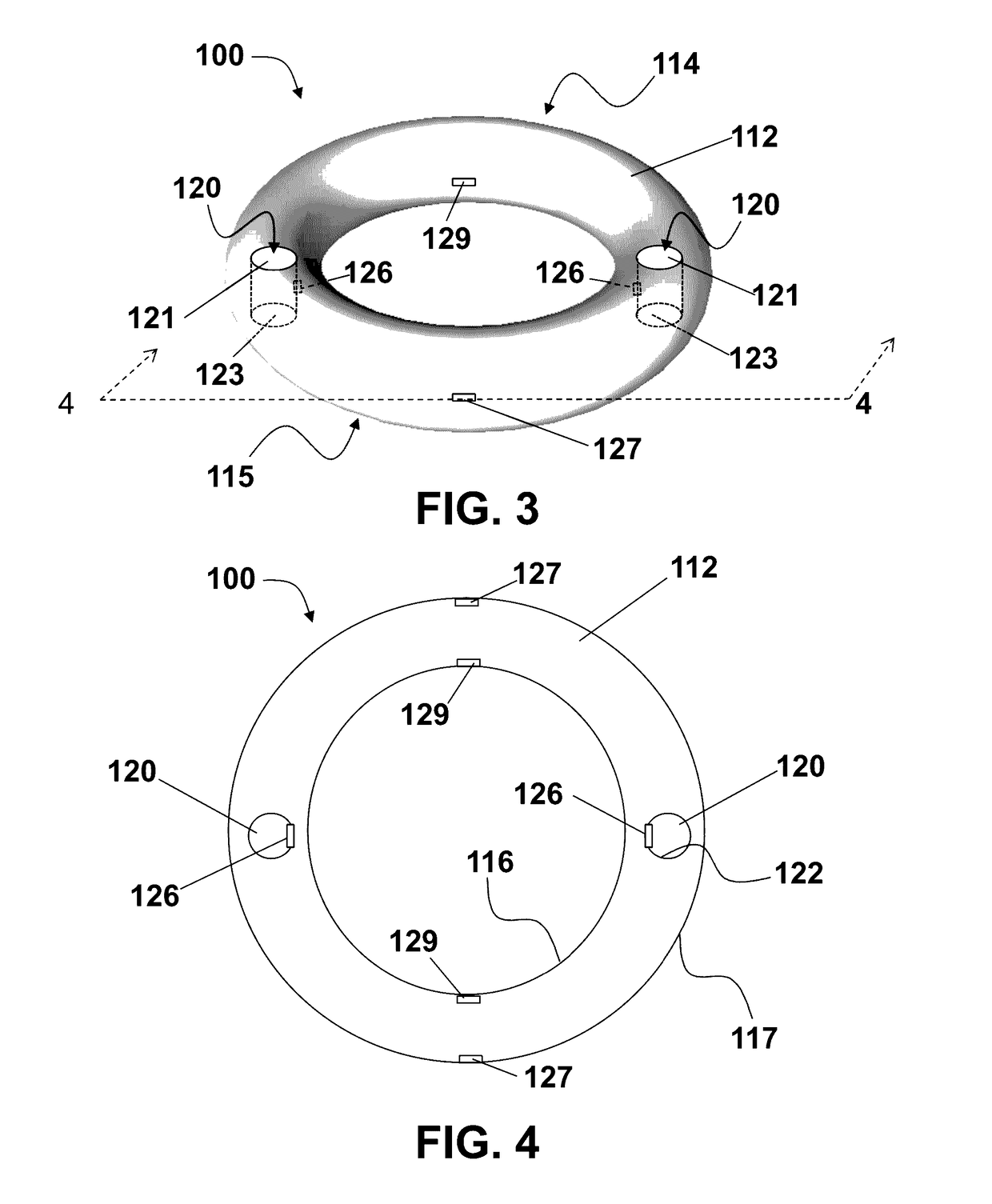
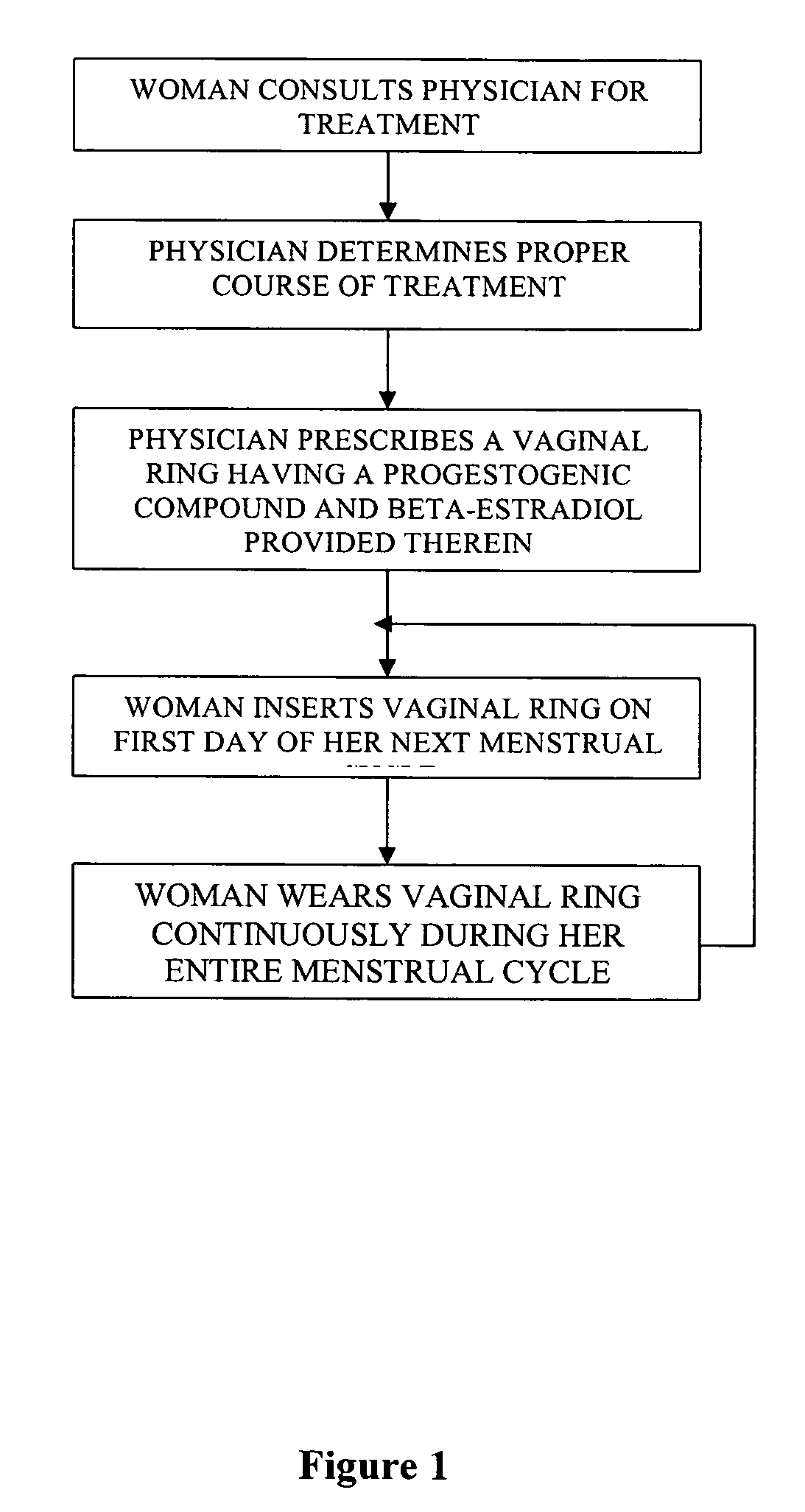
Method of birth control and hormone regulation
InactiveUS20070077269A1Avoid problemsReduce doseOrganic active ingredientsBiocideObstetricsSexual hormones
A method of birth control and hormone regulation comprises utilizing a vaginal ring worn continuously during each menstrual cycle. A vaginal ring delivers predetermined doses of progesterone and beta-estradiol. Beta-estradiol does not cause an increase in sex hormone binding globulin and thereby does not decrease a woman's levels of free testosterone and libido.
Owner:LES MEDECINS
Mifepristone shell-type vaginal ring preparation and application
InactiveCN103505802AImprove solubilityReduce solubilityOrganic active ingredientsSurgeryPhysiologyGYNECOLOGIC DISORDERS
The invention relates to mifepristone shell-type vaginal ring preparation and application. A miferprisone shell-type vaginal ring comprises, by weight, 1.0-6.0% of mifepristone, 0.5-3.0% of medicine dispersion carriers, 0.3-2.0% of additives and 89.0-98.2% of medical polymer controlled release materials. Firstly, a solid dispersion technology is adopted for improving the solubility in water and release degree in vitro of the mifepristone, then a method of mold pressing vulcanization forming is adopted for preparing the mifepristone shell-type vaginal ring, the inner layer is a vacancy silicon rubber skeleton layer, the middle is a medicated layer, and the outmost layer is a controlled release film. By the implementation of the mifepristone shell-type vaginal ring preparation and application, the vaginal ring can release the mifepristone within 21 days at almost constant speed slowly and continuously for a long time, so that the medicine can be released more stably, the first-pass effect and gastrointestinal reaction caused by oral medication of the mifepriston are avoided, and the biological availability of the medicine and medication compliance of a patient are improved. The vaginal ring can be used for routine contraception and treating gynecological diseases such as the endometriosis and the uterine fibroid.
Owner:NAT RES INST FOR FAMILY PLANNING
Device and method for treatment of dysmenorrhea
InactiveCN1263454AIncrease drug concentrationReduce first pass metabolismSuppositories deliveryMedical devicesHigh concentrationMuscle contraction
Methods, devices, and compositions for treatment of dysmenorrhea comprise an intravaginal drug delivery system containing an appropriate pharmaceutical agent incorporated into a pharmaceutically acceptable carrier whereby the pharmaceutical agent is released into the vagina and absorbed through the vaginal mucosa to provide relief of dysmenorrhea. The drug delivery system can be a tampon device (42), vaginal ring, pessary, tablet, suppository, vaginal sponge, bioadhesive tablet, bioadhesive microparticle, cream lotion, foam, ointment, paste solution, or gel. The system delivers a higher concentration to the muscle of the uterus, the primary site for the dyskinetic muscle contraction, which is the pathophysiologic cause of dysmenorrhea.
Owner:UNIVERSITY OF MINNESOTA DULUTH
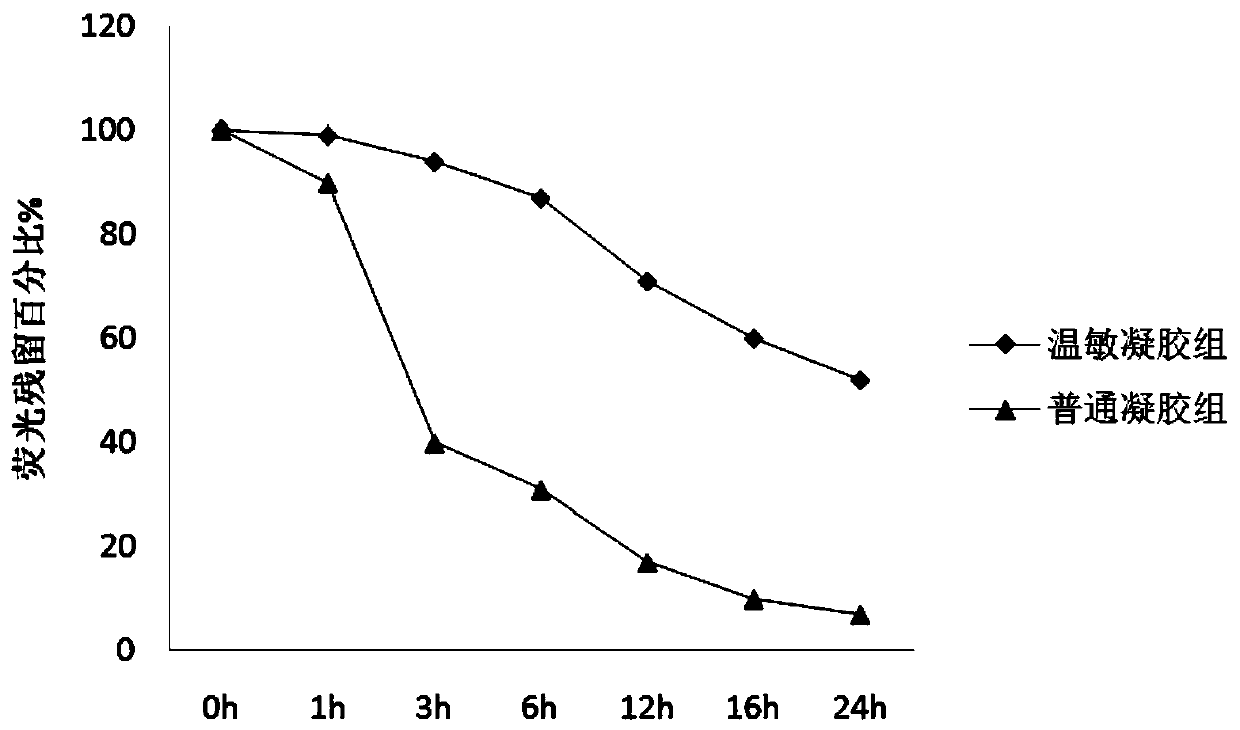
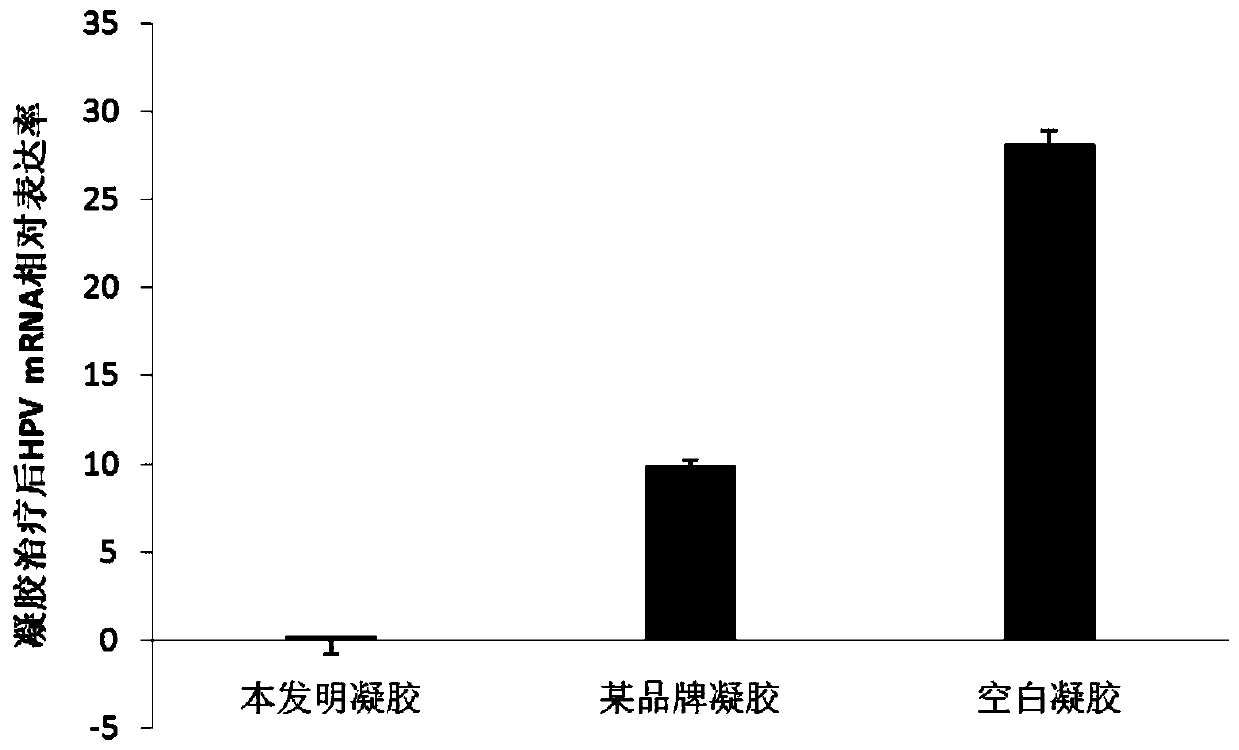
Anti-HPV type vaginal temperature-sensitive gel, preparation method and application thereof.
InactiveCN110743003AGood killing effectEasy to useAntibacterial agentsOrganic active ingredientsDiseaseHuman papillomavirus
The invention provides anti-HPV type vaginal temperature-sensitive gel, a preparation method and application thereof. The temperature-sensitive gel includes poloxamer, chitosan, chlorhexidine gluconate, giant salamander oligosaccharide peptides, recombinant human interferons and cell growth factors at the specific ratio, and on this basis, antiviral drugs, antibacterial drugs, antimycotic drugs, antifungal drugs, antimycoplasma drugs and other functional components can be further added. The temperature-sensitive gel is a sustained release preparation with a pH value consistent with the vaginalenvironment, when the temperature is lower than 28 DEG C, the temperature-sensitive gel is in a liquid state, can penetrate into deep folds of the uterus and the vagina and kill pathogens, gel is formed after making contact with the body temperature for 3-5 minutes to cover the disease part, and is not prone to overflowing, and action time and action concentration of the drug and the drug receiving part are guaranteed to the maximum extent; meanwhile, the gel can block the protein synthesis of human papillomavirus (HPV) E6 and E7, and inhibit the proliferation of HPV; and meanwhile, high bactericidal effects on gram-positive bacteria, gram-negative bacteria and fungi are achieved.
Owner:刘志鹏
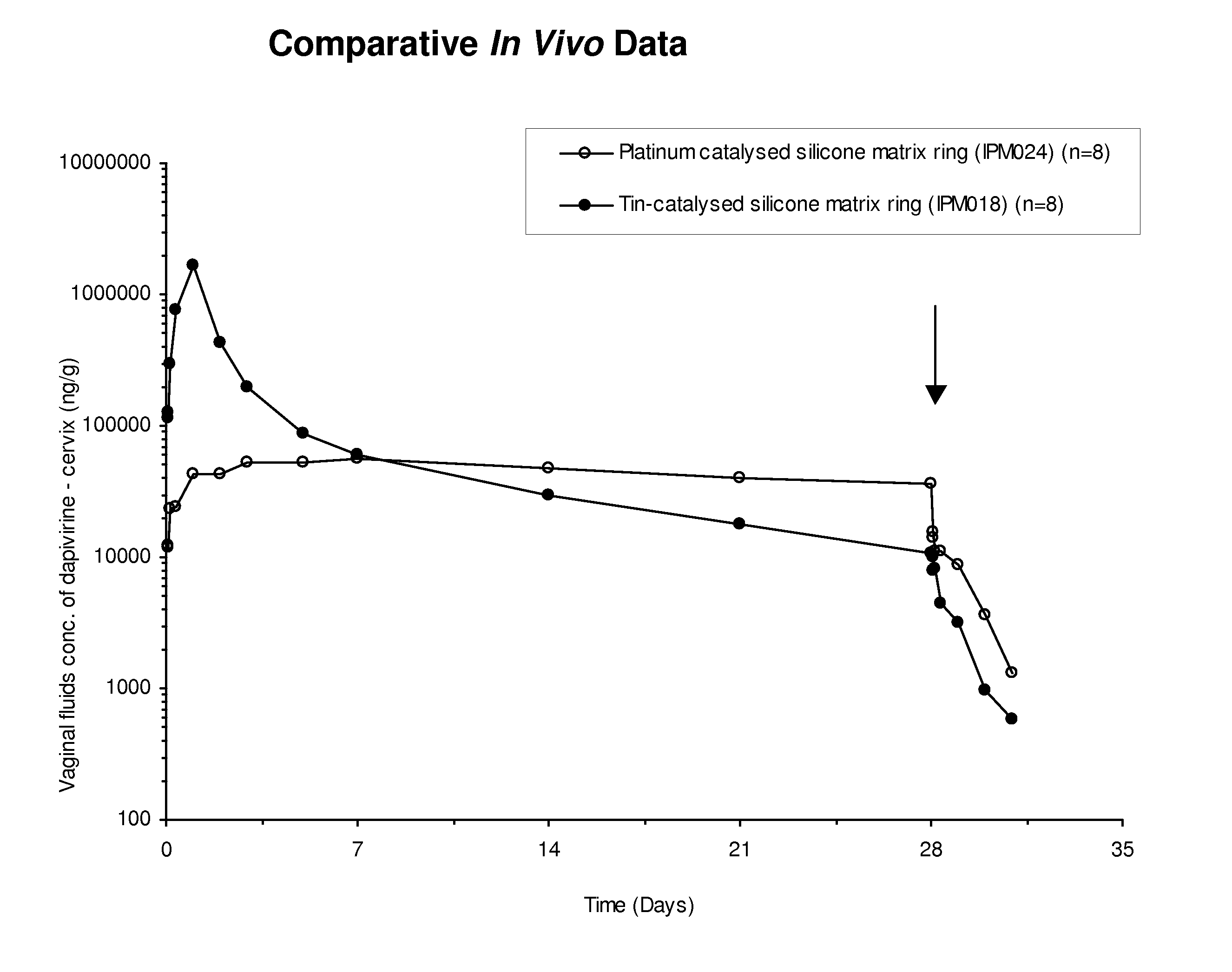
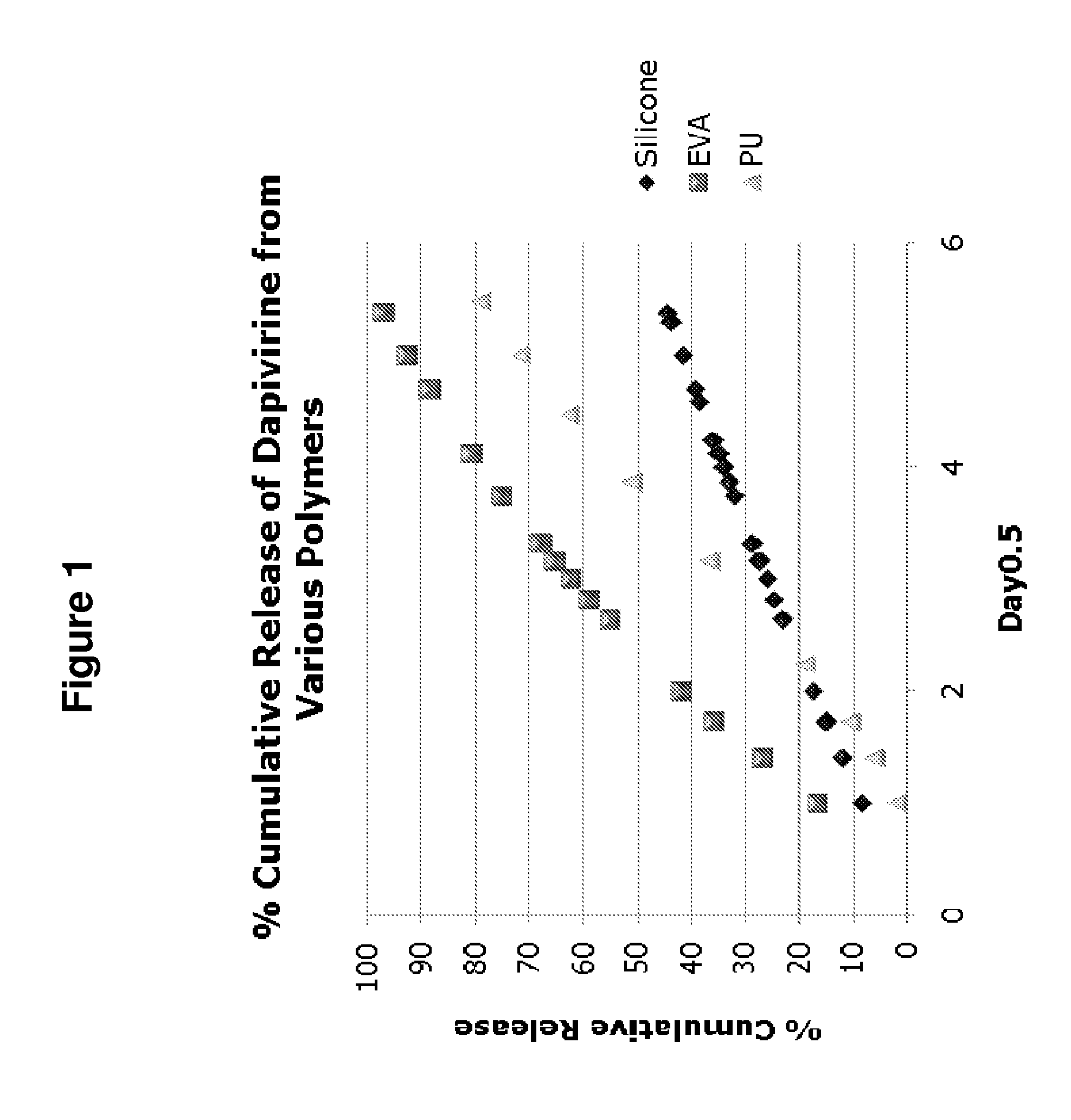
Platinum-catalyzed intravaginal rings
ActiveUS8580294B2High and steady release rateEasy and less-expensive to manufactureBiocideFemale contraceptivesReverse transcriptaseDrug loading dose
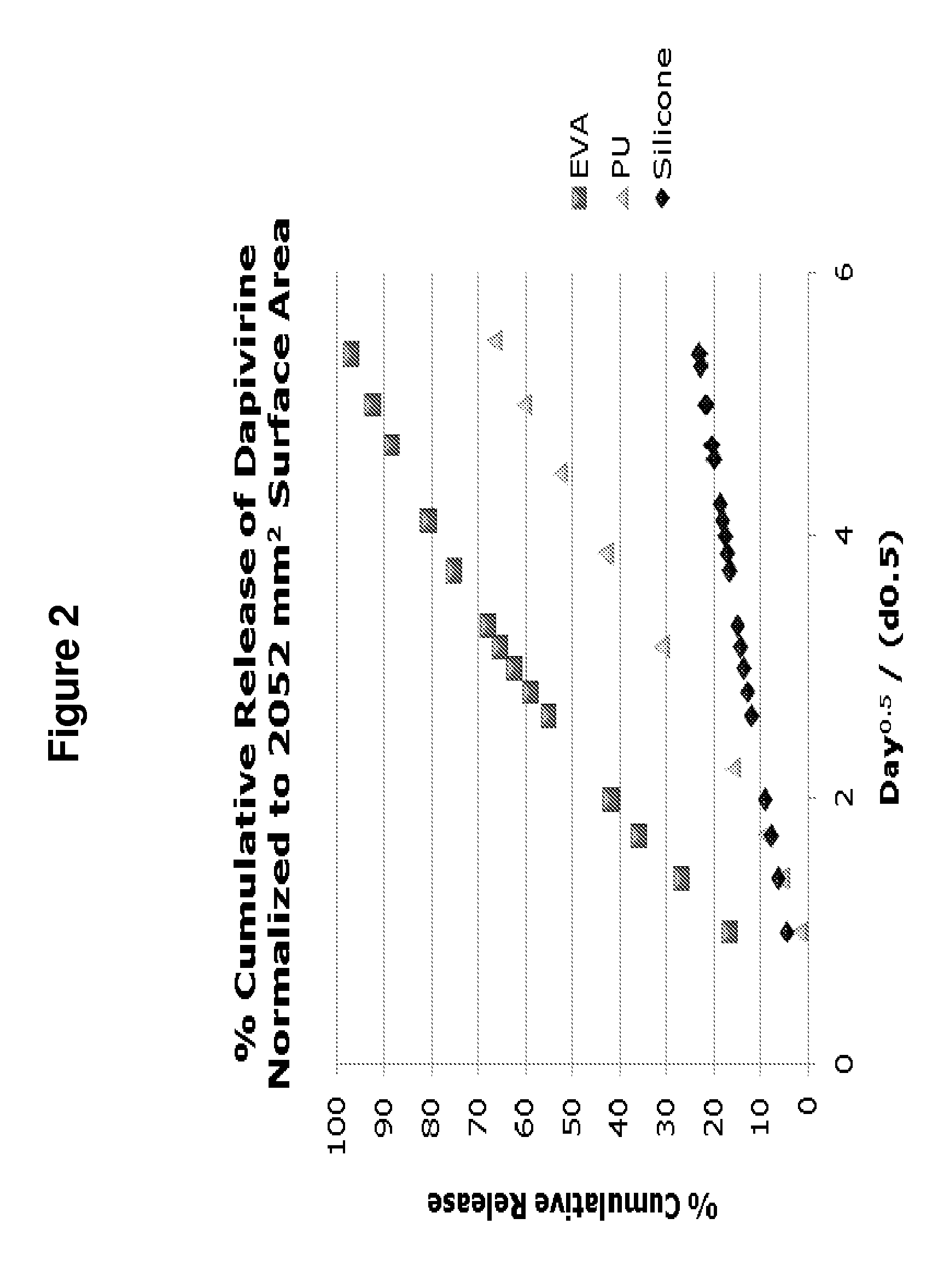
The present invention provides improved intravaginal drug delivery devices, i.e., intravaginal rings, useful for the prophylactic administration of an antimicrobial compound, e.g., Dapivirine, to a human. The intravaginal rings of the invention address previous stability issues by utilizing a platinum catalyst (e.g., in the form of a platinum-siloxane complex) for the cross-linking reaction. The vaginal rings surprisingly achieve relatively high and steady release rates in vivo with a matrix ring containing a relatively small loading dose. While the matrix rings of the present invention have in vivo the steady release rates associated with reservoir rings, they are easier and less expensive to manufacture. The present invention also provides methods of blocking DNA polymerization by an HIV reverse transcriptase enzyme, methods of preventing HIV infection in a female human, methods of treating HIV infection in a female human, and methods of preparing platinum-catalyzed intravaginal rings.
Owner:THE POPULATION COUNCIL INC
Progesterone impregnated vaginal ring/pessary
InactiveUS20160089380A1Organic active ingredientsPharmaceutical delivery mechanismInterior spaceProgesterones
Owner:YALE UNIV
Intravaginal ring devices
PendingUS20210275347A1Less waterHeavy metal active ingredientsPharmaceutical non-active ingredientsSexually transmitted diseaseVaginal ring
Disclosed herein are intravaginal ring (IVR) devices comprising barriers that may be metal, polymeric or combinations of metal and polymeric. The IVRs are useful for contraception, treating and / or preventing sexually transmitted diseases, and treating and / or preventing bacterial infections. The IVR's are resistant to distortion due to absorption of vaginal fluids. This allows the IVRs to perform better over time as compared to other intravaginal rings made with copolymeric silicone.
Owner:DARE BIOSCIENCE INC
A kind of medical vaginal lubricant and preparation method thereof
Owner:GUANGXI XINYE BIOLOGICAL TECH
Vaginal acid-base buffer gel and preparation method thereof
ActiveCN110575431AIncrease the chance of infectionIncrease productivityAerosol deliveryInorganic non-active ingredientsBenzoic acidWhite petrolatum
The invention provides a vaginal acid-base buffer gel and a preparation method thereof, and specifically relates to the pharmaceutical field. The vaginal acid-base buffer gel consists of the followingcomponents (by mass): 1%-39% of an oil phase, 60-99% of a water phase, 0.001-2% of a pH regulator and 0.05-5% of a bacteriostat, wherein the oil phase is white petrolatum or liquid paraffin or glyceryl behenate or Span or Tween or glycerol or propylene glycol; the water phase is hydroxypropyl methylcellulose or carbomer or sodium carboxymethylcellulose or polycarbophil or polyethylene glycol or azone or gelatin or purified water; the pH regulator is citric acid or sodium citrate or sodium hydroxide or hydrochloric acid or triethanolamine or boric acid or borax or sodium dihydrogen phosphate or disodium hydrogen phosphate; the bacteriostat is benzoic acid or sodium benzoate or benzyl alcohol or benzalkonium bromide or benzalkonium chloride or sorbic acid or potassium sorbate or nipalgin. The product prepared by the invention can effectively regulate the original vaginal environment of inflammation, remove vaginal odor and improve symptoms such as increased vaginal secretions or itching.
Owner:NANJING SKY LONG PHARMA
Vaginal ring sensor
A vaginal ring sensor device adapted to be placed within the vaginal vault of a user, the device including a ring body, at least one through hole that passes through the ring body, and at least one biosensor structured and arranged to sense and / or measure a parameter of vaginal fluid as such fluid passes through the at least one through hole.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
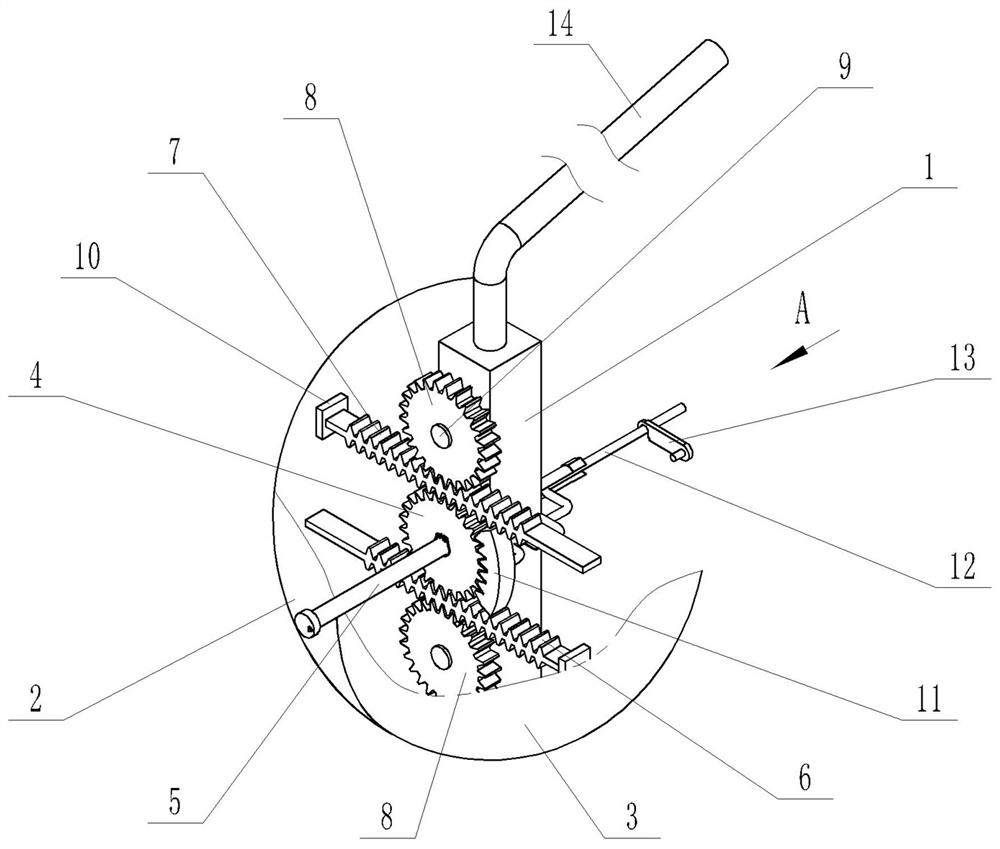
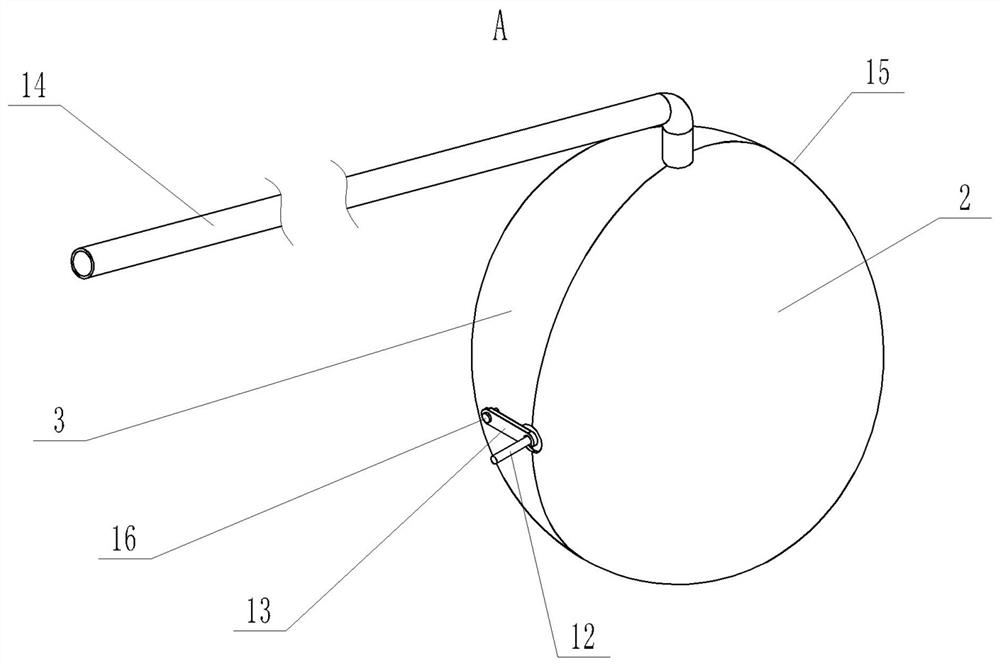
Oval-body-adjustable gynecological close-range back-loading source applicator
PendingCN111840827AReduce financial burdenNo financial burdenX-ray/gamma-ray/particle-irradiation therapySide effectVaginal ring
The invention discloses an oval-body-adjustable gynecological close-range back-loading source applicator, and solves the problems that in the prior art, the source applicator cannot adapt to cervicalcancer patients in various vaginal environments, errors of radiotherapy dosage are easily caused, tumor control fails, and side effects are aggravated. The source applicator comprises a middle connecting frame consisting of a connecting frame main body, and is characterized in that in an elbow mounting hole at the upper end of the middle connecting frame, a source applicator bent is inserted; an oval body left hemisphere and an oval body right hemisphere are symmetrically arranged on the two sides of the middle connecting frame respectively and are each composed of a hemispheric shell, and inwards-concave cavities are formed in the opposite sides of the two hemispheric shells respectively. The oval body left hemisphere and the oval body right hemisphere are connected with the middle connecting frame through the oval body adjusting mechanism. The source applicator is reasonable in design, compact in structure, capable of meeting the treatment requirements of different types of middle and advanced cervical cancer radiotherapy patients, easy and convenient to operate, capable of effectively improving the radiotherapy quality, free of trauma, small in side reaction after treatment, economical, practical and good in patient medical compliance.
Owner:辽宁省肿瘤医院
Therapeutic and Method of Use
InactiveUS20170095456A1Hydroxy compound active ingredientsTetracycline active ingredientsTransdermal patchVaginal ring
Owner:ALBERTSEN HANS M
Medical vagina lubricating fluid and preparation method thereof
ActiveCN104941010AWith viscosityHigh fatty acidOrganic active ingredientsSurgeryGynecologyWater soluble
The invention relates to the technical field of lubricating fluid, in particular to a medical vagina lubricating fluid and a preparation method thereof. A medical vagina lubricating fluid comprises the following components in percent by weight: 7-15% of glycerinum, 7-16% of almond oil, 3-6% of camellia oil, 1.5-2.3% of sodium carboxymethylcellulose, 2.7-3.2% of water-soluble chitosan, 0.3-0.7% of natamycin, 2-4.5% of vitamin E, 0.15-0.3% of ceramide, 0.4-0.7% of mint oil, and the balance of purified water. According to the invention, the vagina environment can be effectively improved, the lubricating and moisturizing effects can be achieved, the vagina mucosa nerve sensitivity is improved, and the lubricating fluid is sanitary and safe; the use effect is excellent.
Owner:GUANGXI XINYE BIOLOGICAL TECH
Preparation and Application of Drug Sustained and Controlled Release System with Liquid Silicone Rubber as Carrier
The invention relates to preparation and application of a drug slow-release and controlled-release system with liquid silicone rubber as a carrier. The slow-release and controlled-release system consists of a controlled-released film and one or more framework sections, wherein at least one framework section contains drugs; one or more additives are contained in the framework and the film, and are used for adjusting the release speed of the drugs; the framework is prepared through injection moulding; the controlled-released film is added through injection moulding, hot-pressing vulcanization or high-temperature vulcanization; the drug contained in the slow-release and controlled-release system can be anti-inflammatory and analgesic non-steroidal anti-inflammatory drugs, imidazoles antifungal drugs and purine nucleoside antiviral drugs which are applied to a genital tract, and also can be steroidal progesterone and estrogen drugs applied to contraception, hormone replacement therapy and gynaecological disease treatment. The slow-release and controlled-release system can be used for preparing a vaginal ring or an IUD and an assembly thereof, and can release the contained drugs at a slow or constant speed.
Owner:国家卫生健康委科学技术研究所
Liquid dressing and preparation method thereof
ActiveCN112274690AProlong the action timeOvercome the defect of short action timeBandagesVitamin CGlycerol
The invention relates to the technical field of medicines, and particularly discloses a liquid dressing and a preparation method thereof. The liquid dressing is prepared from the following componentsin percentage by mass: 0.5-5.0 percent of polyvinylpyrrolidone, 0.5-2.0 percent of sodium alginate, 1.0-5.0 percent of glycerol, 3.0-8.0 percent of vitamin C, 0.5-2.0 percent of a plasticizer, 5.0-10.0 percent of a humectant, 2.0-15.6 percent of an acetate buffer solution and the balance of purified water. The liquid dressing has the advantages of being long in acting time, good in air permeability after film forming, capable of helping wound healing and high in film forming speed, does not cause vaginal environment pH imbalance when being used for gynecological perineum lateral incision wounds or gynecological examination wounds, and has the characteristic of selectively inhibiting harmful microorganisms in vaginal flora.
Owner:SHENZHEN HUAZHONG BIOLOGICAL PHARMA MACHINERY
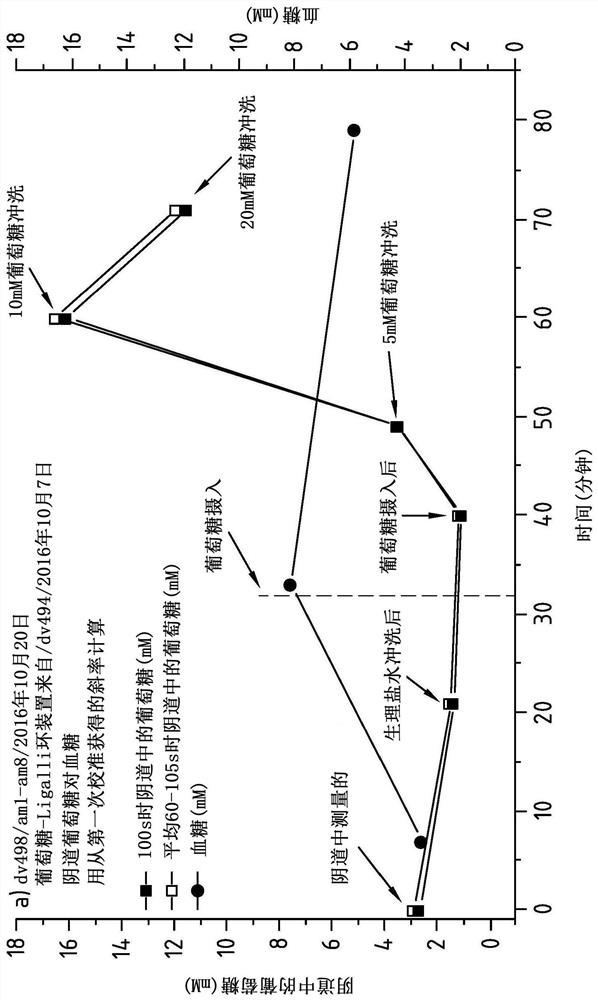
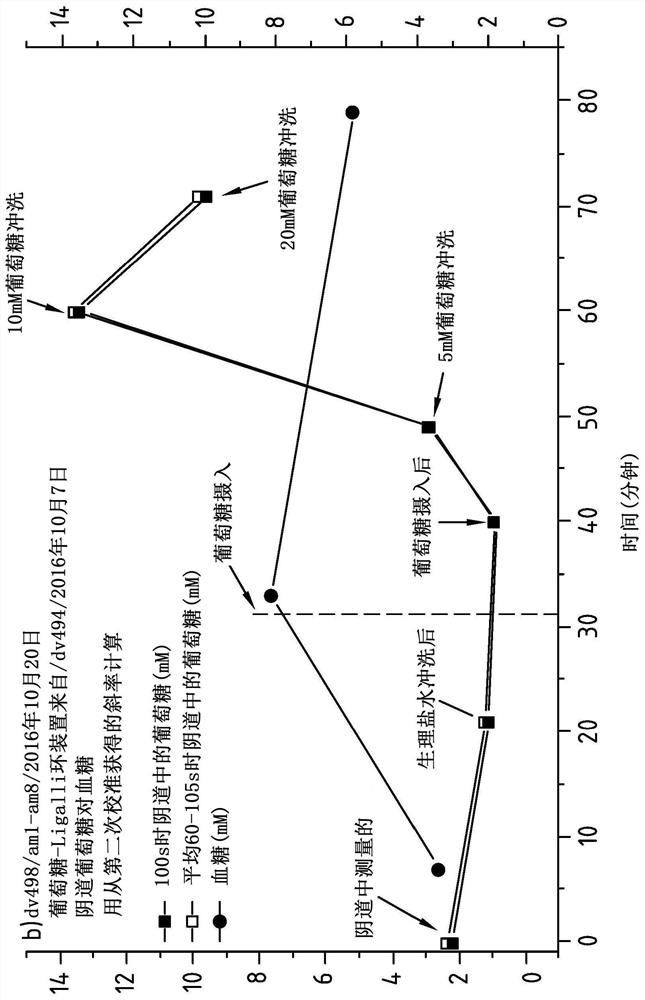
Vaginal Measurements Using a Vaginal Ring
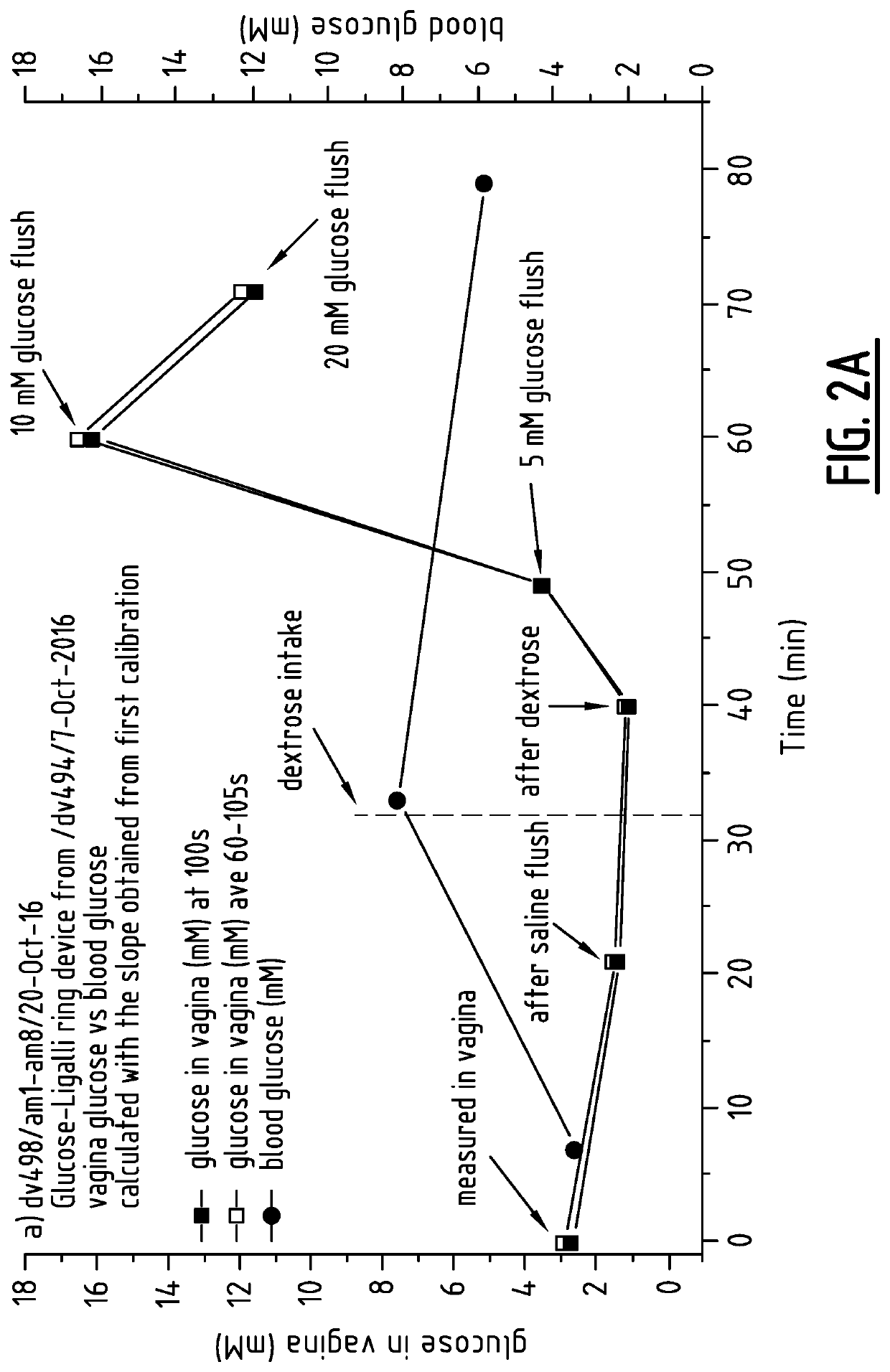
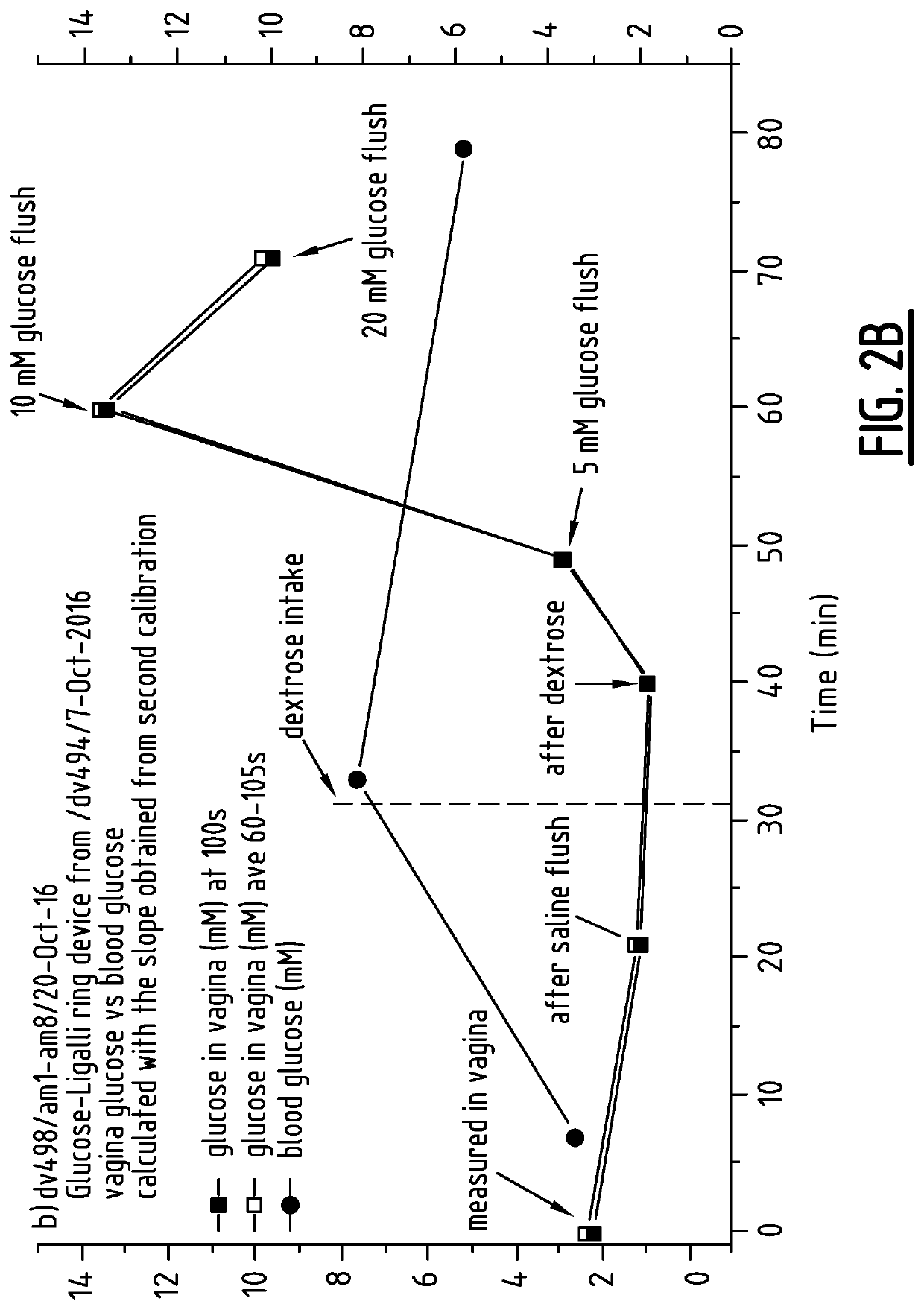
Method for measuring the presence of a compound or a physiological parameter in the human or animal body, comprising positioning an intravaginal ring that comprises a sensor for the compound or parameter to be measured in contact with the vaginal mucosa and recording a signal produced by the sensor. It is further disclosed a method for the combined administration and measurement of compounds in the human or animal body, comprising the administration of one or more biologically active compounds and the simultaneous and / or subsequent measurement of the presence and / or concentration of the same and / or another compound and / or physiological parameter that is the result of the administration of the one or more biologically active compounds, wherein the measurement is performed with a sensor that is in contact with the vaginal mucosa.
Owner:LIGALLI BV
Targeted delivery of progestins and estrogens via vaginal ring devices for fertility control and hrt products
InactiveUS20190328656A1Inhibit ovulationEasy cycle controlOrganic active ingredientsSuppositories deliveryDrug deliveryDrug
A variety of different intravaginal drug delivery devices are described for the delivery of estrogens and progestins. The release rate of estrogens and progestins can be controlled by varying the matrix material or by the application of a thin coating. The intravaginal drug delivery devices may be composed of one or more individual compartments. By controlling various physical and chemical parameters, non-zero release rates of the estrogen or progestins may be achieved.
Owner:EVESTRA
Intelligent compound gestodene vaginal ring preparation with memory effect and application of intelligent compound gestodene vaginal ring preparation
InactiveCN102525722AHave memory effectImprove complianceOrganic active ingredientsFemale contraceptivesOral medicationControl release
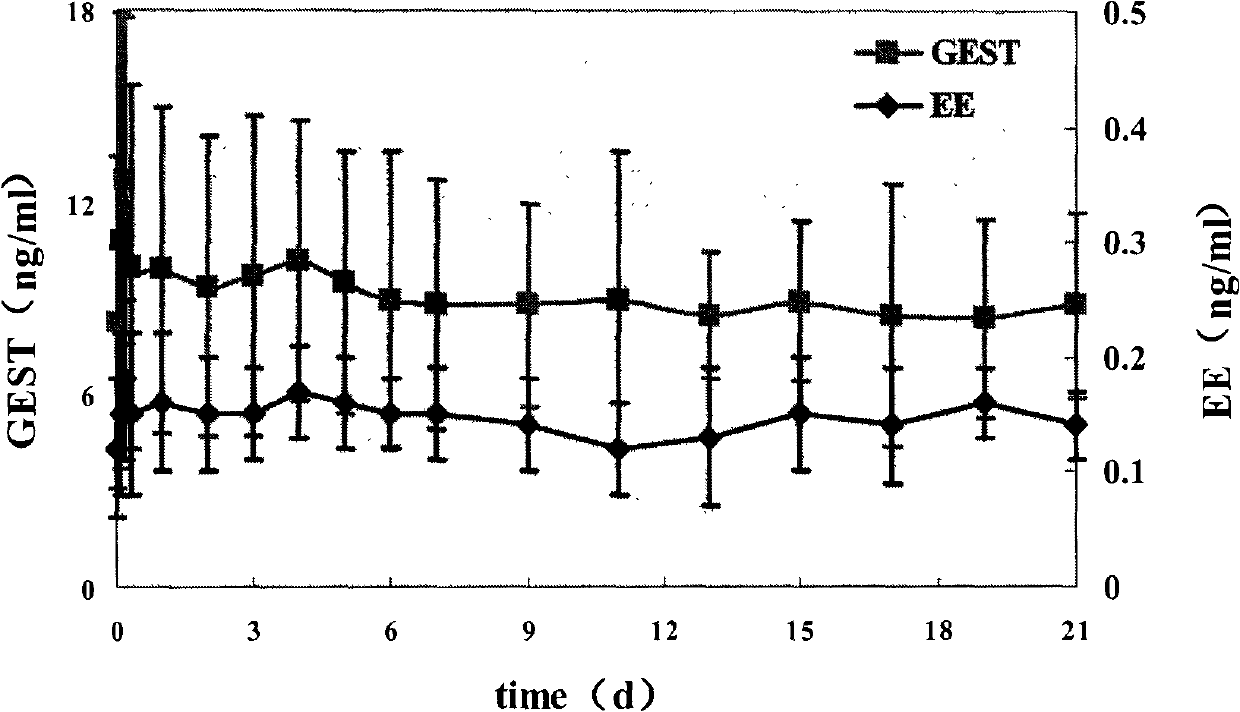
The invention relates to a contraceptive, in particular to an intelligent compound gestodene vaginal ring preparation with a memory effect. A vaginal ring consists of a controlled release membrane, a gestodene / ethinyloestradiol-containing carrier and a shape memory material, and can slowly release a contraceptive at constant speed within 21 days. By implementing the preparation, the remarkable contraception effect can be achieved, the inconvenience caused by the oral administration of the contraceptive is avoided, and the appliance of the administration is improved.
Owner:LIAONING AIMU MEDICAL SCI&TECH +1
Vaginal ring containing non-nucleoside reverse transcriptase inhibitor and its preparation method
ActiveCN106267234BPharmaceutical delivery mechanismAntiviralsNucleoside Reverse Transcriptase InhibitorCyclodextrin
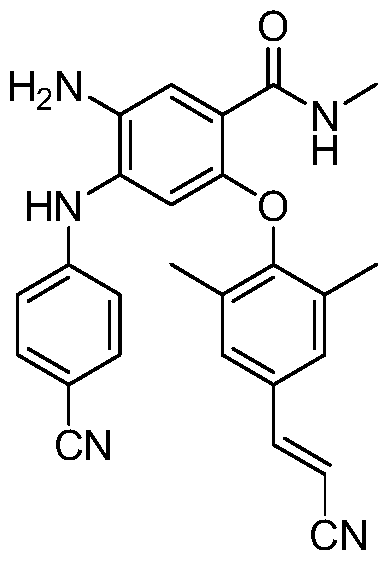
The invention relates to a pessulum containing a non-nucleoside reverse transcriptase inhibitor and a preparation method of the pessulum. The pessulum comprises N1-(4'-cyanophenyl)-5-(4''-propylene cyano-2'',6''-dimethyl phenol)-4-(N-methyl formamido)-1,2-phenylenediamine, hydroxypropyl betadex and lauryl sodium sulfate. The weight ratio of silicone rubber to N1-(4'-cyanophenyl)-5-(4''-propylene cyano-2'',6''-dimethyl phenol)-4-(N-methyl formamido)-1,2-phenylenediamine to hydroxypropyl betadex to lauryl sodium sulfate is (30-80):(1-5):(3-8):(8-15). The pessulum can precisely release the non-nucleoside reverse transcriptase inhibitor in a controlled mode, and the purpose of efficiently, easily and conveniently treating Aids is achieved.
Owner:SHANGHAI INST OF PLANNED PARENTHOOD RES +1
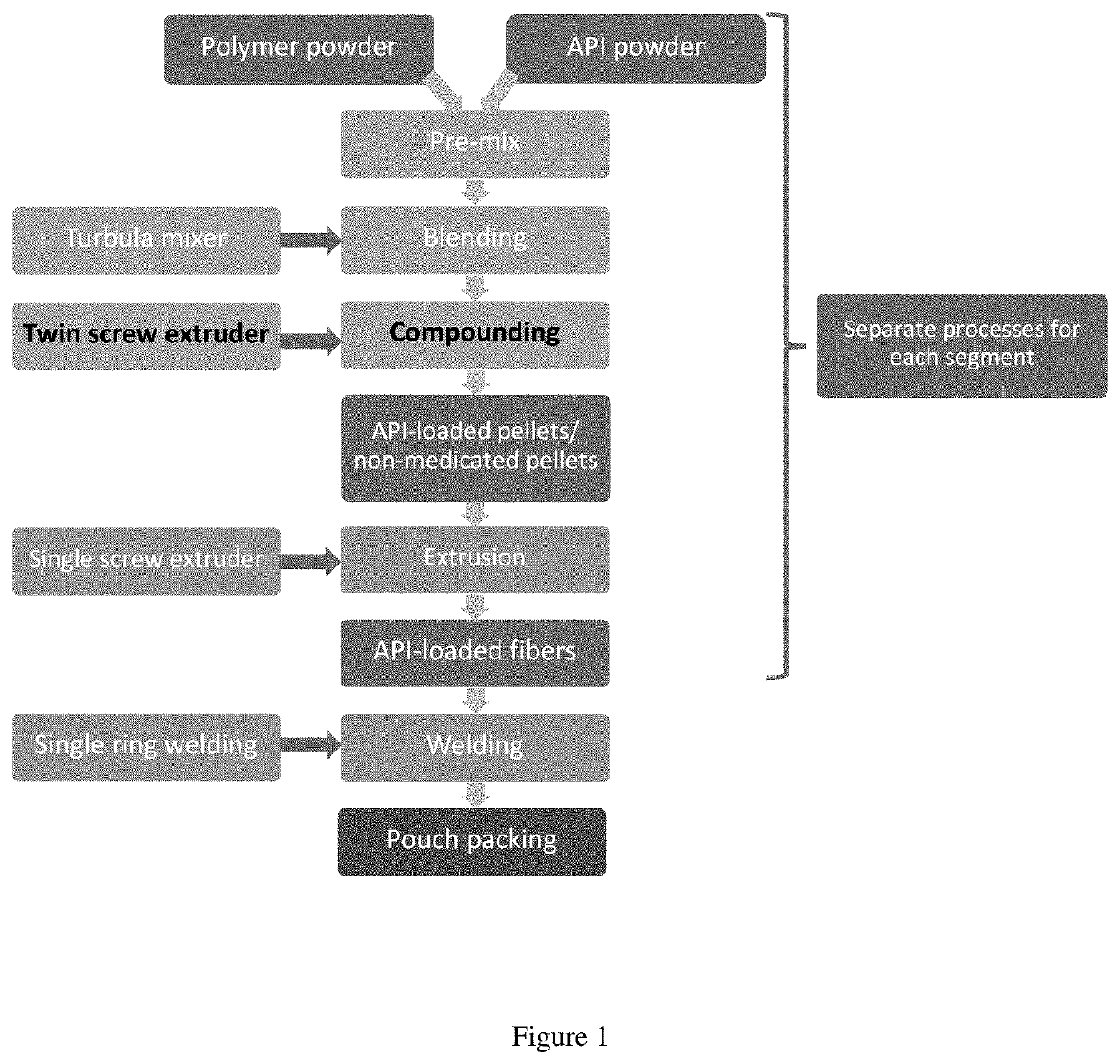
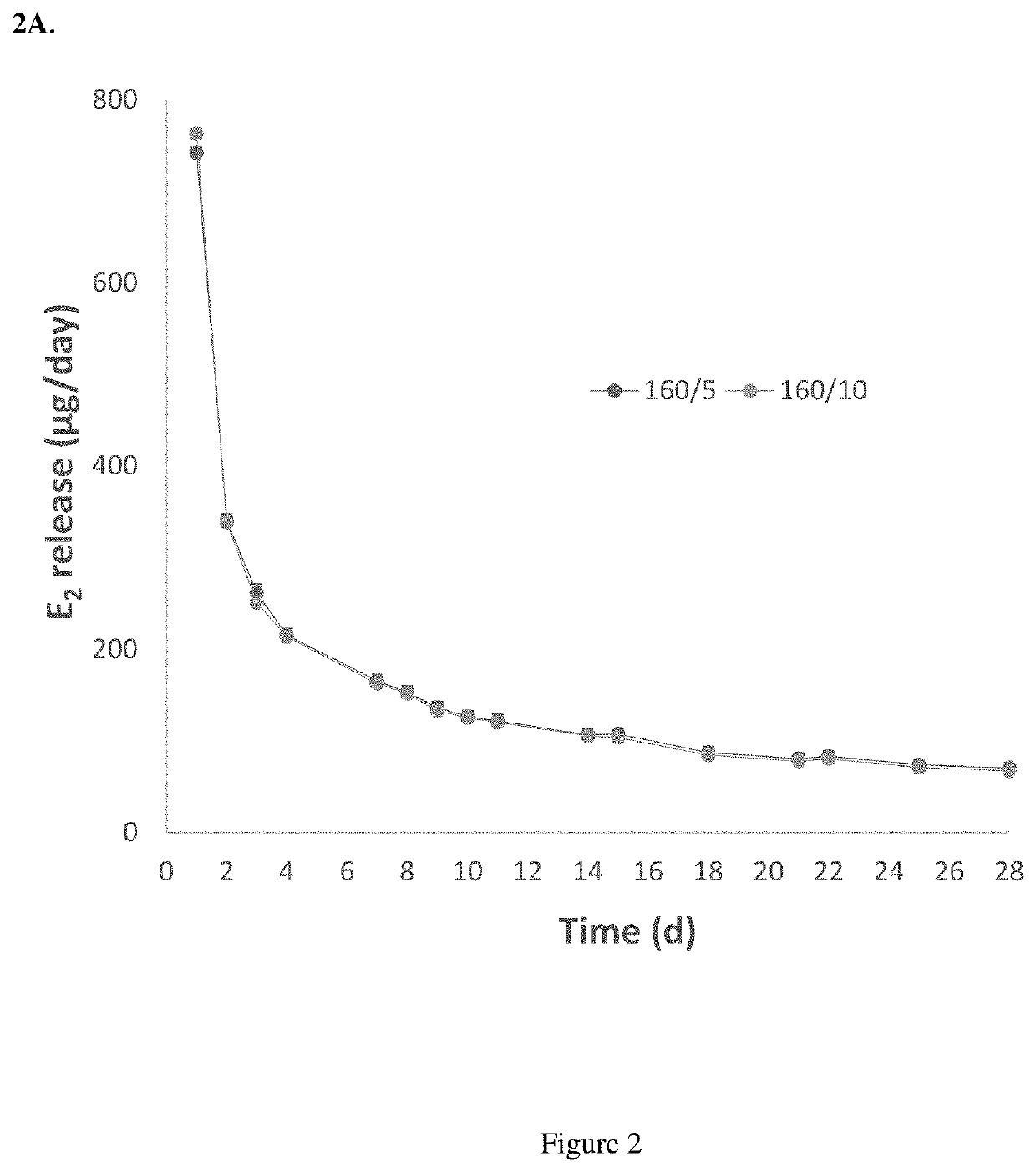
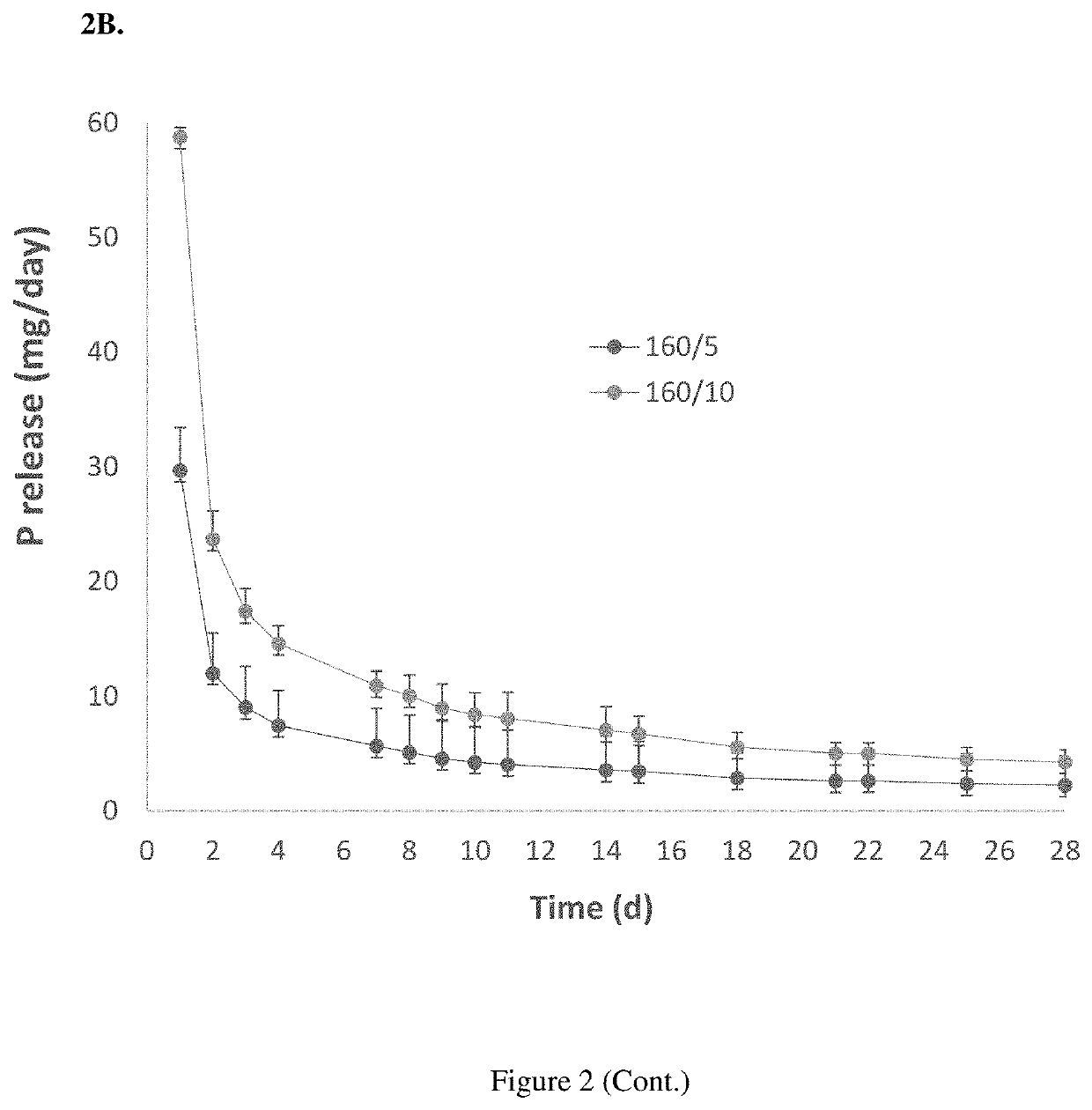
Segmented EVA Intravaginal Rings
PendingUS20210007976A1Increase infectionReduce lubricationOrganic active ingredientsSurgeryPregnanetrioloneVaginal ring
Disclosed herein are segmented, EVA intravaginal rings that release 17β-estradiol and progesterone with specific pharmacokinetics useful for treating, ameliorating, and preventing symptoms associated with menopause and vulvar and vaginal atrophy.
Owner:JUNIPER PHARM INC N K A CATALENT JNP INC
Intra-Vaginal Ring with Pressure Sensor
PendingUS20220287623A1Sufficiently long antennaReliable resultsElectrotherapyEndoradiosondesIntra abdominal pressureVaginal ring
The present invention relates to a device for determining intra-abdominal pressure in a subject, comprising an intravaginal ring that is provided with a pressure sensor, means for registering the intra-abdominal pressure value measured by the pressure sensor and means for generating an alert signal when the intra-abdominal pressure exceeds a reference value. The present invention also relates to a method for determining intra-abdominal pressure in a subject, comprising the steps of: a) measuring the intra-abdominal pressure in the vagina by means of a device of the invention, b) comparing the intra-abdominal pressure value measured with a reference value; and c) in case the intra-abdominal pressure value exceeds the reference value transmitting an alert signal to the device that triggers a vibration element in the device or an external device, such as a smartphone or smart watch, to provide a warning to change the subjects behaviour in order to lower the intra-abdominal pressure value.
Owner:LIGALLI BV
Vaginal sustained and controlled release drug delivery system containing aromatase inhibitor and preparation method thereof
ActiveCN112315898BEasy to useUse long-actingOrganic active ingredientsAntipyreticAromatase inhibitorPharmaceutical drug
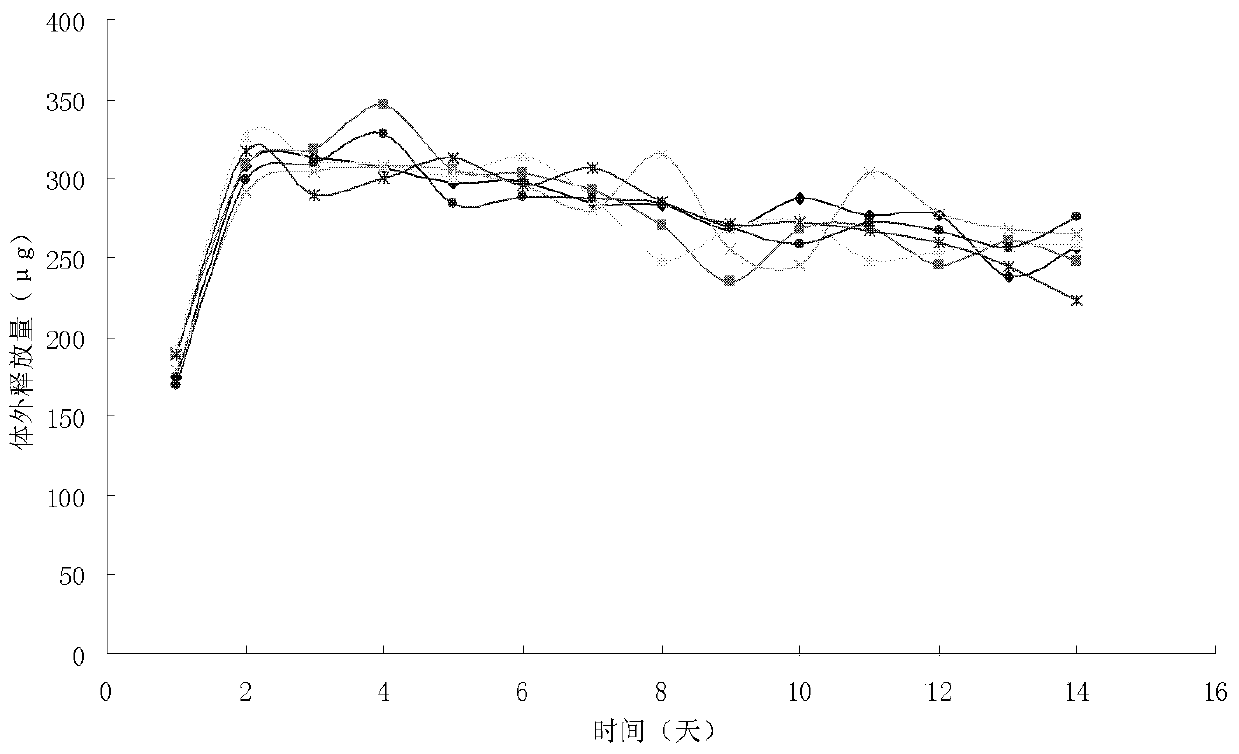
The invention provides a vaginal sustained and controlled release drug delivery system containing an aromatase inhibitor and a preparation method thereof. The drug delivery system is an aromatase inhibitor reservoir-type vaginal ring prepared by loading aromatase inhibitor drugs with EVA as a carrier material. The vaginal ring includes a core layer and a film layer wrapped outside the core layer. The core layer It is formed after the aromatase inhibitor drug is evenly mixed with the EVA material; the film layer is formed of the EVA material. The release period of the vaginal sustained and controlled release drug delivery system of the present invention is 28 days to 42 days, the use process is more flexible, the preparation cost is also lower, and there is no obvious burst release effect, and the desired release can be achieved in a short time Therefore, the drug release is more stable and the application is safer.
Owner:国家卫生健康委科学技术研究所
Hormonal contraception using a vaginal ring which releases estriol and trimegestone
InactiveUS20200383906A1Prevent/reduce intermenstrual bleedingReduce riskOrganic active ingredientsPharmaceutical delivery mechanismIntravaginal administrationEstriol
The invention relates to an intravaginal drug delivery device for hormonal contraception with prevention / reduction of intermenstrual bleeding and menopausal cycle disorders. The intravaginal drug delivery device includes estriol or a precursor of this compound and a progestogen or a precursor thereof.
Owner:EVESTRA
Vaginal measurements using vaginal ring
Method for measuring the presence of a compound or a physiological parameter in the human or animal body, comprising positioning an intravaginal ring that comprises a sensor for the compound or parameter to be measured in contact with the vaginal mucosa and recording a signal produced by the sensor. It is further disclosed a method for the combined administration and measurement of compounds in the human or animal body, comprising the administration of one or more biologically active compounds and the simultaneous and / or subsequent measurement of the presence and / or concentration of the same and / or another compound and / or physiological parameter that is the result of the administration of the one or more biologically active compounds, wherein the measurement is performed with a sensor thatis in contact with the vaginal mucosa.
Owner:里加利私人有限公司
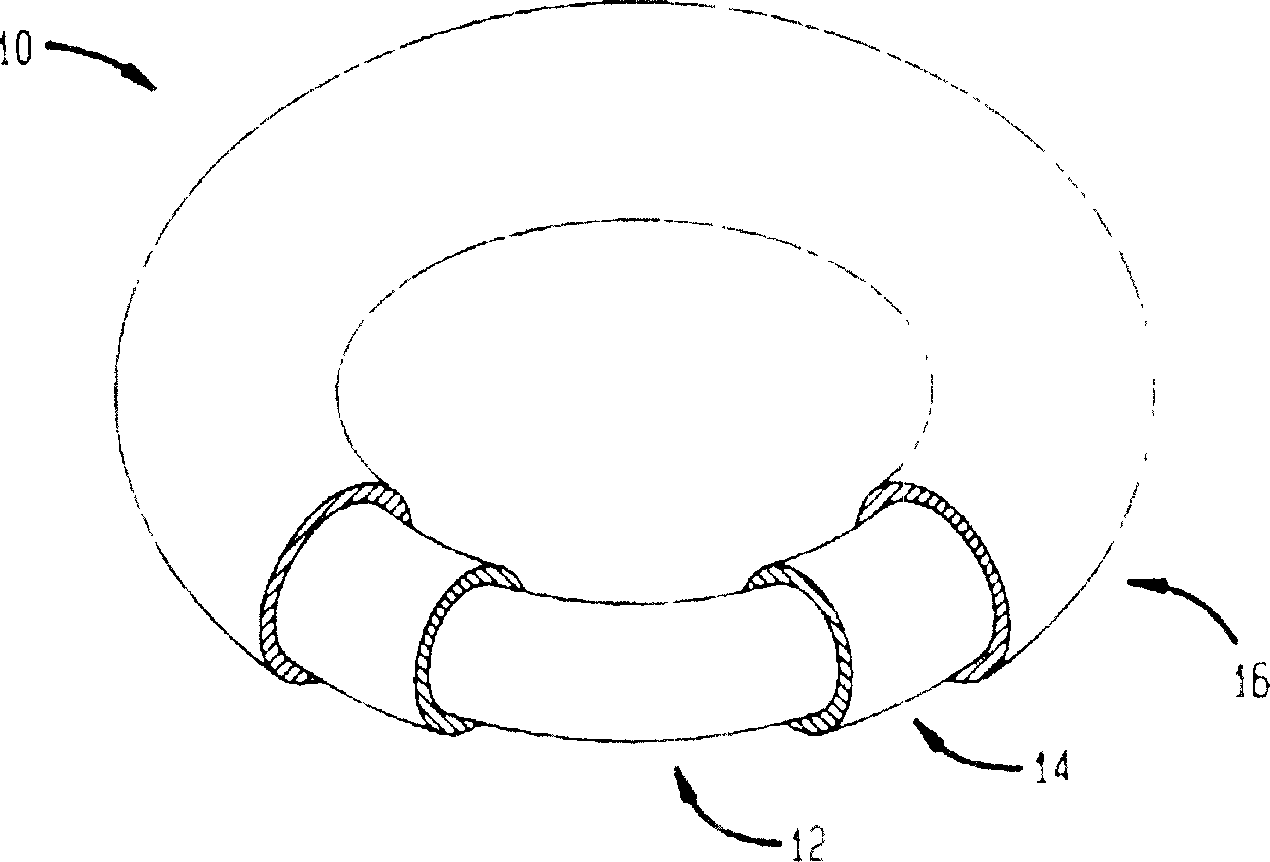
Intravaginal rings with insertable drug-containing core
InactiveCN1154455CTreating Vaginal InfectionsPharmaceutical delivery mechanismPharmaceutical non-active ingredientsIntravaginal administrationGynecology
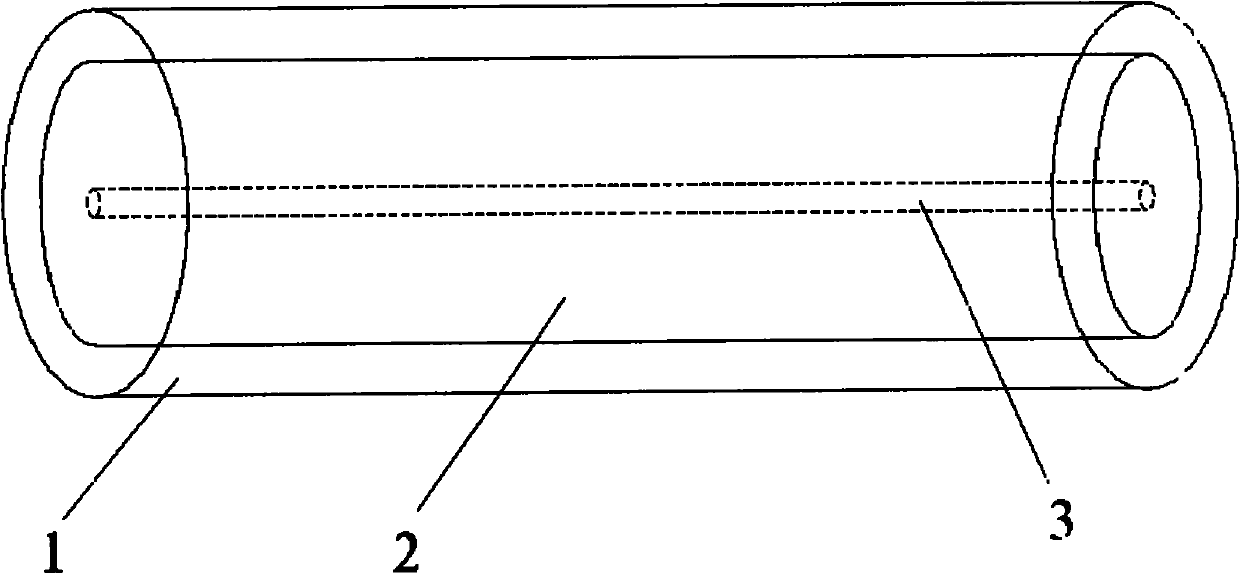
Disclosed is a vaginal ring containing a ring body made of a first polymeric material having at least one hollow internal channel defining an opening to the exterior of said body and which channel is adapted to receive a drug-containing core through said opening, and a core containing at least one intravaginally administerable drug dispersed in a second polymeric material disposed in the channel. The core is positioned in the vaginal ring body suitably prior to use in order to substantially avoid initial bursts of drug into the tissues of the subject and resultant side effects such as nausea and vomiting. Representative drugs include contraceptive agents and other steroidal substances for use in hormone replacement therapy. Also disclosed are methods for preparing the vaginal rings, kits for assembling the vaginal rings, and methods of using the vaginal rings to achieve intravaginal delivery of drugs to a female.
Owner:THE POPULATION COUNCIL INC
Hormonal contraception using a vaginal ring which releases estriol
InactiveUS20190328657A1Inhibit ovulationEasy cycle controlOrganic active ingredientsSuppositories deliveryEstriolMedicine
A variety of different intravaginal drug delivery devices are described for the delivery of estrogens and progestins. The release rate of estrogens and progestins can be controlled by varying the matrix material or by the application of a thin coating. The intravaginal drug delivery devices may be composed of one or more individual compartments. By controlling various physical and chemical parameters, non-zero release rates of the estrogen or progestins may be achieved.
Owner:EVESTRA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com