Vaginal sustained and controlled release drug delivery system containing aromatase inhibitor and preparation method thereof
A technology of aromatase and drug delivery system, which is applied in the field of pharmacy, can solve the problems of serious burst release phenomenon, unstable drug release, poor curative effect, etc., and achieve the solution of burst release effect, reduction of adverse reactions, and stable drug release Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
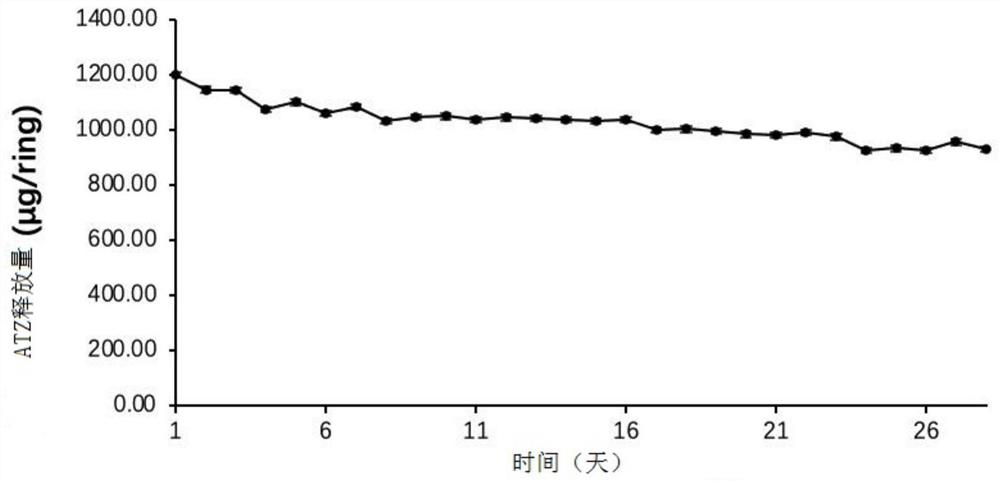
Embodiment 1
[0043] In this example, the raw materials for preparing the vaginal sustained and controlled release drug delivery system are shown in Table 1 below.
[0044] Table 1
[0045] name level batch number Manufacturer anastrozole API HSF1903001 Shanghai Hushi Pharmaceutical Technology Co., Ltd. EVA600^28% medical grade 56492201 Korea Lotte Chemical EVA810^33% medical grade E19222127 Korea Lotte Chemical
[0046] Take ATZ 12.5g, EVA28 (containing 28% VA) blank powder 100g, fully stir for 1min, then transfer the mixture to a twin-screw extruder (parameters: the temperature of the first zone, the second zone and the third zone are all 83 °C, the speed of the main engine is 76rpm, and the feeding rotating speed 50rpm) to extrude into thin tubular lines, and then use a granulator to granulate (with a particle diameter of about 3mm to 5mm) to obtain an EVA particle matrix with a drug loading of 12.5%. Add the drug-containing EVA particl...
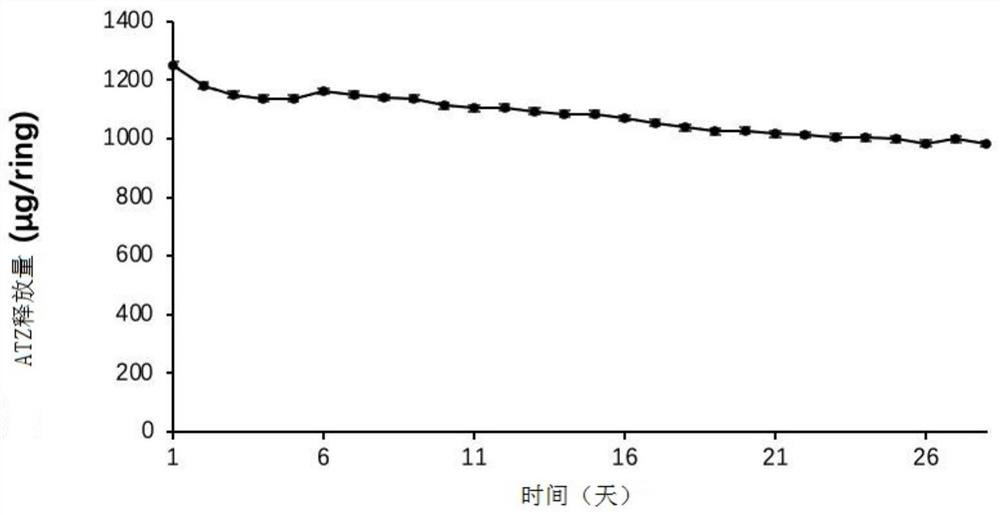
Embodiment 2
[0048] In this example, the raw materials for preparing the vaginal sustained and controlled release drug delivery system are shown in Table 2 below. Table 2
[0049] name level batch number Manufacturer anastrozole API HSF1903001 Shanghai Hushi Pharmaceutical Technology Co., Ltd. EVA810^33% medical grade E19222127 Korea Lotte Chemical EVA40-45^40% medical grade 77625022 Arkema, France
[0050] Take ATZ 15g, EVA33 (containing 33% VA) blank powder 100g, fully stir for 1min, then transfer the mixture to a twin-screw extruder (parameters: the temperature in the first zone, the second zone and the third zone is 73°C, the speed of the main engine is 76rpm, and the speed of feeding 50rpm) to extrude into a thin tubular line, and then use a granulator to granulate (the particle diameter is about 3mm to 5mm) to obtain an EVA particle matrix with a drug loading of 15%. The drug-containing EVA particle matrix is added to the single-s...
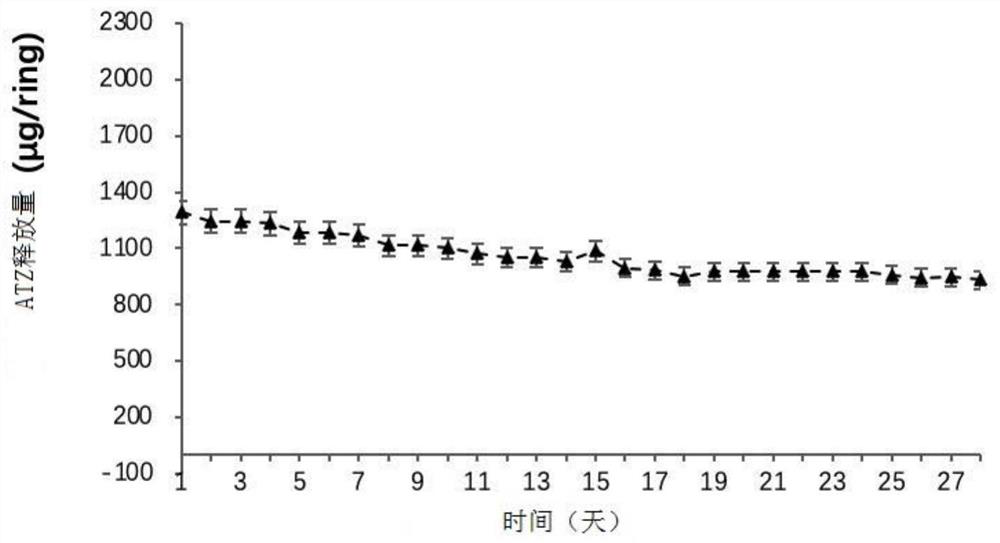
Embodiment 3
[0052] In this example, the raw materials for preparing the vaginal sustained and controlled release drug delivery system are shown in Table 3 below. table 3
[0053] name level batch number Manufacturer anastrozole API HSF1903001 Shanghai Hushi Pharmaceutical Technology Co., Ltd. EVA25-28^28% medical grade 56492201 Arkema, France EVA810^33% medical grade E19222127 Korea Lotte Chemical
[0054]Take 15g of ATZ, 100g of EVA28 (containing 28% VA) blank powder, fully stir for 1min, and then transfer the mixture to a twin-screw extruder (parameters: the temperature of the first zone is 76°C, the temperature of the second zone is 73°C, and the temperature of the third zone is 73°C, The main engine speed is 50rpm, the feeding speed is 50rpm) and extruded into thin tubular lines, and then granulated by a granulator (particle size is about 3mm-5mm) to obtain an EVA particle matrix with a drug loading of 15%. The drug-containing EVA pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com