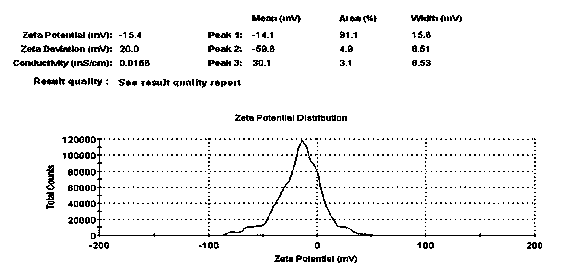
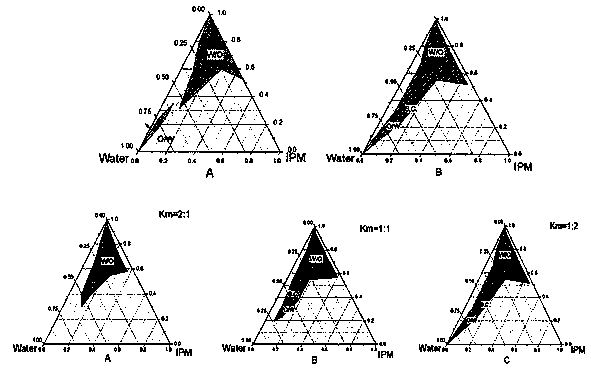
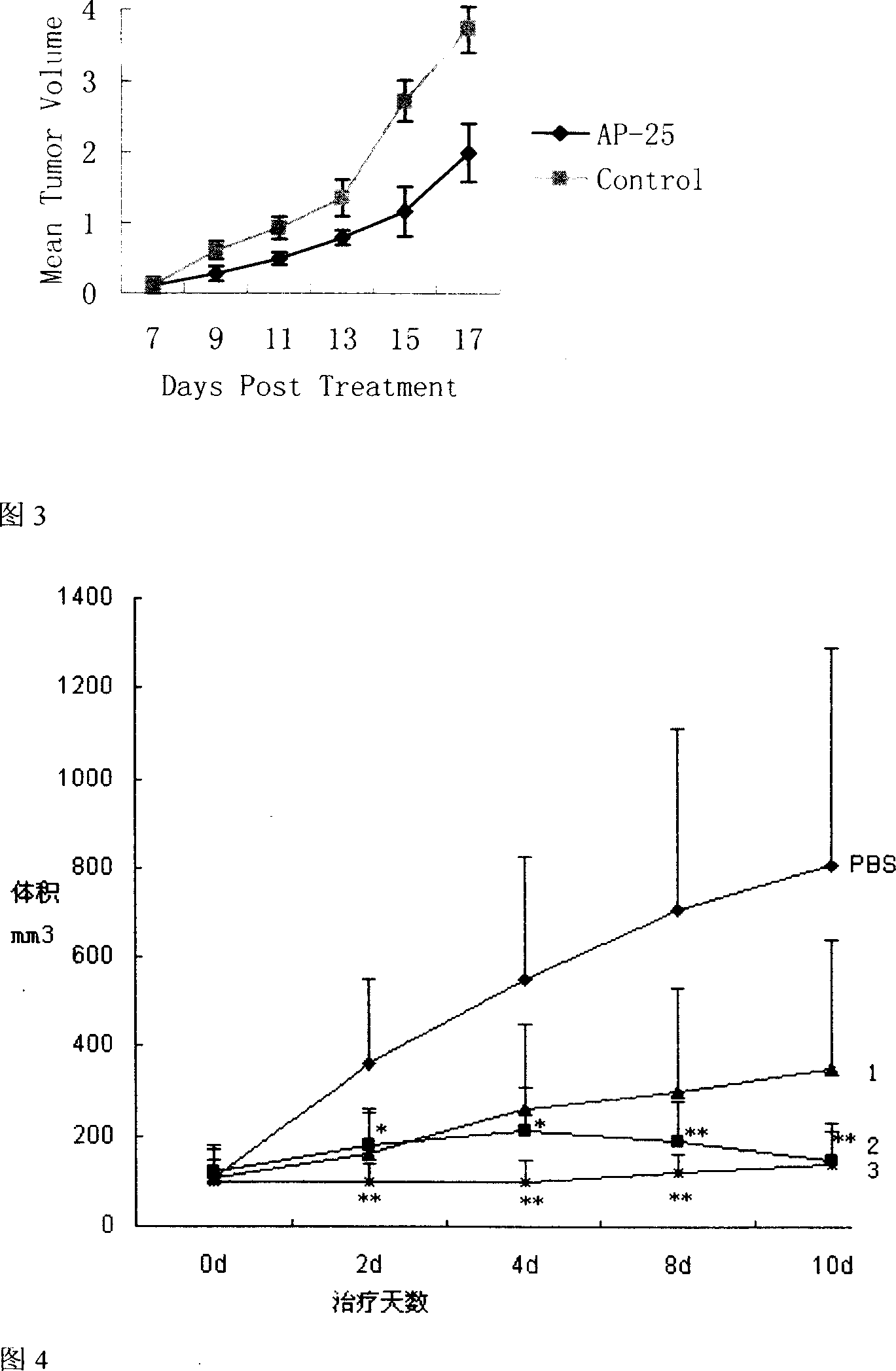
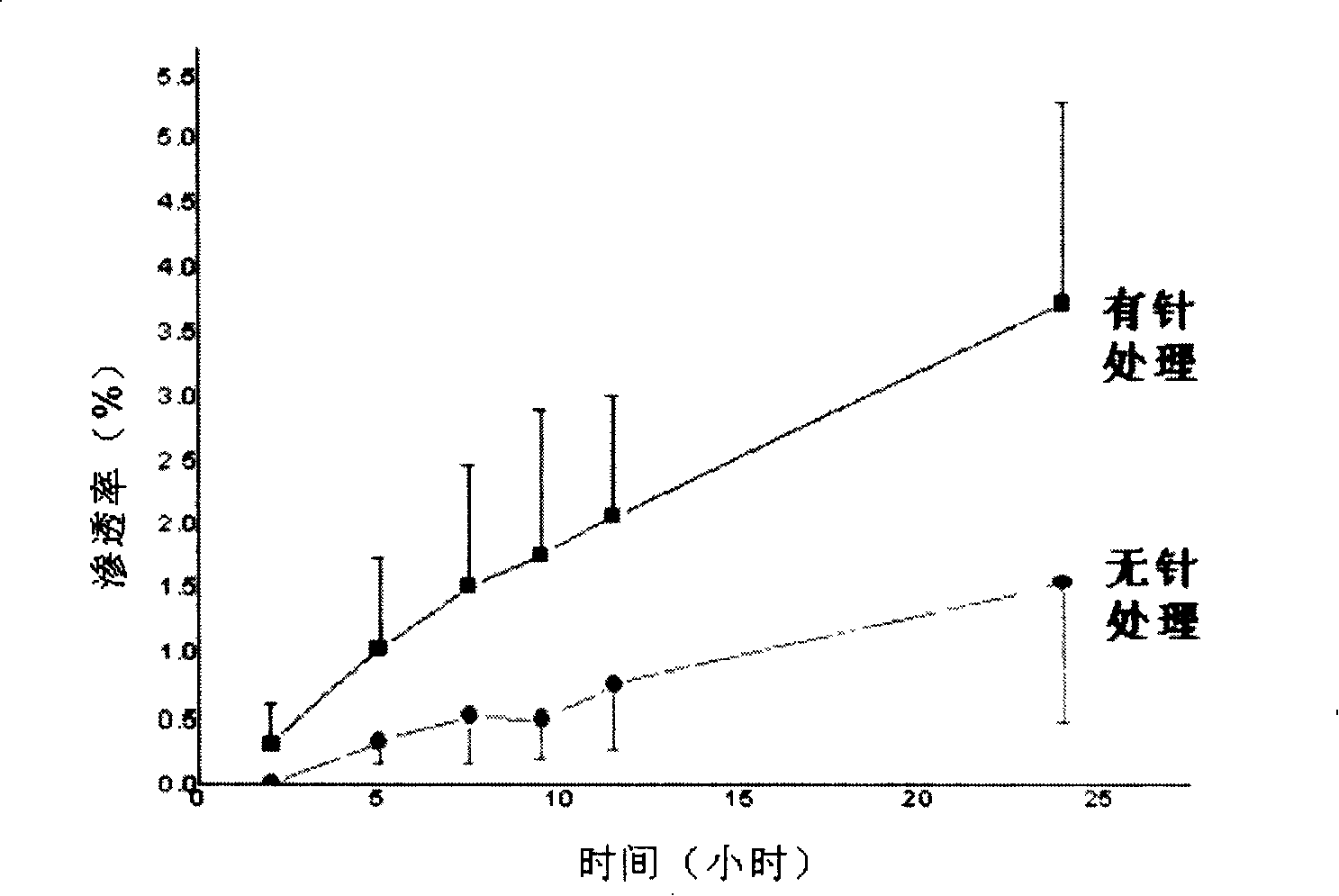
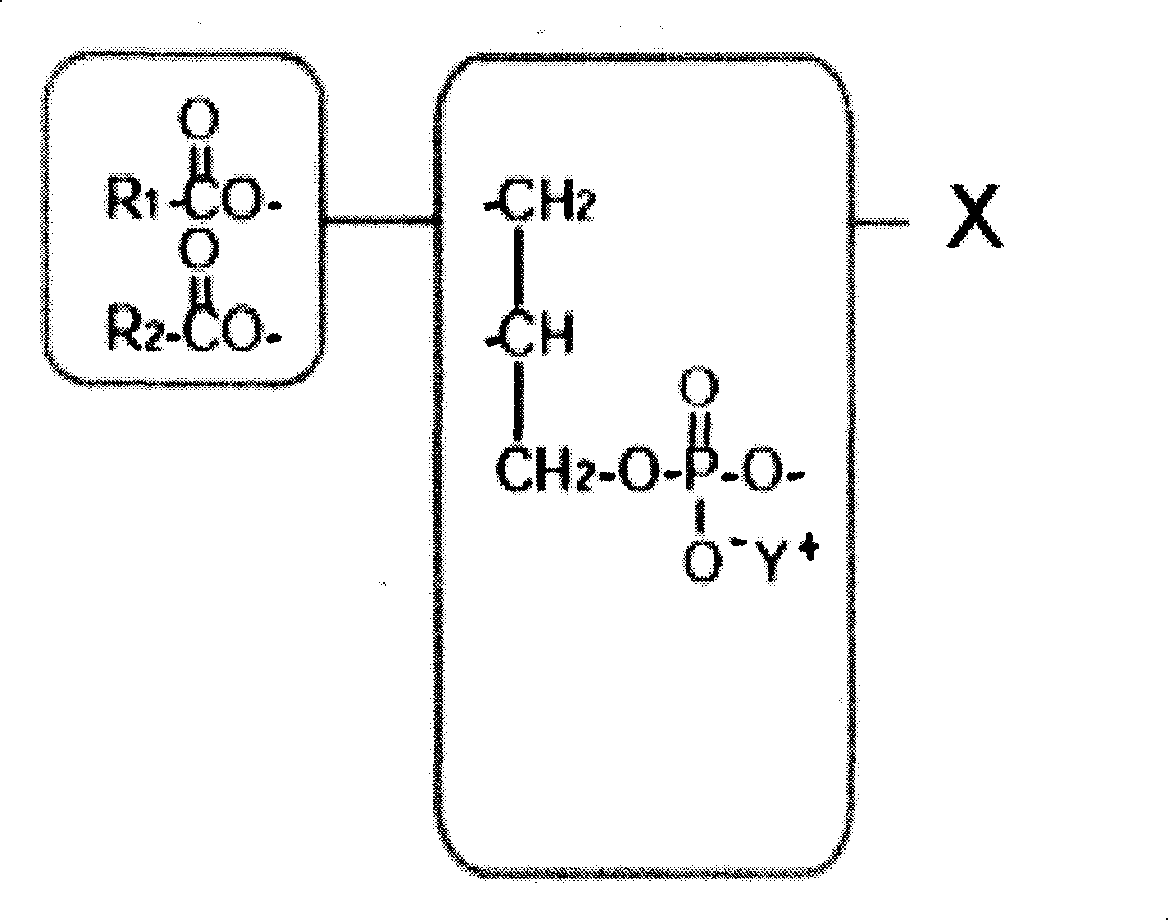
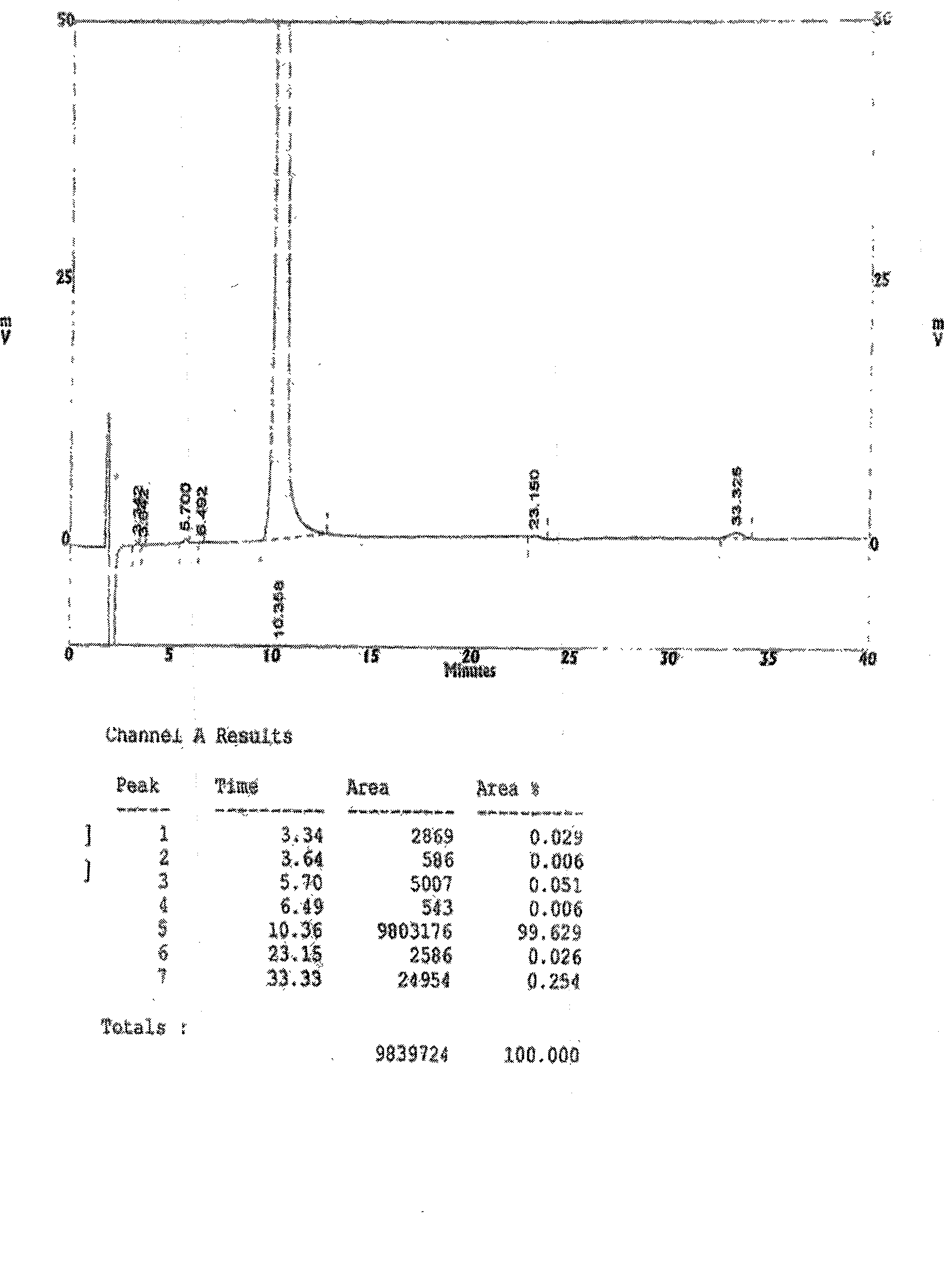
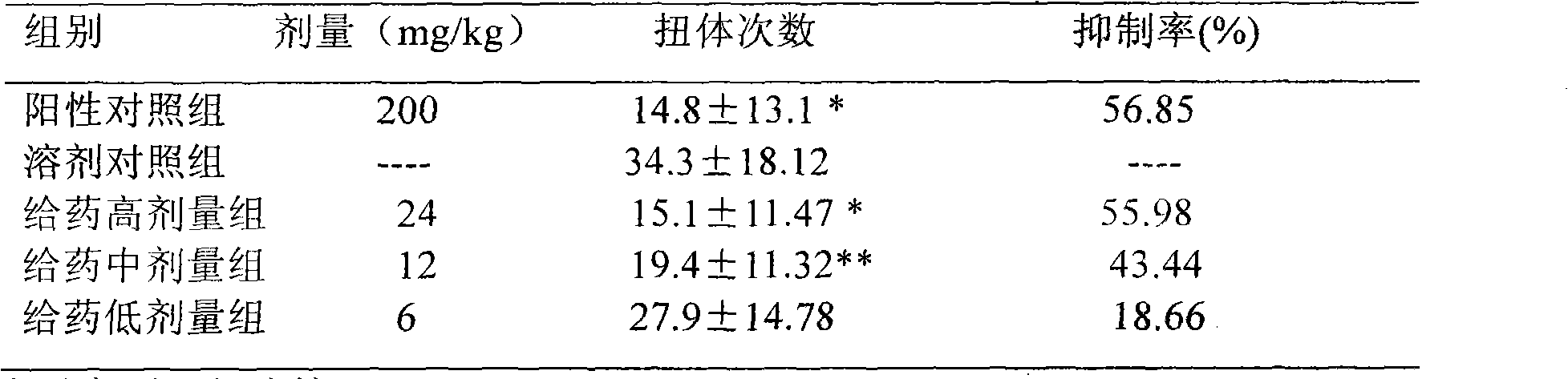
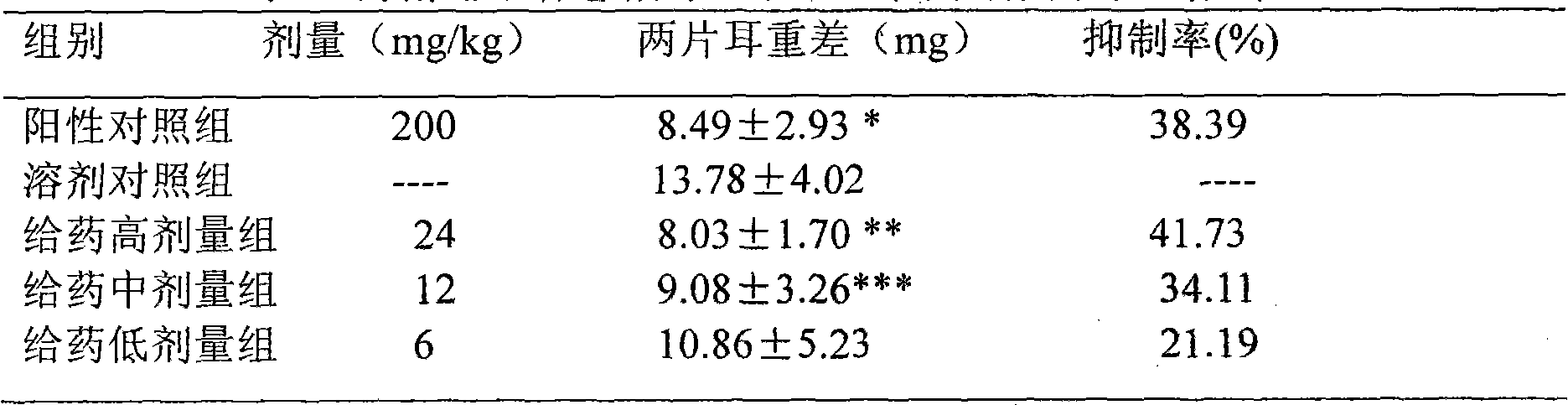
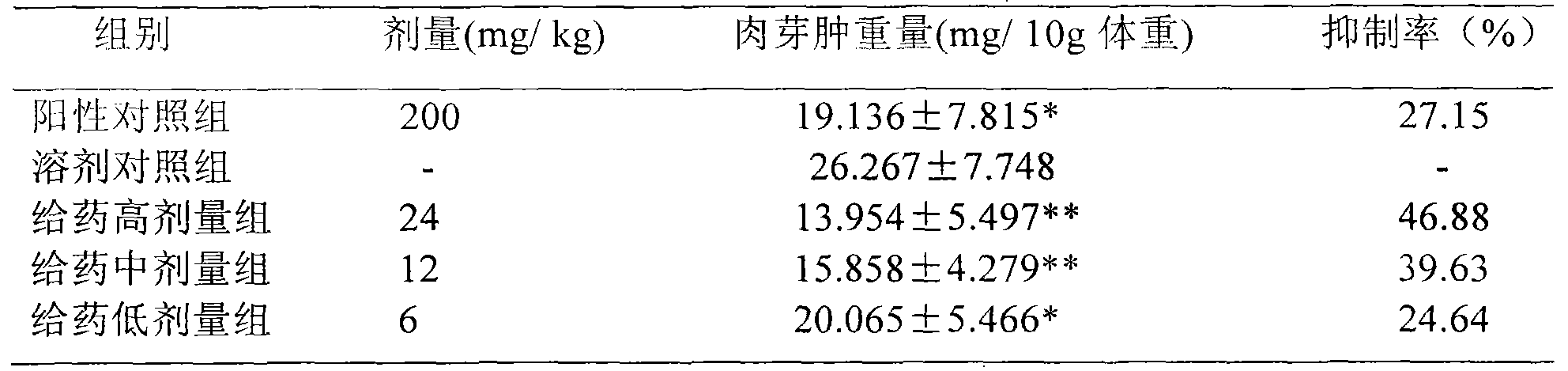
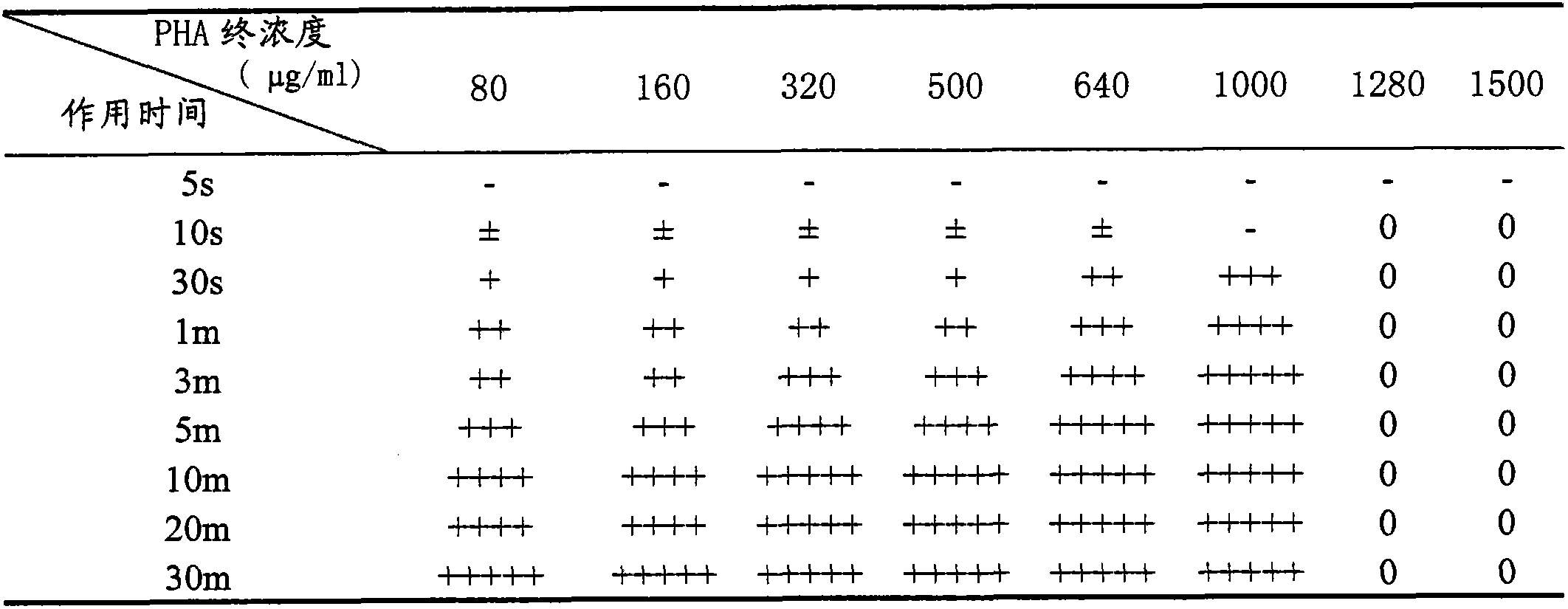
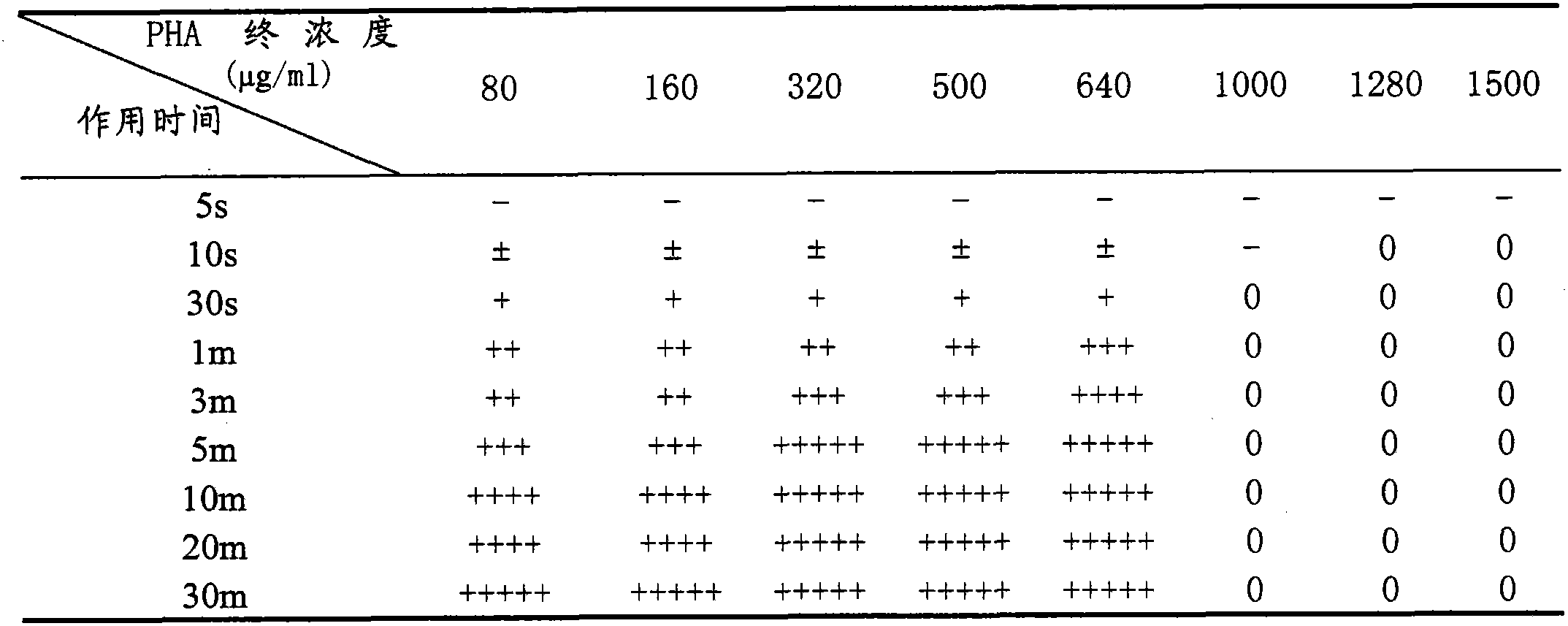
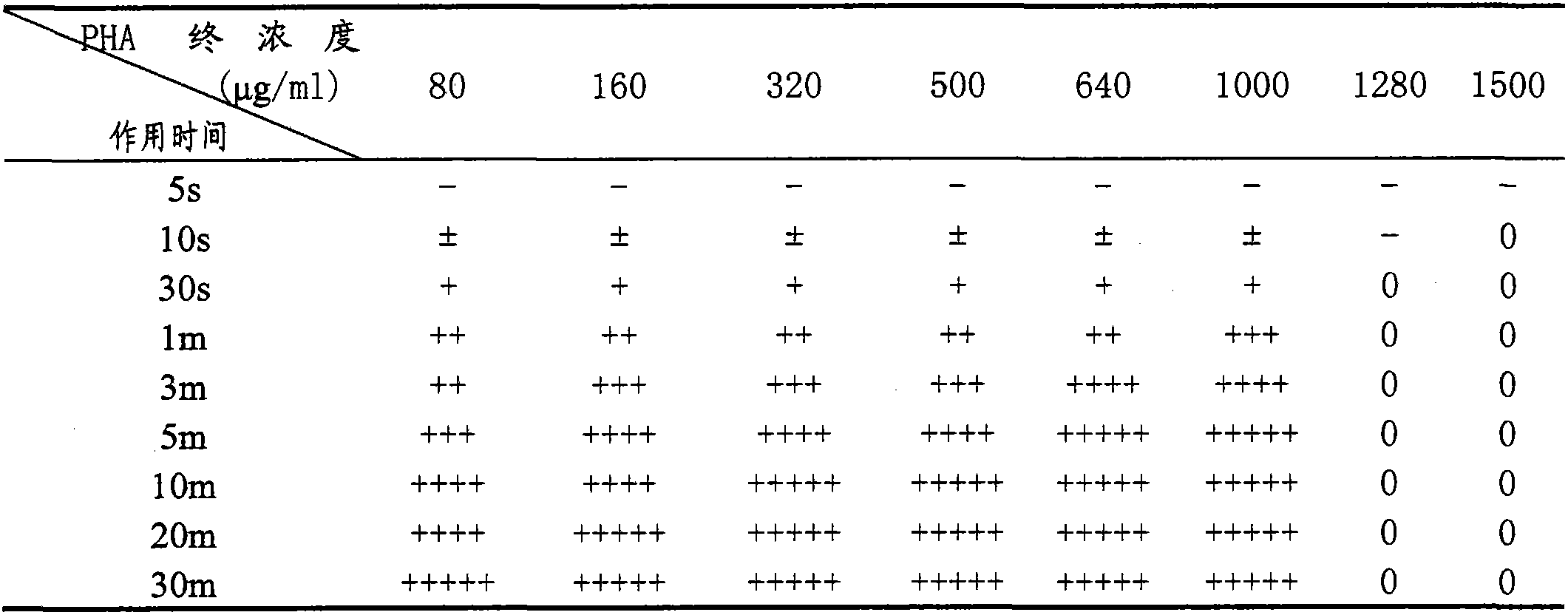
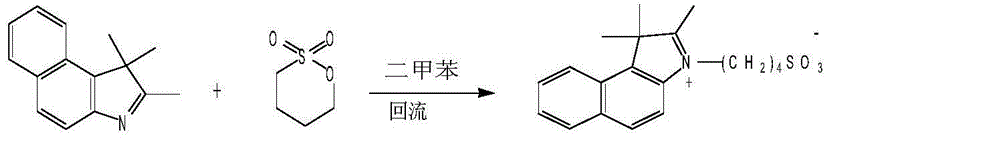
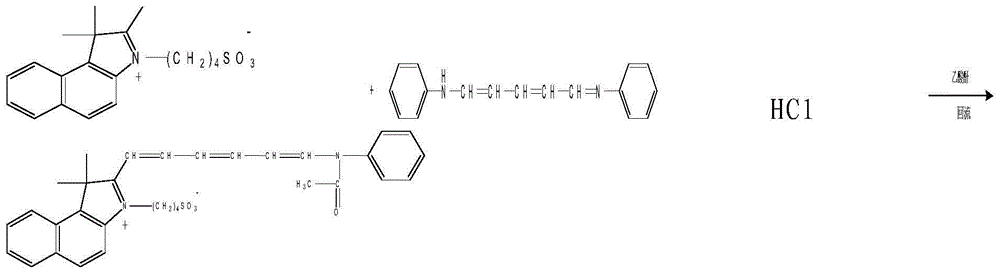
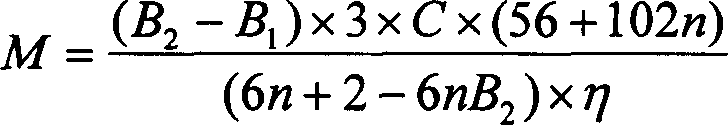Patents
Literature
867 results about "Pharmaceutical technology" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS7175854B2Improve stabilityUniform deliveryBiocidePowder deliveryEngineeringBULK ACTIVE INGREDIENT
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohol, triglyceride, partial glyceride and fatty acid ester.
Owner:ASTRAZENECA AB
Novel crystal form of ibrutinib and preparation method thereof
InactiveCN105085529AOrganic active ingredientsOrganic chemistryCombinatorial chemistryPharmaceutical technology
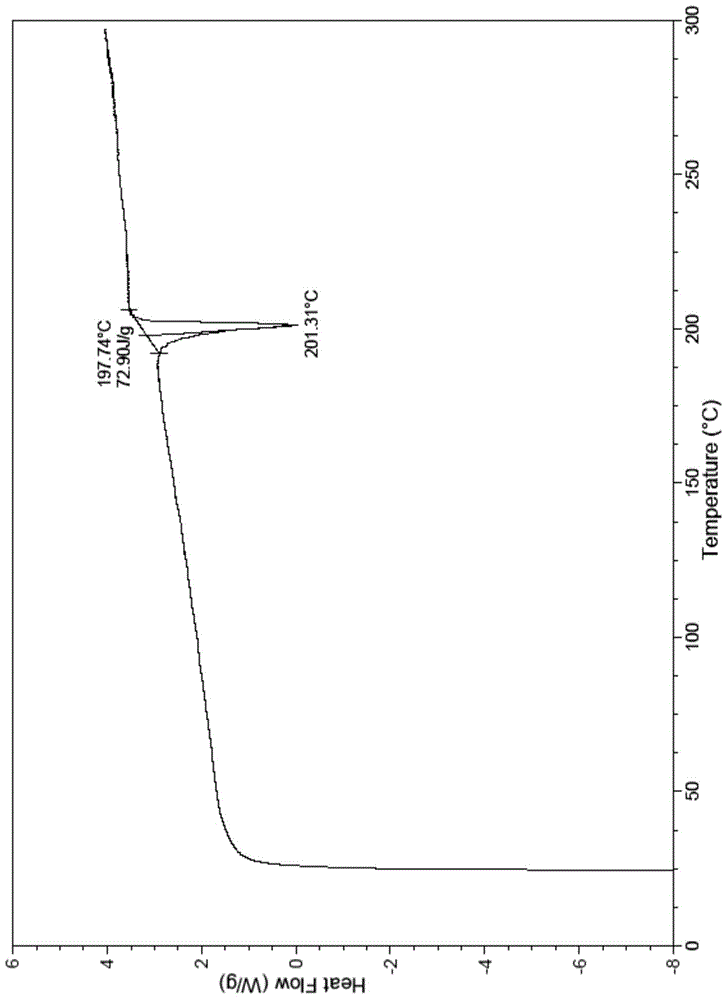
The present invention relates to a novel crystal form of ibrutinib and a preparation method thereof, and belongs to the technical field of pharmacy. The differential scanning calorimetry curve of the novel crystal form has endothermic peak at 194-204 DEG C; and the novel crystal form has good physical and chemical properties, is conducive to the production operation, and can be used in the preparation of pharmaceutical preparations.
Owner:SUNSHINE LAKE PHARM CO LTD
Ginseng-based whitening and anti-aging nanoemulsion essence and preparation method thereof
InactiveCN103462846AEasy to prepareGood repeatabilityCosmetic preparationsToilet preparationsBiotechnologyFruit juice
The invention relates to the technical field of pharmaceutical nanoemulsion drug delivery systems and transdermal absorption, in particular relates to ginseng-containing whitening and anti-aging nanoemulsion essence and a preparation method thereof, and belongs to a new pharmaceutical technology and the field of study on new formulations applied to cosmetics. The nanoemulsion essence is characterized in that a natural or food-grade emulsifier and an oil phase are selected, wherein double distilled water containing fruit juice, vegetable juice or honey is used as an oil phase. The nanoemulsion essence comprises the following components in percent: 10.30 to 80.60% of emulsifier / co-emulsifier, 1.0 to 40.80% of oil phase, 0.10 to 14.0% of ginseng, and the balance of the water phase. The confocal microscopy result shows that the transdermal absorption mechanism of the essence is the mechanism of majorly dispersing among cells of cutaneous appendages at the beginning and then dispersing and penetrating through cytomembrane. The nanoemulsion essence has high absorption, is innocuous, unpoisonous and pollution-free, does not damage the skin, and can completely realize the green, health, whitening, anti-aging and beautifying makeup concept.
Owner:JILIN UNIV
Highly effective polypeptide for inhibiting angiogenesis, physical chemistry modifying method and application thereof
InactiveCN101143894APrevent proliferationLittle side effectsPeptide/protein ingredientsPeptidesIon exchangeGenetic engineering
The invention relates to a high-performance angiogenesis inhibitor and a production method, which belongs to the field of the biological engineering pharmaceutical technology or protein polypeptide drugs. The invention designs a high-performance angiogenesis inhibitor RGD-ED with integrin compatibility, the inhibitor contains angiogenesis inhibition polypeptide isoleucine-valine-arginine-arginine-alanine-aspartate-arginine-alanine-alanine-valine-proline, and one or two ends of the inhibitor are respectively connected with polypeptides containing arginine-glycin-aspartate sequence. The RGD-ED of the invention can be synthesized. By the method of genetic engineering, the invention also expresses one of RGD-Eds in escherichia coli or other eukaryotic cells, and the RGD-ED is obtained by carrying out the separation, dissolution and renaturation of inclusion body protein and separation and purification by ion exchange chromatography. All the polypeptide sequences of the invention are modified by Polyethylene Glycol (PEG), heparin, dextran, polyvinylpyrrolidone (PVP), polyglycol-poly (amino acid) copolymer, palmitic acid, colominic acid and liposome, which includes liposome (REV), drying liposomal (DRV) and multivesicular liposome (Mvl). In vivo and in vitro experiments, the synthetic polypeptide sequence, product of genetic engineering and modified product of the invention can notably increase the effects of inhibiting the growth of endothelial cells, inhibiting angiogenesis and resisting tumor of the present angiogenesis inhibitors, and moreover, the high-performance angiogenesis inhibitor can be used as a drug curing solid tumors and rheumatoid arthritis.
Owner:CHINA PHARM UNIV
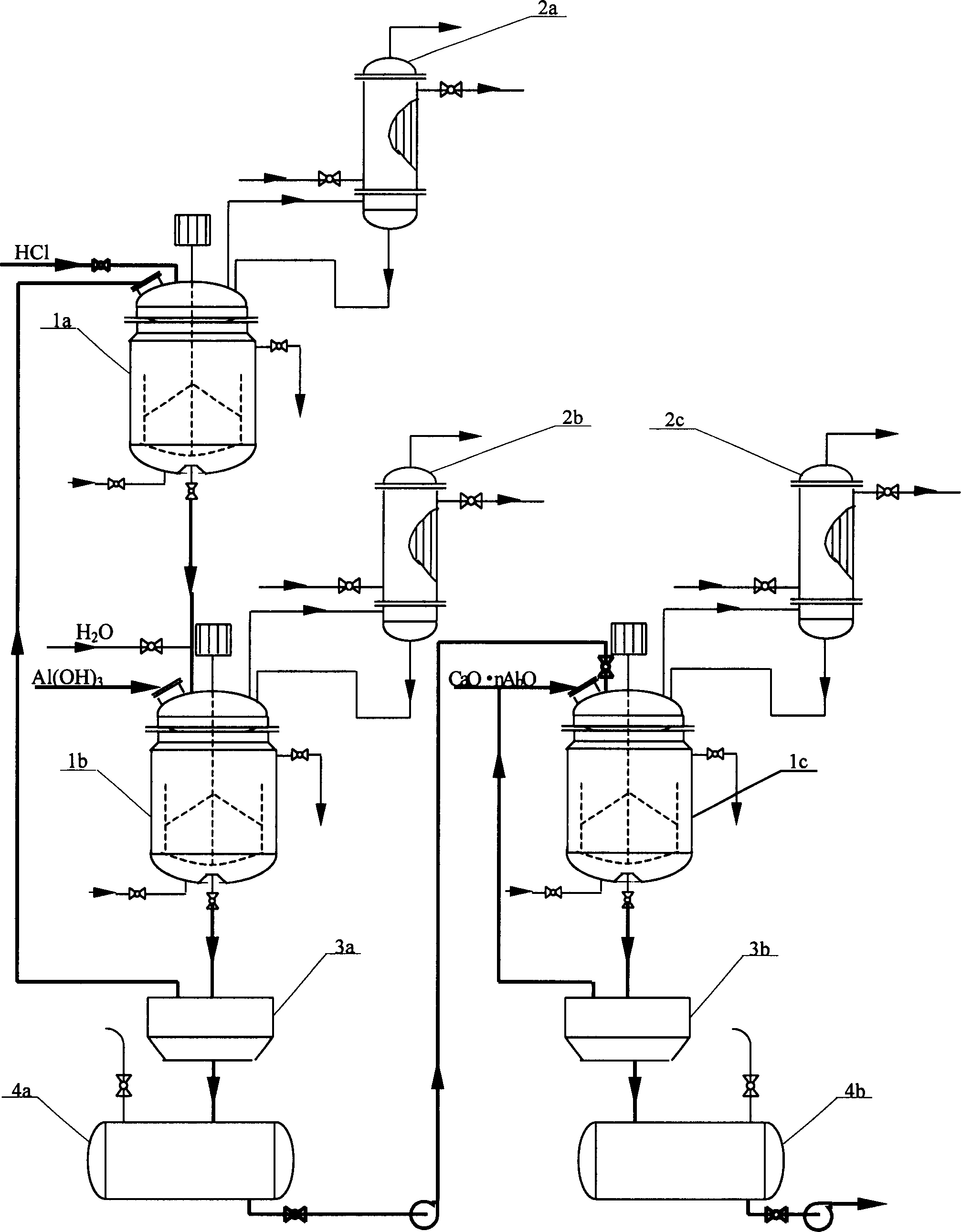
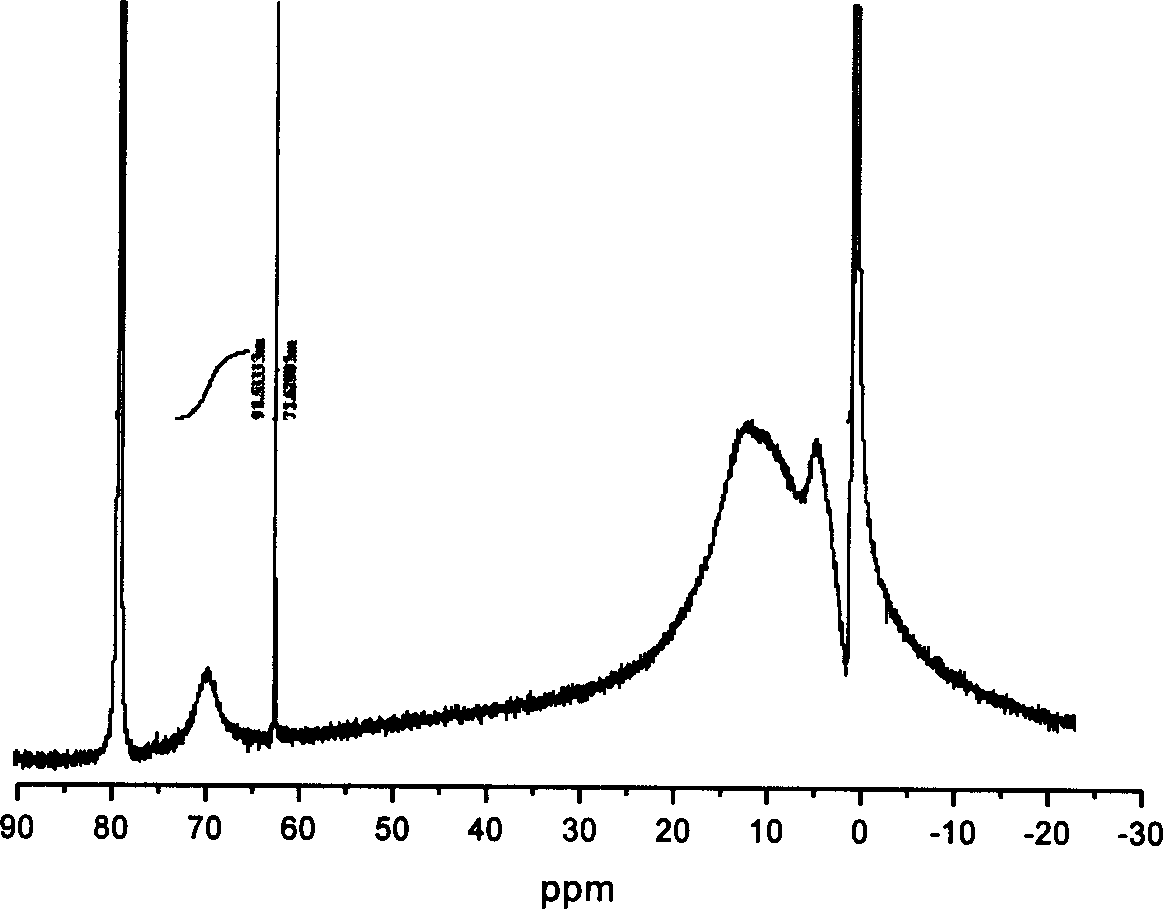
Method and technique for preparing Nano type sol of polyaluminium chloride in high purity
This invention describes a process for preparing high-purity nanoscale polyalumina sols. This invention uses active aluminum hydroxide and industrial hydrochloric acid as raw materials, and adopts a two-stage reverse stripping process to produce a solution of polyaluminum chloride with an alkalinity higher than 50%, which is then increased to 70% or higher using high-purity calcium aluminate as the alkalizer. The process ensures complete stripping of the aluminum hydroxide raw material at normal atmosphere and a low temperature, thus obtaining a high-purity product. In addition, in the produced polyaluminum chloride sols the total content of nanoscale Al13 and Al30 having Keggin structure is higher than 70%, the alkalinity is 70-85% and the aluminum concentration is 10-19% (measured as Al2O3). The product is widely used as raw material for modern fine chemical binders, gluing agent for neutral paper manufacturing, intermediate or additive for modern pharmaceutical technology, coupling agent and catalyst for chemical production and flocculant for water treatment, and has potential applications in such emerging fields as inorganic nanocomposites and inorganic membrane materials.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Elastic nano vesicle carrier and preparation and application thereof
InactiveCN101209348AGood percutaneous penetrationImproves transdermal penetrationElectrotherapyAntipyreticLipid formationNatural barrier
The invention pertains to the field of pharmaceutical technology, which more particularly relates to an elastic nano-vesicle carrier which can transport the active ingredients of the drug to penetrate the natural permeability barrier or pores (such as skin, mucosa, and organs, etc.) and the preparation method, as well as the usage. The vesicle has lipid double molecular layers for transporting one or more active ingredients to penetrate the natural barrier. The vesicle at least contains three dressing components of phospholipid, membrane softener and alcohol, and the three have different physical and chemical properties. The diameter of the typical nano-vesicle is less than 200nm. The vesicle carrier containing the active ingredients of the drug can be applied in the injection, spray and the transdermal preparation.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
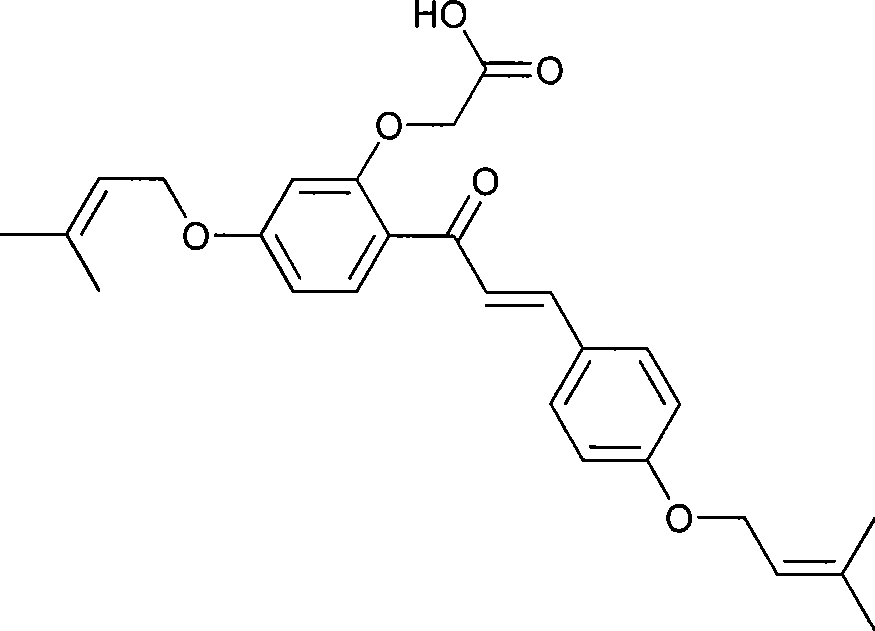
Novel preparation of sofalcone
InactiveCN101434533ASimple processing methodHigh yieldOrganic compound preparationCarboxylic compound preparationOxygenAcetophenone
The invention pertains to the technical field of chemical pharmaceutical production and particularly provides a novel preparation method of sofalcone which has a general formula (I) structure and is mainly applied to the treatment of gastric ulcer and chronic gastritis. The preparation method adopts 2-hydroxy-4-(3-methyl-2-butene oxygen) hypnone as a starting material and a target compound sofalcone (I) is finally prepared after reactions with four steps. Compared with synthesis technologies provided by the existing literatures, the method simplifies the processing method of intermediate products, improves the quality and yield of products and reduces costs.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Oral disintegrative tablet of sildenafil citrate and preparation method thereof
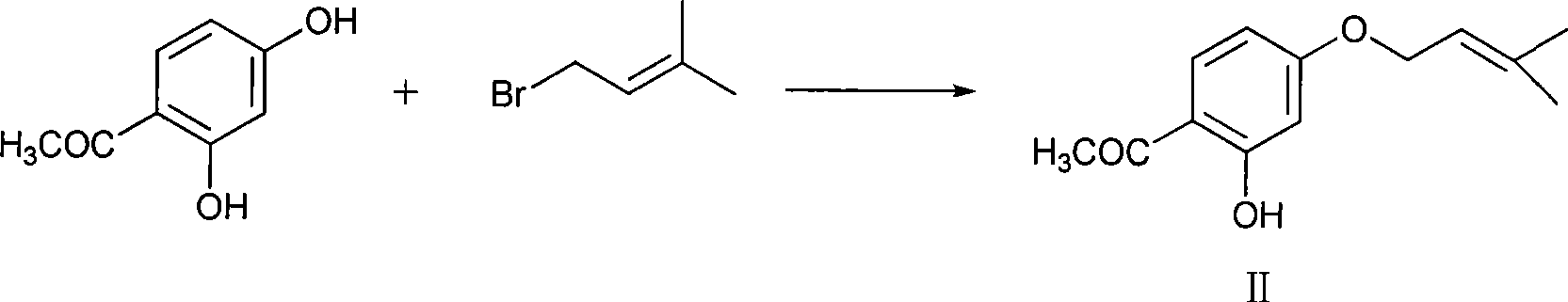
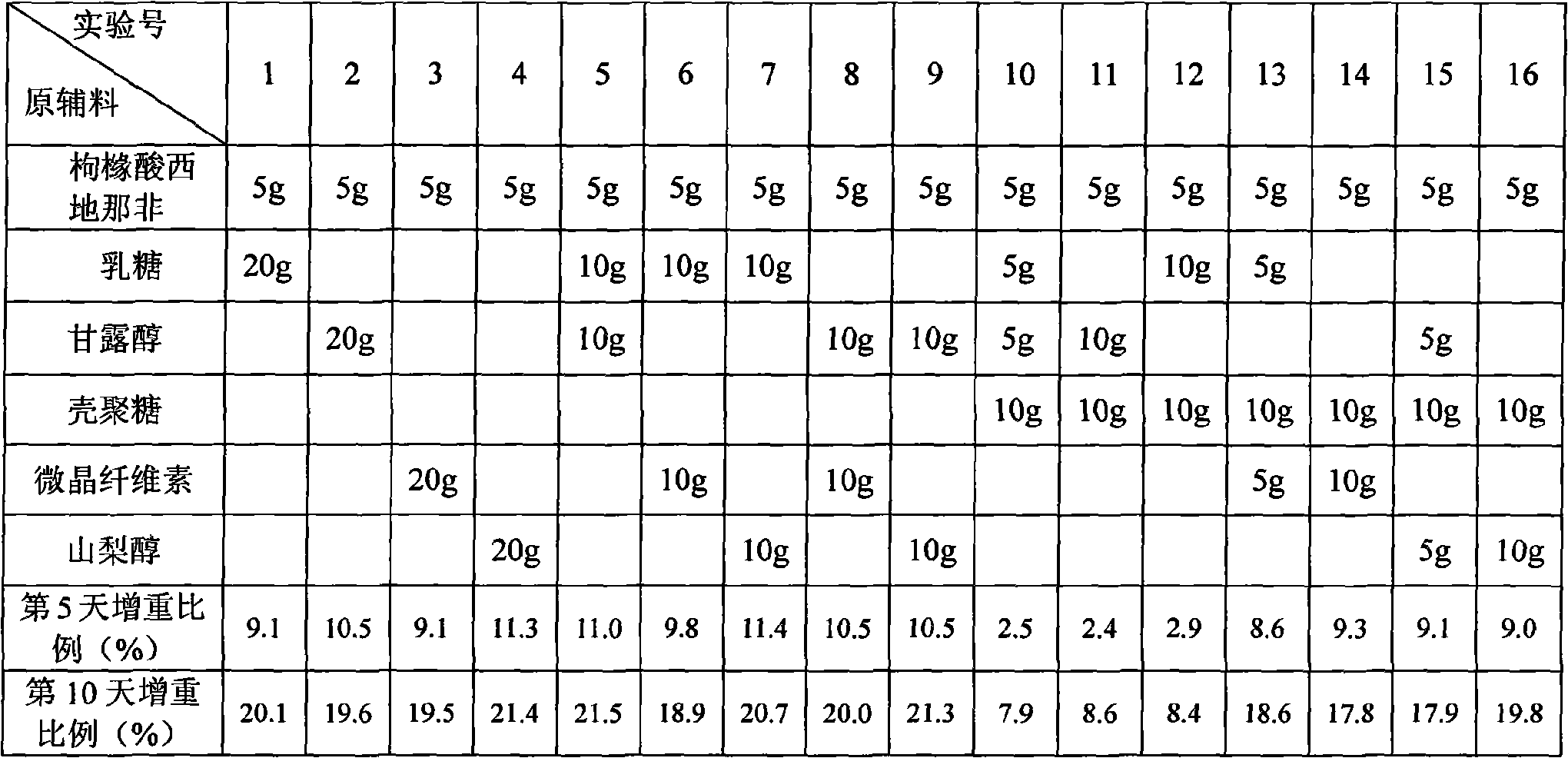
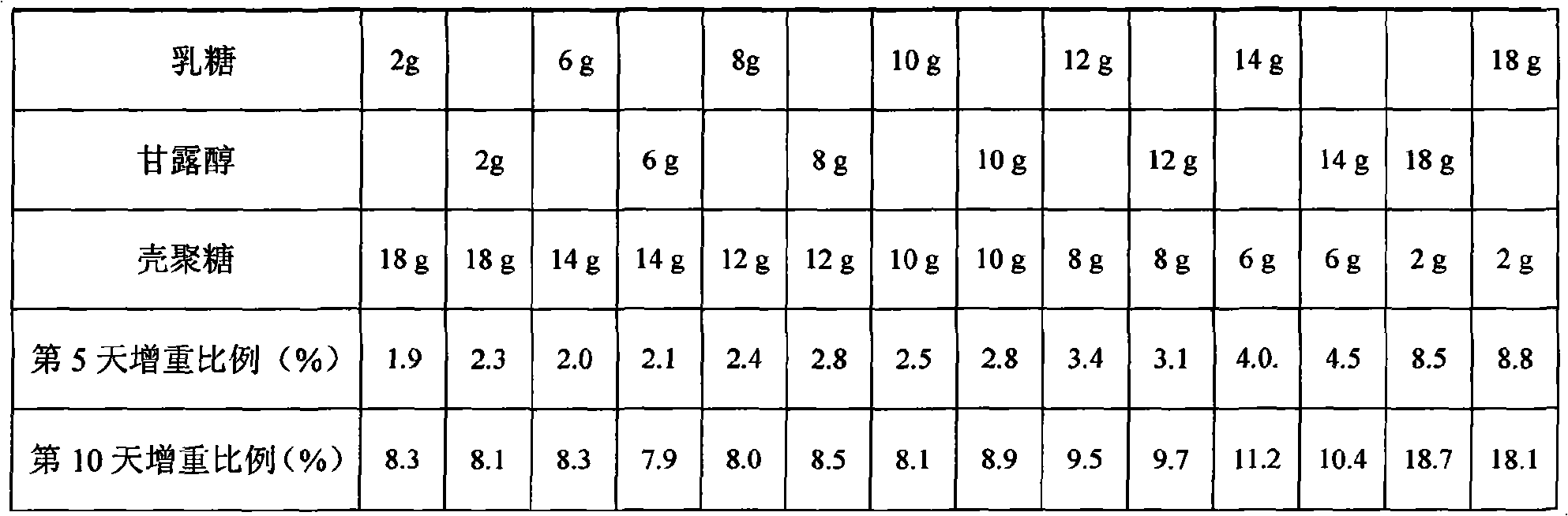
InactiveCN101683324AImprove moisture resistanceReduce contentOrganic active ingredientsPill deliveryCITRATE ESTERMANNITOL/SORBITOL
The invention belongs to the technical field of pharmacy, in particular to an oral disintegrative tablet for treating male erection dysfunction by using sildenafil citrate as an active component and apreparation method thereof. The oral disintegrative tablet of sildenafil citrate comprises the following components in parts by weight: 2.5-10 sildenafil citrate, 10-20 diluent, 0.5-1 disintegrant, 0.1-0.2 lubricant, 0.05-0.1 taste modifier and 0.01-0.02 bonder (accounted by povidone K30), wherein the diluent is a compound jointly formed by chitosan and one or two of lactose and mannitol. The oral disintegrative tablet remarkably improves the humidity resistance and is beneficial to improving the stability of the preparation and ensuring the quality in the production and storage processes.
Owner:张晓芳
Pharmaceutical composition containing ligustrazine and effective component of ginkgo leaf and formulation thereof
InactiveCN1965870AAddressing Medication Safety IssuesOrganic active ingredientsGinkgophyta medical ingredientsDiseaseGinkgo biloba
The invention relates to pharmaceutical composition and relevant preparation containing active constituents of ligustrazine and ginkgo leaves, wherein the compound preparation can act on human body by employing the synergism between the two constituents, and is effective in activating blood circulation, removing stasis, resisting platelet conglomeration, expanding blood vessel, improving microcirculation, thus can be used for preparing medicament for treating ischemic cardiovascular and cerebrovascular diseases.
Owner:北京天新园医药科技开发有限公司
Artificially synthesized antimicrobial peptide, preparation method and application thereof
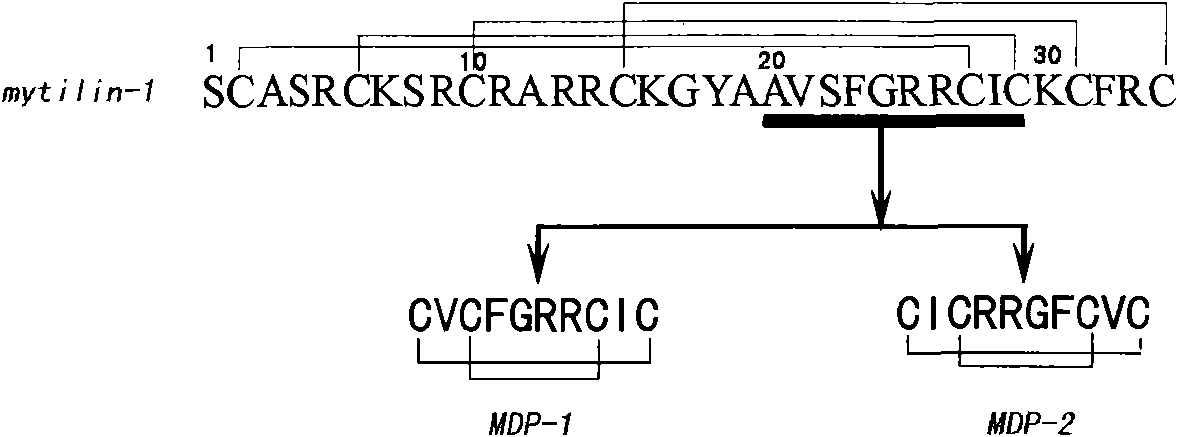
InactiveCN102079777ASmall molecular weightStable structureAntibacterial agentsAntimycoticsAntimicrobial peptidesGram-positive bacterium
The invention discloses an artificially synthesized antimicrobial peptide, which belongs to the field of biological pharmaceutical technology. The amino acid sequence of the artificially synthesized antimicrobial peptide is Cys-Val-Cys-Phe-Gly-Arg-ARG-Cys-Ile-Cys or Cys-Ile-Cys-Arg-Arg-Gly-Phe-Cys-Val-Cys. The invention also discloses a method for artificially synthesizing the antimicrobial peptide and application of the artificially synthesized antimicrobial peptide in development of preparing medicaments for treating Gram-positive bacteria and Gram-negative bacteria or feed additives. Compared with the prior art, the artificially synthesized antimicrobial peptide has the following advantages and effects, such as small molecular weight as compared with the natural ones, stable structure, strong antimicrobial activity, short sequence, less disulfide bond number, low cost and the like.
Owner:ZHEJIANG OCEAN UNIV
Targeting preparation for resisting drug-resistant tumor, as well as preparation method and application thereof
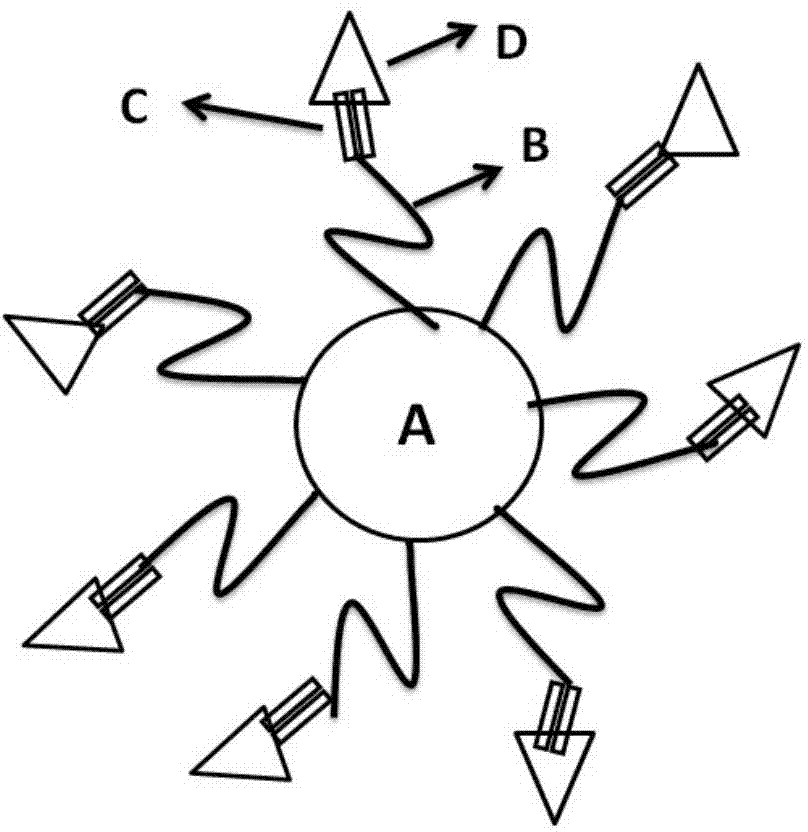
ActiveCN103768080ASmall toxicityHas acid sensitive propertiesOrganic active ingredientsPharmaceutical non-active ingredientsSide effectPolyethylene glycol
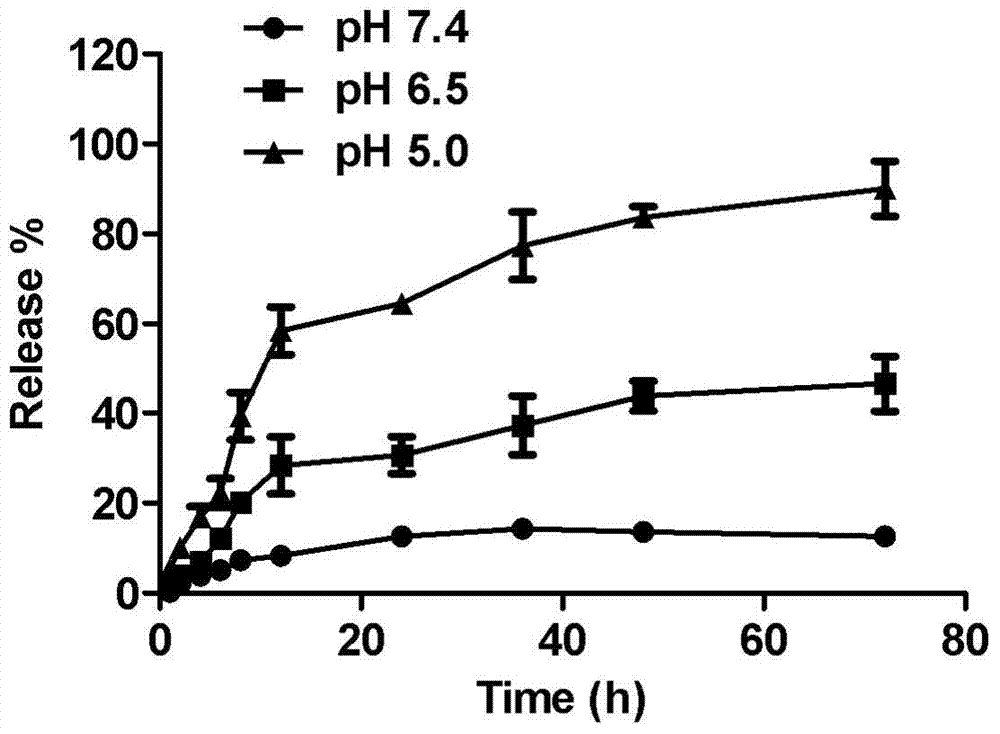
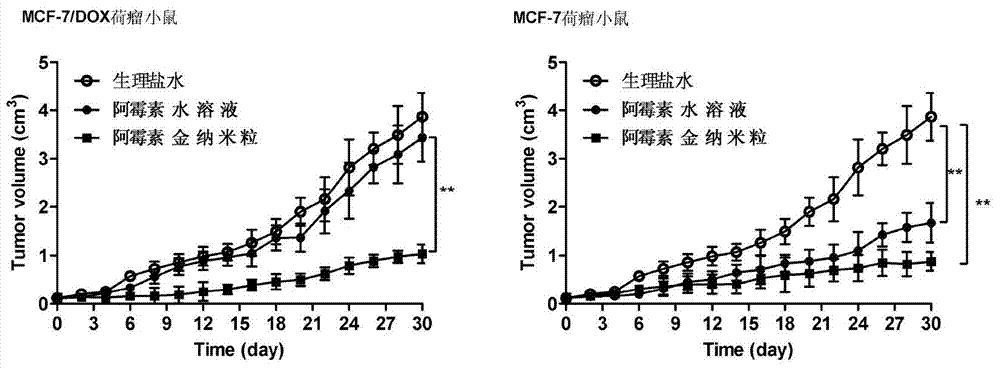
The invention relates to a medicinal preparation, particularly relates to a targeting preparation for resisting drug-resistant tumor, as well as a preparation method and application thereof, and belongs to the technical field of pharmaceuticals. The targeting preparation for resisting drug-resistant tumor comprises a part A which is gold nanoparticles, a part B which is polyethylene glycol, a part C which is a hydrazone bond, and a part D which is a chemotherapeutic drug. The targeting gold nanoparticle drug delivery system prepared by linking amycin and gold nanoparticles via hydrazone bond has acid sensitivity, and can specifically release amycin to an environment with pH less than 6 to realize specific response to microenvironment (lysosome and endosome, pH less than 6) in drug-resistant tumor. The targeting preparation provided by the invention meets the needs for drug-resistant tumor therapy and can be used for reducing toxic and adverse side effects of amycin.
Owner:哈尔滨医大药业股份有限公司
Pharmaceutical preparation comprising an active dispersed on a matrix
InactiveUS20070122474A1Improve stabilityUniform deliveryPowder deliveryOrganic non-active ingredientsTriglycerideEngineering
The present invention relates to the field of pharmaceutical technology and describes a novel advantageous preparation for an active ingredient. The novel preparation is suitable for producing a large number of pharmaceutical dosage forms. In the new preparation, an active ingredient is present essentially uniformly dispersed in an excipient matrix composed of one or more excipients selected from the group of fatty alcohols, triglycerides, partial triglycerides and fatty acid esters.
Owner:ASTRAZENECA AB
Antioxidant and anti-aging medicinal and health-care product
InactiveCN104855957ACompatibility scienceAccurate dosageFood ingredient as antioxidantNatural extract food ingredientsGrape seedFood culture
The present invention relates to an antioxidant and anti-aging astaxanthin health-care composition and preparation method thereof. The composition comprises the following components in parts by weight: astaxanthin 120-160 parts, lycopene 83-133 parts, and grape seed extracts 67-89 parts. The composition can be processed into hard capsules, soft capsules and oral liquid by adding accessories accepted by the pharmaceutical technology. By using astaxanthin, lycopene, and grape seed extracts as raw materials, aiming at the physical signs and food culture features of Chinese people, and scientifically quantifying and blending the raw materials, the composition maximally exerts the synergistic effect of anti-oxidation, and makes the functional features of these three varieties complement each other, thus leading to comprehensive antioxidant effect and remarkable anti-aging effect.
Owner:李燕铭 +1
Edible health collocryst and its preparation method
InactiveCN101283783AThe preparation process is concise and practicalEasy to useAerosol deliveryOintment deliveryAdjuvantPlasticizer
An edible healthy gelatin crystal and the preparation method thereof belong to the field of Chinese healthy product processing technology. The edible healthy gelatin crystal is prepared from one, two, three, or four of preprocessed donkey skin, deer horn, velvet and turtle shell as main materials or from one, two or three of donkey-hide gelatin, deer-horn gelatin and turtle shell gelatin as main materials by soaking in water, cutting into small pieces, decocting with water, filtering, reducing water to 20 to 35% by concentration with slow fire to obtain gelatin paste for standby, adding adjuvant, cooling, shaping, and drying in the shade to obtain the product. Based on the prior pharmaceutical technology, the invention can prepare a directly-edible functional food mainly containing donkey-hide gelatin, deer-horn gelatin, velvet, turtle shell gelatin, etc. by decocting animal skin, bone, shell or horn with water to obtain gelatin or drying the gelatin, and adding plasticizer and other additional components. The functional food can be made into 'soft capsule' that can be applied directly, and has simple and practical preparation process and convenient usage. The soft capsule preparation has the advantages of convenient usage, good portability and wide application range, and can be taken orally, swallowed directly or applied externally.
Owner:钟世杰
Eye drop used for releasing eye fatigue and preparation method thereof
InactiveCN103393969ARelieve conjunctival congestionRelieve symptoms such as eye itchingSenses disorderPharmaceutical delivery mechanismEye/ear dropsSide effect
The invention relates to an eye drop used for releasing eye fatigue and a preparation method thereof. The eye drop comprises following raw materials: radix rehmanniae, rhizoma atractylodis, crab grass, serissa japonica, jasmine, red-spotted stonecrop, wolfberry, astragalus smicus, python gall, cocklebur, chamomile, concha haliotidis, Taiwan beautyberry, globe amaranth, pearl and weathered sodium sulfate powder. The eye drop is a pure traditional Chinese medicine preparation; is prepared by modern advanced pharmaceutical technologies; has efficacies of clearing away heat and purging pathogenic fire, relieving spasm and removing eyewinker, and nourishing the liver and improving visual acuity; and can be used for treating diminution of vision and pseudomyopia of teenagers, and reliving visual fatigue of eyes, and symptoms caused by visual fatigue such as conjunctival injection and eye itch. Compatibility of the selected raw materials is appropriate, curative effects are significant, safety performance is excellent, no side effects exist, cost is low, and the eye drop is worth of wide popularization and application.
Owner:吴春子
Method for preparing microspheres with solid-in-oil-in-hydrophilic oil-in-ethanol
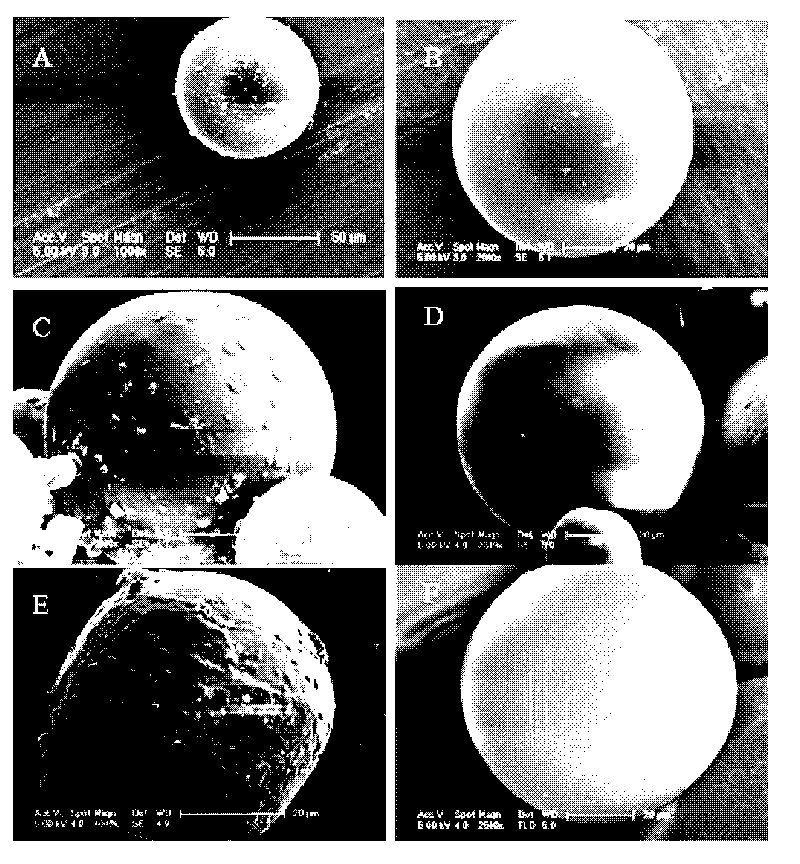
InactiveCN101721377AAvoid the effects of treatmentDisadvantages of Avoiding PollutionGranular deliveryMacromolecular non-active ingredientsDispersityControlled release
The invention relates to a method for preparing microspheres with solid-in-oil-in-hydrophilic oil-in-ethanol, belonging to the field of the pharmaceutical technology and comprising the following steps: (1) adding and stirring or swirling medicament particles to the organic solution, i.e. the oil phase of sustained-release or controlled-release material; (2) adding mixed suspension to another hydrophilic organic solution, i.e. hydrophilic oil phase and stirring the mixed suspension to the hydrophilic organic solution, i.e. the hydrophilic oil phase for 0.1-5 min to form spheres of 1-500 Mum; (3) transferring the mixed suspension containing the microspheres to ethanol and solidifying the mixed suspension for 1-4h; (4) and drying and freezing the sample to obtain the dry microspheres. The diameter of the microspheres can be controlled and adjusted from 1 Mum to 500 Mum according to the need; the microspheres can not cause pollution to the environment; the surfaces of the microspheres aresmooth and round; the microspheres are consistent without being adhered; the frozen-dried powder of the microspheres is white, fine and loose, can not collapse, can not be adhered and has good dispersity. The method can be used for preparing various sustained-release or controlled-release microspheres of various medicament or preparing the adjuvant of vaccines.
Owner:SHANGHAI JIAO TONG UNIV
Method for preparing purine derivatives
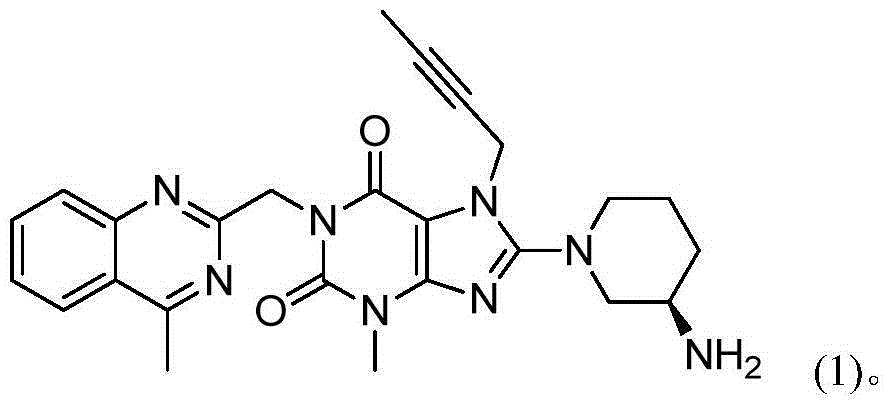
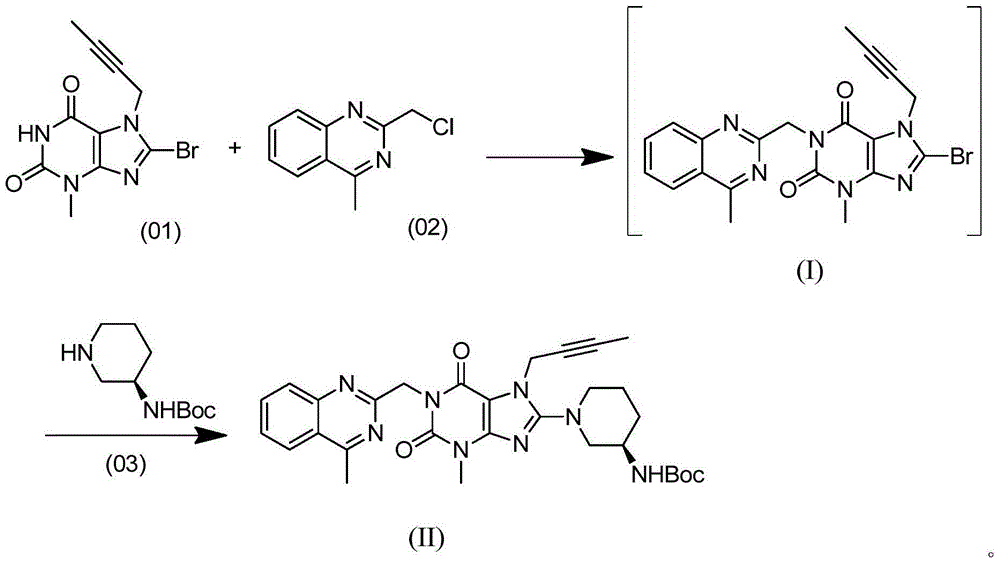
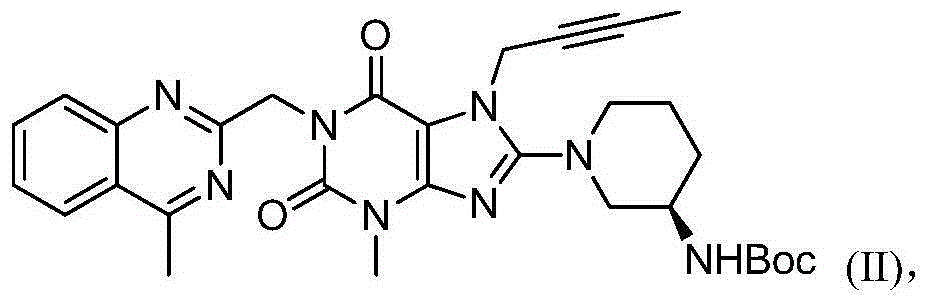
The invention provides a method for preparing an intermediate of trajenta and belongs to the technical field of pharmacy. The method comprises the following steps: reacting 8-bromo-7-(2-butynyl)-3-methyl-1-purine-2,6-dione with 2-chloromethyl-4-methylquinazoline to obtain a reaction liquid; reacting the reaction liquid with (R)-3-tert-butyloxycarboryl-aminopiperidine to obtain 8-[(3R)-3-(tert-butyloxycarboryl-amino)-1-piperidyl]-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)-methyl]-1H-purine-2,6-dione. In the reacting process, an intermediate product is not separated. According to the preparation method, the intermediate product does not need to be separated or purified, the operation is simplified, the yield is high, the production cost is reduced, and the industrial production is benefited.
Owner:SUNSHINE LAKE PHARM CO LTD
Air purification device and method for clean pharmaceutical workshop
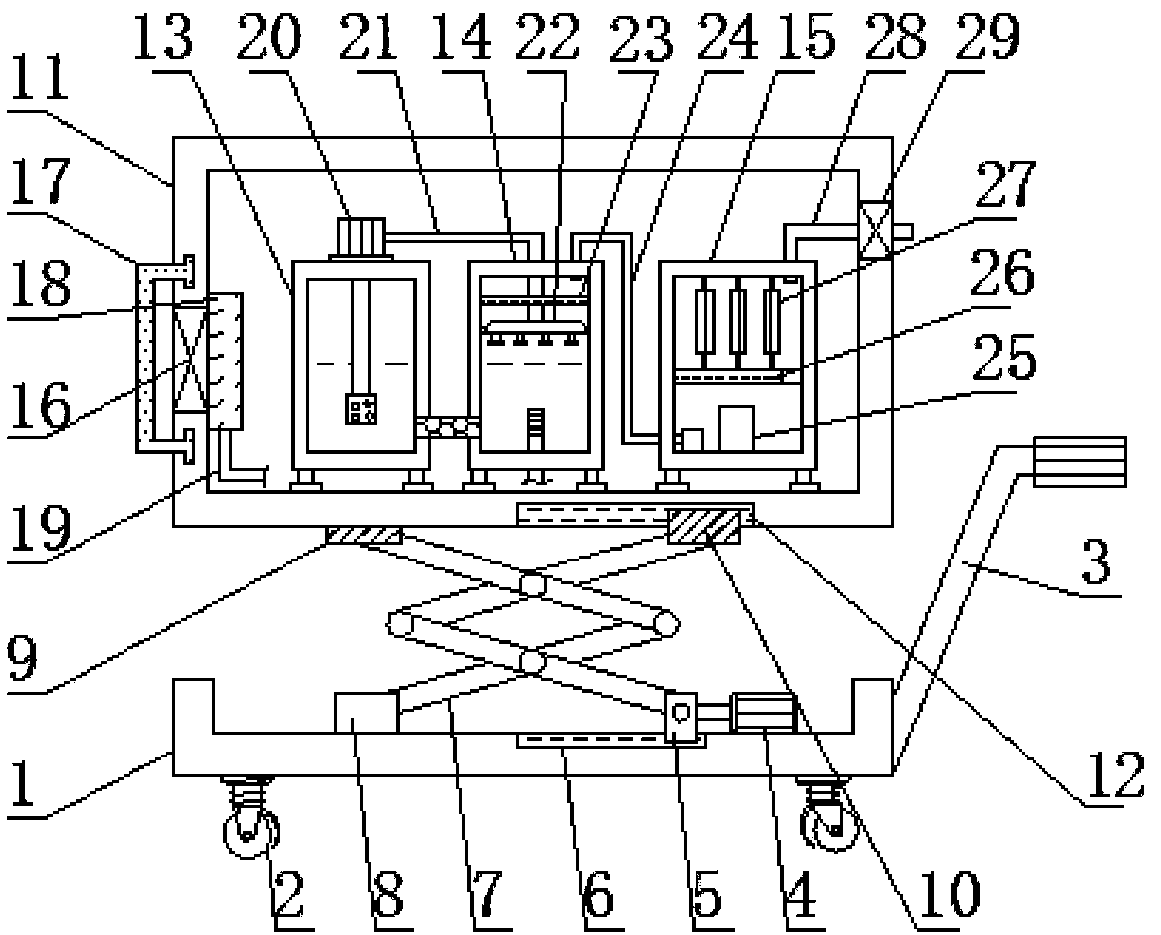
InactiveCN108332301AImprove comfortIncrease frictionMechanical apparatusLighting and heating apparatusActivated carbonHazardous substance
The invention discloses an air purification device for a clean pharmaceutical workshop in the technical field of pharmacy. The air purification device comprises a base, wherein a hydraulic device is mounted on the right side of the top of the base; an upper fixed block and an upper slider are mounted at tops of left and right ends of a scissor-type lifting frame respectively; a water storage tank,a purifying tank and a drying and sterilizing tank are arranged at the bottom of an inner chamber of a casing from left to right sequentially; an intake fan is mounted on the outer wall of the left side of the casing; the right end of an exhaust pipe is connected with an exhaust fan. The an air purification device is simple to operate and high in purifying efficiency, dust and impurities with larger particles in the air are filtered out preliminarily by a filter screen plate, hazardous substances in the air are adsorbed effectively by activated carbon bars, the air purified by the purifying tank is leached with clear water again through water spray holes in a spray plate, the air is further purified, the air is finally discharged after being sterilized by an ultraviolet sterilization lampgroup, and secondary pollution of the air by dust and impurities in the pharmaceutical workshop due to incomplete purification is avoided.
Owner:苏州万君筑天科技有限公司
Preparation method of citalopram intermediate
InactiveCN105294496ACarboxylic acid nitrile preparationOrganic compound preparationGrignard reagentNitrogen
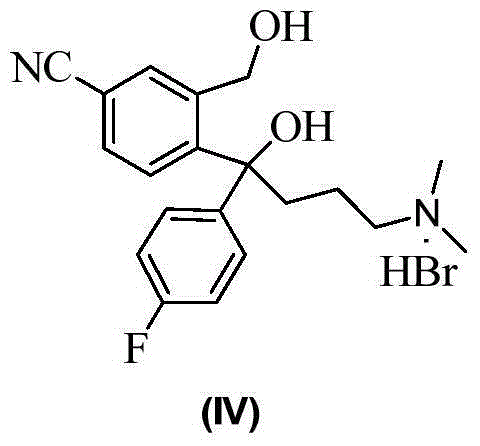
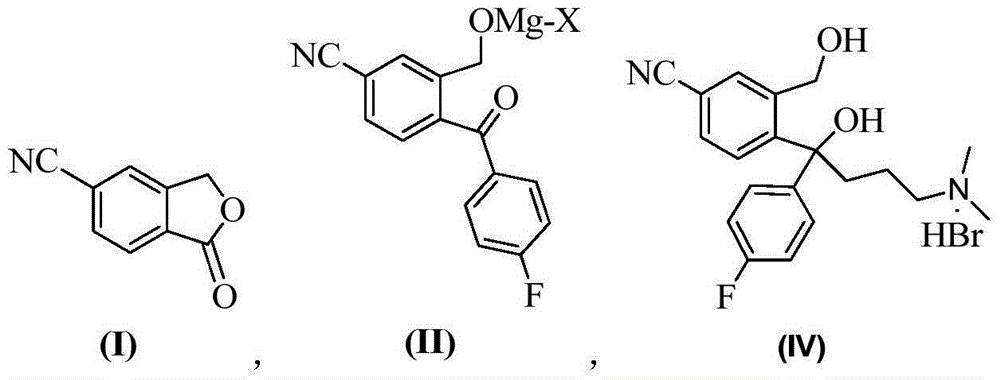
The invention provides a preparation method of a citalopram intermediate, and belongs to the technical field of pharmaceuticals. In the method, 2-methyltetrahydrofuran is taken as a reaction solvent. Under the protection of nitrogen, a 4-fluorophenylmagnesium bromide solution Grignard reagent, 5-cyanophthalein and a N,N-dimethylpropyl magnesium chloride Grignard reagent are taken as raw materials. A reaction is carried out to synthesize 4-(4-(dimethylamino)-1-(4-fluorophenyl)-1-hydroxybutyl)-3-(hydroxymethyl)benzonitrilehydrobromide. The method is characterized by high yield and high purity, and is suitable for industrial production.
Owner:SUN YAT SEN UNIV +1
Pharmaceutical preparation in the form of a suspension comprising an acid-labile active ingredient
The present invention relates to the field of pharmaceutical technology and describes a novel pharmaceutical preparation in the form of a suspension comprising an acid-labile active ingredient, in particular an acid-labile proton pump inhibitor. The invention also relates to processes for producing the suspension. The suspension is particularly suitable for administering acid-labile active ingredients to people who have difficulty taking solid dosage forms such as tablets or capsules.
Owner:TAKEDA GMBH
Method for preparing (+)-(S-)-clopidogrel hydrosulfate high melting point crystal I
ActiveCN101100471AAvoid it happening againSufficient precipitationOrganic chemistryHydrogen SulfateAlcohol
Preparation of (+)-(S)-chlorpyrroline hydrogen sulfate high-smelting point crystal Iis carried out by dissolving chlorpyrroline free alkali into ketone solvent, adding into concentrated sulfuric acid, eluting crystal out, adding into alcohol solvent, agitating while crystallizing, filtering, washing for filter cake by ketone solvent, pumping and vacuum drying to obtain final product. It's simple and efficient, has gentle reactive condition and can be used for industrial production.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Stephania hainanensis total alkaloid extract and preparation method thereof
The invention belongs to the technical field of pharmacy, and particularly discloses a stephania hainanensis total alkaloid extract extracted from stephania hainanensis tubers and a preparation method thereof. The preparation process is as follows: material (stephania hainanensis dry tubers); grinding; soaking in a moderate amount of 0.01 to 10 percent of hydrochloric acid or sulfuric acid; 1 to 4 times of ultrasonic extraction; filtration; storng-acid cation exchange resin purification; solvent recovery for drying; and stephania hainanensis total alkaloid extract. The extract mainly comprises L-tetrahydropalmatine, palmatine, fangchinoline, hanfangchin and other active ingredients, the percentage by weight of stephania hainanensis total alkaloid is greater than or equal to 70 percent, the percentage by weight of the palmatine is greater than or equal to 20 percent, and the percentage by weight of the fangchinoline is greater than or equal to 40 percent. Reports on the stephania hainanensis total alkaloid extraction process are not found in related literatures so far, the operation flow of the method is simple, the purity of the product is high, the efficacy is remarkable, and the method disclosed by the invention is applicable to industrial production and the exploitation and utilization of local stephania hainanensis resources.
Owner:朱毅
A kind of compound preparation for human body and application thereof
InactiveCN102274486AHigh activityEffective fertilizationCosmetic preparationsPeptide/protein ingredientsHuman usePhytohemagglutinins
A compound preparation for human external use and its application belong to the technical field of human preparations. The lectin and the white fungus polysaccharide are used as active components, and the relative weight ratio between the two active components contained in the preparation is: 0.5-1.0 parts of the lectin and 1.5-3.0 parts of the white fungus polysaccharide. Said lectin is preferably kidney bean lectin. The dosage form is preferably a gel solution. 100ml of the gel solution contains 0.5-1.0 grams of plant lectin, 1.5-3.0 grams of tremella polysaccharide, 0.8-0.9 grams of NaCl, and pH buffers, stabilizers, and preservatives. The preparation is mainly used for external use by humans during sexual behavior, and it also has the functions of contraception, prevention and treatment of sexually transmitted diseases and lubrication; it can also be used as skin care products. Its principle of action is brand new and will not affect the human endocrine system; it can interfere and prevent HIV and other pathogens from invading normal cells in the body; it is extremely safe.
Owner:YUNNAN NORMAL UNIV
Method of preparing traditional Chinese medicine unction formulated product for treating cervical erosion, colpitis
InactiveCN101108222AEvenly dispersedIncrease coating areaHydroxy compound active ingredientsAluminium/calcium/magnesium active ingredientsWide areaOil phase
The invention relates to a TCM ointment preparation method for cervical erosion and vaginitis, which belongs to the field of pharmaceutical technology. The preparation process is shown below: catechu and dry alum are extracted by adding water, and the mixed extraction solution are of the centrifugation, decompression, concentrating, spray and drying to get the dry extract powder for stand-by; porphyrizate borneol for stand-by; Boil phellodendron, sophora flavescens ait and lithospermum are decocted for three times by adding water, mix the water decoction to concentrate and alcohol precipitate, keep it static and get the ethanol from the supernatant liquid, and concentrate to get the thick paste for stand-by; screen ginsenosides head of over 80 to 120 for sieving; extract oil phase to melt and stir uniformity with maintained temperature for stand-by; extract water phase to stir uniformity with maintained temperature for stand-by; add oil phase to the water phase while stirring the mixture until the mixture shapes into a uniform and delicate paste; finally pack to get the invention. The medicine prepared by the invention can disperse uniformly in the vaginal cavity and coats wide area so that the medicine can be effectively infiltrates into mucosal folds, thus increasing the bioavailability and ensuring the efficacy. The invention is quite convenient for patients to take without contaminating the clothing.
Owner:修涞贵
Industrialized synthesis method of medicinal indocyanine green
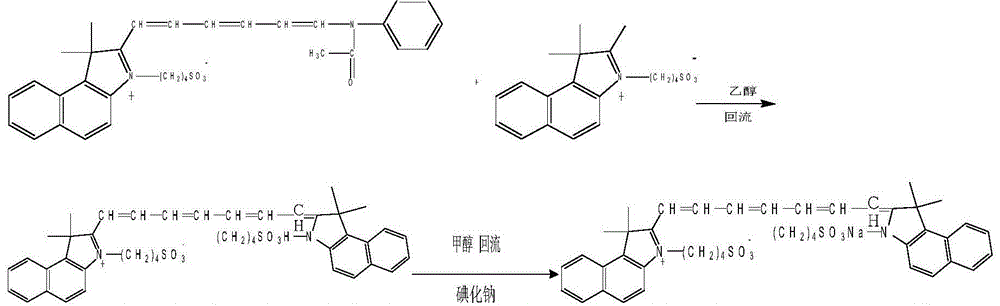
InactiveCN104130178AHigh yieldMild reaction conditionsOrganic chemistryAcetic anhydrideSynthesis methods
The invention belongs to the pharmaceutical technical field, and in particular relate to an industrialized synthesis method of medicinal indocyanine green. The synthesis method is as follows: 2, 3, 3-trimethyl-4, 5-benzo indole benzene and 1, 4-butyl sultone are mixed in the weight ratio of 1: 1.6 to 1.7 for reflux for 2-3 hours in the presence of xylene to obtain a light blue solid; the light blue solid and 2-glutacondianil hydrochloride are mixed in the weight ratio of 1: 0.9-1.1 weight ratio for reflux in the presence of acetic anhydride to obtain a blue black solid; the obtained light blue solid and the blue black solid are mixed in the weight ratio of 1: 1.3 to 1.7 for mixing and reflux reaction for 15-20min by addition of triethylamine in presence of anhydrous ethanol, after the reaction liquid is cooled, diethyl ether is added for extraction, the extraction liquid is filtered, filtrate is collected, a sodium iodide methanol solution is dripped, after the reaction, methanol is evaporated out, and the medicinal indocyanine green is obtained by drying. The synthesis method is mild in reaction conditions, improves yield, reduces cost, and reduces the product impurity content.
Owner:卫材(辽宁)制药有限公司
Sterilization disinfectant used for clinical laboratory and preparation method of sterilization disinfectant
InactiveCN103705649AImprove work efficiencyGuaranteed accuracyBiocideAntisepticsCudrania cochinchinensisSide effect
The invention relates to a sterilization disinfectant used for a clinical laboratory and a preparation method of the sterilization disinfectant. Raw materials for preparing effective constituents of the disinfectant comprises the bark of ash, sculellaria barbata, fisheye grass, saururus chinensis, prismatomeria tetrandra, large-leaved gentian, oriental wormwood, spina gleditsiae, cudrania cochinchinensis kudo et masam, eupatorium, fructus ulmi, Japan clover herb, witloof, cottonrose hibiscus, folium sennae and wikstroemia indica. Compatibility of medicinal materials selected by the disinfectant is appropriate, and the disinfectant is prepared by adopting modern advanced medicine pharmaceutical technology, strong in sterilization ability, fast in action speed and strong in stability, and the like, does not have stimulation on human skin, is soluble in water, does not have residue after use, is safe and doe not have toxic and side effects. The sterilization disinfectant can be widely applied to disinfection of staff skin, instruments, and the like of the clinical laboratory. By utilizing the sterilization disinfectant, the work efficiency of clinical laboratory staff is improved, the accuracy of inspection result is ensured, and the sterilization disinfectant is an ideal disinfectant worthy to be popularized.
Owner:聂俊杰
Medicinal composition containing bilobalide B and its preparation process and usage
The invention discloses a medicinal composition containing bilobalide B, which comprises Ligustrazine phosphate and bilobalide B by the weight ratio of 10-500 : 1. the invention also discloses the process for preparing the pharmaceutical composition.
Owner:弘和制药有限公司
Anti-human DLL4 monoclonal antibody and aplysiatoxin derivative MMAE conjugate
InactiveCN107375941AImprove Affinity VitalityRetain Affinity VitalityTetrapeptide ingredientsPharmaceutical non-active ingredientsAntiendomysial antibodiesTherapeutic effect
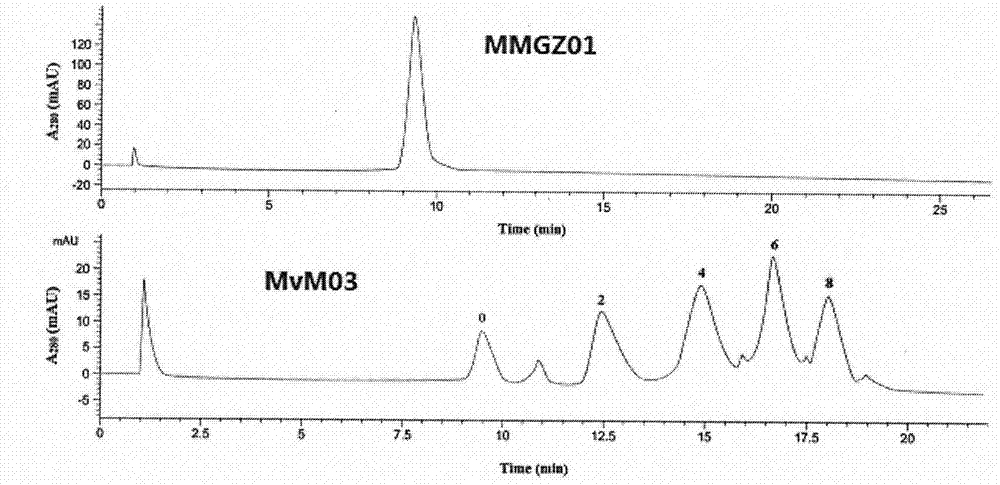
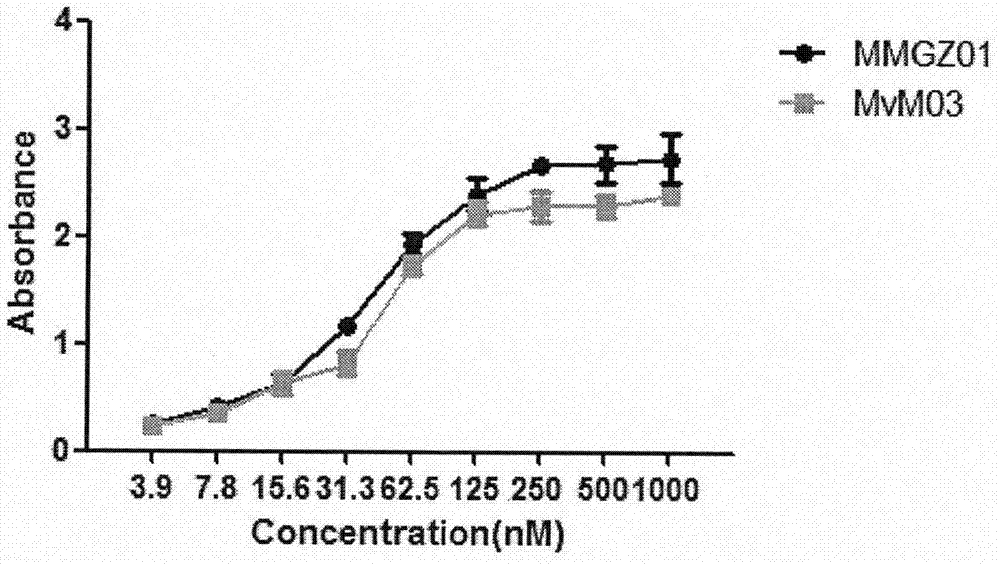
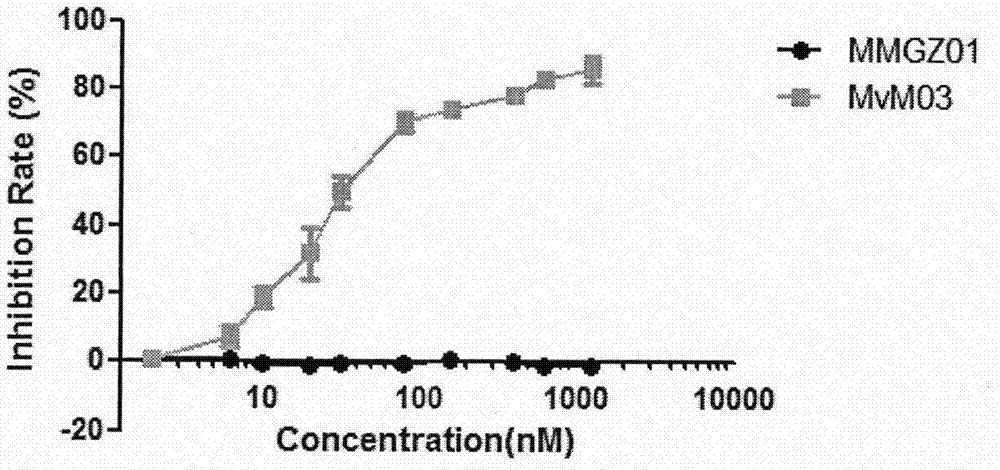
The invention discloses an anti-human DLL4 monoclonal antibody and aplysiatoxin derivative MMAE conjugate, which relates to the technical field of biological pharmacy. The invention is characterized by comprising a preparation method of the conjugate of micromolecule toxin aplysiatoxin derivative MMAE and the anti-human DLL4 monoclonal antibody MMG201 as well as a purpose thereof. The method employs anti-human DLL4 monoclonal antibody MMG201 and selects a vcMMAE joint and a pharmaceutical composition, a phosphine reducing agent tricarboxyethyl phosphine TCEP is used for partially reducing the antibody MMG201 during a coupling technology, then the antibody MMG201 is subjected to coupling with vcMMAE and is subjected to conjugate purifying, through optimization of the process, a novel anti-human DLL4 monoclonal antibody medical conjugate MvM03 is prepared, the novel anti-human DLL4 monoclonal antibody medical conjugate MvM03 has the beneficial effect that the tumour targeting of toxin molecule MMAE is promoted, the poisoning effect on body normal cell is reduced, and the good treatment effect can be achieved.
Owner:CHINA PHARM UNIV
Traditional Chinese medicine for preventing swine influenza
InactiveCN101983665AAdjustment functionImprove immunityPowder deliveryAntiviralsSide effectCimicifuga foetida
The invention relates to a traditional Chinese medicine for preventing swine influenza, which belongs to the field of pharmaceutical technology. The invention mainly solves the following problems in preventing swine influenza: Swines are difficult to catch; toxic heat can not be completely discharged out of swines; the cost is high. In addition, the invention helps to improve the immunity of swines and prevent the second infection of swine influenza effectively. The above traditional Chinese medicine comprises, by weight, 2-6 parts of forsythia fruit, 1-3 parts of dandelion, 2-6 parts of radix isatidis, 1-3 parts of isatis leaf, 1-2 parts of lobelia chinensis, 1-3 parts of licorice, 1-2 parts of root of large-flowered skullcap, 2-6 parts of Yedo violet, 1-2 parts of windproof and 1-2 parts of cimicifuga foetida. Compatibility of Chinese traditional medicine gives a full play in the invention. As a result, toxic heat is completely discharged out of swines and the immunity of swines is improved. The invention also has the advantages of low cost, few toxic or side effect and free of catching swines.
Owner:沁源县灵兴养殖专业合作社
Quickly disintegrating tablet containing cadotril
InactiveCN1506043ASolve the inconvenienceTreat diarrheaDigestive systemPill deliveryActive componentPharmaceutical technology
The present invention belongs to the field of pharmaceutical technology, and is one kind fast disintegrating tablet with active component cadotril. The tablet features its fast disintegrating in one minute after contacting saliva in oral cavity. It is prepared with active component cadotril as active component, disintegrating agent, stuffing, corrective, lubricant, adhesive and coloring agent and through mixing and tabletting. It has the features of fast disintegrating, convenient use and high leaching rate. The preparation has excipient mixture in 10-100 times the weight of the active component, disintegrating agent in 1-20 % of tablet weight and cosolvent in 0-20 % of tablet weight.
Owner:CHENGDU BOLING PHARMA TECH DEV CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com