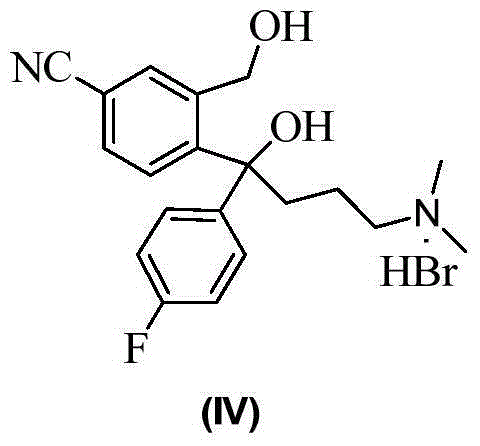
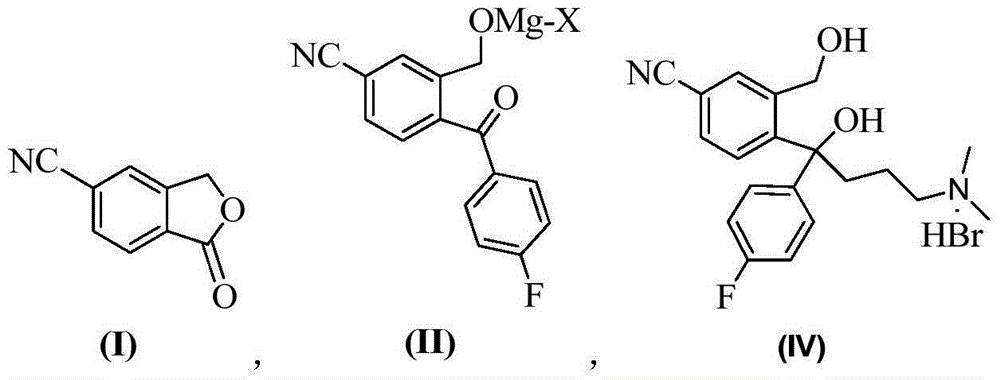
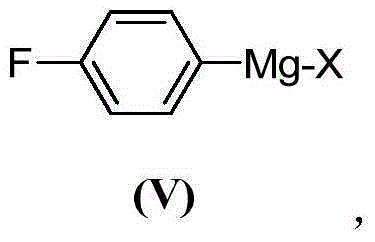
Preparation method of citalopram intermediate
A technology of time and volume ratio, applied in the field of preparation of citalopram intermediates, can solve the problems of poor reaction selectivity and difficulty in obtaining
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0048] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0049] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0050] The synthetic route is as follows:
[0051]
Embodiment 14
[0052] Preparation of embodiment 14-fluorophenylmagnesium bromide Grignard reagent
[0053] 2-Methyltetrahydrofuran as solvent:
[0054]
[0055] reaction process:
[0056] At room temperature, Mg scraps (5.37g, 220.90mmol, 1.10equiv.) and iodine particles (539.3mg, 2.12mmol, 0.01equiv.) were suspended in 2-methyltetrahydrofuran (200mL), and the reaction mixture was evacuated and replaced with nitrogen four times. The reaction mixture was heated to 30°C, BFB (35.07g, 200.40mmol) diluted in 2-methyltetrahydrofuran (100mL) was added dropwise to the reaction mixture, the addition was completed after 85min, and the reaction mixture was reacted at 30°C under nitrogen protection for 0.5h.
[0057] Post-reaction processing:
[0058] The reaction mixture was sampled under nitrogen protection, and saturated NH 4 Cl solution (2 mL) was used to quench the reaction, and 5 drops of the 2-methyltetrahydrofuran layer were diluted with chromatographic acetonitrile / purified water 1.5 mL ...
Embodiment 2
[0085] Preparation of Example 2 (5-cyano-2-(4-fluorobenzoyl) phenyl) magnesium bromide
[0086] 2-Methyltetrahydrofuran as solvent:
[0087]
[0088] reaction process:
[0089] At room temperature, the formula (I) (24.11g, 151.50mmol) was suspended in 2-methyltetrahydrofuran (241mL), the reaction mixture was evacuated and replaced with nitrogen 4 times, the reaction mixture was cooled to -20°C, and the formula (V) Grignard reagent ( 200mmol, dissolved in 300mL 2-methyltetrahydrofuran) was added dropwise to the above reaction mixture, and the dropwise addition was completed after 140min. The reaction mixture was stirred and reacted at -20°C under the protection of nitrogen for 1h and 2h, respectively, and samples were sent for inspection.
[0090] Post-reaction processing:
[0091] Sampling with saturated NH 4 The Cl solution was used to quench the reaction, and the 2-methyltetrahydrofuran layer was taken for HPLC detection. The reaction mixture was cooled to -20°C and u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com