Preparation method of acridine compounds
A compound, acridine technology, applied in the new preparation field, can solve the problems of difficult industrialized production, long reaction time, high process cost, achieve mild preparation conditions, avoid high temperature and high pressure reaction and the use of expensive heavy metal catalyst effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
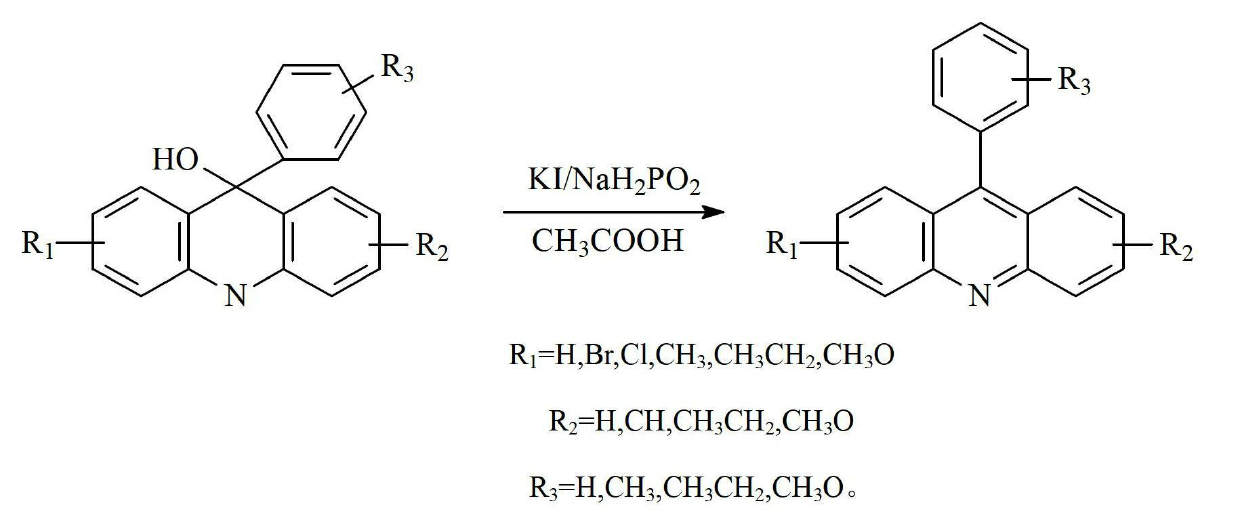
[0033] The preparation of embodiment 1 9-phenylacridine
[0034]
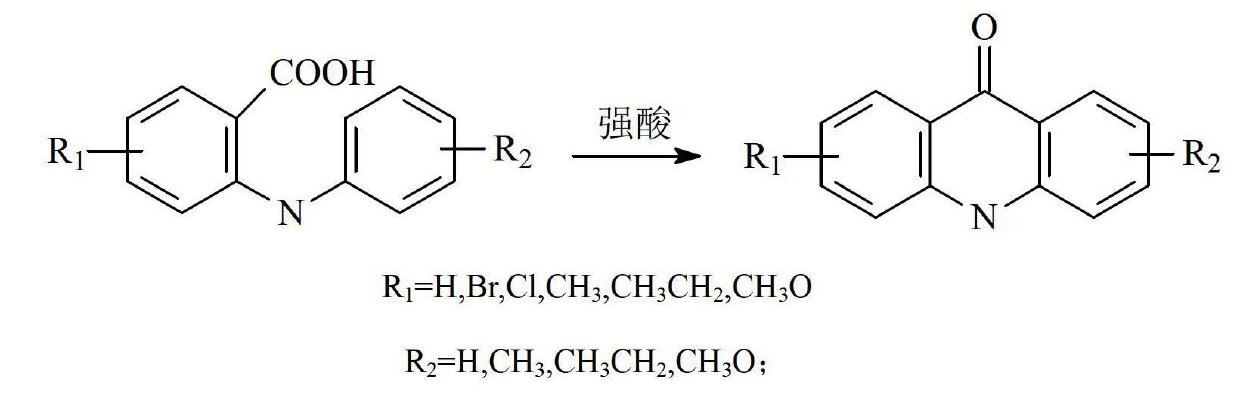
[0035] The first step: the preparation of acridone
[0036] Add 15g of N-phenylanthranilic acid into a 250ml four-necked flask, add 50ml of polyphosphoric acid as a solvent, heat the oil bath to 100°C, and stop the reaction for 3 hours. Slowly add 10% NaHCO dropwise after the reaction solution is cooled 3 PPA was removed from the solution, the reaction solution was adjusted to neutrality, filtered, washed with water and dried to obtain a pale yellow product with a yield of 89.2%. The effect of solvent dosage on the reaction is shown in Table 1.
[0037] Table 1 The influence of solvent consumption on reaction
[0038] experiment
Solvent usage / ml
Yield %
Experiment 1
40
82.9
Experiment 2
50
89.2
Experiment 3
60
88.5
Experiment 4
70
81.3
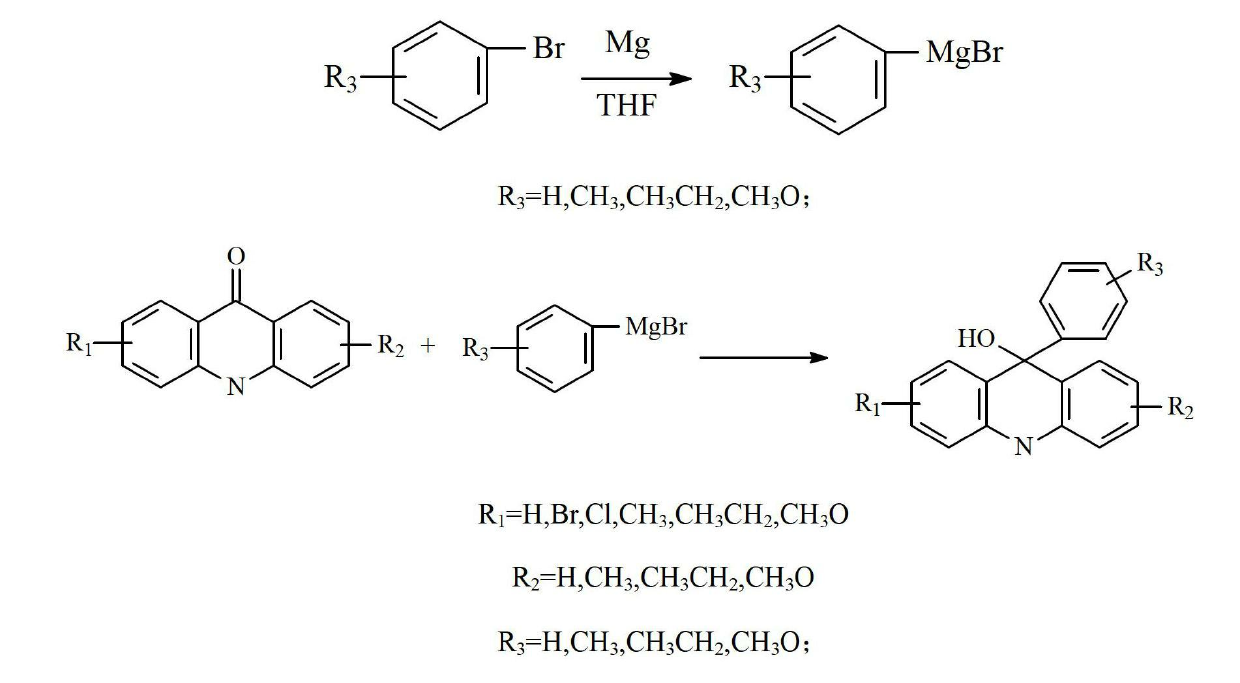
[0039] The second step: the preparation of 9-hydroxyl-9-phenylacridin...
Embodiment 2
[0046] The preparation of embodiment 2 9-phenylacridine
[0047]
[0048] The first step: the preparation of acridone
[0049] Add 15g of N-phenylanthranilic acid into a 250ml four-neck flask, add 50ml of polyphosphoric acid, heat the oil bath to 100°C, and stop the reaction for 3 hours. Slowly add 10% NaHCO dropwise after the reaction solution is cooled 3 PPA was removed from the solution, the reaction solution was adjusted to neutrality, filtered, washed with water and dried to obtain a pale yellow product with a yield of 89.2%. The effect of different solvents on the reaction is shown in Table 2.
[0050] The impact of different solvents in table 2 on the reaction
[0051] experiment
solvent
Yield %
Experiment 1
concentrated sulfuric acid
81.2
Experiment 2
polyphosphoric acid
89.2
Experiment 3
p-Toluenesulfonic acid
66.5
[0052] The second step: the preparation of 9-hydroxyl-9-phenylacridine...
Embodiment 3
[0059] The preparation of embodiment 3 9-tolyl acridine
[0060]
[0061] The first step: the preparation of acridone
[0062] Add 15g of N-phenylanthranilic acid into a 250ml four-neck flask, add 10ml of water and 20ml of concentrated sulfuric acid mixture, heat the oil bath to 100°C, slowly add 30ml of concentrated sulfuric acid dropwise, and stop stirring after 3 hours of reaction. Slowly add 10% NaHCO dropwise after the reaction solution is cooled 3 The concentrated sulfuric acid was removed from the solution, and the reaction solution was adjusted to be neutral. After filtration, the product was washed with water and dried to obtain a yellow product with a yield of 81.2%. The effect of the amount of concentrated sulfuric acid on the reaction is shown in Table 3.
[0063] The impact of the concentrated sulfuric acid consumption of table 3 on reaction
[0064] experiment
Amount of concentrated sulfuric acid / ml
Yield %
Experiment 1
30
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com