Preparation method of 16 Alpha-methyl steroidal compound
A compound, the technology of methylmagnesium bromide, applied in the field of preparation of steroid compounds, can solve the problems of difficult purification of impurities, low yield of 16α-methyl steroid compounds, easy dissociation of ester bonds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
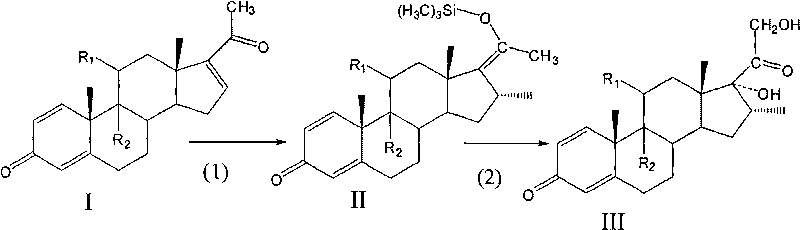
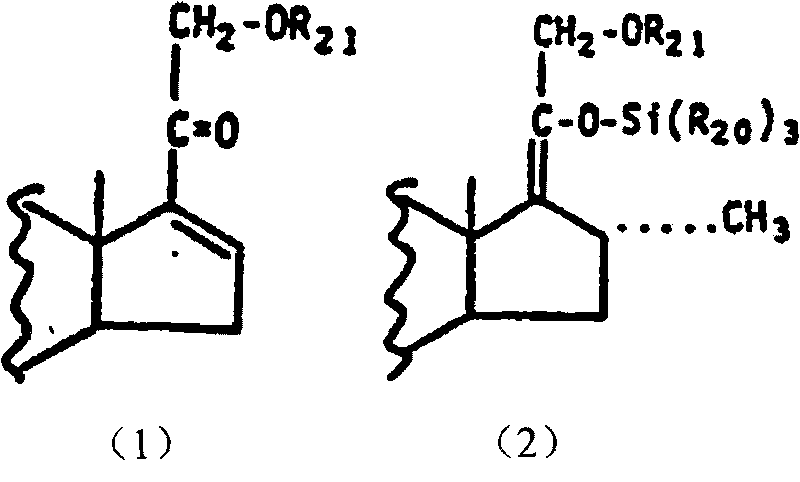
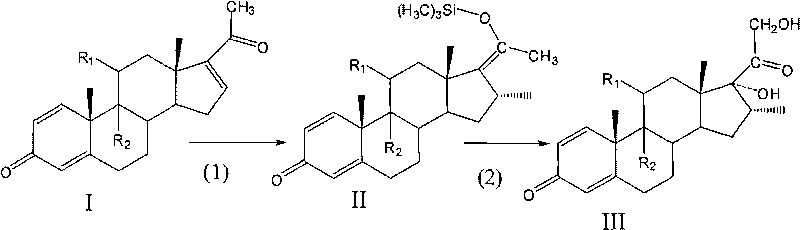
[0017] 1) Combine 5.0 g of Pregna-1,4,16-triene-3,11,20-trione (compound (I-1)) with 0.02 times of cuprous dimethyl sulfide bromide, 1.2 times of trimethyl Add chlorosilane and 2 times hexamethylphosphoric triamide to 60ml tetrahydrofuran, slowly add 1.2 times methylmagnesium bromide dissolved in tetrahydrofuran to carry out the Grignard reaction, the reaction temperature is -30~-20℃, the reaction time N 2 After the reaction, it is diluted with water, separated and dried to obtain Grignard compound (Compound (II-1));
[0018] 2) The above-mentioned Grignard is reacted with 3 times potassium bicarbonate and 1.5 times m-chloroperoxybenzoic acid in dichloromethane at a reaction temperature of -30 to -25° C., and the reaction is complete. Use hydrochloric acid to adjust the pH of the solution to 1, concentrate under reduced pressure, cool, separate, and dry to obtain compound (III-1) with a total yield of 78.2%
[0019]
Embodiment 2
[0021] 1) Combine pregna-1,4,16-triene-3,20-dione (compound (I-2)) with 0.05 times cuprous bromide, 1.5 times trimethylchlorosilane, 3 times hexamethyl Phosphorus triamide was dissolved in 40ml tetrahydrofuran, and 1.4 times methylmagnesium bromide dissolved in tetrahydrofuran was slowly added to carry out the Grignard reaction. 2 After the reaction, it is diluted with water, separated and dried to obtain Grignard compound (compound (II-2));
[0022] 2) Reacting the above-mentioned Grignard compound with 1.1 times sodium hydroxide and 2.5 times m-chloroperoxybenzoic acid in chloroform at a reaction temperature of -25 to -20°C, and the reaction is complete. Adjust the pH of the solution to 2 with hydrochloric acid, concentrate and separate under reduced pressure, cool and separate, and dry to obtain compound (III-3) with a total yield of 80.5%
[0023]
Embodiment 3
[0025] 1) Pregnancy-1,4,9(11),16-tetraene-3,20-dione (compound (I-3)) 5.0g, 0.08 times cuprous chloride, 1.5 times trimethyl chloride Silane and 4-fold hexamethylphosphoric triamide were dissolved in 30ml tetrahydrofuran, and 1.4-fold methylmagnesium chloride dissolved in tetrahydrofuran was slowly added for Grignard reaction. The reaction temperature was -25~-15℃, and N was passed during the reaction. 2 After the reaction, it is diluted with water, separated and dried to obtain Grignard compound (Compound (II-3));
[0026] 2) Reacting the above-mentioned Grignard compound with 1.5 times sodium carbonate and 3 times m-chloroperoxybenzoic acid in chloroform at a reaction temperature of -20 to -15°C, and the reaction is complete. The pH value of the solution was adjusted to 3 with sulfuric acid, separated and dried to obtain compound (III-3) with a total yield of 77.9%.
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com