Synthesis and surface modification method of lithium excessive laminar oxide anode material
A lithium-rich cathode material and lithium-rich material technology, applied in the field of lithium-ion batteries and electrochemistry, can solve the problems of poor rate performance, low charge-discharge efficiency, and large irreversible capacity loss, so as to improve the charge-discharge efficiency and increase the volume. Energy density, the effect that is conducive to rapid de-embedding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1. Manganese sulfate, nickel sulfate, and cobalt sulfate are used to prepare a mixed salt solution 1 with a total concentration of 1.0 mol / L, wherein the molar ratio of manganese salt: nickel salt: cobalt salt is 0.54:0.13:0.13.
[0032] Step 2, prepare a 1.0mol / L sodium carbonate solution, add a certain amount of concentrated ammonia water dropwise to the sodium carbonate solution, and record it as solution 2.
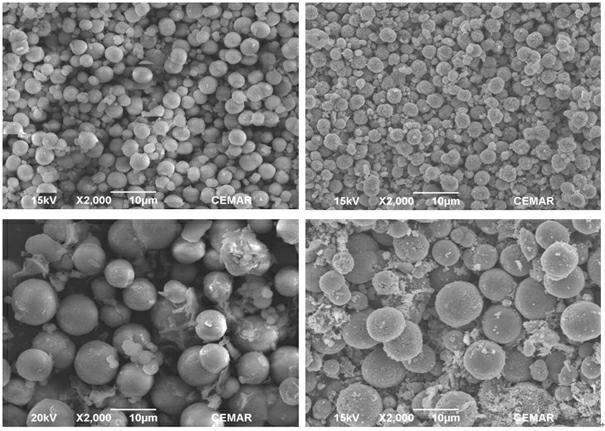
[0033] Step 3. Pump solution 1 and solution 2 into the reaction kettle at the same time for co-precipitation reaction, control the reaction temperature to 60°C, control the pH value of the solution by adding ammonia water to 8, and react for 12 hours. -Washing-drying yields carbonate precursor precipitates of two different particle sizes (see figure 1 ). The SEM pattern shows that the carbonate precursor and Li(Li 0.2 mn 0.54 Ni 0.13 co 0.13 )O 2 They are spherical particles with uniform size, the particle sizes are about 4um and 10um respectively, ...
Embodiment 2
[0039] Steps 1-5 are the same as in Example 1.
[0040] Step 6. Dissolve sodium persulfate and potassium persulfate with a mass fraction of 40% and 50% of the lithium-rich material in deionized water, then add the lithium-rich material, and stir at 80°C until the solution is completely volatilized. The obtained powder was calcined at 200°C for 10 h, filtered, washed and dried to obtain Li 1..2-x mn 0.54 Ni 0.13 co 0.13 o 2 .
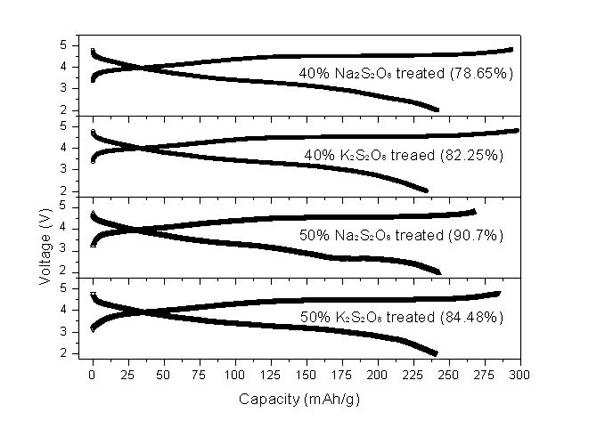
[0041] Electrochemical tests show that the first charge and discharge efficiency of the material is improved by removing part of the lithium in advance (see image 3 ). In the 2-4.8V interval. At a rate of 0.1C (25mA / g), the lithium-rich materials treated with 40% sodium persulfate and potassium persulfate had an initial discharge capacity of 242 mAh / g and 234 mAh / g, and a charge-discharge efficiency of 82.3% and 78.65 %; 50% mass fraction of sodium persulfate and potassium persulfate treated lithium-rich materials, the first discharge capacity o...
Embodiment 3
[0043] Steps 1-5 are the same as in Example 1.
[0044] Step 6. Dissolve potassium persulfate and ammonium sulfate with a mass fraction of 48% and 23% of the lithium-rich material in deionized water, then add the lithium-rich material, and stir at 80°C until the solution is completely volatilized. The obtained powder was calcined at 300°C for 10 h, filtered, washed and dried to obtain Li 1..2-x mn 0.54 Ni 0.13 co 0.13 o 2 .
[0045] Electrochemical tests showed that (see Figure 4 ), at a discharge rate of 0.1C (25mA / g), the initial discharge capacity of the material pretreated with 48% potassium persulfate in the 2-4.8V range is 250mAh / g, and the initial charge-discharge efficiency is 85.6%. ; while the 23% mass fraction of ammonium sulfate pretreated material is better, the first discharge capacity in the range of 2-4.8V and 2-4.6V is 270mAh / g and 257mAh / g, and the charge and discharge efficiency can reach 88.6% respectively and 92.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com