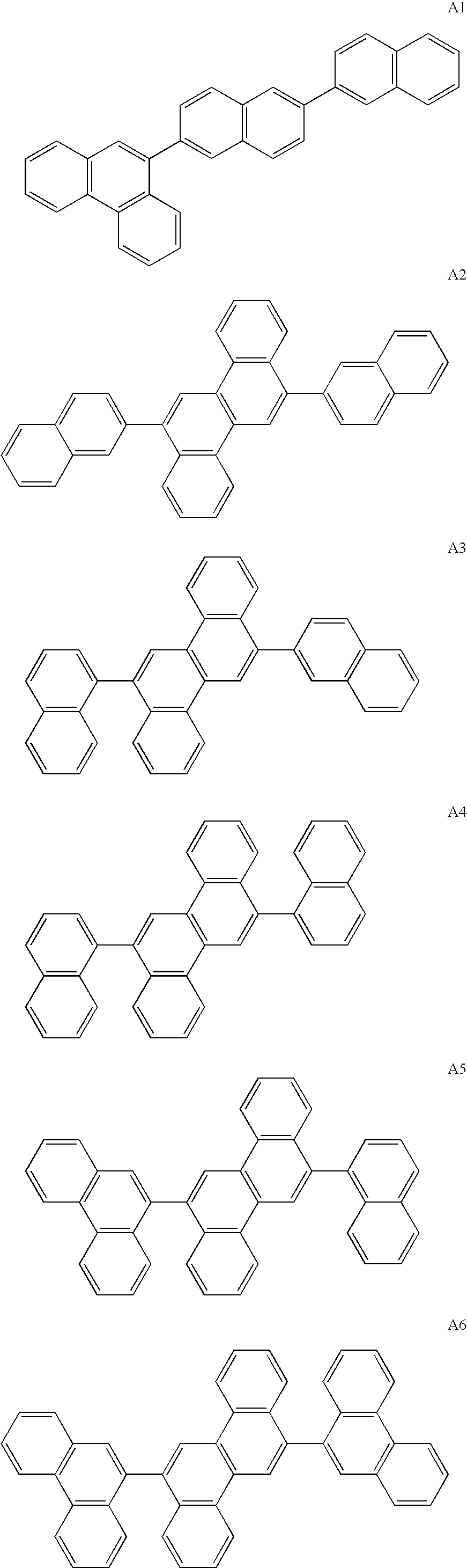
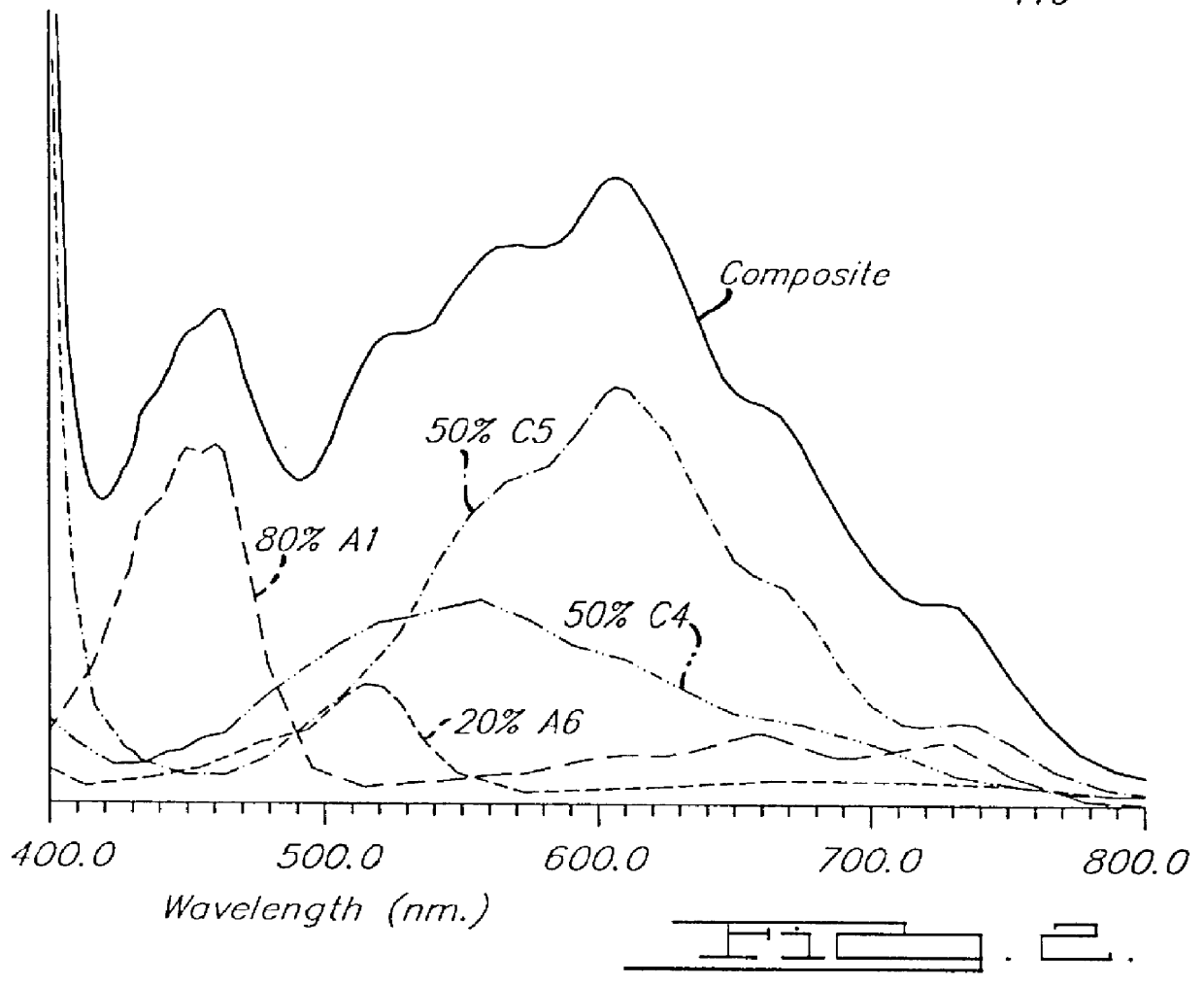
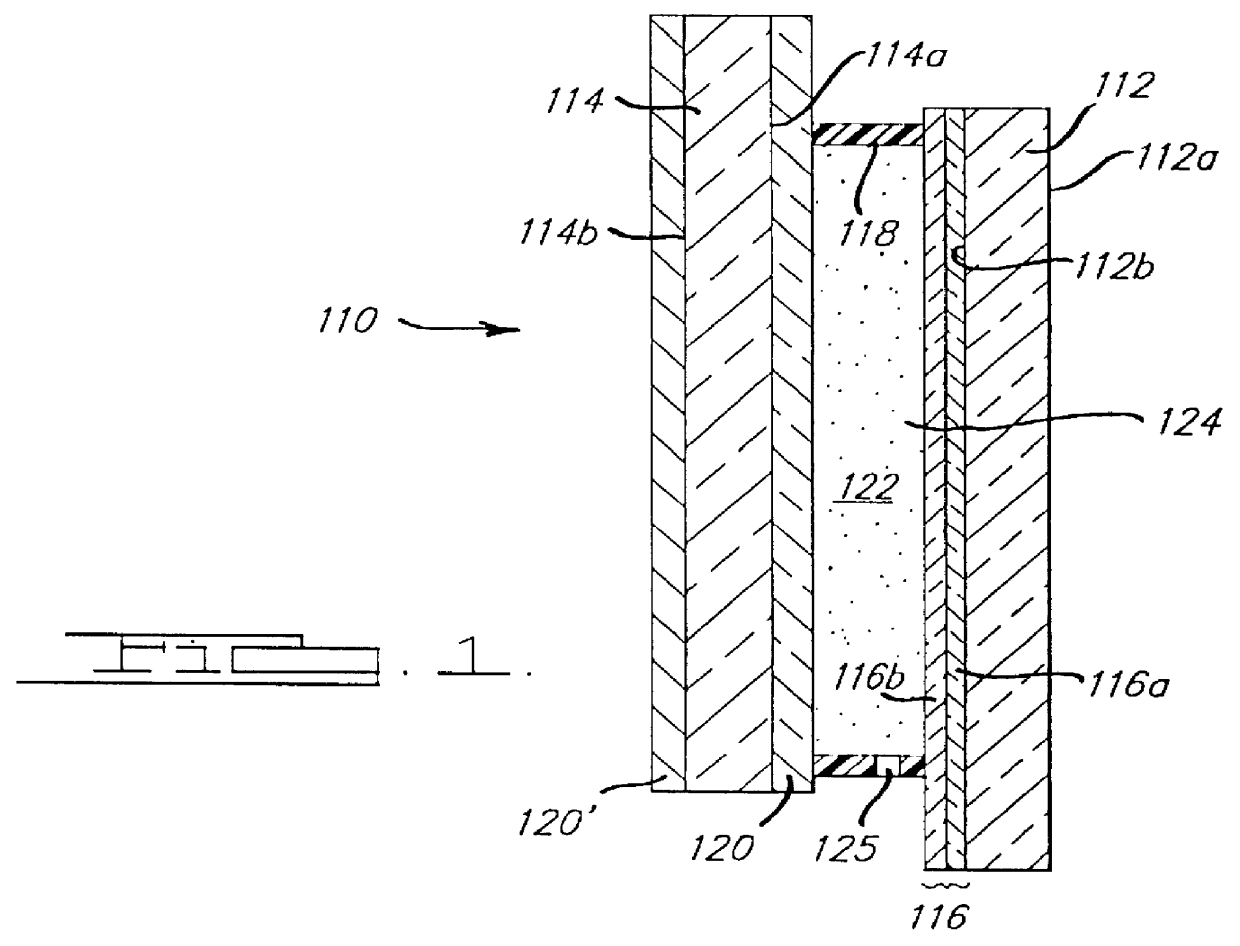
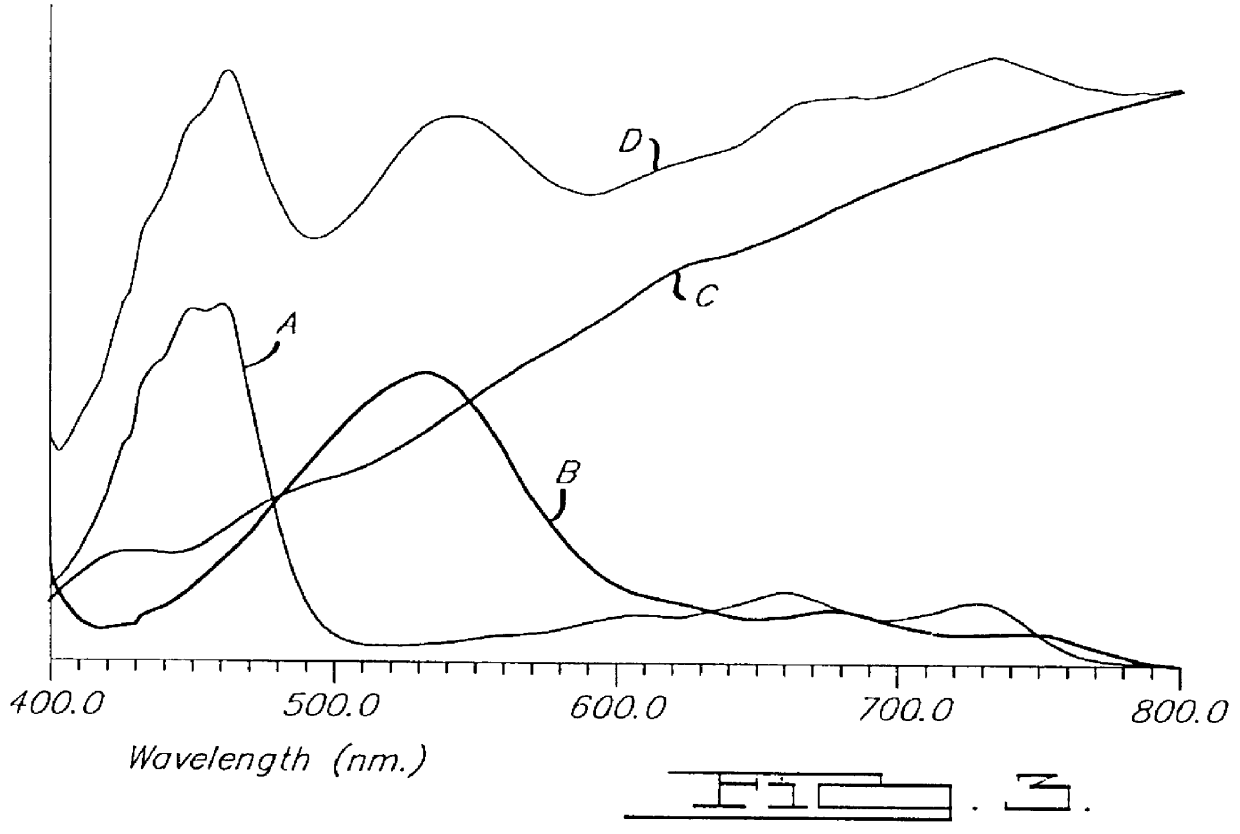
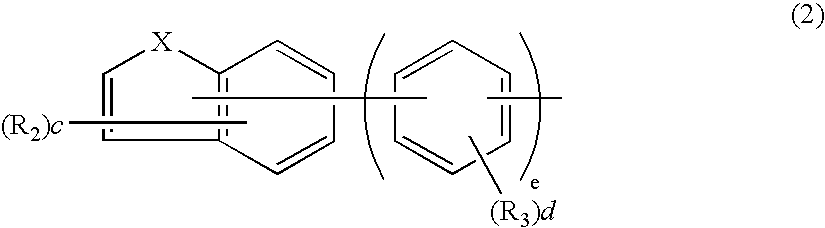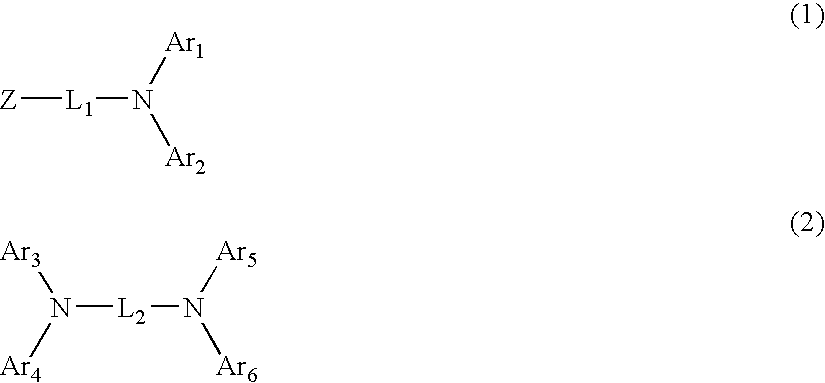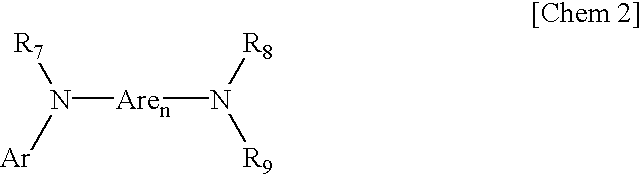Patents
Literature
64990 results about "Anode" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An anode is an electrode through which the conventional current enters into a polarized electrical device. This contrasts with a cathode, an electrode through which conventional current leaves an electrical device. A common mnemonic is ACID, for "anode current into device". The direction of conventional current (the flow of positive charges) in a circuit is opposite to the direction of electron flow, so (negatively charged) electrons flow out the anode into the outside circuit. In a galvanic cell, the anode is the electrode at which the oxidation reaction occurs.
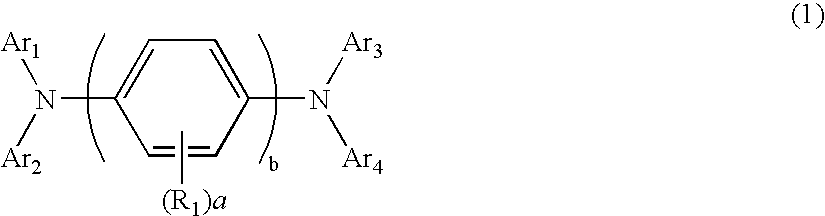
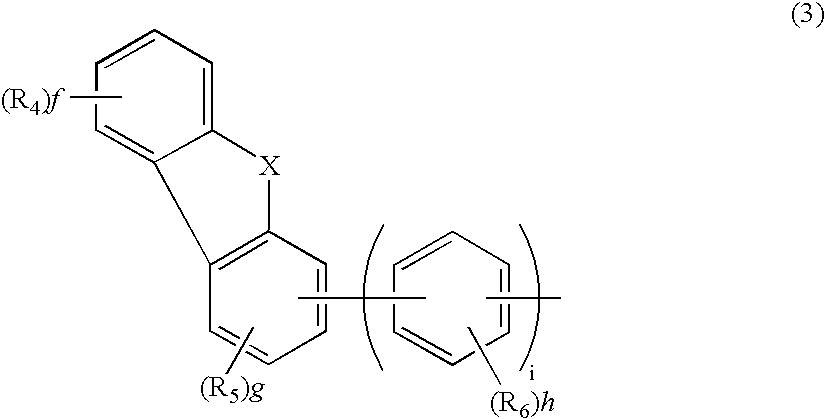
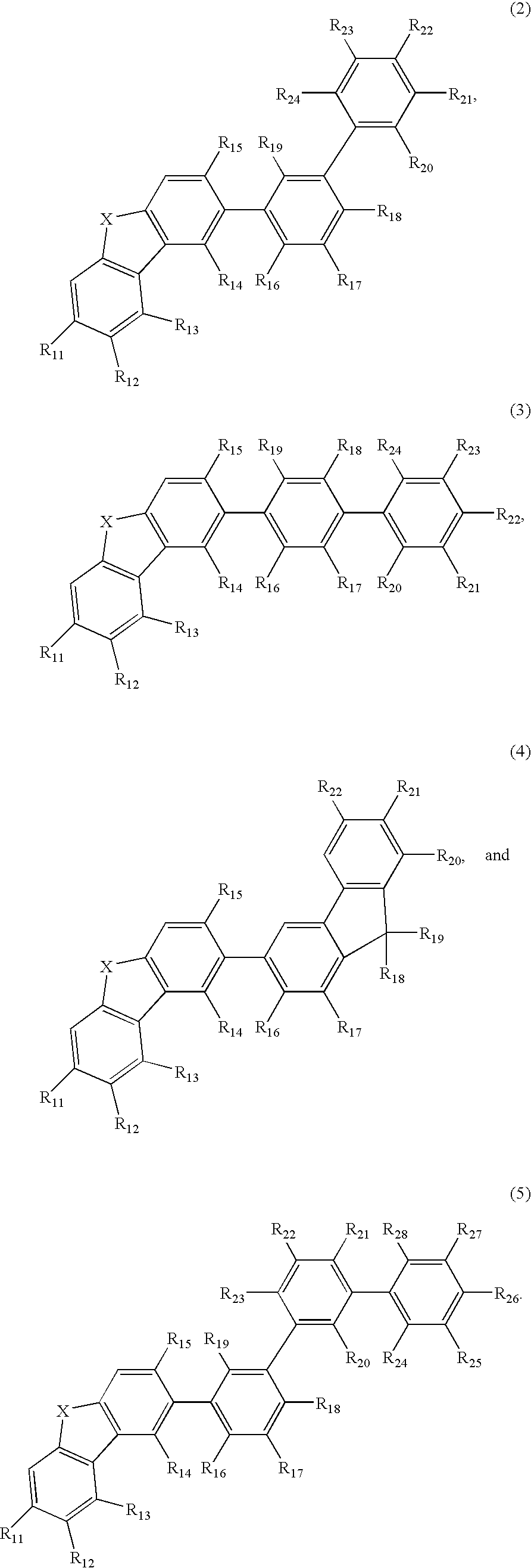
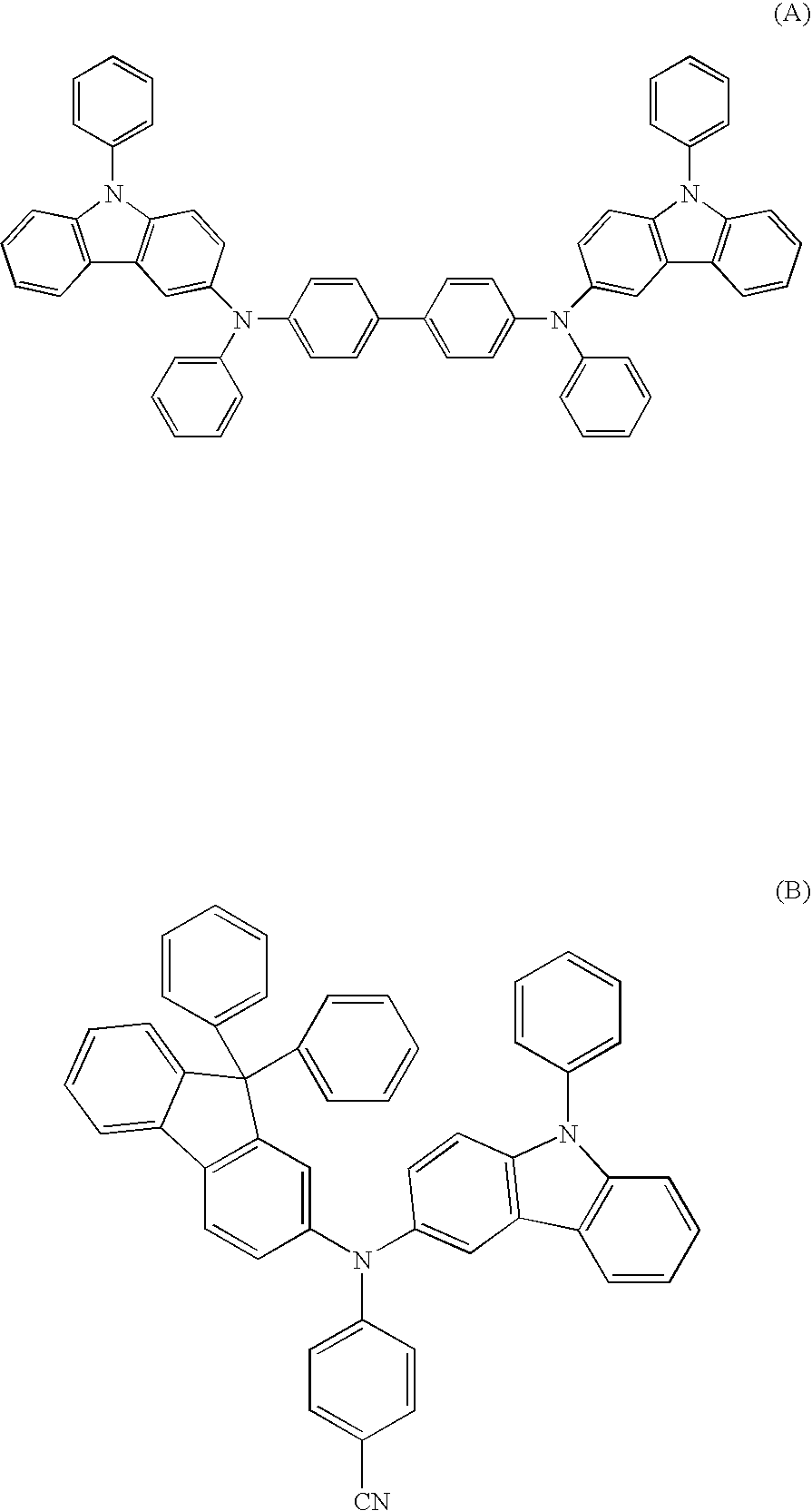
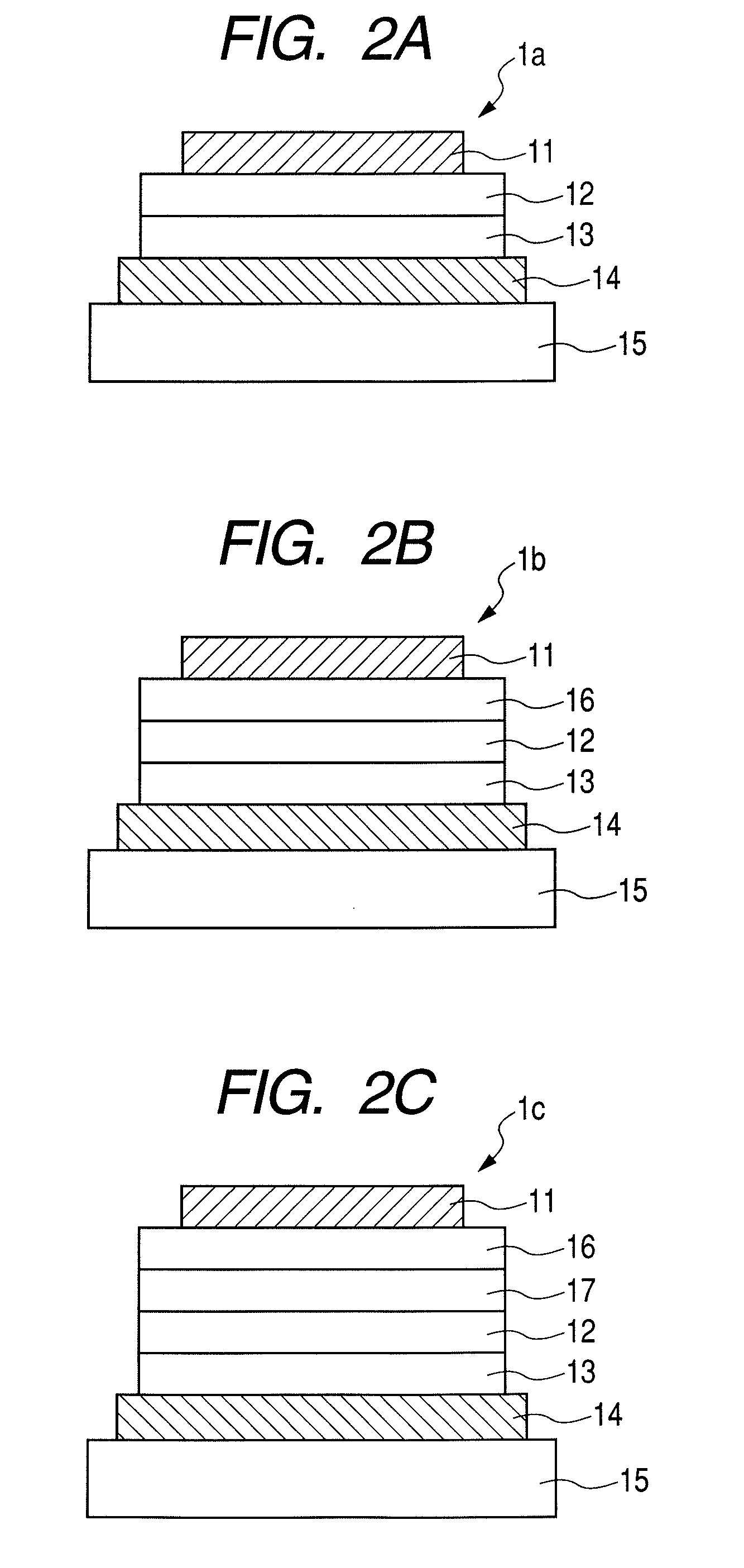
Aromatic amine derivative and electroluminescence device using the same
ActiveUS20070278938A1Improved in yield in producingLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic electroluminescencePerylene derivatives
Provided are a novel aromatic amine derivative having a specific structure and an organic electroluminescence device in which an organic thin layer comprising a single layer or plural layers including a light emitting layer is interposed between a cathode and an anode, wherein at leas one layer of the above organic thin layer contains the aromatic amine derivative described above in the form of a single component or a mixed component. Thus, the organic electroluminescence device is less liable to be crystallized in molecules, improved in a yield in producing the organic electroluminescence device and extended in a lifetime.
Owner:IDEMITSU KOSAN CO LTD
Organometallic compounds for use in electroluminescent devices
ActiveUS7534505B2Discharge tube luminescnet screensGroup 8/9/10/18 element organic compoundsOrganic light emitting deviceOrganic layer
An organic light emitting device having an anode, a cathode and an organic layer between the anode and the cathode is provided. The organic layer comprises a carbene-metal complex having the structure:
Owner:UNIVERSAL DISPLAY +1
Material for organic electroluminescent element and organic electroluminescent element employing the same
ActiveUS20090030202A1Improve efficiencyImprove heat resistanceOrganic chemistryDischarge tube luminescnet screensHeat resistanceHost material
A material for organic electroluminescence devices for use as a host material in combination with at least one phosphorescent metal complex, which comprises a compound having a specific heterocyclic structure, is described. Also described is an organic electroluminescence device having an anode, a cathode and an organic thin film layer having one or more layers. The organic thin film layer is interposed between the anode and cathode and has a light emitting layer containing a host material in combination with at least one phosphorescent metal complex. At least one layer of the organic thin film layer contains the material for organic electroluminescence devices. The material for organic electroluminescence devices provides an organic electroluminescence device which has a high emitting efficiency, causes little pixel defects, is excellent in heat resistance, and show a long lifetime.
Owner:IDEMITSU KOSAN CO LTD
Aromatic amine derivative and organic electroluminescence device using the same
ActiveUS20080124572A1Improve efficiencyImprove recombination efficiencyOrganic chemistrySolid-state devicesArylCarbazole
An aromatic amine derivative with a specific structure having a carbazole skeleton to which a diarylamino group bonds via a bonding group. An organic electroluminescence device which is composed of one or more organic thin film layers including at least one light emitting layer sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers contains the aromatic amine derivative singly or as its mixture component. Organic electroluminescence devices with enhanced efficiency of light emission and a compound realizing the devices are provided.
Owner:IDEMITSU KOSAN CO LTD
Cyclometallated iridium carbene complexes for use as hosts
ActiveUS7154114B2Electrolysis componentsElectroluminescent light sourcesDopantOrganic light emitting device
An organic light emitting device is provided. The device has an anode, a cathode and an organic layer disposed between the anode and the cathode. The organic layer comprises a host and a dopant, and the host comprises a compound having at least one carbene atom coordinated to iridium, and the compound has the structure:
Owner:UNIVERSAL DISPLAY +1
Luminescent compounds with carbene ligands
ActiveUS20050260441A1Way stableIndium organic compoundsDischarge tube luminescnet screensOrganic light emitting deviceOrganic layer
An organic light emitting device is provided. The device has an anode, a cathode and an organic layer disposed between the anode and the cathode. The organic layer comprises a compound further comprising one or more carbene ligands coordinated to a metal center.
Owner:UNIV OF SOUTHERN CALIFORNIA
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009065A1Improve efficiencyLong lastingSilicon organic compoundsDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescence device and material for organic electroluminescence device
InactiveUS20090045731A1Improve efficiencyLong life-timeDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmFluoranthene
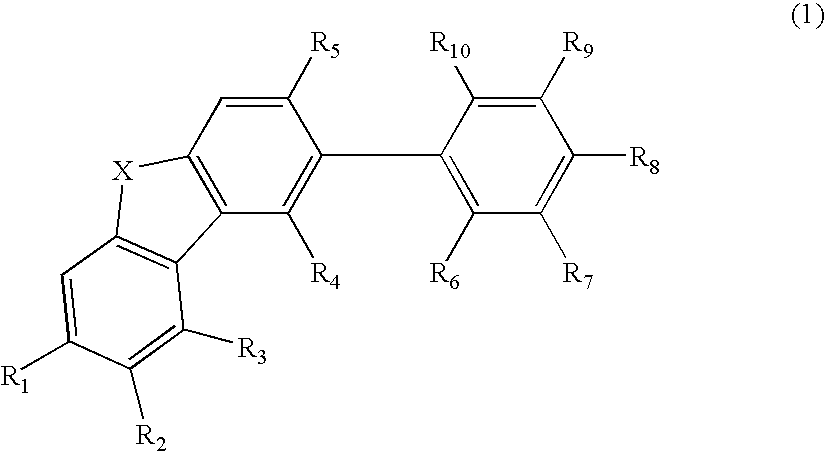
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).In the formula, Ar1, Ar2, Ar3, B1, B2, B3 and B4 each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon ring selected from a naphthalene ring, a chrysene ring, a fluoranthene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a triphenylene ring, a benzo[a]triphenylene ring, a benzochrysene ring, a benzo[b]fluoranthene ring and a picene ring. p is 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
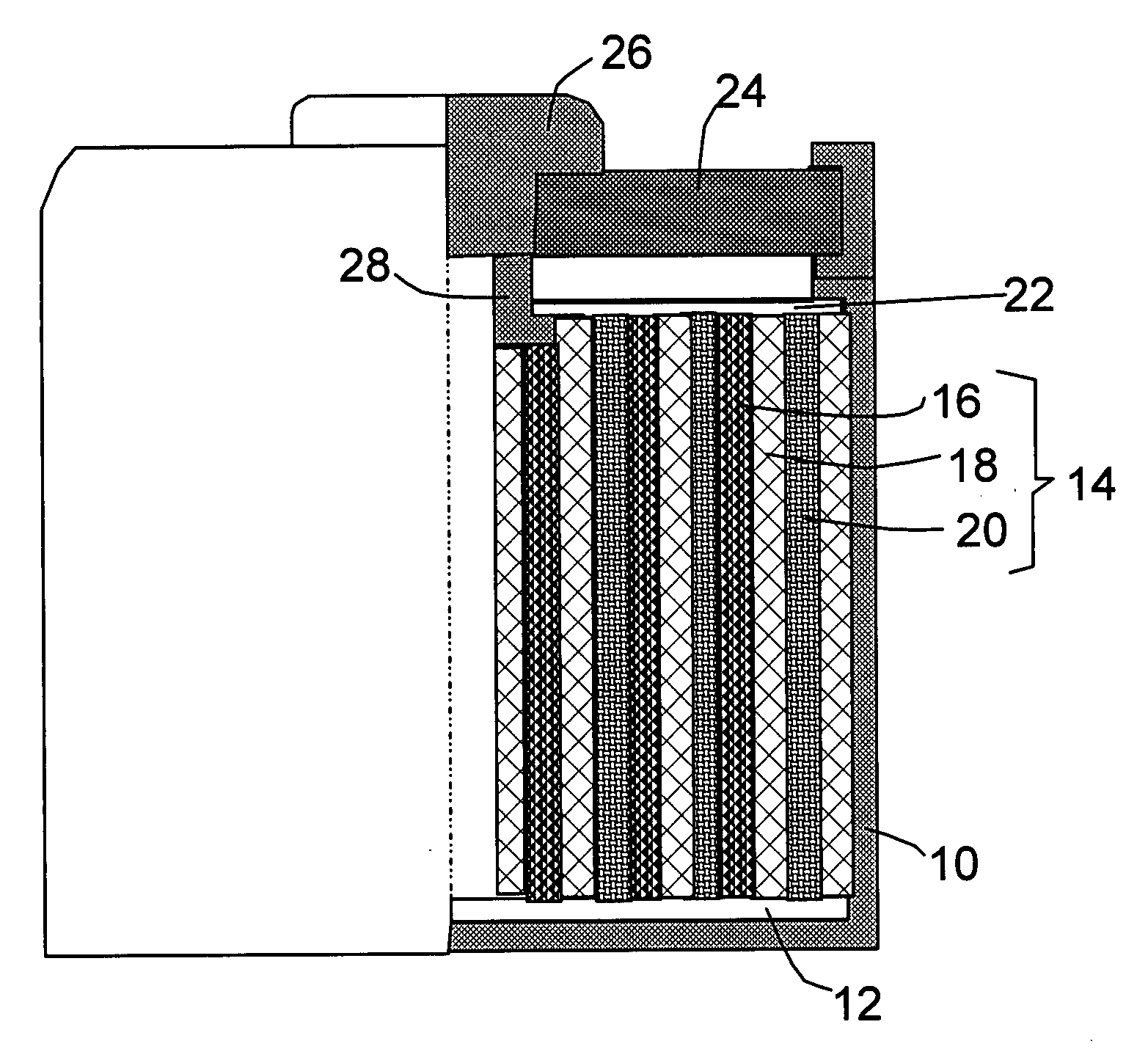
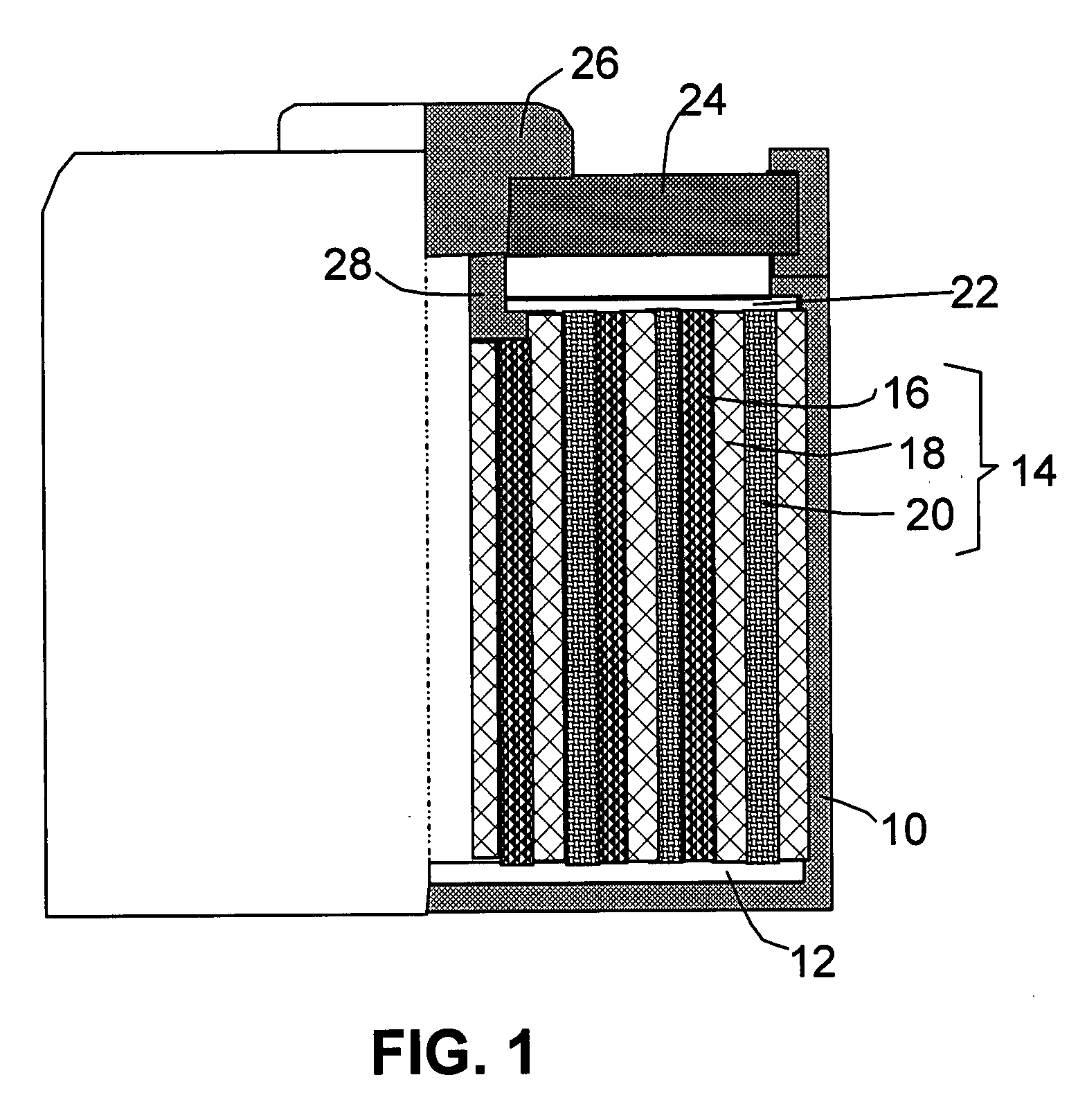
Lithium-Ion Secondary Battery
InactiveUS20110129706A1High levelIncrease battery capacityFinal product manufactureCell temperature controlLithiumEngineering
A lithium-ion secondary battery includes: a winding body in a coil formation at a battery container, the winding body wrapping a cathode film in which lithium ions store and from which lithium ions extract and a anode film in which lithium ions store and from which lithium ions extract, and the cathode film and the anode film being electrically separated from each other via a porous separator; and a heat sink disposed inside the battery container, which contacts the battery container and transmits heat inside the winding body to the battery container.
Owner:HITACHI LTD
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090045730A1Improve efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
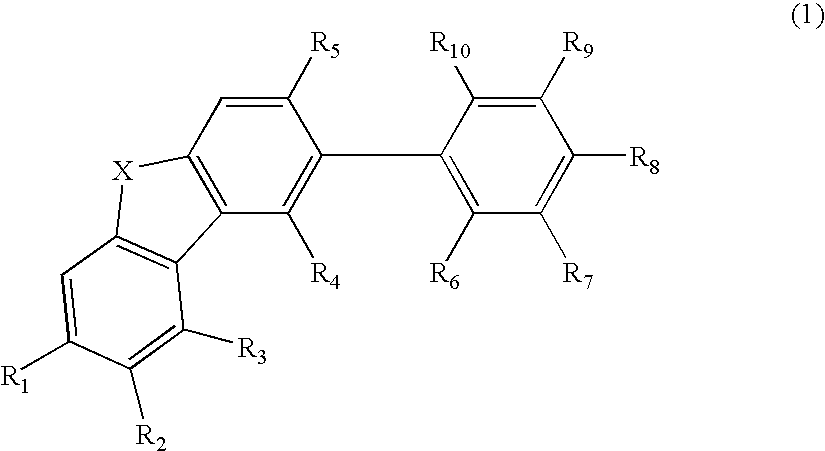
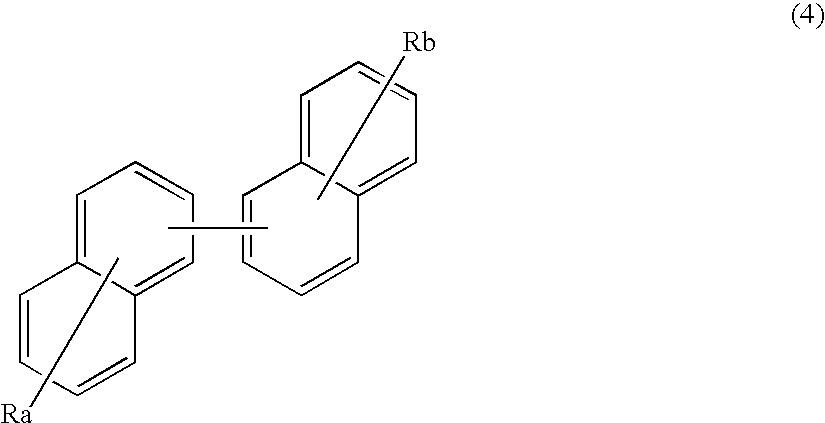
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).Ra-Ar1-Rb (1)In the formula, Ar1, Ra and Rb each represent a substituted or unsubstituted benzene ring or a condensed aromatic hydrocarbon ring selected from a substituted or unsubstituted naphthalene ring, a substituted or unsubstituted chrysene ring, a substituted or unsubstituted fluoranthene ring, a substituted or unsubstituted phenanthrene ring, a substituted or unsubstituted benzophenanthrene ring, a substituted or unsubstituted dibenzophenanthrene ring, a substituted or unsubstituted triphenylene ring, a substituted or unsubstituted benzo[a]triphenylene ring, a substituted or unsubstituted benzochrysene ring, a substituted or unsubstituted benzo[b]fluoranthene ring and a substituted or unsubstituted picene ring. Substituents for Ra and Rb are not aryl groups.
Owner:IDEMITSU KOSAN CO LTD
Electrochromic medium capable of producing a pre-selected color
An improved electrochromic device, the device incorporating an electrochromic medium that comprises at least three electroactive materials having absorption spectra that add together such that the color of the electrochromic medium can be pre-selected by individually choosing the concentrations of the at least three electroactive materials. The electrochromic medium generally maintains the pre-selected perceived color throughout its normal range of voltages when used in an electrochromic device. The at least three electroactive materials include at least one electrochemically reducible material (cathodic material), at least one electrochemically oxidizable material (anodic material) and at least one additional electroactive material which may be either an anodic or cathodic material. Thus, there are always three electroactive materials present in the medium, with at least two either being anodic or cathodic materials. The pre-selected color may be chosen from a wide variety of colors and may be, for example, red, orange, yellow, green, blue, purple. For electrochromic mirrors for motor vehicles, a presently preferred color is gray.
Owner:GENTEX CORP
Organic electroluminescent device using aryl amine derivative containing heterocycle
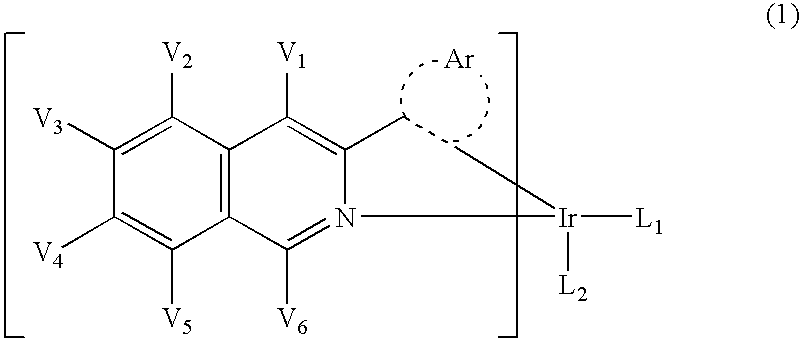
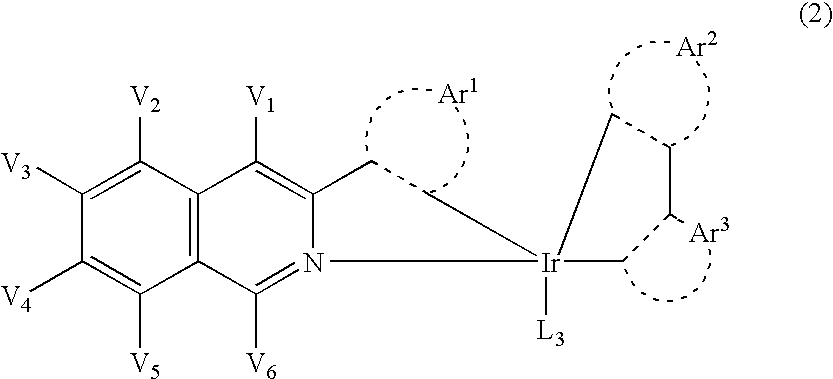
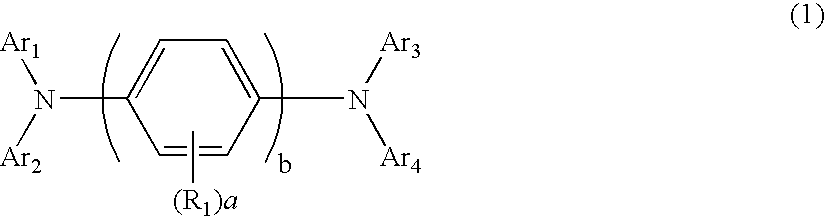
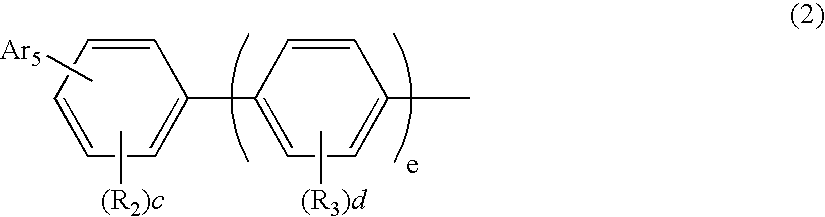
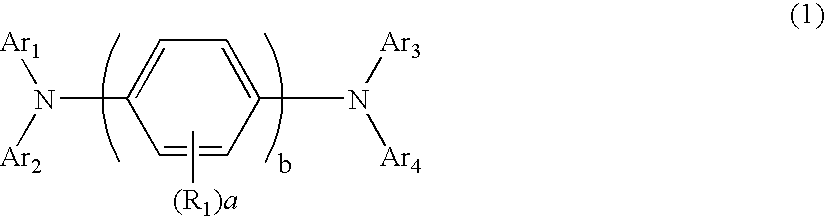
An organic electroluminescent device including: an anode, a cathode, an emitting layer formed of an organic compound and interposed between the cathode and the anode, and two or more layers provided in a hole-injecting / hole-transporting region between the anode and the emitting layer; of the layers which are provided in the hole-injecting / hole-transporting region, a layer which is in contact with the emitting layer containing a compound represented by the formula (1); and of the layers which are provided in the hole-injecting / hole-transporting region, a layer which is interposed between the anode and the layer which is in contact with the emitting layer containing an amine derivative represented by the formula (2).
Owner:IDEMITSU KOSAN CO LTD +1
Material for organic electroluminescence device and organic electroluminescence device using the same
ActiveUS20090309488A1Solve low luminous efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic filmThin membrane
Provided are an organic electroluminescence device, which shows high luminous efficiency, is free of any pixel defect, and has a long lifetime, and a material for an organic electroluminescence device for realizing the device. The material for an organic electroluminescence device is a compound having a n-conjugated heteroacene skeleton crosslinked with a carbon atom, nitrogen atom, oxygen atom, or sulfur atom. The organic electroluminescence device has one or more organic thin film layers including a light emitting layer between a cathode and an anode, and at least one layer of the organic thin film layers contains the material for an organic electroluminescence device.
Owner:IDEMITSU KOSAN CO LTD
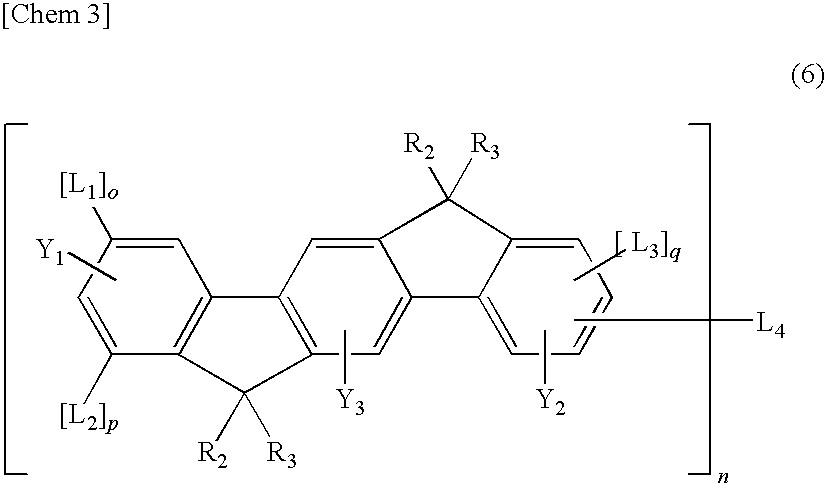
Polycyclic compounds and organic electroluminescence device employing the same
ActiveUS20100012931A1Solve low luminous efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensPolycyclic compoundBenzene
Provided are a polycyclic compound of a compound having such a structure that two benzene rings bond to a central benzene ring each other to form a fused ring and another fused ring bonds to a terminal thereof, and an organic electroluminescence device including one or more organic thin film layers containing a light emitting layer between a cathode and an anode, in which at least one of the organic thin film layers includes the polycyclic compound of the present invention. The organic electroluminescence device has high luminous efficiency, no defect in pixels, and long lifetime. In addition, provided is a polycyclic compound realizing the organic electroluminescence device.
Owner:IDEMITSU KOSAN CO LTD
Coupled electrochromic compounds with photostable dication oxidation states
InactiveUS6249369B1High photochemical stabilityTenebresent compositionsNon-linear opticsAction spectrumElectronic communication
Coupling of anodic electrochromic compounds by a covalent bond or a bridge link which provides for electronic communication between the coupled electrochromic compounds results in coupled electrochromic compounds which exhibit greater stability as well as electrochromic activity that differs from the monomeric electrochromic compounds. Extension of the absorption spectrum into the near-infrared region of the spectrum is frequently observed. The coupled electrochromic compounds are highly suitable for use in electrochromic media used to produce electrochromic devices.
Owner:GENTEX CORP
Programmable metallization cell structure and method of making same
InactiveUS6084796ASolid-state devicesSemiconductor/solid-state device manufacturingCapacitanceElectrical conductor
A programmable metallization cell ("PMC") comprises a fast ion conductor such as a chalcogenide-metal ion and a plurality of electrodes (e.g., an anode and a cathode) disposed at the surface of the fast ion conductor and spaced a set distance apart from each other. Preferably, the fast ion conductor comprises a chalcogenide with Group IB or Group IIB metals, the anode comprises silver, and the cathode comprises aluminum or other conductor. When a voltage is applied to the anode and the cathode, a non-volatile metal dendrite grows from the cathode along the surface of the fast ion conductor towards the anode. The growth rate of the dendrite is a function of the applied voltage and time. The growth of the dendrite may be stopped by removing the voltage and the dendrite may be retracted by reversing the voltage polarity at the anode and cathode. Changes in the length of the dendrite affect the resistance and capacitance of the PMC. The PMC may be incorporated into a variety of technologies such as memory devices, programmable resistor / capacitor devices, optical devices, sensors, and the like. Electrodes additional to the cathode and anode can be provided to serve as outputs or additional outputs of the devices in sensing electrical characteristics which are dependent upon the extent of the dendrite.
Owner:AXON TECH +1
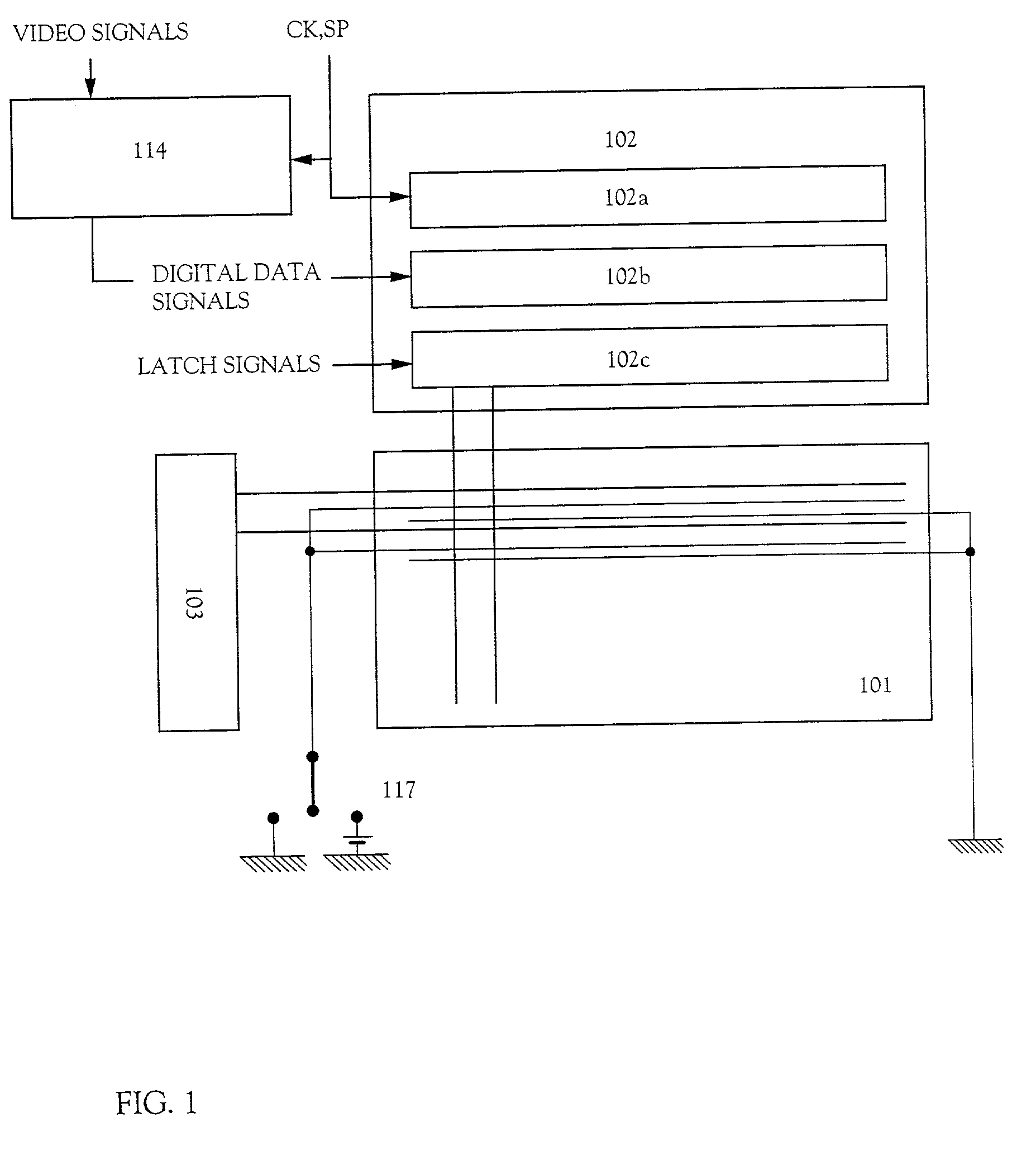
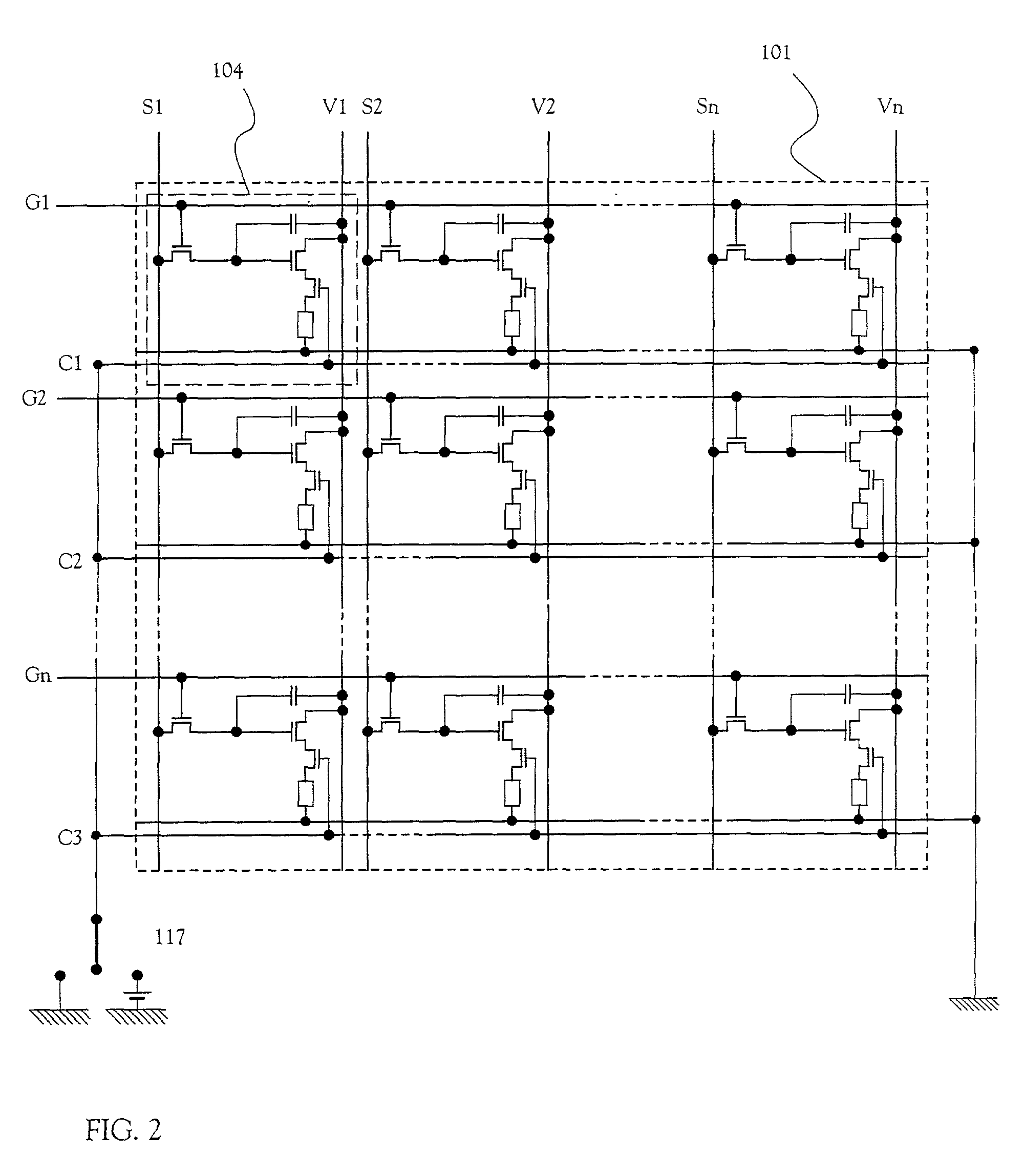
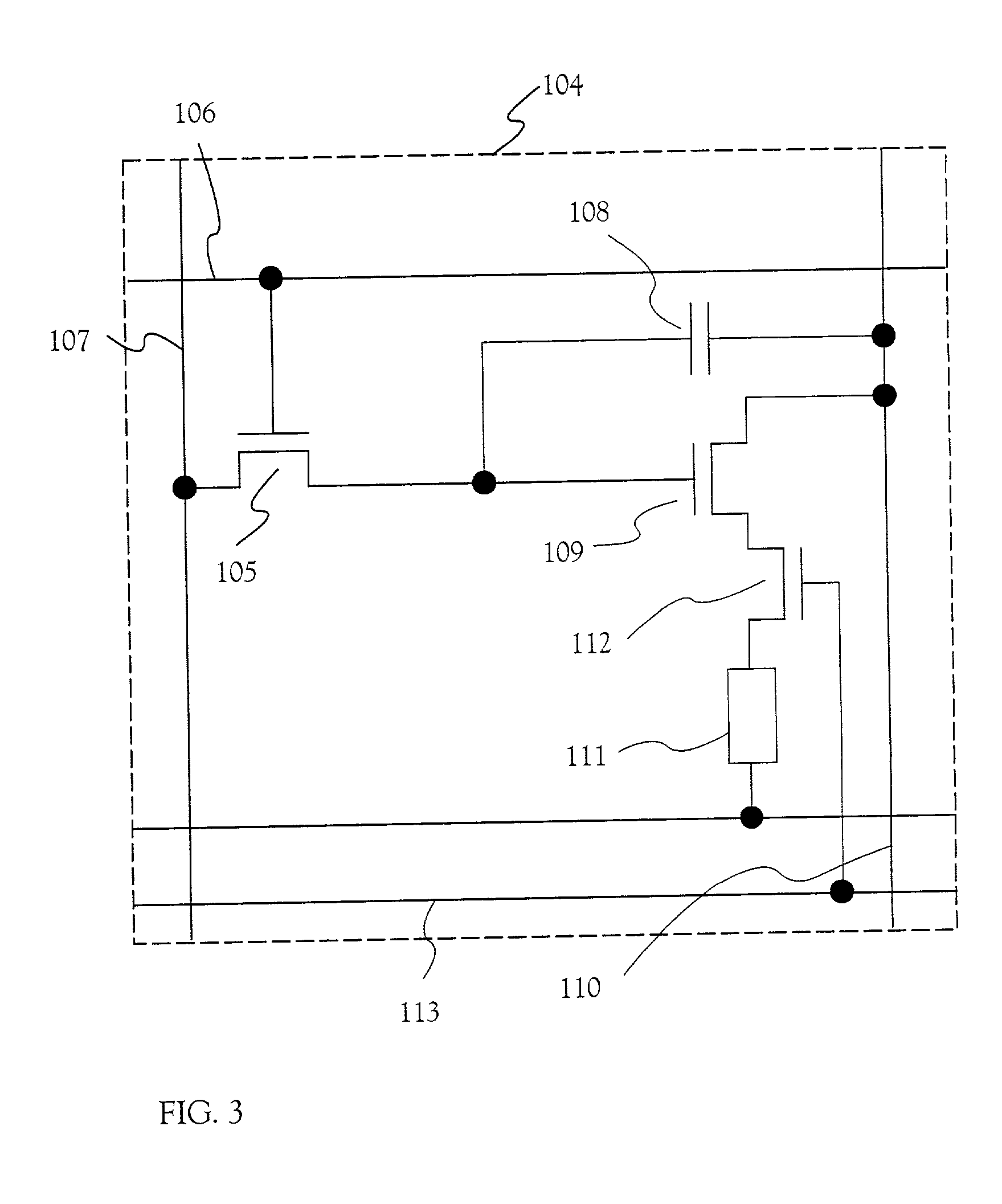
Electric device
InactiveUS20010002703A1Static indicating devicesSolid-state devicesPotential differenceElectrical devices
There is provided an electric device which can prevent a deterioration in a frequency characteristic due to a large electric power external switch connected to an opposite electrode and can prevent a decrease in the number of gradations. The electric device includes a plurality of source signal lines, a plurality of gate signal lines, a plurality of power source supply lines, a plurality of power source control lines, and a plurality of pixels. Each of the plurality of pixels includes a switching TFT, an EL driving TFT, a power source controlling TFT, and an EL element, and the power source controlling TFT controls a potential difference between a cathode and an anode of the EL element.
Owner:SEMICON ENERGY LAB CO LTD
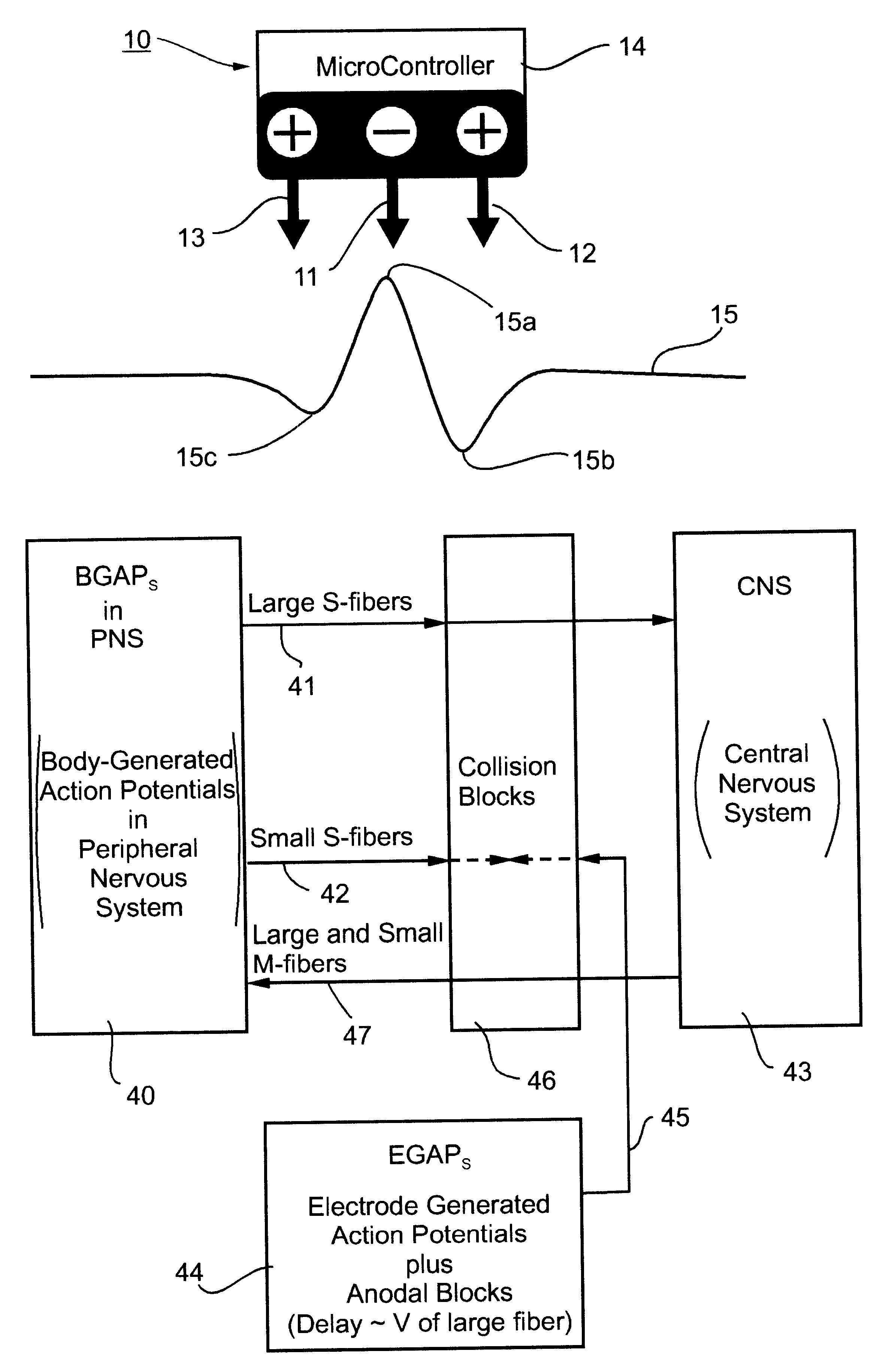
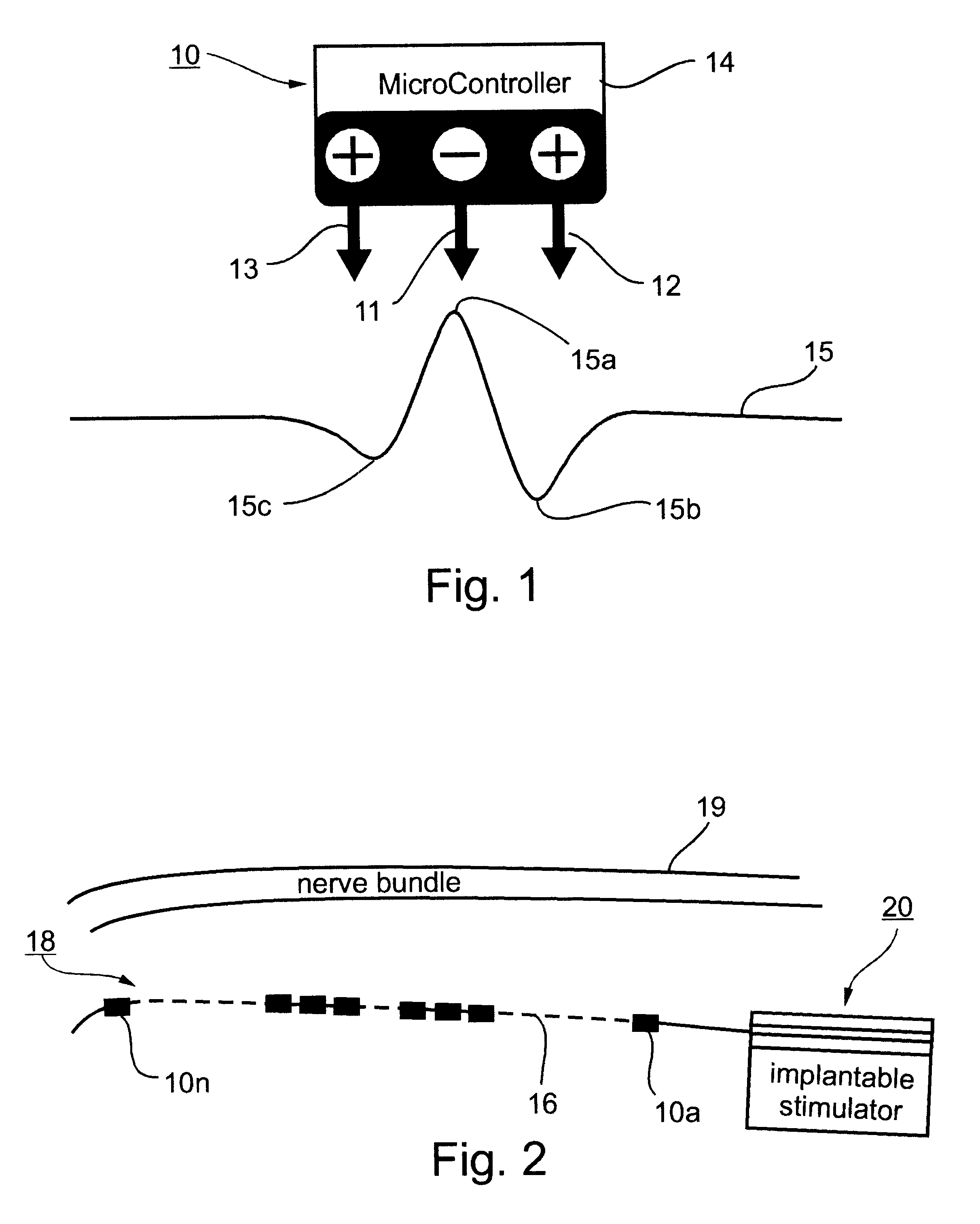
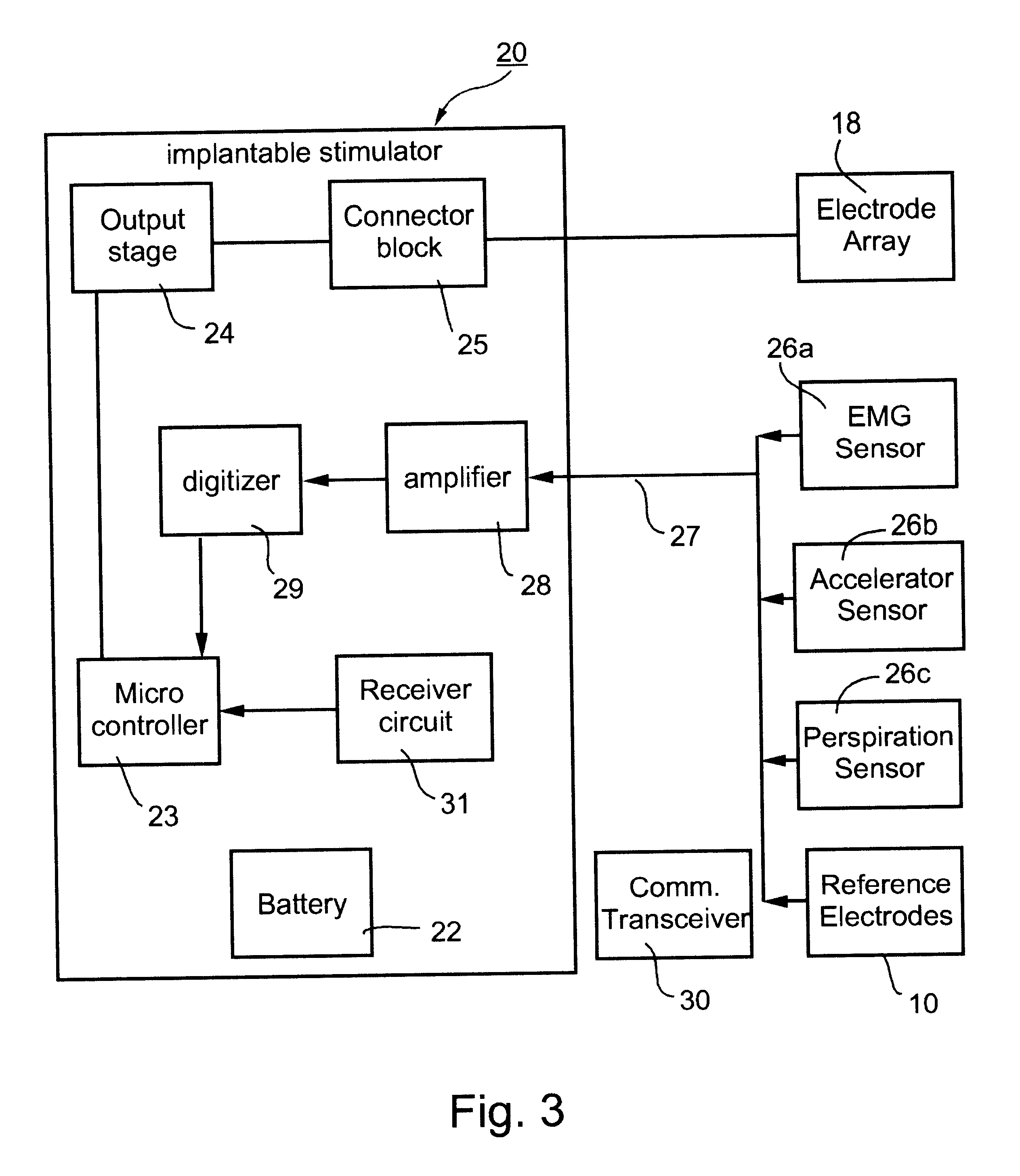
Method and apparatus for selective control of nerve fibers
InactiveUS6600954B2Pain reliefReduced sensationElectrotherapyArtificial respirationFiberNerve fiber bundle
A method and apparatus particularly useful for pain control by selectively blocking the propagation of body-generated action potentials travelling through a nerve bundle by using a tripolar electrode device to generate unidirectional action potentials to serve as collision blocks with the body-generated action potentials representing pain sensations in the small-diameter sensory fibers. In the described preferred embodiments there are a plurality of electrode devices spaced along the length of the nerve bundle which are sequentially actuated with delays corresponding to the velocity of propagation of the body-generated action potentials through the large-diameter fibers to produce a "green wave" effect which minimizes undesired anodal blocking of the large-diameter fibers while maximizing the collision blocking of the small-diameter fibers.
Owner:MEDTRONIC INC
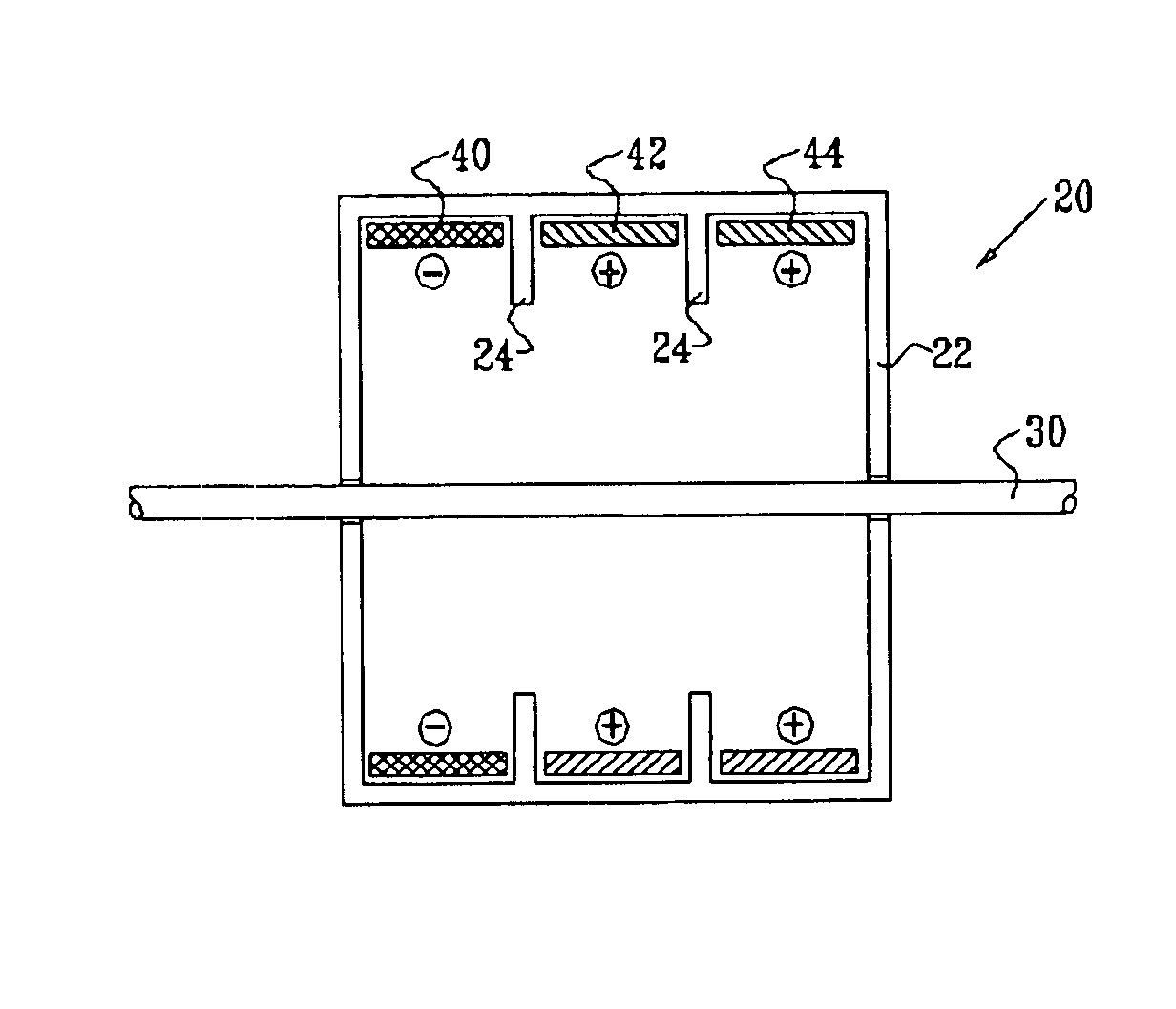
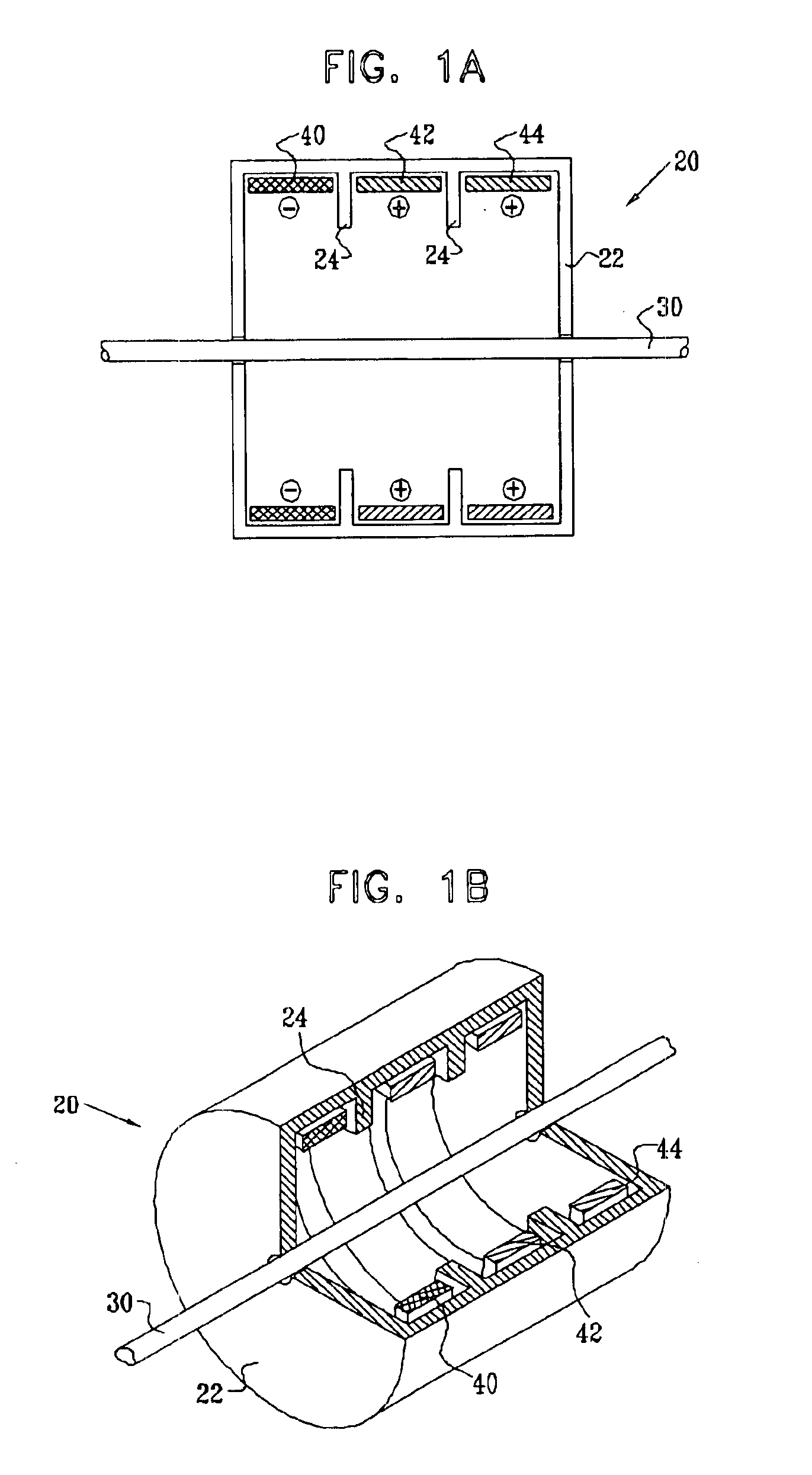
Electrode assembly for nerve control
InactiveUS6907295B2Minimize cathode effectWeakening rangeSpinal electrodesHeart stimulatorsPower flowAnatomy
Apparatus is provided for applying current to a nerve. A cathode is adapted to be placed in a vicinity of a cathodic longitudinal site of the nerve and to apply, a cathodic current to the nerve. A primary inhibiting anode is adapted to be placed in a vicinity of a primary anodal longitudinal site of the nerve and to apply a primary anodal current to the nerve. A secondary inhibiting anode is adapted to be placed in a vicinity of a secondary anodal longitudinal site of the nerve and to apply a secondary anodal current to the nerve, the secondary anodal longitudinal site being closer to the primary anodal longitudinal site than to the cathodic longitudinal site.
Owner:MEDTRONIC INC
Organic electroluminescent devices
InactiveUS20050123791A1Useful white light emissionSolid-state devicesSemiconductor/solid-state device manufacturingIridiumIsoquinoline
Disclosed is an electroluminescent device comprising a cathode and anode, and therebetween, at least two light-emitting layers wherein the first layer, layer A, comprises a phosphorescent light-emitting organometallic compound comprising iridium and an isoquinoline group and a second layer, layer B, comprising a light-emitting material. Such devices provide useful white light emissions.
Owner:EASTMAN KODAK CO
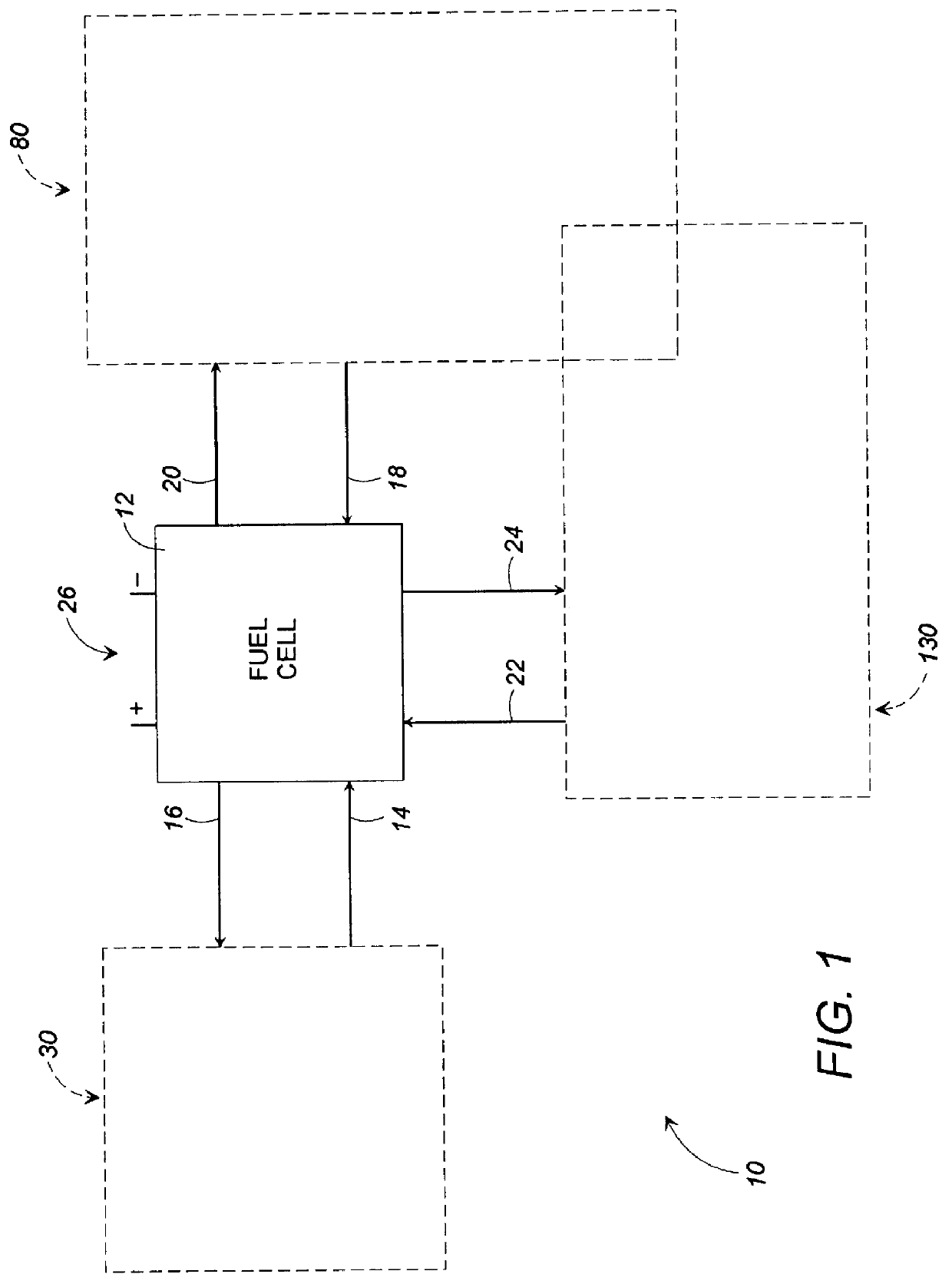
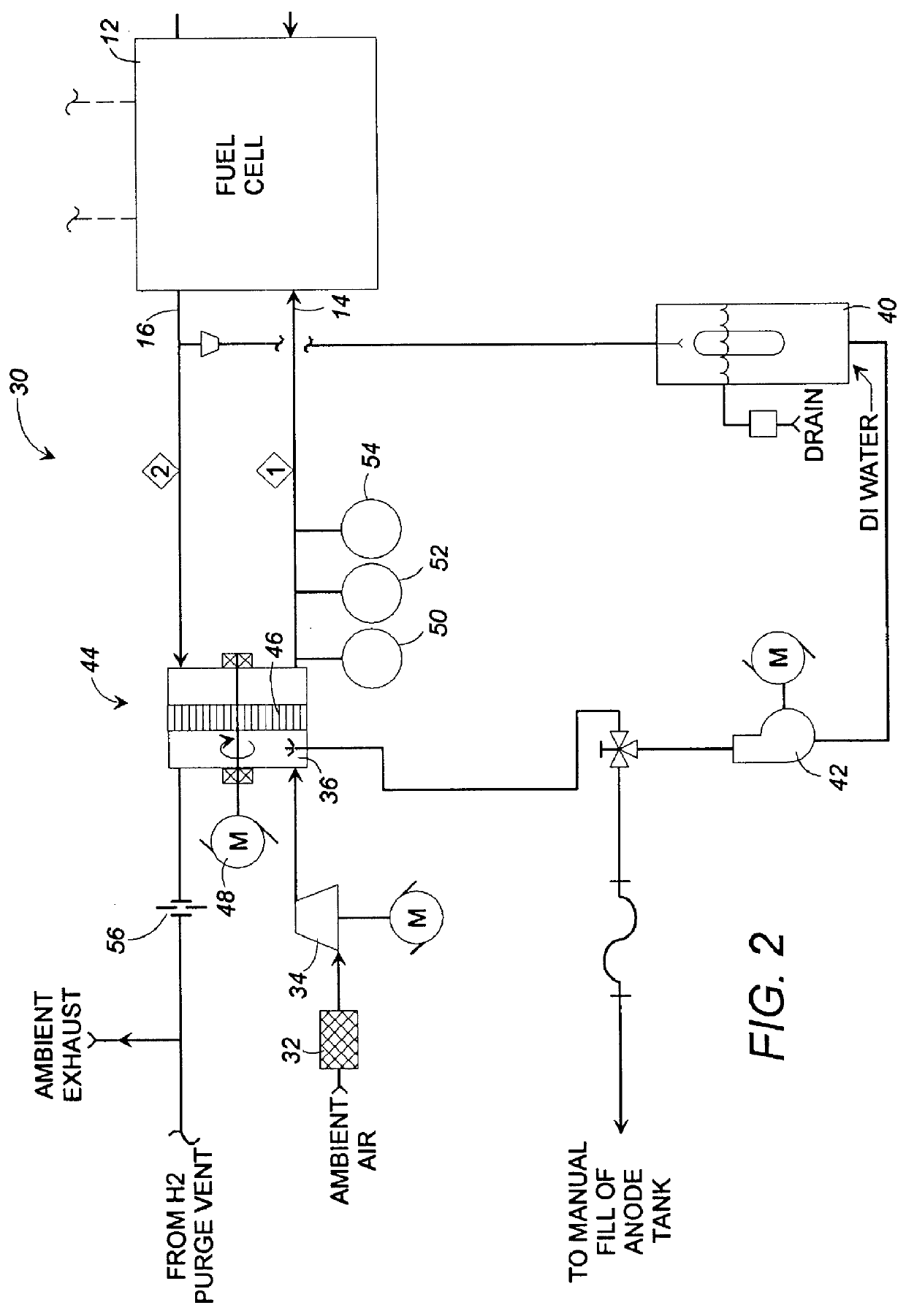
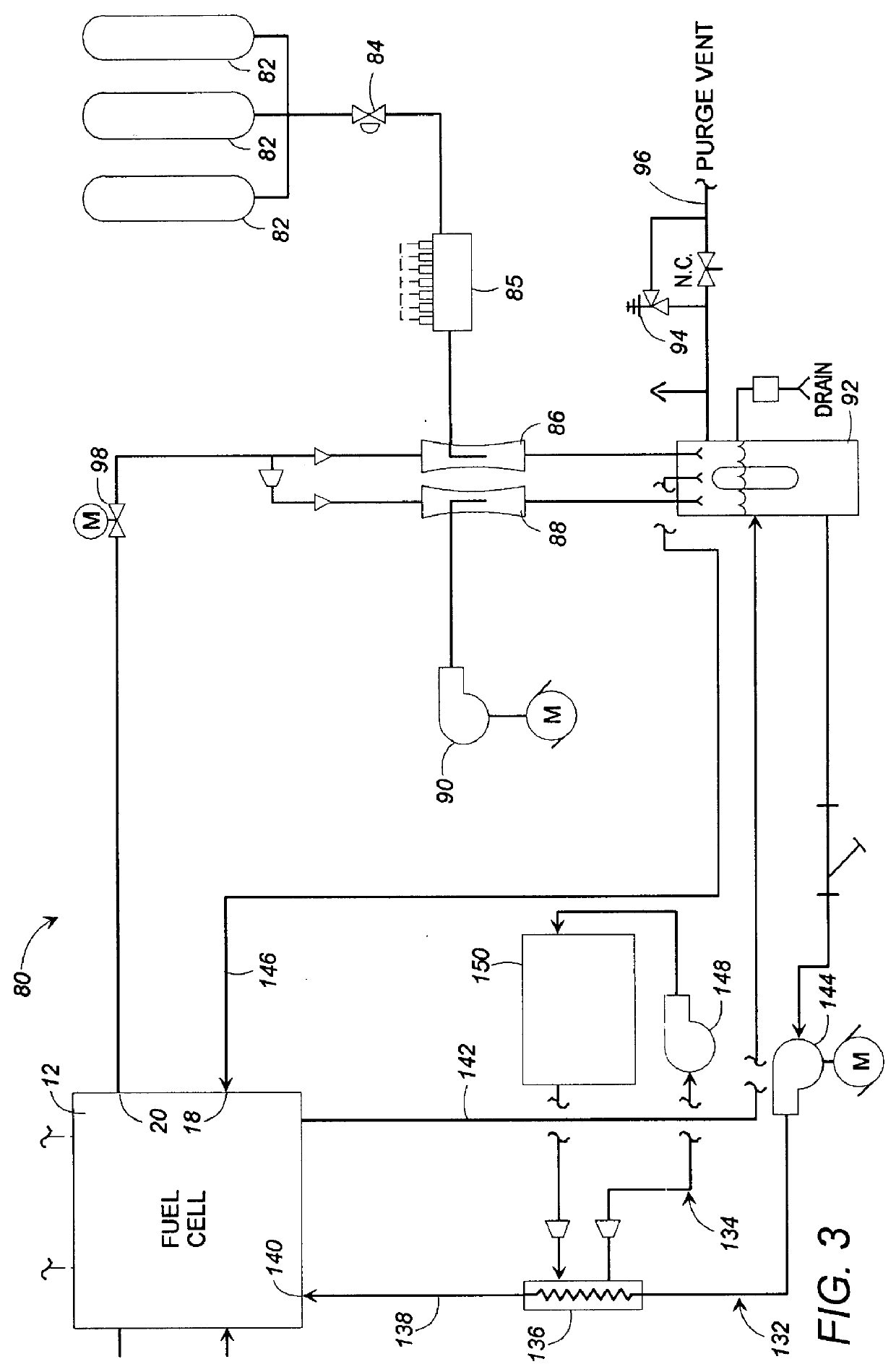
Fuel cell gas management system
InactiveUS6013385AImprove battery efficiencyMinimize the possibilityFuel cell heat exchangeFuel cell controlIonEnthalpy
A fuel cell gas management system including a cathode humidification system for transferring latent and sensible heat from an exhaust stream to the cathode inlet stream of the fuel cell; an anode humidity retention system for maintaining the total enthalpy of the anode stream exiting the fuel cell equal to the total enthalpy of the anode inlet stream; and a cooling water management system having segregated deionized water and cooling water loops interconnected by means of a brazed plate heat exchanger.
Owner:EMPRISE TECH ASSOC
Electronic devices having a header and antiparallel connected light emitting diodes for producing light from AC current
InactiveUS7009199B2Improve light outputEliminate needSolid-state devicesElectric light circuit arrangementEngineeringLight-emitting diode
A light engine comprises a pair of LED active elements mounted on a common header having first and second terminals. The first terminal is connected to the cathode of the first LED active element and the anode of the second LED active element, while the second terminal is connected to the anode of the first LED active element and the cathode of the second LED active element, thereby connecting the LEDs in an anti-parallel arrangement. A light engine having a single insulating or semi-insulating substrate having formed thereon plural LED active elements with associated p- and n-type contacts forming cathode and anode contacts, respectively, for each LED active element is also provided. The LED active elements may be mounted in a flip-chip configuration on a header having a plurality of leads. The header may include a pair of leads adapted such that two LEDs may be flip-mounted thereon with the anode of the first LED and the cathode of the second LED contacting one lead, while the cathode of the first LED and the anode of the second LED contact the other lead. In addition, the header may be adapted to permit a substrate having multiple active elements to be mounted thereon.
Owner:IDEAL IND LIGHTING LLC
Organic element for low voltage electroluminescent devices
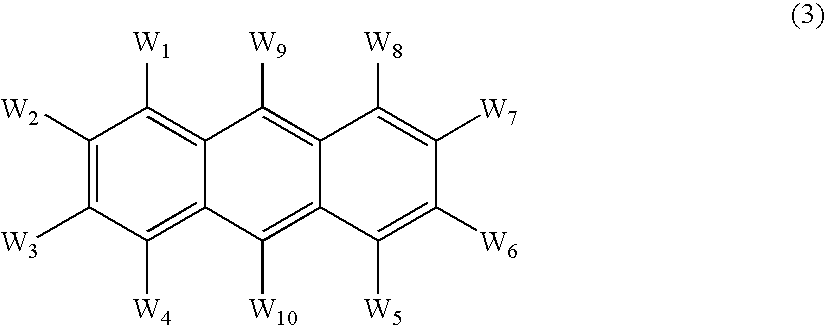
InactiveUS20070092753A1Reduce the driving voltageIncrease brightnessDischarge tube luminescnet screensLamp detailsAnthraceneHydrogen
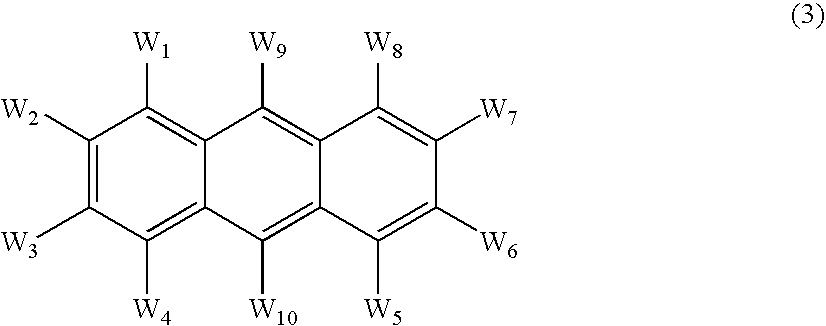
An OLED device comprises a cathode, a light emitting layer and an anode, in that order, wherein (i) the light-emitting layer comprises up to 10 volume % of a light emitting compound and at least one anthracene host compound of Formula (3): wherein W1-W10 independently represents hydrogen or an independently selected substituent, and (ii) a further layer located between the cathode and the light emitting layer, contains (a) 10-volume % or more of an anthracene compound of Formula (3) and (b) at least one salt or complex of an element selected from Group IA, IIA, IIIA and IIB of the Periodic Table. Such devices exhibit reduced drive voltage while maintaining good luminance.
Owner:EASTMAN KODAK CO
Programmable sub-surface aggregating metallization structure and method of making same
InactiveUS6418049B1Low costHighly manufacturableNanoinformaticsSolid-state devicesCapacitanceElectrical conductor
A programmable sub-surface aggregating metallization sructure ("PSAM") includes an ion conductor such as a chalcogenide-glass which includes metal ions and at least two electrodes disposed at opposing surfaces of the ion conductor. Preferably, the ion conductor includes a chalcogenide material with Group IB or Group IIB metals. One of the two electrodes is preferably configured as a cathode and the other as an anode. When a voltage is applied between the anode and cathode, a metal dendrite grows from the cathode through the ion conductor towards the anode. The growth rate of the dendrite may be stopped by removing the voltage or the dendrite may be retracted back towards the cathode by reversing the voltage polarity at the anode and cathode. When a voltage is applied for a sufficient length of time, a continuous metal dendrite grows through the ion conductor and connects the electrodes, thereby shorting the device. The continuous metal dendrite then can be broken by applying another voltage. The break in the metal dendrite can be reclosed by applying yet another voltage. Changes in the length of the dendrite or the presence of a break in the dendrite affect the resistance, capacitance, and impedance of the PSAM.
Owner:THE ARIZONA BOARD OF REGENTS ON BEHALF OF THE UNIV OF ARIZONA +1
Separator for a high energy rechargeable lithium battery
InactiveUS6432586B1Cell seperators/membranes/diaphragms/spacersNon-aqueous electrolyte accumulator electrodesCeramic compositeMetallurgy
The instant invention is directed to a separator for a high energy rechargeable lithium battery and the corresponding battery. The separator includes a ceramic composite layer and a polymeric microporous layer. The ceramic layers includes a mixture of inorganic particles and a matrix material. The ceramic layer is adapted, at least, to block dendrite growth and to prevent electronic shorting. The polymeric layer is adapted, at least, to block ionic flow between the anode and the cathode in the event of thermal runaway.
Owner:CELGARD LLC
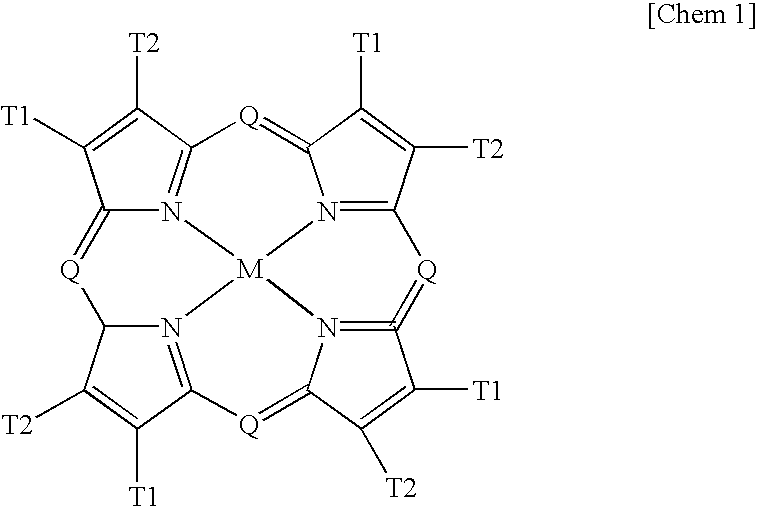
Phosphorescent material, and organic electroluminescent device and image display apparatus using same
InactiveUS8067099B2Improve efficiencyIncreased durabilityIndium organic compoundsDischarge tube luminescnet screensOrganic electroluminescenceOrganic compound
Provided is an organic electroluminescent device which has a high efficiency and high durability. The organic electroluminescent device includes an anode and a cathode; and a layer including an organic compound interposed between the anode and the cathode, in which the layer includes a phosphorescent material including an Ir complex or Pt complex having at least one ligand represented by any one of the following general formulae (1) to (4):
Owner:CANON KK
Aromatic amine derivatives and organic electroluminescence device using the same
InactiveUS20070145888A1Increase productionLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic electroluminescenceAmine derivatives
Provided are: a novel aromatic amine derivative having an asymmetric structure; and an organic electroluminescence device having one or multiple organic thin film layers including at least a light emitting layer, the one or multiple organic thin film layers being interposed between a cathode and an anode. The aromatic amine derivative realizes the organic EL device capable of suppressing the crystallization of a molecule, improving yields upon production of the organic EL device, and having a long lifetime when at least one layer of the one or more multiple organic thin film layers contains the aromatic amine derivative alone or as a component of a mixture.
Owner:IDEMITSU KOSAN CO LTD
Nano graphene platelet-based composite anode compositions for lithium ion batteries
ActiveUS20090117467A1Improve conductivityLower internal resistanceElectrolytic capacitorsSecondary cellsGraphene flakeGraphite
The present invention provides a nano-scaled graphene platelet-based composite material composition for use as an electrode, particularly as an anode of a lithium ion battery. The composition comprises: (a) micron- or nanometer-scaled particles or coating which are capable of absorbing and desorbing lithium ions; and (b) a plurality of nano-scaled graphene platelets (NGPs), wherein a platelet comprises a graphene sheet or a stack of graphene sheets having a platelet thickness less than 100 nm; wherein at least one of the particles or coating is physically attached or chemically bonded to at least one of the graphene platelets and the amount of platelets is in the range of 2% to 90% by weight and the amount of particles or coating in the range of 98% to 10% by weight. Also provided is a lithium secondary battery comprising such a negative electrode (anode). The battery exhibits an exceptional specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:SAMSUNG ELECTRONICS CO LTD
Electro-optical device and electronic device
InactiveUS6689492B1Reduce heatReduce releaseDischarge tube luminescnet screensSemiconductor/solid-state device detailsDisplay deviceEngineering
An object of the present invention is to provide an EL display device, which has a high operating performance and reliability. A third passivation film 45 is disposed under an EL element 203 which comprises a pixel electrode (anode) 46, and EL layer 47 and a cathode 48, to make a structure in which heat generated by the EL element 203 is radiated. Further, the third passivation film 45 prevents alkali metals within the EL element 203 from diffusing into the TFTs side, and prevents moisture and oxygen of the TFTs side from penetrating into the EL element 203. More preferably, heat radiating effect is given to a fourth passivation film 50 to make the EL element 203 to be enclosed by heat radiating layers.
Owner:SEMICON ENERGY LAB CO LTD
Programmable conductor memory cell structure
In programmable conductor memory cells, metal ions precipitate out of a glass electrolyte element in response to an applied electric field in one direction only, causing a conductive pathway to grow from cathode to anode. The amount of conductive pathway growth, and therefore the programming, depends, in part, on the availability of metal ions. It is important that the metal ions come only from the solid solution of the memory cell body. If additional metal ions are supplied from other sources, such as the sidewall edge at the anode interface, the amount of metal ions may not be directly related to the strength of the electric field, and the programming will not respond consistently from cell to cell. The embodiments described herein provide new and novel structures that block interface diffusion paths for metal ions, leaving diffusion from the bulk glass electrolyte as the only supply of metal ions for conductive pathway formation.
Owner:OVONYX MEMORY TECH LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com