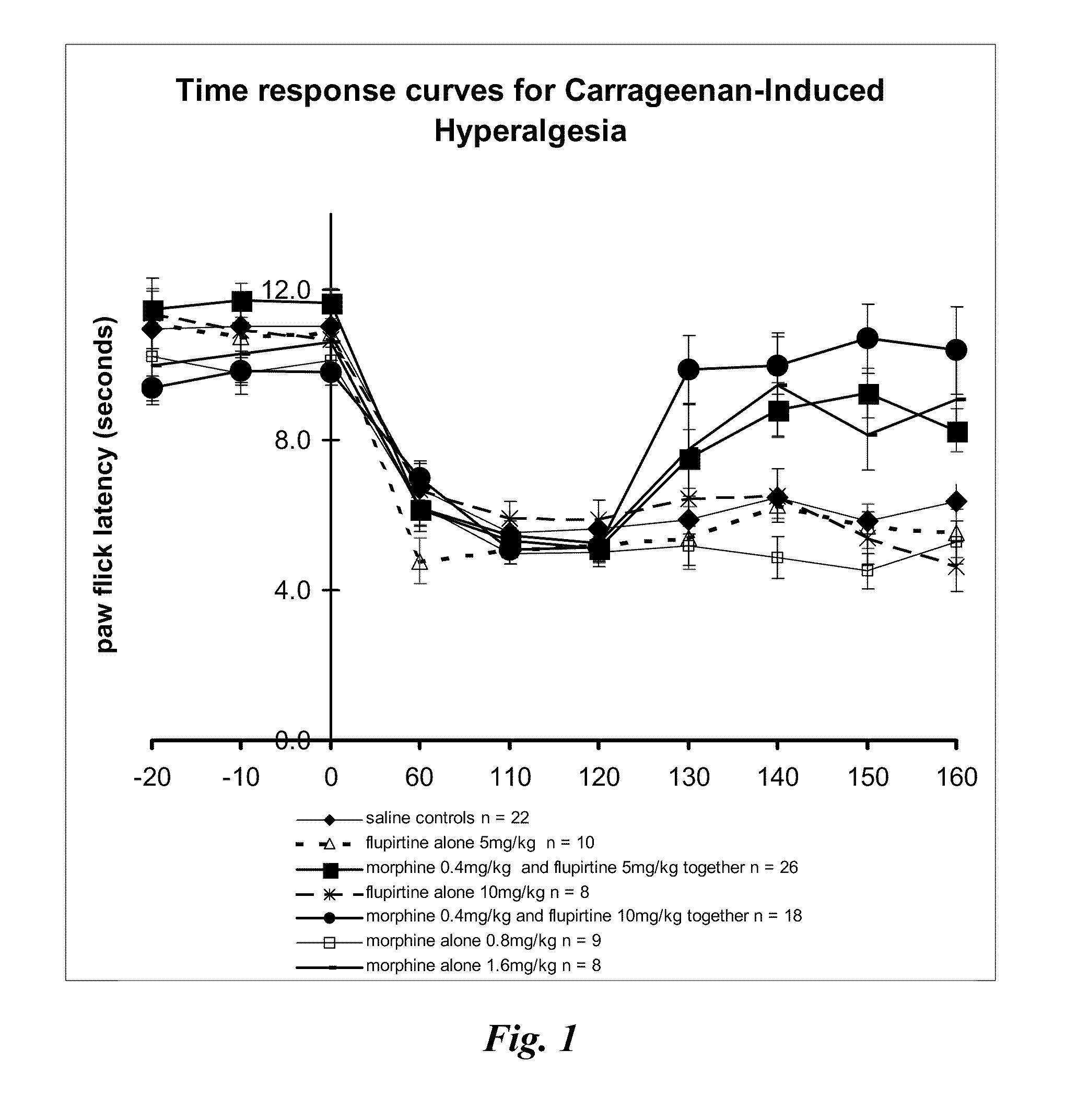
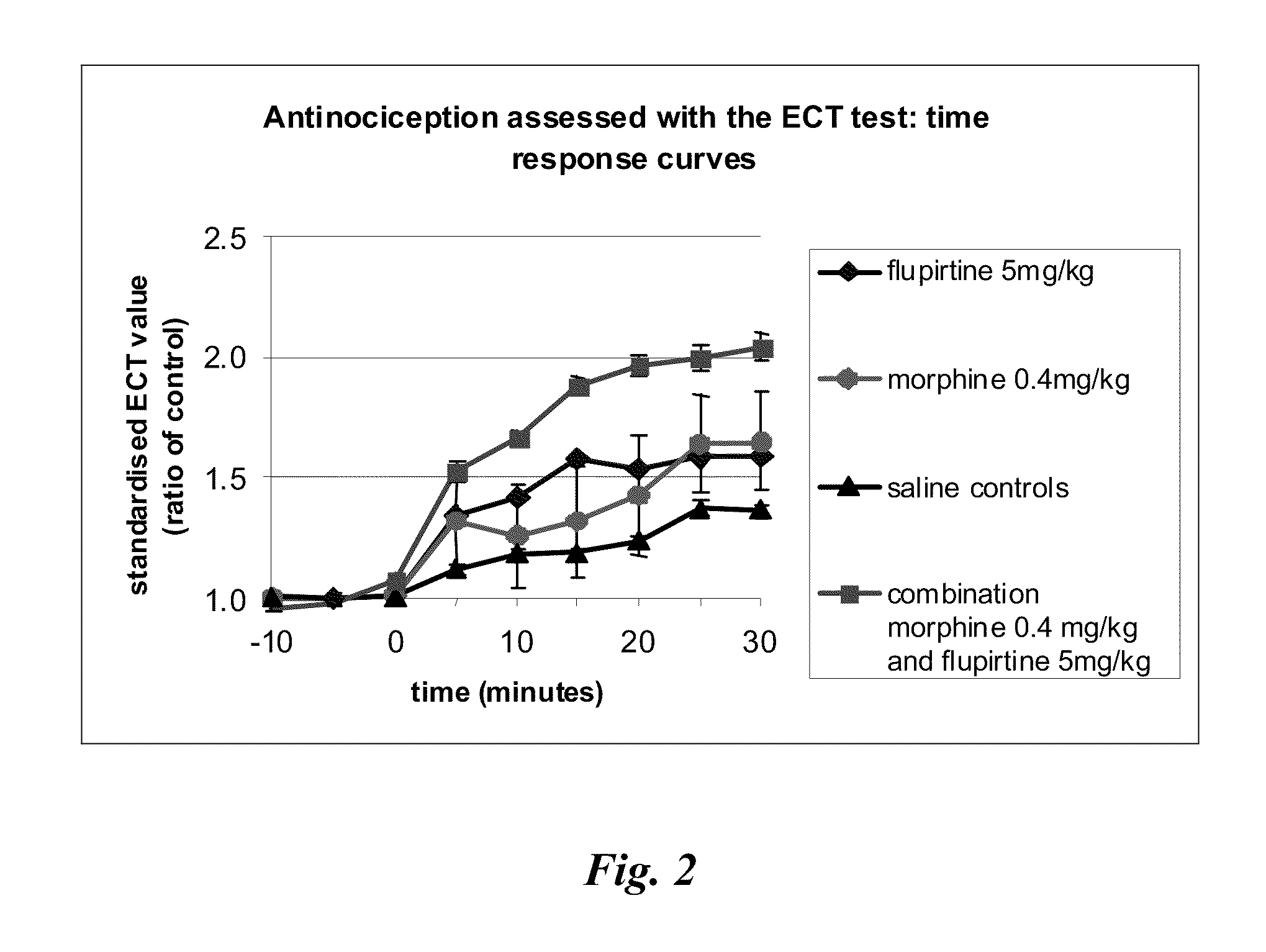
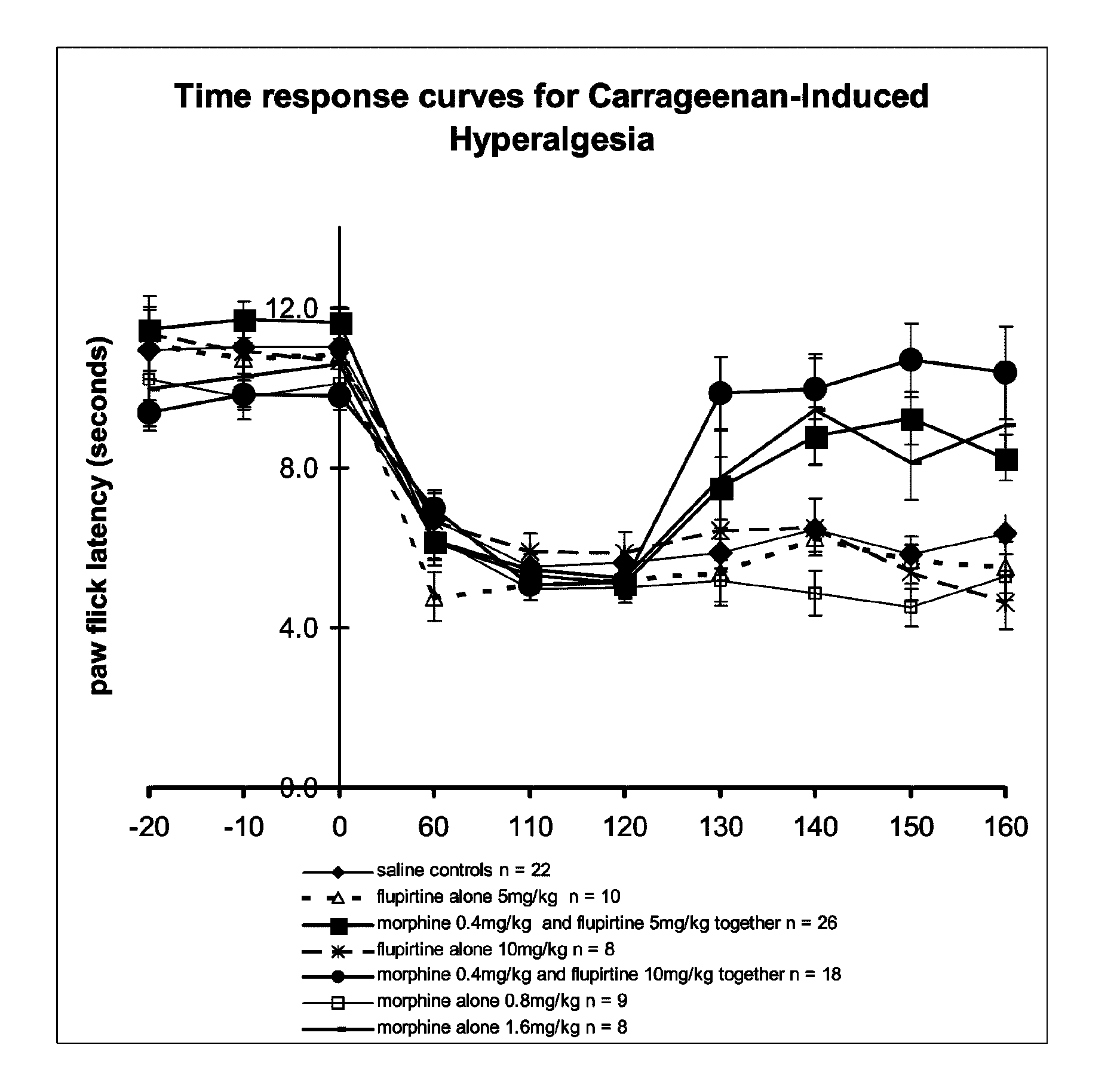
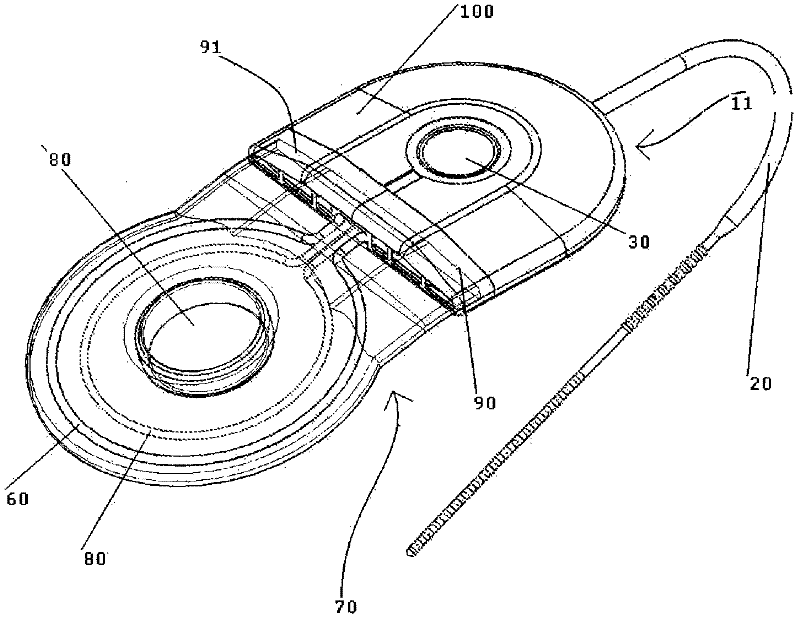
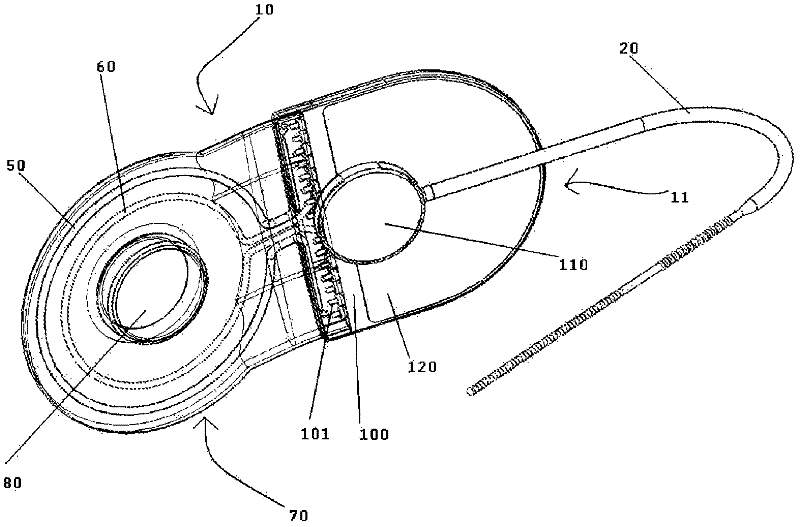
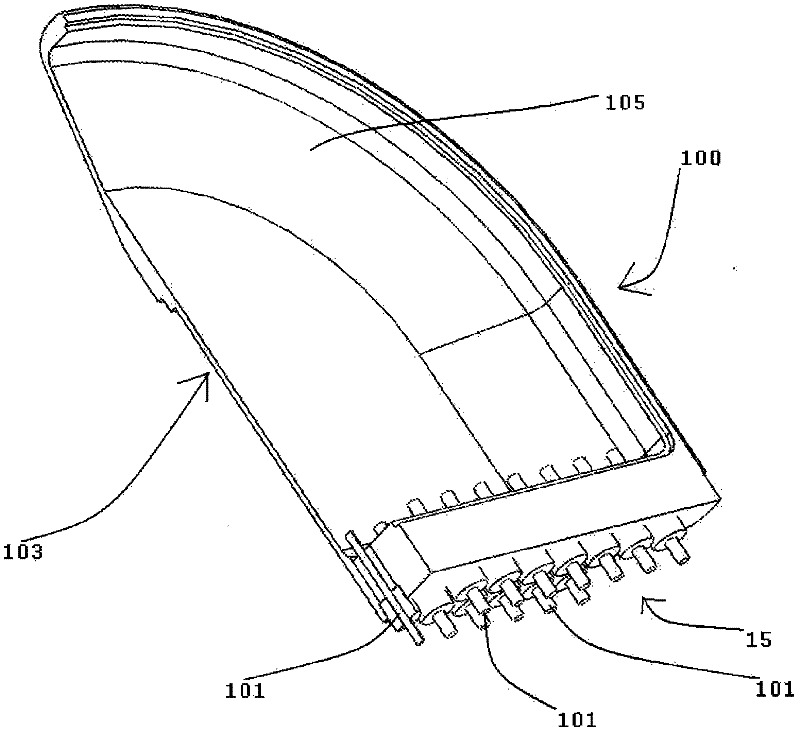
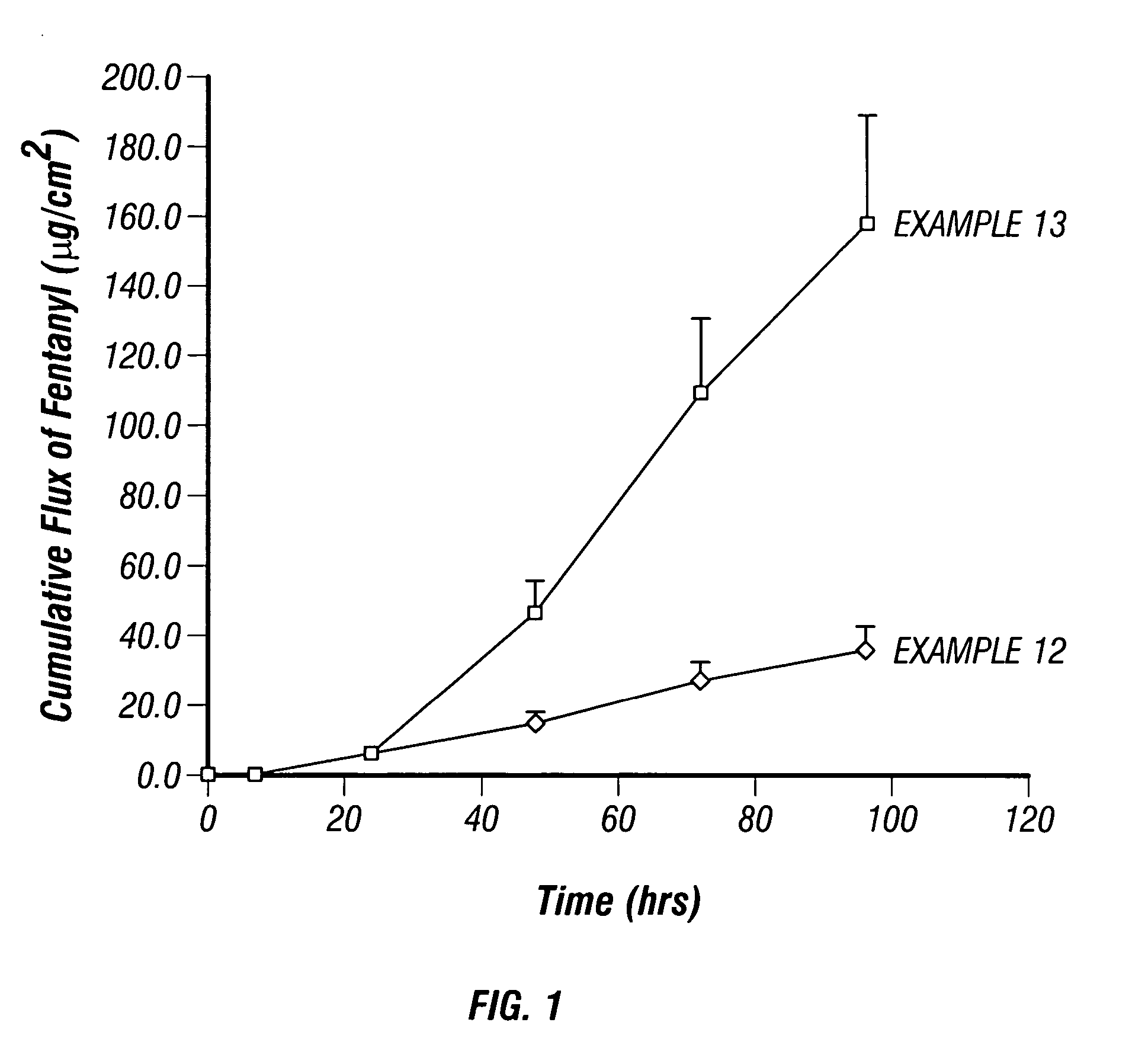
Patents
Literature
64 results about "Pain control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pain management, pain medicine, pain control or algiatry, is a branch of medicine employing an interdisciplinary approach for easing the suffering and improving the quality of life of those living with chronic pain The typical pain management team includes medical practitioners, pharmacists, clinical psychologists, physiotherapists, occupational ...
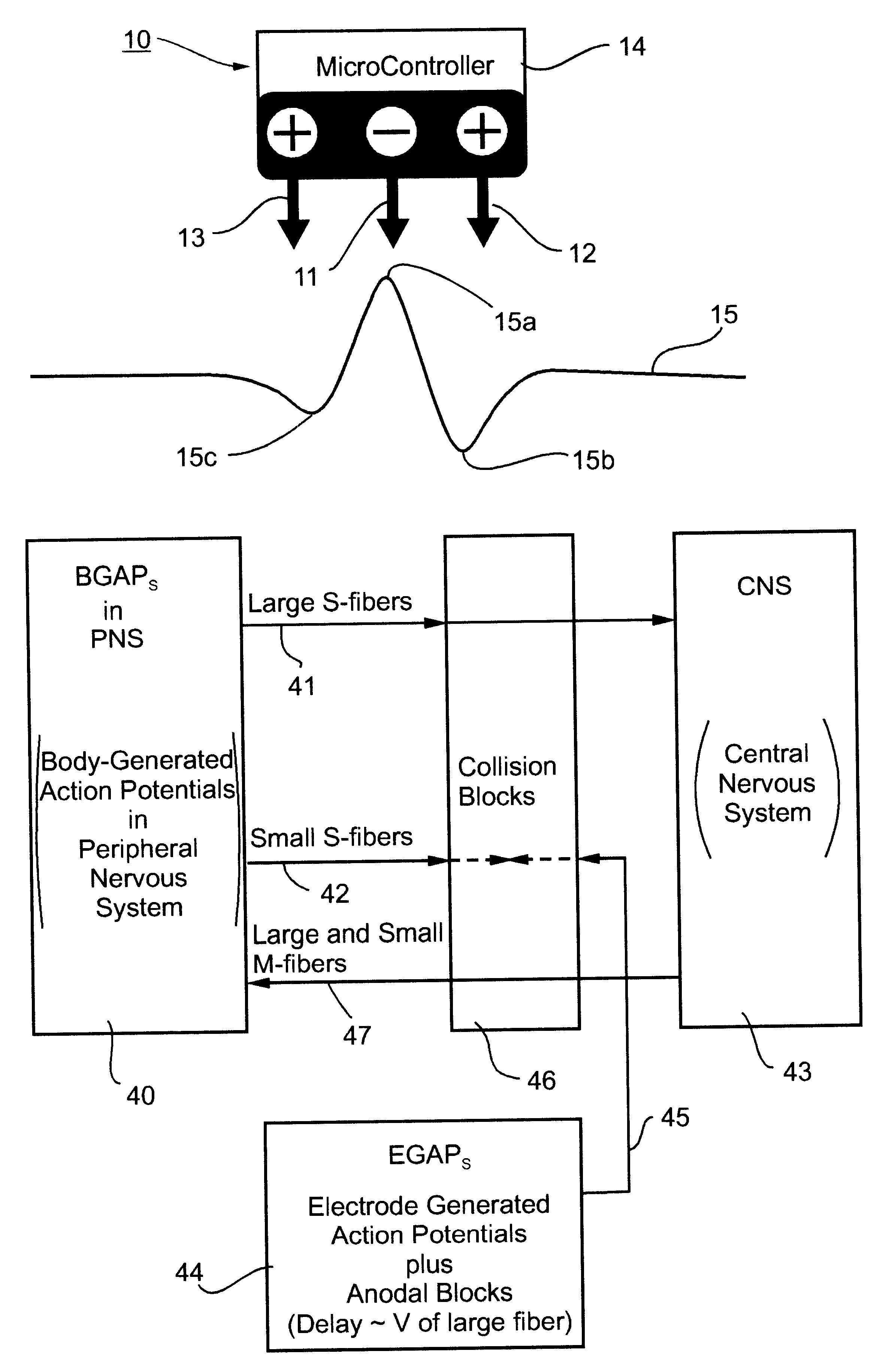
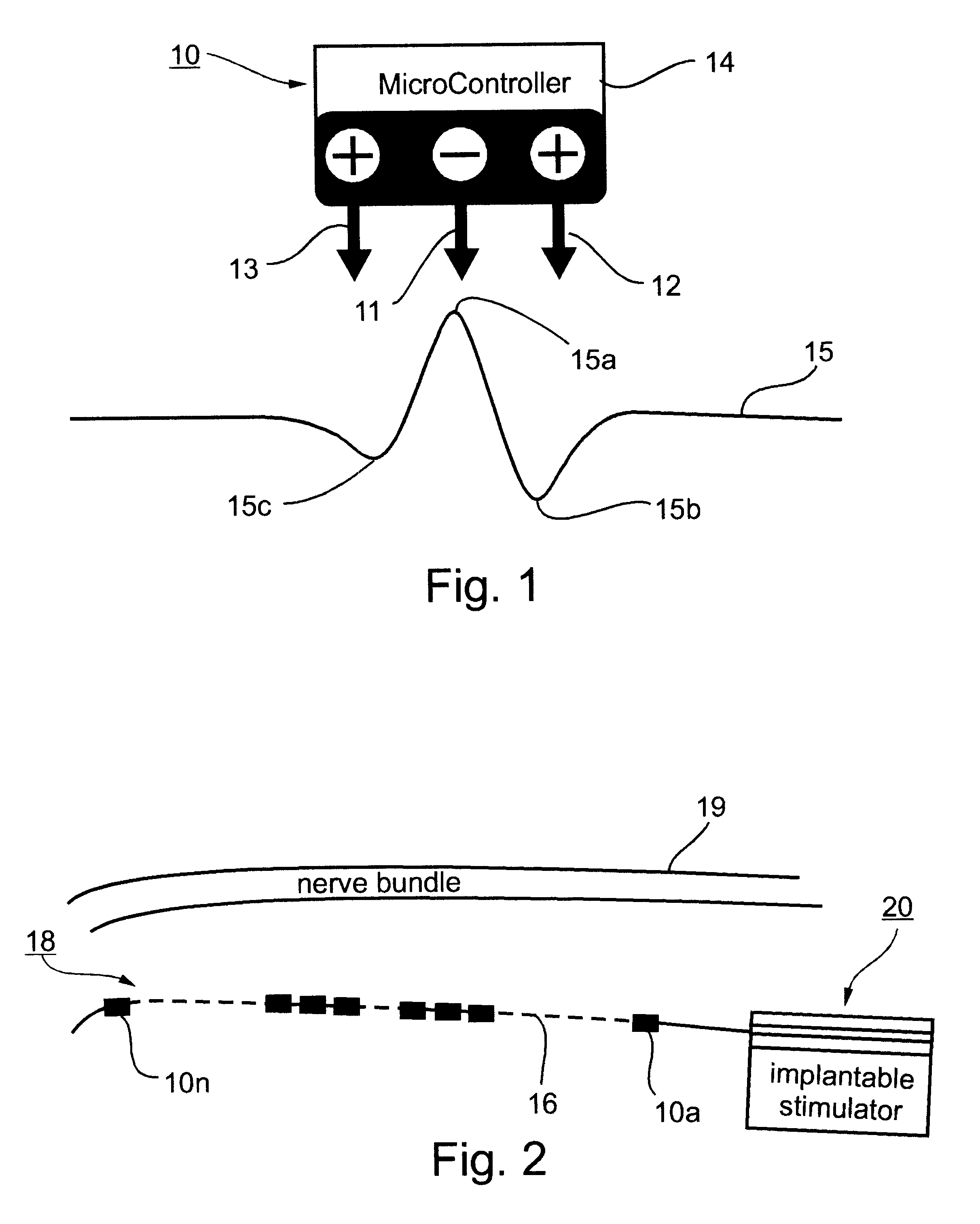
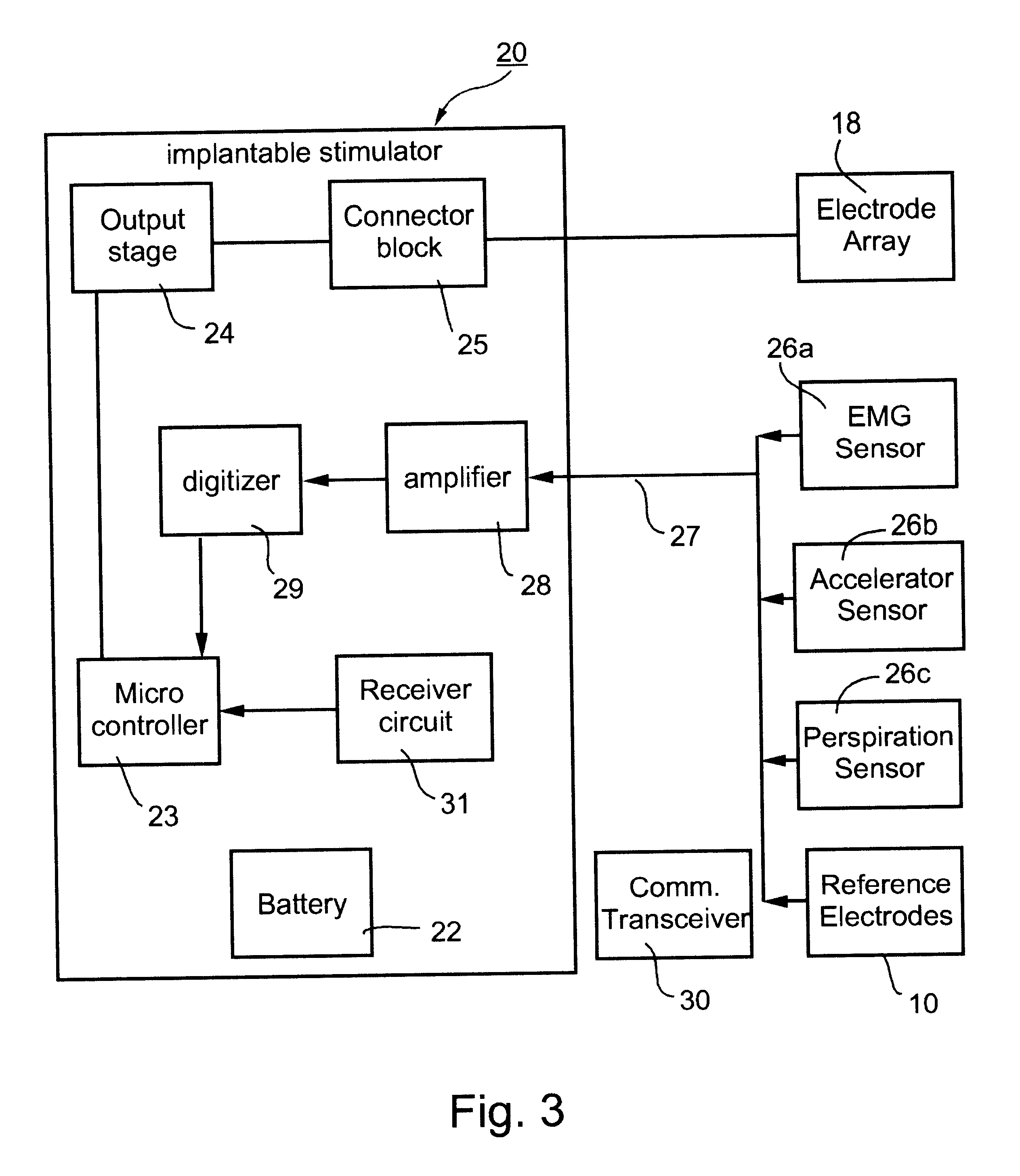
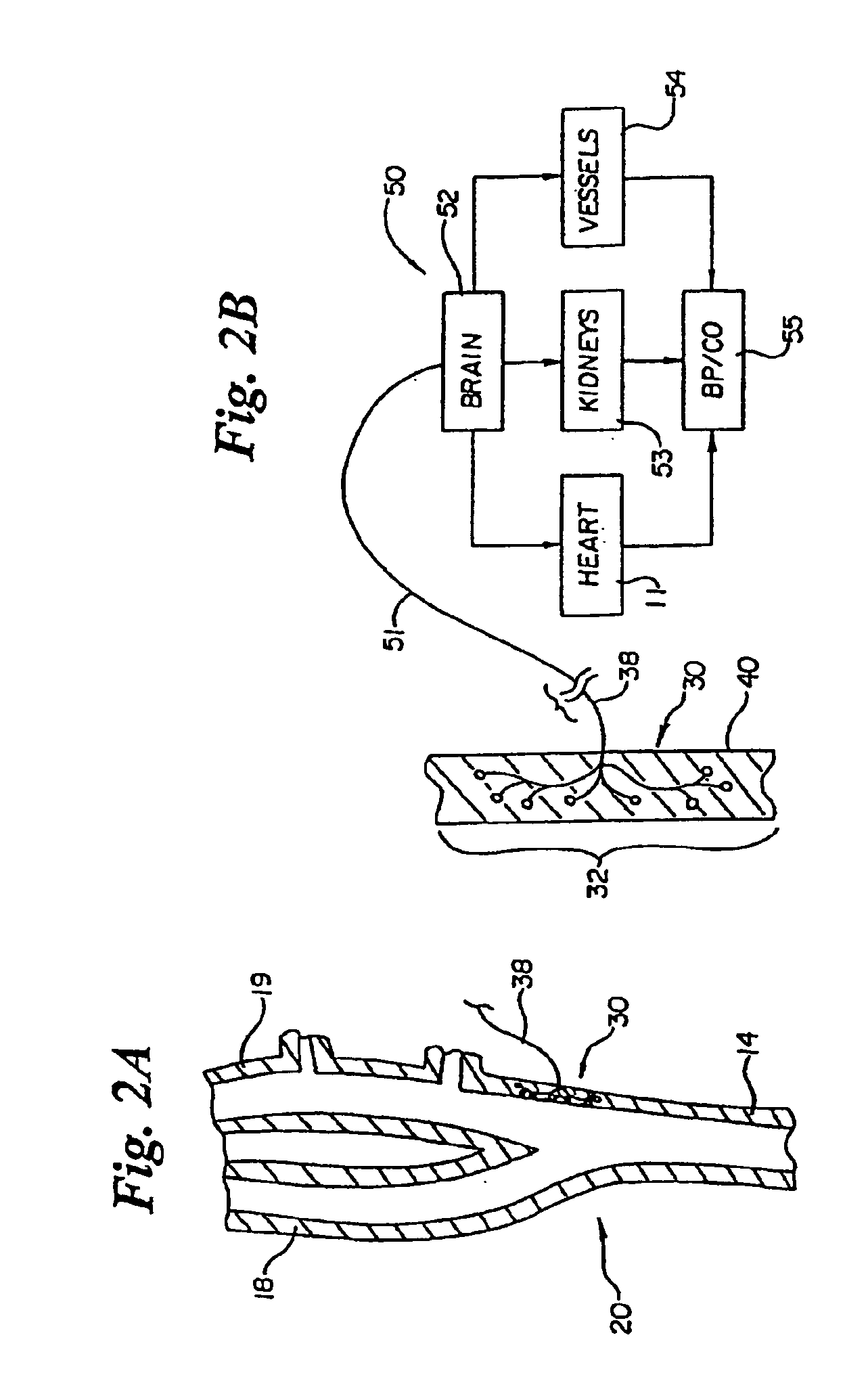
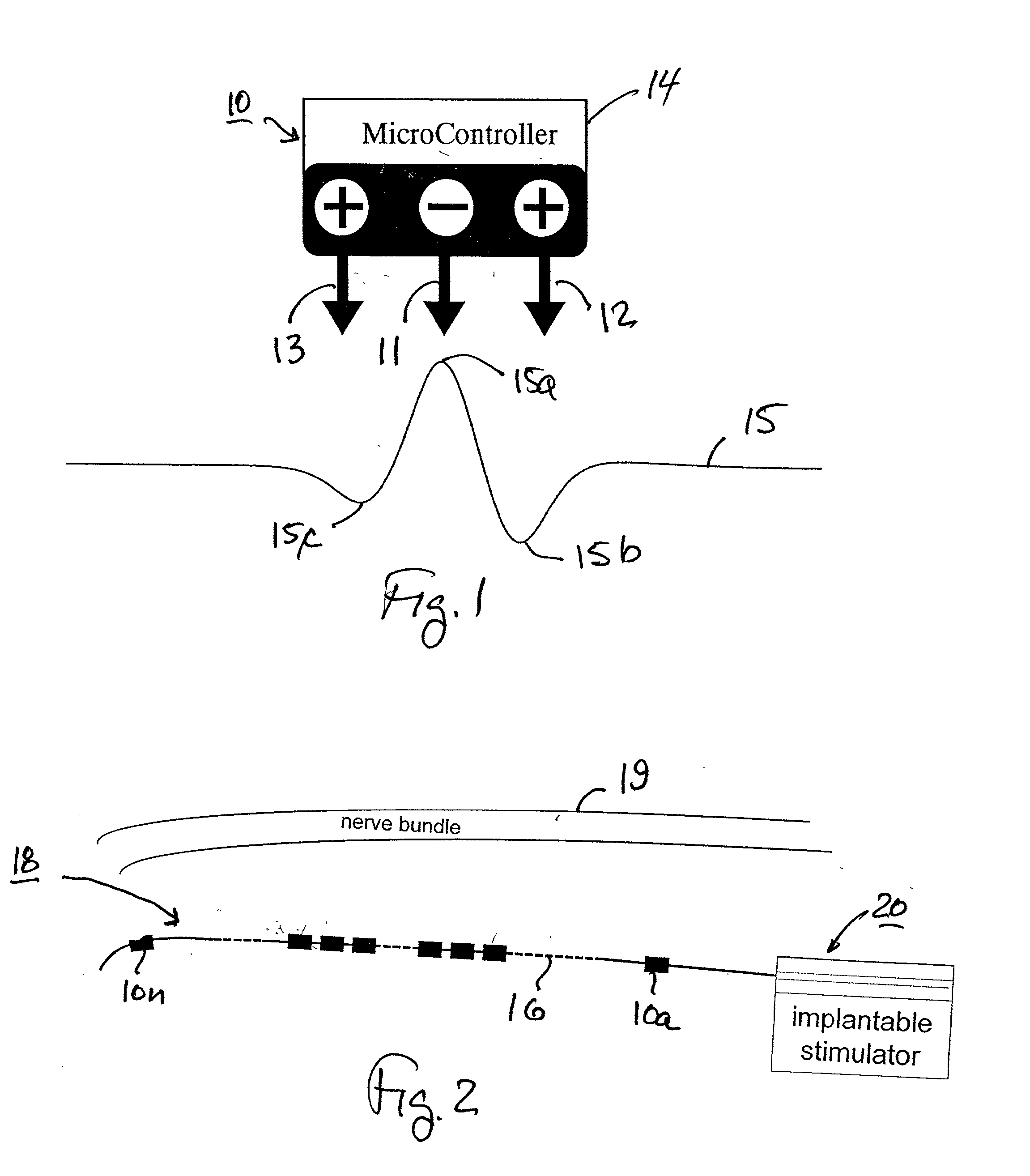
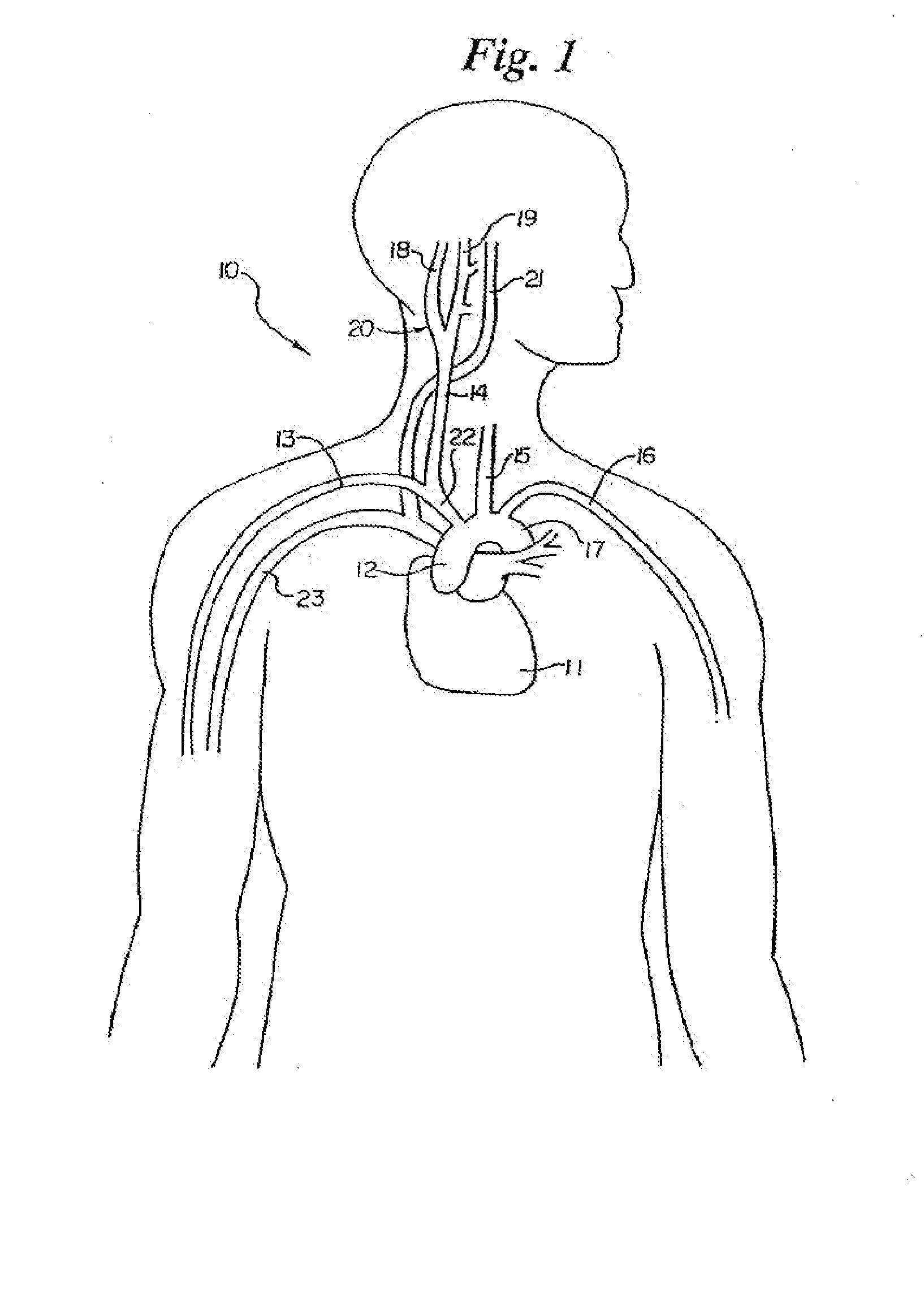
Method and apparatus for selective control of nerve fibers
InactiveUS6600954B2Pain reliefReduced sensationElectrotherapyArtificial respirationFiberNerve fiber bundle
A method and apparatus particularly useful for pain control by selectively blocking the propagation of body-generated action potentials travelling through a nerve bundle by using a tripolar electrode device to generate unidirectional action potentials to serve as collision blocks with the body-generated action potentials representing pain sensations in the small-diameter sensory fibers. In the described preferred embodiments there are a plurality of electrode devices spaced along the length of the nerve bundle which are sequentially actuated with delays corresponding to the velocity of propagation of the body-generated action potentials through the large-diameter fibers to produce a "green wave" effect which minimizes undesired anodal blocking of the large-diameter fibers while maximizing the collision blocking of the small-diameter fibers.
Owner:MEDTRONIC INC
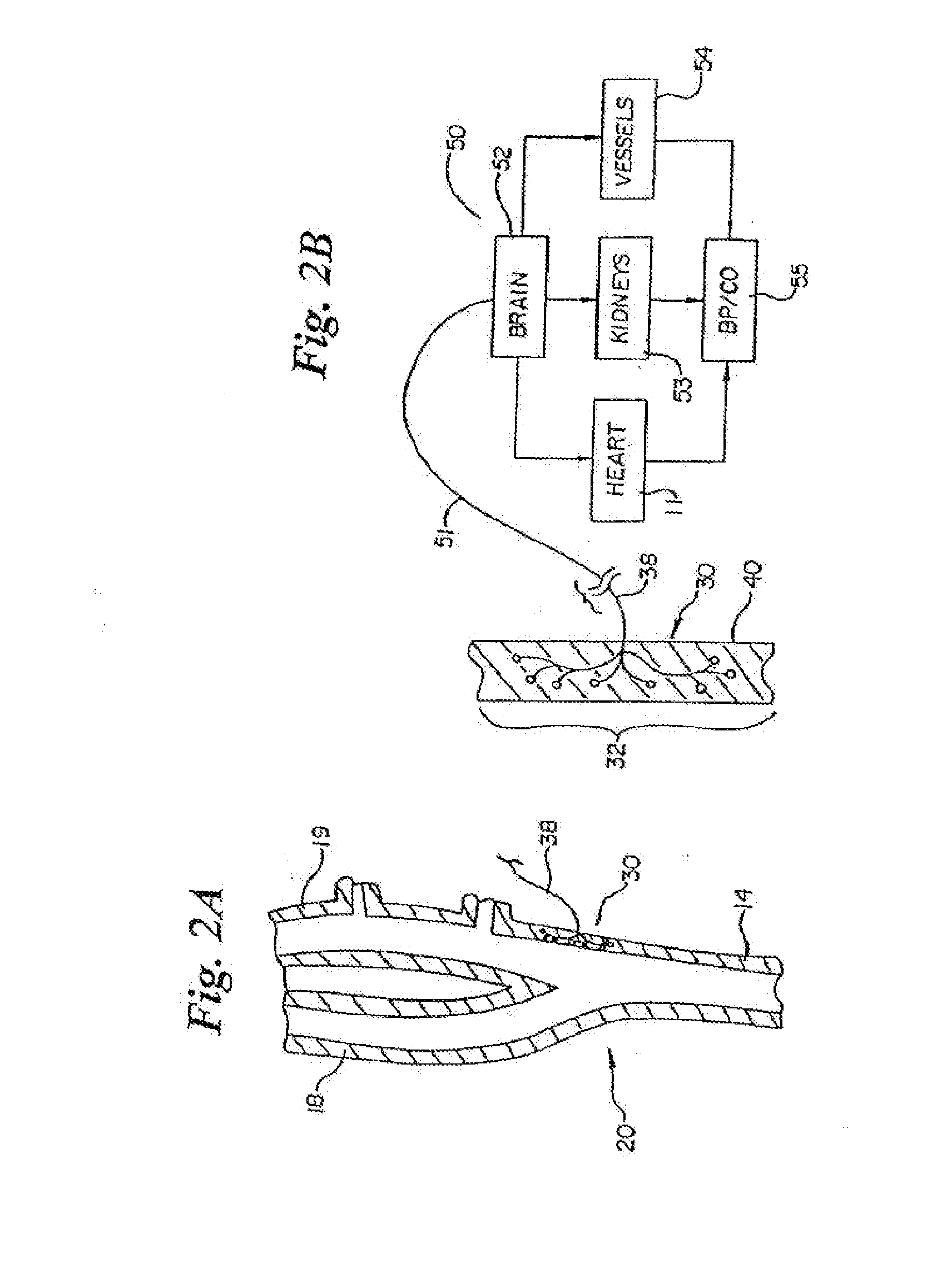
Baroreflex activation for pain control, sedation and sleep
ActiveUS20050154418A1Treat and reduces painRelieve painSpinal electrodesArtificial respirationSensing dataSedation
Systems and methods provide baroreflex activation to treat or reduce pain and / or to cause or enhance sedation or sleep. Methods involve activating the baroreflex system to provide pain reduction, sedation, improved sleep or some combination thereof. Systems include at least one baroreflex activation device, at least one sensor for sensing physiological activity of the patient, and a processor coupled with the baroreflex activation device(s) and the sensor(s) for processing sensed data received from the sensor and for activating the baroreflex activation device. In some embodiments, the system is fully implantable within a patient, such as in an intravascular, extravascular or intramural location.
Owner:CVRX
Method and apparatus for selective control of nerve fibers
InactiveUS20020099419A1Pain reliefReduced sensationElectrotherapyArtificial respirationFiberNerve fiber bundle
A method and apparatus particularly useful for pain control by selectively blocking the propagation of body-generated action potentials travelling through a nerve bundle by using a tripolar electrode device to generate unidirectional action potentials to serve as collision blocks with the body-generated action potentials representing pain sensations in the small-diameter sensory fibers. In the described preferred embodiments there are a plurality of electrode devices spaced along the length of the nerve bundle which are sequentially actuated with delays corresponding to the velocity of propagation of the body-generated action potentials through the large-diameter fibers to produce a "green wave" effect which minimizes undesired anodal blocking of the large-diameter fibers while maximizing the collision blocking of the small-diameter fibers.
Owner:MEDTRONIC INC
Electrical stimulation device and method for therapeutic treatment and pain management
ActiveUS20100042180A1Easily and tactilely differentiateAvoid accidental activationExternal electrodesElectricityManaged pain
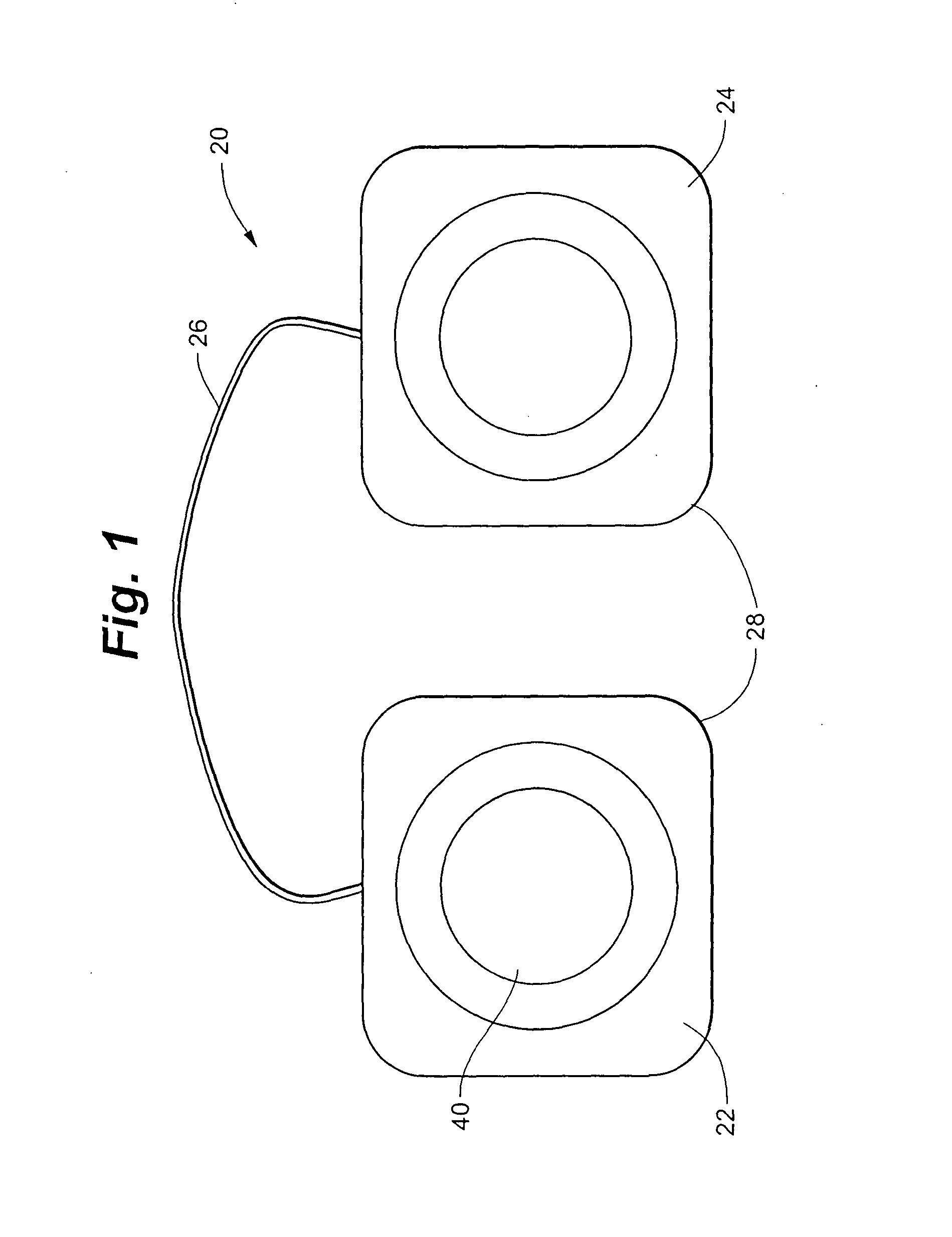
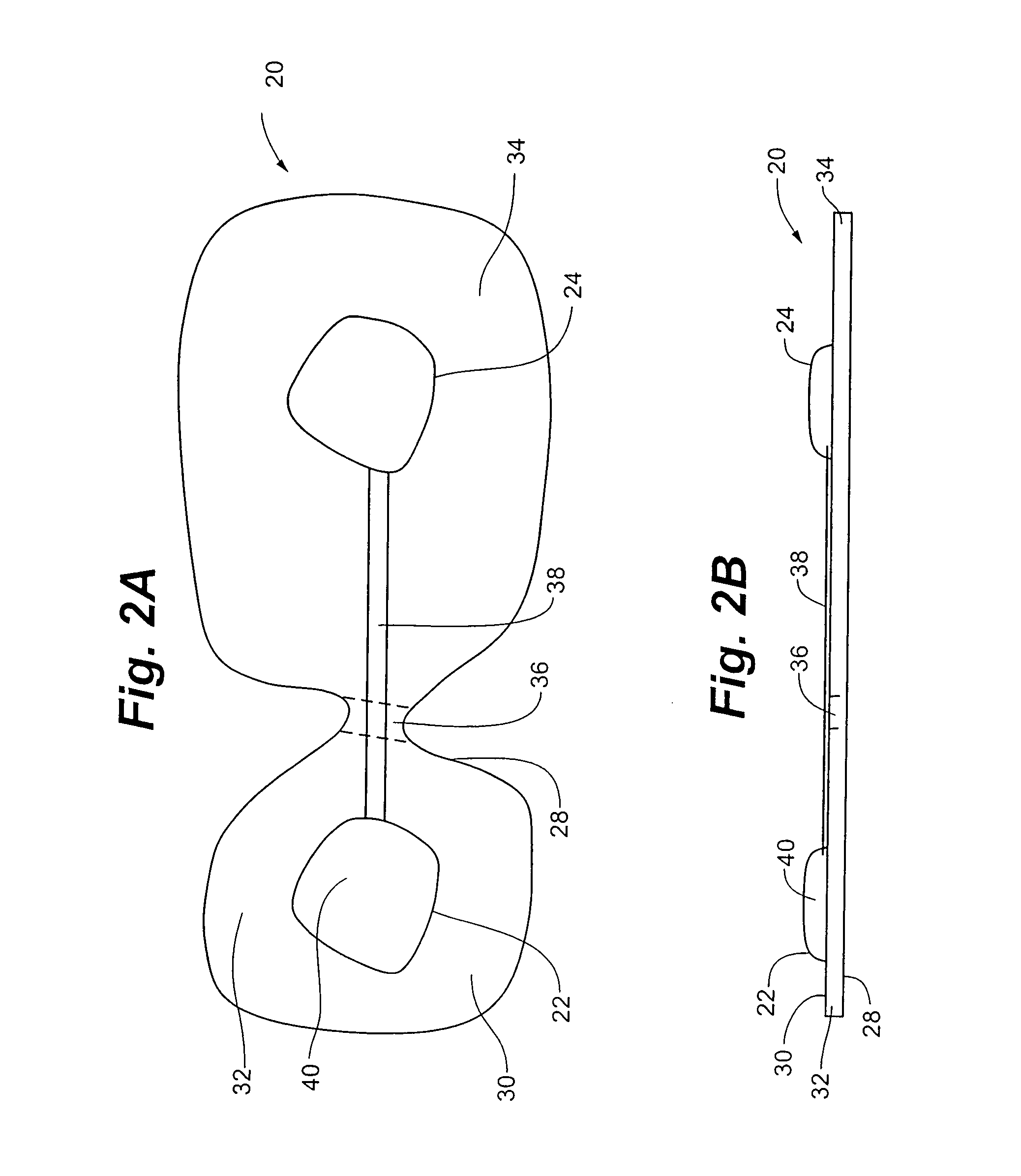
A disposable electrical stimulation device and method for providing therapeutic treatment and pain management in a convenient, compact configuration. Electrode size and shape and relative configuration can be varied according to an intended application and use, or a universal configuration can be provided for use on almost any area of the body. The common structure of communicatively coupled dual electrodes including control circuitry and a power source accommodates a range of different sizes, configurations, stimulation treatment intensities, and other physical and electrical characteristics that can be pre-customized and packaged for specific, limited time use. The device can therefore be used in methods of providing therapy, managing pain, and achieving other treatment goals by electrical stimulation.
Owner:ENCORE MEDICAL ASSET CORP +1
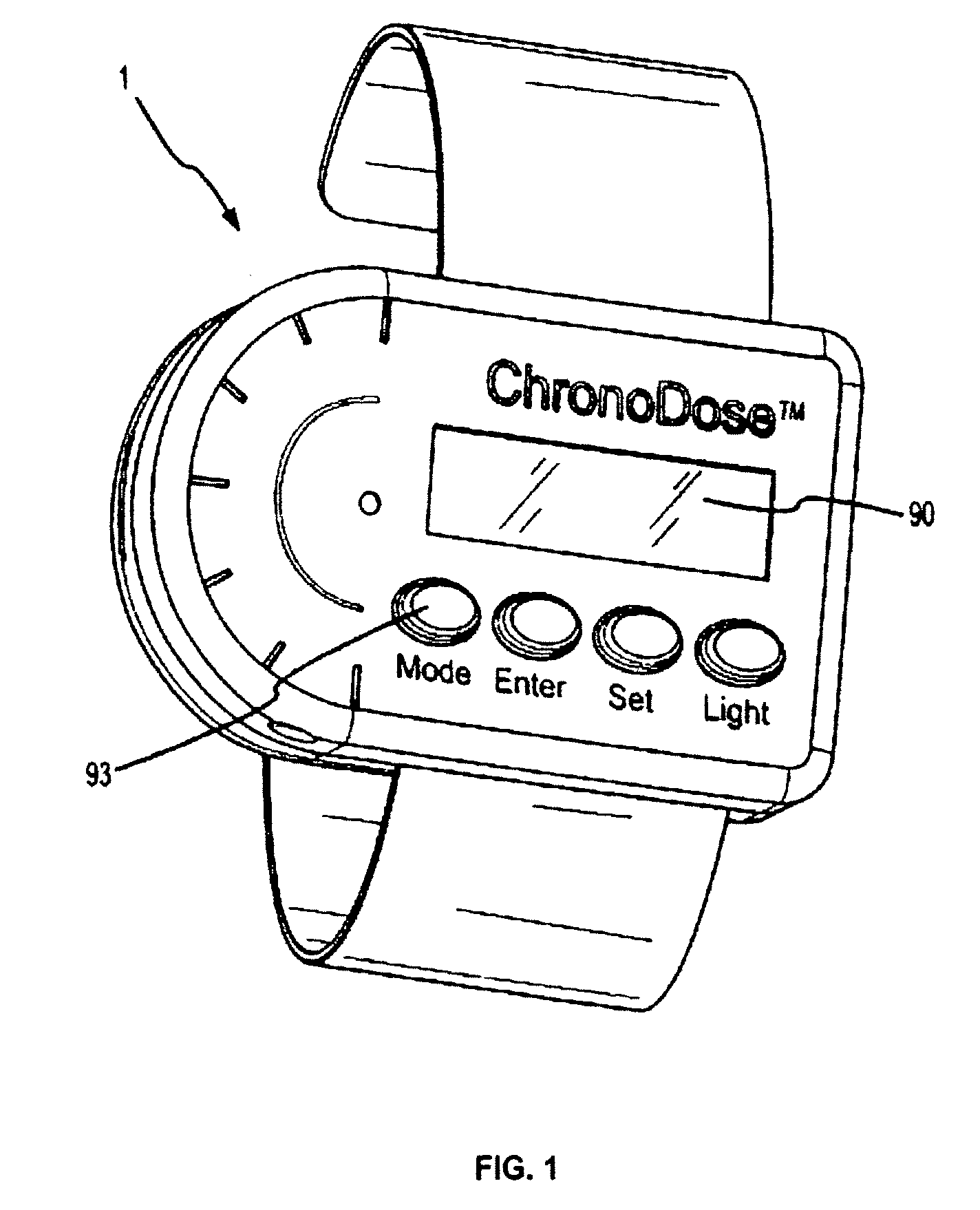
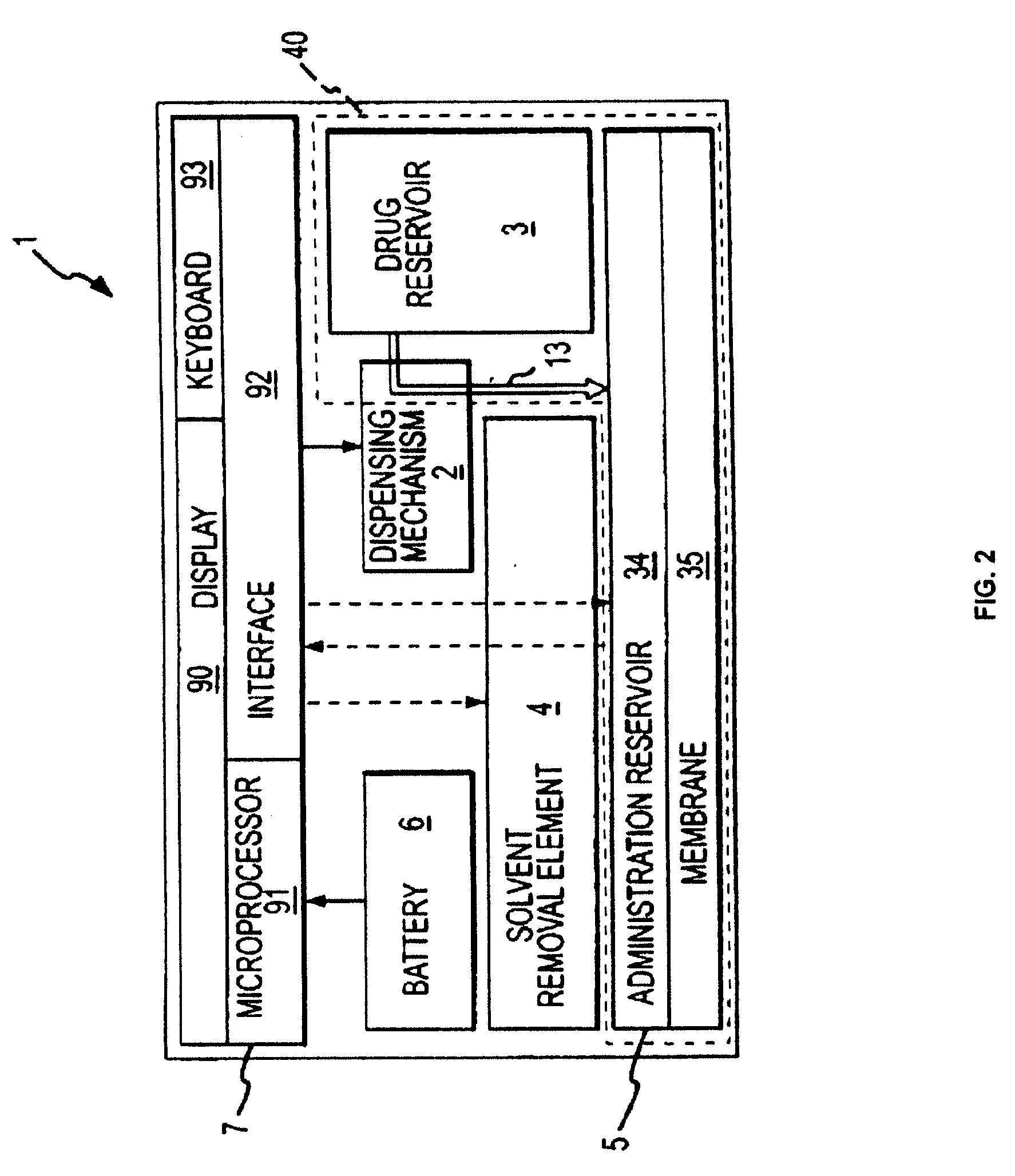
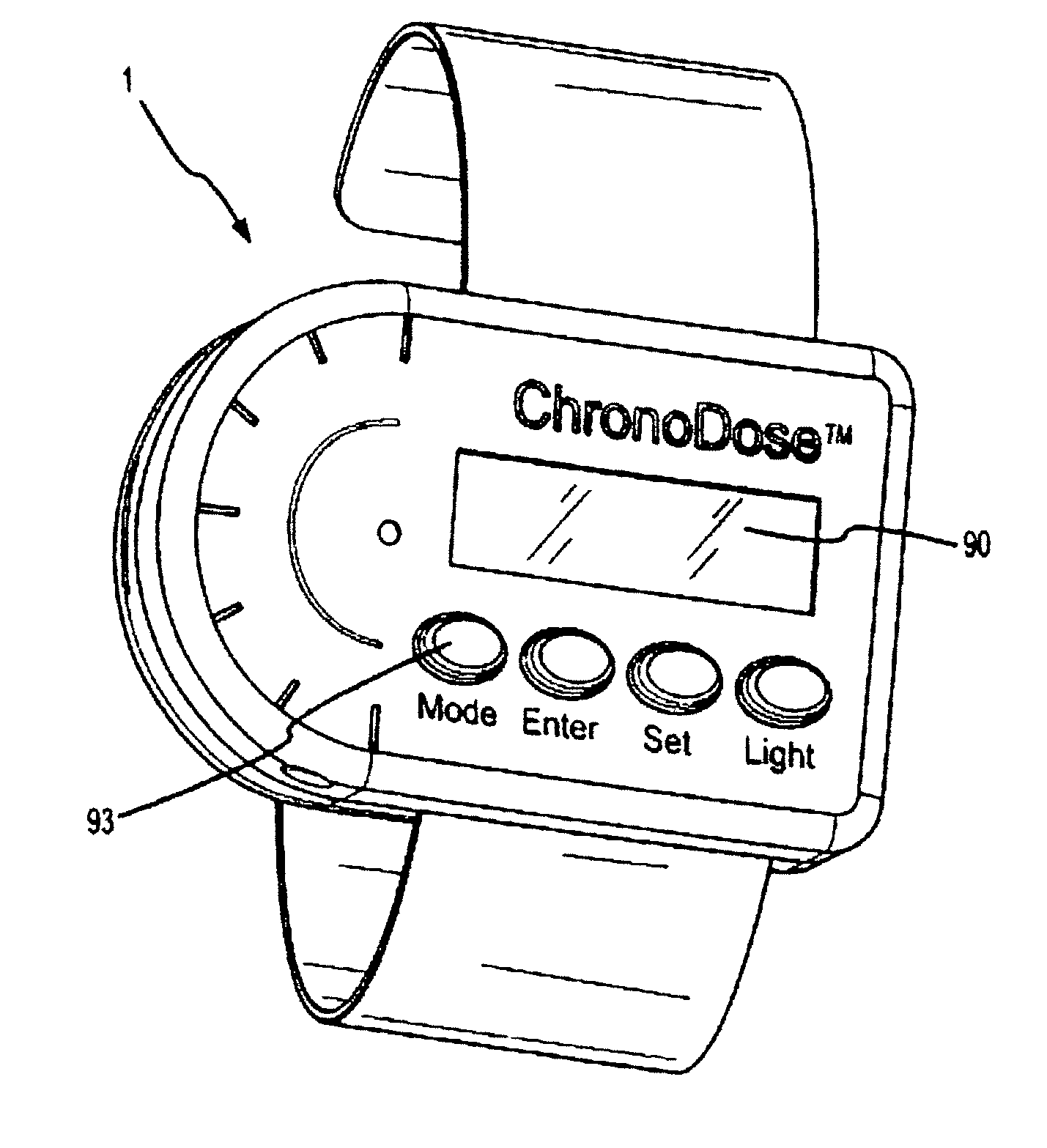
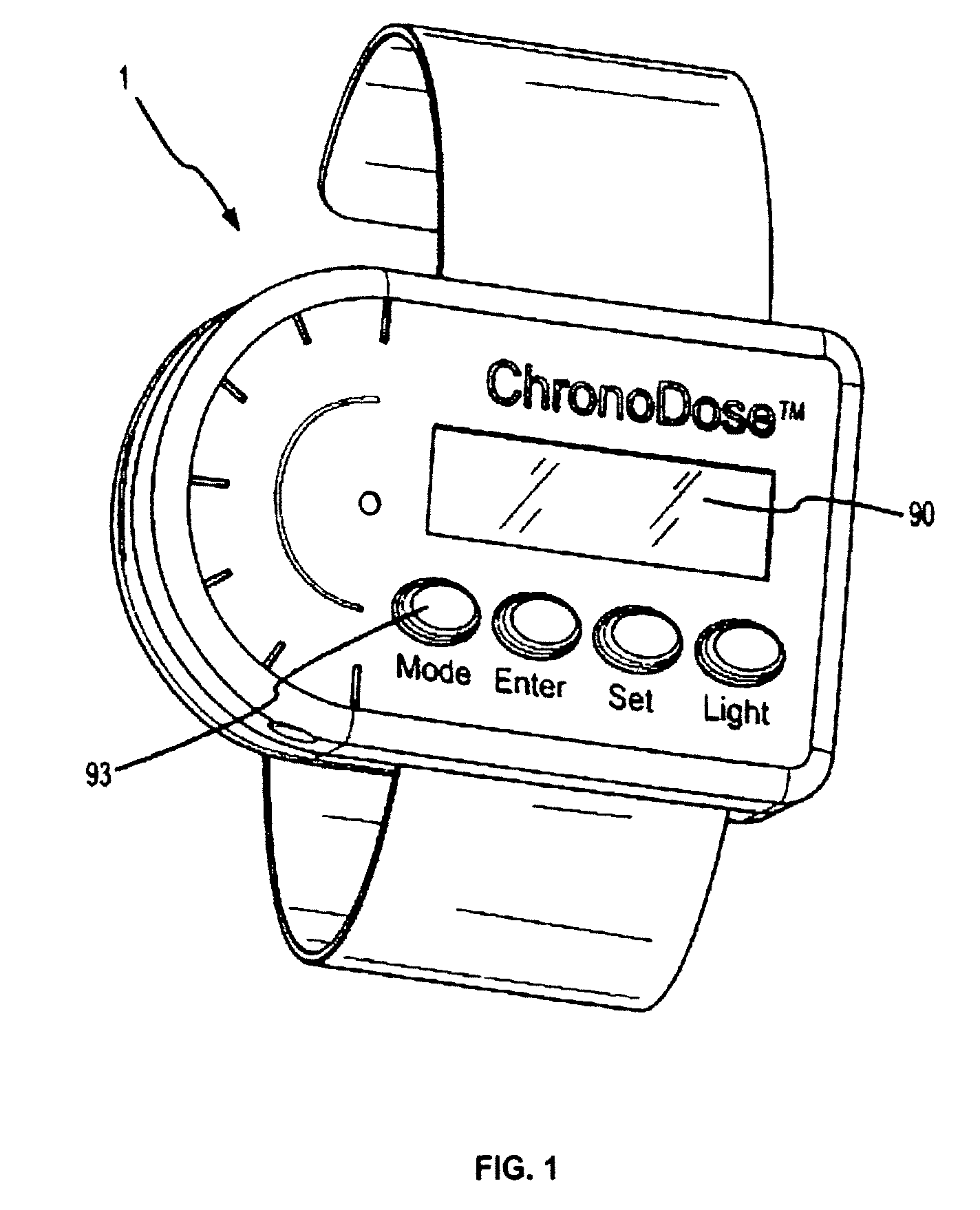
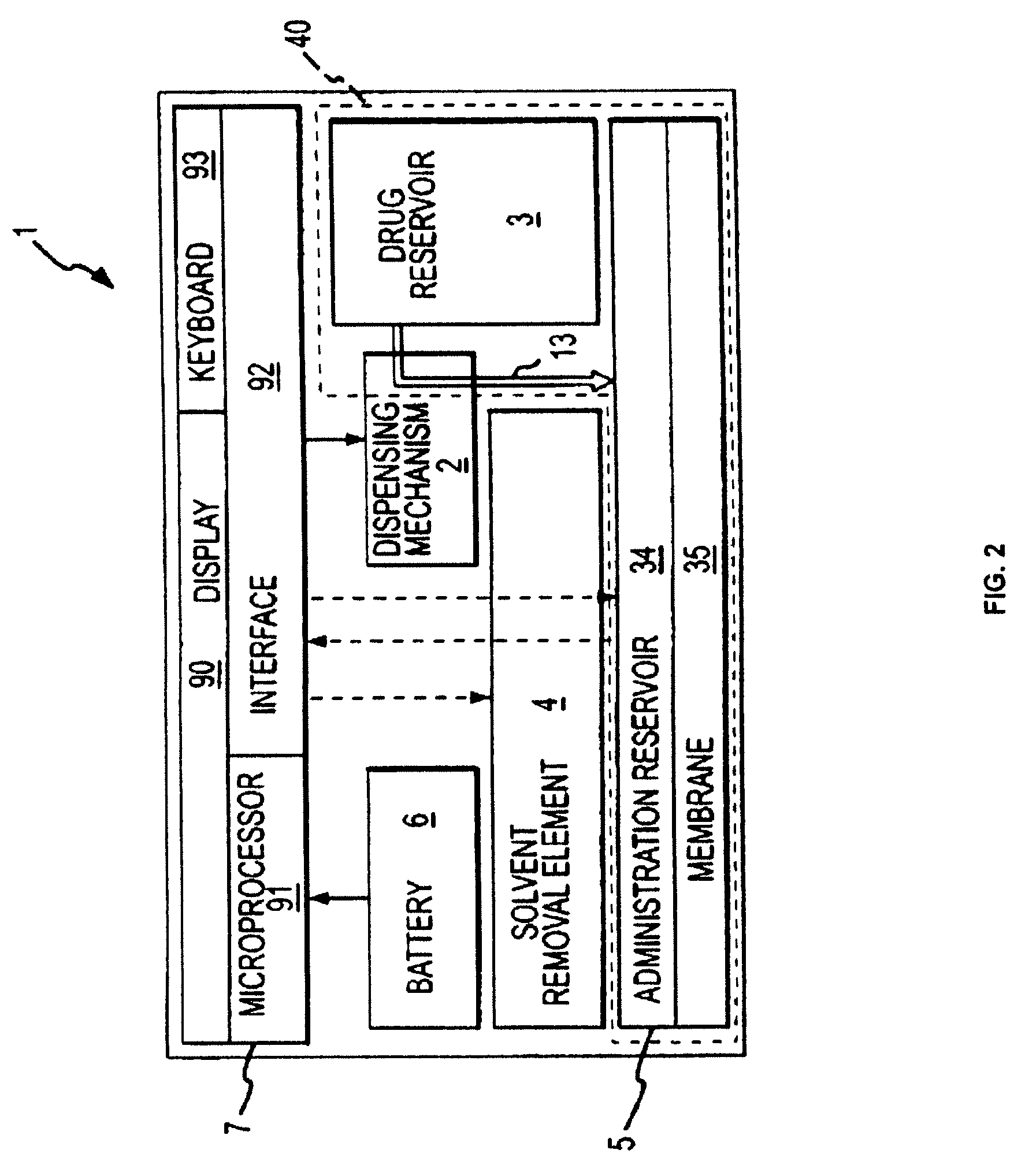
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
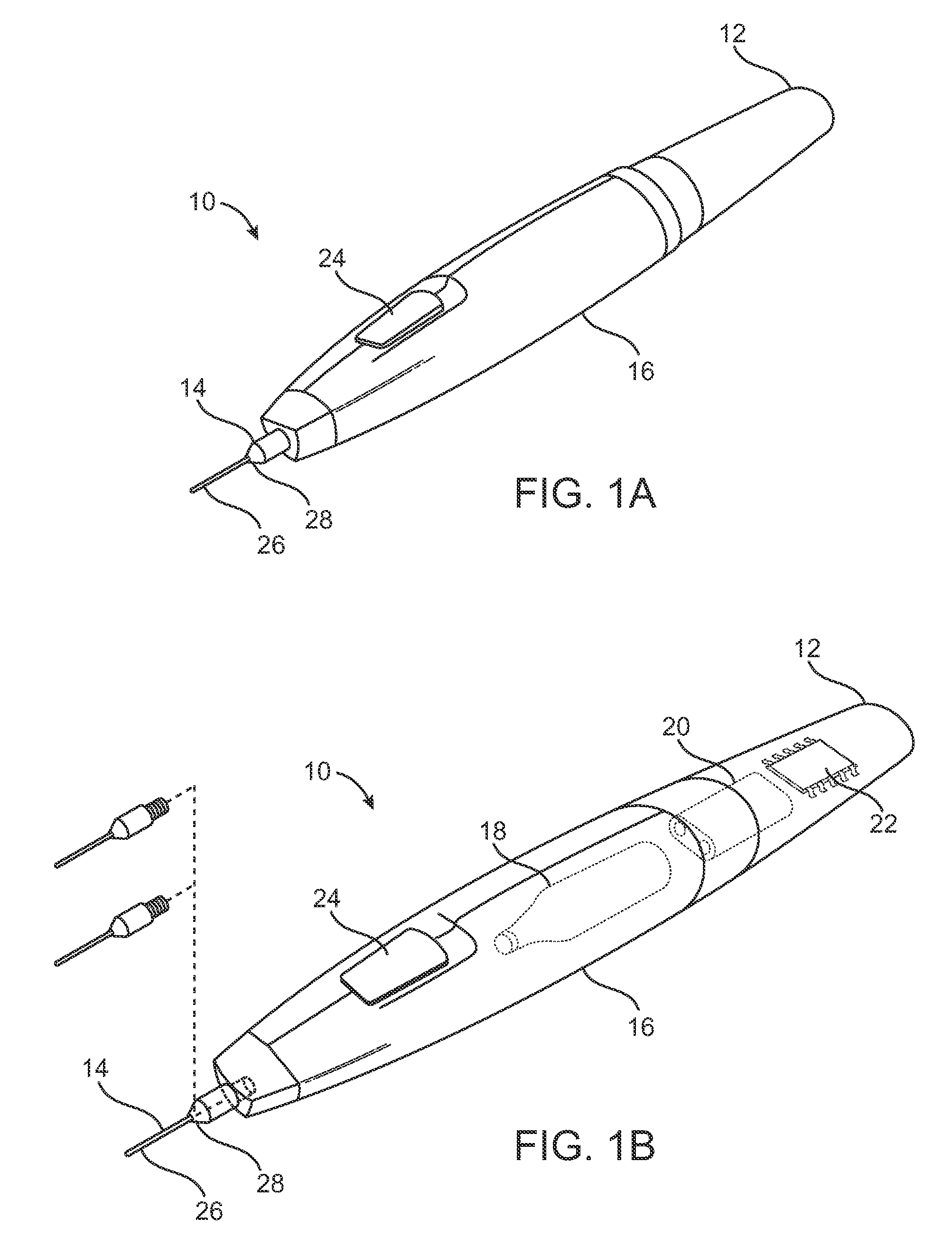
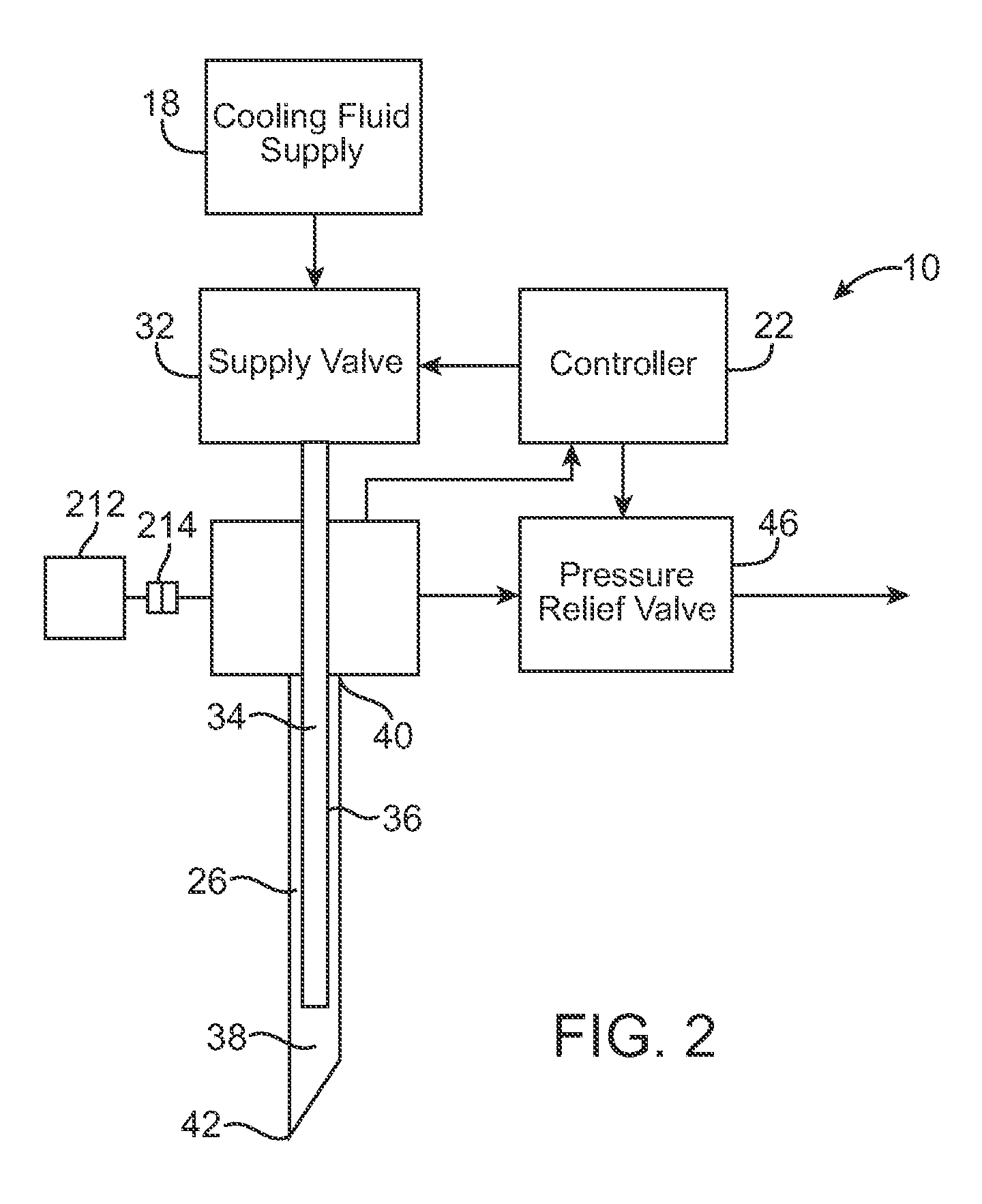
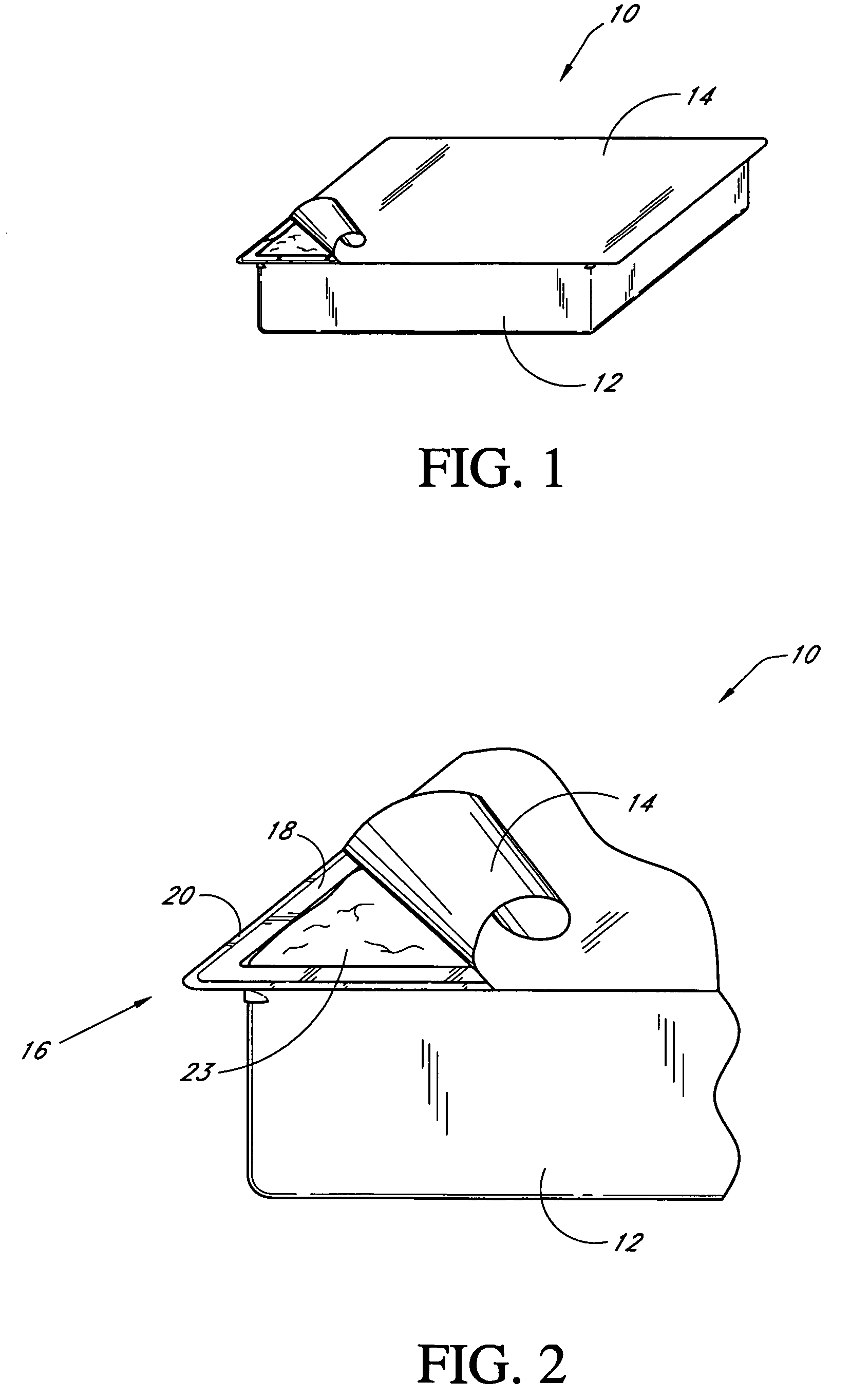
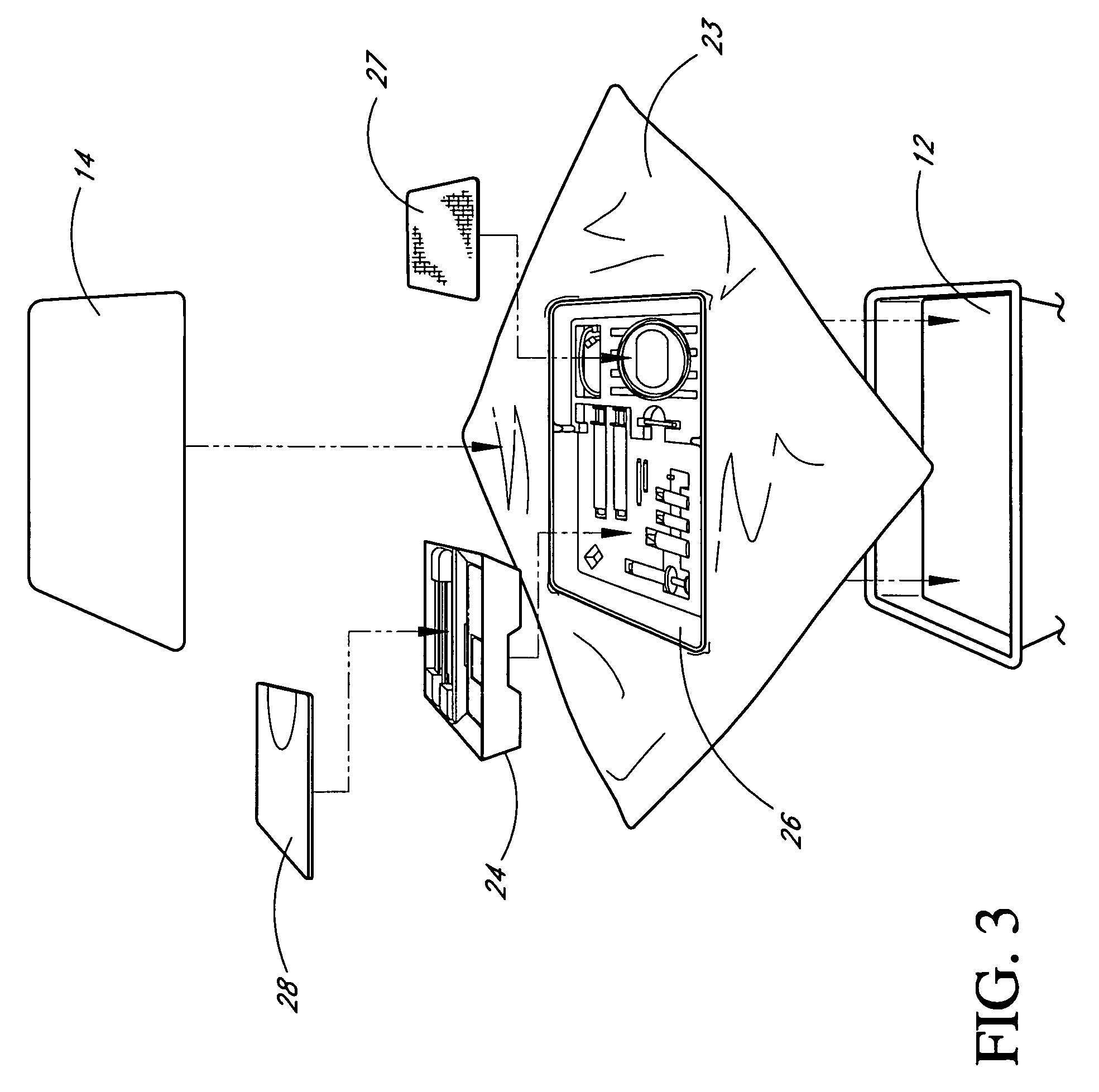
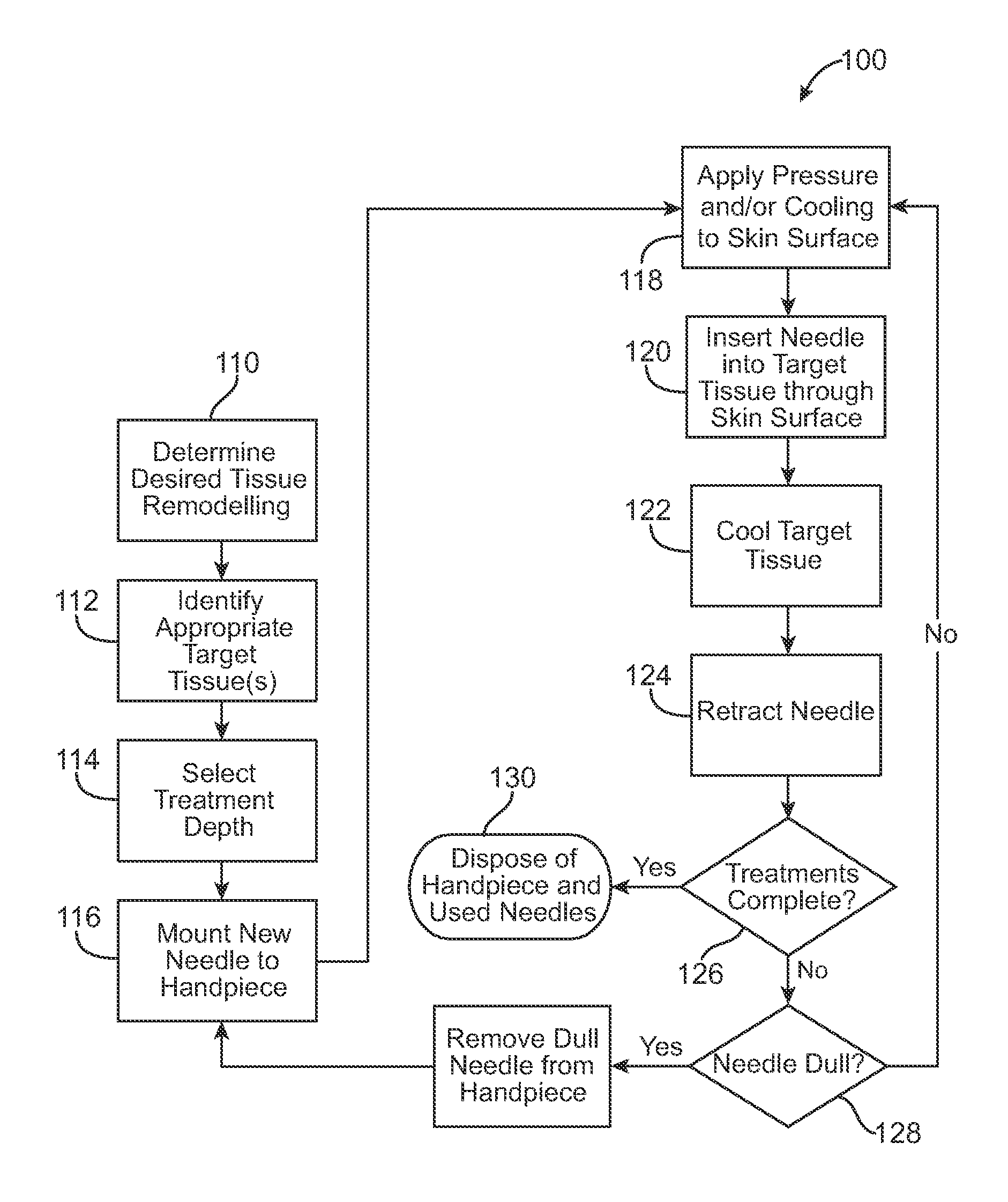
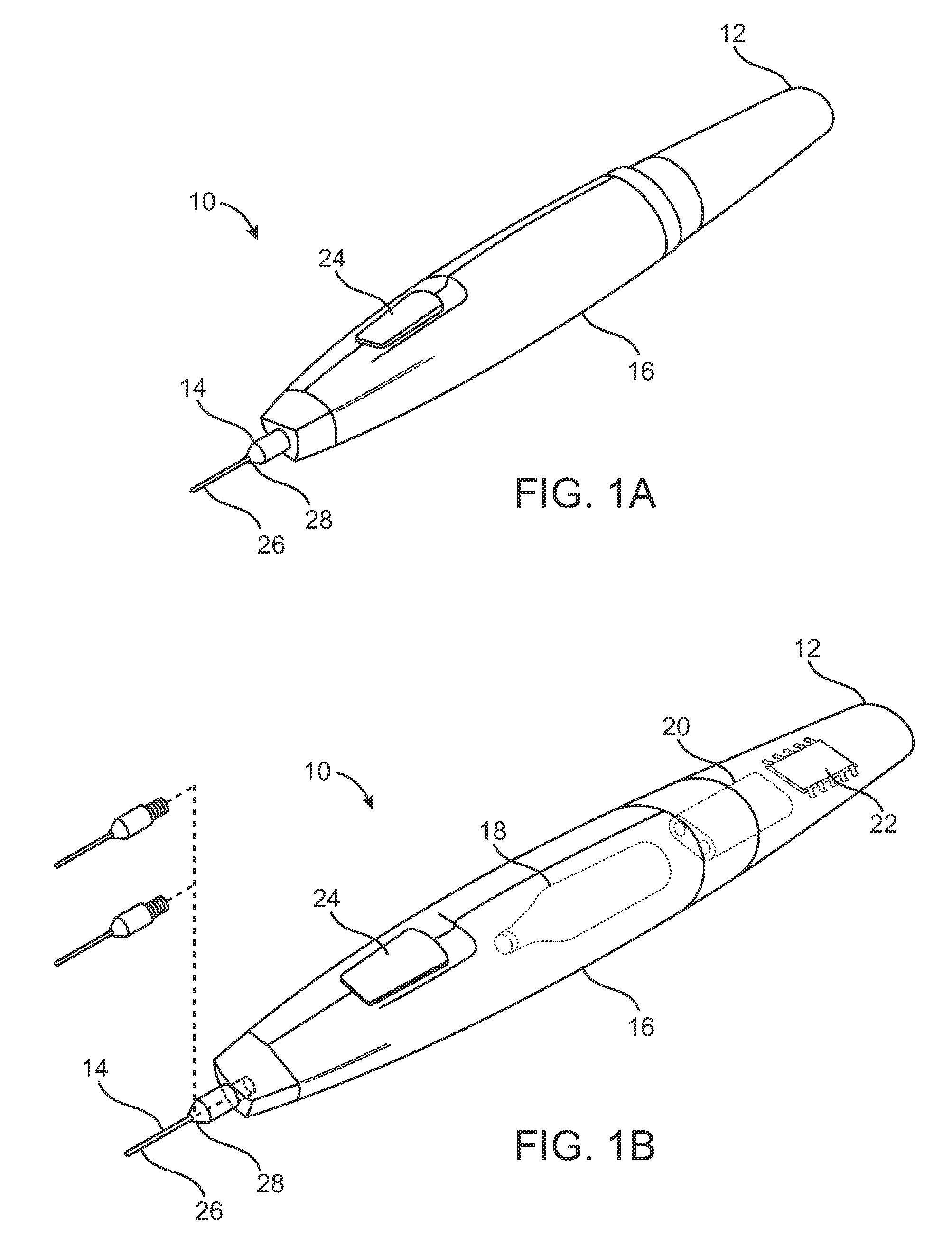
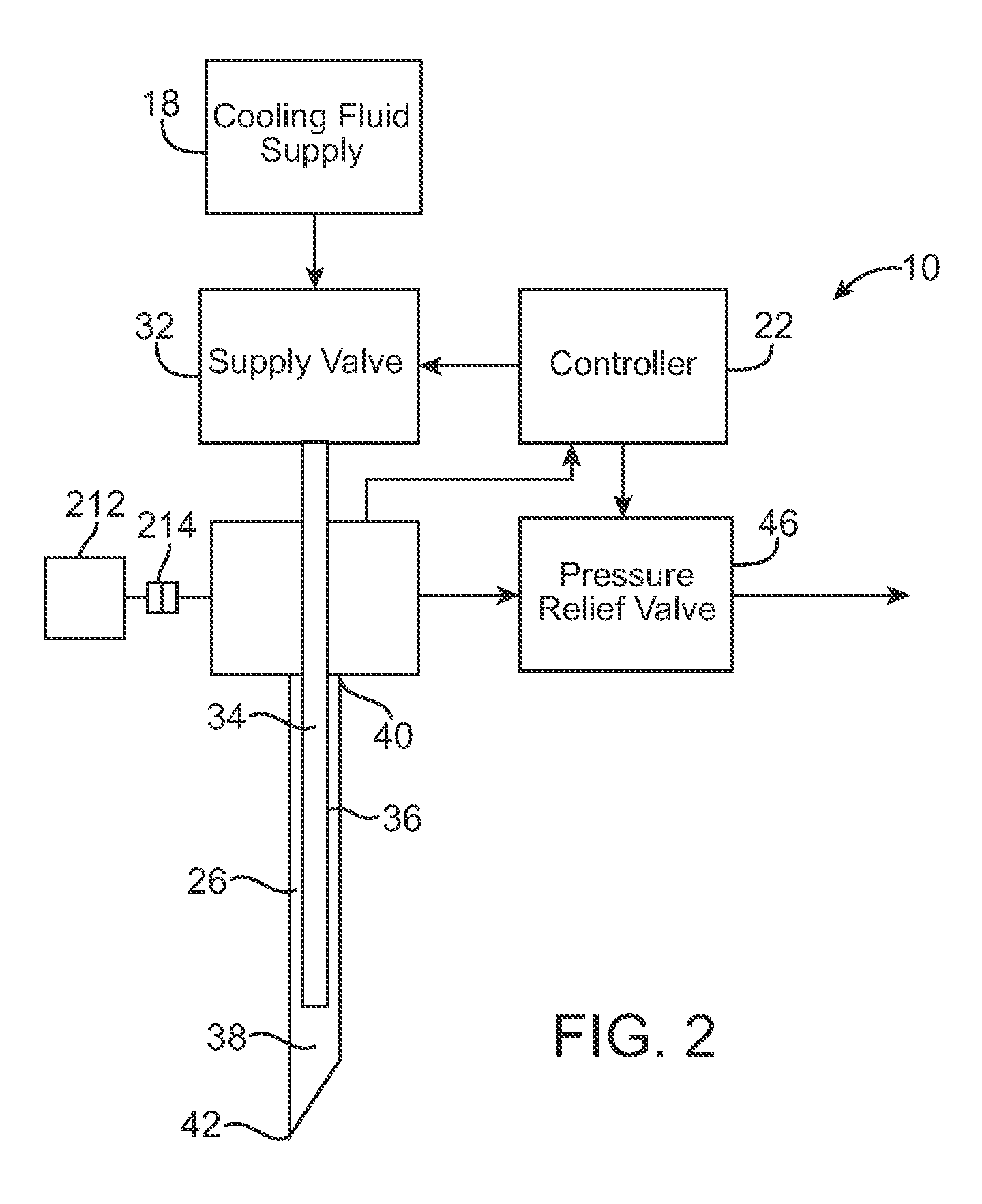
Pain management using cryogenic remodeling
ActiveUS20090248001A1Avoid ablationInduce apoptosis of the nerveSurgical instruments for coolingTherapeutic coolingMuscle contractionPain management
Medical devices, systems, and methods for pain management and other applications may apply cooling with at least one probe inserted through an exposed skin surface of skin. The cooling may remodel one or more target tissues so as to effect a desired change in composition of the target tissue and / or a change in its behavior, often to interfere with transmission of pain signals along sensory nerves. Alternative embodiments may interfere with the function of motor nerves, the function of contractile muscles, and / or some other tissue included in the contractile function chain so as to inhibit muscle contraction and thereby alleviate associated pain. In some embodiments, other sources of pain such as components of the spine (optionally including herniated disks) may be treated.
Owner:PACIRA CRYOTECH INC
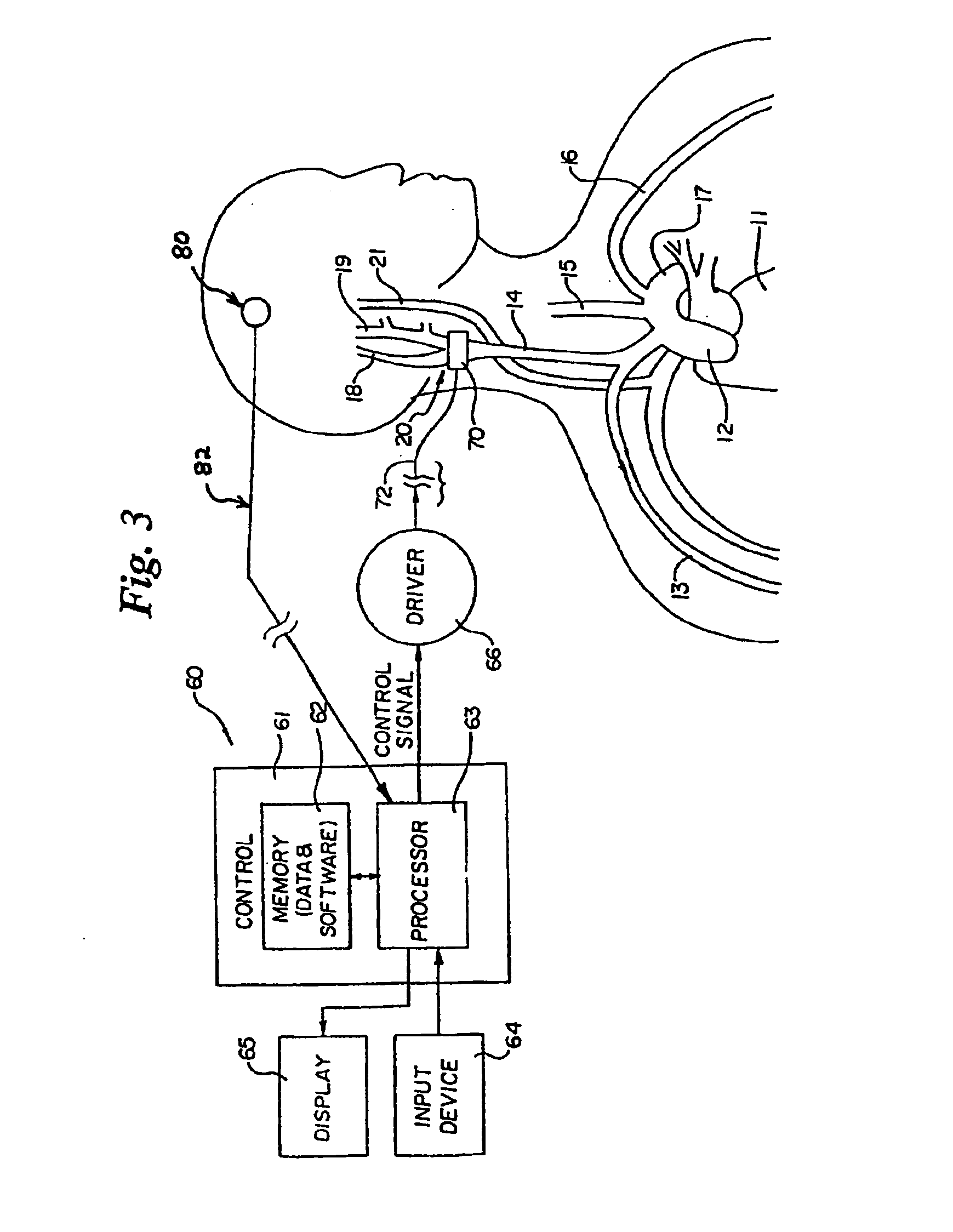
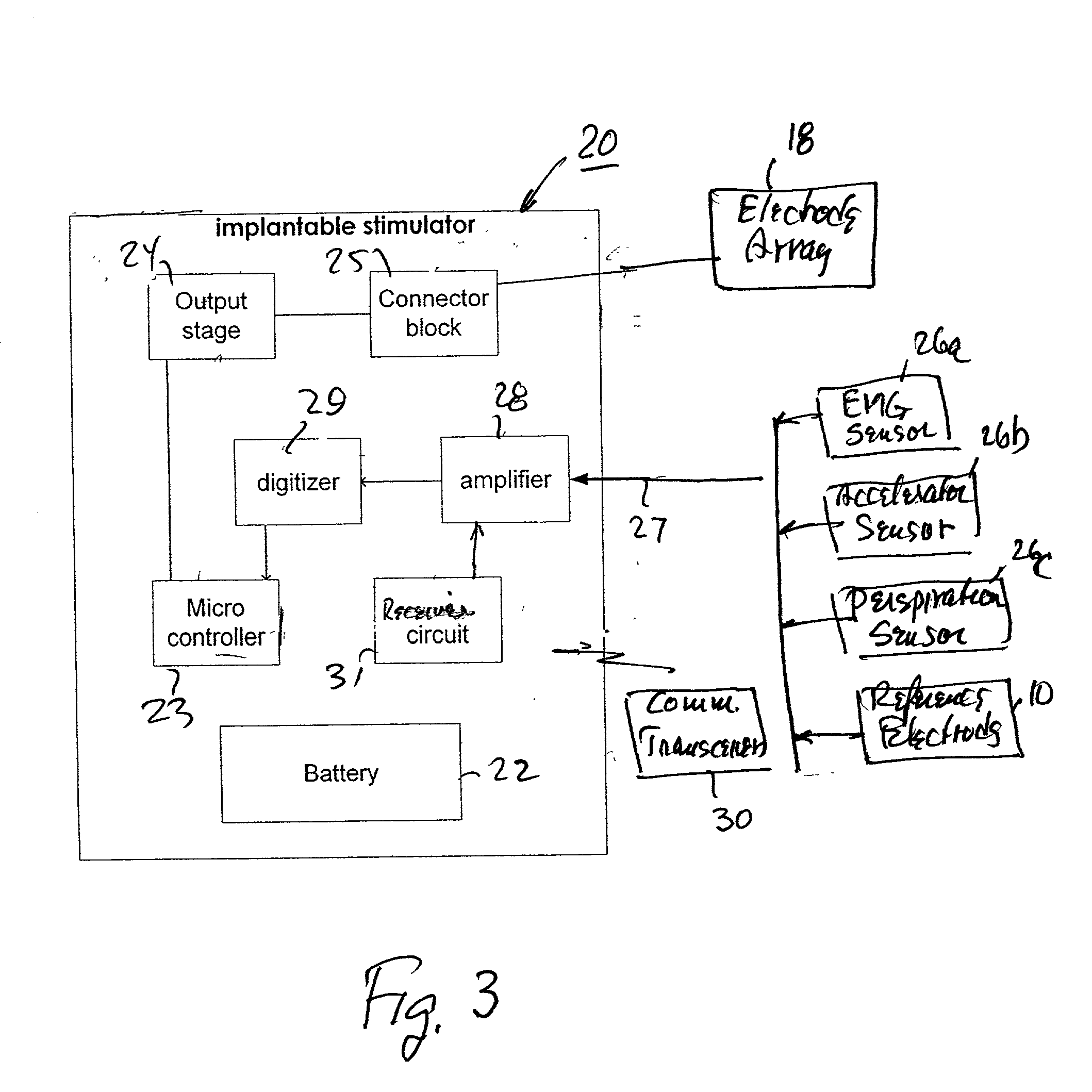
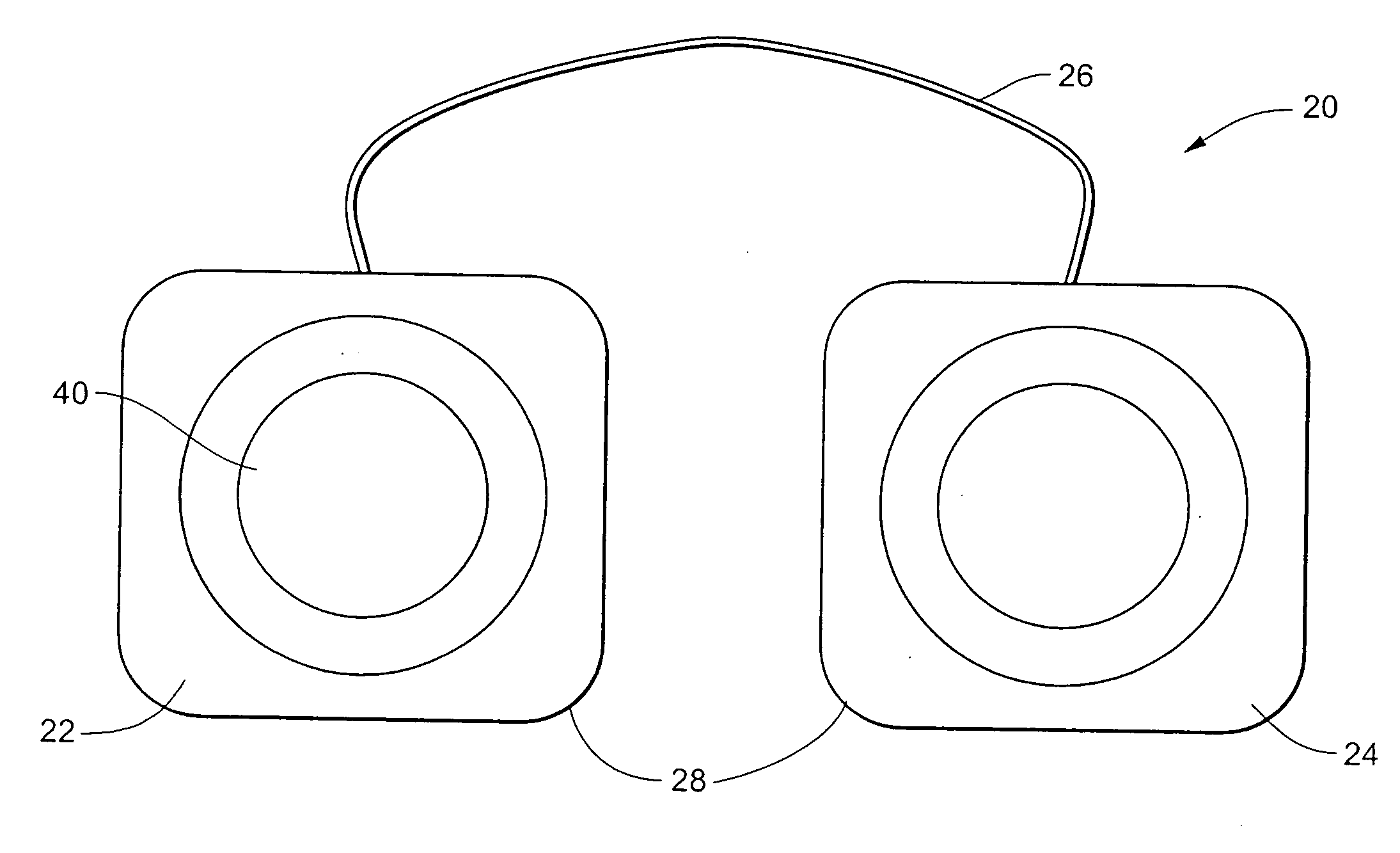
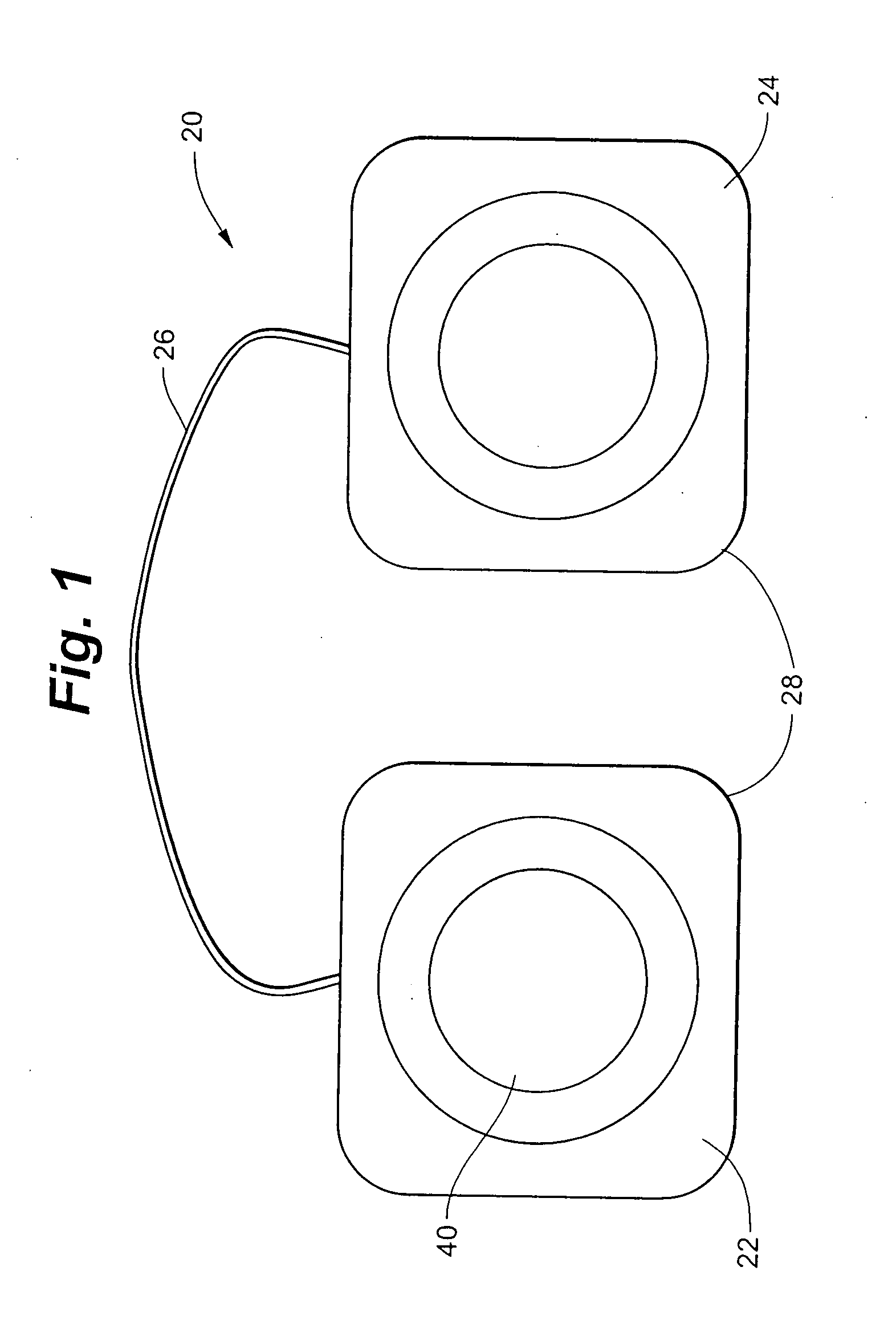
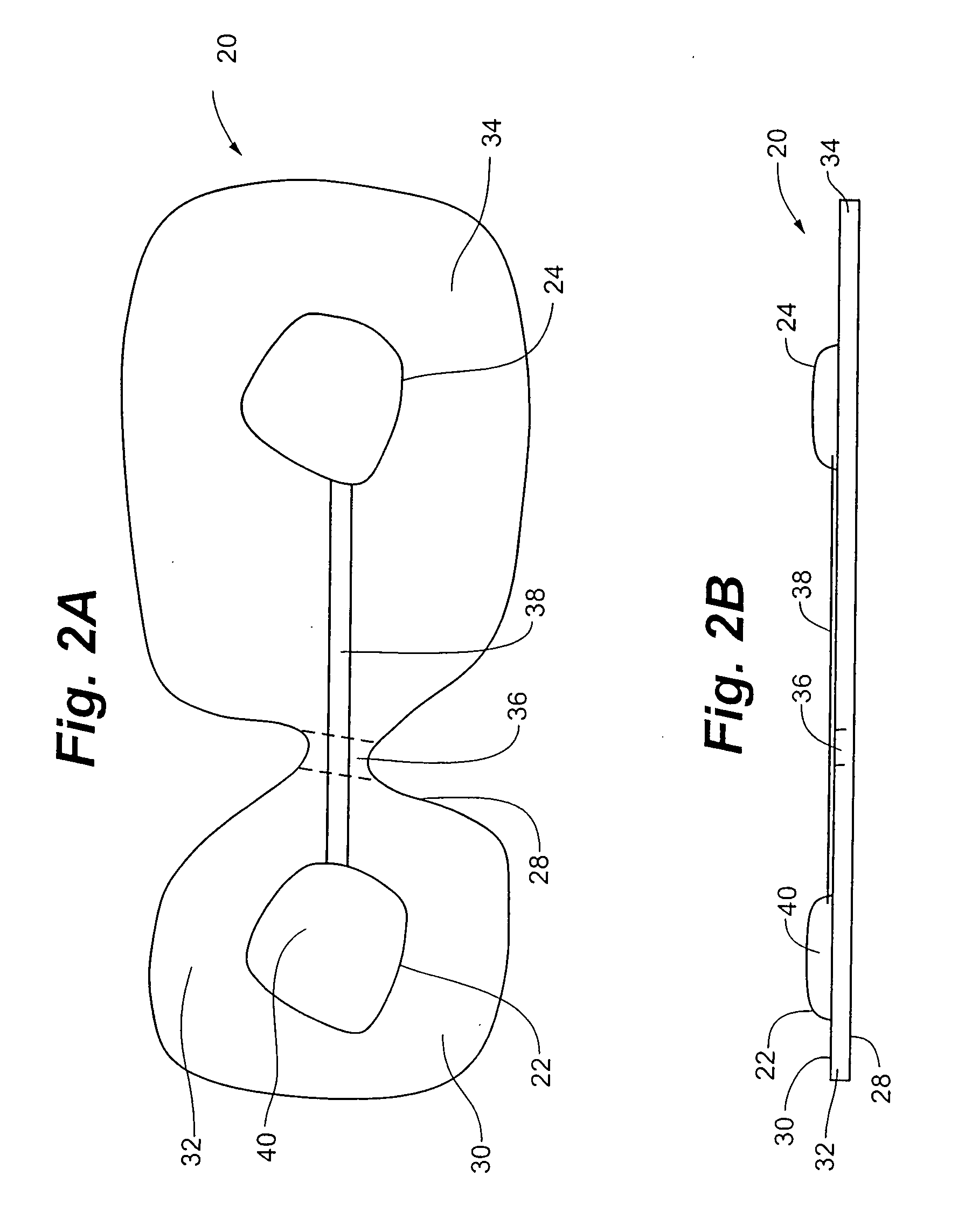
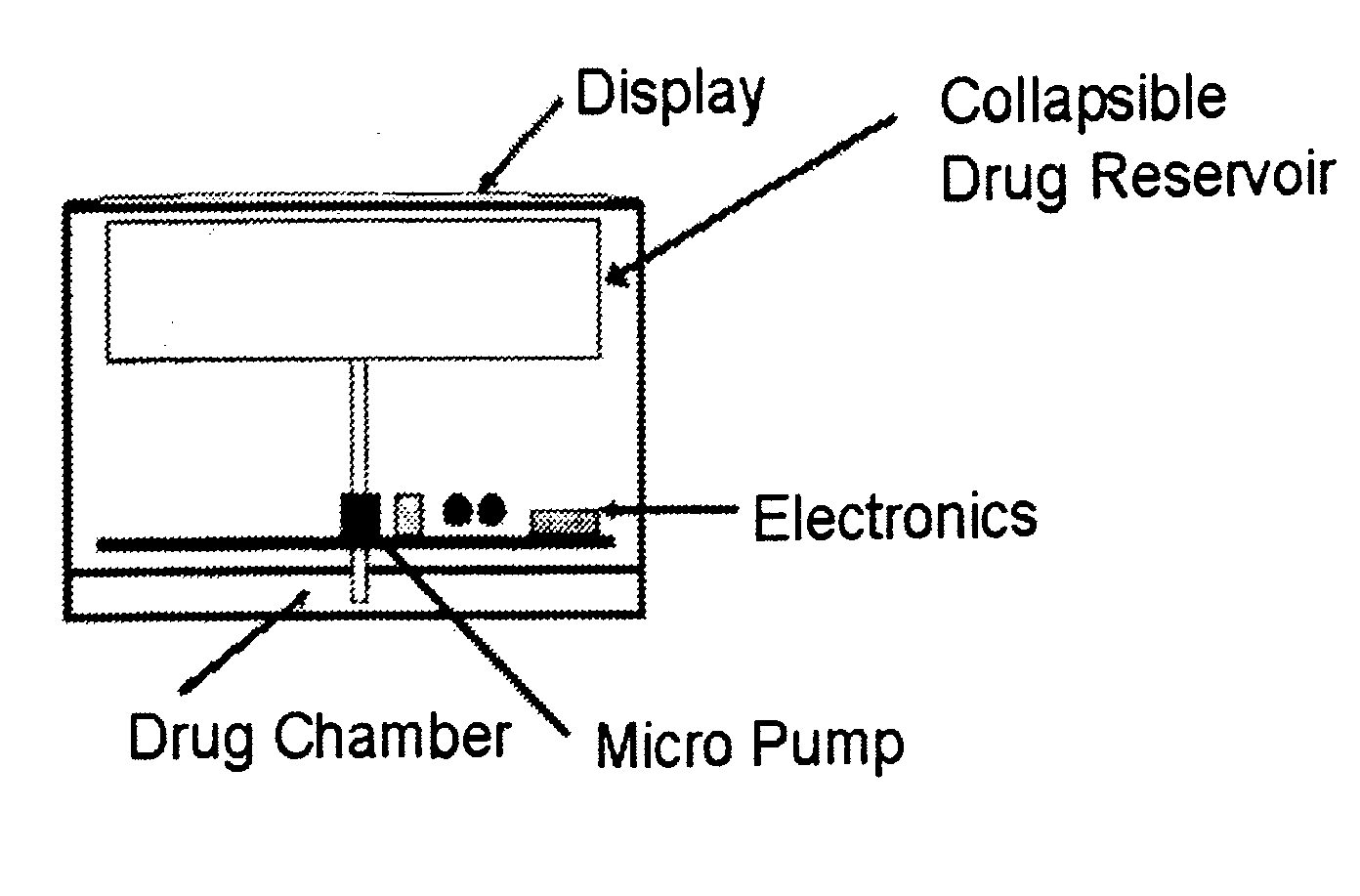
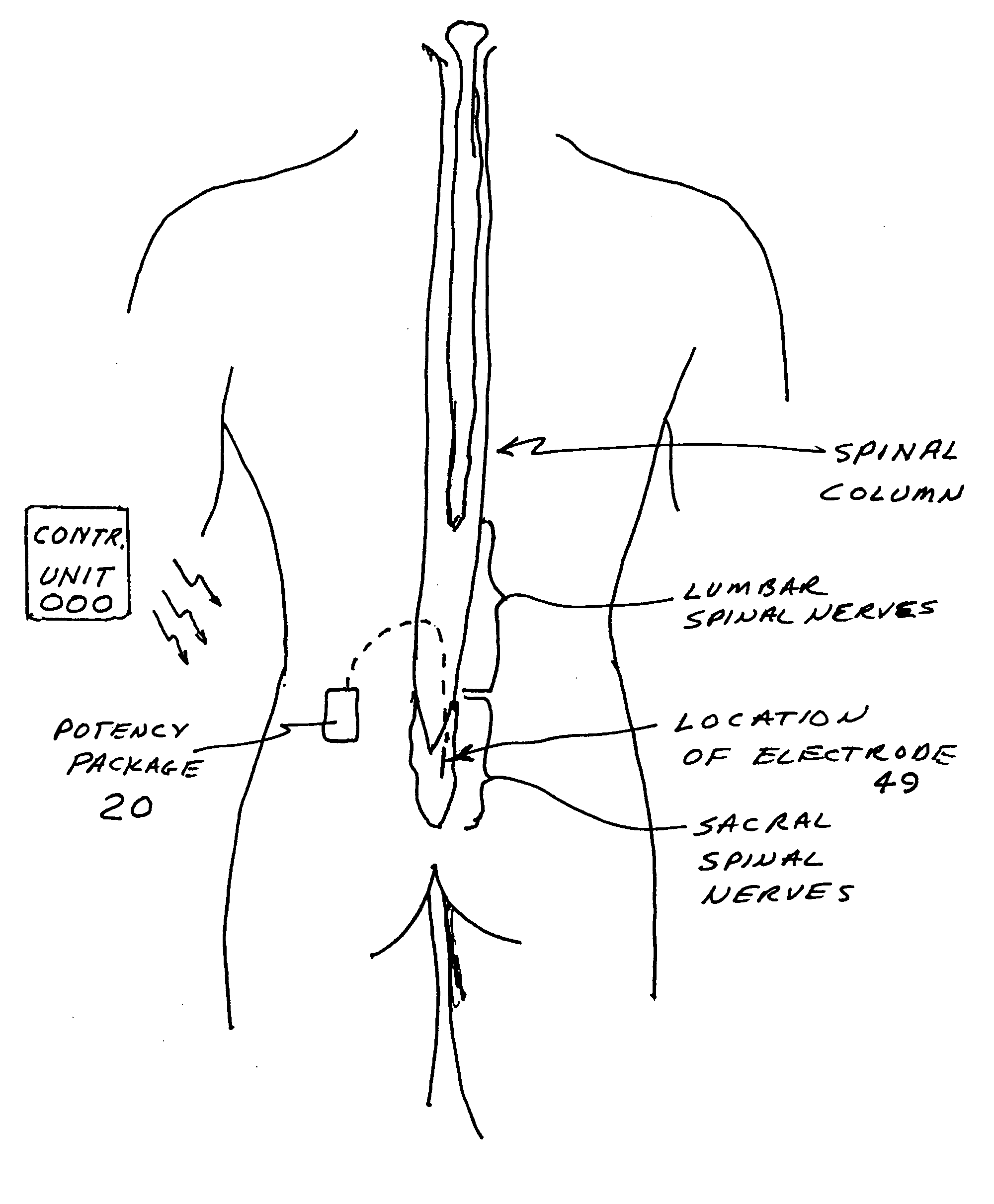
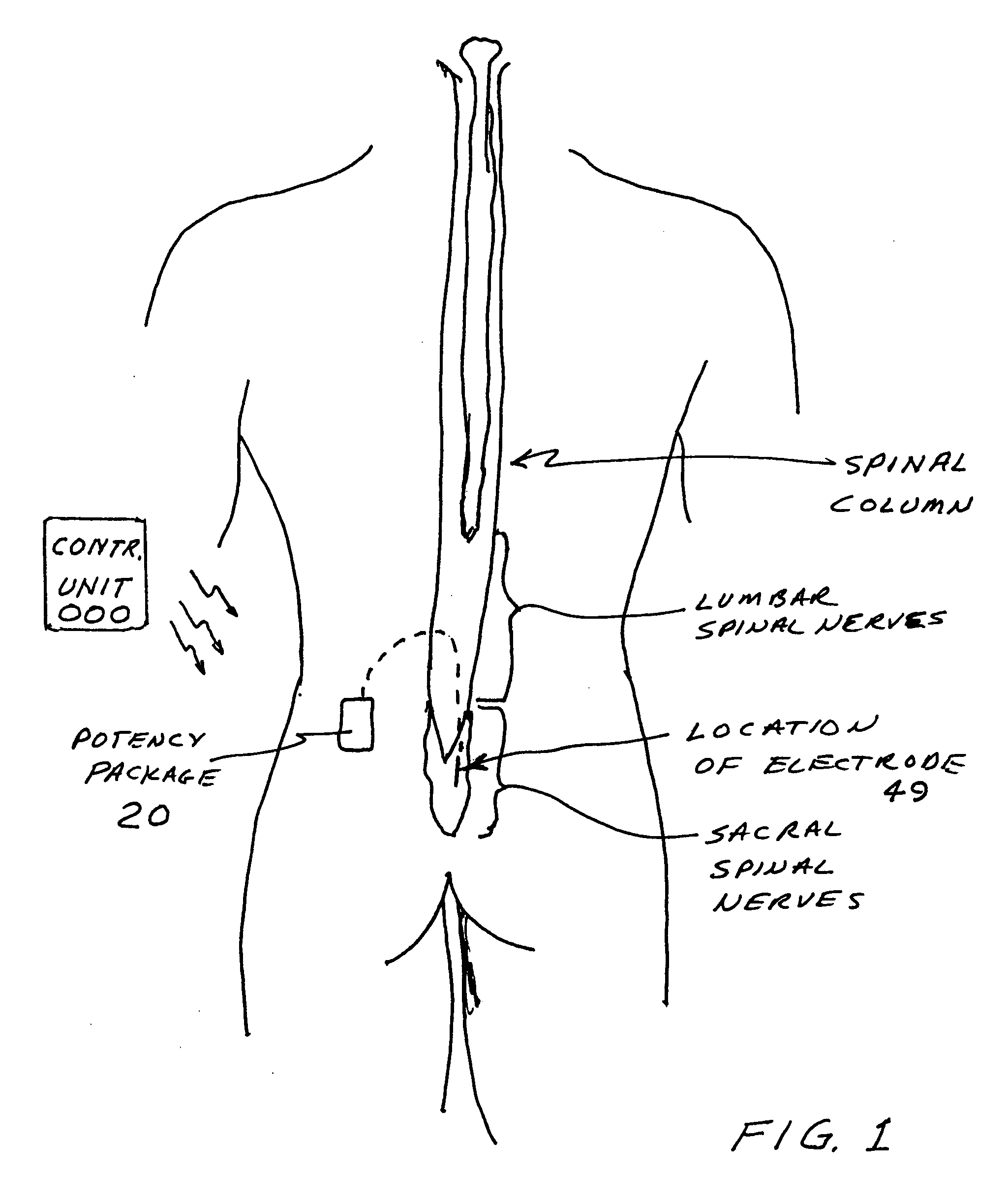
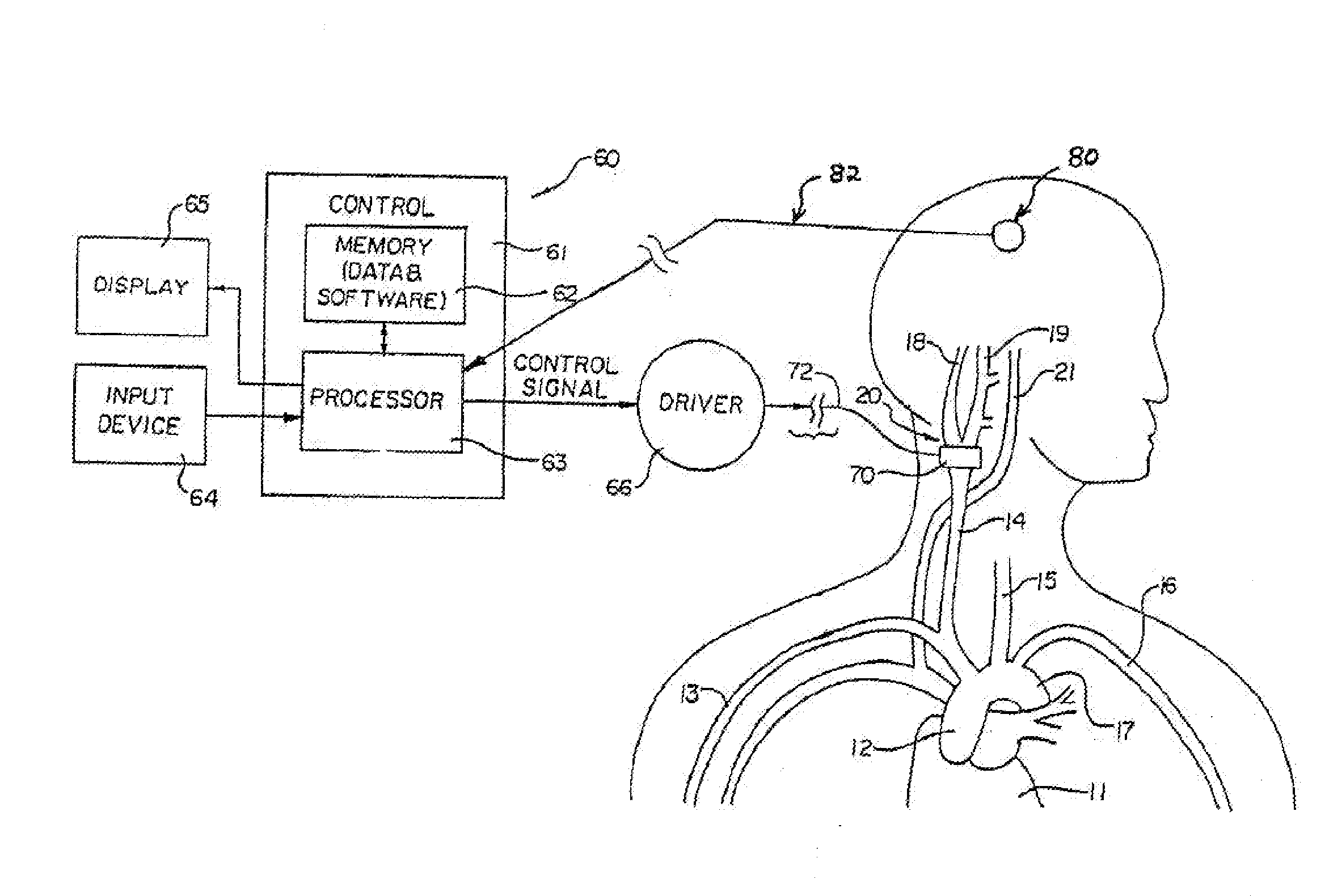
Implantable device for pain control and other medical treatments
InactiveUS20050222628A1Stimulates arousalEscalationElectrotherapyArtificial respirationControl signalImplanted device
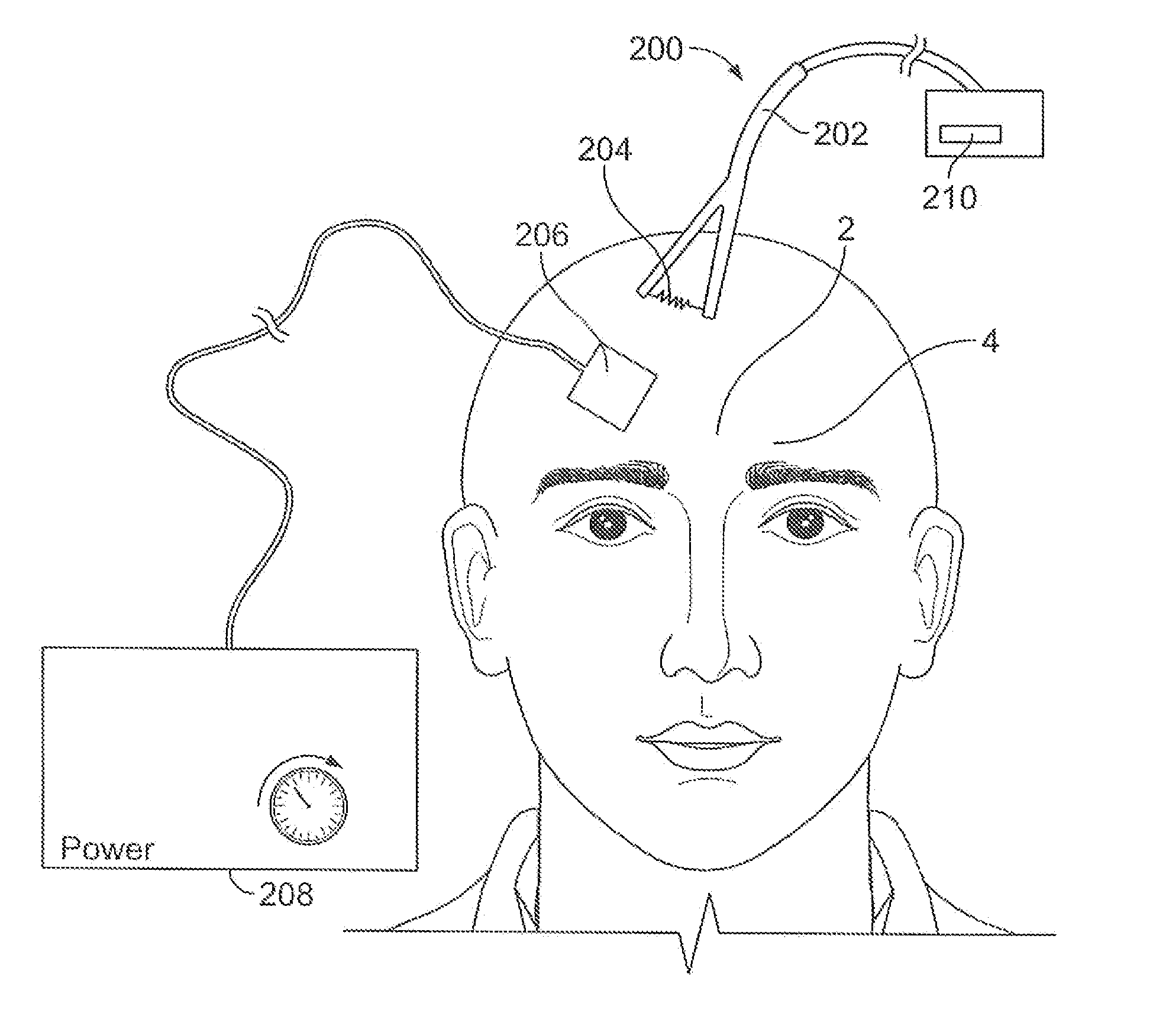
A device for pain control or other medical treatments of a patient. The device includes an implantable unit for implantation under the skin of a patient. The implantable unit includes a drug chamber containing a fluid drug, a tube for delivering the fluid drug to a location in the patient's body, an electronic pump for pumping the drug through the tube to the location, a programmable processor for controlling the pump, and an electronic receiver for receiving control signals from outside the patient's body and delivering the control signals to the processor. The device also includes a controller located outside the patient's body for transmitting control signals to said receiver. In preferred embodiments the implantable device also comprises an electrical pulse generator and at least one electrode implanted at a location in said patient's body and connected to said pulse generator wherein said processor is programmed to control said pulse generator to deliver electrical pulsed to said electrode. In a preferred embodiment for the control of pain, the implantable chamber contains a refillable pain-relieving drug that is placed inside the chamber.
Owner:KRAKOUSKY ALEXANDER A
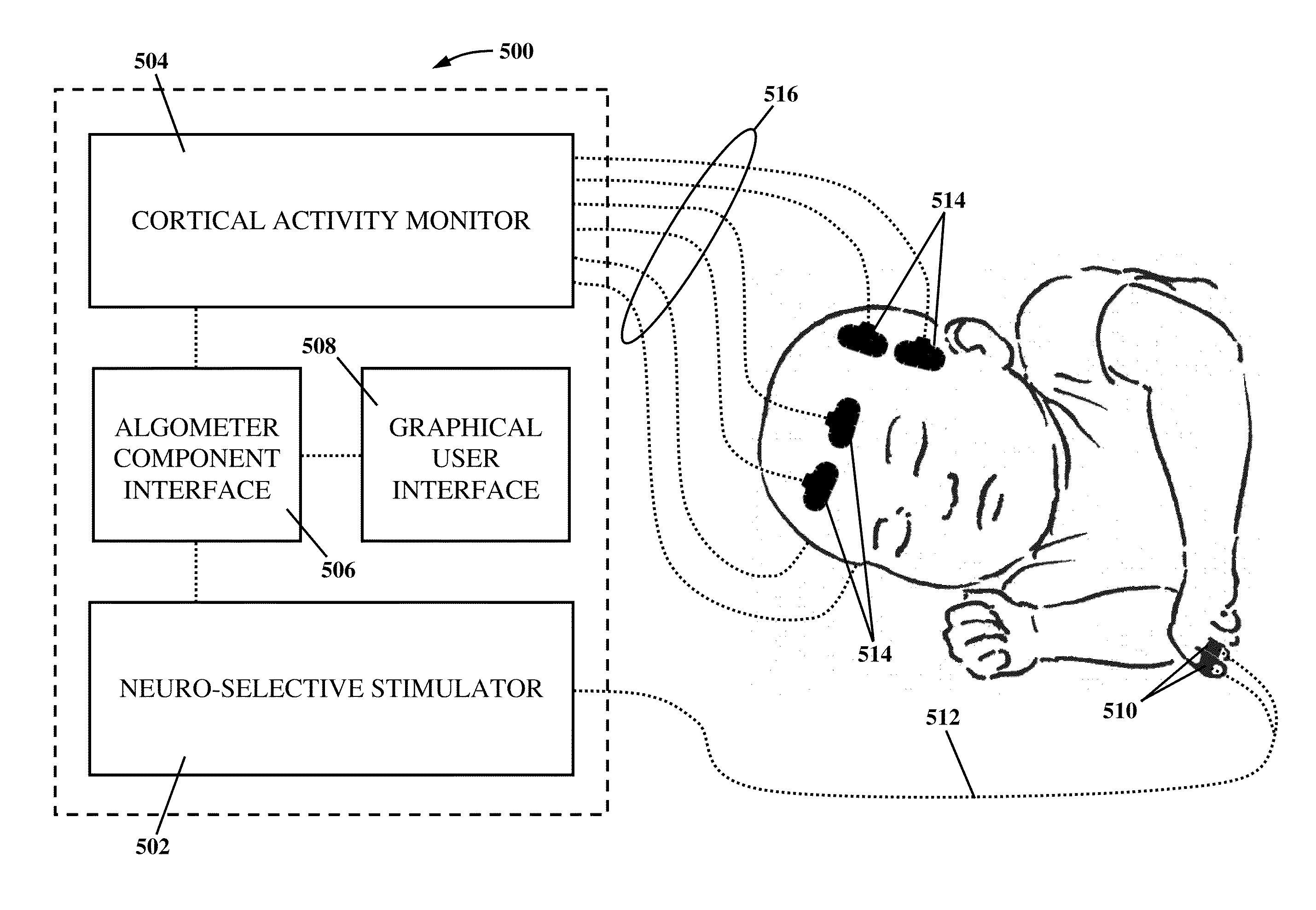
Apparatus and method for human algometry
An apparatus and method for performing human algometry are disclosed. They include a stimulator configured to apply electrical stimulation of variable intensity to an area of a patient's body, a monitoring device configured to measure a. level of cortical activity in one or more regions of the patient's brain, and a microprocessor connected to the stimulator and the monitoring device that is configured to correlate the intensity of the electrical stimulation with the level of activity in the one or more regions of the patient's brain and to determine at least one of a measurement of pain intensity, a measurement of a sensory detection threshold (SDT), a measurement of a drug's analgesic impact, an indication of an onset of tolerance to a drug, an indication of an onset of analgesic-induced hyperalgesia, an indication of conditions of allodynia, a measurement of dose-response characteristics of pain management drugs, and a characterization of a pain condition.
Owner:CHILDRENS NAT MEDICAL CENT
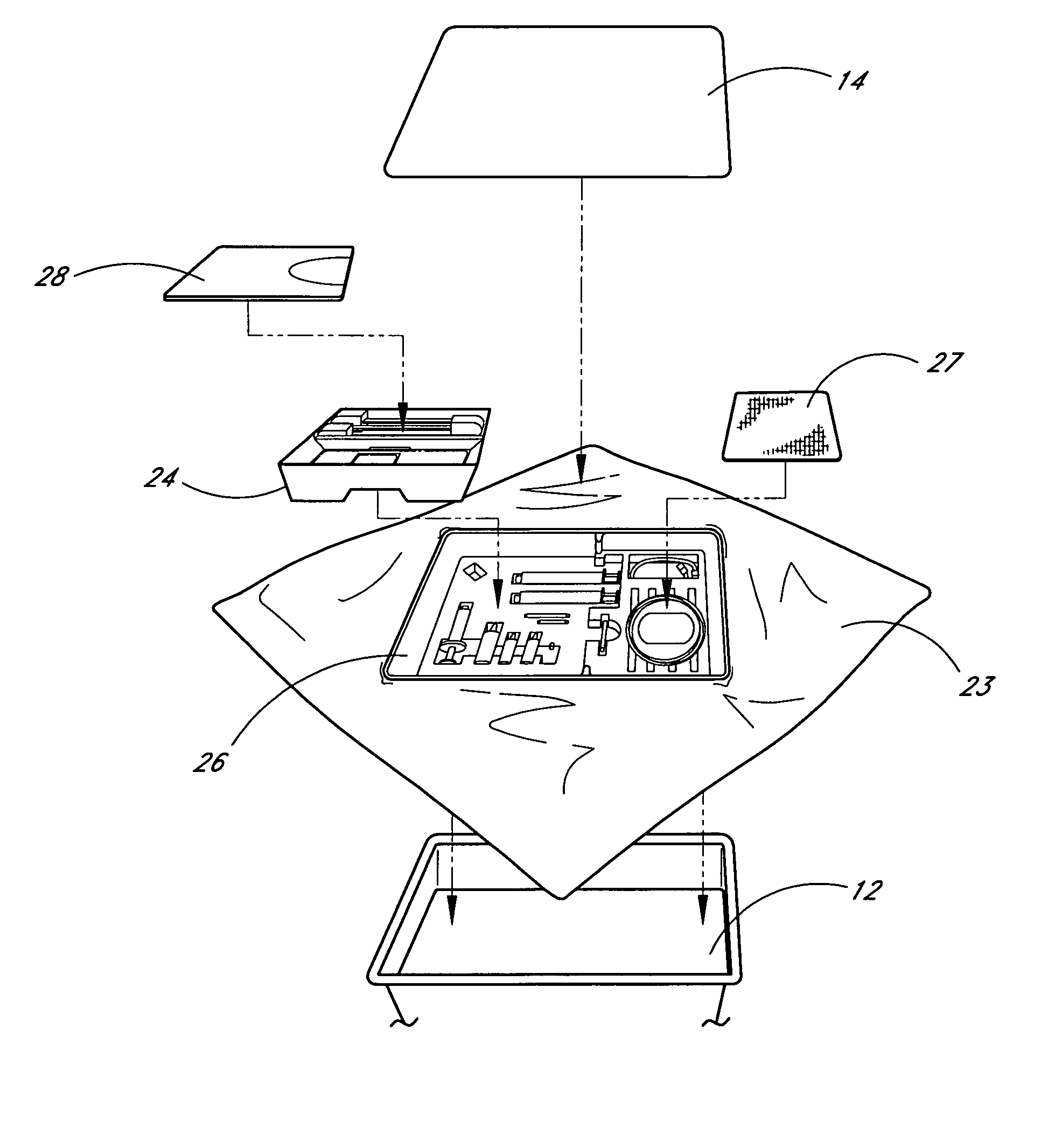
Pain management kit for administration of medication
InactiveUS7100771B2Easy to storeEasy to transportSurgical furnitureDispensing apparatusNerve block procedureSTERILE FIELD
A pain management kit for the administration of medication and a related method of use is disclosed. The pain management kit includes the primary medical supplies for performing a continuous nerve block procedure, preferably in a single, sterile container. Specifically, the kit may contain medical supplies to create a sterile field, perform a local anesthetic procedure and to perform a continuous nerve block procedure. Preferably, the contents of the kit are arranged in the general order of their use.
Owner:AVENT INC
Pain management using cryogenic remodeling
ActiveUS8298216B2Avoid ablationInduce apoptosis of the nerveSurgical instruments for coolingTherapeutic coolingMuscle contractionSkin surface
Medical devices, systems, and methods for pain management and other applications may apply cooling with at least one probe inserted through an exposed skin surface of skin. The cooling may remodel one or more target tissues so as to effect a desired change in composition of the target tissue and / or a change in its behavior, often to interfere with transmission of pain signals along sensory nerves. Alternative embodiments may interfere with the function of motor nerves, the function of contractile muscles, and / or some other tissue included in the contractile function chain so as to inhibit muscle contraction and thereby alleviate associated pain. In some embodiments, other sources of pain such as components of the spine (optionally including herniated disks) may be treated.
Owner:PACIRA CRYOTECH INC
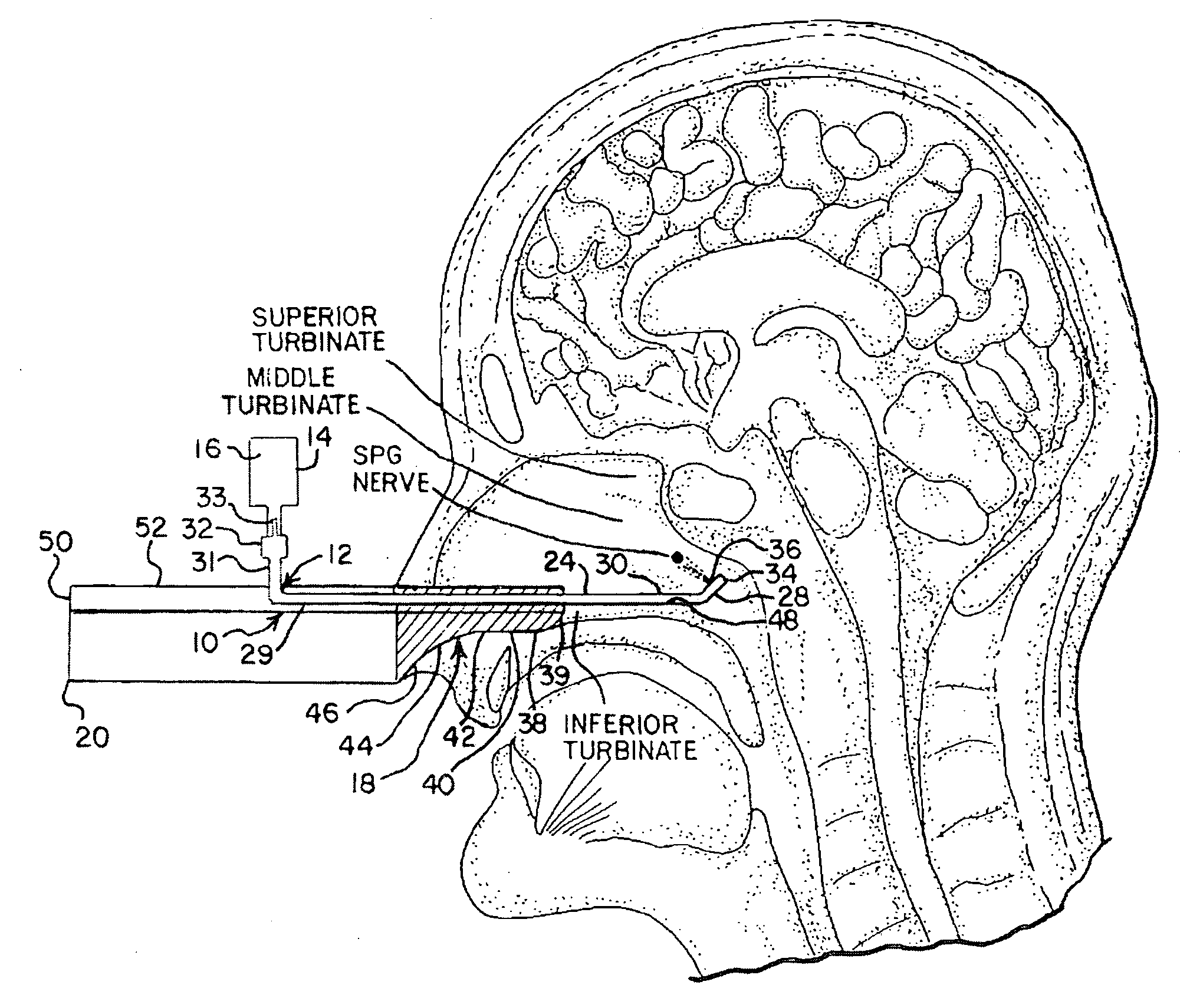
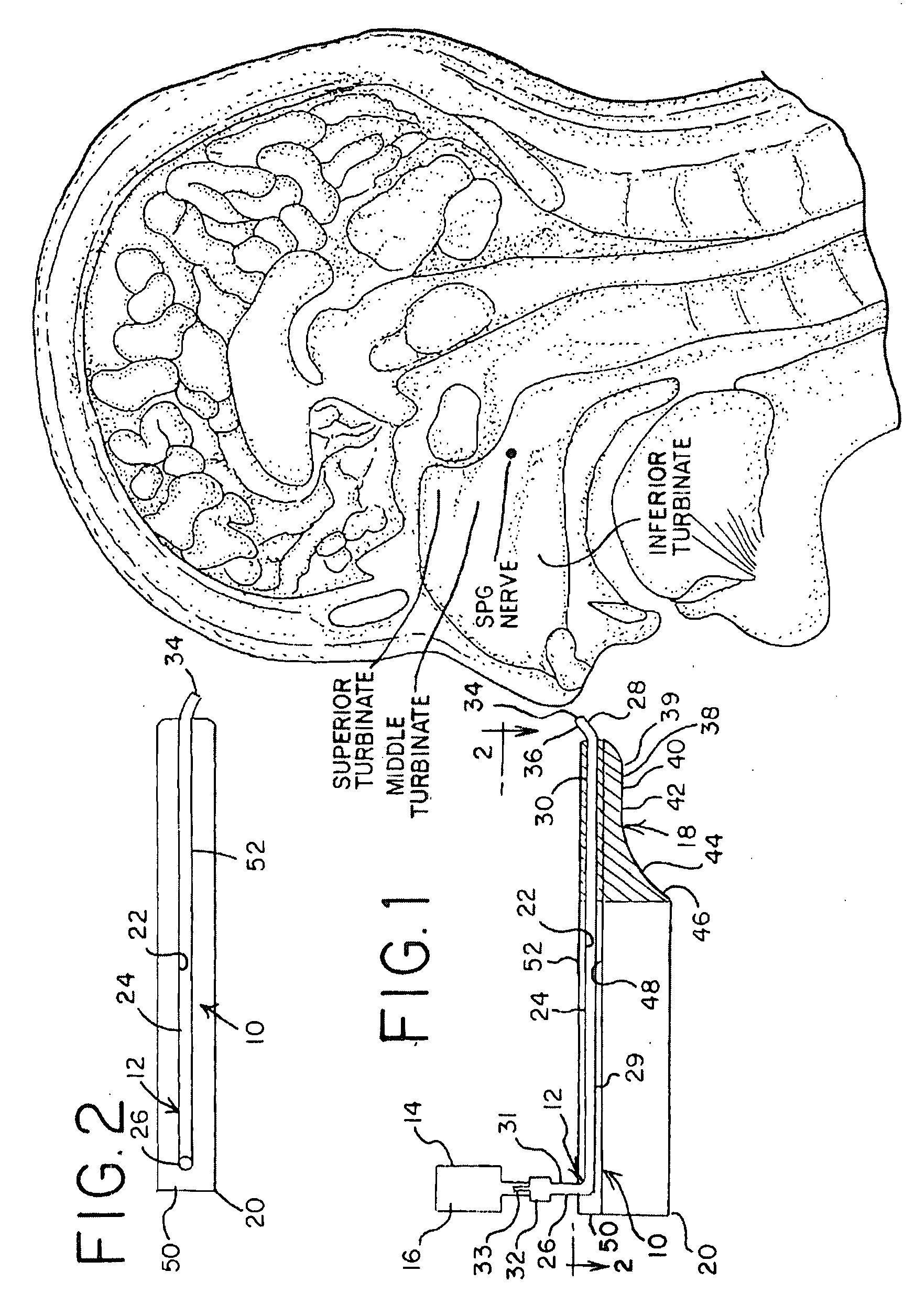
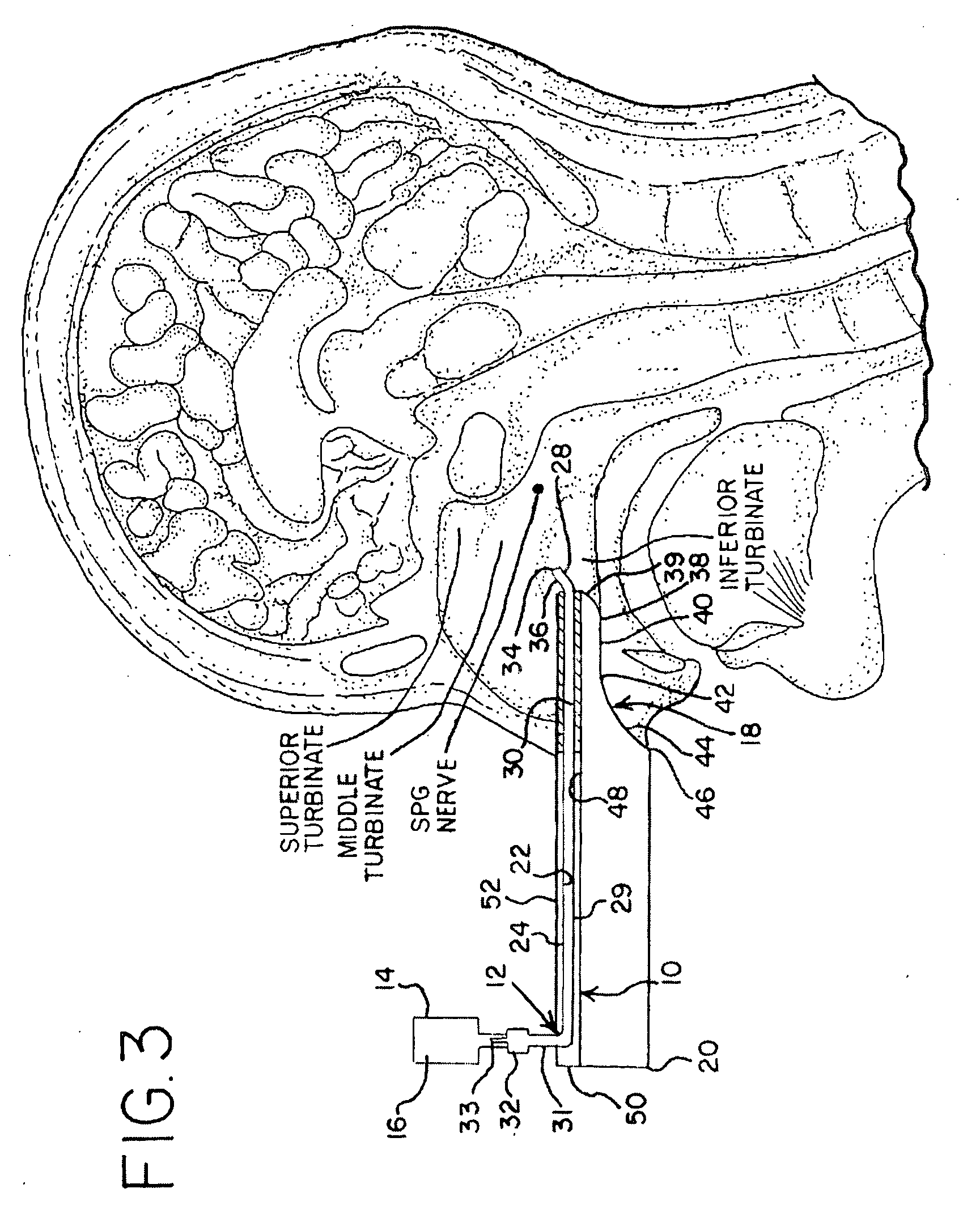
Apparatus and Method for Controlling Headaches
InactiveUS20100030187A1Effective and easy to useEffective pain controlMedical devicesMedical atomisersNostrilHeadaches
Owner:XIA TIAN
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS8252321B2Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocideDiseasePhytochemical
Owner:MORNINGSIDE VENTURE INVESTMENTS
Electrical stimulation device and method for therapeutic treatment and pain management
ActiveUS8958883B2Easily and tactilely differentiateAvoid accidental activationExternal electrodesArtificial respirationElectricityManaged pain
A disposable electrical stimulation device and method for providing therapeutic treatment and pain management in a convenient, compact configuration. Electrode size and shape and relative configuration can be varied according to an intended application and use, or a universal configuration can be provided for use on almost any area of the body. The common structure of communicatively coupled dual electrodes including control circuitry and a power source accommodates a range of different sizes, configurations, stimulation treatment intensities, and other physical and electrical characteristics that can be pre-customized and packaged for specific, limited time use. The device can therefore be used in methods of providing therapy, managing pain, and achieving other treatment goals by electrical stimulation.
Owner:ENCORE MEDICAL ASSET CORP +1
Location and deactivation of muscles
InactiveUS20070255342A1High resolutionEliminate needSpinal electrodesUltrasound therapyHeadachesBiological activation
Methods and devices for treatment to at least interfere with the function of a muscle are described herein. These methods and devices may have application in cosmetic and plastic surgery, dermatology, suppression of tension and / or migraine-type headaches, pain management, one particular application of the subject matter deals primarily with reducing wrinkles caused by ongoing muscular activation. The devices and methods described herein allow medical practitioners to effectively identify selective nerves for paralyzingmuscles, without the need for injections of agents such as botulism toxin. Moreover, the devices and methods herein, may allow for artificial generation of signals in nerves that were otherwise damaged by stimulating transmission of nerve signals across damaged nerves.
Owner:LAUFER MICHAEL D
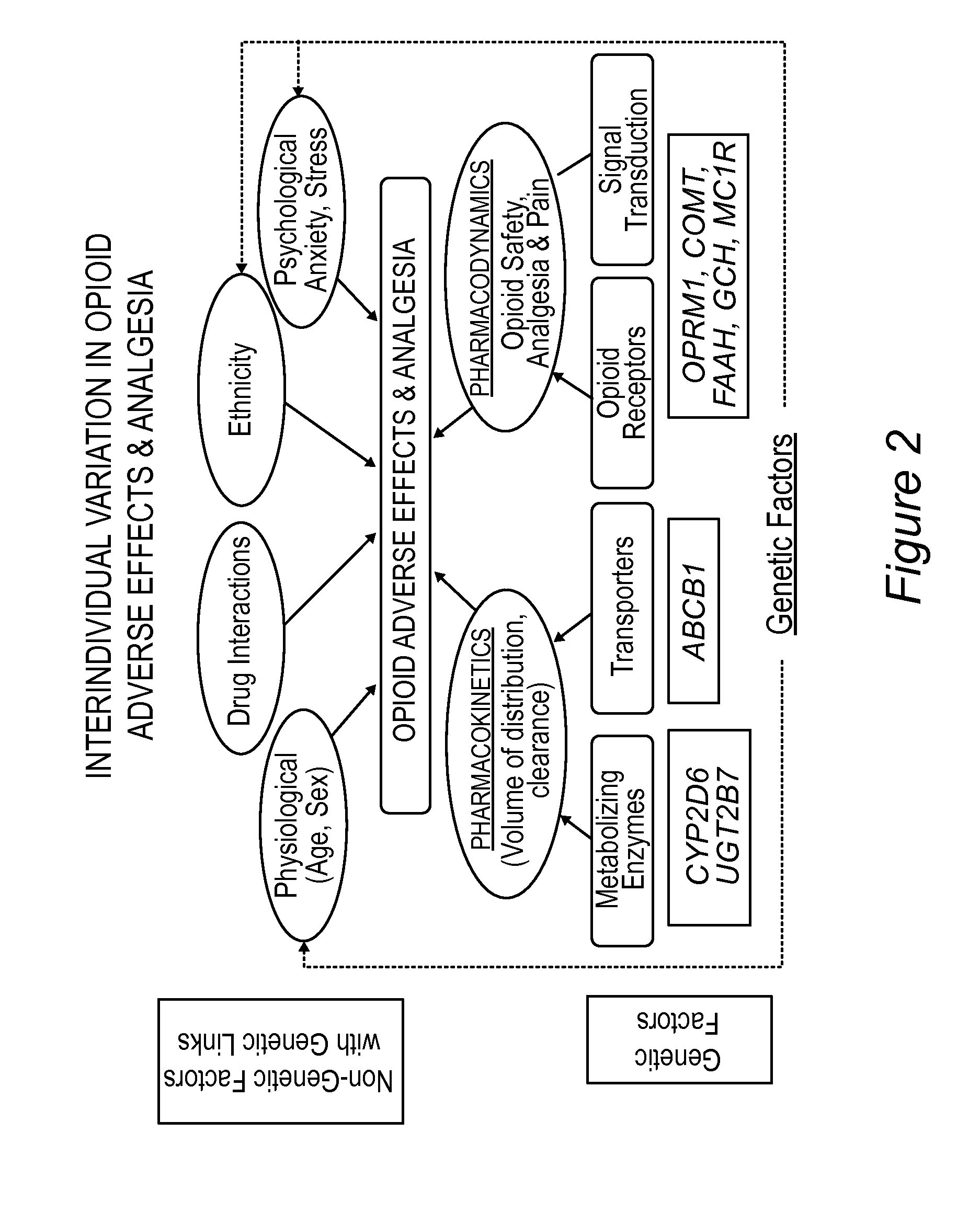
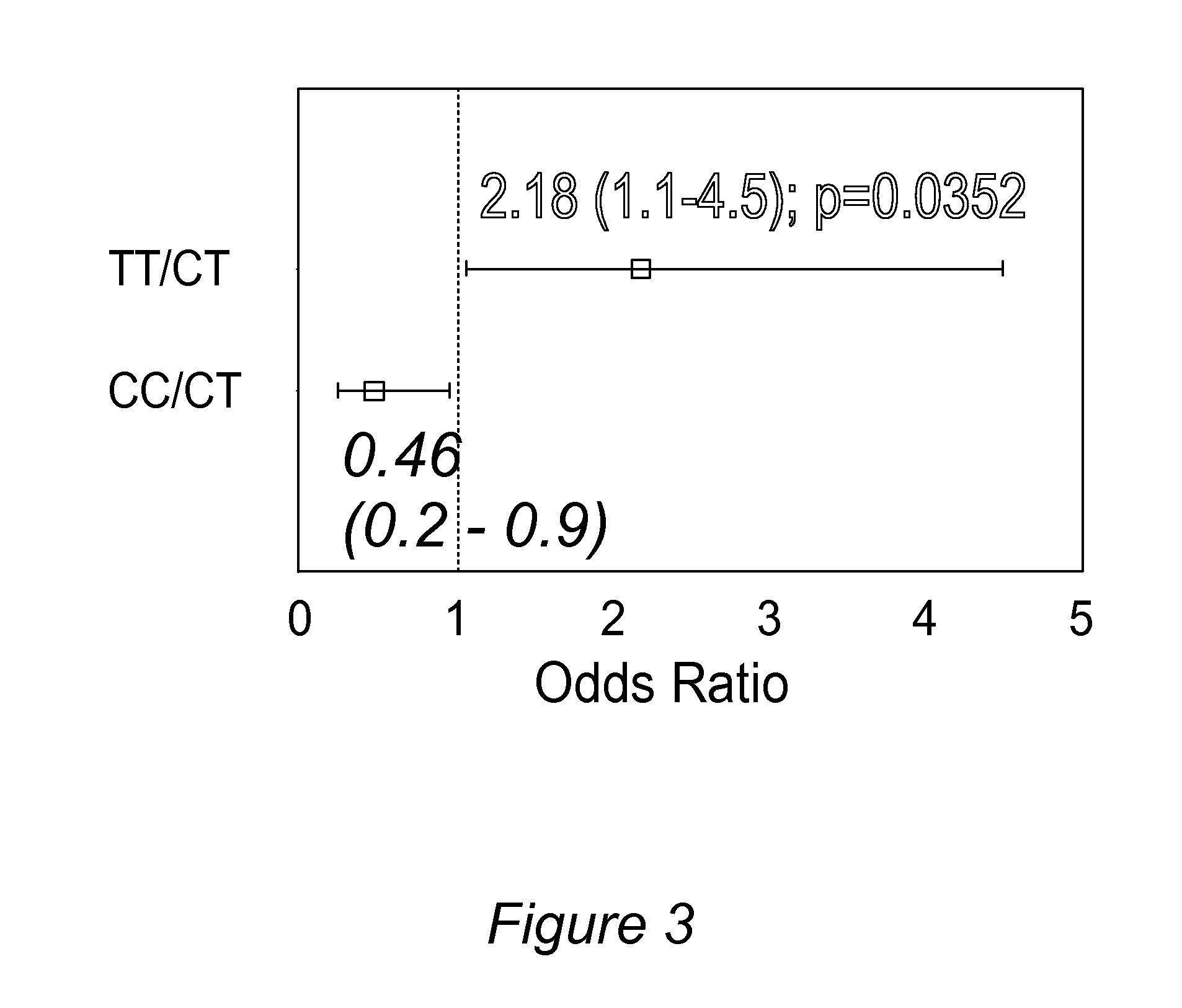
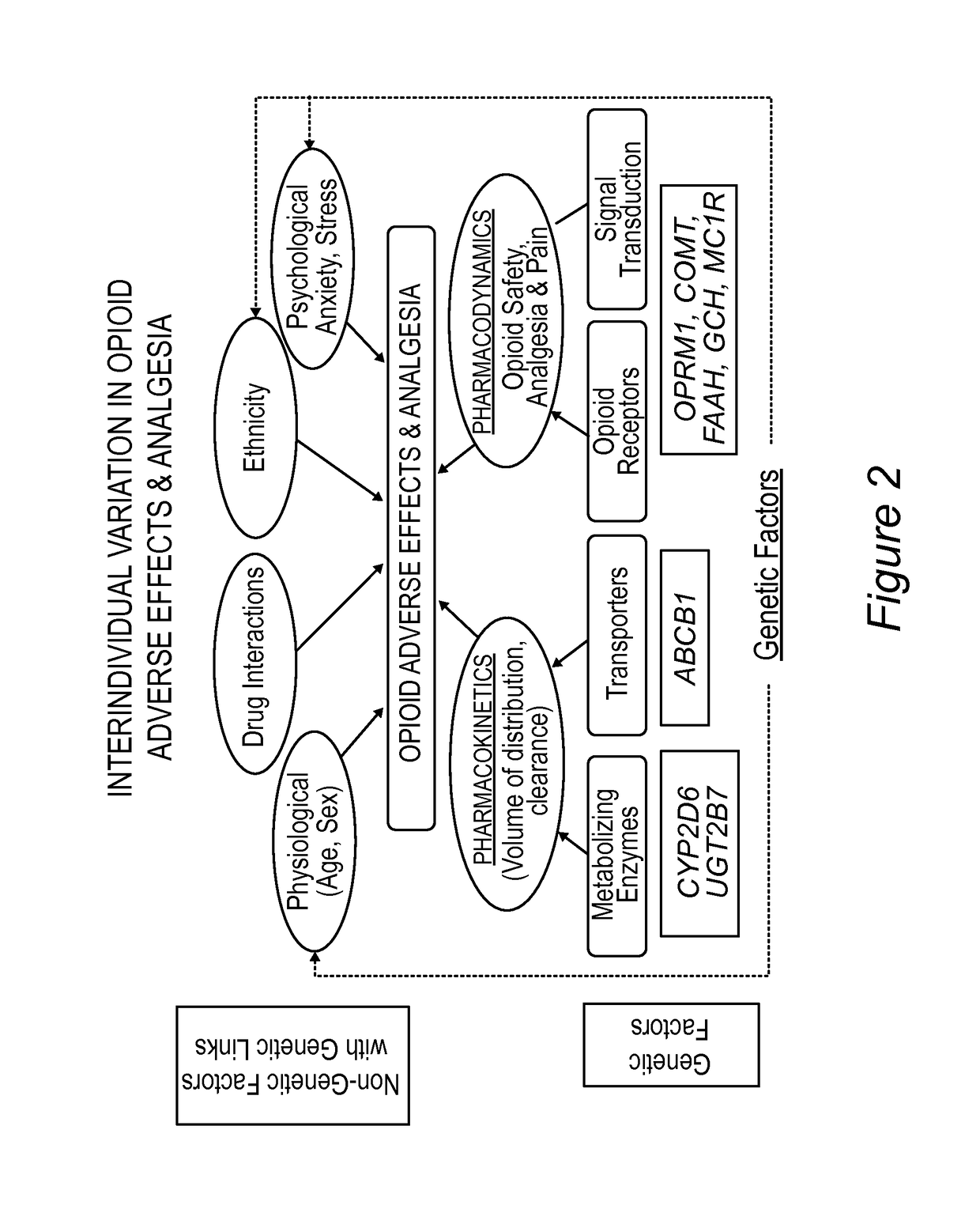
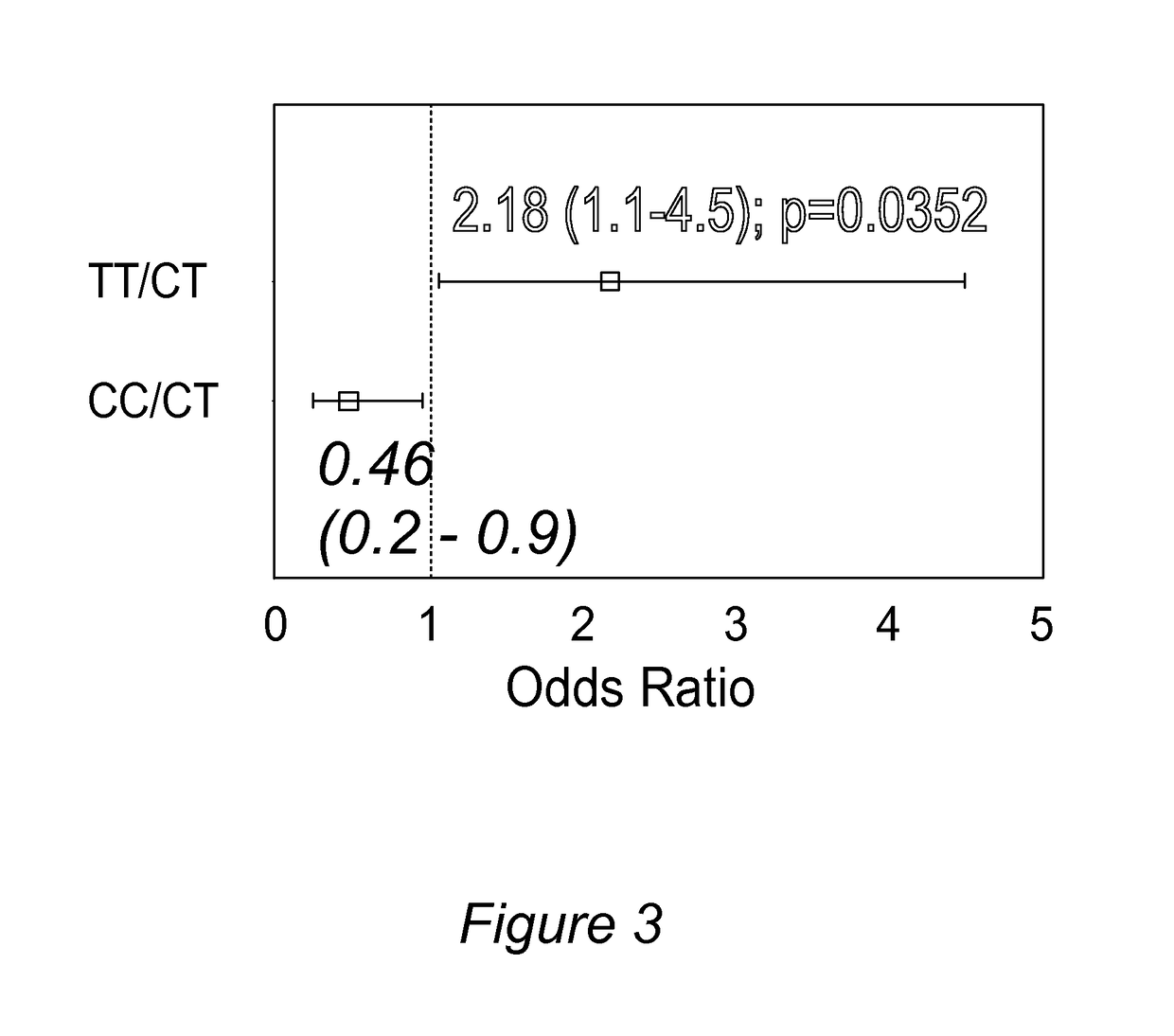
Personalized pain management and anesthesia: preemptive risk identification and therapeutic decision support
ActiveUS20140371256A1Maximize pain reliefAdverse effect in subjectBiocideMicrobiological testing/measurementPersonalizationSide effect
Methods and compositions disclosed herein generally relate to methods of improving clinical and economic outcomes to address adverse effects related to anesthesia, analgesics, opioids, and inadequate pain relief. Embodiments of the invention relate to the association between genes, specific polymorphisms of genes, and non-genetic factors with inadequate pain relief and anesthesia-, analgesic, and / or opioid-related adverse effects. Embodiments of the invention can be used to determine and manage patient risk factors for development of adverse perioperative effects and can allow for personalized anesthesia and pain management for improvement of pain control and reduction of anesthesia-, analgesic-, and opioid-related adverse outcomes. These methods and compositions apply to non-surgical pain management with opioids. Therefore, patients who are genetically predisposed to risk of inadequate pain relief and / or serious side effects from anesthesia, analgesics, and / or opioids can be identified and individualized treatment plans developed for implementation by the clinician to improve clinical and economic outcomes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
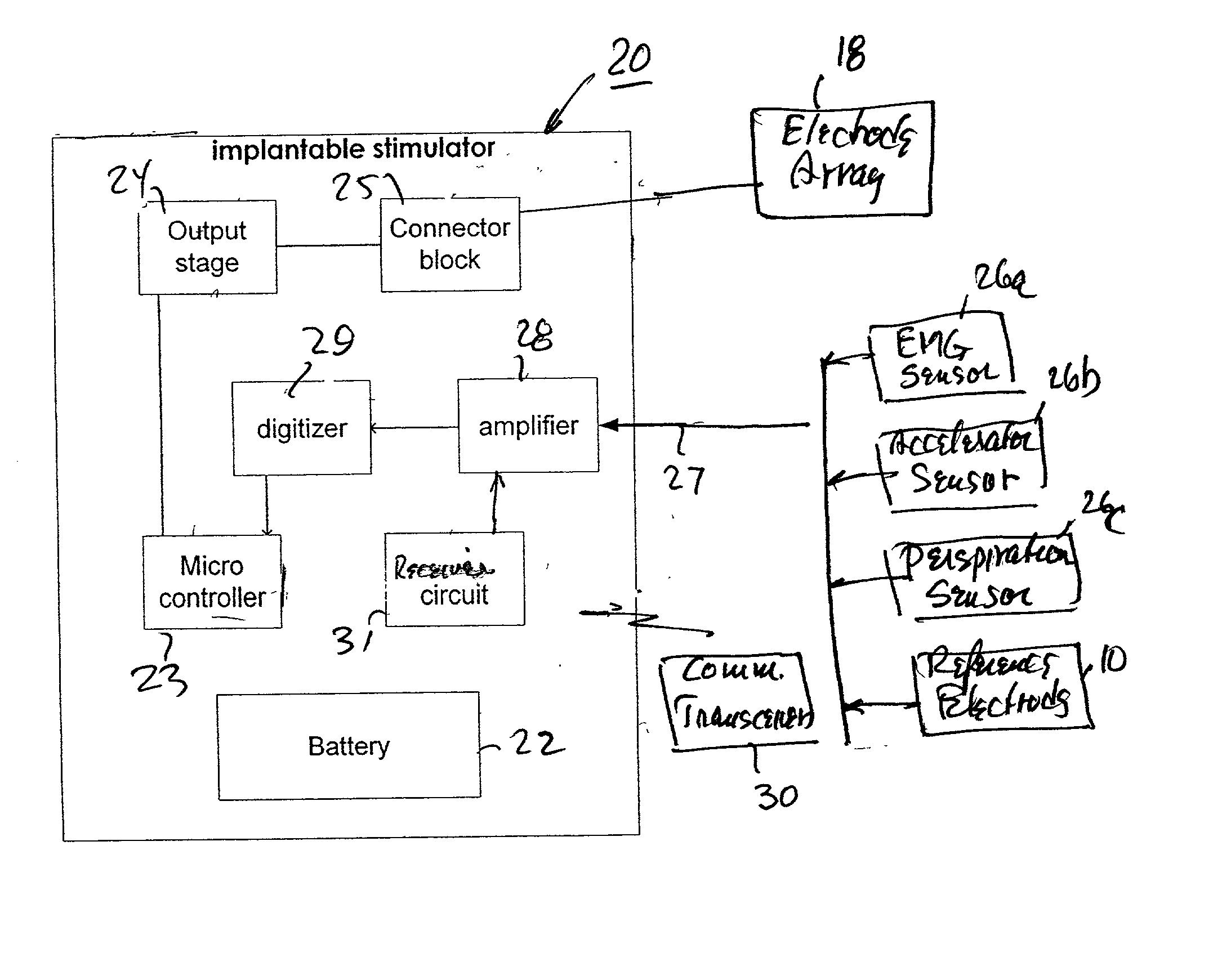
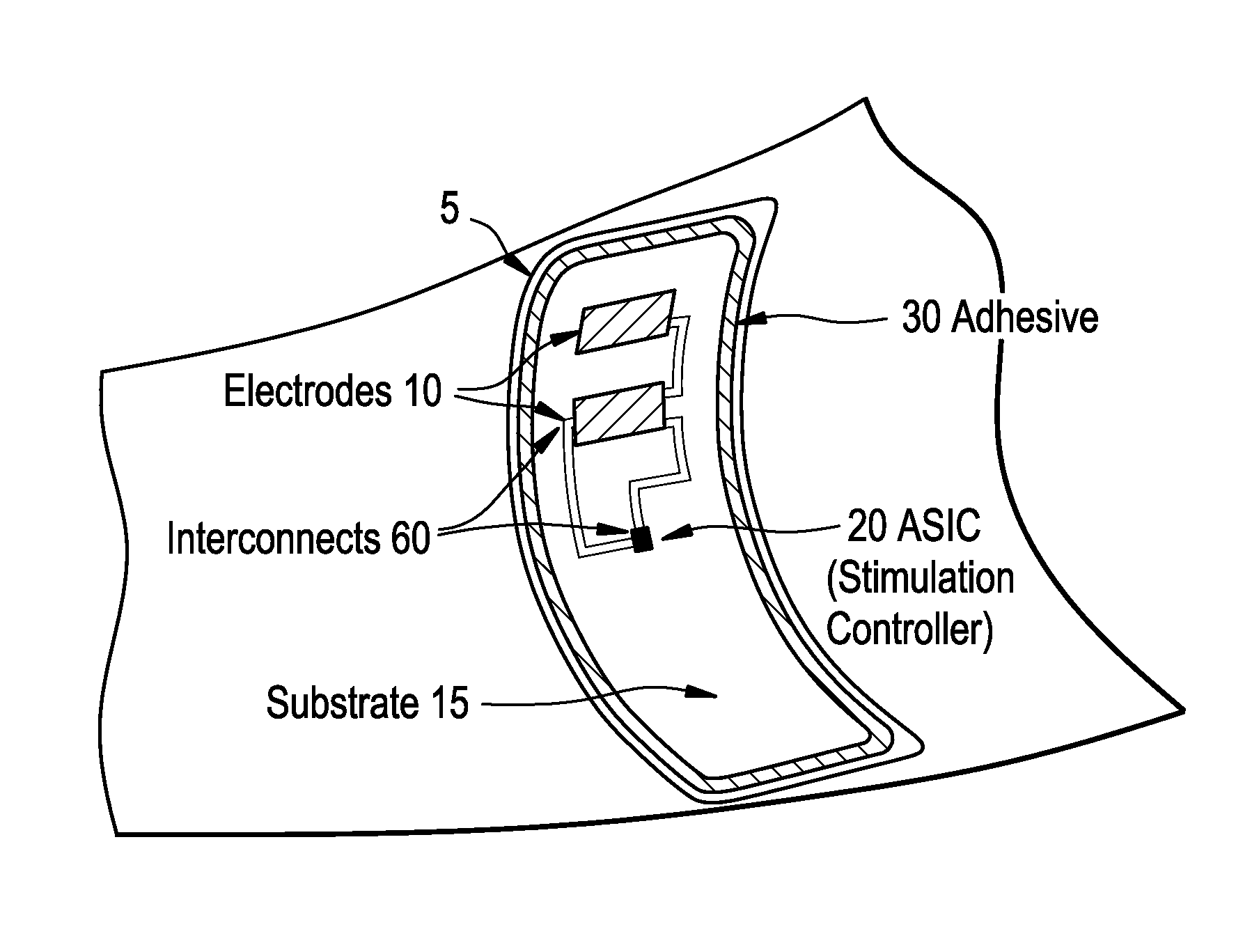
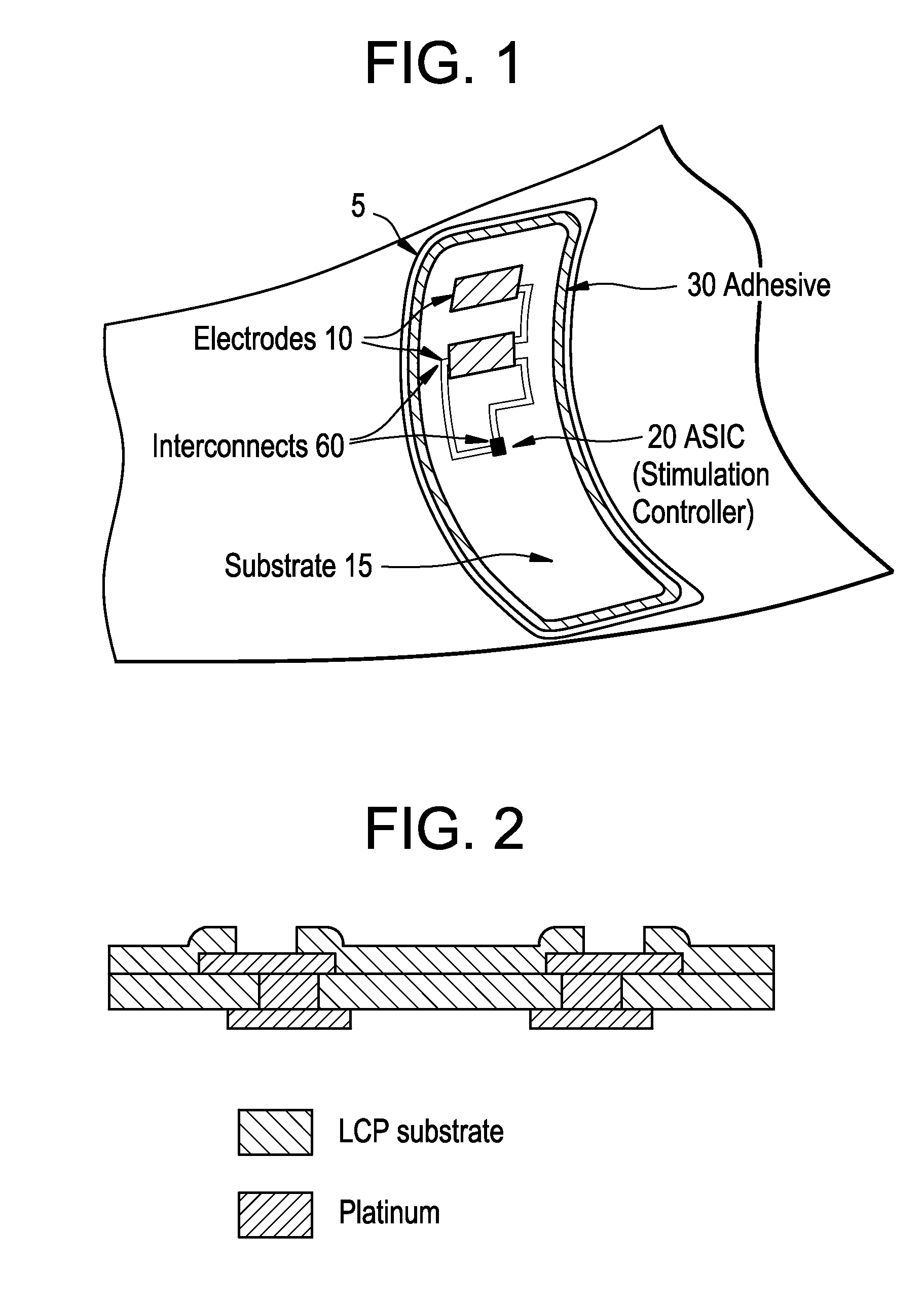
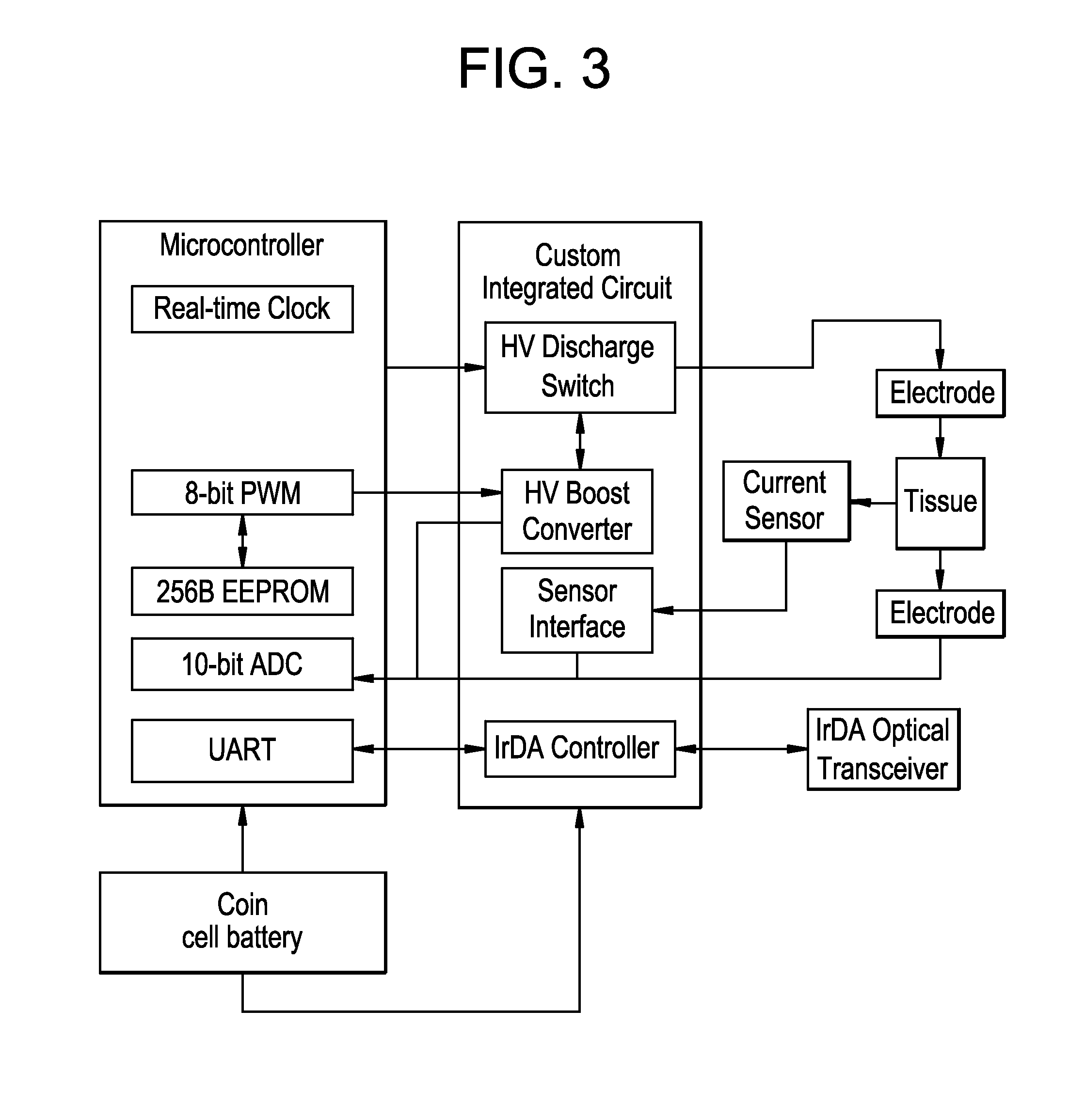
Integrated surface stimulation device for pain management and wound therapy
ActiveUS9320907B2Easy to manageSimple interfaceExternal electrodesArtificial respirationWound healingControl system
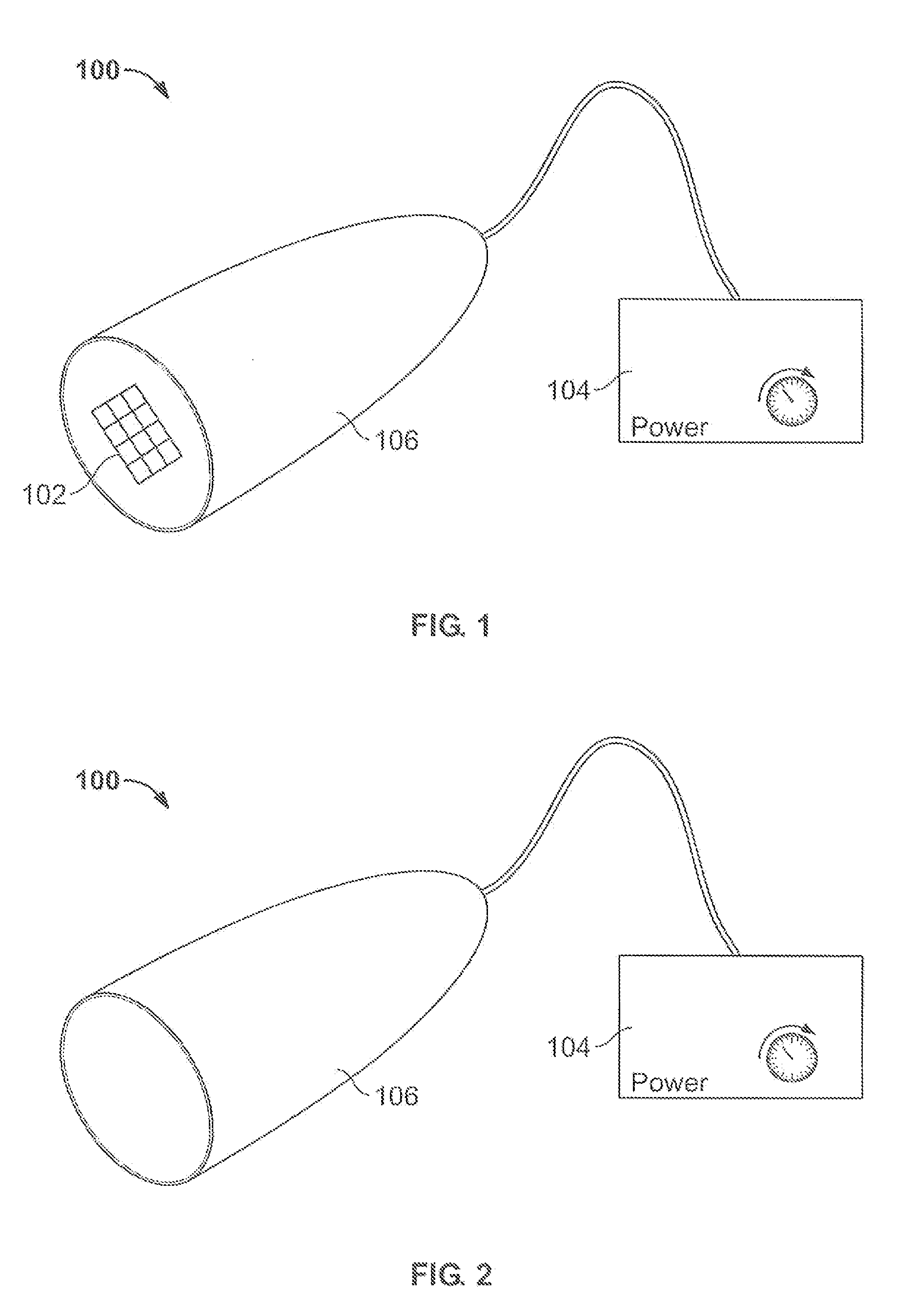
The present invention provides a thin and flexible device and method of use thereof for pain and wound treatment of a subject. The integrated surface stimulation device may comprise a complete wireless stimulation system in a disposable and / or reusable flexible device for widespread use in multiple therapeutic applications. The invention would be situated on the skin surface of a patient and would be activated so as to reduce the overall occurrence of pain and / or increase wound healing rates. As provided, the device will comprise an integrated power supply and pre-programmable stimulator / control system mounted on the upper face of a flexible polymeric substrate layer. The lower face of the substrate layer will comprise areas of stimulating electrodes, applied using thin film coating techniques. The device can then be applied to the user with a medical grade pressure sensitive adhesive coating provided on the lower face of the substrate layer.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
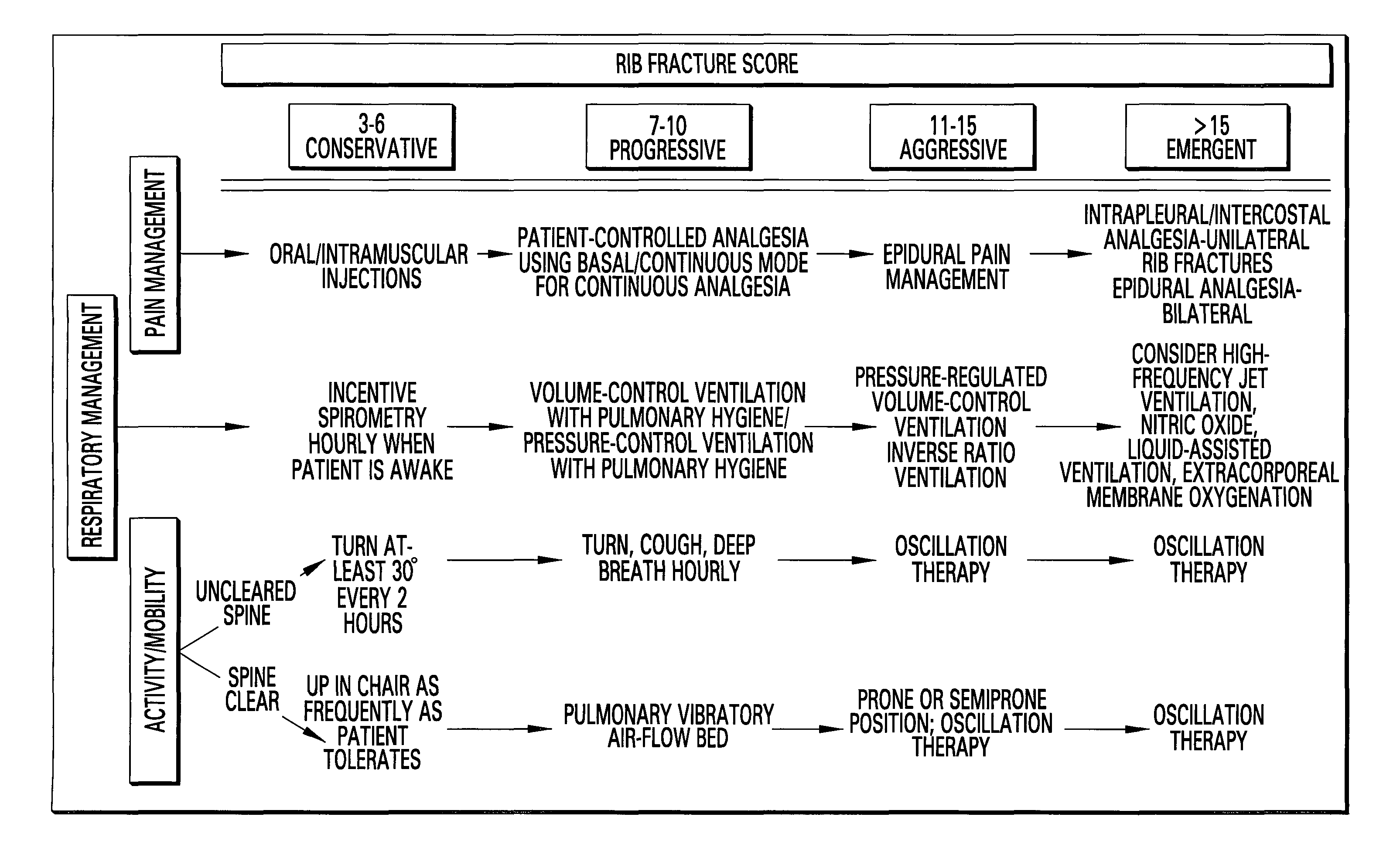
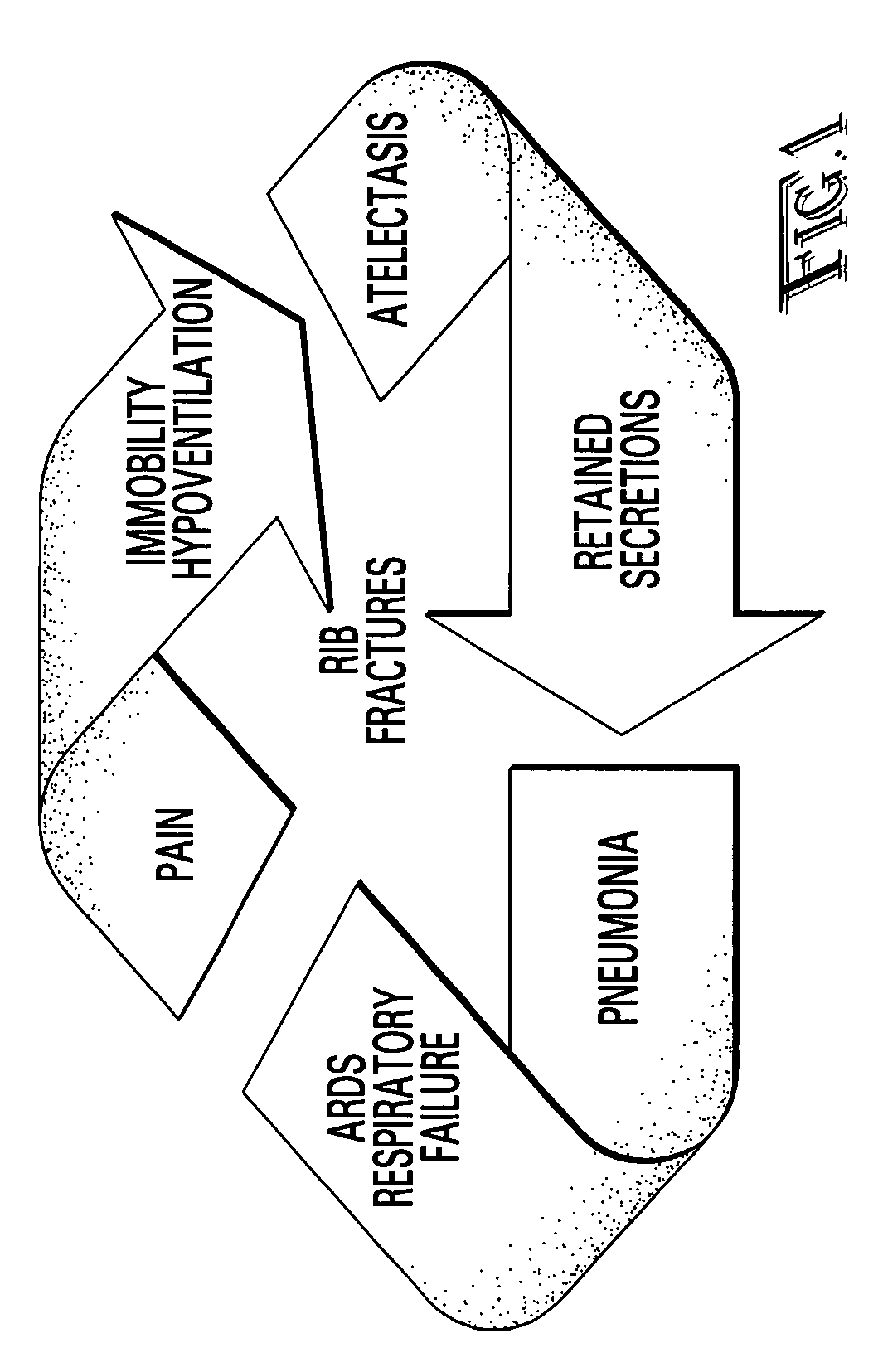
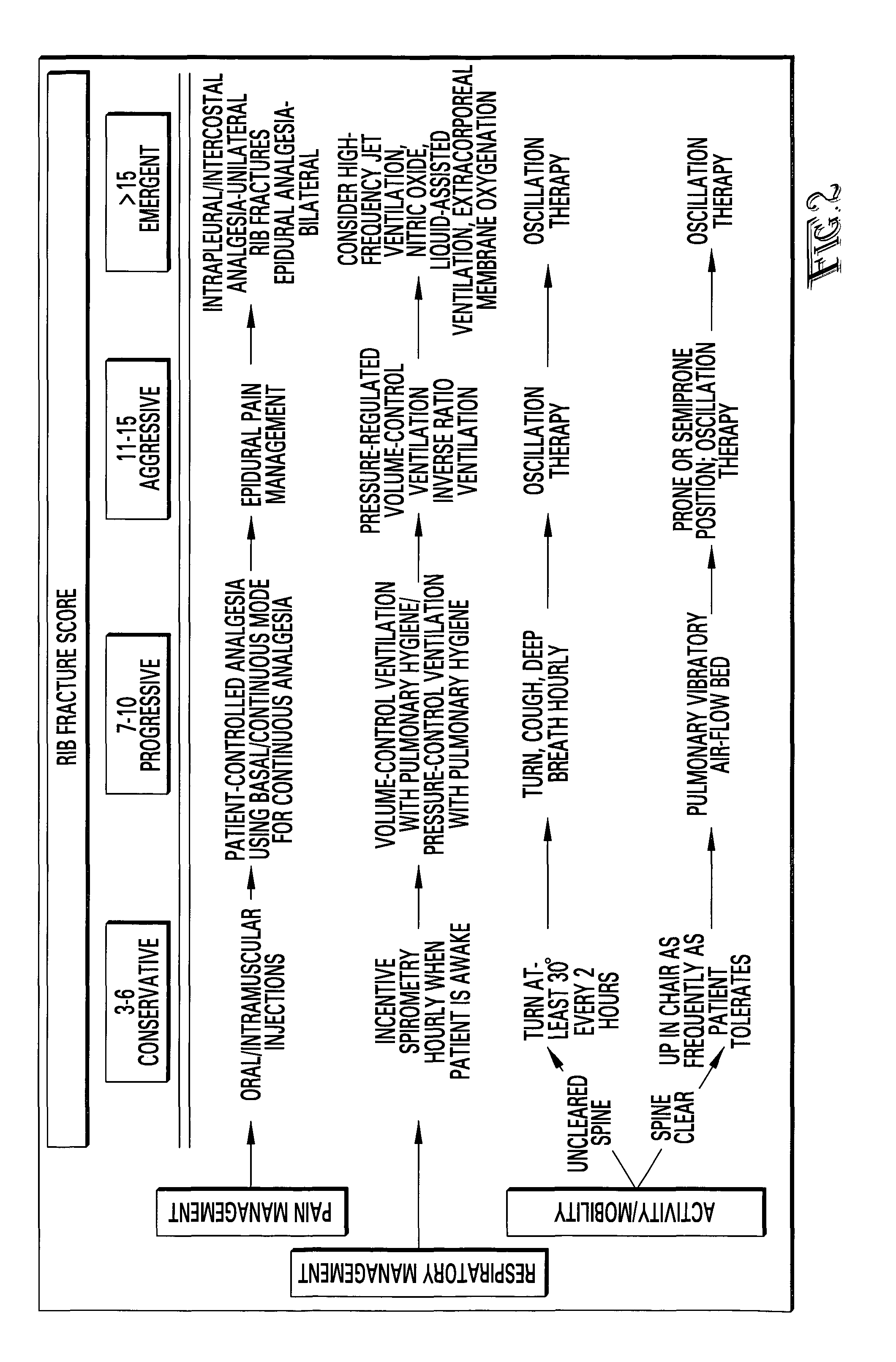
Rib fracture score and protocol
InactiveUS7225813B2Promote recoverySurgeryDiagnostic recording/measuringRespiratory careMortality rate
Multiple rib fractures in trauma patients are associated with significant morbidity and mortality. Delayed morbidity for patients with rib fractures is often a result of hypoventilation leading to atelectasis, pneumonia and respiratory failure. Pain management was first recognized as an important factor in preventing complications in these patients. Later, management of the respiratory system became more widely recognized as a major factor in, patients' care. It is now known that patients with multiple rib fractures benefit most from adequate pain control, rapid mobilization, and meticulous respiratory care to prevent complications. A rib fracture score and protocol based on a synthesis of the existing literature is developed. The protocol is directed to decisions about rapid mobilization, respiratory support, and pain management interventions to decrease the length of patients' stay in intensive care units.
Owner:BIOVENTURES LLC
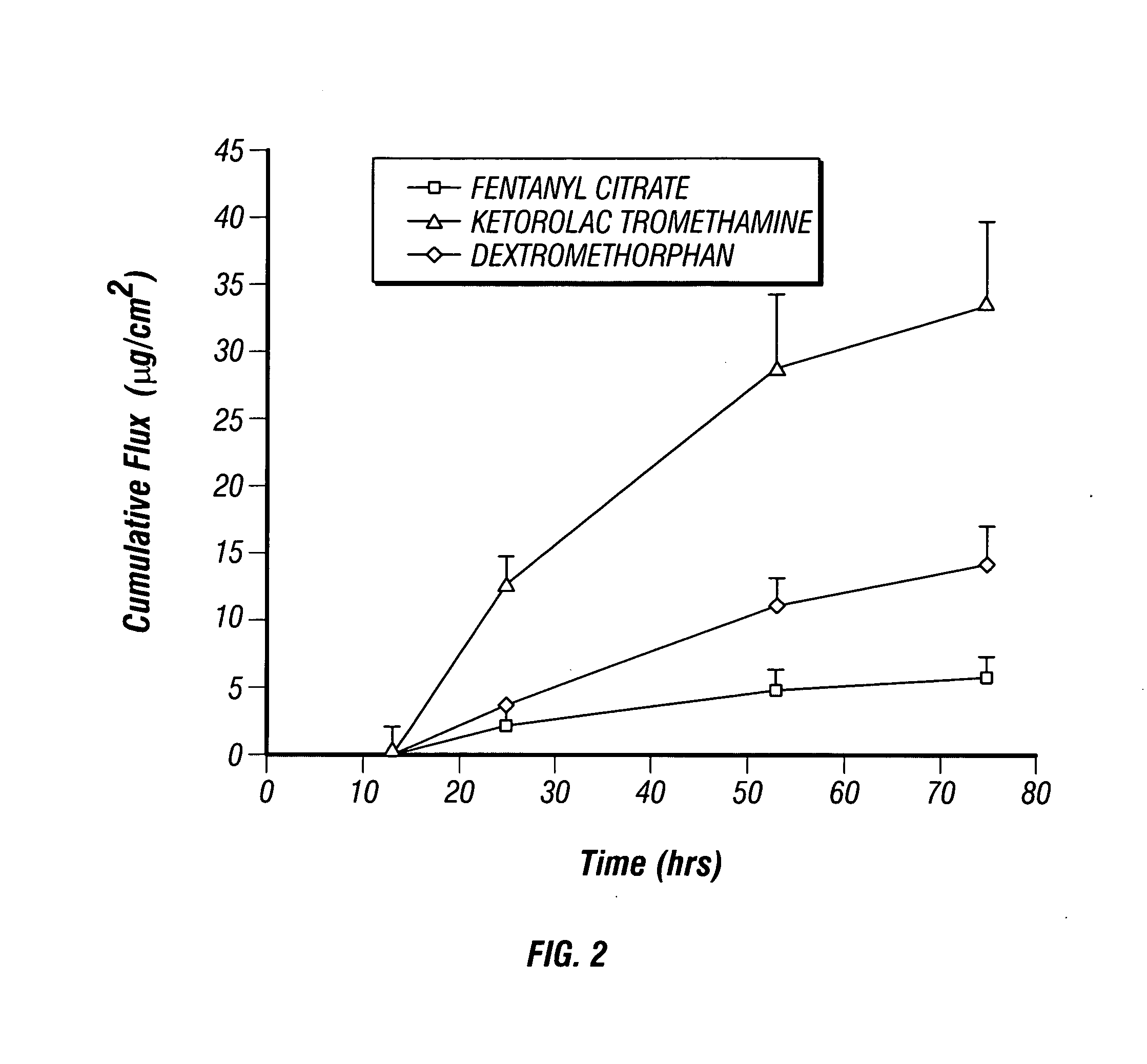
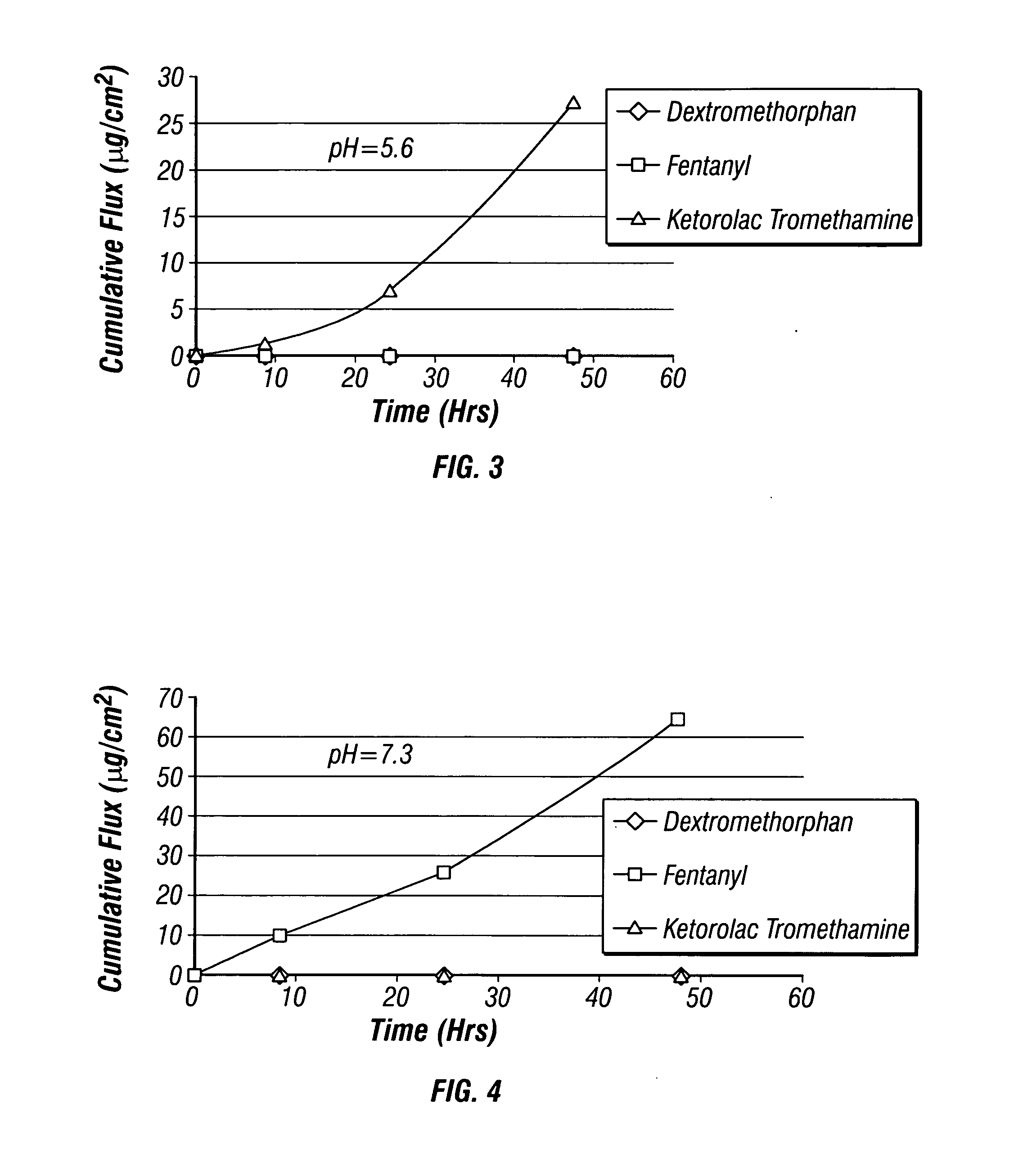
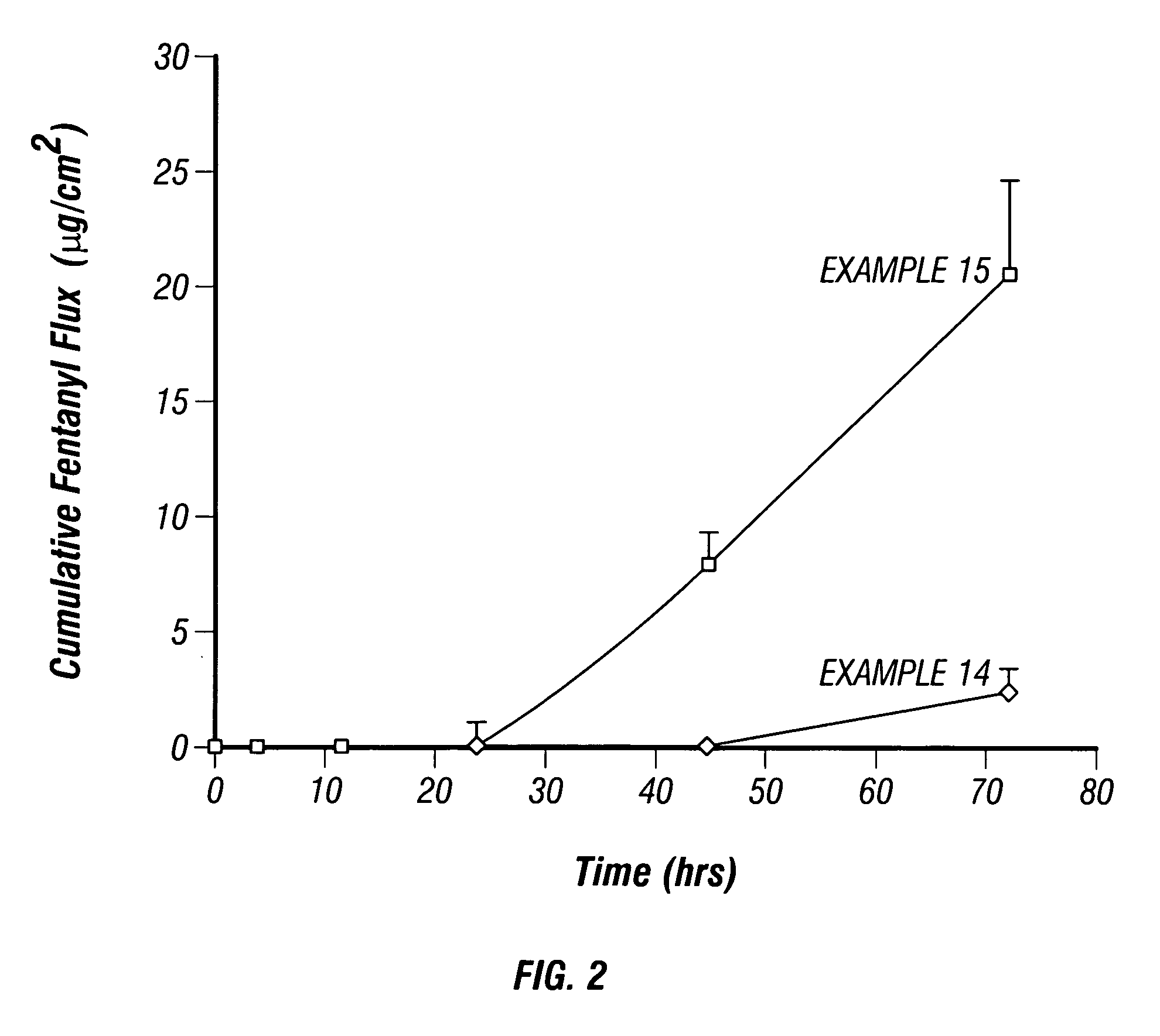
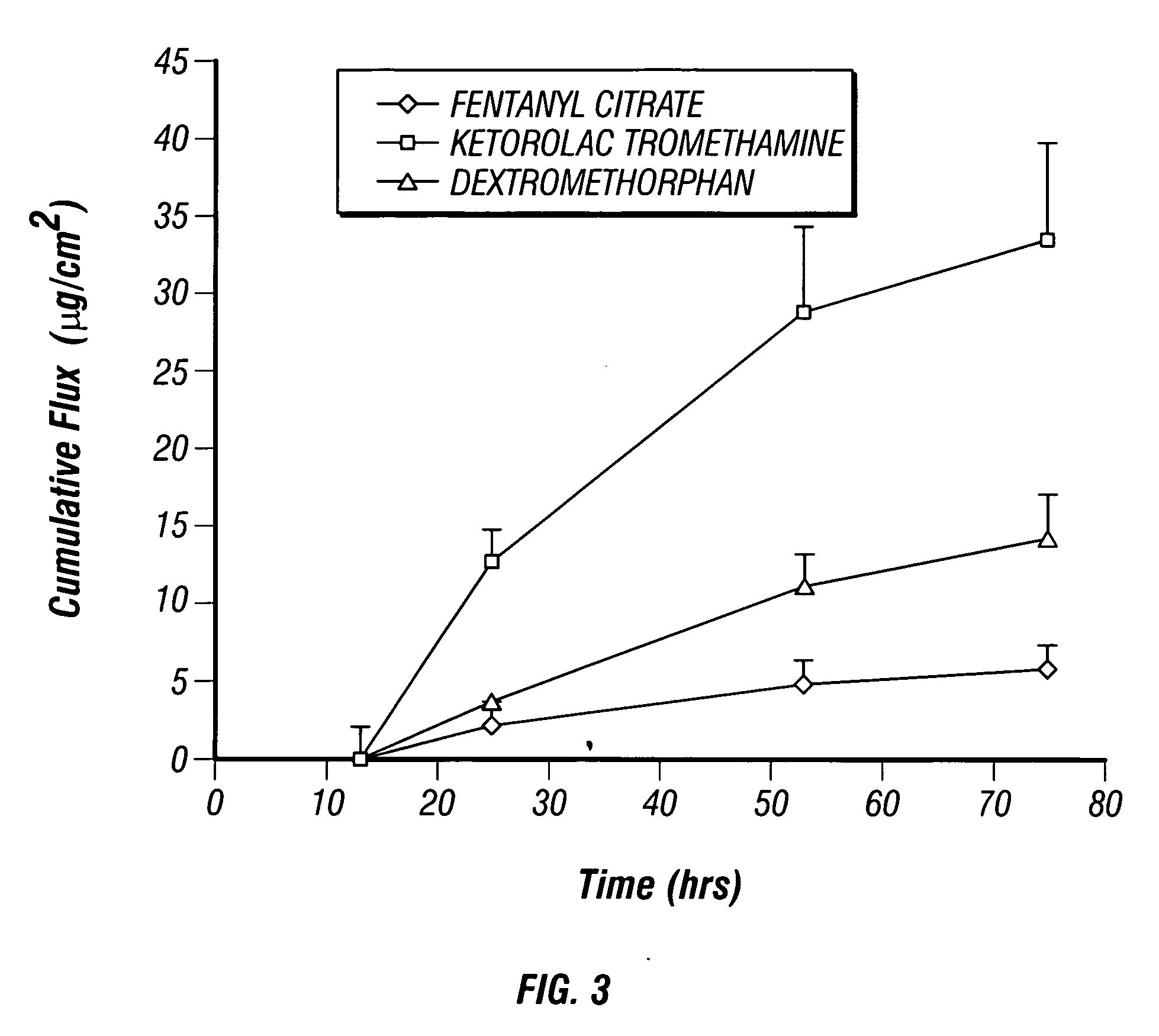
Multi-compartment transdermal pain control device
InactiveUS20070248657A1Relief the painIncrease transdermal fluxBiocideAnimal repellantsNR1 NMDA receptorNMDA receptor
Multi-compartment patches containing skin-permeable forms of pharmaceutically effective amounts of an opioid agonist, an NMDA receptor antagonist and an anti-inflammatory are useful for the transdermal delivery of the active ingredients to alleviate pain.
Owner:INNOVATIVE PHARMA
Baroreflex activation for pain control, sedation and sleep
Systems and methods provide baroreflex activation to treat or reduce pain and / or to cause or enhance sedation or sleep. Methods involve activating the baroreflex system to provide pain reduction, sedation, improved sleep or some combination thereof. Systems include at least one baroreflex activation device, at least one sensor for sensing physiological activity of the patient, and a processor coupled with the baroreflex activation device(s) and the sensor(s) for processing sensed data received from the sensor and for activating the baroreflex activation device. In some embodiments, the system is fully implantable within a patient, such as in an intravascular, extravascular or intramural location.
Owner:CVRX
Extended release analgesic for pain control
InactiveUS6939538B2Electrostatic shieldingAvoid accessSalicyclic acid active ingredientsBiocideControlled releaseAbdominal cavity
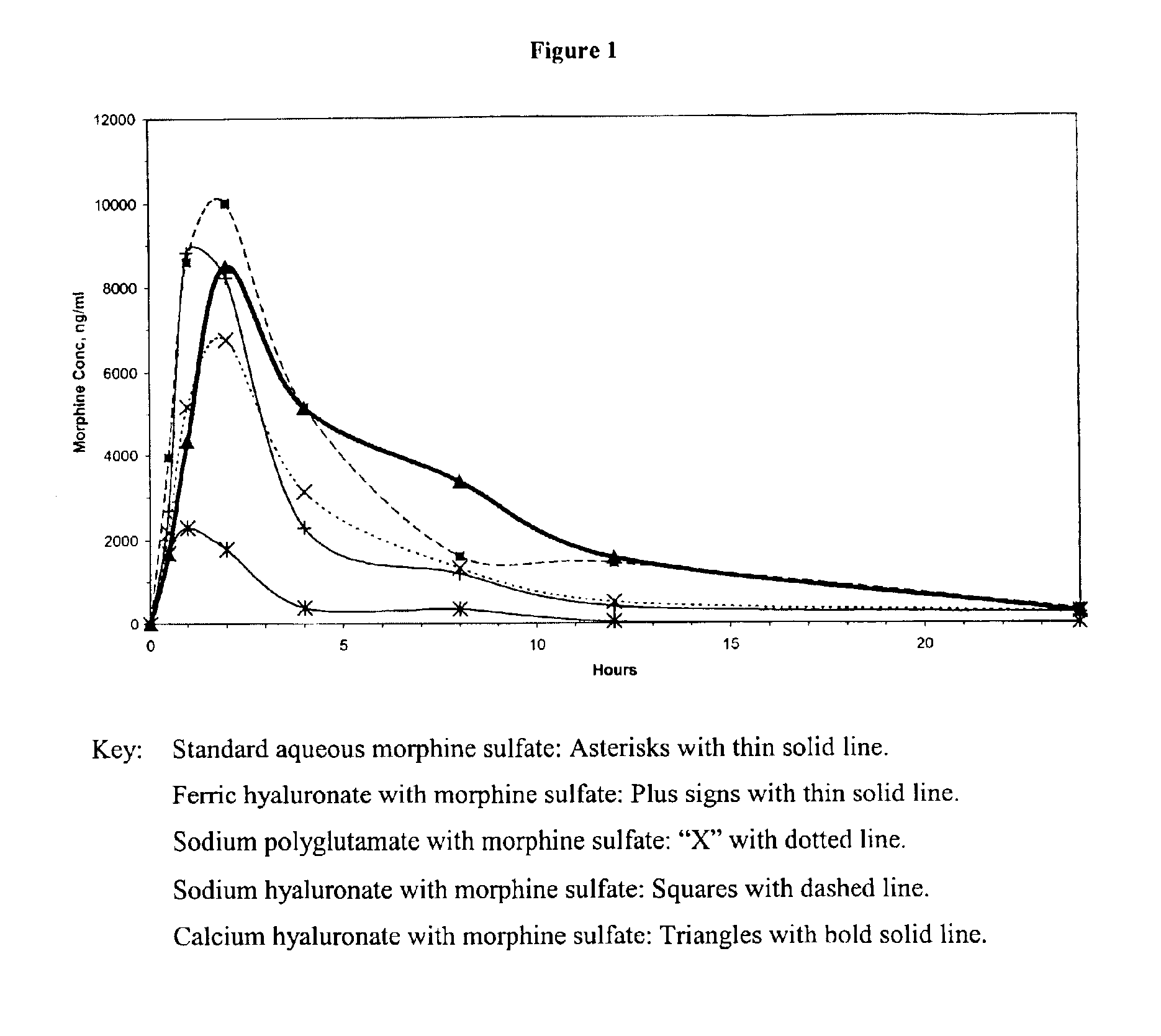
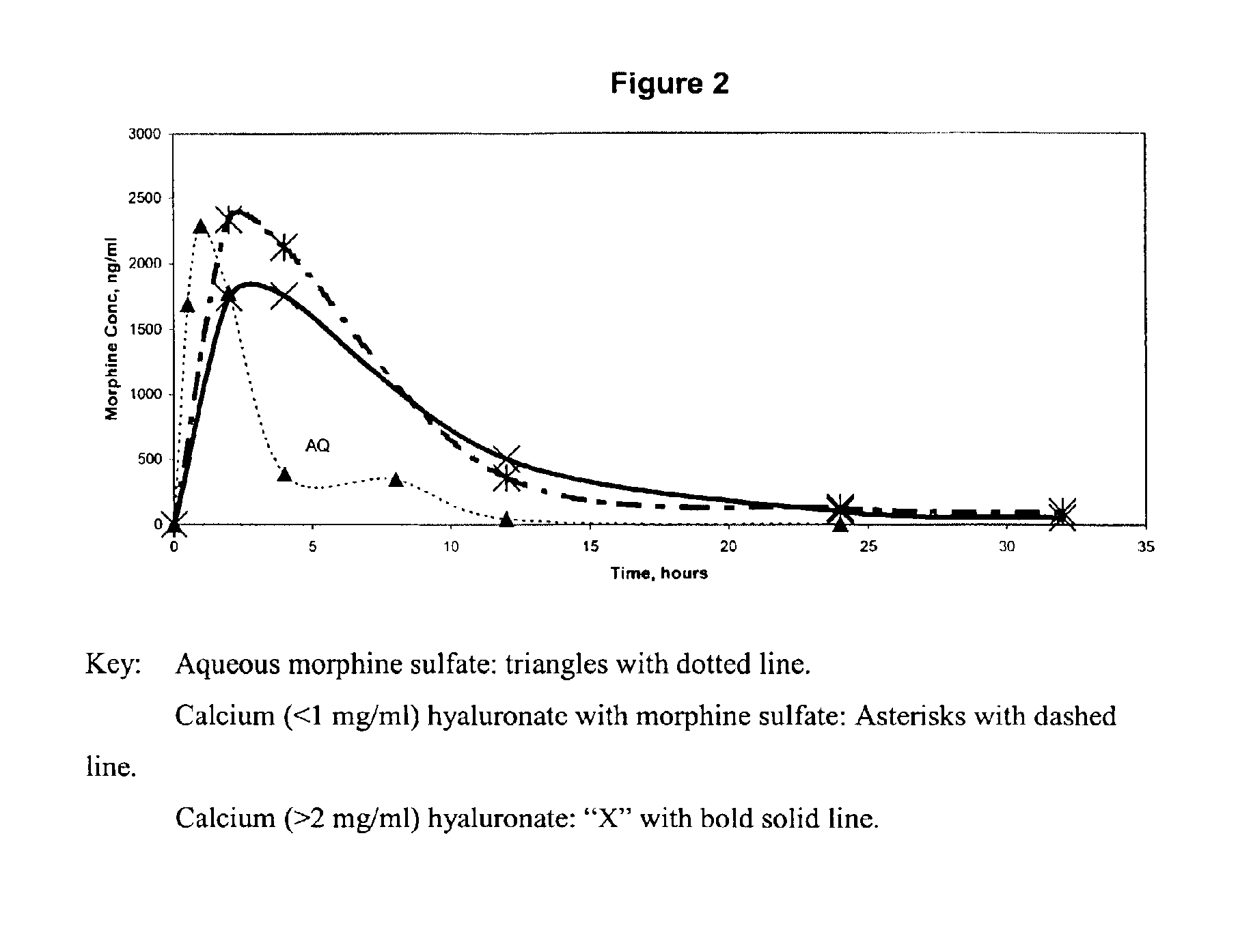
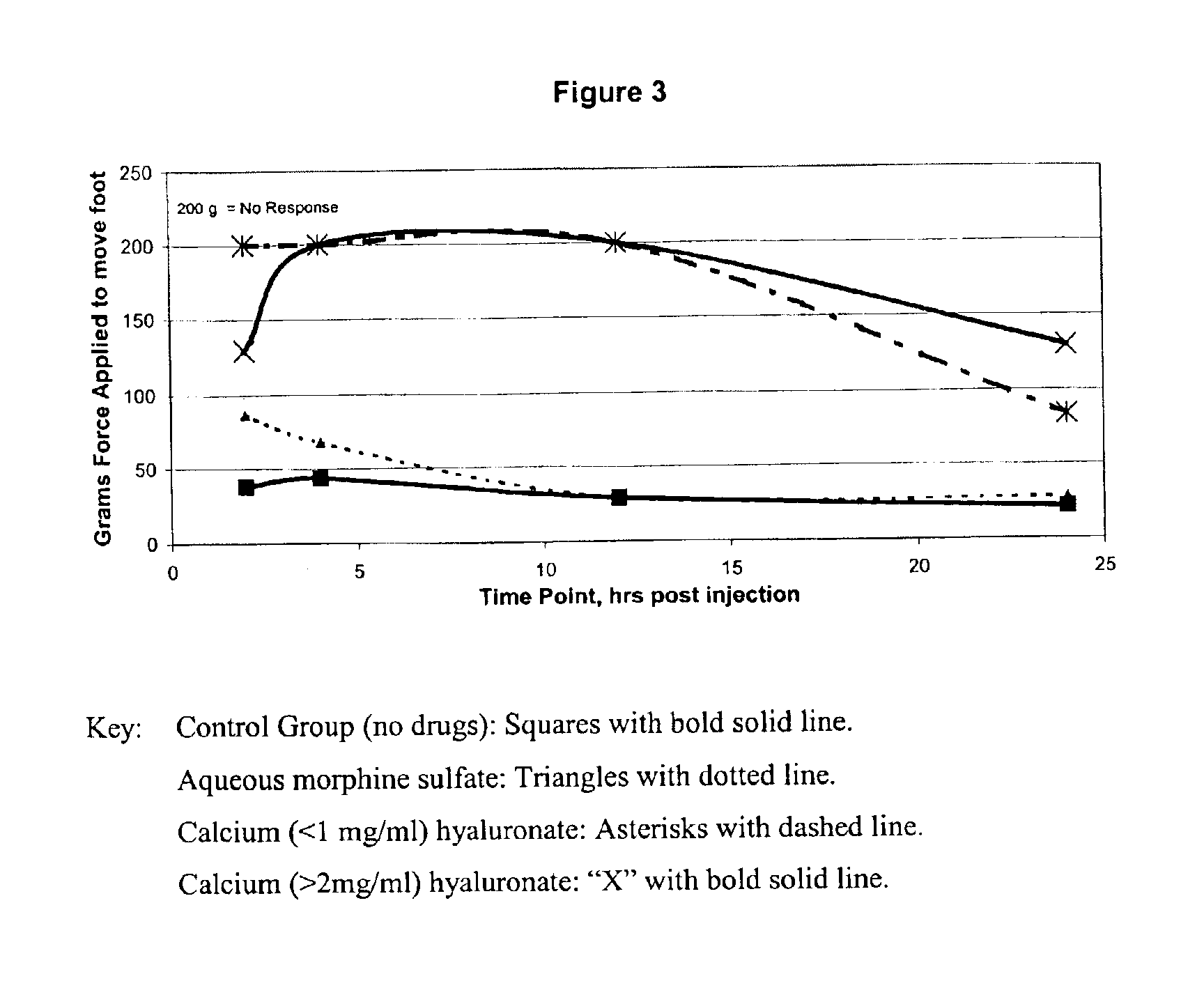
An extended release analgesic for controlling pain comprised of an opioid or non-opioid analgesic drug ionically bound to hyaluronic acid, poly-γ-glutamic acid or other ionic polymers, and injected into a body either subcutaneously, intramuscularly or intraperitoneally, utilizing counter-ions of different valences to control the rate of release into the body.
Owner:BIOMEDICAL RES MODELS
Methods and compositions
InactiveUS20110092482A1Lower Level RequirementsReduce of sensationBiocideNervous disorderDiseaseSlow Release Formulation
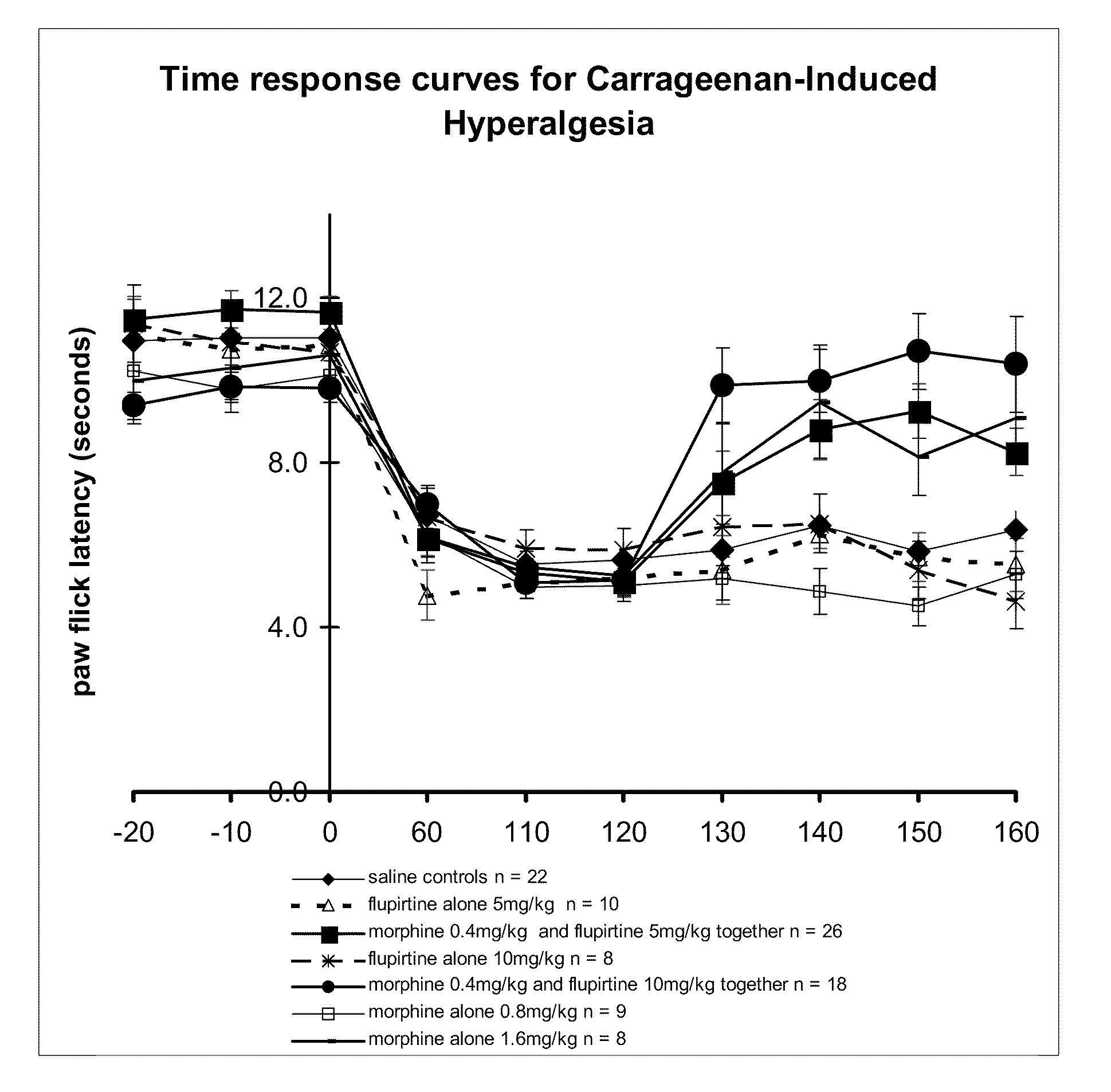
The present invention relates generally to the field of pain management, and in particular, the management of neuropathic or inflammatory pain including a neuropathic or inflammatory component of nociceptive pain. More particularly, the present invention provides methods and compositions which treat, alleviate, prevent, diminish or otherwise ameliorate the symptoms of neuropathic or inflammatory pain. The present invention further contemplates combination therapy involved in the treatment of pain in association with the treatment of a particular disease condition or pathology. The present invention further also provides sustained and slow release formulations, tamper-proof deliver systems and stents, catheters and other mechanical devices coated with formulations which permit sustained or slow release of active ingredients involved in pain management.
Owner:RELEVARE AUST
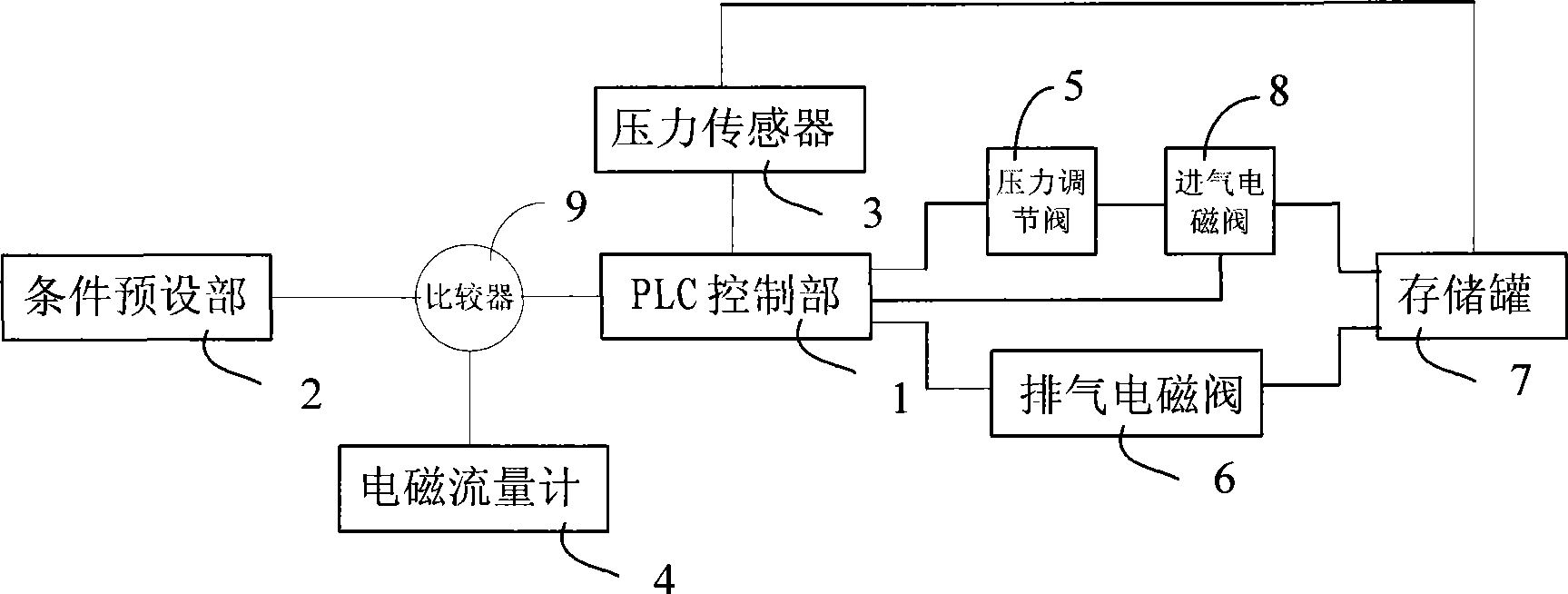
Spray paining control method on mineral wool sound-absorbing panel and control system thereof
ActiveCN101386005AEliminate fluctuationsHigh control precisionLiquid surface applicatorsSpraying apparatusControl systemStreamflow
The invention discloses a control method and a control system used for spraying the surface of a mineral wool sound absorbing board. The spraying control method of the mineral wool sound adsorbing board comprises the steps as follows: a. given quantity of the spraying flux is arranged in advance at the condition pre-arranging part; practical spraying flux is detected in real-time by an electromagnetic flowmeter and the detection result is transmitted to a PLC control part; b. PLC control part compares the prearranged value with the real-time detection valve and adjusts the magnitude of the spraying flux by controlling a pressure adjusting valve and an exhaust electromagnetic valve according to the comparison result. The control system used for the control method comprises a condition prearranging part, a PLC control part and an electromagnetic flowmeter. The mineral wool sound absorbing board disposed by the method and the system completely eliminates the color difference generated on the press-typed spraying process control method.
Owner:BEIJING NEW BUILDING MATERIAL
Methods and compositions
InactiveUS8268821B2Lower Level RequirementsReduce of sensationBiocideNervous disorderDiseaseSlow Release Formulation
The present invention relates generally to the field of pain management, and in particular, the management of neuropathic or inflammatory pain including a neuropathic or inflammatory component of nociceptive pain. More particularly, the present invention provides methods and compositions which treat, alleviate, prevent, diminish or otherwise ameliorate the symptoms of neuropathic or inflammatory pain. The present invention further contemplates combination therapy involved in the treatment of pain in association with the treatment of a particular disease condition or pathology. The present invention further also provides sustained and slow release formulations, tamper-proof deliver systems and stents, catheters and other mechanical devices coated with formulations which permit sustained or slow release of active ingredients involved in pain management.
Owner:RELEVARE AUST
Heat generating biocompatible ceramic materials
An injectable heat generating biocompatible ceramic compositions based on hydraulic calcium aluminate, which can be used for therapeutic treatment in vivo, such as tumour treatment, pain control, vascular treatment, drug activation etc, when curing in situ, and which form a biocompatible solid material that can be left in the body for prolonged periods of time without causing negative health effects, and can also be used to restore the mechanical properties of the skeleton after cancerous diseases as well as in a medical implant, an orthopaedic implant, and a dental implant as a dental filling material.
Owner:DOXA AB
Personalized pain management and anesthesia: preemptive risk identification and therapeutic decision support
ActiveUS9944985B2Maximize pain reliefAdverse effect in subjectSugar derivativesMicrobiological testing/measurementPersonalizationSide effect
Methods and compositions disclosed herein generally relate to methods of improving clinical and economic outcomes to address adverse effects related to anesthesia, analgesics, opioids, and inadequate pain relief. Embodiments of the invention relate to the association between genes, specific polymorphisms of genes, and non-genetic factors with inadequate pain relief and anesthesia-, analgesic, and / or opioid-related adverse effects. Embodiments of the invention can be used to determine and manage patient risk factors for development of adverse perioperative effects and can allow for personalized anesthesia and pain management for improvement of pain control and reduction of anesthesia-, analgesic-, and opioid-related adverse outcomes. These methods and compositions apply to non-surgical pain management with opioids. Therefore, patients who are genetically predisposed to risk of inadequate pain relief and / or serious side effects from anesthesia, analgesics, and / or opioids can be identified and individualized treatment plans developed for implementation by the clinician to improve clinical and economic outcomes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
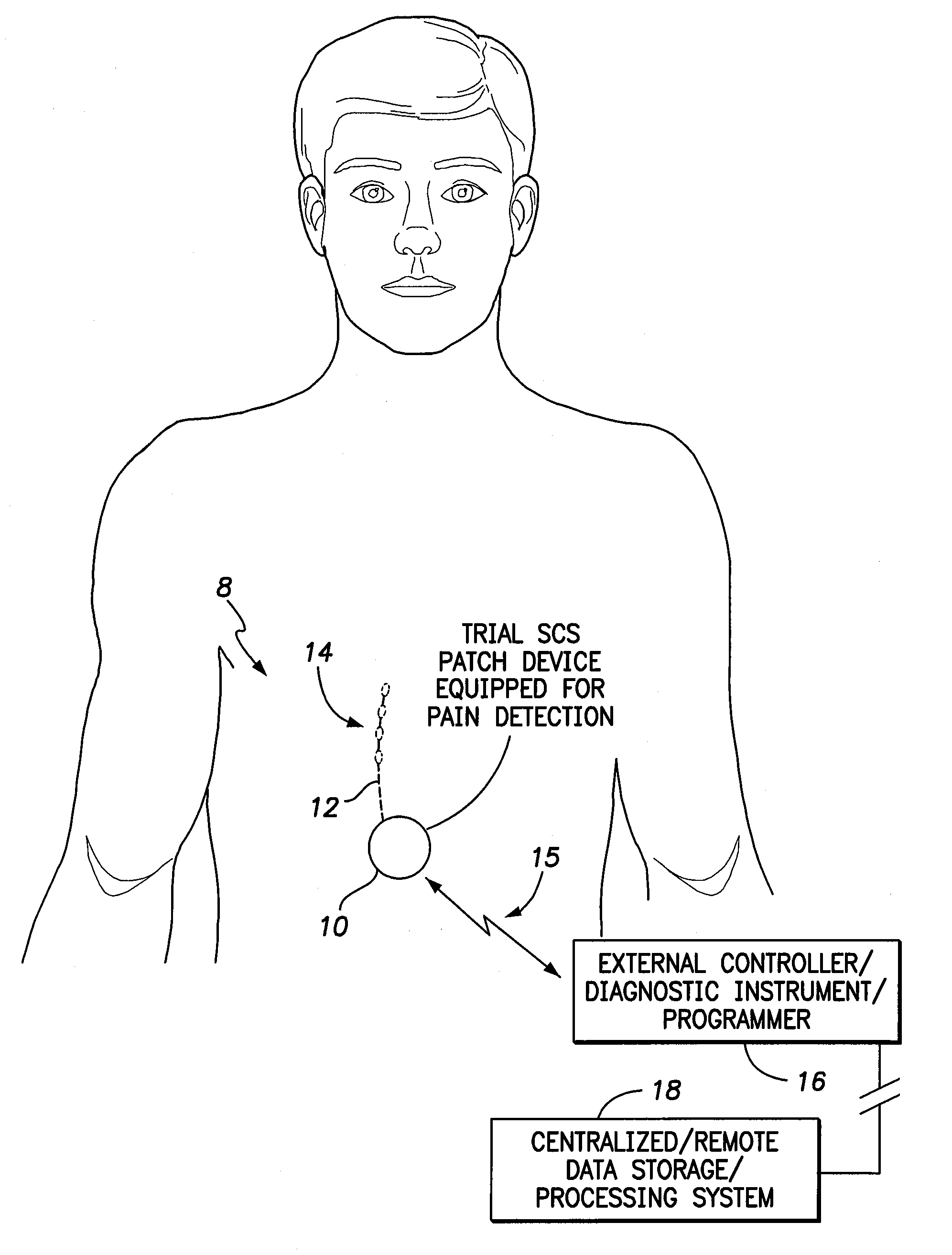
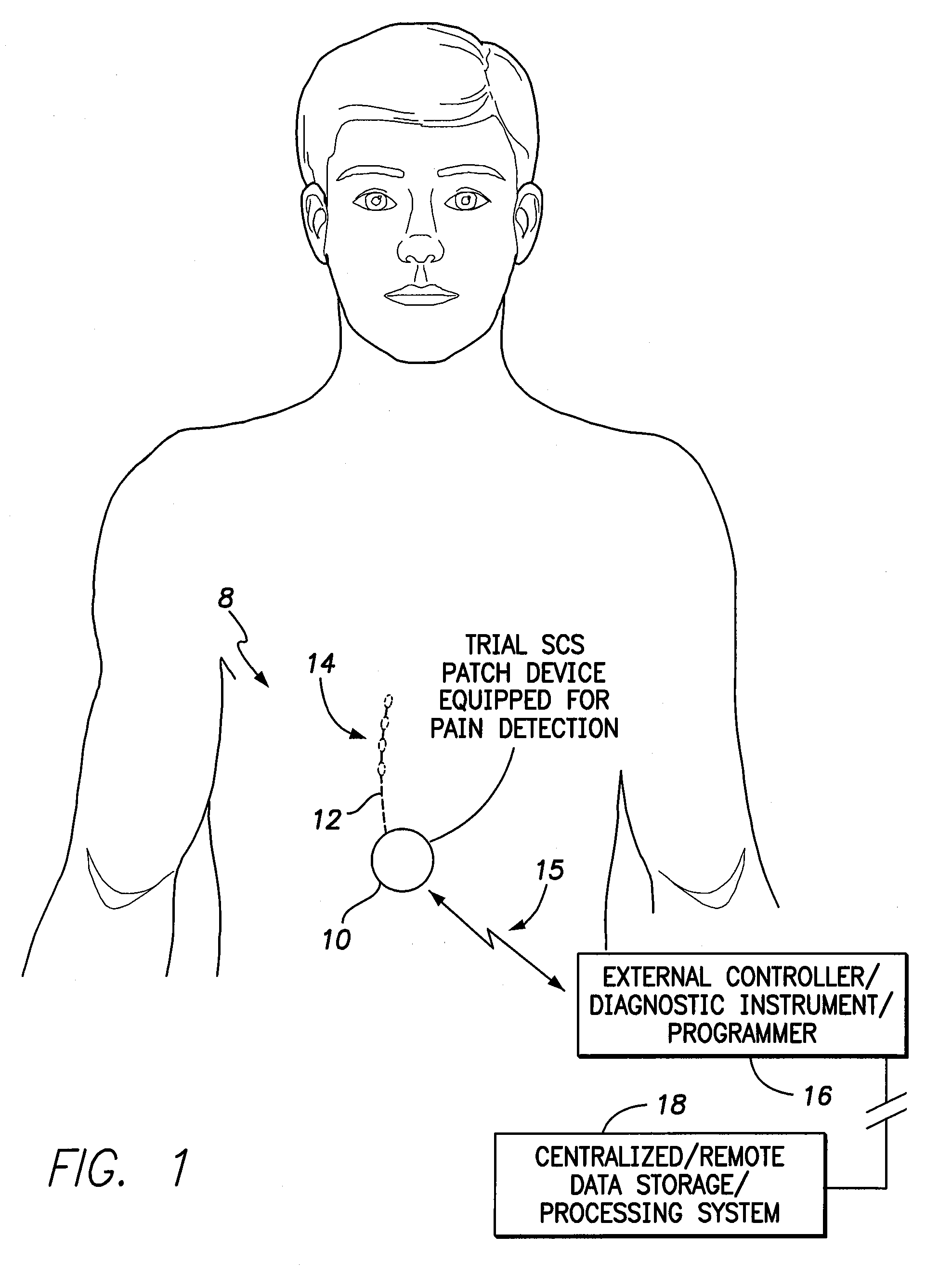
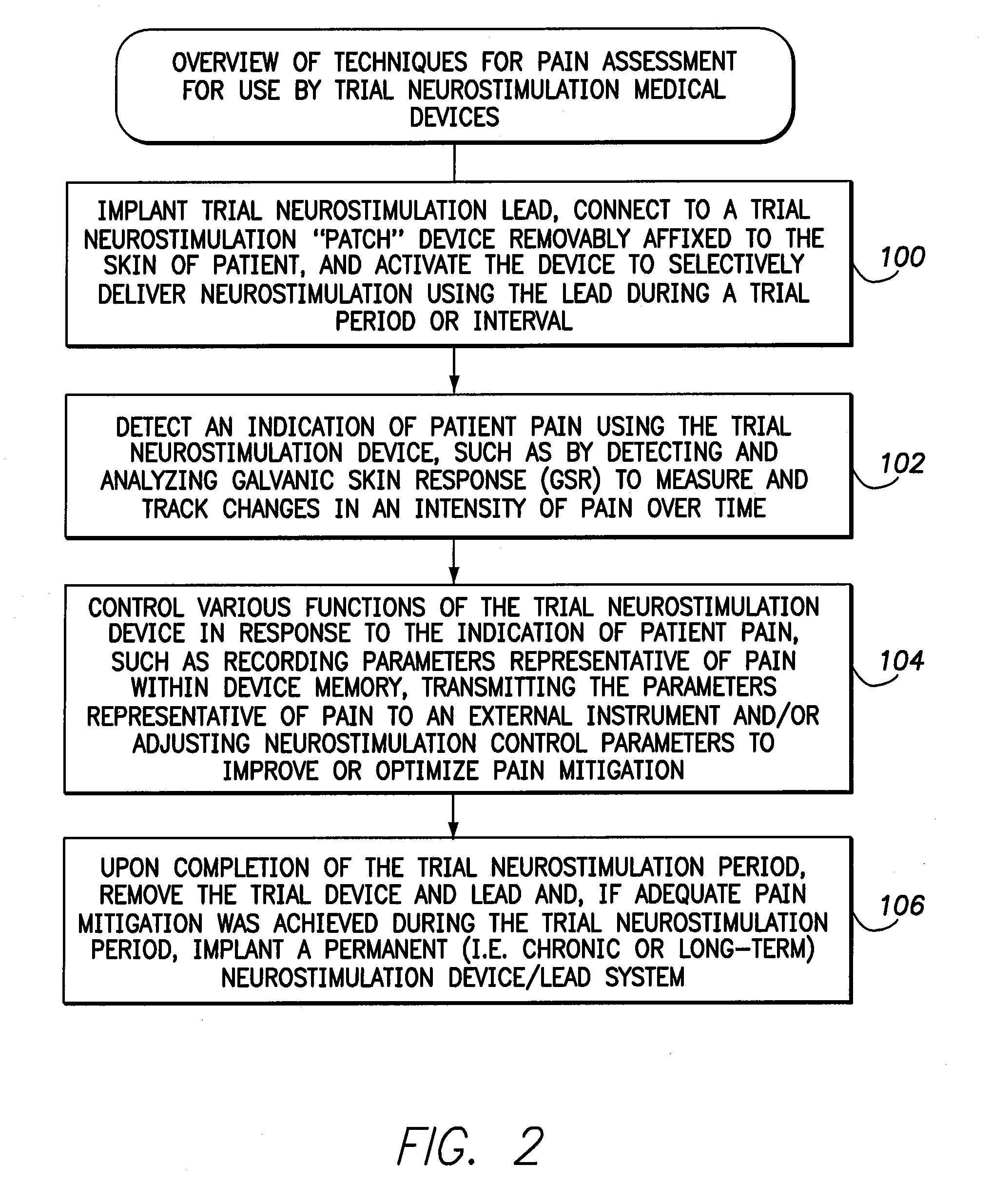
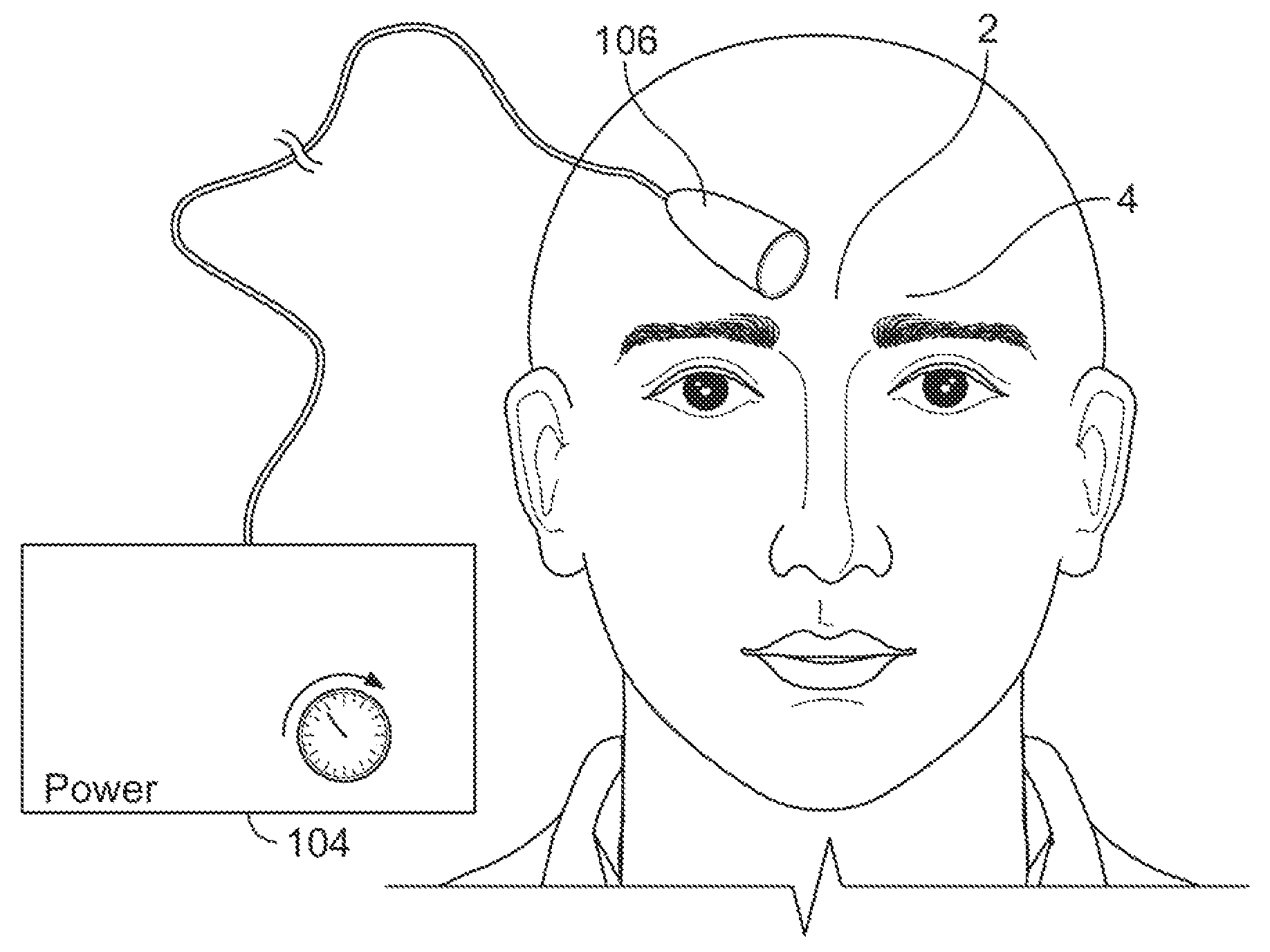
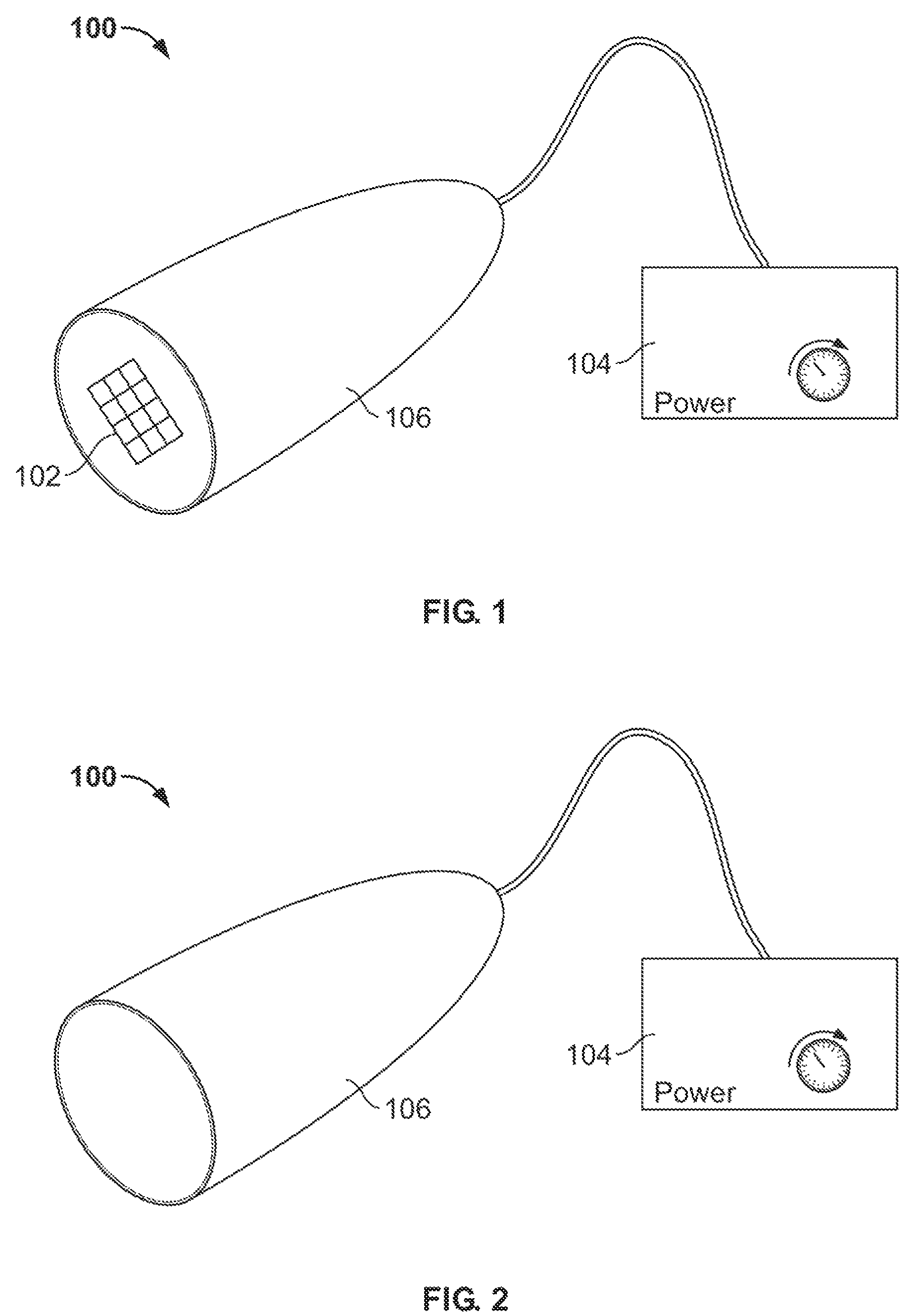
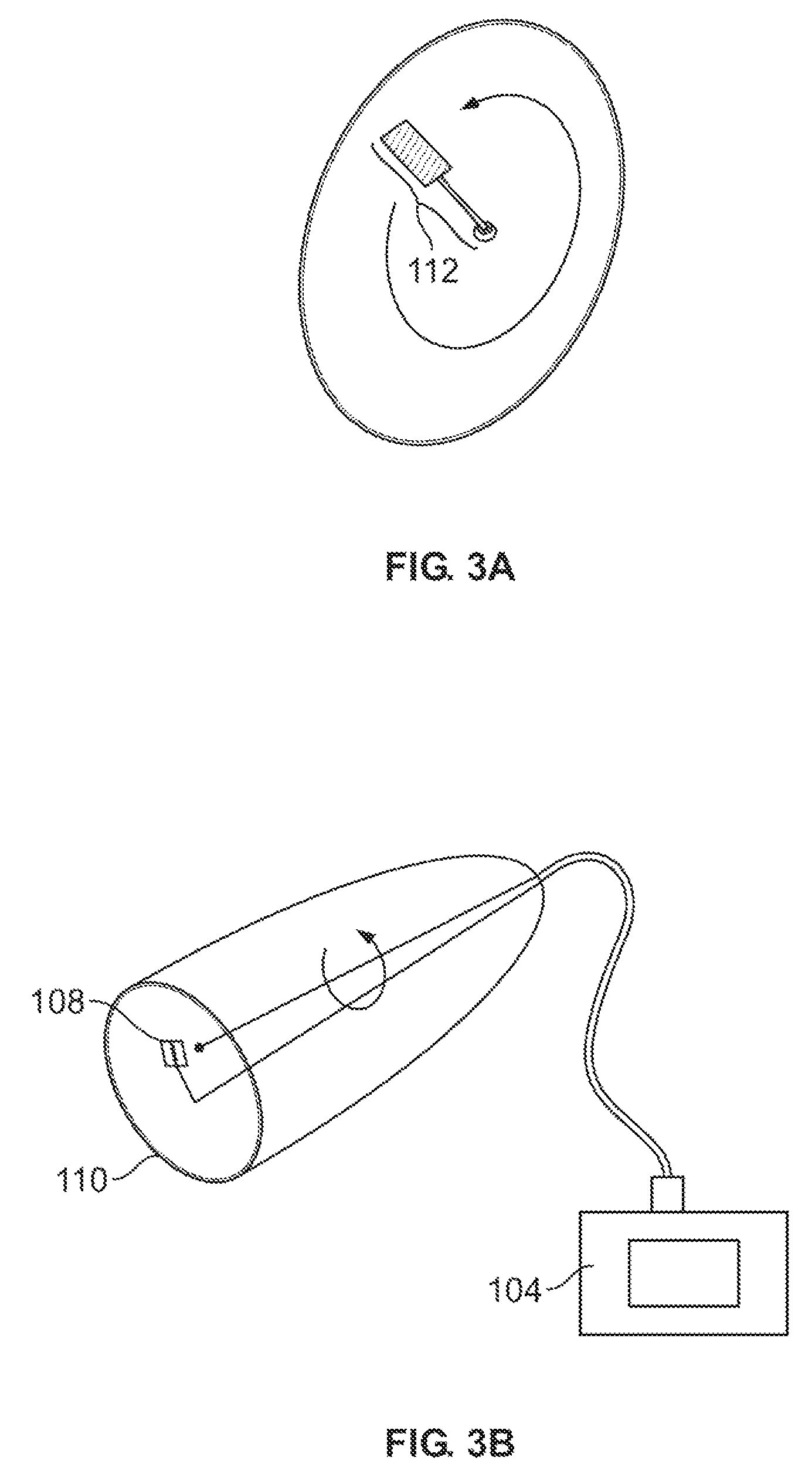
Systems and methods for assessment of pain and other parameters during trial neurostimulation
InactiveUS20150273215A1Reduce and minimize painConveniently detected and assessedSpinal electrodesEvaluation of blood vesselsDiagnostic informationPain reduction
Techniques are provided for use with a trial neurostimulation device having a lead for implant within a patient. In one example, neurostimulation is delivered using the lead while an indication of patient pain is detected. Various functions of the trial device are then controlled in response to patient pain, such as by adjusting neurostimulation control parameters to improve pain reduction, recording diagnostic information representative of patient pain or transmitting such parameters to a separate external instrument for analysis. In this manner, patient pain is automatically detected to provide objective feedback as to the efficacy of trial neurostimulation. Various embodiments of flexible trial neurostimulation device patches are described herein, including patches that are adhesively mounted over the point of entry of the trial lead into the patient, thus providing a comfortable patch that hygienically isolates the point of entry of the lead.
Owner:PACESETTER INC
Location and deactivation of muscles
InactiveUS8521295B2High resolutionEliminate needUltrasound therapySpinal electrodesHeadachesBiological activation
Methods and devices for treatment to at least interfere with the function of a muscle are described herein. These methods and devices may have application in cosmetic and plastic surgery, dermatology, suppression of tension and / or migraine-type headaches, pain management, one particular application of the subject matter deals primarily with reducing wrinkles caused by ongoing muscular activation. The devices and methods described herein allow medical practitioners to effectively identify selective nerves for paralyzingmuscles, without the need for injections of agents such as botulism toxin. Moreover, the devices and methods herein, may allow for artificial generation of signals in nerves that were otherwise damaged by stimulating transmission of nerve signals across damaged nerves.
Owner:LAUFER MICHAEL D
Electrical neurostimulator package
Described herein is technology relevant to the field of implantable electrical neurostimulators. For example, embodiments include but are not limited to cochlear implants, implantable hearing aids, deep brain stimulators and pain control devices, as well as constituent components of such devices, methods for their manufacture, and methods for their use. For the present purposes, embodiments are described particularly by reference to exemplary cochlear implants, although that should not be regarded as necessarily limiting on the underlying concepts.
Owner:NEUROSTIMULATION DEVICES & TECH
Transdermal pain control method and device
Compositions comprising skin-permeable pharmaceutically effective amounts of an opioid agonist; an N-methyl-D-aspartate receptor antagonist and an anti-inflammatory may be incorporated into formulations and devices suitable for transdermal delivery of the active ingredients to alleviate pain.
Owner:INNOVATIVE PHARMA
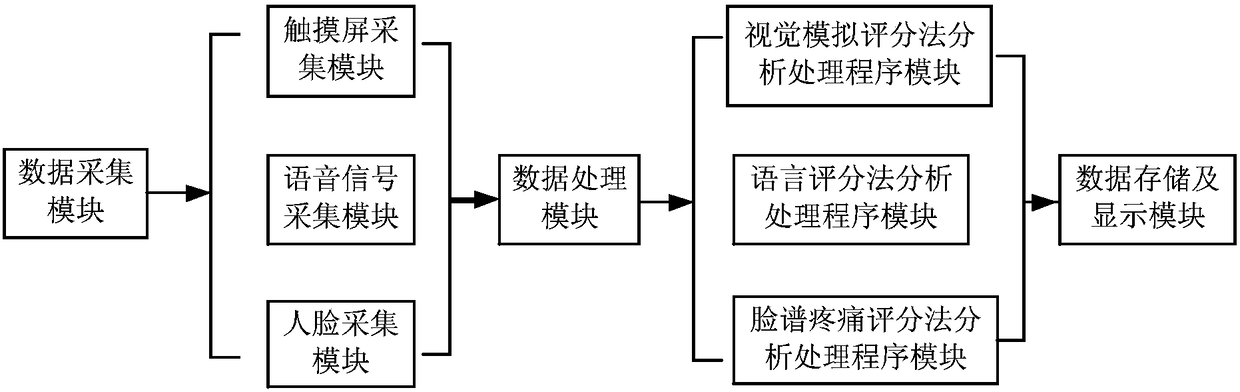
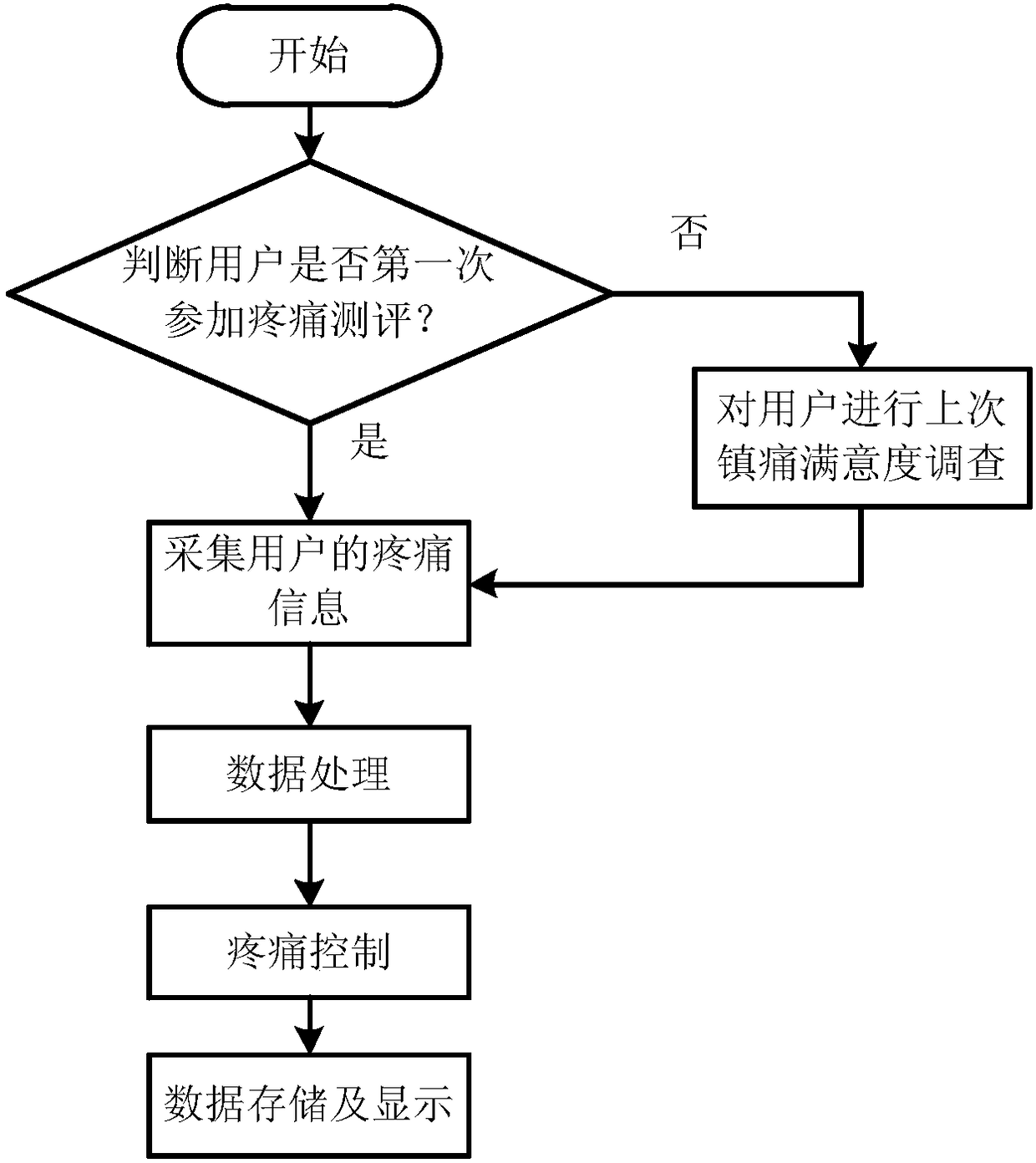
Intelligent and electronic pains coring system and method
InactiveCN108427917AFacilitate statistical communicationEasy to operateMedical communicationSemantic analysisRating systemData information
The invention discloses an intelligent and electronic pain scoring system and method. The system comprises a data acquisition module, a data processing module and a data storage and display module, wherein the data acquisition module is used for acquiring pain data information of a user; the data processing module is used for receiving the pain data information from the data acquisition module, and analyzing the pain data information so as to obtain a pain evaluated value and a pain control requirement of the user; and the data storage and display module is used for storing, displaying and outputting the pain evaluated value and pain control requirement, obtained by the data processing module, of the user and an analgesia control degree. According to the system and method, large-scale evaluation in hospitals and evaluation methods of different hospitals are unified, so that corresponding unnecessary errors are eliminated to facilitate the statistical communication. The system is easy to operate, time-saving, labor-saving and stronger in intuition, is capable of decreasing corresponding workloads, improving the working efficiency, preventing interferences of human factors, and can be directly displayed on display screens.
Owner:NANJING DRUM TOWER HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com