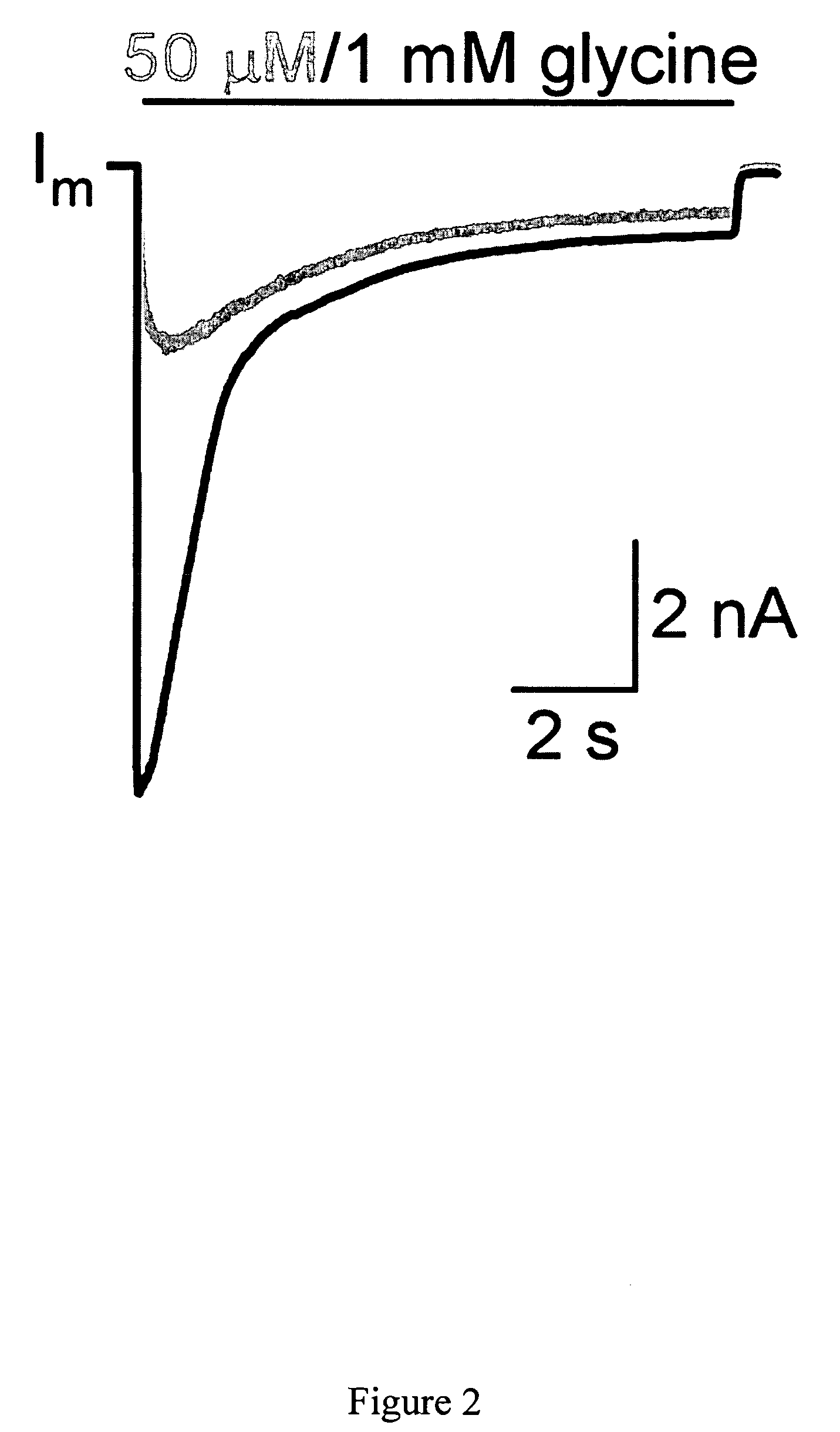Patents
Literature
64 results about "Controlling pain" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Treating Pain with Small Changes Use something that makes you feel better. Check the temperature of your environment. Stay somewhere that is quiet, calm and peaceful. Dress comfortably. Use breathing techniques to control pain. Use distraction as a way to reduce the pain. Relieve immediate pain through distraction. Play a game.
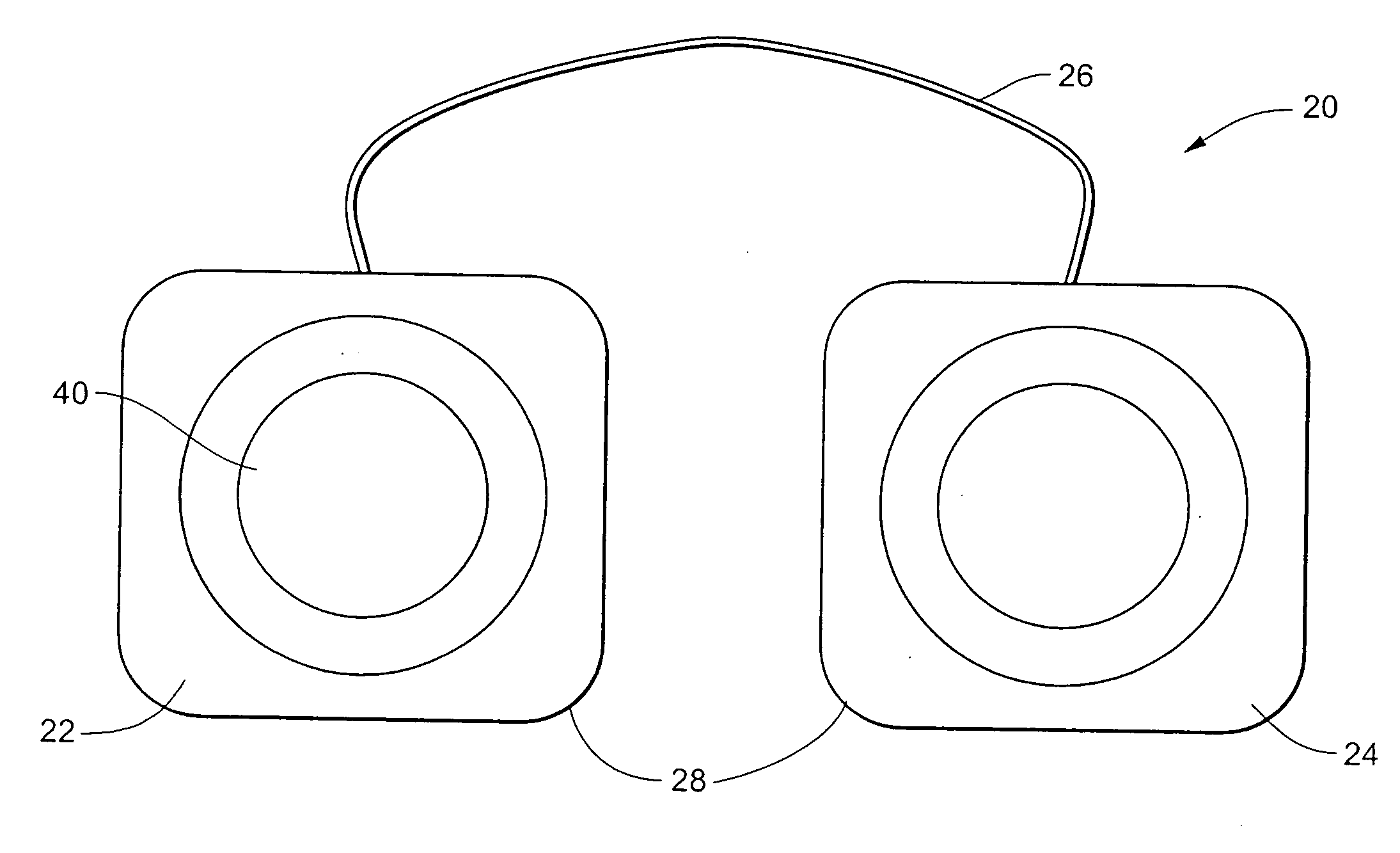
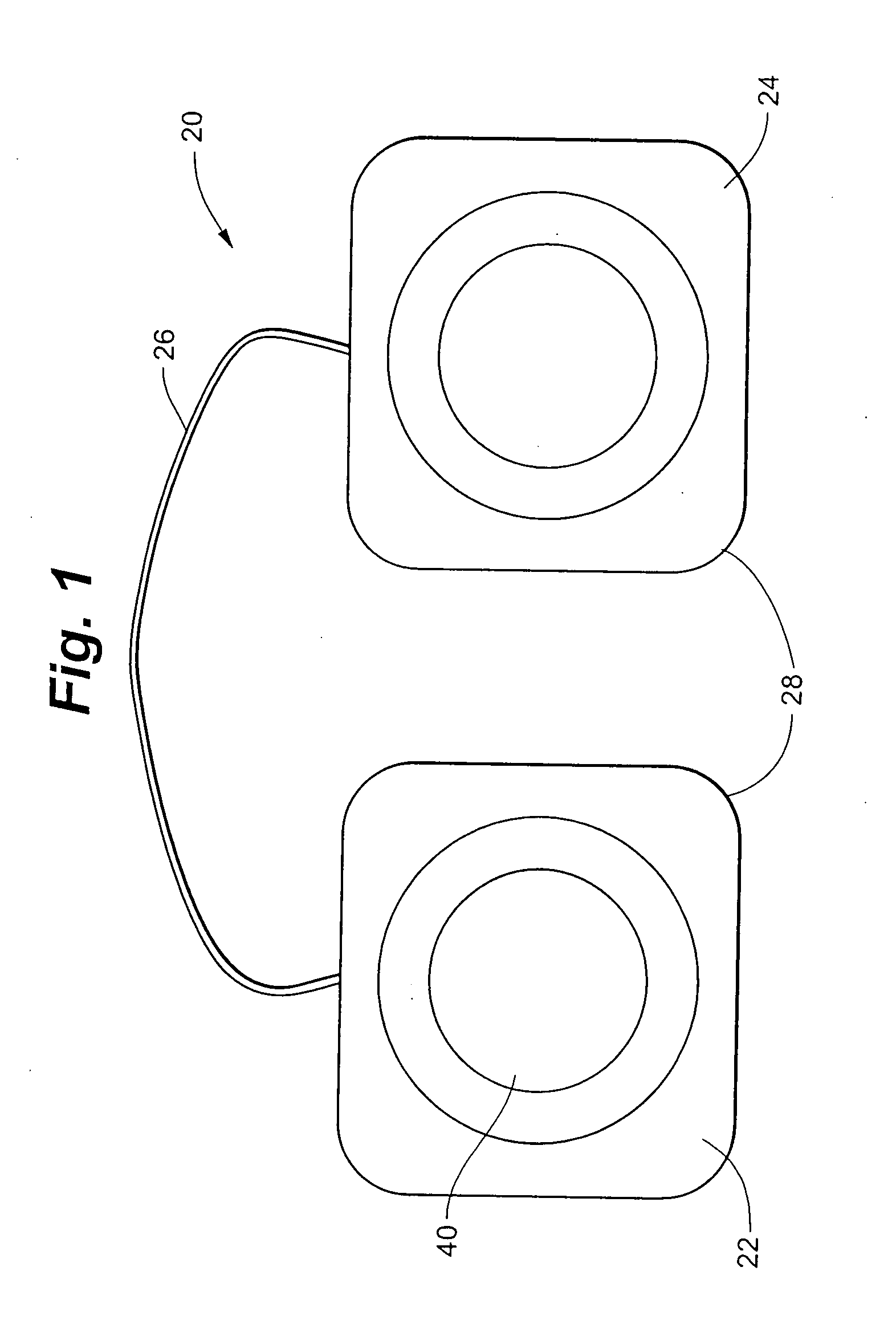
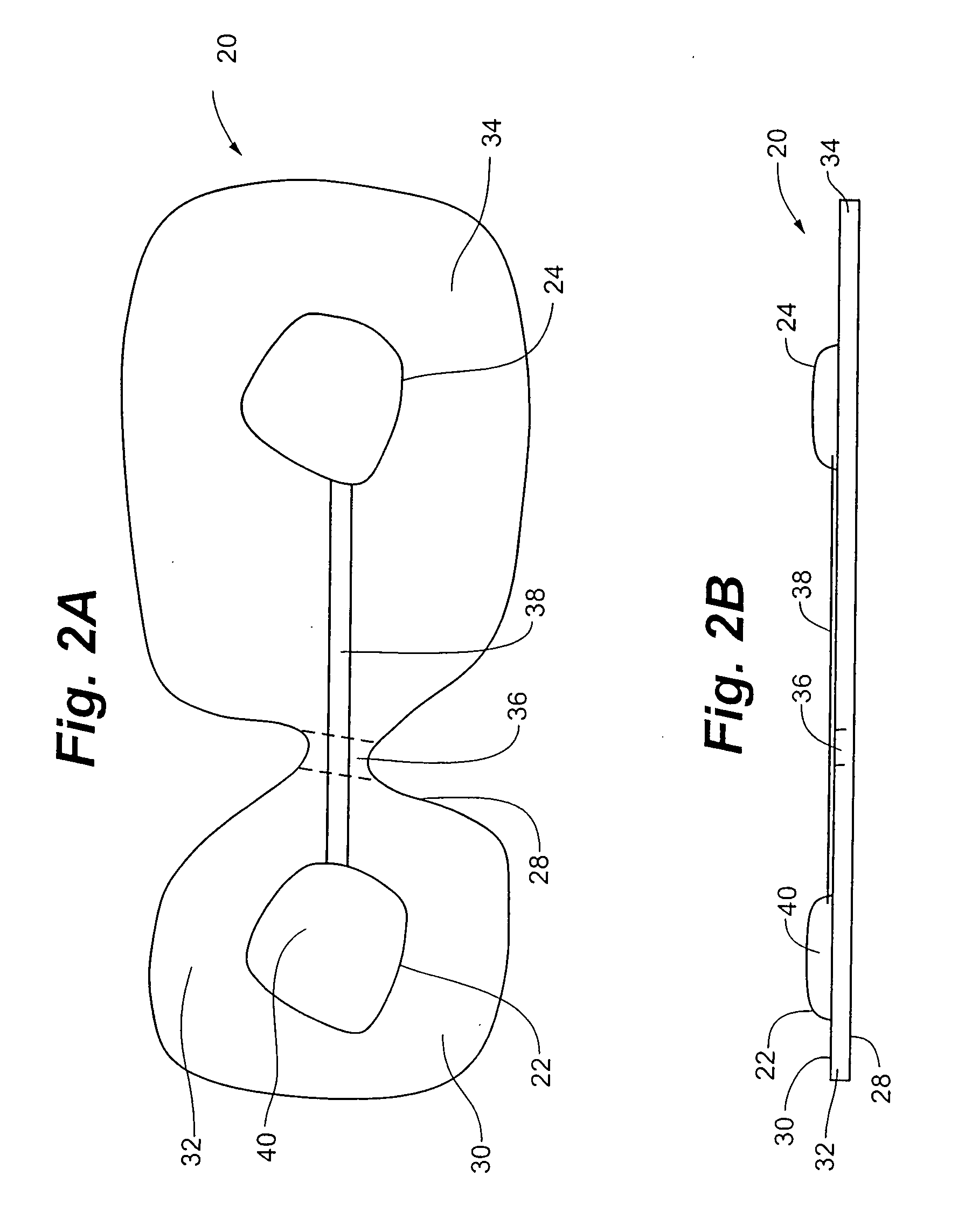
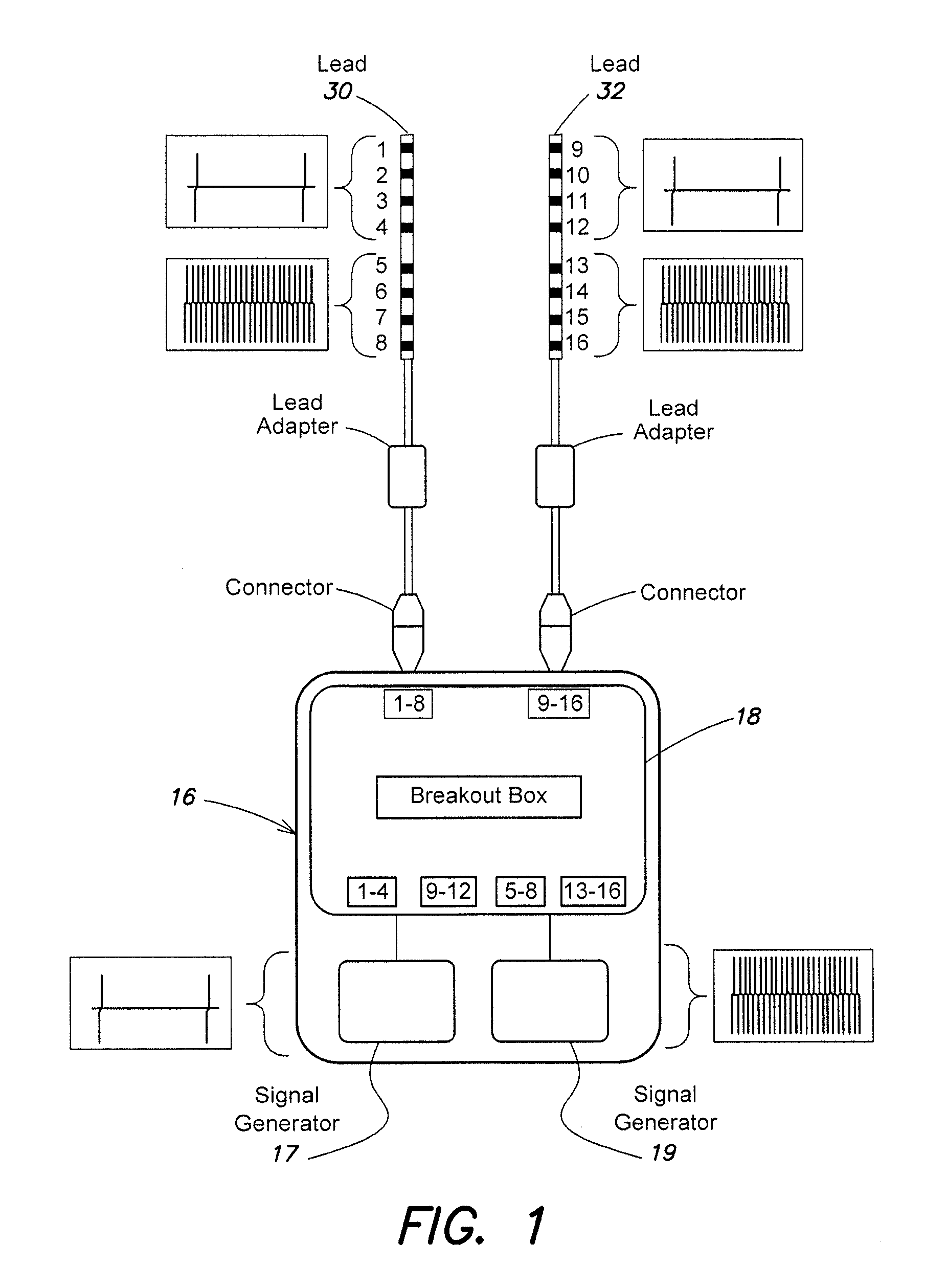
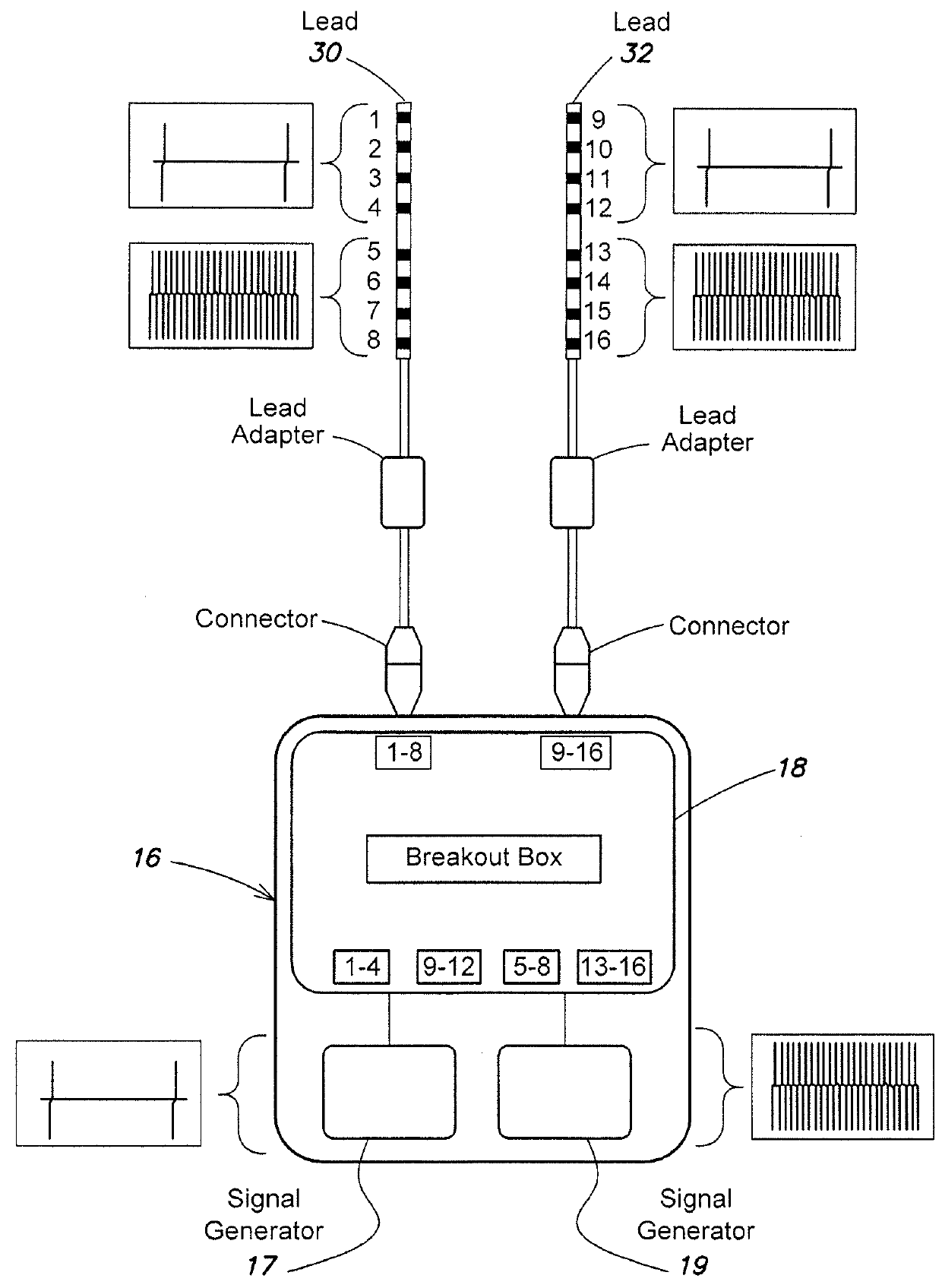
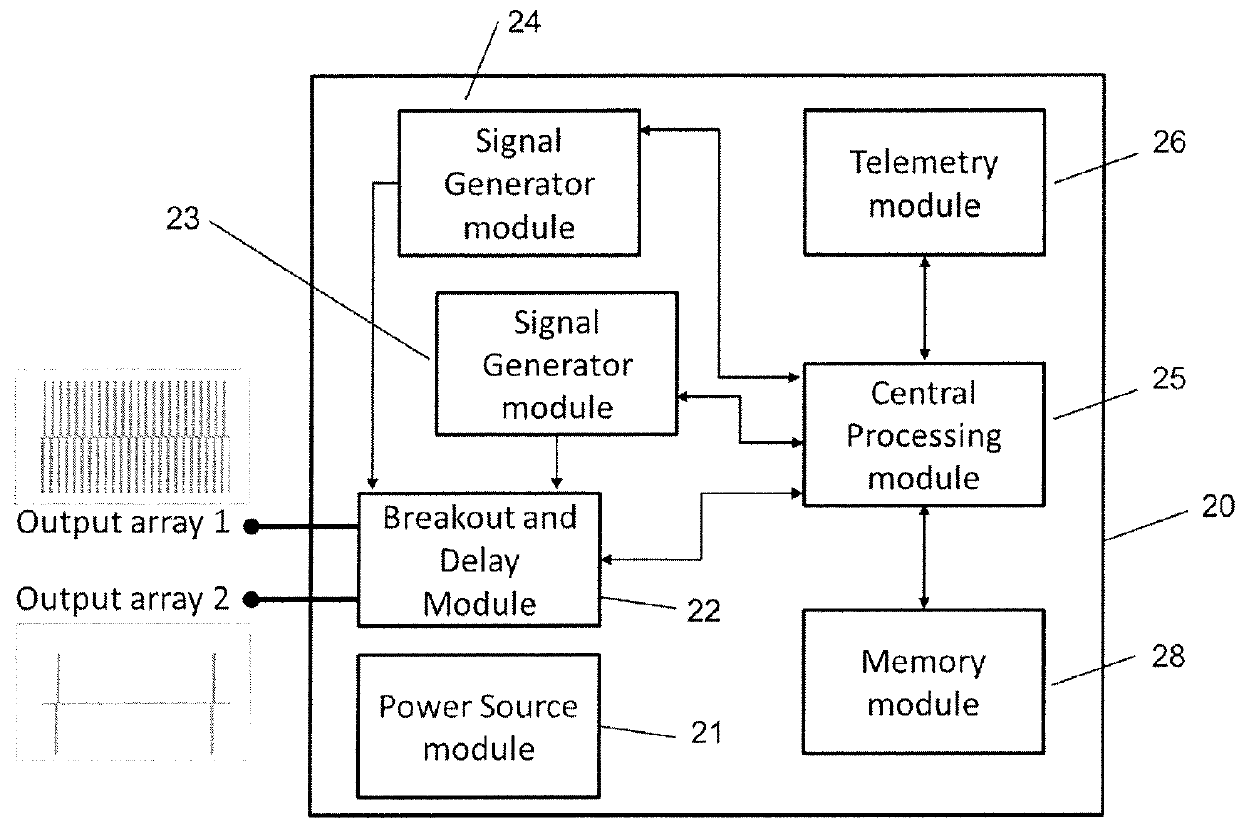
Electrical stimulation device and method for therapeutic treatment and pain management
ActiveUS20100042180A1Easily and tactilely differentiateAvoid accidental activationExternal electrodesElectricityManaged pain
A disposable electrical stimulation device and method for providing therapeutic treatment and pain management in a convenient, compact configuration. Electrode size and shape and relative configuration can be varied according to an intended application and use, or a universal configuration can be provided for use on almost any area of the body. The common structure of communicatively coupled dual electrodes including control circuitry and a power source accommodates a range of different sizes, configurations, stimulation treatment intensities, and other physical and electrical characteristics that can be pre-customized and packaged for specific, limited time use. The device can therefore be used in methods of providing therapy, managing pain, and achieving other treatment goals by electrical stimulation.
Owner:ENCORE MEDICAL ASSET CORP +1
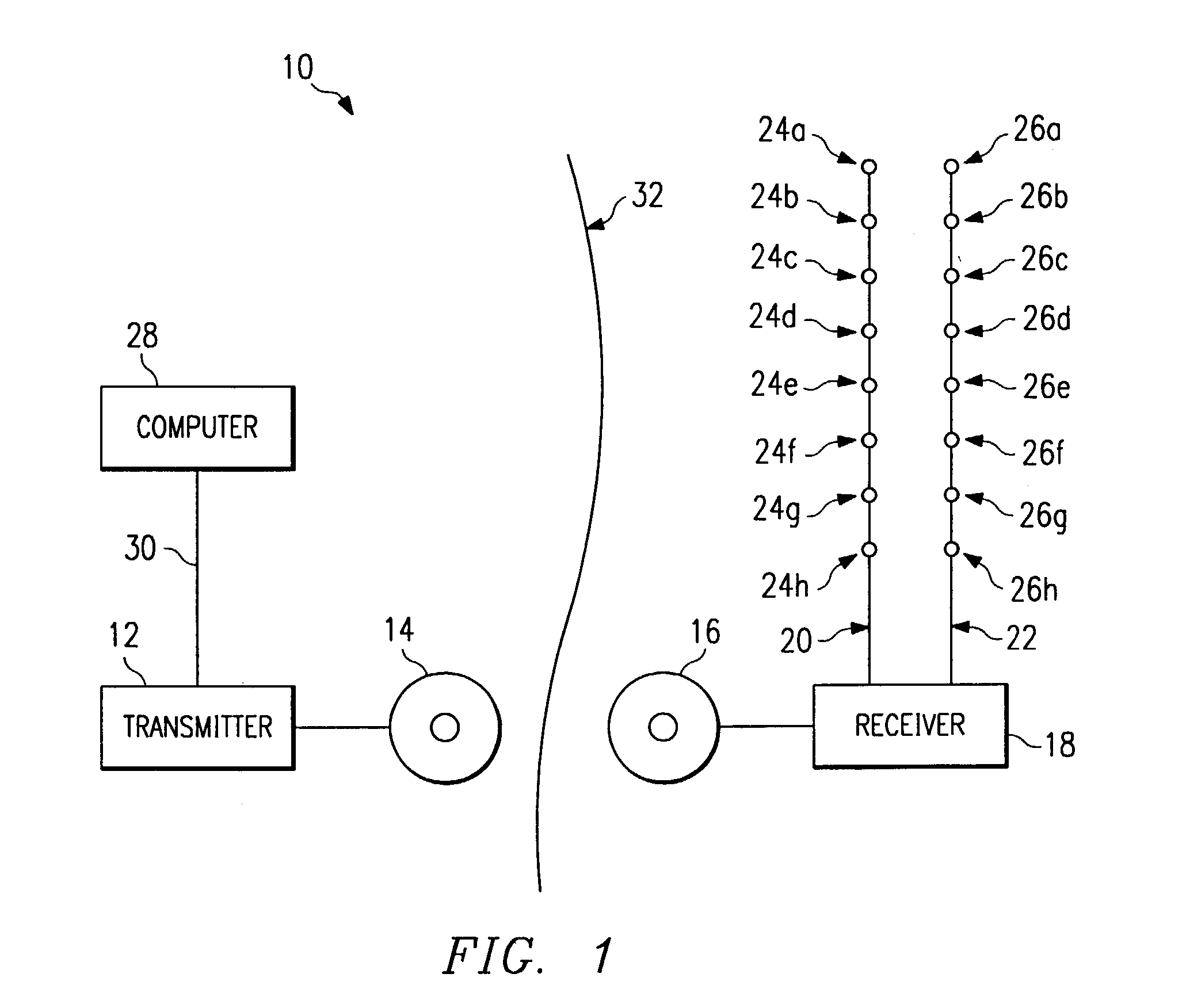
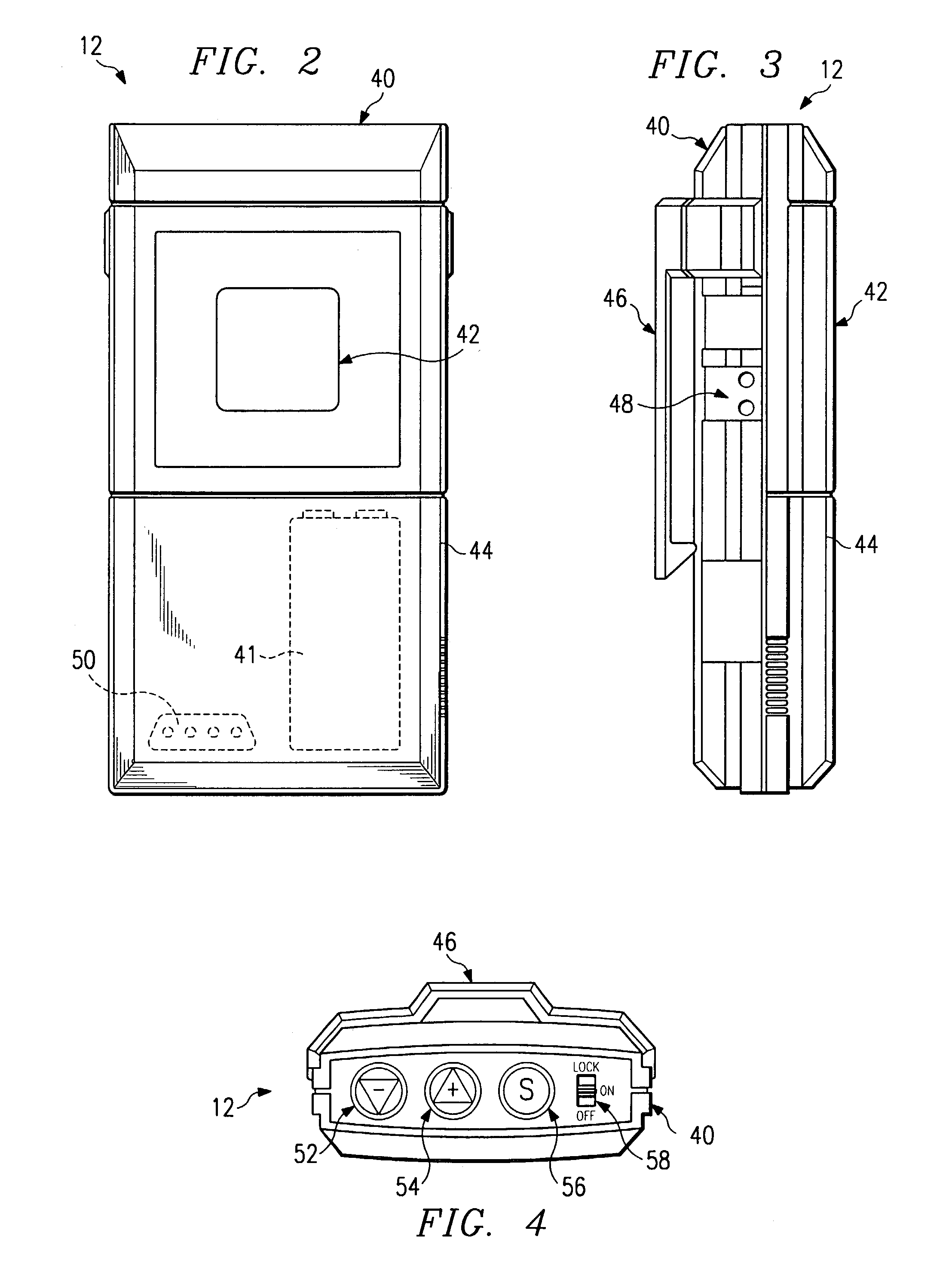
Multiprogrammable tissue stimulator and method
An electronic stimulation system is used to control pain over multiple regions of a patient's body. The system has one or more percutaneous leads, each having multiple electrodes, implanted within the patient's epidural space parallel to the axis of the spinal cord. The leads are connected to either a totally implanted system or a radio frequency system. The system is able to treat pain over different regions of a patient's body by “simultaneously” stimulating the patient with at least three different stimulation settings. “Simultaneous” stimulation involves sequentially stimulating the patient with the multiple stimulation settings such that the patient receives the cumulative effect of each stimulation setting, while not perceiving the transition from one stimulation setting to another.
Owner:ADVANCED NEUROMODULATION SYST INC
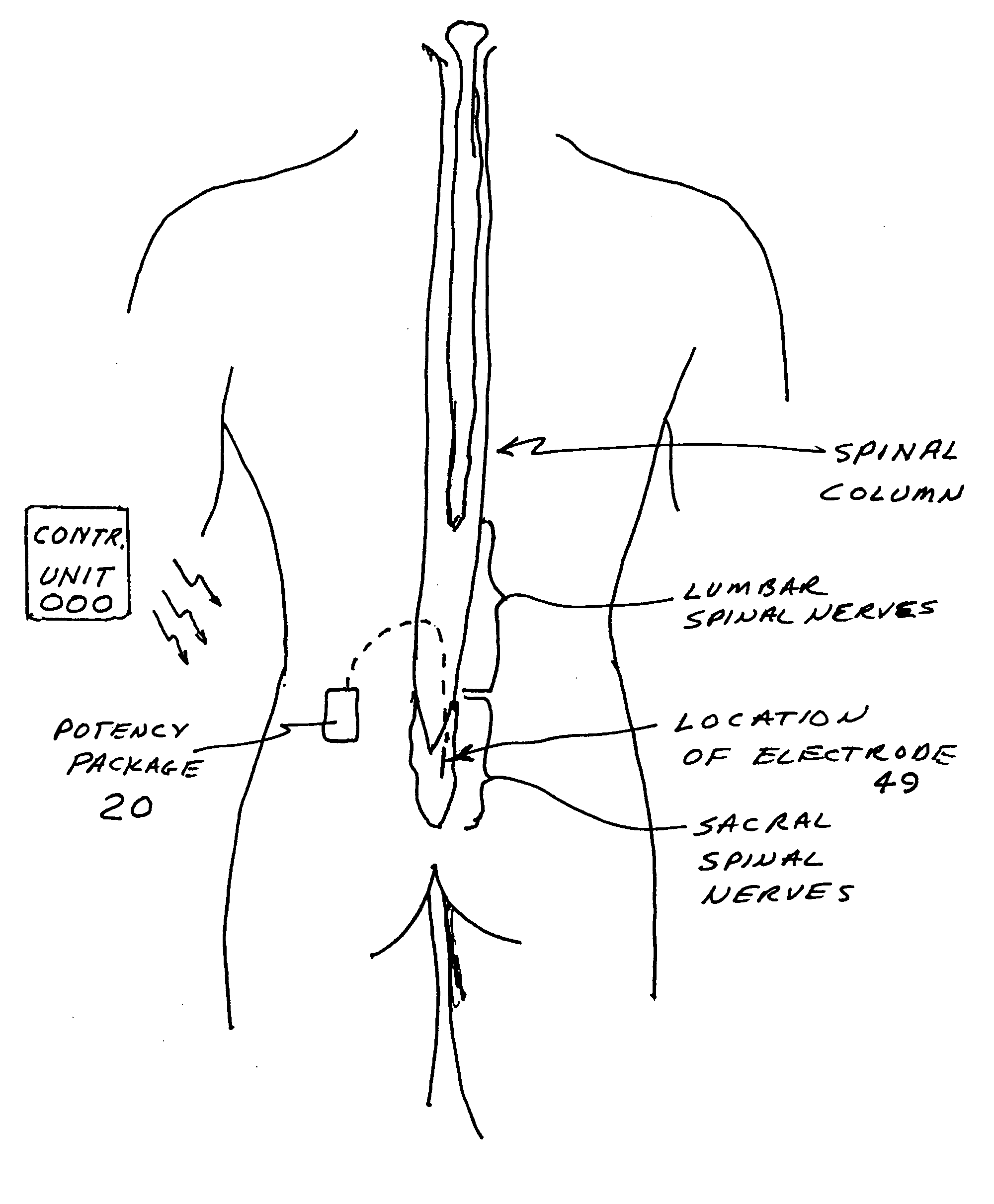
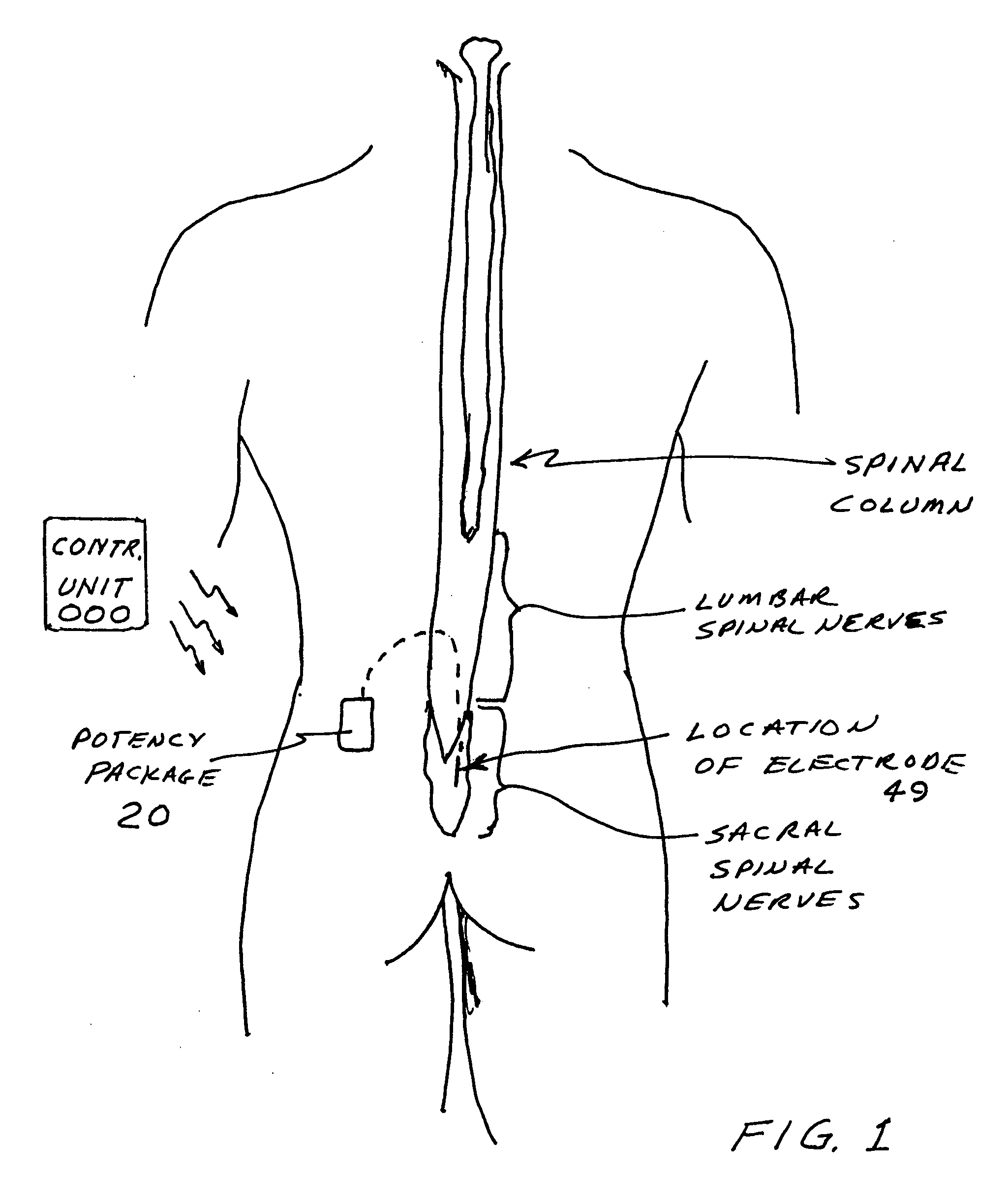
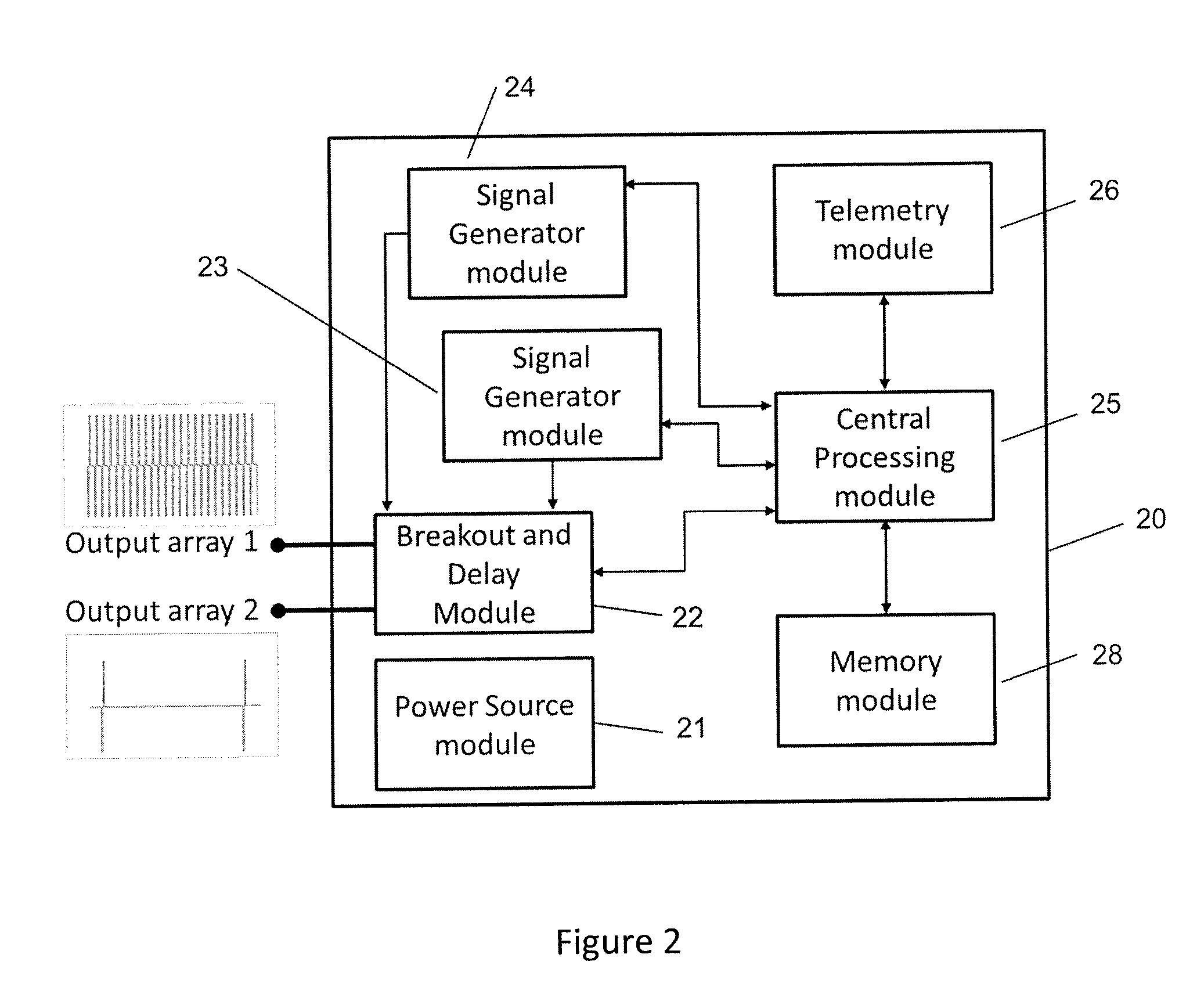
Implantable device for pain control and other medical treatments
InactiveUS20050222628A1Stimulates arousalEscalationElectrotherapyArtificial respirationControl signalImplanted device
A device for pain control or other medical treatments of a patient. The device includes an implantable unit for implantation under the skin of a patient. The implantable unit includes a drug chamber containing a fluid drug, a tube for delivering the fluid drug to a location in the patient's body, an electronic pump for pumping the drug through the tube to the location, a programmable processor for controlling the pump, and an electronic receiver for receiving control signals from outside the patient's body and delivering the control signals to the processor. The device also includes a controller located outside the patient's body for transmitting control signals to said receiver. In preferred embodiments the implantable device also comprises an electrical pulse generator and at least one electrode implanted at a location in said patient's body and connected to said pulse generator wherein said processor is programmed to control said pulse generator to deliver electrical pulsed to said electrode. In a preferred embodiment for the control of pain, the implantable chamber contains a refillable pain-relieving drug that is placed inside the chamber.
Owner:KRAKOUSKY ALEXANDER A
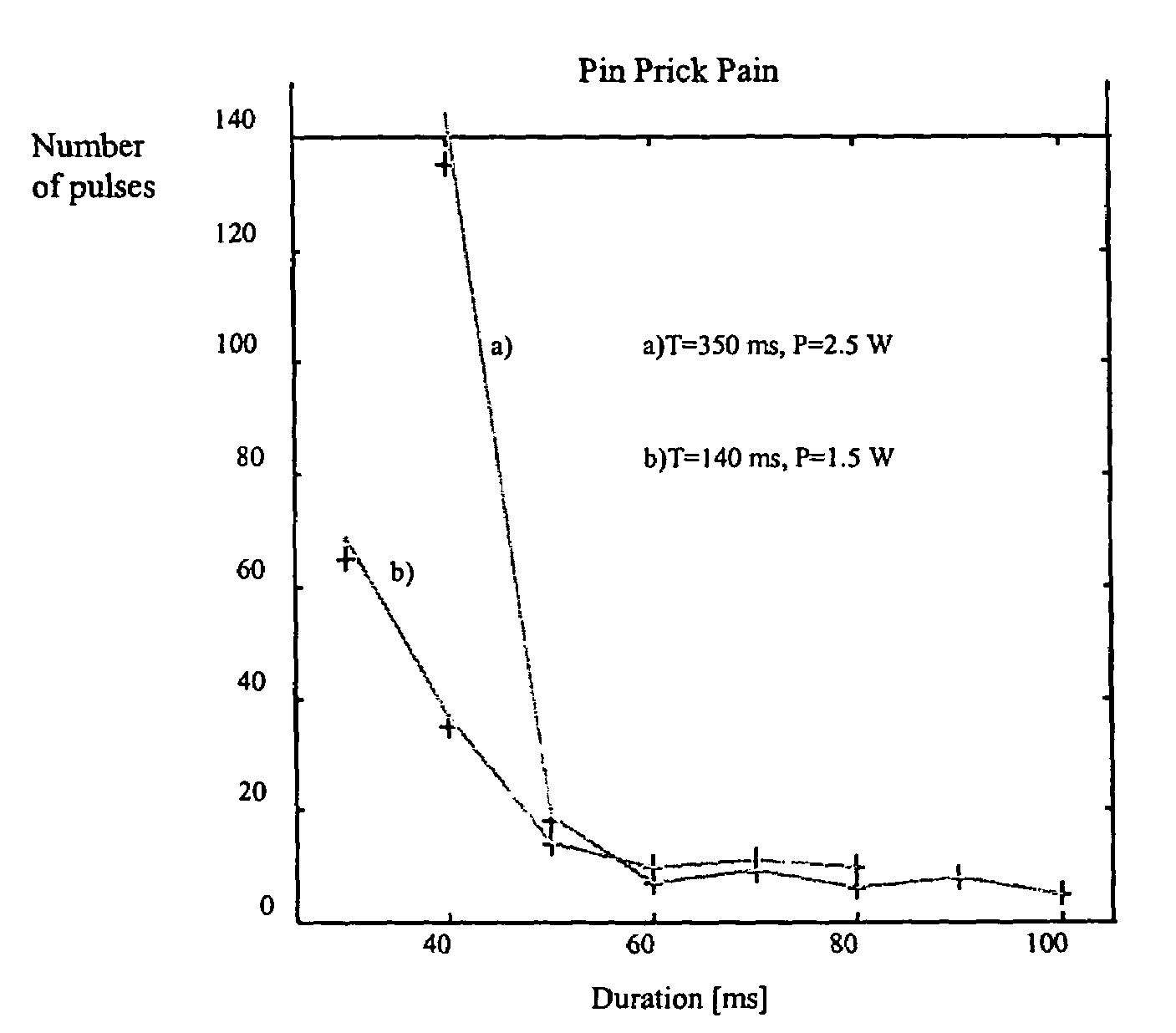
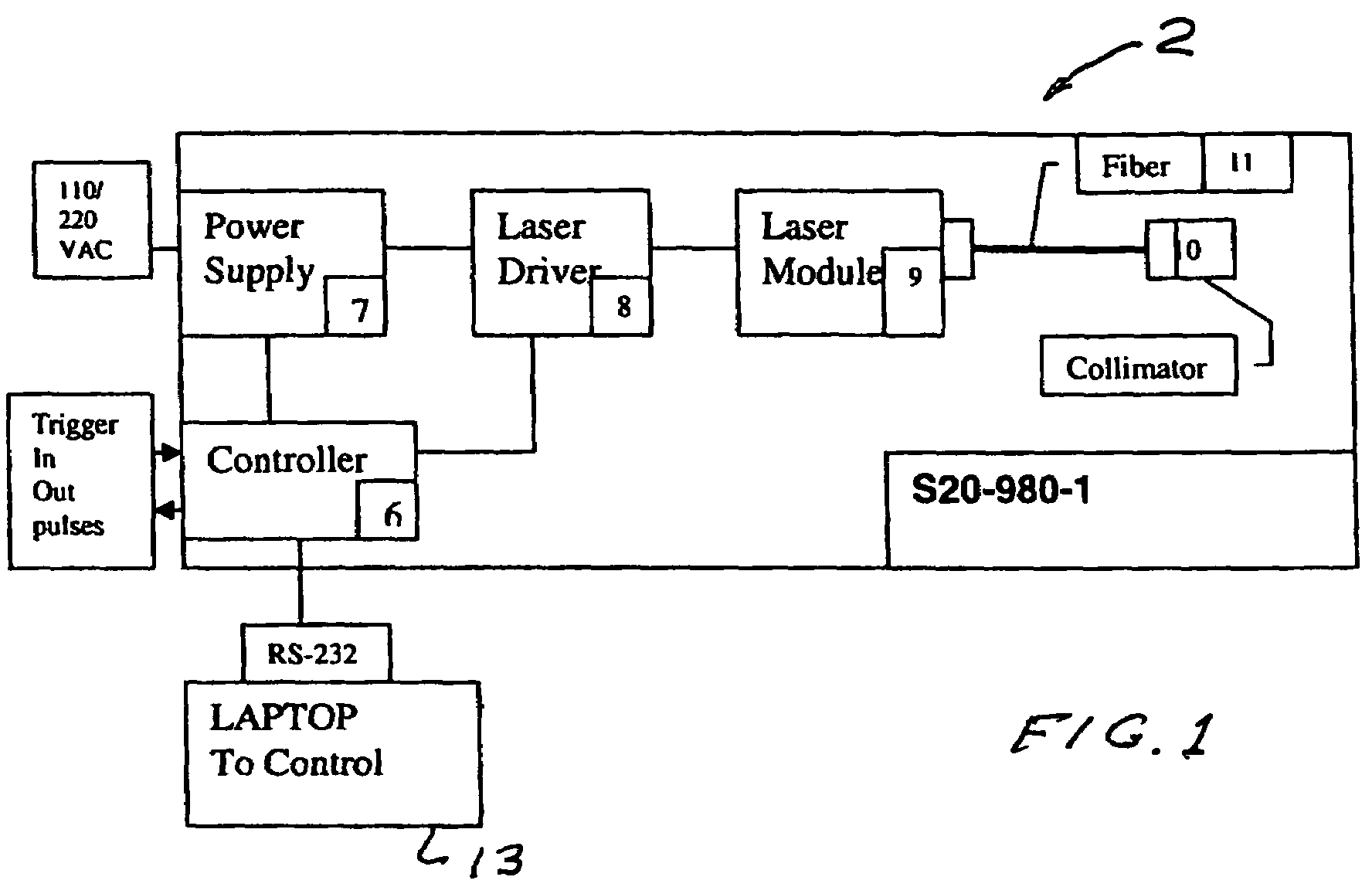
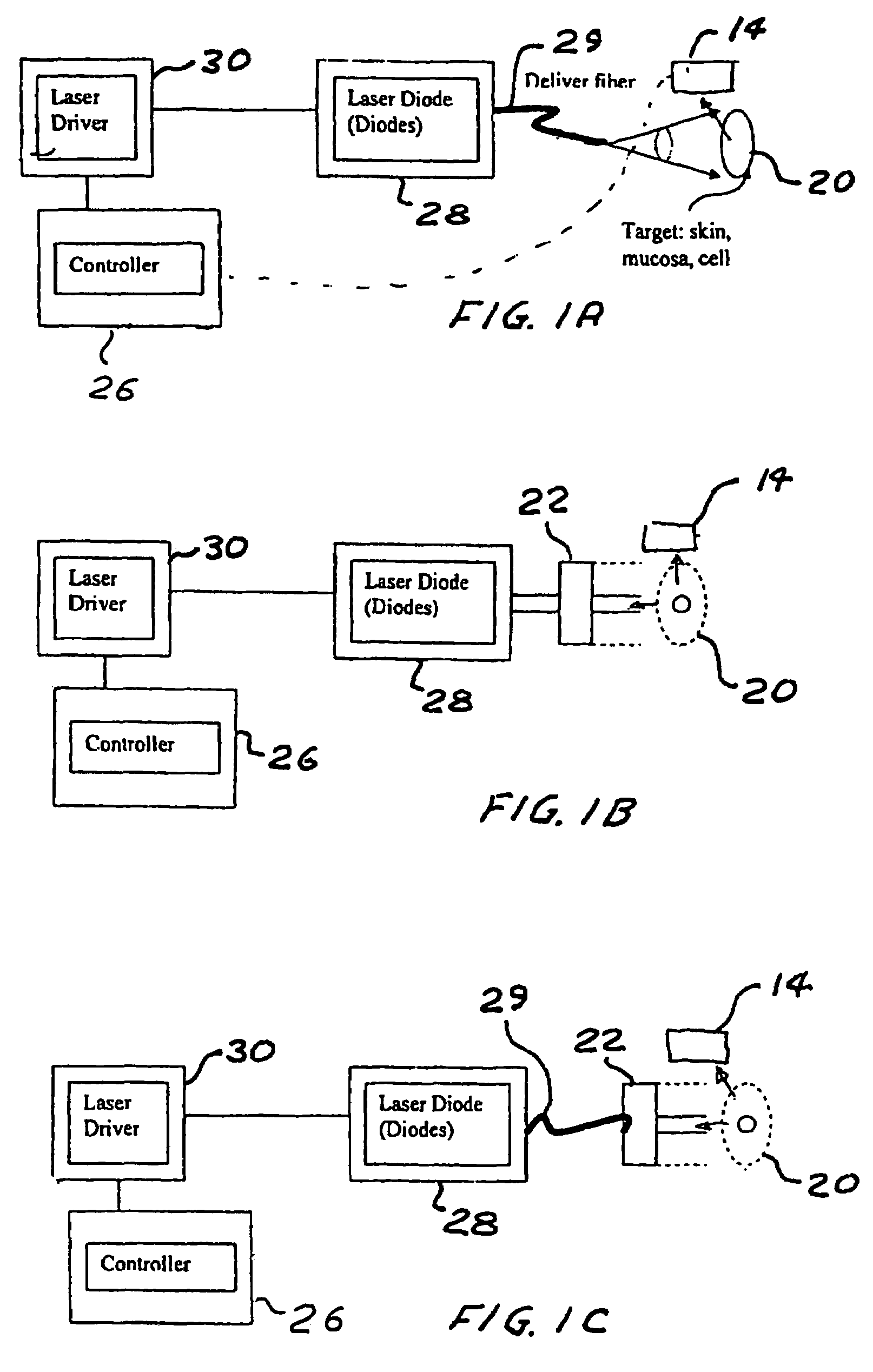
Portable laser and process for producing controlled pain
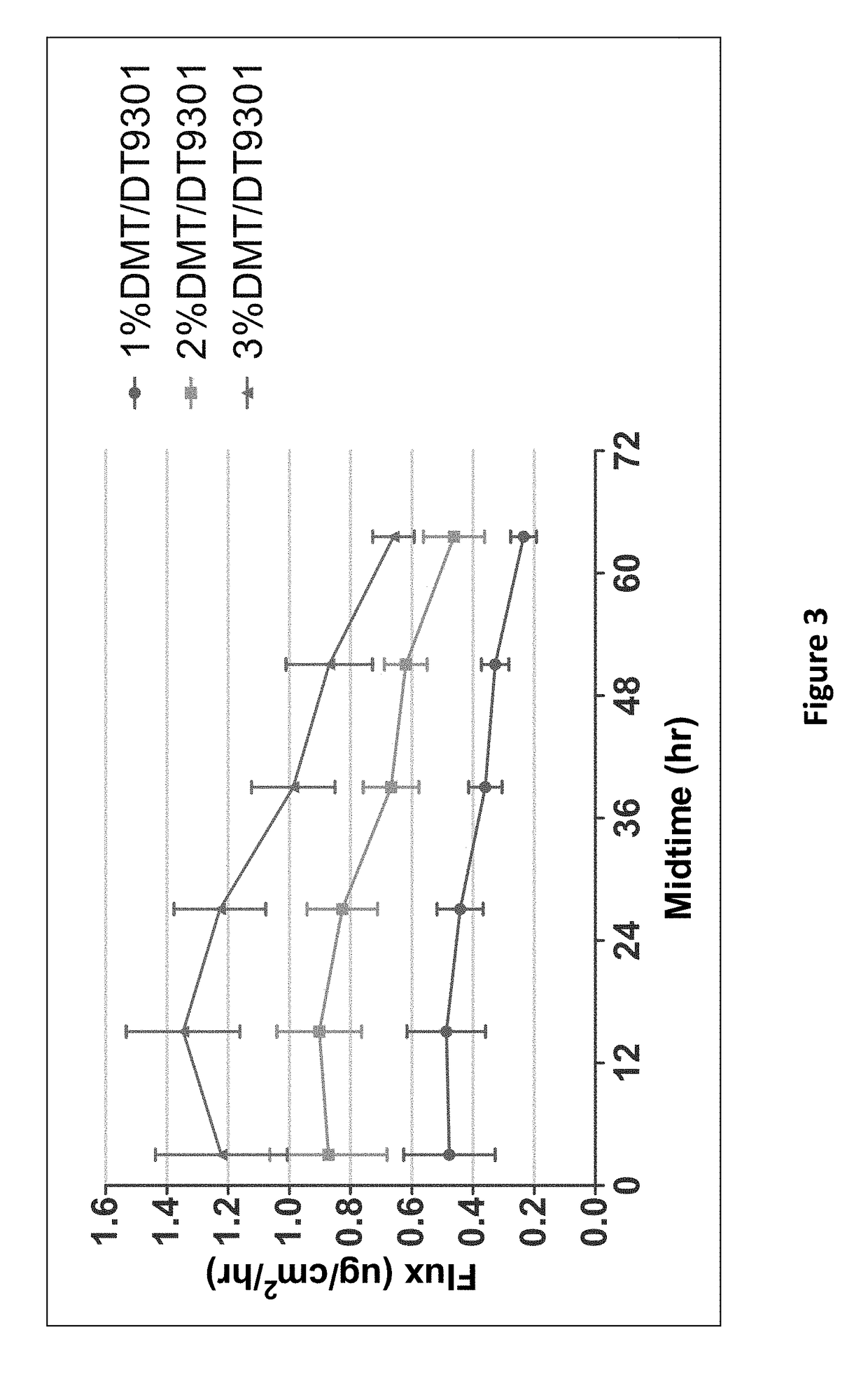
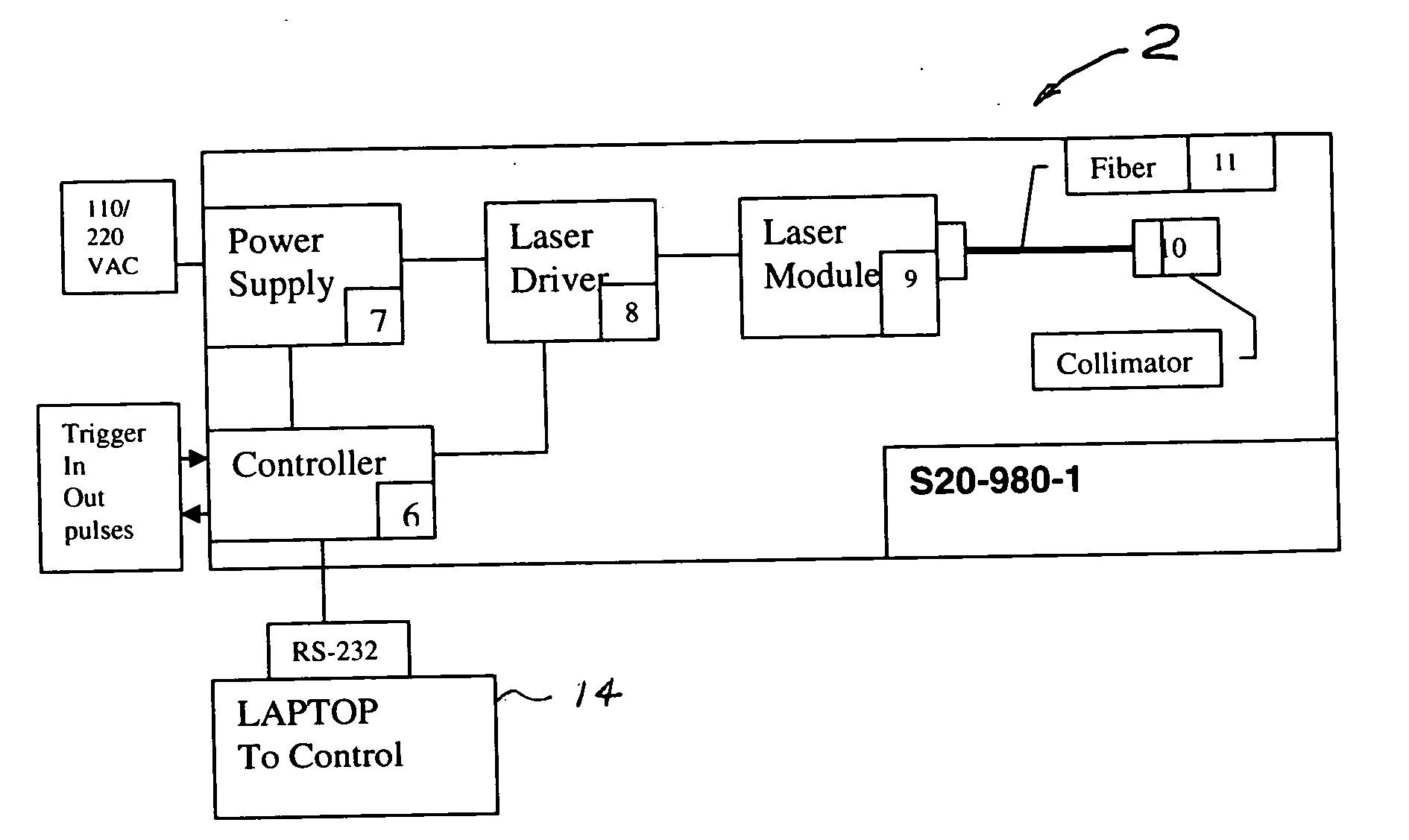
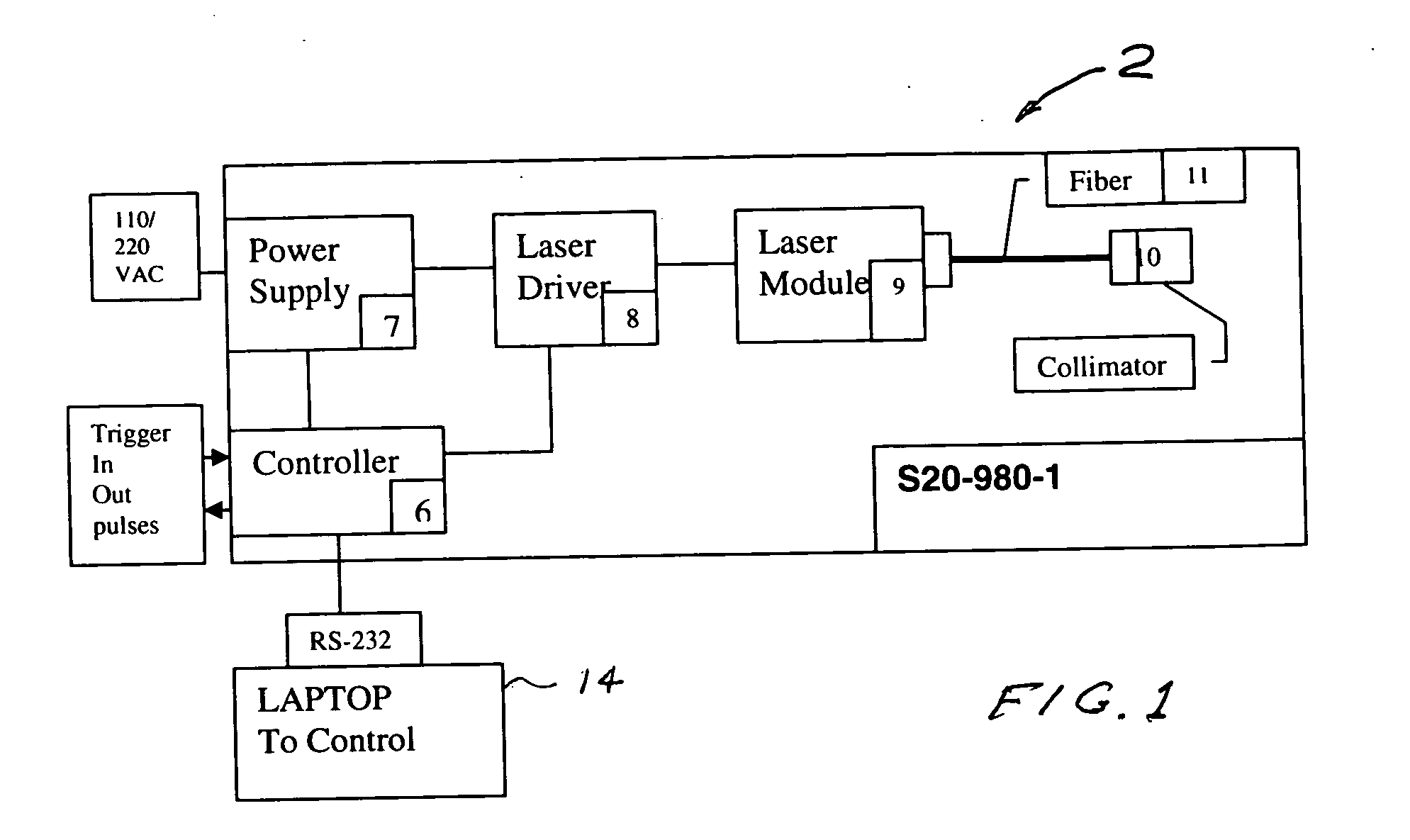
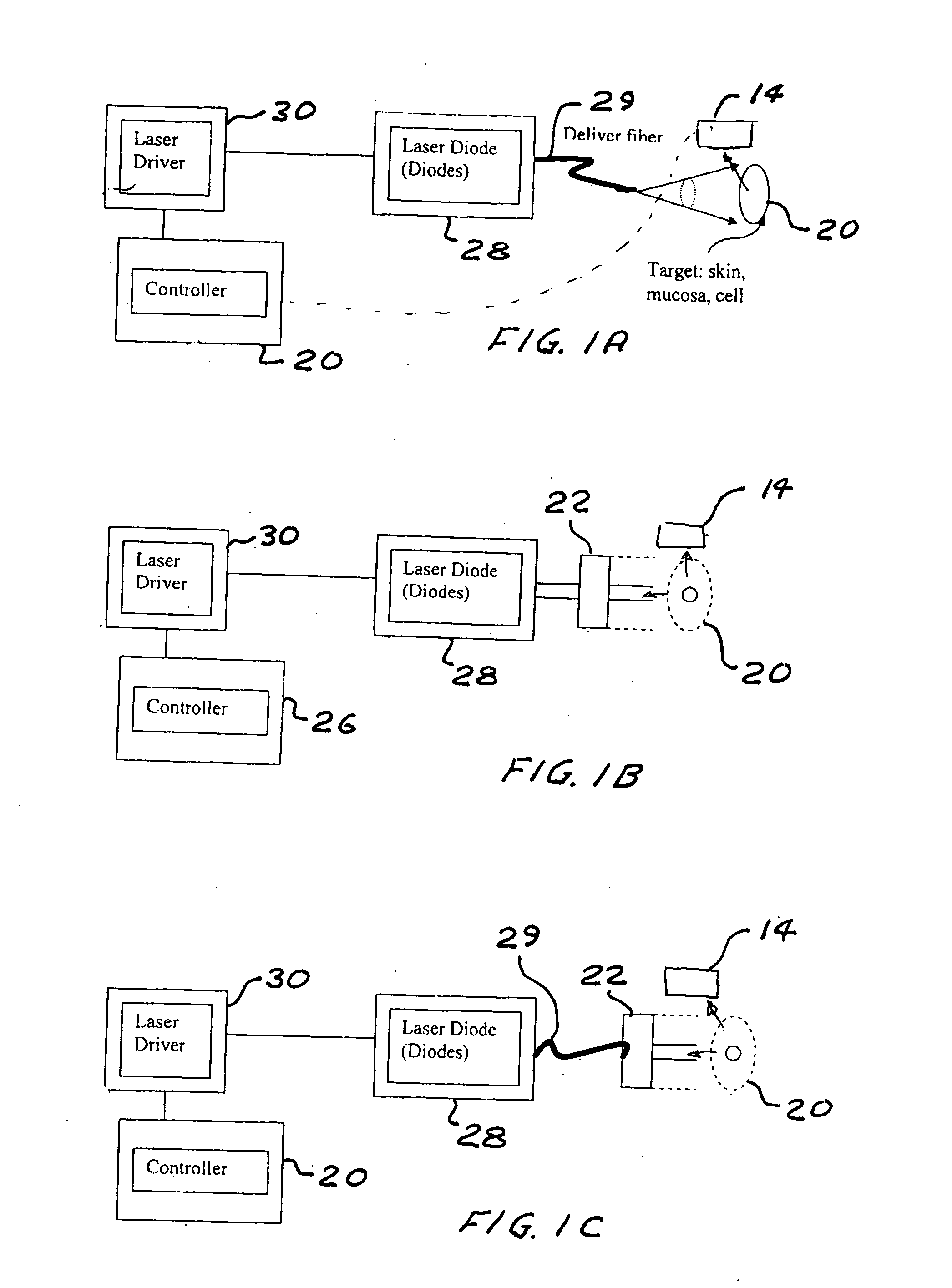
A process and laser system for in vitro and in vivo pain research, pain clinical testing and pain management. In preferred embodiments of the present invention a diode laser operating at a 980 nm wavelength is used to produce warmth, tickling, itching, touch, burning, hot pain or pin-prick pain. The device and methods can be used for stimulation of a single nerve fiber, groups of nerve fibers, nerve fibers of single type only as well as more the one type of nerve fibers simultaneously. The present invention is especially useful for research of human / animal sensitivity, pain management, drug investigation and testing, and psychophysiology / electrophysiology studies. The device and methods permit non-contact, reproducible and controlled tests that avoid risk of skin damage. Applicant an his fellow workers have shown that tests with human subjects with the process and laser of the present invention correlate perfectly with laboratory tests with nerve fibers of rats. The device and the methods can be applied in a wide variety of situations involving the study and treatment of pain. Preferred embodiments of the present invention provide laser systems and techniques that permit mapping and single mode activation of C fibers and A-delta fibers.
Owner:NEMENOV MIKHAIL
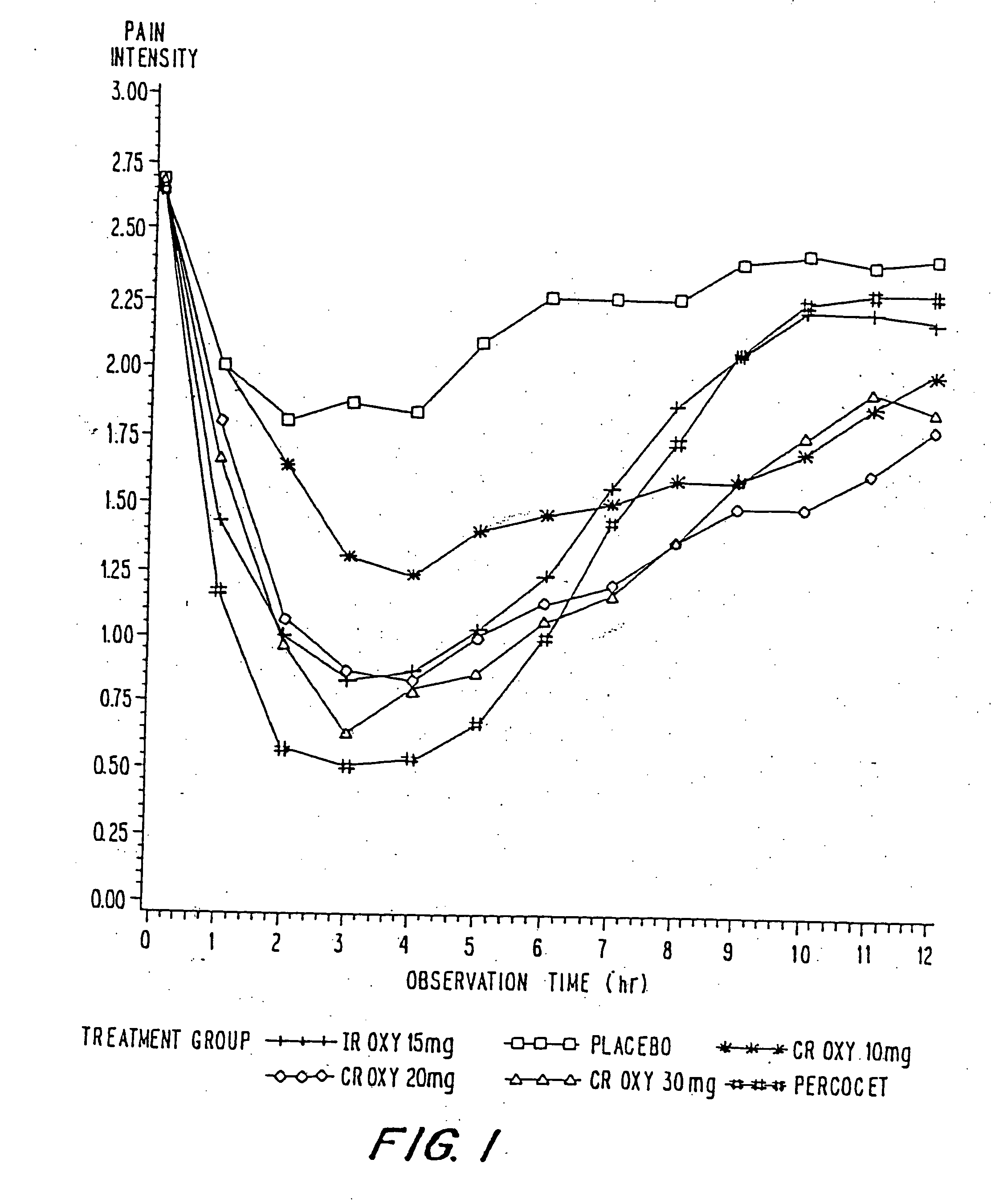
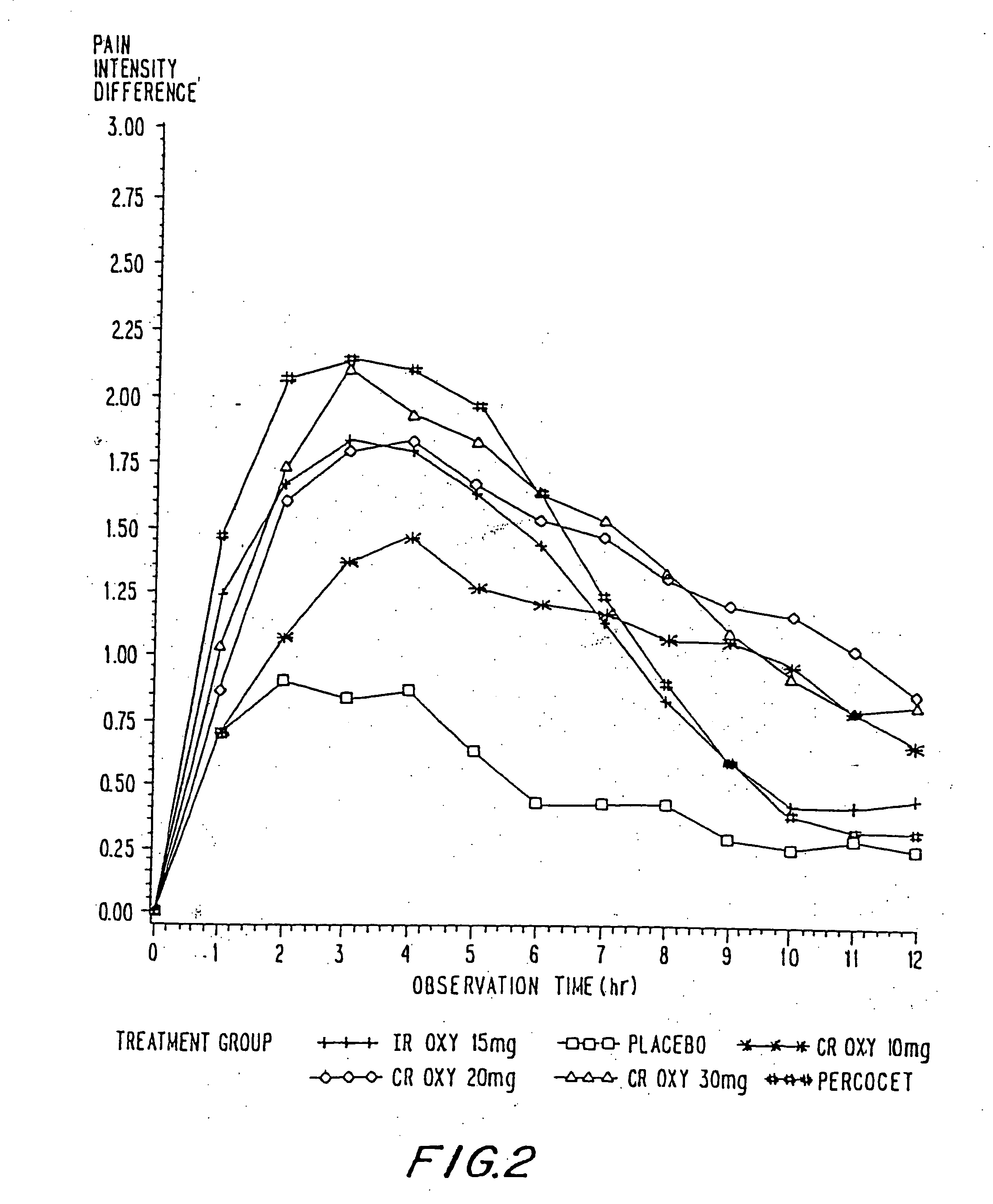
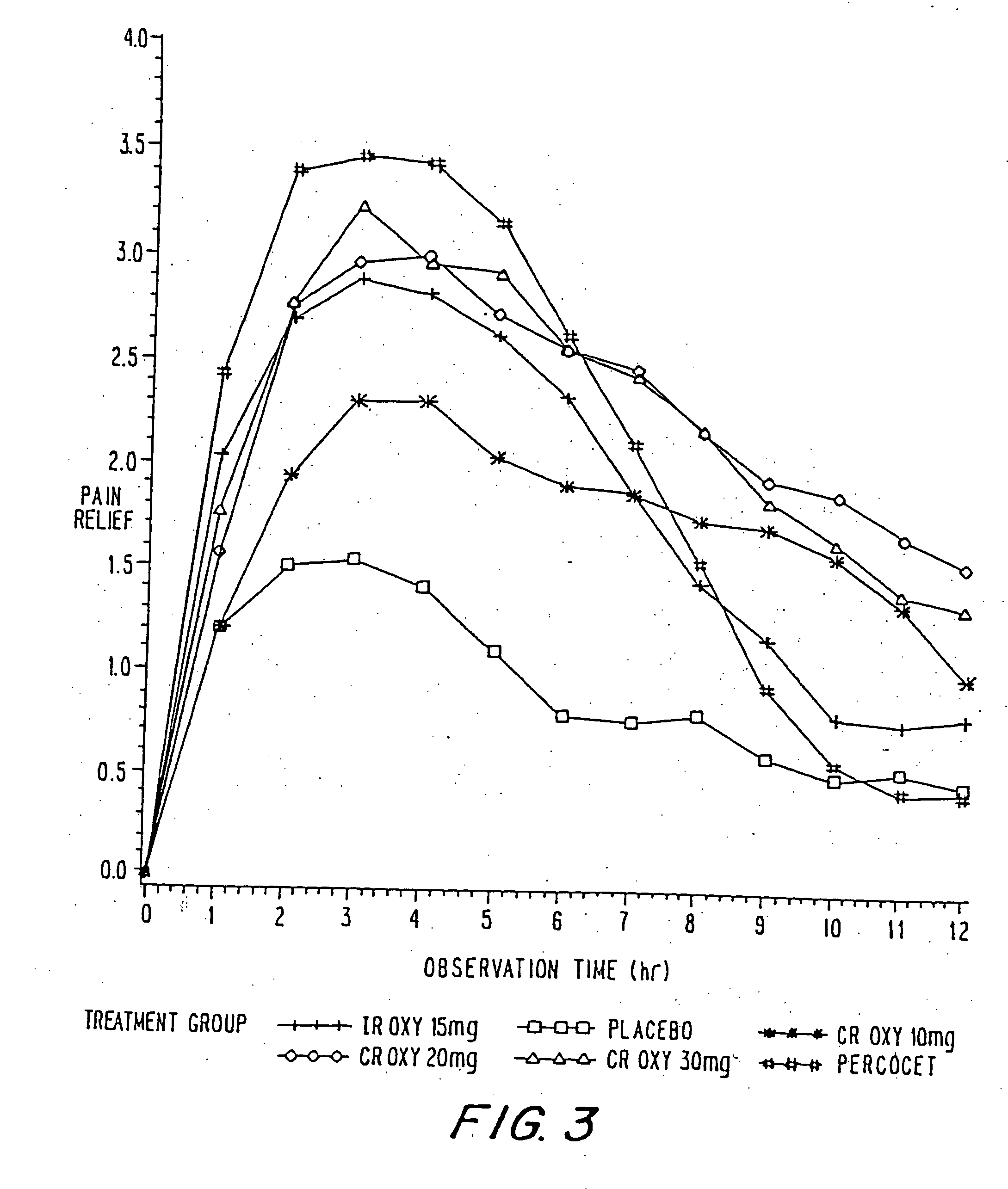
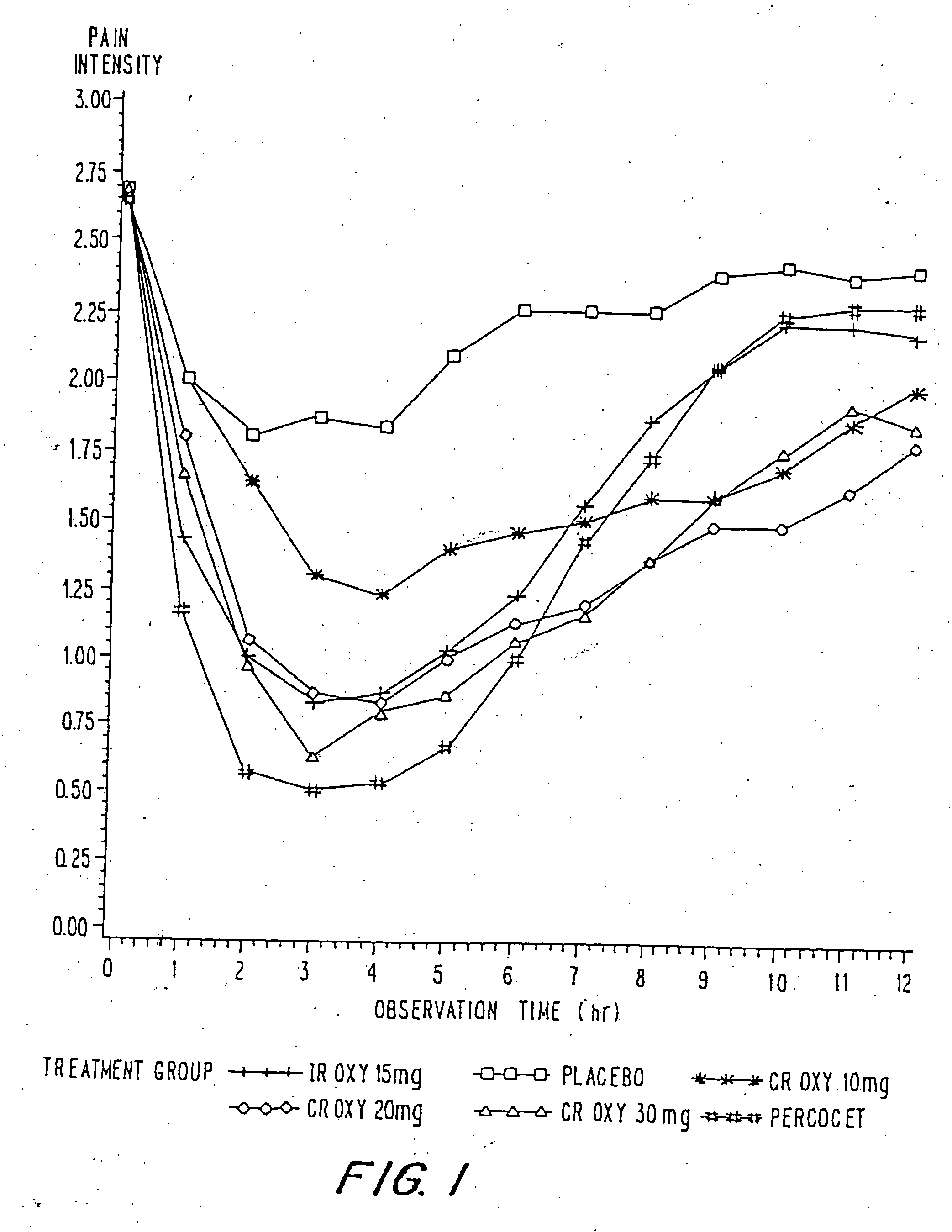
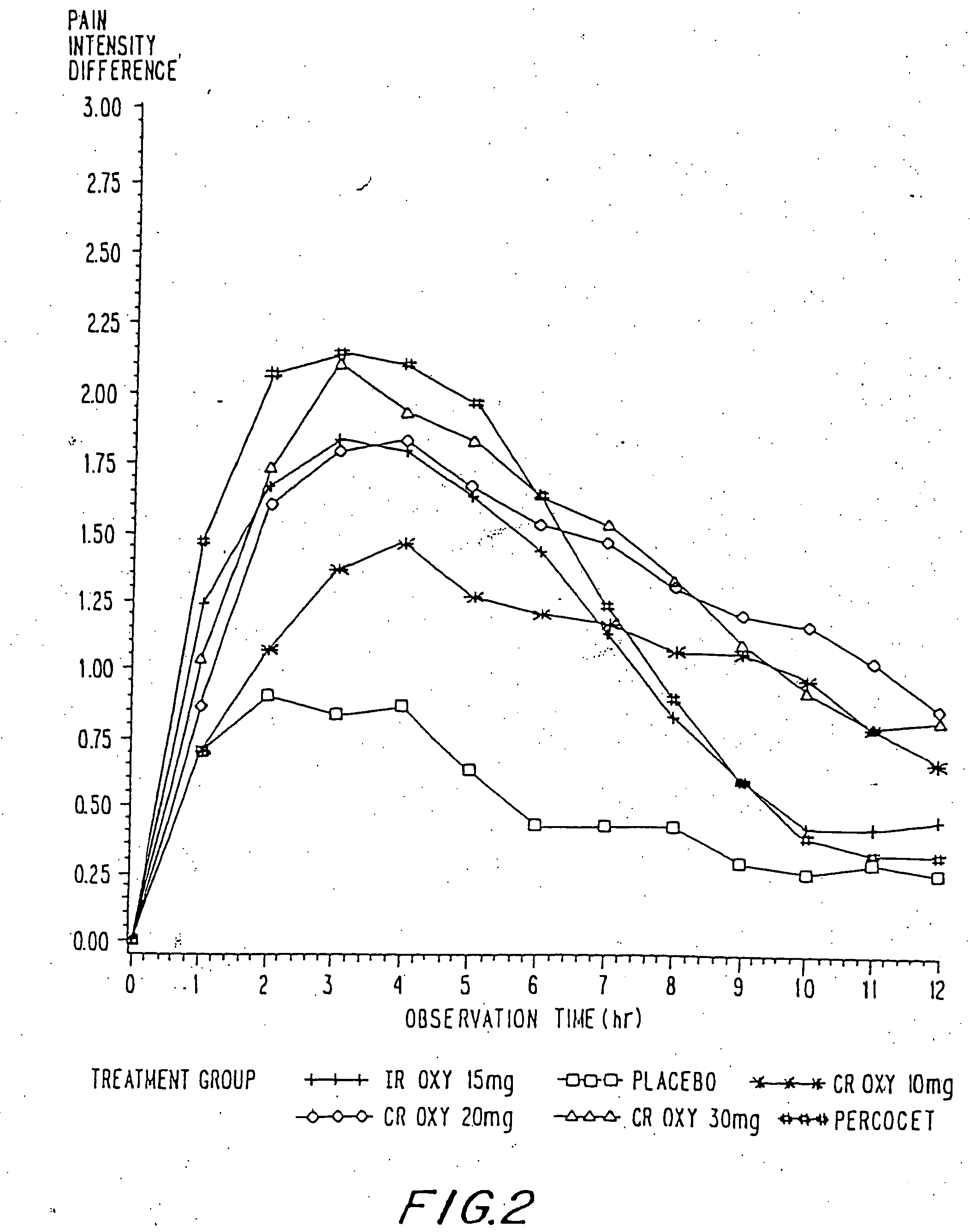
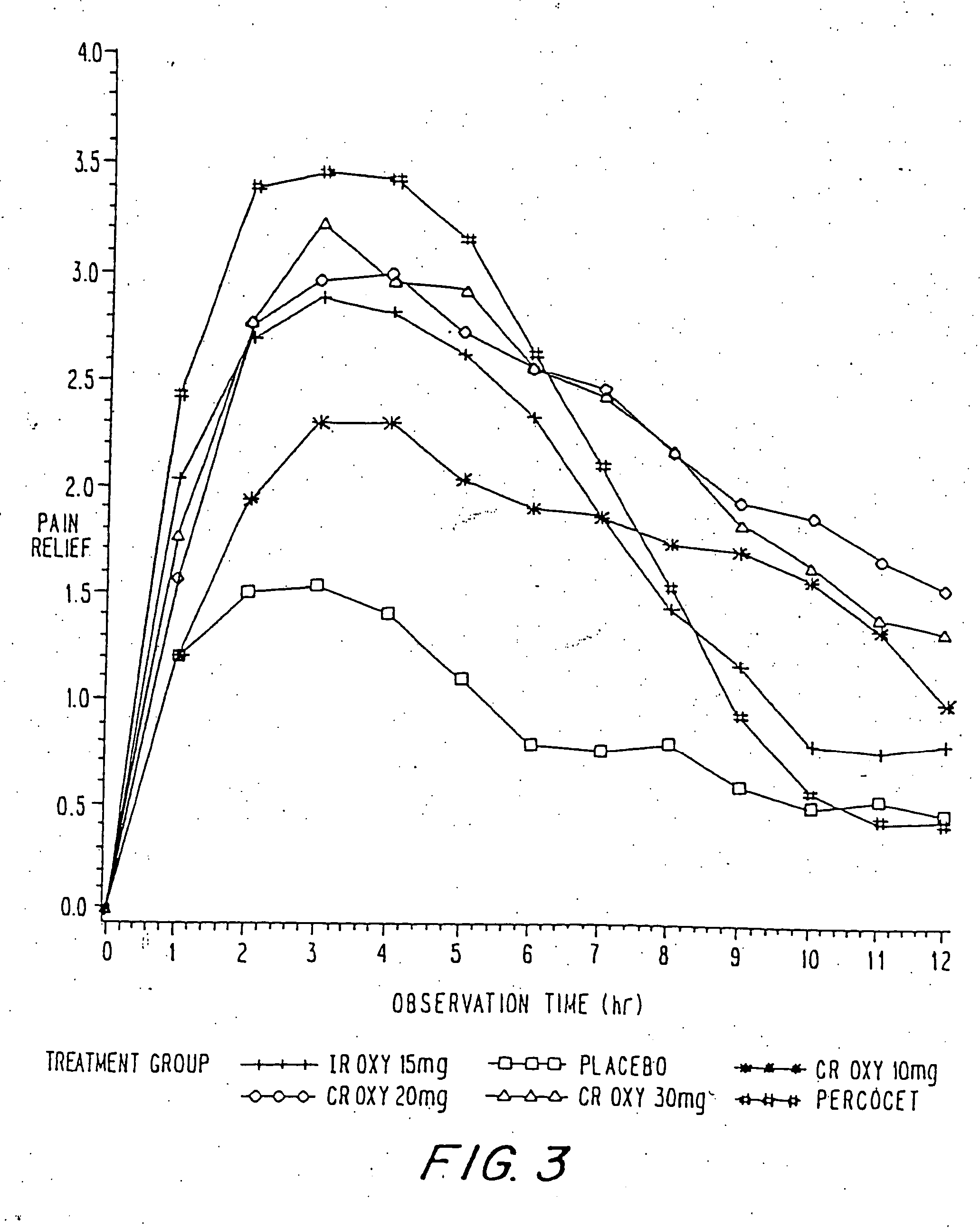
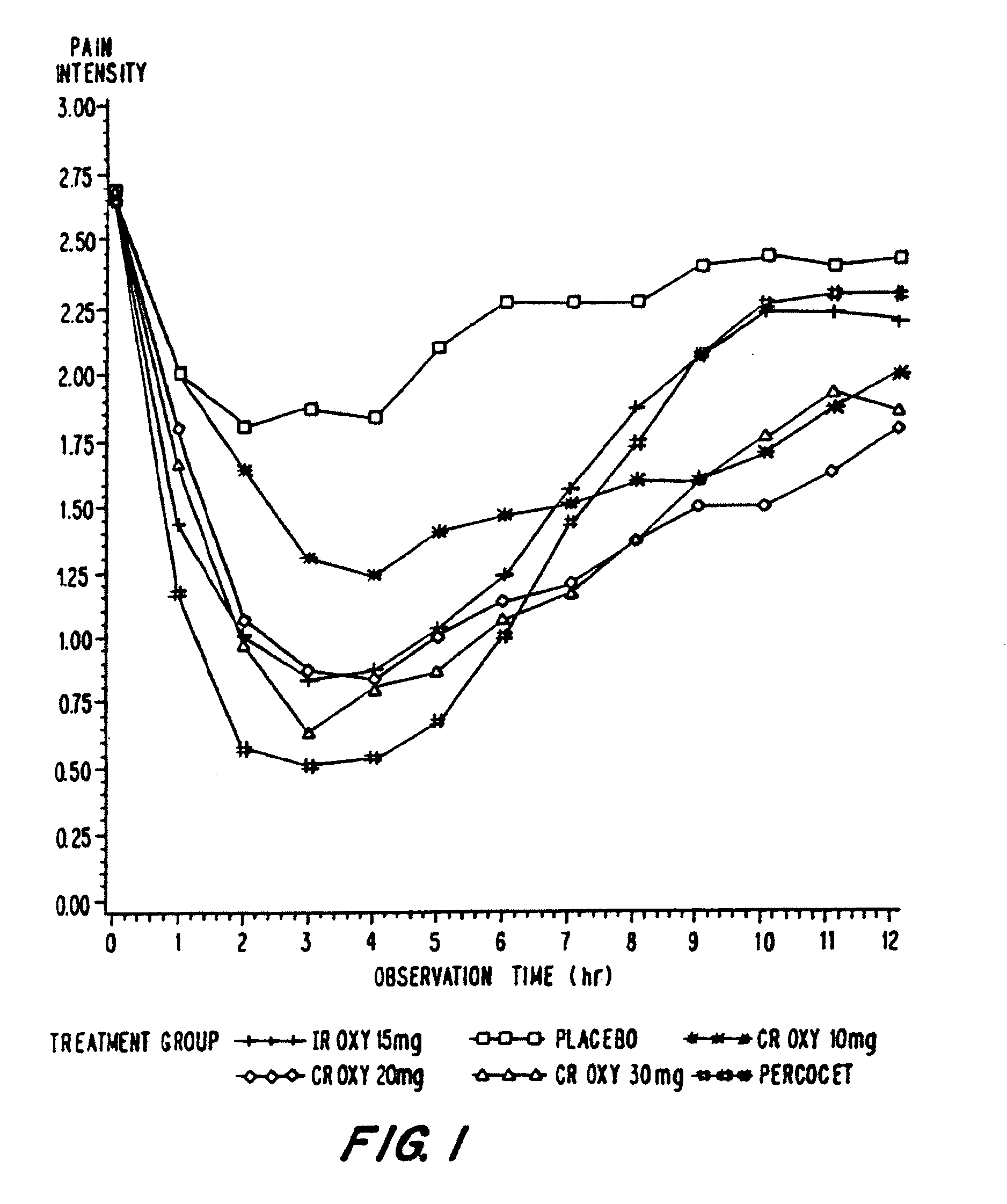
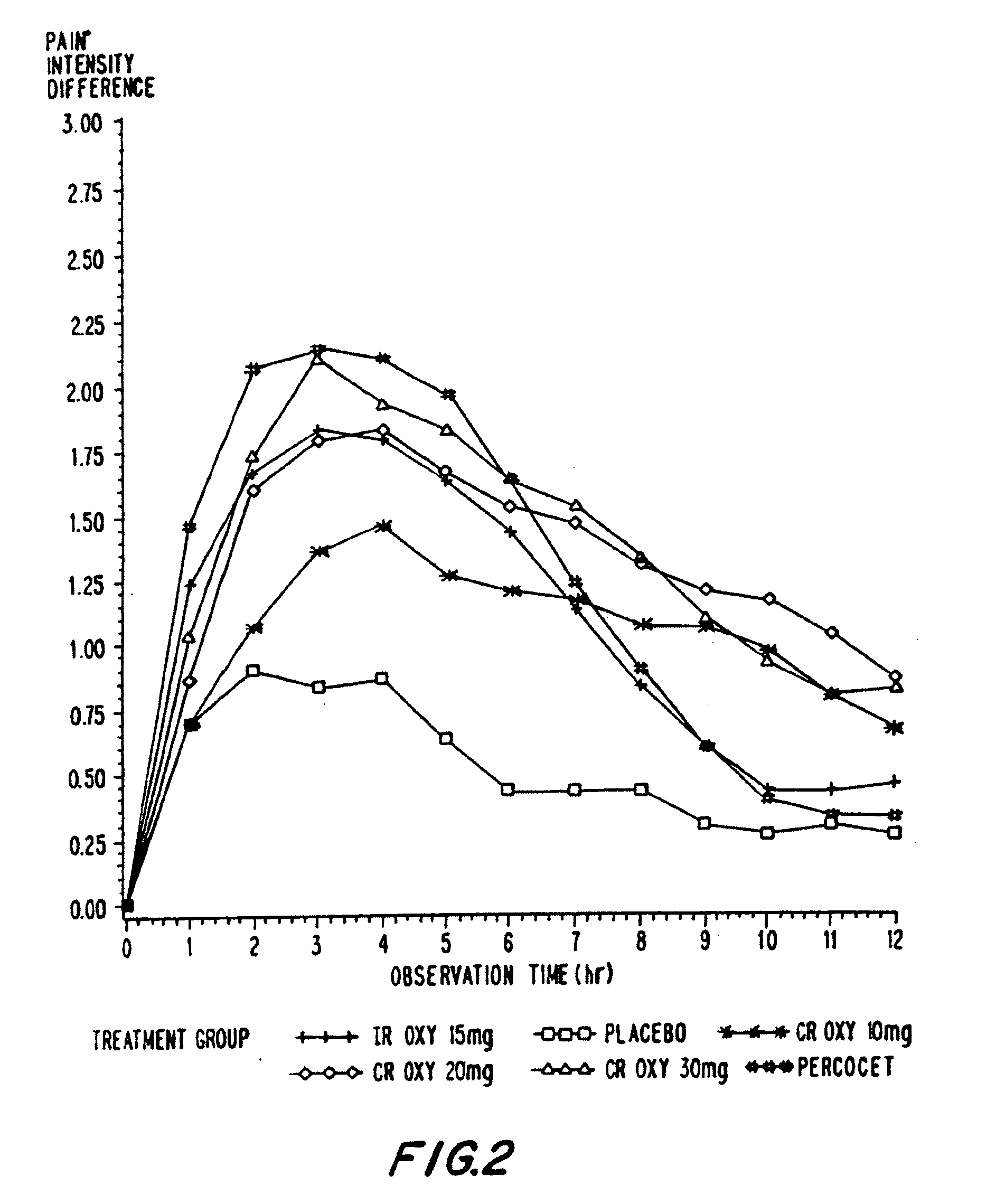
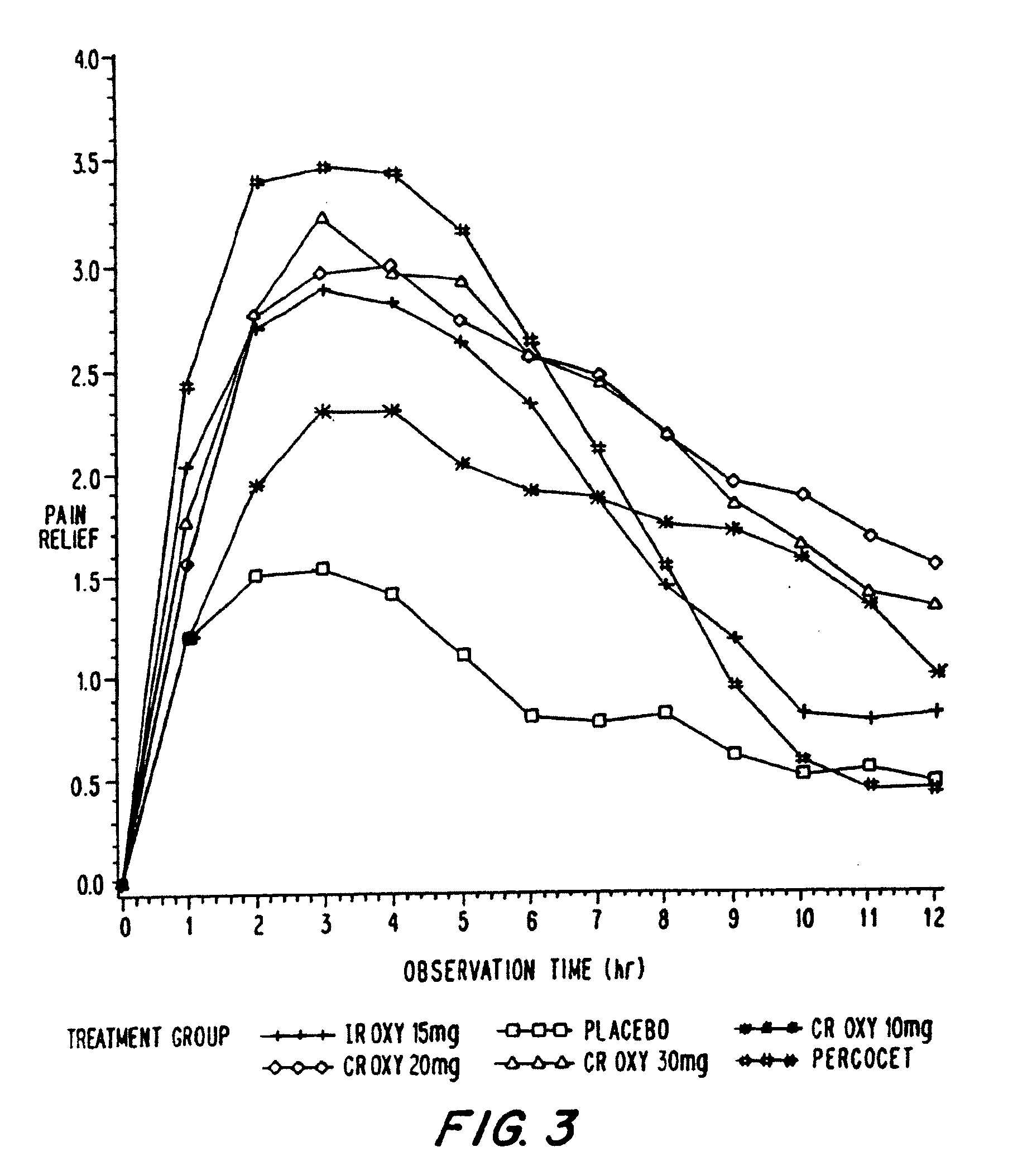
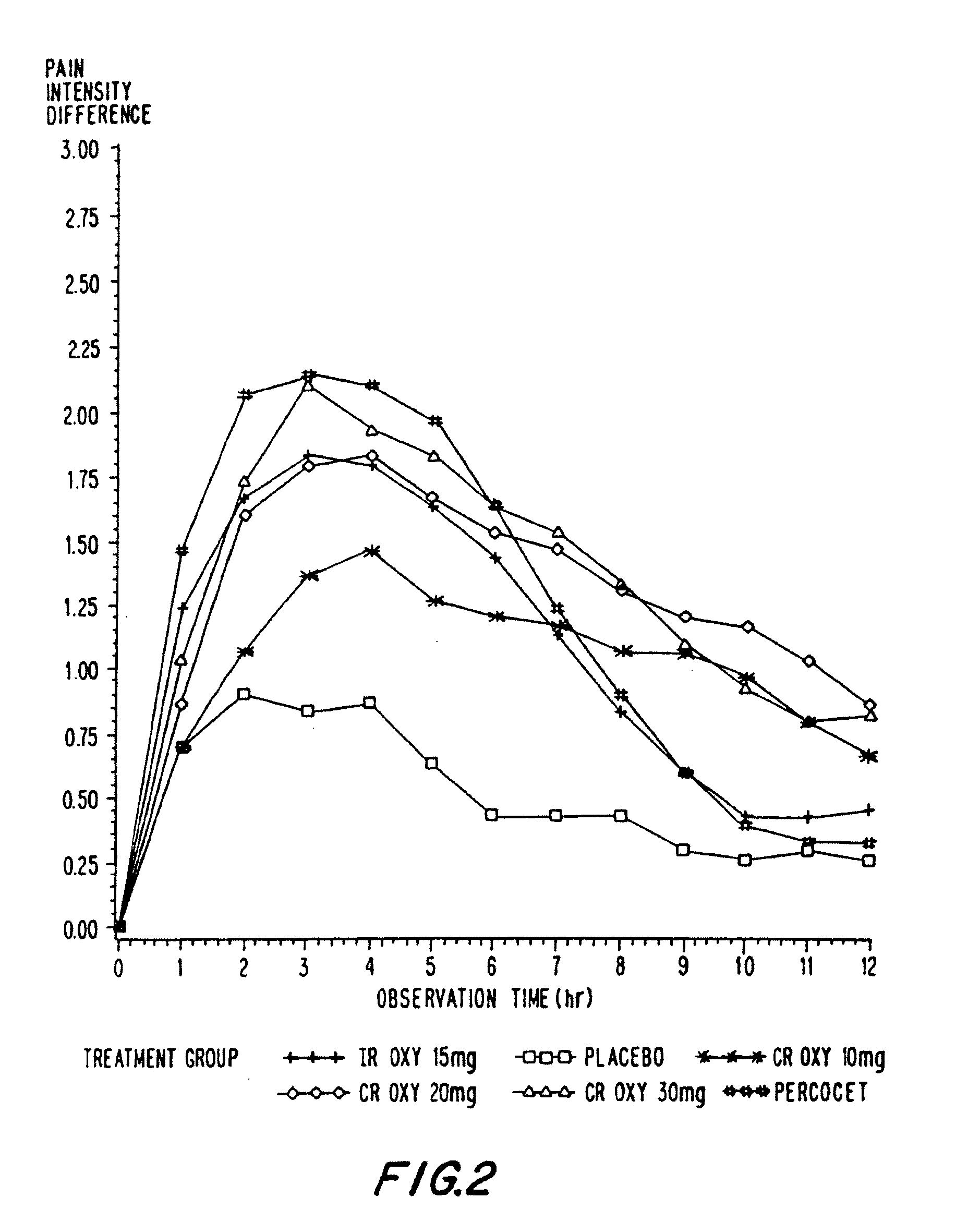
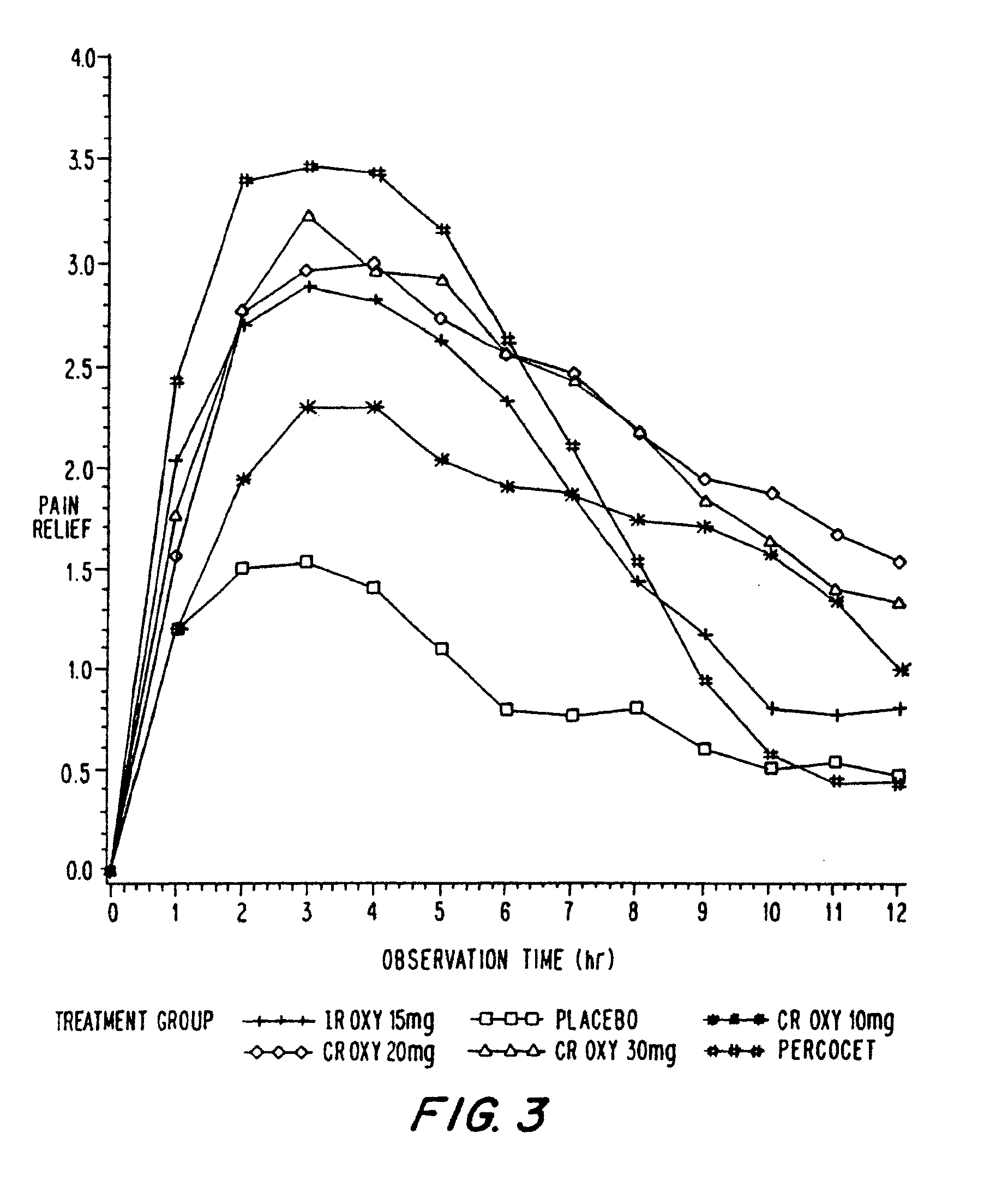
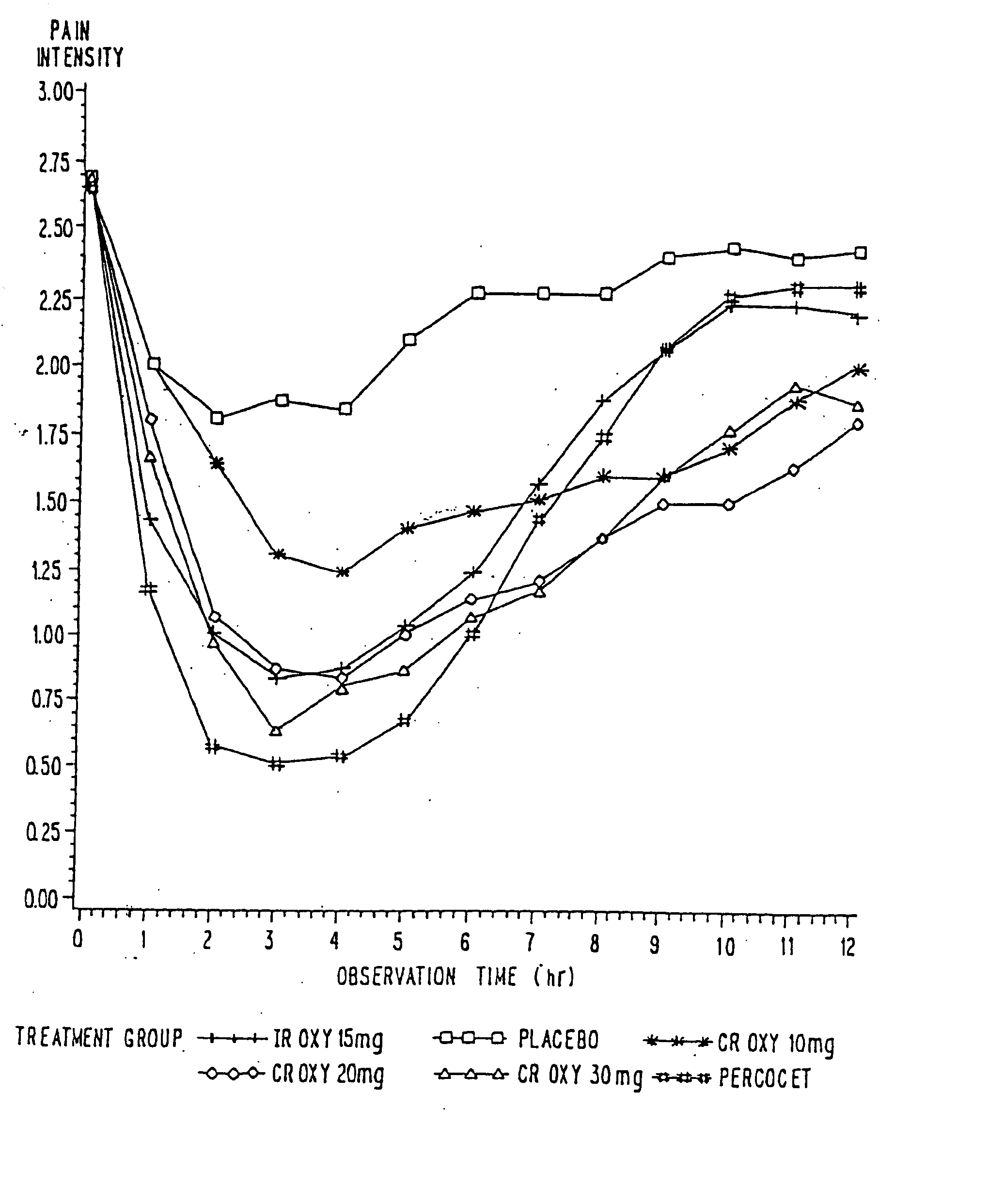
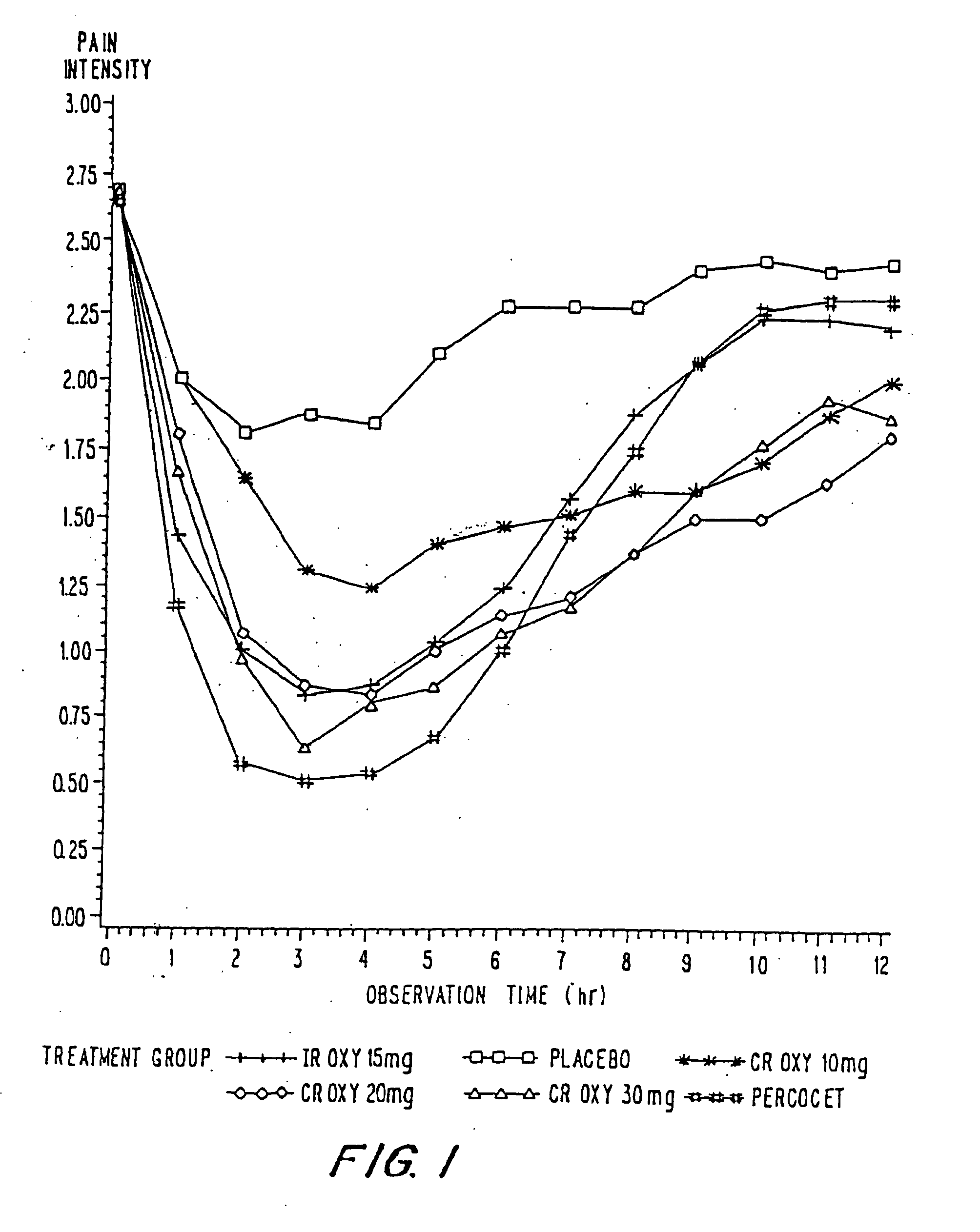
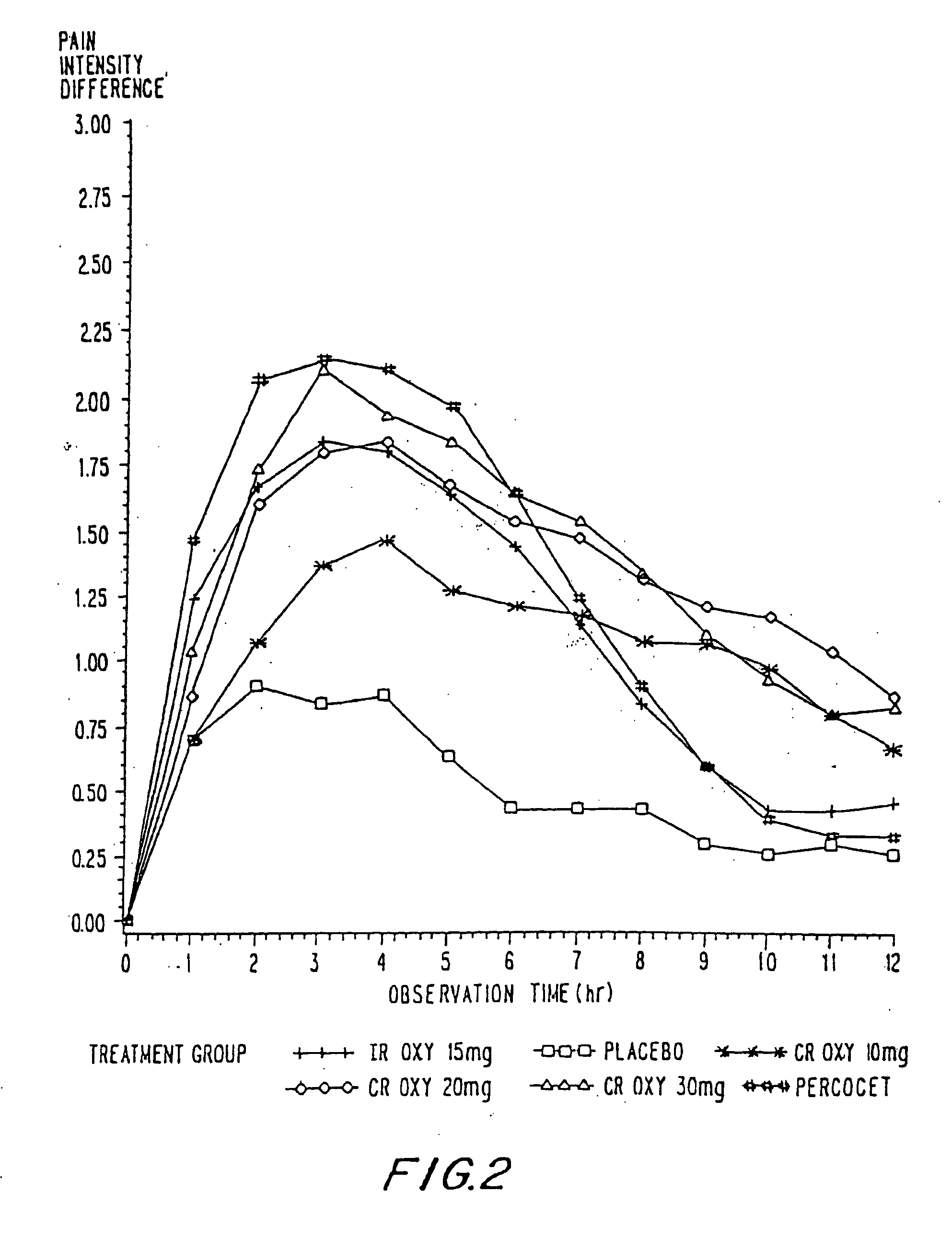
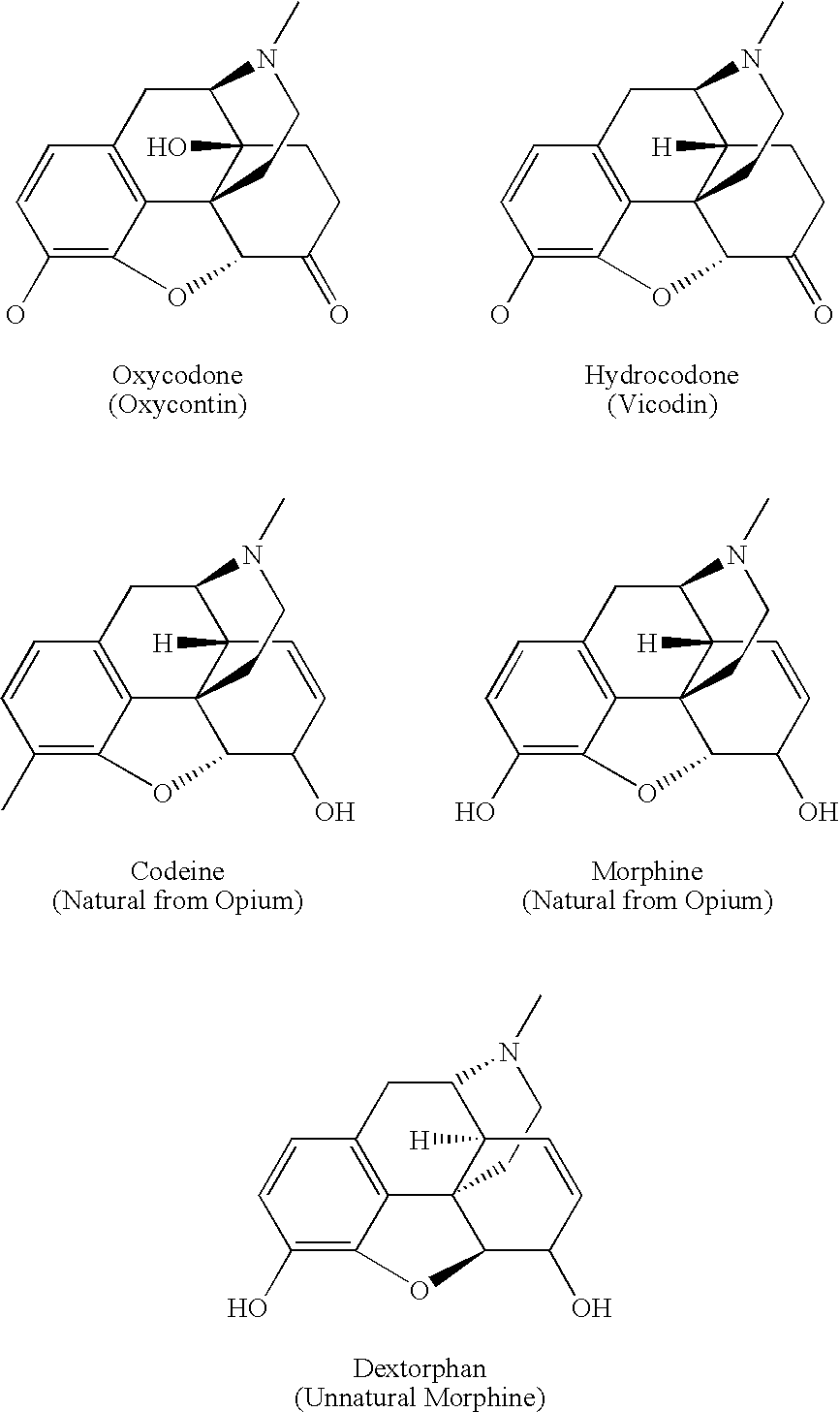
Controlled release oxycodone compositions
InactiveUS20060057210A1Improve efficiencyQuality improvementBiocidePowder deliveryControlled releaseBlood plasma
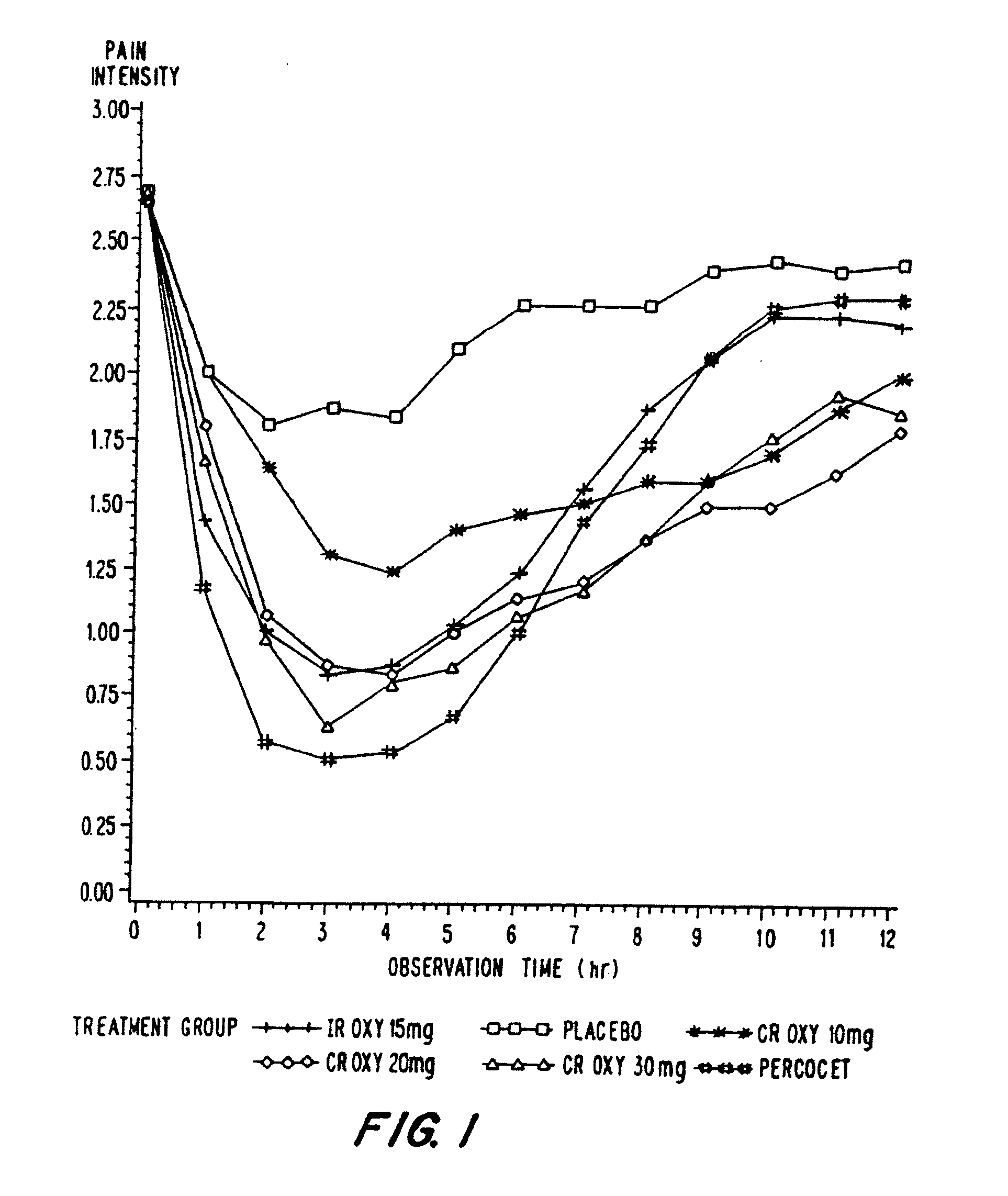
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Electrical stimulation device and method for therapeutic treatment and pain management
ActiveUS8958883B2Easily and tactilely differentiateAvoid accidental activationExternal electrodesArtificial respirationElectricityManaged pain
A disposable electrical stimulation device and method for providing therapeutic treatment and pain management in a convenient, compact configuration. Electrode size and shape and relative configuration can be varied according to an intended application and use, or a universal configuration can be provided for use on almost any area of the body. The common structure of communicatively coupled dual electrodes including control circuitry and a power source accommodates a range of different sizes, configurations, stimulation treatment intensities, and other physical and electrical characteristics that can be pre-customized and packaged for specific, limited time use. The device can therefore be used in methods of providing therapy, managing pain, and achieving other treatment goals by electrical stimulation.
Owner:ENCORE MEDICAL ASSET CORP +1
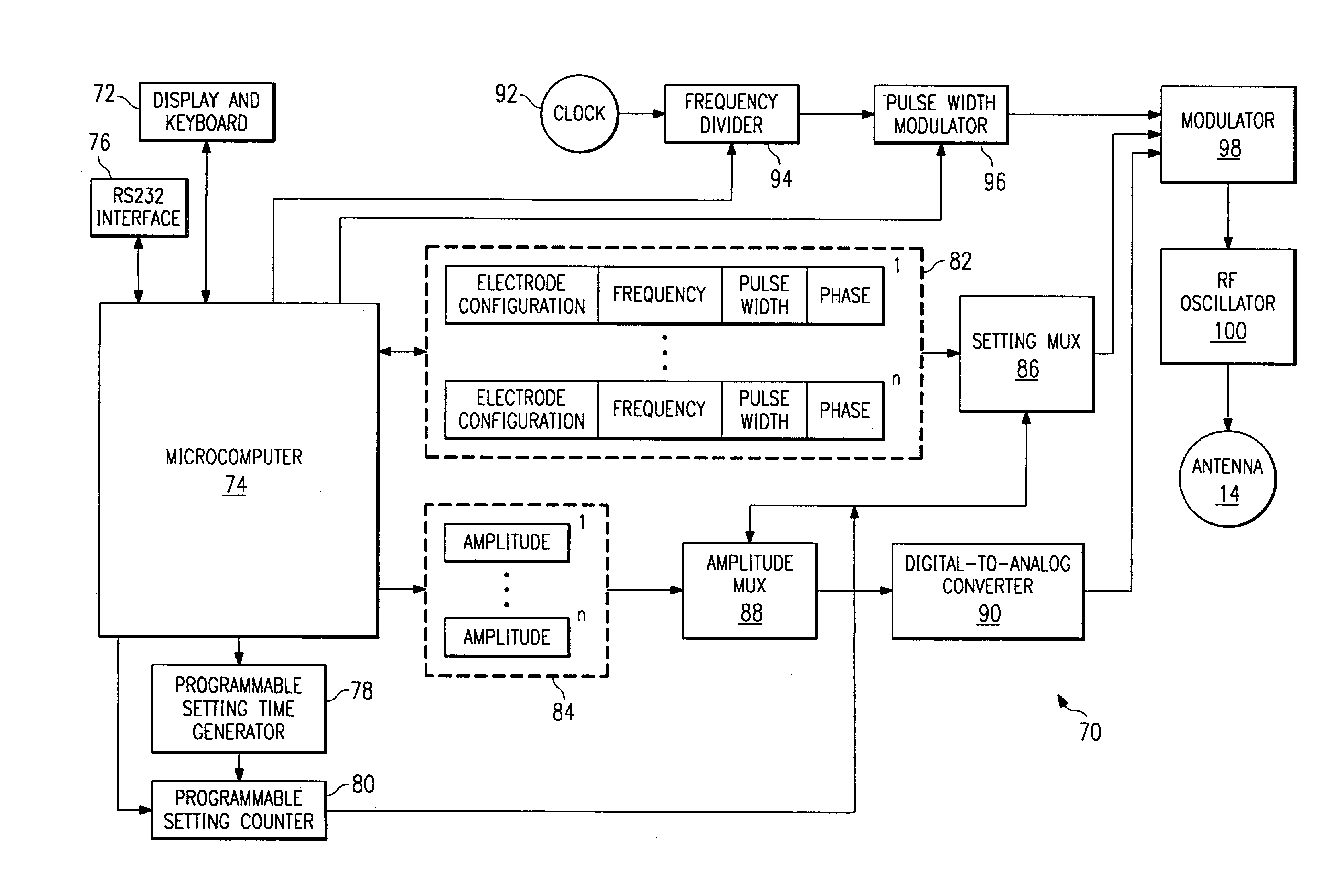
Method and apparatus for multimodal electrical modulation of pain
ActiveUS20160271392A1Good pain reliefChronic painSpinal electrodesExternal electrodesElectromagnetic fieldControlling pain
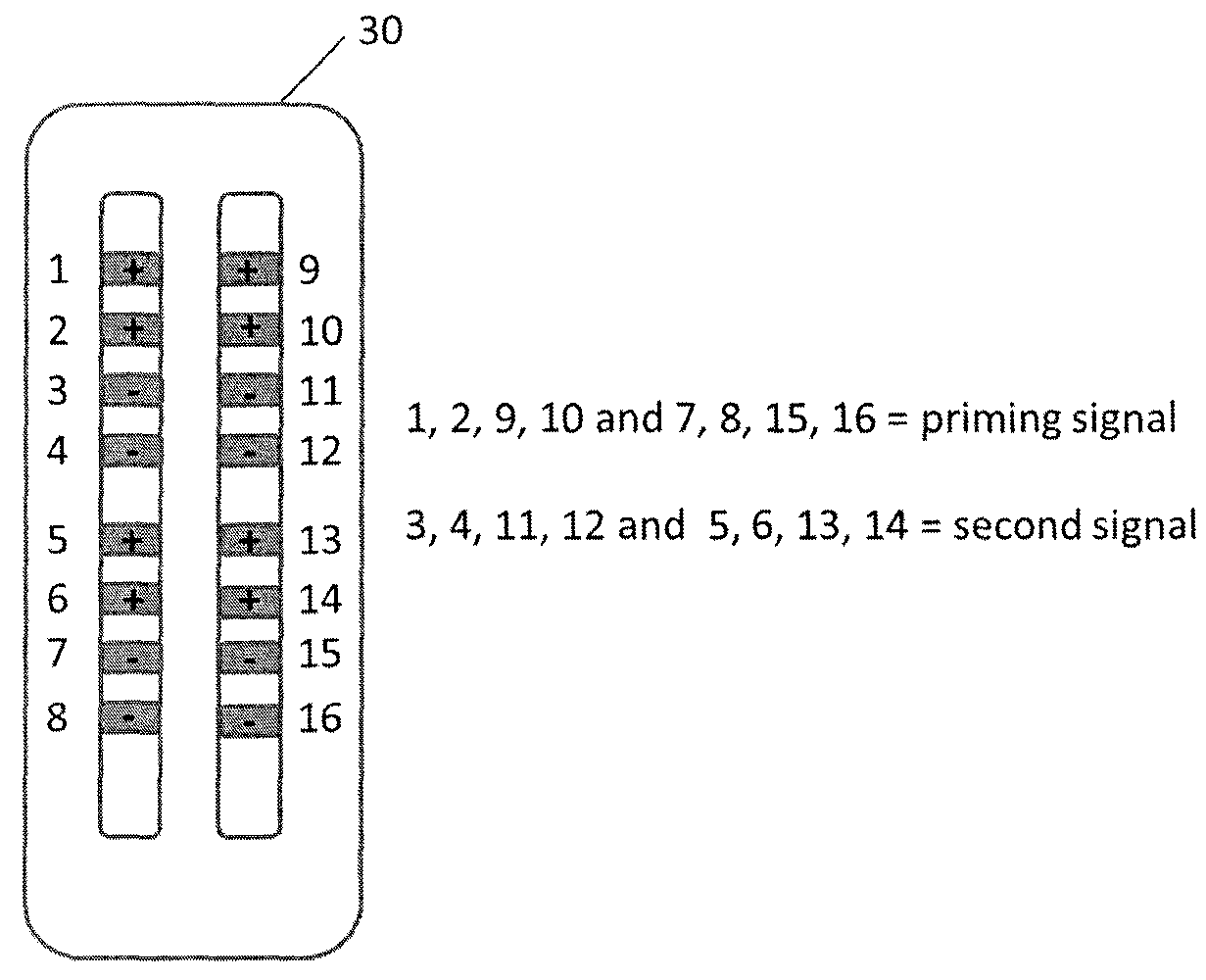
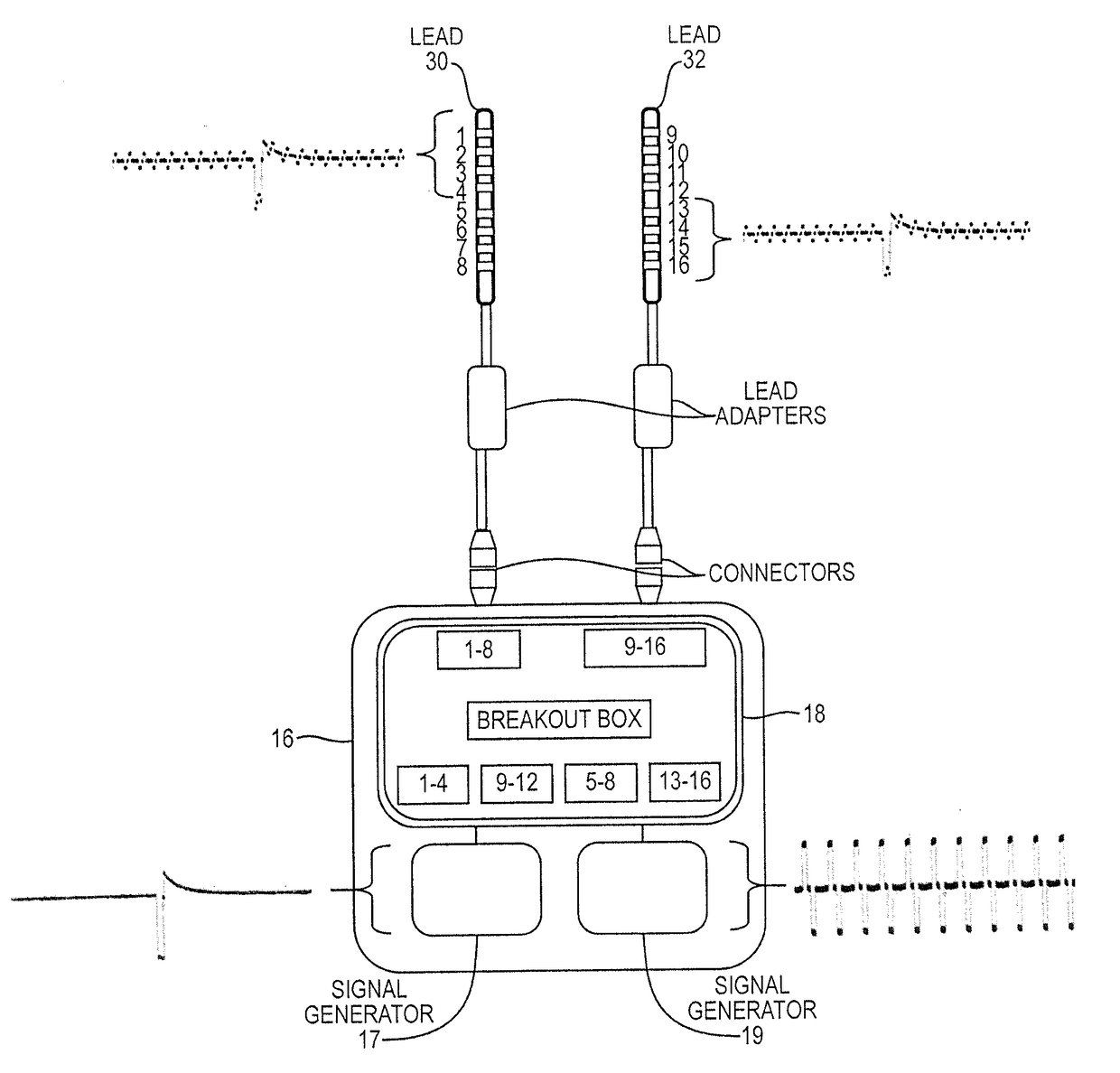
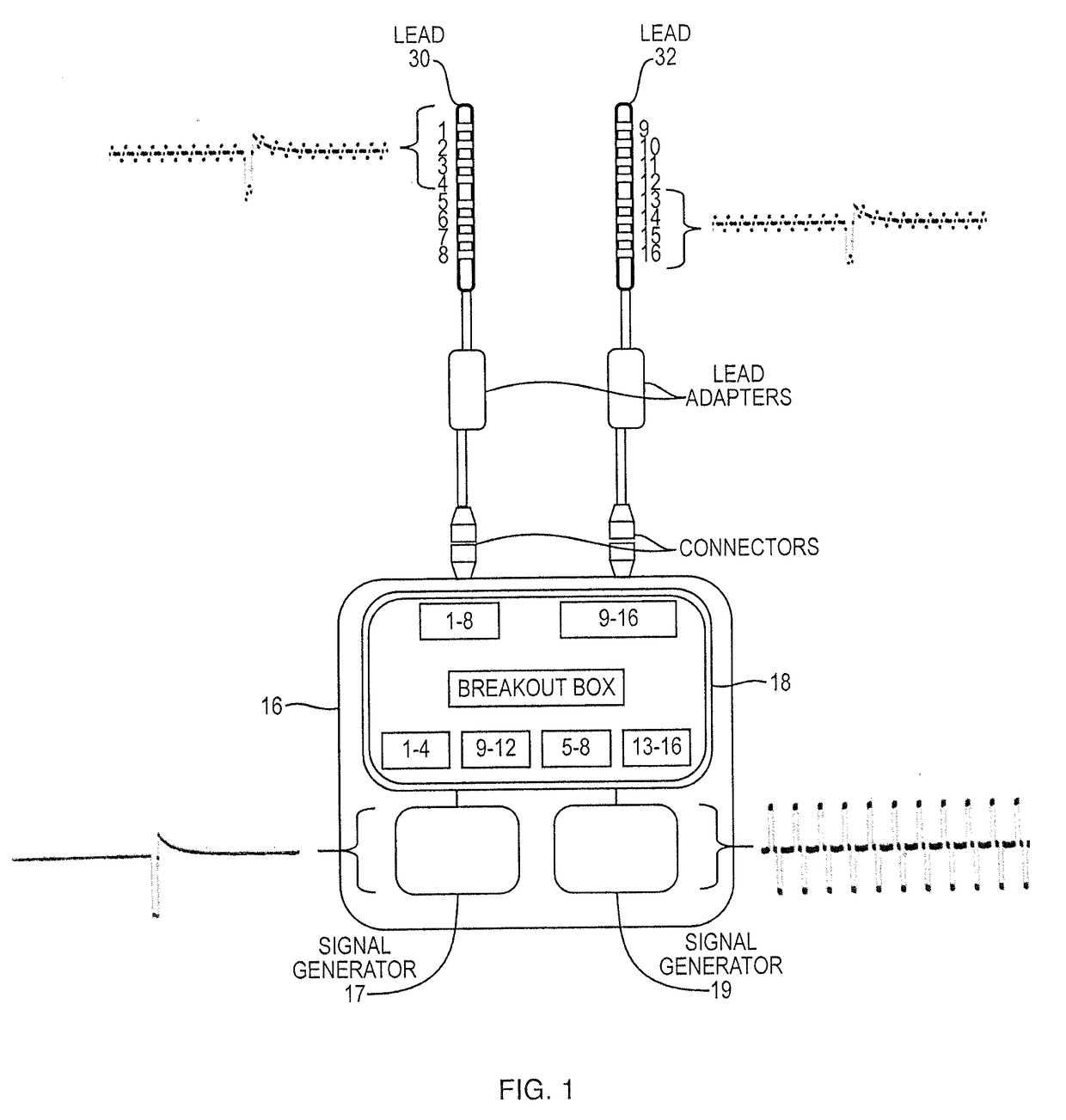
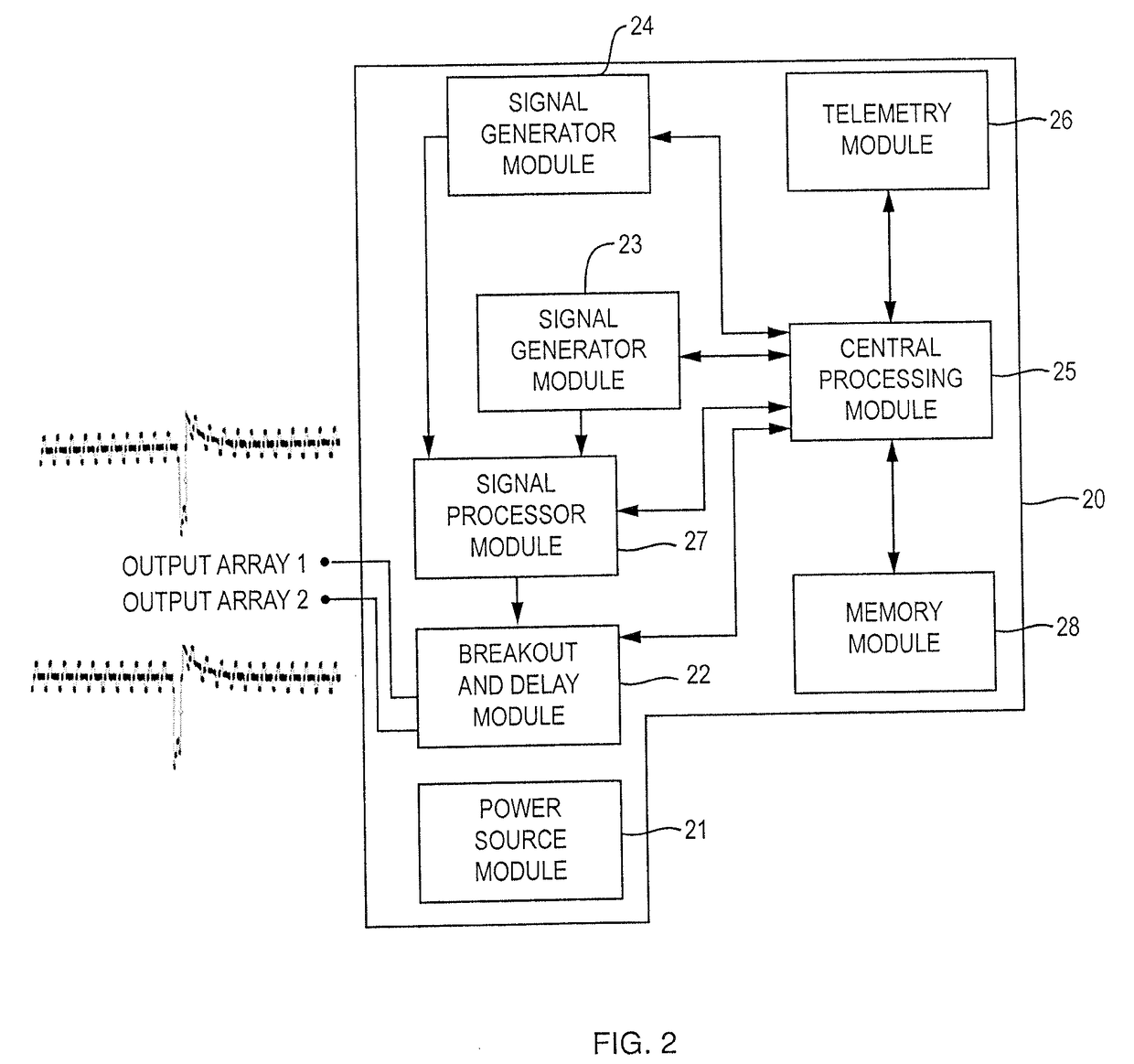
Apparatus and methods for managing pain uses separate varying electromagnetic fields, with a variety of temporal and amplitude characteristics, which are applied to a particular neural structure to modulate glial and neuronal interactions as a mechanism for relieving chronic pain. In another embodiment, a single composite modulation / stimulation signal which has rhythmically varying characteristics is used to achieve the same results as separate varying electromagnetic fields. Also, disclosed is an apparatus and method for modulating the expression of genes involved in diverse pathways including inflammatory / immune system mediators, ion channels and neurotransmitters, in both the Spinal Cord (SC) and Dorsal Root Ganglion (DRG) where such expression modulation is caused by spinal cord stimulation or peripheral nerve stimulation using the disclosed apparatus and techniques. In one embodiment of multimodal modulation therapy, the prime signal may be monophasic, or biphasic, in which the polarity of the first phase of the biphasic prime signal may be either cathodic or anodic while the tonic signal may be either monophasic, or biphasic, with the polarity of the first phase of the biphasic tonic signal being either cathodic or anodic.
Owner:MEDTRONIC SG LLC
Controlled release oxycodone compositions
InactiveUS20060099255A1Improve efficiency and qualityOrganic active ingredientsNervous disorderControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:OSHLACK BENJAMIN +3
Method and apparatus for multimodal electrical modulation of pain
ActiveUS20160256689A1Good pain reliefChronic painSpinal electrodesExternal electrodesElectromagnetic fieldControlling pain
Apparatus and methods for managing pain uses separate varying electromagnetic fields, with a variety of temporal and amplitude characteristics, which are applied to a particular neural structure to modulate glial and neuronal interactions as a mechanism for relieving chronic pain. In another embodiment, a single composite modulation / stimulation signal which has rhythmically varying characteristics is used to achieve the same results as separate varying electromagnetic fields. Also, disclosed is an apparatus and method for modulating the expression of genes involved in diverse pathways including inflammatory / immune system mediators, ion channels and neurotransmitters, in both the Spinal Cord (SC) and Dorsal Root Ganglion (DRG) where such expression modulation is caused by spinal cord stimulation or peripheral nerve stimulation using the disclosed apparatus and techniques. In one embodiment of multimodal modulation therapy, the prime signal may be monophasic, or biphasic, in which the polarity of the first phase of the biphasic prime signal may be either cathodic or anodic while the tonic signal may be either monophasic, or biphasic, with the polarity of the first phase of the biphasic tonic signal being either cathodic or anodic.
Owner:MEDTRONIC SG LLC
Controlled release oxycodone compositions
InactiveUS20100092570A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControl releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +1
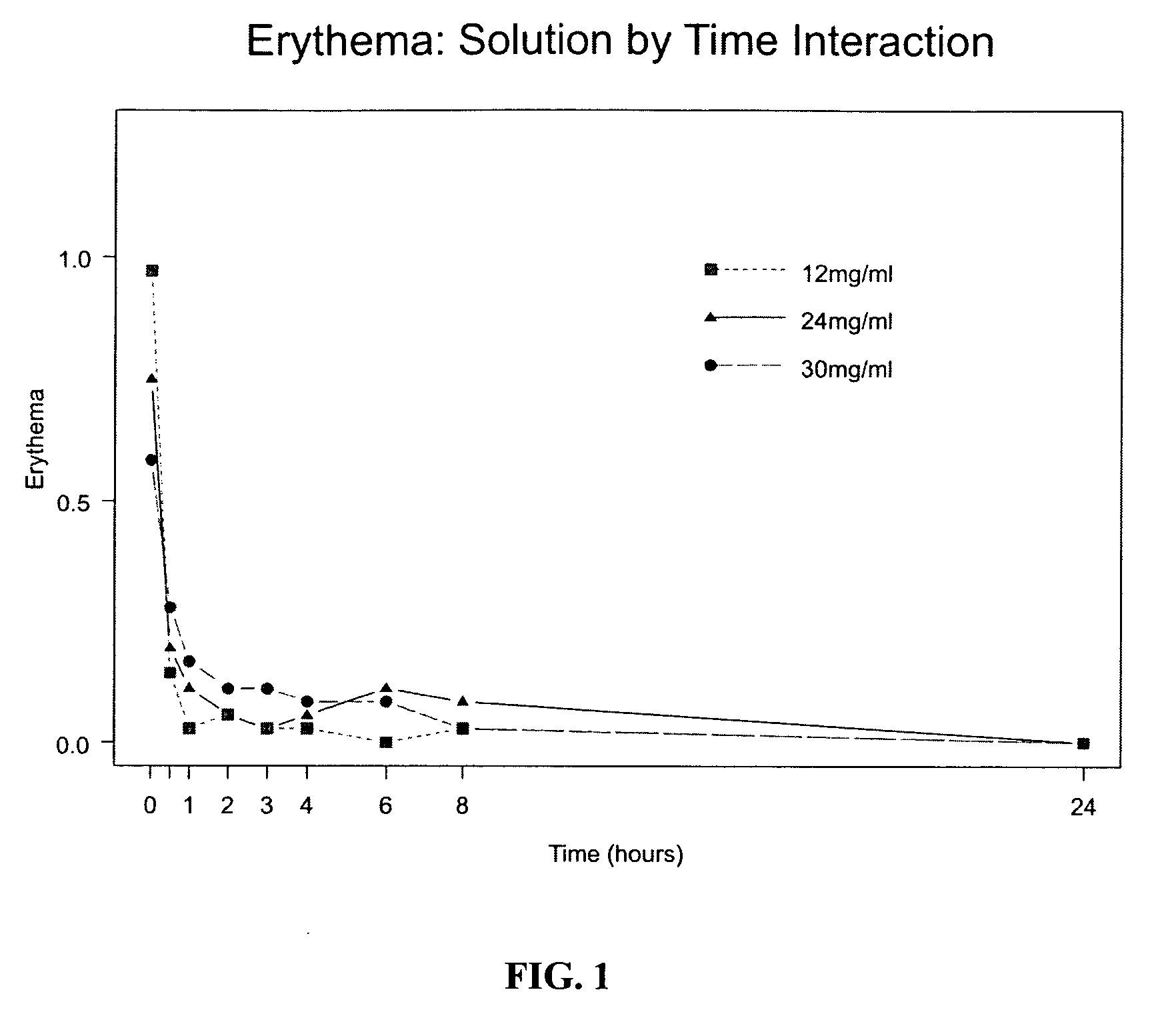
Method of inducing topical anesthesia and transdermal patch
InactiveUS20090123527A1Avoid the needLower potentialPowder deliveryBiocideTransdermal patchPhysiological fluid
Disclosed is a method of inducing topical anesthesia in a tissue or organ of an animal comprising providing an aqueous gel formulation comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed are a transdermal patch comprising the aqueous gel formulation suitable for applying on the skin of a patient and a method of controlling pain therewith.
Owner:AKORN
Methods of using and compositions comprising selective cytokine inhibitory drugs for treatment, modification and management of pain
InactiveUS20040087558A1Extension of timeAvoid managementBiocideNervous disorderCytokine SuppressionPhysical Therapy Modality
Methods of treating, preventing, modifying and managing various types of pain are disclosed. Specific methods comprise the administration of a selective cytokine inhibitory drug, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery, psychological or physical therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Method and system for managing pain
A method of managing pain using neurophysiological data acquired from the brain of a subject is disclosed. The method comprises: identifying activity-related features in the data; parceling the data according to the activity-related features to define a plurality of capsules, each representing a spatiotemporal activity region in the brain; comparing at least some of the defined capsules to at least one reference capsule; and assessing the likelihood that the subject is experiencing pain responsively to the comparison.
Owner:ELMINDA LTD
Controlled release oxycodone compositions
InactiveUS20100034876A1Improve efficiency and qualityBiocidePowder deliveryControlled releaseMedicine
Owner:PURDUE PHARMA LP +1
Method and apparatus for multimodal electrical modulation of pain
ActiveUS10039930B2Chronic painGood pain reliefSpinal electrodesExternal electrodesElectromagnetic fieldControlling pain
Apparatus and methods for managing pain uses separate varying electromagnetic fields, with a variety of temporal and amplitude characteristics, which are applied to a particular neural structure to modulate glial and neuronal interactions as a mechanism for relieving chronic pain. In another embodiment, a single composite modulation / stimulation signal which has rhythmically varying characteristics is used to achieve the same results as separate varying electromagnetic fields. Also, disclosed is an apparatus and method for modulating the expression of genes involved in diverse pathways including inflammatory / immune system mediators, ion channels and neurotransmitters, in both the Spinal Cord (SC) and Dorsal Root Ganglion (DRG) where such expression modulation is caused by spinal cord stimulation or peripheral nerve stimulation using the disclosed apparatus and techniques. In one embodiment of multimodal modulation therapy, the prime signal may be monophasic, or biphasic, in which the polarity of the first phase of the biphasic prime signal may be either cathodic or anodic while the tonic signal may be either monophasic, or biphasic, with the polarity of the first phase of the biphasic tonic signal being either cathodic or anodic.
Owner:MEDTRONIC SG LLC
Method and apparatus for multimodal electrical modulation of pain using composite electromagnetic fields
ActiveUS20180028812A1Chronic painGood pain reliefSpinal electrodesExternal electrodesVariable CharacteristicElectromagnetic field
Apparatus and methods for managing pain uses a single composite modulation / stimulation signal with variable characteristics to achieve the same results as separate varying electromagnetic signals. The composite signal is utilized for modulating the expression of genes involved in diverse pathways including inflammatory / immune system mediators, ion channels and neurotransmitters, in both the Spinal Cord (SC) and Dorsal Root Ganglion (DRG) where such expression modulation is caused by spinal cord stimulation or peripheral nerve stimulation using the disclosed apparatus and techniques.
Owner:MEDTRONIC SG LLC
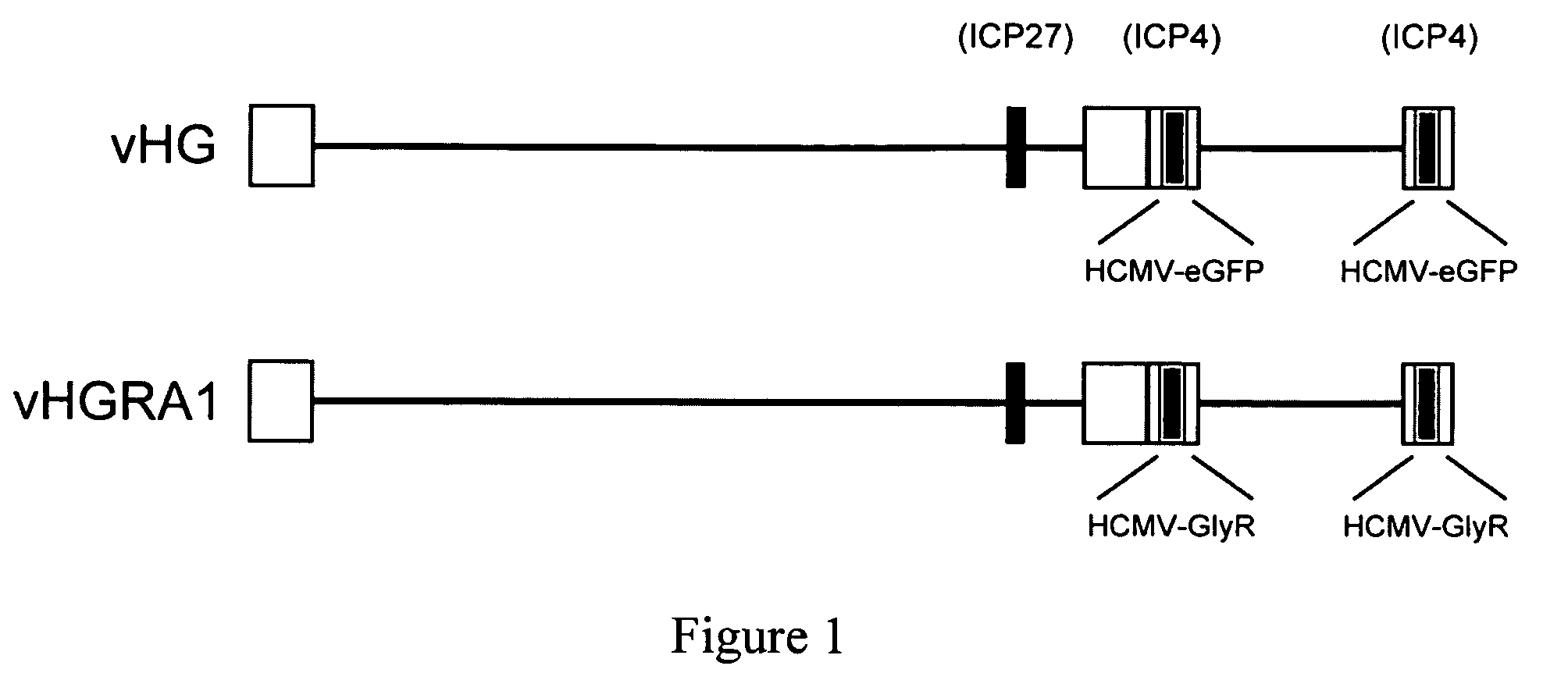
Targeted delivery of glycine receptors to excitable cells
InactiveUS20080289058A1Reduce decreaseReduces formalin-induced nociceptive behaviorOrganic active ingredientsNervous disorderGlycine receptorAllosteric modulator
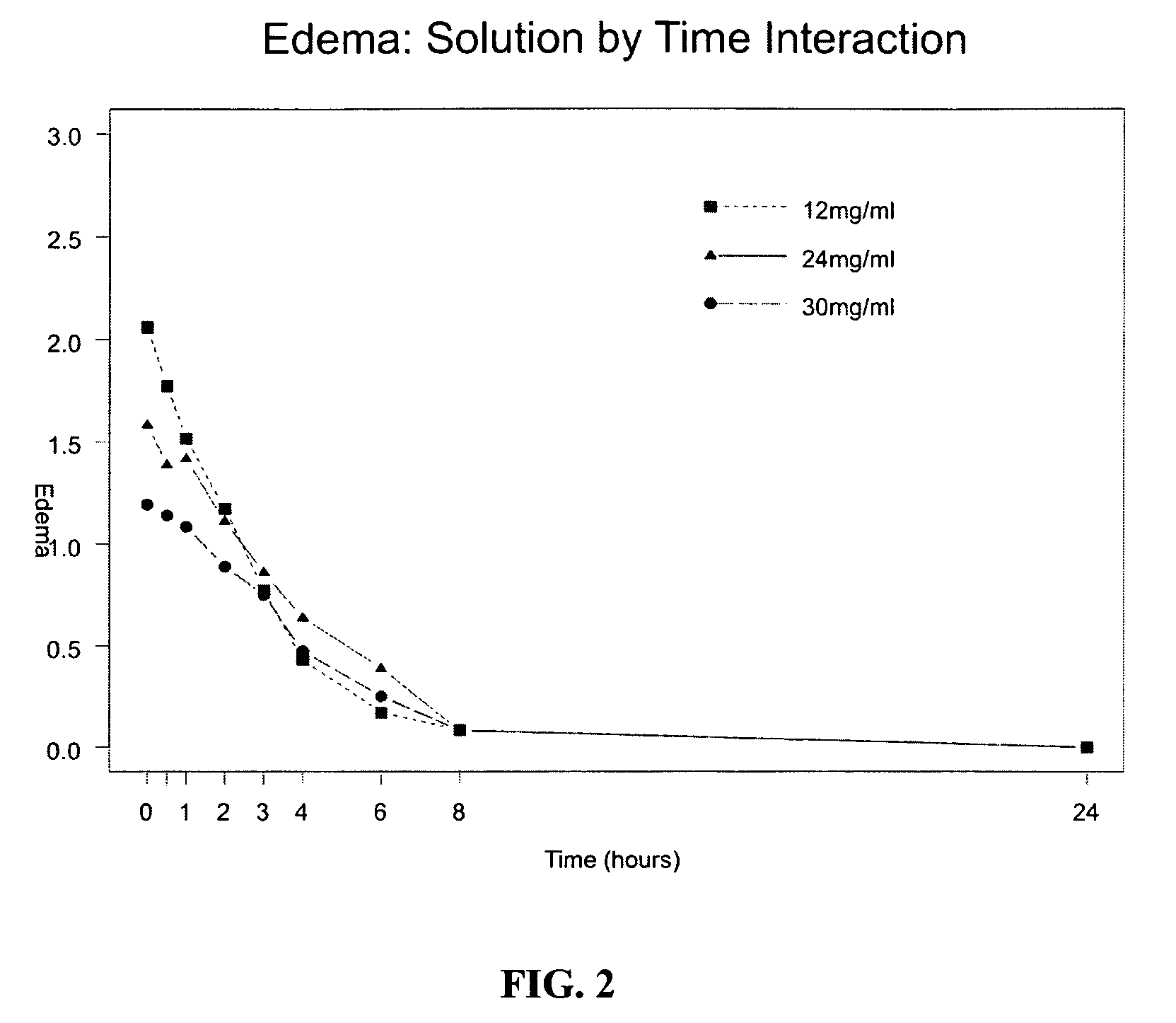
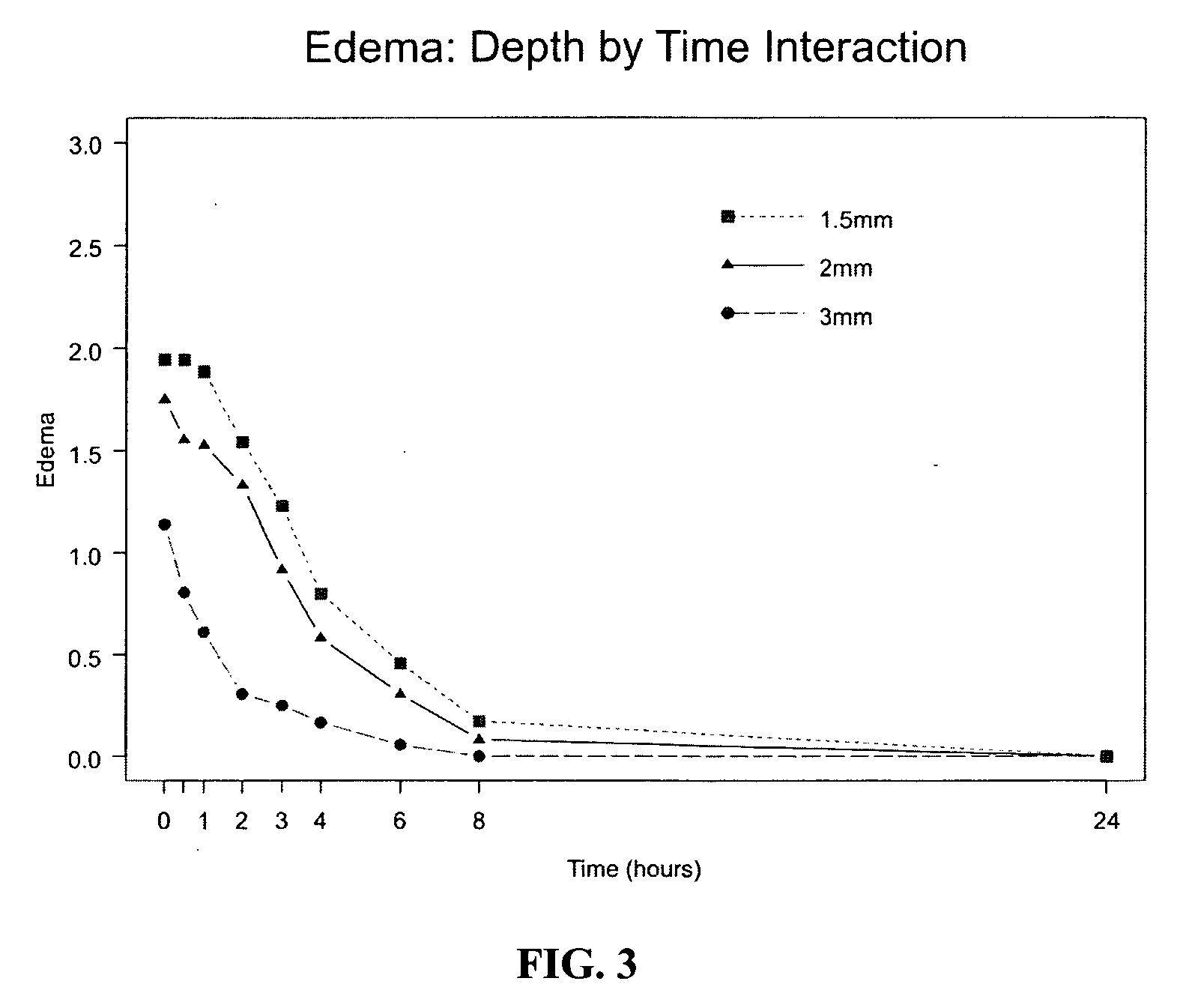
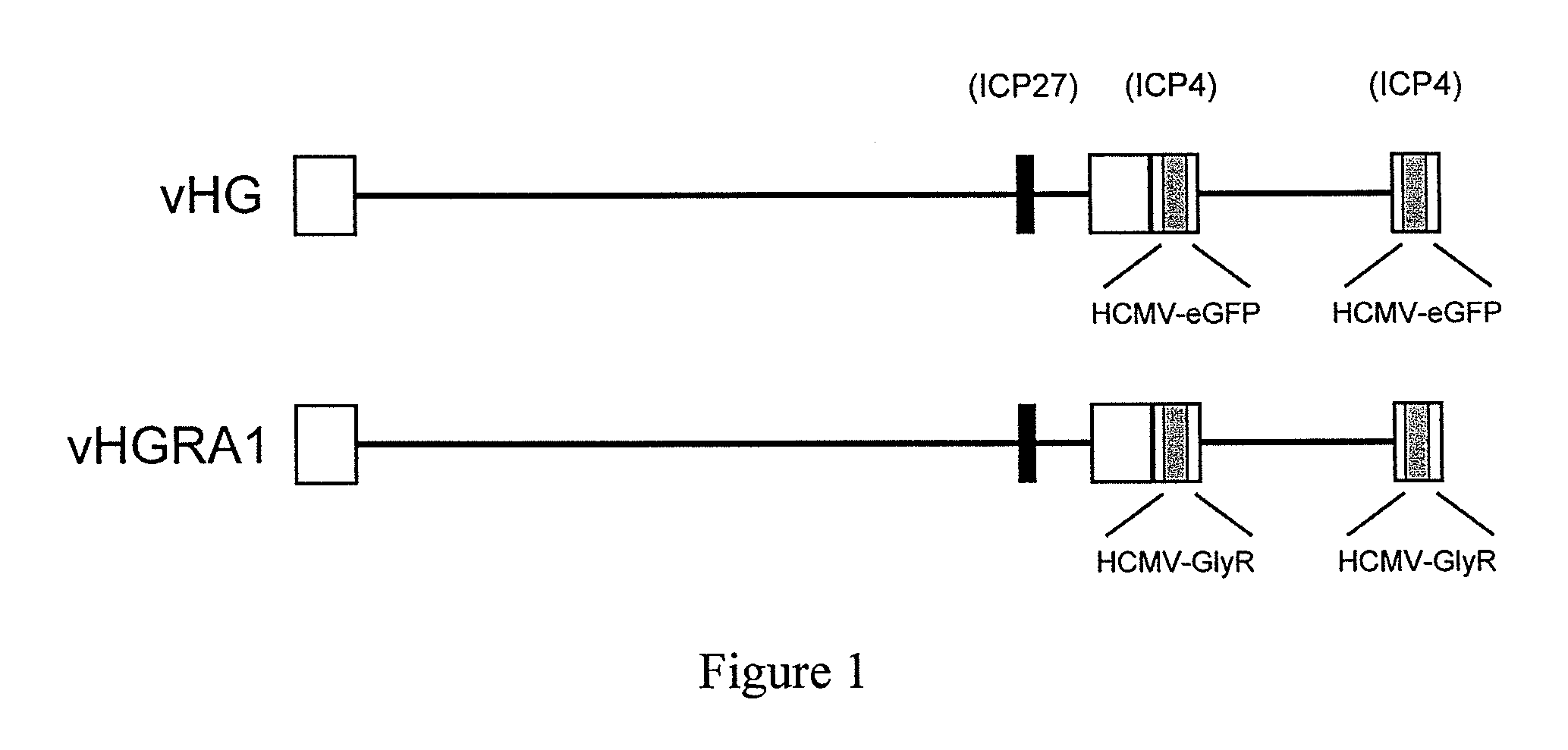
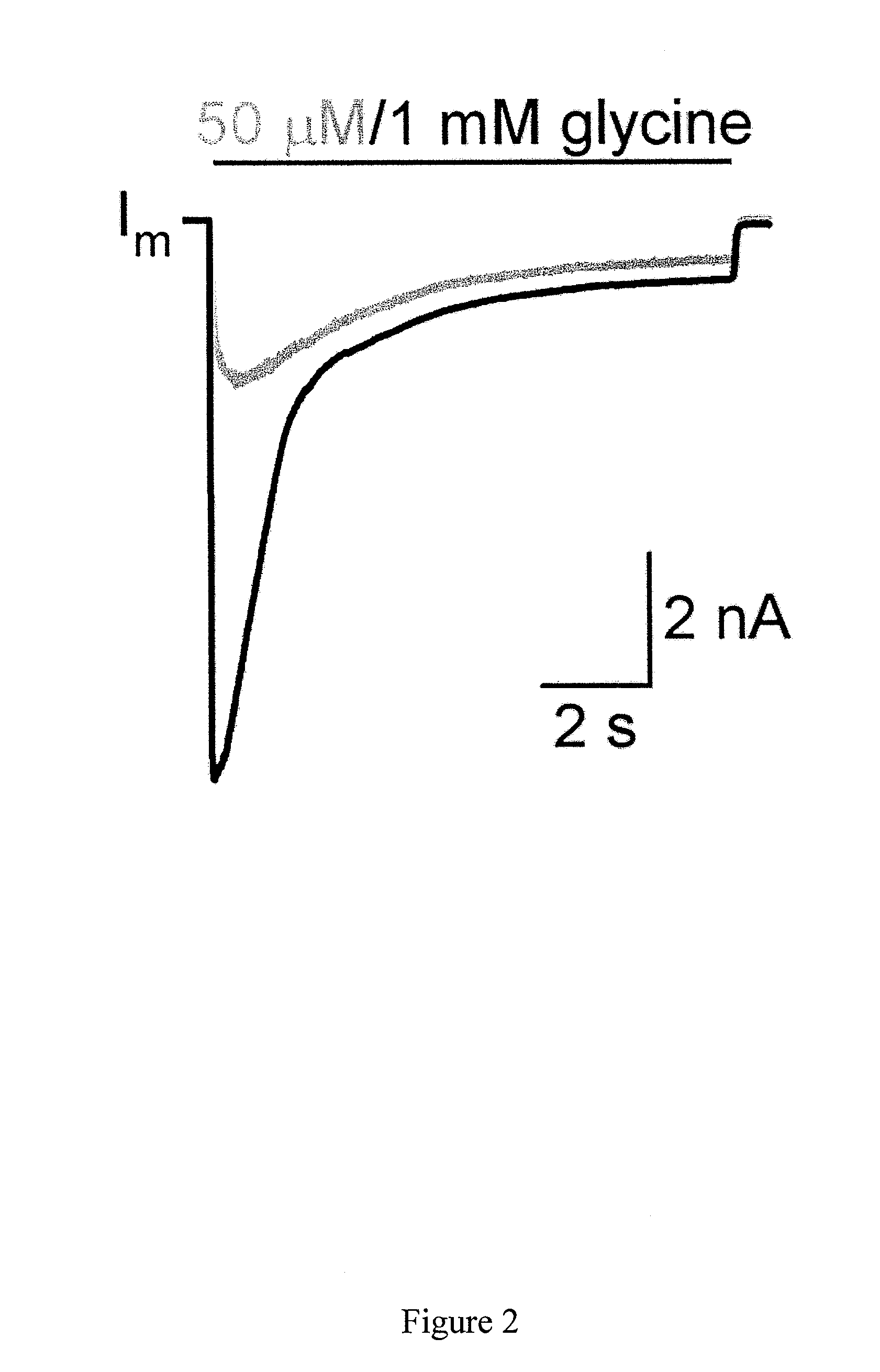
The invention provides a method of modulating electrophysiological activity of an excitable cell. The method involves causing exogenous expression of a glycine receptor (GlyR) protein in an excitable cell of a subject. Thereafter, the excitable cell is exposed to an allosteric modulator of the GlyR protein. Modulation of the exogenous GlyR protein (an ion channel) in response to the allosteric modulator modulates the electrophysiological activity of the excitable cell. The method can be used to control pain in a subject. The invention further provides a replication-defective HSV vector comprising an expression cassette encoding a GlyR protein, stocks and pharmaceutical compositions containing such vectors, and a transgenic animal.
Owner:UNIVERSITY OF PITTSBURGH
Selective ablation of pain-sensing neurons by administration of a vanilloid receptor agonist
The present invention provides methods and kits for the selective ablation of pain-sensing neurons. The methods comprise administration of a vanilloid receptor agonist to a ganglion in an amount that causes death of vanilloid receptor-bearing neurons. Accordingly, the present invention provides methods of controlling pain and inflammatory disorders that involve activation of vanilloid receptor-bearing neurons.
Owner:UNITED STATES OF AMERICA
Combination of effective substances causing synergistic effects of multiple targeting and use thereof
InactiveUS20150290181A1Good curative effectPrevention and improvementBiocideCosmetic preparationsWrinkle skinDisease
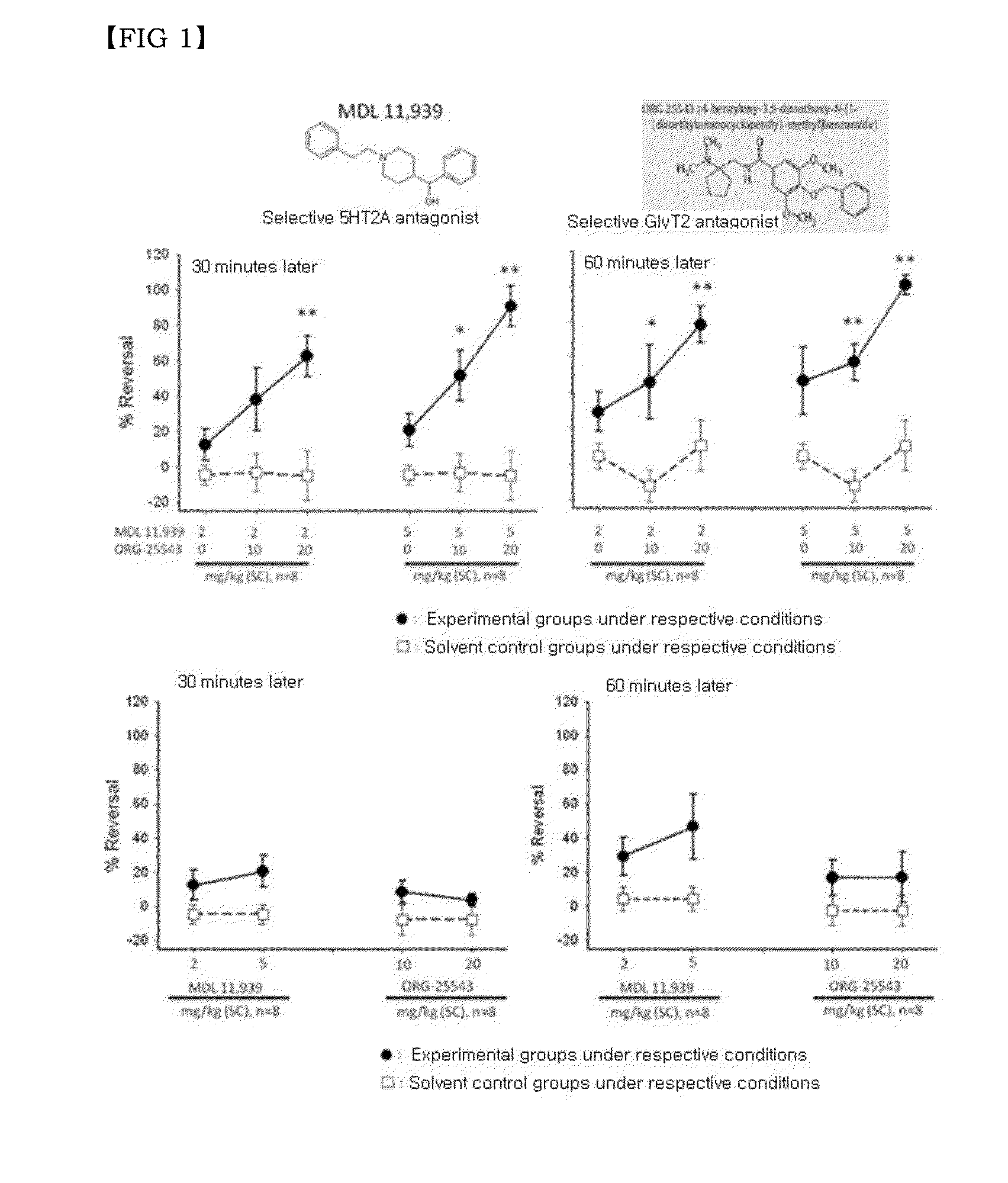
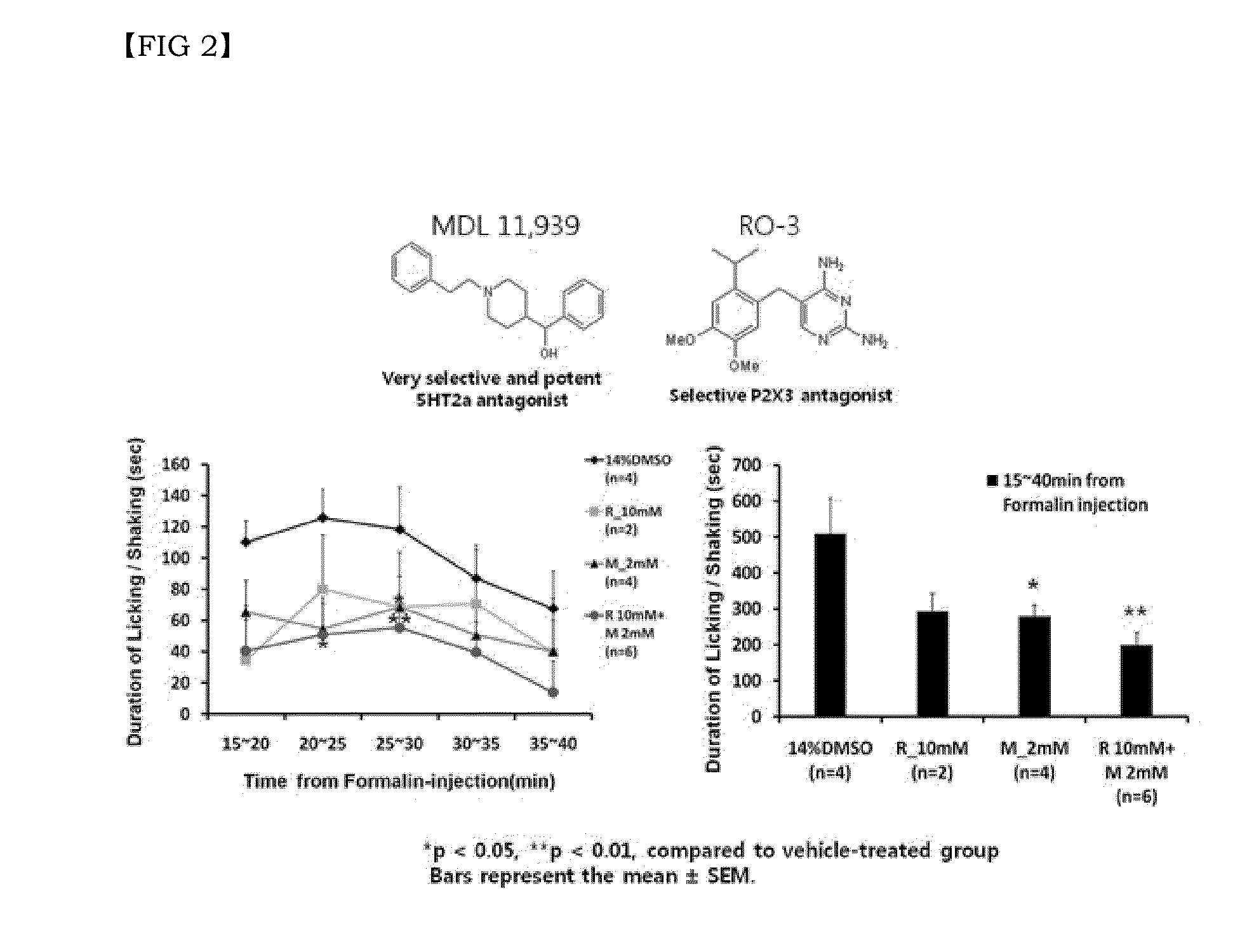
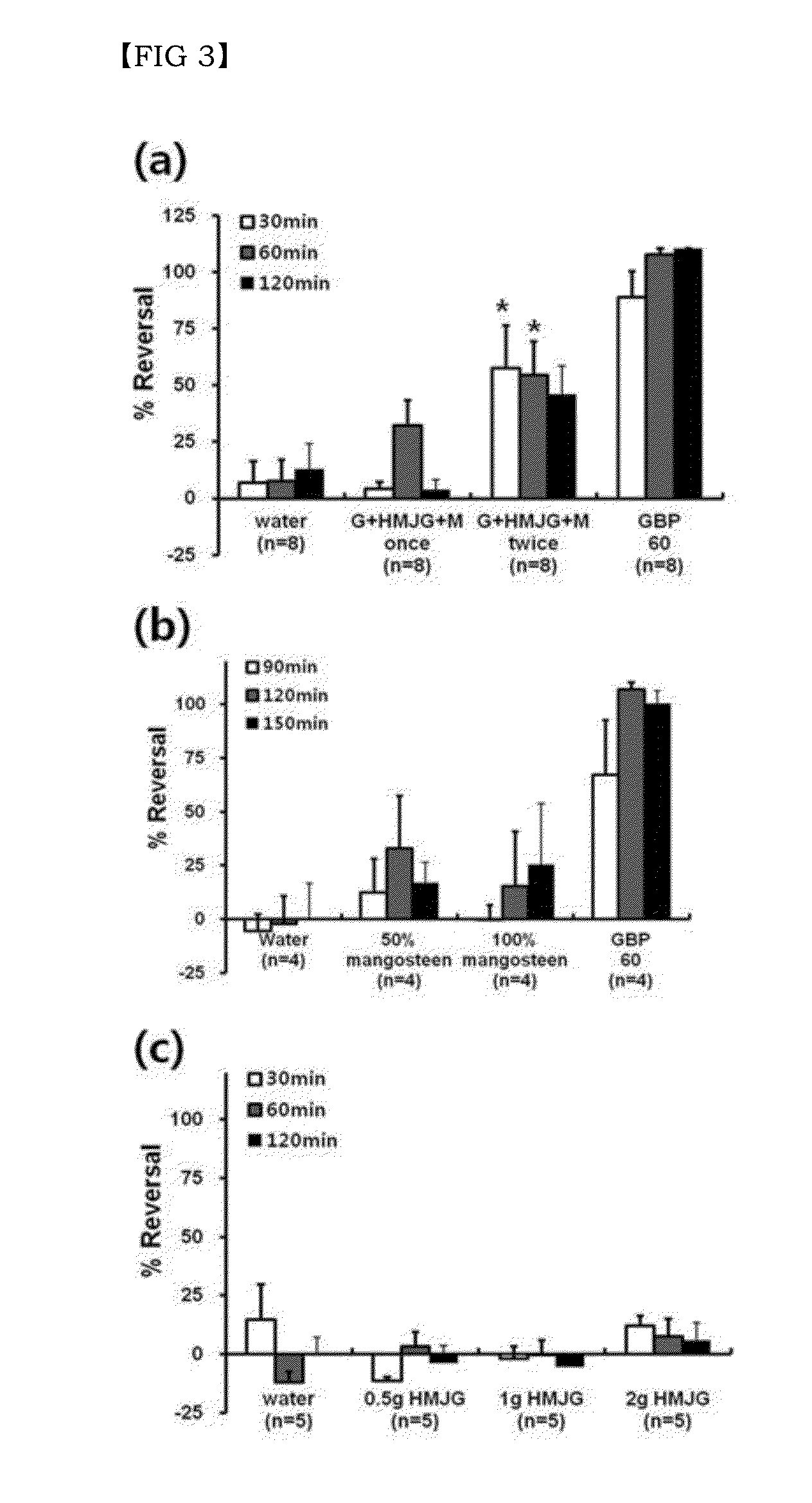
Disclosed are a combination of active components inducing synergistic effects of multi-targeting and a use thereof. More particularly, disclosed are a functional food composition, a cosmetic composition, a pain-suppressive composition, and a composition for treatment or prevention of pruritus or atopic dermatitis, which comprise as active components, two or more components selected from a group consisting of (a) a 5-hydroxytryptamine subtype 2 (5-HT2) receptor antagonist; (b) a P2X receptor antagonist; and (c) any one of a glycine receptor agonist, a glycine transporter (GlyT) antagonist, a gamma-aminobutyric acid (GABA) receptor agonist, and a GABA transporter 1 (GAT1) antagonist. The multi-targeting composite composition (specifically, natural substances composite composition) has synergistic effects, and thus a treatment using a combination of respective components may achieve increased biological effects, in which mechanisms targeted by respective components are involved. Thus, the multi-targeting composite composition may not only remarkably control pain but may also increase effects of: alleviating symptoms of skin diseases such as itching and atopic dermatitis; preventing or improving depression, refreshment, pore minimization, improving wrinkles, skin regeneration, skin health, recovery of skin condition, skin whitening, preventing or improving athlete's foot, recovery of scalp health and regeneration of scalp, promoting hair growth, preventing gray hair, and improving dental and periodontal diseases, etc.
Owner:VIVOZON
Controlled release oxycodone compositions
InactiveUS20070275065A1Improve efficiencyQuality improvementBiocidePill deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately. 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean,of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour): administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Dermal delivery of anti-pain agents and methods useful thereof
The present invention relates to methods and devices for delivery of therapeutically and / or prophylactically effective amounts of agents for management of pain, particularly anti-migraine agents, more particularly triptan compounds, by depositing the agent into the intradermal or junctional compartment of a subject's skin. Agents delivered in accordance with the methods of the invention have an improved clinical utility and therapeutic efficacy relative to other drug delivery methods, including intraperitoneal, intramuscular and subcutaneous delivery. The methods of the present invention provide benefits and improvements over conventional drug delivery methods including dose sparing, increased drug efficacy, reduced side effects.
Owner:BECTON DICKINSON & CO
Targeted delivery of glycine receptors to excitable cells
ActiveUS20110213017A1Nervous disorderCell receptors/surface-antigens/surface-determinantsGlycine receptorAllosteric modulator
The invention provides a method of modulating electrophysiological activity of an excitable cell. The method involves causing exogenous expression of a glycine receptor (GlyR) protein in an excitable cell of a subject. Thereafter, the excitable cell is exposed to an allosteric modulator of the GlyR protein. Modulation of the exogenous GlyR protein (an ion channel) in response to the allosteric modulator modulates the electrophysiological activity of the excitable cell. The method can be used to control pain in a subject. The invention further provides a replication-defective HSV vector comprising an expression cassette encoding a GlyR protein, stocks and pharmaceutical compositions containing such vectors, and a transgenic animal.
Owner:UNIVERSITY OF PITTSBURGH
Compositions and methods for controlling pain
ActiveUS20160199663A1Relieve painNervous disorderPeptide/protein ingredientsControlling painControlled pain
The present disclosure provides compositions and methods for controlling pain. The present disclosure provides methods for identifying agents that control pain.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Extended release analgesic for pain control
InactiveUS6939538B2Electrostatic shieldingAvoid accessSalicyclic acid active ingredientsBiocideControlled releaseAbdominal cavity
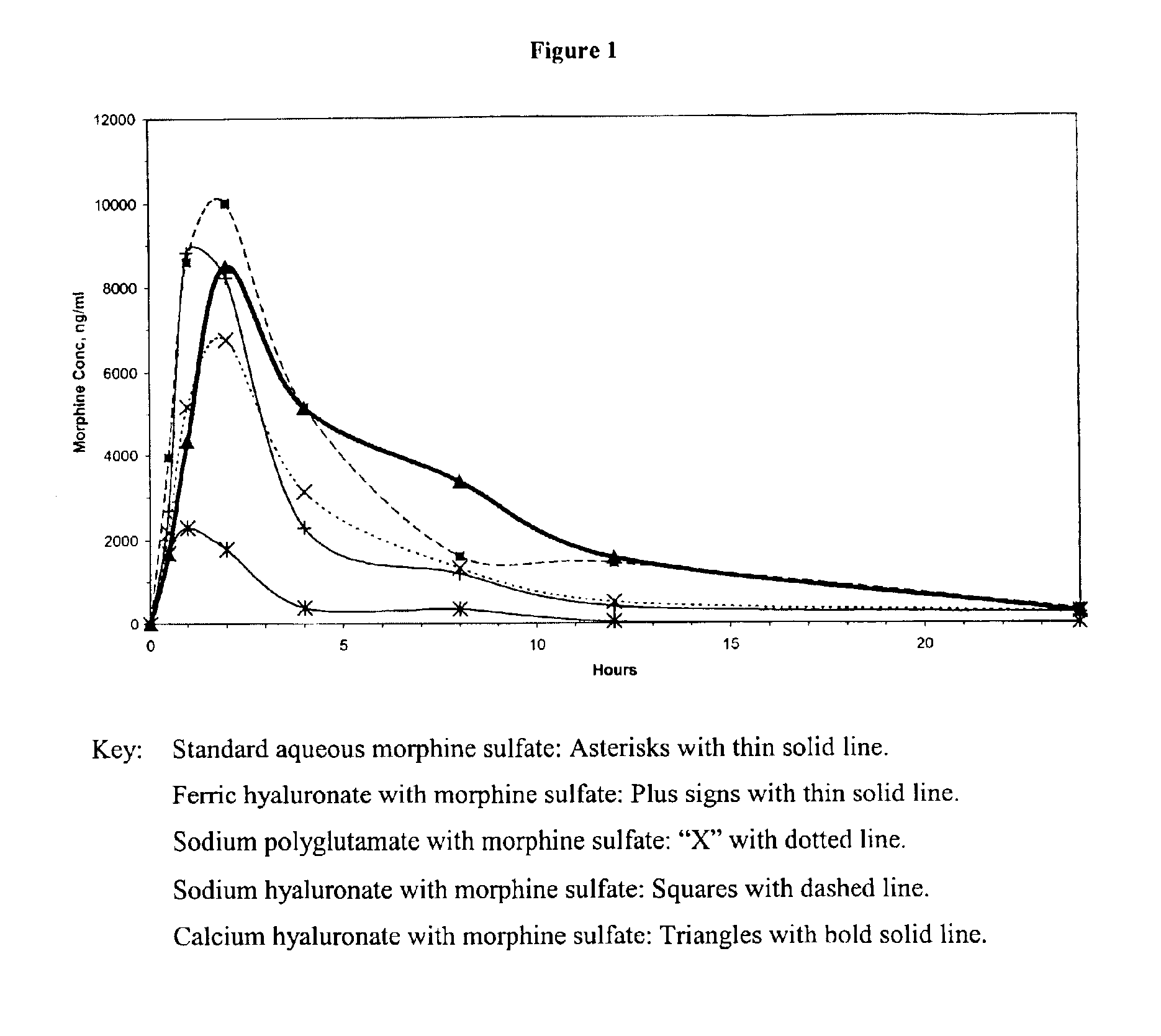
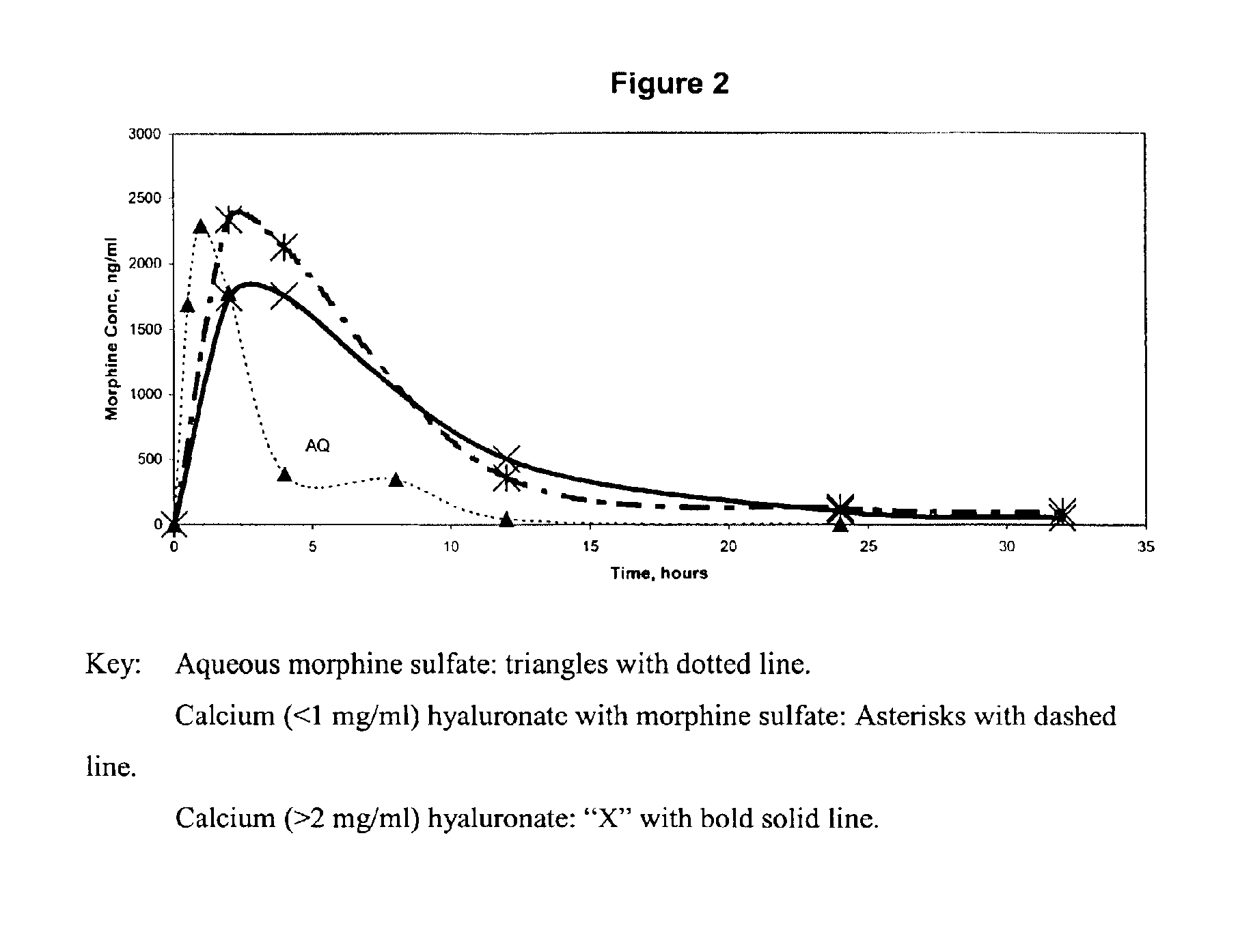
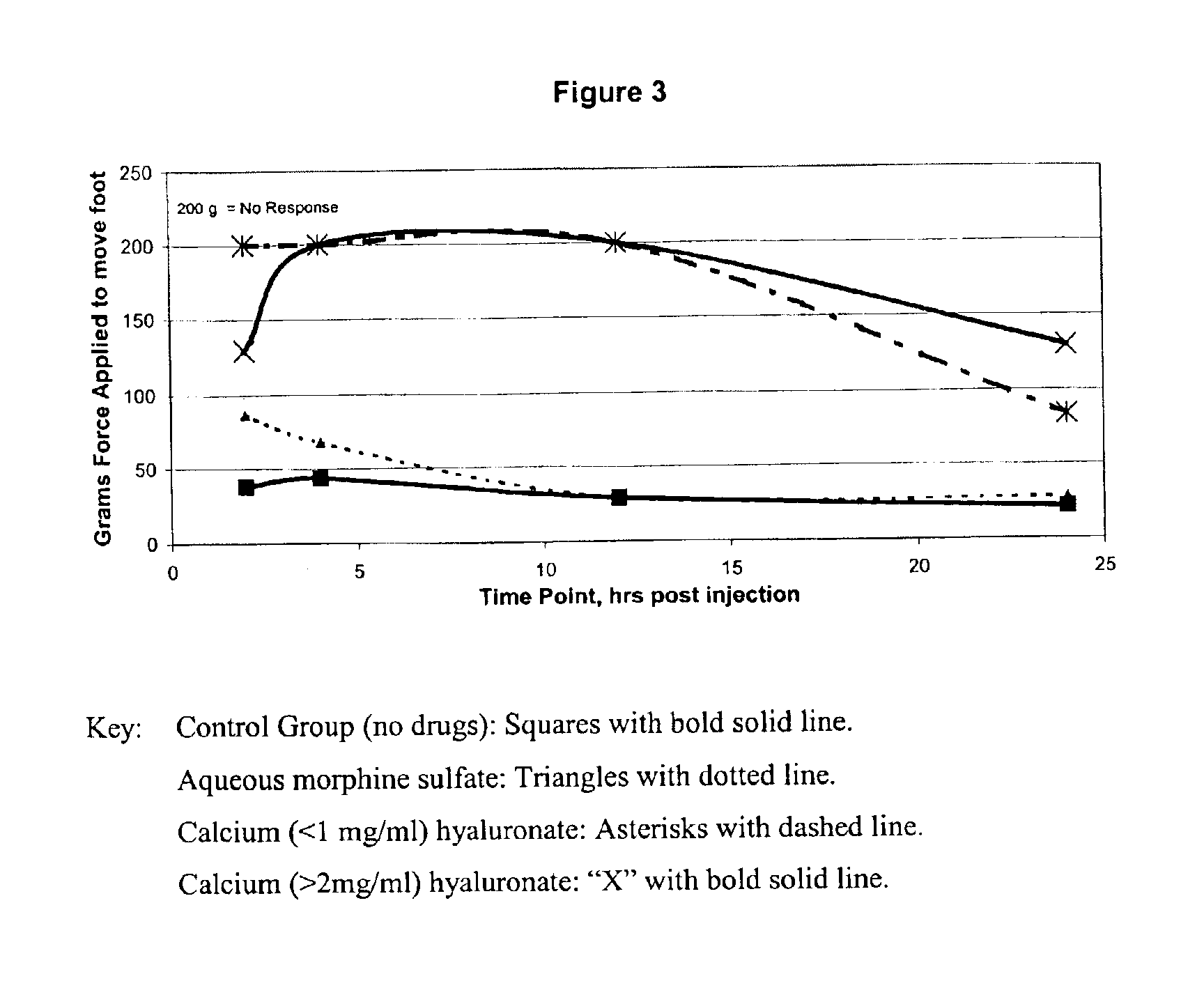
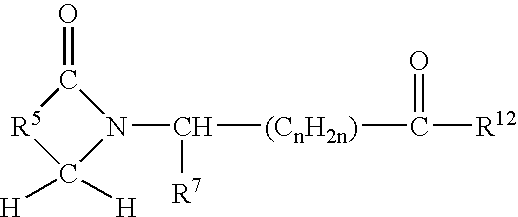
An extended release analgesic for controlling pain comprised of an opioid or non-opioid analgesic drug ionically bound to hyaluronic acid, poly-γ-glutamic acid or other ionic polymers, and injected into a body either subcutaneously, intramuscularly or intraperitoneally, utilizing counter-ions of different valences to control the rate of release into the body.
Owner:BIOMEDICAL RES MODELS
Methods of using and compositions comprising selective cytokine inhibitory drugs for treatment, modification and management of pain
InactiveUS20070161696A1Extension of timeAvoid managementBiocideSalicyclic acid active ingredientsCytokine SuppressionPhysical Therapy Modality
Methods of treating, preventing, modifying and managing various types of pain are disclosed. Specific methods comprise the administration of a selective cytokine inhibitory drug, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or surgery, psychological or physical therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Methods of Managing Pain Using Dexmedetomidine Transdermal Delivery Devices
PendingUS20180117012A1Sufficient amountPractical methodOrganic active ingredientsAntipyreticManaged painDexmedetomidine
Aspects of the invention include methods of managing pain in a subject by applying a transdermal delivery device containing a dexmedetomidine composition formulated to deliver a pain relieving effective amount of dexmedetomidine to a subject. In practicing methods according to certain embodiments, a transdermal delivery device having a dexmedetomidine composition is applied to a subject and is maintained in contact with the subject in a manner sufficient to deliver an amount of dexmedetomidine effective to manage pain in the subject. In some embodiments, methods include hydrating the subject, such as by administering a hydration fluid composition to the subject. Methods according to certain embodiments may also include co-administering an opioid to the subject. Also provided is a transdermal delivery device configured to deliver dexmedetomidine sufficient for practicing the subject methods, as well as kits containing the transdermal delivery device.
Owner:TEIKOKU PHARMA USA INC
Portable laser and process for producing controlled pain
ActiveUS20050027336A1Avoid disagreementReduce beam divergenceDiagnostics using lightSurgical instrument detailsElectrophysiology studySingle type
A process and laser system for in vitro and in vivo pain research, pain clinical testing and pain management. In preferred embodiments of the present invention a diode laser operating at a 980 nm wavelength is used to produce warmth, tickling, itching, touch, burning, hot pain or pin-prick pain. The device and methods can be used for stimulation of a single nerve fiber, groups of nerve fibers, nerve fibers of single type only as well as more the one type of nerve fibers simultaneously. The present invention is especially useful for research of human / animal sensitivity, pain management, drug investigation and testing, and psychophysiology / electrophysiology studies. The device and methods permit non-contact, reproducible and controlled tests that avoid risk of skin damage. Applicant an his fellow workers have shown that tests with human subjects with the process and laser of the present invention correlate perfectly with laboratory tests with nerve fibers of rats. The device and the methods can be applied in a wide variety of situations involving the study and treatment of pain. Preferred embodiments of the present invention provide laser systems and techniques that permit mapping and single mode activation of C fibers and A-delta fibers.
Owner:NEMENOV MIKHAIL
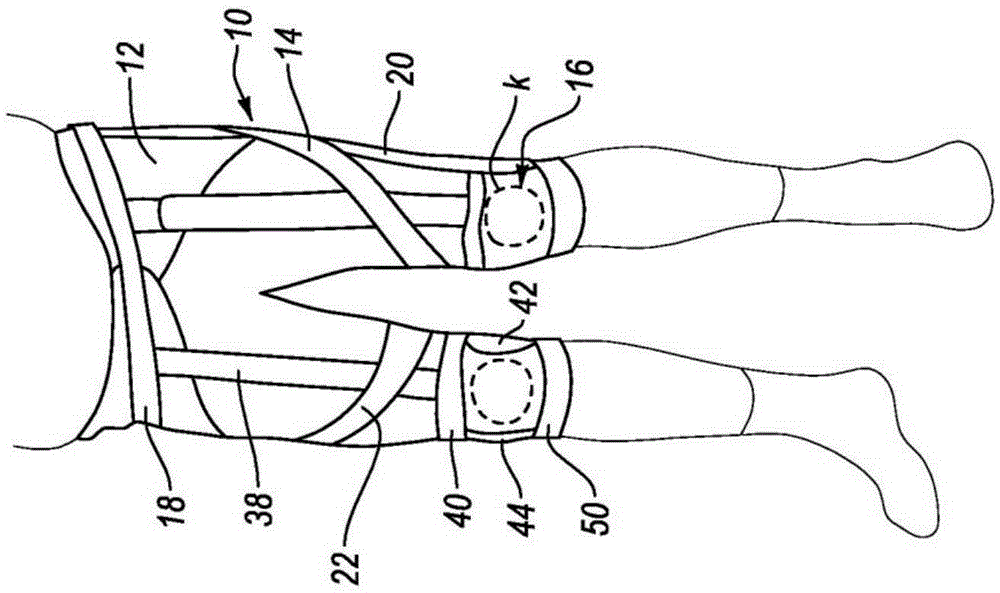
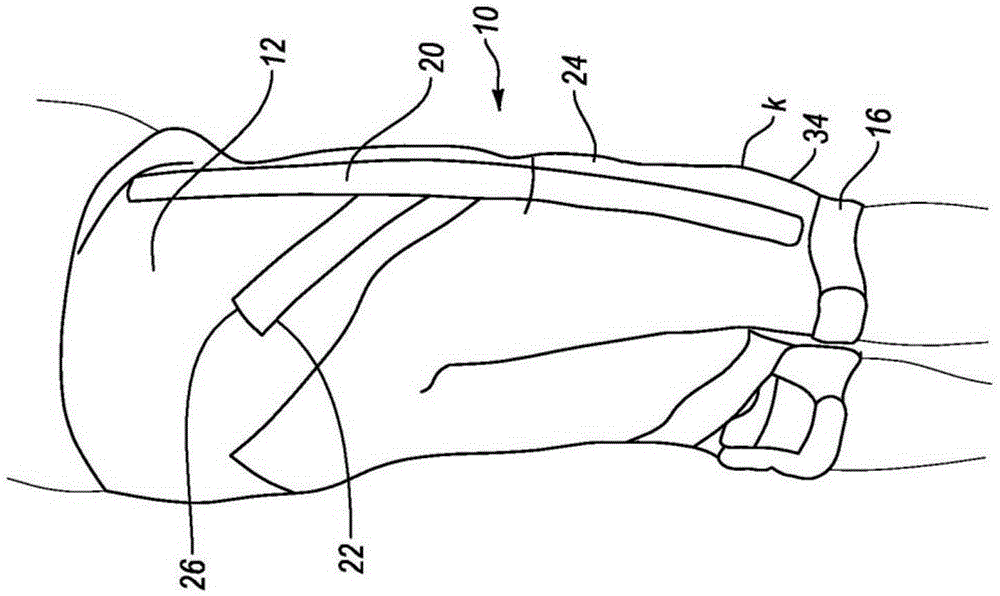
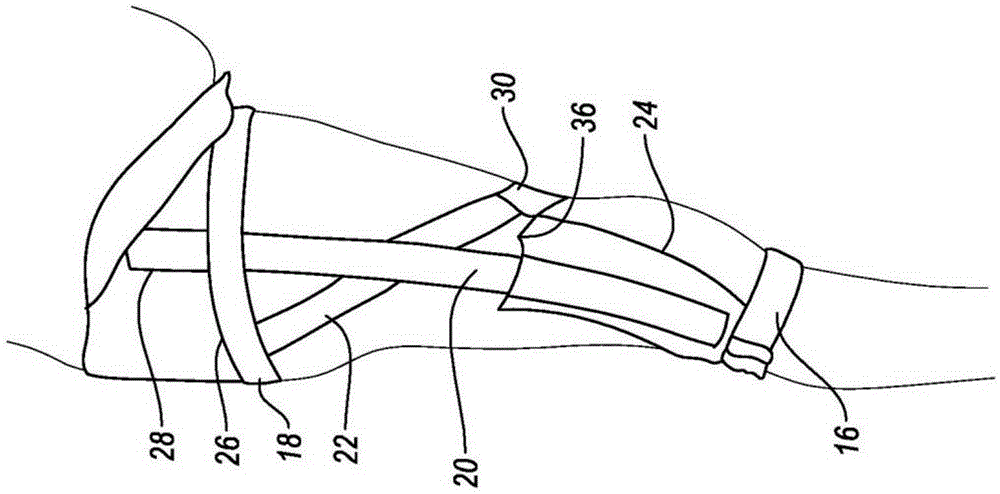
Orthopedic device for treating complications of the hip
An orthopedic device (200) is provided for treating complications of the hip and has means for trochanter compression, pelvis support, lumbar compression, variously directed straps, and thigh support. The trochanter compression and an internal / external rotation strap (217) provide pain relief through compression and skin protection, unloading of joints through compression and sealing, and unloading by load transfer. Means (220) for adjustably dosing of straps enables pain management and ease of use.
Owner:OSSUR HF
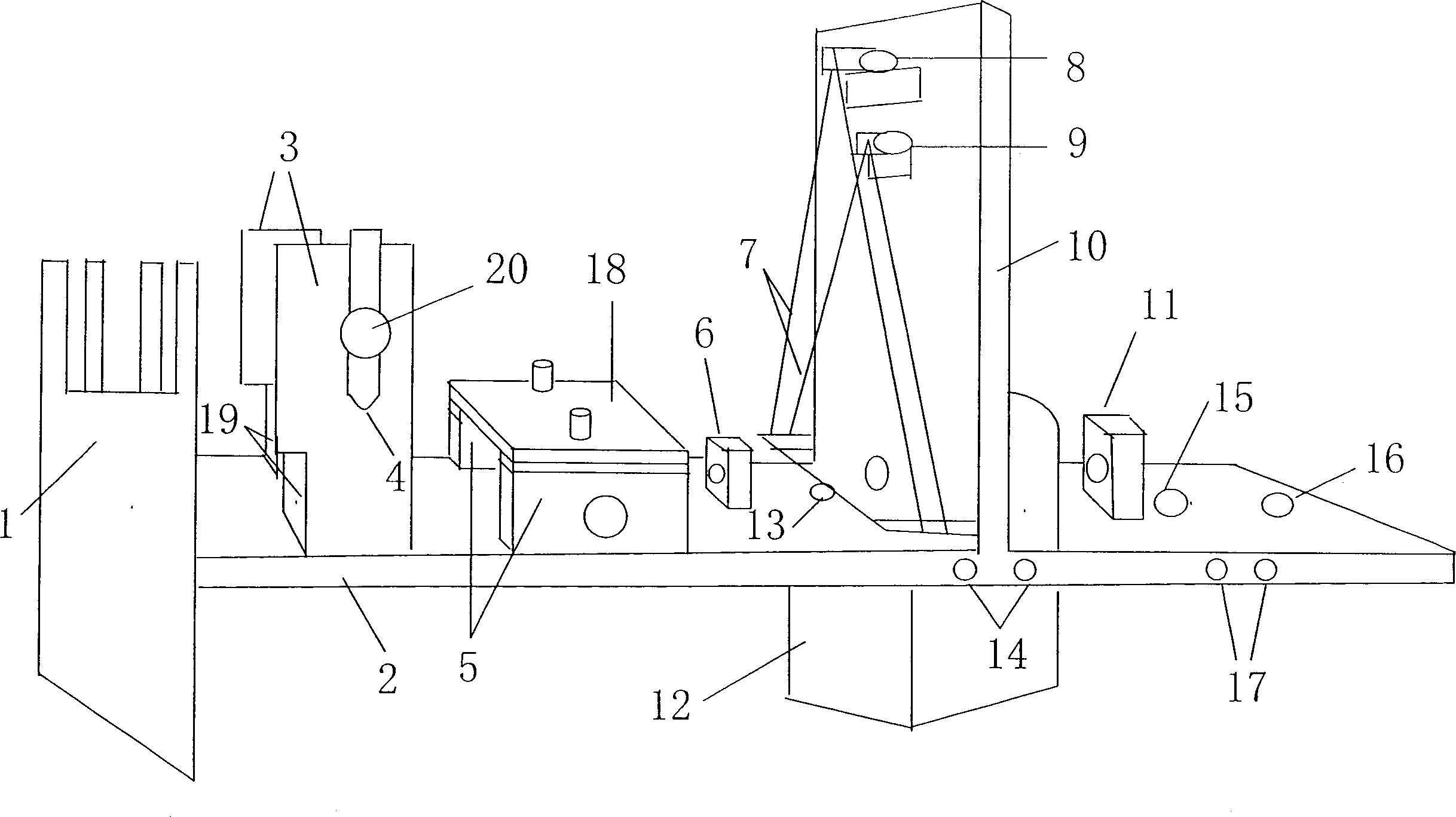
Fixing device for experiment of stimulates of senses of pain and heat
InactiveCN1915186AAvoid damageSolve the problem of damage fixationDiagnostic recording/measuringSensorsNMR - Nuclear magnetic resonanceControlling pain
A fixing apparatus for the remotely controlled pain sense / warmth sense experiment of rat or mouse in functional nuclear magnetic resonance imaging environment is composed of animal fixer, coil fixer, beater for pain sense, and stimulating box for wormth sense.
Owner:徐杰 +1
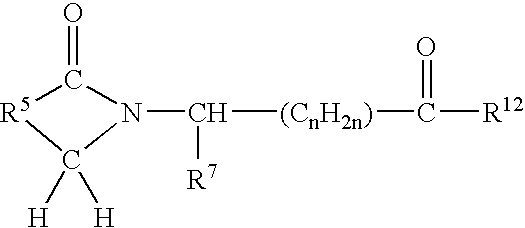
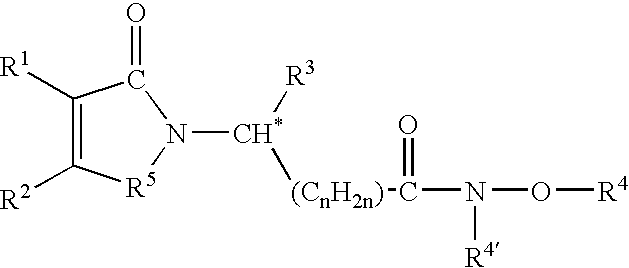
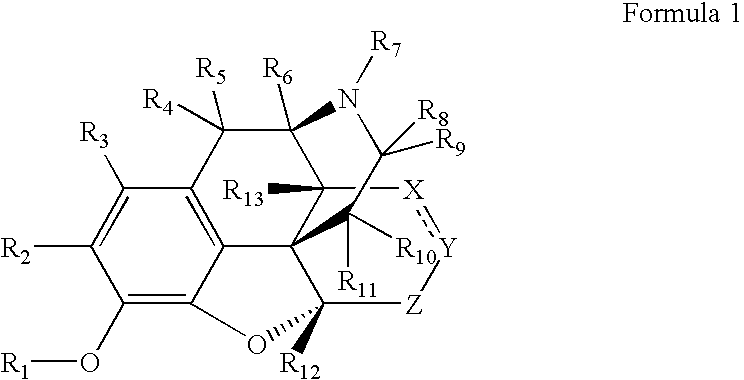
Preparation and utility of opioid analgesics
The present disclosure is directed to modulators of opiate- and / or NMDA receptors and pharmaceutically acceptable salts and prodrugs thereof, the chemical synthesis thereof, and the use of such compounds for the treatment and / or management of pain, anxiety, neurodegeneration, drug dependence, coughing, muscular tension, and / or glaucoma and any other condition in which it is beneficial to modulate an opiate- and / or NMDA receptor.
Owner:AUSPEX PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com