Patents
Literature
456results about How to "Improve efficiency and quality" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
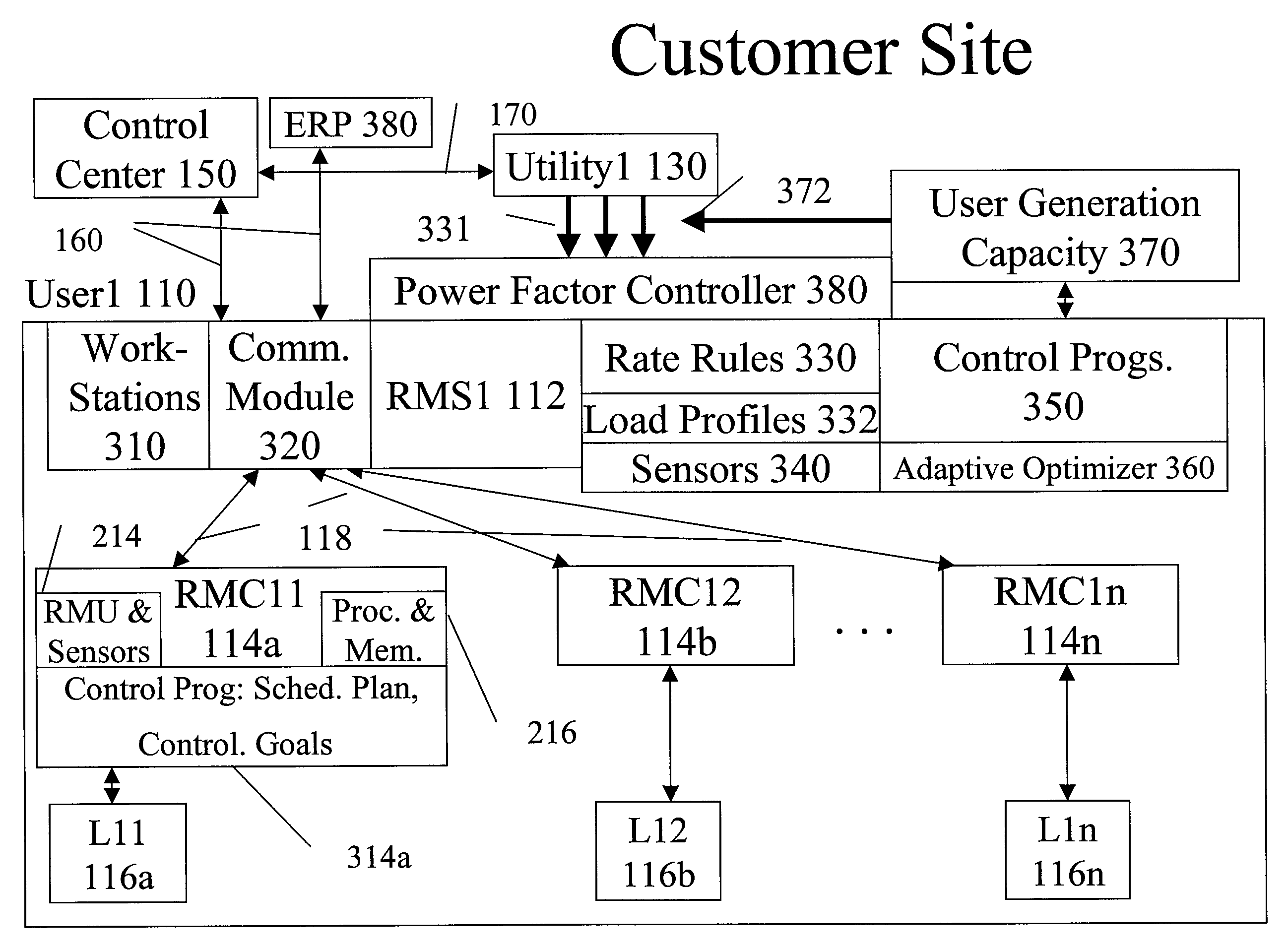
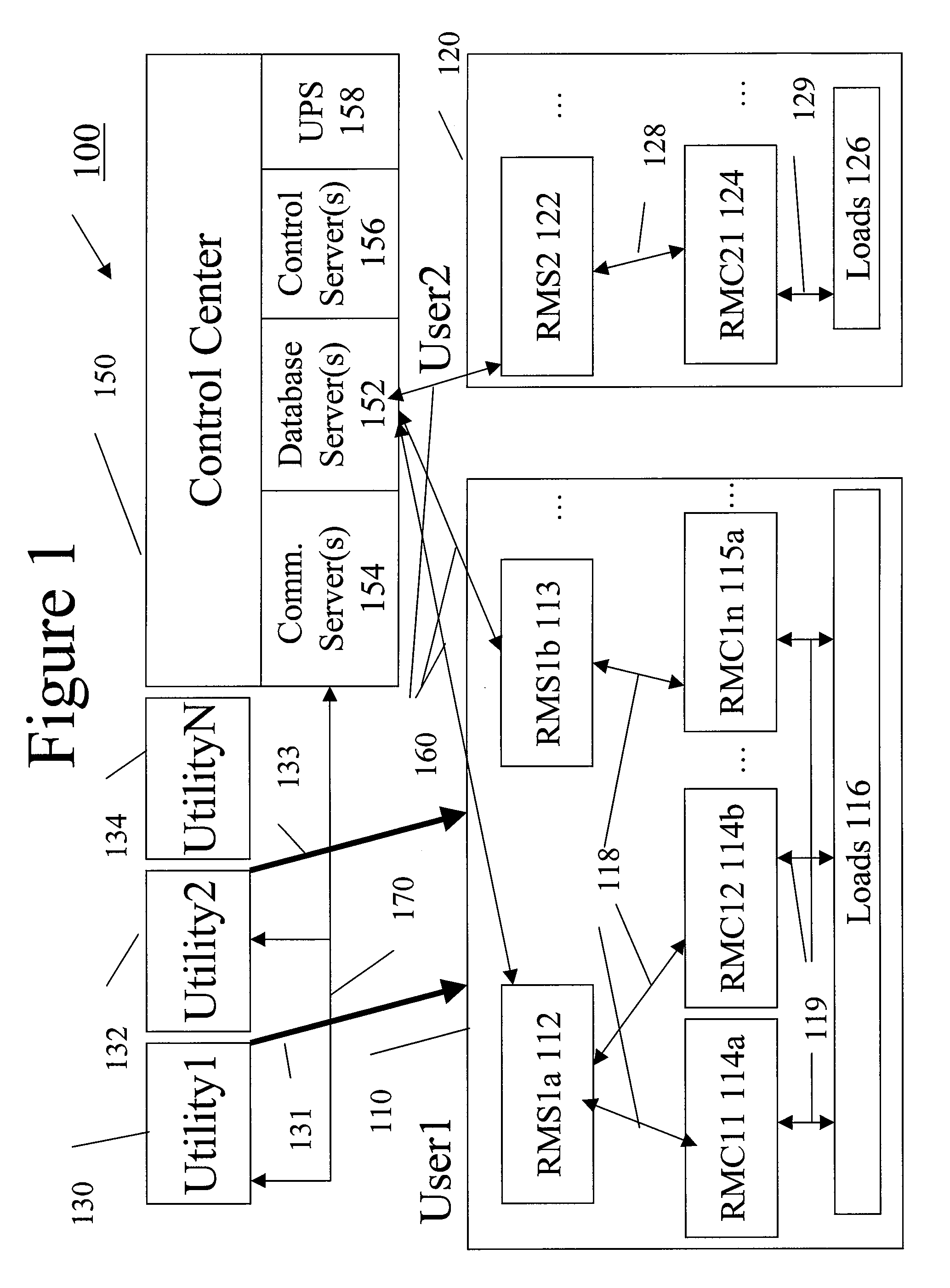
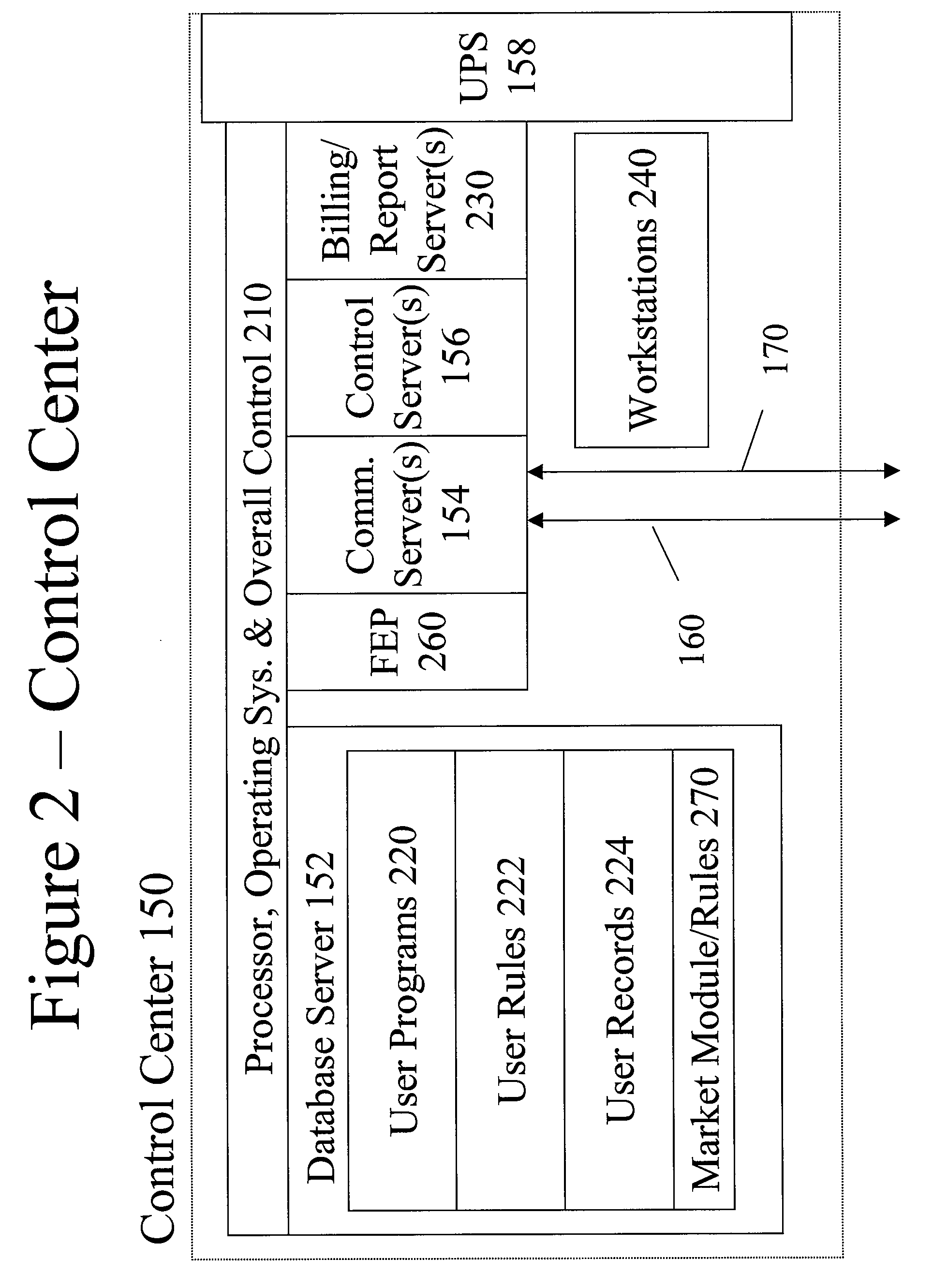
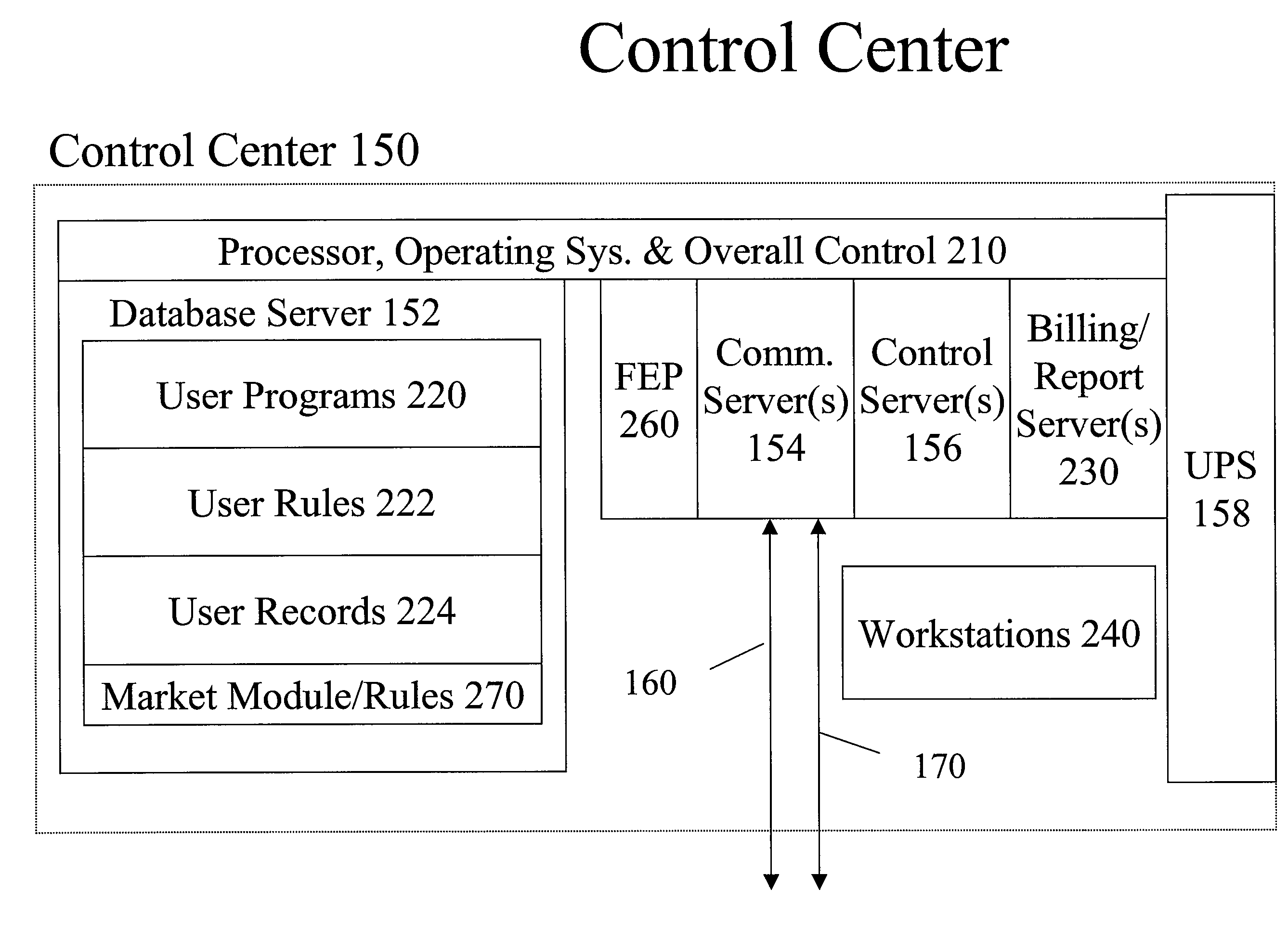
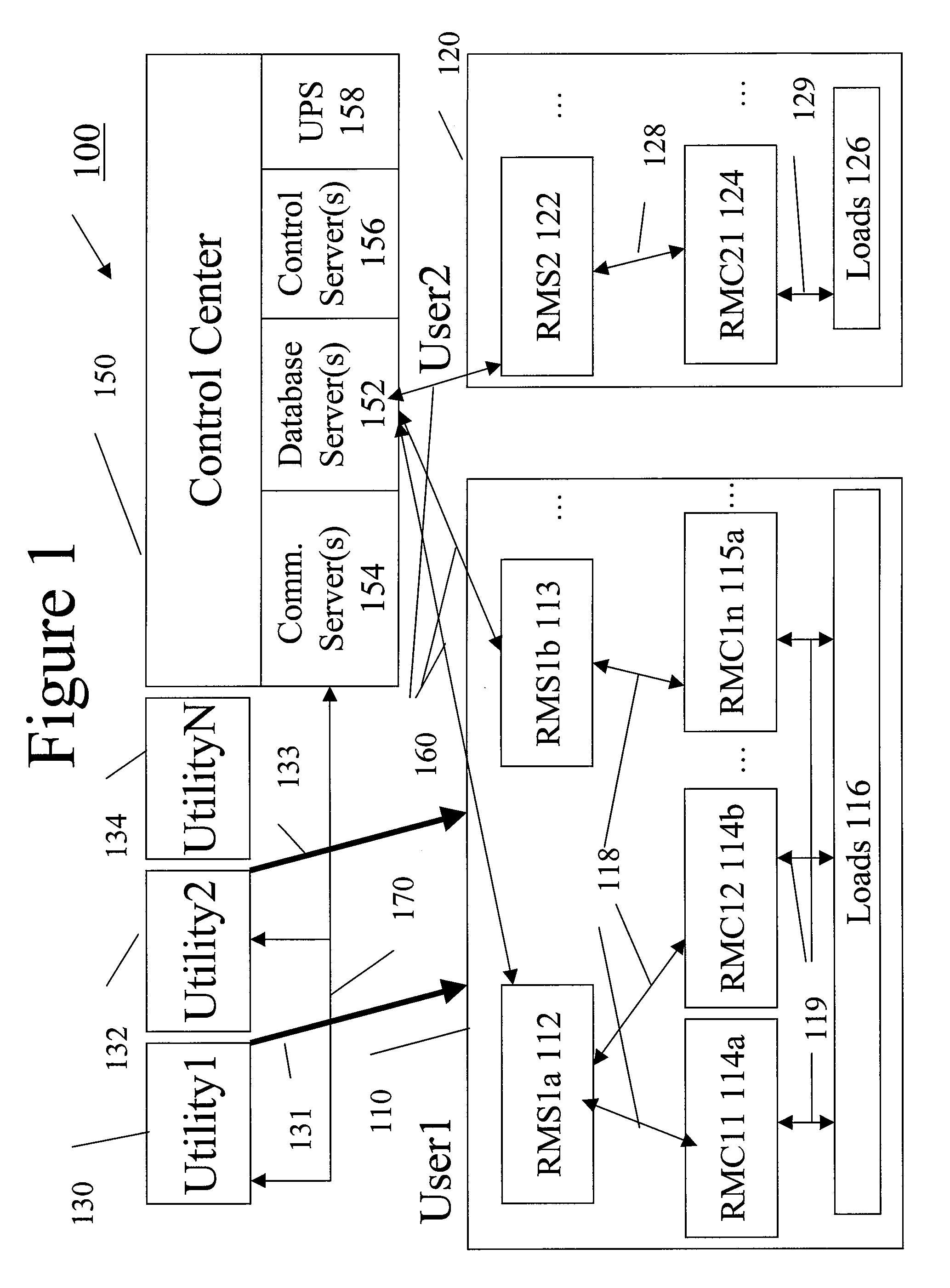
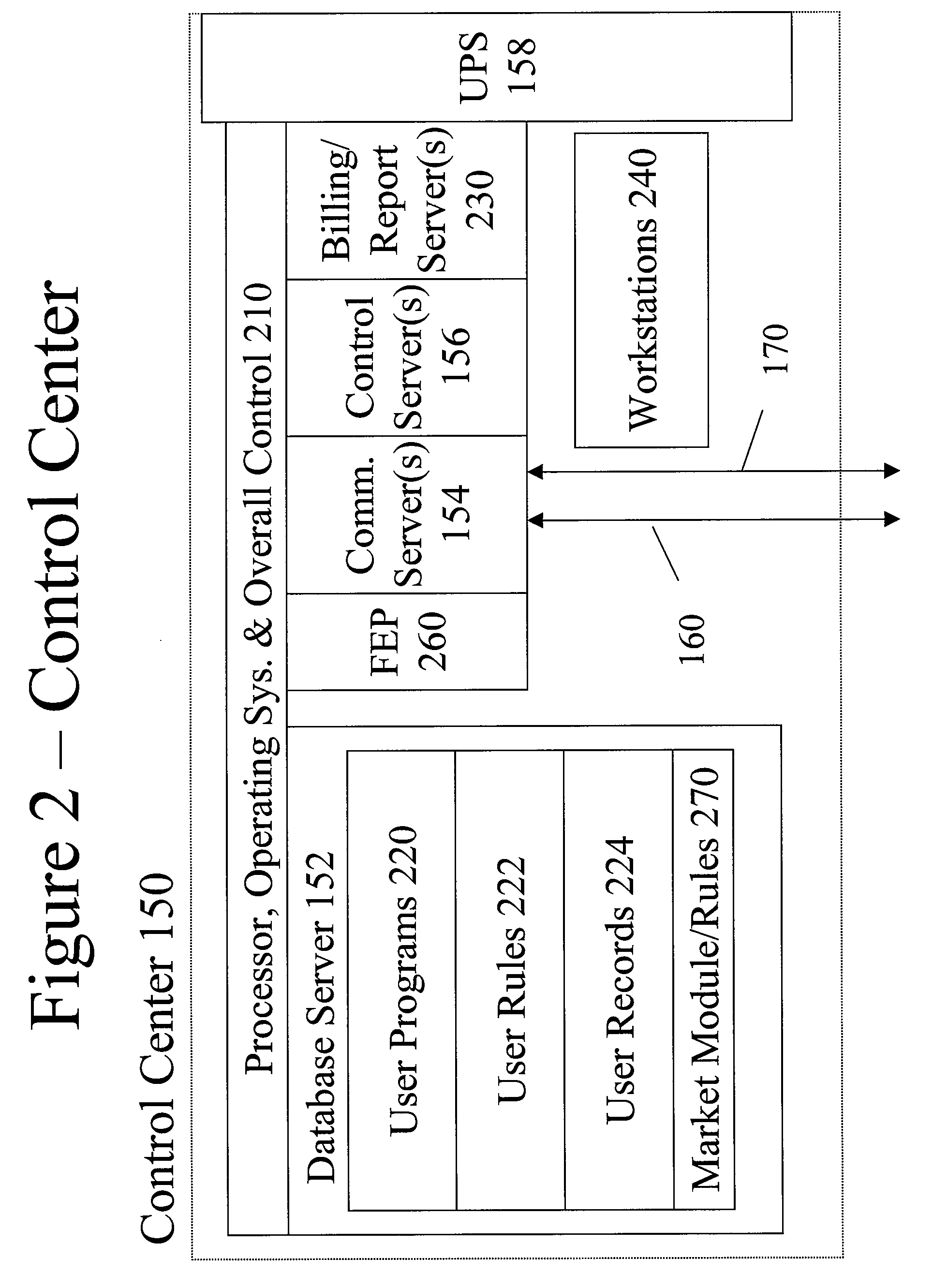
System and method for resource management
InactiveUS20080172312A1Low costImprove efficiencyElectric signal transmission systemsLevel controlResource managementSystem usage
A system uses an intelligent load controller for managing use of a consumable resource at an associated load. The controller has a resource measuring component for measuring the rate of use of the resource by the associated load, including measuring at least one of an instantaneous usage rate and a usage rate over an integration period and a load status component for receiving load status data for the associated load. The controller also has a communication component for receiving control messages from and sending load status messages to other associated controllers; a memory for storing a load control goal set; and a load control computer program responsive to the resource measuring component, the load status component, the control messages from other associated controllers and the load control goal set, to determine a load operating level for, and provide control commands to, the associated load.
Owner:SYNESIOU ANDREAS JOANNI +1
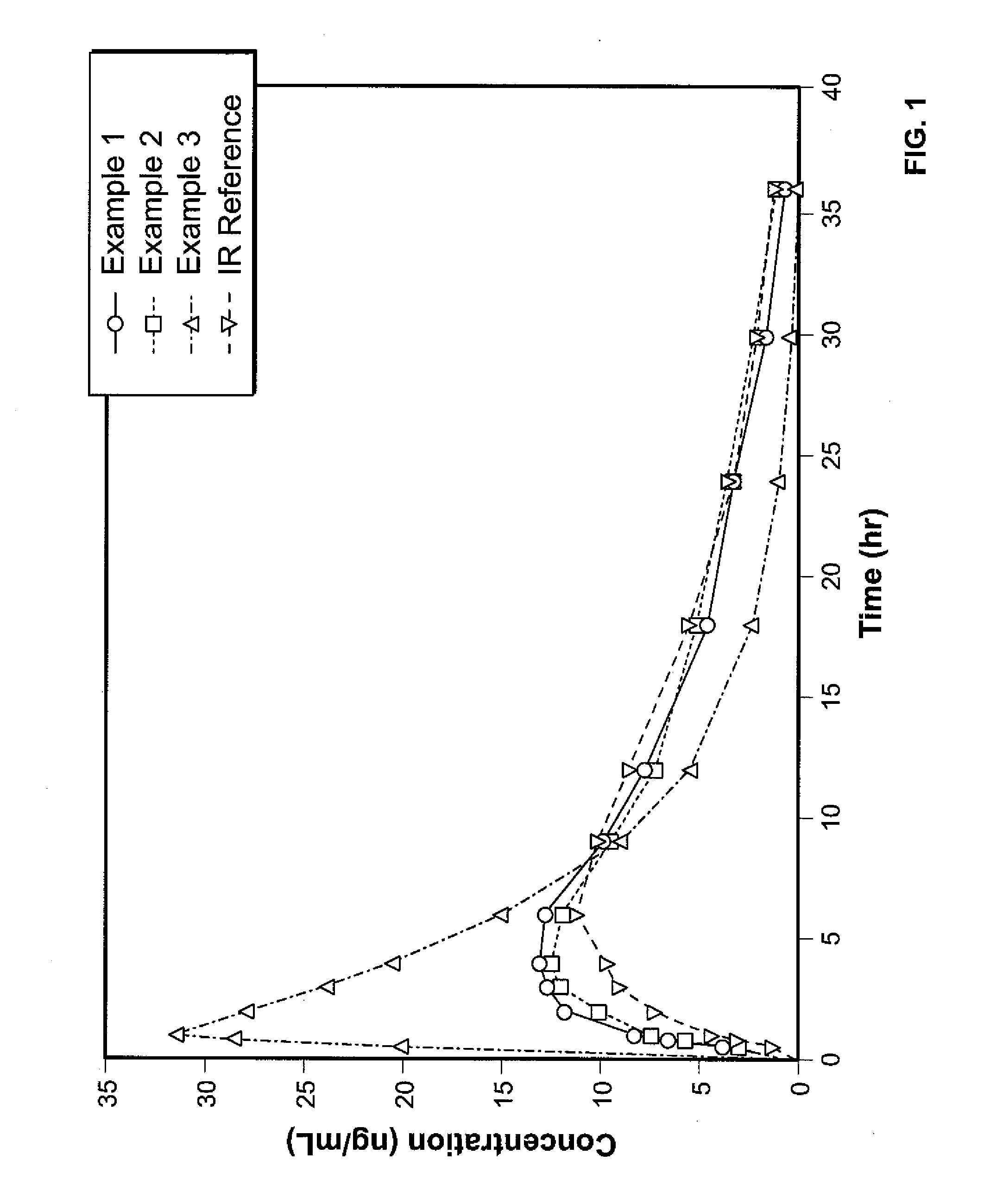
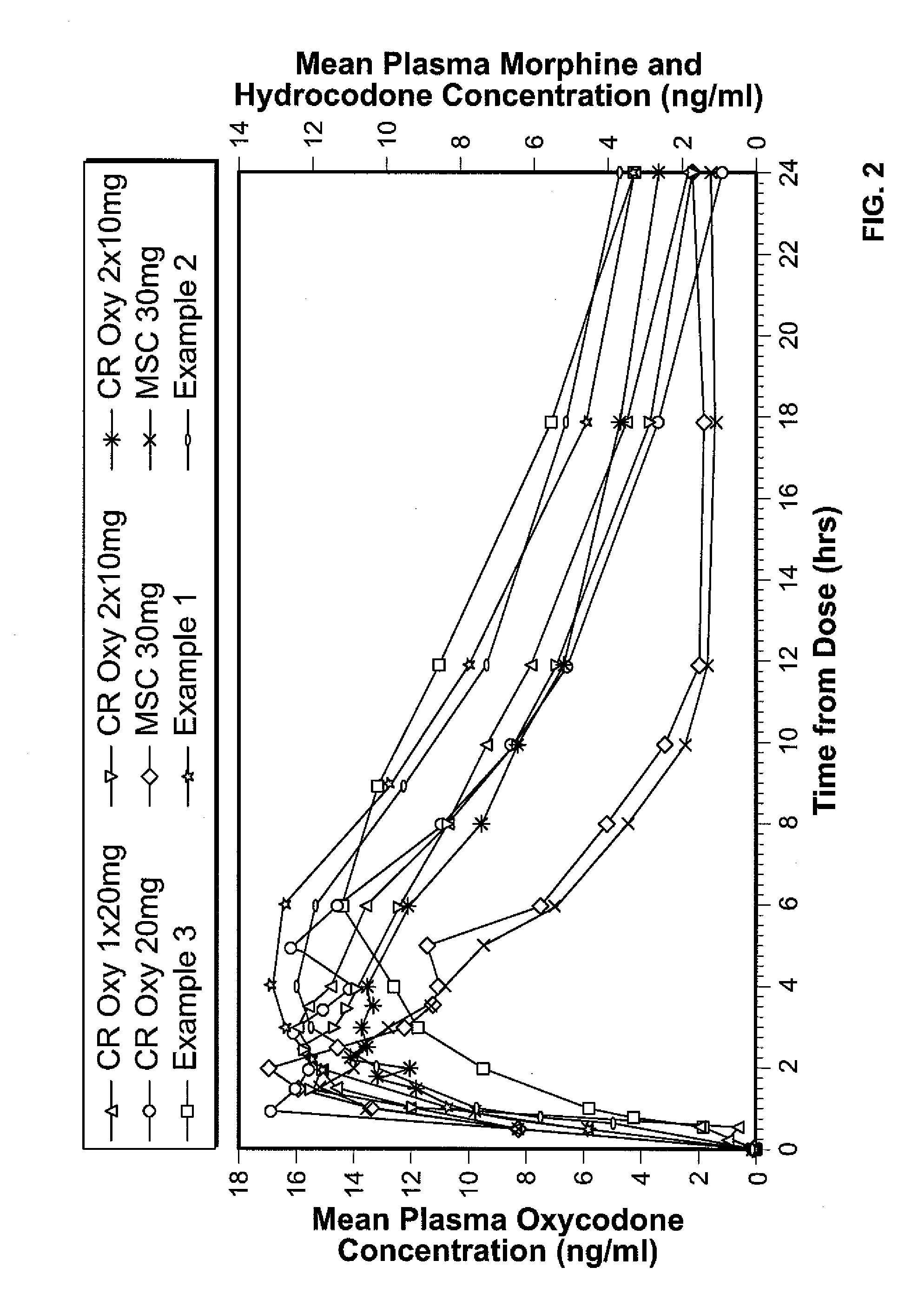
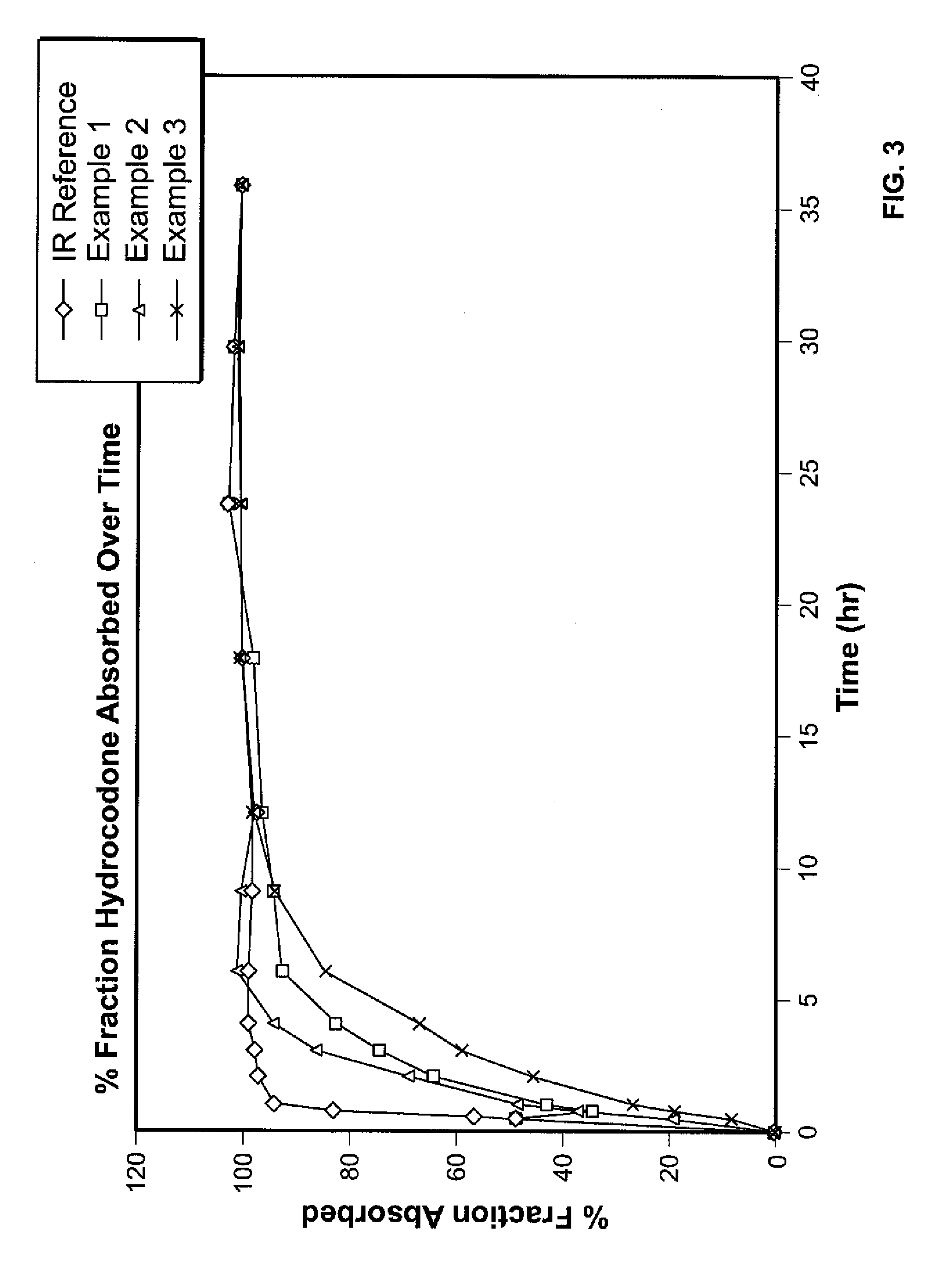
Controlled release hydrocodone formulations
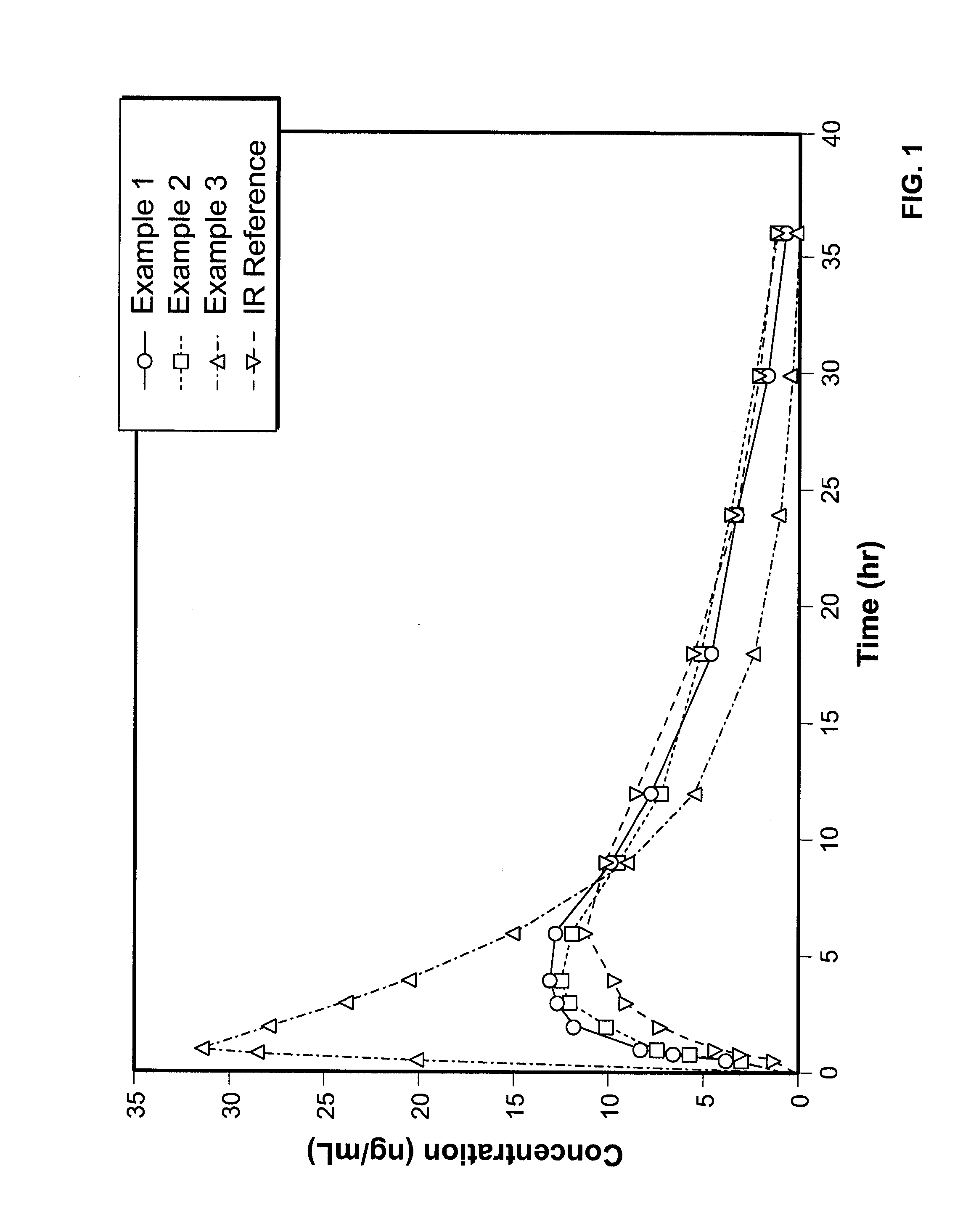
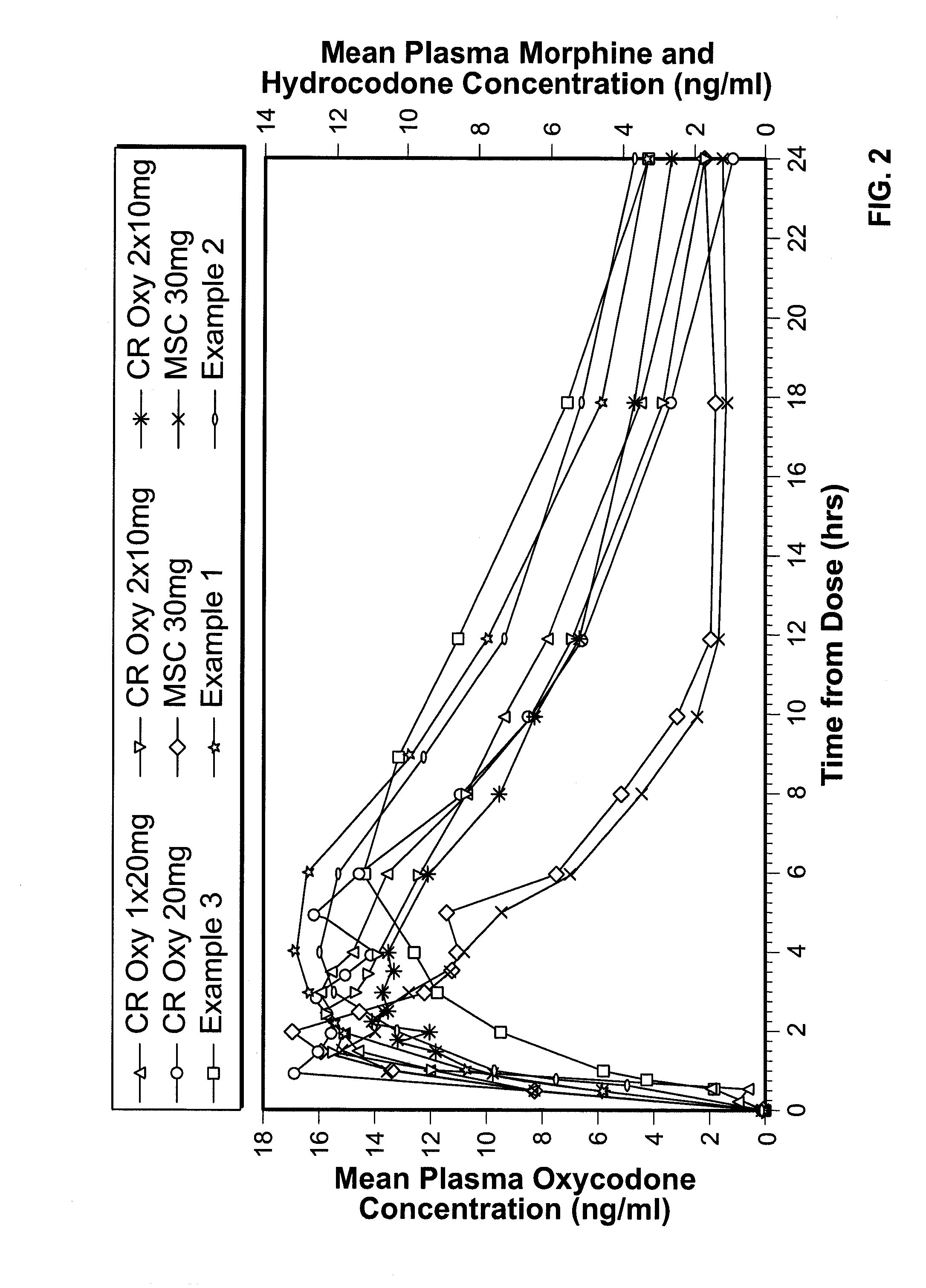
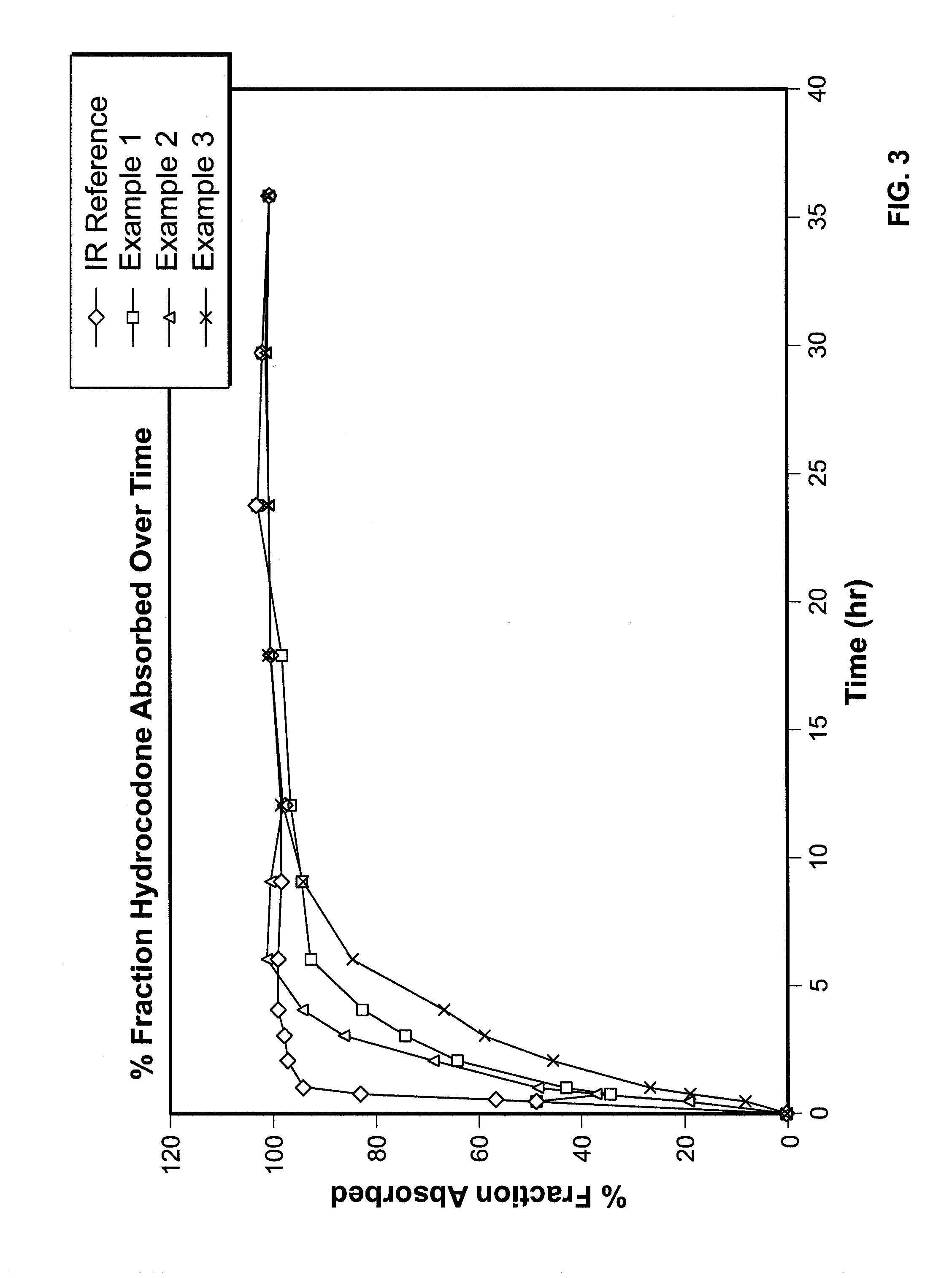
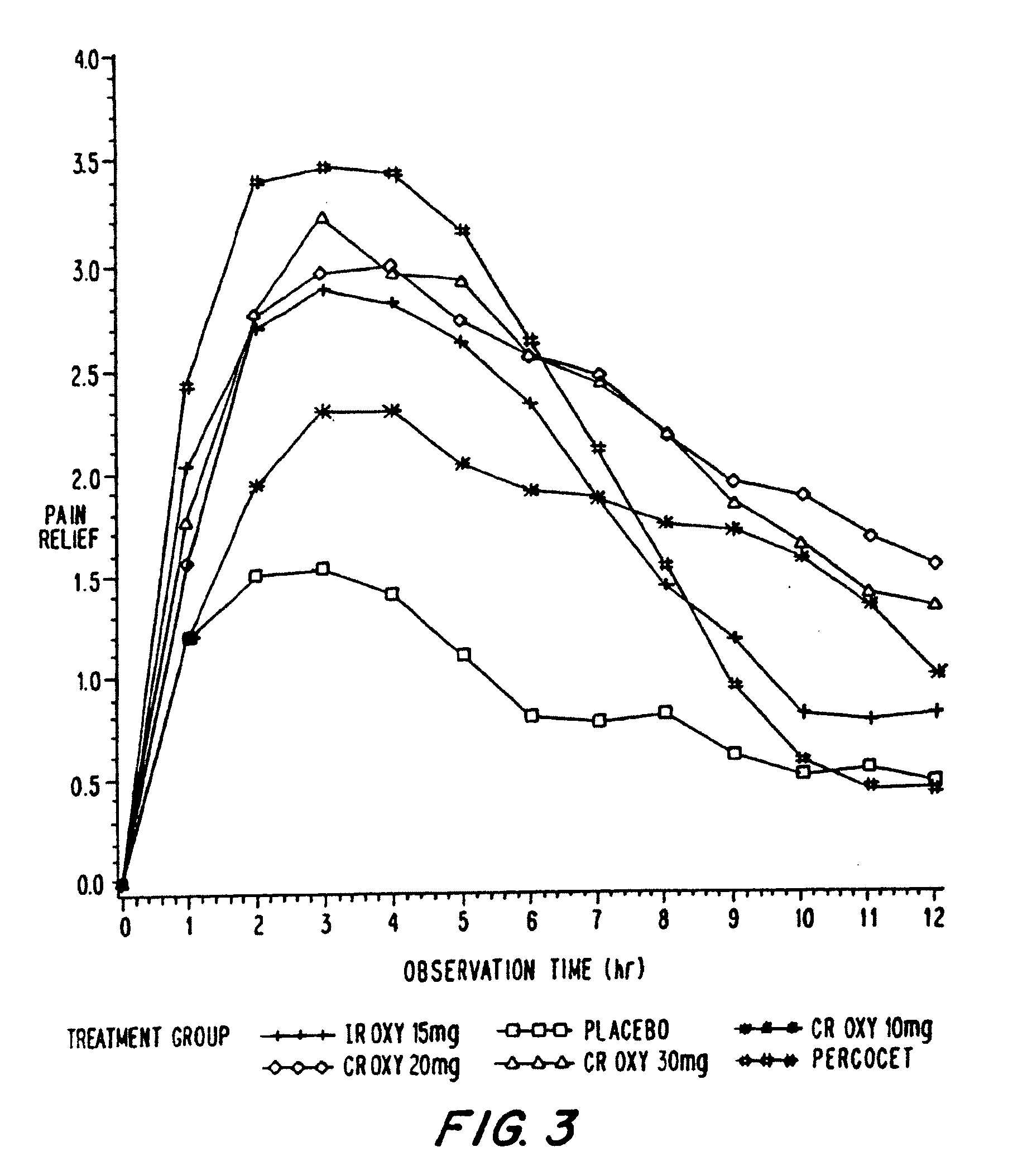
InactiveUS7943174B2No difference in analgesic efficacyGreat efficacyBiocidePowder deliveryTreatment effectDosage form
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
System for execution of a load operating plan for load control
InactiveUS7873441B2Improve efficiency and qualityImprove stabilityElectric signal transmission systemsLevel controlComputer programReal-time computing
Owner:SYNESIOU ANDREAS JOANNI +1
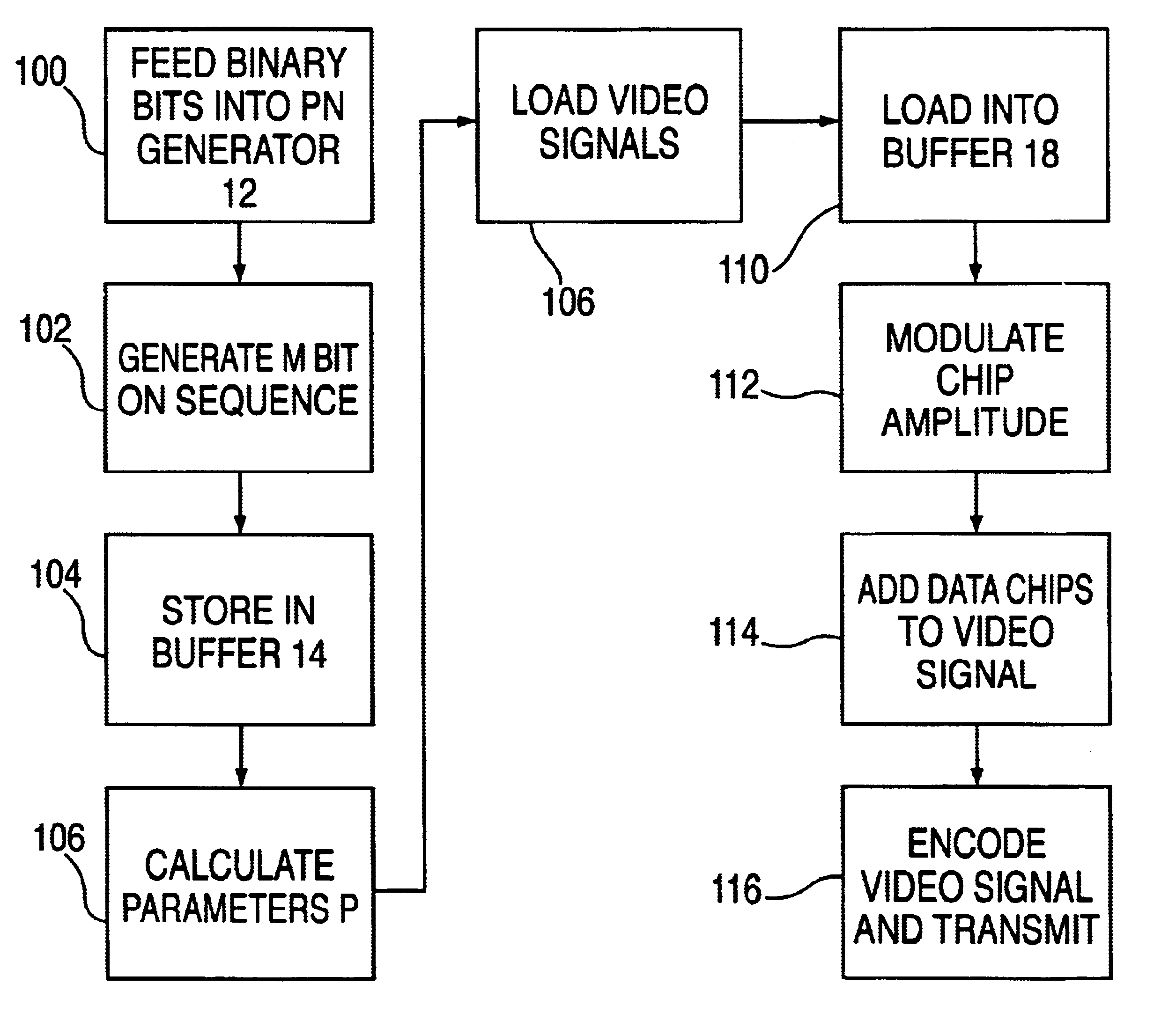
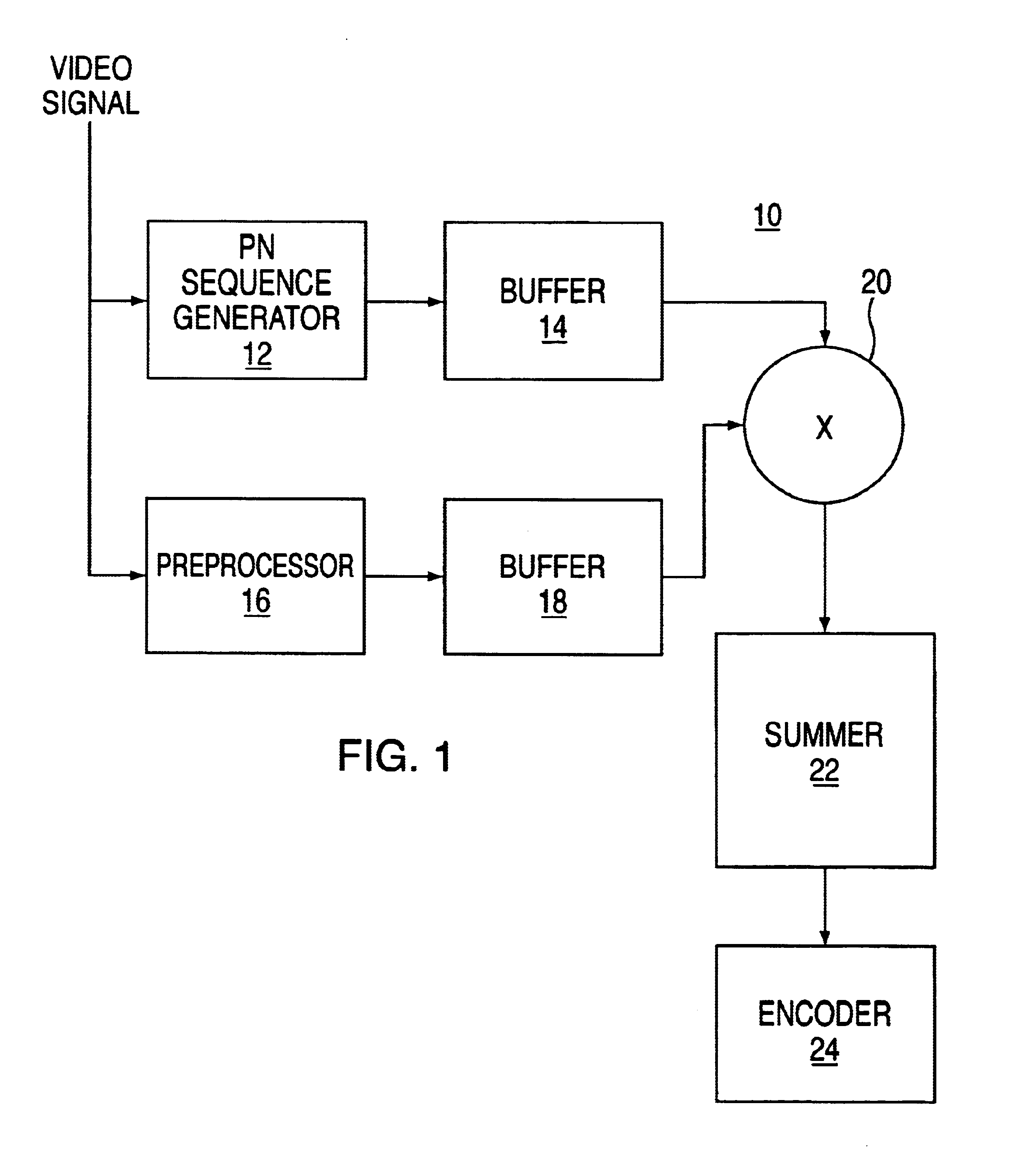
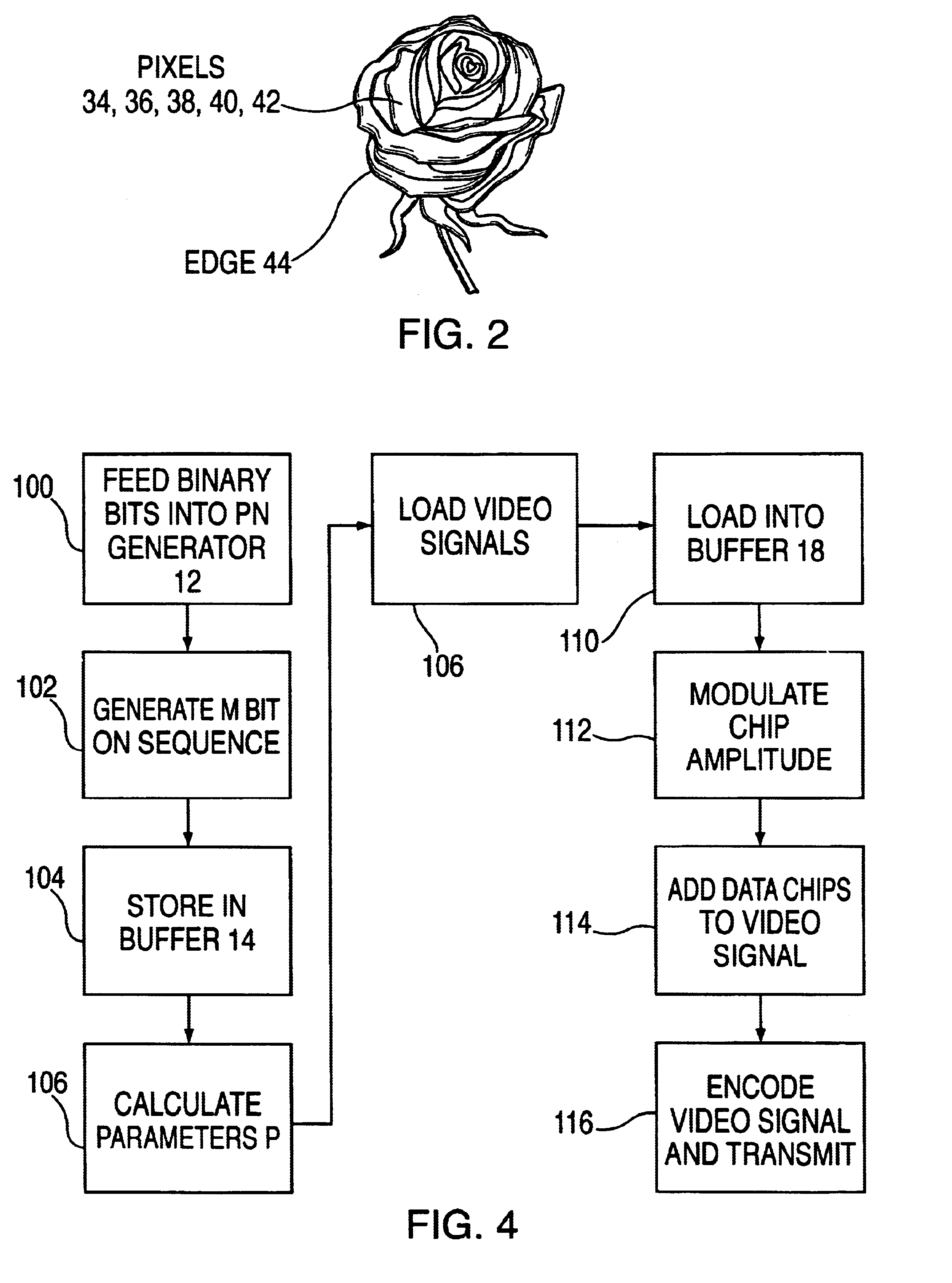
Method for transmitting data on a viewable portion of a video signal
InactiveUS6661905B1Improve reliabilityImprove efficiency and qualityCharacter and pattern recognitionSimultaneous/sequential multiple television signal transmissionComputer scienceLine scan
A method of encoding data in the visible portion of a transmitted video signal without degrading display of the received video signal, and for decoding the data in the received video signal. Each group of data bits to be transmitted, referred to a data symbol, is associated with one of a number of longer predetermined sequences of chips. Each chip sequence is divided into a multiplicity of lines of chips, and each line of chips together with its inverse are embedded, in pairwise fashion, in respective pairs of line scans of the video signal prior to its transmission. Received pairs of line scans are operated upon to detect the lines of chips they represent, and each of the number of chip sequences is correlated with the detected line of chips to derive a correlation magnitude. The chip sequence with the largest correlation magnitude is selected as the chip sequence whose data symbol was transmitted. The number of data lines exceeds the number of video lines required to define a video framer. In addition, each line has an amplitude which is modulated in accordance with a data carrying parameter determined by analyzing spatial and / or temporal characteristics of the video signal.
Owner:KOPLAR INTERACTIVE SYST INT
Controlled Release Hydrocodone Formulations
InactiveUS20110262532A1Improve efficiency and qualityGood effectPowder deliveryBiocideControlled releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
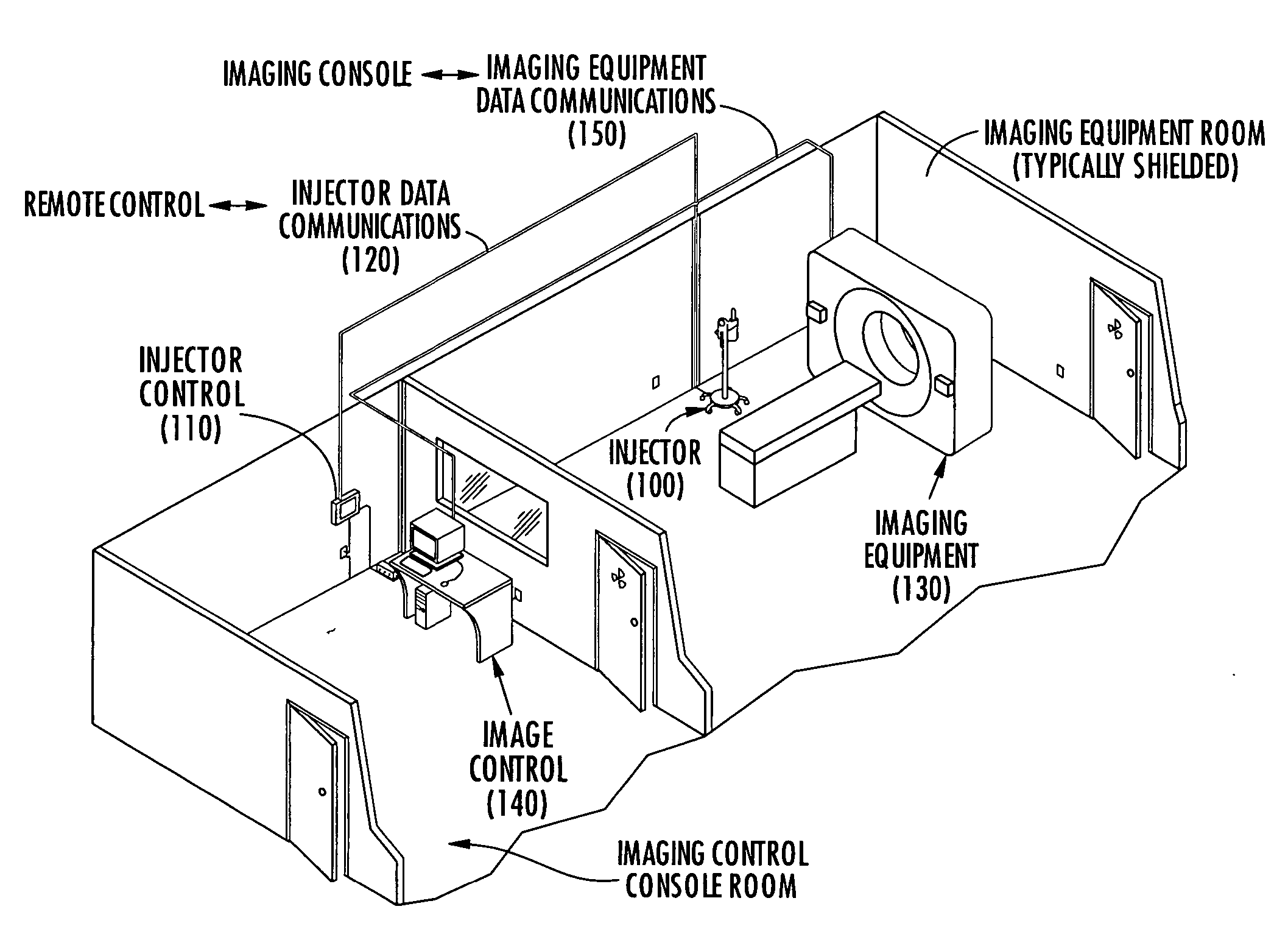
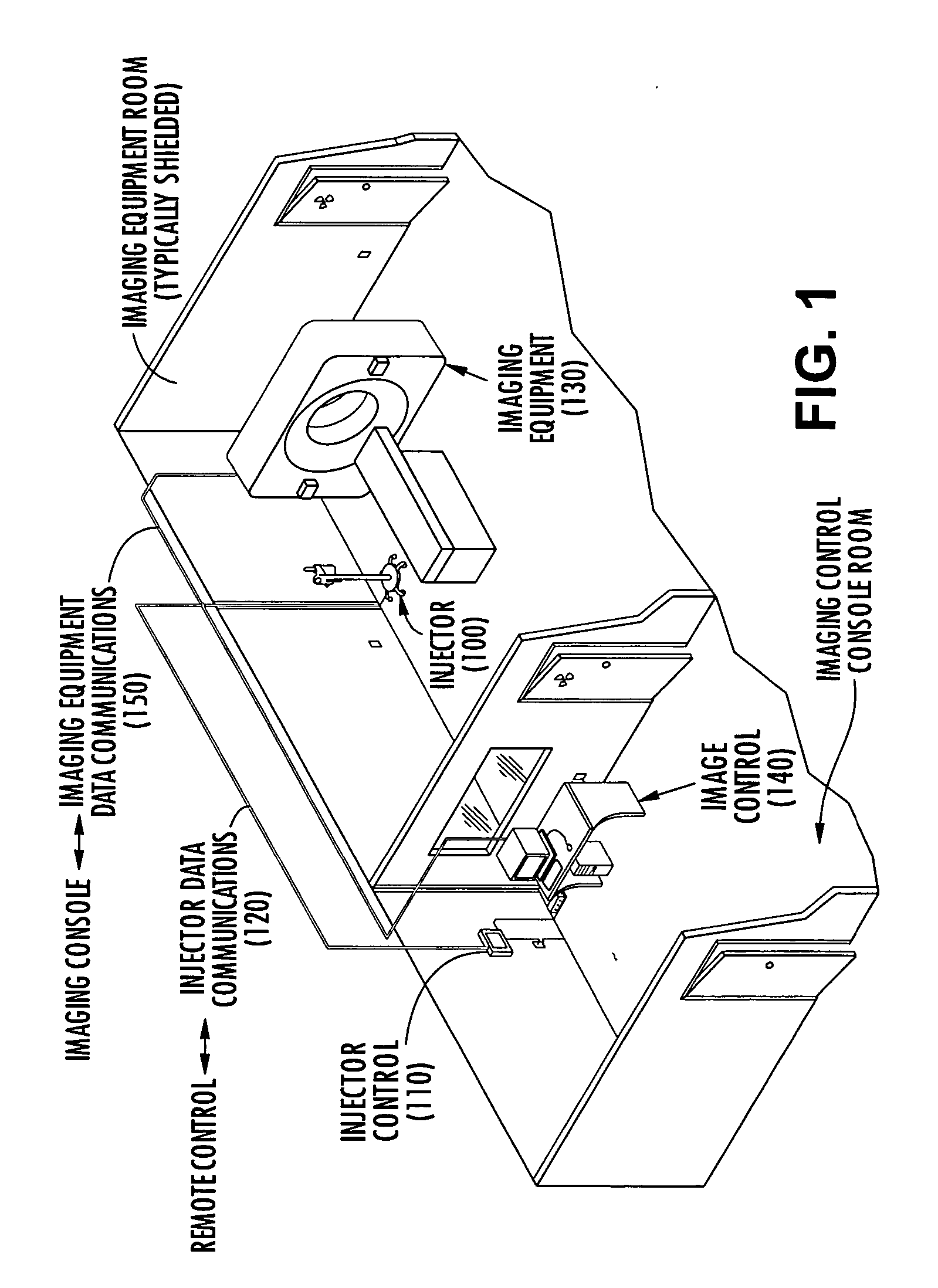
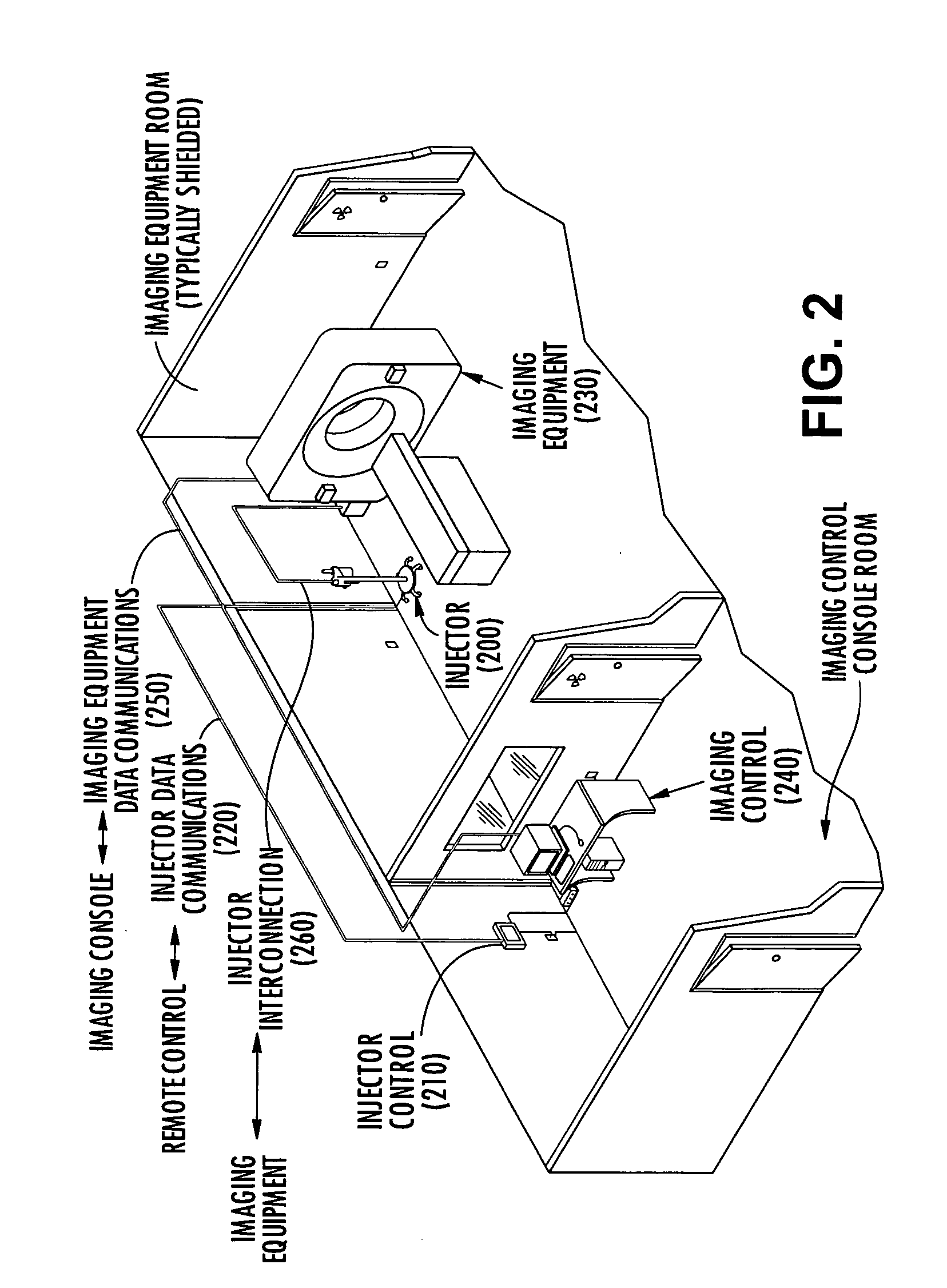
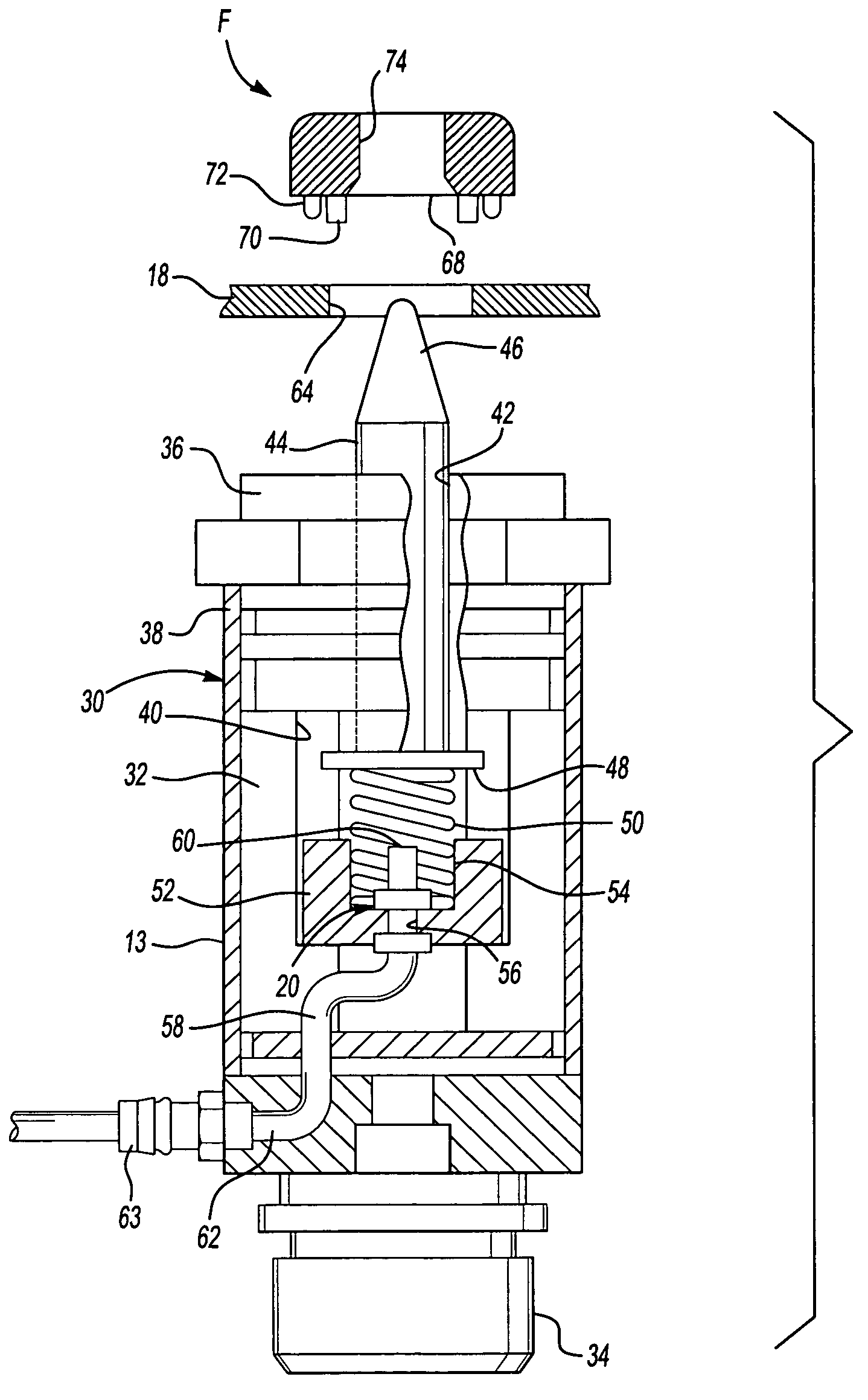
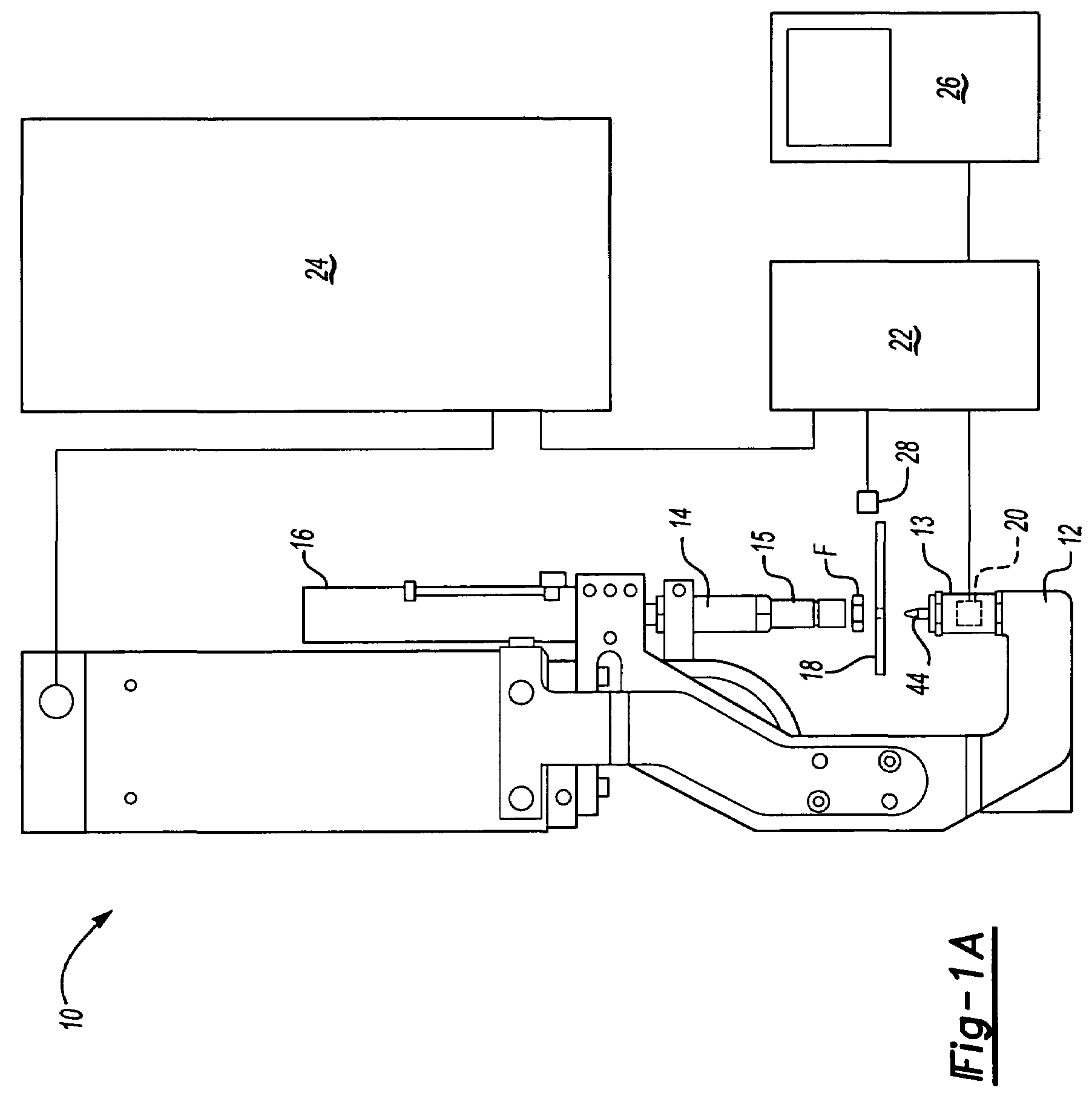
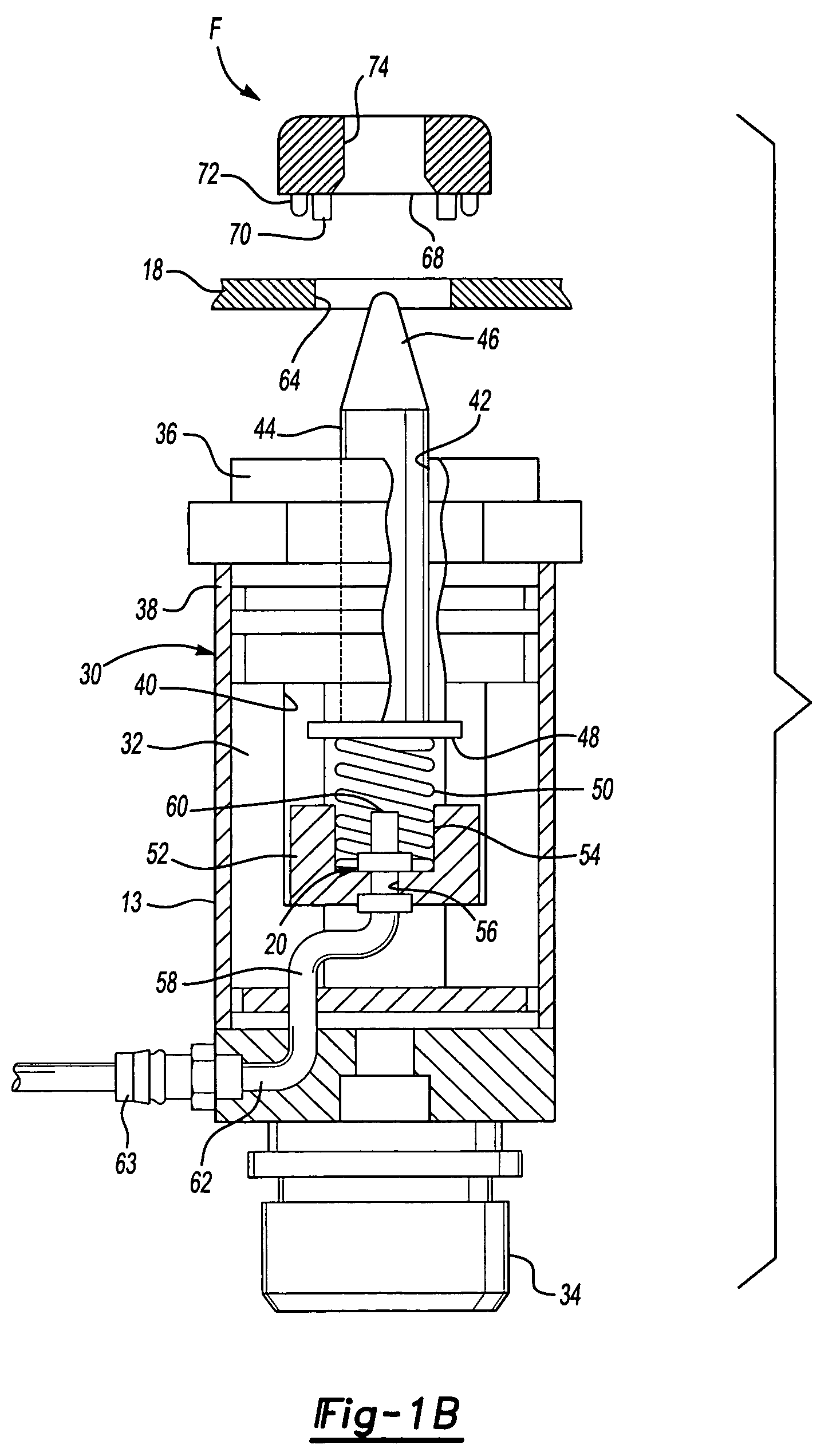
Method system and apparatus for operating a medical injector and diagnostic imaging device
InactiveUS20050203389A1Effectively managed and controlledImprove efficiency and qualityInfusion syringesSurgeryEngineeringImaging equipment
The invention is generally directed, but not limited to, a method system and apparatus that allows an operator to control an injection device and imaging equipement from a common control console. The injection device may be used to administer a contrast medium into a patient so that imaging equipment can acquire internal images of the patient. The invention may include an injection system that can be bundled with software and / or hardware that can be used to modify an existing imaging control console so that it can be used to operate both the injection device and imaging device. In one embodiment, the common control console can access stored protocols that can contain operational parameters for the injection device, the imaging device, or both. Consequently, the efficiency of the test and final quality of the images can be improved. Additionally, the combined control console will aid in the overall process of caring out the imaging tests.
Owner:MEDTRONIC INC +1
Resistance welding fastener electrode and monitor and method of using same
InactiveUS7564005B2Improved feedback and controlShorten cycle timeElectrode supporting devicesWelding monitoring devicesElectrical resistance and conductanceEngineering
Owner:DOBEN LTD
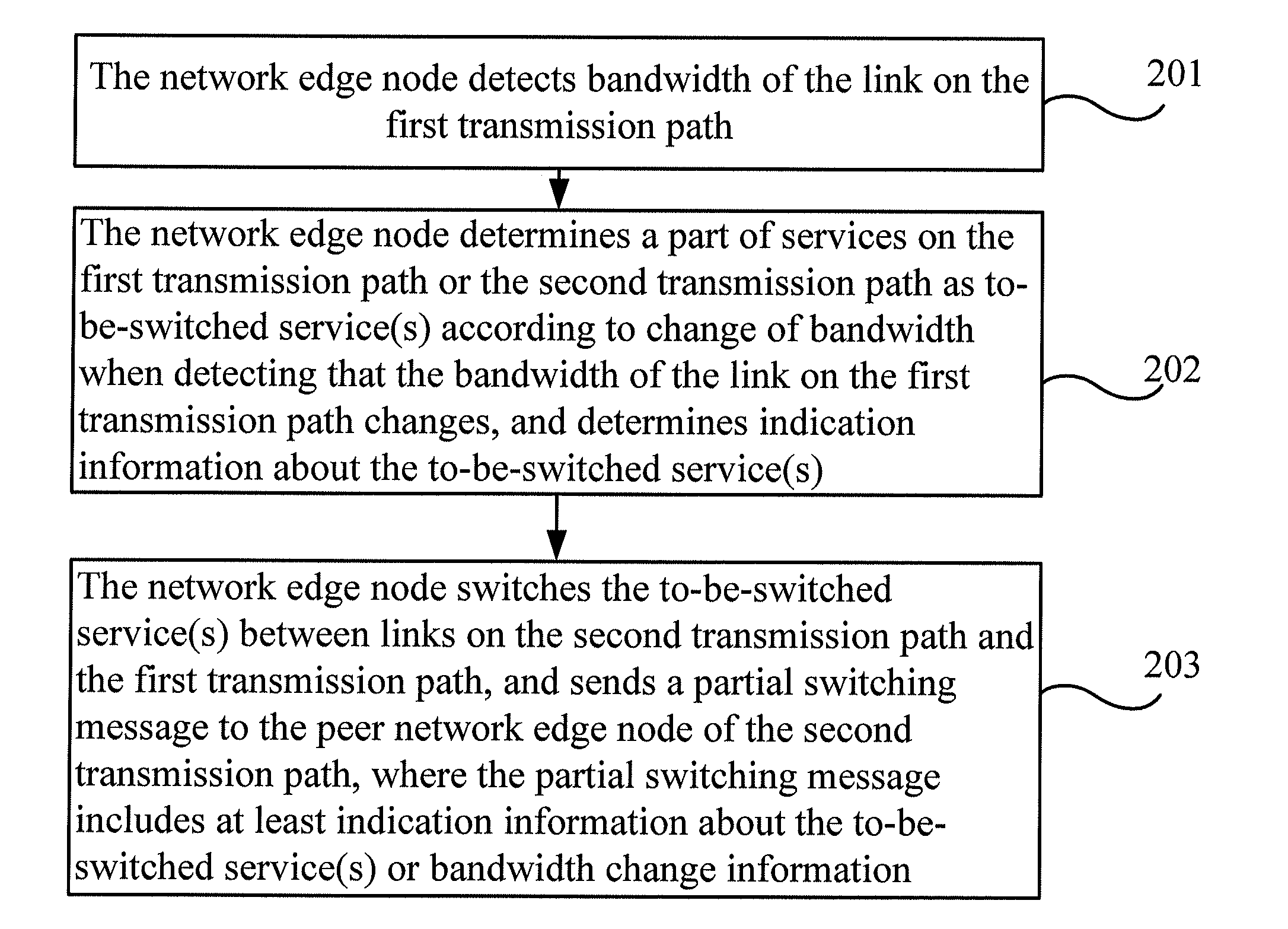
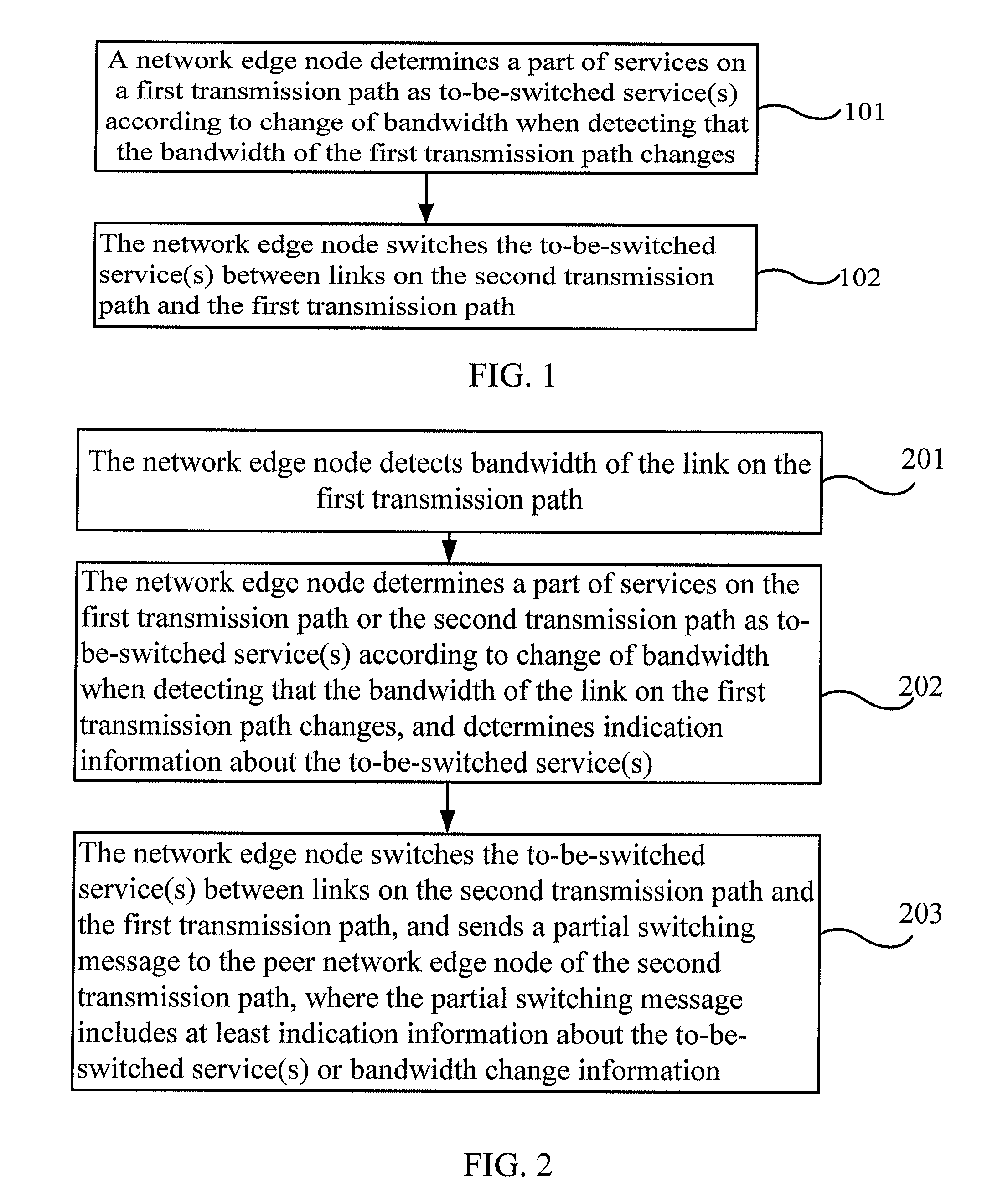
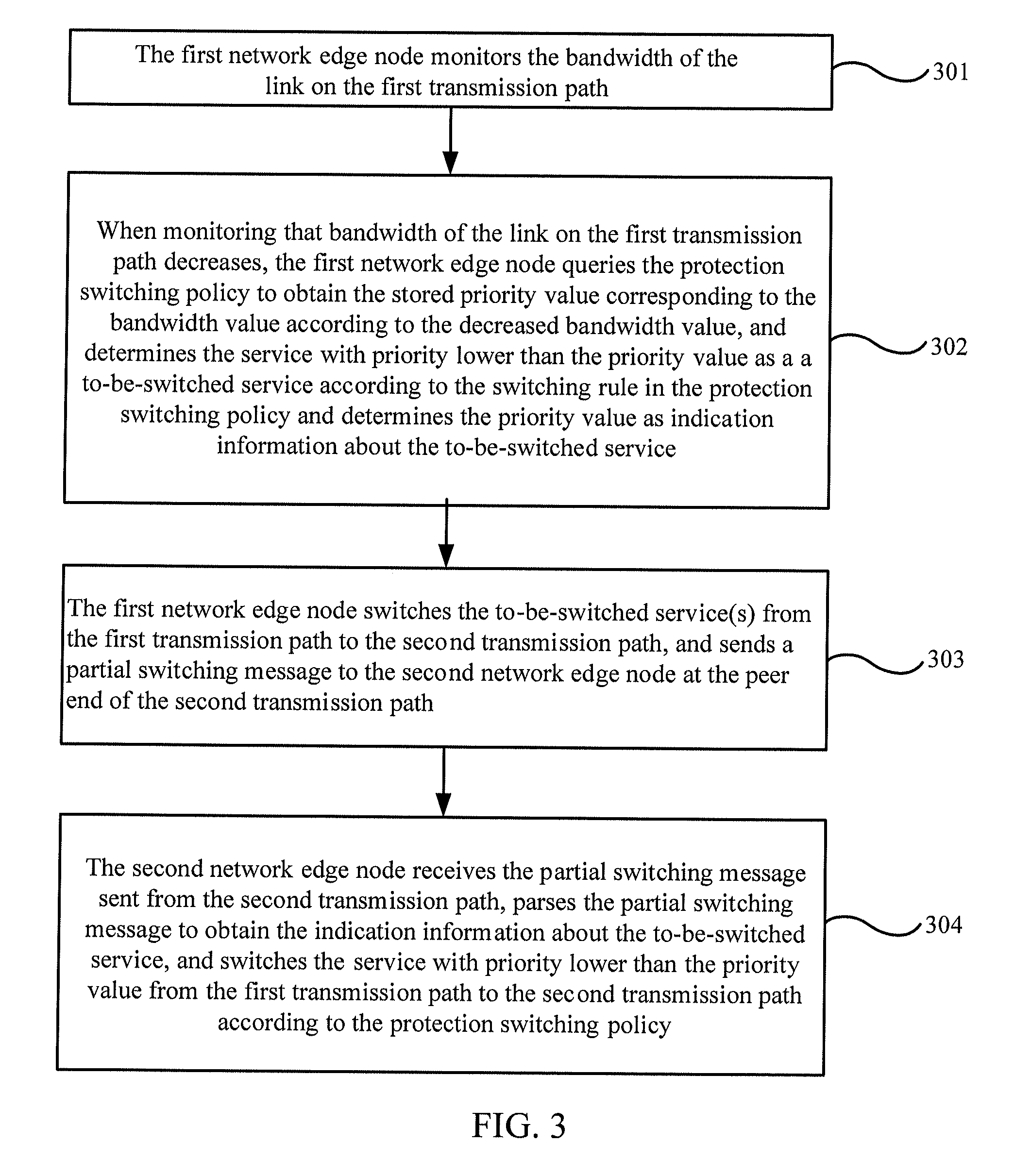
Automatic protection switching method, device and system
ActiveUS20120163224A1Lower the volumeImprove efficiencyError preventionTransmission systemsAutomatic protection switchingEdge node
Embodiments of the present invention provide an automatic protection switching method, device and system. The method includes: determining a part of services as to-be-switched service(s) according to change of bandwidth when monitoring that the bandwidth of the first transmission path changes; and switching the to-be-switched service(s) between links on the second transmission path and the first transmission path. Another method includes: receiving a partial switching message from a peer network edge node through the first transmission path or the second transmission path; and determining the to-be-switched service(s) according to indication information about the to-be-switched service(s) or bandwidth change information in the partial switching message, and switching the to-be-switched service(s) between links on the first transmission path and the second transmission path.
Owner:HUAWEI TECH CO LTD
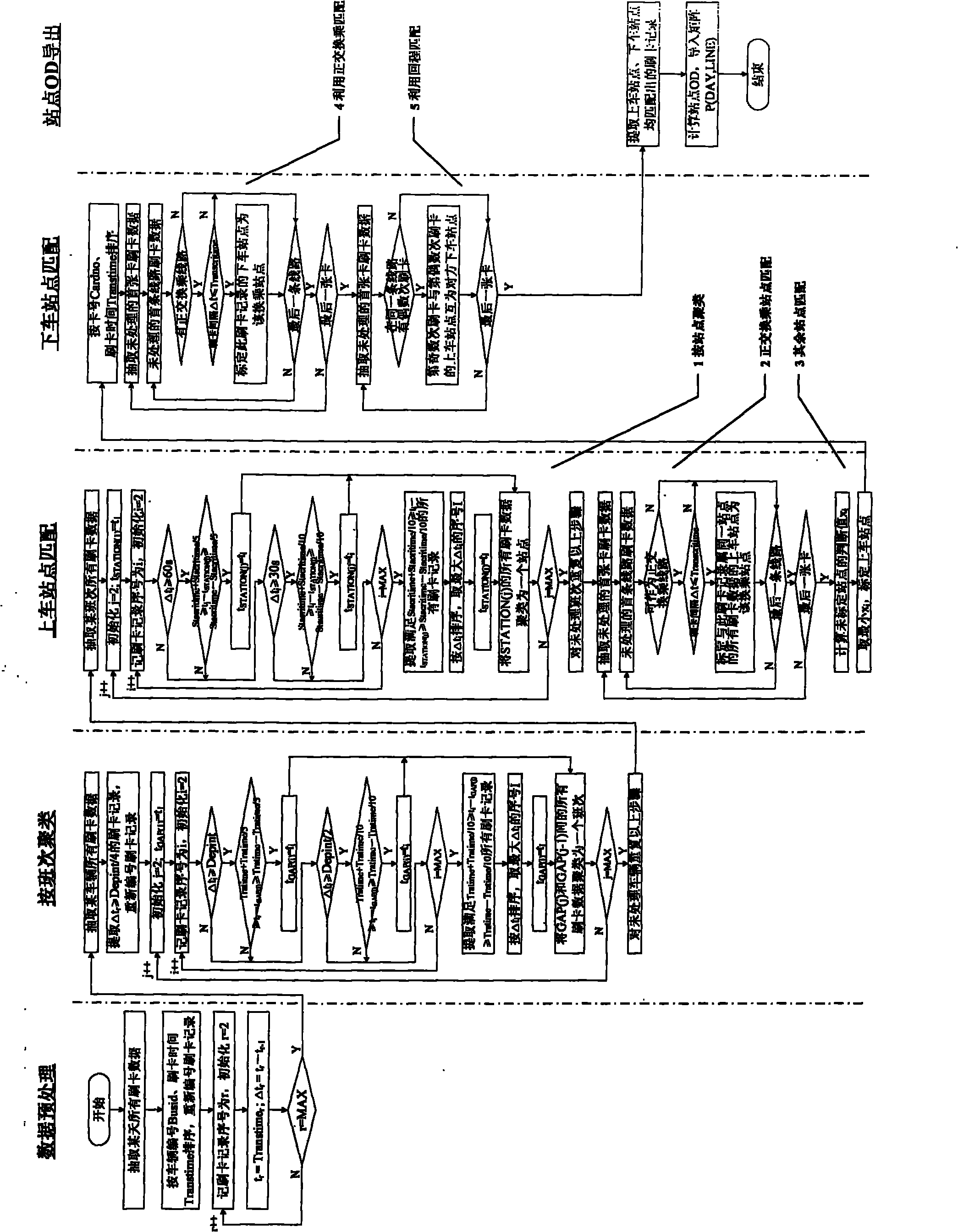
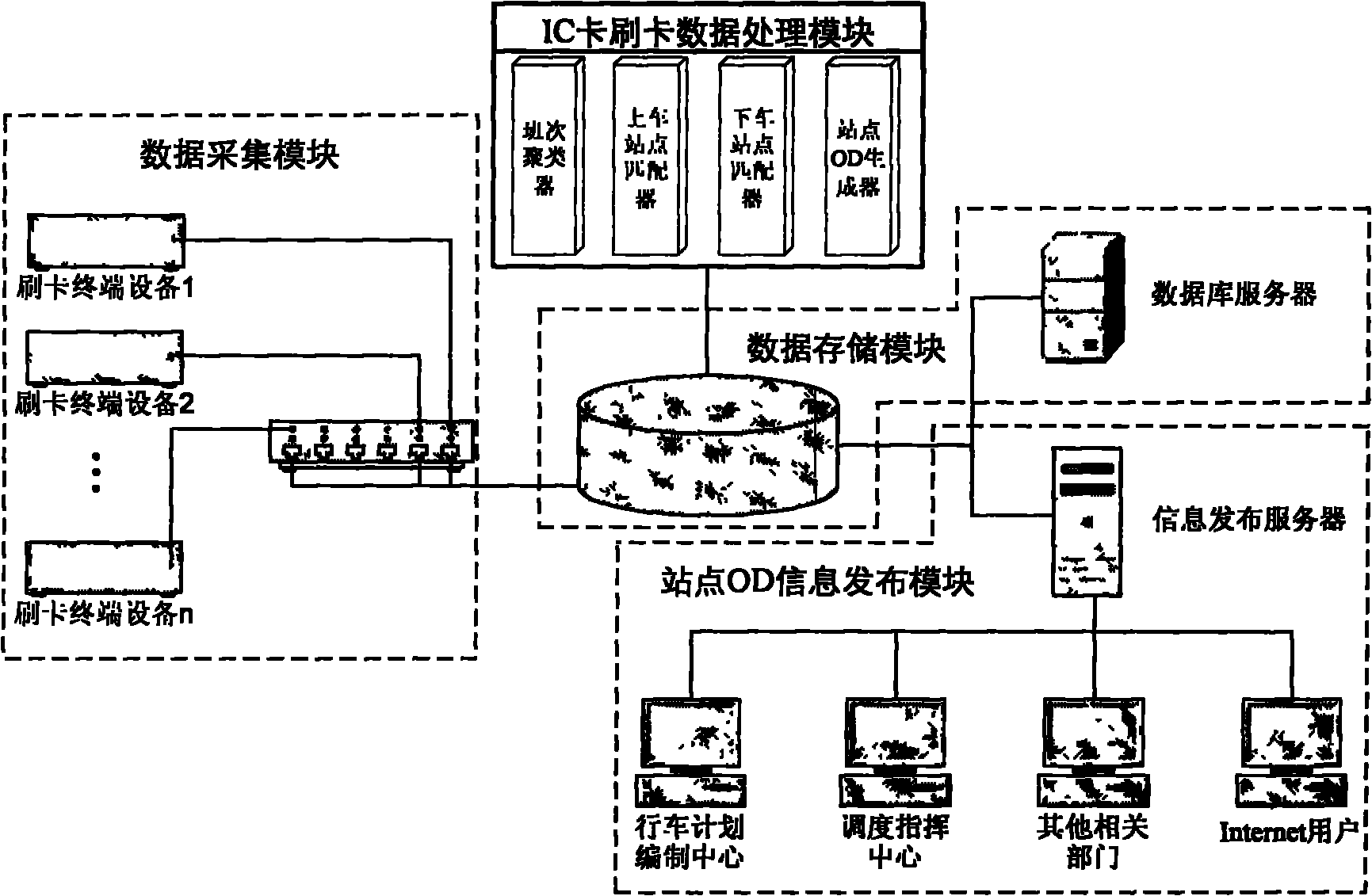
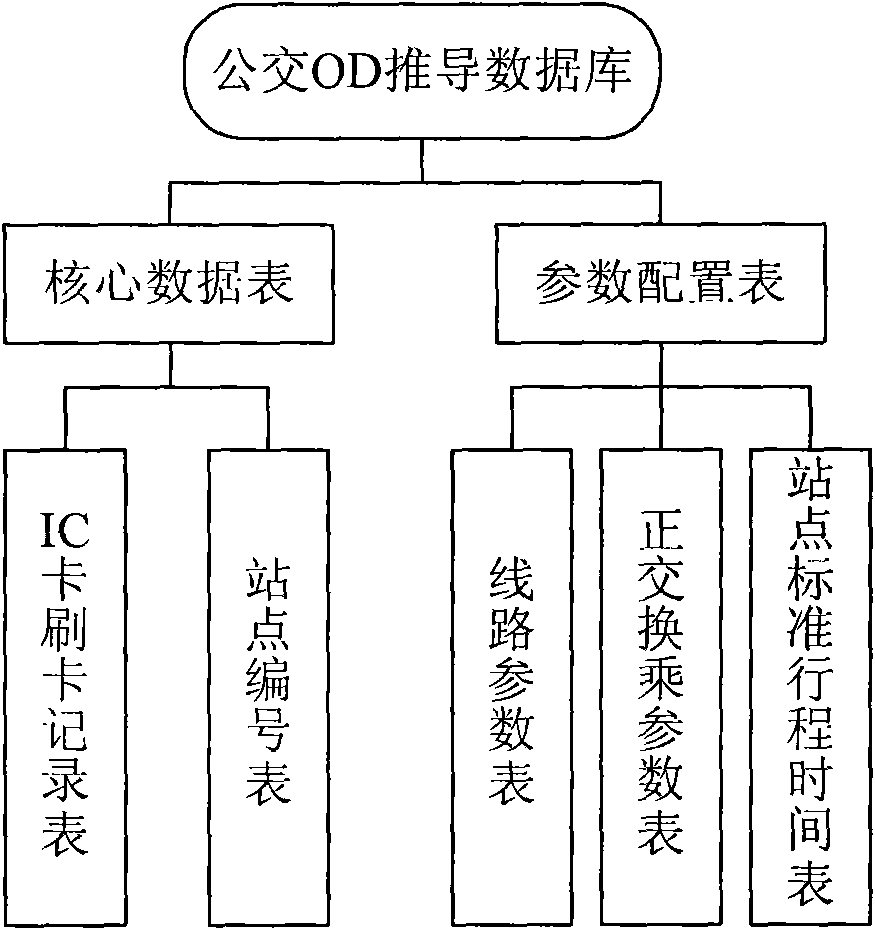
Method and system for acquiring bus stop OD based on IC card data
InactiveCN102097002AImprove efficiency and qualityImprove efficiencyDetection of traffic movementData acquisition moduleData processing
The invention discloses a method and system for acquiring a bus stop OD based on IC card data. The method comprises the following step of carrying out runs number clustering, stop clustering, getting-on stop matching, getting-off stop matching and the like on acquired IC card swiping data to obtain a bus stop OD array. The system comprises a data acquisition module, a data storage module, an IC card swiping data processing module and a stop OD information issuing module. The invention provides timely stop OD information for bus dispatching and modeling and has the characteristics of accuracy and real time compared with the traditional stop OD acquiring technology.
Owner:SOUTHEAST UNIV
Sustained release formulations containing acetaminophen and tramadol
InactiveUS7374781B2Improve efficiency and qualityProvide clinical efficiencyOrganic active ingredientsNervous disorderDrugSustained Release Formulations
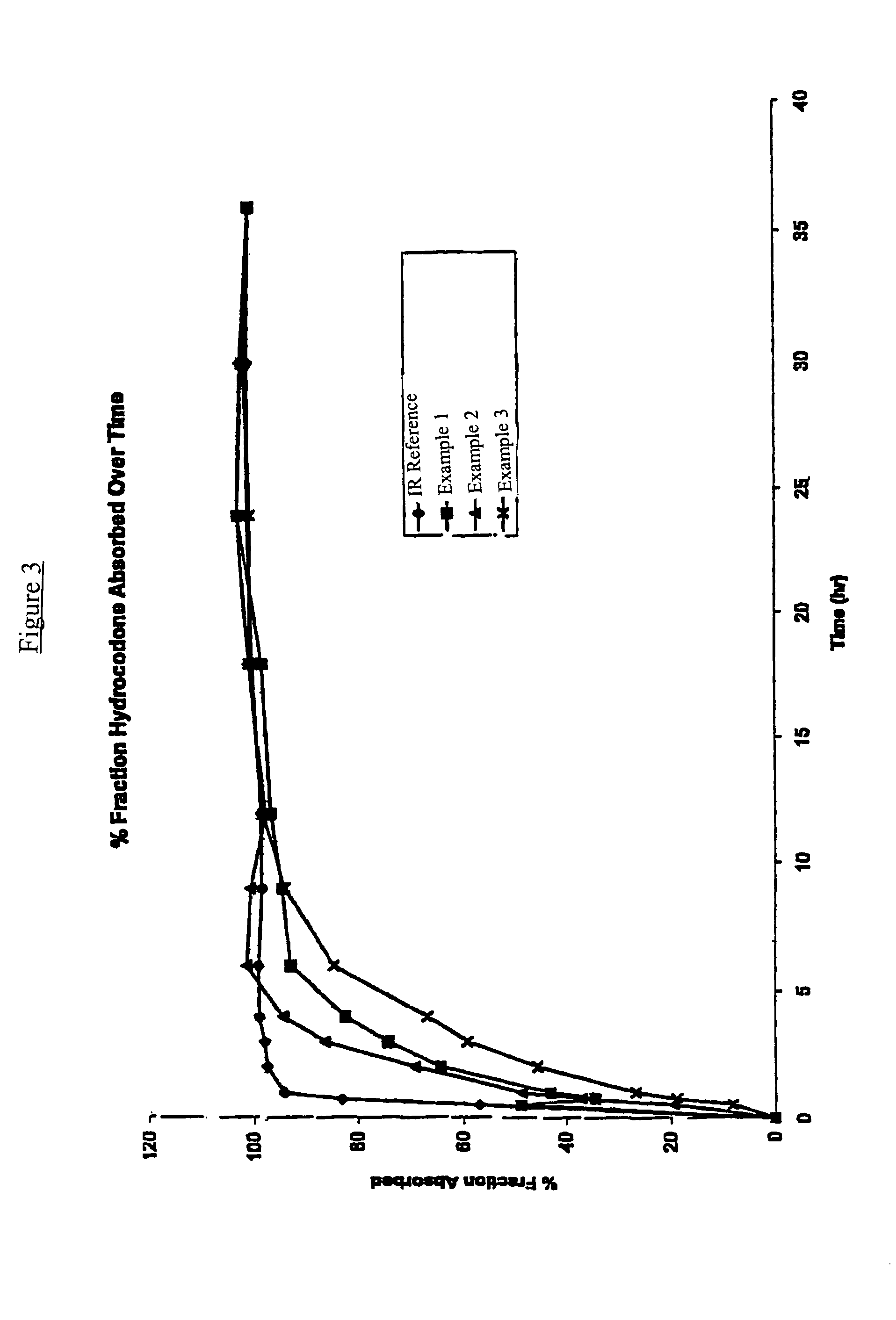
A sustained release formulation as a unit dose contains 100 mg-1000 mg of Acetaminophen and 15 mg-150 mg of tramadol hydrochloride, which comprises of 1) an immediate release portion comprising of 25%-75% of the total effective amount of drug in the dosage form and 2) a sustained release portion comprising of a) 25%-75% of the total effective amount of drugs in the dosage form; b) 6%-50% of gelling polymers of the total formulation, and c) optionally an enteric coating at a level of 5%-40% of the total formulation. The set forth formulation dissolves 25%-60% of the total drug in the first hour, 50%-90% of the total drug in the first four hours and not less than 80% of the total drug in the first 12 hours using USP dissolution method II at 50 rpm.
Owner:ZHANG SHUYI +1
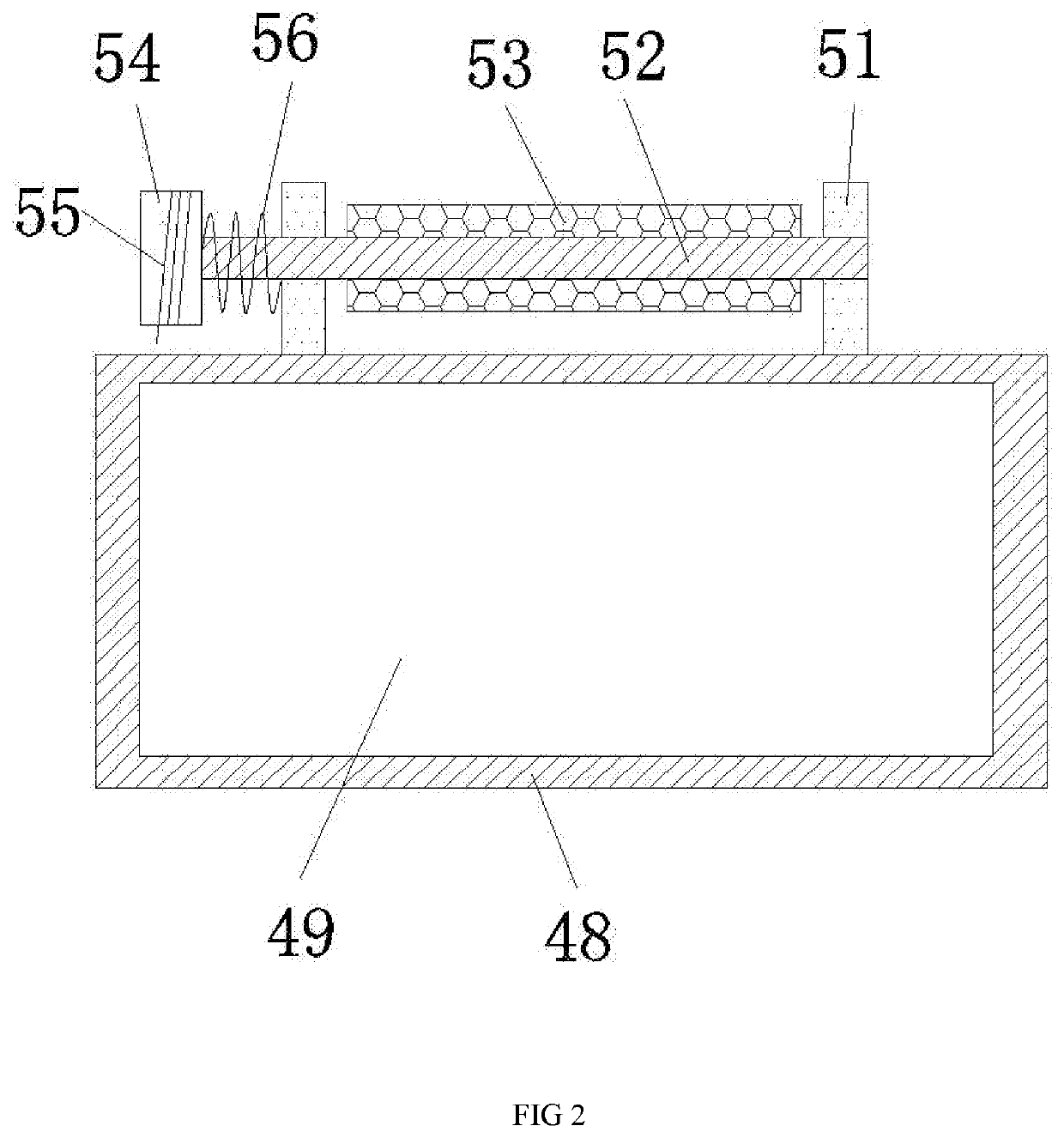
Quick cleaning apparatus for large display screen
InactiveUS20200188963A1Reduce riskEasy to cleanDrying gas arrangementsCleaning using toolsElectric machineryTruck
The invention discloses a large-scale quick-cleaning device for a display screen, which includes a storage box fixed on a truck, and an upper end surface of the storage box is provided with a lifting cavity with an upward opening, and the lifting cavity is provided with a lifting device for lifting. The device comprises a first motor fixedly arranged at the center of the lower wall of the lifting cavity, and a lifting platform can be slid up and down above the first motor, and a support block is fixedly arranged on the rear side of the upper end surface of the lifting platform. The upper end surface is provided with a turning groove, and a turning plate is rotatably provided between the left and right walls of the turning groove.
Owner:YE MENGJIE
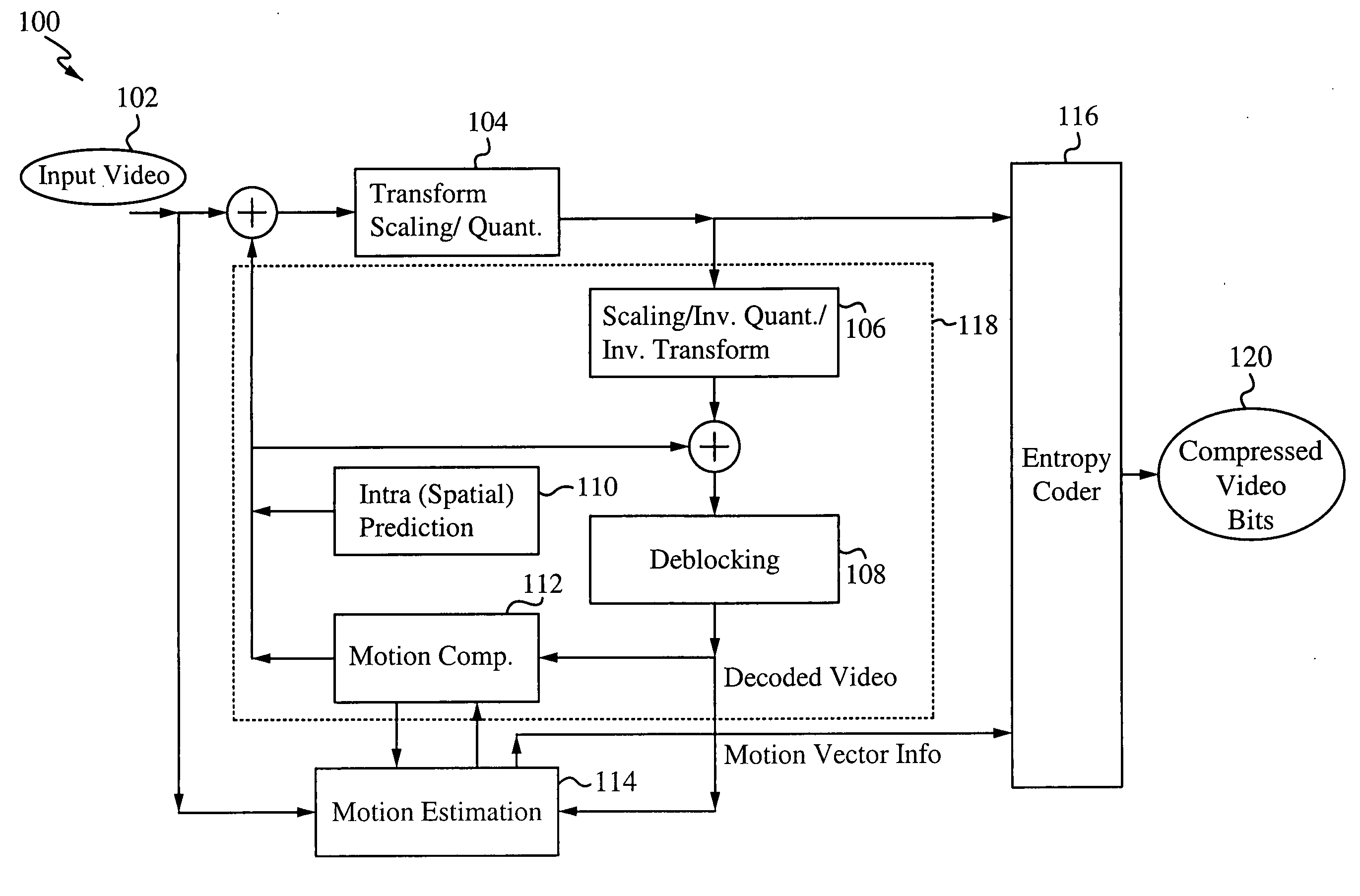
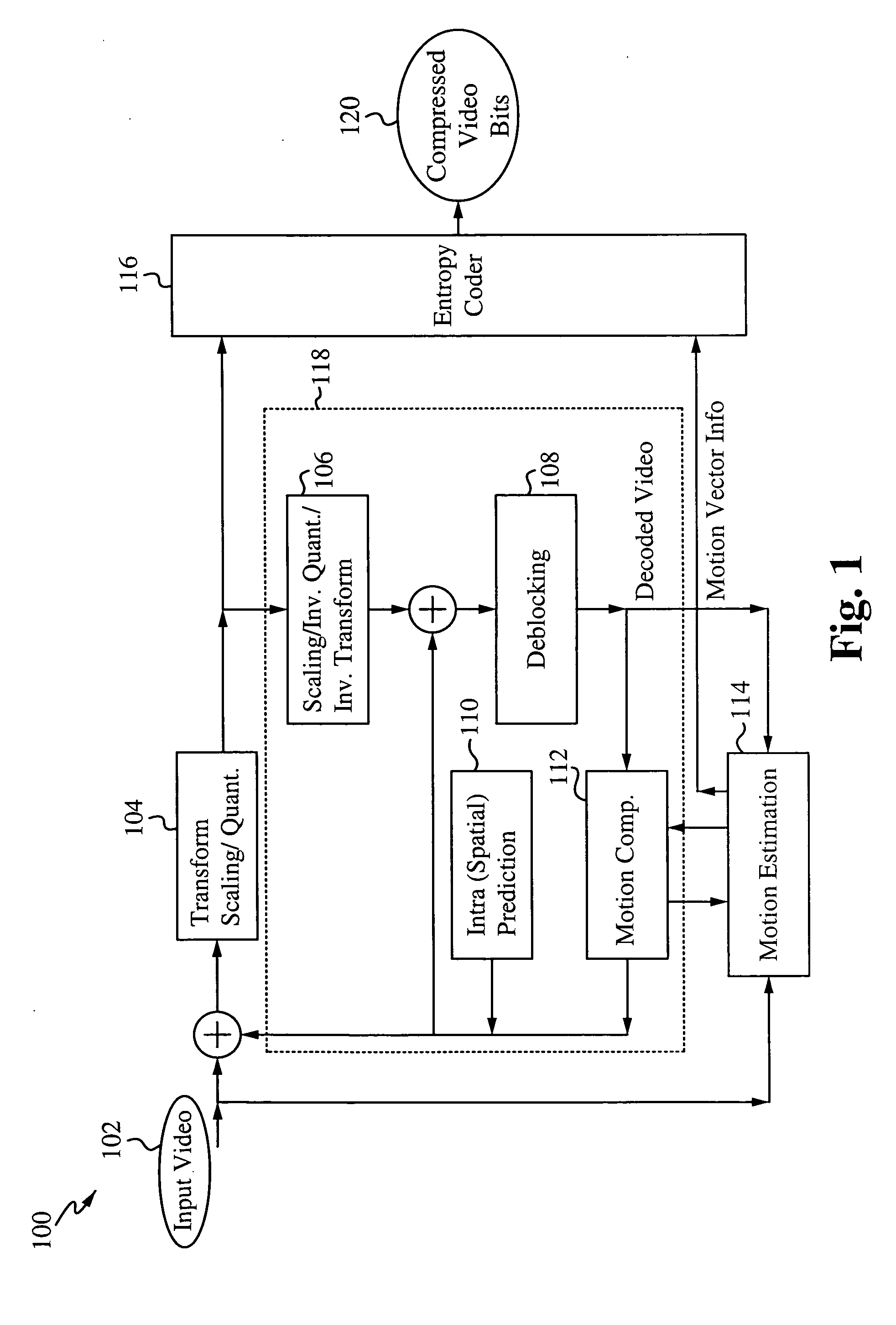
Speculative start point selection for motion estimation iterative search
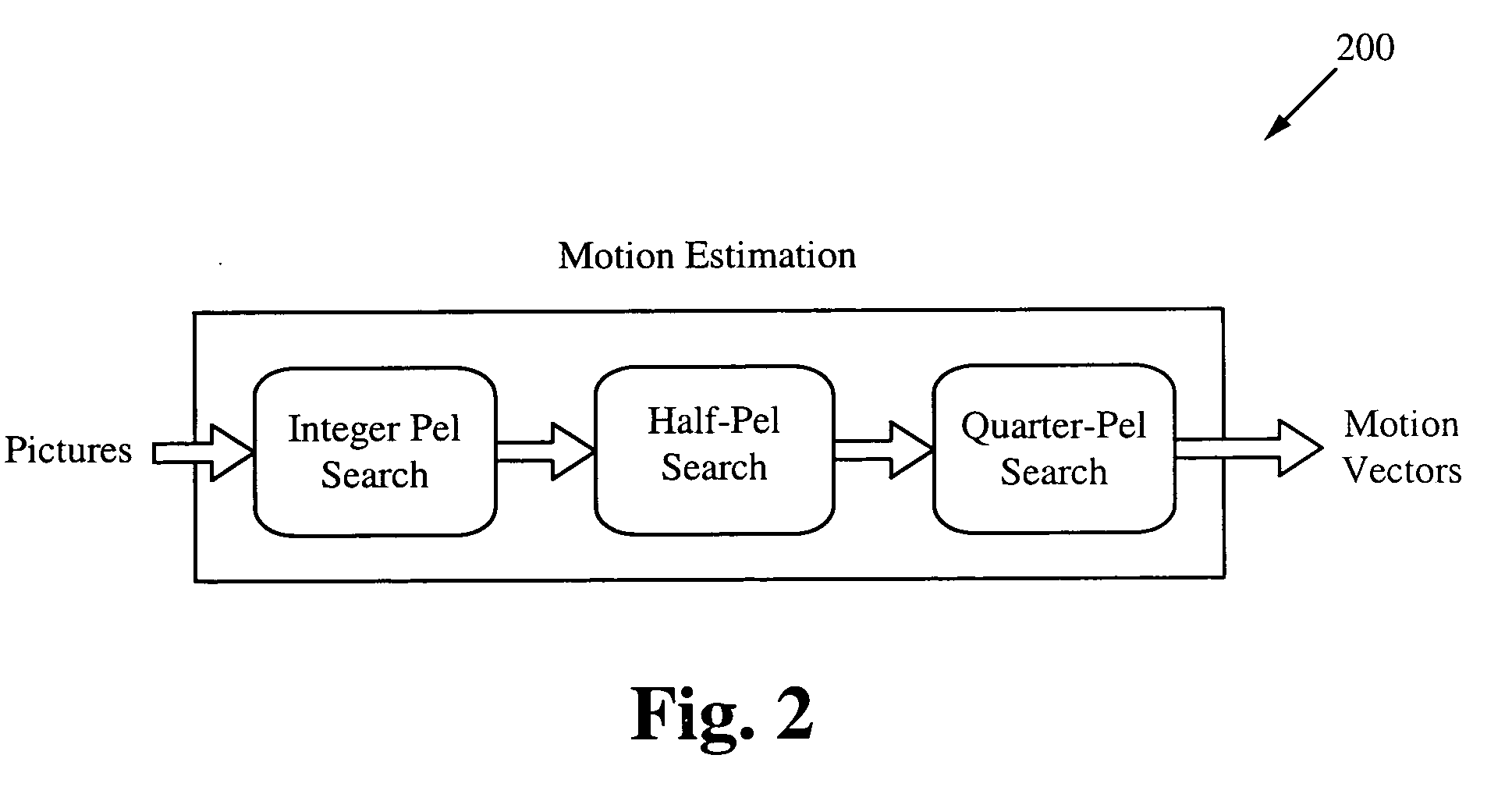
InactiveUS20100014588A1Improve efficiencyQuality improvementColor television with pulse code modulationColor television with bandwidth reductionIterative searchMotion vector
A speculative start point selection for motion estimation iterative search improves the efficiency and quality of the integer-pel motion estimation iterative search by speculatively selecting the start position of the iteration. The start position is selected by comparing the Sum of Absolute Differences (SAD) value of a 0 motion vector, a predicted motion vector and a global motion vector (GMV) and selecting the position with the smallest SAD value. A refinement scheme with a threshold improves the efficiency and quality of the motion estimation iterative search by performing several comparisons to ensure the proper motion vector is selected. Applications of this improved motion estimation search include stabilizing an image as well as many other applications where motion vectors are used.
Owner:SONY CORP +1
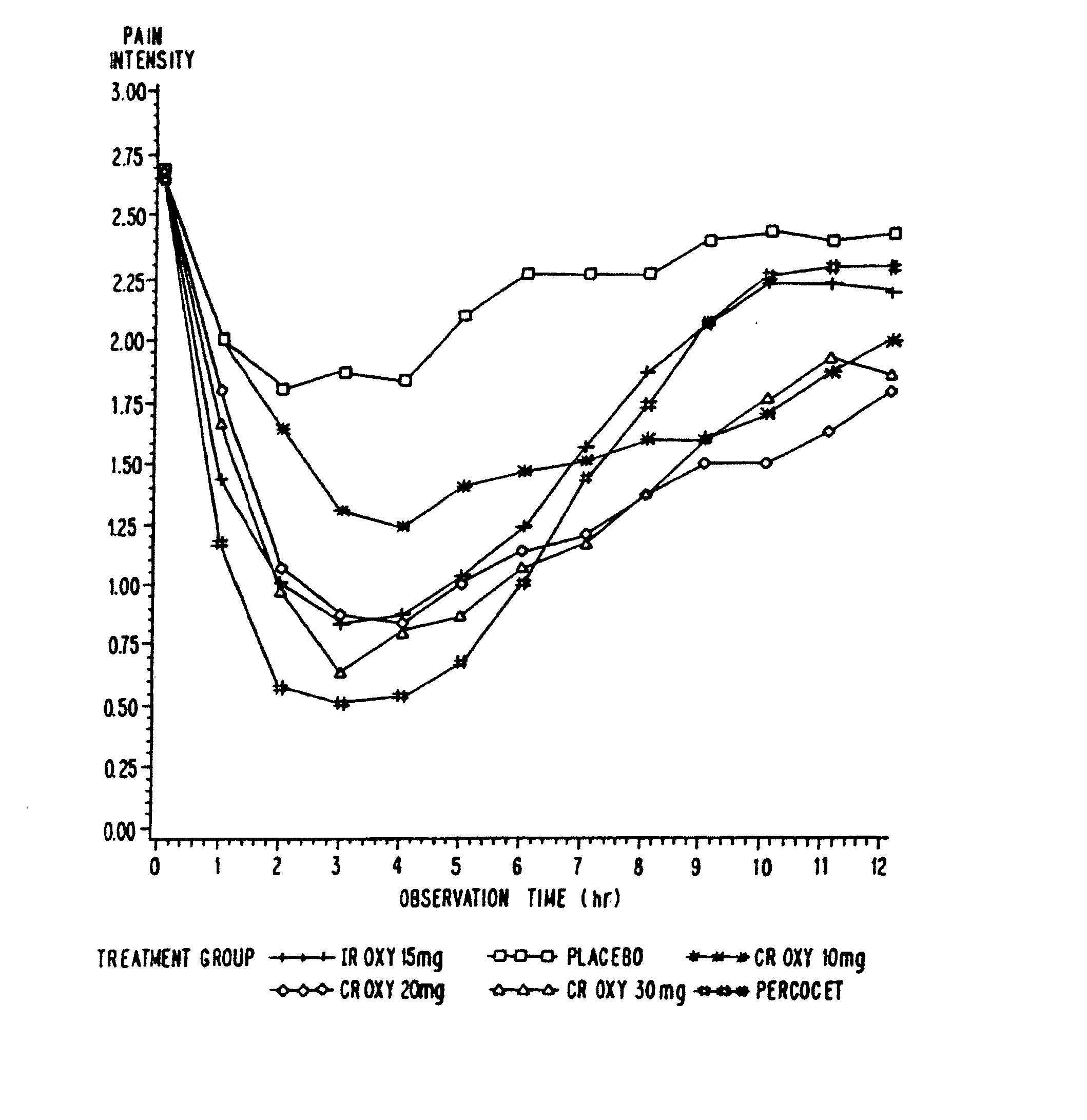
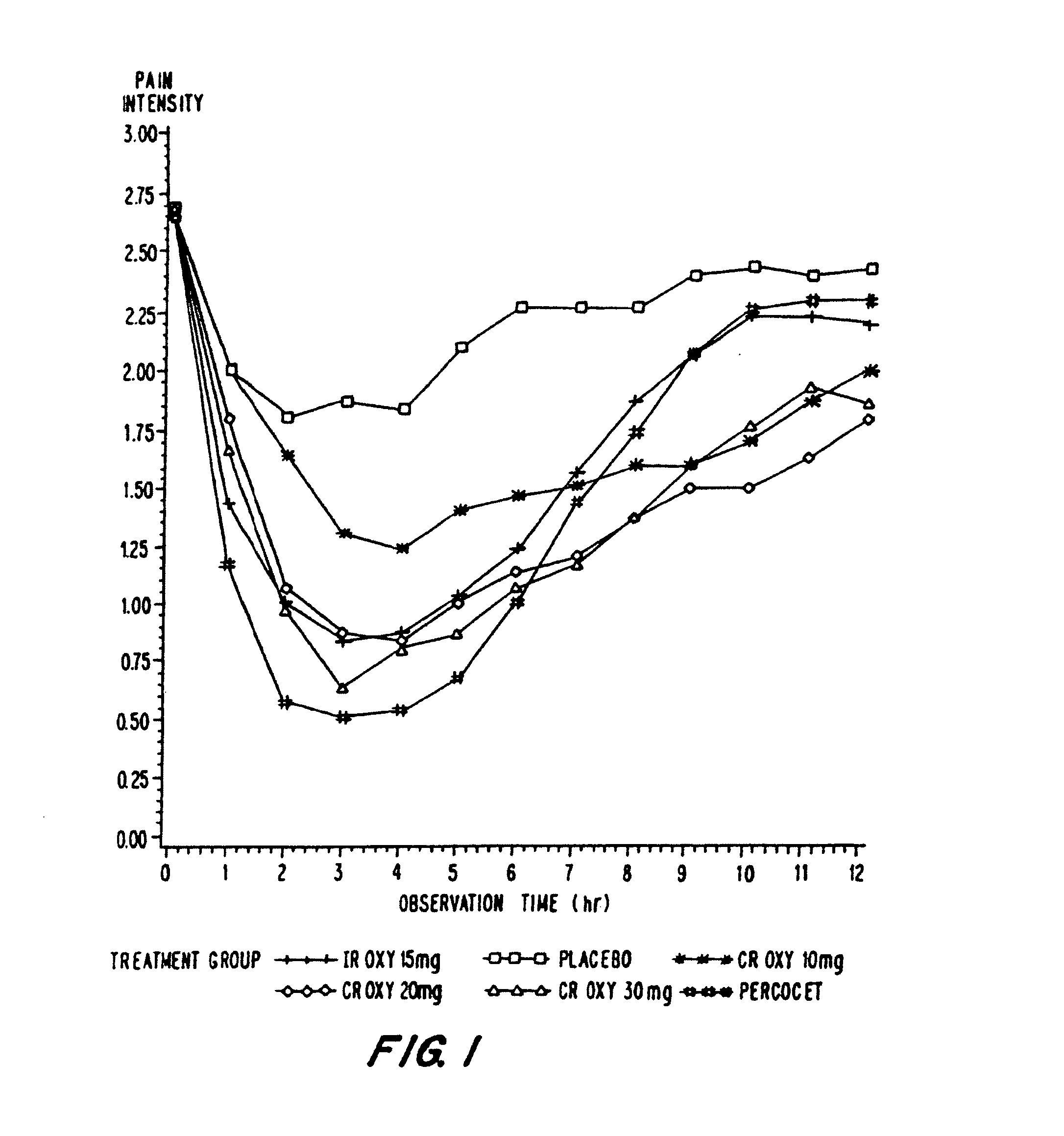
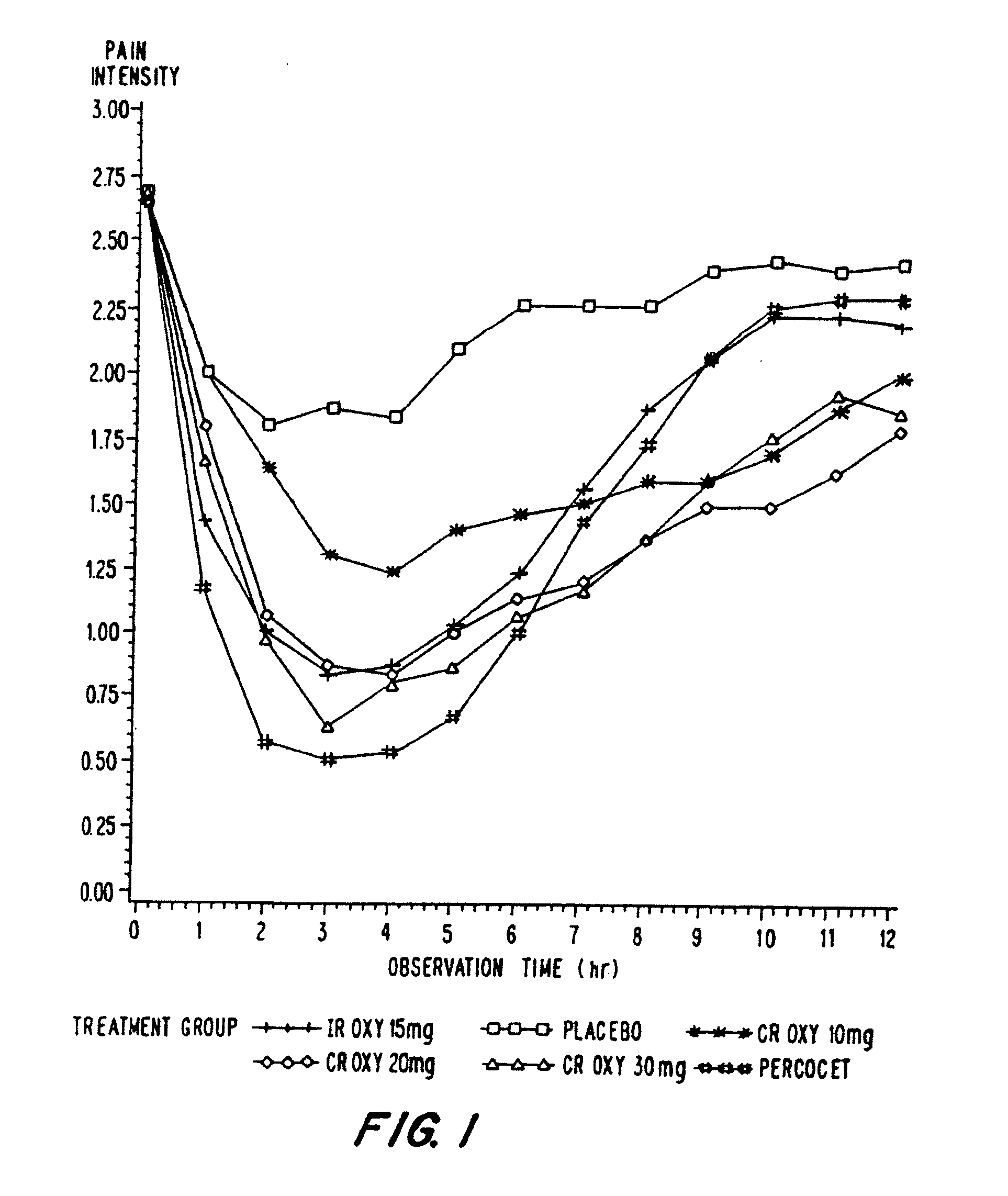
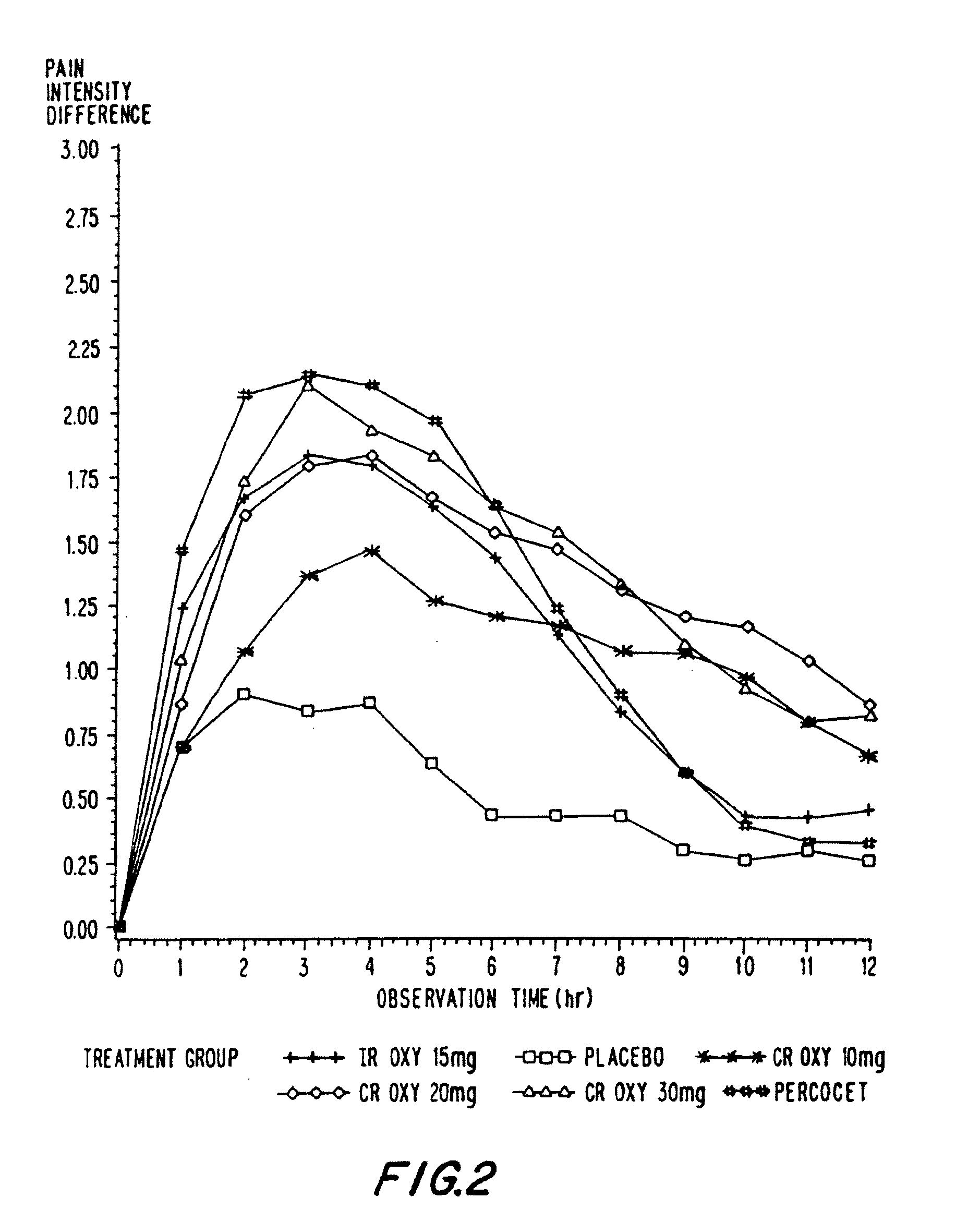
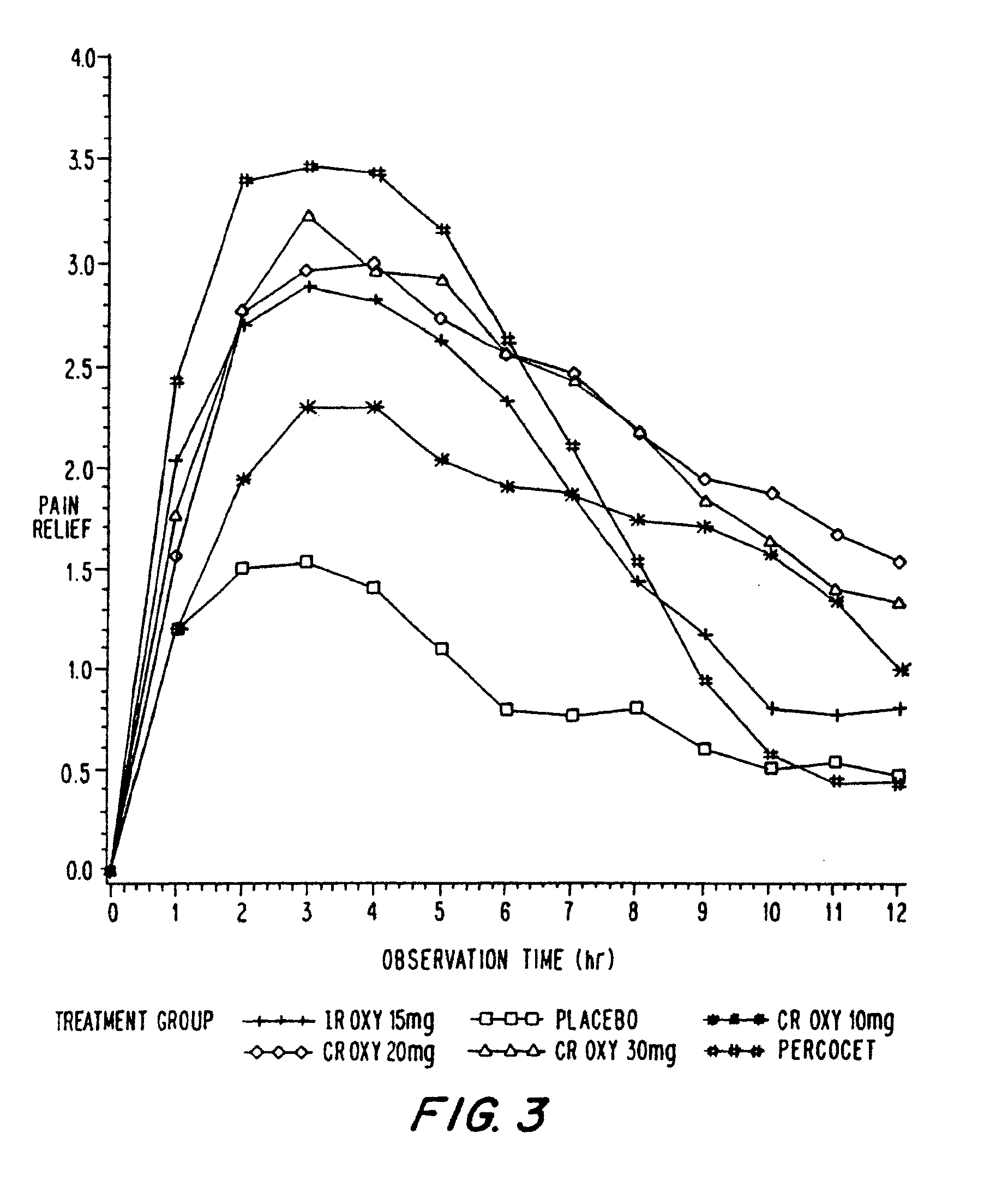
Controlled release oxycodone compositions
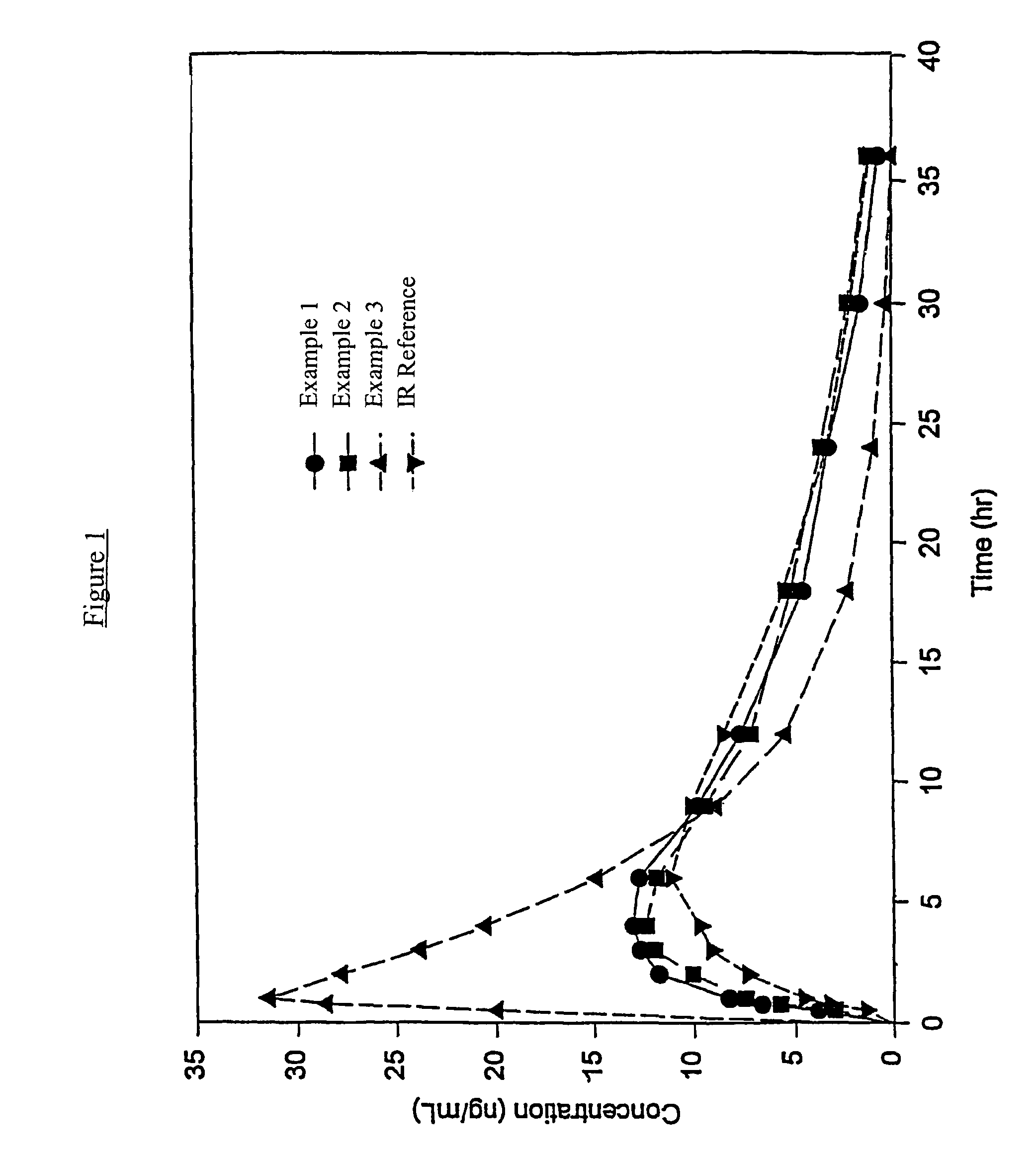
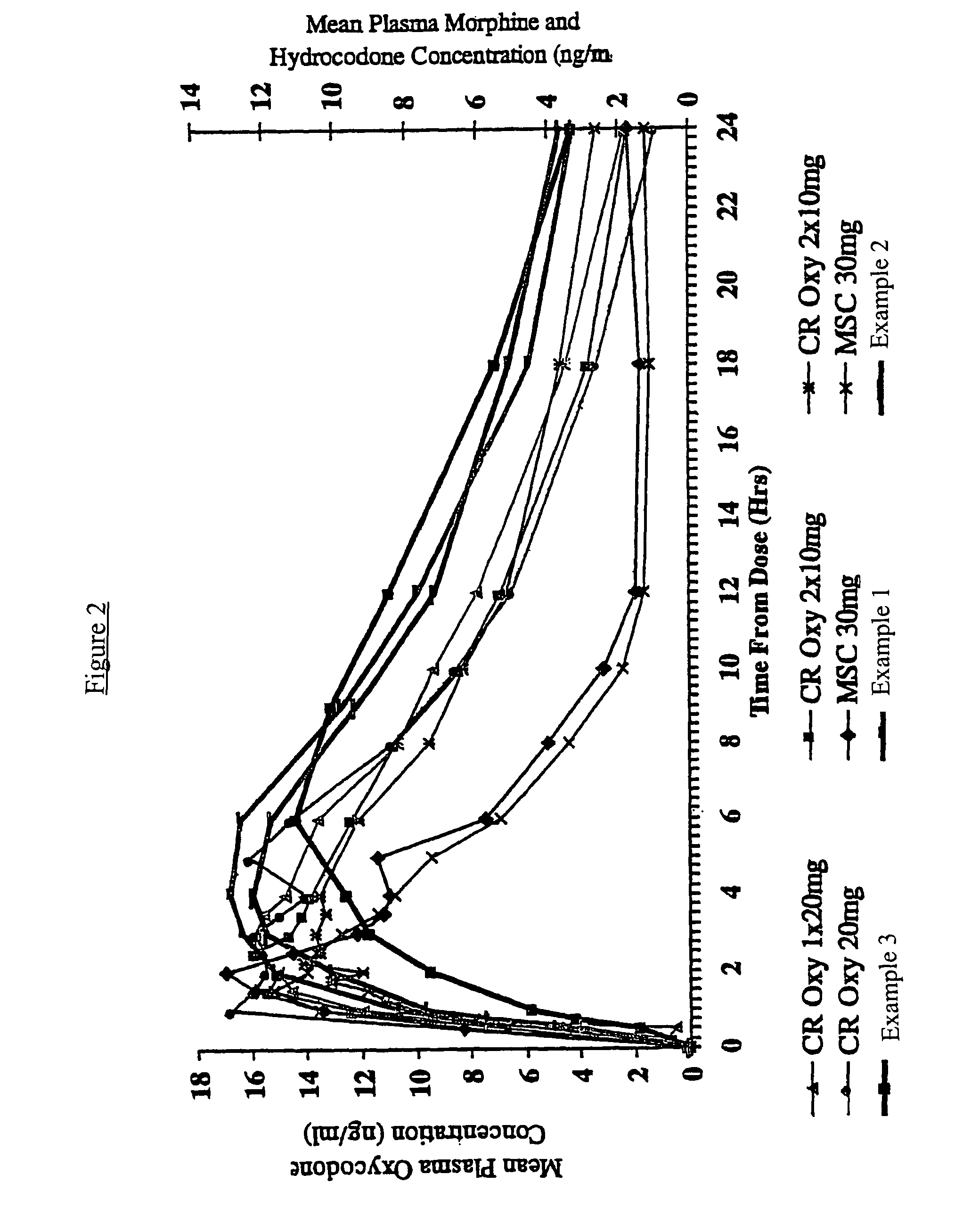
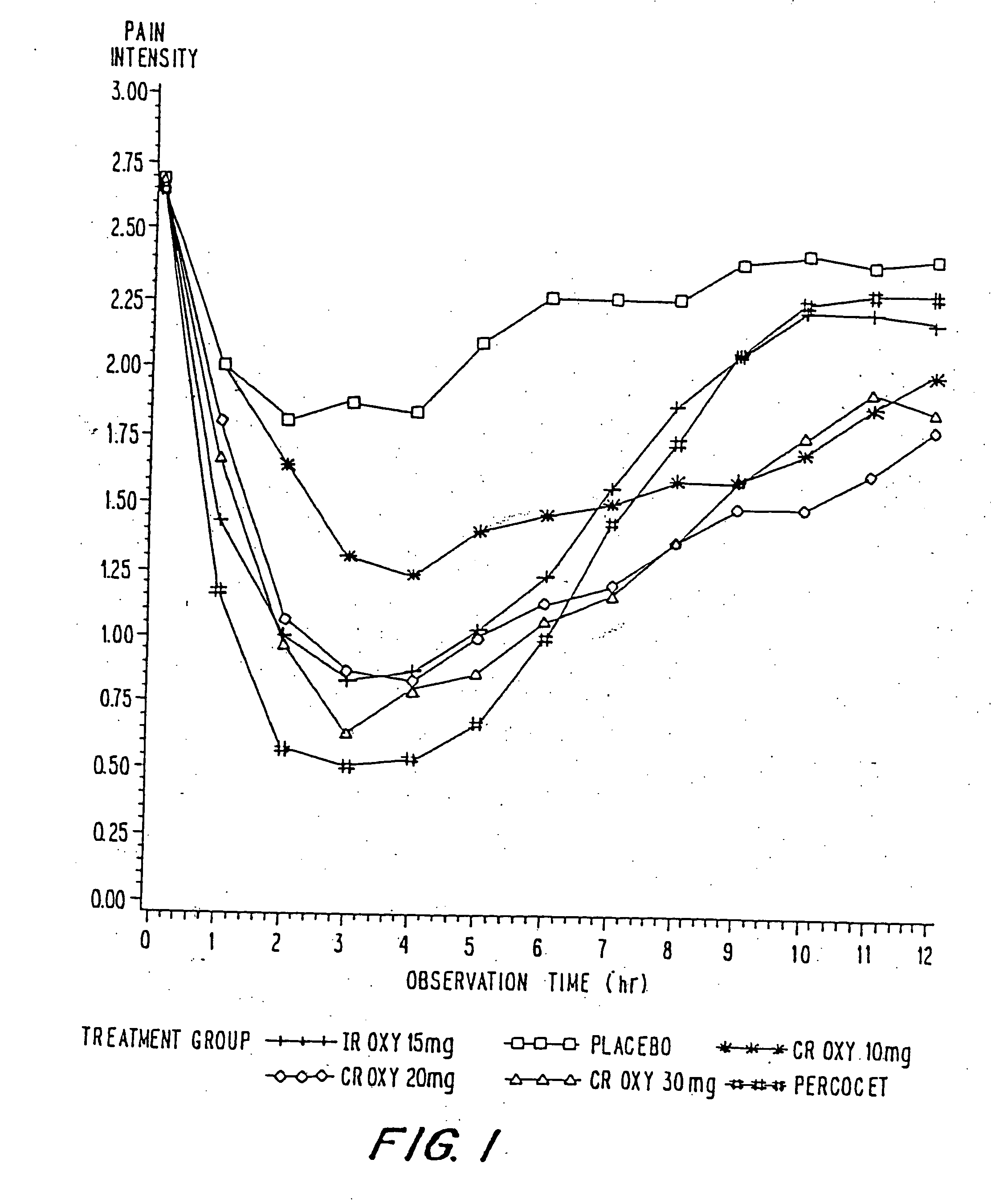
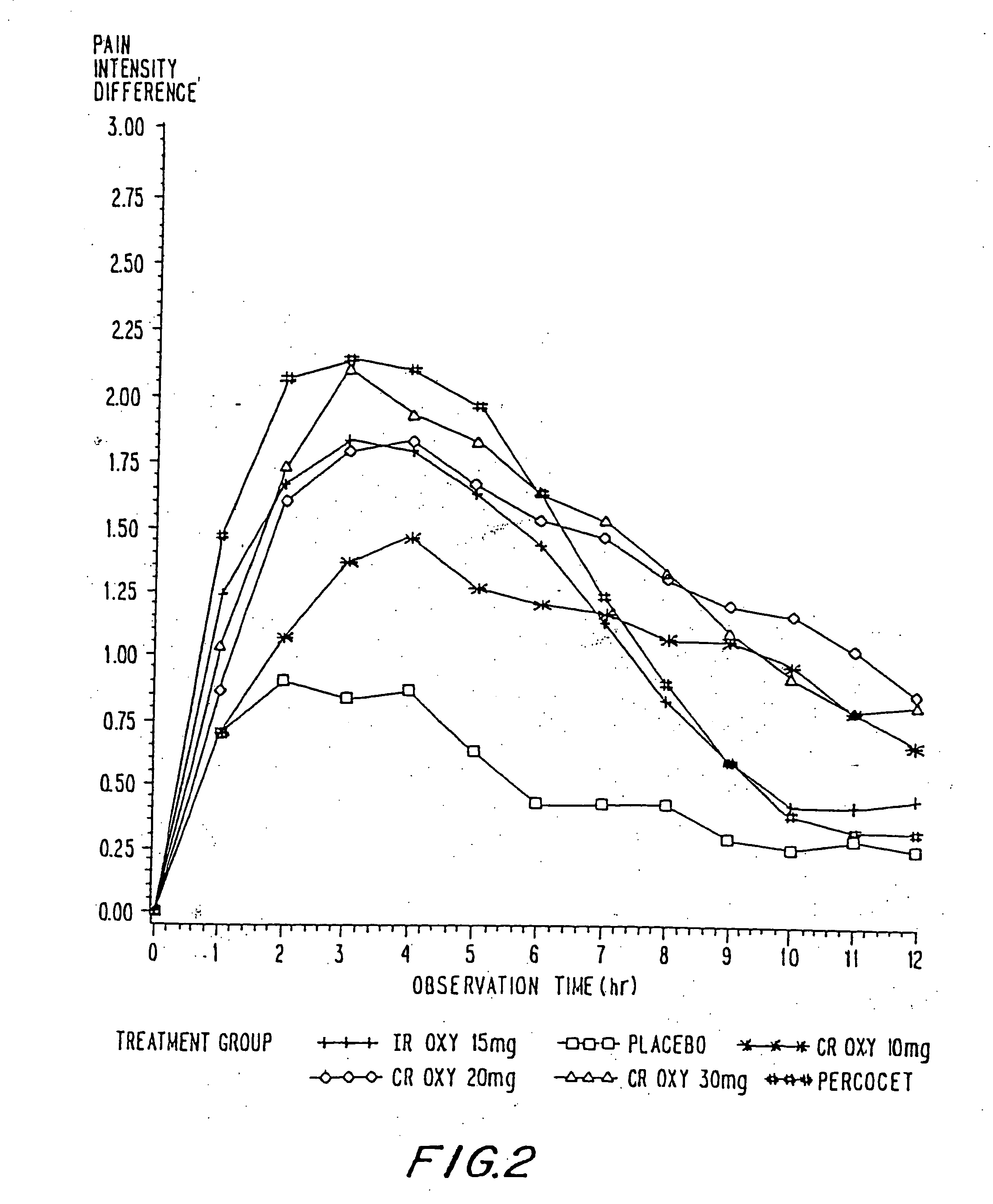
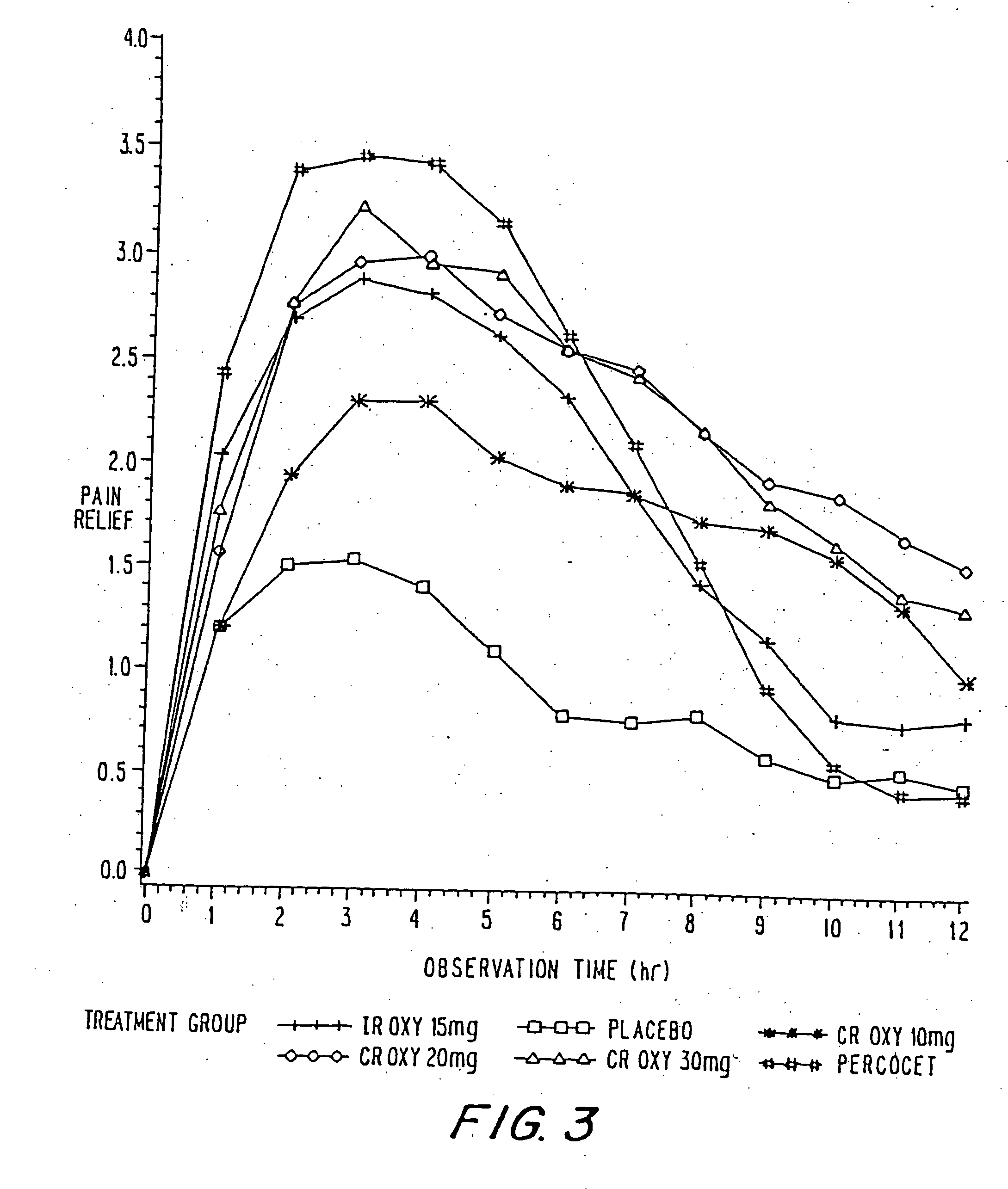
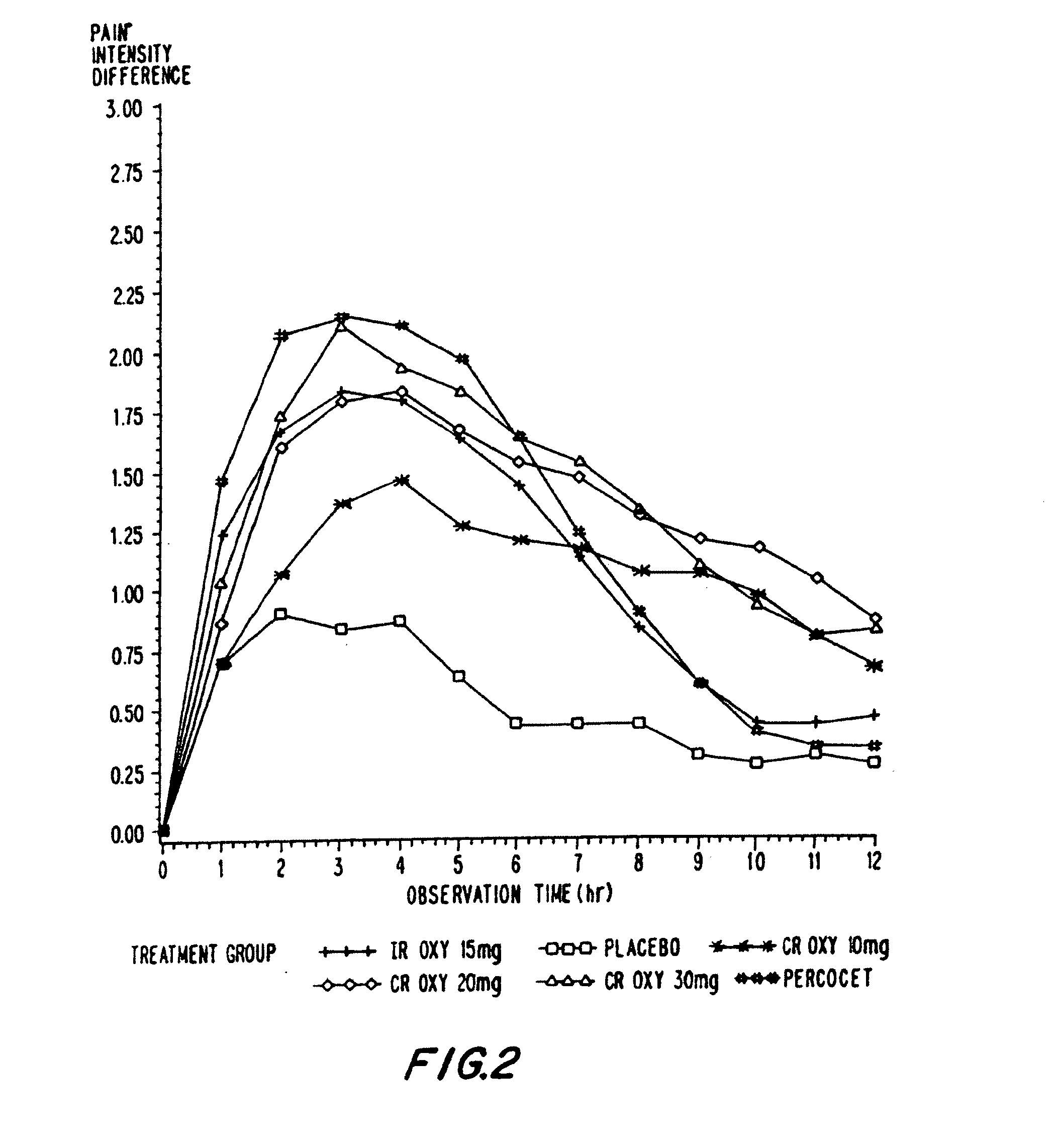
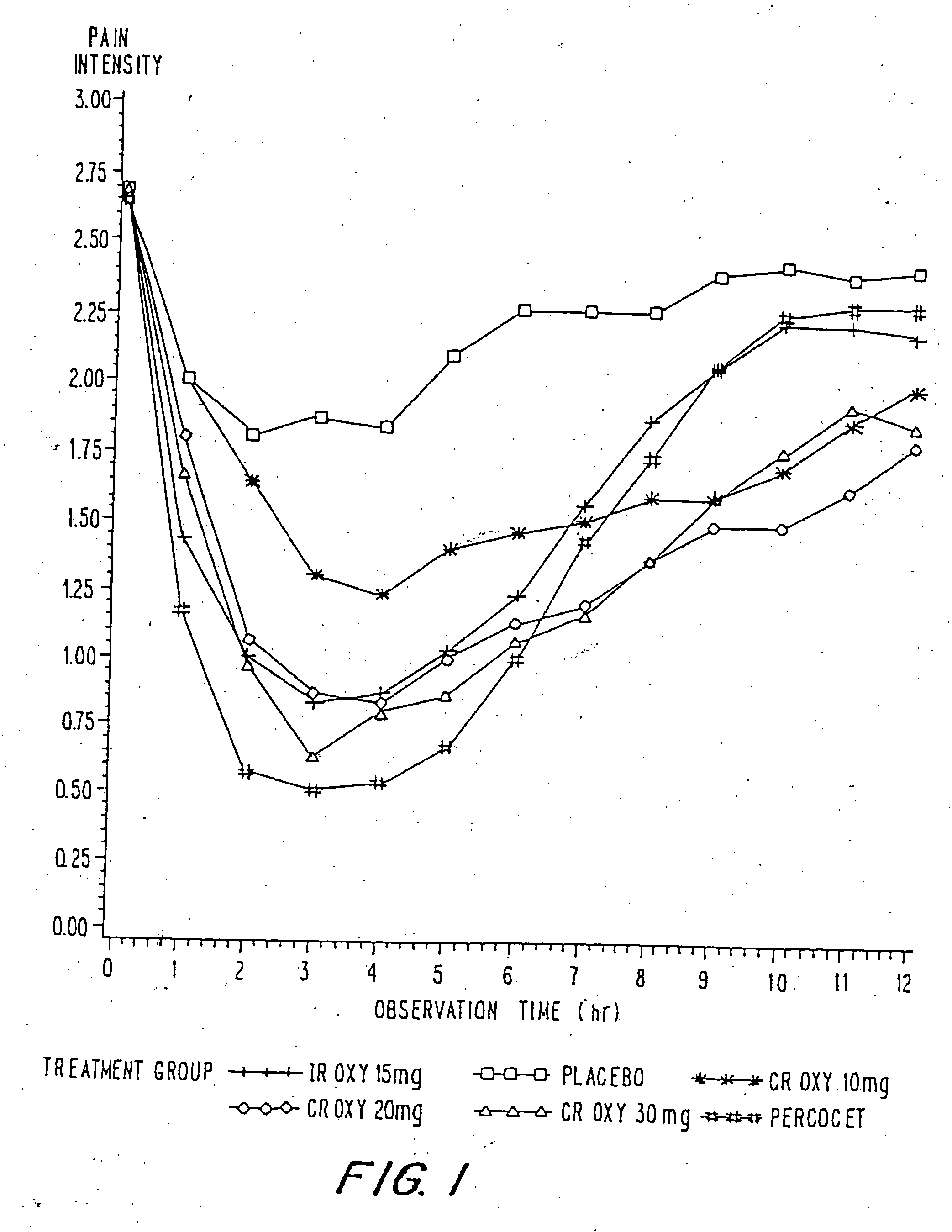
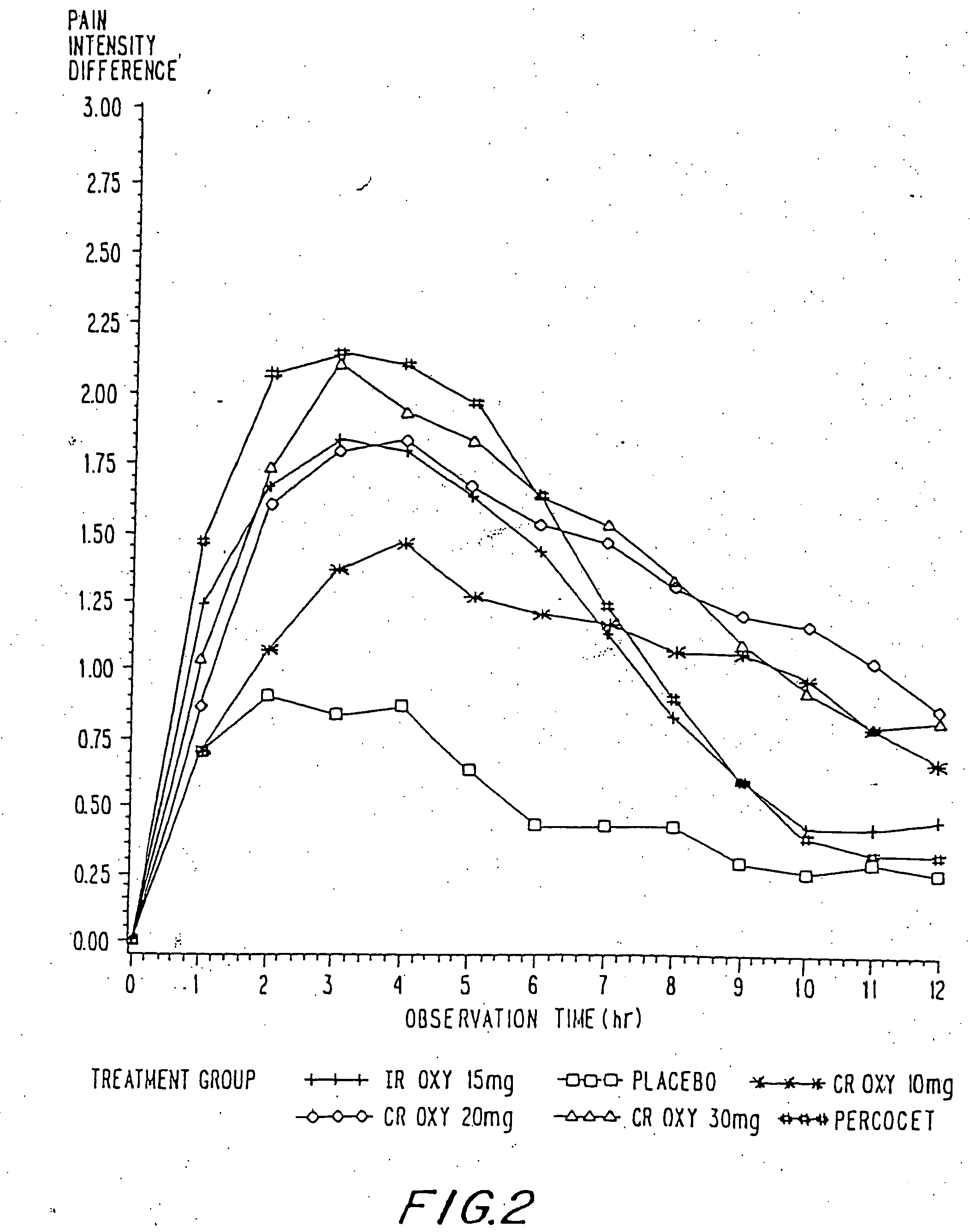
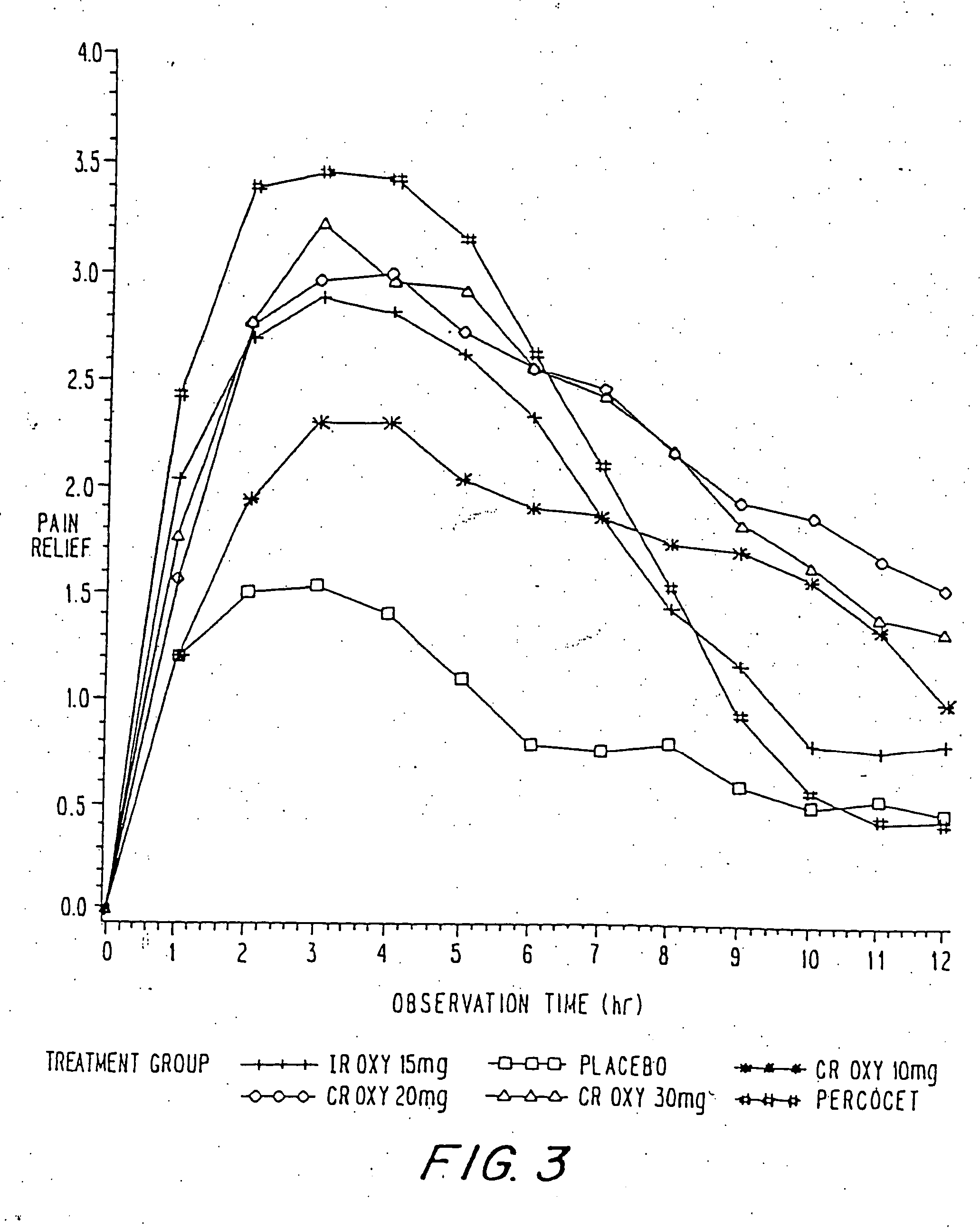
InactiveUS20060057210A1Improve efficiencyQuality improvementBiocidePowder deliveryControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +2
Controlled release hydrocodone formulations
InactiveUS20140271840A1Improve efficiency and qualityGood effectPowder deliveryBiocideControl releaseHuman patient
A solid oral controlled-release oral dosage form of hydrocodone is disclosed. The dosage form comprising an analgesically effective amount of hydrocodone or a pharmaceutically acceptable salt thereof, and a sufficient amount of a controlled release material to render the dosage form suitable for twice-a-day administration to a human patient, the dosage form providing a C12 / Cmax ratio of 0.55 to 0.85, said dosage form providing a therapeutic effect for at least about 12 hours.
Owner:PURDUE PHARMA LP
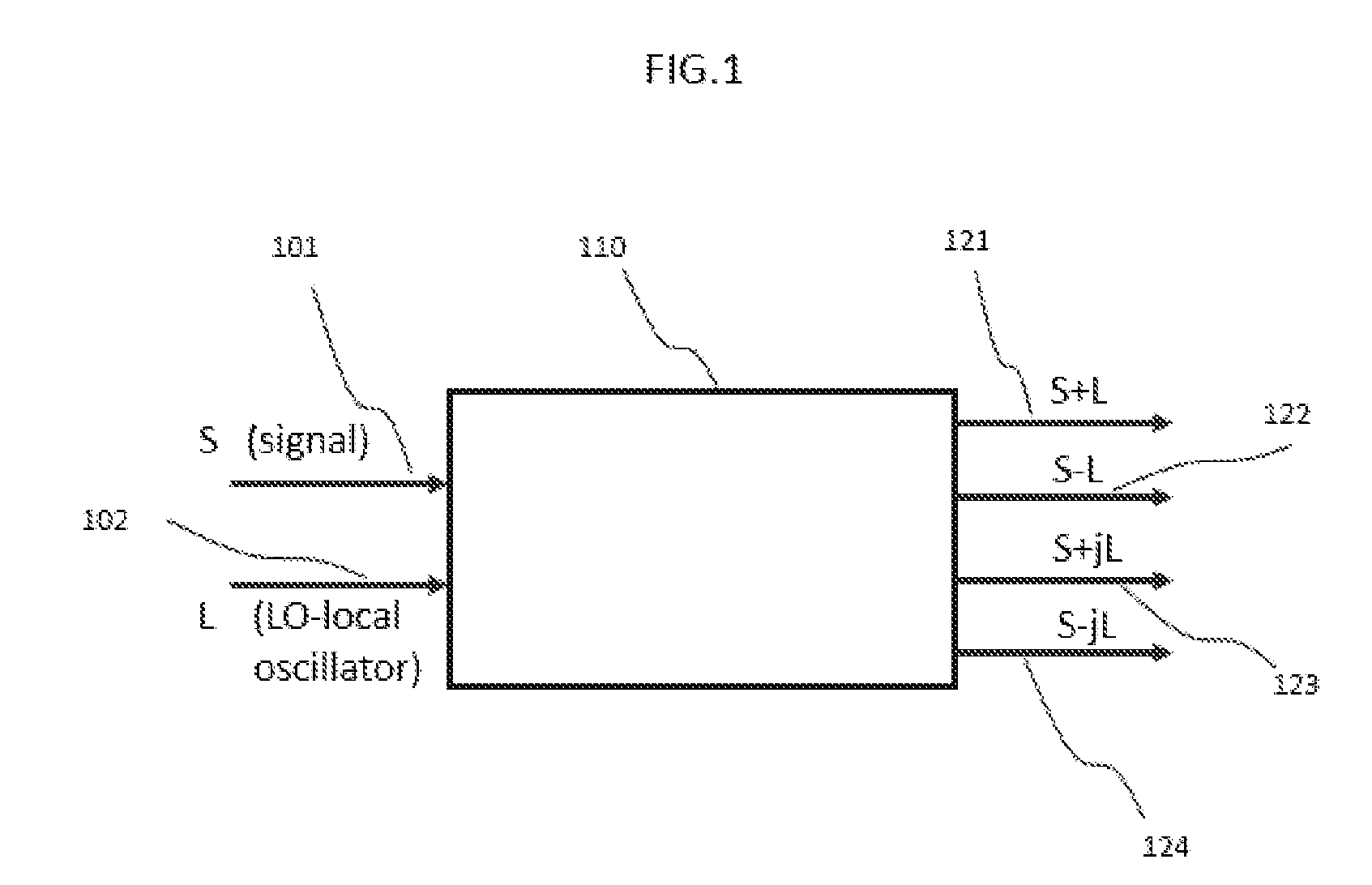
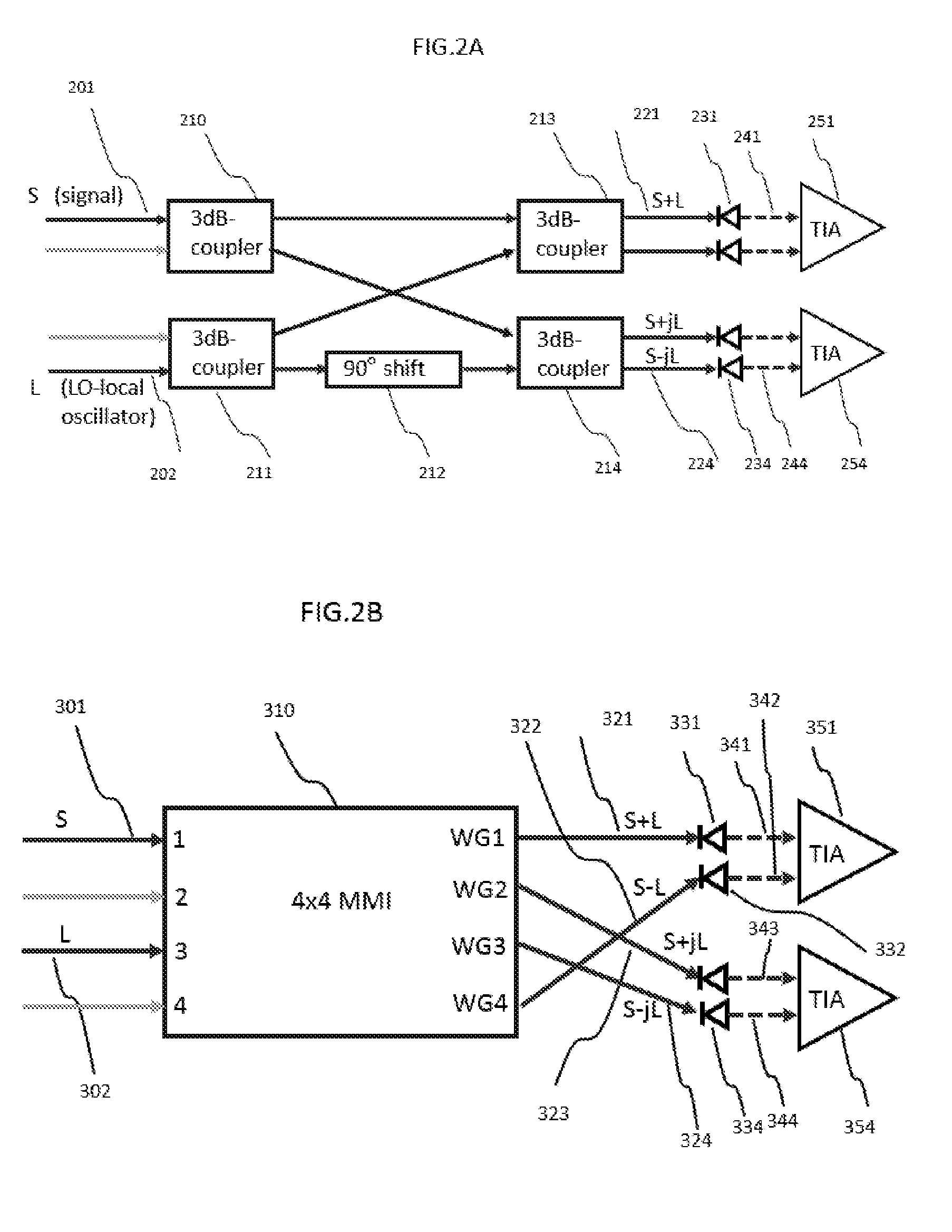
Integrated Coherent Receiver Having a Geometric Arrangement for Improved Device Efficiency
ActiveUS20160285561A1Efficient and reliable assembly processIssue to overcomeFibre transmissionCoupling light guidesPhase shiftedComputer module
Disclosed herein is a monolithically integrated coherent receiver chip which has a geometric arrangement of the on-chip components that significantly improves the performance and the manufacturability of a coherent receiver module for Dual Polarization Quadrature Phase Shift Keyed (DP-QPSK) applications and other optical coherent detection systems. The coherent receiver chip comprises two optical hybrids, three optical inputs and eight electrical outputs with the two optical hybrids oriented perpendicular to the optical inputs and the electrical outputs which are widely spaced and arranged in a co-linear fashion that simplifies module design and assembly. The proposed geometric arrangement also replaces any optical waveguide crossings with vertical electrical-optical crossings and includes electrical transmissions which are used to minimize channel skew. The proposed configuration also has the additional benefit of improved thermal management by separating the module's trans-impedance amplifiers.
Owner:O NET COMM (SHENZHEN) LTD
Controlled release oxycodone compositions
InactiveUS20080075781A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControlled painBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +3
Controlled release oxycodone compositions
InactiveUS20060099255A1Improve efficiency and qualityOrganic active ingredientsNervous disorderControlled releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:OSHLACK BENJAMIN +3
Controlled release opioid analgesic formulation
InactiveUS20050226929A1Improve efficiencyQuality improvementGranular deliveryCoatingsControlled releaseSustained Release Formulations
A pharmaceutical sustained release formulation for opioid analgesics which can be administered every 12 hours for control of pain in patients suffering from chronic pain.
Owner:ANDRX PHARMA INC
Winged venous catheter
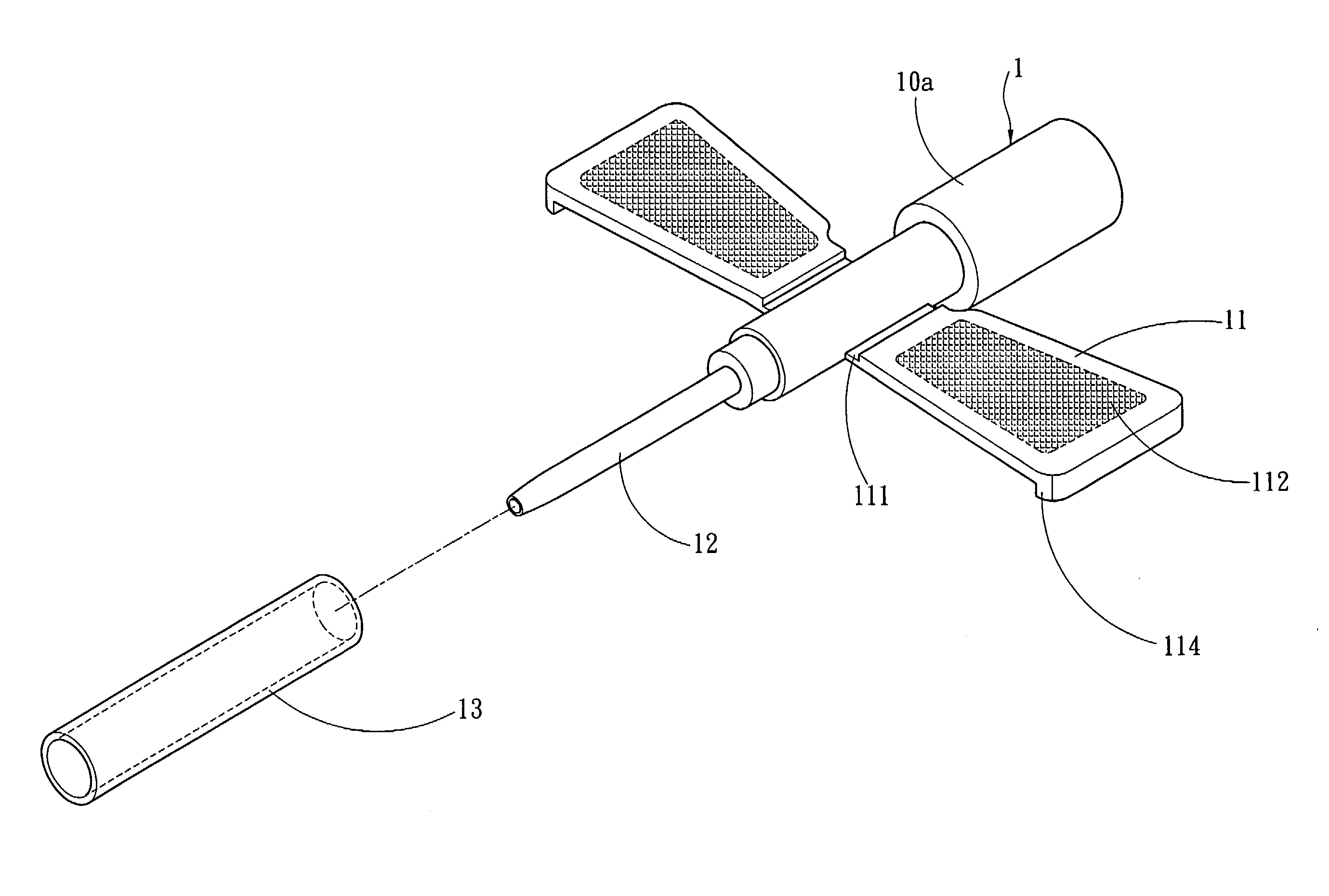
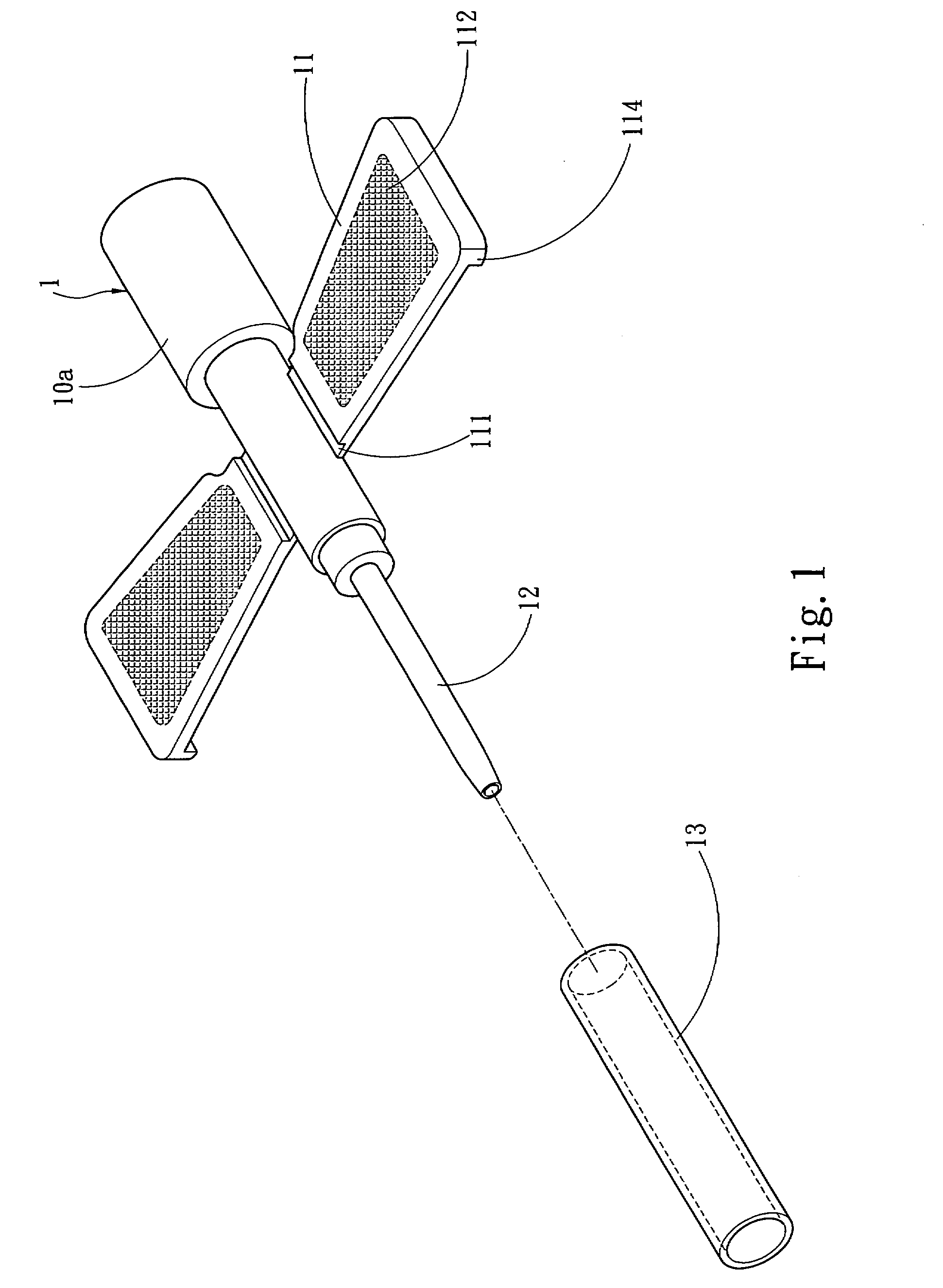
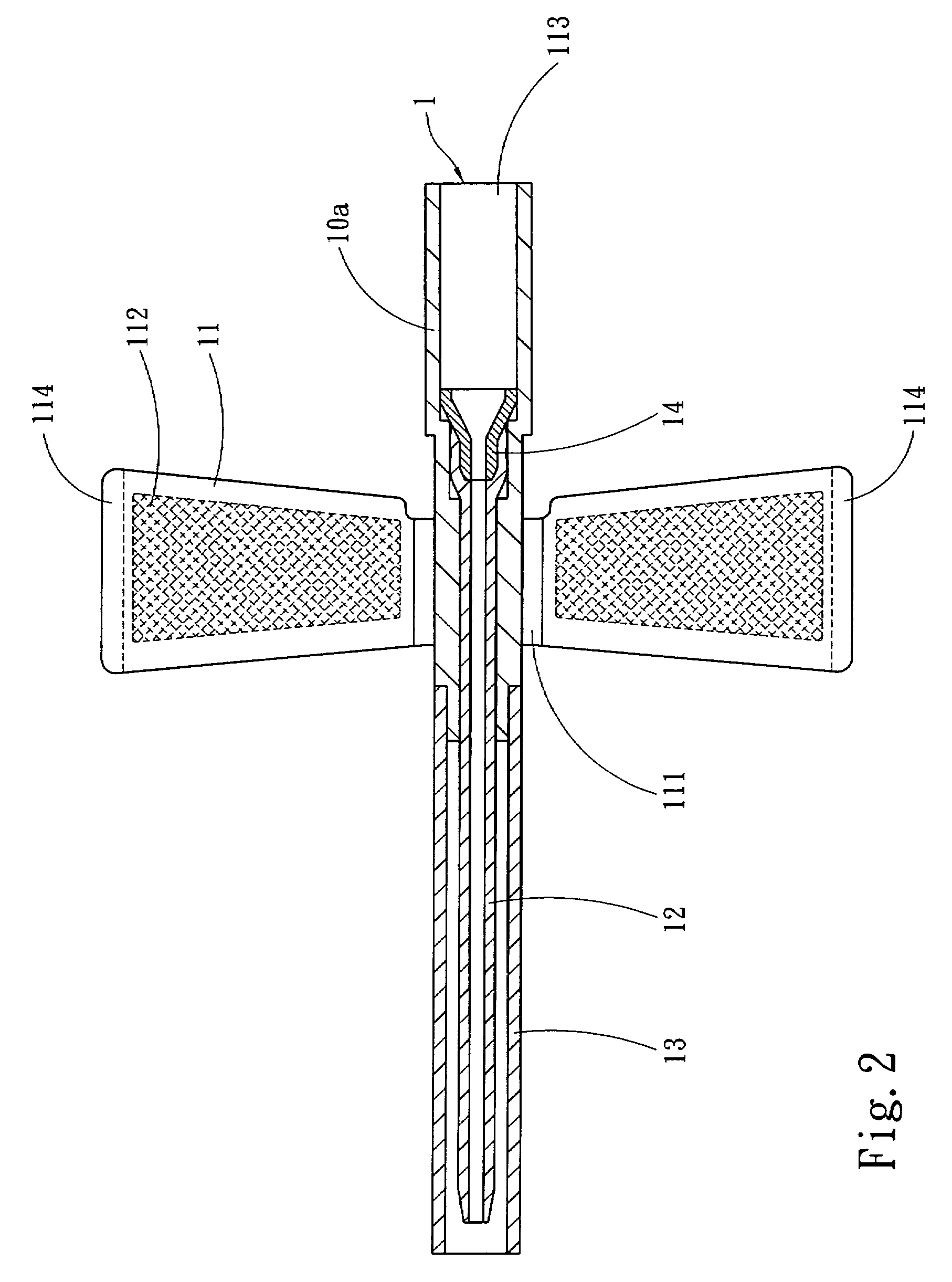
InactiveUS20070250011A1Simplify medical treatment procedureReduce harmInfusion syringesCatheterFixed wingMedical treatment
A winged venous catheter capable of simplifying a medical treatment procedure for medical professionals includes a barrel having a set of fixed wings, a soft tube having a tapered diameter and extended from an end of the barrel, a connecting opening extended from another end of the barrel without the soft tube. The barrel includes a position limiting portion disposed on an internal wall and coupled to the soft tube for preventing accidental pierces. The invention provides medical professionals to insert a syringe needle into the soft tube through the barrel, use the clamped fixed wing to insert the syringe needle into the vein, and pull the syringe needle out from the vein but let the soft tube remain in the vein for facilitating liquid medicines to continuously flow from the connecting opening into the vein through the soft tube.
Owner:LEE DAVID
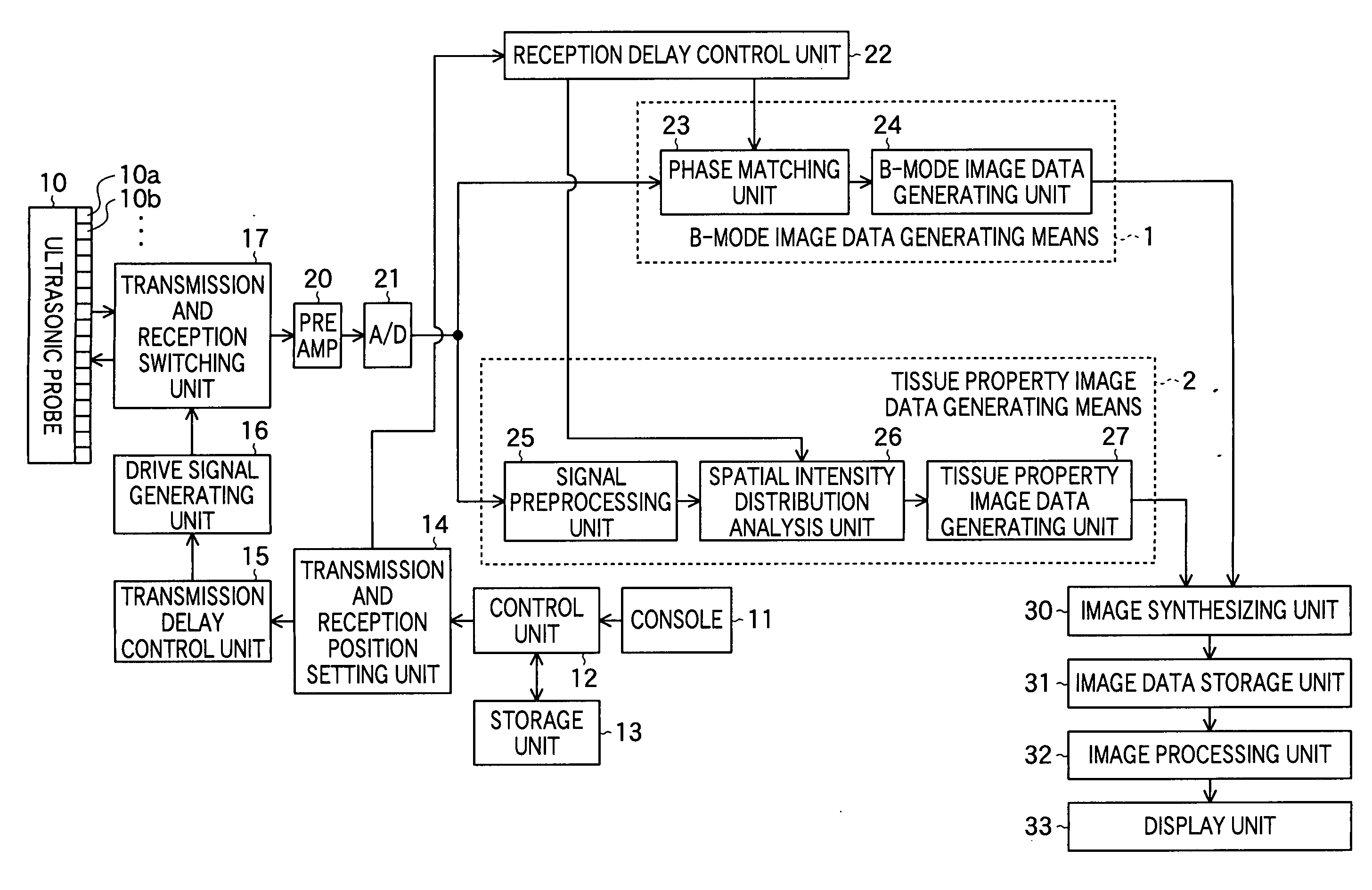
Ultrasonic imaging apparatus
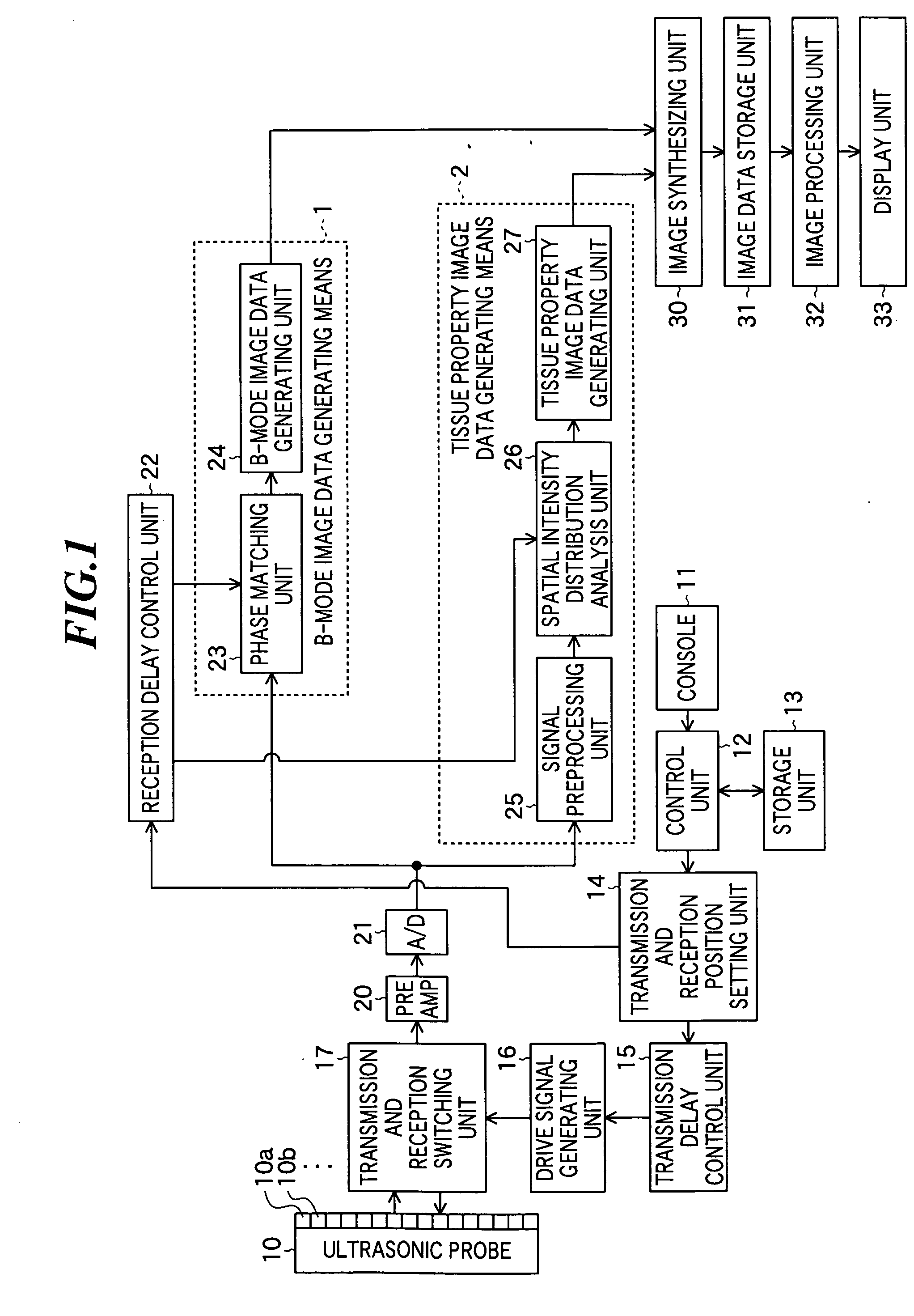
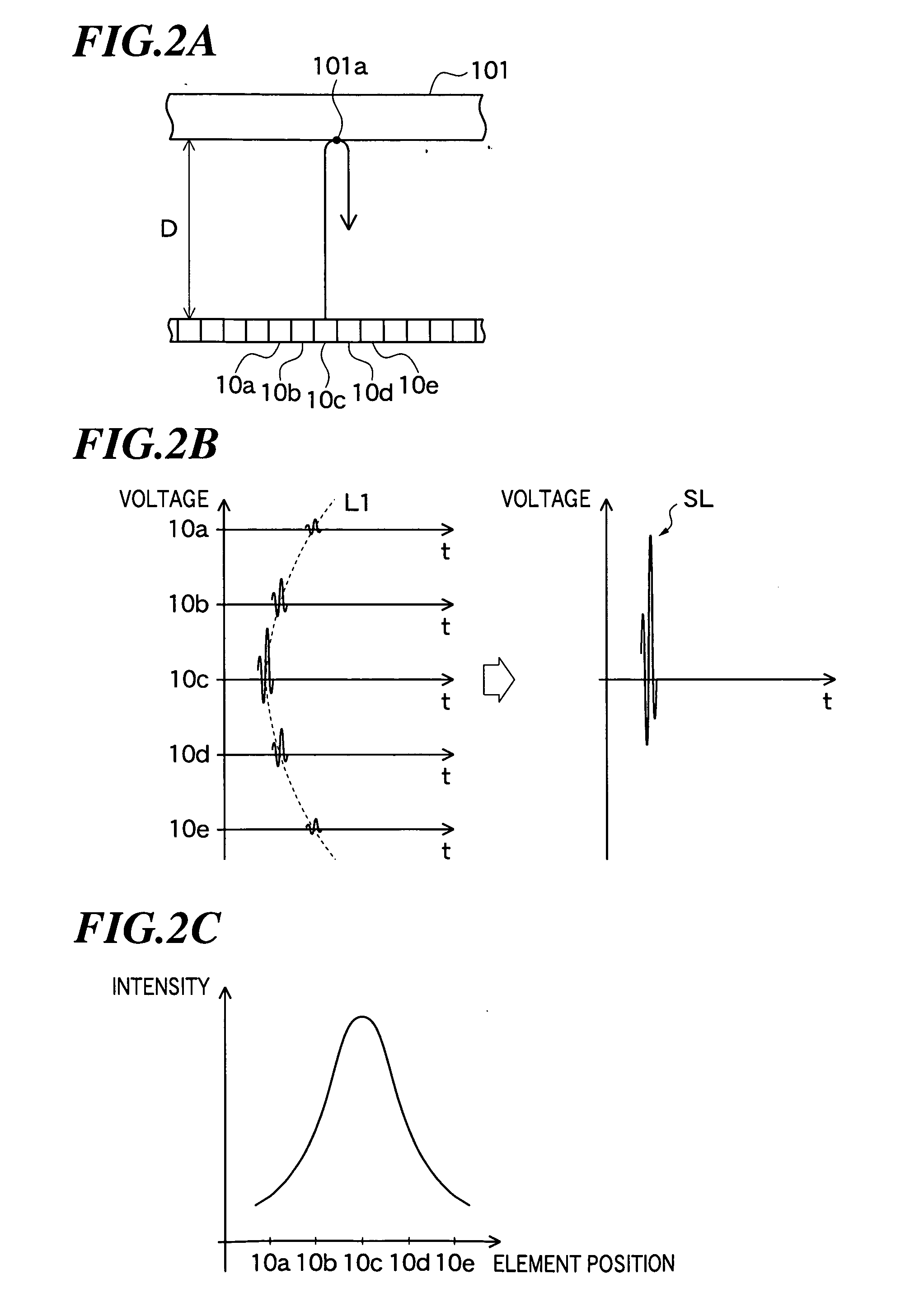
ActiveUS20060079776A1Improve efficiency and qualityImprove efficiencyUltrasonic/sonic/infrasonic diagnosticsInfrasonic diagnosticsSignal onGenerating unit
Tissue property of a reflector is detected based on plural reception signals outputted from plural ultrasonic transducers by receiving ultrasonic echoes. An ultrasonic imaging apparatus includes: an ultrasonic probe including plural ultrasonic transducers for transmitting ultrasonic waves toward an object to be inspected and receiving ultrasonic waves reflected from the object to output plural reception signals; and a tissue property image generating unit for generating information on tissue property in a region within the object based on interrelationship among at least one group of reception signals on the region from among the plural reception signals respectively outputted from the plural ultrasonic transducers.
Owner:FUJIFILM CORP +1
Controlled release oxycodone compositions
InactiveUS20100092570A1Improve efficiency and qualityLow variabilityBiocidePowder deliveryControl releaseBlood plasma
A method for substantially reducing the range in daily dosages required to control pain in approximately 90% of patients is disclosed whereby an oral solid controlled release dosage formulation having from about 10 to about 40 mg of oxycodone or a salt thereof is administered to a patient. The formulation provides a mean maximum plasma concentration of oxycodone from about 6 to about 60 ng / ml from a mean of about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration from about 3 to about 30 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions. Another embodiment is directed to a method for substantially reducing the range in daily dosages required to control pain in substantially all patients by administering an oral solid controlled release dosage formulation comprising up to about 160 mg of oxycodone or a salt thereof, such that a mean maximum plasma concentration of oxycodone up to about 240 ng / ml from a mean of up to about 2 to about 4.5 hours after administration, and a mean minimum plasma concentration up to about 120 ng / ml from about 10 to about 14 hours after repeated “q12h” (i.e., every 12 hour) administration through steady-state conditions are achieved. Controlled release oxycodone formulations for achieving the above are also disclosed.
Owner:PURDUE PHARMA LP +1
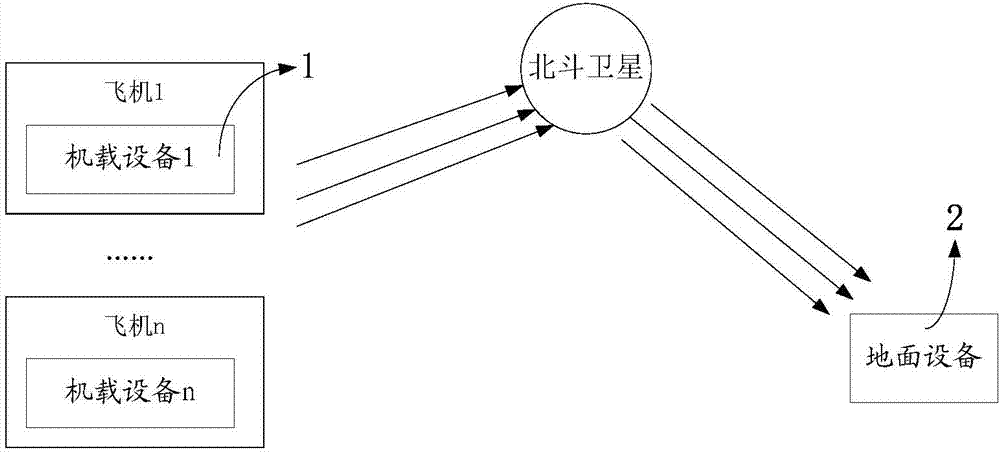
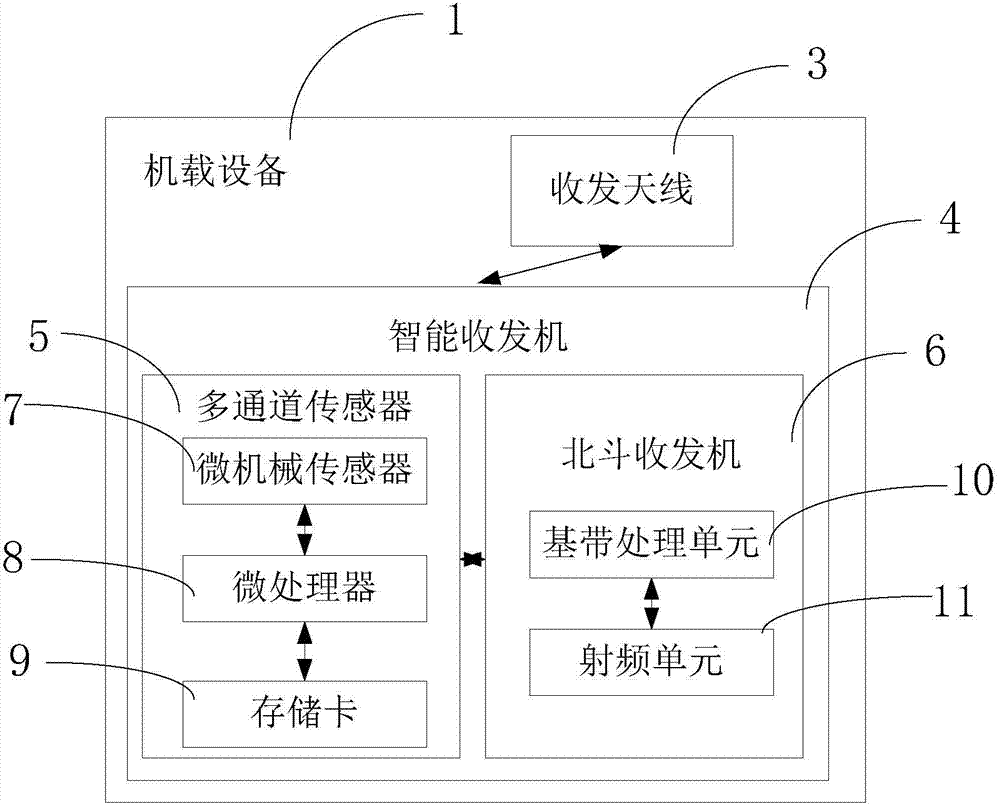
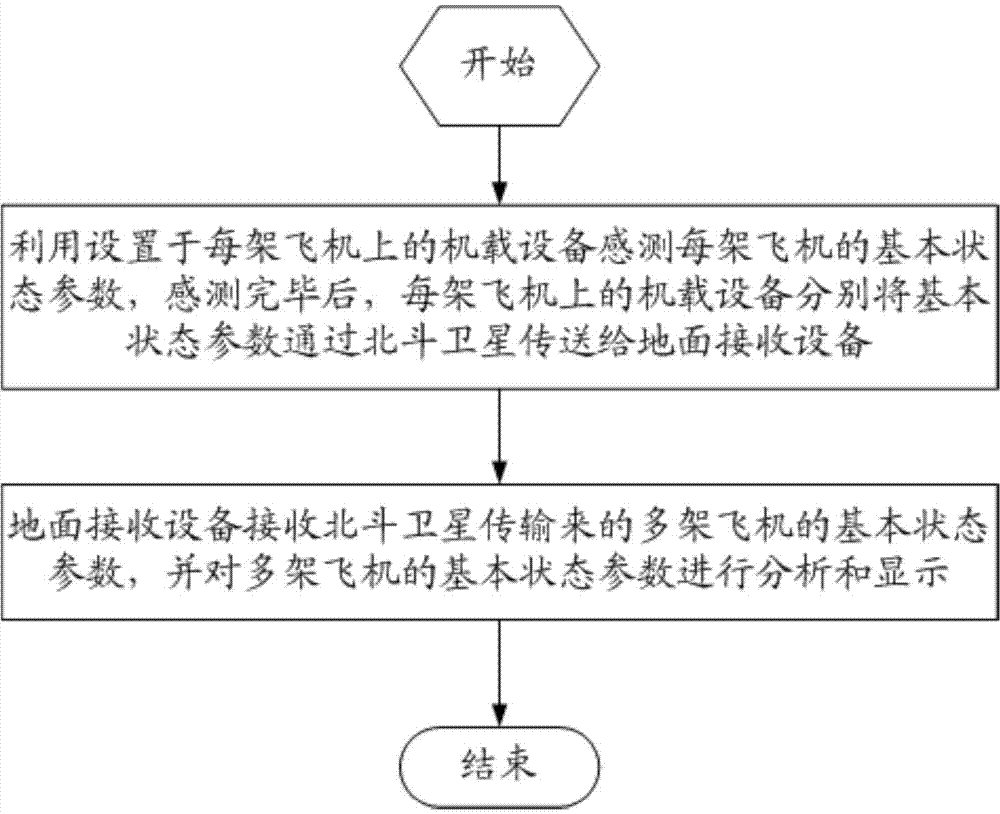
Beidou-based flight safety real-time monitoring system and method
InactiveCN104267417AImprove securityImprove efficiency and qualityTransmission systemsNetwork topologiesAirplaneFlight data
The invention relates to a Beidou-based flight safety real-time monitoring system and method. The system comprises a plurality of airborne devices and a ground receiving device; each airborne device is correspondingly arranged on one airplane and used for sensing basic state parameters of the airplane provided with the airborne device; the basic state parameters of the multiple airplanes are transmitted to the ground receiving device through a Beidou satellite; the ground receiving device is used for receiving the basic state parameters, transmitted by the Beidou satellite, of the multiple airplanes and analyzing and displaying the basic state parameters of the multiple airplanes. The main parameters of the airplanes are measured through the airborne parts; the airborne parts are not hinged to electronic parts of the airplanes; a plurality of flight data segments are designed in one Beidou short message, so that the problem that as the transmission rate of a Beidou system is low, the continuity is poor is solved; pictures are displayed in real time, and the system and method are friendly to commanders.
Owner:XIAN KEYUAN MEASUREMENT & CONTROL TECH
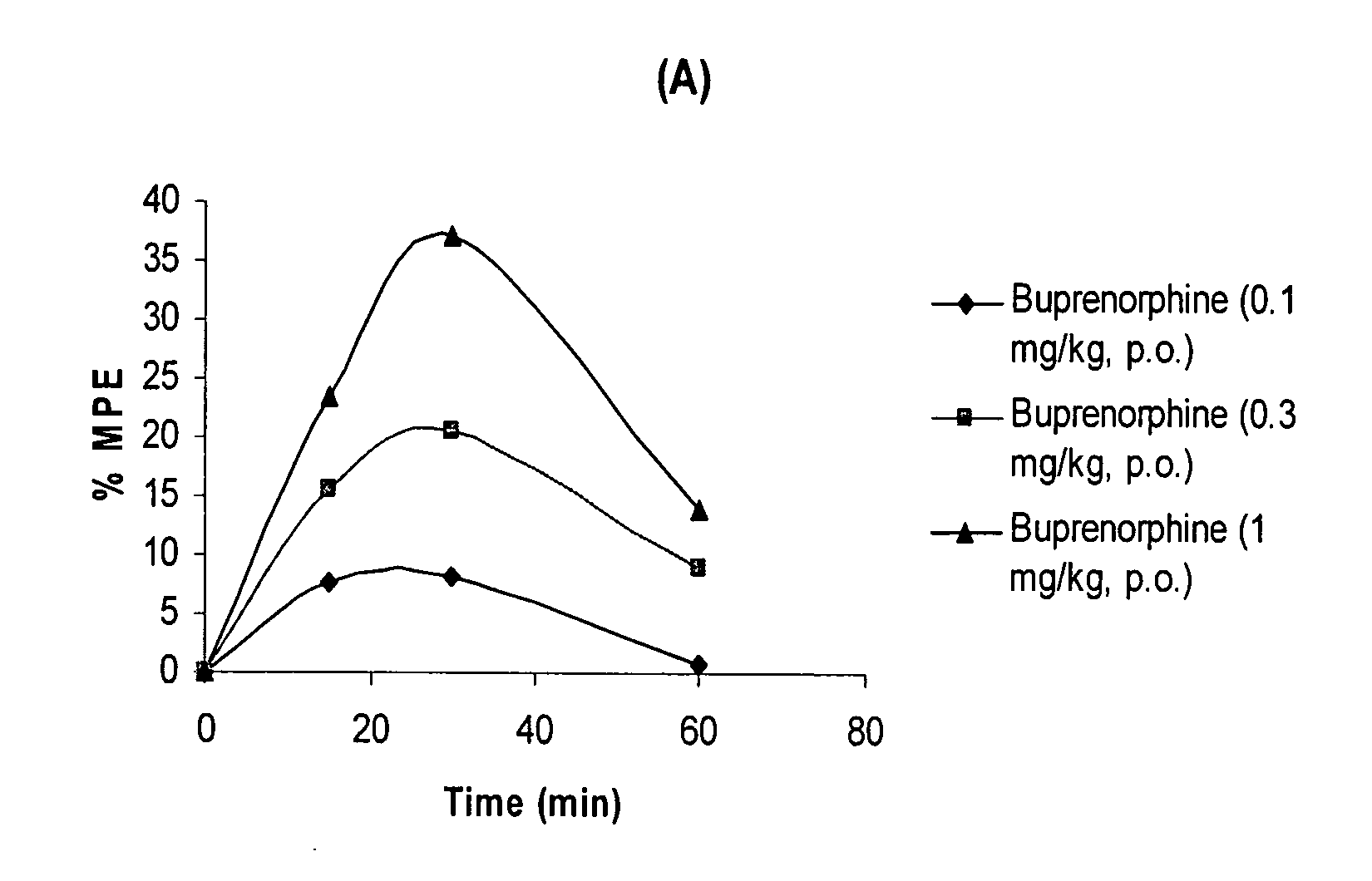
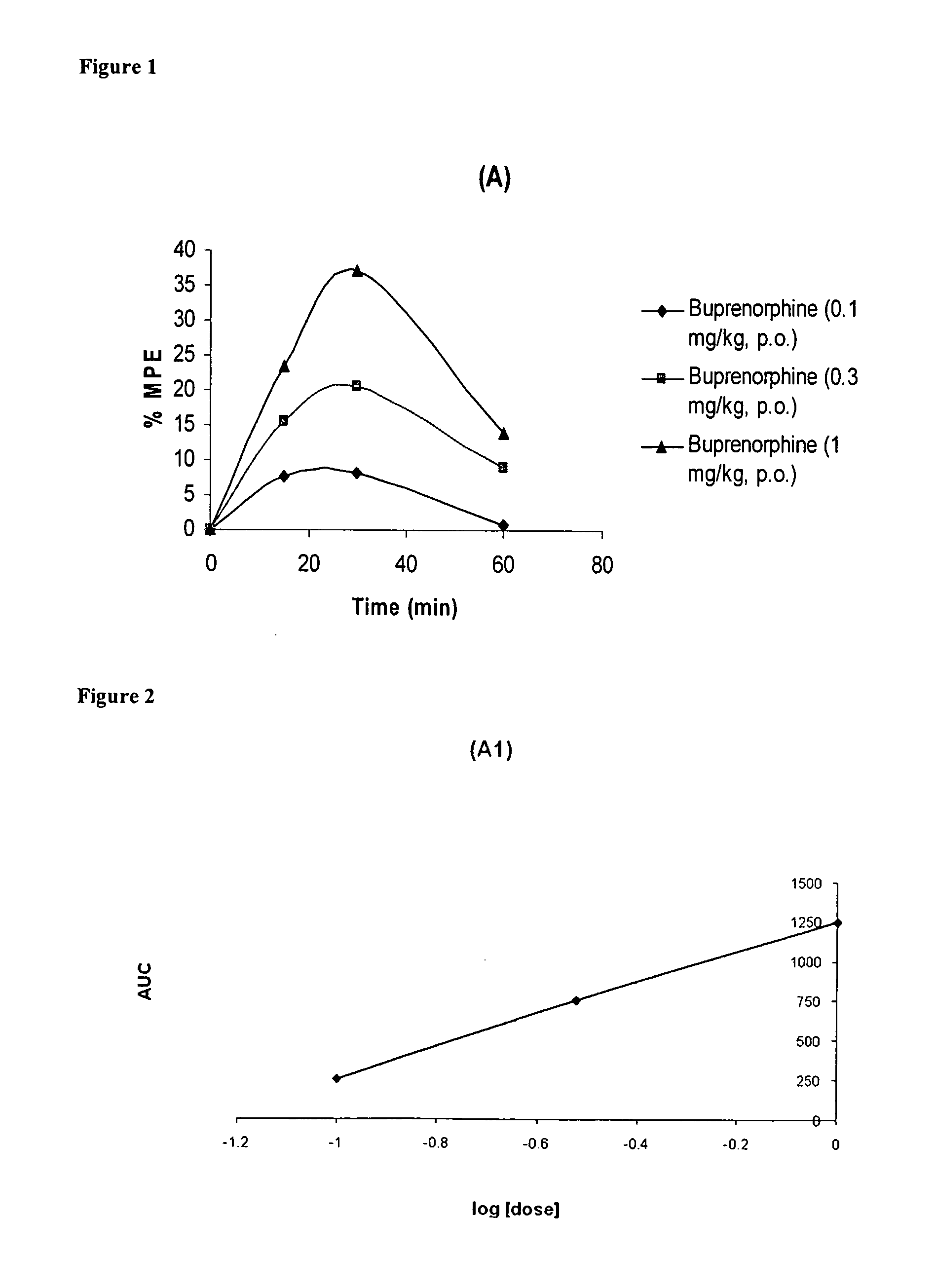
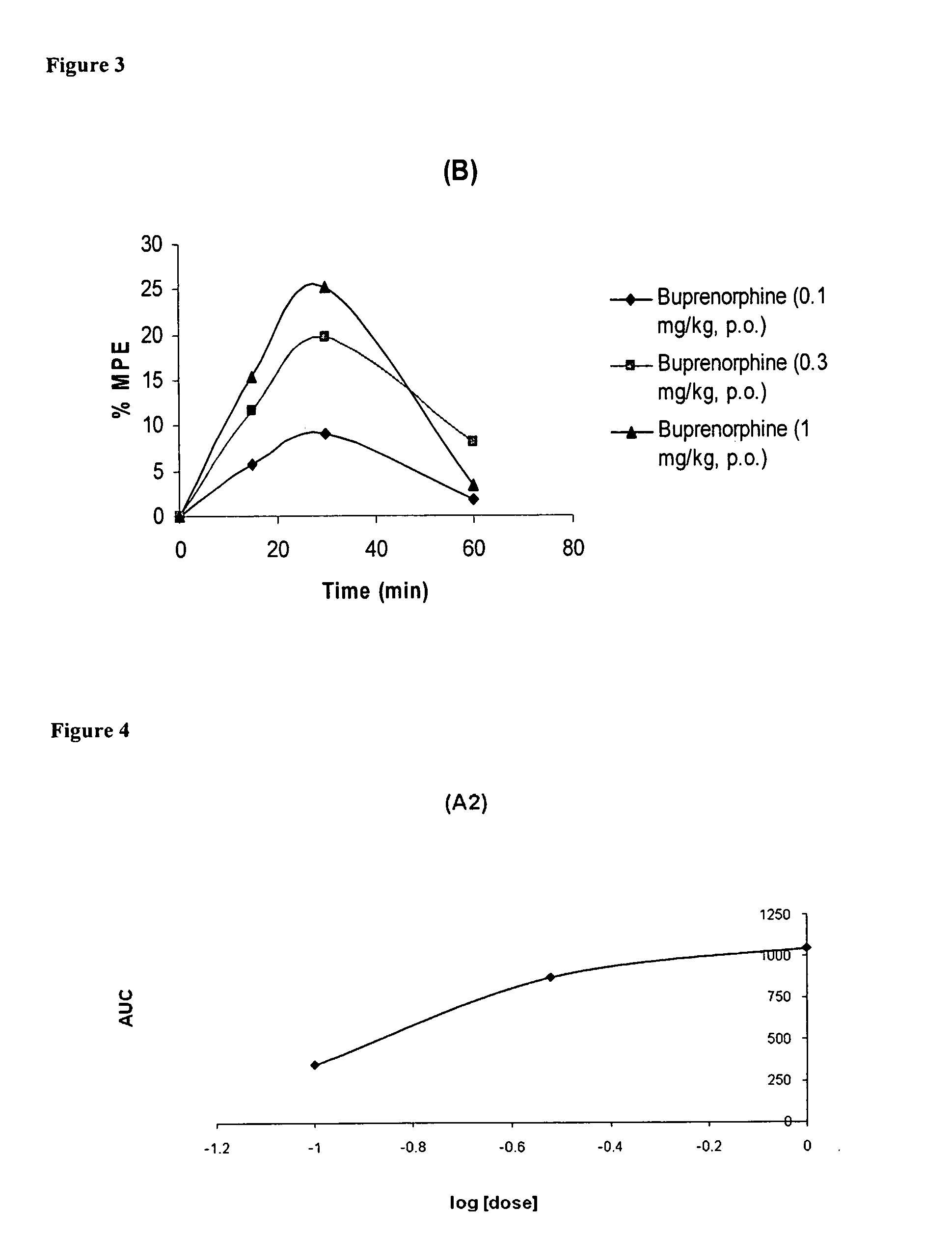
Oral Pharmaceutical Compositions of Buprenorphine and Method of Use
InactiveUS20110097395A1Improve efficiency and qualityDiscourages improper usagePowder deliveryBiocideBuprenorphineDrug
The present invention is directed to oral pharmaceutical compositions of buprenorphine and it pharmaceutically acceptable salts and the use thereof.
Owner:RELMADA THERAPEUTICS
Composite material vacuum bag forming method
The invention belongs to the technical field of resin group composite material liquid moulding manufacture, and relates to a composite material vacuum bag forming method. According to the composite material vacuum bag forming method provided by the invention, a multilayer metal fiber mesh grid can be used as a rigid resin flow distributor, and technologic advantages such as resin film impregnation molding (RFI), vacuum auxiliary resin impregnation molding (VARI) and vacuum bag autoclave molding (Autoclave) are combined; the composite material vacuum bag forming method can be used for effectively promoting resin to flow along the thickness direction of a fiber perform body so as to guarantee the structural support of the preform body, and overcoming the disadvantages that a large-area composite material liquid moulding workpiece brought by the flexible distributor is low in superficial dimension precision and poor in thickness uniformity at present; and the moulding efficiency and dimensional accuracy of the composite material workpiece are improved, and a more rapid, more flexible and low-cost liquid molding technical scheme for manufacturing a high-performance composite material structure piece is provided.
Owner:AVIC BEIJING INST OF AERONAUTICAL MATERIALS
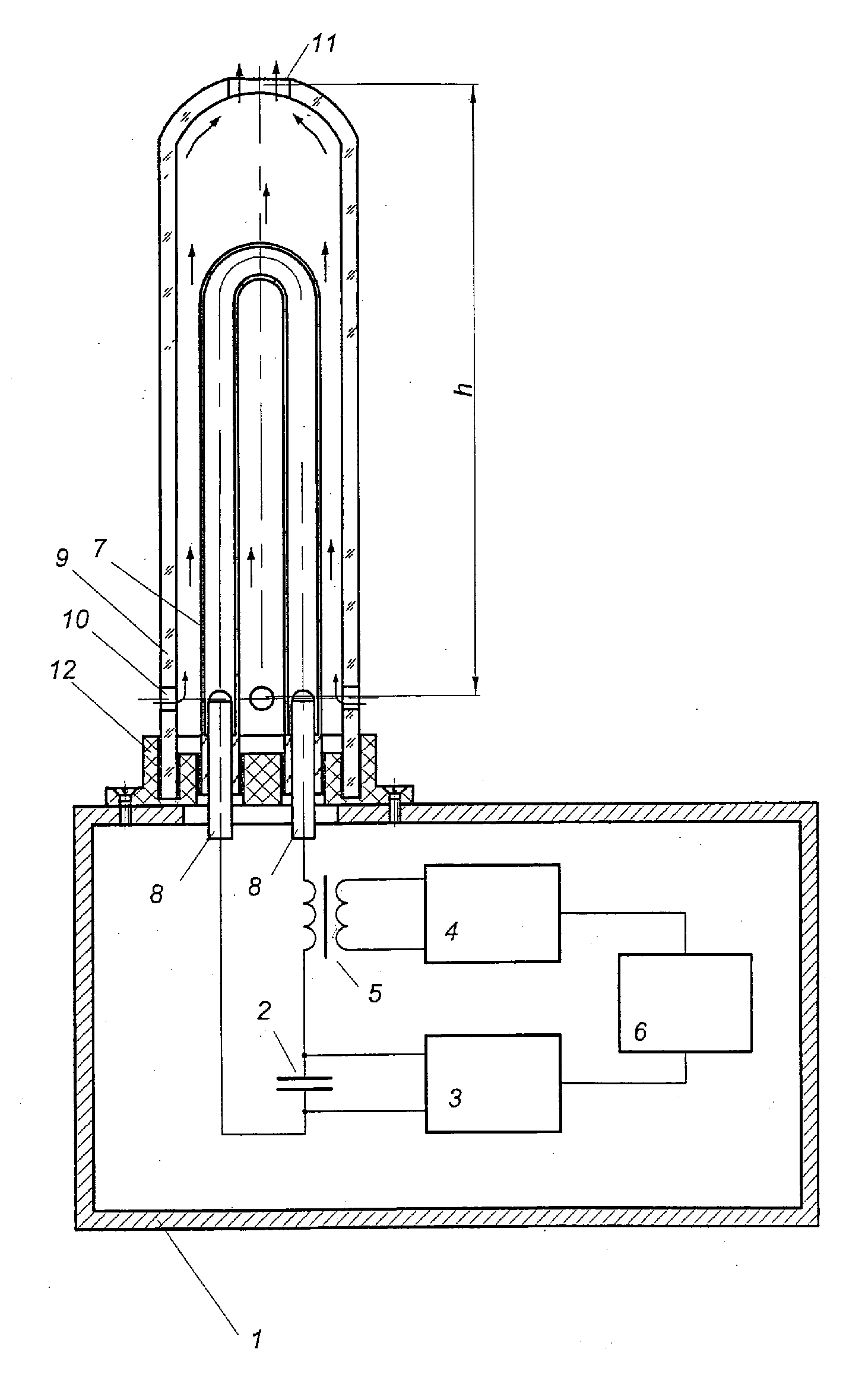
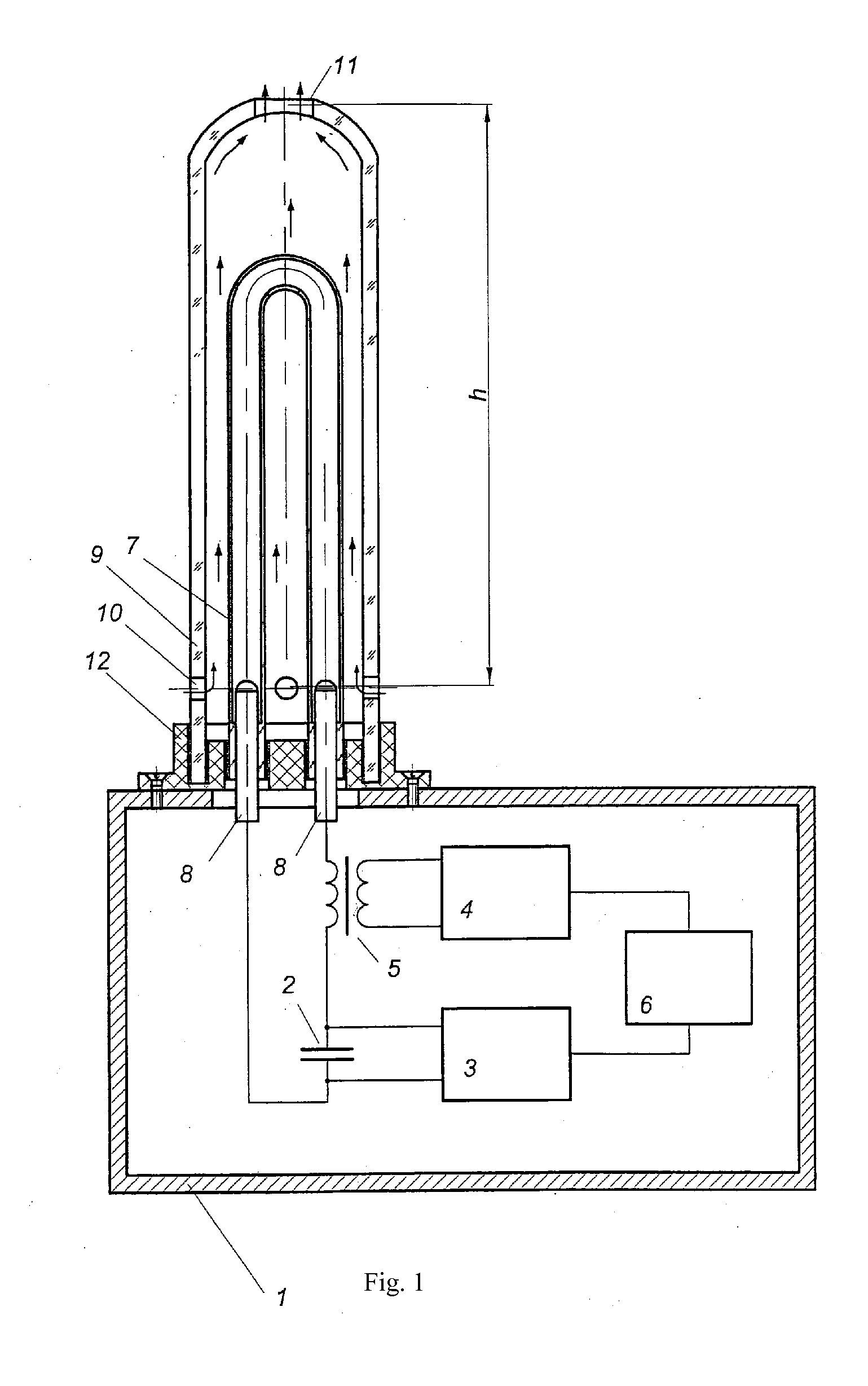
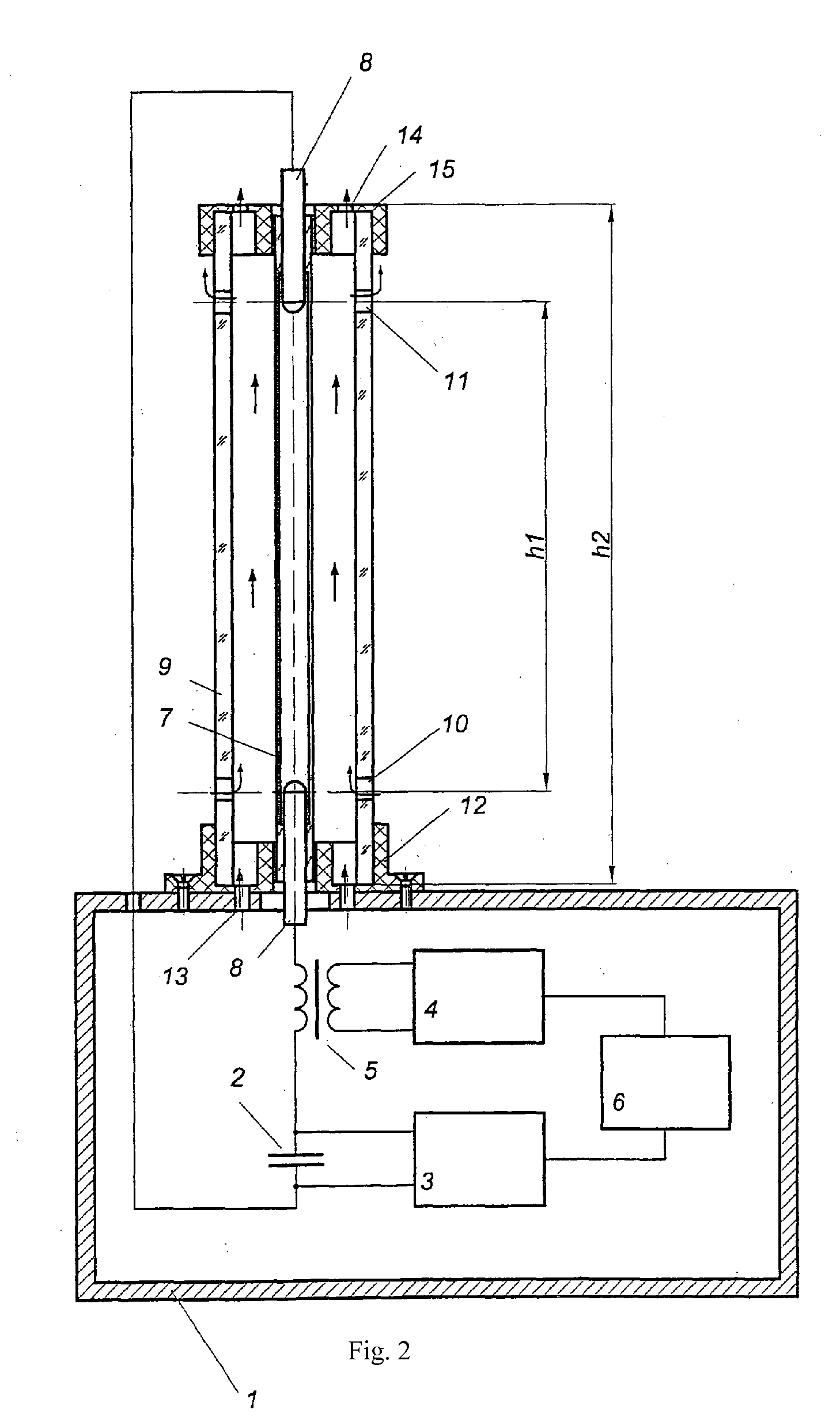
Air sterilizing assembly
ActiveUS20120119108A1Simplifying unit designImprove efficiencyScattering properties measurementsMaterial analysis by transmitting radiationHigh-voltage direct currentEngineering
Air disinfection device comprises a body that houses the power supply and control unit comprising a storage capacitor, a high-voltage DC power supply, an ignition pulse generator, a ferrite-core pulse transformer and a program control unit, and an ultraviolet radiation source mounted on the body in the form of a pulse gas discharge lamp enclosed in a tubular cooled casing transparent to bactericidal radiation. The storage capacitor and the pulse gas discharge lamp form a discharge circuit connected to the ignition pulse generator through the ferrite-core pulse transformer. The pulse gas discharge lamp is installed in a casing transparent to bactericidal radiation with convection air cooling capabilities provided by natural draft produced inside the casing, wherein the casing has one or several upper-level orifices in its upper part and some lower-level orifices in its lower part, with the following ratio of parameters:h·Supper2·Slower2Supper2+Slower2=1A·C2U04F2where h is the distance between the orifices of the upper and lower levels, m; Supper—total surface area of the orifice in the upper level, m2; Slower—total surface area of the orifice in the lower level, m2; A=(2÷30)·1013J2 / m5s2—power correlation coefficient; C—capacity of energy storage capacitor, F; U0—charge voltage of the energy storage capacitor, V; F—pulse repetition rate of the ignition pulse generator, Hz.
Owner:NAUCHNO PROIZVODSTVENNOE PREDPRIYATIE MELITTA LLC
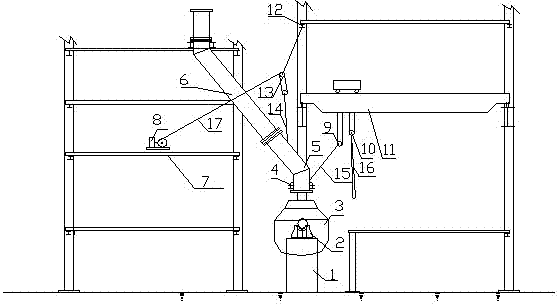
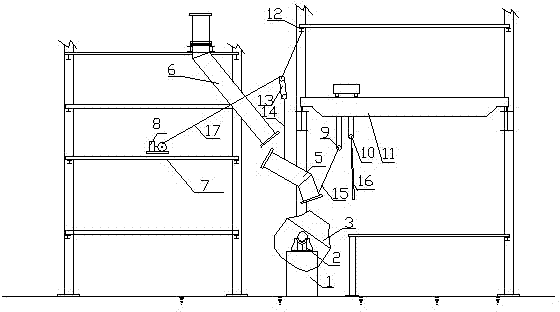
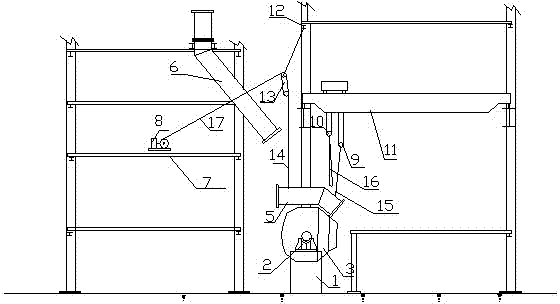
Hoisting method for overhauling and dismantling vaporization flue fixed at first section of converter
ActiveCN102816891AImprove efficiency and qualityReduce construction costsManufacturing convertersFlueVaporization
Owner:CHINA MCC17 GRP
Rapid configuration and test method for programmable logic device in system programming
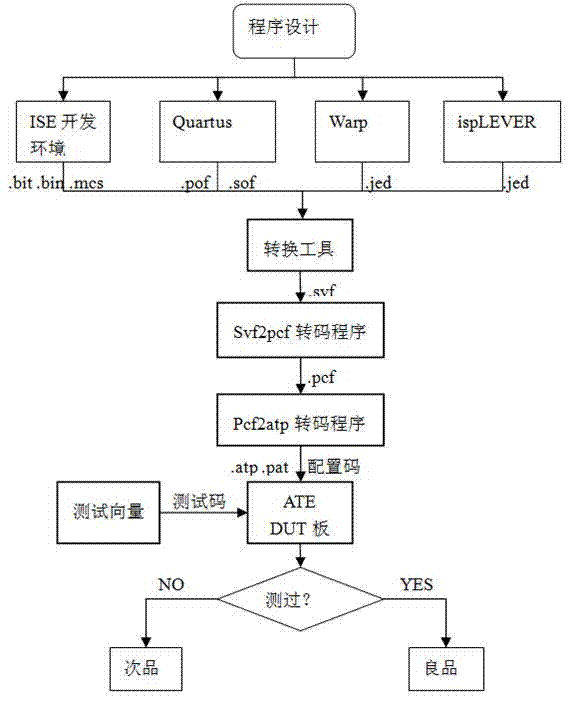
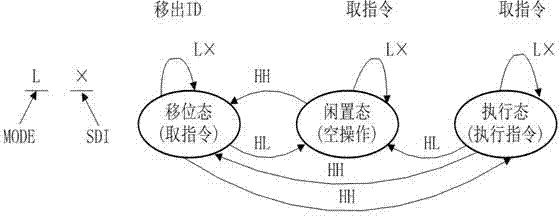
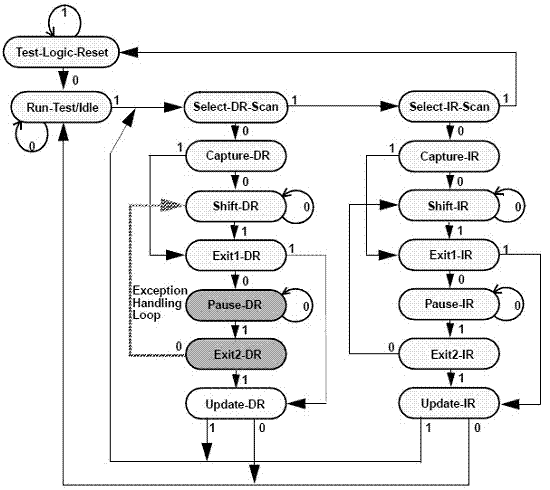
InactiveCN104515947AImprove test fault coverageImprove efficiency and qualityElectronic circuit testingSoftware testing/debuggingEngineeringDevelopment environment
The invention discloses a rapid configuration and test method for a programmable logic device in system programming. According to the method, ISP (in-system programmable) state machine configuration codes are acquired by one-time programming and four-time transcoding. The rapid configuration and test method includes the steps of conducting test configuration program development in a corresponding development environment of the programmable logic device to acquire original configuration codes; converting the original configuration codes into an SVF (serial vector format) file through a conversion tool; converting the SVF file into a PCF (portable compiled format) file; generating an ATP file by a C-language transcoding program; converting the ATP file into a Pattern file, and using an ATE (automatic test equipment) automatic test system for rapid configuration and test. The rapid configuration and test method for the programmable logic device in system programming has the advantages that the ISP state machine configuration codes can be generated automatically, and multi-time configuration and test operation can be conducted, so that test fault coverage rate is increased greatly, and the test problem of the programmable logic device is solved; the rapid configuration and test method is universal.
Owner:58TH RES INST OF CETC
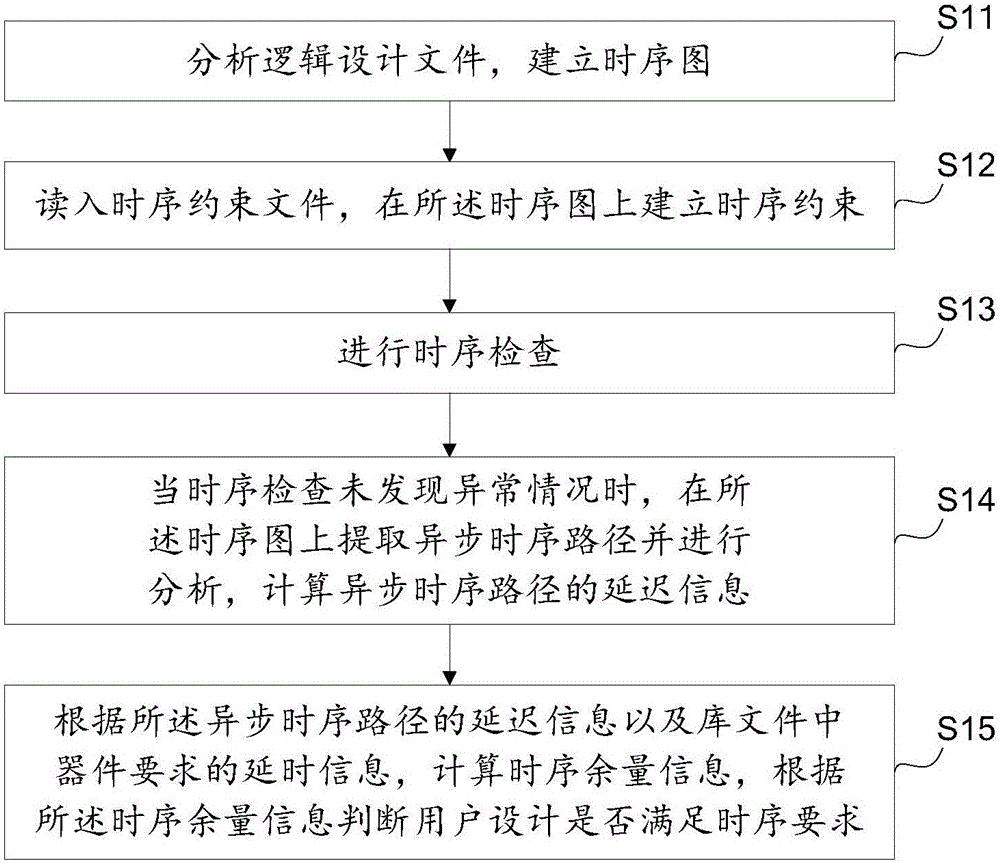
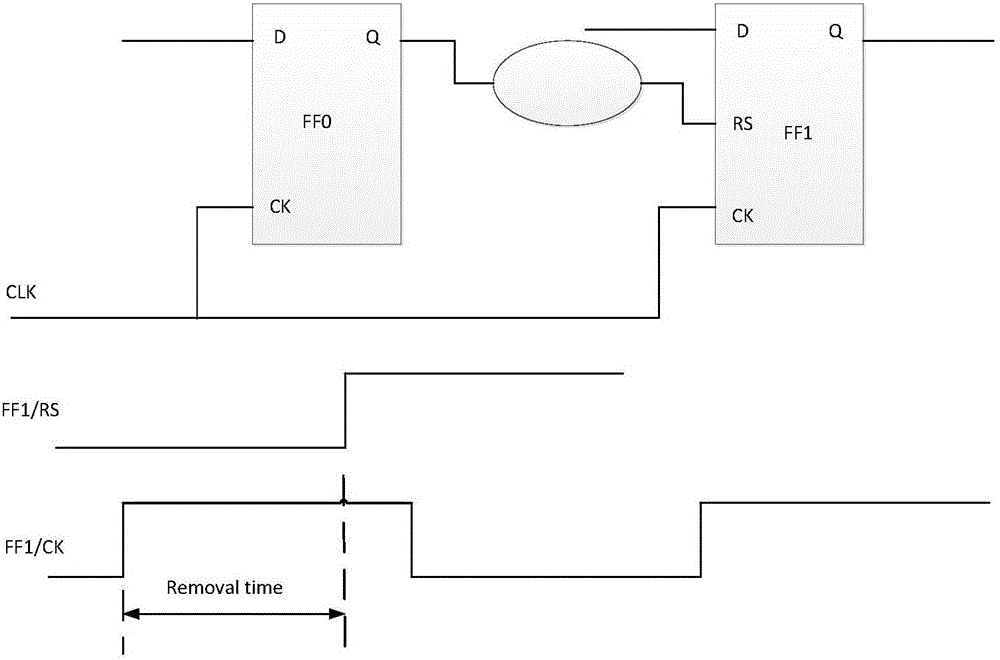
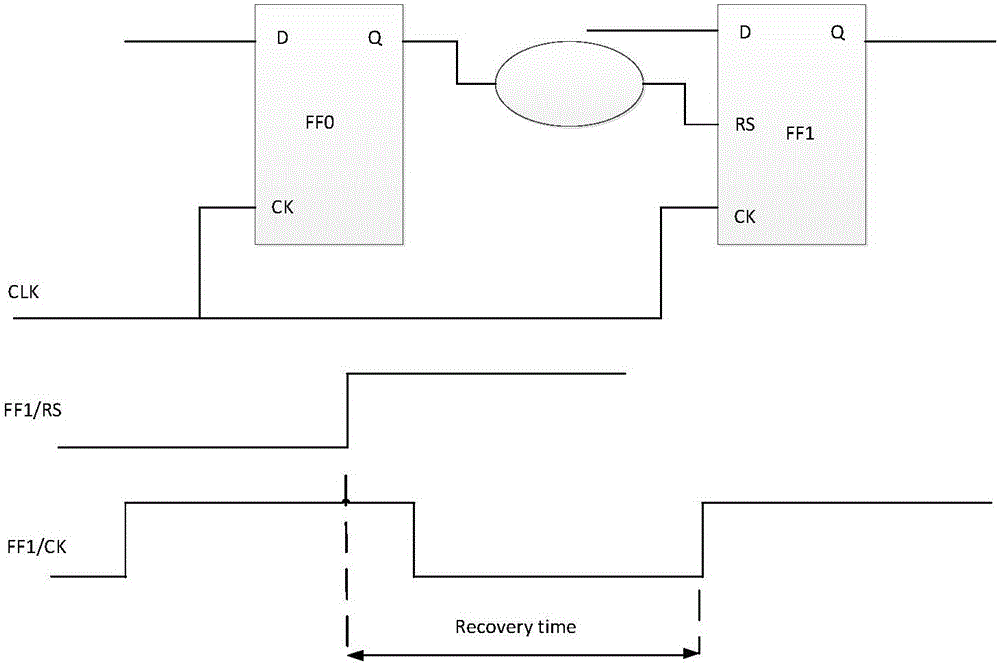
Asynchronous circuit timing sequence checking method based on static analysis
InactiveCN106096171AImprove efficiency and qualityTiming issues do not ariseCAD circuit designSpecial data processing applicationsUser designIntegrated circuit
The invention provides an asynchronous circuit timing sequence checking method based on static analysis. The method includes the steps that a logic design file is analyzed, and a timing sequence diagram is established; a timing sequence constraint file is read in, and timing sequence constraint is established on the timing sequence diagram; timing sequence inspection is conducted; when abnormity is not found in timing sequence inspection, an asynchronous timing sequence path is extracted out of the timing sequence diagram and analyzed, and the delay information of the asynchronous timing sequence path is calculated; timing sequence allowance information is calculated according to the delay information of the asynchronous timing sequence path and delay information required by a device in a library file, and whether user design meets the timing sequence requirement or not is judged according to the timing sequence allowance information. By means of the asynchronous circuit timing sequence checking method, whether a timing sequence problem occurs in an asynchronous circuit or not can be judged, a reference is provided for integrated circuit designers, and design quality is guaranteed.
Owner:SHENZHEN PANGO MICROSYST CO LTD
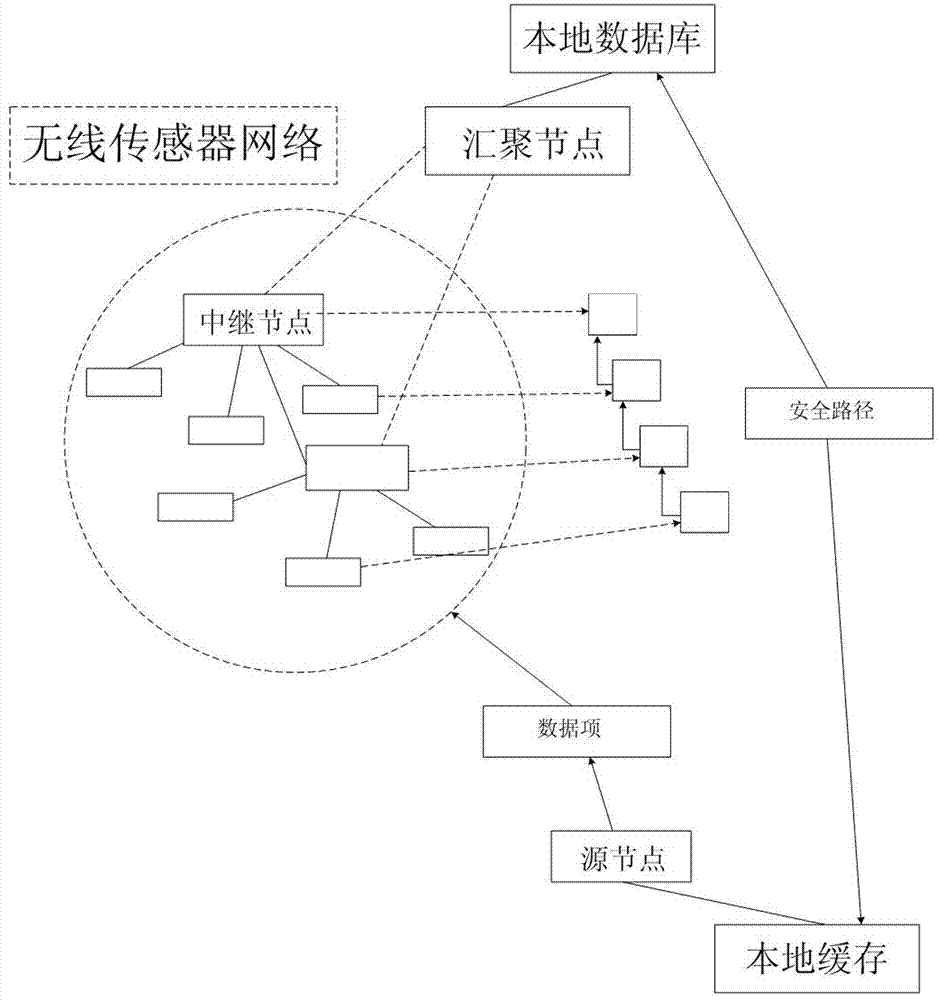
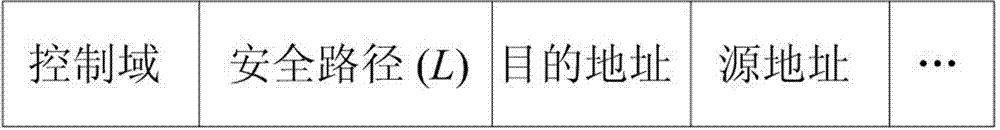
Feedback information and multi-path routing based wireless sensor network data transmission method
InactiveCN103037465AReduce chances of interception and theftImprove efficiency and qualityKey distribution for secure communicationNetwork topologiesMulti path routingData transmission
The invention discloses a feedback information and multi-path routing based wireless sensor network data transmission method which comprises a feedback information based safety routing structure, multi-path routing establishment and multi-path routing transmission application. According to the feedback information and multi-path routing based wireless sensor network data transmission method, in the multi-path routing aspect, the multi-path routing protocol of on-demand routing is mainly adopted, and multiple maximum disjoint routing paths are established, and therefore network congestion is prevented, and available wireless sensor network resources are effectively initialized. Through the feedback information based safety routing structure, feedback paths can be obtained from Sink node for an originating node of data transmission. When the originating node receives enough feedback paths from the Sink node, the paths can be utilized for multi-path data transmission with combination of multi-path routing.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Controlled release oxycodone compositions
InactiveUS20100034876A1Improve efficiency and qualityBiocidePowder deliveryControlled releaseMedicine
Owner:PURDUE PHARMA LP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com