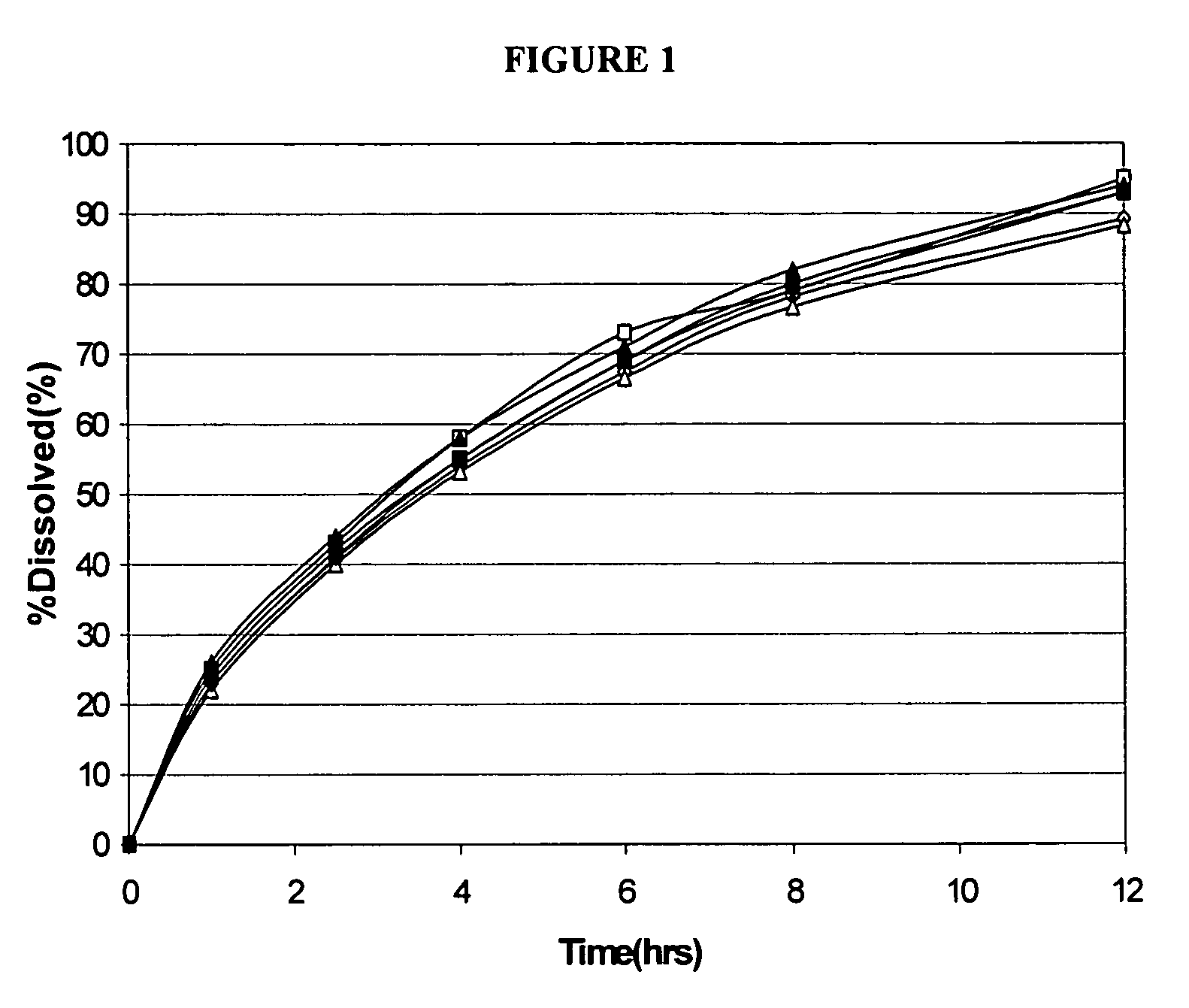
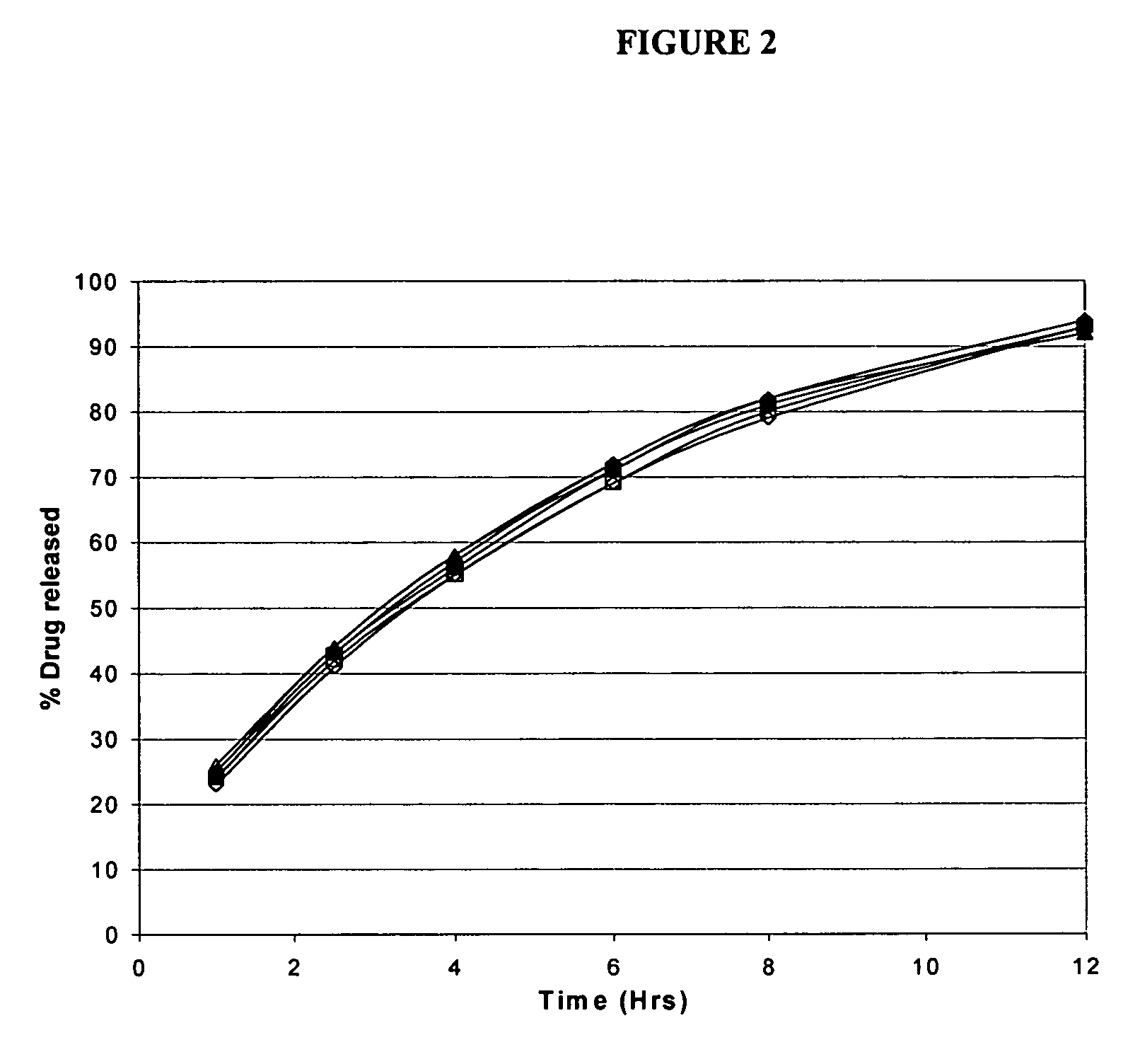
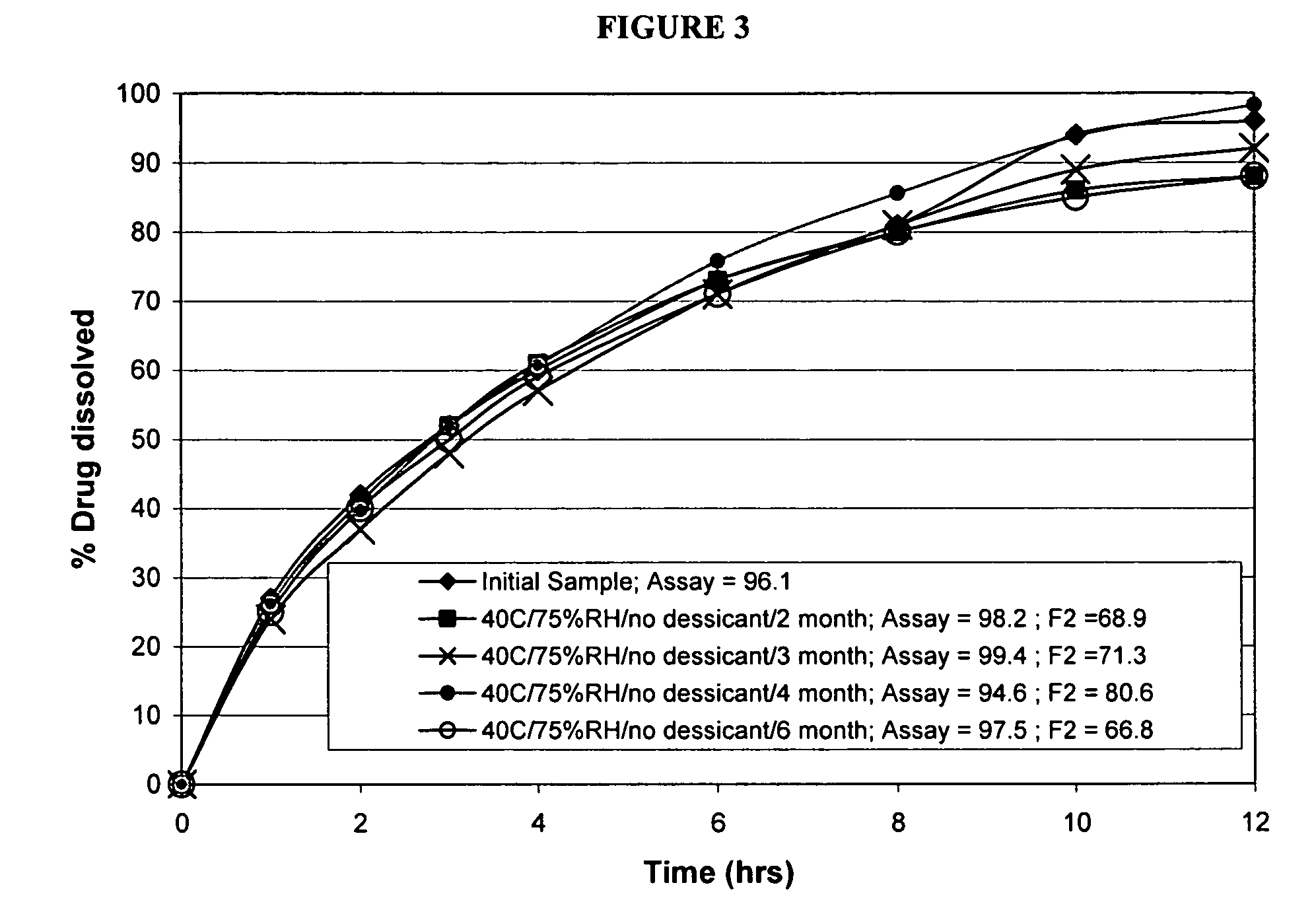
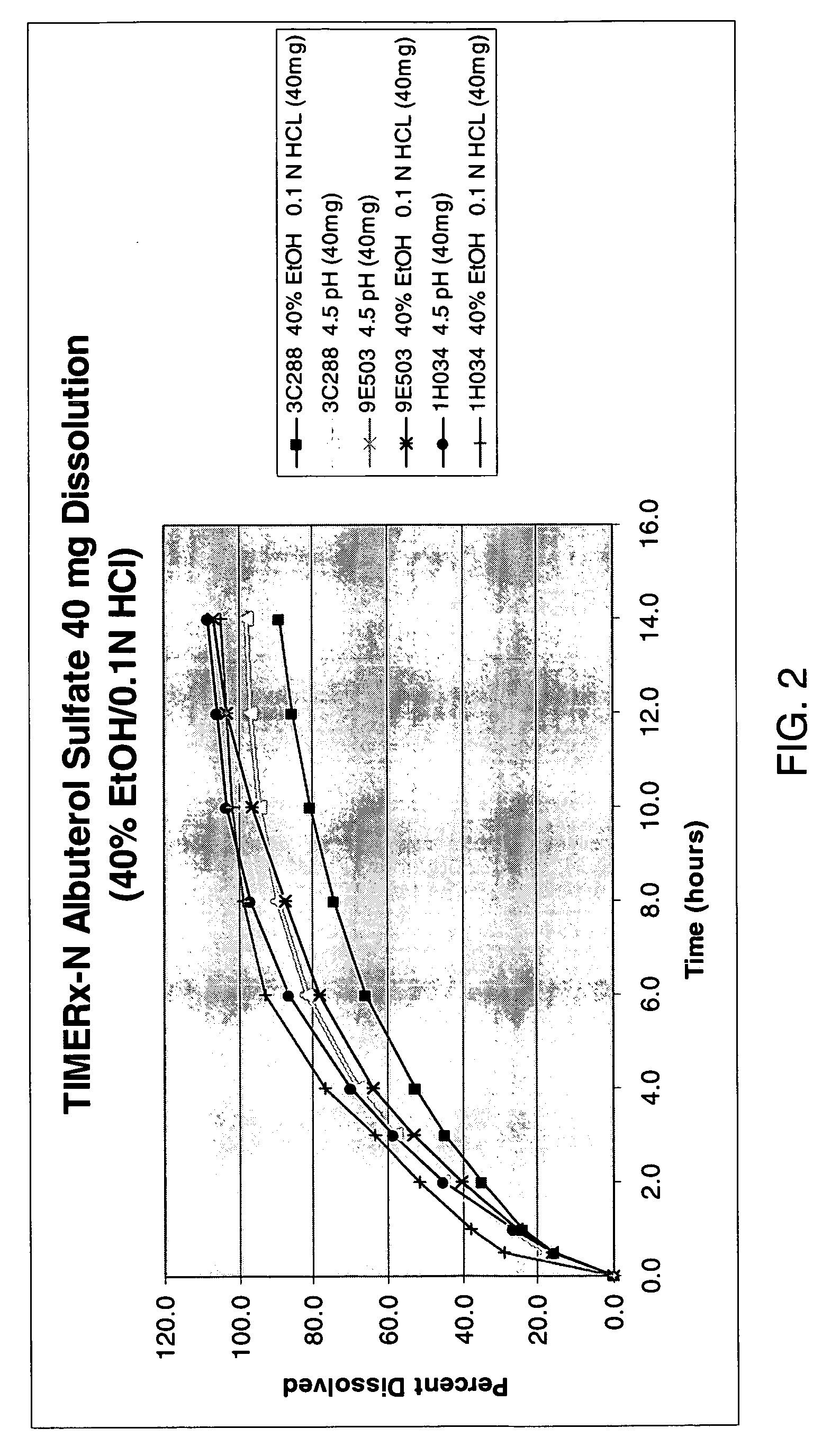
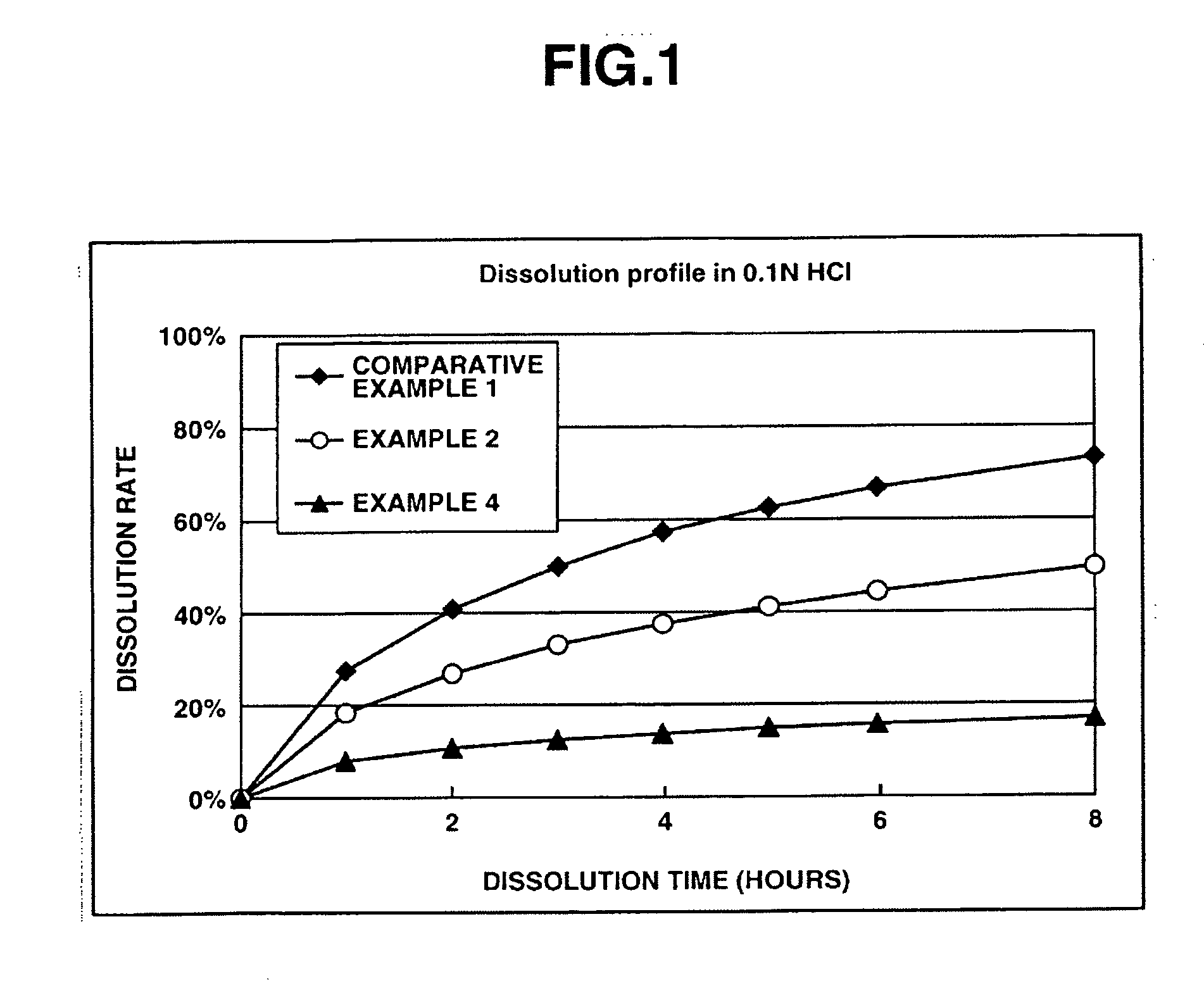
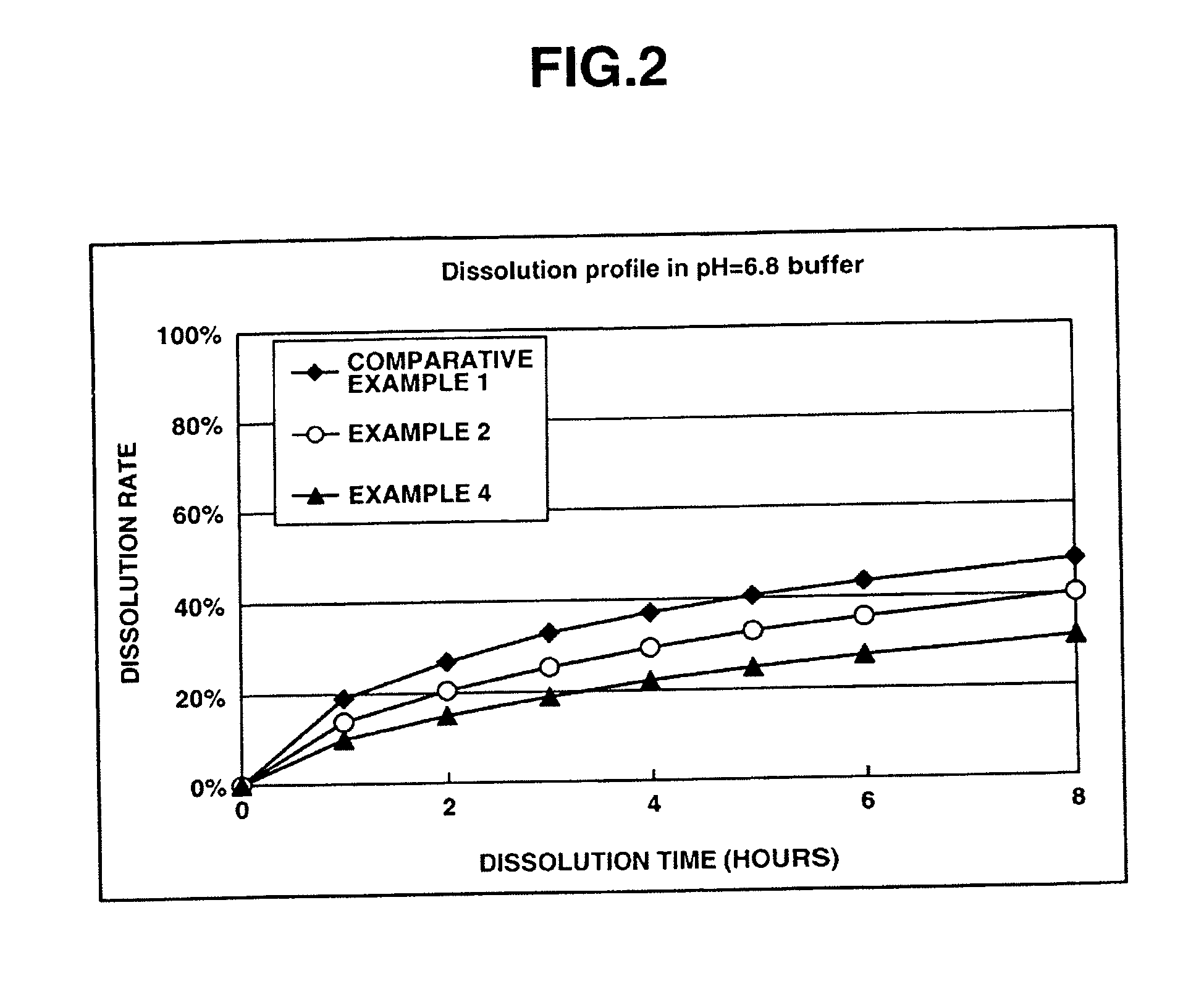
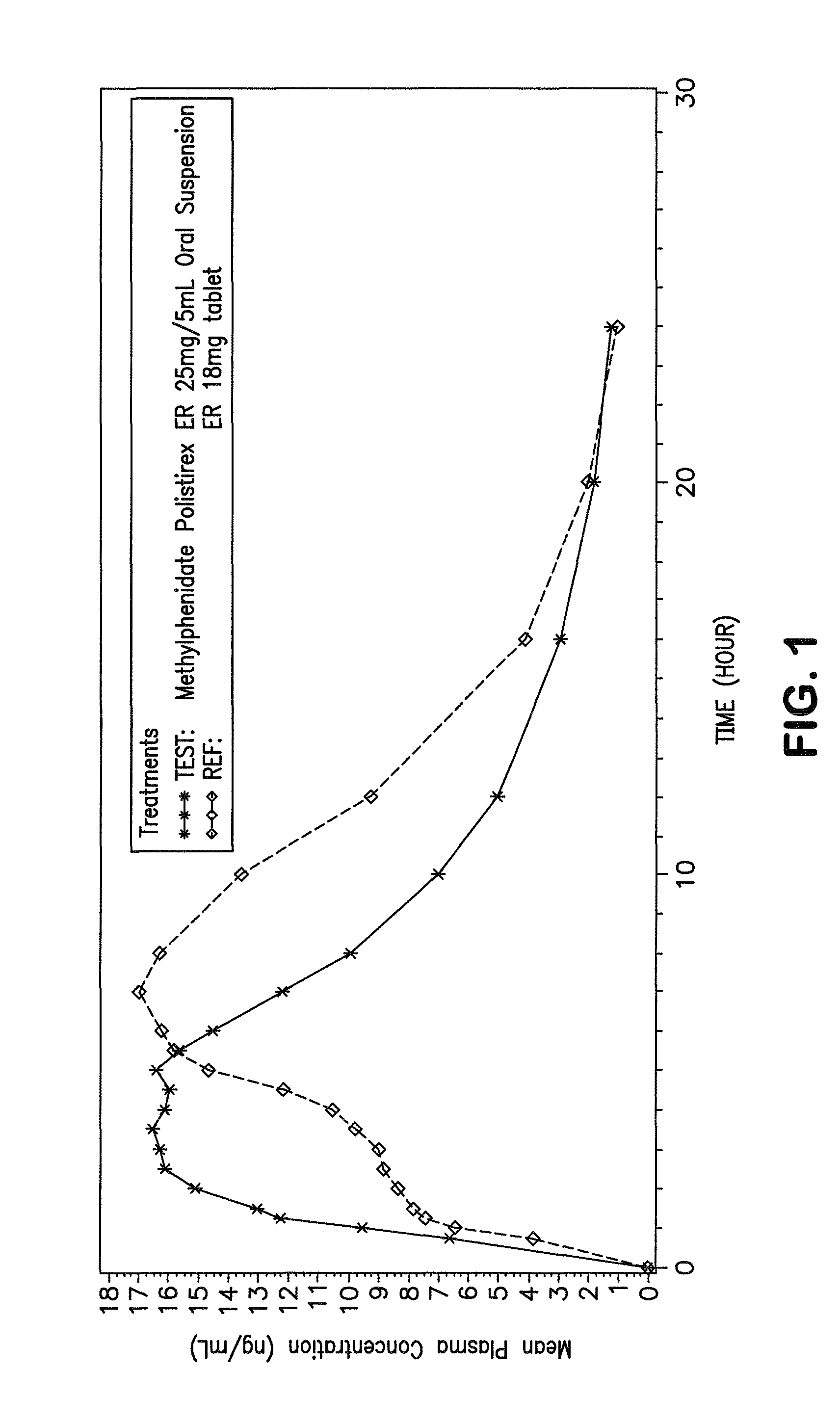
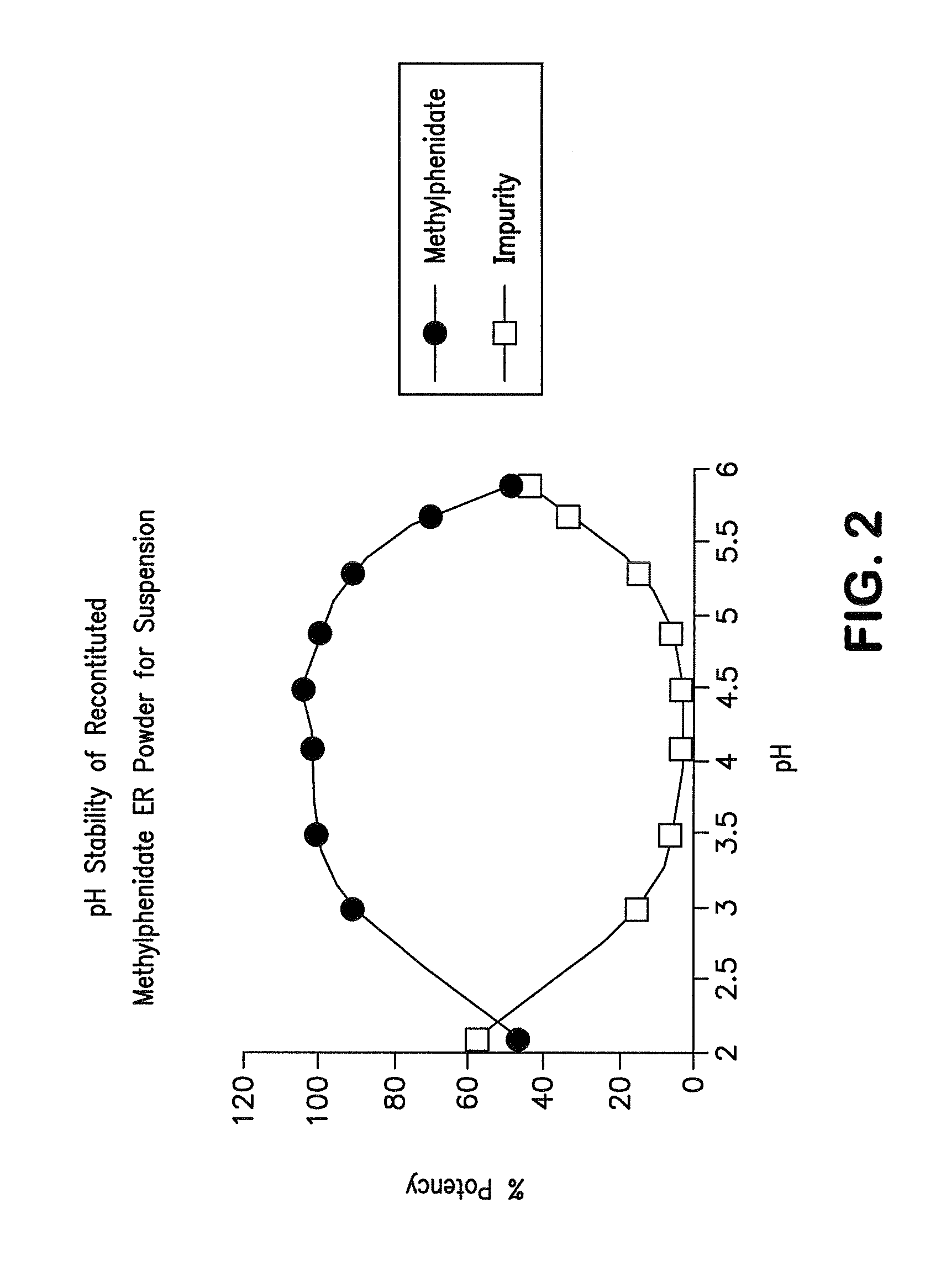
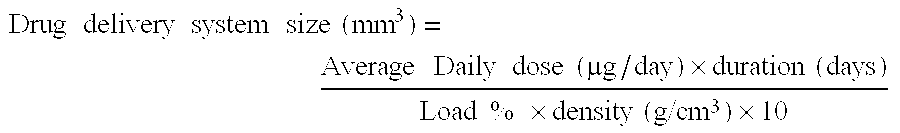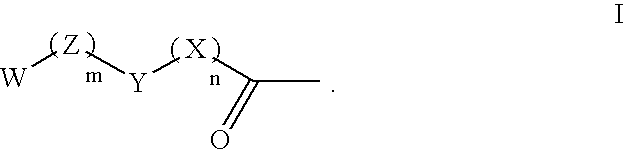Patents
Literature
463 results about "Sustained Release Formulations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and drug delivery systems for the treatment of orofacial diseases
This invention relates to methods of treating various orofacial diseases involving inflammation, infection and / or pain, using intratissue controlled release drug delivery systems. More particularly, the invention relates to methods for localized or targeted administration of a sustained release formulation of an agent such as an anti-inflammatory agent to a specified tissue location within the orofacial environment.
Owner:HALLUX
Modified release formulations containing drug-ion exchange resin complexes
ActiveUS20070215511A1Reduction of undesirable tasteConvenient coatingPowder deliverySmall article dispensingDrugPlasticizer
A coated drug-ion exchange resin complex comprising a core composed of a drug complexed with a pharmaceutically acceptable ion-exchange resin is provided. The drug-ion exchange resin complex is in admixture with a release retardant. The coating is a polyvinyl acetate polymer and a plasticizer. Methods of making and products containing this coated complex are described.
Owner:TRIS PHARMA
Modified release formulations of memantine oral dosage forms
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
Stent coated with a sustained-release drug delivery and method for use thereof
ActiveUS7279175B2Reduce deliveryReduce solubilitySuture equipmentsAntibacterial agentsSustained release drugDrug compound
An intraluminal medical device comprises a stent having a coating applied to at least part of an interior surface, an exterior surface, or both. The coating comprises a sustained release formulation of a combination of pharmaceutical compounds dispersed within a biologically tolerated polymer composition. The choice of the combination of pharmaceutical compounds are intended to reduce neointimal hyperplasia restenosis.
Owner:PSIVIDA US INC
Donepezil formulations
InactiveUS20050232990A1Highly preventive effectEasy to openPowder deliveryBiocideDonepezilSustained Release Formulations
Owner:ACTAVIS GRP PTC EHF
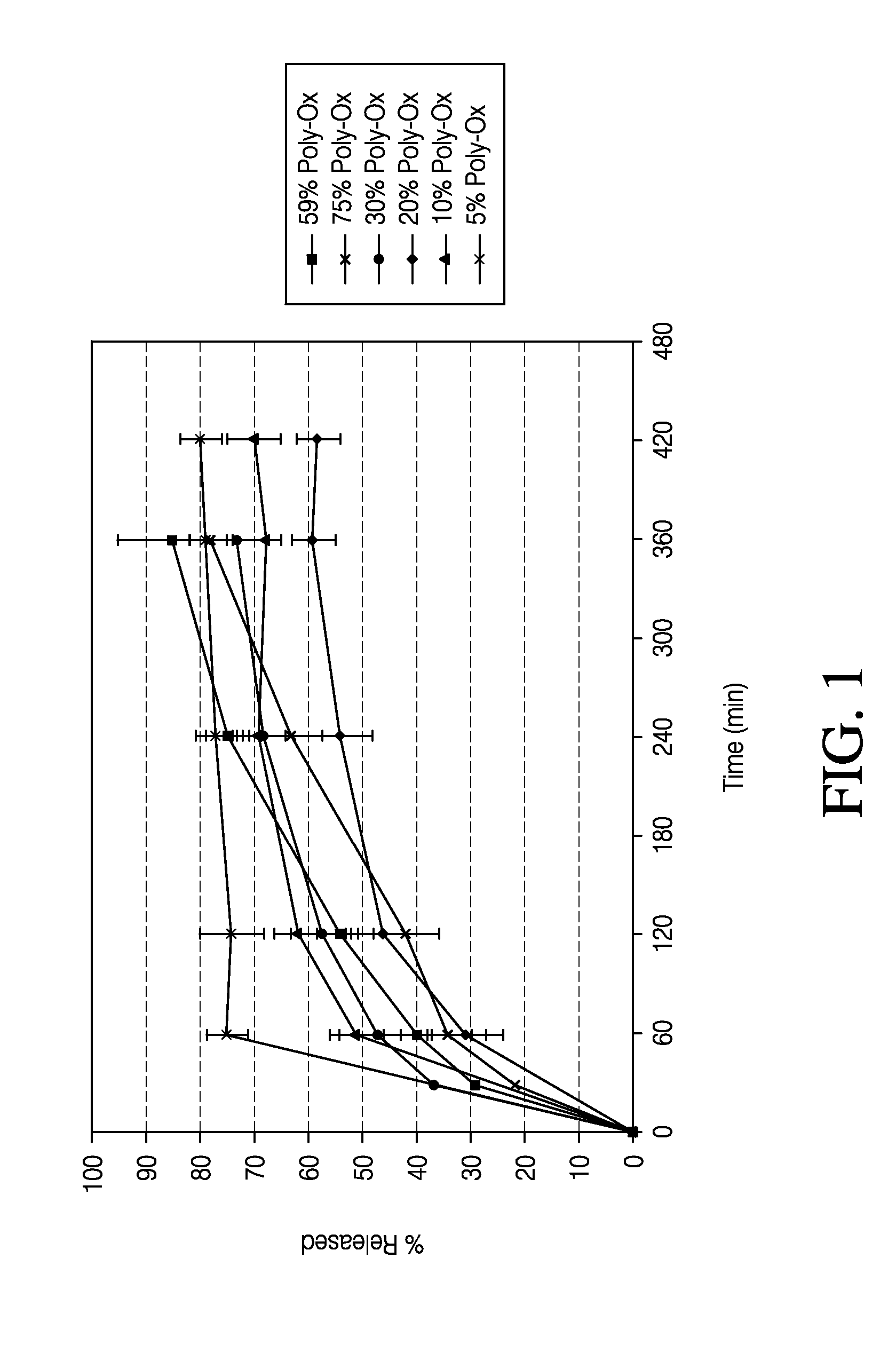
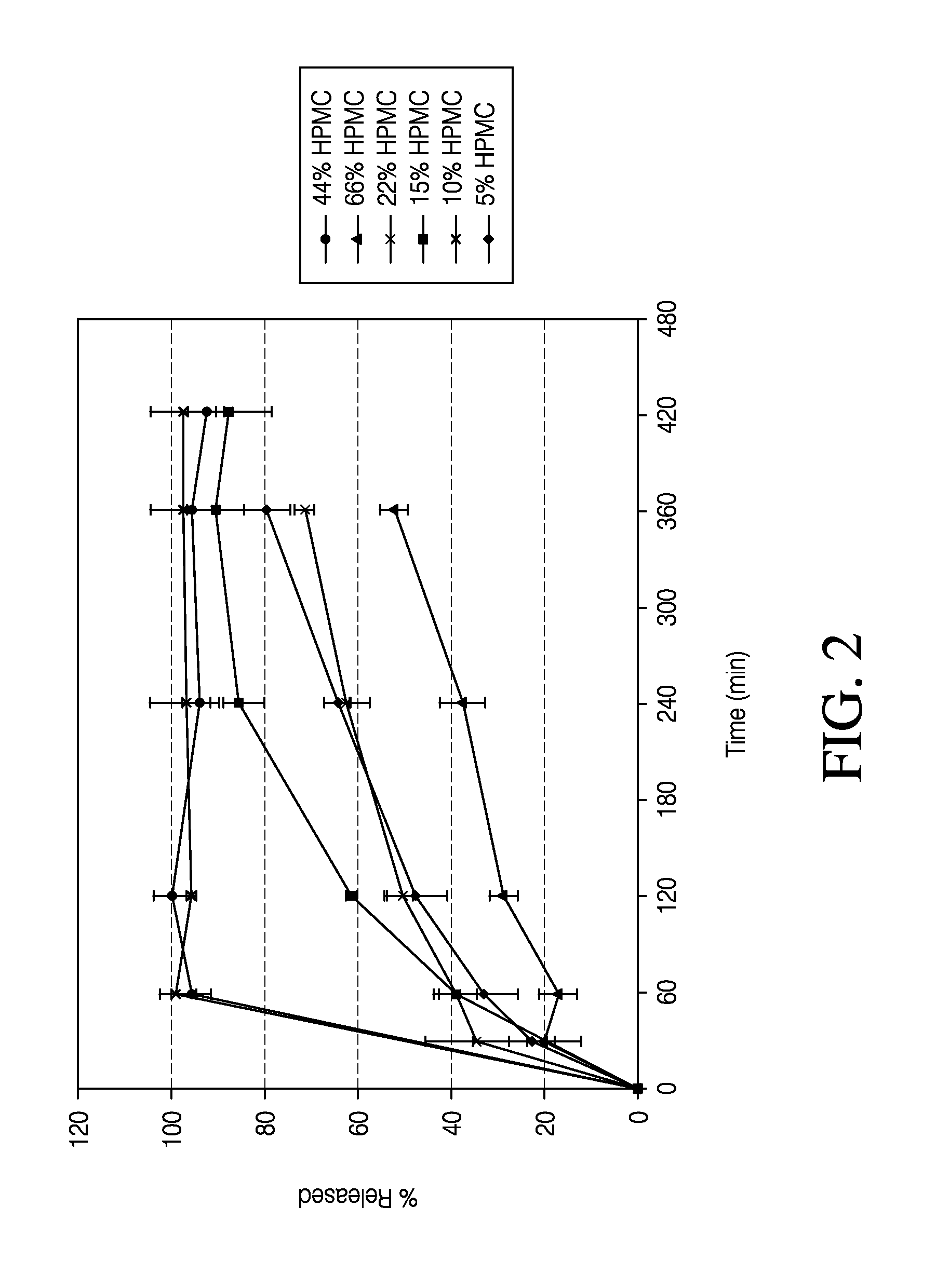
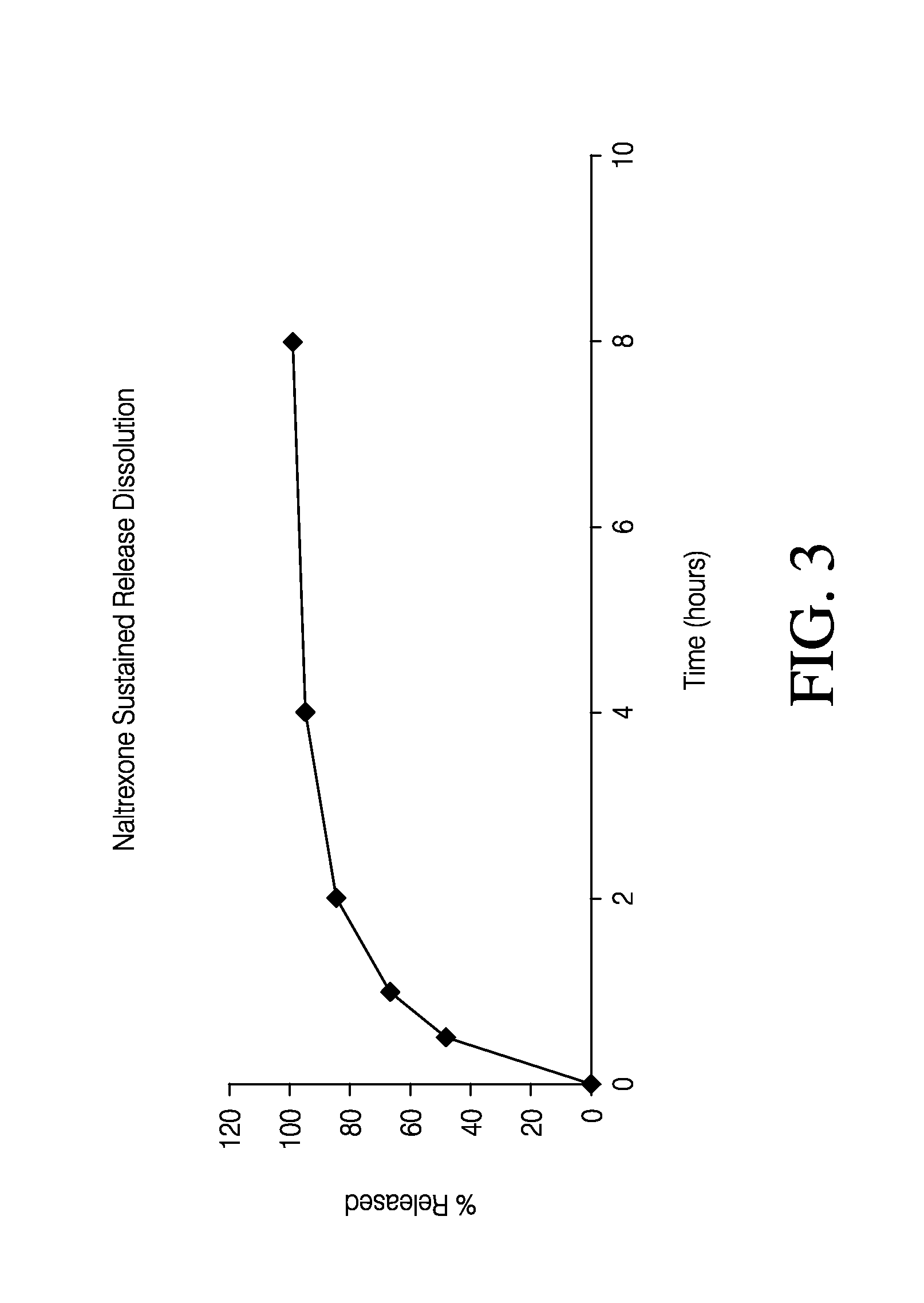
Sustained release formulation of naltrexone
ActiveUS20070281021A1Cause weight lossAvoid weight gainBiocideKetone active ingredientsSide effectCompound (substance)
A sustained-release oral dosage form of naltrexone or a pharmaceutically acceptable salt thereof is provided. The oral dosage form may be administered with another compound. Administration of the oral dosage form may reduce a side effect, which may be a side effect at least partially attributable to a weight-loss treatment. The oral dosage form may be administered to treat a weight-loss condition.
Owner:OREXIGEN THERAPEUTICS INC
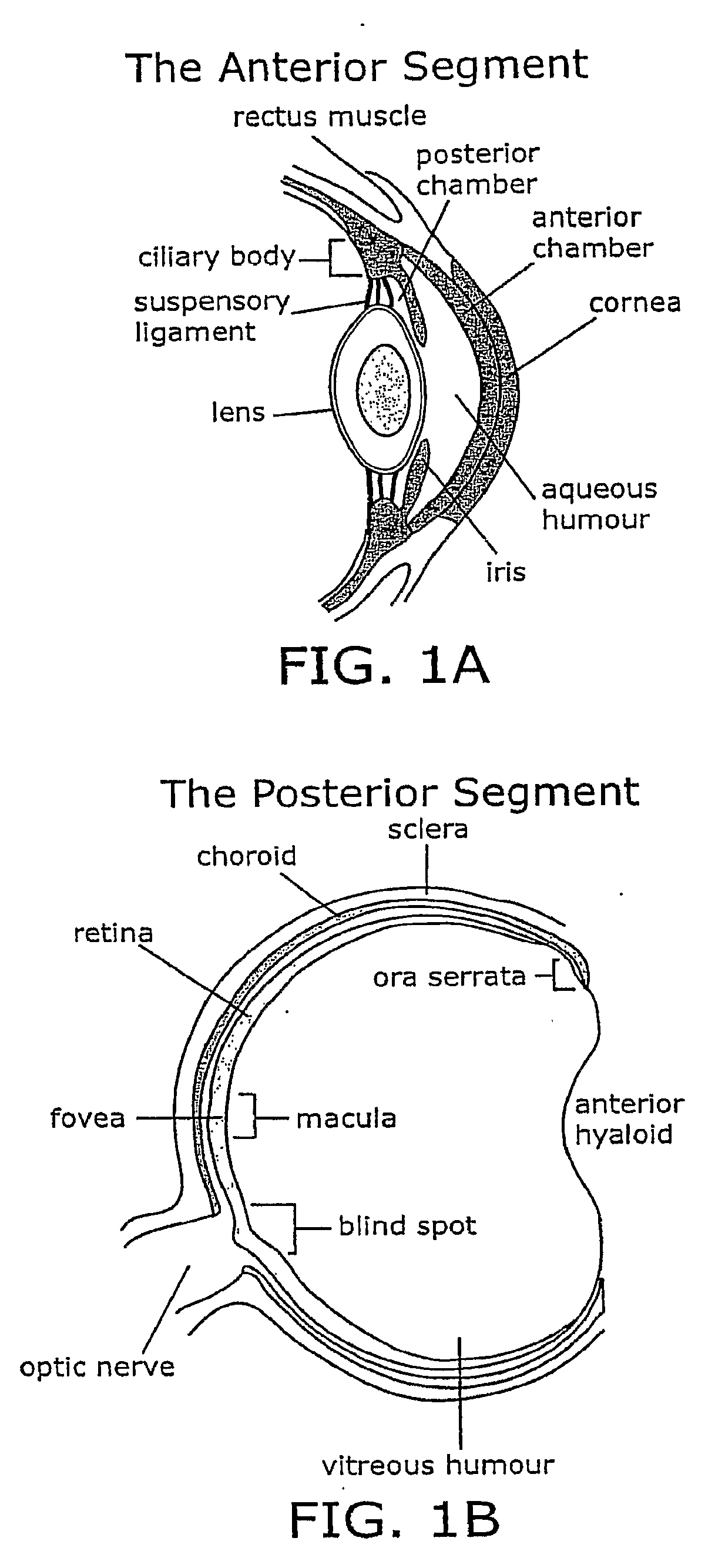
Injectable Combination Therapy for Eye Disorders
InactiveUS20090220572A1Compounds screening/testingPowder deliveryLiquid mediumRetinal neovascularization
The present invention provides composition, methods, and articles of manufacture for treating an eye disorder, e.g., a disorder characterized by macular degeneration, choroidal neovascularization, or retinal neovascularization. One method of the invention comprises the step of: administering first and second therapeutic agents to the subject's eye in a single procedure, wherein the first therapeutic agent provides rapid improvement in the condition of the subject's eye and the second therapeutic agent is administered as a sustained release formulation of the second therapeutic agent. For example, the first and second therapeutic agents are administered by intravitreal injection. The first therapeutic agent may be dissolved in a liquid medium located in the syringe and the sustained formulation of the second therapeutic agent may comprise an ocular implant or plurality of particles located in the needle. The therapeutic agents may be selected from the group consisting of angiogenesis inhibitors and complement inhibitors.
Owner:POTENTIA PHARMA INC
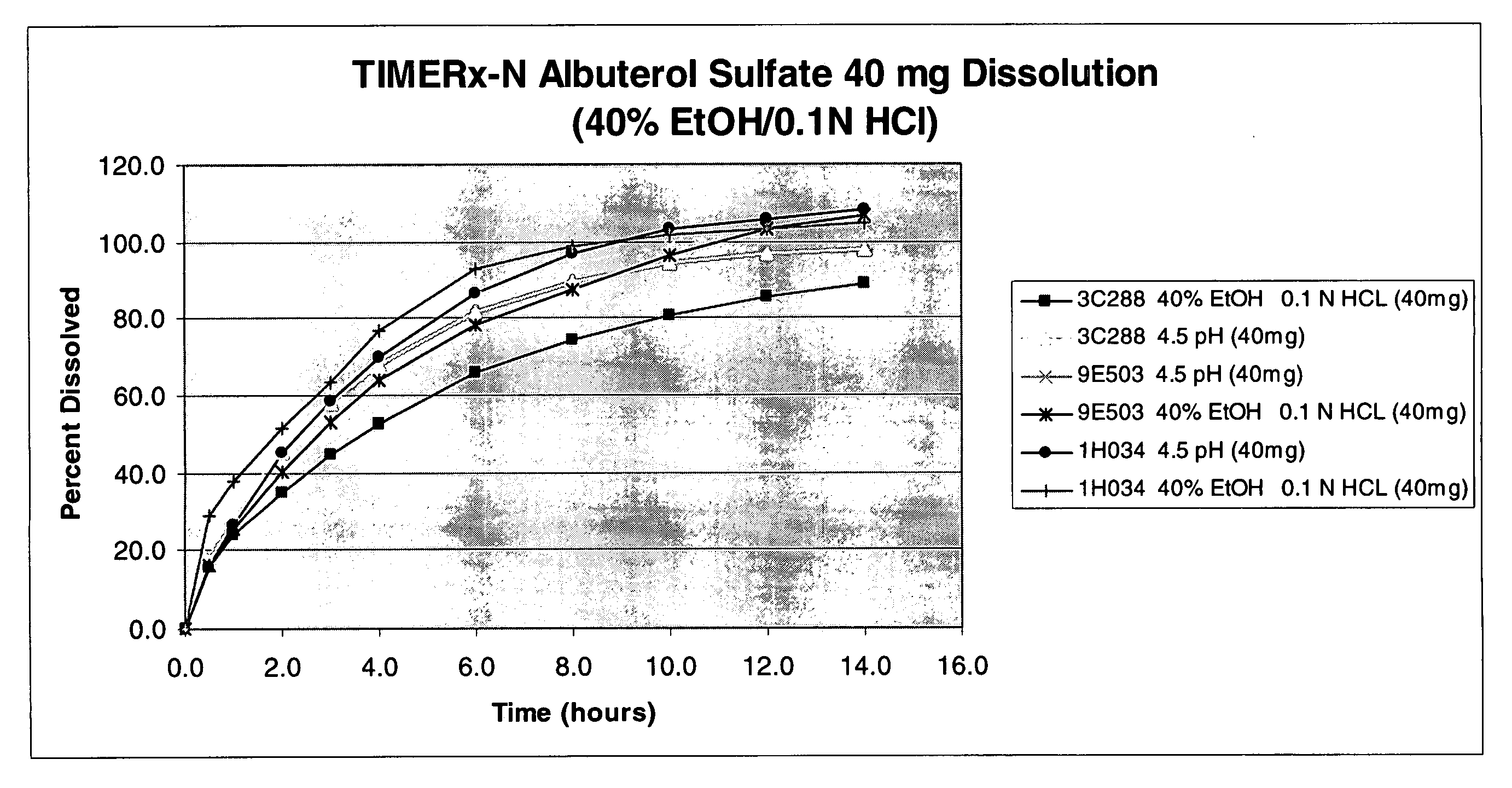
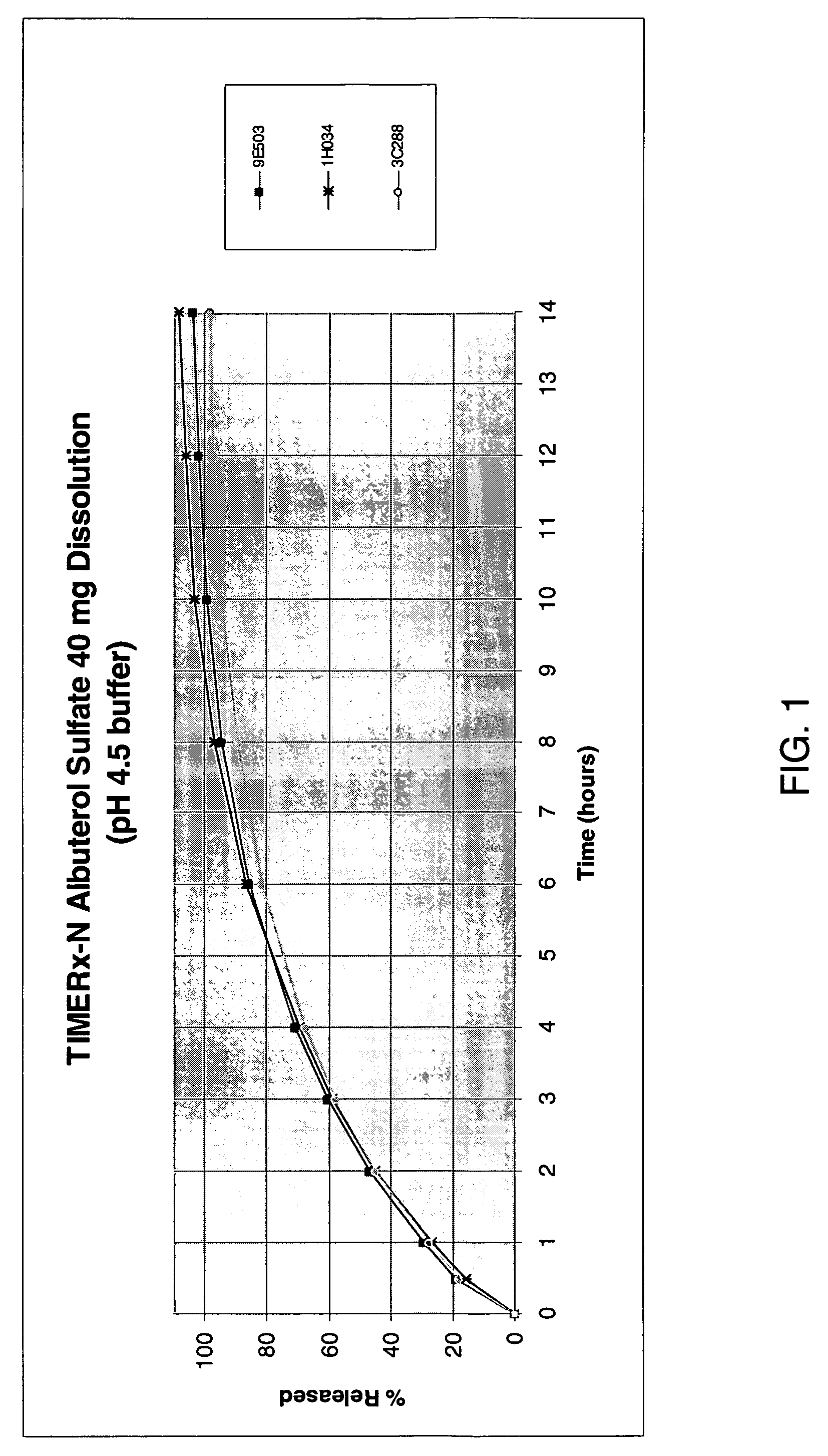
Ethanol-resistant sustained release formulations
InactiveUS20070212414A1High drug safetyLower potentialBiocideNervous disorderSustained Release FormulationsDelivery system
The invention provides formulations that resist dose dumping in the presence of ethanol and methods of use thereof. The formulations can be used to prevent dose dumping, to increase safety of drugs, and to reduce abuse of drugs prone to such abuse. The formulations comprise at least one drug and a sustained release delivery system. In one embodiment, the drug is an opioid.
Owner:ENDO PHARMA INC
Robust sustained release formulations
InactiveUS20080085304A1Avoid dose dumpingHigh drug safetyPowder deliveryPill deliverySolid Dose FormOxymorphone
Robust sustained release formulations, solid dosage forms comprising robust sustained release formulations, and methods for making and using these formulations and solid dosage forms are provided. Robustness of the sustained release formulation is related to the particle size of the hydrophilic gum. Sustained release formulations resist dose-dumping when ingested with alcohol. The formulations are useful for treating a patient suffering from a condition, e.g., pain. The formulations comprise at least one drug. In one embodiment, the drug is an opioid, e.g., oxymorphone.
Owner:ENDO PHARMA INC
Sustained release formulation
A composition comprises a protein, a polyol, and a metal. The protein is stabilized by the polyol and the metal, and is protected from denaturing when in contact with an organic solvent. The polyol may be a hydrocarbon containing two or more hydroxyl groups (—OH) bonded to carbon. The metal may be divalent.
Owner:GENENTECH INC
Compounds and methods for lowering the abuse potential and extending the duration of action of a drug
ActiveUS7230005B2Low abuse potentialProlong the action timeBiocideNervous disorderNasal cavityEster prodrug
The abuse potential of a bioavailable drug such as an opiate analgesic agent is reduced and its duration of action is extended by converting it to a poorly absorbed ester prodrug or other prodrug derivative prior to formulation. Unlike many existing sustained release formulations of active pharmaceutical agents wherein an active pharmaceutical agent can be released by chewing, crushing, or otherwise breaking tablets or capsule beads containing the active pharmaceutical agent, such mechanical processing of tablets or capsule beads containing a prodrug of this invention neither releases the active drug nor compromises the controlled conversion of prodrug to drug. Moreover, tablets and capsule beads containing prodrugs of this invention or other drugs can be formulated with a sufficient amount of a thickening agent such as hydroxypropylmethylcellulose or carboxymethylcellulose to impede inappropriate intravenous and nasal administration of formulations that are not indicated for these modes of administration.
Owner:CONTROLLED CHEM INC
Sustained release topiramate
InactiveUS20030072802A1Avoid degradationInhibit growthBiocideCarbohydrate active ingredientsDepressantTopiramate
The present invention is an improvement in the treatment of mania and depression by administering topiramate in a sustained-release formulation. The sustained-release formulation of the present invention may also be co-administered with anti-psychotics and anti-depressants.
Owner:R T ALAMO VENTURES
Sustained release formulation
InactiveUS20030045454A1Easy to packHigh activityPowder deliveryPeptide/protein ingredientsOrganic solventPolyol
A composition comprises a protein, a polyol, and a metal. The protein is stabilized by the polyol and the metal, and is protected from denaturing when in contact with an organic solvent. The polyol may be a hydrocarbon containing two or more hydroxyl groups (-OH) bonded to carbon. The metal may be divalent.
Owner:GENENTECH INC
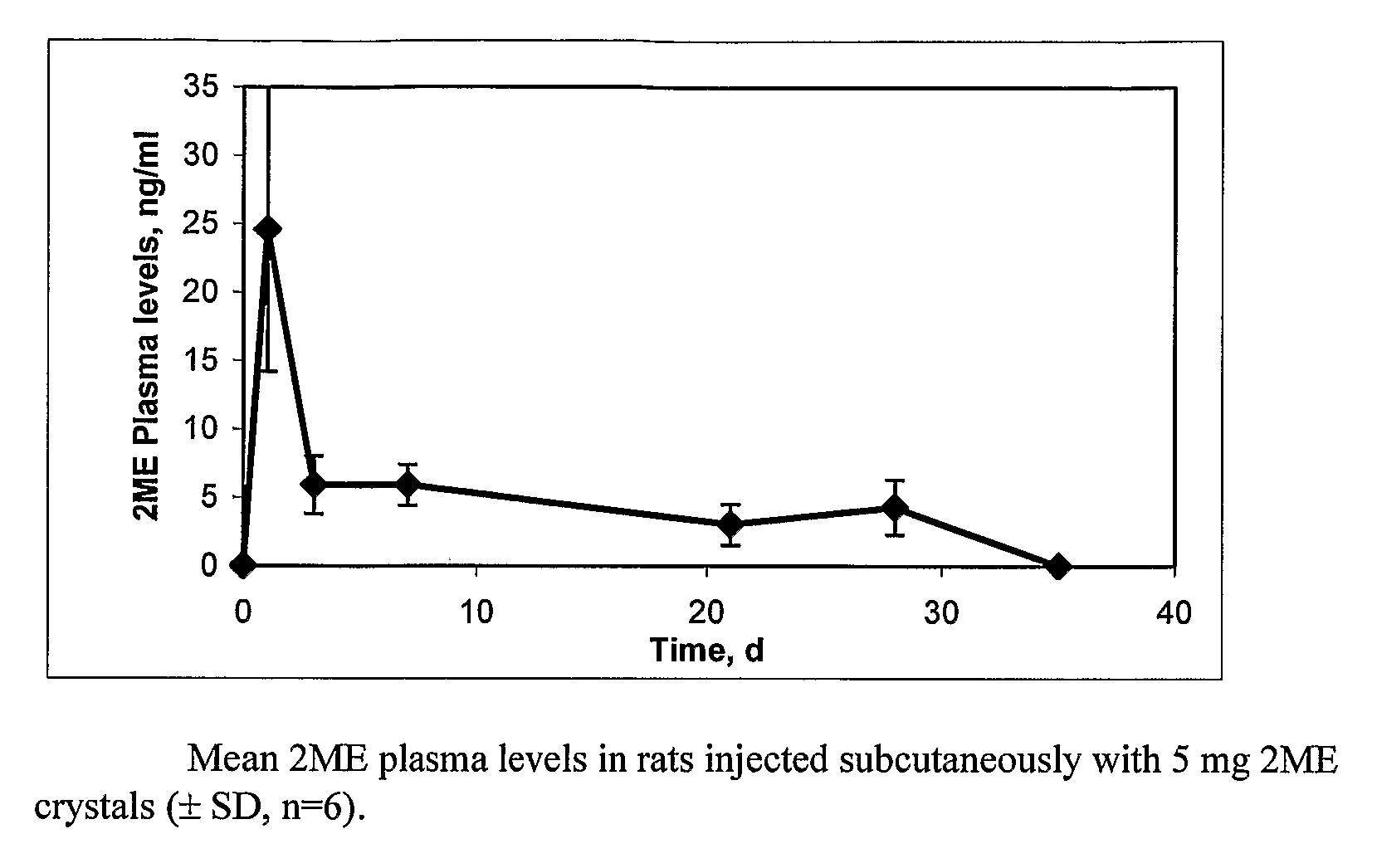
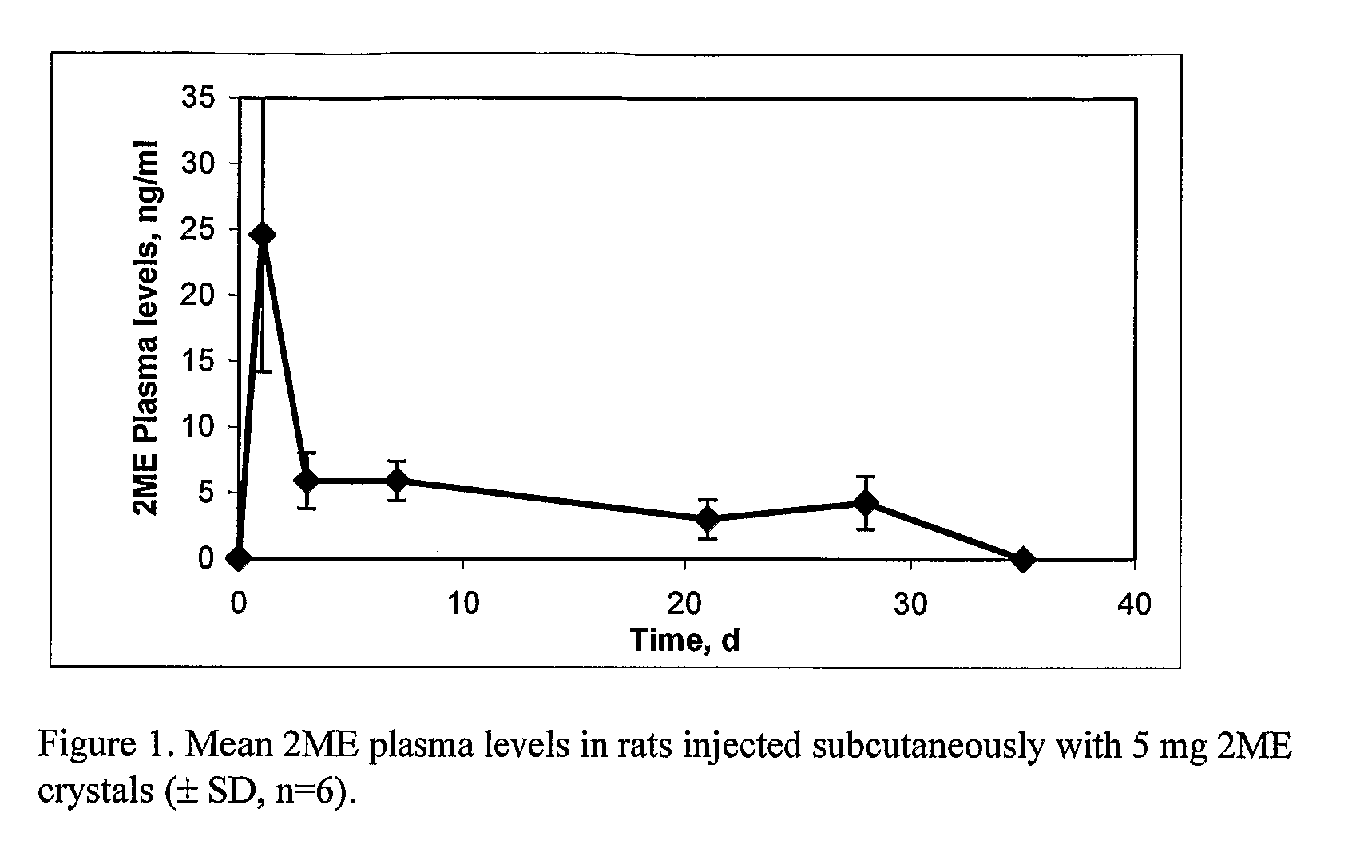
Long Acting Injectable Crystal Formulations of Estradiol Metabolites and Methods of Using Same
InactiveUS20080220069A1Simpler and less-expensive to manufactureEasy to managePowder deliveryOrganic active ingredientsDiseaseMetabolite
The present invention provides sustained release formulations of estradiol metabolites whereby the in vivo pharmacokinetics are manipulated by a method selected from the group consisting of chemical modification, crystal packing formation, particle size or a combination thereof. Such compositions are useful in the long-term treatment of a wide variety of diseases.
Owner:PR PHARMA
Nanoscale particle formulations and methods
InactiveUS20140205546A1Reduce the populationSustained releasePowder deliveryMaterial nanotechnologyDermatologySustained Release Formulations
Provided herein in some embodiments is a formulation comprising (a) one or more nanoscale particle; and (b) a film-forming polymer. In some instances, the formulation is an immediate and sustained released formulation suitable for topical administration or administration to surfaces. Also provided here in certain embodiments is a method of reducing the population of pathogenic microorganisms on skin or surfaces, the method comprising applying to the skin or surface a composition, the composition comprising (a) one or more nanoscale particle; and (b) a film-forming polymer.
Owner:ANNUARY HEALTHCARE
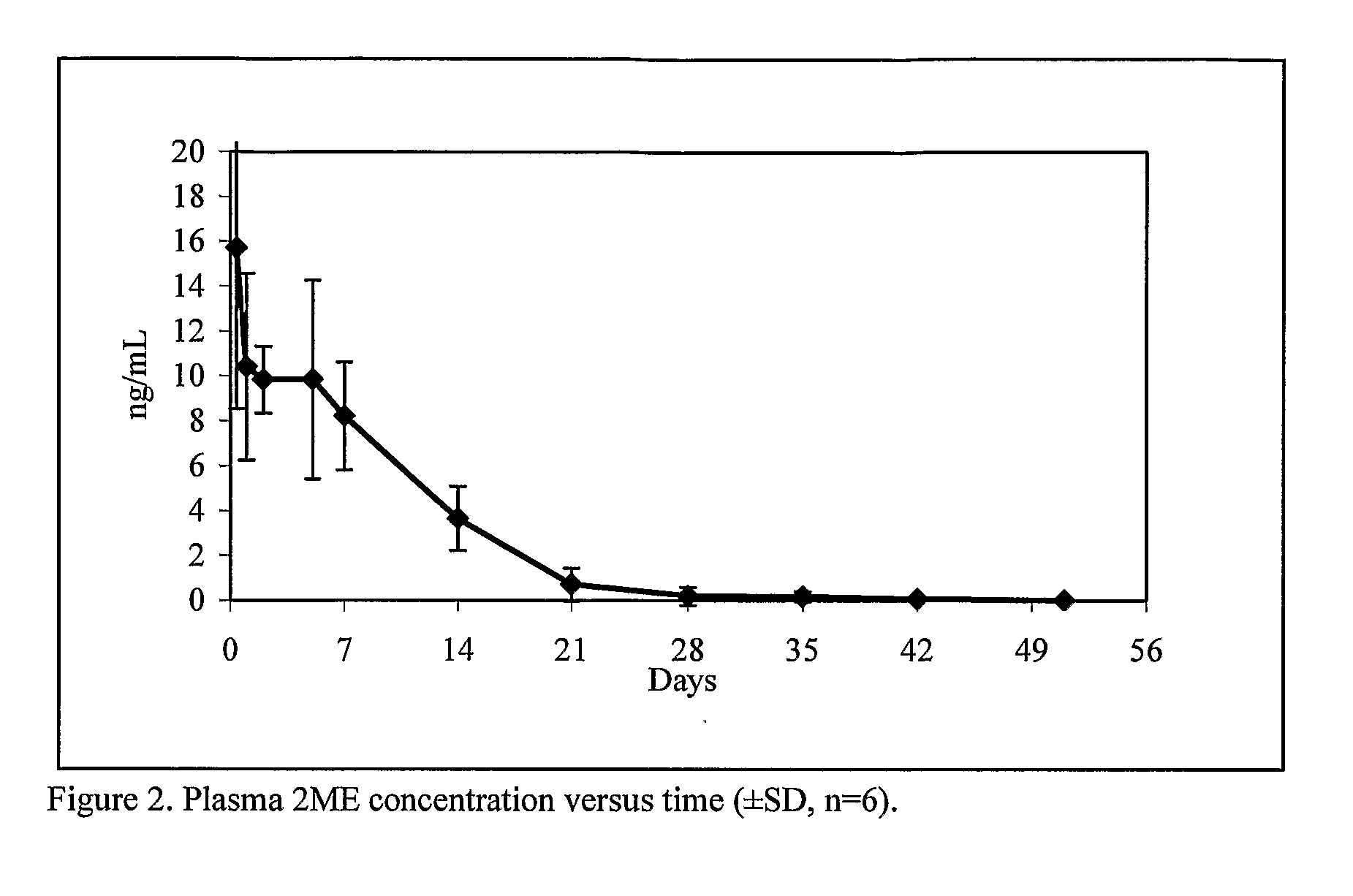
Controlled release compositions of estradiol metabolites
The present invention provides improved sustained release formulations of estradiol metabolites, including 2-hydroxyestradiol, 2-methoxyestradiol, 4-hydroxyestradiol and 4-methoxyestradiol, useful for therapeutic treatments. The invention also provides methods of producing sustained release forms of estradiol metabolites. The compositions of the present invention include microparticles, nanoparticles, patches, crystals, gels, rods, stints, pallets, discs, lozenges, wafers, capsules, films, microcapsules nanocapsules, hydrogels, liposomes, implants and vaginal rings. Compositions also include formulations for transdermal and intravenous delivery of estradiol metabolites. The present invention provides numerous improvements over previous forms of estradiol metabolites, such advantages including the sustained release of normally short half-life compounds to maintain therapeutic blood levels.
Owner:PR PHARMA
Sustained release formulations of oxymorphone
Sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; methods for making the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof; and methods for using the sustained release formulations of oxymorphone or pharmaceutically acceptable salts thereof to treat patients suffering from pain are provided.
Owner:ENDO PHARMA INC
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
Abuse resistant opioid drug-ion exchange resin complexes having hybrid coatings
InactiveUS20120148672A1Improved resistance characteristicsPowder deliveryNervous disorderMedicineIon-exchange resin
A sustained release formulation for opioid drugs is described. The formulation contains an opioid-ion exchange resin complex having a hybrid coating. The hybrid coating contains a cured polyvinylacetate polymer and a pH-dependent enteric coating layer mixed therein. Also provided are methods of making and using same.
Owner:TRIS PHARMA
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
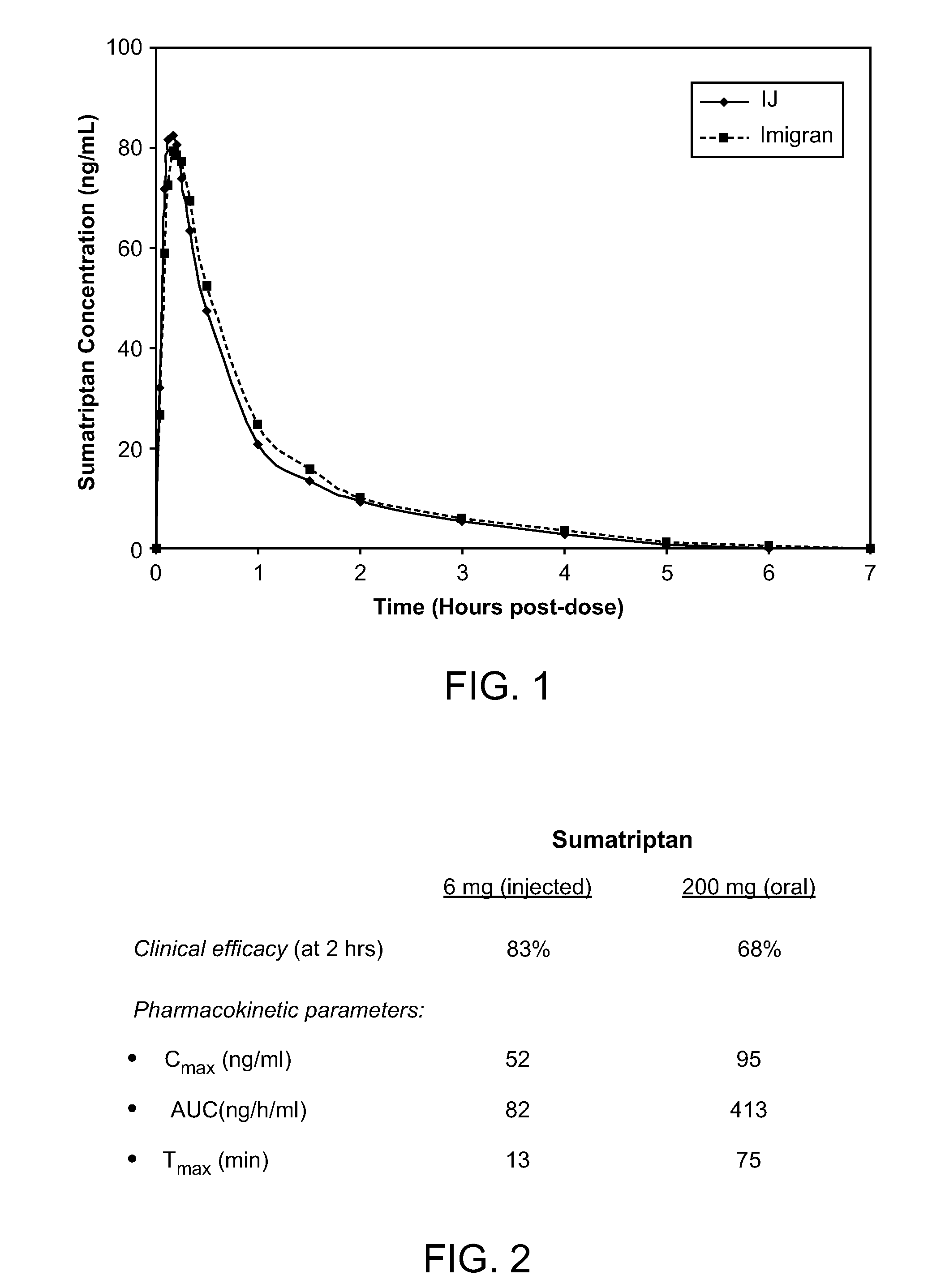
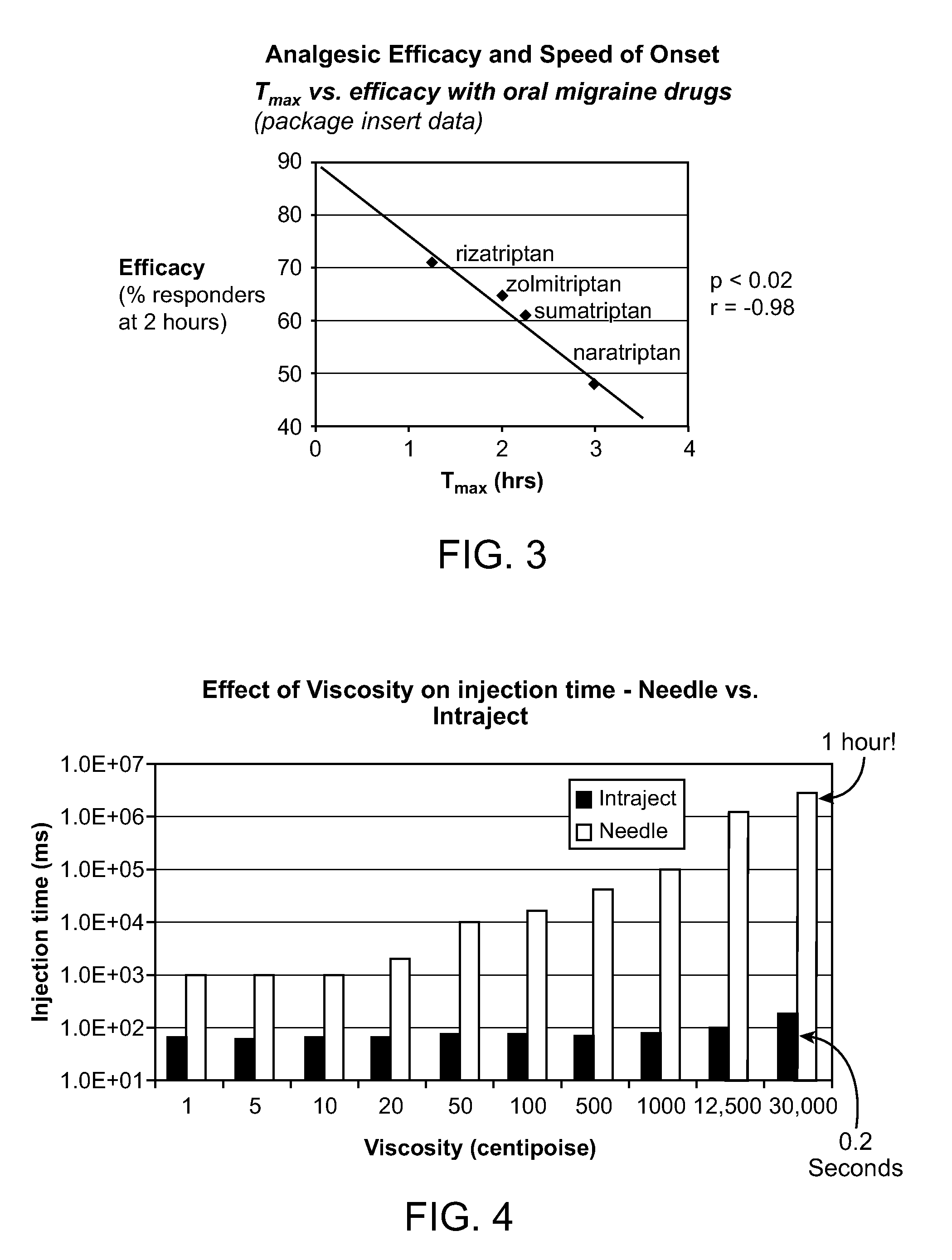
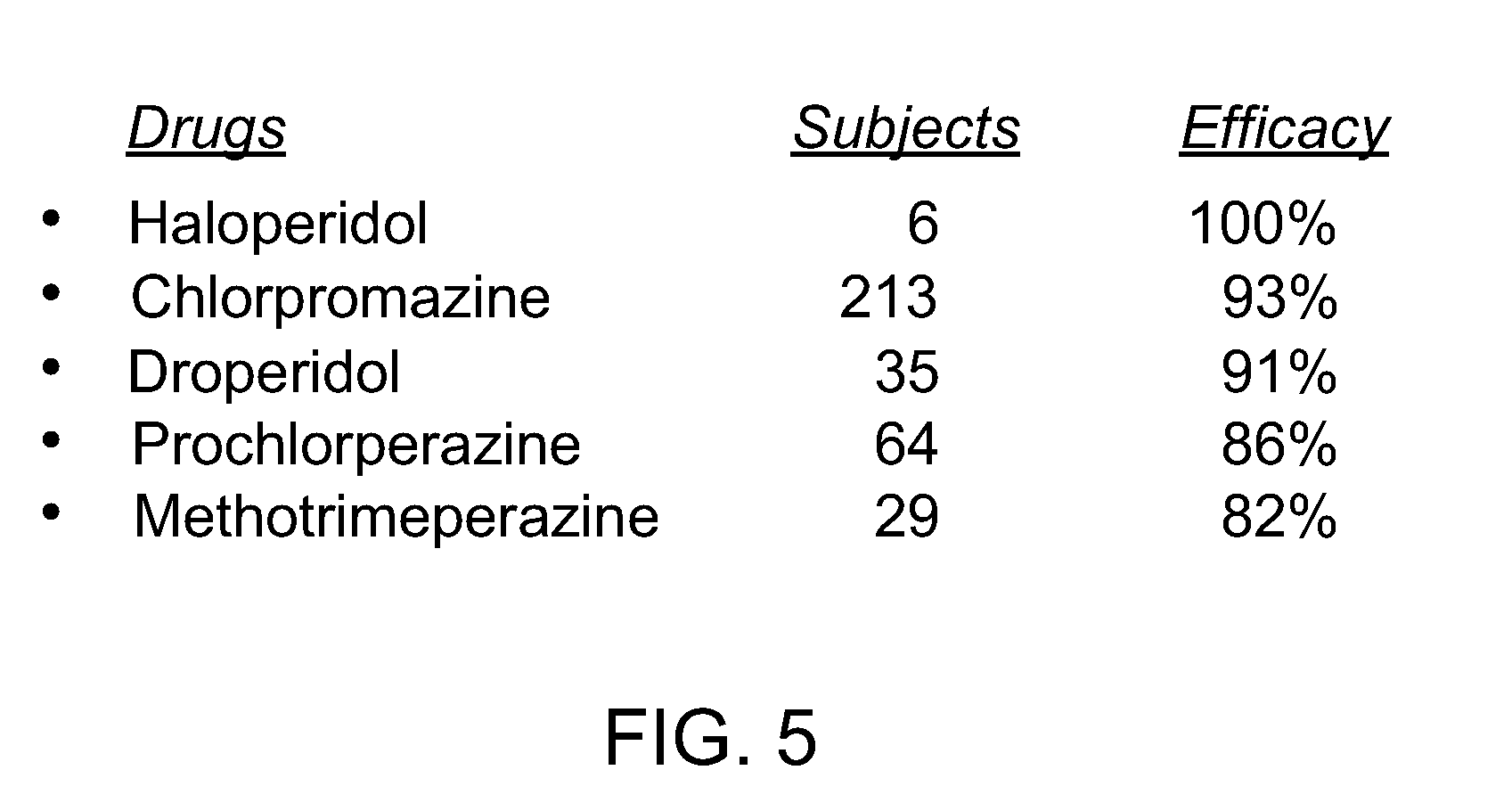
Novel formulations for treatment of migraine
InactiveUS20110118189A1Immediate pharmacological effectLonger effectBiocideSenses disorderNeedle freeHeadaches
Systems and methods are described for treating un-met medical needs in migraine and related conditions such as cluster headache. Included are treatments that are both rapid onset and long acting, which include sustained release formulations, and combination products. Also included are treatments for multiple symptoms of migraine, especially headache and nausea and vomiting. Systems that are self contained, portable, prefilled, and simple to self administer at the onset of a migraine attack are disclosed, and preferably include a needle-free injector and a high viscosity formulation, to eliminate such issues as fear of self administration with needles, and needle stick and cross contamination.
Owner:ZOGENIX INC
Loadable polymeric particles for therapeutic and/or diagnostic applications and methods of preparing and using the same
ActiveUS20060088476A1Minimize blood flowMinimizing agglomeration and aggregationPowder deliveryX-ray constrast preparationsActive agentVolumetric Mass Density
Particles are provided for use in therapeutic and / or diagnostic procedures. The particles include poly[bis(trifluoroethoxy) phosphazene] and / or a derivatives thereof which may be present throughout the particles or within an outer coating of the particles. The particles can also include a core having a hydrogel formed from an acrylic-based polymer. Barium sulfate may also be provided to the core of the particles as a coating or absorbed within the core of the particles. The particles can be used to minimize blood flow to mammalian tissues by occluding at least a portion of a blood vessel of the mammal, or to deliver an active agent to a localized area within a body of a mammal by contacting a localized area with at least one of the particles. Further, the particles are useful in sustained release formulations including active agent(s) for oral administration, as tracer particles for injection into the bloodstream of a mammal or for use in enhanced ultrasound imaging. The particles may include agents for increasing density for achieving useful buoyancy levels in suspension.
Owner:VARIAN MEDICAL SYSTEMS
Sustained drug release compositions
ActiveUS20070128269A1Accurately and precisely deliver the appropriate amount of drugBiocideNervous disorderCross-linkActive agent
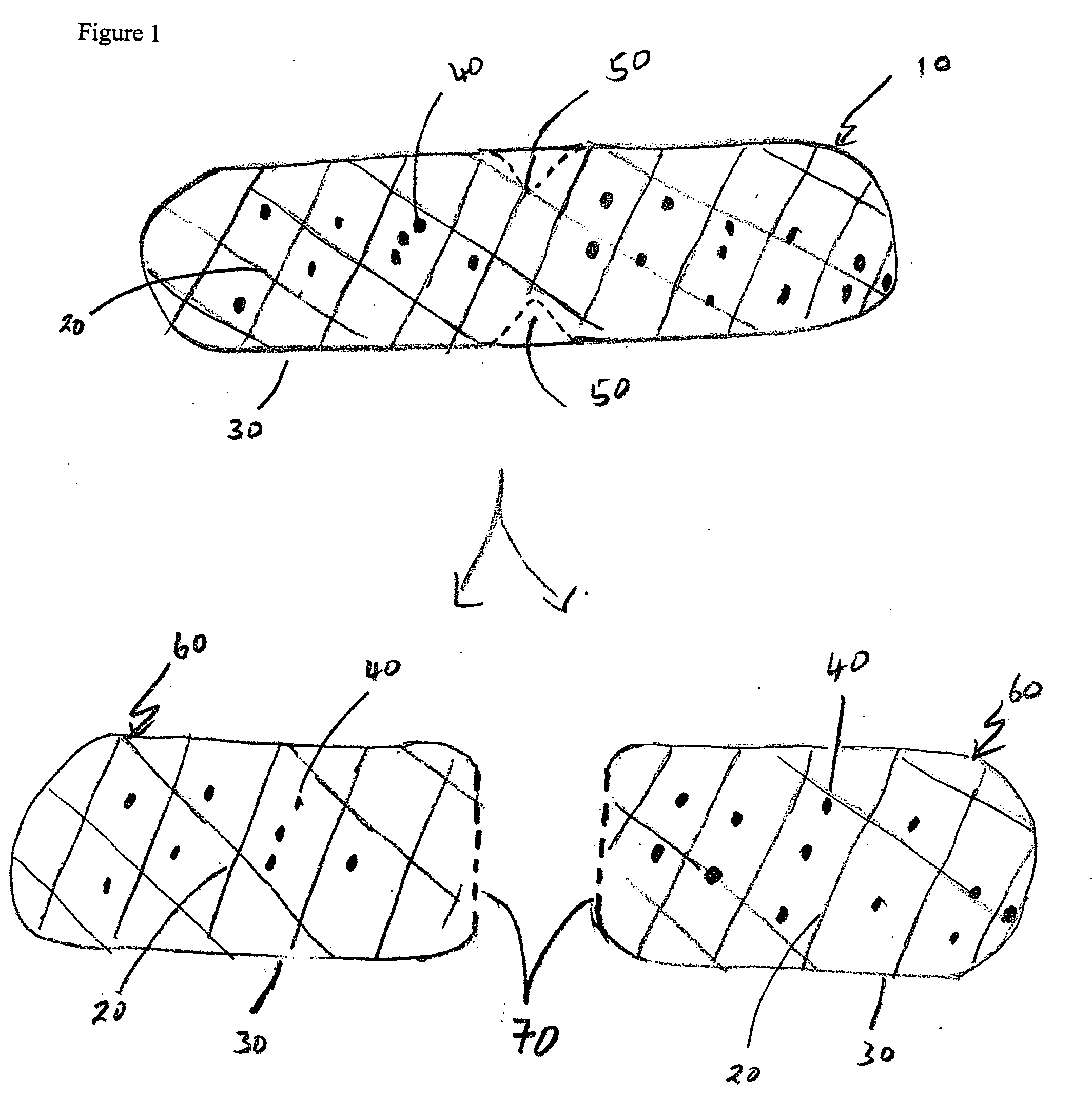
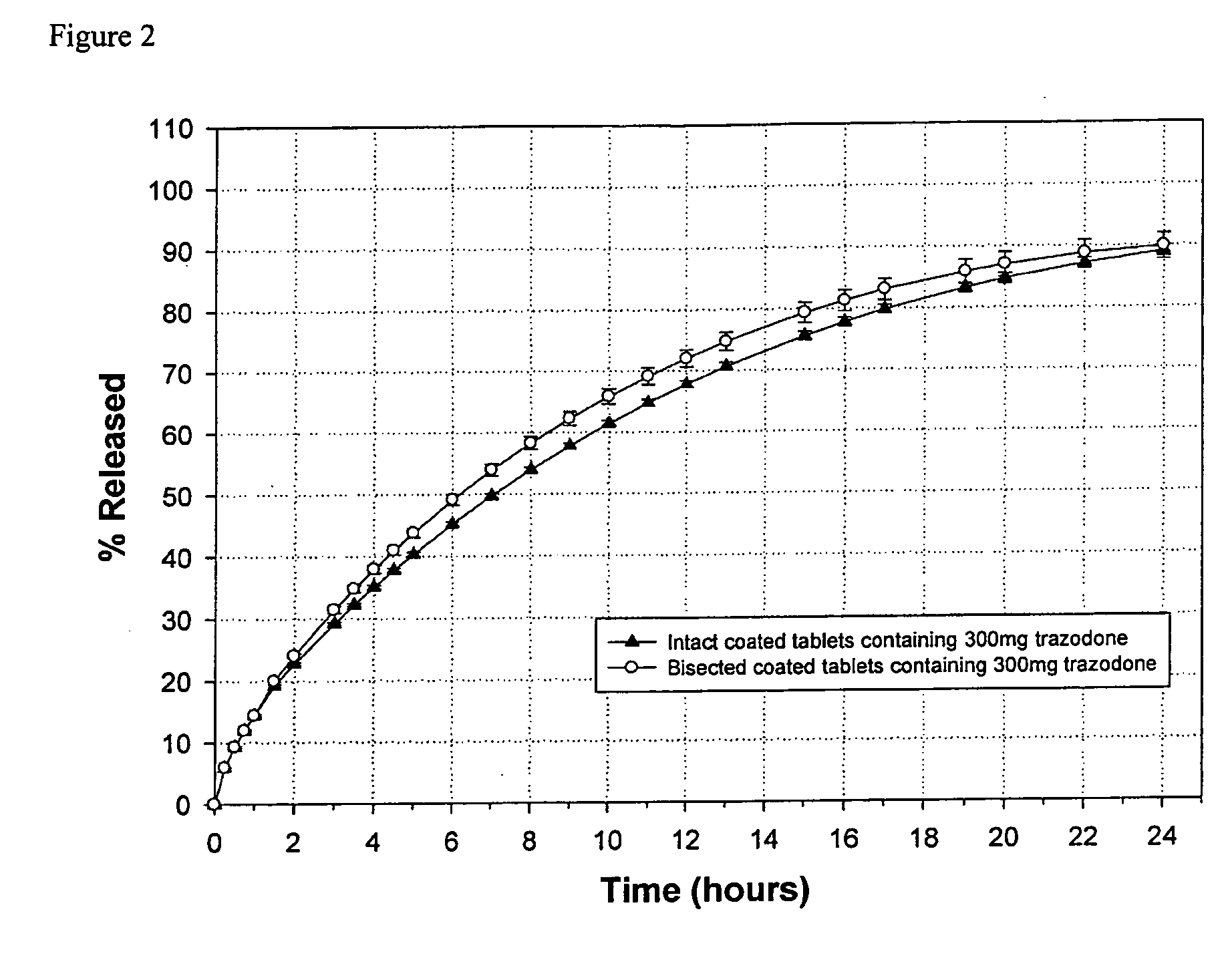
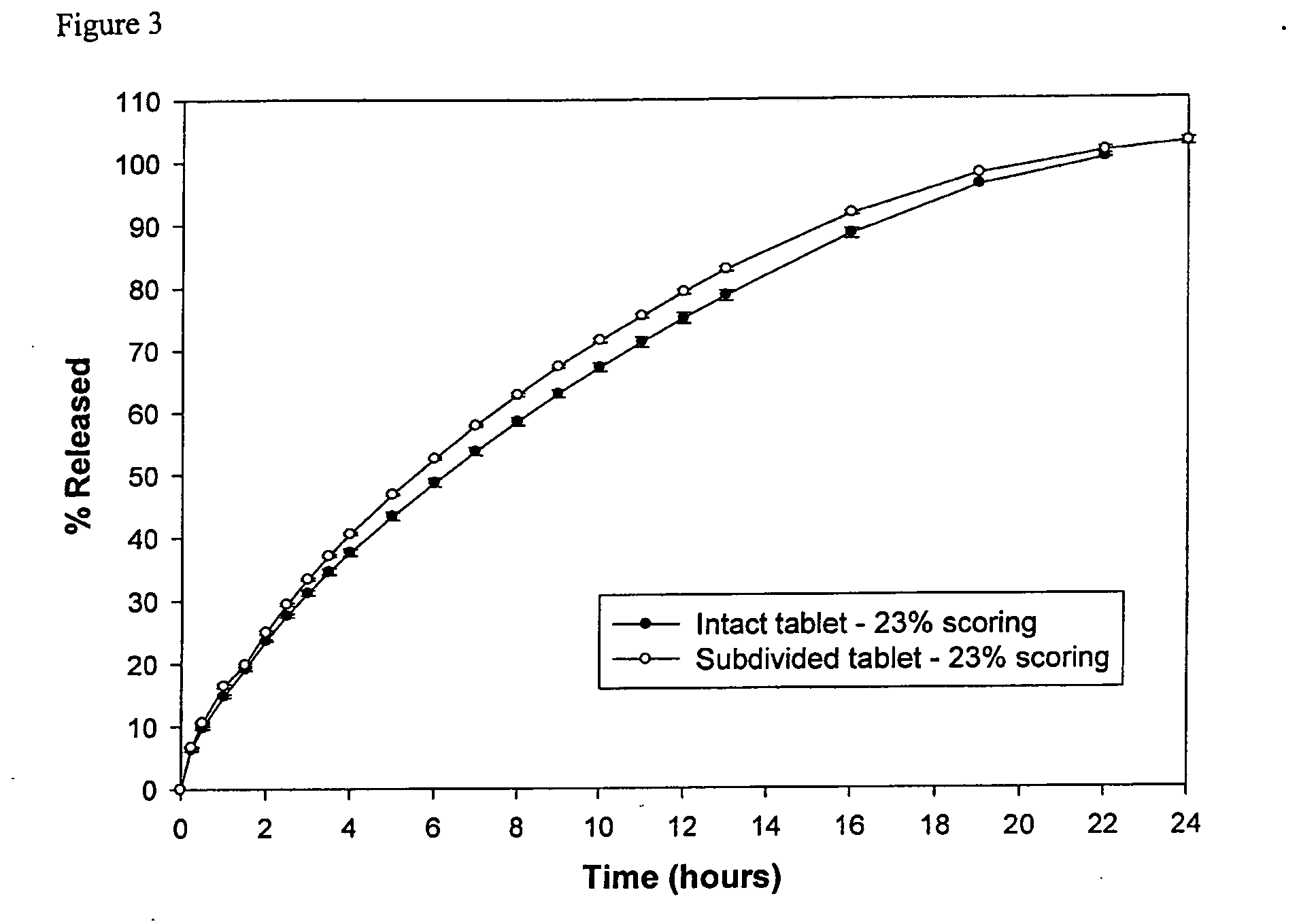
The invention relates to a sustained release formulation for delivering one or more pharmaceutically active agents. The formulation comprises cross-linked high amylose starch and at least one pharmaceutically active agent, and optionally can be subdivided into smaller dosage forms where the smaller dosage forms have substantially the same sustained release properties as the formulation from which they were derived. The formulations can provide sustained release for up to at least 24 hours, and because of their divisability permits a recipient of the active agent or the person administering the active agent to titrate the dosage of the agent.
Owner:ANGELINI PHARM INC
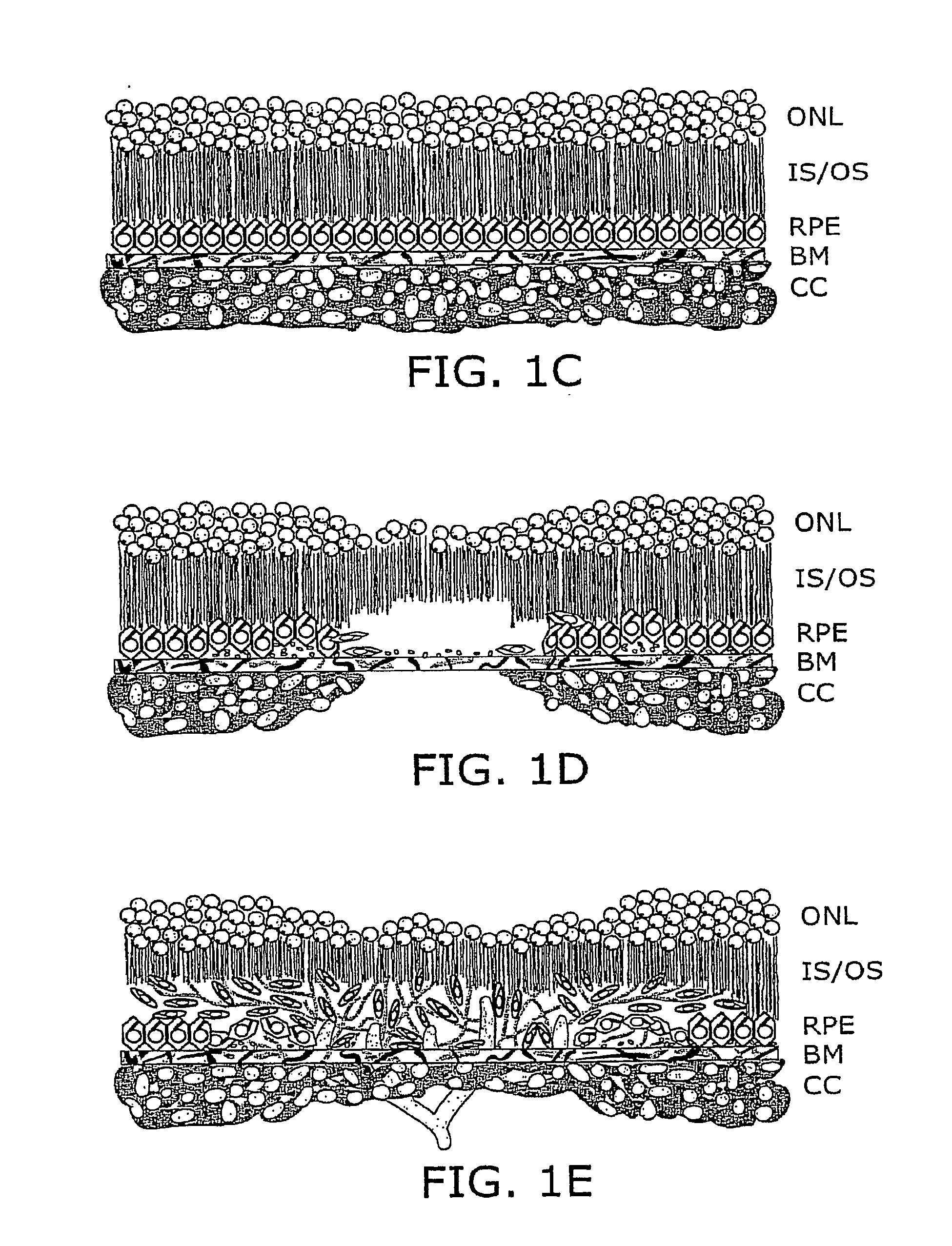
Local Complement Inhibition for Treatment of Complement-Mediated Disorders
ActiveUS20100166862A1Improve in vivo stabilityLow failure rateOrganic active ingredientsPowder deliveryDiseaseComplement Inhibitors
The present invention features the local administration of complement inhibitors for treatment of complement-mediated disorders. In certain embodiments the invention features inhibiting activation of one or more locally produced complement proteins. The invention provides sustained release formulations and devices comprising a complement inhibitor and methods of use thereof.
Owner:APELLIS PHARMA
Sustained release formulations
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
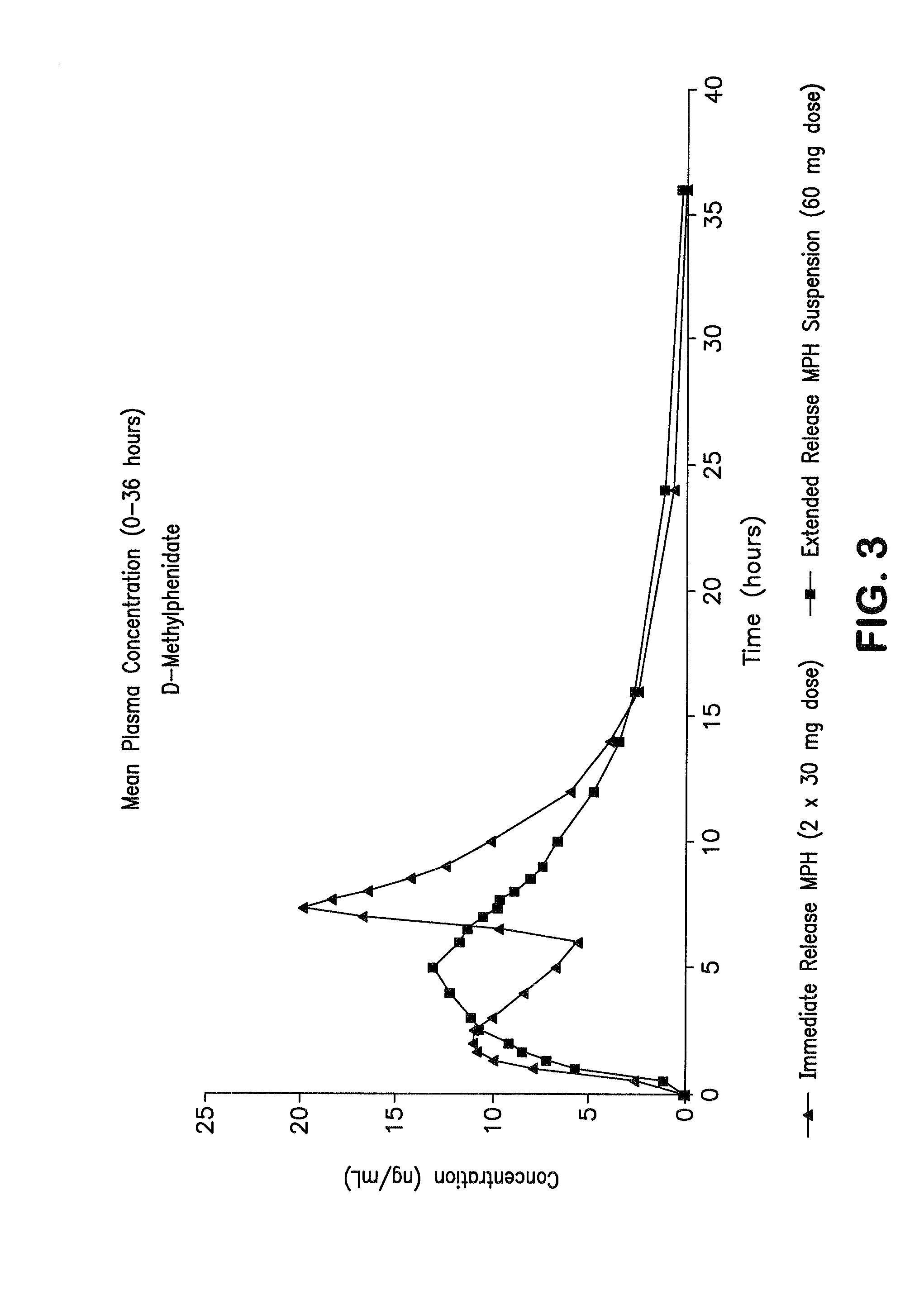
Orally effective methylphenidate extended release powder and aqueous suspension product
An oral methylphenidate powder which is reconstitutable into a final oral aqueous sustained release formulation containing at least about 50%, or at least about 80% by weight water based on the total weight of the suspension, is provided. The powder is a blend containing a combination of an uncoated methylphenidate-ion exchange resin complex, a barrier coated methylphenidate-ion exchange resin complex-matrix, and a water soluble buffering agent such that upon formed into an aqueous liquid formulation, the formulation has a pH in the range of about 3.5 to about 5, or about 4 to about 4.5. Following administration of a single dose of the oral aqueous methylphenidate suspension, a therapeutically effective amount of methylphenidate is reached in less than one hour and the composition provides a twelve-hour extended release profile.
Owner:TRIS PHARMA
Dimethicone-containing sustained release injection formulation
InactiveUS20070053943A1Prevention and treatment of problemBiocidePharmaceutical delivery mechanismDrugAnalgesic agents
A sustained release formulation by using dimeticone as the dispersion medium, which includes active ingredient (e.g., drugs against parasites, insecticides, NSAIDs, antibiotics, sex hormone like agents or oily soluble vitamins) and dimeticone as the medium. Suitable stabilizer, antioxidant, local analgesics and material for sustained release may be added. The formulation is bio-compatible, stable and injectable.
Owner:WANG YUWAN +2
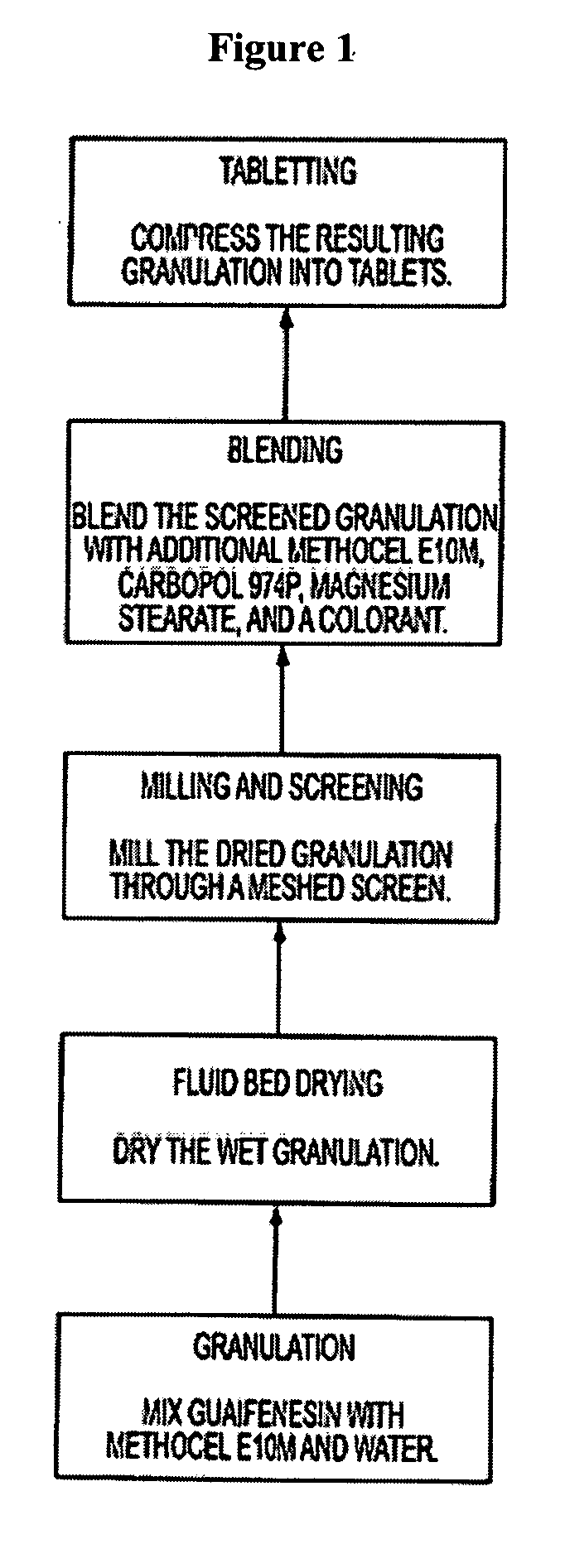
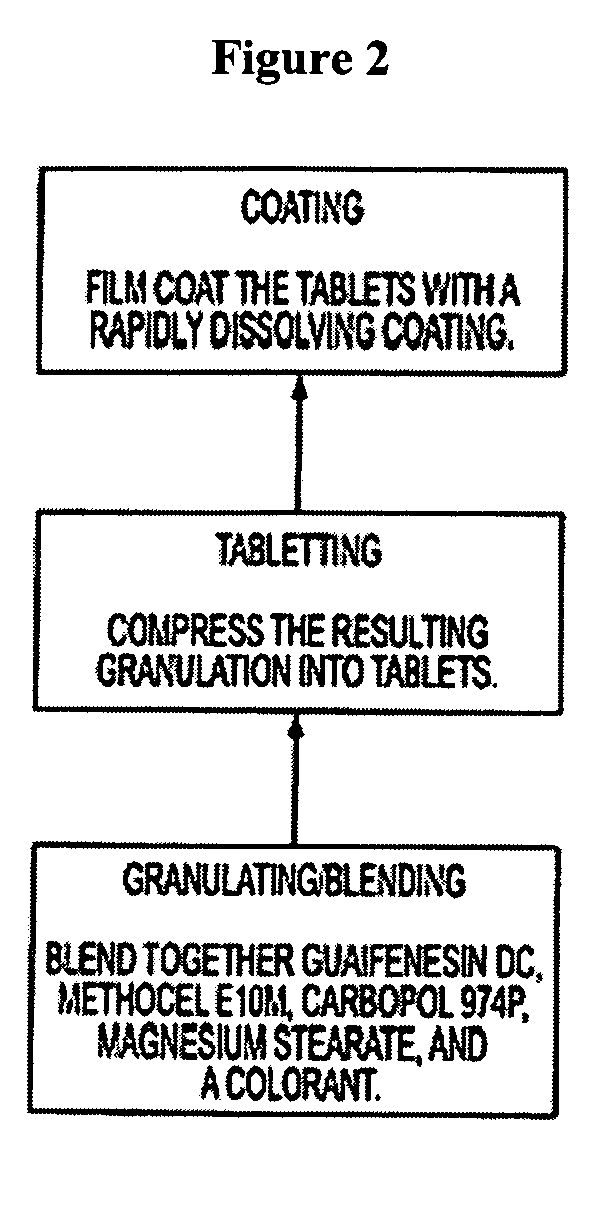
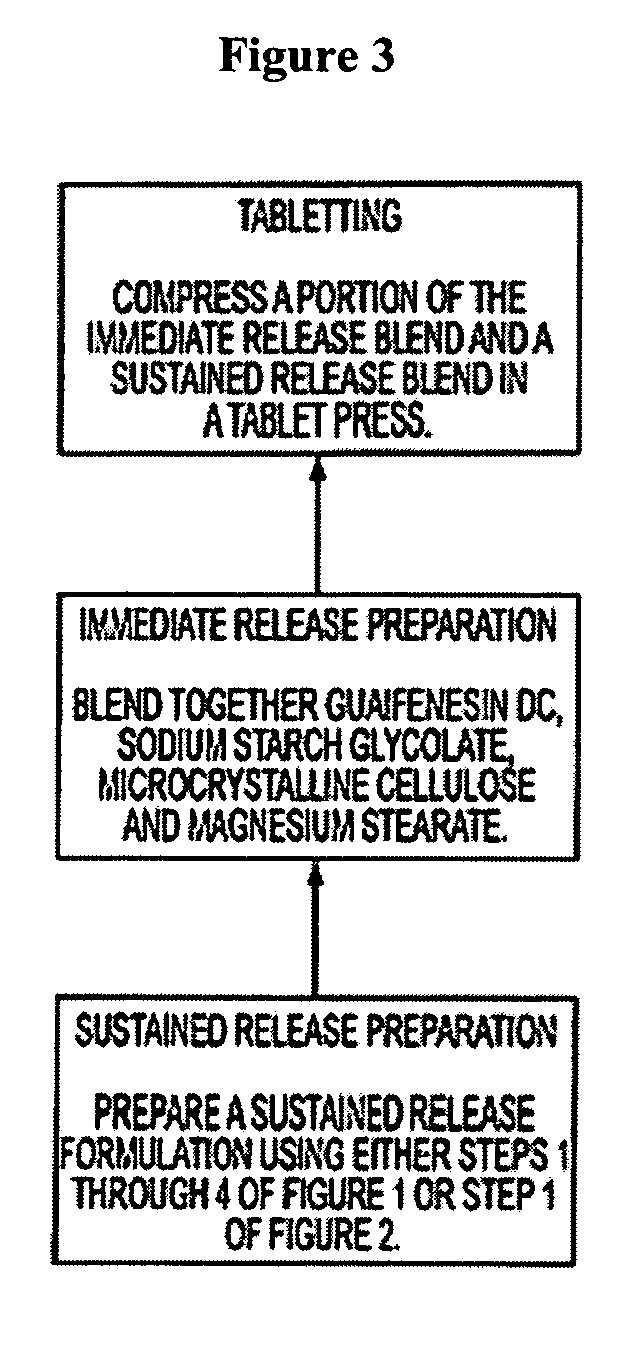
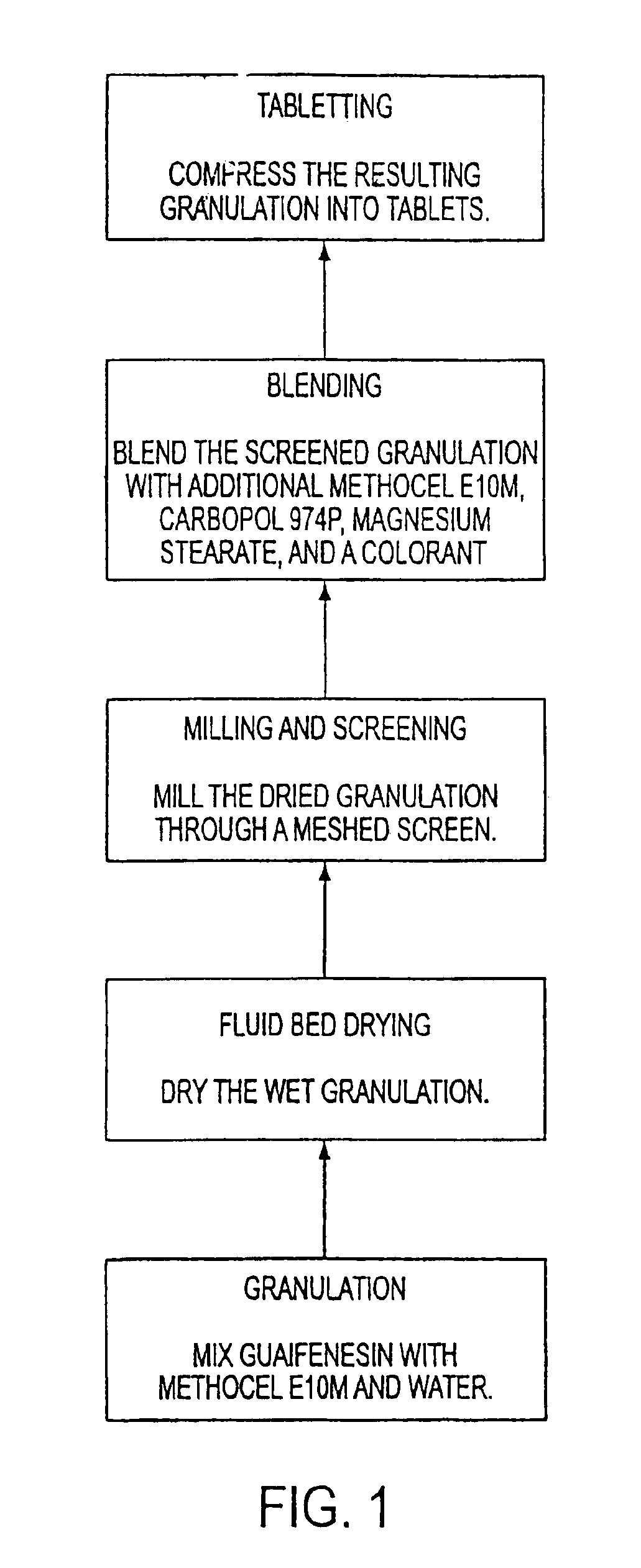
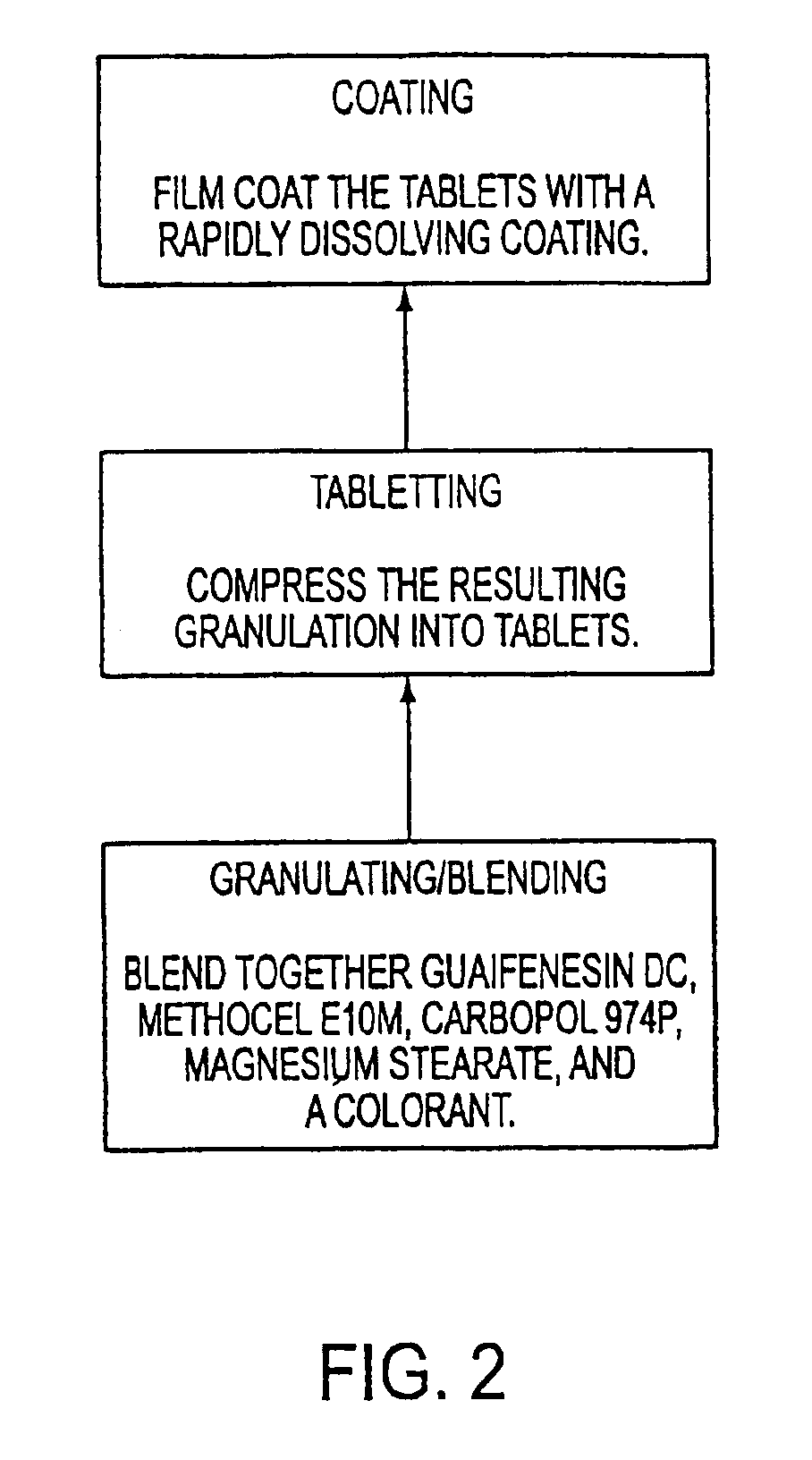
Sustained release formulations of guaifenesin and additional drug ingredients
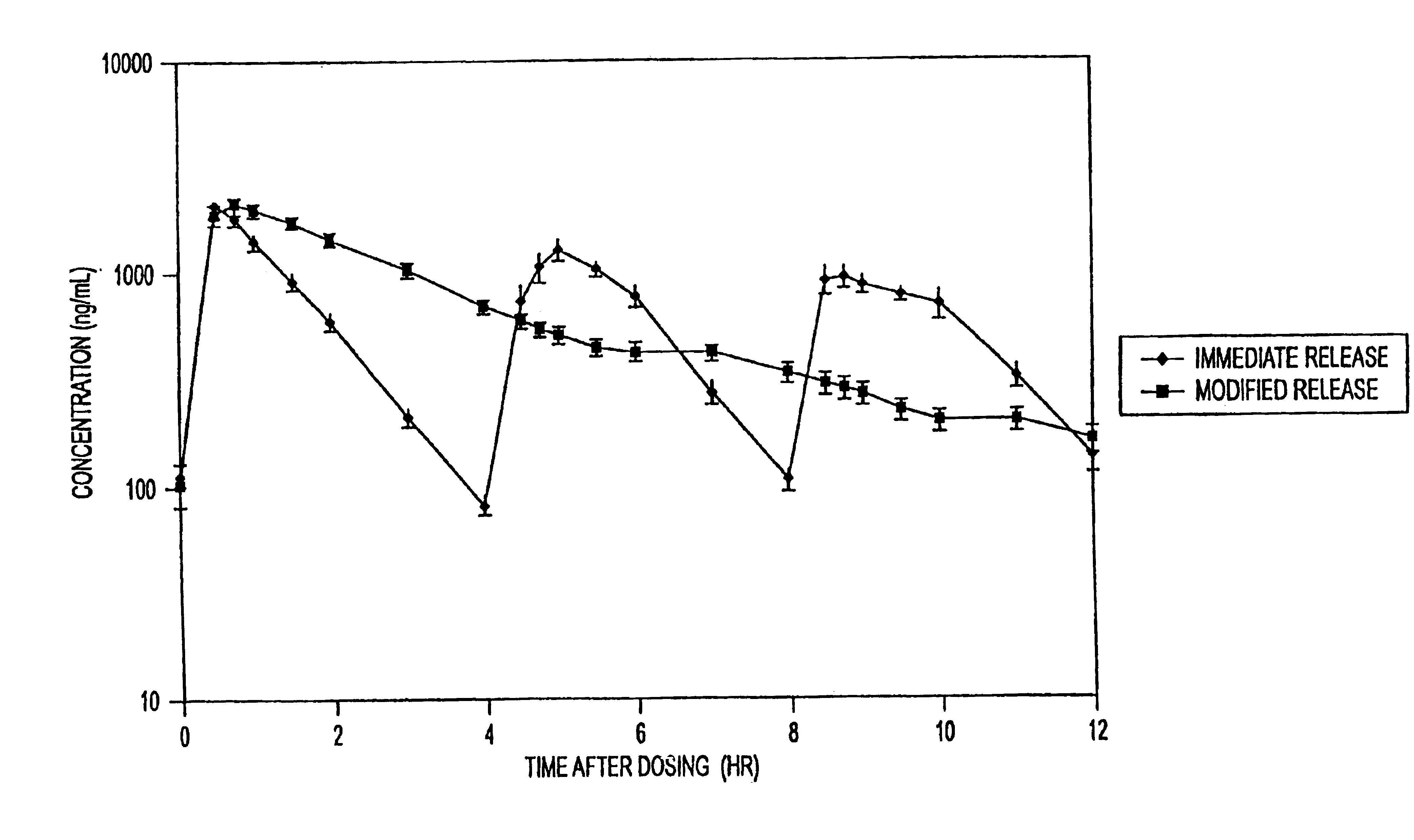
InactiveUS20050276852A1Increase in drug strengthSustained releaseOrganic non-active ingredientsEther/acetal active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Directly compressible sustained release formulation containing microcrystalline cellulose
The present invention provides an improved process for the preparation of a agglomerated solid dosage form, comprising: (1) preparing an aqueous slurry of (a) microcrystalline cellulose; (b) a microcrystalline cellulose compressibility augmenting agent which (i) physically restricts the proximity of the interface between adjacent cellulose surfaces; (ii) inhibits interactions between adjacent cellulose surfaces, for example, via the creation of a hydrophobic boundary at cellulose surfaces; or (iii) accomplishes both (i) and (ii) above; and (c) an active agent; (2) thereafter drying the resultant aqueous slurry in a manner which inhibits quasi-hornification, thereby obtaining an agglomerated material which is directly compressible into a solid dosage form.
Owner:J RETTENMAIER & SOEHNE GMBH CO KG ROSENBERG
Sustained release formulations of guaifenesin and additional drug ingredients
InactiveUS6955821B2Increase in drug strengthSustained releaseEther/acetal active ingredientsOrganic non-active ingredientsSerum concentrationImmediate release
The invention relates to a novel pharmaceutical sustained release formulation of guaifenesin and at least one additional drug ingredient. The formulation may comprise a hydrophilic polymer, preferably a hydroxypropyl methylcellulose, and a water-insoluble polymer, preferably an acrylic resin, in a ratio range of about one-to-one (1:1) to about nine-to-one (9:1), more preferably a range of about three-to-two (3:2) to about six-to-one (6:1), and most preferably in a range of about two-to-one (2:1) to about four-to-one (4:1) by weight. This formulation capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject. The invention also relates to a modified release product which has two portions: a first portion having an immediate release formulation of guaifenesin and a second portion having a sustained release formulation of guaifenesin, wherein one or both portions has at least one additional drug ingredient. The modified release product has a maximum guaifenesin serum concentration equivalent to that of an immediate release guaifenesin tablet, and is capable of providing therapeutically effective bioavailability of guaifenesin for at least twelve hours after dosing in a human subject.
Owner:RB HEALTH US LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com