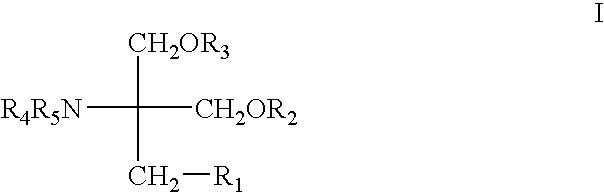Patents
Literature
77 results about "Complement inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
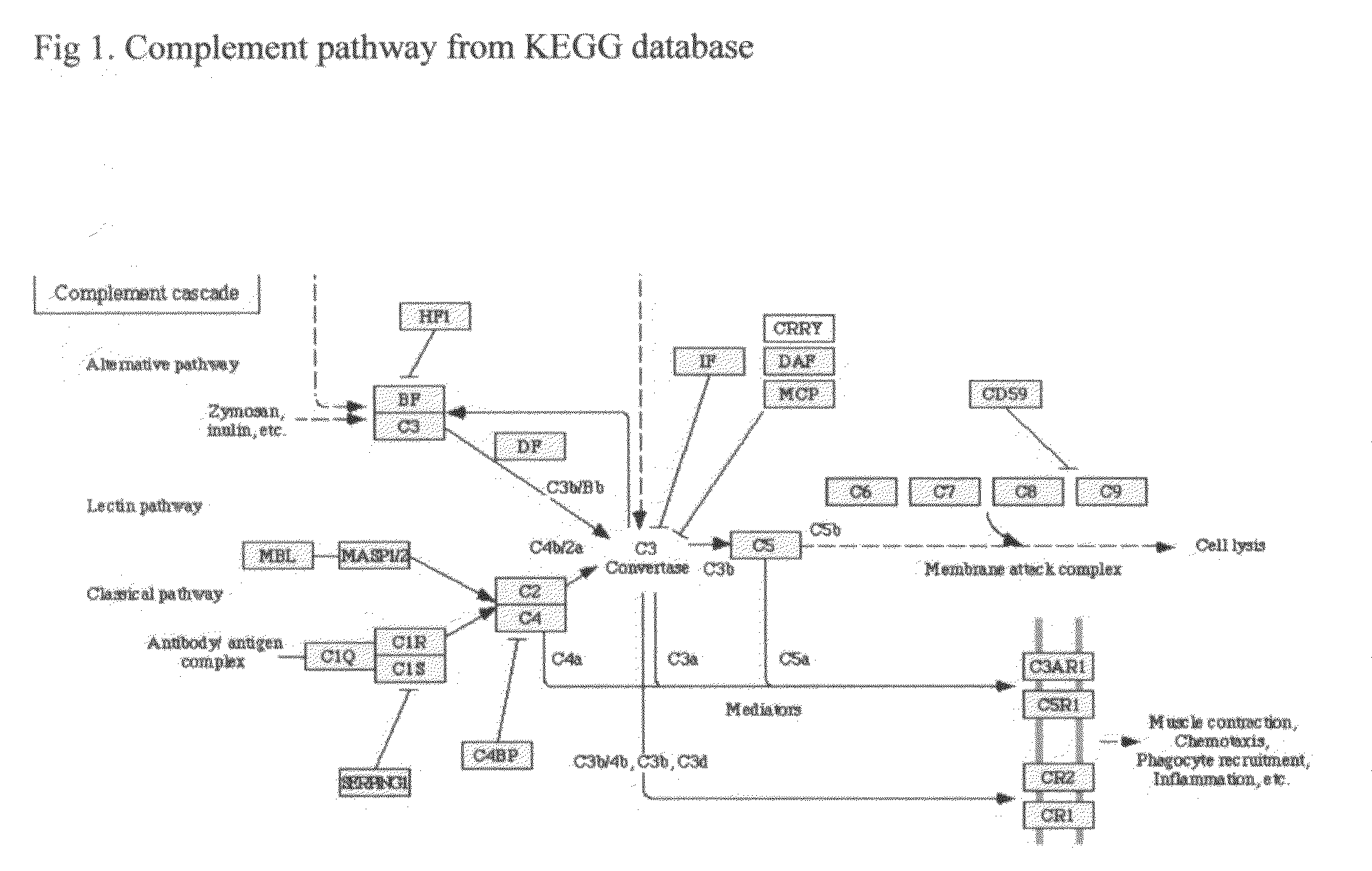
Complement Inhibitors. Complement inhibitors are a type of monoclonal antibody that are used to treat paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS). They prevent a part of the immune system from harming healthy cells and tissue.
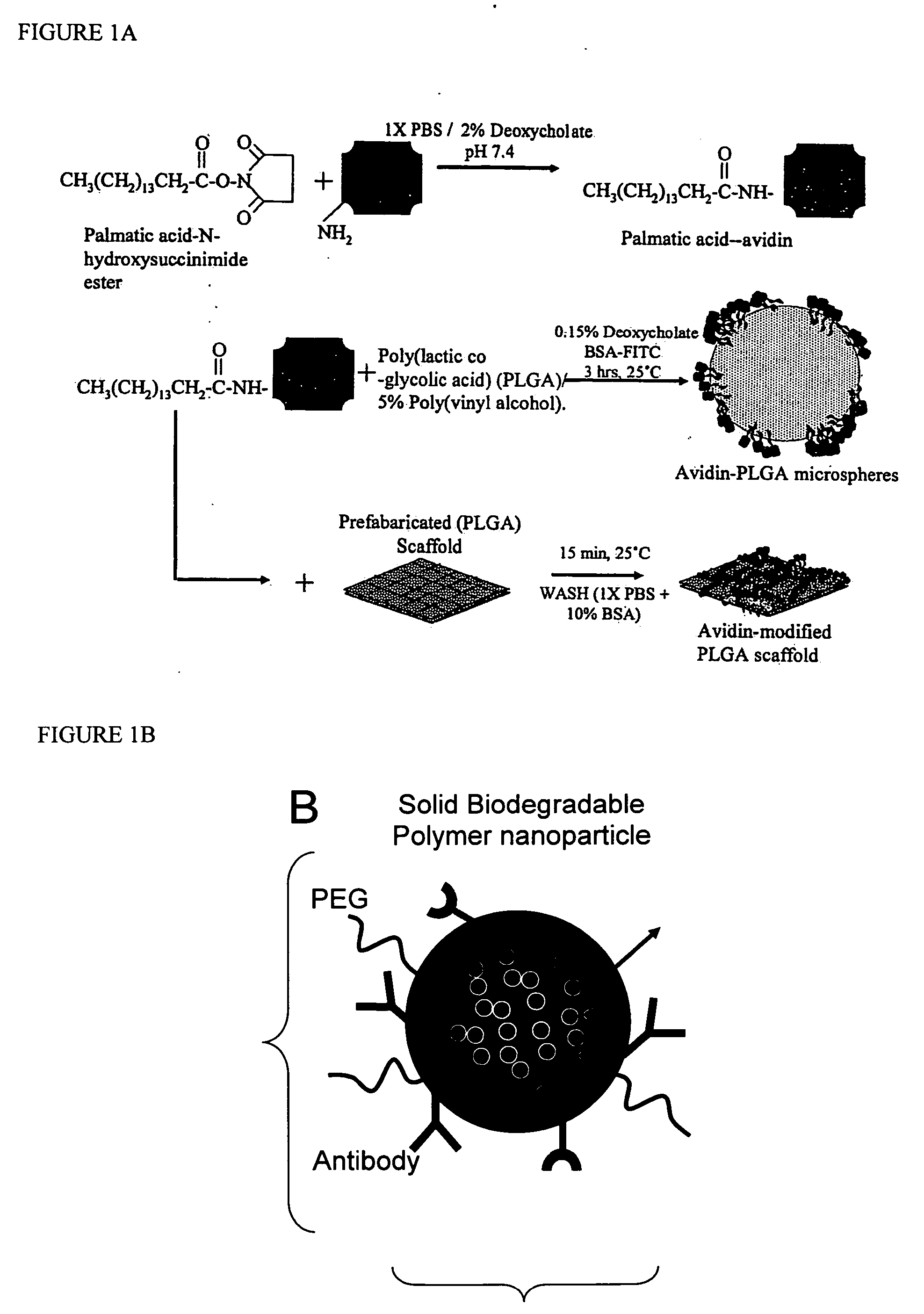
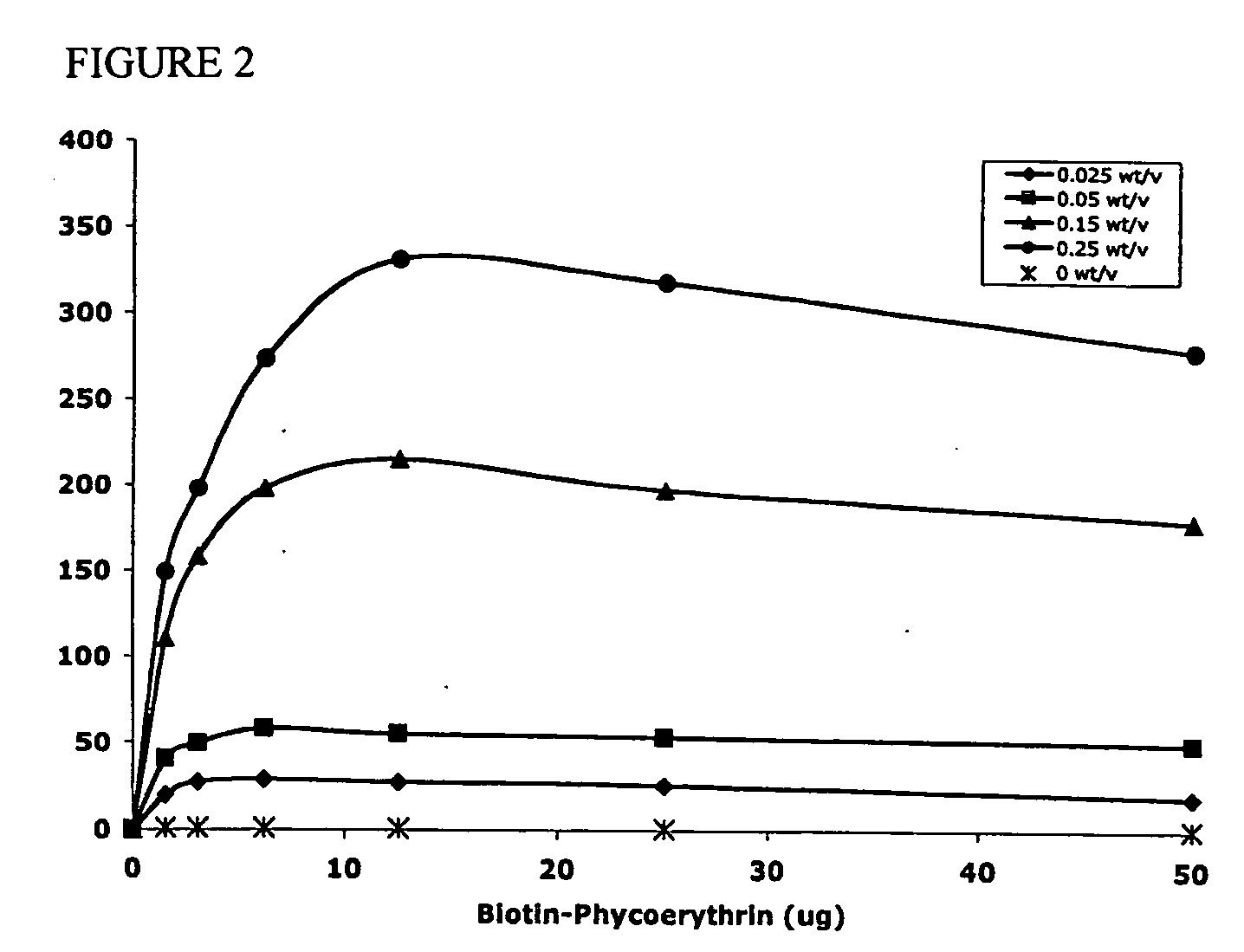
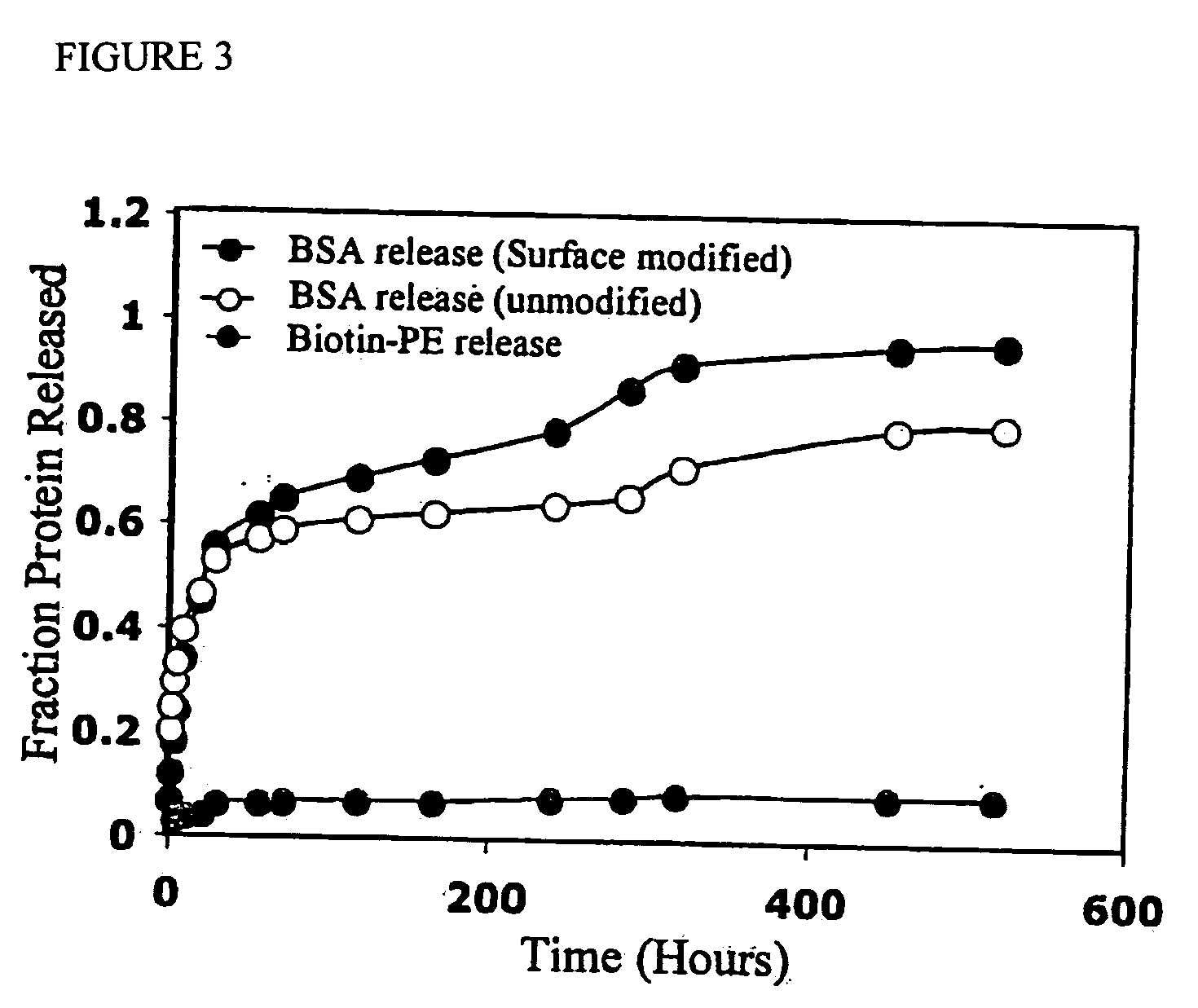
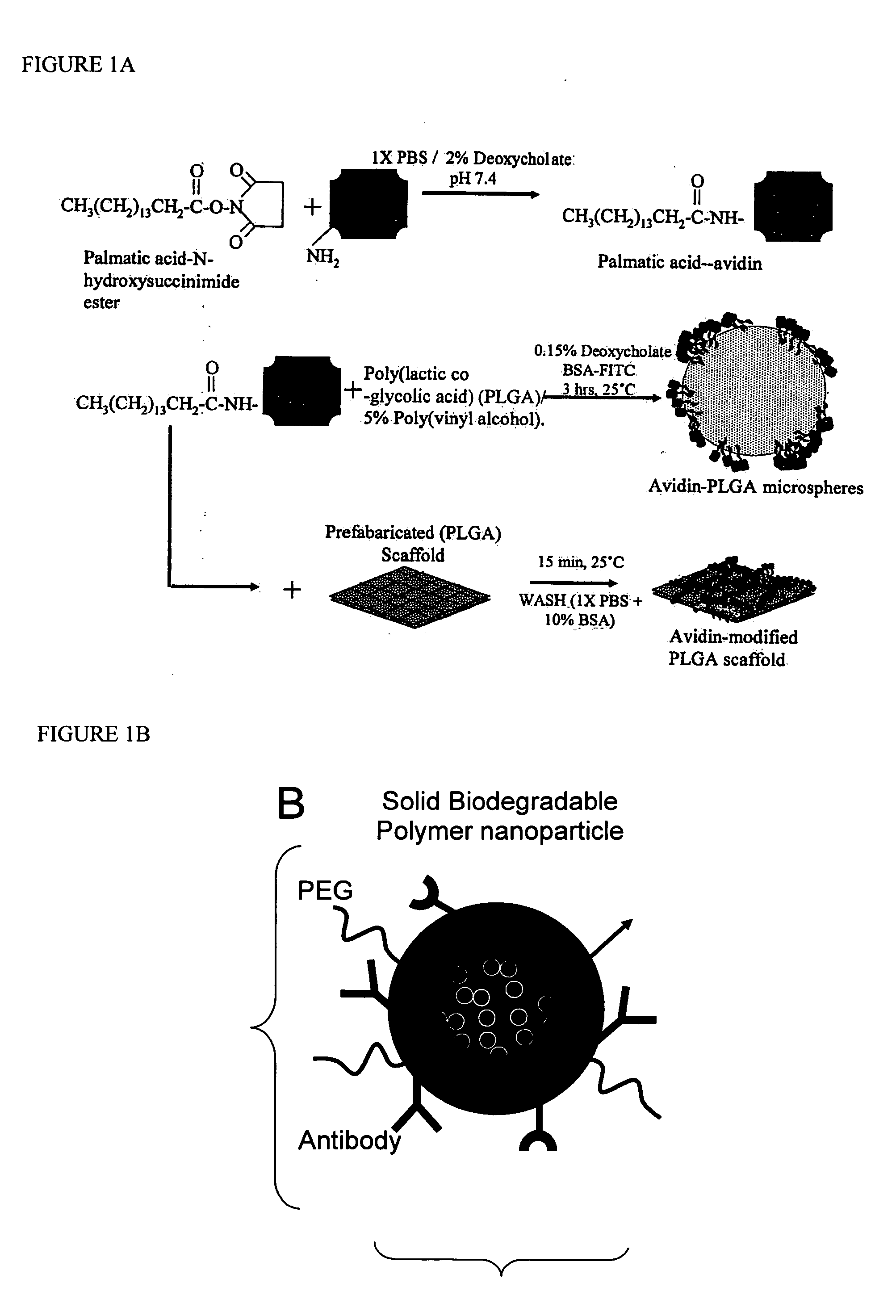
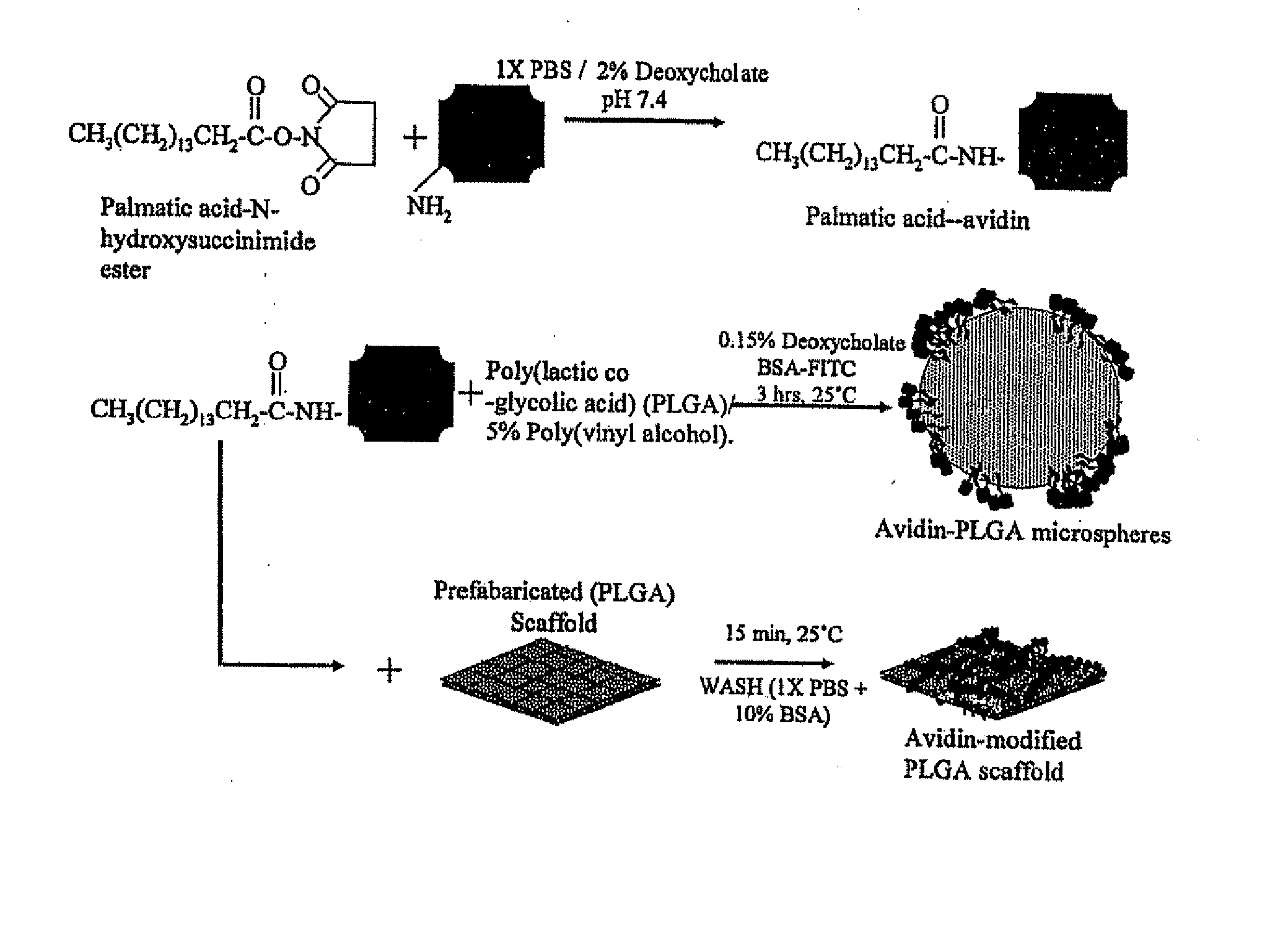
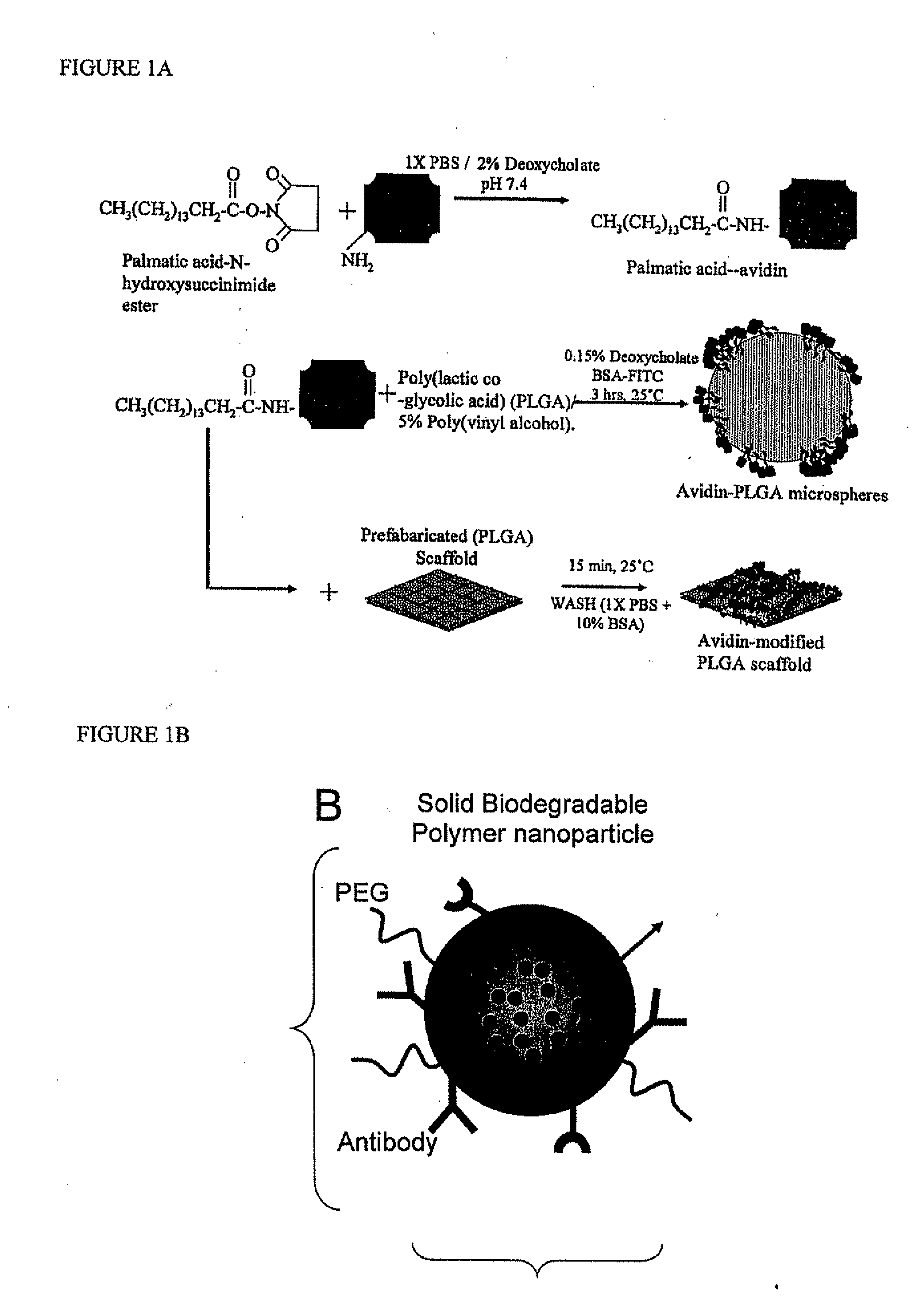
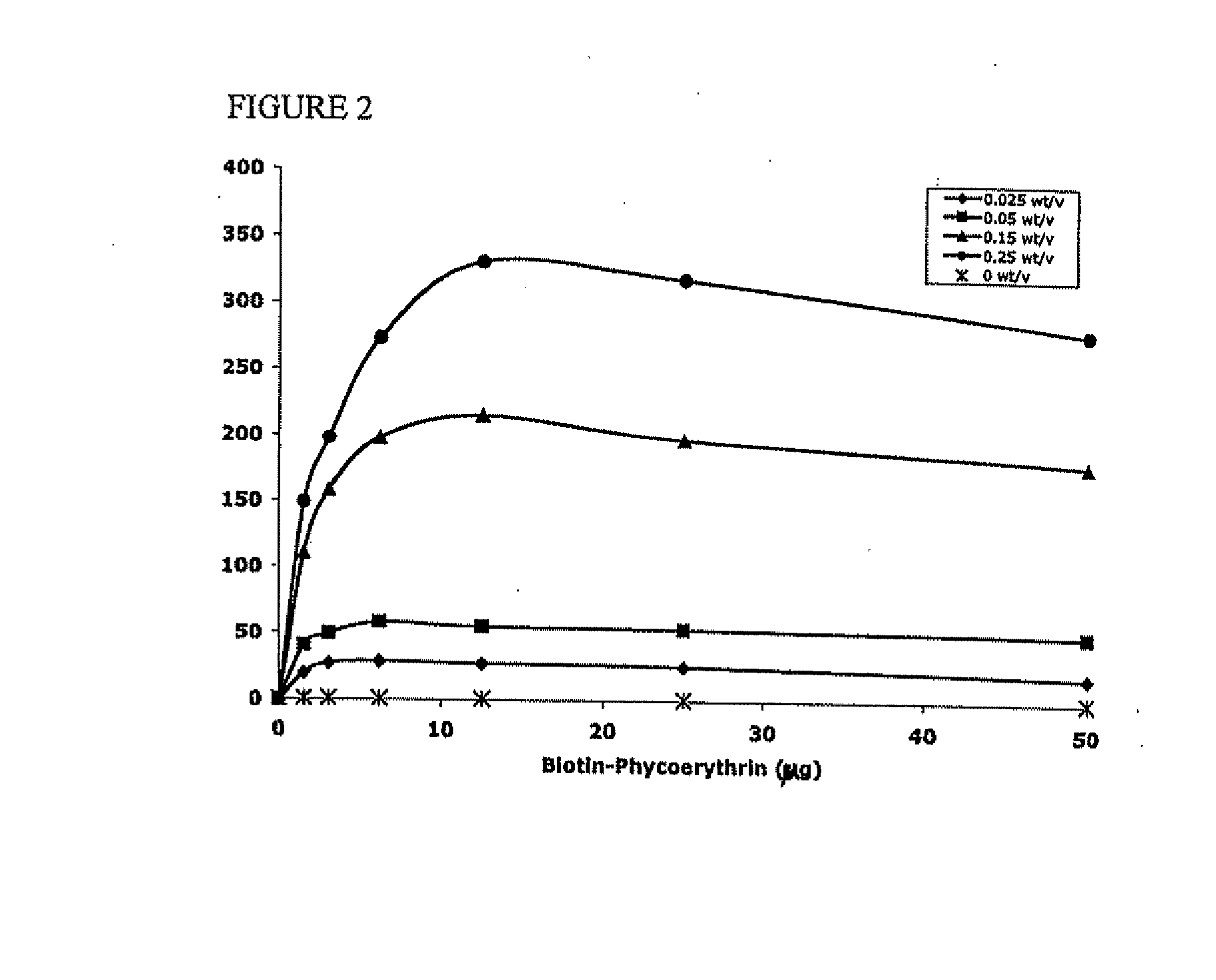
Targeted and high density drug loaded polymeric materials
ActiveUS20060002852A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
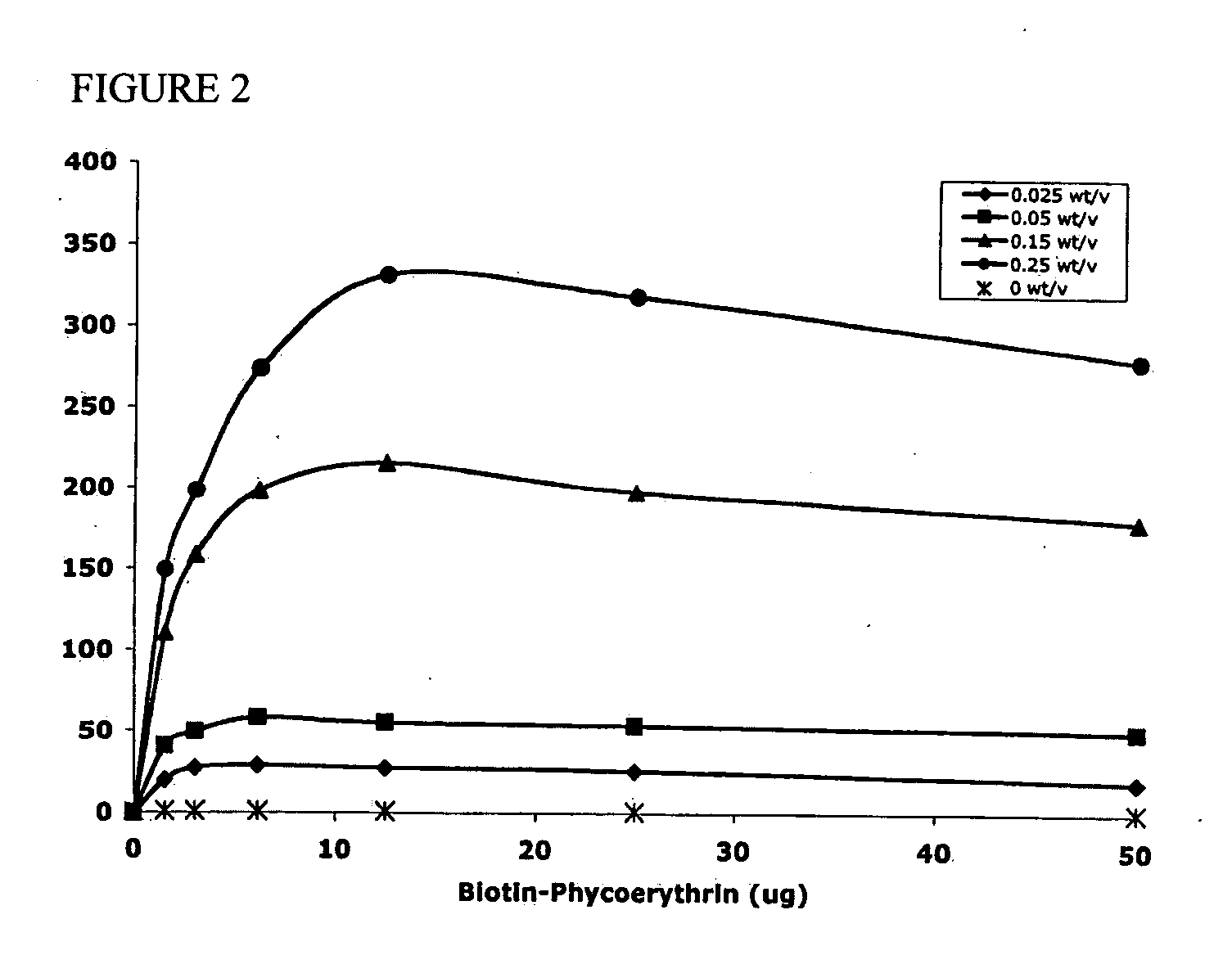
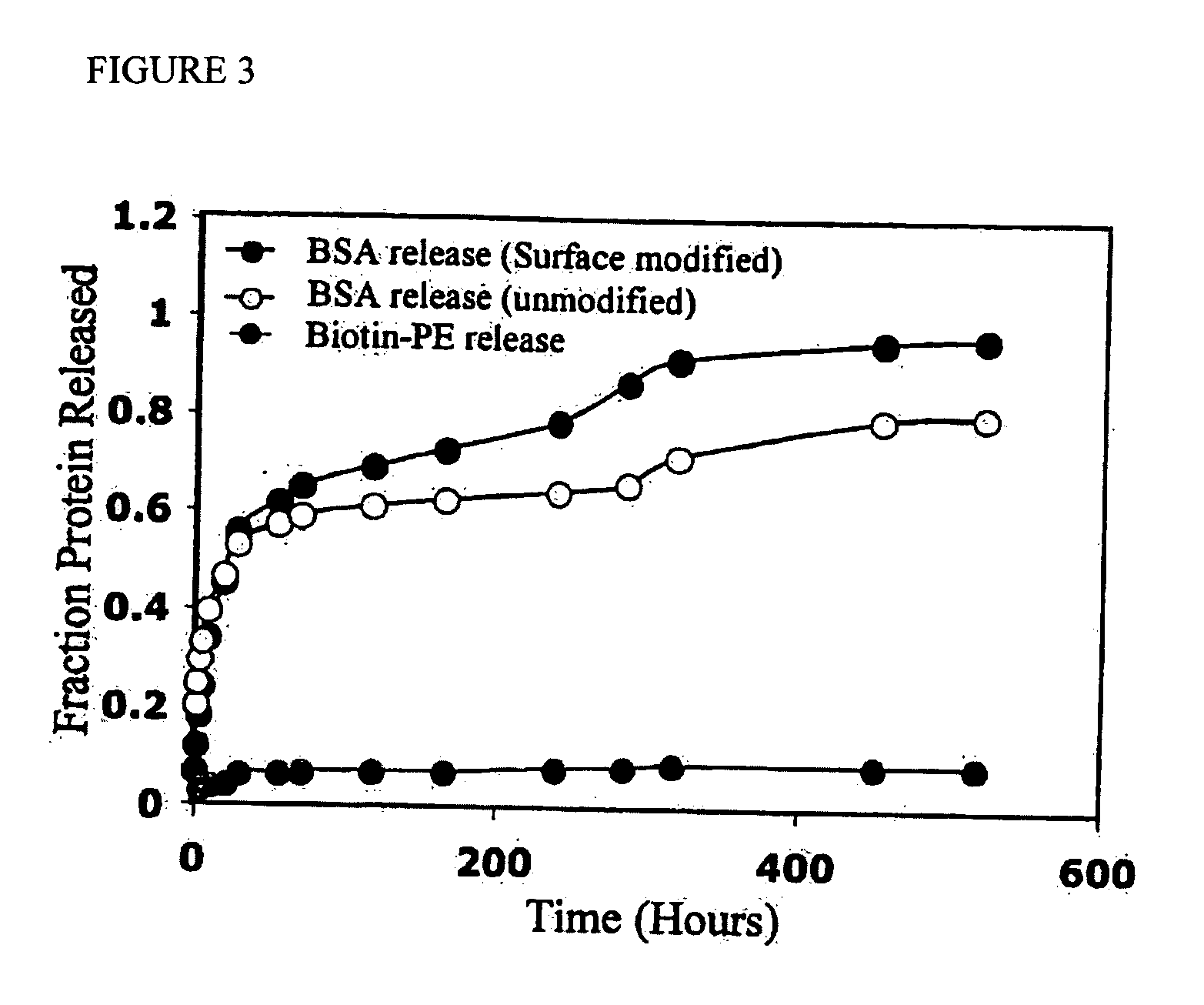
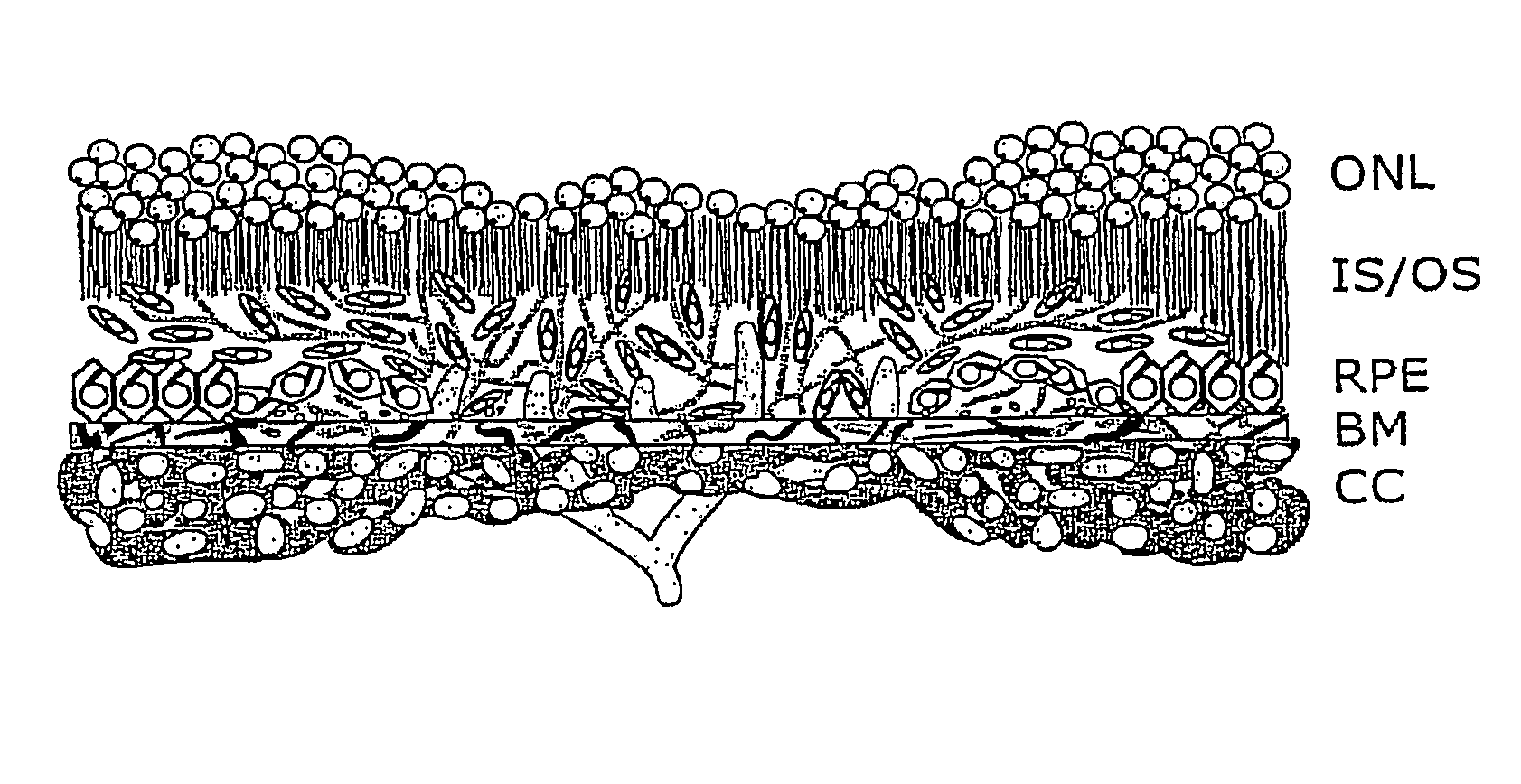
Polymeric delivery devices have been developed which combine high loading / high density of molecules to be delivered with the option of targeting. As used herein, “high density” refers to microparticles having a high density of ligands or coupling agents, which is in the range of 1000-10,000,000, more preferably between 10,000 and 1,000,000 ligands per square micron of microparticle surface area. A general method for incorporating molecules into the surface of biocompatible polymers using materials with an HLB of less than 10, more preferably less than 5, such as fatty acids, has been developed. Because of its ease, generality and flexibility, this method has widespread utility in modifying the surface of polymeric materials for applications in drug delivery and tissue engineering, as well other other fields. Targeted polymeric microparticles have also been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Methods of treatment with drug loaded polymeric materials
ActiveUS20060002971A1Increase molecular densityHigh densityPowder deliveryBiocideAntigenWound dressing
Polymeric microparticles have been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and can have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, bone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Injectable Combination Therapy for Eye Disorders
InactiveUS20090220572A1Compounds screening/testingPowder deliveryLiquid mediumRetinal neovascularization
The present invention provides composition, methods, and articles of manufacture for treating an eye disorder, e.g., a disorder characterized by macular degeneration, choroidal neovascularization, or retinal neovascularization. One method of the invention comprises the step of: administering first and second therapeutic agents to the subject's eye in a single procedure, wherein the first therapeutic agent provides rapid improvement in the condition of the subject's eye and the second therapeutic agent is administered as a sustained release formulation of the second therapeutic agent. For example, the first and second therapeutic agents are administered by intravitreal injection. The first therapeutic agent may be dissolved in a liquid medium located in the syringe and the sustained formulation of the second therapeutic agent may comprise an ocular implant or plurality of particles located in the needle. The therapeutic agents may be selected from the group consisting of angiogenesis inhibitors and complement inhibitors.
Owner:POTENTIA PHARMA INC
Methods of treating chronic disorders with complement inhibitors
ActiveUS20140371133A1Organic active ingredientsSenses disorderObstructive Pulmonary DiseasesDisease cause
In some aspects, the invention provides methods of treating a subject in need of treatment for a chronic complement-mediated disorder. In some aspects, the invention provides methods of treating a subject in need of treatment for a Th17-associated disorder. In some aspects, the invention provides methods of treating a subject in need of treatment for a chronic respiratory system disorder. In some aspects, the invention provides methods of administering a complement inhibitor to a subject. In some embodiments, a method of treating a subject comprises administering multiple doses of a complement inhibitor to the subject according to a dosing schedule that leverages the prolonged effect of complement inhibition in chronic respiratory disorders. In some embodiments, a subject has chronic obstructive pulmonary disease. In some embodiments, a subject has asthma.
Owner:APELLIS PHARMA
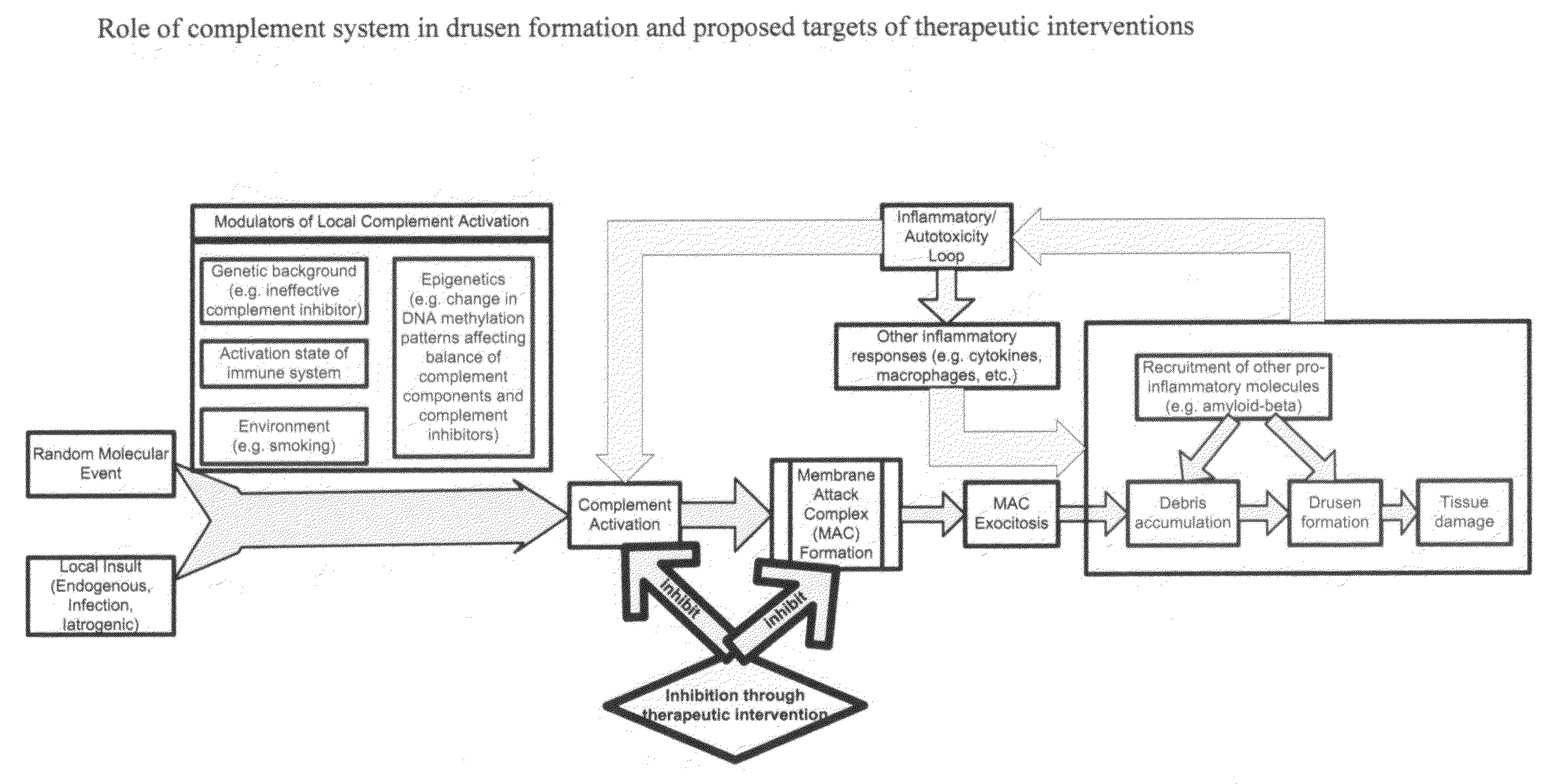
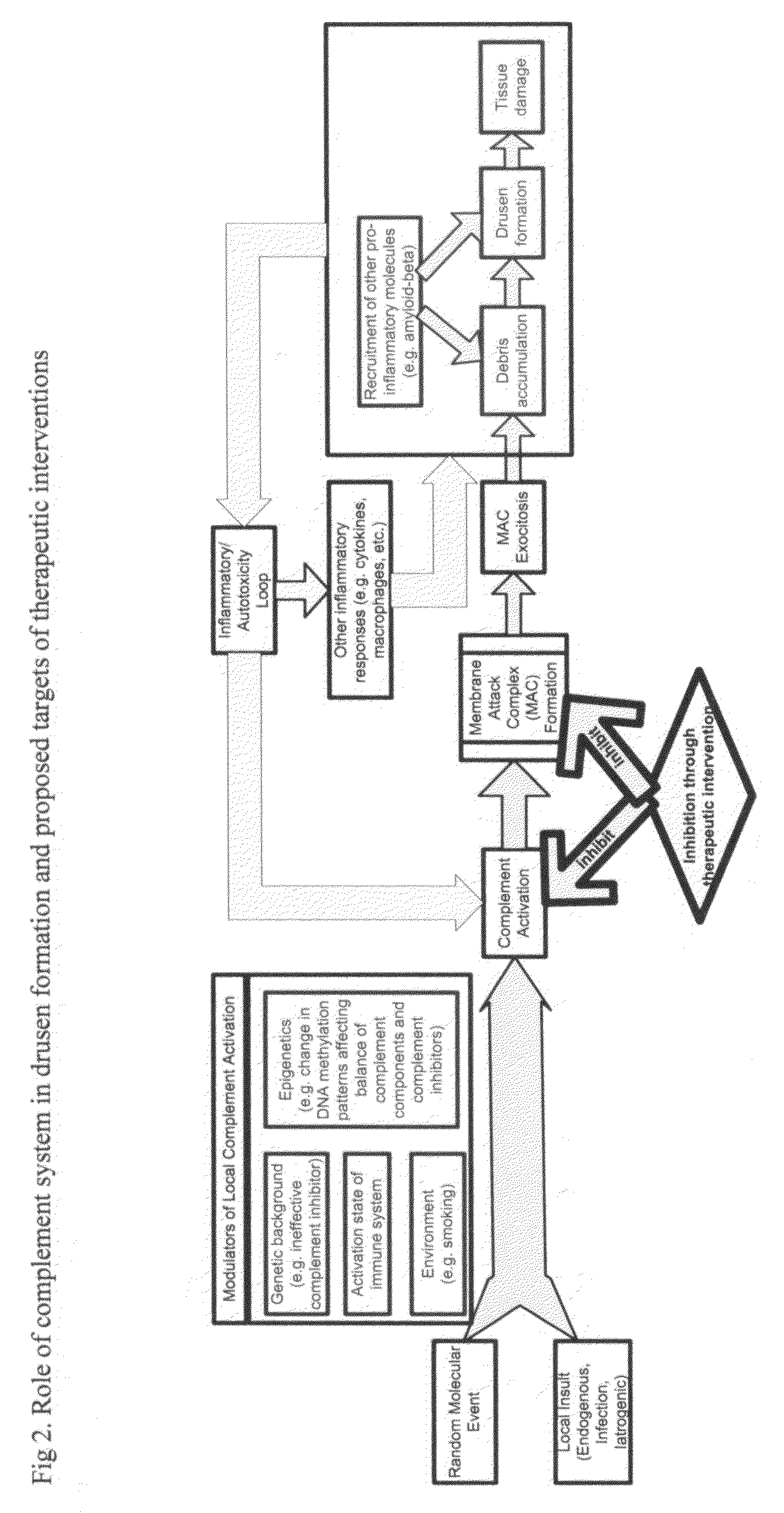
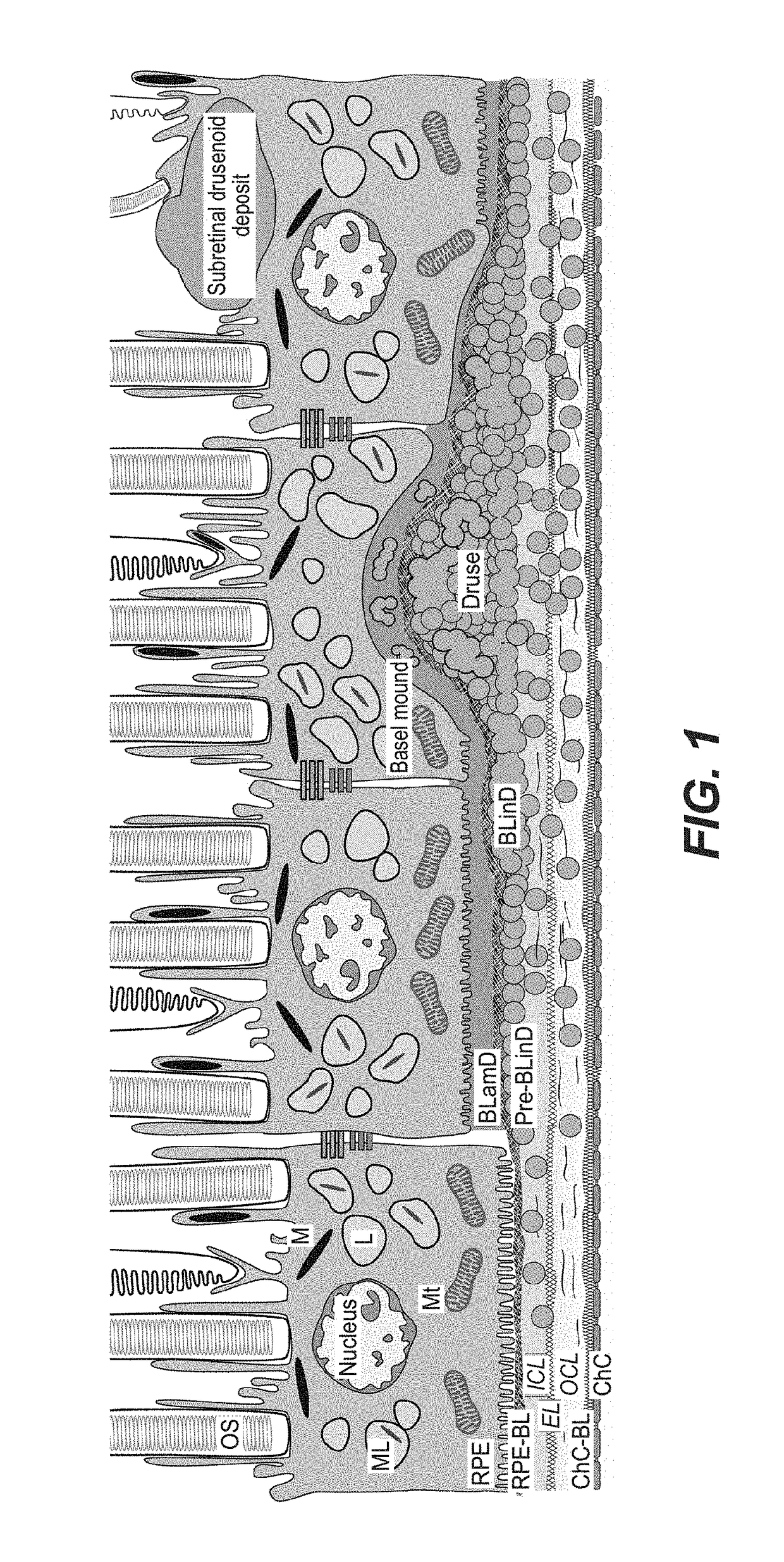
Methods of preventing and treating Alzheimer's disease, age related macular degeneration and other diseases involving extra-cellular debris through the inhibition of the complement system
This invention proposes that the best therapeutic strategy for treating and / or preventing Alzheimer's disease (AD), age related macular degeneration (AMD) and other diseases that exhibit extra-cellular debris deposits, such as atherosclerosis, is the inhibition of the complement pathway. A model for the accumulation of extra-cellular debris through the activation of the complement pathway is presented, and the primary pathogenic role of the debris in the etiology of the disorders is explained. Previously identified complement inhibitors are identified as therapeutic agents for the treatment and / or prevention of AD, AMD and other diseases that exhibit extra-cellular debris deposits.
Owner:DINU VALENTIN
Methods of Treatment with Drug Loaded Polymeric Materials
Polymeric microparticles have been developed which encapsulate therapeutic compounds such as drugs, cellular materials or components, and antigens, and can have targeting ligands directly bound to the microparticle surface. Preferred applications include use in tissue engineering matrices, wound dressings, hone repair or regeneration materials, and other applications where the microparticles are retained at the site of application or implantation. Another preferred application is in the use of microparticles to deliver anti-proliferative agents to the lining of blood vessels following angioplasty, transplantation or bypass surgery to prevent or decrease restenosis, and in cancer therapy. In still another application, the microparticles are used to treat or prevent macular degeneration when administered to the eye, where agents such as complement inhibitors are administered.
Owner:YALE UNIV
Use of complement inhibitors to treat ocular diseases
Owner:GENENTECH INC
Methods and compositions for the prevention and treatment of sepsis
The present invention includes compositions comprising one or more complement inhibitors and one or more CD14 pathway inhibitors for the prevention or treatment of sepsis. The complement inhibitors may be antibodies that bind to and inhibit complement proteins such as C5a and the CD14 pathway inhibitors may be antibodies that bind to and inhibit CD14 pathway components, such as CD14 and LPS. The invention also relates to methods of treating subjects suffering from sepsis comprising administering these compositions, as well as kits for supplying the compositions for treatment.
Owner:GENENTECH INC
Treatment Of Sepsis Using Complement Inhibitors
Methods for the treatment of sepsis with complement inhibitors are disclosed. In particular, C3 inhibitors, such as Compstatin and Compstatin analogs, are administered at various times following the onset of sepsis to alleviate tissue damage and organ failure, which are hallmarks of the second, extravascular stage of sepsis. Combination therapies for comprehensive treatment of sepsis are also disclosed. Pharmaceutical compositions and kits for use in the methods are disclosed as well.
Owner:OKLAHOMA MEDICAL RES FOUND +2
Methods of distributing complement-inhibiting drugs to patients receiving a complement inhibitor
InactiveUS20130246083A1Data processing applicationsDrug and medicationsDrug treatmentFamily medicine
This disclosure relates to methods of authorizing distribution of complement-inhibiting drugs to patients who have a complement-associated disorder in a manner to ensure that the patients are aware of the possible dangers of discontinuing treatment with the drugs. A database is prepared comprising patient information including experiencing adverse clinical events after discontinuing the drug treatment. The information in the database is collected and may be reported. The patients are given a warning as to adverse events that may occur if treatment with the complement inhibiting drugs is discontinued.
Owner:ALEXION PHARMA INC
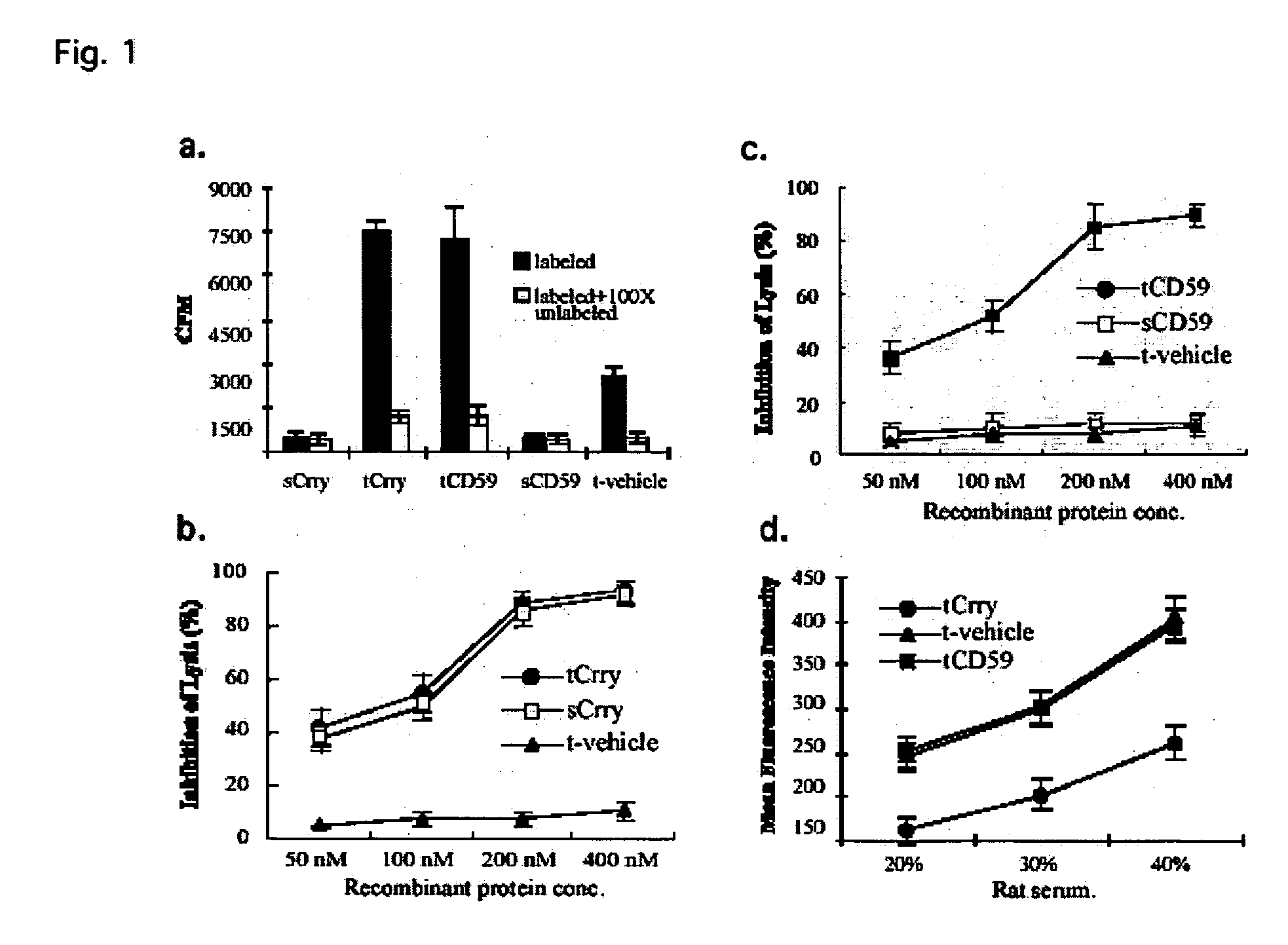
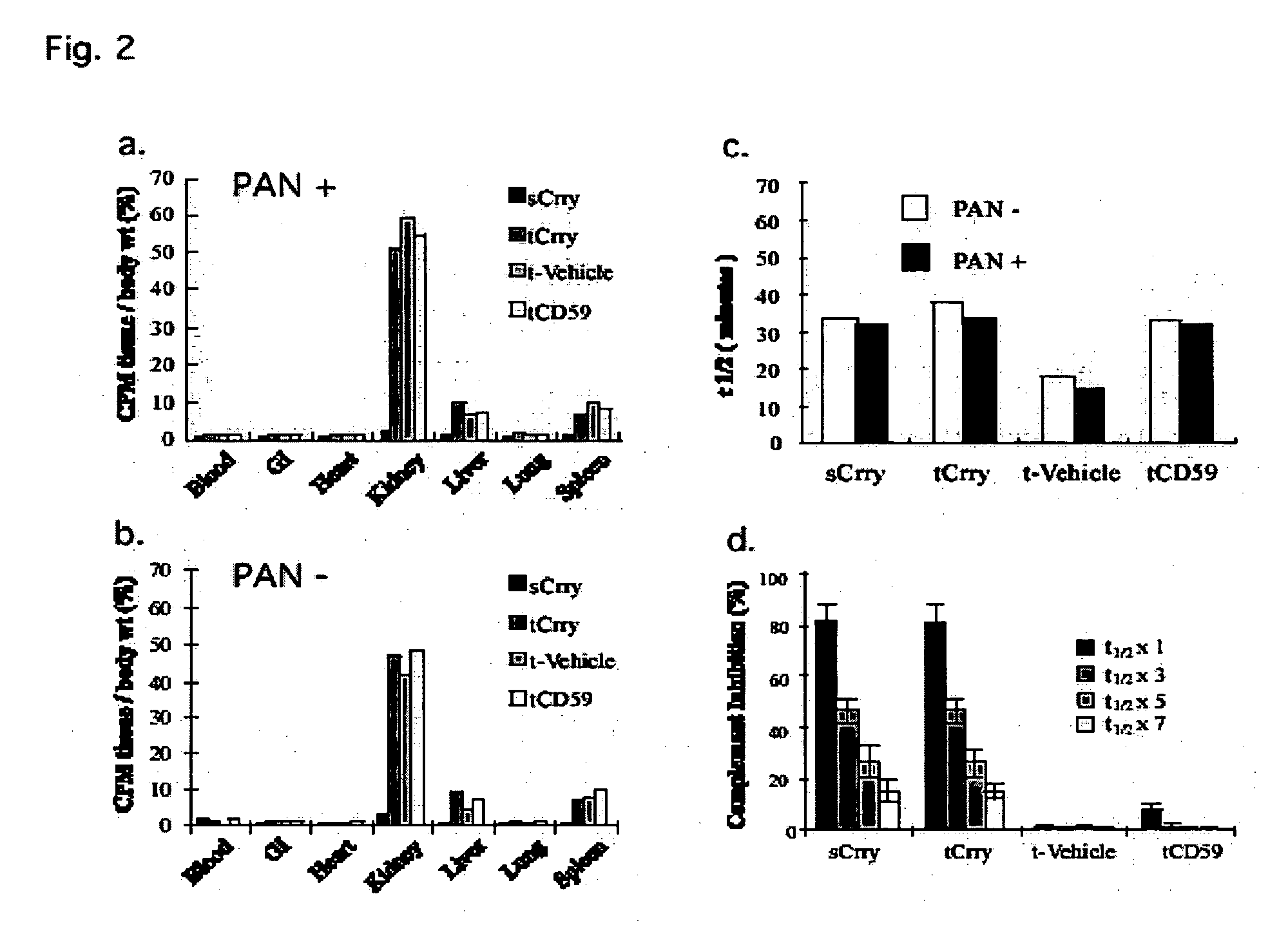
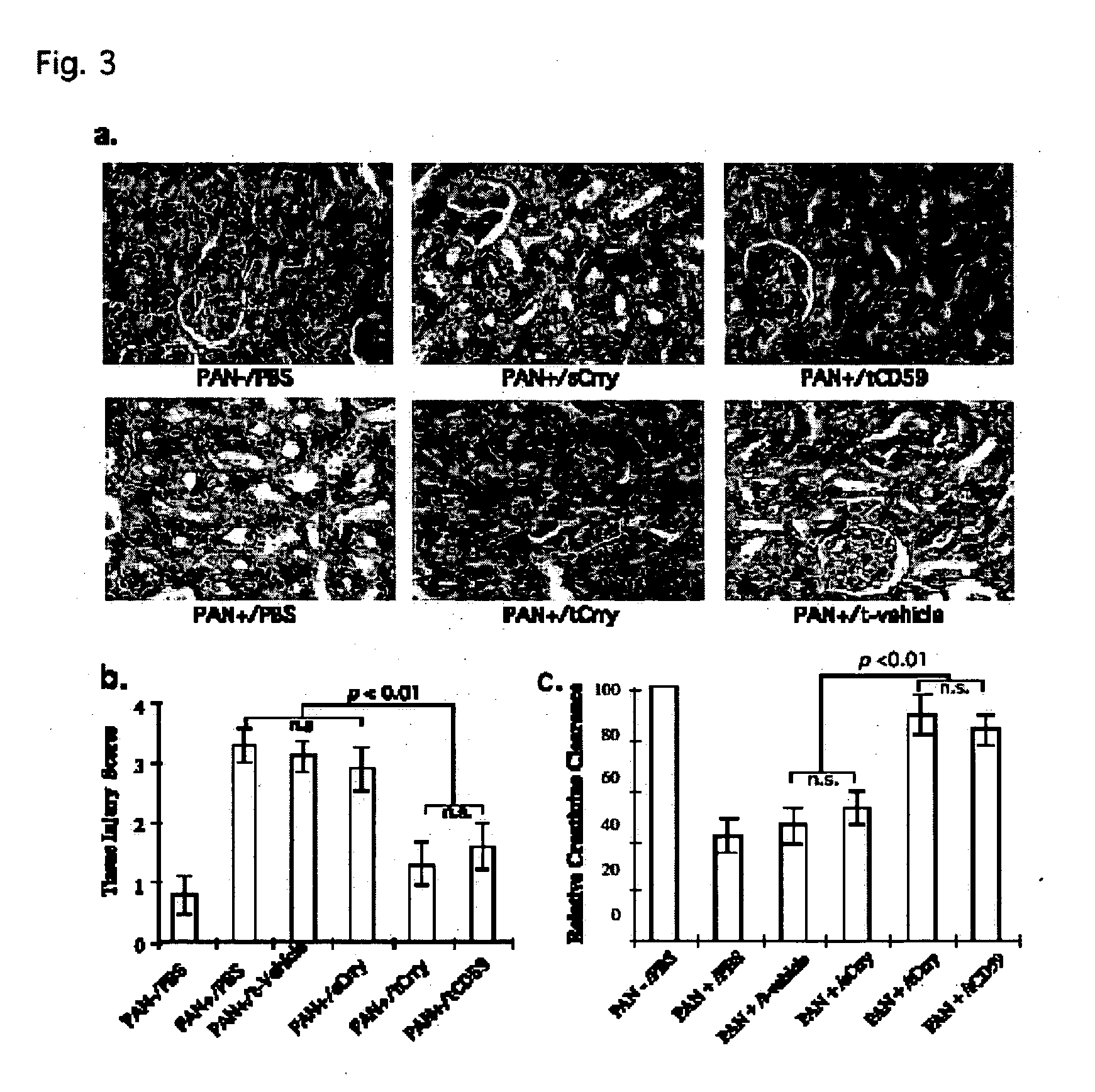
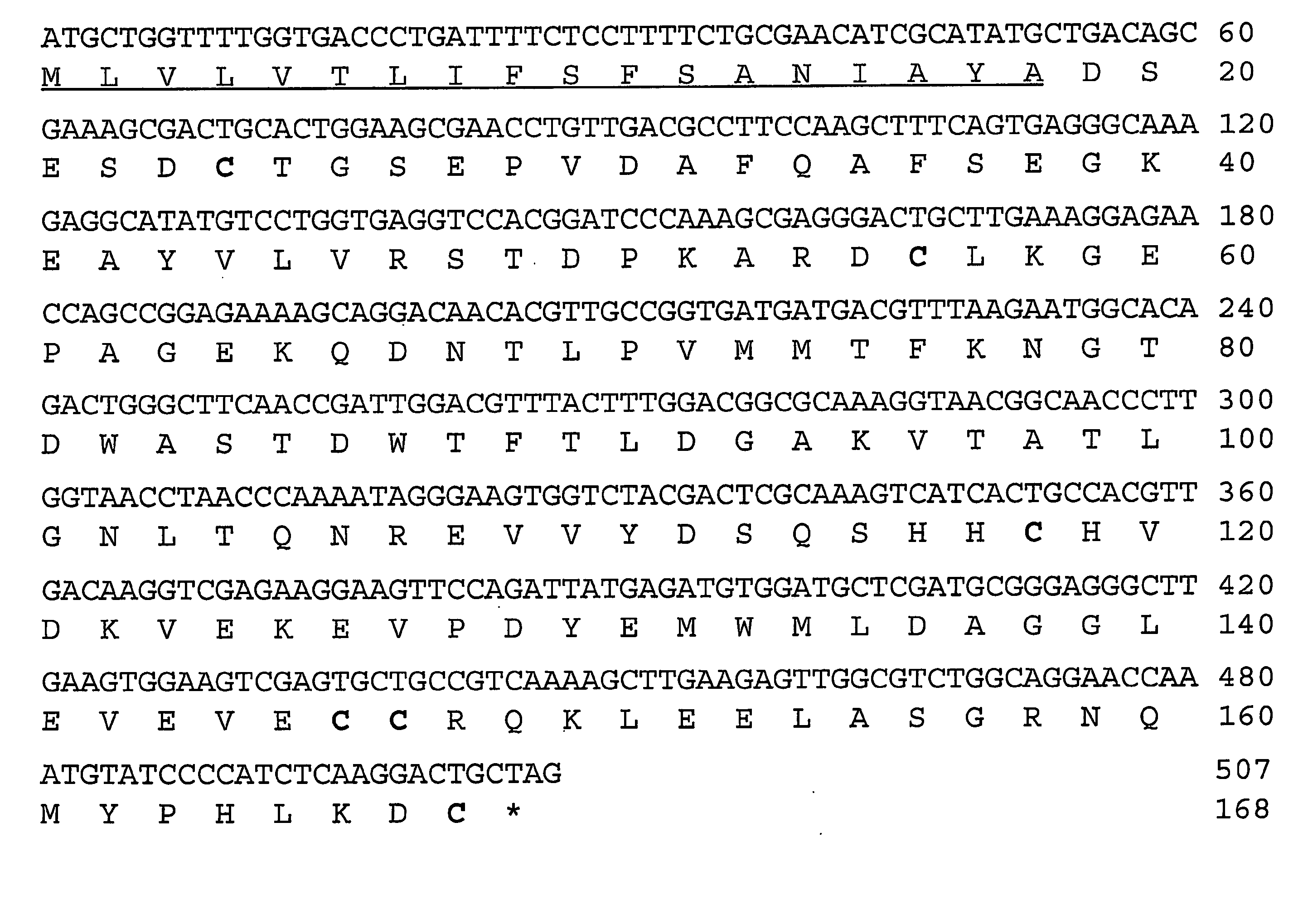
Tissue targeted complement modulators
InactiveUS20050265995A1Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsEpitheliumWhole body
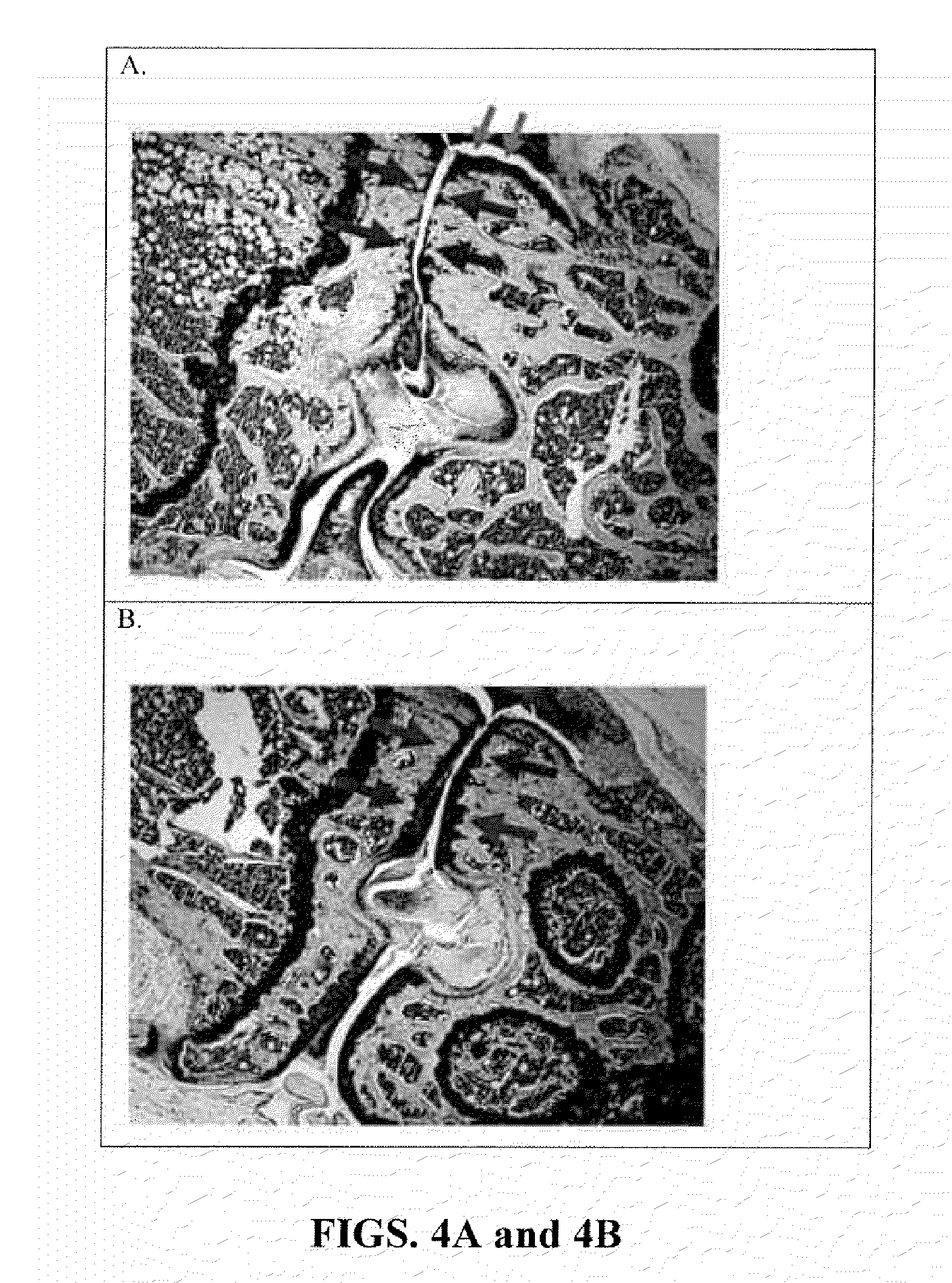
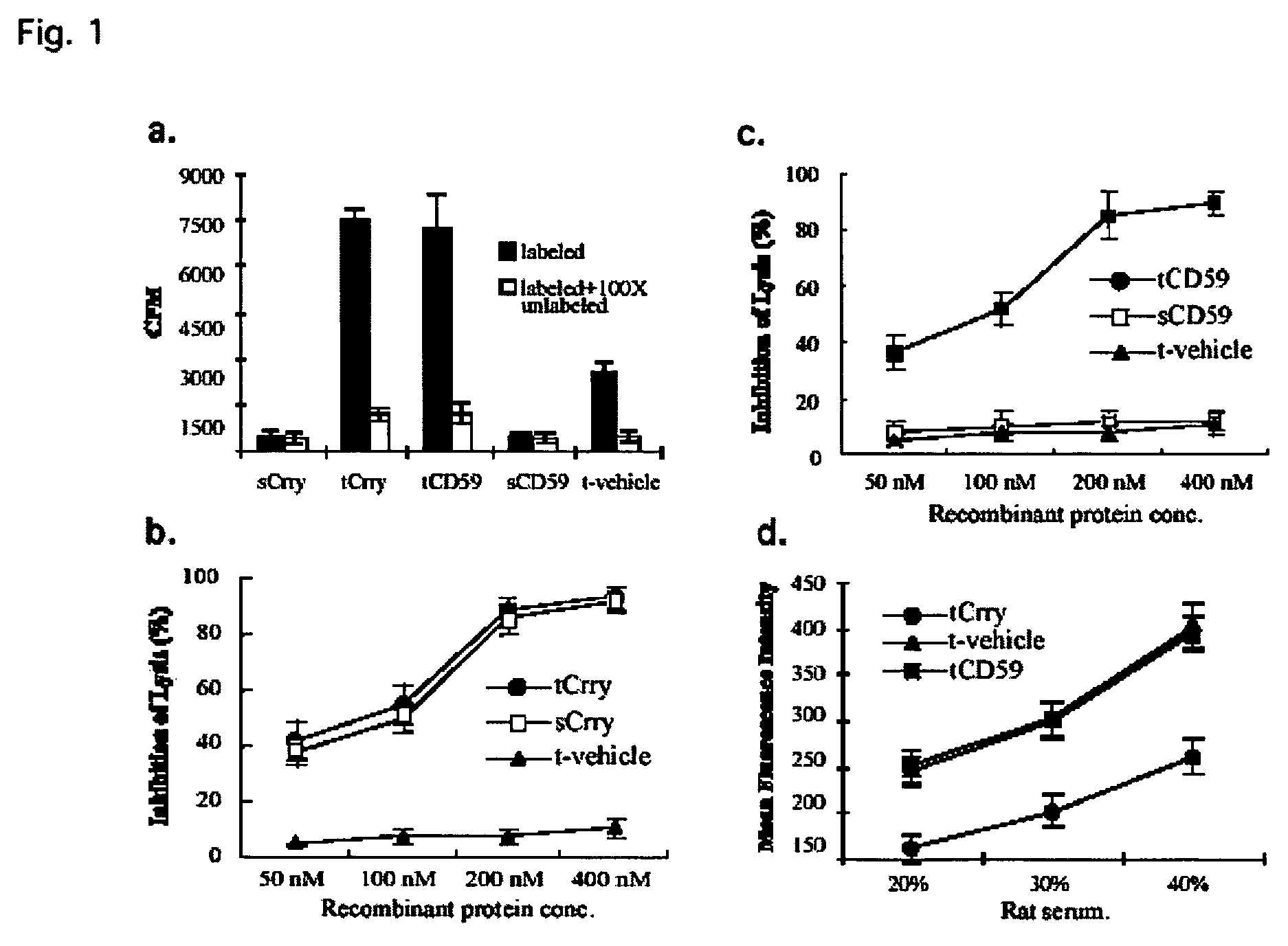
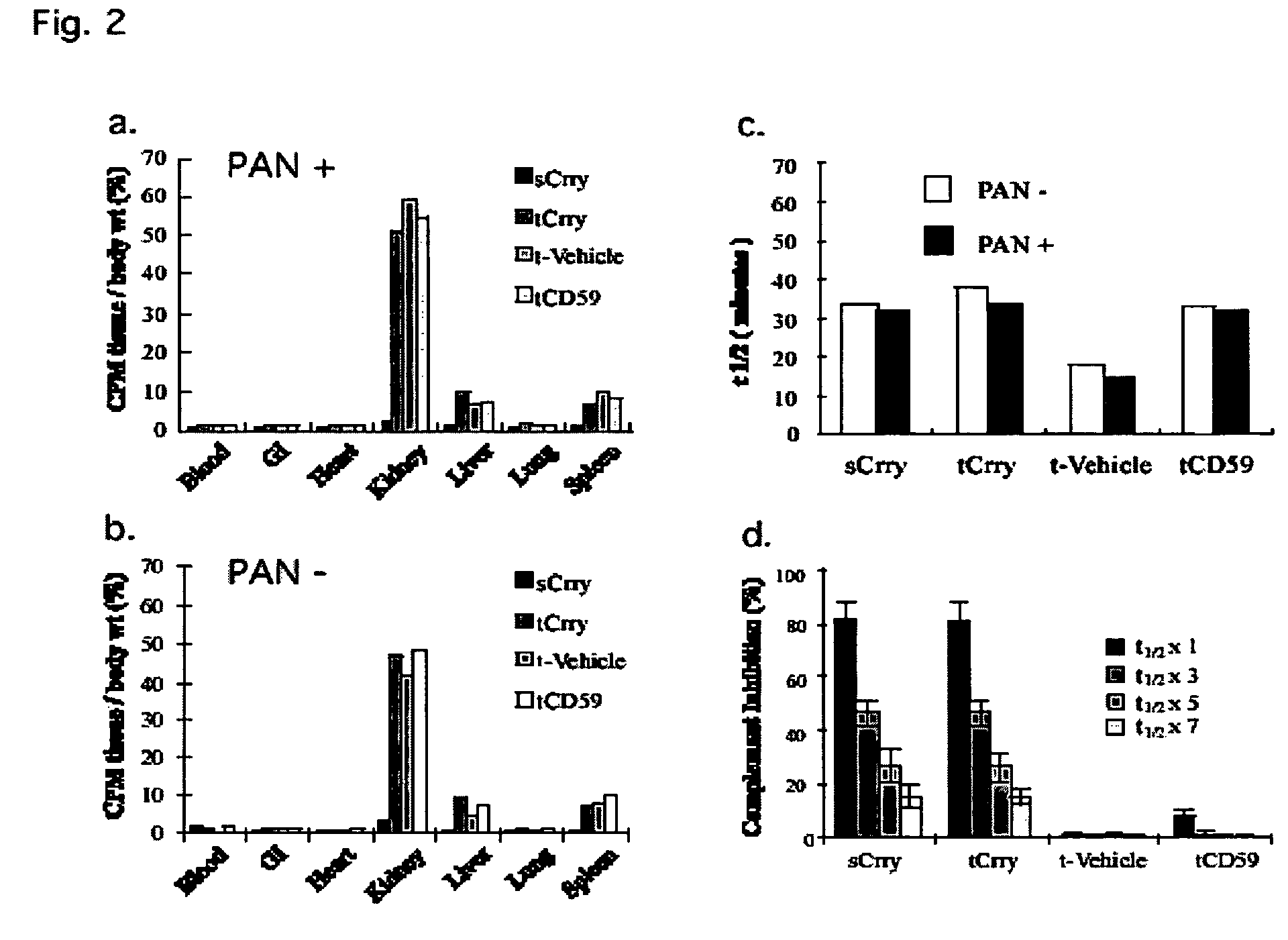
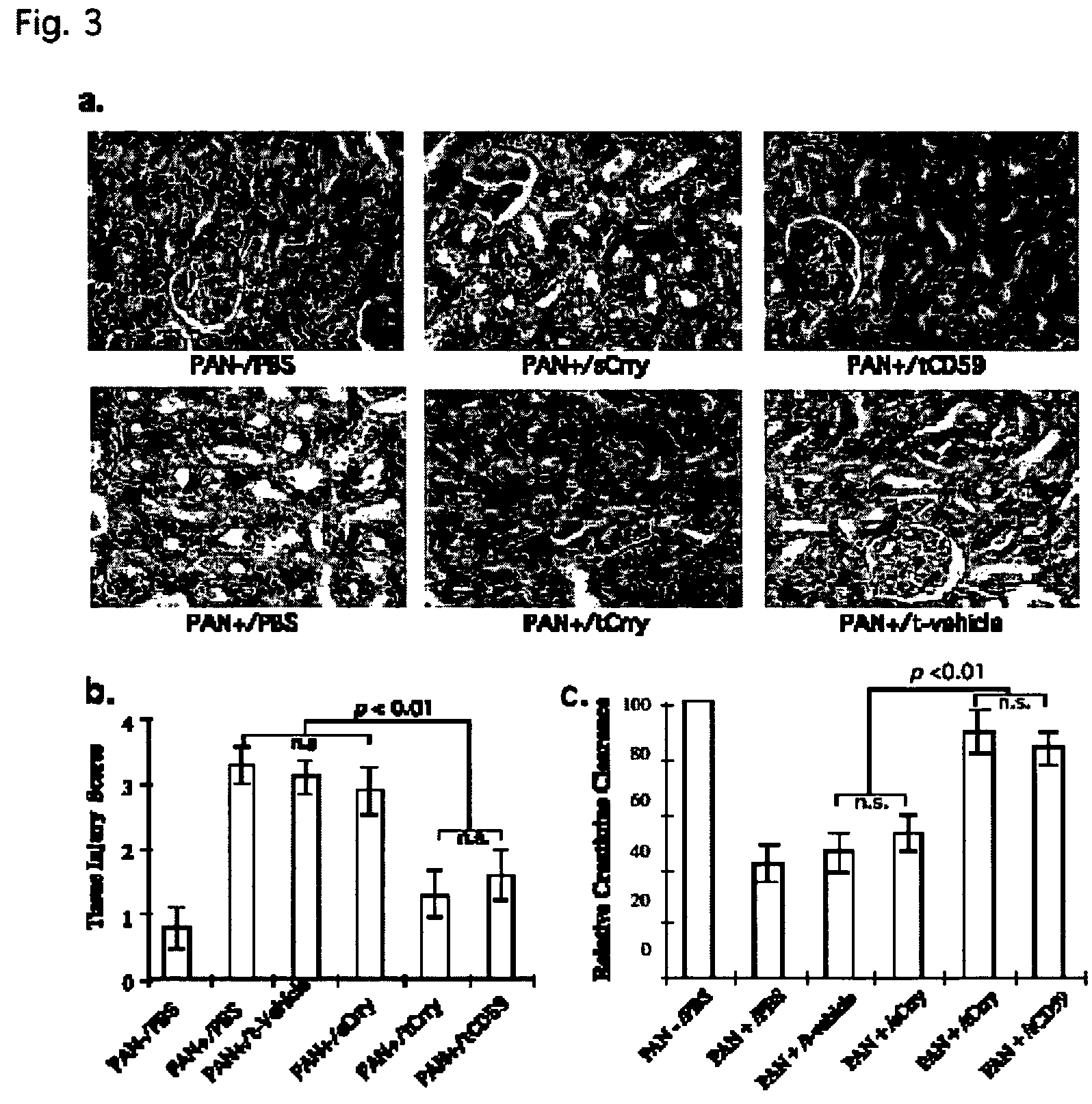
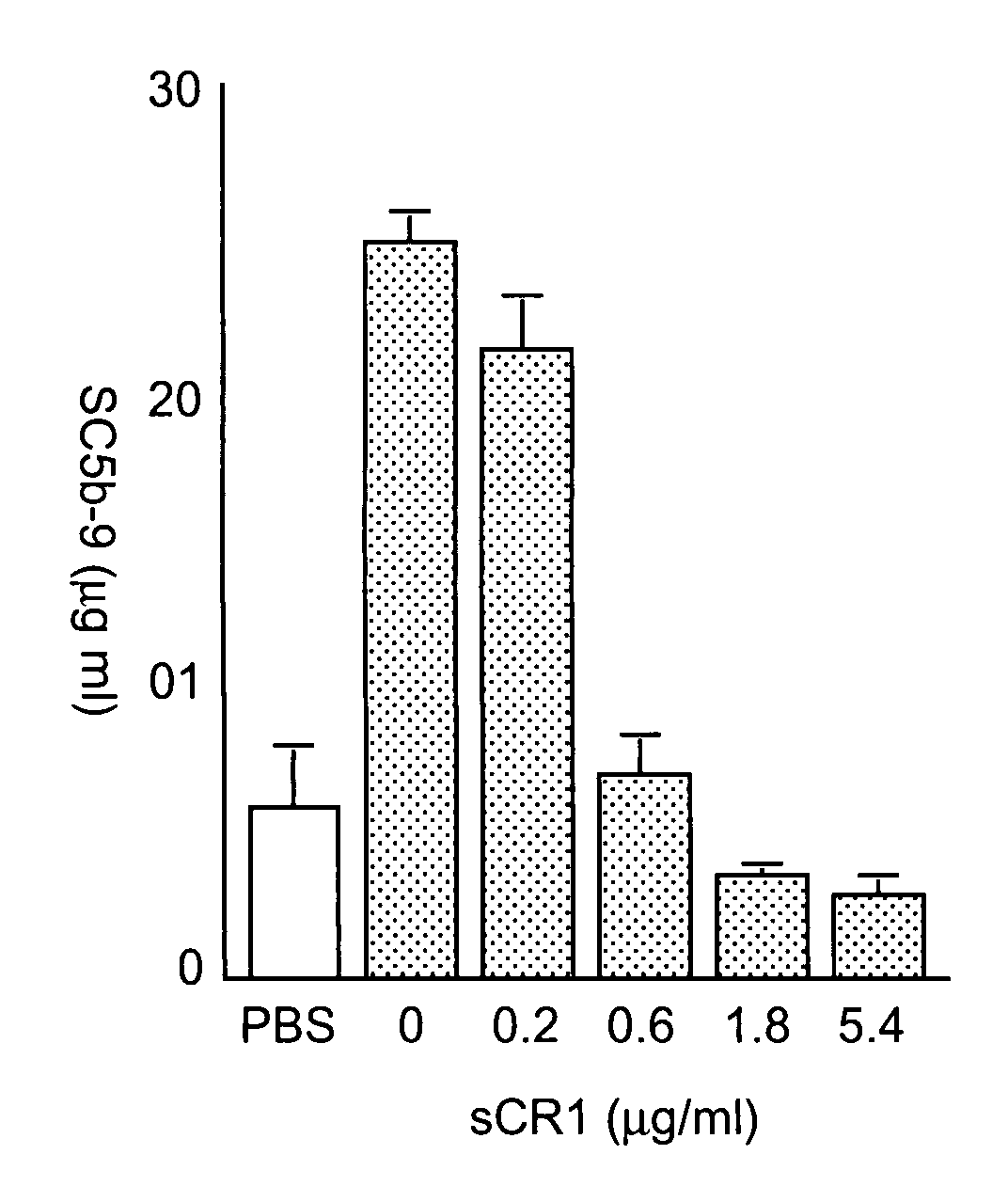
Systemic suppression of the complement system has been shown to be effective to treat inflammatory disease, yet at the potential cost of compromising host defense and immune homeostasis. Herein disclosed are methods for antigen-specific targeting of complement inhibitors that show that complement inhibitors targeted to the proximal tubular epithelium protect against tubulointerstitial injury and renal dysfunction in a rat model of nephrosis. It is shown that appropriate targeting of a systemically administered complement inhibitor to a site of disease markedy enhances efficacy and obviates the need to systemically inhibit complement. Additionally, it is shown by specifically inhibiting the terminal pathway of complement, that the membrane attack complex (MAC) plays a key role in proteinuria-induced tubulointerstitial injury, thus establishing the MAC as a valid target for pharmacological intervention in proteinuric disorders. The disclosed are compositions can be used in methods of treating pathogenic diseases and inflammatory conditions by modulating the complement system.
Owner:UNIVERSITY OF CHICAGO +1
Methods and compositions for the treatment of meconium aspiration syndrome
InactiveUS20070065433A1Reduce morbidityReduce mortalityImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntiendomysial antibodiesComplement S-Protein
A method for preventing or treating meconium aspiration syndrome (“MAS”) by administering a meconium aspiration syndrome preventing or treating amount of one or more complement inhibitors to a patient likely to develop or suffering from MAS. The complement inhibitors are preferably antibodies that bind to and inhibit complement proteins involved in the formation of the membrane attach complex, preferably anti-Factor D or anti-C5 antibodies. The complement inhibitors can be used alone or in combination with other MAS therapies to decrease the morbidity and mortality caused by MAS.
Owner:MOLLNES TOM EIRIK +1
C5 Antigens and Uses Thereof
InactiveUS20100166748A1Shorten the progressInhibits function of proteinSenses disorderPeptide/protein ingredientsAntigenEye disease
The present invention pertains to the use of a complement inhibitor in methods of treatment of ocular disorders and the use of a complement inhibitor in the manufacture of a medicament in the treatment of an ocular disorder.
Owner:NOVARTIS AG
Compstatin analogs for treatment of rhinosinusitis and nasal polyposis
In some aspects, the present invention provides methods treating a subject in need of treatment for chronic rhinosinusitis or nasal polyposis, the methods comprising administering a complement inhibitor such as a compstatin analog to the subject. In some embodiments, the complement inhibitor is administered intranasally, e.g., in a nasal spray.
Owner:APELLIS PHARMA
Methods and Compositions for the Prevention and Treatment of Sepsis
The present invention includes compositions comprising one or more complement inhibitors and one or more CD14 pathway inhibitors for the prevention or treatment of sepsis. The complement inhibitors may be antibodies that bind to and inhibit complement proteins such as C5a and the CD14 pathway inhibitors may be antibodies that bind to and inhibit CD14 pathway components, such as CD14 and LPS. The invention also relates to methods of treating subjects suffering from sepsis comprising administering these compositions, as well as kits for supplying the compositions for treatment.
Owner:GENENTECH INC
Compositions and methods for treatment of trauma
InactiveUS20110092446A1Increased riskReduce the possibilityMicrobiological testing/measurementImmunoglobulinsGenotypeIncreased risk
The present invention features the use of a complement inhibitor, e.g., a compstatin analog for treating an individual who has suffered a severe injury. In some embodiments, the complement inhibitor may be administered within 24 hours following the injury and optionally also at later time points. The complement inhibitor may, for example, be administered prior to transporting the patient to a health care facility, during transport of the patient to a health care facility, or in the emergency department. Further provided are methods of selecting individuals for such therapy. Further provided are methods of identifying individuals at increased risk of poor outcome following trauma. In certain embodiments the methods comprise determining whether the genotype of the patient includes an allele of a polymorphism in or near a complement-related gene, wherein said allele is associated with risk of poor outcome following trauma.
Owner:APELLIS PHARMA +1
Complement inhibitors
ActiveUS20070141573A1Increase deposition rateConvenient treatmentAntibacterial agentsPeptide/protein ingredientsDiseaseMedicine
The invention relates to complement inhibitors that inhibit both the classical and alternative complement pathways. In particular, the invention relates to complement inhibitors derived from the salivary glands of haematophagous arthropods that inhibit both the classical and alternative complement pathways. The invention also relates to the use of such complement inhibitors in the treatment and prevention of diseases.
Owner:VOLUTION IMMUNO PHARMA
Complement inhibitory agents as therapeutics in posttraumatic and degenerative arthritis
InactiveUS20090324585A1Peptide/protein ingredientsImmunoglobulins against animals/humansMedicineDegenerative arthropathy
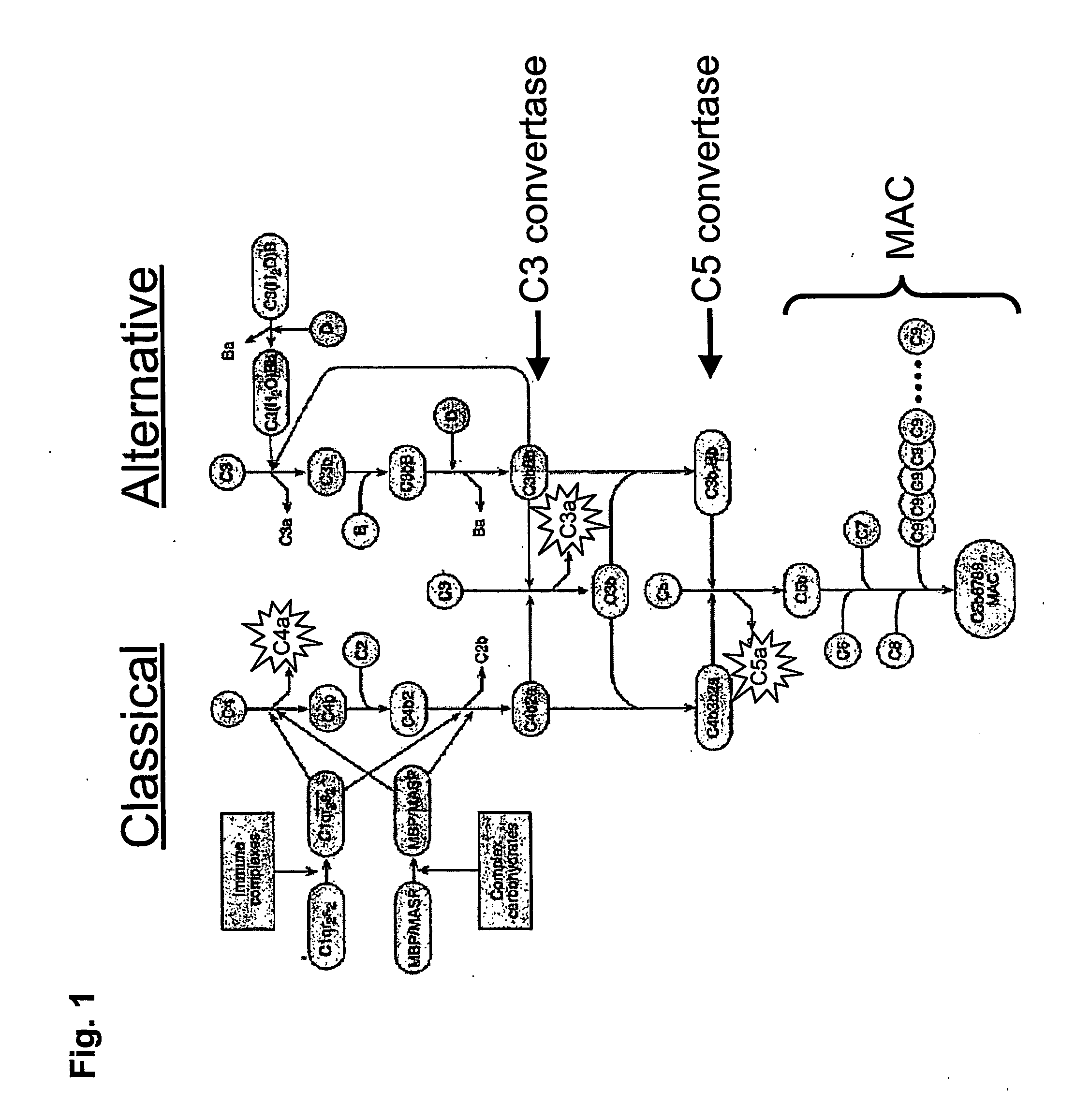
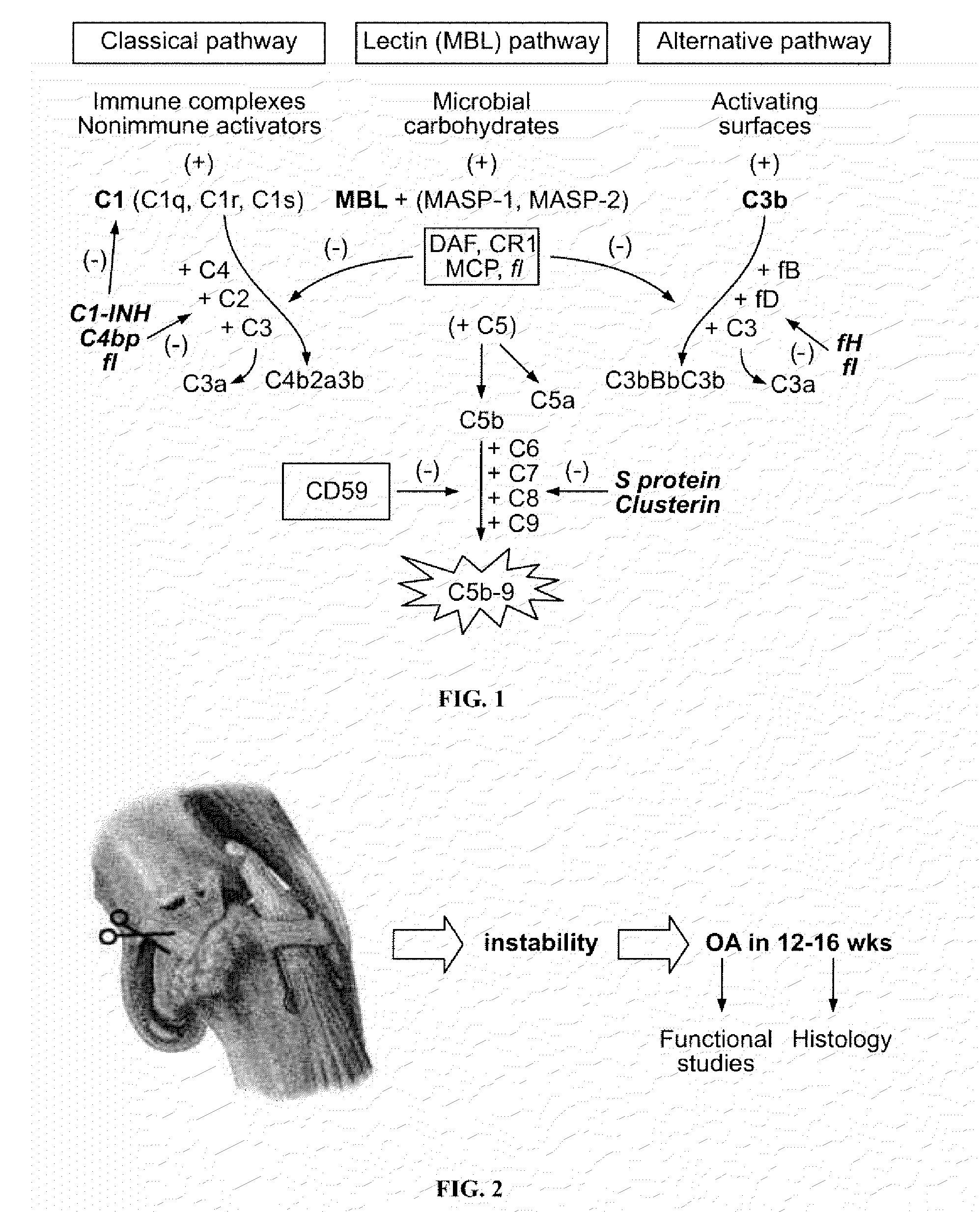
The present disclosure is directed to methods and compositions for treating osteoarthritis and preventing osteoarthritis, by administering a compound that modulates one or more components in the complement system. In one embodiment, a compound that inhibits a component in the complement system is administered to prevent, delay the progression of, or treat osteoarthritis. In other embodiments, compounds with specific inhibition of a component in a particular pathway in the complement system, such as the alternative, mannose binding lectin, and / or classical pathway, are administered to a subject having osteoarthritis or at risk of developing osteoarthritis.
Owner:UNIV OF COLORADO THE REGENTS OF +2
Tissue targeted complement modulators
InactiveUS8454963B2Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsTissue targetingEpithelium
Systemic suppression of the complement system has been shown to be effective to treat inflammatory disease, yet at the potential cost of compromising host defense and immune homeostasis. Herein disclosed are methods for antigen-specific targeting of complement inhibitors that show that complement inhibitors targeted to the proximal tubular epithelium protect against tubulointerstitial injury and renal dysfunction in a rat model of nephrosis. It is shown that appropriate targeting of a systemically administered complement inhibitor to a site of disease markedy enhances efficacy and obviates the need to systemically inhibit complement. Additionally, it is shown by specifically inhibiting the terminal pathway of complement, that the membrane attack complex (MAC) plays a key role in proteinuria-induced tubulointerstitial injury, thus establishing the MAC as a valid target for pharmacological intervention in proteinuric disorders. The disclosed are compositions can be used in methods of treating pathogenic diseases and inflammatory conditions by modulating the complement system.
Owner:UNIVERSITY OF CHICAGO +1
Complement inhibitors as therapeutic agents for treatment of cancer
ActiveUS20110044983A1Preventing and reducing and delaying growth of tumorOrganic active ingredientsPeptide/protein ingredientsTumor microenvironmentOncology
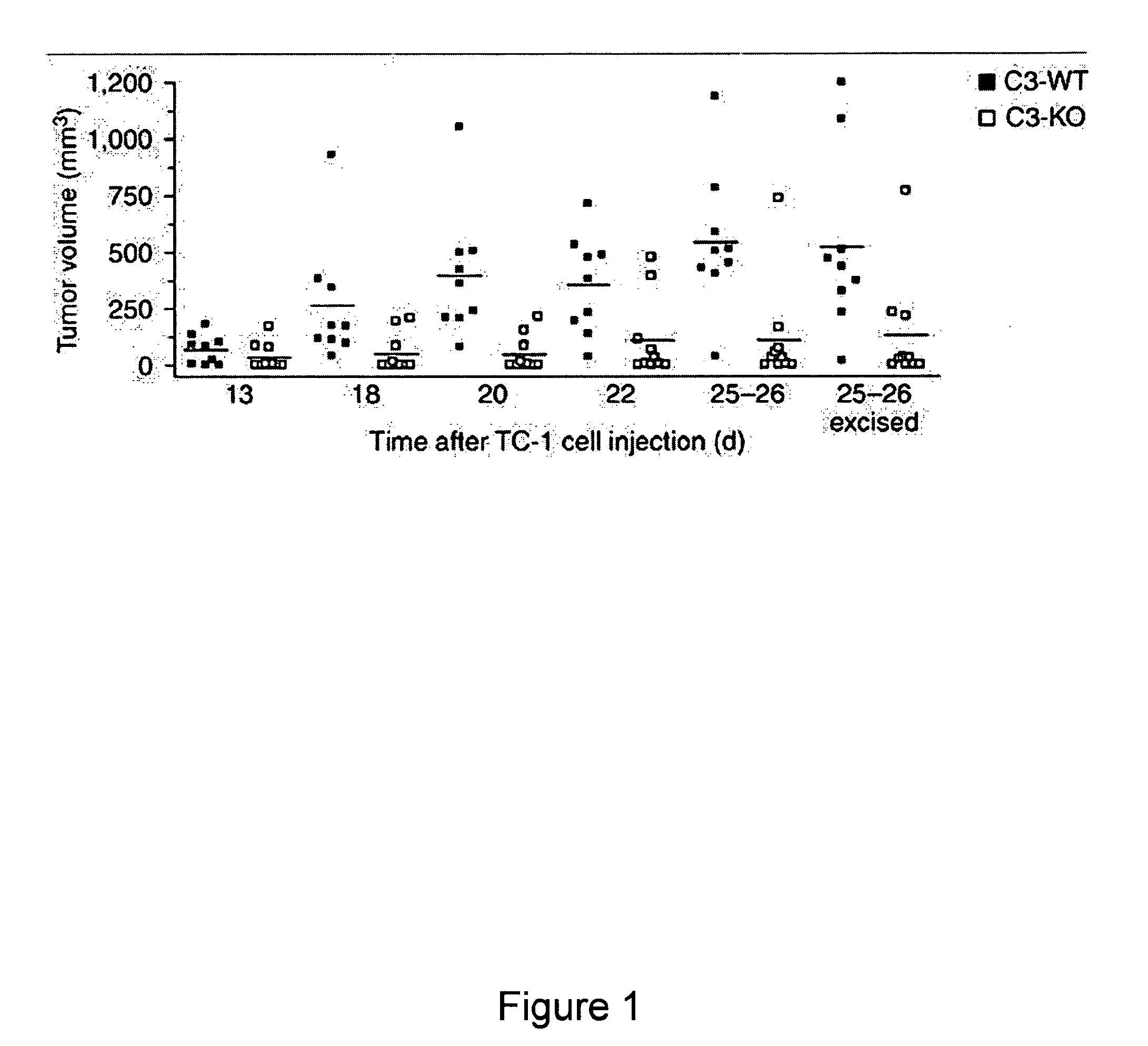
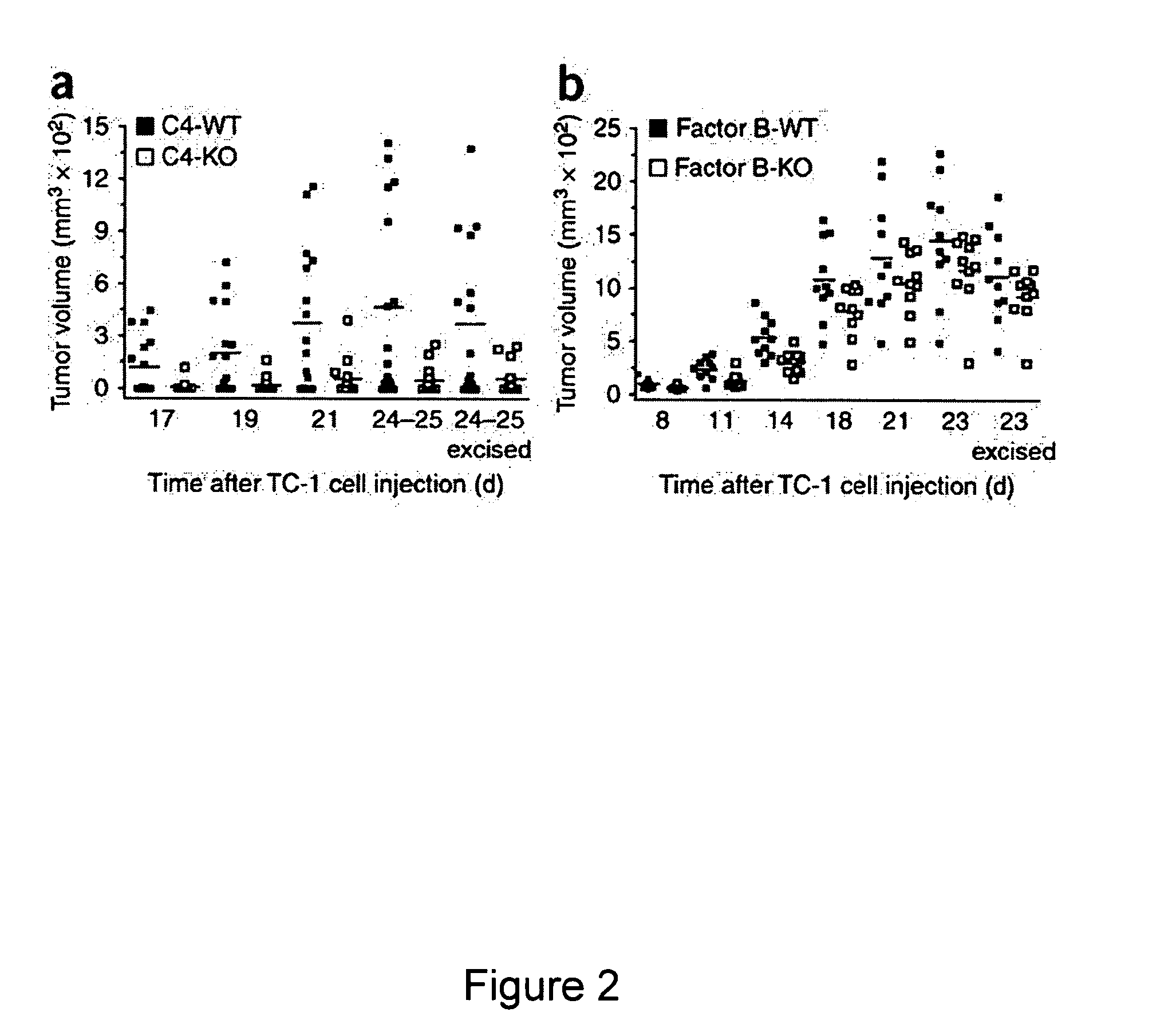
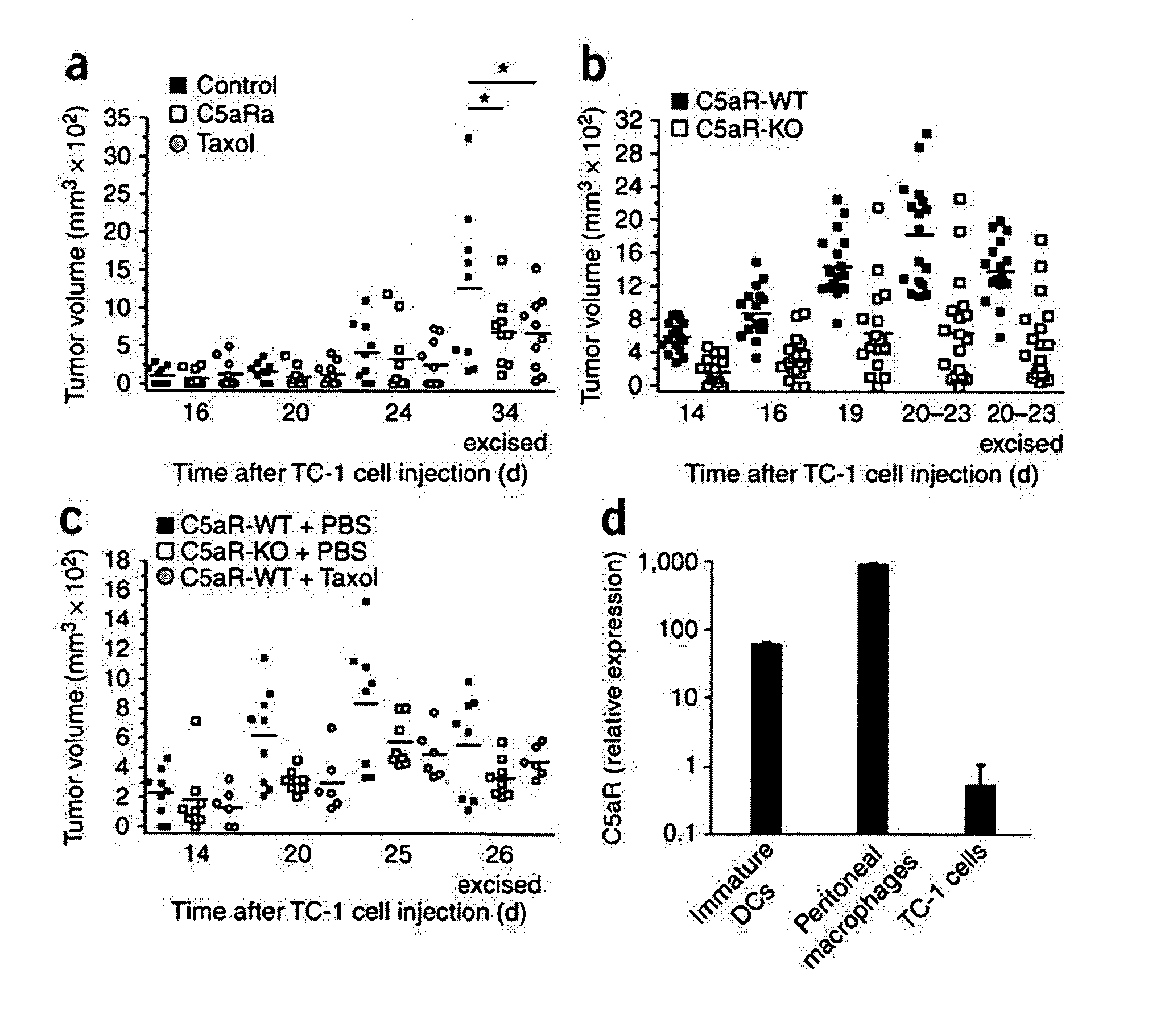
Methods for treating, preventing or delaying onset of tumor formation and other forms of cancer are disclosed. The methods involve administration of a complement inhibitor to inhibit C5a receptor signaling in the tumor microenvironment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
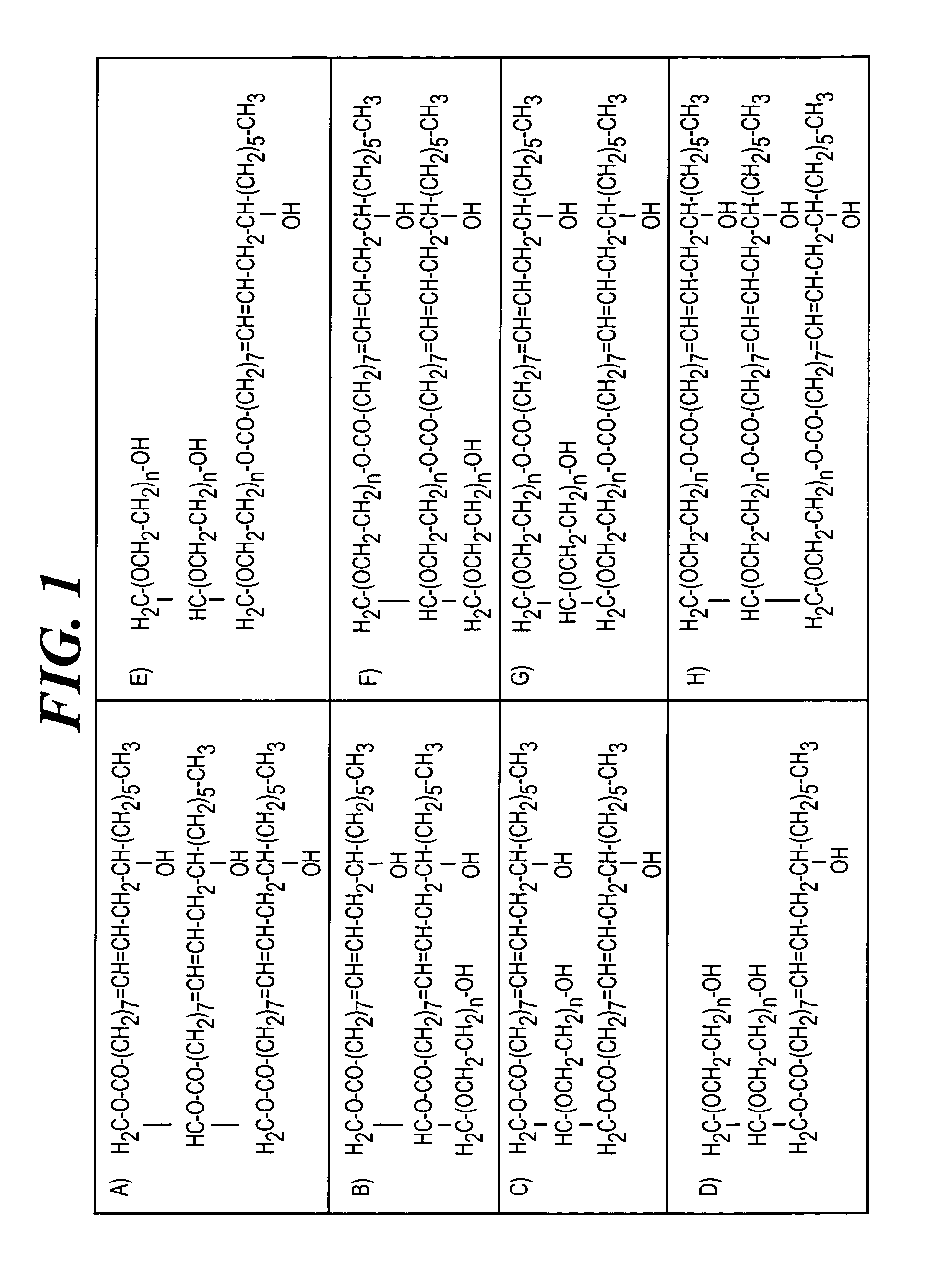
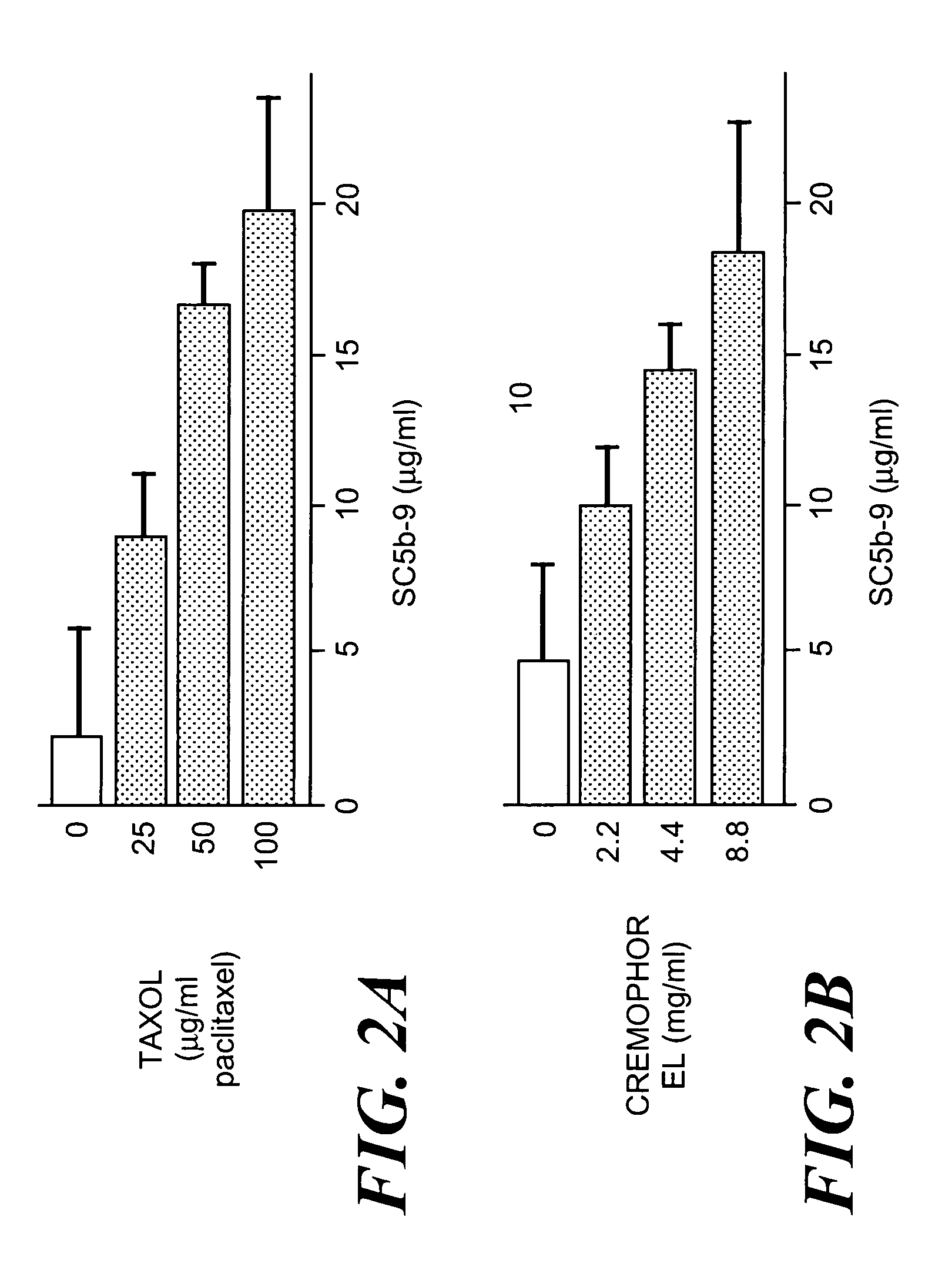
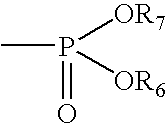
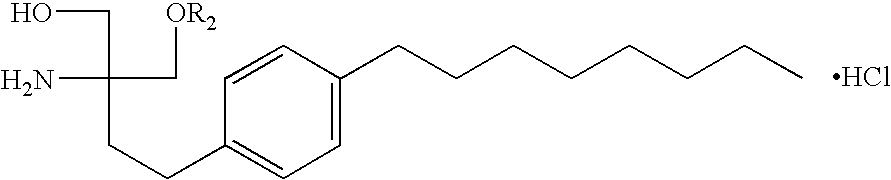
Method of inhibiting side effects of pharmaceutical compositions containing amphiphilic vehicles or drug carrier molecules
A method is provided for inhibiting or preventing toxicity and other unwanted effects (a) caused by solvents for pharmaceuticals which solvents or emulsifier which contain amphiphilic molecules such as polyethoxylated oils or a derivative thereof, or (b) caused by a drug in a vehicle containing amphiphilic molecules such as phopholipids or derivative thereof, emptying a complement inhibitor. Drug compositions containing amphiphilic molecules, or derivatives thereof and a complement inhibitor, and pharmaceutical compositions including a drug, solvent or carrier containing amphiphilic molecules or derivatives thereof, and a complement inhibitor are also provided.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Immunosuppressive combination and its use in the treatment or prophylaxis or insulin-producing cell graft rejection
InactiveUS20060153842A1Effective treatmentExtended insulin independenceBiocidePeptide/protein ingredientsCellular graft rejectionFree form
A pharmaceutical combination comprising an accelerated lymphocyte homing agent in free form or in pharmaceutically acceptable salt form, and one or more compounds selected from the group consisting of an antibody to the IL-2 receptor, an immunosuppressive macrocyclic lactone and a soluble human complement inhibitor is used to treat or prevent insulin-producing cell graft rejection.
Owner:LAKE PHILIP +1
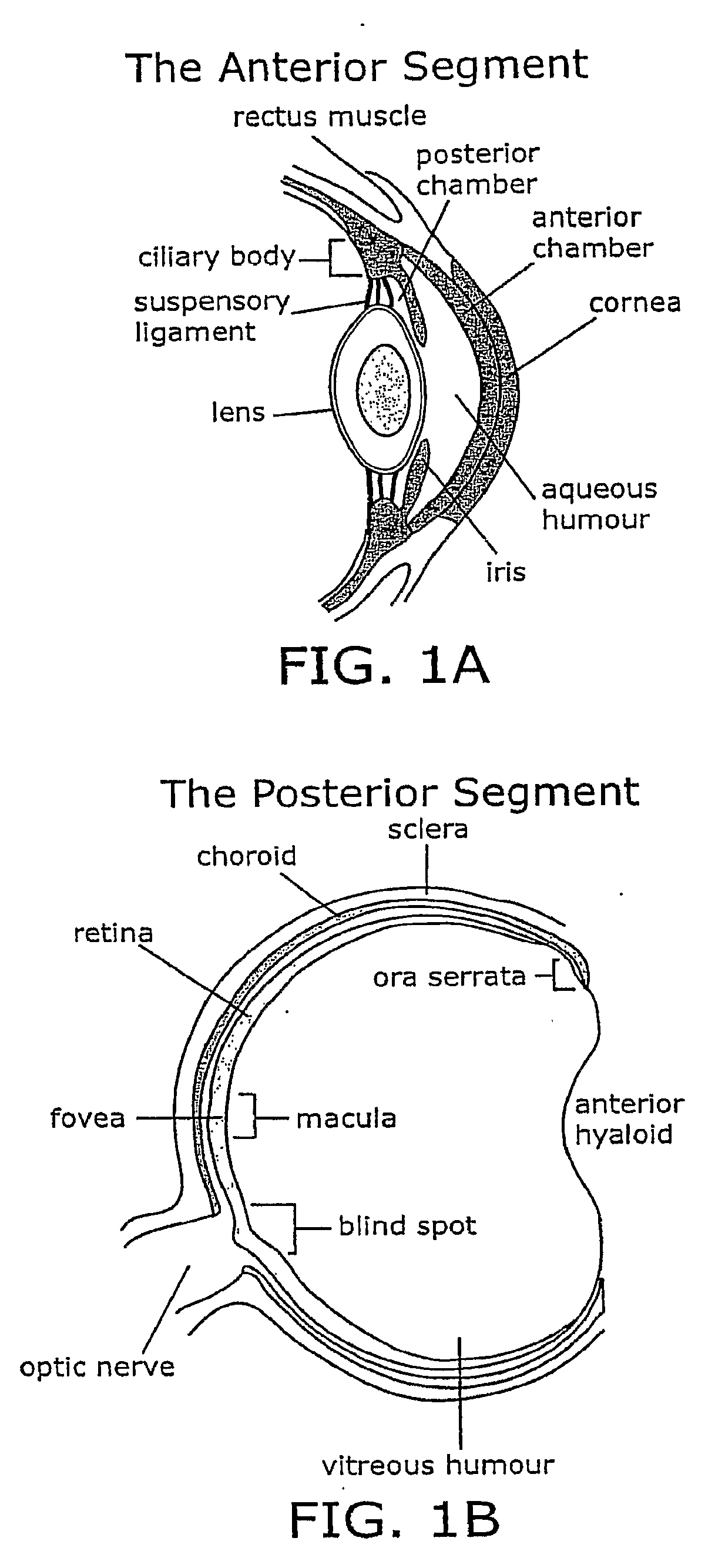
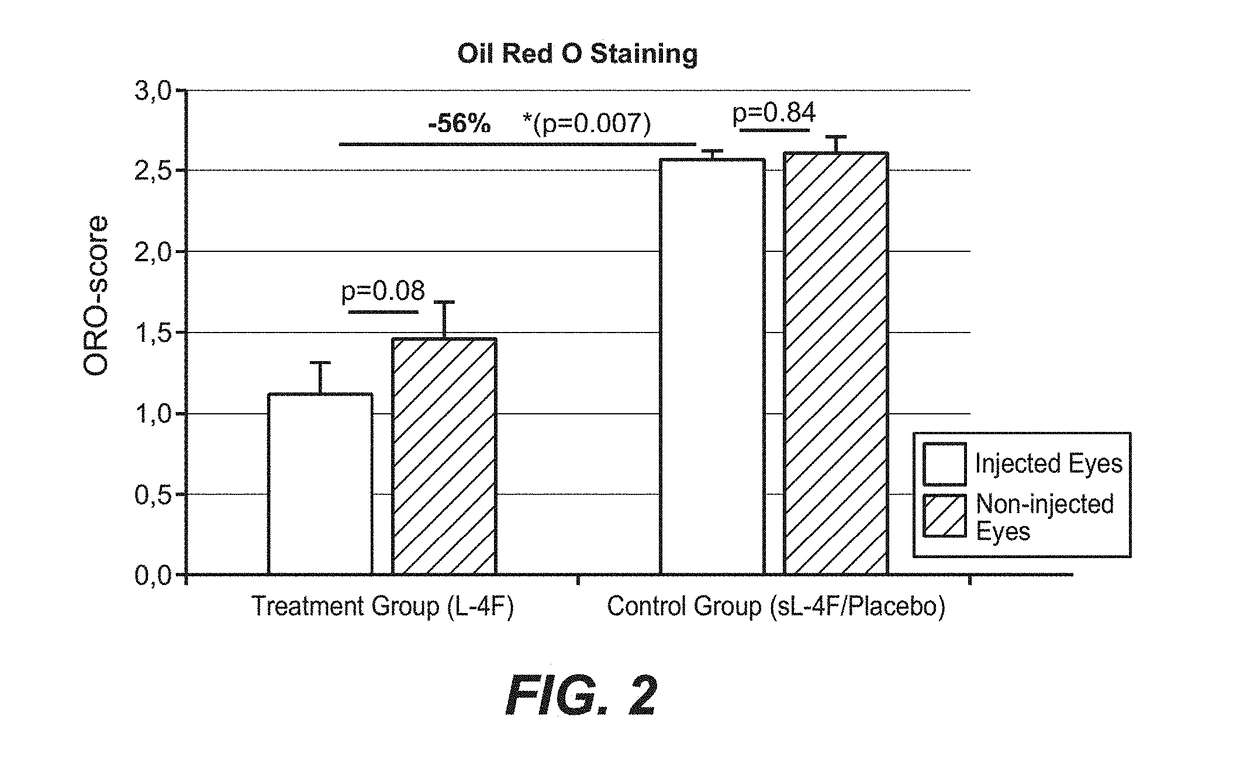
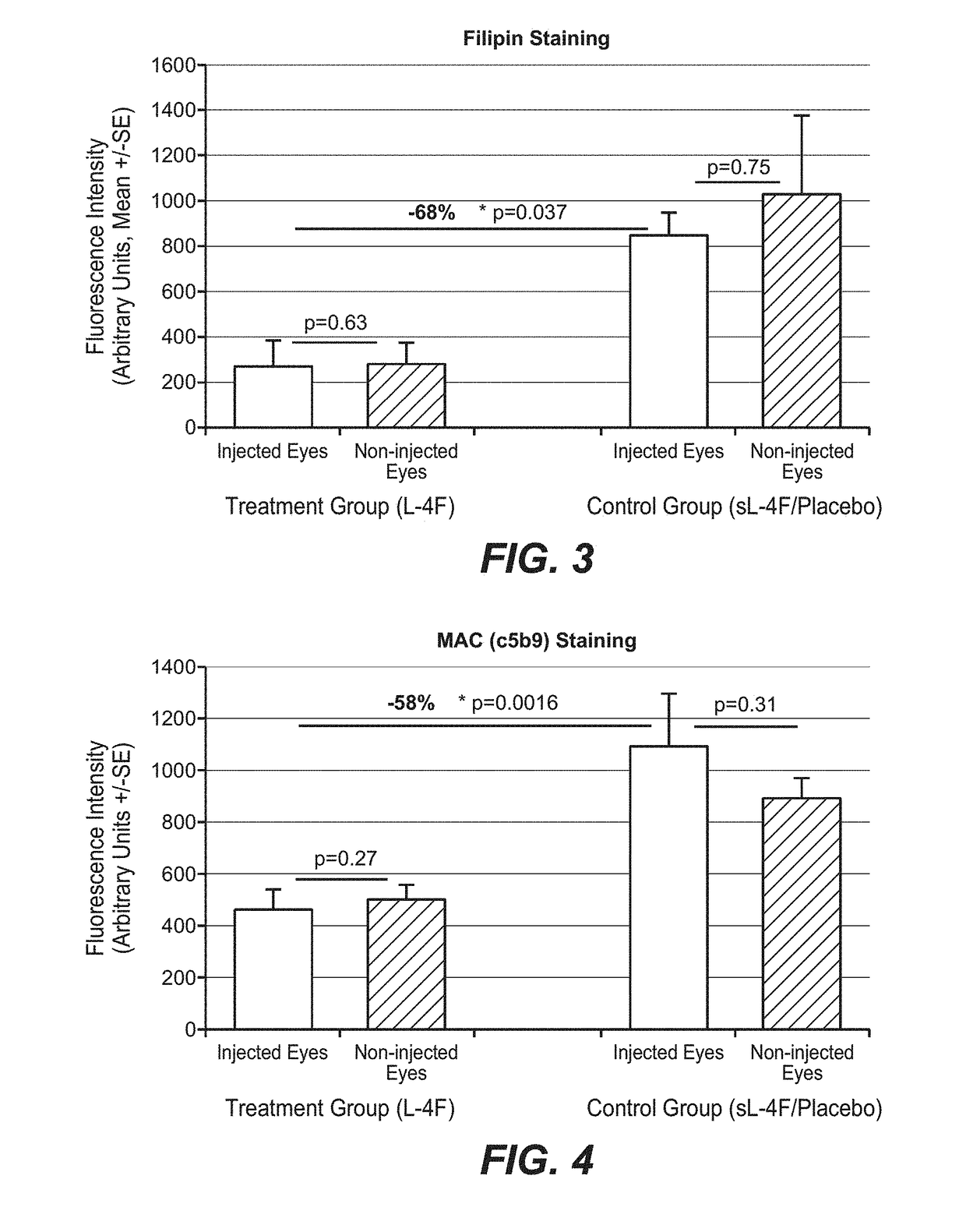
Treatment of age-related macular degeneration and other eye diseases with one or more therapeutic agents
InactiveUS20180296525A1Prevent and slow progressionSlow onsetSenses disorderPeptide/protein ingredientsDyslipidemiaAntioxidant
The present disclosure provides therapeutic agents for the treatment of age-related macular degeneration (AMD) and other eye disorders. One or more therapeutic agents can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (including non-central GA and central GA) and neovascularization (including types 1, 2 and 3 NV). In certain embodiments, an anti-dyslipidemic agent (e.g., an apolipoprotein mimetic and / or a statin) is used alone to treat or slow the progression of atrophic AMD (including early AMD and intermediate AMD), and / or to prevent or delay the onset of AMD, advanced AMD and / or neovascular AMD. In further embodiments, two or more therapeutic agents (e.g., any combinations of an anti-dyslipidemic agent, an antioxidant, an anti-inflammatory agent, a complement inhibitor, a neuroprotector and an anti-angiogenic agent) that target multiple underlying factors of AMD (e.g., formation of lipid-rich deposits, oxidative stress, local inflammation, cell death and neovascularization) are used to treat or slow the progression of atrophic AMD (including non-central GA and central GA) or neovascular AMD (including types 1, 2 and 3 NV), and / or to prevent or delay the onset of AMD, advanced AMD and / or neovascular AMD.
Owner:MACREGEN INC
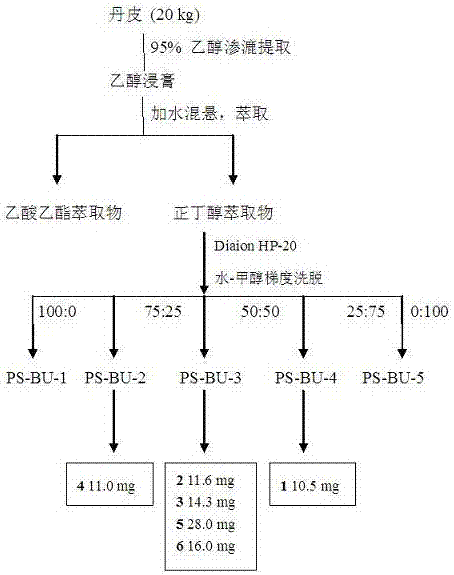
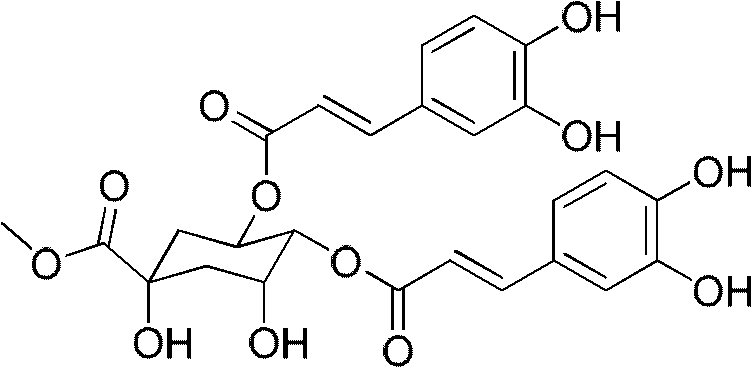
Application of phenolic glycoside compounds in anticomplement medicine preparation
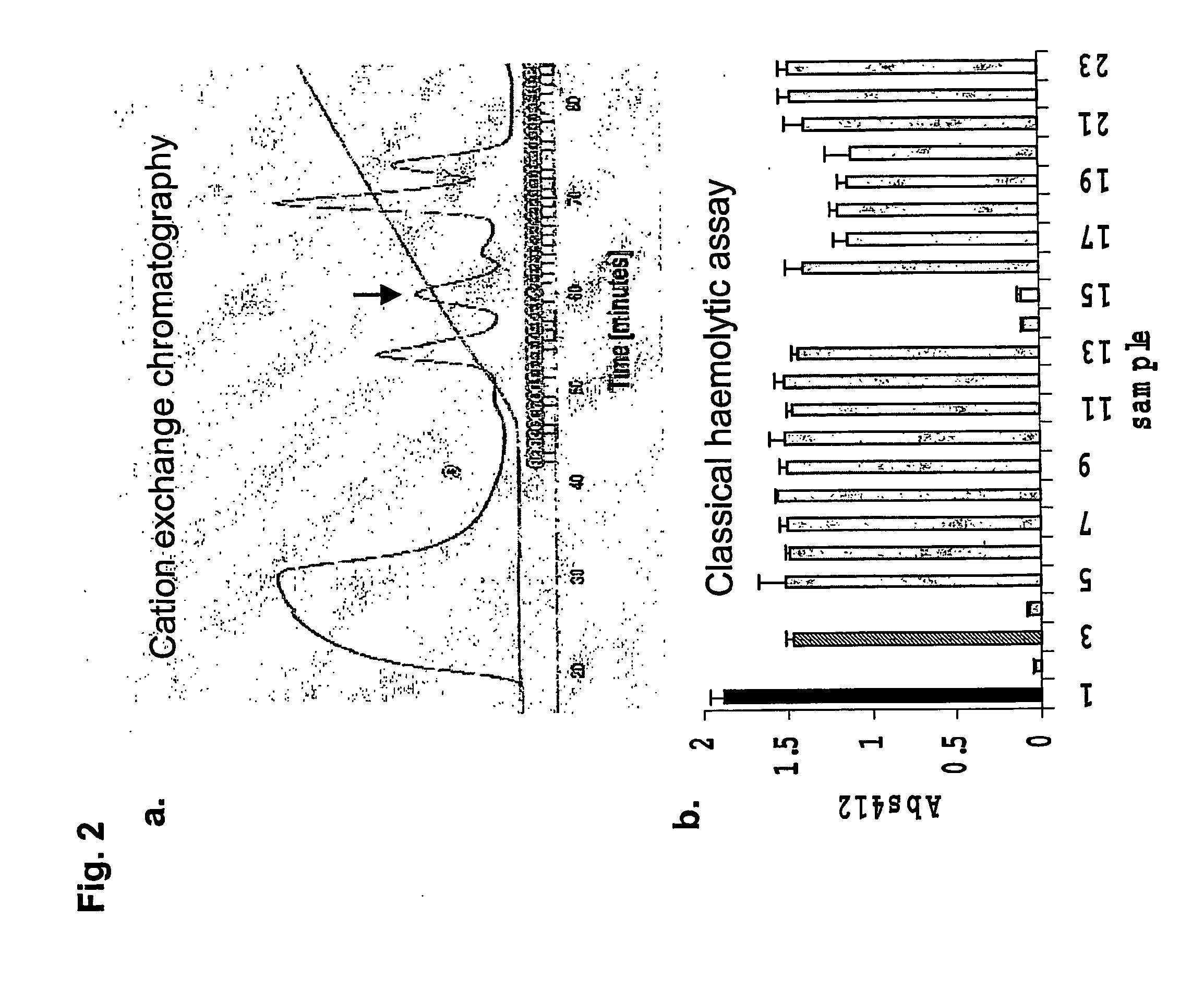
The invention belongs to the field of manufacturing of traditional Chinese medicines, and relates to phenolic glycoside compounds in Paeonia suffruticosa Andr. velamen and novel application of the compounds in anticomplement medicine preparation. According to the invention, a modern pharmacological research method is adopted, dried velamen anticomplement active materials of Paeonia suffruticosa Andr. belonging to Paeonia L. are researched, six phenolic glycoside compounds are separated at an n-butyl alcohol extraction position of a 95% ethanol extract, and the phenolic glycoside compounds are verified to have inhibiting effects on a complement system classical pathway and an alternative pathway. The inhibiting effect CH<50> of the compounds on the complement system classical pathway is 0.062-0.364 mg / mL, and the inhibiting effect AP<50> on the alternative pathway is 0.102-0.566 mg / mL. The compounds can be used to prepare complement inhibitors.
Owner:FUDAN UNIV
Application of caffeoylquinic acid and its derivatives in the preparation of anti-complement drugs
InactiveCN102266318ASignificant anticomplement activityLow effective concentrationOrganic active ingredientsImmunological disordersDiseaseCaffeoylquinic acid
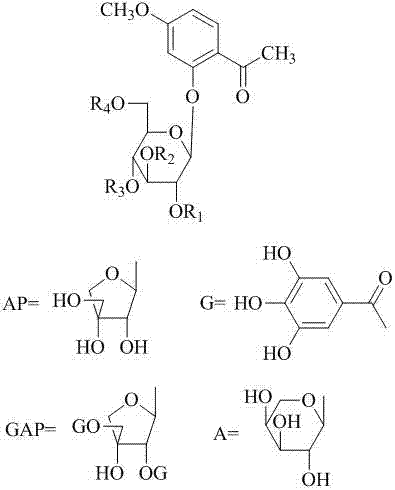
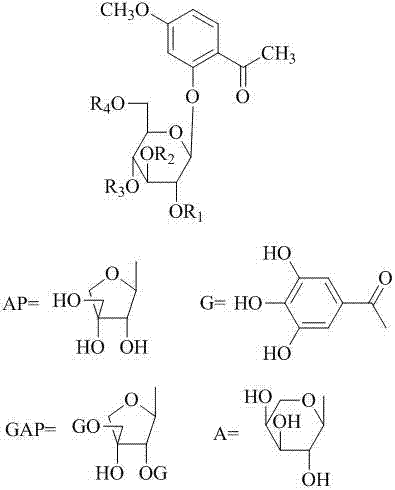
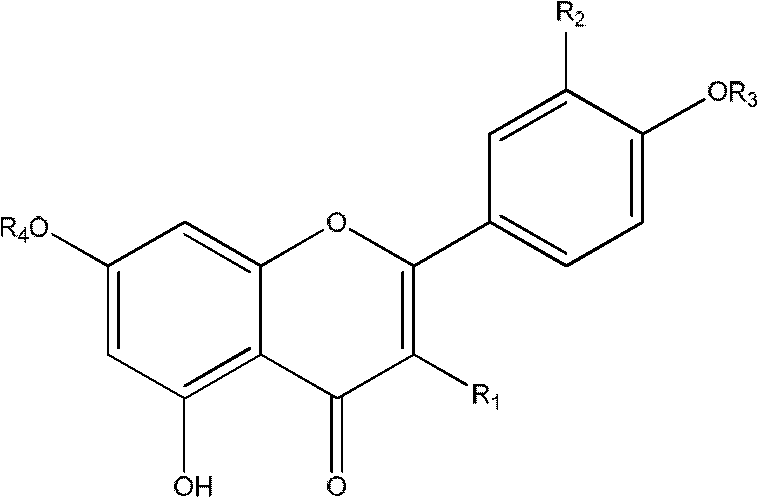
The invention provides the application of caffeoylquinic acid and its derivatives in the preparation of anti-complement drugs. The present invention extracts and separates the caffeoylquinic acid compounds obtained from Shamrock, through in vitro experiments, it is confirmed that the cell hemolysis caused by the activation of the classic pathway of the complement system is inhibited, indicating that there is an anti-complement effect. The activity is stronger than that of the total extract of S. sageris, and it is a kind of good complement inhibitor, which can be used to prepare new anti-complement drugs and treat various diseases caused by abnormal activation of complement, and has low effective concentration and low toxicity. The drug is safe, the source of raw materials is abundant, and it has great clinical application value. Caffeoylquinic acid and its derivatives are shown in the following structural formula I: Formula I wherein R1, R2, R3, or R4 can be the same or different, and they are H or caffeoyl respectively, provided that they cannot be H at the same time; R5 is H , CH3, CH2CH3 or CH2CH2CH2CH3.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Risk evaluation and management strategy involving patient follow-ups relating to the use or discontinuation of a complement inhibitor
ActiveUS20160140298A1Reduce morbidityData processing applicationsDrug and medicationsComplement InhibitorsMedicine
This invention provides, inter alia, a complement-inhibitor-based treatment plan coupled with a risk evaluation and management strategy (“REMS”) and a safety support program (“SSP”) for reinforcing the REMS. The REMS and SPP are implemented using one or more computer devices with software tools programmed to enforce conditions of the REMS and / or prompt follow-ups by registered nurses enrolled in the SSP. The software tool(s) determines whether a prescriber requesting the complement inhibitor has agreed to abide by the REMS, and can prompt a provider of the complement inhibitor to provide updated educational materials to the prescriber at predetermined times or intervals, to monitor the prescriber for compliance with the REMS, and / or to monitor patients for signs of adverse events. Using exemplary embodiments described herein, a risk of adverse events (especially, but not limited to, meningococcal infections) can be managed and an incidence of the adverse events can be reduced.
Owner:ALEXION PHARMA INC
Treatment of graft rejection by administering a complement inhibitor to an organ prior to transplant
InactiveUS20160184391A1Easily penetrate organMinimize impactPeptide/protein ingredientsAntibody mimetics/scaffoldsIMMUNE SUPPRESSANTSAntiendomysial antibodies
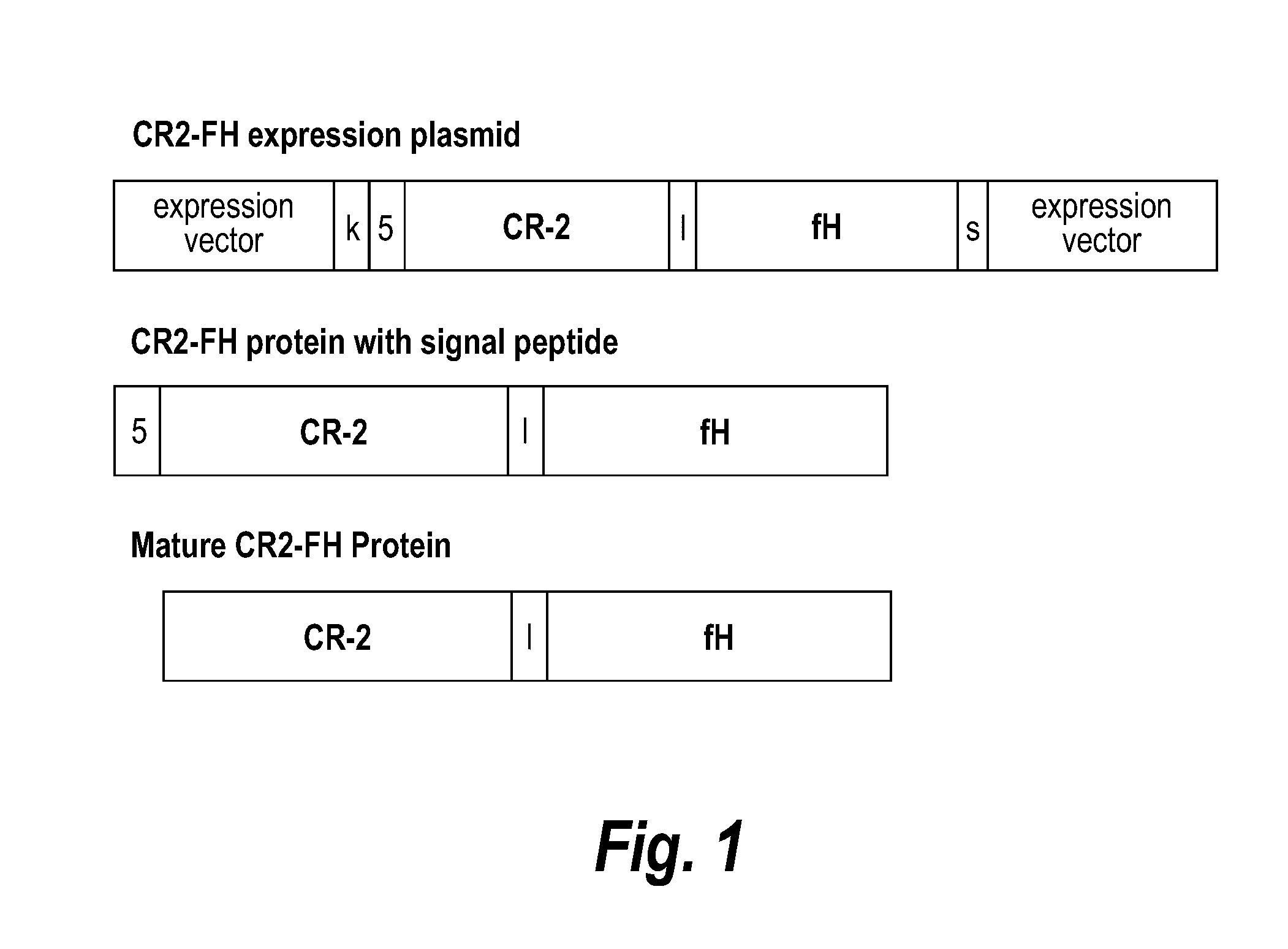
Methods of prolonging survival of a transplanted organ, as well as methods of preventing or attenuating rejection of a transplanted organ are provided. These methods involve contacting the organ with an inhibitor of complement activity (e.g., a complement inhibitor that has a maximum molecular weight of 70 kDa and / or a half-life shorter than 10 days, such as a CR2-FH fusion protein or a single chain anti-C5 antibody), prior to transplantation The methods also include administering to the allotransplant recipient an inhibitor of complement activity together with one or more immunosuppressants. A pretreatment with an alternative complement inhibitor was found to be effective in improving graft survival and decreasing ischemia-reperfusion injury in animal.
Owner:ALEXION PHARMA INC
Acylated flavonoid glycoside compounds and application thereof in preparation of complement inhibitor medicines
InactiveCN102304158ASignificant anticomplement activityHigh activityOrganic active ingredientsSugar derivativesDiseaseHemolysis
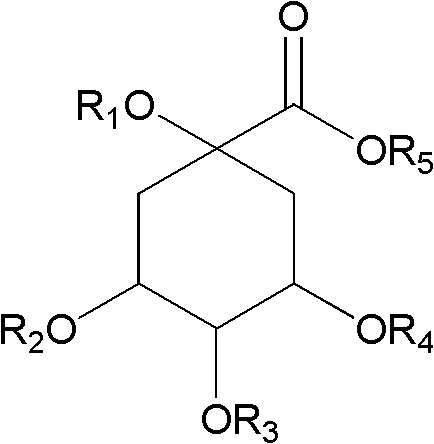
The invention discloses acylated flavonoid glycoside compounds shown as a formula I and application thereof in preparation of complement inhibitor medicines. Acylated flavonoid glycosides extracted and separated from cudweed herb are proved to inhibit hemolysis caused by activation of a complement system in a classical pathway through in vitro experiments, and have complement inhibition effect; the activity of each monomericcompound is higher than that of a total extract of cudweed herb; and the acylated flavonoid glycoside compounds are good complement inhibitors and have low effective concentration, can serve as active ingredients to prepare novel complement inhibitor medicines for treating various diseases caused by abnormal activation of complements, are low in toxicity, safe in administration and rich in raw material sources and have great clinical application value. The structure of the formula I is shown as the specifications, wherein R1 and R2 are same or different, and respectively H, OH or OCH3; and R3 and R4 are respectively H or groups shown in the specifications.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
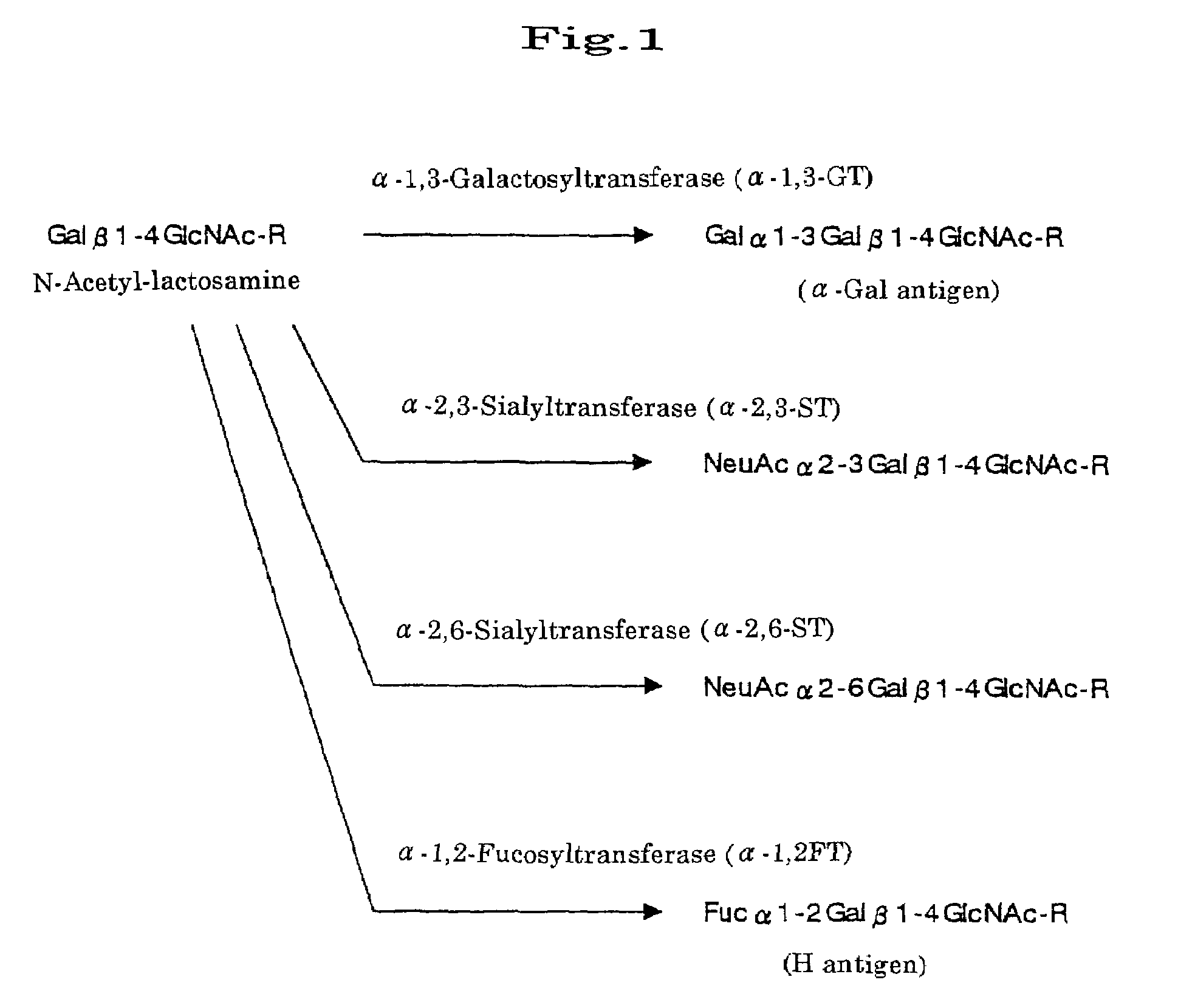
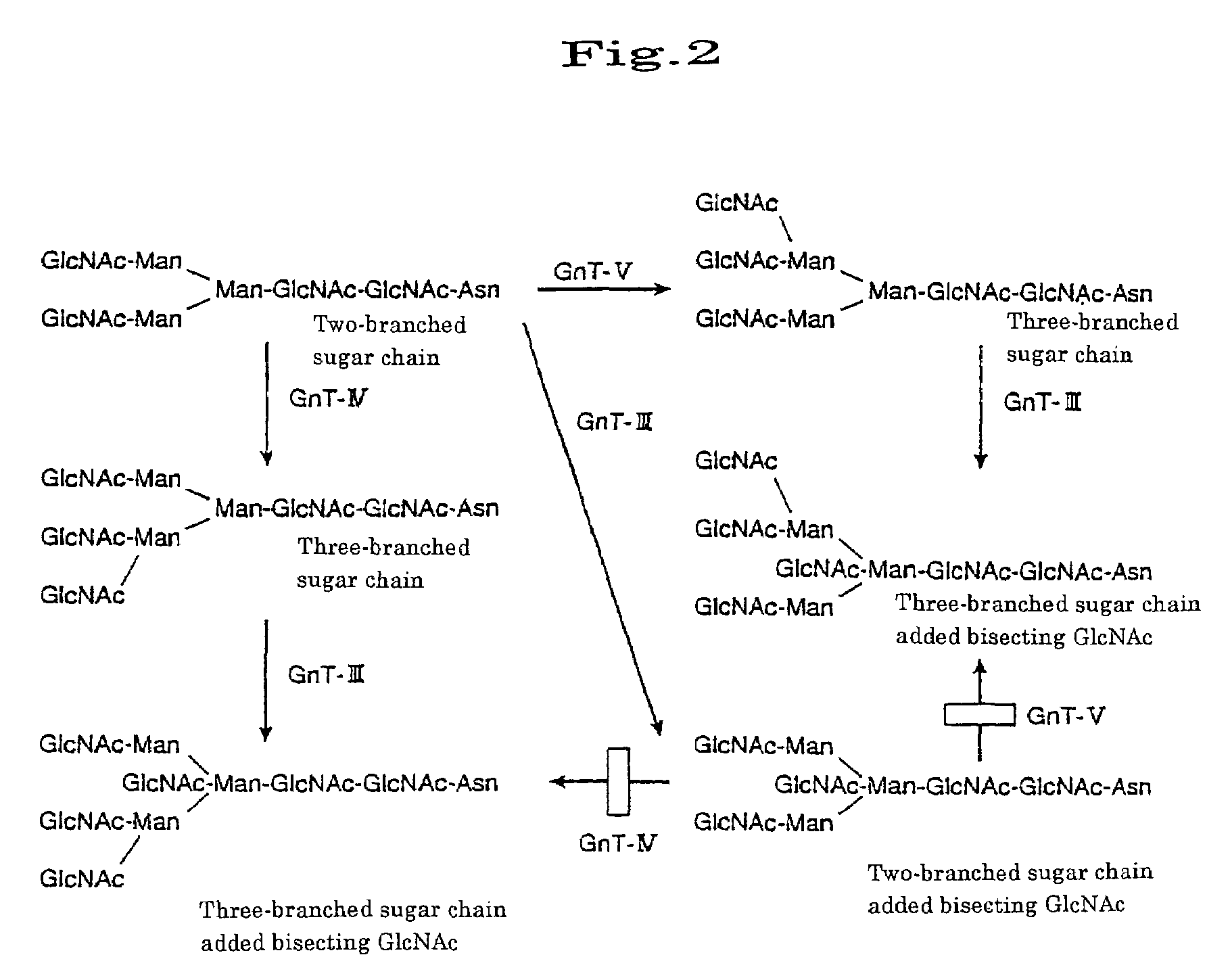
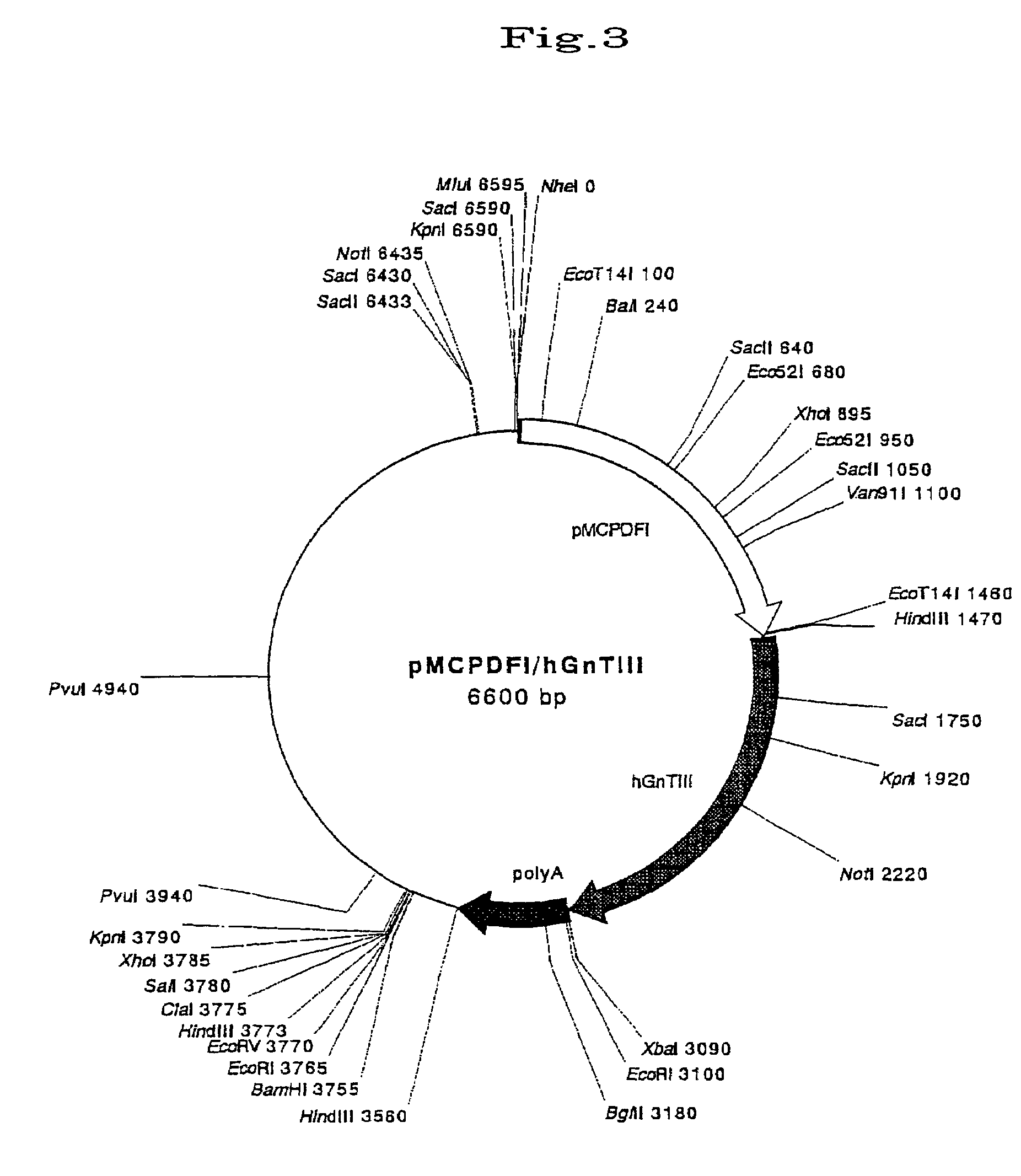
Transgenic mammals
This invention provides a nonhuman transgenic mammal carrying transgene comprising the regulatory genes capable of functioning in the hyperacute rejection-occurring local cells and gene encoding human N-acetylglucosaminyltransferase III (GnT-III), or a nonhuman transgenic mammal carrying transgene comprising the regulatory genes and genes encoding GnT-III and the human complement inhibitor. Because of reduced α-Gal antigens in the hyperacute rejection-occurring local cells or because of both reduced α-Gal antigens and expression of the human complement inhibitor, the transgenic mammal of this invention can effectively inhibit the hyperacute rejection caused by discordant xenotransplantation. Consequently, this invention provides the transgenic mammal suitable for organ transplantation.
Owner:NIPPON HAM
Immunosuppressive combination and its use in the treatment or prophylaxis of insulin-producing cell graft rejection
InactiveUS20080199465A1Effective treatmentIncrease independenceBiocidePeptide/protein ingredientsCellular graft rejectionFree form
A pharmaceutical combination comprising an accelerated lymphocyte homing agent in free form or in pharmaceutically acceptable salt form, and one or more compounds selected from the group consisting of an antibody to the IL-2 receptor, an immunosuppressive macrocyclic lactone and a soluble human complement inhibitor is used to treat or prevent insulin-producing cell graft rejection.
Owner:LAKE PHILIP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com