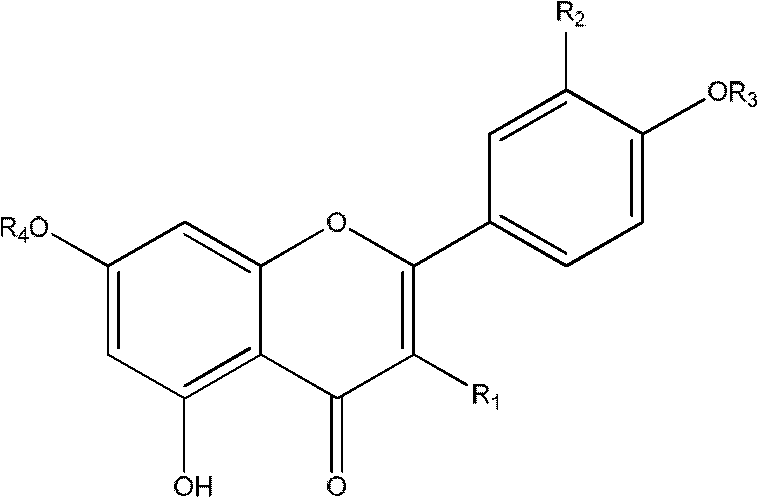
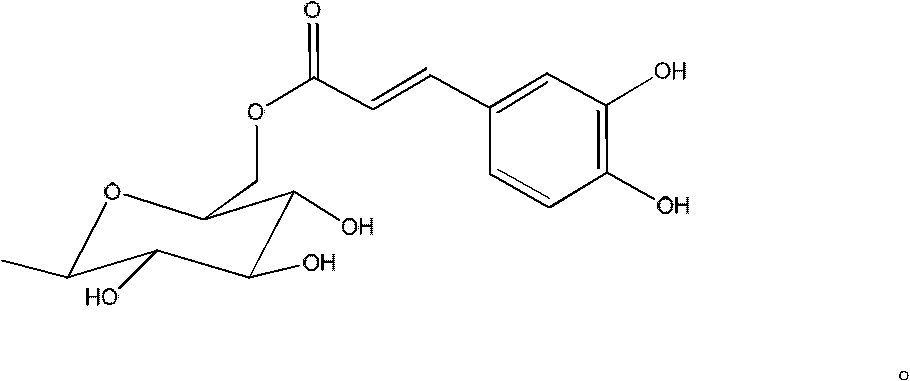
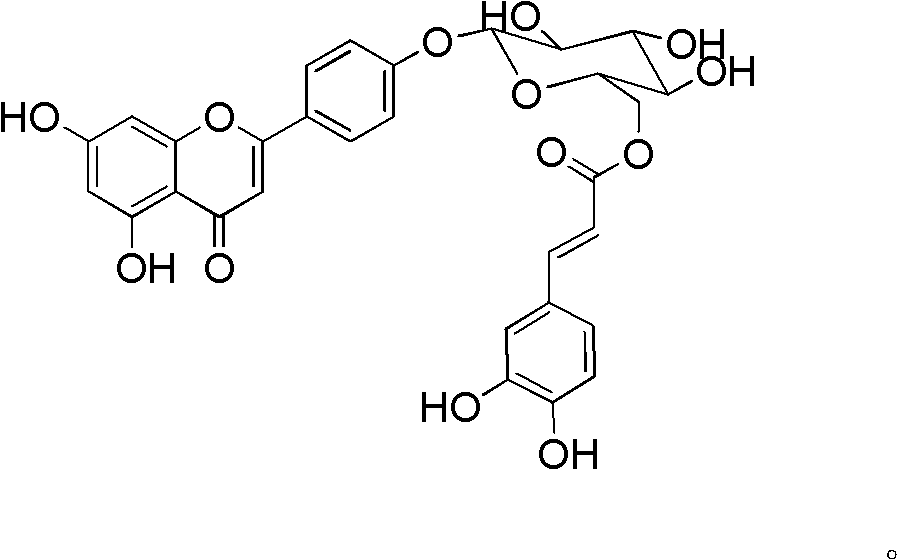
Acylated flavonoid glycoside compounds and application thereof in preparation of complement inhibitor medicines
A technology of acylated flavone glycoside compounds and compounds, which is applied in the application field of acylated flavone glycoside compounds and preparation of anti-complement drugs, can solve the problems such as no anti-complement reports of acylated flavone glycosides, and achieve great clinical application value and raw materials The effect of abundant sources and low effective concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of acylated flavonoid glycoside compounds
[0038] 25 kg of dry whole herb of S. sageii (purchased from Bozhou medicinal material market, Anhui, China) was pulverized, extracted three times under 80% ethanol hot reflux (200L×3), combined the extracts to recover ethanol, and concentrated to dryness under reduced pressure at 60°C to obtain total S. Extract 2.9kg of extract, take 2.5kg of total extract and suspend with water (15L), extract with equal volume of petroleum ether, ethyl acetate, n-butanol for 3 times in turn, combine ethyl acetate extract and concentrate at 60°C under reduced pressure To dryness, 450 g of ethyl acetate extract was obtained. Take 400 g of the ethyl acetate extract and pass through a silica gel column (4 kg, 100-200 mesh, 10 cm × 100 cm) chromatography to dichloromethane: methanol (70:1, 50:1, 30:1, 20:1, 10:1, 5:1, 3:1, 1:1) gradient elution, 20L of each gradient elution, collected dichloromethane:methanol (10:1) elution ...
Embodiment 2
[0044] Example 2 Classical pathway complement inhibition test
[0045] 1 Instruments and reagents
[0046] Low-temperature high-speed centrifuge (Jouan MR22i), microplate reader (Thermo Labsystems, Well scanMK3), sheep red blood cells, anti-sheep red blood cell antibodies (sigma company), human serum, barbiturate-barbital sodium buffered saline (BBS 2+ , pH=7.4, containing 0.5mM Mg 2+ and 0.15mM Ca 2+ ), triple distilled water, heparin sodium, and a constant temperature water bath.
[0047] 2 Test drugs
[0048] The total sage extract prepared in Example 1 and the acylated flavonoid glycosides isolated therefrom
[0049] 3 Experimental methods
[0050] Take human serum to VBS 2+ Buffer (barbital buffer, pH=7.4, containing 0.5mM Mg 2+ and 0.15mM Ca 2+ ) diluted 1:10, as a source of "complement" in the classical pathway. Antibodies against goat erythrocytes in VBS 2+ The buffer was diluted 1:1000 as hemolysin; the sheep red blood cells were treated with VBS 2+ The buf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com