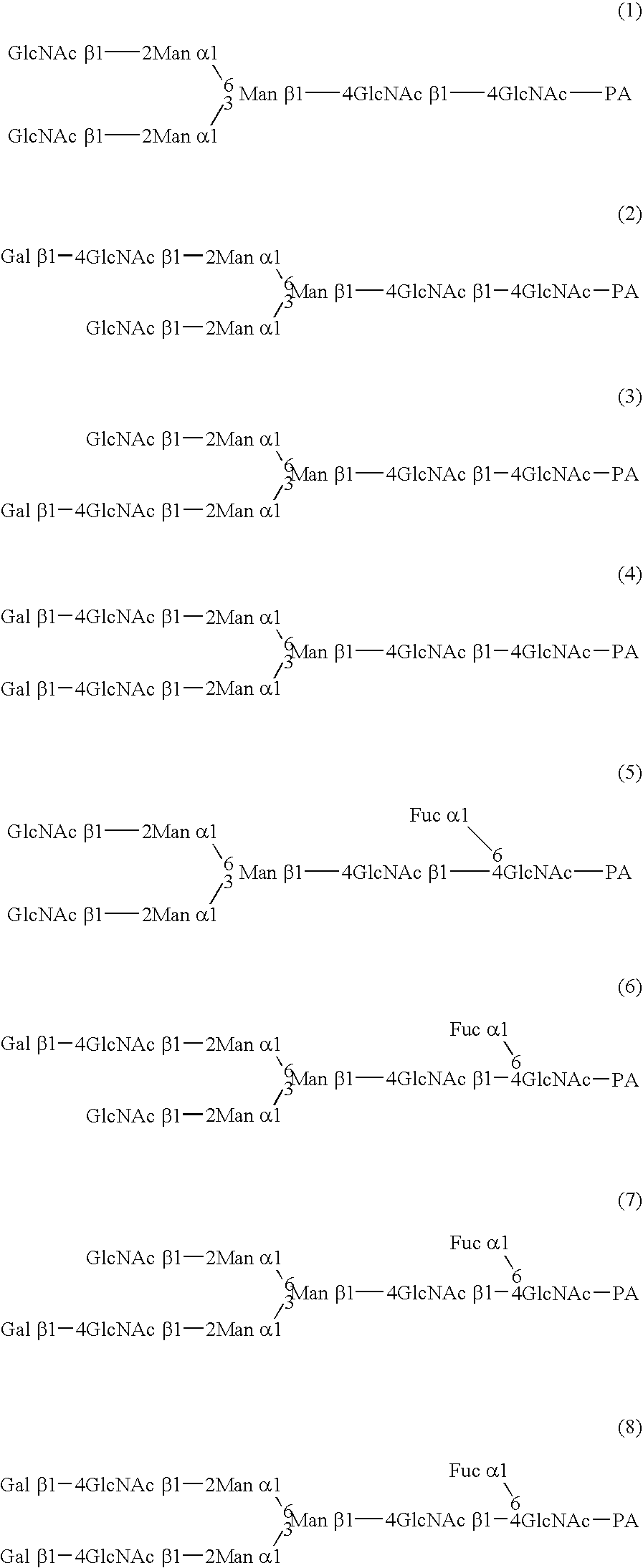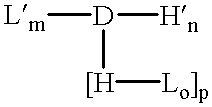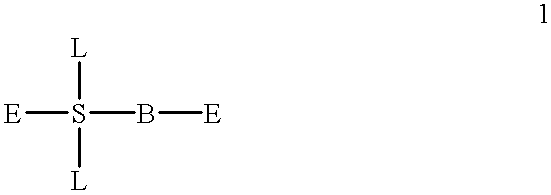Patents
Literature
50975 results about "Sugar" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules composed of two monosaccharides joined by a glycosidic bond. Common examples are sucrose (glucose + fructose), lactose (glucose + galactose), and maltose (two molecules of glucose). In the body, compound sugars are hydrolysed into simple sugars. Table sugar, granulated sugar or regular sugar refers to sucrose, a disaccharide composed of glucose and fructose.
Cells of which genome is modified
InactiveUS20040110704A1Raise the ratioDecreased and deleted activityAntibacterial agentsAntipyreticGlycosideN-Acetylglucosamine
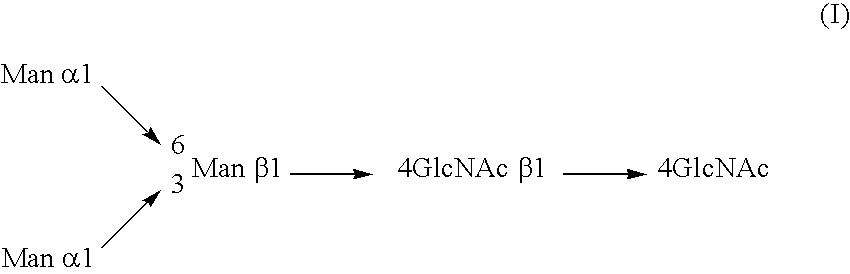
A cell in which genome is modified so as to have a more decreased or deleted activity of an enzyme relating to modification of a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain than its parent cell, and a process for producing an antibody composition using the cell.
Owner:KYOWA HAKKO KOGYO CO LTD
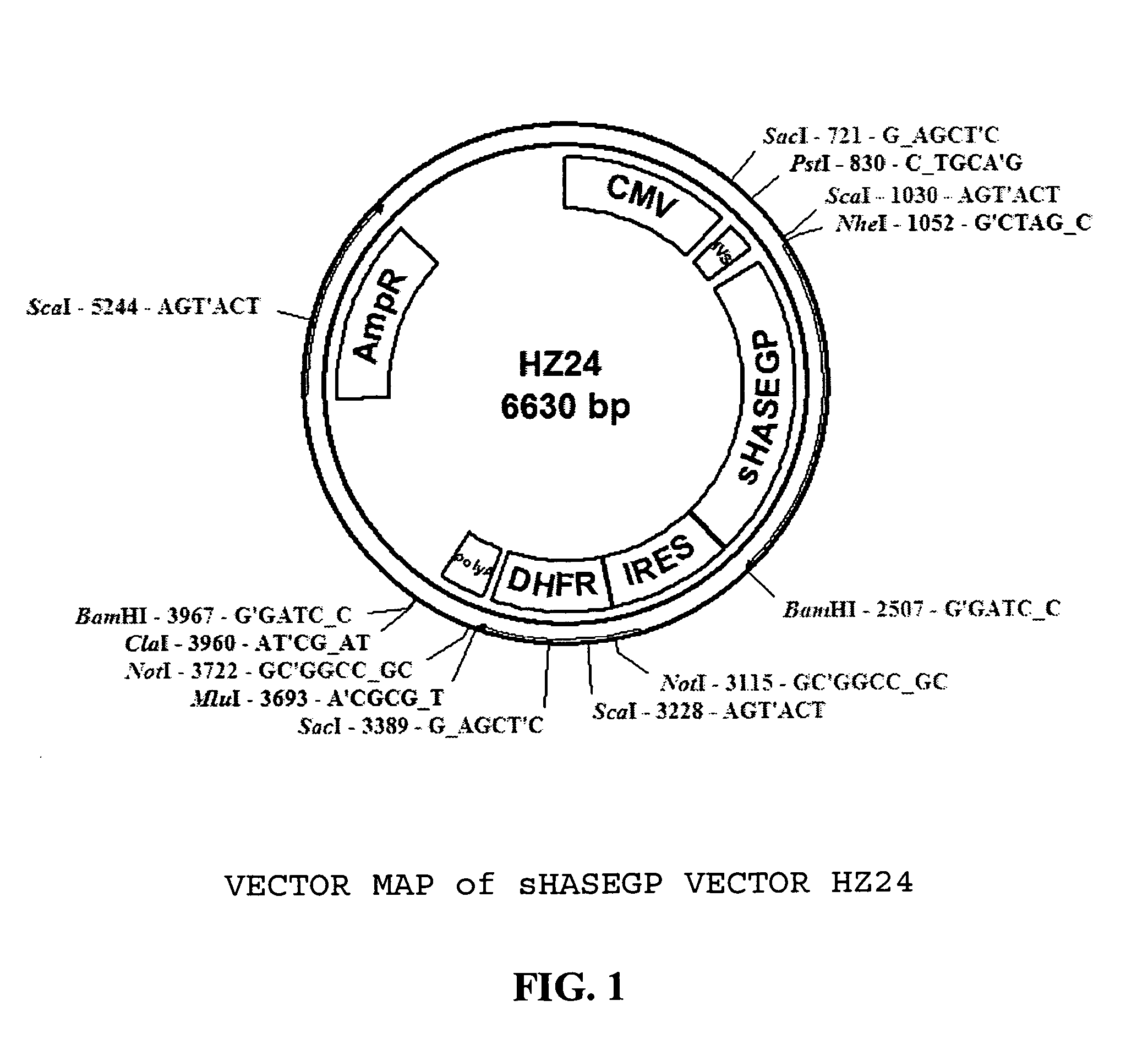
Soluble glycosaminoglycanases and methods of preparing and using soluble glycosaminoglycanases
ActiveUS20050260186A1Improve extentIncrease ratingsAntibacterial agentsSenses disorderHyaluronidasePathology diagnosis
The invention relates to the discovery of novel soluble neutral active Hyaluronidase Glycoproteins (sHASEGPs), methods of manufacture, and their use to facilitate administration of other molecules or to alleviate glycosaminoglycan associated pathologies. Minimally active polypeptide domains of the soluble, neutral active sHASEGP domains are described that include asparagine-linked sugar moieties required for a functional neutral active hyaluronidase domain. Included are modified amino-terminal leader peptides that enhance secretion of sHASEGP. The invention further comprises sialated and pegylated forms of a recombinant sHASEGP to enhance stability and serum pharmacokinetics over naturally occurring slaughterhouse enzymes. Further described are suitable formulations of a substantially purified recombinant sHASEGP glycoprotein derived from a eukaryotic cell that generate the proper glycosylation required for its optimal activity.
Owner:HALOZYME
Polycyclic sugar surrogate-containing oligomeric compounds and compositions for use in gene modulation
Compositions comprising first and second oligomers are provided wherein at least a portion of the first oligomer is capable of hybridizing with at least a portion of the second oligomer, at least a portion of the first oligomer is complementary to and capable of hybridizing to a selected target nucleic acid, and at least one of the first or second oligomers includes a modification comprising a polycyclic sugar surrogate. Oligomer / protein compositions are also provided comprising an oligomer complementary to and capable of hybridizing to a selected target nucleic acid and at least one protein comprising at least a portion of an RNA-induced silencing complex (RISC), wherein at least one nucleoside of the oligomer has a polycyclic sugar surrogate modification.
Owner:ALLERSON CHARLES +6
Devices and methods for pyloric anchoring
ActiveUS20050055039A1Avoiding erosion and ulcerationSuture equipmentsElectrotherapyPylorusPatient characteristics
A device for performing one or more functions in a gastrointestinal tract of a patient includes an anchoring member and at least one actuator, sensor, or combination of both coupled with the anchoring device. The anchoring device is adapted to maintain at least part of the device within a pyloric portion of the patient's stomach and to intermittently engage, without directly attaching to, stomach tissue. Actuators perform any suitable function, such as transmitting energy to tissue, acting as a sleeve to reduce nutrient absorption, occupying space in the stomach, eluting a drug and / or the like. Sensors may be adapted to sense any suitable patient characteristic within the patient's gastrointestinal tract, such as pH, temperature, bile content, nutrient content, fats, sugars, alcohol, opiates, drugs, analytes, electrolytes and / or hemoglobin.
Owner:BARONOVA
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Breed-specific canine food formulations
InactiveUS6156355AUnique shapeManaged fat levelMilk preparationAnimal feeding stuffFood formulationAdditive ingredient
Breed-specific dog food formulations that comprise chicken meat as the major ingredient, rice as the predominant (or sole) grain source, fruit and / or vegetable fiber as the primary or sole fiber source, unique fat and antioxidant blend, vitamins, herbs and spices, carotenoids, and no corn or artificial colors, preservatives, flavors or sugars are provided.
Owner:BIG HEART PET INC
Cyanine dyes as labeling reagents for detection of biological and other materials by luminescence methods
Cyanine and related dyes, such as merocyanine, styryl and oxonol dyes, are strongly light-absorbing and highly luminescent. Cyanine and related dyes having functional groups make them reactive with amine, hydroxy and sulfhydryl groups are covalently attached to proteins, nucleic acids, carbohydrates, sugars, cells and combinations thereof, and other biological and nonbiological materials, to make these materials fluorescent so that they can be detected. The labeled materials can then be used in assays employing excitation light sources and luminescence detectors. For example, fluorescent cyanine and related dyes can be attached to amine, hydroxy or sulfhydryl groups of avidin and to antibodies and to lectins. Thereupon, avidin labeled with cyanine type dyes can be used to quantify biotinylated materials and antibodies conjugated with cyanine-type dyes can be used to detect and measure antigens and haptens. In addition, cyanine-conjugated lectins can be used to detect specific carbohydrate groups. Also, cyanine-conjugated fragments of DNA or RNA can be used to identify the presence of complementary nucleotide sequences in DNA or RNA.
Owner:CARNEGIE MELLON UNIV
Nano-chem-FET based biosensors
ActiveUS7303875B1Facilitate membrane potential studiesBioreactor/fermenter combinationsBiological substance pretreatmentsNanowireGlucose polymers
Methods of detecting components of interest, e.g., nucleic acids and sugars, are provided. The methods comprise contacting one or more nanowires comprising a functional group with a sample containing the component or components of interest. In one embodiment, the functional group comprises a hairpin oligonucleotide, e.g., a hairpin that changes conformation upon binding the component of interest, e.g., a nucleic acid. The change in conformation produces a change in charge that is detected. In another embodiment, the functional group comprises an enzyme, e.g., glucose oxidase, which produces a change in pH when glucose is present in a sample.
Owner:SHOEI CHEM IND CO LTD
Purification of biologically-produced 1,3-propanediol
InactiveUS20050069997A1Reduce the amount of waterFermented solutions distillation/rectificationMembranesEscherichia coliDistillation
A process for purifying 1,3-propanediol from the fermentation broth of a cultured E. coli that has been bioengineered to synthesize 1,3-propanediol from sugar is provided. The basic process entails filtration, ion exchange and distillation of the fermentation broth product stream, preferably including chemical reduction of the product during the distillation procedure. Also provided are highly purified compositions of 1,3-propanediol.
Owner:DUPONT IND BIOSCIENCES USA LLC +1
Compound for detecting and modulating RNA activity and gene expression
InactiveUS6262241B1Tightly boundEfficient executionSugar derivativesPeptide/protein ingredientsBond cleavageMinor groove
Compositions and methods for modulating the activity of RNA and DNA are disclosed. In accordance with preferred embodiments, antisense compositions are prepared comprising targeting and reactive portions. Reactive portions which act, alternatively, through phosphorodiester bond cleavage, through backbone sugar bond cleavage or through base modification are preferrably employed. Groups which improve the pharmacodynamic and pharmacokinetic properties of the oligonucleotides are also useful in accordance with certain embodiments of this invention. Delivery of the reactive or non-reactive functionalities into the minor groove formed by the hybridization of the composition with the target RNA is also preferrably accomplished. Therapeutics, diagnostics and research methods and also disclosed. Synthetic nucleosides and nucleoside fragments are also provided useful for elaboration of oligonucleotides and oligonucleotide analogs for such purposes.
Owner:IONIS PHARMA INC
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
Combination rawhide and formulated food pet chew
InactiveUS6277420B1Limited toughnessChew life increaseProtein composition from fishMeat/fish preservationWater activityCapillary action
A highly palatable and long lasting dog chew for pets has been developed by combining a formulation and processing sequence which results in a highly palatable meat based filling being incorporated into the center of a preformed rawhide stick or rawhide roll. Such outside rawhide fraction is extremely tough and chewy which results in a dog chew which takes a long period of time for the dog to consume. The inside meat filling is highly palatable which results in the animal maintaining interest in the treat until nearly the entire chew has been consumed. The interior meat filling is preserved by reduced water activity to below 0.85 as a result of incorporating of salt, sugars and natural humectants. Said filling is formulated and processed in such a manner that the water phase is bound within the filling and does not pass by capillary action to the outside rawhide fraction. This results in the outer rawhide shell maintaining a tough and chewable texture until such point as the dog is offered the finished chew.
Owner:ANDERSEN DAVID B +1
Topologically segregated, encoded solid phase libraries comprising linkers having an enzymatically susceptible bond
The invention relates to libraries of synthetic test compound attached to separate phase synthesis supports. In particular, the invention relates to libraries of synthetic test compound attached to separate phase synthesis supports that also contain coding molecules that encode the structure of the synthetic test compound. The molecules may be polymers or multiple nonpolymeric molecules. Each of the solid phase synthesis support beads contains a single type of synthetic test compound. The synthetic test compound can have backbone structures with linkages such as amide, urea, carbamate (i.e., urethane), ester, amino, sulfide, disulfide, or carbon-carbon, such as alkane and alkene, or any combination thereof. Examples of subunits suited for the different linkage chemistries are provided. The synthetic test compound can also be molecular scaffolds, such as derivatives of monocyclic of bicyclic carbohydrates, steroids, sugars, heterocyclic structures, polyaromatic structures, or other structures capable of acting as a scaffolding. Examples of suitable molecular scaffolds are provided. The invention also relates to methods of synthesizing such libraries and the use of such libraries to identify and characterize molecules of interest from among the library of synthetic test compound.
Owner:AVENTIS PHARMA INC
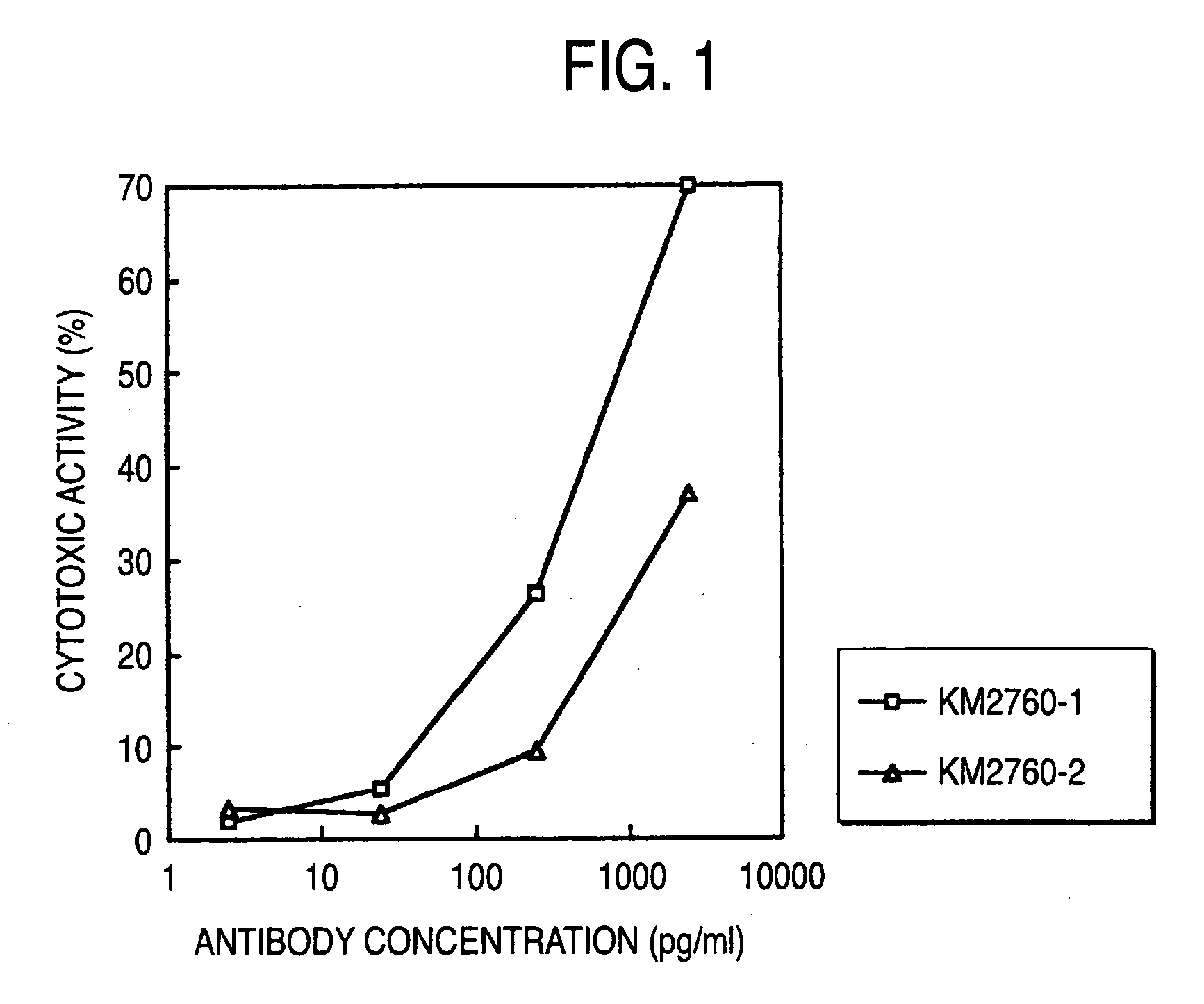
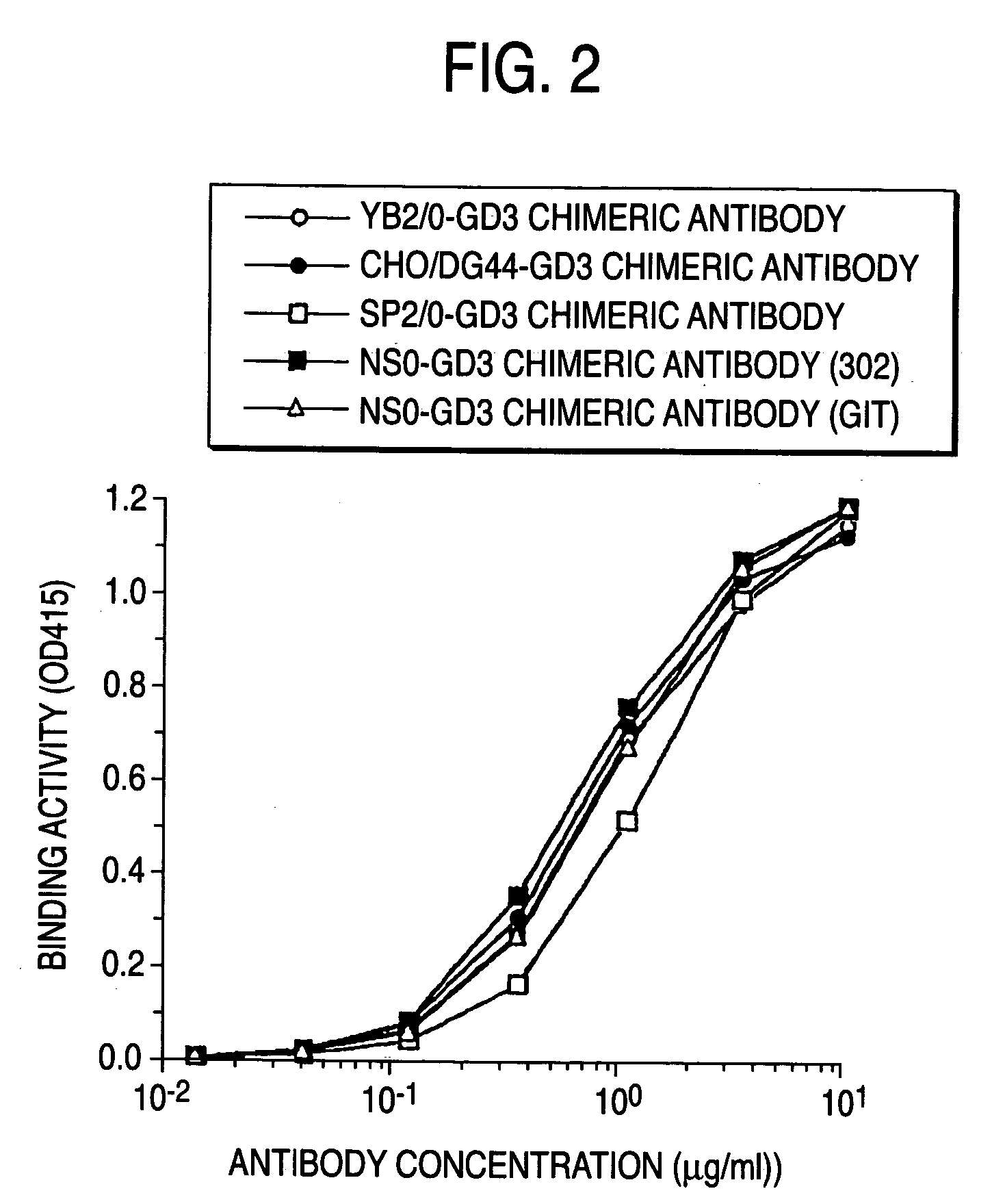
Method of enhancing of binding activity of antibody composition to Fcgamma receptor IIIa
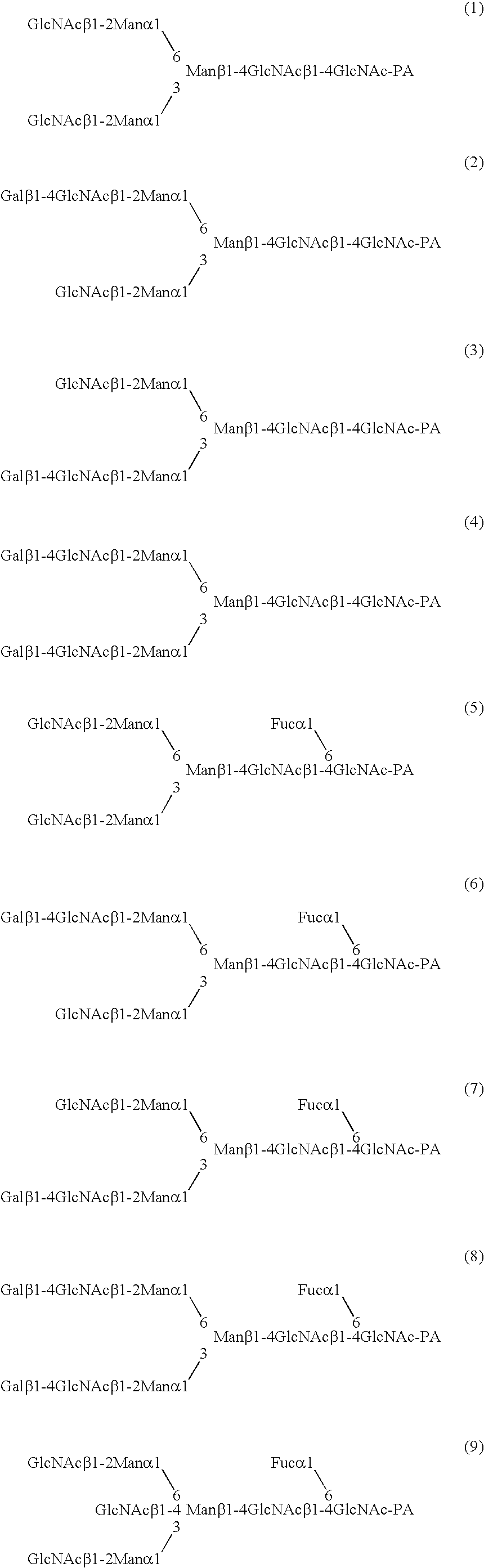
A method for enhancing a binding activity of an antibody composition to Fcgamma receptor IIIa, which comprises modifying a complex N-glycoside-linked sugar chain which is bound to the Fc region of an antibody molecule; a method for enhancing an antibody-dependent cell-mediated cytotoxic activity of an antibody composition; a process for producing an antibody composition having an enhanced binding activity to Fcgamma receptor IIIa; a method for detecting the ratio of a sugar chain in which fucose is not bound to N-acetylglucosamine in the reducing end in the sugar chain among total complex N-glycoside-linked sugar chains bound to the Fc region in an antibody composition; an Fc fusion protein composition produced by using a cell resistant to a lectin which recognizes a sugar chain in which 1-position of fucose is bound to 6-position of N-acetylglucosamine in the reducing end through alpha-bond in a complex N-glycoside-linked sugar chain; and a process for producing the same.
Owner:KYOWA HAKKO KIRIN CO LTD
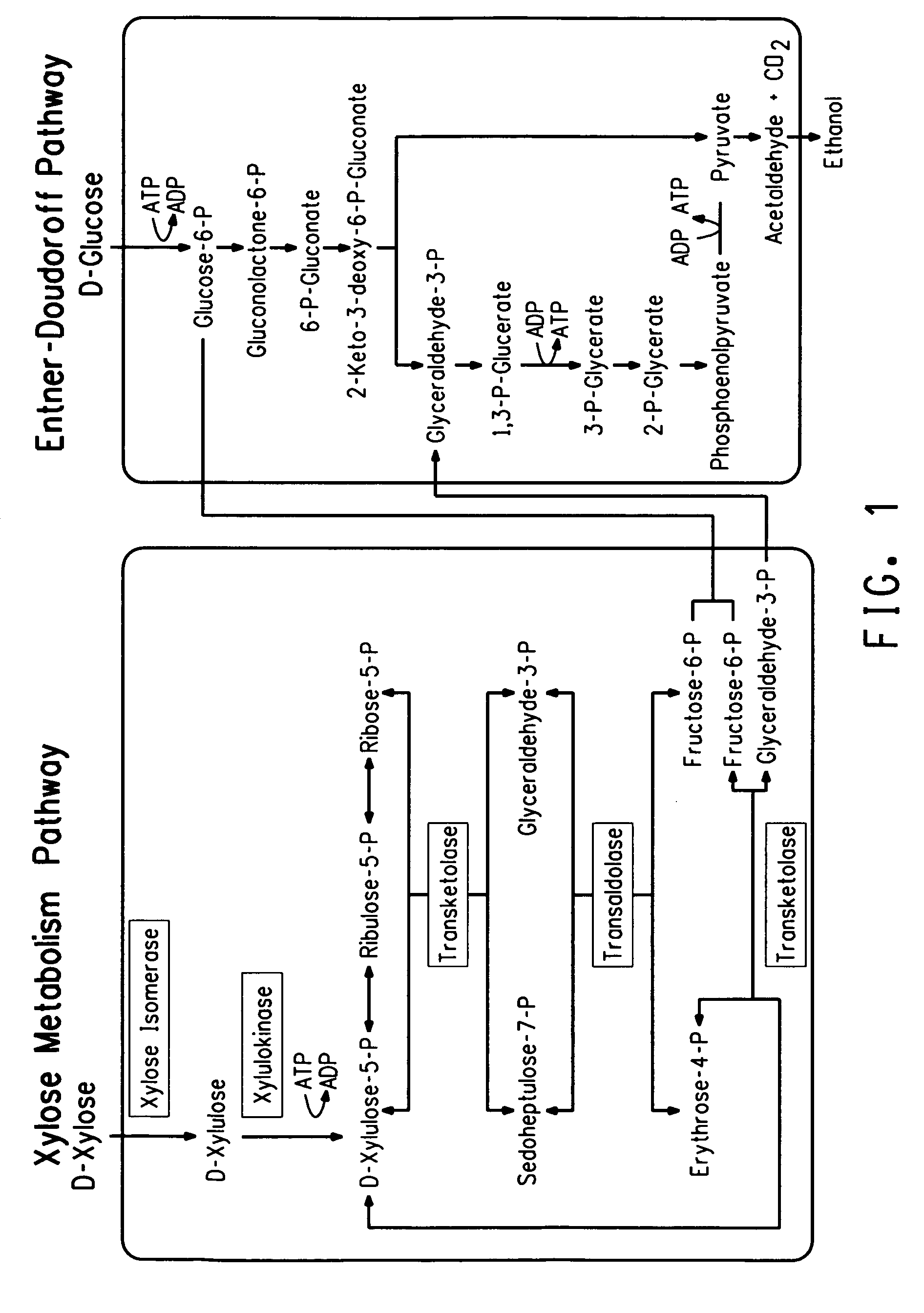
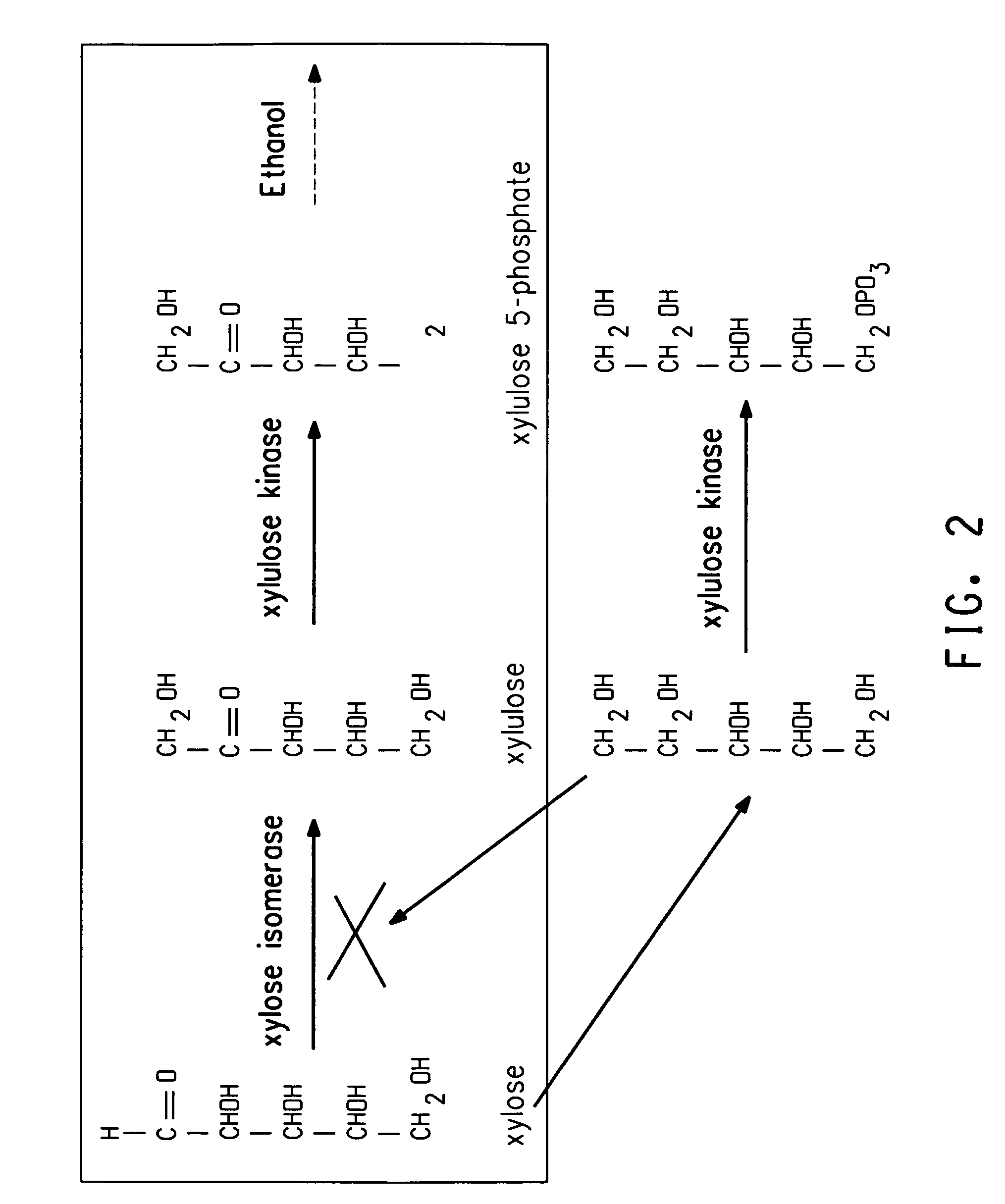
Xylitol synthesis mutant of xylose-utilizing zymomonas for ethanol production
InactiveUS7741119B2Reduce productionIncreased ethanol productionBacteriaUnicellular algaeFructoseOxidoreductase Gene
A strain of xylose-utilizing Zymomonas was engineered with a genetic modification to the glucose-fructose oxidoreductase gene resulting in reduced expression of GFOR enzyme activity. The engineered strain exhibits reduced production of xylitol, a detrimental by-product of xylose metabolism. It also consumes more xylose and produces more ethanol during mixed sugar fermentation under process-relevant conditions.
Owner:SUSTAINABLE TECH CORP +1
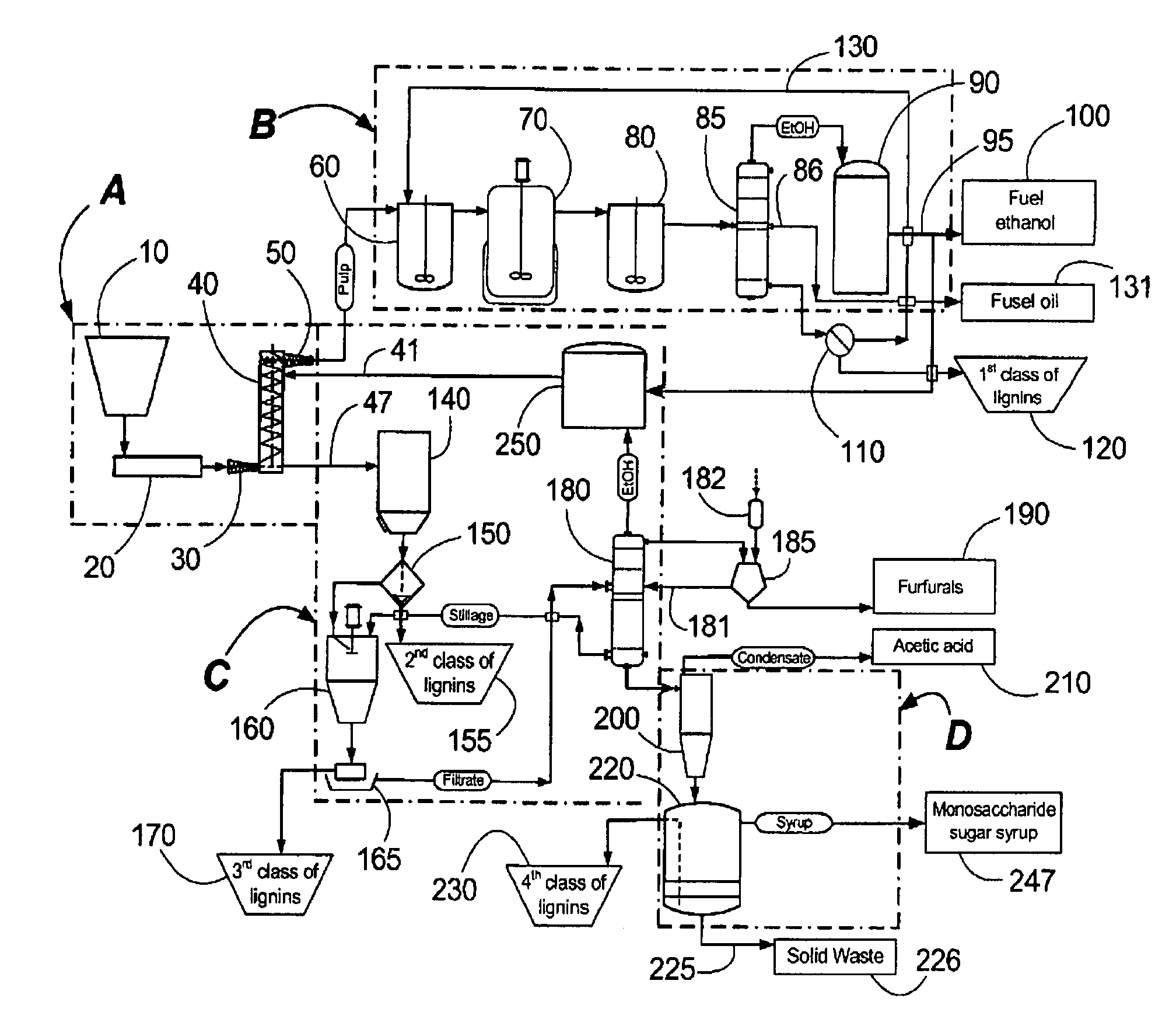
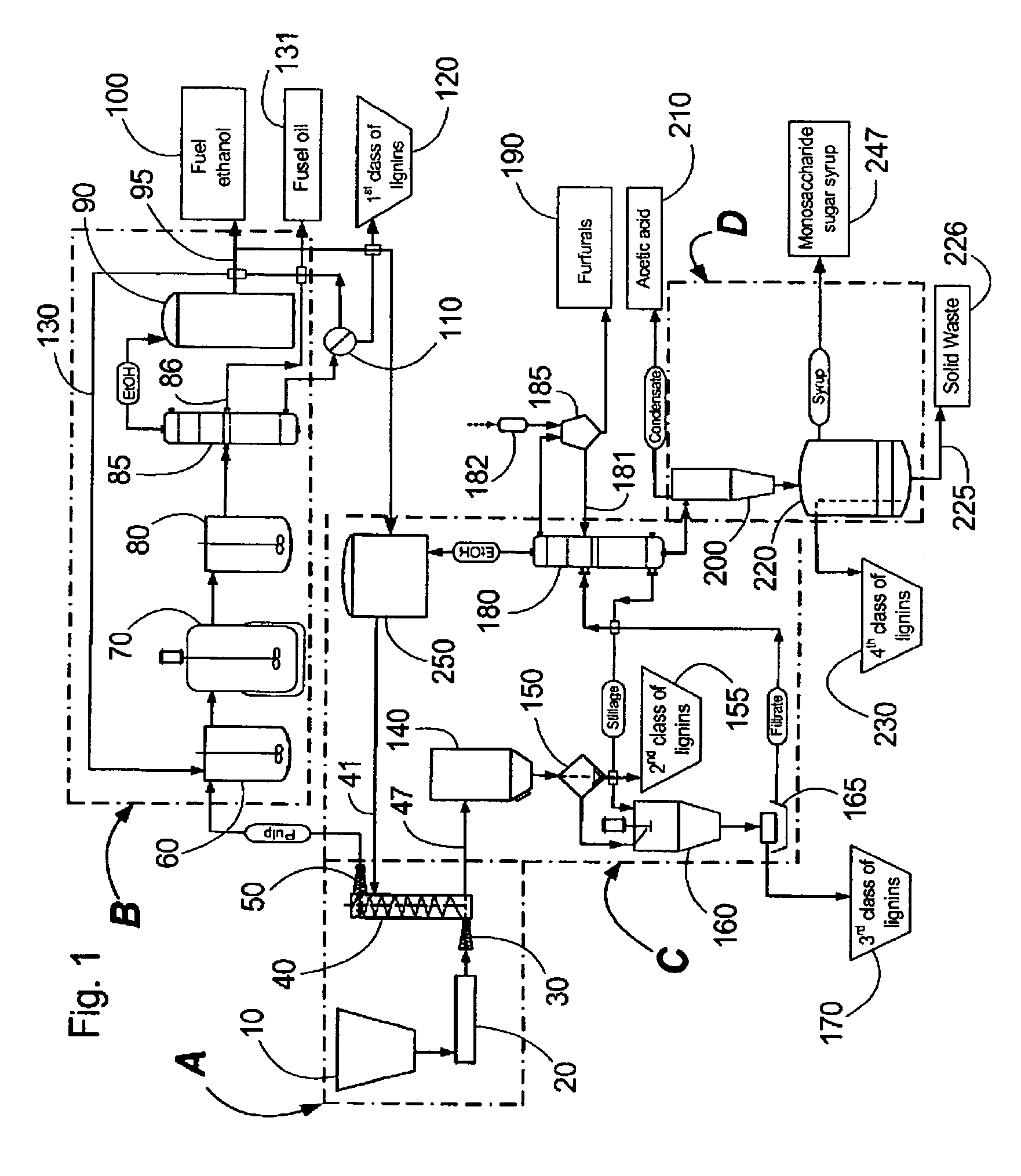
Continuous counter-current organosolv processing of lignocellulosic feedstocks
InactiveUS7465791B1Low viscosityNon-fibrous pulp additionBiological substance pretreatmentsFractionationOrganosolv
A modular process for organosolv fractionation of lignocellulosic feedstocks into component parts and further processing of said component parts into at least fuel-grade ethanol and four classes of lignin derivatives. The modular process comprises a first processing module configured for physico-chemically digesting lignocellulosic feedstocks with an organic solvent thereby producing a cellulosic solids fraction and a liquid fraction, a second processing module configured for producing at least a fuel-grade ethanol and a first class of novel lignin derivatives from the cellulosic solids fraction, a third processing module configured for separating a second class and a third class of lignin derivatives from the liquid fraction and further processing the liquid fraction to produce a distillate and a stillage, a fourth processing module configured for separating a fourth class of lignin derivatives from the stillage and further processing the stillage to produce a sugar syrup.
Owner:SUZANO CANADA INC
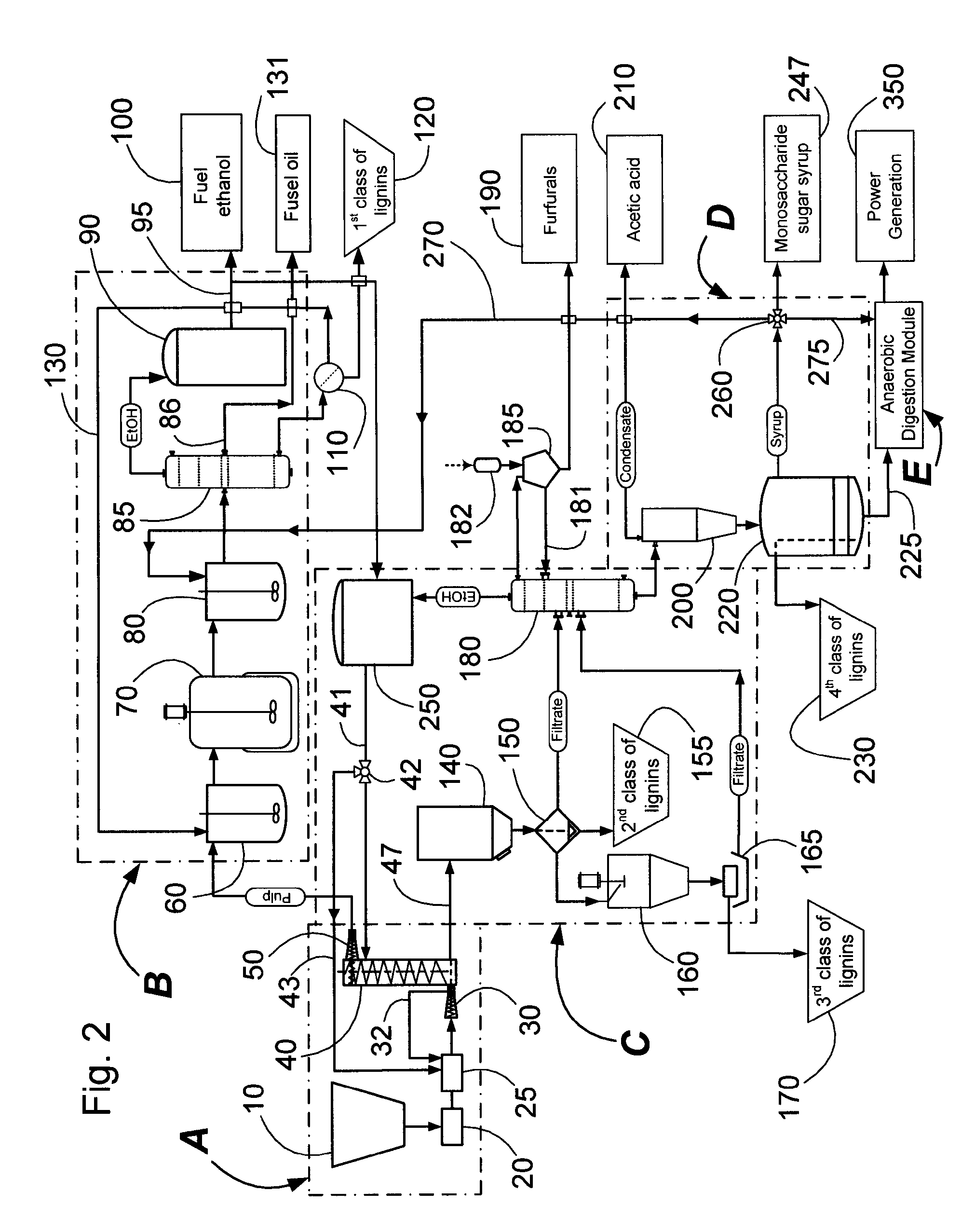
Inorganic salt recovery during processing of lignocellulosic feedstocks
InactiveUS20080102502A1Reduce inhibitionOvercome disadvantagesBiofuelsFermentationInorganic saltsCellulose
A method for recovering inorganic salt during processing of a lignocellulosic feedstock is provided. The method comprises pretreating the lignocellulosic feedstock by adding an acid or a base to the feedstock to produce a pretreated lignocellulosic feedstock. A soluble base or acid is then added to the pretreated lignocellulosic feedstock to adjust the pH and produce a neutralized feedstock. The neutralized feedstock is then hydrolyzed to produce an hydrolyzed feedstock and a sugar stream. Inorganic salt is recovered from a wash stream obtained from the pretreated lignocellulosic feedstock, a stream obtained from the neutralized feedstock, a stream obtained from the sugar stream, or a combination of these streams. The inorganic salt may be concentrated, clarified, recovered and purified by crystallization, electrodialysis, drying, or agglomeration and granulation, and then used as desired, for example, as a fertilizer.
Owner:IOGEN ENERGY CORP
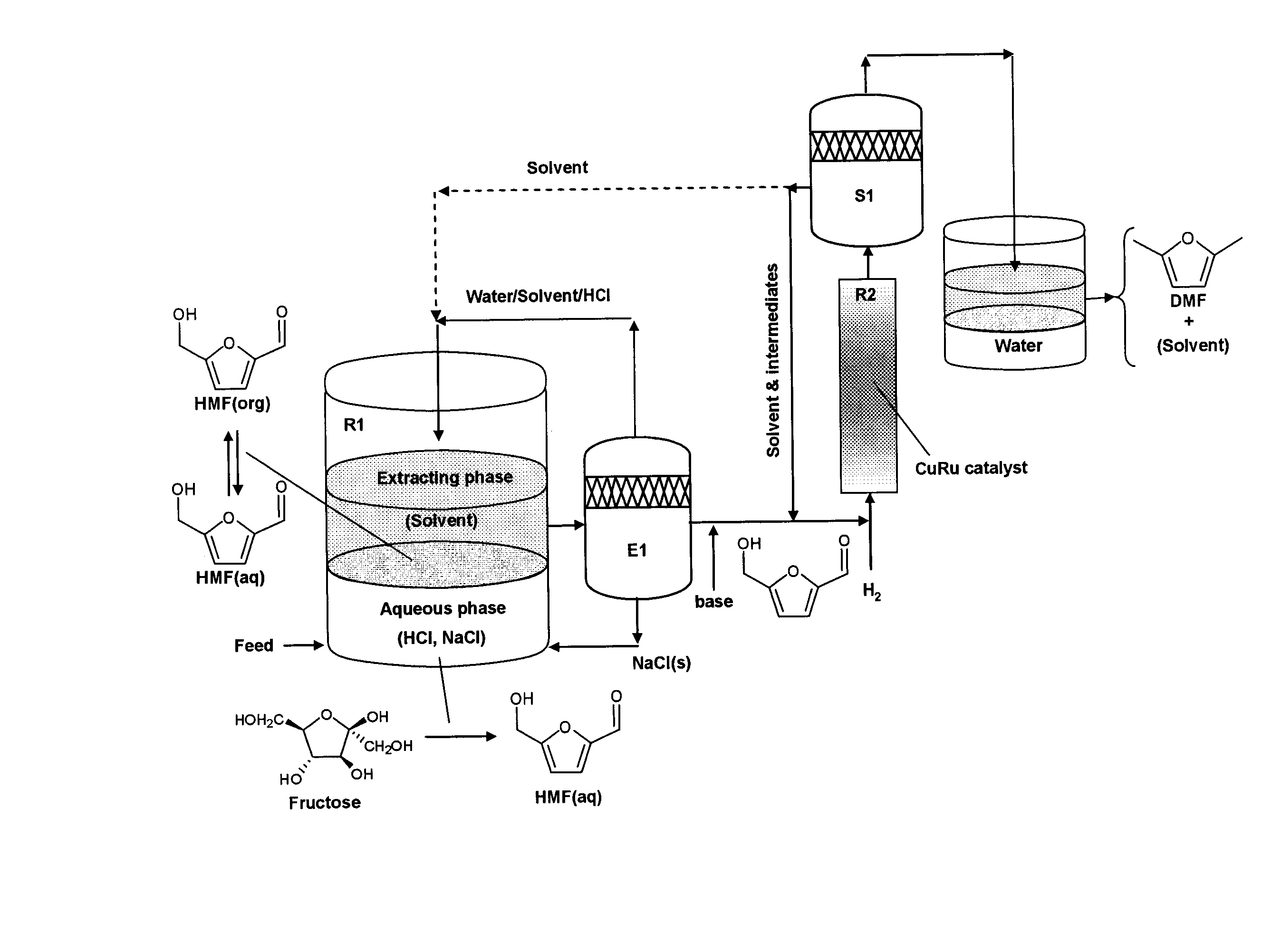
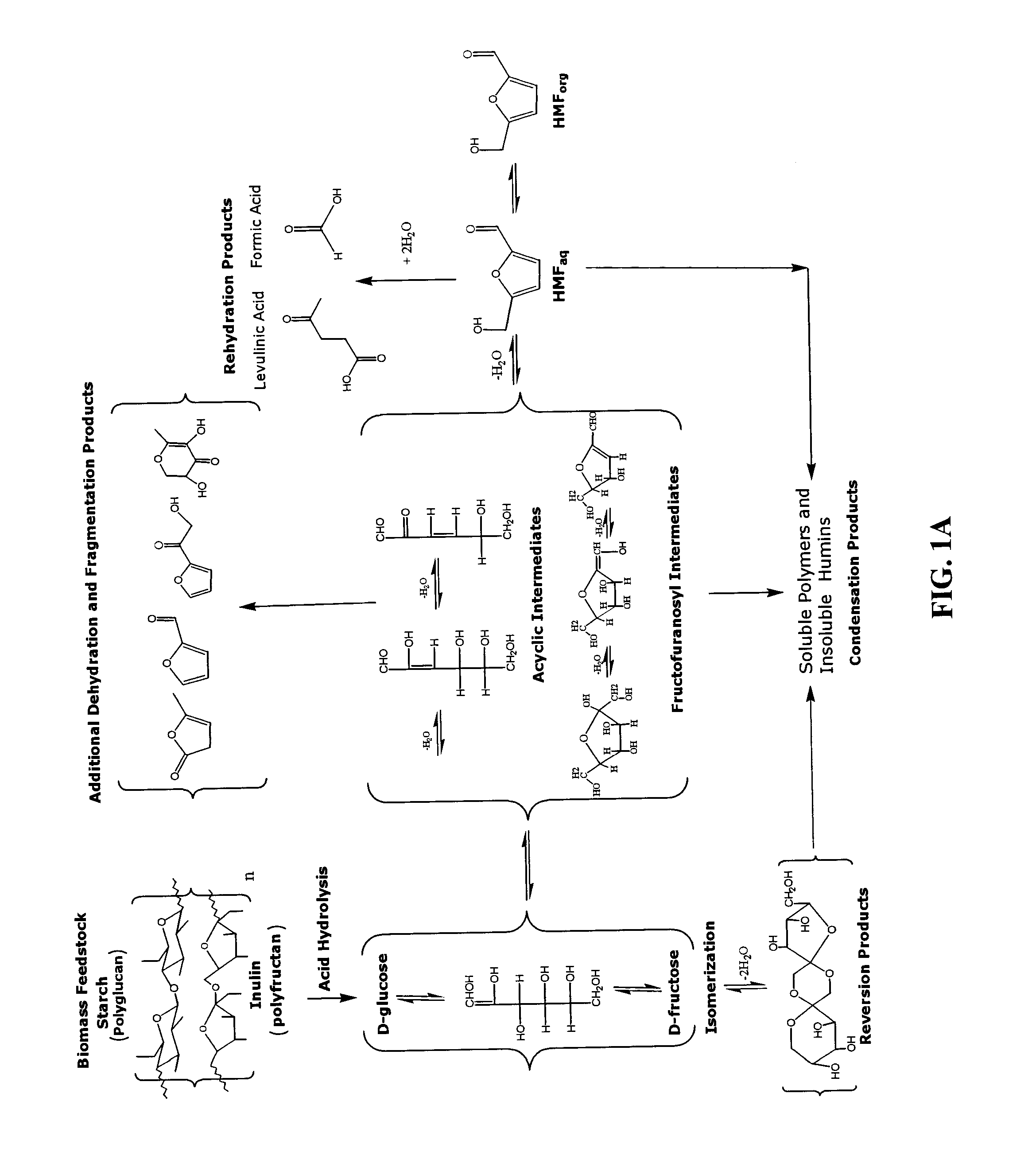
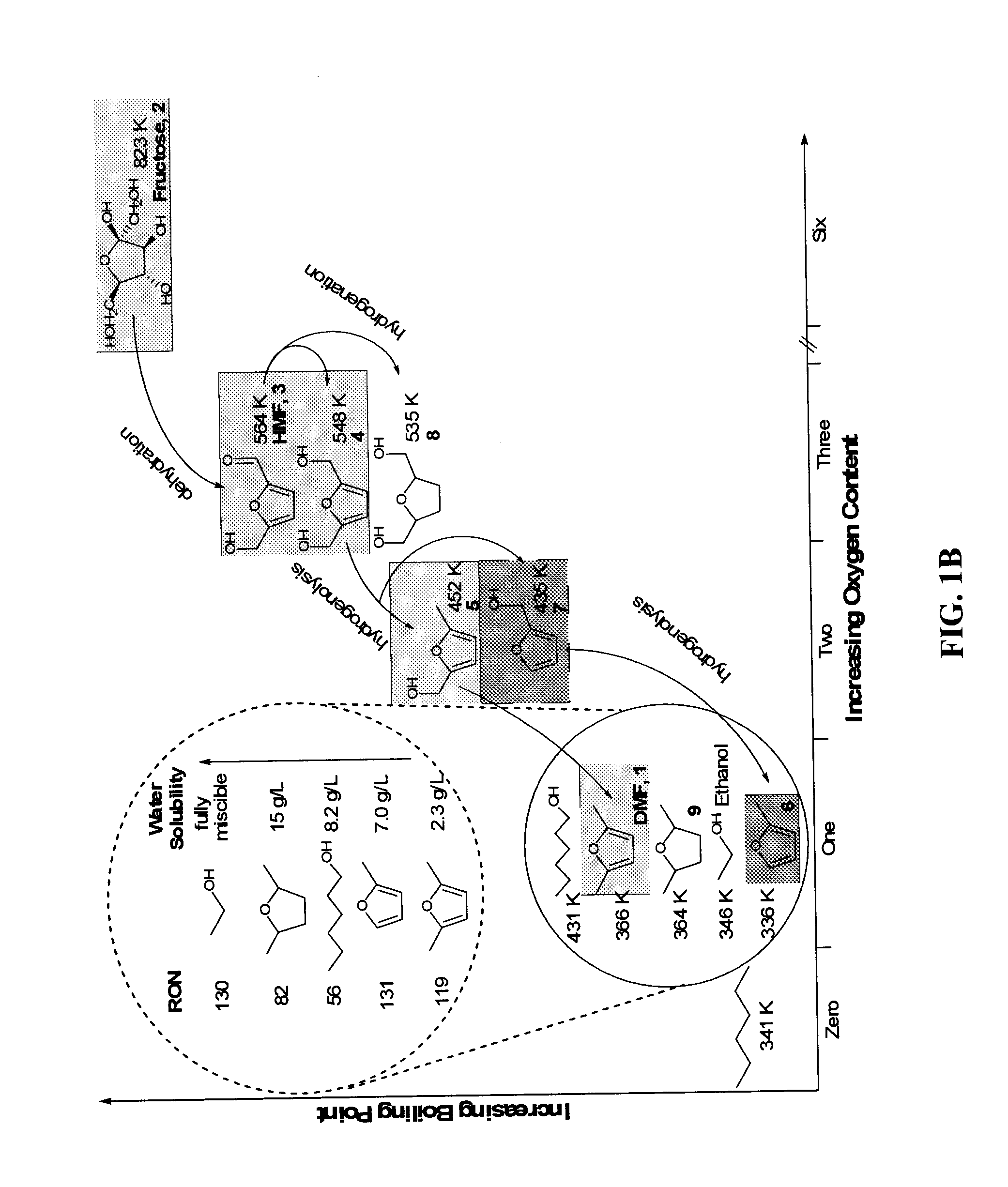
Catalytic process for producing furan derivatives in a biphasic reactor
Described is a catalytic process for converting sugars to furan derivatives (e.g. 5-hydroxymethylfurfural, furfural, dimethylfuran, etc.) using a biphasic reactor containing a reactive aqueous phase and an organic extracting phase. The process provides a cost-effective route for producing di-substituted furan derivatives. The furan derivatives are useful as value-added intermediates to produce polymers, as precursors to diesel fuel, and as fuel additives.
Owner:WISCONSIN ALUMNI RES FOUND
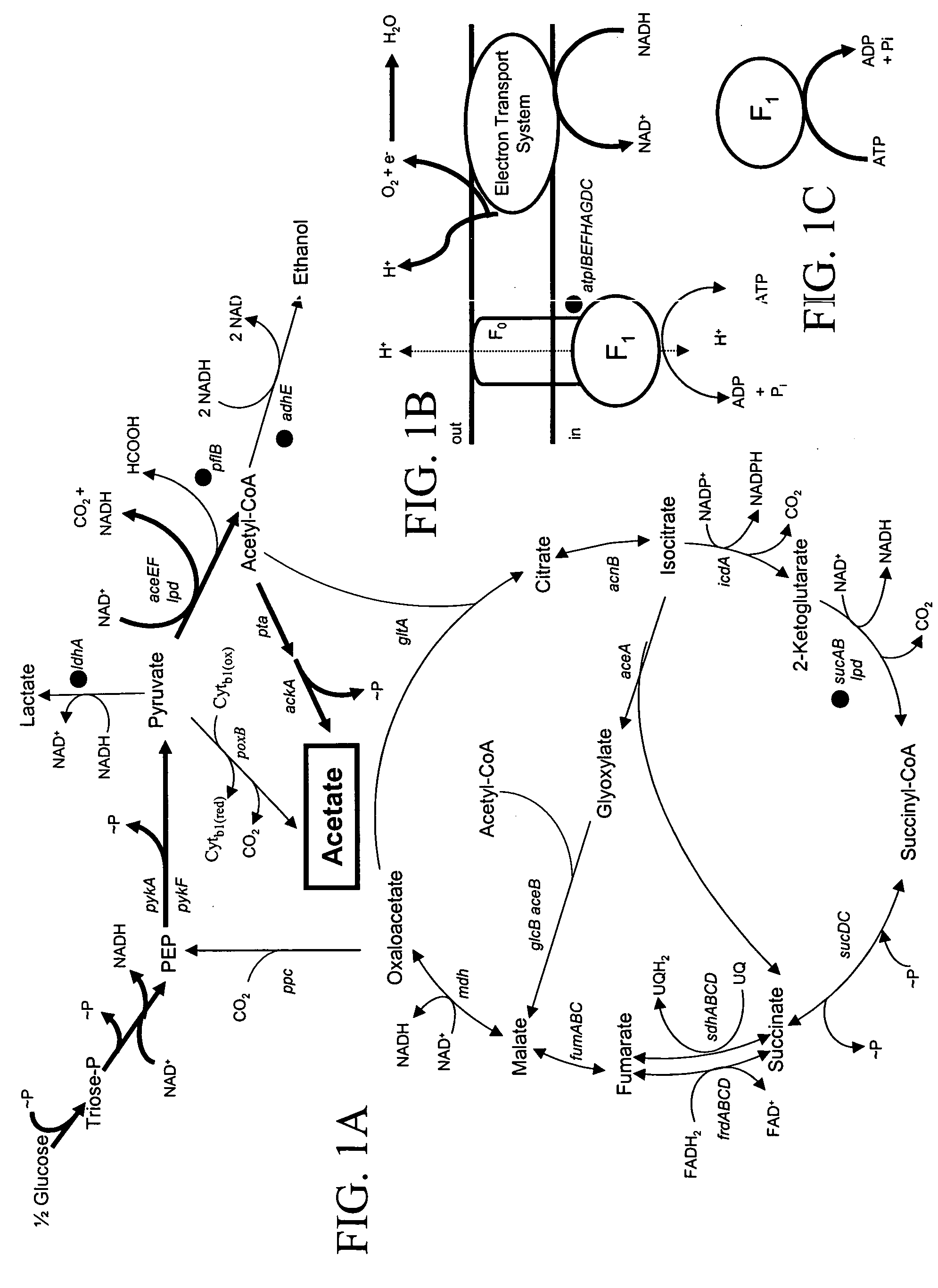
Materials and methods for the efficient production of acetate and other products
InactiveUS20040152159A1Increase productionIncrease acetate productionBacteriaHydrolasesGenes mutationAcetic acid
The subject invention provides materials and methods wherein unique and advantageous combinations of gene mutations are used to direct carbon flow from sugars to a single product. The techniques of the subject invention can be used to obtain products from native pathways as well as from recombinant pathways. In preferred embodiments, the subject invention provides new materials and methods for the efficient production of acetate and pyruvic acid.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Oligonucleotides comprising a modified or non-natural nucleobase
One aspect of the present invention relates to a double-stranded oligonucleotide comprising at least one non-natural nucleobase. In certain embodiments, the non-natural nucleobase is difluorotolyl, nitroindolyl, nitropyrrolyl, or nitroimidazolyl. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, only one of the two oligonucleotide strands comprising the double-stranded oligonucleotide contains a non-natural nucleobase. In certain embodiments, both of the oligonucleotide strands comprising the double-stranded oligonucleotide independently contain a non-natural nucleobase. In certain embodiments, the oligonucleotide strands comprise at least one modified sugar moiety. Another aspect of the present invention relates to a single-stranded oligonucleotide comprising at least one non-natural nucleobase. In a preferred embodiment, the non-natural nucleobase is difluorotolyl. In certain embodiments, the ribose sugar moiety that occurs naturally in nucleosides is replaced with a hexose sugar, polycyclic heteroalkyl ring, or cyclohexenyl group. In certain embodiments, at least one phosphate linkage in the oligonucleotide has been replaced with a phosphorothioate linkage.
Owner:ALNYLAM PHARM INC
High-throughput nucleic acid polymerase devices and methods for their use
High throughput lyophilized polymerase devices and methods for their use in the production of nucleic acids using template dependent polymerase reactions are provided. The subject devices are typically made up of a multi-well substrate that includes in a least one well a lyophilized nucleic acid polymerase composition. The subject nucleic acid polymerase compositions include at least one polymerase and a carbohydrate stabilizing composition that is made up of at least one low molecular weight sugar and a starch. In many embodiments, the compositions also include buffer components and nucleotides, as well as a temperature dependent polymerase inhibitor, e.g., a polymerase specific antibody. Also provided are kits that include the subject devices.
Owner:CLONTECH LAB
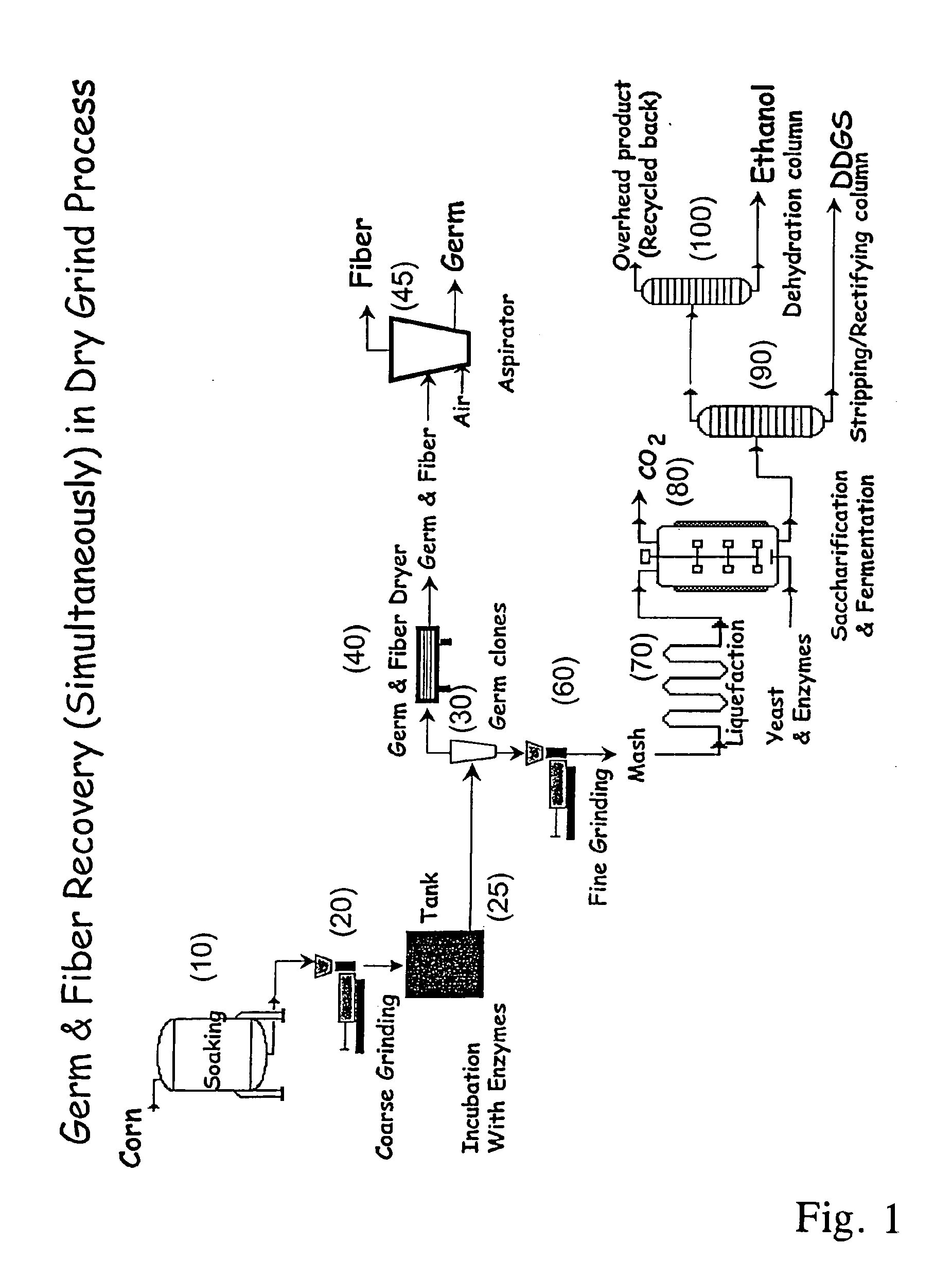
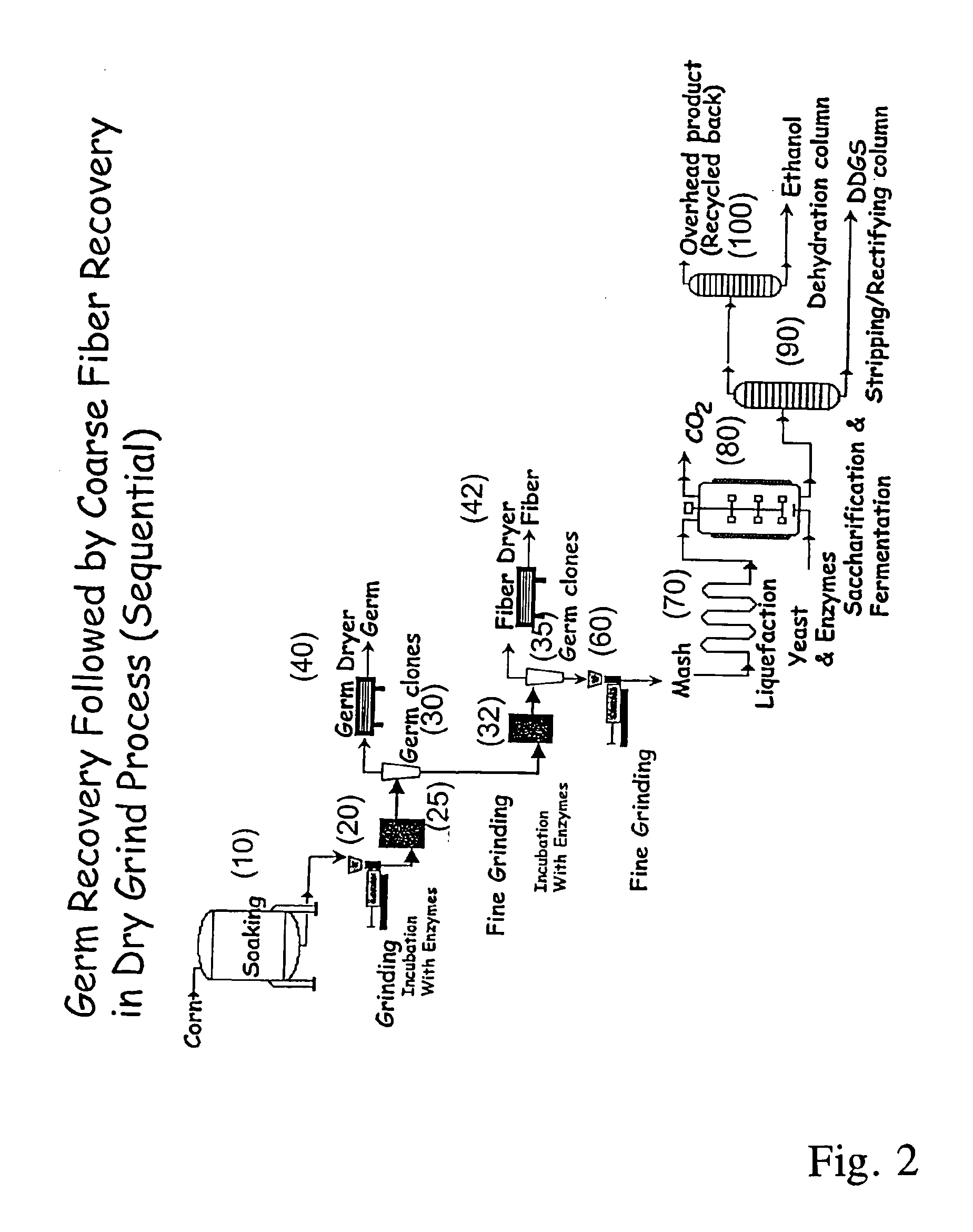
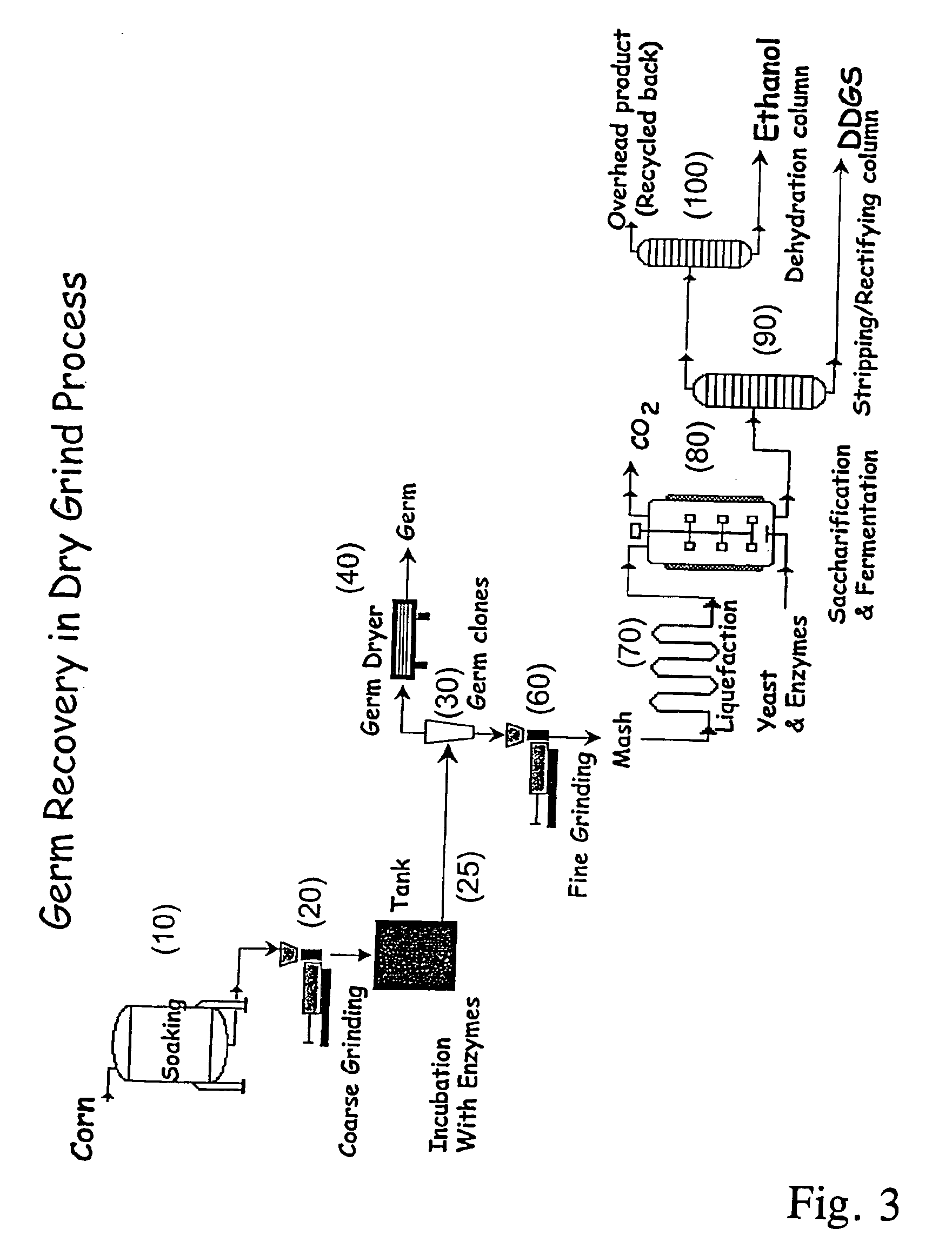
Processes for recovery of corn germ and optionally corn coarse fiber (pericarp)
A process for recovering corn germ and corn coarse fiber from corn in a dry grind process, involving soaking corn kernels in water to produce soaked corn kernels, grinding the soaked corn kernels to produce a ground corn slurry, and incubating the ground corn slurry with at least one enzyme (amylase(s), protease(s), cell wall degrading enzyme(s), or mixtures thereof, and optionally other enzyme(s)) to increase the specific gravity of the slurry to about 10-about 16 Baume so that the corn germ and corn coarse fiber floats to the top of the slurry, recovering the corn germ and the corn coarse fiber, and optionally producing ethanol from the slurry no longer containing the corn germ and corn coarse fiber. The process does not involve the addition of starch, a salt, a sugar syrup, or mixtures thereof to the slurry.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Nucleotide mimics and their prodrugs
The present invention relates to nucleoside diphosphate mimics and nucleoside triphosphate mimics, which contain diphosphate or triphosphate moiety mimics and optionally sugar-modifications and / or base-modifications. The nucleotide mimics of the present invention, in a form of a pharmaceutically acceptable salt, a pharmaceutically acceptable prodrug, or a pharmaceutical formulation, are useful as antiviral, antimicrobial, and anticancer agents. The present invention provides a method for the treatment of viral infections, microbial infections, and proliferative disorders. The present invention also relates to pharmaceutical compositions comprising the compounds of the present invention optionally in combination with other pharmaceutically active agents.
Owner:BIOTA SCI MANAGEMENT PTI LTD
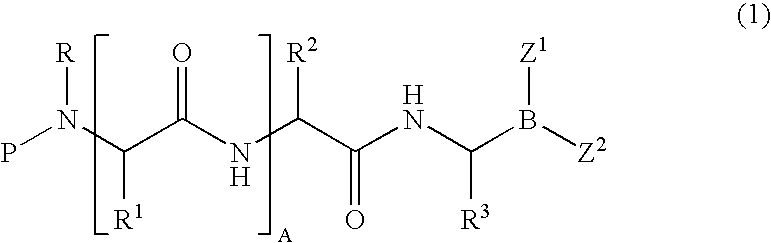
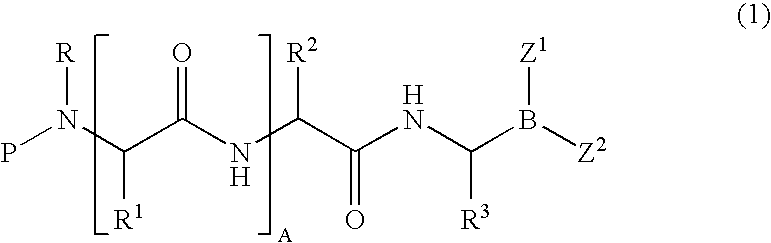
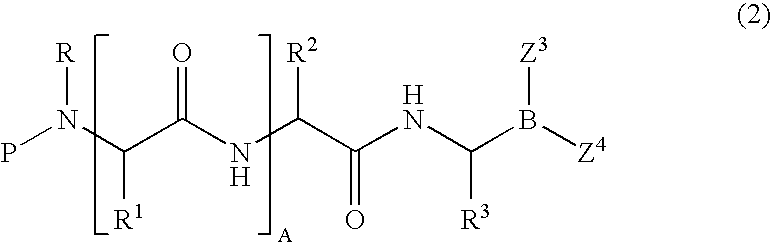
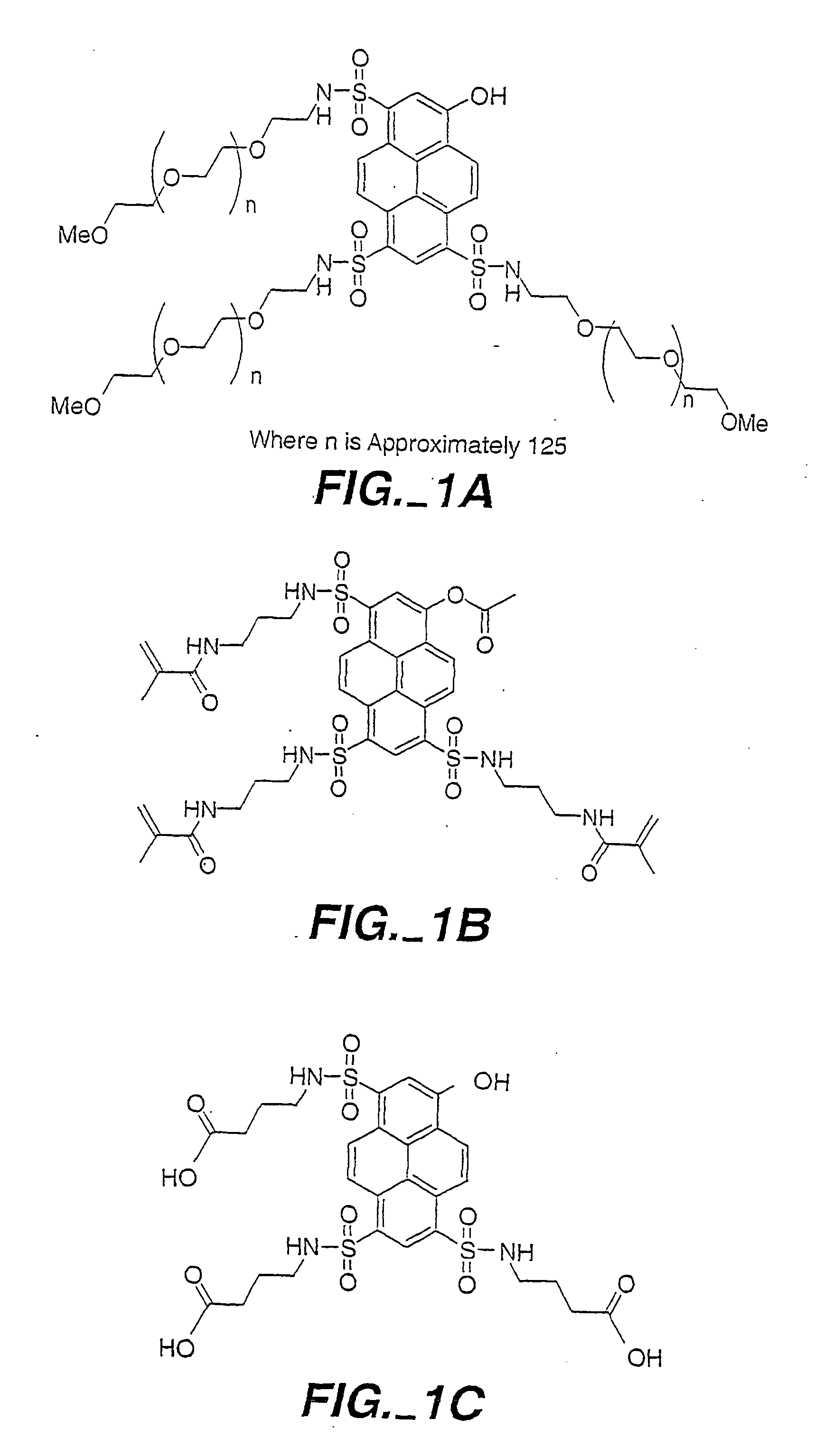
Formulation of boronic acid compounds
The present invention provides stable compounds prepared from boronic acid and lyophilized compounds thereof of the formula (1): in which Z1 and Z2 are moieties derived from sugar. The invention also provides methods for preparing such compounds. Lyophilizing a mixture comprising a boronic acid compound and a moiety derived from sugar produces a stable composition that readily releases the boronic acid compound upon reconstitution in aqueous media.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
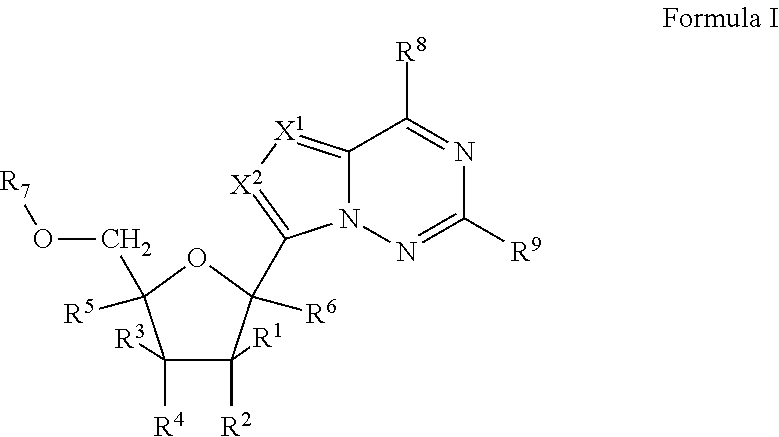
2'-fluoro substituted carba-nucleoside analogs for antiviral treatment
Provided are select imidazo[1,2-f][1,2,4]triazinyl nucleosides, nucleoside phosphates and prodrugs thereof, wherein the 2′ position of the nucleoside sugar is substituted with halogen and carbon substituents. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections caused by both wild type and mutant strains of HCV.
Owner:GILEAD SCI INC
pH-triggered microparticles
InactiveUS20050123596A1Efficient deliveryGood biocompatibilityPowder deliveryMicroencapsulation basedPolyesterLipid formation
Microparticles that are designed to release their payload when exposed to acidic conditions are provided as a vehicle for drug delivery. Any therapeutic, diagnostic, or prophylatic agent may be encapsulated in a lipid-protein-sugar or polymeric matrix including a pH triggering agent to form pH triggerable microparticles. Preferably the diameter of the pH triggered microparticles ranges from 50 nm to 10 micrometers. The matrix of the particles may be prepared using any known lipid (e.g., DPPC), protein (e.g., albumin), or sugar (e.g., lactose). The matrix of the particles may also be prepared using any synthetic polymers such as polyesters. Methods of preparing and administering the particles are provided. Methods of immunization, transfection, and gene therapy are also provided by administering pH triggerable microparticles.
Owner:DANA FARBER CANCER INST INC +2
Optical determination of glucose utilizing boronic acid adducts
InactiveUS20060083688A1Efficient excitationHigh selectivityUltrasonic/sonic/infrasonic diagnosticsMaterial nanotechnologyIn vivoAdduct
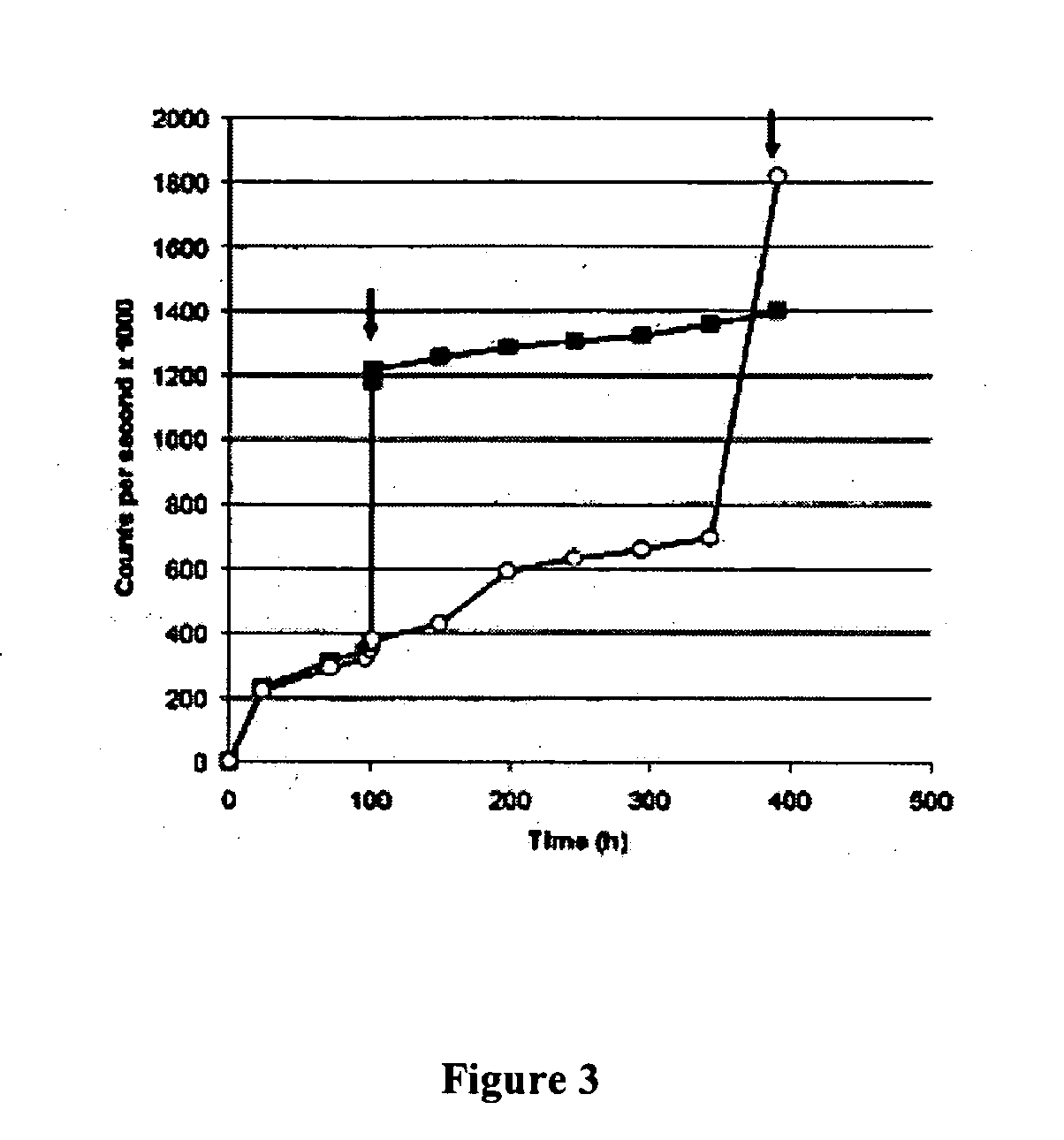
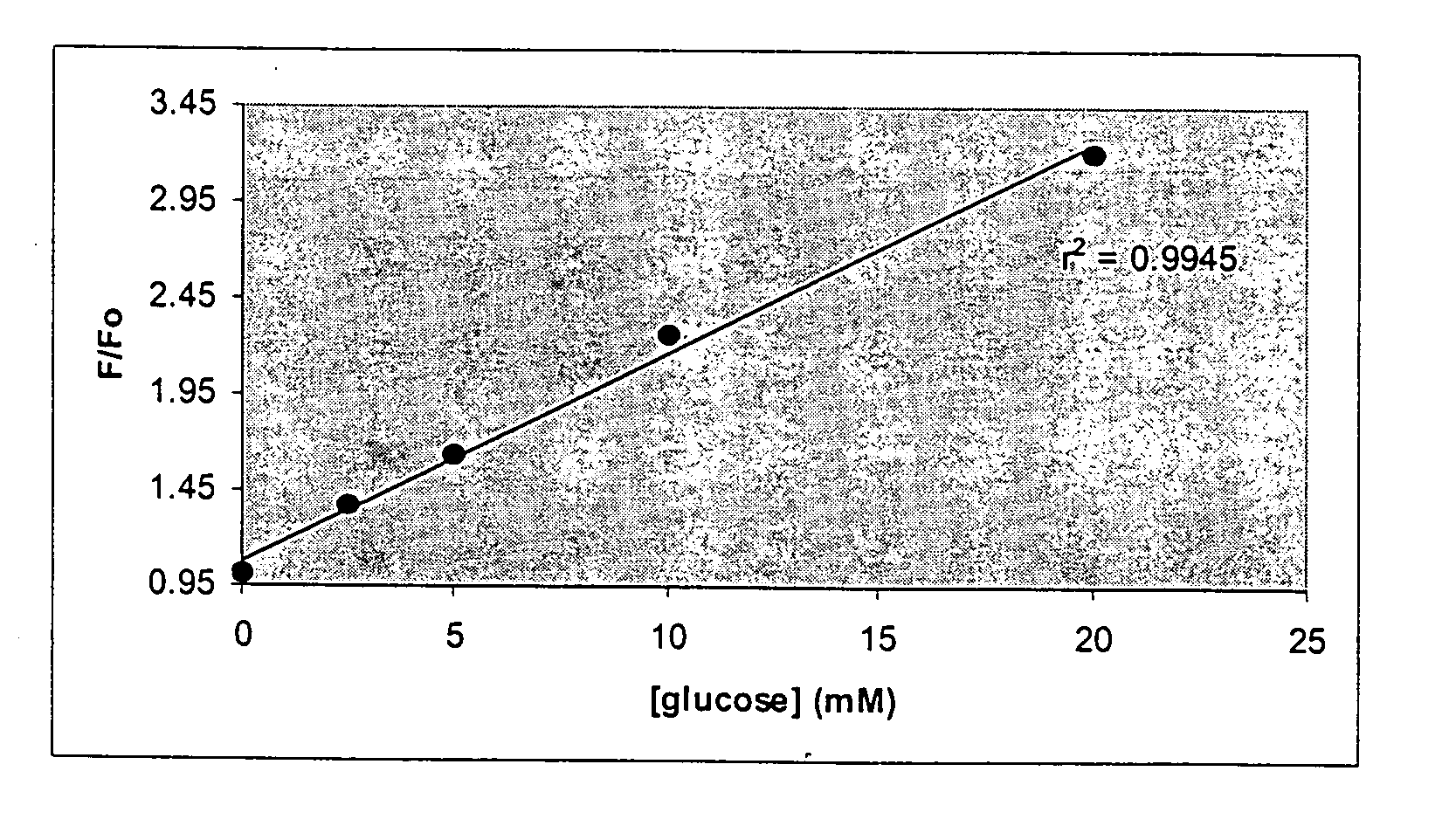
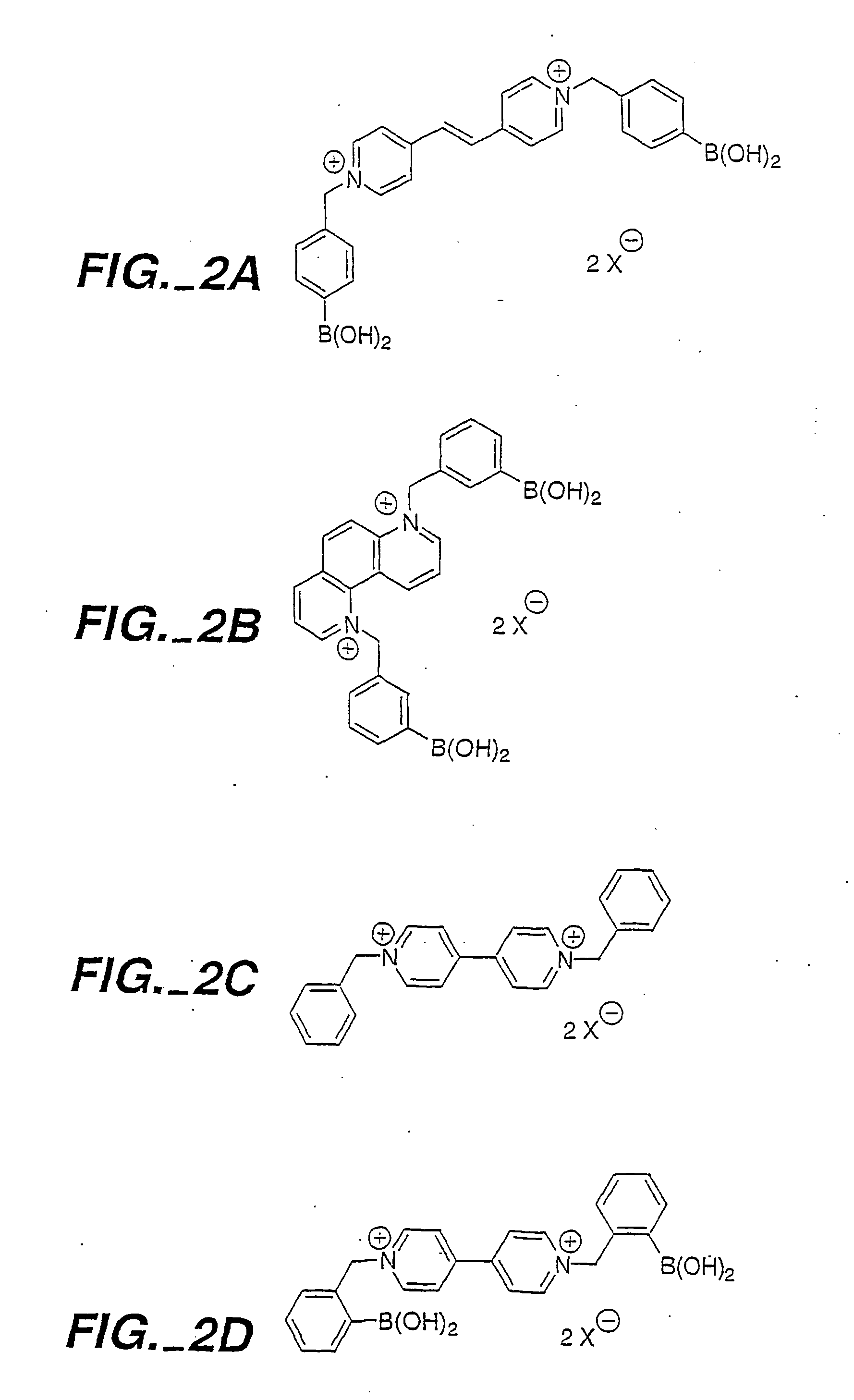
The present invention concerns an improved optical method and optical sensing device for determining the levels of polyhydroxyl-substituted organic molecules in vitro and / or in vivo in aqueous media. The range of detection is between about 400 and 800 nm. In particular, a sensory devise is implemented in a mammal to determine sugar levels. Specifically, a dye is combined with a conjugated nitrogen-containing heterocyclic aromatic boronic acid-substituted bis-onium compound in the presence of a sugar, such as fructose or glucose. The viologens are preferred as the aromatic conjugated nitrogen-containing boronic acid substituted compounds. The method is useful to determine sugar levels in a human being.
Owner:RGT UNIV OF CALIFORNIA
Sugar modified oligonucleotides that detect and modulate gene expression
Compositions and methods are provided for the treatment and diagnosis of diseases amenable to modulation of the production of selected proteins. In accordance with preferred embodiments, oligonucleotides and oligonucleotide analogs are provided which are specifically hybridizable with a selected sequence of RNA or DNA wherein at least one of the 2'-deoxyfuranosyl moieties of the nucleoside unit is modified. Treatment of HIV, herpes virus, papillomavirus and other infections is provided.
Owner:IONIS PHARMA INC
Use of lignocellulosics solvated in ionic liquids for production of biofuels
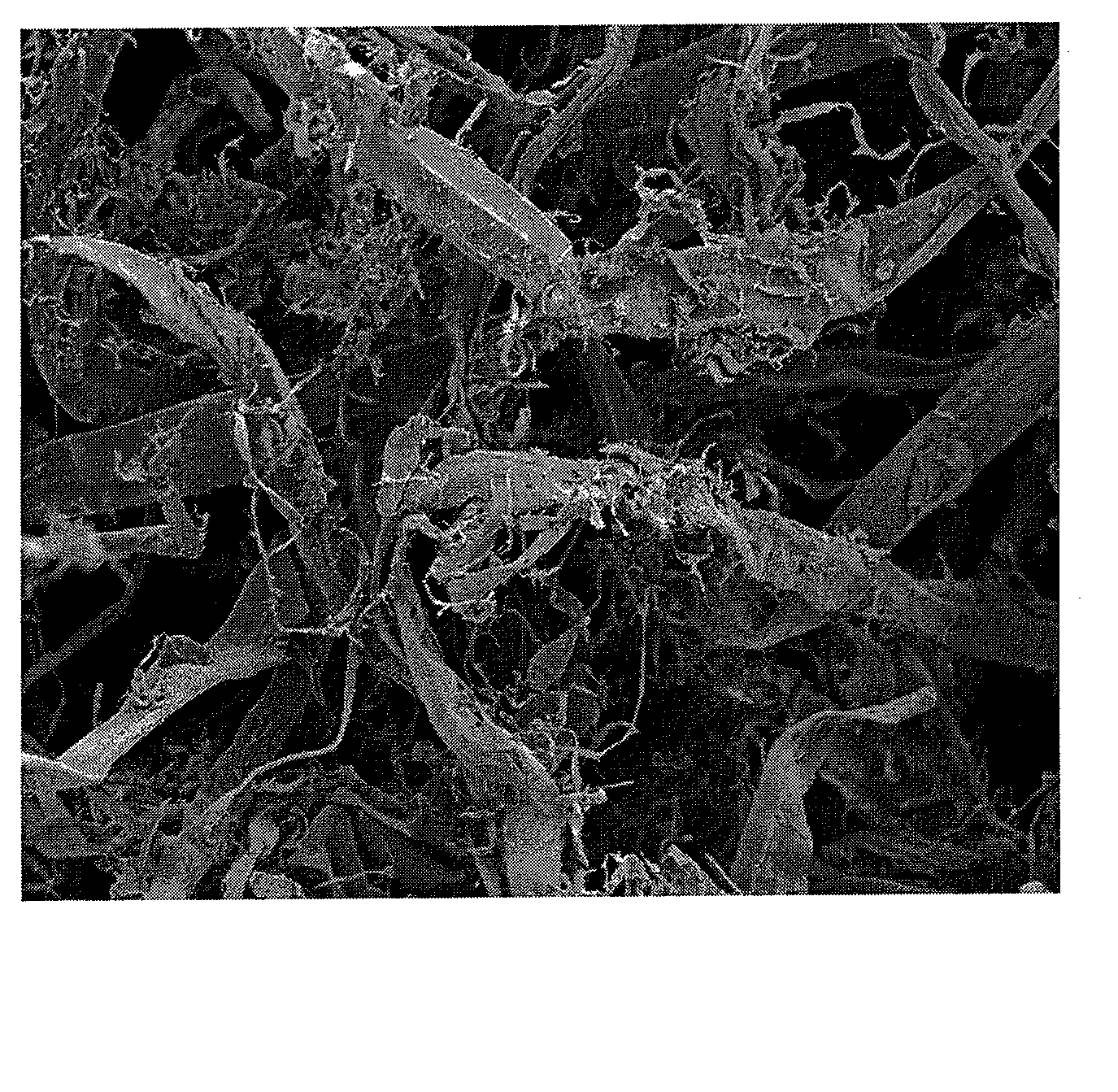
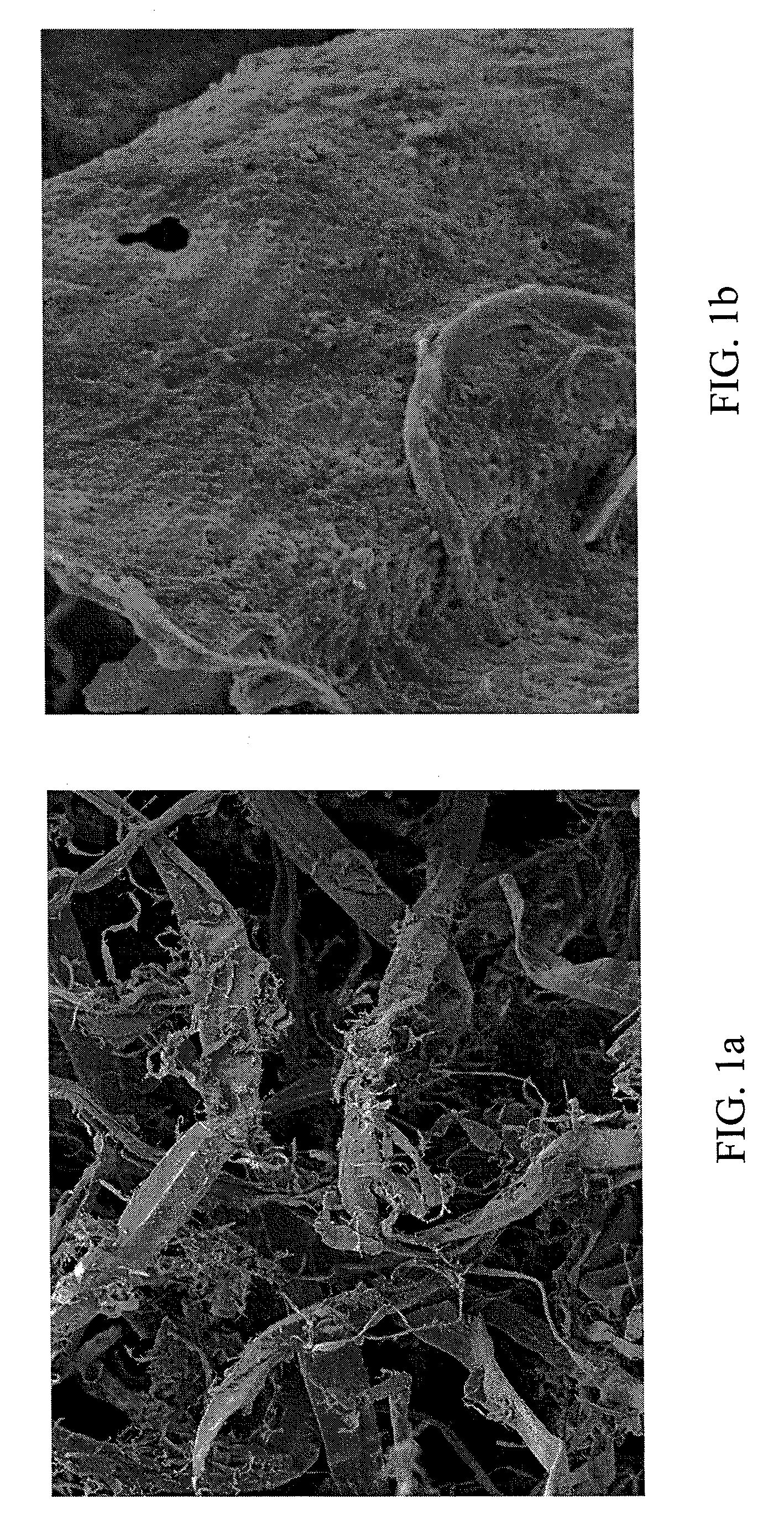
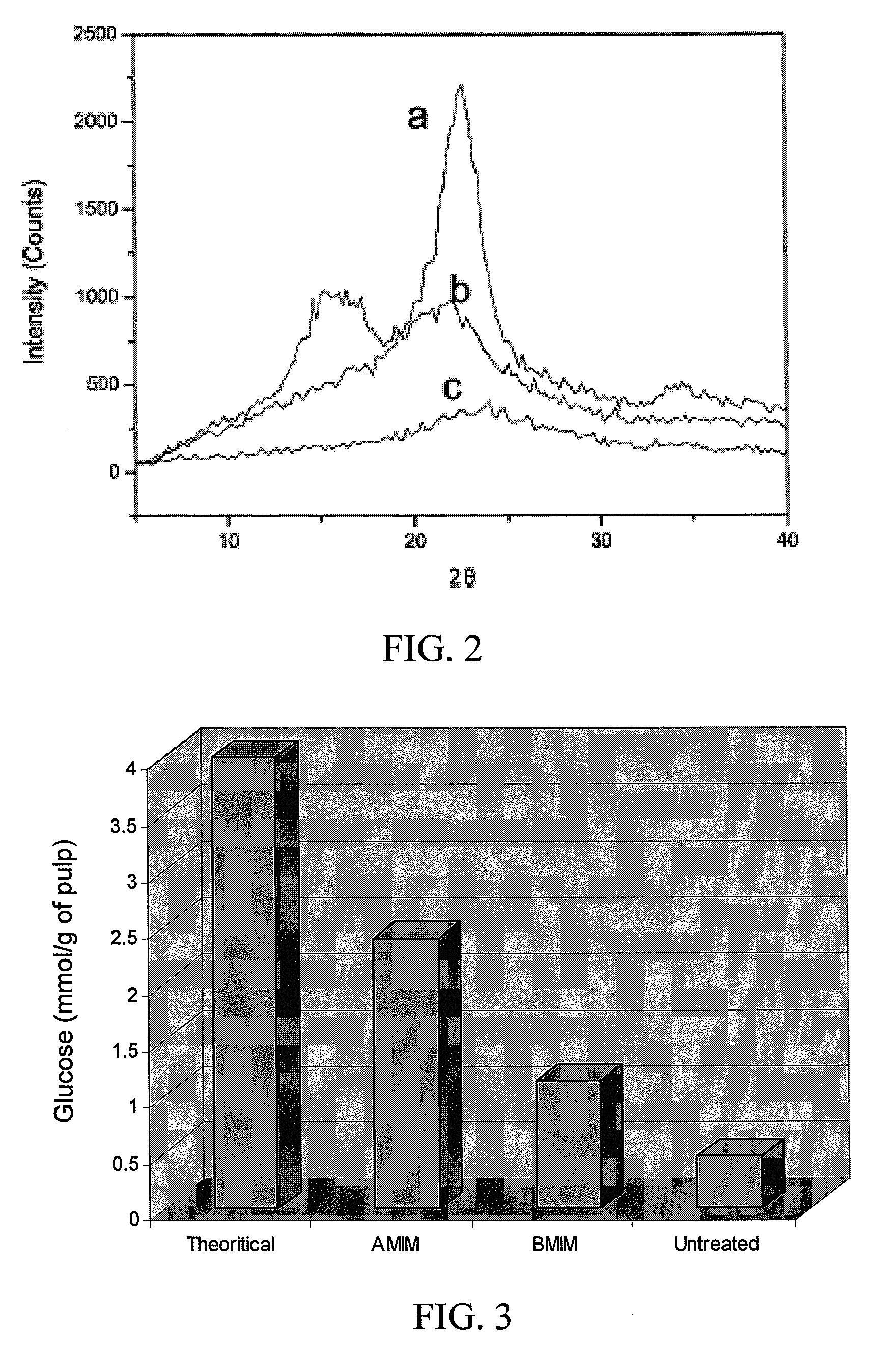
InactiveUS20080190013A1Guaranteed economic efficiencyReduced availabilityCellulosic pulp after-treatmentSugar productsBiofuelEvaporation
The present invention provides a method for converting lignocellulosic material into biofuel. In particular embodiments, the method comprises pre-treating lignocellulosic material by dissolving the material in ionic liquids. The pretreated lignocellulosic material can be isolated, such as by precipitation with a regenerating solvent (e.g., water), and be used directly in the formation of biofuel, including undergoing hydrolysis to form sugar and fermentation to form fuel, such as bioethanol. The ionic liquid can be recycled for further use, such as by evaporation of the water introduced during precipitation, and the recycling provides a route to a hemicellulose rich fraction and an ionic liquid of consistent quality and wood dissolution characteristics. The recovered hemicelluloses are of significant utilization potential toward commodity and specialty applications.
Owner:NORTH CAROLINA STATE UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com