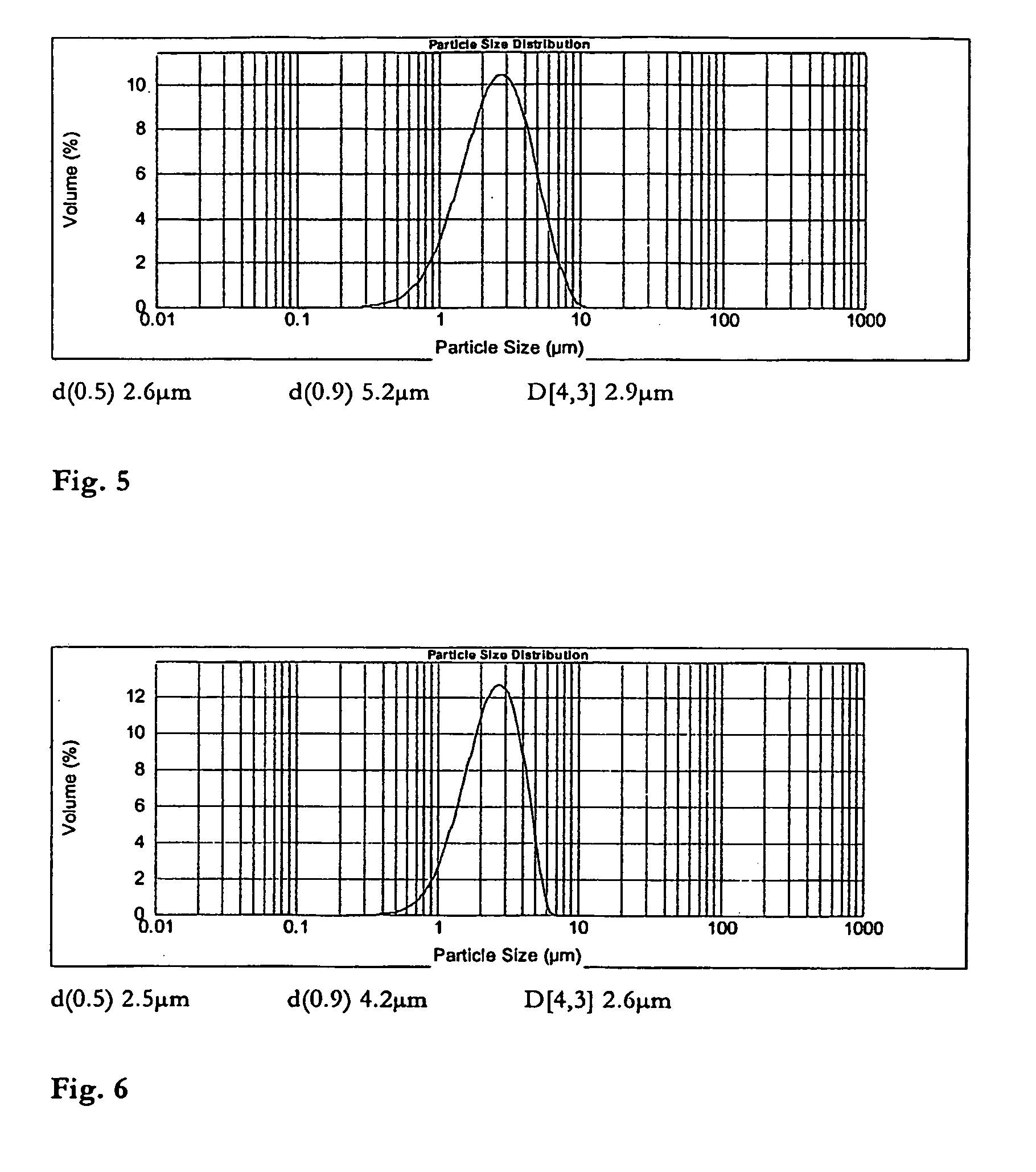
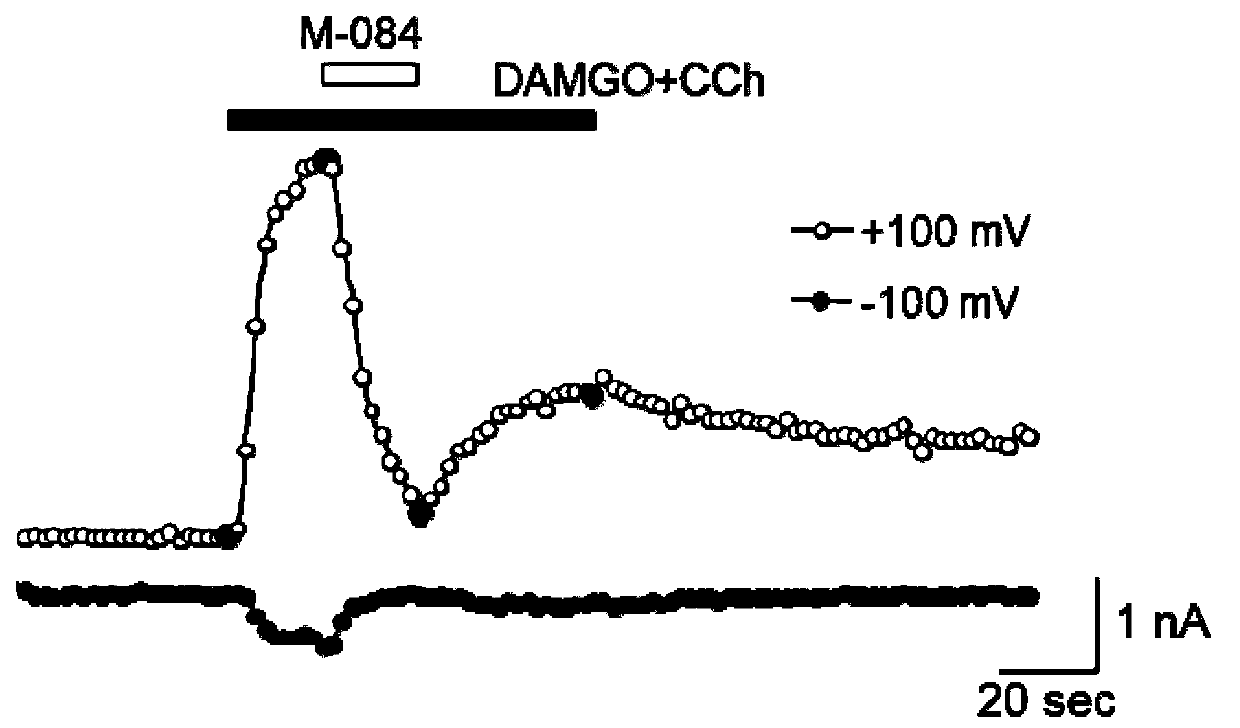
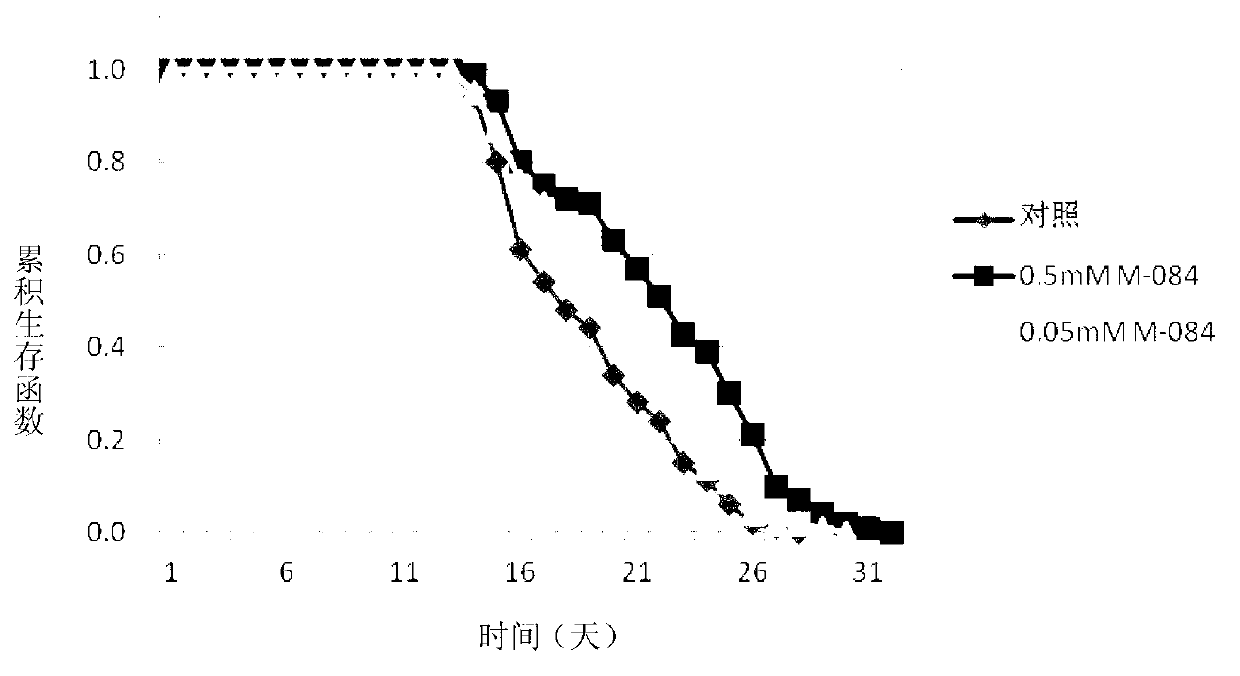
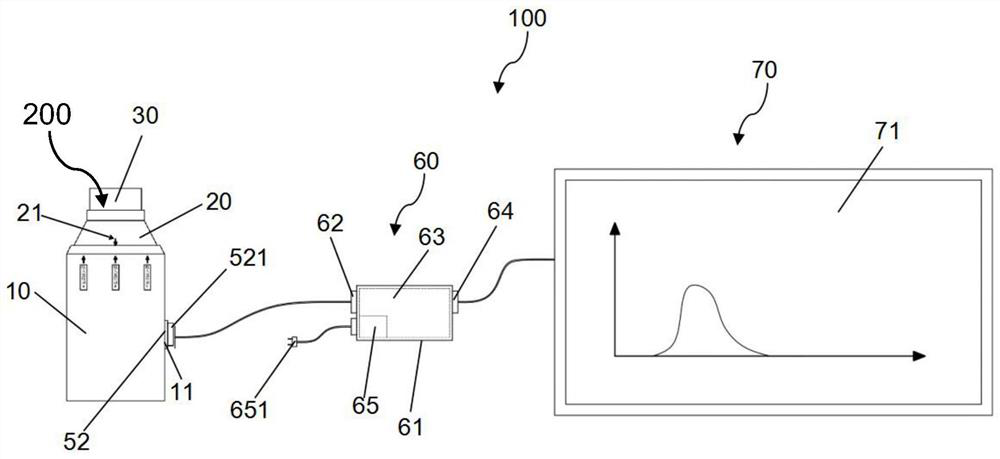
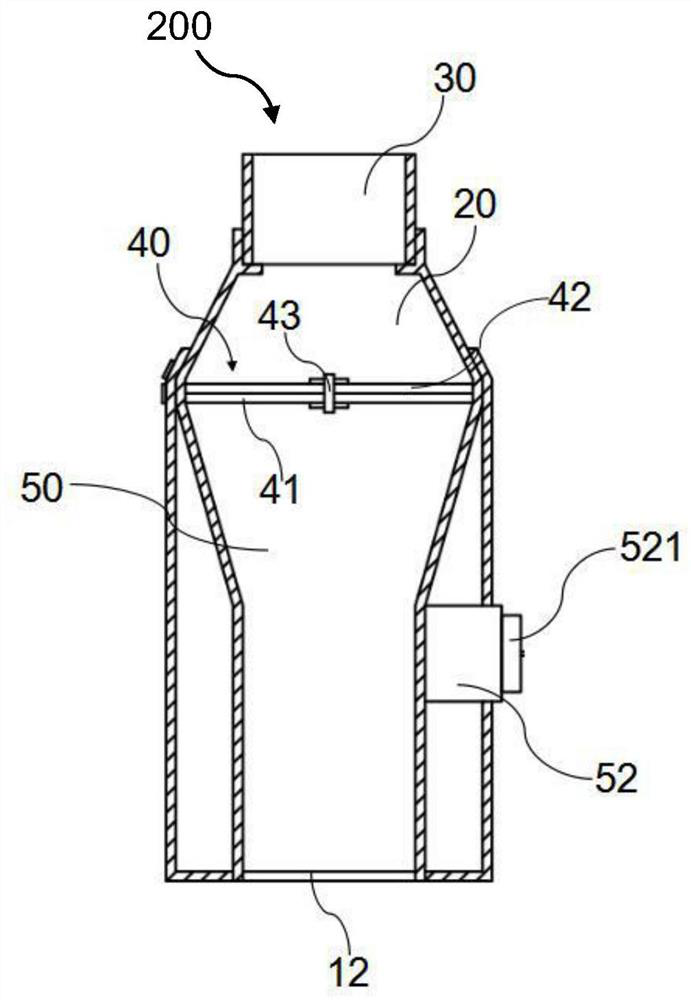
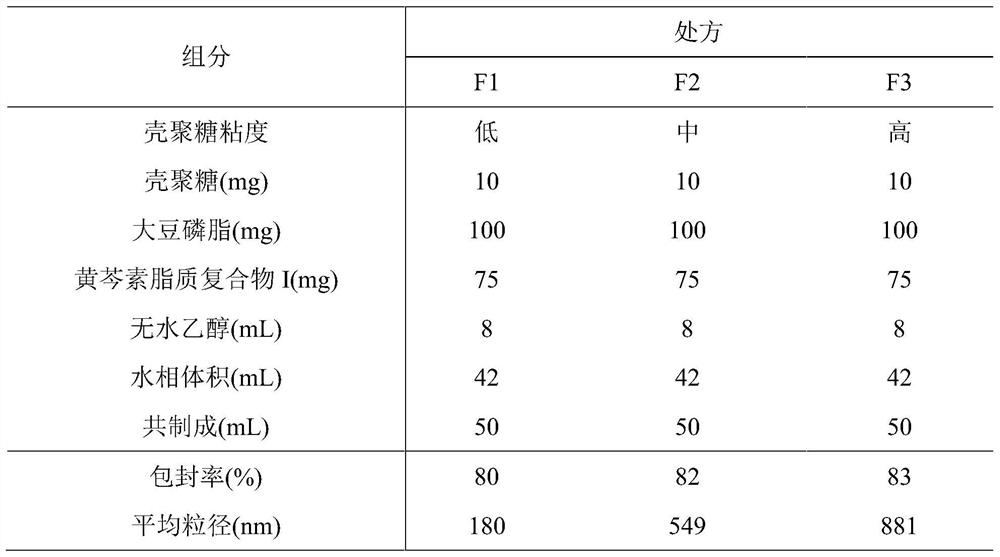
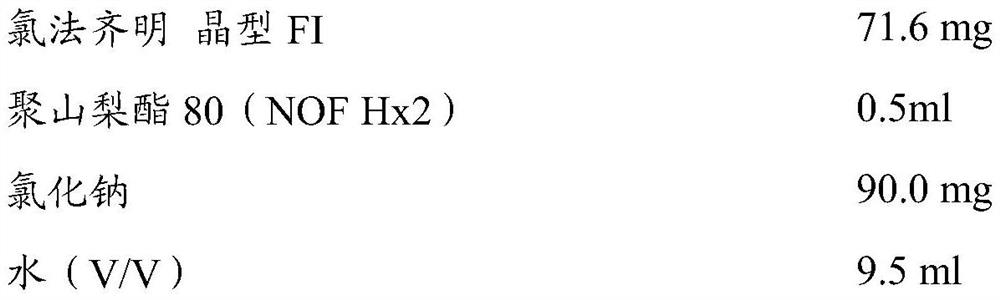Patents
Literature
45 results about "Pulmonary inhalation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Parenteral delivery systems
Hypertonic sugar compositions administered by other than ingestion and swallowing or intravascular injection, such as by intranasal spray or drops, intraocular drops or ointment, oral spray, intraotic spray or drops, lozenges, chewable tablet, chewing gum, or gargle, pulmonary inhalation, vaginal or rectal suppositories, or transdermal creams, ointments, lotions, or patches, are effective to open the blood-brain barrier to permit entry into the central nervous system of a co-administered chemical compound, such as a nutrient or a therapeutic or diagnostic agent. In this way, the compositions and methods of the invention increase the therapeutic or diagnostic efficacy of such chemical compounds.
Owner:NAITO ALBERT T
Medical product
InactiveUS20060239933A1Powder deliveryPeptide/protein ingredientsPulmonary inhalationMedical product
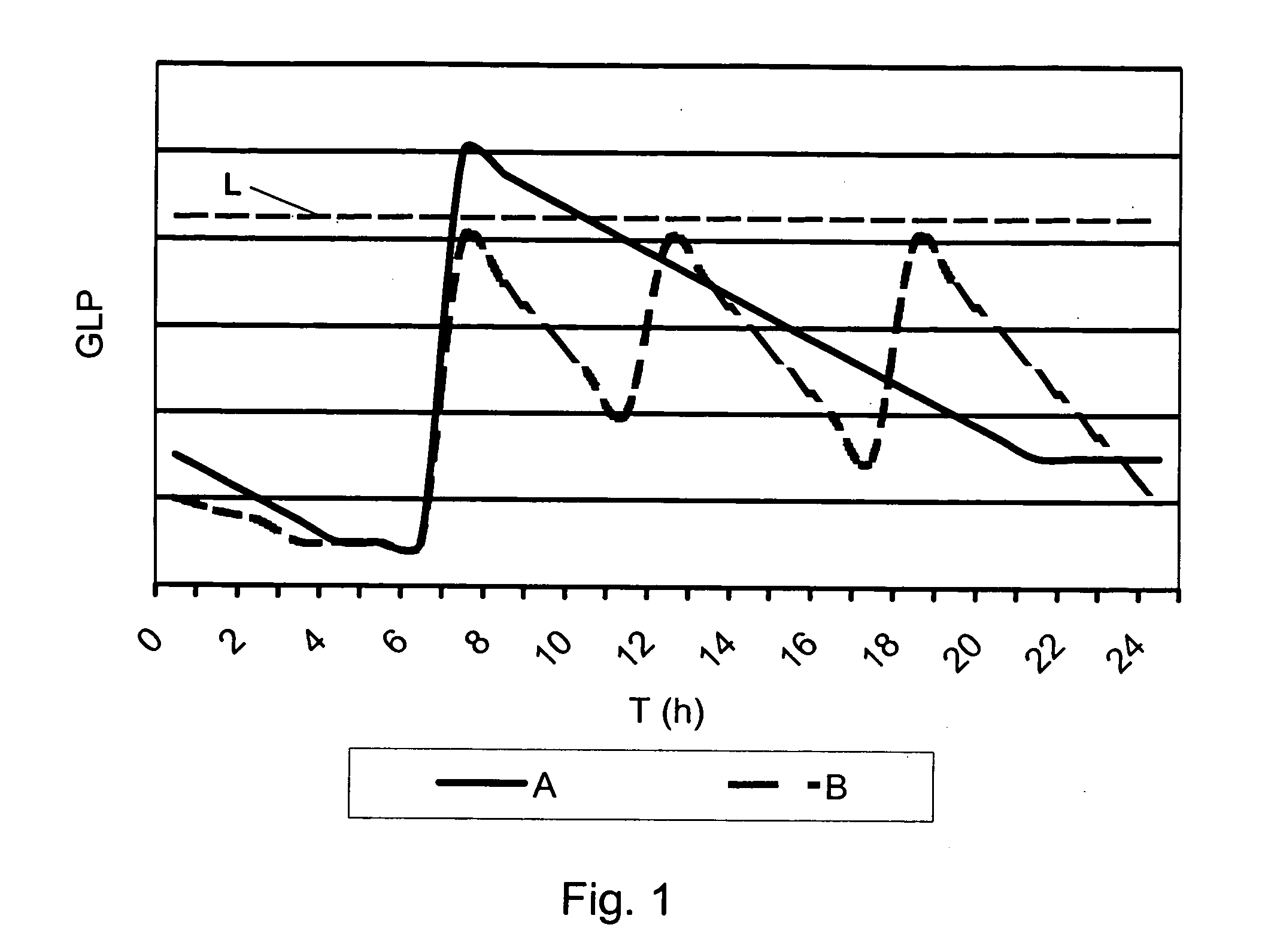
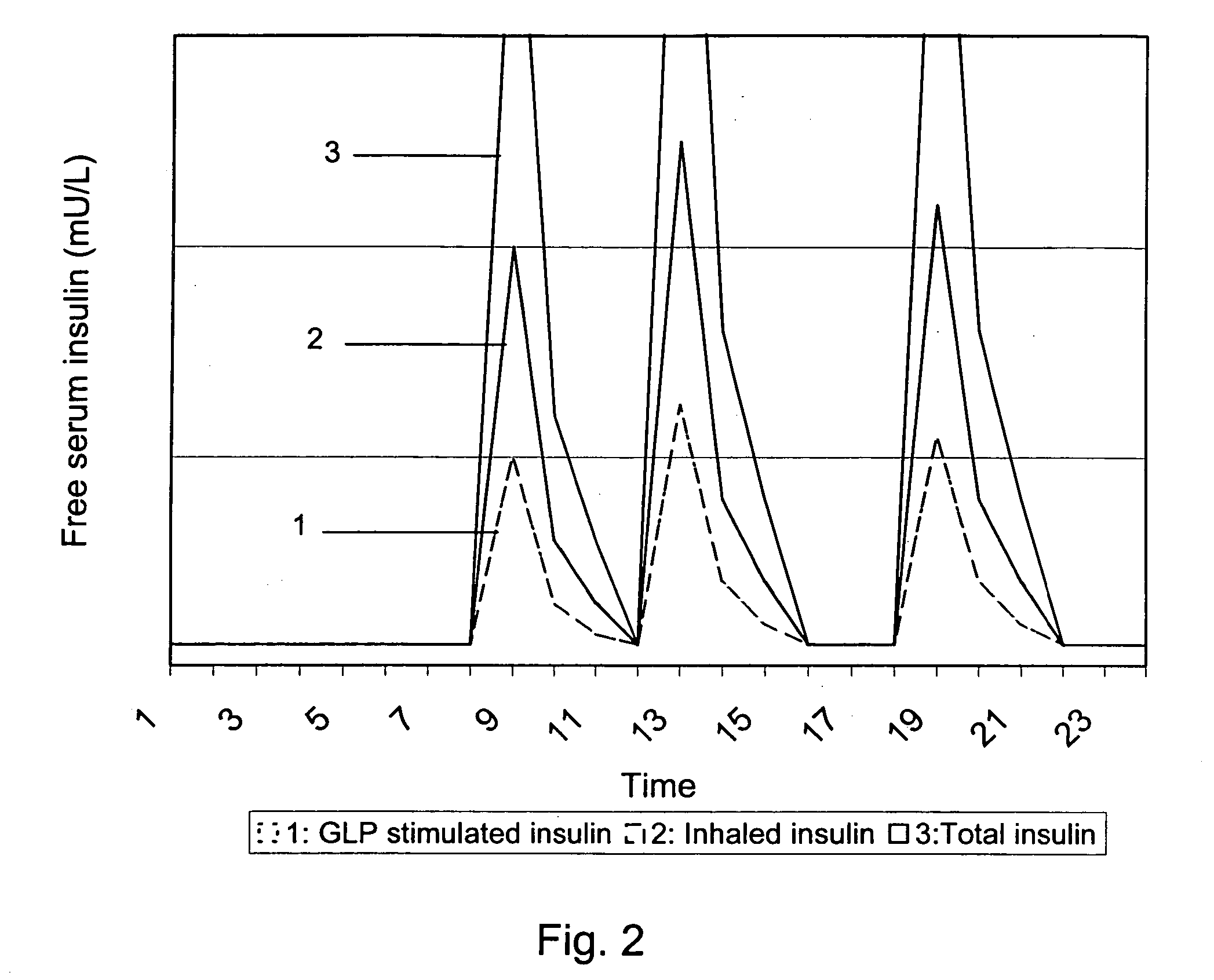
A medical product is disclosed. The medical product contains an accurately metered dose of at least one GLP medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Suspension formulations
ActiveUS20110182997A1Reduced tendency to flocculateLong shelf lifeBiocideDispersion deliveryPulmonary inhalationNasal cavity
The present invention relates to suspension formulations, especially those for delivering a pharmaceutically active agent in aerosol form using a spray or aerosol device, such as a pressurised metered dose inhaler (pMDI). The formulations may be for pulmonary, nasal, buccal or topical administration, but are preferably for pulmonary inhalation.
Owner:INNOVATA BIOMED +1
Compositions for treating parkinson's disease
The present invention relates to improved treatment of diseases and disorders of the central nervous system by administration of apomorphine. In particular, the administration is via pulmonary inhalation. The invention provides the means for improving the treatment of a number of conditions, including Parkinson's Disease.
Owner:VECTURA LTD
Pyrazolopyrimidine compound and pharmaceutical composition thereof as well as pharmaceutical application of pyrazolopyrimidine compound
The invention provides a pyrazolopyrimidine compound shown as a structural formula (I), a pharmaceutical composition taking the pyrazolopyrimidine compound as an active component, a preparation method of the pyrazolopyrimidine compound and the pharmaceutical composition as well as an application of the pyrazolopyrimidine compound and the pharmaceutical composition in preparation of a TRPC6 (Transient Receptor Potential Channel 6) adjustor probe medicine and related medicines for preventing and treating glomerulopathy and myocardial hypertrophy. The pyrazolopyrimidine compound and derivatives provided by the invention can be used to prepare medical preparations in various forms which comprise oral liquids, injections, pulmonary inhalation preparations and transdermal preparations, specifically injections, oral liquids, troches, capsules, granules, aerosols, dry powder inhalation, patches and the like.
Owner:泸州天演生物医药科技有限公司
Mucoactive agents for treating a pulmonary disease
InactiveUS7928089B2Decrease mucus viscoelasticityReduce viscoelasticityPowder deliveryOrganic active ingredientsPulmonary inhalationObstructive Pulmonary Diseases
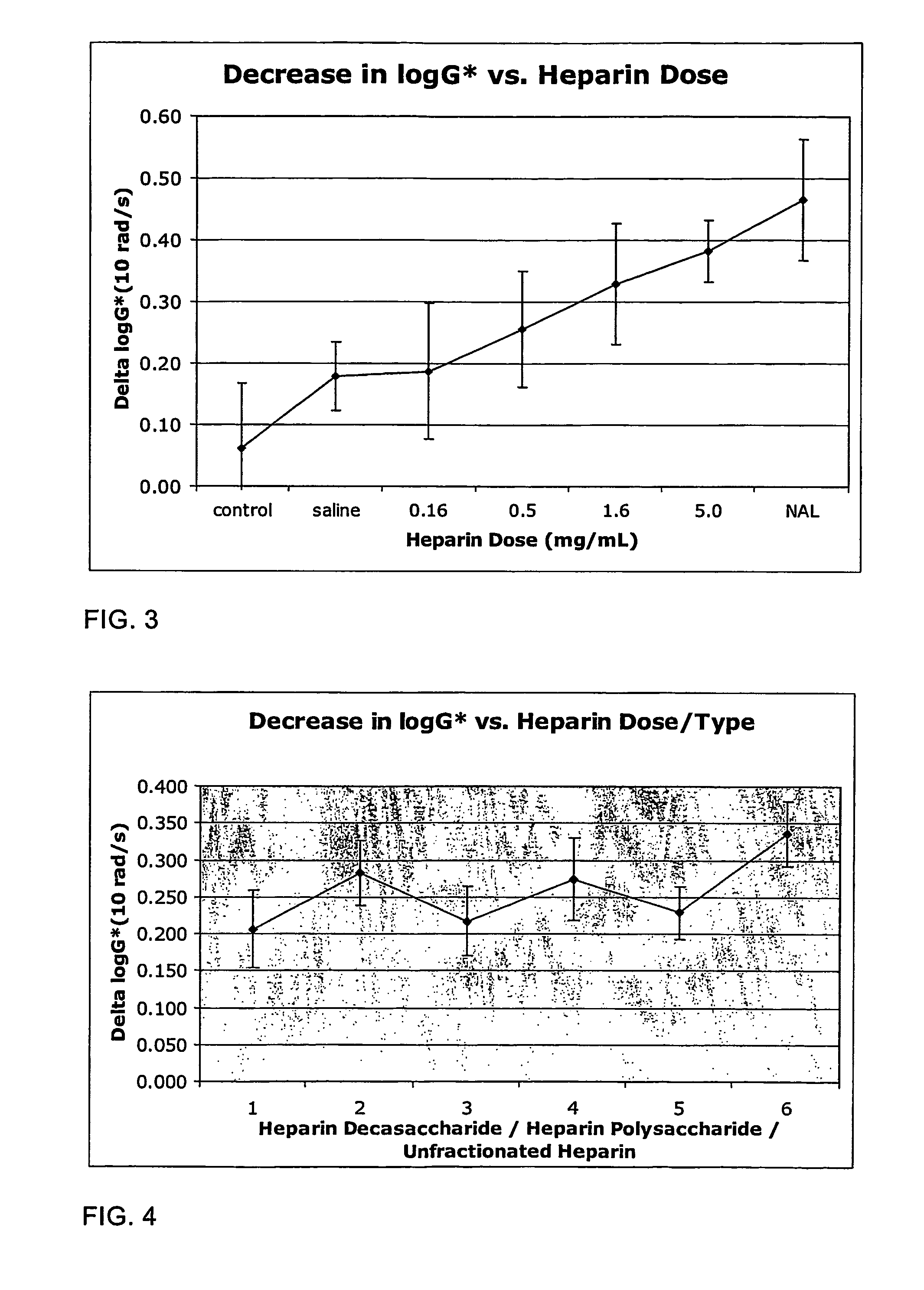
The present invention relates to mucoactive agents, such as heparin which are useful in the treatment of diseases where excess mucus is present in the respiratory tract, such as cystic fibrosis and chronic obstructive pulmonary disease. In particular, the invention relates to pharmaceutical compositions for administration by pulmonary inhalation. It also relates to methods for producing particles suitable for pulmonary inhalation, such as spray drying or jet milling.
Owner:VECTURA LTD
Breakthrough pain management
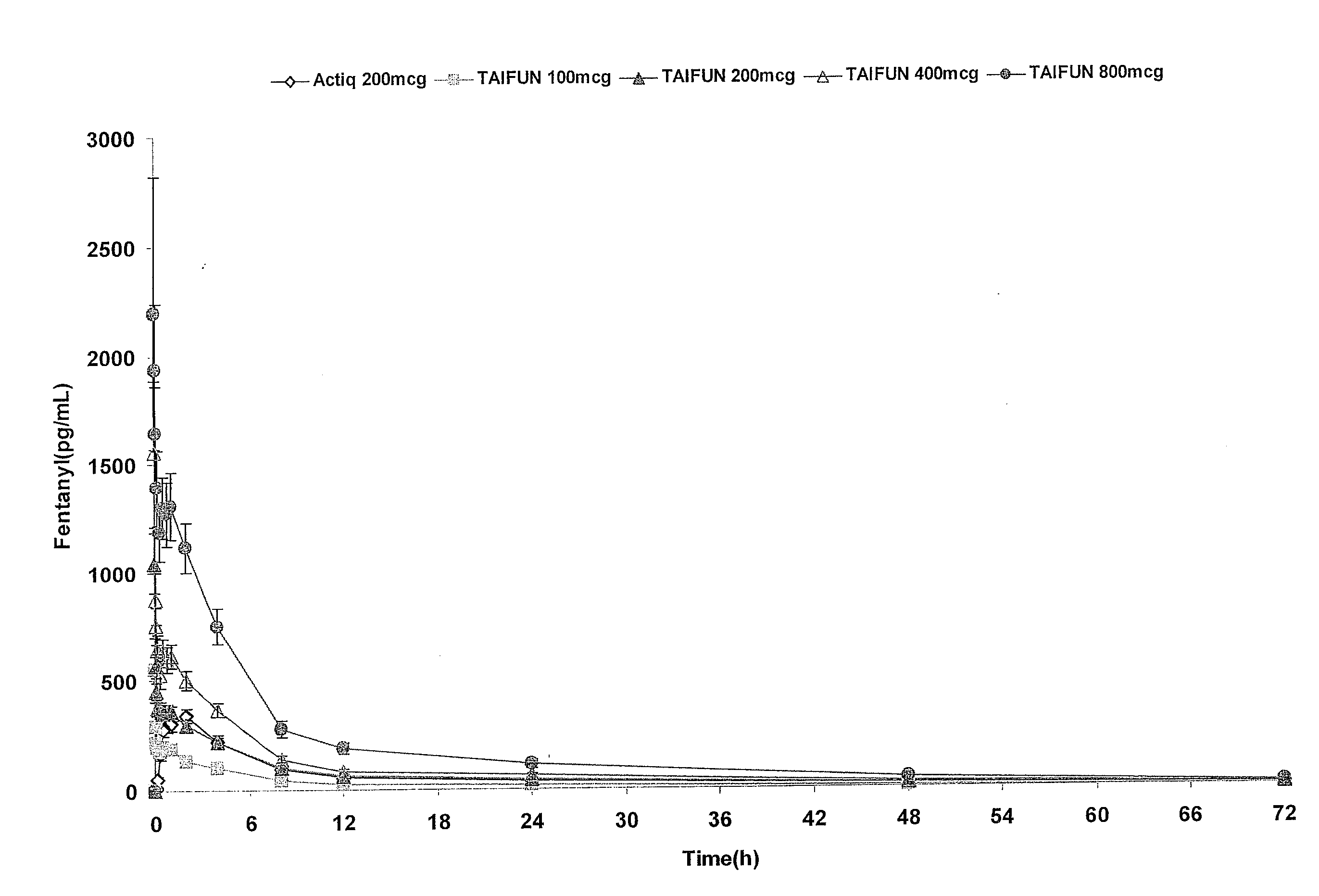
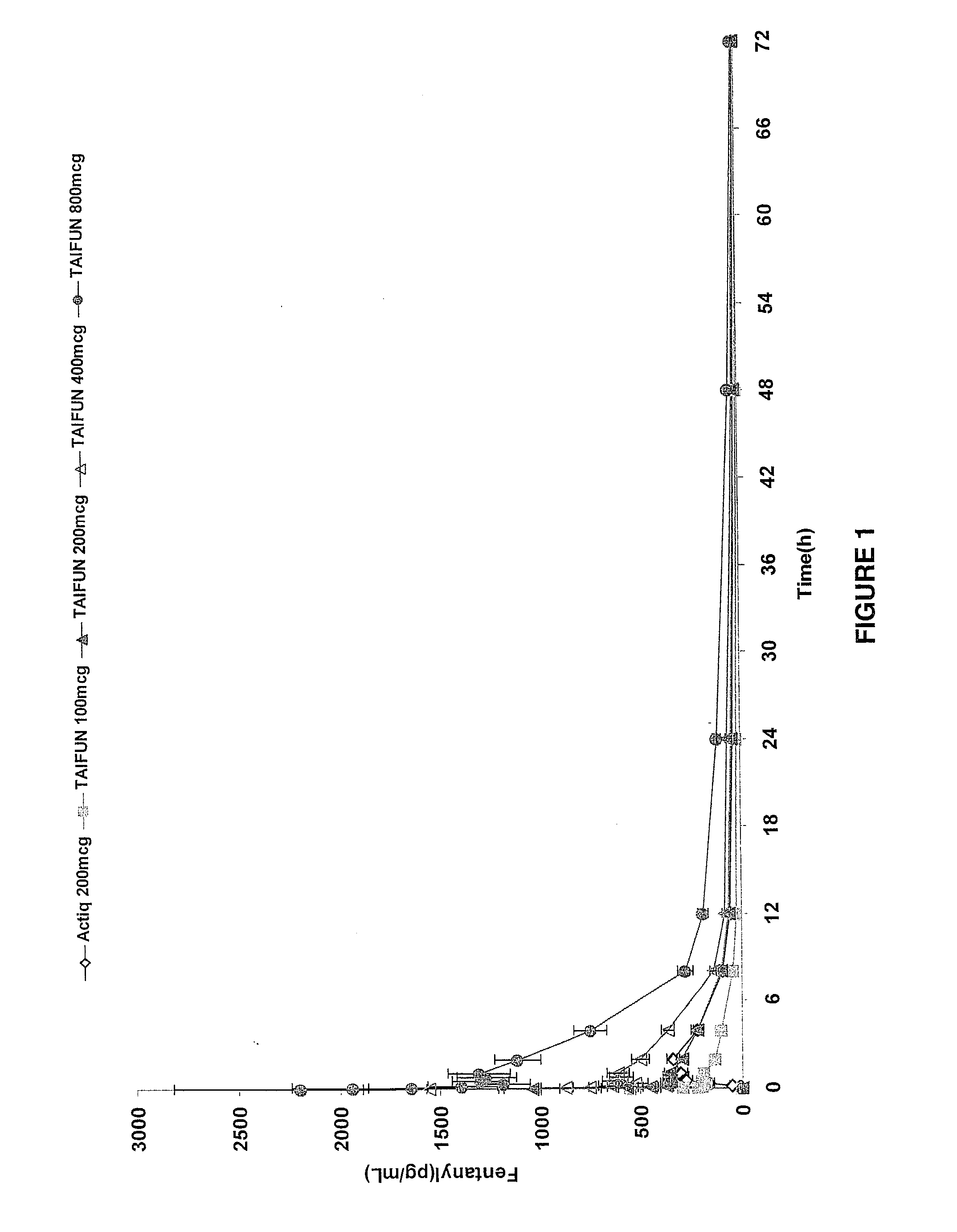
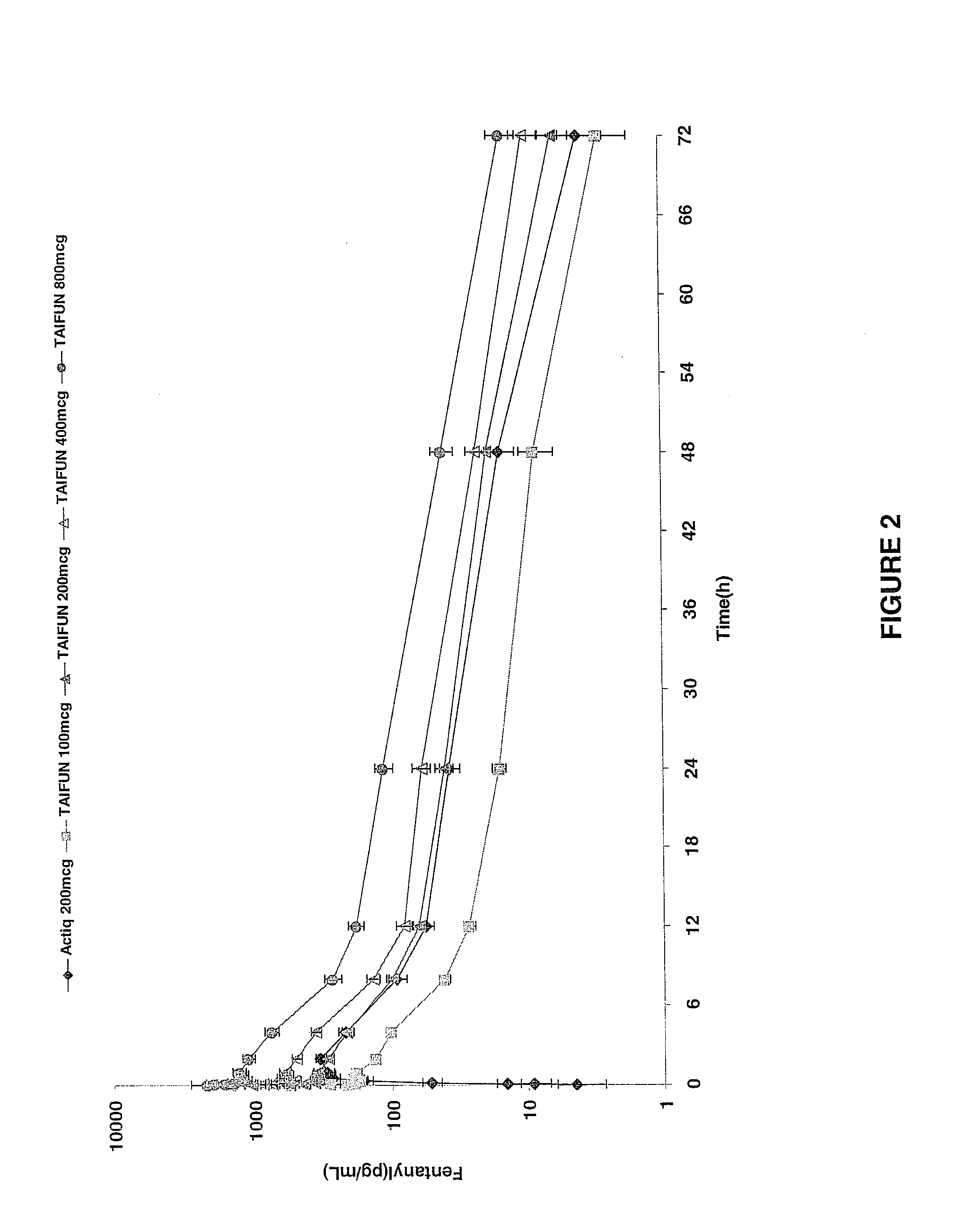
InactiveUS20090011030A1Treatment and alleviation of painAct quicklyBiocidePowder deliveryPulmonary inhalationFentanyl
The present invention is directed to a powdered formulation comprising an analgesic, preferably fentanyl, for use in pulmonary inhalation administration for the rapid analgesic titration of pain, in particular breakthrough pain. Upon administration, the powdered formulation is able to provide a narrower titration range in patients suffering from pain, as well as effective analgesic amounts of fentanyl in a shorter time and at lower dose levels of administered fentanyl when compared to fentanyl administered by an oral transmucosal route.
Owner:LAB INT
Dry powder composition comprising co-jet milled particles for pulmonary inhalation
ActiveUS8182838B2Great dosing efficiencyEasy dosePowder deliveryDispersion deliveryPulmonary inhalationMedicine
The present invention relates to particles and to methods of making particles. In particular, the invention relates to methods of making composite active particles comprising a pharmaceutically active material for pulmonary inhalation, the method comprising a jet milling process.
Owner:VECTURA LTD
Pharmaceutical compositions comprising apomorphine for pulmonary inhalation
InactiveUS20060178394A1Improve performanceRapid blood levelBiocidePowder deliveryPulmonary inhalationSexual dysfunction
The present invention relates to inhalable formulations of apomorphine or its pharmaceutically acceptable salts or esters for use in treating sexual dysfunction. The present invention also relates to methods for preparing the apomorphine formulations as well as to methods for treatment of sexual dysfunction using said formulations and inhalers including said formulations. The present invention further relates to the use of apomorphine in the manufacture of a medicament for treating sexual dysfunction.
Owner:VECTURA LTD
Phospholipid chitosan drug delivery system, preparation method and uses thereof
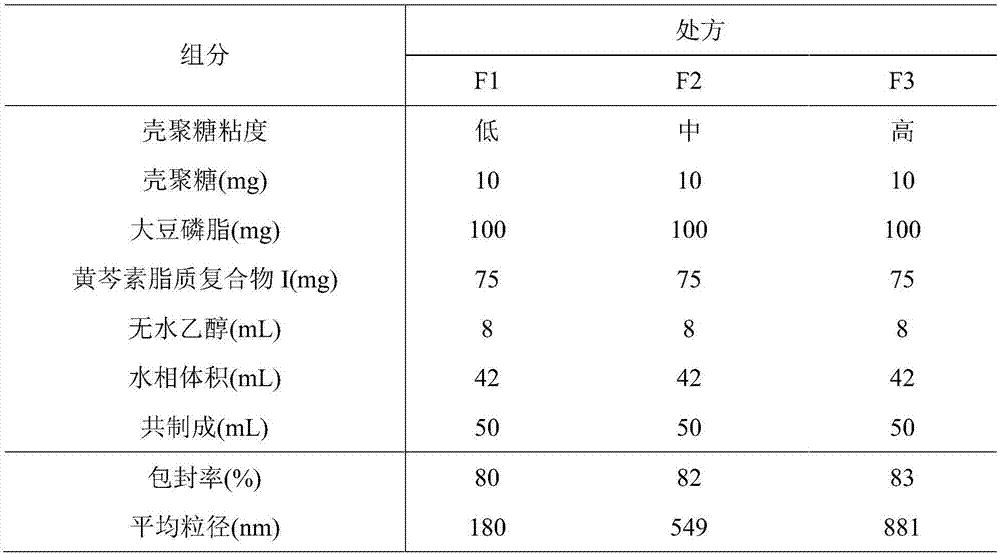
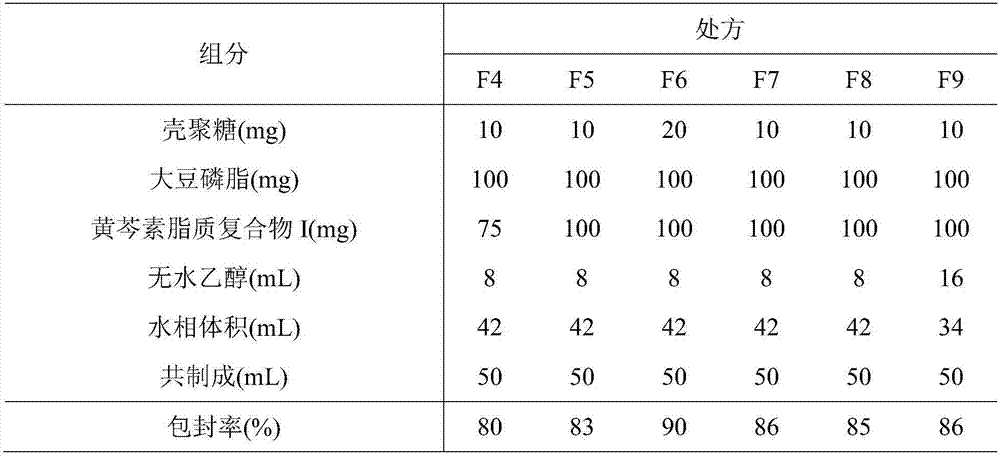
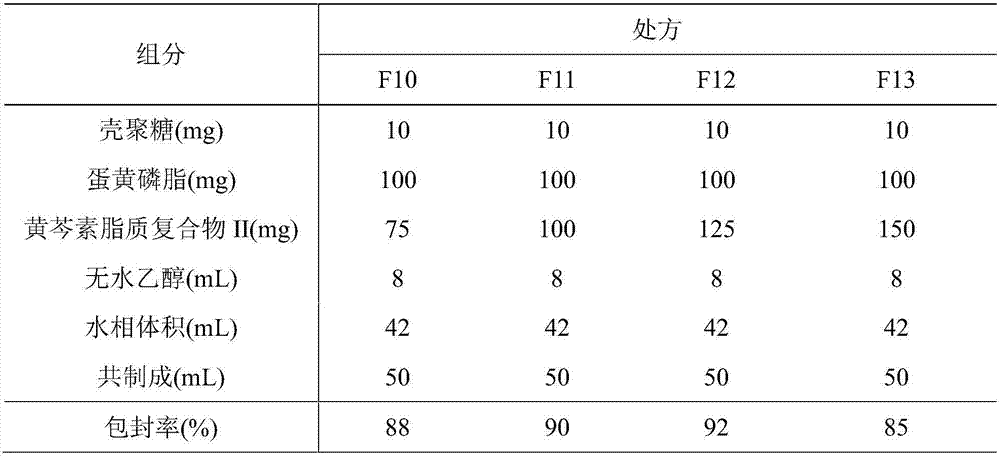
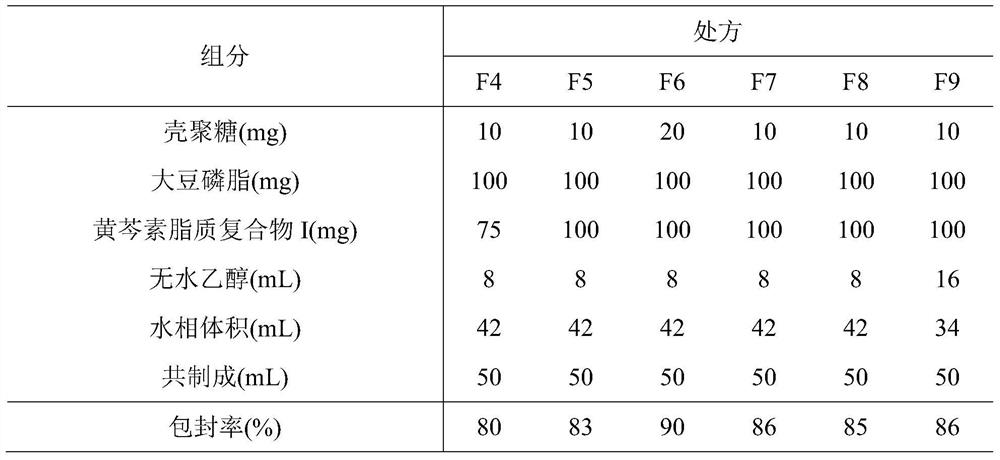
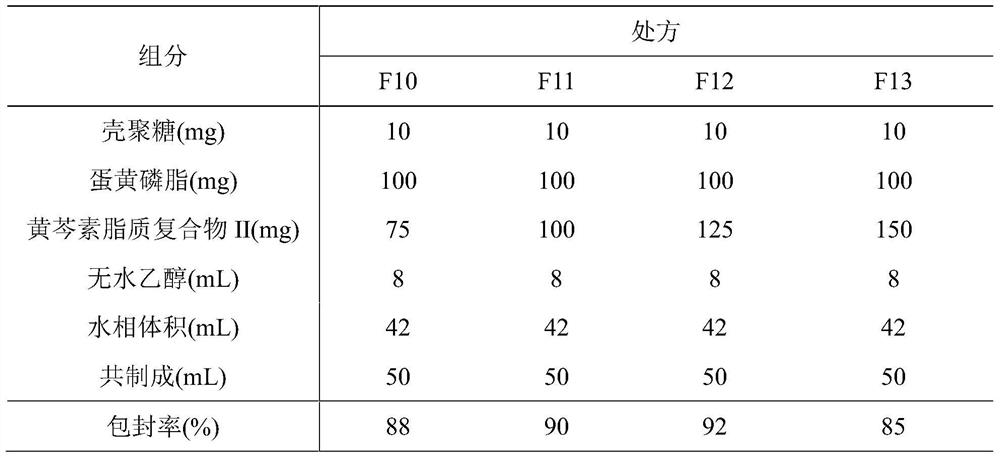
ActiveCN107308132AHigh encapsulation efficiencyHigh encapsulation efficiency and stabilityAntibacterial agentsOrganic active ingredientsPulmonary inhalationOral medication
The invention relates to a phospholipid chitosan drug delivery system, a preparation method and uses thereof, wherein the phospholipid chitosan drug delivery system comprises a drug-lipid complex and chitosan, the phospholipid in the phospholipid chitosan drug delivery system is derived from free phospholipid and / or the phospholipid in a lipid complex, and the drug in the drug-lipid complex does not contain insulin. The drug delivery system of the present invention can be used for oral administration systems, transdermal administration systems, mucosal administration systems, pulmonary inhalation administration systems and other administration systems.
Owner:BEIJING WEHAND BIO PHARMA CO LTD +1
Proanthocyanidin sustained release nano-micro spheres, as well as preparation method and application thereof
ActiveCN103536583AOvercome environmental problemsOvercome the conditionsPowder deliveryOrganic active ingredientsPulmonary inhalationPhospholipid
The invention relates to proanthocyanidin sustained release nano-micro spheres. The proanthocyanidin sustained release nano-micro spheres comprise 1-5 parts of proanthocyanidin, 1-12 parts of chitosan or derivatives thereof, 1-12 parts of phospholipid or derivatives thereof and 1-10 parts of excipients, wherein the chitosan and the derivatives thereof are one or more of high, medium and low-molecular weight chitosan, chitosan oligosaccharide, chitosan quaternary ammonium salt and carboxymethyl chitosan. The particle size of the proanthocyanidin sustained release nano-micro spheres is 10nm-0.3 mu m; the proanthocyanidin is prepared into the powder sustained release nano-micro spheres, and the proanthocyanidin sustained release nano-micro spheres can directly play a role in the lung as a pulmonary inhalation medicine-carrying formulation, improve the bioavailability of the proanthocyanidin and reduce the dosage by sustained release; the proanthocyanidin nano-micro spheres can also be applied as an oral sustained release formulation; the proanthocyanidin nano-micro spheres have appropriate particle size and morphology and other powder properties, as well as sustained release; furthermore, the preparation method has the advantages of simplicity and convenience in operation, stable process and easiness in industrial production.
Owner:WEIFANG MEDICAL UNIV
Processes for taste-masking of inhaled formulations
InactiveUS20080138397A1Minimizing bitter tasteMinimizing cough creationBiocideDispersion deliveryPulmonary inhalationThroat irritation
The present invention provides novel processes and methodologies to minimize the bitter or otherwise unpleasant taste, to minimize the tendency to stimulate the cough reflex, or to minimize oropharyngeal deposition of medically-active compounds administered by the pulmonary / inhalation route and to deliver hydroxychloroquine (HCQ) either singularly or in combination with an antimalarial and aminoquinolone by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the bitter or otherwise unpleasant taste of HCQ or any potential to stimulate the cough reflex, and to deliver a dopaminergic compound or its prodrug, including ABT-431 by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate the cough reflex, and to deliver a lantibiotic, including duramycin by the pulmonary / inhalation route in a sustained release or other formulation that minimizes the unpleasant taste of the drug or any potential to stimulate throat irritation.
Owner:ARADIGM
Suspension formulations
The present invention relates to suspension formulations, especially those for delivering a pharmaceutically active agent in aerosol form using a spray or aerosol device, such as a pressurised metered dose inhaler (pMDI). The formulations may be for pulmonary, nasal, buccal or topical administration, but are preferably for pulmonary inhalation.
Owner:INNOVATA BIOMED +1
Pulmonary inhalation curcumin-phospholipid complex chitosan microspheres and preparation method thereof
InactiveCN105250244AGood dispersionNo obvious aggregation etc.Pharmaceutical delivery mechanismKetone active ingredientsPulmonary inhalationMedicine
The present invention discloses pulmonary inhalation curcumin-phospholipid complex chitosan microspheres and a preparation method thereof, wherein the pulmonary inhalation curcumin-phospholipid complex chitosan microspheres comprise, by weight, 1 part of a curcumin compound, 2-4 parts of phospholipid, and 4-10 parts of chitosan, and are prepared by using a spray-drying method. According to the present invention, the microspheres have good dispersion property, do not have significant aggregation and other phenomena, have the particle size of 1-10 [mu]m, and are suitable for the dry powder inhalation agent so as to achieve the pulmonary administration purpose, wherein the effective site deposition rate determined by a two-stage glass impactor is 20-60%.
Owner:SOUTHWEST UNIVERSITY
Benzimidazole and derivative thereof, and medicinal composition and application thereof in preparation of antidepressant medicaments
The invention provides benzimidazole and derivative thereof, an antidepressant medicament which contains the benzimidazole and derivative thereof and a pharmaceutical carrier and excipient, and application thereof in preparation of antidepressant medicaments and preparation of functional foods. The benzimidazole and derivative thereof provided by the invention can be prepared into pharmaceutical preparations of various forms, including oral administration, injection, lung inhalation and transdermal preparations, and specifically including injections, oral liquids, tablets, capsules, granules, aerosols, dry powder inhalers, sprays, plasters and the like.
Owner:泸州天演生物医药科技有限公司
Drug for treating acute lung injury and acute respiratory distress syndrome, and uses thereof
InactiveCN108904511AOrganic active ingredientsRespiratory disorderPulmonary inhalationCorosolic acid
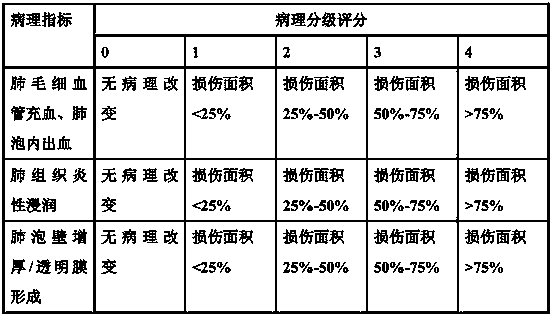
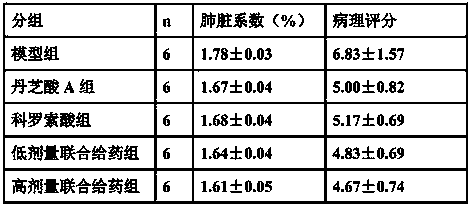
The invention belongs to the field of medicine, and relates to a drug for treating acute lung injury and acute respiratory distress syndrome, wherein the active components of the drug is ganolucidic acid A or corosolic acid or a composition of ganolucidic acid A and corosolic acid, and the dosage form of the drug is one selected from an oral liquid preparation, a pulmonary inhalation preparation and an injection. According to the present invention, the drug can reduce the pulmonary edema of acute lung injury and acute respiratory distress syndrome, reduce lung hyaline membrane formation and other lung lesions, and easily improve the symptoms of acute lung injury and acute respiratory distress syndrome.
Owner:天威英利
Application of 3-(3,4-dihydroxycinnamoyl) quinic acid to preparation of medicines or food for preventing and treating ovulation disorder
The invention relates to application of 3-(3,4-dihydroxycinnamoyl) quinic acid to the preparation of medicines and functional food for protecting an ovarian function, promoting ovulation and treating ovulation disorder. The 3-(3,4-dihydroxycinnamoyl) quinic acid can be prepared into medicinal preparations of various forms which comprise oral administration, injection and pulmonary inhalation preparations and transdermal preparations and particularly comprise injections, oral liquid, tablets, capsules, granules, aerosols, powder aerosols, pressurized sprays, patches and the like.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
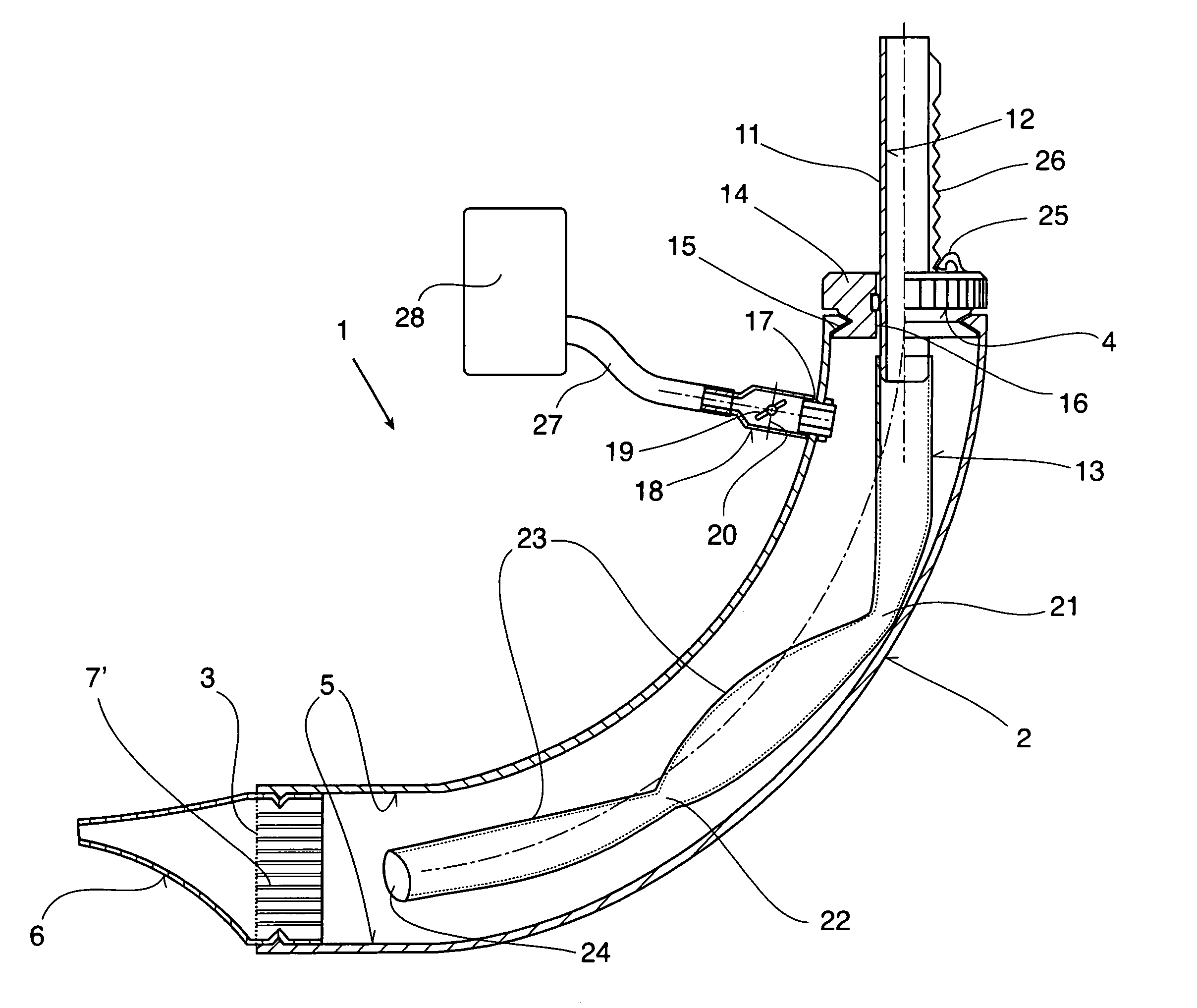
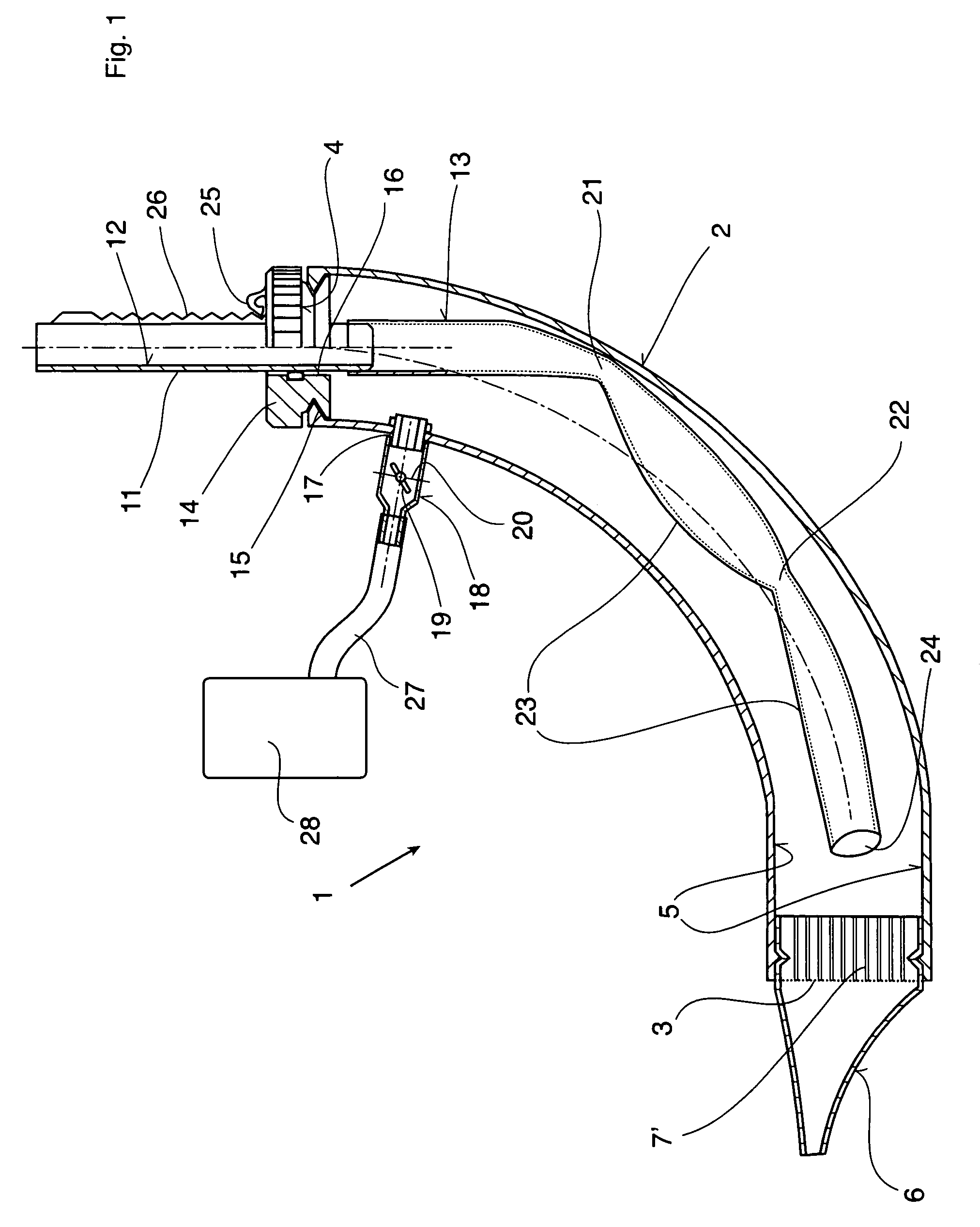
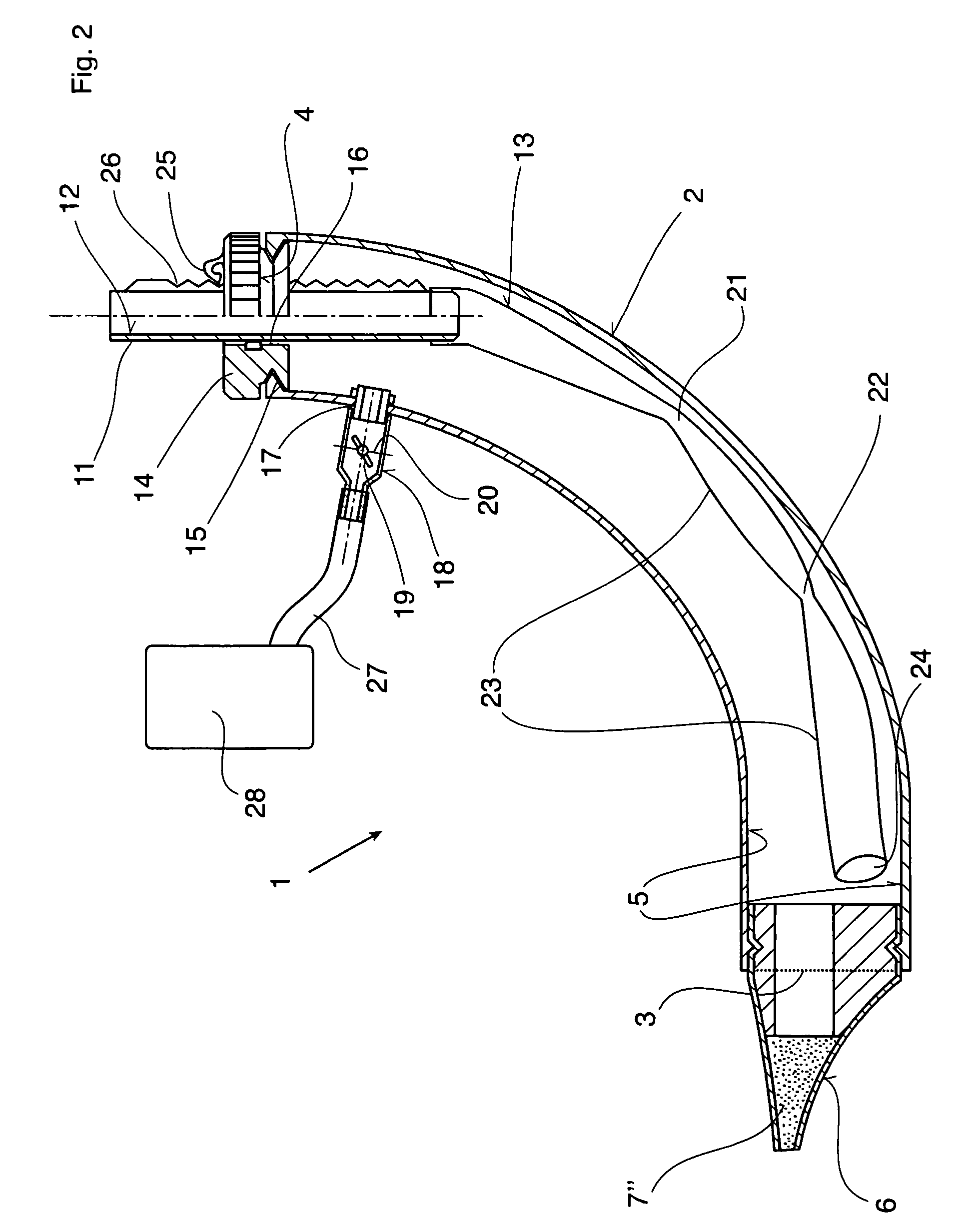
Therapeutic device
ActiveUS8267090B2Easy to handleClear the patient's airwaysRespiratorsBreathing masksPulmonary inhalationTherapeutic Devices
A therapeutic device for improving the respiration of a patient, with a curved or bent pipe section and a mouthpiece inserted in a first end of the mouthpiece and adapted to provide an easy-to-handle medicinal device by means of which diseases of the airway can be treated, or the pulmonary volume, as well as the pulmonary inhalation performance, of a patient can be improved. This is achieved in that a holding peg connected to the pipe section can be pushed into a second end of the pipe section, and a passage channel disposed in the holding peg which penetrates into the inside of the pipe section, completely or in part, and the holding peg has a flexible hose is attached to it which runs inside the pipe section, the free end of which can move freely in the area of the mouthpiece between inner walls of the pipe section.
Owner:R CEGLA
Insulin powder spray for lung inhalation and preparation method thereof
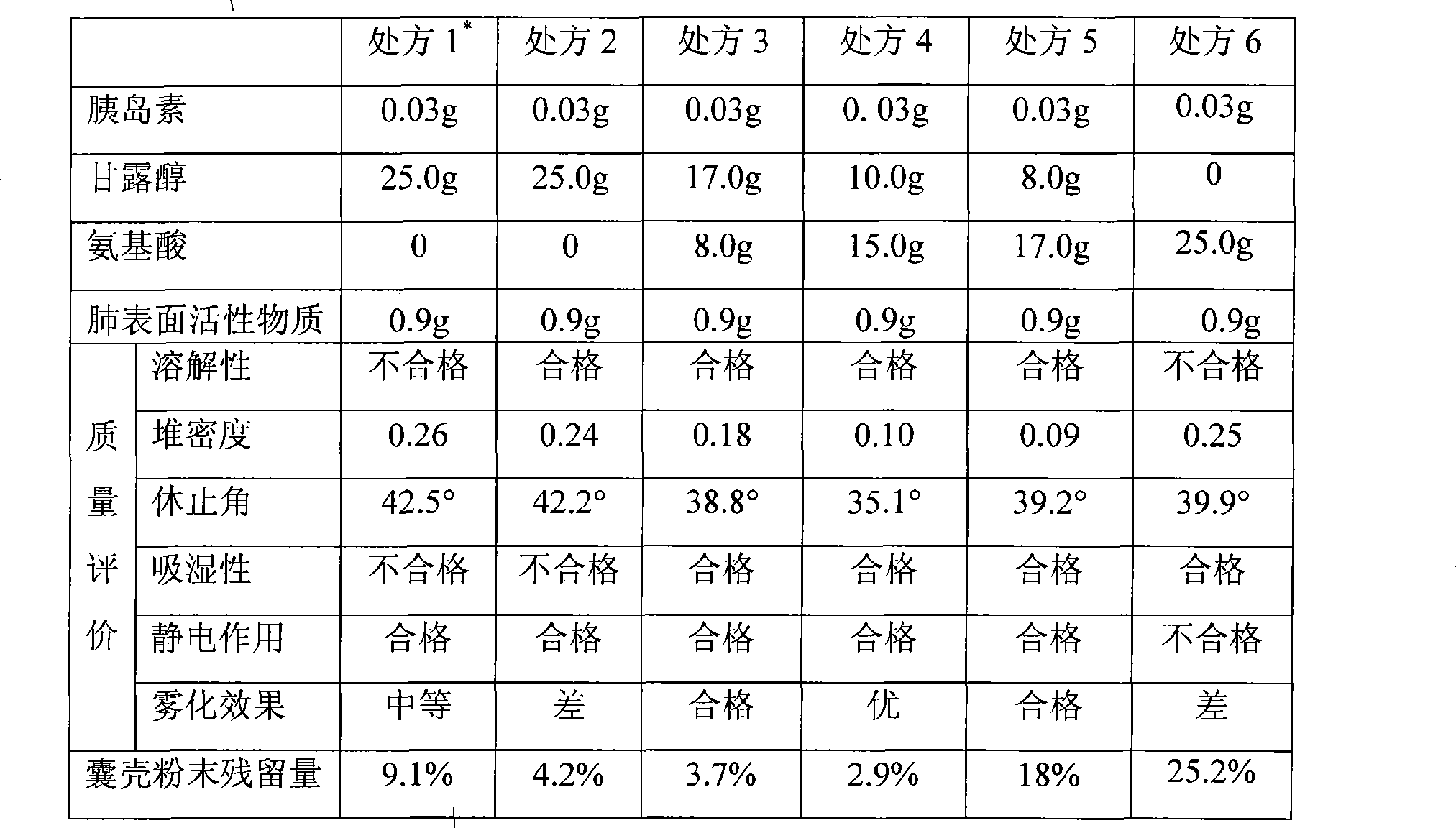
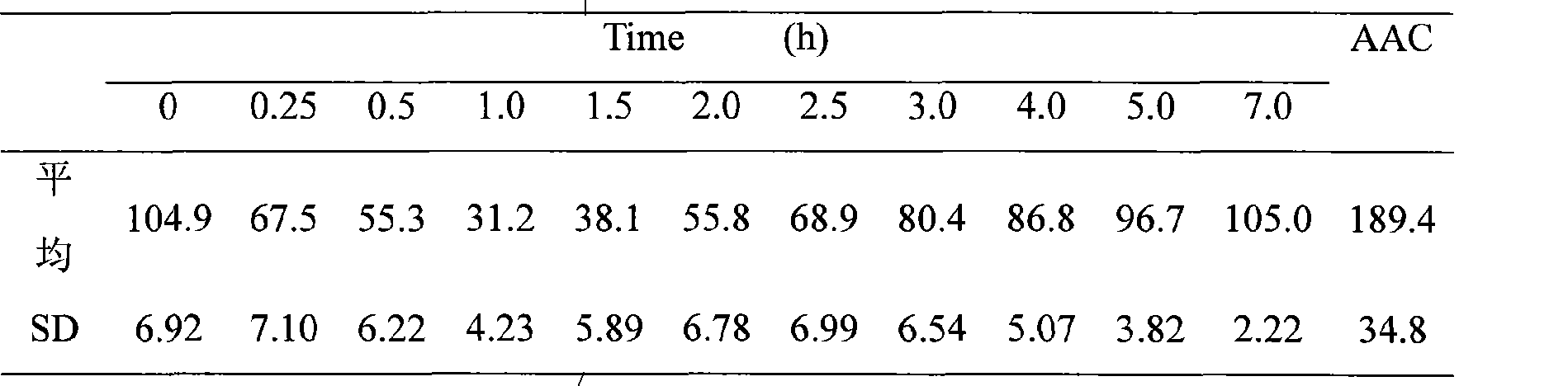
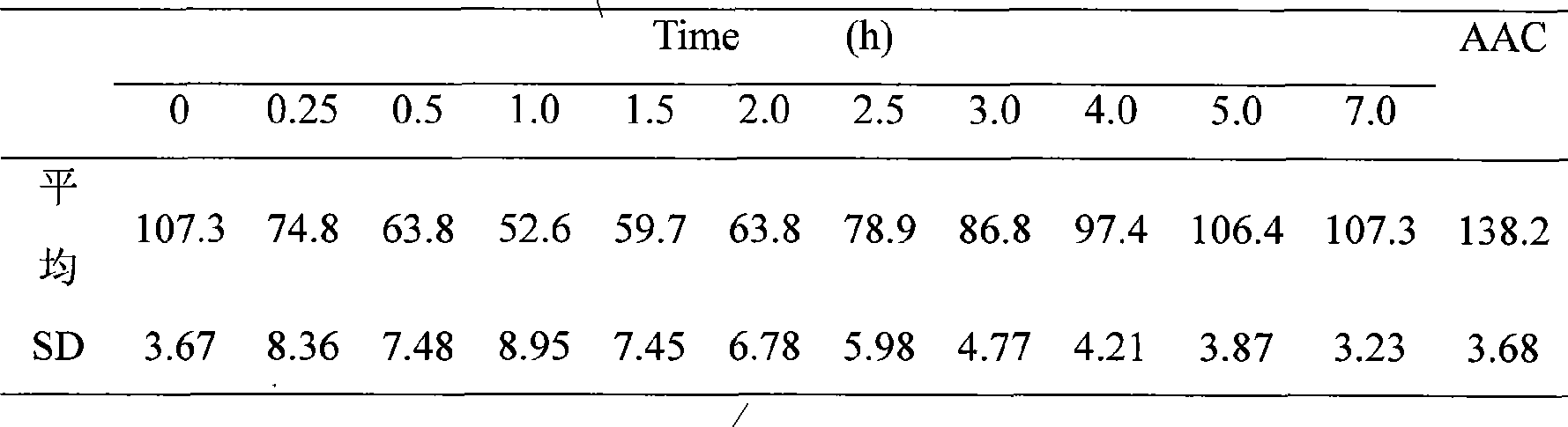
InactiveCN101474399AEnhance hypoglycemic functionPowder deliveryPeptide/protein ingredientsAngle of reposeSolubility
The invention relates to the field of pharmaceutical preparation, in particular to insulin powder inhalation absorbed in lung through mouth. The invention is characterized in that the insulin powder inhalation consists of insulin, mannite, amino acid and lung surface active substances. The insulin powder inhalation has the advantages of high bioavailability, good safety, and better solubility, bulk density, angle of repose and atomizing property, and is not easy to absorb moisture.
Owner:CHINA PHARM UNIV
Benzimidazole and derivative thereof, and medicinal composition and application thereof
The invention provides benzimidazole and a derivative thereof, a medicinal composition which contains the benzimidazole and derivative thereof as active components and is used for preventing and treating neurological and psychotic diseases, and application thereof in preparation of medicaments and functional foods for resisting senility, senile dementia and other neurological and psychotic diseases. The benzimidazole and derivative thereof provided by the invention can be prepared into pharmaceutical preparations of various forms, including oral administration, injection, lung inhalation and transdermal preparations, and specifically including injections, oral liquids, tablets, capsules, granules, aerosols, dry powder inhalers, sprays, plasters and the like.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
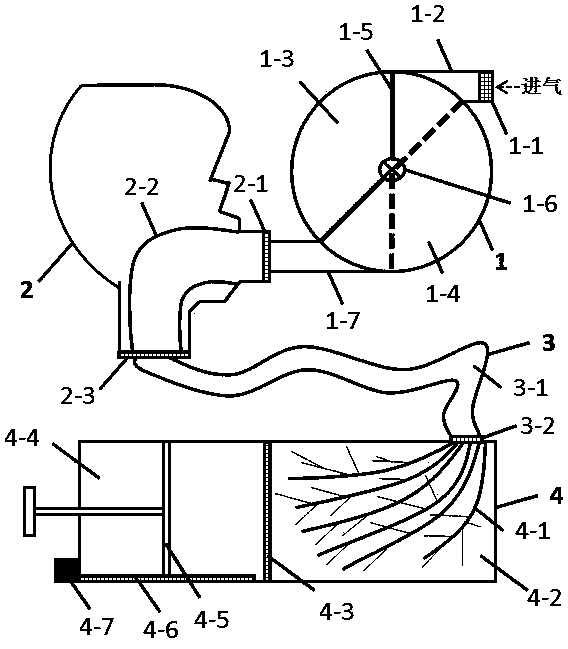
Inhalation training instrument for pulmonary inhalation administration
PendingCN112206386AGood treatment effectIncrease deposition rateInhalatorsPulmonary inhalationFlow transducer
The invention belongs to the technical field of medical instruments and provides an inhalation training instrument for pulmonary inhalation administration. The inhalation training instrument comprisesa tank, an inhalation cylinder, a resistance regulator, a flow sensor, a signal converter and a data analysis part. The inhalation cylinder can rotate around the axis of the tank. Along with rotationof the inhalation cylinder, fan-shaped regulating holes in the resistance regulator coincide with different vent holes, so that resistance borne by airflow is regulated, the flow sensor measures actual flow, the actual flow is converted by the signal converter and then transmitted to the data analysis part, the data analysis part calculates inhalation acceleration, inhalation peak flow velocity and inhalation volume according to flow data, and the data are displayed through a displayer of the data analysis part. According to the inhalation training instrument for pulmonary inhalation administration, the data results such as the inhalation acceleration, the inhalation peak flow velocity and the inhalation volume are visualized, a patient is helped to train the inhalation mode, the pulmonary deposition rate of medicine and the uniformity of delivery dosage are improved, and the patient can obtain a better treatment effect in inhalation medicine treatment.
Owner:张玮
Tea tree oil clathrate powder inhalation and application thereof to treatment of infected pneumonia
The invention discloses a tea tree oil clathrate powder inhalation and application thereof to treatment of infected pneumonia. Tea tree oil is prepared into clathrate to improve stability and reduce irritation, and water solubility is also improved to achieve suitableness for pulmonary inhalation. The tea tree oil clathrate powder inhalation is excellent in treatment effect on infected pneumonia including bacterial pneumonia and fungal pneumonia and is convenient to carry and use.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Mucoactive agents for treating a pulmonary disease
The present invention relates to pharmaceutical compositions which are useful in the treatment of diseases where excess mucus is present in the respiratory tract, such as cystic fibrosis and chronic obstructive pulmonary disease. In particular, the invention relates to pharmaceutical compositions for administration by pulmonary inhalation.
Owner:VECTURA LTD
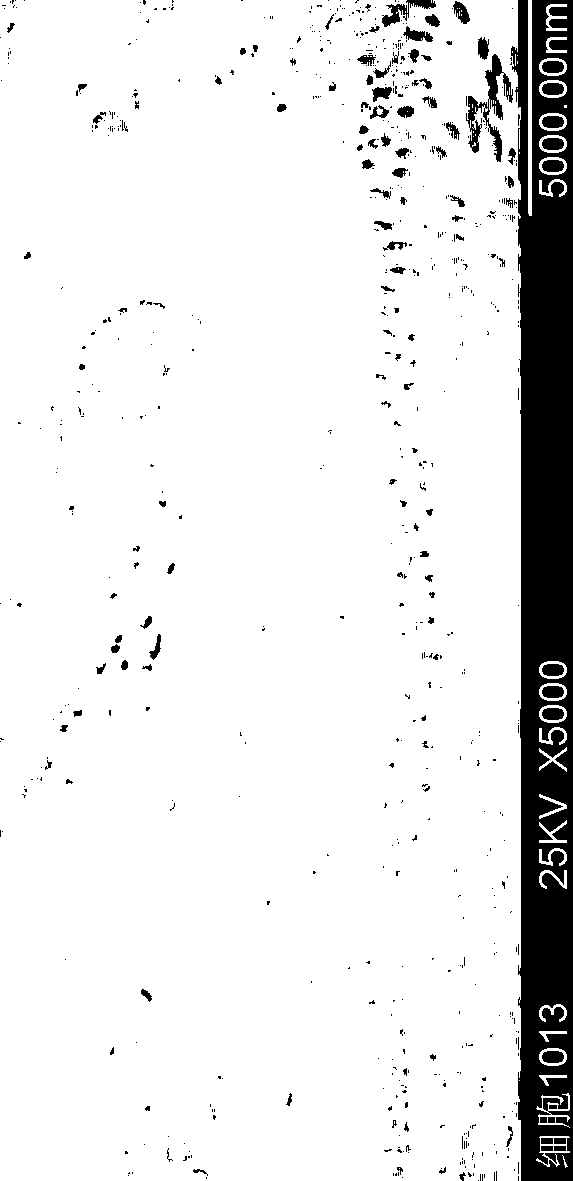
Insulin flexible particles and preparation thereof
ActiveCN110464835AImprove flexibilityHigh transmucosal transport capacityPeptide/protein ingredientsMetabolism disorderPulmonary inhalationOral medication
The invention discloses insulin flexible particles and a preparation thereof. The insulin flexible particles contain an insulin / phospholipid composite and an ionic surfactant and are vesicles of circular or oval structures, the particle size is 5000nm or below, and the drug encapsulation rate is 60 percent or above. The insulin flexible particles of the invention can further contain a nonionic surfactant, free phospholipid or cholesterol. The insulin flexible particles of the invention differ in composition from insulin contained vesicles or flexible vesicles reported in the prior literatures,is also different from novel insulin contained nanovesicles as reported in the prior literatures, thus having better flexibility and higher transmucosal transport capacity. The insulin flexible particles of the invention can be prepared into clinically acceptable dosage forms by being added with an excipient for insulin parenteral administration delivery including intranasal administration, oraladministration, transdermal administration and pulmonary inhalation.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
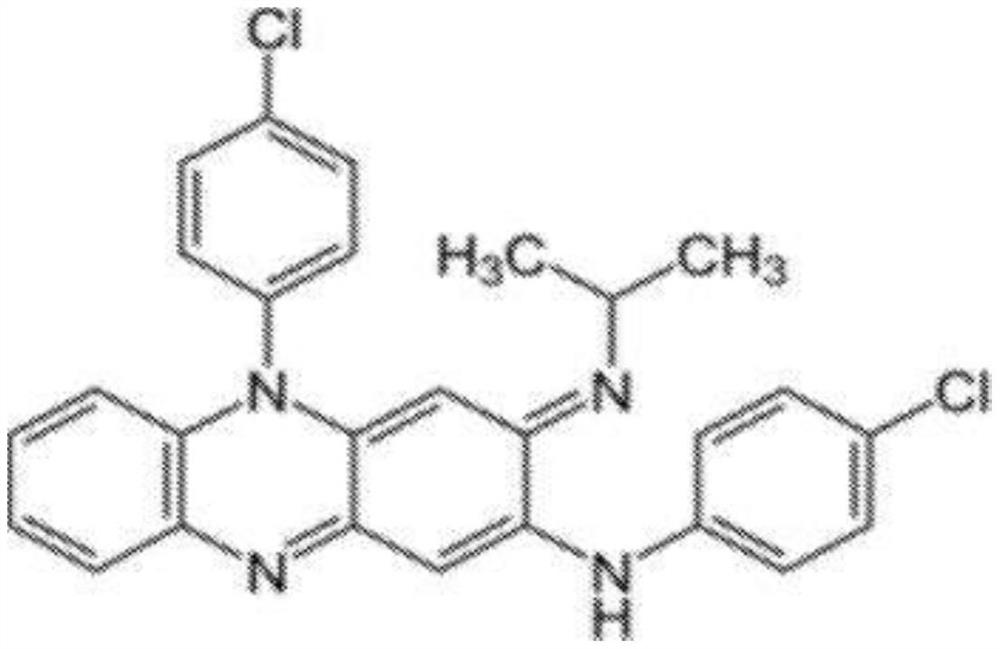
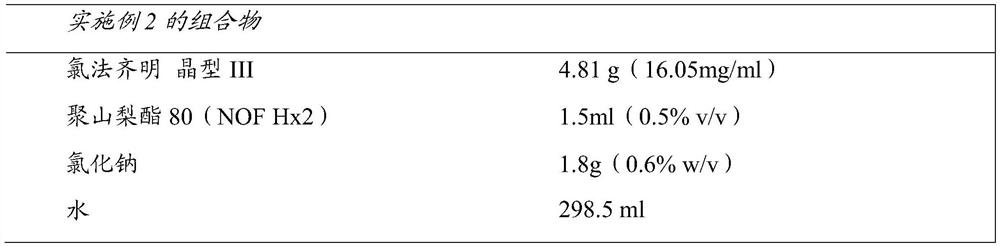
Compositions of clofazimine, combinations comprising them, processes for their preparation, uses and methods comprising them
The present invention relates to pharmaceutical compositions for inhalation comprising a therapeutically effective dose of clofazimine wherein the clofazimine is provided in the form of a suspension, and processes for their preparation. Furthermore, the present invention provides pharmaceutical combinations comprising clofazimine in the form of an aerosol for pulmonary inhalation. The combinations and compositions provided by the present invention may be used in the treatment and / or prophylaxis of pulmonary infections caused by mycobacteria and other gram-positive bacteria, and of pulmonary fungal infections.
Owner:MANNKIND CORP
Medicine for treating children bronchial asthma and preparation method thereof
ActiveCN112274497AImprove stabilityUniform particle sizeOrganic active ingredientsPharmaceutical non-active ingredientsPulmonary inhalationFormulary
The invention relates to a medicine for treating bronchial asthma in children. The medicine is characterized by containing salbutamol, budesonide and soybean lecithin. By reasonably designing the formula, the medicine for treating children bronchial asthma has the advantages of being good in stability and uniform in particle size, and particle size distribution and average particle size both meetthe requirements of pulmonary inhalation preparations.
Owner:QINGDAO CENT HOSPITAL
A kind of phospholipid chitosan drug delivery system and its preparation method and application
ActiveCN107308132BHigh encapsulation efficiencyHigh encapsulation efficiency and stabilityAntibacterial agentsOrganic active ingredientsPulmonary inhalationPhospholipid
A phospholipid chitosan drug delivery system and its preparation method and application, the drug delivery system includes drug lipid complex and chitosan, the phospholipids in the drug delivery system come from free phospholipids and / or lipid complexes The phospholipids in the drug lipid complex do not include insulin. The drug delivery system can be used in oral, transdermal, mucosal, pulmonary inhalation and other drug delivery systems.
Owner:BEIJING WEHAND BIO PHARMA CO LTD +1
Cyclosporin A pulmonary inhaled macroporous microspheres and preparation method thereof
ActiveCN102429875BLess irritatingReduce dosageCyclic peptide ingredientsGranular deliveryPulmonary inhalationMicrosphere
The invention discloses cyclosporin A pulmonary inhaled macroporous microspheres and a preparation method thereof. The cyclosporin A pulmonary inhaled macroporous microspheres consist of the following components in part by weight: 1 to 3 parts of cyclosporin A, 1 to 10 parts of chitosan derivative, 0.5 to 5 parts of beta-cyclodextrin or a derivative thereof and 0.5 to 5 parts of porogen, and havethe particle size of 1 to 10mu m. The preparation method mainly comprises the steps of filtering and spray-drying. The invention has the advantages that: the pulmonary inhaled microspheres have a macroporous structure, can be used for pulmonary administration, have the particle size of 1 to 10mu m, the medicine loading capacity of 5.28 to 7.28 percent, the encapsulating rate of 0.85 to 1.19 percent, and high biocompatibility, can reduce irritation of a respiratory mucosa, and is particularly suitable for a dry powder inhalant to fulfill the aim of pulmonary administration, the medicine is sustained-release, the using amount and side effects of the medicine can be reduced, the preparation method is easy and convenient to operate, the process is stable, and industrial production is easy to implement.
Owner:WEIFANG MEDICAL UNIV
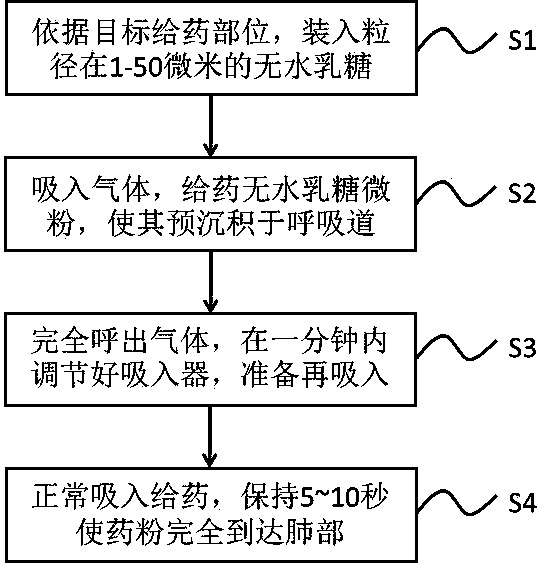
Integrated device and method for improving pulmonary inhalation medication through lactose micropowder pre-deposition
ActiveCN109771397AIncrease inhalationEnhance the efficiency of subsequent drug administrationPharmaceutical delivery mechanismPharmaceutical non-active ingredientsPulmonary inhalationThroat
The invention relates to an integrated device and method for improving pulmonary inhalation medication through lactose micropowder pre-deposition, wherein the integrated device mainly comprises an inhaler, a head and throat part, a respiratory pipeline model device and a lung model device; the inhaler comprises two modes of pre-deposition lactose administration and normal administration; and the respiratory pipeline model device can simulate the real environment of a human body by wetting the pipe wall during measurement. According to the novel administration method, a lactose micro-powder pre-deposition step is arranged one minute before prior inhalation administration, and 20-200 milligrams of anhydrous lactose micro-powder such as flower-shaped lactose, amorphous coated lactose and thelike are pre-deposited in the moist and viscous head and throat part, the respiratory pipeline and part of a lung trachea, so that a smooth inhalation environment is provided for the inhalation of subsequent medicinal powder, and the deposition rate of the medicinal powder in a target area of the lung trachea is improved by 30-200%.
Owner:湖南致雅生物科技有限公司
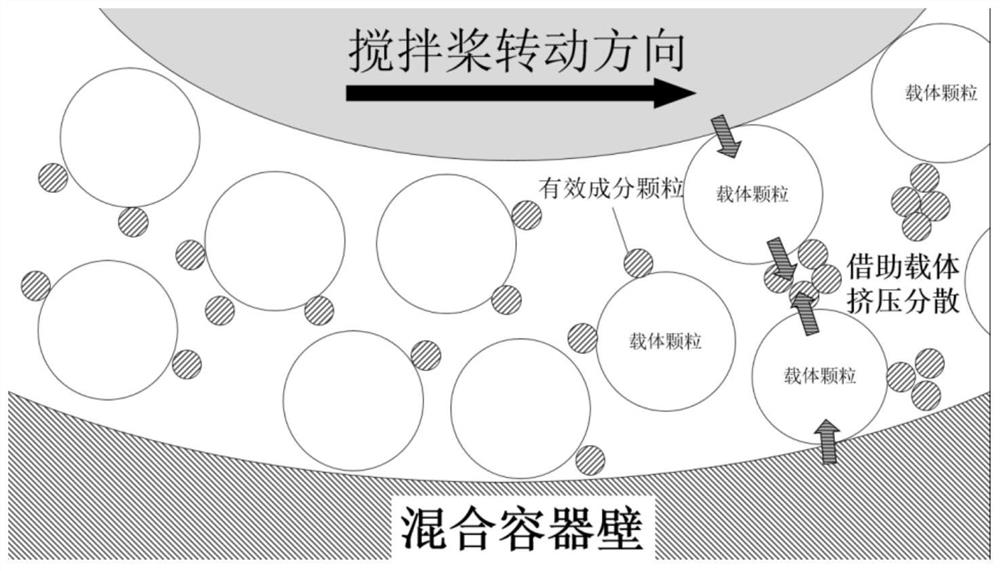
A kind of preparation method of dry powder inhaler
ActiveCN111297837BImproved inhalation characteristicsGood content uniformityPowder deliveryOrganic active ingredientsPulmonary inhalationPowder Inhaler
The invention provides a method for preparing a dry powder inhaler, which comprises dispersing active ingredients in a carrier through a specific mixing method, wherein the specific mixing method is extrusion shear mixing. The dry powder inhaler is prepared by the method provided by the invention, the particles of the active ingredient are not easily crushed during the mixing process, and the prepared dry powder inhaler has suitable aerodynamic fine particles when it is administered to the lungs through a dry powder inhalation device Fraction (FPF) and good content uniformity, excellent inhalation characteristics and other characteristics.
Owner:SHANGHAI FANGYU HEALTH PHARMA TECH CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com