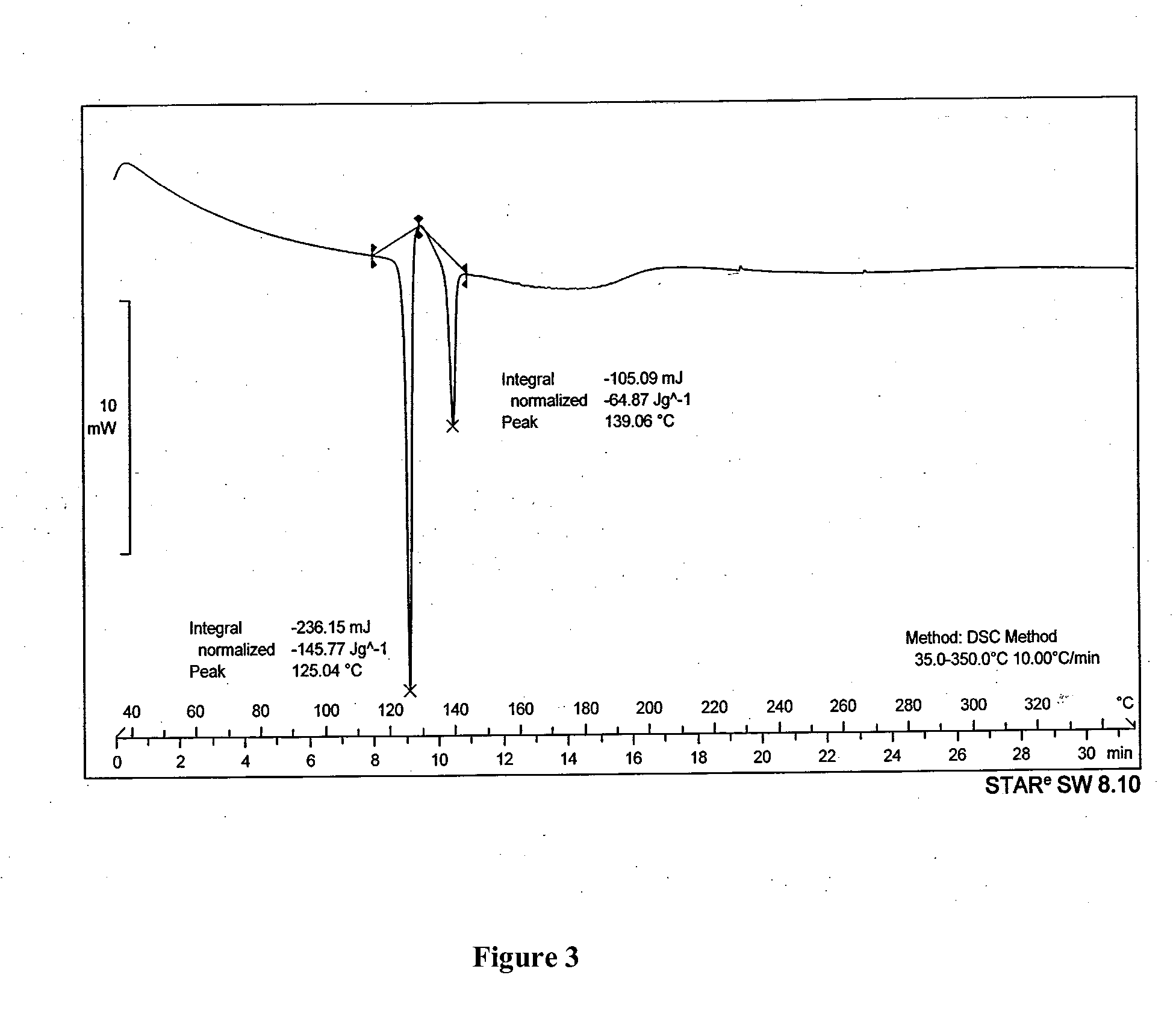
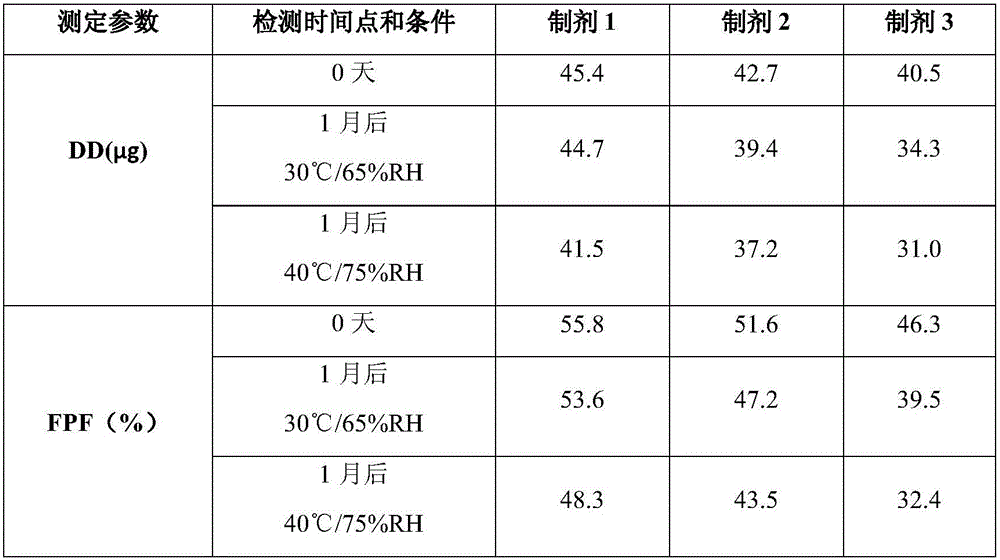
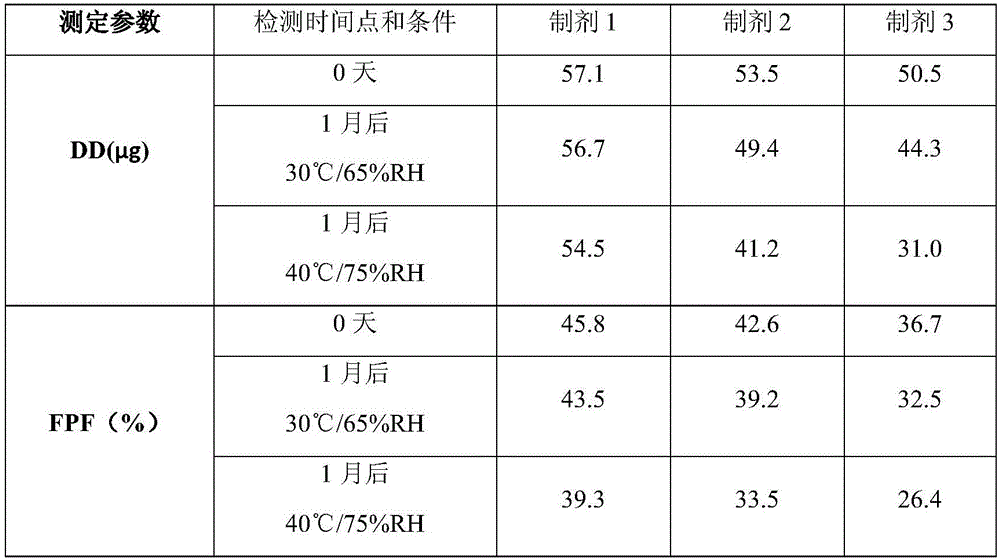
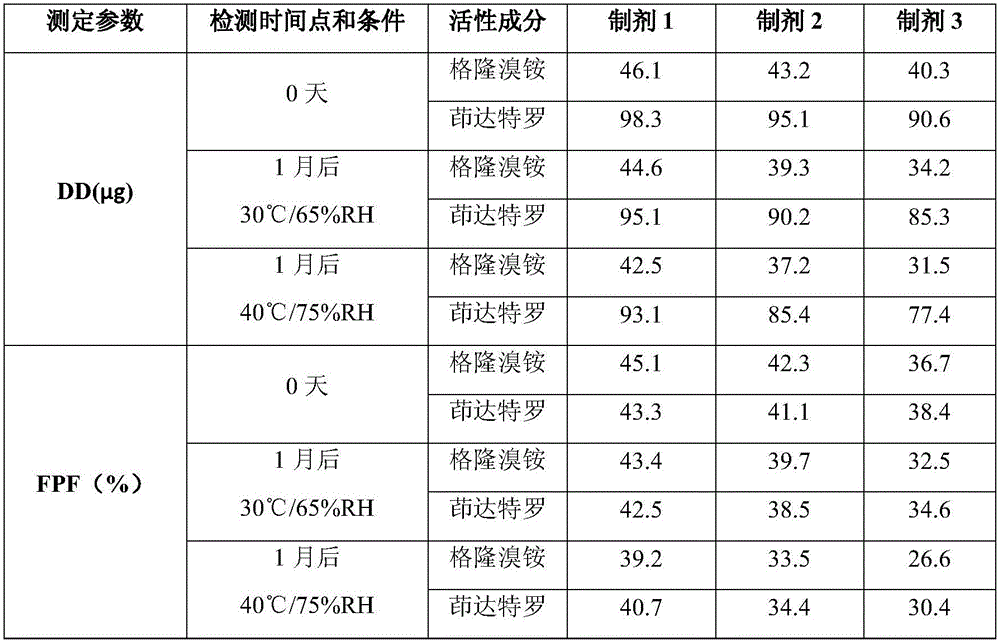
Patents
Literature
213 results about "Dry powder inhalation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
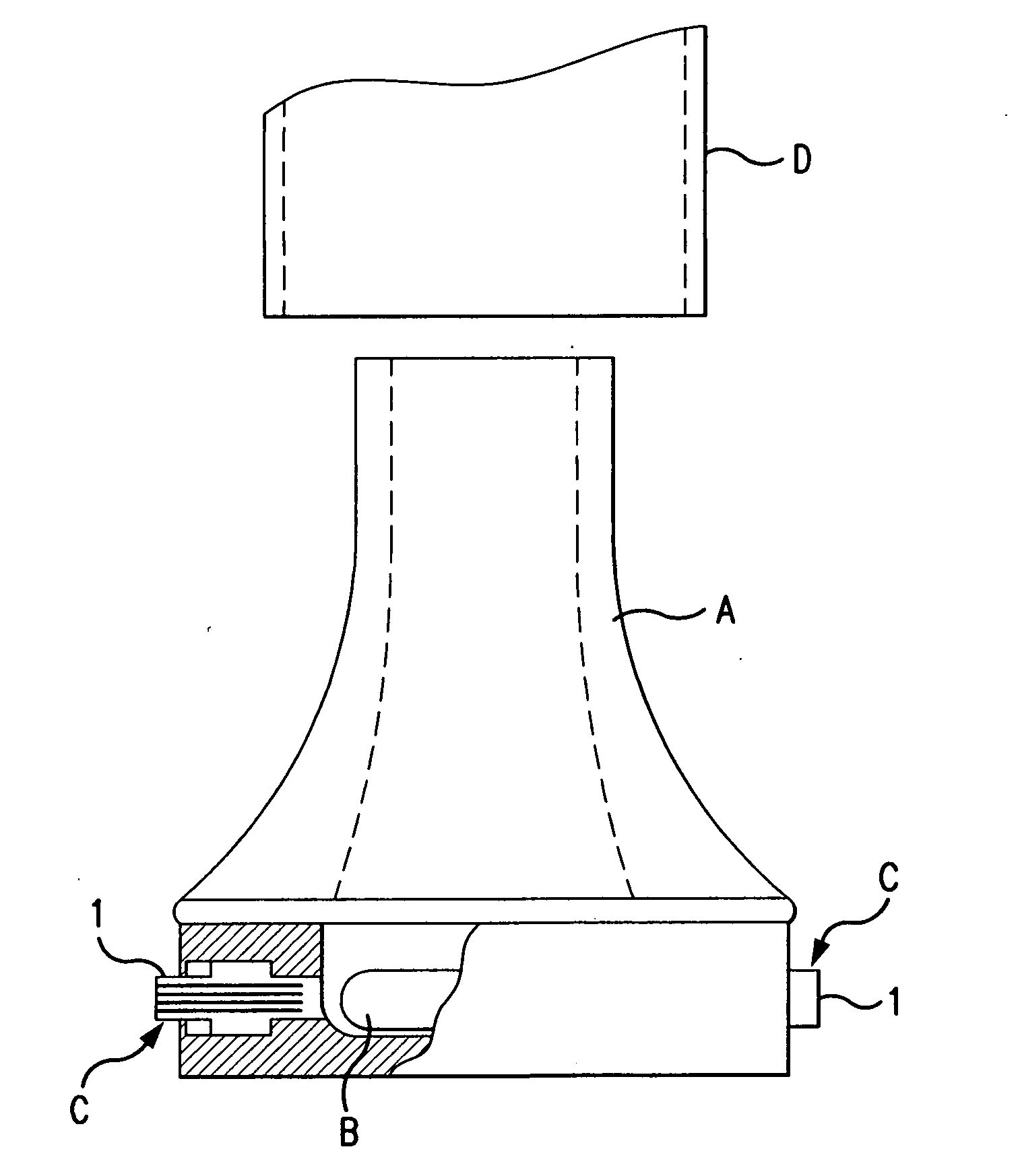
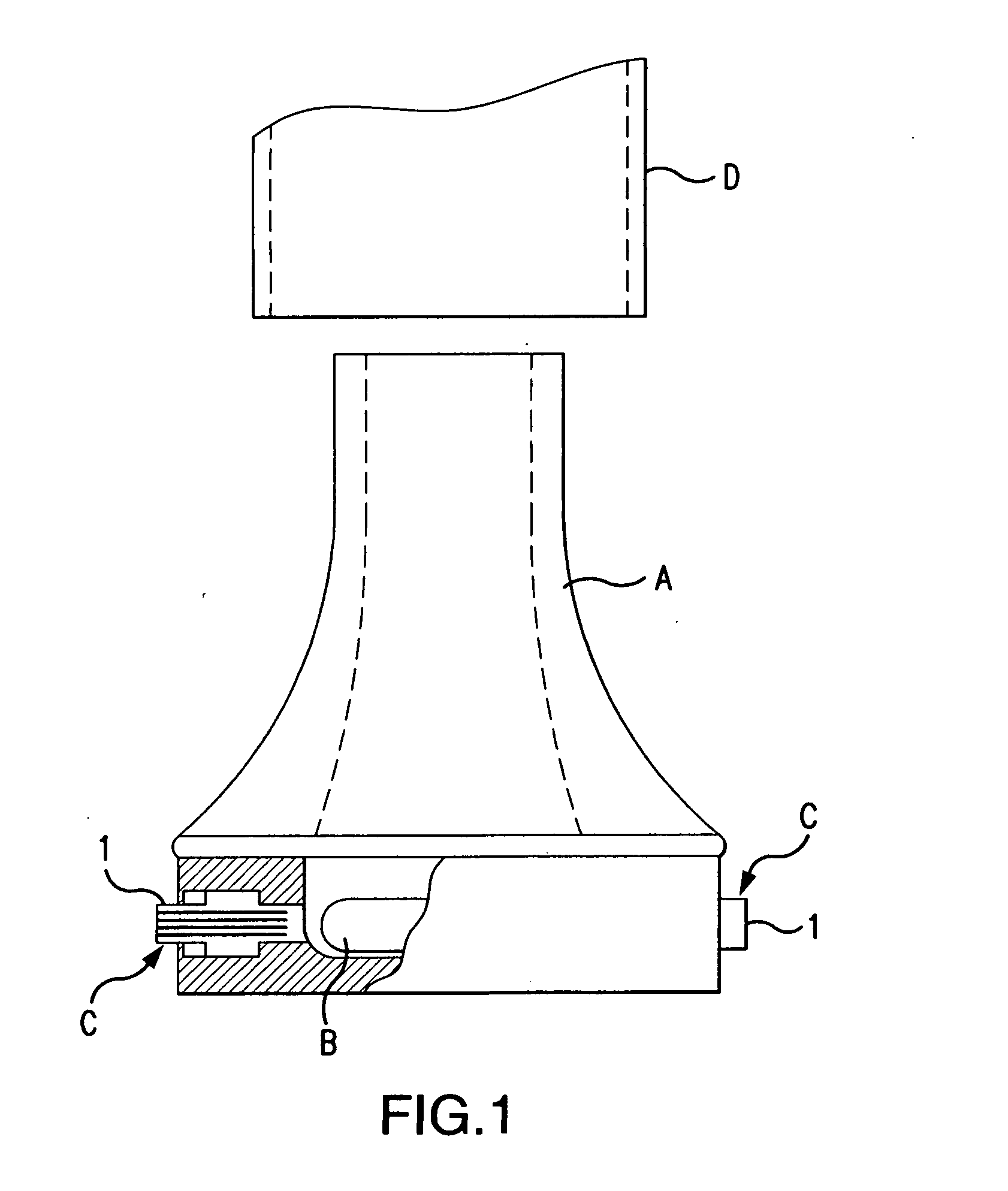
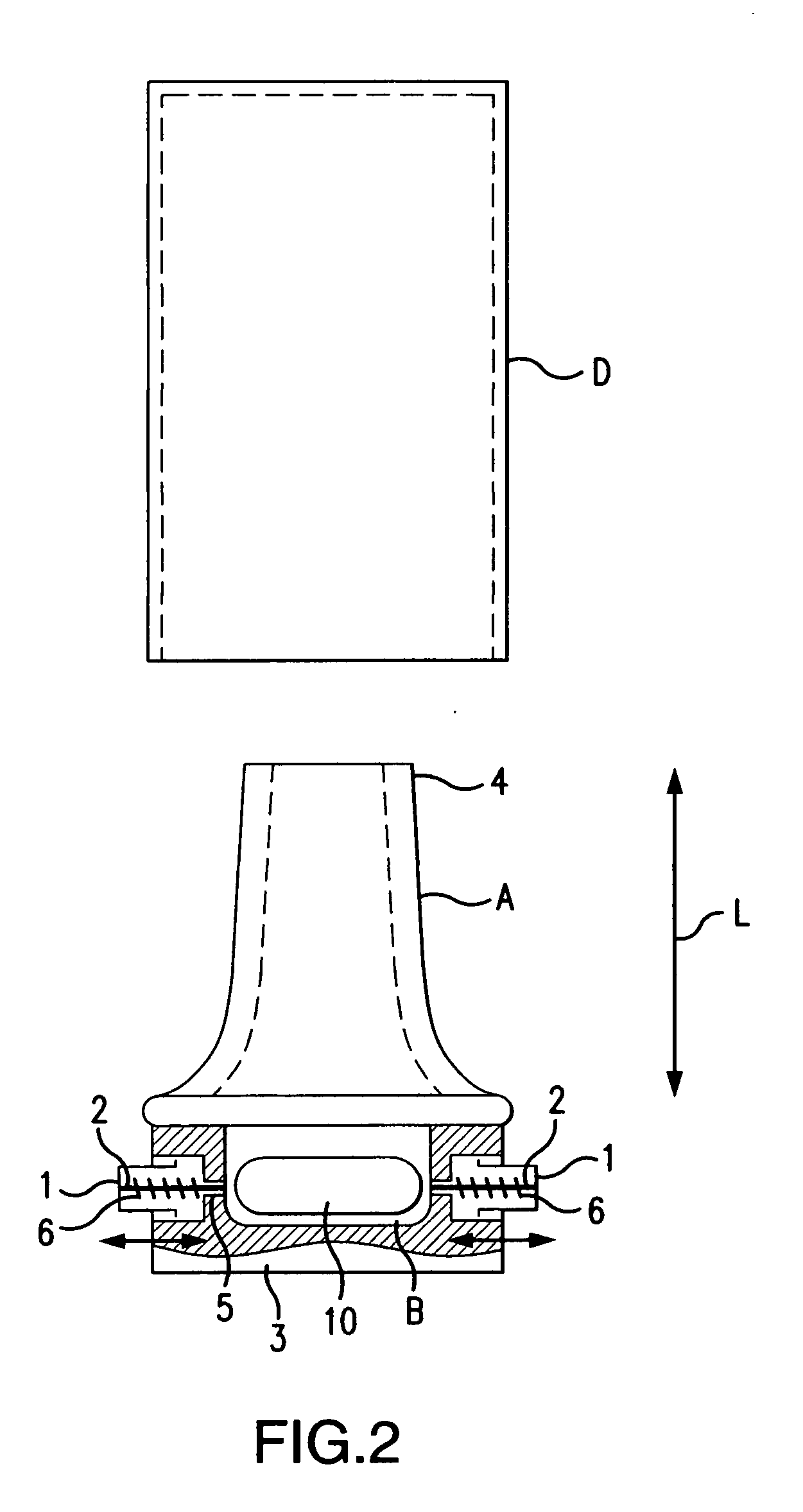
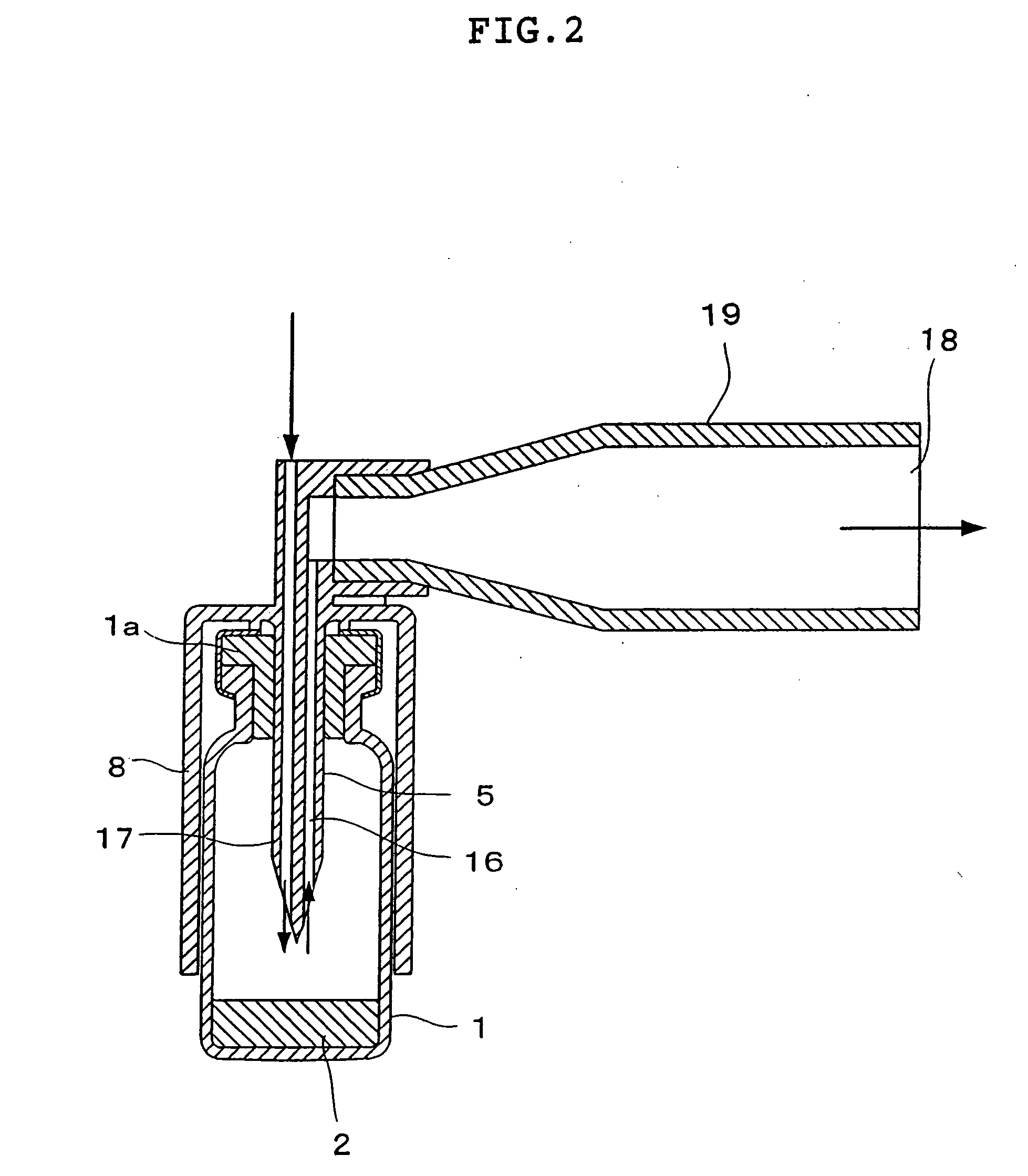
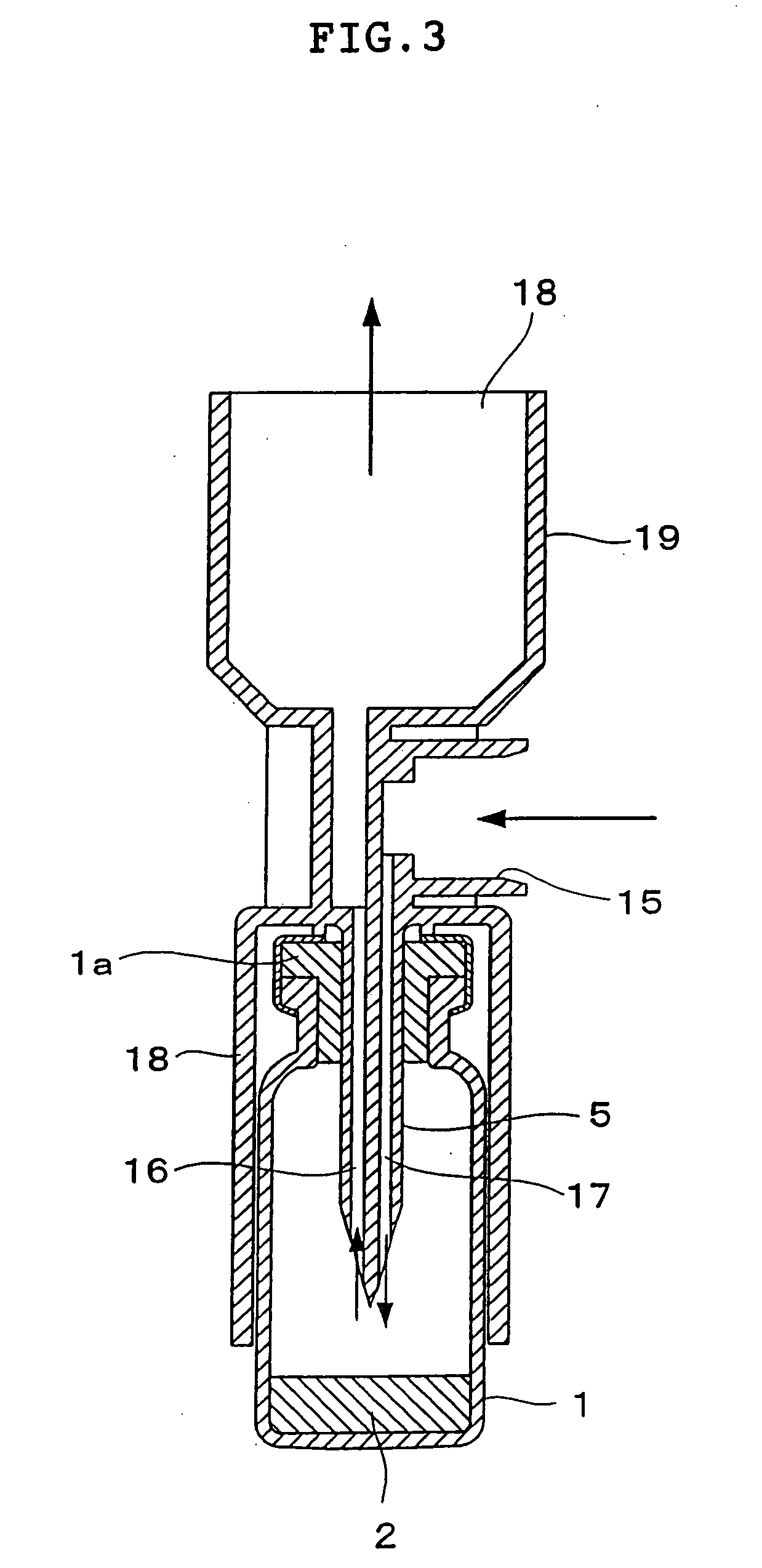
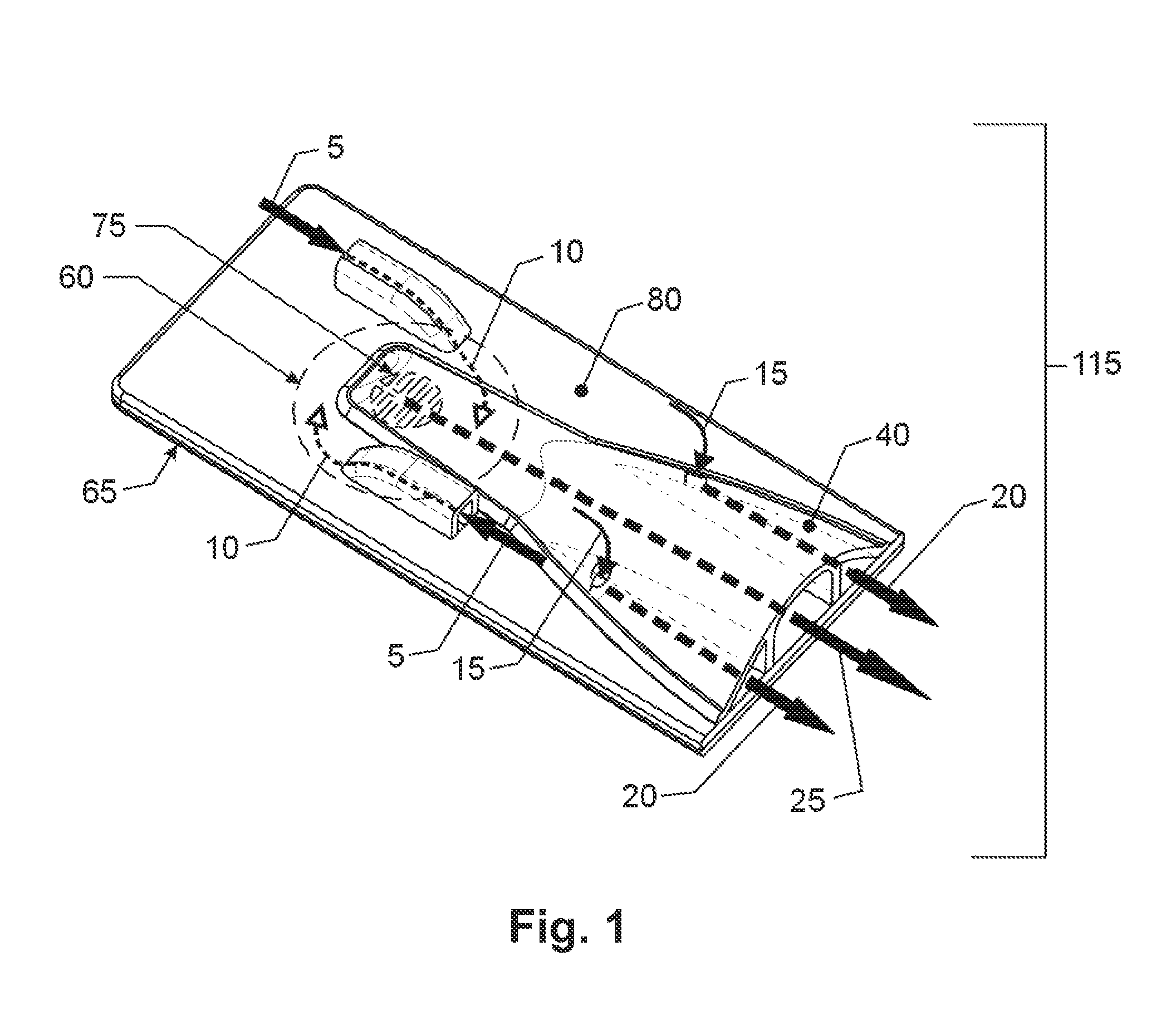
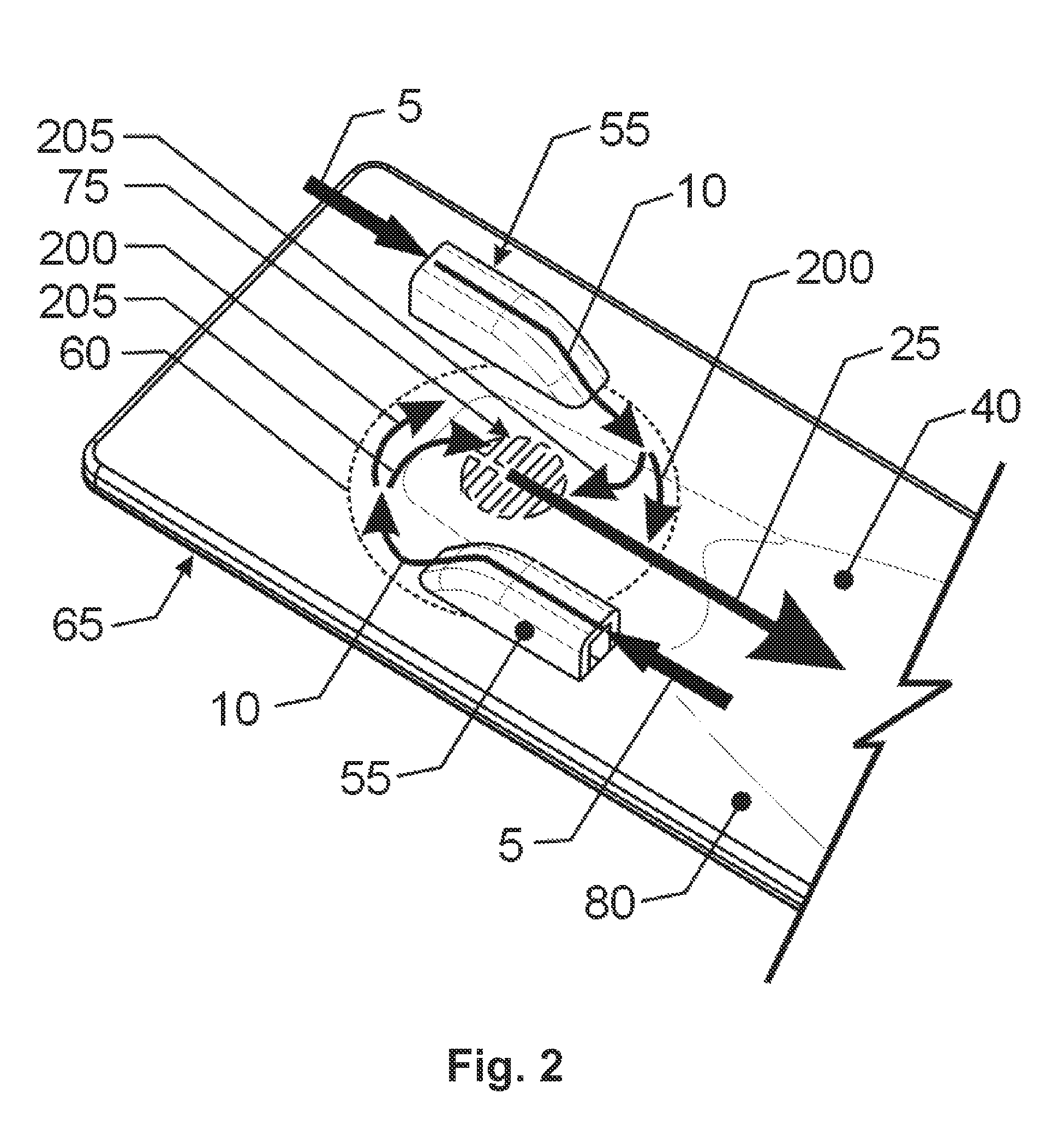
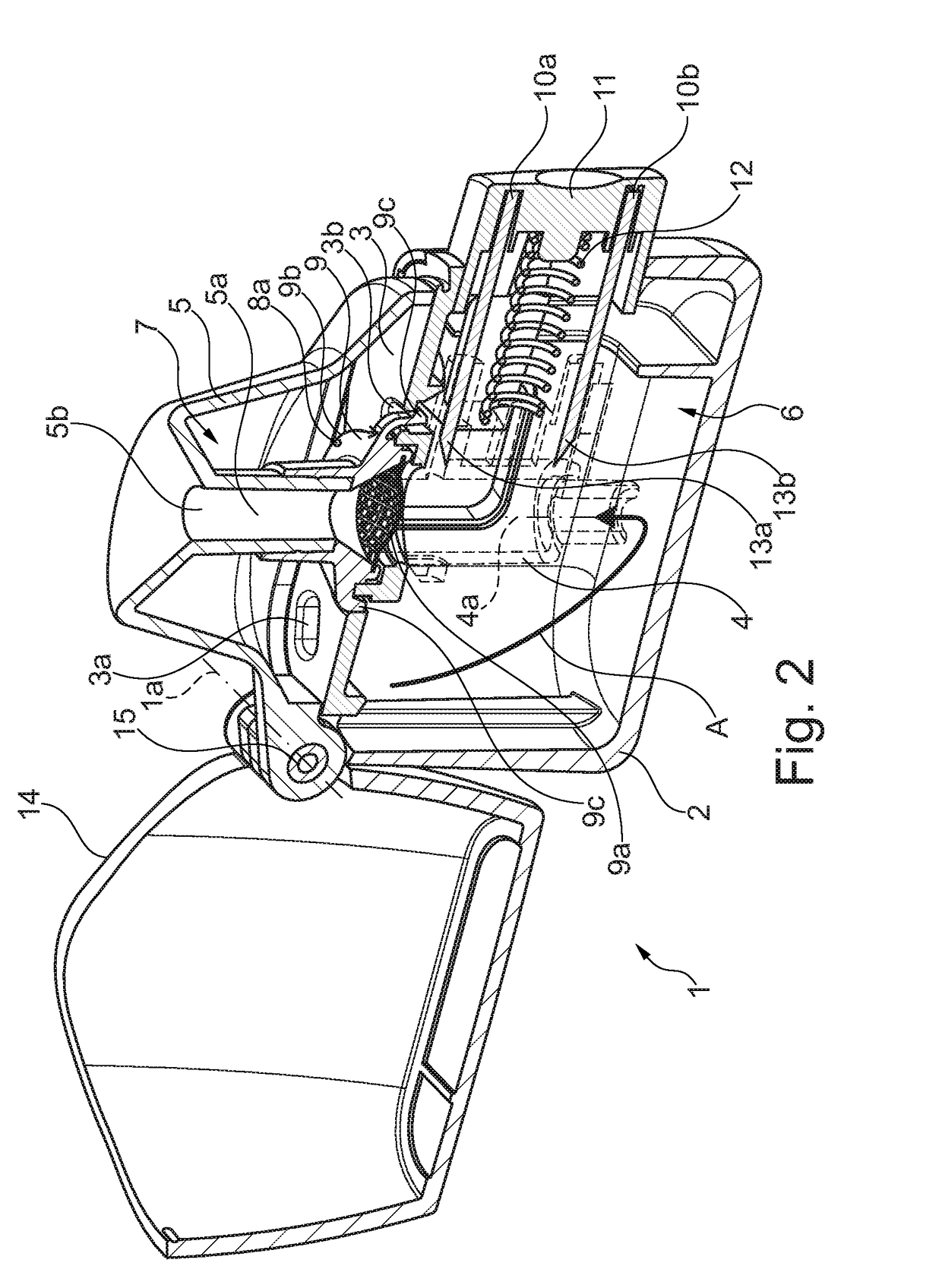
Dry powder inhaler system
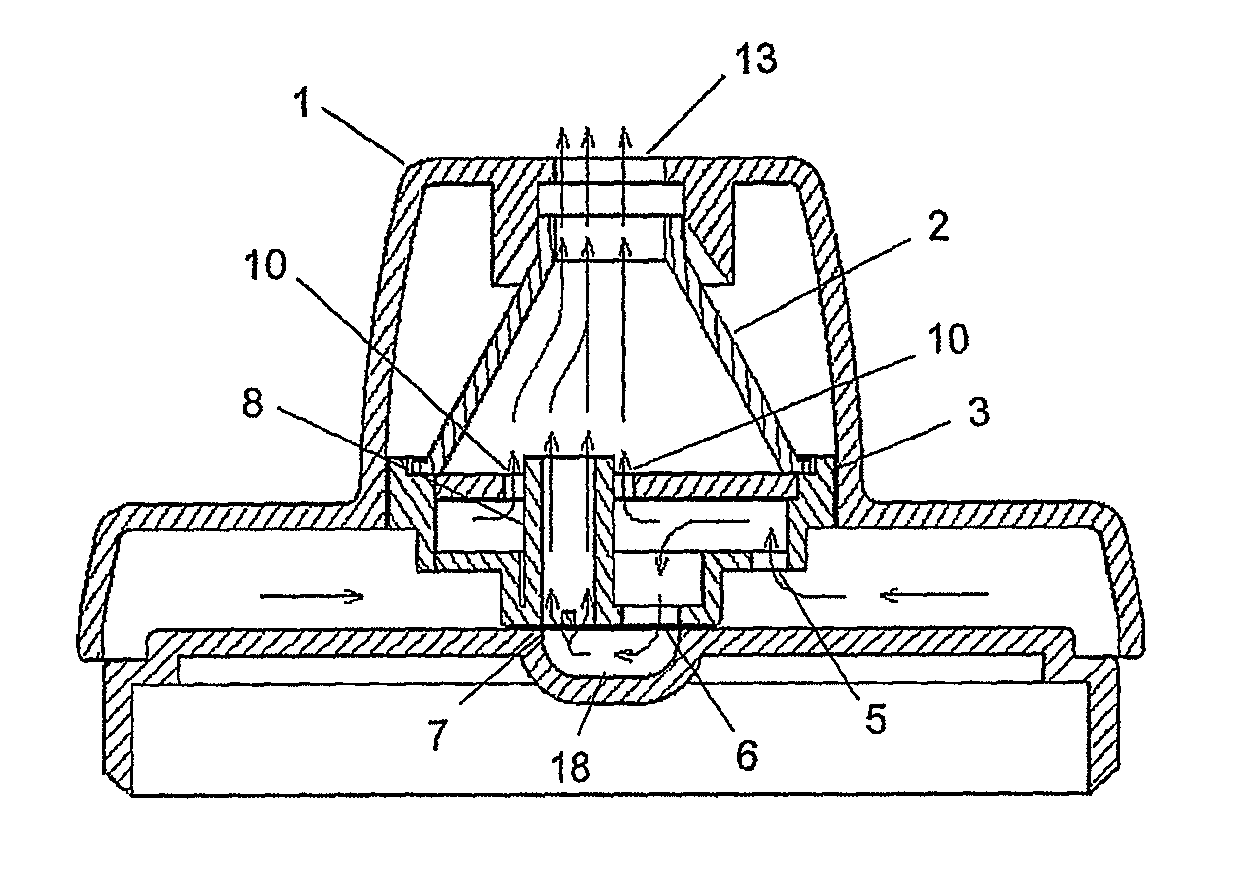
InactiveUS20060254583A1Easy piercingRespiratorsPowder deliveryBULK ACTIVE INGREDIENTActive ingredient
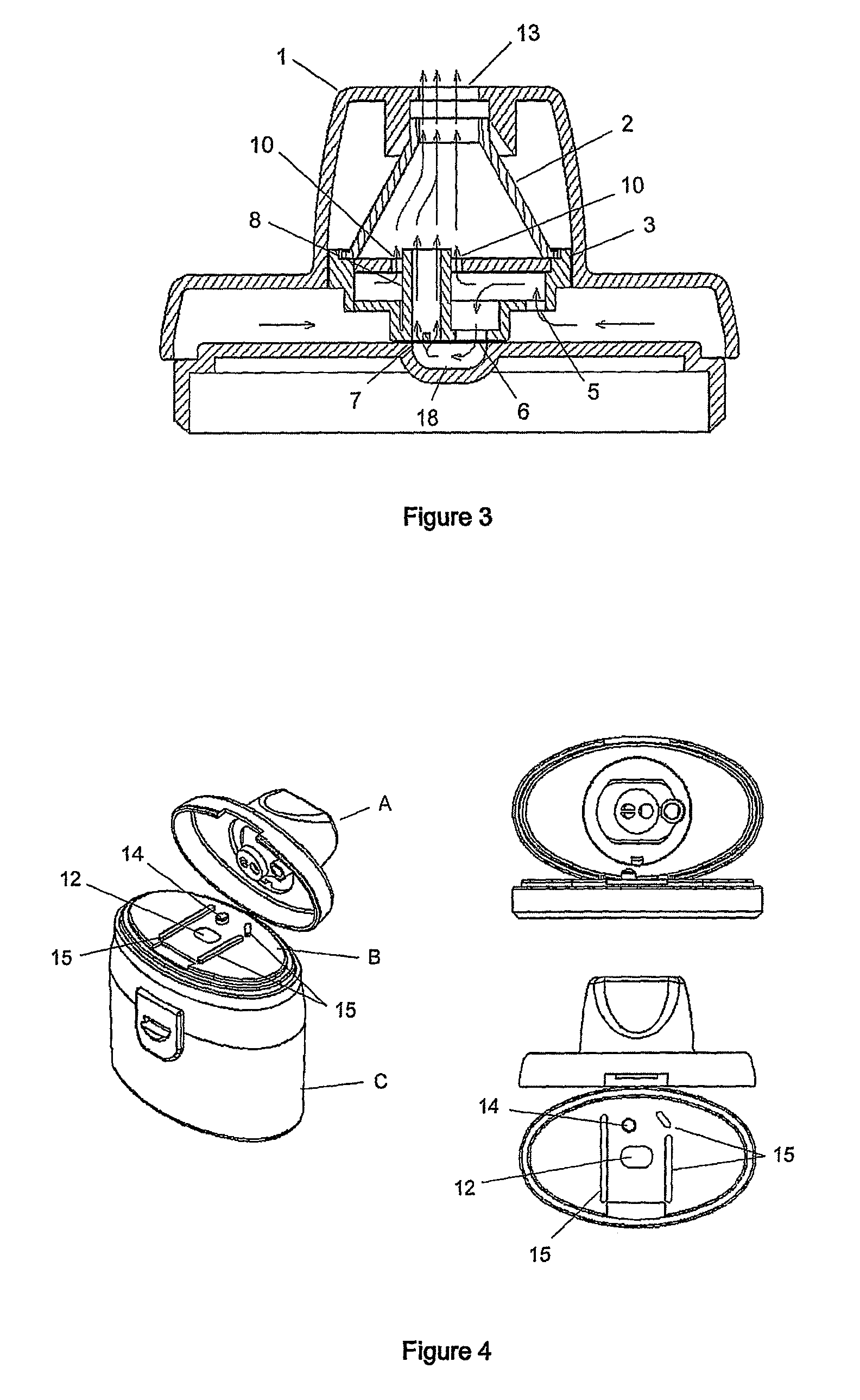
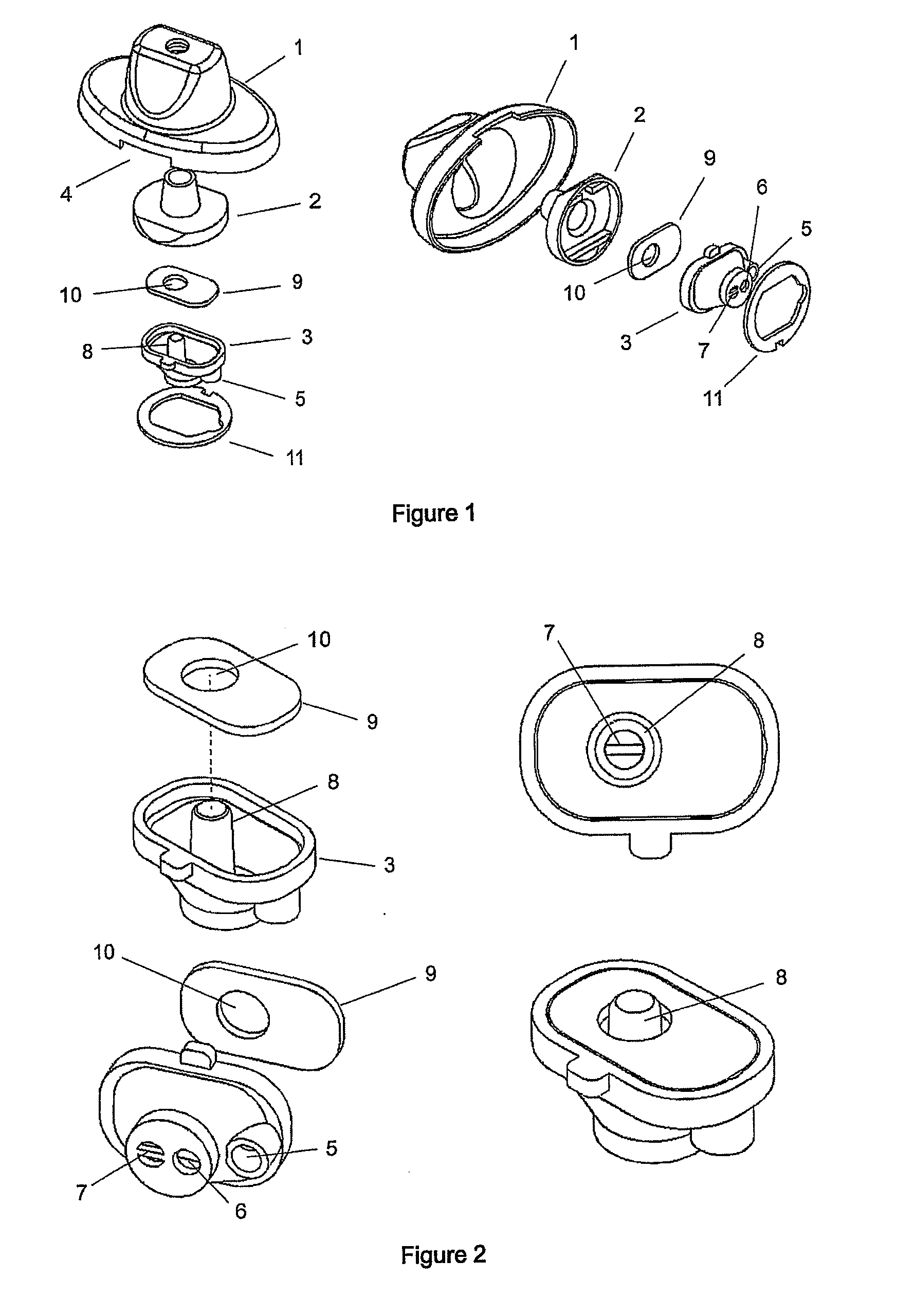
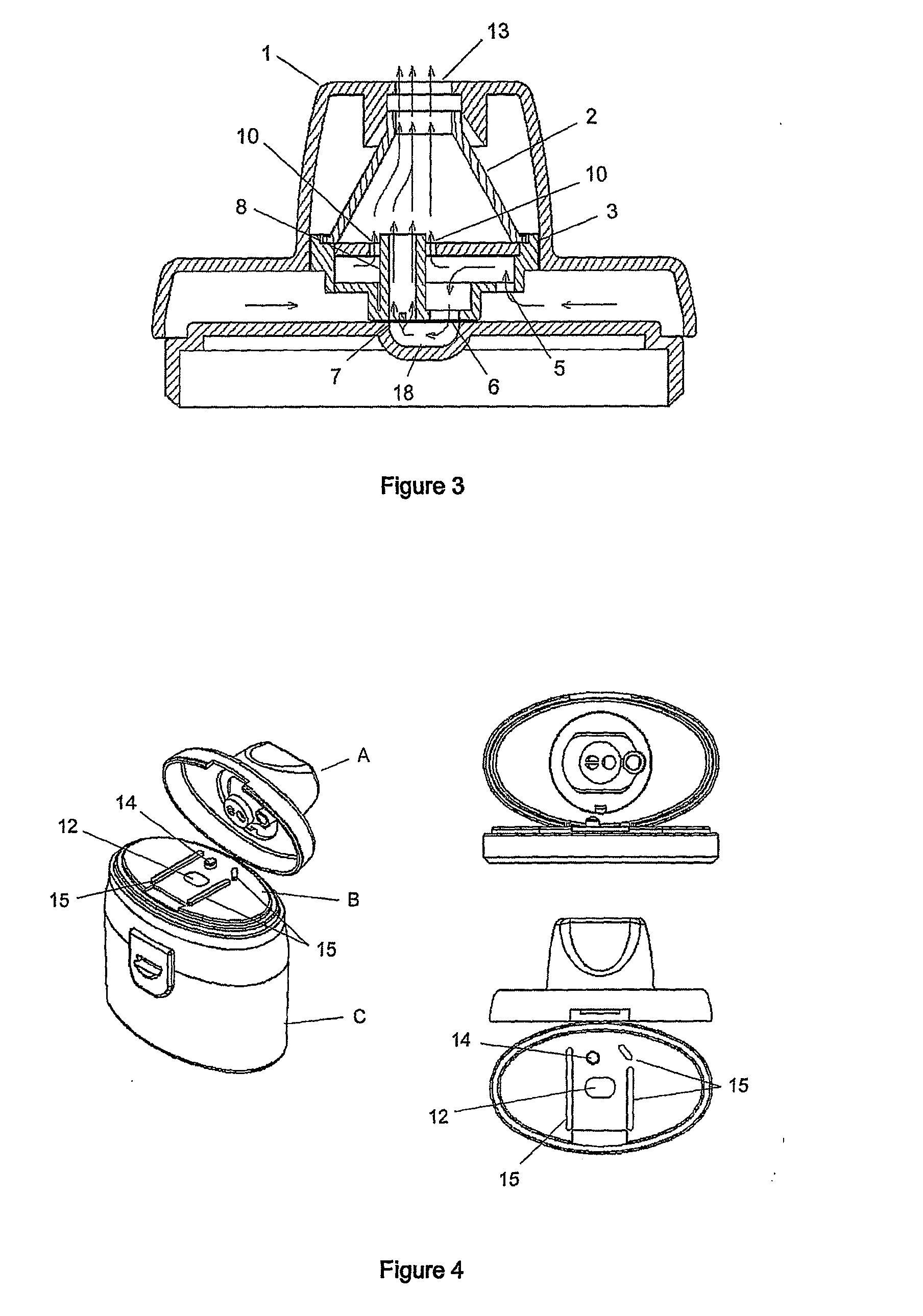
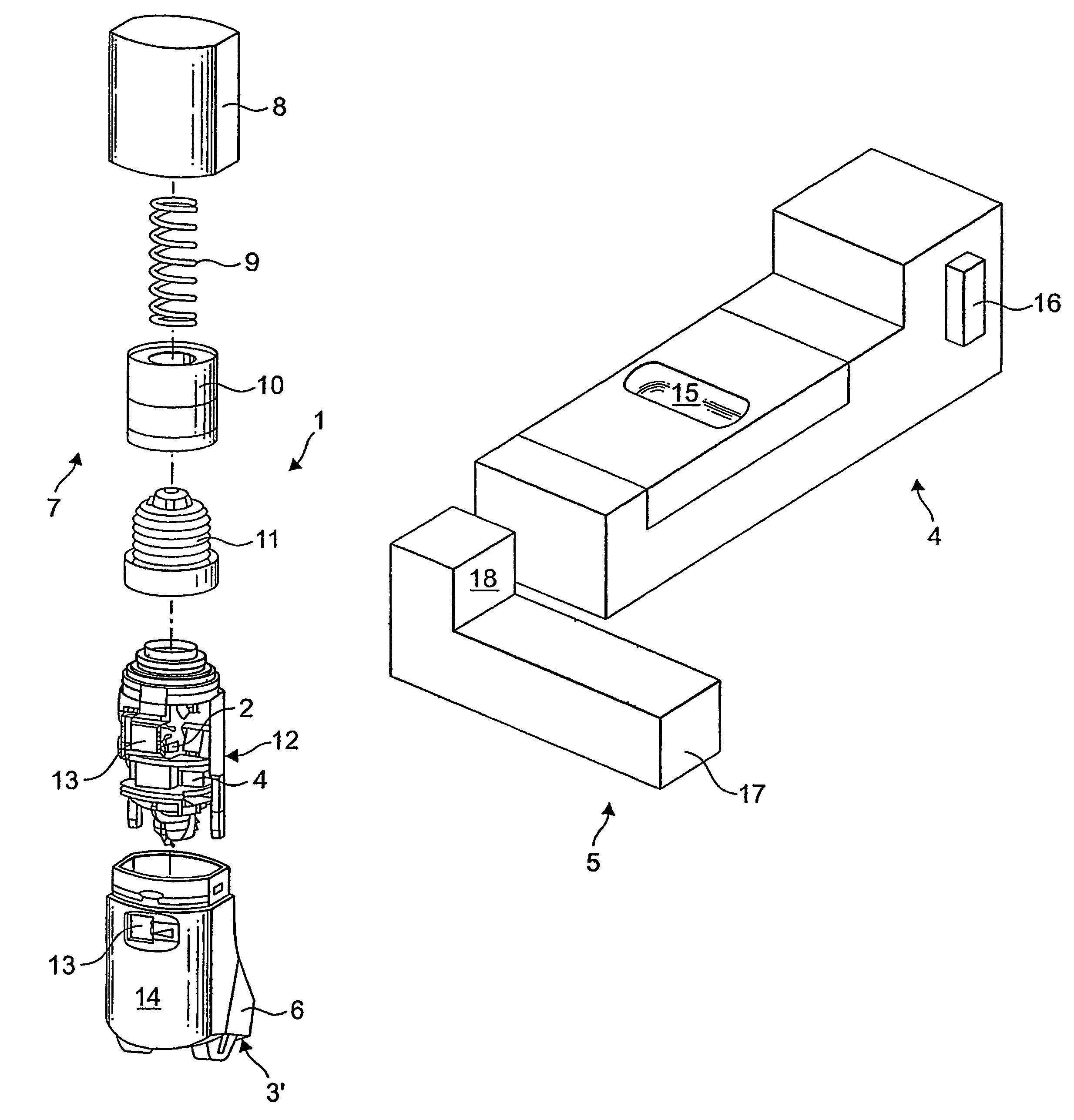
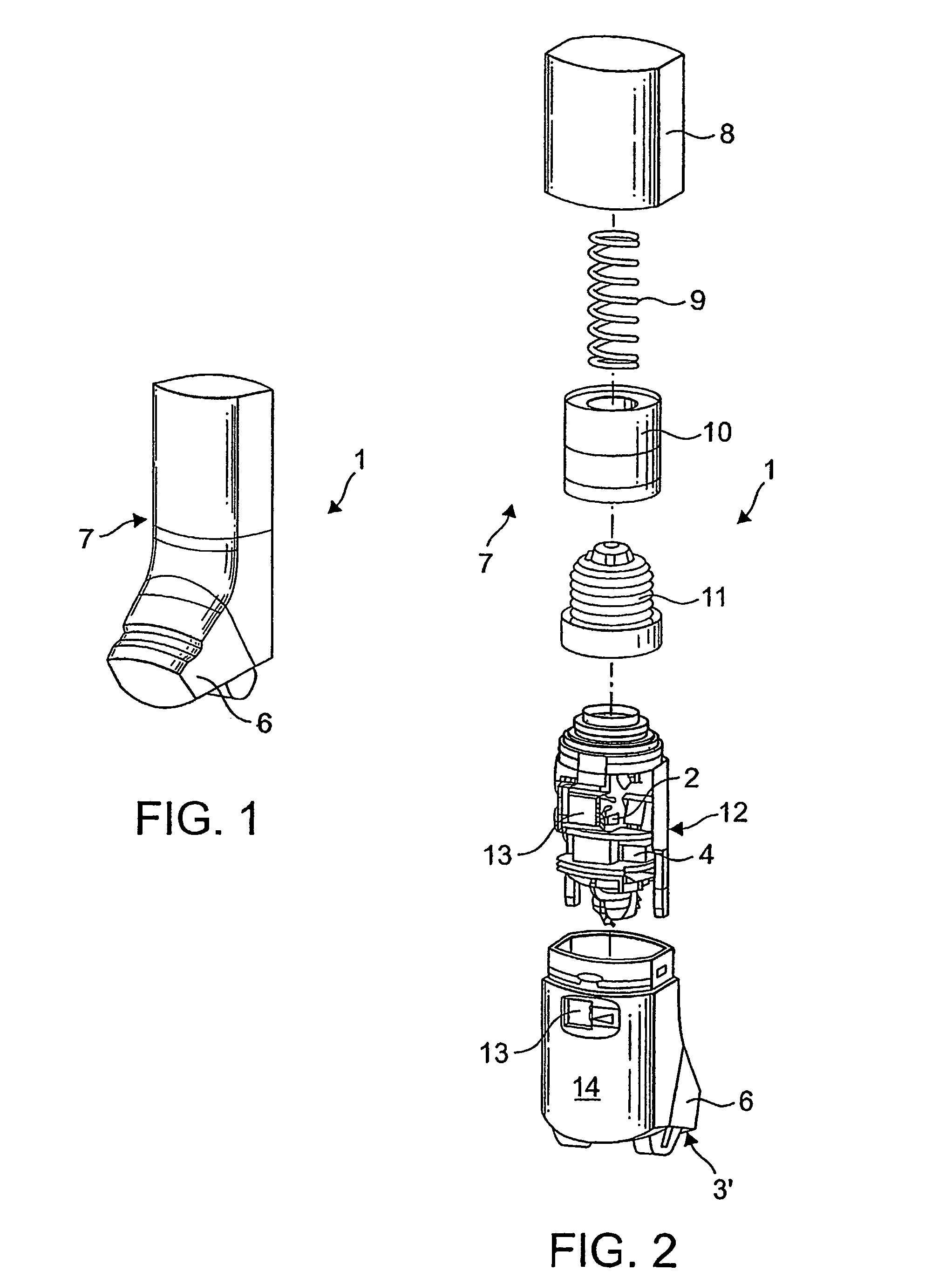
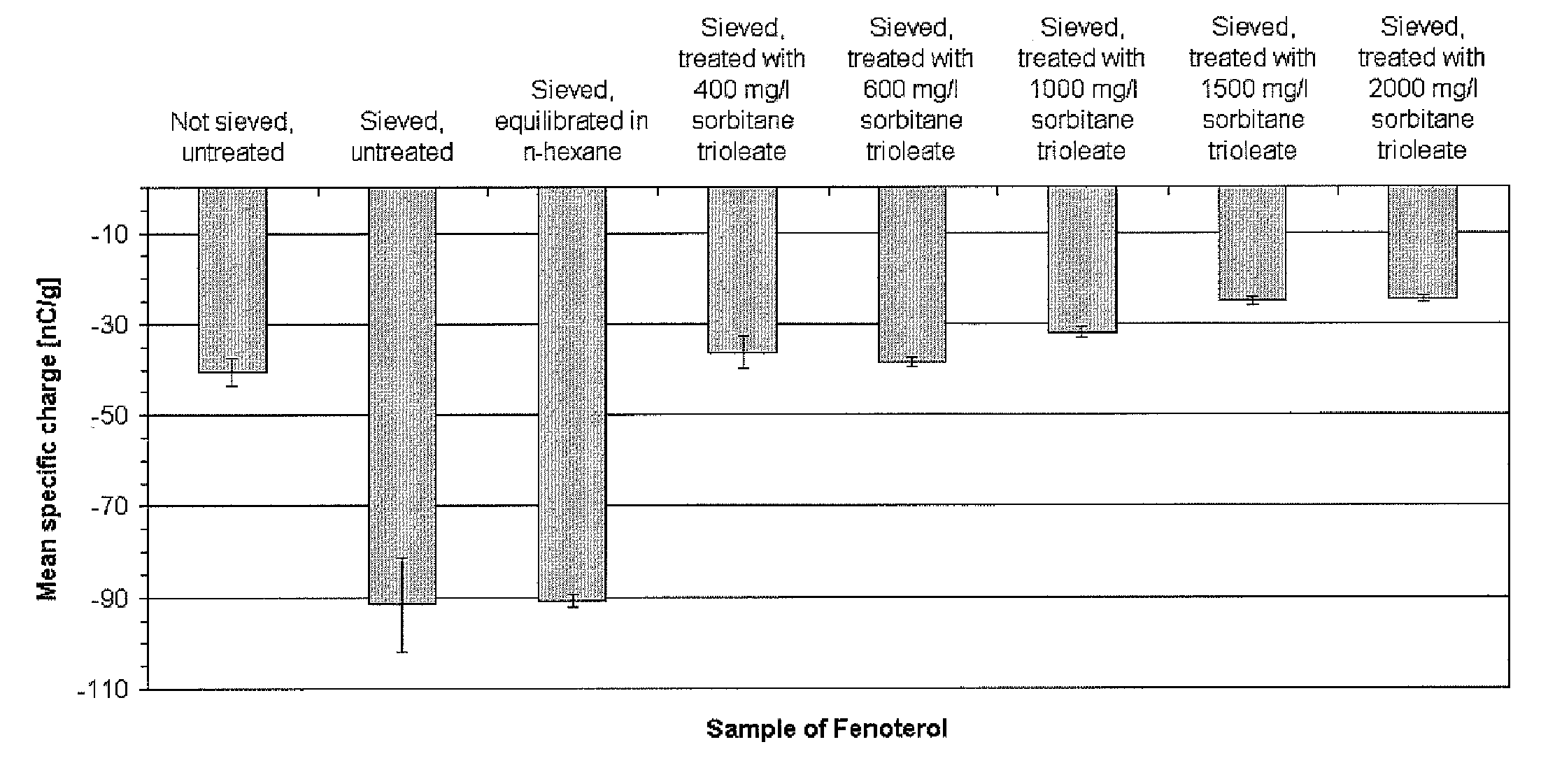
An improved dry powder inhalation system comprising: at least one micronized active ingredient in an hydroxypropylmethylcellulose capsule (10), and an dry powder inhaler device equipped with piercing systems (12) having an equivalent diameter of not less than 0.8 mm.
Owner:GALEPHAR PHARMA RES
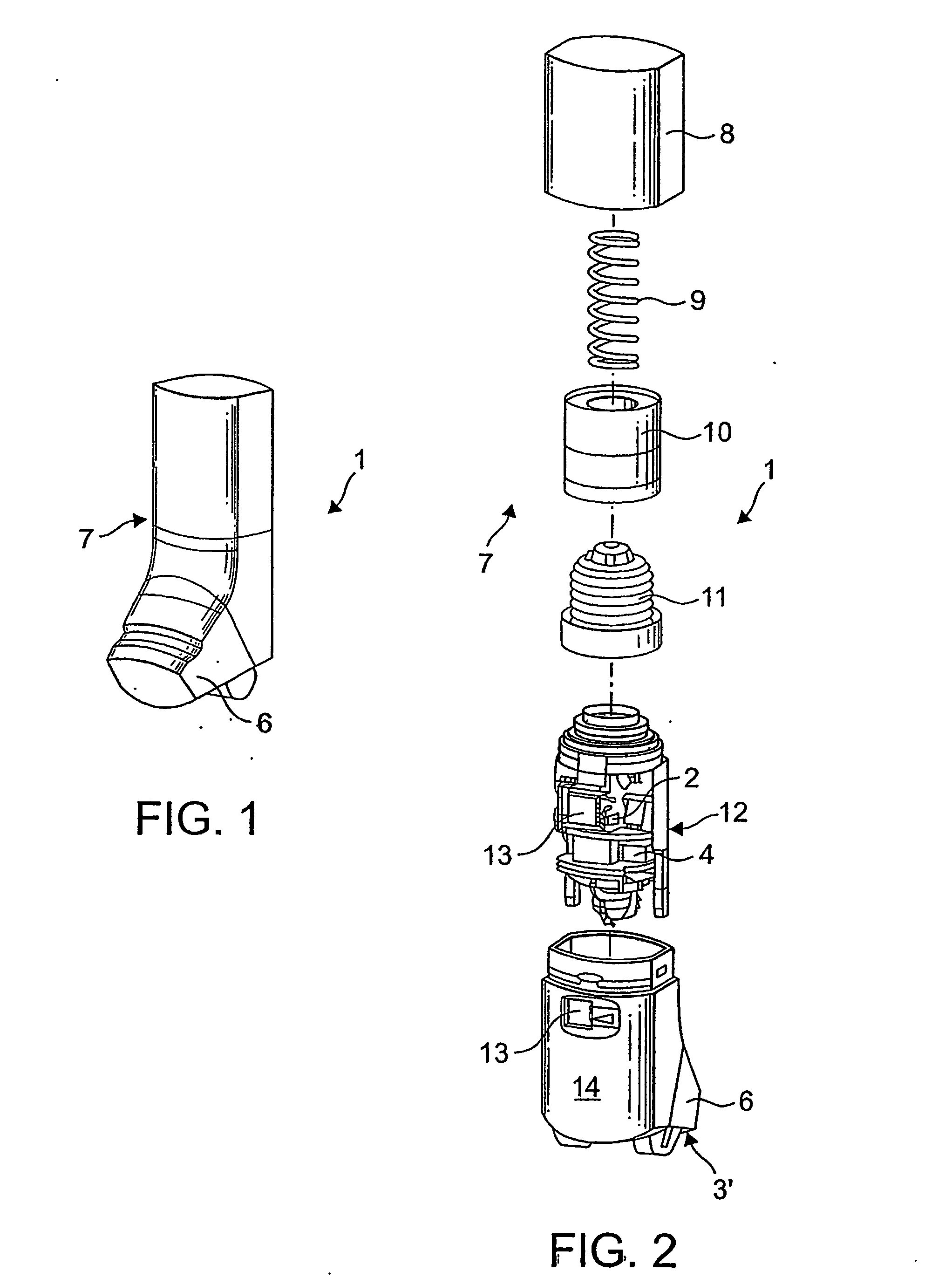
Dry Powder Inhalation Apparatus
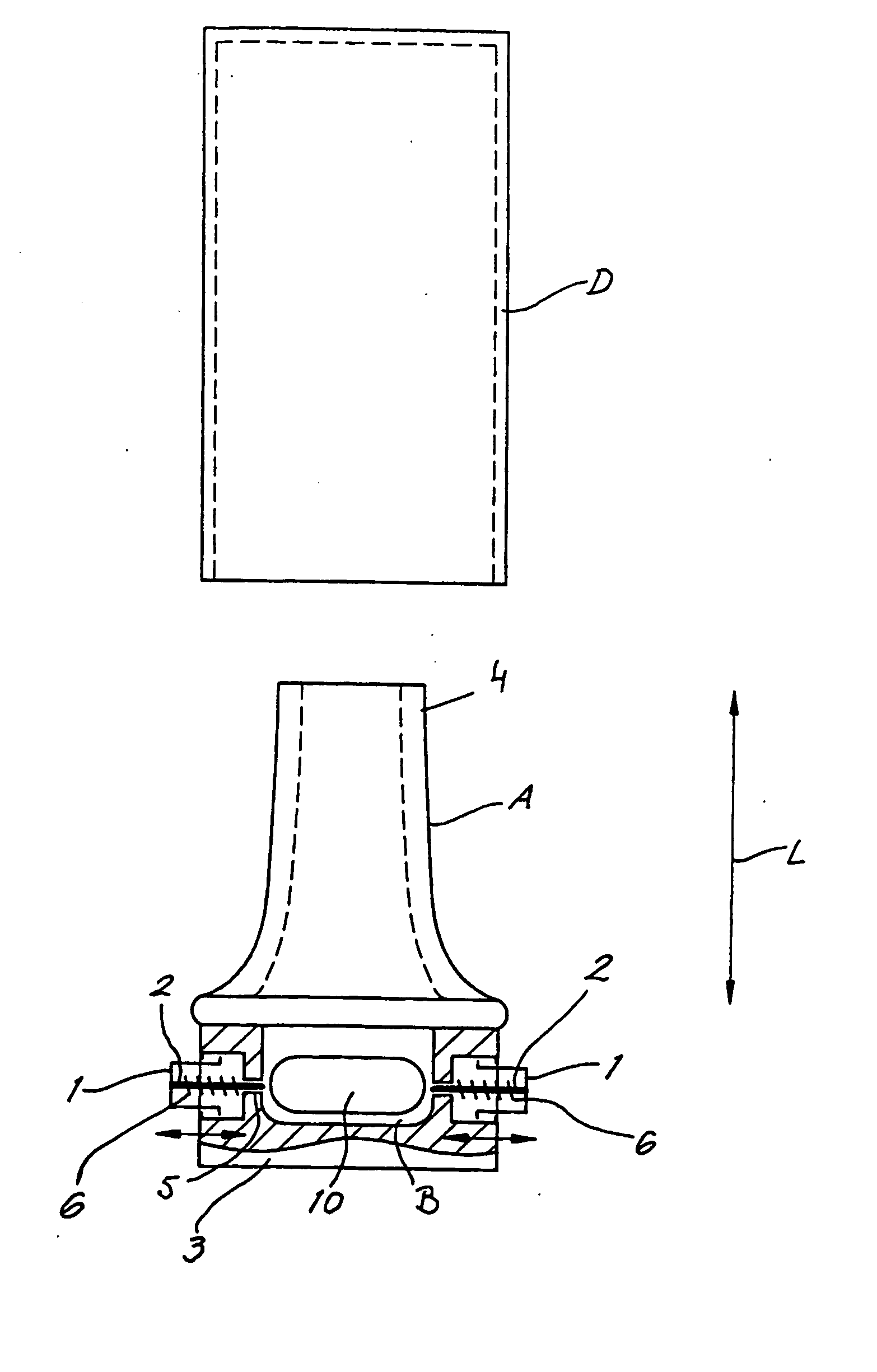
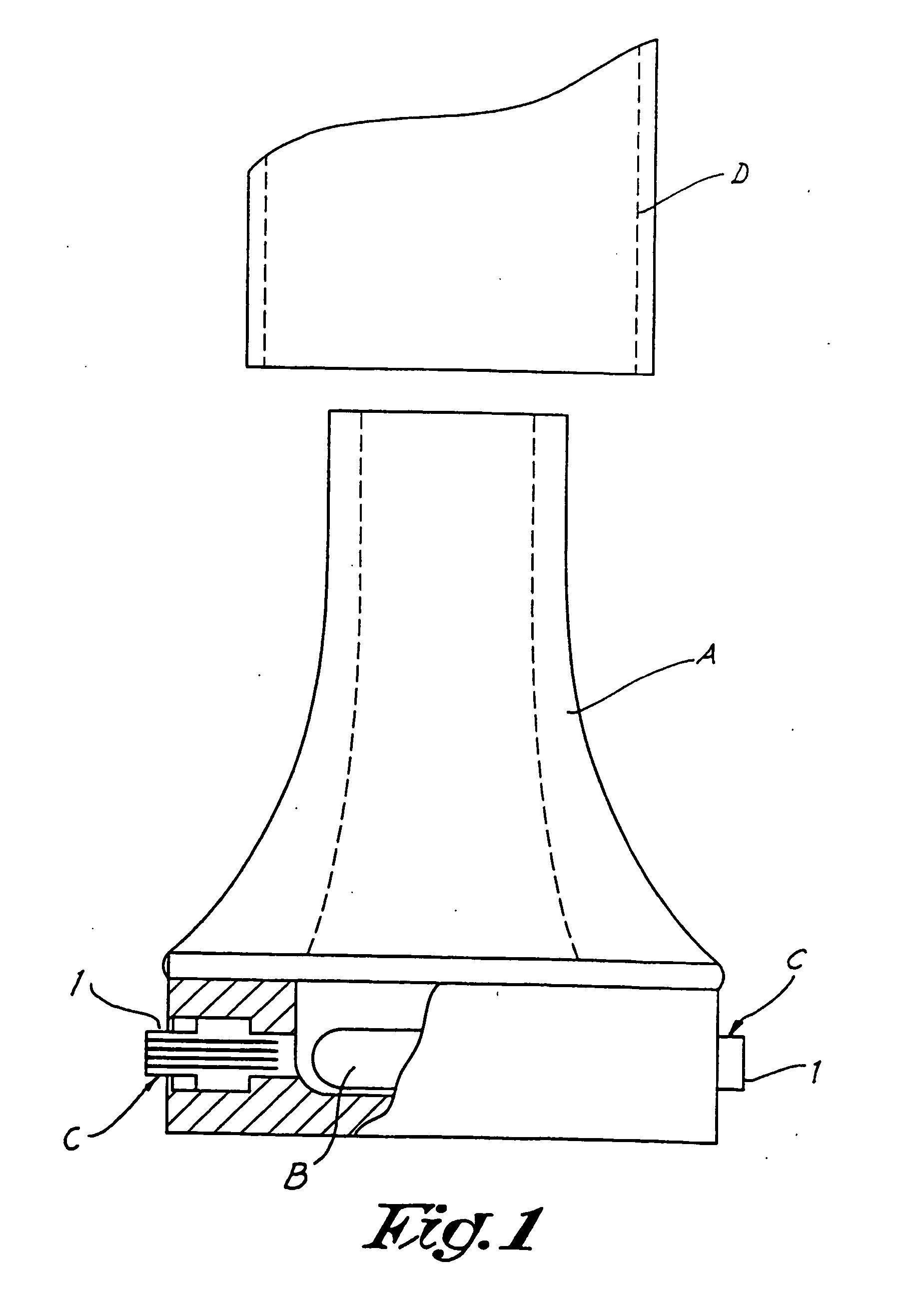
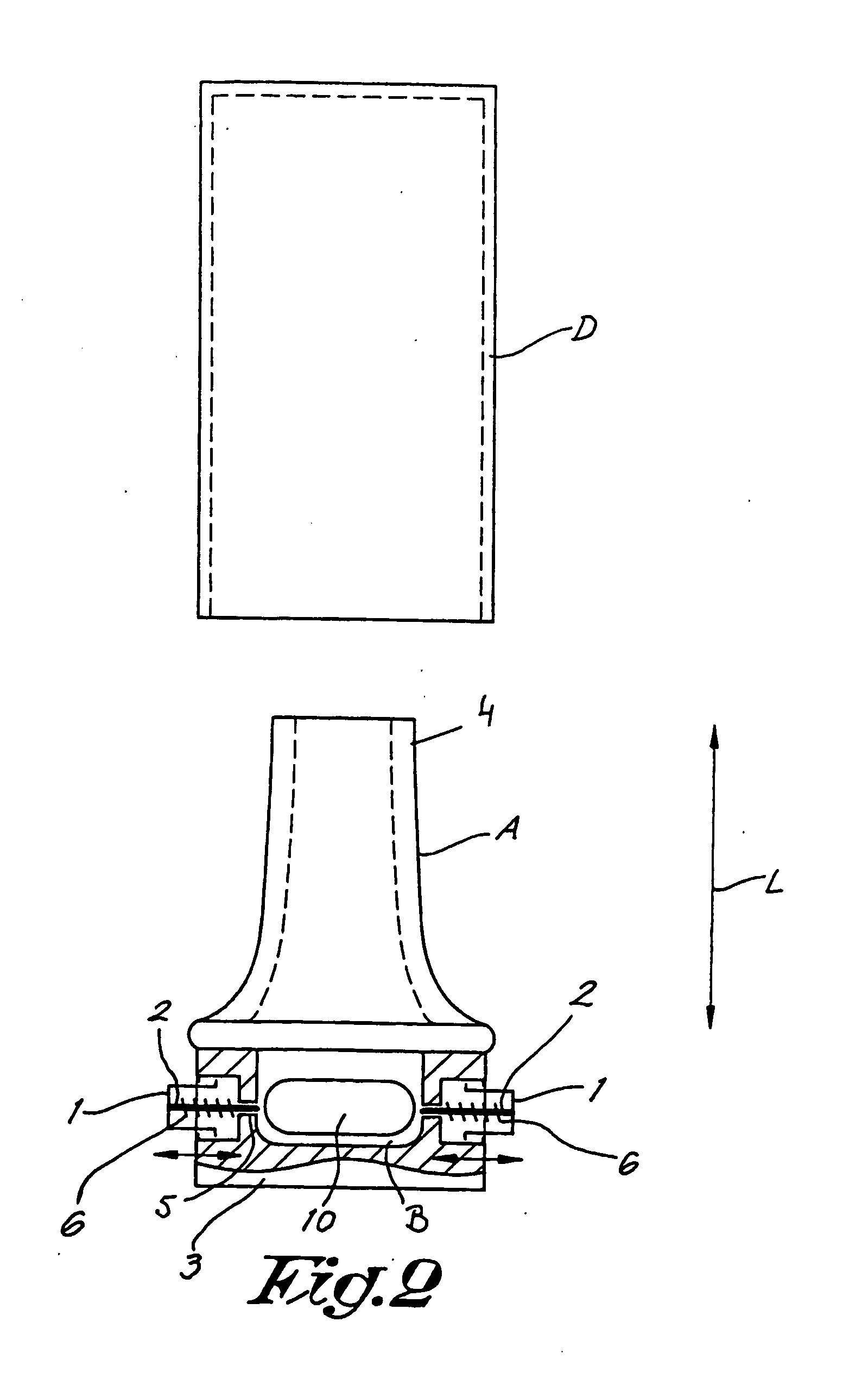
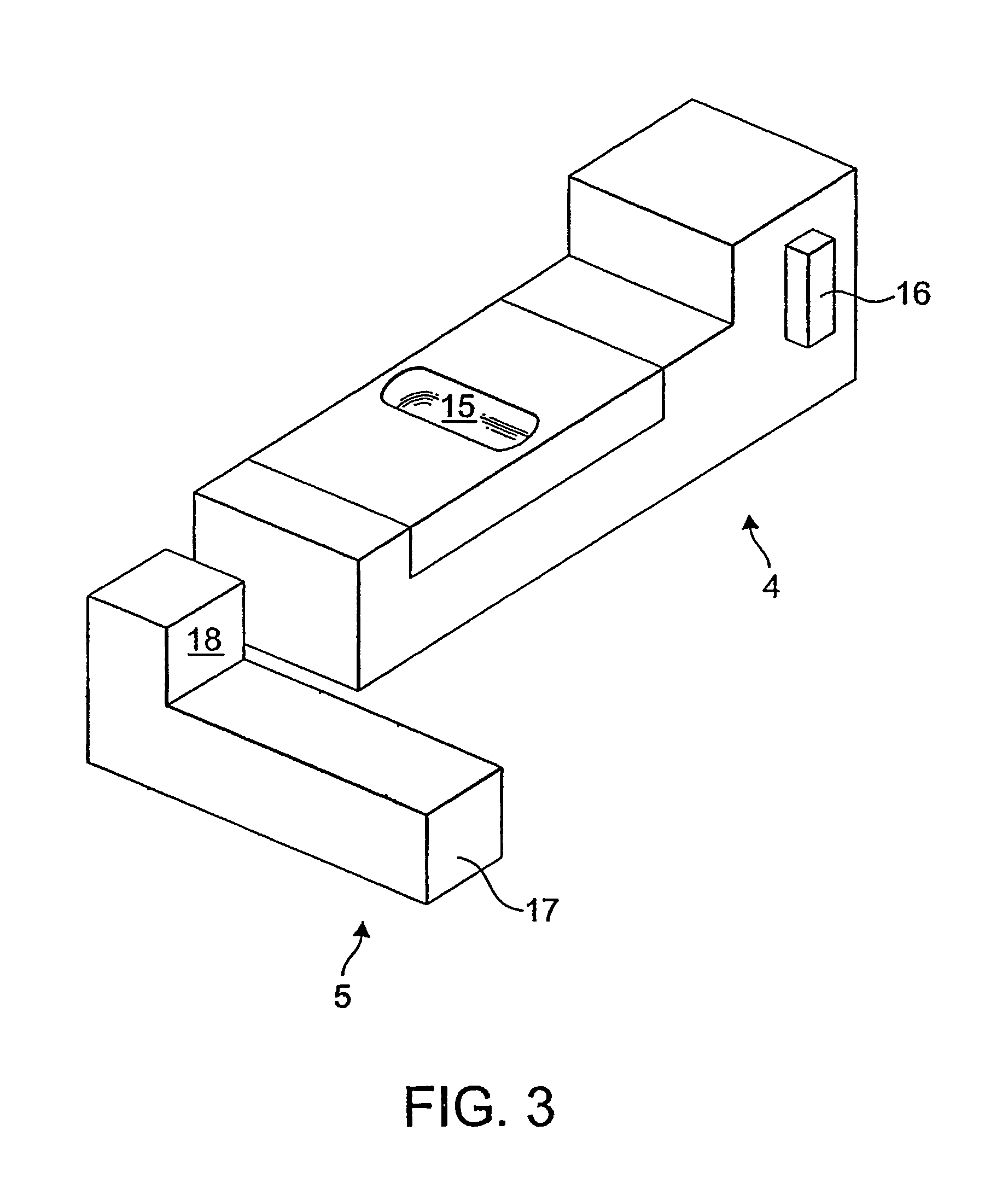
The invention relates to dry powder inhalation apparatus usually operable by breath of a user which provides for controlled and smooth transfer of medicament during multiple actuations by a user. A mechanism of the apparatus for achieving this controlled and smooth transfer includes a device (4) normally held adjacent a reservoir for receiving medicament in a cup or receptacle (15) and which is generally movable transversely of a longitudinal axis of the apparatus to delivery channels of the apparatus. This bodily shifting of the device (4) is achieved by a yoke acting on an abutment (16) thereof. Spillage of medicament in the apparatus is avoided.
Owner:NORTON HEALTHCARE
Dry powder inhaler
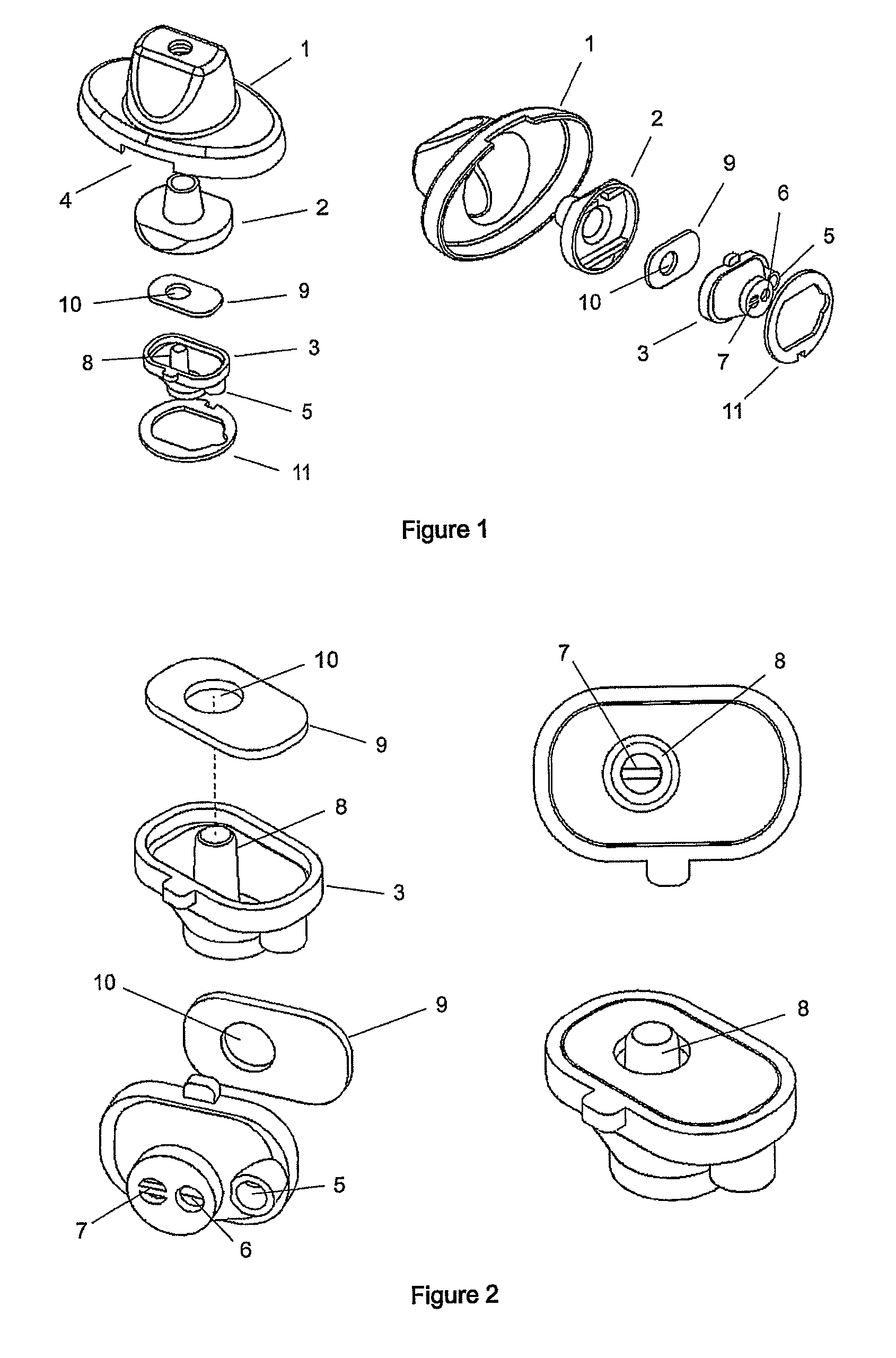
InactiveUS8037881B2Increase resistanceReduce resistanceRespiratorsLiquid surface applicatorsMedicineBlisters
Owner:PENTAFRAGAS DIMITRIOS
Dry powder inhaler
InactiveUS20100154795A1Increase resistanceReduce resistanceRespiratorsLiquid surface applicatorsPhysical therapySurgery
The present invention relates to an improvement of the mouthpiece of a dry powder inhalation device wherein the medicament is packed in the blisters of single dose blister strips. According to the invention a portion of the air which enters the mouthpiece does not pass through the powder containing blister, but follows an alternative path through the mouthpiece, enabling therefore the modification of the resistance of the device in a simple and cost effective manner.
Owner:PENTAFRAGAS DIMITRIOS
Dry powder inhaler system
Owner:GALEPHAR PHARMA RES
Dry powder inhalation, preparation method and application thereof
InactiveCN101669925AImprove efficacyLittle side effectsPowder deliveryAntineoplastic agentsSide effectMedicine
The invention relates to a dry powder inhalation with low-density or ultralow-density inhalable medicinal powder, a method for preparing the dry powder inhalation and application of the dry powder inhalation in preparing medicament for treating tumor, in particular lung tumor. The dry powder inhalation can deliver the tumor medicament to the lung directly so as to improve the medicament effect andreduce side effect; and the 'foaming' technology adopted for preparing the dry powder inhalation optimizes the micromeritic properties of the medicinal powder, and greatly improves the deposition performance of the tumor medicinal dry powder inhalation. The dry powder inhalation, the preparation method and the application thereof are also suitable for preparing non-vector dry powder inhalation toovercome the defects of uneven mixture between medicament and vector easily occurring in the dry powder inhalation.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Particles for inhalation having rapid release properties
InactiveUS20080226730A1Facilitated releaseShorten the timePowder deliveryOrganic active ingredientsActive agentPhospholipid
The invention generally relates to formulations having particles comprising phospholipids, bioactive agent and excipients and the pulmonary delivery thereof. Dry powder inhaled insulin formulations are disclosed. Improved formulations comprising DPPC, insulin and sodium citrate which are useful in the treatment of diabetes are disclosed. Also, the invention relates to a method of for the pulmonary delivery of a bioactive agent comprising administering to the respiratory tract of a patient in need of treatment, or diagnosis an effective amount of particles comprising a bioactive agent or any combination thereof in association, wherein release of the agent from the administered particles occurs in a rapid fashion.
Owner:CIVITAS THERAPEUTICS
Pyrazolopyrimidine compound and pharmaceutical composition thereof as well as pharmaceutical application of pyrazolopyrimidine compound
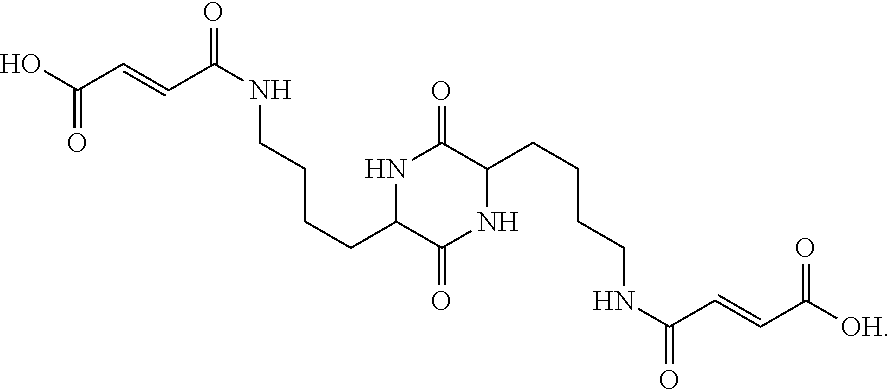
The invention provides a pyrazolopyrimidine compound shown as a structural formula (I), a pharmaceutical composition taking the pyrazolopyrimidine compound as an active component, a preparation method of the pyrazolopyrimidine compound and the pharmaceutical composition as well as an application of the pyrazolopyrimidine compound and the pharmaceutical composition in preparation of a TRPC6 (Transient Receptor Potential Channel 6) adjustor probe medicine and related medicines for preventing and treating glomerulopathy and myocardial hypertrophy. The pyrazolopyrimidine compound and derivatives provided by the invention can be used to prepare medical preparations in various forms which comprise oral liquids, injections, pulmonary inhalation preparations and transdermal preparations, specifically injections, oral liquids, troches, capsules, granules, aerosols, dry powder inhalation, patches and the like.
Owner:泸州天演生物医药科技有限公司
Powdery inhalational preparations and process for producing the same
The present invention is aimed at providing a dry powder inhalation with minimal adhesive-agglomerative property during storage and with a good inhalation behavior for a pharmaceutically active ingredient, and is a dry powder inhalation wherein at least an active ingredient is adhered to the surface of a carrier particle comprised of erythritol and / or trehalose. It is also a dry powder inhalation wherein at least S-36496 and / or Pralmorelin dihydrochloride is adhered to the surface of the carrier particle. It is further a dry powder inhalation wherein at least an active ingredient and a surface modifier are adhered to the surface of the carrier particle. It is also a preparation method of these. Said dry powder inhalation may be applied to the capsules for use in an inhaler device. In addition to the achievement of above objectives, the present invention provides a dry powder inhalation with improved taste in inhalation or with reduced discomfort in the oral cavity and the throat, and a preparation method for enabling an easy preparation thereof without complicated processes.
Owner:KAKEN PHARMA CO LTD
Dry powder inhalation apparatus
The invention relates to dry powder inhalation apparatus usually operable by breath of a user which provides for controlled and smooth transfer of medicament during multiple actuations by a user. A mechanism of the apparatus for achieving this controlled and smooth transfer includes a device (4) normally held adjacent a reservoir for receiving medicament in a cup or receptacle (15) and which is generally movable transversely of a longitudinal axis of the apparatus to delivery channels of the apparatus. This bodily shifting of the device (4) is achieved by a yoke acting on an abutment (16) thereof. Spillage of medicament in the apparatus is avoided.
Owner:NORTON HEALTHCARE
Novel dry powder inhalation system for transpulmonary administration
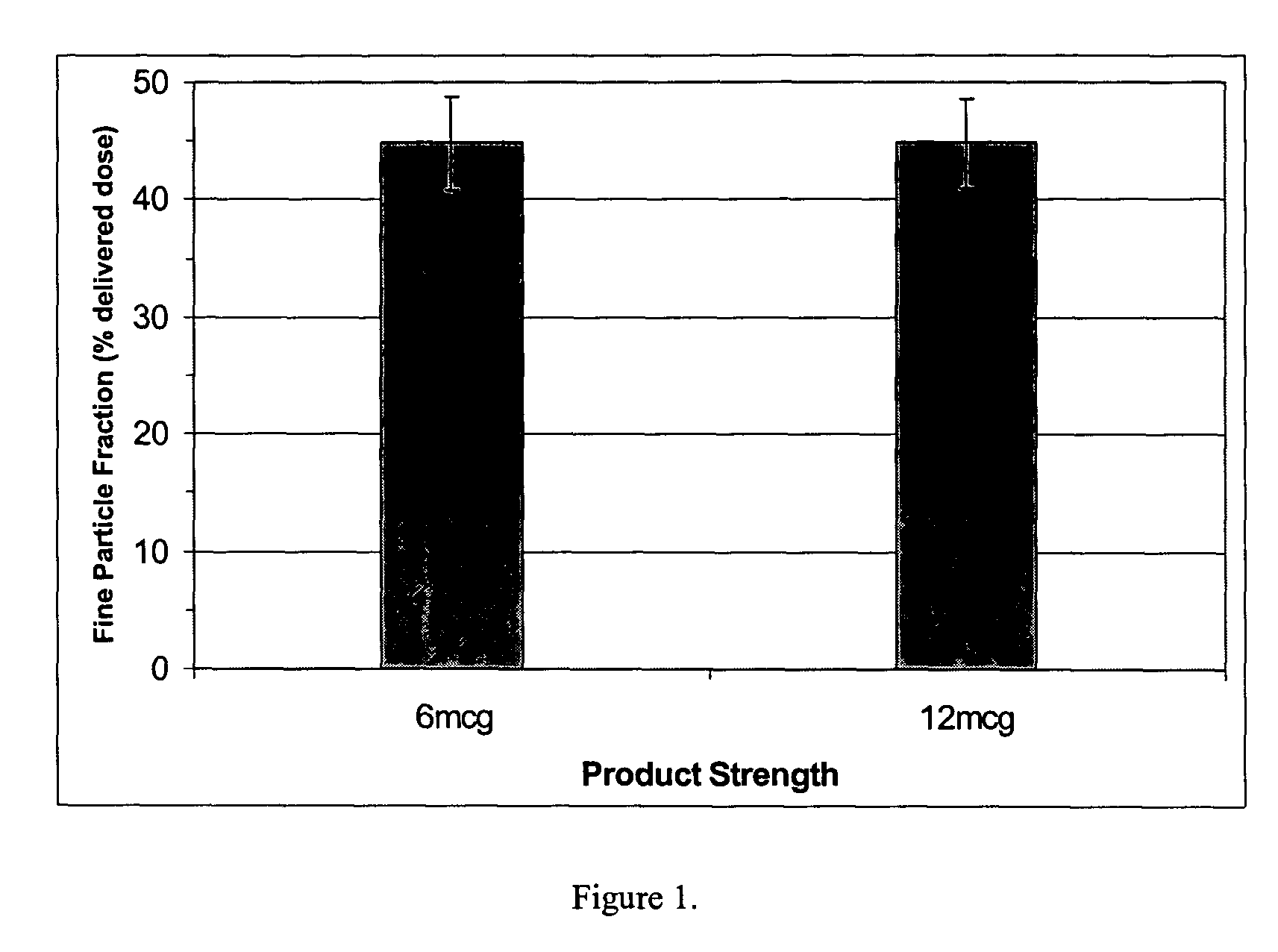
InactiveUS20060073105A1Reduce manufacturing costHigh fine particle fractionPowder deliverySpray deliveryFreeze-dryingMedicine
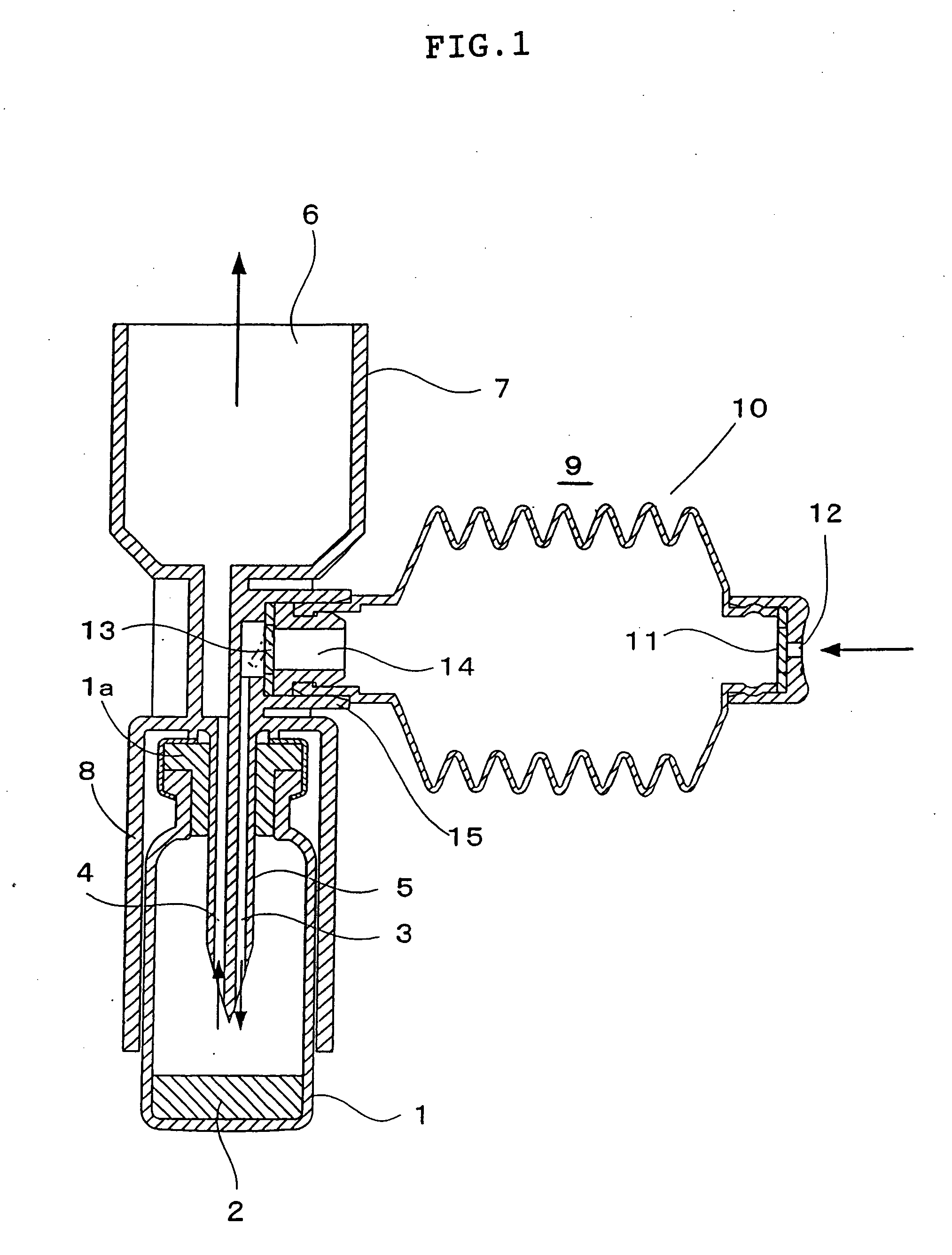
The present invention provides a novel dry powder inhalation system suitable for transpulmonary administration. The dry powder inhalation system of the present invention characterized by using a combination of: (1) a vessel housing a freeze-dried composition prepared by freeze-drying a composition liquid containing ingredients in a non-dissolved form, and has: (i) a non-powder cake-like form, (ii) a disintegration index of 0.05 or more, and (iii) a property of becoming fine particles having a mean particle diameter (mass median aerodynamic diameter) of 10 microns or less or a fine particle fraction of 10% or more upon receipt of an air impact having an air speed of at least 1 m / sec and an air flow rate of at least 17 ml / sec; and (2) a device comprising a member capable of applying said air impact to the freeze-dried composition in said vessel, and a member for discharging the powder-form freeze-dried composition that has been made into fine particles.
Owner:OTSUKA PHARM CO LTD
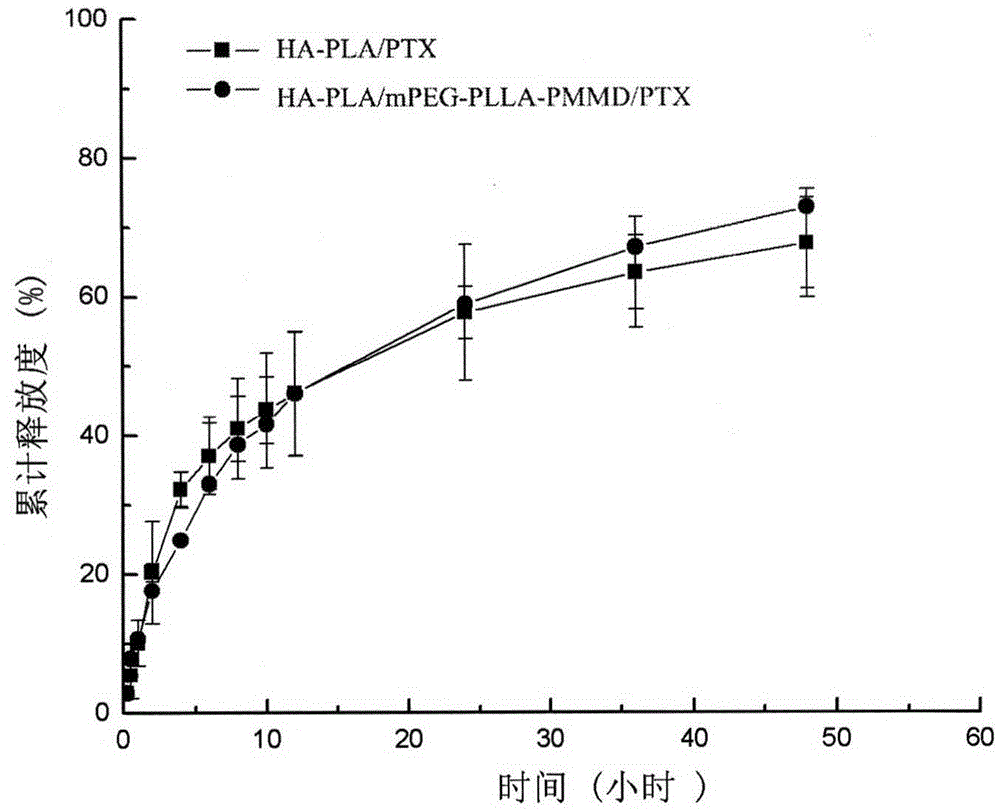
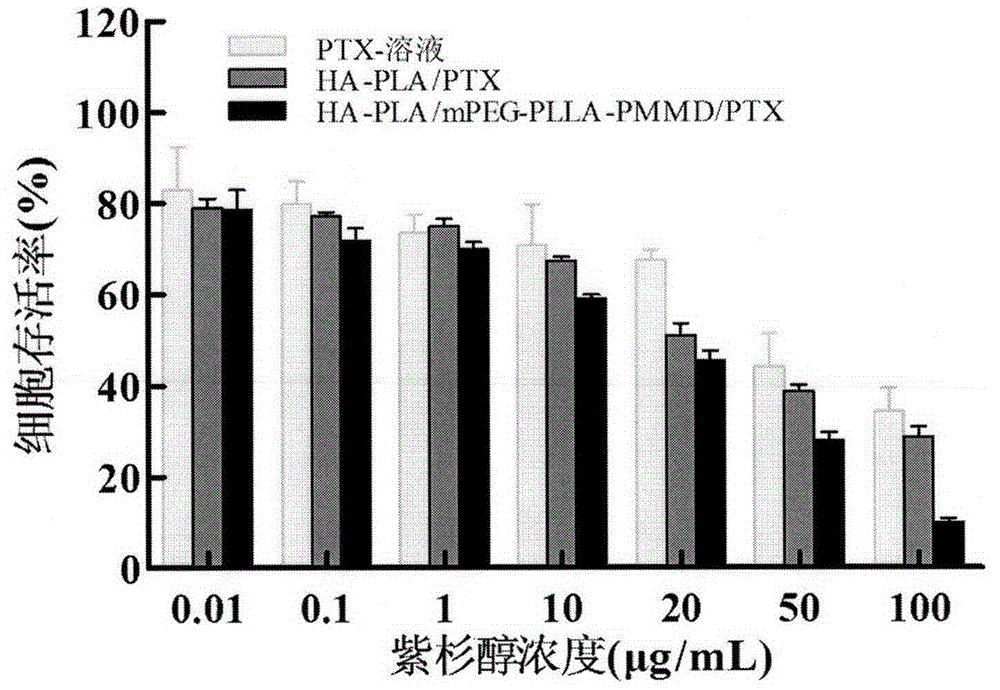
Method for synthesizing multifunctional active targeted hyaluronic acid-polylactic acid carrier and preparing anti-tumor medicinal micelle of multifunctional active targeted hyaluronic acid-polylactic acid carrier
ActiveCN104056275AExtend cycle timeSmall toxicityPharmaceutical non-active ingredientsEmulsion deliverySolubilityPolyester
The invention belongs to the fields of polymer chemistry and medicinal preparations, and particularly relates to a method for synthesizing an active targeted hyaluronic acid-polylactic acid carrier, a method for preparing an anti-tumor medicinal micelle of the active targeted hyaluronic acid-polylactic acid carrier and an application thereof. By adopting a novel self-assembly technology, amphipathic PEG (polyethylene glycol) block polyester copolymer and tumor targeted ligand hyaluronic acid-polylactic acid copolymer are self-assembled by means of the electrostatic interaction to form a multifunctional composite micelle; the solubility of insoluble tumor medicaments and the drug loading capacity and encapsulation efficiency of water-soluble anti-tumor medicines can be remarkably improved by virtue of the anti-cancer drug-loaded micelle and composite micelle composition, the medicines can be biodegraded in a body, phagocytosis of a reticuloendothelial system (RES) and excretion of a kidney can be avoided. The active targeted hyaluronic acid-polylactic acid carrier has a long-circulating effect, the multifunctional composition has a prominent advantage of tumor active targeting effect, and parameters of pharmacodynamics in vitro and in vivo of the micelle are remarkably superior to those of common anti-tumor injections. Clinically acceptable administration means of the micelle includes injection administration or mucosal administration, and preparations of the micelle can be injection, transfusion, injection lyophilized powder injections or dry powder inhalation.
Owner:CHINA PHARM UNIV
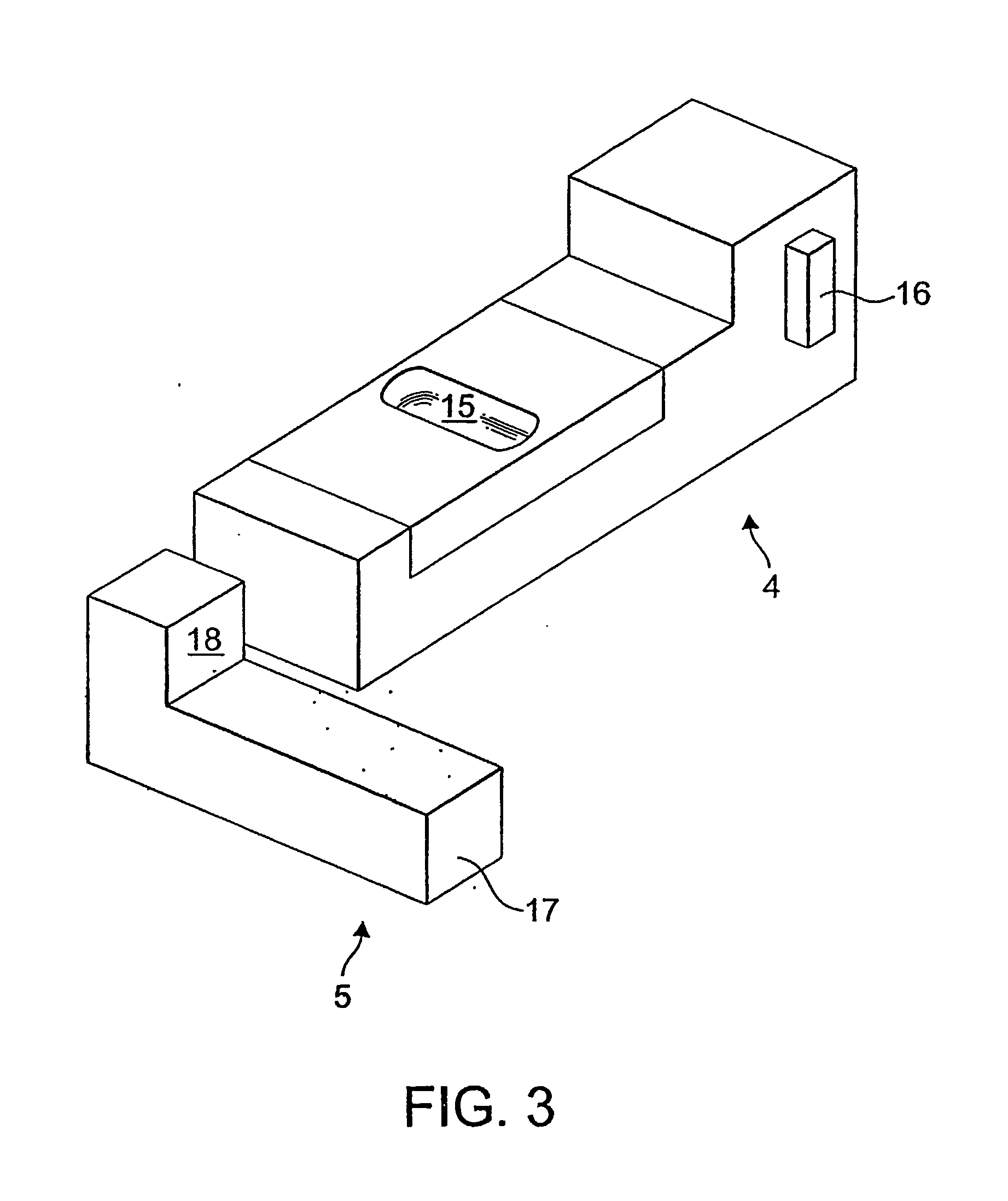
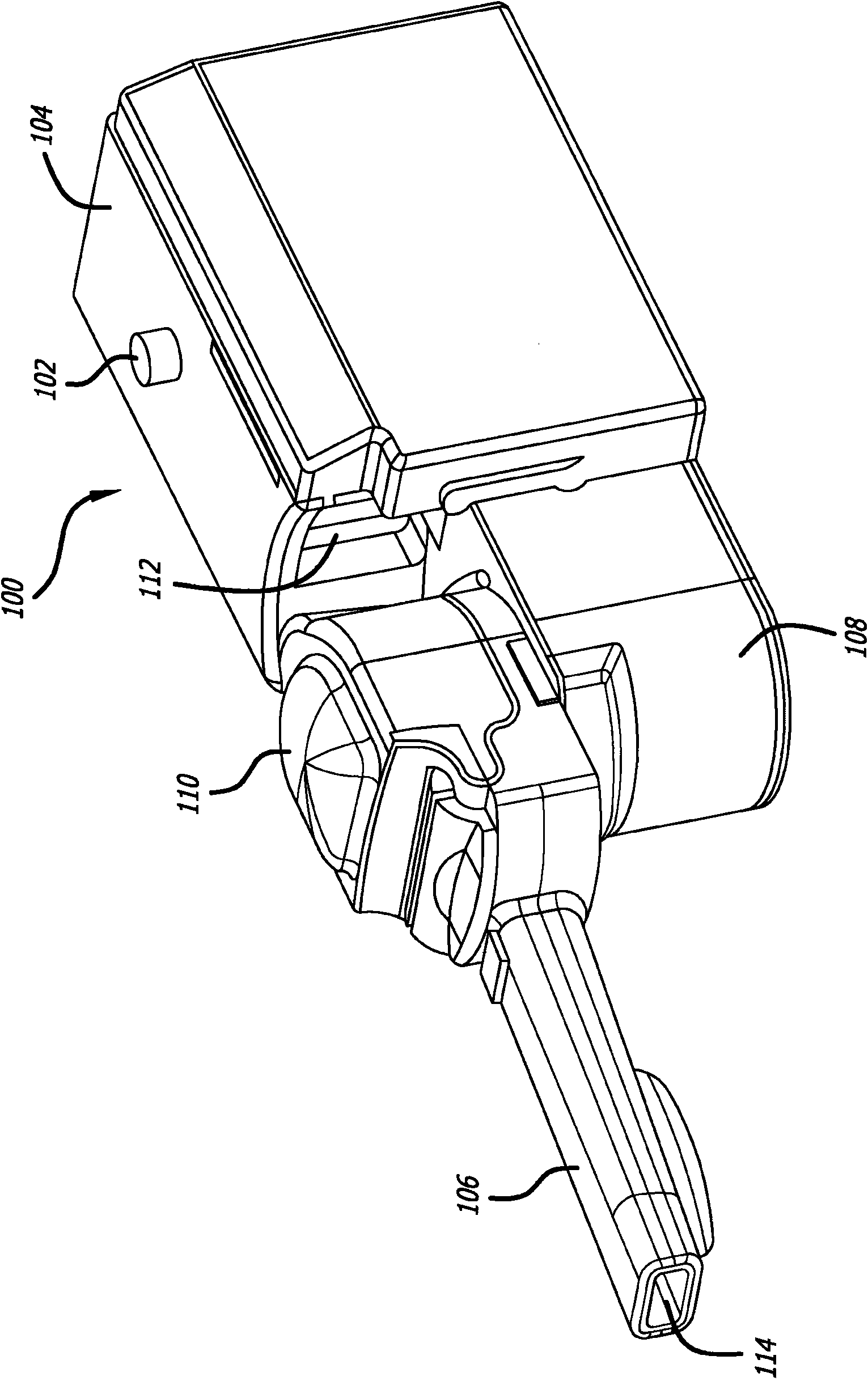
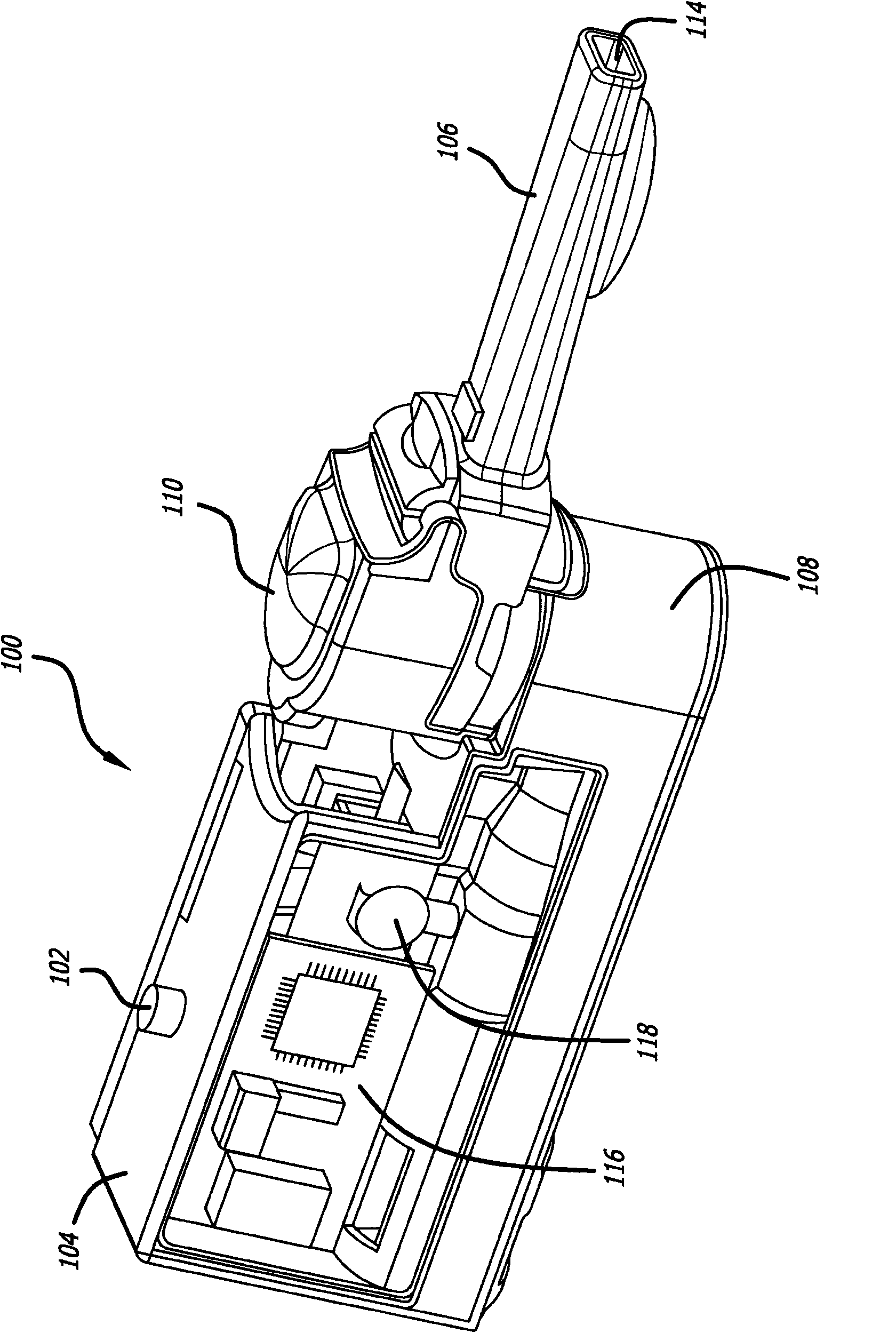
Dry powder inhalation device
ActiveUS20140230817A1Minimal sizeMinimal weightRespiratorsLiquid surface applicatorsMedicineDrug Storage
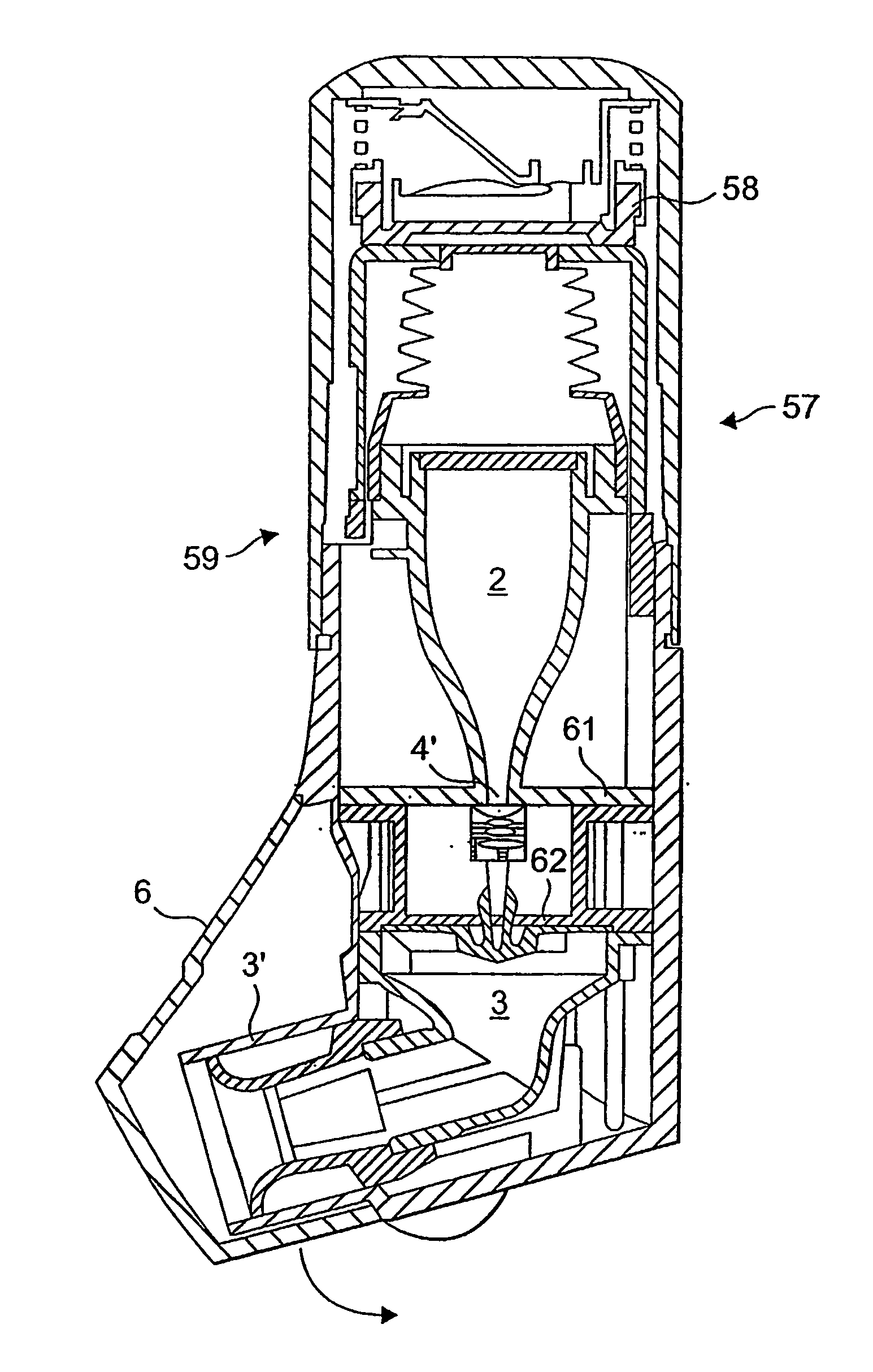
Taught herein is a disposable breath actuated dry powder drug inhalation device having a powderized drug storage chamber with integral toroidal geometry and air flow pathways for entraining and breaking up powder aggregates prior to delivery to the patient. The toroidal chamber is fluidly connected by one or more air inlets directed in a non-tangent manner toward the powder to loft and set up an irregular-rotational flow pattern. Also, in fluid connection with the toroidal chamber is a centrally or near centrally located air and powder outlet consisting of one or more holes forming a grid in fluid connection with a channel providing a passageway for powder flow to the patient.
Owner:CONCENTRX PHARMA
Powder inhaler formulations
The present invention relates to new methods for the surface modification of powders. Furthermore the present invention relates to new, improved pharmaceutical dosage forms obtainable by the new methods for surface modification of drugs according to the invention and to the use of these pharmaceutical dosage forms within dry powder inhalation devices (DPI).
Owner:BECHTOLD PETERS KAROLINE +2
Insulin intranasal inhalation powder spray
InactiveCN101428009AImprove stabilityImprove bioavailabilityPeptide/protein ingredientsMetabolism disorderNasal cavityLymphatic vessel
The invention provides insulin nasal dry powder inhalation which comprises the following components by contents (weight percentages): 1% to 100% of insulin freeze-dry powder with self emulsifying effect and 0% to 99% of carrier. In the insulin nasal dry powder inhalation, the dosage of grease is determined according to the surface area and the grain size of grease drops, and a large quantity of animal experiments prove that under the condition and in the proportion, the optimal drug treatment effect can be achieved. Compared with the liquid preparation, the stability of the dry powder is increased, and the dry powder can be automatically re-dissolved into nano-sized emulsion after being in contact with water; after the drug-containing compound enters the nasal cavity, the nano-size emulsion easily passes by the barrier of the nasal mucosa and enters the body via the rich capillaries and lymphatic vessels in the nasal mucosa to exert the efficacy, thereby remarkably improving the bioavailability of the drug and being rapidly absorbed, without stimulation to the nasal mucosa; in addition, the adoption of bio-adhesive increases the retention time of the drug-containing powder on the nasal mucosa, so that the absorption and the utilization of the drug are more complete.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Dry powder inhaler and system for drug delivery
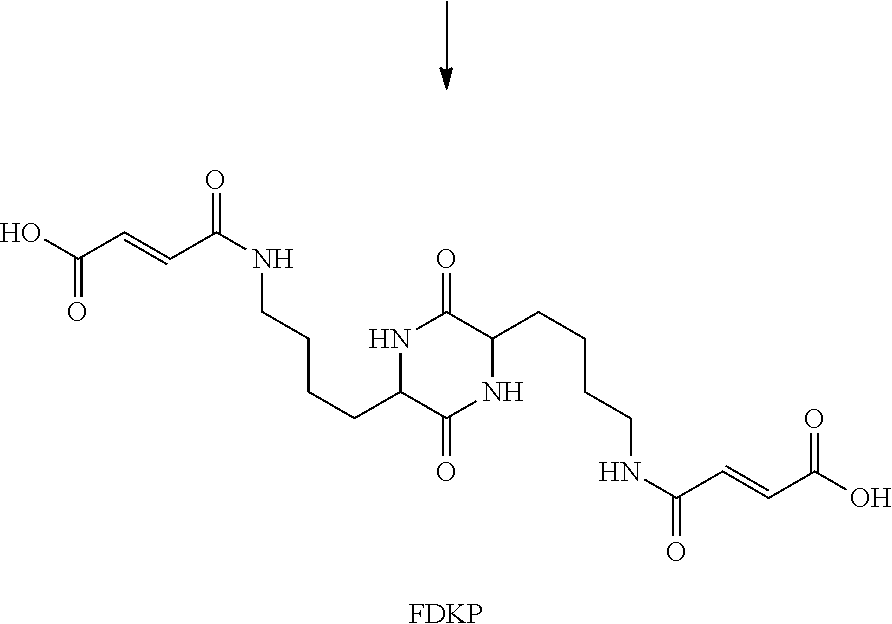
ActiveUS20160228659A1Maximize deagglomerationPowder deliveryOrganic active ingredientsDiketopiperazinesGlucagon-like peptide-1
A breath-powered, dry powder inhaler, a cartridge, and a pulmonary drug delivery system are provided. The dry powder inhaler can be provided with or without a unit dose cartridge for using with the inhaler. The inhaler and / or cartridge can be provided with a drug delivery formulation comprising, for example, a diketopiperazine and an active ingredient, including, peptides and proteins such as insulin and glucagon-like peptide 1 for the treatment of diabetes and / or obesity. The dry powder inhaler is compact; can be provided in various shapes and sizes, colors, and comprises a housing, a mouthpiece, a cartridge placement area, and a mechanism for opening and closing the medicament cartridge. The device is easy to manufacture, provides a pre-metered single unit dose, it is relatively easy to use, and can be reusable or disposable.
Owner:MANNKIND CORP
Pharmaceutical composition for dry powder inhalation and preparation method of composition
ActiveCN105412049AAvoid stimulationReduce surface adsorptionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsLACTOSE MONOHYDRATEMagnesium stearate
The invention provides a pharmaceutical composition for a dry powder inhalation and a preparation method of the composition. The composition comprises a coating agent, lactose monohydrate for a carrier and micronized active pharmaceutical ingredients, wherein the coating agent is inhaled magnesium stearate or a combination of the inhaled magnesium stearate and micronized lactose monohydrate. The pharmaceutical composition for the dry powder inhalation and the preparation method of the composition have the advantages that the inhaled magnesium stearate with specific particle size characteristics or the combination of the inhaled magnesium stearate and the micronized lactose monohydrate serves as the coating agent to be applied to the composition for the dry powder inhalation, stimulus to the lung caused by deposition of magnesium stearate on the lung or other side effects can be avoided, in-vitro determining parameters of the active ingredients can be increased, fine particle proportion of the active ingredients can be increased, and the hydrothermal stability of the product can be improved.
Owner:SICHUAN HAISCO PHARMA CO LTD
Dry powder inhalation device for the simultaneous administration of more than one medicament
InactiveUS20090250056A1Enables the adjustment of the inhaler's resistanceLower resistanceRespiratorsSmall article dispensingAdditive ingredientBlisters
The present invention relates to a dry powder inhalation device which is suitable for the simultaneous administration of a combination of pharmaceutical ingredients, wherein each pharmaceutical ingredient is packed in a separate blister of the same single dose blister strip. The medicaments that form the combination come into contact just before their exit from the mouthpiece of the device.
Owner:PENTAFRAGAS DIMITRIOS
Dry powder inhalation device
Owner:CONCENTRX PHARMA
An interactive apparatus and method for real-time profiling of inhalation efforts
ActiveCN102065942AMonitor Mechanical IntegrityLarge flow distributionRespiratorsPharmaceutical containersEnvironmental healthDisplay device
Described herein are interactive apparatus and methods for sensing and measuring real-time characteristic patterns of a subject's use of a dry powder inhalation system. The devices can be used in a wired or wireless communication mode to communicate with a display to assess the subject's usage of the inhalation system, to evaluate the performance of the inhalation system and / or to detect the characteristics profile of a dry powder formulation emitted from the inhalation system in use.
Owner:MANNKIND CORP
Dry powder inhaler system
The present invention relates to a pharmaceutical composition for inhalation, consisting in a combination of (A) a dry powder formulation containing a micronized active ingredient, alone or mixed with an inactive ingredient, said powder being filled in a hydroxypropylmethylcellulose (HPMC) capsule and (B) a single dose dry powder inhaler device especially adapted to said capsule to provide a high respiratory dose of said active ingredient when said drug is inhaled by the mouth through said device. Said device being characterized in that he is equipped with piercing needles or pins (in order to pierce the capsule) of diameter of not less than 0.8 mm preferably not less than 1 mm.
Owner:GALEPHAR PHARMA RES
Dry powder inhaler with constituent rubescensin A as well as preparation method and application thereof
InactiveCN102058566APromote absorptionNo first pass effectPowder deliveryOrganic active ingredientsOral medicationAdditive ingredient
The invention relates to a dry powder inhaler with a constituent rubescensin A as well as a preparation method and application thereof. The dry powder inhaler is a medicinal composition formed by mixing the constituent rubescensin A as an active constituent with pharmaceutically acceptable excipients. Compared with orally administrated medicines, the dry powder inhaler developed by using the technical scheme provided by the invention greatly improves the effect of the constituent rubescensin A on treating esophagus cancers, lung tumors and leukemia.
Owner:CHINA PHARM UNIV
Particles for inhalation having rapid release properties
InactiveUS20080227690A1High releaseReduce amount of timePowder deliveryOrganic active ingredientsCitrate sodiumActive agent
The invention generally relates to formulations having particles comprising phospholipids, bioactive agent and excipients and the pulmonary delivery thereof. Dry powder inhaled insulin formulations are disclosed. Formulations comprising DPPC, insulin and sodium citrate which are useful in the treatment of diabetes are disclosed. Also, the invention relates to a method of for the pulmonary delivery of a bioactive agent comprising administering to the respiratory tract of a patient in need of treatment, or diagnosis an effective amount of particles comprising a bioactive agent or any combination thereof in association, wherein release of the agent from the administered particles occurs in a rapid fashion.
Owner:CIVITAS THERAPEUTICS
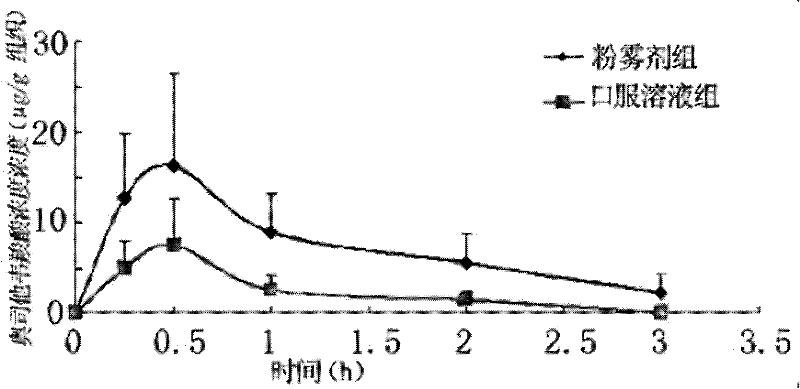
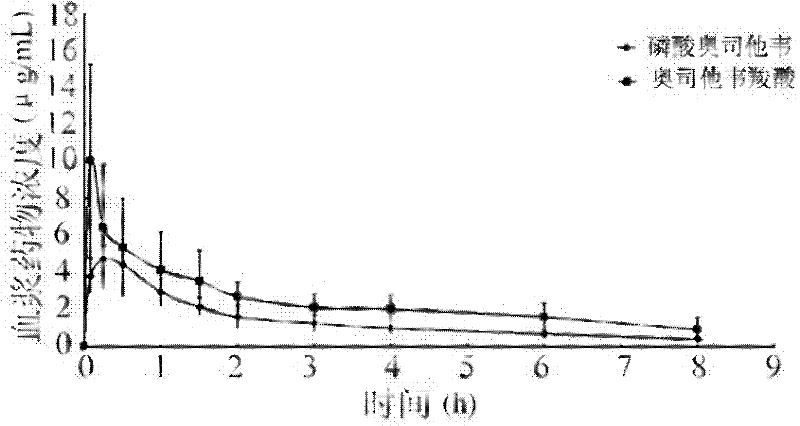
Oseltamivir phosphate dry powder inhalations and preparation method thereof
The invention relates to the field of pharmaceutical preparations, and particularly relates to oseltamivir phosphate dry powder inhalations for treating influenza A and influenza B and a preparation method thereof. The oseltamivir phosphate dry powder inhalations are characterized in that the inhalations comprise oseltamivir phosphate and a co-atomizing agent in a weight ratio of (8:2)-(6:4). The oseltamivir phosphate dry powder inhalations provided by the invention have a simple preparation method, good fluidity, and good stability. After administration, the medicine can be metabolized to metabolites with anti-influenza virus activity, which can take an antibacterial effect and be used for preventing and treating influenza A and influenza B.
Owner:CHINA PHARM UNIV
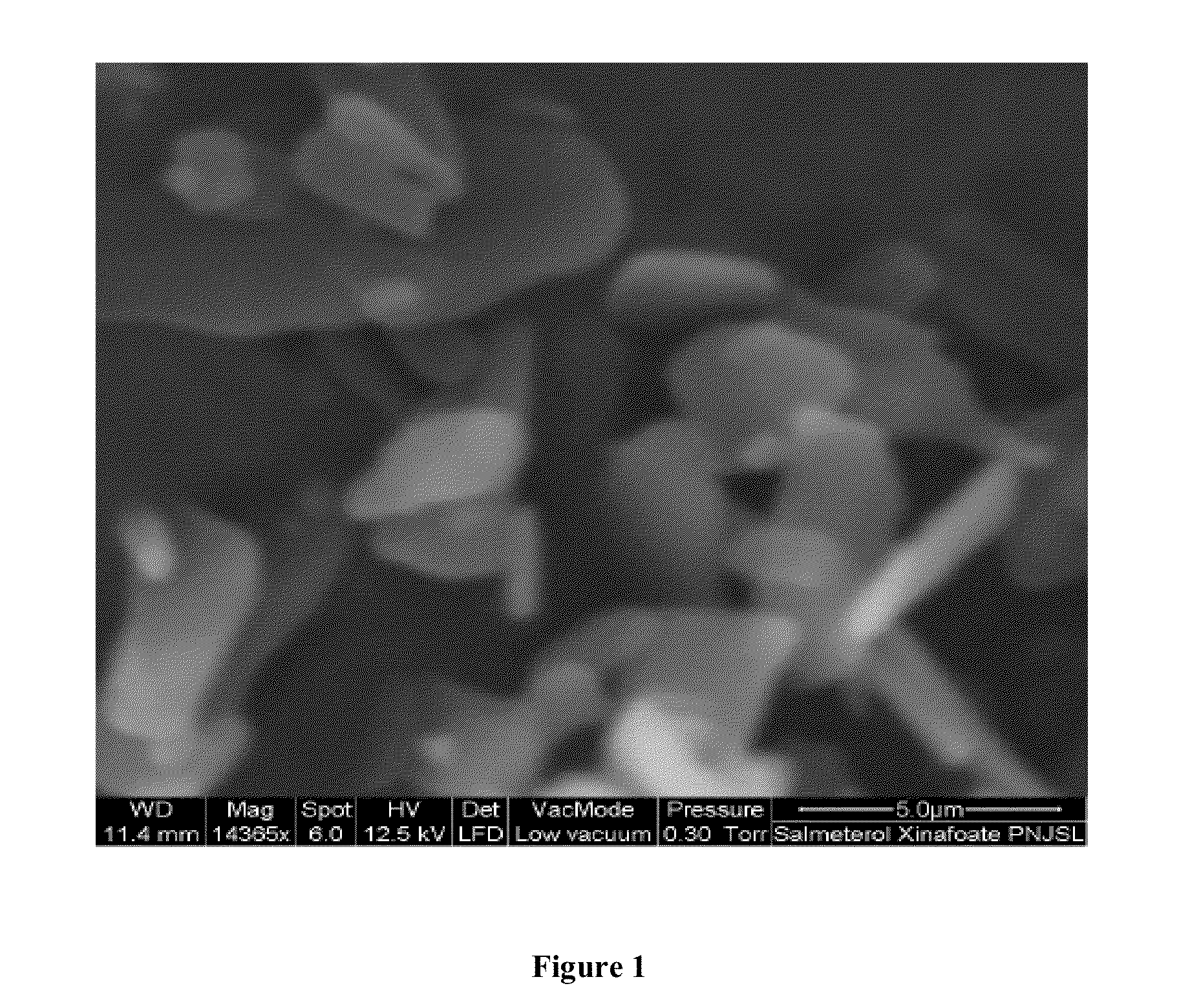
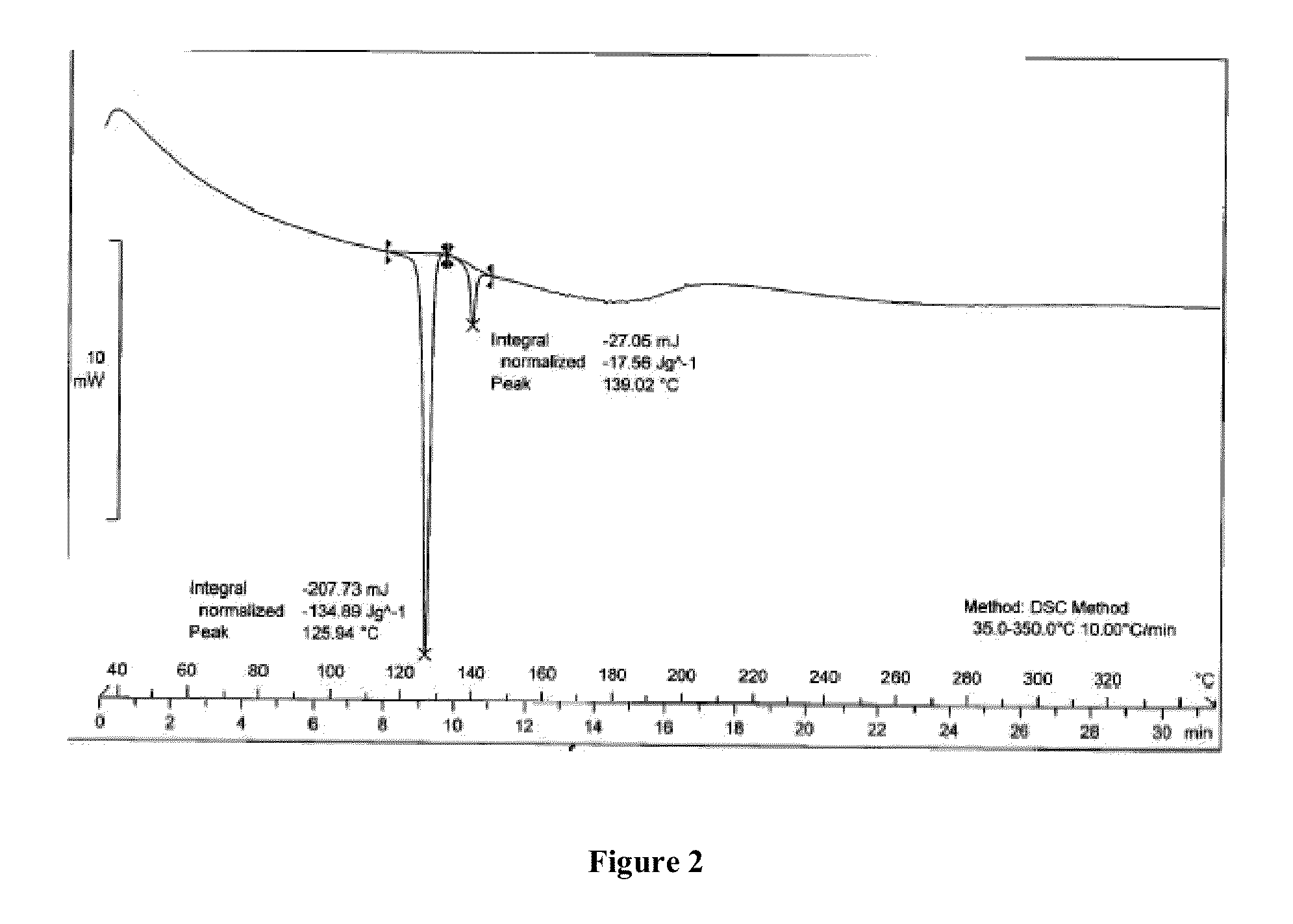
Dry powder inhalation composition
InactiveUS20130064870A1Predictable dissolution patternGood curative effectBiocideOrganic active ingredientsPhysiologyBULK ACTIVE INGREDIENT
The dry powder inhalation composition comprising(1) salmeterol xinafoate having mean particle size in range of 2.0μ-6μ microns and a tapped density in the range of 0.20 g·cm−3 to 0.45 g·cm−3 and(2) optionally, one or more other active ingredientsand pharmaceutically acceptable carrier.
Owner:SUN PHARMA INDS
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Method of preparing dry powder inhalation compositions
InactiveUS20090264389A1Even doseSmall proportionPowder deliveryOrganic active ingredientsParticulatesDry powder inhalation
The invention provides a method of preparing a dry powder inhalation composition comprising a pharmaceutically acceptable particulate carrier, a first particulate inhalant medicament and a second particulate inhalant medicament. Also provided are dry powder compositions and methods of using them with a dry powder inhalation device.
Owner:NORTON HEALTHCARE
Application of dry curcumin nano-powder inhalant in treatment of acute lung injury
The invention discloses an application of a dry curcumin nano-powder inhalant in treatment of acute lung injury. The dry curcumin nano-powder inhalant is prepared from a curcumin nanometer administration system. The dosage form of the curcumin nanometer administration system is selected from a liposome, a nano-particle, a nano-emulsion and a micro-emulsion. The curcumin nanometer administration system with or without auxiliary materials is dried to form a solid powder, and the solid powder is mixed with a carrier to obtain the dry curcumin nano-powder inhalant. The dry curcumin nano-powder inhalant has the advantages of stable drugs, easy entrance of the deep portion of lung tissues, targeted positioning, carrying and use convenience, and benefiting for the treatment of the acute lung injury. The dry curcumin nano-powder inhalant is used for treating acute lung injury caused by infection, shock, smoking, wounds, poisoning, irritating gas inhalation, radiation, high oxygen and low oxygen. Irritating gases comprise phosgene, diphosgene, triphosgene, chlorine, nitrogen oxide, formaldehyde, dimethyl sulfate, hydrogen chloride, hydrogen bromide, hydrogen fluoride, ammonia, ozone and sulfur dioxide.
Owner:ACADEMY OF MILITARY MEDICAL SCI
Dry powder inhaler with reproducible flow resistance
A dry powder inhalation device is disclosed. In one optional aspect, the device includes a housing, a base plate, a receptacle for a medicament and a mouthpiece. The base plate is engageable with the housing to form a main space with a main air inlet. The mouthpiece includes an inner conduit connected to its outlet and is engageable with the base plate to fluidly connect the inner conduit to the receptacle. The receptacle is fluidly connected to the main space, so that upon inhalation by a user, air can be drawn through the main air inlet into the main space and onward through the receptacle into the inner conduit. The mouthpiece and the base plate form an auxiliary space with an auxiliary air inlet, wherein the auxiliary space is fluidly connected to the main space.
Owner:ALFRED E TIEFENBACHER
Inhalation compositions with high drug ratios
InactiveUS20060292083A1Easy to handleAccurate measurementPowder deliveryOrganic active ingredientsParticulatesTherapeutic treatment
The invention provides a dry powder inhalation composition comprising, at least 0.25% by weight of the composition of an active ingredient with a particle size of less than 10 microns in diameter and a pharmaceutically acceptable particulate carrier with a particle size of less than 250 microns in diameter. Also disclosed are methods for use of the compositions of the invention with dry powder inhalers for therapeutic treatments.
Owner:NORTON HEALTHCARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com