Patents
Literature
83 results about "Glycopyrronium bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glycopyrrolate is used with other drugs to treat a certain type of stomach/intestinal ulcer (peptic ulcer).
Topical glycopyrrolate formulations
ActiveUS20100276329A1Hyperhidrosis is substantially reducedEasy to carryBiocideCosmetic preparationsHydrophilic polymersHydrophobic polymer
Individually packaged topical formulations comprising about 0.25 to about 6% w / w of glycopyrrolate for the treatment of hyperhidrosis, wherein said wipe is contained within a pouch resistant to leakage. The formulations may further comprise ethanol, a buffering agent and water. In addition, the formulations may further comprise a polymer system comprising a hydrophobic polymer in combination with a hydrophilic polymer.
Owner:ROSE U
Topical glycopyrrolate product
InactiveUS7060289B2Easy to closeEasy to openOrganic active ingredientsCosmetic preparationsSide effectEndoscopic thoracic sympathectomy
This invention relates to a convenient and safe product and method of applying glycopyrrolate topically in order to reduce excessive sweating in localized areas for those who suffer from this condition. This invention also relates to combining oral and topical delivery of glycopyrrolate to reduce excessive sweating and minimize side effects. This invention also relates to a convenient and safe product and method of applying glycopyrrolate topically to areas of compensatory sweating after endoscopic thoracic sympathectomy.
Owner:PUREPHARM
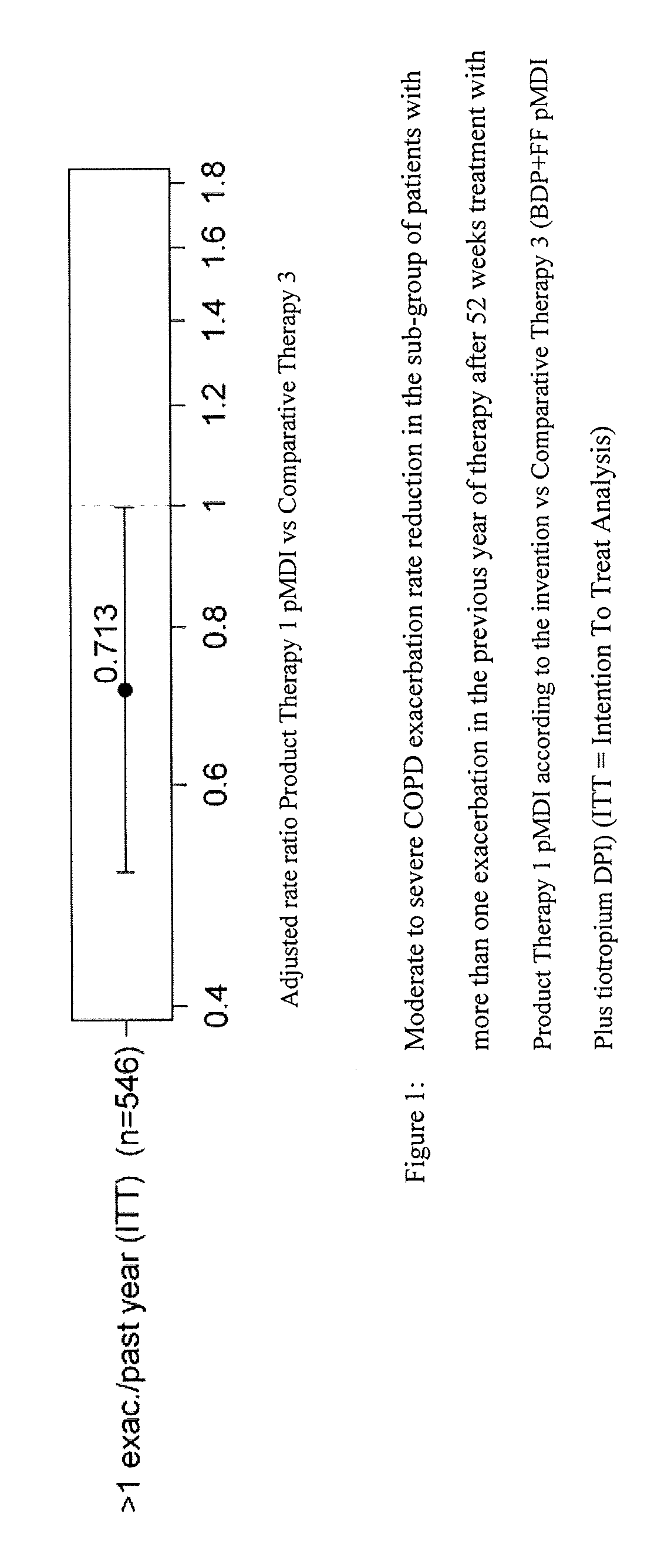
Combination therapy for COPD
InactiveUS20110150782A1Reduce usageUseful therapyDispersion deliveryAerosol deliveryObstructive Pulmonary DiseasesBeclometasone dipropionate
Aerosol formulations comprising glycopyrronium bromide in combination with formoterol are useful for the prevention or treatment of chronic obstructive pulmonary disease. The formulation further comprises a HFA propellant, a co-solvent, and an amount of inorganic acid sufficient to stabilize both the glycopyrronium bromide and the formoterol components. Optionally the formulation may further comprise beclometasone dipropionate.
Owner:CHIESI FARM SPA
Glycopyrrolate in cosmetic preparations
Glycopyrronium bromide, derivatives and / or isomers thereof in combination with one or more active substances selected from a list of substances as recited in the claims and / or in the form of a W / Si emulsion, an O / W gel, a soap gel stick, and / or a surfactant-containing cleansing formula, and corresponding cosmetic preparations, in particular deodorant / antiperspirant preparations.
Owner:BEIERSDORF AG
Stable pressurised aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof or a solvate of said salt, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in a can internally coated by a resin comprising a fluorinated ethylene propylene (FEP) polymer.
Owner:CHIESI FARM SPA
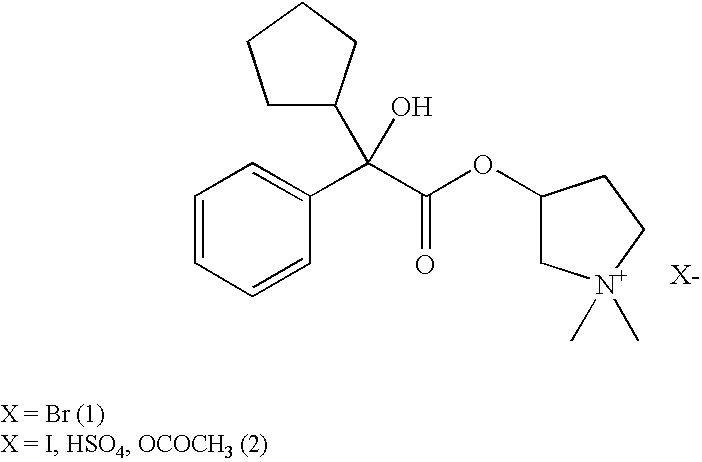
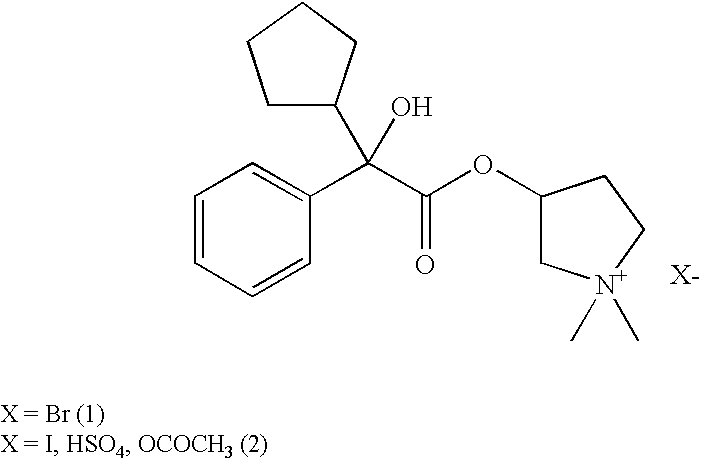
Glycopyrronium salts and their therapeutic use
A glycopyrronium salt such as glycopyrronium iodide has a lower glass transition temperature than glycopyrronium bromide. It is therefore more suitable for formulation.
Owner:HEPTARES THERAPEUTICS
Crystallisation and Purification of Glycopyrronium Bromide
ActiveUS20080227988A1High purityReduce cooling rateOrganic chemistryDigestive systemEnantiomerDiastereomer
A method for the production of crystalline glycopyrronium bromide, comprises the reaction of glycopyrronium base with methyl bromide in a solvent, in which the solvent is selected such that the diastereoisomeric ratio of the product favours the R, S and S, R diastereoisomers over the R, R, and S, S diastereoisomers, and separating the desired diastereoisomers by one or more controlled crystallisation steps. This method gives a product having a particle size of narrow distribution.
Owner:HEPTARES THERAPEUTICS
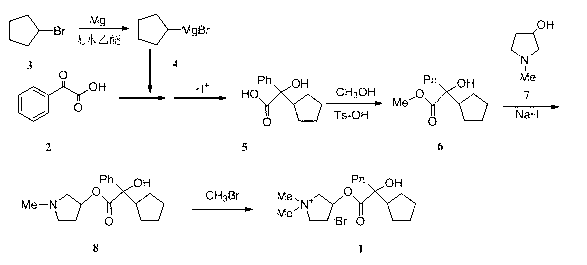
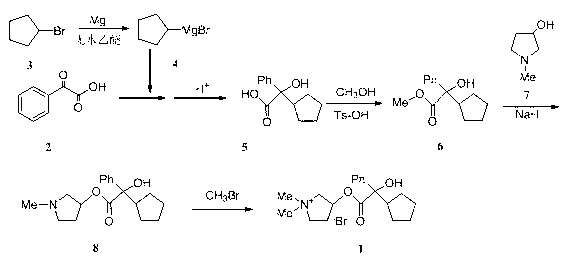
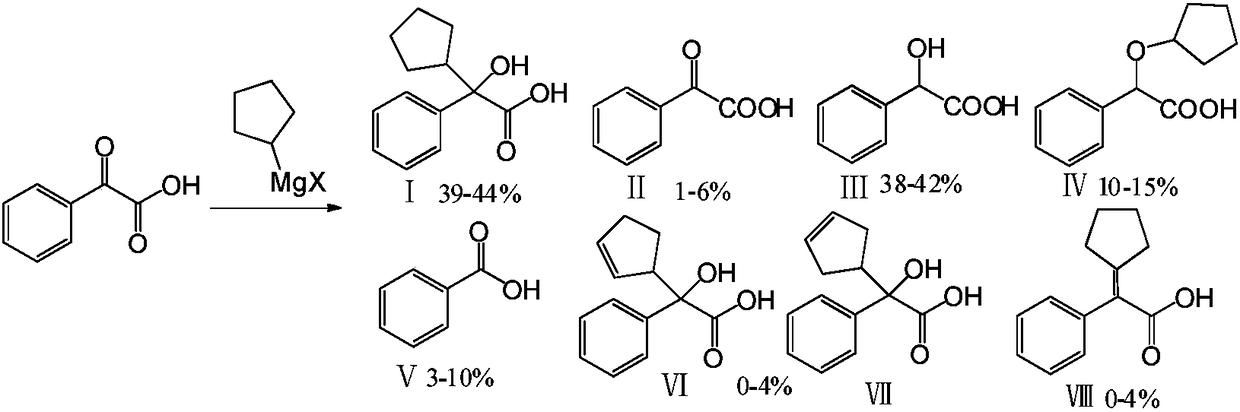
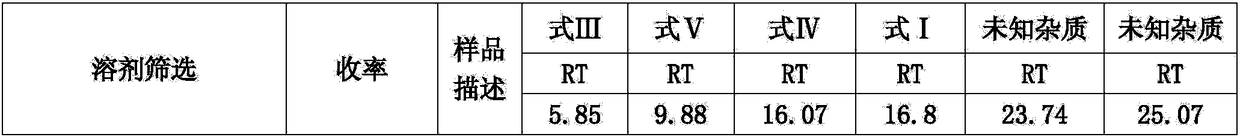
Preparation method and application of glycopyrronium bromide chiral antipode
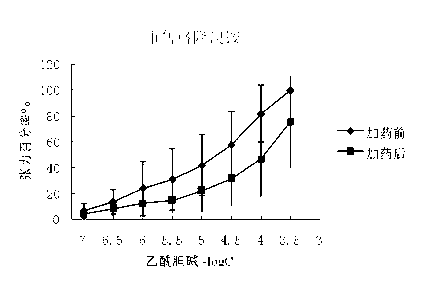
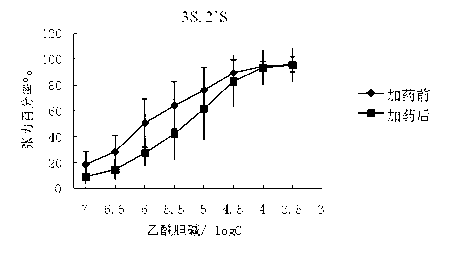
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
Stable pressurised aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in an aerosol can provided with a metering valve having at least a butyl rubber gasket.
Owner:CHIESI FARM SPA
Aerosol formulation for COPD
Stable aerosol solution formulations comprising glycopyrronium bromide are useful for administration to patients with COPD and other respiratory conditions.
Owner:CHIESI FARM SPA
Combination therapy for COPD
ActiveUS20150328144A1Dispersion deliveryAerosol deliveryObstructive Pulmonary DiseasesCombination therapy
Owner:CHIESI FARM SPA
Glycopyrronium Salts and Their Therapeutic Use
A glycopyrronium salt such as glycopyrronium iodide has a lower glass transition temperature than glycopyrronium bromide. It is therefore more suitable for formulation.
Owner:HEPTARES THERAPEUTICS
Topical glycopyrrolate product
InactiveUS20030211134A1Reduce sweatingReduced strengthOrganic active ingredientsCosmetic preparationsSide effectEndoscopic thoracic sympathectomy
This invention relates to a convenient and safe product and method of applying glycopyrrolate topically in order to reduce excessive sweating in localized areas for those who suffer from this condition. This invention also relates to combining oral and topical delivery of glycopyrrolate to reduce excessive sweating and minimize side effects. This invention also relates to a convenient and safe product and method of applying glycopyrrolate topically to areas of compensatory sweating after endoscopic thoracic sympathectomy.
Owner:PUREPHARM
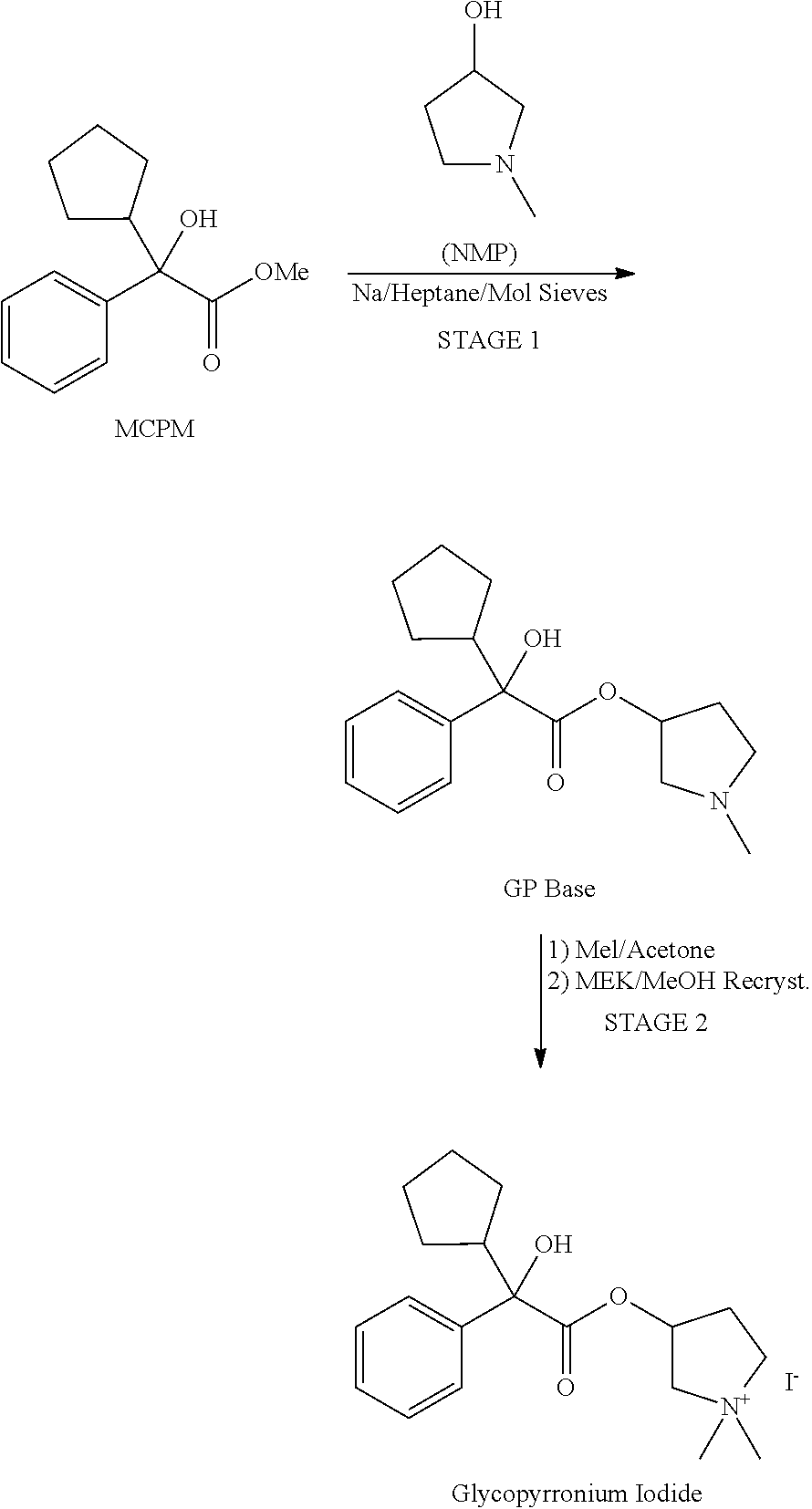
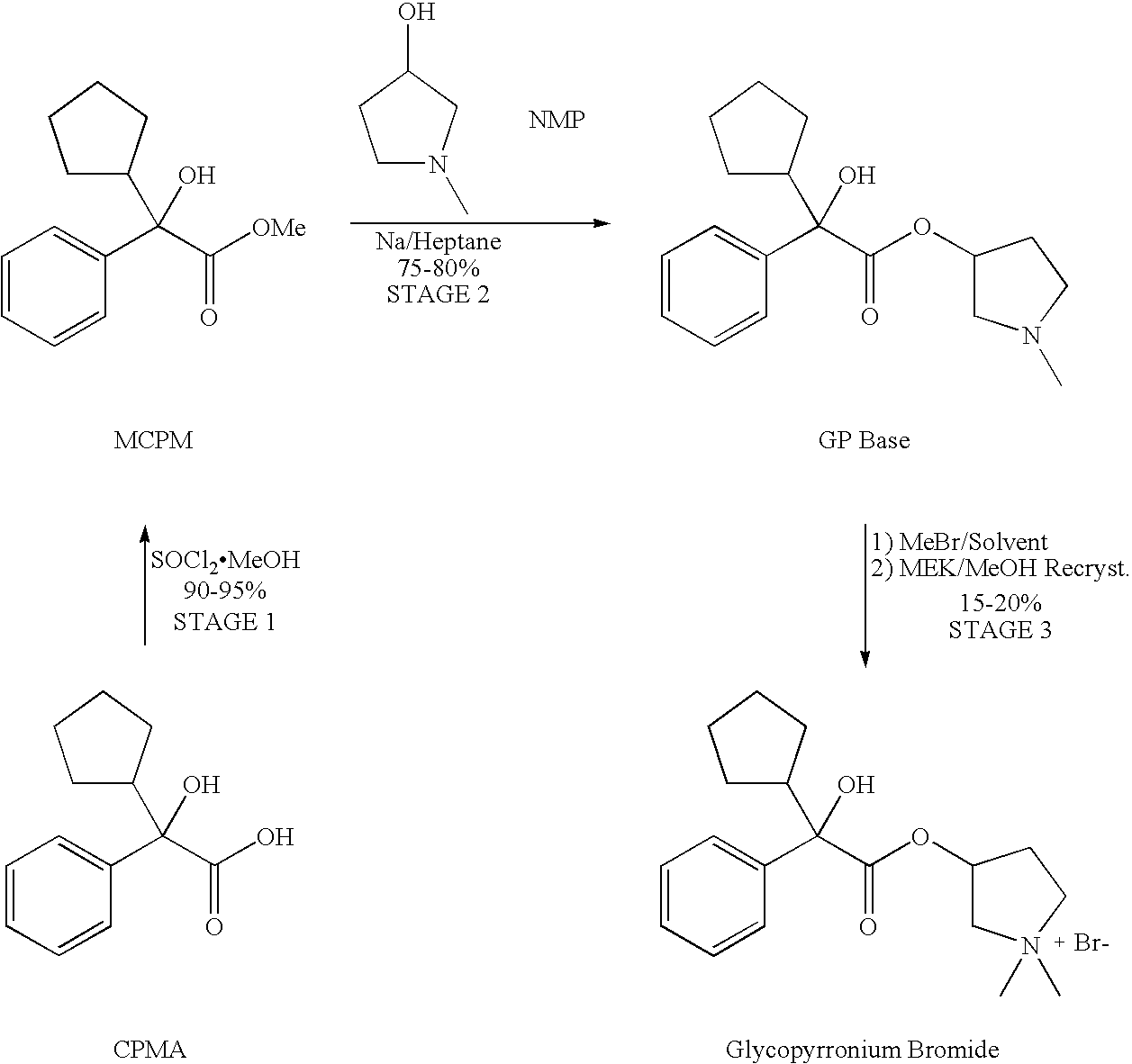
Preparation method of muscarinic receptor antagonist glycopyrronium bromide
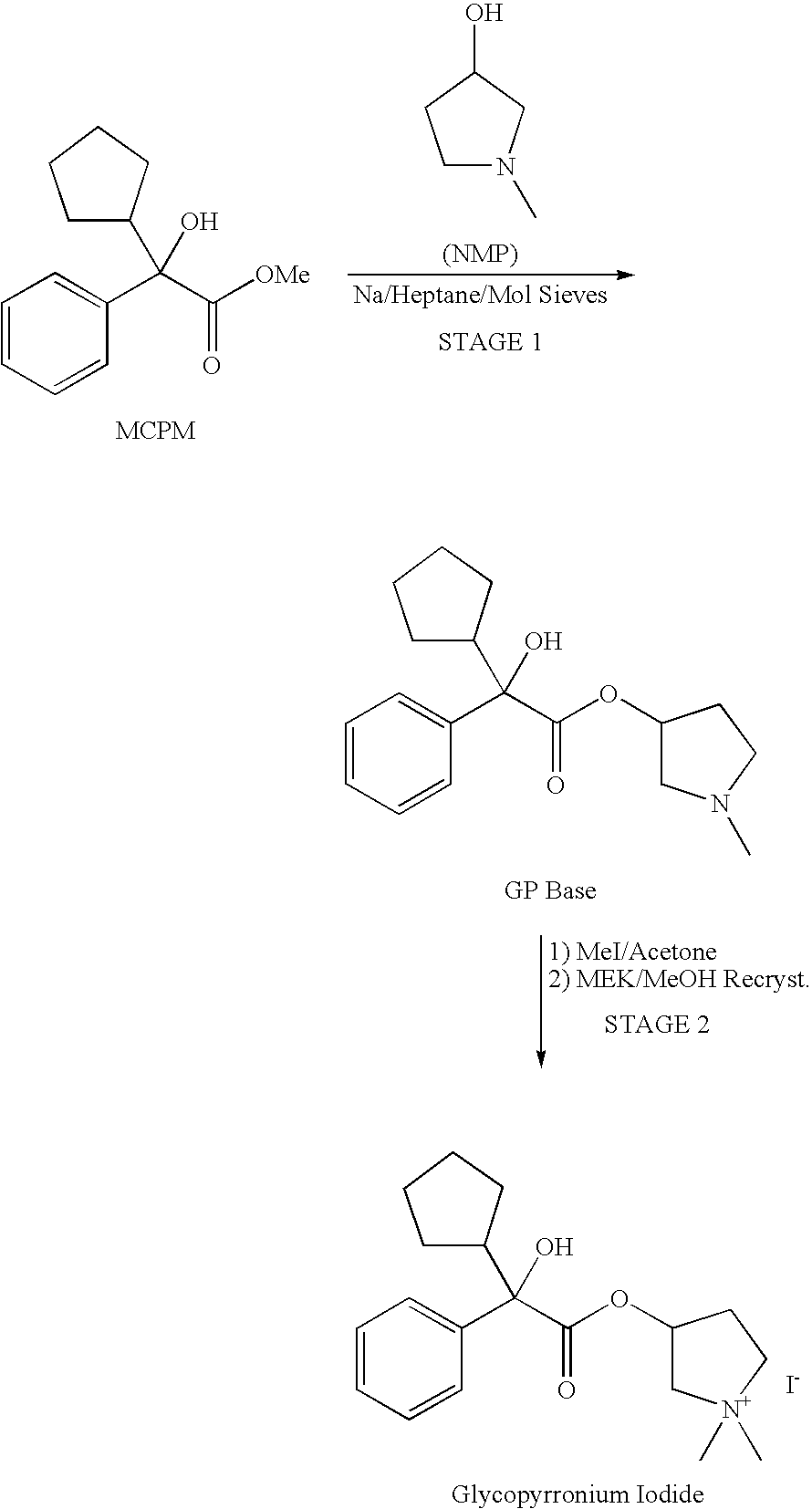
The invention belongs to the technical field of medicine and discloses a preparation method of muscarinic receptor antagonist glycopyrronium bromide. The preparation method of the muscarinic receptor antagonist glycopyrronium bromide is characterized in that Alpha-cyclopentyl mandelic acid methyl ester is obtained by aceptophenone ketonic acid Grignard reaction and esterification reaction, and then glycopyrronium bromide is obtained by N-methyl pyrrolidine-3-alcohol ester exchange and quaterisation.
Owner:SHENYANG PHARMA UNIVERSITY +1
Dry powder inhalation medicine composition and preparation method thereof
InactiveCN105982880AAvoid stimulationImproving In Vitro Assay ParametersPowder deliveryPharmaceutical non-active ingredientsIndacaterolAdditive ingredient
The invention provides a dry powder inhalation medicine composition and a preparation method thereof. The composition is prepared from a coating agent with a specific particle size characteristic, lactose monohydrate with a specific particle size characteristic for a carrier and a micronized medicinal active ingredient, wherein the coating agent is an inhaling magnesium stearate or a mixture of the inhaling magnesium stearate and micronized lactose monohydrate; and the medicinal active ingredient is selected from at least one of glycopyrronium bromide, umeclidinium, indacaterol, formoterol, vilanterol, fluticasone and pharmaceutically available salt of the active ingredients. The preparation method comprises the following steps of sufficiently mixing and coating the coating agent and the lactose monohydrate, and uniformly mixing with the micronized medicinal active ingredients.
Owner:SICHUAN HAISCO PHARMA CO LTD
Aerosol formulation for COPD
InactiveUS20110150783A1Prevention and therapyReduce usageDispersion deliveryAerosol deliveryGlycopyrronium bromidePharmacology
Stable aerosol solution formulations comprising glycopyrronium bromide are useful for administration to patients with COPD and other respiratory conditions.
Owner:CHIESI FARM SPA
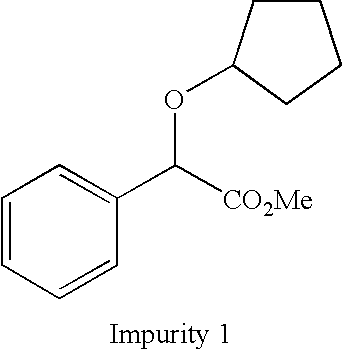
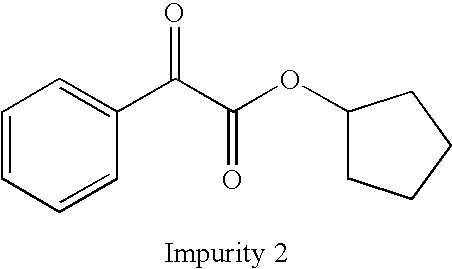
Method for preparation of glycopyrronium bromide
InactiveCN102627595AReduce pollutionRaw materials are easy to getOrganic chemistryChemical synthesisState of art
The invention belongs to the field of chemical synthesis, relates to a method for preparation of glycopyrronium bromide, and especially relates to enriched high-purity (3R, 2'S,) and (3S, 2'R,) glycopyrronium bromide and a preparation method of a novel intermediate involved in the synthesis method of glycopyrronium bromide. The method provided by the invention overcomes the defect that the prior art has a low yield and can produce large pollution, can realize preparation of glycopyrronium bromide shown in the formula I, has a high yield, produces low environmental pollution and is convenient for purification. Glycopyrronium bromide obtained by the method has a melting point of 195 to 198 DEG C.
Owner:CUREGEN JIANGSU PHARMA
Stable pressurized aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof or a solvate of said salt, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in a can internally coated by a resin comprising a fluorinated ethylene propylene (FEP) polymer.
Owner:CHIESI FARM SPA
Atomizing agent with arformoterol and glycopyrronium bromide serving as active components and preparation method of fogging agent
ActiveCN107233311ANo need to diluteRealize nebulized drug deliveryPowder deliverySpray deliveryActive componentBULK ACTIVE INGREDIENT
The invention relates to an atomizing agent with arformoterol and glycopyrronium bromide serving as active components and a preparation method of the atomizing agent. The invention further relates to an application of glycopyrronium bromide in improving the stability of an arformoterol water solution. Arformoterol and glycopyrronium bromide can perform a synergistic function pharmacologically while glycopyrronium bromide is used for improving the stability of arformoterol, and accordingly, the local treatment function on the lung is improved.
Owner:CF PHARMTECH
Stable pressurized aerosol solution composition of glycopyrronium bromide and formoterol combination
Aerosol solution compositions intended for use with a pressurized metered dose inhaler, comprising glycopyrronium bromide and formoterol, or a salt thereof, optionally in combination with one or more additional active ingredients, and stabilized by a selected amount of a mineral acid, exhibit improved stability when contained in an aerosol can provided with a metering valve having at least a butyl rubber gasket.
Owner:CHIESI FARM SPA
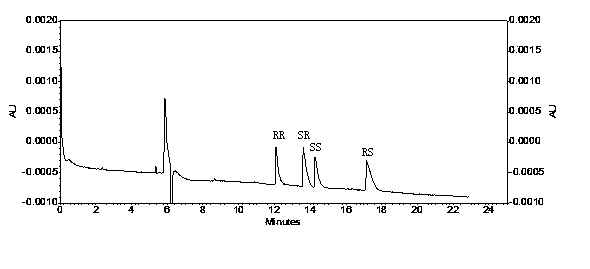
Method for separating glycopyrronium bromide enantiomers through capillary electrophoresis technique, and inspecting impurities of enantiomers
InactiveCN104280446AReduce dosageSatisfied with the separation effectMaterial analysis by electric/magnetic meansCapillary electrophoresisEnantiomer
The invention relates to a method for separating glycopyrronium bromide enantiomers through a capillary electrophoresis technique, and inspecting impurities of the enantiomers. The glycopyrronium bromide enantiomers are separated through capillary electrophoresis, the positions of the peaks of four glycopyrronium bromide enantiomer monomers are confirmed, and the limit of enantiomers in glycopyrronium bromide is calculated according to an impurity control technique; and water is used to prepare all tested samples, a capillary electrophoresis buffer solution is a sodium dihydrogen phosphate buffer solution, and sulfonated-beta-cyclodextrin (S-beta-C-D) is added to the above buffer salt. The method adopting the capillary electrophoresis technique to separate glycopyrronium bromide has the advantages of good separation effect, low amount of the samples, no pollution and low cost, and is suitable for separating racemic glycopyrronium bromide.
Owner:LIAONING YAOLIAN PHARMA
Solution-type metered dose inhalation aerosol for treating respiratory diseases and preparation method thereof
InactiveCN103784401AEasy to expandGood against airway inflammationAerosol deliveryRespiratory disorderDiseaseAerosol drugs
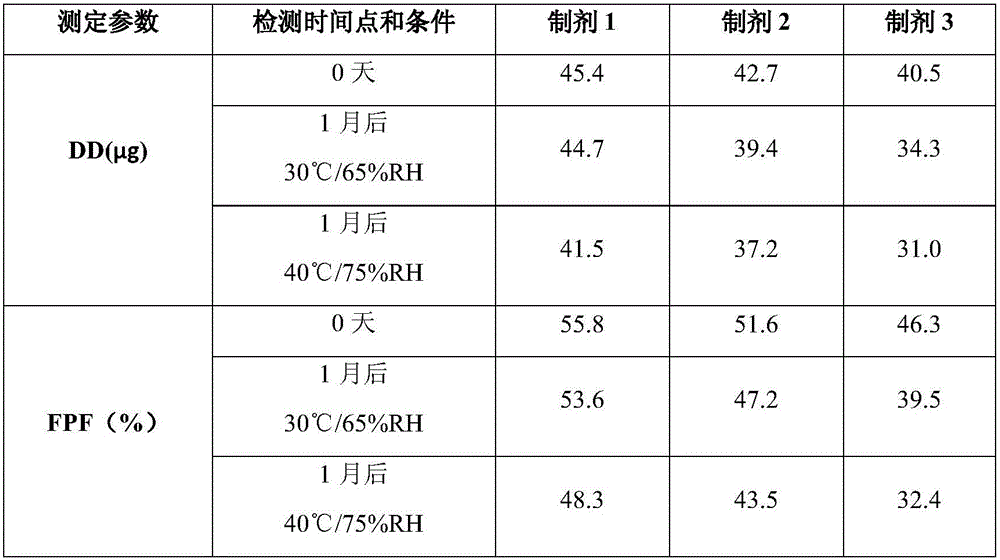
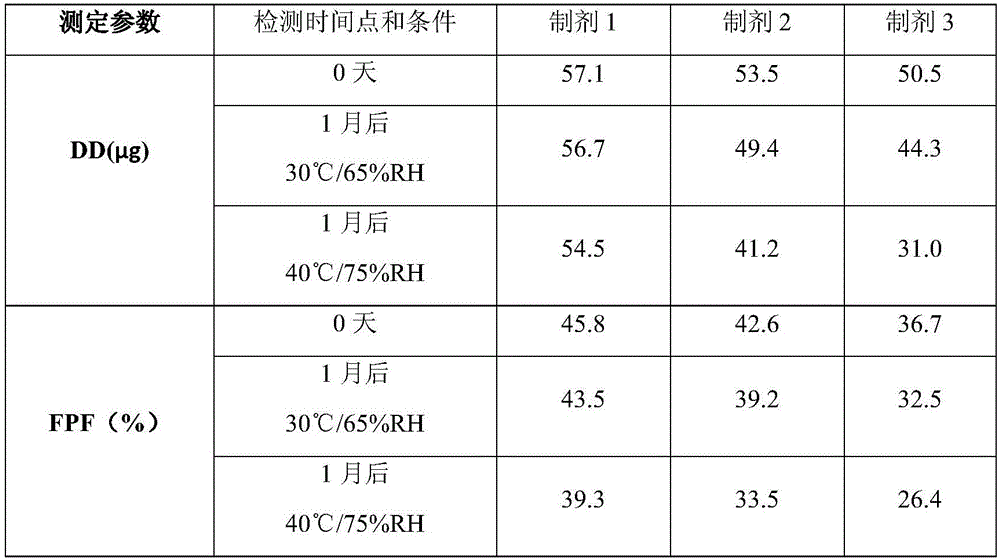
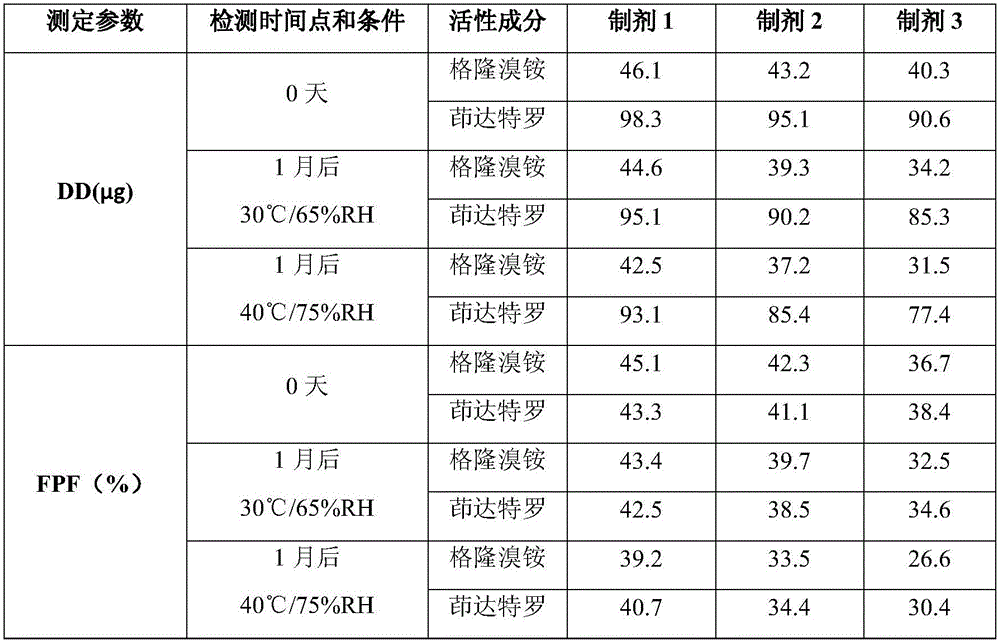
The invention discloses a solution type metered dose inhalation aerosol of glycopyrronium bromide or derivatives thereof and a preparation method thereof. The aerosol comprises a main drug, a cosolvent, a surfactant and a propellant. The aerosol is high in fine particle fraction (FPF) and good in placement stability under long-term reserving and accelerating conditions, and the FPF does not have remarkable variation. Pharmacodynamic test results indicate that the aerosol is quick in response, can be used for treating bronchial asthma and chronic obstructive pulmonary disease, and has excellent effects of resisting airway inflammation, airway fibrosis and airway remodeling.
Owner:BEIJING FSWELCOME TECH DEV +1
The treatment of respiratory disease
Glycopyrrate or an analogue thereof is useful for the treatment of bronchospasm or as a rescue medication.
Owner:SOSEI R&D LTD
Pharmaceutical Composition
Owner:CIPLA LTD
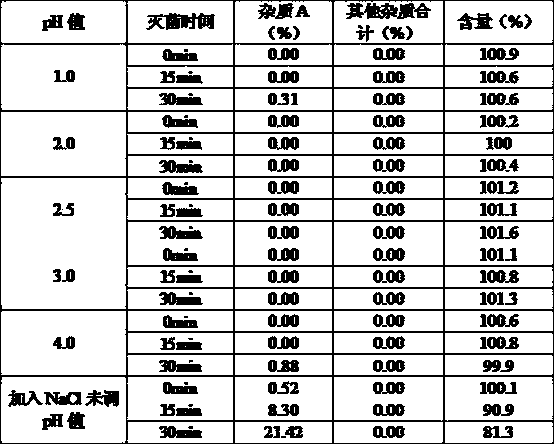
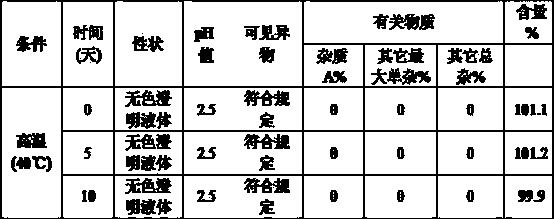
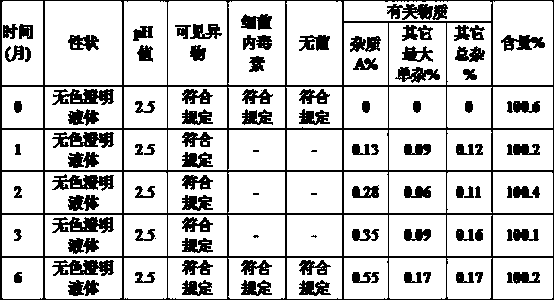
Glycopyrronium bromide injection and preparation method thereof
ActiveCN103690479AReduce heat sourceImprove efficacyDigestive systemInorganic non-active ingredientsFiltration membraneGlycopyrrolate Injection
The invention discloses glycopyrronium bromide injection and a preparation method thereof. The preparation method disclosed by the invention comprises the following steps of: (1), adding 50-90% of injection water into a preparation tank, adding sodium chloride, adding a pH adjusting agent after dissolving to adjust the pH value to 1.0-4.0, and then, diluting to total weight by adding injection water to obtain solution A; (2), adding glycopyrronium bromide into the solution A at the constant temperature of 30-40 DEG C, stirring till dissolving completely, crudely filtering in a filtrate tank through a milliporous filtration membrane which is 0.45 mu m, and finally filtering through a milliporous filtration membrane which is 0.22 mu m to obtain an intermediate product; (3), filling the filtrate tank into a neutral boron-silicon container, sealing, and sterilizing through steam at 115-121 DEG C for 8-30 min to obtain the glycopyrronium bromide injection. The glycopyrronium bromide injection disclosed by the invention has the characteristics of being good in stability, high in safety and the like.
Owner:GUANGDONG JIABO PHARM CO LTD
Combination therapy for COPD
ActiveUS10098837B2RespiratorsPowder deliverySevere chronic obstructive pulmonary diseaseBeclometasone dipropionate
Aerosol formulations comprising glycopyrronium bromide, formoterol or a salt thereof, and beclometasone dipropionate are useful for the prevention or treatment of moderate / severe chronic obstructive pulmonary disease.
Owner:CHIESI FARM SPA
Combination therapy for COPD
PendingCN109562061ARespiratorsPowder deliverySevere chronic obstructive pulmonary diseaseGlycopyrronium bromide
Aerosol formulations comprising glycopyrronium bromide, formoterol or a salt thereof, and beclometasone dipropionate are useful for the prevention or treatment of moderate / severe chronic obstructive pulmonary disease.
Owner:CHIESI FARM SPA
Combination therapy for COPD
ActiveUS20180028439A1Novel methodRespiratorsPowder deliverySevere chronic obstructive pulmonary diseaseGlycopyrronium bromide
Aerosol formulations comprising glycopyrronium bromide, formoterol or a salt thereof, and beclometasone dipropionate are useful for the prevention or treatment of moderate / severe chronic obstructive pulmonary disease.
Owner:CHIESI FARM SPA
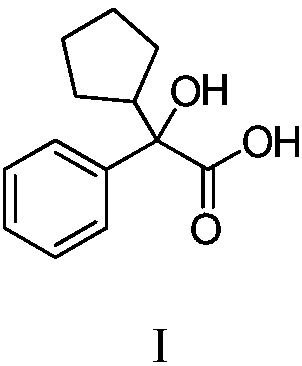
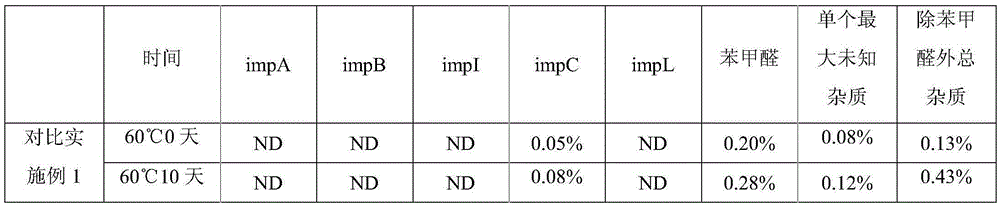
Method for purifying glycopyrronium bromide intermediate 2-cyclopentyl-2-hydroxyphenylacetic acid
InactiveCN108976114AEfficient removalEasy to removeCarboxylic compound separation/purificationSolventToluene
The invention discloses a method for purifying a glycopyrronium bromide intermediate 2-cyclopentyl-2-hydroxyphenylacetic acid. The method comprises the following steps: (1) heating and dissolving crude 2-cyclopentyl-2-hydroxyphenylacetic acid in a mixed solvent of ethanol and water, cooling, crystallizing, and filtering to obtain a solid; (2) heating and dissolving the solid obtained in the step (1), cooling, crystallizing, and filtering, thereby obtaining the solid 2-cyclopentyl-2-hydroxyphenylacetic acid. According to the method, the various impurities can be effectively removed, the productpurity is obviously improved, the purity of HPLC (High Performance Liquid Chromatography) is more than 98.0%, the loss of the 2-cyclopentyl-2-hydroxyphenylacetic acid is reduced to the greatest degree, the yield is improved, and the method is suitable for industrial production.
Owner:SHANGHAI AOBO PHARMTECH INC LTD +1
Glycopyrronium bromide injection and preparation method thereof
InactiveCN105362218AImprove product qualitySimple production processDigestive systemInorganic non-active ingredientsGlycopyrrolate InjectionBENZYL ALCOHOL/WATER
The invention discloses glycopyrronium bromide injection and a preparation method thereof. The glycopyrronium bromide injection comprises glycopyrronium bromide, a pH adjusting agent and water for injection, and no benzyl alcohol or sodium chloride is contained. The glycopyrronium bromide injection prepared through the preparation method is controllable in quality, good in stability and suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com